Multiple Autism-Linked Genes Mediate Synapse Elimination via Proteasomal Degradation of a Synaptic Scaffold PSD-95
|
|
|
- Diane Merritt
- 6 years ago
- Views:
Transcription
1 Multiple Autism-Linked Genes Mediate Synapse Elimination via Proteasomal Degradation of a Synaptic Scaffold PSD-95 Nien-Pei Tsai, 1,4 Julia R. Wilkerson, 1,4 Weirui Guo, 1 Marina A. Maksimova, 1 George N. DeMartino, 2 Christopher W. Cowan, 3,5 and Kimberly M. Huber 1, * 1 Department of Neuroscience 2 Department of Physiology 3 Departments of Psychiatry and Ophthalmology University of Texas Southwestern Medical Center, Dallas, TX 75390, USA 4 These authors contributed equally to this work 5 Present address: Department of Psychiatry, Harvard Medical School, McLean Hospital, Belmont, MA 02478, USA *Correspondence: kimberly.huber@utsouthwestern.edu SUMMARY The activity-dependent transcription factor myocyte enhancer factor 2 (MEF2) induces excitatory synapse elimination in mouse neurons, which requires fragile X mental retardation protein (FMRP), an RNA-binding protein implicated in human cognitive dysfunction and autism. We report here that protocadherin 10 (Pcdh10), an autism-spectrum disorders gene, is necessary for this process. MEF2 and FMRP cooperatively regulate the expression of Pcdh10. Upon MEF2 activation, PSD-95 is ubiquitinated by the ubiquitin E3 ligase murine double minute 2 (Mdm2) and then binds to Pcdh10, which links it to the proteasome for degradation. Blockade of the Pcdh10- proteasome interaction inhibits MEF2-induced PSD-95 degradation and synapse elimination. In FMRP-lacking neurons, elevated protein levels of eukaryotic translation elongation factor 1 a (EF1a), an Mdm2-interacting protein and FMRP target mrna, sequester Mdm2 and prevent MEF2-induced PSD- 95 ubiquitination and synapse elimination. Together, our findings reveal roles for multiple autism-linked genes in activity-dependent synapse elimination. INTRODUCTION Sensory experience, learning, and neuronal activity stabilize or eliminate select excitatory synapses and sculpt the mature neuronal circuits that mediate sensory processing and memory (Xu et al., 2009; Yang et al., 2009). The cellular mechanisms that underlie activity or experience-dependent synapse elimination in the central nervous system are largely unknown. The discovery that activation of the myocyte enhancer factor 2 (MEF2) family of transcription factors suppresses synapse number provides an important molecular link to understand how neuronal activity leads to synapse elimination (Flavell et al., 2006; Pfeiffer et al., 2010; Tian et al., 2010). MEF2 is activated upon neuronal depolarization and calcium influx, which induces the expression of MEF2 target genes that are thought to lead to synapse elimination (Flavell et al., 2006, 2008; McKinsey et al., 2002; Pulipparacharuvil et al., 2008). The identity of the MEF2 target genes that promote synapse elimination or their mechanisms of action in synapse elimination is unknown. We recently demonstrated that MEF2-induced synapse elimination requires fragile X mental retardation protein (FMRP) (Pfeiffer et al., 2010), an RNA-binding protein that regulates translation of its target mrnas (Bassell and Warren, 2008). FMRP is encoded by the Fmr1 gene, which is transcriptionally silenced in patients with fragile X syndrome (FXS), the most common inherited form of intellectual disability and autism (Abrahams and Geschwind, 2008; Kelleher and Bear, 2008). In neurons of the mouse model of FXS, Fmr1 knockout (KO), MEF2-triggered synapse elimination is absent (Pfeiffer et al., 2010), which may contribute to the observed excess of dendritic spines on cortical neurons of FXS patients and the Fmr1 KO mouse (Bagni and Greenough, 2005). The mechanisms that underlie the deficiencies in synapse elimination associated with FXS are unknown. Our previous results indicated that FMRP functions downstream of MEF2-induced transcription to eliminate synapses (Pfeiffer et al., 2010). Based on the known function of FMRP, we hypothesized that FMRP regulated translation of MEF2-generated transcripts. To identify candidate transcripts involved in synapse elimination, we compared the known MEF2 target genes (Flavell et al., 2008) with FMRP-interacting mrnas (Darnell et al., 2011). Of the many common MEF2- and FMRP-interacting transcripts, we focused on protocadherin 10 (Pcdh10), also known as OL-protocadherin, a member of the cadherin superfamily of calcium-dependent cell adhesion molecules (Morishita and Yagi, 2007). Importantly, homologous deletion of Pcdh10 in humans is associated with autism (Morrow et al., 2008). Pcdh10 and other family members of the d2 nonclustered protocadherin family (Pcdh8 and 19) demonstrate weak homophilic Cell 151, , December 21, 2012 ª2012 Elsevier Inc. 1581
2 binding activity relative to the classical cadherins (Hirano et al., 1999). Furthermore, the diverse structure of protocadherins cytoplasmic C termini is consistent with the multifunctional roles of protocadherins in cell signaling and function, in addition to cell adhesion (Kim et al., 2011; Yasuda et al., 2007). Pcdh10 is primarily expressed in the brain (Hirano et al., 1999), where it is required for the growth of striatal axons and patterning of thalamocortical and corticothalamic projections during embryonic development (Uemura et al., 2007). Interestingly, Pcdh10 is highly expressed in mature cortical neurons, where nothing is known of its function (Kim et al., 2011). Here, we demonstrate that Pcdh10 is required for MEF2-induced synapse elimination and functions to deliver ubiquitinated postsynaptic density protein 95 (PSD-95), a critical synaptic scaffolding molecule to the proteasome. Fmr1 KO neurons have deficits in ubiquitination and degradation of PSD-95, and we uncover the molecular basis of this defect. Our results reveal mechanisms by which the activity-dependent transcription factor MEF2 refines synaptic connections in wild-type neurons and identify the mechanisms that underlie abnormal synapse refinement in fragile X syndrome. Importantly, this work demonstrates the necessity and distinct cellular functions of three genes linked to autism in the same synapse elimination process (Pcdh10, Fmr1. and Mef2; Novara et al., 2010) and thus provides evidence for a common synaptic deficit among different genetic causes of autism. RESULTS MEF2 and FMRP Regulate Pcdh10 Expression in Dendrites To study the role of Pcdh10 in MEF2 and FMRP regulation of synapse number, we first validated the association of FMRP and Pcdh10 mrna (Darnell et al., 2011). We performed an RNA immunoprecipitation with an anti-fmrp antibody and pulled down Pcdh10 mrna from hippocampal lysates of wildtype (WT) mice, but not Fmr1 KO mice (Figure 1A), providing evidence for Pcdh10 as an mrna target of FMRP. We next examined whether Pcdh10 expression is regulated by MEF2 and FMRP, using lentivirus to transfect tamoxifen-inducible, constitutively active MEF2 (MEF2-VP16ERtm) (Flavell et al., 2006; Pfeiffer et al., 2010) into dissociated cortical neuron cultures, which are amenable to biochemical assays. Basal levels of Pcdh10 mrna and another MEF2 target gene Nurr77 were not different between WT and Fmr1 KO neurons (vehicle; white bars, Figure 1B). After 6 hr of 4-hydroxytamoxifen (tamoxifen, 1 mm) treatment, which induces translocation of MEF2- VP16ERtm to the nucleus to activate target genes (Flavell et al., 2006), both Pcdh10 and Nurr77 mrnas were elevated in WT and Fmr1 KO neurons (black bars, Figure 1B), indicating that FMRP is not required for MEF2-induced transcription of target genes. Surprisingly, the level of Pcdh10 mrna induced with MEF2 activation in Fmr1 KO neurons was greater than in WT neurons, perhaps reflecting a role of FMRP in RNA stability (Zhang et al., 2007). In contrast, Pcdh10 protein is basally higher in Fmr1 KO neurons and is unresponsive to MEF2 activation (Figure 1C). Similar to cultured neurons, Pcdh10 protein is elevated in hippocampal lysates of Fmr1 KO mice (Figures 1D and S1A). One function of FMRP is to suppress translation of its mrna targets (Darnell et al., 2011; Napoli et al., 2008), suggesting that elevated Pcdh10 in Fmr1 KO neurons is due to increased translation rate. To test this possibility, we performed metabolic labeling under basal and MEF2 activated conditions. 35 S-Met/ Cys was added with vehicle or tamoxifen for 6 hr to MEF2- VP16ERtm-transfected cultures followed by immunoprecipitation of Pcdh10 or Tubulin (normalization control). We observed enhanced newly synthesized Pcdh10 in WT neurons upon tamoxifen treatment (Figure 1E). In Fmr1 KO neurons, basal translation rates of Pcdh10 were elevated and MEF2 activation failed to further increase Pcdh10 synthesis. To determine whether MEF2 regulates the half-life of Pcdh10, we applied a 2 hr pulse of 35 S-Met/Cys label to neuronal cultures and chased this with fresh medium containing vehicle or tamoxifen for the time points indicated in Figure 1F. The half-life of Pcdh10 was not affected by genotype or MEF2 activation (Figure 1F). This data indicates that the elevated basal levels of Pcdh10 in Fmr1 KO neurons are due to elevated protein synthesis rates and not reduced turnover. To determine whether dendritic Pcdh10 protein levels are elevated in Fmr1 KO neurons and to assess the cell autonomous role of FMRP in regulation of Pcdh10, we performed immunohistochemistry for Pcdh10 and microtubule-associated protein 2 (MAP2), a dendritic marker, in dissociated neuron cultures prepared from GFP/Fmr1 mosaic mice (i.e., GFP+ cells are wild type and GFP cells are Fmr1 KO; see Extended Experimental Procedures available online). Immunohistochemistry confirmed that FMRP and GFP are coexpressed in hippocampal and cortical neurons of GFP/Fmr1 mosaic mice (Niere et al., 2012) (Figure S1B). Pcdh10, but not MAP2, were elevated in both the soma and dendrites of Fmr1 KO neurons (Figure 1G) in comparison to neighboring GFP+, WT neurons. These results support a role for FMRP in translational suppression of Pcdh10, which leads to elevated Pcdh10 in Fmr1 KO neurons. Immunohistochemistry for Pcdh10 in WT cultures revealed that Pcdh10 was present in dendritic spines where PSD-95, the postsynaptic excitatory synapse marker, was also enriched (Figure 1H). To validate the specificity of the antibody used for immunohistochemistry, we performed staining on hippocampal neurons transfected with two independent shrnas against Pcdh10 (Figure S1C). The results showed similar knockdown efficiency (>50%) by shrnas as observed with western blot (Figures 3F and S3B), validating the fluorescence signal of Pcdh10 seen in Figure 1H. Therefore, Pcdh10 is present in spines at the site of excitatory synapses and may function in MEF2 regulation of excitatory synapse number. Pcdh10 Is Required for MEF2-Induced Synapse Elimination Because the expression of Pcdh10 is regulated by both MEF2 and FMRP, we hypothesized that Pcdh10 is required for MEF2- and FMRP-dependent synapse elimination (Pfeiffer et al., 2010). We first determined whether manipulation of Pcdh10 alone was sufficient to affect excitatory synaptic transmission. To do this, we overexpressed mouse Pcdh10 in WT organotypic hippocampal slice cultures using biolistic transfection (Figures S2A and S2B) (Pfeiffer et al., 2010). At hr after 1582 Cell 151, , December 21, 2012 ª2012 Elsevier Inc.
3 Figure 1. MEF2 and FMRP Coregulate Pcdh10 Expression (A) Quantitative real-time RT-PCR of Pcdh10 precipitated with anti-fmrp antibody or IgG from wild-type (WT) or Fmr1 KO mouse brains. Plotted is average of Pcdh10/Gapdh mrna. n = 5 mice per genotype. (B and C) (B) Quantitative real-time RT-PCR of Pcdh10/Gapdh and Nurr77/Gapdh mrna and (C) Western blots of Pcdh10 from vehicle (V)- or 4-hydroxytamoxifen-treated (tamoxifen or T ) WT or Fmr1 KO cortical neurons transfected with MEF2-VP16ERtm. n = 5 independent cultures for real-time RT-PCR and n = 3 for western blots. (D) Western blots of Pcdh10 from WT or Fmr1 KO hippocampi. n = 4 mice per genotype. (E) 35 S-Met/Cys metabolic labeling from vehicle-treated or tamoxifen-treated (T) WT or Fmr1 KO cortical neurons transfected with MEF2-VP16ERtm followed by immunoprecipitation of Pcdh10 and Tubulin. Plotted is average of Pcdh10/Tubulin. n = 3 cultures. (F) 35 S-Met/Cys pulse-chase assay of Pcdh10 from vehicle- or T-treated WT or Fmr1 KO cortical neurons transfected with MEF2-VP16ERtm. Plotted is average half-life of Pcdh10. n = 3 cultures. (G) Immunohistochemistry of GFP with Pcdh10 or MAP2 from hippocampal neurons of GFP/Fmr1 mosaic mouse. Selected GFP-positive (WT) and GFP-negative (Fmr1 KO) areas are enlarged in the middle. Quantification of fluorescence intensity from soma and dendrites is shown on the right. Scale bar, 20 mm. n = 21 cells for both WT and Fmr1 KO. (H) Immunohistochemistry of Pcdh10 and PSD-95 from dissociated hippocampal neurons of WT or Fmr1 KO mice. GFP was used to visualize dendritic spines. Arrows indicate spines. Error bars represent SEM. Scale bar, 5 mm. *p < 0.05, **p < 0.01, ***p < See also Figure S1. transfection, we performed simultaneous whole-cell patch clamp recordings from Pcdh10-transfected and neighboring untransfected CA1 pyramidal neurons. Pcdh10 overexpression had no effect on excitatory synaptic transmission, as measured by the frequency or amplitude of miniature (m) EPSCs or evoked EPSC amplitude (Figure S2B and Table S1). Similarly, there was no effect of Pcdh10 on paired-pulse facilitation (PPF) of evoked EPSCs, an indicator of presynaptic release probability (Table S1). In the converse experiment, we knocked down endogenous Pcdh10 in WT hippocampal slice cultures using either one of two shrnas targeting different regions of Pcdh10 mrna. After hr transfection, neither shrna against Pcdh10 nor a control nontarget shrna had any effect on the aforementioned measurements of excitatory synaptic function (Figures S2C, S2D, and S2E and Table S1). Similarly, transfection of dissociated hippocampal cultures with either Flag-Pcdh10 or shrna against Pcdh10 (Figures S2F and S2G) had no effect on synapse number, as measured by coclusters of the pre- and postsynaptic proteins synapsin-1 and PSD-95, or PSD-95 puncta number and size. These results indicate that acute changes in Pcdh10 levels are not sufficient to affect excitatory synaptic function. To test whether Pcdh10 is required for MEF2-induced synapse elimination, we cotransfected WT hippocampal slice cultures with plasmids generating either one of two shrnas against Pcdh10 together with MEF2-VP16ERtm. Sister cultures were cotransfected with a control shrna together with MEF2- VP16ERtm. After 24 hr, tamoxifen was applied to the slices to activate MEF2 for another hr, followed by simultaneous Cell 151, , December 21, 2012 ª2012 Elsevier Inc. 1583
4 Figure 2. Pcdh10 Is Required for MEF2-Induced Functional Synapse Elimination in WT Neurons (A) Average evoked EPSC amplitude (A 1 ) and mepsc frequency (A 2 ) from untransfected and control shrna with MEF2-VP16ERtm-transfected CA1 neurons in slice culture. n = 13 for both A 1 and A 2. (B) Average evoked EPSC amplitude (B 1 ) and mepsc frequency (B 2 ) from untransfected and Pcdh10 shrna with MEF2-VP16ERtm-transfected cells. n = 16 for both B 1 and B 2. (C) Average evoked EPSC amplitude (C 1 ) and mepsc frequency (C 2 ) from untransfected and Pcdh10 shrna with MEF2-VP16ERtm and shrna-insensitive Pcdh10 construct-transfected cells. n = 24 for both C 1 and C 2. Error bars represent SEM. *p < Representative evoked EPSC and mepsc traces are shown with the dot plots of individual cell pairs and on the right of each figure, respectively. See also Figure S2 and Table S1. recordings of transfected and neighboring untransfected CA1 neurons. As shown, neurons transfected with MEF2-VP16ERtm and control shrna had a decrease in mepsc frequency and evoked EPSC amplitude but no change in PPF (Figure 2A and Table S1). These changes are consistent and correlate with structural synapse elimination that we previously demonstrated with MEF2-VP16ERtm in this preparation (Flavell et al., 2006; Pfeiffer et al., 2010). However, MEF2-induced functional synapse elimination was blocked in neurons transfected with shrna against Pcdh10 (Figures 2B and S2H and Table S1) and rescued by cotransfection of a shrna-insensitive Pcdh10 (Figure 2C and Table S1) together with the Pcdh10 shrna. shrna-mediated knockdown of Pcdh10 did not affect MEF2-induced transcription of Nurr77 as measured with real-time RT-PCR (Figure S2I), suggesting that Pcdh10 functions in synapse elimination downstream of MEF2 activated transcription. MEF2 Induces Pcdh10-Dependent Degradation of PSD-95 To determine the cellular mechanism by which Pcdh10 mediates MEF2-induced synapse elimination, we turned to dissociated cortical neuron cultures, which are amenable to biochemical methods. We hypothesized that MEF2 activation causes rapid 1584 Cell 151, , December 21, 2012 ª2012 Elsevier Inc.
5 Figure 3. MEF2 Induces Pcdh10-Dependent Degradation of PSD-95 (A) Western blots of postsynaptic proteins from dissociated WT or Fmr1 KO cortical neurons transfected with MEF2-VP16ERtm and treated with either vehicle or tamoxifen for 6 hr. Quantification of PSD-95 is shown on the right. n = 3 cultures. (B) Immunohistochemistry of PSD-95 from dissociated WT or Fmr1 KO hippocampal neurons transfected with MEF2-VP16ERtm and treated with either vehicle or tamoxifen for 6 hr. Bicistronic GFP from MEF2-VP16ERtm plasmid and endogenous MAP2 serve as transfection control and quantification control, respectively. Scale bars, 5 mm. Quantifications of total PSD-95 intensity, PSD-95 puncta number, PSD-95 puncta size, and GFP intensity are shown on the right. n = 21 cells/condition. (C) 35 S-Met/Cys metabolic labeling from vehicle- or tamoxifen-treated WT or Fmr1 KO cortical neurons transfected with MEF2-VP16ERtm followed by immunoprecipitation of PSD-95 and Tubulin. Plotted is average of Pcdh10/Tubulin. n = 3 cultures. (D) 35 S-Met/Cys pulse-chase assay of Pcdh10 from vehicle- or tamoxifen-treated WT or Fmr1 KO cortical neurons transfected with MEF2-VP16ERtm. Plotted is the average half-life of Pcdh10. n = 3 cultures. (E) Western blots of PSD-95 and Tubulin from dissociated WT or Fmr1 KO cortical neurons transfected with MEF2-VP16ERtm and treated with vehicle, tamoxifen, and/or proteasome inhibitor MG132, as indicated for 6 hr. Quantification is shown below. n = 4 cultures. (F) Western blots of PSD-95, Pcdh10, and Tubulin from dissociated cortical neurons of WT or Fmr1 KO mice transfected with MEF2-VP16ERtm and Pcdh10 shrna. Quantification is shown below. n = 3 cultures. Error bars represent SEM. **p < See also Figure S3. synapse elimination by the regulated degradation of synaptic scaffolding proteins. To investigate this possibility, we monitored the level of several postsynaptic scaffolding proteins in MEF2- VP16ERtm transfected cortical neuron cultures after 6 hr of tamoxifen treatment. As shown in Figure 3A, PSD-95 levels were significantly decreased in WT neurons, whereas other synaptic proteins (mglur5, Homer, and CaMKIIa) remained unchanged. Consistent with the deficit in MEF2-induced synapse elimination in Fmr1 KO neurons, PSD-95 levels were unchanged upon MEF2 activation in Fmr1 KO neurons. Similar results were observed with immunohistochemistry for PSD-95 in dissociated hippocampal neurons (Figure 3B). This result also suggests that the downregulation of PSD-95 is a common consequence of MEF2 activation in both cortical and hippocampal neurons. To determine whether the downregulation of PSD-95 resulted from reduced synthesis or increased degradation, we performed metabolic labeling with 35 S-Met/Cys in dissociated cortical neuron cultures. After 6 hr of MEF2 activation and 35 S-Met/Cys label, there was no change in newly synthesized PSD-95 from either WT or Fmr1 KO neurons (Figure 3C). However, using the pulse-chase 35 S-Met/Cys labeling assay, the half-life of Cell 151, , December 21, 2012 ª2012 Elsevier Inc. 1585
6 1586 Cell 151, , December 21, 2012 ª2012 Elsevier Inc. (legend continued on next page)
7 PSD-95 was reduced by 35% after MEF2 activation in WT neurons (Figure 3D). Like PSD-95 protein levels (Figures 3A and 3B), PSD-95 half-life was unaffected by MEF2 activation in Fmr1 KO neurons. To investigate whether MEF2-induced degradation of PSD-95 in WT neurons is proteasome dependent, we applied proteasome inhibitors MG132 (5 mm) or lactacystin (5 mm), together with tamoxifen (Figures 3E and S3A). Both MG132 and lactacystin blocked MEF2-induced downregulation of PSD-95 in WT neurons, and no changes in PSD-95 were observed under any condition in Fmr1 KO neurons. To determine whether MEF2-induced degradation of PSD-95 is mediated by Pcdh10, we used lentivirus to deliver MEF2- VP16ERtm along with one of two different shrnas against Pcdh10 into dissociated cortical neuron cultures. Knocking down Pcdh10 with either shrna inhibited MEF2-induced PSD-95 degradation (Figures 3F and S3B). PSD-95 degradation is rescued by lentiviral coexpression of shrna-insensitive Pcdh10 together with the Pcdh10 shrna (Figure S3C). These findings indicate that Pcdh10 is required for MEF2-induced degradation of PSD-95 and suggest a molecular mechanism for Pcdh10 in MEF2-induced synapse elimination. Pcdh10 Facilitates the Proteasomal Deposition of Ubiquitinated PSD-95 To better determine the role of Pcdh10 in MEF2-induced regulation of PSD-95, we investigated whether Pcdh10 physically associated with PSD-95 and whether this changed with MEF2 activation. Using coimmunoprecipitation of Pcdh10 with PSD- 95 from cortical neuron cultures (in the presence of MG132), we found that Pcdh10 could associate with PSD-95 (Figure 4A). Activation of MEF2 for 6 hr drastically increased the association between Pcdh10 and PSD-95 in WT neurons, but not in Fmr1 KO neurons (Figure 4A). Similar results were obtained in hippocampal neuron cultures (Figure S4A). Given that MEF2 increases the expression of Pcdh10 in WT cultures (Figure 1D), we considered whether the MEF2-induced association of Pcdh10 and PSD-95 is due to enhanced production of Pcdh10. We used lentivirus to transfect WT or Fmr1 KO cortical cultures with a Flag-tagged Pcdh10 driven by the cytomegalovirus (CMV) promoter whose transcription is not regulated by MEF2. Although total Flag-Pcdh10 levels were unchanged upon MEF2 activation, immunoprecipitation of PSD-95 revealed an increase in Flag-Pcdh10 association with PSD-95 (Figure 4B, in the presence of MG132). This suggests that MEF2 promotes the association of Pcdh10 with PSD-95 independent of regulating Pcdh10 levels. In Fmr1 KO cultures, MEF2 activation did not stimulate an interaction of PSD-95 with Flag-Pcdh10, suggesting that the deficit in PSD95 degradation in Fmr1 KO neurons is not due to the lack of MEF2-triggered Pcdh10 translation but instead on a distinct function of FMRP. To determine the mechanism by which Pcdh10 regulates MEF2-dependent PSD-95 proteasomal degradation, we examined ubiquitination of PSD-95. MEF2 activation in WT cultures resulted in PSD-95 ubiquitination, as observed by immunoprecipitation of PSD-95 followed by western blotting with an antiubiquitin (Ub) antibody (in the presence of MG132, Figure 4C, top). Similar to previous reports (Colledge et al., 2003; Rezvani et al., 2007), polyubiquitinated PSD-95 was observed as multiple, distinct high-molecular-weight bands ( kda). Knockdown of Pcdh10 with a lentiviral-expressed shrna had a slight effect on MEF2-induced PSD-95 ubiquitination. However, using GST-Ubl (ubiquitin-like domain from Rad23) (Bingol et al., 2010) to pull down the proteasome and proteasome-interacting proteins (in the presence of MG132), we could only detect ubiquitinated PSD-95 in neurons transfected with MEF2 plus control shrna, but not Pcdh10 shrna (Figure 4C, second panel from the top, and S4B). These results suggest that the predominant role of Pcdh10 is to associate ubiquitinated PSD-95 with the proteasome. Because Pcdh10 is important for association of ubiquitinated PSD-95 with the proteasome, we next asked whether PSD-95 ubiquitination or proteasome association is deficient in Fmr1 KO neurons. In contrast to WT cultures, MEF2 activation in Fmr1 KO cultures failed to increase PSD-95 ubiquitination (Figure 4C). Consistent with these results, basal ubiquitination of PSD-95 is reduced in vivo in Fmr1 KO hippocampi (Figure S4C). Taken together, these findings suggest that FMRP and Pcdh10 have distinct roles in MEF2-induced PSD-95 degradation: (1) FMRP is required for MEF2-induced ubiquitination of PSD-95, and (2) Pcdh10 is primarily required for linking ubiquitinated PSD-95 to the proteasomal machinery. Based on results in WT cultures, we hypothesize that Pcdh10 associates ubiquitinated PSD-95 with the proteasome. To test this idea, we determined whether Pcdh10 interacts with ubiquitinated PSD-95 in vitro. We prepared native and ubiquitinated GST-PSD-95 by in vitro ubiquitination and observed that Figure 4. Pcdh10 Facilitates the Proteasomal Degradation of Ubiquitinated PSD-95 (A and B) Western blots of (A) endogenous Pcdh10 or (B) Flag-Pcdh10 coimmunoprecipitated by anti-psd-95 antibody. Protein samples were from dissociated WT or Fmr1 KO cortical neurons transfected with (A) MEF2-VP16ERtm or (B) MEF2-VP16ERtm plus Flag-Pcdh10 and treated with either vehicle or tamoxifen for 6 hr. Inputs and quantification results are shown on the bottom and on the right. n = 4 cultures. (C) Western blots of ubiquitin after immunoprecipitation of PSD-95 (top). Western blots of PSD-95 and Rpt1 after pull-down with GST-Ubl (second and third panels from the top). Western blots of PSD-95 and Rpt1 after pull-down with GST alone (fourth and fifth panels from the top). Protein samples were from dissociated WT or Fmr1 KO cortical neurons transfected with MEF2-VP16ERtm with either control shrna or Pcdh10 shrna. Treatment is as indicated, and inputs are shown on the bottom. (D) Western blots of Pcdh10 from WT brain lysates pulled down by increasing amount of native GST-PSD-95 or in vitro ubiquitinated GST-PSD-95. GST-PSD-95 used for the assay was detected by anti-psd-95 antibody shown in the middle panel, whereas ubiquitinated GST-PSD-95 was detected by anti-ha antibody shown on the bottom. Arrowhead and arrows indicate native and ubiquitinated PSD-95, respectively. (E) Western blots of Pcdh10, Rpt1, CaMKIIa, Pcdh9, and Tubulin from WT or Fmr1 KO mice brains after pull-down with either GST or GST-Ubl. GST proteins used are shown on the bottom after Coomassie blue staining on a SDS-PAGE gel. (F) Western blots of GST or GST-Pcdh10-C terminus pulled down by purified proteasome from Flag-Rpt6 stably expressing HEK293 cells. Experiments in (A), (B), and (C) were done in the presence of MG132. Error bars represent SEM. **p < See also Figure S4. Cell 151, , December 21, 2012 ª2012 Elsevier Inc. 1587
8 Pcdh10 from WT brain lysates preferentially interacts with ubiquitinated, but not native, GST-PSD-95 (Figure 4D). Ubiquitinated GST-PSD-95 failed to interact with in-vitro-translated Pcdh10 (Figure S4D), suggesting that posttranslational modifications of Pcdh10 or other interacting proteins are necessary. To test whether Pcdh10 interacts with the proteasome, we used GST-Ubl to pull down the proteasome and find that Pcdh10, the proteasome component Rpt1 (Demartino and Gillette, 2007), and the proteasome-interacting protein CaMKIIa (Bingol et al., 2010) were pulled down from both WT and Fmr1 KO mouse brain (Figure 4E). In contrast, another protocadherind family member, Pcdh9 (Morishita and Yagi, 2007), was not pulled down by GST-Ubl. The interaction of Pcdh10 and the proteasome was also confirmed by the demonstration that the GST-tagged C terminus of Pcdh10 interacts with a purified proteasome preparation from Flag-Rpt6 stably expressing HEK293 cells (Kim and DeMartino, 2011)(Figures 4F and S4E). Together, these results suggest that Pcdh10 interacts with ubiquitinated PSD-95 and the proteasome, and this interaction may not be conserved in other protocadherin family members. We next determined whether MEF2-induced PSD-95 degradation and synapse elimination required Pcdh10 association with the proteasome. Toward this goal, we first mapped the minimum region of Pcdh10 that is required for interaction with the proteasome. We focused on the C terminus of Pcdh10 (266 amino acid) because it is predicted to be a cytoplasmic domain (Morishita and Yagi, 2007). We made various deletion constructs of the C terminus of Pcdh10 tagged with GST and tested their ability to pull down proteasomal complexes from brain lysates, as determined by association with the essential proteasome protein component Rpt1 (Bingol et al., 2010) (Figure 5A). Using this assay, we identified a 102 amino acid region (amino acids ) of Pcdh10 required for the interaction with the proteasome. Amino acids are highly conserved in Pcdh10 across species (Figure S5A), and we termed this sequence the proteasome interacting region (PIR). We aimed to determine whether Pcdh10 association with the proteasome is necessary for MEF2-induced PSD-95 degradation and synapse elimination. In preparation for these experiments, we expressed a Flag-tagged PIR in WT cultured neurons and found that it distributes in dendrites and spines but did not alter general proteasome activity (Figures S5B and S5C). Importantly, Flag-PIR expression effectively competed with Pcdh10 and greatly reduced the association of endogenous Pcdh10 with the proteasome in neurons, as assessed by GST-Ubl pull-down (Figure 5B). Coexpression of Flag-PIR and MEF2- VP16ERtm in WT cortical neuron cultures blocked MEF2- induced degradation of PSD-95 (Figure 5C). Similarly, biolistic cotransfection of Flag-PIR and MEF2-VP16 into CA1 neurons of WT mouse hippocampal slice cultures blocked MEF2-induced functional synapse elimination (Figures 5D and 5E and Table S1) and MEF2-induced reduction in dendritic spine density (Figure 5F). Expression of Flag-PIR alone did not alter synaptic function (Figure S5D), spine density (Figure 5F), or MEF2-induced transcription (Figure S2I). These data demonstrate that Pcdh10 association with the proteasome is necessary for MEF2-induced PSD-95 degradation, as well as functional and structural synapse elimination. And based on these and our biochemical data (Figure 4), we propose that one role of Pcdh10 in synapse elimination is to deliver ubiquitinated PSD-95 to the proteasome for degradation. The Ubiquitin E3 Ligase for PSD-95, Mdm2, Is Dysregulated in Fmr1 KO Neurons In Fmr1 KO neurons, MEF2-induced ubiquitination of PSD-95 is absent; a process that is independent of Pcdh10. To investigate the deficit in PSD-95 ubiquitination in Fmr1 KO neurons, we examined the substrate-specific step of the ubiquitination process, which involves the ubiquitin E3 ligase for PSD-95, murine double minute 2 (Mdm2) (Colledge et al., 2003). Using shrnas against Mdm2, we observed that Mdm2 is required for MEF2-induced PSD-95 ubiquitination (Figure 6A) and degradation (Figures 6B, S6A, S6B, and S6C) in WT cortical neurons, thus confirming that Mdm2 is the relevant E3 ligase for PSD- 95. To investigate whether Mdm2 is dysregulated in Fmr1 KO neurons, we performed coimmunoprecipitation and observed reduced interaction of Mdm2 and PSD-95 in Fmr1 KO hippocampus in contrast to WT hippocampus (Figure 6C). Using immunohistochemistry for Mdm2 in dissociated hippocampal neurons, we also observed lower colocalization of Mdm2 with PSD-95 in dendrites of Fmr1 KO neurons compared to WT neurons (Figure 6D). Although total Mdm2 levels were normal in Fmr1 KO hippocampus (Figure 6C), Mdm2 levels in synaptoneurosome fractions of Fmr1 KO hippocampi were reduced to about 40% of WT levels (Figure 6E). We hypothesized that MEF2 may enhance the synaptic localization of Mdm2, and this may be deficient in Fmr1 KO neurons. To test this possibility, we activated MEF2 in dissociated cortical neuron cultures and examined Mdm2 levels in synaptoneurosome fractions and in coimmunoprecipitates with PSD-95. In WT neurons, MEF2 activation increased Mdm2 levels in synaptoneurosomes (Figure 6F), as well as the interaction between PSD-95 and Mdm2 (Figure 6G). In contrast, in Fmr1 KO neurons, MEF2 failed to increase Mdm2 levels in synaptoneurosomes or the association of Mdm2 with PSD-95. MEF2 activation did not alter total Mdm2 levels in either WT or Fmr1 KO cultures (Figures 6B and 6F). Together, these results indicate that MEF2 stimulates the localization of Mdm2 to the synaptic compartment and to PSD-95, and this process is defective in Fmr1 KO neurons. Elevated EF1a Inhibits Mdm2 from Interacting with PSD-95 in Fmr1 KO Neurons In Fmr1 KO neurons, Mdm2 localization at the synapse is reduced, and MEF2-induced redistribution of Mdm2 to synapses is deficient (Figure 6). We hypothesized that Mdm2 may have an abnormal interactome in Fmr1 KO neurons. A previous proteomic study to identify human Mdm2 (Hdm2)-interacting proteins discovered a robust interaction of eukaryotic translation elongation factor 1-a (EF1a) and Hdm2 (Frum et al., 2007). EF1a is an FMRP target mrna, and EF1a protein levels are upregulated in Fmr1 KO brain (Darnell et al., 2011; Sung et al., 2003). We hypothesized that elevated EF1a in Fmr1 KO neurons sequesters Mdm2 and prevents it from ubiquitinating PSD-95. In support of this hypothesis, immunoprecipitation of Mdm2 from hippocampal lysates revealed an increased interaction of EF1a and Mdm2 in vivo in Fmr1 KO mice (Figure 7A). This increased 1588 Cell 151, , December 21, 2012 ª2012 Elsevier Inc.
9 Figure 5. Blocking Interaction of Pcdh10 and Proteasome Inhibits MEF2-Induced PSD-95 Degradation and Synapse Elimination (A) Western blots of Rpt1 from WT mouse brain after pull-down by GST alone or different GST-Pcdh10 carboxyl terminus proteins. Schematic diagram of Pcdh10- C-terminal deletions are shown on top. GST proteins used are shown on the right with Coomassie blue staining on a SDS-PAGE gel. (B) Western blots of Pcdh10, Rpt1, and Flag from dissociated WT cortical neurons transfected with Flag-PIR after pull-down with either GST or GST-Ubl. Input of each protein and quantification are shown on the bottom and right, respectively. n = 3 cultures. (C) Western blots of PSD-95, Flag, and Tubulin from dissociated WT cortical neurons transfected with MEF2-VP16ERtm and Flag-PIR with vehicle or tamoxifen treatment as indicated. Quantification of PSD-95 level is shown on the right. n = 4 cultures. (D and E) Average evoked EPSC amplitude (D 1 ) and mepsc frequency (D 2 ) from untransfected and control vector plus MEF2-VP16-transfected cells. n = 14 for both D 1 and D 2. (E) Average evoked EPSC amplitude (E 1 ) and mepsc frequency (E 2 ) from untransfected and PIR plus MEF2-VP16-transfected cells. n = 16 for both E 1 and E 2. Representative evoked EPSC and mepsc traces are shown with the dot plots and on the right of each figure, respectively. (F) Quantification and representative images of apical secondary dendrites from WT CA1 pyramidal neurons transfected with GFP plus other plasmids as indicated. n = cells in each condition. Scale bar, 5 mm. Error bars represent SEM. *p < 0.05, ***p < See also Figure S5 and Table S1. interaction may be due to the observed increase in total EF1a protein in Fmr1 KO (Figure 7A). To determine whether EF1a and PSD-95 directly compete for interactions with Mdm2, we obtained recombinant His-Mdm2, GST-EF1a, and GST-PSD- 95. To Ni beads with bound His-Mdm2, we first added increasing amounts of GST-EF1a. After washing off unbound GST-EF1a, we incubated the complex with GST-PSD-95. Because a possible degraded product of His-Mdm2 runs at the same position of GST- EF1a on a SDS-PAGE, we used western blotting to detect the composition of the complex. As shown (Figure 7B), the binding of Mdm2 to EF1a occurs with a stoichiometry of 1:1, and EF1a-bound Mdm2 lost the ability to bind PSD-95. His-Mdm2 also runs at a position approximately doubled to its predicted molecular weight, suggesting formation of homodimer (Dolezelova et al., 2012). The GST tag does not interact with His- Mdm2 nor interfere with the binding of His-Mdm2 to GST-EF1a or GST-PSD-95 (Figure S7A). To test the affinity of Mdm2 toward binding EF1a or PSD-95, we premixed GST- EF1a (1 pmol) and Cell 151, , December 21, 2012 ª2012 Elsevier Inc. 1589
10 Figure 6. Mdm2 and Mdm2-Mediated Degradation of PSD-95 Are Dysregulated in Fmr1 KO Neurons (A) Western blots of ubiquitin after immunoprecipitation with PSD-95 (top) or IgG (second panel from the top). Protein samples were from dissociated WT or Fmr1 KO cortical neurons transfected with either control shrna or Mdm2 shrna together with MEF2-VP16ERtm. Treatment was as indicated, and input was shown on the bottom. (B) Western blots of PSD-95, Mdm2, and Tubulin from dissociated WT or Fmr1 KO cortical neurons transfected with either control shrna or Mdm2 shrna together with MEF2-VP16ERtm. Quantification is shown on the right. n = 3 cultures. (C) Western blots of Mdm2 immunoprecipitated with PSD-95 from WT or Fmr1 KO hippocampi. n = 4 mice per genotype. (D) Immunohistochemistry of dendritic Mdm2 with PSD-95 from dissociated WT or Fmr1 KO hippocampal neurons. Colocalization images are shown on the bottom. Scale bars, 5 mm. Quantification of colocalization is shown on the right. n = 21 cells. (E) Western blots of Mdm2, PSD-95, GluR1 (GluA1), and Tubulin in synaptoneurosomes prepared from WT or Fmr1 KO hippocampi. Quantification of Mdm2 in supernatant or synaptoneurosome is shown on the right. n = 4 mice per genotype. (F) Western blots of Mdm2, PSD-95, GluR1 (GluA1), and Tubulin after synaptoneurosome preparation of dissociated WT or Fmr1 KO cortical neurons transfected with MEF2-VP16ERTm. Quantification of Mdm2 in synaptoneurosome is shown on the right. n = 3 cultures. (G) Western blots of Mdm2 immunoprecipitated with PSD-95 from dissociated WT or Fmr1 KO cortical neurons transfected with MEF2-VP16ERtm. Quantification is shown on the right. n = 3 cultures. Error bars represent SEM. *p < 0.05, **p < 0.01, ***p < Experiments in (A), (F), and (G) were done in the presence of MG132. See also Figure S6. GST-PSD-95 (1 pmol), followed by adding Ni beads with bound His-Mdm2 (0.5 pmol). As shown in Figure 7C, Mdm2 has higher affinity toward binding EF1a over PSD-95. Together, these results indicate that EF1a can effectively compete with PSD-95 for binding to Mdm2. We hypothesized that reducing EF1a levels in Fmr1 KO neurons may release Mdm2, allow interactions with PSD-95, and rescue MEF2-induced PSD-95 degradation. To test this possibility, we applied a lentivirus expressing a shrna against EF1a that reduced EF1a in Fmr1 KO neurons to WT levels but did not affect Pcdh10 protein levels (Figure S7B). Knocking down EF1a in Fmr1 KO neurons did not affect MEF2 activation (Figure S7C) but restored MEF2-induced synaptic localization of Mdm2 (Figure 7D) and the interaction between Mdm2 and PSD-95 (Figure 7E). Knocking down EF1a also restored MEF2-induced PSD-95 degradation in Fmr1 KO neurons, which required Mdm2 (Figures 7F and S7D). Coexpression of a shrna-insensitive EF1a together with the EF1a shrna inhibited MEF2-induced synaptic accumulation of Mdm2 (Figure S7E), the interaction between PSD-95 and Mdm2 (Figure S7F), and PSD-95 degradation (Figure S7G) in Fmr1 KO neurons, demonstrating the specificity of the EF1a shrna. These results demonstrate that elevated EF1a in Fmr1 KO neurons inhibits Mdm2 from interacting with PSD- 95 and blocks the subsequent degradation of PSD-95. To determine whether knocking down EF1a in Fmr1 KO neurons could also restore MEF2-induced synapse elimination, we biolistically transfected Fmr1 KO hippocampal slice cultures with a plasmid encoding a shrna for EF1a or a control shrna Cell 151, , December 21, 2012 ª2012 Elsevier Inc.
11 Expression of either shrna alone had no effect on synaptic function (Figures S7H and S7I and Table S1). We then cotransfected Fmr1 KO slice cultures with either control shrna or EF1a shrna together with MEF2-VP16ERtm and subsequently treated the cultures with tamoxifen. Fmr1 KO neurons transfected with control shrna had a deficit in MEF2-induced functional synapse elimination (Figure 7G), consistent with our previous report (Pfeiffer et al., 2010). As observed with PSD-95 degradation, EF1a shrna transfection rescued robust functional synapse elimination in response to MEF2 activation (Figure 7H). Again, coexpression of a shrna-insensitive EF1a together with the EF1a shrna blocked MEF2-induced functional synapse elimination (Figure 7I). The shrna-insensitive EF1a on its own did not affect excitatory synaptic function (Figure S7J). Taken together, our results indicate that elevated EF1a in Fmr1 KO neurons inhibits Mdm2 from triggering PSD-95 degradation and subsequent synapse elimination by MEF2 in the Fmr1 KO neurons. By knocking down EF1a, we were able to rescue the following in response to MEF2 activation: (1) synaptic redistribution of Mdm2, (2) the interaction between Mdm2 and PSD-95, (3) PSD-95 degradation, and (4) synapse elimination. DISCUSSION MEF2 Triggers Synapse Elimination by Promoting Pcdh10-Dependent Degradation of a Synaptic Scaffolding Protein Here we identified a mechanism by which the activity-dependent transcription factor, MEF2, promotes synapse elimination as well as the molecular basis of the deficit in synapse elimination observed in fragile X syndrome, a prevalent cognitive and autistic disorder. In wild-type neurons, MEF2 activation induces transcription of Pcdh10, which mediates degradation of the synaptic scaffold PSD-95, and synapse elimination by associating ubiquitinated PSD-95 with the proteasome (Figure 7J). Although translation of Pcdh10, an FMRP target mrna, is dysregulated in Fmr1 KO neurons, we find a deficit in MEF2-stimulated ubiquitination of PSD-95, a step that is upstream and independent of Pcdh10. The deficit in PSD-95 ubiquitination in Fmr1 KO neurons is caused by elevated levels of EF1a protein, an FMRP target mrna, which sequesters Mdm2 from the synapse and PSD-95 (Figure 7J). In support of this conclusion, knockdown of EF1a levels rescues MEF2-induced PSD-95 degradation and synapse elimination in Fmr1 KO neurons. Whether MEF2 regulates the interaction of EF1a and Mdm2 to regulate PSD-95 degradation in WT neurons is unknown. Protocadherins and Synapse Elimination The protocadherins are ancestors of the classical cadherins, the more well-known members of the Ca 2+ -dependent cell adhesion molecule family (Giagtzoglou et al., 2009). Although the extracellular domains of protocadherins share homology with the traditional cadherins, they lack a hydrophobic pocket within extracellular domain 1 that is important for strong homophilic adhesion (Morishita et al., 2006; Morishita and Yagi, 2007). Consistent with this prediction, Pcdh10, when expressed in cell lines, clusters at points of cell contact and stimulates cell aggregation but less robustly than classical cadherins (Hirano et al., 1999). A recent study demonstrated that another d2 nonclustered protocadherin family member, Pcdh8 or Arcadlin, is induced in neurons in response to seizures and interacts in cis with N-cadherin (a classical cadherin) in postsynaptic membranes. Homophilic interactions of Pcdh8, in cis or trans, recruit p38 MAPK to its C terminus, which in turn, stimulates the coendocytosis of Pcdh8 and N-cadherin and decreases dendritic spine density (Yasuda et al., 2007). An interesting possibility is that the induction of the d2 Pcdhs, such as Pcdh8 and Pcdh10, functions to increase the membrane concentration and probability of homophilic Pcdh interactions, which activates key signaling pathways and their endocytosis. Such endocytosis could facilitate interactions of Pcdh10 with ubiquitinated PSD-95 and the proteasome. MEF2 Promotes Degradation of a Postsynaptic Scaffold to Eliminate Synapses MEF2 activation is associated with a rapid reduction of synapse number, suggesting that MEF2 eliminates pre-existing synapses (Flavell et al., 2006; Pfeiffer et al., 2010). However, MEF2 may also inhibit synapse formation rates. In support of an elimination mechanism, MEF2 activation caused a rapid and selective degradation of PSD-95 and a decrease in functional synapse number effects that both required the MEF2 transcript, Pcdh10. Disrupting the association of Pcdh10 with the proteasome using the PIR peptide blocked MEF2-induced PSD-95 degradation, as well as functional and structural synapse elimination, suggesting that ubiquitination and Pcdh10-dependent degradation of PSD-95 are key steps that couple MEF2-driven transcription to synapse elimination. PSD-95 stabilizes synaptic AMPARs, increases synaptic function, and results in larger and more stable spines (Keith and El-Husseini, 2008; Opazo et al., 2011). Conversely, reductions in PSD-95 can lead to diffusion and endocytosis of synaptic AMPARs, weaker synapses, smaller spines, and spine elimination (Colledge et al., 2003; Haas et al., 2007; Keith and El-Husseini, 2008; Opazo et al., 2011; Woods et al., 2011). Specifically, in response to NMDAR activation, PSD-95 is ubiquitinated by Mdm2, degraded by the proteasome, removed from spines, and required for endocytosis of AMPARs (Colledge et al., 2003; Sturgill et al., 2009). Therefore, MEF2- triggered degradation of PSD-95 may cause destabilization and removal of synaptic AMPARs that precedes elimination of the structural synapse. Of note, knockdown of Pcdh10 prevented MEF2-induced functional synapse elimination, but not ubiquitination of PSD-95. This suggests that ubiquitination of PSD-95 is not sufficient to mediate functional synapse elimination, as Pcdh10-dependent targeting of ubiquitinated PSD-95 to the proteasome is also required. Recapitulating Synapse Elimination in Fragile X Syndrome Fragile X syndrome patients and Fmr1 KO mice display elevated dendritic spine number in mature cortical neurons, which may contribute to the neuronal hyperexcitability, sensory hypersensitivity, and epileptic seizures observed in patients and the mouse model (Berry-Kravis, 2002; Dölen et al., 2010). A deficit in activity and MEF2-dependent synapse elimination could lead to excess spines. In support of this idea, experience-dependent spine Cell 151, , December 21, 2012 ª2012 Elsevier Inc. 1591
12 Figure 7. Elevated EF1a in Fmr1 KO Neurons Inhibits Mdm2 from Interacting with PSD-95 and Subsequent PSD-95 Degradation (A) Western blots of EF1a from WT or Fmr1 KO hippocampi after immunoprecipitation by anti-mdm2 antibody or IgG. Quantifications on total and immunoprecipitated EF1a with Mdm2 are shown on the right. n = 3 mice per genotype. (B) Western blots of Mdm2, EF1a, and PSD-95 after in vitro sequential binding with recombinant proteins. Quantifications of binding and competition are shown in the middle (n = 3), whereas the purity of recombinant proteins is shown on the right with Coomassie-blue-stained SDS-PAGE gel. (C) Western blots of GST- EF1a and GST-PSD-95 after in vitro binding with His-Mdm2. Quantification of relative amount of GST proteins pulled down by His-Mdm2 is on the right. n = 4. (D) Western blots of Mdm2, PSD-95, EF1a, GluR1 (GluA1), and Tubulin after synaptoneurosome preparation of dissociated Fmr1 KO cortical neurons transfected with MEF2-VP16ERtm and either control shrna or EF1a shrna. Quantification of Mdm2 in synaptoneurosome is shown on the right. n = 3 cultures. (E) Western blots of Mdm2 immunoprecipitated with PSD-95 from dissociated Fmr1 KO cortical neurons transfected with MEF2-VP16ERtm and either control shrna or EF1a shrna. Quantification is shown on the right. n = 3 cultures. Experiments in (D) and (E) were done in the presence of MG132. (F) Western blots of PSD-95, Mdm2, EF1a, and Tubulin from dissociated Fmr1 KO cortical neurons transfected with MEF2-VP16ERtm and either control shrna, EF1a shrna, and/or Mdm2 shrna as indicated. Treatment with vehicle or tamoxifen is also as indicated. Quantification is shown on the bottom. n = 4 cultures. (G) Average evoked EPSC amplitude (G 1 ) and mepsc frequency (G 2 ) from untransfected and control shrna with MEF2-VP16ERtm-transfected Fmr1 KO cells. n = 11 for both G 1 and G 2. (H) Average evoked EPSC amplitude (H 1 ) and mepsc frequency (H 2 ) from untransfected and EF1a shrna with MEF2-VP16ERtm-transfected Fmr1 KO cells. n = 9 for both H 1 and H 2. (legend continued on next page) 1592 Cell 151, , December 21, 2012 ª2012 Elsevier Inc.
13 elimination in the somatosensory barrel cortex is deficient in Fmr1 KO mice (Pan et al., 2010). The molecular mechanisms that lead to elevated dendritic spine number or the deficits in synapse elimination associated with fragile X are unknown. Current evidence suggests that FMRP functions as a translational switch of its target mrnas. At steady state, FMRP suppresses translation of its mrna targets in dendrites, and it derepresses or stimulates their translation upon synaptic stimulation dephosphorylation and/or degradation (Bassell and Warren, 2008; Niere et al., 2012). Consistent with this model, we observed elevated Pcdh10 translation rates in Fmr1 KO neurons that were not stimulated by MEF2 despite induction of Pcdh10 mrna. Alternatively, Pcdh10 translation rates in Fmr1 KO neurons may be saturated and cannot be further increased by elevated mrna. We would expect that translational control of other FMRP target mrnas that are induced in response to MEF2 would be similarly affected in Fmr1 KO neurons. Although translational control of Pcdh10 is abnormal in Fmr1 KO neurons, our results suggest that this does not underlie the deficit in MEF2-induced synapse elimination. Knocking down EF1a restores MEF2-induced synapse elimination in Fmr1 KO mice without affecting Pcdh10 protein levels. Therefore, one requirement of FMRP in MEF2-induced synapse elimination is to suppress steady-state translation of EF1a and allow appropriate MEF2-regulated synaptic targeting of Mdm2 and PSD- 95 ubiquitination. This highlights the importance of translational suppression and regulation of protein concentration in dendrites for proper synaptic plasticity. Loss-of-function mutations in the MEF2 isoform, Mef2C, Fmr1, or Pcdh10 are all associated with autism, with or without intellectual disability (Abrahams and Geschwind, 2008; Morrow et al., 2008; Novara et al., 2010). Here, we have described distinct roles for all three genes in regulated degradation of PSD-95 and synapse elimination. This work suggests a common deficit in activity-dependent synapse elimination among different genetic causes of autism. EXPERIMENTAL PROCEDURES Organotypic Slice Culture and Dissociated Neuron Culture Organotypic hippocampal slice cultures were made from postnatal day (P) 6 7 WT or Fmr1 KO mice bred from the congenic C57BL/6 mouse strain and transfected as described (Pfeiffer et al., 2010)(Extended Experimental Procedures). Dissociated hippocampal or cortical cultures were prepared from P0-1 WT and Fmr1 KO mice as described (Niere et al., 2012). Electrophysiology Dual whole-cell patch recordings were obtained from CA1 pyramidal neurons in slice cultures using IR-DIC and GFP fluorescence to identify nontransfected and transfected neurons as described (Pfeiffer et al., 2010) (Extended Experimental Procedures). Synaptoneurosome Preparation Hippocampal synaptoneurosomes were prepared as previously described (Waung et al., 2008). For synaptoneurosomes of dissociated cultures, cells were scraped off in buffer: 118 mm NaCl, 4.7 mm KCl, 1.2 mm MgSO 4, 2.5 mm CaCl 2, 1.53 mm KH 2 PO 4, mm glucose, 1 mm DTT (ph 7.4), and protease inhibitor cocktail (Calbiochem) followed by same procedures as described (Waung et al., 2008). Statistical Analysis For multiple comparisons, two-way ANOVA and Bonferroni posthoc tests were performed. Paired t tests were used for electrophysiology assays. Independent t test was used for Figures 1D, 5B, 5F, 6C, and 7A. In all figures, error bars represent SEM; *p < 0.05, **p < 0.01, ***p < SUPPLEMENTAL INFORMATION Supplemental Information includes Extended Experimental Procedures, seven figures, and one table and can be found with this article at /j.cell ACKNOWLEDGMENTS This research was supported by grants from the National Institutes of Health NS045711, HD (K.M.H.), F32HD (N.-P.T.), F32HD (J.R.W.) and from the Simons Foundations (K.M.H. and C.W.C.). We would also like to thank L. Ormazabal, N. Cabalo, F. Niere, K. Loerwald, T. Zang, D. Thompson, X. Li, and B. Chen for technical assistance; Dr. M. Rosen for the GST-Pcdh10 constructs; Dr. J. Gibson for helpful discussions; and Drs. Robert and Jennifer Darnell for sharing their FMRP-CLIP target list prior to publication. Received: February 25, 2012 Revised: August 29, 2012 Accepted: November 20, 2012 Published: December 20, 2012 REFERENCES Abrahams, B.S., and Geschwind, D.H. (2008). Advances in autism genetics: on the threshold of a new neurobiology. Nat. Rev. Genet. 9, Bagni, C., and Greenough, W.T. (2005). From mrnp trafficking to spine dysmorphogenesis: the roots of fragile X syndrome. Nat. Rev. Neurosci. 6, Bassell, G.J., and Warren, S.T. (2008). Fragile X syndrome: loss of local mrna regulation alters synaptic development and function. Neuron 60, Berry-Kravis, E. (2002). Epilepsy in fragile X syndrome. Dev. Med. Child Neurol. 44, Bingol, B., Wang, C.F., Arnott, D., Cheng, D., Peng, J., and Sheng, M. (2010). Autophosphorylated CaMKIIalpha acts as a scaffold to recruit proteasomes to dendritic spines. Cell 140, Colledge, M., Snyder, E.M., Crozier, R.A., Soderling, J.A., Jin, Y., Langeberg, L.K., Lu, H., Bear, M.F., and Scott, J.D. (2003). Ubiquitination regulates PSD- 95 degradation and AMPA receptor surface expression. Neuron 40, Darnell, J.C., Van Driesche, S.J., Zhang, C., Hung, K.Y., Mele, A., Fraser, C.E., Stone, E.F., Chen, C., Fak, J.J., Chi, S.W., et al. (2011). FMRP stalls ribosomal translocation on mrnas linked to synaptic function and autism. Cell 146, Demartino, G.N., and Gillette, T.G. (2007). Proteasomes: machines for all reasons. Cell 129, Dölen, G., Carpenter, R.L., Ocain, T.D., and Bear, M.F. (2010). Mechanismbased approaches to treating fragile X. Pharmacol. Ther. 127, (I) Average evoked EPSC amplitude (I 1 ) and mepsc frequency (I 2 ) from untransfected and EF1a shrna with MEF2-VP16ERtm and shrna-insensitive EF1a-transfected Fmr1 KO cells (n = 13 for both I 1 and I 2 ). Error bars represent SEM. Representative evoked EPSC and mepsc traces are shown with the dot plots and on the right of each figure, respectively. *p < 0.05, **p < (J) Working model of MEF2-induced synapse elimination in WT neurons and the molecular basis of the deficit in synapse elimination in Fmr1 KO neurons. See also Figure S7 and Table S1. Cell 151, , December 21, 2012 ª2012 Elsevier Inc. 1593
14 Dolezelova, P., Cetkovska, K., Vousden, K.H., and Uldrijan, S. (2012). Mutational analysis of Mdm2 C-terminal tail suggests an evolutionarily conserved role of its length in Mdm2 activity toward p53 and indicates structural differences between Mdm2 homodimers and Mdm2/MdmX heterodimers. Cell Cycle 11, Flavell, S.W., Cowan, C.W., Kim, T.K., Greer, P.L., Lin, Y., Paradis, S., Griffith, E.C., Hu, L.S., Chen, C., and Greenberg, M.E. (2006). Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science 311, Flavell, S.W., Kim, T.K., Gray, J.M., Harmin, D.A., Hemberg, M., Hong, E.J., Markenscoff-Papadimitriou, E., Bear, D.M., and Greenberg, M.E. (2008). Genome-wide analysis of MEF2 transcriptional program reveals synaptic target genes and neuronal activity-dependent polyadenylation site selection. Neuron 60, Frum, R., Busby, S.A., Ramamoorthy, M., Deb, S., Shabanowitz, J., Hunt, D.F., and Deb, S.P. (2007). HDM2-binding partners: interaction with translation elongation factor EF1alpha. J. Proteome Res. 6, Giagtzoglou, N., Ly, C.V., and Bellen, H.J. (2009). Cell adhesion, the backbone of the synapse: vertebrate and invertebrate perspectives. Cold Spring Harb. Perspect. Biol. 1, a Haas, K.F., Miller, S.L., Friedman, D.B., and Broadie, K. (2007). The ubiquitinproteasome system postsynaptically regulates glutamatergic synaptic function. Mol. Cell. Neurosci. 35, Hirano, S., Yan, Q., and Suzuki, S.T. (1999). Expression of a novel protocadherin, OL-protocadherin, in a subset of functional systems of the developing mouse brain. J. Neurosci. 19, Keith, D., and El-Husseini, A. (2008). Excitation Control: Balancing PSD-95 Function at the Synapse. Front. Mol. Neurosci. 1, 4. Kelleher, R.J., III, and Bear, M.F. (2008). The autistic neuron: troubled translation? Cell 135, Kim, Y.C., and DeMartino, G.N. (2011). C termini of proteasomal ATPases play nonequivalent roles in cellular assembly of mammalian 26 S proteasome. J. Biol. Chem. 286, Kim, S.Y., Yasuda, S., Tanaka, H., Yamagata, K., and Kim, H. (2011). Nonclustered protocadherin. Cell Adhes. Migr. 5, McKinsey, T.A., Zhang, C.L., and Olson, E.N. (2002). MEF2: a calciumdependent regulator of cell division, differentiation and death. Trends Biochem. Sci. 27, Morishita, H., and Yagi, T. (2007). Protocadherin family: diversity, structure, and function. Curr. Opin. Cell Biol. 19, Morishita, H., Umitsu, M., Murata, Y., Shibata, N., Udaka, K., Higuchi, Y., Akutsu, H., Yamaguchi, T., Yagi, T., and Ikegami, T. (2006). Structure of the cadherin-related neuronal receptor/protocadherin-alpha first extracellular cadherin domain reveals diversity across cadherin families. J. Biol. Chem. 281, Morrow, E.M., Yoo, S.Y., Flavell, S.W., Kim, T.K., Lin, Y., Hill, R.S., Mukaddes, N.M., Balkhy, S., Gascon, G., Hashmi, A., et al. (2008). Identifying autism loci and genes by tracing recent shared ancestry. Science 321, Napoli, I., Mercaldo, V., Boyl, P.P., Eleuteri, B., Zalfa, F., De Rubeis, S., Di Marino, D., Mohr, E., Massimi, M., Falconi, M., et al. (2008). The fragile X syndrome protein represses activity-dependent translation through CYFIP1, a new 4E-BP. Cell 134, Niere, F., Wilkerson, J.R., and Huber, K.M. (2012). Evidence for a fragile X mental retardation protein-mediated translational switch in metabotropic glutamate receptor-triggered Arc translation and long-term depression. J. Neurosci. 32, Novara, F., Beri, S., Giorda, R., Ortibus, E., Nageshappa, S., Darra, F., Dalla Bernardina, B., Zuffardi, O., and Van Esch, H. (2010). Refining the phenotype associated with MEF2C haploinsufficiency. Clin. Genet. 78, Opazo, P., Sainlos, M., and Choquet, D. (2011). Regulation of AMPA receptor surface diffusion by PSD-95 slots. Curr. Opin. Neurobiol. 22, Pan, F., Aldridge, G.M., Greenough, W.T., and Gan, W.B. (2010). Dendritic spine instability and insensitivity to modulation by sensory experience in a mouse model of fragile X syndrome. Proc. Natl. Acad. Sci. USA 107, Pfeiffer, B.E., Zang, T., Wilkerson, J.R., Taniguchi, M., Maksimova, M.A., Smith, L.N., Cowan, C.W., and Huber, K.M. (2010). Fragile X mental retardation protein is required for synapse elimination by the activity-dependent transcription factor MEF2. Neuron 66, Pulipparacharuvil, S., Renthal, W., Hale, C.F., Taniguchi, M., Xiao, G., Kumar, A., Russo, S.J., Sikder, D., Dewey, C.M., Davis, M.M., et al. (2008). Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron 59, Rezvani, K., Teng, Y., Shim, D., and De Biasi, M. (2007). Nicotine regulates multiple synaptic proteins by inhibiting proteasomal activity. J. Neurosci. 27, Sturgill, J.F., Steiner, P., Czervionke, B.L., and Sabatini, B.L. (2009). Distinct domains within PSD-95 mediate synaptic incorporation, stabilization, and activity-dependent trafficking. J. Neurosci. 29, Sung, Y.J., Dolzhanskaya, N., Nolin, S.L., Brown, T., Currie, J.R., and Denman, R.B. (2003). The fragile X mental retardation protein FMRP binds elongation factor 1A mrna and negatively regulates its translation in vivo. J. Biol. Chem. 278, Tian, X., Kai, L., Hockberger, P.E., Wokosin, D.L., and Surmeier, D.J. (2010). MEF-2 regulates activity-dependent spine loss in striatopallidal medium spiny neurons. Mol. Cell. Neurosci. 44, Uemura, M., Nakao, S., Suzuki, S.T., Takeichi, M., and Hirano, S. (2007). OL- Protocadherin is essential for growth of striatal axons and thalamocortical projections. Nat. Neurosci. 10, Waung, M.W., Pfeiffer, B.E., Nosyreva, E.D., Ronesi, J.A., and Huber, K.M. (2008). Rapid translation of Arc/Arg3.1 selectively mediates mglur-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron 59, Woods, G.F., Oh, W.C., Boudewyn, L.C., Mikula, S.K., and Zito, K. (2011). Loss of PSD-95 enrichment is not a prerequisite for spine retraction. J. Neurosci. 31, Xu, T., Yu, X., Perlik, A.J., Tobin, W.F., Zweig, J.A., Tennant, K., Jones, T., and Zuo, Y. (2009). Rapid formation and selective stabilization of synapses for enduring motor memories. Nature 462, Yang, G., Pan, F., and Gan, W.B. (2009). Stably maintained dendritic spines are associated with lifelong memories. Nature 462, Yasuda, S., Tanaka, H., Sugiura, H., Okamura, K., Sakaguchi, T., Tran, U., Takemiya, T., Mizoguchi, A., Yagita, Y., Sakurai, T., et al. (2007). Activityinduced protocadherin arcadlin regulates dendritic spine number by triggering N-cadherin endocytosis via TAO2beta and p38 MAP kinases. Neuron 56, Zhang, M., Wang, Q., and Huang, Y. (2007). Fragile X mental retardation protein FMRP and the RNA export factor NXF2 associate with and destabilize Nxf1 mrna in neuronal cells. Proc. Natl. Acad. Sci. USA 104, Cell 151, , December 21, 2012 ª2012 Elsevier Inc.
15 Supplemental Information EXTENDED EXPERIMENTAL PROCEDURES Electrophysiology Data Acquisition and Analysis As described (Pfeiffer et al., 2010), recordings were performed at 30 C in a submersion chamber perfused at 3 ml/min with artificial cerebrospinal fluid (ACSF) containing: 119 mm NaCl, 2.5 mm KCl, 26 mm NaHCO 3, 1 mm NaH 2 PO 4, 11 mm D-Glucose, 4 mm CaCl 2, 4 mm MgCl 2, 0.1 mm picrotoxin, mm 2-chloro-adenosine; 0.1% DMSO ph 7.28, 300 mosm and saturated with 95% O 2 /5% CO 2. For mepsc measurements, the ACSF was supplemented with 1 mm TTX. For evoked EPSC and mepsc recordings, neurons were voltage clamped at 60 mv through whole cell recording pipettes (3-7 MU) filled with an intracellular solution containing: 0.2 mm EGTA, 130 mm K-Gluconate, 6 mm KCl, 3 mm NaCl, 10 mm HEPES, 2 mm QX-314, 4 mm ATP-Mg, 0.4 mm GTP-Na, 14 mm phosphocreatine-tris, ph7.2 adjusted by KOH, 285 mosm. Synaptic responses were evoked by single bipolar electrode placed in stratum radiatum of area CA1 (along the Schaffer collaterals) mm from the recorded neurons with monophasic current pulses (1-60 ma, ms). Series and input resistance were measured in voltage clamp with a 400-ms, 10 mv step from a 60 mv holding potential (filtered at 30 khz, sampled at 50 khz). Cells were only used for analysis if they met the following criteria: Series resistance < 25 MU and stable throughout the experiment, resting membrane potential < 35mV; and input resistance > 75 MU ; evoked EPSC > 20pA (for the untransfected cell). Synaptic currents were filtered at 2 khz, acquired and digitized at 10 khz on a PC using custom software (Labview; National Instruments, Austin, TX). mepscs were detected off-line using an automatic detection program (MiniAnalysis; Synaptosoft Inc, Decatur, Ga.) with a detection threshold set at a value greater than at least 2 SD of the noise values, followed by a subsequent round of visual confirmation. The detection threshold remained constant for the duration of each experiment. For evoked EPSCs shown in figures the stimulation artifact has been digitally removed for clarity. Significant differences between transfected and non-transfected neurons were determined using a paired t test. Plasmid Constructions Pcdh10 cdna expression clone was purchased from Origene and Flag- Pcdh10 was purchased from GeneCopoeia. Gene-specific shrnas were purchased from Sigma: Pcdh10 (TRCN and TRCN ), Mdm2 (TRCN and TRCN ), EF1a (TRCN and TRCN ). Control shrnas were from Sigma (Non-target shrna) and GeneCopoeia (CSHCTR001-HIVU6). GST-fused Pcdh10 are made by PCR-amplifying various Pcdh10 carboxyl-terminal regions into pgst-2t vector. Flag-PIR was made by PCR-amplifying PIR region from full-length Pcdh10 into BamHI/EcoRI digested pcdna3.1 vector (Invitrogen) or FUGW vector (Waung et al., 2008). shrna-insensitive Pcdh10, Mdm2 and EF1a were made into pcdna3.1 vector for biolistic transfection in slice cultures and into FUGW for lentiviral transfection in dissociated neuronal cultures. Full-length cdna templates of these genes were purchased from Origene. The shrna targeting sequences and mutated sequences introduced into cdnas are as followed. Pcdh10, targeting: GCCACGGTCAACATCTTGATA, mutated: GCAACCGTAAATATAC TAATC; Mdm2, targeting: CCAATCCAAATGATTGTGCT, mutated: CCTATTCAGATGATCGTATT; EF1a, targeting: GCGTGGTATCA CTATTGAC, mutated: ACGAGGAATTACAATCGAT. GFP/Fmr1 Mosaic Mice GFP/Fmr1 mosaic mice were generated by crossing Fmr1 KO mice with mice that harbor a GFP vector on the X chromosome (Hadjantonakis et al., 1998; Hanson and Madison, 2007). Due to random X-inactivation, cells of heterozygous females are mosaic; that is, GFP (+) cells are wild type and express FMRP and are intermingled with GFP (-) cells that are Fmr1 KO (Figure S1B). Transfection Transfection in HEK293 cells is done by FuGENE reagent (Promega). All neuronal transfections in this study were done by either lentivirus (for dissociated cultures) or biolistic gene gun transfection (for slice cultures). Lentiviral particles were generated by transfecting HEK293 cells with packaging vectors (VSVG, pmdl and RSV-REV) and either lentiviral shrna plasmid or overexpression plasmid by FuGENE transfection reagent (Promega). After 6 hr transfection, the culture medium was replaced with Neurobasal A medium plus B-27 supplement (Invitrogen). Virus-containing media were harvested 60 hr post-transfection and applied onto dissociated cultured neuron directly. Biolistic transfection was performed when slice cultures were at 3 DIV. mcherry was used as transfection control. The procedure and gold bullet preparation were performed with the Helios Gene Gun system (BioRad) according to the manufacturer s protocols (McAllister, 2004). Immunohistochemistry and Image Acquisition Immunohistochemistry was done as previously described (Tsai et al., 2010). In brief, primary neurons grown on poly-d-lysine coated coverslips were fixed at DIV with ice-cold buffer (4% paraformaldehyde and 5% sucrose in PBS) for 15 min followed by permeabilization with 0.5% Triton X-100 in PBS for 5 min. After blocking (1% BSA in PBS) for 30 min, primary antibodies were diluted 1:200 in blocking buffer and applied to the fixed cells overnight at 4 C. After washing three times with PBS, fluorescence-conjugated secondary antibodies were applied to the cells at room temperature for 4 hr. After additionally washing the cells three times with PBS, the coverslips were mounted and observed on Zeiss LSM 510 Confocal Microscope. Image co-localization assay was performed by Cell 151, , December 21, 2012 ª2012 Elsevier Inc. S1
16 ImageJ software (National Institute of Health). Quantification of fluorescence intensity and puncta numbers were done by MetaMorph software (Molecular Devices). The co-clusters obtained from overlapping of PSD-95 and synapsin-1 immunostained signals were obtained and analyzed as previously described (Flavell et al., 2006). Dendritic Spine Imaging Hippocampal slice cultures were prepared as described for electrophysiology and biolistically transfected (at 5-6 DIV) with pa1-gfp that expresses GFP and a myristolated form of GFP from two transcriptional start sites to better fill the dendritic spines (Pfeiffer et al., 2010) together with relevant MEF2 and PIR constructs as indicated in Figure 5F. After 24-30hr, secondary apical dendrites ( mm from the cell body) from transfected CA1 neurons were imaged live using a Zeiss LSM 510 two-photon laser scanning microscope equipped with a Chameleon-Ti: sapphire standard laser at an excitation wavelength of 920 nm using a 63X 0.9 NA waterimmersion objective. Z-stacks were acquired at a z-step of 0.3 mm at 2048x2048 pixel resolution yielding an image with pixel dimensions of 0.07x0.07x0.3 mm. Images were processed using NIH ImageJ (version 1.45 s) and dendritic spine density was quantified using NeuronStudio software (Rodriguez et al., 2008; Rodriguez et al., 2006). 1-3 dendritic regions of interest were analyzed per cell (minimum of 3 independent slice cultures). Image acquisition and analysis were performed by an observer blind to treatment and/or genotype. Pulse-Chase Assay and Metabolic Labeling For pulse-chase assays, MEF2-VP16ERtm lentivirus-transfected cortical neurons were cultured in the media supplemented with 35 S-Met/Cys. After 2hrs, the medium was replaced with fresh culture media containing either vehicle or tamoxifen. Cells were harvested at the time points indicated. For metabolic labeling, MEF2-VP16ERtm lentivirus-transfected cortical neurons were cultured in the media containing 35 S-Met/Cys along with vehicle or tamoxifen for the time points indicated. For both methods, 150 mg of lysates proteins from harvested cells were used for immunoprecipitation. Samples were resolved by 8% SDS-PAGE and exposed to a PhosphorImager (Amersham Biosciences) for quantification. Immunoprecipitation, GST Pull-Down, and Western Blotting For immunoprecipitation, cells were harvested and sonicated in Co-IP buffer (50 mm Tris, ph 7.4, 120 mm NaCl, 0.5% NP-40). 150 mg protein was incubated with antibodies overnight at 4 C and then with protein A/G beads for 1 hr. After washing three times, the samples were subjected to SDS-PAGE analysis followed by western blotting. In the assays, IgG was used as a control antibody. For GST pull-down, GST- Pcdh10 or GST-Ubl proteins were bound to glutathione beads and incubated with total protein extract of mouse brain for 1 hr at 4 C. After washing, the samples were subjected to SDS-PAGE and western blotting analysis. For western blotting after SDS-PAGE, the gel was transferred onto a polyvinylidene fluoride (PVDF) membrane. After blocking with 5% milk in TBS, the membrane was incubated with the appropriate primary antibody for 4 hr at room temperature, followed by a 30 min wash with TBS. The membrane was then incubated with an HRP-conjugated secondary antibody for 1 hr, followed by another 30 min wash. The membrane was then developed with an ECL (enhanced chemiluminescence) solution as described (Waung et al., 2008). Primary antibodies used in this study were anti- Pcdh10 (Sigma), anti-gfp (AVES), anti-map2 (Chemicon), anti-fmrp (Abcam), anti-tubulin (Abcam), anti-vp16 (Santa Cruz Biotechnology), anti-psd-95 (Santa Cruz Biotechnology), anti-synapsin1 (Abcam), anti-flag (Sigma), anti-shank1 (Abcam), anti-homer (Santa Cruz Biotechnology), anti-mglur5 (Millipore), anti-camkiia (Santa Cruz Biotechnology), anti-ub (Santa Cruz Biotechnology), anti-rpt1 (Santa Cruz Biotechnology), anti- Pcdh9 (Santa Cruz Biotechnology), anti-mdm2 (Santa Cruz Biotechnology), anti-glur1 (GluA1) (Millipore), anti-gst (Cell Signaling), anti-ha (Roche), anti-neun (Millipore) and anti-ef1a (Abcam). RNA Immunoprecipitation, RNA Extraction, and Quantitative Real-Time RT-PCR For RNA-IP, 500 mg of total lysates of brain was mixed with anti-fmrp antibody (Abcam) or control IgG (Santa Cruz Biotechnology). After shaking for 1 hr at 4 C, protein A/G beads were added for another hour. After washing, 500 ml of TRIZol (Invitrogen) was added to extract the RNA co-precipitated with FMRP or IgG. RNA was extracted with TRIzol reagent as previously described (Pfeiffer et al., 2010). The reverse transcription reaction was conducted by Omniscript Reverse Transcription Kit (QIAGEN). Briefly, 2 mg of total RNA was used for one RT reaction with random hexamer as starting primer. After obtaining the first strand cdna, real-time PCR was conducted by using Fast SYBR Green Master Mix reagent (Applied Biosystems) in Applied Biosystems 7500 Fast Real-Time PCR System. Gene specific primers used for PCR reactions are as below: Pcdh10, 5 0 -GCTCGCAGGTTTCAGACGGTG-3 0 and 5 0 -CGTAGTCTCGCAAGGAGTTGG-3 0 ; Nurr77, 5 0 -CCGGTGACGTGCAGCAATTTTAT GAC-3 0 and 5 0 -GGCTAGAATGTTGTCTATCCAGTCACC-3 0 ; Gapdh, 5 0 -GGTATTCAAGAGAGTAGGGAG-3 0 and 5 0 -GGGTGCAGC GAACTTTATTG-3 0. Proteasome Purification Proteasome from mouse brain was isolated by using GST-Ubl (Calbiochem) with 250 mg total brain lysate according to manufacturer s protocol. GST alone protein was served as negative control. Proteasome purification from Flag-Rpt6 stably expressed- HEK cells was performed as previously described (Kim and DeMartino, 2011). S2 Cell 151, , December 21, 2012 ª2012 Elsevier Inc.
17 In Vitro Ubiquitination Mdm2 (E3) (Abcam), HA-Ub (Boston Biochem), Ubiquitin Activating Enzyme (UBE1) (Boston Biochem) and UbcH5b/UBE2D2 (Boston Biochem) were purchased as indicated. GST-PSD-95 (Novus Biologicals) was used as substrate for in vitro ubiquitination with a protocol previously described (Woelk et al., 2006). Protein-Protein Interaction with Recombinant Proteins His-Mdm2 (Abcam) was first incubated with Ni-beads (Millipore). After brief washing with PBS, increasing amounts of GST-EF1a (Abcam) (0, 1, 2, 4 and 8 pmol) was added to incubate with His-Mdm2 for 1 hr at 4 C. After three-times washing with PBS, GST- PSD-95 was added to incubate with the complex for 1 hr at 4 C. After three-times washing with PBS, the samples were processed and analyzed by western blotting. In Vitro Synthesis of Pcdh10 35 S-labeled full-length Pcdh10 was made by TNT transcription/translation coupled system (Promega) using T7 promoter-driven Pcdh10 construct as a template. The reaction mixture after pull-down was subjected to SDS-PAGE and analyzed by PhosphorImager. Proteasome Activity Assay Proteasome activity of cultured cortical neurons was measured by the hydrolysis of Suc-Leu-Leu-Val-Tyr-amino methyl coumarin (Proteasome activity assay kit, Millipore) according to manufacturer s protocol. Sequence Alignment The sequence alignment of PIRs from multiple animal models was done by UniProt software ( SUPPLEMENTAL REFERENCES Hadjantonakis, A.K., Gertsenstein, M., Ikawa, M., Okabe, M., and Nagy, A. (1998). Generating green fluorescent mice by germline transmission of green fluorescent ES cells. Mech. Dev. 76, Hanson, J.E., and Madison, D.V. (2007). Presynaptic FMR1 genotype influences the degree of synaptic connectivity in a mosaic mouse model of fragile X syndrome. J. Neurosci. 27, McAllister, A.K. (2004). Biolistic transfection of cultured organotypic brain slices. Methods Mol. Biol. 245, Rodriguez, A., Ehlenberger, D.B., Hof, P.R., and Wearne, S.L. (2006). Rayburst sampling, an algorithm for automated three-dimensional shape analysis from laser scanning microscopy images. Nat. Protoc. 1, Rodriguez, A., Ehlenberger, D.B., Dickstein, D.L., Hof, P.R., and Wearne, S.L. (2008). Automated three-dimensional detection and shape classification of dendritic spines from fluorescence microscopy images. PLoS One 3, e1997. Tsai, N.P., Lin, Y.L., Tsui, Y.C., and Wei, L.N. (2010). Dual action of epidermal growth factor: extracellular signal-stimulated nuclear-cytoplasmic export and coordinated translation of selected messenger RNA. J. Cell Biol. 188, Woelk, T., Oldrini, B., Maspero, E., Confalonieri, S., Cavallaro, E., Di Fiore, P.P., and Polo, S. (2006). Molecular mechanisms of coupled monoubiquitination. Nat. Cell Biol. 8, Cell 151, , December 21, 2012 ª2012 Elsevier Inc. S3
18 Figure S1. Validation of Antibodies and Fmr1 Mosaic Cultures Used in This Study, Related to Figure 1 (A) Full sample western blots with antibodies against critical proteins (Pcdh10, Mdm2, PSD-95, EF1a and FMRP) examined in this study. (B) Immunohistochemistry of GFP, FMRP and NeuN from dissociated hippocampal neuron culture of GFP/Fmr1 mosaic mice. (C) Immunohistochemistry of Pcdh10 and GFP from two Pcdh10 shrnas transfected hippocampal neurons. In this case, a bicistronic GFP was expressed within the Pcdh10 shrna plasmid to identify transfected cells. Scale bars: 10 mm. Quantification of Pcdh10 signal from GFP positive and negative dendrites in a same image (n = 21) is shown on the right. S4 Cell 151, , December 21, 2012 ª2012 Elsevier Inc.
19 (legend continued on next page) Cell 151, , December 21, 2012 ª2012 Elsevier Inc. S5
20 Figure S2. Manipulation of Pcdh10 Levels Alone Is Not Sufficient to Affect Excitatory Synaptic Function or Number, Related to Figure 2 (A) Experimental recording configuration. Organotypic hippocampal slice cultures from p6 7 WT mice were biolistically transfected with plasmids as indicated. Synaptic function was measured using dual simultaneous whole-cell patch clamp recordings of transfected and neighboring untransfected CA1 neurons. (B) Average evoked EPSC amplitude (B 1 ) and mepsc frequency (B 2 ) from untransfected and Pcdh10 transfected cells (n = 27 and 26 for B 1 and B 2 respectively). An example of average mepsc response is shown on top of each bar. (C) Average evoked EPSC amplitude (C 1 ) and mepsc frequency (C 2 ) from untransfected and control shrna transfected cells (n = 21 and 18 for C 1 and C 2 respectively). (D) Average evoked EPSC amplitude (D 1 ) and mepsc frequency (D 2 ) from untransfected and Pcdh10 shrna#1 transfected cells (n = 14 and 19 for D 1 and D 2 respectively). (E) Average evoked EPSC amplitude (E 1 ) and mepsc frequency (E 2 ) from untransfected and Pcdh10 shrna#2 transfected cells (n = 14 and 16 for E 1 and E 2 respectively). (F and G) Immunohistochemistry of synapsin-1 and PSD-95, quantification of co-clusters, and PSD-95 puncta number and size in untransfected cultured hippocampal neurons and neurons transfected with (F) Flag-Pcdh10 or (G) control shrna (left) or Pcdh10 shrna (right) Scale bar: 20 mm. (n = 30 cells from 3 independent cultures). (H) Average evoked EPSC amplitude (H 1 ) and mepsc frequency (H 2 ) from untransfected and Pcdh10 shrna#2 with MEF-2VP16ERtm transfected cells (n = 15 for both evoked EPSC amplitude and mepsc frequency). Representative evoked EPSC and mepsc traces are shown with the dot plots of individual cell pairs and on the right of each figure, respectively. (I) Quantitative real-time RT-PCR of MEF2 target gene, Nurr77, in WT cortical neuron cultures transfected with MEF2-VP16ERtm plus PIR or shrna plasmids indicate that PIR or the shrnas do not affect MEF2-induced transcription. In all figures, error bars represent SEM. S6 Cell 151, , December 21, 2012 ª2012 Elsevier Inc.
21 Figure S3. Validation of MEF2-Induced, Pcdh10-Dependent Degradation of PSD-95 through Proteasome, Related to Figure 3 (A) Western blots of PSD-95 and Tubulin from dissociated WT cortical neurons transfected with MEF2-VP16ERtm and treated with vehicle, tamoxifen and/or the proteasome inhibitor, Lactacystin, for 6 hr demonstrate that an independent proteasome inhibitor blocks MEF2-induced PSD-95 degradation. (B) Western blots of PSD-95, Pcdh10 and Tubulin from dissociated cortical neurons of WT mice transfected with MEF2-VP16ERtm and a second Pcdh10 shrna (Pcdh10 shrna #2) confirm that Pcdh10 is necessary for MEF2-induced PSD-95 degradation. Treatment with vehicle or tamoxifen is as indicated. (C) Western blots of PSD-95, Pcdh10 and Tubulin from dissociated cortical neurons of WT mice transfected with MEF2-VP16ERtm, the first Pcdh10 shrna (Pcdh10 shrna #1) and a shrna-insensitive Pcdh10 construct confirm that the effect of Pcdh10 shrna #1 on MEF2-induced PSD-95 degradation is specific for Pcdh10. In all figures, quantification is shown on the right. (n = 3 cultures) Error bars represent SEM (*p < 0.05, **p < 0.01). Cell 151, , December 21, 2012 ª2012 Elsevier Inc. S7
22 Figure S4. Supporting Data for MEF2-Induced PSD-95 Ubiquitination and Pcdh10-Dependent Proteasomal Degradation of PSD-95, Related to Figure 4 (A) Reproducible results of western blots of endogenous Pcdh10 co-immunoprecipitated with PSD-95 from dissociated WT hippocampal neurons transfected with MEF2-VP16ERtm and treated with either vehicle or tamoxifen for 6 hr. Inputs are shown on the bottom and quantification results are shown on the right. (n =3 cultures, **p < 0.01). (B) Western blotting results corresponding to Figures 4C and 6A (WT cortical cultures) repeated on the same gel. shrna knock-down efficiency is shown on the right. (C) Western blots of Ubiquitin after immunoprecipitation of PSD-95 (top) from WT or Fmr1 KO hippocampi show that PSD-95 is less ubiquitinated in vivo in the Fmr1 KO hippocampus (Similar results have been observed from 4 sets of mice). Inputs are shown on the bottom. (D) PhosphoImager gel scan from in vitro synthesized 35 S-Pcdh10 after attempted pull down by GST-PSD-95 or ubiquitinated-gst-psd-95 demonstrate that PSD-95 does not interact with in vitro translated Pcdh10. GST proteins used are shown on the right after western blotting with anti-psd-95 antibody. (E) Left: Western blots of purified proteasome detecting Flag-Rpt6 from Flag-Rpt6 stably expressing HEK293 cells. Right: Proteasome activity assay of proteasome purified from Flag-Rpt6 stably expressing HEK293 cells by Flag-magnetic beads. S8 Cell 151, , December 21, 2012 ª2012 Elsevier Inc.
23 Figure S5. Additional Information Related to PIR, Related to Figure 5 (A) Alignment of PIR-containing Pcdh10 sequence across species. PIR (highlighted in yellow) demonstrates conservation across species. (B) Immunohistochemistry of Flag-PIR with MAP2 and GFP in WT mouse hippocampal neurons demonstrates that transfected PIR is expressed in dendrites and spines. Scale bars: 20 mm. (C) Proteasome activities from control or Flag-PIR transfected dissociated cortical neurons demonstrate that PIR does not affect general proteasome activity. (D) Average evoked EPSC amplitude (D 1 ) and mepsc frequency (D 2 ) from untransfected and Flag-PIR transfected cells (n = 14 and 16 for D 1 and D 2 respectively). In all figures, error bars represent SEM. Representative evoked EPSC and mepsc traces are shown with the dot plots and on the right of each figure, respectively. Cell 151, , December 21, 2012 ª2012 Elsevier Inc. S9
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1. Normal AMPAR-mediated fepsp input-output curve in CA3-Psen cdko mice. Input-output curves, which are plotted initial slopes of the evoked fepsp as function of the amplitude of the
Supplementary Figure 1. Normal AMPAR-mediated fepsp input-output curve in CA3-Psen cdko mice. Input-output curves, which are plotted initial slopes of the evoked fepsp as function of the amplitude of the
SUPPLEMENTARY FIGURE LEGENDS
 SUPPLEMENTARY FIGURE LEGENDS Supplemental FIG. 1. Localization of myosin Vb in cultured neurons varies with maturation stage. A and B, localization of myosin Vb in cultured hippocampal neurons. A, in DIV
SUPPLEMENTARY FIGURE LEGENDS Supplemental FIG. 1. Localization of myosin Vb in cultured neurons varies with maturation stage. A and B, localization of myosin Vb in cultured hippocampal neurons. A, in DIV
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/6/283/ra57/dc1 Supplementary Materials for JNK3 Couples the Neuronal Stress Response to Inhibition of Secretory Trafficking Guang Yang,* Xun Zhou, Jingyan Zhu,
www.sciencesignaling.org/cgi/content/full/6/283/ra57/dc1 Supplementary Materials for JNK3 Couples the Neuronal Stress Response to Inhibition of Secretory Trafficking Guang Yang,* Xun Zhou, Jingyan Zhu,
293T cells were transfected with indicated expression vectors and the whole-cell extracts were subjected
 SUPPLEMENTARY INFORMATION Supplementary Figure 1. Formation of a complex between Slo1 and CRL4A CRBN E3 ligase. (a) HEK 293T cells were transfected with indicated expression vectors and the whole-cell
SUPPLEMENTARY INFORMATION Supplementary Figure 1. Formation of a complex between Slo1 and CRL4A CRBN E3 ligase. (a) HEK 293T cells were transfected with indicated expression vectors and the whole-cell
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2566 Figure S1 CDKL5 protein expression pattern and localization in mouse brain. (a) Multiple-tissue western blot from a postnatal day (P) 21 mouse probed with an antibody against CDKL5.
DOI: 10.1038/ncb2566 Figure S1 CDKL5 protein expression pattern and localization in mouse brain. (a) Multiple-tissue western blot from a postnatal day (P) 21 mouse probed with an antibody against CDKL5.
Disrupting GluA2-GAPDH Interaction Affects Axon and Dendrite Development
 Disrupting GluA2-GAPDH Interaction Affects Axon and Dendrite Development 1 Frankie Hang Fung Lee, 1 Ping Su, 1 Yu Feng Xie, 1 Kyle Ethan Wang, 2 Qi Wan and 1,3 Fang Liu 1 Campbell Research Institute, Centre
Disrupting GluA2-GAPDH Interaction Affects Axon and Dendrite Development 1 Frankie Hang Fung Lee, 1 Ping Su, 1 Yu Feng Xie, 1 Kyle Ethan Wang, 2 Qi Wan and 1,3 Fang Liu 1 Campbell Research Institute, Centre
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/7/308/ra4/dc1 Supplementary Materials for Antipsychotics Activate mtorc1-dependent Translation to Enhance Neuronal Morphological Complexity Heather Bowling, Guoan
www.sciencesignaling.org/cgi/content/full/7/308/ra4/dc1 Supplementary Materials for Antipsychotics Activate mtorc1-dependent Translation to Enhance Neuronal Morphological Complexity Heather Bowling, Guoan
Schwarz et al. Activity-Dependent Ubiquitination of GluA1 Mediates a Distinct AMPAR Endocytosis
 Schwarz et al Activity-Dependent Ubiquitination of GluA1 Mediates a Distinct AMPAR Endocytosis and Sorting Pathway Supplemental Data Supplemental Fie 1: AMPARs undergo activity-mediated ubiquitination
Schwarz et al Activity-Dependent Ubiquitination of GluA1 Mediates a Distinct AMPAR Endocytosis and Sorting Pathway Supplemental Data Supplemental Fie 1: AMPARs undergo activity-mediated ubiquitination
supplementary information
 DOI: 10.1038/ncb1875 Figure S1 (a) The 79 surgical specimens from NSCLC patients were analysed by immunohistochemistry with an anti-p53 antibody and control serum (data not shown). The normal bronchi served
DOI: 10.1038/ncb1875 Figure S1 (a) The 79 surgical specimens from NSCLC patients were analysed by immunohistochemistry with an anti-p53 antibody and control serum (data not shown). The normal bronchi served
Part 11: Mechanisms of Learning
 Neurophysiology and Information: Theory of Brain Function Christopher Fiorillo BiS 527, Spring 2012 042 350 4326, fiorillo@kaist.ac.kr Part 11: Mechanisms of Learning Reading: Bear, Connors, and Paradiso,
Neurophysiology and Information: Theory of Brain Function Christopher Fiorillo BiS 527, Spring 2012 042 350 4326, fiorillo@kaist.ac.kr Part 11: Mechanisms of Learning Reading: Bear, Connors, and Paradiso,
Supplemental information Acid-sensing ion channel 1a contributes to hippocampal LTP inducibility through multiple mechanisms
 Supplemental information Acid-sensing ion channel 1a contributes to hippocampal LTP inducibility through multiple mechanisms Ming-Gang Liu, Hu-Song Li, Wei-Guang Li, Yan-Jiao Wu, Shi-Ning Deng, Chen Huang,
Supplemental information Acid-sensing ion channel 1a contributes to hippocampal LTP inducibility through multiple mechanisms Ming-Gang Liu, Hu-Song Li, Wei-Guang Li, Yan-Jiao Wu, Shi-Ning Deng, Chen Huang,
Supplementary Information
 Supplementary Information D-Serine regulates cerebellar LTD and motor coordination through the 2 glutamate receptor Wataru Kakegawa, Yurika Miyoshi, Kenji Hamase, Shinji Matsuda, Keiko Matsuda, Kazuhisa
Supplementary Information D-Serine regulates cerebellar LTD and motor coordination through the 2 glutamate receptor Wataru Kakegawa, Yurika Miyoshi, Kenji Hamase, Shinji Matsuda, Keiko Matsuda, Kazuhisa
Zhu et al, page 1. Supplementary Figures
 Zhu et al, page 1 Supplementary Figures Supplementary Figure 1: Visual behavior and avoidance behavioral response in EPM trials. (a) Measures of visual behavior that performed the light avoidance behavior
Zhu et al, page 1 Supplementary Figures Supplementary Figure 1: Visual behavior and avoidance behavioral response in EPM trials. (a) Measures of visual behavior that performed the light avoidance behavior
Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2)
 Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
Reversing the Effects of Fragile X Syndrome
 CLINICAL IMPLICATIONS OF BASIC RESEARCH Paul J. Lombroso, M.D., Marilee P. Ogren, Ph.D. Assistant Editors Reversing the Effects of Fragile X Syndrome MARILEE P. OGREN, PH.D., AND PAUL J. LOMBROSO, M.D.
CLINICAL IMPLICATIONS OF BASIC RESEARCH Paul J. Lombroso, M.D., Marilee P. Ogren, Ph.D. Assistant Editors Reversing the Effects of Fragile X Syndrome MARILEE P. OGREN, PH.D., AND PAUL J. LOMBROSO, M.D.
RAW264.7 cells stably expressing control shrna (Con) or GSK3b-specific shrna (sh-
 1 a b Supplementary Figure 1. Effects of GSK3b knockdown on poly I:C-induced cytokine production. RAW264.7 cells stably expressing control shrna (Con) or GSK3b-specific shrna (sh- GSK3b) were stimulated
1 a b Supplementary Figure 1. Effects of GSK3b knockdown on poly I:C-induced cytokine production. RAW264.7 cells stably expressing control shrna (Con) or GSK3b-specific shrna (sh- GSK3b) were stimulated
Supplemental Materials Molecular Biology of the Cell
 Supplemental Materials Molecular Biology of the Cell Garcia-Alvarez et al. Supplementary Figure Legends Figure S1.Expression and RNAi-mediated silencing of STIM1 in hippocampal neurons (DIV, days in vitro).
Supplemental Materials Molecular Biology of the Cell Garcia-Alvarez et al. Supplementary Figure Legends Figure S1.Expression and RNAi-mediated silencing of STIM1 in hippocampal neurons (DIV, days in vitro).
BIPN 140 Problem Set 6
 BIPN 140 Problem Set 6 1) The hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
BIPN 140 Problem Set 6 1) The hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
BIPN 140 Problem Set 6
 BIPN 140 Problem Set 6 1) Hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
BIPN 140 Problem Set 6 1) Hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
How Nicotinic Signaling Shapes Neural Networks
 How Nicotinic Signaling Shapes Neural Networks Darwin K. Berg Division of Biological Sciences University of California, San Diego Nicotinic Cholinergic Signaling Uses the transmitter ACh to activate cation-selective
How Nicotinic Signaling Shapes Neural Networks Darwin K. Berg Division of Biological Sciences University of California, San Diego Nicotinic Cholinergic Signaling Uses the transmitter ACh to activate cation-selective
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:1.138/nature9814 a A SHARPIN FL B SHARPIN ΔNZF C SHARPIN T38L, F39V b His-SHARPIN FL -1xUb -2xUb -4xUb α-his c Linear 4xUb -SHARPIN FL -SHARPIN TF_LV -SHARPINΔNZF -SHARPIN
SUPPLEMENTARY INFORMATION doi:1.138/nature9814 a A SHARPIN FL B SHARPIN ΔNZF C SHARPIN T38L, F39V b His-SHARPIN FL -1xUb -2xUb -4xUb α-his c Linear 4xUb -SHARPIN FL -SHARPIN TF_LV -SHARPINΔNZF -SHARPIN
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells
 Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
SUPPLEMENTARY INFORMATION
 doi: 1.138/nature6416 Supplementary Notes Spine Ca 2+ signals produced by glutamate uncaging We imaged uncaging-evoked [Ca 2+ ] transients in neurons loaded with a green Ca 2+ - sensitive indicator (G;
doi: 1.138/nature6416 Supplementary Notes Spine Ca 2+ signals produced by glutamate uncaging We imaged uncaging-evoked [Ca 2+ ] transients in neurons loaded with a green Ca 2+ - sensitive indicator (G;
Supplementary Figure 1. mir124 does not change neuron morphology and synaptic
 Supplementary Figure 1. mir124 does not change neuron morphology and synaptic density. Hippocampal neurons were transfected with mir124 (containing DsRed) or DsRed as a control. 2 d after transfection,
Supplementary Figure 1. mir124 does not change neuron morphology and synaptic density. Hippocampal neurons were transfected with mir124 (containing DsRed) or DsRed as a control. 2 d after transfection,
Synaptic plasticityhippocampus. Neur 8790 Topics in Neuroscience: Neuroplasticity. Outline. Synaptic plasticity hypothesis
 Synaptic plasticityhippocampus Neur 8790 Topics in Neuroscience: Neuroplasticity Outline Synaptic plasticity hypothesis Long term potentiation in the hippocampus How it s measured What it looks like Mechanisms
Synaptic plasticityhippocampus Neur 8790 Topics in Neuroscience: Neuroplasticity Outline Synaptic plasticity hypothesis Long term potentiation in the hippocampus How it s measured What it looks like Mechanisms
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk
 Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
Supplementary Materials for VAMP4 directs synaptic vesicles to a pool that selectively maintains asynchronous neurotransmission
 Supplementary Materials for VAMP4 directs synaptic vesicles to a pool that selectively maintains asynchronous neurotransmission Jesica Raingo, Mikhail Khvotchev, Pei Liu, Frederic Darios, Ying C. Li, Denise
Supplementary Materials for VAMP4 directs synaptic vesicles to a pool that selectively maintains asynchronous neurotransmission Jesica Raingo, Mikhail Khvotchev, Pei Liu, Frederic Darios, Ying C. Li, Denise
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2988 Supplementary Figure 1 Kif7 L130P encodes a stable protein that does not localize to cilia tips. (a) Immunoblot with KIF7 antibody in cell lysates of wild-type, Kif7 L130P and Kif7
DOI: 10.1038/ncb2988 Supplementary Figure 1 Kif7 L130P encodes a stable protein that does not localize to cilia tips. (a) Immunoblot with KIF7 antibody in cell lysates of wild-type, Kif7 L130P and Kif7
Supporting Information
 ATP from synaptic terminals and astrocytes regulates NMDA receptors and synaptic plasticity through PSD- 95 multi- protein complex U.Lalo, O.Palygin, A.Verkhratsky, S.G.N. Grant and Y. Pankratov Supporting
ATP from synaptic terminals and astrocytes regulates NMDA receptors and synaptic plasticity through PSD- 95 multi- protein complex U.Lalo, O.Palygin, A.Verkhratsky, S.G.N. Grant and Y. Pankratov Supporting
Authors: K. L. Arendt, M. Royo, M. Fernández-Monreal, S. Knafo, C. N. Petrok, J.
 SUPPLEMENTARY INFORMATION Title: PIP 3 controls synaptic function by maintaining AMPA receptor clustering at the postsynaptic membrane Authors: K. L. Arendt, M. Royo, M. Fernández-Monreal, S. Knafo, C.
SUPPLEMENTARY INFORMATION Title: PIP 3 controls synaptic function by maintaining AMPA receptor clustering at the postsynaptic membrane Authors: K. L. Arendt, M. Royo, M. Fernández-Monreal, S. Knafo, C.
The Nogo Receptor Family Restricts Synapse Number in the Developing Hippocampus
 Article The Nogo Receptor Family Restricts Synapse Number in the Developing Hippocampus Zachary P. Wills,, Caleigh Mandel-Brehm, Alan R. Mardinly, Alejandra E. McCord, Roman J. Giger, 3,4 and Michael E.
Article The Nogo Receptor Family Restricts Synapse Number in the Developing Hippocampus Zachary P. Wills,, Caleigh Mandel-Brehm, Alan R. Mardinly, Alejandra E. McCord, Roman J. Giger, 3,4 and Michael E.
SUPPLEMENTARY INFORMATION. Supplementary Figure 1
 SUPPLEMENTARY INFORMATION Supplementary Figure 1 The supralinear events evoked in CA3 pyramidal cells fulfill the criteria for NMDA spikes, exhibiting a threshold, sensitivity to NMDAR blockade, and all-or-none
SUPPLEMENTARY INFORMATION Supplementary Figure 1 The supralinear events evoked in CA3 pyramidal cells fulfill the criteria for NMDA spikes, exhibiting a threshold, sensitivity to NMDAR blockade, and all-or-none
The clathrin adaptor Numb regulates intestinal cholesterol. absorption through dynamic interaction with NPC1L1
 The clathrin adaptor Numb regulates intestinal cholesterol absorption through dynamic interaction with NPC1L1 Pei-Shan Li 1, Zhen-Yan Fu 1,2, Ying-Yu Zhang 1, Jin-Hui Zhang 1, Chen-Qi Xu 1, Yi-Tong Ma
The clathrin adaptor Numb regulates intestinal cholesterol absorption through dynamic interaction with NPC1L1 Pei-Shan Li 1, Zhen-Yan Fu 1,2, Ying-Yu Zhang 1, Jin-Hui Zhang 1, Chen-Qi Xu 1, Yi-Tong Ma
Myelin suppresses axon regeneration by PIR-B/SHPmediated inhibition of Trk activity
 Manuscript EMBO-2010-76298 Myelin suppresses axon regeneration by PIR-B/SHPmediated inhibition of Trk activity Yuki Fujita, Shota Endo, Toshiyuki Takai and Toshihide Yamashita Corresponding author: Toshihide
Manuscript EMBO-2010-76298 Myelin suppresses axon regeneration by PIR-B/SHPmediated inhibition of Trk activity Yuki Fujita, Shota Endo, Toshiyuki Takai and Toshihide Yamashita Corresponding author: Toshihide
Psychiatric Risk Factor ANK3/Ankyrin-G Nanodomains Regulate the Structure and Function of Glutamatergic Synapses
 Neuron Article Psychiatric Risk Factor ANK3/Ankyrin-G Nanodomains Regulate the Structure and Function of Glutamatergic Synapses Katharine R. Smith, 1 Katherine J. Kopeikina, 1 Jessica M. Fawcett-Patel,
Neuron Article Psychiatric Risk Factor ANK3/Ankyrin-G Nanodomains Regulate the Structure and Function of Glutamatergic Synapses Katharine R. Smith, 1 Katherine J. Kopeikina, 1 Jessica M. Fawcett-Patel,
Nature Structural and Molecular Biology: doi: /nsmb Supplementary Figure 1
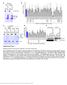 Supplementary Figure 1 Mutational analysis of the SA2-Scc1 interaction in vitro and in human cells. (a) Autoradiograph (top) and Coomassie stained gel (bottom) of 35 S-labeled Myc-SA2 proteins (input)
Supplementary Figure 1 Mutational analysis of the SA2-Scc1 interaction in vitro and in human cells. (a) Autoradiograph (top) and Coomassie stained gel (bottom) of 35 S-labeled Myc-SA2 proteins (input)
Supplementary Figure 1. SybII and Ceb are sorted to distinct vesicle populations in astrocytes. Nature Neuroscience: doi: /nn.
 Supplementary Figure 1 SybII and Ceb are sorted to distinct vesicle populations in astrocytes. (a) Exemplary images for cultured astrocytes co-immunolabeled with SybII and Ceb antibodies. SybII accumulates
Supplementary Figure 1 SybII and Ceb are sorted to distinct vesicle populations in astrocytes. (a) Exemplary images for cultured astrocytes co-immunolabeled with SybII and Ceb antibodies. SybII accumulates
pt305-camkii stabilizes a learning-induced increase in AMPA receptors for ongoing memory consolidation after classical conditioning
 Received 6 Nov 213 Accepted 25 Apr 214 Published 3 May 214 pt35- stabilizes a learning-induced increase in AMPA receptors for ongoing memory consolidation after classical conditioning Souvik Naskar 1,,
Received 6 Nov 213 Accepted 25 Apr 214 Published 3 May 214 pt35- stabilizes a learning-induced increase in AMPA receptors for ongoing memory consolidation after classical conditioning Souvik Naskar 1,,
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1. Behavioural effects of ketamine in non-stressed and stressed mice. Naive C57BL/6 adult male mice (n=10/group) were given a single dose of saline vehicle or ketamine (3.0 mg/kg,
Supplementary Figure 1. Behavioural effects of ketamine in non-stressed and stressed mice. Naive C57BL/6 adult male mice (n=10/group) were given a single dose of saline vehicle or ketamine (3.0 mg/kg,
TGF-β Signaling Regulates Neuronal C1q Expression and Developmental Synaptic Refinement
 Supplementary Information Title: TGF-β Signaling Regulates Neuronal C1q Expression and Developmental Synaptic Refinement Authors: Allison R. Bialas and Beth Stevens Supplemental Figure 1. In vitro characterization
Supplementary Information Title: TGF-β Signaling Regulates Neuronal C1q Expression and Developmental Synaptic Refinement Authors: Allison R. Bialas and Beth Stevens Supplemental Figure 1. In vitro characterization
S1a S1b S1c. S1d. S1f S1g S1h SUPPLEMENTARY FIGURE 1. - si sc Il17rd Il17ra bp. rig/s IL-17RD (ng) -100 IL-17RD
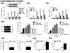 SUPPLEMENTARY FIGURE 1 0 20 50 80 100 IL-17RD (ng) S1a S1b S1c IL-17RD β-actin kda S1d - si sc Il17rd Il17ra rig/s15-574 - 458-361 bp S1f S1g S1h S1i S1j Supplementary Figure 1. Knockdown of IL-17RD enhances
SUPPLEMENTARY FIGURE 1 0 20 50 80 100 IL-17RD (ng) S1a S1b S1c IL-17RD β-actin kda S1d - si sc Il17rd Il17ra rig/s15-574 - 458-361 bp S1f S1g S1h S1i S1j Supplementary Figure 1. Knockdown of IL-17RD enhances
CONTEXT. LTP (long term potentiation) definition. LTP as a interesting mechanism for learning and memory
 CONTEXT LTP (long term potentiation) definition LTP as a interesting mechanism for learning and memory LTP is due primarily to a pre or post- synaptic modification? (Increased Glut release or increased
CONTEXT LTP (long term potentiation) definition LTP as a interesting mechanism for learning and memory LTP is due primarily to a pre or post- synaptic modification? (Increased Glut release or increased
Structural basis for the role of inhibition in facilitating adult brain plasticity
 Structural basis for the role of inhibition in facilitating adult brain plasticity Jerry L. Chen, Walter C. Lin, Jae Won Cha, Peter T. So, Yoshiyuki Kubota & Elly Nedivi SUPPLEMENTARY FIGURES 1-6 a b M
Structural basis for the role of inhibition in facilitating adult brain plasticity Jerry L. Chen, Walter C. Lin, Jae Won Cha, Peter T. So, Yoshiyuki Kubota & Elly Nedivi SUPPLEMENTARY FIGURES 1-6 a b M
Chapter 9: Biochemical Mechanisms for Information Storage at the Cellular Level. From Mechanisms of Memory, second edition By J. David Sweatt, Ph.D.
 Chapter 9: Biochemical Mechanisms for Information Storage at the Cellular Level From Mechanisms of Memory, second edition By J. David Sweatt, Ph.D. Chapter 9: Dendritic Spine Figure 1 Summary: Three Primary
Chapter 9: Biochemical Mechanisms for Information Storage at the Cellular Level From Mechanisms of Memory, second edition By J. David Sweatt, Ph.D. Chapter 9: Dendritic Spine Figure 1 Summary: Three Primary
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS)
 Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
RNA interference induced hepatotoxicity results from loss of the first synthesized isoform of microrna-122 in mice
 SUPPLEMENTARY INFORMATION RNA interference induced hepatotoxicity results from loss of the first synthesized isoform of microrna-122 in mice Paul N Valdmanis, Shuo Gu, Kirk Chu, Lan Jin, Feijie Zhang,
SUPPLEMENTARY INFORMATION RNA interference induced hepatotoxicity results from loss of the first synthesized isoform of microrna-122 in mice Paul N Valdmanis, Shuo Gu, Kirk Chu, Lan Jin, Feijie Zhang,
Ube3a is required for experience-dependent maturation of the neocortex
 Ube3a is required for experience-dependent maturation of the neocortex Koji Yashiro, Thorfinn T. Riday, Kathryn H. Condon, Adam C. Roberts, Danilo R. Bernardo, Rohit Prakash, Richard J. Weinberg, Michael
Ube3a is required for experience-dependent maturation of the neocortex Koji Yashiro, Thorfinn T. Riday, Kathryn H. Condon, Adam C. Roberts, Danilo R. Bernardo, Rohit Prakash, Richard J. Weinberg, Michael
Supplementary Figure 1. PD-L1 is glycosylated in cancer cells. (a) Western blot analysis of PD-L1 in breast cancer cells. (b) Western blot analysis
 Supplementary Figure 1. PD-L1 is glycosylated in cancer cells. (a) Western blot analysis of PD-L1 in breast cancer cells. (b) Western blot analysis of PD-L1 in ovarian cancer cells. (c) Western blot analysis
Supplementary Figure 1. PD-L1 is glycosylated in cancer cells. (a) Western blot analysis of PD-L1 in breast cancer cells. (b) Western blot analysis of PD-L1 in ovarian cancer cells. (c) Western blot analysis
Supplementary Figure 1
 Supplementary Figure 1 Supplementary Figure 1 Schematic depiction of the tandem Fc GDF15. Supplementary Figure 2 Supplementary Figure 2 Gfral mrna levels in the brains of both wild-type and knockout Gfral
Supplementary Figure 1 Supplementary Figure 1 Schematic depiction of the tandem Fc GDF15. Supplementary Figure 2 Supplementary Figure 2 Gfral mrna levels in the brains of both wild-type and knockout Gfral
Supplementary Figure 1. Spatial distribution of LRP5 and β-catenin in intact cardiomyocytes. (a) and (b) Immunofluorescence staining of endogenous
 Supplementary Figure 1. Spatial distribution of LRP5 and β-catenin in intact cardiomyocytes. (a) and (b) Immunofluorescence staining of endogenous LRP5 in intact adult mouse ventricular myocytes (AMVMs)
Supplementary Figure 1. Spatial distribution of LRP5 and β-catenin in intact cardiomyocytes. (a) and (b) Immunofluorescence staining of endogenous LRP5 in intact adult mouse ventricular myocytes (AMVMs)
Supplementary Figures
 Supplementary Figures Supplementary Figure 1 Characterization of stable expression of GlucB and sshbira in the CT26 cell line (a) Live cell imaging of stable CT26 cells expressing green fluorescent protein
Supplementary Figures Supplementary Figure 1 Characterization of stable expression of GlucB and sshbira in the CT26 cell line (a) Live cell imaging of stable CT26 cells expressing green fluorescent protein
SUPPLEMENTARY INFORMATION
 Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Lrig1 is a cell-intrinsic modulator of hippocampal dendrite complexity and BDNF signaling
 Article Lrig1 is a cell-intrinsic modulator of hippocampal dendrite complexity and BDNF signaling Fernando Cruz Alsina 1,, Francisco Javier Hita 1,, Paula Aldana Fontanet 1, Dolores Irala 1, Håkan Hedman
Article Lrig1 is a cell-intrinsic modulator of hippocampal dendrite complexity and BDNF signaling Fernando Cruz Alsina 1,, Francisco Javier Hita 1,, Paula Aldana Fontanet 1, Dolores Irala 1, Håkan Hedman
NNZ-2566 in Rett Syndrome and Autism Spectrum Disorders Role and Update
 NNZ-2566 in Rett Syndrome and Autism Spectrum Disorders Role and Update 1 Overview The natural growth factor IGF-1 is broken down in the body to IGF-1[1-3] NNZ-2566 is an analogue of IGF-1[1-3] developed
NNZ-2566 in Rett Syndrome and Autism Spectrum Disorders Role and Update 1 Overview The natural growth factor IGF-1 is broken down in the body to IGF-1[1-3] NNZ-2566 is an analogue of IGF-1[1-3] developed
Ionotropic glutamate receptors (iglurs)
 Ionotropic glutamate receptors (iglurs) GluA1 GluA2 GluA3 GluA4 GluN1 GluN2A GluN2B GluN2C GluN2D GluN3A GluN3B GluK1 GluK2 GluK3 GluK4 GluK5 The general architecture of receptor subunits Unique properties
Ionotropic glutamate receptors (iglurs) GluA1 GluA2 GluA3 GluA4 GluN1 GluN2A GluN2B GluN2C GluN2D GluN3A GluN3B GluK1 GluK2 GluK3 GluK4 GluK5 The general architecture of receptor subunits Unique properties
Supplementary Fig. 1. GPRC5A post-transcriptionally down-regulates EGFR expression. (a) Plot of the changes in steady state mrna levels versus
 Supplementary Fig. 1. GPRC5A post-transcriptionally down-regulates EGFR expression. (a) Plot of the changes in steady state mrna levels versus changes in corresponding proteins between wild type and Gprc5a-/-
Supplementary Fig. 1. GPRC5A post-transcriptionally down-regulates EGFR expression. (a) Plot of the changes in steady state mrna levels versus changes in corresponding proteins between wild type and Gprc5a-/-
Supplemental Information. Menin Deficiency Leads to Depressive-like. Behaviors in Mice by Modulating. Astrocyte-Mediated Neuroinflammation
 Neuron, Volume 100 Supplemental Information Menin Deficiency Leads to Depressive-like Behaviors in Mice by Modulating Astrocyte-Mediated Neuroinflammation Lige Leng, Kai Zhuang, Zeyue Liu, Changquan Huang,
Neuron, Volume 100 Supplemental Information Menin Deficiency Leads to Depressive-like Behaviors in Mice by Modulating Astrocyte-Mediated Neuroinflammation Lige Leng, Kai Zhuang, Zeyue Liu, Changquan Huang,
When cells are already maximally potentiated LTP is occluded.
 When cells are already maximally potentiated LTP is occluded. Stein, V et al., (2003) J Neurosci, 23:5503-6606. Also found in Rat Barrel Cortex Ehrlich & Malinow (2004) J. Neurosci. 24:916-927 Over-expression
When cells are already maximally potentiated LTP is occluded. Stein, V et al., (2003) J Neurosci, 23:5503-6606. Also found in Rat Barrel Cortex Ehrlich & Malinow (2004) J. Neurosci. 24:916-927 Over-expression
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11095 Supplementary Table 1. Summary of the binding between Angptls and various Igdomain containing receptors as determined by flow cytometry analysis. The results were summarized from
doi:10.1038/nature11095 Supplementary Table 1. Summary of the binding between Angptls and various Igdomain containing receptors as determined by flow cytometry analysis. The results were summarized from
SUPPLEMENTARY FIGURES
 SUPPLEMENTARY FIGURES 1 Supplementary Figure 1, Adult hippocampal QNPs and TAPs uniformly express REST a-b) Confocal images of adult hippocampal mouse sections showing GFAP (green), Sox2 (red), and REST
SUPPLEMENTARY FIGURES 1 Supplementary Figure 1, Adult hippocampal QNPs and TAPs uniformly express REST a-b) Confocal images of adult hippocampal mouse sections showing GFAP (green), Sox2 (red), and REST
crossmark Ca V subunits interact with the voltage-gated calcium channel
 crossmark THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 291, NO. 39, pp. 20402 20416, September 23, 2016 Author s Choice 2016 by The American Society for Biochemistry and Molecular Biology, Inc. Published in
crossmark THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 291, NO. 39, pp. 20402 20416, September 23, 2016 Author s Choice 2016 by The American Society for Biochemistry and Molecular Biology, Inc. Published in
Supplementary Figure 1. Microglia do not show signs of classical immune activation following MD a-b. Images showing immunoreactivity for MHCII (a)
 1 Supplementary Figure 1. Microglia do not show signs of classical immune activation following MD a-b. Images showing immunoreactivity for MHCII (a) and CD45 (b) in fixed sections of binocular visual cortex
1 Supplementary Figure 1. Microglia do not show signs of classical immune activation following MD a-b. Images showing immunoreactivity for MHCII (a) and CD45 (b) in fixed sections of binocular visual cortex
Social deficits in Shank3-deficient mouse models of autism are rescued by histone deacetylase (HDAC) inhibition
 SUPPLEMENTARY INFORMATION Articles https://doi.org/10.1038/s41593-018-0110-8 In the format provided by the authors and unedited. Social deficits in Shank3-deficient mouse models of autism are rescued by
SUPPLEMENTARY INFORMATION Articles https://doi.org/10.1038/s41593-018-0110-8 In the format provided by the authors and unedited. Social deficits in Shank3-deficient mouse models of autism are rescued by
Lecture 7: Roles for MAGUKS in Activity-dependent Synaptogenesis MCP
 Lecture 7: Roles for MAGUKS in Activity-dependent Synaptogenesis MCP 9.013 04 Po st -S yn ap (P ti SD c ) De ns it y PSD site en face.25 µm From: Kennedy (2000) Science MEMBRANE ASSOCIATED GUANYLATE KINASES
Lecture 7: Roles for MAGUKS in Activity-dependent Synaptogenesis MCP 9.013 04 Po st -S yn ap (P ti SD c ) De ns it y PSD site en face.25 µm From: Kennedy (2000) Science MEMBRANE ASSOCIATED GUANYLATE KINASES
SUPPLEMENTAL FIGURE LEGENDS
 SUPPLEMENTAL FIGURE LEGENDS Supplemental Figure S1: Endogenous interaction between RNF2 and H2AX: Whole cell extracts from 293T were subjected to immunoprecipitation with anti-rnf2 or anti-γ-h2ax antibodies
SUPPLEMENTAL FIGURE LEGENDS Supplemental Figure S1: Endogenous interaction between RNF2 and H2AX: Whole cell extracts from 293T were subjected to immunoprecipitation with anti-rnf2 or anti-γ-h2ax antibodies
BISP194: MOLECULAR MECHANISM OF SYNAPTIC PLASTICITY Spring Quarter
 BISP194: MOLECULAR MECHANISM OF SYNAPTIC PLASTICITY Spring Quarter 2011 Instructor: Class Website: Gentry N. Patrick (gpatrick@ucsd.edu) http://www.biology.ucsd.edu/classes/bisp194.sp11 Class Meetings:
BISP194: MOLECULAR MECHANISM OF SYNAPTIC PLASTICITY Spring Quarter 2011 Instructor: Class Website: Gentry N. Patrick (gpatrick@ucsd.edu) http://www.biology.ucsd.edu/classes/bisp194.sp11 Class Meetings:
BIPN140 Lecture 12: Synaptic Plasticity (II)
 BIPN140 Lecture 12: Synaptic Plasticity (II) 1. Early v.s. Late LTP 2. Long-Term Depression 3. Molecular Mechanisms of Long-Term Depression: NMDA-R dependent 4. Molecular Mechanisms of Long-Term Depression:
BIPN140 Lecture 12: Synaptic Plasticity (II) 1. Early v.s. Late LTP 2. Long-Term Depression 3. Molecular Mechanisms of Long-Term Depression: NMDA-R dependent 4. Molecular Mechanisms of Long-Term Depression:
supplementary information
 DOI: 10.1038/ncb2153 Figure S1 Ectopic expression of HAUSP up-regulates REST protein. (a) Immunoblotting showed that ectopic expression of HAUSP increased REST protein levels in ENStemA NPCs. (b) Immunofluorescent
DOI: 10.1038/ncb2153 Figure S1 Ectopic expression of HAUSP up-regulates REST protein. (a) Immunoblotting showed that ectopic expression of HAUSP increased REST protein levels in ENStemA NPCs. (b) Immunofluorescent
Nature Structural & Molecular Biology: doi: /nsmb Supplementary Figure 1. Generation and validation of mtef4-knockout mice.
 Supplementary Figure 1 Generation and validation of mtef4-knockout mice. (a) Alignment of EF4 (E. coli) with mouse, yeast and human EF4. (b) Domain structures of mouse mtef4 compared to those of EF4 (E.
Supplementary Figure 1 Generation and validation of mtef4-knockout mice. (a) Alignment of EF4 (E. coli) with mouse, yeast and human EF4. (b) Domain structures of mouse mtef4 compared to those of EF4 (E.
Silencing neurotransmission with membrane-tethered toxins
 nature methods Silencing neurotransmission with membrane-tethered toxins Sebastian Auer, Annika S Stürzebecher, René Jüttner, Julio Santos-Torres, Christina Hanack, Silke Frahm, Beate Liehl & Inés Ibañez-Tallon
nature methods Silencing neurotransmission with membrane-tethered toxins Sebastian Auer, Annika S Stürzebecher, René Jüttner, Julio Santos-Torres, Christina Hanack, Silke Frahm, Beate Liehl & Inés Ibañez-Tallon
Type of file: PDF Title of file for HTML: Supplementary Information Description: Supplementary Figures
 Type of file: PDF Title of file for HTML: Supplementary Information Description: Supplementary Figures Type of file: MOV Title of file for HTML: Supplementary Movie 1 Description: NLRP3 is moving along
Type of file: PDF Title of file for HTML: Supplementary Information Description: Supplementary Figures Type of file: MOV Title of file for HTML: Supplementary Movie 1 Description: NLRP3 is moving along
Supplementary Materials
 Supplementary Materials Supplementary Figure S1 Regulation of Ubl4A stability by its assembly partner A, The translation rate of Ubl4A is not affected in the absence of Bag6. Control, Bag6 and Ubl4A CRISPR
Supplementary Materials Supplementary Figure S1 Regulation of Ubl4A stability by its assembly partner A, The translation rate of Ubl4A is not affected in the absence of Bag6. Control, Bag6 and Ubl4A CRISPR
SUPPLEMENTARY INFORMATION. Supplementary Figures S1-S9. Supplementary Methods
 SUPPLEMENTARY INFORMATION SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane Jian Huang 1,2#, Jie Yan 1,2#, Jian Zhang 3#, Shiguo Zhu 1, Yanli Wang
SUPPLEMENTARY INFORMATION SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane Jian Huang 1,2#, Jie Yan 1,2#, Jian Zhang 3#, Shiguo Zhu 1, Yanli Wang
supplementary information
 DOI: 1.138/ncb1 Control Atg7 / NAC 1 1 1 1 (mm) Control Atg7 / NAC 1 1 1 1 (mm) Lamin B Gstm1 Figure S1 Neither the translocation of into the nucleus nor the induction of antioxidant proteins in autophagydeficient
DOI: 1.138/ncb1 Control Atg7 / NAC 1 1 1 1 (mm) Control Atg7 / NAC 1 1 1 1 (mm) Lamin B Gstm1 Figure S1 Neither the translocation of into the nucleus nor the induction of antioxidant proteins in autophagydeficient
Synaptic Plasticity and the NMDA Receptor
 Synaptic Plasticity and the NMDA Receptor Lecture 4.2 David S. Touretzky November, 2015 Long Term Synaptic Plasticity Long Term Potentiation (LTP) Reversal of LTP Long Term Depression (LTD) Reversal of
Synaptic Plasticity and the NMDA Receptor Lecture 4.2 David S. Touretzky November, 2015 Long Term Synaptic Plasticity Long Term Potentiation (LTP) Reversal of LTP Long Term Depression (LTD) Reversal of
NMDAR-dependent Argonaute 2 phosphorylation regulates mirna activity and dendritic spine plasticity
 Article NMDAR-dependent Argonaute 2 phosphorylation regulates mirna activity and dendritic spine plasticity Dipen Rajgor 1, Thomas M Sanderson 2, Mascia Amici 2, Graham L Collingridge 2,3,4 & Jonathan
Article NMDAR-dependent Argonaute 2 phosphorylation regulates mirna activity and dendritic spine plasticity Dipen Rajgor 1, Thomas M Sanderson 2, Mascia Amici 2, Graham L Collingridge 2,3,4 & Jonathan
Supplemental Information. NRF2 Is a Major Target of ARF. in p53-independent Tumor Suppression
 Molecular Cell, Volume 68 Supplemental Information NRF2 Is a Major Target of ARF in p53-independent Tumor Suppression Delin Chen, Omid Tavana, Bo Chu, Luke Erber, Yue Chen, Richard Baer, and Wei Gu Figure
Molecular Cell, Volume 68 Supplemental Information NRF2 Is a Major Target of ARF in p53-independent Tumor Suppression Delin Chen, Omid Tavana, Bo Chu, Luke Erber, Yue Chen, Richard Baer, and Wei Gu Figure
PKCλ Is Critical in AMPA Receptor Phosphorylation and Synaptic Incorporation during LTP
 Manuscript EMBO-2012-82900 PKCλ Is Critical in AMPA Receptor Phosphorylation and Synaptic Incorporation during LTP Si-Qiang Ren, Jing-Zhi Yan, Xiao-Yan Zhang, Yun-Fei Bu, Wei-Wei Pan, Wen Yao, Tian Tian
Manuscript EMBO-2012-82900 PKCλ Is Critical in AMPA Receptor Phosphorylation and Synaptic Incorporation during LTP Si-Qiang Ren, Jing-Zhi Yan, Xiao-Yan Zhang, Yun-Fei Bu, Wei-Wei Pan, Wen Yao, Tian Tian
Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory
 (2011) 16, 156 170 & 2011 Macmillan Publishers Limited All rights reserved 1359-4184/11 www.nature.com/mp ORIGINAL ARTICLE Mechanisms for acute stress-induced enhancement of glutamatergic transmission
(2011) 16, 156 170 & 2011 Macmillan Publishers Limited All rights reserved 1359-4184/11 www.nature.com/mp ORIGINAL ARTICLE Mechanisms for acute stress-induced enhancement of glutamatergic transmission
Increased Threshold for Spike-Timing-Dependent Plasticity Is Caused by Unreliable Calcium Signaling in Mice Lacking Fragile X Gene Fmr1
 Article Increased Threshold for Spike-Timing-Dependent Plasticity Is Caused by Unreliable Calcium Signaling in Mice Lacking Fragile X Gene Fmr1 Rhiannon M. Meredith, 1,3 Carl D. Holmgren, 1,3 Meredith
Article Increased Threshold for Spike-Timing-Dependent Plasticity Is Caused by Unreliable Calcium Signaling in Mice Lacking Fragile X Gene Fmr1 Rhiannon M. Meredith, 1,3 Carl D. Holmgren, 1,3 Meredith
Contribution of mglur5 to hippocampal pathophysiology in a mouse model of human chromosome 16p11.2 microdeletion
 Contribution of mglur5 to hippocampal pathophysiology in a mouse model of human chromosome 16p11.2 microdeletion The Harvard community has made this article openly available. Please share how this access
Contribution of mglur5 to hippocampal pathophysiology in a mouse model of human chromosome 16p11.2 microdeletion The Harvard community has made this article openly available. Please share how this access
Supplemental Information. Differential Regulation. of Evoked and Spontaneous Release. by Presynaptic NMDA Receptors
 Neuron, Volume 96 Supplemental Information Differential Regulation of Evoked and Spontaneous Release by Presynaptic NMDA Receptors Therése Abrahamsson, hristina You hien hou, Si Ying Li, Adamo Mancino,
Neuron, Volume 96 Supplemental Information Differential Regulation of Evoked and Spontaneous Release by Presynaptic NMDA Receptors Therése Abrahamsson, hristina You hien hou, Si Ying Li, Adamo Mancino,
Reviewers' comments: Reviewer #1 (Remarks to the Author):
 Reviewers' comments: Reviewer #1 (Remarks to the Author): In this manuscript, Song et al. identified FBXW7 as a new positive regulator for RIG-Itriggered type I IFN signaling pathway. The authors observed
Reviewers' comments: Reviewer #1 (Remarks to the Author): In this manuscript, Song et al. identified FBXW7 as a new positive regulator for RIG-Itriggered type I IFN signaling pathway. The authors observed
Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the
 Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the location of the transmembrane (TM), FRM binding (FB)
Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the location of the transmembrane (TM), FRM binding (FB)
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11700 Figure 1: RIP3 as a potential Sirt2 interacting protein. Transfected Flag-tagged Sirt2 was immunoprecipitated from cells and eluted from the Sepharose beads using Flag peptide.
doi:10.1038/nature11700 Figure 1: RIP3 as a potential Sirt2 interacting protein. Transfected Flag-tagged Sirt2 was immunoprecipitated from cells and eluted from the Sepharose beads using Flag peptide.
Astrocyte signaling controls spike timing-dependent depression at neocortical synapses
 Supplementary Information Astrocyte signaling controls spike timing-dependent depression at neocortical synapses Rogier Min and Thomas Nevian Department of Physiology, University of Berne, Bern, Switzerland
Supplementary Information Astrocyte signaling controls spike timing-dependent depression at neocortical synapses Rogier Min and Thomas Nevian Department of Physiology, University of Berne, Bern, Switzerland
Supplementary Figure S1 Supplementary Figure S2
 Supplementary Figure S A) The blots shown in Figure B were qualified by using Gel-Pro analyzer software (Rockville, MD, USA). The ratio of LC3II/LC3I to actin was then calculated. The data are represented
Supplementary Figure S A) The blots shown in Figure B were qualified by using Gel-Pro analyzer software (Rockville, MD, USA). The ratio of LC3II/LC3I to actin was then calculated. The data are represented
Neuronal Differentiation: CNS Synapse Formation
 Neuronal Differentiation: CNS Synapse Formation Comparison of the NMJ and Central Synapses Sanes and Lichtman 2001 Comparison of the NMJ and Central Synapses (1) Each neuron in the CNS is innervated by
Neuronal Differentiation: CNS Synapse Formation Comparison of the NMJ and Central Synapses Sanes and Lichtman 2001 Comparison of the NMJ and Central Synapses (1) Each neuron in the CNS is innervated by
Nature Neuroscience: doi: /nn Supplementary Figure 1. Diverse anorexigenic signals induce c-fos expression in CEl PKC-δ + neurons
 Supplementary Figure 1 Diverse anorexigenic signals induce c-fos expression in CEl PKC-δ + neurons a-c. Quantification of CEl c-fos expression in mice intraperitoneal injected with anorexigenic drugs (a),
Supplementary Figure 1 Diverse anorexigenic signals induce c-fos expression in CEl PKC-δ + neurons a-c. Quantification of CEl c-fos expression in mice intraperitoneal injected with anorexigenic drugs (a),
File name: Supplementary Information Description: Supplementary Figures, Supplementary Table and Supplementary References
 File name: Supplementary Information Description: Supplementary Figures, Supplementary Table and Supplementary References File name: Supplementary Data 1 Description: Summary datasheets showing the spatial
File name: Supplementary Information Description: Supplementary Figures, Supplementary Table and Supplementary References File name: Supplementary Data 1 Description: Summary datasheets showing the spatial
SUPPLEMENTARY INFORMATION
 Supplementary Table 1. Cell sphingolipids and S1P bound to endogenous TRAF2. Sphingolipid Cell pmol/mg TRAF2 immunoprecipitate pmol/mg Sphingomyelin 4200 ± 250 Not detected Monohexosylceramide 311 ± 18
Supplementary Table 1. Cell sphingolipids and S1P bound to endogenous TRAF2. Sphingolipid Cell pmol/mg TRAF2 immunoprecipitate pmol/mg Sphingomyelin 4200 ± 250 Not detected Monohexosylceramide 311 ± 18
Supplementary Information
 Supplementary Information mediates STAT3 activation at retromer-positive structures to promote colitis and colitis-associated carcinogenesis Zhang et al. a b d e g h Rel. Luc. Act. Rel. mrna Rel. mrna
Supplementary Information mediates STAT3 activation at retromer-positive structures to promote colitis and colitis-associated carcinogenesis Zhang et al. a b d e g h Rel. Luc. Act. Rel. mrna Rel. mrna
Comparison of open chromatin regions between dentate granule cells and other tissues and neural cell types.
 Supplementary Figure 1 Comparison of open chromatin regions between dentate granule cells and other tissues and neural cell types. (a) Pearson correlation heatmap among open chromatin profiles of different
Supplementary Figure 1 Comparison of open chromatin regions between dentate granule cells and other tissues and neural cell types. (a) Pearson correlation heatmap among open chromatin profiles of different
Nature Neuroscience: doi: /nn Supplementary Figure 1
 Supplementary Figure 1 Subcellular segregation of VGluT2-IR and TH-IR within the same VGluT2-TH axon (wild type rats). (a-e) Serial sections of a dual VGluT2-TH labeled axon. This axon (blue outline) has
Supplementary Figure 1 Subcellular segregation of VGluT2-IR and TH-IR within the same VGluT2-TH axon (wild type rats). (a-e) Serial sections of a dual VGluT2-TH labeled axon. This axon (blue outline) has
hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This
 SUPPLEMENTAL FIGURE LEGEND Fig. S1. Generation and characterization of. (A) Coomassie staining of soluble hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This protein was expressed
SUPPLEMENTAL FIGURE LEGEND Fig. S1. Generation and characterization of. (A) Coomassie staining of soluble hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This protein was expressed
Palmitoylation regulates glutamate receptor distributions in postsynaptic densities through control of PSD95 conformation and orientation
 Palmitoylation regulates glutamate receptor distributions in postsynaptic densities through control of PSD95 conformation and orientation Okunola Jeyifous a,b,1, Eric I. Lin a,b,1, Xiaobing Chen b,c, Sarah
Palmitoylation regulates glutamate receptor distributions in postsynaptic densities through control of PSD95 conformation and orientation Okunola Jeyifous a,b,1, Eric I. Lin a,b,1, Xiaobing Chen b,c, Sarah
(a) Significant biological processes (upper panel) and disease biomarkers (lower panel)
 Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Nature Methods: doi: /nmeth.4257
 Supplementary Figure 1 Screen for polypeptides that affect cellular actin filaments. (a) Table summarizing results from all polypeptides tested. Source shows organism, gene, and amino acid numbers used.
Supplementary Figure 1 Screen for polypeptides that affect cellular actin filaments. (a) Table summarizing results from all polypeptides tested. Source shows organism, gene, and amino acid numbers used.
SUPPLEMENTARY FIGURES
 SUPPLEMENTARY FIGURES Figure S1. Clinical significance of ZNF322A overexpression in Caucasian lung cancer patients. (A) Representative immunohistochemistry images of ZNF322A protein expression in tissue
SUPPLEMENTARY FIGURES Figure S1. Clinical significance of ZNF322A overexpression in Caucasian lung cancer patients. (A) Representative immunohistochemistry images of ZNF322A protein expression in tissue
