Laura Bonnett, Catrin Tudur Smith, David Smith, Paula Williamson, David Chadwick, Anthony G Marson
|
|
|
- Elfreda Henry
- 5 years ago
- Views:
Transcription
1 Prognostic factors for time to treatment failure and time to 1 months of remission for patients with focal epilepsy: post-hoc, subgroup analyses of data from the SANAD trial Laura Bonnett, Catrin Tudur Smith, David Smith, Paula Williamson, David Chadwick, Anthony G arson Summary Background Epilepsy is a heterogeneous disorder, with outcomes ranging from immediate remission after taking a first antiepileptic drug to frequent unremitting seizures with multiple treatment failures. ew prognostic models enable prediction of outcome; we therefore aimed to use data from the SANAD study to predict outcome overall and for patients receiving specific treatments. ethods The SANAD study was a randomised controlled trial in which standard antiepileptic drugs were compared with new treatments. Arm A included patients for whom carbamazepine was considered the first-line treatment, most of whom were newly diagnosed with focal epilepsy. Patients were randomly assigned to receive carba mazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate. Outcomes were time to treatment failure overall, because of inadequate seizure control, and because of adverse events, and time to 1 months of remission from seizures. In this post-hoc study we used regression multivariable modelling to investigate how clinical factors affect the probability of treatment failure and the probability of achieving 1 months of remission. indings or time to treatment failure, we identified several significant risk factors: sex (male vs female, hazard ratio [HR] 0 86, 95% CI ), treatment history (taking non-sanad antiepileptic drugs [other than those listed above] vs treatment naive, 1 7, ), age (eg, older than 71 years vs years or younger, 0 68, ), total number of seizures (eg, four to 11 seizures vs two or fewer, 8, ), electroencephalogram results (epileptiform abnormality vs normal, 1 6, ), seizure type (eg, secondary generalised vs simple or complex partial only, 0 78, ), site of onset (not localised vs temporal lobe, 1 5, ), and treatment (lamotrigine vs carbamazepine, 0 76, ). Significant factors for time to 1 months of remission were sex (male vs female, 1 19, ), treatment history (taking a non-sanad antiepileptic drug vs treatment naive, 0 64, ), age (eg, older than 71 years vs years or younger, 1 60, ), time from first seizure (60 39 months vs months, 1 14, 1 1 9; > months vs months, 1 39, ), neurological insult (presentv absent, 0, ), total number of seizures before randomisation (eg, four to 11 vs two or fewer, 0 87, ), and treatment (gabapentin vs carbamazepine, 0 71, ; topiramate vs carbamazepine, 0 81, ). Lancet Neurol 01; 4: 331 Published Online ebruary 8, 01 DOI:.16/S (1) See Comment page 98 Department of Biostatistics (L Bonnett mathstat, C Tudur Smith PhD, P Williamson PhD) and Department of olecular and Clinical Pharmacology (Prof A G arson D), University of Liverpool, Liverpool, UK; and The Walton Centre oundation NHS Trust, Liverpool, UK (D Smith D, Prof D Chadwick D) Correspondence to: Prof Anthony G arson, Department of olecular and Clinical Pharmacology, University of Liverpool, Clinical Sciences Centre for Research and Education, azakerley, Liverpool L9 7LJ, UK a.g.marson@liverpool.ac.uk Interpretation We present a thorough investigation of prognostic factors from a large randomised controlled trial in patients starting antiepileptic monotherapy. If validated, our models could aid in individual patient risk stratification and the design and analysis of epilepsy trials. unding National Institute for Health Research (UK). Introduction ew epilepsy trials have been designed to be pragmatic, to recruit a broad heterogeneous population representative of clinical practice, and to provide data that will inform everyday decision making. Arm A of the Standard and New Antiepileptic Drug (SANAD) trial 1 recruited 171 patients for whom clinicians considered carbamazepine to be the first-line standard treatment, 89% of whom had a focal epilepsy. Patients were randomly assigned to receive treatment with carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate. The results showed that lamotrigine was a potential first-line treatment because it was significantly superior to carbamazepine for time to treatment failure (hazard ratio [HR] 0 78, 95% CI ), but not significantly different to carbamazepine for time to 1 months of remission. Gabapentin and topiramate were identified as unsuitable first-line treatments, gabapentin because of poor efficacy and topiramate because of poor efficacy and poor tolerability. Oxcarbazepine was not significantly different from carbamazepine for either outcome. The epilepsies are a heterogeneous group of disorders, which consist of numerous seizure types and epilepsy syndromes 3 with differing causes, severities, and ages of onset. Although the SANAD results provide overall estimates of treatment effect, 1 a large heterogeneous group of patients was recruited, providing an opportunity to use prognostic modelling to investigate which clinical factors might affect outcome. Previous prognostic models have been derived from the National General Practice Survey of Epilepsy, 4 the edical Research Council antiepileptic drug Vol 11 April
2 withdrawal study, 5 and the multicentre study of early epilepsy and single seizures. 6 These models identified patient charac teristics that modify seizure recurrence risks, informing decisions about whether to stop antiepileptic drug treatment for patients with seizures in remission or whether to start antiepileptic drug treatment for patients with one or few seizures. However, few prognostic models based on data from prospective cohorts or randomised controlled trials have been published, and none represent an epilepsy cohort accrued at the start of antiepileptic drug treatment. In this Article we have modelled data from SANAD arm A to identify clinical factors that affect outcome when antiepileptic drug treatment is started. ethods Patients and procedures Patients were eligible for inclusion in arm A of the SANAD study if, in the previous year, they had had at least two clinically definite unprovoked epileptic seizures, if they were at least 5 years old, and if the recruiting clinician deemed carbamazepine, not valproate, to be the optimum standard treatment. Between Dec 1, 1999, and June 1, 001, patients were allocated in a ratio of 1:1:1:1 to receive carbamazepine, gabapentin, lamotrigine, or topiramate. rom June 1, 001, to Aug 31, 004, an oxcarbazepine group was added to the trial and patients were randomly allocated in a ratio of 1:1:1:1:1 to receive carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate. SANAD had two primary outcomes. The first was time to treatment failure from randomisation. Treatment failure can be split into two categories: inadequate seizure control for which the randomly assigned drug might be withdrawn or a second treatment added, or failure because of unacceptable adverse events. Patients were categorised into these two main failure groups as was done in the original SANAD analyses. 1,7 The second outcome was time to the first period of 1 months of remission from seizures. The methods for the SANAD study have been published in full. 1 SANAD received appropriate multicentre research committee approvals, and was managed according to the edical Research Council good clinical practice guidelines. 8 Patients provided informed written consent for inclusion and long-term follow-up. SANAD is registered with the International Standard Randomized Controlled Trial Number Register, number ISRCTN Prognostic modelling Our aim was to identify two sets of factors one that predicted time to 1 months of remission, and one that predicted time to treatment failure. On the basis of clinical consensus and our knowledge of previous prognostic studies in epilepsy, 9, we compiled a list of potential prognostic factors: sex, febrile seizure history, first degree relative with epilepsy, CT or RI scan results, treatment history, age, time from first seizure to randomisation, neurological insult (eg, hemiparesis), total number of seizures before randomisation, electroencephalogram (EEG) results, seizure type, and epilepsy type. or the CT and RI scans, results were classified as normal, abnormal, or not done. Patients were classified as having had a neurological insult if they had learning disabilities or a neurological deficit. EEGs were classified as normal, not done, non-specific 1836 patients assessed for eligibility 115 excluded (did not provide consent) 171 randomly assigned 378 allocated to receive carbamazepine 377 allocated to receive gabapentin 378 allocated to receive lamotrigine allocated to receive oxcarbazepine 378 allocated to receive topiramate removed not epilepsy 9 extremes* 3 generalised 19 removed not epilepsy 8 extremes* 1 generalised 0 removed 8 not epilepsy 7 extremes* 5 generalised 16 removed 8 not epilepsy 3 extremes* 5 generalised 4 removed 8 not epilepsy 7 extremes* 9 generalised 7 missing outcome data 1 for treatment failure 6 for 1 month remission 9 missing outcome data for treatment failure 7 for 1 month remission 6 missing outcome data 1 for treatment failure 5 for 1 month remission 3 missing outcome data 1 for treatment failure for 1 month remission 19 missing outcome data 7 for treatment failure 1 for 1 month remission 355 analysed for treatment failure 350 analysed for 1 month remission 356 analysed for treatment failure 351 analysed for 1 month remission 357 analysed for treatment failure 353 analysed for 1 month remission 193 analysed for treatment failure 19 analysed for 1 month remission 347 analysed for treatment failure 34 analysed for 1 month remission igure 1: Trial profile *Patients with a time from first seizure to randomisation in the first or last 1% of the variable were removed from the data set. 1 Patients with generalised epilepsy were removed from analyses Vol 11 April 01
3 abnormality, or epileptiform abnor mality (focal or generalised spikes or spike and slow wave activity). Seizure types were classified according to the International League Against Epilepsy seizure classification. Epilepsy type was first classified as focal, generalised, or unclassified. When there was uncertainty between focal onset and generalised onset seizures, patients were recorded as having had unclassified convulsive or other unclassified seizures. ocal epilepsy was further classified as temporal lobe, frontal lobe, parietal lobe, occipital lobe, benign focal epilepsy, or focal epilepsy not localised. or the regression modelling, because of the small numbers of participants, occipital lobe, parietal lobe, and benign focal epilepsy were combined into one group ( other ). Only 9 patients were classified as having generalised epilepsy; hence, they were removed from the dataset. Variables associated with an increased chance of treatment failure and an increased chance of achieving 1 months of remission were determined after adjust ment for multiple variables with Cox proportional hazards modelling. Because oxcarbazepine was included only after June 1, 001, all analyses were stratified by randomisation period to account for the reduced number of patients who took this drug. Variables were centred to reduce multicolinearity 11 and tested with the likelihood ratio test. 1 Bestfitting, parsimonious, multivariable models were produced with backwards elimination. rom the multivariable model we calculated the probability of the event for combinations of risk factors of age, sex, and total number of seizures. The proportional hazards assumption was tested with Schoenfeld residual plots 13 and in corporation of time-dependent covariate effects. Internal validity was (n=355) Gabapentin (n=356) (n=357) Oxacarbaepine (n=193) Topiramate (n=347) Total (n=1608) ales 196 (55%) 197 (55%) 199 (56%) (53%) 193 (56%) 887 (55%) History of febrile seizures 4 (7%) 15 (4%) (6%) 7 (4%) 13 (4%) 81 (5%) irst degree relative with epilepsy 36 (%) 41 (1%) 33 (9%) 1 (11%) 34 (%) 165 (%) Treatment history Treatment naive 90 (8%) 89 (81%) 9 (8%) 167 (87%) 8 (81%) 130 (8%) Taking non-sanad antiepileptic drug* 57 (16%) 56 (16%) 58 (16%) 3 (1%) 57 (16%) 51 (16%) Seizures after remission 8 (%) 11 (3%) 7 (%) (1%) 9 (3%) 37 (%) Age at randomisation (years; median [IQR]) 38 (5 53) 36 (4 50) 34 ( 51) (7 56) 37 (5 5) 38 (4 5) Time from first seizure to randomisation 1 4 ( ) 1 3 ( ) 1 4 ( ) 1 3 ( ) 1 4 ( ) 1 4 ( ) (years; median [IQR]) Neurological insult 4 (1%) 41 (1%) 41 (11%) 16 (8%) 43 (1%) 113 (7%) Total seizures before randomisation 1 (4 ) 14 (4 70) 1 (4 60) 11 (4 53) 1 (4 0) 1 (4 70) (median [IQR]) Seizure type Simple or complex partial only 1 (34%) 11 (31%) 5 (9%) 55 (8%) 116 (33%) 5 (3%) Secondary generalised tonic-clonic 0 (57%) 09 (59%) 16 (61%) 117 (61%) 193 (56%) 937 (58%) Uncertain 31 (9%) 35 (%) 36 (%) 1 (11%) 38 (11%) 161 (%) Epilepsy type ocal 35 (9%) 33 (91%) 31 (90%) 173 (90%) 311 (90%) 1453 (90%) Temporal 150 (46%) 10 (37%) 7 (33%) 56 (3%) 114 (37%) 547 (38%) rontal 0 (6%) 17 (5%) 34 (11%) 5 (3%) 31 (%) 7 (7%) Other 0 (6%) 8 (9%) 17 (5%) 13 (8%) 1 (6%) 99 (7%) Not localised 135 (4%) 158 (49%) 163 (51%) 99 (57%) 145 (47%) 700 (48%) Unclassified 30 (8%) 33 (9%) 36 (%) 0 (%) 36 (%) 155 (%) EEG results Normal 150 (4%) 177 (50%) 169 (47%) 84 (44%) 144 (41%) 74 (45%) Non-specific abnormality 59 (17%) 48 (13%) 56 (16%) 3 (17%) 59 (17%) 54 (16%) Epileptiform abnormality 113 (3%) 0 (8%) 0 (8%) 54 (8%) 4 (30%) 471 (9%) Not done 33 (9%) 31 (9%) 3 (9%) 3 (1%) (1%) 159 (%) CT or RI results Normal 05 (58%) 5 (63%) 07 (58%) 9 (56%) 188 (54%) 934 (58%) Abnormal 99 (8%) 8 (3%) 83 (3%) 53 (7%) 3 (30%) (6%) Not done 51 (14%) 49 (14%) 67 (19%) 31 (16%) 56 (16%) 54 (16%) Data are n (%), unless otherwise stated. EEG=electroencephalogram. SANAD=Standard and New Antiepileptic Drug trial. *Antiepileptic drugs other than those that were randomly allocated in SANAD. Table 1: Baseline characteristics of patients included in the analysis of time to treatment failure Vol 11 April
4 Overall HR (95% CI) Inadequate seizure control HR (95% CI) Unacceptable adverse events HR (95% CI) Sex emale ale 0 86 (0 0 99) 6 ( ) 0 81 ( ) Treatment history Treatment naive Seizures after remission 1 35 (0 87 ) 0 48 ( ) 13 (1 3 73) Taking non-sanad antiepileptic drugs 1 7 (5 1 53) 1 56 ( 03) 0 (0 1 33) Age (years) ( ) 0 85 ( ) 6 (0 1 1) ( ) 0 68 ( ) 1 14 (1 1 30) ( ) 0 55 ( ) 1 3 (1 1 49) ( ) 0 4 ( ) 1 35 ( 1 79) ( ) 0 31 ( ) 1 50 ( 0) Total number of seizures before randomisation (1 3) 4 (3 5) 0 (0 99 1) (5 1 11) 1 14 (9 1 18) 0 99 (0 95 4) (1 1 3) 1 9 ( ) 0 99 (0 91 7) ( ) 1 51 ( ) 0 98 ( ) ( ) 00 (1 6 47) 0 97 ( ) EEG result Normal Not done 1 5 ( ) 0 79 ( ) 1 34 ( ) Non-specific abnormality ( ) 1 ( ) 1 1 ( ) Epileptiform abnormality 1 6 (7 1 50) 1 1 ( ) 1 11 ( ) Seizure type Simple or complex partial only Secondary generalised tonic-clonic 0 78 ( ) 1 ( ) 0 69 ( ) Uncertain 0 33 ( ) 0 76 ( ) Could not be estimated ocal epilepsy site of onset Temporal Not localised 1 5 (6 1 47) 1 18 ( ) 1 17 ( ) rontal 1 18 ( ) 4 ( ) 1 4 ( ) Other 0 9 ( ) 4 ( ) 0 76 ( ) Unclassified 69 ( ) 1 8 ( ) Could not be estimated Treatment Gabapentin 1 3 (0 1 51) 45 ( ) 0 59 ( ) 0 76 ( ) 5 (0 1 48) 0 6 ( ) Oxcarbazepine 0 94 ( ) 1 1 ( ) 0 84 (0 59 ) Topiramate 1 3 (0 1 5) 1 44 (3 00) 1 ( ) HR=hazard ratio. SANAD=Standard and New Antiepileptic Drug. EEG=electroencephalogram. Table : ultivariable model hazard ratios for time to overall treatment failure, treatment failure because of inadequate seizure control, and treatment failure because of unacceptable adverse events, by prognostic factor See Online for appendix assessed by the c statistic, which assesses the discriminatory power and the predictive accuracy of nonlinear statistical models. 14 Assessment of the different reasons for treatment withdrawal requires a competing risks analysis that includes the probability of one of several different events occurring. Therefore, we did cumulative incidence analyses to assess the probability of one of the two treatment failure events occurring (inadequate seizure control and unacceptable adverse events), with covariates tested by Gray s method. 15 We investigated continuous variables with log and fractional polynomial transformations The results for the continuous variables are presented as categorical variables defined post hoc with categories chosen according to knot positions for a spline model fit to the data. 0 The time from first seizure to randomisation includes extreme values. Therefore people with a time from first seizure to randomisation in the first or last 1% of the variable were removed from the dataset 1 this applied to 34 patients. Role of the funding source The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. Results igure 1 shows the flow of the 171 patients recruited into arm A of SANAD patients were included in the analysis of time to treatment failure and 1588 were included in the analysis of time to 1 months of remission. The baseline demographic data for patients included in the analysis of time to treatment failure are summarised in table 1. Data were much the same for time to 1 months of remission (data not shown). The parsimonious model for overall treatment failure included variables for sex, treatment history, age, total number of seizures before randomisation, EEG result, seizure type, focal epilepsy site of onset, and treatment, which was forced in the model regardless of significance, using the backward elimination process. Table shows the multivariable HRs (see appendix for regression coefficients and standard errors); HR greater than 1 indicates that treatment failure is more likely. The c statistic for the model was 0 6, indicating that the model accurately discriminates patients 60% of the time. Because the aim is to predict patients with poor outcomes, our c statistic suggests that the internal validation of the model is acceptable. Treatment failure decreased as age increased and patients aged years or younger were significantly more likely to have treatment failure. Treatment failure increased as the total number of seizures before randomisation increased and patients with two or fewer seizures before randomisation were significantly less likely to experience treatment failure. Treatment failure was significantly more likely in women than in men, in patients taking a non-sanad antiepileptic drug (ie, not carbamazepine, gabapentin, lamotrigine, oxcarbazepine, Vol 11 April 01
5 Age (years) Sex Seizures (n) Treatment Treatment failure at 1 year (%) Treatment failure at 3 years (%) 0 igure : Combination of risk factors for time to treatment failure Bars show 95% CIs. =male. =female. or topiramate) immediately before randomisation than in patients who were treatment naive, in patients with an epileptiform EEG abnormality than in patients with a normal EEG, in patients with simple or complex partial seizures without secondary generalisation than in patients with secondary gener alised tonic-clonic seizures, in patients with epilepsy that was not localised than in patients with temporal lobe epilepsy, and in patients taking carbamazepine than in patients taking lamotrigine (table ). To show the range of treatment failure rates predicted by the multivariable model, figure shows estimates of the proportion of patients treated with either carbamazepine or lamotrigine having treatment failure 1 year and 3 years after randomisation (see appendix for numerical results). 1 year and 3 years were chosen to represent medium-term and long-term timepoints. Patients were assumed to be treatment naive and to have normal EEG results, simple or complex partial seizures, and temporal lobe epilepsy, which were the most common characteristics in the SANAD dataset. The other variables were altered according to categories of interest (eg, age as years, years, and years; figure ). and carbamazepine were chosen because carbamazepine is the standard treatment for focal epilepsy and lamotrigine was suggested by the results of the SANAD trial as a potential first line treatment after the publication of the initial results of SANAD. Generally, overall treatment failure rates were lower in patients treated with lamotrigine (figure ). The risk of failure decreased slightly with an increase in age and men had a slightly lower chance of treatment failure than did women. An increase in total number of seizures before randomisation also slightly increased the chance of treatment failure. The competing risks model has been fitted to match the model for overall time to treatment failure results (table ). The appendix includes regression coeffi cients and results for the multivariable model including all variables ie, without variable selection. The significant variables in the model for treatment failure because of inadequate seizure control were treatment history, age, total number of seizures before randomisation, and treatment. Compared with treat ment naive patients, patients already taking a non-sanad antiepileptic drug had a high rate of treatment failure because of inadequate seizure control (table ). igure 3 shows relative hazard plots for the continuous Vol 11 April
6 A 1 4 B C Inadequate seizure control Unacceptable adverse events D 5 Inadequate seizure control Unacceptable adverse events Age (years) Total number of seizures before randomisation igure 3: plots for age and total number of seizures before randomisation for the time to treatment failure Hazard ratio estimates with 95% CIs are shown for overall time to treatment failure, for age (A) and total number of seizures (B), and for time to treatment failure because of inadequate seizure control and because of unacceptable adverse events, for age (C) and total number of seizures (D). variables (for associated regression coefficients, see appendix). A linear effect was noted for age, with older patients significantly less likely to have a treatment failure because of inadequate seizure control than were younger patients. The number of seizures before randomisation was positively associated with the overall risk of treatment failure, which was largely caused by an increasing chance of failure because of inadequate seizure control. The significant variables in the model of treatment failure because of unacceptable adverse events were sex, treatment history, age, seizure type, and treatment (table ). Women were more likely to have treatment failure than were men. Patients restarting treatment after remission had a higher treatment failure rate than treatment naive patients. A linear effect was noted for age, with older patients significantly more likely to have a treatment failure because of unacceptable adverse events than were younger patients (figure 3). Patients with simple or complex partial seizures were significantly more likely to have a treatment failure attributable to adverse events than were patients with secondary generalised tonic-clonic seizures (table ). Although the chance of treatment failure for any reason decreased with age, in the competing risks analysis an X-shaped relation was seen (figure 3), in which younger patients have a higher chance of treatment failure because of inadequate seizure control and a lower chance of treatment failure because of adverse events than do older patients, who have a lower chance of treatment Vol 11 April 01
7 failure because of inadequate seizure control and a higher chance of treatment withdrawal because of unacceptable adverse events. Table 3 and the appendix show results for time to 1 months of remission. The factors significantly associated with time to 1 months of remission were sex, treatment history, age, time from first seizure to randomisation, neurological insult, total number of seizures before randomisation, CT or RI scan results, and treatment. The c statistic for the model is 0 7, indicating that the model accurately discriminates patients 70% of the time, which is an acceptable internal validation.,3 or age, the relation is U-shaped with patients aged years or less, or more than 71 years, having a significantly higher chance of remission than that of patients in the middle of the age range (figure 4). Time to 1 months of remission was higher for men than for women, and for treatment naive patients than for patients already taking a non-sanad antiepileptic drug (table 3). Time to 1 months of remission decreased with increasing number of seizures before randomisation (figure 4, table 3). igure 4 shows relative hazard plots for the continuous variables (for associated regression coeffi cients, see appendix). Rates of 1 months of remission were significantly higher for patients on carbamazepine than for those on gabapentin or topiramate (table 3). To show the range of rates of 1 months of remission predicted by the model, figure 5 shows estimates of the proportion of patients achieving a remission 1 year and 3 years after randomisation for patients treated with either carbamazepine or lamotrigine (see appendix for numerical results). Patients were assumed to be treatment naive, to have been randomised 6 months after their first seizure, to not have neurological insult, to have normal CT or RI results, and to have temporal lobe epilepsy, because those were the most common patient characteristics. In the subset of combinations included, remission rates were highest in older patients. en were slightly more likely to achieve remission than were women, as were patients with fewer seizures compared with patients with many seizures. The probability of remission in patients taking carbamazepine increased slightly compared with those taking lamotrigine, but not all the comparisons were significant. Discussion Treatment failure because of unacceptable adverse effects was significantly less likely in men than in women, but no significant difference existed for failure because of inadequate seizure control. This finding could explain the lower time to 1 months of remission in women, if women are less likely to remain on treatment for long enough to achieve therapeutic doses. urther analyses of dose data in SANAD show no difference between the mean doses of carbamazepine, oxcarbazepine, or topiramate taken by men and women, but women did Sex emale 0 ale 1 19 (5 1 35) CT or RI scan result Normal 0 Abnormal 0 88 (0 76 3) Not done 1 1 ( ) Treatment history Treatment naive 0 Seizures after remission 6 ( ) Taking a non-sanad antiepileptic drug 0 64 ( ) Age (years) ( ) ( ) (0 68 7) ( ) (1 6 03) Time from first seizure (months) (0 1) (0 ) (0 7) (1 1 9) 1 39 (4 1 86) Neurological insult Absent 0 Present 0 ( ) Total number of seizures ( ) ( ) ( ) ( ) ( ) ocal epilepsy site of onset Temporal 0 Not localised 0 87 (0 ) rontal 1 1 ( ) Other ( ) Unclassified 1 15 ( ) Treatment 0 Gabapentin 0 71 ( ) 0 90 (0 8) Oxcarbazepine 0 97 ( ) Topiramate 0 81 ( ) HR=hazard ratio. receive lower doses of gabapentin and lamotrigine (data not shown). Baseline bodyweight was not measured in SANAD and therefore cannot be adjusted for. Time to 1 months of remission HR (95% CI) Table 3: ultivariable model hazard ratios for prognostic factors for time to 1 months of remission Vol 11 April
8 A B C Age (years) Time from first seizure (months) Log (total number of seizures) 8 igure 4: plots for age, time from first seizure to randomisation, and total number of seizures for time to 1 months of remission Hazard ratio estimates with 95% CIs are shown for time to 1 months of remission, for age (A), time from first seizure (B), and total number of seizures (C). Age (years) Sex Seizures (n) Treatment Remission at 1 year (%) Remission at 3 years (%) 0 igure 5: Combination of risk factors for time to 1 months of remission Bars show 95% CIs. =male. =female. Patients with an abnormal EEG were more likely to experience treatment failure than were those with a normal EEG, predominantly because of inadequate seizure control. Patients who did not have an EEG done had a lower chance of treatment failure because of inadequate seizure control and a higher chance of failure because of unacceptable adverse events. Patients who did not have an EEG tended to be older (mean age 4 years vs 38 years) and therefore there might have been more clinical certainty that they had focal epilepsy Vol 11 April 01
9 en were more likely to achieve 1 months of remission than were women, which might be because women had a higher rate of treatment withdrawal because of adverse events, which precedes a change in treatment and a delay in controlling seizures. The relation between age and 1 months of remission is U-shaped the chance of 1 months of remission is higher in children and elderly people than in those in the mid-age range (approximately 0 50 years old). This relation might be because of the different aetiologies in different age groups, pharmacokinetics, or other factors, and should be investigated further. As might be anticipated, patients who were already taking an anti epileptic drug but needed to change treatment had a lower chance of having 1 months of remission than did treatment naive patients. The chance of remission that lasted 1 months increased with time between first seizure and randomisation but decreased with increasing number of seizures before randomisation, indicating that patients with a larger number of seizures over a shorter period before starting treatment had a reduced chance of remission. Of the antiepileptic drugs, carbamazepine was associated with the highest rates of remission that lasted for 1 months. The SANAD study is the largest randomised controlled trial in epilepsy and includes data for long-term treatment outcomes, which is essential to inform the management of this chronic condition (panel). A heterogeneous group of patients was recruited, which some have criticised 6,7 but we argue that this is a strength, as shown by this Article, because such a group enables thorough investigation of the factors that affect treatment outcome; the multivariable models in this Article include up to nine clinical factors. The SANAD investigators were not masked to treatment allocation, which could have affected outcome assess ment for example, decisions about whether a treatment had failed although dosing data indicate that reasonable doses were tried to give each treatment the best chance before it was decided that treatment had failed. Although randomised controlled trials are the best method for assessing treatment outcomes, they recruit a selected population, which might affect estimates of prognosis. Ideally SANAD would have recruited a greater proportion of children and elderly patients; nonetheless, the analysis has clearly shown the effect of age on outcome. Participants were predominantly examined by neurologists experienced at identifying and classifying seizures, but a further challenge in outpatient studies of seizures and epilepsy, such as SANAD, is that seizures are reported to the clinician by the patient, and patients might under-report the occurrence of seizures. Validation of patient reporting in an outpatient population with infrequent seizures is difficult and has not been done to date. Recruiting clinicians were asked to estimate the likely site of seizure onset or if uncertain they were able to categorise the patient as such. This approach might lead to some imprecision, although it does allow patients Panel: Research in context Systematic review We identified studies published between Dec 1, 1946, and Jan 0, 01, by searching edline with the terms prognostic model, prognostic factor, predictive model, predictive factor, epilepsy, and seizures. Searches were restricted to human studies. Any prognostic factor studies or prognostic models reporting a seizure outcome were included. All included clinical trials were assessed by LB for methodological quality for randomisation, masking, and proportion of patients lost to follow-up. Interpretation Although other prognostic models have been constructed for epilepsy 9,4,5 no other epilepsy monotherapy trial has had sufficient power to investigate prognostic factors thoroughly. 6 Analysis of the National General Practice Study of Epilepsy 4 a large prospective population-based observational study identified only one independent predictor of 1 year and year remission: the number of seizures the patient had had in the 6 months after the first seizure. However, many patients in the National General Practice Study of Epilepsy were not prescribed antiepileptic drugs. Our Article extends previous studies because it is a thorough investigation of prognostic factors with data from a large randomised controlled trial in which patients were prescribed antiepileptic drugs. We have identified numerous prognostic factors for treatment failure and 1 months of remission from seizures and have produced models that should enable the estimation of these outcomes for individual patients. to be categorised in a way that clinicians are familiar with and allows assessment of whether this categorisation is of prognostic value. We have presented models that have the potential to inform patient counselling and treatment decisions, and internal validation of our two models suggests an adequate model fit. However, these models should be validated in other similar datasets and their predictive power should be tested. Unfortunately, no other datasets that are similar to SANAD exist. The best match is a set of individual participant data we have collected. 8 However, these data do not include covariates that are significant in the multivariable model and the treatments to which patients were randomly allocated do not always coincide with the drugs tested in SANAD. Therefore more work should be done to determine how best to overcome these difficulties. Our results show the heterogeneity of outcome in epilepsy and the complex interplay between the factors that affect the condition. Patients with different risks of treatment failure and achieving 1 months of remission could be identified when antiepileptic drug treatment is initiated. If validated, our results might improve predictions of outcome for patients and enable identification of patients more likely to have a poor treatment outcome, who might need to be followed up more regularly. The models might also help with identification of patients with poor seizure control outcomes who might be eligible to participate in trials of new treatments, particularly treatments that might have a greater risk of adverse events than do conventional antiepileptic drugs, such as anti-inflammatory and other potential disease-modifying treatments. Vol 11 April
10 Although we have identified clinical predictors of outcome, the mechanism by which these factors affect outcome are still poorly understood and some variability is unexplained. Personalised medicine and pharmacogenetics hold much interest and predictors of rare hypersensitivity reactions have been identified, 9 but predictors of seizure control or common adverse effects have not yet been established. Contributors LJB analysed the data and drafted and revised the Article. CTS and PRW provided support for statistical analyses and drafted and revised the Article. AG, DS, and DWC coordinated the SANAD trial, provided clinical input, and drafted and revised the Article. Conflicts of interest We declare that we have no conflicts of interest. Acknowledgments The SANAD trial was funded by the Health Technology Assessment programme (HTA). Our Article received financial support from the National Institute for Health Research (NIHR) Programme Grants for Applied Research funding scheme (RP-PG ). The views and opinions expressed in this Article do not necessarily represent those of the NHS, the HTA, or the Department of Health. References 1 arson AG, Al-Kharusi A, Alwaidh, et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial. Lancet 007; 369: Berg AT, Berkovic S, Brodie J, et al. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, Epilepsia 0; 51: Engel JJ. ILAE classification of epilepsy syndromes. Epilepsy Res 006; 70 (suppl 1): 5. 4 Cockerell OC, Sander JWAS, Hart Y, Shorvon SD, Johnson AL. Remission of epilepsy: results from the National General-Practice Study of Epilepsy. Lancet 1995; 346: edical Research Council Antiepileptic Drug Withdrawal Study Group. Randomised study of antiepileptic drug withdrawal in patients in remission. Lancet 1991; 337: arson A, Jacoby A, Johnson A, Kim L, Gamble C, Chadwick D, on behalf of the edical Research Council ESS Study Group. Immediate versus deferred antiepileptic drug treatment for early epilepsy and single seizures: a randomised controlled trial. Lancet 005; 365: arson AG, Al-Kharusi A, Alwaidh, et al. The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for generalised and unclassifiable epilepsy: an unblinded randomised controlled trial. Lancet 007; 369: edical Research Council. Guidelines for Good Clinical Practice in Clinical Trials. London: edical Research Council, Kim LG, Johnson TL, arson AG, Chadwick DW. Prediction of risk of seizure recurrence after a single seizure and early epilepsy: further results from the ESS trial. Lancet Neurol 006; 5: 317. acdonald BK, Johnson AL, Goodridge D, Cockerell OC, Sander JWAS, Shorvon SD. actors predicting prognosis of epilepsy after presentation with seizures. Ann Neurol 000; 48: Kraemer HC, Blasey C. Centring in regression analyses: a strategy to prevent errors in statistical inference. Int J ethods Psychiatr Res 004; 13: Collett D. odelling Survival Data in edical Research. Boca Raton: Chapman & Hall/CRC, Schoenfeld D. Partial residuals for the proportional hazards regression-model. Biometrika 198; 69: Harrell E, Lee KL, ark DB. ultivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat ed 1996; 15: Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988; 16: Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol 1999; 8: Royston P, Altman DG. Regression using fractional polynomials of continuous covariates parsimonious parametric modeling. J R Stat Soc Ser C Appl Stat 1994; 43: Royston P, Sauerbrei W. ultivariable odel-building a pragmatic approach to regression analysis based on fractional polynomials for modelling continuous variables. Chichester: Wiley, Royston P, Sauerbrei W. Building multivariable regression models with continuous covariates in clinical epidemiology with an emphasis on fractional polynomials. ethods Inf ed 005; 44: Stone CJ. Comment: generalized additive models. Stat Sci 1986; 1: 3. 1 Royston P, Sauerbrei W. Improving the robustness of fractional polynomial models by preliminary covariate transformation: a pragmatic approach. Comput Stat Data Anal 007; 51: Hosmer DW, Lemeshow S. Applied Logistic Regression. nd edn. New York: John Wiley & Sons, Harrell E Jr. Regression modeling stategies: with applications to linear models, logistic regression, and survival analysis. New York: Springer, Bonnett LJ, Tudur-Smith C, Williamson PR, arson AG. Risk of recurrence after a first seizure and implications for driving: further analysis of the ulticentre study of early Epilepsy and Single Seizures. BJ 0; 341: c Bonnett LJ, Shukralla A, Tudur-Smith C, Williamson PR, arson AG. Seizure recurrence after antiepileptic drug withdrawal and the implications for driving: further results from the RC Antiepileptic Drug Withdrawal Study and a systematic review. J Neurol Neurosurg Psychiatry 011; 8: Panayiotopoulos CP. Old versus new antiepileptic drugs: the SANAD study. Lancet 007; 370: Panayiotopoulos CP. Evidence-based epileptology, randomized controlled trials, and SANAD: a critical clinical view. Epilepsia 007; 48: Tudur Smith C, arson A, Chadwick D, Williamson P. ultiple treatment comparisons in epilepsy monotherapy trials. Trials 007; 8:34. 9 ccormack, Alfirevic A, Bourgeois S, et al. HLA-A*31 and carbamazepine-induced hypersensitivity reactions in Europeans. N Engl J ed 011; 364: Vol 11 April 01
Treatment outcome after failure of a first antiepileptic drug
 Treatment outcome after failure of a first antiepileptic drug Laura J. Bonnett, PhD Catrin Tudur Smith, PhD Sarah Donegan, PhD Anthony G. Marson, PhD Correspondence to Prof. Marson: A.G.Marson@liverpool.ac.uk
Treatment outcome after failure of a first antiepileptic drug Laura J. Bonnett, PhD Catrin Tudur Smith, PhD Sarah Donegan, PhD Anthony G. Marson, PhD Correspondence to Prof. Marson: A.G.Marson@liverpool.ac.uk
L J Bonnett, 1 G A Powell, 2 C Tudur Smith, 1 AG Marson 2. Research. Open Access
 To cite: Bonnett LJ, Powell GA, Tudur Smith C, et al. Risk of a seizure recurrence after a breakthrough seizure and the implications for driving: further analysis of the standard versus new antiepileptic
To cite: Bonnett LJ, Powell GA, Tudur Smith C, et al. Risk of a seizure recurrence after a breakthrough seizure and the implications for driving: further analysis of the standard versus new antiepileptic
Prediction of risk of seizure recurrence after a single seizure and early epilepsy: further results from the MESS trial
 Prediction of risk of seizure recurrence after a single seizure and early epilepsy: further results from the MESS trial Lois G Kim, Tony L Johnson, Anthony G Marson, David W Chadwick on behalf of the MRC
Prediction of risk of seizure recurrence after a single seizure and early epilepsy: further results from the MESS trial Lois G Kim, Tony L Johnson, Anthony G Marson, David W Chadwick on behalf of the MRC
Keywords: treatment; epilepsy; population based cohort Institute of Neurology, University College London, London WC1N 3BG, UK
 632 Institute of Neurology, University College London, London WC1N 3BG, UK S D Lhatoo JWASSander S D Shorvon Correspondence to: Professor J W Sander, Department of Clinical and Experimental Epilepsy, Institute
632 Institute of Neurology, University College London, London WC1N 3BG, UK S D Lhatoo JWASSander S D Shorvon Correspondence to: Professor J W Sander, Department of Clinical and Experimental Epilepsy, Institute
Statistical Models for Censored Point Processes with Cure Rates
 Statistical Models for Censored Point Processes with Cure Rates Jennifer Rogers MSD Seminar 2 November 2011 Outline Background and MESS Epilepsy MESS Exploratory Analysis Summary Statistics and Kaplan-Meier
Statistical Models for Censored Point Processes with Cure Rates Jennifer Rogers MSD Seminar 2 November 2011 Outline Background and MESS Epilepsy MESS Exploratory Analysis Summary Statistics and Kaplan-Meier
p ผศ.นพ.ร งสรรค ช ยเสว ก ล คณะแพทยศาสตร ศ ร ราชพยาบาล
 Natural Course and Prognosis of Epilepsy p ผศ.นพ.ร งสรรค ช ยเสว ก ล คณะแพทยศาสตร ศ ร ราชพยาบาล Introduction Prognosis of epilepsy generally means probability of being seizure-free after starting treatment
Natural Course and Prognosis of Epilepsy p ผศ.นพ.ร งสรรค ช ยเสว ก ล คณะแพทยศาสตร ศ ร ราชพยาบาล Introduction Prognosis of epilepsy generally means probability of being seizure-free after starting treatment
Staging of Seizures According to Current Classification Systems December 10, 2013
 Staging of Seizures According to Current Classification Systems December 10, 2013 Elinor Ben-Menachem, M.D.,Ph.D, Instituet of Clinical Neuroscience and Physiology, Sahlgren Academy, Goteborg University,
Staging of Seizures According to Current Classification Systems December 10, 2013 Elinor Ben-Menachem, M.D.,Ph.D, Instituet of Clinical Neuroscience and Physiology, Sahlgren Academy, Goteborg University,
Review of health related quality of life evidence in the TLoC population
 Appendix H Review of health related quality of life evidence in the TLoC population The aim of this review is to summarise the available evidence on healthrelated quality of life (HRQoL) that could be
Appendix H Review of health related quality of life evidence in the TLoC population The aim of this review is to summarise the available evidence on healthrelated quality of life (HRQoL) that could be
When to start, which drugs and when to stop
 When to start, which drugs and when to stop Dr. Suthida Yenjun, MD. PMK Epilepsy Annual Meeting 2016 The main factors to consider in making the decision The risk for recurrent seizures, which varies based
When to start, which drugs and when to stop Dr. Suthida Yenjun, MD. PMK Epilepsy Annual Meeting 2016 The main factors to consider in making the decision The risk for recurrent seizures, which varies based
Improving trial methodology: Examples from epilepsy. Tony Marson University of Liverpool
 Improving trial methodology: Examples from epilepsy Tony Marson University of Liverpool This talk Examples of trial methodology research Focus on epilepsy but Examples are relevant to any field Epilepsy
Improving trial methodology: Examples from epilepsy Tony Marson University of Liverpool This talk Examples of trial methodology research Focus on epilepsy but Examples are relevant to any field Epilepsy
BIBLIOGRAPHIC REFERENCE TABLE FOR SODIUM VALPROATE IN CHILDHOOD EPILEPSY
 BIBLIOGRAPHIC REFERENCE TABLE FOR SODIUM VALPROATE IN CHILDHOOD EPILEPSY Bibliographic Marson AG et al. for (Review). The Cochrane 2000 De Silva M et al. Romised or for childhood. Lancet, 1996; 347: 709-713
BIBLIOGRAPHIC REFERENCE TABLE FOR SODIUM VALPROATE IN CHILDHOOD EPILEPSY Bibliographic Marson AG et al. for (Review). The Cochrane 2000 De Silva M et al. Romised or for childhood. Lancet, 1996; 347: 709-713
[ ]). 2 4 (95% CI)
![[ ]). 2 4 (95% CI) [ ]). 2 4 (95% CI)](/thumbs/77/75812901.jpg) The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial Anthony G Marson, Asya
The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial Anthony G Marson, Asya
Joint Modelling of Event Counts and Survival Times: Example Using Data from the MESS Trial
 Joint Modelling of Event Counts and Survival Times: Example Using Data from the MESS Trial J. K. Rogers J. L. Hutton K. Hemming Department of Statistics University of Warwick Research Students Conference,
Joint Modelling of Event Counts and Survival Times: Example Using Data from the MESS Trial J. K. Rogers J. L. Hutton K. Hemming Department of Statistics University of Warwick Research Students Conference,
LMMG New Medicine Recommendation
 LMMG New Medicine Recommendation Oxcarbazepine (Trileptal ) for the treatment of epilepsy LMMG Recommendation: Amber 0: Oxcarbazepine (Trileptal ) is recommended for use as monotherapy or adjunctive therapy
LMMG New Medicine Recommendation Oxcarbazepine (Trileptal ) for the treatment of epilepsy LMMG Recommendation: Amber 0: Oxcarbazepine (Trileptal ) is recommended for use as monotherapy or adjunctive therapy
Seizure remission in adults with long-standing intractable epilepsy: An extended follow-up
 Epilepsy Research (2010) xxx, xxx xxx journal homepage: www.elsevier.com/locate/epilepsyres Seizure remission in adults with long-standing intractable epilepsy: An extended follow-up Hyunmi Choi a,, Gary
Epilepsy Research (2010) xxx, xxx xxx journal homepage: www.elsevier.com/locate/epilepsyres Seizure remission in adults with long-standing intractable epilepsy: An extended follow-up Hyunmi Choi a,, Gary
Topiramate in clinical practice: first year s postlicensing experience in a specialist epilepsy clinic
 J Neurol Neurosurg Psychiatry 1999;66:759 763 759 The Walton Centre for Neurology and Neurosurgery, Lower Lane, Liverpool L9 7LJ, UK M W Kellett D F Smith P A Stockton D W Chadwick Correspondence to: Dr
J Neurol Neurosurg Psychiatry 1999;66:759 763 759 The Walton Centre for Neurology and Neurosurgery, Lower Lane, Liverpool L9 7LJ, UK M W Kellett D F Smith P A Stockton D W Chadwick Correspondence to: Dr
Epilepsy Specialist Symposium Treatment Algorithms in the Diagnosis and Treatment of Epilepsy
 Epilepsy Specialist Symposium Treatment Algorithms in the Diagnosis and Treatment of Epilepsy November 30, 2012 Fred Lado, MD, Chair Montefiore Medical Center Albert Einstein College of Medicine Bronx,
Epilepsy Specialist Symposium Treatment Algorithms in the Diagnosis and Treatment of Epilepsy November 30, 2012 Fred Lado, MD, Chair Montefiore Medical Center Albert Einstein College of Medicine Bronx,
CHILDHOOD OCCIPITAL EPILEPSY OF GASTAUT: A LONG-TERM PROSPECTIVE STUDY
 Acta Medica Mediterranea, 2017, 33: 1175 CHILDHOOD OCCIPITAL EPILEPSY OF GASTAUT: A LONG-TERM PROSPECTIVE STUDY MURAT GÖNEN ¹, EMRAH AYTAǹ, BÜLENT MÜNGEN¹ University of Fırat, Faculty of medicine, Neurology
Acta Medica Mediterranea, 2017, 33: 1175 CHILDHOOD OCCIPITAL EPILEPSY OF GASTAUT: A LONG-TERM PROSPECTIVE STUDY MURAT GÖNEN ¹, EMRAH AYTAǹ, BÜLENT MÜNGEN¹ University of Fırat, Faculty of medicine, Neurology
Zonisamide Should Be Considered a First-Line Antiepileptic Drug for Patients with Newly Diagnosed Partial Epilepsy
 Current Literature In Clinical Science Zonisamide Should Be Considered a First-Line Antiepileptic Drug for Patients with Newly Diagnosed Partial Epilepsy Efficacy and Tolerability of Zonisamide Versus
Current Literature In Clinical Science Zonisamide Should Be Considered a First-Line Antiepileptic Drug for Patients with Newly Diagnosed Partial Epilepsy Efficacy and Tolerability of Zonisamide Versus
levetiracetam 250,500,750 and 1000mg tablets and levetiracetam oral solution 100mg/1ml (Keppra ) (No. 397/07) UCB Pharma Ltd
 Scottish Medicines Consortium Resubmission levetiracetam 250,500,750 and 1000mg tablets and levetiracetam oral solution 100mg/1ml (Keppra ) (No. 397/07) UCB Pharma Ltd 11 January 2008 The Scottish Medicines
Scottish Medicines Consortium Resubmission levetiracetam 250,500,750 and 1000mg tablets and levetiracetam oral solution 100mg/1ml (Keppra ) (No. 397/07) UCB Pharma Ltd 11 January 2008 The Scottish Medicines
Intervention [5] Comparison [6]
![Intervention [5] Comparison [6] Intervention [5] Comparison [6]](/thumbs/86/94005273.jpg) Cowan LD. The epidemiology of the epilepsies in children. Mental Retardation and Developmental Disabilities Res Reviews, 2002; 8: 171-181 Review article Paediatric patients Discusses incidence and prevalence
Cowan LD. The epidemiology of the epilepsies in children. Mental Retardation and Developmental Disabilities Res Reviews, 2002; 8: 171-181 Review article Paediatric patients Discusses incidence and prevalence
What do we know about prognosis and natural course of epilepsies?
 What do we know about prognosis and natural course of epilepsies? Dr. Chusak Limotai, MD., M.Sc., CSCN (C) Chulalongkorn Comprehensive Epilepsy Center of Excellence (CCEC) The Thai Red Cross Society First
What do we know about prognosis and natural course of epilepsies? Dr. Chusak Limotai, MD., M.Sc., CSCN (C) Chulalongkorn Comprehensive Epilepsy Center of Excellence (CCEC) The Thai Red Cross Society First
Treatment of epilepsy in adults
 Treatment of epilepsy in adults Review 33 Treatment of epilepsy in adults S B Gunatilake 1, A Arasalingam 2 Sri Lanka Journal of Neurology, 2012, 1, 33-38 Case vignettes 1. A 60-year old patient with long
Treatment of epilepsy in adults Review 33 Treatment of epilepsy in adults S B Gunatilake 1, A Arasalingam 2 Sri Lanka Journal of Neurology, 2012, 1, 33-38 Case vignettes 1. A 60-year old patient with long
Q9. In adults and children with convulsive epilepsy in remission, when should treatment be discontinued?
 updated 2012 When to discontinue antiepileptic drug treatment in adults and children Q9. In adults and children with convulsive epilepsy in remission, when should treatment be discontinued? Background
updated 2012 When to discontinue antiepileptic drug treatment in adults and children Q9. In adults and children with convulsive epilepsy in remission, when should treatment be discontinued? Background
MODEL SELECTION STRATEGIES. Tony Panzarella
 MODEL SELECTION STRATEGIES Tony Panzarella Lab Course March 20, 2014 2 Preamble Although focus will be on time-to-event data the same principles apply to other outcome data Lab Course March 20, 2014 3
MODEL SELECTION STRATEGIES Tony Panzarella Lab Course March 20, 2014 2 Preamble Although focus will be on time-to-event data the same principles apply to other outcome data Lab Course March 20, 2014 3
2018 American Academy of Neurology
 Practice Guideline Update Efficacy and Tolerability of the New Antiepileptic Drugs I: Treatment of New-Onset Epilepsy Report by: Guideline Development, Dissemination, and Implementation Subcommittee of
Practice Guideline Update Efficacy and Tolerability of the New Antiepileptic Drugs I: Treatment of New-Onset Epilepsy Report by: Guideline Development, Dissemination, and Implementation Subcommittee of
Supplementary appendix
 Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Pujar SS, Martinos MM, Cortina-Borja M, et
Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Pujar SS, Martinos MM, Cortina-Borja M, et
A systematic review of treatment of typical absence seizures in children and adolescents with ethosuximide, sodium valproate or lamotrigine.
 Seizure (2005) 14, 117 122 www.elsevier.com/locate/yseiz A systematic review of treatment of typical absence seizures in children and adolescents with ethosuximide, sodium valproate or lamotrigine Ewa
Seizure (2005) 14, 117 122 www.elsevier.com/locate/yseiz A systematic review of treatment of typical absence seizures in children and adolescents with ethosuximide, sodium valproate or lamotrigine Ewa
Risk of seizure recurrence after antiepileptic drug withdrawal, an Indian study
 Neurology Asia 2006; 11 : 19 23 Risk of seizure recurrence after antiepileptic drug withdrawal, an Indian study Archana VERMA DM (Neurology) MD, Surendra MISRA DM (Neurology) FRCP (Edin) Department of
Neurology Asia 2006; 11 : 19 23 Risk of seizure recurrence after antiepileptic drug withdrawal, an Indian study Archana VERMA DM (Neurology) MD, Surendra MISRA DM (Neurology) FRCP (Edin) Department of
Trials. Open Access. Abstract
 Trials BioMed Central Research Multiple treatment comparisons in epilepsy monotherapy trials Catrin Tudur Smith* 1, Anthony G Marson 2, David W Chadwick 2 and Paula R Williamson1 1 Open Access Address:
Trials BioMed Central Research Multiple treatment comparisons in epilepsy monotherapy trials Catrin Tudur Smith* 1, Anthony G Marson 2, David W Chadwick 2 and Paula R Williamson1 1 Open Access Address:
Birth Rate among Patients with Epilepsy: A Nationwide Population-based Cohort Study in Finland
 American Journal of Epidemiology Copyright 2004 by the Johns Hopkins Bloomberg School of Public Health All rights reserved Vol. 159, No. 11 Printed in U.S.A. DOI: 10.1093/aje/kwh140 Birth Rate among Patients
American Journal of Epidemiology Copyright 2004 by the Johns Hopkins Bloomberg School of Public Health All rights reserved Vol. 159, No. 11 Printed in U.S.A. DOI: 10.1093/aje/kwh140 Birth Rate among Patients
Children with Rolandic spikes and ictal vomiting: Rolandic epilepsy or Panayiotopoulos syndrome?
 Original article Epileptic Disord 2003; 5: 139-43 Children with Rolandic spikes and ictal vomiting: Rolandic epilepsy or Panayiotopoulos syndrome? Athanasios Covanis, Christina Lada, Konstantinos Skiadas
Original article Epileptic Disord 2003; 5: 139-43 Children with Rolandic spikes and ictal vomiting: Rolandic epilepsy or Panayiotopoulos syndrome? Athanasios Covanis, Christina Lada, Konstantinos Skiadas
Diagnosing refractory epilepsy: response to sequential treatment schedules
 European Journal of Neurology 6, 13: 277 282 Diagnosing refractory epilepsy: response to sequential treatment schedules R. Mohanraj and M. J. Brodie Epilepsy Unit, Division of Cardiovascular and Medical
European Journal of Neurology 6, 13: 277 282 Diagnosing refractory epilepsy: response to sequential treatment schedules R. Mohanraj and M. J. Brodie Epilepsy Unit, Division of Cardiovascular and Medical
Antiepileptic drug monotherapy for epilepsy: a network meta-analysis of individual participant data(review)
 Cochrane Database of Systematic Reviews Antiepileptic drug monotherapy for epilepsy: a network metaanalysis of individual participant data(review) NevittSJ,SudellM,WestonJ,TudurSmithC,MarsonAG NevittSJ,SudellM,WestonJ,TudurSmithC,MarsonAG.
Cochrane Database of Systematic Reviews Antiepileptic drug monotherapy for epilepsy: a network metaanalysis of individual participant data(review) NevittSJ,SudellM,WestonJ,TudurSmithC,MarsonAG NevittSJ,SudellM,WestonJ,TudurSmithC,MarsonAG.
Double-blind, placebo controlled, crossover study
 448 48ournal of Neurology, Neurosurgery, and Psychiatry 1993;56:448-453 PAPERS Department of Clinical Pharmacology, Queen Elizabeth Hospital, Woodville, SA, G J Schapel Liverpool Hospital, Liverpool, NSW,
448 48ournal of Neurology, Neurosurgery, and Psychiatry 1993;56:448-453 PAPERS Department of Clinical Pharmacology, Queen Elizabeth Hospital, Woodville, SA, G J Schapel Liverpool Hospital, Liverpool, NSW,
Seizure. Early prediction of refractory epilepsy in childhood. J. Ramos-Lizana *, P. Aguilera-López, J. Aguirre-Rodríguez, E.
 Seizure 18 (2009) 412 416 Contents lists available at ScienceDirect Seizure journal homepage: www.elsevier.com/locate/yseiz Early prediction of refractory epilepsy in childhood J. Ramos-Lizana *, P. Aguilera-López,
Seizure 18 (2009) 412 416 Contents lists available at ScienceDirect Seizure journal homepage: www.elsevier.com/locate/yseiz Early prediction of refractory epilepsy in childhood J. Ramos-Lizana *, P. Aguilera-López,
Downloaded from jssu.ssu.ac.ir at 0:37 IRST on Sunday February 17th 2019
 -2384 2 *. : 4 :. 2 / 4 3 6/. ( /) : 6 /4. 6. 00 92 6. 0 :. :. 0 :. International league Against Epilepsy (ILAE) First Unprovoked Seizure (FUS) 24 () (2) 20.. 2 3-4. (). : -* - 0 626024: 0 626024 : E-mial:
-2384 2 *. : 4 :. 2 / 4 3 6/. ( /) : 6 /4. 6. 00 92 6. 0 :. :. 0 :. International league Against Epilepsy (ILAE) First Unprovoked Seizure (FUS) 24 () (2) 20.. 2 3-4. (). : -* - 0 626024: 0 626024 : E-mial:
Monotherapy in Newly Diagnosed Epilepsy: Levetiracetam Versus Standard Anticonvulsants
 Monotherapy in Newly Diagnosed Epilepsy: Levetiracetam Versus Standard Anticonvulsants Current Literature In Clinical Science KOMET: An Unblinded, Randomised, Two Parallel-Group, Stratified Trial Comparing
Monotherapy in Newly Diagnosed Epilepsy: Levetiracetam Versus Standard Anticonvulsants Current Literature In Clinical Science KOMET: An Unblinded, Randomised, Two Parallel-Group, Stratified Trial Comparing
An introduction to individual patient data (IPD) meta-analysis
 An introduction to individual patient data (IPD) meta-analysis Dr Sarah Nevitt Department of Biostatistics University of Liverpool Email: sjn16@liverpool.ac.uk : @sjn_16 Outline of the webinar IPD meta-analysis
An introduction to individual patient data (IPD) meta-analysis Dr Sarah Nevitt Department of Biostatistics University of Liverpool Email: sjn16@liverpool.ac.uk : @sjn_16 Outline of the webinar IPD meta-analysis
PROTOCOL. Francesco Brigo, Luigi Giuseppe Bongiovanni
 COMMON REFERENCE-BASED INDIRECT COMPARISON META-ANALYSIS OF INTRAVENOUS VALPROATE VERSUS INTRAVENOUS PHENOBARBITONE IN GENERALIZED CONVULSIVE STATUS EPILEPTICUS PROTOCOL Francesco Brigo, Luigi Giuseppe
COMMON REFERENCE-BASED INDIRECT COMPARISON META-ANALYSIS OF INTRAVENOUS VALPROATE VERSUS INTRAVENOUS PHENOBARBITONE IN GENERALIZED CONVULSIVE STATUS EPILEPTICUS PROTOCOL Francesco Brigo, Luigi Giuseppe
Scottish Medicines Consortium
 Scottish Medicines Consortium levetiracetam, 250, 500, 750 and 1000mg tablets and levetiracetam oral solution 100mg/ml (Keppra ) No. (394/07) UCB Pharma Limited 10 August 2007 The Scottish Medicines Consortium
Scottish Medicines Consortium levetiracetam, 250, 500, 750 and 1000mg tablets and levetiracetam oral solution 100mg/ml (Keppra ) No. (394/07) UCB Pharma Limited 10 August 2007 The Scottish Medicines Consortium
The New England Journal of Medicine EARLY IDENTIFICATION OF REFRACTORY EPILEPSY. Patients
 EARLY IDENTIFICATION OF REFRACTORY EPILEPSY PATRICK KWAN, M.D., AND MARTIN J. BRODIE, M.D. ABSTRACT Background More than 30 percent of patients with epilepsy have inadequate control of seizures with drug
EARLY IDENTIFICATION OF REFRACTORY EPILEPSY PATRICK KWAN, M.D., AND MARTIN J. BRODIE, M.D. ABSTRACT Background More than 30 percent of patients with epilepsy have inadequate control of seizures with drug
NHS services for epilepsy from the patient s perspective: a survey of primary, secondary and tertiary care access throughout the UK
 Seizure 2000; 9: 559 565 doi:0.053/seiz.2000.045, available online at http://www.idealibrary.com on NHS services for epilepsy from the patient s perspective: a survey of primary, secondary and tertiary
Seizure 2000; 9: 559 565 doi:0.053/seiz.2000.045, available online at http://www.idealibrary.com on NHS services for epilepsy from the patient s perspective: a survey of primary, secondary and tertiary
Background. Correlation between epilepsy and attention deficit hyperactivity disorder. Background. Epidemiology of ADHD among children with epilepsy
 Correlation between epilepsy and attention deficit hyperactivity disorder I-Ching Chou M.D. Director, Department of Pediatric Neurology China Medical University Hospital Taiwan Background Attention deficit/hyperactivity
Correlation between epilepsy and attention deficit hyperactivity disorder I-Ching Chou M.D. Director, Department of Pediatric Neurology China Medical University Hospital Taiwan Background Attention deficit/hyperactivity
Defining refractory epilepsy
 Defining refractory epilepsy Pasiri S, PMK Hospital @ 8.30 9.00, 23/7/2015 Nomenclature Drug resistant epilepsy Medically refractory epilepsy Medical intractable epilepsy Pharmacoresistant epilepsy 1 Definition
Defining refractory epilepsy Pasiri S, PMK Hospital @ 8.30 9.00, 23/7/2015 Nomenclature Drug resistant epilepsy Medically refractory epilepsy Medical intractable epilepsy Pharmacoresistant epilepsy 1 Definition
Template 1 for summarising studies addressing prognostic questions
 Template 1 for summarising studies addressing prognostic questions Instructions to fill the table: When no element can be added under one or more heading, include the mention: O Not applicable when an
Template 1 for summarising studies addressing prognostic questions Instructions to fill the table: When no element can be added under one or more heading, include the mention: O Not applicable when an
CLINICIAN INTERVIEW AS i M: When you examine a clinical trial in new- onset epilepsy, how relevant are the results to your daily clinical practice?
 FROM CLINICAL TRIALS TO CLINICAL PRACTICE: TRANSLATING EPILEPSY RESEARCH INTO PATIENT CARE Interview with Jacqueline A. French, MD Dr Jacqueline A. French is a Professor in the Department of Neurology
FROM CLINICAL TRIALS TO CLINICAL PRACTICE: TRANSLATING EPILEPSY RESEARCH INTO PATIENT CARE Interview with Jacqueline A. French, MD Dr Jacqueline A. French is a Professor in the Department of Neurology
June 30 (Fri), Teaching Session 1. New definition & epilepsy classification. Chairs Won-Joo Kim Ran Lee
 June 30 (Fri), 2017 Teaching Session 1 New definition & epilepsy classification Chairs Won-Joo Kim Ran Lee Teaching Session 1 TS1-1 Introduction of new definition of epilepsy Sung Chul Lim Department of
June 30 (Fri), 2017 Teaching Session 1 New definition & epilepsy classification Chairs Won-Joo Kim Ran Lee Teaching Session 1 TS1-1 Introduction of new definition of epilepsy Sung Chul Lim Department of
Refractory epilepsy: treatment with new antiepileptic drugs
 Seizure 2000; 9: 51 57 doi: 10.1053/seiz.1999.0348, available online at http://www.idealibrary.com on Refractory epilepsy: treatment with new antiepileptic drugs P. K. DATTA & P. M. CRAWFORD Department
Seizure 2000; 9: 51 57 doi: 10.1053/seiz.1999.0348, available online at http://www.idealibrary.com on Refractory epilepsy: treatment with new antiepileptic drugs P. K. DATTA & P. M. CRAWFORD Department
Is it epilepsy? Does the patient need long-term therapy?
 Is it a seizure? Definition Transient occurrence of signs and/or symptoms due to abnormal excessive or synchronous neuronal activity in the brain Is it provoked or unprovoked? Is it epilepsy? Does the
Is it a seizure? Definition Transient occurrence of signs and/or symptoms due to abnormal excessive or synchronous neuronal activity in the brain Is it provoked or unprovoked? Is it epilepsy? Does the
Supplementary appendix
 Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Callegaro D, Miceli R, Bonvalot S, et al. Development
Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Callegaro D, Miceli R, Bonvalot S, et al. Development
Auras and the risk of seizures with impaired consciousness following epilepsy surgery: implications for driving
 Auras and the risk of seizures with impaired consciousness following epilepsy surgery: implications for driving S Fairclough MBChB MSc, AG O Keeffe PhD*, J de Tisi BA, JS Duncan FRCP FMedSci Dept. of Clinical
Auras and the risk of seizures with impaired consciousness following epilepsy surgery: implications for driving S Fairclough MBChB MSc, AG O Keeffe PhD*, J de Tisi BA, JS Duncan FRCP FMedSci Dept. of Clinical
Epilepsy management What, when and how?
 Epilepsy management What, when and how? J Helen Cross UCL-Institute of Child Health, Great Ormond Street Hospital for Children, London, & National Centre for Young People with Epilepsy, Lingfield, UK What
Epilepsy management What, when and how? J Helen Cross UCL-Institute of Child Health, Great Ormond Street Hospital for Children, London, & National Centre for Young People with Epilepsy, Lingfield, UK What
Stay, Hit, or Fold? What Do You Do If the Treatment May Be as Bad as the Problem Results of a Q-PULSE Survey
 It s Current Epilepsy Resources and Updates Stay, Hit, or Fold? What Do You Do If the Treatment May Be as Bad as the Problem Results of a Q-PULSE Survey Chad Carlson, MD Associate Professor of Neurology,
It s Current Epilepsy Resources and Updates Stay, Hit, or Fold? What Do You Do If the Treatment May Be as Bad as the Problem Results of a Q-PULSE Survey Chad Carlson, MD Associate Professor of Neurology,
Perampanel Benefit assessment according to 35a Social Code Book V 1
 IQWiG Reports Commission No. A14-16 Perampanel Benefit assessment according to 35a Social Code Book V 1 Extract 1 Translation of Sections 2.1 to 2.6 of the dossier assessment Perampanel Nutzenbewertung
IQWiG Reports Commission No. A14-16 Perampanel Benefit assessment according to 35a Social Code Book V 1 Extract 1 Translation of Sections 2.1 to 2.6 of the dossier assessment Perampanel Nutzenbewertung
RISK OF RECURRENT SEIZURES AFTER TWO UNPROVOKED SEIZURES RISK OF RECURRENT SEIZURES AFTER TWO UNPROVOKED SEIZURES. Patients
 RISK OF RECURRENT SEIZURES AFTER TWO UNPROVOKED SEIZURES RISK OF RECURRENT SEIZURES AFTER TWO UNPROVOKED SEIZURES W. ALLEN HAUSER, M.D., STEPHEN S. RICH, PH.D., JU R.-J. LEE, PH.D., JOHN F. ANNEGERS, PH.D.,
RISK OF RECURRENT SEIZURES AFTER TWO UNPROVOKED SEIZURES RISK OF RECURRENT SEIZURES AFTER TWO UNPROVOKED SEIZURES W. ALLEN HAUSER, M.D., STEPHEN S. RICH, PH.D., JU R.-J. LEE, PH.D., JOHN F. ANNEGERS, PH.D.,
Prescribing and Monitoring Anti-Epileptic Drugs
 Prescribing and Monitoring Anti-Epileptic Drugs Mark Granner, MD Clinical Professor and Vice Chair for Clinical Programs Director, Iowa Comprehensive Epilepsy Program Department of Neurology University
Prescribing and Monitoring Anti-Epileptic Drugs Mark Granner, MD Clinical Professor and Vice Chair for Clinical Programs Director, Iowa Comprehensive Epilepsy Program Department of Neurology University
Early add-on treatment vs alternative monotherapy in patients with partial epilepsy
 Original article Epileptic Disord 2014; 16 (2): 165-74 Early add-on treatment vs alternative monotherapy in patients with partial epilepsy Franck Semah 1, Pierre Thomas 2, Safia Coulbaut 3, Philippe Derambure
Original article Epileptic Disord 2014; 16 (2): 165-74 Early add-on treatment vs alternative monotherapy in patients with partial epilepsy Franck Semah 1, Pierre Thomas 2, Safia Coulbaut 3, Philippe Derambure
SEIZURE OUTCOME AFTER EPILEPSY SURGERY
 SEIZURE OUTCOME AFTER EPILEPSY SURGERY Prakash Kotagal, M.D. Head, Pediatric Epilepsy Cleveland Clinic Epilepsy Center LEFT TEMPORAL LOBE ASTROCYTOMA SEIZURE OUTCOME 1 YEAR AFTER EPILEPSY SURGERY IN ADULTS
SEIZURE OUTCOME AFTER EPILEPSY SURGERY Prakash Kotagal, M.D. Head, Pediatric Epilepsy Cleveland Clinic Epilepsy Center LEFT TEMPORAL LOBE ASTROCYTOMA SEIZURE OUTCOME 1 YEAR AFTER EPILEPSY SURGERY IN ADULTS
When choosing an antiepileptic ... PRESENTATION... Pharmacokinetics of the New Antiepileptic Drugs. Based on a presentation by Barry E.
 ... PRESENTATION... Pharmacokinetics of the New Antiepileptic Drugs Based on a presentation by Barry E. Gidal, PharmD Presentation Summary A physician s choice of an antiepileptic drug (AED) usually depends
... PRESENTATION... Pharmacokinetics of the New Antiepileptic Drugs Based on a presentation by Barry E. Gidal, PharmD Presentation Summary A physician s choice of an antiepileptic drug (AED) usually depends
Appendix M Health Economic Evidence Extractions
 Appendix M Health Economic Evidence Extractions Which AEDs are clinically effective and cost-effective for people with focal epilepsy with or without secondary generalisation seizures? Frew E, Sandercock
Appendix M Health Economic Evidence Extractions Which AEDs are clinically effective and cost-effective for people with focal epilepsy with or without secondary generalisation seizures? Frew E, Sandercock
Recommendations. for Care of Adults with Epilepsy. Seeking the best treatment from the right doctor at the right time!
 Recommendations for Care of Adults with Epilepsy Seeking the best treatment from the right doctor at the right time! Contents This booklet is to help adults and their caregivers know when it is appropriate
Recommendations for Care of Adults with Epilepsy Seeking the best treatment from the right doctor at the right time! Contents This booklet is to help adults and their caregivers know when it is appropriate
The EEG and Epilepsy in Kelantan --- A Hospital/laboratory... Based Study
 The EEG and Epilepsy in Kelantan --- A Hospital/laboratory... Based Study M.N. Wm, FRCP Department of Medicine, Hospital Universiti Sains Malaysia, Kubang Kerian, 75990 Kelantan Darul Nairn Introduction
The EEG and Epilepsy in Kelantan --- A Hospital/laboratory... Based Study M.N. Wm, FRCP Department of Medicine, Hospital Universiti Sains Malaysia, Kubang Kerian, 75990 Kelantan Darul Nairn Introduction
Epilepsy in a children's hospital: an out-patient survey
 Seizure 1995; 4:279-285 Epilepsy in a children's hospital: an out-patient survey A.P. HUGHES & R.E. APPLETON Roald Dahl E.E.G. Unit, Royal Liverpool Children's NHS Trust Address for correspondence: Dr
Seizure 1995; 4:279-285 Epilepsy in a children's hospital: an out-patient survey A.P. HUGHES & R.E. APPLETON Roald Dahl E.E.G. Unit, Royal Liverpool Children's NHS Trust Address for correspondence: Dr
BLISTER STATISTICAL ANALYSIS PLAN Version 1.0
 The Bullous Pemphigoid Steroids and Tetracyclines (BLISTER) Study EudraCT number: 2007-006658-24 ISRCTN: 13704604 BLISTER STATISTICAL ANALYSIS PLAN Version 1.0 Version Date Comments 0.1 13/01/2011 First
The Bullous Pemphigoid Steroids and Tetracyclines (BLISTER) Study EudraCT number: 2007-006658-24 ISRCTN: 13704604 BLISTER STATISTICAL ANALYSIS PLAN Version 1.0 Version Date Comments 0.1 13/01/2011 First
MARIE DE ZÉLICOURT, LAURENT BUTEAU, FRANCIS FAGNANI & PIERRE JALLON
 Seizure 2000; 9: 88 95 doi: 10.1053/seiz.1999.0364, available online at http://www.idealibrary.com on The contributing factors to medical cost of epilepsy: an estimation based on a French prospective cohort
Seizure 2000; 9: 88 95 doi: 10.1053/seiz.1999.0364, available online at http://www.idealibrary.com on The contributing factors to medical cost of epilepsy: an estimation based on a French prospective cohort
Update in Clinical Guidelines in Epilepsy
 Why We Need Clinical Guidelines? Clinician needs advice! Update in Clinical Guidelines in Epilepsy Charcrin Nabangchang, M.D. Phramongkutklao College of Medicine Tiamkao S, Neurology Asia2013 Why We Need
Why We Need Clinical Guidelines? Clinician needs advice! Update in Clinical Guidelines in Epilepsy Charcrin Nabangchang, M.D. Phramongkutklao College of Medicine Tiamkao S, Neurology Asia2013 Why We Need
eslicarbazepine acetate 800mg tablet (Zebinix) SMC No. (592/09) Eisai Ltd
 eslicarbazepine acetate 800mg tablet (Zebinix) SMC No. (592/09) Eisai Ltd 8 October 2010 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises NHS Boards
eslicarbazepine acetate 800mg tablet (Zebinix) SMC No. (592/09) Eisai Ltd 8 October 2010 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises NHS Boards
Epilepsy: problems of diagnosis and recommended treatment Nicola Cooper MRCP and Morgan Feely MD, FRCP, FRCP(I)
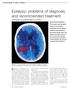 Epilepsy: problems of diagnosis and recommended treatment Nicola Cooper MRCP and Morgan Feely MD, FRCP, FRCP(I) VM Our series Prescribing in gives practical advice for successful management of the special
Epilepsy: problems of diagnosis and recommended treatment Nicola Cooper MRCP and Morgan Feely MD, FRCP, FRCP(I) VM Our series Prescribing in gives practical advice for successful management of the special
Clinical characteristics, seizure spread patterns and prognosis of seizures associated with a single small cerebral calcific CT lesion
 Seizure 1998; 7:153-157 Clinical characteristics, seizure spread patterns and prognosis of seizures associated with a single small cerebral calcific CT lesion J. M. K. MURTHY & V. SREENIVAS REDDY Department
Seizure 1998; 7:153-157 Clinical characteristics, seizure spread patterns and prognosis of seizures associated with a single small cerebral calcific CT lesion J. M. K. MURTHY & V. SREENIVAS REDDY Department
The epilepsies: the diagnosis and management of the epilepsies in adults and children in primary and secondary care
 The epilepsies: the diagnosis and management of the epilepsies in adults and children in primary and secondary care Issued: January 2012 guidance.nice.org.uk/cg137 NHS Evidence has accredited the process
The epilepsies: the diagnosis and management of the epilepsies in adults and children in primary and secondary care Issued: January 2012 guidance.nice.org.uk/cg137 NHS Evidence has accredited the process
Does a diagnosis of epilepsy commit patients to lifelong therapy? Not always. Here s how to taper AEDs safely and avoid relapse.
 Does a diagnosis of epilepsy commit patients to lifelong therapy? Not always. Here s how to taper AEDs safely and avoid relapse. T he epilepsy specialist always has two equally important endpoints in mind
Does a diagnosis of epilepsy commit patients to lifelong therapy? Not always. Here s how to taper AEDs safely and avoid relapse. T he epilepsy specialist always has two equally important endpoints in mind
Predictors of Intractable Childhood Epilepsy
 ORIGINAL ARTICLE Predictors of Intractable Childhood Epilepsy Muhammad Akbar Malik 1, Muhammad Haroon Hamid 2, Tahir Masood Ahmed 2 and Qurban Ali 3 ABSTRACT Objective: To determine the prognosis of seizures
ORIGINAL ARTICLE Predictors of Intractable Childhood Epilepsy Muhammad Akbar Malik 1, Muhammad Haroon Hamid 2, Tahir Masood Ahmed 2 and Qurban Ali 3 ABSTRACT Objective: To determine the prognosis of seizures
Epilepsy T.I.A. Cataplexy. Nonepileptic seizure. syncope. Dystonia. Epilepsy & other attack disorders Overview
 : Clinical presentation and management Markus Reuber Professor of Clinical Neurology Academic Neurology Unit University of Sheffield, Royal Hallamshire Hospital. Is it epilepsy? Overview Common attack
: Clinical presentation and management Markus Reuber Professor of Clinical Neurology Academic Neurology Unit University of Sheffield, Royal Hallamshire Hospital. Is it epilepsy? Overview Common attack
NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE. Final Appraisal Determination. Newer drugs for epilepsy in children
 NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE 1 Guidance 1.1 The newer antiepileptic drugs gabapentin, lamotrigine, oxcarbazepine, tiagabine, topiramate, and vigabatrin (as an adjunctive therapy for partial
NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE 1 Guidance 1.1 The newer antiepileptic drugs gabapentin, lamotrigine, oxcarbazepine, tiagabine, topiramate, and vigabatrin (as an adjunctive therapy for partial
Levetiracetam monotherapy in juvenile myoclonic epilepsy
 Seizure (2008) 17, 64 68 www.elsevier.com/locate/yseiz Levetiracetam monotherapy in juvenile myoclonic epilepsy Deron V. Sharpe *, Anup D. Patel, Bassel Abou-Khalil, Gerald M. Fenichel Vanderbilt University
Seizure (2008) 17, 64 68 www.elsevier.com/locate/yseiz Levetiracetam monotherapy in juvenile myoclonic epilepsy Deron V. Sharpe *, Anup D. Patel, Bassel Abou-Khalil, Gerald M. Fenichel Vanderbilt University
Lacosamide (Vimpat) for partial-onset epilepsy monotherapy. December 2011
 Lacosamide (Vimpat) for partial-onset epilepsy monotherapy This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a
Lacosamide (Vimpat) for partial-onset epilepsy monotherapy This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a
 PATCH Analysis Plan v1.2.doc Prophylactic Antibiotics for the Treatment of Cellulitis at Home: PATCH Analysis Plan for PATCH I and PATCH II Authors: Angela Crook, Andrew Nunn, James Mason and Kim Thomas,
PATCH Analysis Plan v1.2.doc Prophylactic Antibiotics for the Treatment of Cellulitis at Home: PATCH Analysis Plan for PATCH I and PATCH II Authors: Angela Crook, Andrew Nunn, James Mason and Kim Thomas,
Distribution of Epilepsy Syndromes in a Cohort of Children Prospectively Monitored from the Time of Their First Unprovoked Seizure
 Epilepsiu, 4( ):378-383, 999 Lippincott Williams & Wilkins, Inc., Philadelphia International League Against Epilepsy Clinical Research Distribution of Epilepsy Syndromes in a Cohort of Children Prospectively
Epilepsiu, 4( ):378-383, 999 Lippincott Williams & Wilkins, Inc., Philadelphia International League Against Epilepsy Clinical Research Distribution of Epilepsy Syndromes in a Cohort of Children Prospectively
The epilepsies: the diagnosis and management of the epilepsies in adults and children in primary and secondary care
 The epilepsies: the diagnosis and management of the epilepsies in adults and children in primary and secondary care Issued: January 2012 last modified: January 2015 guidance.nice.org.uk/cg137 NICE has
The epilepsies: the diagnosis and management of the epilepsies in adults and children in primary and secondary care Issued: January 2012 last modified: January 2015 guidance.nice.org.uk/cg137 NICE has
T he diagnosis and classification of a first seizure in
 241 PAPER Interrater agreement of the diagnosis and classification of a first seizure in childhood. The Dutch Study of Epilepsy in Childhood H Stroink, C A van Donselaar, A T Geerts, A C B Peters, O F
241 PAPER Interrater agreement of the diagnosis and classification of a first seizure in childhood. The Dutch Study of Epilepsy in Childhood H Stroink, C A van Donselaar, A T Geerts, A C B Peters, O F
All patients with a diagnosis of treatment resistant (intractable) epilepsy.* Denominator Statement
 MEASURE #7 Referral to Comprehensive Epilepsy Center Measure Description Percent of all patients with a diagnosis of treatment resistant (intractable) epilepsy who were referred for consultation to a comprehensive
MEASURE #7 Referral to Comprehensive Epilepsy Center Measure Description Percent of all patients with a diagnosis of treatment resistant (intractable) epilepsy who were referred for consultation to a comprehensive
Epilepsy. Seizures and Epilepsy. Buccal Midazolam vs. Rectal Diazepam for Serial Seizures. Epilepsy and Seizures 6/18/2008
 Seizures and Epilepsy Paul Garcia, M.D. UCSF Epilepsy Epileptic seizure: the physical manifestation of aberrant firing of brain cells Epilepsy: the tendency to recurrent, unprovoked epileptic seizures
Seizures and Epilepsy Paul Garcia, M.D. UCSF Epilepsy Epileptic seizure: the physical manifestation of aberrant firing of brain cells Epilepsy: the tendency to recurrent, unprovoked epileptic seizures
Epilepsy 101. Russell P. Saneto, DO, PhD. Seattle Children s Hospital/University of Washington November 2011
 Epilepsy 101 Russell P. Saneto, DO, PhD Seattle Children s Hospital/University of Washington November 2011 Specific Aims How do we define epilepsy? Do seizures equal epilepsy? What are seizures? Seizure
Epilepsy 101 Russell P. Saneto, DO, PhD Seattle Children s Hospital/University of Washington November 2011 Specific Aims How do we define epilepsy? Do seizures equal epilepsy? What are seizures? Seizure
Management of Epilepsy in Primary Care and the Community. Carrie Burke, Epilepsy Specialist Nurse
 Management of Epilepsy in Primary Care and the Community Carrie Burke, Epilepsy Specialist Nurse Epilepsy & Seizures Epilepsy is a common neurological disorder characterised by recurring seizures (NICE,
Management of Epilepsy in Primary Care and the Community Carrie Burke, Epilepsy Specialist Nurse Epilepsy & Seizures Epilepsy is a common neurological disorder characterised by recurring seizures (NICE,
Prognosis for New-Onset Epilepsy Dec. 6th, 2013
 Prognosis for New-Onset Epilepsy Dec. 6th, 2013 Scott Mintzer, MD Jefferson Comprehensive Epilepsy Center Thomas Jefferson University Philadelphia, PA American Epilepsy Society Annual Meeting Disclosure
Prognosis for New-Onset Epilepsy Dec. 6th, 2013 Scott Mintzer, MD Jefferson Comprehensive Epilepsy Center Thomas Jefferson University Philadelphia, PA American Epilepsy Society Annual Meeting Disclosure
2018 American Academy of Neurology
 Practice Guideline Update Efficacy and Tolerability of the New Antiepileptic Drugs II: Treatment-Resistant Epilepsy Report by: Guideline Development, Dissemination, and Implementation Subcommittee of the
Practice Guideline Update Efficacy and Tolerability of the New Antiepileptic Drugs II: Treatment-Resistant Epilepsy Report by: Guideline Development, Dissemination, and Implementation Subcommittee of the
The Effectiveness of Monotheraby in Epileptic Sudanese Patients
 The Effectiveness of Monotheraby in Epileptic Sudanese Patients Amel Elmahi Mohamed (1) Sawsan A Aldeaf (1) Alsadig Gassoum (1) Alnada Abdalla Mohamed (2) Mohamed A Arbab (1,3) and Alamin Ebrahim (2) Abstract
The Effectiveness of Monotheraby in Epileptic Sudanese Patients Amel Elmahi Mohamed (1) Sawsan A Aldeaf (1) Alsadig Gassoum (1) Alnada Abdalla Mohamed (2) Mohamed A Arbab (1,3) and Alamin Ebrahim (2) Abstract
The epilepsies: pharmacological treatment by epilepsy syndrome
 The epilepsies: pharmacological treatment by epilepsy syndrome This table provides a summary reference guide to pharmacological treatment. Anti-epileptic drug (AED) options by epilepsy syndrome Childhood
The epilepsies: pharmacological treatment by epilepsy syndrome This table provides a summary reference guide to pharmacological treatment. Anti-epileptic drug (AED) options by epilepsy syndrome Childhood
Seizure 18 (2009) Contents lists available at ScienceDirect. Seizure. journal homepage:
 Seizure 18 (2009) 620 624 Contents lists available at ScienceDirect Seizure journal homepage: www.elsevier.com/locate/yseiz Response to sequential treatment schedules in childhood epilepsy Risk for development
Seizure 18 (2009) 620 624 Contents lists available at ScienceDirect Seizure journal homepage: www.elsevier.com/locate/yseiz Response to sequential treatment schedules in childhood epilepsy Risk for development
Economic Evaluation of AEDs used as monotherapy in the treatment of adults with newly diagnosed focal epilepsy
 0 0 APPENDIX P Cost-effectiveness analyses Five economic models were developed as part of the guideline development, one for each of the following clinical areas:. Monotherapy for adults with newly diagnosed
0 0 APPENDIX P Cost-effectiveness analyses Five economic models were developed as part of the guideline development, one for each of the following clinical areas:. Monotherapy for adults with newly diagnosed
Prognosis of chronic epilepsy with complex partial
 Journal of Neurology, Neurosurgery, and Psychiatry 1984;47:1274-1278 279/83 Prognosis of chronic epilepsy with complex partial seizures D SCHMIDT From the Department ofneurology, University of Berlin,
Journal of Neurology, Neurosurgery, and Psychiatry 1984;47:1274-1278 279/83 Prognosis of chronic epilepsy with complex partial seizures D SCHMIDT From the Department ofneurology, University of Berlin,
M. Sillanpää a, D. Schmidt b, * Received 27 January 2006; revised 28 February 2006; accepted 28 February 2006 Available online 17 April 2006
 Epilepsy & Behavior 8 (2006) 713 719 www.elsevier.com/locate/yebeh Prognosis of seizure recurrence after stopping antiepileptic drugs in seizure-free patients: A long-term population-based study of childhood-onset
Epilepsy & Behavior 8 (2006) 713 719 www.elsevier.com/locate/yebeh Prognosis of seizure recurrence after stopping antiepileptic drugs in seizure-free patients: A long-term population-based study of childhood-onset
Risk Factors of Poorly Controlled Childhood Epilepsy - A Study in A Tertiary Care Hospital
 44 BANGLADESH J CHILD HEALTH 2010; VOL 34 (2): 44-50 Risk Factors of Poorly Controlled Childhood Epilepsy - A Study in A Tertiary Care Hospital AKM MOINUDDIN 1, MD. MIZANUR RAHMAN 2, SHAHEEN AKHTER 3,
44 BANGLADESH J CHILD HEALTH 2010; VOL 34 (2): 44-50 Risk Factors of Poorly Controlled Childhood Epilepsy - A Study in A Tertiary Care Hospital AKM MOINUDDIN 1, MD. MIZANUR RAHMAN 2, SHAHEEN AKHTER 3,
Anti-epileptic drugs: a guide for the non-neurologist
 OCCASIONAL PAPERS Clinical Medicine 2010, Vol 10, No 1: 54 8 Anti-epileptic drugs: a guide for the non-neurologist Joseph Anderson and Carl-Christian Moor ABSTRACT Epilepsy is the most common serious chronic
OCCASIONAL PAPERS Clinical Medicine 2010, Vol 10, No 1: 54 8 Anti-epileptic drugs: a guide for the non-neurologist Joseph Anderson and Carl-Christian Moor ABSTRACT Epilepsy is the most common serious chronic
APPENDIX S. Removed sections from original guideline. 1.1 Pharmacological treatment Introduction
 00 0 APPENDIX S Removed sections from original guideline. Pharmacological treatment.. Introduction The evidence base for the newer AEDs (gabapentin, lamotrigine, levetiracetam, oxcarbazepine, tiagabine,
00 0 APPENDIX S Removed sections from original guideline. Pharmacological treatment.. Introduction The evidence base for the newer AEDs (gabapentin, lamotrigine, levetiracetam, oxcarbazepine, tiagabine,
London, 07 August 2006 Product name: Keppra Procedure No. EMEA/H/C/277/II/63 SCIENTIFIC DISCUSSION
 London, 07 August 2006 Product name: Keppra Procedure No. EMEA/H/C/277/II/63 SCIENTIFIC DISCUSSION 1/15 EMEA 2006 1. Introduction Epilepsy is one of the most common and challenging neurological disorders.
London, 07 August 2006 Product name: Keppra Procedure No. EMEA/H/C/277/II/63 SCIENTIFIC DISCUSSION 1/15 EMEA 2006 1. Introduction Epilepsy is one of the most common and challenging neurological disorders.
ORIGINAL CONTRIBUTION
 Epilepsy in Childhood An Audit of Clinical Practice ORIGINAL CONTRIBUTION Hans A. Carpay, MD; Willem F. M. Arts, MD, PhD; Ada T. Geerts, MSc; Hans Stroink, MD; Oebele F. Brouwer, MD, PhD; A. C. Boudewyn
Epilepsy in Childhood An Audit of Clinical Practice ORIGINAL CONTRIBUTION Hans A. Carpay, MD; Willem F. M. Arts, MD, PhD; Ada T. Geerts, MSc; Hans Stroink, MD; Oebele F. Brouwer, MD, PhD; A. C. Boudewyn
License to Ill: Playing the Odds After Withdrawing and Restarting Antiepileptic Drugs
 License to Ill: Playing the Odds After Withdrawing and Restarting Antiepileptic Drugs Current Literature In Clinical Science Seizure Recurrence After Antiepileptic Drug Withdrawal and the Implications
License to Ill: Playing the Odds After Withdrawing and Restarting Antiepileptic Drugs Current Literature In Clinical Science Seizure Recurrence After Antiepileptic Drug Withdrawal and the Implications
