FDA Approves New Novo Nordisk Treatment for Patients with Hemophilia
|
|
|
- Adele Wright
- 5 years ago
- Views:
Transcription
1 FDA Approves New Novo Nordisk Treatment for Patients with Hemophilia PLAINSBORO, N.J., May 31, 2017 Novo Nordisk today announced that the U.S. Food and Drug Administration (FDA) has approved the Biologics License Application for REBINYN (Coagulation Factor IX (Recombinant), GlycoPEGylated) for the treatment of adults and children with hemophilia B. Hemophilia B is a chronic and inherited bleeding disorder that affects approximately 5,000 people in the U.S. 1 People with hemophilia B have deficient blood clotting factor IX activity that results in prolonged or spontaneous bleeding, especially into the muscles, joints or internal organs. 2 REBINYN is the brand name for nonacog beta pegol, N9-GP. REBINYN is indicated for on-demand treatment and control of bleeding episodes, and the perioperative management of bleeding in adults and children with hemophilia B. REBINYN is not indicated for routine prophylaxis or for immune tolerance induction in patients with hemophilia B. The efficacy and safety evaluation was based on 115 patients across the four paradigm TM clinical trials, and the approval follows the Blood Products Advisory Committee meeting held on April 4, We would like to thank the patients who participated in the clinical studies that led to this decision. Thanks to their commitment, we are able to continue to provide new medicines for people with hemophilia, said Bill Breitenbach, Vice President, Biopharmaceuticals Portfolio, Novo Nordisk. We are committed to the hemophilia community and will continue on our path to bring this new extended half-life treatment to patients who need it. Novo Nordisk expects to launch REBINYN in the U.S. in the first half of Patient Product Information REBINYN (reh-bē-nine) Coagulation Factor IX (Recombinant), GlycoPEGylated Read the Patient Product Information and the Instructions For Use that come with REBINYN before you start taking this medicine and each time you get a refill. There may be new information. This Patient Product Information does not take the place of talking with your healthcare provider about your medical condition or treatment. If you have questions about REBINYN after reading this information, ask your healthcare provider. What is the most important information I need to know about REBINYN? Do not attempt to do an infusion yourself unless you have been taught how by your healthcare provider or hemophilia treatment center.
2 Page 2 of 6 You must carefully follow your healthcare provider's instructions regarding the dose and schedule for infusing REBINYN so that your treatment will work best for you. What is REBINYN? REBINYN is an injectable medicine used to replace clotting Factor IX that is missing in patients with hemophilia B. Hemophilia B is an inherited bleeding disorder in all age groups that prevents blood from clotting normally. REBINYN is used to treat and control bleeding in people with hemophilia B. Your healthcare provider may give you REBINYN when you have surgery. Who should not use REBINYN? You should not use REBINYN if you are allergic to Factor IX or any of the other ingredients of REBINYN if you are allergic to hamster proteins If you are not sure, talk to your healthcare provider before using this medicine. Tell your healthcare provider if you are pregnant or nursing because REBINYN might not be right for you. What should I tell my healthcare provider before I use REBINYN? You should tell your healthcare provider if you Have or have had any medical conditions. Take any medicines, including non-prescription medicines and dietary supplements. Are nursing. Are pregnant or planning to become pregnant. Have been told that you have inhibitors to Factor IX. How should I use REBINYN? Treatment with REBINYN should be started by a healthcare provider who is experienced in the care of patients with hemophilia B. REBINYN is given as an infusion into the vein. You may infuse REBINYN at a hemophilia treatment center, at your healthcare provider's office or in your home. You should be trained on how to do infusions by your hemophilia treatment center or healthcare provider. Many people with hemophilia B learn to infuse the medicine by themselves or with the help of a family member. Your healthcare provider will tell you how much REBINYN to use based on your weight, the severity of your hemophilia B, and where you are bleeding. Your dose will be calculated in international units, IU.
3 Page 3 of 6 Call your healthcare provider right away if your bleeding does not stop after taking REBINYN. If your bleeding is not adequately controlled, it could be due to the development of Factor IX inhibitors. This should be checked by your healthcare provider. You might need a higher dose of REBINYN or even a different product to control bleeding. Do not increase the total dose of REBINYN to control your bleeding without consulting your healthcare provider. Use in children REBINYN can be used in children. Your healthcare provider will decide the dose of REBINYN you will receive. If you forget to use REBINYN If you forget a dose, infuse the missed dose when you discover the mistake. Do not infuse a double dose to make up for a forgotten dose. Proceed with the next infusions as scheduled and continue as advised by your healthcare provider. If you stop using REBINYN Do not stop using REBINYN without consulting your healthcare provider. If you have any further questions on the use of this product, ask your healthcare provider. What if I take too much REBINYN? Always take REBINYN exactly as your healthcare provider has told you. You should check with your healthcare provider if you are not sure. If you infuse more REBINYN than recommended, tell your healthcare provider as soon as possible. What are the possible side effects of REBINYN? Common Side Effects Include: swelling, pain, rash or redness at the location of infusion itching Other Possible Side Effects: You could have an allergic reaction to coagulation Factor IX products. Call your healthcare provider right away or get emergency treatment right away if you get any of the following signs of an allergic reaction: hives, chest tightness, wheezing, difficulty breathing, and/or swelling of the face. Your body can also make antibodies called inhibitors against REBINYN, which may stop REBINYN from working properly. Your healthcare provider may need to test your blood for inhibitors from time to time.
4 Page 4 of 6 You may be at an increased risk of forming blood clots in your body, especially if you have risk factors for developing blood clots. Call your healthcare provider if you have chest pain, difficulty breathing, leg tenderness or swelling. These are not all of the possible side effects from REBINYN. Ask your healthcare provider for more information. You are encouraged to report side effects to FDA at FDA Tell your healthcare provider about any side effect that bothers you or that does not go away. What are the REBINYN dosage strengths? REBINYN comes in three different dosage strengths. The actual number of international units (IU) of Factor IX in the vial will be imprinted on the label and on the box. The three different strengths are as follows: Cap Color Indicator Red Green Yellow Nominal Strength 500 IU per vial 1000 IU per vial 2000 IU per vial Always check the actual dosage strength printed on the label to make sure you are using the strength prescribed by your healthcare provider. How should I store REBINYN? Prior to Reconstitution (mixing the dry powder in the vial with the diluent): Store in original package in order to protect from light. Do not freeze REBINYN. REBINYN vials can be stored in the refrigerator (36-46 F [2 C 8 C]) for up to 24 months until the expiration date, or at room temperature (up to 86 F [30 C]) for a single period not more than 6 months. If you choose to store REBINYN at room temperature: Note the date that the product is removed from refrigeration on the box. The total time of storage at room temperature should not be more than 6 months. Do not return the product to the refrigerator. Do not use after 6 months from this date or the expiration date listed on the vial, whichever is earlier. Do not use this medicine after the expiration date which is on the outer carton and the vial. The expiration date refers to the last day of that month. After Reconstitution: The reconstituted (the final product once the powder is mixed with the diluent) REBINYN should appear clear without visible particles.
5 Page 5 of 6 The reconstituted REBINYN should be used immediately. If you cannot use the reconstituted REBINYN immediately, it should be used within 4 hours when stored at or below 86ºF (30 C). Store the reconstituted product in the vial. Keep this medicine out of the sight and out of reach of children. What else should I know about REBINYN and hemophilia B? Medicines are sometimes prescribed for purposes other than those listed here. Do not use REBINYN for a condition for which it is not prescribed. Do not share REBINYN with other people, even if they have the same symptoms that you have. For more information about REBINYN, please call Novo Nordisk at REB- INYN. Revised: 05/2017 REBINYN is a trademark of Novo Nordisk A/S. For Patent Information, refer to: Novo Nordisk Manufactured by: Novo Nordisk A/S Novo Allé DK-2880 Bagsværd, Denmark For information about REBINYN contact: Novo Nordisk Inc. 800 Scudders Mill Road Plainsboro, NJ 08536, USA Please click here for full REBINYN Prescribing Information. About Novo Nordisk Novo Nordisk is a global healthcare company with more than 90 years of innovation and leadership in diabetes care. This heritage has given us experience and capabilities that also enable us to help people defeat other serious chronic conditions: hemophilia, growth disorders and obesity. With U.S. headquarters in Plainsboro, N.J., Novo Nordisk Inc. has nearly 5,000 employees in the United States. For more information, visit novonordisk.us or follow us on References 1. Community Counts: The HTC Population Profile. Available at:
6 Page 6 of 6 c-population-profile-report-5-rev2_508compliant.pdf. Accessed May 31, Konkle BA, Josephson NC, Nakaya Fletcher S. Hemophilia B Oct 2 [Updated 2014 Jun 5]. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews [Internet]. Seattle (WA): University of Washington, Seattle; Available from: 3. REBINYN [package insert]. Plainsboro, NJ: Novo Nordisk Inc; May Further information Media: Marisa Sharkey mzsx@novonordisk.com Investors: Kasper Veje kpvj@novonordisk.com Novo Nordisk is a registered trademark of Novo Nordisk A/S Novo Nordisk All rights reserved. USA17BIO01874 May 2017
Instructional Guide RECONSTITUTION OF IDELVION USING THE MIX2VIAL TRANSFER SET
 Instructional Guide RECONSTITUTION OF IDELVION USING THE MIX2VIAL TRANSFER SET The only FDA-approved treatment for hemophilia B with up to 14-day dosing* * In appropriate people 12 years and older. Talk
Instructional Guide RECONSTITUTION OF IDELVION USING THE MIX2VIAL TRANSFER SET The only FDA-approved treatment for hemophilia B with up to 14-day dosing* * In appropriate people 12 years and older. Talk
Before you use NovoThirteen. When NovoThirteen should not be used
 NovoThirteen 2500 U catridecacog (rys) Recombinant coagulation factor X Consumer Medicine nformation What is in this leaflet What NovoThirteen is used for 1 Before you use NovoThirteen 1 Using NovoThirteen...
NovoThirteen 2500 U catridecacog (rys) Recombinant coagulation factor X Consumer Medicine nformation What is in this leaflet What NovoThirteen is used for 1 Before you use NovoThirteen 1 Using NovoThirteen...
Recombinant Treatments for Bleeding Disorders. An overview of treatments that are considered to have a low risk of viral transmission
 Recombinant Treatments for Bleeding Disorders An overview of treatments that are considered to have a low risk of viral transmission History of bleeding disorder treatments Recombinant products: A significant
Recombinant Treatments for Bleeding Disorders An overview of treatments that are considered to have a low risk of viral transmission History of bleeding disorder treatments Recombinant products: A significant
IMPORTANT: PLEASE READ
 PART III: CONSUMER INFORMATION ALPROLIX [pronounced all prō liks] Coagulation Factor IX (Recombinant), Fc Fusion Protein This leaflet is part III of a three-part "Product Monograph" published when ALPROLIX
PART III: CONSUMER INFORMATION ALPROLIX [pronounced all prō liks] Coagulation Factor IX (Recombinant), Fc Fusion Protein This leaflet is part III of a three-part "Product Monograph" published when ALPROLIX
IMPORTANT: PLEASE READ
 PART III: CONSUMER INFORMATION ALPROLIX [pronounced all prō liks] Coagulation Factor IX (Recombinant), Fc Fusion Protein This leaflet is part III of a three-part "Product Monograph" published when ALPROLIX
PART III: CONSUMER INFORMATION ALPROLIX [pronounced all prō liks] Coagulation Factor IX (Recombinant), Fc Fusion Protein This leaflet is part III of a three-part "Product Monograph" published when ALPROLIX
Important News Regarding Helixate FS, Antihemophilic Factor (Recombinant):
 Important News Regarding Helixate FS, Antihemophilic Factor (Recombinant): Availability and what comes next Please see Important Safety Information on pages 10 11 and accompanying full prescribing information,
Important News Regarding Helixate FS, Antihemophilic Factor (Recombinant): Availability and what comes next Please see Important Safety Information on pages 10 11 and accompanying full prescribing information,
Package leaflet: Information for the user
 Package leaflet: Information for the user ADVATE 250 IU powder and solvent for solution for injection ADVATE 500 IU powder and solvent for solution for injection ADVATE 1000 IU powder and solvent for solution
Package leaflet: Information for the user ADVATE 250 IU powder and solvent for solution for injection ADVATE 500 IU powder and solvent for solution for injection ADVATE 1000 IU powder and solvent for solution
RMHBDA in Washington. Education Weekend RMHBDA Newsletter
 spring/summer 2018 RMHBDA Newsletter RMHBDA is a 501(c)(3) nonprofit organization founded in 2000 and is a chartered chapter of the National Hemophilia Foundation. Education Weekend 2018 Our mission is
spring/summer 2018 RMHBDA Newsletter RMHBDA is a 501(c)(3) nonprofit organization founded in 2000 and is a chartered chapter of the National Hemophilia Foundation. Education Weekend 2018 Our mission is
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICATION PATIENT MEDICATION INFORMATION. KOVALTRY Antihemophilic Factor (Recombinant)
 READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICATION PATIENT MEDICATION INFORMATION Pr KOVALTRY Antihemophilic Factor (Recombinant) Read this carefully before you start taking KOVALTRY and each time
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICATION PATIENT MEDICATION INFORMATION Pr KOVALTRY Antihemophilic Factor (Recombinant) Read this carefully before you start taking KOVALTRY and each time
Package leaflet: Information for the patient. NEGABAN 1g, powder for solution for injection or infusion Temocillin
 Package leaflet: Information for the patient NEGABAN 1g, powder for solution for injection or infusion Temocillin Read all of this leaflet carefully before you start using this medicine because it contains
Package leaflet: Information for the patient NEGABAN 1g, powder for solution for injection or infusion Temocillin Read all of this leaflet carefully before you start using this medicine because it contains
Package leaflet: Information for the user. RIXUBIS 500 IU powder and solvent for solution for injection
 Package leaflet: Information for the user RIXUBIS 250 IU powder and solvent for solution for injection RIXUBIS 500 IU powder and solvent for solution for injection RIXUBIS 1000 IU powder and solvent for
Package leaflet: Information for the user RIXUBIS 250 IU powder and solvent for solution for injection RIXUBIS 500 IU powder and solvent for solution for injection RIXUBIS 1000 IU powder and solvent for
Package leaflet: Information for the user. GlucaGen HypoKit 1 mg Powder and solvent for solution for injection Glucagon
 Package leaflet: Information for the user GlucaGen HypoKit 1 mg Powder and solvent for solution for injection Glucagon Read all of this leaflet carefully before you are given this injection because it
Package leaflet: Information for the user GlucaGen HypoKit 1 mg Powder and solvent for solution for injection Glucagon Read all of this leaflet carefully before you are given this injection because it
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PART III: PATIENT MEDICATION INFORMATION. ixekizumab
 READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PART III: PATIENT MEDICATION INFORMATION Pr TALTZ ixekizumab www.lilly.ca Lilly Read this carefully before you start taking TALTZ and each time you
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PART III: PATIENT MEDICATION INFORMATION Pr TALTZ ixekizumab www.lilly.ca Lilly Read this carefully before you start taking TALTZ and each time you
STEP-BY-STEP GUIDE TO SELF-INFUSION. Subcutaneous Administration of GAMMAGARD LIQUID
 STEP-BY-STEP GUIDE TO SELF-INFUSION Subcutaneous Administration of GAMMAGARD LIQUID This handy guide will help you manage your subcutaneous administration of GAMMAGARD LIQUID. If you have questions on
STEP-BY-STEP GUIDE TO SELF-INFUSION Subcutaneous Administration of GAMMAGARD LIQUID This handy guide will help you manage your subcutaneous administration of GAMMAGARD LIQUID. If you have questions on
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. (em-plis-city)
 READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr Empliciti TM (em-plis-city) (elotuzumab 300 & 400 mg powder for concentrate for solution for infusion) Read this
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr Empliciti TM (em-plis-city) (elotuzumab 300 & 400 mg powder for concentrate for solution for infusion) Read this
PART III: CONSUMER INFORMATION
 IMPORTANT: PLEASE READ PART III: CONSUMER INFORMATION NiaStase RT (eptacog alfa, activated) Activated Recombinant Human Blood Coagulation Factor VII Room Temperature Stable This leaflet is Part III of
IMPORTANT: PLEASE READ PART III: CONSUMER INFORMATION NiaStase RT (eptacog alfa, activated) Activated Recombinant Human Blood Coagulation Factor VII Room Temperature Stable This leaflet is Part III of
PATIENT INFORMATION. Auvi-Q (epinephrine injection) Auto-Injector For allergic emergencies (anaphylaxis)
 PATIENT INFORMATION Auvi-Q (epinephrine injection) Auto-Injector For allergic emergencies (anaphylaxis) Read this Patient Information Leaflet before you have to use Auvi-Q and each time you get a refill.
PATIENT INFORMATION Auvi-Q (epinephrine injection) Auto-Injector For allergic emergencies (anaphylaxis) Read this Patient Information Leaflet before you have to use Auvi-Q and each time you get a refill.
INSTRUCTIONS FOR USE. EMGALITY TM (em-gal-it-ē) (galcanezumab-gnlm)
 1 INSTRUCTIONS FOR USE EMGALITY TM (em-gal-it-ē) (galcanezumab-gnlm) injection, for subcutaneous use Prefilled Pen For subcutaneous injection only. Before you use the EMGALITY prefilled pen (Pen), read
1 INSTRUCTIONS FOR USE EMGALITY TM (em-gal-it-ē) (galcanezumab-gnlm) injection, for subcutaneous use Prefilled Pen For subcutaneous injection only. Before you use the EMGALITY prefilled pen (Pen), read
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. SYLVANT pronounced SILL-vant siltuximab for injection
 READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr SYLVANT pronounced SILL-vant siltuximab for injection Read this carefully before you start taking SYLVANT and each
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr SYLVANT pronounced SILL-vant siltuximab for injection Read this carefully before you start taking SYLVANT and each
Glatopa is prescription medicine used for the treatment of people with relapsing forms of multiple sclerosis (MS).
 PATIENT INFORMATION Glatopa (gluh-toh-puh) (Glatiramer Acetate Injection) for Subcutaneous Use Read this Patient Information before you start using Glatopa and each time you get a refill. There may be
PATIENT INFORMATION Glatopa (gluh-toh-puh) (Glatiramer Acetate Injection) for Subcutaneous Use Read this Patient Information before you start using Glatopa and each time you get a refill. There may be
Pfizer, BeneFIX R2 Recombinant Factor IX
 Pfizer, BeneFIX R2 Recombinant Factor IX BeneFIX, Recombinant Factor IX, is indicated for the prevention and control of bleeding episodes in people with hemophilia B (Christmas Disease). BeneFIX is also
Pfizer, BeneFIX R2 Recombinant Factor IX BeneFIX, Recombinant Factor IX, is indicated for the prevention and control of bleeding episodes in people with hemophilia B (Christmas Disease). BeneFIX is also
RELISTOR. Methylnaltrexone bromide, Subcutaneous solution for injection. Consumer Medicine Information
 RELISTOR Methylnaltrexone bromide, Subcutaneous solution for injection Consumer Medicine Information What is in this leaflet? This leaflet answers some common questions about RELISTOR. It does not contain
RELISTOR Methylnaltrexone bromide, Subcutaneous solution for injection Consumer Medicine Information What is in this leaflet? This leaflet answers some common questions about RELISTOR. It does not contain
paracetamol Please read this leaflet carefully before you are given Paracetamol BNM.
 1 Consumer Medicine Information paracetamol 1000 mg/100 ml solution for injection What is in this leaflet Please read this leaflet carefully before you are given. This leaflet answers some common questions
1 Consumer Medicine Information paracetamol 1000 mg/100 ml solution for injection What is in this leaflet Please read this leaflet carefully before you are given. This leaflet answers some common questions
Summary of the risk management plan (RMP) for Rixubis (nonacog gamma)
 EMA/677082/2014 Summary of the risk management plan (RMP) for Rixubis (nonacog gamma) This is a summary of the risk management plan (RMP) for Rixubis, which details the measures to be taken in order to
EMA/677082/2014 Summary of the risk management plan (RMP) for Rixubis (nonacog gamma) This is a summary of the risk management plan (RMP) for Rixubis, which details the measures to be taken in order to
Idelvion. (coagulation factor IX [recombinant], albumin fusion protein) New Product Slideshow
![Idelvion. (coagulation factor IX [recombinant], albumin fusion protein) New Product Slideshow Idelvion. (coagulation factor IX [recombinant], albumin fusion protein) New Product Slideshow](/thumbs/95/122489856.jpg) Idelvion (coagulation factor IX [recombinant], albumin fusion protein) New Product Slideshow Introduction Brand name: Idelvion Generic name: Coagulation factor IX (recombinant), albumin fusion protein
Idelvion (coagulation factor IX [recombinant], albumin fusion protein) New Product Slideshow Introduction Brand name: Idelvion Generic name: Coagulation factor IX (recombinant), albumin fusion protein
Getting started on MYALEPT (metreleptin) for injection
 For problems of leptin deficiency in people with congenital or acquired generalized lipodystrophy Getting started on MYALEPT (metreleptin) for injection MEET LORELEI, A YOUNG GIRL WITH ACQUIRED GENERALIZED
For problems of leptin deficiency in people with congenital or acquired generalized lipodystrophy Getting started on MYALEPT (metreleptin) for injection MEET LORELEI, A YOUNG GIRL WITH ACQUIRED GENERALIZED
BayCuff : Self-infusion training made simple
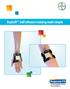 BayCuff : Self-infusion training made simple Contents Introduction to the program Introduction to the program 3 Features of BayCuff 4 Self-infusion training with BayCuff 5 Welcome to the BayCuff self-infusion
BayCuff : Self-infusion training made simple Contents Introduction to the program Introduction to the program 3 Features of BayCuff 4 Self-infusion training with BayCuff 5 Welcome to the BayCuff self-infusion
Before you are given CYRAMZA. When you must not be given it
 Ramucirumab (rmc) Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about It does not contain all the available information. It does not take the place of
Ramucirumab (rmc) Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about It does not contain all the available information. It does not take the place of
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la]
![READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la] READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la]](/thumbs/81/83994292.jpg) READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr NUCALA [new-ka la] mepolizumab lyophilized powder for subcutaneous injection Read this carefully before you start
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr NUCALA [new-ka la] mepolizumab lyophilized powder for subcutaneous injection Read this carefully before you start
HIGHLIGHTS OF PRESCRIBING INFORMATION WARNINGS AND PRECAUTIONS
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Novoeight safely and effectively. See full prescribing information for Novoeight. Novoeight, Antihemophilic
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Novoeight safely and effectively. See full prescribing information for Novoeight. Novoeight, Antihemophilic
Patient Information. EGRIFTA (eh-grif-tuh) (tesamorelin for injection) for subcutaneous use
 Patient Information EGRIFTA (eh-grif-tuh) (tesamorelin for injection) for subcutaneous use Read the Patient Information that comes with EGRIFTA before you start to take it and each time you get a refill.
Patient Information EGRIFTA (eh-grif-tuh) (tesamorelin for injection) for subcutaneous use Read the Patient Information that comes with EGRIFTA before you start to take it and each time you get a refill.
PACKAGE LEAFLET: INFORMATION FOR THE USER Pulmicort Turbohaler budesonide. 1. What Pulmicort Turbohaler is and what it is used for
 PACKAGE LEAFLET: INFORMATION FOR THE USER Pulmicort Turbohaler budesonide Read all of this leaflet carefully before you start taking this medicine. Keep this leaflet. You may need to read it again. If
PACKAGE LEAFLET: INFORMATION FOR THE USER Pulmicort Turbohaler budesonide Read all of this leaflet carefully before you start taking this medicine. Keep this leaflet. You may need to read it again. If
Instructions For Use PRALUENT (PRAHL-u-ent) (alirocumab) Injection, for Subcutaneous Injection Single-Dose Pre-Filled Syringe (75 mg/ml)
 Instructions For Use PRALUENT (PRAHL-u-ent) (alirocumab) Injection, for Subcutaneous Injection Single-Dose Pre-Filled Syringe (75 mg/ml) Important Information The device is a single-dose pre-filled syringe.
Instructions For Use PRALUENT (PRAHL-u-ent) (alirocumab) Injection, for Subcutaneous Injection Single-Dose Pre-Filled Syringe (75 mg/ml) Important Information The device is a single-dose pre-filled syringe.
Package Leaflet: Information for the User
 Package Leaflet: Information for the User REPLENINE -VF 250 IU, 500 IU, 1000 IU POWDER FOR SOLUTION FOR INJECTION HIGH PURITY FACTOR IX FJL14 Please read all of this leaflet carefully before you start
Package Leaflet: Information for the User REPLENINE -VF 250 IU, 500 IU, 1000 IU POWDER FOR SOLUTION FOR INJECTION HIGH PURITY FACTOR IX FJL14 Please read all of this leaflet carefully before you start
1. What Miacalcic is and what it is used for
 Package leaflet: Information for the user Miacalcic 100 IU/ml solution for injection and infusion Calcitonin (salmon, synthetic) Read all of this leaflet carefully before you start using this medicine
Package leaflet: Information for the user Miacalcic 100 IU/ml solution for injection and infusion Calcitonin (salmon, synthetic) Read all of this leaflet carefully before you start using this medicine
Perjeta Contains the active ingredient pertuzumab (rch)
 Perjeta Contains the active ingredient pertuzumab (rch) Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Perjeta. It does not contain all the available
Perjeta Contains the active ingredient pertuzumab (rch) Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Perjeta. It does not contain all the available
Package leaflet: Information for the user. Pharmalgen Bee Venom Pharmalgen Wasp Venom. 100μg/ml. powder and solvent for solution for injection
 Package leaflet: Information for the user Pharmalgen Bee Venom Pharmalgen Wasp Venom 100μg/ml powder and solvent for solution for injection Read all of this leaflet carefully before you start using this
Package leaflet: Information for the user Pharmalgen Bee Venom Pharmalgen Wasp Venom 100μg/ml powder and solvent for solution for injection Read all of this leaflet carefully before you start using this
COMPANY CORE PACKAGE INSERT CCPI (PI/CORE/ENGLISH)
 COMPANY CORE PACKAGE INSERT CCPI (PI/CORE/ENGLISH) CLUVOT 250 IU Rev.: 08-MAY-2014 / New license CSL Behring Page 1 of 10 Package leaflet: Information for the user Powder and solvent for solution for injection/infusion.
COMPANY CORE PACKAGE INSERT CCPI (PI/CORE/ENGLISH) CLUVOT 250 IU Rev.: 08-MAY-2014 / New license CSL Behring Page 1 of 10 Package leaflet: Information for the user Powder and solvent for solution for injection/infusion.
ELAPRASE Idursulfase 6 mg/3 ml, Concentrate for Solution for Infusion
 ELAPRASE Idursulfase 6 mg/3 ml, Concentrate for Solution for Infusion Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Elaprase. It does not contain
ELAPRASE Idursulfase 6 mg/3 ml, Concentrate for Solution for Infusion Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Elaprase. It does not contain
DALACIN C PHOSPHATE Injection Clindamycin phosphate
 DALACIN C PHOSPHATE Injection Clindamycin phosphate Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Dalacin C Phosphate injection. It does not contain
DALACIN C PHOSPHATE Injection Clindamycin phosphate Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Dalacin C Phosphate injection. It does not contain
70/30 HUMAN Novo Nordisk Patient Information for Novolin 70/30
 70/30 HUMAN Novo Nordisk Patient Information for Novolin 70/30 NOVOLIN 70/30 (NO-voe-lin) 70% NPH, Human Insulin Isophane Suspension and 30% Regular, Human Insulin Injection (recombinant DNA origin) 100
70/30 HUMAN Novo Nordisk Patient Information for Novolin 70/30 NOVOLIN 70/30 (NO-voe-lin) 70% NPH, Human Insulin Isophane Suspension and 30% Regular, Human Insulin Injection (recombinant DNA origin) 100
MEDICATION GUIDE ANORO ELLIPTA
 MEDICATION GUIDE ANORO ELLIPTA [a-nor oh e-lip-ta] (umeclidinium and vilanterol inhalation powder) for oral inhalation What is the most important information I should know about ANORO ELLIPTA? ANORO ELLIPTA
MEDICATION GUIDE ANORO ELLIPTA [a-nor oh e-lip-ta] (umeclidinium and vilanterol inhalation powder) for oral inhalation What is the most important information I should know about ANORO ELLIPTA? ANORO ELLIPTA
METALYSE Tenecteplase
 METALYSE Tenecteplase Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Metalyse. It does not contain all the available information. It does not take
METALYSE Tenecteplase Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Metalyse. It does not contain all the available information. It does not take
Before you use the TALTZ autoinjector, read and carefully follow all the step-by-step instructions.
 1 INSTRUCTIONS FOR USE TALTZ (tȯl(t)s) (ixekizumab) injection, for subcutaneous use Autoinjector Before you use the TALTZ autoinjector, read and carefully follow all the step-by-step instructions. Important
1 INSTRUCTIONS FOR USE TALTZ (tȯl(t)s) (ixekizumab) injection, for subcutaneous use Autoinjector Before you use the TALTZ autoinjector, read and carefully follow all the step-by-step instructions. Important
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. AIM-oh-vig Pr AIMOVIG (erenumab injection)
 READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION AIM-oh-vig Pr AIMOVIG (erenumab injection) Single-Use Pre-filled SureClick Autoinjector Read this carefully before you
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION AIM-oh-vig Pr AIMOVIG (erenumab injection) Single-Use Pre-filled SureClick Autoinjector Read this carefully before you
What Biostate is used for
 Biostate Human coagulation factor VIII (FVIII) and human von Willebrand factor (VWF) complex, powder for injection. Consumer Medicine Information Biostate 250 IU FVIII/500 IU VWF: AUST R 73032 Biostate
Biostate Human coagulation factor VIII (FVIII) and human von Willebrand factor (VWF) complex, powder for injection. Consumer Medicine Information Biostate 250 IU FVIII/500 IU VWF: AUST R 73032 Biostate
Package leaflet: Information for the user
 Package leaflet: Information for the user ReFacto AF 250 IU powder and solvent for solution for injection in pre-filled syringe ReFacto AF 500 IU powder and solvent for solution for injection in pre-filled
Package leaflet: Information for the user ReFacto AF 250 IU powder and solvent for solution for injection in pre-filled syringe ReFacto AF 500 IU powder and solvent for solution for injection in pre-filled
MEDICATION GUIDE ELIQUIS (ELL eh kwiss) (apixaban) tablets
 MEDICATION GUIDE ELIQUIS (ELL eh kwiss) (apixaban) tablets What is the most important information I should know about ELIQUIS? For people taking ELIQUIS for atrial fibrillation: People with atrial fibrillation
MEDICATION GUIDE ELIQUIS (ELL eh kwiss) (apixaban) tablets What is the most important information I should know about ELIQUIS? For people taking ELIQUIS for atrial fibrillation: People with atrial fibrillation
PACKAGE LEAFLET: INFORMATION FOR THE USER. Cisatracurium 2 mg/ml solution for injection/infusion Cisatracurium 5 mg/ml solution for injection/infusion
 PACKAGE LEAFLET: INFORMATION FOR THE USER Cisatracurium 2 mg/ml solution for injection/infusion Cisatracurium 5 mg/ml solution for injection/infusion Cisatracurium Read all of this leaflet carefully before
PACKAGE LEAFLET: INFORMATION FOR THE USER Cisatracurium 2 mg/ml solution for injection/infusion Cisatracurium 5 mg/ml solution for injection/infusion Cisatracurium Read all of this leaflet carefully before
AIMOVIG TM solution for injection in pre-filled pen erenumab Consumer Medicine Information
 This medicine is subject to additional monitoring in Australia. This will allow quick identification of new safety information. You can help by reporting any side effects you may get. You can report side
This medicine is subject to additional monitoring in Australia. This will allow quick identification of new safety information. You can help by reporting any side effects you may get. You can report side
This leaflet answers some common questions about HERCEPTIN SC. It does not contain all the available information.
 Consumer Medicine Information HERCEPTIN SC Trastuzumab 600 mg/5 ml solution for subcutaneous injection What is in this leaflet This leaflet answers some common questions about HERCEPTIN SC. It does not
Consumer Medicine Information HERCEPTIN SC Trastuzumab 600 mg/5 ml solution for subcutaneous injection What is in this leaflet This leaflet answers some common questions about HERCEPTIN SC. It does not
Package leaflet: Information for the user. Norditropin SimpleXx 5 mg/1.5 ml solution for injection in cartridge somatropin
 Package leaflet: Information for the user Norditropin SimpleXx 5 mg/1.5 ml solution for injection in cartridge somatropin Read all of this leaflet carefully before you start using this medicine because
Package leaflet: Information for the user Norditropin SimpleXx 5 mg/1.5 ml solution for injection in cartridge somatropin Read all of this leaflet carefully before you start using this medicine because
AIMOVIG solution for injection in pre-filled pen
 This medicine is subject to additional monitoring. This will allow quick identification of new safety information. You can help by reporting any side effects you may get. You can report side effects to
This medicine is subject to additional monitoring. This will allow quick identification of new safety information. You can help by reporting any side effects you may get. You can report side effects to
Package leaflet: Information for the user
 Package leaflet: Information for the user ReFacto AF 250 IU powder and solvent for solution for injection ReFacto AF 500 IU powder and solvent for solution for injection ReFacto AF 1000 IU powder and solvent
Package leaflet: Information for the user ReFacto AF 250 IU powder and solvent for solution for injection ReFacto AF 500 IU powder and solvent for solution for injection ReFacto AF 1000 IU powder and solvent
ALPROLIX Coagulation Factor IX (Recombinant), Fc Fusion Protein INSTRUCTIONS FOR USE Do not Do not YOUR KIT CONTAINS:
 ALPROLIX Coagulation Factor IX (Recombinant), Fc Fusion Protein INSTRUCTIONS FOR USE Read the Instructions for Use before you start using ALPROLIX and each time you get a refill. There may be new information.
ALPROLIX Coagulation Factor IX (Recombinant), Fc Fusion Protein INSTRUCTIONS FOR USE Read the Instructions for Use before you start using ALPROLIX and each time you get a refill. There may be new information.
Package leaflet: Information for the user. Elaprase 2 mg/ml concentrate for solution for infusion idursulfase
 Package leaflet: Information for the user Elaprase 2 mg/ml concentrate for solution for infusion idursulfase This medicine is subject to additional monitoring. This will allow quick identification of new
Package leaflet: Information for the user Elaprase 2 mg/ml concentrate for solution for infusion idursulfase This medicine is subject to additional monitoring. This will allow quick identification of new
Normal Immunoglobulin (Human) 16%, solution for subcutaneous administration.
 Normal Immunoglobulin (Human) 16%, solution for subcutaneous administration. Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about. It does not contain
Normal Immunoglobulin (Human) 16%, solution for subcutaneous administration. Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about. It does not contain
Pemetrexed APOTEX Powder for Injection Contains the active ingredient pemetrexed (as disodium)
 Pemetrexed APOTEX Powder for Injection Contains the active ingredient pemetrexed (as disodium) Consumer Medicine Information For a copy of a large print leaflet, Ph: 1800 195 055 What is in this leaflet
Pemetrexed APOTEX Powder for Injection Contains the active ingredient pemetrexed (as disodium) Consumer Medicine Information For a copy of a large print leaflet, Ph: 1800 195 055 What is in this leaflet
PACKAGE LEAFLET: INFORMATION FOR THE USER Rhinocort Aqua, 64 micrograms, Nasal Spray Budesonide
 PACKAGE LEAFLET: INFORMATION FOR THE USER Rhinocort Aqua, 64 micrograms, Nasal Spray Budesonide Read all of this leaflet carefully before you start using this medicine. Keep this leaflet. You may need
PACKAGE LEAFLET: INFORMATION FOR THE USER Rhinocort Aqua, 64 micrograms, Nasal Spray Budesonide Read all of this leaflet carefully before you start using this medicine. Keep this leaflet. You may need
Package leaflet: Information for the user
 Package leaflet: Information for the user Aimovig 70 mg solution for injection in pre-filled pen erenumab This medicine is subject to additional monitoring. This will allow quick identification of new
Package leaflet: Information for the user Aimovig 70 mg solution for injection in pre-filled pen erenumab This medicine is subject to additional monitoring. This will allow quick identification of new
OXEZE TURBUHALER formoterol fumarate dihydrate dry powder for oral inhalation
 IMPORTANT: PLEASE READ PART III: CONSUMER INFORMATION OEZE TURBUHALER formoterol fumarate dihydrate dry powder for oral inhalation This leaflet is part III of a three-part Product Monograph published when
IMPORTANT: PLEASE READ PART III: CONSUMER INFORMATION OEZE TURBUHALER formoterol fumarate dihydrate dry powder for oral inhalation This leaflet is part III of a three-part Product Monograph published when
Package leaflet: Information for the user. Bricanyl Turbohaler 0.5 mg/dose terbutaline sulfate
 Package leaflet: Information for the user Bricanyl Turbohaler 0.5 mg/dose terbutaline sulfate Read all of this leaflet carefully before you start using this medicine because it contains important information
Package leaflet: Information for the user Bricanyl Turbohaler 0.5 mg/dose terbutaline sulfate Read all of this leaflet carefully before you start using this medicine because it contains important information
PATIENT INFORMATION INCRUSE ELLIPTA
 PATIENT INFORMATION INCRUSE ELLIPTA [IN-cruise e-lip-ta] (umeclidinium inhalation powder) for oral inhalation What is INCRUSE ELLIPTA? INCRUSE ELLIPTA is an anticholinergic medicine. Anticholinergic medicines
PATIENT INFORMATION INCRUSE ELLIPTA [IN-cruise e-lip-ta] (umeclidinium inhalation powder) for oral inhalation What is INCRUSE ELLIPTA? INCRUSE ELLIPTA is an anticholinergic medicine. Anticholinergic medicines
INFORMATION FOR THE CONSUMER
 INFORMATION FOR THE CONSUMER This insert provides a summary of the information about CETROTIDE (cetrorelix for injection). If you have any questions or concerns, or want more information about CETROTIDE,
INFORMATION FOR THE CONSUMER This insert provides a summary of the information about CETROTIDE (cetrorelix for injection). If you have any questions or concerns, or want more information about CETROTIDE,
Package leaflet: Information for the user Zinforo 600 mg powder for concentrate for solution for infusion Ceftaroline fosamil
 Package leaflet: Information for the user Zinforo 600 mg powder for concentrate for solution for infusion Ceftaroline fosamil Read all of this leaflet carefully before you start using this medicine because
Package leaflet: Information for the user Zinforo 600 mg powder for concentrate for solution for infusion Ceftaroline fosamil Read all of this leaflet carefully before you start using this medicine because
o Your healthcare provider should test you for TB before starting CIMZIA.
 Medication Guide CIMZIA (CIM-zee-uh) (certolizumab pegol) lyophilized powder or solution for subcutaneous use Read the Medication Guide that comes with CIMZIA before you start using it, and before each
Medication Guide CIMZIA (CIM-zee-uh) (certolizumab pegol) lyophilized powder or solution for subcutaneous use Read the Medication Guide that comes with CIMZIA before you start using it, and before each
What do I need to tell the doctor BEFORE my child takes this drug? If your child has an allergy to this drug or any part of this drug.
 PATIENT & CAREGIVER EDUCATION Magnesium Carbonate Brand Names: US Mag onate [OTC] What is this drug used for? It is used to treat or prevent low magnesium levels. What do I need to tell the doctor BEFORE
PATIENT & CAREGIVER EDUCATION Magnesium Carbonate Brand Names: US Mag onate [OTC] What is this drug used for? It is used to treat or prevent low magnesium levels. What do I need to tell the doctor BEFORE
1.0mg, 2.0mg, 5.0mg and 8.0mg
 NovoSeven RT 1.0mg, 2.0mg, 5.0mg and 8.0mg eptacog alfa (activated) (bhk) Recombinant coagulation factor VIIa Consumer Medicine Information What is in this leaflet What is in this leaflet... 1 What NovoSeven
NovoSeven RT 1.0mg, 2.0mg, 5.0mg and 8.0mg eptacog alfa (activated) (bhk) Recombinant coagulation factor VIIa Consumer Medicine Information What is in this leaflet What is in this leaflet... 1 What NovoSeven
CIPROXIN HC Ear Drops
 CIPROXIN HC Ear Drops Ciprofloxacin and Hydrocortisone CONSUMER MEDICINE INFORMATION What is in this leaflet Please read this leaflet carefully before you use. This leaflet answers some common questions
CIPROXIN HC Ear Drops Ciprofloxacin and Hydrocortisone CONSUMER MEDICINE INFORMATION What is in this leaflet Please read this leaflet carefully before you use. This leaflet answers some common questions
PACKAGE LEAFLET: INFORMATION FOR THE USER. Bricanyl Turbohaler 0.5 mg/dose terbutaline sulphate
 PACKAGE LEAFLET: INFORMATION FOR THE USER Bricanyl Turbohaler 0.5 mg/dose terbutaline sulphate Read all of this leaflet carefully before you start taking this medicine. Keep this leaflet. You may need
PACKAGE LEAFLET: INFORMATION FOR THE USER Bricanyl Turbohaler 0.5 mg/dose terbutaline sulphate Read all of this leaflet carefully before you start taking this medicine. Keep this leaflet. You may need
NovoSeven RT 1.0mg, 2.0mg, 5.0mg and 8.0mg eptacog alfa (activated) (bhk) Consumer Medicine Information
 NovoSeven RT 1.0mg, 2.0mg, 5.0mg and 8.0mg eptacog alfa (activated) (bhk) Consumer Medicine Information Recombinant coagulation factor VIIa What is in this leaflet What is in this leaflet What NovoSeven
NovoSeven RT 1.0mg, 2.0mg, 5.0mg and 8.0mg eptacog alfa (activated) (bhk) Consumer Medicine Information Recombinant coagulation factor VIIa What is in this leaflet What is in this leaflet What NovoSeven
DUKORAL. Oral inactivated cholera and ETEC vaccine. Consumer Medicine Information. It does not take the place of talking to your doctor or pharmacist.
 What is in this leaflet DUKORAL Oral inactivated cholera and ETEC vaccine Consumer Medicine Information This leaflet answers some common questions about Dukoral. It does not contain all the available information.
What is in this leaflet DUKORAL Oral inactivated cholera and ETEC vaccine Consumer Medicine Information This leaflet answers some common questions about Dukoral. It does not contain all the available information.
Package leaflet: Information for the user
 Package leaflet: Information for the user Meropenem Milpharm 500 mg powder for solution for injection or infusion Meropenem Milpharm 1 g powder for solution for injection or infusion meropenem Read all
Package leaflet: Information for the user Meropenem Milpharm 500 mg powder for solution for injection or infusion Meropenem Milpharm 1 g powder for solution for injection or infusion meropenem Read all
MEDICATION GUIDE PRADAXA (pra dax a) (dabigatran etexilate mesylate) capsules
 MEDICATION GUIDE PRADAXA (pra dax a) (dabigatran etexilate mesylate) capsules Read this Medication Guide before you start taking PRADAXA and each time you get a refill. There may be new information. This
MEDICATION GUIDE PRADAXA (pra dax a) (dabigatran etexilate mesylate) capsules Read this Medication Guide before you start taking PRADAXA and each time you get a refill. There may be new information. This
Package leaflet: Information for the user. Novastan 100 mg/ml concentrate for solution for infusion. argatroban monohydrate
 Package leaflet: Information for the user 100 mg/ml concentrate for solution for infusion argatroban monohydrate Read all of this leaflet carefully before you start using this medicine because it contains
Package leaflet: Information for the user 100 mg/ml concentrate for solution for infusion argatroban monohydrate Read all of this leaflet carefully before you start using this medicine because it contains
Actemra concentrate for intravenous infusion
 Consumer Medicine Information Actemra concentrate for intravenous infusion Tocilizumab 80 mg in 4 ml, 200 mg in 10 ml and 400 mg in 20 ml concentrate for solution for infusion What is in this leaflet This
Consumer Medicine Information Actemra concentrate for intravenous infusion Tocilizumab 80 mg in 4 ml, 200 mg in 10 ml and 400 mg in 20 ml concentrate for solution for infusion What is in this leaflet This
Package leaflet: Information for the user
 Package leaflet: Information for the user Cisatracurium 2 mg/ml Solution for injection/ infusion Cisatracurium 5 mg/ml Solution for injection/ infusion Cisatracurium The name of your medicine are Cisatracurium
Package leaflet: Information for the user Cisatracurium 2 mg/ml Solution for injection/ infusion Cisatracurium 5 mg/ml Solution for injection/ infusion Cisatracurium The name of your medicine are Cisatracurium
your breathing problems worsen quickly. you use your rescue inhaler, but it does not relieve your breathing problems.
 MEDICATION GUIDE ADVAIR DISKUS [ad vair disk us] (fluticasone propionate and salmeterol inhalation powder) for oral inhalation What is the most important information I should know about ADVAIR DISKUS?
MEDICATION GUIDE ADVAIR DISKUS [ad vair disk us] (fluticasone propionate and salmeterol inhalation powder) for oral inhalation What is the most important information I should know about ADVAIR DISKUS?
MEDICATION GUIDE. BENLYSTA (ben-list-ah) (belimumab) Injection for intravenous use
 MEDICATION GUIDE BENLYSTA (ben-list-ah) (belimumab) Injection for intravenous use Read this Medication Guide before you start receiving BENLYSTA and before each treatment. There may be new information.
MEDICATION GUIDE BENLYSTA (ben-list-ah) (belimumab) Injection for intravenous use Read this Medication Guide before you start receiving BENLYSTA and before each treatment. There may be new information.
2. What you need to know before you are given Konakion MM. Package Leaflet: Information for the patient
 Package Leaflet: Information for the patient Konakion MM Ampoules 10mg/ml solution for injection Phytomenadione (vitamin K 1 ) Roche Read all of this leaflet carefully before you start taking this medicine
Package Leaflet: Information for the patient Konakion MM Ampoules 10mg/ml solution for injection Phytomenadione (vitamin K 1 ) Roche Read all of this leaflet carefully before you start taking this medicine
Mycamine Micafungin (as sodium)
 Mycamine Micafungin (as sodium) Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Mycamine. It does not contain all the available information. It does
Mycamine Micafungin (as sodium) Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Mycamine. It does not contain all the available information. It does
PACKAGE LEAFLET: INFORMATION FOR THE PATIENT. Estracyt Capsules Estramustine phosphate 140mg (as estramustine sodium phosphate)
 PACKAGE LEAFLET: INFORMATION FOR THE PATIENT Estracyt Capsules Estramustine phosphate 140mg (as estramustine sodium phosphate) Pfizer Logo Read all of this leaflet carefully before you start taking this
PACKAGE LEAFLET: INFORMATION FOR THE PATIENT Estracyt Capsules Estramustine phosphate 140mg (as estramustine sodium phosphate) Pfizer Logo Read all of this leaflet carefully before you start taking this
HEMLIBRA (emicizumab-kxwh) IN HEMOPHILIA A WITH OR WITHOUT FACTOR VIII INHIBITORS
 HEMLIBRA (emicizumab-kxwh) IN HEMOPHILIA A WITH OR WITHOUT FACTOR VIII INHIBITORS Media Inquiries: (650) 467-6800 About Hemlibra Hemlibra (emicizumab-kxwh) is approved by the FDA as a prophylactic (preventative)
HEMLIBRA (emicizumab-kxwh) IN HEMOPHILIA A WITH OR WITHOUT FACTOR VIII INHIBITORS Media Inquiries: (650) 467-6800 About Hemlibra Hemlibra (emicizumab-kxwh) is approved by the FDA as a prophylactic (preventative)
Package leaflet: Information for the patient Tranexamic acid 100 mg/ml Solution for Injection tranexamic acid
 Package leaflet: Information for the patient Tranexamic acid 100 mg/ml Solution for Injection tranexamic acid Read all of this leaflet carefully before you are given this medicine because it contains important
Package leaflet: Information for the patient Tranexamic acid 100 mg/ml Solution for Injection tranexamic acid Read all of this leaflet carefully before you are given this medicine because it contains important
OXEZE TURBUHALER formoterol fumarate dihydrate
 IMPORTANT: PLEASE READ PART III: CONSUMER INFORMATION OEZE TURBUHALER formoterol fumarate dihydrate This leaflet is part III of a three-part Product Monograph published when OEZE TURBUHALER was approved
IMPORTANT: PLEASE READ PART III: CONSUMER INFORMATION OEZE TURBUHALER formoterol fumarate dihydrate This leaflet is part III of a three-part Product Monograph published when OEZE TURBUHALER was approved
Cofact can be used for: The treatment of haemorrhages or the prevention of peri-operative haemorrhages as the result of
 Package leaflet: Information for the user Cofact 250 IU, human prothrombin complex, 250 IU factor IX per vial, powder and solvent for solution for injection. Cofact 500 IU, human prothrombin complex, 500
Package leaflet: Information for the user Cofact 250 IU, human prothrombin complex, 250 IU factor IX per vial, powder and solvent for solution for injection. Cofact 500 IU, human prothrombin complex, 500
PATIENT INFORMATION INCRELEX (EENK-RUH-LEX)
 PATIENT INFORMATION INCRELEX (EENK-RUH-LEX) (mecasermin) injection for subcutaneous use Read the Patient Information that comes with INCRELEX before your child starts receiving INCRELEX and each time your
PATIENT INFORMATION INCRELEX (EENK-RUH-LEX) (mecasermin) injection for subcutaneous use Read the Patient Information that comes with INCRELEX before your child starts receiving INCRELEX and each time your
PACKAGE LEAFLET: INFORMATION FOR THE USER Meronem IV 500 mg and 1 g Powder for solution for injection or infusion meropenem
 PACKAGE LEAFLET: INFORMATION FOR THE USER Meronem IV 500 mg and 1 g Powder for solution for injection or infusion meropenem Read all of this leaflet carefully before you start using this medicine because
PACKAGE LEAFLET: INFORMATION FOR THE USER Meronem IV 500 mg and 1 g Powder for solution for injection or infusion meropenem Read all of this leaflet carefully before you start using this medicine because
Sensipar Cinacalcet hydrochloride
 Sensipar Cinacalcet hydrochloride Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Sensipar. It does not contain all the available information. It
Sensipar Cinacalcet hydrochloride Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Sensipar. It does not contain all the available information. It
HEMLIBRA (emicizumab-kxwh) IN HEMOPHILIA A WITH INHIBITORS
 HEMLIBRA (emicizumab-kxwh) IN HEMOPHILIA A WITH INHIBITORS Media Inquiries: (650) 467-6800 About HEMLIBRA HEMLIBRA (emicizumab-kxwh) is approved by the FDA as a prophylactic medicine used to prevent or
HEMLIBRA (emicizumab-kxwh) IN HEMOPHILIA A WITH INHIBITORS Media Inquiries: (650) 467-6800 About HEMLIBRA HEMLIBRA (emicizumab-kxwh) is approved by the FDA as a prophylactic medicine used to prevent or
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la] Mepolizumab for Injection
![READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la] Mepolizumab for Injection READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la] Mepolizumab for Injection](/thumbs/93/111890101.jpg) READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr NUCALA [new-ka la] Mepolizumab for Injection Read this carefully before you start taking NUCALA and each time you
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr NUCALA [new-ka la] Mepolizumab for Injection Read this carefully before you start taking NUCALA and each time you
READY. SET. DOPTELET. HELP GET READY FOR YOUR UPCOMING PROCEDURE
 HELP GET READY FOR YOUR UPCOMING PROCEDURE READY. SET. DOPTELET. Full Prescribing Information for DOPTELET (avatrombopag), including Patient For adults with chronic liver disease and a low platelet count
HELP GET READY FOR YOUR UPCOMING PROCEDURE READY. SET. DOPTELET. Full Prescribing Information for DOPTELET (avatrombopag), including Patient For adults with chronic liver disease and a low platelet count
Medication Information for Parents and Teachers
 Medication Information for Parents and Teachers Modafinil Provigil Armodafinil Nuvigil General Information About Medication Each child and adolescent is different. No one has exactly the same combination
Medication Information for Parents and Teachers Modafinil Provigil Armodafinil Nuvigil General Information About Medication Each child and adolescent is different. No one has exactly the same combination
Sodium Zirconium Cyclosilicate
 PATIENT & CAREGIVER EDUCATION Sodium Zirconium Cyclosilicate Brand Names: US Lokelma What is this drug used for? It is used to treat high potassium levels. What do I need to tell my doctor BEFORE I take
PATIENT & CAREGIVER EDUCATION Sodium Zirconium Cyclosilicate Brand Names: US Lokelma What is this drug used for? It is used to treat high potassium levels. What do I need to tell my doctor BEFORE I take
MERIEUX INACTIVATED RABIES VACCINE (MIRV) Rabies Vaccine
 MERIEUX INACTIVATED RABIES VACCINE (MIRV) Rabies Vaccine Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Merieux Inactivated Rabies Vaccine (MIRV).
MERIEUX INACTIVATED RABIES VACCINE (MIRV) Rabies Vaccine Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Merieux Inactivated Rabies Vaccine (MIRV).
Ventolin Accuhaler 200 micrograms salbutamol sulfate
 Package Leaflet: Information for the User Ventolin Accuhaler 200 micrograms salbutamol sulfate Read all of this leaflet carefully before you start taking this medicine because it contains important information
Package Leaflet: Information for the User Ventolin Accuhaler 200 micrograms salbutamol sulfate Read all of this leaflet carefully before you start taking this medicine because it contains important information
It is used to treat or prevent upset stomach and throwing up. What do I need to tell my doctor BEFORE I take this drug?
 PATIENT & CAREGIVER EDUCATION Ondansetron Brand Names: US Zofran; Zofran ODT [DSC]; Zuplenz Brand Names: Canada Ondissolve ODF; Zofran ODT What is this drug used for? It is used to treat or prevent upset
PATIENT & CAREGIVER EDUCATION Ondansetron Brand Names: US Zofran; Zofran ODT [DSC]; Zuplenz Brand Names: Canada Ondissolve ODF; Zofran ODT What is this drug used for? It is used to treat or prevent upset
Before you take. Tell your doctor if you have any of the conditions mentioned below or if you have ever experienced any of these conditions.
 This medicine is subject to additional monitoring. This will allow quick identification of new safety information. You can help by reporting any side effects you may get. You can report side effects to
This medicine is subject to additional monitoring. This will allow quick identification of new safety information. You can help by reporting any side effects you may get. You can report side effects to
