Aspira* Peritoneal Drainage Catheter
|
|
|
- Osborne Marsh
- 6 years ago
- Views:
Transcription
1 Aspira* Peritoneal Drainage Catheter Instructions For Use Access Systems
2
3 Product Description: The Aspira* Peritoneal Drainage Catheter is a tunneled, long-term catheter used to drain accumulated fluid from the peritoneal cavity to relieve symptoms associated with malignant ascites. The catheter is implanted in the patient s peritoneal cavity enabling the patient to perform intermittent malignant ascites drainage at home. Drainage is achieved using the Aspira* Peritoneal Drainage System. The primary components of the system are the Aspira* Peritoneal Drainage Catheter and the Aspira* Drainage Kit. The proximal end of the catheter has a valve that prevents fluid or air from moving in or out of the peritoneal cavity until the valve is activated. The valve can be activated by the Aspira* Drainage Kit or by connecting the catheter to a wall suction unit, water seal drainage system, glass vacuum bottle, syringe, or other appropriate method using the Luer Adapter or Universal Tubing Adapter. Fluid drains by connecting the drainage bag to the catheter, pumping the in-line siphon chamber to initiate flow, and allowing gravity to drain the fluid. When draining fluid using a wall suction (glass vacuum bottle), syringe or other appropriate method, fluid drains by connecting the drainage unit to the Luer Adapter or Universal Tubing Adapter and then to the catheter. The peritoneal drainage catheter provides patients with a convenient and compassionate way to relieve malignant ascites symptoms at home. Indications For Use: The Aspira* Drainage System is indicated for intermittent drainage of recurrent and symptomatic malignant ascites. The catheter is intended for long-term access of the peritoneal cavity in order to relieve symptoms such as dyspnea or other symptoms associated with malignant ascites. Contraindications, Warnings and Precautions: Contraindications: This device is contraindicated under the following conditions: Known or suspected peritoneal cavity infection. Known or suspected coagulopathy or other hemorrhagic tendency. Peritoneal cavity fluid is multi-loculated in a way that drainage from a single location is not expected to effectively relieve related symptoms. Patient medical condition including their anatomy is insufficient to accommodate an indwelling drainage catheter. Patient is known or suspected to be allergic to materials contained in the device. Patient has a medical history of symptom palliation failure by peritoneal drainage. 1
4 Warnings: Intended for Single Use. DO NOT REUSE. Reuse and/or repackaging may create a risk of patient or user infection, compromise the structural integrity and/or essential material and design characteristics of the device, which may lead to device failure, and/or lead to injury, illness or death of the patient. Do not use excessive force on the valve or catheter. Excessive force or incorrect usage may damage the device, or cause accidental catheter dislodgement. Accessing the catheter valve with anything other than the Aspira* Drainage Bag, Universal Tubing Adapter or Luer Adapter may damage the valve. Dispose of the used product in accordance with accepted medical practice and applicable local, state and federal regulations. Used product may present a potential biohazard. When using the Luer Adapter or Universal Tubing Adapter to access the catheter, attach the adapter to the syringe or wall suction line prior to attachment to the catheter. Do not attempt to pass a wire, needle or other device through the valve. The Luer Adapter and Universal Tubing Adapter create an open pathway into or out of the catheter; to close the pathway when not in use, tighten the pinch clamp. Do not flush or attempt to clear an occluded catheter with a syringe smaller than 10 ml. Precautions: Federal (USA) law restricts this device to sale by or on the order of a physician. Carefully read and follow instructions prior to using this device. Insertion or removal of this device is only to be done by qualified health professionals. Follow aseptic techniques when inserting the catheter. Sterilized using ethylene oxide. Do not resterilize. Avoid puncturing or lacerating the liver, bowel or any abdominal organs with the introducer needle. If guidewire must be withdrawn while the needle is inserted, remove both the needle and guidewire as a unit to prevent the needle from damaging or shearing the guidewire. Sutures should not be tied around the catheter itself. The provided suture wings will secure the catheter without compromising catheter patency. Use only the Luer Adapter or Universal Tubing Adapter to access the catheter with a syringe, wall suction unit, water seal drainage system or glass vacuum bottle per instructions below. Prior to Placement: Check the package for damage before opening. Ensure the expiration date has not passed. Inspect kit to ensure all components are included. During Placement: Do not allow the device to contact sharp instruments. Mechanical damage may occur. Use only smooth edged atraumatic clamps or forceps. Care must be taken to avoid damaging the peritoneal wall or organs contained in the peritoneum. Do not use the catheter if it is damaged. Carefully follow the catheter valve connection technique described in the 2
5 instructions to ensure proper connection and avoid catheter damage. If guidewire must be withdrawn while the needle is inserted, remove both the needle and guidewire as a unit to prevent the needle from damaging or shearing the guidewire. After Placement: Do not use the catheter if it is damaged. Do not attempt to repair the catheter if damage has occurred within 5 cm of the exit site. Use only the Luer Adapter, Universal Tubing Adapter, or Aspira* Drainage Bag to access the catheter valve. Be careful not to dislodge the catheter when assembling the valve. Possible Complications: Inserting the catheter and draining the ascitic fluid may result in any of the following complications: Infection Exposure to bodily fluids Peritonitis Leakage Hypotension subsequent to drainage Skin irritation or infection Splenic or hepatic laceration Occlusion Pain during fluid removal Catheter malposition Accidental catheter dislodgement, breakage or removal Tumor seeding Catheter or cuff erosion through skin Hemoperitoneum Bowel damage Abdominal wall cellulitis Electrolyte imbalance Sepsis Protein depletion Loculation of peritoneal cavity Hematoma Insertion Instructions: Before beginning this procedure, read the Contraindications, Warnings and Precautions and Possible Complications sections of this manual. There are three possible placement techniques: antegrade, retrograde and over-thewire. The following are common steps that apply to all three placement techniques listed above. Common Steps: 1. Select the site for catheter insertion. 2. Create sterile field and open tray. Surgically prep and drape the operative site. 3. Perform adequate anesthesia. 3
6 4. Flush catheter through Y-connector to hydrate stylet. Allow catheter to soak in saline. (fig. 1a) 5. Attach the introducer needle to the syringe. (fig. 1b) 6. Insert the introducer needle into the peritoneal cavity and aspirate fluid to confirm proper positioning. (fig. 1b) CAUTION: Avoid puncturing or lacerating the liver, bowel or any abdominal organs with the introducer needle. 7. Remove the syringe from the introducer needle. 8. Insert the guidewire through the introducer needle into the peritoneal cavity. (fig. 1c) NOTE: If using over-the-wire technique, select a guidewire that is approximately 1 1/2 times the length of the catheter. 9. Remove the introducer needle over the guidewire and discard it. (fig. 1c) CAUTION: If guidewire must be withdrawn while the needle is inserted, remove both the needle and guidewire as a unit to prevent the needle from damaging or shearing the guidewire. 10. Estimate desired length of catheter. Trim catheter if necessary. NOTE: If fenestrated section is too long for the patient, it may be trimmed to length by cutting between the fenestrations. (Fig. 1a) (fig. 1b) (fig. 1c) Antegrade Tunnel Insertion Procedure: 1. Make an incision at the desired catheter insertion site. Make another incision superior and medial to the insertion site at a distance selected for tunnel length (generally 5 to 8 cm). (fig. 2a) 2. Create tunnel between the 2 incision sites. (fig. 2b) 3. Attach distal end of the catheter to the tunneler. 4. Thread tunneler and catheter from superior incision or catheter exit site to incision at the insertion site. 5. Pull the catheter through the tunnel until the cuff is appropriately positioned. 6. Separate the catheter from the tunneler. 7. Dilate the insertion site, guiding the dilators over the wire. (fig. 2c) 8. Thread the peel-apart introducer sheath over the guidewire into the peritoneal cavity. (fig. 2d) 9. Remove the guidewire and dilator as a unit, leaving the peel-apart introducer sheath in place. (fig. 2d) NOTE: Do not pinch the introducer sheath. Instead, place thumb over the sheath hub to prevent excess fluid from draining out of the peritoneal cavity. 4
7 10. Pass the distal tip of the catheter into the peel-apart introducer sheath ensuring that all fenestrations are within the peritoneal cavity. (fig. 2e) NOTE: The most proximal fenestration is placed through the barium stripe to enable verification of the catheter placement using fluoroscopy or x-ray. 11. Peel away the introducer sheath keeping the catheter in place. 12. Remove stylet from catheter. (fig. 2f) 13. Place slide clamp on the catheter immediately proximal to the exit site. 14. Cut catheter below Y-connector. (fig. 2a) (fig. 2b) (fig. 2c) (fig. 2d) (fig. 2e) (fig. 2f) Retrograde Tunnel Insertion Procedure: 1. Make an incision at the desired catheter insertion site. (fig. 3a) 2. Dilate the insertion site, guiding the dilators over the wire. (fig. 3b) 3. Thread the peel-apart introducer sheath over the guidewire into the peritoneal cavity. (fig. 3c) 4. Remove the guidewire and dilator as a unit. (fig. 3c) NOTE: Do not pinch the introducer sheath. Instead, place thumb over the sheath hub to prevent excess fluid from draining out of the peritoneal cavity. 5. Pass the distal tip of the catheter into the peel-apart introducer sheath ensuring that all fenestrations are within the peritoneal space. (fig. 3d) NOTE: The most proximal fenestration is placed through the barium stripe to enable verification of catheter placement using fluoroscopy or x-ray. 6. Peel away the introducer sheath keeping the catheter in place. NOTE: Keep the catheter in place. 7. Make an incision superior and medial to the insertion site at a distance selected for 5
8 tunnel length (generally 5 to 8 cm). 8. Create a tunnel between the 2 incision sites. (fig. 3e) 9. Remove the stylet from the catheter. (fig. 3f) 10. Cut catheter below Y-connector. 11. Attach proximal end of the catheter to tunneler. 12. Thread tunneler and catheter from insertion site to incision at catheter exit site. 13. Pull the catheter through the tunnel until the cuff is appropriately positioned. 14. Place slide clamp on the catheter immediately proximal to the exit site. 15. Separate the catheter from the tunneler. (fig. 3a) (fig. 3b) (fig. 3c) (fig. 3d) (fig. 3e) (fig. 3f) Over the Wire Insertion Procedure: 1. Make an incision at the desired catheter insertion site. (fig. 4a) 2. Dilate the insertion site, guiding the dilators over the wire. (fig. 4b) 3. Thread the peel-apart introducer sheath over the guidewire into the peritoneal cavity. (fig. 4c) 4. Remove dilator leaving the wire and peel-apart introducer in place. (fig. 4c) 5. Pass catheter over the guidewire and through peel-apart introducer. Ensure that all fenestrations lay within the peritoneal cavity. (fig. 4d) NOTE: The most proximal fenestration is placed through the barium stripe to enable verification of placement using fluoroscopy or x-ray. 6. Peel away the sheath keeping the catheter in place. 7. Make an incision superior and medial to the insertion site at a distance selected for tunnel length (generally 5-8 cm). 8. Create a tunnel between the 2 incision sites. (fig. 4e) 6
9 9. Remove the guidewire and stylet from the catheter as a unit. (fig. 4f) 10. Cut catheter below Y-connector. Attach proximal end to tunneler. 11. Thread tunneler and catheter from insertion site to incision at catheter exit site. 12. Pull the catheter through the tunnel until cuff is appropriately positioned. 13. Place slide clamp on catheter immediately proximal to exit site. 14. Separate catheter from tunneler. (fig. 4a) (fig. 4b) (fig. 4c) (fig. 4d) (fig. 4e) (fig. 4f) Catheter to Valve Connection Procedure: 1. Advance catheter over valve stem until it is past the shoulder. (fig. 1) NOTE: Once the catheter and valve assembly are connected, they cannot be removed and reused. To replace valve assembly, trim catheter below the valve assembly and attach a new valve assembly to ensure a secure connection. 2. Remove slide clamp from the catheter. 3. Ensure patency using the Luer Adapter. (see using a syringe) WARNING: When using the Luer Adapter or the Universal Tubing Adapter to access the catheter, the adapter must be attached to the syringe or wall suction prior to attaching to the catheter. 4. Palpate the catheter along the tunnel track to ensure proper positioning without kinks. 5. Suture the incision sites as needed. 6. Secure the catheter to the skin near the exit site using the provided suture wings or as instructed by institution protocol. CAUTION: Sutures should not be tied around the catheter itself. The provided suture wings will secure the catheter without compromising catheter patency. 7
10 (fig. 1) Initial Drainage Procedure: After catheter placement, perform fluid drainage using an Aspira* Drainage Bag, syringe, standard wall suction unit, water seal drainage system, glass vacuum bottle or other appropriate method. CAUTION: Use only the Luer Adapter or Universal Tubing Adapter to access the catheter with a syringe, wall suction unit, water seal drainage system or glass vacuum bottle per instructions below. NOTE: When using the Aspira* Drainage Kit, follow instructions for use supplied. WARNING: The Luer Adapter and Universal Tubing Adapter create an open pathway into or out of the catheter; to close the pathway when not in use, tighten pinch clamp. Drainage Line - Luer Adapter - Catheter Luer Adapter - Catheter 8
11 Using a Syringe: 1. Connect supplied Luer Adapter to the syringe. 2. Push the Luer Adapter and syringe onto the catheter until you hear or feel a click. Tug gently to ensure connection is secure. 3. Pull back on the syringe plunger to draw fluid out of the peritoneal cavity. 4. When drainage is complete, disconnect Luer Adapter and syringe by squeezing the wings on the Luer Adapter and gently pulling to separate it from the catheter valve. NOTE: If necessary to repeat procedure, disconnect Luer Adapter from catheter valve between drainages. Using a Wall Suction Unit: 1. Connect the Luer Adapter or Universal Tubing Adapter to a Medi-Vac* Non-Conductive Suction Tube. 2. Attach the opposite end of the tubing to wall suction. Activate the pinch clamp. 3. Push the Luer Adapter or Universal Tubing Adapter onto the catheter valve until you hear or feel a click. Tug gently to ensure connection is secure. Open the pinch clamp. 4. Initiate Drain. 5. When ready to disconnect wall suction, pinch the wings on the Luer Adapter or Universal Tubing Adapter until it easily comes away from the catheter valve. NOTE: Continuous or intermittent wall suction is acceptable. WARNING: The Luer Adapter creates an open pathway into or out of the catheter; to close the pathway when not in use, tighten pinch clamp. Using a Glass Vacuum Bottle: 1. Attach male-to-male vinyl connecting tube to the Luer Adapter. 2. Attach the other end of the tubing to an 18 G percutaneous entry needle. Activate the pinch clamp. 3. Push the Luer Adapter onto the catheter valve until you hear or feel a click. Tug gently to ensure connection is secure. 4. Puncture the vacuum bottle seal with needle. Open the pinch clamp. 5. When drainage is complete, pinch the wings of the Luer Adapter until it easily comes away from the catheter valve. WARNING: The Luer Adapter or Universal Tubing Adapter creates an open pathway into or out of the catheter; to close the pathway when not in use, tighten pinch clamp. 9
12 Dressing the Catheter: Weekly Dressing Procedure (option #1): 1. Wipe the end of the catheter valve with an alcohol pad. 2. Place the valve protective cap on the catheter valve. 3. Place a split gauze pad on the skin around the catheter. Lay the catheter straight down toward the waist and place gauze on top of split gauze pad. 4. Hold gauze and split gauze pad in position. 5. Place the transparent dressing over the gauze. 6. Tape catheter to patient s skin. Alternative Dressing Procedure (with every drainage procedure) (option #2): 1. Wipe the end of the catheter valve with an alcohol pad. 2. Place the valve protective cap on the catheter valve. 3. Place a split gauze pad on the skin around the catheter. Coil the catheter on top of the pad and place gauze on top of the coiled catheter. 4. Hold gauze, coiled catheter and split gauze pad in position. 5. Place the transparent dressing over the catheter and gauze. Catheter Maintenance: See Dressing Kit and Drainage Kit instructions for use or patient guide for regular peritoneal drainage and catheter maintenance information. Catheters that present resistance to flushing and aspiration may be partially or completely occluded. Do not flush against resistance. If the lumen will neither flush nor aspirate, and it has been determined that the catheter is occluded, a declotting procedure may be followed per institution protocol. WARNING: Do not flush or attempt to clear an occluded catheter with a syringe smaller than 10 ml. In the case of valve or catheter damage, the Aspira* Valve Assembly / Repair Kit may be used to replace the valve. Catheter Removal: The retention cuff facilitates tissue in-growth. The catheter must be surgically removed. Free the cuff from the tissue and pull the catheter gently and smoothly. 10
13 References: Belfort, M.A.; Stevens, P. J.; DeHaek, K.; Soeters, R.; Krige, J.E.J. A New Approach to the Management of Malignant Ascites; a Permanently Implanted Abdominal Drain, European Journal of Surgical Oncology, Vol. 16, No. 1, February 1990, pp Bui, C.; Martin, C. J.; Currow, D. J. Effective Community Palliation of Intractable Malignant Ascites with a Permanently Implanted Abdominal Drain, Journal of Palliative Medicine, Vol. 2, No. 3, Fall 1999, pp Richard III, H. M.; Coldwell, D. M.; Boyd-Kranis, R. L.; Murthy, R; Van Echo, D. A. Pleurx Tunneled Catheter in the Management of Malignant Ascites, Journal of Vascular and Interventional Radiology, Vol. 12 No. 3, March 2001, pp Rosenberg, S.; Coutney, A.; Nemcek jr., A. A.; Omary, R. A. Comparison of Percutaneous Management Techniques for Recurrent Malignant Ascites, Journal of Vascular and Interventional Radiology, Vol. 15, No. 10, October 2004; pp
14
15
16 An issued or revision date for these instructions is included for the user s information. In the event two years have elapsed between this date and product use, the user should contact Bard Access Systems, Inc. to see if additional product information is available. Revised Date: May 2011 * Bard and Aspira are trademarks and/or registered trademarks of C. R. Bard, Inc. All other trademarks are the property of their respective owners C. R. Bard, Inc. All rights reserved. Bard Access Systems, Inc. 605 North 5600 West Salt Lake City, UT U.S.A (Ordering Information) (Clinical Information) R
ASEPT Pleural Drainage System
 ORDERING INFORMATION ASEPT Drainage PRODUCTS (Provided separately, see package label for contents) ASEPT Pleural Drainage System 622289 (1 each) (includes ASEPT Pleural drainage catheter and insertion
ORDERING INFORMATION ASEPT Drainage PRODUCTS (Provided separately, see package label for contents) ASEPT Pleural Drainage System 622289 (1 each) (includes ASEPT Pleural drainage catheter and insertion
ASEPT. Pleural Drainage System INSTRUCTIONS FOR USE REF LOT STERILE EO. Manufactured for: 824 Twelfth Avenue Bethlehem, PA
 ASEPT Pleural Drainage System LS-00116-01-AB 017-11 INSTRUCTIONS FOR USE Do not reuse STERILIZE Do not resterilize 30 C Rx only 15 C REF Store at controlled room temperature 15-30 C (59-86 F) Keep away
ASEPT Pleural Drainage System LS-00116-01-AB 017-11 INSTRUCTIONS FOR USE Do not reuse STERILIZE Do not resterilize 30 C Rx only 15 C REF Store at controlled room temperature 15-30 C (59-86 F) Keep away
ASEPT. Pleural Drainage System INSTRUCTIONS FOR USE. Rx only REF LOT. STERILE EO Sterilized using ethylene oxide
 ASEPT Pleural Drainage System INSTRUCTIONS FOR USE L1171/D REV 2014-02 2 Do not reuse 2 STERILIZE Do not resterilize Store at room temperature STERILE EO Sterilized using ethylene oxide Rx only Do not
ASEPT Pleural Drainage System INSTRUCTIONS FOR USE L1171/D REV 2014-02 2 Do not reuse 2 STERILIZE Do not resterilize Store at room temperature STERILE EO Sterilized using ethylene oxide Rx only Do not
Peritoneal Drainage System
 ASEPT Drainage Parts and Accessories (Provided separately, see package label for contents.) ASEPT Pleural Drainage System 622289 (1 each) (includes ASEPT drainage catheter and insertion kit) ASEPT Peritoneal
ASEPT Drainage Parts and Accessories (Provided separately, see package label for contents.) ASEPT Pleural Drainage System 622289 (1 each) (includes ASEPT drainage catheter and insertion kit) ASEPT Peritoneal
DISCOVER NEW HORIZONS IN FLUID DRAINAGE. Bringing Safety and Convenience to Fluid Drainage Management
 DISCOVER NEW HORIZONS IN FLUID DRAINAGE Bringing Safety and Convenience to Fluid Drainage Management DRAIN ASEPT Pleural and Peritoneal Drainage Catheter System 600mL or 1,000mL Evacuated Drainage Bottle
DISCOVER NEW HORIZONS IN FLUID DRAINAGE Bringing Safety and Convenience to Fluid Drainage Management DRAIN ASEPT Pleural and Peritoneal Drainage Catheter System 600mL or 1,000mL Evacuated Drainage Bottle
Peel-Apart Percutaneous Introducer Kits for
 Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Per-Q-Cath* PICC Catheters with Excalibur Introducer* System
 Bard Access Systems Per-Q-Cath* PICC and Catheters with Excalibur Introducer* System Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description, Indications & Contraindications
Bard Access Systems Per-Q-Cath* PICC and Catheters with Excalibur Introducer* System Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description, Indications & Contraindications
Groshong Catheters with Introcan Safety Peripheral IV Catheter Introducer
 Bard Access Systems Groshong Catheters with Introcan Safety Peripheral IV Catheter Introducer Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description 2 Product Description,
Bard Access Systems Groshong Catheters with Introcan Safety Peripheral IV Catheter Introducer Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description 2 Product Description,
Groshong* PICC and Catheters
 Bard Access Systems Groshong* PICC and Catheters Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description 2 Product Description, Indications & Contraindications 3 Contraindications,
Bard Access Systems Groshong* PICC and Catheters Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description 2 Product Description, Indications & Contraindications 3 Contraindications,
CATHETER ACCESS KIT. For use with Prometra Programmable Infusion Systems
 CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
FLEXIC ATH LTD. Peripherally Inserted. Instructions n For Use.
 FLEXIC ATH LTD * M/29M Peripherally Inserted Catheter Instructions n For Use This leaflet contains instructions for both stan- dard needle-introducer er and protection con- tained M/29 models, i.e., with
FLEXIC ATH LTD * M/29M Peripherally Inserted Catheter Instructions n For Use This leaflet contains instructions for both stan- dard needle-introducer er and protection con- tained M/29 models, i.e., with
Drainage Frequency: PATIENT GUIDE. Dressing Frequency: Every Drainage Weekly Drainage. Physician Contact Information. Dr. Phone:
 Drainage Frequency: PATIENT GUIDE Dressing Frequency: Every Drainage Weekly Drainage Physician Contact Information Dr. Phone: CHEST DRAINAGE Pleural Space Insertion Site Cuff Exit Site Catheter Valve Connector
Drainage Frequency: PATIENT GUIDE Dressing Frequency: Every Drainage Weekly Drainage Physician Contact Information Dr. Phone: CHEST DRAINAGE Pleural Space Insertion Site Cuff Exit Site Catheter Valve Connector
Vascu-PICC WITH CUFF PERIPHERALLY INSERTED CENTRAL VEIN ACCESS CATHETER INSTRUCTIONS FOR USE
 Vascu-PICC WITH CUFF PERIPHERALLY INSERTED CENTRAL VEIN ACCESS CATHETER INSTRUCTIONS FOR USE INDICATIONS FOR USE: The Vascu-PICC with cuff Peripherally Inserted Central Vein Catheters are designed for
Vascu-PICC WITH CUFF PERIPHERALLY INSERTED CENTRAL VEIN ACCESS CATHETER INSTRUCTIONS FOR USE INDICATIONS FOR USE: The Vascu-PICC with cuff Peripherally Inserted Central Vein Catheters are designed for
Home Health Foundation, Inc. To create more permanent IV access for patients undergoing long term IV therapy.
 PROCEDURE ORIGINAL DATE: 06/99 Revised Date: 09/02 Home Health Foundation, Inc. SUBJECT: PURPOSE: MIDLINE CATHETER INSERTION To create more permanent IV access for patients undergoing long term IV therapy.
PROCEDURE ORIGINAL DATE: 06/99 Revised Date: 09/02 Home Health Foundation, Inc. SUBJECT: PURPOSE: MIDLINE CATHETER INSERTION To create more permanent IV access for patients undergoing long term IV therapy.
BARD. Instructions For Use
 BARD Instructions For Use 1 Product Description PowerMidline Catheters are a family of peripherally placed catheters made from radiopaque body-softening polyurethane materials. Each PowerMidline Catheter
BARD Instructions For Use 1 Product Description PowerMidline Catheters are a family of peripherally placed catheters made from radiopaque body-softening polyurethane materials. Each PowerMidline Catheter
Initial placement 24FR Pull PEG kit REORDER NO:
 Initial placement 24FR Pull PEG kit REORDER NO: 00710805 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 24FR Pull PEG kit is
Initial placement 24FR Pull PEG kit REORDER NO: 00710805 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 24FR Pull PEG kit is
Groshong Central Venous Catheters
 Bard Access Systems Groshong Central Venous Catheters Long Term Instructions For Use Table of Contents Contents Page Introduction... 1 Description Placement Schematics Groshong Valve Function Indications
Bard Access Systems Groshong Central Venous Catheters Long Term Instructions For Use Table of Contents Contents Page Introduction... 1 Description Placement Schematics Groshong Valve Function Indications
Ponsky * PEG Safety System - "Pull" Bard * PEG Safety System - "Guidewire" Information for Use
 Ponsky * PEG Safety System - "Pull" Bard * PEG Safety System - "Guidewire" Information for Use Rx only Single patient use DEHP-Free This product and package do not contain natural rubber latex STERILE
Ponsky * PEG Safety System - "Pull" Bard * PEG Safety System - "Guidewire" Information for Use Rx only Single patient use DEHP-Free This product and package do not contain natural rubber latex STERILE
Initial placement 20FR Guidewire PEG kit REORDER NO:
 Initial placement 20FR Guidewire PEG kit REORDER NO: 00710802 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 20FR Guidewire
Initial placement 20FR Guidewire PEG kit REORDER NO: 00710802 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 20FR Guidewire
Radiology Polyurethane Catheter
 Radiology Polyurethane Catheter with Microintroducer Revised date: January 2018 Bard, PowerPICC, Provena and StatLock are trademarks and/or registered trademarks of C. R. Bard, Inc. All other trademarks
Radiology Polyurethane Catheter with Microintroducer Revised date: January 2018 Bard, PowerPICC, Provena and StatLock are trademarks and/or registered trademarks of C. R. Bard, Inc. All other trademarks
ER REBOA Catheter. Instructions for Use
 ER REBOA Catheter Instructions for Use Prytime Medical Devices, Inc. 229 N. Main Street Boerne, TX 78006, USA feedback@prytimemedical.com www.prytimemedical.com US 1 210 340 0116 U.S. and Foreign Patents
ER REBOA Catheter Instructions for Use Prytime Medical Devices, Inc. 229 N. Main Street Boerne, TX 78006, USA feedback@prytimemedical.com www.prytimemedical.com US 1 210 340 0116 U.S. and Foreign Patents
Polyurethane Radiology PICC
 Polyurethane Radiology PICC with Microintroducer Revised date: February 2016 Bard, PowerPICC, StatLock, and The Power of Purple are trademarks and/or registered trademarks of C. R. Bard, Inc. All other
Polyurethane Radiology PICC with Microintroducer Revised date: February 2016 Bard, PowerPICC, StatLock, and The Power of Purple are trademarks and/or registered trademarks of C. R. Bard, Inc. All other
Document No. BMB/IFU/40 Rev No. & Date 00 & 15/11/2017 Issue No & Date 01 & 15/11/2017
 Central Venous Catheter Device Description Multi-lumen catheters incorporate separate, non-communicating vascular access lumens within a single catheter body. Minipunctur Access Sets And Trays: Used for
Central Venous Catheter Device Description Multi-lumen catheters incorporate separate, non-communicating vascular access lumens within a single catheter body. Minipunctur Access Sets And Trays: Used for
ATI Skills Modules Checklist for Central Venous Access Devices
 For faculty use only Educator s name Score Date ATI Skills Modules Checklist for Central Venous Access Devices Student s name Date Verify order Patient record Assess for procedure need Identify, gather,
For faculty use only Educator s name Score Date ATI Skills Modules Checklist for Central Venous Access Devices Student s name Date Verify order Patient record Assess for procedure need Identify, gather,
for the ideal insertion of peritoneal dialysis catheter (patent pending)
 for the ideal insertion of peritoneal dialysis catheter (patent pending) CAUTION: U.S. federal law restricts this device to sale by or on the order of a properly licensed practitioner. DEVICE DESCRIPTION
for the ideal insertion of peritoneal dialysis catheter (patent pending) CAUTION: U.S. federal law restricts this device to sale by or on the order of a properly licensed practitioner. DEVICE DESCRIPTION
Better Post-Op Pain Control Starts Here
 Better Post-Op Pain Control Starts Here POST-OP PAIN CONTROL PUMP It s Easy to Get Started About the ACCUFUSER Pump Thank you for considering the This brochure makes it easy for For complete information
Better Post-Op Pain Control Starts Here POST-OP PAIN CONTROL PUMP It s Easy to Get Started About the ACCUFUSER Pump Thank you for considering the This brochure makes it easy for For complete information
AXS Catalyst Distal Access Catheter
 AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
ARROW ENDURANCE. Extended Dwell Peripheral Catheter System. Rx only.
 ARROW ENDURANCE Extended Dwell Peripheral Catheter System Rx only. Product Description: The ARROW Endurance catheter system is a sterile, single use peripheral intravascular device designed to permit access
ARROW ENDURANCE Extended Dwell Peripheral Catheter System Rx only. Product Description: The ARROW Endurance catheter system is a sterile, single use peripheral intravascular device designed to permit access
Exceptional Performance and Ease of Placement
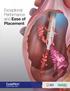 Exceptional Performance and Ease of Placement S IMU LATED FLO W PERFO RMANC E Higher Flow Rates Higher is Better GLIDEPATH catheters demonstrated on average in forward Precision catheters Lower Pressures
Exceptional Performance and Ease of Placement S IMU LATED FLO W PERFO RMANC E Higher Flow Rates Higher is Better GLIDEPATH catheters demonstrated on average in forward Precision catheters Lower Pressures
Designed to Reduce the Risk of Occlusion (1)
 Designed to Reduce the Risk of Occlusion (1) more efficient flushing better outcomes The Vortex* ports feature the Vortex Technology for a more efficient flushing. The Vortex Technology sets up efficient
Designed to Reduce the Risk of Occlusion (1) more efficient flushing better outcomes The Vortex* ports feature the Vortex Technology for a more efficient flushing. The Vortex Technology sets up efficient
CLARIVEIN INFUSION CATHETER
 CLARIVEIN INFUSION CATHETER General Product Description Overview The ClariVein Infusion Catheter (ClariVein -IC) is an infusion catheter system designed to introduce physician-specified medicaments into
CLARIVEIN INFUSION CATHETER General Product Description Overview The ClariVein Infusion Catheter (ClariVein -IC) is an infusion catheter system designed to introduce physician-specified medicaments into
Instructions For Use. Implantable Ports. with Open-Ended and Groshong Catheters
 Instructions For Use Implantable Ports with Open-Ended and Groshong Catheters 3 Implantable Ports with Open-Ended and Groshong Catheters 1 Description The BardPort, SlimPort, and X-Port implantable ports
Instructions For Use Implantable Ports with Open-Ended and Groshong Catheters 3 Implantable Ports with Open-Ended and Groshong Catheters 1 Description The BardPort, SlimPort, and X-Port implantable ports
DIGESTIVE HEALTH ENTERAL FEEDING PRODUCTS
 DIGESTIVE HEALTH ENTERAL FEEDING PRODUCTS HALYARD* MIC* AND MIC-KEY* INTRODUCER KITS HALYARD* MIC* and MIC-KEY* Introducer Kits provide physicians with an innovative solution to facilitate the primary
DIGESTIVE HEALTH ENTERAL FEEDING PRODUCTS HALYARD* MIC* AND MIC-KEY* INTRODUCER KITS HALYARD* MIC* and MIC-KEY* Introducer Kits provide physicians with an innovative solution to facilitate the primary
Xcela Hybrid PICC with PASV Valve Technology
 Xcela Hybrid PICC with PASV Valve Technology Directions For Use...3 Navilyst Medical, Master DFU Template 8in x 8in Global, 146T0808 Rev/Ver. A, DFU, XCELA HYBRID PICC w/ PASV VALVE TECHNOLOGY, 14600125-01A
Xcela Hybrid PICC with PASV Valve Technology Directions For Use...3 Navilyst Medical, Master DFU Template 8in x 8in Global, 146T0808 Rev/Ver. A, DFU, XCELA HYBRID PICC w/ PASV VALVE TECHNOLOGY, 14600125-01A
Polyurethane Catheter
 References 1. Aitken, D.R. and Minton, J.P. The Pinch-Off Sign: A Subclavian Catheters, American Journal of Surgery, Vol. 148, Nov. 1984, pp. 633-636. 2. Rubenstein, R.B., Alberty, R.E., et al. Hickman
References 1. Aitken, D.R. and Minton, J.P. The Pinch-Off Sign: A Subclavian Catheters, American Journal of Surgery, Vol. 148, Nov. 1984, pp. 633-636. 2. Rubenstein, R.B., Alberty, R.E., et al. Hickman
CARE OF A TUNNELED CATHETER (HICKMAN & BROVIAC ) with a Needleless Connector (MicroClave Clear)
 CARE OF A TUNNELED CATHETER (HICKMAN & BROVIAC ) with a Needleless Connector (MicroClave Clear) Table of Contents Part 1 Learning about the Catheter...2 Part 2 Caring for Your Child s Catheter...3 A. Preventing
CARE OF A TUNNELED CATHETER (HICKMAN & BROVIAC ) with a Needleless Connector (MicroClave Clear) Table of Contents Part 1 Learning about the Catheter...2 Part 2 Caring for Your Child s Catheter...3 A. Preventing
1 Description. 2 Indications. 3 Warnings ASPIRATION CATHETER
 Page 1 of 5 ASPIRATION CATHETER Carefully read all instructions prior to use, observe all warnings and precautions noted throughout these instructions. Failure to do so may result in complications. STERILE.
Page 1 of 5 ASPIRATION CATHETER Carefully read all instructions prior to use, observe all warnings and precautions noted throughout these instructions. Failure to do so may result in complications. STERILE.
The Power of Purple* Polyurethane PICC. Patient Guide. Access Systems
 The Power of Purple* Polyurethane PICC Patient Guide Access Systems Preamble Your doctor is giving you a PowerPICC* catheter so that you can easily get the intravenous (IV) medicines you need. This catheter
The Power of Purple* Polyurethane PICC Patient Guide Access Systems Preamble Your doctor is giving you a PowerPICC* catheter so that you can easily get the intravenous (IV) medicines you need. This catheter
BioFlo PICC. with Endexo and PASV Valve Technology. Clinician Reference Tool
 BioFlo PICC with Endexo and PASV Valve Technology Clinician Reference Tool BioFlo * PICC with Endexo * and PASV * Valve Technology The FIRST and only power injectable PICC that integrates Endexo Technology
BioFlo PICC with Endexo and PASV Valve Technology Clinician Reference Tool BioFlo * PICC with Endexo * and PASV * Valve Technology The FIRST and only power injectable PICC that integrates Endexo Technology
Title: EZ-IO. Effective Date: January SOG Number: EMS Rescinds:
 S O G Title: EZ-IO Effective Date: January 2010 SOG Number: EMS - 25 Rescinds: Scope: Providers Authorized are AIC s in the following certifications EMT-I and EMT-P who have been trained and cleared by
S O G Title: EZ-IO Effective Date: January 2010 SOG Number: EMS - 25 Rescinds: Scope: Providers Authorized are AIC s in the following certifications EMT-I and EMT-P who have been trained and cleared by
ICY Intravascular Heat Exchange Catheter (Custom Luer) Instructions for Use Model IC-3893A/
 Caution: Federal law restricts this device to sale by or on the order of a physician. ICY Intravascular Heat Exchange Catheter (ICY Catheter) Kit includes: Quantity 1 English ICY Catheter 3-Luer 9.3 French
Caution: Federal law restricts this device to sale by or on the order of a physician. ICY Intravascular Heat Exchange Catheter (ICY Catheter) Kit includes: Quantity 1 English ICY Catheter 3-Luer 9.3 French
E S C C R I A L T E A R. One great catheter + maximum barrier insertion. c k. p a. h e. a t. a l. r i t e. A r
 T E A R r i t e R I A L a l A c a t C C h e E S t e r S p a c k s One great catheter + maximum barrier insertion A r Difficult Arterial Access The problem Direct insertion technique Arterial cannulation
T E A R r i t e R I A L a l A c a t C C h e E S t e r S p a c k s One great catheter + maximum barrier insertion A r Difficult Arterial Access The problem Direct insertion technique Arterial cannulation
Split-Stream LONG-TERM HEMODIALYSIS INSTRUCTIONS FOR USE
 Split-Stream LONG-TERM HEMODIALYSIS INSTRUCTIONS FOR USE INDICATIONS FOR USE: INSTRUCTIONS FOR USE The Medcomp Split-Stream is indicated for use in attaining Long-Term vascular access for Hemodialysis
Split-Stream LONG-TERM HEMODIALYSIS INSTRUCTIONS FOR USE INDICATIONS FOR USE: INSTRUCTIONS FOR USE The Medcomp Split-Stream is indicated for use in attaining Long-Term vascular access for Hemodialysis
NON-COMPLIANT PTCA RAPID EXCHANGE DILATATION CATHETER
 Page 1 of 5 NON-COMPLIANT PTCA RAPID EXCHANGE DILATATION CATHETER STERILE. SINGLE USE ONLY. Sterilized with ethylene oxide gas. Non pyrogenic. Do not resterilize. Do not use opened or damaged packages.
Page 1 of 5 NON-COMPLIANT PTCA RAPID EXCHANGE DILATATION CATHETER STERILE. SINGLE USE ONLY. Sterilized with ethylene oxide gas. Non pyrogenic. Do not resterilize. Do not use opened or damaged packages.
Education for Self Administration of Intravenous Therapy HOME IV THERAPY PICC. Portacath
 HOME IV THERAPY PICC Portacath Who To contact Cardio-Respiratory Integrated Specialist Services (CRISS) Office hours 0800 1630 hours Ph: 364 0167 Weekends and after hours, phone Christchurch Hospital operator
HOME IV THERAPY PICC Portacath Who To contact Cardio-Respiratory Integrated Specialist Services (CRISS) Office hours 0800 1630 hours Ph: 364 0167 Weekends and after hours, phone Christchurch Hospital operator
FAST1 Intraosseous Infusion System. Training Session
 FAST1 Intraosseous Infusion System Training Session Why IO? Peripheral IV is often difficult to obtain Requires an average of 3-12 minutes Failure rate ranges between 10-40% AHA & ILCOR guidelines now
FAST1 Intraosseous Infusion System Training Session Why IO? Peripheral IV is often difficult to obtain Requires an average of 3-12 minutes Failure rate ranges between 10-40% AHA & ILCOR guidelines now
ARROW EZ-IO Intraosseous Vascular Access System Procedure Template
 ARROW EZ-IO Intraosseous Vascular Access System Procedure Template PURPOSE To provide procedural guidance for establishment of intraosseous vascular access using the ARROW EZ-IO Intraosseous Vascular Access
ARROW EZ-IO Intraosseous Vascular Access System Procedure Template PURPOSE To provide procedural guidance for establishment of intraosseous vascular access using the ARROW EZ-IO Intraosseous Vascular Access
Directions For Use. All directions should be read before use
 Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
A CATHETER FAMILY FOR ALL YOUR PATIENT NEEDS
 A CATHETER FAMILY FOR ALL YOUR PATIENT NEEDS MAHURKAR * Elite acute dialysis catheter family EFFICIENCY OF DIALYSIS IN REVERSE FLOW Less than one percent recirculation Symmetrical tip design minimizes
A CATHETER FAMILY FOR ALL YOUR PATIENT NEEDS MAHURKAR * Elite acute dialysis catheter family EFFICIENCY OF DIALYSIS IN REVERSE FLOW Less than one percent recirculation Symmetrical tip design minimizes
Peripherally Inserted Central Catheter & Midline Placement with ECG Confirmation of Tip Placement
 Title/Description: Peripherally Inserted Central Catheter & Midline Placement with ECG Confirmation of Tip Placement Department: Patient Care Services Personnel: Nursing Services Effective Date: April
Title/Description: Peripherally Inserted Central Catheter & Midline Placement with ECG Confirmation of Tip Placement Department: Patient Care Services Personnel: Nursing Services Effective Date: April
SYMBOLOGY. = Manufacturer. = Manufacturing Serial Number. = Manufacturing Lot Number = Catalog Number LOT REF. = Do not resterilize Single Use Only
 damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
Dignity Dual Power Injectable Implantable Infusion Port INSTRUCTIONS FOR USE
 DESCRIPTION: Dignity Dual Power Injectable Implantable Infusion Port INSTRUCTIONS FOR USE The Dignity Dual power injectable implantable infusion port is an implantable access device designed to provide
DESCRIPTION: Dignity Dual Power Injectable Implantable Infusion Port INSTRUCTIONS FOR USE The Dignity Dual power injectable implantable infusion port is an implantable access device designed to provide
Echo PS Positioning System with Ventralight ST Mesh or Composix L/P Mesh
 Ventralight ST Mesh with Echo PS Positioning System Catalog Number Shape Mesh Size 5955450 Circle 4.5" (11.4 cm) 5955460 Ellipse 4" x 6" (10.2 cm x 15.2 cm) 5955600 Circle 6" (15.2 cm) 5955680 Ellipse
Ventralight ST Mesh with Echo PS Positioning System Catalog Number Shape Mesh Size 5955450 Circle 4.5" (11.4 cm) 5955460 Ellipse 4" x 6" (10.2 cm x 15.2 cm) 5955600 Circle 6" (15.2 cm) 5955680 Ellipse
Quattro Intravascular Heat Exchange Catheter (Custom Luer) Instructions for Use Model IC4593/
 Caution: Federal law restricts this device to sale by or on the order of a physician. includes: Quantity English 1 Quattro Catheter 3-Luer 9.3 French x 45cm Triple Infusion Luer Extension Line Clamps Radiopaque
Caution: Federal law restricts this device to sale by or on the order of a physician. includes: Quantity English 1 Quattro Catheter 3-Luer 9.3 French x 45cm Triple Infusion Luer Extension Line Clamps Radiopaque
IV Catheter Placement
 Year Group: BVSc3 + Document number: CSL_A06 Equipment for this station: Equipment list: IV catheter model, with giving set and red fluid bag IV catheter Bung or T-port Tape two strips cut to size before
Year Group: BVSc3 + Document number: CSL_A06 Equipment for this station: Equipment list: IV catheter model, with giving set and red fluid bag IV catheter Bung or T-port Tape two strips cut to size before
Cat B / PleurX Peritoneal Catheter Kit
 Cat. 50-9000B / 50-9900 PleurX Peritoneal Catheter Kit For Peritoneal Placement Only Sterile For Single Use Only LATEX Not made with natural rubber latex. Instructions for Use USA Rx Only For product inquiries
Cat. 50-9000B / 50-9900 PleurX Peritoneal Catheter Kit For Peritoneal Placement Only Sterile For Single Use Only LATEX Not made with natural rubber latex. Instructions for Use USA Rx Only For product inquiries
NexSite HD, Hemodialysis Symmetric Tip Catheter for long term use. Directions for Use. Contents
 NexSite HD, Hemodialysis Symmetric Tip Catheter for long term use Directions for Use Contents Note to Physicians: Please consult the on-line training video on www.marvaomedical.com for information on the
NexSite HD, Hemodialysis Symmetric Tip Catheter for long term use Directions for Use Contents Note to Physicians: Please consult the on-line training video on www.marvaomedical.com for information on the
with Blom-Singer Gel Cap Insertion System
 with Blom-Singer Gel Cap Insertion System MEDICAL PROFESSIONAL Instructions For Use R2 37269-02G 37269-02G Effective January 2016 Blom-Singer and InHealth Technologies are registered trademarks of Freudenberg
with Blom-Singer Gel Cap Insertion System MEDICAL PROFESSIONAL Instructions For Use R2 37269-02G 37269-02G Effective January 2016 Blom-Singer and InHealth Technologies are registered trademarks of Freudenberg
LONG-TERM HEMODIALYSIS CATHETER INSTRUCTIONS FOR USE
 LONG-TERM HEMODIALYSIS CATHETER INSTRUCTIONS FOR USE LONG-TERM HEMODIALYSIS CATHETER INSTRUCTIONS FOR USE INDICATIONS FOR USE: The Centros and CentrosFLO long-term hemodialysis catheter are indicated
LONG-TERM HEMODIALYSIS CATHETER INSTRUCTIONS FOR USE LONG-TERM HEMODIALYSIS CATHETER INSTRUCTIONS FOR USE INDICATIONS FOR USE: The Centros and CentrosFLO long-term hemodialysis catheter are indicated
Aus Artificial Uretheral Sphincter Port System
 NORFOLK VET PRODUCTS the AUS for the long-term relief of incontinence in dogs and cats making life easier for pets Speciality Medical Devices For The Veterinary Community the Aus Artificial Uretheral Sphincter
NORFOLK VET PRODUCTS the AUS for the long-term relief of incontinence in dogs and cats making life easier for pets Speciality Medical Devices For The Veterinary Community the Aus Artificial Uretheral Sphincter
Endorectal Balloon (ERB) Endorectal Balloon (ERB) Instructions for Use
 Endorectal Balloon (ERB) Instructions for Use GRF-0004-00 Rev. 08 Page 1 of 9 Contents Instructions for Use... 3 Indications for Use... 3 Contraindications... 3 Storage... 4 Warnings... 4 Accessories Provided...
Endorectal Balloon (ERB) Instructions for Use GRF-0004-00 Rev. 08 Page 1 of 9 Contents Instructions for Use... 3 Indications for Use... 3 Contraindications... 3 Storage... 4 Warnings... 4 Accessories Provided...
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE
 CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
Parts identification:
 TRANSPARENT CATHETERIZATION SIMULATOR Parts identification: Clamp Urethra channel Prostate velcro strap (male genitalia only) Transparent Simulator Bladder tubing and luer locks Female genitalia Male genitalia
TRANSPARENT CATHETERIZATION SIMULATOR Parts identification: Clamp Urethra channel Prostate velcro strap (male genitalia only) Transparent Simulator Bladder tubing and luer locks Female genitalia Male genitalia
Radux StandTall Instructions for Use Sheath Extender and Securement Clasp
 Radux StandTall Instructions for Use Sheath Extender and Securement Clasp USA Caution Federal (USA) law restricts this device to sale by or on the order of a health care professional. Caution The Radux
Radux StandTall Instructions for Use Sheath Extender and Securement Clasp USA Caution Federal (USA) law restricts this device to sale by or on the order of a health care professional. Caution The Radux
Central Venous Catheter Care and Maintenance (includes catheter troubleshooting guide)
 Central Venous Catheter Care and Maintenance (includes catheter troubleshooting guide) A Guide for Patients in the Home Phone Number: Nurse/Contact: Central Venous Catheters This manual is a guide for
Central Venous Catheter Care and Maintenance (includes catheter troubleshooting guide) A Guide for Patients in the Home Phone Number: Nurse/Contact: Central Venous Catheters This manual is a guide for
Curraheen, Co. Cork. Guidelines on the Management and Care of Central Venous Access Devices
 Curraheen, Co. Cork. Guidelines on the Management and Care of Central Venous Access Devices Date re-approved: 27 th Jan 2015. Version No: 2 Revision Due: 2018 Index code: CLIN028 Disclaimer: The information
Curraheen, Co. Cork. Guidelines on the Management and Care of Central Venous Access Devices Date re-approved: 27 th Jan 2015. Version No: 2 Revision Due: 2018 Index code: CLIN028 Disclaimer: The information
The SplitWire Percutaneous Transluminal Angioplasty Scoring Device. Instructions for Use
 The SplitWire Percutaneous Transluminal Angioplasty Scoring Device Instructions for Use Contents Contains one (1) SplitWire device. Sterile. Sterilized with ethylene oxide gas. Radiopaque. For single use
The SplitWire Percutaneous Transluminal Angioplasty Scoring Device Instructions for Use Contents Contains one (1) SplitWire device. Sterile. Sterilized with ethylene oxide gas. Radiopaque. For single use
Central Line Care and Management
 Central Line Care and Management What is a Central Line/ CVAD? (central venous access device) A vascular infusion device that terminates at or close to the heart or in one of the great vessels (aorta,
Central Line Care and Management What is a Central Line/ CVAD? (central venous access device) A vascular infusion device that terminates at or close to the heart or in one of the great vessels (aorta,
The High-Flow Port Designed & Indicated for Apheresis
 The High-Flow Port Designed & Indicated for Apheresis Advancing Lives and the Delivery of Health Care Bard is proud to introduce the first and only high-flow, power-injectable port designed and indicated
The High-Flow Port Designed & Indicated for Apheresis Advancing Lives and the Delivery of Health Care Bard is proud to introduce the first and only high-flow, power-injectable port designed and indicated
St. Jude Medical 8-Channel Adapter. Clinician's Manual
 St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
CATHETERIZATION TRAINER ON A STAND
 CATHETERIZATION TRAINER ON A STAND Parts identification: Clamp Prostate velcro strap (male genitalia only) Genitalia stand male female Urethra channel Bladder tubing and luer locks Female genitalia Male
CATHETERIZATION TRAINER ON A STAND Parts identification: Clamp Prostate velcro strap (male genitalia only) Genitalia stand male female Urethra channel Bladder tubing and luer locks Female genitalia Male
ASEPT System ASEPT Pleural/Peritoneal. ASEPT Drainage Kits (600 ml ml) Accessories. Quality and Experience
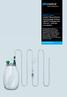 Quality and Experience ASEPT System ASEPT Pleural/Peritoneal Drainage System ASEPT Drainage Kits (600 ml + 000 ml) Accessories Recurring pleural effusions or malignant ascites can be treated on an outpatient
Quality and Experience ASEPT System ASEPT Pleural/Peritoneal Drainage System ASEPT Drainage Kits (600 ml + 000 ml) Accessories Recurring pleural effusions or malignant ascites can be treated on an outpatient
Single Use Curlew TM Multiple Biopsy Forceps
 Single Use Curlew TM Multiple Biopsy Forceps 13 SPECIMEN WITH METAL STORAGE CYLINDER With In Situ Fixation, Batch or Single Specimen Collection US Patents 5,782,747; 5,980,468; 6,071,248; foreign patents
Single Use Curlew TM Multiple Biopsy Forceps 13 SPECIMEN WITH METAL STORAGE CYLINDER With In Situ Fixation, Batch or Single Specimen Collection US Patents 5,782,747; 5,980,468; 6,071,248; foreign patents
ASEPT -System ASEPT Pleural/Peritoneal. ASEPT Drainage Kits (600 ml + 1,200 ml) Accessories. Quality and Experience
 Quality and Experience ASEPT -System ASEPT Pleural/Peritoneal Drainage System ASEPT Drainage Kits (600 ml + 1,200 ml) Accessories Recurring pleural effusions or malignant ascites can be treated on an outpatient
Quality and Experience ASEPT -System ASEPT Pleural/Peritoneal Drainage System ASEPT Drainage Kits (600 ml + 1,200 ml) Accessories Recurring pleural effusions or malignant ascites can be treated on an outpatient
Quick Start Guide. Venipuncture and Intramuscular Injection Training Arm
 Venipuncture and Intramuscular Injection Training Arm Quick Start Guide Realistic training arm for demonstrating and practicing proper venipuncture procedure and deltoid intramuscular injections. The venous
Venipuncture and Intramuscular Injection Training Arm Quick Start Guide Realistic training arm for demonstrating and practicing proper venipuncture procedure and deltoid intramuscular injections. The venous
BD Eclipse Needle for Luer Lock Syringes - How to Use the BD Eclipse Needle for Luer Lock Syringes 3. Injection 1. Attachment
 BD Eclipse Needle How to Use the BD Eclipse Needle for Luer Lock Syringes 1. Attachment 1a. Firmly attach needle onto a Luer Lock syringe with a push and clockwise twist. 1b. Pull back on the safety cover
BD Eclipse Needle How to Use the BD Eclipse Needle for Luer Lock Syringes 1. Attachment 1a. Firmly attach needle onto a Luer Lock syringe with a push and clockwise twist. 1b. Pull back on the safety cover
EL DORADO COUNTY EMS AGENCY FIELD PROCEDURES
 EL DORADO COUNTY EMS AGENCY FIELD PROCEDURES Effective: July 1, 2017 Reviewed: November 9, 2016 Revised: November 9, 2016 EMS Agency Medical Director INTRAOSSEOUS INFUSION PURPOSE: To establish immediate
EL DORADO COUNTY EMS AGENCY FIELD PROCEDURES Effective: July 1, 2017 Reviewed: November 9, 2016 Revised: November 9, 2016 EMS Agency Medical Director INTRAOSSEOUS INFUSION PURPOSE: To establish immediate
LESSON ASSIGNMENT. Urinary System Diseases/Disorders. After completing this lesson, you should be able to:
 LESSON ASSIGNMENT LESSON 4 Urinary System Diseases/Disorders LESSON ASSIGNMENT Paragraphs 4-1 through 4-8. LESSON OBJECTIVES After completing this lesson, you should be able to: 4-1. Identify the purposes
LESSON ASSIGNMENT LESSON 4 Urinary System Diseases/Disorders LESSON ASSIGNMENT Paragraphs 4-1 through 4-8. LESSON OBJECTIVES After completing this lesson, you should be able to: 4-1. Identify the purposes
Department Policy. Code: D:PC Entity: Fairview Pharmacy Services. Department: Fairview Home Infusion. Manual: Policy and Procedure Manual
 Entity: Fairview Pharmacy Services Department: Fairview Home Infusion Manual: Policy and Procedure Manual Category: Home Infusion Subject: Midline (Extended Dwell Peripheral) Catheter Care and Management
Entity: Fairview Pharmacy Services Department: Fairview Home Infusion Manual: Policy and Procedure Manual Category: Home Infusion Subject: Midline (Extended Dwell Peripheral) Catheter Care and Management
PRODUCTS FOR THE DIFFICULT AIRWAY. Courtesy of Cook Critical Care
 PRODUCTS FOR THE DIFFICULT AIRWAY Courtesy of Cook Critical Care EMERGENCY CRICOTHYROTOMY Thyroid Cartilage Access Site Cricoid Cartilage Identify the cricothyroid membrane between the cricoid and thyroid
PRODUCTS FOR THE DIFFICULT AIRWAY Courtesy of Cook Critical Care EMERGENCY CRICOTHYROTOMY Thyroid Cartilage Access Site Cricoid Cartilage Identify the cricothyroid membrane between the cricoid and thyroid
2. Need for serial arterial blood gas determinations. 2. Anticipation of the initiation of thrombolytic therapy
 I. Subject: Arterial Cannulation II. Policy: Arterial cannulation will be performed upon a physician's order by Cardiopulmonary and Respiratory Therapy personnel certified in the arterial catheterization
I. Subject: Arterial Cannulation II. Policy: Arterial cannulation will be performed upon a physician's order by Cardiopulmonary and Respiratory Therapy personnel certified in the arterial catheterization
Integra Flowable Wound Matrix
 Integra Flowable Wound Matrix Table of Contents How Supplied...2 Storage...2 Product Information Disclosure...2 Symbols Used On Labeling...2 Instructions for Use... 3 Instructions for Use (Continued)...
Integra Flowable Wound Matrix Table of Contents How Supplied...2 Storage...2 Product Information Disclosure...2 Symbols Used On Labeling...2 Instructions for Use... 3 Instructions for Use (Continued)...
Emergency clamp should always be readily available in case of accidental catheter fracture
 Note: Please see individual policies for further information. Flushing best practice: Always use a 10 diameter syringe or larger when first accessing and when flushing vascular access device (VAD) Use
Note: Please see individual policies for further information. Flushing best practice: Always use a 10 diameter syringe or larger when first accessing and when flushing vascular access device (VAD) Use
Mary Lou Garey MSN EMT-P MedFlight of Ohio
 Mary Lou Garey MSN EMT-P MedFlight of Ohio Function Prolonged and frequent access to venous circulation Allows for patient to carry on normal life; decrease number of needle sticks Medications, parenteral
Mary Lou Garey MSN EMT-P MedFlight of Ohio Function Prolonged and frequent access to venous circulation Allows for patient to carry on normal life; decrease number of needle sticks Medications, parenteral
Standard Operating Procedure for cannulation
 Standard Operating Procedure for cannulation Effective date: 26.07.2017 Review due date: 31.03.2019 Original Author Name: Richard Metcalfe Position: PhD Student Date: 05.12.2012 Reviewer Name: Pippa Heath
Standard Operating Procedure for cannulation Effective date: 26.07.2017 Review due date: 31.03.2019 Original Author Name: Richard Metcalfe Position: PhD Student Date: 05.12.2012 Reviewer Name: Pippa Heath
RECOMMENDED INSTRUCTIONS FOR USE
 Rapid Exchange PTCA Dilatation Catheter RECOMMENDED INSTRUCTIONS FOR USE Available in diameters 1.25mm to 4.5mm and in lengths 09mm to 40mm Caution: This device should be used only by physicians trained
Rapid Exchange PTCA Dilatation Catheter RECOMMENDED INSTRUCTIONS FOR USE Available in diameters 1.25mm to 4.5mm and in lengths 09mm to 40mm Caution: This device should be used only by physicians trained
Advancing Lives and the Delivery of Health Care. The High-Flow Port Designed for Apheresis
 Advancing Lives and the Delivery of Health Care The High-Flow Port Designed for Apheresis Optimized for Long Device Life Bench Tested up to 1,000 Accesses 1 Bard is proud to introduce the first and only
Advancing Lives and the Delivery of Health Care The High-Flow Port Designed for Apheresis Optimized for Long Device Life Bench Tested up to 1,000 Accesses 1 Bard is proud to introduce the first and only
Central Venous Catheter Insertion: Assisting
 Approved by: Central Venous Catheter Insertion: Assisting Gail Cameron Senior Director, Operations, Maternal, Neonatal & Child Health Programs Dr. Santiago Ensenat Medical Director, Neonatology Neonatal
Approved by: Central Venous Catheter Insertion: Assisting Gail Cameron Senior Director, Operations, Maternal, Neonatal & Child Health Programs Dr. Santiago Ensenat Medical Director, Neonatology Neonatal
If viewing a printed copy of this policy, please note it could be expired. Got to to view current policies.
 If viewing a printed copy of this policy, please note it could be expired. Got to www.fairview.org/fhipolicies to view current policies. Department Policy Code: D: PC-5555 Entity: Fairview Pharmacy Services
If viewing a printed copy of this policy, please note it could be expired. Got to www.fairview.org/fhipolicies to view current policies. Department Policy Code: D: PC-5555 Entity: Fairview Pharmacy Services
Cat B / PleurX Pleural Catheter Kit. Instructions for Use. For Pleural Placement Only Sterile For Single Use Only USA.
 Cat. 50-7000B / 50-7700 PleurX Pleural Catheter Kit For Pleural Placement Only Sterile For Single Use Only Instructions for Use LATEX Not made with natural rubber latex. USA Rx Only For product inquiries
Cat. 50-7000B / 50-7700 PleurX Pleural Catheter Kit For Pleural Placement Only Sterile For Single Use Only Instructions for Use LATEX Not made with natural rubber latex. USA Rx Only For product inquiries
ompanionport Speciality Medical Devices For The Veterinary Community Surgical Suggestions
 Speciality Medical Devices For The Veterinary Community suture holes Place the CompanionPort in the subcutaneous port pocket off to one side so that the septum of the port will not lie directly beneath
Speciality Medical Devices For The Veterinary Community suture holes Place the CompanionPort in the subcutaneous port pocket off to one side so that the septum of the port will not lie directly beneath
INNOVATIVE PRODUCTS G, J AND GJ-TUBES WITH ENFit TM CONNECTION
 MIC * & MIC-KEY * Enterostomy Tubes INNOVATIVE PRODUCTS G, J AND GJ-TUBES WITH ENFit TM CONNECTION DIGESTIVE HEALTH PRODUCT CATALOGUE INNOVATIVE PRODUCTS - G, J AND GJ-TUBES WITH ENFit TM CONNECTION DIGESTIVE
MIC * & MIC-KEY * Enterostomy Tubes INNOVATIVE PRODUCTS G, J AND GJ-TUBES WITH ENFit TM CONNECTION DIGESTIVE HEALTH PRODUCT CATALOGUE INNOVATIVE PRODUCTS - G, J AND GJ-TUBES WITH ENFit TM CONNECTION DIGESTIVE
INSTRUCTIONS FOR USE FOR:
 INSTRUCTIONS FOR USE FOR: en English INSTRUCTIONS FOR USE GORE ENFORM PREPERITONEAL BIOMATERIAL Carefully read all instructions prior to use. Observe all instructions, warnings, and precautions noted throughout.
INSTRUCTIONS FOR USE FOR: en English INSTRUCTIONS FOR USE GORE ENFORM PREPERITONEAL BIOMATERIAL Carefully read all instructions prior to use. Observe all instructions, warnings, and precautions noted throughout.
PLASTIC POWER INJECTABLE IMPLANTABLE INFUSION PORT
 PLASTIC POWER INJECTABLE IMPLANTABLE INFUSION PORT INSTRUCTIONS FOR USE DESCRIPTION: The Power Injectable Implantable Infusion Port is an implantable access device designed to provide repeated access to
PLASTIC POWER INJECTABLE IMPLANTABLE INFUSION PORT INSTRUCTIONS FOR USE DESCRIPTION: The Power Injectable Implantable Infusion Port is an implantable access device designed to provide repeated access to
REFILL KIT For use with Prometra Programmable Infusion Systems
 REFILL KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3 Warnings...
REFILL KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3 Warnings...
CHEST DRAIN PROTOCOL
 CHEST DRAIN PROTOCOL Rationale The pleural membranes have an important role in effective lung expansion. The visceral pleura is a thin, smooth, serous membrane covering the surface of the lungs and is
CHEST DRAIN PROTOCOL Rationale The pleural membranes have an important role in effective lung expansion. The visceral pleura is a thin, smooth, serous membrane covering the surface of the lungs and is
Sterile Technique & IJ/Femoral Return Demonstration
 Sterile Technique & IJ/Femoral Return Demonstration Sterile Technique Description: This is a return demonstration checklist used to evaluate participants in the simulated hands on skills portions for certification
Sterile Technique & IJ/Femoral Return Demonstration Sterile Technique Description: This is a return demonstration checklist used to evaluate participants in the simulated hands on skills portions for certification
Peripheral Intravascular Lithotripsy (IVL) Catheter Instructions for Use (IFU)
 Peripheral Intravascular Lithotripsy (IVL) Catheter Instructions for Use (IFU) For use with the Shockwave Medical, Inc. IVL Generator and Connector Cable Indication for Use The Shockwave Medical IVL System
Peripheral Intravascular Lithotripsy (IVL) Catheter Instructions for Use (IFU) For use with the Shockwave Medical, Inc. IVL Generator and Connector Cable Indication for Use The Shockwave Medical IVL System
Hospital of the University of Pennsylvania NURSING. Insertion of Peripherally Inserted Central Catheter (PICC) and Midline Catheter (MLC)
 Page 1 of 11 KEYWORDS: Central Catheter Sterility REFER TO: 4B-02-09 Nursing Management of the Patient with a Central Venous Access Device HUP 1-12-24 Consent to Health Care Services SCOPE Registered Nurses
Page 1 of 11 KEYWORDS: Central Catheter Sterility REFER TO: 4B-02-09 Nursing Management of the Patient with a Central Venous Access Device HUP 1-12-24 Consent to Health Care Services SCOPE Registered Nurses
