Forigerimod plus standard of care for systemic lupus erythematosus
|
|
|
- Sydney Randall
- 5 years ago
- Views:
Transcription
1 NIHR Innovation Observatory Evidence Briefing: March 2018 Forigerimod plus standard of care for systemic lupus erythematosus NIHRIO (HSRIC) ID: 2313 NICE ID: 9732 LAY SUMMARY Systemic lupus erythematosus (SLE) is a long-term condition causing inflammation to the joints, skin and other organs. Symptoms presented are usually very general: fever, joint pain, skin rash but can progress to the most severe: kidney failure. SLE typically has patterns of flare-ups where the condition gets worse for a period of time. The disease is likely to be caused by a combination of genetic and lifestyle factors and most commonly affects middle-aged women and those who belong to an ethnic minority group such as African-Caribbean. Treatment for SLE is currently aimed at controlling or easing the symptoms associated with the disease. Forigerimod is being developed for the treatment of SLE in combination with standard of care. Forigerimod is being developed as an injection given every 4 weeks and may be considered beneficial compared to current treatments. It is expected to fight the body s dysfunctional immune system by the earlier activation of T-cells. Therefore if licensed it will be a potential additional treatment for SLE. This briefing reflects the evidence available at the time of writing. A version of the briefing was sent to the company for a factual accuracy check. The company was unavailable to provide comment. It is not intended to be a definitive statement on the safety, efficacy or effectiveness of the health technology covered and should not be used for commercial purposes or commissioning without additional information. 1 This briefing presents independent research funded by the National Institute for Health Research (NIHR). The views expressed are those of the author and not necessarily those of the NHS, the NIHR or the Department of Health.
2 TARGET GROUP Systemic lupus erythematosus (SLE) TECHNOLOGY DESCRIPTION Forigerimod (Lupuzur; IPP ; P140) is a 21-mer linear peptide which comes from the small nuclear ribonucleoprotein U1-70K and is phosphorylated at the Ser140 position. 1 Its unique mechanism of action involves modulating the activation of auto-reactive T-cells. In targeting upstream T-cell activation, forigerimod presents a novel approach in modulating this unwelcome autoimmune reaction. This targeted approach marks a paradigm shift in treating autoimmune disease. Instead of shutting down otherwise healthy immune responses the T-cells are suppressed, leaving the immune system deleted from unwanted deleterious cells but intact as normal, beneficial immune cells are concerned. 2 Current treatment for SLE is often palliative rather than curative as corticosteroids and other immunosuppressive agents are generally given to control and ease the symptoms associated with condition. 1,3 Forigerimod is currently in phase III clinical trial development (pivotal and extension) for the treatment of SLE. 4,5 In the pivotal phase III trial forigerimod is administered as a subcutaneous injection, in combination with standard of care at a dose of 200 mcg every 4 weeks, for 48 weeks in total. A total of 13 doses is expected to be administered. 4 Forigerimod does not currently have Marketing Authorisation in the EU for any indication and is not being developed for any other indication. INNOVATION and/or ADVANTAGES Forigerimod has a novel mechanism of action which involves auto-reactive T-cell activation, specifically targeting the upstream of T-cell activation. This helps combat the body s compromised immune system. 2 Additionally forigerimod is distinctly different from other approved treatments for SLE as it is a small peptide as opposed to a monoclonal antibody and its dosing is relatively small and is only required every few weeks. 6 Current treatments for SLE consists of drugs which have many side-effects and limited efficacy, 2 therefore if licensed, forigerimod could offer an additional treatment for SLE. ImmuPharma DEVELOPER 2
3 PATIENT GROUP BACKGROUND SLE is a complex autoimmune disease that affects numerous bodily systems and organs. 3,7 Mild SLE is marked by generalised symptoms such as headaches, fever, muscle and joint pain and skin rash, whilst severe SLE is characterised by significant morbidity such as renal damage, haemolytic anaemia, thrombocytopenic purpura and central nervous system abnormalities. 3, 8 SLE can involve various relapsing and remitting episodes which results in flare-ups that cause an accumulation of damage to the body s tissues, increasing the risk of infection, cardiovascular disease and even death. 3,8 Patients with late-onset SLE ( 50 years) tend to have a more insidious onset of disease with severe manifestations being infrequent, however they are also more likely to have greater damage at diagnosis, a higher frequency of comorbidities and a higher risk of premature mortality than those with an earlier onset of SLE. 9 Severe SLE may manifest into lupus nephritis, which is difficult to treat and can lead to permanent kidney damage, and end-stage renal disease. 8 Treatment aimed at controlling nephritis involves cytotoxic therapeutic regimes which consequently leads to significant morbidity and mortality. 1 Whilst the cause of SLE is not fully understood, genetics is considered to have a strong role in its development along with other lifestyle and environmental factors. 10 SLE is genetically complex and is characterised by the production of multiple autoantibodies which may contribute to the similarities being drawn between its own clinical manifestations and those of other inflammatory conditions such as arthritis. 2,11 This can create difficulty in the diagnosis and monitoring of the condition. 7 The role of gender and socioeconomic factors are further identified as being strong risk factors for developing SLE, with the disease being more prominent in ethnic minority groups and in women. 12 CLINICAL NEED and BURDEN OF DISEASE Gender is considered the strongest risk factor for SLE development, with most studies containing predominantly all-female (90%) populations. 12 Furthermore, the incidence in females is approximately 5.8 times that of males (8.34 per 100,000) per year compared to 1.44 per 100,000 per year. The peak age of incidence for females was between years whilst SLE occurred much later in life in males at years. In the UK, for , the incidence rate of SLE was 4.91 per 100,000 people and the prevalence rate was per 100,000 people. As gender is a strong risk factor in the development of SLE, the highest incidence rates are observed in those of African-Caribbean descent: 31.4 per 100,000 people per year, compared with 6.7 per 100,000 per year for those of white European descent. 13 In the UK, death from active lupus is rare, however a 10% mortality rate and a mean age of death at 53 years old, has recently been reported. Approximately one-third of patients with SLE go on to develop lupus nephritis. 9 In there were 4,734 admissions for SLE (ICD 10: M32) which resulted in 5,299 finished consultant episodes (FCE) and 7,608 FCE bed days. 14 3
4 PATIENT PATHWAY RELEVANT GUIDANCE NICE GUIDANCE NICE technology appraisal guidance in development. Systemic lupus erythematosus prasterone (ID392). Expected date of issue to be confirmed. NICE technology appraisal guidance. Belimumab for treating active autoantibody-positive systemic lupus erythematosus (TA397). June NHS ENGLAND and POLICY GUIDANCE NHS England. 2013/14 NHS Standard Contract for Specialised Rheumatology Services (Adult). A13/S/a. NHS England. 2013/14 NHS Interim Clinical Commissioning Policy Statement: Rituximab for the treatment of Systemic Lupus Erythematosus in adults. A13/PS/a. OTHER GUIDANCE British Society for Rheumatology (BSR). Guideline for the management of adults with Systemic Lupus Erythematosus CURRENT TREATMENT OPTIONS Currently there is no cure for SLE, therefore the aim of treatment is to control and ease the symptoms associated with the disease. 3,15 Treatment is more successful when initiated at the earliest stage of the disease, and is dictated by the severity of the disease, the part of the body affected and depending on flare-ups. 15 Standard therapy includes the use of non-steroidal anti-inflammatory drugs; corticosteroids such as prednisolone; disease-modifying drugs such as hydroxychloroquine; and immunosuppressants such as azathioprine, cyclophosphamide, methotrexate and mycophenolate mofetil. Rituximab is also considered as a treatment option, particularly in the case of more severe disease, and is covered by an interim clinical commissioning policy statement by NHS England for certain people with the disease. Belimumab is licensed for add-on therapy in adults with active, autoantibody-positive systemic lupus erythematosus with a high degree of disease activity. 3 It is important to be able to manage immunosuppressive therapies and their potential toxicities in SLE patients, although it can be a considerable challenge due to the risk of infection, difficulties with attribution of cytopenias to lupus or cytotoxic drugs, and difficulties in distinguishing manifestations of lupus disease activity from damage and co-morbid conditions. 8 4
5 Trial EFFICACY and SAFETY LUPUZOR, NCT ; forigerimod plus standard of care vs placebo plus standard of care; phase III IP-006, NCT : forigerimod plus standard of care; phase III extension Sponsor ImmuPharma ImmuPharma Status Complete but unpublished Ongoing Source of Trial registry 4 Trial registry 5 Information Location EU (not incl UK), USA and other countries EU (not incl UK), USA and other countries Design Participants Schedule Randomised, double-blind, placebocontrolled study n=200; aged years; active systemic lupus erythematosus Randomised to forigerimod at a dose of 200 mcg every 4 weeks in combination with standard of care for 48 weeks in total or placebo in combination with standard of care for 48 weeks in total. A total of 13 doses will be administered with the experimental or placebo product. Non-randomised, open-label study n=100 (planned); 18 years; active systemic lupus erythematosus Receive forigerimod at a dose of 200 mcg every 4 weeks in combination with standard of care for 24 weeks. Follow-up Active treatment period for 48 weeks. Primary Assessment of SLE Responder Index Outcomes (SRI) at week 52 Active treatment period for 24 weeks. Occurrence of adverse events throughout the study [Time Frame: 7 months] Clinical laboratory test results at extension period [Time Frame: 7 months] Body weight measurements at period [Time Frame: 7 months] Temperature measurements at period [Time Frame: 7 months] Pulse measurements at each [Time Frame: 7 months] Systolic and diastolic blood pressures measurements at each 5
6 Secondary Outcomes SLE Responder Index (SRI) response at each visit during the study [Time Frame: baseline, weeks 4, 8, 12, 16, 20, 24, 28, 32, 36, 40, 44, 48 and Reduction in the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) total score by at least 4 points at each [Time Frame: baseline, weeks 4, 8, 12, 16, 20, 24, 28, 32, 36, 40, 44, 48 and Assessment of the British Isles Lupus Assessment Group (BILAG- 2004) disease activity index, at period [Time Frame: baseline, weeks 4, 8, 12, 16, 20, 24, 28, 32, 36, 40, 44, 48 and Status of disease by Physician's Global Assessment (PhGA scale) at each visit during the treatment period [Time Frame: baseline, weeks 4, 8, 12, 16, 20, 24, 28, 32, 36, 40, 44, 48 and Reduction of the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) total score by at least 5 points at each [Time Frame: baseline, weeks 4, 8, 12, 16, 20, 24, 28, 32, 36, 40, 44, 48 and Reduction of the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) total [Time Frame: 7 months] 2-lead electrocardiogram (ECG) findings at week 28 (or final assessment) [Time Frame: 7 months] Physical examination findings, at specified time points at each visit during the treatment extension period [Time Frame: 7 months] Concomitant medication usage throughout the study extension [Time Frame: 7 months] Effect in the Clinical SLEDAI-2K total score by at final visit compared to initial visit [Time Frame: at week 28] Remission of the disease (i.e reduction of clinical SLEDAI-2K score to 0) [Time Frame: at week 28] 6
7 score by at least 6 points at each [Time Frame: baseline, weeks 4, 8, 12, 16, 20, 24, 28, 32, 36, 40, 44, 48 and Systemic Lupus Erythematosus Responder Index (SRI-5) response at each visit during the treatment period [Time Frame: baseline, weeks 4, 8, 12, 16, 20, 24, 28, 32, 36, 40, 44, 48 and Systemic Lupus Erythematosus RI-6 response at each visit during the treatment period [Time Frame: baseline, weeks 4, 8, 12, 16, 20, 24, 28, 32, 36, 40, 44, 48 and Assessment by the 28-joint count examination for pain and tenderness at each visit during the treatment period [Time Frame: baseline, weeks 4, 8, 12, 16, 20, 24, 28, 32, 36, 40, 44, 48 and Incidence of disease flares at period [Time Frame: baseline, weeks 4, 8, 12, 16, 20, 24, 28, 32, 36, 40, 44, 48 and Occurrence of Systemic Lupus Erythematosus-induced organ damage at visits at weeks 24 and 52 (or final assessment) [Time Frame: baseline, weeks 24 and Assessment of health-related quality of life by completion of the Medical Outcome Survey Short Form 36 (SF-36) at visits at weeks 12, 24, 36, and 52 (or final assessment) [Time Frame: baseline, weeks 12, 24, 36 and Steroid dose over time throughout the study. [Time Frame: baseline, weeks 4, 8, 12, 16, 20, 24, 28, 32, 36, 40, 44, 48 and activity assessment at each visit during the treatment period 7
8 [Time Frame: baseline, weeks 4, 8, 12, 16, 20, 24, 28, 32, 36, 40, 44, 48 and activity assessment at visits at weeks 4, 12, 24, 36, and 52 (or final assessment) [Time Frame: activity assessment at visits at weeks 4, 12, 24, 36, and 52 (or final assessment) [Time Frame: activity assessment at visits at weeks 4, 12, 24, 36, and 52 (or final assessment) [Time Frame: activity assessment at visits at weeks 4, 12, 24, 36, and 52 (or final assessment) [Time Frame: activity assessment at visits at weeks 4, 12, 24, 36, and 52 (or final assessment) [Time Frame: Assessment of fatigue using the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) scale at visits at weeks 12, 24, 36, and 52 (or final assessment) [Time Frame: In vitro intracellular and cytokine response [Time Frame: baseline, weeks 4, 24 and Occurrence of adverse events throughout the study [Time Frame: baseline, weeks 2, 4, 8, 12, 16, 20, 24, 28, 32, 36, 40, 44, 48 and Clinical laboratory test results at 8
9 period [Time Frame: baseline, weeks 4, 8, 12, 16, 20, 24, 28, 32, 36, 40, 44, 48 and Vital signs assessment at each visit [Time Frame: baseline, weeks 2, 4, 8, 12, 16, 20, 24, 28, 32, 36, 40, 44, 48 and 52 ] 12-lead electrocardiogram (ECG) findings at week 52 (or final assessment) [Time Frame: baseline and week Physical examination findings [Time Frame: baseline, weeks 2, 4, 8, 12, 16, 20, 24, 28, 32, 36, 40, 44, 48 and Evaluation for suicidality at each using the Columbia-Suicide Severity Rating Scale (C-SSRS) [Time Frame: baseline, weeks 4, 8, 12, 16, 20, 24, 28, 32, 36, 40, 44, 48 and Concomitant medication usage throughout the study [Time Frame: baseline, weeks 2, 4, 8, 12, 16, 20, 24, 28, 32, 36, 40, 44, 48 and Key Results Not reported - Adverse effects (AEs) Expected reporting date Not reported - Results expected to be reported in Q Primary completion date reported as October 2018 ESTIMATED COST and IMPACT COST The cost of forigerimod is not yet known. IMPACT SPECULATIVE IMPACT ON PATIENTS AND CARERS Reduced mortality/increased length of survival Reduced symptoms or disability 9
10 Other No impact identified IMPACT ON HEALTH and SOCIAL CARE SERVICES Increased use of existing services Decreased use of existing services Re-organisation of existing services Need for new services Other None identified IMPACT ON COSTS and OTHER RESOURCE USE Increased drug treatment costs Reduced drug treatment costs Other increase in costs Other reduction in costs Other: uncertain unit cost compared to existing treatments None identified OTHER ISSUES Clinical uncertainty or other research question identified None identified 10
11 REFERENCES 1 Zimmer R, Scherbarth HR, Rillo OL, Gomez-Reino JJ, Muller S. Lupuzor/P140 peptide in patients with systemic lupus erythematosus: a randomised, double-blind, placebo-controlled phase IIb clinical trial. Annals of the rheumatic diseases Nov 1;72(11): ImmuPharma. Lupuzor. Available from: [Accessed 01 March 2018] 3 National Institute for Health and Care Excellence. Systemic lupus erythematosus: oral mycophenolate. Available from: [Accessed 01 March 2018] 4 ClinicalTrials.gov. A 52-Week, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Study to Evaluate the Efficacy and Safety of a 200-mcg Dose of IPP Plus Standard of Care in Patients With Systemic Lupus Erythematosus (LUPUZOR). Available from: [Accessed 01 March 2018] 5 ClinicalTrials.gov. Study of Repeated Administration of a 200-mcg Dose of IPP Plus Standard of Care in Patients With Systemic Lupus Erythematosus (IP-006). Available from: [Accessed 01 March 2018] 6 Lupuzor. Lupuzor. Available from: [Accessed 01 March 2018] 7 Gordon C, Amissah-Arthur MB, Gayed M et al. The British Society for Rheumatology guideline for the management of systemic lupus erythematosus in adults. Rheumatology Oct;57:1-45. Advance access publication available from: [Accessed 21 February 2018] 8 Fellner C. Immunotherapies in Late-Stage Development for Patients With Severe SLE and/or Lupus Nephritis. Pharmacy and Therapeutics Jun;42(6): Yee CS, Su L, Toescu V, Hickman R, Situnayake D, Bowman S, Farewell V, Gordon C. Birmingham SLE cohort: outcomes of a large inception cohort followed for up to 21 years. Rheumatology Oct 15;54(5): Rare Diseases. Systemic lupus erythematosus. Available from: [Accessed 01 March 2018] 11 Taylor KE, Chung SA, Graham RR, Ortmann WA, Lee AT, Langefeld CD, Jacob CO, Kamboh MI, Alarcón- Riquelme ME, Tsao BP, Moser KL. Risk alleles for systemic lupus erythematosus in a large case-control collection and associations with clinical subphenotypes. PLOS genetics Feb 17;7(2): Pons-Estel GJ, Alarcón GS, Scofield L, Reinlib L, Cooper GS. Understanding the epidemiology and progression of systemic lupus erythematosus. Seminars in arthritis and rheumatism 2010 Feb 1;39(4): Rees F, Doherty M, Grainge M, Davenport G, Lanyon P, Zhang W. The incidence and prevalence of systemic lupus erythematosus in the UK, Annals of the rheumatic diseases Sep;0: Office of National Statistics. Hospital Episodes Statistics Primary diagnosis: 3 character. NHS Digital. Available from: epis stat admi diag tab [Accessed 01 March 2018] 15 Arthritis Research UK. Lupus. Available from: [Accessed 01 March 2018] 11
AR101 peanut allergy immunotherapy for adult and paediatric patients
 AR101 peanut allergy immunotherapy for adult and paediatric patients NIHRIO (HSRIC) ID: 11815 NIHR Innovation Observatory Evidence Briefing: May 2017 NICE ID: 8773 LAY SUMMARY Food allergy occurs when
AR101 peanut allergy immunotherapy for adult and paediatric patients NIHRIO (HSRIC) ID: 11815 NIHR Innovation Observatory Evidence Briefing: May 2017 NICE ID: 8773 LAY SUMMARY Food allergy occurs when
Certolizumab pegol (Cimzia) for psoriatic arthritis second line
 Certolizumab pegol (Cimzia) for psoriatic arthritis second line This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
Certolizumab pegol (Cimzia) for psoriatic arthritis second line This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
Horizon Scanning Centre November Secukinumab for active and progressive psoriatic arthritis. SUMMARY NIHR HSC ID: 5330
 Horizon Scanning Centre November 2012 Secukinumab for active and progressive psoriatic arthritis. SUMMARY NIHR HSC ID: 5330 Secukinumab is a high-affinity fully human monoclonal antibody that antagonises
Horizon Scanning Centre November 2012 Secukinumab for active and progressive psoriatic arthritis. SUMMARY NIHR HSC ID: 5330 Secukinumab is a high-affinity fully human monoclonal antibody that antagonises
KTE-C19 for relapsed or refractory mantle cell lymphoma
 NIHR Innovation Observatory Evidence Briefing: July 2017 KTE-C19 for relapsed or refractory mantle cell lymphoma NIHRIO (HSRIC) ID: 11846 NICE ID: 9122 LAY SUMMARY Mantle cell lymphoma is an uncommon type
NIHR Innovation Observatory Evidence Briefing: July 2017 KTE-C19 for relapsed or refractory mantle cell lymphoma NIHRIO (HSRIC) ID: 11846 NICE ID: 9122 LAY SUMMARY Mantle cell lymphoma is an uncommon type
Horizon Scanning Centre May Tabalumab for systemic lupus erythematosus SUMMARY NIHR HSC ID: 5581
 Horizon Scanning Centre May 2014 Tabalumab for systemic lupus erythematosus SUMMARY NIHR HSC ID: 5581 This briefing is based on information available at the time of research and a limited literature search.
Horizon Scanning Centre May 2014 Tabalumab for systemic lupus erythematosus SUMMARY NIHR HSC ID: 5581 This briefing is based on information available at the time of research and a limited literature search.
Clinical Commissioning Policy Statement: Rituximab For Systemic Lupus Erythematosus (SLE) December Reference : NHSCB/A3C/1b
 Clinical Commissioning Policy Statement: Rituximab For Systemic Lupus Erythematosus (SLE) December 2012 Reference : NHSCB/A3C/1b NHS Commissioning Board Clinical Commissioning Policy Statement: Rituximab
Clinical Commissioning Policy Statement: Rituximab For Systemic Lupus Erythematosus (SLE) December 2012 Reference : NHSCB/A3C/1b NHS Commissioning Board Clinical Commissioning Policy Statement: Rituximab
Certolizumab pegol (Cimzia) for chronic plaque psoriasis in adults
 NIHR Innovation Observatory Evidence Briefing: April 2017 Certolizumab pegol (Cimzia) for chronic plaque psoriasis in adults NIHRIO (HSRIC) ID: 2406 NICE ID: 9112 LAY SUMMARY Plaque psoriasis is the most
NIHR Innovation Observatory Evidence Briefing: April 2017 Certolizumab pegol (Cimzia) for chronic plaque psoriasis in adults NIHRIO (HSRIC) ID: 2406 NICE ID: 9112 LAY SUMMARY Plaque psoriasis is the most
Ustekinumab (Stelara) for psoriatic arthritis second line after disease modifying anti rheumatic drugs (DMARDs)
 Ustekinumab (Stelara) for psoriatic arthritis second line after disease modifying anti rheumatic drugs (DMARDs) January 2010 This technology summary is based on information available at the time of research
Ustekinumab (Stelara) for psoriatic arthritis second line after disease modifying anti rheumatic drugs (DMARDs) January 2010 This technology summary is based on information available at the time of research
Committee Approval Date: May 9, 2014 Next Review Date: May 2015
 Medication Policy Manual Policy No: dru248 Topic: Benlysta, belimumab Date of Origin: May 13, 2011 Committee Approval Date: May 9, 2014 Next Review Date: May 2015 Effective Date: June 1, 2014 IMPORTANT
Medication Policy Manual Policy No: dru248 Topic: Benlysta, belimumab Date of Origin: May 13, 2011 Committee Approval Date: May 9, 2014 Next Review Date: May 2015 Effective Date: June 1, 2014 IMPORTANT
Rituximab (MabThera) maintenance therapy for granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA)
 NIHR Innovation Observatory Evidence Briefing: August 2017 Rituximab (MabThera) maintenance therapy for granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) NIHRIO (HSRIC) ID: 12979
NIHR Innovation Observatory Evidence Briefing: August 2017 Rituximab (MabThera) maintenance therapy for granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) NIHRIO (HSRIC) ID: 12979
Risankizumab (by subcutaneous injection) for moderate to severe chronic plaque psoriasis
 NIHR Innovation Observatory Evidence Briefing: November 2017 Risankizumab (by subcutaneous injection) for moderate to severe chronic plaque psoriasis NIHRIO (HSRIC) ID: 9708 NICE ID: 9191 LAY SUMMARY Plaque
NIHR Innovation Observatory Evidence Briefing: November 2017 Risankizumab (by subcutaneous injection) for moderate to severe chronic plaque psoriasis NIHRIO (HSRIC) ID: 9708 NICE ID: 9191 LAY SUMMARY Plaque
Abatacept (Orencia) for active rheumatoid arthritis. August 2009
 Abatacept (Orencia) for active rheumatoid arthritis August 2009 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
Abatacept (Orencia) for active rheumatoid arthritis August 2009 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
Horizon Scanning Technology Summary. Abatacept (Orencia) for juvenile idiopathic arthritis. National Horizon Scanning Centre.
 Horizon Scanning Technology Summary National Horizon Scanning Centre Abatacept (Orencia) for juvenile idiopathic arthritis June 2007 This technology summary is based on information available at the time
Horizon Scanning Technology Summary National Horizon Scanning Centre Abatacept (Orencia) for juvenile idiopathic arthritis June 2007 This technology summary is based on information available at the time
Horizon Scanning Centre November Enobosarm (Ostarine) for cachexia in patients with advanced non-small cell lung cancer first line
 Horizon Scanning Centre November 2012 Enobosarm (Ostarine) for cachexia in patients with advanced non-small cell lung cancer first line SUMMARY NIHR HSC ID: 5206 This briefing is based on information available
Horizon Scanning Centre November 2012 Enobosarm (Ostarine) for cachexia in patients with advanced non-small cell lung cancer first line SUMMARY NIHR HSC ID: 5206 This briefing is based on information available
Darbepoetin alfa (Aranesp) for treatment of anaemia in adults with low or intermediate-1-risk myelodysplastic syndromes
 NIHR Innovation Observatory Evidence Briefing: August 2017 Darbepoetin alfa (Aranesp) for treatment of anaemia in adults with low or intermediate-1-risk myelodysplastic syndromes NIHRIO (HSRIC) ID: 13763
NIHR Innovation Observatory Evidence Briefing: August 2017 Darbepoetin alfa (Aranesp) for treatment of anaemia in adults with low or intermediate-1-risk myelodysplastic syndromes NIHRIO (HSRIC) ID: 13763
Horizon Scanning Technology Summary. Adalimumab (Humira) for juvenile idiopathic arthritis. National Horizon Scanning Centre.
 Horizon Scanning Technology Summary National Horizon Scanning Centre Adalimumab (Humira) for juvenile idiopathic arthritis June 2007 This technology summary is based on information available at the time
Horizon Scanning Technology Summary National Horizon Scanning Centre Adalimumab (Humira) for juvenile idiopathic arthritis June 2007 This technology summary is based on information available at the time
Technology appraisal guidance Published: 22 June 2016 nice.org.uk/guidance/ta397
 Belimumab for treating active autoantibody-positive systemic lupus erythematosus Technology appraisal guidance Published: 22 June 2016 nice.org.uk/guidance/ta397 NICE 2018. All rights reserved. Subject
Belimumab for treating active autoantibody-positive systemic lupus erythematosus Technology appraisal guidance Published: 22 June 2016 nice.org.uk/guidance/ta397 NICE 2018. All rights reserved. Subject
National Horizon Scanning Centre. Irbesartan (Aprovel) for heart failure with preserved systolic function. August 2008
 Irbesartan (Aprovel) for heart failure with preserved systolic function August 2008 This technology summary is based on information available at the time of research and a limited literature search. It
Irbesartan (Aprovel) for heart failure with preserved systolic function August 2008 This technology summary is based on information available at the time of research and a limited literature search. It
Belinostat (Beleodaq) with CHOP for treatment of patients with peripheral T cell lymphoma, 1st line
 NIHR Innovation Observatory Evidence Briefing: September 2017 Belinostat (Beleodaq) with CHOP for treatment of patients with peripheral T cell lymphoma, 1st line NIHRIO (HSRIC) ID: 12674 NICE ID: 9532
NIHR Innovation Observatory Evidence Briefing: September 2017 Belinostat (Beleodaq) with CHOP for treatment of patients with peripheral T cell lymphoma, 1st line NIHRIO (HSRIC) ID: 12674 NICE ID: 9532
NEOD001 for amyloid light-chain (AL) amyloidosis
 NIHR Innovation Observatory Evidence Briefing: October 2017 NEOD001 for amyloid light-chain (AL) amyloidosis NIHRIO (HSRIC) ID: 10617 NICE ID: 8803 LAY SUMMARY Amyloid light-chain (AL) amyloidosis is caused
NIHR Innovation Observatory Evidence Briefing: October 2017 NEOD001 for amyloid light-chain (AL) amyloidosis NIHRIO (HSRIC) ID: 10617 NICE ID: 8803 LAY SUMMARY Amyloid light-chain (AL) amyloidosis is caused
Botulinum toxin A (Dysport) for hyperhidrosis of the axillae
 April 2016 Horizon Scanning Research & Intelligence Centre Botulinum toxin A (Dysport) for hyperhidrosis of the axillae LAY SUMMARY This briefing is based on information available at the time of research
April 2016 Horizon Scanning Research & Intelligence Centre Botulinum toxin A (Dysport) for hyperhidrosis of the axillae LAY SUMMARY This briefing is based on information available at the time of research
Cabozantinib for medullary thyroid cancer. February 2012
 Cabozantinib for medullary thyroid cancer February 2012 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a definitive
Cabozantinib for medullary thyroid cancer February 2012 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a definitive
Lenti D for adrenoleukodystrophy in boys NIHRIO (HSRIC) ID: 7992 NICE ID: 8732
 NIHR Innovation Observatory Evidence Briefing: May 2017 Lenti D for adrenoleukodystrophy in boys NIHRIO (HSRIC) ID: 7992 NICE ID: 8732 LAY SUMMARY Adrenoleukodystrophy (ALD), otherwise known as Lorenzo
NIHR Innovation Observatory Evidence Briefing: May 2017 Lenti D for adrenoleukodystrophy in boys NIHRIO (HSRIC) ID: 7992 NICE ID: 8732 LAY SUMMARY Adrenoleukodystrophy (ALD), otherwise known as Lorenzo
Horizon Scanning Centre November Faldaprevir with BI for chronic hepatitis C infection, genotype 1 SUMMARY NIHR HSC ID: 7688
 Horizon Scanning Centre November 2012 Faldaprevir with BI 207127 for chronic hepatitis C infection, genotype 1 SUMMARY NIHR HSC ID: 7688 This briefing is based on information available at the time of research
Horizon Scanning Centre November 2012 Faldaprevir with BI 207127 for chronic hepatitis C infection, genotype 1 SUMMARY NIHR HSC ID: 7688 This briefing is based on information available at the time of research
Liraglutide (Victoza) in combination with basal insulin for type 2 diabetes
 Liraglutide (Victoza) in combination with basal insulin for type 2 diabetes May 2011 This technology summary is based on information available at the time of research and a limited literature search. It
Liraglutide (Victoza) in combination with basal insulin for type 2 diabetes May 2011 This technology summary is based on information available at the time of research and a limited literature search. It
B-cell lymphoma vaccine (BiovaxID) for follicular non-hodgkin s lymphoma
 B-cell lymphoma vaccine (BiovaxID) for follicular non-hodgkin s lymphoma May 2010 This technology summary is based on information available at the time of research and a limited literature search. It is
B-cell lymphoma vaccine (BiovaxID) for follicular non-hodgkin s lymphoma May 2010 This technology summary is based on information available at the time of research and a limited literature search. It is
Efficacy and Safety of Belimumab in the treatment of Systemic Lupus Erythematosus: a Prospective Multicenter Study.
 1. Title Efficacy and Safety of Belimumab in the treatment of Systemic Lupus Erythematosus: a Prospective Multicenter Study. 2. Background Systemic Lupus Erythematosus (SLE) is a chronic, autoimmune and
1. Title Efficacy and Safety of Belimumab in the treatment of Systemic Lupus Erythematosus: a Prospective Multicenter Study. 2. Background Systemic Lupus Erythematosus (SLE) is a chronic, autoimmune and
Certolizumab pegol (Cimzia) for the treatment of ankylosing spondylitis second or third line
 Certolizumab pegol (Cimzia) for the treatment of ankylosing spondylitis second or third line August 2011 This technology summary is based on information available at the time of research and a limited
Certolizumab pegol (Cimzia) for the treatment of ankylosing spondylitis second or third line August 2011 This technology summary is based on information available at the time of research and a limited
NIHR Innovation Observatory Evidence Briefing: November 2017
 NIHR Innovation Observatory Evidence Briefing: November 2017 Upadacitinib for adults with moderate to severe active rheumatoid arthritis after conventional synthetic disease-modifying anti-rheumatic drugs
NIHR Innovation Observatory Evidence Briefing: November 2017 Upadacitinib for adults with moderate to severe active rheumatoid arthritis after conventional synthetic disease-modifying anti-rheumatic drugs
Horizon Scanning Centre January Apremilast for psoriatic arthritis SUMMARY NIHR HSC ID: 3716
 Horizon Scanning Centre January 2013 Apremilast for psoriatic arthritis SUMMARY NIHR HSC ID: 3716 This briefing is based on information available at the time of research and a limited literature search.
Horizon Scanning Centre January 2013 Apremilast for psoriatic arthritis SUMMARY NIHR HSC ID: 3716 This briefing is based on information available at the time of research and a limited literature search.
Setmelanotide for pro-opiomelanocortin deficiency obesity
 NIHR Innovation Observatory Evidence Briefing: September 2017 Setmelanotide for pro-opiomelanocortin deficiency obesity NIHRIO (HSRIC) ID: 8496 NICE ID: 9505 LAY SUMMARY Body fat and food intake are regulated
NIHR Innovation Observatory Evidence Briefing: September 2017 Setmelanotide for pro-opiomelanocortin deficiency obesity NIHRIO (HSRIC) ID: 8496 NICE ID: 9505 LAY SUMMARY Body fat and food intake are regulated
Emapalumab for primary haemophagocytic lymphohistiocytosis
 NIHR Innovation Observatory Evidence Briefing: February 2018 Emapalumab for primary haemophagocytic lymphohistiocytosis NIHRIO (HSRIC) ID: 8727 NICE ID: 8735 LAY SUMMARY Haemophagocytic lymphohistiocytosis
NIHR Innovation Observatory Evidence Briefing: February 2018 Emapalumab for primary haemophagocytic lymphohistiocytosis NIHRIO (HSRIC) ID: 8727 NICE ID: 8735 LAY SUMMARY Haemophagocytic lymphohistiocytosis
Axicabtagene ciloleucel IV for relapsed/refractory acute lymphoblastic leukaemia in children and adolecents (aged 2-21 years) second line
 NIHR Innovation Observatory Evidence Briefing: August 2017 Axicabtagene ciloleucel IV for relapsed/refractory acute lymphoblastic leukaemia in children and adolecents (aged 2-21 years) second line NIHRIO
NIHR Innovation Observatory Evidence Briefing: August 2017 Axicabtagene ciloleucel IV for relapsed/refractory acute lymphoblastic leukaemia in children and adolecents (aged 2-21 years) second line NIHRIO
Olesoxime for amyotrophic lateral sclerosis first line
 Olesoxime for amyotrophic lateral sclerosis first line May 2011 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
Olesoxime for amyotrophic lateral sclerosis first line May 2011 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
Insulin degludec/insulin aspart (DegludecPlus) for type 1 diabetes
 Insulin degludec/insulin aspart (DegludecPlus) for type 1 diabetes This technology summary is based on information available at the time of research and a limited literature search. It is not intended
Insulin degludec/insulin aspart (DegludecPlus) for type 1 diabetes This technology summary is based on information available at the time of research and a limited literature search. It is not intended
Horizon Scanning Centre March Denosumab for glucocorticoidinduced SUMMARY NIHR HSC ID: 6329
 Horizon Scanning Centre March 2014 Denosumab for glucocorticoidinduced osteoporosis SUMMARY NIHR HSC ID: 6329 This briefing is based on information available at the time of research and a limited literature
Horizon Scanning Centre March 2014 Denosumab for glucocorticoidinduced osteoporosis SUMMARY NIHR HSC ID: 6329 This briefing is based on information available at the time of research and a limited literature
Horizon Scanning Centre March Tildrakizumab for moderate to severe plaque psoriasis SUMMARY NIHR HSC ID: 6798
 Horizon Scanning Centre March 2015 Tildrakizumab for moderate to severe plaque psoriasis SUMMARY NIHR HSC ID: 6798 This briefing is based on information available at the time of research and a limited
Horizon Scanning Centre March 2015 Tildrakizumab for moderate to severe plaque psoriasis SUMMARY NIHR HSC ID: 6798 This briefing is based on information available at the time of research and a limited
Clinical Commissioning Policy Proposition: Rituximab for cytopaenia complicating primary immunodeficiency
 Clinical Commissioning Policy Proposition: Rituximab for cytopaenia complicating primary immunodeficiency Reference: NHS England F06X02/01 Information Reader Box (IRB) to be inserted on inside front cover
Clinical Commissioning Policy Proposition: Rituximab for cytopaenia complicating primary immunodeficiency Reference: NHS England F06X02/01 Information Reader Box (IRB) to be inserted on inside front cover
Rilonacept for cryopyrin associated periodic syndromes
 Rilonacept for cryopyrin associated periodic syndromes August 2009 This technology summary is based on information available at the time of research and a limited literature search. It is not intended
Rilonacept for cryopyrin associated periodic syndromes August 2009 This technology summary is based on information available at the time of research and a limited literature search. It is not intended
National Horizon Scanning Centre. Rituximab (MabThera) for chronic lymphocytic leukaemia. September 2007
 Rituximab (MabThera) for chronic lymphocytic leukaemia This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a definitive
Rituximab (MabThera) for chronic lymphocytic leukaemia This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a definitive
Horizon Scanning Centre November Spheroids of human autologous matrix-associated chondrocytes (Chondrosphere) for articular cartilage defects
 Horizon Scanning Centre November 2014 Spheroids of human autologous matrix-associated chondrocytes (Chondrosphere) for articular cartilage defects SUMMARY NIHR HSC ID: 8515 This briefing is based on information
Horizon Scanning Centre November 2014 Spheroids of human autologous matrix-associated chondrocytes (Chondrosphere) for articular cartilage defects SUMMARY NIHR HSC ID: 8515 This briefing is based on information
Enasidenib for relapsed or refractory acute myeloid leukaemia (AML) with an isocitrate dehydrogenase 2 (IDH2) mutation
 NIHR Innovation Observatory Evidence Briefing: March 2018 Enasidenib for relapsed or refractory acute myeloid leukaemia (AML) with an isocitrate dehydrogenase 2 (IDH2) mutation NIHRIO (HSRIC) ID: 11750
NIHR Innovation Observatory Evidence Briefing: March 2018 Enasidenib for relapsed or refractory acute myeloid leukaemia (AML) with an isocitrate dehydrogenase 2 (IDH2) mutation NIHRIO (HSRIC) ID: 11750
Belimumab for the treatment of autoantibody-active systemic lupus erythematosus
 National Institute for Health and Clinical Excellence Comment 1: the draft remit Single Technology Appraisal (STA) Belimumab for the treatment of autoantibody-active systemic lupus erythematosus Response
National Institute for Health and Clinical Excellence Comment 1: the draft remit Single Technology Appraisal (STA) Belimumab for the treatment of autoantibody-active systemic lupus erythematosus Response
Benlysta (belimumab) Prior Authorization Criteria Program Summary
 Benlysta (belimumab) Prior Authorization Criteria Program Summary This prior authorization applies to Commercial, NetResults A series, NetResults F series and Health Insurance Marketplace formularies.
Benlysta (belimumab) Prior Authorization Criteria Program Summary This prior authorization applies to Commercial, NetResults A series, NetResults F series and Health Insurance Marketplace formularies.
Voxelotor for sickle cell disease
 NIHR Innovation Observatory Evidence Briefing: November 2017 Voxelotor for sickle cell disease NIHRIO (HSRIC) ID: 10748 NICE ID: 9700 LAY SUMMARY Sickle cell disease (SCD) describes a group of inherited
NIHR Innovation Observatory Evidence Briefing: November 2017 Voxelotor for sickle cell disease NIHRIO (HSRIC) ID: 10748 NICE ID: 9700 LAY SUMMARY Sickle cell disease (SCD) describes a group of inherited
Horizon Scanning Centre March Cholic acid (Orphacol) for inborn errors of primary bile acid synthesis first line SUMMARY NIHR HSC ID: 9468
 Horizon Scanning Centre March 2014 Cholic acid (Orphacol) for inborn errors of primary bile acid synthesis first line SUMMARY NIHR HSC ID: 9468 This briefing is based on information available at the time
Horizon Scanning Centre March 2014 Cholic acid (Orphacol) for inborn errors of primary bile acid synthesis first line SUMMARY NIHR HSC ID: 9468 This briefing is based on information available at the time
Horizon Scanning Centre March Ixekizumab for moderate to severe chronic plaque psoriasis SUMMARY NIHR HSC ID: 5209
 Horizon Scanning Centre March 2015 Ixekizumab for moderate to severe chronic plaque psoriasis SUMMARY NIHR HSC ID: 5209 This briefing is based on information available at the time of research and a limited
Horizon Scanning Centre March 2015 Ixekizumab for moderate to severe chronic plaque psoriasis SUMMARY NIHR HSC ID: 5209 This briefing is based on information available at the time of research and a limited
RE: FINAL APPRAISAL DETERMINATION BELIMUMAB FOR THE TREATMENT OF SYSTEMIC LUPUS ERYTHEMATOSUS (SLE)
 GlaxoSmithKline UK Ltd Stockley Park West Uxbridge Middlesex UB11 1BT Tel: +44 (0)20 8990 9000 Fax: +44 (0)20 8990 4321 www.gsk.com BY E-MAIL Friday, May 11, 2012 XXXXXXXXXXX Chair, Appeal Committee National
GlaxoSmithKline UK Ltd Stockley Park West Uxbridge Middlesex UB11 1BT Tel: +44 (0)20 8990 9000 Fax: +44 (0)20 8990 4321 www.gsk.com BY E-MAIL Friday, May 11, 2012 XXXXXXXXXXX Chair, Appeal Committee National
Cilengitide (Impetreve) for glioblastoma multiforme. February 2012
 Cilengitide (Impetreve) for glioblastoma multiforme February 2012 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
Cilengitide (Impetreve) for glioblastoma multiforme February 2012 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
rhngf for neurotrophic keratitis first line
 September 2015 Horizon Scanning Research & Intelligence Centre rhngf for neurotrophic keratitis first line LAY SUMMARY This briefing is based on information available at the time of research and a limited
September 2015 Horizon Scanning Research & Intelligence Centre rhngf for neurotrophic keratitis first line LAY SUMMARY This briefing is based on information available at the time of research and a limited
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: belimumab_benlysta 6/2011 2/2018 2/2019 3/2018 Description of Procedure or Service Belimumab (Benlysta) is
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: belimumab_benlysta 6/2011 2/2018 2/2019 3/2018 Description of Procedure or Service Belimumab (Benlysta) is
Horizon Scanning Centre November Vinflunine (Javlor) monotherapy for advanced breast cancer SUMMARY NIHR HSC ID: 7887
 Horizon Scanning Centre November 2012 Vinflunine (Javlor) monotherapy for advanced breast cancer SUMMARY NIHR HSC ID: 7887 This briefing is based on information available at the time of research and a
Horizon Scanning Centre November 2012 Vinflunine (Javlor) monotherapy for advanced breast cancer SUMMARY NIHR HSC ID: 7887 This briefing is based on information available at the time of research and a
Cabazitaxel (XRP-6258) for hormone refractory, metastatic prostate cancer second line after docetaxel
 Cabazitaxel (XRP-6258) for hormone refractory, metastatic prostate cancer second line after docetaxel April 2009 This technology summary is based on information available at the time of research and a
Cabazitaxel (XRP-6258) for hormone refractory, metastatic prostate cancer second line after docetaxel April 2009 This technology summary is based on information available at the time of research and a
Lacosamide (Vimpat) for partial-onset epilepsy monotherapy. December 2011
 Lacosamide (Vimpat) for partial-onset epilepsy monotherapy This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a
Lacosamide (Vimpat) for partial-onset epilepsy monotherapy This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a
Anakinra for Still s disease
 NIHR Innovation Observatory Evidence Briefing: April 2018 Anakinra for Still s disease NIHRIO (HSRIC) ID: 13328 NICE ID: 9818 LAY SUMMARY Still s disease is a rare auto inflammatory disease which can affect
NIHR Innovation Observatory Evidence Briefing: April 2018 Anakinra for Still s disease NIHRIO (HSRIC) ID: 13328 NICE ID: 9818 LAY SUMMARY Still s disease is a rare auto inflammatory disease which can affect
Darolutamide for non metastatic, castrationresistant
 NIHR Innovation Observatory Evidence Briefing: February 2018 Darolutamide for non metastatic, castrationresistant prostate cancer NIHRIO (HSRIC) ID: 7531 NICE ID: 9152 LAY SUMMARY Prostate cancer is cancer
NIHR Innovation Observatory Evidence Briefing: February 2018 Darolutamide for non metastatic, castrationresistant prostate cancer NIHRIO (HSRIC) ID: 7531 NICE ID: 9152 LAY SUMMARY Prostate cancer is cancer
Denosumab (AMG 162) for bone metastases from solid tumours and multiple myeloma
 Denosumab (AMG 162) for bone metastases from solid tumours and multiple myeloma September 2008 This technology summary is based on information available at the time of research and a limited literature
Denosumab (AMG 162) for bone metastases from solid tumours and multiple myeloma September 2008 This technology summary is based on information available at the time of research and a limited literature
Horizon Scanning Research & Intelligence Centre. Baricitinib for moderate to severe rheumatoid arthritis. May 2015 SUMMARY NIHR HSRIC ID: 5270
 May 2015 Horizon Scanning Research & Intelligence Centre Baricitinib for moderate to severe rheumatoid arthritis SUMMARY NIHR HSRIC ID: 5270 This briefing is based on information available at the time
May 2015 Horizon Scanning Research & Intelligence Centre Baricitinib for moderate to severe rheumatoid arthritis SUMMARY NIHR HSRIC ID: 5270 This briefing is based on information available at the time
Volixibat for non-alcoholic steatohepatitis (NASH)
 NIHR Innovation Observatory Evidence Briefing: October 2017 Volixibat for non-alcoholic steatohepatitis (NASH) NIHRIO (HSRIC) ID: 10240 NICE ID: 9524 LAY SUMMARY Non-alcoholic steatohepatitis (NASH) is
NIHR Innovation Observatory Evidence Briefing: October 2017 Volixibat for non-alcoholic steatohepatitis (NASH) NIHRIO (HSRIC) ID: 10240 NICE ID: 9524 LAY SUMMARY Non-alcoholic steatohepatitis (NASH) is
SAGE-547 for super-refractory status epilepticus
 NIHR Innovation Observatory Evidence Briefing: April 2017 SAGE-547 for super-refractory status epilepticus NIHRIO (HSRIC) ID: 10866 NICE ID: 8456 Status epilepticus is a single epileptic seizure lasting
NIHR Innovation Observatory Evidence Briefing: April 2017 SAGE-547 for super-refractory status epilepticus NIHRIO (HSRIC) ID: 10866 NICE ID: 8456 Status epilepticus is a single epileptic seizure lasting
Appendix to Notification Letter for rituximab and eltrombopag dated 20 February 2014
 Appendix to Notification Letter for rituximab and eltrombopag dated 20 February 2014 The notification letter which contains details of the decision to widen the restriction criteria for rituximab and eltrombopag
Appendix to Notification Letter for rituximab and eltrombopag dated 20 February 2014 The notification letter which contains details of the decision to widen the restriction criteria for rituximab and eltrombopag
Inhaled Lipid complexed Cisplatin for pulmonary relapse of osteosarcoma
 NIHR Innovation Observatory Evidence Briefing: February 2018 Inhaled Lipid complexed Cisplatin for pulmonary relapse of osteosarcoma NIHRIO (HSRIC) ID: 9204 NICE ID: 9673 LAY SUMMARY Osteosarcoma is a
NIHR Innovation Observatory Evidence Briefing: February 2018 Inhaled Lipid complexed Cisplatin for pulmonary relapse of osteosarcoma NIHRIO (HSRIC) ID: 9204 NICE ID: 9673 LAY SUMMARY Osteosarcoma is a
29/10. Treatment (brand name, manufacturer): For the treatment of: Background: Rituximab (MabThera, Roche) SLE in adults (unlicensed)
 GENERAL POLICY Policy Ref: 29/10 Treatment (brand name, manufacturer): For the treatment of: Background: Commissioning position: Rituximab (MabThera, Roche) SLE in adults (unlicensed) There are frequent
GENERAL POLICY Policy Ref: 29/10 Treatment (brand name, manufacturer): For the treatment of: Background: Commissioning position: Rituximab (MabThera, Roche) SLE in adults (unlicensed) There are frequent
NICE: Single technology appraisal: Belimumab for the treatment of active auto-antibody systemic lupus erythematosus (April 2012)
 LUPUS UK, St James House, Eastern Road, Romford Essex RM1 3NH Tel: 01708 731251 visit our website www.lupusuk.org.uk NICE: Single technology appraisal: Belimumab for the treatment of active auto-antibody
LUPUS UK, St James House, Eastern Road, Romford Essex RM1 3NH Tel: 01708 731251 visit our website www.lupusuk.org.uk NICE: Single technology appraisal: Belimumab for the treatment of active auto-antibody
Roflumilast (Daxas) for chronic obstructive pulmonary disease
 Roflumilast (Daxas) for chronic obstructive pulmonary disease August 2009 This technology summary is based on information available at the time of research and a limited literature search. It is not intended
Roflumilast (Daxas) for chronic obstructive pulmonary disease August 2009 This technology summary is based on information available at the time of research and a limited literature search. It is not intended
Erlotinib (Tarceva) for non small cell lung cancer advanced or metastatic maintenance monotherapy
 Erlotinib (Tarceva) for non small cell lung cancer advanced or metastatic maintenance monotherapy September 2008 This technology summary is based on information available at the time of research and a
Erlotinib (Tarceva) for non small cell lung cancer advanced or metastatic maintenance monotherapy September 2008 This technology summary is based on information available at the time of research and a
Lupus. Fast facts. What is lupus? What causes lupus? Who gets lupus?
 Lupus Systemic lupus erythematosus, referred to as SLE or lupus, is sometimes called the "great imitator." Why? Because of its wide range of symptoms, people often confuse lupus with other health problems.
Lupus Systemic lupus erythematosus, referred to as SLE or lupus, is sometimes called the "great imitator." Why? Because of its wide range of symptoms, people often confuse lupus with other health problems.
Summary of Risk Minimization Measures
 Table 6.1.4-1: Summary of Risk Minimization Measures Safety Concern Vaccination Hepatic and renal impairment Combination therapy Elderly Routine Risk Minimization Measures Specific subsection on vaccination
Table 6.1.4-1: Summary of Risk Minimization Measures Safety Concern Vaccination Hepatic and renal impairment Combination therapy Elderly Routine Risk Minimization Measures Specific subsection on vaccination
Riociguat for chronic thromboembolic pulmonary hypertension
 Riociguat for chronic thromboembolic pulmonary hypertension This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a
Riociguat for chronic thromboembolic pulmonary hypertension This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a
National Horizon Scanning Centre. Pregabalin (Lyrica) for fibromyalgia. September 2007
 Pregabalin (Lyrica) for fibromyalgia September 2007 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a definitive
Pregabalin (Lyrica) for fibromyalgia September 2007 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a definitive
9/25/2013 SYSTEMIC LUPUS ERYTHEMATOSUS (SLE)
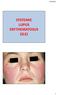 SYSTEMIC LUPUS ERYTHEMATOSUS (SLE) 1 Other Types of Lupus Discoid Lupus Erythematosus Lupus Pernio --- Sarcoidosis Lupus Vulgaris --- Tuberculosis of the face Manifestations of SLE Fever Rashes Arthritis
SYSTEMIC LUPUS ERYTHEMATOSUS (SLE) 1 Other Types of Lupus Discoid Lupus Erythematosus Lupus Pernio --- Sarcoidosis Lupus Vulgaris --- Tuberculosis of the face Manifestations of SLE Fever Rashes Arthritis
Avacopan in addition to standard of care for ANCA-associated vasculitis
 EVIDENCE BRIEFING DECEMBER 2018 Avacopan in addition to standard of care for ANCA-associated vasculitis NIHRIO ID 24160 NICE ID 10023 Developer/Company ChemoCentryx and Vifor Pharma UK Ltd UKPS ID N/A
EVIDENCE BRIEFING DECEMBER 2018 Avacopan in addition to standard of care for ANCA-associated vasculitis NIHRIO ID 24160 NICE ID 10023 Developer/Company ChemoCentryx and Vifor Pharma UK Ltd UKPS ID N/A
Gilteritinib for relapsed or refractory acute myeloid
 NIHR Innovation Observatory Evidence Briefing: May 2018 Gilteritinib for relapsed or refractory acute myeloid leukaemia (AML) with an FLT3 mutation second line NIHRIO (HSRIC) ID: 11486 NICE ID: 8749 LAY
NIHR Innovation Observatory Evidence Briefing: May 2018 Gilteritinib for relapsed or refractory acute myeloid leukaemia (AML) with an FLT3 mutation second line NIHRIO (HSRIC) ID: 11486 NICE ID: 8749 LAY
Pasireotide Long-Acting Repeatable (Signifor) for acromegaly first and second line
 Pasireotide Long-Acting Repeatable (Signifor) for acromegaly first and second line December 2010 This technology summary is based on information available at the time of research and a limited literature
Pasireotide Long-Acting Repeatable (Signifor) for acromegaly first and second line December 2010 This technology summary is based on information available at the time of research and a limited literature
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Proposed Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Secukinumab for treating ankylosing spondylitis after inadequate response to non-steroidal anti-inflammatory drugs
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Secukinumab for treating ankylosing spondylitis after inadequate response to non-steroidal anti-inflammatory drugs
National Horizon Scanning Centre. Mepolizumab (Bosatria) for hypereosinophilic syndrome first line in combination with corticosteroids.
 Mepolizumab (Bosatria) for hypereosinophilic syndrome first line in combination with corticosteroids May 2008 This technology summary is based on information available at the time of research and a limited
Mepolizumab (Bosatria) for hypereosinophilic syndrome first line in combination with corticosteroids May 2008 This technology summary is based on information available at the time of research and a limited
Lupuzor/P140 peptide in patients with systemic lupus erythematosus: a randomised, double-blind, placebo-controlled phase IIb clinical trial
 ARD Online First, published on November 21, 2012 as 10.1136/annrheumdis-2012-202460 Clinical and epidemiological research 1 ImmuPharma, Mulhouse, France 2 Servicio de Reumatología, Hospital Interzonal
ARD Online First, published on November 21, 2012 as 10.1136/annrheumdis-2012-202460 Clinical and epidemiological research 1 ImmuPharma, Mulhouse, France 2 Servicio de Reumatología, Hospital Interzonal
Tenapanor for irritable bowel syndrome with constipation
 NIHR Innovation Observatory Evidence Briefing: February 2018 Tenapanor for irritable bowel syndrome with constipation NIHRIO (HSRIC) ID: 6704 NICE ID: 9736 LAY SUMMARY Irritable bowel syndrome with constipation
NIHR Innovation Observatory Evidence Briefing: February 2018 Tenapanor for irritable bowel syndrome with constipation NIHRIO (HSRIC) ID: 6704 NICE ID: 9736 LAY SUMMARY Irritable bowel syndrome with constipation
National Horizon Scanning Centre. Mannitol dry powder for inhalation (Bronchitol) for cystic fibrosis. April 2008
 Mannitol dry powder for inhalation (Bronchitol) for cystic fibrosis April 2008 This technology summary is based on information available at the time of research and a limited literature search. It is not
Mannitol dry powder for inhalation (Bronchitol) for cystic fibrosis April 2008 This technology summary is based on information available at the time of research and a limited literature search. It is not
Pembrolizumab in combination with epacadostat for unresectable or metastatic melanoma
 NIHR Innovation Observatory Evidence Briefing: January 2018 Pembrolizumab in combination with epacadostat for unresectable or metastatic melanoma NIHRIO (HSRIC) ID: 7812 NICE ID: 9396 LAY SUMMARY Melanoma
NIHR Innovation Observatory Evidence Briefing: January 2018 Pembrolizumab in combination with epacadostat for unresectable or metastatic melanoma NIHRIO (HSRIC) ID: 7812 NICE ID: 9396 LAY SUMMARY Melanoma
Apixaban for stroke prevention in atrial fibrillation. August 2010
 Apixaban for stroke prevention in atrial fibrillation August 2010 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
Apixaban for stroke prevention in atrial fibrillation August 2010 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
Everolimus (Votubia) for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis first line or post surgery
 Everolimus (Votubia) for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis first line or post surgery April 2011 This technology summary is based on information
Everolimus (Votubia) for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis first line or post surgery April 2011 This technology summary is based on information
Clinical Commissioning Policy Proposition:
 Clinical Commissioning Policy Proposition: Rituximab for the treatment of dermatomyositis and polymyositis (Adults) Reference: NHS England A13X05/01 Information Reader Box (IRB) to be inserted on inside
Clinical Commissioning Policy Proposition: Rituximab for the treatment of dermatomyositis and polymyositis (Adults) Reference: NHS England A13X05/01 Information Reader Box (IRB) to be inserted on inside
* Autoantibody positive (i.e. antinuclear antibody or ANA titre 1:80 or anti-double stranded (ds) DNA antibody 30 IU/mL)
 Benlysta (belimumab) Policy Number: 5.02.509 Last Review: 05/2018 Origination: 06/2011 Next Review: 05/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Benlysta
Benlysta (belimumab) Policy Number: 5.02.509 Last Review: 05/2018 Origination: 06/2011 Next Review: 05/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Benlysta
Horizon Scanning Centre July Ocrelizumab for relapsing-remitting multiple sclerosis SUMMARY NIHR HSC ID: 3711
 Horizon Scanning Centre July 2014 Ocrelizumab for relapsing-remitting multiple sclerosis SUMMARY NIHR HSC ID: 3711 This briefing is based on information available at the time of research and a limited
Horizon Scanning Centre July 2014 Ocrelizumab for relapsing-remitting multiple sclerosis SUMMARY NIHR HSC ID: 3711 This briefing is based on information available at the time of research and a limited
National Horizon Scanning Centre. Saxagliptin (BMS ) for type 2 diabetes. April 2008
 Saxagliptin (BMS 477118) for type 2 diabetes This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a definitive statement
Saxagliptin (BMS 477118) for type 2 diabetes This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a definitive statement
National Horizon Scanning Centre. Temsirolimus (Torisel) for mantle cell lymphoma - relapsed and/or refractory. January 2008
 Temsirolimus (Torisel) for mantle cell lymphoma - relapsed and/or refractory January 2008 This technology summary is based on information available at the time of research and a limited literature search.
Temsirolimus (Torisel) for mantle cell lymphoma - relapsed and/or refractory January 2008 This technology summary is based on information available at the time of research and a limited literature search.
SYSTEMIC LUPUS ERYTHEMATOSUS: CURRENT CONCEPTS AND CLINICAL PEARLS. Dr Sheila Vasoo Consultant Division of Rheumatology NUHS
 SYSTEMIC LUPUS ERYTHEMATOSUS: CURRENT CONCEPTS AND CLINICAL PEARLS Dr Sheila Vasoo Consultant Division of Rheumatology NUHS Listen to the Patient Concepts Diagnosis Immunopathogenesis Clinical Pearls Disease
SYSTEMIC LUPUS ERYTHEMATOSUS: CURRENT CONCEPTS AND CLINICAL PEARLS Dr Sheila Vasoo Consultant Division of Rheumatology NUHS Listen to the Patient Concepts Diagnosis Immunopathogenesis Clinical Pearls Disease
National Horizon Scanning Centre. Irbesartan (Aprovel) for prevention of cardiovascular complications in patients with persistent atrial fibrillation
 Irbesartan (Aprovel) for prevention of cardiovascular complications in patients with persistent atrial fibrillation August 2008 This technology summary is based on information available at the time of
Irbesartan (Aprovel) for prevention of cardiovascular complications in patients with persistent atrial fibrillation August 2008 This technology summary is based on information available at the time of
National Horizon Scanning Centre. Azacitidine (Vidaza) for myelodysplastic syndrome. September 2007
 Azacitidine (Vidaza) for myelodysplastic syndrome September 2007 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
Azacitidine (Vidaza) for myelodysplastic syndrome September 2007 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
Lupus. and the Kidneys
 Lupus and the Kidneys LupusuK 2015 This information booklet has been produced by LUPUS UK 2015 LUPUS UK LUPUS UK is the national charity caring for those with systemic lupus erythematosus (SLE) and discoid
Lupus and the Kidneys LupusuK 2015 This information booklet has been produced by LUPUS UK 2015 LUPUS UK LUPUS UK is the national charity caring for those with systemic lupus erythematosus (SLE) and discoid
AUTOIMMUNE DISORDERS IN THE ACUTE SETTING
 AUTOIMMUNE DISORDERS IN THE ACUTE SETTING Diagnosis and Treatment Goals Aimee Borazanci, MD BNI Neuroimmunology Objectives Give an update on the causes for admission, clinical features, and outcomes of
AUTOIMMUNE DISORDERS IN THE ACUTE SETTING Diagnosis and Treatment Goals Aimee Borazanci, MD BNI Neuroimmunology Objectives Give an update on the causes for admission, clinical features, and outcomes of
Ticagrelor (Brilique) for peripheral arterial disease
 July 2016 Horizon Scanning Research & Intelligence Centre Ticagrelor (Brilique) for peripheral arterial disease LAY SUMMARY This briefing is based on information available at the time of research and a
July 2016 Horizon Scanning Research & Intelligence Centre Ticagrelor (Brilique) for peripheral arterial disease LAY SUMMARY This briefing is based on information available at the time of research and a
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Proposed Highly Specialised Technology Evaluation
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Highly Specialised Technology Evaluation Drisapersen for treating Duchenne muscular Draft scope (pre-referral) Draft remit/evaluation objective
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Highly Specialised Technology Evaluation Drisapersen for treating Duchenne muscular Draft scope (pre-referral) Draft remit/evaluation objective
Otamixaban for non-st-segment elevation acute coronary syndrome
 Otamixaban for non-st-segment elevation acute coronary syndrome September 2011 This technology summary is based on information available at the time of research and a limited literature search. It is not
Otamixaban for non-st-segment elevation acute coronary syndrome September 2011 This technology summary is based on information available at the time of research and a limited literature search. It is not
Elevated BLyS levels in patients with systemic lupus erythematosus: Associated factors and responses to belimumab
 (2016) 25, 346 354 http://lup.sagepub.com PAPER Elevated BLyS levels in patients with systemic lupus erythematosus: Associated factors and responses to belimumab DA Roth 1, A Thompson 2, Y Tang 2*, AE
(2016) 25, 346 354 http://lup.sagepub.com PAPER Elevated BLyS levels in patients with systemic lupus erythematosus: Associated factors and responses to belimumab DA Roth 1, A Thompson 2, Y Tang 2*, AE
Sotagliflozin tablets as an adjuct therapy to insulin for Type 1 diabetes mellitus
 NIHR Innovation Observatory Evidence Briefing: September 2017 Sotagliflozin tablets as an adjuct therapy to insulin for Type 1 diabetes mellitus NIHRIO (HSRIC) ID: 8655 NICE ID: 9521 LAY SUMMARY Type 1
NIHR Innovation Observatory Evidence Briefing: September 2017 Sotagliflozin tablets as an adjuct therapy to insulin for Type 1 diabetes mellitus NIHRIO (HSRIC) ID: 8655 NICE ID: 9521 LAY SUMMARY Type 1
Horizon Scanning Centre November 2012
 Horizon Scanning Centre November 2012 Cangrelor to reduce platelet aggregation and thrombosis in patients undergoing percutaneous coronary intervention99 SUMMARY NIHR HSC ID: 2424 This briefing is based
Horizon Scanning Centre November 2012 Cangrelor to reduce platelet aggregation and thrombosis in patients undergoing percutaneous coronary intervention99 SUMMARY NIHR HSC ID: 2424 This briefing is based
Nivolumab (Opdivo) with ipilimumab (Yervoy) for recurrent, metastatic, squamous cell head and neck cancer first line
 NIHR Innovation Observatory Evidence Briefing: September 2017 Nivolumab (Opdivo) with ipilimumab (Yervoy) for recurrent, metastatic, squamous cell head and neck cancer first line NIHRIO (HSRIC) ID: 12568
NIHR Innovation Observatory Evidence Briefing: September 2017 Nivolumab (Opdivo) with ipilimumab (Yervoy) for recurrent, metastatic, squamous cell head and neck cancer first line NIHRIO (HSRIC) ID: 12568
Horizon Scanning Technology Briefing. Sutent (Sunitinib) for first-line and adjuvant treatment of renal cell carcinoma
 Horizon Scanning Technology Briefing National Horizon Scanning Centre Sutent (Sunitinib) for first-line and adjuvant treatment of renal cell carcinoma August 2006: Updated October 2006 This technology
Horizon Scanning Technology Briefing National Horizon Scanning Centre Sutent (Sunitinib) for first-line and adjuvant treatment of renal cell carcinoma August 2006: Updated October 2006 This technology
