The estimated absorbed doses from a bolus intravenous
|
|
|
- Allison Marsh
- 6 years ago
- Views:
Transcription
1 BASIC SCIENCE INVESTIGATIONS MIRD Dose Estimate Report No. 19: Radiation Absorbed Dose Estimates from F-FDG Marguerite T. Hays, MD 1,2 ; Evelyn E. Watson, BA 3 ; Stephen R. Thomas, PhD 4 ; and Michael Stabin, PhD 5 (Task Group), for the MIRD Committee 1 Nuclear Medicine Service, VA Palo Alto Health Care System, Palo Alto, California; 2 Department of Radiology, Stanford University, Palo Alto, California; 3 Oak Ridge, Tennessee; 4 Department of Radiology, College of Medicine, University of Cincinnati, Cincinnati, Ohio; and 5 Department of Radiology and Radiological Sciences, Vanderbilt University, Nashville, Tennessee Key Words: F-FDG; MIRD dose estimate report J Nucl Med 2002; 43: The estimated absorbed doses from a bolus intravenous administration of F-FDG are given in Table 1. The data and assumptions used in these calculations are presented as follows. RADIOPHARMACEUTICAL F-FDG is formed through radiochemical synthesis from cyclotron-produced F (K. Breslow, written communication, June 2000). Production of F is through proton bombardment of enriched O-water. F-fluoride is bound to 1,3,4,6-tetra-O-acetyl-2-O-trifluoromethanesulfonyl- -Dmannopyranose (mannose triflate) under conditions of a stereospecific second-order nucleophilic substitution reaction, which produces no-carrier-added F-FDG. The F- FDG is injected intravenously as an isotonic, sterile, pyrogen-free, clear, colorless solution. NUCLEAR DATA F decays to stable O by positron emission with a half-life of min. Physical data are given in Table 2(1). BIOLOGIC DATA Residence time ( ), as used here, refers to the area under the time activity curve for the organ of interest, divided by the activity injected as an intravenous bolus at time zero. The residence times that form the basis for the calculations Received Mar. 20, 2001; revision accepted Oct. 24, For correspondence contact: Marguerite T. Hays, MD, 270 Campesino Ave., Palo Alto, CA ritahays19@yahoo.com in this report were derived from the 4 sources described below. Published Residence Times for F-FDG Calculated Using Mathematical Model for Distribution in Healthy Humans For this study (2) conducted at the VA Medical Center in Palo Alto, CA, all patients recruited (6 men, 1 woman; age range, y; 13 studies) had previously undergone cardiac stress studies, requested for the usual clinical indications, that had been interpreted as normal. Heart, liver, lung, whole blood, and plasma time activity data were acquired for 90 min after intravenous F-FDG administration. Accumulated F-FDG activity in the urine was assayed at 100 min. Cardiac uptake of F-FDG had been expected to be enhanced by glucose loading. However, paired sessions in 5 of these subjects comparing the fasting state with the glucose-loaded state showed no significant differences; therefore, studies are included in this summary regardless of the subject s glucose status. Three studies on 2 subjects are included here that were omitted from the analysis presented in the study of Hays and Segall (2) because they did not meet the criteria for paired samples required in that analysis. The observed time activity data (corrected for physical decay) for F activity in the heart, liver, lungs, plasma, erythrocytes, and urine were fitted simultaneously to a multicompartmental model using the SAAM 30 program and methodology as described in Hays and Segall (2). The physiologic model was solved, and the kinetic parameters were calculated for each study. Model-generated time activity curves (incorporating physical decay) were used to determine the residence time for each source organ. Brain time activity data were not directly observed in this study. Instead, brain residence times were calculated using the observed plasma data, incorporating published model parameters for brain F-FDG transport (3) into this model. Because direct observational data were unavailable for red marrow, the residence time for this organ was calculated assuming that its F-FDG concentration and kinetics are the same as those of whole blood. 210 THE JOURNAL OF NUCLEAR MEDICINE Vol. 43 No. 2 February 2002
2 TABLE 1 Estimated Absorbed Doses from Intravenous Administration of F-FDG (Mean SD) Target organ Absorbed dose per unit of administered activity mgy/mbq rad/mci Brain Heart wall Kidneys Liver Lungs Pancreas Red marrow Spleen Urinary bladder wall* Ovaries Testes Whole body *Dose to urinary bladder wall is based on 120-min void intervals, starting 120 min after dosing, using traditional static MIRD model. Doses to ovaries and testes include doses from residence times in urinary bladder and remainder of body as calculated from data in Hays and Segall (2). Time activity curves projected from this model using mean parameter values derived from the individual studies are shown in Figure 1 for brain, heart, lungs, liver, and urine. In addition, urine data from the SAAM 30 output were used to provide biologic parameters for input into the MIRD dynamic bladder model (4) for calculation of the dose to the surface of the urinary bladder wall under a variety of circumstances. The results of this calculation were validated against the traditional (static 200 ml) MIRD bladder dose calculations. Table 3 presents the radiation dose per administered activity to the surface of the urinary bladder wall (mean and range) as provided by the dynamic bladder model for the 13 studies from the investigation of Hays and Segall (2). Published Residence Times for F-FDG in Healthy Japanese Subjects The results of Mejia et al. (5) were based on analysis of quantitative organ time activity curves for 1 h after bolus intravenous F-FDG injection (2 h in 2 of the brain studies). Radionuclide Physical half-life Decay constant Decay mode Principal radiation TABLE 2 Nuclear Data E i (kev) F min min 1, electron capture n i rad g/ Ci h i Gy kg/ Bq s Photon E E-14 Data are from Weber et al. (1). They also recorded bladder activity for 2 h by external counting, normalized to the activity in the cumulated urine at 2 h. Because of the smaller size of the average Japanese adult, these authors used S tables devised for Japanese subjects (6) based on a model of Japanese reference man (7). To make these data comparable with the American data, values for residence times from this study were normalized to the standard MIRD model by multiplying by the ratio of the organ weight in the MIRD reference man to that in the Japanese reference man. (The logic of this adjustment is that tissue concentrations as a function of blood concentration would be expected to be the same regardless of body size or relative organ size. Thus, adjusting for the differences in sizes in the MIRD and the Japanese standard man models would make the Japanese data usable for dose calculations with the MIRD standard man.) Adjusted residence times for brain (6 subjects), heart (5 subjects), liver (4 subjects), pancreas (3 subjects), spleen (3 subjects), kidneys (4 subjects), and lungs (6 subjects) were used in the dose estimates presented here. Bladder residence times (8 subjects) using the static MIRD model in this study were comparable with those calculated by Hays and Segall (2). Published Residence Times for F-FDG in Bladder In the study by Jones et al. (8), bladder residence times were based on continuous external counting of bladder F activity in 10 patients, normalized to the activity in the cumulated urine at 2 h. Published Residence Times for F-FDG in Brain In the study by Niven et al. (9), brain residence times in patients undergoing clinical PET studies were derived from 1-h brain F-FDG dynamic studies in which data were acquired at 5-min intervals and integrated numerically using the trapezoidal rule. The authors assumed that no biologic removal occurred after the 1hofdata collection. Eight men F-FDG DOSE ESTIMATE REPORT Hays et al. 211
3 FIGURE 1. Time activity curves for decay-corrected FDG activity in normal human brain, heart, lungs, liver, and urine. These curves were projected by model presented in report by Hays and Segall (2), using geometric means of model parameters derived from fits of data from 13 individual studies. and 6 women, aged y, were studied, and duplicate studies were done on 6 of the men and all of the women (26 studies total). Because there were no statistically significant sequential differences in residence time, data on each individual study (provided by E. Niven, written communication, July 2001) are considered separately in the current report. The authors found a minor difference (P 0.05) in residence times between sexes, with residence times for women 4.8% 5.2% (mean SD) greater than those for men. In pooling data for the current report, this difference has been ignored. Summary statistics for the residence times used in the dose estimates are presented in Table 4. ABSORBED DOSE ESTIMATES Residence times calculated from data from individual subjects were used with S values to calculate radiation absorbed dose estimates for each person. The source organs included brain, heart wall, liver, kidneys, pancreas, spleen, urinary bladder, red marrow, lungs and whole body, the organs for which observed or inferred residence time data TABLE 3 Radiation Dose per Administered Activity to Surface of Urinary Bladder Wall as Provided by Dynamic Bladder Model Initial bladder volume (ml) Initial void time (min) Mean Range Mean Range Mean Range Mean Range Data show mean and range (in mgy/mbq) of doses for the 13 studies from investigation by Hays and Segall (2), as function of selected initial bladder volumes and initial void times. Data indicate variability between individual studies and importance of initial bladder volume and timing of initial void. Calculations assumed day/night bladder filling rate of 1.0/0.5 ml/min, with administration of radiopharmaceutical at 9:00 AM. Voiding schedule was every 3 h until midnight, with 6-h nighttime gap between midnight and 6:00 AM. Dynamic bladder model is that described in Thomas et al. (4). 212 THE JOURNAL OF NUCLEAR MEDICINE Vol. 43 No. 2 February 2002
4 Organ TABLE 4 Residence Times, in Hours, Used in Absorbed Dose Estimates Data source Hays and Segall (2) Mejia et al. (5) Jones et al. (8) Niven et al. (9) Weighted mean Brain (n 33) Heart (n ) Bladder, 2 h (n 28) Liver (n 17) Lungs * 0.06 (n 19) Kidneys (n 4) Pancreas (n 3) Spleen (n 3) Whole blood (n 13) Whole body (n 13) *SD Data are mean SD for each study. were available. Absorbed doses were calculated for these organs and also for the gonads. In this calculation, it was assumed that the gonads had the same F-FDG concentration as the remainder of the body. The dose to each target organ was calculated according to the procedures outlined in MIRD Pamphlet No. 1, Revised (10). The dose per unit administered activity for an organ is the sum of the products obtained from multiplying the residence time in the source organ by the appropriate S value. With the exception of brain, the S values were those published in MIRD Pamphlet No. 11 (11). Because the brain is not included in MIRD Pamphlet No. 11, the S value for brain irradiating brain was calculated from the absorbed fractions given in MIRD Pamphlet No. 5 (12). A mass of 1,400 g was assigned to the brain of the adult man. The radiation dose to the brain includes only the dose from activity in the brain because the fraction of radiation emitted from other source organs that would be absorbed in the brain is negligible. The individual dose estimates were averaged, and these averaged results are shown in Table 1. The number of subjects whose data were included in the calculation for each organ is shown in Table 4. Bladder doses for a typical subject under various conditions of initial urine volume and void times are presented in Figure 2. These were calculated using the MIRD dynamic bladder model (4), incorporating data from a subject reported by Hays and Segall (2). Table 3 presents the means and ranges of the results of these calculations in the 13 studies from the data of Hays and FIGURE 2. Dose per unit administered activity to bladder-wall surface as calculated by MIRD dynamic bladder model (4) for typical subject from study of Hays and Segall (2) for 1.0/0.5 ml/min (daytime/nighttime) bladder filling rate. Dose depends on initial bladder (urine) volume, V 0, and time of first void, T 1. F-FDG DOSE ESTIMATE REPORT Hays et al. 213
5 Segall (2), with the bladder fill rate taken to be 1 ml/min during waking hours and 0.5 ml/min during sleeping hours. DISCUSSION As a MIRD dose estimate report, this study incorporates only data from well-documented human studies of F-FDG kinetics done independently in more than one laboratory and providing time activity data with sufficient time points to project cumulated activities. In particular, the brain data from the study by Jones et al. (8) were not incorporated in this report because they were based on a single observation. Similarly, the data from a 1998 study by Deloar et al. (13) were not included because their residence times were projected from only 3 time points. Although F-FDG is widely used clinically and scientifically, there have been few studies that provide the type of human kinetic data needed for dosimetry calculations. The International Commission on Radiological Protection (ICRP), in its publications 53 (14) and 80 (15), presents tables of F-FDG doses derived from a model assuming specific uptake of F-FDG by the brain and heart with the further assumption that all other activity is distributed uniformly in the body. The ICRP authors used the kinetic data on urinary excretion from the study of Jones et al. (8) to calculate the kinetics of total-body F-FDG retention and assumed that 4% and 6% of the administered tracer were taken up by the myocardium and brain, respectively. They were not specific about the source of those figures. The radiation dose values for F-FDG presented in ICRP 80 differ from those in ICRP 53, but the database for the calculations presented in ICRP 80 appears to be the same as that used for the ICRP 53 report. Several differences exist between the results provided in ICRP 80 (15) and those presented here. Although the wholebody residence time in the ICRP publication (2.13 h) is similar to that reported here (2.38 h), residence times for some source organs are notably different. This MIRD report finds a brain residence time of 0.23 h, which is higher than the ICRP value of 0.15 h, resulting in a correspondingly greater dose to the brain (0.046 mgy/mbq vs mgy/ MBq). For the liver, ICRP 80 gives the dose as mgy/mbq, whereas this report lists the mean liver dose as mgy/mbq. This difference reflects the observed specific liver uptake found in the human studies that form the basis of this MIRD dose-estimate report, whereas the ICRP authors assumed that the human liver had no specific F- FDG uptake (12). The MIRD Committee reports the total-body dose (based on the total energy deposited in the body divided by its total mass), whereas the ICRP reports effective dose (a value estimated by applying risk-based weighting factors to individual organ doses, to estimate a uniform whole-body dose that in theory gives the same risk as the nonuniform dose pattern that actually occurred). These values are not directly comparable, being based on different concepts. It has been shown that effective dose for many diagnostic radiopharmaceuticals is generally higher than total-body dose by a factor of (16). For F-FDG, using the same kinetic data as input, effective dose is estimated to be higher than total-body dose by approximately a factor of 2. CONCLUSION This dose estimate report presents estimated radiation doses to human organs after a bolus intravenous injection of F-FDG, based on review of the published literature as interpreted by members of the MIRD Committee. The absorbed dose estimates are summarized in Table 1. ACKNOWLEDGMENTS The members of the MIRD Committee of the Society of Nuclear Medicine are Wesley E. Bolch, A. Bertrand Brill, N. David Charkes, Darrel R. Fisher, Marguerite T. Hays, Ruby F. Meredith, George Sgouros, Jeffry A. Siegel, Stephen R. Thomas, and Evelyn E. Watson (chair). The activities of the MIRD Committee are partially supported by the Society of Nuclear Medicine. REFERENCES 1. Weber DA, Eckerman KF, Dillman LT, Ryman JC. MIRD Radionuclide Data and Decay Schemes. New York, NY: Society of Nuclear Medicine; 1989: Hays MT, Segall GM. A mathematical model for the distribution of fluorodeoxyglucose in humans. J Nucl Med. 1999;40: Huang S-C, Phelps ME, Hoffman EJ, Sideris K, Selin CJ, Kuhl DE. Noninvasive determination of local cerebral metabolic rate of glucose in man. Am J Physiol. 1980;238:E69 E Thomas SR, Stabin MG, Chen C-T, Samaratunga RC. MIRD pamphlet no. 14 revised: a dynamic urinary bladder model for radiation dose calculations. J Nucl Med. 1999;40:102S 123S. 5. Mejia AA, Nakamura T, Masatoshi I, Hatazawa J, Mazaki M, Watanuki S. Estimation of absorbed doses in humans due to intravenous administration of fluorine--fluorodeoxyglucose in PET studies. J Nucl Med. 1991;32: Yamaguchi H, Nishizawa K, Maruyama T, Chiba M, Fukuhisa K, Hashizumi T. A computer program to calculate MIRD tables for Japanese physiques. Hoken Butsuri. 1983;: Tanaka G. Japanese reference man 1988-III: masses of organs and tissues and other physical properties. Nippon Acta Radiol. 1988;48: Jones SC, Alavi A, Christman D, Montanez I, Wolf AP, Reivich M. The radiation dosimetry of 2-[F-]fluoro-2-deoxy-D-glucose in man. J Nucl Med. 1982;23: Niven E, Thompson M, Nahmias C. Absorbed dose to the adult male and female brain from F-fluorodeoxyglucose. Health Phys. 2001;80: Loevinger R, Berman M. A revised schema for calculating the absorbed dose from biologically distributed radiopharmaceuticals. In: MIRD Pamphlet No. 1, Revised. Reston, VA: Society of Nuclear Medicine; Snyder WS, Ford MR, Warner GG, Watson SB. S absorbed dose per unit cumulated activity for selected radionuclides and organs. In: MIRD Pamphlet No. 11. Reston, VA: Society of Nuclear Medicine; Snyder WS, Ford MR, Warner GG, Fisher HL Jr. Estimates of absorbed fractions for monoenergetic photon sources uniformly distributed in various organs of a heterogeneous phantom: MIRD pamphlet no. 5. J Nucl Med. 1969;10(suppl 3): Deloar HM, Fujiwara T, Shidahara M, et al. Estimation of absorbed dose for 2-[F-]fluoro-2-deoxy-D-glucose using whole-body positron emission tomography and magnetic resonance imaging. Eur J Nucl Med. 1998;25: Radiation Dose to Patients from Radiopharmaceuticals. Oxford, U.K.: Pergamon Press; 1988: ICRP Publication Radiation Dose to Patients from Radiopharmaceuticals. Oxford, U.K.: Pergamon Press; 1999:76. ICRP Publication 80, Addendum 2 to ICRP Publication Toohey RE, Stabin MG. Comparative analysis of dosimetry parameters for nuclear medicine. In: Stelson A, Stabin M, Sparks R, eds. Sixth International Radiopharmaceutical Dosimetry Symposium, May 7 10, Gatlinburg, TN: Oak Ridge Associated Universities; 1999: THE JOURNAL OF NUCLEAR MEDICINE Vol. 43 No. 2 February 2002
A Real-Time Monte Carlo System for Internal Dose Evaluation Using an Anthropomorphic Phantom with Different Shapes of Tumors Inserted
 A Real-Time Monte Carlo System for Internal Dose Evaluation Using an Anthropomorphic Phantom with Different Shapes of Tumors Inserted J. Wu, S. J. Chang, B. J. Chang, I. J. Chen, J. H. Chiu Health Physics
A Real-Time Monte Carlo System for Internal Dose Evaluation Using an Anthropomorphic Phantom with Different Shapes of Tumors Inserted J. Wu, S. J. Chang, B. J. Chang, I. J. Chen, J. H. Chiu Health Physics
Standardization of Radiopharmaceutical Dosimetry
 Standardization of Radiopharmaceutical Dosimetry Jonathon A. Nye, PhD Department of Radiology and Imaging Sciences Emory University SEAAPM 2011 Myrtle Beach, SC Review of Dosimetry Nomenclature Dose Gray
Standardization of Radiopharmaceutical Dosimetry Jonathon A. Nye, PhD Department of Radiology and Imaging Sciences Emory University SEAAPM 2011 Myrtle Beach, SC Review of Dosimetry Nomenclature Dose Gray
GALLIUM CITRATE Ga 67 INJECTION
 511945-0903 September 2003 USA Bristol-Myers Squibb Medical Imaging 331 Treble Cove Road N. Billerica, MA 01862 USA GALLIUM CITRATE Ga 67 INJECTION FOR DIAGNOSTIC USE DESCRIPTION: Gallium Citrate Ga 67
511945-0903 September 2003 USA Bristol-Myers Squibb Medical Imaging 331 Treble Cove Road N. Billerica, MA 01862 USA GALLIUM CITRATE Ga 67 INJECTION FOR DIAGNOSTIC USE DESCRIPTION: Gallium Citrate Ga 67
Research Article Kidney Modelling for FDG Excretion with PET
 Biomedical Imaging Volume 2007, Article ID 63234, 4 pages doi:10.1155/2007/63234 Research Article Kidney Modelling for FDG Excretion with PET Huiting Qiao, 1 Jing Bai, 1 Yingmao Chen, 2 and Jiahe Tian
Biomedical Imaging Volume 2007, Article ID 63234, 4 pages doi:10.1155/2007/63234 Research Article Kidney Modelling for FDG Excretion with PET Huiting Qiao, 1 Jing Bai, 1 Yingmao Chen, 2 and Jiahe Tian
Several different radiopharmaceuticals have been used in
 SPECIAL CONTRIBUTION Radiopharmaceuticals for Nuclear Cardiology: Radiation Dosimetry, Uncertainties, and Risk Michael G. Stabin Department of Radiology and Radiological Sciences, Vanderbilt University,
SPECIAL CONTRIBUTION Radiopharmaceuticals for Nuclear Cardiology: Radiation Dosimetry, Uncertainties, and Risk Michael G. Stabin Department of Radiology and Radiological Sciences, Vanderbilt University,
Colour on-line figures None Colour print figures None
 Journal: Article id: Raddos ncs128 Colour on-line figures None Colour print figures None Radiation Protection Dosimetry (2012), pp. 1 9 doi:10.1093/rpd/ncs128 5 10 15 20 25 30 35 40 45 50 55 TEDE PER CUMULATED
Journal: Article id: Raddos ncs128 Colour on-line figures None Colour print figures None Radiation Protection Dosimetry (2012), pp. 1 9 doi:10.1093/rpd/ncs128 5 10 15 20 25 30 35 40 45 50 55 TEDE PER CUMULATED
Dose Estimates for Nuclear Medicine Procedures: What are they? Where do they come from?
 Dose Estimates for Nuclear Medicine Procedures: What are they? Where do they come from? SNM Continuing Education Lecture Salt Lake City, UT -- June 6, 2010 Darrell R. Fisher Pacific Northwest National
Dose Estimates for Nuclear Medicine Procedures: What are they? Where do they come from? SNM Continuing Education Lecture Salt Lake City, UT -- June 6, 2010 Darrell R. Fisher Pacific Northwest National
Impact of ICRP-89 Based Models on Dose Estimates for Radiopharmaceuticals and CT Exams. Stabin MG, Kost SD, Clark JH, Pickens DR, Price RR, Carver DE
 Impact of ICRP-89 Based Models on Dose Estimates for Radiopharmaceuticals and CT Exams Stabin MG, Kost SD, Clark JH, Pickens DR, Price RR, Carver DE Vanderbilt University, Nashville, TN, USA Abstract New
Impact of ICRP-89 Based Models on Dose Estimates for Radiopharmaceuticals and CT Exams Stabin MG, Kost SD, Clark JH, Pickens DR, Price RR, Carver DE Vanderbilt University, Nashville, TN, USA Abstract New
Optimization of a routine method for bone marrow dose estimation in
 Optimization of a routine method for bone marrow dose estimation in 177 Lu-EDTMP therapy- Experience in Uruguay. Teran. M 1, Paolino.A 2, Coppe.F 2, Nuñez M 2, Hermida J C 2, Gaudiano.J 2 1 Cátedra de
Optimization of a routine method for bone marrow dose estimation in 177 Lu-EDTMP therapy- Experience in Uruguay. Teran. M 1, Paolino.A 2, Coppe.F 2, Nuñez M 2, Hermida J C 2, Gaudiano.J 2 1 Cátedra de
Radiation Dose to Patients from Radiopharmaceuticals
 2001-08-20 RADIATION PROTECTION ADDENDUM 4 TO ICRP PUBLICATION 53 Radiation Dose to Patients from Radiopharmaceuticals A report of a Task Group of Committees 2 and 3 of the International Commission on
2001-08-20 RADIATION PROTECTION ADDENDUM 4 TO ICRP PUBLICATION 53 Radiation Dose to Patients from Radiopharmaceuticals A report of a Task Group of Committees 2 and 3 of the International Commission on
Health Concerns Related to Radiation Exposure. of the Female Nuclear Medicine Patient. Michael G. Stabin
 Health Concerns Related to Radiation Exposure of the Female Nuclear Medicine Patient Michael G. Stabin Radiation Internal Dose Information Center Oak Ridge Institute for Science and Education P.O. Box
Health Concerns Related to Radiation Exposure of the Female Nuclear Medicine Patient Michael G. Stabin Radiation Internal Dose Information Center Oak Ridge Institute for Science and Education P.O. Box
The Management of Imaging Procedure Dose Nuclear Medicine Dose Indices
 The Management of Imaging Procedure Dose Nuclear Medicine Dose Indices Wesley E. Bolch, PhD, PE, DABHP, FHPS, FAAPM Director, Advanced Laboratory for Radiation Dosimetry Studies Department of Biomedical
The Management of Imaging Procedure Dose Nuclear Medicine Dose Indices Wesley E. Bolch, PhD, PE, DABHP, FHPS, FAAPM Director, Advanced Laboratory for Radiation Dosimetry Studies Department of Biomedical
Click Here to Continue. Click Here to Return to Table of Contents
 Hippuran I 131 Injection Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions Click Here to Continue Click Here to Return to Table
Hippuran I 131 Injection Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions Click Here to Continue Click Here to Return to Table
PRODUCT MONOGRAPH. F-Fluorodeoxyglucose ( 18 F-FDG) Parenteral Solution, > 0.5 GBq /ml. Diagnostic Radiopharmaceutical
 PRODUCT MONOGRAPH 18 F-Fluorodeoxyglucose ( 18 F-FDG) Parenteral Solution, > 0.5 GBq /ml Diagnostic Radiopharmaceutical Isologic Innovative Radiopharmaceuticals Ltd. Date of Preparation: 1855 32 nd Avenue,
PRODUCT MONOGRAPH 18 F-Fluorodeoxyglucose ( 18 F-FDG) Parenteral Solution, > 0.5 GBq /ml Diagnostic Radiopharmaceutical Isologic Innovative Radiopharmaceuticals Ltd. Date of Preparation: 1855 32 nd Avenue,
Radiopharmaceuticals used in diagnostic and therapeutic
 MIRD Pamphlet No. 19: Absorbed Fractions and Radionuclide S Values for Six Age-Dependent Multiregion Models of the Kidney* Lionel G. Bouchet, PhD 1, ; Wesley E. Bolch, PhD,3 ; H. Pablo Blanco, MS 3 ; Barry
MIRD Pamphlet No. 19: Absorbed Fractions and Radionuclide S Values for Six Age-Dependent Multiregion Models of the Kidney* Lionel G. Bouchet, PhD 1, ; Wesley E. Bolch, PhD,3 ; H. Pablo Blanco, MS 3 ; Barry
Amira K. Brown, Ph.D. Molecular Imaging Branch, NIMH Bldg. 1 Rm. B3-10
 Whole-body biodistribution and radiation dosimetry estimates for the metabotropic glutamate receptor subtype 5 (mglur5) radioligand [ 18 F]SP203 in nonhuman primates Amira K. Brown, Ph.D. Molecular Imaging
Whole-body biodistribution and radiation dosimetry estimates for the metabotropic glutamate receptor subtype 5 (mglur5) radioligand [ 18 F]SP203 in nonhuman primates Amira K. Brown, Ph.D. Molecular Imaging
GLUCEPTATE. Technetium Tc 99m Gluceptate Kit DIAGNOSTIC DESCRIPTION
 27194 0001E m TM GLUCEPTATE Technetium Tc 99m Gluceptate Kit DIAGNOSTIC DESCRIPTION The kit consists of reaction vials which contain the sterile, non-pyrogenic, nonradioactive ingredients necessary to
27194 0001E m TM GLUCEPTATE Technetium Tc 99m Gluceptate Kit DIAGNOSTIC DESCRIPTION The kit consists of reaction vials which contain the sterile, non-pyrogenic, nonradioactive ingredients necessary to
Use Of MCNP With Voxel-Based Image Data For Internal Dosimetry Applications
 Use Of MCNP With Voxel-Based Image Data For Internal Dosimetry Applications The Monte Carlo Method: Versatility Unbounded In A Dynamic Computing World Chattanooga, TN, USA, April 17-21, 2005 M. G. Stabin
Use Of MCNP With Voxel-Based Image Data For Internal Dosimetry Applications The Monte Carlo Method: Versatility Unbounded In A Dynamic Computing World Chattanooga, TN, USA, April 17-21, 2005 M. G. Stabin
Citation for the original published paper (version of record):
 http://www.diva-portal.org This is the published version of a paper published in EJNMMI Research. Citation for the original published paper (version of record): Andersson, M., Johansson, L., Eckerman,
http://www.diva-portal.org This is the published version of a paper published in EJNMMI Research. Citation for the original published paper (version of record): Andersson, M., Johansson, L., Eckerman,
Click Here to Continue. Click Here to Return to Table of Contents
 TechneScan Gluceptate Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions. Click Here to Continue Click Here to Return to Table
TechneScan Gluceptate Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions. Click Here to Continue Click Here to Return to Table
Downloaded from by guest on 18 November 2018
 Radiation Protection Dosimetry Vol. 105, No. 1 4, pp. 581 586 (2003) Published by Nuclear Technology Publishing 2003 Nuclear Technology Publishing ASSESSMENTS FOR HIGH DOSE RADIONUCLIDE THERAPY TREATMENT
Radiation Protection Dosimetry Vol. 105, No. 1 4, pp. 581 586 (2003) Published by Nuclear Technology Publishing 2003 Nuclear Technology Publishing ASSESSMENTS FOR HIGH DOSE RADIONUCLIDE THERAPY TREATMENT
Tracking Doses in the Pediatric Population
 Tracking Doses in the Pediatric Population Frederic H. Fahey DSc Boston Children s Hospital Harvard Medical School frederic.fahey@childrens.harvard.edu Disclosures Sadly, none that pay me any money! SNMMI
Tracking Doses in the Pediatric Population Frederic H. Fahey DSc Boston Children s Hospital Harvard Medical School frederic.fahey@childrens.harvard.edu Disclosures Sadly, none that pay me any money! SNMMI
Whole-body biodistribution and radiation dosimetry estimates for the β-amyloid radioligand [ 11 C]MeS-IMPY in non-human primates
![Whole-body biodistribution and radiation dosimetry estimates for the β-amyloid radioligand [ 11 C]MeS-IMPY in non-human primates Whole-body biodistribution and radiation dosimetry estimates for the β-amyloid radioligand [ 11 C]MeS-IMPY in non-human primates](/thumbs/87/95592963.jpg) Whole-body biodistribution and radiation dosimetry estimates for the β-amyloid radioligand [ 11 C]MeS-IMPY in non-human primates Molecular Imaging Branch, NIMH Bldg. 1 Rm. B3-10 September 6 th, 2006 The
Whole-body biodistribution and radiation dosimetry estimates for the β-amyloid radioligand [ 11 C]MeS-IMPY in non-human primates Molecular Imaging Branch, NIMH Bldg. 1 Rm. B3-10 September 6 th, 2006 The
INDICATIONS AND USAGE
 1. INDICATIONS AND USAGE a) Axumin is indicated for positron emission tomography (PET) in men with suspected prostate cancer recurrence based on elevated blood prostate specific antigen (PSA) levels following
1. INDICATIONS AND USAGE a) Axumin is indicated for positron emission tomography (PET) in men with suspected prostate cancer recurrence based on elevated blood prostate specific antigen (PSA) levels following
Sodium Fluoride F 18 Injection* PET/CT Imaging
 Sodium Fluoride F 18 Injection* PET/CT Imaging for Prostate Cancer Sodium Fluoride F 18 Injection* ( 18 F NaF) PET/CT Imaging of Bone Metastases in Prostate Cancer About Prostate Cancer According to the
Sodium Fluoride F 18 Injection* PET/CT Imaging for Prostate Cancer Sodium Fluoride F 18 Injection* ( 18 F NaF) PET/CT Imaging of Bone Metastases in Prostate Cancer About Prostate Cancer According to the
PHYSICAL CHARACTERISTICS
 BRACCO DIAGNOSTICS L/4739/0 1 CHOLETEC Kit for the Preparation of Technetium Tc 99m Mebrofenin For Diagnostic Use DESCRIPTION Each reaction vial contains a nonradioactive, sterile, nonpyrogenic mixture
BRACCO DIAGNOSTICS L/4739/0 1 CHOLETEC Kit for the Preparation of Technetium Tc 99m Mebrofenin For Diagnostic Use DESCRIPTION Each reaction vial contains a nonradioactive, sterile, nonpyrogenic mixture
Individualised Treatment Planning for Radionuclide therapy (Molecular Radiotherapy)
 Individualised Treatment Planning for Radionuclide therapy (Molecular Radiotherapy) FMU/ICRP workshop 2017 Glenn Flux Royal Marsden Hospital & Institute of Cancer Research Sutton UK ICRP Task Group 101
Individualised Treatment Planning for Radionuclide therapy (Molecular Radiotherapy) FMU/ICRP workshop 2017 Glenn Flux Royal Marsden Hospital & Institute of Cancer Research Sutton UK ICRP Task Group 101
Impact of ICRP-89 Based Models on Dose Estimates for Radiopharmaceuticals and CT Exams
 Impact of ICRP-89 Based Models on Dose Estimates for Radiopharmaceuticals and CT Exams Stabin MG, Kost SD, Clark JH, Pickens DR, Price RR, Carver DE Vanderbilt University Nashville, TN, USA 13th International
Impact of ICRP-89 Based Models on Dose Estimates for Radiopharmaceuticals and CT Exams Stabin MG, Kost SD, Clark JH, Pickens DR, Price RR, Carver DE Vanderbilt University Nashville, TN, USA 13th International
ICRP Perspective on Internal Dosimetry OIR and Radiopharmaceuticals
 ICRP Perspective on Internal Dosimetry OIR and Radiopharmaceuticals Dietmar Noßke dnosske@web.de 1 Disclaimer The information and views set out in this presentation are those of the author and do not necessarily
ICRP Perspective on Internal Dosimetry OIR and Radiopharmaceuticals Dietmar Noßke dnosske@web.de 1 Disclaimer The information and views set out in this presentation are those of the author and do not necessarily
Sodium Iodide I 131 Solution. Click Here to Continue. Click Here to Return to Table of Contents
 Sodium Iodide I 131 Solution Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions Click Here to Continue Click Here to Return to
Sodium Iodide I 131 Solution Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions Click Here to Continue Click Here to Return to
Quantitation of Cerebral Glucose Utilization using the Arterial Input Function or the Standardized Uptake Value (SUV)
 Quantitation of Cerebral Glucose Utilization using the Arterial Input Function or the Standardized Uptake Value (SUV) Brian R. Moyer BRMoyer & Associates, LLC, Amherst, NH 03031 http://www.brmassocllc-org.com/
Quantitation of Cerebral Glucose Utilization using the Arterial Input Function or the Standardized Uptake Value (SUV) Brian R. Moyer BRMoyer & Associates, LLC, Amherst, NH 03031 http://www.brmassocllc-org.com/
Austin Radiological Association Nuclear Medicine Procedure PET SODIUM FLUORIDE BONE SCAN (F-18 NaF)
 Austin Radiological Association Nuclear Medicine Procedure PET SODIUM FLUORIDE BONE SCAN (F-18 NaF) Overview Indication Sodium Fluoride F18 injection is a radioactive diagnostic agent for positron emission
Austin Radiological Association Nuclear Medicine Procedure PET SODIUM FLUORIDE BONE SCAN (F-18 NaF) Overview Indication Sodium Fluoride F18 injection is a radioactive diagnostic agent for positron emission
Lu-DOTATATE PRRT dosimetry:
 177 Lu-DOTATATE PRRT dosimetry: From theory to practice Silvano Gnesin Medical Physics department Institute of Radiation Physics, Lausanne University Hospital, Lausanne, Switzerland Gwennaëlle Marin Medical
177 Lu-DOTATATE PRRT dosimetry: From theory to practice Silvano Gnesin Medical Physics department Institute of Radiation Physics, Lausanne University Hospital, Lausanne, Switzerland Gwennaëlle Marin Medical
R: March, E D T P A. Kit for the Preparation of Technetium Tc 99m Pentetate Injection. DIAGNOSTIC - For Intravenous Use
 R: March, 1998 27227 0002E m TM DESCRIPTION D T P A Kit for the Preparation of Technetium Tc 99m Pentetate Injection DIAGNOSTIC - For Intravenous Use Each kit consists of reaction vials which contain the
R: March, 1998 27227 0002E m TM DESCRIPTION D T P A Kit for the Preparation of Technetium Tc 99m Pentetate Injection DIAGNOSTIC - For Intravenous Use Each kit consists of reaction vials which contain the
Page 1 of CONTRAINDICATIONS None (4)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use AXUMIN safely and effectively. See full prescribing information for AXUMIN. AXUMIN (fluciclovine
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use AXUMIN safely and effectively. See full prescribing information for AXUMIN. AXUMIN (fluciclovine
Journal of Radiation Research and Applied Sciences 8 (2015) 317e322. Available online at ScienceDirect
 Journal of Radiation Research and Applied Sciences 8 (2015) 317e322 HOSTED BY Available online at www.sciencedirect.com ScienceDirect Journal of Radiation Research and Applied Sciences journal homepage:
Journal of Radiation Research and Applied Sciences 8 (2015) 317e322 HOSTED BY Available online at www.sciencedirect.com ScienceDirect Journal of Radiation Research and Applied Sciences journal homepage:
Internal Doslmetry in Nuclear Medicine: A Summary of its Development, Applications and Current Limitations
 Internal Doslmetry in Nuclear Medicine: A Summary of its Development, Applications and Current Limitations by M. Lyra and P. Phinou Introduction In this article, a historical presentation of the development
Internal Doslmetry in Nuclear Medicine: A Summary of its Development, Applications and Current Limitations by M. Lyra and P. Phinou Introduction In this article, a historical presentation of the development
Austin Radiological Association Ga-68 NETSPOT (Ga-68 dotatate)
 Austin Radiological Association Ga-68 NETSPOT (Ga-68 dotatate) Overview Ga-68 dotatate binds to somatostatin receptors, with highest affinity for subtype 2 receptors (sstr2). It binds to cells that express
Austin Radiological Association Ga-68 NETSPOT (Ga-68 dotatate) Overview Ga-68 dotatate binds to somatostatin receptors, with highest affinity for subtype 2 receptors (sstr2). It binds to cells that express
Comparison of absorbed fraction of Gamma and Beta rays of I-124 and I-131radio-isotopes in thyroid gland with Monte Carlo Simulation
 Available online at http://www.ijabbr.com International journal of Advanced Biological and Biomedical Research Volume 1, Issue 9, 2013: 993-998 Comparison of absorbed fraction of Gamma and Beta rays of
Available online at http://www.ijabbr.com International journal of Advanced Biological and Biomedical Research Volume 1, Issue 9, 2013: 993-998 Comparison of absorbed fraction of Gamma and Beta rays of
Table 1 Principal Radiation Emission Data * Disintegration. Gamma Ka 1 X-ray
 DESCRIPTIONGeneralGlofil-125 (Sodium Iothalamate I-125 Injection) is a sterile, nonpyrogenic aqueous injection containing approximately 1 mg sodium iothalamate per ml, and 0.9 percent benzyl alcohol as
DESCRIPTIONGeneralGlofil-125 (Sodium Iothalamate I-125 Injection) is a sterile, nonpyrogenic aqueous injection containing approximately 1 mg sodium iothalamate per ml, and 0.9 percent benzyl alcohol as
Austin Radiological Association Nuclear Medicine Procedure THYROID UPTAKE MEASUREMENT (I-123 or I-131 as Sodium Iodide)
 Austin Radiological Association Nuclear Medicine Procedure THYROID UPTAKE MEASUREMENT (I-123 or I-131 as Sodium Iodide) Overview Indications The Thyroid Uptake Measurement measures the metabolic activity
Austin Radiological Association Nuclear Medicine Procedure THYROID UPTAKE MEASUREMENT (I-123 or I-131 as Sodium Iodide) Overview Indications The Thyroid Uptake Measurement measures the metabolic activity
Value of PET/CT in Initial Staging and Subsequent Treatment Strategy for Metastatic Breast Cancer
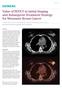 Case Study Value of PET/CT in Initial Staging and Subsequent Treatment Strategy for Metastatic Breast Cancer By Dustin Osborne, PhD, and Yong Bradley, MD, University of Tennessee, Knoxville, TN, USA Data
Case Study Value of PET/CT in Initial Staging and Subsequent Treatment Strategy for Metastatic Breast Cancer By Dustin Osborne, PhD, and Yong Bradley, MD, University of Tennessee, Knoxville, TN, USA Data
STANDARDIZED RADIOGENIC CANCER RISK COEFFICIENTS: A REVIEW OF THE METHODOLOGY PRESENTED IN FEDERAL GUIDANCE REPORT NO. 13
 STANDARDIZED RADIOGENIC CANCER RISK COEFFICIENTS: A REVIEW OF THE METHODOLOGY PRESENTED IN FEDERAL GUIDANCE REPORT NO. 13 ABSTRACT Michael Boyd and Christopher Nelson, U.S. Environmental Protection, Office
STANDARDIZED RADIOGENIC CANCER RISK COEFFICIENTS: A REVIEW OF THE METHODOLOGY PRESENTED IN FEDERAL GUIDANCE REPORT NO. 13 ABSTRACT Michael Boyd and Christopher Nelson, U.S. Environmental Protection, Office
Internal Dosimetry of Human Brain for 99m tc and 131 I Using Nuclear Imaging in Bangladesh
 Sri Lankan Journal of Physics, Vol. 6 (2005) 33-41 Institute of Physics - Sri Lanka Internal Dosimetry of Human Brain for 99m tc and 131 I Using Nuclear Imaging in Bangladesh M. M. Alam a, M. I. Kabir
Sri Lankan Journal of Physics, Vol. 6 (2005) 33-41 Institute of Physics - Sri Lanka Internal Dosimetry of Human Brain for 99m tc and 131 I Using Nuclear Imaging in Bangladesh M. M. Alam a, M. I. Kabir
To report suspected adverse reactions to Axumin, call AXUMIN1 ( ) or contact FDA at FDA-1088 or
 An Axumin scan can detect and localize recurrent prostate cancer Axumin is an FDA-approved diagnostic imaging agent, also known as a tracer, which may help your physician determine if and where your prostate
An Axumin scan can detect and localize recurrent prostate cancer Axumin is an FDA-approved diagnostic imaging agent, also known as a tracer, which may help your physician determine if and where your prostate
Detection of abscesses and infection in soft tissues, particularly in patients without localizing findings.
 NUCLEAR MEDICINE SERVICES SUBJECT: WHITE BLOOD CELL STUDY Overview Indications The White Blood Cell Study demonstrates the distribution of labeled autologous white blood cells within the body at various
NUCLEAR MEDICINE SERVICES SUBJECT: WHITE BLOOD CELL STUDY Overview Indications The White Blood Cell Study demonstrates the distribution of labeled autologous white blood cells within the body at various
Nuclear Medicine and PET. D. J. McMahon rev cewood
 Nuclear Medicine and PET D. J. McMahon 150504 rev cewood 2018-02-15 Key Points Nuclear Medicine and PET: Imaging: Understand how Nuc Med & PET differ from Radiography & CT by the source of radiation. Be
Nuclear Medicine and PET D. J. McMahon 150504 rev cewood 2018-02-15 Key Points Nuclear Medicine and PET: Imaging: Understand how Nuc Med & PET differ from Radiography & CT by the source of radiation. Be
Measurement of organ dose in abdomen-pelvis CT exam as a function of ma, KV and scanner type by Monte Carlo method
 Iran. J. Radiat. Res., 2004; 1(4): 187-194 Measurement of organ dose in abdomen-pelvis CT exam as a function of ma, KV and scanner type by Monte Carlo method M.R. Ay 1, M. Shahriari 2, S. Sarkar 3, P.
Iran. J. Radiat. Res., 2004; 1(4): 187-194 Measurement of organ dose in abdomen-pelvis CT exam as a function of ma, KV and scanner type by Monte Carlo method M.R. Ay 1, M. Shahriari 2, S. Sarkar 3, P.
FP/1-61//- p-o37 A mb. Precision Nuclear, LLC. July 7, 2011
 211 JUL Ili A II 5b Precision Nuclear, LLC July 7, 211 Amendment to Citizen Petition docket no. FDA-211-P-337-1/CP filed on 5/6/211 I, Jon L. McReynolds, PharmD, representing Precision Nuclear, LLC submit
211 JUL Ili A II 5b Precision Nuclear, LLC July 7, 211 Amendment to Citizen Petition docket no. FDA-211-P-337-1/CP filed on 5/6/211 I, Jon L. McReynolds, PharmD, representing Precision Nuclear, LLC submit
45 Hr PET Registry Review Course
 45 HR PET/CT REGISTRY REVIEW COURSE Course Control Document Timothy K. Marshel, MBA, R.T. (R), (N)(CT)(MR)(NCT)(PET)(CNMT) The PET/CT Training Institute, Inc. SNMMI-TS 028600-028632 45hr CEH s Voice Credits
45 HR PET/CT REGISTRY REVIEW COURSE Course Control Document Timothy K. Marshel, MBA, R.T. (R), (N)(CT)(MR)(NCT)(PET)(CNMT) The PET/CT Training Institute, Inc. SNMMI-TS 028600-028632 45hr CEH s Voice Credits
Skyscan 1076 in vivo scanning: X-ray dosimetry
 Skyscan 1076 in vivo scanning: X-ray dosimetry DOSIMETRY OF HIGH RESOLUTION IN VIVO RODENT MICRO-CT IMAGING WITH THE SKYSCAN 1076 An important distinction is drawn between local tissue absorbed dose in
Skyscan 1076 in vivo scanning: X-ray dosimetry DOSIMETRY OF HIGH RESOLUTION IN VIVO RODENT MICRO-CT IMAGING WITH THE SKYSCAN 1076 An important distinction is drawn between local tissue absorbed dose in
DRAXIMAGE SODIUM IODIDE I 131 CAPSULES, USP DIAGNOSTIC. For Oral Use DESCRIPTION
 DRAXIMAGE SODIUM IODIDE I 131 CAPSULES, USP DIAGNOSTIC For Oral Use DESCRIPTION Sodium Iodide I 131 Capsules, USP are color-coded capsules containing sodium iodide I 131 for diagnostic use by oral administration.
DRAXIMAGE SODIUM IODIDE I 131 CAPSULES, USP DIAGNOSTIC For Oral Use DESCRIPTION Sodium Iodide I 131 Capsules, USP are color-coded capsules containing sodium iodide I 131 for diagnostic use by oral administration.
Radiopharmacy. Prof. Dr. Çetin ÖNSEL. CTF Nükleer Tıp Anabilim Dalı
 Prof. Dr. Çetin ÖNSEL CTF Nükleer Tıp Anabilim Dalı What is Nuclear Medicine? Nuclear Medicine is the branch of medicine concerned with the use of radionuclides in the study and the diagnosis of diseases.
Prof. Dr. Çetin ÖNSEL CTF Nükleer Tıp Anabilim Dalı What is Nuclear Medicine? Nuclear Medicine is the branch of medicine concerned with the use of radionuclides in the study and the diagnosis of diseases.
Joint ICTP-IAEA Advanced School on Internal Dosimetry. Trieste, April 2010
 Joint ICTP-IAEA Advanced School on Internal Dosimetry Trieste, 12-16 April 2010 Dosimetry in PRRT: what for Dosimetry has the purpose to address %&!"# '!&!"#!(%)* $ *!$+ Most Used Radiopeptides for PRRT
Joint ICTP-IAEA Advanced School on Internal Dosimetry Trieste, 12-16 April 2010 Dosimetry in PRRT: what for Dosimetry has the purpose to address %&!"# '!&!"#!(%)* $ *!$+ Most Used Radiopeptides for PRRT
TABLE 1 Principal Radiation Emission Data Radiation Mean % per Disintegration Mean Energy (kev) Gamma
 KIT FOR THE PREPARATION OF TECHNETIUM TC 99M MEBROFENIN - mebrofenin injection, powder, lyophilized, for solution ---------- Kit for the Preparation of Technetium Tc 99m Mebrofenin DESCRIPTION Each multidose
KIT FOR THE PREPARATION OF TECHNETIUM TC 99M MEBROFENIN - mebrofenin injection, powder, lyophilized, for solution ---------- Kit for the Preparation of Technetium Tc 99m Mebrofenin DESCRIPTION Each multidose
Quantitative Theranostics in Nuclear Medicine
 Quantitative Theranostics in Nuclear Medicine M. Lassmann Klinik und Poliklinik für Nuklearmedizin Direktor: Prof. Dr. A. Buck Contents What is Theranostics? Potential Targets Basic Principles of Quantitative
Quantitative Theranostics in Nuclear Medicine M. Lassmann Klinik und Poliklinik für Nuklearmedizin Direktor: Prof. Dr. A. Buck Contents What is Theranostics? Potential Targets Basic Principles of Quantitative
Theragnostics for bone metastases. Glenn Flux Royal Marsden Hospital & Institute of Cancer Research Sutton UK
 Theragnostics for bone metastases Glenn Flux Royal Marsden Hospital & Institute of Cancer Research Sutton UK NPL 2015 Ra-223 Biodistribution & dosimetry Ra-223: 11.4 days half-life, range of 100 µm Six
Theragnostics for bone metastases Glenn Flux Royal Marsden Hospital & Institute of Cancer Research Sutton UK NPL 2015 Ra-223 Biodistribution & dosimetry Ra-223: 11.4 days half-life, range of 100 µm Six
CARDIAC PET PERFUSION IMAGING with RUBIDIUM-82
 CARDIAC PET PERFUSION IMAGING with RUBIDIUM-82 Pr Denis AGOSTINI Président du Groupe de Cardiologie Nucléaire et IRM CHU Caen Bordeaux 2006 Cardiac Perfusion-Metabolism Mismatch with PET Cumulative Survival
CARDIAC PET PERFUSION IMAGING with RUBIDIUM-82 Pr Denis AGOSTINI Président du Groupe de Cardiologie Nucléaire et IRM CHU Caen Bordeaux 2006 Cardiac Perfusion-Metabolism Mismatch with PET Cumulative Survival
Figure 3. DS02 point-wise kerma coefficients (see Table 2) and DS02 fine group kerma coefficients (see Table 5) for photons in soft tissue.
 841 Figure 3. DS02 point-wise kerma coefficients (see Table 2) and DS02 fine group kerma coefficients (see Table 5) for photons in soft tissue. by a factor of two depending on whether the location was
841 Figure 3. DS02 point-wise kerma coefficients (see Table 2) and DS02 fine group kerma coefficients (see Table 5) for photons in soft tissue. by a factor of two depending on whether the location was
THYROID IMAGING STUDY (Tc-99m as Sodium Pertechnetate)
 THYROID IMAGING STUDY (Tc-99m as Sodium Pertechnetate) Overview Indications The Thyroid Imaging Study with Tc-99m-pertechnetate demonstrates the distribution of tissues that take up anions. Such tissues
THYROID IMAGING STUDY (Tc-99m as Sodium Pertechnetate) Overview Indications The Thyroid Imaging Study with Tc-99m-pertechnetate demonstrates the distribution of tissues that take up anions. Such tissues
Application of 3D Printing to Molecular Radiotherapy Phantoms. Nick Calvert Nuclear Medicine Group The Christie NHS Foundation Trust, Manchester
 Application of 3D Printing to Molecular Radiotherapy Phantoms Nick Calvert Nuclear Medicine Group The Christie NHS Foundation Trust, Manchester Molecular Radiotherapy Radionuclide administered to patient
Application of 3D Printing to Molecular Radiotherapy Phantoms Nick Calvert Nuclear Medicine Group The Christie NHS Foundation Trust, Manchester Molecular Radiotherapy Radionuclide administered to patient
POSITRON EMISSION TOMOGRAPHY (PET)
 Status Active Medical and Behavioral Health Policy Section: Radiology Policy Number: V-27 Effective Date: 08/27/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members should
Status Active Medical and Behavioral Health Policy Section: Radiology Policy Number: V-27 Effective Date: 08/27/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members should
Nuclear Medicine: Manuals. Nuclear Medicine. Nuclear imaging. Emission imaging: study types. Bone scintigraphy - technique
 Nuclear Medicine - Unsealed radioactive preparations the tracer mixes with the patients body fluids on a molecular level (e.g. after intravenous injection) - 3 main fields: - In vitro : measuring concentrations
Nuclear Medicine - Unsealed radioactive preparations the tracer mixes with the patients body fluids on a molecular level (e.g. after intravenous injection) - 3 main fields: - In vitro : measuring concentrations
DRAXIMAGE SODIUM IODIDE I 131 SOLUTION USP DIAGNOSTIC. For Oral Use DESCRIPTION
 DRAXIMAGE SODIUM IODIDE I 131 SOLUTION USP DIAGNOSTIC For Oral Use DESCRIPTION Sodium Iodide I 131 Solution is an aqueous solution of sodium iodide I-131 for diagnostic use by oral administration. The
DRAXIMAGE SODIUM IODIDE I 131 SOLUTION USP DIAGNOSTIC For Oral Use DESCRIPTION Sodium Iodide I 131 Solution is an aqueous solution of sodium iodide I-131 for diagnostic use by oral administration. The
How to assess doses from internal emitters
 How to assess doses from internal emitters in Radiation Protection and Medicine PD Dr. Bastian Breustedt, Safety and Environment (SUM) KIT Die Forschungsuniversität in der Helmholtz-Gemeinschaft www.kit.edu
How to assess doses from internal emitters in Radiation Protection and Medicine PD Dr. Bastian Breustedt, Safety and Environment (SUM) KIT Die Forschungsuniversität in der Helmholtz-Gemeinschaft www.kit.edu
Society of Nuclear Medicine Procedure Guideline for Tumor Imaging Using F-18 FDG
 Society of Nuclear Medicine Procedure Guideline for Tumor Imaging Using F-18 FDG version 2.0, approved February 7, 1999 Authors: Heinrich R. Schelbert, MD, PhD (UCLA School of Medicine, Los Angeles, CA);
Society of Nuclear Medicine Procedure Guideline for Tumor Imaging Using F-18 FDG version 2.0, approved February 7, 1999 Authors: Heinrich R. Schelbert, MD, PhD (UCLA School of Medicine, Los Angeles, CA);
AN INTRODUCTION TO NUCLEAR MEDICINE
 AN INTRODUCTION TO NUCLEAR MEDICINE WITH RESPECT TO THYROID DISORDERS By: B.Shafiei MD Nuclear Physician Taleghani Medical Center Radioactive: An element with Unstable Nucleus (Excess Energy), can achieve
AN INTRODUCTION TO NUCLEAR MEDICINE WITH RESPECT TO THYROID DISORDERS By: B.Shafiei MD Nuclear Physician Taleghani Medical Center Radioactive: An element with Unstable Nucleus (Excess Energy), can achieve
ICRP Recommendations Evolution or Revolution? John R Cooper Main Commission
 ICRP Recommendations Evolution or Revolution? John R Cooper Main Commission 3 September 2009 ICRP Recommendations 1. Reasons for new Recommendations 2. Summary of health risks 3. Summary of changes to
ICRP Recommendations Evolution or Revolution? John R Cooper Main Commission 3 September 2009 ICRP Recommendations 1. Reasons for new Recommendations 2. Summary of health risks 3. Summary of changes to
Estimated cumulative radiation dose from PET/CT in children with malignancies: a 5-year retrospective review
 Pediatr Radiol (1) :681 686 DOI 1.17/s247-9-1434-z ORIGINAL ARTICLE Estimated cumulative radiation dose from PET/CT in children with malignancies: a 5-year retrospective review Soni C. Chawla & Noah Federman
Pediatr Radiol (1) :681 686 DOI 1.17/s247-9-1434-z ORIGINAL ARTICLE Estimated cumulative radiation dose from PET/CT in children with malignancies: a 5-year retrospective review Soni C. Chawla & Noah Federman
Amyloid plaques are one of the pathologic hallmarks of
 Radiation Dosimetry of b-amyloid Tracers C-PiB and F-BAY94-9172 Graeme J. O Keefe 1, Timothy H. Saunder 1, Steven Ng 1, Uwe Ackerman 1, Henri J. Tochon-Danguy 1, J. Gordon Chan 1, Sylvia Gong 1, Thomas
Radiation Dosimetry of b-amyloid Tracers C-PiB and F-BAY94-9172 Graeme J. O Keefe 1, Timothy H. Saunder 1, Steven Ng 1, Uwe Ackerman 1, Henri J. Tochon-Danguy 1, J. Gordon Chan 1, Sylvia Gong 1, Thomas
Calculation methods in Hermes Medical Solutions dosimetry software
 Calculation methods in Hermes Medical Solutions dosimetry software Helena McMeekin MSc. Clinical Applications Scientist, Hermes Medical Solutions MRTDosimetry Scientific Workshop The Principals and Clinical
Calculation methods in Hermes Medical Solutions dosimetry software Helena McMeekin MSc. Clinical Applications Scientist, Hermes Medical Solutions MRTDosimetry Scientific Workshop The Principals and Clinical
Ibritumomab tiuxetan (Zevalin; Cell Therapeutics, Inc.)
 MIRD Dose Estimate Report No. 20: Radiation Absorbed-Dose Estimates for In- and Y-Ibritumomab Tiuxetan Darrell R. Fisher 1, Sui Shen 2, and Ruby F. Meredith 2 1 Radioisotopes Program, Pacific Northwest
MIRD Dose Estimate Report No. 20: Radiation Absorbed-Dose Estimates for In- and Y-Ibritumomab Tiuxetan Darrell R. Fisher 1, Sui Shen 2, and Ruby F. Meredith 2 1 Radioisotopes Program, Pacific Northwest
Core SPC for Fludeoxyglucose ( 18 F) March 2005
 Core SPC for Fludeoxyglucose ( 18 F) March 2005 This FDG Core SPC has been prepared on the basis, and taking into account the available published scientific literature dated from more than 10 years. Then
Core SPC for Fludeoxyglucose ( 18 F) March 2005 This FDG Core SPC has been prepared on the basis, and taking into account the available published scientific literature dated from more than 10 years. Then
Comparison of organ doses estimations in radiology with PCXMC application based on MIRD phantoms and CALDose-X application based on voxel phantoms
 Comparison of organ doses estimations in radiology with PCXMC application based on MIRD phantoms and CALDose-X application based on voxel phantoms G. Gialousis 1, Z. Pappouli 2, A. Dimitriadis 2, E. Karavassilis
Comparison of organ doses estimations in radiology with PCXMC application based on MIRD phantoms and CALDose-X application based on voxel phantoms G. Gialousis 1, Z. Pappouli 2, A. Dimitriadis 2, E. Karavassilis
Radiation Doses in Radiology: Influence of Standards and Regulations
 Radiation Doses in Radiology: Influence of Standards and Regulations Beebe Symposium National Academy of Sciences December 9, 2009 Washington D.C. Orhan H Suleiman MS PhD, FAAPM Senior Science Policy Adviser
Radiation Doses in Radiology: Influence of Standards and Regulations Beebe Symposium National Academy of Sciences December 9, 2009 Washington D.C. Orhan H Suleiman MS PhD, FAAPM Senior Science Policy Adviser
Internal dosimetry for radioembolization therapy with Yttrium-90 microspheres
 Received: 13 July 2016 Accepted: 21 December 2016 DOI: 10.1002/acm2.12042 MEDICAL IMAGING Internal dosimetry for radioembolization therapy with Yttrium-90 microspheres Maryam Fallahpoor 1 Mehrshad Abbasi
Received: 13 July 2016 Accepted: 21 December 2016 DOI: 10.1002/acm2.12042 MEDICAL IMAGING Internal dosimetry for radioembolization therapy with Yttrium-90 microspheres Maryam Fallahpoor 1 Mehrshad Abbasi
Nuclear medicine dosimetry
 INSTITUTE OF PHYSICS PUBLISHING Phys. Med. Biol. 51 (2006) R187 R202 PHYSICS IN MEDICINE AND BIOLOGY doi:10.1088/0031-9155/51/13/r12 REVIEW Nuclear medicine dosimetry Michael Stabin Department of Radiology
INSTITUTE OF PHYSICS PUBLISHING Phys. Med. Biol. 51 (2006) R187 R202 PHYSICS IN MEDICINE AND BIOLOGY doi:10.1088/0031-9155/51/13/r12 REVIEW Nuclear medicine dosimetry Michael Stabin Department of Radiology
Michael Lassmann, PhD, and S. Ted Treves, MD, for the EANM/SNMMI pediatric dosage harmonization working group*
 Pediatric Radiopharmaceutical Administration: Harmonization of the 2007 EANM Paediatric Dosage Card (Version 1.5.2008) and the 2010 North America Consensus guideline Michael Lassmann, PhD, and S. Ted Treves,
Pediatric Radiopharmaceutical Administration: Harmonization of the 2007 EANM Paediatric Dosage Card (Version 1.5.2008) and the 2010 North America Consensus guideline Michael Lassmann, PhD, and S. Ted Treves,
Biokinetics and radiation dosimetry for [4-14 C] cholesterol in humans
![Biokinetics and radiation dosimetry for [4-14 C] cholesterol in humans Biokinetics and radiation dosimetry for [4-14 C] cholesterol in humans](/thumbs/87/96641951.jpg) NUKLEONIKA 2012;57(4):607 613 ORIGINAL PAPER Biokinetics and radiation dosimetry for [4-14 C] cholesterol in humans Larissa A. Marcato, Margarida M. Hamada, Carlos H. de Mesquita Abstract. This study proposes
NUKLEONIKA 2012;57(4):607 613 ORIGINAL PAPER Biokinetics and radiation dosimetry for [4-14 C] cholesterol in humans Larissa A. Marcato, Margarida M. Hamada, Carlos H. de Mesquita Abstract. This study proposes
Coronary artery disease is a major cause of death in
 Phase I, First-in-Human Study of BMS747158, a Novel F-Labeled Tracer for Myocardial Perfusion PET: Dosimetry, Biodistribution, Safety, and Imaging Characteristics After a Single Injection at Rest Jamshid
Phase I, First-in-Human Study of BMS747158, a Novel F-Labeled Tracer for Myocardial Perfusion PET: Dosimetry, Biodistribution, Safety, and Imaging Characteristics After a Single Injection at Rest Jamshid
Dosimetry (Dose Estimation) of Internal Emitters. Outline. For Radiation Effects, is Dose the only Answer? Estimation of Dose and not Dosimetry
 Dosimetry (Dose Estimation) of Internal Emitters. Lawrence E. Williams, PhD City of Hope National Medical Center Duarte CA 91010 lwilliams@coh.org Outline 1. Dose Estimation Formula D = S*Ã 2. Determination
Dosimetry (Dose Estimation) of Internal Emitters. Lawrence E. Williams, PhD City of Hope National Medical Center Duarte CA 91010 lwilliams@coh.org Outline 1. Dose Estimation Formula D = S*Ã 2. Determination
Austin Radiological Association Nuclear Medicine Procedure WHITE BLOOD CELL MIGRATION STUDY (In-111-WBCs, Tc-99m-HMPAO-WBCs)
 Austin Radiological Association Nuclear Medicine Procedure WHITE BLOOD CELL MIGRATION STUDY (In-111-WBCs, Tc-99m-HMPAO-WBCs) Overview Indications The White Blood Cell Migration Study demonstrates the distribution
Austin Radiological Association Nuclear Medicine Procedure WHITE BLOOD CELL MIGRATION STUDY (In-111-WBCs, Tc-99m-HMPAO-WBCs) Overview Indications The White Blood Cell Migration Study demonstrates the distribution
Summary of Patient Release after Radioiodine Therapy Research Review
 Summary of Patient Release after Radioiodine Therapy Research Review Introduction This report provides a summary of the Office of Research (RES) staff s efforts to evaluate radiation exposure to members
Summary of Patient Release after Radioiodine Therapy Research Review Introduction This report provides a summary of the Office of Research (RES) staff s efforts to evaluate radiation exposure to members
KEYWORDS: nuclear medicine; gamma camera; radiopharmaceutical activities.
 Radiopharmaceutical Activities Administered for Diagnostic Procedures in Nuclear Medicine in the First Six Months of the Gamma Camera Use in the Clinical Center of Montenegro - Podgorica Nevenka Antovic
Radiopharmaceutical Activities Administered for Diagnostic Procedures in Nuclear Medicine in the First Six Months of the Gamma Camera Use in the Clinical Center of Montenegro - Podgorica Nevenka Antovic
The sentinel lymph node (SLN) technique has proven
 and Fetal Absorbed Dose Estimates from 99m Tc-Sulfur Colloid Lymphoscintigraphy and Sentinel Node Localization in Breast Cancer Patients Neeta Pandit-Taskar 1, Lawrence T. Dauer 2, Leslie Montgomery 3,
and Fetal Absorbed Dose Estimates from 99m Tc-Sulfur Colloid Lymphoscintigraphy and Sentinel Node Localization in Breast Cancer Patients Neeta Pandit-Taskar 1, Lawrence T. Dauer 2, Leslie Montgomery 3,
RADIONUCLIDE THERAPY FOR PALLIATION OF PAIN FROM BONY METASTASES
 RADIONUCLIDE THERAPY FOR PALLIATION OF PAIN FROM BONY METASTASES Overview and Topics to Be Covered Reviews the use of radionuclide therapy in nuclear medicine for palliation of pain due to bony metastases.
RADIONUCLIDE THERAPY FOR PALLIATION OF PAIN FROM BONY METASTASES Overview and Topics to Be Covered Reviews the use of radionuclide therapy in nuclear medicine for palliation of pain due to bony metastases.
VASCULOCIS 10 mg kit for radiopharmaceutical preparation
 VASCULOCIS 10 mg kit for radiopharmaceutical preparation SUMMARY OF PRODUCT CHARACTERISTICS T0200nH 12/2012 CIS bio international, Member of IBA Molecular group of companies 1. NAME OF THE MEDICINAL PRODUCT
VASCULOCIS 10 mg kit for radiopharmaceutical preparation SUMMARY OF PRODUCT CHARACTERISTICS T0200nH 12/2012 CIS bio international, Member of IBA Molecular group of companies 1. NAME OF THE MEDICINAL PRODUCT
Physical Bases : Which Isotopes?
 Physical Bases : Which Isotopes? S. Gnesin Institute of Radiation Physics, Lausanne University Hospital, Lausanne, Switzerland 1/53 Theranostic Bruxelles, 2 Octobrer 2017 Theranostic : use of diagnostic
Physical Bases : Which Isotopes? S. Gnesin Institute of Radiation Physics, Lausanne University Hospital, Lausanne, Switzerland 1/53 Theranostic Bruxelles, 2 Octobrer 2017 Theranostic : use of diagnostic
SUMMARY OF PRODUCT CHARACTERISTICS. for. BRIDATEC, kit for radiopharmaceutical preparation
 February 9, 2010 SUMMARY OF PRODUCT CHARACTERISTICS for BRIDATEC, kit for radiopharmaceutical preparation 1. NAME OF THE MEDICINAL PRODUCT BRIDATEC 2. QUALITATIVE AND QUANTITATIVE COMPOSITION N-(3-bromo-2,4,6-trimethylphenylcarbamoyl
February 9, 2010 SUMMARY OF PRODUCT CHARACTERISTICS for BRIDATEC, kit for radiopharmaceutical preparation 1. NAME OF THE MEDICINAL PRODUCT BRIDATEC 2. QUALITATIVE AND QUANTITATIVE COMPOSITION N-(3-bromo-2,4,6-trimethylphenylcarbamoyl
Austin Radiological Association Nuclear Medicine Procedure PROSTATE CANCER STUDY (In-111-Capromab Pendetide [ProstaScint ])
![Austin Radiological Association Nuclear Medicine Procedure PROSTATE CANCER STUDY (In-111-Capromab Pendetide [ProstaScint ]) Austin Radiological Association Nuclear Medicine Procedure PROSTATE CANCER STUDY (In-111-Capromab Pendetide [ProstaScint ])](/thumbs/81/82771892.jpg) Austin Radiological Association Nuclear Medicine Procedure PROSTATE CANCER STUDY (In-111-Capromab Pendetide [ProstaScint ]) Overview Indications The Prostate Cancer Study with an indium-111 labeled murine
Austin Radiological Association Nuclear Medicine Procedure PROSTATE CANCER STUDY (In-111-Capromab Pendetide [ProstaScint ]) Overview Indications The Prostate Cancer Study with an indium-111 labeled murine
Molecular Imaging and the Brain
 Molecular imaging technologies are playing an important role in neuroimaging, a branch of medical imaging, by providing a window into the living brain. Where CT and conventional MR imaging provide important
Molecular imaging technologies are playing an important role in neuroimaging, a branch of medical imaging, by providing a window into the living brain. Where CT and conventional MR imaging provide important
Clinical Implementation of patient-specific dosimetry, comparison with absorbed fraction-based method
 Clinical Implementation of patient-specific dosimetry, comparison with absorbed fraction-based method George Sgouros, Ph.D. Russell H. Morgan Dept of Radiology & Radiological Science Johns Hopkins University,
Clinical Implementation of patient-specific dosimetry, comparison with absorbed fraction-based method George Sgouros, Ph.D. Russell H. Morgan Dept of Radiology & Radiological Science Johns Hopkins University,
Radionuclides in Medical Imaging. Danielle Wilson
 Radionuclides in Medical Imaging Danielle Wilson Outline Definitions History and development Radionuclide applications & techniques in imaging Conclusion Definition #1 : Radionuclide An unstable nucleus
Radionuclides in Medical Imaging Danielle Wilson Outline Definitions History and development Radionuclide applications & techniques in imaging Conclusion Definition #1 : Radionuclide An unstable nucleus
Option D: Medicinal Chemistry
 Option D: Medicinal Chemistry Basics - unstable radioactive nuclei emit radiation in the form of smaller particles alpha, beta, positron, proton, neutron, & gamma are all used in nuclear medicine unstable
Option D: Medicinal Chemistry Basics - unstable radioactive nuclei emit radiation in the form of smaller particles alpha, beta, positron, proton, neutron, & gamma are all used in nuclear medicine unstable
EANM Procedure Guideline For Therapy with Iodine-131
 EANM Procedure Guideline For Therapy with Iodine-131 I. PURPOSE The purpose of this guideline is to assist nuclear medicine practitioners in 1. evaluating patients who might be candidates for therapy with
EANM Procedure Guideline For Therapy with Iodine-131 I. PURPOSE The purpose of this guideline is to assist nuclear medicine practitioners in 1. evaluating patients who might be candidates for therapy with
Unusual Radiation Safety Issues in Nuclear Medicine From Alphas to Fetuses
 Unusual Radiation Safety Issues in Nuclear Medicine From Alphas to Fetuses 59 th Annual Meeting Southwestern Chapter Society of Nuclear Medicine and Molecular Imaging Stephen Lokitz Center for Molecular
Unusual Radiation Safety Issues in Nuclear Medicine From Alphas to Fetuses 59 th Annual Meeting Southwestern Chapter Society of Nuclear Medicine and Molecular Imaging Stephen Lokitz Center for Molecular
INTRACAVITARY THERAPY FOR FLUID OR NEOPLASM (P-32 as Chromic Phosphate Colloid)
 Overview Indications INTRACAVITARY THERAPY FOR FLUID OR NEOPLASM (P-32 as Chromic Phosphate Colloid) P-32 radiocolloids may be injected into body cavities that are lined with metastases that are producing
Overview Indications INTRACAVITARY THERAPY FOR FLUID OR NEOPLASM (P-32 as Chromic Phosphate Colloid) P-32 radiocolloids may be injected into body cavities that are lined with metastases that are producing
Reevaluation of the Standardized Uptake Value for FDG: Variations with Body Weight and Methods for Correction 1
 Nuclear Medicine Yoshifumi Sugawara, MD Kenneth R. Zasadny, PhD Alex W. Neuhoff, BS Richard L. Wahl, MD Index terms: Breast neoplasms, PET, 00.12163, 00.32 Breast neoplasms, radionuclide studies, 00.12163,
Nuclear Medicine Yoshifumi Sugawara, MD Kenneth R. Zasadny, PhD Alex W. Neuhoff, BS Richard L. Wahl, MD Index terms: Breast neoplasms, PET, 00.12163, 00.32 Breast neoplasms, radionuclide studies, 00.12163,
