ORIGINAL INVESTIGATION. Effects of Weight Loss With Orlistat on Glucose Tolerance and Progression to Type 2 Diabetes in Obese Adults
|
|
|
- Martin O’Connor’
- 6 years ago
- Views:
Transcription
1 ORIGINAL INVESTIGATION Effects of Weight Loss With Orlistat on Glucose Tolerance and Progression to Type 2 Diabetes in Obese Adults Steven B. Heymsfield, MD; Karen R. Segal, PhD; Jonathan Hauptman, MD; Charles P. Lucas, MD; Mark N. Boldrin, MS; Aila Rissanen, MD; John P. H. Wilding, MD; Lars Sjöström, MD Background: Orlistat is a gastrointestinal lipase inhibitor that reduces dietary fat absorption by approximately 30%, promotes weight loss, and may reduce the risk of developing impaired glucose tolerance and type 2 diabetes in obese subjects. Objective: To test the hypothesis that orlistat combined with dietary intervention improves glucose tolerance status and prevents worsening of diabetes status more effectively than placebo. Methods: We pooled data from 675 obese (body mass index, kg/m 2 ) adults at 39 US and European research centers in 3 randomized, double-blind, placebocontrolled multicenter clinical trials. Subjects received placebo plus a low-energy diet during a 4-week lead-in period. On study day 1, the diet was continued, and subjects were randomized to receive placebo 3 times a day (n=316) or treatment with orlistat, 120 mg 3 times a day (n=359), for 104 weeks. A standard 3-hour oral glucose tolerance test was performed on day 1 and at the end of treatment. Main Outcome Measures: The categorical assessment of glucose tolerance status (normal, impaired, diabetic) and changes in status from randomization to end of treatment were the primary efficacy measures. The secondary measures were fasting and postchallenge glucose and insulin levels. Results: The mean length of follow-up was 582 days. Subjects who were treated with orlistat lost more weight (mean±sem, 6.72±0.41 kg from initial weight) than subjects who received placebo (3.79±0.38 kg; P.001). A smaller percentage of subjects with impaired glucose tolerance at baseline progressed to diabetic status in the orlistat (3.0%) vs placebo (7.6%) group. Conversely, among subjects with impaired glucose tolerance at baseline, glucose levels normalized in more subjects after orlistat treatment (71.6%) vs placebo (49.1%; P=.04). Conclusions: The addition of orlistat to a conventional weight loss regimen significantly improved oral glucose tolerance and diminished the rate of progression to the development of impaired glucose tolerance and type 2 diabetes. Arch Intern Med. 2000;160: From Columbia University College of Physicians and Surgeons, St Luke s-roosevelt Hospital Center, New York, NY (Dr Heymsfield); Hoffmann- La Roche Inc, Nutley, NJ (Drs Segal, Hauptman, and Lucas and Mr Boldrin); Helsinki University Central Hospital, Helsinki, Finland (Dr Rissanen); University Hospital Aintree, Liverpool, England (Dr Wilding); and Sahlgrenska University Hospital, Göteborg, Sweden (Dr Sjöström). MORE THAN 50% of Americans are overweight or obese, with 20% classified as obese (body mass index [BMI] [calculated as weight in kilograms divided by the square of height in meters] [BMI] 30), according to recently established National Institutes of Health body weight guidelines. 1 An important consequence of excessive adiposity is type 2 diabetes mellitus, 2,3 a condition that is associated with an increased risk of mortality and morbidity. 4 Furthermore, the incidence and prevalence of obesity and diabetes are increasing worldwide, especially in developing and newly industrialized nations. The estimated 80 million persons with diabetes in 1990 is expected to double by the year 2000, becoming a global epidemic, and the major part of the increase will occur in developing countries. 3 Over 12 million Americans are now overweight or obese and have type 2 diabetes. 3 The primary treatment for type 2 diabetes is weight loss. 1 Weight reduction of 5% to 10% improves serum glucose and insulin levels, 5,6 and intentional weight loss of a similar degree lowers diabetes-related mortality risk. 7 Although an untested hypothesis, a widely held view among diabetes and obesity experts is that a relatively small long-term loss of body weight might lower rates of deterioration in glucose tolerance and the development of new diabetes in high-risk obese subjects. While the evidence for the short-term beneficial effects of weight loss on carbohydrate metabolism is compelling, firm experimental 1321
2 SUBJECTS AND METHODS SUBJECTS The present study was a pooled analysis of three 2-year randomized placebo-controlled clinical trials 9,10 in which 675 obese individuals (BMI, 30-43) received either orlistat, 120 mg 3 times daily, or placebo 3 times daily with a mildly lowenergy diet for 1 year; subjects who were treated for 2 years had a weight maintenance diet in the second year. Data from these clinical trials were pooled to increase the sample size to attain greater statistical power: the individual studies were powered to test the statistical significance of differences in weight loss between treatments rather than secondary measures of efficacy, such as glucose tolerance. Subjects were recruited, evaluated, and monitored at 39 clinical research centers in the United States and Europe between 1992 and Entry criteria included age greater than 18 years, BMI of 30 to 43, adequate contraception in women of childbearing potential, and absence of weight loss ( 4 kg) in the previous 3 months. Subjects were excluded if they had stopped smoking within the past 6 months; had significant cardiac, renal, hepatic, gastrointestinal, psychiatric, or endocrine disorders; had drug-treated type 2 diabetes; had a history or presence of substance abuse; had excessive intake of alcohol; or concomitantly used medications that alter appetite or lipid levels. The initial demographic characteristics of the subjects were similar in all 3 studies. STUDY DESIGN The effects of weight loss and orlistat on measures of glucose metabolism were analyzed retrospectively from pooled data from three 2-year, double-blind, randomized, placebo-controlled clinical trials. The protocols from these investigations differed minimally and are described in greater detail elsewhere Only those subjects assigned to receive either orlistat, 120 mg 3 times daily, or placebo for 2 full years were included. Thus, subjects who were switched from one treatment to another at the end of year 1 were not included in the analysis. Subjects were placed on a low-energy diet that provided 30% of energy intake as fat during a 4-week, single-blind placebo lead-in period. Energy intake in year 1 was prescribed for each patient on the basis of his or her estimated daily maintenance energy requirement (1.3 calculated basal metabolic rate) minus 2083 to 3333 kj/d ( kcal/d). During year 2, a weightmaintaining diet was prescribed. Weight change during the 4-week lead-in period was used as a measure of the potential to lose weight, and subjects were stratified accordingly at randomization to ensure an even distribution between treatment groups of individuals who lost less than 2 kg vs 2 kg or more during the run-in period. After the 4-week placebo lead-in period, subjects were randomized on study day 1 to receive placebo or orlistat, 120-mg capsules 3 times per day with their 3 main meals for 52 or 104 weeks. Each subject provided written informed consent before entry into the trial. The study protocol was reviewed and approved by the institutional review boards or research ethics committees of each investigational site. ASSESSMENTS The initial screening visit (ie, week 4) included a medical history, physical examination, body weight evaluation, evidence in support of the hypothesis that effective medical treatment of obesity delays or prevents the onset of type 2 diabetes is lacking. Testing this hypothesis requires a large and thoroughly evaluated obese cohort combined with effective dietary and medical therapies that are applied over long periods. The recent US 8 and European 9 multicenter evaluations of the lipase inhibitor orlistat demonstrated that in obese subjects partial inhibition (approximately 30%) of dietary fat absorption combined with behavioral treatment promotes significant weight loss and weight maintenance over 2 years. The aim of the present study was to evaluate critically, in the pooled multicenter orlistat clinical trial population, the hypothesis that relatively small long-term body weight loss significantly improves glucose tolerance and reduces the rate of diabetes onset in obese subjects. RESULTS SUBJECTS The study design and disposition of the subjects over 2 years are shown in the Figure. A total of 675 subjects completed the 4-week placebo lead-in period and were randomized to receive double-blind treatment with placebo (n=316) or orlistat, 120 mg (n=359); each subject had at least 1 follow-up visit, including a repeated OGTT. Since the present study is a meta-analysis of subjects who had at least 1 follow-up assessment of glucose tolerance, consideration of the dropout rate from the initiation of doubleblind treatment is more relevant than that from enrollment into the study. Reasons for withdrawal from the study included entry violations (clinical or laboratory findings incompatible with study entry that were not available until after the patient had begun the study), lack of cooperation, administrative considerations (changes in subjects lives making continuation impractical or impossible), adverse events (clinical adverse events or adverse laboratory findings), protocol violations, loss to follow-up, and refusal of treatment. There were no significant differences in the withdrawal rates across the individual studies or across the possible treatment end points. The mean duration of treatment was 587 and 577 days for the orlistat and placebo groups, respectively. The end point of treatment was 2 years for 217 subjects (68.7%) and 246 subjects (68.5%) in the placebo and orlistat groups, respectively. The characteristics of the study population at randomization were similar in the 2 treatment groups (Table 1). The characteristics of the study populations for whom treatment end point occurred at 26, 52, or 104 weeks were also similar. 1322
3 electrocardiogram, and clinical chemistry, thyroid function, hematology, and urinalysis laboratory tests. A 3-hour oral glucose tolerance test (OGTT) with a 75-g oral glucose load was performed at the time of randomization (day 1), 26 weeks (one study only), 52 weeks, and/or 104 weeks. The OGTT that most closely coincided with the time at which the patient s participation in the study ended was used for the end-point analysis. Glucose tolerance was classified according to the 2-hour serum glucose values as described by the World Health Organization. 11 The criterion for normal glucose tolerance was a 2-hour glucose value of less than 7.8 mmol/l (140 mg/dl). Impaired glucose tolerance (IGT) was defined as a 2-hour glucose level of 7.8 to 11.0 mmol/l ( mg/dl), and diabetes was defined as a 2-hour glucose level of 11.1 mmol/l (200 mg/dl) or higher. For classification purposes, 2-hour post glucose-challenge serum glucose values are more robust than fasting glucose levels owing to variation among subjects in the number of hours the subjects fasted prior to the test. A recent study also reports greater sensitivity of the glucose tolerance test compared with fasting glucose levels in predicting progression to type 2 diabetes. 12 Body weight was evaluated every 2 weeks until week 16, every 4 weeks until the end of year 1, and every 8 weeks thereafter for the 2-year studies. Measurements taken after the subjects last OGTT measurements were excluded. STATISTICAL ANALYSIS An end-point analysis was applied in which each subject s last OGTT at 26, 52, or 104 weeks was taken to be the treatment end point. An analysis of the intent-totreat population was applied, as recommended in the Consolidated Standard of Reporting Trials guidelines. 13 Since the analysis of the impact of treatment depended on the results of the OGTT performed at the end of treatment, statistical analyses were performed on those subjects who had baseline and follow-up OGTT and body weight measurements. Prior to pooling the data from the individual studies and for the specific treatment end points, the initial characteristics as well as the outcomes were checked for similarity across cohorts. The characteristics of the subjects in the 3 individual studies as well as the characteristics of subjects whose treatment end points occurred at 26, 52, and 104 weeks were not significantly different. The hypothesis that changes from baseline in body weight and oral glucose tolerance were greater in the orlistat treatment group was tested using analysis of variance models with the study protocol (the 3 individual studies) and treatment as factors. 14 This analysis was applied to the fasting glucose and insulin values and the integrated areas under the curves (AUCs) for the 3-hour glucose and insulin responses to oral glucose, which were calculated according to the trapezoidal rule. Data are presented as mean ± SEM. Categorical analyses of the frequency distributions of weight loss and shift in OGTT status were performed with use of 2 analysis. 15 The glucose and insulin AUCs were compared across the treatment groups by 2-way analyses of covariance using treatment (placebo or orlistat) and diabetes status (normal, impaired, or diabetic) as grouping variables, with baseline values as covariates. Additional analyses are described in the Results section. For all statistical analyses, P.05 was considered statistically significant. No adjustments were made for multiple comparisons. WEIGHT LOSS Obese subjects in the orlistat group achieved a weight loss of 6.72±0.41 kg over the study period (ie, from week 4) compared with a weight loss of 3.79±0.38 kg for obese subjects in the placebo group (P.001). Expressed as percentage of weight change from initial body weight, the orlistat group lost significantly more weight than the placebo group (6.8%±0.4% vs 3.9%±0.4%; P.001). Analysis of the frequency distribution of weight loss indicated that 52.9% of subjects in the orlistat group lost 5% or more of initial body weight and 30.1% lost 10% or more of initial weight, while 37.7% of subjects in the placebo group lost 5% or more of initial body weight and 16.5% lost 10% or more of initial weight (P.001 for orlistat vs placebo for both 5% and 10% weight loss). GLUCOSE TOLERANCE On day 1 (randomization), 5.3% of the orlistat group and 4.4% of the placebo group met the diagnostic criteria for diabetes mellitus. Impaired glucose tolerance at baseline was present in 18.7% and 16.8% of subjects in the orlistat and placebo groups, respectively. Thus, the baseline distribution among the categories of glucose tolerance was similar in the 2 treatment groups. The baseline distributions were also similar across the 3 individual studies and among the cohorts for whom the treatment end point occurred at 26, 52, or 104 weeks. Changes in glucose tolerance status are shown in Table 2. The distribution of changes in glucose tolerance status was not significantly different in the cohorts for whom the treatment end point occurred at 26, 52, or 104 weeks. Changes in glucose tolerance status were significantly different between the subjects who were categorized as normal or impaired at baseline (P=.04). For subjects with abnormal glucose tolerance at baseline, a greater proportion who received orlistat vs placebo had improved glucose tolerance status after treatment, whereas deterioration in glucose tolerance (normal to IGT or diabetes, or IGT to diabetes) occurred in a greater proportion of subjects receiving placebo. For example, of the subjects with IGT at baseline, 71.6% who were treated with orlistat had normal glucose tolerance at the end of treatment compared with 49.1% in the placebo group (P=.04), whereas 3.0% who were treated with orlistat progressed to diabetic status vs 7.6% in the placebo group. SERUM GLUCOSE AND INSULIN LEVELS Fasting serum glucose levels and glucose AUCs at baseline were progressively greater in subjects with IGT vs 1323
4 35 Withdrawn After 6-Month Follow-up 64 Withdrawn Placebo 3 Times Daily (n = 316) Placebo Completed Year 2 (n = 217) Randomization to Double-blind Treatment Plus Low-Energy Diet (n = 675) Completed Year 1 (n = 281) Orlistat, 120 mg 3 Times Daily (n = 359) Completed Year 1 (n = 333) Weight Maintenance Diet Year 2 Study design and disposition of subjects. Orlistat, 120 mg Completed Year 2 (n = 246) 26 Withdrawn After 6-Month Follow-up 87 Withdrawn Table 1. Demographic Characteristics for the Intent-to-Treat Population From the Start of the Placebo Lead-in Period (Week 4) Characteristic Placebo (n = 316) Orlistat, 120 mg (n = 359) Male/female, No. 49/267 69/290 Race, No. White African American Hispanic 9 5 Asian 1 2 Age, mean ± SEM, y 44.3 ± ± 0.6 Weight, mean ± SEM, kg 99.8 ± ± 0.6 Body mass index, mean ± SEM, kg/m ± ± 0.1 Table 2. Change in Oral Glucose Tolerance Status From Baseline to End of Treatment Status at Baseline Status at End Point, No. of Subjects (%) Treatment Normal Impaired Diabetic Normal Placebo 219 (88.0) 27 (10.8) 3 (1.2) Orlistat 255 (93.4) 18 (6.6) 0 (0) Impaired Placebo 26 (49.1) 23 (43.4) 4 (7.6) Orlistat 48 (71.6) 17 (25.4) 2 (3.0) Diabetic Placebo 2 (14.3) 2 (14.3) 10 (71.4) Orlistat 3 (15.8) 8 (42.1) 8 (42.1) *Refers to significance of 2 for distribution of end-point status within each category of baseline status. P * normal subjects, and greater in subjects with diabetes vs subjects with IGT. Fasting glucose levels decreased more among those subjects receiving orlistat vs placebo, whether subjects were normal or had IGT at baseline (Table 3). Fasting serum insulin levels and the insulin AUCs at baseline were progressively greater in subjects with IGT vs normal subjects. Analysis of the insulin levels in the orlistat and placebo groups revealed significant interaction terms for the baseline-by-treatment interaction. Baseline insulin levels tended to be higher among nondiabetic subjects in the placebo vs orlistat group. However, within the group of subjects categorized as diabetic at baseline, the mean fasting insulin level was roughly 2-fold lower among subjects receiving orlistat vs placebo. This suggests more advanced beta-cell dysfunction and more severe diabetes at baseline in the orlistat group. 16,17 With this baseline difference taken into account, the magnitude of change in the insulin AUC was comparable among subjects with diabetes in the placebo and orlistat groups. The improvement in the insulin AUC was significantly greater in the orlistat vs placebo group in the subjects with normal glucose tolerance at baseline (P=.03; Table 3). Fasting glucose levels decreased significantly more in both the normal and impaired groups treated with orlistat compared with placebo (Table 3), while the glucose AUC decreased significantly more only in the normal group treated with orlistat vs placebo (P.001). Further analyses were applied to the changes in glucose tolerance in order to evaluate the extent to which observed treatment effects are dependent on the greater weight loss produced by orlistat vs placebo. Analysis of covariance was applied to the 2-hour serum glucose values (which are the basis for glucose tolerance classification) using weight change as the covariate. Within the impaired group, the treatment difference was not significant after adjustment for weight change, whereas within the normal group a significant treatment effect persisted (P=.003) after adjusting for differences in weight loss between the treatment groups. COMMENT The clinical and public health implications of the obesityrelated risk for diabetes are important: over 16.6 million Americans have type 2 diabetes, and twice as many have IGT, a condition that progresses to type 2 diabetes at a rate of 6% per year. 18 Measures that prevent diabetes or improve glucose homeostasis are therefore central to reducing morbidity and mortality as well as augmenting quality of life and reducing national health care costs. In the present analysis, we pooled data from 3 similar multicenter, randomized, double-blind orlistat trials to test the hypothesis that relatively small long-term body weight loss significantly improves glucose tolerance and reduces the rate of diabetes onset in obese subjects. Our observations strongly support this hypothesis. Compared with the placebo group, the orlistat group lost more weight (mean difference from placebo, approximately 3 kg), experienced improved categorical glucose tolerance status, and showed improved serum insulin and glucose levels. The lowering of serum insulin levels observed in the present investigation may be clinically important since earlier studies have linked fasting serum insulin levels to ischemic heart disease risk, insulin resistance, and obesity-related hypertension. 19 The trend for reduced fasting and postchallenge serum insulin levels with treatment thus suggests that weight loss produced by orlistat combined with dietary and lifestyle intervention may improve the insulin resistance syndrome
5 Table 3. Fasting Serum Insulin and Glucose Levels and Oral Glucose Response Areas Under the Curve (AUCs) at Randomization and After Treatment* Insulin, pmol/l Glucose, mmol/l (mg/dl) Insulin Glucose Categorical Status Study Period Fasting AUC Fasting AUC Fasting AUC Fasting AUC Normal Placebo Baseline (day 1) 85 ± ± ± 0.03 (94 ± 1) 37.1 ± 0.4 (668 ± 7) Follow-up 89 ± ± ± 0.04 (94 ± 1) 37.7 ± 0.5 (679 ± 9) Change +4 ± ± ± 0.04 ( 1 ± 1) +0.7 ± 0.4 (+13 ± 7) Orlistat Baseline (day 1) 81 ± ± ± 0.04 (95 ± 1) 37.9 ± 0.4 (683 ± 7) Follow-up 74 ± ± ± 0.04 (92 ± 1) 36.6 ± 0.5 (659 ± 9) Change 7 ± ± ± 0.04 ( 3 ± 1) 1.4 ± 0.4 ( 25 ± 7) Impaired Placebo Baseline (day 1) 110 ± ± ± 0.09 (101 ± 2) 51.0 ± 0.7 (919 ± 13) Follow-up 130 ± ± ± 0.13 (103 ± 2) 48.7 ± 1.6 (877 ± 29) Change +20 ± ± ± 0.04 (+2 ± 1) 2.3 ± 1.3 ( 414 ± 23) Orlistat Baseline (day 1) 102 ± ± ± 0.11 (104 ± 2) 50.3 ± 0.7 (906 ± 13) Follow-up 88 ± ± ± 0.07 (97 ± 1) 44.5 ± 1.0 (802 ± 18) Change 14 ± ± ± 0.05 ( 8 ± 1) 5.7 ± 1.0 ( 103 ± 18) Diabetic Placebo Baseline (day 1) 179 ± ± ± 0.30 (124 ± 5) 71.4 ± 2.5 (1286 ± 45) Follow-up 129 ± ± ± 0.32 (127 ± 6) 69.9 ± 3.5 (1259 ± 63) Change 50 ± ± ± 0.16 (+3 ± 2) 1.5 ± 2.1 ( 27 ± 38) Orlistat Baseline (day 1) 97 ± ± ± 0.27 (127 ± 5) 72.0 ± 2.3 (1297 ± 41) Follow-up 131 ± ± ± 0.58 (130 ± 10) 68.3 ± 4.8 (1230 ± 86) Change +34 ± ± ± 0.42 (+3 ± 8) 3.8 ± 3.6 ( 68 ± 64) *All values are expressed as mean ± SEM. The AUC units are picomoles per liter per 3 hours and millimoles per liter per 3 hours for insulin and glucose, respectively. The difference between placebo and orlistat in the change from baseline to follow-up (least square means). P The data from the present study suggest that improvements in glucose tolerance depend on relatively small changes in body weight. The improved glycemic control secondary to weight loss and the difficulty of maintaining weight loss are highlighted by the recent study of Wing et al. 21 Lifestyle interventions, including diet, exercise, and behavior modification, produced a mean 4.5-kg weight loss at 6 months of treatment and lowered the risk of developing type 2 diabetes in subjects who either were normal or had IGT. However, the weight loss achieved in the first 6 months of treatment was largely regained by the end of the second year, and along with this relapse all physiological measures of glucose metabolism had returned to or exceeded their baseline levels. In the present study, the orlistat group maintained weight loss that was 3 kg greater than that in the placebo group at the end of year 2, which was statistically significant. Although this difference in weight loss was modest, it was associated with significantly improved glycemic control. Moreover, within the group of subjects who had normal glucose tolerance, the effect of orlistat treatment was statistically significant after adjusting for the effect of weight change alone. Few earlier intervention studies carried out in a medical setting have directly tested the hypothesis that intervention fostering weight loss in obese individuals diminishes the progression from IGT to diabetes. Long et al 22 reported that morbidly obese persons with IGT who underwent gastric bypass surgery developed diabetes at a reduced rate of 0.15 cases per 100 person-years vs the expected rate of 5% to 6%. Preliminary analysis of data from another study of the surgical treatment of obesity, the Swedish Obesity Study, 23 indicated that in the surgically treated group, 69% of cases of diabetes diagnosed at baseline were reclassified as nondiabetic at 2-year follow-up compared with 16% of cases reclassified in the control group, and 0.5% of subjects in the treated group who did not have diabetes at baseline developed diabetes over 2 years compared with 7.8% in the control group. However, results of these obesity surgery interventions that produced large weight loss ( 20% of initial weight) in morbidly obese subjects may have limited practical application to the treatment of less severe obesity. The Diabetes Prevention Program is an ongoing prospective multicenter randomized clinical trial to assess the impact of several interventions on reducing the rate of onset of diabetes, 24 but the results of this trial are not yet available. A limitation of the present study is the fact that the baseline OGTT was performed on day 1 (randomization) after a 4-week lead-in period of placebo plus a lowenergy diet. It is very likely that changes in oral glucose tolerance, both categorical and quantitative, would have been even greater if the test had been performed at the initial visit. However, both treatment groups lost similar amounts of weight during this lead-in period, and there is no evidence that use of the baseline test from the point at which subjects were randomized is a source of bias, except to the extent that the number of subjects with IGT or diabetes prior to treatment was probably underestimated. Furthermore, subjects with significant existing heart disease and uncontrolled hypertension were excluded, which would lower the number of obese sub- 1325
6 jects at risk of developing IGT or type 2 diabetes. Nevertheless, significant improvements were seen in both quantitative and categorical measures of glucose tolerance, which supports the concept that modest weight loss may reduce the risk of developing diabetes in obese subjects. Finally, this report is a retrospective meta-analysis of glucose tolerance data, and none of the individual studies was powered to study shifts in glucose tolerance. Shifts in glucose tolerance status were significantly different between the orlistat and placebo groups for subjects who were normal (P=.04) and impaired (P=.04) at baseline, but the number of subjects who progressed to diabetic status was very small. Accordingly, confirmation of the results of this meta-analysis by prospective trials specifically designed to address this issue is warranted. CONCLUSIONS Our findings indicate that even modest pharmacologically facilitated weight loss produces important metabolic benefits. Antiobesity pharmacotherapy may therefore offer an additional therapeutic option for the prevention of diabetes, although further long-term studies are required. This study demonstrates that partial inhibition of fat absorption by orlistat in obese subjects produces sustained weight loss, significantly impacts glucose and insulin metabolism, and can significantly diminish the risk of deterioration of glucose tolerance. These observations collectively suggest that pharmacologic treatment with orlistat may be a useful adjunct to dietary and lifestyle interventions in preventing or delaying the onset of glucose intolerance or type 2 diabetes in obese subjects who are at risk. Accepted for publication September 23, This study was supported by a research grant from Hoffmann-La Roche Inc, Nutley, NJ. Presented in part at meetings of the Endocrine Society, Minneapolis, Minn, June 11, 1997; American Diabetes Association, Chicago, Ill, June 13, 1998; European Society of Cardiology, Stockholm, Sweden, August 24, 1997; American Heart Association, Orlando, Fla, November 9, 1997; North American Association for the Study of Obesity, Cancun, Mexico, November 1997; and European Association for the Study of Diabetes, Barcelona, Spain, September 8, Corresponding author: Steven B. Heymsfield, MD, Obesity Research Center, St Luke s-roosevelt Hospital, New York, NY ( SBH2@Columbia.edu). REFERENCES 1. Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Am J Clin Nutr. 1998;68: Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122: World Health Organization. The World Health Report, 1997: Conquering Suffering, Enriching Humanity. Geneva, Switzerland: World Health Organization; Fuller JH. Mortality trends and causes of death in diabetic patients. Diabetes Metab. 1993;19: Wing R, Blair E, Bononi P, Marcus M, Watanabe R, Bergman R. Caloric restriction per se is a significant factor in improvements in glycemic control and insulin sensitivity during weight loss in obese NIDDM patients. Diabetes Care. 1994; 17: Watts NB, Spanheimer RG, Digirolamo M, et al. Prediction of glucose response to weight loss in patients with non-insulin dependent diabetes mellitus. Arch Intern Med. 1990;150: Williamson D. Intentional weight loss: patterns in the general population and its association with morbidity and mortality. Int J Obes Relat Metab Disord. 1997; 21(suppl 1):S14-S Davidson MH, Hauptman J, DiGirolamo M, et al. Weight control and risk factor reduction in obese subjects treated for 2 years with orlistat: a randomized controlled trial. JAMA. 1999;281: Sjöström L, Rissanen A, Andersen T, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. Lancet. 1998;352: Hauptman J, Lucas C, Boldrin MN, Collins H, for the Orlistat Primary Care Study Group, Segal KR. Orlistat in the long-term treatment of obesity in primary care settings. Arch Fam Med. 2000;9: Diabetes mellitus: report of a WHO study group. World Health Organ Tech Rep Ser. 1985;727: Shaw JE, Zimmet PZ, de Courten M, et al. Impaired fasting glucose or impaired glucose tolerance: what best predicts future diabetes in Mauritius? Diabetes Care. 1999;22: Begg C, Cho M, Eastwood S, et al. Improving the quality of reporting of randomized controlled trials: the CONSORT statement. JAMA. 1996;276: Winer BJ. Statistical Principles in Experimental Design. 2nd ed. New York, NY: McGraw-Hill Co; Agresti A. Categorical Data Analysis. New York, NY: John Wiley & Sons Inc; Saad MF, Knowler WC, Pettitt DJ, Nelson RG, Charles MA, Bennett PH. A twostep model for development of non-insulin-dependent diabetes. Am J Med. 1991; 90: Saad MF, Knowler WC, Pettitt DJ, Nelson RG, Mott DM, Bennett PH. The natural history of impaired glucose tolerance in the Pima Indians. N Engl J Med. 1988; 319: Edelstein SL, Knowler WC, Bain RP, et al. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes. 1997;46: Despres J, Lamarche B, Mauriege P, et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med. 1996;334: Landsberg L. Hyperinsulinemia: possible role in obesity-induced hypertension. Hypertension. 1992;19(1 suppl):i61-i Wing R, Venditti E, Jakicic J, Polley B, Lang W. Lifestyle intervention in overweight individuals with a family history of diabetes. Diabetes Care. 1998;21: Long SD, O Brien K, MacDonald KG, et al. Weight loss in severely obese subjects prevents the progression of impaired glucose tolerance to type II diabetes. Diabetes Care. 1994;17: Bray GA. Coherent, preventive and management strategies for obesity. Ciba Found Symp. 1996;201: Diabetes Prevention Program Research Group. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care. 1999;22:
Energy Balance Equation
 Energy Balance Equation Intake Expenditure Hunger Satiety Nutrient Absorption Metabolic Rate Thermogenesis Activity Eat to Live! Live to Eat! EAT TO LIVE Intake = Expenditure Weight Stable LIVE TO EAT
Energy Balance Equation Intake Expenditure Hunger Satiety Nutrient Absorption Metabolic Rate Thermogenesis Activity Eat to Live! Live to Eat! EAT TO LIVE Intake = Expenditure Weight Stable LIVE TO EAT
Obesity Management in Patients with Diabetes Jamy D. Ard, MD Sunday, February 11, :15 a.m. 11:00 a.m.
 Obesity Management in Patients with Diabetes Jamy D. Ard, MD Sunday, February 11, 2018 10:15 a.m. 11:00 a.m. Type 2 diabetes mellitus (T2DM) is closely associated with obesity, primarily through the link
Obesity Management in Patients with Diabetes Jamy D. Ard, MD Sunday, February 11, 2018 10:15 a.m. 11:00 a.m. Type 2 diabetes mellitus (T2DM) is closely associated with obesity, primarily through the link
Prevalence of Diabetes Mellitus among Non-Bahraini Workers Registered in Primary Health Care in Bahrain
 Prevalence of Diabetes Mellitus among Non-Bahraini Workers Page 1 of 10 Bahrain Medical Bulletin, Vol.25, No.1, March 2003 Prevalence of Diabetes Mellitus among Non-Bahraini Workers Registered in Primary
Prevalence of Diabetes Mellitus among Non-Bahraini Workers Page 1 of 10 Bahrain Medical Bulletin, Vol.25, No.1, March 2003 Prevalence of Diabetes Mellitus among Non-Bahraini Workers Registered in Primary
International Journal of Pharma and Bio Sciences COMPARISON OF EFFICACY AND SAFETY OF RIMONABANT WITH ORLISTAT IN OBESE AND OVERWEIGHT PATIENTS
 International Journal of Pharma and Bio Sciences RESEARCH ARTICLE PHARMACOLOGY COMPARISON OF EFFICACY AND SAFETY OF RIMONABANT WITH ORLISTAT IN OBESE AND OVERWEIGHT PATIENTS Corresponding Author DR.JAIN
International Journal of Pharma and Bio Sciences RESEARCH ARTICLE PHARMACOLOGY COMPARISON OF EFFICACY AND SAFETY OF RIMONABANT WITH ORLISTAT IN OBESE AND OVERWEIGHT PATIENTS Corresponding Author DR.JAIN
The Effects of Orlistat Treatment Interruption on Weight and Associated Metabolic Parameters
 394) Prague Medical Report / Vol. 107 (2006) No. 4, p. 394 400 The Effects of Orlistat Treatment Interruption on Weight and Associated Metabolic Parameters Owen K., Svačina S. Third Medical Department
394) Prague Medical Report / Vol. 107 (2006) No. 4, p. 394 400 The Effects of Orlistat Treatment Interruption on Weight and Associated Metabolic Parameters Owen K., Svačina S. Third Medical Department
Supplementary Online Content
 Supplementary Online Content Larsen JR, Vedtofte L, Jakobsen MSL, et al. Effect of liraglutide treatment on prediabetes and overweight or obesity in clozapine- or olanzapine-treated patients with schizophrenia
Supplementary Online Content Larsen JR, Vedtofte L, Jakobsen MSL, et al. Effect of liraglutide treatment on prediabetes and overweight or obesity in clozapine- or olanzapine-treated patients with schizophrenia
Treating Type 2 Diabetes by Treating Obesity. Vijaya Surampudi, MD, MS Assistant Professor of Medicine Center for Human Nutrition
 Treating Type 2 Diabetes by Treating Obesity Vijaya Surampudi, MD, MS Assistant Professor of Medicine Center for Human Nutrition 2 Center Stage Obesity is currently an epidemic in the United States, with
Treating Type 2 Diabetes by Treating Obesity Vijaya Surampudi, MD, MS Assistant Professor of Medicine Center for Human Nutrition 2 Center Stage Obesity is currently an epidemic in the United States, with
Risks and benefits of weight loss: challenges to obesity research
 European Heart Journal Supplements (2005) 7 (Supplement L), L27 L31 doi:10.1093/eurheartj/sui083 Risks and benefits of weight loss: challenges to obesity research Donna Ryan* Pennington Biomedical Research
European Heart Journal Supplements (2005) 7 (Supplement L), L27 L31 doi:10.1093/eurheartj/sui083 Risks and benefits of weight loss: challenges to obesity research Donna Ryan* Pennington Biomedical Research
Other Ways to Achieve Metabolic Control
 Other Ways to Achieve Metabolic Control Nestor de la Cruz- Muñoz, MD, FACS Associate Professor of Clinical Surgery Chief, Division of Laparoendoscopic and Bariatric Surgery DeWitt Daughtry Family Department
Other Ways to Achieve Metabolic Control Nestor de la Cruz- Muñoz, MD, FACS Associate Professor of Clinical Surgery Chief, Division of Laparoendoscopic and Bariatric Surgery DeWitt Daughtry Family Department
Optimizing Postpartum Maternal Health to Prevent Chronic Diseases
 Optimizing Postpartum Maternal Health to Prevent Chronic Diseases Amy Loden, MD, FACP, NCMP Disclosures Research: None Financial: none applicable to this presentation PRIUM QEssentials Market Research
Optimizing Postpartum Maternal Health to Prevent Chronic Diseases Amy Loden, MD, FACP, NCMP Disclosures Research: None Financial: none applicable to this presentation PRIUM QEssentials Market Research
Obesity the global epidemic
 Obesity the global epidemic Obesity the global epidemic 35% 35% 35% 34% 34% 33% 33% 33% 32% 43% Top 10 obese countries Smoking Obesity Alcohol Inf. Diseases Toxins Vehicle Collisions Firearms Death Sexual
Obesity the global epidemic Obesity the global epidemic 35% 35% 35% 34% 34% 33% 33% 33% 32% 43% Top 10 obese countries Smoking Obesity Alcohol Inf. Diseases Toxins Vehicle Collisions Firearms Death Sexual
Weight Management: Obesity to Diabetes
 Weight Management: Obesity to Diabetes Marion J. Franz Nutrition Concepts by Franz, Minneapolis, MN Corresponding author: Marion J. Franz, MarionFranz@aol.com https://doi.org/10.2337/ds17-0011 2017 by
Weight Management: Obesity to Diabetes Marion J. Franz Nutrition Concepts by Franz, Minneapolis, MN Corresponding author: Marion J. Franz, MarionFranz@aol.com https://doi.org/10.2337/ds17-0011 2017 by
Gestational Diabetes. Gestational Diabetes:
 Gestational Diabetes Detection and Management Steven Gabbe, MD The Ohio State University Medical Center Gestational Diabetes: Detection and Management Learning Objectives: At the conclusion of this presentation,
Gestational Diabetes Detection and Management Steven Gabbe, MD The Ohio State University Medical Center Gestational Diabetes: Detection and Management Learning Objectives: At the conclusion of this presentation,
Overweight is defined as a body mass
 THE DANGEROUS LIAISON: WEIGHT GAIN AND ITS ASSOCIATED COMORBIDITIES * Zachary T. Bloomgarden, MD ABSTRACT Overweight and obesity have tangible physical consequences that affect mortality and economics,
THE DANGEROUS LIAISON: WEIGHT GAIN AND ITS ASSOCIATED COMORBIDITIES * Zachary T. Bloomgarden, MD ABSTRACT Overweight and obesity have tangible physical consequences that affect mortality and economics,
WEIGHT LOSS/MANAGEMENT IS IT JUST ANOTHER PIPE DREAM?
 WEIGHT LOSS/MANAGEMENT IS IT JUST ANOTHER PIPE DREAM? THE OBESITY MEDICINE ASSOCIATION S DEFINITION OF OBESITY Obesity is defined as a chronic, relapsing, multi-factorial, neurobehavioral disease, wherein
WEIGHT LOSS/MANAGEMENT IS IT JUST ANOTHER PIPE DREAM? THE OBESITY MEDICINE ASSOCIATION S DEFINITION OF OBESITY Obesity is defined as a chronic, relapsing, multi-factorial, neurobehavioral disease, wherein
Normal Fasting Plasma Glucose and Risk of Type 2 Diabetes Diagnosis
 CLINICAL RESEARCH STUDY Normal Fasting Plasma Glucose and Risk of Type 2 Diabetes Diagnosis Gregory A. Nichols, PhD, Teresa A. Hillier, MD, MS, Jonathan B. Brown, PhD, MPP Center for Health Research, Kaiser
CLINICAL RESEARCH STUDY Normal Fasting Plasma Glucose and Risk of Type 2 Diabetes Diagnosis Gregory A. Nichols, PhD, Teresa A. Hillier, MD, MS, Jonathan B. Brown, PhD, MPP Center for Health Research, Kaiser
Donna Tomky, MSN, C-ANP, CDE, FAADE Albuquerque, New Mexico
 Donna Tomky, MSN, C-ANP, CDE, FAADE Albuquerque, New Mexico Presented in Collaboration with New Mexico Health Care Takes On Diabetes Discuss the burden and challenges prediabetes presents in New Mexico.
Donna Tomky, MSN, C-ANP, CDE, FAADE Albuquerque, New Mexico Presented in Collaboration with New Mexico Health Care Takes On Diabetes Discuss the burden and challenges prediabetes presents in New Mexico.
Discussion points. The cardiometabolic connection. Cardiometabolic Risk Management in the Primary Care Setting
 Session #5 Cardiometabolic Risk Management in the Primary Care Setting Sonja Reichert, MD MSc FCFP FACPM Betty Harvey, RNEC BScN MScN Amanda Mikalachki, RN BScN CDE S Discussion points Whom should we be
Session #5 Cardiometabolic Risk Management in the Primary Care Setting Sonja Reichert, MD MSc FCFP FACPM Betty Harvey, RNEC BScN MScN Amanda Mikalachki, RN BScN CDE S Discussion points Whom should we be
This study may not be disseminated, reproduced in whole or in part without the written permission of Roduve Healthcare Solutions.
 This study may not be disseminated, reproduced in whole or in part without the written permission of Roduve Healthcare Solutions. A Randomized Controlled Trial for Roduve Healthcare Solutions on the Efficacy
This study may not be disseminated, reproduced in whole or in part without the written permission of Roduve Healthcare Solutions. A Randomized Controlled Trial for Roduve Healthcare Solutions on the Efficacy
Secular Trends in Birth Weight, BMI, and Diabetes in the Offspring of Diabetic Mothers
 Epidemiology/Health Services/Psychosocial Research O R I G I N A L A R T I C L E Secular Trends in Birth Weight, BMI, and Diabetes in the Offspring of Diabetic Mothers ROBERT S. LINDSAY, MB, PHD ROBERT
Epidemiology/Health Services/Psychosocial Research O R I G I N A L A R T I C L E Secular Trends in Birth Weight, BMI, and Diabetes in the Offspring of Diabetic Mothers ROBERT S. LINDSAY, MB, PHD ROBERT
Study Code: Date: 27 July 2007
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Generic drug name:
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Generic drug name:
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP)
 European Medicines Agency London, 15 November 2007 Doc. Ref. EMEA/CHMP/EWP/517497/2007 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON CLINICAL EVALUATION OF MEDICINAL PRODUCTS USED
European Medicines Agency London, 15 November 2007 Doc. Ref. EMEA/CHMP/EWP/517497/2007 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON CLINICAL EVALUATION OF MEDICINAL PRODUCTS USED
BARIATRIC SURGERY AND TYPE 2 DIABETES MELLITUS
 BARIATRIC SURGERY AND TYPE 2 DIABETES MELLITUS George Vl Valsamakis European Scope Fellow Obesity Visiting iti Associate Prof Warwick Medical School Diabetes is an increasing healthcare epidemic throughout
BARIATRIC SURGERY AND TYPE 2 DIABETES MELLITUS George Vl Valsamakis European Scope Fellow Obesity Visiting iti Associate Prof Warwick Medical School Diabetes is an increasing healthcare epidemic throughout
O besity is associated with increased risk of coronary
 134 RESEARCH REPORT Overweight and obesity and weight change in middle aged men: impact on cardiovascular disease and diabetes S Goya Wannamethee, A Gerald Shaper, Mary Walker... See end of article for
134 RESEARCH REPORT Overweight and obesity and weight change in middle aged men: impact on cardiovascular disease and diabetes S Goya Wannamethee, A Gerald Shaper, Mary Walker... See end of article for
Bariatric Surgery versus Intensive Medical Therapy for Diabetes 3-Year Outcomes
 The new england journal of medicine original article Bariatric Surgery versus Intensive Medical for Diabetes 3-Year Outcomes Philip R. Schauer, M.D., Deepak L. Bhatt, M.D., M.P.H., John P. Kirwan, Ph.D.,
The new england journal of medicine original article Bariatric Surgery versus Intensive Medical for Diabetes 3-Year Outcomes Philip R. Schauer, M.D., Deepak L. Bhatt, M.D., M.P.H., John P. Kirwan, Ph.D.,
FAMILY SUPPORT IS ASSOCIATED WITH SUCCESS IN ACHIEVING WEIGHT LOSS IN A GROUP LIFESTYLE INTERVENTION FOR DIABETES PREVENTION IN ARAB AMERICANS
 FAMILY SUPPORT IS ASSOCIATED WITH SUCCESS IN ACHIEVING WEIGHT LOSS IN A GROUP LIFESTYLE INTERVENTION FOR DIABETES PREVENTION IN ARAB AMERICANS Objective: We have recently shown the feasibility of a community-based,
FAMILY SUPPORT IS ASSOCIATED WITH SUCCESS IN ACHIEVING WEIGHT LOSS IN A GROUP LIFESTYLE INTERVENTION FOR DIABETES PREVENTION IN ARAB AMERICANS Objective: We have recently shown the feasibility of a community-based,
Review of Pharmacologic Weight Loss Medications in a Patient-Centered Medical Home
 604858PMTXXX10.1177/8755122515604858Journal of Pharmacy TechnologyCostello et al research-article2015 Case report Review of Pharmacologic Weight Loss Medications in a Patient-Centered Medical Home Journal
604858PMTXXX10.1177/8755122515604858Journal of Pharmacy TechnologyCostello et al research-article2015 Case report Review of Pharmacologic Weight Loss Medications in a Patient-Centered Medical Home Journal
Disclosures OBESITY. Overview. Obesity: Definition. Prevalence of Obesity is Rising. Obesity as a Risk Factor. None
 Disclosures None OBESITY Florencia Halperin, M.D. Medical Director, Program for Management Brigham and Women s Hospital Instructor in Medicine, Harvard Medical School Overview Obesity: Definition Definition
Disclosures None OBESITY Florencia Halperin, M.D. Medical Director, Program for Management Brigham and Women s Hospital Instructor in Medicine, Harvard Medical School Overview Obesity: Definition Definition
Low glycaemic index diet is effective in managing weight among obese postpartum women
 548 RESEARCH ARTICLE Low glycaemic index diet is effective in managing weight among obese postpartum women Shahnai Basharat, 1 Syed Amir Gilani, 2 Amjad Iqbal Burq, 3 Shahid Bashir 4 Abstract Objective:
548 RESEARCH ARTICLE Low glycaemic index diet is effective in managing weight among obese postpartum women Shahnai Basharat, 1 Syed Amir Gilani, 2 Amjad Iqbal Burq, 3 Shahid Bashir 4 Abstract Objective:
OBESITY IN PRIMARY CARE
 OBESITY IN PRIMARY CARE Obesity- definition Is a chronic disease In ICD 10 E66 Overweight and obesity are defined as abnormal or excessive fat accumulation that may impair health. Obesity is a leading
OBESITY IN PRIMARY CARE Obesity- definition Is a chronic disease In ICD 10 E66 Overweight and obesity are defined as abnormal or excessive fat accumulation that may impair health. Obesity is a leading
How do we adapt diet approaches for patients with obesity with or without diabetes? Therese Coleman Dietitian
 How do we adapt diet approaches for patients with obesity with or without diabetes? Therese Coleman Dietitian Developing a specialist weight management programme How did we adapt dietary approaches for
How do we adapt diet approaches for patients with obesity with or without diabetes? Therese Coleman Dietitian Developing a specialist weight management programme How did we adapt dietary approaches for
Gestational Diabetes: Long Term Metabolic Consequences. Outline 5/27/2014
 Gestational Diabetes: Long Term Metabolic Consequences Gladys (Sandy) Ramos, MD Associate Clinical Professor Maternal Fetal Medicine Outline Population rates of obesity and T2DM Obesity and metabolic syndrome
Gestational Diabetes: Long Term Metabolic Consequences Gladys (Sandy) Ramos, MD Associate Clinical Professor Maternal Fetal Medicine Outline Population rates of obesity and T2DM Obesity and metabolic syndrome
Individual Study Table Referring to Item of the Submission: Volume: Page:
 2.0 Synopsis Name of Company: Abbott Laboratories Name of Study Drug: Meridia Name of Active Ingredient: Sibutramine hydrochloride monohydrate Individual Study Table Referring to Item of the Submission:
2.0 Synopsis Name of Company: Abbott Laboratories Name of Study Drug: Meridia Name of Active Ingredient: Sibutramine hydrochloride monohydrate Individual Study Table Referring to Item of the Submission:
PATIENT GROUP DIRECTION FOR THE SUPPLY OF ORLISTAT BY COMMUNITY PHARMACISTS
 PATIENT GROUP DIRECTION FOR THE SUPPLY OF ORLISTAT BY COMMUNITY PHARMACISTS November 2009 Orlistat PGD Page 1 of 7 Rationale Patient Group Direction For Supply Of Orlistat By Community Pharmacists To enable
PATIENT GROUP DIRECTION FOR THE SUPPLY OF ORLISTAT BY COMMUNITY PHARMACISTS November 2009 Orlistat PGD Page 1 of 7 Rationale Patient Group Direction For Supply Of Orlistat By Community Pharmacists To enable
R. Leibel Naomi Berrie Diabetes Center 19 March 2010
 Pathophysiology of type 2 diabetes mellitus R. Leibel Naomi Berrie Diabetes Center 19 March 2010 Body Mass Index Chart 25-29.9 29.9 = overweight; 30-39.9= 39.9 obese; >40= extreme obesity 5'0" 5'2" 52
Pathophysiology of type 2 diabetes mellitus R. Leibel Naomi Berrie Diabetes Center 19 March 2010 Body Mass Index Chart 25-29.9 29.9 = overweight; 30-39.9= 39.9 obese; >40= extreme obesity 5'0" 5'2" 52
Objectives. Objectives. Alejandro J. de la Torre, MD Cook Children s Hospital May 30, 2015
 Alejandro J. de la Torre, MD Cook Children s Hospital May 30, 2015 Presentation downloaded from http://ce.unthsc.edu Objectives Understand that the obesity epidemic is also affecting children and adolescents
Alejandro J. de la Torre, MD Cook Children s Hospital May 30, 2015 Presentation downloaded from http://ce.unthsc.edu Objectives Understand that the obesity epidemic is also affecting children and adolescents
The prevalence of obesity in adults has doubled over the past 30 years
 Obesity in America: Facts and Fiction MICHAEL G. PERRI, PhD Professor, Clinical and Health Psychology Interim Dean, College of Public Health and Health Professions University of Florida Overview: Key Questions
Obesity in America: Facts and Fiction MICHAEL G. PERRI, PhD Professor, Clinical and Health Psychology Interim Dean, College of Public Health and Health Professions University of Florida Overview: Key Questions
Diabetes: Staying Two Steps Ahead. The prevalence of diabetes is increasing. What causes Type 2 diabetes?
 Focus on CME at the University of University Manitoba of Manitoba : Staying Two Steps Ahead By Shagufta Khan, MD; and Liam J. Murphy, MD The prevalence of diabetes is increasing worldwide and will double
Focus on CME at the University of University Manitoba of Manitoba : Staying Two Steps Ahead By Shagufta Khan, MD; and Liam J. Murphy, MD The prevalence of diabetes is increasing worldwide and will double
UnitedHealthcare Pharmacy Clinical Pharmacy Programs
 UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2018 P 1172-3 Program Prior Authorization - California and New York Regulatory Program - Weight Loss Medication Includes both brand and
UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2018 P 1172-3 Program Prior Authorization - California and New York Regulatory Program - Weight Loss Medication Includes both brand and
13/09/2012. Therapeutic Options in Obesity. Clinical scenario (1) Clinical Symposium. Clinical scenario (2) Clinical scenario (3)
 2 nd AME Italian Meeting Associazione Medici Endocrinologi Joint Meeting with AACE American Association of Clinical Endocrinologists Reggio Emilia, Italy - November 8-10, 2002 Clinical Symposium Therapeutic
2 nd AME Italian Meeting Associazione Medici Endocrinologi Joint Meeting with AACE American Association of Clinical Endocrinologists Reggio Emilia, Italy - November 8-10, 2002 Clinical Symposium Therapeutic
Pre-diabetes. Pharmacological Approaches to Delay Progression to Diabetes
 Pre-diabetes Pharmacological Approaches to Delay Progression to Diabetes Overview Definition of Pre-diabetes Risk Factors for Pre-diabetes Clinical practice guidelines for diabetes Management, including
Pre-diabetes Pharmacological Approaches to Delay Progression to Diabetes Overview Definition of Pre-diabetes Risk Factors for Pre-diabetes Clinical practice guidelines for diabetes Management, including
Obesity D R. A I S H A H A L I E K H Z A I M Y
 Obesity D R. A I S H A H A L I E K H Z A I M Y Objectives Definition Pathogenesis of obesity Factors predisposing to obesity Complications of obesity Assessment and screening of obesity Management of obesity
Obesity D R. A I S H A H A L I E K H Z A I M Y Objectives Definition Pathogenesis of obesity Factors predisposing to obesity Complications of obesity Assessment and screening of obesity Management of obesity
Fasting Glucose, Obesity, and Metabolic Syndrome as Predictors of Type 2 Diabetes: The Cooper Center Longitudinal Study
 Fasting Glucose, Obesity, and Metabolic Syndrome as Predictors of Type 2 Diabetes: The Cooper Center Longitudinal Study Laura F. DeFina, MD,* Gloria Lena Vega, PhD,Þ David Leonard, PhD,Þ and Scott M. Grundy,
Fasting Glucose, Obesity, and Metabolic Syndrome as Predictors of Type 2 Diabetes: The Cooper Center Longitudinal Study Laura F. DeFina, MD,* Gloria Lena Vega, PhD,Þ David Leonard, PhD,Þ and Scott M. Grundy,
Diabesity. Metabolic dysfunction that ranges from mild blood glucose imbalance to full fledged Type 2 DM Signs
 Diabesity Metabolic dysfunction that ranges from mild blood glucose imbalance to full fledged Type 2 DM Signs Abdominal obesity Low HDL, high LDL, and high triglycerides HTN High blood glucose (F>100l,
Diabesity Metabolic dysfunction that ranges from mild blood glucose imbalance to full fledged Type 2 DM Signs Abdominal obesity Low HDL, high LDL, and high triglycerides HTN High blood glucose (F>100l,
Diabetes mellitus is diagnosed and characterized by chronic hyperglycemia. The effects of
 Focused Issue of This Month Early Diagnosis of Diabetes Mellitus Hyun Shik Son, MD Department of Internal Medicine, The Catholic University of Korea College of Medicine E - mail : sonhys@gmail.com J Korean
Focused Issue of This Month Early Diagnosis of Diabetes Mellitus Hyun Shik Son, MD Department of Internal Medicine, The Catholic University of Korea College of Medicine E - mail : sonhys@gmail.com J Korean
Sponsor Novartis. Generic Drug Name Vildagliptin/Metformin. Therapeutic Area of Trial Type 2 diabetes. Approved Indication Type 2 diabetes
 Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Vildagliptin/Metformin Therapeutic Area of Trial Type 2 diabetes Approved Indication Type 2 diabetes Study Number CLMF237A2309
Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Vildagliptin/Metformin Therapeutic Area of Trial Type 2 diabetes Approved Indication Type 2 diabetes Study Number CLMF237A2309
Diabetes Mellitus Type 2 Evidence-Based Drivers
 This module is supported by an unrestricted educational grant by Aventis Pharmaceuticals Education Center. Copyright 2003 1 Diabetes Mellitus Type 2 Evidence-Based Drivers Driver One: Reducing blood glucose
This module is supported by an unrestricted educational grant by Aventis Pharmaceuticals Education Center. Copyright 2003 1 Diabetes Mellitus Type 2 Evidence-Based Drivers Driver One: Reducing blood glucose
Dietary Treatment and Long-Term Weight Loss and Maintenance in Type 2 Diabetes
 Dietary Treatment and Long-Term Weight Loss and Maintenance in Type 2 Diabetes Donald D. Hensrud Abstract HENSRUD, DONALD D. Dietary treatment and long-term weight loss and maintenance in type 2 diabetes.
Dietary Treatment and Long-Term Weight Loss and Maintenance in Type 2 Diabetes Donald D. Hensrud Abstract HENSRUD, DONALD D. Dietary treatment and long-term weight loss and maintenance in type 2 diabetes.
SCIENTIFIC STUDY REPORT
 PAGE 1 18-NOV-2016 SCIENTIFIC STUDY REPORT Study Title: Real-Life Effectiveness and Care Patterns of Diabetes Management The RECAP-DM Study 1 EXECUTIVE SUMMARY Introduction: Despite the well-established
PAGE 1 18-NOV-2016 SCIENTIFIC STUDY REPORT Study Title: Real-Life Effectiveness and Care Patterns of Diabetes Management The RECAP-DM Study 1 EXECUTIVE SUMMARY Introduction: Despite the well-established
Innovate. Discover. Cure. Type 2 Diabetes what you and your family need to know
 1 Innovate. Discover. Cure. Type 2 Diabetes what you and your family need to know Opening comments Steven R. Smith, MD Founding Scientific Director - TRI Professor, metabolic diseases program, Sanford-Burnham
1 Innovate. Discover. Cure. Type 2 Diabetes what you and your family need to know Opening comments Steven R. Smith, MD Founding Scientific Director - TRI Professor, metabolic diseases program, Sanford-Burnham
Orlistat. How Alli really affects you during weight loss. JD Welch
 Orlistat How Alli really affects you during weight loss JD Welch HE 426 Tom Kelly November 24, 2008 Classification and Usage Orlistat is the first drug approved by the Food and Drug Administration (FDA)
Orlistat How Alli really affects you during weight loss JD Welch HE 426 Tom Kelly November 24, 2008 Classification and Usage Orlistat is the first drug approved by the Food and Drug Administration (FDA)
ACTIVO-I Study Report A Retrospective Prescription Event Monitoring for Safety and Efficacy of Bioactives in the Management of Overweight and Obesity
 96 Clinical Study Indian Medical Gazette MARCH 2014 ACTIVO-I Study Report A Retrospective Prescription Event Monitoring for Safety and Efficacy of Bioactives in the Management of Overweight and Obesity
96 Clinical Study Indian Medical Gazette MARCH 2014 ACTIVO-I Study Report A Retrospective Prescription Event Monitoring for Safety and Efficacy of Bioactives in the Management of Overweight and Obesity
23-Aug-2011 Lixisenatide (AVE0010) - EFC6014 Version number: 1 (electronic 1.0)
 SYNOPSIS Title of the study: A randomized, double-blind, placebo-controlled, parallel-group, multicenter 24-week study followed by an extension assessing the efficacy and safety of AVE0010 on top of metformin
SYNOPSIS Title of the study: A randomized, double-blind, placebo-controlled, parallel-group, multicenter 24-week study followed by an extension assessing the efficacy and safety of AVE0010 on top of metformin
8/27/2012. Mississippi s Big Problem. An Epidemic Now Reaching Our Children. What Can We Do?
 Mississippi s Big Problem. An Epidemic Now Reaching Our Children What Can We Do? Richard D. deshazo, MD Billy S. Guyton Distinguished Professor Professor of Medicine & Pediatrics University of Mississippi
Mississippi s Big Problem. An Epidemic Now Reaching Our Children What Can We Do? Richard D. deshazo, MD Billy S. Guyton Distinguished Professor Professor of Medicine & Pediatrics University of Mississippi
It s Never Too Early To Prevent Diabetes: The Lasting Impact of Gestational Diabetes on Mothers and Children
 It s Never Too Early To Prevent Diabetes: The Lasting Impact of Gestational Diabetes on Mothers and Children Robert Ratner, M.D., F.A.C.P. Vice President for Scientific Affairs, Medstar Research Institute
It s Never Too Early To Prevent Diabetes: The Lasting Impact of Gestational Diabetes on Mothers and Children Robert Ratner, M.D., F.A.C.P. Vice President for Scientific Affairs, Medstar Research Institute
Clinical Trial Synopsis TL-OPI-518, NCT#
 Clinical Trial Synopsis, NCT# 00225264 Title of Study: A Double-Blind, Randomized, Comparator-Controlled Study in Subjects With Type 2 Diabetes Mellitus Comparing the Effects of Pioglitazone HCl vs Glimepiride
Clinical Trial Synopsis, NCT# 00225264 Title of Study: A Double-Blind, Randomized, Comparator-Controlled Study in Subjects With Type 2 Diabetes Mellitus Comparing the Effects of Pioglitazone HCl vs Glimepiride
How to Achieve Medical Weight Loss in 2012
 How to Achieve Medical Weight Loss in 2012 Gary D. Foster, Ph.D. Laure H. Carnell Professor of Medicine, Public Health, and Psychology Director, Center for Obesity Research and Education Temple University
How to Achieve Medical Weight Loss in 2012 Gary D. Foster, Ph.D. Laure H. Carnell Professor of Medicine, Public Health, and Psychology Director, Center for Obesity Research and Education Temple University
Pasta: A High-Quality Carbohydrate Food
 Pasta: A High-Quality Carbohydrate Food Cyril W.C. Kendall Department of Nutritional Sciences, Faculty of Medicine, University of Toronto; Clinical Nutrition & Risk Factor Modification Center, St. Michael
Pasta: A High-Quality Carbohydrate Food Cyril W.C. Kendall Department of Nutritional Sciences, Faculty of Medicine, University of Toronto; Clinical Nutrition & Risk Factor Modification Center, St. Michael
Metformin is the only drug. Sustained release metformin where standard metformin is not tolerated. Julie Brake
 Sustained release metformin where standard metformin is not tolerated Julie Brake Article points 1. Few people will continue taking medication while experiencing side effects. 2. Hypoglycaemia does not
Sustained release metformin where standard metformin is not tolerated Julie Brake Article points 1. Few people will continue taking medication while experiencing side effects. 2. Hypoglycaemia does not
The prevalence of obesity has increased markedly in
 Brief Communication Use of Prescription Weight Loss Pills among U.S. Adults in 1996 1998 Laura Kettel Khan, PhD; Mary K. Serdula, MD; Barbara A. Bowman, PhD; and David F. Williamson, PhD Background: Pharmacotherapy
Brief Communication Use of Prescription Weight Loss Pills among U.S. Adults in 1996 1998 Laura Kettel Khan, PhD; Mary K. Serdula, MD; Barbara A. Bowman, PhD; and David F. Williamson, PhD Background: Pharmacotherapy
GRACE C. PAGUIA, MD DPPS DPBCN
 Nutrition Dilemmas, WEIGHT MANAGEMENT IN CHILDREN AND ADOLESCENTS: THE EXISTING GUIDELINES GRACE C. PAGUIA, MD DPPS DPBCN Overweight & Obesity in Pediatrics Nutrition Dilemmas, q results from a chronic
Nutrition Dilemmas, WEIGHT MANAGEMENT IN CHILDREN AND ADOLESCENTS: THE EXISTING GUIDELINES GRACE C. PAGUIA, MD DPPS DPBCN Overweight & Obesity in Pediatrics Nutrition Dilemmas, q results from a chronic
Original Article. Keiko KOHNO 1), Kazuhiko HOSHI 1), Motoi TAKIZAWA 1), Takashi KANEKO 2), and Shuji HIRATA 1)
 Yamanashi Med. J. 21(3), 53 ~ 58, 2006 Original Article Usefulness of the 50-g Glucose Challenge Test for Screening of Patients with Gestational Diabetes Mellitus and an Analysis of the Timing of Administration
Yamanashi Med. J. 21(3), 53 ~ 58, 2006 Original Article Usefulness of the 50-g Glucose Challenge Test for Screening of Patients with Gestational Diabetes Mellitus and an Analysis of the Timing of Administration
Diabetes Care 24:89 94, 2000
 Pathophysiology/Complications O R I G I N A L A R T I C L E Insulin Resistance and Insulin Secretory Dysfunction Are Independent Predictors of Worsening of Glucose Tolerance During Each Stage of Type 2
Pathophysiology/Complications O R I G I N A L A R T I C L E Insulin Resistance and Insulin Secretory Dysfunction Are Independent Predictors of Worsening of Glucose Tolerance During Each Stage of Type 2
Investigating Massage as a Complementary Therapy for Weight Loss By Morgan J Lawless, LMT
 Investigating Massage as a Complementary Therapy for Weight Loss By Morgan J Lawless, LMT Obesity and excess body fat in The United States is a growing health risk to many Americans. The Center for Disease
Investigating Massage as a Complementary Therapy for Weight Loss By Morgan J Lawless, LMT Obesity and excess body fat in The United States is a growing health risk to many Americans. The Center for Disease
Management of Obesity. Objectives. Background Impact and scope of Obesity. Control of Energy Homeostasis Methods of treatment Medications.
 Medical Management of Obesity Ben O Donnell, MD 1 Objectives Background Impact and scope of Obesity Control of Energy Homeostasis Methods of treatment Medications 2 O'Donnell 1 Impact of Obesity According
Medical Management of Obesity Ben O Donnell, MD 1 Objectives Background Impact and scope of Obesity Control of Energy Homeostasis Methods of treatment Medications 2 O'Donnell 1 Impact of Obesity According
Clinical Trial Synopsis TL-OPI-525, NCT#
 Clinical Trial Synopsis, NCT#00762736 Title of Study: A Phase II, Double-Blind, Randomized, Placebo-Controlled, Proof-of-Concept Study of the Efficacy, Safety, and Tolerability of Pioglitazone HCl (ACTOS
Clinical Trial Synopsis, NCT#00762736 Title of Study: A Phase II, Double-Blind, Randomized, Placebo-Controlled, Proof-of-Concept Study of the Efficacy, Safety, and Tolerability of Pioglitazone HCl (ACTOS
Clinical Guidelines for the Hospitalized Adult Patient with Obesity
 Clinical Guidelines for the Hospitalized Adult Patient with Obesity 1 Definition of obesity: Obesity is characterized by an excess storage of adipose tissue that is related to an imbalance between energy
Clinical Guidelines for the Hospitalized Adult Patient with Obesity 1 Definition of obesity: Obesity is characterized by an excess storage of adipose tissue that is related to an imbalance between energy
Why Do We Care About Prediabetes?
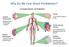 Why Do We Care About Prediabetes? Complications of Diabetes Diabetic Retinopathy Leading cause of blindness in adults 1,2 Diabetic Nephropathy Leading cause of Kidney failure Stroke 2- to 4-fold increase
Why Do We Care About Prediabetes? Complications of Diabetes Diabetic Retinopathy Leading cause of blindness in adults 1,2 Diabetic Nephropathy Leading cause of Kidney failure Stroke 2- to 4-fold increase
STATE OF THE STATE: TYPE II DIABETES
 STATE OF THE STATE: TYPE II DIABETES HENRY DRISCOLL, MD, CHIEF of ENDOCRINOLOGY MARSHALL U, CHERTOW DIABETES CENTER, HUNTINGTON VAMC HEATHER VENOY, RD, LD, CDE DIETITIAN, DIABETES EDUCATOR, CHERTOW DIABETES
STATE OF THE STATE: TYPE II DIABETES HENRY DRISCOLL, MD, CHIEF of ENDOCRINOLOGY MARSHALL U, CHERTOW DIABETES CENTER, HUNTINGTON VAMC HEATHER VENOY, RD, LD, CDE DIETITIAN, DIABETES EDUCATOR, CHERTOW DIABETES
A study of waist hip ratio in identifying cardiovascular risk factors at Government Dharmapuri College Hospital
 Original Research Article A study of waist hip ratio in identifying cardiovascular risk factors at Government Dharmapuri College Hospital M. Arivumani * Assistant Professor of General Medicine, Government
Original Research Article A study of waist hip ratio in identifying cardiovascular risk factors at Government Dharmapuri College Hospital M. Arivumani * Assistant Professor of General Medicine, Government
Body Mass Index Chart = overweight; = obese; >40= extreme obesity
 Pathophysiology of type 2 diabetes mellitus R. Leibel Naomi Berrie Diabetes Center 25 February 2008 Body Mass Index Chart 25-29.9 = overweight; 30-39.9= obese; >40= extreme obesity 5'0" 5'2" Weight (lbs)
Pathophysiology of type 2 diabetes mellitus R. Leibel Naomi Berrie Diabetes Center 25 February 2008 Body Mass Index Chart 25-29.9 = overweight; 30-39.9= obese; >40= extreme obesity 5'0" 5'2" Weight (lbs)
programme. The DE-PLAN follow up.
 What are the long term results and determinants of outcomes in primary health care diabetes prevention programme. The DE-PLAN follow up. Aleksandra Gilis-Januszewska, Noël C Barengo, Jaana Lindström, Ewa
What are the long term results and determinants of outcomes in primary health care diabetes prevention programme. The DE-PLAN follow up. Aleksandra Gilis-Januszewska, Noël C Barengo, Jaana Lindström, Ewa
ORIGINAL INVESTIGATION. Portion Control Plate for Weight Loss in Obese Patients With Type 2 Diabetes Mellitus
 ORIGINAL INVESTIGATION Portion Control Plate for Weight Loss in Obese Patients With Type 2 Diabetes Mellitus A Controlled Clinical Trial Sue D. Pedersen, MD, FRCPC; Jian Kang, MSc; Gregory A. Kline, MD,
ORIGINAL INVESTIGATION Portion Control Plate for Weight Loss in Obese Patients With Type 2 Diabetes Mellitus A Controlled Clinical Trial Sue D. Pedersen, MD, FRCPC; Jian Kang, MSc; Gregory A. Kline, MD,
THE PERENNIAL STRUGGLE TO LOSE WEIGHT AND MAINTAIN: WHY IS IT SO DIFFICULT?
 THE PERENNIAL STRUGGLE TO LOSE WEIGHT AND MAINTAIN: WHY IS IT SO DIFFICULT? Robert Ferraro, MD Medical Director Southwest Endocrinology Associates, PA Diabetes and Weight Management Center OBESITY The
THE PERENNIAL STRUGGLE TO LOSE WEIGHT AND MAINTAIN: WHY IS IT SO DIFFICULT? Robert Ferraro, MD Medical Director Southwest Endocrinology Associates, PA Diabetes and Weight Management Center OBESITY The
Faculty/Presenter Disclosure
 Weight loss & Obesity WHAT S NEW & EXCITING? Tina Korownyk Dept of Family Medicine, UofA Faculty/Presenter Disclosure Faculty/Presenter: Tina Korownyk Relationships with commercial interests: None 1 Drowning
Weight loss & Obesity WHAT S NEW & EXCITING? Tina Korownyk Dept of Family Medicine, UofA Faculty/Presenter Disclosure Faculty/Presenter: Tina Korownyk Relationships with commercial interests: None 1 Drowning
Supplementary Item 1. Study design
 Supplementary Item 1. Study design Arrows indicate time points for measurement of body weight, body composition, and resting energy expenditure (REE) in the continuous (CON) and intermittent (INT) groups.
Supplementary Item 1. Study design Arrows indicate time points for measurement of body weight, body composition, and resting energy expenditure (REE) in the continuous (CON) and intermittent (INT) groups.
Isolated Post-challenge Hyperglycemia: Concept and Clinical Significance
 CLINICAL PRACTICE Isolated Post-challenge Hyperglycemia: Concept and Clinical Significance John MF. Adam*, Daniel Josten** ABSTRACT The American Diabetes Association has strongly recommended that fasting
CLINICAL PRACTICE Isolated Post-challenge Hyperglycemia: Concept and Clinical Significance John MF. Adam*, Daniel Josten** ABSTRACT The American Diabetes Association has strongly recommended that fasting
RELATIONS AMONG OBESITY, ADULT WEIGHT STATUS AND CANCER IN US ADULTS. A Thesis. with Distinction from the School of Allied Medical
 RELATIONS AMONG OBESITY, ADULT WEIGHT STATUS AND CANCER IN US ADULTS A Thesis Presented in Partial Fulfillment of the Requirements to Graduate with Distinction from the School of Allied Medical Professions
RELATIONS AMONG OBESITY, ADULT WEIGHT STATUS AND CANCER IN US ADULTS A Thesis Presented in Partial Fulfillment of the Requirements to Graduate with Distinction from the School of Allied Medical Professions
Metabolic Syndrome. Shon Meek MD, PhD Mayo Clinic Florida Endocrinology
 Metabolic Syndrome Shon Meek MD, PhD Mayo Clinic Florida Endocrinology Disclosure No conflict of interest No financial disclosure Does This Patient Have Metabolic Syndrome? 1. Yes 2. No Does This Patient
Metabolic Syndrome Shon Meek MD, PhD Mayo Clinic Florida Endocrinology Disclosure No conflict of interest No financial disclosure Does This Patient Have Metabolic Syndrome? 1. Yes 2. No Does This Patient
Objectives 10/11/2013. Diabetes- The Real Cost of Sugar. Diabetes 101: What is Diabetes. By Ruth Nekonchuk RD CDE LMNT
 Diabetes- The Real Cost of Sugar By Ruth Nekonchuk RD CDE LMNT Objectives To explain diabetes To explain the risks of diabetes To enumerate the cost of diabetes to our country To enumerate the cost of
Diabetes- The Real Cost of Sugar By Ruth Nekonchuk RD CDE LMNT Objectives To explain diabetes To explain the risks of diabetes To enumerate the cost of diabetes to our country To enumerate the cost of
Page: 1 / 5 Produced by the Centre for Reviews and Dissemination Copyright 2018 University of York
 Weight management using a meal replacement strategy: meta and pooling analysis from six studies Heymsfield S B, van Mierlo C A, van der Knaap H C, Heo M, Frier H I CRD summary The review assessed partial
Weight management using a meal replacement strategy: meta and pooling analysis from six studies Heymsfield S B, van Mierlo C A, van der Knaap H C, Heo M, Frier H I CRD summary The review assessed partial
These results are supplied for informational purposes only.
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
XENical in the Prevention of Diabetes in Obese Subjects (XENDOS) Study
 Emerging Treatments and Technologies O R I G I N A L A R T I C L E XENical in the Prevention of Diabetes in Obese Subjects (XENDOS) Study A randomized study of orlistat as an adjunct to changes for the
Emerging Treatments and Technologies O R I G I N A L A R T I C L E XENical in the Prevention of Diabetes in Obese Subjects (XENDOS) Study A randomized study of orlistat as an adjunct to changes for the
Sitagliptin: first DPP-4 inhibitor to treat type 2 diabetes Steve Chaplin MSc, MRPharmS and Andrew Krentz MD, FRCP
 Sitagliptin: first DPP-4 inhibitor to treat type 2 diabetes Steve Chaplin MSc, MRPharmS and Andrew Krentz MD, FRCP KEY POINTS sitagliptin (Januvia) is a DPP-4 inhibitor that blocks the breakdown of the
Sitagliptin: first DPP-4 inhibitor to treat type 2 diabetes Steve Chaplin MSc, MRPharmS and Andrew Krentz MD, FRCP KEY POINTS sitagliptin (Januvia) is a DPP-4 inhibitor that blocks the breakdown of the
Physical Activity and the Prevention of Type 2 Diabetes Mellitus How Much for How Long?
 CURRENT OPINION Sports Med 2000 Mar; 29 (3): 147-151 0112-1642/00/0003-0147/$20.00/0 Adis International Limited. All rights reserved. Physical Activity and the Prevention of Type 2 Diabetes Mellitus How
CURRENT OPINION Sports Med 2000 Mar; 29 (3): 147-151 0112-1642/00/0003-0147/$20.00/0 Adis International Limited. All rights reserved. Physical Activity and the Prevention of Type 2 Diabetes Mellitus How
SYNOPSIS 2/198 CSR_BDY-EFC5825-EN-E02. Name of company: TABULAR FORMAT (For National Authority Use only)
 SYNOPSIS Title of the study: A randomized, double-blind, placebo-controlled, parallel-group, fixed-dose (rimonabant 20 mg) multicenter study of long-term glycemic control with rimonabant in treatment-naïve
SYNOPSIS Title of the study: A randomized, double-blind, placebo-controlled, parallel-group, fixed-dose (rimonabant 20 mg) multicenter study of long-term glycemic control with rimonabant in treatment-naïve
Elevated Incidence of Type 2 Diabetes in San Antonio, Texas, Compared With That of Mexico City, Mexico
 Epidemiology/Health Services/Psychosocial Research O R I G I N A L A R T I C L E Elevated Incidence of Type 2 Diabetes in San Antonio, Texas, Compared With That of Mexico City, Mexico JAMES P. BURKE, PHD
Epidemiology/Health Services/Psychosocial Research O R I G I N A L A R T I C L E Elevated Incidence of Type 2 Diabetes in San Antonio, Texas, Compared With That of Mexico City, Mexico JAMES P. BURKE, PHD
Type 2 DM in Adolescents: Use of GLP-1 RA. Objectives. Scope of Problem: Obesity. Background. Pathophysiology of T2DM
 Type 2 DM in Adolescents: Use of GLP-1 RA Objectives Identify patients in the pediatric population with T2DM that would potentially benefit from the use of GLP-1 RA Discuss changes in glycemic outcomes
Type 2 DM in Adolescents: Use of GLP-1 RA Objectives Identify patients in the pediatric population with T2DM that would potentially benefit from the use of GLP-1 RA Discuss changes in glycemic outcomes
Effect of Weight Gain and Weight Loss on Risk of Diabetes amongst Over Weight Subjects: A Hospital Based Study
 Original article: Effect of Weight Gain and Weight Loss on Risk of Diabetes amongst Over Weight Subjects: A Hospital Based Study Hanuman Ram Choudhary Junior Specialist (General Medicine), Government Hospital,
Original article: Effect of Weight Gain and Weight Loss on Risk of Diabetes amongst Over Weight Subjects: A Hospital Based Study Hanuman Ram Choudhary Junior Specialist (General Medicine), Government Hospital,
Hypertension and obesity. Dr Wilson Sugut Moi teaching and referral hospital
 Hypertension and obesity Dr Wilson Sugut Moi teaching and referral hospital No conflict of interests to declare Obesity Definition: excessive weight that may impair health BMI Categories Underweight BMI
Hypertension and obesity Dr Wilson Sugut Moi teaching and referral hospital No conflict of interests to declare Obesity Definition: excessive weight that may impair health BMI Categories Underweight BMI
Anti-Obesity Agents Drug Class Prior Authorization Protocol
 Anti-Obesity Agents Drug Class Prior Authorization Protocol Line of Business: Medicaid P & T Approval Date: February 21, 2018 Effective Date: March 1, 2018 This policy has been developed through review
Anti-Obesity Agents Drug Class Prior Authorization Protocol Line of Business: Medicaid P & T Approval Date: February 21, 2018 Effective Date: March 1, 2018 This policy has been developed through review
SYNOPSIS. Administration: subcutaneous injection Batch number(s):
 SYNOPSIS Title of the study: A randomized, double-blind, placebo-controlled, 2-arm parallel-group, multicenter 24-week study followed by an extension assessing the efficacy and safety of AVE0010 on top
SYNOPSIS Title of the study: A randomized, double-blind, placebo-controlled, 2-arm parallel-group, multicenter 24-week study followed by an extension assessing the efficacy and safety of AVE0010 on top
The relation between obesity and several
 Clinical Care/Education/Nutrition O R I G I N A L A R T I C L E Effect of Orlistat on Weight Regain and Cardiovascular Risk Factors Following a Very-Low-Energy Diet in Abdominally Obese Patients A 3-year
Clinical Care/Education/Nutrition O R I G I N A L A R T I C L E Effect of Orlistat on Weight Regain and Cardiovascular Risk Factors Following a Very-Low-Energy Diet in Abdominally Obese Patients A 3-year
Diabetes and Weight Management: Tools to Affect Patient Outcomes
 Diabetes and Weight Management: Tools to Affect Patient Outcomes Today s discussion Review the problem of diabetes and the importance of lifestyle intervention Identify current research supporting the
Diabetes and Weight Management: Tools to Affect Patient Outcomes Today s discussion Review the problem of diabetes and the importance of lifestyle intervention Identify current research supporting the
The Global Agenda for the Prevention of Diabetes: Research Opportunities
 The Global Agenda for the Prevention of Diabetes: Research Opportunities William H. Herman, MD, MPH Stefan S. Fajans/GlaxoSmithKline Professor of Diabetes Professor of Internal Medicine and Epidemiology
The Global Agenda for the Prevention of Diabetes: Research Opportunities William H. Herman, MD, MPH Stefan S. Fajans/GlaxoSmithKline Professor of Diabetes Professor of Internal Medicine and Epidemiology
OBESITY 2008: DIET, EXERCISE, DRUGS, AND SURGERY
 OBESITY 2008: DIET, EXERCISE, DRUGS, AND SURGERY Robert B. Baron MD MS Professor and Associate Dean UCSF School of Medicine Declaration of full disclosure: No conflict of interest CLASSIFICATION OF OVERWEIGHT
OBESITY 2008: DIET, EXERCISE, DRUGS, AND SURGERY Robert B. Baron MD MS Professor and Associate Dean UCSF School of Medicine Declaration of full disclosure: No conflict of interest CLASSIFICATION OF OVERWEIGHT
Obesity, Weight Loss and Obstructive Sleep Apnea
 Obesity, Weight Loss and Obstructive Sleep Apnea Gary D. Foster, Ph.D. Center for Obesity Research and Education Temple University School of Medicine Overview Sociocultural context Obesity: Prevalence
Obesity, Weight Loss and Obstructive Sleep Apnea Gary D. Foster, Ph.D. Center for Obesity Research and Education Temple University School of Medicine Overview Sociocultural context Obesity: Prevalence
OBESITY: The Growing Epidemic and its Medical Impact
 OBESITY: The Growing Epidemic and its Medical Impact Ray Plodkowski, MD Co-Chief, Chief, of Division of Medical Nutrition, University of Nevada School of Medicine. Chief, Endocrinology & Metabolism, Sachiko
OBESITY: The Growing Epidemic and its Medical Impact Ray Plodkowski, MD Co-Chief, Chief, of Division of Medical Nutrition, University of Nevada School of Medicine. Chief, Endocrinology & Metabolism, Sachiko
Initiating Insulin in Primary Care for Type 2 Diabetes Mellitus. Dr Manish Khanolkar, Diabetologist, Auckland Diabetes Centre
 Initiating Insulin in Primary Care for Type 2 Diabetes Mellitus Dr Manish Khanolkar, Diabetologist, Auckland Diabetes Centre Outline How big is the problem? Natural progression of type 2 diabetes What
Initiating Insulin in Primary Care for Type 2 Diabetes Mellitus Dr Manish Khanolkar, Diabetologist, Auckland Diabetes Centre Outline How big is the problem? Natural progression of type 2 diabetes What
WORLDWIDE, THE PREVAlence
 ORIGINAL CONTRIBUTION Relation of Impaired Fasting and Postload Glucose With Incident Type 2 Diabetes in a Dutch Population The Hoorn Study Femmie de Vegt, PhD Jacqueline M. Dekker, PhD Agnes Jager, MD,
ORIGINAL CONTRIBUTION Relation of Impaired Fasting and Postload Glucose With Incident Type 2 Diabetes in a Dutch Population The Hoorn Study Femmie de Vegt, PhD Jacqueline M. Dekker, PhD Agnes Jager, MD,
