Frequently Asked Questions for Ontario Pharmacists: Blood Glucose Test Strip Reimbursement Policy
|
|
|
- Charles Harrell
- 6 years ago
- Views:
Transcription
1 Ontario Public Drug Programs, Ministry of Health and Long-Term Care Frequently Asked Questions for Ontario Pharmacists: Blood Glucose Test Strip Reimbursement Policy 1. Why is the ministry introducing changes to the reimbursement of Blood Glucose Test Strips (BGTS)? Research has indicated that BGTS have a limited benefit for many patients who do not take insulin to manage their diabetes. Based on best evidence, the government is introducing changes to the number of BGTS it will reimburse people with diabetes while ensuring those who need test strips to help manage diabetes, will continue to have access to them. The new changes on the reimbursement of blood glucose test strips are effective August 1, 2013, and are aligned with the Canadian Diabetes Association (CDA) to encourage proper testing practices for optimal patient outcomes. A July, 2009 report from the Canadian Agency for Drugs and Technologies in Health (CADTH), stated that in practice, blood glucose test strips have a limited clinical benefit for many patients who are not on insulin. The CDA also published a commentary for healthcare providers recognizing that some limits on the number of blood glucose test strips reimbursed for patients on oral anti-diabetes medications may be reasonable. 2. How often should patients be self-monitoring their blood glucose? The ministry s BGTS reimbursement policy is in alignment with the CDA recommendations to test according to the current medication profile. The CDA has made several recommendations about Self-Monitoring of Blood Glucose (SMBG) in their 2013 Clinical Practice Guidelines: For individuals using insulin more than once a day, SMBG should be used as an essential part of diabetes self-management [Grade A, Level 1, for type 1 diabetes; Grade C, Level 3, for type 2 diabetes] and should be undertaken at least three times per day [Grade C, Level 3] and include both pre- and postprandial measurements [Grade C, Level 3] In those with type 2 diabetes on once-daily insulin in addition to oral antihyperglycemic agents, testing at least once a day at variable times is recommended [Grade D]. For individuals with type 2 diabetes not receiving insulin therapy, SMBG recommendations should be individualized depending on type of antihyperglycemic agents, level of glycemic control and risk of hypoglycemia [Grade D]. When glycemic control is not being achieved, SMBG should be instituted [Grade B, Level 2] and should include periodic pre- and postprandial 1
2 measurements and training of healthcare providers and patients on methods to modify lifestyle and medications in response to SMBG values [Grade B, Level 2]. If achieving glycemic targets or receiving medications not associated with hypoglycemia, infrequent SMBG is appropriate [Grade D]. For a complete version of the 2013 Clinical Practice Guidelines: 3. When will changes in reimbursement of BGTS be in effect? The changes for BGTS reimbursement are effective on or after August 1, 2013 and will be applied on the first claim for test strips after this date. 4. How many diabetes test strips are ODB recipients eligible to receive under the ODB Program? As of August 1, 2013, the Health Network System (HNS) will track and determine appropriate levels of reimbursement of BGTS based on the current diabetes therapy used by eligible ODB recipients. When a claim is submitted for BGTS for eligible ODB recipients, the HNS will automatically review the anti-diabetes medications claims within the previous six months to identify claims for insulin products and other anti-diabetes medications. The HNS will then apply a maximum number of self-monitoring BGTS that may be reimbursed for the recipient in the following 365 days, based on both online and paper claims as follows: Diabetes Treatment History Number of BGTS allowed within a 365-day period Patients managing diabetes with insulin 3,000 Patients managing diabetes with anti-diabetes medication with high risk of causing hypoglycemia* Patients managing diabetes using anti-diabetes medication with low risk of causing hypoglycemia Patients managing diabetes through diet/lifestyle therapy only (no insulin or anti-diabetes medications) (*low blood sugar) For additional information regarding treatment classes, please refer to question 7 below. Recipients will be allotted the indicated number of test strips for use over the course of a 365-day period. The test strip allotment will apply to both online and paper claims. 2
3 When submitting a claim for insulin or anti-diabetes medication along with a claim for BGTS, pharmacists should submit all anti-diabetes medications prior to entering the BGTS claim. This ensures that the most current drug profile is included in the historical treatment review and patients are allocated the proper number of test strips. Similarly, all related paper claims should be submitted for processing as soon as possible. When a claim is submitted, the HNS will look back over the prior 365 days, and will calculate whether the recipient has met their allotted maximum during that time. If the recipient has not reached their maximum number of allotted test strips over the previous 365 days, they will be eligible to receive test strips up to the maximum amount. 5. When does the new policy for reimbursement start for each patient? The application of a maximum number of test strips provided to an individual in a 365- day period begins when a pharmacist submits the first claim for BGTS for the patient on or after August 1, How should simultaneous claims for test strips and insulin/antidiabetes medications be processed? When submitting a claim for insulin or anti-diabetes medication along with a claim for BGTS, pharmacists should submit all anti-diabetes medications prior to entering the test strip claim. This ensures that the most current drug profile is included in the historical treatment review and patients are allocated the proper number of test strips. All related paper claims should be submitted for processing as soon as possible. The test strip allotment will apply to both online and paper claims. 7. What insulin or anti-diabetes medications will the HNS use to determine treatment categories for ODB recipients? The HNS system will determine the treatment category for an ODB recipient based upon claims for insulin products or other anti-diabetes medications available on the ODB Formulary. Treatment Class Patients managing diabetes with anti-diabetes medication with HIGH risk of causing hypoglycemia Patients managing diabetes using anti-diabetes medication with LOW risk of causing hypoglycemia Medication Name or Class Sulfonylureas (e.g. glyburide) Meglitinides (e.g. nateglinide) Metformin Acarbose Thiazolidinediones (e.g. pioglitazone) DPP-4 inhibitors (e.g. saxagliptin) 3
4 GLP-1 agonists (e.g. exenatide) 8. What should I do if an ODB recipient has received insulin or antidiabetes medications that were not reimbursed under the ODB program? The intervention code NF Override Quantity Appropriate may be used for patients who require more than 200 or 400 test strips in a given year because they had claims for insulin and/or anti-diabetes medications at high risk for hypoglycemia in the recent six months that were not reimbursed under the ODB program. The identified anti-diabetes medications that were reimbursed by private drug insurance plan or paid by the patient must be documented and readily available for audit purposes. 9. How would I know how many strips a patient is entitled? Pharmacists can determine the overall annual allotment for test strips that patients are entitled to by assessing the patient s medications in the past six months and the number of strips already reimbursed in the past 365 days at any point in time. In order for patients to track the remaining number of strips available for reimbursement, patients should be encouraged to have their prescriptions filled at one pharmacy to ensure that they have a complete history of all the medications and test strips that they have received in the past. 4
5 10. What happens when an ODB recipient reaches their annual allotment of test strips? If the maximum number of test strips is exceeded in a 365-day period for a given patient, a response code is provided to the pharmacist indicating that the recipient has reached their allotment and the claim is rejected. If the claim can be accepted by reducing the quantity of strips dispensed, a response code and message line will be returned to the pharmacy (OC Quantity Reduction Required) to advise the pharmacist of the allowable number of test strips for the recipient before they reach their limit. If the response code LO Maximum Benefit Exceeded is returned, the recipient has exceeded their maximum benefit. 11. What if an ODB recipient requires more test strips annually than they are allowed under the new limits? A maximum of 100 additional test strips may be reimbursed for non-insulin dependent patients who have been directed by a healthcare professional, for clinical reasons, to monitor blood glucose levels more closely than what would be required over a one year period as determined by their anti-diabetic medication profile. Pharmacists can enter the intervention code MG Override Clinical Reasons for patients with documented clinical reasons for requiring test strips beyond the prescribed annual limit for a given 365-day period and submit a claim of up to 100 test strips. Documentation should include the reason for the higher than recommended monitoring schedule, specific testing frequency (if not indicated on the prescription) and the name of the referring healthcare professional. 12. What are some clinical reasons for which a person may require additional BGTS? Patients may require more frequent testing for a variety of reasons. Some common reasons for which an individual may require more frequent testing include the following: Patient has experienced acute illness or infection that affected blood glucose control over a sustained period of time Issues related to drug interactions which have impacted blood glucose control Patient has gestational diabetes Patient has an occupation that requires strict avoidance of hypoglycemia (e.g., pilots, air-traffic controllers, critical positions in railways) Patient is not meeting glycemic targets for 3 months or greater 5
6 Documentation should include reason for the higher than recommended monitoring schedule, specific testing frequency (if not indicated on the prescription) and the name of the referring healthcare professional. 13. My patient indicates they have not received their maximum allotment but I am receiving a maximum limit code. What should I do? If a patient provides information that differs from what is being indicated in the HNS, the patient medication profile may be out of date or incomplete. If there is a discrepancy is the pharmacist should: 1. Reconfirm the patient s allotment based on the patient history to ensure that the limit indicated in the HNS is correct. All prescription information should be confirmed as up-to-date. 2. Review what order prescriptions were entered - if a prescription was entered into the system after the claim for the test strips, the limit indicated for the patient may not be accurate. 3. Inquire to see if the patient has received any medications outside of the ODB program within the last six months that would entitle them to a higher allotment. If it is determined that the patient is wrong about their allotment (change in medication profile, etc.), an override is not allowed. 14. Can a pharmacist prescribe or authorise a renewal for BGTS? BGTS are designated listed substances as defined by ODBA and require a prescription by a physician in order to be eligible for reimbursement under the ODB Program. Prescriptions and prescription extensions by pharmacists for BGTS are not eligible for reimbursement under the ODB program. The ministry will continue to monitor the impact of this policy. 15. How does the HNS system determine the allotment if a patient changes to a medication from a different treatment class? When a BGTS claim is submitted, the HNS determines the patient s diabetic medication use in the previous six months and calculates the number of test strips already reimbursed in the previous 365 days. Thus a patient s allotment can change if a patient originally uses a medication with a high risk of hypoglycaemia, but then only uses medications with a low risk of hypoglycaemia or vice versa. For example: A physician discontinues glyburide, a medication with a high risk of hypoglycaemia, and the patient remains on medications with a low risk of hypoglycaemia. The patient s allotment was originally 400 annual test strips, but will change to 200 annual test strips six months after the last fill of glyburide. The 6
7 total number of strips reimbursed in the past 365 days is still calculated in the same manner. Education of the patient s monitoring frequency is important when there are changes with the patient s medications. 16. A patient changed their diabetic testing device and their current test strips are not compatible with their new device. Will they receive the full allocation of test strips for their device? Changing to a new device does not qualify as a clinical reason for additional test strips. If the patient has not reached their allotment, they can receive test strips for their new device, but the total allocation includes the test strips from their previous device. It is recommended that the patient use a device that will be compatible with their current test strips to ensure the patient does not exceed their allocated maximum number of test strips for the year. If there are malfunctions with their current device, it is recommended that the patient use a new device that remains compatible with their test strips. 7
8 17. How can I ensure that my patient appropriately manages their blood glucose monitoring? We re asking that you discuss the new limits with your patients and speak to them about proper testing practices and frequency of testing that s right for them. Informing patients to monitor their blood sugar as directed by their health care professional can help ensure that patients monitor their blood glucose effectively and prevent patients from running out of test strips. Patients should be reminded that BGTS do have an expiry date and that refilling their test strips judiciously can ensure that they are not left with expired test strips. A blood glucose log sheet is available on the Canadian Diabetes Association website ( that a patient can use to keep track of their blood glucose levels. Health care professionals may use this tracking sheet to assess the patient s blood glucose control and determine if they are monitoring their blood glucose as recommended by their healthcare provider. Printable documents for sharing with your patients are available on the ministry website at How does the HNS administer claims for BGTS that exceed the usual 50 or 100 strips per pack? Changes to the HNS for the reimbursement of blood glucose test strips for eligible ODB recipients have been made to accommodate test strip packaging that exceeds the usual 50 or 100 strips per pack. Test strip allotments for ODB recipients take into account diabetic test strip products that contain 51 and 102 test strips per package. The revised limits are administered automatically without requiring the use of an override code. 8
Notice from the Executive Officer: Promoting Compliance with the Existing Limited Use Criteria for Fentanyl Transdermal Patch
 Notice from the Executive Officer: Promoting Compliance with the Existing Limited Use Criteria for Fentanyl Transdermal Patch Frequently Asked Questions 1. What is the new Health Network System feature
Notice from the Executive Officer: Promoting Compliance with the Existing Limited Use Criteria for Fentanyl Transdermal Patch Frequently Asked Questions 1. What is the new Health Network System feature
INCRETIN THERAPY ROUNDTABLE: Putting recommendations into practice. Program guide
 INCRETIN THERAPY ROUNDTABLE: Putting recommendations into practice Program guide 1 TABLE OF CONTENTS Objectives. 2 Case study and interactive questions... 3 Top 5 clinical tips..... 11 OBJECTIVES After
INCRETIN THERAPY ROUNDTABLE: Putting recommendations into practice Program guide 1 TABLE OF CONTENTS Objectives. 2 Case study and interactive questions... 3 Top 5 clinical tips..... 11 OBJECTIVES After
Canadian Diabetes Association 2013
 Spring 2014 Canadian Diabetes Association 2013 clinical practice guidelines - Do claims data align to the guidelines? Canadian Diabetes Association 2013 clinical practice guidelines - Do claims data align
Spring 2014 Canadian Diabetes Association 2013 clinical practice guidelines - Do claims data align to the guidelines? Canadian Diabetes Association 2013 clinical practice guidelines - Do claims data align
Alabama Medicaid Pharmacy Override
 Alabama Medicaid Pharmacy Override Therapeutic Duplication, Early Refill, Maximum Unit, Prescription Limit Switchover, Dispense as Written, Accumulation Edit, Maintenance Supply Opt Out, and Maximum Cost
Alabama Medicaid Pharmacy Override Therapeutic Duplication, Early Refill, Maximum Unit, Prescription Limit Switchover, Dispense as Written, Accumulation Edit, Maintenance Supply Opt Out, and Maximum Cost
Self Monitoring of Blood Glucose (SMBG)
 Self Monitoring of Blood Glucose (SMBG) DCPNS Provincial Workshop April 28, 2011 Presenter: Pam McLean-Veysey Team Leader, Drug Evaluation Unit, Capital Health 1 Disclosure Pam McLean-Veysey provides drug
Self Monitoring of Blood Glucose (SMBG) DCPNS Provincial Workshop April 28, 2011 Presenter: Pam McLean-Veysey Team Leader, Drug Evaluation Unit, Capital Health 1 Disclosure Pam McLean-Veysey provides drug
Overview. Ontario Public Drug Programs, Ministry of Health and Long-Term Care
 Ontario Public Drug Programs, Ministry of Health and Long-Term Care Frequently Asked Questions for Pharmacists October 2015 Pharmacist Administration of the Publicly Funded Influenza Vaccine and Claims
Ontario Public Drug Programs, Ministry of Health and Long-Term Care Frequently Asked Questions for Pharmacists October 2015 Pharmacist Administration of the Publicly Funded Influenza Vaccine and Claims
Joslin Diabetes Center Joslin Diabetes Forum 2013: The Impact of Comorbidities on Glucose Control Scenario 2: Reduced Renal Function
 Scenario 2: Reduced Renal Function 62 y.o. white man with type 2 diabetes for 18 years Hypertension and hypercholesterolemia Known proliferative retinopathy Current medications: Metformin 1000 mg bid Glyburide
Scenario 2: Reduced Renal Function 62 y.o. white man with type 2 diabetes for 18 years Hypertension and hypercholesterolemia Known proliferative retinopathy Current medications: Metformin 1000 mg bid Glyburide
1. When are dispensers able to provide publicly funded naloxone emergency kits for Ontarians?
 Ontario Public Drug Programs Division Ontario Naloxone Program for Pharmacies (ONPP) Frequently Asked Questions for Pharmacy Dispensers: Providing Publicly Funded Naloxone Kits and Claims Submission Using
Ontario Public Drug Programs Division Ontario Naloxone Program for Pharmacies (ONPP) Frequently Asked Questions for Pharmacy Dispensers: Providing Publicly Funded Naloxone Kits and Claims Submission Using
Ministry of Health and Long-Term Care Ontario Public Drug Programs. 1. What changes are occurring to the ONPP?
 Funding of Naloxone Nasal Spray Kits through the Ontario Naloxone Program for Pharmacies (ONPP) and Updates to the Existing Program: Frequently Asked Questions for Pharmacists 1. What changes are occurring
Funding of Naloxone Nasal Spray Kits through the Ontario Naloxone Program for Pharmacies (ONPP) and Updates to the Existing Program: Frequently Asked Questions for Pharmacists 1. What changes are occurring
Collaborative Practice Agreement
 Collaborative Practice Agreement [community pharmacy name] [address] [phone number] [physician practice] [address] [phone number] Effective: [date] Expiration: [date] 1 Table of Contents 1.0 Introduction...4
Collaborative Practice Agreement [community pharmacy name] [address] [phone number] [physician practice] [address] [phone number] Effective: [date] Expiration: [date] 1 Table of Contents 1.0 Introduction...4
This Frequently Asked Questions document contains various aspects of UIIP in the following sections. Overview Eligibility...
 Ontario Public Drug Programs, Ministry of Health and Long-Term Care Frequently Asked Questions for Pharmacists October 2017: Pharmacist Administration of the Publicly Funded Influenza Vaccine and Claims
Ontario Public Drug Programs, Ministry of Health and Long-Term Care Frequently Asked Questions for Pharmacists October 2017: Pharmacist Administration of the Publicly Funded Influenza Vaccine and Claims
CADTH Optimal use report
 Canadian Agency for Drugs and Technologies in Health Agence canadienne des médicaments et des technologies de la santé CADTH Optimal use report Volume 3, Issue 1D July 2013 Optimal Use Recommendations
Canadian Agency for Drugs and Technologies in Health Agence canadienne des médicaments et des technologies de la santé CADTH Optimal use report Volume 3, Issue 1D July 2013 Optimal Use Recommendations
CASE A2 Managing Between-meal Hypoglycemia
 Managing Between-meal Hypoglycemia 1 I would like to discuss this case of a patient who, overall, was doing well on her therapy until she made an important lifestyle change to lose weight. This is a common
Managing Between-meal Hypoglycemia 1 I would like to discuss this case of a patient who, overall, was doing well on her therapy until she made an important lifestyle change to lose weight. This is a common
PEER REVIEW HISTORY ARTICLE DETAILS TITLE (PROVISIONAL)
 PEER REVIEW HISTORY BMJ Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form (see an example) and are provided with free text boxes to
PEER REVIEW HISTORY BMJ Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form (see an example) and are provided with free text boxes to
PHARMACISTS INTERACTIVE EDUCATION CASE STUDIES
 PHARMACISTS INTERACTIVE EDUCATION CASE STUDIES Disclaimer: The information in this document is not a substitute for clinical judgment in the care of a particular patient. CADTH is not liable for any damages
PHARMACISTS INTERACTIVE EDUCATION CASE STUDIES Disclaimer: The information in this document is not a substitute for clinical judgment in the care of a particular patient. CADTH is not liable for any damages
Chronic Hepatitis B Drug Therapies: Frequently Asked Questions
 Chronic Hepatitis B Drug Therapies: Frequently Asked Questions 1. What is the funding status for drugs used in the of chronic Hepatitis B infection? In Ontario, current s for chronic Hepatitis B infection
Chronic Hepatitis B Drug Therapies: Frequently Asked Questions 1. What is the funding status for drugs used in the of chronic Hepatitis B infection? In Ontario, current s for chronic Hepatitis B infection
Technician Tutorial: Dispensing Insulin and Other Injectable Meds
 (Page 1 of 6) Technician Tutorial: Dispensing Insulin and Other Injectable Meds Insulin is a hormone secreted by the pancreas. It helps the body use glucose as an energy source. Insulin acts like a key
(Page 1 of 6) Technician Tutorial: Dispensing Insulin and Other Injectable Meds Insulin is a hormone secreted by the pancreas. It helps the body use glucose as an energy source. Insulin acts like a key
This program applies to Commercial, GenPlus and Health Insurance Marketplace formularies.
 OBJECTIVE The intent of the GLP-1 (glucagon-like peptide-1) Agonists [Adlyxin (lixisenatide), Byetta (exenatide), Bydureon (exenatide extended-release), Tanzeum (albiglutide), Trulicity (dulaglutide),
OBJECTIVE The intent of the GLP-1 (glucagon-like peptide-1) Agonists [Adlyxin (lixisenatide), Byetta (exenatide), Bydureon (exenatide extended-release), Tanzeum (albiglutide), Trulicity (dulaglutide),
Dipeptidyl-Peptidase 4 (DPP-4) Inhibitors Drug Class Prior Authorization Protocol
 Dipeptidyl-Peptidase 4 (DPP-4) Inhibitors Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed
Dipeptidyl-Peptidase 4 (DPP-4) Inhibitors Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed
PHARMACISTS INTERACTIVE EDUCATION CASE STUDIES
 PHARMACISTS INTERACTIVE EDUCATION CASE STUDIES Disclaimer: The information in this document is not a substitute for clinical judgment in the care of a particular patient. CADTH is not liable for any damages
PHARMACISTS INTERACTIVE EDUCATION CASE STUDIES Disclaimer: The information in this document is not a substitute for clinical judgment in the care of a particular patient. CADTH is not liable for any damages
The Many Faces of T2DM in Long-term Care Facilities
 The Many Faces of T2DM in Long-term Care Facilities Question #1 Which of the following is a risk factor for increased hypoglycemia in older patients that may suggest the need to relax hyperglycemia treatment
The Many Faces of T2DM in Long-term Care Facilities Question #1 Which of the following is a risk factor for increased hypoglycemia in older patients that may suggest the need to relax hyperglycemia treatment
APIDRA (insulin glulisine) injection vial APIDRA SOLOSTAR (insulin glulisine) subcutaneous solution pen-injector
 APIDRA SOLOSTAR (insulin glulisine) subcutaneous solution pen-injector Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific
APIDRA SOLOSTAR (insulin glulisine) subcutaneous solution pen-injector Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific
Feidhmeannacht na Seirbhíse Sláinte
 Feidhmeannacht na Seirbhíse Sláinte Health Service Executive Feidhmeannacht na Seirbhíse Sláinte Seirbhís Aisíocaíochta Cúraim Phríomhúil Bealach amach 5 an M50 An Bóthar Thuaidh Fionnghlas Baile Átha
Feidhmeannacht na Seirbhíse Sláinte Health Service Executive Feidhmeannacht na Seirbhíse Sláinte Seirbhís Aisíocaíochta Cúraim Phríomhúil Bealach amach 5 an M50 An Bóthar Thuaidh Fionnghlas Baile Átha
Disclosure. Learning Objectives. Case. Diabetes Update: Incretin Agents in Diabetes-When to Use Them? I have no disclosures to declare
 Disclosure Diabetes Update: Incretin Agents in Diabetes-When to Use Them? I have no disclosures to declare Spring Therapeutics Update 2011 CSHP BC Branch Anar Dossa BScPharm Pharm D CDE April 20, 2011
Disclosure Diabetes Update: Incretin Agents in Diabetes-When to Use Them? I have no disclosures to declare Spring Therapeutics Update 2011 CSHP BC Branch Anar Dossa BScPharm Pharm D CDE April 20, 2011
Reviewing Diabetes Guidelines. Newsletter compiled by Danny Jaek, Pharm.D. Candidate
 Reviewing Diabetes Guidelines Newsletter compiled by Danny Jaek, Pharm.D. Candidate AL AS KA N AT IV E DI AB ET ES TE A M Volume 6, Issue 1 Spring 2011 Dia bet es Dis pat ch There are nearly 24 million
Reviewing Diabetes Guidelines Newsletter compiled by Danny Jaek, Pharm.D. Candidate AL AS KA N AT IV E DI AB ET ES TE A M Volume 6, Issue 1 Spring 2011 Dia bet es Dis pat ch There are nearly 24 million
Canadian Society of Internal Medicine Annual Meeting 2016 Montreal, QC
 Canadian Society of Internal Medicine Annual Meeting 2016 Montreal, QC Choosing the Right Agent for your Patient with diabetes: Individualizing type 2 diabetes management in light of the expanding therapies
Canadian Society of Internal Medicine Annual Meeting 2016 Montreal, QC Choosing the Right Agent for your Patient with diabetes: Individualizing type 2 diabetes management in light of the expanding therapies
CADTH THERAPEUTIC REVIEW New Drugs for Type 2 Diabetes: Second-Line Therapy Recommendations Report
 CADTH THERAPEUTIC REVIEW New Drugs for Type 2 Diabetes: Second-Line Therapy Recommendations Report Product Line: Therapeutic Review Recommendations Volume: Volume 4 Issue: No. 1c Publication Date: May
CADTH THERAPEUTIC REVIEW New Drugs for Type 2 Diabetes: Second-Line Therapy Recommendations Report Product Line: Therapeutic Review Recommendations Volume: Volume 4 Issue: No. 1c Publication Date: May
Oral and Injectable Non-insulin Antihyperglycemic Agents
 Appendix 5: Diabetes Education and Medical Management in Adults with Diabetes Oral and Injectable Non-insulin s This directive will be implemented by RPhs, RNs or RDs who have been deemed authorized implementers.
Appendix 5: Diabetes Education and Medical Management in Adults with Diabetes Oral and Injectable Non-insulin s This directive will be implemented by RPhs, RNs or RDs who have been deemed authorized implementers.
GLYXAMBI (empagliflozin-linagliptin) oral tablet
 GLYXAMBI (empagliflozin-linagliptin) oral tablet Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This
GLYXAMBI (empagliflozin-linagliptin) oral tablet Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This
Cardiovascular Health and Diabetes Screening for People with Schizophrenia
 Cardiovascular Health and Diabetes Screening for People with Schizophrenia The percentage of members 25 years and older with a schizophrenia diagnosis and who were prescribed any antipsychotic medication,
Cardiovascular Health and Diabetes Screening for People with Schizophrenia The percentage of members 25 years and older with a schizophrenia diagnosis and who were prescribed any antipsychotic medication,
Dispensing and administration of emergency opioid antagonist without a
 68-7-23. Dispensing and administration of emergency opioid antagonist without a prescription. (a) A pharmacist may dispense an FDA-approved emergency opioid antagonist and the necessary medical supplies
68-7-23. Dispensing and administration of emergency opioid antagonist without a prescription. (a) A pharmacist may dispense an FDA-approved emergency opioid antagonist and the necessary medical supplies
LEVEMIR (insulin detemir) subcutaneous solution LEVEMIR FLEXTOUCH (insulin detemir) subcutaneous solution pen-injector
 LEVEMIR FLEXTOUCH (insulin detemir) subcutaneous solution pen-injector Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific
LEVEMIR FLEXTOUCH (insulin detemir) subcutaneous solution pen-injector Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific
Next Steps for Clinicians
 Controversies in the Management of Patients with Type 2 Diabetes The New England Comparative Effectiveness Public Advisory Council An Action Guide for Type 2 Diabetes Management Next Steps for Clinicians
Controversies in the Management of Patients with Type 2 Diabetes The New England Comparative Effectiveness Public Advisory Council An Action Guide for Type 2 Diabetes Management Next Steps for Clinicians
Pharmacy Audit Recovery Guidelines
 1/1E DAW 1 DAW 1 Error The prescription order does not indicate the prescriber ordered brand name. 2 /2E DAW 2 DAW 2 Not Documented The documentation does not specify the patient s request for brand. Pharmacy
1/1E DAW 1 DAW 1 Error The prescription order does not indicate the prescriber ordered brand name. 2 /2E DAW 2 DAW 2 Not Documented The documentation does not specify the patient s request for brand. Pharmacy
ADMELOG, NOVOLIN, NOVOLOG, and FIASP
 ADMELOG, NOVOLIN, NOVOLOG, and FIASP Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy
ADMELOG, NOVOLIN, NOVOLOG, and FIASP Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy
TABLE 1A : Formulary Coverage of Insulin Therapies & Indications for Use in Various Populations
 177 TABLE 1A : Formulary Coverage of Insulin Therapies & Indications for Use in Various Populations Formulary Coverage Indication for use with: INSULIN THERAPY NS NB NL PE ADULTS PEDIATRICS PREGNANCY BOLUS
177 TABLE 1A : Formulary Coverage of Insulin Therapies & Indications for Use in Various Populations Formulary Coverage Indication for use with: INSULIN THERAPY NS NB NL PE ADULTS PEDIATRICS PREGNANCY BOLUS
Flu Vaccination Clinics
 Flu Vaccination Clinics June 18, 2009 Shepell fgi Contact: Paula Allen Vice President Organizational Solutions & Training 416-355-5495 1-800-461-9722 ext. 5495 pallen@shepellfgi.com www.shepellfgi.com
Flu Vaccination Clinics June 18, 2009 Shepell fgi Contact: Paula Allen Vice President Organizational Solutions & Training 416-355-5495 1-800-461-9722 ext. 5495 pallen@shepellfgi.com www.shepellfgi.com
TABLE 1A: Formulary Coverage of Insulin Therapies & Indications for Use in Various Populations
 177 TABLE 1A: Formulary Coverage of Insulin Therapies & Indications for Use in Various Populations TABLE 1A : Formulary Coverage of Insulin Therapies & Indications for Use in Various Populations Formulary
177 TABLE 1A: Formulary Coverage of Insulin Therapies & Indications for Use in Various Populations TABLE 1A : Formulary Coverage of Insulin Therapies & Indications for Use in Various Populations Formulary
In-Hospital Management of Diabetes. Dr Benjamin Schiff Assistant Professor McGill University
 In-Hospital Management of Diabetes Dr Benjamin Schiff Assistant Professor McGill University No conflict of interest to declare CLINICAL SCENARIO 62 y/o male with hx of DM 2, COPD, and HT is admitted with
In-Hospital Management of Diabetes Dr Benjamin Schiff Assistant Professor McGill University No conflict of interest to declare CLINICAL SCENARIO 62 y/o male with hx of DM 2, COPD, and HT is admitted with
Glyceamic control is indicated by 1. Fasting blood sugar less than 126 mg/dl 2. Random blood sugar 3. HbA1c less than 6.5 % Good glycaemic control
 Glyceamic control is indicated by 1. Fasting blood sugar less than 126 mg/dl 2. Random blood sugar 3. HbA1c less than 6.5 % Good glycaemic control can prevent many of early type 1 DM(in DCCT trail ). UK
Glyceamic control is indicated by 1. Fasting blood sugar less than 126 mg/dl 2. Random blood sugar 3. HbA1c less than 6.5 % Good glycaemic control can prevent many of early type 1 DM(in DCCT trail ). UK
GLP-1 (glucagon-like peptide-1) Agonists (Byetta, Bydureon, Tanzeum, Trulicity, Victoza ) Step Therapy and Quantity Limit Criteria Program Summary
 OBJECTIVE The intent of the GLP-1 (glucagon-like peptide-1) s (Byetta/exenatide, Bydureon/ exenatide extended-release, Tanzeum/albiglutide, Trulicity/dulaglutide, and Victoza/liraglutide) Step Therapy
OBJECTIVE The intent of the GLP-1 (glucagon-like peptide-1) s (Byetta/exenatide, Bydureon/ exenatide extended-release, Tanzeum/albiglutide, Trulicity/dulaglutide, and Victoza/liraglutide) Step Therapy
Scottish Medicines Consortium
 Scottish Medicines Consortium saxagliptin, 5mg film-coated tablet (Onglyza ) No. (603/10) Bristol-Myers Squibb Pharmaceuticals Ltd 05 February 2010 The Scottish Medicines Consortium (SMC) has completed
Scottish Medicines Consortium saxagliptin, 5mg film-coated tablet (Onglyza ) No. (603/10) Bristol-Myers Squibb Pharmaceuticals Ltd 05 February 2010 The Scottish Medicines Consortium (SMC) has completed
BLOOD GLUCOSE METER TEST STRIP STEP THERAPY CRITERIA
 BLOOD GLUCOSE METER TEST STRIP STEP THERAPY CRITERIA Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan.
BLOOD GLUCOSE METER TEST STRIP STEP THERAPY CRITERIA Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan.
YOU HAVE DIABETES. Angie O Connor Community Diabetes Nurse Specialist 25th September 2013
 YOU HAVE DIABETES Angie O Connor Community Diabetes Nurse Specialist 25th September 2013 Predicated 2015 figures are already met 1 in 20 have diabetes:1in8 over 60years old Definite Diagnosis is key Early
YOU HAVE DIABETES Angie O Connor Community Diabetes Nurse Specialist 25th September 2013 Predicated 2015 figures are already met 1 in 20 have diabetes:1in8 over 60years old Definite Diagnosis is key Early
Guide to Starting and Adjusting Insulin for Type 2 Diabetes*
 Guide to Starting and Adjusting Insulin for Type 2 Diabetes* www.cadth.ca * Adapted from Guide to Starting and Adjusting Insulin for Type 2 Diabetes, 2008 International Diabetes Center, Minneapolis, MN.
Guide to Starting and Adjusting Insulin for Type 2 Diabetes* www.cadth.ca * Adapted from Guide to Starting and Adjusting Insulin for Type 2 Diabetes, 2008 International Diabetes Center, Minneapolis, MN.
Pharmacy Coverage Guidelines are subject to change as new information becomes available.
 Metformin tablet SR 24-hour modified release oral tablet Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit
Metformin tablet SR 24-hour modified release oral tablet Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit
New Drugs for Type 2 Diabetes: Second-Line Therapy Recommendations Report
 CADTH THERAPEUTIC REVIEW New Drugs for Type 2 Diabetes: Second-Line Therapy Recommendations Report Product Line: Volume: Issue: Publication Date: Report Length: Therapeutic Review Recommendations Disclaimer:
CADTH THERAPEUTIC REVIEW New Drugs for Type 2 Diabetes: Second-Line Therapy Recommendations Report Product Line: Volume: Issue: Publication Date: Report Length: Therapeutic Review Recommendations Disclaimer:
Society for Ambulatory Anesthesia Consensus Statement on Perioperative Blood Glucose Management in Diabetic Patients Undergoing Ambulatory Surgery
 Society for Ambulatory Anesthesia Consensus Statement on Perioperative Blood Glucose Management in Diabetic Patients Undergoing Ambulatory Surgery Girish P. Joshi, MB BS, MD, FFARCSI Anesthesia & Analgesia
Society for Ambulatory Anesthesia Consensus Statement on Perioperative Blood Glucose Management in Diabetic Patients Undergoing Ambulatory Surgery Girish P. Joshi, MB BS, MD, FFARCSI Anesthesia & Analgesia
Soliqua (insulin glargine and lixisenatide), Xultophy (insulin degludec and liraglutide)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.48 Subject: Insulin GLP-1 Combinations Page: 1 of 5 Last Review Date: September 15, 2017 Insulin GLP-1
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.48 Subject: Insulin GLP-1 Combinations Page: 1 of 5 Last Review Date: September 15, 2017 Insulin GLP-1
Choosing a Diabetes Strategy Where to Start and Where to Go
 Choosing a Diabetes Strategy Where to Start and Where to Go Erin Keely, MD, FRCPC; and Sharon Brez, RN, BScN, MA(Ed), CDE As presented at the University of Ottawa's 52nd Annual Refresher Course for Family
Choosing a Diabetes Strategy Where to Start and Where to Go Erin Keely, MD, FRCPC; and Sharon Brez, RN, BScN, MA(Ed), CDE As presented at the University of Ottawa's 52nd Annual Refresher Course for Family
Type 2 Diabetes: Where Do We Start with Treatment? DIABETES EDUCATION. Diabetes Mellitus: Complications and Co-Morbid Conditions
 Diabetes Mellitus: Complications and Co-Morbid Conditions ADA Guidelines for Glycemic Control: 2016 Retinopathy Between 2005-2008, 28.5% of patients with diabetes 40 years and older diagnosed with diabetic
Diabetes Mellitus: Complications and Co-Morbid Conditions ADA Guidelines for Glycemic Control: 2016 Retinopathy Between 2005-2008, 28.5% of patients with diabetes 40 years and older diagnosed with diabetic
DIABETES DEBATE - IS NEW BETTER?
 DIABETES DEBATE - IS NEW BETTER? WHAT MEDICATION CLASS AFTER METFORMIN TO CONTROL BLOOD SUGAR Dr. Lydia Hatcher, MD, CCFP, FCFP, CHE, D-CAPM Associate Clinical Professor of Family Medicine, McMaster Chief
DIABETES DEBATE - IS NEW BETTER? WHAT MEDICATION CLASS AFTER METFORMIN TO CONTROL BLOOD SUGAR Dr. Lydia Hatcher, MD, CCFP, FCFP, CHE, D-CAPM Associate Clinical Professor of Family Medicine, McMaster Chief
New Measure Recommended for Endorsement by PQA
 New Measure Recommended for Endorsement by PQA Measure: Statin Use in Persons with Diabetes Description: The percentage of patients ages 40 75 years who were dispensed a medication for diabetes that receive
New Measure Recommended for Endorsement by PQA Measure: Statin Use in Persons with Diabetes Description: The percentage of patients ages 40 75 years who were dispensed a medication for diabetes that receive
LOUISIANA MEDICAID PROGRAM ISSUED: 02/01/12 REPLACED: 02/01/94 CHAPTER 5: PROFESSIONAL SERVICES SECTION 5.1: COVERED SERVICES PAGE(S) 6
 Diabetes Education Management Training Diabetes self management training (DSMT) is a collaborative process through which recipients with diabetes gain knowledge and skills needed to modify behavior and
Diabetes Education Management Training Diabetes self management training (DSMT) is a collaborative process through which recipients with diabetes gain knowledge and skills needed to modify behavior and
SECTION 2. Diabetes Management Challenges Facing the Patient, Physician, and Payer
 n report n SECTION 2 Diabetes Management Challenges Facing the Patient, Physician, and Payer The content in this section is based on a presentation at the roundtable meeting by Bruce Niebylski, MD Guidelines,
n report n SECTION 2 Diabetes Management Challenges Facing the Patient, Physician, and Payer The content in this section is based on a presentation at the roundtable meeting by Bruce Niebylski, MD Guidelines,
Changes to the National Diabetes Services Scheme (NDSS)
 Changes to the National Diabetes Services Scheme (NDSS) June 2016 FREQUENTLY ASKED QUESTIONS 1. What are the changes from 1 July 2016? 2. Why are the supply changes happening? 3. What is the role of Diabetes
Changes to the National Diabetes Services Scheme (NDSS) June 2016 FREQUENTLY ASKED QUESTIONS 1. What are the changes from 1 July 2016? 2. Why are the supply changes happening? 3. What is the role of Diabetes
Abbreviations DPP-IV dipeptidyl peptidase IV DREAM Diabetes REduction Assessment with ramipril and rosiglitazone
 Index Abbreviations DPP-IV dipeptidyl peptidase IV DREAM Diabetes REduction Assessment with ramipril and rosiglitazone Medication GAD glutamic acid decarboxylase GLP-1 glucagon-like peptide 1 NPH neutral
Index Abbreviations DPP-IV dipeptidyl peptidase IV DREAM Diabetes REduction Assessment with ramipril and rosiglitazone Medication GAD glutamic acid decarboxylase GLP-1 glucagon-like peptide 1 NPH neutral
HARVARD PILGRIM HEALTH CARE RECOMMENDED MEDICATION REQUEST GUIDELINES
 Generic Brand HICL GCN Exception/Other BLOOD SUGAR DIAGNOSTIC DIABETIC TEST STRIPS 25200 NOTE: Requests for preferred blood glucose (diabetic) test strips manufactured by Abbott Diabetes Care (such as
Generic Brand HICL GCN Exception/Other BLOOD SUGAR DIAGNOSTIC DIABETIC TEST STRIPS 25200 NOTE: Requests for preferred blood glucose (diabetic) test strips manufactured by Abbott Diabetes Care (such as
For the 2017/2018 Influenza Immunization Season. Patient Eligibility. Description. Ontario Public Drug Programs, Ministry of Health and Long-Term Care
 Ontario Public Drug Programs, Ministry of Health and Long-Term Care Notice from the Executive Officer: Pharmacist Administration of Publicly Funded Influenza Vaccines and Claims Submission using the Health
Ontario Public Drug Programs, Ministry of Health and Long-Term Care Notice from the Executive Officer: Pharmacist Administration of Publicly Funded Influenza Vaccines and Claims Submission using the Health
This case study is supported by an educational grant from Abbott.
 Program Name: Planning Committee: When and How to Start or Intensify Insulin Therapy in Your Patients with Type 2 Diabetes Alice Cheng, MD, FRCPC Jean-Francois Yale, MD, CSPQ Lori Berard, RN, CDE Sol Stern,
Program Name: Planning Committee: When and How to Start or Intensify Insulin Therapy in Your Patients with Type 2 Diabetes Alice Cheng, MD, FRCPC Jean-Francois Yale, MD, CSPQ Lori Berard, RN, CDE Sol Stern,
Medicines Optimisation Team Standard Operating procedure for Alogliptin Audit:
 Medicines Optimisation Team Standard Operating procedure for Alogliptin Audit: To review type 2 diabetic patients prescribed dipedtidylpeptidase-4 (DPP-4) inhibitors (linagliptin (Trajenta, saxagliptin
Medicines Optimisation Team Standard Operating procedure for Alogliptin Audit: To review type 2 diabetic patients prescribed dipedtidylpeptidase-4 (DPP-4) inhibitors (linagliptin (Trajenta, saxagliptin
Note: Mandatory measures are those measures that are a requirement of accreditation and must be reported to URAC on an annual basis.
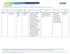 Note: Mandatory measures are those measures that are a requirement of accreditation and must be reported to URAC on an annual basis. DESCRIPTION NUMERATOR DENOMINATOR DM2012-12 Portion of Days Covered
Note: Mandatory measures are those measures that are a requirement of accreditation and must be reported to URAC on an annual basis. DESCRIPTION NUMERATOR DENOMINATOR DM2012-12 Portion of Days Covered
GUIDELINE FOR SELF MONITORING OF BLOOD GLUCOSE IN ADULTS WITH DIABETES
 It is recognised that self- monitoring of blood glucose (SMBG) may have an important role to play for some patients with. It can allow individuals to see what impact particular behaviours, such as dietary
It is recognised that self- monitoring of blood glucose (SMBG) may have an important role to play for some patients with. It can allow individuals to see what impact particular behaviours, such as dietary
New: Health Incentive Credits In 2012, Health Incentive Credits can be earned to use toward the calendar year deductible.
 Core s LifeStyles Management Program is a program which identifies and assists individuals with known and unknown chronic illnesses. The purpose of the LifeStyles Management Program is to promote member
Core s LifeStyles Management Program is a program which identifies and assists individuals with known and unknown chronic illnesses. The purpose of the LifeStyles Management Program is to promote member
PRESCRIPTION PAD. The Newsletter of the Cumbria Area Prescribing Committee. February 2012 No. 18. Click here to find more. News from the MHRA
 PRESCRIPTION PAD The Newsletter of the Cumbria Area Prescribing Committee February 2012 No. 18 Click here to find more Clinical policy and Formulary news Lothian changes Targeted Medication Use Reviews
PRESCRIPTION PAD The Newsletter of the Cumbria Area Prescribing Committee February 2012 No. 18 Click here to find more Clinical policy and Formulary news Lothian changes Targeted Medication Use Reviews
DRUG PRODUCT INTERCHANGEABILITY AND PRICING ACT
 c t DRUG PRODUCT INTERCHANGEABILITY AND PRICING ACT PLEASE NOTE This document, prepared by the Legislative Counsel Office, is an office consolidation of this Act, current to September 22, 2014. It is intended
c t DRUG PRODUCT INTERCHANGEABILITY AND PRICING ACT PLEASE NOTE This document, prepared by the Legislative Counsel Office, is an office consolidation of this Act, current to September 22, 2014. It is intended
medicines_management/correspondence/pathway-for-the-managed- Access-of-FreeStyle-Libre.
 Pathway for the Managed Access of FreeStyle Libre (Flash Glucose monitoring) for Adults and Children in the care of Trust Specialist Diabetes Clinics in Northern Ireland www.hscboard.hscni.net/download/publications/pharmacy_and_
Pathway for the Managed Access of FreeStyle Libre (Flash Glucose monitoring) for Adults and Children in the care of Trust Specialist Diabetes Clinics in Northern Ireland www.hscboard.hscni.net/download/publications/pharmacy_and_
Ontario Public Drug Programs, Ministry of Health and Long-Term Care. For the Influenza Immunization Season
 Ontario Public Drug Programs, Ministry of Health and Long-Term Care Notice from the Executive Officer: Pharmacist Administration of Publicly Funded Influenza Vaccines and Claims Submission using the Health
Ontario Public Drug Programs, Ministry of Health and Long-Term Care Notice from the Executive Officer: Pharmacist Administration of Publicly Funded Influenza Vaccines and Claims Submission using the Health
DEPARTMENT OF HEALTH MEDICAID PAYMENTS FOR DIABETIC TESTING SUPPLIES. Report 2008-S-123 OFFICE OF THE NEW YORK STATE COMPTROLLER
 Thomas P. DiNapoli COMPTROLLER OFFICE OF THE NEW YORK STATE COMPTROLLER DIVISION OF STATE GOVERNMENT ACCOUNTABILITY Audit Objective... 2 Audit Results - Summary... 2 DEPARTMENT OF HEALTH Background...
Thomas P. DiNapoli COMPTROLLER OFFICE OF THE NEW YORK STATE COMPTROLLER DIVISION OF STATE GOVERNMENT ACCOUNTABILITY Audit Objective... 2 Audit Results - Summary... 2 DEPARTMENT OF HEALTH Background...
PATIENT-IMPACT SCORECARD
 UNDERSTANDING THE GSC PATIENT-IMPACT SCORECARD What is the GSC Patient-Impact Scorecard? The GSC Patient-Impact Scorecard shows pharmacy performance scores for GSC patients during the period indicated.
UNDERSTANDING THE GSC PATIENT-IMPACT SCORECARD What is the GSC Patient-Impact Scorecard? The GSC Patient-Impact Scorecard shows pharmacy performance scores for GSC patients during the period indicated.
Frequent Dispensing Frequently Asked Questions
 What is the Frequent Dispensing Policy? The frequent dispensing policy covers a maximum number of dispensing fees for prescriptions filled daily or in 2 to 27 day supplies. The current policies regulating
What is the Frequent Dispensing Policy? The frequent dispensing policy covers a maximum number of dispensing fees for prescriptions filled daily or in 2 to 27 day supplies. The current policies regulating
Table 1. Antihyperglycemic agents for use in type 2 diabetes
 Table 1. Antihyperglycemic agents for use in type 2 diabetes DRUG IN ALPHA-GLUCOSIDASE INHIBITOR: inhibits pancreatic alpha-amyle and intestinal alpha-glucoside Acarbose (Glucobay) 0.6% Negligible Not
Table 1. Antihyperglycemic agents for use in type 2 diabetes DRUG IN ALPHA-GLUCOSIDASE INHIBITOR: inhibits pancreatic alpha-amyle and intestinal alpha-glucoside Acarbose (Glucobay) 0.6% Negligible Not
A BULLETIN FOR PHARMACY SERVICE PROVIDERS FROM ALBERTA BLUE CROSS
 Pharmacy Benefact A BULLETIN FOR PHARMACY SERVICE PROVIDERS FROM ALBERTA BLUE CROSS Number 757 September 2018 Alberta Public Health Activities Program (APHAP) Influenza Immunization Program 2018/2019 This
Pharmacy Benefact A BULLETIN FOR PHARMACY SERVICE PROVIDERS FROM ALBERTA BLUE CROSS Number 757 September 2018 Alberta Public Health Activities Program (APHAP) Influenza Immunization Program 2018/2019 This
Starting and Helping People with Type 2 Diabetes on Insulin
 Starting and Helping People with Type 2 Diabetes on Insulin Elaine Cooke, BSc(Pharm), RPh, CDE Pharmacist and Certified Diabetes Educator Maple Ridge, BC Objectives After attending this session, participants
Starting and Helping People with Type 2 Diabetes on Insulin Elaine Cooke, BSc(Pharm), RPh, CDE Pharmacist and Certified Diabetes Educator Maple Ridge, BC Objectives After attending this session, participants
Mirus Metrics Process Companion
 Mirus Metrics Process Companion Contents Getting Started... 1 Executive Summary... 2 Key Functions... 3 Glossary... 4 Care Revenue... 5 Performance... 7 Planning (Mandatory Assessments)... 13 Planning
Mirus Metrics Process Companion Contents Getting Started... 1 Executive Summary... 2 Key Functions... 3 Glossary... 4 Care Revenue... 5 Performance... 7 Planning (Mandatory Assessments)... 13 Planning
Management of Type 2 Diabetes Mellitus. Heather Corn, MD, MS Endocrinology, Diabetes, and Metabolism
 Management of Type 2 Diabetes Mellitus Heather Corn, MD, MS Endocrinology, Diabetes, and Metabolism Disclosures Working for Intermountain Healthcare Some of the views represented are the opinion of ABIM-certified
Management of Type 2 Diabetes Mellitus Heather Corn, MD, MS Endocrinology, Diabetes, and Metabolism Disclosures Working for Intermountain Healthcare Some of the views represented are the opinion of ABIM-certified
Multiple Small Feedings of the Mind: Diabetes. Sonja K Fredrickson, MD, BC-ADM March 7, 2014
 Multiple Small Feedings of the Mind: Diabetes Sonja K Fredrickson, MD, BC-ADM March 7, 2014 Question 1: Setting A1c Goals Describe the evidence based approach to determining the target HgbA1c in different
Multiple Small Feedings of the Mind: Diabetes Sonja K Fredrickson, MD, BC-ADM March 7, 2014 Question 1: Setting A1c Goals Describe the evidence based approach to determining the target HgbA1c in different
Comparative Effectiveness and Safety of Diabetes Medications for Adults with Type 2 Diabetes
 Draft Comparative Effectiveness Review Comparative Effectiveness and Safety of Diabetes Medications for Adults with Type Diabetes Prepared for: Agency for Healthcare Research and Quality U.S. Department
Draft Comparative Effectiveness Review Comparative Effectiveness and Safety of Diabetes Medications for Adults with Type Diabetes Prepared for: Agency for Healthcare Research and Quality U.S. Department
第十五章. Diabetes Mellitus
 Diabetes-1/9 第十五章 Diabetes Mellitus 陳曉蓮醫師 2/9 - Diabetes 羅東博愛醫院 Management of Diabetes mellitus A. DEFINITION OF DIABETES MELLITUS Diabetes Mellitus is characterized by chronic hyperglycemia with disturbances
Diabetes-1/9 第十五章 Diabetes Mellitus 陳曉蓮醫師 2/9 - Diabetes 羅東博愛醫院 Management of Diabetes mellitus A. DEFINITION OF DIABETES MELLITUS Diabetes Mellitus is characterized by chronic hyperglycemia with disturbances
Comprehensive support for your patients on MYALEPT
 Comprehensive support for your patients on MYALEPT Insurance and financial assistance options (see page 3) Fulfillment support (see page 6) Co-pay assistance a,b (see page 4) Your patient Injection training
Comprehensive support for your patients on MYALEPT Insurance and financial assistance options (see page 3) Fulfillment support (see page 6) Co-pay assistance a,b (see page 4) Your patient Injection training
FlexRx 6-Tier. SM Pharmacy Benefit Guide
 FlexRx 6-Tier SM Pharmacy Benefit Guide Welcome to FlexRx The AllWays Health Partners FlexRx SM program is built for choice, savings, and convenience with benefits including: Low-cost drug tier for many
FlexRx 6-Tier SM Pharmacy Benefit Guide Welcome to FlexRx The AllWays Health Partners FlexRx SM program is built for choice, savings, and convenience with benefits including: Low-cost drug tier for many
Wayne Gravois, MD August 6, 2017
 Wayne Gravois, MD August 6, 2017 Americans with Diabetes (Millions) 40 30 Source: National Diabetes Statistics Report, 2011, 2017 Millions 20 10 0 1980 2009 2015 2007 - $174 Billion 2015 - $245 Billion
Wayne Gravois, MD August 6, 2017 Americans with Diabetes (Millions) 40 30 Source: National Diabetes Statistics Report, 2011, 2017 Millions 20 10 0 1980 2009 2015 2007 - $174 Billion 2015 - $245 Billion
PBMs: Impact on Cost and Quality of Pharmaceutical Care in the U.S.
 Speaker Brian K. Solow, MD, FAAFP Optum Life Sciences Irvine, CA, USA PBMs: Impact on Cost and Quality of Pharmaceutical Care in the U.S. Brian K. Solow, MD, FAAFP Chief Medical Officer, Optum Life Sciences
Speaker Brian K. Solow, MD, FAAFP Optum Life Sciences Irvine, CA, USA PBMs: Impact on Cost and Quality of Pharmaceutical Care in the U.S. Brian K. Solow, MD, FAAFP Chief Medical Officer, Optum Life Sciences
Thiazolidinedione Step Therapy Program
 Thiazolidinedione Step Therapy Program Policy Number: 5.01.580 Last Review: 7/2018 Origination: 07/2014 Next Review: 7/2019 LoB: ACA Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
Thiazolidinedione Step Therapy Program Policy Number: 5.01.580 Last Review: 7/2018 Origination: 07/2014 Next Review: 7/2019 LoB: ACA Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
Guidance on the Self-Monitoring of Blood Glucose in Adults with Diabetes
 Introduction Guidance on the Self-Monitoring of Blood Glucose in Adults with Diabetes This guideline is designed to offer guidance for primary and secondary care on the use of selfmonitoring of blood glucose
Introduction Guidance on the Self-Monitoring of Blood Glucose in Adults with Diabetes This guideline is designed to offer guidance for primary and secondary care on the use of selfmonitoring of blood glucose
DIABETES AND RAMADAN FASTING
 DIABETES AND RAMADAN FASTING Dr. A. Nigam, M.B.B.S., M.D. (Medicine) Specialist Internal Medicine Al Zahrawi Hospital, Ras Al Khaimah, U.A.E. It is estimated that UAE s population currently stands at approximately
DIABETES AND RAMADAN FASTING Dr. A. Nigam, M.B.B.S., M.D. (Medicine) Specialist Internal Medicine Al Zahrawi Hospital, Ras Al Khaimah, U.A.E. It is estimated that UAE s population currently stands at approximately
An Overview of Medicare Covered Diabetes Supplies and Services
 News Flash - Understanding the Remittance Advice: A Guide for Medicare Providers, Physicians, Suppliers, and Billers serves as a resource on how to read and understand a Remittance Advice (RA). Inside
News Flash - Understanding the Remittance Advice: A Guide for Medicare Providers, Physicians, Suppliers, and Billers serves as a resource on how to read and understand a Remittance Advice (RA). Inside
Quick Reference Guide
 2013 Clinical Practice Guidelines Quick Reference Guide (Updated November 2016) 416569-16 guidelines.diabetes.ca diabetes.ca 1-800-BANTING (226-8464) Copyright 2016 Canadian Diabetes Association SCREENING
2013 Clinical Practice Guidelines Quick Reference Guide (Updated November 2016) 416569-16 guidelines.diabetes.ca diabetes.ca 1-800-BANTING (226-8464) Copyright 2016 Canadian Diabetes Association SCREENING
SGLT2 Inhibitors
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: SGLT2 Inhibitors Page: 1 of 7 Last Review Date: November 30, 2018 SGLT2 Inhibitors Description
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: SGLT2 Inhibitors Page: 1 of 7 Last Review Date: November 30, 2018 SGLT2 Inhibitors Description
SGLT2 Inhibitors
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: SGLT2 Inhibitors Page: 1 of 7 Last Review Date: June 22, 2018 SGLT2 Inhibitors Description Invokana
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: SGLT2 Inhibitors Page: 1 of 7 Last Review Date: June 22, 2018 SGLT2 Inhibitors Description Invokana
Early treatment for patients with Type 2 Diabetes
 Israel Society of Internal Medicine Kibutz Hagoshrim, June 22, 2012 Early treatment for patients with Type 2 Diabetes Eduard Montanya Hospital Universitari Bellvitge-IDIBELL CIBERDEM University of Barcelona
Israel Society of Internal Medicine Kibutz Hagoshrim, June 22, 2012 Early treatment for patients with Type 2 Diabetes Eduard Montanya Hospital Universitari Bellvitge-IDIBELL CIBERDEM University of Barcelona
CADTH Optimal Use Report
 Canadian Agency for Drugs and Technologies in Health Agence canadienne des médicaments et des technologies de la santé CADTH Optimal Use Report Volume 3, Issue 1B July 2013 Third-Line Pharmacotherapy for
Canadian Agency for Drugs and Technologies in Health Agence canadienne des médicaments et des technologies de la santé CADTH Optimal Use Report Volume 3, Issue 1B July 2013 Third-Line Pharmacotherapy for
New patients approved for the Novo Nordisk PAP may only be eligible for insulin vials. For a full list of available products, please visit:
 The Novo Nordisk Diabetes Patient Assistance Program (PAP) provides medication to qualifying applicants at no charge. If the applicant qualifies under the Novo Nordisk Diabetes PAP guidelines, a 120-day
The Novo Nordisk Diabetes Patient Assistance Program (PAP) provides medication to qualifying applicants at no charge. If the applicant qualifies under the Novo Nordisk Diabetes PAP guidelines, a 120-day
Rethink. Adherence. David D. Pope, PharmD, CDE Editor-in-Chief, CreativePharmacist.com Brands
 Rethink. Adherence. David D. Pope, PharmD, CDE Editor-in-Chief, CreativePharmacist.com Brands Objectives Identify patient barriers to medication adherence. Describe the clinical and economic impact of
Rethink. Adherence. David D. Pope, PharmD, CDE Editor-in-Chief, CreativePharmacist.com Brands Objectives Identify patient barriers to medication adherence. Describe the clinical and economic impact of
CBR201609: Diabetic Testing Supplies
 Stay Tuned for Webinar Audio dial-in: 323 920 0091; PIN: 256-7691# For technical assistance, send email to support@anymeeting.com : Diabetic Testing Supplies 3:00 P.M. ET July 27, 2016 : Diabetic Testing
Stay Tuned for Webinar Audio dial-in: 323 920 0091; PIN: 256-7691# For technical assistance, send email to support@anymeeting.com : Diabetic Testing Supplies 3:00 P.M. ET July 27, 2016 : Diabetic Testing
Access Points: Frequently Asked Questions 15 June 2016
 Access Points: Frequently Asked Questions 15 June 2016 Access Point Interim Agreement Q. Why have I received an Interim Agreement? The Commonwealth and Diabetes Australia have entered into a new NDSS Agreement
Access Points: Frequently Asked Questions 15 June 2016 Access Point Interim Agreement Q. Why have I received an Interim Agreement? The Commonwealth and Diabetes Australia have entered into a new NDSS Agreement
A1. Does your government have a formal, written diabetes policy or strategy?
 Survey of Diabetes Programs and Services in Canada Canadian Diabetes Association Diabetes Progress Report A1. Does your government have a formal, written diabetes policy or strategy? Yes. In October 2002,
Survey of Diabetes Programs and Services in Canada Canadian Diabetes Association Diabetes Progress Report A1. Does your government have a formal, written diabetes policy or strategy? Yes. In October 2002,
Very Practical Tips for Managing Type 2 Diabetes
 Very Practical Tips for Managing Type 2 Diabetes Jean-François Yale, MD, FRCPC McGill University Health Centre, Montreal, Canada Jean-francois.yale@mcgill.ca www.dryale.ca OBJECTIVES DISCLOSURES The participant
Very Practical Tips for Managing Type 2 Diabetes Jean-François Yale, MD, FRCPC McGill University Health Centre, Montreal, Canada Jean-francois.yale@mcgill.ca www.dryale.ca OBJECTIVES DISCLOSURES The participant
Louisiana. Prescribing and Dispensing Profile. Research current through November 2015.
 Prescribing and Dispensing Profile Louisiana Research current through November 2015. This project was supported by Grant No. G1599ONDCP03A, awarded by the Office of National Drug Control Policy. Points
Prescribing and Dispensing Profile Louisiana Research current through November 2015. This project was supported by Grant No. G1599ONDCP03A, awarded by the Office of National Drug Control Policy. Points
