Over one hundred years ago Hans Chiari first published
|
|
|
- Allyson Lang
- 5 years ago
- Views:
Transcription
1 J Neurosurg Pediatrics 8: , 8: , 2011 Complications following decompression of Chiari malformation Type I in children: dural graft or sealant? Clinical article Stephen R. Parker, M.D., M.P.H., 1 Peggy Harris, P.A.-C, 1 Thomas J. Cummings, M.D., 1 Timothy George, M.D., 2 Herbert Fuchs, M.D., Ph.D., 1 and Gerald Grant, M.D. 1 1 Division of Neurosurgery, Department of Surgery, Duke University Medical Center, Durham, North Carolina; and 2 Pediatric Neurosurgery Center of Central Texas at Dell Children s Hospital, Austin, Texas Object. Posterior fossa decompression with duraplasty for Chiari malformation Type I (CM-I) is a common pediatric neurosurgery procedure. Published series report a complication rate ranging from 3% to 40% for this procedure. Historically, many dural substitutes have been used, including bovine grafts, human cadaveric pericardium, synthetic dura, and autologous pericranium. The authors hypothesized that a recently observed increase in complications was dependent on the graft used. Methods. Between January 2004 and January 2008, 114 consecutive patients 18 years old underwent primary CM-I decompression using duraplasty. Records were retrospectively reviewed for short- and intermediate-term complications and operative technique, focusing on the choice of duraplasty graft with or without application of a tissue sealant. Results. The average age of the patients was 8.6 years. The dural graft used was variable: 15 were treated with cadaveric pericardium, 12 with Durepair, and 87 with EnDura. Tisseel was used in 75 patients, DuraSeal in 12, and no tissue sealant was used in 27 patients. The overall complication rate was 21.1%. The most common complications included aseptic meningitis, symptomatic pseudomeningocele, or a CSF leak requiring reoperation. The overall complication rates were as follows: cadaveric pericardium 26.7%, Durepair 41.7%, and EnDura 17.2%; reoperation rates were 13%, 25%, and 8.1%, respectively. Prior to adopting a different graft product, the overall complication rate was 18.1%; following the change the rate increased to 35%. Complication rates for tissue sealants were 14.8% for no sealant, 18.7% for Tisseel, and 50% for DuraSeal. Nine patients were treated with the combination of Durepair and DuraSeal and this subgroup had a 56% complication rate. Conclusions. Complication rates after CM-I decompression may be dependent on the dural graft with or without the addition of tissue sealant. The complication rate at the authors institution approximately doubled following the adoption of a different graft product. Tissue sealants used in combination with a dural substitute to augment a duraplasty may increase the risk of aseptic meningitis and/or CSF leak. The mechanism of the apparent increased inflammation with this combination remains under investigation. (DOI: / PEDS10362) Key Words aseptic meningitis CSF leak Chiari complication pseudomeningocele decompression Over one hundred years ago Hans Chiari first published his observations of autopsy patients with cerebellar ectopia involving the spinal canal. 6 Since his initial observations, our understanding of Chiari malformation Type I (CM-I) has continued to evolve. Most CM-I are believed to be congenital and patients typically present with symptoms related to posterior fossa compression and/or spinal cord dysfunction. 1,13 The number of pediatric patients presenting with CM-I appears to be increasing. 21,22,28,29 Surgical intervention has been shown to change the clinical course of CM-I. 28,31 Many different Abbreviation used in this paper: CM-I = Chiari malformation Type I. surgical treatment strategies have been described, including bone decompression with or without dural expansion, along with various algorithms for deciding whether dural expansion should be undertaken. 1,5,9,11 15,17,19,20 24,26 28,30,32 However, the majority of pediatric neurosurgeons surveyed in 2000 recommended using bone decompression along with dural grafting for CM-I. 18 Tissue sealants are also commonly used to augment the dural graft in an effort to obtain a watertight closure. 10 A large range of complications has been reported in the literature following surgery for CM-I. Most published series have a postoperative complication rate between 3% and 40% consisting primarily of aseptic meningitis, pseudomeningocele, CSF leak, and wound infections. 2,8,9,13,19,22,30,32 177
2 S. R. Parker et al. In 2007, the dural graft substitute commonly used by the pediatric neurosurgeons at our institution was changed due to the removal of EnDura No-React Dural Substitute (Shelhigh, Inc., Integra LifeSciences) from the market. 33 Subsequent to the product removal, our postoperative complication rate appeared to increase dramatically. We hypothesized that the complication rate following surgery for CM-I was contingent upon the choice of expansile duraplasty material and the graft sealant used. To evaluate our hypothesis, we retrospectively reviewed 4 years of records from consecutive pediatric patients who underwent primary surgical decompression for CM-I, investigating operative technique and postoperative complications. Methods We present data from 114 consecutive patients 18 years old who underwent primary decompression for CM-I between January 1, 2004, and January 1, 2008, at Duke University Medical Center. Following institutional review board approval the medical records of the patients were retrospectively reviewed for short- and intermediate-term complications and operative technique, consisting of duraplasty with expansile graft with or without application of a tissue sealant. Surgical Technique All patients were treated by 1 of 3 established pediatric neurosurgeons (H.F., T.G., and G.G.) at our institution using a standardized operative technique. All 3 surgeons applied the same surgical approach and technique to patients with CM-I. All patients were placed prone. A suboccipital decompression and C-1 laminectomy were performed. The craniectomy extended from the foramen magnum to the inferior nuchal line, taking care to provide adequate decompression of the rim of the foramen. The size of the suboccipital decompression typically measured 3 cm 3 cm in width and height. The dura mater was opened in all cases in a Y-shaped fashion prior to the expansile duraplasty. Intradural arachnoid adhesions were sharply dissected and the pial surface of the tonsils was coagulated to restore normal CSF flow. Dural closure was accomplished with a 6-0 Prolene suture using an expansile triangular graft of cadaveric pericardium (LifeNet Health), EnDura, or Durepair Dura Regeneration Matrix (TEI Biosciences, Medtronic Neurosurgery). EnDura was used in all cases except 7 prior to its removal from the market. In the 7 cases in which EnDura was not used prior to its removal from the market, it was not in stock in the hospital, thus necessitating use of an alternative graft product. Once EnDura was removed from the market, cadaveric pericardium and Durepair were used in an approximate 1:1 ratio. The application of a tissue sealant Tisseel (Baxter Healthcare) or DuraSeal (Covidien, Inc.) was used on a case-by-case basis, with the overwhelming majority of grafts augmented with a tissue sealant. Follow-Up and Complications The patients returned routinely for postoperative visits at 2 weeks, 8 weeks, and 1 year. All patients underwent MR imaging of the brain and a cine CSF flow study was performed to evaluate the craniocervical junction at the 8-week visit. For this study, a postoperative complication was defined as any symptomatic patient who returned to the emergency department or pediatric neurosurgery clinic for medical attention. Possible complications included aseptic meningitis, a pseudomeningocele, or CSF leak. Aseptic meningitis was diagnosed most often following CSF obtained through a lumbar puncture that showed an elevated opening pressure (> 20 cm H 2 O), mononuclear pleocytosis, elevated protein, and a negative gram stain and culture. Two patients arrived with severe frontal headaches and neck stiffness without fever, and began treatment with steroid tapers without a spinal tap for presumed aseptic meningitis; these 2 patients improved. Statistical Analysis We evaluated the overall responder characteristics focusing on postoperative complications as our outcome variable of interest. Bivariate analysis of postoperative complications and dural graft/dural sealant was performed using the Pearson chi-square test. Bivariate analysis of the need for reoperation with graft type was also performed using the Pearson chi-square test. All analyses were performed using the statistical software StataIC version 10 (StataCorp LP). Results Patient Population Our retrospective review of postoperative complications in patients 18 years of age after decompression for CM-I showed an average patient age of 8.6 ± 5.3 years. The dural graft used was variable: 13.2% (15 of 114) received cadaveric pericardium, 10.5% (12 of 114) Durepair, and 76.3% (87 of 114) EnDura. The tissue sealant Tisseel was used in 65.8% of patients, DuraSeal in 10.5%, and no tissue sealant in 23.7% (Table 1). Complication Outcome The overall complication rate was 21.1%. Results from our bivariate analyses showed complication rates for the TABLE 1: Characteristics of the 114 children who underwent decompression surgery for CM-I Characteristic Value age (yrs) 8.6 ± 5.3 graft EnDura 76.3% Durepair 10.5% cadaveric pericardium 13.2% tissue sealant Tisseel 65.8% DuraSeal 10.5% none 23.7% complication postop day 25.5 ±
3 Complications following surgery for Chiari malformation Type I graft products to be 26.7% for cadaveric pericardium, 41.7% for Durepair, and 17.2% for EnDura (p = 0.128). Reoperation rates were 13%, 25%, and 8.1%, respectively. There were no infections reported in this cohort. The most common complications encountered for the graft products were aseptic meningitis (EnDura 5.7%, Durepair 41.7%, cadaveric pericardium 20%), symptomatic pseudomeningocele (EnDura 8%, Durepair 33.3%, cadaveric pericardium 13.3%), and CSF leak (EnDura 3.4%, Durepair 8.3%, cadaveric pericardium 13%). The complication rates for tissue sealants were 14.8% for no sealant, 18.7% for Tisseel, and 50% for DuraSeal (p < 0.05). The highest complication percentage for the combination group of graft and sealant in which there was more than 1 patient was 55.6% (p = 0.081), with the combination of DuraSeal and Durepair used in 9 patients (Table 2). No patients received the combination of EnDura and DuraSeal because DuraSeal was not available at our institution until after EnDura was removed from the market. However, when DuraSeal was used with either cadaveric pericardium or Durepair there was a 33.3% reoperation rate (p = 0.361; Table 3). Of interest, prior to the institutional switch from EnDura, the complication rate was 18.1% but this rate increased to 35% following the adoption of a different graft and sealant product (Table 4). All patients in the Durepair group that required reoperation secondary to chemical meningitis and elevated lumbar pressures, which did not resolve with a course of steroids, had their grafts replaced with autologous pericranium without further complications. Timing of Postoperative Complications Patients on average presented with postoperative complications on postoperative Day 25 (range 3 63; Table 1). Although the majority of the complications occurred within the first 21 days of surgery, there were outliers that were unexpected up to 2 months following the initial procedure. In the patients who underwent reoperation, there were no postoperative complications reported. Discussion In choosing a dural graft for duraplasty, the ideal material would be available in abundance, relatively inexpensive, easy to handle, non-immunogenic, and biodegradable. 8 Historically, many different dural substitutes have been used for the expansile graft including bovine grafts, human cadaveric pericardium, synthetic dura, and autologous pericranium. All of these various substitutes have been shown to be potentially effective for dural grafting. 3,7,8,26,36 Xenogeneic collagen-based dural TABLE 2: Complication rates Complications Type of Graft DuraSeal Tisseel No Augmentation EnDura 0 62 (19.4) 25 (12) Durepair 9 (55.6)* 2 (0) 1 (0) cadaveric pericardium 3 (33.3) 11 (18.2) 1 (100) * p = 0.081, Pearson chi-square test. TABLE 3: Association between graft/sealant combinations and reoperation Graft/Sealant Combination % w/ Reoperation EnDura 8 Durepair 0 cadaveric pericardium 0 EnDura/Tisseel 8.1 Durepair/Tisseel 0 cadaveric pericardium/tisseel 9.1 EnDura/DuraSeal none w/ this combination Durepair/DuraSeal 33.3* cadaveric pericardium/duraseal 33.3* * p = 0.361, Pearson chi-square test. graft substitutes have become increasingly popular and are typically composed of animal collagens processed to remove cellular and other immunogenic components (for example, Durepair). 12 The materials used for duraplasty at our institution during the review included EnDura, Durepair, cadaveric pericardium, and autologous pericranium. No new surgical techniques were required to be learned to appropriately use the graft products, thus reducing the possibility of a lead-in learning bias. There were also 2 dural sealants used in this study, Tisseel and DuraSeal. EnDura EnDura is a glutaraldehyde cross-linked bovine pericardium. 21 According to the product information provided at the time on EnDura, glutaraldehyde treated tissue may deteriorate, cause inflammatory reaction, adhesions, and calcification mineralization. 21 Because of the possibility of inflammatory reaction the graft product was washed vigorously in sterile saline for at least 2 minutes prior to implantation. This dural substitute is detoxified, which is intended to prevent glutaraldehyde leaching. However, if the washing procedure was not conducted according to the instructions and leaching occured, there is a possibility that glutaraldehyde-treated tissue could cause a local inflammatory reaction and increase postoperative complications. TABLE 4: Complication rates before and after dural graft change Time Period Total No. of Grafts Total Complications Complications/ Total Grafts (%) prior to EnDura s removal from the market cadaveric pericardium /94 (18.1) EnDura Durepair 1 0 after EnDura s removal from the market cadaveric pericardium 9 2 7/20 (35) Durepair
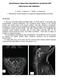
4 S. R. Parker et al. Durepair Durepair is a collagenous implant derived from fetal bovine dermis and is processed to remove all cellular components but is not modified with cross-linking chemicals. Durepair is composed of an array of collagen fibers that form a porous matrix into which host cells and supporting blood vessels can penetrate. According to the manufacturer: animal study results suggest that the foreign body response associated with the use of sealants and hemostatic agents in conjunction with Durepair may be more pronounced than use of Durepair alone. This response may increase the incident rates of known risks of dura substitutes, particularly in high pressure gradient applications. If intending to use ancillary products with Durepair, ensure the products are applied in accordance with their instructions for use. 16 Cadaveric Pericardium Cadaveric pericardium is an allograft product made from human cadaveric pericardium and subsequently freeze dried. Cadaveric pericardium contains the risk of a potential immunogenic reaction or transmission of disease. Historically, the concern for infectious disease transmission was much higher as even Creutzfeldt-Jacob disease has been reported following implantation of allograft cadaveric dural substitutes. 4 Autologous Pericranium Autologous pericranium was used in our series only as a dural graft substitute at the time of the second procedure. This was a personal preference of the authors due to the increased surgical exposure required to obtain an adequate amount of pericranium in a child. Tisseel Tisseel, a human fibrin sealant, contains fibrinogen as the main active ingredient and also contains thrombin (derived from pooled human plasma), calcium chloride, and fibrinolysis activator (aprotinin). Although Tisseel is not approved for use in neurosurgery, it has been widely adopted for off-label use. According to the manufacturer s insert, contraindications include avoiding injection into the circulatory system, not using with hypersensitivity to aprotinin, and avoidance of use with brisk arterial bleeding. Because Tisseel contains pooled human plasma, there are potential risks for viral transmission. DuraSeal DuraSeal is 100% synthetic and consists of 2 dilute aqueous precursor liquids that cross-link within 1 2 seconds of spraying to form a hydrogel network. This sealant has the potential to absorb fluid and swell following application. The hydrogel matrix then breaks down by hydrolysable linkages in the polyethylene glycol matrix and is absorbed within 4 8 weeks. According to the manufacturer s insert, contraindications to its use include a history of allergy, penetration of an air sinus, renal/hepatic/immune dysfunction, head trauma, and infection. It is also to be avoided in cases of hydrocephalus, and those involving a ventricular drain or lumbar drain. DuraSeal is FDA-approved for use in cranial and spinal surgery, and has been evaluated in several animal-based and clinical studies, but was associated with quadriplegia and cauda equina syndrome in 2 patients. Characterization of Postoperative Complications With Combined Dural Graft and Sealant A retrospective review 25 of a single-institution experience with DuraSeal sealant in combination with nonautologous duraplasty materials revealed 44 cases in which DuraSeal was combined with a collagen matrix allograft, and 10 cases in combination with bovine pericardium. Overall, in 154 cases, there were 13 cases of CSF leak (8.4%), 6 cases of pseudomeningocele (3.9%), and 5 surgical infections (3.2%). There were 5 cases of CSF leak (11.4%), 3 pseudomeningoceles (6.8%), and 1 infection (0.6%) in the collagen matrix subgroup, and there was 1 CSF leak (10%), 1 pseudomeningocele (10%), and no infections in the bovine pericardium group. In a multidisciplinary historical cohort study, a retrospective review was performed of 66 patients (> 15 years of age) who underwent cranial operations that were closed using DuraSeal with nonautologous duraplasty and compared with 50 patients who were enrolled in the DuraSeal Pivotal Trial treated with autologous material. The complications from both groups were similar within 90 days of surgery, although there were very few patients with Chiari malformations in this adult series. The incidence of postoperative CSF leakage was 7.6% in the study group (retrospective population) and 6.0% in the Pivotal Trial population. The incidence of meningitis was 0% and 4.0% in the retrospective and Pivotal Trial groups, respectively. 35 In our retrospective review of postoperative complications in 114 consecutive patients 18 years old undergoing surgical decompression for CM-I, we found that the complication rate may be dependent on the dural graft with or without the addition of tissue sealant used. Because of the relatively few number of patients in some of the combination groups of graft/sealant, it was difficult for any of our analyses to reach a significance level of p < 0.05; although even with such small group sizes, there are trends toward significance that should be investigated further. The results from this series suggest that the association of a higher complication rate for the combination of Durepair and DuraSeal, although not statistically significant, might be clinically significant. The reasons behind this are unclear. Foy et al. 12 reported a severe allergic reaction to Durepair in the lumbar spine associated with a positive antigen skin test, along with eosinophilic and chronic lymphocytic graft infiltration. This inflammation resolved after removal of the graft. Following this report, one of the patients in the current series developed a severe postoperative aseptic meningitis and symptomatic pseudomeningocele. His lumbar puncture revealed spinal pressures that were markedly elevated (> 50 cm H 2 O), and CSF analysis revealed a profound lymphocytosis. Pathology showed nongranulomatous inflammation (Fig. 1), but without eosinophilic graft infiltration. The initial Chiari malformation repair in this patient was made using a Durepair graft augmented with DuraSeal. This patient had a 180
5 Complications following surgery for Chiari malformation Type I negative antigen skin test to Durepair placed on the skin. A complete reversal of the elevated lumbar pressures and chemical meningitis occurred rapidly with the replacement of the dural graft with autologous pericranium. Other series of duraplasties for CM-I report CSF leak rates of 0% 12%. Among 74 combined patients from 3 different series undergoing duraplasty with xenogeneic pericardium, 1 patient developed a CSF leak and no patients developed a pseudomeningocele or required reoperation. 8,11,32 Fischer 11 and Vanaclocha and Saiz-Sapena 34 combined reported no pseudomeningocle or CSF leak in 32 patients who underwent a duraplasty with pericranial autograft. In another study by Danish et al., 8 56 patients underwent a duraplasty with a suturable synthetic collagen graft (DuraGen) and 5 patients developed a pseudomeningocele, 1 patient developed a CSF leak, and 4 required reoperation. In the same series, 45 patients underwent a duraplasty with acellular human dermis, 5 developed a pseudomeningocele, and 1 had a CSF leak. Finally, Attenello et al. 3 reported on 77 patients who underwent a duraplasty with pericranial autograft (40 patients) or polytetrafluoroethylene graft (27 patients). No patient in either group developed a CSF leak or symptomatic pseudomeningocele. In the current series, however, there was a 21.1% overall complication rate, which included symptomatic pseudomeningocele and CSF leak. Time Course of Postoperative Complications It was also of interest to evaluate the time course of postoperative complication presentation. In comparing the various dural graft products and tissue sealants, those patients who received Durepair and DuraSeal did not present until after postoperative Day 16 (Fig. 2). It is difficult to evaluate whether this relates to Durepair alone, DuraSeal alone, or the combination, because our series had only 3 patients who received Durepair but not DuraSeal, and only 3 patients who received DuraSeal but not Durepair. Biocompatible hydrogels such as polyethylene glycol hydrogel in DuraSeal do not typically allow cells to migrate through, which may actually delay postsurgical inflammation and healing. This delay in cell migration may explain why the Fig. 1. Photomicrograph of the junction of synthetic dura graft (upper) and native dura (lower) showing moderate chronic nongranulomatous inflammation. H & E, original magnification 20. Fig. 2. Bar graphs of postoperative complications for graft type (upper) and sealant type (lower). CSF leaks presented much later in the patients who underwent closure using Durepair and DuraSeal. On February 2, 2009, Medtronic issued a recall of Durepair in Canada, stating that preliminary data suggests that the foreign body reaction of concomitant use of some ancillary products with Durepair is more pronounced than Durepair alone and may increase rates of other known risks. 16 While evaluating the use of tissue sealants, we were surprised that the lowest complication rate occurred when no tissue sealant was used (14.8%) compared with DuraSeal (50%) or Tisseel (18.7%). Although the majority of patients in our series underwent graft augmentation using a tissue sealant, it is unclear as to why a sealant was not used in those cases involving no sealant. It is possible the surgeon believed an excellent watertight primary closure was obtained in those cases, and thus, no sealant augmentation was warranted. This result questions the need and efficacy of using tissue sealants to augment a watertight closure. It appears that a good primary dural closure is just as effective and may have less morbidity than the addition of a tissue sealant. At the current time, pediatric neurosurgeons at our institution have switched to using cadaveric or autologous pericranium with Tisseel, and the complication rate dropped to 5% in the next 40 consecutive decompressions of CM-I, which is comparable to the historical rate. Conclusions Complication rates from decompression of CM-I in children may be dependent on the dural graft with or without the addition of tissue sealant. Our complication rate approximately doubled following the adoption of a different graft product. The use of tissue sealants to aug- 181
6 S. R. Parker et al. ment duraplasty may not provide any additional benefit. A complete reversal of the chemical meningitis can occur with replacement of the dural graft, although the source of the increased inflammation and complication rate, graft versus sealant, or combination of the two, remains under investigation. Disclosure The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper. Author contributions to the study and manuscript preparation include the following. Conception and design: Parker. Acquisition of data: Parker, Cotton, Cummings, George, Fuchs. Analysis and interpretation of data: Parker. Drafting the article: Parker. Critically revising the article: Grant. Reviewed submitted version of manuscript: all authors. Statistical analysis: Parker. Administrative/technical/material support: Cummings. Study supervision: Grant. References 1. Alden TD, Ojemann JG, Park TS: Surgical treatment of Chiari I malformation: indications and approaches. Neurosurg Focus 11(1):E2, Alzate JC, Kothbauer KF, Jallo GI, Epstein FJ: Treatment of Chiari I malformation in patients with and without syringomyelia: a consecutive series of 66 cases. Neurosurg Focus 11(1):E3, Attenello FJ, McGirt MJ, Garcés-Ambrossi GL, Chaichana KL, Carson B, Jallo GI: Suboccipital decompression for Chiari I malformation: outcome comparison of duraplasty with expanded polytetrafluoroethylene dural substitute versus pericranial autograft. Childs Nerv Syst 25: , Brown P, Cervenáková L, Goldfarb LG, McCombie WR, Rubenstein R, Will RG, et al: Iatrogenic Creutzfeldt-Jakob disease: an example of the interplay between ancient genes and modern medicine. Neurology 44: , Caldarelli M, Novegno F, Vassimi L, Romani R, Tamburrini G, Di Rocco C: The role of limited posterior fossa craniectomy in the surgical treatment of Chiari malformation Type I: experience with a pediatric series. J Neurosurg 106 (3 Suppl): , Chiari H: Über die Veränderungen des Kleinhirns infolge von Hydrocephalie des Geßhirns. Dtsch Med Wochenschr 17: , Collins RL, Christiansen D, Zazanis GA, Silver FH: Use of collagen film as a dural substitute: preliminary animal studies. J Biomed Mater Res 25: , Danish SF, Samdani A, Hanna A, Storm P, Sutton L: Experience with acellular human dura and bovine collagen matrix for duraplasty after posterior fossa decompression for Chiari malformations. J Neurosurg 104 (1 Suppl):16 20, Durham SR, Fjeld-Olenec K: Comparison of posterior fossa decompression with and without duraplasty for the surgical treatment of Chiari malformation Type I in pediatric patients: a meta-analysis. J Neurosurg Pediatr 2:42 49, Epstein NE: Dural repair with four spinal sealants: focused review of the manufacturers inserts and the current literature. Spine 10: , Fischer EG: Posterior fossa decompression for Chiari I deformity, including resection of the cerebellar tonsils. Childs Nerv Syst 11: , Foy AB, Giannini C, Raffel C: Allergic reaction to a bovine dural substitute following spinal cord untethering. Case report. J Neurosurg Pediatr 1: , Galarza M, Sood S, Ham S: Relevance of surgical strategies for the management of pediatric Chiari type I malformation. Childs Nerv Syst 23: , Gambardella G, Caruso G, Caffo M, Germanò A, La Rosa G, Tomasello F: Transverse microincisions of the outer layer of the dura mater combined with foramen magnum decompression as treatment for syringomyelia with Chiari I malformation. Acta Neurochir (Wien) 140: , Genitori L, Peretta P, Nurisso C, Macinante L, Mussa F: Chiari type I anomalies in children and adolescents: minimally invasive management in a series of 53 cases. Childs Nerv Syst 16: , Government of Canada HC, Health Products and Food Branch, HPFB Inspectorate, Medical Recall Device Listings (From January 2009 to March 2009). manu-fab_jan-march_2009-eng.php#tphp. [Accessed March 30, 2009] 17. Guyotat J, Bret P, Jouanneau E, Ricci AC, Lapras C: Syringomyelia associated with type I Chiari malformation. A 21-year retrospective study on 75 cases treated by foramen magnum decompression with a special emphasis on the value of tonsils resection. Acta Neurochir (Wien) 140: , Haroun RI, Guarnieri M, Meadow JJ, Kraut M, Carson BS: Current opinions for the treatment of syringomyelia and chiari malformations: survey of the Pediatric Section of the American Association of Neurological Surgeons. Pediatr Neurosurg 33: , Hayhurst C, Richards O, Zaki H, Findlay G, Pigott TJ: Hindbrain decompression for Chiari-syringomyelia complex: an outcome analysis comparing surgical techniques. Br J Neurosurg 22:86 91, Isu T, Sasaki H, Takamura H, Kobayashi N: Foramen magnum decompression with removal of the outer layer of the dura as treatment for syringomyelia occurring with Chiari I malformation. Neurosurgery 33: , Klekamp J, Batzdorf U, Samii M, Bothe HW: The surgical treatment of Chiari I malformation. Acta Neurochir (Wien) 138: , Krieger MD, McComb JG, Levy ML: Toward a simpler surgical management of Chiari I malformation in a pediatric population. Pediatr Neurosurg 30: , Kumar R, Kalra SK, Vaid VK, Mahapatra AK: Chiari I malformation: surgical experience over a decade of management. Br J Neurosurg 22: , Limonadi FM, Selden NR: Dura-splitting decompression of the craniocervical junction: reduced operative time, hospital stay, and cost with equivalent early outcome. J Neurosurg 101 (2 Suppl): , Litvack ZN, Anderson VC, Delashaw JB Jr: Neurosurgical Applications for a PEG Based Hydrogel (DuraSeal ): Effectiveness and Safety Across the Spectrum of Neurosurgical Procedures. ( php?function=public_view&id=37662) [Accessed May 16, 2011] 26. McGirt MJ, Attenello FJ, Datoo G, Gathinji M, Atiba A, Weingart JD, et al: Intraoperative ultrasonography as a guide to patient selection for duraplasty after suboccipital decompression in children with Chiari malformation Type I. J Neurosurg Pediatr 2:52 57, Milhorat TH, Bolognese PA: Tailored operative technique for Chiari type I malformation using intraoperative color Doppler ultrasonography. Neurosurgery 53: , Navarro R, Olavarria G, Seshadri R, Gonzales-Portillo G, McLone DG, Tomita T: Surgical results of posterior fossa decompression for patients with Chiari I malformation. Childs Nerv Syst 20: , Park JK, Gleason PL, Madsen JR, Goumnerova LC, Scott RM: Presentation and management of Chiari I malformation in children. Pediatr Neurosurg 26: , Perrini P, Benedetto N, Tenenbaum R, Di Lorenzo N: Extraarachnoidal cranio-cervical decompression for syringomyelia associated with Chiari I malformation in adults: tech- 182
7 Complications following surgery for Chiari malformation Type I nique assessment. Acta Neurochir (Wien) 149: , Tubbs RS, Lyerly MJ, Loukas M, Shoja MM, Oakes WJ: The pediatric Chiari I malformation: a review. Childs Nerv Syst 23: , Tubbs RS, McGirt MJ, Oakes WJ: Surgical experience in 130 pediatric patients with Chiari I malformations. J Neurosurg 99: , US Food and Drug Administration: Integra LifeSciences Corporation (also known as Integra NeuroSciences) EnDura No-React Dural Substitute. Class 1 Recall. ( gov/medicaldevices/safety/recallscorrectionsremovals/ ListofRecalls/ucm htm) [Accessed May 16, 2011] 34. Vanaclocha V, Saiz-Sapena N: Duraplasty with freeze-dried cadaveric dura versus occipital pericranium for Chiari type I malformation: comparative study. Acta Neurochir (Wien) 139: , Weinstein JS, Liu KC, Delashaw JB Jr, Burchiel KJ, van Loveren HR, Vale FL, et al: The safety and effectiveness of a dural sealant system for use with nonautologous duraplasty materials. Clinical article. J Neurosurg 112: , Zerris VA, James KS, Roberts JB, Bell E, Heilman CB: Repair of the dura mater with processed collagen devices. J Biomed Mater Res B Appl Biomater 83: , 2007 Manuscript submitted August 16, Accepted May 10, This work was presented in part as proceedings at the CNS Annual Meeting, Orlando, Florida, September 2008, and the American So ciety of Pediatric Neurosurgeons Annual Meeting, Hawaii, Janu ary Address correspondence to: Gerald Grant, M.D., Neurosurgery and Pediatrics, Duke University Medical Center, Box 3272, Durham, North Carolina gerald.grant@duke.edu. 183
A case report on result of posterior fossa decompression on syringomyelia in a case of chiary type I malformation
 Romanian Neurosurgery Volume XXXII Number 1 2018 January-March Article A case report on result of posterior fossa decompression on syringomyelia in a case of chiary type I malformation Asheesh Kumar Gupta,
Romanian Neurosurgery Volume XXXII Number 1 2018 January-March Article A case report on result of posterior fossa decompression on syringomyelia in a case of chiary type I malformation Asheesh Kumar Gupta,
Suboccipital decompression for Chiari malformation associated scoliosis: risk factors and time course of deformity progression
 J Neurosurg Pediatrics 1:456 460, 2008 Suboccipital decompression for Chiari malformation associated scoliosis: risk factors and time course of deformity progression FRANK J. ATTENELLO, M.S., MATTHEW J.
J Neurosurg Pediatrics 1:456 460, 2008 Suboccipital decompression for Chiari malformation associated scoliosis: risk factors and time course of deformity progression FRANK J. ATTENELLO, M.S., MATTHEW J.
Minimally invasive subpial tonsillectomy for Chiari I decompression
 Acta Neurochir (2016) 158:1807 1811 DOI 10.1007/s00701-016-2877-2 HOW I DO IT - NEUROSURGICAL TECHNIQUES Minimally invasive subpial tonsillectomy for Chiari I decompression Jeffrey S. Beecher 1 Yong Liu
Acta Neurochir (2016) 158:1807 1811 DOI 10.1007/s00701-016-2877-2 HOW I DO IT - NEUROSURGICAL TECHNIQUES Minimally invasive subpial tonsillectomy for Chiari I decompression Jeffrey S. Beecher 1 Yong Liu
Experience with acellular human dura and bovine collagen matrix for duraplasty after posterior fossa decompression for Chiari malformations
 J Neurosurg (1 Suppl Pediatrics) 104:16 20, 2006 Experience with acellular human dura and bovine collagen matrix for duraplasty after posterior fossa decompression for Chiari malformations SHABBAR F. DANISH,
J Neurosurg (1 Suppl Pediatrics) 104:16 20, 2006 Experience with acellular human dura and bovine collagen matrix for duraplasty after posterior fossa decompression for Chiari malformations SHABBAR F. DANISH,
DuraMatrix Collagen Dura Substitute Membrane. Manufactured by: Collagen Matrix, Inc., Franklin Lakes, New Jersey
 DuraMatrix Collagen Dura Substitute Membrane Manufactured by: Collagen Matrix, Inc., Franklin Lakes, New Jersey What is DuraMatrix? Onlay or Sutured Implantation Balanced Implant Resorption vs. Host Tissue
DuraMatrix Collagen Dura Substitute Membrane Manufactured by: Collagen Matrix, Inc., Franklin Lakes, New Jersey What is DuraMatrix? Onlay or Sutured Implantation Balanced Implant Resorption vs. Host Tissue
Neurosurgery is complex. DuraGen is the simple choice.
 Neurosurgery is complex. is the simple choice. Family of Grafts Dural Regeneration Matrix Keep Closure Simple. A Breakthrough in Dural Repair A Pioneer in Dural Regeneration Matrices For almost forty years,
Neurosurgery is complex. is the simple choice. Family of Grafts Dural Regeneration Matrix Keep Closure Simple. A Breakthrough in Dural Repair A Pioneer in Dural Regeneration Matrices For almost forty years,
Chiari malformation Type I (CM-I) is typically defined
 PEDIATRICS clinical article J Neurosurg Pediatr 16:150 158, 2015 Outcomes after suboccipital decompression without dural opening in children with Chiari malformation Type I Benjamin C. Kennedy, MD, 1 Kathleen
PEDIATRICS clinical article J Neurosurg Pediatr 16:150 158, 2015 Outcomes after suboccipital decompression without dural opening in children with Chiari malformation Type I Benjamin C. Kennedy, MD, 1 Kathleen
After a great surgery, why leave anything to chance?
 HyperBranch Medical Technology, Inc. creates innovative medical devices using synthetic hydrogels with novel tunable properties for use as surgical sealants in general and specialty surgery. The products
HyperBranch Medical Technology, Inc. creates innovative medical devices using synthetic hydrogels with novel tunable properties for use as surgical sealants in general and specialty surgery. The products
Pediatric and adult Chiari malformation Type I surgical series : a review of demographics, operative treatment, and outcomes
 PEDIATRICS literature review Pediatric and adult Chiari malformation Type I surgical series 1965 2013: a review of demographics, operative treatment, and outcomes aska arnautovic, bsc, 1 bruno splavski,
PEDIATRICS literature review Pediatric and adult Chiari malformation Type I surgical series 1965 2013: a review of demographics, operative treatment, and outcomes aska arnautovic, bsc, 1 bruno splavski,
What Every Spine Surgeon Should Know About Neurosurgical Issues
 What Every Spine Surgeon Should Know About Neurosurgical Issues Amer Samdani, MD Chief of Surgery Shriners Hospitals for Children Philadelphia, PA Objectives Main intraspinal lesions Chiari malformation
What Every Spine Surgeon Should Know About Neurosurgical Issues Amer Samdani, MD Chief of Surgery Shriners Hospitals for Children Philadelphia, PA Objectives Main intraspinal lesions Chiari malformation
OUTCOME OF SUBOCCIPITAL DECOMPRESSION WITH AND WITHOUT DURAPLASTY IN ADULTS WITH CHIARI I MALFORMATION
 OUTCOME OF SUBOCCIPITAL DECOMPRESSION WITH AND WITHOUT DURAPLASTY IN ADULTS WITH CHIARI I MALFORMATION Mushtaq 1, Zia ur Rehman 1, M Mukhtar Khan 2 ABSTRACT Objectives: To investigate outcome for ACM1
OUTCOME OF SUBOCCIPITAL DECOMPRESSION WITH AND WITHOUT DURAPLASTY IN ADULTS WITH CHIARI I MALFORMATION Mushtaq 1, Zia ur Rehman 1, M Mukhtar Khan 2 ABSTRACT Objectives: To investigate outcome for ACM1
Idiopathic cervical syringomyelia can be associated. Pediatric Chiari malformation Type 0: a 12-year institutional experience.
 J Neurosurg J Neurosurg Pediatrics Pediatrics 8:000 000, 8:1 5, 2011 Pediatric Chiari malformation Type 0: a 12-year institutional experience Clinical article Joshua J. Chern, M.D., Ph.D., Amber J. Gordon,
J Neurosurg J Neurosurg Pediatrics Pediatrics 8:000 000, 8:1 5, 2011 Pediatric Chiari malformation Type 0: a 12-year institutional experience Clinical article Joshua J. Chern, M.D., Ph.D., Amber J. Gordon,
SYNTHECEL Dura Repair. Assurance and Versatility for Dura Reconstruction.
 SYNTHECEL Dura Repair Assurance and Versatility for Dura Reconstruction. SYNTHECEL Dura Repair is an assured and versatile solution for your dura reconstruction needs. Clinically proven One product choice
SYNTHECEL Dura Repair Assurance and Versatility for Dura Reconstruction. SYNTHECEL Dura Repair is an assured and versatile solution for your dura reconstruction needs. Clinically proven One product choice
Role of MRI in Selection of Patients for Surgery and Assessing the Post Operative Outcome in Chiari 1 Malformation
 DOI: 10.7860/IJARS/2017/13599:2222 Radiology Section Original Article Role of MRI in Selection of Patients for Surgery and Assessing the Post Operative Outcome in Chiari 1 Malformation Rajesh Kumar V,
DOI: 10.7860/IJARS/2017/13599:2222 Radiology Section Original Article Role of MRI in Selection of Patients for Surgery and Assessing the Post Operative Outcome in Chiari 1 Malformation Rajesh Kumar V,
Restoration not just repair
 DURAGEN ENGLISH Restoration not just repair r e g e n e r at i v e t e c h n o l o g y PRODUCTS FOR SALE IN EUROPE, MIDDLE-EAST and AFRICA ONLY. More than a graft DuraGen is a matrix for repair of the
DURAGEN ENGLISH Restoration not just repair r e g e n e r at i v e t e c h n o l o g y PRODUCTS FOR SALE IN EUROPE, MIDDLE-EAST and AFRICA ONLY. More than a graft DuraGen is a matrix for repair of the
DuraMatrix portfolio. Restore with confidence
 DuraMatrix portfolio Restore with confidence History Since 2003, Stryker has partnered with Collagen Matrix, Inc. to provide our customers with quality products that are safe and effective in the restoration
DuraMatrix portfolio Restore with confidence History Since 2003, Stryker has partnered with Collagen Matrix, Inc. to provide our customers with quality products that are safe and effective in the restoration
RESEARCH HUMAN CLINICAL STUDIES
 TOPIC RESEARCH HUMAN CLINICAL STUDIES RESEARCH HUMAN CLINICAL STUDIES A Novel Scoring System for Assessing Chiari Malformation Type I Treatment Outcomes Leonardo Aliaga, BFA Katherine E. Hekman, BA Reza
TOPIC RESEARCH HUMAN CLINICAL STUDIES RESEARCH HUMAN CLINICAL STUDIES A Novel Scoring System for Assessing Chiari Malformation Type I Treatment Outcomes Leonardo Aliaga, BFA Katherine E. Hekman, BA Reza
DIANE MUELLER, N.D., R.N., C-F.N.P., AND JOHN J. ORO, M.D.
 Neurosurg Focus 18 (2):ECP2, 2005 Prospective analysis of self-perceived quality of life before and after posterior fossa decompression in 112 patients with Chiari malformation with or without syringomyelia
Neurosurg Focus 18 (2):ECP2, 2005 Prospective analysis of self-perceived quality of life before and after posterior fossa decompression in 112 patients with Chiari malformation with or without syringomyelia
Chiari Malformations. Google. Objectives Seventh Annual NKY TBI Conference 3/22/13. Kerry R. Crone, M.D.
 Chiari Malformations Kerry R. Crone, M.D. Professor of Neurosurgery and Pediatrics University of Cincinnati College of Medicine University of Cincinnati Medical Center Cincinnati Children s Hospital Medical
Chiari Malformations Kerry R. Crone, M.D. Professor of Neurosurgery and Pediatrics University of Cincinnati College of Medicine University of Cincinnati Medical Center Cincinnati Children s Hospital Medical
Moderators: Dr Manmohan Singh Dr Pankaj Singh Presentor : Dr Kanwaljeet Garg
 Moderators: Dr Manmohan Singh Dr Pankaj Singh Presentor : Dr Kanwaljeet Garg History! Hans Chiari (1851 1916) was born in Vienna, Austria! He was Professor of pathology at Prague, Czechoslovakia! His initial
Moderators: Dr Manmohan Singh Dr Pankaj Singh Presentor : Dr Kanwaljeet Garg History! Hans Chiari (1851 1916) was born in Vienna, Austria! He was Professor of pathology at Prague, Czechoslovakia! His initial
Surgical Neurology International
 Surgical Neurology International OPEN ACCESS For entire Editorial Board visit : http://www.surgicalneurologyint.com Editor: James I. Ausman, MD, PhD University of California, Los Angeles, CA, USA Original
Surgical Neurology International OPEN ACCESS For entire Editorial Board visit : http://www.surgicalneurologyint.com Editor: James I. Ausman, MD, PhD University of California, Los Angeles, CA, USA Original
D. Andrew Wilkinson, MD, Kyle Johnson, BS, Hugh J. L. Garton, MD, MSc, Karin M. Muraszko, MD, and Cormac O. Maher, MD
 clinical article J Neurosurg Pediatr 19:208 216, 2017 Trends in surgical treatment of Chiari malformation Type I in the United States D. Andrew Wilkinson, MD, Kyle Johnson, BS, Hugh J. L. Garton, MD, MSc,
clinical article J Neurosurg Pediatr 19:208 216, 2017 Trends in surgical treatment of Chiari malformation Type I in the United States D. Andrew Wilkinson, MD, Kyle Johnson, BS, Hugh J. L. Garton, MD, MSc,
A NOVEL CAUSE FOR CAUDA- EQUINA SYNDROME WITH A NEW RADIOLOGICAL SIGN
 A NOVEL CAUSE FOR CAUDA- EQUINA SYNDROME WITH A NEW RADIOLOGICAL SIGN W Singleton, D Ramnarine, N Patel, C Wigfield Department of Neurological Surgery, Frenchay Hospital, Bristol, UK Introduction We present
A NOVEL CAUSE FOR CAUDA- EQUINA SYNDROME WITH A NEW RADIOLOGICAL SIGN W Singleton, D Ramnarine, N Patel, C Wigfield Department of Neurological Surgery, Frenchay Hospital, Bristol, UK Introduction We present
Outcome of Two Surgical Options Used in Treatment of Chiari Type-One Malformation
 ISPUB.COM The Internet Journal of Neurosurgery Volume 11 Number 1 Outcome of Two Surgical Options Used in Treatment of Chiari Type-One Malformation M G Ammar Citation M G Ammar. Outcome of Two Surgical
ISPUB.COM The Internet Journal of Neurosurgery Volume 11 Number 1 Outcome of Two Surgical Options Used in Treatment of Chiari Type-One Malformation M G Ammar Citation M G Ammar. Outcome of Two Surgical
ISSN X (Print) Original Research Article. DOI: /sjams
 DOI: 10.21276/sjams Scholars Journal of Applied Medical Sciences (SJAMS) Sch. J. App. Med. Sci., 2017; 5(8E):3349-3365 Scholars Academic and Scientific Publisher (An International Publisher for Academic
DOI: 10.21276/sjams Scholars Journal of Applied Medical Sciences (SJAMS) Sch. J. App. Med. Sci., 2017; 5(8E):3349-3365 Scholars Academic and Scientific Publisher (An International Publisher for Academic
Chiari FAQ's. 1. What is a Chiari Malformation?
 Chiari FAQ's These FAQ's are for informational purposes only and in no way represent an attempt to provide medical advice. This information may or may not apply to your case and anyone with a question
Chiari FAQ's These FAQ's are for informational purposes only and in no way represent an attempt to provide medical advice. This information may or may not apply to your case and anyone with a question
Durotomies with CSF leakage are one of the most
 CASE REPORT J Neurosurg Spine 28:181 185, 2018 A novel duraplasty technique following fenestration of a massive lumbar arachnoid cyst in a patient with scoliosis: technical case report Matthew T. Neal,
CASE REPORT J Neurosurg Spine 28:181 185, 2018 A novel duraplasty technique following fenestration of a massive lumbar arachnoid cyst in a patient with scoliosis: technical case report Matthew T. Neal,
Comparative study of duraplasty and non duraplasty in Chiari 1 malformation with syringomyleia our institute experience
 International Journal of Research in Medical Sciences Naik BDBS et al. Int J Res Med Sci. 2017 Apr;5(4):1325-1330 www.msjonline.org pissn 2320-6071 eissn 2320-6012 Original Research Article DOI: http://dx.doi.org/10.18203/2320-6012.ijrms20170976
International Journal of Research in Medical Sciences Naik BDBS et al. Int J Res Med Sci. 2017 Apr;5(4):1325-1330 www.msjonline.org pissn 2320-6071 eissn 2320-6012 Original Research Article DOI: http://dx.doi.org/10.18203/2320-6012.ijrms20170976
The Role of Cine Flow Magnetic Resonance Imaging in Patients with Chiari 0 Malformation
 DOI: 10.5137/1019-5149.JTN.19049-16.2 Received: 11.11.2016 / Accepted: 14.12.2016 Published Online: 16.01.2017 Original Investigation The Role of Cine Flow Magnetic Resonance Imaging in Patients with Chiari
DOI: 10.5137/1019-5149.JTN.19049-16.2 Received: 11.11.2016 / Accepted: 14.12.2016 Published Online: 16.01.2017 Original Investigation The Role of Cine Flow Magnetic Resonance Imaging in Patients with Chiari
The Chiari malformation I (CM I) is a disorder that has been
 DOI: 10.5137/1019-5149.JTN.18349-16.1 Received: 31.05.2016 / Accepted: 01.07.2016 Published Online: 22.08.2016 Original Investigation Are Herniated Cerebellar Tonsils the Main Culprit of Chiari Malformation
DOI: 10.5137/1019-5149.JTN.18349-16.1 Received: 31.05.2016 / Accepted: 01.07.2016 Published Online: 22.08.2016 Original Investigation Are Herniated Cerebellar Tonsils the Main Culprit of Chiari Malformation
Iatrogenic lumbar Pseudomeningocele: A case report and review of literature
 Available online at Available online at: www.ijmrhs.com ISSN No: 2319-5886 International Journal of Medical Research & Health Sciences, 2016, 5, 1:153-157 Iatrogenic lumbar Pseudomeningocele: A case report
Available online at Available online at: www.ijmrhs.com ISSN No: 2319-5886 International Journal of Medical Research & Health Sciences, 2016, 5, 1:153-157 Iatrogenic lumbar Pseudomeningocele: A case report
Fleece-Bound Tissue Sealing in Microvascular Decompression
 DOI: 10.5137/1019-5149.JTN.17462-16.2 Received: 01.03.2016 / Accepted: 20.04.2016 Published Online: 23.08.2016 Original Investigation Fleece-Bound Tissue Sealing in Microvascular Decompression Levent TANRIKULU
DOI: 10.5137/1019-5149.JTN.17462-16.2 Received: 01.03.2016 / Accepted: 20.04.2016 Published Online: 23.08.2016 Original Investigation Fleece-Bound Tissue Sealing in Microvascular Decompression Levent TANRIKULU
Persistent/Recurrent Syringomyelia after Chiari Decompression Natural History and Management Strategies: A Systematic Review
 116 Systematic Review Persistent/Recurrent Syringomyelia after Chiari Decompression Natural History and Management Strategies: A Systematic Review James M. Schuster 1 Fangyi Zhang 2,3 Daniel C. Norvell
116 Systematic Review Persistent/Recurrent Syringomyelia after Chiari Decompression Natural History and Management Strategies: A Systematic Review James M. Schuster 1 Fangyi Zhang 2,3 Daniel C. Norvell
Chiari 1 malformations: an Indian hospital experience
 O r i g i n a l A r t i c l e Singapore Med J 2008; 49 (12) : 1029 Chiari 1 malformations: an Indian hospital experience Ramnarayan R, Praharaj M S, Jayakumar P N Department of Neurosurgery, The National
O r i g i n a l A r t i c l e Singapore Med J 2008; 49 (12) : 1029 Chiari 1 malformations: an Indian hospital experience Ramnarayan R, Praharaj M S, Jayakumar P N Department of Neurosurgery, The National
Surgical Management of the Chiari Malformation Stephen A. Fletcher, D.O. Associate Professor Division of Pediatric Neurosurgery
 Surgical Management of the Chiari Malformation Stephen A. Fletcher, D.O. Associate Professor Division of Pediatric Neurosurgery Historical Descriptions Antoine Portal -1803 described the post-mortem spinal
Surgical Management of the Chiari Malformation Stephen A. Fletcher, D.O. Associate Professor Division of Pediatric Neurosurgery Historical Descriptions Antoine Portal -1803 described the post-mortem spinal
Cranio-cervical decompression. Information for patients Neurosurgery
 Cranio-cervical decompression Information for patients Neurosurgery page 2 of 12 What is a cranio-cervical decompression? A cranio-cervical decompression is an operation involving the back of the head
Cranio-cervical decompression Information for patients Neurosurgery page 2 of 12 What is a cranio-cervical decompression? A cranio-cervical decompression is an operation involving the back of the head
Developments in the treatment of Chiari type 1 malformations over the past decade
 Original Study Developments in the treatment of Chiari type 1 malformations over the past decade Peter G. Passias 1, Alexandra Pyne 1, Samantha R. Horn 1, Gregory W. Poorman 1, Muhammad B. Janjua 1, Dennis
Original Study Developments in the treatment of Chiari type 1 malformations over the past decade Peter G. Passias 1, Alexandra Pyne 1, Samantha R. Horn 1, Gregory W. Poorman 1, Muhammad B. Janjua 1, Dennis
got you covered. Codman s The global leader in neurosurgery is your one-stop, comprehensive source for proven, market-leading dural repair solutions.
 Codman s got you covered. The global leader in neurosurgery is your one-stop, comprehensive source for proven, market-leading dural repair solutions. AN INTEGRA LIFESCIENCES COMPANY The Leader in Dural
Codman s got you covered. The global leader in neurosurgery is your one-stop, comprehensive source for proven, market-leading dural repair solutions. AN INTEGRA LIFESCIENCES COMPANY The Leader in Dural
5.5. RETROSIGMOID APPROACH
 5.5. RETROSIGMOID APPROACH The retrosigmoid approach provides good access to the cerebellopontine angle. It is by far simpler and faster with much less need for bone removal than other more extensive lateral
5.5. RETROSIGMOID APPROACH The retrosigmoid approach provides good access to the cerebellopontine angle. It is by far simpler and faster with much less need for bone removal than other more extensive lateral
Prior Authorization Review Panel MCO Policy Submission
 Prior Authorization Review Panel MCO Policy Submission A separate copy of this form must accompany each policy submitted for review. Policies submitted without this form will not be considered for review.
Prior Authorization Review Panel MCO Policy Submission A separate copy of this form must accompany each policy submitted for review. Policies submitted without this form will not be considered for review.
Application of a hydrogel sealant improves watertight closures of duraplasty onlay grafts in a canine craniotomy model
 J Neurosurg 107:642 650, 2007 Application of a hydrogel sealant improves watertight closures of duraplasty onlay grafts in a canine craniotomy model MARK C. PREUL, M.D., 1 PATRICK K. CAMPBELL, PH.D., 2
J Neurosurg 107:642 650, 2007 Application of a hydrogel sealant improves watertight closures of duraplasty onlay grafts in a canine craniotomy model MARK C. PREUL, M.D., 1 PATRICK K. CAMPBELL, PH.D., 2
Introduction to Neurosurgical Subspecialties:
 Introduction to Neurosurgical Subspecialties: Pediatric Neurosurgery Brian L. Hoh, MD 1 and Gregory J. Zipfel, MD 2 1 University of Florida, 2 Washington University Pediatric Neurosurgery Pediatric neurosurgeons
Introduction to Neurosurgical Subspecialties: Pediatric Neurosurgery Brian L. Hoh, MD 1 and Gregory J. Zipfel, MD 2 1 University of Florida, 2 Washington University Pediatric Neurosurgery Pediatric neurosurgeons
Chiari malformations. A fact sheet for patients and carers
 A fact sheet for patients and carers Chiari malformations This fact sheet provides information on Chiari malformations. It focuses on Chiari malformations in adults. Our fact sheets are designed as general
A fact sheet for patients and carers Chiari malformations This fact sheet provides information on Chiari malformations. It focuses on Chiari malformations in adults. Our fact sheets are designed as general
At the conclusion of craniotomy procedures, it is
 J Neurosurg 120:278 284, 2014 AANS, 2014 The incidence of complications in elective cranial neurosurgery associated with dural closure material Clinical article Brian P. Walcott, M.D., 1 Jonathan B. Neal,
J Neurosurg 120:278 284, 2014 AANS, 2014 The incidence of complications in elective cranial neurosurgery associated with dural closure material Clinical article Brian P. Walcott, M.D., 1 Jonathan B. Neal,
Ventricles, CSF & Meninges. Steven McLoon Department of Neuroscience University of Minnesota
 Ventricles, CSF & Meninges Steven McLoon Department of Neuroscience University of Minnesota 1 Coffee Hour Thursday (Sept 14) 8:30-9:30am Surdyk s Café in Northrop Auditorium Stop by for a minute or an
Ventricles, CSF & Meninges Steven McLoon Department of Neuroscience University of Minnesota 1 Coffee Hour Thursday (Sept 14) 8:30-9:30am Surdyk s Café in Northrop Auditorium Stop by for a minute or an
Pichayen Duangthongpon MD*, Chaiwit Thanapaisal MD*, Amnat Kitkhuandee MD*, Kowit Chaiciwamongkol MD**, Vilaiwan Morthong MD**
 The Relationships between Asterion, the Transverse-Sigmoid Junction, the Superior Nuchal Line and the Transverse Sinus in Thai Cadavers: Surgical Relevance Pichayen Duangthongpon MD*, Chaiwit Thanapaisal
The Relationships between Asterion, the Transverse-Sigmoid Junction, the Superior Nuchal Line and the Transverse Sinus in Thai Cadavers: Surgical Relevance Pichayen Duangthongpon MD*, Chaiwit Thanapaisal
Decompression of the spinal subarachnoid space as a solution for syringomyelia without Chiari malformation
 (2002) 40, 501 ± 506 ã 2002 International Society All rights reserved 1362 ± 4393/02 $25.00 www.nature.com/sc Original Article Decompression of the spinal subarachnoid space as a solution for syringomyelia
(2002) 40, 501 ± 506 ã 2002 International Society All rights reserved 1362 ± 4393/02 $25.00 www.nature.com/sc Original Article Decompression of the spinal subarachnoid space as a solution for syringomyelia
Creutzfeldt-Jakob Disease Transmitted by Dura mater Graft Dr. Manuel Clavel, C/ Margenat 19, E Bellaterra (Barcelona) (Spain)
 / Short Report Eur Neurol 1996;36:239-240 M. Manuel Clavel P. Pablo Clavel Neurosurgery Service, Hospital General de Cataluna, Barcelona, Spain Creutzfeldt-Jakob Disease Transmitted by Dura mater Graft
/ Short Report Eur Neurol 1996;36:239-240 M. Manuel Clavel P. Pablo Clavel Neurosurgery Service, Hospital General de Cataluna, Barcelona, Spain Creutzfeldt-Jakob Disease Transmitted by Dura mater Graft
Djamila Kafoufi Al Galaa Military Hospital Cairo
 Djamila Kafoufi Al Galaa Military Hospital Cairo Herniation cerebellar tonsils below the foramen magnum, Hans Chiari 4 types Chiari I less than 5mm,HDC rare,syringomyelia often present. Chiari II,protrusion
Djamila Kafoufi Al Galaa Military Hospital Cairo Herniation cerebellar tonsils below the foramen magnum, Hans Chiari 4 types Chiari I less than 5mm,HDC rare,syringomyelia often present. Chiari II,protrusion
Brain Imaging. Bearbeitet von Klaus Sartor, Stefan Hähnel, Bodo Kress
 Brain Imaging Bearbeitet von Klaus Sartor, Stefan Hähnel, Bodo Kress 1. Auflage 2007. Taschenbuch. 312 S. Paperback ISBN 978 3 13 143961 1 Format (B x L): 12,5 x 19 cm Weitere Fachgebiete > Medizin > Sonstige
Brain Imaging Bearbeitet von Klaus Sartor, Stefan Hähnel, Bodo Kress 1. Auflage 2007. Taschenbuch. 312 S. Paperback ISBN 978 3 13 143961 1 Format (B x L): 12,5 x 19 cm Weitere Fachgebiete > Medizin > Sonstige
Kiyoshi Ito, MD, Tatsuro Aoyama, MD, Takuya Nakamura, MD, Yoshiki Hanaoka, MD, Tetsuyoshi Horiuchi, MD, and Kazuhiro Hongo, MD
 technical note J Neurosurg Spine 25:620 625, 2016 Novel dural incision and closure procedure for preventing postoperative cerebrospinal fluid leakage during the surgical removal of dumbbell-shaped spinal
technical note J Neurosurg Spine 25:620 625, 2016 Novel dural incision and closure procedure for preventing postoperative cerebrospinal fluid leakage during the surgical removal of dumbbell-shaped spinal
A review of the disagreements in the prevalence and treatment of the tethered cord syndromes with chiari 1 malformations
 SNI: Spine OPEN ACCESS For entire Editorial Board visit : http://www.surgicalneurologyint.com Editor: Nancy E. Epstein, MD Winthrop Hospital, Mineola, NY, USA Review Article A review of the disagreements
SNI: Spine OPEN ACCESS For entire Editorial Board visit : http://www.surgicalneurologyint.com Editor: Nancy E. Epstein, MD Winthrop Hospital, Mineola, NY, USA Review Article A review of the disagreements
Brain Meninges, Ventricles and CSF
 Brain Meninges, Ventricles and CSF Lecture Objectives Describe the arrangement of the meninges and their relationship to brain and spinal cord. Explain the occurrence of epidural, subdural and subarachnoid
Brain Meninges, Ventricles and CSF Lecture Objectives Describe the arrangement of the meninges and their relationship to brain and spinal cord. Explain the occurrence of epidural, subdural and subarachnoid
Multimodal Evaluation Of Cerebrospinal Fluid (csf ) Dynamics Following Extradural Decompression For Chiari I Malformation
 Yale University EliScholar A Digital Platform for Scholarly Publishing at Yale Yale Medicine Thesis Digital Library School of Medicine January 2015 Multimodal Evaluation Of Cerebrospinal Fluid (csf ) Dynamics
Yale University EliScholar A Digital Platform for Scholarly Publishing at Yale Yale Medicine Thesis Digital Library School of Medicine January 2015 Multimodal Evaluation Of Cerebrospinal Fluid (csf ) Dynamics
Integra. TenoGlide Tendon Protector Sheet SURGICAL TECHNIQUE
 Integra TenoGlide Tendon Protector Sheet SURGICAL TECHNIQUE Table of Contents Description...2 Indications...2 Contraindications...2 Surgical Technique...4 Step 1 Surgical Approach...4 Step 2 Remove TenoGlide
Integra TenoGlide Tendon Protector Sheet SURGICAL TECHNIQUE Table of Contents Description...2 Indications...2 Contraindications...2 Surgical Technique...4 Step 1 Surgical Approach...4 Step 2 Remove TenoGlide
Application Guide for Full-Thickness Wounds
 Application Guide for Full-Thickness Wounds PriMatrix Dermal Repair Scaffold PriMatrix Ag Antimicrobial Dermal Repair Scaffold Application Guide for Full Thickness Wounds PriMatrix is a unique dermal repair
Application Guide for Full-Thickness Wounds PriMatrix Dermal Repair Scaffold PriMatrix Ag Antimicrobial Dermal Repair Scaffold Application Guide for Full Thickness Wounds PriMatrix is a unique dermal repair
OHSU Neurological Surgery Education. Neurosurgical Basics. Copyright Oregon Health & Science University Department of Neurological Surgery
 OHSU Neurological Surgery Education Neurosurgical Basics Chapter 1 Basic Principles: Cranial Three-Point Head Fixation Proper and safe application of a three-point rigid cranial fixation device provides
OHSU Neurological Surgery Education Neurosurgical Basics Chapter 1 Basic Principles: Cranial Three-Point Head Fixation Proper and safe application of a three-point rigid cranial fixation device provides
The natural history of the Chiari Type I anomaly
 See the corresponding editorial in this issue, pp 177 178. J Neurosurg Pediatrics 2:179 187, 2008 The natural history of the Chiari Type I anomaly FEDERICA NOVEGNO, M.D., MASSIMO CALDARELLI, M.D., ANTONIO
See the corresponding editorial in this issue, pp 177 178. J Neurosurg Pediatrics 2:179 187, 2008 The natural history of the Chiari Type I anomaly FEDERICA NOVEGNO, M.D., MASSIMO CALDARELLI, M.D., ANTONIO
Unilateral extended suboccipital approach for a C1 dumbbell schwanoma
 38 Gorgan et al Unilateral extended suboccipital approach for a C1 dumbbell schwanoma Unilateral extended suboccipital approach for a C1 dumbbell schwanoma R.M. Gorgan, Angela Neacşu, A. Giovani Clinical
38 Gorgan et al Unilateral extended suboccipital approach for a C1 dumbbell schwanoma Unilateral extended suboccipital approach for a C1 dumbbell schwanoma R.M. Gorgan, Angela Neacşu, A. Giovani Clinical
Surgical treatment of Chiari I malformation: indications and approaches
 Neurosurg Focus 11 (1):Article 2, 2001, Click here to return to Table of Contents Surgical treatment of Chiari I malformation: indications and approaches TORD D. ALDEN, M.D., JEFFREY G. OJEMANN, M.D.,
Neurosurg Focus 11 (1):Article 2, 2001, Click here to return to Table of Contents Surgical treatment of Chiari I malformation: indications and approaches TORD D. ALDEN, M.D., JEFFREY G. OJEMANN, M.D.,
Incidental durotomy during spine surgery occurs in. Computed tomography guided epidural patching of postoperative cerebrospinal fluid leaks
 J Neurosurg Spine 21:805 810, 2014 AANS, 2014 Computed tomography guided epidural patching of postoperative cerebrospinal fluid leaks Clinical article Frank Mihlon, M.D., Peter G. Kranz, M.D., Andreia
J Neurosurg Spine 21:805 810, 2014 AANS, 2014 Computed tomography guided epidural patching of postoperative cerebrospinal fluid leaks Clinical article Frank Mihlon, M.D., Peter G. Kranz, M.D., Andreia
Chiari bridges review Chiari Treatments & Potential Pitfalls
 Chiari bridges review Chiari Treatments & Potential Pitfalls Once diagnosed, you will usually be referred to a specialist (not a Chiari Specialist, but an everyday, run-of-the-mill neurologist or neurosurgeon).
Chiari bridges review Chiari Treatments & Potential Pitfalls Once diagnosed, you will usually be referred to a specialist (not a Chiari Specialist, but an everyday, run-of-the-mill neurologist or neurosurgeon).
The Synthetic Self-Adhesive Barrier to Cerebrospinal Fluid Leaks
 The Synthetic SelfAdhesive Barrier to Cerebrospinal Fluid Leaks TissuePatchl has a fused multilaminate structure containing patented reactive polymers for chemical bonding to tissue surface, and a nonadhesive
The Synthetic SelfAdhesive Barrier to Cerebrospinal Fluid Leaks TissuePatchl has a fused multilaminate structure containing patented reactive polymers for chemical bonding to tissue surface, and a nonadhesive
Occipital flattening in the infant skull
 Occipital flattening in the infant skull Kant Y. Lin, M.D., Richard S. Polin, M.D., Thomas Gampper, M.D., and John A. Jane, M.D., Ph.D. Departments of Plastic Surgery and Neurological Surgery, University
Occipital flattening in the infant skull Kant Y. Lin, M.D., Richard S. Polin, M.D., Thomas Gampper, M.D., and John A. Jane, M.D., Ph.D. Departments of Plastic Surgery and Neurological Surgery, University
Morphometric Analysis of Left & Right Tonsils in Adult Symptomatic Type 1 Chiari Patients and Healthy Controls
 The University of Akron IdeaExchange@UAkron Honors Research Projects The Dr. Gary B. and Pamela S. Williams Honors College Spring 2015 Morphometric Analysis of Left & Right Tonsils in Adult Symptomatic
The University of Akron IdeaExchange@UAkron Honors Research Projects The Dr. Gary B. and Pamela S. Williams Honors College Spring 2015 Morphometric Analysis of Left & Right Tonsils in Adult Symptomatic
Chiari malformation Type I (CM-I) in children is
 PEDIATRICS clinical article J Neurosurg Pediatr 16:159 166, 2015 A prospective natural history study of nonoperatively managed Chiari I malformation: does follow-up MRI surveillance alter surgical decision
PEDIATRICS clinical article J Neurosurg Pediatr 16:159 166, 2015 A prospective natural history study of nonoperatively managed Chiari I malformation: does follow-up MRI surveillance alter surgical decision
Published clinical papers
 Published clinical papers Published clinical papers (Neuro) Use of a new absorbable sealing film for preventing postoperative cerebrospinal fluid leaks: remarks on a new approach. (12 cranial cases) A
Published clinical papers Published clinical papers (Neuro) Use of a new absorbable sealing film for preventing postoperative cerebrospinal fluid leaks: remarks on a new approach. (12 cranial cases) A
DELINEATION OF PRIVILEGES - NEUROSURGERY
 KALEIDA HEALTH Name Date DELINEATION OF PRIVILEGES - NEUROSURGERY PLEASE NOTE: Please check the box for each privilege requested. Do not use an arrow or line to make selections. We will return applications
KALEIDA HEALTH Name Date DELINEATION OF PRIVILEGES - NEUROSURGERY PLEASE NOTE: Please check the box for each privilege requested. Do not use an arrow or line to make selections. We will return applications
Case Log Mapping Update: April 2018 Review Committee for Neurological Surgery
 Case Log Mapping Update: April 2018 Review Committee for Neurological Surgery The Review Committee has made the following changes to the CPT code mappings: The following previously untracked CPT codes
Case Log Mapping Update: April 2018 Review Committee for Neurological Surgery The Review Committee has made the following changes to the CPT code mappings: The following previously untracked CPT codes
Curriculum Vitae. Date March 1, Contact Information
 Curriculum Vitae Mark S. Greenberg, MD, FAANS Associate Professor, Department of Neurosurgery and Brain Repair University of South Florida, Morsani College of Medicine Date March 1, 2018 Contact Information
Curriculum Vitae Mark S. Greenberg, MD, FAANS Associate Professor, Department of Neurosurgery and Brain Repair University of South Florida, Morsani College of Medicine Date March 1, 2018 Contact Information
NewBridge. Laminoplasty Fixation INTERNATIONAL EDITION
 NewBridge L A M I N O P L A S T Y F I X A T I O N S Y S T E M Laminoplasty Fixation INTERNATIONAL EDITION Table of Contents 1 INTRODUCTION 2 PRE-OPERATIVE 3 OPERATIVE 10 INSTRUCTIONS FOR USE 12 PART NUMBERS
NewBridge L A M I N O P L A S T Y F I X A T I O N S Y S T E M Laminoplasty Fixation INTERNATIONAL EDITION Table of Contents 1 INTRODUCTION 2 PRE-OPERATIVE 3 OPERATIVE 10 INSTRUCTIONS FOR USE 12 PART NUMBERS
Safety and efficacy of a novel polyethylene glycol hydrogel sealant for watertight dural repair
 J Neurosurg 106:52 58, 2007 Safety and efficacy of a novel polyethylene glycol hydrogel sealant for watertight dural repair G. REES COSGROVE, M.D., F.R.C.S.C., 1 JOHNNY B. DELASHAW, M.D., 2 J. ANDRE GROTENHUIS,
J Neurosurg 106:52 58, 2007 Safety and efficacy of a novel polyethylene glycol hydrogel sealant for watertight dural repair G. REES COSGROVE, M.D., F.R.C.S.C., 1 JOHNNY B. DELASHAW, M.D., 2 J. ANDRE GROTENHUIS,
Lumbar puncture. Invasive procedure: diagnostic or therapeutic. The subarachnoid space 4-13 ys: ml Replenished: 4-6 h Routine LP (3-5 ml): <1h
 Lumbar puncture Lumbar puncture Invasive procedure: diagnostic or therapeutic. The subarachnoid space 4-13 ys: 65-150ml Replenished: 4-6 h Routine LP (3-5 ml):
Lumbar puncture Lumbar puncture Invasive procedure: diagnostic or therapeutic. The subarachnoid space 4-13 ys: 65-150ml Replenished: 4-6 h Routine LP (3-5 ml):
KEY WORDS algorithm; predictive model analysis; postsurgical improvement; outcome; Chiari Type I malformation; congenital; syringomyelia
 CLINICAL ARTICLE J Neurosurg Spine 28:23 32, 2018 A points-based algorithm for prognosticating clinical outcome of Chiari malformation Type I with syringomyelia: results from a predictive model analysis
CLINICAL ARTICLE J Neurosurg Spine 28:23 32, 2018 A points-based algorithm for prognosticating clinical outcome of Chiari malformation Type I with syringomyelia: results from a predictive model analysis
PARADIGM SPINE. Minimally Invasive Lumbar Fusion. Interlaminar Stabilization
 PARADIGM SPINE Minimally Invasive Lumbar Fusion Interlaminar Stabilization 2 A UNIQUE MIS ALTERNATIVE TO PEDICLE SCREW FIXATION The Gold Standard The combined use of surgical decompression and different
PARADIGM SPINE Minimally Invasive Lumbar Fusion Interlaminar Stabilization 2 A UNIQUE MIS ALTERNATIVE TO PEDICLE SCREW FIXATION The Gold Standard The combined use of surgical decompression and different
ProDisc-C versus fusion with Cervios chronos prosthesis in cervical degenerative disc disease: Is there a difference at 12 months?
 Original research ProDisc-C versus fusion with Cervios chronos prosthesis in cervical degenerative disc ( ) 51 51 56 ProDisc-C versus fusion with Cervios chronos prosthesis in cervical degenerative disc
Original research ProDisc-C versus fusion with Cervios chronos prosthesis in cervical degenerative disc ( ) 51 51 56 ProDisc-C versus fusion with Cervios chronos prosthesis in cervical degenerative disc
Case Report Primary Dural Repair in Minimally Invasive Spine Surgery
 Case Reports in Medicine Volume 2013, Article ID 876351, 6 pages http://dx.doi.org/10.1155/2013/876351 Case Report Primary Dural Repair in Minimally Invasive Spine Surgery Raqeeb M. Haque, Sohaib Z. Hashmi,
Case Reports in Medicine Volume 2013, Article ID 876351, 6 pages http://dx.doi.org/10.1155/2013/876351 Case Report Primary Dural Repair in Minimally Invasive Spine Surgery Raqeeb M. Haque, Sohaib Z. Hashmi,
Lyoplant Onlay. Adding another layer of confidence to every procedure. Aesculap Neurosurgery
 Adding another layer of confidence to every procedure Aesculap Neurosurgery Adding another layer of confidence to every procedure For more than 140 years, Aesculap Neurosurgery has developed products that
Adding another layer of confidence to every procedure Aesculap Neurosurgery Adding another layer of confidence to every procedure For more than 140 years, Aesculap Neurosurgery has developed products that
ADVANCED BONE GRAFT SYSTEM OVERVIEW
 ADVANCED BONE GRAFT SYSTEM OVERVIEW NANOSS BIOACTIVE ADVANCED BONE GRAFT Table Of Contents INTRODUCTION System Overview... 1 NANOSS BIOACTIVE COMPONENTS Comparison of nanoss Bioactive and Human Bone...
ADVANCED BONE GRAFT SYSTEM OVERVIEW NANOSS BIOACTIVE ADVANCED BONE GRAFT Table Of Contents INTRODUCTION System Overview... 1 NANOSS BIOACTIVE COMPONENTS Comparison of nanoss Bioactive and Human Bone...
Inion BioRestore. Bone Graft Substitute. Product Overview
 Inion BioRestore Bone Graft Substitute Product Overview Inion BioRestore Introduction Inion BioRestore is a synthetic bone graft substitute, which remodels into bone and is easy to use. Inion BioRestore
Inion BioRestore Bone Graft Substitute Product Overview Inion BioRestore Introduction Inion BioRestore is a synthetic bone graft substitute, which remodels into bone and is easy to use. Inion BioRestore
Integra TenoGlide Tendon Protector Sheet
 Integra Use of TenoGlide Tendon Protector Sheet as an Interface to Protect Extensor Tendons after Removal of Hardware from Multiple Metacarpal Fractures 1 CASE STUDY Use of Integra TenoGlide Tendon Protector
Integra Use of TenoGlide Tendon Protector Sheet as an Interface to Protect Extensor Tendons after Removal of Hardware from Multiple Metacarpal Fractures 1 CASE STUDY Use of Integra TenoGlide Tendon Protector
CorMatrix ECM Bioscaffold
 CorMatrix ECM Bioscaffold REMODEL. REGROW. RESTORE. CorMatrix ECM Bioscaffold provides a natural bioscaffold matrix that enables the body s own cells to repair and remodel damaged cardio-vascular tissue.
CorMatrix ECM Bioscaffold REMODEL. REGROW. RESTORE. CorMatrix ECM Bioscaffold provides a natural bioscaffold matrix that enables the body s own cells to repair and remodel damaged cardio-vascular tissue.
Enhancement of Cranial US: Utility of Supplementary Acoustic Windows and Doppler Harriet J. Paltiel, MD
 Enhancement of Cranial US: Utility of Supplementary Acoustic Windows and Doppler Harriet J. Paltiel, MD Boston Children s Hospital Harvard Medical School None Disclosures Conventional US Anterior fontanelle
Enhancement of Cranial US: Utility of Supplementary Acoustic Windows and Doppler Harriet J. Paltiel, MD Boston Children s Hospital Harvard Medical School None Disclosures Conventional US Anterior fontanelle
PATIENT INFORMATION BROCHURE
 PATIENT INFORMATION BROCHURE PATIENT INFORMATION BROCHURE This brochure is designed to help you make an informed decision about your surgery. Please read this entire document carefully. Keep this document
PATIENT INFORMATION BROCHURE PATIENT INFORMATION BROCHURE This brochure is designed to help you make an informed decision about your surgery. Please read this entire document carefully. Keep this document
SMARTBRANE SIMPLE RELIABLE PURE NEW! Resorbable Pericardium Membrane MORE ECONOMIC. THE SMALLEST MEMBRANE 10 x 10 mm. 10 x10
 SMARTBRANE Resorbable Pericardium Membrane 10 x10 NEW! THE SMALLEST MEMBRANE 10 x 10 mm SIMPLE RELIABLE PURE MORE ECONOMIC SIMPLE Optimized handling properties ensuring straight-forward application The
SMARTBRANE Resorbable Pericardium Membrane 10 x10 NEW! THE SMALLEST MEMBRANE 10 x 10 mm SIMPLE RELIABLE PURE MORE ECONOMIC SIMPLE Optimized handling properties ensuring straight-forward application The
Spontaneous intracranial hypotension syndrome with extracranial vein dilatation
 Spontaneous intracranial hypotension syndrome with extracranial vein dilatation C. Heine 1, H. Altinova 1, T. Pietilä 1, LC Stavrinou 1 1. Department of Neurosurgery, Evangelical Hospital Bielefeld, Germany
Spontaneous intracranial hypotension syndrome with extracranial vein dilatation C. Heine 1, H. Altinova 1, T. Pietilä 1, LC Stavrinou 1 1. Department of Neurosurgery, Evangelical Hospital Bielefeld, Germany
Managing life with neurological symptoms
 Managing life with neurological symptoms Petra M Klinge Professor of Neurosurgery Director, Division of Pediatric Neurosurgery Director, CSF center of the brain and Spine Rhode Island Hospital and Hasbro
Managing life with neurological symptoms Petra M Klinge Professor of Neurosurgery Director, Division of Pediatric Neurosurgery Director, CSF center of the brain and Spine Rhode Island Hospital and Hasbro
NeuroMend. Collagen Wrap Conduits. Operative Technique
 NeuroMend Collagen Wrap Conduits Operative Technique Stryker NeuroMend Collagen Wrap Conduits Wrap a protective environment around your patients injured peripheral nerves Common Indications The NeuroMend
NeuroMend Collagen Wrap Conduits Operative Technique Stryker NeuroMend Collagen Wrap Conduits Wrap a protective environment around your patients injured peripheral nerves Common Indications The NeuroMend
Unintended durotomy during degenerative lumbar spine surgery (Incidence and management)
 Original Article Unintended durotomy during degenerative lumbar spine surgery * Ali T. AbdulWahid** Ammar Salah*** FRCS MBChB, FIBMS MBChB, FIBMS Fac Med Baghdad 2014 Vol.56, No.4 Received: Sept., 2014
Original Article Unintended durotomy during degenerative lumbar spine surgery * Ali T. AbdulWahid** Ammar Salah*** FRCS MBChB, FIBMS MBChB, FIBMS Fac Med Baghdad 2014 Vol.56, No.4 Received: Sept., 2014
Spine Surgery: Techniques, Complication Avoidance, and Management. 2 Volume Set
 Spine Surgery: Techniques, Complication Avoidance, and Management. 2 Volume Set Benzel, E ISBN-13: 9781437705874 Table of Contents SECTION 1 - HISTORY 1 - History 2 - History of Spine Instrumentation -
Spine Surgery: Techniques, Complication Avoidance, and Management. 2 Volume Set Benzel, E ISBN-13: 9781437705874 Table of Contents SECTION 1 - HISTORY 1 - History 2 - History of Spine Instrumentation -
Patient Information Brochure
 Patient Information Brochure Patient Information Brochure This brochure is designed to help you make an informed decision about your surgery. Please read this entire document carefully. Keep this document
Patient Information Brochure Patient Information Brochure This brochure is designed to help you make an informed decision about your surgery. Please read this entire document carefully. Keep this document
IIH, previously known as pseudotumor cerebri, is a syndrome
 ORIGINAL RESEARCH A.H. Aiken J.A. Hoots A.M. Saindane P.A. Hudgins Incidence of Cerebellar Tonsillar Ectopia in Idiopathic Intracranial Hypertension: A Mimic of the Chiari I Malformation BACKGROUND AND
ORIGINAL RESEARCH A.H. Aiken J.A. Hoots A.M. Saindane P.A. Hudgins Incidence of Cerebellar Tonsillar Ectopia in Idiopathic Intracranial Hypertension: A Mimic of the Chiari I Malformation BACKGROUND AND
Product Information. MIS Corporation. All Rights Reserved.
 Product Information MIS Corporation. All Rights Reserved. MIS Warranty: MIS exercises great care and effort in maintaining the superior quality of its products. All MIS products are guaranteed to be free
Product Information MIS Corporation. All Rights Reserved. MIS Warranty: MIS exercises great care and effort in maintaining the superior quality of its products. All MIS products are guaranteed to be free
Intracranial hypotension secondary to spinal CSF leak: diagnosis
 Intracranial hypotension secondary to spinal CSF leak: diagnosis Spinal cerebrospinal fluid (CSF) leak is an important and underdiagnosed cause of new onset headache that is treatable. Cerebrospinal fluid
Intracranial hypotension secondary to spinal CSF leak: diagnosis Spinal cerebrospinal fluid (CSF) leak is an important and underdiagnosed cause of new onset headache that is treatable. Cerebrospinal fluid
Long-term outcome after surgery for Chiari I malformation
 Acta Neurol Scand 2009: 120: 295 299 DOI: 10.1111/j.1600-0404.2009.01183.x Copyright Ó 2009 The Authors Journal compilation Ó 2009 Blackwell Munksgaard ACTA NEUROLOGICA SCANDINAVICA Long-term outcome after
Acta Neurol Scand 2009: 120: 295 299 DOI: 10.1111/j.1600-0404.2009.01183.x Copyright Ó 2009 The Authors Journal compilation Ó 2009 Blackwell Munksgaard ACTA NEUROLOGICA SCANDINAVICA Long-term outcome after
Guidelines in the management of neural tube defects and hydrocephalus
 Guidelines in the management of neural tube defects and hydrocephalus Dominic Venne, MD, MSc, FRCSC, Division of Neurosurgery Sheikh Khalifa Medical City Abu Dhabi, UAE 1. Introduction: Neural tube defects
Guidelines in the management of neural tube defects and hydrocephalus Dominic Venne, MD, MSc, FRCSC, Division of Neurosurgery Sheikh Khalifa Medical City Abu Dhabi, UAE 1. Introduction: Neural tube defects
Patient Guide to Neck Surgery
 The following is a sampling of products offered by Zimmer Spine for use in Posterior Cervical Fusion procedures. Patient Guide to Neck Surgery Posterior Cervical Fusion NexPosure NexPosure MIS Access provides
The following is a sampling of products offered by Zimmer Spine for use in Posterior Cervical Fusion procedures. Patient Guide to Neck Surgery Posterior Cervical Fusion NexPosure NexPosure MIS Access provides
Complications in Adult Spinal Deformity Surgery
 Complications in Adult Spinal Deformity Surgery Jacob M. Buchowski, M.D., M.S. Professor of Orthopaedic and Neurological Surgery Director, Washington University Spine Fellowship Director, Center for Spinal
Complications in Adult Spinal Deformity Surgery Jacob M. Buchowski, M.D., M.S. Professor of Orthopaedic and Neurological Surgery Director, Washington University Spine Fellowship Director, Center for Spinal
Osteoarthrosis, unspecified whether generalized or localized, lower leg. Osteoarthrosis, localized, not specified whether primary or secondary, pelvic
 Page 1 Appendix TABLE E-1 Codes (and Definitions) in Humana Database Used for Study Inclusion and Exclusion of Patients Who Underwent,, or 1 to 2-Level Inclusion ICD-9-P-8154 Total knee replacement ICD-9-D-71596
Page 1 Appendix TABLE E-1 Codes (and Definitions) in Humana Database Used for Study Inclusion and Exclusion of Patients Who Underwent,, or 1 to 2-Level Inclusion ICD-9-P-8154 Total knee replacement ICD-9-D-71596
ENCORE, a Study to Investigate the Durability of Polymer EVAR with Ovation A Contemporary Review of 1296 Patients
 ENCORE, a Study to Investigate the Durability of Polymer EVAR with Ovation A Contemporary Review of 1296 Patients The Ovation System is approved to treat infrarenal abdominal aortic aneurysms and is not
ENCORE, a Study to Investigate the Durability of Polymer EVAR with Ovation A Contemporary Review of 1296 Patients The Ovation System is approved to treat infrarenal abdominal aortic aneurysms and is not
