Japan Lifeline Co., Ltd.
|
|
|
- Shauna Morton
- 5 years ago
- Views:
Transcription
1 7575 Tokyo Stock Exchange First Section Analyst Kimiteru Miyata
2 Index Summary Business overview Business description Distribution structure Condition by product category Business outlook The turning point Trends in FY3/ Sales trends by product in FY3/ FY3/19 outlook Outlook of net sales by product in FY3/ The medium-term management plan The medium-term management plan Developments for medium-term growth Shareholder return policy Information security
3 Summary Stable, high growth of 20% in FY3/18 and the same forecast for FY3/19 also, based on actual capabilities <7575> (hereafter, also the Company ) is both an import trading company and a manufacturer, and it is an independent, hybrid trading company specializing in medical devices. Japan s medical devices market is gradually expanding against the backdrop of the aging population. Within it, the cardiovascular field has shown higher growth than other fields due to the expansion of indicated cases resulting from the advancements being made in medical devices, in addition to the aging of the population. In this sort of market environment, the Company is expanding its business by increasing the overseas products it handles as an import trading company specializing in medical devices, while also strengthening the development of its in-house products as a manufacturer. In Japan s medical devices market, except for some diagnostic equipment such as magnetic resonance imaging (MRI) and endoscopes, the level of dependence on foreign manufacturers, particularly from the United States and Europe, is high for advanced medical devices, including cardiac pacemakers used for direct treatment. Since its establishment, the Company has built a network of domestic medical sites by concluding exclusive sales agreements with overseas manufacturers and introducing cutting-edge medical devices into these sites in Japan. Moreover, on behalf of these overseas manufacturers, it engages in all of the processes other than manufacturing, such as domestic regulatory applications, marketing, education, and sales promotions. On the other hand, the Company s in-house manufactured products include many with high market shares domestically, including vascular grafts and catheters for use in internal atrial cardioversion systems, and it has an excellent reputation as a manufacturer. In this way, it does not simply buy and sell products, it also plays the role of a manufacturer or close to that of a manufacturer, so one of its features is high profitability. Currently, as a specialist in the cardiovascular field with a unique business form from handling both highly original purchased products and in-house manufactured products, it can be counted among the companies that are driving the industry. The FY3/18 results were positive, with net sales of 42,298mn (up 13.8% year-on-year (YoY)) and operating income of 10,671mn (up 38.9%). In the cardiovascular field, in addition to the increase in the number of cases of disease due to the aging of the population, through the advancement of medical devices, it has become possible to treat cases that were previously difficult to treat. In particular, the number of cases of atrial fibrillation treatment continues to increase at a high level, and alongside this, sales have especially grown of the highly profitable only-one products, which was a factor behind the positive results. Also, following the absorption merger of the consolidated subsidiary JUNKEN MEDICAL Co., Ltd., the adjustment of an unrealized gain for inventory purchased before the merger became a temporary factor pushing-up operating income. The forecasts for the FY3/19 results are for net sales of 49,411mn (up 16.8% YoY) and operating income of 11,202mn (up 5.0%). Continuing from before, the number of cases of atrial fibrillation treatment is expected to increase, and in addition, the Company will launch highly anticipated large-scale products, so net sales are forecast to grow significantly. However, in addition to the end of the temporary profits that were recorded in FY3/18 following the merger with the subsidiary, profits seem to be slowing, including due to the revisions to the insurance reimbursement prices, promotions costs for new large-scale products, and development costs for in-house manufactured products. However, even when deducting temporary profits and converting the results to on an actual-capabilities basis, we find that in FY3/18, operating income was still 9,201mn and that operating income is continuing to grow at the high rate of around 20% for 2 consecutive years, of 19.7% in FY3/18 and 21.7% in FY3/
4 Summary On May 29, 2018, the Company updated its medium-term management plan released in 2017, and the Company is now aiming for net sales of 77.7bn and an operating income margin of 25% in FY3/23. On considering factors such as the aging of the population in Japan, the advancements being made in medical devices, and the Company s sales and regulatory structures and its production system, these targets would seem to be fully within an achievable range. In addition, it is working on expanding its business domains, such as by entering into new business fields other than the cardiovascular field and strengthening exports supported by the construction of factories overseas. Its profits at the current time are also a strength that we can expect a further medium-term growth above the forecast. Key Points A hybrid import trading company and manufacturer specializing in medical devices. It is benefitting from the external environment, including the aging of the population and the advancements being made in medical devices Its features include its sales and regulatory structures, purchased products with exclusive sales agreements, and in-house manufactured products that meet the needs of medical sites. It has a highly profitable structure Profits may seem to slow in FY3/19, but this will be due to the recording of temporary profits in FY3/18. On an actual-capabilities basis, operating income will grow by around 20% for the second consecutive year. Source: Prepared by FISCO from the Company s financial results 02 18
5 Business overview Features include a sales system, a regulatory system, the domestic launches of advanced products, and products developed according to needs 1. Business description From the time it was founded for the sales of imported cardiac pacemakers in 1981 up to the present day, the Company has expanded its sales bases nationwide, while importing into Japan the latest medical devices from overseas. It has advanced expertise as a specialist trading company in medical devices and communicates closely with doctors to cultivate the power of discernment for products. Furthermore, it is utilizing its network of doctors who are active on the medical frontline to develop medical products precisely tailored to meet the needs of medical sites. Also, regulatory approval is required to introduce a medical device, and the Company is strengthening its regulatory department structure based on its experience of introducing devices over many years, and it is able to smoothly progress this process, including for the acquisition of data showing a product s safety and effectiveness and negotiations with the administration. In addition to its features of having a nationwide sales structure, an enhanced regulatory structure, domestic introduction of advanced product and a development system for products meeting needs, it is independent. Therefore, for overseas manufacturers that do not have sales channels in Japan, the Company can be said to be extremely trustworthy and appealing as a sales partner. The Company's growth foundation Source: The Company's medium-term management plan briefing materials 03 18
6 Business overview As a specialist trading company and manufacturer, it plays many roles in the cardiovascular medical devices industry 2. Distribution structure As its sales structure, the Company has 47 sales bases (as of April 2018) throughout Japan, from Hokkaido to Okinawa, with staff who possess high-level specialist knowledge and who support medical practitioners. Its customers include medical institutions and sales agencies, although it sells few devices directly to medical institutions and most are sold via sales agencies. Sales representative focus on specialist operations, including providing product information to medical institutions, and the cooperation they obtain from sales agencies, such as for supplementing product inventory and for sales, enables them to conduct sales efficiently. For purchased products, the Company concludes exclusive sales agreements with overseas manufacturers, mainly in Europe and the United States, which means it ties up with a single partner in a particular product category. Also, it takes the same position as that of manufacturers for aspects such as the acquisition of regulatory approval within Japan, marketing smoothly to spread the use of medical devices through academic societies and related, and education for medical institutions. Further, as there is a commercial practice unique to the medical devices industry of deposit sales, which entails depositing a product at a medical facility and recording it as sales when it is used, manufacturers have to bear the inventory burden instead of sales agencies. However, as a result of handling products with an exclusive sales contract, the Company s gross profit margin on its purchased products averages from 40-45%, which is extremely high compared to the margins of trading companies. In general, sales agencies conducting secondary distribution in Japan handle the products of multiple companies, and although their stock burden is light, many of these companies have a gross profit margin of below 20%. Based on this, there is a clear differentiation between the business structures of the Company and general trading companies. The Company develops and manufactures in-house products as a manufacturer. It currently has one R&D base (the Research Center) and three manufacturing bases (the Toda Factory, Oyama Factory, and Ichihara Factory) in Japan. The products that the Company develops and manufactures in-house are guide wires, EP catheters, and ablation catheters, while it has also expanded its portfolio through M&A. The gross profit margin of the Company s in-house products is relatively high, and of the Company s in-house products, those that are one-of-a-kind products have an even higher gross profit margin. In 2009, it acquired Ube Junken Co., Ltd. (name subsequently changed to JUNKEN MEDICAL Co. Ltd.), which at that time was the only manufacturer of vascular grafts in Japan, and it incorporated these vascular grafts into its lineup of in-house manufactured products. Also, in 2010, it made subsidiaries of the SYNEXMED Group (companies in Hong Kong and Shenzhen), which manufactured guide wires and balloon catheters, and newly added balloon catheters to its lineup of in-house manufactured products. JUNKEN MEDICAL merged with the Company in April 2017 through an absorption-type merger toward realizing synergies and improved efficiency
7 Business overview Schematic diagram of the business Source: The Company s results briefing materials An abundant product lineup specializing in cardiovascular diseases 3. Condition by product category The Company has four product categories; Cardiac Rhythm Management, EP/Ablation, Cardiovascular & Vascular Surgery, and Transvascular Intervention. In terms of the percentages of sales, in FY3/18, Cardiac Rhythm Management contributed 17.1%, EP/Ablation 48.1%, Cardiovascular & Vascular Surgery 27.1%, and Transvascular Intervention 7.6%. ) Note: In line with the subsidiary merger, the product category Others has been reclassified as Cardiovascular & Vascular Surgery starting from FY3/18. Source: Prepared by FISCO from the Company s results briefing materials 05 18
8 Business overview a) Cardiac Rhythm Management Cardiac Rhythm Management handles for treating cardiac arrhythmia. The main products are implantable devices that use electrical stimulation to ensure that the heart beats normally, including cardiac pacemakers, ICDs (implantable cardioverter defibrillators), and CRT-Ds (cardiac resynchronization therapy defibrillators). It also handles AEDs (automated external defibrillators). In the cardiac pacemaker market, MRI-compliant pacemakers have rapidly become the mainstream product in recent years, so the Company has also launched the KORA 250 and other MRI-compliant products. All of the items in this segment are purchased products. The cardiac pacemaker (LivaNova) is an implantable medical device that is used when the pulse is less than normal. It constantly monitors the heart beat and when it senses that the pulse has been interrupted, it sends out an electrical stimulus and returns the heart beat to normal. The ICD (implantable cardioverter defibrillator, LivaNova) automatically senses high-risk arrhythmias, such as sudden ventricular fibrillation and ventricular tachycardia, and also returns the heart beat to normal by performing electrical therapy. In the event of severe heart failure, the CRT-D (cardiac resynchronization therapy defibrillator, LivaNova) prepares for ventricular dyssynchrony and improves the heart s pump function by electrically stimulating both the left and right ventricles of the heart, while it also has a defibrillation function, the same as the ICD. The pacemaker lead (LivaNova) is a conducting wire used to connect to the cardiac pacemaker in order to transmit an electrical stimulation to the myocardium. The ICD lead (LivaNova) for transmitting the electrical stimulation emitted by the ICD to the myocardium is equipped with a coil for treatment by electric shock. The event recorder (LivaNova) is an extracorporeal electrocardiograph that detects and records cardiac events. It can confirm changes in an electrocardiogram and its uses include the analysis of arrhythmia. The AED (automated external defibrillator, NANOOMTECH) automatically determines the state of the heart, and when it senses it is in a convulsive state, such as ventricular fibrillation, it gives it an electric shock and returns it to a normal state. The Company is supplied with cardiac pacemakers and related products by LivaNova. The number of units sold had declined due to a delay in launching pacemakers compliant with MRI, but in FY3/18, its market share recovered to 15%. However, in FY3/19, as the number of insurance points for remote medical treatment has increased following the revisions to medical fees in April, the needs of medical facilities have shifted toward remote monitoring. But there is a problem with the supply capabilities of LivaNova PLC for remote monitoring devices, and it estimated that sales volume in FY3/19 would decline YoY. Cardiac Rhythm Management Source: The Company's results briefing materials b) EP/Ablation In EP/Ablation, the Company handles disposable electrode-equipped catheter for the examination and treatment of arrhythmia. In FY3/18, the number of cases increased 22% YoY, and in recent years, the number of cases of atrial fibrillation treated with ablation has been rapidly increasing. A catheter broadly refers to a medical device that is a hollow, soft, and thin tube that is inserted into a blood vessel from the surface of the skin to provide treatment
9 Business overview Forecast number of cases of EP/ABL ablation Source: The Company's medium-term management plan briefing materials The EP catheter (Japan Lifeline) is a thin catheter with an electrode used to measure the electric potential in the heart and to identify the part causing the arrhythmia. In order to be able to inspect the various parts within the heart, it comes in an abundance of types, including with a curve-shaped tip and also in terms of the number and positions of the electrodes and the thinness. There is also a single-directional type (turns to one side), in which the tip is curved and there is a grip lever for the hand, and a bi-directional type (turns to both sides), and the lineups of both types come with a variety of curves. The ablation catheter (Japan Lifeline) is an electrode catheter that locally cauterizes and treats the stimulation conduction pathway causing local arrhythmia (tachycardia) with a high frequency current. The same as the EP catheter, it comes in an abundance of types so that various parts of the heart can be cauterized. In addition, the irrigation catheter is one type of ablation catheter, and at the tip of the catheter there is a hole that enables the injection of a physiological saline solution. It has a function to reduce the occurrence of thrombus in the body by performing cauterization, while cooling the tip electrode. The internal atrial cardioversion system (Japan Lifeline) is a system that performs defibrillation within the heart, such as for atrial fibrillation that can occur during ablation treatment. Since it has effects with lower energy compared to defibrillation from outside of the body, it can be performed less invasively (reducing the physical burden on the patient). Compared to other ablations, ablation treatment for atrial fibrillation has a high rate of incidence of complications of esophageal esophagitis and esophageal ulcers, especially in the left atrium with a high mortality rate and the esophageal fistula through which the esophagus penetrates. The esophageal temperature monitoring system (Japan Lifeline) prevents these complications by continuously monitoring the temperature of the esophagus during ablation treatment. EP/Ablation catheter Source: The Company's results briefing materials 07 18
10 Business overview c) Cardiovascular & Vascular Surgery Cardiovascular & Vascular Surgery handles medical devices used for surgical treatments to replace with artificial organs those blood vessels or heart valves that have lost their functioning. Main in-house manufactured products are vascular grafts and open stent grafts, both of which had been manufactured by JUNKEN MEDICAL, which the Company merged with and absorbed in April Main purchased products include thoracic and abdominal stent grafts, prosthetic heart valves, and annuloplasty rings. The AFX Stent Graft System for the abdomen has proven popular, and it has acquired over 15% market share in FY3/18. Stent grafts are medical devices used to treat aortic aneurysms, the same as vascular grafts. In contrast to a vascular graft requiring open-chest surgery, a stent graft is a medical device made up of spring-like metal tubes called stents, which remain in a contracted state within the catheter from the blood vessel at the base of the foot until inserted at the treatment site, at which point the force of the springs press against the blood vessel to fix it in place. The vascular graft (Japan Lifeline) is a medical device for the treatment of conditions such as thoracic and abdominal aortic aneurysms, and it is used for the replacement of blood vessels and to bypass blocked blood vessels. The open stent graft (Japan Lifeline) is a medical device to treat thoracic aortic disease. When replacing thoracic aorta with vascular grafts over a wide range, in the case of treatment only using conventional vascular grafts, two open-chest surgeries are required. But by using the open stent grafts, the treatment can be completed with just one open-chest surgery, which shortens the surgery time and reduces the physical burden on the patient. The thoracic stent graft (Bolton Medical) is a medical device for treating thoracic aortic aneurysms. Unlike vascular grafts, open chest-surgery is not required, and they are a treatment in which a vascular graft, onto which is sewn metallic stents that are able to expand, is carried from the blood vessel at the base of the foot to the treatment site in the chest through the catheter, where it is deployed to block the blood flow to the aortic aneurysm. The abdominal stent graft (Endologix) is a medical device for treating abdominal aortic aneurysm, and similar to the thoracic stent, it is carried to the treatment site in the abdomen where it is deployed to block the blood flow to the aortic aneurysm. Other products include the prosthetic heart valve (LivaNova), which is a medical device used to replace a valve in the heart that has ceased its original function due to conditions such as stenosis and valve dysfunction. Products for Cardiovascular & Vascular Surgery Source: The Company's results briefing materials 08 18
11 Business overview d) Transvascular Intervention Transvascular Intervention mainly handles medical devices to treat conditions such as myocardial infarction and angina pectoris. Main in-house manufactured products are guide wires and balloon catheters used to treat blood vessels (coronary arteries) in cases of myocardial infarction and related conditions. Main purchased products include penetration catheters that are used in the same way when treating myocardial infarction, especially balloon catheters used in treatment of cases of complete closure, and atrial septal defect closing devices that are used when treating congenital structural heart disease. The percutaneous transluminal angioplasty balloon catheters market for peripheral blood vessels is growing, and it seems that the Company is working to capture this growth by meeting needs in this expanding field. The guide wire (Japan Lifeline) is a wire-like medical device used to guide a balloon catheter, stent, or other device through the blood vessels to the treatment site. It is inserted into the blood vessel, such as via the thigh, and passed through the stenosis in the coronary arteries and peripheral arteries, and the device is carried along this wire. The balloon catheter (Japan Lifeline) is a medical device for treating myocardial infarction and angina caused by the narrowing or the blocking of the coronary arteries. They are treated by inflating the balloon on the catheter (thin tube) from inside the blood vessel and expanding the blood vessel. The penetration catheter (Vascular Solutions) is a medical device used to support the passage of guide wires through lesions in coronary arteries and peripheral arteries. The atrial septal defect closing device (Occlutech) is a medical device for the treatment of atrial septal defect, which is a congenital disease in which a hole called a septal defect hole is found in the atrial septal, which is the wall separating the left and right atria of the heart. It does not require surgery and it is said to be extremely minimally invasive because it uses a catheter to close and treat the septal defect hole with a disk-shaped device called a closing plug. Transvascular Intervention Source: The Company's results briefing materials 09 18
12 Performance trends Learned a lot from major decreases in sales due to the loss of sales rights in the past 1. The turning point Many Japanese medical device-related companies are either manufacturers or specialist trading companies. However, while the Company started as a trading company, it subsequently added manufacturing functions. Currently, it has two business forms, import and sales of products manufactured overseas and sales of products manufactured in-house, and it has established a hybrid business model. The trigger for its incorporation of manufacturing functions was a major decline in sales in FY3/00. At that time, Arterial Vascular Engineering Inc., which up to then had supplied the Company with coronary bare metal stents, was acquired by a competitor, which meant that the Company lost the sales rights to this product within Japan. It has experienced similar cases on several occasions in the past, and so as a means of preparing for the risk of losing sales rights, it launched the Research Center in 1999 and started to develop in-house manufactured products. In 2001, it launched guide wires as its first in-house manufactured product, and subsequently expanded its lineup to include EP catheters and ablation catheters. In FY3/09, the Company acquired Ube Junken, which was a subsidiary of Ube Industries, Ltd. <4208> and the only manufacturer of vascular grafts in Japan. Manufacturing capabilities were also triggered by the fact that, at that time, Vascutek Ltd. was acquired by a competitor, and this acquisition subsequently led to the Company to launch in-house manufactured products, such as the J-Graft series of vascular grafts and only-one product, open stent grafts. * Ube Junken was subsequently renamed JUNKEN MEDICAL and absorbed by the Company in April The product that spurred the growth of in-house manufactured products, lineup of which has expanded in this way, was BeeAT, which is an only-one catheter product with an internal atrial cardioversion system that was launched in October This product is used in approximately 80% of ablation treatments of atrial fibrillation, and its sales volume has grown rapidly along with the increase in the number of cases of this condition, contributing greatly to improvement in the Company s profit level. The development of in-house manufactured products started as a means of hedging against the risk of losing sales rights to purchased products, but today, their sales scale has grown to exceed that of purchased products. However, for the time being from FY3/19, the ratio of in-house products is forecast to decrease slightly due to multiple sales of large purchased products
13 Performance trends Sales of in-house group products Source: The Company s results briefing materials Major increase in profits in FY3/18, even on an actual-capabilities basis excluding the unrealized gain 2. Trends in FY3/18 In the FY3/18 results, net sales were 42,298mn (up 13.8% YoY), operating income was 10,671mn (up 38.9%), ordinary income was 10,730mn (up 34.0%), and net income attributable to owners of the parent was 7,478mn (up 39.8%). The number of cases in the cardiovascular field, which is the Company s foundation, continued to grow significantly, and alongside this, results were strong for both in-house manufactured products and purchased products. In addition, gross profit grew from the adjustment of an unrealized gain, greatly raising the gross profit margin. Therefore, the Company was able to absorb the increases in travel costs, development costs, and SG&A expenses such as payment commissions, and also the costs of relocating a subsidiary s factory and a disposal loss, to achieve significantly higher profits. One of the reasons for the extremely high profit growth was the adjustment of an unrealized gain. This was following the absorption merger of the consolidated subsidiary JUNKEN MEDICAL on April 1, 2017, after which the Company adjusted the unrealized gain relating to inventory purchased before the merger and recorded negative costs of sales of 1,170mn. This overlapped with factors including the contribution to profits of products manufactured in-house, and so the gross profit margin rose by 3.6 percentage points. As this unrealized gain is not part of the Company s original, actual capabilities, when provisionally calculating the results excluding this unrealized gain, even then profits increased considerably by around 20%, for operating income of 9,201mn (up 19.7%), ordinary income of 9,560mn (up 19.4%), and net income attributable to owners of the parent of 6,423mn (up 20.0%). So even when based on its actual capabilities, the Company s results grew significantly
14 Performance trends FY3/18 results ( mn) FY3/17 % of sales FY3/18 % of sales Change rate Net sales 37, , Gross profit 21, , SG&A expenses 14, , Operating income 7, , Ordinary income 8, , Net income attributable to owners of the parent 5, , Source: Prepared by FISCO from the Company's results briefing materials The strong sales of existing products covered for the delays in the releases of large-scale products 3. Sales trends by product in FY3/18 In sales conditions by product, each product sold strongly and exceed the initial forecasts. In Cardiac Rhythm Management, as the Company was able to offer a full lineup of cardiac pacemakers compliant with MRI, including the leads, its share of the cardiac pacemaker market recovered greatly to 15%. In EP/Ablation, since the number of cases of ablation treatment for atrial fibrillation increased significantly, rising 22%, which was higher than the initial assumption of a rise of 17%, sales increased of BeeAT, an intracardiac defibrillation catheter and only-one product, and also of products related to atrial fibrillation treatment. In Cardiovascular & Vascular Surgery, sales grew of the abdominal stent graft AFX2, and also of FROZENIX, an open stent graft that was highly evaluated for its contribution to realizing minimally invasive treatment. In Transvascular Intervention, sales increased of balloon catheters for peripheral blood vessels and of atrial septal defect closing devices. Also, following the launch in March 2018 of the drug-eluting coronary stent Orsiro, it had a sales period of 1 month and thereby contributed to the increase in sales. In addition, the Company is taking on the challenge of entering-into a new area, the gastrointestinal area, with JENTLLY, a colonic stent that it released in a limited way in June 2017, and then began its fully fledged sales in January The launches of the endoscopic ablation system HeartLight and the sutureless bio-prosthetic valve PERCEVAL were later than initially forecast, but this was covered by the strong sales of existing products
15 Performance trends Net sales by product FY3/17 % of sales FY3/18 % of sales Change rate Pacemaker-related 5, , ICD related Others Cardiac Rhythm Management 6, , EP catheter 13, , ABL catheter 1, , Others 3, , EP/ABL total 17, , Heart valve related 1, , Cardiopulmonary related Vascular graft related 7, , Blood purification 1, , Other Cardiovascular Surgery related 10, , Balloon catheter Guide wire Others 1, , Transvascular Intervention 2, , Others 1, Total 37, , Note: In line with the subsidiary merger, the product category Others has been re-classified as Cardiovascular & Vascular Surgery starting from FY3/18. Source: Prepared by FISCO from the Company's results briefing materials ( mn) The outlook for the FY3/19 results is for profits to again increase by more than 20% on an actual-capabilities basis 4. FY3/19 outlook For the FY3/19 results, the Company is forecasting net sales of 49,411mn (up 16.8% YoY), operating income of 11,202mn (up 5.0%), ordinary income of 11,482mn (up 7.0%), and net income attributable to owners of the parent of 7,825mn (up 4.6%). Results in FY3/18 were strongly affected by the recording of an unrealized gain of 1,170mn, which meant each profit item was calculated lower in the range of 1 digit. But on conducting provisional calculations excluding this item (FISCO s provisional calculations), we find that the increases in profits become 21.7% for operating income, 20.1% for ordinary income, and 21.8% for net income attributable to owners of the parent, and all profit items grew strongly by more than 20%. So an actual-capabilities basis, we can say that the Company is continuing to maintain high growth. In sales, as the number of cases of ablation treatment for atrial fibrillation is expected to continue to increase significantly, sales of related products are forecast to grow. In addition, the full fiscal year contribution of Orsiro, which was launched in March 2018 as a product for which there are high expectations, is forecast to be net sales of 5bn. The April revisions to the insurance reimbursement prices had a negative effect on the Company s net sales, but the extent of the decline will be small for the products that are driving sales, such as only-one products, and will be 5.8% for all the products it handles, so it seems their impact on results will be limited. On the other hand, in Cardiac Rhythm Management, demand for remote monitoring devices is increasing due to the receipt of medical fee incentives for remote treatment, but as the Company s supplier manufacturers are experiencing supply-side problems, the outlook is for a decline in sales
16 Performance trends In profits, the gross profit margin is forecast to decline due to the end of the adjustment of the temporary unrealized gain that was recorded in FY3/18, the lowering of insurance reimbursement prices, and a change to the product mix through Orsiro, a large-scale purchased product with major sales. Conversely, SG&A expenses are expected to rise because of an increase in advertising expenses for the promotion of large-scale products and the establishment of a logistics base in Kansai, and also due to increases in other costs, including development-related costs to expand the lineup of in-house manufactured products, and clinical trial expenses and examination expenses toward obtain drug regulatory approval for new products. Therefore, the outlook is for profits to increase only by a single digit. But when excluding the effects of above-described unrealized gain, the forecast is actually for a major rise in profits, with each profit item to increase by more than 20%. FY3/19 outlook ( mn) FY3/18 % of sales FY3/19 E % of sales Change rate Net sales 42, , Gross profit 26, , SG&A expenses 15, , Operating income 10, , Ordinary income 10, , Net income attributable to owners of the parent 7, , Source: Prepared by FISCO from the Company s results briefing materials In FY3/19, will launch 3 large-scale products for which there are major expectations 5. Outlook of net sales by product in FY3/19 By product, in Cardiac Rhythm Management, while demand for remote monitoring devices is increasing, there remain supply problems, so the forecast is that the sales volume of pacemakers will decline. In EP/Ablation, assuming that the number of cases of atrial fibrillation will increase by 18%, sales of BeeAT and other related products will continue to grow, while the endoscopic ablation system HeartLight is set to be added as a new product. The Company has already launched its in-house manufactured open stent graft in Taiwan in February 2018, and the first case occurred in April, while it plans to also develop it in Europe in the 2H. In Cardiovascular & Vascular Surgery, sales of the open stent graft FROZENIX and the abdominal stent graft AFX2 are expected to continue to increase. In Transvascular Intervention, the forecast is for sales to decrease due to the reduction in the insurance reimbursement prices of balloon catheters and guide wires. But overall, sales are expected to increase greatly because of the contribution of Orsiro
17 Performance trends Outlook of net sales by product in FY3/19 ( mn) FY3/18 % of sales FY3/19 E % of sales Change rate Pacemaker-related 6, , ICD related Others Cardiac Rhythm Management 7, , EP catheter 15, , ABL catheter 1, , Others 3, , EP/ABL total 20, , Heart valve related 1, , Cardiopulmonary related Vascular graft related 8, , Blood purification 1, , Other Cardiovascular Surgery related 11, , Balloon catheter Guide wire Others 1, , Transvascular Intervention 3, , Total 42, , Source: Prepared by FISCO from the Company's results briefing materials The three large-scale products, for which there are great expectations, are set to be rolled-out during this fiscal year. Needs for each at medical sites are thought to be high, and they are considered to be a product Group that will drive the Company s medium-term growth. First is the drug-eluting coronary stent Orsiro, which has already been launched in March and which will be a major device for coronary artery disease. As it is a new entry into the market, preparing consignment stock for hospitals of a certain size seems to be a burdensome task. But its features include that it has the world s thinnest strut to inhibit restenosis and prevention of thrombus, that it uses nano-coating to prevent elution of metallic ions, and that it is extremely durable. Therefore, it was highly evaluated in the clinical trials and was forecast to obtain a 10% share of the drug-eluting coronary stent market in its first fiscal year (FY3/19), but this has been upwardly revised to 15%. Second is the endoscopic ablation system HeartLight, which is scheduled to be released in July. It is a new product that incorporates the technology of laser cauterization into balloon technology, to make possible accurate cauterization with a laser while observing the endoscopic images. After its launch, the Company plans to progress the launch of a next-generation model with even more advanced functions. Third is the sutureless bio-prosthetic valve PERCEVAL, which is scheduled to be released in the 2H. Its features include that it can be expected to reduce the surgery time and the burden on the patient because sutures are not required and that is has excellent hemodynamics as it has a structure without a valve ring, so it is optimal for complex surgeries and keyhole surgeries. It is considered to be product that can be expected to create a new market for surgeons
18 Performance trends Large-scale products for which there are high expectations Source: The Company's results briefing materials The medium-term management plan Even if the product mix is changed, strong growth is expected in the medium term 1. The medium-term management plan The heart disease medical devices market continues to expand against the backdrop of the aging of the Japanese population and the advancement of medical devices. In this sort of environment, there is no other company in Japan that can provide sales and regulatory structures at the same levels as the Company, which means that collaborations with it are the best possible choice for overseas manufacturers. As a result, the Company will have an abundance of products in the pipeline in the future. Moreover, it is considering expanding its R&D bases and constructing overseas factories, and it is thought to be advancing joint development not only for in-house manufactured products, but for purchased products also. Both the external and internal environments are in place, and it seems it will continue to grow as in the past. In its medium-term management plan, the Company is aiming for net sales of 77.7bn and an operating income margin of 25% in FY3/23, and these targets can be said to be fully within range. On the other hand, through the successive market launches of large-scale products, the ratio of in-house manufactured products, which was 55.4% in FY3/18, is expected to decline slightly to a level of around 50% in the medium term. In this way, it is inevitable that the Company s product mix will change and the gross profit margin will rise or fall depending on the timing of the product launches and the needs of medical sites. However, in many cases, purchased products target larger and more advanced categories, and moreover do not have any development costs. Therefore, as their operating income amount is not considered to be greatly different to that of in-house manufactured products, the strong profit growth can be expected to continue. Of course, the Company will not change its policy of strengthening its manufacturer functions, and from a long-term perspective, it intends to conduct development in fields closer to basic research. So its image for the medium-to-long term is an approach of strengthening both purchased products and in-house manufactured products 16 18
19 The medium-term management plan Steadily strengthening medium-term growth through equity finance 2. Developments for medium-term growth From the viewpoint of medium- to long-term growth, an issue for the Company is expanding its business areas. At the present time, its growth potential for the cardiovascular field in Japan is forecast to be strong for the foreseeable future, while it also plans to expand its business areas, including by entering-into new areas. In June 2017, it market launched a colonic stent, which is an in-house manufactured product, and took its first step into the gastrointestinal field. Although it is still at the stage of trial operations as a new field, the Company s policy is to gradually increase sales and to accumulate expertise in this field. It also plans to build a new factory in Malaysia, which initially will manufacture balloon catheters as back-up to the Shenzhen factory. But in the future, the intention is for it to manufacture EP catheters and to expand the sales areas to overseas, including to Asia and Europe. Most of the contribution to profits of this expansion of business areas has not been incorporated into the targets in the medium-term management plan. On November 30, 2017, the Company issued share acquisition rights through a third-party allocation in order to strengthen medium-term growth and its management base. The issue is expected to raise funds of approximately 17,484mn, while the exercise period for the share acquisition rights is January 2018 to December In terms of the specific uses of the funds, 5,900mn will to be to secure the pipeline for new products, 5,300mn to strengthen the development and production structure, 4,200mn as working funds for the sales of large-scale new products, and 2,084mn as standby funds assuming, for example, M&A and other usages, and to repay borrowings. The expenditure periods for all of these items are scheduled to be from January 2018 to December The Company continues to conduct active management in the growth market of cardiovascular-related medical devices, while also entering-into new business areas, such as the gastrointestinal field and overseas. For a strongly growth-oriented enterprise such as the Company, we welcome the use of equity finance as it increases the likelihood of stronger growth in the medium term. Source: Prepared by FISCO from the Company's results briefing materials 17 18
20 Shareholder return policy Dividends are expected to continue to increase alongside the profit growth The Company s basic policy for returning profits to shareholders is to consider the various factors, such as the results and the demand for capital for business development in the future, and to implement appropriate measures to return profits to shareholders, mainly through continuously and stably paying a dividend, while retaining the necessary internal reserves. In addition, its policy for internal reserves is to increase enterprise value by aiming to raise results and invest in developing and manufacturing products in-house that utilize our strengths, and going forward, it will raise the dividend in line with profit growth. It has carried out equity finance, but on considering the Company s medium-term management targets up to the present time, we estimate that its free cash flow will continue to expand in the future. Accordingly, while maintaining a balance between returning profits to shareholders and securing internal reserves, the Company s investment and dividend capabilities have been improving, and it can be said to have reached a growth stage where it can return profit growth to shareholders. ( ) Notes; the amounts have been retroactively adjusted in relation to the 2-for-1 share splits in 2015, 2016, and 2018 Source: Prepared by FISCO from the Company's results briefing materials Information security The Company is implementing various information security measures, such as using remote servers, implementing encryption measures and measures to defend against malware, and conducting network monitoring to detect unauthorized access. Recently, it has started collaborating with a company specializing in security and it is regularly conducting operations evaluations and ascertaining points to improve on, and in such ways it is working to improve the information-security management level
21 Disclaimer (the terms FISCO, we, mean ) has legal agreements with the Tokyo Stock Exchange, the Osaka Exchange,and Nikkei Inc. as to the usage of stock price and index information. The trademark and value of the JASDAQ INDEX are the intellectual properties of the Tokyo Stock Exchange, and therefore all rights to them belong to the Tokyo Stock Exchange. This report is based on information that we believe to be reliable, but we do not confirm or guarantee its accuracy, timeliness,or completeness, or the value of the securities issued by companies cited in this report. Regardless of purpose,investors should decide how to use this report and take full responsibility for such use. We shall not be liable for any result of its use. We provide this report solely for the purpose of information, not to induce investment or any other action. This report was prepared at the request of its subject company using information provided by the company in interviews, but the entire content of the report, including suppositions and conclusions, is the result of our analysis. The content of this report is based on information that was current at the time the report was produced, but this information and the content of this report are subject to change without prior notice. All intellectual property rights to this report, including copyrights to its text and data, are held exclusively by FISCO. Any alteration or processing of the report or duplications of the report, without the express written consent of FISCO, is strictly prohibited. Any transmission, reproduction, distribution or transfer of the report or its duplications is also strictly prohibited. The final selection of investments and determination of appropriate prices for investment transactions are decisions for the recipients of this report.
Results Presentation 2Q FY March Win-Partners Co., Ltd. (3183)
 Results Presentation 2Q FY March 2018 Win-Partners Co., Ltd. (3183) 2Q results ending September 2017 Consolidated results summary ( mil)2q to Sep 2016 Sep 2017 YoY OE Sales 27,713 29,753 +7.4% 29,500 Operating
Results Presentation 2Q FY March 2018 Win-Partners Co., Ltd. (3183) 2Q results ending September 2017 Consolidated results summary ( mil)2q to Sep 2016 Sep 2017 YoY OE Sales 27,713 29,753 +7.4% 29,500 Operating
Results Presentation 2Q FY March Win-Partners Co., Ltd. (3183)
 Results Presentation 2Q FY March 2019 Win-Partners Co., Ltd. (3183) 2Q results ending September 2019 3 Consolidated results summary ( mil)2q to Sep 2017 Sep 2018 YoY OE Sales 29,753 31,863 +7.1% 31,700
Results Presentation 2Q FY March 2019 Win-Partners Co., Ltd. (3183) 2Q results ending September 2019 3 Consolidated results summary ( mil)2q to Sep 2017 Sep 2018 YoY OE Sales 29,753 31,863 +7.1% 31,700
Results Presentation FY March Win-Partners Co., Ltd. (3183)
 Results Presentation FY March 2018 Win-Partners Co., Ltd. (3183) Full year results ending March 2018 Consolidated results summary ( mil) Mar 2017 Mar 2018 YoY OE Sales 57,760 62,832 +8.8% 63,100 Operating
Results Presentation FY March 2018 Win-Partners Co., Ltd. (3183) Full year results ending March 2018 Consolidated results summary ( mil) Mar 2017 Mar 2018 YoY OE Sales 57,760 62,832 +8.8% 63,100 Operating
Second Quarter and First Half 2011 Conference Call. 29 July, SORIN GROUP Presentation 1
 Second Quarter and First Half 2011 Conference Call 29 July, 2011 SORIN GROUP Presentation 1 DISCLAIMER This presentation contains management preliminary estimates and forward-looking statements, including
Second Quarter and First Half 2011 Conference Call 29 July, 2011 SORIN GROUP Presentation 1 DISCLAIMER This presentation contains management preliminary estimates and forward-looking statements, including
ST. JUDE MEDICAL / THORATEC TRANSACTION HIGHLIGHTS July 22, 2015
 ST. JUDE MEDICAL / THORATEC TRANSACTION HIGHLIGHTS July 22, 2015 1 FORWARD LOOKING STATEMENT This presentation contains forward-looking statements within the meaning of the Private Securities Litigation
ST. JUDE MEDICAL / THORATEC TRANSACTION HIGHLIGHTS July 22, 2015 1 FORWARD LOOKING STATEMENT This presentation contains forward-looking statements within the meaning of the Private Securities Litigation
SUPPLEMENTAL MATERIAL
 SUPPLEMENTAL MATERIAL Table S1. Patient Selection Codes, CIED Generator Procedures Code Type Code Description ICD9 Proc 00.51 Implantation of cardiac resynchronization defibrillator, total system [CRT-D]
SUPPLEMENTAL MATERIAL Table S1. Patient Selection Codes, CIED Generator Procedures Code Type Code Description ICD9 Proc 00.51 Implantation of cardiac resynchronization defibrillator, total system [CRT-D]
Sumitomo Pharmaceuticals reaches a basic agreement to merge with Dainippon Pharmaceutical
 For immediate release November 25, 2004 Sumitomo Pharmaceuticals reaches a basic agreement to merge with Dainippon Pharmaceutical Sumitomo Chemical Co., Ltd. announced today that its subsidiary, Sumitomo
For immediate release November 25, 2004 Sumitomo Pharmaceuticals reaches a basic agreement to merge with Dainippon Pharmaceutical Sumitomo Chemical Co., Ltd. announced today that its subsidiary, Sumitomo
Implantable Defibrillators Market by Product Type [Transvenous Implantable Cardioverter-Defibrillator (T-ICDs), Subcutaneous Implantable Cardioverter
 Implantable Defibrillators Market by Product Type [Transvenous Implantable Cardioverter-Defibrillator (T-ICDs), Subcutaneous Implantable Cardioverter Defibrillators (S-ICDS), and Cardiac Resynchronization
Implantable Defibrillators Market by Product Type [Transvenous Implantable Cardioverter-Defibrillator (T-ICDs), Subcutaneous Implantable Cardioverter Defibrillators (S-ICDS), and Cardiac Resynchronization
1Q Fornebu, April 29, 2015 Luis Araujo and Svein Stoknes
 1Q 2015 Fornebu, April 29, 2015 Luis Araujo and Svein Stoknes Forward-Looking Statements and Copyright This Presentation includes and is based, inter alia, on forward-looking information and statements
1Q 2015 Fornebu, April 29, 2015 Luis Araujo and Svein Stoknes Forward-Looking Statements and Copyright This Presentation includes and is based, inter alia, on forward-looking information and statements
Newer pacemakers also can monitor your blood temperature, breathing, and other factors and adjust your heart rate to changes in your activity.
 Pacemakers & Defibrillators A pacemaker system consists of a battery, a computerized generator and wires with sensors called electrodes on one end. The battery powers the generator, and both are surrounded
Pacemakers & Defibrillators A pacemaker system consists of a battery, a computerized generator and wires with sensors called electrodes on one end. The battery powers the generator, and both are surrounded
FY2009 First Quarter Financial Results. SUZUKI MOTOR CORPORATION August 3, 2009
 FY2009 First Quarter Financial Results SUZUKI MOTOR CORPORATION August 3, 2009 Consolidated: Financial Summary Page 2 FY2009 1Q FY2008 4Q 09/4-6 09/1-3 Change FY2008 1Q 08/4-6 Change (Billion Yen) (A)
FY2009 First Quarter Financial Results SUZUKI MOTOR CORPORATION August 3, 2009 Consolidated: Financial Summary Page 2 FY2009 1Q FY2008 4Q 09/4-6 09/1-3 Change FY2008 1Q 08/4-6 Change (Billion Yen) (A)
Fiscal Year (FY) 2019 Hospital Inpatient Proposed Rule Interventional Cardiology, Peripheral Interventions & Rhythm Management
 Fiscal Year (FY) 2019 Hospital Inpatient Proposed Rule Interventional Cardiology, Peripheral Interventions & Rhythm Management On April 24, 2018, the Centers for Medicare & Medicaid Services (CMS) released
Fiscal Year (FY) 2019 Hospital Inpatient Proposed Rule Interventional Cardiology, Peripheral Interventions & Rhythm Management On April 24, 2018, the Centers for Medicare & Medicaid Services (CMS) released
Asia-Pacific Electrophysiology Market Outlook to 2020
 Asia-Pacific Electrophysiology Market Outlook to 2020 Reference Code: GDMECR0114DB Publication Date: May 2014 Page 1 1 Table of Contents 1 Table of Contents... 2 1.1 List of Tables... 5 1.2 List of Figures...
Asia-Pacific Electrophysiology Market Outlook to 2020 Reference Code: GDMECR0114DB Publication Date: May 2014 Page 1 1 Table of Contents 1 Table of Contents... 2 1.1 List of Tables... 5 1.2 List of Figures...
Structural Heart Disease Patient Guide. Guide contents: 2 What Is Structural Heart Disease? 2 What Happens During Structural Heart Disease?
 Guide contents: 2 What Is Structural Heart Disease? 2 What Happens During Structural Heart Disease? 3 Diagnosis 3 Treatment Options 4 Managing the Condition 5 Surgery and Insurance 6 Symptom Tracker Form
Guide contents: 2 What Is Structural Heart Disease? 2 What Happens During Structural Heart Disease? 3 Diagnosis 3 Treatment Options 4 Managing the Condition 5 Surgery and Insurance 6 Symptom Tracker Form
Corporate Update Simon Hubbert, CEO Bill Kullback, CFO June, 2015
 (Nasdaq:EVAR) Corporate Update Simon Hubbert, CEO Bill Kullback, CFO June, 2015 Safe Harbor This presentation contains forward-looking statements as that term is defined in the Private Securities Litigation
(Nasdaq:EVAR) Corporate Update Simon Hubbert, CEO Bill Kullback, CFO June, 2015 Safe Harbor This presentation contains forward-looking statements as that term is defined in the Private Securities Litigation
INPATIENT REIMBURSEMENT PROSPECTUS
 2018 CARDIOVASCULAR SERVICE LINE INPATIENT REIMBURSEMENT PROSPECTUS Increasing financial risk to U.S. health care providers, including physicians and hospitals, has been centered on outcomes-based modifiers
2018 CARDIOVASCULAR SERVICE LINE INPATIENT REIMBURSEMENT PROSPECTUS Increasing financial risk to U.S. health care providers, including physicians and hospitals, has been centered on outcomes-based modifiers
AORTIC STENOSIS HENRY FORD HOSPITAL CENTER FOR STRUCTURAL HEART DISEASE
 AORTIC STENOSIS HENRY FORD HOSPITAL CENTER FOR STRUCTURAL HEART DISEASE WHAT IS AORTIC STENOSIS? THE AORTIC VALVE The aorta is the major vessel that carries oxygenated blood out of the left side of the
AORTIC STENOSIS HENRY FORD HOSPITAL CENTER FOR STRUCTURAL HEART DISEASE WHAT IS AORTIC STENOSIS? THE AORTIC VALVE The aorta is the major vessel that carries oxygenated blood out of the left side of the
View Report Details. Global Coronary Stent Market
 View Report Details Global Coronary Stent Market ------------------------------------------------- 2013 View Report Details Executive Summary Coronary heart diseases are nowadays becoming more and more
View Report Details Global Coronary Stent Market ------------------------------------------------- 2013 View Report Details Executive Summary Coronary heart diseases are nowadays becoming more and more
Arrhythmias. Pulmonary Artery
 Arrhythmias Introduction Cardiac arrhythmia is an irregularity of the heart beat that causes the heart to beat too slowly, too fast, or irregularly. There are different types of arrhythmias. Most arrhythmias
Arrhythmias Introduction Cardiac arrhythmia is an irregularity of the heart beat that causes the heart to beat too slowly, too fast, or irregularly. There are different types of arrhythmias. Most arrhythmias
Consolidated: Financial Summary
 FY2010 First Quarter Financial Results SUZUKI MOTOR CORPORATION 3 August 2010 Consolidated: Financial Summary Page2 FY2010 1Q FY2009 1Q (2010/4-6) (2009/4-6) Change Net sales 656.3 577.1 +79.2 +13.7% Operating
FY2010 First Quarter Financial Results SUZUKI MOTOR CORPORATION 3 August 2010 Consolidated: Financial Summary Page2 FY2010 1Q FY2009 1Q (2010/4-6) (2009/4-6) Change Net sales 656.3 577.1 +79.2 +13.7% Operating
DIEBOLD NIXDORF AG AT GERMANY EQUITY FORUM
 November 28, 2017 DIEBOLD NIXDORF AG AT GERMANY EQUITY FORUM Dr. Jürgen Wunram Agenda Diebold Nixdorf AG History and shareholder structure Market trends, strategy and progress of integration Diebold Nixdorf
November 28, 2017 DIEBOLD NIXDORF AG AT GERMANY EQUITY FORUM Dr. Jürgen Wunram Agenda Diebold Nixdorf AG History and shareholder structure Market trends, strategy and progress of integration Diebold Nixdorf
Interventions in Congenital & Structural Heart Disease: Who Drives New Techniques and Devices?
 Interventions in Congenital & Structural Heart Disease: Who Drives New Techniques and Devices? Lee Benson MD Professor Pediatrics (Cardiology) Director, Cardiac Diagnostic & Interventional Unit The Hospital
Interventions in Congenital & Structural Heart Disease: Who Drives New Techniques and Devices? Lee Benson MD Professor Pediatrics (Cardiology) Director, Cardiac Diagnostic & Interventional Unit The Hospital
Walgreens (WAG) Analyst: Juan Fabres Fall 2014
 Recommendation: Buy Target Price August 31, 2016: $77.57 1. Reasons for the Recommendation With the acquisition of Alliance Boots in Europe, Walgreens will be the first US pharmacy to operate retail stores
Recommendation: Buy Target Price August 31, 2016: $77.57 1. Reasons for the Recommendation With the acquisition of Alliance Boots in Europe, Walgreens will be the first US pharmacy to operate retail stores
ATRIAL FIBRILLATION ANSWERS. A Patient Education Handbook on Electrophysiology
 ATRIAL FIBRILLATION ANSWERS A Patient Education Handbook on Electrophysiology MY LIFE HAS TAKEN A TURN FOR THE BETTER. -Emie Bishop ARRHYTHMIAANSWERS.COM AF ANSWERS. A PATIENT EDUCATION HANDBOOK ON ELECTROPHYSIOLOGY
ATRIAL FIBRILLATION ANSWERS A Patient Education Handbook on Electrophysiology MY LIFE HAS TAKEN A TURN FOR THE BETTER. -Emie Bishop ARRHYTHMIAANSWERS.COM AF ANSWERS. A PATIENT EDUCATION HANDBOOK ON ELECTROPHYSIOLOGY
Itamar Medical. December Investors Presentation.
 Itamar Medical December 2017. Investors Presentation. Disclaimer Itamar Medical Ltd. (the "Company") is furnishing this presentation and any information given during this presentation, solely for the consideration
Itamar Medical December 2017. Investors Presentation. Disclaimer Itamar Medical Ltd. (the "Company") is furnishing this presentation and any information given during this presentation, solely for the consideration
NEIL CISPER TECHNICAL FIELD ENGINEER ICD/CRTD BASICS
 NEIL CISPER TECHNICAL FIELD ENGINEER ICD/CRTD BASICS OBJECTIVES Discuss history of ICDs Review the indications for ICD and CRT therapy Describe basic lead and device technology Discuss different therapies
NEIL CISPER TECHNICAL FIELD ENGINEER ICD/CRTD BASICS OBJECTIVES Discuss history of ICDs Review the indications for ICD and CRT therapy Describe basic lead and device technology Discuss different therapies
North America Cardiac Rhythm Management (CRM) Procedures Outlook to 2021
 North America Cardiac Rhythm Management (CRM) Procedures Outlook to 2021 North America Cardiac Rhythm Management (CRM) Procedures Outlook to 2021 BioPortfolio has been marketing business and market research
North America Cardiac Rhythm Management (CRM) Procedures Outlook to 2021 North America Cardiac Rhythm Management (CRM) Procedures Outlook to 2021 BioPortfolio has been marketing business and market research
Russia Cardiac Assist Devices Market Outlook to 2021
 Russia Cardiac Assist Devices Market Outlook to 2021 Russia Cardiac Assist Devices Market Outlook to 2021 BioPortfolio has been marketing business and market research reports from selected publishers for
Russia Cardiac Assist Devices Market Outlook to 2021 Russia Cardiac Assist Devices Market Outlook to 2021 BioPortfolio has been marketing business and market research reports from selected publishers for
Sorin Group. Marco Chiadò Piat, VP Corporate Development. Goldman Sachs European Medtech and Healthcare Services 5-6 September 2007
 Sorin Group Marco Chiadò Piat, VP Corporate Development Goldman Sachs European Medtech and Healthcare Services 5-6 September 2007 Sorin FY 2006 results by Business Area FY 06 ( Mn) FY 06 vs FY 05 as reported
Sorin Group Marco Chiadò Piat, VP Corporate Development Goldman Sachs European Medtech and Healthcare Services 5-6 September 2007 Sorin FY 2006 results by Business Area FY 06 ( Mn) FY 06 vs FY 05 as reported
For personal use only
 HY18 Result Presentation 13 February 2018 Dig Howitt Brent Cubis CEO & President CFO HY18 Result highlights Strong momentum across developed markets continues Developed market unit growth up 12% Strengthening
HY18 Result Presentation 13 February 2018 Dig Howitt Brent Cubis CEO & President CFO HY18 Result highlights Strong momentum across developed markets continues Developed market unit growth up 12% Strengthening
Cardiac Resynchronisation Therapy Patient Information
 Melbourne Heart Rhythm Cardiac Resynchronisation Therapy Patient Information Normal Heart Function The heart is a pump responsible for maintaining blood supply to the body. It has four chambers. The two
Melbourne Heart Rhythm Cardiac Resynchronisation Therapy Patient Information Normal Heart Function The heart is a pump responsible for maintaining blood supply to the body. It has four chambers. The two
Cardiovascular Nursing Practice: A Comprehensive Resource Manual and Study Guide for Clinical Nurses 2 nd Edition
 Cardiovascular Nursing Practice: A Comprehensive Resource Manual and Study Guide for Clinical Nurses 2 nd Edition Table of Contents Volume 1 Chapter 1: Cardiovascular Anatomy and Physiology Basic Cardiac
Cardiovascular Nursing Practice: A Comprehensive Resource Manual and Study Guide for Clinical Nurses 2 nd Edition Table of Contents Volume 1 Chapter 1: Cardiovascular Anatomy and Physiology Basic Cardiac
Do Now. Get out work from last class to be checked
 Do Now Get out work from last class to be checked Heart Actions Cardiac Cycle: One complete heartbeat. The contraction of a heart chamber is called systole and the relaxation of a chamber is called diastole.
Do Now Get out work from last class to be checked Heart Actions Cardiac Cycle: One complete heartbeat. The contraction of a heart chamber is called systole and the relaxation of a chamber is called diastole.
Heart Circulatory System
 Heart Circulatory System 25 26 Prof. Dr. med. Walter Eichinger Department for Cardiac Surgery > Bogenhausen Hospital Englschalkinger Straße 77 81925 München Phone +49 89 9270-2630 walter.eichinger@klinikum-muenchen.de
Heart Circulatory System 25 26 Prof. Dr. med. Walter Eichinger Department for Cardiac Surgery > Bogenhausen Hospital Englschalkinger Straße 77 81925 München Phone +49 89 9270-2630 walter.eichinger@klinikum-muenchen.de
Cardiac Rhythm Management Coder 2018
 Cardiac Rhythm Management Coder 2018 An easy-to-use tool for coding and reimbursement compliance Prepared and Published By: MedLearn Publishing, A Division of MedLearn Media, Inc. 445 Minnesota Street,
Cardiac Rhythm Management Coder 2018 An easy-to-use tool for coding and reimbursement compliance Prepared and Published By: MedLearn Publishing, A Division of MedLearn Media, Inc. 445 Minnesota Street,
IHCP bulletin INDIANA HEALTH COVERAGE PROGRAMS BT JANUARY 24, 2012
 IHCP bulletin INDIANA HEALTH COVERAGE PROGRAMS BT201203 JANUARY 24, 2012 The IHCP to reimburse implantable cardioverter defibrillators separately from outpatient implantation Effective March 1, 2012, the
IHCP bulletin INDIANA HEALTH COVERAGE PROGRAMS BT201203 JANUARY 24, 2012 The IHCP to reimburse implantable cardioverter defibrillators separately from outpatient implantation Effective March 1, 2012, the
CY2015 Hospital Outpatient: Endovascular Procedure APCs and Complexity Adjustments
 CY2015 Hospital Outpatient: Endovascular Procedure APCs Complexity Adjustments Comprehensive Ambulatory Payment Classifications (c-apcs) CMS finalized the implementation of 25 Comprehensive APC to further
CY2015 Hospital Outpatient: Endovascular Procedure APCs Complexity Adjustments Comprehensive Ambulatory Payment Classifications (c-apcs) CMS finalized the implementation of 25 Comprehensive APC to further
China Portable Medical Electronic Devices Industry Report, 2010
 China Portable Medical Electronic Devices Industry Report, 2010 Portable medical electronic devices can be mainly divided into home type and professional type, of which, the former covers electronic sphygmomanometer,
China Portable Medical Electronic Devices Industry Report, 2010 Portable medical electronic devices can be mainly divided into home type and professional type, of which, the former covers electronic sphygmomanometer,
Summary of Results for the First Half of FY2015/3
 Summary of Results for the First Half of FY2015/3 November 10, 2014 Tokyu Corporation (9005) http://www.tokyu.co.jp/ Contents Ⅰ.Executive Summary 2 Ⅱ.Conditions in Each Business 5 Ⅲ.Details of Financial
Summary of Results for the First Half of FY2015/3 November 10, 2014 Tokyu Corporation (9005) http://www.tokyu.co.jp/ Contents Ⅰ.Executive Summary 2 Ⅱ.Conditions in Each Business 5 Ⅲ.Details of Financial
January 30, 2018 Dow Wilson President and Chief Executive Officer
 Acquisition of SIRTeX January 30, 2018 Dow Wilson President and Chief Executive Officer This presentation is intended exclusively for investors. It is not intended for use in Sales or Marketing. Forward-Looking
Acquisition of SIRTeX January 30, 2018 Dow Wilson President and Chief Executive Officer This presentation is intended exclusively for investors. It is not intended for use in Sales or Marketing. Forward-Looking
About atrial fibrillation (AFib) Atrial Fibrillation (AFib) What is AFib? What s the danger? Who gets AFib?
 Understanding AFib Atrial Fibrillation (AFib) About AFib 3 How Your Heart Works 4 Types of AFib 5 Symptoms 5 Risk Factors 5 How is AFib Diagnosed? 6 Treatment 6 What to Ask Your Doctor 7 A normal heartbeat
Understanding AFib Atrial Fibrillation (AFib) About AFib 3 How Your Heart Works 4 Types of AFib 5 Symptoms 5 Risk Factors 5 How is AFib Diagnosed? 6 Treatment 6 What to Ask Your Doctor 7 A normal heartbeat
Acquisition of Novartis Influenza Vaccines Business. 27 th October 2014
 1 Acquisition of Novartis Influenza Vaccines Business 27 th October 2014 2 Legal Notice Forward looking statements The materials in this presentation speak only as of the date of these materials, and include
1 Acquisition of Novartis Influenza Vaccines Business 27 th October 2014 2 Legal Notice Forward looking statements The materials in this presentation speak only as of the date of these materials, and include
Slide 1. Financial update and closing remarks. Jesper Brandgaard EVP and CFO. RAFAEL DE JESÚS FLORES, Mexico Rafael has haemophilia A
 Slide 1 Financial update and closing remarks Jesper Brandgaard EVP and CFO RAFAEL DE JESÚS FLORES, Mexico Rafael has haemophilia A Slide 2 Forward-looking statements Novo Nordisk s reports filed with or
Slide 1 Financial update and closing remarks Jesper Brandgaard EVP and CFO RAFAEL DE JESÚS FLORES, Mexico Rafael has haemophilia A Slide 2 Forward-looking statements Novo Nordisk s reports filed with or
The World s Smallest Heart Pump
 Transcript Details This is a transcript of an educational program accessible on the ReachMD network. Details about the program and additional media formats for the program are accessible by visiting: https://reachmd.com/programs/clinicians-roundtable/the-worlds-smallest-heart-pump/3367/
Transcript Details This is a transcript of an educational program accessible on the ReachMD network. Details about the program and additional media formats for the program are accessible by visiting: https://reachmd.com/programs/clinicians-roundtable/the-worlds-smallest-heart-pump/3367/
Tamsulosin Hydrochloride 0.4 mg Capsule
 Tamsulosin Hydrochloride 0.4 mg Capsule, Tamsulosin Hydrochloride 0.4 mg Capsule India, Tamsulosin Hydrochloride 0.4 mg Capsule manufacturers India, side effects Tamsulosin Hydrochloride 0.4 mg Capsule
Tamsulosin Hydrochloride 0.4 mg Capsule, Tamsulosin Hydrochloride 0.4 mg Capsule India, Tamsulosin Hydrochloride 0.4 mg Capsule manufacturers India, side effects Tamsulosin Hydrochloride 0.4 mg Capsule
Company Profile 2011
 Company Profile 2011 Disclaimer This presentation contains forward-looking statements based on the currently held beliefs and assumptions of our management, which are expressed in good faith and in their
Company Profile 2011 Disclaimer This presentation contains forward-looking statements based on the currently held beliefs and assumptions of our management, which are expressed in good faith and in their
Welcome to LG Electronics
 Welcome to LG Electronics April 21, 2004 As a note, this presentation has been prepared based on internal audited figures and final figures may change due to the results of independent auditors review.
Welcome to LG Electronics April 21, 2004 As a note, this presentation has been prepared based on internal audited figures and final figures may change due to the results of independent auditors review.
Asia-Pacific Bariatric Surgery Devices Market Outlook to 2020
 Asia-Pacific Bariatric Surgery Devices Market Outlook to 2020 Reference Code: GDMECR0195DB Publication Date: July 2014 Page 1 1 Table of Contents 1 Table of Contents... 2 1.1 List of Tables... 4 1.2 List
Asia-Pacific Bariatric Surgery Devices Market Outlook to 2020 Reference Code: GDMECR0195DB Publication Date: July 2014 Page 1 1 Table of Contents 1 Table of Contents... 2 1.1 List of Tables... 4 1.2 List
DS-8201 Strategic Collaboration
 DS-8201 Strategic Collaboration DAIICHI SANKYO CO., LTD George Nakayama Chairman and CEO March 29, 2019 Forward-Looking Statements Management strategies and plans, financial forecasts, future projections
DS-8201 Strategic Collaboration DAIICHI SANKYO CO., LTD George Nakayama Chairman and CEO March 29, 2019 Forward-Looking Statements Management strategies and plans, financial forecasts, future projections
Coloplast A/S. Investor presentation 1H 2005/06
 Coloplast A/S Investor presentation 1H 2005/06 2 Coloplast Coloplast products and services help patients achieve greater independence from medical challenges in 4 areas: Ostomy care, continence care, wound
Coloplast A/S Investor presentation 1H 2005/06 2 Coloplast Coloplast products and services help patients achieve greater independence from medical challenges in 4 areas: Ostomy care, continence care, wound
ASX Investor Presentation
 ASX Investor Presentation Sydney, Australia Dr Peter Neustadt Executive Chairman and CEO November 2014 Copyright and registered trademark notice ASX Investor Presentation Page 0 Who we are SomnoMed Limited
ASX Investor Presentation Sydney, Australia Dr Peter Neustadt Executive Chairman and CEO November 2014 Copyright and registered trademark notice ASX Investor Presentation Page 0 Who we are SomnoMed Limited
The Adolescent and Adult Congenital Heart Disease Program
 The Adolescent and Adult Congenital Heart Disease Program The Heart Center at Nationwide Children s Hospital & The Ohio State University D- Transposition of the Great Vessels D- transposition of the great
The Adolescent and Adult Congenital Heart Disease Program The Heart Center at Nationwide Children s Hospital & The Ohio State University D- Transposition of the Great Vessels D- transposition of the great
Information for patients. The Cambridge Heart Clinic. World class private patient cardiology services in partnership with Addenbrooke s Hospital
 Information for patients The Cambridge Heart Clinic World class private patient cardiology services in partnership with Addenbrooke s Hospital This information can also be provided in different languages,
Information for patients The Cambridge Heart Clinic World class private patient cardiology services in partnership with Addenbrooke s Hospital This information can also be provided in different languages,
Cardiac Cycle. Each heartbeat is called a cardiac cycle. First the two atria contract at the same time.
 The Heartbeat Cardiac Cycle Each heartbeat is called a cardiac cycle. First the two atria contract at the same time. Next the two ventricles contract at the same time. Then all the chambers relax. http://www.youtube.com/watch?v=frd3k6lkhws
The Heartbeat Cardiac Cycle Each heartbeat is called a cardiac cycle. First the two atria contract at the same time. Next the two ventricles contract at the same time. Then all the chambers relax. http://www.youtube.com/watch?v=frd3k6lkhws
Understanding Atrial Fibrillation A guide for patients
 Understanding Atrial Fibrillation A guide for patients Your doctor has determined that you have atrial fibrillation (AF), a common disturbance of the heart s rhythm. This pamphlet will answer many of your
Understanding Atrial Fibrillation A guide for patients Your doctor has determined that you have atrial fibrillation (AF), a common disturbance of the heart s rhythm. This pamphlet will answer many of your
2006 Annual Report. Anastomosis Made Simple
 2006 Annual Report R Anastomosis Made Simple Cardica designs and manufactures proprietary automated anastomosis systems used by surgeons to perform rapid, reliable and consistent connections, or anastomoses,
2006 Annual Report R Anastomosis Made Simple Cardica designs and manufactures proprietary automated anastomosis systems used by surgeons to perform rapid, reliable and consistent connections, or anastomoses,
DELICA D:5 ACTIVE GEAR 0
 DELICA D:5 ACTIVE GEAR 0 1 1. FY2017 1Q Results 2. FY2017 1Q Results by Region 3. FY2017 Forecast 4. Initiatives for the Future FY2017 1Q Results Summary (vs. FY2016 1Q) (Apr-Jun 2016) (Apr-Jun 2017) (billion
DELICA D:5 ACTIVE GEAR 0 1 1. FY2017 1Q Results 2. FY2017 1Q Results by Region 3. FY2017 Forecast 4. Initiatives for the Future FY2017 1Q Results Summary (vs. FY2016 1Q) (Apr-Jun 2016) (Apr-Jun 2017) (billion
Blood Flow Measurement Devices Market - Global Industry Analysis, Size, Share, Growth, Trends and Forecast, Single User License: US $ 4595
 Transparency Market Research Blood Flow Measurement Devices Market - Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2013-2019 Single User License: US $ 4595 Buy Now Request Sample
Transparency Market Research Blood Flow Measurement Devices Market - Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2013-2019 Single User License: US $ 4595 Buy Now Request Sample
National Imaging Associates, Inc. Clinical guidelines CARDIAC CATHETERIZATION -LEFT HEART CATHETERIZATION. Original Date: October 2015 Page 1 of 5
 National Imaging Associates, Inc. Clinical guidelines CARDIAC CATHETERIZATION -LEFT HEART CATHETERIZATION CPT Codes: 93451, 93452, 93453, 93454, 93455, 93456, 93457, 93458, 93459, 93460, 93461 LCD ID Number:
National Imaging Associates, Inc. Clinical guidelines CARDIAC CATHETERIZATION -LEFT HEART CATHETERIZATION CPT Codes: 93451, 93452, 93453, 93454, 93455, 93456, 93457, 93458, 93459, 93460, 93461 LCD ID Number:
1. CARDIOLOGY. These listings cannot be correctly interpreted without reference to the Preamble. Anes. $ Level
 1. CARDIOLOGY These listings cannot be correctly interpreted without reference to the Preamble. Anes. Referred Cases 33010 Consultation: To consist of examination, review of history, laboratory, X-ray
1. CARDIOLOGY These listings cannot be correctly interpreted without reference to the Preamble. Anes. Referred Cases 33010 Consultation: To consist of examination, review of history, laboratory, X-ray
Adult Cardiology Clinical Privileges
 Name: Effective from / / to / / Initial privileges (initial appointment) (reappointment) Renewal of privileges All new applicants should meet the following requirements as approved by the governing body,
Name: Effective from / / to / / Initial privileges (initial appointment) (reappointment) Renewal of privileges All new applicants should meet the following requirements as approved by the governing body,
Cardiac Rhythm Management Coder 2017
 Cardiac Rhythm Management Coder 2017 An easy-to-use tool for coding and reimbursement compliance Prepared and Published By: MedLearn Publishing, A Division of Panacea Healthcare Solutions, Inc. 287 East
Cardiac Rhythm Management Coder 2017 An easy-to-use tool for coding and reimbursement compliance Prepared and Published By: MedLearn Publishing, A Division of Panacea Healthcare Solutions, Inc. 287 East
Patient Resources: Arrhythmias and Congenital Heart Disease
 Patient Resources: Arrhythmias and Congenital Heart Disease Overview Arrhythmias (abnormal heart rhythms) can develop in patients with congenital heart disease (CHD) due to thickening/weakening of their
Patient Resources: Arrhythmias and Congenital Heart Disease Overview Arrhythmias (abnormal heart rhythms) can develop in patients with congenital heart disease (CHD) due to thickening/weakening of their
Interventional procedures guidance Published: 26 September 2014 nice.org.uk/guidance/ipg504
 Transcatheter valve-in-valve e implantation for aortic bioprosthetic valve dysfunction Interventional procedures guidance Published: 26 September 2014 nice.org.uk/guidance/ipg504 Your responsibility This
Transcatheter valve-in-valve e implantation for aortic bioprosthetic valve dysfunction Interventional procedures guidance Published: 26 September 2014 nice.org.uk/guidance/ipg504 Your responsibility This
A world leader in allergy immunotherapy
 A world leader in allergy immunotherapy Investor Relations presentation June 2017 1 I Investor Relations presentation I June 2017 ALK: Towards redefining treatment of severe allergies The commercial leader
A world leader in allergy immunotherapy Investor Relations presentation June 2017 1 I Investor Relations presentation I June 2017 ALK: Towards redefining treatment of severe allergies The commercial leader
Your heart is a muscular pump about the size of your fist, located
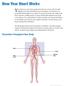 How Your Heart Works Your heart is a muscular pump about the size of your fist, located slightly to the left and behind your breastbone. Its function is to pump blood throughout your body. As your heart
How Your Heart Works Your heart is a muscular pump about the size of your fist, located slightly to the left and behind your breastbone. Its function is to pump blood throughout your body. As your heart
2.02 Understand the functions and disorders of the circulatory system
 2.02 Understand the functions and disorders of the circulatory system 2.02 Understand the functions and disorders of the circulatory system Essential questions: What are the functions of blood? What are
2.02 Understand the functions and disorders of the circulatory system 2.02 Understand the functions and disorders of the circulatory system Essential questions: What are the functions of blood? What are
Unit 1: Human Systems. The Circulatory System
 Unit 1: Human Systems The Circulatory System nourish all cells with oxygen, glucose, amino acids and other nutrients and carry away carbon dioxide, urea and other wastes Purposes Transport chemical messengers
Unit 1: Human Systems The Circulatory System nourish all cells with oxygen, glucose, amino acids and other nutrients and carry away carbon dioxide, urea and other wastes Purposes Transport chemical messengers
Company Overview February 26, 2019
 Company Overview February 26, 2019 SAFE HARBOR Cautionary Note Regarding Forward-Looking Statements Certain statements in this presentation constitute forward-looking statements, including, without limitation,
Company Overview February 26, 2019 SAFE HARBOR Cautionary Note Regarding Forward-Looking Statements Certain statements in this presentation constitute forward-looking statements, including, without limitation,
Oral semaglutide and production expansion. Henrik Wulff EVP Product Supply. Peter Kristensen SVP Global Development
 YASMIN FIEDLER Germany Yasmin has type 1 diabetes Slide 1 Oral semaglutide and production expansion Henrik Wulff EVP Product Supply Peter Kristensen SVP Global Development Slide 2 Forward-looking statements
YASMIN FIEDLER Germany Yasmin has type 1 diabetes Slide 1 Oral semaglutide and production expansion Henrik Wulff EVP Product Supply Peter Kristensen SVP Global Development Slide 2 Forward-looking statements
Global Remote Cardiac Monitoring Market: Size, Trends & Forecasts ( )
 Global Remote Cardiac Monitoring Market: Size, Trends & Forecasts (2016-2020) #910680 $800 55 pages In Stock Report Description Scope of the Report The report entitled Global Remote Cardiac Monitoring
Global Remote Cardiac Monitoring Market: Size, Trends & Forecasts (2016-2020) #910680 $800 55 pages In Stock Report Description Scope of the Report The report entitled Global Remote Cardiac Monitoring
ICD Implantation Patient Information
 Melbourne Heart Rhythm ICD Implantation Patient Information The Heart The heart is a pump responsible for maintaining blood supply to the body. It has four chambers. The two upper chambers (the right atrium
Melbourne Heart Rhythm ICD Implantation Patient Information The Heart The heart is a pump responsible for maintaining blood supply to the body. It has four chambers. The two upper chambers (the right atrium
Trans-catheter aortic valve implantation (TAVI) work up
 Trans-catheter aortic valve implantation (TAVI) work up You have been referred for an assessment known as a TAVI work up because you have been diagnosed with aortic stenosis. This factsheet explains the
Trans-catheter aortic valve implantation (TAVI) work up You have been referred for an assessment known as a TAVI work up because you have been diagnosed with aortic stenosis. This factsheet explains the
2015 Facility and Physician Billing Guide Heart Valve Technologies
 2015 Facility and Physician Billing Guide Heart Valve Technologies PHYSICIAN BILLING CODES Clinicians use Current Procedural Terminology (CPT 1 ) codes to bill for procedures and services. Each CPT code
2015 Facility and Physician Billing Guide Heart Valve Technologies PHYSICIAN BILLING CODES Clinicians use Current Procedural Terminology (CPT 1 ) codes to bill for procedures and services. Each CPT code
Heart Failure. Symptoms and Treatments. FloridaHospital.com
 Heart Failure Symptoms and Treatments FloridaHospital.com Understanding Heart Failure According to the American Heart Association, one in five people over age 40 will develop heart failure. Right now,
Heart Failure Symptoms and Treatments FloridaHospital.com Understanding Heart Failure According to the American Heart Association, one in five people over age 40 will develop heart failure. Right now,
Key Trends for Ambulatory Surgery Centers in 2018
 Key Trends for Ambulatory Surgery Centers in 2018 Don Phalen Vice President Business Development, Regent Surgical Health Mark Murphy Chief Strategy Officer, St Joseph s Hospital MOVING TOWARDS VALUE-BASED
Key Trends for Ambulatory Surgery Centers in 2018 Don Phalen Vice President Business Development, Regent Surgical Health Mark Murphy Chief Strategy Officer, St Joseph s Hospital MOVING TOWARDS VALUE-BASED
There are different types of ICDs:
 Guidelines for Patients with Implantable Devices o Implantable Cardioverter Defibrillator (ICD) Your ICD is approximately the size of a pager and includes the following parts: The ICD: A battery powered
Guidelines for Patients with Implantable Devices o Implantable Cardioverter Defibrillator (ICD) Your ICD is approximately the size of a pager and includes the following parts: The ICD: A battery powered
CPH Chemie + Papier Holding AG. Investor Day. Perlen, 13 th September 2018
 CPH Chemie + Papier Holding AG Investor Day Perlen, 13 th September 2018 Agenda 2.00 pm Welcome 2.05 pm Presentation Course of Business 2018 2.50 pm Break 3.00 pm Presentation Perlen Packaging 3.30 pm
CPH Chemie + Papier Holding AG Investor Day Perlen, 13 th September 2018 Agenda 2.00 pm Welcome 2.05 pm Presentation Course of Business 2018 2.50 pm Break 3.00 pm Presentation Perlen Packaging 3.30 pm
Q Investor Kit JANUARY-MARCH 2014
 Q1 2014 Investor Kit JANUARY-MARCH 2014 Swedish Match reporting segments Snus and moist snuff Snus (Scandinavia and US) Moist snuff (US) SMPM International Other tobacco products Cigars (US) Chewing tobacco
Q1 2014 Investor Kit JANUARY-MARCH 2014 Swedish Match reporting segments Snus and moist snuff Snus (Scandinavia and US) Moist snuff (US) SMPM International Other tobacco products Cigars (US) Chewing tobacco
Peripheral and Cardiology Coder 2018
 Peripheral and Cardiology Coder 2018 Cardiovascular Services and Procedures Prepared and Published By: MedLearn Publishing A Division of MedLearn Media, Inc. 445 Minnesota Street, Suite 514 St. Paul, MN
Peripheral and Cardiology Coder 2018 Cardiovascular Services and Procedures Prepared and Published By: MedLearn Publishing A Division of MedLearn Media, Inc. 445 Minnesota Street, Suite 514 St. Paul, MN
Abnormal Heart Rhythms (Arrhythmias)
 Abnormal Heart Rhythms (Arrhythmias) Page 1 of 5 The heart needs a small electrical current to pass through the heart in a very set pattern. This is called the heart's conduction system. This leaflet explains
Abnormal Heart Rhythms (Arrhythmias) Page 1 of 5 The heart needs a small electrical current to pass through the heart in a very set pattern. This is called the heart's conduction system. This leaflet explains
Diagnostic & Therapeutic Cardiac Catheterization Coder 2017
 Diagnostic & Therapeutic Cardiac Catheterization Coder 2017 Including peripheral and cardiovascular services and procedures Prepared and Published By: MedLearn Publishing A Division of Panacea Healthcare
Diagnostic & Therapeutic Cardiac Catheterization Coder 2017 Including peripheral and cardiovascular services and procedures Prepared and Published By: MedLearn Publishing A Division of Panacea Healthcare
CPT Code Details
 CPT Code 93572 Details Code Descriptor Intravascular Doppler velocity and/or pressure derived coronary flow reserve measurement (coronary vessel or graft) during coronary angiography including pharmacologically
CPT Code 93572 Details Code Descriptor Intravascular Doppler velocity and/or pressure derived coronary flow reserve measurement (coronary vessel or graft) during coronary angiography including pharmacologically
View Report Details. Global Aortic Aneurysm Market: Trends and Opportunities ( )
 View Report Details Global Aortic Aneurysm Market: Trends and Opportunities (2014-2019) View Report Details Scope of the Report The report titled Global Aortic Aneurysm Market: Trends and Opportunities
View Report Details Global Aortic Aneurysm Market: Trends and Opportunities (2014-2019) View Report Details Scope of the Report The report titled Global Aortic Aneurysm Market: Trends and Opportunities
Transcatheter Aortic Valve Implantation Procedure (TAVI)
 Page 1 of 5 Procedure (TAVI) Introduction Aortic stenosis (AS) is a common heart valve problem associated with heart failure and death. Surgical valve repair or replacement is recommended if AS patients
Page 1 of 5 Procedure (TAVI) Introduction Aortic stenosis (AS) is a common heart valve problem associated with heart failure and death. Surgical valve repair or replacement is recommended if AS patients
For personal use only
 Cochlear Limited Results for the half year ended 31 December 2011 (H1 F12) Chris Roberts, CEO Neville Mitchell, CFO Cochlear Overview Cochlear Limited (ASX:COH): global leader in implantable devices for
Cochlear Limited Results for the half year ended 31 December 2011 (H1 F12) Chris Roberts, CEO Neville Mitchell, CFO Cochlear Overview Cochlear Limited (ASX:COH): global leader in implantable devices for
Personalized Care One Heart at a Time
 Personalized Care One Heart at a Time You can have confidence in your care at The James Family Heart Center at YRMC West, where our highly qualified surgeons, nurses and technologists provide a hearthealthy
Personalized Care One Heart at a Time You can have confidence in your care at The James Family Heart Center at YRMC West, where our highly qualified surgeons, nurses and technologists provide a hearthealthy
Slide 1. Investor presentation. London 5 February 2019
 Slide Investor presentation London 5 February 209 Slide 2 Forward-looking statements Novo Nordisk s reports filed with or furnished to the US Securities and Exchange Commission (SEC), including this presentation
Slide Investor presentation London 5 February 209 Slide 2 Forward-looking statements Novo Nordisk s reports filed with or furnished to the US Securities and Exchange Commission (SEC), including this presentation
Asia-Pacific Endoscopic Retrograde Cholangiopancreatography (ERCP) Procedures Outlook to 2020
 Asia-Pacific Endoscopic Retrograde Cholangiopancreatography (ERCP) Reference Code: GDMECR0107PDB Publication Date: July 2014 Page 1 1 Table of Contents 1 Table of Contents... 2 1.1 List of Tables... 3
Asia-Pacific Endoscopic Retrograde Cholangiopancreatography (ERCP) Reference Code: GDMECR0107PDB Publication Date: July 2014 Page 1 1 Table of Contents 1 Table of Contents... 2 1.1 List of Tables... 3
A PATIENT S GUIDE TO THE LEFT ATRIAL APPENDAGE CLOSURE. Reducing the risk of stroke in atrial fibrillation
 A PATIENT S GUIDE TO THE LEFT ATRIAL APPENDAGE CLOSURE Reducing the risk of stroke in atrial fibrillation TABLE OF CONTENTS IMPORTANT Please Note: Information provided by Boston Scientific Corporation
A PATIENT S GUIDE TO THE LEFT ATRIAL APPENDAGE CLOSURE Reducing the risk of stroke in atrial fibrillation TABLE OF CONTENTS IMPORTANT Please Note: Information provided by Boston Scientific Corporation
Supplemental Material
 Supplemental Material 1 Table S1. Codes for Patient Selection Cohort Codes Primary PM CPT: 33206 or 33207 or 33208 (without 33225) ICD-9 proc: 37.81, 37.82, 37.83 Primary ICD Replacement PM Replacement
Supplemental Material 1 Table S1. Codes for Patient Selection Cohort Codes Primary PM CPT: 33206 or 33207 or 33208 (without 33225) ICD-9 proc: 37.81, 37.82, 37.83 Primary ICD Replacement PM Replacement
AP2 Lab 3 Coronary Vessels, Valves, Sounds, and Dissection
 AP2 Lab 3 Coronary Vessels, Valves, Sounds, and Dissection Project 1 - BLOOD Supply to the Myocardium (Figs. 18.5 &18.10) The myocardium is not nourished by the blood while it is being pumped through the
AP2 Lab 3 Coronary Vessels, Valves, Sounds, and Dissection Project 1 - BLOOD Supply to the Myocardium (Figs. 18.5 &18.10) The myocardium is not nourished by the blood while it is being pumped through the
The Dental Corporation Opportunity
 The Dental Corporation Opportunity for Practice Principals. It s the perfect professional collaboration. You focus on dentistry, we look after the paperwork. In recognition of what you, the Practice Principal,
The Dental Corporation Opportunity for Practice Principals. It s the perfect professional collaboration. You focus on dentistry, we look after the paperwork. In recognition of what you, the Practice Principal,
Slide 1. International Operations update. Mike Doustdar EVP International Operations. YASMIN FIEDLER, Germany Yasmin has type 1 diabetes
 Slide 1 International Operations update Mike Doustdar EVP International Operations YASMIN FIEDLER, Germany Yasmin has type 1 diabetes Slide 2 Forward-looking statements Novo Nordisk s reports filed with
Slide 1 International Operations update Mike Doustdar EVP International Operations YASMIN FIEDLER, Germany Yasmin has type 1 diabetes Slide 2 Forward-looking statements Novo Nordisk s reports filed with
FY2007 Consolidated Financial Overview
 FY2007 Consolidated Financial Overview CHUGAI PHARMACEUTICAL CO., LTD. Executive Vice President and CFO Ryuzo Kodama January 30/31, 2008 Forward-Looking Statements This presentation may include forward-looking
FY2007 Consolidated Financial Overview CHUGAI PHARMACEUTICAL CO., LTD. Executive Vice President and CFO Ryuzo Kodama January 30/31, 2008 Forward-Looking Statements This presentation may include forward-looking
2015 ST. JUDE MEDICAL THERAPY CODING GUIDE Cardiac Pacemakers
 2015 ST. JUDE MEDICAL THERAPY CODING GUIDE Cardiac Pacemakers This guide provides physician and hospital coding and reimbursement information for cardiac pacemaker procedures. In addition, St. Jude Medical
2015 ST. JUDE MEDICAL THERAPY CODING GUIDE Cardiac Pacemakers This guide provides physician and hospital coding and reimbursement information for cardiac pacemaker procedures. In addition, St. Jude Medical
REBEL. Platinum Chromium Coronary Stent System. Patient Information Guide
 REBEL Patient Information Guide REBEL PATIENT INFORMATION GUIDE You have recently had a REBEL bare metal stent implanted in the coronary arteries of your heart. The following information is important for
REBEL Patient Information Guide REBEL PATIENT INFORMATION GUIDE You have recently had a REBEL bare metal stent implanted in the coronary arteries of your heart. The following information is important for
Unit 6: Circulatory System. 6.2 Heart
 Unit 6: Circulatory System 6.2 Heart Functions of Circulatory System 1. The heart is the pump necessary to circulate blood to all parts of the body 2. Arteries, veins and capillaries are the structures
Unit 6: Circulatory System 6.2 Heart Functions of Circulatory System 1. The heart is the pump necessary to circulate blood to all parts of the body 2. Arteries, veins and capillaries are the structures
Structural Heart Devices Market by Product (Structural Heart Repair Devices and Replacement Valves), Age Group (Pediatric and Adult), and Indication
 Structural Heart Devices Market by Product (Structural Heart Repair Devices and Replacement Valves), Age Group (Pediatric and Adult), and Indication (Atrial Septal Defect (ASD), Patent Foramen Ovale (PFO),
Structural Heart Devices Market by Product (Structural Heart Repair Devices and Replacement Valves), Age Group (Pediatric and Adult), and Indication (Atrial Septal Defect (ASD), Patent Foramen Ovale (PFO),
Introduction What Causes Peripheral Vascular Disease? How Do Doctors Treat Peripheral Vascular Disease?... 9
 Patient Information Table of Contents Introduction... 3 What is Peripheral Vascular Disease?... 5 What Are Some of the Symptoms of Peripheral Vascular Disease?... 7 What Causes Peripheral Vascular Disease?...
Patient Information Table of Contents Introduction... 3 What is Peripheral Vascular Disease?... 5 What Are Some of the Symptoms of Peripheral Vascular Disease?... 7 What Causes Peripheral Vascular Disease?...
