The medical and economic effect of heart disease in
|
|
|
- Ambrose Golden
- 6 years ago
- Views:
Transcription
1 New Innovations in Circulatory Support With Ventricular Assist Device and Extracorporeal Membrane Oxygenation Therapy Robert N. Sladen, MBChB, FCCM E SYSTEMATIC REVIEW ARTICLE The past decade has seen an exponential increase in the application and development of durable long-term as well as nondurable short-term mechanical circulatory support for cardiogenic shock and acute or chronic heart failure. Support has evolved from bridge-to-transplant to destination therapy, bridge to rescue, bridge to decision making, and bridge to a bridge. Notable trends include device miniaturization, minimally invasive and/or percutaneous insertion, and efforts to superimpose pulsatility on continuous flow. We can certainly anticipate that innovation will accelerate in the months and years to come. However, despite or perhaps because of the enhanced equipment now available, mechanical circulatory support is an expensive, complex, resource-intensive modality. It requires considerable expertise that should preferably be centralized to highly specialized centers. Formidable challenges remain: systemic inflammatory response syndromes and vasoplegia after device insertion; postoperative sepsis; optimal anticoagulation regimens to prevent device-induced thrombosis and cerebral thromboembolism; wound site, intracranial, and gastrointestinal bleeding; multisystem injury and failure; patient dissatisfaction (even when providers consider the procedure a success ); and ethical decision making in conditions of futility. (Anesth Analg 2017;124: ) The medical and economic effect of heart disease in the United States is enormous. Today, an estimated 5.8 million Americans suffer from heart failure, representing 650,000 new cases annually with more than 1 million hospital admissions, more than 300,000 deaths, and a health care cost approaching $40 million per year. 1 The latter part of the twentieth century saw steady development of mechanical circulatory support (MCS) for end-stage heart disease or circulatory shock. However, in the past decade, its utilization and applications have exponentially increased (Figure 1 and Table 1). 2 This progress has been driven by the quarter million patients with advanced heart failure (New York Heart Association [NYHA] class IIIb or IV), of which a substantial proportion become refractory to medical management (American Heart Association stage D). Development of the Total Artificial Heart In the 1960s, Congress approved the goal of developing a total artificial heart (TAH) for advanced heart failure. In 1967, the world s attention focused on what was thought to be an exciting future for orthotopic heart transplantation (OHT), and, in 1969, Cooley et al 5 used the first TAH, the Liotta-Heart, as a 3-day bridge to transplant (BTT). The From the Department of Anesthesiology, Columbia University Medical Center, New York, New York. Accepted for publication August 24, Funding: None. The author declares no conflicts of interest. Reprints will not be available from the author. Address correspondence to Robert N. Sladen, MBChB, FCCM, Department of Anesthesiology, Columbia University Medical Center, College of Physicians & Surgeons of Columbia University, 630 West 168th St, PH 527-B, New York, NY Address to rs543@columbia.edu. Copyright 2016 International Anesthesia Research Society DOI: /ANE obstacle of acute rejection stunted the application of OHT for the next decade, but with the introduction of cyclosporine in the early 1980s, transplantation reemerged as a viable therapeutic option. However, the supply of donor hearts was far less than the demand, to the extent that more than half of potential recipients died while awaiting a transplant. This drove the development of durable MCS to sustain recipients as a BTT. In 1982, the Jarvik-7 TAH kept a 61-yearold patient alive for 112 days, 6 and, in 1985, Copeland et al 7 achieved the first successful BTT with this device. The Jarvik-7 TAH underwent considerable modification before being approved as the Syncardia TAH for BTT in Development of the Left Ventricular Assist Device The early 1990s saw the introduction of the first-generation left ventricular assist device (LVAD), the HeartMate XVE, which provided pulsatile circulatory support in series with the left ventricle (LV). A major watershed was the publication of the REMATCH study in 2001, which demonstrated that pulsatile LVAD support achieved a 1-year survival twice that of optimal medical management (OMM), approximately 50% vs 25%. 8 However, the pulsatile LVAD was limited by its large size, extensive subdiaphragmatic pocket, loud noise, potential for bleeding and pocket infection, and unidirectional inflow and outflow porcine valves that deteriorated after about 12 months. It is no longer in use. In 2007, the second-generation and first continuous flow LVAD (CF-LVAD) was introduced as the HeartMate II. This valveless impeller device provides axial flow from the LV apex to the ascending aorta, usually in parallel with the native circulation flowing through the aortic valve. It has a much smaller (although still subdiaphragmatic) profile than the XVE, and a dramatic improvement in durability. In a pivotal study that established the superiority of a April 2017 Volume 124 Number
2 E SYSTEMATIC REVIEW ARTICLE continuous versus pulsatile LVAD, the HeartMate II provided significantly greater 2-year survival (58% vs 24%) and freedom from disabling stroke or device malfunction (46% vs 11%) than the XVE. 9 This opened the door for durable LVAD support as destination therapy (DT) for patients who were not candidates for OHT. The third-generation CF-LVADs introduced a miniaturized centrifugal device implanted intrapericardially into the apex of the LV. Examples include the HeartWare HVAD, which achieved a 180-day survival of 91% as BTT, 10 and the HeartMate III, currently undergoing trials for BTT and DT. A comparison between axial flow and centrifugal flow devices is summarized in Table 2 and Figure 2. A fourthgeneration CF-VAD is represented by a further miniaturized intrapericardial device, the MVAD, that combines both axial and centrifugal flow; and a so-called fifth-generation supradiaphragmatic impeller device, the HeartAssist 5, provides remote wireless monitoring via an integrated cell phone. By 2015, more than 15,000 durable implantable devices had been placed, with a current 1-year survival rate of more than 80%. 4 The current status of durable CF-VADs is summarized in Table 3. Some patients qualifying for a durable long-term device still have potential for myocardial recovery. Ventricular chamber volume unloading may promote reverse myocardial remodeling with a shift of the end-diastolic pressure volume relationship back toward normal. Recovery may be further enhanced by neurohormonal blockade with angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and α-β antagonist therapy. 1 Unresolved Challenges of LVAD Implantation Notwithstanding the dramatic advances in CF-LVAD therapy, formidable challenges remain. These include acute surgical bleeding, vasoplegic shock and systemic inflammatory response syndrome, acute right heart failure, device thrombosis, thromboembolic stroke, platelet dysfunction from acquired von Willebrand syndrome 11, and recurrent gastrointestinal bleeding from the development of arteriovenous malformations. 12 Although gastrointestinal bleeding is seldom fatal, it is responsible for frequent hospital readmissions and impaired quality of life (QOL). Stroke is the most devastating complication of MCS, especially when it occurs in young patients who are doing well as candidates for heart transplantation. As a consequence of these complications, a certain proportion of patients will have a disproportionally prolonged intensive care unit (ICU) and hospital length of stay; recurrent readmissions to the hospital and ICU; and impaired QOL after hospital discharge. These are discussed Figure 1. Exponential growth of patient enrolment and hospital activation ( ). The INTERMACS (International Registry of Mechanical Assistance of Circulatory Support) was first established in and is updated annually. 4 This graph demonstrates the exponential increase in patient enrollment and number of hospitals activated to implant durable long-term ventricular assist devices (VADs) by the end of By 2015, more than 15,000 patients were on the registry as having received VADs. Table 1. Indications for Mechanical Circulatory Support Objective Abbreviation Rationale Rescue Rescue during a life-threatening crisis to provide temporary circulatory support Bridge to recovery BTR Rescue with anticipation of ventricular recovery, device weaning, and discontinuation Bridge to decision BTD Rescue during a life-threatening crisis to provide circulatory support until a decision can be made regarding further definitive therapy Bridge to a bridge BTB A short-term rescue device that is emergently placed to provide support until a longer-term, larger device can be placed Bridge to transplant BTT To temporarily support patients awaiting heart transplant Destination therapy DT To permanently support patients with ESHD who are not candidates for heart transplantation Abbreviation: ESHD, end-stage heart disease ANESTHESIA & ANALGESIA
3 New Innovations in VAD and ECMO Therapy Table 2. Comparison of Axial and Centrifugal Pump Flow 1 Axial (Impeller) Pump Centrifugal Pump Factors governing blood flow Rotor speed, inflow/outflow pressure differential Hydrodynamic relationship to rotor speed Steep, inverse linear Flatter, variable, more preload, and afterload sensitive Flow dependence on loading conditions Less dependent More dependent Flow range 3 7 L/min 0 10 L/min Waveform Less pulsatile More pulsatile Flow estimation Less accurate More accurate Suction events More likely Less likely Figure 2. Continuous flow durable left ventricular assist devices. The diagram on the left (A) illustrates the typical components of an implanted durable CF-LVAD. The surgically implanted pump draws blood from a left ventricular inflow cannula and pumps it to an outflow graft in the ascending aorta, creating a parallel circuit to the native circulation. The pump is connected by a percutaneous driveline via a system controller to its power source, electrically powered batteries with a life span up to 12 h. On the right, the basic types of continuous flow are illustrated: (B) axial or impeller flow (HeartMate II, Jarvik 2000); (C) centrifugal flow (HVAD, HeartMate III); and (D) hybrid flow, where the pump generates axial flow but blood exits perpendicularly as in a centrifugal pump (MVAD). CF-LVAD, continuous flow left ventricular assist device. From Mancini et al. 1 Used with permission. Table 3. Current and Investigational Long-term Durable CF-VADs Device Pocket Pump Flow Weight (g) BTT DT Thoratec HeartMate II Subdiaphragmatic Axial 281 A A Jarvik-2000 Intrapericardial Axial 85 I I HeartWare HVAD Intrapericardial Centrifugal 145 A I Thoratec HeartMate III Intrapericardial Centrifugal a I I HeartWare MVAD Intrapericardial Centrifugal/axial 85 A I Reliant HeartAssist 5 Supradiaphragmatic Axial 92 I I Abbreviations: A, approved; BTT, bridge to transplant; CF-VAD, continuous flow ventricular assist device; DT, destination therapy; I, investigational. a The weight was not provided by manufacturer. in more detail in the section on The Continuous Flow Left Ventricular Assist Device. Development of Short-term, Nondurable Devices In recent years, there has also been exponential development of short-term, nondurable devices for rescue therapy in cardiogenic shock, or to temporarily decompress the ventricles and enhance their recovery in acute heart failure. They may thereby serve as a bridge to recovery, or bridge to a decision regarding viability, transplantation, or a longterm VAD. Short-term, nondurable MCS includes extracorporeal membrane oxygenation (ECMO); surgically implanted external centrifugal devices such as the CentriMag; and an increasing number of percutaneous devices (Table 4). Placement of these devices is currently transitioning from invasive sternotomy or minimally invasive thoracotomy performed by surgeons, to peripheral percutaneous placement increasingly done by cardiologists in the cardiac catheterization laboratory or at the bedside. Prioritization for long-term device placement is based upon a consensus functional risk assessment profile April 2017 Volume 124 Number
4 E SYSTEMATIC REVIEW ARTICLE called INTERMACS (International Registry of Mechanical Assistance of Circulatory Support), first established in 2007, 3 and since updated annually (Table 5). 4 By 2015, more than 15,000 patients were on the registry as having received durable long-term VADs. Patients in cardiogenic shock (INTERMACS 1) or cardiogenic shock with intractable ventricular arrhythmias (INTERMACS 1A) may undergo percutaneous institution of ECMO with or without a short-term nondurable device as a bridge to recovery or decision. Currently accepted indications for a durable LVAD placement include patients at INTERMACS level 1 to 3, but, as device safety and longevity improve, there is an increasing hope that earlier placement (INTERMACS 4 or even higher) may become justified. LONG-TERM DURABLE DEVICES The Total Artificial Heart Syncardia TAH. The first TAH, the Jarvik-7, was implanted in Subsequently, the device was enhanced and marketed as CardioWest, and currently as the Syncardia TAH. It comprises 2 pneumatically driven ventricles with 2 mechanical valves sewn into the atria and is capable of generating cardiac outputs of up to 9 L/min. It is primarily indicated for patients who are not candidates for an LVAD because of complications such as intractable ventricular tachyarrhythmias, an ischemic ventricular septal defect (VSD), intracardiac thrombus, congenital heart disease, or severe right heart failure (RHF). The TAH obviously cannot be used as a bridge to recovery. In 2004, the Syncardia TAH was approved by the Food and Drug Administration (FDA) for BTT, and trials for DT are underway. The limitations of the Syncardia TAH have been its large volume displacement, which makes it largely unsuitable for female and small patients, and its massive console. A small portable console was introduced in 2012, and an option for smaller chambers (50 ml vs 70 ml) is in progress. In 2012 Copeland et al 14 published the results achieved at the University of Arizona in 101 patients who received the Syncardia TAH as BTT. Despite the fact that 90% of the patients were at INTERMACS 1 or 1A, the total group had n almost 70% survival to transplant and a less than 10% incidence of stroke, although there was a 25% incidence of take-back for bleeding. The authors concluded that the TAH provides a real alternative for patients who are not candidates for an LVAD, and who otherwise would be assigned to terminal hospice care. Nonetheless, implantation of the Syncardia TAH is largely confined to 3 major centers, 2 in Europe and 1 in the United States. About 1300 of these devices have been implanted over the last 3 decades, which represents less than 10% of all durable MCS. CARMAT TAH Development of the CARMAT TAH was initiated by Carpentier as long ago as 1993, and since 2008 has been developed by CARMAT SAS as a second-generation TAH. 15 It consists of two 65-mL chambers covered by a flexible compliance bag with actuating fluid. Enhancements over the Syncardia TAH include noiseless electrohydraulic pumps that produce pulsatile flow; biocompatible processed bovine pericardial tissue membranes to decrease contact activation, thrombosis, and bleeding; and ventricular pressure sensors that govern a sophisticated control algorithm responding to changes in preload and afterload with a variable cardiac output (CO) range of 2 to 9 L/min. Limitations of the CARMAT TAH are its large (750 ml) displacement, suitable for only 15% of female candidates; and its unknown long-term durability. If the sensors fail, the entire device must be replaced. By January 2016, a 3-year feasibility study had been completed on 4 patients, who survived between 2 and 8 months, and a pivotal study is underway. 16 The Continuous Flow Left Ventricular Assist Device Advances in Hemodynamic Management. The CF-VAD provides assisted flow that is in parallel to the native circulation, such that a variable proportion of the LV stroke volume is ejected via the native aorta. As a consequence, aortic valve function must be preserved, and the valve repaired or replaced if there is significant aortic stenosis or regurgitation. Continuous or intermittent opening of the aortic valve is an important safeguard against valve thrombosis, which may be exacerbated by inadvertently excessive oversewing of an incompetent valve. Conversely, Table 4. Short-term Nondurable Devices 13 Device Placement Pump Flow CO max (LPM) IABP Femoral artery to descending thoracic aorta Pulsatile 0.5 (axillary artery to descending thoracic aorta) CentriMag a Left atrium to aorta (LVAD) Centrifugal 6 Right atrium to pulmonary artery (RVAD) VA-ECMO Femoral vein-ivc to femoral artery (see text) Centrifugal 8 TandemHeart a Femoral vein across foramen ovale to LV Centrifugal 4.5 Impella 2.5 Femoral artery across aortic valve Axial 2.5 Impella CF (4.0) Femoral artery across aortic valve Axial 4.0 Impella 5.0 Femoral artery across aortic valve Axial 5.0 Impella RP a Femoral vein-ivc to pulmonary artery Axial 4.0 HeartMate PHP Femoral artery across aortic valve Axial 5 Aortix Femoral artery to descending abdominal aorta Axial 4 b Abbreviations: CO max, maximum pump flow in LPM superadded to native cardiac output; IABP, intraaortic balloon pump; IVC, inferior vena cava; LPM, liters per minute; LV, left ventricle; LVAD, left ventricular assist device; RVAD, right ventricular assist device; VA-ECMO, veno-arterial extracorporeal membrane oxygenation. a Capable of right ventricular support. b Partial assist only ANESTHESIA & ANALGESIA
5 New Innovations in VAD and ECMO Therapy Table 5. INTERMACS a Functional Risk Stratification of Severity of Heart Failure Risk Level Colloquial Description Defining Factors 7 Placeholder Advanced NYHA Class III HF 6 Walking wounded Recent fatigue at rest (NYHA IIIb) 5 Housebound Consistent fatigue at rest 4 Frequent flyer Recurrent episodes of ADHF 3 Dependent stability Stable but inotropic agent dependent 2 Sliding on inotropes Progressive decline with multiorgan dysfunction 1 Crash and burn Critical cardiogenic shock b INTERMACS levels 1 3 (shaded) are the current indications for durable LVAD support. Abbreviations: ADHF, advanced decompensated heart failure; HF, heart failure; LVAD, left ventricular assist device; NYHA, New York Heart Association. a INTERMACS: Interagency Registry for Mechanical Assisted Circulatory Support. b INTERMACS 1A: Critical cardiogenic shock with intractable ventricular arrhythmias. if there is a known aortic valve thrombus (not uncommon in chronic advanced heart failure), it may be prudent to keep pump speed (rpm) at a level low enough to avoid aortic valve opening. The proportion of ventricular ejection that flows through the device versus the aortic valve is dependent on the pump speed (rpm), and the pressure differential between the device inflow at the LV apex, and the device outflow into the aorta. Thus, the CF-VAD is both preload and afterload dependent. VAD preload (ie, LV filling) is determined by intravascular volume, right ventricular (RV) function, and pump speed. An important hemodynamic variable monitored by most devices is the pulsatility index (PI), a unitless measure of systolic pump flow created by contraction of the native LV. The PI is the averaged magnitude of flow pulses over a defined time period, for example, 15 seconds. An increase in pump speed increases LV unloading and PI decreases; conversely, a decrease in pump speed decreases LV unloading and PI increases. Other displayed parameters include set pump speed in rpm, pump power (watts), and pump flow, which may be calculated from rpm and power (HeartWare II) and therefore unreliable or directly measured with centrifugal pumps (HeartWare HVAD and HeartMate III). A suction event is a potentially devastating consequence of inadequate LV filling that results in acute obstruction of the VAD inflow by inward septal shift that in turn compromises RV outflow, triggers ventricular arrhythmias, and potentially results in circulatory collapse. 17 It may or may not be heralded by sudden loss of power and PI. An urgent immediate intervention is to decrease pump speed and LV unloading, facilitating septal return and RV recovery. The most effective means of anticipating or diagnosing a suction event is by transthoracic or transesophageal echocardiography (TTE or TEE), which reveals conversion of the short-axis round LV to a D -shape induced by the flattened or convex intraventricular septum. 18 Suction events are more likely to occur with axial than centrifugal flow devices (Table 2). The pressure differential between inflow and outflow at low flow states increases much more with axial pumps, so that they pull harder with a greater risk of ventricular suck down. 19 The HeartMate II (an axial pump) has internal device algorithms that detect suction events when they occur, automatically decrease the pump speed, and allow time for providers to address the underlying cause. In contrast, because centrifugal pumps have greater accuracy in flow estimation, they can detect low-flow states and alarm before an actual suction event occurs. 19 Advances in the Management of Elevated Pulmonary Vascular Resistance and Right Heart Failure. Cardiac modeling has demonstrated that the RV free wall comprises transverse fibers that contract with a bellows action and contribute only about 20% of the RV stroke volume. 20 In contrast, the RV septum comprises oblique fibers shared with the LV septum, whose contraction contributes 80% of the RV stroke volume (systolic ventricular interaction). Using TEE, RV contractility can be quantitated by calculating the distance the tricuspid annulus moves toward the RV apex with systolic LV septal contraction, known as tricuspid annular plane systolic excursion (TAPSE). A TAPSE of <1.6 cm is indicative of RV systolic dysfunction. Progressive LV failure increases left atrial pressure, which is indirectly measured by pulmonary artery occlusion pressure. This in turn increases pulmonary artery pressure and RV afterload, further increasing RV dependence on the interventricular septum for its ejection. Impaired LV septal contraction increases the effect of the increased pulmonary vascular resistance (PVR) in diminishing RV ejection fraction, leading to a vicious cycle of progressive RHF. LV septal contraction is inevitably compromised in patients requiring a durable LVAD, so that RV output is highly dependent on the maintenance of low RV afterload, that is, a low PVR. Elevated PVR contributes to the acute RHF and impairs LVAD filling and output. Perioperative factors causing acute increases in PVR include hypoxemia, hypercarbia, endogenous or exogenous catecholamines, inflammatory responses to cardiopulmonary bypass (CPB), transfusion or protamine reactions, or acute respiratory distress syndrome induced by transfusion-associated circulatory overload or immunologic-mediated transfusionrelated acute lung injury. Maintenance of effective RV function is essential to achieve a good outcome after LVAD placement. 18 Acute RHF may occur in up to 40% of patients receiving an LVAD 21 and is associated with an increased risk of reoperation for bleeding and postoperative acute kidney injury, increased ICU length of stay, impaired LVAD success as BTT, and early mortality. About a third of the patients with RHF ultimately require a right ventricular assist device (RVAD). The principles of the management of RV function include maintenance of RV coronary perfusion pressure by appropriate vasopressor therapy; prevention of RV volume overload by controlling right atrial pressure; avoidance of excessive RV afterload induced by iatrogenic pulmonary vasoconstriction; timely administration of inhaled pulmonary vasodilators such as nitric oxide and prostacyclins; and combined inodilator therapy with milrinone and dobutamine. 18 There is as yet no long-term RVAD, and the primary long-term option for intractable right heart or biventricular April 2017 Volume 124 Number
6 E SYSTEMATIC REVIEW ARTICLE failure remains the TAH. A more recent but as yet uncertain innovation is the combination of 2 miniaturized devices such as the HVAD simultaneously implanted into the left and right ventricle (Figure 5B). 22 Advances in Anticoagulation and Prevention and Diagnosis of Pump Thrombosis. Pump thrombosis is an uncommon but potentially devastating complication of a durable LVAD, especially within the first 2 years of implantation. Predisposing factors may be related to the pump itself nonpulsatile flow and sheer stress are inherently prothrombotic or there may be inflow cannula malposition or kinking, or low pump flow. They may be exacerbated by inadequate anticoagulation and/ or antiplatelet therapy because of patient noncompliance, genetic resistance, or suboptimal dosing. Intracardiac thrombosis may be induced by preexisting stasis or atrial fibrillation. Hypercoagulable states may be genetic (low levels of endogenous anticoagulant factors) or triggered by surgical stress, shock, or sepsis. Early diagnosis hinges on looking for signs of hemolysis heralded by increasing lactate dehydrogenase levels above 500 IU/L, and progressive or intermittent increases in LVAD pump power. When the diagnosis is missed or delayed, patients may present with rapidly progressive LV failure or even cardiogenic shock. 23 A definitive diagnosis can be achieved with a ramp study, where the pump speed is ramped up to its maximum with simultaneous echocardiographic and hemodynamic monitoring. 24 Normally ramped pump speed results in progressive LV emptying and decrease in LV end-diastolic diameter, decrease in central venous and pulmonary artery occlusion pressure, and loss of aortic valve opening. Attenuation of these responses to speed ramping is highly suggestive of pump thrombosis. Certain risk factors have been defined for pump thrombosis, including younger age, large body mass index, severe RHF, and noncompliance with medications. 25 Once it occurs, there is a high likelihood of recurrence. The risk of pump thrombosis is decreased by strict compliance to a preventative protocol. 26 There should be assiduous attention to pump pocket size and device placement, with careful positioning of the inflow cannula and outflow graft. Strict anticoagulation protocols should be followed, including heparin titrated to achieve a therapeutic partial thromboplastin time within 48 hours of surgery; early antiplatelet therapy with aspirin (plus dipyridamole in younger patients); and warfarin therapy titrated to an international normalized ratio of 2 to 3, depending on the device. Slow pump speeds, for example, <8600 rpm for the HeartMate II, should be avoided. Strict maintenance of mean arterial pressure <90 mm Hg has been shown to be especially important to prevent stroke induced by the HeartWare HVAD. Despite all this, on the basis of an individual patient, the yin and yang between bleeding and thrombosis remains unsolved. Innovations in the Continuous Flow Left Ventricular Device Development HeartMate II. The HeartMate II is a valveless pencil-like impeller pump that rotates at 8000 to 10,000 rpm and creates axial flow (Figure 3A). Although it is implanted in a subdiaphragmatic preperitoneal pocket, it is less than a quarter of the size of the pulsatile HeartMate XVE. It is FDA approved for both BTT and DT and is currently the most widely used LVAD with some DT recipients surviving 7 years or more. In operating the HeartMate II, there is no built-in variability of pump speed, so rpm must be adjusted to allow some aortic valve opening to prevent thrombus formation. Ideally, the PI should be kept between 4.0 and 6.0. Pump flow is calculated, not measured, and is based on the power (watt) and rpm. At the extremes of flow, this calculation is subjected to error and may indicate normal flow in lowflow states such as cardiac tamponade. 18 Between 2010 and 2013, a higher incidence of pump thrombosis (8.4% vs 2.2%) and earlier onset (3 vs 18 months) was noted with the HeartMate II in 3 major implantation centers. 27 Medical management alone with aggressive heparin anticoagulation and/or thrombolysis with tissue plasminogen activator was associated with a 50% mortality rate in the ensuing 6 months, and improved survival required urgent OHT or VAD replacement. However, by 2014, this trend had started to reverse, perhaps reflecting earlier diagnosis. 25 Subsequently, preliminary findings of the PREVENT study demonstrated a substantial reduction in the 3-month incidence of VAD thrombosis, using a protocol that paid careful attention to inflow cannula and outflow graft positioning, anticoagulation, adequacy of pump speed (>8600 rpm), and tight blood pressure control. 26 Jarvik The Jarvik 2000 is a unique miniaturized titanium axial flow device with an integrated inflow placed directly into the apex of the LV, with an efferent conduit placed either into the ascending or descending aorta. 28 The latter facilitates placement via a left minithoracotomy, sparing sternotomy. The driveline is threaded within the thoracic cavity to emerge at an occipital retroauricular skull-mounted pedestal, which substantially decreases the risk of VAD pocket infection and increases the patient mobility. 29 The Jarvik 2000 controller is extremely simple, consisting of a watt meter and a five gear rpm setting adjustable by the patient between 1 and 5 (8000 to 12,000 rpm). Every minute the device decreases its output for 8 seconds to allow aortic ejection, maintain aortic valve function, and minimize thrombus formation. One limitation of the Jarvik 2000 is that its peak outflow is about 7 L/min, so it is suited for smaller patients or those with greater intrinsic LV function. A nonrandomized pivotal clinical trial on 150 patients in the United States was completed in 2012 (ClinicalTrials. gov NCT ), and the prospective, controlled RELIVE trial comparing with to the HeartMate II in 350 patients for DT is currently in progress (ClinicalTrials.gov NCT ). HeartWare HVAD. The HeartWare HVAD is a miniaturized centrifugal device in which the inflow cannula is cored directly into the LV apex so that the device is intrapericardial and entirely above the diaphragm (Figure 3B). 30 It has a driveline that is exteriorized and attached to small portable ANESTHESIA & ANALGESIA
7 New Innovations in VAD and ECMO Therapy Figure 3. Radiographic appearance of second- and third-generation CF-LVADs. The radiographic footprint of (A) a second-generation subdiaphragmatic axial flow device (HeartMate II), and 2 third-generation LV apical centrifugal flow devices, the HVAD (B) and HeartMate III (C). CF-LVAD indicates continuous flow left ventricular assist device. battery packs. A major advance is that through magnetic or hydrodynamic levitation, there is no contact between the impeller and the drive mechanism, with minimal blood contact. The impeller rotates centrifugally more slowly than the rotary devices, at 2750 to 3000 rpm. The advantages claimed are decreased hemolysis and thrombogenesis and greater mechanical durability. The HVAD was FDA-approved for BTT in 2012, and its use has been associated with a 91% 6-month survival and 84% 1-year survival. 10 However, the ENDURANCE trial, 31 which compared the HVAD with the HeartMate II for DT approval, demonstrated a 3-fold higher incidence of ischemic and hemorrhagic stroke, 28.7% vs 12.1% (data presented at the 35th ISHLT Meeting in 2015). The major risk factor identified was a mean arterial pressure >90 mm Hg. Other factors associated with an increased risk of stroke included the use of low-dose aspirin 81 mg/day and an international normalized ratio <2. In the original HVAD, the inflow cannula is made of a highly polished titanium alloy that promotes tissue ingrowth that may be prothrombogenic. Subsequently, titanium microspheres were sintered (heated and coalesced without liquefaction) onto the external surface, and markedly impede the tissue ingrowth. 32 A follow-up study, the ENDURANCE Supplemental Trial (ClinicalTrials.gov NCT ) is currently investigating the effect of tight blood pressure control on stroke incidence with the HVAD. HeartMate III. The HeartMate III is a third-generation centrifugal-flow pump seated in an intrapericardial pocket and is essentially an enhanced, miniaturized CentriMag pump (Figures 3C and 4). Its major advantage is its fully magnetically levitated rotor with wider blood flow gaps that theoretically minimize red blood cell damage, thrombus formation, and von Willebrand syndrome. 33 Rotor speeds that alternate every 2 seconds promote pulsatile flow that contributes to these benefits. Sintered titanium microspheres on surfaces making contact with blood promote endothelial ingrowth to diminish the probability of contact-activated thrombus formation, much like the HeartMate XVE. The controller display is identical to the HeartMate II, except that pump speed is typically kept 3000 to 5000 rpm; pump flow is directly measured and therefore reliable, and the PI goal is lower, between 3.0 and 5.0. The U.S. MOMENTUM 3 study (ClinicalTrials.gov NCT ) is actively randomly assigning more than 1000 patients to HeartMate III or HeartMate II to study its efficacy and safety as for BTT and DT. HeartWare MVAD. The MVAD is an innovative, miniaturized (22-mL volume displacement) VAD device that combines April 2017 Volume 124 Number
8 E SYSTEMATIC REVIEW ARTICLE centrifugal and axial flow into a tiny longitudinal pump implanted via a minithoracotomy into the LV apex without requiring CPB, and with an outflow cannula that passes through the aortic valve to just proximal to the innominate Figure 4. Magnetically levitated centrifugal pump. The principle of a fully magnetically levitated centrifugal pump is illustrated by the Heart Mate III LVAD. The full magnetically levitated rotor allows large pump gaps, which minimize red blood cell damage, hemolysis and the potential for thrombus formation. LVAD indicates left ventricular assist device. From Schmitto et al. 33 Used with permission. artery. As such it provides antegrade ejection alongside the native flow that can be varied from full to partial assist by virtue of a pump speed between 12,000 and 22,000 rpm. 34 The platinum alloy impeller is suspended in a ceramic tube by passive magnetic and hydrodynamic forces. A gimbal sewing ring allows modification of device direction and pump depth, with the potential for RV placement and biventricular assist device (BiVAD) support. Introduction of the MVAD has been somewhat beset by technical problems, including pump thrombosis. The nonrandomized MVAdvantage Trial (ClinicalTrials.gov NCT ) was designed to study MVAD safety and performance in 70 subjects with NYHA IIIB or IV heart failure over 24 months, with a primary end point of survival at 6 months. It is currently on hold. HeartAssist 5. The HeartAssist 5 (for fifth generation ) is an axial flow impeller device modified from the MicroMed DeBakey Noon VAD developed as long ago as It was first implanted in Berlin in 1998 for BTT, and in the United States in Since then it has undergone numerous technical modifications to attempt to decrease thrombus formation. It is an external device similar in configuration to the HeartMate II, but considerably smaller (92 vs 282 g) and is placed in an extrapericardial supradiaphragmatic pocket. The most sophisticated aspect of HeartAssist 5 technology is that it incorporates a wireless transmitter that provides continuous data acquisition that can be captured by a computer or smartphone, with a patient home support system called HeartAttendant that can transmit alarm alerts to a support center (VADLink.com). A randomized prospective study on just under 200 patients is active, comparing 180- day success among the HeartAssist 5, HeartMate II, and HVAD (ClinicalTrials.gov NCT ). Figure 5. Radiographic appearance of BiVADs. A, Jarvik Flowmaker with LVAD in LV apex and RVAD in right atrium. BiVAD indicates biventricular assist device. From Frazier et al. 37 Used with permission. B, HeartWare HVAD with RVAD in anterior free wall of RV (on left) and in right atrium (on right). RVAD indicates right ventricular assist device. From Krabatsch et al. 22 Used with permission ANESTHESIA & ANALGESIA
9 New Innovations in VAD and ECMO Therapy Challenges and Further Innovations in the Continuous Flow Left Ventricular Device Support Biventricular Assist Device (BiVAD) Support. Refractory RHF is estimated to occur in as many as 30% of patients who have a durable CF-LVAD. 36 Short-term RV support can be provided by an increasing array of surgically placed or percutaneous devices (see below), but until very recently, the primary option for durable RV support has been the placement of a TAH. 14 There have been isolated case reports of BiVAD application of the Jarvik LVAD. The first, in 2004, utilized a previous version, the Jarvik Flowmaker, to provide support with an apical LVAD coupled with a right atrial device, but the patient succumbed after 12 days (Figure 5A). 37 In 2014, a Japanese group reported a successful BiVAD with the Jarvik 2000 placed in the left and right ventricles. 38 After a 2-year course, the RVAD started to fail, but the patient was able to undergo life-saving OHT. Most recently, Brenner et al 39 reported a case series of 3 severely ill patients who received biventricular Jarvik support. Two patients died of other causes at 3 and 50 days postoperatively, and 1 died a year later after RVAD pump shutdown. The most successful experience thus far is that of the group at the Berlin Heart Center, using 2 HVAD devices, with the RVAD implanted into the RV free wall (Figure 5B). 22 In 2011, they reported on their experience with 17 patients, who had an 82% 30-day survival, and 59% were discharged from hospital. Two key technical innovations were to avoid excessive pulmonary blood flow and pulmonary edema by reducing the graft outflow diameter to increase RVAD afterload, and 2 silicon suture rings were used to shorten the length of the inflow cannula. The RVAD speed was adjusted between 2400 and 3800 rpm based on central venous pressure, with the LVAD adjusted to provide maximal flow. In 1 patient, who had a compressed RV, the RVAD was successfully repositioned in the right atrium. Two patients had delayed RV recovery and underwent successful RVAD decannulation. In a case report from Germany, a patient with cardiogenic shock secondary to a massive ischemic ventricular septal defect was successfully bridged to transplant 14 weeks after BiVAD implantation of 2 HVAD. 40 Maintenance of Arterial Pulsatility. Nonpulsatile continuous flow appears to promote the development of arteriovenous malformations throughout the gastrointestinal tract that predispose to recurrent gastrointestinal bleeding. 1 Bleeding is exacerbated by acquired Von Willebrand syndrome, which appears to be because of the sheer stress induced activation of a metaloprotease, ADAMTS-13 that cleaves off the high-molecular-weight multimers essential for platelet activation by von Willebrand factor. 41 Continuous flow with the absence of aortic valve opening predisposes to valve thrombosis. Over time, the aortic valve commissure starts to fuse, and about 30% of all longterm VAD patients develop some degree of aortic regurgitation that may require surgical intervention. A number of strategies have been developed to enhance pulsatility and intermittent aortic valve opening, which may prevent valve or chamber thrombus formation by inhibiting stasis or recirculation zones. These include periodic rpm slowing (Jarvik 2000) or pump speed modulation that generates intrinsic pulsatile flow from the LVAD (HeartMate III). Pump speed manipulation may be asynchronous or synchronous, that is, independent of or consistent with the native heart rate. 1 Synchronous pump speed manipulation can maximize LVAD flow during LV systole (copulsation) or diastole (counterpulsation). The latter favors LV unloading, diminished LV pressure work, and the potential for remodeling and recovery. In the near future, devices will incorporate speed modulation algorithms that adjust device output during exercise, fluctuations in blood pressure, and arrhythmias. Transcutaneous Energy Transfer. The development of a safe, effective transcutaneous energy transfer (TET) system for durable ventricular support devices has been in progress for more than 3 decades. 42 The TET system uses electromagnetic induction to transfer power from a transmitter coil belt worn by the patient to a small power system implanted alongside the VAD. By eliminating the driveline and its potential for infection, the TET system could provide total device implantability and markedly improve patient acceptance and QOL. A number of challenges remain, including the potential for electrical burns, infection of implanted material, and component durability. Although many devices have been patented, at this stage, investigations remain confined to animal studies. 43 SHORT-TERM NONDURABLE DEVICES Nondurable devices are placed for emergent short-term rescue of acute heart failure or cardiogenic shock; as bridge to recovery, for example, for postcardiotomy shock, acute myocarditis, or acute myocardial infarction; or as a bridge to decision if recovery is not immediate (Table 4). Surgical implantation is rapidly being supervened by percutaneous placement in the cardiac catheterization laboratory. Decision making is often complex because these devices are inserted urgently or emergently, but consideration must be taken of advance directives (do not resuscitate, do not intubate), terminal illness, rescue after protracted cardiopulmonary resuscitation, severe neurologic injury, or severe sepsis. Intraaortic Balloon Pump The intraaortic balloon pump (IABP) is a short-term counterpulsation device introduced a half-century ago. It augments cardiac output by not more than 0.5 L/min, and its primary function is to enhance myocardial oxygen balance during acute myocardial ischemia. Placed percutaneously via the femoral artery into the descending aorta, balloon inflation provides diastolic pressure augmentation that increases coronary perfusion pressure (CPP) and myocardial oxygen supply, whereas subsequent deflation decreases LV afterload and myocardial oxygen supply. Major limitations include the requirement for a competent aortic valve and stable heart rate and rhythm; inability to mobilize the patient; and complications related to leg ischemia, bleeding, or thrombosis. Although the IABP is often a successful first line of support for post-cpb shock because of the short-lived ischemia reperfusion injury, cardiologists still frequently place an IABP as primary support for cardiogenic shock. However, April 2017 Volume 124 Number
10 E SYSTEMATIC REVIEW ARTICLE the large (600 patient) Shock II trial demonstrated no difference in 1-year survival whether or not an IABP was placed in patients with cardiogenic shock complicating acute myocardial infarction where early percutaneous or surgical coronary revascularization was planned. 44 Femoral veno-arterial (VA) ECMO increases LV afterload because its arterial output is retrograde to native aortic outflow. Counterpulsation by IABP placement could be an important means of decreasing LV afterload. Although there is evidence that the combination of IABP with ECMO enhances coronary graft flow, 45 and decreases pulmonary artery pressures and LV dimensions, no improvements in microcirculation 46 or outcome 47 have been demonstrated. Another innovation is the placement of the IABP via the subclavian or axillary artery, which allows full patient mobilization, and may provide a much longer-term support as a bridge to recovery, VAD, or transplant. 47,48 Thoratec CentriMag The Thoratec CentriMag, introduced in 2007, is an external, short-term device, whose magnetically levitated centrifugal pump was the forerunner for that incorporated into the HeartMate III. Separate circuits and pumps may be utilized as an isolated LVAD (inflow from left atrium, outflow to aorta), or RVAD (inflow from right atrium, outflow to pulmonary artery) or BiVAD. 49 The small cannulas (7 mm) facilitate surgical rapid placement during cardiac surgery or via a new sternotomy. A bedside console displays rpm and ultrasound-sensed flow rate. A membrane oxygenator is easily placed in the RVAD circuit to support acute lung injury. 50 Cannula vibration or chatter may reveal hypovolemia or excessive ventricular emptying, although it may also be induced by return of ventricular contractility. The CentriMag LVAD is FDA approved for 6 hours only, but in clinical practice, it is left in place for considerably longer days to weeks. 51 The CentriMag RVAD is FDA approved for up to 30 days for acute RHF. Since its introduction in 2003, the CentriMag has become established as the predominant medium-term device for pre- and postcardiotomy cardiogenic shock; posttransplant graft rejection or failure; and acute RHF after LVAD placement, with survival rates between 60% and 80%. The CentriMag may serve as a bridge to recovery, or a bridge to decision, that is, whether the patient is a candidate for a long-term LVAD as BTT or DT. In about 25% of cases, it is placed as a part of an ECMO circuit (see Veno-Arterial Extracorporeal Membrane Oxygenation). Veno-Arterial Extracorporeal Membrane Oxygenation In the half-decade between 2006 and 2011, application of adult ECMO has increased more than 4-fold in the United States. 52 VA-ECMO is analogous to CPB, in which the function of the heart is replaced or supplemented by the ECMO pump, with or without lung replacement. VA-ECMO is primarily instituted as rescue therapy in cardiogenic shock; as a bridge to recovery; or, more often, as a bridge to a decision. It supports circulatory resuscitation and stabilization, allows assessment of neurologic status, facilitates myocardial recovery, and evaluation of patient candidacy for VAD or transplant. 53,54 There is an increasing application of VA-ECMO for refractory cardiac arrest, so-called extracorporeal cardiopulmonary resuscitation or E-CPR. When combined with mechanical CPR, therapeutic hypothermia, and, when indicated, primary percutaneous coronary intervention (PCI), good neurologic outcomes and survival rates as high as 54% were reported in the CHEER Trial. 55 However, best outcomes are predicated on initiation of VA-ECMO within 30 minutes of cardiac arrest. For many years, the provision of postoperative VA-ECMO essentially required a CPB machine at the bedside, staffed full-time by a perfusionist. However, in the past decade, there has been remarkable miniaturization and improvement in safety. The development of a polymethylpentene (PMP) membrane oxygenator (Quadrox-D) 56 driven by a small centrifugal pump reduces the footprint of the device to one that can be mounted on an IV stand. The PMP membrane avoids the plasma leakage associated with conventional hollow-fiber oxygenators and decreases but does not eliminate the risk of thrombogenesis. Indeed, thrombocytopenia, coagulopathy, disseminated intravascular coagulation, bleeding, blood and blood product transfusion, stroke, sepsis, and dialysis-dependent acute renal failure continue to be formidable risks in these very sick patients. Depending on the circumstances, overall inhospital mortality with VA-ECMO varies between 35% and 80%. 57,58 Central VA-ECMO is provided for intractable cardiac failure in the operating room (OR) and is essentially a continuation of the CPB circuit, that is, venous cannulation of the atria or superior vena cava with arterial cannulation of the aorta. The latter provides high anterograde flow of oxygenated blood to the heart and brain, with LV afterload reduction. However, the sternum has to be left open, so within 24 to 48 hours, the patient either has to recover sufficiently to be weaned off central ECMO or transitioned to another modality such as peripheral ECMO or CentriMag. Peripheral VA-ECMO can be placed relatively quickly via femoral venous and arterial cannulas in the cardiac catheterization laboratory, OR, ICU, or emergency department. However, there are a number of potentially adverse effects, not the least of which is the inability to mobilize the patient. ECMO flow is limited by the arterial cannula diameter, but the greater the size relative to the native femoral artery, the greater the risk of arterial occlusion and limb ischemia. In the worst case, this may result in muscle edema, a fascial compartment syndrome, severe rhabdomyolysis, and acute renal failure. Prevention requires the placement of a reperfusion catheter adjacent to the arterial cannula or in the posterior popliteal artery. 59 Retrograde oxygenated arterial inflow via a femoral cannula may not reach the heart and brain, especially via the right carotid artery, because it competes with the native aortic output. Arterial oxygenation (Spo 2 ) should always be monitored by a pulse oximeter on the right hand, to better reflect the Spo 2 of blood reaching the brain. In an extreme case, the patient s head may appear blue, whereas the legs appear red, the so-called Harlequin syndrome. 13 This situation becomes dire with acute hypoxemic respiratory failure, ANESTHESIA & ANALGESIA
11 New Innovations in VAD and ECMO Therapy necessitating additional venovenous circuits (VAV-ECMO) to the upper vasculature to ameliorate this situation. 60 Retrograde femoral arterial ECMO flow increases LV afterload, LV end-diastolic volume (LVEDV), and LV enddiastolic pressure (LVEDP). It may thereby induce pulmonary congestion and edema, adversely affect myocardial oxygen balance, and decrease the potential for LV recovery. This effect is exaggerated when LV function is severely impaired and the chamber is distended. 13 Increased LV afterload may be ameliorated by decreasing ECMO flow, but this compromises circulatory support. Inotropic support with drugs such as dobutamine and/or milrinone may improve intrinsic LV contractility but increases the risk of ventricular tachyarrhythmias. Concomitant use of an IABP or ventricular decompression by an Impella device (Abiomed, Danvers, MA) may decrease LV afterload and chamber distension but can impede blood flow and oxygenation to the head. The most expeditious solution is to decompress the LV by an apical vent that is then spliced into the ECMO circuit. 61 Innovative circuitry systems provide useful alternative approaches to VA-ECMO. 62,63 As illustrated in Figure 6A, the arterial inflow cannula can be grafted percutaneously into the right axillary and subclavian artery. This ensures superior oxygenation of the head and brain, as well as anterograde flow into the aortic arch. Care must be taken not to flood the arm with blood, but this can be ameliorated by placing a snare on the cannula. The venous cannula drains deoxygenated blood from the inferior vena cava, as per standard VA-ECMO, and then returns it to the arterial circulation via a pump and oxygenator. A major innovation is placing an arterial outflow cannula as an LV vent into the ventricular apex via a minithoracotomy and splicing it into the VA-ECMO circuit just before the pump. With the aid of the oxygenator, the patient can be weaned from mechanical ventilatory support and proceed to tracheal extubation. When lung function improves, the femoral venous cannula and oxygenator are removed, leaving what is essentially a peripheral CentriMag LVAD circuit in place (Figure 6B). This facilitates early mobilization and ambulation and minimizes the potential for ICU-related complications such as ventilator-associated lung injury, delirium, muscle weakness, and bedsores. The circuit may serve as a bridge to recovery or to a long-term VAD. If the latter is required, the percutaneous minithoracotomy approach decreases the bleeding and inflammatory response associated with reopening a previous median sternotomy to place a durable LVAD. TandemHeart The TandemHeart is a versatile percutaneous VAD (pvad) that utilizes an external centrifugal pump. It can be placed percutaneously or by open surgical cannulation and can support the LV, RV, or both. 64 For LVAD support, a femoral vein cannula is passed into the right atrium and thence across the intraatrial septum into the left atrium, from which it withdraws oxygenated blood and pumps it into a femoral artery cannula. Support of the RV may be provided by a surgically placed right atrial cannula that drains venous blood and pumps it via an oxygenator into a surgically placed pulmonary artery cannula. Alternatively, using imaging, a specialized triple lumen cannula can be passed percutaneously into the pulmonary artery. Right atrial blood is drawn into the cannula via 2 orifices and then pumped via an oxygenator through a channel that terminates in the pulmonary artery. The TandemHeart provides several advantages. It decompresses the left atrium and LV, and decreases LVEDV, LVEDP and myocardial oxygen demand, but this may be offset by its retrograde aortic flow. Compared with the IABP in cardiogenic shock, the TandemHeart provides enhanced end-organ perfusion with decreased lactate levels, but no difference in 30-day mortality (45% vs 43%). Although introduced as a short-term rescue device, the TandemHeart has met with some success as bridge to a durable VAD or transplantation. 65,66 The Impella System The Impella system comprises a family of short-term, nondurable multiport pigtail axial devices to provide LV rescue in cardiogenic shock, support hemodynamically unstable patients in the cardiac catheterization laboratory during PCIs, or to decompress a distended LV during VA-ECMO. 13 The catheter is placed via the femoral artery across the aortic valve and pumps in series with the LV outflow. Unlike the IABP or Tandem Heart, the Impella decreases LV preload without increasing afterload, thereby enhancing myocardial oxygen balance. A series of increasingly large devices named for the maximal flow augmentation in liters per min (Impella 2.5, 4.0/ CP, 5.0) have been designed to provide increasingly greater flow and LV support. The device has also been placed via the axillary artery to facilitate patient mobilization. 67 A right-sided device (Impella RP) has been introduced that is advanced up the femoral vein and across the pulmonary valve to enhance RV flow in acute RHF. A number of studies support the Impella s ability to decrease mortality in acute myocardial infarction with cardiogenic shock 68 and to enhance the completeness of revascularization, mapping, or arrhythmia ablation during PCI. 69 In a small case series of Impella RP placement in 30 patients with acute RHF complicating LVAD implantation, cardiotomy, or acute myocardial infarction, hemodynamic improvement was immediate, and overall 30-day survival rate was 73.3%. 70 As with all percutaneous arterial devices, there is a risk of bleeding and limb ischemia. Management of the Impella requires close attention and considerable skill. Although the monitor provides continuous information about ventricular pressure, power, and flow, frequent echocardiography is required to ensure proper central positioning of the axial pump across the aortic valve, as well as ventricular chamber filling, thrombus formation, and ventricular recovery. 71 Impella flow may be acutely impaired or completely obstructed by catheter migration, malposition, kinking, or thrombus. As with a durable LVAD, mural pump inflow obstruction and complete cessation of flow (a suckdown or suction event) may be precipitated by acute LV emptying caused excessive pump speed, hypovolemia or acute pulmonary hypertension, or RHF. April 2017 Volume 124 Number
12 E SYSTEMATIC REVIEW ARTICLE Figure 6. A, Hybrid veno-arterial ECMO (VA-ECMO) circuit with left ventricular apical vent. The circuit is set up as a conventional veno-arterial ECMO circuit with a venous cannula drawing deoxygenated blood from the inferior vena cava, which is then pumped through a membrane oxygenator. However, the arterial outflow is grafted into the axillary rather than femoral artery, which enhances the delivery of oxygenated blood to the head and brain. A second arterial circuit has its inflow from the LV apex, providing chamber venting and is spliced into the venous line. (Figure courtesy of Koji Takeda, MD). B, Minimally invasive CentriMag. When the patient has recovered sufficient lung function, the membrane oxygenator is weaned off and venous circuit is decannulated. This leaves an LV apex to axillary arterial circuit that constitutes a minimally invasive CentriMag LVAD that facilitates patient mobilization and can serve as a bridge to recovery, or durable LVAD without requiring reoperation via a previous sternotomy. (Figure courtesy of Koji Takeda, MD). HeartMate Percutaneous Heart Pump The HeartMate percutaneous heart pump (PHP) is a new percutaneous axial flow pump that appears analogous to the Impella. However, it has a distal collapsible elastomeric impeller that passes through a relatively small 14F introducer; once through the aortic valve, the impeller expands to 24F, capable of generating a flow of 4 to 5 L/min at approximately 20,000 rpm. The HeartMate PHP is approved for PCI support in the European Union, and, in the United States, it is currently undergoing a large randomized openlabel noninferiority trial versus the Impella 2.5 for support during PCI (SHIELD II, ClinicalTrials.gov NCT ). Aortix System The Aortix system is a miniaturized axial flow pump that represents a radical departure from current percutaneous devices in that it is intended to provide partial circulatory assist in less-sick patients (NYHA III). Passed through the femoral artery, it deploys expandable side anchors that secure the pump in the supradiaphragmatic descending aorta above the renal arteries. It has entraining jets that entrain blood to augment flow ( jet pumping ) in series with the circulation, thus decreasing LV afterload while minimizing central thromboembolic risk. In an ischemic heart failure sheep model, the Aortix system not only decreased myocardial oxygen consumption and improved LV ejection fraction and CO, but also increased renal blood flow and urine output. 72 Clinical studies are planned in 2016 and OVERVIEW OF CLINICAL PATHWAYS FOR MECHANICAL CIRCULATORY SUPPORT Figure 7 illustrates some generic clinical pathways for mechanical circulatory support, although every patient must be evaluated individually for their own specific management plan. Patients who are at INTERMACS 1, that is, who are in life-threatening cardiogenic shock, may be rescued by a short-term device, which in the majority of cases will be VA-ECMO. In some situations, particularly in the catheterization laboratory or coronary care unit, rescue with alternate short-term devices (eg, IABP, Impella, or TandemHeart) may be attempted but inevitably converted to VA-ECMO on site or in the OR. If patients are deemed to be viable from a neurologic standpoint, they will be supported and closely monitored for possible cardiac recovery and VA-ECMO weaning. If cardiac recovery is negligible or slow, they may be converted to a CentriMag device to allow organ recovery and possible circulatory weaning. If this appears unlikely, the patient will be evaluated for a durable VAD or TAH as BTT or DT. Patients who are at INTERMACS 2, that is, who are developing progressive organ dysfunction, are generally evaluated for a durable VAD or TAH as BTT or DT. If they deteriorate more rapidly, they may undergo interim support with a CentriMag device pending evaluation for a durable VAD or TAH as BTT or DT. Patients who are at INTERMACS 3, that is, who are stable but dependent on inotropic support, are generally evaluated for a durable VAD or TAH as BTT or DT ab initio. It should be noted that in some patients with a durable VAD as DT, conditions may improve to allow them to enter the BTT pathway. Conversely, in some patients with a durable VAD as BTT, conditions may deteriorate to preclude OHT and force a change to DT. In either situation, complications may occur that cause consideration of device deactivation (see below) ANESTHESIA & ANALGESIA
13 New Innovations in VAD and ECMO Therapy PATIENT-CENTERED CONSIDERATIONS IN DURABLE DEVICE PLACEMENT Ethical and Psychosocial Issues in Durable Device Placement Durable device placement is increasingly considered as a standard of care for older patients with intractable endstage heart failure who are not candidates for heart transplantation. The most recent INTERMACS report on more than 15,000 patients reveals an 80% 1-year and 70% 2-year survival with durable CF-VADs. 4 However, what caregivers may interpret as a therapeutic success may not necessarily translate into improved QOL for the patient, which after all is the primary goal of durable device implantation. Ethical issues in device placement include appropriate patient evaluation and selection; fully informed consent for device initiation; and end-of-life decision making, that is, device deactivation. 73 The Centers for Medicare and Medicaid Services (CMS) have clearly defined medical criteria for durable device placement, but psychosocial criteria unlike those well established for heart transplantation remain generic and institution dependent. Key issues of focus must include drug, alcohol, or tobacco abuse or psychiatric illness; capacity for learning and problem solving; previous compliance with medical regimens; the quality of psychosocial, family, and financial support after implantation; and the adequacy of health insurance coverage. 74 These concerns particularly apply to older patients with impaired functional or cognitive reserve (frailty) that affects their ability to take care of their device. Understanding of their full implications of device insertion among patients and families remains extremely limited. Patient acceptance may be automatic ( There was no other choice ) or reflective ( I thought about it an awful lot ) and inform their subsequent postoperative expectations, including rejection versus acceptance of device deactivation in the context of their QOL. 75 Careful discharge planning is essential with regard to device and battery management, anticoagulation regimen, 24/7 emergency contacts, and the potential for complications such as device malfunction, bleeding, infection, and stroke. 74 In this regard, routine palliative care consultation at the time of evaluation can be very helpful in establishing patient and family goals of care and expectations. 76 Predictive outcome models tend to focus on mortality rather than (uncertain) patient QOL or its economic effect, measured by quality-adjusted life years. Patient perception of good QOL relates to successful hospital discharge, ambulation, adequate sleep, and acceptance of their artificial support; the obverse is associated with increasing anxiety and depression. In interviews, patients express VAD insertion as a new lease on life, while acknowledging its limitations on their lifestyle, the care burden placed on the spouse or caregiver (especially during the early postdischarge period), time spent in follow-up care, and anxiety about access to VAD-trained providers. They also emphasize the importance of developing support groups with other VAD recipients. 76 Durable Device Withdrawal The ethics of durable device withdrawal remain controversial, even in situations where advance care planning has been well documented or discontinuation is requested by the patient. 77 Current advanced directives or living wills do not specify no mechanical circulatory support as opposed to do not resuscitate or do not intubate. Relatives of incapacitated loved ones who are placed in a situation of substituted judgment may feel that they have no choice but to allow durable VAD placement even though the patient may previously have expressed a desire not to be on life support. 78 It has been suggested that, in uncertain situations, a time-limited trial of device support could be offered, that is, reevaluated based on whether the condition of the patient improves or deteriorates a judgment that should be made by the patient as well as the medical team. 78 The fundamental question is whether device deactivation represents physician-assisted death (an act of commission) versus allowing natural death to proceed (an act of Figure 7. Overview of clinical pathways for mechanical circulatory support. A schematic of potential generic pathways for patients presenting in INTERMACS 1, 2, or 3 are presented, although every patient will have a unique course. * indicates the potential for cardiac recovery and weaning from mechanical circulatory support. For a full explanation, see text. BiVAD indicates biventricular assist device; BTT, bridge to transplant; DT, destination therapy; LVAD, left ventricular assist device; RVAD, right ventricular assist device; TAH, total artificial heart; VA-ECMO, veno-arterial extracorporeal membrane oxygenation. April 2017 Volume 124 Number
Ventricular Assisting Devices in the Cathlab. Unrestricted
 Ventricular Assisting Devices in the Cathlab Unrestricted What is a VAD? A single system device that is surgically attached to the left ventricle of the heart and to the aorta for left ventricular support
Ventricular Assisting Devices in the Cathlab Unrestricted What is a VAD? A single system device that is surgically attached to the left ventricle of the heart and to the aorta for left ventricular support
Ventricular Assist Devices for Permanent Therapy: Current Status and Future
 Ventricular Assist Devices for Permanent Therapy: Current Status and Future Prospects Francis D. Pagani MD PhD Professor of Cardiac Surgery University of Michigan April 28 th, 2012 Disclosures NHLBI and
Ventricular Assist Devices for Permanent Therapy: Current Status and Future Prospects Francis D. Pagani MD PhD Professor of Cardiac Surgery University of Michigan April 28 th, 2012 Disclosures NHLBI and
Implantable Ventricular Assist Devices and Total Artificial Hearts. Policy Specific Section: June 13, 1997 March 29, 2013
 Medical Policy Implantable Ventricular Assist Devices and Total Artificial Hearts Type: Medical Necessity and Investigational / Experimental Policy Specific Section: Surgery Original Policy Date: Effective
Medical Policy Implantable Ventricular Assist Devices and Total Artificial Hearts Type: Medical Necessity and Investigational / Experimental Policy Specific Section: Surgery Original Policy Date: Effective
Mechanical Cardiac Support in Acute Heart Failure. Michael Felker, MD, MHS Associate Professor of Medicine Director of Heart Failure Research
 Mechanical Cardiac Support in Acute Heart Failure Michael Felker, MD, MHS Associate Professor of Medicine Director of Heart Failure Research Disclosures Research Support and/or Consulting NHLBI Amgen Cytokinetics
Mechanical Cardiac Support in Acute Heart Failure Michael Felker, MD, MHS Associate Professor of Medicine Director of Heart Failure Research Disclosures Research Support and/or Consulting NHLBI Amgen Cytokinetics
เอกราช อร ยะช ยพาณ ชย
 30 July 2016 เอกราช อร ยะช ยพาณ ชย Heart Failure and Transplant Cardiology aekarach.a@chula.ac.th Disclosure Speaker, CME service: Merck, Otsuka, Servier Consultant, non-cme service: Novartis, Menarini
30 July 2016 เอกราช อร ยะช ยพาณ ชย Heart Failure and Transplant Cardiology aekarach.a@chula.ac.th Disclosure Speaker, CME service: Merck, Otsuka, Servier Consultant, non-cme service: Novartis, Menarini
Left Ventricular Assist Devices (LVADs): Overview and Future Directions
 Left Ventricular Assist Devices (LVADs): Overview and Future Directions FATIMA KARAKI, M.D. PGY-3, DEPARTMENT OF MEDICINE WASHINGTON UNIVERSITY IN ST. LOUIS ST. LOUIS, MISSOURI, USA St. Louis, Missouri,
Left Ventricular Assist Devices (LVADs): Overview and Future Directions FATIMA KARAKI, M.D. PGY-3, DEPARTMENT OF MEDICINE WASHINGTON UNIVERSITY IN ST. LOUIS ST. LOUIS, MISSOURI, USA St. Louis, Missouri,
ECMO as a bridge to durable LVAD therapy. Jonathan Haft, MD Department of Cardiac Surgery University of Michigan
 ECMO as a bridge to durable LVAD therapy Jonathan Haft, MD Department of Cardiac Surgery University of Michigan Systolic Heart Failure Prevalence 4.8 million U.S. 287,000 deaths per year $39 billion spent
ECMO as a bridge to durable LVAD therapy Jonathan Haft, MD Department of Cardiac Surgery University of Michigan Systolic Heart Failure Prevalence 4.8 million U.S. 287,000 deaths per year $39 billion spent
Modern Left Ventricular Assist Devices (LVAD) : An Intro, Complications, and Emergencies
 Modern Left Ventricular Assist Devices (LVAD) : An Intro, Complications, and Emergencies ERIC T. ROME D.O. HEART FAILURE, MECHANICAL ASSISTANCE AND TRANSPLANTATION CVI Left Ventricular Assist Device An
Modern Left Ventricular Assist Devices (LVAD) : An Intro, Complications, and Emergencies ERIC T. ROME D.O. HEART FAILURE, MECHANICAL ASSISTANCE AND TRANSPLANTATION CVI Left Ventricular Assist Device An
Understanding the Pediatric Ventricular Assist Device
 Understanding the Pediatric Ventricular Assist Device W. James Parks, MSc., MD Pediatric Cardiologist Assistant Professor of Pediatrics and Radiology Children s Healthcare of Atlanta Sibley Heart Center
Understanding the Pediatric Ventricular Assist Device W. James Parks, MSc., MD Pediatric Cardiologist Assistant Professor of Pediatrics and Radiology Children s Healthcare of Atlanta Sibley Heart Center
Further devices to treat heart failure
 Postgraduate Course Heart Failure Further devices to treat heart failure Pr. Matthias Kirsch Department of Cardiac Surgery Centre Hospitalo-Universitaire Vaudois Université de Lausanne e-mail: matthias.kirsch@chuv.ch
Postgraduate Course Heart Failure Further devices to treat heart failure Pr. Matthias Kirsch Department of Cardiac Surgery Centre Hospitalo-Universitaire Vaudois Université de Lausanne e-mail: matthias.kirsch@chuv.ch
Echo assessment of patients with an ECMO device
 Echo assessment of patients with an ECMO device Evangelos Leontiadis Cardiologist 1st Cardiology Dept. Onassis Cardiac Surgery Center Athens, Greece Gibbon HLM 1953 Goldstein DJ et al, NEJM 1998; 339:1522
Echo assessment of patients with an ECMO device Evangelos Leontiadis Cardiologist 1st Cardiology Dept. Onassis Cardiac Surgery Center Athens, Greece Gibbon HLM 1953 Goldstein DJ et al, NEJM 1998; 339:1522
Management of Cardiogenic Shock. Dr Stephen Pettit, Consultant Cardiologist
 Dr Stephen Pettit, Consultant Cardiologist Cardiogenic shock Management of Cardiogenic Shock Outline Definition, INTERMACS classification Medical management of cardiogenic shock PA catheters and haemodynamic
Dr Stephen Pettit, Consultant Cardiologist Cardiogenic shock Management of Cardiogenic Shock Outline Definition, INTERMACS classification Medical management of cardiogenic shock PA catheters and haemodynamic
End Stage Heart Failure - Time to Bring the Hammer Down
 End Stage Heart Failure - Time to Bring the Hammer Down Eric R. Skipper, MD, FACS Chief, Adult Cardiovascular Surgery Surgical Director of Cardiac Transplantation and Mechanical Circulatory Support 2 3
End Stage Heart Failure - Time to Bring the Hammer Down Eric R. Skipper, MD, FACS Chief, Adult Cardiovascular Surgery Surgical Director of Cardiac Transplantation and Mechanical Circulatory Support 2 3
The Role of Mechanical Circulatory Support in Cardiogenic Shock: When to Utilize
 The Role of Mechanical Circulatory Support in Cardiogenic Shock: Presented by Nancy Scroggins ACNP, CNS-CC CV Surgery ACNP Bayshore Medical Center The Role of Mechanical Circulatory Support in Cardiogenic
The Role of Mechanical Circulatory Support in Cardiogenic Shock: Presented by Nancy Scroggins ACNP, CNS-CC CV Surgery ACNP Bayshore Medical Center The Role of Mechanical Circulatory Support in Cardiogenic
None. Declaration of conflict of interest
 None Declaration of conflict of interest New Long Term Circulatory Support Technology and Treatment Strategies Stephen Westaby Oxford, UK Cardiac Transplantation: Facts from the UNOS Database Median survival
None Declaration of conflict of interest New Long Term Circulatory Support Technology and Treatment Strategies Stephen Westaby Oxford, UK Cardiac Transplantation: Facts from the UNOS Database Median survival
Pediatric Mechanical Circulatory Support - What to Use
 Pediatric Mechanical Circulatory Support - What to Use Ronald K. Woods, MD, PhD Associate Professor Medical College of Wisconsin Pediatric Cardiothoracic Surgery Children s Hospital of Wisconsin Disclosure
Pediatric Mechanical Circulatory Support - What to Use Ronald K. Woods, MD, PhD Associate Professor Medical College of Wisconsin Pediatric Cardiothoracic Surgery Children s Hospital of Wisconsin Disclosure
New ventricular assist devices. FW Mohr Clinical seminar: Devices for severe heart failure ESC congress Stockholm 2010
 New ventricular assist devices FW Mohr Clinical seminar: Devices for severe heart failure ESC congress Stockholm 2010 The real world of CHF Prevalence 1-3% in europe, in the age of 70-80 years up to 10-20%
New ventricular assist devices FW Mohr Clinical seminar: Devices for severe heart failure ESC congress Stockholm 2010 The real world of CHF Prevalence 1-3% in europe, in the age of 70-80 years up to 10-20%
Overview of MCS in Bruce B Reid, MD Surgical Director Artificial Heart Program/Heart Transplantation
 Overview of MCS in 2017 Bruce B Reid, MD Surgical Director Artificial Heart Program/Heart Transplantation Technology Embracing Progress Technology Adoption Internet Adoption of Technology Pioneer in the
Overview of MCS in 2017 Bruce B Reid, MD Surgical Director Artificial Heart Program/Heart Transplantation Technology Embracing Progress Technology Adoption Internet Adoption of Technology Pioneer in the
Planned, Short-Term RVAD During Durable LVAD Implant: Indications and Management
 Planned, Short-Term RVAD During Durable LVAD Implant: Indications and Management Yoshifumi Naka, MD, PhD Columbia University Medical Center New York, NY Disclosure Abbott/St. Jude Med./Thoratec Consultant
Planned, Short-Term RVAD During Durable LVAD Implant: Indications and Management Yoshifumi Naka, MD, PhD Columbia University Medical Center New York, NY Disclosure Abbott/St. Jude Med./Thoratec Consultant
Mechanical Support in the Failing Fontan-Kreutzer
 Mechanical Support in the Failing Fontan-Kreutzer Stephanie Fuller MD, MS Thomas L. Spray Endowed Chair in Congenital Heart Surgery Associate Professor, The Perelman School of Medicine at the University
Mechanical Support in the Failing Fontan-Kreutzer Stephanie Fuller MD, MS Thomas L. Spray Endowed Chair in Congenital Heart Surgery Associate Professor, The Perelman School of Medicine at the University
Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy with HeartMate 3 (MOMENTUM 3) Long Term Outcomes
 Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy with (MOMENTUM 3) Long Term Outcomes Mandeep R. Mehra, MD, Daniel J. Goldstein, MD, Nir Uriel, MD, Joseph
Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy with (MOMENTUM 3) Long Term Outcomes Mandeep R. Mehra, MD, Daniel J. Goldstein, MD, Nir Uriel, MD, Joseph
UNIVERSITY OF UTAH HEALTH CARE HOSPITALS AND CLINICS
 UNIVERSITY OF UTAH HEALTH CARE HOSPITALS AND CLINICS CARDIAC MECHANICAL SUPPORT PROGRAM GUIDELINES CARDIAC MECHANICAL SUPPORT: LVAD BASICS FREQUENT SCENARIOS AND TROUBLESHOOTING Review Date: July 2011
UNIVERSITY OF UTAH HEALTH CARE HOSPITALS AND CLINICS CARDIAC MECHANICAL SUPPORT PROGRAM GUIDELINES CARDIAC MECHANICAL SUPPORT: LVAD BASICS FREQUENT SCENARIOS AND TROUBLESHOOTING Review Date: July 2011
HeartWare ADVANCE Bridge to Transplant Trial and Continued Access Protocol Update
 HeartWare ADVANCE Bridge to Transplant Trial and Continued Access Protocol Update Mark S. Slaughter, MD University of Louisville, KY, USA HeartWare Users Meeting 29 October 2012 Barcelona, Spain HEARTWARE,
HeartWare ADVANCE Bridge to Transplant Trial and Continued Access Protocol Update Mark S. Slaughter, MD University of Louisville, KY, USA HeartWare Users Meeting 29 October 2012 Barcelona, Spain HEARTWARE,
Acute heart failure: ECMO Cardiology & Vascular Medicine 2012
 Acute heart failure: ECMO Cardiology & Vascular Medicine 2012 Lucia Jewbali cardiologist-intensivist 14 beds/8 ICU beds Acute coronary syndromes Heart failure/ Cardiogenic shock Post cardiotomy Heart
Acute heart failure: ECMO Cardiology & Vascular Medicine 2012 Lucia Jewbali cardiologist-intensivist 14 beds/8 ICU beds Acute coronary syndromes Heart failure/ Cardiogenic shock Post cardiotomy Heart
ORIGINAL ARTICLE. Alexander M. Bernhardt a, *, Theo M.M.H. De By b, Hermann Reichenspurner a and Tobias Deuse a. Abstract INTRODUCTION
 European Journal of Cardio-Thoracic Surgery 48 (2015) 158 162 doi:10.1093/ejcts/ezu406 Advance Access publication 29 October 2014 ORIGINAL ARTICLE Cite this article as: Bernhardt AM, De By TMMH, Reichenspurner
European Journal of Cardio-Thoracic Surgery 48 (2015) 158 162 doi:10.1093/ejcts/ezu406 Advance Access publication 29 October 2014 ORIGINAL ARTICLE Cite this article as: Bernhardt AM, De By TMMH, Reichenspurner
Extra Corporeal Life Support for Acute Heart failure
 Extra Corporeal Life Support for Acute Heart failure Benjamin Medalion, MD Director Heart and Lung Transplantation Department of Cardiothoracic Surgery Rabin Medical Center, Beilinson Campus, Israel Mechanical
Extra Corporeal Life Support for Acute Heart failure Benjamin Medalion, MD Director Heart and Lung Transplantation Department of Cardiothoracic Surgery Rabin Medical Center, Beilinson Campus, Israel Mechanical
Mechanical Circulatory Support in the Management of Heart Failure
 Mechanical Circulatory Support in the Management of Heart Failure Feras Bader, MD, MS, FACC Associate Professor of Medicine Director, Heart Failure and Transplant Cleveland Clinic Abu Dhabi Chairman, Heart
Mechanical Circulatory Support in the Management of Heart Failure Feras Bader, MD, MS, FACC Associate Professor of Medicine Director, Heart Failure and Transplant Cleveland Clinic Abu Dhabi Chairman, Heart
Innovative ECMO Configurations in Adults
 Innovative ECMO Configurations in Adults Practice at a Single Center with Platinum Level ELSO Award for Excellence in Life Support Monika Tukacs, BSN, RN, CCRN Columbia University Irving Medical Center,
Innovative ECMO Configurations in Adults Practice at a Single Center with Platinum Level ELSO Award for Excellence in Life Support Monika Tukacs, BSN, RN, CCRN Columbia University Irving Medical Center,
Hemodynamic Monitoring and Circulatory Assist Devices
 Hemodynamic Monitoring and Circulatory Assist Devices Speaker: Jana Ogden Learning Unit 2: Hemodynamic Monitoring and Circulatory Assist Devices Hemodynamic monitoring refers to the measurement of pressure,
Hemodynamic Monitoring and Circulatory Assist Devices Speaker: Jana Ogden Learning Unit 2: Hemodynamic Monitoring and Circulatory Assist Devices Hemodynamic monitoring refers to the measurement of pressure,
Management of Acute Shock and Right Ventricular Failure
 Management of Acute Shock and Right Ventricular Failure Nader Moazami, MD Department of Thoracic and Cardiovascular Surgery and Biomedical Engineering, Cleveland Clinic NONE Disclosures CARDIOGENIC SHOCK
Management of Acute Shock and Right Ventricular Failure Nader Moazami, MD Department of Thoracic and Cardiovascular Surgery and Biomedical Engineering, Cleveland Clinic NONE Disclosures CARDIOGENIC SHOCK
Artificial Heart Program
 Artificial Heart Program Provider Review: General VAD Overview Indications for VAD Bridge to transplant (BTT) historically most common (~80%) allow rehab from severe CHF while awaiting donor Bridge to
Artificial Heart Program Provider Review: General VAD Overview Indications for VAD Bridge to transplant (BTT) historically most common (~80%) allow rehab from severe CHF while awaiting donor Bridge to
Surgical Options for Advanced Heart Failure
 Surgical Options for Advanced Heart Failure Benjamin Medalion, MD Director, Transplantation and Heart Failure Surgery Department of Cardiothoracic Surgery Rabin Medical Center, Beilinson Hospital Heart
Surgical Options for Advanced Heart Failure Benjamin Medalion, MD Director, Transplantation and Heart Failure Surgery Department of Cardiothoracic Surgery Rabin Medical Center, Beilinson Hospital Heart
I have nothing to disclose.
 I have nothing to disclose. Right ventricular failure and need for biventricular support Friedrich Wilhelm Mohr, MD, PhD Munich, August 27, 2012 Male; date of birth: 19.07.1984 Out clinic visit 10/ 2004:
I have nothing to disclose. Right ventricular failure and need for biventricular support Friedrich Wilhelm Mohr, MD, PhD Munich, August 27, 2012 Male; date of birth: 19.07.1984 Out clinic visit 10/ 2004:
Update on Mechanical Circulatory Support. AATS May 5, 2010 Toronto, ON Canada
 Update on Mechanical Circulatory Support AATS May 5, 2010 Toronto, ON Canada Disclosures NONE Emergency Circulatory Support ECMO Tandem Heart Impella Assessment Cardiac Function Pulmonary function Valvular
Update on Mechanical Circulatory Support AATS May 5, 2010 Toronto, ON Canada Disclosures NONE Emergency Circulatory Support ECMO Tandem Heart Impella Assessment Cardiac Function Pulmonary function Valvular
Cath Lab Essentials : LV Assist Devices for Hemodynamic Support (IABP, Impella, Tandem Heart, ECMO)
 Cath Lab Essentials : LV Assist Devices for Hemodynamic Support (IABP, Impella, Tandem Heart, ECMO) Michael A. Gibson, MD Assistant Professor of Medicine University of California, Irvine Division of Cardiology
Cath Lab Essentials : LV Assist Devices for Hemodynamic Support (IABP, Impella, Tandem Heart, ECMO) Michael A. Gibson, MD Assistant Professor of Medicine University of California, Irvine Division of Cardiology
Echocardiography as a diagnostic and management tool in medical emergencies
 Echocardiography as a diagnostic and management tool in medical emergencies Frank van der Heusen MD Department of Anesthesia and perioperative Care UCSF Medical Center Objective of this presentation Indications
Echocardiography as a diagnostic and management tool in medical emergencies Frank van der Heusen MD Department of Anesthesia and perioperative Care UCSF Medical Center Objective of this presentation Indications
DECLARATION OF CONFLICT OF INTEREST
 DECLARATION OF CONFLICT OF INTEREST Cardiogenic Shock Mechanical Support Eulàlia Roig FESC Heart Failure and HT Unit Hospital Sant Pau - UAB Barcelona. Spain No conflics of interest Mechanical Circulatory
DECLARATION OF CONFLICT OF INTEREST Cardiogenic Shock Mechanical Support Eulàlia Roig FESC Heart Failure and HT Unit Hospital Sant Pau - UAB Barcelona. Spain No conflics of interest Mechanical Circulatory
The Balancing Act Bleeding and Thrombosis in MCS. Muhammad Adil Soofi
 The Balancing Act Bleeding and Thrombosis in MCS Muhammad Adil Soofi Road Map Survival and complications with LVAD What is the Burden of thrombosis and bleeding Why Bleeding and Thrombosis happen When
The Balancing Act Bleeding and Thrombosis in MCS Muhammad Adil Soofi Road Map Survival and complications with LVAD What is the Burden of thrombosis and bleeding Why Bleeding and Thrombosis happen When
Implantable Ventricular Assist Devices and Total Artificial Hearts
 Implantable Ventricular Assist Devices and Total Artificial Hearts Policy Number: Original Effective Date: MM.06.017 05/21/1999 Line(s) of Business: Current Effective Date: PPO; HMO; QUEST Integration
Implantable Ventricular Assist Devices and Total Artificial Hearts Policy Number: Original Effective Date: MM.06.017 05/21/1999 Line(s) of Business: Current Effective Date: PPO; HMO; QUEST Integration
Right Heart Failure in LVAD patients: Prevention and Management.
 Christian Bermudez MD. Associate Professor Director Thoracic Transplantation Division Cardiac Surgery Department of Surgery University of Pennsylvania Right Heart Failure in LVAD patients: Prevention and
Christian Bermudez MD. Associate Professor Director Thoracic Transplantation Division Cardiac Surgery Department of Surgery University of Pennsylvania Right Heart Failure in LVAD patients: Prevention and
Mechanics of Cath Lab Support Devices
 Mechanics of Cath Lab Support Devices Issam D. Moussa, MD Professor of Medicine Mayo Clinic College of Medicine Chair, Division of Cardiovascular Diseases Mayo Clinic Jacksonville, Florida DISCLOSURE Presenter:
Mechanics of Cath Lab Support Devices Issam D. Moussa, MD Professor of Medicine Mayo Clinic College of Medicine Chair, Division of Cardiovascular Diseases Mayo Clinic Jacksonville, Florida DISCLOSURE Presenter:
Mechanical circulatory support in cardiogenic shock The Cardiologist s view ACCA Masterclass 2017
 Mechanical circulatory support in cardiogenic shock The Cardiologist s view ACCA Masterclass 2017 Pascal Vranckx MD, PhD. Medical director Cardiac Critical Care Services Hartcentrum Hasselt Belgium Disclosure
Mechanical circulatory support in cardiogenic shock The Cardiologist s view ACCA Masterclass 2017 Pascal Vranckx MD, PhD. Medical director Cardiac Critical Care Services Hartcentrum Hasselt Belgium Disclosure
Name of Policy: Ventricular Assist Devices and Total Artificial Hearts
 Name of Policy: Ventricular Assist Devices and Total Artificial Hearts Policy #: 033 Latest Review Date: February 2014 Category: Surgery Policy Grade: A Background/Definitions: As a general rule, benefits
Name of Policy: Ventricular Assist Devices and Total Artificial Hearts Policy #: 033 Latest Review Date: February 2014 Category: Surgery Policy Grade: A Background/Definitions: As a general rule, benefits
University of Florida Department of Surgery. CardioThoracic Surgery VA Learning Objectives
 University of Florida Department of Surgery CardioThoracic Surgery VA Learning Objectives This service performs coronary revascularization, valve replacement and lung cancer resections. There are 2 faculty
University of Florida Department of Surgery CardioThoracic Surgery VA Learning Objectives This service performs coronary revascularization, valve replacement and lung cancer resections. There are 2 faculty
Disclosures. No disclosures to report
 Disclosures No disclosures to report Update on MOMENTUM 3 Trial: The Final Word? Francis D. Pagani MD PhD Otto Gago MD Professor of Cardiac Surgery University of Michigan Ann Arbor, Michigan, USA LVAD
Disclosures No disclosures to report Update on MOMENTUM 3 Trial: The Final Word? Francis D. Pagani MD PhD Otto Gago MD Professor of Cardiac Surgery University of Michigan Ann Arbor, Michigan, USA LVAD
Total Artificial Hearts and Implantable Ventricular Assist Devices
 Total Artificial Hearts and Implantable Ventricular Assist Devices Policy Number: 7.03.11 Last Review: 12/2013 Origination: 12/2001 Next Review: 12/2014 Policy Blue Cross and Blue Shield of Kansas City
Total Artificial Hearts and Implantable Ventricular Assist Devices Policy Number: 7.03.11 Last Review: 12/2013 Origination: 12/2001 Next Review: 12/2014 Policy Blue Cross and Blue Shield of Kansas City
To ECMO Or Not To ECMO Challenges of venous arterial ECMO. Dr Emily Granger St Vincent s Hospital Darlinghurst NSW
 To ECMO Or Not To ECMO Challenges of venous arterial ECMO Dr Emily Granger St Vincent s Hospital Darlinghurst NSW The Start: 1972 St Vincent s Hospital The Turning Point ECMO program restarted in 2004
To ECMO Or Not To ECMO Challenges of venous arterial ECMO Dr Emily Granger St Vincent s Hospital Darlinghurst NSW The Start: 1972 St Vincent s Hospital The Turning Point ECMO program restarted in 2004
LVAD Complications, Recovery
 LVAD Complications, Recovery Abbas Ardehali, M.D., F.A.C.S. Professor of Surgery and Medicine, Division of Cardiac Surgery William E. Connor Chair in Cardiothoracic Transplantation Director, UCLA Heart,
LVAD Complications, Recovery Abbas Ardehali, M.D., F.A.C.S. Professor of Surgery and Medicine, Division of Cardiac Surgery William E. Connor Chair in Cardiothoracic Transplantation Director, UCLA Heart,
Jennifer A. Brown The Cleveland Clinic School of Perfusion Cleveland, Ohio
 Biventricular Heart Failure Advanced Treatment Options at The Cleveland Clinic Jennifer A. Brown The Cleveland Clinic School of Perfusion Cleveland, Ohio I have no disclosures. Examine respiratory and
Biventricular Heart Failure Advanced Treatment Options at The Cleveland Clinic Jennifer A. Brown The Cleveland Clinic School of Perfusion Cleveland, Ohio I have no disclosures. Examine respiratory and
Implantable Ventricular Assist Devices and Total Artificial Hearts
 Implantable Ventricular Assist Devices and Total Artificial Hearts Policy Number: Original Effective Date: MM.06.017 05/21/1999 Line(s) of Business: Current Effective Date: PPO; HMO; QUEST Integration
Implantable Ventricular Assist Devices and Total Artificial Hearts Policy Number: Original Effective Date: MM.06.017 05/21/1999 Line(s) of Business: Current Effective Date: PPO; HMO; QUEST Integration
Ventricular Assist Devices (VADs) and Percutaneous Cardiac Support Systems
 Medical Coverage Policy Effective Date... 2/15/2018 Next Review Date... 2/15/2019 Coverage Policy Number... 0054 Ventricular Assist Devices (VADs) and Percutaneous Cardiac Support Systems Table of Contents
Medical Coverage Policy Effective Date... 2/15/2018 Next Review Date... 2/15/2019 Coverage Policy Number... 0054 Ventricular Assist Devices (VADs) and Percutaneous Cardiac Support Systems Table of Contents
ECMO as a Bridge to Heart Transplant in the Era of LVAD s.
 Christian Bermudez MD. Associate Professor Director Thoracic Transplantation Division Cardiac Surgery Department of Surgery University of Pennsylvania ECMO as a Bridge to Heart Transplant in the Era of
Christian Bermudez MD. Associate Professor Director Thoracic Transplantation Division Cardiac Surgery Department of Surgery University of Pennsylvania ECMO as a Bridge to Heart Transplant in the Era of
Evaluation of the Right Ventricle in Candidates for Right Ventricular Assist Device Implantation.
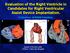 Evaluation of the Right Ventricle in Candidates for Right Ventricular Assist Device Implantation. Evaluation of RVAD Function. Ioannis A Paraskevaidis Attikon University Hospital Historical Perspective
Evaluation of the Right Ventricle in Candidates for Right Ventricular Assist Device Implantation. Evaluation of RVAD Function. Ioannis A Paraskevaidis Attikon University Hospital Historical Perspective
Mechanics of Cath Lab Support Devices
 Mechanics of Cath Lab Support Devices Issam D. Moussa, MD Chief Medical Officer First Coast Cardiovascular Institute, Jacksonville, FL Professor of Medicine, UCF, Orlando, FL None DISCLOSURE Percutaneous
Mechanics of Cath Lab Support Devices Issam D. Moussa, MD Chief Medical Officer First Coast Cardiovascular Institute, Jacksonville, FL Professor of Medicine, UCF, Orlando, FL None DISCLOSURE Percutaneous
MEDICAL POLICY Ventricular Assist Devices
 POLICY: PG0070 ORIGINAL EFFECTIVE: 02/28/06 LAST REVIEW: 02/22/18 MEDICAL POLICY Ventricular Assist Devices GUIDELINES This policy does not certify benefits or authorization of benefits, which is designated
POLICY: PG0070 ORIGINAL EFFECTIVE: 02/28/06 LAST REVIEW: 02/22/18 MEDICAL POLICY Ventricular Assist Devices GUIDELINES This policy does not certify benefits or authorization of benefits, which is designated
Mechanical assist patient selection, device selection, and outcomes
 Mechanical assist patient selection, device selection, and outcomes Josef Stehlik, MD, MPH Associate Professor of Medicine Medical Director, Heart Transplant Program University of Utah School of Medicine
Mechanical assist patient selection, device selection, and outcomes Josef Stehlik, MD, MPH Associate Professor of Medicine Medical Director, Heart Transplant Program University of Utah School of Medicine
Echo in Heart Failure
 Echo in Heart Failure Karima Addetia, MD Heart Failure: Definition A clinical syndrome that results from impairment of ventricular filling or ejection of blood. Manifestations include dyspnea and fatigue,
Echo in Heart Failure Karima Addetia, MD Heart Failure: Definition A clinical syndrome that results from impairment of ventricular filling or ejection of blood. Manifestations include dyspnea and fatigue,
Section 6 Intra Aortic Balloon Pump
 Section 6 Intra Aortic Balloon Pump The Intra Aortic Balloon Pump (IABP) The balloon is synthetic and is made for single use only. It is threaded into the aorta, usually via a femoral approach. The balloon
Section 6 Intra Aortic Balloon Pump The Intra Aortic Balloon Pump (IABP) The balloon is synthetic and is made for single use only. It is threaded into the aorta, usually via a femoral approach. The balloon
Implantable Ventricular Assist Devices and Total Artificial Hearts
 Implantable Ventricular Assist Devices and Total Artificial Hearts Policy Number: Original Effective Date: MM.06.017 05/21/1999 Line(s) of Business: Current Effective Date: PPO; HMO; QUEST Integration
Implantable Ventricular Assist Devices and Total Artificial Hearts Policy Number: Original Effective Date: MM.06.017 05/21/1999 Line(s) of Business: Current Effective Date: PPO; HMO; QUEST Integration
Mechanical Circulatory Support (MCS): What Every Pharmacist Needs to Know!
 Mechanical Circulatory Support (MCS): What Every Pharmacist Needs to Know! Matthew A. Wanat, PharmD, BCPS, BCCCP, FCCM Clinical Assistant Professor University of Houston College of Pharmacy Clinical Pharmacy
Mechanical Circulatory Support (MCS): What Every Pharmacist Needs to Know! Matthew A. Wanat, PharmD, BCPS, BCCCP, FCCM Clinical Assistant Professor University of Houston College of Pharmacy Clinical Pharmacy
Do we really need an Artificial Heart? No!! John V. Conte, MD, Professor of Surgery Johns Hopkins University School of Medicine
 Do we really need an Artificial Heart? No!! John V. Conte, MD, Professor of Surgery Johns Hopkins University School of Medicine Division of Cardiac Surgery The Johns Hopkins Medical Institutions Conflict
Do we really need an Artificial Heart? No!! John V. Conte, MD, Professor of Surgery Johns Hopkins University School of Medicine Division of Cardiac Surgery The Johns Hopkins Medical Institutions Conflict
Complications of VAD therapy - RV failure
 Complications of VAD therapy - RV failure Nana Afari-Armah, MD Advanced heart failure and transplant cardiology Temple University Hospital 3/24/18 Goals Understand the role of the right ventricle in LVAD
Complications of VAD therapy - RV failure Nana Afari-Armah, MD Advanced heart failure and transplant cardiology Temple University Hospital 3/24/18 Goals Understand the role of the right ventricle in LVAD
Ventricular Assist Device. Lauren Bartlett 10/5/16 BME 281, section 1
 Ventricular Assist Device Lauren Bartlett 10/5/16 BME 281, section 1 What is a Ventricular Assist Device (VAD)? Electromechanical device for assisting cardiac circulation Used to partially or completely
Ventricular Assist Device Lauren Bartlett 10/5/16 BME 281, section 1 What is a Ventricular Assist Device (VAD)? Electromechanical device for assisting cardiac circulation Used to partially or completely
Left Ventricular Assist Device: What Should I Report?
 2017 SOTA, Tucson, AZ February 21, 2017 11:15 11:40 AM 25 min Left Ventricular Assist Device: What Should I Report? Muhamed Sarić MD, PhD, MPA Director of Noninvasive Cardiology Echo Lab Associate Professor
2017 SOTA, Tucson, AZ February 21, 2017 11:15 11:40 AM 25 min Left Ventricular Assist Device: What Should I Report? Muhamed Sarić MD, PhD, MPA Director of Noninvasive Cardiology Echo Lab Associate Professor
CENTRAL ECMO WHEN AND HOW? RANJIT JOHN, MD UNIVERSITY OF MINESOTA
 CENTRAL ECMO WHEN AND HOW? RANJIT JOHN, MD UNIVERSITY OF MINESOTA Background How to do Case reports When to do Managing complications Post operative management strategies CASE PRESENTATION 46 year old
CENTRAL ECMO WHEN AND HOW? RANJIT JOHN, MD UNIVERSITY OF MINESOTA Background How to do Case reports When to do Managing complications Post operative management strategies CASE PRESENTATION 46 year old
 CASE PRESENTATION Ravi Dhanisetty, M.D. SUNY Downstate 23 July 2009 CASE PRESENTATION xx yr old female with chest pain for 3 days. Initially taken to outside hospital 3 days history of chest pain, shortness
CASE PRESENTATION Ravi Dhanisetty, M.D. SUNY Downstate 23 July 2009 CASE PRESENTATION xx yr old female with chest pain for 3 days. Initially taken to outside hospital 3 days history of chest pain, shortness
Percutaneous Mechanical Circulatory Support Devices
 Percutaneous Mechanical Circulatory Support Devices Daniel Vazquez RN, RCIS Miami Cardiac & Vascular Institute FINANCIAL DISCLOSURES none CASE STUDY CASE STUDY 52 year old gentlemen Complaining of dyspnea
Percutaneous Mechanical Circulatory Support Devices Daniel Vazquez RN, RCIS Miami Cardiac & Vascular Institute FINANCIAL DISCLOSURES none CASE STUDY CASE STUDY 52 year old gentlemen Complaining of dyspnea
Mechanical Cardiac Support and Cardiac Transplant: The Role for Echocardiography
 Mechanical Cardiac Support and Cardiac Transplant: The Role for Echocardiography David Langholz, M.D., F.A.C.C. Co-Director Cardiovascular Imaging Fredrick Meijer Heart and Vascular Institute Spectrum
Mechanical Cardiac Support and Cardiac Transplant: The Role for Echocardiography David Langholz, M.D., F.A.C.C. Co-Director Cardiovascular Imaging Fredrick Meijer Heart and Vascular Institute Spectrum
VAD come Destination therapy nell adulto con Scompenso Cardiaco
 VAD come Destination therapy nell adulto con Scompenso Cardiaco Francesco Santini Division of Cardiac Surgery, IRCCS San Martino IST University of Genova Medical School, Italy Heart Transplantation is
VAD come Destination therapy nell adulto con Scompenso Cardiaco Francesco Santini Division of Cardiac Surgery, IRCCS San Martino IST University of Genova Medical School, Italy Heart Transplantation is
Total Artificial Hearts and Implantable Ventricular Assist Devices
 Total Artificial Hearts and Implantable Ventricular Assist Devices Policy Number: 7.03.11 Last Review: 12/2018 Origination: 12/2001 Next Review: 12/2019 Policy Blue Cross and Blue Shield of Kansas City
Total Artificial Hearts and Implantable Ventricular Assist Devices Policy Number: 7.03.11 Last Review: 12/2018 Origination: 12/2001 Next Review: 12/2019 Policy Blue Cross and Blue Shield of Kansas City
3/1/2017. Chronic Mechanical Support for Heart Failure. Heart Failure is a major driver of morbidity and mortality in the US 1-7
 Chronic Mechanical Support for Heart Failure Margarita Camacho MD, FACS Surgical Director Cardiac Transplant and Mechanical Assist Device Program RWJ/Barnabas Health Heart Centers at Newark Beth Israel
Chronic Mechanical Support for Heart Failure Margarita Camacho MD, FACS Surgical Director Cardiac Transplant and Mechanical Assist Device Program RWJ/Barnabas Health Heart Centers at Newark Beth Israel
Introduction to Acute Mechanical Circulatory Support
 Introduction to Acute Mechanical Circulatory Support Navin K. Kapur, MD, FACC, FSCAI, FAHA Associate Professor, Department of Medicine Interventional Cardiology & Advanced Heart Failure Programs Executive
Introduction to Acute Mechanical Circulatory Support Navin K. Kapur, MD, FACC, FSCAI, FAHA Associate Professor, Department of Medicine Interventional Cardiology & Advanced Heart Failure Programs Executive
Total Artificial Hearts and Implantable Ventricular Assist Devices
 Total Artificial Hearts and Implantable Ventricular Assist Devices Policy Number: 7.03.11 Last Review: 12/2017 Origination: 12/2001 Next Review: 12/2018 Policy Blue Cross and Blue Shield of Kansas City
Total Artificial Hearts and Implantable Ventricular Assist Devices Policy Number: 7.03.11 Last Review: 12/2017 Origination: 12/2001 Next Review: 12/2018 Policy Blue Cross and Blue Shield of Kansas City
HEARTWARE HVAD WAVEFORM APP INSTRUCTIONS
 HEARTWARE HVAD WAVEFORM APP INSTRUCTIONS TABLE OF CONTENTS Welcome... 3 HVAD Waveforms 1. Characteristics... 4 2. Theory of Operation... 5 3. Ao & LV Pressure... 6 4. HQ Curve... 7 5. PV Loops... 8 Home
HEARTWARE HVAD WAVEFORM APP INSTRUCTIONS TABLE OF CONTENTS Welcome... 3 HVAD Waveforms 1. Characteristics... 4 2. Theory of Operation... 5 3. Ao & LV Pressure... 6 4. HQ Curve... 7 5. PV Loops... 8 Home
EMS and Nursing Considerations in VAD Patient Care
 EMS and Nursing Considerations in VAD Patient Care B R I T T A N Y B U T Z L E R B S N R N V A D C O O R D I N A T O R F R O E D T E R T A N D T H E M E D I C A L C O L L E G E O F W I 1 0 / 2 5 / 1 8
EMS and Nursing Considerations in VAD Patient Care B R I T T A N Y B U T Z L E R B S N R N V A D C O O R D I N A T O R F R O E D T E R T A N D T H E M E D I C A L C O L L E G E O F W I 1 0 / 2 5 / 1 8
Which mechanical assistance for cardiogenic shock?
 Which mechanical assistance for cardiogenic shock? Alain Combes, MD, PhD, Hôpital Pitié-Salpêtrière, AP-HP Inserm UMRS 1166, ican, Institute of Cardiometabolism and Nutrition Pierre et Marie Curie Sorbonne
Which mechanical assistance for cardiogenic shock? Alain Combes, MD, PhD, Hôpital Pitié-Salpêtrière, AP-HP Inserm UMRS 1166, ican, Institute of Cardiometabolism and Nutrition Pierre et Marie Curie Sorbonne
Complications in CHD with a little help from our friends
 Complications in CHD with a little help from our friends Systemic ventricular failure how can the surgeon help? Assist device or transplantation? Michael Huebler (Zurich, CH) Survivors of CHD Curative
Complications in CHD with a little help from our friends Systemic ventricular failure how can the surgeon help? Assist device or transplantation? Michael Huebler (Zurich, CH) Survivors of CHD Curative
Challenges to MCS Use in the Middle East
 Challenges to MCS Use in the Middle East Feras Khaliel, MD, Ph.D Consultant Cardiac Surgeon Associate Professor of Surgery, Alfaisal Univerisity King Faisal Specialist Hospital & Research Center Dr. Michael
Challenges to MCS Use in the Middle East Feras Khaliel, MD, Ph.D Consultant Cardiac Surgeon Associate Professor of Surgery, Alfaisal Univerisity King Faisal Specialist Hospital & Research Center Dr. Michael
Index. K Knobology, TTE artifact, image resolution, ultrasound, 14
 A Acute aortic regurgitation (AR), 124 128 Acute aortic syndrome (AAS) classic aortic dissection diagnosis, 251 263 evolutive patterns, 253 255 pathology, 250 251 classifications, 247 248 incomplete aortic
A Acute aortic regurgitation (AR), 124 128 Acute aortic syndrome (AAS) classic aortic dissection diagnosis, 251 263 evolutive patterns, 253 255 pathology, 250 251 classifications, 247 248 incomplete aortic
Initial experience with Imacor htee-guided management of patients following transplant and mechanical circulatory support.
 Thomas Jefferson University Jefferson Digital Commons Department of Cancer Biology Faculty Papers Department of Cancer Biology Fall 11-1-2012 Initial experience with Imacor htee-guided management of patients
Thomas Jefferson University Jefferson Digital Commons Department of Cancer Biology Faculty Papers Department of Cancer Biology Fall 11-1-2012 Initial experience with Imacor htee-guided management of patients
From Recovery to Transplant: One Patient's Journey
 From Recovery to Transplant: One Patient's Journey Tonya Elliott, RN, MSN Assist Device and Thoracic Transplant Coordinator Inova Transplant Center at Inova Fairfax Hospital Falls Church, VA Introduction
From Recovery to Transplant: One Patient's Journey Tonya Elliott, RN, MSN Assist Device and Thoracic Transplant Coordinator Inova Transplant Center at Inova Fairfax Hospital Falls Church, VA Introduction
Description. Section: Surgery Effective Date: April 15, Subsection: Transplant Original Policy Date: September 13, 2012 Subject:
 Last Review Status/Date: March 2016 Page: 1 of 30 Description Mechanical devices to assist or replace a failing heart have been developed over many decades of research. A ventricular assist device (VAD)
Last Review Status/Date: March 2016 Page: 1 of 30 Description Mechanical devices to assist or replace a failing heart have been developed over many decades of research. A ventricular assist device (VAD)
Outpatient Treatment of MCS Patient. F. Bennett Pearce, MD Professor of Pediatrics Med Director Heart Transplant COA
 Outpatient Treatment of MCS Patient F. Bennett Pearce, MD Professor of Pediatrics Med Director Heart Transplant COA Disclosure Statement I DO NOT HAVE ANY RELEVANT FINANCIAL RELATIONSHIPS WITH ANY COMMERCIAL
Outpatient Treatment of MCS Patient F. Bennett Pearce, MD Professor of Pediatrics Med Director Heart Transplant COA Disclosure Statement I DO NOT HAVE ANY RELEVANT FINANCIAL RELATIONSHIPS WITH ANY COMMERCIAL
Pediatric Mechanical Circulatory Support (MCS)
 Pediatric Mechanical Circulatory Support (MCS) Ivan Wilmot, MD Heart Failure, Transplant, MCS Assistant Professor The Heart Institute Cincinnati Children s Hospital Medical Center The University of Cincinnati
Pediatric Mechanical Circulatory Support (MCS) Ivan Wilmot, MD Heart Failure, Transplant, MCS Assistant Professor The Heart Institute Cincinnati Children s Hospital Medical Center The University of Cincinnati
Intra-operative Echocardiography: When to Go Back on Pump
 Intra-operative Echocardiography: When to Go Back on Pump GREGORIO G. ROGELIO, MD., F.P.C.C. OUTLINE A. Indications for Intraoperative Echocardiography B. Role of Intraoperative Echocardiography C. Criteria
Intra-operative Echocardiography: When to Go Back on Pump GREGORIO G. ROGELIO, MD., F.P.C.C. OUTLINE A. Indications for Intraoperative Echocardiography B. Role of Intraoperative Echocardiography C. Criteria
12/19/2017. Learning Objectives. Mechanical Circulatory Support. Mechanical Aids to External Massage. Noninvasive Mechanical Support Devices
 Learning Objectives Mechanical Circulatory Support Explain the indications, function, & complications for selected mechanical circulatory support devices Describe examples of ventricular assist devices
Learning Objectives Mechanical Circulatory Support Explain the indications, function, & complications for selected mechanical circulatory support devices Describe examples of ventricular assist devices
Left Ventricular Assist Devices LVAD. North Country EMS Program Agency 3/21/12
 Left Ventricular Assist Devices LVAD North Country EMS Program Agency 3/21/12 Objectives Describe indications for and functions of ventricular assist devices (LVAD) Differentiate assessment findings of
Left Ventricular Assist Devices LVAD North Country EMS Program Agency 3/21/12 Objectives Describe indications for and functions of ventricular assist devices (LVAD) Differentiate assessment findings of
Case - Advanced HF and Shock (INTERMACS 1)
 Case - Advanced HF and Shock (INTERMACS 1) Navin K. Kapur, MD, FACC, FSCAI, FAHA Associate Professor, Department of Medicine Interventional Cardiology & Advanced Heart Failure Programs Executive Director,
Case - Advanced HF and Shock (INTERMACS 1) Navin K. Kapur, MD, FACC, FSCAI, FAHA Associate Professor, Department of Medicine Interventional Cardiology & Advanced Heart Failure Programs Executive Director,
Giving your heart strength. Ventricular Assist Device.
 Giving your heart strength. Ventricular Assist Device. 1 National leader in Ventricular Assist Devices Although you may be nervous when considering heart surgery, you can rest assured knowing that UR Medicine
Giving your heart strength. Ventricular Assist Device. 1 National leader in Ventricular Assist Devices Although you may be nervous when considering heart surgery, you can rest assured knowing that UR Medicine
CHANGING THE WAY HEART FAILURE IS TREATED. VAD Therapy
 CHANGING THE WAY HEART FAILURE IS TREATED VAD Therapy VAD THERAPY IS BECOMING AN ESSENTIAL PART OF HEART FAILURE PROGRAMS AROUND THE WORLD. Patients with advanced heart failure experience an impaired quality
CHANGING THE WAY HEART FAILURE IS TREATED VAD Therapy VAD THERAPY IS BECOMING AN ESSENTIAL PART OF HEART FAILURE PROGRAMS AROUND THE WORLD. Patients with advanced heart failure experience an impaired quality
Bridging With Percutaneous Devices: Tandem Heart and Impella
 Bridging With Percutaneous Devices: Tandem Heart and Impella DAVID A. BARAN, MD, FACC, FSCAI SYSTEM DIRECTOR, ADVANCED HEART FAILURE, TX AND MCS SENTARA HEART HOSPITAL NORFOLK, VA PROFESSOR OF MEDICINE
Bridging With Percutaneous Devices: Tandem Heart and Impella DAVID A. BARAN, MD, FACC, FSCAI SYSTEM DIRECTOR, ADVANCED HEART FAILURE, TX AND MCS SENTARA HEART HOSPITAL NORFOLK, VA PROFESSOR OF MEDICINE
HEARTMATE 3 LEFT VENTRICULAR ASSIST SYSTEM
 HEARTMATE 3 LEFT VENTRICULAR ASSIST SYSTEM A New Milestone in LVAD Therapy HeartMate 3 Left Ventricular Assist Device Introducing the new HEARTMATE 3 LVAD WITH FULL MAGLEV FLOW TECHNOLOGY HeartMate 3 LVAD
HEARTMATE 3 LEFT VENTRICULAR ASSIST SYSTEM A New Milestone in LVAD Therapy HeartMate 3 Left Ventricular Assist Device Introducing the new HEARTMATE 3 LVAD WITH FULL MAGLEV FLOW TECHNOLOGY HeartMate 3 LVAD
Disclosures. Objectives 10/11/17. Short Term Mechanical Circulatory Support for Advanced Cardiogenic Shock. I have no disclosures to report
 Short Term Mechanical Circulatory Support for Advanced Cardiogenic Shock Christopher K. Gordon MSN, ACNP-BC Disclosures I have no disclosures to report 1. Pathophysiology 2. Epidemiology 3. Assessment
Short Term Mechanical Circulatory Support for Advanced Cardiogenic Shock Christopher K. Gordon MSN, ACNP-BC Disclosures I have no disclosures to report 1. Pathophysiology 2. Epidemiology 3. Assessment
Medical Policy. MP Total Artificial Hearts and Implantable Ventricular Assist Devices
 Medical Policy MP 7.03.11 BCBSA Ref. Policy: 7.03.11 Last Review: 08/20/2018 Effective Date: 08/20/2018 Section: Surgery Related Policies 7.03.08 Heart/Lung Transplant 7.03.09 Heart Transplant 8.01.60
Medical Policy MP 7.03.11 BCBSA Ref. Policy: 7.03.11 Last Review: 08/20/2018 Effective Date: 08/20/2018 Section: Surgery Related Policies 7.03.08 Heart/Lung Transplant 7.03.09 Heart Transplant 8.01.60
DEMYSTIFYING VADs. Nicolle Choquette RN MN Athabasca University
 DEMYSTIFYING VADs Nicolle Choquette RN MN Athabasca University Objectives odefine o Heart Failure o VAD o o o o Post Operative Complications Acute Long Term Nursing Interventions What is Heart Failure?
DEMYSTIFYING VADs Nicolle Choquette RN MN Athabasca University Objectives odefine o Heart Failure o VAD o o o o Post Operative Complications Acute Long Term Nursing Interventions What is Heart Failure?
Description. Section: Surgery Effective Date: January 15, 2015 Subsection: Transplant Original Policy Date: September 13, 2012 Subject:
 Last Review Status/Date: December 2014 Page: 1 of 26 Description Mechanical devices to assist or replace a failing heart have been developed over many decades of research. A ventricular assist device (VAD)
Last Review Status/Date: December 2014 Page: 1 of 26 Description Mechanical devices to assist or replace a failing heart have been developed over many decades of research. A ventricular assist device (VAD)
The era of mechanical circulatory support (MCS) began in
 AHA Scientific Statement Recommendations for the Use of Mechanical Circulatory Support: Device Strategies and Patient Selection A Scientific Statement From the American Heart Association Jennifer L. Peura,
AHA Scientific Statement Recommendations for the Use of Mechanical Circulatory Support: Device Strategies and Patient Selection A Scientific Statement From the American Heart Association Jennifer L. Peura,
Rhondalyn C. McLean. 2 ND YEAR RESEARCH ELECTIVE RESIDENT S JOURNAL Volume VII, A. Study Purpose and Rationale
 A Randomized Clinical Study To Compare The Intra-Aortic Balloon Pump To A Percutaneous Left Atrial-To-Femoral Arterial Bypass Device For Treatment Of Cardiogenic Shock Following Acute Myocardial Infarction.
A Randomized Clinical Study To Compare The Intra-Aortic Balloon Pump To A Percutaneous Left Atrial-To-Femoral Arterial Bypass Device For Treatment Of Cardiogenic Shock Following Acute Myocardial Infarction.
Bridge to Heart Transplantation
 Bridge to Heart Transplantation Ulf Kjellman MD, PhD Senior Consultant Surgeon Heart Centre KFSH&RC 1 Disclosure Appointed for Proctorship by Thoratec/St.Jude/Abbott 2 To run a full overall covering transplant
Bridge to Heart Transplantation Ulf Kjellman MD, PhD Senior Consultant Surgeon Heart Centre KFSH&RC 1 Disclosure Appointed for Proctorship by Thoratec/St.Jude/Abbott 2 To run a full overall covering transplant
Index. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A Ablation, radiofrequency, anesthetic considerations for, 479 489 Acute aortic syndrome, thoracic endovascular repair of, 457 462 aortic
Index Note: Page numbers of article titles are in boldface type. A Ablation, radiofrequency, anesthetic considerations for, 479 489 Acute aortic syndrome, thoracic endovascular repair of, 457 462 aortic
