SVEIKATOS TECHNOLOGIJOS VERTINIMAS: DEŠINIAJAME SKILVELYJE IMPLANTUOJAMAS BELAIDIS ŠIRDIES STIMULIATORIUS
|
|
|
- Norah Bridges
- 5 years ago
- Views:
Transcription
1 SVEIKATOS TECHNOLOGIJOS VERTINIMAS: DEŠINIAJAME SKILVELYJE IMPLANTUOJAMAS BELAIDIS ŠIRDIES STIMULIATORIUS HEALTH TECHNOLOGY ASSESSMENT: LEADLESS PACEMAKERS FOR RIGHT VENTRICLE PACING 2016 VILNIUS 1
2 Valstybinė akreditavimo sveikatos priežiūros veiklai tarnyba prie Sveikatos apsaugos ministerijos Autoriai: Medicinos technologijų skyriaus vyr.specialistės: Kristina Grigaitė Vitalija Mazgelė Valstybinė akreditavimo sveikatos priežiūros veiklai tarnyba prie Sveikatos apsaugos ministerijos Jeruzalės g. 21, LT Vilnius Tel. (8 5) , Faks. (8 5) , El. paštas: Sveikatos technologijos vertinimo santrauką galima rasti interneto svetainėje: Interesų konfliktas: Visi autoriai ir recenzentai, įtraukti į sveikatos priežiūros technologijos vertinimą, deklaravo neturintys interesų konflikto. Sveikatos priežiūros technologijos vertinimo turiniui ir/ ar struktūrai buvo naudotas EUnetHTA ( sveikatos priežiūros technologijų vertinimo šerdinis modelis (HTA Core Model ). State Health Care Accreditation Agency under the Ministry of Health Authors: Chief specialists of Medical Technology division: Kristina Grigaitė Vitalija Mazgelė State Health Care Accreditation Agency under the Ministry of Health Jeruzalės st. 21, LT Vilnius Tel. (370 5) , Fax. (370 5) , E. mail: Health technology assessment is available on the website: Conflict of interest: All authors and the reviewers involved in the production of this report have declared they have no conflicts of interest in relation to the technology assessed. The HTA Core Model, developed within EUnetHTA ( has been utilised when producing the contents and/ or structure of this work. 2
3 TABLE OF CONTENTS TABLE OF CONTENTS... 3 ABBREVIATIONS... 6 SANTRAUKA... 7 Sveikatos technologijos vertinimo metodika... 7 Tikslinė būklė... 9 Tikslinė populiacija Šiuolaikinis būklės valdymas Kompensavimas Pagrindinės technologijos charakteristikos Investicijos ir prietaisai, reikalingi technologijos naudojimui Pacientų saugumas Mirštamumas Organizmo funkcijos Gyvenimo kokybė SVEIKATOS TECNOLOGIJOS FUNKCINĖ VERTĖ IŠVADOS REKOMENDACIJOS SUMMARY Scope Target condition Target population Current clinical management of the disease or health condition Regulatory & reimbursement status Features of the technology Investments and tools required to use the technology Patient safety Mortality Function Quality of life HEALTH PROBLEM AND CURRENT USE OF THE TECHNOLOGY Research questions Overview of the disease or health condition A0001. For which health conditions, and for what purposes are leadless pacemakers used? A0002. What is the disease or health condition in the scope of this assessment? A0003. What are the known risk factors for cardiac arrhythmias? A0004. What is the natural course of cardiac arrhythmias? Effects of the disease or health condition on the individual and society A0005. What is the burden of disease for patients with cardiac arrhythmias? A0006. What are the consequences of cardiac arrhythmias for the society? Current clinical management of the disease or health condition A0024. How are cardiac arrhythmias currently diagnosed according to published guidelines and in practice? A0025. How are cardiac arrhythmias currently managed according to published guidelines and in practice? Target population A0007. What is the target population in this assessment? A0023. How many people belong to the target population?
4 A0011. How much are leadless pacemakers utilised? Regulatory & reimbursement status A0020. What is the marketing authorisation status of leadless pacemakers? A0021. What is the reimbursement status of leadless pacemakers? Discussion DESCRIPTION AND TECHNICAL CHARACTERISTICS OF THE LEADLESS PACEMAKERS.. 28 Research questions Features of the technology and comparators B0001. What are leadless pacemakers and conventional single-chamber ventricular pacemakers? 28 B0002. What is the claimed benefit of leadless pacemakers in relation to conventional singlechamber ventricular pacemakers? B0003. What is the phase of development and implementation of leadless pacemakers and conventional single-chamber ventricular pacemakers? Administration, Investments, personnel and tools required to use the technology and the comparator(s) B0004. Who administers leadless pacemakers and conventional single-chamber ventricular pacemakers and in what context and level of care are they provided? B0008. What kind of special premises are needed to use leadless pacemakers and conventional single-chamber ventricular pacemakers? B0009. What supplies are needed to use leadless pacemakers and conventional single-chamber ventricular pacemakers? Discussion SAFETY Research questions Patient safety C0008. How safe are leadless pacemakers in comparison to conventional single-chamber ventricular pacemakers? C0005. What are the susceptible patient groups that are more likely to be harmed through the use of the technology? C0007. Are leadless pacemakers and conventional single-chamber ventricular pacemakers associated with user-dependent harms? Discussion CLINICAL EFFECTIVENESS Research questions Mortality D0001. What is the expected beneficial effect of leadless pacemakers on mortality? D0003. What is the effect of leadless pacemakers on the mortality due to causes other than cardiac arrhythmia? Morbidity D0005. How do leadless pacemakers affect symptoms and findings (severity, frequency) of cardiac arrhythmias? D0006. How do leadless pacemakers affect progression (or recurrence) of cardiac arrhythmias?.. 36 Function D0011. What is the effect of leadless pacemakers on patients body functions? D0016. How does the use of leadless pacemakers affect activities of daily living? Health-related quality of life D0012. What is the effect of leadless pacemakers on generic health-related quality of life? D0013. What is the effect of leadless pacemakers on disease-specific quality of life? Patient satisfaction D0017. Was the use of leadless pacemakers worthwhile?
5 Discussion CONCLUSIONS RECOMMENDATIONS REFERENCES APPENDIX 1: METHODOLOGY AND DESCRIPTION OF THE EVIDENCES USED Reporting of results Study characteristics Quality assessment Limitations ADAPTATION TOOLKIT Documentation of the basic search strategies Flow charts of study selection Questions used from HTA Core Model Application for Rapid Relative Effectiveness assessment (version 4.2) Health problem and current use of the technology Description and technical characteristics of technology Safety Clinical effectiveness APPENDIX 2: DESCRIPTION OF THE EVIDENCE USED Evidence tables of individual studies included Included studies Excluded studies APPENDIX 3: QUALITY ASSESSMENT OF SELECTED STUDIES Quality assessment of the selected case series The IHE checklist for case series Quality assessment of the selected systematic reviews The AMSTAR checklist for systematic reviews Checklist for potential ethical, organisational, social and legal aspects
6 ABBREVIATIONS % percent; ACC American College of Cardiology; AF atrial fibrillation; AHA American Heart Association; ATP anti-tachycardia pacing; AV atrioventricular; BBB bundle branch block; CE Conformité Européene (European Conformity); CRT cardiac resynchronization therapy; DRG diagnosis-related group; ECG electrocardiogram; ESC European Society of Cardiology; etc. et cetera; EU European Union; EUR euro; FDA Food and Drug Administration; Fr French units; HRS Heart Rhythm Society; i.e. in essence; ICD implantable cardioverter-defibrillator; ICD-10-AM International Classification of Diseases, 10th Revision, Australian Modification; LCP leadless cardiac pacemaker; MCRD monolithic controlled release device; MRI magnetic resonance imaging; NBG North American Society of Pacing and Electrophysiology (NASPE) and the British Pacing and Electrophysiology Group (BPEG); PFO patent foramen ovale; SB sinus bradycardia; SND sinus node disease (also sick sinus syndrome); TGA Therapeutic Goods Administration; TPS transcatheter pacing system; USA United States of America; VVI single-chamber ventricular pacing; VVIR single-chamber ventricular pacing with response modulation; WiCS -LV system Wireless Cardiac Stimulation System. 6
7 SANTRAUKA Sveikatos technologijos vertinimo metodika Šis sveikatos priežiūros technologijos vertinimas yra Austrijos Liudviko Boltzmano instituto sveikatos technologijų vertinimui (angl. Ludwig Boltzmann Institute-Health Technology Assessment, Austria) atlikto vertinimo Dešiniajame skilvelyje implantuojamas belaidis širdies stimuliatorius (angl. Leadless pacemakers for right ventricle pacing ) atnaujinimas ir adaptavimas Lietuvos kontekstui pagal EUnetHTA metodikas. Europos Komisija inicijuoja ir remia EUnetHTA bei kitų šalių atliktų sveikatos priežiūros technologijų vertinimų naudojimą ir adaptavimą nacionaliniams Europos šalių poreikiams. Pirminis šaltinis, kurio pagalba buvo atrinkti pagrindiniai vertinimo elementai Sveikatos technologijų vertinimo šerdinis modelis greitam santykinio veiksmingumo vertinimui, versija 4.2 (angl. HTA Core Model for Rapid Relative Effectiveness Assessments (version 4.2 )). Be to, kiti EUnetHTA šerdinio modelio dokumentai (Sveikatos technologijų vertinimo šerdinis modelis medicininių ir chirurginių intervencijų vertinimui, versija 3.0 (angl. HTA Core Model for Medical and Surgical Interventions (version 3.0 ))) buvo peržiūrėti ir, esant poreikiui, papildomi vertinimo elementai įtraukti ųjų metų rugpjūčio mėn. vykdyta sisteminė literatūros paieška buvo tikslinama naudojant duomenų filtrą publikacijos išspausdintos laikotarpyje nuo 2015-ųjų metų gruodžio mėn. 10 d. iki 2016-ųjų metų rugpjūčio mėn. 25 d., imtinai. Dalis informacijos buvo atnaujinta ir panaudota remiantis Austrijos Liudviko Boltzmano instituto sveikatos technologijų vertinimui (LBI-HTA, Austrija) atliktu sveikatos technologijų vertinimu, įvardinamu kaip sprendimų paramos dokumentas Nr. 97 Leadless pacemakers for right ventricle pacing. Belaidžio širdies stimuliatoriaus (BŠS) vertinimo analizė atlikta remiantis mokslinės literatūros šaltiniais, esančiais: The Cochrane Library duomenų bazėje; PubMed (Medline) duomenų bazėje; CRD duomenų bazėje; Gamintojų internetiniuose puslapiuose, kurių ieškota rankiniu būdu viešai prieinamoje erdvėje (internete). Straipsniai, skirti Saugumo ir Klinikinio efektyvumo skyrių adaptavimui, buvo atrinkti VASPVT (Valstybinė akreditavimo sveikatos priežiūros veiklai tarnyba prie Sveikatos apsaugos ministerijos, Lietuva) Medicinos technologijų skyriaus specialistų. Papildomi moksliniai straipsniai buvo įtraukti arba atmesti vadovaujantis PICO lentele, kuri pateikta santraukoje. Nė vienas randomizuotas kontroliuojamas tyrimas bei sisteminė literatūros apžvalga nebuvo rastas/ įtrauktas į vertinimą. Mokslinių straipsnių įtraukimo ir atmetimo procesą vykdė du tyrėjai. Jei ta pati informacija dubliavosi keliuose straipsniuose, į vertinimą įtraukti tik tie, kuriuose rezultatai pateikti išsamiausiai arba straipsniai buvo naujausi. Visais atvejais, tiek atmetant, tiek įtraukiant tyrimus į vertinimą buvo siekiama bendro sutarimo. Vertinime naudojamų nekontroliuojamų tyrimų (angl. case series) kokybė buvo įvertinta specialiu, nekontroliuojamiems tyrimams skirtu, Sveikatos Ekonomikos instituto kontrolės klausimynu (angl. The IHE checklist), kurio rezultatus galima rasti Austrijos LBI-HTA instituto sveikatos technologijų vertinime, įvardintame kaip sprendimų paramos dokumentas Nr. 97 Leadless pacemakers for right ventricle pacing ; klausimynus individualiai pildė du specialistai. Išsiskyrus nuomonėms, trečias specialistas buvo įtrauktas į tyrimų kokybės vertinimo procesą. Palyginamoji analizė buvo negalima, kadangi į vertinimą įtraukti tik nekontroliuojami tyrimai. LBI-HTA agentūros specialistai Saugumo ir Klinikinio efektyvumo skyriuose pateiktų duomenų kokybę įvertino remiantis tarptautinėmis Rekomendacijų lygių Vertinimo ir Nustatymo Grupės 7
8 rekomendacijomis (angl. The Grading of Recommendations Assessment, Development and Evaluation, GRADE). Trijų nekontroliuojamų į vertinimą įtrauktų tyrimų (iš viso 5 straipsniai) pagrindinių charakteristikų lentelės yra Austrijos LBI-HTA instituto sveikatos technologijų vertinime, kuris įvardinamas kaip sprendimų paramos dokumentas Nr. 97 Leadless pacemakers for right ventricle pacing. Atsakant į Sveikatos problema ir dabartinis technologijos naudojimas bei Techninės charakteristikos skyrių klausimus, į vertinimą įtrauktiems tyrimams jokie apribojimai netaikyti, informacijos ieškota rankiniu būdu viešai prieinamoje erdvėje (internete). Į vertinime analizuojamus klausimus atsakyta tekstiniu formatu. Kadangi nėra palyginamųjų grupių ir duomenys yra heterogeniški, atlikta analizė yra ne kiekybinė, bet kokybinė. Populiacija PICO lentelė Pirmojo pasirinkimo gydymo metodas pacientams, turintiems vienos kameros (vienkamerinio) širdies stimuliatoriaus indikacijas [2,4]: Pacientai, kuriems diagnozuotas lėtinis prieširdžių virpėjimas (TLK-10-AM: I48) ir reikalingas širdies stimuliatorius dėl bradikardijos, kuri išsivystė dėl atrioventrikulinės (AV) blokados (TLK-10-AM: I44); Pacientai, kuriems diagnozuota bradikardija dėl AV blokados arba sinusinio mazgo silpnumo sindromo (TLK-10-AM: I49.5) 1. Kontraindikacijos: Pacientai, kuriems reikalinga ilgalaikė stimuliacija, viršijanti numatytą prietaiso veikimo laiką (pvz., vaikai); Pacientai, kuriems reikalingas vienos kameros (prieširdžio) stimuliatorius arba dviejų kamerų stimuliatorius arba pacientai, kuriems reikalinga širdies resinchronizavimo terapija. Intervencija MESH term: Arrhythmias, Cardiac [C ] and Arrhythmias, Cardiac [C ]. Belaidis autonominis ir visiškai implantuojamas vienkamerinis (dešiniojo skilvelio) širdies stimuliatorius. Kontekstas: kraujagyslių chirurgija, intervencinė kardiologija; specializuota ligoninė. Prietaisai: Micra, gamintojas Medtronic Inc.; Nanostim, gamintojas St. Jude Medical. Alternatyvos Rezultatai Efektyvumas MESH term: Pacemaker, Artificial [E ] Įprastinis (tradicinis) implantuojamas vienkamerinis dešiniojo širdies skilvelio stimuliatorius. MESH term: Pacemaker, Artificial [E ] Mirtingumas dėl širdies kraujagyslių sistemos ligų; Sergamumas širdies kraujagyslių sistemos ligomis; 1 Tik tais atvejais, kai kiti stimuliavimo režimai (dvikamerinis stimuliavimas, prieširdžių stimuliavimas) nėra rekomenduojami. 8
9 Pacientų gyvenimo kokybė; Fizinis pajėgumas; Stimuliavimo veikla. Saugumas Nepageidaujamų įvykių dažnis. Tyrimų tipas Efektyvumas Randomizuoti kontroliuojami tyrimai 2 ; Prospektyviniai nerandomizuoti kontroliuojami tyrimai. Saugumas Randomizuoti kontroliuojami tyrimai; Prospektyviniai nerandomizuoti kontroliuojami tyrimai; Prospektyviniai nekontroliuojami tyrimai ar atvejų registrai, kuriuose daugiau nei 100 pacientų. PICO klausimas: Ar dešiniajame skilvelyje implantuojami belaidžiai širdies stimuliatoriai, lyginant juos su įprastiniais (tradiciniais) širdies stimuliatoriais, yra tokie pat efektyvūs atsižvelgiant į mirtingumą ir sergamumą širdies kraujagyslių sistemos ligomis, fizinį pajėgumą bei ar yra dar efektyvesni ir saugesni atsižvelgiant į gyvenimo kokybės aspektą ir nepageidaujamų įvykių dažnį? Tikslinė būklė Belaidžiai širdies stimuliatoriai (BŠS) yra alternatyva tradiciniams širdies stimuliatoriams (ŠS) įvairių širdies aritmijų gydymui. Natūralus širdies stimuliatorius organizme yra dešiniajame prieširdyje esantis sinusinis mazgas. Širdies bradiaritmija (bradikardija, susijusi su aritmija) išsivysto dėl sinusinio mazgo silpnumo (sinusinio mazgo silpnumo sindromas, SND) arba dėl atrioventrikulinės blokados (širdies laidumo sutrikimai). Taip pat bradikardija gali būti susijusi su prieširdžių virpėjimu. (A0001) Širdies stimuliacijos tikslas užtikrinti tinkamą širdies ritmą ir širdies atsaką atkuriant efektyvią cirkuliaciją bei hemodinamiką, sutrikusią dėl lėto širdies plakimo (bradikardija ar bradiaritmija: <60 širdies susitraukimų per minutę). Manoma, kad nuolatinė širdies stimuliacija gali sumažinti simptomus, susijusius su bradikardija (pvz.: galvos svaigimą, nuovargį, alpimą, prastą ištvermę), arba apsaugoti nuo galimo ritmo sutrikimo blogėjimo. (A0001) Šio vertinimo tikslinė būklė širdies aritmijos suaugusiems, kuriems indikuotinas vienkamerinis širdies skilvelio stimuliavimas. Toks stimuliacijos režimas gali būti taikomas pacientams, sergantiems lėtiniu prieširdžių virpėjimu dėl kurio reikalingas širdies stimuliatorius skilvelio atsako koregavimui. (A0002) Bradiaritmija, kuriai valdyti reikalingas ŠS, gali būti sukelta daugelio veiksnių. Esminės priežastys: idiopatinė (senėjimo) degeneracija; išeminė širdies liga; infiltracinės ligos (pvz.: sarkoidozė, amiloidozė, hemochromatozė); kolageno ir kraujagyslių ligos (pvz.: sisteminė raudonoji vilkligė, reumatoidinis artritas, sklerodermija); įgimtos ligos, įskaitant SND bei atriventrikulinę blokadą; infekcinės ligos (pvz.: Laimo liga); chirurginės traumos, tokios kaip vožtuvas keitimas, širdies transplantacija. (A0003) Priklausomai nuo bradiaritmijos tipo skiriasi širdies stimuliavimo režimai. Pacientai, kuriems atrioventrikulinė blokada negydyta, gali mirti dėl širdies nepakankamumo arba dėl skilvelinės tachiaritmijos juos gali ištikti staigi mirtis. Pacientų, sergančių SND, bendras išgyvenamumas bei staigios mirties rizika yra panaši kaip bendros populiacijos. Vis dėlto, vieningai sutariama, kad pacientams, sergantiems SND, širdies stimuliavimas gali turėti įtakos simptomų palengvinimui. (A0004) 2 Randomizuoti kontroliuojami tyrimai, kuriuose lyginami belaidžiai ir įprastiniai širdies stimuliatoriai, yra pageidautini, jei jie yra tinkami (pakankamas pacientų skaičius, intervencija nėra skubi), etiški (klinikinė atsvara, pacientai gali duoti sutikimą) ir būtini dėl galimo poveikio. Tyrimo vykdytojai bei pacientai negali būti maskuoti, o lyginimas su placebu yra neetiškas dėl efektyvaus gydymo prieinamumo. 9
10 Tikslinė populiacija BŠS yra skirtas naudoti kaip įprastų vienkamerinių dešiniojo širdies skilvelio stimuliatorių pakaitalas. Tikslinė populiacija yra tie pacientai, kuriems yra indikuojamas vienkamerinis dešiniojo širdies skilvelio stimuliacijos režimas (VVI). (A0007; A0023) Kai bradikardija kelia grėsmę normaliai kraujotakai, pasireiškia tokie simptomai: nuovargis, galvos svaigimas, alpimas, dusulys, krūtinės skausmas, silpnumas, sumažėjęs fizinis pajėgumas. Per pirmuosius 6 mėn. po širdies elektroninių prietaisų (visų tipų) implantacijos mažiau nei 6% pacientų patiria sunkias komplikacijas, dažniausia iš jų pakartotinė intervencija dėl laidų sukeltų komplikacijų. (A0005) Pacientų, kuriems reikalinga vienkamerinio širdies stimuliatoriaus implantacija, skaičius lieka neaiškus. Lietuvoje 2015 m. užregistruota daugiau nei 110,000 pacientų, kuriems diagnozuota širdies aritmija m. Lietuvoje buvo atlikta daugiau nei 1,500 širdies stimuliatorių implantacijų operacijų; šis skaičius didėja kiekvienais metais. (A0006; A0011) Šiuolaikinis būklės valdymas Nėra apibrėžta, kokia turėtų būti širdies ritmas riba, žemiau kurios gydymas būtų sindikuotinas. Sprendžiant širdies stimuliacijos reikalingumą, reikia nustatyti sąsają tarp simptomų ir bradikardijos. Nuolatinė bradiaritmija nustatoma pagal standartinę elektrokardiogramą (EKG), o protarpinė pagal standartinę EKG arba pagal prailgintą EKG. Jei bradikardija yra įtariama, tačiau nediagnozuota, gali prireikti provokuojančių testų arba elektrofiziologinio tyrimo. (A0024) Sprendimas dėl širdies stimuliatoriaus implantacijos ir ritmo režimo grindžiamas pagal tris klinikinius veiksnius: širdies laidumo sutrikimai, simptomų buvimas ir jų sąsaja su bradikardija, priežasties negrįžtamumas. VVI stimuliavimo režimas gali būti taikomas pacientams, sergantiems prieširdžių virpėjimu, dėl kurio reikalingas širdies stimuliatorius skilvelio atsako (atrioventrikulinei blokadai) koregavimui. Pacientams, su nustatyta atrioventrikuline blokada (be prieširdžių virpėjimo) arba SND, gali būti taikomas vienkamerinis arba dvikamerinis širdies stimuliatorius. Simptominė bradikardija yra indikacija širdies stimuliatoriaus implantacijos operacijai, jei simptomai yra susiję su bradikardija ir bradikardijos atsiradimo priežastis yra negrįžtama. (A0025) Kompensavimas Nanostim : Po LEADLESS I tyrimo, baigto 2013 m. spalio mėn., BŠS gamintojas St. Jude Medical gavo patvirtinimą, kad Nanostim BŠS gavo CE ženklą ir gali būti naudojamas Europos Sąjungoje. Tyrimas, skirtas patikrinti Nanostim BŠS Jungtinėse Amerikos Valstijose, buvo pradėtas 2014 m. Medicinos priemonės (prietaiso) vis dar nepatvirtino nei JAV Maisto ir vaistų administracija (FDA), nei Kanados federalinis sveikatos apsaugos departamentas Health Canada, nei Australijos Terapinių prekių administracija (TGA). Planuojama, kad FDA patvirtinimą Nanostim BŠS gaus (A0020; A0021) Micra Transcatheter Pacing System: 2015 m. BŠS gamintojas Medtronic medicinos priemonei (prietaisui) Micra Transcatheter Pacing System (TPS) gavo CE ženklą. Tyrimas, skirtas įvertinti Micra BŠS klinikinius rezultatus JAV, buvo pradėtas 2013 m. lapkričio mėn., o JAV Maisto ir vaistų administracija medicinos priemonę (prietaisą) patvirtino 2016 m. balandžio mėn. (A0020; A0021) Nėra tikslių duomenų apie šių įrenginių kainą, tačiau akivaizdu, kad vienkameriniai belaidžiai širdies stimuliatoriai kainuoja brangiau nei įprastiniai (Australijoje jų kainos yra 11,300 EUR, palyginus su įprastiniais, kurių kainos 4,200 EUR). Italijos sveikatos technologijų vertinimo agentūros AGENAS ataskaitoje skelbiama, kad Nanostim prietaiso (gamintojas St. Jude Medical) ir jo implantavimo kaina 11,500 EUR. Gamintojas Medtronic Inc. nepateikia prietaiso Micra TPS kainos. (A0020; A0021) 10
11 (Belaidžiai) širdies stimuliatoriai Lietuvoje yra kompensuojami atsižvelgiant į DRG kodus F12A bei F12B (Širdies stimuliatoriaus (visos sistemos) implantavimas ar pakeitimas), kurių kainos atitinkamai yra 2, EUR ir 1, EUR. (A0020; A0021) Pagrindinės technologijos charakteristikos Belaidžiai širdies stimuliatoriai yra autonominiai (savarankiški) intrakardialiniai širdies prietaisai, atliekantys tas pačias funkcijas kaip įprasti ŠS, tačiau gerokai mažesnio dydžio, todėl su įvedimo kateteriu gali būti visiškai implantuoti dešiniajame širdies skilvelyje. (B0001) Pirmasis visiškai į endokardą implantuojamas stimuliatoriaus prototipas buvo pristatytas 1970 m. Nuo to laiko buvo kuriamos kelios skirtingos belaidės sistemos su skirtingais energijos šaltiniais bei skirtingais prisitvirtinimo mechanizmais; galiausiai CE ženklą gavo dvi belaidės vienkamerinės dešiniojo skilvelio stimuliavimo sistemos: Nanostim belaidis širdies stimuliatorius ir Micra transkateterinio stimuliavimo sistema. Šių dviejų prietaisų techninės charakteristikos panašios: tai vienos kameros stimuliatoriai, įmontuoti hermetiškai sandarioje kapsulėje, abiejų nustatymai programuojami. Dydis maždaug dešimt kartų mažesnis nei įprasto vienos kameros ŠS ~1cm³, sveria ~2 gramus. Numatomas prietaisų baterijos ilgaamžiškumas ~10 m. (kaip ir įprastinio ŠS). Implantacija į dešinįjį skilvelį atliekama per šlaunies veną, naudojant 18 Fr (Nanostim ) ir 23 Fr (Micra ) dydžio kateterius; prietaiso proksimalinį galą galima prijungti prie įvedimo ir išėmimo kateterių, todėl skilvelyje galima pakeisti prietaiso padėtį arba jį išimti (eksplantuoti). Teoriškai, abi sistemos suteikia galimybę prietaisą eksplantuoti ar pakeisti jo padėtį po implantacijos; apie eksplantaciją yra ir praktiniųmokslinių duomenų iš tyrimų su gyvūnais bei žmonėmis. Vis dėlto, šiuo metu turimų duomenų trūksta ir manoma, jog, esant atitinkamoms aplinkybėms, yra racionaliau į širdies skilvelį šalia išjungto prietaiso implantuoti naują stimuliatorių. (B0001; B0003) Pagrindinis dviejų BŠS sistemų skirtumas skirtingi prisitvirtinimo endokarde mechanizmai: Nanostim BŠS turi įsukamą vienos vijos spiralę, be to, papildomai prietaisas tvirtinamas trimis siūlėmis; Micra BŠS prisitvirtina savaime išsiskleidžiančiais (angl. self-expanding) kabliukais pagamintais iš nikelio ir titano lydinio (nitinolio). (B0001) Įprastinis ŠS, kurį sudaro maitinimo elementas ir elektroninė schema, atsakinga už energijos iš maitinimo elemento transformavimą į elektros impulsus, stimuliuojančius širdį (schema kontroliuoja siunčiamų impulsų dažnį bei elektros impulsų tiekiamų į širdį intensyvumą), yra implantuojama į po raktikauliu suformuotą odos kišenę (angl. pectoral subcutaneous pocket). Stimuliavimo laidas užbaigia elektrinį kelią tarp ŠS ir širdies, per jį elektriniai impulsai siunčiami į širdį. Atsižvelgiant į būklės sudėtingumą, ŠS gali stimuliuoti tiek vieną (vienkameriniai), tiek dvi kameras (dvikameriniai); dvikameriniams ŠS reikalingi du laidai. (B0001) Lyginant su įprastiniu ŠS, tam, jog užtikrintų savo funkcijas, BŠS nereikalinga suformuota odos kišenė kairėje krūtinės pusėje ir nereikalingi į kraujagysles įvesti stimuliavimo laidai, per kuriuos impulsai pasiekia širdies prieširdžius ar skilvelius. Minėti faktai leidžia išvengti su šiomis sudedamosiomis prietaiso dalimis susijusių komplikacijų. Operacinio pjūvio vietoje (kišenėje) būdingos vietinės komplikacijos odos erozija, hematoma, paraudimas, patinimas, žaizdos infekcija. 6-iems pacientams iš 10-ies, šios komplikacijos apriboja peties regiono, kuriame įsodintas ŠS, judesius. Su stimuliavimo laidais susiję nepageidaujami įvykiai: venų obstrukcija (nepraeinamumas), įtrūkimai stimuliavimo laidų paviršiuje (izoliacijos įtrūkimai), stimuliavimo laidų atsijungimas nuo ŠS, elektrinių signalų trukdžiai, stimuliavimo laidų lūžiai (nutrūkimas), infekcija. Pavojingiausi nepageidaujami įvykiai yra tie, kuriems pašalinti reikalinga chirurginė intervencija, ypač pavojingos infekcijos, dėl kurių reikia atlikti ŠS eksplantaciją. (B0001; B0002) Dar vienas BŠS implantacijos privalumas susijęs su trumpesne procedūros ir gijimo laikotarpio trukme bei geresne gyvenimo kokybe, kadangi po procedūros nelieka rando, nėra iškilimo, peties judesiai išlieka neapriboti. (B0002) 11
12 Apskaičiuota, jog apytiksliai 75% pacientų, kuriems implantuotas ŠS, per laiką atsiranda indikacijų, kai reikia atlikti magnetinio rezonanso tomografijos tyrimus. Vis dėlto, yra žinoma, jog tokie tyrimai pacientams, su implantuotu įprastu ŠS, gali būti žalingi paciento sveikatai ir sutrikdyti prietaiso veiklą. Micra BŠS suteikia galimybę pacientams saugiai atlikti magnetinę tomografiją su 1.5 ir 3 Tesla galingumo magnetinio rezonanso tomografais, neseniai (2016 m. kovo mėn.) ir Nanostim BŠS gamintojai paskelbė apie tokią galimybę (tik su 1.5 Tesla galingumo prietaisais). (B0002) Investicijos ir prietaisai, reikalingi technologijos naudojimui Tiek BŠS, tiek įprastinio vienkamerinio ŠS implantavimo procedūros gali būti atliekamos tik specializuotuose intervencinės kardiologijos skyriuose. BŠS implantacijos metu reikalingi fluoroskopijos procedūrai naudojami prietaisai, kad stimuliatorius būtų tinkamai implantuotas į dešinįjį skilvelį. Kaip ir prieš atliekant bet kokią naują procedūrą, prieš BŠS implantaciją reikalingas specialus pasiruošimas bei papildomi mokymai. Specialistai kardiologai gali atlikti abi minėtas procedūras, tačiau specialus pasiruošimas yra privalomas. (B0004; B0008; B0009) Pacientų saugumas Sunkių, su prietaiso naudojimu susijusių, nepageidaujamų įvykių dažnis, trijuose į vertinimą įtrauktuose nekontroliuojamuose tyrimuose, varijavo nuo 4 iki 6.5%. Vis dėlto, svarbu atkreipti dėmesį, jog rezultatai gauti remiantis 3-mis nerandomizuotais moksliniais tyrimais, todėl duomenys apie belaidžių širdies stimuliatorių saugumo aspektą yra preliminarūs. (C0008) LEADLESS I tyrime širdies sužalojimas (širdies tamponada (angl. cardiac tamponade)) nustatytas vienam pacientui, LEADLESS II tyrime tokio pobūdžio sužalojimai (širdies perforacijos su/be tamponados ar perikardo efuzijos 3 (angl. cardiac perforations with/without tamponade or pericardial effusions)) nustatyti 8 pacientams, o Micra Transcatheter Pacemaker Study tyrime širdies sužalojimai (širdies perforacijos ar efuzijos (angl. cardiac perforations or effusions)) diagnozuoti 11 pacientų. 6 pacientams, kuriems buvo implantuotas Nanostim BŠS, nustatytas prietaiso pasislinkimas iš implantavimo vietos; tokie nepageidaujami įvykiai nenustatyti pacientams, kuriems buvo implantuoti Micra BŠS. Nustatyti ir kiti, su prietaisu ar su implantacijos procedūra susiję, paciento gyvybei pavojų keliantys nepageidaujami įvykiai: kraujagyslių komplikacijos, širdies ritmo sutrikimai (aritmija) procedūros metu, padidėjusios ribinės (slenkstinės) stimuliavimo vertės ir dėl to reikalinga prietaiso eksplantacija bei naujo prietaiso implantacija. (C0008) Šiuo metu turimų duomenų nepakanka, jog būtų nustatytos pacientų grupės, kurioms yra didžiausia rizika patirti nepageidaujamus įvykius. Taip pat nėra visiškai aišku ar ilgalaikėje perspektyvoje, praėjus keleriems metams po implantacijos, bus įmanoma prietaisą saugiai eksplantuoti. Tokiu atveju, alternatyvi stimuliatoriaus pakeitimo strategija galėtų būti šalia neveikiančio prietaiso, implantuoti naująjį BŠS. BŠS yra nedidelis (užima ~1cm³), todėl įvertinus širdies skilvelio anatominius parametrus, tai reali galimybė. Remiantis moksliniais skaičiavimais, dešiniajame skilvelyje įmanoma implantuoti bent 3 Micra BŠS, tačiau tiksliai šiuos klausimus galėtų atsakyti tik tolimesni moksliniai tyrimai. (C0005; C diskusija) Tiek BŠS, tiek įprastinio vienkamerinio ŠS implantavimo procedūros yra susijusios su nepageidaujamais įvykiais, kurie įvyksta dėl specialisto nepatyrimo, kompetencijos stokos. Viename moksliniame tyrime buvo analizuota, kaip komplikacijų dažnis susijęs su specialisto patirtimi; rezultatai rodo, jog atliekant 10 pirmųjų implantacijos procedūrų, su procedūra susijusių nepageidaujamų įvykių dažnis siekia 6.8%, o atliekant vėlesnes procedūras sumažėja 3.6% (p=0.56). (C0007) 3 Perikardo efuzija kraujo išsiliejimas į perikardą. 12
13 Mirštamumas Nėra tikimasi, kad BŠS galėtų turėti didesnį teigiamą poveikį mirtingumui negu įprastiniai VVI širdies stimuliatoriai. Bendras mirtingumo dažnis svyravo nuo 3 iki 5%, o mirtingumas, susijęs su širdies-kraujagyslių ligomis, nuo 0.8 iki 1%. (D0001) Su procedūra susijęs mirtingumas buvo aprašytas visuose trijuose tyrimuose. LEADLESS I tyrime vienam pacientui implantacijos metu pasireiškė perforacija, pacientas mirė dėl masinio smegenų arterijų išeminio infarkto. LEADLESS II tyrime įvardintos dvi su procedūra susijusios mirtys: vienam pacientui BŠS implantacija komplikavosi kirkšnies hematoma, o kitas pacientas dėl nesėkmingos BŠS implantacijos patyrė komplikuotą perikardo eksudaciją. Micra Transcatheter Pacemaker Study tyrime vienas pacientas mirė dėl su procedūra susijusių komplikacijų: implantacijos procedūra užtruko ilgiau nei įprasta dėl atrioventikulinio mazgo abliacijos bei inkstų ligos, vis dėlto mirties priežastis buvo metabolinė acidozė. (D0003) Organizmo funkcijos BŠS sistema buvo sukurta siekiant išvengti ŠS generatoriaus kišenės ir transveninių laidų (BŠS sistemoje elektrodai išdėstyti kapsulėje), tokiu būdu panaikinant svarbiausias komplikacijų priežastis, susijusias su įprastomis ŠS sistemomis, ir gaunant panašią naudą. (D diskusija) Pažymėtina, kad per didelė skilvelių stimuliacija gali būti susijusi su blogesniais širdies kraujagyslių rodmenų rezultatais. Kai kurie autoriai pastebėjo, kad BŠS gali padidinti aritmijos riziką dėl didesnio BŠS kontakto endokarde, lyginant su įprastinėmis širdies stimuliatorių sistemomis; vis dėlto, prospektyviniuose tyrimuose papildomų aritmijos atvejų nebuvo nustatyta. (D diskusija). Gyvenimo kokybė Generatoriaus kišenės nebuvimas turi tam tikrų privalumų, susijusių tiek su pacientų komfortu (gyvenimo kokybe), tiek su mažesne infekcinių įvykių rizika. Su sveikata susijusi gyvenimo kokybė nebuvo vertinta nė viename tyrime, o, be to, neaišku, ar laidų/ generatoriaus sukeliamų komplikacijų išvengimas duoda atitinkamos naudos pacientams. (D diskusija) 13
14 SVEIKATOS TECNOLOGIJOS FUNKCINĖ VERTĖ Vadovaujantis Ligų, vaistinių preparatų ir medicinos pagalbos priemonių įrašymo į kompensavimo sąrašus ir jų keitimo tvarkos aprašu, patvirtintu Lietuvos Respublikos sveikatos apsaugos ministro 2002 m. balandžio 5 d. įsakymu Nr. 159 Dėl Ligų, vaistinių preparatų ir medicinos pagalbos priemonių įrašymo į kompensavimo sąrašus ir jų keitimo tvarkos aprašo patvirtinimo, buvo įvertinta šios sveikatos technologijos dešiniajame skilvelyje implantuojamo belaidžio širdies stimuliatoriaus kaip medicinos pagalbos priemonės (MPP), funkcinė vertė. Belaidžio širdies stimuliatoriaus funkcinė vertė buvo vertinta bradikardijos (kartu su prieširdžių virpėjimu ir plazdėjimu (pagal TLK-10-AM: I48)), atrioventrikulinės blokados (pagal TLK-10-AM: I44) bei sinusinio mazgo silpnumo sindromo (pagal TLK-10-AM: I49.5) atvejais (1 lentelė). 1 lentelė. Dešiniajame skilvelyje implantuojamo belaidžio širdies stimuliatoriaus funkcinė vertė. Funkcinės vertės kriterijai Balai Pastabos Prieširdžių virpėjimas ir plazdėjimas bei Ligos įtaka sveikatai 2 sinusinio mazgo silpnumo sindromas daro įtaką neįgalumui/ darbingumui ir gyvenimo kokybei. Ligai progresuojant gali kilti grėsmė paciento gyvybei. Didžiąja dalimi (daugiau nei 50%) gali atkurti Socialinė MPP svarba 2 prarastas funkcijas, sumažinti neįgalumą/ atkurti darbingumą. MPP inovatyvumas 1 MPP iš dalies pakeis šiuo metu naudojamą alternatyvią MPP: MPP bus naudojama kartu su šiuo metu naudojama alternatyvia MPP toms pačioms indikacijoms. MPP klinikinis efektyvumas panašus į MPP klinikinis 1 alternatyvios MPP, nors mokslinių įrodymų efektyvumas trūksta. MPP ekonominis efektyvumas Galutinis balas 6 *Ekonominio efektyvumo aspektas nebuvo vertintas. 0* MPP klinikinis efektyvumas panašus į alternatyvios MPP, tačiau vertinamos MPP kaina yra aukštesnė. 14
15 IŠVADOS 1. BŠS siūlomas kaip alternatyva vienkameriniams širdies stimuliatoriams. Vienkamerinis skilvelio stimuliacijos režimas gali būti taikomas pacientams, kuriems diagnozuotas prieširdžių virpėjimas (arba ne) ar sinusinio mazgo silpnumo sindromas; dėl šių indikacijų atsiradusios atrioventrikulinės blokados koregavimui reikalingas širdies stimuliatorius, tačiau jis skiriamas tik simptomų, susijusių su bradiaritmija, palengvinimui. 2. Rinkoje yra dvi belaidžių širdies stimuliatorių sistemos Nanostim ir Micra ; abu prietaisai yra vienkameriniai širdies stimuliatoriai, turintys panašias technines charakteristikas bei CE ženklą. Pagrindinis dviejų BŠS sistemų skirtumas skirtingi prisitvirtinimo endokarde mechanizmai: Nanostim BŠS turi įsukamą vienos vijos spiralę, be to, papildomai prietaisas tvirtinamas trimis siūlėmis; Micra BŠS prisitvirtina savaime išsiskleidžiančiais kabliukais pagamintais iš nikelio ir titano lydinio (nitinolio). 3. BŠS technologijos saugumas nėra pakankamai moksliškai pagrįstas duomenys preliminarūs, grindžiami tik 3 nekontroliuojamų tyrimų rezultatais. Sunkių, su prietaiso naudojimu susijusių, nepageidaujamų įvykių dažnis į vertinimą įtrauktuose tyrimuose varijavo nuo 4 iki 6.5%. Dažniausiai pacientams nustatyti širdies sužalojimai (širdies perforacijos su/be tamponados, perikardo efuzijos, širdies tamponados). Nustatyti 6 (0.95%) atvejai, kai prietaisas pasislinko iš implantavimo vietos; visi šie atvejai įvyko pacientams, kuriems buvo implantuotas Nanostim BŠS, o ne Micra BŠS. 4. Mirštamumo rodikliai, lyginant BŠS su įprastais vienkameriniais stimuliatoriais, reikšmingai nesiskiria. Vis dėlto, manoma, kad generatoriaus kišenės nebuvimas turi privalumų, lemiančių didesnį paciento komfortą (gyvenimo kokybę) ir mažesnę infekcijų riziką, nors nei klinikinis efektyvumas, nei gyvenimo kokybė tyrimuose nebuvo analizuoti. REKOMENDACIJOS 1. Prieš priimant sprendimus dėl belaidžio širdies stimuliatoriaus naudojimo, rekomenduojama įvertinti galimų nepageidaujamų įvykių riziką, nes, nepaisant BŠS sistemų pranašumų, informacija apie šios sveikatos priežiūros technologijos saugumą ir efektyvumą yra ribota. Reikalingi papildomi moksliniai įrodymai (randomizuoti kontroliuojami tyrimai), kurie nustatytų/ palygintų klinikinę bei ekonominę vertinamos sveikatos priežiūros technologijos ir įprastinių širdies stimuliatorių naudą. 15
16 SUMMARY Scope Population PICO for leadless pacemakers for right ventricle pacing First line treatment of patients with indications for single-chamber ventricular pacemakers [2,4]: Patients with chronic atrial fibrillation (AF; ICD-10 I.48) who require a pacemaker for persistent or intermittent bradycardia due to slow ventricular response (atrioventricular (AV) block, ICD-10 I.44); Patients with persistent or intermittent bradycardia due to AV block or symptomatic sinus node disease (SND, ICD-10 I.49.5) 4. Contraindications: Patients requiring long-term pacing exceeding estimated device longevity (NB. children); Patients with indications for atrial single-chamber pacemakers or dual-chamber pacemakers or with indications for cardiac resynchronisaton therapy. Intervention MESH term: Arrhythmias, Cardiac [C ] and Arrhythmias, Cardiac [C ]. Leadless self-contained and fully implantable VVI(R) pacemaker. Setting: Vascular surgery, Interventional cardiology; specialist hospital, general hospital. Products: Micra TPS, Medtronic Inc.; Nanostim, St. Jude Medical. Comparison MESH term: Pacemaker, Artificial [E ] Conventional VVI(R) pacemaker. Outcomes Efficacy MESH term: Pacemaker, Artificial [E ] Cardiovascular mortality; Cardiovascular morbidity; Patient related quality of life; Exercise capacity; Pacing performance. Safety Complication rate. Study design Efficacy Randomised controlled trials (Non-inferiority) 5 ; 4 Only in specific instances, where other pacing modes (dual-pacing, atrial pacing) are not recommended 5 Randomised controlled trials comparing leadless pacemakers with traditional pace-makers are desired, since they are appropriate (adequate number of patients, inter-vention not urgent) and ethical (clinical equipoise, patients able to give consent) and necessary due to small plausible effect sizes. Blinding of operators and patients how-ever is not possible, and placebo-controlled trials would be unethical due to the avail-ability of an effective treatment 16
17 Prospective non-randomised controlled trials. Safety Randomised controlled trials; Prospective non-randomised controlled trials; Prospective case series or registries with >100 patients. PICO research question: Are leadless pacemakers in comparison to conventional pacemakers in patients with indications for right ventricle pacing as effective concerning cardiovascular morbidity and mortality, exercise capacity, and more effective and safe concerning patient-related quality of life and complication rate? ESC European Society of Cardiology; AV atrioventricular; TPS transcatheter pacing system; VVIR Single-chamber ventricular pacing with response modulation. Target condition Leadless pacemakers are developed as alternatives for traditional permanent cardiac pacemakers for the treatment of a variety of cardiac arrhythmias. The natural pacemaker of the heart is the sinus node located in the right atrium. Cardiac bradyarrhythmias (bradycardia associated with arrhythmia) are mainly due to either the incapacity of the sinus node to produce enough number of impulses per minute (sinus node disease) or the disturbance in atrioventricular (AV) conduction. Also, bradycardia can be associated with atrial fibrillation (AF), which is an abnormal heart rhythm characterized by rapid and irregular beating. (A0001) The purpose of cardiac pacing is to provide an appropriate heart rate and heart response to reestablish effective circulation and more normal haemodynamics that are compromised by a slow heart rate (bradycardia or bradyarrhythmia: <60 beats per minute). Permanent pacemaker implantation is further considered to alleviate symptoms associated with a bradyarrhythmia (e.g. dizziness, lightheadedness, syncope, fatigue, poor exercise tolerance) or to prevent the possible worsening of the rhythm disturbance. (A0001) In the scope of this assessment are cardiac arrhythmias in adults for which single-chamber ventricular pacing (VVI) is indicated. VVI pacing mode is the method of choice for patients with chronic atrial fibrillation who require a pacemaker due to slow ventricular response (atrioventricular block, Class I recommendation). (A0002) Bradyarrhythmias requiring cardiac pacing can be caused by a variety of aetiologies. Intrinsic causes are: idiopathic (ageing) degeneration; ischaemic heart disease; infiltrative diseases (e.g. sarcoidosis, amyloidosis, haemochromatosis); collagen vascular diseases (e.g. systemic lupus erythematosus, rheumatoid arthritis, scleroderma); congenital diseases, including sinus node and AV node disease; infective diseases (e.g. Lyme disease); surgical trauma: valve replacement (including percutaneous aortic replacement), heart transplantation. (A0003) The natural history and the role of pacing differ depending on the type of bradyarrhythmia. In patients with untreated AV block, death can occur due to heart failure secondary to low cardiac output or to sudden cardiac death caused by prolonged asystole or bradycardia-triggered ventricular tachyarrhythmia. Total survival and the risk of sudden cardiac death of patients with sinus node disease (SND) are similar to the general population. There is a strong consensus that patients with SND will benefit from cardiac pacing for symptom relief (only). (A0004) Target population Leadless pacemakers are intended to be used as replacement for conventional single-chamber right ventricular pacemakers. The target population consists of the patients in which this pacing mode is indicated. (A0007; A0023) Symptoms are present if bradycardia is severe enough to compromise blood flow: they may comprise fatigue, dizziness, syncope (fainting), dyspnoea, chest pain, weakness and a reduced exercise 17
18 capacity. Up to 6% of patients experience major complications within the first 6 months following implantation of cardiac electronic devices (all types), with lead-related reintervention being the single most common complication. (A0005) The prevalence of indications requiring single-chamber pacemaker implantation is unclear. In 2015 over 110,000 patients with cardiac arrhythmias were recorded in Lithuania. There were about 1,500 pacemaker implantations in Lithuania in 2005, and the number is growing each year. (A0006; A0011) Current clinical management of the disease or health condition There is no defined heart rate below which treatment is indicated. When deciding on the need for cardiac pacing, the correlation between symptoms and bradyarrhythmia needs to be established. The diagnosis of bradyarrhythmia is usually made from a standard electrocardiogram (ECG) when persistent, and from a standard ECG or more prolonged ECG recordings when intermittent. Provocative testing or an electrophysiological study may be required when a bradycardia is suspected but not documented. (A0024) The decision regarding pacemaker implantation and choice of pacing mode is based on three clinical factors: the location of the conduction abnormality, the presence of symptoms and their association with the bradyarrhythmia, and the absence of a reversible cause. VVI pacing mode is the method of choice for patients with chronic atrial fibrillation who require a pacemaker due to slow ventricular response (atrioventricular block, Class I recommendation). In patients with acquired AV block (but no AF) or sinus node disease, the condition can be managed with either a single or dual chamber pacemaker. Also, sinus bradycardia is only an indication for pacing if bradycardia is symptomatic, if the symptoms can be attributed to sinus bradycardia and if a reversible cause can be excluded. (A0025) Regulatory & reimbursement status Nanostim : After the LEADLESS trial in October 2013, St. Jude Medical received CE mark approval (for European commercialization) to market the Nanostim leadless pacemaker in the European Union. A trial designed to investigate Nanostim for the United States Food and Drug Administration (FDA) approval was initiated in February The device has not yet been approved by FDA, Health Canada or Therapeutic Goods Administration (Australia). FDA approval of the device is sought in (A0020; A0021) Micra Transcatheter Pacing System: Medtronic received CE mark for Micra Transcatheter Pacing System (TPS) in An FDA approval study on Micra TPS was initiated in November 2013 and based on the favorable clinical results of the study, the FDA approved the Micra Transcatheter Pacing System for clinical use on April (A0020; A0021) As yet there are no data available about the cost of these devices. Leadless pacemakers in Australia are, however, significantly more expensive in comparison to conventional single chamber pacemakers (11,300 EUR vs. 4,200 EUR). In a Horizon Scanning report published by the Italian group AGENAS, the reported cost of the Nanostim device and implantation was 11,500 EUR, according to St Jude Medical; however, costs were not available for the Micra TPS device from Medtronic Inc. (Leadless) cardiac pacemakers are currently reimbursed in Lithuania via the DRG code F12A and F12B Implantation or changing of a pacemaker (prices are 2, EUR and 1, EUR, respectively). (A0020; A0021) 18
19 Features of the technology Leadless cardiac pacemakers (LCP) are self-contained intracardiac devices that are designed to have the same function as traditional cardiac pacemakers, but are miniaturized and can be implanted entirely inside the right ventricle of the heart via a steerable catheter. (B0001) The first prototype of a completely endocardial implantable pacemaker was presented in Since then, different leadless systems with different energy sources and myocardial fixation systems have been developed, resulting in the CE certification of two right ventricular single-chamber pacemaker systems: the Nanostim leadless cardiac pacemaker and the Micra TPS. These devices share common characteristics, as they are both programmable, single-chamber ventricular pacemakers, self-contained in a hermetically sealed capsule. Both have a volume of up to 1cm³, weight just 2 g and thus are approximately ten times smaller than conventional VVI pacemakers. The devices have an estimated battery longevity of approximately ten years, which is comparable to conventional pacemakers. Both systems are delivered through the femoral vein, via 18 Fr (Nanostim LCP) and 23 Fr (Micra TPS) catheters, and have a docking feature which allows attachment to a catheter for delivery, repositioning and retrieval. In theory, both systems offer a device retrieval option, allowing repositioning or retrieval of the devices following implantation. There are some data on the removal of implanted systems in animal studies and in humans. However, retrieval of the device requires confirmation and rationale is to add another pacemaker into the right ventricle and this is possible due to the small size of the device. (B0001; B0003) Main differences between the systems are related to the fixation mechanism: Nanostim LCP uses a screw-in helix and a secondary fixation mechanism of three nylon tines, whereas the Micra TPS uses four self-expanding nitinol tines. (B0001) In conventional pacemakers, a separate pulse generator containing the battery and the machinery for sensing and timing of the electrical impulses is placed in a (most commonly) pectoral subcutaneous pocket. The electrical impulses are delivered from the generator directly to the heart through one or more transvenous leads, depending on the desired pacing mode. The majority of conventional pacemakers are capable of several pacing modes. (B0001) In contrast to traditional pacemakers, leadless pacemakers do not require the placement of an external pulse generator in a surgical pocket in the chest and the transmission of impulses through transvenous leads. The claimed benefit is accordingly the avoidance of complications associated with these two components of traditional pacemaker implantation. The subcutanous pocket has a potential for local complications such as skin erosion, pocket haematoma and pocket infection. In up to six out of ten patients, it causes reduced mobility in the shoulder region where the pulse generator is placed. Leadrelated complications include venous obstructions, insulation breaks, lead dislodgements, electrical malfunction, lead fractures and infection. Of particular concern are infections requiring lead extraction, as the procedure is associated with a high risk of complications. The longer the leads are in place, the greater the risk. (B0001; B0002) Additional benefits are expected with regards to shorter procedure and recovery times and a better quality of life as a result of the maintenance of shoulder mobility and the absence of a lump or scar. (B0002) Moreover, it is estimated that up to 75% of pacemaker patients are expected to develop an indication for an MRI scan over the lifetime of their device. Pre-clinical MRI testing has consistently shown the incompatibility of traditional pacing systems and the MRI environment, which may compromise the patient s health and/or damage the device. In addition, the Micra Transcatheter Pacing System is designed to allow patients to safely undergo MRI scans, in both 1.5 and 3 Tesla MRI scanners; the Nanostim device allows patients to safely undergo MRI scans in 1.5 Tesla MRI scanners. (B0002) 19
20 Investments and tools required to use the technology Both LCP and traditional pacemakers are provided in specialised centres with interventional cardiology in the cardiac catheterization, laboratory sedative medication and local anaesthesia. LCP implantation further requires fluoroscopy to guide positioning of the device. Setting and staff required for LCP implantation do not differ from traditional pacemaker procedures. As with any novel implantation method or procedure, there is a learning curve for implantation of the leadless pacemaker and additional training for medical specialists will be required. (B0004; B0008; B0009) Patient safety The rates of serious adverse device effects ranged between 4% and 6.5% in the three case series. However, data on the safety of transcatheter pacing are preliminary and have been limited to a few reports from nonrandomized studies. (C0008) There was one patient with cardiac injury (cardiac tamponade) in the LEADLESS I trial, eight injuries (cardiac perforations with/without tamponade or pericardial effusions) in LEADLESS II and 11 injuries (cardiac perforations or effusions) in the Micra Transcatheter Pacemaker Study. Six dislodgements were reported with the Nanostim device, but none with the Micra TPS system. Other serious adverse events that were attributable either to the device or the procedure included vascular complications, arrhythmia during device implantation and elevated pacing thresholds requiring retrieval and implantation of a new device. (C0008) There are not enough data to answer the question about the susceptible patient groups that are more likely to be harmed through the use of the technology. Also, it is unknown whether long-term retrieval of the pacemaker after several years of implantation in patients will be possible. An alternative replacement strategy could be to place an additional device next to the initial device, without compromising the right ventricular volume capacity and overall function. For this strategy, it is important to realize that the LCP only takes up 1.0 ml of volume in the right ventricle, but also that evidence of these strategies is currently lacking. (C0005; C discussion) Leadless pacemakers and conventional single-chamber ventricular pacemakers are associated with user-dependent harms due to the risk of serious adverse events related with the implantation procedure. The influence of operator experience on the rate of device-related serious adverse events was assessed in one case serie; The rate of device related serious adverse events was 6.8% for the initial 10 cases versus 3.6% for the subsequent implants (p=0.56). (C0007) Mortality Leadless pacemakers are not expected to have a beneficial effect on mortality compared to conventional VVI pacemakers. Overall mortality ranged from 3 to 5% and cardiac mortality ranged from 0.8 to 1%. (D0001) Procedural mortality was reported in all three studies. In LEADLESS I trial, one patient had a perforation during the implantation procedure and died of a massive cerebral artery ischaemic infarct. Two procedure-related (but classified as non-device-related) deaths were reported in the LEADLESS II cohort: in one patient LCP implantation was complicated by a large groin haematoma; the second subject underwent an unsuccessful LCP implant complicated by pericardial effusion. In the Micra Transcatheter Pacemaker Study cohort, one death was adjudicated as related to the transcatheter implantation procedure: the patient had a prolonged procedure time due to a concomitant AV node ablation and end stage renal disease and the cause of death was perceived to be metabolic acidosis. (D0003) 20
21 Function A leadless intracardiac transcatheter pacing system has been designed to avoid the need for a pacemaker pocket and transvenous lead (the electrodes are positioned on the pacemaker capsule), thereby eliminating an important source of complications associated with traditional pacing systems while providing similar benefits. (D discussion) Notably, excessive ventricular pacing has been associated with worse cardiovascular outcomes. Some have proposed that LCPs may increase risk of arrhythmia due to their larger point of endocardial contact when compared to conventional pacing systems, although excessive arrhythmic events have not been noted in prospective trials. (D discussion) Quality of life The lack of a generator pocket has some advantages as well both in terms of patient comfort (and quality of life) and infectious risk. However, health-related quality of life was not assessed in the studies. Also, it is unclear, if the avoidance of lead/ generator complications translates in a relevant benefit for the patients. (D discussion) 21
22 HEALTH PROBLEM AND CURRENT USE OF THE TECHNOLOGY Research questions ID Question A0001 For which health conditions, and for what purposes are leadless pacemakers used? A0002 What is the disease or health condition in the scope of this assessment? A0003 What are the known risk factors for cardiac arrhythmias? A0004 What is the natural course of cardiac arrhythmias? A0005 What are the consequences of cardiac arrhythmias for the society? A0006 What is the burden of disease for patients with cardiac arrhythmias? A0007 What is the target population in this assessment? A0023 How many people belong to the target population? A0011 How much are leadless pacemakers utilised? A0020 What is the marketing authorisation status of leadless pacemakers? A0021 What is the reimbursement status of leadless pacemakers? A0024 How are cardiac arrhythmias currently diagnosed according to published guidelines and in practice? A0025 How are cardiac arrhythmias currently managed according to published guidelines and in practice? Overview of the disease or health condition A0001. For which health conditions, and for what purposes are leadless pacemakers used? Leadless pacemakers are developed as alternatives for traditional permanent cardiac pacemakers for the treatment of a variety of cardiac arrhythmias. The natural pacemaker of the heart is the sinus node located in the right atrium. It generates around 70 regular electrical impulses per minute (at rest), that are conducted across the rest of the heart. This triggers contraction of the atriums followed by the contraction of the ventricles allowing the blood flow. Cardiac bradyarrhythmias (bradycardia associated with arrhythmia) are mainly due to either the incapacity of the sinus node to produce enough number of impulses per minute (sinus node disease) or the disturbance in atrioventricular (AV) conduction. Atrial fibrillation is an abnormal heart rhythm characterized by rapid and irregular beating, but can be associated with bradycardia. The principal reason to place a pacemaker in a patient with atrial fibrillation (AF) is to treat symptomatic bradycardia. Pacing has not been shown to prevent the development of AF. The purpose of cardiac pacing is to provide an appropriate heart rate and heart response to reestablish effective circulation and more normal haemodynamics that are compromised by a slow heart rate (bradycardia or bradyarrhythmia: <60 beats per minute). Permanent pacemaker implantation is further considered to alleviate symptoms associated with a bradyarrhythmia (e.g. dizziness, lightheadedness, syncope, fatigue, poor exercise tolerance) or to prevent the possible worsening of the rhythm disturbance [1]. A0002. What is the disease or health condition in the scope of this assessment? In the scope of this assessment are cardiac arrhythmias in adults for which single-chamber ventricular pacing (VVI) is indicated. Guidelines for implantation of permanent pacemakers have been 22
23 established by the American College of Cardiology, the American Heart Association and the Heart Rhythm Society (ACC/AHA/HRS) [2,3] and by the European Society of Cardiology (ESC) [4]. VVI pacing mode is the method of choice for patients with chronic atrial fibrillation (AF; ICD- 10-AM: I44) who require a pacemaker due to slow ventricular response (atrioventricular (AV) block, Class I recommendation [4]). This pacing mode may be considered for patients with AV block, even in the absence of AF, on an individual basis, but in general is not considered the first choice [4]. In patients with sinus node disease (SND, also sick sinus syndrome) as well as in patients with atrial fibrillation, pacing is only indicated if bradycardia causes symptoms. Dual-chamber pacing is recommended over VVI pacing [2,3,4]. A0003. What are the known risk factors for cardiac arrhythmias? Bradyarrhythmias requiring cardiac pacing can be caused by a variety of aetiologies [4]. Intrinsic causes are: Idiopathic (ageing) degeneration; Ischaemic heart disease; Infiltrative diseases (e.g. sarcoidosis, amyloidosis, haemochromatosis); Collagen vascular diseases (e.g. systemic lupus erythematosus, rheumatoid arthritis, scleroderma); Congenital diseases, including sinus node and AV node disease; Infective diseases (e.g. Lyme disease); Rare genetic diseases; Surgical trauma: valve replacement (including percutaneous aortic replacement), heart transplantation; Intended or unintended AV block due to catheter ablation procedure. Extrinsic causes are: Physical training (sports); Vagal reflex: vasovagal, situational, carotid sinus syndrome; Idiopathic paroxysmal AV block; (Adverse) drug effects; Cocaine abuse and other recreational drugs; Electrolyte imbalance: hypokalaemia, hyperkalaemia; Metabolic disorders: hypothyroidism, hypothermia, anorexia nervosa; Neurological disorders; Obstructive sleep apnoea. A0004. What is the natural course of cardiac arrhythmias? The natural history and the role of pacing differ depending on the type of bradyarrhythmia. In patients with untreated AV block, death can occur due to heart failure secondary to low cardiac output or to sudden cardiac death caused by prolonged asystole or bradycardia-triggered ventricular tachyarrhythmia. Several observational studies indicate that pacing prevents recurrence of syncope and improves survival [4]. Total survival and the risk of sudden cardiac death of patients with SND are similar to the general population [5,6]. There is a strong consensus that patients with SND will benefit from cardiac pacing for symptom relief (only) [4]. 23
24 Effects of the disease or health condition on the individual and society A0005. What is the burden of disease for patients with cardiac arrhythmias? Symptoms are present if bradycardia is severe enough to compromise blood flow: they may comprise fatigue, dizziness, syncope (fainting), dyspnoea, chest pain, weakness and a reduced exercise capacity. Major complications associated with the implantation of a single-chamber right ventricular pacemaker include lead-related reinterventions, local infections requiring reintervention, device-related systemic infections, endocarditis, pneumothorax requiring drainage, cardiac perforation, pocket revisions because of pain, generator-lead interface problems requiring reintervention, haematomas requiring reintervention, deep venous thrombosis, Twiddler s syndrome, wound revisions, stroke, myocardial infarctions, and procedure-related deaths. Minor complications include haematomas resulting in a prolonged hospital stay, hospital readmissions, additional outpatient visits, wound infections treated with antibiotics, conservatively treated pneumothorax, and lead dislodgements without reintervention [7,8]. Up to 6% of patients experience major complications within the first 6 months following implantation of cardiac electronic devices (all types), with lead-related reintervention being the single most common complication, followed by infections, pneumothorax and cardiac perforation. For singlechamber pacemakers, this risk is however significantly lower, with 3.3% experiencing any major complication [8]. Also the risk of lead complications is lower for single-chamber right ventricular pacemakers compared to other pacemaker types [9]. A0006. What are the consequences of cardiac arrhythmias for the society? The prevalence of indications requiring single-chamber pacemaker implantation is unclear. In 2015 over 110,000 patients with cardiac arrhythmias were recorded in Lithuania [10]. Each year there are about 6,000 pacemaker implantations in Austria, of which approximately one third are singlechamber pacemakers [11,12]. There were about 1,500 pacemaker implantations in Lithuania in 2005, and the number is growing each year [13]. Current clinical management of the disease or health condition A0024. How are cardiac arrhythmias currently diagnosed according to published guidelines and in practice? There is no defined heart rate below which treatment is indicated. When deciding on the need for cardiac pacing, the correlation between symptoms and bradyarrhythmia needs to be established. The diagnosis of bradyarrhythmia is usually made from a standard electrocardiogram (ECG) when persistent, and from a standard ECG or more prolonged ECG recordings (ambulatory monitoring or implantable loop recorder) when intermittent. Provocative testing or an electrophysiological study may be required when a bradycardia is suspected but not documented. This strategy is based on the assumption that provoked abnormalities will have the same mechanism as spontaneous episodes. (Longterm) ECG monitoring has the advantage of high diagnostic accuracy, whereas provocative testing is faster, but more prone to misdiagnosis [4]. 24
25 A0025. How are cardiac arrhythmias currently managed according to published guidelines and in practice? The decision regarding pacemaker implantation and choice of pacing mode is based on three clinical factors: the location of the conduction abnormality, the presence of symptoms and their association with the bradyarrhythmia, and the absence of a reversible cause (Figure 1). SND sinus node disease; AV atrioventricular; AF atrial fibrillation; AVM AV delay management. For nomenclature of pacing modes see Table 1 (question B0001): Revised NBG code for pacing nomenclature [14] Figure 1. Choice of the pacing mode (ESC guidelines, [4]) VVI pacing mode is the method of choice for patients with chronic atrial fibrillation (AF; ICD- 10-AM: I44) who require a pacemaker due to slow ventricular response (atrioventricular (AV) block, Class I recommendation [4]). Atrioventricular (AV) block is defined as a delay or interruption in the transmission of an impulse from the atria to the ventricles due to an anatomical or functional impairment in the conduction system. The conduction can be delayed, intermittent, or absent. The commonly used classification includes first degree (slowed conduction without loss of atrioventricular synchrony), second degree (intermittent loss of atrioventricular conduction, often in a regular pattern, e.g., 2:1, 3:2, or higher degrees of block), and third degree or complete AV block [1]. In patients with acquired AV block (but no AF) or sinus node disease (SND), the condition can be managed with either a single or dual-chamber pacemaker. Dual-chamber pacing is recommended over single chamber ventricular pacing for avoiding pacemaker syndrome, lowering the risk of developing AF and improving quality of life (class IIa recommendation, [4]). However, the decision should take into account the increased complication risk and costs of dual-chamber pacing. Sinus bradycardia (SB) is a rhythm in which fewer impulses than the normal number arise from the sinoatrial node. It is caused by a primary sinus node dysfunction or by other conditions (drugs, acute myocardial infarction, obstructive sleep apnoea, etc.). In general, SB is only an indication for pacing if bradycardia is symptomatic, if the symptoms can be attributed to SB and if a reversible cause can be 25
SVEIKATOS PRIEŽIŪROS TECHNOLOGIJOS VERTINIMAS:
 SVEIKATOS PRIEŽIŪROS TECHNOLOGIJOS VERTINIMAS: TRANSKATETERINIŲ IMPLANTUOJAMŲJŲ PRIEMONIŲ TAIKYMAS MITRALINIO VOŽTUVO KOREKCIJAI SUAUGUSIEMS PACIENTIEMS, KURIEMS DIAGNOZUOTA LĖTINĖ MITRALINIO VOŽTUVO REGURGITACIJA
SVEIKATOS PRIEŽIŪROS TECHNOLOGIJOS VERTINIMAS: TRANSKATETERINIŲ IMPLANTUOJAMŲJŲ PRIEMONIŲ TAIKYMAS MITRALINIO VOŽTUVO KOREKCIJAI SUAUGUSIEMS PACIENTIEMS, KURIEMS DIAGNOZUOTA LĖTINĖ MITRALINIO VOŽTUVO REGURGITACIJA
New generations pacemakers and ICDs: an update
 Advances in Cardiac Arrhythmias and Great Innovations in Cardiology XXVII Giornate Cardiologiche Torinesi New generations pacemakers and ICDs: an update Prof. Fiorenzo Gaita, MD Division of Cardiology
Advances in Cardiac Arrhythmias and Great Innovations in Cardiology XXVII Giornate Cardiologiche Torinesi New generations pacemakers and ICDs: an update Prof. Fiorenzo Gaita, MD Division of Cardiology
SVEIKATOS TECHNOLOGIJOS VERTINIMAS:
 SVEIKATOS TECHNOLOGIJOS VERTINIMAS: ULTRAGARSINIO SIGNALO ALGORITMINIO ANALIZATORIAUS PROSTATOS AUDINIO DIFERENCIACIJAI PROSTATE HISTOSCANNING NAUDOJIMAS PROSTATOS VĖŽIO DIAGNOSTIKAI BEI LOKALIZACIJAI
SVEIKATOS TECHNOLOGIJOS VERTINIMAS: ULTRAGARSINIO SIGNALO ALGORITMINIO ANALIZATORIAUS PROSTATOS AUDINIO DIFERENCIACIJAI PROSTATE HISTOSCANNING NAUDOJIMAS PROSTATOS VĖŽIO DIAGNOSTIKAI BEI LOKALIZACIJAI
Leadless Pacing. Osama Diab Assistant Prof. of Cardiology Ain Shams University Egypt
 Leadless Pacing Osama Diab Assistant Prof. of Cardiology Ain Shams University Egypt The weakest link in Pacemaker system the lead. The more the leads the more the complications Dislodgement Fracture Insulation
Leadless Pacing Osama Diab Assistant Prof. of Cardiology Ain Shams University Egypt The weakest link in Pacemaker system the lead. The more the leads the more the complications Dislodgement Fracture Insulation
SVEIKATOS TECHNOLOGIJOS VERTINIMAS: KRŪTIES VĖŽIO DIAGNOSTIKA NAUDOJANT VAKUUMINĘ KRŪTIES BIOPSIJĄ
 SVEIKATOS TECHNOLOGIJOS VERTINIMAS: KRŪTIES VĖŽIO DIAGNOSTIKA NAUDOJANT VAKUUMINĘ KRŪTIES BIOPSIJĄ HEALTH TECHNOLOGY ASSESSMENT: VACUUM-ASSISTED BREAST BIOPSY IN THE DIAGNOSIS OF BREAST CANCER 2017 VILNIUS
SVEIKATOS TECHNOLOGIJOS VERTINIMAS: KRŪTIES VĖŽIO DIAGNOSTIKA NAUDOJANT VAKUUMINĘ KRŪTIES BIOPSIJĄ HEALTH TECHNOLOGY ASSESSMENT: VACUUM-ASSISTED BREAST BIOPSY IN THE DIAGNOSIS OF BREAST CANCER 2017 VILNIUS
NOVEL DEVICE TECHNOLOGIES
 NOVEL DEVICE TECHNOLOGIES Leadless Pacemakers and Subcutaneous ICDs Do the Benefits Outweigh MRI Incompatibility? Disclosures None Background PPMs and ICDs are very effective therapy for treating bradyarrhythmias
NOVEL DEVICE TECHNOLOGIES Leadless Pacemakers and Subcutaneous ICDs Do the Benefits Outweigh MRI Incompatibility? Disclosures None Background PPMs and ICDs are very effective therapy for treating bradyarrhythmias
Different indications for pacemaker implantation are the following:
 Patient Resources: ICD/Pacemaker Overview ICD/Pacemaker Overview What is a pacemaker? A pacemaker is a device that uses low energy electrical pulses to prompt the heart to beat whenever a pause in the
Patient Resources: ICD/Pacemaker Overview ICD/Pacemaker Overview What is a pacemaker? A pacemaker is a device that uses low energy electrical pulses to prompt the heart to beat whenever a pause in the
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE GUIDANCE EXECUTIVE (GE)
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE GUIDANCE EXECUTIVE (GE) Review of TA88; Dual-chamber pacemakers for symptomatic bradycardia due to sick sinus syndrome and/or atrioventricular block
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE GUIDANCE EXECUTIVE (GE) Review of TA88; Dual-chamber pacemakers for symptomatic bradycardia due to sick sinus syndrome and/or atrioventricular block
MEET MICRA. Micra TM ACTUAL SIZE. Transcatheter Pacing System
 MEET MICRA ACTUAL SIZE Micra TM Transcatheter Pacing System MEET MICRA The world s smallest pacemaker 1 MINIATURIZED. 93% smaller than modern-day pacemakers 6 Completely self contained within the heart,
MEET MICRA ACTUAL SIZE Micra TM Transcatheter Pacing System MEET MICRA The world s smallest pacemaker 1 MINIATURIZED. 93% smaller than modern-day pacemakers 6 Completely self contained within the heart,
Prostatos vėžys: samprata apie riziką. Ramūnas Mickevičius Urologijos klinika LSMU KK Druskininkai
 Prostatos vėžys: samprata apie riziką Ramūnas Mickevičius Urologijos klinika LSMU KK Druskininkai 2014-01-31 02-01 Terminas PROSTATOS VĖŽYS pacientui kelia nerimą, įtampą, baimę mirčiai. Kas metai Lietuvoje
Prostatos vėžys: samprata apie riziką Ramūnas Mickevičius Urologijos klinika LSMU KK Druskininkai 2014-01-31 02-01 Terminas PROSTATOS VĖŽYS pacientui kelia nerimą, įtampą, baimę mirčiai. Kas metai Lietuvoje
MEET MICRA. Micra TM ACTUAL SIZE. Transcatheter Pacing System
 MEET MICRA ACTUAL SIZE Micra TM Transcatheter Pacing System MEET MICRA The world s smallest pacemaker 1 MINIATURIZED. 93% smaller than modern-day pacemakers 7 Completely self contained within the heart,
MEET MICRA ACTUAL SIZE Micra TM Transcatheter Pacing System MEET MICRA The world s smallest pacemaker 1 MINIATURIZED. 93% smaller than modern-day pacemakers 7 Completely self contained within the heart,
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of a leadless cardiac pacemaker implantation for bradyarrhythmias Bradyarrhythmias
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of a leadless cardiac pacemaker implantation for bradyarrhythmias Bradyarrhythmias
NEIL CISPER TECHNICAL FIELD ENGINEER ICD/CRTD BASICS
 NEIL CISPER TECHNICAL FIELD ENGINEER ICD/CRTD BASICS OBJECTIVES Discuss history of ICDs Review the indications for ICD and CRT therapy Describe basic lead and device technology Discuss different therapies
NEIL CISPER TECHNICAL FIELD ENGINEER ICD/CRTD BASICS OBJECTIVES Discuss history of ICDs Review the indications for ICD and CRT therapy Describe basic lead and device technology Discuss different therapies
Cardiac Implanted Electronic Devices Pacemakers, Defibrillators, Cardiac Resynchronization Devices, Loop Recorders, etc.
 Cardiac Implanted Electronic Devices Pacemakers, Defibrillators, Cardiac Resynchronization Devices, Loop Recorders, etc. The Miracle of Living February 21, 2018 Matthew Ostrom MD,FACC,FHRS Division of
Cardiac Implanted Electronic Devices Pacemakers, Defibrillators, Cardiac Resynchronization Devices, Loop Recorders, etc. The Miracle of Living February 21, 2018 Matthew Ostrom MD,FACC,FHRS Division of
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of leadless cardiac pacemaker implantation for bradyarrhythmias Bradyarrhythmias
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of leadless cardiac pacemaker implantation for bradyarrhythmias Bradyarrhythmias
Review of Pacemakers and ICD Therapy: Overview and Patient Management
 Review of Pacemakers and ICD Therapy: Overview and Patient Management Pacing Systems Charles J. Love, MD FACC FAHA FHRS CCDS Professor of Medicine Director, Cardiac Rhythm Device Services OSU Division
Review of Pacemakers and ICD Therapy: Overview and Patient Management Pacing Systems Charles J. Love, MD FACC FAHA FHRS CCDS Professor of Medicine Director, Cardiac Rhythm Device Services OSU Division
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Implantable cardioverter defibrillators for the treatment of arrhythmias and cardiac resynchronisation therapy for the treatment of heart failure (review
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Implantable cardioverter defibrillators for the treatment of arrhythmias and cardiac resynchronisation therapy for the treatment of heart failure (review
Essentials of Pacemakers and ICD s. Rajesh Banker, MD, MPH
 Essentials of Pacemakers and ICD s Rajesh Banker, MD, MPH Pacemakers have 4 basic functions: Stimulate cardiac depolarization Sense intrinsic cardiac function Respond to increased metabolic demand by providing
Essentials of Pacemakers and ICD s Rajesh Banker, MD, MPH Pacemakers have 4 basic functions: Stimulate cardiac depolarization Sense intrinsic cardiac function Respond to increased metabolic demand by providing
Analysis of prognostic factors for melanoma patients
 ACTA MEDICA LITUANICA. 2017. Vol. 24. No. 1. P. 25 34 Lietuvos mokslų akademija, 2017 Analysis of prognostic factors for melanoma patients Andrė Lideikaitė 1, Julija Mozūraitienė 2, Simona Letautienė 1,
ACTA MEDICA LITUANICA. 2017. Vol. 24. No. 1. P. 25 34 Lietuvos mokslų akademija, 2017 Analysis of prognostic factors for melanoma patients Andrė Lideikaitė 1, Julija Mozūraitienė 2, Simona Letautienė 1,
CHANGES RELATED TO INPATIENT MORTALITY FROM ACUTE STROKE IN THE STROKE UNIT OF THE KLAIPEDA UNIVERSITY HOSPITAL IN
 74 BIOMEDICINA / BIOMEDICINE SVEIKATOS MOKSLAI / HEALTH SCIENCES IN EASTERN EUROPE ISSN 1392-6373 print / 2335-867X online 2016, 26 tomas, Nr. 5, p. 74-78 DOI: http://doi.org/10.5200/sm-hs.2016.075 CHANGES
74 BIOMEDICINA / BIOMEDICINE SVEIKATOS MOKSLAI / HEALTH SCIENCES IN EASTERN EUROPE ISSN 1392-6373 print / 2335-867X online 2016, 26 tomas, Nr. 5, p. 74-78 DOI: http://doi.org/10.5200/sm-hs.2016.075 CHANGES
Cardiac Pacemakers» 2013 HOSPITAL REIMBURSEMENT GUIDE
 Cardiac Pacemakers» 2013 HOSPITAL REIMBURSEMENT GUIDE 2 Contents Page Introduction Medicare Coding and Payment Overview Hospital Inpatient Hospital Outpatient HCPCS Device Category C-Codes Coverage for
Cardiac Pacemakers» 2013 HOSPITAL REIMBURSEMENT GUIDE 2 Contents Page Introduction Medicare Coding and Payment Overview Hospital Inpatient Hospital Outpatient HCPCS Device Category C-Codes Coverage for
Transvenous Pacemaker Procedures
 Cardiology: Pacemaker and Defibrillator Coding Presented By: Moderate Sedation 2017 99151 : under age 5, initial 15 minutes by MD performing intervention 99152: age 5 or older, initial 15 minutes by MD
Cardiology: Pacemaker and Defibrillator Coding Presented By: Moderate Sedation 2017 99151 : under age 5, initial 15 minutes by MD performing intervention 99152: age 5 or older, initial 15 minutes by MD
REVIEW ARTICLE. Leadless Cardiac Pacemaker Therapy. An Overview for the Hospitalist Richard Weachter 1
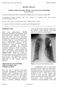 Leadless Cardiac Pacemaker Therapy. An Overview for the Hospitalist Richard Weachter 1 1 Division of Cardiovascular Medicine, Department of Medicine, University of Missouri, Columbia, MO Correspondence:
Leadless Cardiac Pacemaker Therapy. An Overview for the Hospitalist Richard Weachter 1 1 Division of Cardiovascular Medicine, Department of Medicine, University of Missouri, Columbia, MO Correspondence:
Q & A: How to Safely Scan Patients with a SureScan MRI Pacemaker
 Q & A: How to Safely Scan Patients with a SureScan MRI Pacemaker Patient Scheduling and Screening Q: Is there any special (other than regular MRI consent) consent form that should be signed by the patient?
Q & A: How to Safely Scan Patients with a SureScan MRI Pacemaker Patient Scheduling and Screening Q: Is there any special (other than regular MRI consent) consent form that should be signed by the patient?
Leadless pacemakers a panacea for bradyarrhythmias?
 Leadless pacemakers a panacea for bradyarrhythmias? Peysh A Patel Take Home Messages Why may leadless systems be required? Where the cessation of vital action is very complete, and continues long, we ought
Leadless pacemakers a panacea for bradyarrhythmias? Peysh A Patel Take Home Messages Why may leadless systems be required? Where the cessation of vital action is very complete, and continues long, we ought
Recent Advances in Pacing and Defibrillation Harish Doppalapudi, MD
 Recent Advances in Pacing and Defibrillation Harish Doppalapudi, MD Harish Doppalapudi, MD Assistant Professor of Medicine Director, Clinical Cardiac Electrophysiology Training Program University of Alabama
Recent Advances in Pacing and Defibrillation Harish Doppalapudi, MD Harish Doppalapudi, MD Assistant Professor of Medicine Director, Clinical Cardiac Electrophysiology Training Program University of Alabama
Antroji (ir tolesnės) autologinės KKLT mielominei ligai gydyti. Indrė Klimienė, Vilniaus Universiteto Ligoninė Santariškių Klinikos.
 Antroji (ir tolesnės) autologinės KKLT mielominei ligai gydyti. Indrė Klimienė, Vilniaus Universiteto Ligoninė Santariškių Klinikos. 2 Gydymo Evoliucija Milestone 1962 Melphalan-prednisone (MP) Notes Introduction
Antroji (ir tolesnės) autologinės KKLT mielominei ligai gydyti. Indrė Klimienė, Vilniaus Universiteto Ligoninė Santariškių Klinikos. 2 Gydymo Evoliucija Milestone 1962 Melphalan-prednisone (MP) Notes Introduction
Leadless Cardiac Pacemaker
 Leadless Cardiac Pacemaker Policy Number: Original Effective Date: MM.02.042 XXXMay 1, 2019 Lines of Business: Current Effective Date: HMO; PPO; QUEST Integration; FED87 XXXMay 1, 2019 Section: Medicine
Leadless Cardiac Pacemaker Policy Number: Original Effective Date: MM.02.042 XXXMay 1, 2019 Lines of Business: Current Effective Date: HMO; PPO; QUEST Integration; FED87 XXXMay 1, 2019 Section: Medicine
SJM MRI ACTIVATOR HANDHELD DEVICE WORKFLOW Model: EX4000. SJM-EDTR Item approved for U.S. use only.
 SJM MRI ACTIVATOR HANDHELD DEVICE WORKFLOW Model: EX4000 1 APPROVED MRI SCAN CONDITIONS 1.5 TESLA FULL BODY 2 SETUP SJM MRI ACTIVATOR HANDHELD DEVICE The SJM MRI Activator handheld device must be enabled
SJM MRI ACTIVATOR HANDHELD DEVICE WORKFLOW Model: EX4000 1 APPROVED MRI SCAN CONDITIONS 1.5 TESLA FULL BODY 2 SETUP SJM MRI ACTIVATOR HANDHELD DEVICE The SJM MRI Activator handheld device must be enabled
Yes, out of sight out of mind.
 Micra TRANSCATHETER PACING SYSTEM 25.9 mm Yes, out of sight out of mind. cardiocapsule Meet Micra The Invisible Pacemaker Micra is the world s smallest pacemaker 1. It leaves no bump under the skin, no
Micra TRANSCATHETER PACING SYSTEM 25.9 mm Yes, out of sight out of mind. cardiocapsule Meet Micra The Invisible Pacemaker Micra is the world s smallest pacemaker 1. It leaves no bump under the skin, no
Changes in health-related quality of life among patients with coronary artery disease: a 2-year follow-up
 Medicina (Kaunas) 2010;46(12):843-50 Changes in health-related quality of life among patients with coronary artery disease: a 2-year follow-up nstitute of Psychophysiology and Rehabilitation, Lithuanian
Medicina (Kaunas) 2010;46(12):843-50 Changes in health-related quality of life among patients with coronary artery disease: a 2-year follow-up nstitute of Psychophysiology and Rehabilitation, Lithuanian
EXAMPLE PROTOCOL FOR PATIENT SCANNING PROCESS
 EXAMPLE PROTOCOL FOR PATIENT SCANNING PROCESS MRI SureScan Pacing, ICD, and CRT (CRT-D and CRT-P) Transvenous Implantable Cardiac Systems Timothy S. E. Albert, M.D. President CVMR Solutions, Inc. Carmel
EXAMPLE PROTOCOL FOR PATIENT SCANNING PROCESS MRI SureScan Pacing, ICD, and CRT (CRT-D and CRT-P) Transvenous Implantable Cardiac Systems Timothy S. E. Albert, M.D. President CVMR Solutions, Inc. Carmel
Figure 2. Normal ECG tracing. Table 1.
 Figure 2. Normal ECG tracing that navigates through the left ventricle. Following these bundle branches the impulse finally passes to the terminal points called Purkinje fibers. These Purkinje fibers are
Figure 2. Normal ECG tracing that navigates through the left ventricle. Following these bundle branches the impulse finally passes to the terminal points called Purkinje fibers. These Purkinje fibers are
Pediatric pacemakers & ICDs:
 Pediatric pacemakers & ICDs: perioperative management Manchula Navaratnam Clinical Assistant Professor LPCH, Stanford SPA 2016 Conflict of interest: none Objectives Indications in pediatrics Components
Pediatric pacemakers & ICDs: perioperative management Manchula Navaratnam Clinical Assistant Professor LPCH, Stanford SPA 2016 Conflict of interest: none Objectives Indications in pediatrics Components
Call Medtronic at 1 (800) to verify the patient s current implanted system
 MRI SURESCAN SYSTEMS Patient Scanning Process Transvenous Implantable Cardiac Systems PATIENT PRESCREENING SureScan Pacing, Defibrillation, and CRT (CRT-D and CRT-P) Systems Verification Verify that patient
MRI SURESCAN SYSTEMS Patient Scanning Process Transvenous Implantable Cardiac Systems PATIENT PRESCREENING SureScan Pacing, Defibrillation, and CRT (CRT-D and CRT-P) Systems Verification Verify that patient
The study of cancer patients distress
 ACTA MEDICA LITUANICA. 2014. Vol. 21. No. 2. P. 51 56 Lietuvos mokslų akademija, 2014 The Second International Conference on Psychosocial Oncology Psychosocial Support and Communication in Cancer Care:
ACTA MEDICA LITUANICA. 2014. Vol. 21. No. 2. P. 51 56 Lietuvos mokslų akademija, 2014 The Second International Conference on Psychosocial Oncology Psychosocial Support and Communication in Cancer Care:
Temporary pacemaker 삼성서울병원 심장혈관센터심장검사실 박정왜 RN, CCDS
 Temporary pacemaker 삼성서울병원 심장혈관센터심장검사실 박정왜 RN, CCDS NBG Codes 1st Letter 2nd Letter 3rd Letter A V D Chamber(s) Paced = atrium = ventricle = dual (both atrium and ventricle) Chamber(s) Sensed A = atrium
Temporary pacemaker 삼성서울병원 심장혈관센터심장검사실 박정왜 RN, CCDS NBG Codes 1st Letter 2nd Letter 3rd Letter A V D Chamber(s) Paced = atrium = ventricle = dual (both atrium and ventricle) Chamber(s) Sensed A = atrium
Pacing Codes and Modes Concepts
 Pacing Codes and Modes Concepts Pacing codes and modes concepts Objectives Upon completion of this program the participant will be able to: State what the first four positions of the NBG code represent.
Pacing Codes and Modes Concepts Pacing codes and modes concepts Objectives Upon completion of this program the participant will be able to: State what the first four positions of the NBG code represent.
Influence of enteral nutrition on the frequency of complications in case of major burns
 957 Influence of enteral nutrition on the frequency of complications in case of major burns Daiva Gudavičienė, Rytis Rimdeika, Kęstutis Adamonis 1 Division of Plastic Surgery and Burns, 1 Clinic of Gastroenterology,
957 Influence of enteral nutrition on the frequency of complications in case of major burns Daiva Gudavičienė, Rytis Rimdeika, Kęstutis Adamonis 1 Division of Plastic Surgery and Burns, 1 Clinic of Gastroenterology,
National Coverage Determination (NCD) for Cardiac Pacemakers (20.8)
 Page 1 of 12 Centers for Medicare & Medicaid Services National Coverage Determination (NCD) for Cardiac Pacemakers (20.8) Tracking Information Publication Number 100-3 Manual Section Number 20.8 Manual
Page 1 of 12 Centers for Medicare & Medicaid Services National Coverage Determination (NCD) for Cardiac Pacemakers (20.8) Tracking Information Publication Number 100-3 Manual Section Number 20.8 Manual
Demographic-clinical profile of the patients with Myasthenia gravis
 611 Demographic-clinical profile of the patients with Myasthenia gravis Daiva Rastenytė, Antanas Vaitkus 1, Rimas Neverauskas 1, Valerijus Pauza 2 Institute of Cardiology, Kaunas University of Medicine,
611 Demographic-clinical profile of the patients with Myasthenia gravis Daiva Rastenytė, Antanas Vaitkus 1, Rimas Neverauskas 1, Valerijus Pauza 2 Institute of Cardiology, Kaunas University of Medicine,
FREQUENTLY ASKED QUESTIONS
 FREQUENTLY ASKED QUESTIONS Micra TPS U.S. Private Payer Prior Authorization Micra Transcatheter Pacing System Most commercial payers in the United States do not have a positive coverage policy for Micra
FREQUENTLY ASKED QUESTIONS Micra TPS U.S. Private Payer Prior Authorization Micra Transcatheter Pacing System Most commercial payers in the United States do not have a positive coverage policy for Micra
EHRA Accreditation Exam - Sample MCQs Cardiac Pacing and ICDs
 EHRA Accreditation Exam - Sample MCQs Cardiac Pacing and ICDs Dear EHRA Member, Dear Colleague, As you know, the EHRA Accreditation Process is becoming increasingly recognised as an important step for
EHRA Accreditation Exam - Sample MCQs Cardiac Pacing and ICDs Dear EHRA Member, Dear Colleague, As you know, the EHRA Accreditation Process is becoming increasingly recognised as an important step for
Pediatric Pacemaker Implantation Endocardial or Epicardial
 Pediatric Pacemaker Implantation Endocardial or Epicardial HAITHAM BADRAN, MD, FEHRA CONSULTANT OF INTERVENTIONAL CARDIOLOGY CONSULTANT OF CARDIAC PACING AND ELECTROPHYSIOLOGY LECTURER OF CARDIOLOGY AIN
Pediatric Pacemaker Implantation Endocardial or Epicardial HAITHAM BADRAN, MD, FEHRA CONSULTANT OF INTERVENTIONAL CARDIOLOGY CONSULTANT OF CARDIAC PACING AND ELECTROPHYSIOLOGY LECTURER OF CARDIOLOGY AIN
CHANGES IN CARE MANAGEMENT AFTER FAST TRACK PROTOCOL INTRODUCTION FOR HIP FRACTURE PATIENTS
 126 BIOMEDICINA / BIOMEDICINE SVEIKATOS MOKSLAI / HEALTH SCIENCES IN EASTERN EUROPE ISSN 1392-6373 print / 2335-867X online 215, 25 tomas, Nr. 5, p. 126-13 DOI: http://doi.org/1.52/sm-hs.215.99 CHANGES
126 BIOMEDICINA / BIOMEDICINE SVEIKATOS MOKSLAI / HEALTH SCIENCES IN EASTERN EUROPE ISSN 1392-6373 print / 2335-867X online 215, 25 tomas, Nr. 5, p. 126-13 DOI: http://doi.org/1.52/sm-hs.215.99 CHANGES
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists. International authors and editors
 We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 4,000 116,000 120M Open access books available International authors and editors Downloads Our
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 4,000 116,000 120M Open access books available International authors and editors Downloads Our
Pacing Without Wires: Leadless Cardiac Pacing
 REVIEWS AND CONTEMPORARY UPDATES Ochsner Journal 16:238 242, 2016 Ó Academic Division of Ochsner Clinic Foundation Pacing Without Wires: Leadless Cardiac Pacing Michael L. Bernard, MD, PhD Department of
REVIEWS AND CONTEMPORARY UPDATES Ochsner Journal 16:238 242, 2016 Ó Academic Division of Ochsner Clinic Foundation Pacing Without Wires: Leadless Cardiac Pacing Michael L. Bernard, MD, PhD Department of
Trombozinė trombocitopeninė purpura (TTP) R. Petrauskaitė
 Trombozinė trombocitopeninė purpura (TTP) R. Petrauskaitė Pentada klinikinių simptomų Trombocitopenija Mikroangiopatinė hemolizinė anemija (MAHA) Fliuktuojantys neurologiniai simptomai Inkstų funkcijos
Trombozinė trombocitopeninė purpura (TTP) R. Petrauskaitė Pentada klinikinių simptomų Trombocitopenija Mikroangiopatinė hemolizinė anemija (MAHA) Fliuktuojantys neurologiniai simptomai Inkstų funkcijos
11/21/18. EKG Pop Quiz. Michael Giocondo, MD Cardiac Electrophysiology Saint Luke s Cardiovascular Consultants
 EKG Pop Quiz Michael Giocondo, MD Cardiac Electrophysiology Saint Luke s Cardiovascular Consultants 1 Disclosures No financial relationships to disclose. EKG #1 75 y/o woman with a dual-chamber pacemaker
EKG Pop Quiz Michael Giocondo, MD Cardiac Electrophysiology Saint Luke s Cardiovascular Consultants 1 Disclosures No financial relationships to disclose. EKG #1 75 y/o woman with a dual-chamber pacemaker
Leadless pacing: Technical challenges and concerns. Panos Vardas President Elect of the ESC, Prof. of Cardiology, Heraklion University Hospital
 Leadless pacing: Technical challenges and concerns Panos Vardas President Elect of the ESC, Prof. of Cardiology, Heraklion University Hospital Modest consultancy fees/honoraria from Bayer Boehringer Ingelheim
Leadless pacing: Technical challenges and concerns Panos Vardas President Elect of the ESC, Prof. of Cardiology, Heraklion University Hospital Modest consultancy fees/honoraria from Bayer Boehringer Ingelheim
MICRA TRANSCATHETER PACING SYSTEM (TPS) REIMBURSEMENT OVERVIEW
 MICRA TRANSCATHETER PACING SYSTEM (TPS) REIMBURSEMENT OVERVIEW MARCH 20, 2017 DISCLAIMER This presentation is intended only for educational use. Any duplication is prohibited without written consent of
MICRA TRANSCATHETER PACING SYSTEM (TPS) REIMBURSEMENT OVERVIEW MARCH 20, 2017 DISCLAIMER This presentation is intended only for educational use. Any duplication is prohibited without written consent of
INNOVATIONS IN DEVICE THERAPY:
 INNOVATIONS IN DEVICE THERAPY: Subcutaneous ICDs, Leadless Pacemakers, CRT Indications David J Wilber MD Loyola University Medical Center Disclosures: ACC Foundation: Consultant; Biosense / Webster: Consultant,
INNOVATIONS IN DEVICE THERAPY: Subcutaneous ICDs, Leadless Pacemakers, CRT Indications David J Wilber MD Loyola University Medical Center Disclosures: ACC Foundation: Consultant; Biosense / Webster: Consultant,
Leadless pacemakers and subcutaneous ICD s: will we use them for most of our patients? K.-H. Kuck Asklepios Klinik St. Georg Hamburg, Germany
 Leadless pacemakers and subcutaneous ICD s: will we use them for most of our patients? K.-H. Kuck Asklepios Klinik St. Georg Hamburg, Germany Disclosure Statement Research Grants Consultant / Advisory
Leadless pacemakers and subcutaneous ICD s: will we use them for most of our patients? K.-H. Kuck Asklepios Klinik St. Georg Hamburg, Germany Disclosure Statement Research Grants Consultant / Advisory
SERGANČIŲJŲ POINSULTINE PNEUMONIJA IR UROINFEKCIJA KLINIKINIŲ CHARAKTERISTIKŲ, GYDYMO TRUKMĖS IR KAŠTŲ SĄSAJOS
 92 SVEIKATOS EKONOMIKA IR VADYBA / HEALTH ECONOMICS AND MANAGEMENT SVEIKATOS MOKSLAI / HEALTH SCIENCES ISSN 2335-867X 2013, 23 tomas, Nr. 3, p. 92-97 doi:10.5200/sm-hs.2013.083 SERGANČIŲJŲ POINSULTINE
92 SVEIKATOS EKONOMIKA IR VADYBA / HEALTH ECONOMICS AND MANAGEMENT SVEIKATOS MOKSLAI / HEALTH SCIENCES ISSN 2335-867X 2013, 23 tomas, Nr. 3, p. 92-97 doi:10.5200/sm-hs.2013.083 SERGANČIŲJŲ POINSULTINE
Patient Resources: Arrhythmias and Congenital Heart Disease
 Patient Resources: Arrhythmias and Congenital Heart Disease Overview Arrhythmias (abnormal heart rhythms) can develop in patients with congenital heart disease (CHD) due to thickening/weakening of their
Patient Resources: Arrhythmias and Congenital Heart Disease Overview Arrhythmias (abnormal heart rhythms) can develop in patients with congenital heart disease (CHD) due to thickening/weakening of their
Cardiac Rhythm Management Coder 2018
 Cardiac Rhythm Management Coder 2018 An easy-to-use tool for coding and reimbursement compliance Prepared and Published By: MedLearn Publishing, A Division of MedLearn Media, Inc. 445 Minnesota Street,
Cardiac Rhythm Management Coder 2018 An easy-to-use tool for coding and reimbursement compliance Prepared and Published By: MedLearn Publishing, A Division of MedLearn Media, Inc. 445 Minnesota Street,
Update on Device Innovation (S-ICD, Wearable, Leadless)
 Update on Device Innovation (S-ICD, Wearable, Leadless) C. W. Israel Dept. of Medicine - Cardiology Evangelical Hospital Bielefeld J. W. Goethe University Frankfurt Carsten.Israel@em.uni-frankfurt.de Conflicts
Update on Device Innovation (S-ICD, Wearable, Leadless) C. W. Israel Dept. of Medicine - Cardiology Evangelical Hospital Bielefeld J. W. Goethe University Frankfurt Carsten.Israel@em.uni-frankfurt.de Conflicts
Key words: atrioventricular nodal reentrant tachycardia; ablation.
 632 Changes in electrophysiologic properties of the conductive system of the heart in children with atrioventricular nodal reentrant tachycardia after 2 8 years following radiofrequency catheter ablation
632 Changes in electrophysiologic properties of the conductive system of the heart in children with atrioventricular nodal reentrant tachycardia after 2 8 years following radiofrequency catheter ablation
MEDTRONIC CARELINK NETWORK FOR PACEMAKERS. Comparison between the Medtronic CareLink Network for Pacemakers and Transtelephonic Monitoring
 MEDTRONIC CARELINK NETWORK FOR PACEMAKERS Comparison between the Medtronic CareLink Network for Pacemakers and Transtelephonic Monitoring Transtelephonic Monitoring Transmission What can you determine
MEDTRONIC CARELINK NETWORK FOR PACEMAKERS Comparison between the Medtronic CareLink Network for Pacemakers and Transtelephonic Monitoring Transtelephonic Monitoring Transmission What can you determine
ICD: Basics, Programming and Trouble-shooting
 ICD: Basics, Programming and Trouble-shooting Amir AbdelWahab, MD Electrophysiology and Pacing Service Cardiology Department Cairo University Feb 2013 Evolution of ICD Technology ICD Evolution Indications
ICD: Basics, Programming and Trouble-shooting Amir AbdelWahab, MD Electrophysiology and Pacing Service Cardiology Department Cairo University Feb 2013 Evolution of ICD Technology ICD Evolution Indications
Index. cardiology.theclinics.com. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A ACHD. See Adult congenital heart disease (ACHD) Adult congenital heart disease (ACHD), 503 512 across life span prevalence of, 504 506
Index Note: Page numbers of article titles are in boldface type. A ACHD. See Adult congenital heart disease (ACHD) Adult congenital heart disease (ACHD), 503 512 across life span prevalence of, 504 506
2009 CPT Codes for Cardiac Device Monitoring
 2009 CPT Codes for Cardiac Device Monitoring December 2008 Notices Current Procedural Terminology (CPT ) is copyright 2008 American Medical Association. All Rights reserved. No fee schedules, basic units,
2009 CPT Codes for Cardiac Device Monitoring December 2008 Notices Current Procedural Terminology (CPT ) is copyright 2008 American Medical Association. All Rights reserved. No fee schedules, basic units,
Supplemental Material
 Supplemental Material 1 Table S1. Codes for Patient Selection Cohort Codes Primary PM CPT: 33206 or 33207 or 33208 (without 33225) ICD-9 proc: 37.81, 37.82, 37.83 Primary ICD Replacement PM Replacement
Supplemental Material 1 Table S1. Codes for Patient Selection Cohort Codes Primary PM CPT: 33206 or 33207 or 33208 (without 33225) ICD-9 proc: 37.81, 37.82, 37.83 Primary ICD Replacement PM Replacement
CARDIAC DEVICE MR-CONDITIONAL PRODUCT SUMMARY CHART
 CARDIAC DEVICE MR-CONDITIONAL PRODUCT SUMMARY CHART December 2015 This chart encompasses all Medtronic cardiac devices FDA-Approved as MR Conditional and included in the MRI SureScan portfolio. If a device
CARDIAC DEVICE MR-CONDITIONAL PRODUCT SUMMARY CHART December 2015 This chart encompasses all Medtronic cardiac devices FDA-Approved as MR Conditional and included in the MRI SureScan portfolio. If a device
ATRIAL FIBRILLATION ANSWERS. A Patient Education Handbook on Electrophysiology
 ATRIAL FIBRILLATION ANSWERS A Patient Education Handbook on Electrophysiology MY LIFE HAS TAKEN A TURN FOR THE BETTER. -Emie Bishop ARRHYTHMIAANSWERS.COM AF ANSWERS. A PATIENT EDUCATION HANDBOOK ON ELECTROPHYSIOLOGY
ATRIAL FIBRILLATION ANSWERS A Patient Education Handbook on Electrophysiology MY LIFE HAS TAKEN A TURN FOR THE BETTER. -Emie Bishop ARRHYTHMIAANSWERS.COM AF ANSWERS. A PATIENT EDUCATION HANDBOOK ON ELECTROPHYSIOLOGY
Newer pacemakers also can monitor your blood temperature, breathing, and other factors and adjust your heart rate to changes in your activity.
 Pacemakers & Defibrillators A pacemaker system consists of a battery, a computerized generator and wires with sensors called electrodes on one end. The battery powers the generator, and both are surrounded
Pacemakers & Defibrillators A pacemaker system consists of a battery, a computerized generator and wires with sensors called electrodes on one end. The battery powers the generator, and both are surrounded
The prevalence and risk factors of low-energy fractures among postmenopausal women with osteoporosis in Belarus
 Gerontologija 2014; 15(3): 143 147 GERONTOLOGIJA Original article The prevalence and risk factors of low-energy fractures among postmenopausal women with osteoporosis in Belarus Ema Rudenka 1, Natalya
Gerontologija 2014; 15(3): 143 147 GERONTOLOGIJA Original article The prevalence and risk factors of low-energy fractures among postmenopausal women with osteoporosis in Belarus Ema Rudenka 1, Natalya
CRF procedure PROCEDURE FLOW. PATIENT ASSESSMENT Symptoms and indication. Pacemaker & ICD registration. Procedures. Procedure ICD.
 Pacemaker & ICD registration Procedures CRF procedure PROCEDURE FLOW Procedure ICD ICD PM ICM lead only PATIENT ASSESSMENT Symptoms and indication Symptoms and events (multiple possibilities) Asymptomatic
Pacemaker & ICD registration Procedures CRF procedure PROCEDURE FLOW Procedure ICD ICD PM ICM lead only PATIENT ASSESSMENT Symptoms and indication Symptoms and events (multiple possibilities) Asymptomatic
Cardiac Rhythm Management Coder 2017
 Cardiac Rhythm Management Coder 2017 An easy-to-use tool for coding and reimbursement compliance Prepared and Published By: MedLearn Publishing, A Division of Panacea Healthcare Solutions, Inc. 287 East
Cardiac Rhythm Management Coder 2017 An easy-to-use tool for coding and reimbursement compliance Prepared and Published By: MedLearn Publishing, A Division of Panacea Healthcare Solutions, Inc. 287 East
UnitedHealthcare Medicare Advantage Cardiology Prior Authorization Program
 Electrophysiology Implant Classification Table The table below contains the codes that apply to our UnitedHealthcare Medicare Advantage cardiology prior Description Includes Generator Placement Includes
Electrophysiology Implant Classification Table The table below contains the codes that apply to our UnitedHealthcare Medicare Advantage cardiology prior Description Includes Generator Placement Includes
PACING AT THE BUNDLE OF HIS
 PACING AT THE BUNDLE OF HIS Cardiac Rhythm Heart Failure Coding Corner Disclaimer While Medtronic offers opinions about how to code when using our technology according to its FDA-approved labeling, these
PACING AT THE BUNDLE OF HIS Cardiac Rhythm Heart Failure Coding Corner Disclaimer While Medtronic offers opinions about how to code when using our technology according to its FDA-approved labeling, these
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Medical technology guidance FINAL SCOPE ENDURALIFE-powered CRT-D devices for the treatment of heart failure 1 Technology 1.1 Description of the technology
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Medical technology guidance FINAL SCOPE ENDURALIFE-powered CRT-D devices for the treatment of heart failure 1 Technology 1.1 Description of the technology
PACEMAKER INTERPRETATION AND DEVICE MANAGEMENT PART I
 1 PACEMAKER INTERPRETATION AND DEVICE MANAGEMENT PART I Cynthia Webner DNP, RN, CCNS, CCRN-CMC Karen Marzlin DNP, RN, CCNS, CCRN-CMC 2 PROFESSIONAL NURSING PRACTICE CAN ONLY ADVANCE AS MUCH AS INDIVIDUAL
1 PACEMAKER INTERPRETATION AND DEVICE MANAGEMENT PART I Cynthia Webner DNP, RN, CCNS, CCRN-CMC Karen Marzlin DNP, RN, CCNS, CCRN-CMC 2 PROFESSIONAL NURSING PRACTICE CAN ONLY ADVANCE AS MUCH AS INDIVIDUAL
MRI SURESCAN SYSTEMS. Patient Scanning Process PATIENT PRESCREENING. SureScan Pacing, Defibrillation, and CRT-D Systems Verification
 MRI SURESCAN SYSTEMS Patient Scanning Process PATIENT PRESCREENING SureScan Pacing, Defibrillation, and CRT-D Systems Verification Verify that patient has a complete SureScan pacing, defibrillation, or
MRI SURESCAN SYSTEMS Patient Scanning Process PATIENT PRESCREENING SureScan Pacing, Defibrillation, and CRT-D Systems Verification Verify that patient has a complete SureScan pacing, defibrillation, or
Model 5392 EPG Temporary Pacer
 Model 5392 EPG Temporary Pacer Compatible Components Reference Card 5392 Surgical Cables 5487 Disposable, short 5487L Disposable, long 5832S Reusable, small clip 5833S 5833SL Disposable, small clip, short
Model 5392 EPG Temporary Pacer Compatible Components Reference Card 5392 Surgical Cables 5487 Disposable, short 5487L Disposable, long 5832S Reusable, small clip 5833S 5833SL Disposable, small clip, short
Cardiac Pacing. Learning outcomes. Introduction. The cardiac impulse - its formation and its failure CHAPTER. To understand:
 Cardiac Pacing CHAPTER 10 Learning outcomes To understand: The indications for cardiac pacing in the peri-arrest setting How to perform percussion pacing How to apply non-invasive, transcutaneous electrical
Cardiac Pacing CHAPTER 10 Learning outcomes To understand: The indications for cardiac pacing in the peri-arrest setting How to perform percussion pacing How to apply non-invasive, transcutaneous electrical
Cardiovascular Nursing Practice: A Comprehensive Resource Manual and Study Guide for Clinical Nurses 2 nd Edition
 Cardiovascular Nursing Practice: A Comprehensive Resource Manual and Study Guide for Clinical Nurses 2 nd Edition Table of Contents Volume 1 Chapter 1: Cardiovascular Anatomy and Physiology Basic Cardiac
Cardiovascular Nursing Practice: A Comprehensive Resource Manual and Study Guide for Clinical Nurses 2 nd Edition Table of Contents Volume 1 Chapter 1: Cardiovascular Anatomy and Physiology Basic Cardiac
PATIENT WITH ARRHYTHMIA IN DENTIST S OFFICE. Małgorzata Kurpesa, MD., PhD. Chair&Department of Cardiology
 PATIENT WITH ARRHYTHMIA IN DENTIST S OFFICE Małgorzata Kurpesa, MD., PhD. Chair&Department of Cardiology Medical University of Łódź The heart is made up of four chambers Left Atrium Right Atrium Left Ventricle
PATIENT WITH ARRHYTHMIA IN DENTIST S OFFICE Małgorzata Kurpesa, MD., PhD. Chair&Department of Cardiology Medical University of Łódź The heart is made up of four chambers Left Atrium Right Atrium Left Ventricle
Quality Standards for the Implantation of Cardiac Rhythm Management Devices. Pan- London Arrhythmia Project Group. Version 3 (18 th July 2011)
 Quality Standards for the Implantation of Cardiac Rhythm Management Devices Pan- London Arrhythmia Project Group Version 3 (18 th July 2011) 1 Standards for Implantation of Permanent Pacemakers (including
Quality Standards for the Implantation of Cardiac Rhythm Management Devices Pan- London Arrhythmia Project Group Version 3 (18 th July 2011) 1 Standards for Implantation of Permanent Pacemakers (including
Pediatrics. Arrhythmias in Children: Bradycardia and Tachycardia Diagnosis and Treatment. Overview
 Pediatrics Arrhythmias in Children: Bradycardia and Tachycardia Diagnosis and Treatment See online here The most common form of cardiac arrhythmia in children is sinus tachycardia which can be caused by
Pediatrics Arrhythmias in Children: Bradycardia and Tachycardia Diagnosis and Treatment See online here The most common form of cardiac arrhythmia in children is sinus tachycardia which can be caused by
Implantable Defibrillators Market by Product Type [Transvenous Implantable Cardioverter-Defibrillator (T-ICDs), Subcutaneous Implantable Cardioverter
 Implantable Defibrillators Market by Product Type [Transvenous Implantable Cardioverter-Defibrillator (T-ICDs), Subcutaneous Implantable Cardioverter Defibrillators (S-ICDS), and Cardiac Resynchronization
Implantable Defibrillators Market by Product Type [Transvenous Implantable Cardioverter-Defibrillator (T-ICDs), Subcutaneous Implantable Cardioverter Defibrillators (S-ICDS), and Cardiac Resynchronization
BHRS Prep course Pub style Quiz NOT A
 BHRS Prep course Pub style Quiz NOT A Round 1 Quiz questions Common ECG s in CRM Colin Cunnington Over the past 3 months, a 24-yearold man who works as a truck driver has had frequent episodes of pre-syncope
BHRS Prep course Pub style Quiz NOT A Round 1 Quiz questions Common ECG s in CRM Colin Cunnington Over the past 3 months, a 24-yearold man who works as a truck driver has had frequent episodes of pre-syncope
Cardiac resynchronisation therapy (biventricular pacing) for the treatment of heart failure
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Health Technology Appraisal for the treatment of heart failure Final scope Appraisal objective To appraise the clinical and cost effectiveness of cardiac
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Health Technology Appraisal for the treatment of heart failure Final scope Appraisal objective To appraise the clinical and cost effectiveness of cardiac
Cardiac Imaging in abnormal rhythm Role of MDCT
 Cardiac Imaging in abnormal rhythm Role of MDCT Cardiac Imaging in abnormal rhythm Role of MDCT Scope of the problem CT in Atrial Fibrillation CT and pacing Ventricular arrhythmia Other applications 1
Cardiac Imaging in abnormal rhythm Role of MDCT Cardiac Imaging in abnormal rhythm Role of MDCT Scope of the problem CT in Atrial Fibrillation CT and pacing Ventricular arrhythmia Other applications 1
PERMANENT PACEMAKERS AND IMPLANTABLE DEFIBRILLATORS Considerations for intensivists
 PERMANENT PACEMAKERS AND IMPLANTABLE DEFIBRILLATORS Considerations for intensivists Craig A. McPherson, MD, FACC Associate Professor of Medicine Constantine Manthous, MD, FACP, FCCP Associate Clinical
PERMANENT PACEMAKERS AND IMPLANTABLE DEFIBRILLATORS Considerations for intensivists Craig A. McPherson, MD, FACC Associate Professor of Medicine Constantine Manthous, MD, FACP, FCCP Associate Clinical
ASSESSING THE INCIDENCE RATES OF SUBSTANCE USE DISORDERS AMONG THOSE WITH ANTISOCIAL AND BORDERLINE PERSONALITY DISORDERS IN RURAL SETTINGS
 ASSESSING THE INCIDENCE RATES OF SUBSTANCE USE DISORDERS AMONG THOSE WITH ANTISOCIAL AND BORDERLINE PERSONALITY DISORDERS IN RURAL SETTINGS Jacob X. Chávez, Julie A. Dinsmore 1, David D. Hof University
ASSESSING THE INCIDENCE RATES OF SUBSTANCE USE DISORDERS AMONG THOSE WITH ANTISOCIAL AND BORDERLINE PERSONALITY DISORDERS IN RURAL SETTINGS Jacob X. Chávez, Julie A. Dinsmore 1, David D. Hof University
AF Today: W. For the majority of patients with atrial. are the Options? Chris Case
 AF Today: W hat are the Options? Management strategies for patients with atrial fibrillation should depend on the individual patient. Treatment with medications seems adequate for most patients with atrial
AF Today: W hat are the Options? Management strategies for patients with atrial fibrillation should depend on the individual patient. Treatment with medications seems adequate for most patients with atrial
Evaluation of stereotactic radiosurgery in the treatment of trigeminal neuralgia
 Medical sciences 1 (2017) 1 6 Evaluation of stereotactic radiosurgery in the treatment of trigeminal neuralgia Aivaras Grigonis, Gabija Solovjovaitė Lithuanian university of health sciences, faculty of
Medical sciences 1 (2017) 1 6 Evaluation of stereotactic radiosurgery in the treatment of trigeminal neuralgia Aivaras Grigonis, Gabija Solovjovaitė Lithuanian university of health sciences, faculty of
Puzzling Pacemakers Cheryl Herrmann, APN, CCRN, CCNS-CSC-CMC
 Puzzling Pacemakers Cheryl Herrmann, APN, CCRN, CCNS-CSC-CMC Pacemaker: An electric device implanted in the body to regulate the heart beat. Delivers electrical stimuli over leads with electrodes in contact
Puzzling Pacemakers Cheryl Herrmann, APN, CCRN, CCNS-CSC-CMC Pacemaker: An electric device implanted in the body to regulate the heart beat. Delivers electrical stimuli over leads with electrodes in contact
Summary of the Final 2013 Policy, Payment Changes For Hospital Outpatient Departments
 Summary of the Final 2013 Policy, Payment Changes For Hospital Outpatient Departments A. Introduction On November 1, 2012, the Centers for Medicare & Medicaid Services (CMS) issued a final rule that will
Summary of the Final 2013 Policy, Payment Changes For Hospital Outpatient Departments A. Introduction On November 1, 2012, the Centers for Medicare & Medicaid Services (CMS) issued a final rule that will
Cardiac implantable electronic devices (CIEDs) in children include pacemakers and implantable cardioverter defibrillators (ICDs).
 Management of Children with Cardiac Devices Guideline originally developed by Leann Miles, APRN; Lindsey Pumphrey, RN; Srikant Das, MD, and the ANGELS Team. Last reviewed by Lindsey Pumphrey, RN, Srikant
Management of Children with Cardiac Devices Guideline originally developed by Leann Miles, APRN; Lindsey Pumphrey, RN; Srikant Das, MD, and the ANGELS Team. Last reviewed by Lindsey Pumphrey, RN, Srikant
Is This Thing Working?
 Is This *#@!* Thing Working? Pacemaker (and ICD) ECG and Telemetry Pitfalls Wayne O. Adkisson, MD adki0004@umn.edu Disclosures I currently receive research support from Medtronic, Inc. I have been compensated
Is This *#@!* Thing Working? Pacemaker (and ICD) ECG and Telemetry Pitfalls Wayne O. Adkisson, MD adki0004@umn.edu Disclosures I currently receive research support from Medtronic, Inc. I have been compensated
Subcutaneous implantable cardioverter defibrillator insertion for preventing sudden cardiac death
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Subcutaneous implantable cardioverter defibrillator insertion for preventing sudden cardiac death A subcutaneous
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Subcutaneous implantable cardioverter defibrillator insertion for preventing sudden cardiac death A subcutaneous
UnitedHealthcare, UnitedHealthcare of the River Valley and Neighborhood Health Partnership Cardiology Notification and Prior Authorization Program:
 Notification and Prior Authorization Program: Electrophysiology Implant Classification Table The following chart contains the codes that require notification or prior authorization as part of UnitedHealthcare
Notification and Prior Authorization Program: Electrophysiology Implant Classification Table The following chart contains the codes that require notification or prior authorization as part of UnitedHealthcare
IHCP bulletin INDIANA HEALTH COVERAGE PROGRAMS BT JANUARY 24, 2012
 IHCP bulletin INDIANA HEALTH COVERAGE PROGRAMS BT201203 JANUARY 24, 2012 The IHCP to reimburse implantable cardioverter defibrillators separately from outpatient implantation Effective March 1, 2012, the
IHCP bulletin INDIANA HEALTH COVERAGE PROGRAMS BT201203 JANUARY 24, 2012 The IHCP to reimburse implantable cardioverter defibrillators separately from outpatient implantation Effective March 1, 2012, the
Epidemiology of burns in Lithuania during
 541 Epidemiology of burns in Lithuania during 1991 2004 Department of Plastic and Reconstructive Surgery, Kaunas University of Medicine, Lithuania Key words: burns; epidemiology; Lithuania. Summary. The
541 Epidemiology of burns in Lithuania during 1991 2004 Department of Plastic and Reconstructive Surgery, Kaunas University of Medicine, Lithuania Key words: burns; epidemiology; Lithuania. Summary. The
Peri-operative management of pacemakers and implantable cardiac defibrillators
 Guideline Title: Peri-operative management of patients fitted with Permanent Pacemakers (PPMs) and Implantable Cardioverter Defibrillators (ICDs). Author(s): Jessica Osman (Chief Pacing Physiologist),
Guideline Title: Peri-operative management of patients fitted with Permanent Pacemakers (PPMs) and Implantable Cardioverter Defibrillators (ICDs). Author(s): Jessica Osman (Chief Pacing Physiologist),
Clinical characteristics and long-term outcomes of 35 patients with Wegener s granulomatosis followed up at two rheumatology centers in Lithuania
 256 Medicina (Kaunas) 2010;46(4):256-60 Clinical characteristics and long-term outcomes of 35 patients with Wegener s granulomatosis followed up at two rheumatology centers in Lithuania Jolanta Dadonienė
256 Medicina (Kaunas) 2010;46(4):256-60 Clinical characteristics and long-term outcomes of 35 patients with Wegener s granulomatosis followed up at two rheumatology centers in Lithuania Jolanta Dadonienė
1. CARDIOLOGY. These listings cannot be correctly interpreted without reference to the Preamble. Anes. $ Level
 1. CARDIOLOGY These listings cannot be correctly interpreted without reference to the Preamble. Anes. Referred Cases 33010 Consultation: To consist of examination, review of history, laboratory, X-ray
1. CARDIOLOGY These listings cannot be correctly interpreted without reference to the Preamble. Anes. Referred Cases 33010 Consultation: To consist of examination, review of history, laboratory, X-ray
Increasing attendance in a cervical cancer screening programme by personal invitation: experience of a Lithuanian primary health care centre
 ACTA MEDICA LITUANICA. 2016. Vol. 23. No. 3. P. 180 184 Lietuvos mokslų akademija, 2016 Increasing attendance in a cervical cancer screening programme by personal invitation: experience of a Lithuanian
ACTA MEDICA LITUANICA. 2016. Vol. 23. No. 3. P. 180 184 Lietuvos mokslų akademija, 2016 Increasing attendance in a cervical cancer screening programme by personal invitation: experience of a Lithuanian
