Glucagon-like peptide-2 (GLP-2) is a naturally
|
|
|
- Mark Rodgers
- 5 years ago
- Views:
Transcription
1 PHARMACOKINETICS Pharmacokinetics, Safety, and Tolerability of Teduglutide, a Glucagon-Like Peptide-2 (GLP-2) Analog, Following Multiple Ascending Subcutaneous Administrations in Healthy Subjects Jean-Francois Marier, PhD, FCP, Martin Beliveau, PhD, Mohamad-Samer Mouksassi, PharmD, Paula Shaw, MD, Jane Cyran, PhD, Jothi Kesavan, PhD, MBA, John Wallens, MBA, Hamim Zahir, PhD, David Wells, PhD, and John Caminis, MD Teduglutide, a glucagon-like peptide-2 (GLP-2) analog, is currently being evaluated for the treatment of short-bowel syndrome, Crohn s disease, and other gastrointestinal disorders. The pharmacokinetics, safety, and tolerability of teduglutide in healthy subjects (N = 64) were assessed following daily subcutaneous administrations for 8 days in a doubleblinded, randomized, placebo-controlled, ascending-dose study. Teduglutide treatments were administered as a 5- mg/ml (1, 15, 2, 25, 3, 5, and 8 mg) or 2-mg/mL (2 mg) formulation. Blood samples were collected on days 1 and 8, and plasma concentrations of teduglutide were measured using a liquid chromatography/tandem mass spectrometry method. Mean systemic exposures to teduglutide were very similar on days 1 and 8, suggesting minimal, if any, accumulation following once-daily repeated administrations. The apparent clearance of teduglutide following administration of the 5-mg/mL formulation was constant over the dose range, with mean values in male and female subjects of.155 and.159 L/h/kg, respectively. Peak plasma concentrations and total exposure of teduglutide after subcutaneous injection of a 2-mg/mL formulation (1. ml) were approximately 15% and 78% higher than those observed with the 5-mg/mL formulation (.4 ml), respectively. Teduglutide treatments were safe and well tolerated. All but 1 adverse event was assessed as mild or moderate in severity. No relationship between teduglutide treatments and frequency of adverse events was observed, with the exception of injection site pain, which increased as a function of dose and injected volume. Results from the current study will assist in the dose selection in future efficacy studies. Keywords: Teduglutide; multiple-ascending dose; subcutaneous; pharmacokinetics; safety and tolerability Journal of Clinical Pharmacology, 28;48: the American College of Clinical Pharmacology From Pharsight Corporation, Montreal, QC, Canada (Dr Marier, Dr Beliveau, Dr Mouksassi); Northwest Kinetics Ltd, Tacoma, Washington (Dr Shaw); and NPS Pharmaceuticals, Inc, Parsippany, New Jersey (Dr Cyran, Dr Kesavan, Mr Wallens, Dr Zahir, Dr Wells, Dr Caminis). Dr Zahir is currently at Daiichi-Sankyo, Edison, New Jersey. Dr Wells is currently at Concert Pharmaceuticals, Lexington, Massachusetts. Dr Caminis is currently at Eurand Pharmaceuticals, Yardley, Pennsylvania. Submitted for publication January 19, 28; revised version accepted May 9, 28. Address for correspondence: John L. Wallens, MBA, Director, Clinical Operations, NPS Pharmaceuticals, Bedminster, New Jersey; jwallens@npsp.com. DOI: / Glucagon-like peptide-2 (GLP-2) is a naturally occurring peptide involved in the regeneration, maintenance, and repair of the intestinal epithelium. 1,2 GLP-2 is composed of 33 amino acids and is located at the carboxyterminal end of proglucagon. Although GLP-2 activity is limited by its rapid rate of degradation via circulating dipeptidylpeptidase IV (DPP-IV) enzymes, ongoing research suggests that GLP-2 may be a promising therapeutic adjuvant for the treatment of patients with various forms of J Clin Pharmacol 28;48:
2 MARIER ET AL intestinal failure and/or compromised function. 3,4 Teduglutide (ALX-6, [gly 2 ]-hglp-2) is a synthetic analog of human GLP-2 that contains a single amino acid substitution at the second position of the N- terminus, which confers resistance to enzymatic degradation by DPP-IV. Teduglutide has been shown to elicit a number of pharmacological responses in the gastrointestinal tract, including an increase of small and large intestinal weight in mice, rats, and monkeys. 5 The bioavailability of teduglutide was evaluated following administration of a.12-mg/kg dose level administered intravenously (IV) and subcutaneously (SC) in the abdomen in 12 healthy subjects (Protocol No. CLO6-6). 6 The bioavailability of teduglutide after SC administration was 87.1%, and the mean terminal elimination half-life was 3.2 hours. In another phase I study, the relative bioavailability of an SC injection of 1 mg teduglutide in the thigh and arm, relative to the abdomen, was evaluated in 16 healthy subjects. Relative bioavailability of teduglutide was 86.5% and 89.2%, respectively, for thigh and arm sites relative to abdomen injection. 7 Teduglutide is currently evaluated for the treatment of short-bowel syndrome (SBS) and other gastrointestinal disorders. Short-bowel syndrome typically occurs when there is less than 2 cm of functioning small bowel and manifests as a collection of signs and symptoms such as malabsorption, diarrhea, steatorrhea, fluid and electrolyte disturbances, and malnutrition. Short-bowel syndrome usually results from surgical resection of bowel secondary to Crohn s disease, mesenteric vascular complications, trauma, extensive aganglionosis, or as a congenital condition in infants born with intestinal atresia. Overall, data from ongoing clinical trials suggest that teduglutide may have the ability to enhance the intestinal absorptive capacity in patients with SBS. 5,8 Based on clinical studies performed in SBS patients, the efficacy and safety of teduglutide was demonstrated following daily administrations of doses up to.15 mg/kg/day. 8 Teduglutide is also being evaluated for the management of Crohn s disease, a chronic inflammatory bowel disorder characterized by patchy granulomatous inflammation of any part of the gastrointestinal tract and associated with chronic morbidity. Crohn s disease has a prevalence of about.1% in many developed countries 9 and is thought to result from a combination of influences, including genetics, immune system function, and environmental factors. Crohn s disease is characterized by repeated episodes (remission and relapse) of intestinal mucosal inflammation and is associated with a spectrum of clinical complications such as fissure, fistulae, strictures, and abscesses, even with optimal treatment. Despite intense investigation, the exact etiology of Crohn s disease remains unknown. Crohn s disease is generally managed by a variety of pharmacological agents, including infliximab, azathioprine, 6-mercaptopurine, methotrexate, and corticosteroids. 1 Present therapeutic options for Crohn s disease with both anti-inflammatory and immunosuppressive drugs are limited and may be associated with severe side effects. 11 The current study was designed to determine the pharmacokinetics, safety, and tolerability of teduglutide following multiple subcutaneous administrations in a double-blinded, randomized, placebo- controlled, ascending-dose study. In addition, teduglutide was administered as 2- and 5-mg/mL formulations to evaluate whether this affected its pharmacokinetics, safety, and tolerability profile. The teduglutide dose range started at the highest previously investigated clinical dose up to a maximum of 8 mg, which was considered the highest feasible dose given volume constraints. In nonhuman primate toxicity studies, no organ toxicity was observed up to doses of 25 mg/kg/day. MATERIALS AND METHODS Study Design and Treatments This was a single-center, double-blinded, randomized, placebo-controlled study. Separate cohorts of healthy subjects were administered ascending doses of teduglutide or placebo as once-daily subcutaneous injections in the abdomen over 8 days. In each cohort, 12 subjects were randomized to either active (9) or placebo (3) treatment. Teduglutide (ALX-6, [gly 2 ]-hglp-2) doses and formulations used in each cohort are presented in Table I. The injection site was rotated over the 4 quadrants of the abdomen, and each injection site was used twice. For subjects receiving the 8-mg dose, 2 separate 4-mg injections (considered to be 1 dose) were administered almost simultaneously, within the same quadrant of the abdomen 3 to 4 cm apart from one another. The second injection was administered medial and central to the initial injection. Placebo consisted of the same excipient solution as the teduglutide treatment. Placebo for subcutaneous injection was provided as a lyophilized powder containing L-histidine, mannitol, and monobasic and dibasic sodium phosphate. The injection volume of placebo was the same as that for teduglutide for a specific cohort and was administered subcutaneously. 129 J Clin Pharmacol 28;48:
3 PHARMACOKINETICS, SAFETY, AND TOLERABILITY OF TEDUGLUTIDE Table I Teduglutide Dose and Formulation Used in Each Treatment Group Teduglutide, Formulation, Volume Per Cohort mg/dose mg/ml Injection, ml A B (2 ) a a Each 8-mg dose was administered as 2 injections of.8 ml each administered 3 to 4 cm apart within a single quadrant of the abdomen. Subjects arrived at check-in (day 1) to the clinic the evening prior to the drug administration (at least 12 hours prior to dosing on day 1) and were confined at the clinic for approximately 8.5 days. Following admission on day 1, clinical and laboratory assessments were performed, and subjects meeting all eligibility criteria were dosed on day 1. The original study protocol and subject informed consent forms (ICFs) were approved in writing by an institutional review board/independent ethics committee (IRB/IEC) before the enrollment of any subjects (Aspire IRB, La Mesa, California). Prior to the initiation of the next scheduled cohort(s), safety and tolerability data were reviewed and assessed by an independent safety review panel (ISRP). The study was performed in accordance with International Conference on Harmonization (ICH) good clinical practice (GCP) guideline ICH E6 (May 1, 1996) and applicable local regulatory requirements. The regulatory authorities and/or IRB/IECs were informed of amendments to the protocol, any serious adverse events (SAEs), and any other information arising during the course of the study that was deemed to change the risk or safety of the subjects involved in the study. Inclusion and Exclusion Criteria This study enrolled 95 subjects (69 men and 26 women) who were randomized into 8 cohorts. Subjects who met all of the following criteria were enrolled in this study: adult men and women (not pregnant or nursing) who were between 2 and 55 years of age and had a body mass index of 18 to 35, were able to understand and voluntarily willing to sign an ICF, and were willing and able to be confined at the clinical research center for 8.5 days; women who were postmenopausal, surgically sterilized, or of childbearing potential (WOCBP) who were nonlactating and agreed to use an effective form of birth control for at least 3 days prior to study screening, during the study, and at least 3 days after the treatment period; and medically healthy volunteers with normal or clinically insignificant clinical results (laboratory profiles, medical histories, electrocardiograms [ECGs], and physical examination) at screening (days 28 to 2) and check-in (day 1). Subjects who had any of the following during the screening visit were not eligible for enrollment in this study: (1) donated 1 pint or more of blood or blood products within 56 days prior to the study and/or had a plasma donation within 7 days prior to the study; (2) was pregnant or became pregnant; (3) participated in any other investigational drug trial within 3 days prior to study entry and until study completion; (4) had a physical examination/medical history that indicated a clinical condition or concurrent illness; (5) had a history or presence of clinically significant cardiovascular, pulmonary, hepatic, renal, hematological, gastrointestinal, endocrine, immunologic, dermatologic, neurological, or psychiatric disease; (6) had history or evidence of congenital nonhemolytic hyperbilirubinemia; (7) had history or evidence of gallstone disease or stomach or intestinal surgery, except that appendectomy was allowed; (8) had evidence of colorectal cancer; (9) had a history or evidence of malabsorption, pancreatic disease, gastrointestinal polyps, or gastrointestinal disorders such as irritable bowel syndrome, Crohn s disease, or ulcerative colitis; and (1) had a history or evidence of skin rashes or dermatitis. With the exception of oral contraception, a subject was excluded if taking prescription or over-thecounter medication during the 7 days preceding or during the study. Safety and Tolerability Safety data included treatment-emergent adverse events (AEs), injection site reactions, clinical laboratory assessments, vital signs, ECGs, and physical exams. All AEs were coded using MedDRA and summarized by system-organ-class and by highlevel term and preferred term for each treatment group. Treatment-emergent AEs were defined as AEs whose onset occurs, severity worsens, or intensity increases after receiving double-blind study drug. An interim safety review was conducted by the ISRP PHARMACOKINETICS 1291
4 MARIER ET AL for each completed cohort. Alternate and repeat doses were selected versus those described in the original dosing scheme (up to a maximum of 8 mg of teduglutide) based on a recommendation by the ISRP and approval by the sponsor in conjunction with the principal investigator. Treatment-emergent signs and symptoms (TESSs) were defined as AEs whose onset occurs, severity worsens, or intensity increases after receiving the study medication. Any AE with a start date equal to the date of dosing, where the time of the AE cannot definitively place the start of the AE prior to the time of first dosing, was considered treatment emergent. In accordance with the Declaration of Helsinki, subjects were free to discontinue the study at any time for any or for no reason and without prejudice to further treatment. Adverse events that were not resolved at the end of treatment were to be followed until resolution or until the AE was judged by the investigator to be stabilized. The sponsor and/or designee was notified prior to premature discontinuation of a subject. The occurrence of any of the following events was to necessitate premature discontinuation of a subject from the study: (1) occurrence of an SAE thought to be related to study drug and not alleviated by symptomatic treatment, (2) development of intolerable AEs as evaluated by the investigator, (3) death of the subject, (4) unwillingness to continue in the clinical study, (5) lack of subject compliance with the protocol, (6) investigator decision (investigator must have conferred with the sponsor and/or designee prior to the subject s discontinuation), (7) decision to stop the study or dosing cohort for administrative reasons or in conjunction with the ISRP, and (8) subjects who received study drug and were then prematurely withdrawn from the clinical study were not to be replaced. During the course of dosing, if a subject s aspartate aminotransferase (AST) and/or alanine aminotransferase (ALT) value(s) was 2.5 times the upper limit of normal (ULN), irrespective of the subject s treatment group, the subject was to receive matching placebo for the rest of the dosing period, and values were monitored until return to normal. Subjects remained confined at the site for the scheduled dosing period and remain blinded to the treatment. Blood Sampling and Analytical Assay Blood samples for pharmacokinetic (PK) analysis of teduglutide were collected at hours (within 3 minutes prior to dosing) and at.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 6, 8, 1, 12, and 24 hours after dosing on days 1 and 8. Blood samples were collected in 6-mL-draw, green-top, plastic Vacutainer Hemogard evacuated collection tubes (containing sodium heparin). Following centrifugation, plasma aliquots were transferred into 2 appropriately labeled storage tubes and frozen at 8 C pending analytical assay. Plasma concentrations of teduglutide were assayed using a liquid chromatography/tandem mass spectrometry (LC/MS/MS) assay with an analytical range of 1. to 12 ng/ml (SCIEX API 4, Concord, Canada). Briefly, an aliquot of human plasma containing the internal standard (ALX-6-IS) was extracted using an automated protein precipitation procedure. Multiple-charged positive ions (4+) were monitored in the multiple-reaction monitoring (MRM) model. The following ion transition m/z was monitored for teduglutide and the internal standard: and , respectively. Quantitation was by peak ratio. Inter- and intrabatch precision and accuracy were assessed at low, medium, and high quality control (QC) concentration level (3., 3., and 9. ng/ml, respectively). Interbatch precision (coefficient of variation [CV%]) results were less than 5.%, and accuracy (percent theoretical) results ranged between 97.8% and 17.7%. Intrabatch precision (CV%) results were less than 4.4%, and accuracy (percent theoretical) results ranged between 12.% and 19.%. The lower limit of quantitation (LOQ) of the assay was 1. ng/ml. Actual concentration values exceeding the upper range of the analytical curve were diluted with blank plasma to demonstrate the precision and accuracy of the assay following dilution. A QC sample of 5 ng/ml was quantified following the application of an appropriate dilution factor. The intrabatch precision (CV%) result of the 5-ng/mL QC sample was 6.5%, and accuracy (percent theoretical) was 14.4%. Pharmacokinetics and Statistics The following PK parameters were calculated using noncompartmental methods (WinNonlin Version 5.2, Pharsight Corporation, Mountain View, California): the area under the curve from time to the last measurable concentration (AUC -t ) using the linear trapezoidal rule, the area under the curve extrapolated to infinity (AUC -t + C last /kel, where C last is the last measurable plasma concentration), the maximum plasma concentration (C max ), and the time to maximum plasma concentration (t max ), the terminal rate constant of elimination (k el ), and terminal elimination half-life (t 1/2 ). 12 Teduglutide concentration values below the LOQ of the assay were set to J Clin Pharmacol 28;48:
5 PHARMACOKINETICS, SAFETY, AND TOLERABILITY OF TEDUGLUTIDE In addition, the apparent clearance (CL/F) was calculated as dose/auc - and adjusted for body weight of subjects. Dose proportionality of PK parameters of teduglutide was assessed using a power model. 13 A statistical linear relationship between ln-transformed pharmacokinetic parameters AUC -t, and C max on days 1 and 8 and the ln-transformed dose was fitted. As a first step, the statistical significance of a dose-by-day interaction was verified. Second, the statistical linear relationship between the lntransformed parameters and ln-transformed dose was verified by including cubic and quadratic effects, respectively. The statistical linear relationship was concluded if the quadratic and cubic terms were not statistically significant or if the effects were statistically significant. If the statistical linear relationship was established, 95% confidence intervals for the slope of ln-transformed parameters were to be calculated. Dose proportionality was established if a statistical linear relationship was demonstrated and if the 95% confidence intervals for these parameters include the value of 1. The above assessments were performed using the SAS Mixed procedure. Analyses of variance (ANOVAs) were used to compare ln-transformed AUC -t, and C max of teduglutide between cohorts B1 and B2 (2-mg doses in 2- or 5-mg/mL formulations) on days 1 and The ANOVA model included formulation and day (1 and 8) as a fixed effect and subject as a random effect. The ANOVA included calculation of least squares means (LSMs), the difference between regimen LSMs, and the standard error associated with this difference. Statistical analyses were performed using the SAS Mixed procedure. Ratios of LSMs (cohort B1/B2) were calculated using the exponentiation of the LSM from the analyses on the ln-transformed AUC -t, and C max of teduglutide. Ninety percent confidence intervals for the ratios were derived from the ANOVA. RESULTS Demographics For subjects randomized to placebo and teduglutide treatments, mean (± SD) values for age (34.4 ± 1.6 and 32.1 ± 9.75 years, respectively), weight (8.3 ± 17. and 82. ± 15.2 kg, respectively), height (173.8 ± 7.1 and ± 1.8 cm, respectively), and body mass index (BMI; 26.5 ± 4.95 and 27.5 ± 5.28 kg/m 2, respectively) were similar. A similar percentage of male and female subjects received placebo (29.2% vs 26.8%, respectively) and teduglutide treatments (7.8% vs 73.2%, respectively). Overall, demographic characteristics of subjects in the placebo and teduglutide groups were comparable. Pharmacokinetics Mean plasma concentration-time profiles of teduglutide following multiple ascending subcutaneous administrations of the 5-mg/mL formulation (male and female subjects combined) on days 1 and 8 are presented in Figure 1. Overall, the pharmacokinetics of teduglutide were well characterized on both days because peak plasma concentrations were more than 2-fold higher than the LOQ of the assay. Peak plasma concentrations of teduglutide were observed at approximately 4 hours after drug administration on days 1 and 8. Mean predose plasma concentrations of teduglutide on day 8 were very low, suggesting minimal accumulation of the drug following repeated subcutaneous administrations. Descriptive statistics of PK parameters of teduglutide following administration of the 5- mg/ml formulations (combined gender) on days 1 and 8 are presented in Tables II and III, respectively. Mean AUC -t, and C max values of teduglutide were very similar on days 1 and 8, confirming that minimal accumulation of the drug occurs following repeated once-daily SC administrations. Teduglutide was readily absorbed and then eliminated rapidly, with mean elimination half-life values ranging from 3.17 to 5.53 hours on day 1 and from 2.99 to 4.63 on day 8. These results suggest that the PK of teduglutide is linear because its elimination half-life remained very similar after single and multiple subcutaneous administrations across all dose ranges. Overall, mean CL/F values remained relatively constant over the dose range studied on days 1 and 8. As a result, CL/F values in men and women were pooled across cohorts to assess any gender-related trend. Mean CL/F values in male and female subjects were.155 and.159 L/h/kg, respectively, suggesting no gender-related difference in the apparent clearance of teduglutide. Dose proportionality was evaluated using a power model. 13 Pharmacokinetic parameters (AUC -t, and C max ) on days 1 and 8 were pooled in the analysis because the dose-by-day interaction term was not statistically significant. A linear relationship best described the relationship between dose and PK parameters (AUC -t, and C max ) because cubic and quadratic terms were not statistically significant. Slope estimates (95%) for AUC -last and AUC - PHARMACOKINETICS 1293
6 Table II Arithmetic Mean (± SD) Pharmacokinetic Parameters Following Subcutaneous Administration of Teduglutide in Healthy Subjects on Day 1 Cohort 1 Cohort 2 Cohort 3A Cohort 4 Cohort 5 Cohort 6 Cohort 7 1 mg qd 15 mg qd 2 mg qd 25 mg qd 3 mg qd 5 mg qd 8 mg qd Parameters (n = 9) (n = 9) (n = 9) (n = 9) (n = 9) (n = 9) (n = 8) AUC -t, ng h/ml 774 (128) 1172 (444.4) 1429 (162.2) 1658 (31.) 2189 (628.2) 4166 (864.6) 5113 (2.1) AUC -, ng h/ml 857 (146.1) 1423 (483.2) 1528 (213.2) 1784 (653.1) 2634 (1295.3) 4526 (12.3) 577 (972.2) C max, ng/ml 11 (38.1) 175 (89.3) 152 (54.5) 162 (51.3) 25 (169.3) 456 (96.5) 562 (355.1) t max, h a (3.-6.) ( ) (3.-12.) ( ) (3.-8.) (2.5-8.) (3.-12.) t 1/2, h 3.2 (1.3) 3.17 (1.774) 3.38 (2.31) 3.6 (1.7) 5.53 (6.97) 3.33 (1.28) 5.41(4.992) CL/F, L/h/kg.151 (.167).158 (.355).157 (.255).162 (.264).164 (.486).158 (.153).165 (.363) a Median (range). Table III Arithmetic Mean (± SD) Pharmacokinetic Parameters Following Subcutaneous Administration of Teduglutide in Healthy Subjects on Day 8 Cohort 1 Cohort 2 Cohort 3A Cohort 4 Cohort 5 Cohort 6 Cohort 7 1 mg qd 15 mg qd 2 mg qd 25 mg qd 3 mg qd 5 mg qd 8 mg qd Parameters (n = 8) (n = 8) (n = 9) (n = 9) (n = 8) (n = 8) (n = 8) AUC -t, ng h/ml 74 (144.7) 1135 (565.8) 1418 (288) 1529 (29.3) 2266 (679.3) 457 (987.8) 588 (129) AUC -, ng h/ml 863 (14.1) 1289 (663.1) 179 (364) 1924 (928.7) 2433 (651.4) 4316 (894.8) 5377 (91) C max, ng/ml 14 (36.2) 186 (15.4) 165 (57.4) 15 (47.8) 211 (11.6) 454 (156.6) 555 (329.8) t max, h a (3.-6.) (.52-4.) (2.5-6.) (3.- 1.) ( ) (2.5-6.) (3.- 8.) t 1/2, h 3.4 (1.535) 2.99 (1.147) 4.5 (2.513) 4.63 (2.681) 4.61 (1.93) 3.25 (1.753) 4.49 (3.348) CL/F, L/h/kg.151 (.234).148 (.345).137 (.256).161 (.324).16 (.264).165 (.286).174 (.235) a Median (range). 1294
7 PHARMACOKINETICS, SAFETY, AND TOLERABILITY OF TEDUGLUTIDE Mean Teduglutide Plasma Concentrations (ng/ml) Mean Teduglutide Plasma Concentrations (ng/ml) Day Day 8 Time (h) Time (h) 8 mg sc qd (Cohort 7) 5 mg sc qd (Cohort 6) 3 mg sc qd (Cohort 5) 25 mg sc qd (Cohort 4) 2 mg sc qd (Cohort 3A) 15 mg sc qd (Cohort 2) 1 mg sc qd (Cohort 1) 8 mg sc qd (Cohort7) 5 mg sc qd (Cohort6) 3 mg sc qd (Cohort5) 25 mg sc qd (Cohort4) 2 mg sc qd (Cohort 3A) 15 mg sc qd (Cohort 2) 1 mg sc qd (Cohort 1) Figure 1. Mean plasma concentrations of teduglutide following subcutaneous administration of the 5-mg/mL formulations on days 1 and 8 (combined gender). were.95 ( ) and.91 ( ), respectively. Dose proportionality in the AUC -t and AUC - of teduglutide was therefore confirmed over the 1- to 8-mg dose range because the 95% confidence intervals around the slope values included the value 1. Slope estimates (95%) for C max were.8 ( ), suggesting a less than proportional increase from 1 to 8 mg because the upper confidence interval was below 1.. Following removal of the highest dose level (ie, 8 mg, the only dose level administered as 2 injections of.8 ml), dose proportionality in C max of teduglutide was confirmed, with 95% confidence intervals (.61 to 1.4) including the value 1. An ANOVA model was used to compare lntransformed PK parameters of teduglutide (AUC -t, AUC -, and C max ) after administration of 2 mg in cohort B1 (1.-mL injection of a 2-mg/mL formulation) and cohort B2 (.4-mL injection of a 5-mg/mL formulation) on days 1 and 8. Because the day-byformulation interaction term was not statistically significant for all PK parameters, relative bioavailability was assessed using pooled data from days 1 and 8. Results of the statistical analysis are presented in Table IV. Ratios of LSM for AUC -t, and C max of teduglutide (ratio: 3A/3B) were 122.6%, 115.1%, and 177.7%, with the 9% CI falling outside the 8.% to 125.% range. These results suggest that subcutaneous administration of 1. ml of a 2-mg/mL formulation of teduglutide (cohort A) resulted in a higher total exposure and peak plasma concentrations of the drug as compared with the 5- mg/ml formulation by approximately 15% and 78%, respectively (Figure 2). Safety and Tolerability Results Based on the ISRP review of safety information from cohorts 3A and 3B, the protocol was amended to investigate slightly lower and higher doses levels than 2 mg (ie, 1, 15, and 25 mg, respectively). Following a safety evaluation of these dose levels, the ISRP further allowed dosing with 8 mg. A total of 86 subjects (17 placebo; 69 teduglutide) reported a total of 49 AEs (placebo: n = 7; teduglutide: n = 339). The overall number of subjects reporting AEs and the number of AEs were distributed similarly across all treatment groups. In general, most AEs in each of the treatment groups were rated as mild or moderate in severity. A similar number of events were considered as related and not related to the study drug for all treatment groups. One subject reported a single SAE (left orbital fracture, teduglutide, cohort A) during the study follow-up period (postconfinement) that was not related to study drug. One subject (teduglutide, cohort 3A) reported 1 AE that led to treatment and study discontinuation because the subject displayed a vasovagal-related seizure prior to dosing on day 2 that was rated as moderate. This AE was considered related to the administered treatment. No other significant AEs were noted. The vast majority of AEs were considered related to the study drug across treatment groups. The most frequently reported AEs for teduglutide by preferred term were as follows: abdominal distension (29.6%), abdominal pain (16.9%), constipation (22.5%), nausea (14.1%), injection site erythema (15.5%), injection site pain (35.2%), headache (23.9%), increased ALT (18.3%), and increased AST (14.1%). Although PHARMACOKINETICS 1295
8 MARIER ET AL Table IV Comparison of Pharmacokinetic Parameters of the 2- and 5-mg/mL Formulations of Teduglutide (Combined Gender) Geometric LSM (%CV) ANOVA 3A (2 mg qd) 3B (2 mg qd) Ratio of LSM 9% CI Parameters (2 mg/ml) (5 mg/ml) (3A/3B) for Ratio P Value AUC -t, ng h/ml 1723 (15.2) 148 (15.3) AUC -, ng h/ml 1861 (13.) 1637 (19.6) C max, ng/ml 266 (36.5) 151 (32.5) ANOVA, analysis of variance; CI, confidence interval; LSM, least squares mean. Mean Teduglutide Plasma Concentrations (ng/ml) Mean Teduglutide Plasma Concentrations (ng/ml) Day Day 8 Time (h) Time (h) Cohort 3A - 2 mg sc qd (2 mg/ml) Cohort 3B- 2 mg sc qd (5 mg/ml) Cohort 3A - 2 mg sc qd (2mg/mL) Cohort 3B - 2 mg sc qd (5mg/mL) Figure 2. Mean plasma concentrations of teduglutide following subcutaneous administration of a 2-mg dose level (2- or 5- mg/ml formulations) on days 1 and 8 (combined gender). individual changes in liver function test (LFT) occurred during the study, the changes were transient in nature, and the frequency of subjects displaying changes was similar to that observed in the placebo group, and values returned to normal by the end of the study (ie, day 14). Overall, increases in LFTs were mild, with the exception of 3 subjects whose values increased by more than 2-fold. The transient nature of liver enzymes changes may be related to confinement of subjects in a clinical pharmacology unit rather than treatment with study drug. 15 All other events were reported for less than 1% of subjects in the teduglutide and placebo groups. For AEs pertaining to the gastrointestinal system and general disorders, the frequency of events recorded for the teduglutide groups was generally greater when compared with placebo. A summary figure depicting the frequency of occurrence of AEs for the ascending treatments of teduglutide is presented in Figure 3. Although no obvious relationship with increasing dose levels was observed for any body system organ class, a noticeable decrease in the frequency of AE associated with the general disorders and administration site conditions system class were observed in cohorts 3B, 4, and 5 as compared with those observed in cohorts 3A, 6, and 7. Adverse events observed in the general disorders and administration site conditions system class were mainly injection site pain and appeared to be correlated with the volume injected. A summary figure depicting the frequency of occurrence of injection site pain in each cohort as a function of injected volume is presented in Figure 4. In general, the frequency of injection site pain increased as a function of dose for the 5-mg/mL formulations and correlated with the increase in injection volume ( ml), with the exception of cohort 2, which displayed an unexpectedly high frequency of injection site pain considering the low volume injected J Clin Pharmacol 28;48:
9 PHARMACOKINETICS, SAFETY, AND TOLERABILITY OF TEDUGLUTIDE A B C Frequency of Adverse Events (%) Placebo 1 2 3A 3B Placebo 1 2 3A 3B Placebo 1 2 3A 3B Frequency of Adverse Events (%) D E F Placebo 1 2 3A 3B Placebo 1 2 3A 3B Placebo 1 2 3A 3B Figure 3. Summary of most frequent treatment-emergent adverse events by cohort and system organ class: (A) gastrointestinal disorders, (B) general disorders and administration site conditions, (C) nervous system disorders, (D) investigations, (E) musculoskeletal and connective tissue disorders, and (F) skin and subcutaneous tissue disorders. Frequency of Injection Site Pain (%) Frequency of Injection Site Pain Total Volume Injected Placebo 1 2 3A 3B Figure 4. Frequency of injection site pain in each cohort as a function of total volume injected. Although the source of injection site pain events in cohort 2 remains unknown, all events were rated as Total Volume Injected(mL) mild and did not show time-specific patterns (ie, the percentage of subjects experiencing injection site pain on days 1, 2, 3, 4, 5, 6, 7, and 8 was %, 22.2%, 33.3%, 22.2%,.%, 11.1%, 11.1%, and.%, respectively). Clinical labs, vitals, and ECGs remained relatively unchanged throughout the duration of the study, with very few values above or below normal limits and with very few clinically significant abnormal findings. DISCUSSION Crohn s disease is generally managed by a variety of pharmacological agents, including infliximab, azathioprine, 6-mercaptopurine, methotrexate, and corticosteroids. 1 Present therapeutic options for Crohn s disease with both anti-inflammatory and immunosuppressive drugs are limited and may be associated with severe side effects. 11 Infliximab, a monoclonal PHARMACOKINETICS 1297
10 MARIER ET AL antibody to tumor necrosis factor α (TNF-α), has been found to be effective in the treatment of Crohn s disease. 1 This therapy works by targeting inflammatory pathways but has also been shown to reverse the defect in intestinal permeability found in Crohn s disease patients. 11 However, about 2% of Crohn s disease patients fail to respond to this agent, and a recent report suggested there may be an increased risk of lymphoproliferative disorders in patients treated with TNF-α antagonists. 16,17 Alternatively, recent studies examining growth hormone, which stimulates intestinal growth and repair and decreases intestinal permeability, have shown efficacy in the treatment of Crohn s disease. 18 Although clinical studies in SBS patients supported effective teduglutide doses up to.15 mg/kg/day for the treatment of SBS, preliminary analysis of dose-response data in a proof-of-concept study in Crohn s patients suggests a possible need for higher doses in this population. The teduglutide dose range in this study started at the highest investigated clinical dose up to a maximum of 8 mg, which at this time is thought to be the highest dose for additional studies. At a dose of 8 mg, the margins of safety were estimated to be approximately 3.4- and 2.3-fold, based on teduglutide s exposure observed at the no observed adverse effect level (NOAEL) for nonhuman primates. The NOAELs in the nonhuman primate study were based on injection site reactions; no organ toxicity was observed. The abdomen was chosen as the primary site of injection of teduglutide because subcutaneous administration of 1 mg in the abdomen, thigh, and arm resulted in bioequivalent exposure of teduglutide. As a result, repeated subcutaneous administrations of teduglutide with doses up to 8 mg were investigated in the current study to evaluate its pharmacokinetics, safety, and tolerability in healthy volunteers. In the current study, teduglutide was readily absorbed and declined with mean t 1/2 values ranging from 2.99 to 4.63 hours over the dose range studied. Teduglutide s elimination half-life in healthy humans is in sharp contrast to the very rapid elimination of native GLP-2 by circulating DPP-IV enzymes, which was reported to be approximately 7 minutes. 19 Although providing resistance to enzymatic degradation, the single amino acid substitution of teduglutide was also shown to provide enhanced binding to the GLP-2 receptor as compared with the native GLP-2 (EC 5 of 9.2 ±.6 vs 14 ± 2.9 nmol, respectively). 2 The pharmacokinetics of teduglutide were linear across the dose range studied because apparent clearance values remained relatively constant after single and repeated administrations, and no accumulation of teduglutide was observed following repeated subcutaneous administrations. The total exposure of teduglutide increased proportionally with increasing dose levels on days 1 and 8, whereas peak plasma concentrations of teduglutide increased in a dose-proportional manner over the range of 1 to 5 mg. No gender-related difference was observed in the apparent clearance of teduglutide. Peak plasma concentrations of teduglutide following subcutaneous administration of 2 mg as a 2-mg/mL formulation (cohort 3A) were approximately 78% higher than those observed for the 5- mg/ml formulation (cohort 3B). These data indicate that a less concentrated formulation of teduglutide results in a more rapid uptake and a slightly higher overall bioavailability of the peptide than the more concentrated formulation. A decreased frequency of injection site-related AEs was observed when subjects were administered a 5-mg/mL formulation of teduglutide as compared with that observed with a 2- mg/ml formulation. Because the drug is more rapidly taken up from the injection site following administration of the 2-mg/mL formulation, the higher incidence of injection site reactions with this formulation may be due more to volume of injection than the presence of drug. Evaluations of AEs, clinical laboratory assessments, vital sign measurements, 12-lead ECGs, and physical examinations indicated that subcutaneous injections of 1 to 8 mg of teduglutide given by subcutaneous injection in the abdomen were safe and well tolerated. Overall, pharmacokinetic, safety, and tolerability results from the current study will help to determine optimal doses, formulations, and injected volume in future efficacy studies for the treatment of Crohn s disease and other gastrointestinal indications. Financial disclosure: David Wells and John Caminis are former employees of NPS and own stock in the company. REFERENCES 1. Munroe DG, Gupta AK, Kooshesh F, et al. Prototypic G proteincoupled receptor for the intestinotrophic factor glucagon-like peptide 2. Proc Natl Acad Sci USA. 1999;96: Tavares W, Drucker DJ, Brubaker PL. Enzymatic- and renaldependent catabolism of the intestinotrophic hormone glucagonlike peptide-2 in rats. Am J Physiol Endocrinol Metab. 2;278: E134-E Martin GR, Beck PL, Sigalet DL. Gut hormones, and short bowel syndrome: the enigmatic role of glucagon-like peptide-2 in the 1298 J Clin Pharmacol 28;48:
11 PHARMACOKINETICS, SAFETY, AND TOLERABILITY OF TEDUGLUTIDE regulation of intestinal adaptation. World J Gastroenterol. 26; 12: Wallis K, Walters JR, Forbes A. Review article: glucagon-like peptide 2 current applications and future directions. Aliment Pharmacol Ther. 27;25: Jeppesen PB, Sanguinetti EL, Buchman A, et al. Teduglutide (ALX-6), a dipeptidyl peptidase IV resistant glucagon-like peptide 2 analogue, improves intestinal function in short bowel syndrome patients. Gut. 25;54: PROTOCOL CLO6-6. A phase I, randomized, two-way crossover bioavailability study of.12 mg/kg ALX-6 in normal healthy subjects. September PROTOCOL CL6-15. A phase I, randomized, open-label, 3- way crossover bioavailability study of 1 mg teduglutide in healthy adults. July Ferrone M, Scolapio JS. Teduglutide for the treatment of short bowel syndrome. Ann Pharmacother. 26;4: Ferguson A. Ulcerative colitis and Crohn s disease: important and disabling diseases, still under researched. BMJ. 1994;39: Scribano ML, Prantera C. Review article: medical treatment of active Crohn s disease. Aliment Pharmacol Ther. 22;16(suppl 4): Hanauer SB, Sandborn WJ; The Practice Parameters Committee of the ACG. Management of Crohn s disease in adults. Am J Gastroenterol. 21;96: Rowland M, Tozer TN. Clinical Pharmacokinetics: Concepts and Application. 3rd ed. Baltimore: Williams & Wilkins; Smith BP, Vandenhende FR, Desante KA, et al. Confidence interval criteria for assessment of dose proportionality. Pharm Res. 2;17: Chow SC, Liu JP. Design and Analysis of Bioavailability and Bioequivalence Studies. New York: Marcel Dekker; Rosenzweig P, Miget N, Brohier S. Transaminase elevation on placebo during phase I trials: prevalence and significance. Br J Clin Pharmacol. 1999;48: Shetty A, Forbes A. Pharmacogenomics of response to antitumor necrosis factor therapy in patients with Crohn s disease. Am J Pharmacogenomics. 22;2: Brown SL, Greene MH, Gershon SK, Edwards ET, Braun MM. Tumor necrosis factor antagonist therapy and lymphoma development: twenty-six cases reported to the Food and Drug Administration. Arthritis Rheum. 22;46: Sandborn WJ, Targan SR. Biologic therapy of inflammatory bowel disease. Gastroenterology. 22;122: Hartmann B, Harr MB, Jeppesen PB, et al. In vivo and in vitro degradation of glucagon-like peptide-2 in humans. J Clin Endocrinol Metab. 2;85: DaCambra MP, Yutsa B, Sumner-Smith M, et al. Structural determinants for activity of glucagon-like peptide-2. Biochemistry. 2;39: PHARMACOKINETICS 1299
12 本文献由 学霸图书馆 - 文献云下载 收集自网络, 仅供学习交流使用 学霸图书馆 ( 是一个 整合众多图书馆数据库资源, 提供一站式文献检索和下载服务 的 24 小时在线不限 IP 图书馆 图书馆致力于便利 促进学习与科研, 提供最强文献下载服务 图书馆导航 : 图书馆首页文献云下载图书馆入口外文数据库大全疑难文献辅助工具
Supporting Information. Electrochemiluminescence for Electric-Driven Antibacterial. Therapeutics
 Supporting Information Electrochemiluminescence for Electric-Driven Antibacterial Therapeutics Shanshan Liu, a,b Huanxiang Yuan, a Haotian Bai, a Pengbo Zhang, a Fengting Lv, a Libing Liu, a Zhihui Dai,
Supporting Information Electrochemiluminescence for Electric-Driven Antibacterial Therapeutics Shanshan Liu, a,b Huanxiang Yuan, a Haotian Bai, a Pengbo Zhang, a Fengting Lv, a Libing Liu, a Zhihui Dai,
Racial disparities in the management of acne: evidence from the National Ambulatory Medical Care Survey,
 Journal of Dermatological Treatment ISSN: 0954-6634 (Print) 1471-1753 (Online) Journal homepage: http://www.tandfonline.com/loi/ijdt20 Racial disparities in the management of acne: evidence from the National
Journal of Dermatological Treatment ISSN: 0954-6634 (Print) 1471-1753 (Online) Journal homepage: http://www.tandfonline.com/loi/ijdt20 Racial disparities in the management of acne: evidence from the National
Optimization of Processing Parameters of Stabilizers After Enzymes Hydrolysis for Cloudy Ginkgo Juice
 Optimization of Processing Parameters of Stabilizers After Enzymes Hydrolysis for Cloudy Ginkgo Juice Haifeng Yu, Junyan Liu and Jingxi Yang 1 Introduction Ginkgo biloba, dating back 300 million years,
Optimization of Processing Parameters of Stabilizers After Enzymes Hydrolysis for Cloudy Ginkgo Juice Haifeng Yu, Junyan Liu and Jingxi Yang 1 Introduction Ginkgo biloba, dating back 300 million years,
Pharmacokinetics of a Novel Orodispersible Tablet of Sildenafil in Healthy Subjects
 Clinical Therapeutics/Volume 36, Number 2, 2014 Pharmacokinetics of a Novel Orodispersible Tablet of Sildenafil in Healthy Subjects Bharat Damle, PhD 1 ; Gregory Duczynski, MS 2 ; Barrett W. Jeffers, PhD
Clinical Therapeutics/Volume 36, Number 2, 2014 Pharmacokinetics of a Novel Orodispersible Tablet of Sildenafil in Healthy Subjects Bharat Damle, PhD 1 ; Gregory Duczynski, MS 2 ; Barrett W. Jeffers, PhD
Accepted Manuscript. Hemorrhagic cystitis associated with gefitinib treatment: a case report. Peng Zhang, Jinjing Tu, Tieding Chen, Rubing Li
 Accepted Manuscript Hemorrhagic cystitis associated with gefitinib treatment: a case report Peng Zhang, Jinjing Tu, Tieding Chen, Rubing Li PII: S0090-4295(18)30555-7 DOI: 10.1016/j.urology.2018.05.035
Accepted Manuscript Hemorrhagic cystitis associated with gefitinib treatment: a case report Peng Zhang, Jinjing Tu, Tieding Chen, Rubing Li PII: S0090-4295(18)30555-7 DOI: 10.1016/j.urology.2018.05.035
Fetal Response to Intramuscular Epinephrine for Anaphylaxis during Maternal Penicillin Desensitization for Secondary Syphilis
 The Journal of Maternal-Fetal & Neonatal Medicine ISSN: 1476-7058 (Print) 1476-4954 (Online) Journal homepage: http://www.tandfonline.com/loi/ijmf20 Fetal Response to Intramuscular Epinephrine for Anaphylaxis
The Journal of Maternal-Fetal & Neonatal Medicine ISSN: 1476-7058 (Print) 1476-4954 (Online) Journal homepage: http://www.tandfonline.com/loi/ijmf20 Fetal Response to Intramuscular Epinephrine for Anaphylaxis
Chapter 5 Trimalleolar Ankle Fracture: Posterior Plate for Posterior Malleolus Fractures
 Chapter 5 Trimalleolar Ankle Fracture: Posterior Plate for Posterior Malleolus Fractures Roy I. Davidovitch and Alexander M. Crespo Introduction Trimalleolar ankle fractures with a posterior malleolus
Chapter 5 Trimalleolar Ankle Fracture: Posterior Plate for Posterior Malleolus Fractures Roy I. Davidovitch and Alexander M. Crespo Introduction Trimalleolar ankle fractures with a posterior malleolus
Thinking & Reasoning Publication details, including instructions for authors and subscription information:
 This article was downloaded by: [Umeå University Library] On: 07 October 2013, At: 11:46 Publisher: Routledge Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer
This article was downloaded by: [Umeå University Library] On: 07 October 2013, At: 11:46 Publisher: Routledge Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer
ACCEPTED ARTICLE PREVIEW. Accepted manuscript
 First in Class Angiotensin Receptor Neprilysin Inhibitor in Heart Failure Orly Vardeny, PharmD, MS, Travis Tacheny, Scott D. Solomon, MD Cite this article as: Orly Vardeny, PharmD, MS, Travis Tacheny,
First in Class Angiotensin Receptor Neprilysin Inhibitor in Heart Failure Orly Vardeny, PharmD, MS, Travis Tacheny, Scott D. Solomon, MD Cite this article as: Orly Vardeny, PharmD, MS, Travis Tacheny,
Accepted Manuscript. Red yeast rice preparations: are they suitable substitutions for statins?
 Accepted Manuscript Red yeast rice preparations: are they suitable substitutions for statins? Carlos A. Dujovne, MD, Fellow NLA, Certified Clinical Lipidologist PII: S0002-9343(17)30591-0 DOI: 10.1016/j.amjmed.2017.05.013
Accepted Manuscript Red yeast rice preparations: are they suitable substitutions for statins? Carlos A. Dujovne, MD, Fellow NLA, Certified Clinical Lipidologist PII: S0002-9343(17)30591-0 DOI: 10.1016/j.amjmed.2017.05.013
Accepted Manuscript. Robotics in Orthopedics: A Brave New World. Brian S. Parsley, MD, Associate Professor
 Accepted Manuscript Robotics in Orthopedics: A Brave New World Brian S. Parsley, MD, Associate Professor PII: S0883-5403(18)30163-3 DOI: 10.1016/j.arth.2018.02.032 Reference: YARTH 56428 To appear in:
Accepted Manuscript Robotics in Orthopedics: A Brave New World Brian S. Parsley, MD, Associate Professor PII: S0883-5403(18)30163-3 DOI: 10.1016/j.arth.2018.02.032 Reference: YARTH 56428 To appear in:
SOME PRACTICAL IMPROVEMENTS IN THE CONTINUAL REASSESSMENT METHOD FOR PHASE I STUDIES
 STATISTICS IN MEDICINE, VOL. 14, 1149-1161 (1995) SOME PRACTICAL IMPROVEMENTS IN THE CONTINUAL REASSESSMENT METHOD FOR PHASE I STUDIES STEVEN N. GOODMAN, MARIANNA L. ZAHURAK AND STEVEN PIANTADOSI Johns
STATISTICS IN MEDICINE, VOL. 14, 1149-1161 (1995) SOME PRACTICAL IMPROVEMENTS IN THE CONTINUAL REASSESSMENT METHOD FOR PHASE I STUDIES STEVEN N. GOODMAN, MARIANNA L. ZAHURAK AND STEVEN PIANTADOSI Johns
Journal of Chromatography A 819 (1998)
 Journal of Chromatography A 89 (998) 33 42 Investigation of potential degradation products of a newly synthesised b-lactam antibiotic by multi-stage liquid chromatography electrospray mass spectrometry
Journal of Chromatography A 89 (998) 33 42 Investigation of potential degradation products of a newly synthesised b-lactam antibiotic by multi-stage liquid chromatography electrospray mass spectrometry
Synthetic Tannins Structure by MALDI-TOF Mass Spectroscopy
 Synthetic Tannins Structure by MALDI-TOF Mass Spectroscopy S. Giovando, 1 A. Pizzi, 2 H. Pasch, 3,4 K. Rode 4 1 Centro Ricerche per la Chimica Fine Srl, S.Michele Mondovi, Italy 2 ENSTIB-LERMAB, Nancy
Synthetic Tannins Structure by MALDI-TOF Mass Spectroscopy S. Giovando, 1 A. Pizzi, 2 H. Pasch, 3,4 K. Rode 4 1 Centro Ricerche per la Chimica Fine Srl, S.Michele Mondovi, Italy 2 ENSTIB-LERMAB, Nancy
Effects of idebenone on electroencephalograms of patients with cerebrovascular disorders
 Arch. Gerontol. Geriatr., 8 (1989) 355-366 355 Elsevier AGG 00266 Effects of idebenone on electroencephalograms of patients with cerebrovascular disorders Takashi Nakano a Matu6 Miyasaka a, Katsumi Mori
Arch. Gerontol. Geriatr., 8 (1989) 355-366 355 Elsevier AGG 00266 Effects of idebenone on electroencephalograms of patients with cerebrovascular disorders Takashi Nakano a Matu6 Miyasaka a, Katsumi Mori
uncorrected proof version
 Galley Proof 8/02/2017; 9:17 File: jcm 1-jcm708.tex; BOKCTP/ljl p. 1 Journal of Computational Methods in Sciences and Engineering -1 (2017) 1 10 1 DOI 10.3233/JCM-170708 IOS Press 1 2 3 Comparison of sliding
Galley Proof 8/02/2017; 9:17 File: jcm 1-jcm708.tex; BOKCTP/ljl p. 1 Journal of Computational Methods in Sciences and Engineering -1 (2017) 1 10 1 DOI 10.3233/JCM-170708 IOS Press 1 2 3 Comparison of sliding
Indacaterol, a once-daily beta 2 -agonist, versus twice-daily beta-agonists or placebo for chronic obstructive pulmonary disease (Protocol)
 Indacaterol, a once-daily beta 2 -agonist, versus twice-daily beta-agonists or placebo for chronic obstructive pulmonary disease (Protocol) Geake JB, Dabscheck EJ, Wood-Baker R This is a reprint of a Cochrane
Indacaterol, a once-daily beta 2 -agonist, versus twice-daily beta-agonists or placebo for chronic obstructive pulmonary disease (Protocol) Geake JB, Dabscheck EJ, Wood-Baker R This is a reprint of a Cochrane
Introduction. urinary erythropoietin, and the two are indistinguishable
 BIOPHARMACEUTICS & DRUG DISPOSITION Biopharm. Drug Dispos. 21: 211 219 (2000) DOI: 10.1002/bdd.231 Comparative Pharmacokinetics, Safety, and Tolerability After Subcutaneous Administration of Recombinant
BIOPHARMACEUTICS & DRUG DISPOSITION Biopharm. Drug Dispos. 21: 211 219 (2000) DOI: 10.1002/bdd.231 Comparative Pharmacokinetics, Safety, and Tolerability After Subcutaneous Administration of Recombinant
Efficacy, safety and impact on β
 J Endocrinol Invest (2016) 39:1061 1074 DOI 10.1007/s40618-016-0465-1 ORIGINAL ARTICLE Efficacy, safety and impact on β cell function of dipeptidyl peptidase 4 inhibitors plus metformin combination therapy
J Endocrinol Invest (2016) 39:1061 1074 DOI 10.1007/s40618-016-0465-1 ORIGINAL ARTICLE Efficacy, safety and impact on β cell function of dipeptidyl peptidase 4 inhibitors plus metformin combination therapy
Glucose-lowering activity of the dipeptidyl peptidase-4 inhibitor saxagliptin in drug-naive patients with type 2 diabetes*
 ORIGINAL ARTICLE doi: 10.1111/j.1463-1326.2008.00876.x Glucose-lowering activity of the dipeptidyl peptidase-4 inhibitor saxagliptin in drug-naive patients with type 2 diabetes* J. Rosenstock, 1 S. Sankoh
ORIGINAL ARTICLE doi: 10.1111/j.1463-1326.2008.00876.x Glucose-lowering activity of the dipeptidyl peptidase-4 inhibitor saxagliptin in drug-naive patients with type 2 diabetes* J. Rosenstock, 1 S. Sankoh
How might treatment of ALK-positive non-small cell lung cancer change in the near future?
 Expert Review of Anticancer Therapy ISSN: 1473-7140 (Print) 1744-8328 (Online) Journal homepage: http://www.tandfonline.com/loi/iery20 How might treatment of ALK-positive non-small cell lung cancer change
Expert Review of Anticancer Therapy ISSN: 1473-7140 (Print) 1744-8328 (Online) Journal homepage: http://www.tandfonline.com/loi/iery20 How might treatment of ALK-positive non-small cell lung cancer change
ORIGINAL ARTICLE ABSTRACT SUMMARY AT A GLANCE INTRODUCTION
 bs_bs_banner ORIGINAL ARTICLE Budesonide/formoterol maintenance and reliever therapy via Turbuhaler versus fixed-dose budesonide/formoterol plus terbutaline in patients with asthma: Phase III study results
bs_bs_banner ORIGINAL ARTICLE Budesonide/formoterol maintenance and reliever therapy via Turbuhaler versus fixed-dose budesonide/formoterol plus terbutaline in patients with asthma: Phase III study results
Title: Clinical and histopathological features of immunoglobulin G4-associated autoimmune hepatitis in children
 Title: Clinical and histopathological features of immunoglobulin G4-associated autoimmune hepatitis in children Short title: Immunoglobulin G4-associated autoimmune hepatitis in children Yusuf Aydemir¹,
Title: Clinical and histopathological features of immunoglobulin G4-associated autoimmune hepatitis in children Short title: Immunoglobulin G4-associated autoimmune hepatitis in children Yusuf Aydemir¹,
Journal of Chromatography B, 857 (2007)
 Journal of Chromatography B, 857 (2007) 287 295 Determination of serum uric acid using high-performance liquid chromatography (HPLC)/isotope dilution mass spectrometry (ID-MS) as a candidate reference
Journal of Chromatography B, 857 (2007) 287 295 Determination of serum uric acid using high-performance liquid chromatography (HPLC)/isotope dilution mass spectrometry (ID-MS) as a candidate reference
The role of air plethysmography in the diagnosis of chronic venous insufficiency
 The role of air plethysmography in the diagnosis of chronic venous insufficiency Enrique Criado, MD, Mark A. Farber, MD, William A. Marston, MD, Patty F. Daniel, RN, RVT, Cynthia B. Burnham, RN, RVT, and
The role of air plethysmography in the diagnosis of chronic venous insufficiency Enrique Criado, MD, Mark A. Farber, MD, William A. Marston, MD, Patty F. Daniel, RN, RVT, Cynthia B. Burnham, RN, RVT, and
164 J.A.H. an Laarho en et al. / International Journal of Pharmaceutics 232 (2002) An example of a sustained release system is a contraceptive
 International Journal of Pharmaceutics 232 (2002) 163 173 www.elsevier.com/locate/ijpharm In vitro release properties of etonogestrel and ethinyl estradiol from a contraceptive vaginal ring J.A.H. van
International Journal of Pharmaceutics 232 (2002) 163 173 www.elsevier.com/locate/ijpharm In vitro release properties of etonogestrel and ethinyl estradiol from a contraceptive vaginal ring J.A.H. van
Characterization of a prototype MR-compatible Delta4 QA-system in a 1.5 tesla MR-linac
 Physics in Medicine and Biology ACCEPTED MANUSCRIPT Characterization of a prototype MR-compatible Delta QA-system in a. tesla MR-linac To cite this article before publication: J H Wilfred de Vries et al
Physics in Medicine and Biology ACCEPTED MANUSCRIPT Characterization of a prototype MR-compatible Delta QA-system in a. tesla MR-linac To cite this article before publication: J H Wilfred de Vries et al
NON-NARCOTIC ORALLY EFFECTIVE, CENTRALLY ACTING ANALGESIC FROM AN AYURVEDIC DRUG
 Journal of Ethnopharmocology, ll(l984) 309-317 Elsevier Scientific Publishers Ireland Ltd. 309 NON-NARCOTIC ORALLY EFFECTIVE, CENTRALLY ACTING ANALGESIC FROM AN AYURVEDIC DRUG CX ATAL, M.A. SIDDIQUI, USHA
Journal of Ethnopharmocology, ll(l984) 309-317 Elsevier Scientific Publishers Ireland Ltd. 309 NON-NARCOTIC ORALLY EFFECTIVE, CENTRALLY ACTING ANALGESIC FROM AN AYURVEDIC DRUG CX ATAL, M.A. SIDDIQUI, USHA
The conundrum of hodgkin lymphoma nodes: To be or not to be included in the involved node radiation fields. The EORTC-GELA lymphoma group guidelines
 Radiotherapy and Oncology 88 (2008) 202 210 www.thegreenjournal.com Hodgkin guidelines The conundrum of hodgkin lymphoma nodes: To be or not to be included in the involved node radiation fields. The EORTC-GELA
Radiotherapy and Oncology 88 (2008) 202 210 www.thegreenjournal.com Hodgkin guidelines The conundrum of hodgkin lymphoma nodes: To be or not to be included in the involved node radiation fields. The EORTC-GELA
Accepted Manuscript. Dural arteriovenous fistula between the inferolateral trunk and cavernous sinus draining to the ophthalmic vein: a case report
 Accepted Manuscript Dural arteriovenous fistula between the inferolateral trunk and cavernous sinus draining to the ophthalmic vein: a case report Kan Xu, Kun Hou, Baofeng Xu, Yunbao Guo, Jinlu Yu PII:
Accepted Manuscript Dural arteriovenous fistula between the inferolateral trunk and cavernous sinus draining to the ophthalmic vein: a case report Kan Xu, Kun Hou, Baofeng Xu, Yunbao Guo, Jinlu Yu PII:
How Advertising Slogans
 How Advertising Slogans Can Prime Evaluations of Brand Extensions David M. Boush University of Oregon ABSTRACT Different versions of a brand slogan were presented to each of three treatment groups before
How Advertising Slogans Can Prime Evaluations of Brand Extensions David M. Boush University of Oregon ABSTRACT Different versions of a brand slogan were presented to each of three treatment groups before
Validation of ATS clinical practice guideline cut-points for FeNO in asthma
 Accepted Manuscript Validation of ATS clinical practice guideline cut-points for FeNO in asthma Maria Jeppegaard, Sandra Veidal, Asger Sverrild, Vibeke Backer, Celeste Porsbjerg PII: S0954-6111(18)30296-8
Accepted Manuscript Validation of ATS clinical practice guideline cut-points for FeNO in asthma Maria Jeppegaard, Sandra Veidal, Asger Sverrild, Vibeke Backer, Celeste Porsbjerg PII: S0954-6111(18)30296-8
Cost-Effectiveness of Adding Rh-Endostatin to First-Line Chemotherapy in Patients With Advanced Non-Small-Cell Lung Cancer in China
 Clinical Therapeutics/Volume 33, Number 10, 2011 Cost-Effectiveness of Adding Rh-Endostatin to First-Line Chemotherapy in Patients With Advanced Non-Small-Cell Lung Cancer in China Bin Wu, PhD 1, Huafeng
Clinical Therapeutics/Volume 33, Number 10, 2011 Cost-Effectiveness of Adding Rh-Endostatin to First-Line Chemotherapy in Patients With Advanced Non-Small-Cell Lung Cancer in China Bin Wu, PhD 1, Huafeng
Comparison of Carotid Artery Stenting and Carotid Endarterectomy in Patients with Symptomatic Carotid Artery Stenosis: A Single Center Study
 Adv Ther (2013) 30:845 853 DOI 10.1007/s25-013-0058-8 ORIGINAL RESEARCH Comparison of Carotid Artery Stenting and Carotid Endarterectomy in Patients with Symptomatic Carotid Artery Stenosis: A Single Center
Adv Ther (2013) 30:845 853 DOI 10.1007/s25-013-0058-8 ORIGINAL RESEARCH Comparison of Carotid Artery Stenting and Carotid Endarterectomy in Patients with Symptomatic Carotid Artery Stenosis: A Single Center
Divergent Thinking and Evaluation Skills: Do They Always Go Together?
 Journal of Creative Behavior MAGDALENA GROHMAN ZOFIA WODNIECKA MARCIN KLUSAK Divergent Thinking and Evaluation Skills: Do They Always Go Together? ABSTRACT INTRODUCTION The aim of the present study was
Journal of Creative Behavior MAGDALENA GROHMAN ZOFIA WODNIECKA MARCIN KLUSAK Divergent Thinking and Evaluation Skills: Do They Always Go Together? ABSTRACT INTRODUCTION The aim of the present study was
Effects of regular exercise on asthma control in young adults
 Journal of Asthma ISSN: 0277-0903 (Print) 1532-4303 (Online) Journal homepage: http://www.tandfonline.com/loi/ijas20 Effects of regular exercise on asthma control in young adults Dr Sirpa A.M. Heikkinen,
Journal of Asthma ISSN: 0277-0903 (Print) 1532-4303 (Online) Journal homepage: http://www.tandfonline.com/loi/ijas20 Effects of regular exercise on asthma control in young adults Dr Sirpa A.M. Heikkinen,
Author s Accepted Manuscript
 Author s Accepted Manuscript Rheumatologic symptoms in oncologic patients on PD-1 inhibitors Wilson F. Kuswanto, Lindsey A. MacFarlane, Lydia Gedmintas, Alexandra Mulloy, Toni K. Choueiri, Bonnie Bermas
Author s Accepted Manuscript Rheumatologic symptoms in oncologic patients on PD-1 inhibitors Wilson F. Kuswanto, Lindsey A. MacFarlane, Lydia Gedmintas, Alexandra Mulloy, Toni K. Choueiri, Bonnie Bermas
Effects of Angle of Approach on Cursor Movement with a Mouse: Consideration of Fitts' Law
 Pergamon Computers in Human Behavior, Vol. 12, No. 3, pp. 481-495, 1996 Copyright 1996 Elsevier Science Ltd Printed in Great Britain. All rights reserved 0747-5632/96 $15.00 + 0.00 S0747-5632(96) 00020-9
Pergamon Computers in Human Behavior, Vol. 12, No. 3, pp. 481-495, 1996 Copyright 1996 Elsevier Science Ltd Printed in Great Britain. All rights reserved 0747-5632/96 $15.00 + 0.00 S0747-5632(96) 00020-9
THE RATIONALITY/EMOTIONAL DEFENSIVENESS SCALE- I. INTERNAL STRUCTURE AND STABILITY
 Joouml of Psychosomaric Research, Vol. 35. No. 4/S, pp. 545-554, 1991. 0534-3999191 $3.00+.00 Printed in Great Britain 0 1991 Pergamon Press plc THE RATIONALITY/EMOTIONAL DEFENSIVENESS SCALE- I. INTERNAL
Joouml of Psychosomaric Research, Vol. 35. No. 4/S, pp. 545-554, 1991. 0534-3999191 $3.00+.00 Printed in Great Britain 0 1991 Pergamon Press plc THE RATIONALITY/EMOTIONAL DEFENSIVENESS SCALE- I. INTERNAL
Incidence and predictors of synchronous liver metastases in patients with gastrointestinal stromal tumors (GISTs)
 Accepted Manuscript Incidence and predictors of synchronous liver metastases in patients with gastrointestinal stromal tumors (GISTs) Apostolos Gaitanidis, Michail Alevizakos, Alexandra Tsaroucha, Constantinos
Accepted Manuscript Incidence and predictors of synchronous liver metastases in patients with gastrointestinal stromal tumors (GISTs) Apostolos Gaitanidis, Michail Alevizakos, Alexandra Tsaroucha, Constantinos
Hard-tissue alterations following immediate implant placement in extraction sites
 J Clin Periodontol 2004; 31: 820 828 doi: 10.1111/j.1600-051X.2004.00565.x Copyright r Blackwell Munksgaard 2004 Printed in Denmark. All rights reserved Hard-tissue alterations following immediate implant
J Clin Periodontol 2004; 31: 820 828 doi: 10.1111/j.1600-051X.2004.00565.x Copyright r Blackwell Munksgaard 2004 Printed in Denmark. All rights reserved Hard-tissue alterations following immediate implant
Contrasting timing of virological relapse after discontinuation of. tenofovir or entecavir in hepatitis B e antigen-negative patients.
 Contrasting timing of virological relapse after discontinuation of tenofovir or entecavir in hepatitis B e antigen-negative patients. Running title: Difference after stopping TDF or ETV Christoph Höner
Contrasting timing of virological relapse after discontinuation of tenofovir or entecavir in hepatitis B e antigen-negative patients. Running title: Difference after stopping TDF or ETV Christoph Höner
Efficacy and Safety of Saxagliptin Compared with Acarbose in Chinese. Patients with Type 2 Diabetes Mellitus Uncontrolled on Metformin
 Efficacy and Safety of Saxagliptin Compared with Acarbose in Chinese Patients with Type 2 Diabetes Mellitus Uncontrolled on Metformin Monotherapy: Results of a Phase IV Open-Label Randomized Controlled
Efficacy and Safety of Saxagliptin Compared with Acarbose in Chinese Patients with Type 2 Diabetes Mellitus Uncontrolled on Metformin Monotherapy: Results of a Phase IV Open-Label Randomized Controlled
Parallel Stent Graft Techniques to Facilitate Endovascular Repair in the Aortic Arch
 Parallel Stent Graft Techniques to Facilitate Endovascular Repair in the Aortic Arch 35 Frank J. Criado Introduction Whether using a traditional open-chest approach or endovascular techniques, the arch
Parallel Stent Graft Techniques to Facilitate Endovascular Repair in the Aortic Arch 35 Frank J. Criado Introduction Whether using a traditional open-chest approach or endovascular techniques, the arch
The Use of Transdermal Buprenorphine to Relieve Radiotherapy-Related Pain in Head and Neck Cancer Patients
 Cancer Investigation, 31:412 420, 2013 ISSN: 0735-7907 print / 1532-4192 online Copyright C 2013 Informa Healthcare USA, Inc. DOI: 10.3109/07357907.2013.800094 IMAGING, DIAGNOSIS, PROGNOSIS The Use of
Cancer Investigation, 31:412 420, 2013 ISSN: 0735-7907 print / 1532-4192 online Copyright C 2013 Informa Healthcare USA, Inc. DOI: 10.3109/07357907.2013.800094 IMAGING, DIAGNOSIS, PROGNOSIS The Use of
RAVEN'S COLORED PROGRESSIVE MATRICES AND INTELLECTUAL IMPAIRMENT IN PATIENTS WITH FOCAL BRAIN DAMAGE
 RAVEN'S COLORED PROGRESSIVE MATRICES AND INTELLECTUAL IMPAIRMENT IN PATIENTS WITH FOCAL BRAIN DAMAGE Claudio Villardita (Neuropsychology Unit, Department of Neurology, University of Catania) INTRODUCTION
RAVEN'S COLORED PROGRESSIVE MATRICES AND INTELLECTUAL IMPAIRMENT IN PATIENTS WITH FOCAL BRAIN DAMAGE Claudio Villardita (Neuropsychology Unit, Department of Neurology, University of Catania) INTRODUCTION
Mastering the Initial Dissection and Cannulation: Making Ablation Easy and Safe
 Chapter 5 Mastering the Initial Dissection and Cannulation: Making Ablation Easy and Safe 1 INTRODUCTION A good initial dissection with wide mobilization of the left atrium (LA) by dividing its attachments
Chapter 5 Mastering the Initial Dissection and Cannulation: Making Ablation Easy and Safe 1 INTRODUCTION A good initial dissection with wide mobilization of the left atrium (LA) by dividing its attachments
Nebulized Magnesium for Moderate and Severe Pediatric Asthma: A Randomized Trial
 50:1191 1199 (2015) Nebulized Magnesium for Moderate and Severe Pediatric Asthma: A Randomized Trial Khalid Alansari, MD, FRCPC, FAAP(PEM), 1,2,3 * Wessam Ahmed, 2 Bruce L. Davidson, MD, MPH, 4 Mohamed
50:1191 1199 (2015) Nebulized Magnesium for Moderate and Severe Pediatric Asthma: A Randomized Trial Khalid Alansari, MD, FRCPC, FAAP(PEM), 1,2,3 * Wessam Ahmed, 2 Bruce L. Davidson, MD, MPH, 4 Mohamed
Energy Metabolism in Oreochromis niloticus
 Aquuculture, 79 (1989) 283-291 Eisevier Science Pubhshers B.V., Amsterdam - Printed in The Netherlands 283 Energy Metabolism in Oreochromis niloticus K.-H. MEYER-BURGDORFF, M.F. OSMAN and K.D. GUNTHER
Aquuculture, 79 (1989) 283-291 Eisevier Science Pubhshers B.V., Amsterdam - Printed in The Netherlands 283 Energy Metabolism in Oreochromis niloticus K.-H. MEYER-BURGDORFF, M.F. OSMAN and K.D. GUNTHER
Address: Department of General Surgery, Royal Bolton Hospital, Bolton, UK. ; tel:
 Article type : Systematic Review Accepted Article 875-2017.R1 Systematic Review Effect of mesalazine on recurrence of diverticulitis in patients with symptomatic uncomplicated diverticular disease: a meta-analysis
Article type : Systematic Review Accepted Article 875-2017.R1 Systematic Review Effect of mesalazine on recurrence of diverticulitis in patients with symptomatic uncomplicated diverticular disease: a meta-analysis
Use of Digoxin for Heart Failure and Atrial Fibrillation in Elderly Patients
 J.W.M. Cheng and I. Rybak The American Journal of Geriatric Pharmacotherapy Use of Digoxin for Heart Failure and Atrial Fibrillation in Elderly Patients Judy W.M. Cheng, BS, PharmD, MPH 1,2 ; and Iwona
J.W.M. Cheng and I. Rybak The American Journal of Geriatric Pharmacotherapy Use of Digoxin for Heart Failure and Atrial Fibrillation in Elderly Patients Judy W.M. Cheng, BS, PharmD, MPH 1,2 ; and Iwona
Hepatitis B virus (HBV) infection is a global health
 GASTROENTEROLOGY 2012;142:1140 1149 High Levels of Hepatitis B Surface Antigen Increase Risk of Hepatocellular Carcinoma in Patients With Low HBV Load TAI CHUNG TSENG,*,, ** CHUN JEN LIU,, HUNG CHIH YANG,,#
GASTROENTEROLOGY 2012;142:1140 1149 High Levels of Hepatitis B Surface Antigen Increase Risk of Hepatocellular Carcinoma in Patients With Low HBV Load TAI CHUNG TSENG,*,, ** CHUN JEN LIU,, HUNG CHIH YANG,,#
HYDRONEPHROSIS DUE TO THE INFERIOR POLAR ARTERY :
 HYDRONEPHROSIS DUE TO THE INFERIOR POLAR ARTERY : LATE RESULTS AFTER NEPHROPLICATION. Appendix of Recent Cases By A. WILFRID ADAMS, F.R.C.S. Bristol Royal Infirmary FOR hydronephrosis due to an aberrant,
HYDRONEPHROSIS DUE TO THE INFERIOR POLAR ARTERY : LATE RESULTS AFTER NEPHROPLICATION. Appendix of Recent Cases By A. WILFRID ADAMS, F.R.C.S. Bristol Royal Infirmary FOR hydronephrosis due to an aberrant,
Prevalence of different HIV-1 subtypes in sexual transmission in China: a systematic review and meta-analysis
 Epidemiol. Infect. (2016), 144, 2144 2153. Cambridge University Press 2016 doi:10.1017/s0950268816000212 Prevalence of different HIV-1 subtypes in sexual transmission in China: a systematic review and
Epidemiol. Infect. (2016), 144, 2144 2153. Cambridge University Press 2016 doi:10.1017/s0950268816000212 Prevalence of different HIV-1 subtypes in sexual transmission in China: a systematic review and
LONG-TERM RESULTS OF A PHASE III TRIAL COMPARING ONCE-DAILY RADIOTHERAPY WITH TWICE-DAILY RADIOTHERAPY IN LIMITED- STAGE SMALL-CELL LUNG CANCER
 doi:10.1016/j.ijrobp.2004.01.055 Int. J. Radiation Oncology Biol. Phys., Vol. 59, No. 4, pp. 943 951, 2004 Copyright 2004 Elsevier Inc. Printed in the USA. All rights reserved 0360-3016/04/$ see front
doi:10.1016/j.ijrobp.2004.01.055 Int. J. Radiation Oncology Biol. Phys., Vol. 59, No. 4, pp. 943 951, 2004 Copyright 2004 Elsevier Inc. Printed in the USA. All rights reserved 0360-3016/04/$ see front
SYSTEMATIC REVIEW PROTOCOL
 Effectiveness of Silexan oral lavender essential oil compared to inhaled lavender essential oil aromatherapy on sleep in adults: a systematic review protocol Martha J. Greenberg 1,2 Jason T. Slyer 1,2
Effectiveness of Silexan oral lavender essential oil compared to inhaled lavender essential oil aromatherapy on sleep in adults: a systematic review protocol Martha J. Greenberg 1,2 Jason T. Slyer 1,2
Cytochrome P450 (CYP) enzymes play a role in
 Effects of Commonly Administered Agents and Genetics on Nebivolol Pharmacokinetics: Drug Drug Interaction Studies Charles Lindamood, PhD, Stephan Ortiz, PhD, Andrew Shaw, PhD, Russ Rackley, PhD, and J.
Effects of Commonly Administered Agents and Genetics on Nebivolol Pharmacokinetics: Drug Drug Interaction Studies Charles Lindamood, PhD, Stephan Ortiz, PhD, Andrew Shaw, PhD, Russ Rackley, PhD, and J.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Computerized Quantitative Coronary Angiography Applied to Percutaneous Transluminal Coronary Angioplasty: Advantages and Limitations
 Computerized Quantitative Coronary Angiography Applied to Percutaneous Transluminal Coronary Angioplasty: Advantages and Limitations P.W. Serruys, F. Booman, G.J. Troost, J.H.C. Reiber, J.J. Gerbrands*,
Computerized Quantitative Coronary Angiography Applied to Percutaneous Transluminal Coronary Angioplasty: Advantages and Limitations P.W. Serruys, F. Booman, G.J. Troost, J.H.C. Reiber, J.J. Gerbrands*,
Ovarian cancer is the most lethal gynecologic malignancy
 Original Research Laparoscopy Compared With Laparotomy for Debulking Ovarian Cancer After Neoadjuvant Chemotherapy Alexander Melamed, MD, MPH, Roni Nitecki, MD, David M. Boruta II, MD, Marcela G. del Carmen,
Original Research Laparoscopy Compared With Laparotomy for Debulking Ovarian Cancer After Neoadjuvant Chemotherapy Alexander Melamed, MD, MPH, Roni Nitecki, MD, David M. Boruta II, MD, Marcela G. del Carmen,
Clinical investigation of chronic subdural hematoma with impending brain herniation on arrival
 DOI 10.1007/s10143-017-0861-9 ORIGINAL ARTICLE Clinical investigation of chronic subdural hematoma with impending brain herniation on arrival Hiroaki Matsumoto 1 & Hiroaki Hanayama 1 & Takashi Okada 1
DOI 10.1007/s10143-017-0861-9 ORIGINAL ARTICLE Clinical investigation of chronic subdural hematoma with impending brain herniation on arrival Hiroaki Matsumoto 1 & Hiroaki Hanayama 1 & Takashi Okada 1
A. Alonso-Burgos a, *, E. García-Tutor b, G. Bastarrika a, D. Cano a, A. Martínez-Cuesta a, L.J. Pina a
 Journal of Plastic, Reconstructive & Aesthetic Surgery (2006) 59, 585 593 Preoperative planning of deep inferior epigastric artery perforator flap reconstruction with multislice-ct angiography: imaging
Journal of Plastic, Reconstructive & Aesthetic Surgery (2006) 59, 585 593 Preoperative planning of deep inferior epigastric artery perforator flap reconstruction with multislice-ct angiography: imaging
Natural Course of Peripartum Cardiomyopathy
 Natural Course of Peripartum Cardiomyopathy By JOHN G. DEMAKIS, M.D., SHAHBUDIN H. RAHIMTOOLA, M.B., M.R.C.P.E., GEORGE C. SUTrON, M.D., W. ROBERT MEADOWS, M.D., PAUL B. SZANTO, M.D., JoHN R. TOBIN, M.D.,
Natural Course of Peripartum Cardiomyopathy By JOHN G. DEMAKIS, M.D., SHAHBUDIN H. RAHIMTOOLA, M.B., M.R.C.P.E., GEORGE C. SUTrON, M.D., W. ROBERT MEADOWS, M.D., PAUL B. SZANTO, M.D., JoHN R. TOBIN, M.D.,
Pulmonary Vein Stenosis After Catheter Ablation of Atrial Fibrillation
 Pulmonary Vein Stenosis After Catheter Ablation of Atrial Fibrillation E.B. SAAD Introduction Catheter ablation around the pulmonary veins (PVs) has become the treatment of choice for symptomatic patients
Pulmonary Vein Stenosis After Catheter Ablation of Atrial Fibrillation E.B. SAAD Introduction Catheter ablation around the pulmonary veins (PVs) has become the treatment of choice for symptomatic patients
A Diabetes Mobile App With In-App Coaching From a Certified Diabetes Educator Reduces A1C for Individuals With Type 2 Diabetes
 765650TDEXXX10.1177/0145721718765650Impact of a Diabetes App and Coaching Program on A1CKumar et al research-article2018 Impact of a Diabetes App and Coaching Program on A1C 1 A Diabetes Mobile App With
765650TDEXXX10.1177/0145721718765650Impact of a Diabetes App and Coaching Program on A1CKumar et al research-article2018 Impact of a Diabetes App and Coaching Program on A1C 1 A Diabetes Mobile App With
Pulley lesions in rotator cuff tears: prevalence, etiology, and concomitant pathologies
 Arch Orthop Trauma Surg (2017) 137:1097 1105 DOI 10.1007/s00402-017-2721-z ARTHROSCOPY AND SPORTS MEDICINE Pulley lesions in rotator cuff tears: prevalence, etiology, and concomitant pathologies Nael Hawi
Arch Orthop Trauma Surg (2017) 137:1097 1105 DOI 10.1007/s00402-017-2721-z ARTHROSCOPY AND SPORTS MEDICINE Pulley lesions in rotator cuff tears: prevalence, etiology, and concomitant pathologies Nael Hawi
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
ABSTRACT. questions in the version of NorAQ administered to men (m-noraq) against the interview model.
 GENDER MEDICINE/VOL. 8,NO. 2, 2011 NorVold Abuse Questionnaire for Men (m-noraq): Validation of New Measures of Emotional, Physical, and Sexual Abuse and Abuse in Health Care in Male Patients Katarina
GENDER MEDICINE/VOL. 8,NO. 2, 2011 NorVold Abuse Questionnaire for Men (m-noraq): Validation of New Measures of Emotional, Physical, and Sexual Abuse and Abuse in Health Care in Male Patients Katarina
Low- vs. high-pressure suction drainage after total knee arthroplasty: a double-blind randomized controlled trial
 JAN JOURNAL OF ADVANCED NURSING ORIGINAL RESEARCH Low- vs. high-pressure suction drainage after total knee arthroplasty: a double-blind randomized controlled trial Rafael Calvo, M a José Martínez-Zapata,
JAN JOURNAL OF ADVANCED NURSING ORIGINAL RESEARCH Low- vs. high-pressure suction drainage after total knee arthroplasty: a double-blind randomized controlled trial Rafael Calvo, M a José Martínez-Zapata,
Colchicine for prevention and treatment of cardiac diseases: A meta-analysis
 DOI: 10.1111/1755-5922.12226 ORIGINAL RESEARCH ARTICLE Colchicine for prevention and treatment of cardiac diseases: A meta-analysis Nikolaos Papageorgiou 1,2, Alexandros Briasoulis 3, George Lazaros 2
DOI: 10.1111/1755-5922.12226 ORIGINAL RESEARCH ARTICLE Colchicine for prevention and treatment of cardiac diseases: A meta-analysis Nikolaos Papageorgiou 1,2, Alexandros Briasoulis 3, George Lazaros 2
Marlowe Crowne Social Desirability Scale and Short Form C: Forensic Norms
 Marlowe Crowne Social Desirability Scale and Short Form C: Forensic Norms Paul Andrews Private Practice, Tyler, Texas Robert G. Meyer University of Louisville Marlowe Crowne Social Desirability Scale (MC)
Marlowe Crowne Social Desirability Scale and Short Form C: Forensic Norms Paul Andrews Private Practice, Tyler, Texas Robert G. Meyer University of Louisville Marlowe Crowne Social Desirability Scale (MC)
Splenomegaly and Hemolytic Anemia Induced in Rats by Methylcellulose - An electron microscopic study '
 Splenomegaly and Hemolytic Anemia Induced in Rats by Methylcellulose - An electron microscopic study ' EMMA WENNBERG AND LEON WEISS Department of Anatomy, The johns Hopkins University School of Medicine,
Splenomegaly and Hemolytic Anemia Induced in Rats by Methylcellulose - An electron microscopic study ' EMMA WENNBERG AND LEON WEISS Department of Anatomy, The johns Hopkins University School of Medicine,
Epithelial Barrier Defects in HT-29/B6 Colonic Cell Monolayers Induced by Tumor Necrosis Factor α
 Epithelial Barrier Defects in HT-29/B6 Colonic Cell Monolayers Induced by Tumor Necrosis Factor α ALFRED H. GITTER, a,b KERSTIN BENDFELDT, a HEINZ SCHMITZ, c JÖRG-DIETER SCHULZKE, c CARL J. BENTZEL, d
Epithelial Barrier Defects in HT-29/B6 Colonic Cell Monolayers Induced by Tumor Necrosis Factor α ALFRED H. GITTER, a,b KERSTIN BENDFELDT, a HEINZ SCHMITZ, c JÖRG-DIETER SCHULZKE, c CARL J. BENTZEL, d
Lisfranc Arthrodesis for Chronic Pain: A Cannulated Screw Technique
 Lisfranc Arthrodesis for Chronic Pain: A Cannulated Screw Technique Nine patients with injury to the tarsometatarsal joint underwent fusion with cannulated screw fixation after conservative treatment had
Lisfranc Arthrodesis for Chronic Pain: A Cannulated Screw Technique Nine patients with injury to the tarsometatarsal joint underwent fusion with cannulated screw fixation after conservative treatment had
Small pulmonary nodules in baseline and incidence screening rounds of low-dose CT lung cancer screening
 Review Article Small pulmonary nodules in baseline and incidence screening rounds of low-dose CT lung cancer screening Joan E. Walter, Marjolein A. Heuvelmans, Matthijs Oudkerk University Medical Center
Review Article Small pulmonary nodules in baseline and incidence screening rounds of low-dose CT lung cancer screening Joan E. Walter, Marjolein A. Heuvelmans, Matthijs Oudkerk University Medical Center
Serum mir-182 and mir-331-3p as diagnostic and prognostic markers in patients with hepatocellular carcinoma
 Tumor Biol. (2015) 36:7439 7447 DOI 10.1007/s13277-015-3430-2 RESEARCH ARTICLE Serum mir-182 and mir-331-3p as diagnostic and prognostic markers in patients with hepatocellular carcinoma Lin Chen 1 & Feihu
Tumor Biol. (2015) 36:7439 7447 DOI 10.1007/s13277-015-3430-2 RESEARCH ARTICLE Serum mir-182 and mir-331-3p as diagnostic and prognostic markers in patients with hepatocellular carcinoma Lin Chen 1 & Feihu
Effect of health Baduanjin Qigong for mild to moderate Parkinson s disease
 bs_bs_banner Geriatr Gerontol Int 2016; 16: 911 919 ORIGINAL ARTICLE: EPIDEMIOLOGY, CLINICAL PRACTICE AND HEALTH Effect of health Baduanjin Qigong for mild to moderate Parkinson s disease Chun-Mei Xiao
bs_bs_banner Geriatr Gerontol Int 2016; 16: 911 919 ORIGINAL ARTICLE: EPIDEMIOLOGY, CLINICAL PRACTICE AND HEALTH Effect of health Baduanjin Qigong for mild to moderate Parkinson s disease Chun-Mei Xiao
In vivo diagnosis of early-stage gastric cancer found after. Helicobacter pylori eradication using probe-based confocal laser endomicroscopy
 DR. NAOKI OHMIYA (Orcid ID : 0000-0002-7651-402X) Accepted Article Article type : Original article In vivo diagnosis of early-stage gastric cancer found after Helicobacter pylori eradication using probe-based
DR. NAOKI OHMIYA (Orcid ID : 0000-0002-7651-402X) Accepted Article Article type : Original article In vivo diagnosis of early-stage gastric cancer found after Helicobacter pylori eradication using probe-based
A disease- specific quality of life instrument for non- alcoholic fatty liver disease and non- alcoholic steatohepatitis: CLDQ- NAFLD
 Received: 10 November 2016 Accepted: 8 February 2017 DOI: 10.1111/liv.13391 METABOLIC LIVER DISEASE A disease- specific quality of life instrument for non- alcoholic fatty liver disease and non- alcoholic
Received: 10 November 2016 Accepted: 8 February 2017 DOI: 10.1111/liv.13391 METABOLIC LIVER DISEASE A disease- specific quality of life instrument for non- alcoholic fatty liver disease and non- alcoholic
Electrical Acupoint Stimulation Changes Body Composition and the Meridian Systems in Postmenopausal Women with Obesity
 The American Journal of Chinese Medicine, Vol. 38, No. 4, 683 694 2010 World Scientific Publishing Company Institute for Advanced Research in Asian Science and Medicine DOI: 10.1142/S0192415X10008159 Electrical
The American Journal of Chinese Medicine, Vol. 38, No. 4, 683 694 2010 World Scientific Publishing Company Institute for Advanced Research in Asian Science and Medicine DOI: 10.1142/S0192415X10008159 Electrical
EGC Diagnosis of Paroxysmal Supraventricular Tachycardias in Patients without Preexcitation
 REVIEW ARTICLE EGC Diagnosis of Paroxysmal Supraventricular Tachycardias in Patients without Preexcitation Esteban González-Torrecilla, M.D., Ph.D., Angel Arenal, M.D., Felipe Atienza, M.D., Ph.D., Tomás
REVIEW ARTICLE EGC Diagnosis of Paroxysmal Supraventricular Tachycardias in Patients without Preexcitation Esteban González-Torrecilla, M.D., Ph.D., Angel Arenal, M.D., Felipe Atienza, M.D., Ph.D., Tomás
A Motivational Intervention to Reduce Cigarette
 A Motivational Intervention to Reduce Cigarette Smoking Among College Students: Overview and Exploratory Investigation Keith C. Herman and Beth Fahnlander College counselors can play an important role
A Motivational Intervention to Reduce Cigarette Smoking Among College Students: Overview and Exploratory Investigation Keith C. Herman and Beth Fahnlander College counselors can play an important role
Functional Outcome of Unstable Distal Radius Fractures: ORIF With a Volar Fixed-Angle Tine Plate Versus External Fixation
 Functional Outcome of Unstable Distal Radius Fractures: ORIF With a Volar Fixed-Angle Tine Plate Versus External Fixation Thomas W. Wright, MD, MaryBeth Horodyski, EdD, Gainesville, FL, Dean W. Smith,
Functional Outcome of Unstable Distal Radius Fractures: ORIF With a Volar Fixed-Angle Tine Plate Versus External Fixation Thomas W. Wright, MD, MaryBeth Horodyski, EdD, Gainesville, FL, Dean W. Smith,
A LABORATORY TASK FOR INDUCTION OF MOOD STATES*
 khav. Res. & Therapy. 1968. Vol. 6. pp. 473 to 462. Pergamon Press. Printed in Englmd A LABORATORY TASK FOR INDUCTION OF MOOD STATES* EMMETT VELTEN, JR.? University of Southern California (Received 15
khav. Res. & Therapy. 1968. Vol. 6. pp. 473 to 462. Pergamon Press. Printed in Englmd A LABORATORY TASK FOR INDUCTION OF MOOD STATES* EMMETT VELTEN, JR.? University of Southern California (Received 15
Congenital absence of teeth is a common dental
 CASE REPORT Management of congenitally missing second premolars with orthodontics and single-tooth implants Roy Sabri, DDS, MS Beirut, Lebanon This article describes the treatment of an adolescent girl
CASE REPORT Management of congenitally missing second premolars with orthodontics and single-tooth implants Roy Sabri, DDS, MS Beirut, Lebanon This article describes the treatment of an adolescent girl
Antiproliferative, antimigratory, and anticlonogenic effects of Hedyotis diffusa, Panax ginseng, and their combination on colorectal cancer cell lines
 Journal of Herbs, Spices & Medicinal Plants ISSN: 1049-6475 (Print) 1540-3580 (Online) Journal homepage: http://www.tandfonline.com/loi/whsm20 Antiproliferative, antimigratory, and anticlonogenic effects
Journal of Herbs, Spices & Medicinal Plants ISSN: 1049-6475 (Print) 1540-3580 (Online) Journal homepage: http://www.tandfonline.com/loi/whsm20 Antiproliferative, antimigratory, and anticlonogenic effects
Protective effect of HTK solution on postoperative pulmonary function in infants with CHD and PAH
 Bioscience Reports: this is an Accepted Manuscript, not the final Version of Record. You are encouraged to use the Version of Record that, when published, will replace this version. The most up-to-date
Bioscience Reports: this is an Accepted Manuscript, not the final Version of Record. You are encouraged to use the Version of Record that, when published, will replace this version. The most up-to-date
The European Journal of Contraception & Reproductive Health Care
 The European Journal of Contraception & Reproductive Health Care ISSN: 1362-5187 (Print) 1473-0782 (Online) Journal homepage: http://www.tandfonline.com/loi/iejc20 Inhibition of ovulation by NOMAC/E 2,
The European Journal of Contraception & Reproductive Health Care ISSN: 1362-5187 (Print) 1473-0782 (Online) Journal homepage: http://www.tandfonline.com/loi/iejc20 Inhibition of ovulation by NOMAC/E 2,
Gomputed tomography of the normal temporomaandibular joint
 Gomputed tomography of the normal temporomaandibular joint EDWIN h. GHRISTiANSENi, TERENCE T: CHAN^, JOSEPH R. THOMPSON^ ANTON N. HASSO', DAVID B. HINSHAW JR' AND SIGVARD KOPP* ^Department of Endodontics^
Gomputed tomography of the normal temporomaandibular joint EDWIN h. GHRISTiANSENi, TERENCE T: CHAN^, JOSEPH R. THOMPSON^ ANTON N. HASSO', DAVID B. HINSHAW JR' AND SIGVARD KOPP* ^Department of Endodontics^
Development and psychometric evaluation of the Thirst Distress Scale for patients with heart failure
 728624CNU0010.1177/1474515117728624European Journal of Cardiovascular Nursing 0(0)Waldréus et al. research-article2017 Original Article Development and psychometric evaluation of the Thirst Distress Scale
728624CNU0010.1177/1474515117728624European Journal of Cardiovascular Nursing 0(0)Waldréus et al. research-article2017 Original Article Development and psychometric evaluation of the Thirst Distress Scale
Semaglutide in type 2 diabetes is it the best glucagon-like peptide 1 receptor agonist (GLP-1R agonist)?
 Expert Opinion on Drug Metabolism & Toxicology ISSN: 1742-5255 (Print) 1744-7607 (Online) Journal homepage: http://www.tandfonline.com/loi/iemt20 Semaglutide in type 2 diabetes is it the best glucagon-like
Expert Opinion on Drug Metabolism & Toxicology ISSN: 1742-5255 (Print) 1744-7607 (Online) Journal homepage: http://www.tandfonline.com/loi/iemt20 Semaglutide in type 2 diabetes is it the best glucagon-like
Absolute monocyte count predicts overall survival in mantle cell lymphomas: correlation with tumourassociated
 Hematological Oncology Hematol Oncol 2014; 32: 178 186 Published online 29 October 2013 in Wiley Online Library (wileyonlinelibrary.com).2106 Original Research Article Absolute monocyte count predicts
Hematological Oncology Hematol Oncol 2014; 32: 178 186 Published online 29 October 2013 in Wiley Online Library (wileyonlinelibrary.com).2106 Original Research Article Absolute monocyte count predicts
Endoscopic resection therapies for rectal neuroendocrine tumors: A systematic review and meta-analysis
 bs_bs_banner doi:.1111/jgh.12395 META-ANALYSIS AND SYSTEMATIC REVIEW for rectal neuroendocrine tumors: A systematic review and meta-analysis Xin Zhou,* Haiting Xie,* Lingduo Xie,* Jing Li, Weihua Cao and
bs_bs_banner doi:.1111/jgh.12395 META-ANALYSIS AND SYSTEMATIC REVIEW for rectal neuroendocrine tumors: A systematic review and meta-analysis Xin Zhou,* Haiting Xie,* Lingduo Xie,* Jing Li, Weihua Cao and
Effects of Mattress Material on Body Pressure Profiles in Different Sleeping Postures
 Effects of Mattress Material on Body Pressure Profiles in Different Sleeping Postures Fan-Zhe Low, BEng, Matthew Chin-Heng Chua, PhD, Pan-Yin Lim, BEng, and Chen-Hua Yeow, PhD ABSTRACT Objectives: This
Effects of Mattress Material on Body Pressure Profiles in Different Sleeping Postures Fan-Zhe Low, BEng, Matthew Chin-Heng Chua, PhD, Pan-Yin Lim, BEng, and Chen-Hua Yeow, PhD ABSTRACT Objectives: This
Infectivity of HBV DNA positive donations identified in look-back studies in Hyogo-Prefecture, Japan
 Transfusion Medicine, 2011, 21, 107 115 doi: 10.1111/j.1365-3148.2010.01057.x ORIGINAL ARTICLE Infectivity of HBV DNA positive donations identified in look-back studies in Hyogo-Prefecture, Japan Y. Bouike,
Transfusion Medicine, 2011, 21, 107 115 doi: 10.1111/j.1365-3148.2010.01057.x ORIGINAL ARTICLE Infectivity of HBV DNA positive donations identified in look-back studies in Hyogo-Prefecture, Japan Y. Bouike,
Effects of Grapefruit and Pomegranate Juices on the Pharmacokinetic Properties of Dapoxetine and Midazolam in Healthy Subjects
 Eur J Drug Metab Pharmacokinet DOI 10.1007/s13318-016-0352-3 ORIGINAL RESEARCH ARTICLE Effects of Grapefruit and Pomegranate Juices on the Pharmacokinetic Properties of Dapoxetine and Midazolam in Healthy
Eur J Drug Metab Pharmacokinet DOI 10.1007/s13318-016-0352-3 ORIGINAL RESEARCH ARTICLE Effects of Grapefruit and Pomegranate Juices on the Pharmacokinetic Properties of Dapoxetine and Midazolam in Healthy
Combining ECMO with IABP for the Treatment of Critically Ill Adult Heart Failure Patients
 Heart, Lung and Circulation (2014) 23, 363 368 1443-9506/04/$36.00 http://dx.doi.org/10.1016/j.hlc.2013.10.081 ORIGINAL ARTICLE Combining ECMO with IABP for the Treatment of Critically Ill Adult Heart
Heart, Lung and Circulation (2014) 23, 363 368 1443-9506/04/$36.00 http://dx.doi.org/10.1016/j.hlc.2013.10.081 ORIGINAL ARTICLE Combining ECMO with IABP for the Treatment of Critically Ill Adult Heart
Hong-qi Zhang Min-zhong Lin Jin-song Li Ming-xing Tang Chao-feng Guo Jian-huang Wu Jin-yang Liu
 Arch Orthop Trauma Surg (2013) 133:333 341 DOI 10.1007/s00402-012-1669-2 ORTHOPAEDIC SURGERY One-stage posterior debridement, transforaminal lumbar interbody fusion and instrumentation in treatment of
Arch Orthop Trauma Surg (2013) 133:333 341 DOI 10.1007/s00402-012-1669-2 ORTHOPAEDIC SURGERY One-stage posterior debridement, transforaminal lumbar interbody fusion and instrumentation in treatment of
Memory-based attentional capture by colour and shape contents in visual working memory
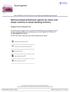 Visual Cognition ISSN: 1350-6285 (Print) 1464-0716 (Online) Journal homepage: http://www.tandfonline.com/loi/pvis20 Memory-based attentional capture by colour and shape contents in visual working memory
Visual Cognition ISSN: 1350-6285 (Print) 1464-0716 (Online) Journal homepage: http://www.tandfonline.com/loi/pvis20 Memory-based attentional capture by colour and shape contents in visual working memory
Tumor Spread Through Air Spaces Identifies a Distinct Subgroup With Poor Prognosis in Surgically Resected Lung Pleomorphic Carcinoma
 1 2 3 4 5 6 7 8 9 1 11 12 13 14 15 16 17 18 19 21 22 23 24 25 26 27 28 29 3 31 32 33 34 35 36 37 38 39 41 42 43 44 45 46 47 48 49 5 51 52 53 54 55 Q15 Q1 Q6 Q2 [ Original Research ] Tumor Spread Through
1 2 3 4 5 6 7 8 9 1 11 12 13 14 15 16 17 18 19 21 22 23 24 25 26 27 28 29 3 31 32 33 34 35 36 37 38 39 41 42 43 44 45 46 47 48 49 5 51 52 53 54 55 Q15 Q1 Q6 Q2 [ Original Research ] Tumor Spread Through
