Elements for a public summary. Overview of disease epidemiology
|
|
|
- Asher Gibson
- 5 years ago
- Views:
Transcription
1 VI.2 VI.2.1 Elements for a public summary Overview of disease epidemiology Glaucoma is an eye disease that can result in damage to the optic nerve and loss of vision (blindness). It is the major cause of blindness world-wide, affecting 2% of individuals of European descent (8). Glaucoma accounts for 20.5 % of causes of blindness in Europe. 25 million European persons are affected by glaucoma. It has been estimated that 21.8 % of European adults (including 18% of those over 50 years of age) have been diagnosed with glaucoma. According to recent epidemiological studies, Germany (14 %) shows the highest prevalence of glaucoma in Europe followed by the European North of Russia (11.9 %). The lowest prevalence of any type of glaucoma has been registered in France (3.4 %) and the UK (3.3 %). A Spanish epidemiological study showed that primary type of glaucoma an open-angle glaucoma (2.1 %) was more prevalent in men (2.4 %) than in women (1.7 %) (16). Strong and consistent evidence regarding the risk of developing glaucoma was found for elevated intraocular pressure (IOP, defined as pressure created by the continual renewal of fluids within the eye), advancing age, non-caucasian ethnicity, family history of glaucoma and changes in the structure of the cornea (greater cup-to-disk ratio and thinner central cornea). There is moderate evidence of an association between glaucoma and migraine, eye injury, Nearsightedness and long-term use of corticosteroids, respectively. There is conflicting evidence for high blood pressure, diabetes and smoking (8, 9, 17, 21). VI.2.2 Summary of treatment benefits Glaucoma cannot be cured and damage caused by the disease cannot be reversed (6). However, adequate treatment can protect subjects at high risk of the disease or patients with early signs of glaucoma from severe visual impairment and blindness. The assessment that elevated intraocular pressure (IOP) is a major risk factor for glaucoma development is corroborated by controlled clinical trials in which substantial benefit of IOP-lowering treatment for patients suspected to have glaucoma was reported before initial damage was seen (4, 12, 17). Three studies have been performed which assessed the IOP lowering efficacy of travoprost. In these studies, patients with open-angle glaucoma (OAG) or ocular hypertension who took travoprost 40 microgram/ml experienced 7-8 mmhg (millimetres of mercury, a measurement of pressure) decreases in IOP. The results have been consistent in all three monotherapy pivotal trials. Travoprost 0.004% dosed once daily produced both clinically relevant and statistically significant IOP reductions when used as a monotherapy. The IOP reductions were maintained over the entire 6 to 12 month treatment period (7). VI.2.3 Unknowns relating to treatment benefits The safety and efficacy in inflammatory ocular conditions, in neovascular, angle-closure, narrow-angle or congenital glaucoma have not been studied. The safety and efficacy in paediatric patients in the age group 2 months to 3 years are limited and in chidren in the age < 2 months has not yet been established.
2 VI.2.4 Summary of safety concerns Important identified risks Risk What is known Preventability Build-up of fluid in the area of the retina that is responsible for sharp vision (Macular oedema) Darkening of eye colour or of skin around the eye (Hyperpigmentation) Excessive growth of hair (Hypertrichosis) Build-up of fluid in the area of the retina that is responsible for sharp vision has been reported in patients using travoprost. Iris darkening (frequency 1/10) and skin darkening around the eyes (frequency >1/100 to <1/10) have been reported with travoprost. They do not pose a known threat to vision or health. Skin changes seem to be reversible after discontinuation of the medicine. However, iris darkening is often irreversible. Excessive growth of hair is considered as a non-serious and mild effect associated with the use of prostaglandin analogues. travoprost in patients who have undergone cataract surgery or other ocular surgery as well as patients with other risk factors for macular oedema, such as ocular (eye) inflammations, diabetes or hypertension (high blood pressure). If travoprost is used in such patients, patients should check their vision frequently and promptly report any change. In case of macular oedema, the medicine should not be used again, to prevent recurrence. The risk of iris darkening appears to depend on eye colour before treatment. Patients with nonhomogenously blue, grey or hazel irises show greater changes. Caution should be exercised when treating glaucoma only in one eye with prostaglandin analogues (class of medicines to which travoprost belongs). Termination of prostaglandin analogue treatment may reverse this effect but conclusive evidence has not been obtained. Patients who have abnormally positioned eyelashes that grow back toward the eye should be monitored for this complication.
3 Risk What is known Preventability Inflammation of certain parts of the eye (Iris and uveal inflammations) Risk for the heart and blood vessels (Cardiac and vascular disorders) Risks for the airways (Respiratory disorders) Allergy (Hypersensitivity) Symptomatic iritis (inflammation of the iris) appears to be an uncommon adverse event associated with all prostaglandin analogues. Its course is generally mild and the inflammation resolves upon discontinuation of the medicine with or without antiinflammatory therapy.. Cardiovascular disorders such as angina pectoris (pains to the chest, jaw and back), bradycardia (slow heart rate), arrhythmia (abnormal heart rhythm), chest pain, hypertension and hypotension (high or low blood pressure) have been reported in association with travoprost administration although they are considered very uncommon. Respiratory disorders such as dyspnoea (difficulty breathing), asthma and worsening of asthma have been associated with the use of prostaglandin analogues. These and other respiratory symptoms have been reported with the use of travoprost. Allergy induced by topical glaucoma treatment is primarily seen in the conjunctiva and around the eye. Serious allergic reactions to travoprost are rare. Yes, by using travoprost with caution in patients with a history of iritis, or with risk factors for iritis. Reinitiating therapy after an episode of iritis may not be advisable. travoprost in patients with preexisting cardiovascular disorders. travoprost in patients with preexisting respiratory disorders. travoprost in patients with hypersensitivity to travoprost or to any of the excipients, or with a tendency to develop allergies and asthma. Also by monitoring for early symptoms.
4 Important potential risks Risk Pigmented skin cancer (Melanoma) Corneal damage due to use of preserved eye drops Use during pregnancy and lactation What is known (including reason why it is considered a potential risk) Prostaglandin analogues are well known to cause pigmentary (colour) changes in iris, eyelashes and skin around the eye. The mechanism by which they increase pigment synthesis is uncertain. Four cases have been reported in the literature with members of the same pharmaceutical class: one eyelid melanoma associated with bimatoprost (another type of prostaglandin analogue) and one choroidal melanoma and two cutaneous melanomas associated with latanoprost (another type of prostaglandin analogue). However, a direct link between prostaglandin analogue use and development of melanoma has never been documented. The product is indicated to decrease elevated intraocular pressure in patients with ocular hypertension or open-angle glaucoma. This is a long-term condition where patients are usually exposed to topical medications for life. The presence of a preservative increases the risk of adverse effects on the corneal surface (cell loss and tear film disruption) and the possibility of hypersensitivity reactions. The damage depends on the agent, the posology and the length of treatment. Clinical trials involving travaprost 40 microgram/ml with a duration of up to 5 years as well as postmarketing experience with travaprost 40 microgram/ml have not confirmed an increased frequency of corneal events. Therefore, this is considered only as a potential risk for Travoprost 40 µg/ml eye drops, solution. Animal studies with travoprost have shown reproductive toxicity. Pregnant women, women of childbearing potential and breastfeeding women were excluded from participation in clinical trials. Travoprost 40 µg/ml eye drops, solution should not be used during pregnancy, breastfeeding, or in women of childbearing potential unless they are using adequate contraceptive methods. Missing information Risk Use in paediatric population aged < 2 months Potential interactions What is known Travoprost can be used in paediatric patients from 2 months to < 18 years at the same posology as in adults. However, data in the age group 2 months to < 3 years is limited. The safety and efficacy of travoprost in children below the age of 2 months have not been established. No data are available. No specific pharmacokinetic drug-drug interactions are known for travoprost. Interaction studies have not been performed for Travoprost 40 µg/ml eye drops, solution.
5 VI.2.5 Summary of additional risk minimisation measures by safety concern All medicines have a Summary of Product Characteristics (SmPC) which provides physicians, pharmacists and other health care professionals with details on how to use the medicine, the risks and recommendations for minimising them. An abbreviated version of this in lay language is provided in the form of the package leaflet (PIL). The measures in these documents are known as routine risk minimisation measures. VI.2.6 Planned post authorisation development plan NA VI.2.7 Summary of changes to the risk management plan over time NA
6
Summary of the risk management plan (RMP) for Izba (travoprost)
 EMA/14138/2014 Summary of the risk management plan (RMP) for Izba (travoprost) This is a summary of the risk management plan (RMP) for Izba, which details the measures to be taken in order to ensure that
EMA/14138/2014 Summary of the risk management plan (RMP) for Izba (travoprost) This is a summary of the risk management plan (RMP) for Izba, which details the measures to be taken in order to ensure that
Elements for a public summary. VI.2.1 Overview of disease epidemiology
 VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Studies estimated that 3-6 million people in the United States alone, including 4-10% of the population older than 40 years, have
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Studies estimated that 3-6 million people in the United States alone, including 4-10% of the population older than 40 years, have
[TRAVOPROST] 40 MICROGRAMS/ML, EYE DROPS, SOLUTION RISK MANAGEMENT PLAN. TRAVOPR-v
![[TRAVOPROST] 40 MICROGRAMS/ML, EYE DROPS, SOLUTION RISK MANAGEMENT PLAN. TRAVOPR-v [TRAVOPROST] 40 MICROGRAMS/ML, EYE DROPS, SOLUTION RISK MANAGEMENT PLAN. TRAVOPR-v](/thumbs/94/119684929.jpg) [TRAVOPROST] 40 MICROGRAMS/ML, EYE DROPS, SOLUTION RISK MANAGEMENT PLAN TRAVOPR-v2-270214 VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Studies estimated that 3-6 million people
[TRAVOPROST] 40 MICROGRAMS/ML, EYE DROPS, SOLUTION RISK MANAGEMENT PLAN TRAVOPR-v2-270214 VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Studies estimated that 3-6 million people
Elements for a Public Summary. Overview of disease epidemiology
 VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Epidemiology of the disease Incidence and prevalence Increased pressure in the eye occurs in more than 100 million people, and
VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Epidemiology of the disease Incidence and prevalence Increased pressure in the eye occurs in more than 100 million people, and
The treatment benefit of latanoprost has not been studied in the following populations/patients:
 6.1. ELEMENTS FOR A PUBLIC SUMMARY 6.1.1. Overview of Disease Epidemiology Glaucoma is the second leading cause of blindness worldwide, and affects about 66.8 million people worldwide i,ii. Glaucoma can
6.1. ELEMENTS FOR A PUBLIC SUMMARY 6.1.1. Overview of Disease Epidemiology Glaucoma is the second leading cause of blindness worldwide, and affects about 66.8 million people worldwide i,ii. Glaucoma can
Summary of the risk management plan (RMP) for Omidria (phenylephrine / ketorolac)
 EMA/368070/2015 Summary of the risk management plan (RMP) for Omidria (phenylephrine / ketorolac) This is a summary of the risk management plan (RMP) for Omidria, which details the measures to be taken
EMA/368070/2015 Summary of the risk management plan (RMP) for Omidria (phenylephrine / ketorolac) This is a summary of the risk management plan (RMP) for Omidria, which details the measures to be taken
Brimonidine/timolol Version 1.2, 16Oct, Elements for a public summary. VI.2.1 Overview of disease epidemiology
 VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology The estimated number of people with vision loss from glaucoma range from 5.2 to 6.7 million. This is approximately 10% of the
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology The estimated number of people with vision loss from glaucoma range from 5.2 to 6.7 million. This is approximately 10% of the
Elements for a public summary. VI.2.1 Overview of disease epidemiology
 VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology The term ocular hypertension usually refers to any situation in which the pressure inside the eye, called intraocular pressure,
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology The term ocular hypertension usually refers to any situation in which the pressure inside the eye, called intraocular pressure,
LOWPROST Eye Drops (Bimatoprost Ophthalmic Solution 0.01%)
 Published on: 14 Apr 2017 LOWPROST Eye Drops (Bimatoprost Ophthalmic Solution 0.01%) For the use of a Registered Medical Practitioner or a Hospital or a Laboratory only Composition Each ml contains: Bimatoprost...
Published on: 14 Apr 2017 LOWPROST Eye Drops (Bimatoprost Ophthalmic Solution 0.01%) For the use of a Registered Medical Practitioner or a Hospital or a Laboratory only Composition Each ml contains: Bimatoprost...
VI.2.2 Summary of treatment benefits
 EU-Risk Management Plan for Bimatoprost V01 aetiology), both OAG and ACG can be secondary conditions. Secondary glaucoma refers to any case in which another disorder (e.g. injury, inflammation, vascular
EU-Risk Management Plan for Bimatoprost V01 aetiology), both OAG and ACG can be secondary conditions. Secondary glaucoma refers to any case in which another disorder (e.g. injury, inflammation, vascular
Package leaflet: Information for the user. Xalatan 50 micrograms/ml Eye drops, solution Latanoprost
 Package leaflet: Information for the user Xalatan 50 micrograms/ml Eye drops, solution Latanoprost Read all of this leaflet carefully before you start using this medicine because it contains important
Package leaflet: Information for the user Xalatan 50 micrograms/ml Eye drops, solution Latanoprost Read all of this leaflet carefully before you start using this medicine because it contains important
Summary of safety concerns Important identified risks
 Valley VI.2 Elements for a public summary NL/H/3661/001-002/DC - Ivabradin Medical VI.2.1 Overview of disease epidemiology Ivabradine is a medicine used for two long-term (chronic) heart conditions: to
Valley VI.2 Elements for a public summary NL/H/3661/001-002/DC - Ivabradin Medical VI.2.1 Overview of disease epidemiology Ivabradine is a medicine used for two long-term (chronic) heart conditions: to
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT LUMIGAN 0.1 mg/ml eye drops, solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One ml of solution contains 0.1 mg bimatoprost.
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT LUMIGAN 0.1 mg/ml eye drops, solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One ml of solution contains 0.1 mg bimatoprost.
Annex I SCIENTIFIC CONCLUSIONS AND GROUNDS FOR THE VARIATION TO THE TERMS OF THE MARKETING AUTHORISATION(S)
 Annex I SCIENTIFIC CONCLUSIONS AND GROUNDS FOR THE VARIATION TO THE TERMS OF THE MARKETING AUTHORISATION(S) Scientific conclusions Taking into account the PRAC Assessment Report on the PSUR for bimatoprost,
Annex I SCIENTIFIC CONCLUSIONS AND GROUNDS FOR THE VARIATION TO THE TERMS OF THE MARKETING AUTHORISATION(S) Scientific conclusions Taking into account the PRAC Assessment Report on the PSUR for bimatoprost,
APPROVED PACKAGE INSERT FOR XALATAN EYE DROPS. Each millilitre contains latanoprost 50 µg and benzalkonium chloride 0,02 % m/v as preservative.
 SCHEDULING STATUS: S4 APPROVED PACKAGE INSERT FOR XALATAN EYE DROPS PROPRIETARY NAME (and dosage form): XALATAN Eye Drops COMPOSITION: Each millilitre contains latanoprost 50 µg and benzalkonium chloride
SCHEDULING STATUS: S4 APPROVED PACKAGE INSERT FOR XALATAN EYE DROPS PROPRIETARY NAME (and dosage form): XALATAN Eye Drops COMPOSITION: Each millilitre contains latanoprost 50 µg and benzalkonium chloride
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology VI.2.2 Summary of treatment benefits
 VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Pulmonary arterial hypertension (a high blood pressure in the blood vessels in the lungs) is a rare disease. Historically, the
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Pulmonary arterial hypertension (a high blood pressure in the blood vessels in the lungs) is a rare disease. Historically, the
Package leaflet: Information for the user. Latanoprost Pfizer 50 micrograms/ml Eye drops, solution Latanoprost
 Package leaflet: Information for the user Latanoprost Pfizer 50 micrograms/ml Eye drops, solution Latanoprost Read all of this leaflet carefully before you start using this medicine because it contains
Package leaflet: Information for the user Latanoprost Pfizer 50 micrograms/ml Eye drops, solution Latanoprost Read all of this leaflet carefully before you start using this medicine because it contains
7.2 Part VI.2 Elements for a Public Summary
 7.2 Part VI.2 Elements for a Public Summary 7.2.1 Part VI.2.1 Overview of disease epidemiology Chronic obstructive pulmonary disease (COPD) is characterized by persistent airflow limitation that is usually
7.2 Part VI.2 Elements for a Public Summary 7.2.1 Part VI.2.1 Overview of disease epidemiology Chronic obstructive pulmonary disease (COPD) is characterized by persistent airflow limitation that is usually
PART VI Summary of the RMP
 PART VI Summary of the RMP Summary of Risk Management Plan for ORKAMBI This is a summary of the risk management plan (RMP) for ORKAMBI. The RMP details important risks of ORKAMBI, how these risks can be
PART VI Summary of the RMP Summary of Risk Management Plan for ORKAMBI This is a summary of the risk management plan (RMP) for ORKAMBI. The RMP details important risks of ORKAMBI, how these risks can be
Summary of the risk management plan (RMP) for Ivabradine Anpharm (ivabradine)
 EMA/518024/2015 Summary of the risk management plan (RMP) for Ivabradine Anpharm (ivabradine) This is a summary of the risk management plan (RMP) for Ivabradine Anpharm, which details the measures to be
EMA/518024/2015 Summary of the risk management plan (RMP) for Ivabradine Anpharm (ivabradine) This is a summary of the risk management plan (RMP) for Ivabradine Anpharm, which details the measures to be
Risk Management Plan
 Management Plan Active substance: VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Hypertension is generally defined as blood pressure higher than 140/90 mmhg. Hypertension may
Management Plan Active substance: VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Hypertension is generally defined as blood pressure higher than 140/90 mmhg. Hypertension may
THE CHRONIC GLAUCOMAS
 THE CHRONIC GLAUCOMAS WHAT IS GLAUCOMA? People with glaucoma have lost some of their field of all round vision. It is often the edge or periphery that is lost. That is why the condition can be missed until
THE CHRONIC GLAUCOMAS WHAT IS GLAUCOMA? People with glaucoma have lost some of their field of all round vision. It is often the edge or periphery that is lost. That is why the condition can be missed until
Summary of the risk management plan (RMP) for Duaklir Genuair (aclidinium / formoterol fumarate dihydrate)
 EMA/605453/2014 Summary of the risk management plan (RMP) for Duaklir Genuair (aclidinium / formoterol fumarate dihydrate) This is a summary of the risk management plan (RMP) for Duaklir Genuair, which
EMA/605453/2014 Summary of the risk management plan (RMP) for Duaklir Genuair (aclidinium / formoterol fumarate dihydrate) This is a summary of the risk management plan (RMP) for Duaklir Genuair, which
East and North Hertfordshire treatment pathway for primary open angle glaucoma and ocular hypertension in adults
 East and North Hertfordshire treatment pathway for primary open angle glaucoma and ocular hypertension in adults Introduction Glaucoma is a group of eye diseases causing optic nerve damage. In most cases
East and North Hertfordshire treatment pathway for primary open angle glaucoma and ocular hypertension in adults Introduction Glaucoma is a group of eye diseases causing optic nerve damage. In most cases
SUMMARY OF PRODUCT CHARACTERISTICS
 Page 1/9 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One ml of solution contains 0.3 mg bimatoprost. One drop contains approximately
Page 1/9 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One ml of solution contains 0.3 mg bimatoprost. One drop contains approximately
The safety concerns defined with the reference product apply to Buprenorphine-Naloxone 2/0.5 mg, 8/2 mg, sublingual tablets.
 These products are indicated in the substitution treatment for opioid drug dependence, within a framework of medical, social and psychological treatment. The intention of the naloxone component is to deter
These products are indicated in the substitution treatment for opioid drug dependence, within a framework of medical, social and psychological treatment. The intention of the naloxone component is to deter
, version 1.1 PUBLIC SUMMARY OF THE RISK MANAGEMENT PLAN
 Docetaxel Orion 20 mg/1 ml concentrate for solution for infusion Docetaxel Orion 80 mg/4 ml concentrate for solution for infusion Docetaxel Orion 160 mg/8 ml concentrate for solution for infusion 11.8.2014,
Docetaxel Orion 20 mg/1 ml concentrate for solution for infusion Docetaxel Orion 80 mg/4 ml concentrate for solution for infusion Docetaxel Orion 160 mg/8 ml concentrate for solution for infusion 11.8.2014,
Package leaflet: Information for the patient. Latanoprost 50 micrograms/ml eye drops, solution latanoprost
 PACKAGE LEAFLET 18 Package leaflet: Information for the patient Latanoprost 50 micrograms/ml eye drops, solution latanoprost Read all of this leaflet carefully before you start using this medicine because
PACKAGE LEAFLET 18 Package leaflet: Information for the patient Latanoprost 50 micrograms/ml eye drops, solution latanoprost Read all of this leaflet carefully before you start using this medicine because
PATIENT INFORMATION LEAFLET
 Page 1 of 6 PATIENT INFORMATION LEAFLET SCHEDULING STATUS Schedule 4 Proprietary name, Strength and Pharmaceutical Form LUMIGAN 0,03 % Eye Drops (each ml contains bimatoprost 0,3 mg) Read all of this leaflet
Page 1 of 6 PATIENT INFORMATION LEAFLET SCHEDULING STATUS Schedule 4 Proprietary name, Strength and Pharmaceutical Form LUMIGAN 0,03 % Eye Drops (each ml contains bimatoprost 0,3 mg) Read all of this leaflet
NEW ZEALAND DATA SHEET. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains 0.1 mg or 0.3 mg of bimatoprost.
 NEW ZEALAND DATA SHEET 1. PRODUCT NAME BIMATOPROST ACTAVIS, Eye drops, solution, 0.1 mg/ml and 0.3 mg/ml 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains 0.1 mg or 0.3 mg of bimatoprost. Excipient
NEW ZEALAND DATA SHEET 1. PRODUCT NAME BIMATOPROST ACTAVIS, Eye drops, solution, 0.1 mg/ml and 0.3 mg/ml 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains 0.1 mg or 0.3 mg of bimatoprost. Excipient
THE CHRONIC GLAUCOMAS
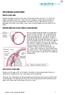 THE CHRONIC GLAUCOMAS WHAT IS GLAUCOMA People with glaucoma have lost some of their field of all round vision. It is often the edge or periphery that is lost. That is why the condition can be missed until
THE CHRONIC GLAUCOMAS WHAT IS GLAUCOMA People with glaucoma have lost some of their field of all round vision. It is often the edge or periphery that is lost. That is why the condition can be missed until
Elements for a Public Summary
 VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Benign prostatic hyperplasia (BPH), also called benign prostatic hypertrophy or benign prostatic obstruction, is a condition in
VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Benign prostatic hyperplasia (BPH), also called benign prostatic hypertrophy or benign prostatic obstruction, is a condition in
DATA SHEET. Clinical studies have shown mean intraocular pressure decreases of up to 9 mmhg.
 DATA SHEET NAME OF MEDICINE LUMIGAN (bimatoprost) 0.3 mg/ml eye drops Presentation LUMIGAN (bimatoprost) eye drops are a clear, isotonic, colourless, sterile ophthalmic solution. Bimatoprost is a white
DATA SHEET NAME OF MEDICINE LUMIGAN (bimatoprost) 0.3 mg/ml eye drops Presentation LUMIGAN (bimatoprost) eye drops are a clear, isotonic, colourless, sterile ophthalmic solution. Bimatoprost is a white
Vildagliptin belongs to a group of medicines called oral antidiabetics.
 VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Vildagliptin belongs to a group of medicines called oral antidiabetics. Vildagliptin is used in a certain form of diabetes (type
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Vildagliptin belongs to a group of medicines called oral antidiabetics. Vildagliptin is used in a certain form of diabetes (type
PATIENT INFORMATION LEAFLET. PROPRIETARY NAME, STRENGTH AND PHARMACEUTICAL FORM LUMIGAN 0,01 %, bimatoprost 0,1 mg/ml eye drops
 Page 1 of 6 SCHEDULING STATUS Schedule 4 PATIENT INFORMATION LEAFLET PROPRIETARY NAME, STRENGTH AND PHARMACEUTICAL FORM LUMIGAN 0,01 %, bimatoprost 0,1 mg/ml eye drops Read all of this leaflet carefully
Page 1 of 6 SCHEDULING STATUS Schedule 4 PATIENT INFORMATION LEAFLET PROPRIETARY NAME, STRENGTH AND PHARMACEUTICAL FORM LUMIGAN 0,01 %, bimatoprost 0,1 mg/ml eye drops Read all of this leaflet carefully
RISK MANAGEMENT PLAN (RMP) PUBLIC SUMMARY ETORICOXIB ORION (ETORICOXIB) 30 MG, 60 MG, 90 MG & 120 MG FILM-COATED TABLET DATE: , VERSION 1.
 RISK MANAGEMENT PLAN (RMP) PUBLIC SUMMARY ETORICOXIB ORION (ETORICOXIB) 30 MG, 60 MG, 90 MG & 120 MG FILM-COATED TABLET DATE: 07-10-2016, VERSION 1.2 VI.2 Elements for a Public Summary Etoricoxib Orion
RISK MANAGEMENT PLAN (RMP) PUBLIC SUMMARY ETORICOXIB ORION (ETORICOXIB) 30 MG, 60 MG, 90 MG & 120 MG FILM-COATED TABLET DATE: 07-10-2016, VERSION 1.2 VI.2 Elements for a Public Summary Etoricoxib Orion
Elements for a Public Summary
 VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Alzheimer's disease is the most common form of dementia, a general term for memory loss and other intellectual abilities serious
VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Alzheimer's disease is the most common form of dementia, a general term for memory loss and other intellectual abilities serious
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Contents METHODS... 2 Inclusion and exclusion criteria... 2 Supplementary table S1... 2 Assessment of abnormal ocular signs and symptoms... 3 Supplementary table S2... 3 Ocular
SUPPLEMENTARY INFORMATION Contents METHODS... 2 Inclusion and exclusion criteria... 2 Supplementary table S1... 2 Assessment of abnormal ocular signs and symptoms... 3 Supplementary table S2... 3 Ocular
Elements for a Public Summary Overview of disease epidemiology
 VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Acute pain usually responds to medication and should settle in less than three months. Inadequate pain relief may lead to other
VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Acute pain usually responds to medication and should settle in less than three months. Inadequate pain relief may lead to other
Package Leaflet: Information for the user. LUMIGAN 0.3 mg/ml, eye drops, solution, in single-dose container Bimatoprost
 Package Leaflet: Information for the user LUMIGAN 0.3 mg/ml, eye drops, solution, in single-dose container Bimatoprost Read all of this leaflet carefully before you start using this medicine because it
Package Leaflet: Information for the user LUMIGAN 0.3 mg/ml, eye drops, solution, in single-dose container Bimatoprost Read all of this leaflet carefully before you start using this medicine because it
Elements for a Public Summary. Overview of disease epidemiology. Cardiovascular Events
 VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Cardiovascular Events Each year cardiovascular disease (CVD) causes 3.9 million deaths in Europe and over 1.8 million deaths in
VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Cardiovascular Events Each year cardiovascular disease (CVD) causes 3.9 million deaths in Europe and over 1.8 million deaths in
Package leaflet: information for the user. OZURDEX 700 micrograms intravitreal implant in applicator dexamethasone
 Package leaflet: information for the user OZURDEX 700 micrograms intravitreal implant in applicator dexamethasone Read all of this leaflet carefully before you are given this medicine because it contains
Package leaflet: information for the user OZURDEX 700 micrograms intravitreal implant in applicator dexamethasone Read all of this leaflet carefully before you are given this medicine because it contains
Summary of the risk management plan (RMP) for Kengrexal (cangrelor)
 EMA/78859/2015 Summary of the risk management plan (RMP) for Kengrexal (cangrelor) This is a summary of the risk management plan (RMP) for Kengrexal, which details the measures to be taken in order to
EMA/78859/2015 Summary of the risk management plan (RMP) for Kengrexal (cangrelor) This is a summary of the risk management plan (RMP) for Kengrexal, which details the measures to be taken in order to
Elements for a public summary
 VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Nevirapine is used for antiretroviral combination therapy of Human Immunodeficiency Virus (HIV) infection. Human immunodeficiency
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Nevirapine is used for antiretroviral combination therapy of Human Immunodeficiency Virus (HIV) infection. Human immunodeficiency
NAPHCON-A Eye Drops naphazoline hydrochloride 0.025% and pheniramine maleate 0.3%.
 NEW ZEALAND DATA SHEET 1. PRODUCT NAME NAPHCON-A Eye Drops naphazoline hydrochloride 0.025% and pheniramine maleate 0.3%. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Naphcon-A contains naphazoline hydrochloride
NEW ZEALAND DATA SHEET 1. PRODUCT NAME NAPHCON-A Eye Drops naphazoline hydrochloride 0.025% and pheniramine maleate 0.3%. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Naphcon-A contains naphazoline hydrochloride
Summary of the risk management plan (RMP) for Opdivo (nivolumab)
 EMA/285771/2015 Summary of the risk management plan (RMP) for Opdivo (nivolumab) This is a summary of the risk management plan (RMP) for Opdivo, which details the measures to be taken in order to ensure
EMA/285771/2015 Summary of the risk management plan (RMP) for Opdivo (nivolumab) This is a summary of the risk management plan (RMP) for Opdivo, which details the measures to be taken in order to ensure
KEY MESSAGES. Details of the evidence supporting these recommendations can be found in the above CPG, available on the following websites:
 QUICK REFERENCE FOR HEALTHCARE PROVIDERS KEY MESSAGES 1. Glaucoma is a chronic eye disease that damages the optic nerve, & can result in serious vision loss and irreversible blindness. 2. Glaucoma diagnosis
QUICK REFERENCE FOR HEALTHCARE PROVIDERS KEY MESSAGES 1. Glaucoma is a chronic eye disease that damages the optic nerve, & can result in serious vision loss and irreversible blindness. 2. Glaucoma diagnosis
SUMMARY OF PRODUCT CHARACTERISTICS
 1. NAME OF THE MEDICINAL PRODUCT SUMMARY OF PRODUCT CHARACTERISTICS Latanoprost Mylan 50 microgram/ml eye drops solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of solution contains 50 micrograms
1. NAME OF THE MEDICINAL PRODUCT SUMMARY OF PRODUCT CHARACTERISTICS Latanoprost Mylan 50 microgram/ml eye drops solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of solution contains 50 micrograms
Elements for a public summary
 VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology High blood pressure, also known as hypertension, occurs in a large percentage of the adult population. Primary (essential) hypertension
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology High blood pressure, also known as hypertension, occurs in a large percentage of the adult population. Primary (essential) hypertension
YOUR VYZULTA TREATMENT GUIDE. Please see Important Safety Information on pages 1, 9, 10, 17 and 18. Please see accompanying Prescribing Information.
 YOUR VYZULTA TREATMENT GUIDE Please see Important Safety Information on pages 1, 9, 10, 17 and 18. Please see accompanying Prescribing Information. INDICATION VYZULTA TM (latanoprostene bunod ophthalmic
YOUR VYZULTA TREATMENT GUIDE Please see Important Safety Information on pages 1, 9, 10, 17 and 18. Please see accompanying Prescribing Information. INDICATION VYZULTA TM (latanoprostene bunod ophthalmic
Summary of the risk management plan (RMP) for Pregabalin Mylan (pregabalin)
 EMA/285074/2015 Summary of the risk management plan (RMP) for Pregabalin Mylan (pregabalin) This is a summary of the risk management plan (RMP) for Pregabalin Mylan, which details the measures to be taken
EMA/285074/2015 Summary of the risk management plan (RMP) for Pregabalin Mylan (pregabalin) This is a summary of the risk management plan (RMP) for Pregabalin Mylan, which details the measures to be taken
Part VI: Summary of the risk management plan by product
 Part VI: Summary of the risk management plan by product VI.1 Elements for summary tables in the EPAR VI.1.1 Summary table of Safety concerns Summary of safety concerns Important identified risks Hepatotoxic
Part VI: Summary of the risk management plan by product VI.1 Elements for summary tables in the EPAR VI.1.1 Summary table of Safety concerns Summary of safety concerns Important identified risks Hepatotoxic
Pregabalin Aristo Version: RMP-Pregabalin0
 VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Epilepsy Epilepsy is a long-term condition affecting the brain and is characterised by recurring seizures (or fits). It is one
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Epilepsy Epilepsy is a long-term condition affecting the brain and is characterised by recurring seizures (or fits). It is one
Summary of the risk management plan (RMP) for Kyprolis (carfilzomib)
 EMA/639793/2015 Summary of the risk management plan (RMP) for Kyprolis (carfilzomib) This is a summary of the risk management plan (RMP) for Kyprolis, which details the measures to be taken in order to
EMA/639793/2015 Summary of the risk management plan (RMP) for Kyprolis (carfilzomib) This is a summary of the risk management plan (RMP) for Kyprolis, which details the measures to be taken in order to
Elements for a Public Summary
 VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Psoriasis (excluding widespread plaque psoriasis) Psoriasis is a common chronic skin disorder. Estimates of the prevalence (proportion
VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Psoriasis (excluding widespread plaque psoriasis) Psoriasis is a common chronic skin disorder. Estimates of the prevalence (proportion
PRODUCT INFORMATION SAFLUTAN
 NAME OF THE MEDICINE Tafluprost Chemical Structure Structural formula: PRODUCT INFORMATION SAFLUTAN CAS Number 209860-87-7 DESCRIPTION The active ingredient in SAFLUTAN is tafluprost, a prostaglandin analogue.
NAME OF THE MEDICINE Tafluprost Chemical Structure Structural formula: PRODUCT INFORMATION SAFLUTAN CAS Number 209860-87-7 DESCRIPTION The active ingredient in SAFLUTAN is tafluprost, a prostaglandin analogue.
PUBLIC SUMMARY OF RISK MANAGEMENT PLAN (RMP) CANDESARTAN/HYDROCHLOROTHIAZIDE ORION 8 MG/12.5 MG, 16 MG/12.5 MG, 32 MG/12.5 MG, 32 MG/25 MG ORION
 PUBLIC SUMMARY OF RISK MANAGEMENT PLAN (RMP) CANDESARTAN/HYDROCHLOROTHIAZIDE ORION 8 MG/12.5 MG, 16 MG/12.5 MG, 32 MG/12.5 MG, 32 MG/25 MG ORION CORPORATION DATE: 17-04-2015, VERSION 2 VI.2 Elements for
PUBLIC SUMMARY OF RISK MANAGEMENT PLAN (RMP) CANDESARTAN/HYDROCHLOROTHIAZIDE ORION 8 MG/12.5 MG, 16 MG/12.5 MG, 32 MG/12.5 MG, 32 MG/25 MG ORION CORPORATION DATE: 17-04-2015, VERSION 2 VI.2 Elements for
Glaucoma. Glaucoma. Optic Disc Cupping
 Glaucoma What is Glaucoma? Bruce James A group of diseases in which damage to the optic nerve occurs as a result of intraocualar pressure being above the physiological norm for that eye Stoke Mandeville
Glaucoma What is Glaucoma? Bruce James A group of diseases in which damage to the optic nerve occurs as a result of intraocualar pressure being above the physiological norm for that eye Stoke Mandeville
Zinplava Swiss Risk Management Plan Summary V1.5. Swiss Summary of the Risk Management Plan (RMP) for. Zinplava. (Bezlotoxumab 1000mg)
 Swiss Summary of the Risk Management Plan (RMP) for (Bezlotoxumab 1000mg) Concentrate for solution for infusion Version 1.5 (November 2016) The Risk Management Plan (RMP) is a comprehensive document submitted
Swiss Summary of the Risk Management Plan (RMP) for (Bezlotoxumab 1000mg) Concentrate for solution for infusion Version 1.5 (November 2016) The Risk Management Plan (RMP) is a comprehensive document submitted
PATIENT INFORMATION LEAFLET. PROPRIETARY NAME, STRENGTH AND PHARMACEUTICAL FORM OZURDEX, dexamethasone 700 μg intravitreal implant
 Page 1 of 6 SCHEDULING STATUS Schedule 4 PATIENT INFORMATION LEAFLET PROPRIETARY NAME, STRENGTH AND PHARMACEUTICAL FORM, dexamethasone 700 μg intravitreal implant Read all of this leaflet carefully before
Page 1 of 6 SCHEDULING STATUS Schedule 4 PATIENT INFORMATION LEAFLET PROPRIETARY NAME, STRENGTH AND PHARMACEUTICAL FORM, dexamethasone 700 μg intravitreal implant Read all of this leaflet carefully before
NEW ZEALAND DATA SHEET 1. PRODUCT NAME
 NEW ZEALAND DATA SHEET 1. PRODUCT NAME Flucon fluorometholone 0.1% Eye Drops Suspension 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of Flucon contains 1.0 mg of fluorometholone (0.1% w/v). Excipient
NEW ZEALAND DATA SHEET 1. PRODUCT NAME Flucon fluorometholone 0.1% Eye Drops Suspension 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of Flucon contains 1.0 mg of fluorometholone (0.1% w/v). Excipient
INDICATIONS ACULAR 0,5 % is indicated for the relief of inflammation following ocular surgery.
 Page 1 of 5 SCHEDULING STATUS Schedule 3 PROPRIETARY NAME (AND DOSAGE FORM) ACULAR 0,5 % COMPOSITION ACULAR 0,5 % contains: Preservatives: Benzalkonium chloride 0,01 % m/v Disodium edetate 0,1 % m/v PHARMACOLOGICAL
Page 1 of 5 SCHEDULING STATUS Schedule 3 PROPRIETARY NAME (AND DOSAGE FORM) ACULAR 0,5 % COMPOSITION ACULAR 0,5 % contains: Preservatives: Benzalkonium chloride 0,01 % m/v Disodium edetate 0,1 % m/v PHARMACOLOGICAL
Science & Technologies. UNWANTED SIDE EFFECTS OF TRAVOPROST Gazepov Strahil 1, Iljaz Ismaili 2, Goshevska Dashtevska Emilija 2
 UNWANTED SIDE EFFECTS OF TRAVOPROST Gazepov Strahil 1, Iljaz Ismaili 2, Goshevska Dashtevska Emilija 2 1 Clinical Hospital, Shtip 2 University Eye Clinic,Skopje Introduction: Glaucoma is a chronical progressive
UNWANTED SIDE EFFECTS OF TRAVOPROST Gazepov Strahil 1, Iljaz Ismaili 2, Goshevska Dashtevska Emilija 2 1 Clinical Hospital, Shtip 2 University Eye Clinic,Skopje Introduction: Glaucoma is a chronical progressive
Zerlinda (MRP DK/H/2265/001)
 Zerlinda (MRP DK/H/2265/001) VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Prevention of bone complications, e.g. fractures, in adult patients with bone metastases (spread
Zerlinda (MRP DK/H/2265/001) VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Prevention of bone complications, e.g. fractures, in adult patients with bone metastases (spread
Etoricoxib STADA 30 mg, 60 mg, 90 mg and 120 mg film-coated tablets , Version V1.2 PUBLIC SUMMARY OF THE RISK MANAGEMENT PLAN
 Etoricoxib STADA 30 mg, 60 mg, 90 mg and 120 mg film-coated tablets 23.5.2016, Version V1.2 PUBLIC SUMMARY OF THE RISK MANAGEMENT PLAN VI.2 Elements for a Public Summary Etoricoxib STADA 30 mg film-coated
Etoricoxib STADA 30 mg, 60 mg, 90 mg and 120 mg film-coated tablets 23.5.2016, Version V1.2 PUBLIC SUMMARY OF THE RISK MANAGEMENT PLAN VI.2 Elements for a Public Summary Etoricoxib STADA 30 mg film-coated
New Zealand Data Sheet APO-BIMATOPROST
 New Zealand Data Sheet APO-BIMATOPROST Presentation APO-BIMATOPROST is a clear colourless solution. Indications APO-BIMATOPROST is indicated as monotherapy for the reduction of elevated intraocular pressure
New Zealand Data Sheet APO-BIMATOPROST Presentation APO-BIMATOPROST is a clear colourless solution. Indications APO-BIMATOPROST is indicated as monotherapy for the reduction of elevated intraocular pressure
ILUVIEN 190 micrograms intravitreal implant in applicator (fluocinolone acetonide)
 ILUVIEN 190 micrograms intravitreal implant in applicator (fluocinolone acetonide) Read all of this information carefully before you are given this medicine because it contains important information for
ILUVIEN 190 micrograms intravitreal implant in applicator (fluocinolone acetonide) Read all of this information carefully before you are given this medicine because it contains important information for
Written by Administrator Wednesday, 13 January :27 - Last Updated Thursday, 21 January :34
 angle closure glaucoma A type of glaucoma caused by a sudden and severe rise in eye pressure. Occurs when the pupil enlarges too much or too quickly, and the outer edge of the iris blocks the eye s drainage
angle closure glaucoma A type of glaucoma caused by a sudden and severe rise in eye pressure. Occurs when the pupil enlarges too much or too quickly, and the outer edge of the iris blocks the eye s drainage
Core Safety Profile. Pharmaceutical form(s)/strength: Sterile eye drops 1%, 2% Date of FAR:
 Core Safety Profile Active substance: Carteolol Pharmaceutical form(s)/strength: Sterile eye drops 1%, 2% P - RMS: SK/H/PSUR/0002/002 Date of FAR: 16.03.2012 4.1 THERAPEUTIC INDICATIONS Ocular hypertension
Core Safety Profile Active substance: Carteolol Pharmaceutical form(s)/strength: Sterile eye drops 1%, 2% P - RMS: SK/H/PSUR/0002/002 Date of FAR: 16.03.2012 4.1 THERAPEUTIC INDICATIONS Ocular hypertension
PATIENT GUIDE TO LUMIGAN 0.01%
 Treatment Tracker enclosed PATIENT GUIDE TO LUMIGAN 0.01% LUMIGAN (bimatoprost ophthalmic solution) 0.01% is used for the treatment of high eye pressure, also called intraocular pressure (IOP), in people
Treatment Tracker enclosed PATIENT GUIDE TO LUMIGAN 0.01% LUMIGAN (bimatoprost ophthalmic solution) 0.01% is used for the treatment of high eye pressure, also called intraocular pressure (IOP), in people
Decrease of elevated intraocular pressure in adult patients with ocular hypertension or open-angle glaucoma (see section 5.1).
 1. NAME OF THE MEDICINAL PRODUCT Travoprost STADA 40 microgram/ml oogdruppels, oplossing 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of solution contains 40 micrograms of travoprost. Excipient(s)
1. NAME OF THE MEDICINAL PRODUCT Travoprost STADA 40 microgram/ml oogdruppels, oplossing 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of solution contains 40 micrograms of travoprost. Excipient(s)
Piazza G, Creager M. Thromboangiitis Obliterans. Circulation Apr 27; 121(16):
 VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Thromboangiitis Obliterans (TAO) 12 TAO, also called Buerger disease, is an inflammatory disease that most commonly affects the
VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Thromboangiitis Obliterans (TAO) 12 TAO, also called Buerger disease, is an inflammatory disease that most commonly affects the
Elements for a Public Summary
 VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Chronic lymphocytic leukaemia 1 Chronic lymphocytic leukemia (CLL) is a condition characterized by a progressive accumulation
VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Chronic lymphocytic leukaemia 1 Chronic lymphocytic leukemia (CLL) is a condition characterized by a progressive accumulation
Sponsor. Generic Drug Name. Trial Indications. Protocol Number. Protocol Title. Clinical Trial Phase. Study Start/End Dates. Reason for Termination
 Sponsor Alcon Research, Ltd. Generic Drug Name Travoprost/timolol maleate Trial Indications Open-angle glaucoma or ocular hypertension Protocol Number C-09-007 Protocol Title An Evaluation of Patient Reported
Sponsor Alcon Research, Ltd. Generic Drug Name Travoprost/timolol maleate Trial Indications Open-angle glaucoma or ocular hypertension Protocol Number C-09-007 Protocol Title An Evaluation of Patient Reported
Risk What is known Preventability Known hypersensitivity to macrolide antibiotic drugs or to any of its excipients.
 VI.2 VI.2.1 Elements for a public summary Overview of disease epidemiology Clarithromycin 500 mg, powder for solution for infusion is indicated when parenteral therapy is required for treatment of severe
VI.2 VI.2.1 Elements for a public summary Overview of disease epidemiology Clarithromycin 500 mg, powder for solution for infusion is indicated when parenteral therapy is required for treatment of severe
Package leaflet: Information for the user. GANFORT 0.3 mg/ml + 5 mg/ml eye drops, solution, in single-dose container Bimatoprost/timolol
 Package leaflet: Information for the user GANFORT 0.3 mg/ml + 5 mg/ml eye drops, solution, in single-dose container Bimatoprost/timolol Read all of this leaflet carefully before you start using this medicine
Package leaflet: Information for the user GANFORT 0.3 mg/ml + 5 mg/ml eye drops, solution, in single-dose container Bimatoprost/timolol Read all of this leaflet carefully before you start using this medicine
SUMMARY OF PRODUCT CHARACTERSITICS
 SUMMARY OF PRODUCT CHARACTERSITICS 1. NAME OF THE MEDICINAL PRODUCT Travoprost Polpharma 40 microgram/ml oogdruppels, oplosing 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of solution contains 40
SUMMARY OF PRODUCT CHARACTERSITICS 1. NAME OF THE MEDICINAL PRODUCT Travoprost Polpharma 40 microgram/ml oogdruppels, oplosing 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of solution contains 40
Elements for a public summary. VI.2.1 Overview of disease epidemiology
 VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Coronary artery disease and as anticoagulant (inhibiting the clotting of the blood) in patients undergoing surgery to treat blockages
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Coronary artery disease and as anticoagulant (inhibiting the clotting of the blood) in patients undergoing surgery to treat blockages
Summary of the risk management plan (RMP) for Budesonide/Formoterol Teva (budesonide / formoterol)
 EMA/639304/2014 Summary of the risk management plan (RMP) for Budesonide/Formoterol Teva (budesonide / formoterol) This is a summary of the risk management plan (RMP) for Budesonide/Formoterol Teva, which
EMA/639304/2014 Summary of the risk management plan (RMP) for Budesonide/Formoterol Teva (budesonide / formoterol) This is a summary of the risk management plan (RMP) for Budesonide/Formoterol Teva, which
9 PM Eye Drops (Latanoprost 0.005%)
 Published on: 10 Jul 2014 9 PM Eye Drops (Latanoprost 0.005%) Composition Each ml contains: Latanoprost... 50 mcg Benzalkonium Chloride, NF... 0.02%w/v (as preservative) aqueous vehicle... q.s. Dosage
Published on: 10 Jul 2014 9 PM Eye Drops (Latanoprost 0.005%) Composition Each ml contains: Latanoprost... 50 mcg Benzalkonium Chloride, NF... 0.02%w/v (as preservative) aqueous vehicle... q.s. Dosage
PUBLIC SUMMARY OF THE RISK MANAGEMENT PLAN
 Cofact 250 IU and 500 IU powder and solvent for solution for injection PUBLIC SUMMARY OF THE RISK MANAGEMENT PLAN VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Cofact contains
Cofact 250 IU and 500 IU powder and solvent for solution for injection PUBLIC SUMMARY OF THE RISK MANAGEMENT PLAN VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Cofact contains
Ocular Hypotensive Efficacy of Netarsudil Ophthalmic Solution 0.02% Over a 24-Hour Period: A Pilot Study
 Ocular Hypotensive Efficacy of Netarsudil Ophthalmic Solution 0.02% Over a 24-Hour Period: A Pilot Study James H. Peace, M.D. 1, Casey K. Kopczynski, Ph.D. 2, and Theresa Heah, M.D. 2 1 Inglewood, CA 2
Ocular Hypotensive Efficacy of Netarsudil Ophthalmic Solution 0.02% Over a 24-Hour Period: A Pilot Study James H. Peace, M.D. 1, Casey K. Kopczynski, Ph.D. 2, and Theresa Heah, M.D. 2 1 Inglewood, CA 2
DATA SHEET. Pharmacotherapeutic group: Antiglaucoma preparations and miotics; other antiglaucoma preparations. ATC code: S01EE03
 DATA SHEET NAME OF MEDICINE LUMIGAN PF (bimatoprost) 0.3 mg/ml eye drops Presentation LUMIGAN PF (bimatoprost) eye drops are a clear, isotonic, colourless, sterile ophthalmic solution. Bimatoprost is a
DATA SHEET NAME OF MEDICINE LUMIGAN PF (bimatoprost) 0.3 mg/ml eye drops Presentation LUMIGAN PF (bimatoprost) eye drops are a clear, isotonic, colourless, sterile ophthalmic solution. Bimatoprost is a
TIMALEN 0.5% eye drops
 PACKAGE LEAFLET: INFORMATION FOR THE USER TIMALEN 0.5% eye drops TIMOLOL (AS TIMOLOL MALEATE) This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine,
PACKAGE LEAFLET: INFORMATION FOR THE USER TIMALEN 0.5% eye drops TIMOLOL (AS TIMOLOL MALEATE) This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine,
LUMIGAN (bimatoprost ophthalmic solution) 0.03% for topical ophthalmic use Initial U.S. Approval: 2001
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LUMIGAN 0.03% safely and effectively. See full prescribing information for LUMIGAN 0.03%. LUMIGAN
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LUMIGAN 0.03% safely and effectively. See full prescribing information for LUMIGAN 0.03%. LUMIGAN
A study conducted in Norway found the combined estimate for panic and generalized anxiety disorder was 1.10 per 1,000 person-years.
 VI.2 Elements for a public summary For sake of completeness, with reference to article 11 of the Directive 2001/83, the applicant retains the option to carve out the patented indication in the national
VI.2 Elements for a public summary For sake of completeness, with reference to article 11 of the Directive 2001/83, the applicant retains the option to carve out the patented indication in the national
OCULAR PHARMACOLOGY GLAUCOMA. increased intraocular pressure. normally mm Hg. when to Tx no fixed level.
 OCULAR PHARMACOLOGY GLAUCOMA increased intraocular pressure normally 12 20 mm Hg. when to Tx no fixed level. literature sets ~21 mm Hg as upper limit of normal. some safe at 30 mm Hg some may have damage
OCULAR PHARMACOLOGY GLAUCOMA increased intraocular pressure normally 12 20 mm Hg. when to Tx no fixed level. literature sets ~21 mm Hg as upper limit of normal. some safe at 30 mm Hg some may have damage
Summary of the risk management plan (RMP) for Vylaer Spiromax (budesonide / formoterol)
 EMA/675937/2014 Summary of the risk management plan (RMP) for Vylaer Spiromax (budesonide / formoterol) This is a summary of the risk management plan (RMP) for Vylaer Spiromax, which details the measures
EMA/675937/2014 Summary of the risk management plan (RMP) for Vylaer Spiromax (budesonide / formoterol) This is a summary of the risk management plan (RMP) for Vylaer Spiromax, which details the measures
NEW ZEALAND DATA SHEET
 NEW ZEALAND DATA SHEET 1. PRODUCT NAME LUMIGAN PF (bimatoprost) 0.3 mg/ml eye drops 2. QUALITATIVE AND QUANTITATIVE COMPOSITION LUMIGAN PF eye drops contains bimatoprost 0.3 mg/ml For full list of excipients,
NEW ZEALAND DATA SHEET 1. PRODUCT NAME LUMIGAN PF (bimatoprost) 0.3 mg/ml eye drops 2. QUALITATIVE AND QUANTITATIVE COMPOSITION LUMIGAN PF eye drops contains bimatoprost 0.3 mg/ml For full list of excipients,
PUBLIC SUMMARY OF RISK MANAGEMENT PLAN (RMP) CIPROFLOXACIN ORION 750 MG FILM-COATED TABLETS ORION OYJ DATE: , VERSION 1.
 PUBLIC SUMMARY OF RISK MANAGEMENT PLAN (RMP) CIPROFLOXACIN ORION 250 MG FILM-COATED TABLETS CIPROFLOXACIN ORION 500 MG FILM-COATED TABLETS CIPROFLOXACIN ORION 750 MG FILM-COATED TABLETS ORION OYJ DATE:
PUBLIC SUMMARY OF RISK MANAGEMENT PLAN (RMP) CIPROFLOXACIN ORION 250 MG FILM-COATED TABLETS CIPROFLOXACIN ORION 500 MG FILM-COATED TABLETS CIPROFLOXACIN ORION 750 MG FILM-COATED TABLETS ORION OYJ DATE:
Revised: 07/2017. LUMIGAN (bimatoprost ophthalmic solution) 0.01% for topical ophthalmic use Initial U.S. Approval: 2001
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LUMIGAN 0.01% safely and effectively. See full prescribing information for LUMIGAN 0.01%. LUMIGAN
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LUMIGAN 0.01% safely and effectively. See full prescribing information for LUMIGAN 0.01%. LUMIGAN
DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC)
 Guidelines for the medical treatment of chronic open angle glaucoma and ocular hypertension Summary: DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC) Diagnosis and management of ocular hypertension (OHT)
Guidelines for the medical treatment of chronic open angle glaucoma and ocular hypertension Summary: DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC) Diagnosis and management of ocular hypertension (OHT)
Glaucoma Disease Progression Role of Intra Ocular Pressure. Is Good Enough, Low Enough?
 Glaucoma Disease Progression Role of Intra Ocular Pressure Is Good Enough, Low Enough? Glaucoma Diseases Progression Key Considerations Good number of patients may be diagnosed only after some damage the
Glaucoma Disease Progression Role of Intra Ocular Pressure Is Good Enough, Low Enough? Glaucoma Diseases Progression Key Considerations Good number of patients may be diagnosed only after some damage the
Eplerenon Medical Valley + Eplerenon Stada
 VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Heart failure is a complex syndrome, clinically characterized by signs and symptoms secondary to abnormal cardiac function. It
VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Heart failure is a complex syndrome, clinically characterized by signs and symptoms secondary to abnormal cardiac function. It
Summary of the risk management plan (RMP) for Adempas (riociguat)
 EMA/76215/2014 Summary of the risk management plan (RMP) for Adempas (riociguat) This is a summary of the risk management plan (RMP) for Adempas, which details the measures to be taken in order to ensure
EMA/76215/2014 Summary of the risk management plan (RMP) for Adempas (riociguat) This is a summary of the risk management plan (RMP) for Adempas, which details the measures to be taken in order to ensure
OCCLUSIVE VASCULAR DISORDERS OF THE RETINA
 OCCLUSIVE VASCULAR DISORDERS OF THE RETINA Learning outcomes By the end of this lecture the students would be able to Classify occlusive vascular disorders (OVD) of the retina. Correlate the clinical features
OCCLUSIVE VASCULAR DISORDERS OF THE RETINA Learning outcomes By the end of this lecture the students would be able to Classify occlusive vascular disorders (OVD) of the retina. Correlate the clinical features
relative s privacy, do not identify your relative by full name in any assignment.
 Overview Do you or a family member have glaucoma? Do you wonder what this diagnosis means? Glaucoma affects tens of millions of people worldwide. Despite its prevalence, many people lack accurate information
Overview Do you or a family member have glaucoma? Do you wonder what this diagnosis means? Glaucoma affects tens of millions of people worldwide. Despite its prevalence, many people lack accurate information
NEPTUNE RED BANK BRICK
 NEPTUNE RED BANK BRICK Diabetes & The Eye Diabetics are more likely to develop Cataracts at a younger age. Diabetics are twice as likely to develop Glaucoma when compared to non-diabetics. The primary
NEPTUNE RED BANK BRICK Diabetes & The Eye Diabetics are more likely to develop Cataracts at a younger age. Diabetics are twice as likely to develop Glaucoma when compared to non-diabetics. The primary
sodium [2-(2,6-dichloroanilino)phenyl] acetate, a phenylacetic acid derivative CH 2 COONa
![sodium [2-(2,6-dichloroanilino)phenyl] acetate, a phenylacetic acid derivative CH 2 COONa sodium [2-(2,6-dichloroanilino)phenyl] acetate, a phenylacetic acid derivative CH 2 COONa](/thumbs/78/77533900.jpg) PRODUCT INFORMATION VOLTAREN * OPHTHA Eye Drop (diclofenac sodium) NAME OF THE MEDICINE Active ingredient: Chemical name: Molecular formula: C 14 H 10 Cl 2 NNaO 2 CAS number: 15307-79-6 Molecular weight:
PRODUCT INFORMATION VOLTAREN * OPHTHA Eye Drop (diclofenac sodium) NAME OF THE MEDICINE Active ingredient: Chemical name: Molecular formula: C 14 H 10 Cl 2 NNaO 2 CAS number: 15307-79-6 Molecular weight:
Summary of the risk management plan (RMP) for Scenesse (afamelanotide)
 EMA/692496/2014 Summary of the risk management plan (RMP) for Scenesse (afamelanotide) This is a summary of the risk management plan (RMP) for Scenesse, which details the measures to be taken in order
EMA/692496/2014 Summary of the risk management plan (RMP) for Scenesse (afamelanotide) This is a summary of the risk management plan (RMP) for Scenesse, which details the measures to be taken in order
