Effect of lanthanum carbonate and calcium acetate in the treatment of hyperphosphatemia in patients of chronic kidney disease
|
|
|
- Kevin Atkins
- 6 years ago
- Views:
Transcription
1 Research Article Effect of lanthanum and calcium acetate in the treatment of hyperphosphatemia in patients of chronic kidney disease P. Thomas Scaria, Reneega Gangadhar 1, Ramdas Pisharody 2 ABSTRACT Departments of Pharmacology and Therapeutics, Government Medical College, Thiruvananthapuram, 1 Pharmacology, Government Medical College, Kottayam, 2 Nephrology, Government Medical College, Thiruvananthapuram, Kerala, India Received: Revised: Accepted: DOI: / Correspondence to: Dr. Thomas P Scaria scariathomasp@rediffmail.com Objectives: The tolerability and efficacy of lanthanum has not been studied in the Indian population. This study was, therefore, undertaken to compare the efficacy and tolerability of lanthanum with calcium acetate in patients with stage 4 chronic kidney disease. Design: A randomized open label two group cross-over study. Materials and Methods: Following Institutional Ethics Committee approval and valid consent, patients with stage 4 chronic kidney disease were randomized to receive either lanthanum 500 mg thrice daily or calcium acetate 667 mg thrice daily for 4 weeks. After a 4-week washout period, the patients were crossed over for another 4 weeks. Serum phosphorous, serum calcium, serum alkaline phosphatase, and serum creatinine were estimated at fixed intervals. Results: Twenty-six patients were enrolled in the study. The mean serum phosphorous concentrations showed a declining trend with lanthanum (from pre-drug levels of 7.88 ± 1.52 mg/dl-7.14 ± 1.51 mg/dl) and calcium acetate (from pre-drug levels of 7.54 ± 1.39 mg/dl-6.51 ± 1.38 mg/dl). A statistically significant difference was seen when comparing the change in serum calcium produced by these drugs (P < 0.05). Serum calcium levels increased with calcium acetate (from pre-drug levels of 7.01 ± ± 0.74 mg dl), while it decreased with lanthanum (from pre-drug levels 7.43 ± ± 0.72 mg/ dl). The incidence of adverse effects was greater with lanthanum. Conclusion: and calcium acetate are equally effective phosphate binders with trends obvious in the first 4 weeks of therapy. The decrease in serum calcium levels with lanthanum when compared to the increase in serum calcium levels due to calcium acetate is statistically significant. The drawback of lanthanum is its high cost and relatively higher incidence of adverse events during treatment. KEY WORDS: Cross-over study, hyperphosphatemia, phosphate binders, lanthanum, serum phosphorous Introduction Hyperphosphatemia is a universal complication in patients with chronic kidney disease (CKD) when the glomerular filtration rate (GFR) falls below 25 ml/min. It is now known to be associated with the development of hyperparathyroidism, renal osteodystrophy, metastatic, and vascular calcification resulting in increased morbidity and mortality. [1-3] Elevated calcium phosphorous product and increased daily intake of calcium are other risk factors for coronary artery calcification. [4] The reduction of serum phosphorus levels in patients with CKD can be achieved through a combination of dietary control, removal by dialysis, and intervention with phosphate binders that prevent the absorption of phosphorus from the intestine. Dietary restriction is not possible beyond a point without risking a negative nitrogen balance. [5] Even regular daily dialysis treatment removes 75% of absorbed phosphorus and not all of the phosphorus ingested. [6] As a result, most CKD patients require phosphate binders to decrease phosphorus absorption from the gastrointestinal tract and maintain the serum phosphorous and calcium phosphorous product at the recommended levels. Aluminium-based phosphate binders are not preferred due to associated toxicities like encephalopathy, microcytic anemia, 187
2 osteomalacia, and arthropathy. [7,8] The calcium salts if given with inappropriate calcitriol treatment can cause hypercalcemia and potentially lead to cardiovascular calcification. [4] It has been observed that long-term, calcium-based phosphate binder therapy can lead to progressive calcification of both coronary and aortic arteries when compared with non-calcium-based agents. [9] Despite these drawbacks, calcium salts especially calcium acetate is the most commonly used phosphate binder due to its efficacy and low cost. The problems associated with the use of aluminium- and calcium-based agents have prompted researchers to develop alternative phosphate binders not containing aluminium or calcium. (La 2 [CO 3 ] 3 ), is a recently developed [10] phosphate binder which does not contain aluminium or calcium. (La) is a naturally occurring rare earth element (atomic number: 57) that can be detected in the tissues of healthy individuals. [11] acts by dissociating in the acid environment of the upper gastrointestinal tract to release lanthanum ions that bind dietary phosphate released from food during digestion to form lanthanum phosphate, an insoluble compound that is poorly absorbed across the gut wall. [12] is well tolerated and the adverse effects were mainly gastrointestinal like nausea and vomiting. [13,14] The present study was undertaken to compare the efficacy and tolerability of lanthanum with the conventional phosphate binder, calcium acetate in our patient population. Materials and Methods This was a randomized open label two-group cross-over study initiated after obtaining Institutional Ethics Committee approval. Patients aged between 18 and 80 years with stage 4 CKD, having serum phosphorous above 5.5 mg/dl, and without significant hypercalcemia or hypocalcemia (serum calcium > 11 mg/dl or < 7.9 mg/dl) were enrolled in the study after obtaining written informed consent. Patients with end-stage renal disease, extensive edema and hypoproteinemia, allergy to any of the medications, or had acute on chronic renal failure were excluded from the study. Pregnant or lactating women, or who were not using appropriate birth control were also excluded. Further, patients with severe gastrointestinal symptoms precluding administration of drugs or gastrointestinal bleeding, malignancy, or exposure to other investigational drugs within 30 days prior to the start of the study were also excluded. Consumption of medications containing calcium, phosphorous, aluminium, or magnesium was not allowed during the study period. Vitamin D supplementation was allowed provided the dose was kept constant during the study period. The total duration of the study for each patient was of 12 weeks, which could be divided into two stages of 4 weeks each with a 4-week washout period in between. The patients meeting the inclusion criteria were randomly allocated into two groups, by using random numbers obtained from a random number table, to receive either lanthanum or calcium acetate for 4 weeks. At the end of the first stage, the respective drug was stopped and the patient entered a 4-week drug-free washout period. After the 4-week washout period, the patients were crossed over to receive the alternate drug for 4 weeks. The patients served as their own controls. The patients were assessed at 4 weekly intervals i.e. at the time of recruitment, after first stage (at 4th week), after washout (at 8 th week), and after second stage (at 12 th week). A detailed history, general examination, vital signs, and relevant systemic examination were carried out at the time of recruitment and at each visit. Serum phosphorous, serum calcium, serum alkaline phosphatase and serum creatinine were estimated at baseline and at each visit. 667 mg tablet was administered thrice daily (667 mg of calcium acetate is equivalent to 169 mg of elemental calcium). It was swallowed along with the meals or immediately after the meals. 500 mg was also taken thrice daily and the tablet was chewed along with the meals. All data were recorded in a specific case record form (CRF) designed for this study. Compliance was estimated by pill count. The drug-related adverse events that occurred during the study period also were noted in the CRF. The costs of 4-week treatments with lanthanum and calcium acetate were calculated from the M.R.P. labels on the respective packages. Statistical considerations The sample size was calculated using the power analysis and sample size (PASS 2005) software. A sample size of 20 would ensure that a two-sided test has 80% power to detect a mean difference of 2 mg/dl between the two treatments. Data were fed into the statistical package 70 of Microsoft Excel and checked for data entry errors. The distribution of variables was noted. Data from the patients who completed the study were analyzed using SPSS software. For comparison of the means between the groups, paired t-test was used. The level of significance was fixed at 5%. Results Out of 26 patients enrolled for the study, 20 patients completed the study [Figure 1]. Of the six patients who did not complete the study, one patient withdrew during phase I (pre-washout) due to adverse effects while on lanthanum ; two patients were withdrawn in phase I due to protocol violation while on calcium acetate; two patients were withdrawn in phase II (post-washout) due to worsening of their renal status while on lanthanum, and one patient was withdrawn in phase II due to worsening of renal status while on calcium acetate. The compliance of the patients during the study was found to be more than 90%. The demographic details of the patients who completed the study are shown in Table 1. The mean serum phosphorous concentrations showed a declining trend during the period in which the phosphate binders were taken. The mean serum phosphorous levels during the intake of lanthanum decreased from 7.88 ± 1.52 mg/ dl-7.14 ± 1.51 mg/dl, while during calcium acetate treatment it decreased from 7.54 ± 1.39 mg/dl-6.51 ± 1.38 mg/ dl. The reduction in serum phosphorous produced by lanthanum and calcium acetate was identical [Figure 2]. The changes in mean serum calcium levels during treatment with lanthanum and calcium acetate showed opposing trends. Serum calcium increased from 7.01 ± ± 0.74 mg/dl with calcium acetate treatment, while it decreased from 7.43 ± ± 0.72 mg/dl with lanthanum. A statistically significant difference was seen when comparing the change in serum calcium produced 188
3 Figure 1: Study ß owchart Figure 2: Changes in S. phosphorous levels during treatment Phase I 500 mg x 4 weeks (n = 13) Patients selected for study (n = 26) Baseline S.Ca, P, Cr, ALP Randomized Phase I 667 mg x 4 weeks (n = 13) serum levels (mg/dl) Figure 3: Changes in S. calcium levels during treatment Completed phase I (n = 12), Washout Completed phase I (n = 11), serum levels (mg/dl) Phase II 500 mg x 4 weeks (n=11) Phase II 667 mg x 4 weeks (n = 12) Figure 4: Changes in Ca x P during treament Completed phase II (n = 10), Table 1 End of study Demographic data of patients Completed phase II (n = 10), End of study (mg 2 /dl 2 ) Feature Treated population No. of patients 20 Mean age (years) 49.9 Mean weight (kg) 55.5 Gender (% male) 65 by these drugs (P < 0.05) [Figure 3]. The mean calcium phosphorous product showed a declining trend during treatment with both the phosphate binders. The mean Ca P product during treatment with lanthanum decreased from ± to ± mg 2 / dl 2 and during treatment with calcium acetate decreased from ± to ± mg 2 /dl 2 [Figure 4]. The changes in alkaline phosphatase levels during treatment with either of the phosphate binders and the difference in the trends of alkaline phosphatase during treatment were not statistically significant. The mean creatinine levels were stable throughout the treatment [Table 2]. Adverse events Over the entire course of the study, four adverse events were reported by patients on lanthanum including abdominal discomfort, burning sensation all over the body and vomiting. One patient was withdrawn from the study due to generalized rashes and small eruptions in the oral mucosa. No adverse event was reported during the intake of calcium acetate. Comparative costs of treatment The cost of a single tablet of lanthanum was Rs.16, resulting into total cost of Rs for 4-week treatment. On the other hand, the cost of a 4-week treatment with calcium acetate was Rs s The cost of treatment with lanthanum is 16 times that of the conventional drug, i.e. calcium acetate. Discussion In this study, the efficacy of the newer non-calcium, non-aluminum phosphate binder i.e. lanthanum was compared with the conventional calcium-containing phosphate binder, calcium acetate, by measuring the changes in the levels of serum phosphorus during treatment with either drugs. It was observed that both drugs were equally good phosphate binders and lower phosphate levels. As both these drugs were given at a fixed dose and only for a limited period (4 weeks), no statistically significant difference was noted in the phosphate-binding ability 189
4 Table 2 Comparison of laboratory parameters in patients treated with lanthanum and calcium acetate (n=20) Parameter Drug N mean (SD) mean (SD) Phosphorous (mg/dl) (1.52) 7.14 (1.51) (1.39) 6.51 (1.38) Calcium (mg/dl) (0.77)* 7.14 (0.72)* (1.07)* 7.46 (0.74)* Calcium phosphorous product (mg 2 /dl 2 ) (13.93) (10.15) (10.32) (11.63) Alkaline phosphatase (IU/L) (25.45) (71.30) (37.25) (30.91) Creatinine (mg/dl) (1.69) 5.04 (1.96) (1.94) 4.86 (2.02) *The difference in serum calcium levels produced by calcium acetate as compared to lanthanum was statistically signiþ cant (P < 0.05) of the drugs. Also, the phosphate levels could not be lowered to the desirable levels in the majority of patients due to the above-mentioned reasons. It seems that adequate control of phosphate levels may be achieved by using doses considerably lower than that prescribed for the western population especially in the case of calcium acetate. A treat-to-goal approach, in which the doses are titrated upward at two weekly intervals till phosphate levels are controlled, is needed to definitely identify a difference in the efficacy of these phosphate binders, if at all they exist. Serum calcium needs to be maintained at high normal levels (9-10 mg/dl) and Ca P product needs to be kept under 55 mg 2 /dl 2 in patients with CKD. When these parameters rise above the levels specified, there is a higher incidence of vascular calcification and cardiovascular morbidity. [4] Even with the limited data obtained from the study, it could be inferred that the usage of calcium acetate as a phosphate binder was associated with a statistically significant rise in serum calcium when compared to the fall in serum calcium produced by lanthanum. The difference in the fall in Ca P product during treatment with the two drugs was not statistically significant. Still the trend of decline in Ca P product was greater with lanthanum when compared to calcium acetate. The level of alkaline phosphatase is an indicator of osteoblast activity. Usually, in CKD, there is hyperphosphatemia and hypocalcemia that produces hyperparathyroidism, which in turn leads to high-turnover bone disease. In such a situation, the alkaline phosphatase levels rise. Since the alkaline phosphatase levels remained fairly uniform throughout the treatment period of both drugs, it may be reasonably assumed that the ability of both these drugs to retard the development of high-turnover bone disease is similar. Further studies are needed, before this assumption is confirmed. One criterion that needs to be satisfied in the case of a crossover study is that the patient should be having a chronic disease and the condition of the patient should remain stable throughout the course of the study. The steady creatinine values observed during the study confirm the stable condition of the 20 patients who completed the study. There are no studies comparing the efficacy of lanthanum and calcium acetate. The only trial that has been conducted comparing lanthanum with a calcium salt (calcium ) by Hutchison et al. concluded that serum phosphate control with lanthanum ( mg/day) is similar to that seen with calcium ( mg/day), but with a significantly reduced incidence of hypercalcemia. [14] It is also mentioned that lanthanum is well tolerated and may be more effective in reducing calcium phosphorous product than calcium. These conclusions are similar to the inferences drawn from the present study. After evaluating the adverse event profile of the drugs, it can be seen that calcium acetate is better tolerated than lanthanum. There were no adverse events when the patients were on calcium acetate, but on lanthanum, four patients had gastrointestinal adverse events and one patient was withdrawn due to generalized rashes and small eruptions in the oral mucosa. A placebo controlled, double blind study by Al-Baaj et al., had also observed that gastrointestinal adverse events were predominant with lanthanum. [15] In a developing country like India, the cost of treatment is a very significant factor influencing treatment. Affordability of the prescribed treatment significantly influences compliance. On calculating the cost of a comparable dosage regimen of lanthanum and calcium acetate, it was seen that the former is approximately 16 times costlier than the latter. The high cost associated with lanthanum treatment makes it beyond the reach of a good proportion of our population. Limitations of the study The study drugs were administered for a limited duration and at a fixed dose. Ideally, the dose should be titrated every 2 weeks for each patient and then the optimal dose has to be maintained for 4-6 weeks or higher. This format was not chosen, as this would put a higher financial burden on the patient due to frequent visits and result in higher dropouts. Dietary phosphorous intake may be different for different patients, as no uniform diet was prescribed due to the difficulty in confirming the patients adherence to the diet. Another limitation of this study was that it was not blinded. The main reason for this was that the parameters of concern were lab values. Also, since the 190
5 comparison was between two active compounds, the double dummy method had to be used and this was not practically feasible. Selection bias was not a major problem as each patient was receiving lanthanum and calcium acetate. Conclusion and calcium acetate are equally effective phosphate binders with trends obvious in the first 4 weeks of therapy. The changes associated with serum calcium levels with both the drugs were statistically significant. The drawback of lanthanum is its high cost and relatively higher incidence of adverse events during treatment. If both the serum calcium and phosphorous levels are elevated, lanthanum can be preferred over calcium acetate if the patient can afford it. If there is hyperphosphatemia with low-normal or low serum calcium levels, calcium acetate is preferred. The selection of phosphate binders for the treatment of hyperphosphatemia in CKD should be made keeping in mind the serum phosphorous, serum calcium, the calcium phosphorous product, and the socioeconomic status of the patient. Further treat to goal studies are needed to establish whether there is a significant difference in efficacy between the two phosphate binders. Acknowledgements The authors wish to thank the staff and post-graduates in the Department of Pharmacology and Therapeutics, and the Department of Nephrology, Medical College, Thiruvananthapuram for their wholehearted support rendered throughout the period of the study. We are grateful to Dr. Sankara Sarma, Associate Professor (Biostatistics), Achutha Menon Centre for Health Science Studies, Sree Chitra Tirunal Institue of Medical Science and Technology, Thiruvananthapuram for his guidance on data entry and statistical analysis. We also acknowledge Panacea Biotech India Limited for providing the samples of lanthanum used in the study and for their financial support. References 1. Malluche HH, Monier-Faugere MC. Understanding and managing hyperphosphatemia in patients with chronic renal disease. Clin Nephrol 1999;52: Block GA, Port FK. Re-evaluation of risks associated with hyperphosphatemia and hyperparathyroidism in dialysis patients:recommendations for a change in management. Am J Kidney Dis 2000;35: lock GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 2004;15: Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, et al. Coronaryartery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 2000;342: Eknoyan G, Levin A, Levin NW; National Kidney Foundation. Bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003;42:S Mallick NP, Gokal R. Haemodialysis. Lancet 1999;353: Alfrey AC, LeGendre GR, Kaehny WD. The dialysis encephalopathy syndrome. Possible aluminium intoxication. N Engl J Med 1976;294: Goodman WG. Bone disease and aluminum: Pathogenic considerations. Am J Kidney Dis 1985;6: Treat to Goal Working Group; Chertow GM, Burke SK, Raggi P. Sevelamer attenuates the progression of coronary and aortic calcifi cation in hemodialysis patients. Kidney Int 2002;62: Behets GJ, Verberckmoes SC, D Haese PC, De Broe ME. : A new phosphate binder. Curr Opin Nephrol Hypertens 2004;13: Evans CH. Biochemistry of the lanthanides. New York, NY: Plenum Press; p Harrison ST, Lesluy SJ.. Drugs 2004;6: Finn WF, Joy MS; LAM-308 Study Group. A long-term, open-label extension study on the safety of treatment with lanthanum, a new phosphate binder, in patients receiving hemodialysis. Curr Med Res Opin 2005;21: Hutchison AJ, Maes B, Vanwalleghem J, Asmus G, Mohamed E, Schmieder R, et al. Long-term effi cacy and tolerability of lanthanum : Results from a 3-year study. Nephron Clin Pract 2006;102:c Al-Baaj F, Speake M, Hutchison AJ. Control of serum phosphate by oral lanthanum in patients undergoing haemodialysis and continuous ambulatory peritoneal dialysis in a short-term, placebo-controlled study. Nephrol Dial Transplant 2005;20: Author Help: Reference checking facility The manuscript system ( allows the authors to check and verify the accuracy and style of references. The tool checks the references with PubMed as per a predefined style. Authors are encouraged to use this facility, before submitting articles to the journal. The style as well as bibliographic elements should be 100% accurate, to help get the references verified from the system. Even a single spelling error or addition of issue number/month of publication will lead to an error when verifying the reference. Example of a correct style Sheahan P, O leary G, Lee G, Fitzgibbon J. Cystic cervical metastases: Incidence and diagnosis using fine needle aspiration biopsy. Otolaryngol Head Neck Surg 2002;127: Only the references from journals indexed in PubMed will be checked. Enter each reference in new line, without a serial number. Add up to a maximum of 15 references at a time. If the reference is correct for its bibliographic elements and punctuations, it will be shown as CORRECT and a link to the correct article in PubMed will be given. If any of the bibliographic elements are missing, incorrect or extra (such as issue number), it will be shown as INCORRECT and link to possible articles in PubMed will be given. 191
Normal kidneys filter large amounts of organic
 ORIGINAL ARTICLE - NEPHROLOGY Effect Of Lanthanum Carbonate vs Calcium Acetate As A Phosphate Binder In Stage 3-4 CKD- Treat To Goal Study K.S. Sajeev Kumar (1), M K Mohandas (1), Ramdas Pisharody (1),
ORIGINAL ARTICLE - NEPHROLOGY Effect Of Lanthanum Carbonate vs Calcium Acetate As A Phosphate Binder In Stage 3-4 CKD- Treat To Goal Study K.S. Sajeev Kumar (1), M K Mohandas (1), Ramdas Pisharody (1),
Hyperphosphatemia is associated with a
 TREATMENT OPTIONS IN THE MANAGEMENT OF PHOSPHATE RETENTION * George A. Porter, MD, FACP, and Hartmut H. Malluche, MD, FACP ABSTRACT Hyperphosphatemia is an independent risk factor for mortality and cardiovascular
TREATMENT OPTIONS IN THE MANAGEMENT OF PHOSPHATE RETENTION * George A. Porter, MD, FACP, and Hartmut H. Malluche, MD, FACP ABSTRACT Hyperphosphatemia is an independent risk factor for mortality and cardiovascular
Secondary Hyperparathyroidism: Where are we now?
 Secondary Hyperparathyroidism: Where are we now? Dylan M. Barth, Pharm.D. PGY-1 Pharmacy Resident Mayo Clinic 2017 MFMER slide-1 Objectives Identify risk factors for the development of complications caused
Secondary Hyperparathyroidism: Where are we now? Dylan M. Barth, Pharm.D. PGY-1 Pharmacy Resident Mayo Clinic 2017 MFMER slide-1 Objectives Identify risk factors for the development of complications caused
Velphoro (sucroferric oxyhydroxide)
 STRENGTH DOSAGE FORM ROUTE GPID 500mg chewable tablet oral 36003 MANUFACTURER Fresenius Medical Care North America INDICATION(S) For the control of serum phosphorus levels in patients with chronic kidney
STRENGTH DOSAGE FORM ROUTE GPID 500mg chewable tablet oral 36003 MANUFACTURER Fresenius Medical Care North America INDICATION(S) For the control of serum phosphorus levels in patients with chronic kidney
TRANSPARENCY COMMITTEE OPINION. 22 July 2009
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 22 July 2009 PHOSPHOSORB 660 mg, film-coated tablet Container of 200 (CIP: 381 466-0) Applicant: FRESENIUS MEDICAL
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 22 July 2009 PHOSPHOSORB 660 mg, film-coated tablet Container of 200 (CIP: 381 466-0) Applicant: FRESENIUS MEDICAL
Improved Assessment of Aortic Calcification in Japanese Patients Undergoing Maintenance Hemodialysis
 ORIGINAL ARTICLE Improved Assessment of Aortic Calcification in Japanese Patients Undergoing Maintenance Hemodialysis Masaki Ohya 1, Haruhisa Otani 2,KeigoKimura 3, Yasushi Saika 4, Ryoichi Fujii 4, Susumu
ORIGINAL ARTICLE Improved Assessment of Aortic Calcification in Japanese Patients Undergoing Maintenance Hemodialysis Masaki Ohya 1, Haruhisa Otani 2,KeigoKimura 3, Yasushi Saika 4, Ryoichi Fujii 4, Susumu
The impact of improved phosphorus control: use of sevelamer hydrochloride in patients with chronic renal failure
 Nephrol Dial Transplant (2002) 17: 340 345 The impact of improved phosphorus control: use of sevelamer hydrochloride in patients with chronic renal failure Naseem Amin Genzyme Corporation, Cambridge, MA,
Nephrol Dial Transplant (2002) 17: 340 345 The impact of improved phosphorus control: use of sevelamer hydrochloride in patients with chronic renal failure Naseem Amin Genzyme Corporation, Cambridge, MA,
Lanthanum Carbonate Reduces Urine Phosphorus Excretion: Evidence of High-Capacity Phosphate Binding
 Renal Failure, 34(3): 263 270, (2012) Copyright Informa Healthcare USA, Inc. ISSN 0886-022X print/1525-6049 online DOI: 10.3109/0886022X.2011.649657 Lanthanum Carbonate Reduces Urine Phosphorus Excretion:
Renal Failure, 34(3): 263 270, (2012) Copyright Informa Healthcare USA, Inc. ISSN 0886-022X print/1525-6049 online DOI: 10.3109/0886022X.2011.649657 Lanthanum Carbonate Reduces Urine Phosphorus Excretion:
Month/Year of Review: September 2012 Date of Last Review: September 2010
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
HYDROCHLORIDE FOR THE TREATMENT OF SECONDARY HYPERPARATHYROIDISM IN PATIENTS WITH END-STAGE RENAL DISEASE ON MAINTENANCE DIALYSIS THERAPY
 UK RENAL PHARMACY GROUP SUBMISSION TO THE NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE on CINACALCET HYDROCHLORIDE FOR THE TREATMENT OF SECONDARY HYPERPARATHYROIDISM IN PATIENTS WITH END-STAGE RENAL DISEASE
UK RENAL PHARMACY GROUP SUBMISSION TO THE NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE on CINACALCET HYDROCHLORIDE FOR THE TREATMENT OF SECONDARY HYPERPARATHYROIDISM IN PATIENTS WITH END-STAGE RENAL DISEASE
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 28 March 2012
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 28 March 2012 OSVAREN 435 mg/235 mg, film-coated tablet Bottle of 180 (CIP: 382 886 3) Applicant: FRESENIUS MEDICAL
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 28 March 2012 OSVAREN 435 mg/235 mg, film-coated tablet Bottle of 180 (CIP: 382 886 3) Applicant: FRESENIUS MEDICAL
The Parsabiv Beginner s Book
 The Parsabiv Beginner s Book A quick guide to help you learn about your treatment with Parsabiv and what to expect Indication Parsabiv (etelcalcetide) is indicated for the treatment of secondary hyperparathyroidism
The Parsabiv Beginner s Book A quick guide to help you learn about your treatment with Parsabiv and what to expect Indication Parsabiv (etelcalcetide) is indicated for the treatment of secondary hyperparathyroidism
Draft Labeling Package Insert Not Actual Size. BRAINTREE LABORATORIES, INC. PhosLo Capsules (Calcium Acetate)
 Draft Labeling Package Insert Not Actual Size BRAINTREE LABORATORIES, INC. PhosLo Capsules (Calcium Acetate) DESCRIPTION: Full Size: Each opaque capsule with a white cap and white body is spin printed
Draft Labeling Package Insert Not Actual Size BRAINTREE LABORATORIES, INC. PhosLo Capsules (Calcium Acetate) DESCRIPTION: Full Size: Each opaque capsule with a white cap and white body is spin printed
The CARI Guidelines Caring for Australasians with Renal Impairment. Serum phosphate GUIDELINES
 Date written: August 2005 Final submission: October 2005 Author: Carmel Hawley Serum phosphate GUIDELINES No recommendations possible based on Level I or II evidence SUGGESTIONS FOR CLINICAL CARE (Suggestions
Date written: August 2005 Final submission: October 2005 Author: Carmel Hawley Serum phosphate GUIDELINES No recommendations possible based on Level I or II evidence SUGGESTIONS FOR CLINICAL CARE (Suggestions
A new era in phosphate binder therapy: What are the options?
 http://www.kidney-international.org & 2006 International Society of Nephrology A new era in phosphate binder therapy: What are the options? IB Salusky 1 1 Department of Pediatrics, David Geffen School
http://www.kidney-international.org & 2006 International Society of Nephrology A new era in phosphate binder therapy: What are the options? IB Salusky 1 1 Department of Pediatrics, David Geffen School
2017 KDIGO Guidelines Update
 2017 KDIGO Guidelines Update Clinic for Hemodialysis Clinical Center University of Sarajevo 13 th Congress of the Balkan cities Association of Nephrology, Dialysis, and Artificial Organs Transplantation
2017 KDIGO Guidelines Update Clinic for Hemodialysis Clinical Center University of Sarajevo 13 th Congress of the Balkan cities Association of Nephrology, Dialysis, and Artificial Organs Transplantation
Benefits and Harms of Phosphate Binders in CKD: A Systematic Review of Randomized Controlled Trials
 Benefits and Harms of Phosphate Binders in CKD: A Systematic Review of Randomized Controlled Trials Sankar D. Navaneethan, MD, MPH, Suetonia C. Palmer, MBChB, Jonathan C. Craig, MBChB, PhD, Grahame J.
Benefits and Harms of Phosphate Binders in CKD: A Systematic Review of Randomized Controlled Trials Sankar D. Navaneethan, MD, MPH, Suetonia C. Palmer, MBChB, Jonathan C. Craig, MBChB, PhD, Grahame J.
Phosphate binders and metabolic acidosis in patients undergoing maintenance hemodialysis sevelamer hydrochloride, calcium carbonate, and bixalomer
 Hemodialysis International 2015; 19:5459 Phosphate binders and metabolic acidosis in patients undergoing maintenance hemodialysis sevelamer hydrochloride, calcium carbonate, and bixalomer Toru SANAI, 1
Hemodialysis International 2015; 19:5459 Phosphate binders and metabolic acidosis in patients undergoing maintenance hemodialysis sevelamer hydrochloride, calcium carbonate, and bixalomer Toru SANAI, 1
Original epidemiologic studies 1 have suggested that approximately
 Factors for Increased Morbidity and Mortality in Uremia: Hyperphosphatemia Nathan W. Levin, Frank A. Gotch, and Martin K. Kuhlmann Hyperphosphatemia is a metabolic abnormality present in the majority of
Factors for Increased Morbidity and Mortality in Uremia: Hyperphosphatemia Nathan W. Levin, Frank A. Gotch, and Martin K. Kuhlmann Hyperphosphatemia is a metabolic abnormality present in the majority of
CKD: Bone Mineral Metabolism. Peter Birks, Nephrology Fellow
 CKD: Bone Mineral Metabolism Peter Birks, Nephrology Fellow CKD - KDIGO Definition and Classification of CKD CKD: abnormalities of kidney structure/function for > 3 months with health implications 1 marker
CKD: Bone Mineral Metabolism Peter Birks, Nephrology Fellow CKD - KDIGO Definition and Classification of CKD CKD: abnormalities of kidney structure/function for > 3 months with health implications 1 marker
Vascular calcification in stage 5 Chronic Kidney Disease patients on dialysis
 Vascular calcification in stage 5 Chronic Kidney Disease patients on dialysis Seoung Woo Lee Div. Of Nephrology and Hypertension, Dept. of Internal Medicine, Inha Unv. College of Medicine, Inchon, Korea
Vascular calcification in stage 5 Chronic Kidney Disease patients on dialysis Seoung Woo Lee Div. Of Nephrology and Hypertension, Dept. of Internal Medicine, Inha Unv. College of Medicine, Inchon, Korea
Sensipar (cinacalcet)
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
2.0 Synopsis. Paricalcitol Capsules M Clinical Study Report R&D/15/0380. (For National Authority Use Only)
 2.0 Synopsis AbbVie Inc. Name of Study Drug: ABT-358/Zemplar (paricalcitol) Capsules Name of Active Ingredient: paricalcitol Individual Study Table Referring to Part of Dossier: Volume: Page: (For National
2.0 Synopsis AbbVie Inc. Name of Study Drug: ABT-358/Zemplar (paricalcitol) Capsules Name of Active Ingredient: paricalcitol Individual Study Table Referring to Part of Dossier: Volume: Page: (For National
ADVERSE REACTIONS The most common (>10%) adverse reactions are hypercalcemia, nausea, and diarrhea. (6.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PHOSLYRA safely and effectively. See full prescribing information for PHOSLYRA. PHOSLYRA (calcium
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PHOSLYRA safely and effectively. See full prescribing information for PHOSLYRA. PHOSLYRA (calcium
The CARI Guidelines Caring for Australasians with Renal Impairment. Biochemical Targets. Calcium GUIDELINES
 Date written: August 2005 Final submission: October 2005 Author: Carmel Hawley Biochemical Targets CARMEL HAWLEY (Woolloongabba, Queensland) GRAHAME ELDER (Westmead, New South Wales) Calcium GUIDELINES
Date written: August 2005 Final submission: October 2005 Author: Carmel Hawley Biochemical Targets CARMEL HAWLEY (Woolloongabba, Queensland) GRAHAME ELDER (Westmead, New South Wales) Calcium GUIDELINES
Tenapanor, a gastrointestinal NHE3 inhibitor, reduces serum phosphate in patients with chronic kidney disease stage 5D and hyperphosphatemia
 , a gastrointestinal NHE3 inhibitor, reduces serum phosphate in patients with chronic kidney disease stage 5D and hyperphosphatemia Geoffrey A Block, 1 David P Rosenbaum, 2 Maria Leonsson-Zachrisson, 3
, a gastrointestinal NHE3 inhibitor, reduces serum phosphate in patients with chronic kidney disease stage 5D and hyperphosphatemia Geoffrey A Block, 1 David P Rosenbaum, 2 Maria Leonsson-Zachrisson, 3
Month/Year of Review: May 2014 Date of Last Review: September New Drug Evaluation: Sucrofferic Oxyhydroxide (Velphoro )
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Effectiveness and tolerability of sevelamer in the treatment of hyperphosphatemia in hemodialysis patients
 Open Access Journal of Journal of Parathyroid Disease 2017,5(1),17 24 http://www.jparathyroid.com Original Effectiveness and tolerability of sevelamer in the treatment of hyperphosphatemia in hemodialysis
Open Access Journal of Journal of Parathyroid Disease 2017,5(1),17 24 http://www.jparathyroid.com Original Effectiveness and tolerability of sevelamer in the treatment of hyperphosphatemia in hemodialysis
SCIENTIFIC DISCUSSION
 European Medicines Agency London, 01 June 2007 Product Name : Renagel Procedure No: EMEA/H/C/000254/II/56 SCIENTIFIC DISCUSSION 1/11 1. Introduction Renagel (sevelamer), a non-absorbed, calcium and metal-free
European Medicines Agency London, 01 June 2007 Product Name : Renagel Procedure No: EMEA/H/C/000254/II/56 SCIENTIFIC DISCUSSION 1/11 1. Introduction Renagel (sevelamer), a non-absorbed, calcium and metal-free
Treatment of hyperphosphatemia in hemodialysis patients: The Calcium Acetate Renagel Evaluation (CARE Study)
 Kidney International, Vol. 65 (2004), pp. 1914 1926 Treatment of hyperphosphatemia in hemodialysis patients: The Calcium Acetate Renagel Evaluation (CARE Study) WAJEH Y. QUNIBI,ROBERT E. HOOTKINS,LAVETA
Kidney International, Vol. 65 (2004), pp. 1914 1926 Treatment of hyperphosphatemia in hemodialysis patients: The Calcium Acetate Renagel Evaluation (CARE Study) WAJEH Y. QUNIBI,ROBERT E. HOOTKINS,LAVETA
Sevelamer hydrochloride: a calcium- and metal-free phosphate binder
 DRUG PROFILE Sevelamer hydrochloride: a calcium- and metal-free phosphate binder Anthony J Bleyer & James Balwit Author for correspondence Wake Forest University School of Medicine, Section on Nephrology,
DRUG PROFILE Sevelamer hydrochloride: a calcium- and metal-free phosphate binder Anthony J Bleyer & James Balwit Author for correspondence Wake Forest University School of Medicine, Section on Nephrology,
Do We Do Too Many Parathyroidectomies in Dialysis? Sagar Nigwekar MD, MMSc Massachusetts General Hospital
 Do We Do Too Many Parathyroidectomies in Dialysis? Sagar Nigwekar MD, MMSc Massachusetts General Hospital E-mail: snigwekar@mgh.harvard.edu March 13, 2017 Disclosures statement: Consultant: Allena, Becker
Do We Do Too Many Parathyroidectomies in Dialysis? Sagar Nigwekar MD, MMSc Massachusetts General Hospital E-mail: snigwekar@mgh.harvard.edu March 13, 2017 Disclosures statement: Consultant: Allena, Becker
PRODUCT MONOGRAPH FOSRENOL *
 PRODUCT MONOGRAPH FOSRENOL * lanthanum carbonate hydrate Chewable tablets 250mg, 500mg, 750mg, 1000mg lanthanum as lanthanum carbonate hydrate Phosphate binder Shire Pharma Canada ULC 22 Adelaide St. West,
PRODUCT MONOGRAPH FOSRENOL * lanthanum carbonate hydrate Chewable tablets 250mg, 500mg, 750mg, 1000mg lanthanum as lanthanum carbonate hydrate Phosphate binder Shire Pharma Canada ULC 22 Adelaide St. West,
Contents. Authors Name: Christopher Wong: Consultant Nephrologist Anne Waddington: Renal Pharmacist Eimear Fegan : Renal Dietitian
 Cheshire and Merseyside Renal Units Guidelines on the Management of Chronic Kidney Disease - Mineral Bone Disorder (adapted from Greater Manchester) Authors Name: Christopher Wong: Consultant Nephrologist
Cheshire and Merseyside Renal Units Guidelines on the Management of Chronic Kidney Disease - Mineral Bone Disorder (adapted from Greater Manchester) Authors Name: Christopher Wong: Consultant Nephrologist
THE IMPACT OF SERUM PHOSPHATE LEVELS IN CKD-MBD PROGRESSION
 THE IMPACT OF SERUM PHOSPHATE LEVELS IN CKD-MBD PROGRESSION Mario Cozzolino, MD, PhD, Fellow of the European Renal Association Department of Health Sciences University of Milan Renal Division & Laboratory
THE IMPACT OF SERUM PHOSPHATE LEVELS IN CKD-MBD PROGRESSION Mario Cozzolino, MD, PhD, Fellow of the European Renal Association Department of Health Sciences University of Milan Renal Division & Laboratory
Incorporating K/DOQI Using a Novel Algorithm Approach: Regina Qu Appelle s Experience
 Incorporating K/DOQI Using a Novel Algorithm Approach: Regina Qu Appelle s Experience Michael Chan, Renal Dietitian Regina Qu Appelle Health Region BC Nephrology Days There is a strong association among
Incorporating K/DOQI Using a Novel Algorithm Approach: Regina Qu Appelle s Experience Michael Chan, Renal Dietitian Regina Qu Appelle Health Region BC Nephrology Days There is a strong association among
New Medicines Profile. December 2013 Issue No. 13/04. Colestilan
 New Medicines Profile December 2013 Issue. 13/04 Concise evaluated information to support the managed entry of new medicines in the NHS Summary (BindRen ) is an oral, non-absorbed, non-calcium, nonmetallic
New Medicines Profile December 2013 Issue. 13/04 Concise evaluated information to support the managed entry of new medicines in the NHS Summary (BindRen ) is an oral, non-absorbed, non-calcium, nonmetallic
Protocol GTC : A Randomized, Open Label, Parallel Design Study of Sevelamer Hydrochloride (Renagel ) in Chronic Kidney Disease Patients.
 Protocol GTC-68-208: A Randomized, Open Label, Parallel Design Study of Sevelamer Hydrochloride (Renagel ) in Chronic Kidney Disease Patients. These results are supplied for informational purposes only.
Protocol GTC-68-208: A Randomized, Open Label, Parallel Design Study of Sevelamer Hydrochloride (Renagel ) in Chronic Kidney Disease Patients. These results are supplied for informational purposes only.
Advances in Peritoneal Dialysis, Vol. 29, 2013
 Advances in Peritoneal Dialysis, Vol. 29, 2013 Takeyuki Hiramatsu, 1 Takahiro Hayasaki, 1 Akinori Hobo, 1 Shinji Furuta, 1 Koki Kabu, 2 Yukio Tonozuka, 2 Yoshiyasu Iida 1 Icodextrin Eliminates Phosphate
Advances in Peritoneal Dialysis, Vol. 29, 2013 Takeyuki Hiramatsu, 1 Takahiro Hayasaki, 1 Akinori Hobo, 1 Shinji Furuta, 1 Koki Kabu, 2 Yukio Tonozuka, 2 Yoshiyasu Iida 1 Icodextrin Eliminates Phosphate
Renal Osteodystrophy. Chapter 6. I. Introduction. Classification of Bone Disease. Eric W. Young
 Chapter 6 Renal Osteodystrophy Eric W. Young I. Introduction Renal osteodystrophy refers to bone disease that occurs in patients with kidney disease. Bone disease occurs across the full spectrum of kidney
Chapter 6 Renal Osteodystrophy Eric W. Young I. Introduction Renal osteodystrophy refers to bone disease that occurs in patients with kidney disease. Bone disease occurs across the full spectrum of kidney
NOTTINGHAMSHIRE AREA PRESCRIBING COMMITTEE SHARED CARE PROTOCOL AGREEMENT
 NOTTINGHAMSHIRE AREA PRESCRIBING COMMITTEE SHARED CARE PROTOCOL AGREEMENT Phosphate Binders for the Treatment of Hyperphosphataemia in adults with Chronic Kidney Disease OBJECTIVES To outline referral
NOTTINGHAMSHIRE AREA PRESCRIBING COMMITTEE SHARED CARE PROTOCOL AGREEMENT Phosphate Binders for the Treatment of Hyperphosphataemia in adults with Chronic Kidney Disease OBJECTIVES To outline referral
Therapeutic golas in the treatment of CKD-MBD
 Therapeutic golas in the treatment of CKD-MBD Hemodialysis clinic Clinical University Center Sarajevo Bantao, 04-08.10.2017, Sarajevo Abbvie Satellite symposium 06.10.2017 Chronic Kidney Disease Mineral
Therapeutic golas in the treatment of CKD-MBD Hemodialysis clinic Clinical University Center Sarajevo Bantao, 04-08.10.2017, Sarajevo Abbvie Satellite symposium 06.10.2017 Chronic Kidney Disease Mineral
Level 1 Strong We recommendyshould A High Moderate Level 2 Weak We suggestymight C Low Very low. K Hyperphosphatemia has been associated with poor
 chapter 4.1 http://www.kidney-international.org & 2009 KDIGO Chapter 4.1: Treatment of CKD MBD targeted at lowering high serum phosphorus and maintaining serum calcium ; doi:10.1038/ki.2009.192 Grade for
chapter 4.1 http://www.kidney-international.org & 2009 KDIGO Chapter 4.1: Treatment of CKD MBD targeted at lowering high serum phosphorus and maintaining serum calcium ; doi:10.1038/ki.2009.192 Grade for
Tenapanor, a gastrointestinal NHE3 inhibitor, reduces serum phosphate in patients with chronic kidney disease stage 5D and hyperphosphatemia
 , a gastrointestinal NHE3 inhibitor, reduces serum phosphate in patients with chronic kidney disease stage 5D and hyperphosphatemia Geoffrey A Block, 1 David P Rosenbaum, 2 Maria Leonsson- Zachrisson,
, a gastrointestinal NHE3 inhibitor, reduces serum phosphate in patients with chronic kidney disease stage 5D and hyperphosphatemia Geoffrey A Block, 1 David P Rosenbaum, 2 Maria Leonsson- Zachrisson,
Calcium x phosphate product
 Date written: August 2005 Final submission: October 2005 Author: Carmel Hawley Calcium x phosphate product GUIDELINES No recommendations possible based on Level I or II evidence SUGGESTIONS FOR CLINICAL
Date written: August 2005 Final submission: October 2005 Author: Carmel Hawley Calcium x phosphate product GUIDELINES No recommendations possible based on Level I or II evidence SUGGESTIONS FOR CLINICAL
Tenapanor, a minimally absorbed NHE3 inhibitor, reduces dietary phosphorus absorption in healthy volunteers
 , a minimally absorbed NHE3 inhibitor, reduces dietary phosphorus absorption in healthy volunteers David Rosenbaum, 1 Susanne Johansson, 2 Björn Carlsson, 2 Andrew G Spencer, 1 Bergur Stefansson, 2 Mikael
, a minimally absorbed NHE3 inhibitor, reduces dietary phosphorus absorption in healthy volunteers David Rosenbaum, 1 Susanne Johansson, 2 Björn Carlsson, 2 Andrew G Spencer, 1 Bergur Stefansson, 2 Mikael
Clinical Guideline Bone chemistry management in adult renal patients on dialysis
 Clinical Guideline Bone chemistry management in adult renal patients on dialysis This guidance covers how to: Maintain serum phosphate 0.8 to 1.7mmol/L 1 Maintain serum corrected calcium 2.1 to 2.5mmol/L
Clinical Guideline Bone chemistry management in adult renal patients on dialysis This guidance covers how to: Maintain serum phosphate 0.8 to 1.7mmol/L 1 Maintain serum corrected calcium 2.1 to 2.5mmol/L
Randomized Clinical Trial of the Iron-Based Phosphate Binder PA21 in Hemodialysis Patients
 Article Randomized Clinical Trial of the Iron-Based Phosphate Binder PA21 in Hemodialysis Patients Rudolf P. Wüthrich,* Michel Chonchol, Adrian Covic, Sylvain Gaillard, Edward Chong, and James A. Tumlin
Article Randomized Clinical Trial of the Iron-Based Phosphate Binder PA21 in Hemodialysis Patients Rudolf P. Wüthrich,* Michel Chonchol, Adrian Covic, Sylvain Gaillard, Edward Chong, and James A. Tumlin
CKD FOR INTERNISTS. Dr Ahmed Hossain Associate professor Medicine Sir Salimullah Medical College
 CKD FOR INTERNISTS Dr Ahmed Hossain Associate professor Medicine Sir Salimullah Medical College INTRODUCTION In 2002, the National Kidney Foundation s Kidney Disease Outcomes Quality Initiative(KDOQI)
CKD FOR INTERNISTS Dr Ahmed Hossain Associate professor Medicine Sir Salimullah Medical College INTRODUCTION In 2002, the National Kidney Foundation s Kidney Disease Outcomes Quality Initiative(KDOQI)
Persistent post transplant hyperparathyroidism. Shiva Seyrafian IUMS-97/10/18-8/1/2019
 Persistent post transplant hyperparathyroidism Shiva Seyrafian IUMS-97/10/18-8/1/2019 normal weight =18-160 mg In HPT= 500-1000 mg 2 Epidemiology Mild 2 nd hyperparathyroidism (HPT) resolve after renal
Persistent post transplant hyperparathyroidism Shiva Seyrafian IUMS-97/10/18-8/1/2019 normal weight =18-160 mg In HPT= 500-1000 mg 2 Epidemiology Mild 2 nd hyperparathyroidism (HPT) resolve after renal
Parsabiv the control of calcimimetic delivery you ve always wanted, the sustained lowering of shpt lab values your patients deserve 1
 Parsabiv the control of calcimimetic delivery you ve always wanted, the sustained lowering of shpt lab values your patients deserve 1 Not an actual Parsabiv vial. The displayed vial is for illustrative
Parsabiv the control of calcimimetic delivery you ve always wanted, the sustained lowering of shpt lab values your patients deserve 1 Not an actual Parsabiv vial. The displayed vial is for illustrative
Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Dietetic Management Protocol
 Nutrition and Dietetic Service Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Dietetic Management Protocol Authors Hilary Mathieson, Renal Dietitian Paul McKeveney, Consultant Nephrologist
Nutrition and Dietetic Service Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Dietetic Management Protocol Authors Hilary Mathieson, Renal Dietitian Paul McKeveney, Consultant Nephrologist
Renal Association Clinical Practice Guideline in Mineral and Bone Disorders in CKD
 Nephron Clin Pract 2011;118(suppl 1):c145 c152 DOI: 10.1159/000328066 Received: May 24, 2010 Accepted: December 6, 2010 Published online: May 6, 2011 Renal Association Clinical Practice Guideline in Mineral
Nephron Clin Pract 2011;118(suppl 1):c145 c152 DOI: 10.1159/000328066 Received: May 24, 2010 Accepted: December 6, 2010 Published online: May 6, 2011 Renal Association Clinical Practice Guideline in Mineral
Amol K Choulwar et al. / Journal of Pharmacy Research 2012,5(1), Available online through
 Research Article ISSN: 0974-6943 Amol K Choulwar et al. / Journal of Pharmacy Research 2012,5(1), Available online through www.jpronline.info Comparison of efficacy and safety of Cinacalcet versus Calcitriol
Research Article ISSN: 0974-6943 Amol K Choulwar et al. / Journal of Pharmacy Research 2012,5(1), Available online through www.jpronline.info Comparison of efficacy and safety of Cinacalcet versus Calcitriol
2.0 Synopsis. ABT-358 M Clinical Study Report R&D/06/099. (For National Authority Use Only) to Item of the Submission: Volume:
 2.0 Synopsis Abbott Laboratories Name of Study Drug: Zemplar Injection Name of Active Ingredient: Paricalcitol Individual Study Table Referring to Item of the Submission: Volume: Page: (For National Authority
2.0 Synopsis Abbott Laboratories Name of Study Drug: Zemplar Injection Name of Active Ingredient: Paricalcitol Individual Study Table Referring to Item of the Submission: Volume: Page: (For National Authority
... . : ... PTH.
 IRMA Email msarookhani@qumsacir * CRD GFR D [1,25OH 2 D 3 ] SHPT GFR» «KDOQI SHPT Ca * P P P Selectra Overt HPT = = Ca * P P > GM II DSL i IRMA ± ± ± Ca * P % % % % % % % % % % % % % % % % pgml = = > ±
IRMA Email msarookhani@qumsacir * CRD GFR D [1,25OH 2 D 3 ] SHPT GFR» «KDOQI SHPT Ca * P P P Selectra Overt HPT = = Ca * P P > GM II DSL i IRMA ± ± ± Ca * P % % % % % % % % % % % % % % % % pgml = = > ±
Cost of applying the K/DOQI guidelines for bone metabolism and disease to a cohort of chronic hemodialysis patients
 original article http://www.kidney-international.org & 2007 International Society of Nephrology Cost of applying the K/DOQI guidelines for bone metabolism and disease to a cohort of chronic hemodialysis
original article http://www.kidney-international.org & 2007 International Society of Nephrology Cost of applying the K/DOQI guidelines for bone metabolism and disease to a cohort of chronic hemodialysis
International Journal of Health Sciences and Research ISSN:
 International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Original Research Article Prevalence and Pattern of Mineral Bone Disorder in Chronic Kidney Disease Patients Using Serum
International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Original Research Article Prevalence and Pattern of Mineral Bone Disorder in Chronic Kidney Disease Patients Using Serum
Literature Scan: Phosphate Binders
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Taking Care of Your Kidneys
 Taking Care of Your Kidneys Part A Roseville & Sacramento Medical Centers Health Promotion Department Nutritional Services Agenda Slide How your kidneys work Explaining chronic kidney disease Protecting
Taking Care of Your Kidneys Part A Roseville & Sacramento Medical Centers Health Promotion Department Nutritional Services Agenda Slide How your kidneys work Explaining chronic kidney disease Protecting
Reversal of adynamic bone disease by lowering of dialysate calcium
 http://www.kidney-international.org & 26 International Society of Nephrology original article Reversal of adynamic bone disease by lowering of dialysate calcium A Haris 1, DJ Sherrard 2 and G Hercz 3 1
http://www.kidney-international.org & 26 International Society of Nephrology original article Reversal of adynamic bone disease by lowering of dialysate calcium A Haris 1, DJ Sherrard 2 and G Hercz 3 1
Metabolic Bone Disease Related to Chronic Kidney Disease
 Metabolic Bone Disease Related to Chronic Kidney Disease Deborah Sellmeyer, MD Director, Johns Hopkins Metabolic Bone Center Dept of Medicine, Division of Endocrinology Disclosure DSMB member for denosumab
Metabolic Bone Disease Related to Chronic Kidney Disease Deborah Sellmeyer, MD Director, Johns Hopkins Metabolic Bone Center Dept of Medicine, Division of Endocrinology Disclosure DSMB member for denosumab
Comparison of Serum Parathyroid Hormone (PTH) Levels in Hemodialysis and Peritoneal Dialysis Patients. Int.J.Curr.Res.Aca.Rev.2016; 4(11):
 Comparison of Serum Parathyroid Hormone (PTH) Levels in Hemodialysis and Peritoneal Dialysis Patients Seyed Seifollah Beladi Mousavi 1, Arman Shahriari 2 and Fatemeh Roumi 3 * 1 Department of Nephrology,
Comparison of Serum Parathyroid Hormone (PTH) Levels in Hemodialysis and Peritoneal Dialysis Patients Seyed Seifollah Beladi Mousavi 1, Arman Shahriari 2 and Fatemeh Roumi 3 * 1 Department of Nephrology,
New biological targets for CKD- MBD: From the KDOQI to the
 New biological targets for CKD- MBD: From the KDOQI to the KDIGO Guillaume JEAN, MD. Centre de Rein Artificiel, 42 avenue du 8 mai 1945, Tassin la Demi-Lune, France. E-mail : guillaume-jean-crat@wanadoo.fr
New biological targets for CKD- MBD: From the KDOQI to the KDIGO Guillaume JEAN, MD. Centre de Rein Artificiel, 42 avenue du 8 mai 1945, Tassin la Demi-Lune, France. E-mail : guillaume-jean-crat@wanadoo.fr
Revised: 11/2017 FULL PRESCRIBING INFORMATION: CONTENTS* 10 OVERDOSAGE 1 INDICATIONS AND USAGE 11 DESCRIPTION
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use AURYXIA safely and effectively. See full prescribing information for AURYXIA. AURYXIA (ferric citrate)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use AURYXIA safely and effectively. See full prescribing information for AURYXIA. AURYXIA (ferric citrate)
Guidelines and new evidence on CKD - MBD treatment
 Guidelines and new evidence on CKD - MBD treatment Goce Spasovski ERBP Advisory Board member University of Skopje, R. Macedonia ERA-EDTA CME course IV Congress of Nephrology of B&H, April 25, 2015, Sarajevo,
Guidelines and new evidence on CKD - MBD treatment Goce Spasovski ERBP Advisory Board member University of Skopje, R. Macedonia ERA-EDTA CME course IV Congress of Nephrology of B&H, April 25, 2015, Sarajevo,
chapter 1 & 2009 KDIGO
 http://www.kidney-international.org chapter 1 & 2009 DIGO Chapter 1: Introduction and definition of CD MBD and the development of the guideline statements idney International (2009) 76 (Suppl 113), S3
http://www.kidney-international.org chapter 1 & 2009 DIGO Chapter 1: Introduction and definition of CD MBD and the development of the guideline statements idney International (2009) 76 (Suppl 113), S3
Effects of Diabetes Mellitus, Age, and Duration of Dialysis on Parathormone in Chronic Hemodialysis Patients. Hamid Nasri 1, Soleiman Kheiri 2
 Saudi J Kidney Dis Transplant 2008;19(4):608-613 2008 Saudi Center for Organ Transplantation Saudi Journal of Kidney Diseases and Transplantation Original Article Effects of Diabetes Mellitus, Age, and
Saudi J Kidney Dis Transplant 2008;19(4):608-613 2008 Saudi Center for Organ Transplantation Saudi Journal of Kidney Diseases and Transplantation Original Article Effects of Diabetes Mellitus, Age, and
Cinacalcet treatment in advanced CKD - is it justified?
 Cinacalcet treatment in advanced CKD - is it justified? Goce Spasovski ERBP Advisory Board member University of Skopje, R. Macedonia TSN Congress October 21, 2017, Antalya Session Objectives From ROD to
Cinacalcet treatment in advanced CKD - is it justified? Goce Spasovski ERBP Advisory Board member University of Skopje, R. Macedonia TSN Congress October 21, 2017, Antalya Session Objectives From ROD to
2.0 Synopsis. ABT-358/Paricalcitol M Clinical Study Report R&D/09/1255. (For National Authority Use Only) to Part of Dossier: Volume:
 2.0 Synopsis Title of Study: Late Phase II Study of Paricalcitol Injection Dose-response study of paricalcitol injection in chronic kidney disease subjects receiving hemodialysis with secondary hyperparathyroidism
2.0 Synopsis Title of Study: Late Phase II Study of Paricalcitol Injection Dose-response study of paricalcitol injection in chronic kidney disease subjects receiving hemodialysis with secondary hyperparathyroidism
BONE AND MINERAL METABOLISM in the PD PATIENT
 BONE AND MINERAL METABOLISM in the PD PATIENT John Burkart, MD Professor of Medicine/Nephrology Wake Forest University Baptist Medical Center Chief Medical Officer Health Systems Management Maria V. DeVita,
BONE AND MINERAL METABOLISM in the PD PATIENT John Burkart, MD Professor of Medicine/Nephrology Wake Forest University Baptist Medical Center Chief Medical Officer Health Systems Management Maria V. DeVita,
Patient Description and Diagnosis: Sarah Jones is a 50-year-old female, 5 4, 131
 Julia Kaesberg Counseling Session KNH 413 February 27 th, 2014 Patient Description and Diagnosis: Sarah Jones is a 50-year-old female, 5 4, 131 pounds and her usual body weight is 125 pounds. Her %UBW
Julia Kaesberg Counseling Session KNH 413 February 27 th, 2014 Patient Description and Diagnosis: Sarah Jones is a 50-year-old female, 5 4, 131 pounds and her usual body weight is 125 pounds. Her %UBW
Treatment Options for Chronic Kidney
 Treatment Options for Chronic Kidney Disease: Metabolic Introduction Bone Disease to Renagel Goce Spasovski, R. Macedonia The 17th Budapest Nephrology School, August 29, 2010 Session Objectives Definition
Treatment Options for Chronic Kidney Disease: Metabolic Introduction Bone Disease to Renagel Goce Spasovski, R. Macedonia The 17th Budapest Nephrology School, August 29, 2010 Session Objectives Definition
PREVALENCE AND PATTERNS OF HYPERPARATHYROIDISM AND MINERAL BONE DISEASE IN PATIENTS WITH CHRONIC KIDNEY DISEASE AT KENYATTA NATIONAL HOSPITAL
 PREVALENCE AND PATTERNS OF HYPERPARATHYROIDISM AND MINERAL BONE DISEASE IN PATIENTS WITH CHRONIC KIDNEY DISEASE AT KENYATTA NATIONAL HOSPITAL DR. ANNE MUGERA The Problem Chronic Kidney disease is a worldwide
PREVALENCE AND PATTERNS OF HYPERPARATHYROIDISM AND MINERAL BONE DISEASE IN PATIENTS WITH CHRONIC KIDNEY DISEASE AT KENYATTA NATIONAL HOSPITAL DR. ANNE MUGERA The Problem Chronic Kidney disease is a worldwide
Use of magnesium as a drug in chronic kidney disease
 Clin Kidney J (2012) 5[Suppl 1]: i62 i70 doi: 10.1093/ndtplus/sfr168 Use of magnesium as a drug in chronic kidney disease Alastair J. Hutchison 1 and Martin Wilkie 2 1 University of Manchester and Manchester
Clin Kidney J (2012) 5[Suppl 1]: i62 i70 doi: 10.1093/ndtplus/sfr168 Use of magnesium as a drug in chronic kidney disease Alastair J. Hutchison 1 and Martin Wilkie 2 1 University of Manchester and Manchester
Drugs for the treatment of secondary hyperparathyroidism and hyperphosphataemia
 NSW Therapeutic Advisory Group Level 5, 376 Victoria Street PO Box 766 Darlinghurst NSW 2010 Phone: 61 2 8382 2852 Fax: 61 2 8382 3529 Email: nswtag@stvincents.com.au www.nswtag.org.au Drugs for the treatment
NSW Therapeutic Advisory Group Level 5, 376 Victoria Street PO Box 766 Darlinghurst NSW 2010 Phone: 61 2 8382 2852 Fax: 61 2 8382 3529 Email: nswtag@stvincents.com.au www.nswtag.org.au Drugs for the treatment
Prescribing Guidelines Prescribing arrangement for the management of patients transferring from Secondary Care to Primary Care
 Berkshire West Integrated Care System Representing Berkshire West Clinical Commisioning Group Royal Berkshire NHS Foundation Trust Berkshire Healthcare NHS Foundation Trust Berkshire West Primary Care
Berkshire West Integrated Care System Representing Berkshire West Clinical Commisioning Group Royal Berkshire NHS Foundation Trust Berkshire Healthcare NHS Foundation Trust Berkshire West Primary Care
Should cinacalcet be used in patients who are not on dialysis?
 Should cinacalcet be used in patients who are not on dialysis? Jorge B Cannata-Andía and José Luis Fernández-Martín Affiliations: Bone and Mineral Research Unit. Hospital Universitario Central de Asturias.
Should cinacalcet be used in patients who are not on dialysis? Jorge B Cannata-Andía and José Luis Fernández-Martín Affiliations: Bone and Mineral Research Unit. Hospital Universitario Central de Asturias.
Ramzi Vareldzis, MD Avanelle Jack, MD Dept of Internal Medicine Section of Nephrology and Hypertension LSU Health New Orleans September 13, 2016
 Ramzi Vareldzis, MD Avanelle Jack, MD Dept of Internal Medicine Section of Nephrology and Hypertension LSU Health New Orleans September 13, 2016 1 MBD + CKD in Elderly patients Our focus for today: CKD
Ramzi Vareldzis, MD Avanelle Jack, MD Dept of Internal Medicine Section of Nephrology and Hypertension LSU Health New Orleans September 13, 2016 1 MBD + CKD in Elderly patients Our focus for today: CKD
Chronic Kidney Disease Mineral Bone Disease
 NHS Logo here Chronic Kidney Disease Mineral Bone Disease (CKD-MBD) Patient Information Health & care information you can trust The Information Standard Certified Member Working together for better patient
NHS Logo here Chronic Kidney Disease Mineral Bone Disease (CKD-MBD) Patient Information Health & care information you can trust The Information Standard Certified Member Working together for better patient
RENAL FAILURE IN CHILDREN Dr. Mai Mohamed Elhassan Assistant Professor Jazan University
 RENAL FAILURE IN CHILDREN Dr. Mai Mohamed Elhassan Assistant Professor Jazan University OBJECTIVES By the end of this lecture each student should be able to: Define acute & chronic kidney disease(ckd)
RENAL FAILURE IN CHILDREN Dr. Mai Mohamed Elhassan Assistant Professor Jazan University OBJECTIVES By the end of this lecture each student should be able to: Define acute & chronic kidney disease(ckd)
Renal Osteodystrophy in Pediatric Patients on Peritoneal Dialysis
 Advances in Peritoneal Dialysis, Vol. 20, 2004 Francisco J. Cano, Marcela Valenzuela, Pedro Zambrano, Marta A. Azocar, Eduardo Wolff, Maria A. Delucchi, Eugenio E. Rodriguez Renal Osteodystrophy in Pediatric
Advances in Peritoneal Dialysis, Vol. 20, 2004 Francisco J. Cano, Marcela Valenzuela, Pedro Zambrano, Marta A. Azocar, Eduardo Wolff, Maria A. Delucchi, Eugenio E. Rodriguez Renal Osteodystrophy in Pediatric
Introduction and rationale. Yoshitaka Isaka 1 Hideki Fujii 2 Yoshihiro Tsujimoto 3 Satoshi Teramukai 4 Takayuki Hamano 5
 https://doi.org/10.1007/s10157-018-1547-5 ORIGINAL ARTICLE Rationale, design, and characteristics of a trial to evaluate the new phosphate iron-based binder sucroferric oxyhydroxide in dialysis patients
https://doi.org/10.1007/s10157-018-1547-5 ORIGINAL ARTICLE Rationale, design, and characteristics of a trial to evaluate the new phosphate iron-based binder sucroferric oxyhydroxide in dialysis patients
Metabolic Bone Disease (Past, Present and Future Challenges in the Management)
 Metabolic Bone Disease 871 151 Metabolic Bone Disease (Past, Present and Future Challenges in the Management) SNA RIZVI INTRODUCTION The past 40 years have seen some important historical events leading
Metabolic Bone Disease 871 151 Metabolic Bone Disease (Past, Present and Future Challenges in the Management) SNA RIZVI INTRODUCTION The past 40 years have seen some important historical events leading
The effect of sevelamer carbonate and lanthanum carbonate on the pharmacokinetics of oral calcitriol
 Nephrol Dial Transplant (2011) 26: 1615 1621 doi: 10.1093/ndt/gfq598 Advance Access publication 4 October 2010 The effect of sevelamer carbonate and lanthanum carbonate on the pharmacokinetics of oral
Nephrol Dial Transplant (2011) 26: 1615 1621 doi: 10.1093/ndt/gfq598 Advance Access publication 4 October 2010 The effect of sevelamer carbonate and lanthanum carbonate on the pharmacokinetics of oral
Management of CKD. Goce Spasovski, R. Macedonia
 Management of CKD complications Introduction Bone disease to Renagel Goce Spasovski, R. Macedonia Istanbul, June 4, 2011 Session Objectives - Mineral and Bone Disorders (MBD) Bone disease a part of CKD
Management of CKD complications Introduction Bone disease to Renagel Goce Spasovski, R. Macedonia Istanbul, June 4, 2011 Session Objectives - Mineral and Bone Disorders (MBD) Bone disease a part of CKD
( ) , (Donabedian, 1980) We would not choose any treatment with poor outcomes
 ..., 2013 Amgen. 1 ? ( ), (Donabedian, 1980) We would not choose any treatment with poor outcomes 1. :, 2. ( ): 3. :.,,, 4. :, [Biomarkers Definitions Working Group, 2001]., (William M. Bennet, Nefrol
..., 2013 Amgen. 1 ? ( ), (Donabedian, 1980) We would not choose any treatment with poor outcomes 1. :, 2. ( ): 3. :.,,, 4. :, [Biomarkers Definitions Working Group, 2001]., (William M. Bennet, Nefrol
Cowen Healthcare Conference March 12, 2018
 Cowen Healthcare Conference March 12, 2018 Forward-Looking Statements Some of the statements included in this presentation, particularly those regarding the commercialization and ongoing clinical development
Cowen Healthcare Conference March 12, 2018 Forward-Looking Statements Some of the statements included in this presentation, particularly those regarding the commercialization and ongoing clinical development
Nuove terapie in ambito Nefrologico: Etelcalcetide (AMG-416)
 Nuove terapie in ambito Nefrologico: Etelcalcetide (AMG-416) Antonio Bellasi, MD, PhD U.O.C. Nefrologia & Dialisi ASST-Lariana, Ospedale S. Anna, Como, Italy Improvement of mineral and bone metabolism
Nuove terapie in ambito Nefrologico: Etelcalcetide (AMG-416) Antonio Bellasi, MD, PhD U.O.C. Nefrologia & Dialisi ASST-Lariana, Ospedale S. Anna, Como, Italy Improvement of mineral and bone metabolism
Cardiovascular Mortality: General Population vs ESRD Dialysis Patients
 Cardiovascular Mortality: General Population vs ESRD Dialysis Patients Annual CVD Mortality (%) 100 10 1 0.1 0.01 0.001 25-34 35-44 45-54 55-64 66-74 75-84 >85 Age (years) GP Male GP Female GP Black GP
Cardiovascular Mortality: General Population vs ESRD Dialysis Patients Annual CVD Mortality (%) 100 10 1 0.1 0.01 0.001 25-34 35-44 45-54 55-64 66-74 75-84 >85 Age (years) GP Male GP Female GP Black GP
HEALTHYSTART TRAINING MANUAL. Living well with Kidney Disease
 HEALTHYSTART TRAINING MANUAL Living well with Kidney Disease KIDNEY DISEASE CAN AFFECT ANYONE! 1 HEALTHYSTART PROGRAMME HEALTHYSTART is a lifestyle management programme to assist you to remain healthy
HEALTHYSTART TRAINING MANUAL Living well with Kidney Disease KIDNEY DISEASE CAN AFFECT ANYONE! 1 HEALTHYSTART PROGRAMME HEALTHYSTART is a lifestyle management programme to assist you to remain healthy
Kobe University Repository : Kernel
 Title Author(s) Citation Issue date 2009-09 Resource Type Resource Version DOI URL Kobe University Repository : Kernel Marked increase in bone formation markers after cinacalcet treatment by mechanisms
Title Author(s) Citation Issue date 2009-09 Resource Type Resource Version DOI URL Kobe University Repository : Kernel Marked increase in bone formation markers after cinacalcet treatment by mechanisms
Ketosteril. Total nitrogen content per tablet
 1. NAME OF THE MEDICINAL PRODUCT Ketosteril film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One film-coated tablet contains: (RS)-3-methyl-2-oxovaleric acid (α-ketoanalogue to DL-isoleucine),
1. NAME OF THE MEDICINAL PRODUCT Ketosteril film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One film-coated tablet contains: (RS)-3-methyl-2-oxovaleric acid (α-ketoanalogue to DL-isoleucine),
Proceedings of the 34th World Small Animal Veterinary Congress WSAVA 2009
 www.ivis.org Proceedings of the 34th World Small Animal Veterinary Congress WSAVA 2009 São Paulo, Brazil - 2009 Next WSAVA Congress : Reprinted in IVIS with the permission of the Congress Organizers STAGED
www.ivis.org Proceedings of the 34th World Small Animal Veterinary Congress WSAVA 2009 São Paulo, Brazil - 2009 Next WSAVA Congress : Reprinted in IVIS with the permission of the Congress Organizers STAGED
The role of calcimimetics in chronic kidney disease
 http://www.kidney-international.org & 2006 International Society of Nephrology The role of calcimimetics in chronic kidney disease A Gal-Moscovici 1,2 and SM Sprague 1 1 Division of Nephrology and Hypertension,
http://www.kidney-international.org & 2006 International Society of Nephrology The role of calcimimetics in chronic kidney disease A Gal-Moscovici 1,2 and SM Sprague 1 1 Division of Nephrology and Hypertension,
TITLE: Sevelamer Hydrochloride for the Treatment of Patients with Chronic Kidney Disease: A Review of the Clinical Effectiveness
 TITLE: Hydrochloride for the Treatment of Patients with Chronic Kidney Disease: A Review of the Clinical Effectiveness DATE: 08 September 2009 CONTEXT AND POLICY ISSUES: Patients with end-stage chronic
TITLE: Hydrochloride for the Treatment of Patients with Chronic Kidney Disease: A Review of the Clinical Effectiveness DATE: 08 September 2009 CONTEXT AND POLICY ISSUES: Patients with end-stage chronic
Case 4 Generalised bone pain
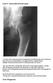 Case 4 Generalised bone pain C A 34- year- old woman presented complaining of multifocal pain in her chest and legs. The pain was intermittent, was aggravated by weight bearing. Initially was alleviated
Case 4 Generalised bone pain C A 34- year- old woman presented complaining of multifocal pain in her chest and legs. The pain was intermittent, was aggravated by weight bearing. Initially was alleviated
White Rose Research Online URL for this paper: Version: Accepted Version
 This is a repository copy of Effect on mortality of elective parathyroid surgery in one hundred and three patients with chronic kidney disease : our experience. White Rose Research Online URL for this
This is a repository copy of Effect on mortality of elective parathyroid surgery in one hundred and three patients with chronic kidney disease : our experience. White Rose Research Online URL for this
Chronic Kidney Disease
 Chronic Kidney Disease Chronic Kidney Disease (CKD) Guideline (2010) Chronic Kidney Disease CKD: Executive Summary of Recommendations (2010) Executive Summary of Recommendations Below are the major recommendations
Chronic Kidney Disease Chronic Kidney Disease (CKD) Guideline (2010) Chronic Kidney Disease CKD: Executive Summary of Recommendations (2010) Executive Summary of Recommendations Below are the major recommendations
