Author's response to reviews
|
|
|
- Alban Baldwin Bond
- 5 years ago
- Views:
Transcription
1 Author's response to reviews Title:Efficacy of 1% carboxymethylcellulose sodium for treating dry eye after phacoemulsification: results from a multicenter, open-label, randomized, controlled study Authors: Ke Yao MD (xlren@zju.edu.cn) Yongzhen Bao MD, PhD (drbaoyz@sina.com) Jian Ye MD (yejian1979@163.com) Yi Lu MD (luyiox@163.com) Hongsheng Bi MD (bihongsheng@126.com) Xin Tang MD (tangprofessor@yahoo.com.cn) Yune Zhao MD ( @qq.com) Jinsong Zhang MD (cmu4h-zjs@126.com) Jinling Yang MD (yang_jinlin@allergan.com) Version:4Date:16 December 2014 Author's response to reviews: see over
2 Sunil Chauhan, MD Associate Editor, Cornea and External Eye Diseases BMC Ophthalmology Re: BMC Ophthalmology - MS: Dear Dr. Chauhan, On behalf of all co-authors, thank you and the reviewers for your interest in our manuscript, Efficacy of 1% carboxymethylcellulose sodium for treating dry eye after phacoemulsification: results from a multicenter, open-label, randomized, controlled study. We have addressed each comment point-by-point in italics below and have amended the manuscript accordingly (highlighted in yellow). Reviewer: Ahmad Kheirkhah 1. Although this study is a multicenter RCT, the patients and researchers were not masked to the treatment regimen. This may significantly affect the findings. This is especially true as the patients in the control group were possibly aware that they are not being treated for dry eye. The open-label design was described as a potential limitation of the study in the discussion, but we have modified the discussion as follows to further emphasize the point (p. 12, lines ): Since neither patients nor investigators were masked to treatment, patients in the control group were likely aware of their treatment status, which could explain the above findings. However, despite this potential limitation of the study, the difference in the percentage of patients with dry eye between the treatment and control groups was not significant by day 30 after surgery, arguing against this possibility. One possible, alternative explanation 2. It is not clear why the authors did not have a control group with placebo or the vehicle of CMC without active ingredient. The study objective was to compare the efficacy and safety of anti-inflammatory + CMC eye drop therapy with anti-inflammatory therapy alone. Use of vehicle as a control tear solution may have had some impact on the symptoms of dry eye and may thus have confounded the results in the control group. 3. In Introduction, the statement on the effects of various factors on ocular surface health after cataract surgery needs to have references. We apologize for the oversight; the following references have been added to support the statement in question (p. 3, line 71). 1
3 1. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 2007, 5(2): Movahedan A, Djalilian AR. Cataract surgery in the face of ocular surface disease. Curr Opin Ophthalmol 2012, 23(1): There is no data to show whether all surgical procedures were standardized regarding, for example, the location and size of incision, intraoperative medications, type of anesthesia. At the reviewer s request, we have added the following information to the manuscript: Overall, 52.3% and 47.7% of patients in the treatment group had surgery in the right and left eye, compared with 55.5% and 44.4% in the control group, respectively. The percentage of patients who had a superior (12 o clock), temporal (9 o clock), tempo-superior (10-11 or o clock), or nasal superior (10-11 or o clock) incision was also similar between groups. The mean incision length (± SD) was 2.8 ± 0.3 cm vs 2.8 ± 0.4 cm in the treatment and control groups, respectively. (p. 8, lines ) We have also added the following information regarding the type of anesthetics used for the surgical procedure: Topical anesthesia with oxybuprocaine hydrochloride 0.4% eye drops (Benoxil, Santen Pharmaceutical Co, Ltd, Osaka, Japan) or proparacaine hydrochloride 0.5% eye drops (Alcaine, Alcon Laboratories, Inc, Ft Worth, TX) was administered before the surgical procedure. (p.5, lines ) No other intraoperative medications were used. 5. It is not clear whether cases with intraoperative or postoperative complications have been excluded from the study. Patients were withdrawn from the study if they experienced complications during surgery, or post-surgical ocular hypertension, endophthalmitis, infectious keratitis, or conjunctivitis. This information has been added to the Methods section (p. 4, lines ). 6. It is not clear whether cases with preop dry eye have been under any treatment for dry eye and whether they have continued it during the course of study. If not, as it seems to be the case, there is a question of ethical issues. Similarly, based on the literature it is obvious that ocular surface will be compromised after the surgery. Therefore, not treating the patients with pre-existing dry eye seems to be unethical. The use of any topical ophthalmologic agents that might have affected the efficacy assessment of the study treatment was prohibited during the study (added to p. 5, lines ). Almost all patients with dry eye at baseline were reported to have mild dry eye symptoms by the clinician using non-standardized criteria. Since there are no usual treatment recommendations for dry eye in these patients, ethics were not in question. Patients undergoing cataract surgery would naturally have tear film instability from the surgery resulting in dry eye syndrome and in this study all patients including those in the 2
4 control group received the usual post-surgical topical corticosteroids and anti-infective care for corneal damage and post-operative inflammation. 7. It is not clear which grading system has been used for nucleus hardness. To address this comment, the following information was added to the Methods (Patients) section: Patients were scheduled to undergo phacoemulsification and IOL implantation and had a lens nucleus hardness of grade 3 or less (based on nucleus color per the standard Lens Opacities Classification System III [12]). (p. 4, lines 96-97) 8. The criteria used in this study for the definition of dry eye needs to have a reference. Otherwise, it is not clear why the authors have used a symptom score of 3 or more and TBUT#5 or Schirmer #3 as their criteria. We apologize for the oversight and have added references to the following sentence (p. 7, lines ): Patients were diagnosed as having dry eye symptoms if they met one of the following conditions: TBUT 5 s, plus a subjective symptom score of 3 or greater,[14, 15] or Schirmer test 3 mm in 5 min, plus a subjective symptom score of 3 or greater. [14, 16] 14. Z Wang, HY Wang, et al. The changes of tear film after phacoemulsification combining with intraocular lens implantation. J Tongji Univer (Medi Sci) 2007, 4: H Li, F Yuan, et al. Chin J Optom Ophthalmol Vis Sci. Effects of phacoemulsification on tear film and eye surface. 2005, 3: Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 2007;5(2): It is not clear how the normality of the data has been tested. To address this comment, the text was modified to include the following: Quantitative data were compared between the two groups using the t-test (normal distribution) and the Wilcoxon rank sum test (non-normal distribution). Similarly, differences before and after surgery within the treatment and control groups were assessed using paired t-tests (normal distribution) or Wilcoxon signed rank test (non-normal distribution). (p. 7, lines ) 10. Although based on sample size calculation the authors needed to have a 31 cases per group, it is not clear why they have included 90 per group. Sample size for the study was determined based on results from an early study, in which the value of TBUT was determined at day 7 after cataract surgery. One-tailed test was conducted with α=0.05 and β=0.2, and it was determined that at least 31 patients per group were required to detect superiority between the treatment groups. However, taking patient discontinuation into consideration, as well as post-surgery follow-up visits at both day 7 and 3
5 day 30, it was estimated that a sample size of 90 patients in each group was required for the intent-to-treat (ITT) population. The underlined portion was added to the text to clarify this point (p. 8, line 185). 11. The authors should mention the exact values for P and avoid stating the P values as >0.05 or <0.05. At the reviewer s request, we have replaced all generic P values with the actual values. 12. In Table 1, the patients with other disease have not been defined. In addition, the duration of cataract should be deleted as there is no precise value for that. The test used for comparison of Schirmer between the two groups in this Table is not clear. We apologize for the apparent oversight. The other diseases were concomitant systemic diseases and information regarding their nature are not available. For clarity, however, other disease has been replaced with concomitant systemic diseases in Table 1. (p. 19) In addition, we have deleted the duration of cataract from Table 1 and have added a footnote to indicate that the results of the Schirmer test were compared between groups using the Wilcoxon rank sum test (p. 19). 13. It is not clear why the authors have used 7-day as a time point for evaluation of dry eye. As at this time point, the patient is still recovering from the surgery with its usual symptoms of postop ocular discomfort, the validity of symptom questionnaire is questionable. Because damage to the ocular surface usually occurs early after phacoemulsification, the day-7 time point was used to assess whether early intervention with carboxymethylcellulose 1% was beneficial for treating post-surgical dry eye. Information was added to the manuscript to clarify this point (p. 6, lines ). 14. The findings have been repeated in text, tables and graphs. The authors should avoid repeating the findings. At the reviewer s request, the Results section has been amended to avoid repetition (p. 8-11). 15. It is not clear why corneal fluorescein staining and conjunctival lissamine green staining have been significantly positive in the patient population at the baseline. As mentioned in the discussion, Aging is an established risk factor for dry eye, [3] and androgen deficiency associated with aging has also been reported to be a contributing factor to dry eye. [20, 21] The patient population enrolled in this study, ages 60 to 80 years with age-related cataract, was high risk of dry eye disease (p. 12, lines ). Moreover, 38.9% and 36.7% of patients in the treatment and control groups were diagnosed with dry 4
6 eye at baseline (p. 19, Table 1), which is consistent with findings of corneal and conjunctival staining in the overall study population at baseline. 16. OSDI at baseline has been very high, probably due to effect of the cataract on the visual part of OSDI. The authors are encouraged to use the non-visual part of OSDI for the study. The presence of cataract can indeed affect the results of the OSDI questionnaire. However, using only the non-visual part of the questionnaire would leave at best- 5 out of 12 questions to be analyzed, which may negate its clinical and statistical value. To circumvent the issue, we instead evaluated 11 additional subjective symptoms to assess dry eye, as described in the Clinical assessments section of the manuscript (p. 6-7, lines ). 17. The methods used for evaluation of the drug safety as well as the definition for adverse events are not clear. For example, the authors have used the symptoms of tearing, foreign body sensation, and photophobia as adverse events (Table 5) while the same items have been used as symptoms of dry eye. To clarify this point, we have modified the last paragraph of the Clinical assessments section as follows: The clinical investigator was responsible for monitoring adverse events (eg, eye swelling, ocular hypertension) and reporting incidents to the study sponsor. Any worsening of pre-existing dry eye symptoms (eg, tearing, foreign body sensation, or photophobia) or new occurrence of such symptoms was also considered an adverse event. (p. 7, lines ) 18. The authors have failed to discuss many of the findings. For example, why patients with preop dry eye had similar TBUT in both treatment and control groups after the surgery; why fluorescein and lissamine staining decreased postop in the control group, why symptoms decreased postop in the control group, etc. It is unclear why post-surgical TBUT values were similar in the treatment and control groups of patients who had dry eye prior to surgery, or why fluorescein staining, lissamine staining and symptoms decreased in the control group after surgery. However, since corticosteroids can reduce inflammation in the injured ocular surface epithelium of patients with dry eye, and prednisolone was part of the post-surgical therapy administered to the entire study population, it is possible that prednisolone prevented the detection of a difference between the treatment and control groups for those parameters. The post-surgical decrease in symptoms observed in the two groups may also be attributable to the enhanced vision due to the phacoemulsification itself. It is also possible that the questionnaires used are not optimally designed to assess dry eye following cataract surgery. This information has been added where appropriate in the Discussion section (p. 12, lines ; p. 13, lines and ). 5
7 In fact, most patients who develop dry eye post-cataract surgery have a mild form of the disease due to the period of tear film instability which usually improves as the eye recovers. This factor may have impacted in the small treatment differences. 19. Discussion has over-emphasized on the efficacy of CMC on postop dry eye while it is not supported with the findings of this study. We understand the reviewer s concern and have revised the discussion and conclusions to avoid over-interpreting and/or over-emphasizing the findings (p ). Reviewer: Tae-im Kim 1. Page 5 line 124 anesthesia (Tianjin Jingming New Technological Development Co, Ltd, Tianjin, 125 China), what is the name of anesthetic medication? It is unclear what is related with Tianjin Jingming New Technological Development Co, Ltd. Does it mean Schirmer strip? Although information regarding the anesthetics used for the Schirmer test had been provided in the second paragraph of the Clinical assessments section, it was not easily recognizable. The description is now included earlier in the first paragraph of the section. In addition, the role of Tianjin Jingming New Technological Development Co, Ltd as the vendor of the test strips has been added. The sentence now reads as follows: At each of the three visits, the following assessments were conducted serially and prior to instillation of eye drops: TBUT, fluorescein and lissamine green ocular staining, and Schirmer test with oxybuprocaine hydrochloride 0.4% eye drops (Benoxil, Santen Pharmaceutical Co, Ltd) or proparacaine hydrochloride 0.5% eye drops (Alcaine, Alcon Laboratories, Inc) as anesthetics (and test strips from Tianjin Jingming New Technological (p. 6, lines ) 2. Schirmer I test usually means the test without anesthesia, Schirmer II test is the test with anesthesia. I recommend use the term Schirmer test and define it as Schirmer test with anesthesia one time. As recommended by the reviewer, the type of Schirmer test used was amended to designate with anesthesia once in the abstract and once in the main text. 3. When do you analyze the patient clinical outcome after artificial tear instillation? Too close application of eye drops may influence on data of TBUT and Schirmer test. To address this comment, the second sentence of the Clinical assessments section was modified as follows: On the day of each of the three visits, administration of CMC was withheld prior to and immediately after study assessments. The following were conducted in sequence: TBUT, fluorescein and lissamine green ocular staining, and Schirmer test with (p. 6, lines ) 4. What is the order of the test was done? Please describe it serially. 6
8 The tests were completed in the following order: Symptom questionnaire, TBUT, fluorescein and lissamine green ocular staining, Schirmer test with anesthesia. The text has been modified to reflect this important point. (p. 6, lines ) 5. In Results session, line 184 Based on the criteria for diagnosis of dry eye. What is the criteria for diagnosis of dry eye in this study? It would be better to describe it. Because this is multicenter study, standardized criteria for diagnosis is critical. This comment is similar to comment #8 from Reviewer 1 and was addressed by adding references to support the statement found on p. 7 (lines ): Patients were diagnosed as having dry eye symptoms if they met one of the following conditions: TBUT 5 s, plus a subjective symptom score of 3 or greater,[14, 15] or Schirmer test 3 mm in 5 min, plus a subjective symptom score of 3 or greater. [14, 16] 14. Discussion and conclusion do not describe separately The discussion and conclusion sections were separated as specified in the instructions to authors provided on the BMC Ophthalmology website. We will, however, gladly combine them if the editor advises so. Thank you for your kind attention. We look forward to hearing from you. Sincerely, Ke Yao, MD Eye Center, Second Affiliated Hospital Zhejiang University School of Medicine Phone: xlren@zju.edu.cn 7
Supplementary Online Content
 Supplementary Online Content Uchino Y, Uchino M, Yokoi N, et al. Alteration of tear mucin 5AC in office workers using visual display terminals: the Osaka Study. JAMA Ophthalmol. Published online June 5,
Supplementary Online Content Uchino Y, Uchino M, Yokoi N, et al. Alteration of tear mucin 5AC in office workers using visual display terminals: the Osaka Study. JAMA Ophthalmol. Published online June 5,
Sponsor. Generic Drug Name. Trial Indications. Protocol Number. Protocol Title. Clinical Trial Phase. Study Start/End Dates. Reason for Termination
 Sponsor Alcon Research, Ltd. Generic Drug Name Travoprost/timolol maleate Trial Indications Open-angle glaucoma or ocular hypertension Protocol Number C-09-007 Protocol Title An Evaluation of Patient Reported
Sponsor Alcon Research, Ltd. Generic Drug Name Travoprost/timolol maleate Trial Indications Open-angle glaucoma or ocular hypertension Protocol Number C-09-007 Protocol Title An Evaluation of Patient Reported
Dry Eye Assessment and Management Study ELIGIBILITY OCULAR EVALUATION FORM
 Page 1 of 13 BEFORE COMPLETING THE OCULAR EXAMINATION, YOU MUST BE ABLE TO ANSWER YES TO THE FOLLOWING QUESTIONS: Have you done MMP9? (SVonly) The Following are done at Baseline: Have you done Tear Osmolarity?
Page 1 of 13 BEFORE COMPLETING THE OCULAR EXAMINATION, YOU MUST BE ABLE TO ANSWER YES TO THE FOLLOWING QUESTIONS: Have you done MMP9? (SVonly) The Following are done at Baseline: Have you done Tear Osmolarity?
Overview & pathophysiology of Dry Eye and the use of cyclosporine eye drops in dry eye...
 Overview & pathophysiology of Dry Eye and the use of cyclosporine eye drops in dry eye... This Allergan sponsored session was held on July 24, 2005, Hotel Satya Ashoka, Jabalpur. The session was followed
Overview & pathophysiology of Dry Eye and the use of cyclosporine eye drops in dry eye... This Allergan sponsored session was held on July 24, 2005, Hotel Satya Ashoka, Jabalpur. The session was followed
The Tear Film! Cataract Surgery in the Diseased Eye: Ocular Surface Disease
 Cataract Surgery in the Diseased Eye: Ocular Surface Disease I am a consultant to Allergan, Alcon, B&L, and Tear Science I have no financial interest in any product discussed herein Parag A. Majmudar,
Cataract Surgery in the Diseased Eye: Ocular Surface Disease I am a consultant to Allergan, Alcon, B&L, and Tear Science I have no financial interest in any product discussed herein Parag A. Majmudar,
HELP HEAL YOUR PATIENTS DRY EYES.
 HELP HEAL YOUR PATIENTS DRY EYES. REFRESH REPAIR promotes healing of the cornea and conjunctival epithelia, and improves visual performance in Dry Eye patients. REF115081_v2 12/18 FIRST AND ONLY ARTIFICIAL
HELP HEAL YOUR PATIENTS DRY EYES. REFRESH REPAIR promotes healing of the cornea and conjunctival epithelia, and improves visual performance in Dry Eye patients. REF115081_v2 12/18 FIRST AND ONLY ARTIFICIAL
nepafenac 1mg/mL eye drops, suspension (Nevanac ) SMC No. (813/12) Alcon Laboratories (UK) Ltd
 nepafenac 1mg/mL eye drops, suspension (Nevanac ) SMC No. (813/12) Alcon Laboratories (UK) Ltd 05 October 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
nepafenac 1mg/mL eye drops, suspension (Nevanac ) SMC No. (813/12) Alcon Laboratories (UK) Ltd 05 October 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
Efficacy of an ocular bandage contact lens for the treatment of dry eye after phacoemulsification
 Chen et al. BMC Ophthalmology (2019) 19:13 https://doi.org/10.1186/s12886-018-1023-8 RESEARCH ARTICLE Open Access Efficacy of an ocular bandage contact lens for the treatment of dry eye after phacoemulsification
Chen et al. BMC Ophthalmology (2019) 19:13 https://doi.org/10.1186/s12886-018-1023-8 RESEARCH ARTICLE Open Access Efficacy of an ocular bandage contact lens for the treatment of dry eye after phacoemulsification
PRODUCT INFORMATION ALCAINE. Proparacaine Hydrochloride Sterile Ophthalmic Solution, USP. 5 mg/ml. Topical Anesthetic
 PRODUCT INFORMATION ALCAINE Proparacaine Hydrochloride Sterile Ophthalmic Solution, USP 5 mg/ml Topical Anesthetic Alcon Canada Inc. 2665 Meadowpine Blvd. Mississauga, ON L5N 8C7 www.alcon.ca Date of Preparation:
PRODUCT INFORMATION ALCAINE Proparacaine Hydrochloride Sterile Ophthalmic Solution, USP 5 mg/ml Topical Anesthetic Alcon Canada Inc. 2665 Meadowpine Blvd. Mississauga, ON L5N 8C7 www.alcon.ca Date of Preparation:
BARRY A. SCHECHTER ABSTRACT
 JOURNAL OF OCULAR PHARMACOLOGY AND THERAPEUTICS Volume 22, Number 2, 2006 Mary Ann Liebert, Inc. The Evaluation of Ketorolac (Acular LS ) to Improve Patient Comfort During the Induction Phase of Cyclosporin-A
JOURNAL OF OCULAR PHARMACOLOGY AND THERAPEUTICS Volume 22, Number 2, 2006 Mary Ann Liebert, Inc. The Evaluation of Ketorolac (Acular LS ) to Improve Patient Comfort During the Induction Phase of Cyclosporin-A
Effects of femtosecond laser assisted cataract surgery on dry eye
 EXPERIMENTAL AND THERAPEUTIC MEDICINE Effects of femtosecond laser assisted cataract surgery on dry eye DEWANG SHAO 1, XIAOQUAN ZHU 1, WEI SUN 1, PENG CHENG 1, WEI CHEN 2 and HUA WANG 2 1 Department of
EXPERIMENTAL AND THERAPEUTIC MEDICINE Effects of femtosecond laser assisted cataract surgery on dry eye DEWANG SHAO 1, XIAOQUAN ZHU 1, WEI SUN 1, PENG CHENG 1, WEI CHEN 2 and HUA WANG 2 1 Department of
Dry eye syndrome in diabetic children
 European Journal of Ophthalmology / Vol. 17 no. 6, 2007 / pp. 873-878 Dry eye syndrome in diabetic children A. AKINCI 1, E. CETINKAYA 2, Z. AYCAN 2 1 Department of Pediatric Ophthalmology 2 Department
European Journal of Ophthalmology / Vol. 17 no. 6, 2007 / pp. 873-878 Dry eye syndrome in diabetic children A. AKINCI 1, E. CETINKAYA 2, Z. AYCAN 2 1 Department of Pediatric Ophthalmology 2 Department
Financial Disclosures. Corneal Problems for the Cataract Surgeon. Four Common Problems. Dry Eye syndrome. Rose-Bengal 3/27/16
 Corneal Problems for the Cataract Surgeon Financial Disclosures Consultant: AMO/VISX Consultant: Angiotech/Sharppoint Michael J Taravella, MD Director: Cornea and Refractive Surgery University of Colorado
Corneal Problems for the Cataract Surgeon Financial Disclosures Consultant: AMO/VISX Consultant: Angiotech/Sharppoint Michael J Taravella, MD Director: Cornea and Refractive Surgery University of Colorado
Ophthalmology Times Case Study Yasmin Mali, MD. Case Study
 Ophthalmology Times Case Study Yasmin Mali, MD Case Study A 57 year old female with presented with ocular irritation and discomfort in both eyes for several months. Patient was previously started on a
Ophthalmology Times Case Study Yasmin Mali, MD Case Study A 57 year old female with presented with ocular irritation and discomfort in both eyes for several months. Patient was previously started on a
Effect of Oral Pilocarpine in Treating Severe Dry Eye in Patients With Sjögren Syndrome. Tetsuya Kawakita, Shigeto Shimmura, and Kazuo Tsubota
 ORIGINAL CLINICAL STUDY Effect of Oral Pilocarpine in Treating Severe Dry Eye in Patients With Sjögren Syndrome Tetsuya Kawakita, Shigeto Shimmura, and Kazuo Tsubota Purpose: The aim of this study is to
ORIGINAL CLINICAL STUDY Effect of Oral Pilocarpine in Treating Severe Dry Eye in Patients With Sjögren Syndrome Tetsuya Kawakita, Shigeto Shimmura, and Kazuo Tsubota Purpose: The aim of this study is to
Dr.Sushil Kumar Tripathi
 Rabamipide:360 Benefits in Dry eye syndrome Dr.Sushil Kumar Tripathi Prevalence of Dry Eye Syndrome: vone in four patients attending ophthalmic clinics report symptoms of dry eye. vprevalence of dry eye
Rabamipide:360 Benefits in Dry eye syndrome Dr.Sushil Kumar Tripathi Prevalence of Dry Eye Syndrome: vone in four patients attending ophthalmic clinics report symptoms of dry eye. vprevalence of dry eye
The Effects of Superior Temporal Small Corneal Incisions on Tear Level
 AASCIT Journal of Medicine 2016; 2(2): 28-32 http://www.aascit.org/journal/medicine ISSN: 2381-1420 (Print); ISSN: 2381-1447 (Online) The Effects of Superior Temporal Small Corneal Incisions on Tear Level
AASCIT Journal of Medicine 2016; 2(2): 28-32 http://www.aascit.org/journal/medicine ISSN: 2381-1420 (Print); ISSN: 2381-1447 (Online) The Effects of Superior Temporal Small Corneal Incisions on Tear Level
ISSN: (Paper) eissn: (Online) JOURNAL OF ADVANCED ACADEMIC RESEARCH (JAAR) April 2017
 Topical proparacaine vs combined topical-intracameral lidocaine anesthesia in phacoemulsification surgery with preoperative counseling about intraoperative visual fear Kiran Shakya 1, Sangita Shakya 2,
Topical proparacaine vs combined topical-intracameral lidocaine anesthesia in phacoemulsification surgery with preoperative counseling about intraoperative visual fear Kiran Shakya 1, Sangita Shakya 2,
Technology appraisal guidance Published: 16 December 2015 nice.org.uk/guidance/ta369
 Ciclosporin for treating dry eye e disease that has not improved despite treatment with artificial tears Technology appraisal guidance Published: 16 December 2015 nice.org.uk/guidance/ta369 NICE 2018.
Ciclosporin for treating dry eye e disease that has not improved despite treatment with artificial tears Technology appraisal guidance Published: 16 December 2015 nice.org.uk/guidance/ta369 NICE 2018.
Optimizing the Ocular Surface. Presentation Title. Charlene M. Grice, Carolina Eyecare Physicians, LLC
 Optimizing the Ocular Surface Presentation Title Presenter Charlene M. Grice, Name MD Carolina Eyecare Physicians, LLC Financial Disclosures I have no financial disclosures. I will discuss off label use
Optimizing the Ocular Surface Presentation Title Presenter Charlene M. Grice, Name MD Carolina Eyecare Physicians, LLC Financial Disclosures I have no financial disclosures. I will discuss off label use
WARNING LETTER DEPARTMENT OF HEALTH & HUMAN SERVICES TRANSMITTED BY FACSIMILE
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville, MD 20857 TRANSMITTED BY FACSIMILE Cary Rayment President and Chief Executive Officer Alcon, Inc. C/O
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville, MD 20857 TRANSMITTED BY FACSIMILE Cary Rayment President and Chief Executive Officer Alcon, Inc. C/O
Eyelid Thermal Pulsation for the Treatment of Dry Eye Syndrome
 Eyelid Thermal Pulsation for the Treatment of Dry Eye Syndrome Policy Number: 9.03.29 Last Review: 5/2018 Origination: 9/2013 Next Review: 11/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue
Eyelid Thermal Pulsation for the Treatment of Dry Eye Syndrome Policy Number: 9.03.29 Last Review: 5/2018 Origination: 9/2013 Next Review: 11/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue
David Clark, MD Chief Medical Officer Aldeyra Therapeutics, Lexington, MA. ARVO: April 30th, 2018
 A Randomized, Double-Masked, Parallel-Group, Phase 2a Dry Eye Disease Clinical Trial to Evaluate the Safety and Efficacy of Topical Ocular ADX-102 (Reproxalap), a Novel Aldehyde Sequestering Agent David
A Randomized, Double-Masked, Parallel-Group, Phase 2a Dry Eye Disease Clinical Trial to Evaluate the Safety and Efficacy of Topical Ocular ADX-102 (Reproxalap), a Novel Aldehyde Sequestering Agent David
Screening of dry eye disease in visual display terminal workers during occupational health examinations: The Moriguchi study
 Motoko KAWASHIMA, et al.: Screening of dry eye in visual display terminal workers J Occup Health 2015; 57: 253 258 253 Journal of Occupational Health Screening of dry eye disease in visual display terminal
Motoko KAWASHIMA, et al.: Screening of dry eye in visual display terminal workers J Occup Health 2015; 57: 253 258 253 Journal of Occupational Health Screening of dry eye disease in visual display terminal
Research Article ABSTRACT INTRODUCTION. Saubhagya Sindhu 1, Shaktibala Dutta 1, Mirza Atif Beg 1, Sanjeev Kumar Mittal 2, Sushobhan Das Gupta 2
 Research Article Comparative evaluation of topical carboxymethyl cellulose either alone or in combination with topical corticosteroid in the treatment of dry eye in a tertiary-care teaching hospital Saubhagya
Research Article Comparative evaluation of topical carboxymethyl cellulose either alone or in combination with topical corticosteroid in the treatment of dry eye in a tertiary-care teaching hospital Saubhagya
Tear Osmolarity and its role in Optometric Practice & Contact Lens Success
 Tear Osmolarity and its role in Optometric Practice & Contact Lens Success Nick Dash: Optometrist Declaration of Association Director: See2wiN Ltd (Accuvision/Visual Edge/SportsVision Institute) Honorary
Tear Osmolarity and its role in Optometric Practice & Contact Lens Success Nick Dash: Optometrist Declaration of Association Director: See2wiN Ltd (Accuvision/Visual Edge/SportsVision Institute) Honorary
Cataract Surgery: Patient Information
 Cataract Surgery: Patient Information How do the Eyes Work? As light enters the eye, it first passes through the cornea the clear window of the eye. Because the cornea is curved, the light rays bend (refract).
Cataract Surgery: Patient Information How do the Eyes Work? As light enters the eye, it first passes through the cornea the clear window of the eye. Because the cornea is curved, the light rays bend (refract).
Eyelid Thermal Pulsation for the Treatment of Dry Eye Syndrome
 Eyelid Thermal Pulsation for the Treatment of Dry Eye Syndrome Policy Number: 9.03.29 Last Review: 12/2017 Origination: 9/2013 Next Review: 5/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue
Eyelid Thermal Pulsation for the Treatment of Dry Eye Syndrome Policy Number: 9.03.29 Last Review: 12/2017 Origination: 9/2013 Next Review: 5/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue
WARNING LETTER. According to the Indications and Usage section of the FDA approved product labeling (PI):
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville, MD 20857 TRANSMITTED BY FACSIMILE David E.I. Pyott President and Chief Executive Officer PO Box 19534
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville, MD 20857 TRANSMITTED BY FACSIMILE David E.I. Pyott President and Chief Executive Officer PO Box 19534
JMSCR Vol 05 Issue 02 Page February 2017
 www.jmscr.igmpublication.org Impact Factor 5.84 Index Copernicus Value: 83.27 ISSN (e)-2347-176x ISSN (p) 2455-0450 DOI: https://dx.doi.org/10.18535/jmscr/v5i2.155 Prevalence of Dry Eye Diseases in the
www.jmscr.igmpublication.org Impact Factor 5.84 Index Copernicus Value: 83.27 ISSN (e)-2347-176x ISSN (p) 2455-0450 DOI: https://dx.doi.org/10.18535/jmscr/v5i2.155 Prevalence of Dry Eye Diseases in the
Ophthalmic Immunomodulators Prior Authorization with Quantity Limit Program Summary
 Ophthalmic Immunomodulators Prior Authorization with Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1,4 Agent Indication Dosage and Administration Restasis (cyclosporine ophthalmic emulsion)
Ophthalmic Immunomodulators Prior Authorization with Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1,4 Agent Indication Dosage and Administration Restasis (cyclosporine ophthalmic emulsion)
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Contents METHODS... 2 Inclusion and exclusion criteria... 2 Supplementary table S1... 2 Assessment of abnormal ocular signs and symptoms... 3 Supplementary table S2... 3 Ocular
SUPPLEMENTARY INFORMATION Contents METHODS... 2 Inclusion and exclusion criteria... 2 Supplementary table S1... 2 Assessment of abnormal ocular signs and symptoms... 3 Supplementary table S2... 3 Ocular
Dry Eye Prescribing Guidelines
 Dry Eye Prescribing Guidelines Amendment History VERSION DATE AMENDMENT HISTORY V1.0 2011 Comments V1.1 2013 Updated V1.1 2017 Draft Updated Thealoz Duo replaces carmellose Optive in severe dry eye on
Dry Eye Prescribing Guidelines Amendment History VERSION DATE AMENDMENT HISTORY V1.0 2011 Comments V1.1 2013 Updated V1.1 2017 Draft Updated Thealoz Duo replaces carmellose Optive in severe dry eye on
Clinical Study Effectiveness and Optical Quality of Topical 3.0% Diquafosol versus 0.05% Cyclosporine A in Dry Eye Patients following Cataract Surgery
 Ophthalmology Volume 216, Article ID 815757, 7 pages http://dx.doi.org/1.1155/216/815757 Clinical Study Effectiveness and Optical Quality of Topical 3.% Diquafosol versus.5% Cyclosporine A in Dry Eye Patients
Ophthalmology Volume 216, Article ID 815757, 7 pages http://dx.doi.org/1.1155/216/815757 Clinical Study Effectiveness and Optical Quality of Topical 3.% Diquafosol versus.5% Cyclosporine A in Dry Eye Patients
Title: Keeping Step with DEWS2: Clinical Applications Lecturer: Scott G. Hauswirth, OD
 Title: Keeping Step with DEWS2: Clinical Applications Lecturer: Scott G. Hauswirth, OD Course Description: The Dry Eye Workshop 2 was an assemblage of experts in dry eye disease from around the world,
Title: Keeping Step with DEWS2: Clinical Applications Lecturer: Scott G. Hauswirth, OD Course Description: The Dry Eye Workshop 2 was an assemblage of experts in dry eye disease from around the world,
Efficacy and Safety of Diquafosol Ophthalmic Solution in Patients with Dry Eye Syndrome: A Japanese Phase 2 Clinical Trial
 Efficacy and Safety of Ophthalmic Solution in Patients with Dry Eye Syndrome: A Japanese Phase 2 Clinical Trial Yukihiro Matsumoto, MD, 1 Yuichi Ohashi, MD, 2 Hitoshi Watanabe, MD, 3 Kazuo Tsubota, MD,
Efficacy and Safety of Ophthalmic Solution in Patients with Dry Eye Syndrome: A Japanese Phase 2 Clinical Trial Yukihiro Matsumoto, MD, 1 Yuichi Ohashi, MD, 2 Hitoshi Watanabe, MD, 3 Kazuo Tsubota, MD,
BREAKING THE VICIOUS CIRCLE OF DRY EYE DISEASE
 BREAKING THE VICIOUS CIRCLE OF DRY EYE DISEASE In this article, Christian Roesky, PhD, Chief Executive Officer, Novaliq, discusses the underserved condition of dry eye disease, and presents two products
BREAKING THE VICIOUS CIRCLE OF DRY EYE DISEASE In this article, Christian Roesky, PhD, Chief Executive Officer, Novaliq, discusses the underserved condition of dry eye disease, and presents two products
Iontophoresis Platform Safely and Effectively Delivers Dexamethasone to Manage Post Operative Inflammation and Pain Following Cataract Surgery.
 Poster #33516 Iontophoresis Platform Safely and Effectively Delivers Dexamethasone to Manage Post Operative Inflammation and Pain Following Cataract Surgery. JEFFREY H. LEVENSON, MD 1 (PRESENTING AUTHOR);
Poster #33516 Iontophoresis Platform Safely and Effectively Delivers Dexamethasone to Manage Post Operative Inflammation and Pain Following Cataract Surgery. JEFFREY H. LEVENSON, MD 1 (PRESENTING AUTHOR);
Does in-office manual expression for Meibomian Gland Dysfunction (MGD) work?
 See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/282816021 Does in-office manual expression for Meibomian Gland Dysfunction (MGD) work? Conference
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/282816021 Does in-office manual expression for Meibomian Gland Dysfunction (MGD) work? Conference
VARIABILITY IS A HALLMARK OF DRY EYE DISEASE
 VARIABILITY IS A HALLMARK OF DRY EYE DISEASE Christopher E. Starr, MD, FACS Associate Professor of Ophthalmology Director, Refractive Surgery Director, Cornea, Cataract & Refractive Surgery Fellowship
VARIABILITY IS A HALLMARK OF DRY EYE DISEASE Christopher E. Starr, MD, FACS Associate Professor of Ophthalmology Director, Refractive Surgery Director, Cornea, Cataract & Refractive Surgery Fellowship
CLINICAL RESEARCH METHODS VISP356. MODULE LEADER: PROF A TOMLINSON B.Sc./B.Sc.(HONS) OPTOMETRY
 DIVISION OF VISION SCIENCES SESSION: 2006/2007 DIET: 1ST CLINICAL RESEARCH METHODS VISP356 LEVEL: MODULE LEADER: PROF A TOMLINSON B.Sc./B.Sc.(HONS) OPTOMETRY MAY 2007 DURATION: 2 HRS CANDIDATES SHOULD
DIVISION OF VISION SCIENCES SESSION: 2006/2007 DIET: 1ST CLINICAL RESEARCH METHODS VISP356 LEVEL: MODULE LEADER: PROF A TOMLINSON B.Sc./B.Sc.(HONS) OPTOMETRY MAY 2007 DURATION: 2 HRS CANDIDATES SHOULD
The first comprehensive definition of DED was published in
 Special Issue Definition and Diagnostic Criteria of Dry Eye Disease: Historical Overview and Future Directions Jun Shimazaki Department of Ophthalmology, Tokyo Dental College, Ichikawa General Hospital,
Special Issue Definition and Diagnostic Criteria of Dry Eye Disease: Historical Overview and Future Directions Jun Shimazaki Department of Ophthalmology, Tokyo Dental College, Ichikawa General Hospital,
1998 DESCRIPTION Evaluation of Subjective and Objective tests for diagnosing tear-film disorders known to cause ocular irritation.
 DEWS DRY EYE: DIAGNOSTIC TEST TEMPLATE RAPPORTEUR A.J.Bron 18 th Oct 2004 TEST Mixed tests TO Ocular Irritation / Dry Eye REFERENCES DIAGNOSE VERSION of TEST Multiple tests DESCRIPTION Evaluation of Subjective
DEWS DRY EYE: DIAGNOSTIC TEST TEMPLATE RAPPORTEUR A.J.Bron 18 th Oct 2004 TEST Mixed tests TO Ocular Irritation / Dry Eye REFERENCES DIAGNOSE VERSION of TEST Multiple tests DESCRIPTION Evaluation of Subjective
Dry Eye After Cataract Surgery and Associated Intraoperative Risk Factors
 접수번호 :08-086 Korean Journal of Ophthalmology 2009;23:65-73 ISSN : 1011-8942 DOI : 10.3341/kjo.2009.23.2.65 Dry Eye After Cataract Surgery and Associated Intraoperative Risk Factors Yang Kyeung Cho, MD,
접수번호 :08-086 Korean Journal of Ophthalmology 2009;23:65-73 ISSN : 1011-8942 DOI : 10.3341/kjo.2009.23.2.65 Dry Eye After Cataract Surgery and Associated Intraoperative Risk Factors Yang Kyeung Cho, MD,
Evaluation of Dry Eye: A Hospital Based Study
 IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 16, Issue 9 Ver. XI (September. 2017), PP 44-49 www.iosrjournals.org Evaluation of : A Hospital Based
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 16, Issue 9 Ver. XI (September. 2017), PP 44-49 www.iosrjournals.org Evaluation of : A Hospital Based
QUALITATIVE INTERPRETATION OF VISUAL ACUITY IN DRY EYE PATIENTS
 Bulletin of the Transilvania University of Braşov Special Issue Series VI: Medical Sciences Vol. 10 (59) No. 2-2017 QUALITATIVE INTERPRETATION OF VISUAL ACUITY IN DRY EYE PATIENTS C. TRIHENEA 1 A. STANILA
Bulletin of the Transilvania University of Braşov Special Issue Series VI: Medical Sciences Vol. 10 (59) No. 2-2017 QUALITATIVE INTERPRETATION OF VISUAL ACUITY IN DRY EYE PATIENTS C. TRIHENEA 1 A. STANILA
New Drug Evaluation: lifitegrast solution, ophthalmic
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
INDICATIONS ACULAR 0,4% ophthalmic solution is indicated for the reduction of ocular pain and burning/stinging following corneal refractive surgery.
 Page 1 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME (and dosage form) ACULAR 0,4% COMPOSITION ACULAR 0,4% ophthalmic solution contains: Ketorolac tromethamine: 4 mg/ml Preservative: Benzalkonium chloride
Page 1 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME (and dosage form) ACULAR 0,4% COMPOSITION ACULAR 0,4% ophthalmic solution contains: Ketorolac tromethamine: 4 mg/ml Preservative: Benzalkonium chloride
Impact of Dry Eye Syndrome on Vision-Related Quality of Life in a Non-Clinic-Based General Population
 Le et al. BMC Ophthalmology 2012, 12:22 RESEARCH ARTICLE Open Access Impact of Dry Eye Syndrome on Vision-Related Quality of Life in a Non-Clinic-Based General Population Qihua Le 1, Xiaodong Zhou 2, Ling
Le et al. BMC Ophthalmology 2012, 12:22 RESEARCH ARTICLE Open Access Impact of Dry Eye Syndrome on Vision-Related Quality of Life in a Non-Clinic-Based General Population Qihua Le 1, Xiaodong Zhou 2, Ling
Ophthalmic Antihistamine Step Therapy Program Summary
 Ophthalmic Antihistamine Step Therapy Program Summary FDA APPROVED INDICATIONS AND DOSAGE 1-8 Drug FDA Indication(s) Administration and Dosing Bepreve Treatment of itching associated Instill one drop into
Ophthalmic Antihistamine Step Therapy Program Summary FDA APPROVED INDICATIONS AND DOSAGE 1-8 Drug FDA Indication(s) Administration and Dosing Bepreve Treatment of itching associated Instill one drop into
Laser-Assisted In Situ Keratomileusis for Patients With Dry Eye
 CLINICAL SCIENCES Laser-Assisted In Situ Keratomileusis for Patients With Dry Eye Ikuko Toda, MD; Naoko Asano-Kato, MD; Yoshiko Hori-Komai, MD; Kazuo Tsubota, MD Objective: To evaluate the efficacy and
CLINICAL SCIENCES Laser-Assisted In Situ Keratomileusis for Patients With Dry Eye Ikuko Toda, MD; Naoko Asano-Kato, MD; Yoshiko Hori-Komai, MD; Kazuo Tsubota, MD Objective: To evaluate the efficacy and
Chairman, Department of Sports Rehabilitation, Shanghai University of Sport
 Author s response to reviews Title: Effects of whole body vibration exercise on neuromuscular function for individuals with knee osteoarthritis: study protocol for a randomized controlled trial Authors:
Author s response to reviews Title: Effects of whole body vibration exercise on neuromuscular function for individuals with knee osteoarthritis: study protocol for a randomized controlled trial Authors:
Original Article The influence of long-term corneal contact lens wearing on the stability and quality of tear film in Qinghai
 Int J Clin Exp Med 2016;9(5):8416-8420 www.ijcem.com /ISSN:1940-5901/IJCEM0020163 Original Article The influence of long-term corneal contact lens wearing on the stability and quality of tear film in Qinghai
Int J Clin Exp Med 2016;9(5):8416-8420 www.ijcem.com /ISSN:1940-5901/IJCEM0020163 Original Article The influence of long-term corneal contact lens wearing on the stability and quality of tear film in Qinghai
Learning Objectives. Disclosures 2/2/ BMT Pharmacists Conference Bandage Contact Lens Therapy for Severe Ocular GVHD
 2015 BMT Pharmacists Conference Bandage Contact Lens Therapy for Severe Ocular GVHD Tueng T. Shen, M.D., Ph.D. Professor of Ophthalmology Adjunct, Bioengineering and Global Health Feb. 13 th, 2015 Learning
2015 BMT Pharmacists Conference Bandage Contact Lens Therapy for Severe Ocular GVHD Tueng T. Shen, M.D., Ph.D. Professor of Ophthalmology Adjunct, Bioengineering and Global Health Feb. 13 th, 2015 Learning
See 17 for PATIENT COUNSELING INFORMATION.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use NEVANAC* safely and effectively. See full prescribing information for NEVANAC. NEVANAC (nepafenac
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use NEVANAC* safely and effectively. See full prescribing information for NEVANAC. NEVANAC (nepafenac
Ocular surface alterations in blepharospasm patients treated with botulinum toxin A injection
 Eur J Ophthalmol 2014; 24 ( 6 ): 830-834 DOI: 10.5301/ejo.5000482 ORIGINAL ARTICLE Ocular surface alterations in blepharospasm patients treated with botulinum toxin A injection Sibel Kocabeyoglu, Hande
Eur J Ophthalmol 2014; 24 ( 6 ): 830-834 DOI: 10.5301/ejo.5000482 ORIGINAL ARTICLE Ocular surface alterations in blepharospasm patients treated with botulinum toxin A injection Sibel Kocabeyoglu, Hande
Vanderbilt Eye Institute Clinical Trials
 April, 2010 Vanderbilt Eye Institute Clinical Trials Ophthalmology Actively Recruiting Studies For information on our clinical trials and other studies, please contact: Sandy Owings, COA, CCRP Clinic Director
April, 2010 Vanderbilt Eye Institute Clinical Trials Ophthalmology Actively Recruiting Studies For information on our clinical trials and other studies, please contact: Sandy Owings, COA, CCRP Clinic Director
Therapeutic effects of 3% diquafosol ophthalmic solution in patients with short tear film break-up time-type dry eye disease
 Mun et al. BMC Ophthalmology (2018) 18:237 https://doi.org/10.1186/s12886-018-0910-3 RESEARCH ARTICLE Open Access Therapeutic effects of 3% diquafosol ophthalmic solution in patients with short tear film
Mun et al. BMC Ophthalmology (2018) 18:237 https://doi.org/10.1186/s12886-018-0910-3 RESEARCH ARTICLE Open Access Therapeutic effects of 3% diquafosol ophthalmic solution in patients with short tear film
NEW ZEALAND DATA SHEET 1. PRODUCT NAME
 NEW ZEALAND DATA SHEET 1. PRODUCT NAME Flucon fluorometholone 0.1% Eye Drops Suspension 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of Flucon contains 1.0 mg of fluorometholone (0.1% w/v). Excipient
NEW ZEALAND DATA SHEET 1. PRODUCT NAME Flucon fluorometholone 0.1% Eye Drops Suspension 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of Flucon contains 1.0 mg of fluorometholone (0.1% w/v). Excipient
Prevalence of dry eye disease in type 2 diabetic patients and its co-relation with the duration, glycemic control and retinopathy
 Al Am een J Med Sci 2015; 8(3):225-229 US National Library of Medicine enlisted journal ISSN 0974-1143 SHORT COMM UN ICAT ION C O D E N : A A J MB G Prevalence of dry eye disease in type 2 diabetic patients
Al Am een J Med Sci 2015; 8(3):225-229 US National Library of Medicine enlisted journal ISSN 0974-1143 SHORT COMM UN ICAT ION C O D E N : A A J MB G Prevalence of dry eye disease in type 2 diabetic patients
Ocular and periocular trauma
 Ocular and periocular trauma No financial disclosures. Tina Rutar M.D. Assistant Professor of Clinical Ophthalmology and Pediatrics Director, Visual Center for the Child University of California San Francisco
Ocular and periocular trauma No financial disclosures. Tina Rutar M.D. Assistant Professor of Clinical Ophthalmology and Pediatrics Director, Visual Center for the Child University of California San Francisco
Ocular Surface Disease. Advanced Treatment in Ocular Surface Disease. Disclosures. Revenue Potential. Chronic Dry Eye Should I Treat It
 Advanced Treatment in Ocular Surface Disease Douglas K. Devries, O.D. Eye Care Associates of Nevada Douglas K. Devries Consultant or Speakers Bureau for Allergan AMO TearLab NicOx BVI B&L Disclosures Chronic
Advanced Treatment in Ocular Surface Disease Douglas K. Devries, O.D. Eye Care Associates of Nevada Douglas K. Devries Consultant or Speakers Bureau for Allergan AMO TearLab NicOx BVI B&L Disclosures Chronic
A Randomized Double-Masked Study of 0.05% Cyclosporine Ophthalmic Emulsion in the Treatment of Meibomian Gland Dysfunction
 CLINICAL SCIENCE A Randomized Double-Masked Study of 0.05% Cyclosporine Ophthalmic Emulsion in the Treatment of Meibomian Gland Dysfunction Pinnita Prabhasawat, MD, Nattaporn Tesavibul, MD, and Wannaree
CLINICAL SCIENCE A Randomized Double-Masked Study of 0.05% Cyclosporine Ophthalmic Emulsion in the Treatment of Meibomian Gland Dysfunction Pinnita Prabhasawat, MD, Nattaporn Tesavibul, MD, and Wannaree
Current Practice Pattern for Dry Eye Patients in South Korea: A Multicenter Study
 pissn: 11-8942 eissn: 92-9382 Korean J Ophthalmol 14;28(2):115-121 http://dx.doi.org/.3341/kjo.14.28.2.115 Original Article Current Practice Pattern for Dry Eye Patients in South Korea: A Multicenter Study
pissn: 11-8942 eissn: 92-9382 Korean J Ophthalmol 14;28(2):115-121 http://dx.doi.org/.3341/kjo.14.28.2.115 Original Article Current Practice Pattern for Dry Eye Patients in South Korea: A Multicenter Study
This indication includes the temporary relief of burning, irritation, and/or discomfort due to dryness of the eye.
 Page 1 of 5 SCHEDULING STATUS Schedule 0 PROPRIETARY NAME AND DOSAGE FORM REFRESH LIQUIGEL COMPOSITION REFRESH LIQUIGEL lubricant eye drops contain carboxymethylcellulose sodium 10 mg/ml. Preservative:
Page 1 of 5 SCHEDULING STATUS Schedule 0 PROPRIETARY NAME AND DOSAGE FORM REFRESH LIQUIGEL COMPOSITION REFRESH LIQUIGEL lubricant eye drops contain carboxymethylcellulose sodium 10 mg/ml. Preservative:
Guide to modern day cataract surgery
 Guide to modern day cataract surgery What are Cataracts? What are Cataracts? Cataracts are a natural clouding of the lens of the eye. Cataracts form usually because of age, but there are eye injuries and
Guide to modern day cataract surgery What are Cataracts? What are Cataracts? Cataracts are a natural clouding of the lens of the eye. Cataracts form usually because of age, but there are eye injuries and
A Clinical Study of Ocular Surface Diseases and their Management
 A Clinical Study of Ocular Surface Diseases and their Management Dr. D. Uday Kumar 1, Dr. G. Manjula 2 1 M.S. Professor of Ophthalmology 2 M.S, D.O. Associate Professor of Ophthalmology Abstract: A Clinical
A Clinical Study of Ocular Surface Diseases and their Management Dr. D. Uday Kumar 1, Dr. G. Manjula 2 1 M.S. Professor of Ophthalmology 2 M.S, D.O. Associate Professor of Ophthalmology Abstract: A Clinical
Long-term impact of dry eye symptoms on vision-related quality of life after phacoemulsification surgery
 https://doi.org/10.1007/s10792-018-0828-z ORIGINAL PAPER Long-term impact of dry eye symptoms on vision-related quality of life after phacoemulsification surgery Wenwen Xue. Ming-ming Zhu. Bi-jun Zhu.
https://doi.org/10.1007/s10792-018-0828-z ORIGINAL PAPER Long-term impact of dry eye symptoms on vision-related quality of life after phacoemulsification surgery Wenwen Xue. Ming-ming Zhu. Bi-jun Zhu.
FUCH S DYSTROPHY & CATARACT SURGERY TREATMENT ALGORITHM
 FUCH S DYSTROPHY & CATARACT SURGERY TREATMENT ALGORITHM ΙΟΑΝΝΙS Α. MALLIAS, MD, PHD Director of the Dept. of Ophthalmology, Mediterraneo Hospital, Glyfada, Athens, Greece Clinical Fellow in Cornea and
FUCH S DYSTROPHY & CATARACT SURGERY TREATMENT ALGORITHM ΙΟΑΝΝΙS Α. MALLIAS, MD, PHD Director of the Dept. of Ophthalmology, Mediterraneo Hospital, Glyfada, Athens, Greece Clinical Fellow in Cornea and
Inflammatory Cytokine and Osmolarity Changes in the Tears of Dry Eye Patients Treated with Topical 1% Methylprednisolone
 Original Article http://dx.doi.org/10.3349/ymj.2014.55.1.203 pissn: 0513-5796, eissn: 1976-2437 Yonsei Med J 55(1):203-208, 2014 Inflammatory Cytokine and Osmolarity Changes in the Tears of Dry Eye Patients
Original Article http://dx.doi.org/10.3349/ymj.2014.55.1.203 pissn: 0513-5796, eissn: 1976-2437 Yonsei Med J 55(1):203-208, 2014 Inflammatory Cytokine and Osmolarity Changes in the Tears of Dry Eye Patients
Innovations in Glaucoma Drug Delivery What the Future Holds. Justin Schweitzer, OD, FAAO Vance Thompson Vision Sioux Falls, South Dakota
 Innovations in Glaucoma Drug Delivery What the Future Holds Justin Schweitzer, OD, FAAO Vance Thompson Vision Sioux Falls, South Dakota Allergan Glaukos Bausch and Lomb Bio- Tissue Alcon TearScience Reichert
Innovations in Glaucoma Drug Delivery What the Future Holds Justin Schweitzer, OD, FAAO Vance Thompson Vision Sioux Falls, South Dakota Allergan Glaukos Bausch and Lomb Bio- Tissue Alcon TearScience Reichert
13 NONCLINICAL TOXICOLOGY 5.1 Increased Bleeding Time Carcinogenesis, Mutagenesis, Impairment of 5.2 Delayed Healing
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ILEVRO* (nepafenac ophthalmic suspension), 0.3% safely and effectively. See full prescribing information
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ILEVRO* (nepafenac ophthalmic suspension), 0.3% safely and effectively. See full prescribing information
Therapeutic Effects of 0.1% Tacrolimus Eye Drops for Refractory Vernal Keratoconjunctivitis
 IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 15, Issue 3 Ver. VI (Mar. 2016), PP 44-48 www.iosrjournals.org Therapeutic Effects of 0.1% Tacrolimus
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 15, Issue 3 Ver. VI (Mar. 2016), PP 44-48 www.iosrjournals.org Therapeutic Effects of 0.1% Tacrolimus
Dry eye syndrome (DES), as defined by
 Signs and Symptoms in Dry Eye Confirm the diagnosis of dry eye before beginning treatment. BY GIANCARLO MONTANI, OPTOM FIACLE, FBCLA; AND UMBERTO BENELLI, MD, PHD Dry eye syndrome (DES), as defined by
Signs and Symptoms in Dry Eye Confirm the diagnosis of dry eye before beginning treatment. BY GIANCARLO MONTANI, OPTOM FIACLE, FBCLA; AND UMBERTO BENELLI, MD, PHD Dry eye syndrome (DES), as defined by
Critical Complication Wonderfully Managed by Vitreoretinal Surgeon
 Critical Complication Wonderfully Managed by Vitreoretinal Surgeon Prof. Dr. Sherif Embabi Consultant of ophthalmology Ain Shams univ. & Alwatany Eye Hospital, MD Dr. Remon Atef Ophthalmology specialist
Critical Complication Wonderfully Managed by Vitreoretinal Surgeon Prof. Dr. Sherif Embabi Consultant of ophthalmology Ain Shams univ. & Alwatany Eye Hospital, MD Dr. Remon Atef Ophthalmology specialist
Lifitegrast Clinical Development Program: Key Findings From 3 Randomized Controlled Trials in Adult Subjects With Dry Eye Disease
 Lifitegrast Clinical Development Program: Key Findings From 3 Randomized Controlled Trials in Adult Subjects With Dry Eye Disease Walter O. Whitley, 1 Edward J. Holland, 2 Kenneth Sall, 3 Stephen S. Lane,
Lifitegrast Clinical Development Program: Key Findings From 3 Randomized Controlled Trials in Adult Subjects With Dry Eye Disease Walter O. Whitley, 1 Edward J. Holland, 2 Kenneth Sall, 3 Stephen S. Lane,
Title: Use of food labels by adolescents to make healthier choices on snacks: a cross sectional study from Sri Lanka
 Author s response to reviews Title: Use of food labels by adolescents to make healthier choices on snacks: a cross sectional study from Sri Lanka Authors: Ishanka Talagala (drmaheshkeerthi@gmail.com;drishanka@gmail.com)
Author s response to reviews Title: Use of food labels by adolescents to make healthier choices on snacks: a cross sectional study from Sri Lanka Authors: Ishanka Talagala (drmaheshkeerthi@gmail.com;drishanka@gmail.com)
Cataract Surgery in Patients with Ocular Surface Disease: An Update in
 Cataract Surgery in Patients with Ocular Surface Disease: An Update in Clinical Diagnosis and Treatment Authors: Neda Afsharkhamseh, Asadolah Movahedan, Hooman Motahari, Ali R. Djalilian Affiliations:
Cataract Surgery in Patients with Ocular Surface Disease: An Update in Clinical Diagnosis and Treatment Authors: Neda Afsharkhamseh, Asadolah Movahedan, Hooman Motahari, Ali R. Djalilian Affiliations:
Title: Can air pollution affect tear film stability? A cross-sectional study in the aftermath of an explosion accident
 Author's response to reviews Title: Can air pollution affect tear film stability? A cross-sectional study in the aftermath of an explosion accident Authors: Bente E Moen (bente.moen@isf.uib.no) Dan Norbäck
Author's response to reviews Title: Can air pollution affect tear film stability? A cross-sectional study in the aftermath of an explosion accident Authors: Bente E Moen (bente.moen@isf.uib.no) Dan Norbäck
Uncovering Contact Lens Discoveries. Joe Barr, OD, MS, FAAO. Key findings of Donald Korb, OD and the presenter s and colleague s work that he inspired
 Uncovering Contact Lens Discoveries Joe Barr, OD, MS, FAAO Dr. Donald R. Korb Award for Excellence Lecture June 30, 2016 Key findings of Donald Korb, OD and the presenter s and colleague s work that he
Uncovering Contact Lens Discoveries Joe Barr, OD, MS, FAAO Dr. Donald R. Korb Award for Excellence Lecture June 30, 2016 Key findings of Donald Korb, OD and the presenter s and colleague s work that he
ALTERNATIVES TO PHAKIC IMPLANT SURGERY
 Visian ICL Consent INTRODUCTION This information is being provided to you so that you can make an informed decision about having eye surgery to reduce or eliminate your nearsightedness. Only you and your
Visian ICL Consent INTRODUCTION This information is being provided to you so that you can make an informed decision about having eye surgery to reduce or eliminate your nearsightedness. Only you and your
1/16/2018. William D. Townsend, O.D., F.A.A.O. Advanced Eye Care Canyon, TX Adjunct Professor UHCO Houston, TX.
 William D. Townsend, O.D., F.A.A.O. Advanced Eye Care Canyon, TX Adjunct Professor UHCO Houston, TX drbilltownsend@gmail.com 1 Alcon CIBA Odyssey Medical Science Based Health Shire TearLab Any product
William D. Townsend, O.D., F.A.A.O. Advanced Eye Care Canyon, TX Adjunct Professor UHCO Houston, TX drbilltownsend@gmail.com 1 Alcon CIBA Odyssey Medical Science Based Health Shire TearLab Any product
Incidence Of Dry Eye Disesase In People Living With Acquired Immuodeficiency Synrome
 IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 15, Issue 9 Ver. X (September). 2016), PP 41-46 www.iosrjournals.org Incidence Of Dry Eye Disesase In
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 15, Issue 9 Ver. X (September). 2016), PP 41-46 www.iosrjournals.org Incidence Of Dry Eye Disesase In
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Medical Science. Research Paper. 2nd yr post graduate, Dept. Of Ophthalmology, SVS Medical College
 Dr. A.Narasimha Rao Dr. N.Deepika Dr. D.Chandrakanth Reddy Dr. M. Vijaya Ramaraju ABSTRACT KEYWORDS Research Paper Medical Science Comparative Evaluation of the Anti- Inflammatory Effect of Topical 1 %
Dr. A.Narasimha Rao Dr. N.Deepika Dr. D.Chandrakanth Reddy Dr. M. Vijaya Ramaraju ABSTRACT KEYWORDS Research Paper Medical Science Comparative Evaluation of the Anti- Inflammatory Effect of Topical 1 %
Combined Medical and Surgical Treatment of Severe Vernal Keratoconjunctivitis
 CLINICAL INVESTIGATIONS Combined Medical and Surgical Treatment of Severe Vernal Keratoconjunctivitis Hiroshi Fujishima,*, Kazumi Fukagawa,* Yoshiyuki Satake,* Ichiro Saito, Jun Shimazaki,* Yoji Takano*,
CLINICAL INVESTIGATIONS Combined Medical and Surgical Treatment of Severe Vernal Keratoconjunctivitis Hiroshi Fujishima,*, Kazumi Fukagawa,* Yoshiyuki Satake,* Ichiro Saito, Jun Shimazaki,* Yoji Takano*,
Author's response to reviews
 Author's response to reviews Title: Pneumococcal vaccination and otitis media in Australian Aboriginal infants: comparison of two birth cohorts before and after introduction of vaccination Authors: Grant
Author's response to reviews Title: Pneumococcal vaccination and otitis media in Australian Aboriginal infants: comparison of two birth cohorts before and after introduction of vaccination Authors: Grant
Phone [850] Fax [850] Web Send s to: Search Millseye to download App Page 1 of 5
![Phone [850] Fax [850] Web Send s to: Search Millseye to download App Page 1 of 5 Phone [850] Fax [850] Web Send s to: Search Millseye to download App Page 1 of 5](/thumbs/81/84009052.jpg) YAG PC (Posterior Capsulotomy) Consent Form 1) I,, hereby authorize: David M. Mills, MD, FACS and/or whomever he may designate as his assistant(s), to perform upon myself the following operation(s): YAG
YAG PC (Posterior Capsulotomy) Consent Form 1) I,, hereby authorize: David M. Mills, MD, FACS and/or whomever he may designate as his assistant(s), to perform upon myself the following operation(s): YAG
AUSTRALIAN PRODUCT INFORMATION FLAREX (FLUOROMETHOLONE ACETATE) EYE DROPS SUSPENSION
 AUSTRALIAN PRODUCT INFORMATION FLAREX (FLUOROMETHOLONE ACETATE) EYE DROPS SUSPENSION 1 NAME OF THE MEDICINE Fluorometholone acetate. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION The active ingredient in
AUSTRALIAN PRODUCT INFORMATION FLAREX (FLUOROMETHOLONE ACETATE) EYE DROPS SUSPENSION 1 NAME OF THE MEDICINE Fluorometholone acetate. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION The active ingredient in
REAL-WORLD UTILIZATION PATTERNS OF CYCLOSPORINE OPHTHALMIC EMULSION 0.05% WITHIN MANAGED CARE
 REAL-WORLD UTILIZATION PATTERNS OF CYCLOSPORINE OPHTHALMIC EMULSION 0.05% WITHIN MANAGED CARE Tina H Chiang 1, John G Walt 1, John P McMahon, Jr. 2, James E Mansfield, Jr. 2, Susan Simonyi 3 1 Allergan
REAL-WORLD UTILIZATION PATTERNS OF CYCLOSPORINE OPHTHALMIC EMULSION 0.05% WITHIN MANAGED CARE Tina H Chiang 1, John G Walt 1, John P McMahon, Jr. 2, James E Mansfield, Jr. 2, Susan Simonyi 3 1 Allergan
Cataract surgery with Dr Wittles
 17 Belair Road Kingswood SA 5062 Phone: 08 8357 8833 Fax: 08 8271 9809 Email: admin@kingswoodeyecentre.com.au Website: www.kingswoodeyecentre.com.au Cataract surgery with Dr Wittles Date of surgery:..
17 Belair Road Kingswood SA 5062 Phone: 08 8357 8833 Fax: 08 8271 9809 Email: admin@kingswoodeyecentre.com.au Website: www.kingswoodeyecentre.com.au Cataract surgery with Dr Wittles Date of surgery:..
PRECISION PROGRAM. Injection Technique Quick-Reference Guide. Companion booklet for the Video Guide to Injection Technique
 Injection Technique Quick-Reference Guide PRECISION PROGRAM Companion booklet for the Video Guide to Injection Technique Available at www.ozurdexprecisionprogram.com Provides step-by-step directions with
Injection Technique Quick-Reference Guide PRECISION PROGRAM Companion booklet for the Video Guide to Injection Technique Available at www.ozurdexprecisionprogram.com Provides step-by-step directions with
A DRY EYE DISEASE DECISION TREE
 A DRY EYE DISEASE DECISION TREE With the DEWS guidelines almost 10 years old, new approaches are needed. BY JASON SCHMIT, OD I am sometimes asked about medical decision-making by students, interns, fellows,
A DRY EYE DISEASE DECISION TREE With the DEWS guidelines almost 10 years old, new approaches are needed. BY JASON SCHMIT, OD I am sometimes asked about medical decision-making by students, interns, fellows,
sodium [2-(2,6-dichloroanilino)phenyl] acetate, a phenylacetic acid derivative CH 2 COONa
![sodium [2-(2,6-dichloroanilino)phenyl] acetate, a phenylacetic acid derivative CH 2 COONa sodium [2-(2,6-dichloroanilino)phenyl] acetate, a phenylacetic acid derivative CH 2 COONa](/thumbs/78/77533900.jpg) PRODUCT INFORMATION VOLTAREN * OPHTHA Eye Drop (diclofenac sodium) NAME OF THE MEDICINE Active ingredient: Chemical name: Molecular formula: C 14 H 10 Cl 2 NNaO 2 CAS number: 15307-79-6 Molecular weight:
PRODUCT INFORMATION VOLTAREN * OPHTHA Eye Drop (diclofenac sodium) NAME OF THE MEDICINE Active ingredient: Chemical name: Molecular formula: C 14 H 10 Cl 2 NNaO 2 CAS number: 15307-79-6 Molecular weight:
Response letter. Dear Reviewers,
 Dear Reviewers, Response letter Thank you for your comments. On behalf of my co-authors, we very much appreciate the time and effort you have put into your comments on our manuscript (NO.35562). Your reviews
Dear Reviewers, Response letter Thank you for your comments. On behalf of my co-authors, we very much appreciate the time and effort you have put into your comments on our manuscript (NO.35562). Your reviews
Effectiveness and relevant factors of 2 % rebamipide ophthalmic suspension treatment in dry eye
 Ueda et al. BMC Ophthalmology (2015) 15:58 DOI 10.1186/s12886-015-0040-0 RESEARCH ARTICLE Open Access Effectiveness and relevant factors of 2 % rebamipide ophthalmic suspension treatment in dry eye Kaori
Ueda et al. BMC Ophthalmology (2015) 15:58 DOI 10.1186/s12886-015-0040-0 RESEARCH ARTICLE Open Access Effectiveness and relevant factors of 2 % rebamipide ophthalmic suspension treatment in dry eye Kaori
Title: Differences between patients' and clinicians' report of sleep disturbance: A field study in mental health care in Norway.
 Author's response to reviews Title: Differences between patients' and clinicians' report of sleep disturbance: A field study in mental health care in Norway. Authors: Håvard Kallestad (havard.kallestad@ntnu.no)
Author's response to reviews Title: Differences between patients' and clinicians' report of sleep disturbance: A field study in mental health care in Norway. Authors: Håvard Kallestad (havard.kallestad@ntnu.no)
Date Initials Comments
 Month 03 Data Processing Cover Sheet ID. No.: - Alpha Code: Visit: 03 FORMS NOTES Check all forms completed: Ocular Surface Disease Index (OSI) Brief Ocular Discomfort Inventory (ODI) Follow-up Health
Month 03 Data Processing Cover Sheet ID. No.: - Alpha Code: Visit: 03 FORMS NOTES Check all forms completed: Ocular Surface Disease Index (OSI) Brief Ocular Discomfort Inventory (ODI) Follow-up Health
Safety of Intracameral Moxifloxacin/ Dexamethasone Fixed-Dose Formulation on the Corneal Endothelium in a Rabbit Model
 Original Article Philippine Journal of OPHTHALMOLOGY Safety of Intracameral Moxifloxacin/ Dexamethasone Fixed-Dose Formulation on the Corneal Endothelium in a Rabbit Model Reginald Robert Tan, MD, Joseph
Original Article Philippine Journal of OPHTHALMOLOGY Safety of Intracameral Moxifloxacin/ Dexamethasone Fixed-Dose Formulation on the Corneal Endothelium in a Rabbit Model Reginald Robert Tan, MD, Joseph
84 Year Old with Rosacea
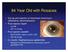 84 Year Old with Rosacea S/p tap and injection of intravitreal vancomycin, ceftazidime, dexamethasone Post-injection day#1 Va HM IOP 14 mmhg Post-injection week#3 BCVA 20/20-3 (plano +0.50 x 180) IOP 23
84 Year Old with Rosacea S/p tap and injection of intravitreal vancomycin, ceftazidime, dexamethasone Post-injection day#1 Va HM IOP 14 mmhg Post-injection week#3 BCVA 20/20-3 (plano +0.50 x 180) IOP 23
