Investor Presentation
|
|
|
- Thomasina Norton
- 6 years ago
- Views:
Transcription
1 Investor Presentation May 1, 2018 Albert Friesen Chair, President & CEO James Kinley CFO
2 Forward Looking Statement This presentation is for informational purposes only and should not be considered as an offer to buy or sell securities. No stock exchange has either approved or disapproved of the information that is contained in this presentation. This presentation may contain forwardlooking statements within the meaning of Canadian Securities legislation and the forwardlooking statements contained herein are made as at the date of this presentation and, accordingly, are subject to change after such date. Undue reliance should not be placed on such statements. These statements involve a number of risks and uncertainties including statements regarding the outlook for Medicure Inc., business and operational results. By nature, these risks and uncertainties could cause actual results to differ materially from what has been indicated. Factors that could cause actual results to differ materially from any forward-looking statement include, but are not limited to, product recalls, competition from similar products and other factors including those risks and uncertainties identified above, and those contained in the Company s most recent MD&A and Form 20F. Medicure Inc. undertakes no obligation to update publicly or otherwise revise any forward-looking information as a result of new information, future results or other such factors which affect this information, except as required by law.
3 Medicure A pharmaceutical company focused on the development and commercialization of therapeutics for the U.S. market. Key Attributes: U.S. hospital sales force with cardio focus Proven success with growth of Aggrastat franchise 2 nd cardio drug, Zypitamag launched 2018 Expanding portfolio through product development and acquisition Growing revenue and cash flow positive Strong balance sheet No debt, presently over $60 million in cash 3
4 AGGRASTAT Net Revenue Growth $35,000,000 AGGRASTAT Net Revenue (CDN Millions) $30,000,000 $25,000,000 $20,000,000 $15,000,000 $10,000,000 $5,000,000 $
5 Adj. EBITDA* Growth $12,000,000 Consolidated Adj. EBITDA (CDN Millions) $9,500,000 $7,000,000 $4,500,000 $2,000,000 -$500,000 -$3,000, * The Company defines EBITDA as "earnings before interest, taxes, depreciation, amortization and other income or expense" and Adjusted EBITDA as "EBITDA adjusted for non-cash and one-time items". The terms "EBITDA" and "Adjusted EBITDA", as it relates to the results prepared using International Financial Reporting Standards ("IFRS"), do not have any standardized meaning according to IFRS. It is therefore unlikely to be comparable to similar measures presented by other companies.
6 Quarterly Net Revenue 10,000,000 Consolidated Quarterly Net Revenue (CDN Millions) 8,000,000 6,000,000 4,000,000 2,000,000 0 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q
7 AGGRASTAT Hospital Demand Total Units Sold 30,000 25,000 20,000 15,000 Label Change Oct ,000 5,000 0 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q
8 AGGRASTAT Patient Market Share 100% Estimated Patient Market Share (GP IIb/IIIa Inhibitors) 80% 60% 40% Aggrastat (tirofiban HCl) Integrilin (eptifibatide) Reopro (abciximab) 53% 20% 0% Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q
9 U.S. GP IIb/IIIa Inhibitor (GPI) Market Aggrastat Market Positioning: Significant platelet inhibition profile Robust data in over 8,000 patients Class 1 guideline recommended 1,2 Numerous administration conveniences Lower per-patient acquisition costs 2017 GPI Market = ~$180 Million USD (WAC* Hospital Sales) Aggrastat $40.5 million ReoPro $38.1 million Integrilin $99.5 million (incl. generics) * WAC = Wholesale Acquisition Costs (no discounts/rebates) 1. Amsterdam EA et al. J Am Coll Cardiol 2014;64: Levine GN et al. J Am Coll Cardiol 2011; 58:e44-e122
10 Acute cardiovascular hospital product I.V. platelet inhibitor; binds to GP IIb/IIIa receptor Indicated for Acute Coronary Syndrome (ACS) 41% reduction in death and MI in high-risk patients 1 Launched by Merck in 1998 U.S. rights acquired by Medicure in 2006 Medicure obtained broader FDA approval in October 2013 for High Dose Bolus regimen Patented until PRISM-PLUS Study Investigators. N Engl J Med. 1998;338:
11 FDA Approval: Bolus Vial Aggrastat is now available as a concentrated bolus vial Pre-mixed, single bolus delivery* Formulated for convenient IV push No pump programming needed Relatively neutral ph Does not require refrigeration * Current 100 ml and 250 ml (both 50 mcg/ml) bag formats provide the infusion dose.
12 Apicore Transaction Summary July 2014 December 2016 July 2017 October 2017 Acquired 5% interest in Apicore for lead role in structuring majority interest purchase and financing of Apicore. Also received option to acquire remaining shares at a fixed price for 3 years. Exercises option to acquire majority interest (60%) of Apicore for US$34.75M after obtaining CDN$60M of long-term debt Acquired additional 32% ownership for US$24.5M after Apicore repays US$9.8M loan from Medicure and advances additional funds to Medicure. Sale of Apicore business for expected net proceeds of US$105M*, including approximately US$55M received on closing. November 2017 Repayment of long-term debt ($60M Crown debt and $1M MDC debt) January 2018 Receipt of second tranche of funds from Apicore sale of approximately US$50M. *Additional payments may be received over the next 18 months relating to holdback funds.
13 Product & Business Development Building a pharmaceutical portfolio focused on the U.S. cardiovascular market Leveraging our sales infrastructure 3 ANDAs in the pipeline for generic cardiovascular drugs Investing in reduced risk - high reward development projects (ANDAs) and acquisitions (Zypitamag) Maintaining focus on profitability
14
15 Zypitamag Overview Branded cardiovascular: Drug for the treatment of patients with primary hyperlipidemia or mixed dyslipidemia. Approved by FDA: 2017 Launch: May 1 st 2018 License Term: On December 14, 2017, obtained an exclusive license from Zydus Cadila, a multinational pharmaceutical company to market and sell in the US for seven years with extensions available. 15
16 Zypitamag (pitavastatin) Key Points Pitavastatin is recommended in the most recent ACC/AHA Statin Intensity Guidelines as a moderate intensity statin (2mg and 4mg). Minimally processed by enzymes of the CYP450 family - decreases the likelihood of CYP-mediated drug-drug interactions. Statistical superiority to Pravachol (pravastatin) in LDL-C reduction, in patients 65 years of age. Comparable efficacy at the 2mg and 4mg doses to commonly prescribed strengths of atorvastatin (10mg, 20mg) and simvastatin (20mg, 40mg). Dosing simplicity with easy to swallow 1 mg (lowest strength), 2 mg (moderate strength) or 4 mg (highest strength) tablets taken once daily with or without food. 16
17 Zypitamag (pitavastatin) Key Points Wealth of post-market surveillance studies in >33,000 patients from diverse ethnicities and with co-morbid conditions. Lowering of LDL-C with non-significant increase in blood glucose level in comparison to Lipitor (atorvastatin) in patients with T2DM. Does not require dosage modifications based on patient race, unlike Crestor (rosuvastatin) and Zocor (simvastatin). Pitavastatin is not associated with headache, nausea, abdominal pain and occurrence of infections, side effects that are commonly experienced with other statins; such as Crestor (rosuvastatin), Lescol (fluvastatin), Mevacor (lovastatin), Pravachol (pravastatin), Zocor (simvastatin) and Lipitor (atorvastatin). 17
18 Zypitamag Launch ACC March 2018
19 Product & Business Development 3 ANDAs in the pipeline for generic cardiovascular drugs Q1 Q2 Q3 Q4 Medicure Product Pipeline Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q Q4 AGGRASTAT ZYPITAMAG ANDA 1 ANDA 2 ANDA 3
20 Key Financial Info MPH: TSXV Capital Structure as at April 27, 2018 Basic Total 15,881,760 Fully Diluted Total 18,453,554 Share Price Market Cap C$6.45 C$102.4M Recent Financial Highlights* 2017 Net Revenue $27.1 M 2017 Adj. EBITDA $4.6 M Current cash >$60 million No debt Focus Continue to grow Aggrastat brand Growing Zypitamag sales Continue diversification through product & business development *Excludes results from Apicore business which was divested on October 2, 2017.
21 Thank you Contact a Product Specialist Investor Relations ir@medicure.com (Ext. 228) For More Information ww.aggrastat.com
22 Aggrastat Prescribing Information Indication: AGGRASTAT is indicated to reduce the rate of thrombotic cardiovascular events (combined endpoint of death, myocardial infarction, or refractory ischemia/repeat cardiac procedure) in patients with non-st elevation acute coronary syndrome (NSTE-ACS). Dosage and Administration: High-Dose Bolus Regimen: Administer intravenously 25 mcg/kg within 5 minutes and then 0.15 mcg/kg/min for up to 18 hours. In patients with CrCl 60 ml/min, use the full bolus and halve the maintenance infusion. Contraindications: Known hypersensitivity to any component of AGGRASTAT; History of thrombocytopenia with prior exposure to AGGRASTAT; Active internal bleeding, or history of bleeding diathesis, major surgical procedure or severe physical trauma within previous month Warnings and Precautions: AGGRASTAT can cause serious bleeding. If bleeding cannot be controlled discontinue AGGRASTAT; Thrombocytopenia: Discontinue AGGRASTAT and heparin Adverse Reactions: Bleeding is the most commonly reported adverse reaction
23 Important Zypitamag Safety Information IMPORTANT SAFETY INFORMATION FOR ZYPITAMAG (pitavastatin) INDICATIONS & USAGE Drug therapy should be one component of multiple-risk-factor intervention in individuals who require modifications of their lipid profile. Lipid-altering agents should be used in addition to a diet restricted in saturated fat and cholesterol only when the response to diet and other nonpharmacological measures has been inadequate. Primary Hyperlipidemia and Mixed Dyslipidemia: ZYPITAMAG is indicated as an adjunctive therapy to diet to reduce elevated total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), apolipoprotein B (Apo B), triglycerides (TG), and to increase HDL-C in adult patients with primary hyperlipidemia or mixed dyslipidemia. Limitations of Use: Doses of ZYPITAMAG greater than 4 mg once daily were associated with an increased risk for severe myopathy in premarketing clinical studies. Do not exceed 4 mg once daily dosing of ZYPITAMAG. The effect of ZYPITAMAG on cardiovascular morbidity and mortality has not been determined. ZYPITAMAG has not been studied in Fredrickson Type I, III, and V dyslipidemias. CONTRAINDICATIONS: ZYPITAMAG is contraindicated in patients with a known hypersensitivity to product components, in patients with active liver disease (which may include unexplained persistent elevations in hepatic transaminase levels), in women who are pregnant or may become pregnant, in nursing mothers, or in coadministration with cyclosporine. WARNINGS & PRECAUTIONS Skeletal Muscle Effects: Cases of myopathy and rhabdomyolysis with acute renal failure secondary to myoglobinuria have been reported with HMG-CoA reductase inhibitors, including pitavastatin. These risks can occur at any dose level, but increase in a dose-dependent manner, with advanced age ( 65 years), renal impairment, and inadequately treated hypothyroidism; administer with caution in these patients, or when used concomitantly with fibrates or lipid-modifying doses of niacin, or colchicine. Avoid concomitant administration with gemfibrozil. Advise patients to promptly report unexplained and/or persistent muscle pain, tenderness, or weakness, particularly if accompanied by malaise or fever; discontinue ZYPITAMAG. If muscle signs and symptoms persist after discontinuation, this may be a sign of immune-mediated necrotizing myopathy (IMNM), an autoimmune myopathy associated with statin use, requiring immediate medical attention. IMNM is characterized by proximal muscle weakness and elevated serum creatine kinase, which persist despite discontinuation of statin treatment; muscle biopsy showing necrotizing myopathy without significant inflammation; improvement with immunosuppressive agents. ZYPITAMAG should be discontinued if markedly elevated creatine kinase levels occur or myopathy is diagnosed or suspected. ZYPITAMAG should also be temporarily withheld in any patient with an acute, serious condition suggestive of myopathy or predisposing to the development of renal failure secondary to rhabdomyolysis (e.g., sepsis, hypotension, dehydration, major surgery, trauma, severe metabolic, endocrine, and electrolyte disorders, or uncontrolled seizures). Liver Enzyme Abnormalities: Persistent elevation in hepatic transaminases can occur. Check liver enzymes before initiating therapy and if signs or symptoms of liver injury occur; advise patients to report fatigue, anorexia, right upper abdominal discomfort, dark urine or jaundice. Fatal and non-fatal hepatic failure can occur. Interrupt ZYPITAMAG if serious liver injury with clinical symptoms and/or hyperbilirubinemia or jaundice occurs. If an alternate etiology is not found do not restart ZYPITAMAG. Use ZYPITAMAG with caution in patients who consume substantial quantities of alcohol and/or have a history of chronic liver disease. Do not use ZYPITAMAG if patient has active liver disease, which may include unexplained persistent transaminase elevations. Endocrine Function: Increases in HbA1c and fasting serum glucose levels have been reported. COMMON ADVERSE REACTIONS: myalgia, back pain, diarrhea, constipation and pain in extremity (rate 2% in at least one marketed dose). This is not a complete list of all reported adverse events. For additional information, refer to full Prescribing Information.
Investor Presentation
 Investor Presentation November 20, 2018 Albert Friesen Chair, President & CEO James Kinley CFO Forward Looking Statement This presentation is for informational purposes only and should not be considered
Investor Presentation November 20, 2018 Albert Friesen Chair, President & CEO James Kinley CFO Forward Looking Statement This presentation is for informational purposes only and should not be considered
Investor Presentation
 Investor Presentation August 15, 2018 Albert Friesen Chair, President & CEO James Kinley CFO Forward Looking Statement This presentation is for informational purposes only and should not be considered
Investor Presentation August 15, 2018 Albert Friesen Chair, President & CEO James Kinley CFO Forward Looking Statement This presentation is for informational purposes only and should not be considered
NEW HEMATOLOGY PARAMETERS
 NEW HEMATOLOGY PARAMETERS CASE STUDIES and IMPLEMENTATION WHAT WE WILL COVER new parameters Ret-He, IPF (and some not so new parameters) - anemias - hemoglobinopathies - problem platelets - uncommon things
NEW HEMATOLOGY PARAMETERS CASE STUDIES and IMPLEMENTATION WHAT WE WILL COVER new parameters Ret-He, IPF (and some not so new parameters) - anemias - hemoglobinopathies - problem platelets - uncommon things
Clinical Policy: Ezetimibe (Zetia) Reference Number: CP.PMN.78 Effective Date: Last Review Date: 02.19
 Clinical Policy: (Zetia) Reference Number: CP.PMN.78 Effective Date: 02.01.17 Last Review Date: 02.19 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Zetia) Reference Number: CP.PMN.78 Effective Date: 02.01.17 Last Review Date: 02.19 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
STATIN UTILIZATION MANAGEMENT CRITERIA
 STATIN UTILIZATION MANAGEMENT CRITERIA DRUG CLASS: HMG Co-A Reductase Inhibitors & Combinations Agents which require prior review: Advicor (niacin extended-release/lovastatin) Crestor (rosuvastatin)(5mg,10mg,
STATIN UTILIZATION MANAGEMENT CRITERIA DRUG CLASS: HMG Co-A Reductase Inhibitors & Combinations Agents which require prior review: Advicor (niacin extended-release/lovastatin) Crestor (rosuvastatin)(5mg,10mg,
Rosuvastatin 5 mg, 10 mg and 20 mg Tablet
 Rosuvastatin 5 mg, 10 mg and 20 mg Tablet Description is a preparation of Rosuvastatin. Rosuvastatin is a member of the drug class of statins, used in combination with exercise, diet, and weight-loss to
Rosuvastatin 5 mg, 10 mg and 20 mg Tablet Description is a preparation of Rosuvastatin. Rosuvastatin is a member of the drug class of statins, used in combination with exercise, diet, and weight-loss to
Clinical Policy: Pitavastatin (Livalo), Ezetimibe/Simvastatin (Vytorin 10/10 mg) Reference Number: CP.CPA.62 Effective Date:
 Clinical Policy: Pitavastatin (Livalo), Ezetimibe/Simvastatin (Vytorin 10/10 mg) Reference Number: CP.CPA.62 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See
Clinical Policy: Pitavastatin (Livalo), Ezetimibe/Simvastatin (Vytorin 10/10 mg) Reference Number: CP.CPA.62 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Reference Number: CP.HNMC.05 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: Reference Number: CP.HNMC.05 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this policy for important
It is the policy of health plans affiliated with PA Health & Wellness that Vytorin is medically necessary when the following criteria are met:
 Clinical Policy: Ezetimibe and Simvastatin (Vytorin) Reference Number: PA.CP.PMN.77 Effective Date: 02.01.17 Last Review Date: 07.18 Revision Log Description Ezetimibe/simvastatin (Vytorin ) contains ezetimibe,
Clinical Policy: Ezetimibe and Simvastatin (Vytorin) Reference Number: PA.CP.PMN.77 Effective Date: 02.01.17 Last Review Date: 07.18 Revision Log Description Ezetimibe/simvastatin (Vytorin ) contains ezetimibe,
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Statin Therapy Page 1 of 10 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Statin Therapy Prime Therapeutics will review Prior Authorization requests. Prior Authorization
Statin Therapy Page 1 of 10 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Statin Therapy Prime Therapeutics will review Prior Authorization requests. Prior Authorization
High ( 50%) Restrictions mg 20-40mg PA; TS ⱡ 15 ⱡ
 MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Cholesterol P&T DATE: 5/9/2017 THERAPEUTIC CLASS: Cardiovascular REVIEW HISTORY: 5/16, 5/15, 2/14, 5/12, LOB AFFECTED: Medi-Cal
MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Cholesterol P&T DATE: 5/9/2017 THERAPEUTIC CLASS: Cardiovascular REVIEW HISTORY: 5/16, 5/15, 2/14, 5/12, LOB AFFECTED: Medi-Cal
Diseases & Conditions
 http://my.clevelandclinic.org Diseases & Conditions About Cholesterol-Lowering Drugs Some people have a genetic predisposition to high blood cholesterol levels. These people may need drug therapy in addition
http://my.clevelandclinic.org Diseases & Conditions About Cholesterol-Lowering Drugs Some people have a genetic predisposition to high blood cholesterol levels. These people may need drug therapy in addition
Joshua Shepherd PA-C, MMS, MT (ASCP)
 Joshua Shepherd PA-C, MMS, MT (ASCP) None What is Cholesterol? Why cholesterol is it important? Review the National Cholesterol Education Programs guidelines (NCEP-ATPIII) Discuss New guidelines from the
Joshua Shepherd PA-C, MMS, MT (ASCP) None What is Cholesterol? Why cholesterol is it important? Review the National Cholesterol Education Programs guidelines (NCEP-ATPIII) Discuss New guidelines from the
Repatha. Repatha (evolocumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.08 Subject: Repatha Page: 1 of 9 Last Review Date: September 15, 2017 Repatha Description Repatha
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.08 Subject: Repatha Page: 1 of 9 Last Review Date: September 15, 2017 Repatha Description Repatha
Repatha. Repatha (evolocumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.08 Subject: Repatha Page: 1 of 9 Last Review Date: November 30, 2018 Repatha Description Repatha (evolocumab)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.08 Subject: Repatha Page: 1 of 9 Last Review Date: November 30, 2018 Repatha Description Repatha (evolocumab)
Repatha. Repatha (evolocumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.16.08 Subject: Repatha Page: 1 of 8 Last Review Date: September 18, 2015 Repatha Description Repatha
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.16.08 Subject: Repatha Page: 1 of 8 Last Review Date: September 18, 2015 Repatha Description Repatha
Clinical Policy: Lomitapide (Juxtapid) Reference Number: ERX.SPA.170 Effective Date:
 Clinical Policy: (Juxtapid) Reference Number: ERX.SPA.170 Effective Date: 01.11.17 Last Review Date: 11.17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Juxtapid) Reference Number: ERX.SPA.170 Effective Date: 01.11.17 Last Review Date: 11.17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Management of Post-transplant hyperlipidemia
 Management of Post-transplant hyperlipidemia B. Gisella Carranza Leon, MD Assistant Professor of Medicine Lipid Clinic - Vanderbilt Heart and Vascular Institute Division of Diabetes, Endocrinology and
Management of Post-transplant hyperlipidemia B. Gisella Carranza Leon, MD Assistant Professor of Medicine Lipid Clinic - Vanderbilt Heart and Vascular Institute Division of Diabetes, Endocrinology and
Clinical Policy: Mipomersen (Kynamro) Reference Number: ERX.SPA.171 Effective Date:
 Clinical Policy: (Kynamro) Reference Number: ERX.SPA.171 Effective Date: 01.11.17 Last Review Date: 08.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Kynamro) Reference Number: ERX.SPA.171 Effective Date: 01.11.17 Last Review Date: 08.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Praluent. Praluent (alirocumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.06 Subject: Praluent Page: 1 of 10 Last Review Date: September 20, 2018 Praluent Description Praluent
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.06 Subject: Praluent Page: 1 of 10 Last Review Date: September 20, 2018 Praluent Description Praluent
SOLVAY GROUP London Morning Meeting. June 26, 2009
 SOLVAY GROUP London Morning Meeting June 2, 9 1 Our fenofibrate franchise Klaus Kirchgassler, MD Sr VP Solvay Pharmaceuticals 2 Summary of lipid lowering market performance in Value Growth vs. previous
SOLVAY GROUP London Morning Meeting June 2, 9 1 Our fenofibrate franchise Klaus Kirchgassler, MD Sr VP Solvay Pharmaceuticals 2 Summary of lipid lowering market performance in Value Growth vs. previous
Repatha. Repatha (evolocumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.08 Subject: Repatha Page: 1 of 8 Last Review Date: December 2, 2016 Repatha Description Repatha (evolocumab)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.08 Subject: Repatha Page: 1 of 8 Last Review Date: December 2, 2016 Repatha Description Repatha (evolocumab)
Cholesterol Management Roy Gandolfi, MD
 Cholesterol Management 2017 Roy Gandolfi, MD Goals Interpreting cholesterol guidelines Cholesterol treatment in diabetics Statin use and side effects therapy Reporting- Comparison data among physicians
Cholesterol Management 2017 Roy Gandolfi, MD Goals Interpreting cholesterol guidelines Cholesterol treatment in diabetics Statin use and side effects therapy Reporting- Comparison data among physicians
Antihyperlipidemic Drugs
 Antihyperlipidemic Drugs Hyperlipidemias. Hyperlipoproteinemias. Hyperlipemia. Hypercholestrolemia. Direct relationship with acute pancreatitis and atherosclerosis Structure Lipoprotein Particles Types
Antihyperlipidemic Drugs Hyperlipidemias. Hyperlipoproteinemias. Hyperlipemia. Hypercholestrolemia. Direct relationship with acute pancreatitis and atherosclerosis Structure Lipoprotein Particles Types
Pharmacy Drug Class Review
 Pharmacy Drug Class Review January 22, 2014 Authored By: Christina Manciocchi, Pharm.D. BCACP Disclaimer: Specific agents may have variations Edited By: Richard J. Kraft, Pharm.D.BCPS NEW CHOLESTEROL GUIDELINES
Pharmacy Drug Class Review January 22, 2014 Authored By: Christina Manciocchi, Pharm.D. BCACP Disclaimer: Specific agents may have variations Edited By: Richard J. Kraft, Pharm.D.BCPS NEW CHOLESTEROL GUIDELINES
Parker & Waichman, LLP Attorneys at Law CRESTOR Information Guide
 Parker & Waichman, LLP Attorneys at Law CRESTOR Information Guide The Case Against CRESTOR: Kidney Problems & Rhabdomyolysis Crestor has been linked to serious side effects including: kidney damage, kidney
Parker & Waichman, LLP Attorneys at Law CRESTOR Information Guide The Case Against CRESTOR: Kidney Problems & Rhabdomyolysis Crestor has been linked to serious side effects including: kidney damage, kidney
2013 ACC/AHA Cholesterol Guidelines JULIE HAMMOND, D.O. PGY-2 MATTHEW PAOLI, D.O. PGY-2
 2013 ACC/AHA Cholesterol Guidelines JULIE HAMMOND, D.O. PGY-2 MATTHEW PAOLI, D.O. PGY-2 GOALS ACC/AHA as publisher of guidelines Determining which patients are appropriate for statin therapy The treatment
2013 ACC/AHA Cholesterol Guidelines JULIE HAMMOND, D.O. PGY-2 MATTHEW PAOLI, D.O. PGY-2 GOALS ACC/AHA as publisher of guidelines Determining which patients are appropriate for statin therapy The treatment
NICE QIPP about Lipitor. Robert Trotter. Clinical Effectiveness Consultant
 NICE QIPP about Lipitor Robert Trotter Clinical Effectiveness Consultant LIP2894c Date of preparation: April 2009 Prescribing information for atorvastatin is available on the last slide Roadmap Background
NICE QIPP about Lipitor Robert Trotter Clinical Effectiveness Consultant LIP2894c Date of preparation: April 2009 Prescribing information for atorvastatin is available on the last slide Roadmap Background
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Caduet) Reference Number: CP.CPA.237 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this policy
Clinical Policy: (Caduet) Reference Number: CP.CPA.237 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this policy
Pharmacology Challenges: Managing Statin Myalgia
 Clinical Case: RM is a 50 year-old African American woman with a past medical history of type diabetes, dyslipidemia, hypertension and peripheral arterial disease. She had been prescribed simvastatin 80
Clinical Case: RM is a 50 year-old African American woman with a past medical history of type diabetes, dyslipidemia, hypertension and peripheral arterial disease. She had been prescribed simvastatin 80
PIEDMONT ACCESS TO HEALTH SERVICES, INC. Guidelines for Screening and Management of Dyslipidemia
 PIEDMONT ACCESS TO HEALTH SERVICES, INC. Policy Number: 01-09-021 SUBJECT: Guidelines for Screening and Management of Dyslipidemia EFFECTIVE DATE: 04/2008 REVIEWED/REVISED: 04/12/10, 03/17/2011, 4/10/2012,
PIEDMONT ACCESS TO HEALTH SERVICES, INC. Policy Number: 01-09-021 SUBJECT: Guidelines for Screening and Management of Dyslipidemia EFFECTIVE DATE: 04/2008 REVIEWED/REVISED: 04/12/10, 03/17/2011, 4/10/2012,
Genomic Health. Kim Popovits, Chairman, CEO and President
 Genomic Health Kim Popovits, Chairman, CEO and President Safe Harbor Statement Various remarks that we make in this presentation that are not historical, including those about our future financial and
Genomic Health Kim Popovits, Chairman, CEO and President Safe Harbor Statement Various remarks that we make in this presentation that are not historical, including those about our future financial and
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Vytorin) Reference Number: CP.PMN.77 Effective Date: 02.01.17 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this policy
Clinical Policy: (Vytorin) Reference Number: CP.PMN.77 Effective Date: 02.01.17 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this policy
Simvastatin 40 mg equivalent
 Simvastatin 40 mg equivalent medications equivalent to Simvastatin is available on the Drugs.com website. Simvastatin (Zocor ): 10 mg : Equivalent Dosages - 3: Atorvastatin (Lipitor. 40 mg : Equivalent
Simvastatin 40 mg equivalent medications equivalent to Simvastatin is available on the Drugs.com website. Simvastatin (Zocor ): 10 mg : Equivalent Dosages - 3: Atorvastatin (Lipitor. 40 mg : Equivalent
Recommendations Contraindicated with VYTORIN
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use VYTORIN safely and effectively. See full prescribing information for VYTORIN. VYTORIN (ezetimibe
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use VYTORIN safely and effectively. See full prescribing information for VYTORIN. VYTORIN (ezetimibe
Primary Prevention Patients aged 85yrs and over
 Rotherham Guideline for the management of Non-Familial Hypercholesterolaemia Type 1 Diabetes Offer lifestyle advice Over 40yrs of age? Diabetic for more than 10 years? Established nephropathy? Other CVD
Rotherham Guideline for the management of Non-Familial Hypercholesterolaemia Type 1 Diabetes Offer lifestyle advice Over 40yrs of age? Diabetic for more than 10 years? Established nephropathy? Other CVD
Pregnancy (4, 8.1, 8.3) Lactation (4, 8.2) Coadministration with cyclosporine (4, 7.1, 12.3)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ZYPITAMAG safely and effectively. See full prescribing information for ZYPITAMAG. ZYPITAMAG (pitavastatin)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ZYPITAMAG safely and effectively. See full prescribing information for ZYPITAMAG. ZYPITAMAG (pitavastatin)
Cardiovascular Drugs and Therapies HMG CoA a REDUCTASE INHIBITORS (available in Canada)
 Trade Name Dosage Forms 10 mg, 20 mg, 40 mg, 80 mg tablets Dosing Range 10-80 mg once daily 2 Dosing Based on Desired LDL Reduction 1,2,3 HMG CoA a REDUCTASE INHIBITORS (available in Canada) LIPITOR, generics
Trade Name Dosage Forms 10 mg, 20 mg, 40 mg, 80 mg tablets Dosing Range 10-80 mg once daily 2 Dosing Based on Desired LDL Reduction 1,2,3 HMG CoA a REDUCTASE INHIBITORS (available in Canada) LIPITOR, generics
Drug Class Review HMG-CoA Reductase Inhibitors (Statins) and Fixed-dose Combination Products Containing a Statin
 Drug Class Review HMG-CoA Reductase Inhibitors (Statins) and Fixed-dose Combination Products Containing a Statin Final Report Update 5 November 2009 This report reviews information about the comparative
Drug Class Review HMG-CoA Reductase Inhibitors (Statins) and Fixed-dose Combination Products Containing a Statin Final Report Update 5 November 2009 This report reviews information about the comparative
AMAG PHARMACEUTICALS. June 2013
 AMAG PHARMACEUTICALS June 2013 FORWARD LOOKING STATEMENTS This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and other federal
AMAG PHARMACEUTICALS June 2013 FORWARD LOOKING STATEMENTS This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and other federal
Elements for a Public Summary
 Rosuvastatin Stada 5 mg film-coated tablets Rosuvastatin Stada 10 mg film-coated tablets Rosuvastatin Stada 20 mg film-coated tablets Rosuvastatin Stada 40 mg film-coated tablets 25.8.2014, V1.1 PUBLIC
Rosuvastatin Stada 5 mg film-coated tablets Rosuvastatin Stada 10 mg film-coated tablets Rosuvastatin Stada 20 mg film-coated tablets Rosuvastatin Stada 40 mg film-coated tablets 25.8.2014, V1.1 PUBLIC
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 6 October 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 6 October 2010 CRESTOR 5 mg, film-coated tablet B/30 (CIP code: 369 853-8) B/90 (CIP code: 391 690-0) CRESTOR 10 mg,
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 6 October 2010 CRESTOR 5 mg, film-coated tablet B/30 (CIP code: 369 853-8) B/90 (CIP code: 391 690-0) CRESTOR 10 mg,
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Kynamro) Reference Number: CP.PHAR.284 Effective Date: 10.16 Last Review Date: 02.19 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this policy
Clinical Policy: (Kynamro) Reference Number: CP.PHAR.284 Effective Date: 10.16 Last Review Date: 02.19 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this policy
Cholesterol. Medicines To Help You
 Medicines To Help You Cholesterol Use this guide to help you talk to your doctor, pharmacist, or nurse about your cholesterol medicines. The guide lists all of the FDA-approved products now available to
Medicines To Help You Cholesterol Use this guide to help you talk to your doctor, pharmacist, or nurse about your cholesterol medicines. The guide lists all of the FDA-approved products now available to
Pravastatin conversion to atorvastatin
 P ford residence southampton, ny Pravastatin conversion to atorvastatin Pravastatin official prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions, pharmacology
P ford residence southampton, ny Pravastatin conversion to atorvastatin Pravastatin official prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions, pharmacology
INVESTOR PRESENTATION
 INVESTOR PRESENTATION May 2018 2 FORWARD-LOOKING INFORMATION The following presentation contains statements that are considered forward-looking information ( FLI ) within the meaning of securities regulation.
INVESTOR PRESENTATION May 2018 2 FORWARD-LOOKING INFORMATION The following presentation contains statements that are considered forward-looking information ( FLI ) within the meaning of securities regulation.
rosuvastatin, 5mg, 10mg, 20mg, film-coated tablets (Crestor ) SMC No. (725/11) AstraZeneca UK Ltd.
 rosuvastatin, 5mg, 10mg, 20mg, film-coated tablets (Crestor ) SMC No. (725/11) AstraZeneca UK Ltd. 09 September 2011 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
rosuvastatin, 5mg, 10mg, 20mg, film-coated tablets (Crestor ) SMC No. (725/11) AstraZeneca UK Ltd. 09 September 2011 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
CENTENE PHARMACY AND THERAPEUTICS DRUG REVIEW 1Q18 January February
 BRAND NAME Prevymis TM GENERIC NAME Letermovir MANUFACTURER Merck & Co., Inc. DATE OF APPROVAL November 9, 2017 PRODUCT LAUNCH DATE TBD REVIEW TYPE Review type 1 (RT1): New Drug Review Full review of new
BRAND NAME Prevymis TM GENERIC NAME Letermovir MANUFACTURER Merck & Co., Inc. DATE OF APPROVAL November 9, 2017 PRODUCT LAUNCH DATE TBD REVIEW TYPE Review type 1 (RT1): New Drug Review Full review of new
David Zaccardelli, PharmD Chief Executive Officer. J.P. Morgan 37 th Annual Healthcare Conference January 8, 2019
 David Zaccardelli, PharmD Chief Executive Officer J.P. Morgan 37 th Annual Healthcare Conference January 8, 2019 Disclaimer Certain information contained in this presentation relates to or is based on
David Zaccardelli, PharmD Chief Executive Officer J.P. Morgan 37 th Annual Healthcare Conference January 8, 2019 Disclaimer Certain information contained in this presentation relates to or is based on
Tamsulosin Hydrochloride 0.4 mg Capsule
 Tamsulosin Hydrochloride 0.4 mg Capsule, Tamsulosin Hydrochloride 0.4 mg Capsule India, Tamsulosin Hydrochloride 0.4 mg Capsule manufacturers India, side effects Tamsulosin Hydrochloride 0.4 mg Capsule
Tamsulosin Hydrochloride 0.4 mg Capsule, Tamsulosin Hydrochloride 0.4 mg Capsule India, Tamsulosin Hydrochloride 0.4 mg Capsule manufacturers India, side effects Tamsulosin Hydrochloride 0.4 mg Capsule
ACTEMRA Risk Mitigation Strategy Presenter Name, Degree
 ACTEMRA Risk Mitigation Strategy Presenter Name, Degree Medical Science Liaison Genentech, Inc. 1 Indications and Dosage Rheumatoid Arthritis (RA) (1 of 2) Indication in RA ACTEMRA (tocilizumab) is indicated
ACTEMRA Risk Mitigation Strategy Presenter Name, Degree Medical Science Liaison Genentech, Inc. 1 Indications and Dosage Rheumatoid Arthritis (RA) (1 of 2) Indication in RA ACTEMRA (tocilizumab) is indicated
Clinical Policy: Evolocumab (Repatha) Reference Number: ERX.SPMN.184 Effective Date: 01/2017
 Clinical Policy: (Repatha) Reference Number: ERX.SPMN.184 Effective Date: 01/2017 Last Review Date: Revision Log See Important Reminder at the end of this policy for important regulatory and legal information.
Clinical Policy: (Repatha) Reference Number: ERX.SPMN.184 Effective Date: 01/2017 Last Review Date: Revision Log See Important Reminder at the end of this policy for important regulatory and legal information.
Major recommendations for statin therapy for ASCVD prevention
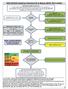 2013 A/AHA Guidelines holesterol Rx to Reduce ASVD Risk in Adults Major recommendations for statin therapy for ASVD prevention *% in LDL can be used as indication of response & adherence to Rx but is not
2013 A/AHA Guidelines holesterol Rx to Reduce ASVD Risk in Adults Major recommendations for statin therapy for ASVD prevention *% in LDL can be used as indication of response & adherence to Rx but is not
Page 1 of 30. SIMVASTATIN tablets, for oral use Initial U.S. Approval: 1991
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use SIMVASTATIN TABLETS safely and effectively. See full prescribing information for SIMVASTATIN TABLETS.
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use SIMVASTATIN TABLETS safely and effectively. See full prescribing information for SIMVASTATIN TABLETS.
Conflicts of interest. What's the Skinny on the Lipid Guidelines? Key Differences. Are you applying the new ACC/AHA Lipid guidelines in your practice?
 Conflicts of interest What's the Skinny on the Lipid Guidelines? The presenter has no relevant conflicts of interest to disclose. Kathleen Vest, PharmD, CDE, BCACP At the end of this presentation, pharmacist
Conflicts of interest What's the Skinny on the Lipid Guidelines? The presenter has no relevant conflicts of interest to disclose. Kathleen Vest, PharmD, CDE, BCACP At the end of this presentation, pharmacist
Scientific conclusions
 Annex II Scientific conclusions and grounds for amendment of the summary of product characteristics, labelling and package leaflet presented by the European Medicines Agency 24 Scientific conclusions Overall
Annex II Scientific conclusions and grounds for amendment of the summary of product characteristics, labelling and package leaflet presented by the European Medicines Agency 24 Scientific conclusions Overall
Elements for a public summary
 VI.2 VI.2.1 Elements for a public summary Overview of disease epidemiology Familial hypercholesterolemia (FH) is a genetic disorder characterized by high cholesterol levels, specifically very high levels
VI.2 VI.2.1 Elements for a public summary Overview of disease epidemiology Familial hypercholesterolemia (FH) is a genetic disorder characterized by high cholesterol levels, specifically very high levels
Eveness Rosuvastatin. Each tablet contains 5mg, 10mg, 20mg, or 40 mg of rosuvastatin (as calcium salt)
 Eveness Rosuvastatin Composition Each tablet contains 5mg, 10mg, 20mg, or 40 mg of rosuvastatin (as calcium salt) Pharmacological properties Pharmacotherapeutic gruoup: HMG CoA reductase inhibitors Pharmacodynamic
Eveness Rosuvastatin Composition Each tablet contains 5mg, 10mg, 20mg, or 40 mg of rosuvastatin (as calcium salt) Pharmacological properties Pharmacotherapeutic gruoup: HMG CoA reductase inhibitors Pharmacodynamic
Investor Presentation
 Investor Presentation February 2018 2 FORWARD-LOOKING INFORMATION The following presentation contains statements that are considered forward-looking information ( FLI ) within the meaning of securities
Investor Presentation February 2018 2 FORWARD-LOOKING INFORMATION The following presentation contains statements that are considered forward-looking information ( FLI ) within the meaning of securities
Clinical Policy: Lomitapide (Juxtapid) Reference Number: ERX.SPA.170 Effective Date:
 Clinical Policy: (Juxtapid) Reference Number: ERX.SPA.170 Effective Date: 01.11.17 Last Review Date: 08.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Juxtapid) Reference Number: ERX.SPA.170 Effective Date: 01.11.17 Last Review Date: 08.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
LIPITOR AND YOU HELPFUL INFORMATION FOR UNDERSTANDING CHOLESTEROL AND RISKS
 LIPITOR AND YOU HELPFUL INFORMATION FOR UNDERSTANDING CHOLESTEROL AND RISKS Learn what your cholesterol levels actually mean, and how you may save money on your prescription each month with the LIPITOR
LIPITOR AND YOU HELPFUL INFORMATION FOR UNDERSTANDING CHOLESTEROL AND RISKS Learn what your cholesterol levels actually mean, and how you may save money on your prescription each month with the LIPITOR
ANCHOR Study Results Overview
 TM ANCHOR Study Results Overview April 18, 2010 Nasdaq: AMRN www.amarincorp.com 1 Forward Looking Statement This presentation contains forward looking statements, including those relating to the Company
TM ANCHOR Study Results Overview April 18, 2010 Nasdaq: AMRN www.amarincorp.com 1 Forward Looking Statement This presentation contains forward looking statements, including those relating to the Company
CRESTOR rosuvastatin calcium tablets
 PART III CONSUMER INFORMATION CRESTOR rosuvastatin calcium tablets This leaflet is part of a "Product Monograph" published when CRESTOR was approved for sale in Canada and is designed specifically for
PART III CONSUMER INFORMATION CRESTOR rosuvastatin calcium tablets This leaflet is part of a "Product Monograph" published when CRESTOR was approved for sale in Canada and is designed specifically for
ANTIHYPERLIPIDEMIA. Darmawan,dr.,M.Kes,Sp.PD
 ANTIHYPERLIPIDEMIA Darmawan,dr.,M.Kes,Sp.PD Plasma lipids consist mostly of lipoproteins Spherical complexes of lipids and specific proteins (apolipoproteins). The clinically important lipoproteins, listed
ANTIHYPERLIPIDEMIA Darmawan,dr.,M.Kes,Sp.PD Plasma lipids consist mostly of lipoproteins Spherical complexes of lipids and specific proteins (apolipoproteins). The clinically important lipoproteins, listed
LIVALO (pitavastatin) Tablet 1 mg, 2 mg, and 4 mg
 (pitavastatin) Tablet 1 mg, 2 mg, and 4 mg Montgomery, AL 36117 Package Insert Product Labeling Version of October 2013 Version 8.0 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include
(pitavastatin) Tablet 1 mg, 2 mg, and 4 mg Montgomery, AL 36117 Package Insert Product Labeling Version of October 2013 Version 8.0 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include
IMPORTANT: PLEASE READ
 PART III: CONSUMER INFORMATION ROSUVASTATIN-5 ROSUVASTATIN-10 ROSUVASTATIN-20 ROSUVASTATIN-40 Rosuvastatin Calcium Tablets This leaflet is part of a oduct Monograph published when ROSUVASTATIN was approved
PART III: CONSUMER INFORMATION ROSUVASTATIN-5 ROSUVASTATIN-10 ROSUVASTATIN-20 ROSUVASTATIN-40 Rosuvastatin Calcium Tablets This leaflet is part of a oduct Monograph published when ROSUVASTATIN was approved
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Repatha) Reference Number: HIM.PA.SP46 Effective Date: 01.01.18 Last Review Date: Line of Business: Health Insurance Marketplace Revision Log See Important Reminder at the end of this
Clinical Policy: (Repatha) Reference Number: HIM.PA.SP46 Effective Date: 01.01.18 Last Review Date: Line of Business: Health Insurance Marketplace Revision Log See Important Reminder at the end of this
Acquisition of Novartis Influenza Vaccines Business. 27 th October 2014
 1 Acquisition of Novartis Influenza Vaccines Business 27 th October 2014 2 Legal Notice Forward looking statements The materials in this presentation speak only as of the date of these materials, and include
1 Acquisition of Novartis Influenza Vaccines Business 27 th October 2014 2 Legal Notice Forward looking statements The materials in this presentation speak only as of the date of these materials, and include
LIPITOR AND YOU HELPFUL INFORMATION FOR UNDERSTANDING CHOLESTEROL AND RISKS
 LIPITOR AND YOU HELPFUL INFORMATION FOR UNDERSTANDING CHOLESTEROL AND RISKS Learn what your cholesterol levels actually mean, and how you may save money on your prescription each month with the LIPITOR
LIPITOR AND YOU HELPFUL INFORMATION FOR UNDERSTANDING CHOLESTEROL AND RISKS Learn what your cholesterol levels actually mean, and how you may save money on your prescription each month with the LIPITOR
Intercept Pharmaceuticals Reports Second Quarter 2017 Financial Results and Provides Business Update
 July 31, 2017 Intercept Pharmaceuticals Reports Second Quarter 2017 Financial Results and Provides Business Update Worldwide net Ocaliva (obeticholic acid or OCA) 2Q 2017 sales of $30.4 million AESOP Phase
July 31, 2017 Intercept Pharmaceuticals Reports Second Quarter 2017 Financial Results and Provides Business Update Worldwide net Ocaliva (obeticholic acid or OCA) 2Q 2017 sales of $30.4 million AESOP Phase
Do not exceed 20 mg simvastatin daily For patients with HoFH, do not exceed 20 mg simvastatin daily* Grapefruit juice
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use simvastatin tablets safely and effectively. See full prescribing information for simvastatin tablets.
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use simvastatin tablets safely and effectively. See full prescribing information for simvastatin tablets.
Media Contacts: Amy Rose Investor Contact: Graeme Bell (908) (908)
 News Release FOR IMMEDIATE RELEASE Media Contacts: Amy Rose Investor Contact: Graeme Bell (908) 423-6537 (908) 423-5185 Tracy Ogden (267) 305-0960 FDA Approves Once-Daily JANUVIA, the First and Only DPP-4
News Release FOR IMMEDIATE RELEASE Media Contacts: Amy Rose Investor Contact: Graeme Bell (908) 423-6537 (908) 423-5185 Tracy Ogden (267) 305-0960 FDA Approves Once-Daily JANUVIA, the First and Only DPP-4
Readers Digest 11/18/04 10:01 AM Page 1 Lowering cholesterol THE STELLAR STUDY Bad cholesterol (LDL-C) lowering effect isn t a game. It s vital
 224174-Readers Digest 11/18/04 10:01 AM Page 1 Lowering cholesterol THE STELLAR STUDY Bad cholesterol (LDL-C) lowering effect isn t a game. It s vital to know not all drugs are the same. Your results may
224174-Readers Digest 11/18/04 10:01 AM Page 1 Lowering cholesterol THE STELLAR STUDY Bad cholesterol (LDL-C) lowering effect isn t a game. It s vital to know not all drugs are the same. Your results may
Study 2 ( ) Pivotal Phase 3 Study Top-Line Results. October 29, 2018
 Study 2 (1002-047) Pivotal Phase 3 Study Top-Line Results October 29, 2018 Safe Harbor Forward-Looking Statements These slides and the accompanying oral presentation contain forward-looking statements
Study 2 (1002-047) Pivotal Phase 3 Study Top-Line Results October 29, 2018 Safe Harbor Forward-Looking Statements These slides and the accompanying oral presentation contain forward-looking statements
How would you manage Ms. Gold
 How would you manage Ms. Gold 32 yo Asian woman with dyslipidemia Current medications: Simvastatin 20mg QD Most recent lipid profile: TC = 246, TG = 100, LDL = 176, HDL = 50 What about Mr. Williams? 56
How would you manage Ms. Gold 32 yo Asian woman with dyslipidemia Current medications: Simvastatin 20mg QD Most recent lipid profile: TC = 246, TG = 100, LDL = 176, HDL = 50 What about Mr. Williams? 56
Synergy Pharmaceuticals TRULANCE (Plecanatide) Receives U.S. FDA Approval for the Treatment of Adults with Chronic Idiopathic Constipation
 January 19, 2017 Synergy Pharmaceuticals TRULANCE (Plecanatide) Receives U.S. FDA Approval for the Treatment of Adults with Chronic Idiopathic Constipation NEW YORK--(BUSINESS WIRE)-- Synergy Pharmaceuticals
January 19, 2017 Synergy Pharmaceuticals TRULANCE (Plecanatide) Receives U.S. FDA Approval for the Treatment of Adults with Chronic Idiopathic Constipation NEW YORK--(BUSINESS WIRE)-- Synergy Pharmaceuticals
Quality ID #438: Statin Therapy for the Prevention and Treatment of Cardiovascular Disease National Quality Strategy Domain: Effective Clinical Care
 Quality ID #438: Statin Therapy for the Prevention and Treatment of Cardiovascular Disease National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS F INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE
Quality ID #438: Statin Therapy for the Prevention and Treatment of Cardiovascular Disease National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS F INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE
CRESTOR 10 mg, 20 mg, 40 mg Film Coated Tablets
 CRESTOR 10 mg, 20 mg, 40 mg Film Coated Tablets COMPOSITION Each film coated tablet contains 10 mg, 20 mg, or 40 mg of rosuvastatin as rosuvastatin calcium. PHARMACEUTICAL FORM Film-coated tablet Round,
CRESTOR 10 mg, 20 mg, 40 mg Film Coated Tablets COMPOSITION Each film coated tablet contains 10 mg, 20 mg, or 40 mg of rosuvastatin as rosuvastatin calcium. PHARMACEUTICAL FORM Film-coated tablet Round,
BioMarin Pharmaceutical Inc. Conference Call to Discuss Approval of
 BioMarin Pharmaceutical Inc. Conference Call to Discuss Approval of For adult patients with PKU who have uncontrolled blood phenylalanine (Phe) concentrations > 600 µmol/l on existing management May 24,
BioMarin Pharmaceutical Inc. Conference Call to Discuss Approval of For adult patients with PKU who have uncontrolled blood phenylalanine (Phe) concentrations > 600 µmol/l on existing management May 24,
HIGHLIGHTS OF PRESCRIBING
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use simvastatin safely and effectively. See full prescribing information for simvastatin. Simvastatin
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use simvastatin safely and effectively. See full prescribing information for simvastatin. Simvastatin
Original paper. Abstract. Abdullah S. Asia 1*, Al-Mahdi A. Modar 2, Hadi M. Ali 3
 Original paper Frequency Of Potential Adverse Effects Of A Semisynthetic Statin (Simvastatin) Compared To A Synthetic Statin (Atorvastatin) Used To Reduce Cardiovascular Risk For Patients In Basra 1*,
Original paper Frequency Of Potential Adverse Effects Of A Semisynthetic Statin (Simvastatin) Compared To A Synthetic Statin (Atorvastatin) Used To Reduce Cardiovascular Risk For Patients In Basra 1*,
Acasti Pharma Provides Business Update for the Third Quarter of Fiscal 2019
 Acasti Pharma Provides Business Update for the Third Quarter of Fiscal 2019 Planned Enrollment targets achieved in both TRILOGY studies with over 74% of patients randomized at more than 150 clinical sites
Acasti Pharma Provides Business Update for the Third Quarter of Fiscal 2019 Planned Enrollment targets achieved in both TRILOGY studies with over 74% of patients randomized at more than 150 clinical sites
4/24/15. AHA/ACC 2013 Guideline Key Points
 Review of the ACC/AHA 2013 Guidelines Anita Ralstin, MS, CNS, CNP Next Step Health Consultant, LLC 1! Discuss the rationale for the change in lipid guidelines and how that affects the decision to implement
Review of the ACC/AHA 2013 Guidelines Anita Ralstin, MS, CNS, CNP Next Step Health Consultant, LLC 1! Discuss the rationale for the change in lipid guidelines and how that affects the decision to implement
DARA Reports Year-End 2012 Financial Results
 April 1, 2013 DARA Reports Year-End 2012 Financial Results Company Provides a Commercial Portfolio and Development Pipeline Business Update RALEIGH, NC -- (MARKETWIRE) -- 04/01/13 -- DARA BioSciences,
April 1, 2013 DARA Reports Year-End 2012 Financial Results Company Provides a Commercial Portfolio and Development Pipeline Business Update RALEIGH, NC -- (MARKETWIRE) -- 04/01/13 -- DARA BioSciences,
Strengthening our global leadership in treatment of addiction. Morgan Stanley Global Healthcare Conference September 13 th and 14 th 2018
 Strengthening our global leadership in treatment of addiction Morgan Stanley Global Healthcare Conference September 13 th and 14 th 2018 Forward Looking Statements This presentation contains certain statements
Strengthening our global leadership in treatment of addiction Morgan Stanley Global Healthcare Conference September 13 th and 14 th 2018 Forward Looking Statements This presentation contains certain statements
Royal Wolverhampton Hospital Adult Lipid Lowering Therapy Guidelines Lipid Lowering Therapy for the Prevention of Cardiovascular Disease
 Royal Wolverhampton Hospital Adult Lipid Lowering Therapy Guidelines 1 This guideline is intended to assist rational and cost-effective prescribing of lipid regulating medications across both primary and
Royal Wolverhampton Hospital Adult Lipid Lowering Therapy Guidelines 1 This guideline is intended to assist rational and cost-effective prescribing of lipid regulating medications across both primary and
Issue date: January Review date: November Statins for the prevention of cardiovascular events. Technology Appraisal 94
 Issue date: January 2006 Review date: November 2008 Statins for the prevention of cardiovascular events Technology Appraisal 94 Technology Appraisal 94 Statins for the prevention of cardiovascular events
Issue date: January 2006 Review date: November 2008 Statins for the prevention of cardiovascular events Technology Appraisal 94 Technology Appraisal 94 Statins for the prevention of cardiovascular events
PART III: CONSUMER INFORMATION
 PART III: CONSUMER INFORMATION Pr SIMVASTATIN 5 Pr SIMVASTATIN 10 Pr SIMVASTATIN 20 Pr SIMVASTATIN 40 Pr SIMVASTATIN 80 Simvastatin Tablets, USP This leaflet is part III of a three-part Product Monograph
PART III: CONSUMER INFORMATION Pr SIMVASTATIN 5 Pr SIMVASTATIN 10 Pr SIMVASTATIN 20 Pr SIMVASTATIN 40 Pr SIMVASTATIN 80 Simvastatin Tablets, USP This leaflet is part III of a three-part Product Monograph
Dyslipidemia in the light of Current Guidelines - Do we change our Practice?
 Dyslipidemia in the light of Current Guidelines - Do we change our Practice? Dato Dr. David Chew Soon Ping Senior Consultant Cardiologist Institut Jantung Negara Atherosclerotic Cardiovascular Disease
Dyslipidemia in the light of Current Guidelines - Do we change our Practice? Dato Dr. David Chew Soon Ping Senior Consultant Cardiologist Institut Jantung Negara Atherosclerotic Cardiovascular Disease
Study 1 ( ) Pivotal Phase 3 Long-Term Safety Study Top-Line Results
 Study 1 (1002-040) Pivotal Phase 3 Long-Term Safety Study Top-Line Results May 2, 2018 1 COPYRIGHT 2018 ESPERION. ALL RIGHTS RESERVED DO NOT COPY OR DISTRIBUTE Safe Harbor Forward-Looking Statements These
Study 1 (1002-040) Pivotal Phase 3 Long-Term Safety Study Top-Line Results May 2, 2018 1 COPYRIGHT 2018 ESPERION. ALL RIGHTS RESERVED DO NOT COPY OR DISTRIBUTE Safe Harbor Forward-Looking Statements These
For personal use only
 HY18 Result Presentation 13 February 2018 Dig Howitt Brent Cubis CEO & President CFO HY18 Result highlights Strong momentum across developed markets continues Developed market unit growth up 12% Strengthening
HY18 Result Presentation 13 February 2018 Dig Howitt Brent Cubis CEO & President CFO HY18 Result highlights Strong momentum across developed markets continues Developed market unit growth up 12% Strengthening
Lipid Panel Management Refresher Course for the Family Physician
 Lipid Panel Management Refresher Course for the Family Physician Objectives Understand the evidence that was evaluated to develop the 2013 ACC/AHA guidelines Discuss the utility and accuracy of the new
Lipid Panel Management Refresher Course for the Family Physician Objectives Understand the evidence that was evaluated to develop the 2013 ACC/AHA guidelines Discuss the utility and accuracy of the new
Diabetes: Use of Adjunctive Therapy ACEs, ARBs, ASA & STATINs --Oh My! Veronica J. Brady, PhD, FNP-BC, BC-ADM, CDE Project ECHO April 19, 2018
 Diabetes: Use of Adjunctive Therapy ACEs, ARBs, ASA & STATINs --Oh My! Veronica J. Brady, PhD, FNP-BC, BC-ADM, CDE Project ECHO April 19, 2018 Points to Ponder ASCVD is the leading cause of morbidity
Diabetes: Use of Adjunctive Therapy ACEs, ARBs, ASA & STATINs --Oh My! Veronica J. Brady, PhD, FNP-BC, BC-ADM, CDE Project ECHO April 19, 2018 Points to Ponder ASCVD is the leading cause of morbidity
Forward-Looking Statements
 Investor Presentation May 2012 Forward-Looking Statements Statements contained herein that are not historical facts are forward-looking statements within the meaning of the Securities Act of 1933, as amended.
Investor Presentation May 2012 Forward-Looking Statements Statements contained herein that are not historical facts are forward-looking statements within the meaning of the Securities Act of 1933, as amended.
ATORVASTATIN CALCIUM tablets, for oral use Initial U.S. Approval: 1996
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ATORVASTATIN CALCIUM TABLETS safely and effectively. See full prescribing information for ATORVASTATIN
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ATORVASTATIN CALCIUM TABLETS safely and effectively. See full prescribing information for ATORVASTATIN
REATA PHARMACEUTICALS, INC. ANNOUNCES SECOND QUARTER 2018 FINANCIAL RESULTS AND AN UPDATE ON DEVELOPMENT PROGRAMS
 REATA PHARMACEUTICALS, INC. ANNOUNCES SECOND QUARTER 2018 FINANCIAL RESULTS AND AN UPDATE ON DEVELOPMENT PROGRAMS IRVING, Texas August 8, 2018 Reata Pharmaceuticals, Inc. (Nasdaq: RETA), a clinical-stage
REATA PHARMACEUTICALS, INC. ANNOUNCES SECOND QUARTER 2018 FINANCIAL RESULTS AND AN UPDATE ON DEVELOPMENT PROGRAMS IRVING, Texas August 8, 2018 Reata Pharmaceuticals, Inc. (Nasdaq: RETA), a clinical-stage
Clinical Policy: Mipomersen (Kynamro) Reference Number: ERX.SPMN.186 Effective Date: 01/2017
 Clinical Policy: (Kynamro) Reference Number: ERX.SPMN.186 Effective Date: 01/2017 Last Review Date: Revision Log See Important Reminder at the end of this policy for important regulatory and legal information.
Clinical Policy: (Kynamro) Reference Number: ERX.SPMN.186 Effective Date: 01/2017 Last Review Date: Revision Log See Important Reminder at the end of this policy for important regulatory and legal information.
