Contractor Information
|
|
|
- Carmel MargaretMargaret Pope
- 5 years ago
- Views:
Transcription
1 Local Coverage Article: Additional Information Required for Coverage and Pricing for Category III CPT s (A55681) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor Name Contract Type Contract Number Jurisdiction State(s) Noridian Healthcare Solutions, LLC A and B MAC MAC A J - F Alaska Noridian Healthcare Solutions, LLC A and B MAC MAC B J - F Alaska Noridian Healthcare Solutions, LLC A and B MAC MAC A J - F Idaho Noridian Healthcare Solutions, LLC A and B MAC MAC B J - F Idaho Noridian Healthcare Solutions, LLC A and B MAC MAC A J - F Oregon Noridian Healthcare Solutions, LLC A and B MAC MAC B J - F Oregon Noridian Healthcare Solutions, LLC A and B MAC MAC A J - F Washington Noridian Healthcare Solutions, LLC A and B MAC MAC B J - F Washington Noridian Healthcare Solutions, LLC A and B MAC MAC A J - F Arizona Noridian Healthcare Solutions, LLC A and B MAC MAC B J - F Arizona Noridian Healthcare Solutions, LLC A and B MAC MAC A J - F Montana Noridian Healthcare Solutions, LLC A and B MAC MAC B J - F Montana Noridian Healthcare Solutions, LLC A and B MAC MAC A J - F North Dakota Noridian Healthcare Solutions, LLC A and B MAC MAC B J - F North Dakota Noridian Healthcare Solutions, LLC A and B MAC MAC A J - F South Dakota Noridian Healthcare Solutions, LLC A and B MAC MAC B J - F South Dakota Noridian Healthcare Solutions, LLC A and B MAC MAC A J - F Utah Noridian Healthcare Solutions, LLC A and B MAC MAC B J - F Utah Noridian Healthcare Solutions, LLC A and B MAC MAC A J - F Wyoming Noridian Healthcare Solutions, LLC A and B MAC MAC B J - F Wyoming Back to Top Article Information General Information Original Article Effective Date 07/01/2017 Article ID A55681 Article Title Additional Information Required for Coverage and Pricing for Category III CPT s AMA CPT / ADA CDT / AHA NUBC Copyright Statement Revision Effective Date 01/01/2018 Revision Ending Date N/A Retirement Date N/A Printed on 1/25/2018. Page 1 of 18
2 CPT only copyright American Medical Association. All Rights Reserved. CPT is a registered trademark of the American Medical Association. Applicable FARS/DFARS Apply to Government Use. Fee schedules, relative value units, conversion factors and/or related components are not assigned by the AMA, are not part of CPT, and the AMA is not recommending their use. The AMA does not directly or indirectly practice medicine or dispense medical services. The AMA assumes no liability for data contained or not contained herein. The on Dental Procedures and Nomenclature () is published in Current Dental Terminology (CDT). Copyright American Dental Association. All rights reserved. CDT and CDT-2016 are trademarks of the American Dental Association. UB-04 Manual. OFFICIAL UB-04 DATA SPECIFICATIONS MANUAL, 2014, is copyrighted by American Hospital Association ( AHA ), Chicago, Illinois. No portion of OFFICIAL UB-04 MANUAL may be reproduced, sorted in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without prior express, written consent of AHA. Health Forum reserves the right to change the copyright notice from time to time upon written notice to Company. Article Guidance Article Text: The CPT(R) Editorial Panel issues Category III codes for services which do not meet the criteria for a Category I code, but do meet the minimal criteria for a Category III code (see criteria listed below). Category III codes are temporary codes created to track new, "emerging" unproven therapies and tests. All Category III s have a sunset date, at which time the CPT(R) Editorial Panel may convert it to a Category I code if the service meets the Category I criteria, or they may delete the code if widespread use of the service has not materialized or they may extend the code as Category III for several more years. The purpose of this article is to indicate for which Category III s Noridian Medical Directors have received sufficient information for making coverage and payment determinations. For the codes listed in Group 1, Noridian Medical Directors have received sufficient information to make these determinations. For Groups 2, 3 and 4, the Noridian Medical Directors have received sufficient information and coverage may be described in one of the Local Coverage Determinations (LCD) (listed elsewhere). If a provider or other interested party believes that a service described by a Category III code falls within one of Medicare s defined benefit categories and is reasonable and medically necessary for a defined group of patients, the provider or party should submit the peer-reviewed medical literature, supporting the safety and effectiveness of the service for Medical Director review. This request for consideration of coverage of the service may be made by submitting full text copies of the supporting literature to "Medical Directors; Noridian Healthcare Solutions, LLC; nd Street South; Fargo, ND, or by to (MedicalPolicy@noridian.com). Alternatively, a provider, after having delivered the service to a beneficiary may use the claims process by submitting a claim; Noridian will send an Additional Documentation Request (ADR) letter requesting specific documentation along with the full text copies of the peer-reviewed medical literature, supporting the safety and effectiveness of the service for Medical Director review. Please send this information to the postal or address noted in the ADR letter within 30 days. Printed on 1/25/2018. Page 2 of 18
3 Coverage: Medicare does not cover items and services that are not reasonable and necessary for the diagnosis or treatment of an illness or injury or to improve the functioning of a malformed body member. Section 1862 (a) (1) of the Social Security Act is the basis for denying payment for types of care, or specific items, services, or procedures that are not excluded by any other statutory clause (such as screening test not specifically approved by the Secretary of DHHS) and meet all technical requirements for coverage but are determined to be any of the following: Not generally accepted in the medical community as safe and effective in the setting and for the condition for which it is used. Not proven to be safe and effective based on peer review or scientific literature. Experimental. Not medically necessary in the particular case. Furnished at a level, duration or frequency that is not medically appropriate. Not furnished in accordance with accepted standards of medical practice. Not furnished in a setting (such as inpatient care at a hospital or SNF, outpatient care through a hospital or physician s office or home care) appropriate to the patient s medical needs and condition. To be considered medically necessary, items and services must have been established as safe and effective. That is, the items and services must be: Consistent with the symptoms or diagnosis of the illness or injury under treatment. Necessary and consistent with generally accepted professional medical standards (e.g., not experimental or investigational). Not furnished primarily for the convenience of the patient, the attending physician or other physician or supplier. Furnished at the most appropriate level that can be provided safely and effectively to the patient. Medicare is a defined benefit program; contractors must initially determine whether a service fits one of the defined benefits categories. Services that this contractor considers non-covered because the service does not fit into a benefit category are also included on a list below. When processing a claim, carriers continue to determine if a service is reasonable and necessary to treat illness or injury. If a service is not reasonable and necessary to treat illness or injury for any reason (including lack of safety and efficacy because it is an experimental procedure, etc.), carriers consider the service noncovered notwithstanding the presence of a payment amount for the service in the Medicare fee schedule. The presence of a payment amount in the MPFS and the Medicare physician fee schedule database (MPFSDB) does not imply that CMS has determined that the service may be covered by Medicare. The nature of the status indicator in the database does not control coverage except where the status is N for noncovered. [Medicare Claims Processing Manual (CMS Pub , Chapter 23, Section 30 A)] Pricing: All Category III s are C Status (carrier-priced) on the Physician Fee Schedule. The information submitted to support coverage must also include information that supports pricing determinations. Such information might include copies of invoices for supplies and equipment, estimates of physician and clinical staff time and intensity of physician work. For surgical procedures please provide documentation of skin-to-skin (intraservice) surgical times. The list below is grouped into those for which this contractor has sufficient information to make coverage and pricing determinations (Group 1), those for which this contractor does have sufficient information to make coverage and pricing determination and has determined that the services are not reasonable and necessary (Group 2), those that do not meet a Medicare Coverage Benefit (Group 3) and those for which this contractor has determined coverage and pricing (Group 4). Printed on 1/25/2018. Page 3 of 18
4 CPT(R) Criteria (from AMA/CPT(R) Website: CPT(R) Category I Criteria: A proposal for a new or revised Category I code must satisfy all of the following criteria: All devices and drugs necessary for performance of the procedure or service have received FDA clearance or approval when such is required for performance of the procedure or service. The procedure or service is performed by many physicians or other qualified health care professionals across the United States. The procedure or service is performed with frequency consistent with the intended clinical use (i.e., a service for a common condition should have high volume, whereas a service commonly performed for a rare condition may have low volume). The procedure or service is consistent with current medical practice. The clinical efficacy of the procedure or service is documented in literature that meets the requirements set forth in the CPT(R) code change application. CPT(R) Category III Criteria: The following criteria are used by the CPT(R)/HCPAC Advisory Committee and the CPT(R) Editorial Panel for evaluating Category III code applications: The procedure or service is currently or recently performed in humans AND At least 1 of the following additional criteria has been met: The application is supported by at least 1 CPT(R) or HCPAC advisor representing practitioners who would use this procedure or service OR The actual or potential clinical efficacy of the specific procedure or service is supported by peer reviewed literature which is available in English for examination by the Editorial Panel OR There is (a) at least 1 Institutional Review Board approved protocol of a study of the procedure or service being performed, (b) a description of a current and ongoing United States trial outlining the efficacy of the procedure or service, or (c) other evidence of evolving clinical utilization. Back to Top Coding Information Bill Type s: Contractors may specify Bill Types to help providers identify those Bill Types typically used to report this service. Absence of a Bill Type does not guarantee that the article does not apply to that Bill Type. Complete absence of all Bill Types indicates that coverage is not influenced by Bill Type and the article should be assumed to apply equally to all claims. N/A Revenue s: Contractors may specify Revenue s to help providers identify those Revenue s typically used to report this service. In most instances Revenue s are purely advisory. Unless specified in the article, services reported under other Revenue s are equally subject to this coverage determination. Complete absence of all Revenue s indicates that coverage is not influenced by Revenue and the article should be assumed to apply equally to all Revenue s. N/A Printed on 1/25/2018. Page 4 of 18
5 s Group 1 Paragraph: For these services, Noridian has not received sufficient information to make coverage and pricing determinations. Most of these codes are included in the Non-Covered Services LCD (L35008); others may not be included due to changes in internal Noridian procedures that are a result of the 21st Century Cures Act. Note: 0470T T will have an ADR letter sent to providers billing for these services. Group 1 s: Group 1 Group 1 Description CEREBRAL PERFUSION ANALYSIS USING COMPUTED TOMOGRAPHY WITH CONTRAST 0042T ADMINISTRATION, INCLUDING POST-PROCESSING OF PARAMETRIC MAPS WITH DETERMINATION OF CEREBRAL BLOOD FLOW, CEREBRAL BLOOD VOLUME, AND MEAN TRANSIT TIME COMPUTER-ASSISTED MUSCULOSKELETAL SURGICAL NAVIGATIONAL ORTHOPEDIC PROCEDURE, 0054T WITH IMAGE-GUIDANCE BASED ON FLUOROSCOPIC IMAGES (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) COMPUTER-ASSISTED MUSCULOSKELETAL SURGICAL NAVIGATIONAL ORTHOPEDIC PROCEDURE, 0055T WITH IMAGE-GUIDANCE BASED ON CT/MRI IMAGES (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) FOCUSED ULTRASOUND ABLATION OF UTERINE LEIOMYOMATA, INCLUDING MR GUIDANCE; 0071T TOTAL LEIOMYOMATA VOLUME LESS THAN 200 CC OF TISSUE FOCUSED ULTRASOUND ABLATION OF UTERINE LEIOMYOMATA, INCLUDING MR GUIDANCE; 0072T TOTAL LEIOMYOMATA VOLUME GREATER OR EQUAL TO 200 CC OF TISSUE REVISION INCLUDING REPLACEMENT OF TOTAL DISC ARTHROPLASTY (ARTIFICIAL DISC), 0098T ANTERIOR APPROACH, EACH ADDITIONAL INTERSPACE, CERVICAL (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) EXTRACORPOREAL SHOCK WAVE INVOLVING MUSCULOSKELETAL SYSTEM, NOT OTHERWISE 0101T SPECIFIED, HIGH ENERGY EXTRACORPOREAL SHOCK WAVE, HIGH ENERGY, PERFORMED BY A PHYSICIAN, REQUIRING 0102T ANESTHESIA OTHER THAN LOCAL, INVOLVING LATERAL HUMERAL EPICONDYLE QUANTITATIVE SENSORY TESTING (QST), TESTING AND INTERPRETATION PER EXTREMITY; 0106T USING TOUCH PRESSURE STIMULI TO ASSESS LARGE DIAMETER SENSATION QUANTITATIVE SENSORY TESTING (QST), TESTING AND INTERPRETATION PER EXTREMITY; 0107T USING VIBRATION STIMULI TO ASSESS LARGE DIAMETER FIBER SENSATION QUANTITATIVE SENSORY TESTING (QST), TESTING AND INTERPRETATION PER EXTREMITY; 0108T USING COOLING STIMULI TO ASSESS SMALL NERVE FIBER SENSATION AND HYPERALGESIA QUANTITATIVE SENSORY TESTING (QST), TESTING AND INTERPRETATION PER EXTREMITY; 0109T USING HEAT-PAIN STIMULI TO ASSESS SMALL NERVE FIBER SENSATION AND HYPERALGESIA QUANTITATIVE SENSORY TESTING (QST), TESTING AND INTERPRETATION PER EXTREMITY; 0110T USING OTHER STIMULI TO ASSESS SENSATION 0111T LONG-CHAIN (C20-22) OMEGA-3 FATTY ACIDS IN RED BLOOD CELL (RBC) MEMBRANES COMMON CAROTID INTIMA-MEDIA THICKNESS (IMT) STUDY FOR EVALUATION OF 0126T ATHEROSCLEROTIC BURDEN OR CORONARY HEART DISEASE RISK FACTOR ASSESSMENT COMPUTER-AIDED DETECTION, INCLUDING COMPUTER ALGORITHM ANALYSIS OF MRI IMAGE DATA FOR LESION DETECTION/CHARACTERIZATION, PHARMACOKINETIC ANALYSIS, WITH 0159T FURTHER PHYSICIAN REVIEW FOR INTERPRETATION, BREAST MRI (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) TOTAL DISC ARTHROPLASTY (ARTIFICIAL DISC), ANTERIOR APPROACH, INCLUDING DISCECTOMY TO PREPARE INTERSPACE (OTHER THAN FOR DECOMPRESSION), EACH 0163T ADDITIONAL INTERSPACE, LUMBAR (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) REVISION INCLUDING REPLACEMENT OF TOTAL DISC ARTHROPLASTY (ARTIFICIAL DISC), 0165T ANTERIOR APPROACH, EACH ADDITIONAL INTERSPACE, LUMBAR (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) 0174T Printed on 1/25/2018. Page 5 of 18
6 Group T 0190T 0195T 0196T 0198T 0202T 0205T 0206T 0207T 0208T 0209T 0210T 0211T 0212T 0219T 0220T 0221T 0222T 0232T 0234T 0235T 0236T Group 1 Description COMPUTER-AIDED DETECTION (CAD) (COMPUTER ALGORITHM ANALYSIS OF DIGITAL IMAGE DATA FOR LESION DETECTION) WITH FURTHER PHYSICIAN REVIEW FOR INTERPRETATION AND REPORT, WITH OR WITHOUT DIGITIZATION OF FILM RADIOGRAPHIC IMAGES, CHEST RADIOGRAPH(S), PERFORMED CONCURRENT WITH PRIMARY INTERPRETATION (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) COMPUTER-AIDED DETECTION (CAD) (COMPUTER ALGORITHM ANALYSIS OF DIGITAL IMAGE DATA FOR LESION DETECTION) WITH FURTHER PHYSICIAN REVIEW FOR INTERPRETATION AND REPORT, WITH OR WITHOUT DIGITIZATION OF FILM RADIOGRAPHIC IMAGES, CHEST RADIOGRAPH(S), PERFORMED REMOTE FROM PRIMARY INTERPRETATION PLACEMENT OF INTRAOCULAR RADIATION SOURCE APPLICATOR (LIST SEPARATELY IN ADDITION TO PRIMARY PROCEDURE) ARTHRODESIS, PRE-SACRAL INTERBODY TECHNIQUE, DISC SPACE PREPARATION, DISCECTOMY, WITHOUT INSTRUMENTATION, WITH IMAGE GUIDANCE, INCLUDES BONE GRAFT WHEN PERFORMED; L5-S1 INTERSPACE ARTHRODESIS, PRE-SACRAL INTERBODY TECHNIQUE, DISC SPACE PREPARATION, DISCECTOMY, WITHOUT INSTRUMENTATION, WITH IMAGE GUIDANCE, INCLUDES BONE GRAFT WHEN PERFORMED; L4-L5 INTERSPACE (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) MEASUREMENT OF OCULAR BLOOD FLOW BY REPETITIVE INTRAOCULAR PRESSURE SAMPLING, WITH INTERPRETATION AND REPORT POSTERIOR VERTEBRAL JOINT(S) ARTHROPLASTY (EG, FACET JOINT[S] REPLACEMENT), INCLUDING FACETECTOMY, LAMINECTOMY, FORAMINOTOMY, AND VERTEBRAL COLUMN FIXATION, INJECTION OF BONE CEMENT, WHEN PERFORMED, INCLUDING FLUOROSCOPY, SINGLE LEVEL, LUMBAR SPINE INTRAVASCULAR CATHETER-BASED CORONARY VESSEL OR GRAFT SPECTROSCOPY (EG, INFRARED) DURING DIAGNOSTIC EVALUATION AND/OR THERAPEUTIC INTERVENTION INCLUDING IMAGING SUPERVISION, INTERPRETATION, AND REPORT, EACH VESSEL (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) COMPUTERIZED DATABASE ANALYSIS OF MULTIPLE CYCLES OF DIGITIZED CARDIAC ELECTRICAL DATA FROM TWO OR MORE ECG LEADS, INCLUDING TRANSMISSION TO A REMOTE CENTER, APPLICATION OF MULTIPLE NONLINEAR MATHEMATICAL TRANSFORMATIONS, WITH CORONARY ARTERY OBSTRUCTION SEVERITY ASSESSMENT EVACUATION OF MEIBOMIAN GLANDS, AUTOMATED, USING HEAT AND INTERMITTENT PRESSURE, UNILATERAL PURE TONE AUDIOMETRY (THRESHOLD), AUTOMATED; AIR ONLY PURE TONE AUDIOMETRY (THRESHOLD), AUTOMATED; AIR AND BONE SPEECH AUDIOMETRY THRESHOLD, AUTOMATED; SPEECH AUDIOMETRY THRESHOLD, AUTOMATED; WITH SPEECH RECOGNITION COMPREHENSIVE AUDIOMETRY THRESHOLD EVALUATION AND SPEECH RECOGNITION (0209T, 0211T COMBINED), AUTOMATED PLACEMENT OF A POSTERIOR INTRAFACET IMPLANT(S), UNILATERAL OR BILATERAL, INCLUDING IMAGING AND PLACEMENT OF BONE GRAFT(S) OR SYNTHETIC DEVICE(S), SINGLE LEVEL; CERVICAL PLACEMENT OF A POSTERIOR INTRAFACET IMPLANT(S), UNILATERAL OR BILATERAL, INCLUDING IMAGING AND PLACEMENT OF BONE GRAFT(S) OR SYNTHETIC DEVICE(S), SINGLE LEVEL; THORACIC PLACEMENT OF A POSTERIOR INTRAFACET IMPLANT(S), UNILATERAL OR BILATERAL, INCLUDING IMAGING AND PLACEMENT OF BONE GRAFT(S) OR SYNTHETIC DEVICE(S), SINGLE LEVEL; LUMBAR PLACEMENT OF A POSTERIOR INTRAFACET IMPLANT(S), UNILATERAL OR BILATERAL, INCLUDING IMAGING AND PLACEMENT OF BONE GRAFT(S) OR SYNTHETIC DEVICE(S), SINGLE LEVEL; EACH ADDITIONAL VERTEBRAL SEGMENT (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) INJECTION(S), PLATELET RICH PLASMA, ANY SITE, INCLUDING IMAGE GUIDANCE, HARVESTING AND PREPARATION WHEN PERFORMED TRANSLUMINAL PERIPHERAL ATHERECTOMY, OPEN OR PERCUTANEOUS, INCLUDING RADIOLOGICAL SUPERVISION AND INTERPRETATION; RENAL ARTERY TRANSLUMINAL PERIPHERAL ATHERECTOMY, OPEN OR PERCUTANEOUS, INCLUDING RADIOLOGICAL SUPERVISION AND INTERPRETATION; VISCERAL ARTERY (EXCEPT RENAL), EACH VESSEL Printed on 1/25/2018. Page 6 of 18
7 Group T 0238T 0253T 0254T 0263T 0264T 0265T 0266T 0267T 0268T 0272T 0273T 0274T 0278T 0290T 0330T 0331T 0332T Group 1 Description TRANSLUMINAL PERIPHERAL ATHERECTOMY, OPEN OR PERCUTANEOUS, INCLUDING RADIOLOGICAL SUPERVISION AND INTERPRETATION; ABDOMINAL AORTA TRANSLUMINAL PERIPHERAL ATHERECTOMY, OPEN OR PERCUTANEOUS, INCLUDING RADIOLOGICAL SUPERVISION AND INTERPRETATION; BRACHIOCEPHALIC TRUNK AND BRANCHES, EACH VESSEL TRANSLUMINAL PERIPHERAL ATHERECTOMY, OPEN OR PERCUTANEOUS, INCLUDING RADIOLOGICAL SUPERVISION AND INTERPRETATION; ILIAC ARTERY, EACH VESSEL INSERTION OF ANTERIOR SEGMENT AQUEOUS DRAINAGE DEVICE, WITHOUT EXTRAOCULAR RESERVOIR, INTERNAL APPROACH, INTO THE SUPRACHOROIDAL SPACE ENDOVASCULAR REPAIR OF ILIAC ARTERY BIFURCATION (EG, ANEURYSM, PSEUDOANEURYSM, ARTERIOVENOUS MALFORMATION, TRAUMA, DISSECTION) USING BIFURCATED ENDOGRAFT FROM THE COMMON ILIAC ARTERY INTO BOTH THE EXTERNAL AND INTERNAL ILIAC ARTERY, INCLUDING ALL SELECTIVE AND/OR NONSELECTIVE CATHETERIZATION(S) REQUIRED FOR DEVICE PLACEMENT AND ALL ASSOCIATED RADIOLOGICAL SUPERVISION AND INTERPRETATION, UNILATERAL INTRAMUSCULAR AUTOLOGOUS BONE MARROW CELL THERAPY, WITH PREPARATION OF HARVESTED CELLS, MULTIPLE INJECTIONS, ONE LEG, INCLUDING ULTRASOUND GUIDANCE, IF PERFORMED; COMPLETE PROCEDURE INCLUDING UNILATERAL OR BILATERAL BONE MARROW HARVEST INTRAMUSCULAR AUTOLOGOUS BONE MARROW CELL THERAPY, WITH PREPARATION OF HARVESTED CELLS, MULTIPLE INJECTIONS, ONE LEG, INCLUDING ULTRASOUND GUIDANCE, IF PERFORMED; COMPLETE PROCEDURE EXCLUDING BONE MARROW HARVEST INTRAMUSCULAR AUTOLOGOUS BONE MARROW CELL THERAPY, WITH PREPARATION OF HARVESTED CELLS, MULTIPLE INJECTIONS, ONE LEG, INCLUDING ULTRASOUND GUIDANCE, IF PERFORMED; UNILATERAL OR BILATERAL BONE MARROW HARVEST ONLY FOR INTRAMUSCULAR AUTOLOGOUS BONE MARROW CELL THERAPY IMPLANTATION OR REPLACEMENT OF CAROTID SINUS BAROREFLEX ACTIVATION DEVICE; TOTAL SYSTEM (INCLUDES GENERATOR PLACEMENT, UNILATERAL OR BILATERAL LEAD PLACEMENT, INTRA-OPERATIVE INTERROGATION, PROGRAMMING, AND REPOSITIONING, WHEN PERFORMED) IMPLANTATION OR REPLACEMENT OF CAROTID SINUS BAROREFLEX ACTIVATION DEVICE; LEAD ONLY, UNILATERAL (INCLUDES INTRA-OPERATIVE INTERROGATION, PROGRAMMING, AND REPOSITIONING, WHEN PERFORMED) IMPLANTATION OR REPLACEMENT OF CAROTID SINUS BAROREFLEX ACTIVATION DEVICE; PULSE GENERATOR ONLY (INCLUDES INTRA-OPERATIVE INTERROGATION, PROGRAMMING, AND REPOSITIONING, WHEN PERFORMED) INTERROGATION DEVICE EVALUATION (IN PERSON), CAROTID SINUS BAROREFLEX ACTIVATION SYSTEM, INCLUDING TELEMETRIC ITERATIVE COMMUNICATION WITH THE IMPLANTABLE DEVICE TO MONITOR DEVICE DIAGNOSTICS AND PROGRAMMED THERAPY VALUES, WITH INTERPRETATION AND REPORT (EG, BATTERY STATUS, LEAD IMPEDANCE, PULSE AMPLITUDE, PULSE WIDTH, THERAPY FREQUENCY, PATHWAY MODE, BURST MODE, THERAPY START/STOP TIMES EACH DAY); INTERROGATION DEVICE EVALUATION (IN PERSON), CAROTID SINUS BAROREFLEX ACTIVATION SYSTEM, INCLUDING TELEMETRIC ITERATIVE COMMUNICATION WITH THE IMPLANTABLE DEVICE TO MONITOR DEVICE DIAGNOSTICS AND PROGRAMMED THERAPY VALUES, WITH INTERPRETATION AND REPORT (EG, BATTERY STATUS, LEAD IMPEDANCE, PULSE AMPLITUDE, PULSE WIDTH, THERAPY FREQUENCY, PATHWAY MODE, BURST MODE, THERAPY START/STOP TIMES EACH DAY); WITH PROGRAMMING PERCUTANEOUS LAMINOTOMY/LAMINECTOMY (INTERLAMINAR APPROACH) FOR DECOMPRESSION OF NEURAL ELEMENTS, (WITH OR WITHOUT LIGAMENTOUS RESECTION, DISCECTOMY, FACETECTOMY AND/OR FORAMINOTOMY), ANY METHOD, UNDER INDIRECT IMAGE GUIDANCE (EG, FLUOROSCOPIC, CT), SINGLE OR MULTIPLE LEVELS, UNILATERAL OR BILATERAL; CERVICAL OR THORACIC TRANSCUTANEOUS ELECTRICAL MODULATION PAIN REPROCESSING (EG, SCRAMBLER THERAPY), EACH TREATMENT SESSION (INCLUDES PLACEMENT OF ELECTRODES) CORNEAL INCISIONS IN THE RECIPIENT CORNEA CREATED USING A LASER, IN PREPARATION FOR PENETRATING OR LAMELLAR KERATOPLASTY (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) TEAR FILM IMAGING, UNILATERAL OR BILATERAL, WITH INTERPRETATION AND REPORT MYOCARDIAL SYMPATHETIC INNERVATION IMAGING, PLANAR QUALITATIVE AND QUANTITATIVE ASSESSMENT; MYOCARDIAL SYMPATHETIC INNERVATION IMAGING, PLANAR QUALITATIVE AND QUANTITATIVE ASSESSMENT; WITH TOMOGRAPHIC SPECT Printed on 1/25/2018. Page 7 of 18
8 Group 1 Group 1 Description 0335T EXTRA-OSSEOUS SUBTALAR JOINT IMPLANT FOR TALOTARSAL STABILIZATION ENDOTHELIAL FUNCTION ASSESSMENT, USING PERIPHERAL VASCULAR RESPONSE TO REACTIVE 0337T HYPEREMIA, NON-INVASIVE (EG, BRACHIAL ARTERY ULTRASOUND, PERIPHERAL ARTERY TONOMETRY), UNILATERAL OR BILATERAL TRANSCATHETER RENAL SYMPATHETIC DENERVATION, PERCUTANEOUS APPROACH INCLUDING ARTERIAL PUNCTURE, SELECTIVE CATHETER PLACEMENT(S) RENAL ARTERY(IES), FLUOROSCOPY, 0338T CONTRAST INJECTION(S), INTRAPROCEDURAL ROADMAPPING AND RADIOLOGICAL SUPERVISION AND INTERPRETATION, INCLUDING PRESSURE GRADIENT MEASUREMENTS, FLUSH AORTOGRAM AND DIAGNOSTIC RENAL ANGIOGRAPHY WHEN PERFORMED; UNILATERAL TRANSCATHETER RENAL SYMPATHETIC DENERVATION, PERCUTANEOUS APPROACH INCLUDING ARTERIAL PUNCTURE, SELECTIVE CATHETER PLACEMENT(S) RENAL ARTERY(IES), FLUOROSCOPY, 0339T CONTRAST INJECTION(S), INTRAPROCEDURAL ROADMAPPING AND RADIOLOGICAL SUPERVISION AND INTERPRETATION, INCLUDING PRESSURE GRADIENT MEASUREMENTS, FLUSH AORTOGRAM AND DIAGNOSTIC RENAL ANGIOGRAPHY WHEN PERFORMED; BILATERAL 0341T QUANTITATIVE PUPILLOMETRY WITH INTERPRETATION AND REPORT, UNILATERAL OR BILATERAL 0342T THERAPEUTIC APHERESIS WITH SELECTIVE HDL DELIPIDATION AND PLASMA REINFUSION 0347T PLACEMENT OF INTERSTITIAL DEVICE(S) IN BONE FOR RADIOSTEREOMETRIC ANALYSIS (RSA) RADIOLOGIC EXAMINATION, RADIOSTEREOMETRIC ANALYSIS (RSA); SPINE, (INCLUDES 0348T CERVICAL, THORACIC AND LUMBOSACRAL, WHEN PERFORMED) RADIOLOGIC EXAMINATION, RADIOSTEREOMETRIC ANALYSIS (RSA); UPPER EXTREMITY(IES), 0349T (INCLUDES SHOULDER, ELBOW, AND WRIST, WHEN PERFORMED) RADIOLOGIC EXAMINATION, RADIOSTEREOMETRIC ANALYSIS (RSA); LOWER EXTREMITY(IES), 0350T (INCLUDES HIP, PROXIMAL FEMUR, KNEE, AND ANKLE, WHEN PERFORMED) OPTICAL COHERENCE TOMOGRAPHY OF BREAST OR AXILLARY LYMPH NODE, EXCISED TISSUE, 0351T EACH SPECIMEN; REAL-TIME INTRAOPERATIVE OPTICAL COHERENCE TOMOGRAPHY OF BREAST OR AXILLARY LYMPH NODE, EXCISED TISSUE, 0352T EACH SPECIMEN; INTERPRETATION AND REPORT, REAL-TIME OR REFERRED OPTICAL COHERENCE TOMOGRAPHY OF BREAST, SURGICAL CAVITY; REAL-TIME 0353T INTRAOPERATIVE OPTICAL COHERENCE TOMOGRAPHY OF BREAST, SURGICAL CAVITY; INTERPRETATION AND 0354T REPORT, REAL-TIME OR REFERRED GASTROINTESTINAL TRACT IMAGING, INTRALUMINAL (EG, CAPSULE ENDOSCOPY), COLON, WITH 0355T INTERPRETATION AND REPORT INSERTION OF DRUG-ELUTING IMPLANT (INCLUDING PUNCTAL DILATION AND IMPLANT 0356T REMOVAL WHEN PERFORMED) INTO LACRIMAL CANALICULUS, EACH 0357T CRYOPRESERVATION; IMMATURE OOCYTE(S) BIOELECTRICAL IMPEDANCE ANALYSIS WHOLE BODY COMPOSITION ASSESSMENT, WITH 0358T INTERPRETATION AND REPORT BEHAVIOR IDENTIFICATION ASSESSMENT, BY THE PHYSICIAN OR OTHER QUALIFIED HEALTH CARE PROFESSIONAL, FACE-TO-FACE WITH PATIENT AND CAREGIVER(S), INCLUDES ADMINISTRATION OF STANDARDIZED AND NON-STANDARDIZED TESTS, DETAILED BEHAVIORAL 0359T HISTORY, PATIENT OBSERVATION AND CAREGIVER INTERVIEW, INTERPRETATION OF TEST RESULTS, DISCUSSION OF FINDINGS AND RECOMMENDATIONS WITH THE PRIMARY GUARDIAN(S)/CAREGIVER(S), AND PREPARATION OF REPORT OBSERVATIONAL BEHAVIORAL FOLLOW-UP ASSESSMENT, INCLUDES PHYSICIAN OR OTHER QUALIFIED HEALTH CARE PROFESSIONAL DIRECTION WITH INTERPRETATION AND REPORT, 0360T ADMINISTERED BY ONE TECHNICIAN; FIRST 30 MINUTES OF TECHNICIAN TIME, FACE-TO-FACE WITH THE PATIENT OBSERVATIONAL BEHAVIORAL FOLLOW-UP ASSESSMENT, INCLUDES PHYSICIAN OR OTHER QUALIFIED HEALTH CARE PROFESSIONAL DIRECTION WITH INTERPRETATION AND REPORT, 0361T ADMINISTERED BY ONE TECHNICIAN; EACH ADDITIONAL 30 MINUTES OF TECHNICIAN TIME, FACE-TO-FACE WITH THE PATIENT (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY SERVICE) EXPOSURE BEHAVIORAL FOLLOW-UP ASSESSMENT, INCLUDES PHYSICIAN OR OTHER QUALIFIED HEALTH CARE PROFESSIONAL DIRECTION WITH INTERPRETATION AND REPORT, ADMINISTERED 0362T BY PHYSICIAN OR OTHER QUALIFIED HEALTH CARE PROFESSIONAL WITH THE ASSISTANCE OF ONE OR MORE TECHNICIANS; FIRST 30 MINUTES OF TECHNICIAN(S) TIME, FACE-TO-FACE WITH THE PATIENT 0363T Printed on 1/25/2018. Page 8 of 18
9 Group T 0365T 0366T 0367T 0368T 0369T 0370T 0371T 0372T 0373T 0374T 0375T 0376T 0381T 0382T 0383T 0384T Group 1 Description EXPOSURE BEHAVIORAL FOLLOW-UP ASSESSMENT, INCLUDES PHYSICIAN OR OTHER QUALIFIED HEALTH CARE PROFESSIONAL DIRECTION WITH INTERPRETATION AND REPORT, ADMINISTERED BY PHYSICIAN OR OTHER QUALIFIED HEALTH CARE PROFESSIONAL WITH THE ASSISTANCE OF ONE OR MORE TECHNICIANS; EACH ADDITIONAL 30 MINUTES OF TECHNICIAN(S) TIME, FACE-TO -FACE WITH THE PATIENT (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) ADAPTIVE BEHAVIOR TREATMENT BY PROTOCOL, ADMINISTERED BY TECHNICIAN, FACE-TO- FACE WITH ONE PATIENT; FIRST 30 MINUTES OF TECHNICIAN TIME ADAPTIVE BEHAVIOR TREATMENT BY PROTOCOL, ADMINISTERED BY TECHNICIAN, FACE-TO- FACE WITH ONE PATIENT; EACH ADDITIONAL 30 MINUTES OF TECHNICIAN TIME (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) GROUP ADAPTIVE BEHAVIOR TREATMENT BY PROTOCOL, ADMINISTERED BY TECHNICIAN, FACE- TO-FACE WITH TWO OR MORE PATIENTS; FIRST 30 MINUTES OF TECHNICIAN TIME GROUP ADAPTIVE BEHAVIOR TREATMENT BY PROTOCOL, ADMINISTERED BY TECHNICIAN, FACE- TO-FACE WITH TWO OR MORE PATIENTS; EACH ADDITIONAL 30 MINUTES OF TECHNICIAN TIME (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) ADAPTIVE BEHAVIOR TREATMENT WITH PROTOCOL MODIFICATION ADMINISTERED BY PHYSICIAN OR OTHER QUALIFIED HEALTH CARE PROFESSIONAL WITH ONE PATIENT; FIRST 30 MINUTES OF PATIENT FACE-TO-FACE TIME ADAPTIVE BEHAVIOR TREATMENT WITH PROTOCOL MODIFICATION ADMINISTERED BY PHYSICIAN OR OTHER QUALIFIED HEALTH CARE PROFESSIONAL WITH ONE PATIENT; EACH ADDITIONAL 30 MINUTES OF PATIENT FACE-TO-FACE TIME (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) FAMILY ADAPTIVE BEHAVIOR TREATMENT GUIDANCE, ADMINISTERED BY PHYSICIAN OR OTHER QUALIFIED HEALTH CARE PROFESSIONAL (WITHOUT THE PATIENT PRESENT) MULTIPLE-FAMILY GROUP ADAPTIVE BEHAVIOR TREATMENT GUIDANCE, ADMINISTERED BY PHYSICIAN OR OTHER QUALIFIED HEALTH CARE PROFESSIONAL (WITHOUT THE PATIENT PRESENT) ADAPTIVE BEHAVIOR TREATMENT SOCIAL SKILLS GROUP, ADMINISTERED BY PHYSICIAN OR OTHER QUALIFIED HEALTH CARE PROFESSIONAL FACE-TO-FACE WITH MULTIPLE PATIENTS EXPOSURE ADAPTIVE BEHAVIOR TREATMENT WITH PROTOCOL MODIFICATION REQUIRING TWO OR MORE TECHNICIANS FOR SEVERE MALADAPTIVE BEHAVIOR(S); FIRST 60 MINUTES OF TECHNICIANS' TIME, FACE-TO-FACE WITH PATIENT EXPOSURE ADAPTIVE BEHAVIOR TREATMENT WITH PROTOCOL MODIFICATION REQUIRING TWO OR MORE TECHNICIANS FOR SEVERE MALADAPTIVE BEHAVIOR(S); EACH ADDITIONAL 30 MINUTES OF TECHNICIANS' TIME FACE-TO-FACE WITH PATIENT (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) TOTAL DISC ARTHROPLASTY (ARTIFICIAL DISC), ANTERIOR APPROACH, INCLUDING DISCECTOMY WITH END PLATE PREPARATION (INCLUDES OSTEOPHYTECTOMY FOR NERVE ROOT OR SPINAL CORD DECOMPRESSION AND MICRODISSECTION), CERVICAL, THREE OR MORE LEVELS INSERTION OF ANTERIOR SEGMENT AQUEOUS DRAINAGE DEVICE, WITHOUT EXTRAOCULAR RESERVOIR, INTERNAL APPROACH, INTO THE TRABECULAR MESHWORK; EACH ADDITIONAL DEVICE INSERTION (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) EXTERNAL HEART RATE AND 3-AXIS ACCELEROMETER DATA RECORDING UP TO 14 DAYS TO ASSESS CHANGES IN HEART RATE AND TO MONITOR MOTION ANALYSIS FOR THE PURPOSES OF DIAGNOSING NOCTURNAL EPILEPSY SEIZURE EVENTS; INCLUDES REPORT, SCANNING ANALYSIS WITH REPORT, REVIEW AND INTERPRETATION BY A PHYSICIAN OR OTHER QUALIFIED HEALTH CARE PROFESSIONAL EXTERNAL HEART RATE AND 3-AXIS ACCELEROMETER DATA RECORDING UP TO 14 DAYS TO ASSESS CHANGES IN HEART RATE AND TO MONITOR MOTION ANALYSIS FOR THE PURPOSES OF DIAGNOSING NOCTURNAL EPILEPSY SEIZURE EVENTS; INCLUDES REPORT, SCANNING ANALYSIS WITH REPORT, REVIEW AND INTERPRETATION BY A PHYSICIAN OR OTHER QUALIFIED HEALTH CARE PROFESSIONAL, REVIEW AND INTERPRETATION ONLY EXTERNAL HEART RATE AND 3-AXIS ACCELEROMETER DATA RECORDING FROM 15 TO 30 DAYS TO ASSESS CHANGES IN HEART RATE TO MONITOR MOTION ANALYSIS FOR THE PURPOSES OF DIAGNOSING NOCTURNAL EPILEPSY SEIZURE EVENTS; INCLUDES REPORT, SCANNING ANALYSIS WITH REPORT, REVIEW AND INTERPRETATION BY A PHYSICIAN OR OTHER QUALIFIED HEALTH CARE PROFESSIONAL Printed on 1/25/2018. Page 9 of 18
10 Group T 0386T 0396T 0397T 0400T 0401T 0402T 0408T 0409T 0410T 0411T 0412T 0413T 0414T 0415T 0416T 0417T 0418T 0419T 0420T Group 1 Description EXTERNAL HEART RATE AND 3-AXIS ACCELEROMETER DATA RECORDING FROM 15 TO 30 DAYS TO ASSESS CHANGES IN HEART RATE TO MONITOR MOTION ANALYSIS FOR THE PURPOSES OF DIAGNOSING NOCTURNAL EPILEPSY SEIZURE EVENTS; INCLUDES REPORT, SCANNING ANALYSIS WITH REPORT, REVIEW AND INTERPRETATION BY A PHYSICIAN OR OTHER QUALIFIED HEALTH CARE PROFESSIONAL, REVIEW AND INTERPRETATION ONLY EXTERNAL HEART RATE AND 3-AXIS ACCELEROMETER DATA RECORDING MORE THAN 30 DAYS TO ASSESS CHANGES IN HEART RATE TO MONITOR MOTION ANALYSIS FOR THE PURPOSES OF DIAGNOSING NOCTURNAL EPILEPSY SEIZURE EVENTS; INCLUDES REPORT, SCANNING ANALYSIS WITH REPORT, REVIEW AND INTERPRETATION BY A PHYSICIAN OR OTHER QUALIFIED HEALTH CARE PROFESSIONAL EXTERNAL HEART RATE AND 3-AXIS ACCELEROMETER DATA RECORDING MORE THAN 30 DAYS TO ASSESS CHANGES IN HEART RATE TO MONITOR MOTION ANALYSIS FOR THE PURPOSES OF DIAGNOSING NOCTURNAL EPILEPSY SEIZURE EVENTS; INCLUDES REPORT, SCANNING ANALYSIS WITH REPORT, REVIEW AND INTERPRETATION BY A PHYSICIAN OR OTHER QUALIFIED HEALTH CARE PROFESSIONAL, REVIEW AND INTERPRETATION ONLY INTRA-OPERATIVE USE OF KINETIC BALANCE SENSOR FOR IMPLANT STABILITY DURING KNEE REPLACEMENT ARTHROPLASTY (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) ENDOSCOPIC RETROGRADE CHOLANGIOPANCREATOGRAPHY (ERCP), WITH OPTICAL ENDOMICROSCOPY (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) MULTI-SPECTRAL DIGITAL SKIN LESION ANALYSIS OF CLINICALLY ATYPICAL CUTANEOUS PIGMENTED LESIONS FOR DETECTION OF MELANOMAS AND HIGH RISK MELANOCYTIC ATYPIA; ONE TO FIVE LESIONS MULTI-SPECTRAL DIGITAL SKIN LESION ANALYSIS OF CLINICALLY ATYPICAL CUTANEOUS PIGMENTED LESIONS FOR DETECTION OF MELANOMAS AND HIGH RISK MELANOCYTIC ATYPIA; SIX OR MORE LESIONS COLLAGEN CROSS-LINKING OF CORNEA (INCLUDING REMOVAL OF THE CORNEAL EPITHELIUM AND INTRAOPERATIVE PACHYMETRY WHEN PERFORMED) INSERTION OR REPLACEMENT OF PERMANENT CARDIAC CONTRACTILITY MODULATION SYSTEM, INCLUDING CONTRACTILITY EVALUATION WHEN PERFORMED, AND PROGRAMMING OF SENSING AND THERAPEUTIC PARAMETERS; PULSE GENERATOR WITH TRANSVENOUS ELECTRODES INSERTION OR REPLACEMENT OF PERMANENT CARDIAC CONTRACTILITY MODULATION SYSTEM, INCLUDING CONTRACTILITY EVALUATION WHEN PERFORMED, AND PROGRAMMING OF SENSING AND THERAPEUTIC PARAMETERS; PULSE GENERATOR ONLY INSERTION OR REPLACEMENT OF PERMANENT CARDIAC CONTRACTILITY MODULATION SYSTEM, INCLUDING CONTRACTILITY EVALUATION WHEN PERFORMED, AND PROGRAMMING OF SENSING AND THERAPEUTIC PARAMETERS; ATRIAL ELECTRODE ONLY INSERTION OR REPLACEMENT OF PERMANENT CARDIAC CONTRACTILITY MODULATION SYSTEM, INCLUDING CONTRACTILITY EVALUATION WHEN PERFORMED, AND PROGRAMMING OF SENSING AND THERAPEUTIC PARAMETERS; VENTRICULAR ELECTRODE ONLY REMOVAL OF PERMANENT CARDIAC CONTRACTILITY MODULATION SYSTEM; PULSE GENERATOR ONLY REMOVAL OF PERMANENT CARDIAC CONTRACTILITY MODULATION SYSTEM; TRANSVENOUS ELECTRODE (ATRIAL OR VENTRICULAR) REMOVAL AND REPLACEMENT OF PERMANENT CARDIAC CONTRACTILITY MODULATION SYSTEM PULSE GENERATOR ONLY REPOSITIONING OF PREVIOUSLY IMPLANTED CARDIAC CONTRACTILITY MODULATION TRANSVENOUS ELECTRODE (ATRIAL OR VENTRICULAR LEAD) RELOCATION OF SKIN POCKET FOR IMPLANTED CARDIAC CONTRACTILITY MODULATION PULSE GENERATOR PROGRAMMING DEVICE EVALUATION (IN PERSON) WITH ITERATIVE ADJUSTMENT OF THE IMPLANTABLE DEVICE TO TEST THE FUNCTION OF THE DEVICE AND SELECT OPTIMAL PERMANENT PROGRAMMED VALUES WITH ANALYSIS, INCLUDING REVIEW AND REPORT, IMPLANTABLE CARDIAC CONTRACTILITY MODULATION SYSTEM INTERROGATION DEVICE EVALUATION (IN PERSON) WITH ANALYSIS, REVIEW AND REPORT, INCLUDES CONNECTION, RECORDING AND DISCONNECTION PER PATIENT ENCOUNTER, IMPLANTABLE CARDIAC CONTRACTILITY MODULATION SYSTEM DESTRUCTION OF NEUROFIBROMA, EXTENSIVE (CUTANEOUS, DERMAL EXTENDING INTO SUBCUTANEOUS); FACE, HEAD AND NECK, GREATER THAN 50 NEUROFIBROMAS DESTRUCTION OF NEUROFIBROMA, EXTENSIVE (CUTANEOUS, DERMAL EXTENDING INTO SUBCUTANEOUS); TRUNK AND EXTREMITIES, EXTENSIVE, GREATER THAN 100 NEUROFIBROMAS Printed on 1/25/2018. Page 10 of 18
11 Group 1 Group 1 Description 0422T TACTILE BREAST IMAGING BY COMPUTER-AIDED TACTILE SENSORS, UNILATERAL OR BILATERAL 0423T SECRETORY TYPE II PHOSPHOLIPASE A2 (SPLA2-IIA) INSERTION OR REPLACEMENT OF NEUROSTIMULATOR SYSTEM FOR TREATMENT OF CENTRAL 0424T SLEEP APNEA; COMPLETE SYSTEM (TRANSVENOUS PLACEMENT OF RIGHT OR LEFT STIMULATION LEAD, SENSING LEAD, IMPLANTABLE PULSE GENERATOR) 0425T INSERTION OR REPLACEMENT OF NEUROSTIMULATOR SYSTEM FOR TREATMENT OF CENTRAL SLEEP APNEA; SENSING LEAD ONLY 0426T INSERTION OR REPLACEMENT OF NEUROSTIMULATOR SYSTEM FOR TREATMENT OF CENTRAL SLEEP APNEA; STIMULATION LEAD ONLY 0427T INSERTION OR REPLACEMENT OF NEUROSTIMULATOR SYSTEM FOR TREATMENT OF CENTRAL SLEEP APNEA; PULSE GENERATOR ONLY 0428T REMOVAL OF NEUROSTIMULATOR SYSTEM FOR TREATMENT OF CENTRAL SLEEP APNEA; PULSE GENERATOR ONLY 0429T REMOVAL OF NEUROSTIMULATOR SYSTEM FOR TREATMENT OF CENTRAL SLEEP APNEA; SENSING LEAD ONLY 0430T REMOVAL OF NEUROSTIMULATOR SYSTEM FOR TREATMENT OF CENTRAL SLEEP APNEA; STIMULATION LEAD ONLY 0431T REMOVAL AND REPLACEMENT OF NEUROSTIMULATOR SYSTEM FOR TREATMENT OF CENTRAL SLEEP APNEA, PULSE GENERATOR ONLY 0432T REPOSITIONING OF NEUROSTIMULATOR SYSTEM FOR TREATMENT OF CENTRAL SLEEP APNEA; STIMULATION LEAD ONLY 0433T REPOSITIONING OF NEUROSTIMULATOR SYSTEM FOR TREATMENT OF CENTRAL SLEEP APNEA; SENSING LEAD ONLY 0434T INTERROGATION DEVICE EVALUATION IMPLANTED NEUROSTIMULATOR PULSE GENERATOR SYSTEM FOR CENTRAL SLEEP APNEA 0435T PROGRAMMING DEVICE EVALUATION OF IMPLANTED NEUROSTIMULATOR PULSE GENERATOR SYSTEM FOR CENTRAL SLEEP APNEA; SINGLE SESSION 0436T PROGRAMMING DEVICE EVALUATION OF IMPLANTED NEUROSTIMULATOR PULSE GENERATOR SYSTEM FOR CENTRAL SLEEP APNEA; DURING SLEEP STUDY IMPLANTATION OF NON-BIOLOGIC OR SYNTHETIC IMPLANT (EG, POLYPROPYLENE) FOR FASCIAL 0437T REINFORCEMENT OF THE ABDOMINAL WALL (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) MYOCARDIAL CONTRAST PERFUSION ECHOCARDIOGRAPHY, AT REST OR WITH STRESS, FOR 0439T ASSESSMENT OF MYOCARDIAL ISCHEMIA OR VIABILITY (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) 0440T ABLATION, PERCUTANEOUS, CRYOABLATION, INCLUDES IMAGING GUIDANCE; UPPER EXTREMITY DISTAL/PERIPHERAL NERVE 0441T ABLATION, PERCUTANEOUS, CRYOABLATION, INCLUDES IMAGING GUIDANCE; LOWER EXTREMITY DISTAL/PERIPHERAL NERVE 0442T ABLATION, PERCUTANEOUS, CRYOABLATION, INCLUDES IMAGING GUIDANCE; NERVE PLEXUS OR OTHER TRUNCAL NERVE (EG, BRACHIAL PLEXUS, PUDENDAL NERVE) REAL-TIME SPECTRAL ANALYSIS OF PROSTATE TISSUE BY FLUORESCENCE SPECTROSCOPY, 0443T INCLUDING IMAGING GUIDANCE (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) 0444T INITIAL PLACEMENT OF A DRUG-ELUTING OCULAR INSERT UNDER ONE OR MORE EYELIDS, INCLUDING FITTING, TRAINING, AND INSERTION, UNILATERAL OR BILATERAL 0445T SUBSEQUENT PLACEMENT OF A DRUG-ELUTING OCULAR INSERT UNDER ONE OR MORE EYELIDS, INCLUDING RE-TRAINING, AND REMOVAL OF EXISTING INSERT, UNILATERAL OR BILATERAL 0446T CREATION OF SUBCUTANEOUS POCKET WITH INSERTION OF IMPLANTABLE INTERSTITIAL GLUCOSE SENSOR, INCLUDING SYSTEM ACTIVATION AND PATIENT TRAINING 0447T REMOVAL OF IMPLANTABLE INTERSTITIAL GLUCOSE SENSOR FROM SUBCUTANEOUS POCKET VIA INCISION REMOVAL OF IMPLANTABLE INTERSTITIAL GLUCOSE SENSOR WITH CREATION OF 0448T SUBCUTANEOUS POCKET AT DIFFERENT ANATOMIC SITE AND INSERTION OF NEW IMPLANTABLE SENSOR, INCLUDING SYSTEM ACTIVATION INSERTION OR REPLACEMENT OF A PERMANENTLY IMPLANTABLE AORTIC COUNTERPULSATION VENTRICULAR ASSIST SYSTEM, ENDOVASCULAR APPROACH, AND PROGRAMMING OF SENSING 0451T AND THERAPEUTIC PARAMETERS; COMPLETE SYSTEM (COUNTERPULSATION DEVICE, VASCULAR GRAFT, IMPLANTABLE VASCULAR HEMOSTATIC SEAL, MECHANO-ELECTRICAL SKIN INTERFACE AND SUBCUTANEOUS ELECTRODES) 0452T Printed on 1/25/2018. Page 11 of 18
12 Group T 0454T 0455T 0456T 0457T 0458T 0459T 0460T 0461T 0462T 0463T 0464T 0465T 0469T 0470T 0471T 0472T 0473T 0475T 0476T 0477T 0478T Group 1 Description INSERTION OR REPLACEMENT OF A PERMANENTLY IMPLANTABLE AORTIC COUNTERPULSATION VENTRICULAR ASSIST SYSTEM, ENDOVASCULAR APPROACH, AND PROGRAMMING OF SENSING AND THERAPEUTIC PARAMETERS; AORTIC COUNTERPULSATION DEVICE AND VASCULAR HEMOSTATIC SEAL INSERTION OR REPLACEMENT OF A PERMANENTLY IMPLANTABLE AORTIC COUNTERPULSATION VENTRICULAR ASSIST SYSTEM, ENDOVASCULAR APPROACH, AND PROGRAMMING OF SENSING AND THERAPEUTIC PARAMETERS; MECHANO-ELECTRICAL SKIN INTERFACE INSERTION OR REPLACEMENT OF A PERMANENTLY IMPLANTABLE AORTIC COUNTERPULSATION VENTRICULAR ASSIST SYSTEM, ENDOVASCULAR APPROACH, AND PROGRAMMING OF SENSING AND THERAPEUTIC PARAMETERS; SUBCUTANEOUS ELECTRODE REMOVAL OF PERMANENTLY IMPLANTABLE AORTIC COUNTERPULSATION VENTRICULAR ASSIST SYSTEM; COMPLETE SYSTEM (AORTIC COUNTERPULSATION DEVICE, VASCULAR HEMOSTATIC SEAL, MECHANO-ELECTRICAL SKIN INTERFACE AND ELECTRODES) REMOVAL OF PERMANENTLY IMPLANTABLE AORTIC COUNTERPULSATION VENTRICULAR ASSIST SYSTEM; AORTIC COUNTERPULSATION DEVICE AND VASCULAR HEMOSTATIC SEAL REMOVAL OF PERMANENTLY IMPLANTABLE AORTIC COUNTERPULSATION VENTRICULAR ASSIST SYSTEM; MECHANO-ELECTRICAL SKIN INTERFACE REMOVAL OF PERMANENTLY IMPLANTABLE AORTIC COUNTERPULSATION VENTRICULAR ASSIST SYSTEM; SUBCUTANEOUS ELECTRODE RELOCATION OF SKIN POCKET WITH REPLACEMENT OF IMPLANTED AORTIC COUNTERPULSATION VENTRICULAR ASSIST DEVICE, MECHANO-ELECTRICAL SKIN INTERFACE AND ELECTRODES REPOSITIONING OF PREVIOUSLY IMPLANTED AORTIC COUNTERPULSATION VENTRICULAR ASSIST DEVICE; SUBCUTANEOUS ELECTRODE REPOSITIONING OF PREVIOUSLY IMPLANTED AORTIC COUNTERPULSATION VENTRICULAR ASSIST DEVICE; AORTIC COUNTERPULSATION DEVICE PROGRAMMING DEVICE EVALUATION (IN PERSON) WITH ITERATIVE ADJUSTMENT OF THE IMPLANTABLE MECHANO-ELECTRICAL SKIN INTERFACE AND/OR EXTERNAL DRIVER TO TEST THE FUNCTION OF THE DEVICE AND SELECT OPTIMAL PERMANENT PROGRAMMED VALUES WITH ANALYSIS, INCLUDING REVIEW AND REPORT, IMPLANTABLE AORTIC COUNTERPULSATION VENTRICULAR ASSIST SYSTEM, PER DAY INTERROGATION DEVICE EVALUATION (IN PERSON) WITH ANALYSIS, REVIEW AND REPORT, INCLUDES CONNECTION, RECORDING AND DISCONNECTION PER PATIENT ENCOUNTER, IMPLANTABLE AORTIC COUNTERPULSATION VENTRICULAR ASSIST SYSTEM, PER DAY VISUAL EVOKED POTENTIAL, TESTING FOR GLAUCOMA, WITH INTERPRETATION AND REPORT SUPRACHOROIDAL INJECTION OF A PHARMACOLOGIC AGENT (DOES NOT INCLUDE SUPPLY OF MEDICATION) RETINAL POLARIZATION SCAN, OCULAR SCREENING WITH ON-SITE AUTOMATED RESULTS, BILATERAL OPTICAL COHERENCE TOMOGRAPHY (OCT) FOR MICROSTRUCTURAL AND MORPHOLOGICAL IMAGING OF SKIN, IMAGE ACQUISITION, INTERPRETATION, AND REPORT; FIRST LESION OPTICAL COHERENCE TOMOGRAPHY (OCT) FOR MICROSTRUCTURAL AND MORPHOLOGICAL IMAGING OF SKIN, IMAGE ACQUISITION, INTERPRETATION, AND REPORT; EACH ADDITIONAL LESION (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) DEVICE EVALUATION, INTERROGATION, AND INITIAL PROGRAMMING OF INTRAOCULAR RETINAL ELECTRODE ARRAY (EG, RETINAL PROSTHESIS), IN PERSON, WITH ITERATIVE ADJUSTMENT OF THE IMPLANTABLE DEVICE TO TEST FUNCTIONALITY, SELECT OPTIMAL PERMANENT PROGRAMMED VALUES WITH ANALYSIS, INCLUDING VISUAL TRAINING, WITH REVIEW AND REPORT BY A QUALIFIED HEALTH CARE PROFESSIONAL DEVICE EVALUATION AND INTERROGATION OF INTRAOCULAR RETINAL ELECTRODE ARRAY (EG, RETINAL PROSTHESIS), IN PERSON, INCLUDING REPROGRAMMING AND VISUAL TRAINING, WHEN PERFORMED, WITH REVIEW AND REPORT BY A QUALIFIED HEALTH CARE PROFESSIONAL RECORDING OF FETAL MAGNETIC CARDIAC SIGNAL USING AT LEAST 3 CHANNELS; PATIENT RECORDING AND STORAGE, DATA SCANNING WITH SIGNAL EXTRACTION, TECHNICAL ANALYSIS AND RESULT, AS WELL AS SUPERVISION, REVIEW, AND INTERPRETATION OF REPORT BY A PHYSICIAN OR OTHER QUALIFIED HEALTH CARE PROFESSIONAL RECORDING OF FETAL MAGNETIC CARDIAC SIGNAL USING AT LEAST 3 CHANNELS; PATIENT RECORDING, DATA SCANNING, WITH RAW ELECTRONIC SIGNAL TRANSFER OF DATA AND STORAGE RECORDING OF FETAL MAGNETIC CARDIAC SIGNAL USING AT LEAST 3 CHANNELS; SIGNAL EXTRACTION, TECHNICAL ANALYSIS, AND RESULT Printed on 1/25/2018. Page 12 of 18
13 Group T 0480T 0481T 0482T 0483T 0484T 0485T 0486T 0487T 0488T 0489T 0490T 0491T 0492T 0493T 0494T 0495T 0496T 0497T 0498T Group 1 Description RECORDING OF FETAL MAGNETIC CARDIAC SIGNAL USING AT LEAST 3 CHANNELS; REVIEW, INTERPRETATION, REPORT BY PHYSICIAN OR OTHER QUALIFIED HEALTH CARE PROFESSIONAL FRACTIONAL ABLATIVE LASER FENESTRATION OF BURN AND TRAUMATIC SCARS FOR FUNCTIONAL IMPROVEMENT; FIRST 100 CM2 OR PART THEREOF, OR 1% OF BODY SURFACE AREA OF INFANTS AND CHILDREN FRACTIONAL ABLATIVE LASER FENESTRATION OF BURN AND TRAUMATIC SCARS FOR FUNCTIONAL IMPROVEMENT; EACH ADDITIONAL 100 CM2, OR EACH ADDITIONAL 1% OF BODY SURFACE AREA OF INFANTS AND CHILDREN, OR PART THEREOF (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) INJECTION(S), AUTOLOGOUS WHITE BLOOD CELL CONCENTRATE (AUTOLOGOUS PROTEIN SOLUTION), ANY SITE, INCLUDING IMAGE GUIDANCE, HARVESTING AND PREPARATION, WHEN PERFORMED ABSOLUTE QUANTITATION OF MYOCARDIAL BLOOD FLOW, POSITRON EMISSION TOMOGRAPHY (PET), REST AND STRESS (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) TRANSCATHETER MITRAL VALVE IMPLANTATION/REPLACEMENT (TMVI) WITH PROSTHETIC VALVE; PERCUTANEOUS APPROACH, INCLUDING TRANSSEPTAL PUNCTURE, WHEN PERFORMED TRANSCATHETER MITRAL VALVE IMPLANTATION/REPLACEMENT (TMVI) WITH PROSTHETIC VALVE; TRANSTHORACIC EXPOSURE (EG, THORACOTOMY, TRANSAPICAL) OPTICAL COHERENCE TOMOGRAPHY (OCT) OF MIDDLE EAR, WITH INTERPRETATION AND REPORT; UNILATERAL OPTICAL COHERENCE TOMOGRAPHY (OCT) OF MIDDLE EAR, WITH INTERPRETATION AND REPORT; BILATERAL BIOMECHANICAL MAPPING, TRANSVAGINAL, WITH REPORT PREVENTIVE BEHAVIOR CHANGE, ONLINE/ELECTRONIC STRUCTURED INTENSIVE PROGRAM FOR PREVENTION OF DIABETES USING A STANDARDIZED DIABETES PREVENTION PROGRAM CURRICULUM, PROVIDED TO AN INDIVIDUAL, PER 30 DAYS AUTOLOGOUS ADIPOSE-DERIVED REGENERATIVE CELL THERAPY FOR SCLERODERMA IN THE HANDS; ADIPOSE TISSUE HARVESTING, ISOLATION AND PREPARATION OF HARVESTED CELLS INCLUDING INCUBATION WITH CELL DISSOCIATION ENZYMES, REMOVAL OF NON-VIABLE CELLS AND DEBRIS, DETERMINATION OF CONCENTRATION AND DILUTION OF REGENERATIVE CELLS AUTOLOGOUS ADIPOSE-DERIVED REGENERATIVE CELL THERAPY FOR SCLERODERMA IN THE HANDS; MULTIPLE INJECTIONS IN ONE OR BOTH HANDS ABLATIVE LASER TREATMENT, NON-CONTACT, FULL FIELD AND FRACTIONAL ABLATION, OPEN WOUND, PER DAY, TOTAL TREATMENT SURFACE AREA; FIRST 20 SQ CM OR LESS ABLATIVE LASER TREATMENT, NON-CONTACT, FULL FIELD AND FRACTIONAL ABLATION, OPEN WOUND, PER DAY, TOTAL TREATMENT SURFACE AREA; EACH ADDITIONAL 20 SQ CM, OR PART THEREOF (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) NEAR-INFRARED SPECTROSCOPY STUDIES OF LOWER EXTREMITY WOUNDS (EG, FOR OXYHEMOGLOBIN MEASUREMENT) SURGICAL PREPARATION AND CANNULATION OF MARGINAL (EXTENDED) CADAVER DONOR LUNG(S) TO EX VIVO ORGAN PERFUSION SYSTEM, INCLUDING DECANNULATION, SEPARATION FROM THE PERFUSION SYSTEM, AND COLD PRESERVATION OF THE ALLOGRAFT PRIOR TO IMPLANTATION, WHEN PERFORMED INITIATION AND MONITORING MARGINAL (EXTENDED) CADAVER DONOR LUNG(S) ORGAN PERFUSION SYSTEM BY PHYSICIAN OR QUALIFIED HEALTH CARE PROFESSIONAL, INCLUDING PHYSIOLOGICAL AND LABORATORY ASSESSMENT (EG, PULMONARY ARTERY FLOW, PULMONARY ARTERY PRESSURE, LEFT ATRIAL PRESSURE, PULMONARY VASCULAR RESISTANCE, MEAN/PEAK AND PLATEAU AIRWAY PRESSURE, DYNAMIC COMPLIANCE AND PERFUSATE GAS ANALYSIS), INCLUDING BRONCHOSCOPY AND X RAY WHEN PERFORMED; FIRST TWO HOURS IN STERILE FIELD INITIATION AND MONITORING MARGINAL (EXTENDED) CADAVER DONOR LUNG(S) ORGAN PERFUSION SYSTEM BY PHYSICIAN OR QUALIFIED HEALTH CARE PROFESSIONAL, INCLUDING PHYSIOLOGICAL AND LABORATORY ASSESSMENT (EG, PULMONARY ARTERY FLOW, PULMONARY ARTERY PRESSURE, LEFT ATRIAL PRESSURE, PULMONARY VASCULAR RESISTANCE, MEAN/PEAK AND PLATEAU AIRWAY PRESSURE, DYNAMIC COMPLIANCE AND PERFUSATE GAS ANALYSIS), INCLUDING BRONCHOSCOPY AND X RAY WHEN PERFORMED; EACH ADDITIONAL HOUR (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY PROCEDURE) EXTERNAL PATIENT-ACTIVATED, PHYSICIAN- OR OTHER QUALIFIED HEALTH CARE PROFESSIONAL-PRESCRIBED, ELECTROCARDIOGRAPHIC RHYTHM DERIVED EVENT RECORDER WITHOUT 24 HOUR ATTENDED MONITORING; IN-OFFICE CONNECTION Printed on 1/25/2018. Page 13 of 18
14 Group T 0500T 0501T 0502T 0503T 0504T Group 2 Paragraph: Group 1 Description EXTERNAL PATIENT-ACTIVATED, PHYSICIAN- OR OTHER QUALIFIED HEALTH CARE PROFESSIONAL-PRESCRIBED, ELECTROCARDIOGRAPHIC RHYTHM DERIVED EVENT RECORDER WITHOUT 24 HOUR ATTENDED MONITORING; REVIEW AND INTERPRETATION BY A PHYSICIAN OR OTHER QUALIFIED HEALTH CARE PROFESSIONAL PER 30 DAYS WITH AT LEAST ONE PATIENT- GENERATED TRIGGERED EVENT CYSTOURETHROSCOPY, WITH MECHANICAL DILATION AND URETHRAL THERAPEUTIC DRUG DELIVERY FOR URETHRAL STRICTURE OR STENOSIS, INCLUDING FLUOROSCOPY, WHEN PERFORMED INFECTIOUS AGENT DETECTION BY NUCLEIC ACID (DNA OR RNA), HUMAN PAPILLOMAVIRUS (HPV) FOR FIVE OR MORE SEPARATELY REPORTED HIGH-RISK HPV TYPES (EG, 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68) (IE, GENOTYPING) NONINVASIVE ESTIMATED CORONARY FRACTIONAL FLOW RESERVE (FFR) DERIVED FROM CORONARY COMPUTED TOMOGRAPHY ANGIOGRAPHY DATA USING COMPUTATION FLUID DYNAMICS PHYSIOLOGIC SIMULATION SOFTWARE ANALYSIS OF FUNCTIONAL DATA TO ASSESS THE SEVERITY OF CORONARY ARTERY DISEASE; DATA PREPARATION AND TRANSMISSION, ANALYSIS OF FLUID DYNAMICS AND SIMULATED MAXIMAL CORONARY HYPEREMIA, GENERATION OF ESTIMATED FFR MODEL, WITH ANATOMICAL DATA REVIEW IN COMPARISON WITH ESTIMATED FFR MODEL TO RECONCILE DISCORDANT DATA, INTERPRETATION AND REPORT NONINVASIVE ESTIMATED CORONARY FRACTIONAL FLOW RESERVE (FFR) DERIVED FROM CORONARY COMPUTED TOMOGRAPHY ANGIOGRAPHY DATA USING COMPUTATION FLUID DYNAMICS PHYSIOLOGIC SIMULATION SOFTWARE ANALYSIS OF FUNCTIONAL DATA TO ASSESS THE SEVERITY OF CORONARY ARTERY DISEASE; DATA PREPARATION AND TRANSMISSION NONINVASIVE ESTIMATED CORONARY FRACTIONAL FLOW RESERVE (FFR) DERIVED FROM CORONARY COMPUTED TOMOGRAPHY ANGIOGRAPHY DATA USING COMPUTATION FLUID DYNAMICS PHYSIOLOGIC SIMULATION SOFTWARE ANALYSIS OF FUNCTIONAL DATA TO ASSESS THE SEVERITY OF CORONARY ARTERY DISEASE; ANALYSIS OF FLUID DYNAMICS AND SIMULATED MAXIMAL CORONARY HYPEREMIA, AND GENERATION OF ESTIMATED FFR MODEL NONINVASIVE ESTIMATED CORONARY FRACTIONAL FLOW RESERVE (FFR) DERIVED FROM CORONARY COMPUTED TOMOGRAPHY ANGIOGRAPHY DATA USING COMPUTATION FLUID DYNAMICS PHYSIOLOGIC SIMULATION SOFTWARE ANALYSIS OF FUNCTIONAL DATA TO ASSESS THE SEVERITY OF CORONARY ARTERY DISEASE; ANATOMICAL DATA REVIEW IN COMPARISON WITH ESTIMATED FFR MODEL TO RECONCILE DISCORDANT DATA, INTERPRETATION AND REPORT For these services, Noridian has received substantial information and has determined that the services do not meet the coverage criteria described above. These codes are listed in the Non-Covered Services LCD (L35008). Group 2 s: Group 2 Group 2 Description PLACEMENT OF A SUBCONJUNCTIVAL RETINAL PROSTHESIS RECEIVER AND PULSE 0100T GENERATOR, AND IMPLANTATION OF INTRAOCULAR RETINAL ELECTRODE ARRAY, WITH VITRECTOMY VAGUS NERVE BLOCKING THERAPY (MORBID OBESITY); LAPAROSCOPIC IMPLANTATION OF NEUROSTIMULATOR ELECTRODE ARRAY, ANTERIOR AND POSTERIOR VAGAL TRUNKS 0312T ADJACENT TO ESOPHAGOGASTRIC JUNCTION (EGJ), WITH IMPLANTATION OF PULSE GENERATOR, INCLUDES PROGRAMMING VAGUS NERVE BLOCKING THERAPY (MORBID OBESITY); LAPAROSCOPIC REVISION OR 0313T REPLACEMENT OF VAGAL TRUNK NEUROSTIMULATOR ELECTRODE ARRAY, INCLUDING CONNECTION TO EXISTING PULSE GENERATOR 0316T VAGUS NERVE BLOCKING THERAPY (MORBID OBESITY); REPLACEMENT OF PULSE GENERATOR VAGUS NERVE BLOCKING THERAPY (MORBID OBESITY); NEUROSTIMULATOR PULSE 0317T GENERATOR ELECTRONIC ANALYSIS, INCLUDES REPROGRAMMING WHEN PERFORMED ULTRASOUND, ELASTOGRAPHY (LIST SEPARATELY IN ADDITION TO CODE FOR PRIMARY 0346T PROCEDURE) 0398T Printed on 1/25/2018. Page 14 of 18
POLICY AND PROCEDURE
 PAGE: Page 1 of 8 SCOPE: This policy applies to any provider furnishing services represented by Category III CPT codes. PURPOSE & IMPORTANT REMINDER: This policy is current at the time of publication.
PAGE: Page 1 of 8 SCOPE: This policy applies to any provider furnishing services represented by Category III CPT codes. PURPOSE & IMPORTANT REMINDER: This policy is current at the time of publication.
Products and Services Considered Experimental and Investigational
 0054T Computer-assisted musculoskeletal surgical navigational orthopedic procedure, with image-guidance based on fluoroscopic images (List separately in addition to code for primary procedure) 0055T Computer-assisted
0054T Computer-assisted musculoskeletal surgical navigational orthopedic procedure, with image-guidance based on fluoroscopic images (List separately in addition to code for primary procedure) 0055T Computer-assisted
Products and Services Considered Experimental and Investigational
 0054T Computer-assisted musculoskeletal surgical navigational orthopedic procedure, with image-guidance based on fluoroscopic images (List separately in addition to code for primary procedure) 0055T Computer-assisted
0054T Computer-assisted musculoskeletal surgical navigational orthopedic procedure, with image-guidance based on fluoroscopic images (List separately in addition to code for primary procedure) 0055T Computer-assisted
00731 Anesthesia for upper gastrointestinal endoscopic procedures, endoscope introduced proximal to duodenum; not otherwise specified 01/01/2018
 00731 Anesthesia for upper gastrointestinal endoscopic procedures, endoscope introduced proximal to duodenum; not otherwise specified 01/01/2018 00732 Anesthesia for upper gastrointestinal endoscopic procedures,
00731 Anesthesia for upper gastrointestinal endoscopic procedures, endoscope introduced proximal to duodenum; not otherwise specified 01/01/2018 00732 Anesthesia for upper gastrointestinal endoscopic procedures,
MEDICAL POLICY New/Experimental Technology Procedure/Services
 POLICY: PG0043 ORIGINAL EFFECTIVE: 07/05/05 LAST REVIEW: 01/25/18 MEDICAL POLICY New/Experimental Technology Procedure/Services GUIDELINES This policy does not certify benefits or authorization of benefits,
POLICY: PG0043 ORIGINAL EFFECTIVE: 07/05/05 LAST REVIEW: 01/25/18 MEDICAL POLICY New/Experimental Technology Procedure/Services GUIDELINES This policy does not certify benefits or authorization of benefits,
MEDICAL POLICY New/Experimental Technology Procedure/Services
 POLICY: PG0043 ORIGINAL EFFECTIVE: 07/05/05 LAST REVIEW: 07/26/18 MEDICAL POLICY New/Experimental Technology Procedure/Services GUIDELINES This policy does not certify benefits or authorization of benefits,
POLICY: PG0043 ORIGINAL EFFECTIVE: 07/05/05 LAST REVIEW: 07/26/18 MEDICAL POLICY New/Experimental Technology Procedure/Services GUIDELINES This policy does not certify benefits or authorization of benefits,
2012 CPT Changes Affecting Radiology REVISIONS
 2012 CPT Changes Affecting Radiology REVISIONS 22520 Percutaneous vertebroplasty (bone biopsy included when performed), 1 vertebral body, unilateral or bilateral injection; thoracic 22521 lumbar 22522
2012 CPT Changes Affecting Radiology REVISIONS 22520 Percutaneous vertebroplasty (bone biopsy included when performed), 1 vertebral body, unilateral or bilateral injection; thoracic 22521 lumbar 22522
Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Jurisdiction Oregon. Retirement Date N/A
 Local Coverage Determination (LCD): Non-Covered Services (L34886) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor
Local Coverage Determination (LCD): Non-Covered Services (L34886) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor
CPT Category III Codes
 CPT Category III Codes Most recent changes to the CPT Category III Codes document Addition of 20 new Category III codes (0543T-0562T) and one revised Category III code (0402T) and guidelines accepted by
CPT Category III Codes Most recent changes to the CPT Category III Codes document Addition of 20 new Category III codes (0543T-0562T) and one revised Category III code (0402T) and guidelines accepted by
Cigna - Prior Authorization Procedure List: Radiology & Cardiology
 Cigna - Prior Authorization Procedure List: Radiology & Cardiology Category CPT Code CPT Code Description 93451 Right heart catheterization 93452 Left heart catheterization 93453 Combined right and left
Cigna - Prior Authorization Procedure List: Radiology & Cardiology Category CPT Code CPT Code Description 93451 Right heart catheterization 93452 Left heart catheterization 93453 Combined right and left
Cigna - Prior Authorization Procedure List Cardiology
 Cigna - Prior Authorization Procedure List Cardiology Category CPT Code CPT Code Description 33206 Insertion of new or replacement of permanent pacemaker with transvenous electrode(s); atrial 33207 Insertion
Cigna - Prior Authorization Procedure List Cardiology Category CPT Code CPT Code Description 33206 Insertion of new or replacement of permanent pacemaker with transvenous electrode(s); atrial 33207 Insertion
Cigna - Prior Authorization Procedure List: Radiology & Cardiology
 Cigna - Prior Authorization Procedure List: Radiology & Cardiology Product Category CPT Code CPT Code Description Radiology MR 70336 MRI Temporomandibular Joint(s), (TMJ) Radiology CT 70450 CT Head or
Cigna - Prior Authorization Procedure List: Radiology & Cardiology Product Category CPT Code CPT Code Description Radiology MR 70336 MRI Temporomandibular Joint(s), (TMJ) Radiology CT 70450 CT Head or
CPT Category III Codes
 CPT Category III Codes Most recent changes to the CPT Category III Codes document Addition of 35 new and/or revised Category III codes (0335T, 0509T-0542T) and guidelines accepted by the CPT Editorial
CPT Category III Codes Most recent changes to the CPT Category III Codes document Addition of 35 new and/or revised Category III codes (0335T, 0509T-0542T) and guidelines accepted by the CPT Editorial
CY2017 Hospital Outpatient: Vascular Procedure APCs and Complexity Adjustments
 CY2017 Hospital Outpatient: Vascular Procedure APCs and Complexity Adjustments Comprehensive Ambulatory Payment Classifications (c-apcs) In CY2015 and in an effort to help pay providers for quality, not
CY2017 Hospital Outpatient: Vascular Procedure APCs and Complexity Adjustments Comprehensive Ambulatory Payment Classifications (c-apcs) In CY2015 and in an effort to help pay providers for quality, not
Jurisdiction New Mexico. Retirement Date N/A
 Local Coverage Determination (LCD): Chiropractic Services (L34816) Contractor Information Contractor Name Novitas Solutions, Inc. opens in new Contract Number 04212 Contract Type A and B MAC J - H LCD
Local Coverage Determination (LCD): Chiropractic Services (L34816) Contractor Information Contractor Name Novitas Solutions, Inc. opens in new Contract Number 04212 Contract Type A and B MAC J - H LCD
Contractor Information
 Local Coverage Determination (LCD): Category III Codes (L35490) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor Name
Local Coverage Determination (LCD): Category III Codes (L35490) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor Name
22110 vertebral segment; cervical vertebral segment; thoracic vertebral segment; lumbar
 The following codes are authorized by Palladian Health for applicable product lines. Visit palladianhealth.com to request authorization and to access guidelines. Palladian Musculoskeletal Program Codes
The following codes are authorized by Palladian Health for applicable product lines. Visit palladianhealth.com to request authorization and to access guidelines. Palladian Musculoskeletal Program Codes
2014 Deleted CPT Codes
 2014 Deleted CPT Codes Surgery 13150 - Repair, complex, eyelids, nose, ears and/or lips; 1.0 cm or less 19102 - Biopsy of breast; percutaneous, needle core, using imaging guidance 19103 - Biopsy of breast;
2014 Deleted CPT Codes Surgery 13150 - Repair, complex, eyelids, nose, ears and/or lips; 1.0 cm or less 19102 - Biopsy of breast; percutaneous, needle core, using imaging guidance 19103 - Biopsy of breast;
Inspire Medical Systems. Physician Billing Guide
 Inspire Medical Systems Physician Billing Guide 2019 Inspire Medical Systems Physician Billing Guide This Physician Billing Guide was developed to help providers correctly bill for Inspire Upper Airway
Inspire Medical Systems Physician Billing Guide 2019 Inspire Medical Systems Physician Billing Guide This Physician Billing Guide was developed to help providers correctly bill for Inspire Upper Airway
CY2015 Hospital Outpatient: Endovascular Procedure APCs and Complexity Adjustments
 CY2015 Hospital Outpatient: Endovascular Procedure APCs Complexity Adjustments Comprehensive Ambulatory Payment Classifications (c-apcs) CMS finalized the implementation of 25 Comprehensive APC to further
CY2015 Hospital Outpatient: Endovascular Procedure APCs Complexity Adjustments Comprehensive Ambulatory Payment Classifications (c-apcs) CMS finalized the implementation of 25 Comprehensive APC to further
Codes Requiring Authorization from MedSolutions (MSI): Updated 3/2014
 s Requiring Authorization from MedSolutions (): Updated 3/2014 0042T Cerebral Perfusion Analysis using CT with contrast 0159T CAD, including computer algorithm analysis, BREAST MRI 0195T prepare interspace,
s Requiring Authorization from MedSolutions (): Updated 3/2014 0042T Cerebral Perfusion Analysis using CT with contrast 0159T CAD, including computer algorithm analysis, BREAST MRI 0195T prepare interspace,
Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Jurisdiction Oregon. Retirement Date N/A
 Local Coverage Determination (LCD): Circulating Tumor Cell Marker Assays (L35096) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information
Local Coverage Determination (LCD): Circulating Tumor Cell Marker Assays (L35096) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information
2012 CPT Coding Update AANS/CNS Joint Section on Disorders of the Spine and Peripheral Nerves
 2012 CPT Coding Update AANS/CNS Joint Section on Disorders of the Spine and Peripheral Nerves Joseph S. Cheng, M.D., M.S. Associate Professor of Neurological Surgery, Orthopedic Surgery, and Rehabilitation
2012 CPT Coding Update AANS/CNS Joint Section on Disorders of the Spine and Peripheral Nerves Joseph S. Cheng, M.D., M.S. Associate Professor of Neurological Surgery, Orthopedic Surgery, and Rehabilitation
Orthopedic Coding Changes for 2012
 Orthopedic Coding Changes for Lynn M. Anderanin, CPC,CPC-I, COSC Vertebroplasty 22520- Percutaneous vertebroplasty, 1 vertebral body, unilateral or bilateral injection; thoracic 22520- Percutaneous vertebroplasty,
Orthopedic Coding Changes for Lynn M. Anderanin, CPC,CPC-I, COSC Vertebroplasty 22520- Percutaneous vertebroplasty, 1 vertebral body, unilateral or bilateral injection; thoracic 22520- Percutaneous vertebroplasty,
CPT 2018 Radiology Code Changes
 CPT 2018 Radiology Code Changes CPT 2018 Radiology Code Changes The following is a listing of new Current Procedural Terminology (CPT ) codes and their descriptors as described in the CPT 2018 codebook.
CPT 2018 Radiology Code Changes CPT 2018 Radiology Code Changes The following is a listing of new Current Procedural Terminology (CPT ) codes and their descriptors as described in the CPT 2018 codebook.
Reimbursement Guide Zenith Fenestrated AAA Endovascular Graft
 MEDICAL Reimbursement Guide Zenith Fenestrated AAA Endovascular Graft Disclaimer: The information provided herein reflects Cook s understanding of the procedure(s) and/or device(s) from sources that may
MEDICAL Reimbursement Guide Zenith Fenestrated AAA Endovascular Graft Disclaimer: The information provided herein reflects Cook s understanding of the procedure(s) and/or device(s) from sources that may
evicore cardiology procedures and services requiring prior authorization
 evicore cardiology procedures and services requiring prior authorization Moda Health Commercial Group and Individual Members* *Check EBT to verify member enrollment in evicore program Radiology Advanced
evicore cardiology procedures and services requiring prior authorization Moda Health Commercial Group and Individual Members* *Check EBT to verify member enrollment in evicore program Radiology Advanced
New and Emerging Medical Technologies and Procedures
 Medical Policy Manual Medicine, Policy No. 149 New and Emerging Medical Technologies and Procedures Next Review: December 2018 Last Review: June 2018 Effective: July 1, 2018 IMPORTANT REMINDER Medical
Medical Policy Manual Medicine, Policy No. 149 New and Emerging Medical Technologies and Procedures Next Review: December 2018 Last Review: June 2018 Effective: July 1, 2018 IMPORTANT REMINDER Medical
CPT Category III Codes
 CPT Category III Codes Most recent changes to the CPT Category III Codes document Addition of Cellular and Gene Therapy guidelines. Addition of 35 new and/or revised Category III codes (0335T, 0509T-0542T)
CPT Category III Codes Most recent changes to the CPT Category III Codes document Addition of Cellular and Gene Therapy guidelines. Addition of 35 new and/or revised Category III codes (0335T, 0509T-0542T)
CERVICAL PROCEDURES PHYSICIAN CODING
 CERVICAL PROCEDURES PHYSICIAN CODING Anterior Cervical Discectomy with Interbody Fusion (ACDF) Anterior interbody fusion, with discectomy and decompression; cervical below C2 22551 first interspace 22552
CERVICAL PROCEDURES PHYSICIAN CODING Anterior Cervical Discectomy with Interbody Fusion (ACDF) Anterior interbody fusion, with discectomy and decompression; cervical below C2 22551 first interspace 22552
Local Coverage Determination (LCD): RAST Type Tests ( L30524 )
 Page 2 of 6 Local Coverage Determination (LCD): RAST Type Tests ( L30524 ) Contractor Information Contractor Name Novitas Solutions, Inc. Contract Number 12502 Contract Type A and B MAC LCD Information
Page 2 of 6 Local Coverage Determination (LCD): RAST Type Tests ( L30524 ) Contractor Information Contractor Name Novitas Solutions, Inc. Contract Number 12502 Contract Type A and B MAC LCD Information
SUPPLEMENTAL MATERIAL
 SUPPLEMENTAL MATERIAL Table S1. Patient Selection Codes, CIED Generator Procedures Code Type Code Description ICD9 Proc 00.51 Implantation of cardiac resynchronization defibrillator, total system [CRT-D]
SUPPLEMENTAL MATERIAL Table S1. Patient Selection Codes, CIED Generator Procedures Code Type Code Description ICD9 Proc 00.51 Implantation of cardiac resynchronization defibrillator, total system [CRT-D]
Jurisdiction Georgia. Retirement Date N/A
 If you wish to save the PDF, please ensure that you change the file extension to.pdf (from.ashx). Local Coverage Determination (LCD): Surgery: Injections of the Spinal Canal (L32112) Contractor Information
If you wish to save the PDF, please ensure that you change the file extension to.pdf (from.ashx). Local Coverage Determination (LCD): Surgery: Injections of the Spinal Canal (L32112) Contractor Information
Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Jurisdiction Oregon. Retirement Date N/A
 Local Coverage Determination (LCD): MolDX: GeneSight Assay for Refractory Depression (L36324) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor
Local Coverage Determination (LCD): MolDX: GeneSight Assay for Refractory Depression (L36324) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor
Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Jurisdiction Texas. Retirement Date N/A
 Local Coverage Determination (LCD): Chiropractic Services (L35424) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor
Local Coverage Determination (LCD): Chiropractic Services (L35424) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor
National Imaging Associates, Inc. Clinical guidelines CARDIAC CATHETERIZATION -LEFT HEART CATHETERIZATION. Original Date: October 2015 Page 1 of 5
 National Imaging Associates, Inc. Clinical guidelines CARDIAC CATHETERIZATION -LEFT HEART CATHETERIZATION CPT Codes: 93451, 93452, 93453, 93454, 93455, 93456, 93457, 93458, 93459, 93460, 93461 LCD ID Number:
National Imaging Associates, Inc. Clinical guidelines CARDIAC CATHETERIZATION -LEFT HEART CATHETERIZATION CPT Codes: 93451, 93452, 93453, 93454, 93455, 93456, 93457, 93458, 93459, 93460, 93461 LCD ID Number:
2019 ABBOTT REIMBURSEMENT GUIDE CMS Physician Fee Schedule
 ABBOTT REIMBURSEMENT GUIDE CMS Physician Fee Schedule This document and the information contained herein is for general information purposes only and is not intended and does not constitute legal, reimbursement,
ABBOTT REIMBURSEMENT GUIDE CMS Physician Fee Schedule This document and the information contained herein is for general information purposes only and is not intended and does not constitute legal, reimbursement,
Medical Policy New Technology Assessment and Non-Covered Services
 Medical Policy New Technology Assessment and Non-Covered Services Subject: New Technology Assessment and Non-Covered Services Background: Harvard Pilgrim Health Care (HPHC) does not cover services or technology
Medical Policy New Technology Assessment and Non-Covered Services Subject: New Technology Assessment and Non-Covered Services Background: Harvard Pilgrim Health Care (HPHC) does not cover services or technology
2017 ICD 10 PCS Code Updates
 2017 ICD 10 PCS Code Updates Kimberly Cunningham CPC, CIC, CCS Copyright/Disclaimer text No part of this presentation may be reproduced or transmitted in any form or by any means (graphically, electronically,
2017 ICD 10 PCS Code Updates Kimberly Cunningham CPC, CIC, CCS Copyright/Disclaimer text No part of this presentation may be reproduced or transmitted in any form or by any means (graphically, electronically,
Primary to non-coronary IVUS
 codes 2018 2018 codes Primary to non-coronary IVUS Page 2 All coding, coverage, billing and payment information provided herein by Philips is gathered from third-party sources and is subject to change.
codes 2018 2018 codes Primary to non-coronary IVUS Page 2 All coding, coverage, billing and payment information provided herein by Philips is gathered from third-party sources and is subject to change.
2011 CPT Code Update. Diagnostic Radiology. Computed Tomography (CT), Abdomen and Pelvis. Deletion of Xeroradiography and Subtraction Codes
 2011 CPT Code Update [The Health Insurance Portability and Accountability Act [HIPAA] transaction and code set rules require the use of the medical code set that is valid at the time a service is provided.
2011 CPT Code Update [The Health Insurance Portability and Accountability Act [HIPAA] transaction and code set rules require the use of the medical code set that is valid at the time a service is provided.
Local Coverage Determination (LCD) for Chiropractic Services (L34816) (Posted for Notice)
 Local Coverage Determination (LCD) for Chiropractic Services (L34816) (Posted for Notice) Print Contractor Information Contractor Name Novitas Solutions, Inc. Contractor Numbers 04911, 07101, 07102, 07201,
Local Coverage Determination (LCD) for Chiropractic Services (L34816) (Posted for Notice) Print Contractor Information Contractor Name Novitas Solutions, Inc. Contractor Numbers 04911, 07101, 07102, 07201,
Inspire Medical Systems. Hospital Billing Guide
 Inspire Medical Systems Hospital Billing Guide Inspire Medical Systems Hospital Billing Guide This Hospital Billing Guide was developed to help centers correctly bill for Inspire Upper Airway Stimulation
Inspire Medical Systems Hospital Billing Guide Inspire Medical Systems Hospital Billing Guide This Hospital Billing Guide was developed to help centers correctly bill for Inspire Upper Airway Stimulation
Local Coverage Article for Chiropractic Services (A47798) Contractor Information. Article Information. Contractor Name. Contractor Numbers
 Local Coverage Article for Chiropractic Services (A47798) Print Contractor Information Contractor Name Novitas Solutions, Inc. Contractor Numbers 12501, 12502, 12101, 12102, 12201, 12202, 12301, 12302,
Local Coverage Article for Chiropractic Services (A47798) Print Contractor Information Contractor Name Novitas Solutions, Inc. Contractor Numbers 12501, 12502, 12101, 12102, 12201, 12202, 12301, 12302,
CPT 2015: Prepare Your Coding Practice For New Codes As Technology Makes An Advance
 2015 Radiology Coding Survival Guide Section X : 2015 Coding Updates CPT 2015: Prepare Your Coding Practice For New Codes As Technology Makes An Advance Watch for changes in Vertebral fracture assessment,
2015 Radiology Coding Survival Guide Section X : 2015 Coding Updates CPT 2015: Prepare Your Coding Practice For New Codes As Technology Makes An Advance Watch for changes in Vertebral fracture assessment,
Cardiovascular nuclear imaging employs non-invasive techniques to assess alterations in coronary artery flow, and ventricular function.
 National Imaging Associates, Inc. Clinical guidelines CARDIOVASCULAR NUCLEAR MEDICINE -MYOCARDIAL PERFUSION IMAGING -MUGA CPT4 Codes: Refer to pages 6-9 LCD ID Number: L33960 J 15 = KY, OH Responsible
National Imaging Associates, Inc. Clinical guidelines CARDIOVASCULAR NUCLEAR MEDICINE -MYOCARDIAL PERFUSION IMAGING -MUGA CPT4 Codes: Refer to pages 6-9 LCD ID Number: L33960 J 15 = KY, OH Responsible
Description MRI, TMJ C T Head Without Contrast C T Head With Contrast C T Head Without & With Contrast
 s Requiring Prior Authorization for the Advanced Imaging 70336 MRI, TMJ 70450 C T Head Without Contrast 70460 C T Head With Contrast 70470 C T Head Without & With Contrast 70480 C T Orbit Without Contrast
s Requiring Prior Authorization for the Advanced Imaging 70336 MRI, TMJ 70450 C T Head Without Contrast 70460 C T Head With Contrast 70470 C T Head Without & With Contrast 70480 C T Orbit Without Contrast
2017 PHYSICIAN PROCEDURE CODE CHANGES
 2017 PHYSICIAN PROCEDURE CODE CHANGES Effective for dates of service on or after 1/1/2017, refer to the New Codes listed below for billing. The discontinued codes are not valid for billing dates of service
2017 PHYSICIAN PROCEDURE CODE CHANGES Effective for dates of service on or after 1/1/2017, refer to the New Codes listed below for billing. The discontinued codes are not valid for billing dates of service
Contractor Information. LCD Information. FUTURE Local Coverage Determination (LCD): Pain Management (L35033) Document Information
 FUTURE Local Coverage Determination (LCD): Pain Management (L35033) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Please note: Future Effective Date.
FUTURE Local Coverage Determination (LCD): Pain Management (L35033) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Please note: Future Effective Date.
Reimbursement Guidelines for Pain Management Procedures 1
 GE Healthcare Reimbursement Guidelines for Pain Management Procedures 1 April 2015 www.gehealthcare.com/reimbursement This overview addresses coding, coverage, and payment for pain management procedures
GE Healthcare Reimbursement Guidelines for Pain Management Procedures 1 April 2015 www.gehealthcare.com/reimbursement This overview addresses coding, coverage, and payment for pain management procedures
b. To facilitate the management decision of a patient with an equivocal stress test.
 National Imaging Associates, Inc. Clinical guidelines EBCT HEART CT & HEART CT CONGENITAL CCTA CPT4 Codes: 75571 EBCT 75572, 75573 Heart CT & Heart CT Congenital 75574 - CCTA LCD ID Number: L33559 J K
National Imaging Associates, Inc. Clinical guidelines EBCT HEART CT & HEART CT CONGENITAL CCTA CPT4 Codes: 75571 EBCT 75572, 75573 Heart CT & Heart CT Congenital 75574 - CCTA LCD ID Number: L33559 J K
Interprovincial Billing Out-Patient Rates Effective for Visits on or after September 1, 2017
 Interprovincial Billing Out-Patient Rates Effective for Visits on or after September 1, 2017 Service Code Description Rate ($) 01 Standard Out-patient Visit, including select discrete high cost diagnostic
Interprovincial Billing Out-Patient Rates Effective for Visits on or after September 1, 2017 Service Code Description Rate ($) 01 Standard Out-patient Visit, including select discrete high cost diagnostic
CPT 2015: Save Your Practice By Shaping Up Your Spinal Procedure Reporting
 2015 Physician Coding Survival Guide CHAPTER 10: NEUROSURGERY CPT 2015: Save Your Practice By Shaping Up Your Spinal Procedure Reporting Sacroplasty codes will now be inclusive of imaging guidance. You
2015 Physician Coding Survival Guide CHAPTER 10: NEUROSURGERY CPT 2015: Save Your Practice By Shaping Up Your Spinal Procedure Reporting Sacroplasty codes will now be inclusive of imaging guidance. You
Arterial Map of the Thorax, Abdomen and Pelvis 2017 Edition
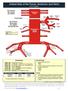 Arterial Map of the Thorax, Abdomen and Pelvis Angiography 75605 (-26) Aortography, thoracic 75625 (-26) Aortography, abdominal by serialography 75630 (-26) Aortography, abdominal + bilat iliofemoral 75705
Arterial Map of the Thorax, Abdomen and Pelvis Angiography 75605 (-26) Aortography, thoracic 75625 (-26) Aortography, abdominal by serialography 75630 (-26) Aortography, abdominal + bilat iliofemoral 75705
Contractor Number 03201
 Local Coverage Article for Bone Mass Measurements Coverage - 2012 CPT Updates (A51577) Contractor Information Contractor Name Noridian Administrative Services, LLC opens in new window Contractor Number
Local Coverage Article for Bone Mass Measurements Coverage - 2012 CPT Updates (A51577) Contractor Information Contractor Name Noridian Administrative Services, LLC opens in new window Contractor Number
Ultrasound and Fluoroscopic Paravertebral Facet Joint Injections
 Policy Number FAC06222011RP Ultrasound and Fluoroscopic Approved By UnitedHealthcare Medicare Committee Current Approval Date 06/25/2014 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy is applicable
Policy Number FAC06222011RP Ultrasound and Fluoroscopic Approved By UnitedHealthcare Medicare Committee Current Approval Date 06/25/2014 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy is applicable
High Tech Imaging Quick Reference Guide
 High Tech Imaging Quick Reference Guide 1 High Tech Imaging Authorizations may now be requested through our secure provider portal, BlueAccess. Getting Started Step 1: Log into BlueAccess from www.bcbst.com
High Tech Imaging Quick Reference Guide 1 High Tech Imaging Authorizations may now be requested through our secure provider portal, BlueAccess. Getting Started Step 1: Log into BlueAccess from www.bcbst.com
2015 Facility and Physician Billing Guide Heart Valve Technologies
 2015 Facility and Physician Billing Guide Heart Valve Technologies PHYSICIAN BILLING CODES Clinicians use Current Procedural Terminology (CPT 1 ) codes to bill for procedures and services. Each CPT code
2015 Facility and Physician Billing Guide Heart Valve Technologies PHYSICIAN BILLING CODES Clinicians use Current Procedural Terminology (CPT 1 ) codes to bill for procedures and services. Each CPT code
Anthem Blue Cross and Blue Shield Virginia Advanced Imaging Procedures Requiring Precertification Revised 02/13/2013
 Anthem Blue Cross and Blue Shield Virginia Advanced Imaging Procedures Requiring Precertification Revised 02/13/2013 Modality and CT Head CTA Head: Cerebrovascular MRI Head MRA Head: Cerebrovascular Functional
Anthem Blue Cross and Blue Shield Virginia Advanced Imaging Procedures Requiring Precertification Revised 02/13/2013 Modality and CT Head CTA Head: Cerebrovascular MRI Head MRA Head: Cerebrovascular Functional
CPT CODING EXAMPLES FUSION PROCEDURES. Anterior Lumbar Interbody Fusion (ALIF)
 CPT CODING EXAMPLES This list represents coding examples for common spine procedures. The information can also be used in conjunction with the Medicare Fee Calculator on http://www.cms.gov/apps/physician-fee-schedule/
CPT CODING EXAMPLES This list represents coding examples for common spine procedures. The information can also be used in conjunction with the Medicare Fee Calculator on http://www.cms.gov/apps/physician-fee-schedule/
Angela Clements, CPC, CEMC, COSC Internal Consultant
 Angela Clements, CPC, CEMC, COSC Internal Consultant aclements@ochsner.org angelaclements0@gmail.com Disclaimer The following information was put together based on my experience, research and expertise
Angela Clements, CPC, CEMC, COSC Internal Consultant aclements@ochsner.org angelaclements0@gmail.com Disclaimer The following information was put together based on my experience, research and expertise
Contractor Information. LCD Information. Local Coverage Determination (LCD): Hospice - Neurological Conditions (L31537) Document Information
 Local Coverage Determination (LCD): Hospice - Neurological Conditions (L31537) Contractor Information Contractor Name Palmetto GBA opens in new window LCD Information Document Information Contract Number
Local Coverage Determination (LCD): Hospice - Neurological Conditions (L31537) Contractor Information Contractor Name Palmetto GBA opens in new window LCD Information Document Information Contract Number
2013 Coding Changes. Diagnostic Radiology. Nuclear Medicine
 2013 Coding Changes The principal coding changes affecting Radiologists in 2013 occur in the Interventional Radiology Section of the AMA/CPT Manual. As in the past, we continue to see the Relative Update
2013 Coding Changes The principal coding changes affecting Radiologists in 2013 occur in the Interventional Radiology Section of the AMA/CPT Manual. As in the past, we continue to see the Relative Update
2015 Procedural Payment Guide
 Contents Introduction Disclaimer (print page 2) Description of Payment Methods (print page 3) Procedural Payment Guide: Cardiac Rhythm Management and Electrophysiology Procedures (print page range: 4-17)
Contents Introduction Disclaimer (print page 2) Description of Payment Methods (print page 3) Procedural Payment Guide: Cardiac Rhythm Management and Electrophysiology Procedures (print page range: 4-17)
PERCUTANEOUS FACET JOINT DENERVATION
 Status Active Medical and Behavioral Health Policy Section: Surgery Policy Number: IV-95 Effective Date: 10/22/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members should
Status Active Medical and Behavioral Health Policy Section: Surgery Policy Number: IV-95 Effective Date: 10/22/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members should
Cardiovascular nuclear imaging employs non-invasive techniques to assess alterations in coronary artery flow, and ventricular function.
 National Imaging Associates, Inc. Clinical guidelines CARDIOVASCULAR NUCLEAR MEDICINE -MYOCARDIAL PERFUSION IMAGING -MUGA Original Date: October 2015 Page 1 of 9 FOR CMS (MEDICARE) MEMBERS ONLY CPT4 Codes:
National Imaging Associates, Inc. Clinical guidelines CARDIOVASCULAR NUCLEAR MEDICINE -MYOCARDIAL PERFUSION IMAGING -MUGA Original Date: October 2015 Page 1 of 9 FOR CMS (MEDICARE) MEMBERS ONLY CPT4 Codes:
April 6, 2017 VIA ELECTRONIC MAIL
 April 6, 2017 VIA ELECTRONIC MAIL Patricia Brooks, RHIA Centers for Medicare and Medicaid Services CMM, HAPG, Division of Acute Care Mail Stop C4-08-06 7500 Security Boulevard Baltimore, Maryland 21244-1850
April 6, 2017 VIA ELECTRONIC MAIL Patricia Brooks, RHIA Centers for Medicare and Medicaid Services CMM, HAPG, Division of Acute Care Mail Stop C4-08-06 7500 Security Boulevard Baltimore, Maryland 21244-1850
Local Coverage Determination (LCD) for Cardiac Catheterization (L29090)
 Local Coverage Determination (LCD) for Cardiac Catheterization (L29090) Contractor Information Contractor Name First Coast Service Options, Inc. Contractor Number 09102 Contractor Type MAC - Part B LCD
Local Coverage Determination (LCD) for Cardiac Catheterization (L29090) Contractor Information Contractor Name First Coast Service Options, Inc. Contractor Number 09102 Contractor Type MAC - Part B LCD
CARDIOLOGY IMAGING PROGRAM
 CARDIOLOGY IMAGING PROGRAM TABLE OF CONTENTS. OVERVIEW............................................................................................. 618..... MEMBERS........... EXEMPT.......... FROM.......
CARDIOLOGY IMAGING PROGRAM TABLE OF CONTENTS. OVERVIEW............................................................................................. 618..... MEMBERS........... EXEMPT.......... FROM.......
Chapter 4 Section 20.1
 Surgery Chapter 4 Section 20.1 Issue Date: August 29, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) Copyright: CPT only 2006 American Medical Association (or such other date of publication of CPT). All
Surgery Chapter 4 Section 20.1 Issue Date: August 29, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) Copyright: CPT only 2006 American Medical Association (or such other date of publication of CPT). All
20983 Ablation therapy for reduction or eradication of 1 or more bone tumors (eg, metastasis) including adjacent soft tissue w
 The following Experimental and Investigational codes will no longer require medical necessity review. These changes are effective July 1, 2017 and subject to change. This update applies to all product
The following Experimental and Investigational codes will no longer require medical necessity review. These changes are effective July 1, 2017 and subject to change. This update applies to all product
Interprovincial Billing Out-Patient Rates Effective for Visits on or After April 1, 2017
 Service Code Interprovincial Billing Out-Patient Rates Effective for Visits on or After April 1, 2017 Description Rate ($) 01 Standard Out-patient Visit, including select discrete high cost diagnostic
Service Code Interprovincial Billing Out-Patient Rates Effective for Visits on or After April 1, 2017 Description Rate ($) 01 Standard Out-patient Visit, including select discrete high cost diagnostic
Transanal Endoscopic Microsurgery (TEMS) Approach for Excision of Rectal Tumor (CPT Code 0184T)
 Medical Coverage Policy Effective Date... 4/15/2018 Next Review Date... 3/15/2019 Coverage Policy Number... 0504 Omnibus Codes Table of Contents Coverage Policy... 1 Overview... 12 General Background...
Medical Coverage Policy Effective Date... 4/15/2018 Next Review Date... 3/15/2019 Coverage Policy Number... 0504 Omnibus Codes Table of Contents Coverage Policy... 1 Overview... 12 General Background...
RadRx Your Prescription for Accurate Coding & Reimbursement Copyright All Rights Reserved.
 Interventional Radiology Coding Case Studies Prepared by Stacie L. Buck, RHIA, CCS-P, RCC, CIRCC, AAPC Fellow President & Senior Consultant INDICATION: Abdominal aortic aneurysm. INTERVENTIONAL RADIOLOGIST:
Interventional Radiology Coding Case Studies Prepared by Stacie L. Buck, RHIA, CCS-P, RCC, CIRCC, AAPC Fellow President & Senior Consultant INDICATION: Abdominal aortic aneurysm. INTERVENTIONAL RADIOLOGIST:
Removal of Total Knee Arthroplasty (TKA) from the Inpatient-Only List (IPO)
 November 7, 2017 Subject: (CMS 1678 F-C) Summary of the Centers for Medicare and Medicaid Services Fiscal Year 2018 Outpatient Prospective Systems () and Ambulatory Surgery System (ASC) Final Rule with
November 7, 2017 Subject: (CMS 1678 F-C) Summary of the Centers for Medicare and Medicaid Services Fiscal Year 2018 Outpatient Prospective Systems () and Ambulatory Surgery System (ASC) Final Rule with
CODING SHEETS CHRONIC INTRACTABLE PAIN MANAGEMENT. Effective January 1, 2009 CODMAN 3000 NEUROMODULATION AND ONCOLOGY REIMBURSEMENT HOTLINE
 CODING SHEETS CHRONIC INTRACTABLE PAIN MANAGEMENT Effective January 1, 2009 CODMAN 3000 NEUROMODULATION AND ONCOLOGY REIMBURSEMENT HOTLINE Phone: 800-609-1108 Email: codmanpump@aol.com Fax: 303-703-1572
CODING SHEETS CHRONIC INTRACTABLE PAIN MANAGEMENT Effective January 1, 2009 CODMAN 3000 NEUROMODULATION AND ONCOLOGY REIMBURSEMENT HOTLINE Phone: 800-609-1108 Email: codmanpump@aol.com Fax: 303-703-1572
If you need additional information, contact our Service Management Department at or at (toll free long distance calls).
 CIRCULAR LETTER #M1801005 December 29, 2017 TO ALL TRIPLE-S SALUD PARTICIPANT PROVIDERS NEW CPT 2018 CODES CCI Integrated Care Center Enclosed is list of new codes, published in the Current Procedure Terminology
CIRCULAR LETTER #M1801005 December 29, 2017 TO ALL TRIPLE-S SALUD PARTICIPANT PROVIDERS NEW CPT 2018 CODES CCI Integrated Care Center Enclosed is list of new codes, published in the Current Procedure Terminology
1. CARDIOLOGY. These listings cannot be correctly interpreted without reference to the Preamble. Anes. $ Level
 1. CARDIOLOGY These listings cannot be correctly interpreted without reference to the Preamble. Anes. Referred Cases 33010 Consultation: To consist of examination, review of history, laboratory, X-ray
1. CARDIOLOGY These listings cannot be correctly interpreted without reference to the Preamble. Anes. Referred Cases 33010 Consultation: To consist of examination, review of history, laboratory, X-ray
Chapter 4 Section 20.1
 Surgery Chapter 4 Section 20.1 Issue Date: August 29, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) 1.0 CPT 1 PROCEDURE CODES 61000-61626, 61680-62264, 62268-62284, 62290-63048, 63055-64484, 64505-64595,
Surgery Chapter 4 Section 20.1 Issue Date: August 29, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) 1.0 CPT 1 PROCEDURE CODES 61000-61626, 61680-62264, 62268-62284, 62290-63048, 63055-64484, 64505-64595,
2 016 HF10 THERAPY HOSPITAL OUTPATIENT DEPARTMENT AND AMBULATORY SURGERY CENTER REIMBURSEMENT REFERENCE GUIDE
 HF10 therapy, delivered by the Nevro Senza System, is a new high-frequency spinal cord stimulation technology designed to aid in the management of chronic intractable pain of the trunk/limbs, including
HF10 therapy, delivered by the Nevro Senza System, is a new high-frequency spinal cord stimulation technology designed to aid in the management of chronic intractable pain of the trunk/limbs, including
INDIANA HEALTH COVERAGE PROGRAMS
 INDIANA HEALTH COVERAGE PROGRAMS PROVIDER CODE TABLES Note: Due to possible changes in Indiana Health Coverage Programs (IHCP) policy or national coding updates, inclusion of a code on the code tables
INDIANA HEALTH COVERAGE PROGRAMS PROVIDER CODE TABLES Note: Due to possible changes in Indiana Health Coverage Programs (IHCP) policy or national coding updates, inclusion of a code on the code tables
Transanal Endoscopic Microsurgery (TEMS) Approach for Excision of Rectal Tumor (CPT Code 0184T)
 Medical Coverage Policy Effective Date... 1/15/2018 Next Review Date... 3/15/2019 Coverage Policy Number... 0504 Omnibus Codes Table of Contents Coverage Policy... 1 Overview... 12 General Background...
Medical Coverage Policy Effective Date... 1/15/2018 Next Review Date... 3/15/2019 Coverage Policy Number... 0504 Omnibus Codes Table of Contents Coverage Policy... 1 Overview... 12 General Background...
Coding Changes for 2018
 Coding Changes for 2018 An overview of changes to interventional CPT coding that you need to know for practicing in 2018. BY KATHARINE L. KROL, MD, FSIR, FACR There are several coding changes for endovascular
Coding Changes for 2018 An overview of changes to interventional CPT coding that you need to know for practicing in 2018. BY KATHARINE L. KROL, MD, FSIR, FACR There are several coding changes for endovascular
Basics of Interventional Radiology Coding 2017
 Basics of Interventional Radiology Coding 2017 Prepared and Published By: MedLearn Publishing A Division of Panacea Healthcare Solutions, Inc. 287 East Sixth Street, Suite 400 St. Paul, MN 55101 1-800-252-1578
Basics of Interventional Radiology Coding 2017 Prepared and Published By: MedLearn Publishing A Division of Panacea Healthcare Solutions, Inc. 287 East Sixth Street, Suite 400 St. Paul, MN 55101 1-800-252-1578
Diagnostic Imaging Prior Review Code List 2 nd Quarter 2018
 Computerized Tomography (CT) Abdomen 6 Abdomen/Pelvis Combination 101 Service 74150 CT abdomen; w/o 74160 CT abdomen; with 74170 CT abdomen; w/o followed by 74176 Computed tomography, abdomen and pelvis;
Computerized Tomography (CT) Abdomen 6 Abdomen/Pelvis Combination 101 Service 74150 CT abdomen; w/o 74160 CT abdomen; with 74170 CT abdomen; w/o followed by 74176 Computed tomography, abdomen and pelvis;
Interprovincial Billing Out-Patient Rates Effective for Visits on or After April 1, 2018
 Service Code Interprovincial Billing Out-Patient Rates Effective for Visits on or After April 1, 2018 Description Rate ($) 01 Standard Out-patient Visit, including select discrete high cost diagnostic
Service Code Interprovincial Billing Out-Patient Rates Effective for Visits on or After April 1, 2018 Description Rate ($) 01 Standard Out-patient Visit, including select discrete high cost diagnostic
Detailed Summary of the Proposed Rule for the Hospital Outpatient Prospective Payment System
 Detailed Summary of the Proposed Rule for the Hospital Outpatient Prospective Payment System The Centers for Medicare and Medicaid Services (CMS) released its proposed rule for calendar year (CY) 2017
Detailed Summary of the Proposed Rule for the Hospital Outpatient Prospective Payment System The Centers for Medicare and Medicaid Services (CMS) released its proposed rule for calendar year (CY) 2017
Sample page. POWER UP YOUR CODING with Optum360, your trusted coding partner for 32 years. Visit optum360coding.com.
 2018 Complete Guide for Interventional Radiology An in-depth guide to interventional radiology coding, billing, and reimbursement for facilities and physicians POWER UP YOUR CODING with Optum360, your
2018 Complete Guide for Interventional Radiology An in-depth guide to interventional radiology coding, billing, and reimbursement for facilities and physicians POWER UP YOUR CODING with Optum360, your
Final MPFS 2014 Summary SIR
 Final MPFS 2014 Summary SIR The CY 2014 PFS CF is $27.2006 (p531) Impact Tables (p1285) Refinement Panel Recommendations (p183) Table 23 presents information on the work RVUs for the codes considered by
Final MPFS 2014 Summary SIR The CY 2014 PFS CF is $27.2006 (p531) Impact Tables (p1285) Refinement Panel Recommendations (p183) Table 23 presents information on the work RVUs for the codes considered by
Chapter 16 Worksheet Code It
 Name: Class: Date: ID: A Chapter 16 Worksheet 3 2 1 Code It True/False Indicate whether the statement is true or false. 1. CT scans generate three-dimensional images. 2. An ultrasound produces images of
Name: Class: Date: ID: A Chapter 16 Worksheet 3 2 1 Code It True/False Indicate whether the statement is true or false. 1. CT scans generate three-dimensional images. 2. An ultrasound produces images of
Spinal and Trigger Point Injections
 Spinal and Trigger Point Injections I. Policy University Health Alliance (UHA) will reimburse for nonsurgical interventional treatment for subacute and chronic spinal pain when determined to be medically
Spinal and Trigger Point Injections I. Policy University Health Alliance (UHA) will reimburse for nonsurgical interventional treatment for subacute and chronic spinal pain when determined to be medically
PROPOSED/DRAFT Local Coverage Determination (LCD): MolDX: Chromosome 1p/19q deletion analysis (DL36483)
 moldx: Chromosome 1p/19q deletion analysis (DL36483) Page 1 of 8 PROPOSED/DRAFT Local Coverage Determination (LCD): MolDX: Chromosome 1p/19q deletion analysis (DL36483) Close Section Navigation
moldx: Chromosome 1p/19q deletion analysis (DL36483) Page 1 of 8 PROPOSED/DRAFT Local Coverage Determination (LCD): MolDX: Chromosome 1p/19q deletion analysis (DL36483) Close Section Navigation
Basics of Interventional Radiology Coding 2018
 Basics of Interventional Radiology Coding 2018 Prepared and Published By: MedLearn Publishing A Division of MedLearn Media, Inc. 445 Minnesota Street, Suite 514 St. Paul, MN 55101 1-800-252-1578 medlearnmedia.com
Basics of Interventional Radiology Coding 2018 Prepared and Published By: MedLearn Publishing A Division of MedLearn Media, Inc. 445 Minnesota Street, Suite 514 St. Paul, MN 55101 1-800-252-1578 medlearnmedia.com
Contractor Information. LCD Information. FUTURE Local Coverage Determination (LCD): Trigger Point Injections (L35010) Document Information
 FUTURE Local Coverage Determination (LCD): Trigger Point Injections (L35010) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Please note: Future Effective
FUTURE Local Coverage Determination (LCD): Trigger Point Injections (L35010) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Please note: Future Effective
Codes for Back and Spinal Procedures
 20930 Allograft for spine surgery only; morselized 20931 Allograft for spine surgery only; structural 20936 Autograft for spine surgery only (includes harvesting the graft); local (eg, ribs, spinous process,
20930 Allograft for spine surgery only; morselized 20931 Allograft for spine surgery only; structural 20936 Autograft for spine surgery only (includes harvesting the graft); local (eg, ribs, spinous process,
Reimbursement Information for Diagnostic Musculoskeletal Ultrasound and Ultrasound-guided Procedures 1
 GE Healthcare Reimbursement Information for Diagnostic Musculoskeletal Ultrasound and Ultrasound-guided Procedures 1 January, 2013 www.gehealthcare.com/reimbursement This overview addresses coding, coverage,
GE Healthcare Reimbursement Information for Diagnostic Musculoskeletal Ultrasound and Ultrasound-guided Procedures 1 January, 2013 www.gehealthcare.com/reimbursement This overview addresses coding, coverage,
Contractor Information. LCD Information. Local Coverage Determination (LCD): Hospice Alzheimer's Disease & Related Disorders (L31539)
 Local Coverage Determination (LCD): Hospice Alzheimer's Disease & Related Disorders (L31539) Contractor Information Contractor Name Palmetto GBA opens in new window LCD Information Document Information
Local Coverage Determination (LCD): Hospice Alzheimer's Disease & Related Disorders (L31539) Contractor Information Contractor Name Palmetto GBA opens in new window LCD Information Document Information
2016 HF10 THERAPY REIMBURSEMENT REFERENCE GUIDE
 206 HF0 THERAPY REIMBURSEMENT REFERENCE GUIDE HF0 therapy, delivered by the Nevro Senza System, is a new high-frequency spinal cord stimulation technology designed to aid in the management of chronic intractable
206 HF0 THERAPY REIMBURSEMENT REFERENCE GUIDE HF0 therapy, delivered by the Nevro Senza System, is a new high-frequency spinal cord stimulation technology designed to aid in the management of chronic intractable
H F 1 0 T H E R A P Y R E I M B U R S E M E N T R E F E R E N C E G U I D E
 HF10 therapy, delivered by the Nevro Senza System, is the high-frequency spinal cord stimulation technology designed to aid in the management of chronic intractable pain of the trunk/limbs without paresthesia.
HF10 therapy, delivered by the Nevro Senza System, is the high-frequency spinal cord stimulation technology designed to aid in the management of chronic intractable pain of the trunk/limbs without paresthesia.
