Country-Specific Glucose Monitor List
|
|
|
- Hector Bishop
- 6 years ago
- Views:
Transcription
1 Country-Specific Glucose Monitor List Country Name: United States Important Information This is a non-comprehensive list, current as of December Absence of a specific glucose monitor or test strips from this list does NOT imply compatibility or incompatibility with EXTRANEAL (icodextrin) Peritoneal Dialysis Solution. Similarly, other glucose-measuring technologies which are not listed below (such as continuous glucose monitoring systems) may or may not be compatible with EXTRANEAL (icodextrin) Solution. Always contact the device manufacturer for current information. If the manufacturer cannot provide information regarding compatibility of the device with icodextrin and maltose, Baxter does NOT recommend that EXTRANEAL (icodextrin) Solution patients use the product. Baxter reserves the right to change this list without notice and does not represent that it includes all potentially incompatible products. The glucose monitor manufacturers listed have certified to Baxter that they have tested (as per ISO 15197) their monitors with maltose and icodextrin to Baxter s recommended limits: 278 mg/dl (maltose) and 1094 mg/dl (icodextrin). The manufacturers certified that their green monitors, below, showed no interference of blood glucose readings under these conditions, with the exception of the green monitors specifically notated 4 for which certification of testing to Baxter s recommended limits has not yet been received. Please note that the compatibility list below is only for brand-name monitors used with the corresponding brand-name test strip. If a brand-name monitor is used with another manufacture s test strip, always contact the test strip manufacturer for current information. This list is compiled from a search via: Internet, literature, Baxter internal studies, information from government agencies, test strip leaflets, safety alerts, and direct information from the product manufacturers. While efforts have been made to provide accurate and current information, Baxter does not manufacture these glucose monitors or test strips and thus does not guarantee the initial or continued accuracy of this information. Please contact the manufacturer(s) of the glucose monitor and test strip to obtain the latest compatibility information before using in conjunction with EXTRANEAL (icodextrin) PD Solution. 1. EXTRANEAL (icodextrin) solution contains icodextrin. Maltose, a metabolite of icodextrin, may interfere with certain glucose monitors or test strips. This interference will result in a falsely elevated glucose reading using these monitors or test strips. 2. This interference may mask true hypoglycemia or lead to the erroneous diagnosis of hyperglycemia. Thus, a blood glucose reading within or above the normal range in a patient on EXTRANEAL (icodextrin) solution, using these monitors or test strips, may mask true low blood sugar. This may cause a patient or health care professional to not take the appropriate steps to bring the blood sugar into a normal range. Alternatively, a falsely-elevated blood glucose reading could cause a patient to get more insulin than needed. Both of these situations can lead to lifethreatening events, including loss of consciousness, coma, neurological damage or death. 3. The dialysis unit or patient should contact the manufacturer of the glucose monitor and/or test strips to determine if the monitor or test strips they are using are subject to interference by icodextrin or maltose. Also, consult the product information included with the glucose monitor and test strips. 4. The following list is for reference only. This list does not imply recommendation of these glucose monitors or test strips. 5. Identified compatibilities are shown in the table below. ONLY glucose-specific monitors and test strips should be used with patients on EXTRANEAL (icodextrin) solution. Contact the manufacturer to verify that the test strips and monitor are glucose-specific. The list provides the contact information of the more common, major brand manufacturers.
2 Glucose Monitor Brand FreeStyle Freedom FreeStyle Freedom Lite FreeStyle InsuLinx FreeStyle Lite FreeStyle Optium 5 FreeStyle Optium H 5 FreeStyle Optium Neo 5 FreeStyle Optium Neo H 5 FreeStyle Papillon InsuLinx 5 FreeStyle Papillon Vision 5 FreeStyle Precision 5 FreeStyle Precision H FreeStyle Precision Neo FreeStyle Precision Neo H FreeStyle Precision Pro Optium 5 OptiumEZ Optium Xido 5 Optium Xido Neo 5 Precision Xceed Pro 5 Precision Xtra ReliOn Ultima GLUCOSE MONITORS Updated December 2017 Compatible with Test Type* EXTRANEAL (icodextrin) PD Solution (Glucosespecific) Manufacturer Abbott Diabetes Care om Phone: Assure Platinum Assure Prism 4 GLUCOCARD 01 GLUCOCARD 01-mini GLUCOCARD 01-mini plus GLUCOCARD Expression GLUCOCARD G Black GLUCOCARD MX GLUCOCARD MyDIA GLUCOCARD Shine 4 GLUCOCARD S GLUCOCARD SM GLUCOCARD Vital GLUCOCARD X-meter 2 GLUCOCARD X-mini 2 GLUCOCARD X-mini plus 2 GLUCOCARD Σ GLUCOCARD Σ-mini ReliOn Confirm ReliOn micro ReliOn Prime Arkray, Inc. Phone:
3 Glucose Monitor Brand Compatible with EXTRANEAL (icodextrin) PD Solution (Glucosespecific) Test Type* Manufacturer Breeze 2 Contour Contour Link Contour Next Contour Next EZ Contour Next Link Contour Next Link 2.4 Contour Next USB Contour Plus Contour Plus Link 2.4 Contour TS Contour USB Contour XT Contour Next One Blood Contour Plus One Blood Ascensia Diabetes Care (formerly Bayer Healthcare) Phone: OneTouch InDuo OneTouch Select OneTouch Select Mini OneTouch Select Simple OneTouch Ultra A OneTouch Ultra 2 OneTouch UltraEasy OneTouch UltraLink OneTouch UltraMini OneTouch SelectPlus OneTouch SelectPlus Flex OneTouch UltraSmart OneTouch UltraVue OneTouch Verio OneTouch VerioFlex OneTouch VerioIQ OneTouch VerioPro OneTouch VerioPro+ OneTouch VerioSync OneTouch VerioVue OneTouch Vita Lifescan, Inc. Phone: Nova Max Plus Nova Max Link StatStrip Hospital Glucose Meter StatStrip Hospital Glucose and Ketone Monitoring System 5 StatStrip Xpress Glucose Meter 5 5 Nova Biomedical Phone: (781)
4 Glucose Monitor Brand StatStrip Xpress Glucose and Ketone Monitoring System 5 StatStrip Xpress2 Glucose and Ketone Monitoring Meter Nova Pro Glucose/Ketone Meter 5 Compatible with EXTRANEAL (icodextrin) PD Solution (Glucosespecific) 5 5 Test Type* 5 5 Manufacturer Accu-Chek Active Accu-Chek Aviva Accu-Chek Aviva Combo Accu-Chek Aviva Connect Accu-Chek Aviva Expert Accu-Chek Aviva Insight Accu-Chek Aviva Nano Accu-Chek Aviva Plus 3 Accu-Chek Compact Plus Accu-Chek Guide Accu-Chek Inform II Accu-Chek Instant Accu-Chek Instant S Accu-Chek Nano 3 Accu-Chek Nano SmartView 3 Accu-Chek Mobile Accu-Chek Performa Accu-Chek Performa Combo Accu-Chek Performa Connect Accu-Chek Performa Insight Accu-Chek Performa Nano Accu-Chek Voicemate Plus System Mut-Q-GDH Roche Diagnostics Phone: Jazz Jazz Wireless 2 Presto ibgstar One Drop AgaMatrix, Inc. Phone : TRUE Metrix 4 TRUE Metrix Air 4 TRUE Track 4 TRUE Balance Trividia Health (formerly Nipro Diagnostics) Phone: Prodigy AutoCode 4 Prodigy Voice 4 Prodigy Pocket 4 Prodigy No Coding Test Strips Embrace Pro Prodigy Diabetes Care Phone: Omnis Health Phone: Two types of compatible test strips for Precision Xtra OK.
5 2 These Arkray monitors/test strips have switched from an incompatible based chemistry to a compatible GDH- FAD based chemistry. Consult manufacturer for additional information. 3 The ACCU-CHEK Nano (not Aviva or Performa) and ACCU-CHEK Aviva Plus glucose systems are available within the United States ONLY, and use test strips branded as ACCU-CHEK Smartview and ACCU-CHEK Aviva Plus, respectively. These systems use the (compatible) strips. Consult manufacturer for additional information. 4 This/These monitor/test strips were not currently certified as having been tested to Baxter s recommended interference limits for maltose or icodextrin when this List was issued. Consult manufacturer for additional information 5 These brand name monitors are not available within United States. Test Type* = glucose oxidase GDH-PQQ = glucose dehydrogenase with pyrroloquinolinequinone (note: GDO, glucose-dye-oxidoreductase, is an incompatible PQQ-based method) = glucose dehydrogenase with nicotinamide-adenine dinucleotide = glucose dehydrogenase with flavin-adenine dinucleotide = glucose dehydrogenase with pyrroloquinolinequinone modified to eliminate maltose interference References: A Baxter report Interim 1, Determination of potential interference of icodextrin and its metabolites on human blood glucose measurement using chosen glucometers. Please see full prescribing information for EXTRANEAL (icodextrin) PD solution.
6 EXTRANEAL (icodextrin) Peritoneal Dialysis Solution Indication and Important Risk Information (IRI) Indication: EXTRANEAL (icodextrin) is indicated for a single daily exchange for the long (8- to 16- hour) dwell during continuous ambulatory peritoneal dialysis (CAPD) or automated peritoneal dialysis (APD) for the management of end-stage renal disease. EXTRANEAL (icodextrin) is also indicated to improve (compared to 4.25% dextrose) long-dwell ultrafiltration and clearance of creatinine and urea nitrogen in patients with high average or greater transport characteristics, as defined using the peritoneal equilibration test (PET). Important Risk Information: WARNING: UNRECOGNIZED HYPOGLYCEMIA RESULTING FROM DRUG-DEVICE INTERACTION Only use glucose-specific monitors and test strips to measure blood glucose levels in patients using EXTRANEAL (icodextrin) Peritoneal Dialysis Solution. Blood glucose monitoring devices using glucose dehydrogenase pyrroloquinolinequinone (GDH-PQQ) or glucose-dye-oxidoreductase (GDO)- based methods must not be used. In addition, some blood glucose monitoring systems using glucose dehydrogenase flavin-adenine dinucleotide (GDH FAD)-based methods must not be used. Use of GDH-PQQ, GDO, and -based glucose monitors and test strips has resulted in falsely elevated glucose readings (due to the presence of maltose). Falsely elevated glucose readings have led patients or health care providers to withhold treatment of hypoglycemia or to administer insulin inappropriately. Both of these situations have resulted in unrecognized hypoglycemia, which has led to loss of consciousness, coma, permanent neurological damage, and death. Plasma levels of EXTRANEAL (icodextrin) and its metabolites return to baseline within approximately 14 days following cessation of EXTRANEAL (icodextrin) administration. Therefore falsely elevated glucose levels may be measured up to two weeks following cessation of EXTRANEAL (icodextrin) therapy when GDH-PQQ, GDO, and -based blood glucose monitors and test strips are used. To avoid improper insulin administration, educate all patients to alert health care providers of this interaction particularly in hospital settings. The manufacturer(s) of the monitor and test strips should be contacted to determine if icodextrin or maltose causes interference or falsely elevated glucose readings. For a list of toll free numbers for glucose monitor and test strip manufacturers, please contact the Baxter Renal Clinical Help Line RENAL-HELP or visit Because of the risk of unrecognized hypoglycemia that could result from a drug-device interaction, EXTRANEAL (icodextrin) is available only through a restricted program.
7 EXTRANEAL (icodextrin) is contraindicated in patients with a known allergy to cornstarch or icodextrin, in patients with maltose or isomaltose intolerance, in patients with glycogen storage disease, and in patients with severe lactic acidosis. EXTRANEAL is intended for intraperitoneal administration only. Not for intravenous injection. Aseptic technique should be used throughout the peritoneal dialysis procedure. Encapsulating peritoneal sclerosis (EPS), sometimes fatal, is a complication of peritoneal dialysis therapy and has been reported in patients using EXTRANEAL. Serious hypersensitivity reactions to EXTRANEAL have been reported such as toxic epidermal necrolysis, angioedema, serum sickness, erythema multiforme and vasculitis. Anaphylactic or anaphylactoid reactions may occur. If a serious reaction is suspected, discontinue EXTRANEAL immediately and institute appropriate therapeutic countermeasures. Overinfusion of peritoneal dialysis solution volume into the peritoneal cavity may be characterized by abdominal distention, feeling of fullness and/or shortness of breath. Drain the peritoneal dialysis solution from the peritoneal cavity to treat overinfusion. Patients with insulin dependent diabetes may require modification of insulin dosage following initiation of treatment with EXTRANEAL. Monitor blood glucose and adjust insulin, if needed. Peritoneal dialysis may affect a patient s protein, water soluble vitamin, potassium, sodium, chloride, bicarbonate, and magnesium levels and volume status. Monitor electrolytes and blood chemistry periodically. Monitor fluid status to avoid hyper or hypovolemia and potentially severe consequences including congestive heart failure, volume depletion, and hypovolemic shock. Abnormalities in any of these parameters should be treated promptly under the care of a physician. In clinical trials, the most frequently reported adverse events occurring in 10% of patients and more common in EXTRANEAL PD Solution patients than in control patients, were peritonitis, upper respiratory infection, hypertension, and rash. The most common treatment related adverse reaction for EXTRANEAL PD Solution patients was skin rash. Please see full Prescribing Information at Baxter and Extraneal are trademarks of Baxter International Inc. Any other trademarks or products appearing herein are the property of their respective owners.
PERITONEAL DIALYSIS PRESCRIPTION MANAGEMENT QUICK REFERENCE GUIDE
 PERITONEAL DIALYSIS PRESCRIPTION MANAGEMENT QUICK REFERENCE GUIDE This quick reference guide will help serve as a reference tool for clinicians setting a patient s Peritoneal Dialysis (PD) prescription.
PERITONEAL DIALYSIS PRESCRIPTION MANAGEMENT QUICK REFERENCE GUIDE This quick reference guide will help serve as a reference tool for clinicians setting a patient s Peritoneal Dialysis (PD) prescription.
Extraneal peritoneal dialysis solution , version 6 PUBLIC SUMMARY OF THE RISK MANAGEMENT PLAN
 Extraneal peritoneal dialysis solution 8.10.2015, version 6 PUBLIC SUMMARY OF THE RISK MANAGEMENT PLAN Elements for a Public Summary Overview of Disease Epidemiology In Europe, it is estimated that more
Extraneal peritoneal dialysis solution 8.10.2015, version 6 PUBLIC SUMMARY OF THE RISK MANAGEMENT PLAN Elements for a Public Summary Overview of Disease Epidemiology In Europe, it is estimated that more
Home Glucose Monitoring
 Home Glucose Monitoring Blood Glucose Monitors Name (Manufacturer/Distributor) Size (inches) Weight (ounces) Active (Roche) 4.6 X 1.07 X 0.9 2.01 without Advantage (Roche) 3.3 X 2.8 X 0.8 1.8 without Test
Home Glucose Monitoring Blood Glucose Monitors Name (Manufacturer/Distributor) Size (inches) Weight (ounces) Active (Roche) 4.6 X 1.07 X 0.9 2.01 without Advantage (Roche) 3.3 X 2.8 X 0.8 1.8 without Test
EXTRANEAL. based methods are used, loss of consciousness, neurological damage and death may occur (See WARNING/Precautions).
 EXTRANEAL Blood glucose management with EXTRANEAL must be done with a glucose-specific method to avoid interference resulting in unrecognised hypoglycemia. If non-specific glucose test methods glucose
EXTRANEAL Blood glucose management with EXTRANEAL must be done with a glucose-specific method to avoid interference resulting in unrecognised hypoglycemia. If non-specific glucose test methods glucose
FDA Perspective - Public Health Notification: Potentially Fatal Errors with GDH-PQQ Glucose Monitoring Technology Courtney C. Harper, Ph.D.
 FDA Perspective - Public Health Notification: Potentially Fatal Errors with GDH-PQQ Glucose Monitoring Technology Courtney C. Harper, Ph.D., Director, Division of Chemistry and Toxicology Devices Office
FDA Perspective - Public Health Notification: Potentially Fatal Errors with GDH-PQQ Glucose Monitoring Technology Courtney C. Harper, Ph.D., Director, Division of Chemistry and Toxicology Devices Office
EXTRANEAL DESCRIPTION. Icodextrin 7.5% peritoneal dialysis solution
 EXTRANEAL Icodextrin 7.5% peritoneal dialysis solution Blood glucose management with EXTRANEAL must be done with a glucosespecific method to avoid interference resulting in unrecognised hypoglycaemia.
EXTRANEAL Icodextrin 7.5% peritoneal dialysis solution Blood glucose management with EXTRANEAL must be done with a glucosespecific method to avoid interference resulting in unrecognised hypoglycaemia.
M E M O R A N D U M. Please share this information as applicable within your organizations/practices.
 ESRD NETWORK 18 M E M O R A N D U M To: From: Subject: Facility Manager Lana Kacherova, QI Director Lisle Mukai, QI Coordinator FDA Recalls & Alerts Date: September 8, 2009 The Network is required to distribute
ESRD NETWORK 18 M E M O R A N D U M To: From: Subject: Facility Manager Lana Kacherova, QI Director Lisle Mukai, QI Coordinator FDA Recalls & Alerts Date: September 8, 2009 The Network is required to distribute
Guidance for the choice of blood glucose testing meters, strips and lancets
 Guidance for the choice of blood glucose testing meters, strips and lancets This guidance is intended to assist healthcare professionals in their selection of appropriate blood glucose meters and testing
Guidance for the choice of blood glucose testing meters, strips and lancets This guidance is intended to assist healthcare professionals in their selection of appropriate blood glucose meters and testing
How to Access A Free Blood Glucose Meter
 How to Access A Free Abbott FreeStyle Lite FreeStyle Lite FreeStyle Freedom Lite FreeStyle InsuLinx How to Access A Free contacting their health care professionals at diabetes clinics; or calling the FreeStyle
How to Access A Free Abbott FreeStyle Lite FreeStyle Lite FreeStyle Freedom Lite FreeStyle InsuLinx How to Access A Free contacting their health care professionals at diabetes clinics; or calling the FreeStyle
Type 2 Diabetes Recommended SMBG
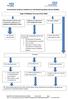 Herefordshire Diabetes Guideline for Self-Monitoring Blood Glucose (SMBG) Type 2 Diabetes Recommended SMBG Diet controlled/ metformin only/ combination of metformin and pioglitazone/dpp4/sglt2 or GLP1
Herefordshire Diabetes Guideline for Self-Monitoring Blood Glucose (SMBG) Type 2 Diabetes Recommended SMBG Diet controlled/ metformin only/ combination of metformin and pioglitazone/dpp4/sglt2 or GLP1
DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS)
 Type 2 Diabetes DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Clinical Guideline Self-monitoring of blood glucose (SMBG) should be considered but not be routinely offered for adults with type 2 diabetes
Type 2 Diabetes DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Clinical Guideline Self-monitoring of blood glucose (SMBG) should be considered but not be routinely offered for adults with type 2 diabetes
SUMMARY OF PRODUCT CHARACTERISTICS
 פורמט עלון זה נקבע ע"י משרד הבריאות ותוכנו נבדק ואושר". עלון מאושר:יוני 2011 This leaflet format has been determined by the Ministry of Health and the content thereof has been checked and approved. Date
פורמט עלון זה נקבע ע"י משרד הבריאות ותוכנו נבדק ואושר". עלון מאושר:יוני 2011 This leaflet format has been determined by the Ministry of Health and the content thereof has been checked and approved. Date
DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS)
 DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Type 2 Diabetes Clinical Guideline Self-monitoring of blood glucose (SMBG) should be considered but not routinely offered for adults with type 2 diabetes
DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Type 2 Diabetes Clinical Guideline Self-monitoring of blood glucose (SMBG) should be considered but not routinely offered for adults with type 2 diabetes
MQ Comparison of glucometers using whole blood
 MQ 2017-1 Comparison of glucometers using whole blood Note: The instrument comparison is structured as a survey. This is a sample test, not a complete evaluation. Introduction Glucose self-testing devices
MQ 2017-1 Comparison of glucometers using whole blood Note: The instrument comparison is structured as a survey. This is a sample test, not a complete evaluation. Introduction Glucose self-testing devices
GUIDELINE FOR SELF MONITORING OF BLOOD GLUCOSE IN ADULTS WITH DIABETES
 It is recognised that self- monitoring of blood glucose (SMBG) may have an important role to play for some patients with. It can allow individuals to see what impact particular behaviours, such as dietary
It is recognised that self- monitoring of blood glucose (SMBG) may have an important role to play for some patients with. It can allow individuals to see what impact particular behaviours, such as dietary
Walsall CCG Supported Blood Glucose Meters April 2018
 Meter Name Picture Type Strips Lancets Information Company PRIMARY CARE INITIATION TEE2+ Uncomplicated Type 2 diabetes patient on TEE2 test CareSens Lancets Easy to use. Large clear display Bluetooth functionality
Meter Name Picture Type Strips Lancets Information Company PRIMARY CARE INITIATION TEE2+ Uncomplicated Type 2 diabetes patient on TEE2 test CareSens Lancets Easy to use. Large clear display Bluetooth functionality
MQ Comparison of glucometers using whole blood
 MQ 2018-2 Comparison of glucometers using whole blood Note: The instrument comparison is structured as a survey. This is a sample test, not a complete evaluation. Introduction Glucose self-testing devices
MQ 2018-2 Comparison of glucometers using whole blood Note: The instrument comparison is structured as a survey. This is a sample test, not a complete evaluation. Introduction Glucose self-testing devices
PRODUCT MONOGRAPH EXTRANEAL* 75 g/l Icodextrin 5.35 g/l Sodium Chloride 4.48 g/l Sodium Lactate 257 mg/l Calcium Chloride 51 mg/l Magnesium Chloride
 PRODUCT MONOGRAPH EXTRANEAL* Icodextrin, Sodium Chloride, Sodium Lactate, Calcium Chloride, Magnesium Chloride 75 g/l Icodextrin 5.35 g/l Sodium Chloride 4.48 g/l Sodium Lactate 257 mg/l Calcium Chloride
PRODUCT MONOGRAPH EXTRANEAL* Icodextrin, Sodium Chloride, Sodium Lactate, Calcium Chloride, Magnesium Chloride 75 g/l Icodextrin 5.35 g/l Sodium Chloride 4.48 g/l Sodium Lactate 257 mg/l Calcium Chloride
MQ Comparison of glucometers using heparin whole blood
 MQ 2014-1 Comparison of glucometers using heparin whole blood Note: The instrument comparison is structured as a survey. This is a sample test, not a complete evaluation. Introduction Glucose self-testing
MQ 2014-1 Comparison of glucometers using heparin whole blood Note: The instrument comparison is structured as a survey. This is a sample test, not a complete evaluation. Introduction Glucose self-testing
DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS)
 Type 2 Diabetes DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Clinical Guideline Self-monitoring of blood glucose should be offered to all newly diagnosed type 2 diabetic patients as an integral part
Type 2 Diabetes DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Clinical Guideline Self-monitoring of blood glucose should be offered to all newly diagnosed type 2 diabetic patients as an integral part
Diabetes Meters: Meter Demo/User Guide link Special Features Test Strips Lancing Device
 Diabetes Meters: Meter Name Active Company /Contact and Web Link Roche https://www. accu- chek.com/mu ltimedia/vide os/products/ metersystem s/aviva_plus/ 00_Aviva_En g_main_secti on.html?tb_i frame=true&
Diabetes Meters: Meter Name Active Company /Contact and Web Link Roche https://www. accu- chek.com/mu ltimedia/vide os/products/ metersystem s/aviva_plus/ 00_Aviva_En g_main_secti on.html?tb_i frame=true&
WHITE PAPER LOOKING PAST THE WINDOW DRESSING: UNDERSTANDING BLOOD GLUCOSE TESTING STRIPS IN A FORMULARY ENVIRONMENT
 WHITE PAPER LOOKING PAST THE WINDOW DRESSING: UNDERSTANDING BLOOD GLUCOSE TESTING STRIPS IN A FORMULARY ENVIRONMENT PURPOSE This paper explores the science behind glucose test strips and proposes the format
WHITE PAPER LOOKING PAST THE WINDOW DRESSING: UNDERSTANDING BLOOD GLUCOSE TESTING STRIPS IN A FORMULARY ENVIRONMENT PURPOSE This paper explores the science behind glucose test strips and proposes the format
PRODUCT MONOGRAPH EXTRANEAL. 75 g/l Icodextrin 5.35 g/l Sodium Chloride 4.48 g/l Sodium Lactate 257 mg/l Calcium Chloride 51 mg/l Magnesium Chloride
 PRODUCT MONOGRAPH EXTRANEAL Icodextrin, Sodium Chloride, Sodium Lactate, Calcium Chloride, Magnesium Chloride 75 g/l Icodextrin 5.35 g/l Sodium Chloride 4.48 g/l Sodium Lactate 257 mg/l Calcium Chloride
PRODUCT MONOGRAPH EXTRANEAL Icodextrin, Sodium Chloride, Sodium Lactate, Calcium Chloride, Magnesium Chloride 75 g/l Icodextrin 5.35 g/l Sodium Chloride 4.48 g/l Sodium Lactate 257 mg/l Calcium Chloride
Supported devices/compatibility list
 Supported devices/compatibility list DIABASS / DIABASS PRO Version:18.11.0.2 / Release date: 29.11.2018 A101-05102017 Blood glucose meters The following blood glucose meters are compatible with DIABASS/DIABASS
Supported devices/compatibility list DIABASS / DIABASS PRO Version:18.11.0.2 / Release date: 29.11.2018 A101-05102017 Blood glucose meters The following blood glucose meters are compatible with DIABASS/DIABASS
Cornerstones4Care Powered by Glooko Android User Guide
 Cornerstones4Care Powered by Glooko Android User Guide March 2018 IM GL+C4C(A) REV C TABLE OF CONTENTS GENERAL INFORMATION...1 Product Description...1 Intended Use...1 Supported Devices...1 Warnings...3
Cornerstones4Care Powered by Glooko Android User Guide March 2018 IM GL+C4C(A) REV C TABLE OF CONTENTS GENERAL INFORMATION...1 Product Description...1 Intended Use...1 Supported Devices...1 Warnings...3
Advances in Diabetes Technology Part II: Analysis of Diabetes Self Management Devices and Support Tools
 Advances in Diabetes Technology Part II: Analysis of Diabetes Self Management Devices and Support Tools Jennifer Trujillo, PharmD, FCCP, BCPS, CDE, BC ADM Associate Professor University of Colorado School
Advances in Diabetes Technology Part II: Analysis of Diabetes Self Management Devices and Support Tools Jennifer Trujillo, PharmD, FCCP, BCPS, CDE, BC ADM Associate Professor University of Colorado School
EXTRANEAL. (i) Name of the Medicine : EXTRANEAL (Icodextrin 7.5%) Peritoneal Dialysis Solution. (ii) Description :
 EXTRANEAL Blood glucose management with EXTRANEAL must be done with a glucose-specific method to avoid interference resulting in unrecognised hypoglycemia. If nonspecific glucose test methods glucose dehydrogenase
EXTRANEAL Blood glucose management with EXTRANEAL must be done with a glucose-specific method to avoid interference resulting in unrecognised hypoglycemia. If nonspecific glucose test methods glucose dehydrogenase
Software Version 2.0. User s Guide
 Software Version 2.0 User s Guide Table of Contents Contents Contents Important Information About Your FreeStyle Auto-Assist Software...1 Intended Use...1 System Requirements...1 Connecting to your Abbott
Software Version 2.0 User s Guide Table of Contents Contents Contents Important Information About Your FreeStyle Auto-Assist Software...1 Intended Use...1 System Requirements...1 Connecting to your Abbott
Prescription Refill List Insulin and Related Supplies
 Insulin and Related Supplies Name Date Local Pharamcy Name/Address : 30 day/ 5 refills 90 day/ 3 refills Mail Away Pharamacy Name/Address: 30 day/ 5 refills 90day / 3 refills *Check only those items that
Insulin and Related Supplies Name Date Local Pharamcy Name/Address : 30 day/ 5 refills 90 day/ 3 refills Mail Away Pharamacy Name/Address: 30 day/ 5 refills 90day / 3 refills *Check only those items that
diabetic Supply Catalogue Easy - Convenient - Life Changing
 diabetic Supply Catalogue Easy - Convenient - Life Changing Prodigy Autocode The Only English - Spanish speaking, No-Coding, blood glucose monitoring system available. Meters - Alternate Site Testing-
diabetic Supply Catalogue Easy - Convenient - Life Changing Prodigy Autocode The Only English - Spanish speaking, No-Coding, blood glucose monitoring system available. Meters - Alternate Site Testing-
Blood Glucose Test Strip Guidelines
 Berkshire West Integrated Care System Representing Berkshire West Clinical Commisioning Group Royal Berkshire NHS Foundation Trust Berkshire Healthcare NHS Foundation Trust Berkshire West Primary Care
Berkshire West Integrated Care System Representing Berkshire West Clinical Commisioning Group Royal Berkshire NHS Foundation Trust Berkshire Healthcare NHS Foundation Trust Berkshire West Primary Care
PERITONEAL EQUILIBRATION TEST. AR. Merrikhi. MD. Isfahan University of Medical Sciences
 PERITONEAL EQUILIBRATION TEST AR. Merrikhi. MD. Isfahan University of Medical Sciences INTRODUCTION The peritoneal equilibration test (PET) is a semiquantitative assessment of peritoneal membrane transport
PERITONEAL EQUILIBRATION TEST AR. Merrikhi. MD. Isfahan University of Medical Sciences INTRODUCTION The peritoneal equilibration test (PET) is a semiquantitative assessment of peritoneal membrane transport
DATE RECEIVED 09/10/2017 SUBJECT Formulary PASSED TO Mary DATE PASSED 09/10/2017 RESPOND BY 27/10/2017 CATEGORY Business FoI NUMBER
 DATE RECEIVED 09/10/2017 SUBJECT Formulary PASSED TO Mary DATE PASSED 09/10/2017 RESPOND BY 27/10/2017 CATEGORY Business FoI NUMBER 2017-404 Question/s to be Answered Can you please provide the following
DATE RECEIVED 09/10/2017 SUBJECT Formulary PASSED TO Mary DATE PASSED 09/10/2017 RESPOND BY 27/10/2017 CATEGORY Business FoI NUMBER 2017-404 Question/s to be Answered Can you please provide the following
Objectives. Peritoneal Dialysis vs. Hemodialysis 02/27/2018. Peritoneal Dialysis Prescription and Adequacy Monitoring
 Peritoneal Dialysis Prescription and Adequacy Monitoring Christine B. Sethna, MD, EdM Division Director, Pediatric Nephrology Cohen Children s Medical Center Associate Professor Hofstra Northwell School
Peritoneal Dialysis Prescription and Adequacy Monitoring Christine B. Sethna, MD, EdM Division Director, Pediatric Nephrology Cohen Children s Medical Center Associate Professor Hofstra Northwell School
Read all of this leaflet carefully before you start using this medicine.
 PACKAGE LEAFLET: INFORMATION FOR THE USER EXTRANEAL Solution for Peritoneal Dialysis Read all of this leaflet carefully before you start using this medicine. Keep this leaflet. You may need to read it
PACKAGE LEAFLET: INFORMATION FOR THE USER EXTRANEAL Solution for Peritoneal Dialysis Read all of this leaflet carefully before you start using this medicine. Keep this leaflet. You may need to read it
DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL
 DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. Uniform Formulary Review Process Under 10 U.S.C. 1074g, as implemented by 32
DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. Uniform Formulary Review Process Under 10 U.S.C. 1074g, as implemented by 32
For more comprehensive information, please refer to the t:connect Application User Guide available online at: Getting Started Guide.
 Congratulations on the purchase of your new insulin pump from Tandem Diabetes Care. Your decision to use insulin pump therapy is a sign of your commitment to actively manage your diabetes. This guide provides
Congratulations on the purchase of your new insulin pump from Tandem Diabetes Care. Your decision to use insulin pump therapy is a sign of your commitment to actively manage your diabetes. This guide provides
time on can extend Summary Icodextrin Sodium Chloride Calcium Chloride Chloride Magnesium 133 mmol/l Calcium Sodium Magnesium Chloride Lactate
 Summary Product Caractestics 1. NAME OF THE MEDICINAL PRODUCT EXTRANEAL (Icodextrin 7.5%) Solution for peritoneal dialysis 2. QUALITATIVE AND QUANTITATIVE COMPOSITION A sterile peritoneal dialysis fluid
Summary Product Caractestics 1. NAME OF THE MEDICINAL PRODUCT EXTRANEAL (Icodextrin 7.5%) Solution for peritoneal dialysis 2. QUALITATIVE AND QUANTITATIVE COMPOSITION A sterile peritoneal dialysis fluid
PHYSIONEAL Risk Management Plan 2016 MAY 13
 VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology In Europe, it is estimated that more than 10% of people are affected by kidney disease. The number of patients with end-stage
VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology In Europe, it is estimated that more than 10% of people are affected by kidney disease. The number of patients with end-stage
CONTRAINDICATIONS Pre-existing severe lactic acidosis (4)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use DIANEAL peritoneal dialysis solutions safely and effectively. See full prescribing information for
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use DIANEAL peritoneal dialysis solutions safely and effectively. See full prescribing information for
510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY ONLY TEMPLATE
 510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY ONLY TEMPLATE A. 510(k) Number: k103542 B. Purpose for Submission: Alternative blood glucose test strips for use with LifeScan OneTouch
510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY ONLY TEMPLATE A. 510(k) Number: k103542 B. Purpose for Submission: Alternative blood glucose test strips for use with LifeScan OneTouch
Evaluation of the state-of-the-art in informatics in glucometers
 Informatics for Health & Social Care, Vol 34, issue 3, September 2009, pp171-179 Evaluation of the state-of-the-art in informatics in glucometers O. Ajai, A. Tiwari, J.R. Alcock School of Applied Sciences,
Informatics for Health & Social Care, Vol 34, issue 3, September 2009, pp171-179 Evaluation of the state-of-the-art in informatics in glucometers O. Ajai, A. Tiwari, J.R. Alcock School of Applied Sciences,
Starting Peritoneal Dialysis
 PD Education Booklet 1 Starting Peritoneal Dialysis What you will learn: What your kidneys do Uremia (kidney disease) and its symptoms How Peritoneal Dialysis (PD) helps The two types of PD: Automated
PD Education Booklet 1 Starting Peritoneal Dialysis What you will learn: What your kidneys do Uremia (kidney disease) and its symptoms How Peritoneal Dialysis (PD) helps The two types of PD: Automated
SENSOR ELECTRODE/PRECISION (Also Medisense Companion)
 DIABETIC PSEUDO-DIN LIST - Revised Mar 2017 For Provinces not listed, please submit using the diabetic pseudo DIN listed in your provincial formulary or the AB/ON pseudo DIN (except for insulin). Pricing
DIABETIC PSEUDO-DIN LIST - Revised Mar 2017 For Provinces not listed, please submit using the diabetic pseudo DIN listed in your provincial formulary or the AB/ON pseudo DIN (except for insulin). Pricing
Approval of Multiple Diabetes Management Products and Mesalazine proposals
 8 June Approval of Multiple Diabetes Management Products and Mesalazine proposals PHARMAC is pleased to announce the approval of five agreements in relation to funding of blood glucose, blood glucose diagnostic
8 June Approval of Multiple Diabetes Management Products and Mesalazine proposals PHARMAC is pleased to announce the approval of five agreements in relation to funding of blood glucose, blood glucose diagnostic
5 BEST PRACTICES To Patient Safety. A Practical Guide to Blood Glucose Testing
 5 BEST PRACTICES To Patient Safety A Practical Guide to Blood Glucose Testing In a seven-year hospital study, most errors (91% 97%) were operator-related rather than instrument-related. Nobels F, et al.,
5 BEST PRACTICES To Patient Safety A Practical Guide to Blood Glucose Testing In a seven-year hospital study, most errors (91% 97%) were operator-related rather than instrument-related. Nobels F, et al.,
BLOOD GLUCOSE METER TEST STRIP STEP THERAPY CRITERIA
 BLOOD GLUCOSE METER TEST STRIP STEP THERAPY CRITERIA Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan.
BLOOD GLUCOSE METER TEST STRIP STEP THERAPY CRITERIA Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan.
Evaluation of Accuracy and User Performance of the TRUE METRIX Self-Monitoring Blood Glucose System
 Evaluation of Accuracy and User Performance of the TRUE METRIX Self-Monitoring Blood Glucose System Summary Objectives: To demonstrate that the TRUE METRIX Self-Monitoring Blood Glucose System*, from Trividia
Evaluation of Accuracy and User Performance of the TRUE METRIX Self-Monitoring Blood Glucose System Summary Objectives: To demonstrate that the TRUE METRIX Self-Monitoring Blood Glucose System*, from Trividia
Determination of Hematocrit Interference in Blood Samples Derived from Patients with Different Blood Glucose Concentrations
 Journal of Diabetes Science and Technology Volume 7, Issue 1, January 2013 Diabetes Technology Society ORIGINAL ARTICLE Determination of Hematocrit Interference in Blood Samples Andreas, M.D., Ph.D., 1
Journal of Diabetes Science and Technology Volume 7, Issue 1, January 2013 Diabetes Technology Society ORIGINAL ARTICLE Determination of Hematocrit Interference in Blood Samples Andreas, M.D., Ph.D., 1
INFUSING. ORENCIA (abatacept) IV INDICATION/USAGE. Your go-to guide for preparing and administering an ORENCIA intravenous (IV) infusion
 INFUSING ORENCIA (abatacept) IV Your go-to guide for preparing and administering an ORENCIA intravenous (IV) infusion INDICATION/USAGE Adult Rheumatoid Arthritis (RA): ORENCIA is indicated for reducing
INFUSING ORENCIA (abatacept) IV Your go-to guide for preparing and administering an ORENCIA intravenous (IV) infusion INDICATION/USAGE Adult Rheumatoid Arthritis (RA): ORENCIA is indicated for reducing
HARVARD PILGRIM HEALTH CARE RECOMMENDED MEDICATION REQUEST GUIDELINES
 Generic Brand HICL GCN Exception/Other BLOOD SUGAR DIAGNOSTIC DIABETIC TEST STRIPS 25200 NOTE: Requests for preferred blood glucose (diabetic) test strips manufactured by Abbott Diabetes Care (such as
Generic Brand HICL GCN Exception/Other BLOOD SUGAR DIAGNOSTIC DIABETIC TEST STRIPS 25200 NOTE: Requests for preferred blood glucose (diabetic) test strips manufactured by Abbott Diabetes Care (such as
Better connected for better decisions
 Introducing the Accu-Chek Connect diabetes management system Better connected for better decisions Help your patients use the power of their mobile devices and the web to manage their diabetes How many
Introducing the Accu-Chek Connect diabetes management system Better connected for better decisions Help your patients use the power of their mobile devices and the web to manage their diabetes How many
" EXTERNAL QUALITY EVALUATION PROGRAM FOR POCT GLUCOSE TESTING years of experience
 Commissie voor Klinische Biologie Commission de Biologie Clinique " EXTERNAL QUALITY EVALUATION PROGRAM FOR POCT GLUCOSE TESTING 8 3 years of experience CM Van Campenhout JC Libeer WIV/ISP/IPH Clinical
Commissie voor Klinische Biologie Commission de Biologie Clinique " EXTERNAL QUALITY EVALUATION PROGRAM FOR POCT GLUCOSE TESTING 8 3 years of experience CM Van Campenhout JC Libeer WIV/ISP/IPH Clinical
Urgent field safety notice
 Accu-Chek Aviva, Accu-Chek Performa Urgent field safety notice May 2018 Important information on selected lots of Accu-Chek Aviva (50s, 10s) and Accu-Chek Performa (10s) strips potentially showing an increased
Accu-Chek Aviva, Accu-Chek Performa Urgent field safety notice May 2018 Important information on selected lots of Accu-Chek Aviva (50s, 10s) and Accu-Chek Performa (10s) strips potentially showing an increased
Urgent Field Safety Notice
 Urgent Field Safety Notice Location, Date 2014 Important notice: Enhanced instructions for the proper handling of the Accu-Chek Mobile system to avoid the potential of falsely elevated
Urgent Field Safety Notice Location, Date 2014 Important notice: Enhanced instructions for the proper handling of the Accu-Chek Mobile system to avoid the potential of falsely elevated
NHS Greater Glasgow and Clyde Adult Blood Glucose Meter and Test Strip Formulary
 NHS Greater Glasgow and Clyde Adult Blood Glucose and Test Strip Formulary 2016-17 Primary Care and Acute Joint Formulary Blood Glucose formulary developed by the Diabetes MCN Blood Glucose Formulary Review
NHS Greater Glasgow and Clyde Adult Blood Glucose and Test Strip Formulary 2016-17 Primary Care and Acute Joint Formulary Blood Glucose formulary developed by the Diabetes MCN Blood Glucose Formulary Review
Product Codes: 5C4474, R5C8320
 12 th November 2014 Field Safety Notice Dear Baxter Healthcare is sending this communication to inform you of additional and updated warnings and cautions being implemented in HomeChoice/HomeChoice
12 th November 2014 Field Safety Notice Dear Baxter Healthcare is sending this communication to inform you of additional and updated warnings and cautions being implemented in HomeChoice/HomeChoice
CHEMICAL REAGENTS 478 CHEMICAL REAGENTS. This document is dated 1 st March 2018
 This document is dated 1 st March 2018 Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS FOR
This document is dated 1 st March 2018 Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS FOR
Guideline on the Choice of Glucometers and Ketometers for ADULTS with TYPE 1 DIABETES (December 2018)
 Guideline on the Choice of Glucometers and Ketometers for ADULTS with TYPE 1 DIABETES (December 2018) This guideline aims to assist healthcare professionals (HCPs) in secondary and primary care (if applicable)
Guideline on the Choice of Glucometers and Ketometers for ADULTS with TYPE 1 DIABETES (December 2018) This guideline aims to assist healthcare professionals (HCPs) in secondary and primary care (if applicable)
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Nutrineal PD4 with 1.1% amino acids. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION L-Tyrosine 0.300 g/l L-Tryptophan 0.270 g/l L-Phenylalanine
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Nutrineal PD4 with 1.1% amino acids. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION L-Tyrosine 0.300 g/l L-Tryptophan 0.270 g/l L-Phenylalanine
Plug-and-play goes online
 The next-generation Accu-Chek T Smart Pix software Plug-and-play goes online Proven tools for efficient and effective diabetes management 1 even for patients who are not in your office How many of these
The next-generation Accu-Chek T Smart Pix software Plug-and-play goes online Proven tools for efficient and effective diabetes management 1 even for patients who are not in your office How many of these
HYDROXYBUTERATE) Gluco Rx Evolve KetoneTest Strips
 Scottish Drug Tariff Part 9 This document is dated 1 st November 2018 Page REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR BLOOD GLUCOSE FOR KETONES (BETA- HYDROXYBUTERATE) 478 DETECTION
Scottish Drug Tariff Part 9 This document is dated 1 st November 2018 Page REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR BLOOD GLUCOSE FOR KETONES (BETA- HYDROXYBUTERATE) 478 DETECTION
TITLE: Blood Glucose Monitors and Test Strips: A Review of the Comparative Clinical Evidence and Cost-Effectiveness
 TITLE: Blood Glucose Monitors and Test Strips: A Review of the Comparative Clinical Evidence and Cost-Effectiveness DATE: 11 April 2011 CONTEXT AND POLICY ISSUES: Blood glucose monitors measure blood glucose
TITLE: Blood Glucose Monitors and Test Strips: A Review of the Comparative Clinical Evidence and Cost-Effectiveness DATE: 11 April 2011 CONTEXT AND POLICY ISSUES: Blood glucose monitors measure blood glucose
Frequently Used PINs for Billing Purposes November 2018
 Diabetic Test Strips PIN Description 97799824 ACCU-CHEK ADVANTAGE STRIPS (100) 97799823 ACCU-CHEK ADVANTAGE STRIPS (50) 97799814 ACCU-CHEK AVIVA TEST STRIPS (100) 97799815 ACCU-CHEK AVIVA TEST STRIPS (50)
Diabetic Test Strips PIN Description 97799824 ACCU-CHEK ADVANTAGE STRIPS (100) 97799823 ACCU-CHEK ADVANTAGE STRIPS (50) 97799814 ACCU-CHEK AVIVA TEST STRIPS (100) 97799815 ACCU-CHEK AVIVA TEST STRIPS (50)
Urgent field safety notice
 Accu-Chek Aviva Urgent field safety notice May 2018 Important information on selected lots of Accu-Chek Aviva (50s, 10s) potentially showing an increased number of strip errors prior to dosing or biased
Accu-Chek Aviva Urgent field safety notice May 2018 Important information on selected lots of Accu-Chek Aviva (50s, 10s) potentially showing an increased number of strip errors prior to dosing or biased
Clinical Accuracy and User Performance of the TRUE METRIX AIR Blood Glucose Monitoring System
 Clinical Accuracy and User Performance of the TRUE METRIX AIR Blood Glucose Monitoring System Summary Objectives: To demonstrate that the TRUE METRIX AIR Blood Glucose Monitoring System, from Trividia
Clinical Accuracy and User Performance of the TRUE METRIX AIR Blood Glucose Monitoring System Summary Objectives: To demonstrate that the TRUE METRIX AIR Blood Glucose Monitoring System, from Trividia
Q99: At what plasma glucose level would glycosuria be expected? Q101: What is the cause of polydipsia in the hypoglycemia?
 Q99: At what plasma glucose level would glycosuria be expected? Q100: What is the cause of polyemia in hyperglycemia? Q101: What is the cause of polydipsia in the hypoglycemia? Q102: What is the effect
Q99: At what plasma glucose level would glycosuria be expected? Q100: What is the cause of polyemia in hyperglycemia? Q101: What is the cause of polydipsia in the hypoglycemia? Q102: What is the effect
Glucometers Gastrostomy Tubes Nasogastric Tubes Insulin pumps Continuous Glucose monitoring system Closed Loop Smart phones/tablets
 Devices Glucometers Gastrostomy Tubes Nasogastric Tubes Insulin pumps Continuous Glucose monitoring system Closed Loop Smart phones/tablets Accuracy Glucometers The ISO (International Organization for
Devices Glucometers Gastrostomy Tubes Nasogastric Tubes Insulin pumps Continuous Glucose monitoring system Closed Loop Smart phones/tablets Accuracy Glucometers The ISO (International Organization for
In humans, self-monitoring of blood glucose is crucial
 J Vet Intern Med 2014;28:1405 1413 ISO-Based Assessment of Accuracy and Precision of Glucose Meters in Dogs Y. Brito-Casillas, P. Figueirinhas, J.C. Wiebe, L. Lopez-Rıos, D. Perez-Barreto, C. Melian, and
J Vet Intern Med 2014;28:1405 1413 ISO-Based Assessment of Accuracy and Precision of Glucose Meters in Dogs Y. Brito-Casillas, P. Figueirinhas, J.C. Wiebe, L. Lopez-Rıos, D. Perez-Barreto, C. Melian, and
Improving Diabetes Care Efficiency: Glucose Meter Download Station in Medical Offices
 18 Osteopathic Family Physician (2017) 18 28 Osteopathic Family Physician Volume 9, No. 6 November/December, 2017 19 REVIEW ARTICLE : Glucose Meter Download Station in Medical Offices Sze Ngong Henry Lo,
18 Osteopathic Family Physician (2017) 18 28 Osteopathic Family Physician Volume 9, No. 6 November/December, 2017 19 REVIEW ARTICLE : Glucose Meter Download Station in Medical Offices Sze Ngong Henry Lo,
Blood glucose test strips (BGTS) evaluation protocol and results. Update April Version 4.1. Note:
 Blood glucose test strips (BGTS) evaluation protocol and results Update April 2016 Version 4.1 Note: December 2017: Prescribing data indicates overall compliance with the GMMMG recommended BGTS of 86%.
Blood glucose test strips (BGTS) evaluation protocol and results Update April 2016 Version 4.1 Note: December 2017: Prescribing data indicates overall compliance with the GMMMG recommended BGTS of 86%.
SUMMARY OF PRODUCT CHARACTERISTICS. Before mixing 1000 ml of electrolyte solution (Small chamber A ) Active substances: Glucose monohydrate
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT PHYSIONEAL 40 Glucose 2.27% w/v / 22.7 mg/ml Solution for peritoneal dialysis 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Before mixing
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT PHYSIONEAL 40 Glucose 2.27% w/v / 22.7 mg/ml Solution for peritoneal dialysis 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Before mixing
CHEMICAL REAGENTS 478 CHEMICAL REAGENTS. This document is dated 1 st October 2017
 This document is dated 1 st October 2017 Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS FOR
This document is dated 1 st October 2017 Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS FOR
Manage Your Pet s Diabetes at Home
 Manage Your Pet s Diabetes at Home Managing your pet s diabetes is easy, accurate and convenient with at-home blood glucose testing with AlphaTRAK 2. If your dog or cat has been diagnosed with diabetes,
Manage Your Pet s Diabetes at Home Managing your pet s diabetes is easy, accurate and convenient with at-home blood glucose testing with AlphaTRAK 2. If your dog or cat has been diagnosed with diabetes,
Dianeal Peritoneal Dialysis Solutions
 Dianeal Peritoneal Dialysis Solutions Name of the medicine Dianeal PD-2, PD-4 and 1 mmol/l Calcium Peritoneal Dialysis Solutions. Description Dianeal PD-2, PD-4 and 1 mmol/l Calcium Peritoneal Dialysis
Dianeal Peritoneal Dialysis Solutions Name of the medicine Dianeal PD-2, PD-4 and 1 mmol/l Calcium Peritoneal Dialysis Solutions. Description Dianeal PD-2, PD-4 and 1 mmol/l Calcium Peritoneal Dialysis
ACCU-CHEK AVIVA TEST STRIPS ACCU-CHEK COMPACT TEST STRIP ACCU-CHEK COMPACT TEST STRIP ACCU-CHEK FASTCLIX 6 LANCET DRUM (102S)
 Appendix H Product Identification Number (PIN) DIN Code Brand Name Canadian Product Name 00999997 99 EXTEMPOR. CPDS - NFLD- 00977062 ACCU-CHEK ADVANTAGE TEST ACCU-CHEK ADVANTAGE TEST 00977124 ACCU-CHEK
Appendix H Product Identification Number (PIN) DIN Code Brand Name Canadian Product Name 00999997 99 EXTEMPOR. CPDS - NFLD- 00977062 ACCU-CHEK ADVANTAGE TEST ACCU-CHEK ADVANTAGE TEST 00977124 ACCU-CHEK
Guideline on the Choice of Blood Glucose Meters, Ketone Meters and Test Strips for children and young people with. TYPE 1 DIABETES (November 2018)
 Guideline on the Choice of Blood Glucose Meters, Ketone Meters and Test Strips for children and young people with TYPE 1 DIABETES (November 2018) Introduction This guideline aims to assist healthcare professionals
Guideline on the Choice of Blood Glucose Meters, Ketone Meters and Test Strips for children and young people with TYPE 1 DIABETES (November 2018) Introduction This guideline aims to assist healthcare professionals
Evaluation of Accuracy and User Performance of the TRUE METRIX Blood Glucose Monitoring System
 Evaluation of Accuracy and User Performance of the TRUE METRIX Blood Glucose Monitoring System Summary Objectives: To demonstrate that the TRUE METRIX Blood Glucose Monitoring System, from Trividia Health,
Evaluation of Accuracy and User Performance of the TRUE METRIX Blood Glucose Monitoring System Summary Objectives: To demonstrate that the TRUE METRIX Blood Glucose Monitoring System, from Trividia Health,
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Physioneal 35 Glucose 2.27% w/v / 22.7 mg/ml Clear-Flex, Solution for peritoneal dialysis. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Physioneal 35 Glucose 2.27% w/v / 22.7 mg/ml Clear-Flex, Solution for peritoneal dialysis. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Quick Reference Guide
 FLASH GLUCOSE MONITORING SYSTEM Quick Reference Guide IMPORTANT USER INFORMATION Before you use your System, review all the product instructions and the Interactive Tutorial. The Quick Reference Guide
FLASH GLUCOSE MONITORING SYSTEM Quick Reference Guide IMPORTANT USER INFORMATION Before you use your System, review all the product instructions and the Interactive Tutorial. The Quick Reference Guide
PERITONEAL DIALYSIS PRESCRIPTION MANAGEMENT GUIDE
 PERITONEAL DIALYSIS PRESCRIPTION MANAGEMENT GUIDE TABLE OF CONTENTS Introduction.... 3 SECTION 1: FUNDAMENTALS OF THE PRESCRIPTION.... 4 Getting Started: Patient Pathway to First Prescription.... 5 Volume
PERITONEAL DIALYSIS PRESCRIPTION MANAGEMENT GUIDE TABLE OF CONTENTS Introduction.... 3 SECTION 1: FUNDAMENTALS OF THE PRESCRIPTION.... 4 Getting Started: Patient Pathway to First Prescription.... 5 Volume
The CARI Guidelines Caring for Australians with Renal Impairment. Monitoring patients on peritoneal dialysis GUIDELINES
 Date written: August 2004 Final submission: July 2005 Monitoring patients on peritoneal dialysis GUIDELINES No recommendations possible based on Level I or II evidence SUGGESTIONS FOR CLINICAL CARE (Suggestions
Date written: August 2004 Final submission: July 2005 Monitoring patients on peritoneal dialysis GUIDELINES No recommendations possible based on Level I or II evidence SUGGESTIONS FOR CLINICAL CARE (Suggestions
DOSING GUIDE. Indications. Important Safety Information. Enable the immune system. RECOGNIZE. RESPOND.
 DOSING GUIDE For patients with unresectable Stage III NSCLC following concurrent CRT For patients with locally advanced or metastatic UC previously treated with platinum-based therapy Enable the immune
DOSING GUIDE For patients with unresectable Stage III NSCLC following concurrent CRT For patients with locally advanced or metastatic UC previously treated with platinum-based therapy Enable the immune
9/11/2012. Chapter 11. Learning Objectives. Learning Objectives. Endocrine Emergencies. Differentiate type 1 and type 2 diabetes
 Chapter 11 Endocrine Emergencies Learning Objectives Differentiate type 1 and type 2 diabetes Explain roles of glucagon, glycogen, and glucose in hypoglycemia Learning Objectives Discuss following medications
Chapter 11 Endocrine Emergencies Learning Objectives Differentiate type 1 and type 2 diabetes Explain roles of glucagon, glycogen, and glucose in hypoglycemia Learning Objectives Discuss following medications
Quick Reference Guide
 FLASH GLUCOSE MONITORING SYSTEM Quick Reference Guide IMPORTANT USER INFORMATION Before you use your System, review all the product instructions and the Interactive Tutorial. The Quick Reference Guide
FLASH GLUCOSE MONITORING SYSTEM Quick Reference Guide IMPORTANT USER INFORMATION Before you use your System, review all the product instructions and the Interactive Tutorial. The Quick Reference Guide
Advances in Diabetes Care Technologies
 1979 Advances in Diabetes Care Technologies 2015 Introduction Insulin pump use: ~ 20% - 30% of patients with T1DM < 1% of insulin-treated patients with T2DM 2007 FDA estimates ~375,000 insulin pumps for
1979 Advances in Diabetes Care Technologies 2015 Introduction Insulin pump use: ~ 20% - 30% of patients with T1DM < 1% of insulin-treated patients with T2DM 2007 FDA estimates ~375,000 insulin pumps for
Understanding Your Medications
 PD Education Booklet 8 Understanding Your Medications What you will learn: Why your medications are important Medications your kidney doctor may prescribe, and what they do Medications that may be added
PD Education Booklet 8 Understanding Your Medications What you will learn: Why your medications are important Medications your kidney doctor may prescribe, and what they do Medications that may be added
Quick Reference Guide
 FLASH GLUCOSE MONITORING SYSTEM Quick Reference Guide FreeStyle LibreLink app A FreeStyle Libre product IMPORTANT USER INFORMATION Before you use your System, review all the product instructions and the
FLASH GLUCOSE MONITORING SYSTEM Quick Reference Guide FreeStyle LibreLink app A FreeStyle Libre product IMPORTANT USER INFORMATION Before you use your System, review all the product instructions and the
Glucophage XR is contra-indicated during breast-feeding.
 Name GLUCOPHAGE XR 1000 mg Prolonged release tablets Active ingredient Metformin hydrochloride Composition Each Glucophage XR 1000 mg prolonged release tablet contains as active ingredient 1000 mg metformin
Name GLUCOPHAGE XR 1000 mg Prolonged release tablets Active ingredient Metformin hydrochloride Composition Each Glucophage XR 1000 mg prolonged release tablet contains as active ingredient 1000 mg metformin
NHS Greater Glasgow and Clyde Adult Blood Glucose and Combined Ketone Meter and Test Strip Formulary
 NHS Greater Glasgow and Clyde Adult Blood Glucose and Combined Ketone Meter and Test Strip Formulary 2018-20 Primary Care and Acute Joint Formulary Blood Glucose Meter formulary developed by the Diabetes
NHS Greater Glasgow and Clyde Adult Blood Glucose and Combined Ketone Meter and Test Strip Formulary 2018-20 Primary Care and Acute Joint Formulary Blood Glucose Meter formulary developed by the Diabetes
Peritoneal Dialysis Prescriptions: A Primer for Nurses
 Peritoneal Dialysis Prescriptions: A Primer for Nurses A Primer ABCs of PD R x Betty Kelman RN-EC MEd CNeph (C) Toronto General Hospital University Health Network Toronto, Ontario, Canada A moment to remember
Peritoneal Dialysis Prescriptions: A Primer for Nurses A Primer ABCs of PD R x Betty Kelman RN-EC MEd CNeph (C) Toronto General Hospital University Health Network Toronto, Ontario, Canada A moment to remember
Renal Self Learning Package INTRODUCTION TO PERITONEAL DIALYSIS
 Renal Self Learning Package INTRODUCTION TO PERITONEAL DIALYSIS St George Hospital Renal Department, reviewed 2017 St George Hospital Renal Department RENAL SELF LEARNING PACKAGE INTRODUCTION TO PERITONEAL
Renal Self Learning Package INTRODUCTION TO PERITONEAL DIALYSIS St George Hospital Renal Department, reviewed 2017 St George Hospital Renal Department RENAL SELF LEARNING PACKAGE INTRODUCTION TO PERITONEAL
GLOOKO FOR ios MIDS USER GUIDE
 GLOOKO FOR ios MIDS USER GUIDE October 2018 IFU-0001 13 Glooko MIDS is cleared for US only Rx only TABLE OF CONTENTS TABLE OF CONTENTS MOBILE INSULIN DOSING SYSTEM (MIDS)... 2 Intended Use... 2 Warnings...
GLOOKO FOR ios MIDS USER GUIDE October 2018 IFU-0001 13 Glooko MIDS is cleared for US only Rx only TABLE OF CONTENTS TABLE OF CONTENTS MOBILE INSULIN DOSING SYSTEM (MIDS)... 2 Intended Use... 2 Warnings...
STARTING PERITONEAL DIALYSIS
 STARTING PERITONEAL DIALYSIS What you will learn: What your kidneys do What uremia (kidney disease) is and what the symptoms are How Peritoneal Dialysis (PD) helps What the two types of PD are: automated
STARTING PERITONEAL DIALYSIS What you will learn: What your kidneys do What uremia (kidney disease) is and what the symptoms are How Peritoneal Dialysis (PD) helps What the two types of PD are: automated
CHEMICAL REAGENTS 478 PART 9 CHEMICAL REAGENTS. This document is dated 1 st March 2016.
 PART 9 This document is dated 1 st March 2016. Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS
PART 9 This document is dated 1 st March 2016. Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS
Physioneal 40 Glucose 1.36% Physioneal 40 Glucose 2.27% Physioneal 40 Glucose 3.86% In Viaflex Plastic Container
 PRESCRIBING INFORMATION Physioneal 40 Glucose 1.36% Physioneal 40 Glucose 2.27% Physioneal 40 Glucose 3.86% In Viaflex Plastic Container Solution for Peritoneal Dialysis Baxter Corporation Mississauga,
PRESCRIBING INFORMATION Physioneal 40 Glucose 1.36% Physioneal 40 Glucose 2.27% Physioneal 40 Glucose 3.86% In Viaflex Plastic Container Solution for Peritoneal Dialysis Baxter Corporation Mississauga,
Self-monitoring of blood glucose: Advice for providers and patients
 REVIEW SHANNON KNAPP, BSN, RN, CDE Manager of Diabetes Education, Department of Endocrinology, Diabetes, and Metabolism, Cleveland Clinic POOJA MANROA, MD Division of Endocrinology, Diabetes, and Metabolism,
REVIEW SHANNON KNAPP, BSN, RN, CDE Manager of Diabetes Education, Department of Endocrinology, Diabetes, and Metabolism, Cleveland Clinic POOJA MANROA, MD Division of Endocrinology, Diabetes, and Metabolism,
