Name of Policy: Transurethral Microwave Thermotherapy
|
|
|
- Rosamund Parks
- 6 years ago
- Views:
Transcription
1 Name of Policy: Transurethral Microwave Thermotherapy Policy #: 449 Latest Review Date: September 2013 Category: Surgery Policy Grade: B Background/Definitions: As a general rule, benefits are payable under Blue Cross and Blue Shield of Alabama health plans only in cases of medical necessity and only if services or supplies are not investigational, provided the customer group contracts have such coverage. The following Association Technology Evaluation Criteria must be met for a service/supply to be considered for coverage: 1. The technology must have final approval from the appropriate government regulatory bodies; 2. The scientific evidence must permit conclusions concerning the effect of the technology on health outcomes; 3. The technology must improve the net health outcome; 4. The technology must be as beneficial as any established alternatives; 5. The improvement must be attainable outside the investigational setting. Medical Necessity means that health care services (e.g., procedures, treatments, supplies, devices, equipment, facilities or drugs) that a physician, exercising prudent clinical judgment, would provide to a patient for the purpose of preventing, evaluating, diagnosing or treating an illness, injury or disease or its symptoms, and that are: 1. In accordance with generally accepted standards of medical practice; and 2. Clinically appropriate in terms of type, frequency, extent, site and duration and considered effective for the patient s illness, injury or disease; and 3. Not primarily for the convenience of the patient, physician or other health care provider; and 4. Not more costly than an alternative service or sequence of services at least as likely to produce equivalent therapeutic or diagnostic results as to the diagnosis or treatment of that patient s illness, injury or disease. Page 1 of 7
2 Description of Procedure or Service: Transurethral microwave thermotherapy (TUMT) has been proposed as an alternative to transurethral resection of the prostate (TURP), or daily medical therapy, for patients who have benign prostatic hyperplasia (BPH). Although TURP has been historically considered definitive treatment, complications from this treatment have encouraged the development of less invasive techniques. Benign prostatic hyperplasia (BPH) is a condition which may lead to lower urinary tract symptoms such as urinary frequency, nocturia, urinary hesitancy and feeling of incomplete voiding. Histologic evidence of BPH is present in approximately 50% of men at age 50 and the prevalence increases with advancing age. Glandular overgrowth causes progressive occlusion of the prostatic portion of the urethra in men, and will cause lower urinary tract symptoms to varying degrees. Transurethral resection of the prostate (TURP) is one of the most commonly performed surgeries and is generally well tolerated, but is not without potential complications, such as blood loss (with or without the need for transfusion), retrograde ejaculation and incontinence. Transurethral resection syndrome is an adverse effect that can occur in up to 2% of cases. The syndrome is caused by absorption of bladder irrigation fluids during the TURP procedure. Depending on the severity, this syndrome can lead to hyponatremia and fluid overload with subsequent neurological and cardiac complications. Less invasive techniques have been investigated using various energy sources, such as laser, direct heat, microwave, radiofrequency and ultrasound. With these techniques, a calculated amount of prostate tissue is destroyed and reabsorbed while leaving the epithelium of the urethral canal relatively intact. This policy addresses the use of microwave energy in the reduction of urethral occlusion and lower urinary tract symptoms. The goal of microwave thermotherapy is destruction of the prostatic adenoma in the lateral lobes of the prostate to achieve improvement in symptoms and voiding. A transurethral catheter containing a microwave antenna that limits microwave radiation is placed at the prostatic level. Water circulating through the catheter cools the urethra. A Foley-type balloon at the end of the catheter inflates to position the catheter, and fiberoptic sensors are positioned to monitor rectal and urethral temperatures. The correct position of the catheter is verified using transrectal ultrasound of the prostate. The electromagnetic waves emit high-energy photons that interact with molecules in prostatic tissue, producing heat. Transurethral microwave thermotherapy (TUMT) produces coagulation necrosis of the lateral lobes of the prostate for a distance up to 17 mm from the urethra with the preservation of the urethral surface, distal sphincter, urethral mucosa, bladder neck, and peripheral prostate. Policy: Effective for dates of service on or after November 3, 2013: Transurethral microwave thermotherapy for benign prostatic hyperplasia meets Blue Cross and Blue Shield of Alabama s medical criteria for coverage for patients who, based on severity of their BPH symptoms, would be candidates for transurethral resection of the prostate. Page 2 of 7
3 Effective for dates of service prior to November 3, 2013: Transurethral microwave thermotherapy for benign prostatic hyperplasia meets Blue Cross and Blue Shield of Alabama s medical criteria for coverage for patients who, based on severity of their BPH symptoms, would be candidates for transurethral resection of the prostate and who have prostatic lengths of mm. Blue Cross and Blue Shield of Alabama does not approve or deny procedures, services, testing, or equipment for our members. Our decisions concern coverage only. The decision of whether or not to have a certain test, treatment or procedure is one made between the physician and his/her patient. Blue Cross and Blue Shield of Alabama administers benefits based on the members' contract and corporate medical policies. Physicians should always exercise their best medical judgment in providing the care they feel is most appropriate for their patients. Needed care should not be delayed or refused because of a coverage determination. Key Points: This policy was originally created in 1996 and was regularly updated with searches of the MEDLINE database through The policy was on no further review status from 2002 until Literature updates resumed in 2010; the most recent MEDLINE search was performed through June Following is a summary of the key literature to date: This policy was initially developed following a 1996 TEC Assessment that evaluated transurethral thermotherapy. The Assessment concluded that the results of studies published at the time provided sufficient evidence on the beneficial and harmful outcomes of transurethral microwave thermotherapy (TUMT). Specifically, two clinical trials, one randomized and one nonrandomized had compared microwave thermotherapy with transurethral resection of the prostate (TURP). The findings of these trials were supplemented with five randomized controlled trials (RCTs) comparing TUMT with a sham procedure, as well as a number of published clinical series. Evidence from a randomized trial showed that both TUMT and TURP produce significant relief of the symptoms of benign prostatic hyperplasia (BPH), although TURP was associated with significantly greater improvement after one year of follow-up. Moreover, while symptom relief following TURP appeared to be greater, fewer major complications were seen with TUMT. In 2012, Hoffman and colleagues published an updated Cochrane Review on transurethral microwave thermotherapy for BPH. The review identified 15 RCTs involving a total of 1,585 patients. No new RCTs were identified since the previous review was published in 2008; the most recent RCT was published in Six trials were comparisons of microwave thermotherapy with TURP, eight were comparisons with sham thermotherapy, and one was a comparison with alpha blocker therapy. The range of duration of the studies was three to 60 months; the mean age of subjects was 66.8 years. Outcomes were reported in urinary flow rate and International Prostate Symptom Scores (IPSS) or converted to IPSS equivalents. The metaanalysis offered the following observations and conclusions: The pooled mean urinary symptom score decreased by 65% with TUMT and 77% with TURP. The weighted mean difference (WMD) for the IPSS was (95% confidence interval [CI]: to -0.03), favoring TURP. The pooled mean peak urinary flow Page 3 of 7
4 increased by 70% with TUMT and 119% with TURP. The WMD for peak urinary flow was 5.08 ml/sec (95% CI: 3.88 to 6.28), favoring TURP. Compared to TURP, TUMT was associated with a decreased risk of retrograde ejaculation, treatment for strictures, hematuria, blood transfusions and the transurethral resection syndrome. TUMT was associated with an increase in dysuria, urinary retention, and retreatment for BPH symptoms. Compared to the sham procedures, microwave thermotherapy also improved symptom scores (WMD: -5.15, 95% CI: to -6.04) on the IPSS and peak urinary flow (WMD: 2.01 ml/s, 95% CI: 0.85 to 3.16). In the single study comparing TUMT to alpha blockade, TUMT led to greater improvement in symptom scores (WMD: -4.20, 95% CI: to -5.25) and peak urinary flow (WMD: 2.30 ml/s, 95% CI: 1.47 to 3.13) than in the group receiving alpha blockers. In 2012, Biester and colleagues published a systematic review of studies comparing standard surgical treatment to minimally invasive procedures in the treatment of BPH. Seven RCTs with a total of 675 patients were identified that evaluated TUMT; six trials compared TUMT to TURP and one trial compared it to transurethral incision of the prostate. In the studies, the mean prostatic size ranged from 34 to 72 ml, and patients were followed for a mean of six to 60 months. The systematic review aimed to determine whether the minimally invasive treatments were non-inferior to surgery. The authors noted that none of the trials investigated noninferiority, and they therefore based their threshold, 0.25 standard deviations (SD), for noninferiority on the published literature. In a meta-analysis of study findings, the authors found that TUMT did not meet their threshold to be considered non-inferior to standard procedures. The pooled SD at months was 0.46 (i.e., greater than 0.25) and the 95% CI was 0.15 to The authors concluded that there is a lack of high-quality RCTs and RCTs that are designed to investigate non-inferiority. Summary Although not clearly superior to standard surgical therapy with transurethral resection of the prostate (TURP); transurethral microwave thermotherapy (TUMT) is an effective therapeutic option and has fewer adverse effects. There is sufficient evidence for patient and providers considering a surgical intervention to make an informed choice between resection of the prostate and transurethral microwave thermotherapy. Practice Guidelines and Position Statements The American Urological Association (AUA) last updated clinical guidelines for BPH in The guideline states that, based on a review of the data and consensus of an expert panel, TUMT is a treatment option for BPH. According to the guideline, TUMT is effective in partially relieving lower urinary tract symptoms secondary to BPH and may be considered for men with moderate or severe symptoms. In 2011, the European Association of Urology (EAU) published guidelines on the treatment of non-neurogenic lower urinary tract symptoms in men. The guidelines included the following statements on TUMT: Page 4 of 7
5 TUMT achieves symptom improvement comparable to TURP, but is associated with decreased morbidity and lower flow improvements and Durability is in favor of TURP with lower retreatment rates compared to TUMT. Key Words: Microwave thermotherapy, transurethral, Thermotherapy, transurethral microwave, benign prostatic hyperplasia, transurethral microwave thermotherapy, TUMT, Prolieve Thermodilation System, Prostalund CoreTherm Approved by Governing Bodies: In December 2002, the Prostalund CoreTherm System (Prostalund Operations AB, Concord, MA) was approved by the U.S. Food and Drug Administration (FDA) through the premarket approval (PMA) process for use in men with BPH who have a prostate size of 30 to 100 grams and prostatic urethra length of 35 mm or greater. Although similar to previous microwave thermotherapy devices, the company sought PMA due to the addition of temperature feedback features intended to address earlier safety concerns related to thermal injuries. The treatment is contraindicated in men with a prostate less than 30 grams or a prostate length less than 35 mm. In February 2004, the Prolieve Thermodilation System (now owned by Medifocus; Columbia, MD) was approved by the FDA for the treatment of BPH through the PMA process in men with a prostate size of 20 to 80 grams and prostatic urethra length between 1.2 and 5.5 cm, and in whom drug therapy is typically indicated. The treatment is contraindicated in men with prostate sizes less than 20 grams and greater than 80 grams. Benefit Application: Coverage is subject to member s specific benefits. Group specific policy will supersede this policy when applicable. ITS: Home Policy provisions apply FEP: Special benefit consideration may apply. Refer to member s benefit plan. Pre-certification requirements: Not applicable Coding: CPT Codes: Transurethral destruction of prostate tissue; by microwave thermotherapy Page 5 of 7
6 References: 1. American Urological Association. Management of BPH, revised Available online at: Last accessed September, Biester K, Skipka G, Jahn R et al. Systematic review of surgical treatments for benign prostatic hyperplasia and presentation of an approach to investigate therapeutic equivalence (non-inferiority). BJU Int 2012; 109(5): Blue Cross and Blue Shield Association. Transurethral microwave thermotherapy for benign prostatic hyperplasia. Technology Evaluation Center (TEC), TEC Assessments 2006: Volume 21, Tab Blute ML, Tomera KM, Hellerstein DK, et al. Transurethral microwave thermotherapy for management of benign prostatic hyperplasia: Results of the United States Prostatron Cooperative Study. J Urol 1993; 150(5 Pt 2): Djavan B, Eckersberger E, Handl MJ et al. Durability and retreatment rates of minimal invasive treatments of benign prostatic hyperplasia: a cross-analysis of the literature. Can J Urol 2010; 17(4): European Association of Urology. Guidelines on the treatment of non-neurogenic male LUTS. Available online at: Last accessed September Food and Drug Administration (FDA). Summary of Safety and Effectiveness Data: ProstaLund CoreTherm. Available online at: Last accessed September, Food and Drug Administration (FDA). Summary of Safety and Effectiveness Data: Prolieve Thermodilatation System (Prolieve ). Available online at: Last accessed September, Hoffman RM, MacDonald R, Monga M, et al. Transurethral microwave thermotherapy vs. transurethral resection for treating benign prostatic hyperplasia: A systematic review. BJU Int 2004; 94(7): Hoffman RM, Monga M, Elliot SP, et al. Microwave thermotherapy for benign prostatic hyperplasia. Cochrane Database Syst Rev 2007; (4):CD Mebust WK, Holtgrewe HL, Cockett AT, et al. Transurethral prostatectomy: Immediate and postoperative complications. Cooperative study of 13 participating institutions evaluating 3,885 patients. J Urol, 141: , J Urol 2002; 167(1): Petrovich Z, Ameye F, Baert L, et al. New trends in the treatment of benign prostatic hyperplasia and carcinoma of the prostate. Am J Clin Oncol 1993; 16(3): Policy History: Medical Policy Group, September 2010 (3) Medical Policy Administration Committee, September 2010 Available for comment September 22-November 5, 2010 Medical Policy Group, September 2011; (3) Updated Key Points and References Medical Policy Group, October 2012 (3): 2012 Update to Key Points and References Medical Policy Panel, August 2013 Medical Policy Group, September 2013 (2), Prostatic lengths of 35-50mm removed from policy statement. Information on the Prolieve Thermodilation System added. Key Points, Approved by Governing Bodies, Key Words, and References updated. Page 6 of 7
7 Medical Policy Administration Committee, September 2013 Available for comment September 19 through November 2, 2013 This medical policy is not an authorization, certification, explanation of benefits, or a contract. Eligibility and benefits are determined on a caseby-case basis according to the terms of the member s plan in effect as of the date services are rendered. All medical policies are based on (i) research of current medical literature and (ii) review of common medical practices in the treatment and diagnosis of disease as of the date hereof. Physicians and other providers are solely responsible for all aspects of medical care and treatment, including the type, quality, and levels of care and treatment. This policy is intended to be used for adjudication of claims (including pre-admission certification, pre-determinations, and pre-procedure review) in Blue Cross and Blue Shield s administration of plan contracts. Page 7 of 7
Original Policy Date
 MP 7.01.39 Transurethral Microwave Thermotherapy Medical Policy Section Surgery Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed with literature search/12:2013 Return to Medical
MP 7.01.39 Transurethral Microwave Thermotherapy Medical Policy Section Surgery Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed with literature search/12:2013 Return to Medical
Policy #: 213 Latest Review Date: September 2012
 Name of Policy: Temporary Prostatic Stent Policy #: 213 Latest Review Date: September 2012 Category: Medicine Policy Grade: Active Policy but no longer scheduled for regular literature reviews and updates.
Name of Policy: Temporary Prostatic Stent Policy #: 213 Latest Review Date: September 2012 Category: Medicine Policy Grade: Active Policy but no longer scheduled for regular literature reviews and updates.
NOTE: This policy is not effective until April 1, Transurethral Water Vapor Thermal Therapy of the Prostate
 NOTE: This policy is not effective until April 1, 2019. Medical Policy Manual Surgery, Policy No. 210 Transurethral Water Vapor Thermal Therapy of the Prostate Next Review: December 2019 Last Review: December
NOTE: This policy is not effective until April 1, 2019. Medical Policy Manual Surgery, Policy No. 210 Transurethral Water Vapor Thermal Therapy of the Prostate Next Review: December 2019 Last Review: December
Policy #: 370 Latest Review Date: April 2017
 Name of Policy: Nerve Graft with Radical Prostatectomy Policy #: 370 Latest Review Date: April 2017 Category: Surgery Policy Grade: B Background/Definitions: As a general rule, benefits are payable under
Name of Policy: Nerve Graft with Radical Prostatectomy Policy #: 370 Latest Review Date: April 2017 Category: Surgery Policy Grade: B Background/Definitions: As a general rule, benefits are payable under
Name of Policy: Computerized Pulse Waveform Analysis
 Name of Policy: Computerized Pulse Waveform Analysis Policy #: 020 Latest Review Date: September 2012 Category: Medical Policy Grade: Active Policy but no longer scheduled for regular literature reviews
Name of Policy: Computerized Pulse Waveform Analysis Policy #: 020 Latest Review Date: September 2012 Category: Medical Policy Grade: Active Policy but no longer scheduled for regular literature reviews
Policy #: 370 Latest Review Date: December 2013
 Name of Policy: Nerve Graft in Association with Radical Prostatectomy Policy #: 370 Latest Review Date: December 2013 Category: Surgery Policy Grade: B Background/Definitions: As a general rule, benefits
Name of Policy: Nerve Graft in Association with Radical Prostatectomy Policy #: 370 Latest Review Date: December 2013 Category: Surgery Policy Grade: B Background/Definitions: As a general rule, benefits
Name of Policy: Yervoy (Ipilimumab)
 Name of Policy: Yervoy (Ipilimumab) Policy #: 335 Latest Review Date: October 2013 Category: Pharmacology Policy Grade: A Background/Definitions: As a general rule, benefits are payable under Blue Cross
Name of Policy: Yervoy (Ipilimumab) Policy #: 335 Latest Review Date: October 2013 Category: Pharmacology Policy Grade: A Background/Definitions: As a general rule, benefits are payable under Blue Cross
Name of Policy: Measurement of Long-Chain Omega-3 Fatty Acids in Red Blood Cell Membranes as a Cardiac Risk Factor
 Name of Policy: Measurement of Long-Chain Omega-3 Fatty Acids in Red Blood Cell Membranes as a Cardiac Risk Factor Policy #: 239 Latest Review Date: July 2010 Category: Laboratory Policy Grade: Active
Name of Policy: Measurement of Long-Chain Omega-3 Fatty Acids in Red Blood Cell Membranes as a Cardiac Risk Factor Policy #: 239 Latest Review Date: July 2010 Category: Laboratory Policy Grade: Active
Policy #: 668 Effective Date: December 1, 2016 Category: Pharmacology Latest Review Date: September 2016
 Name of Policy: Tecentriq (Atezolizumab) Policy #: 668 Effective Date: December 1, 2016 Category: Pharmacology Latest Review Date: September 2016 Background/Definitions: As a general rule, benefits are
Name of Policy: Tecentriq (Atezolizumab) Policy #: 668 Effective Date: December 1, 2016 Category: Pharmacology Latest Review Date: September 2016 Background/Definitions: As a general rule, benefits are
Name of Policy: Sympathetic Therapy and Bioelectrical Nerve Block or Electroanalgesic Nerve Block for the Treatment of Pain
 Name of Policy: Sympathetic Therapy and Bioelectrical Nerve Block or Electroanalgesic Nerve Block for the Treatment of Pain Policy #: 015 Latest Review Date: February 2010 Category: Therapy Policy Grade:
Name of Policy: Sympathetic Therapy and Bioelectrical Nerve Block or Electroanalgesic Nerve Block for the Treatment of Pain Policy #: 015 Latest Review Date: February 2010 Category: Therapy Policy Grade:
Policy #: 049 Latest Review Date: April 2009 Policy Grade: Active policy but no longer scheduled for regular literature reviews and update.
 Name of Policy: Pulsed Irrigation Evacuation (PIE) Policy #: 049 Latest Review Date: April 2009 Category: DME Policy Grade: Active policy but no longer scheduled for regular literature reviews and update.
Name of Policy: Pulsed Irrigation Evacuation (PIE) Policy #: 049 Latest Review Date: April 2009 Category: DME Policy Grade: Active policy but no longer scheduled for regular literature reviews and update.
Policy #: 291 Latest Review Date: February 2013
 Effective for dates of service on or after April 1, 2013, refer to: https://www.bcbsal.org/providers/policies/carecore.cfm Name of Policy: Magnetic Resonance Angiography (MRA) of the Chest (excluding the
Effective for dates of service on or after April 1, 2013, refer to: https://www.bcbsal.org/providers/policies/carecore.cfm Name of Policy: Magnetic Resonance Angiography (MRA) of the Chest (excluding the
Policy #: 069 Latest Review Date: November 2009 Policy Grade: Active Policy but no longer scheduled for regular literature reviews and updates.
 Name of Policy: Ultrasound of the Spinal Canal Policy #: 069 Latest Review Date: November 2009 Category: Radiology Policy Grade: Active Policy but no longer scheduled for regular literature reviews and
Name of Policy: Ultrasound of the Spinal Canal Policy #: 069 Latest Review Date: November 2009 Category: Radiology Policy Grade: Active Policy but no longer scheduled for regular literature reviews and
MODULE 3: BENIGN PROSTATIC HYPERTROPHY
 MODULE 3: BENIGN PROSTATIC HYPERTROPHY KEYWORDS: Prostatic hypertrophy, prostatic hyperplasia, PSA, voiding dysfunction, lower urinary tract symptoms (LUTS) At the end of this clerkship, the medical student
MODULE 3: BENIGN PROSTATIC HYPERTROPHY KEYWORDS: Prostatic hypertrophy, prostatic hyperplasia, PSA, voiding dysfunction, lower urinary tract symptoms (LUTS) At the end of this clerkship, the medical student
Name of Policy: Boniva (Ibandronate Sodium) Infusion
 Name of Policy: Boniva (Ibandronate Sodium) Infusion Policy #: 266 Latest Review Date: April 2010 Category: Pharmacology Policy Grade: Active Policy but no longer scheduled for regular literature reviews
Name of Policy: Boniva (Ibandronate Sodium) Infusion Policy #: 266 Latest Review Date: April 2010 Category: Pharmacology Policy Grade: Active Policy but no longer scheduled for regular literature reviews
Name of Policy: Panitumumab, Vectibix
 Name of Policy: Panitumumab, Vectibix Policy #: 369 Latest Review Date: June 2014 Category: Pharmacology Policy Grade: B Background/Definitions: As a general rule, benefits are payable under Blue Cross
Name of Policy: Panitumumab, Vectibix Policy #: 369 Latest Review Date: June 2014 Category: Pharmacology Policy Grade: B Background/Definitions: As a general rule, benefits are payable under Blue Cross
Name of Policy: Pulsed Dye Laser Treatment of Recalcitrant Verrucae
 Name of Policy: Pulsed Dye Laser Treatment of Recalcitrant Verrucae Policy #: 187 Latest Review Date: July 2010 Category: Surgery Policy Grade: Active Policy but no longer scheduled for regular literature
Name of Policy: Pulsed Dye Laser Treatment of Recalcitrant Verrucae Policy #: 187 Latest Review Date: July 2010 Category: Surgery Policy Grade: Active Policy but no longer scheduled for regular literature
Name of Policy: Reduction Mammaplasty
 Name of Policy: Reduction Mammaplasty Policy #: 056 Latest Review Date: November 2013 Category: Surgery Policy Grade: D Background/Definitions: As a general rule, benefits are payable under Blue Cross
Name of Policy: Reduction Mammaplasty Policy #: 056 Latest Review Date: November 2013 Category: Surgery Policy Grade: D Background/Definitions: As a general rule, benefits are payable under Blue Cross
Name of Policy: Computer-aided Detection (CAD) Mammography
 Name of Policy: Computer-aided Detection (CAD) Mammography Policy #: 112 Latest Review Date: October 2010 Category: Radiology Policy Grade: Active Policy but no longer scheduled for regular literature
Name of Policy: Computer-aided Detection (CAD) Mammography Policy #: 112 Latest Review Date: October 2010 Category: Radiology Policy Grade: Active Policy but no longer scheduled for regular literature
Policy #: 259 Latest Review Date: November 2009
 Name of Policy: Prophylactic Oophorectomy Policy #: 259 Latest Review Date: November 2009 Category: Surgery Policy Grade: Active Policy but no longer scheduled for regular literature reviews and updates.
Name of Policy: Prophylactic Oophorectomy Policy #: 259 Latest Review Date: November 2009 Category: Surgery Policy Grade: Active Policy but no longer scheduled for regular literature reviews and updates.
Policy #: 222 Latest Review Date: March 2009
 Name of Policy: MRI Phase-Contrast Flow Measurement Policy #: 222 Latest Review Date: March 2009 Category: Radiology Policy Grade: Active Policy but no longer scheduled for regular literature reviews and
Name of Policy: MRI Phase-Contrast Flow Measurement Policy #: 222 Latest Review Date: March 2009 Category: Radiology Policy Grade: Active Policy but no longer scheduled for regular literature reviews and
Rezūm procedure for the Prostate
 Rezūm procedure for the Prostate Mr Jas Kalsi Consultant Urological Surgeon This booklet has been provided to help answer the questions you may have with regards to your enlarged prostate and the Rezūm
Rezūm procedure for the Prostate Mr Jas Kalsi Consultant Urological Surgeon This booklet has been provided to help answer the questions you may have with regards to your enlarged prostate and the Rezūm
Policy #: 127 Latest Review Date: June 2011
 Name of Policy: Mohs Micrographic Surgery Policy #: 127 Latest Review Date: June 2011 Category: Surgery Policy Grade: Active Policy but no longer scheduled for regular literature reviews and updates. Background/Definitions:
Name of Policy: Mohs Micrographic Surgery Policy #: 127 Latest Review Date: June 2011 Category: Surgery Policy Grade: Active Policy but no longer scheduled for regular literature reviews and updates. Background/Definitions:
Name of Policy: Magnetic Resonance Neurography
 Name of Policy: Magnetic Resonance Neurography Policy #: 177 Latest Review Date: June 2011 Category: Radiology Policy Grade: C Background/Definitions: As a general rule, benefits are payable under Blue
Name of Policy: Magnetic Resonance Neurography Policy #: 177 Latest Review Date: June 2011 Category: Radiology Policy Grade: C Background/Definitions: As a general rule, benefits are payable under Blue
Name of Policy: Zoledronic Acid (Reclast ) Injection
 Name of Policy: Zoledronic Acid (Reclast ) Injection Policy #: 355 Latest Review Date: May 2011 Category: Pharmacy Policy Grade: Active Policy but no longer scheduled for regular literature reviews and
Name of Policy: Zoledronic Acid (Reclast ) Injection Policy #: 355 Latest Review Date: May 2011 Category: Pharmacy Policy Grade: Active Policy but no longer scheduled for regular literature reviews and
Policy #: 120 Latest Review Date: June 2009
 Name of Policy: Intraductal Biopsy/Breast Duct Endoscopy Policy #: 120 Latest Review Date: June 2009 Category: Surgical Policy Grade: Active Policy but no longer scheduled for regular literature reviews
Name of Policy: Intraductal Biopsy/Breast Duct Endoscopy Policy #: 120 Latest Review Date: June 2009 Category: Surgical Policy Grade: Active Policy but no longer scheduled for regular literature reviews
Transurethral microwave thermotherapy
 Procedure 11 CLINICAL PRIVILEGE WHITE PAPER Transurethral microwave thermotherapy Background Transurethral resection of the prostate (TURP) has become the primary choice for the treatment of benign prostatic
Procedure 11 CLINICAL PRIVILEGE WHITE PAPER Transurethral microwave thermotherapy Background Transurethral resection of the prostate (TURP) has become the primary choice for the treatment of benign prostatic
Subject: Temporary Prostatic Stent and Prostatic Urethral Lift
 02-54000-21 Original Effective Date: 03/15/05 Reviewed: 09/27/18 Revised: 10/15/18 Subject: Temporary Prostatic Stent and Prostatic Urethral Lift THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION,
02-54000-21 Original Effective Date: 03/15/05 Reviewed: 09/27/18 Revised: 10/15/18 Subject: Temporary Prostatic Stent and Prostatic Urethral Lift THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION,
Index. urologic.theclinics.com. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A Ablative therapies, transurethral needle ablation, Adverse events, sexual side effects of BPH Aging, and incidence of BPH associated with
Index Note: Page numbers of article titles are in boldface type. A Ablative therapies, transurethral needle ablation, Adverse events, sexual side effects of BPH Aging, and incidence of BPH associated with
Treating BPH: Comparing Rezum UroLift and HoLEP
 Treating BPH: Comparing Rezum UroLift and HoLEP Scott M. Cheney MD Mayo Clinic Arizona 2018 MFMER slide-1 Welcome to AZ 2018 MFMER slide-2 Outline Background on BPH, Rezum, Urolift, HoLEP AUA Guideline
Treating BPH: Comparing Rezum UroLift and HoLEP Scott M. Cheney MD Mayo Clinic Arizona 2018 MFMER slide-1 Welcome to AZ 2018 MFMER slide-2 Outline Background on BPH, Rezum, Urolift, HoLEP AUA Guideline
Medifocus, Inc. OTCQB: MDFZF TSXV: MFS
 Medifocus, Inc. OTCQB: MDFZF TSXV: MFS www.medifocusinc.com A Leader in Focused Heat Thermotherapy for the Treatment of Benign Prostatic Hyperplasia and Breast Cancer Except for historical information,
Medifocus, Inc. OTCQB: MDFZF TSXV: MFS www.medifocusinc.com A Leader in Focused Heat Thermotherapy for the Treatment of Benign Prostatic Hyperplasia and Breast Cancer Except for historical information,
POLICIES AND PROCEDURE MANUAL
 POLICIES AND PROCEDURE MANUAL Policy: MP048 Section: Medical Benefit Policy Subject: Surgical and Minimally Invasive Therapies for the Treatment of Benign Prostatic Hypertrophy I. Policy: Surgical and
POLICIES AND PROCEDURE MANUAL Policy: MP048 Section: Medical Benefit Policy Subject: Surgical and Minimally Invasive Therapies for the Treatment of Benign Prostatic Hypertrophy I. Policy: Surgical and
Management of LUTS after TURP and MIT
 Management of LUTS after TURP and MIT Hong Sup Kim Konkuk University TURP & MIT TURP : Gold standard MIT TUIP TUNA TUMT HIFU LASER Nd:YAG, ILC, HoLRP, KTP LUTS after TURP and MIT Improved : about 70% Persistent
Management of LUTS after TURP and MIT Hong Sup Kim Konkuk University TURP & MIT TURP : Gold standard MIT TUIP TUNA TUMT HIFU LASER Nd:YAG, ILC, HoLRP, KTP LUTS after TURP and MIT Improved : about 70% Persistent
APPENDIX CLINICAL INPUT RESPONSES
 CLINICAL INPUT RESPONSES AUA: American Urological Association; UCSF Med Ctr: University of California San Francisco Medical Center; PUL: prostatic urethral lift. * Indicates that information on conflicts
CLINICAL INPUT RESPONSES AUA: American Urological Association; UCSF Med Ctr: University of California San Francisco Medical Center; PUL: prostatic urethral lift. * Indicates that information on conflicts
Benign Prostatic Hyperplasia: Update on Innovative Current Treatments
 Benign Prostatic Hyperplasia: Update on Innovative Current Treatments Michael Ferrandino, MD As.soc Professor Director of Minimally Invasive Urologic Surgery Division of Urologic Surgery Duke University
Benign Prostatic Hyperplasia: Update on Innovative Current Treatments Michael Ferrandino, MD As.soc Professor Director of Minimally Invasive Urologic Surgery Division of Urologic Surgery Duke University
Chapter 3: Results of the Treatment Outcomes Analyses
 Chapter 3: Results of the Treatment Outcomes Analyses Introduction To determine the appropriateness of individual therapies, as well as to develop practice recommendations, the American Urological Association
Chapter 3: Results of the Treatment Outcomes Analyses Introduction To determine the appropriateness of individual therapies, as well as to develop practice recommendations, the American Urological Association
Name of Policy: Therapeutic Apheresis, with Extracorporeal Column Immunoadsorption and Plasma Reinfusion
 Name of Policy: Therapeutic Apheresis, with Extracorporeal Column Immunoadsorption and Plasma Reinfusion Policy #: 010 Latest Review Date: December 2008 Category: Surgical Policy Grade: Effective March
Name of Policy: Therapeutic Apheresis, with Extracorporeal Column Immunoadsorption and Plasma Reinfusion Policy #: 010 Latest Review Date: December 2008 Category: Surgical Policy Grade: Effective March
EAU GUIDELINES POCKET EDITION 3
 EAU GUIDELINES POCKET EDITION 3 CONTENTS: BENIGN PROSTATIC HYPERPLASIA URINARY INCONTINENCE UROLITHIASIS 2 3 EAU POCKET GUIDELINES POCKET EDITION 3 This is one of a series of convenient pocket size books
EAU GUIDELINES POCKET EDITION 3 CONTENTS: BENIGN PROSTATIC HYPERPLASIA URINARY INCONTINENCE UROLITHIASIS 2 3 EAU POCKET GUIDELINES POCKET EDITION 3 This is one of a series of convenient pocket size books
Early-Stage Clinical Experiences of Holmium Laser Enucleation of the Prostate (HoLEP)
 JRural Med 2007 ; 2 : 93 97 Original article Early-Stage Clinical Experiences of Holmium Laser Enucleation of the Prostate (HoLEP) Shuzo Hamamoto 1,TakehikoOkamura 1,HideyukiKamisawa 1,KentaroMizuno 1,
JRural Med 2007 ; 2 : 93 97 Original article Early-Stage Clinical Experiences of Holmium Laser Enucleation of the Prostate (HoLEP) Shuzo Hamamoto 1,TakehikoOkamura 1,HideyukiKamisawa 1,KentaroMizuno 1,
A Critical Evaluation of MIST Intervention in LUTS and BPH Treatment
 A Critical Evaluation of MIST Intervention in LUTS and BPH Treatment Claus G. Roehrborn Professor and Chairman Medical 3-9 mo. Min. Invas. 3-9 mo. Surgical 3-9 mo. Medical 1-16 mo. Min. Invas. 1-16 mo.
A Critical Evaluation of MIST Intervention in LUTS and BPH Treatment Claus G. Roehrborn Professor and Chairman Medical 3-9 mo. Min. Invas. 3-9 mo. Surgical 3-9 mo. Medical 1-16 mo. Min. Invas. 1-16 mo.
Overview. Urology Dine and Learn: Erectile Dysfunction & Benign Prostatic Hyperplasia. Iain McAuley September 15, 2014
 Urology Dine and Learn: Erectile Dysfunction & Benign Prostatic Hyperplasia Iain McAuley September 15, 2014 Overview Review of the most recent guidelines for ED and BPH ED Guidelines CUA 2006 AUA 2011
Urology Dine and Learn: Erectile Dysfunction & Benign Prostatic Hyperplasia Iain McAuley September 15, 2014 Overview Review of the most recent guidelines for ED and BPH ED Guidelines CUA 2006 AUA 2011
Benign Prostatic Hyperplasia (BPH)
 Benign Prostatic Hyperplasia (BPH) Definition Prostate gland enlargement is a common condition as men get older. Also called benign prostatic hyperplasia (BPH), prostate gland enlargement can cause bothersome
Benign Prostatic Hyperplasia (BPH) Definition Prostate gland enlargement is a common condition as men get older. Also called benign prostatic hyperplasia (BPH), prostate gland enlargement can cause bothersome
P ROLIEVE. Thermodilatation System. The Prolieve System Patient Information is Directed to You, the Patient. A Transurethral Microwave Therapy Device
 P ROLIEVE Thermodilatation System A Transurethral Microwave Therapy Device The Prolieve System Patient Information is Directed to You, the Patient. Contents Why am I being treated with the Prolieve Thermodilatation
P ROLIEVE Thermodilatation System A Transurethral Microwave Therapy Device The Prolieve System Patient Information is Directed to You, the Patient. Contents Why am I being treated with the Prolieve Thermodilatation
Executive Summary. Non-drug local procedures for treatment of benign prostatic hyperplasia 1. IQWiG Reports - Commission No.
 IQWiG Reports - Commission No. N04-01 Non-drug local procedures for treatment of benign prostatic hyperplasia 1 Executive Summary 1 Translation of the executive summary of the final report Nichtmedikamentöse
IQWiG Reports - Commission No. N04-01 Non-drug local procedures for treatment of benign prostatic hyperplasia 1 Executive Summary 1 Translation of the executive summary of the final report Nichtmedikamentöse
The Enlarged Prostate Symptoms, Diagnosis and Treatment
 The Enlarged Prostate Symptoms, Diagnosis and Treatment MAC00031-01 Rev G Financial support for this seminar has been provided by NeoTract, Inc., the manufacturer of the UroLift System. 1 Today s Agenda
The Enlarged Prostate Symptoms, Diagnosis and Treatment MAC00031-01 Rev G Financial support for this seminar has been provided by NeoTract, Inc., the manufacturer of the UroLift System. 1 Today s Agenda
Chapter 4: Research and Future Directions
 Chapter 4: Research and Future Directions Introduction Many of the future research needs listed in the 1994 Agency for Health Care Policy and Research (AHCPR) clinical practice guideline Benign Prostatic
Chapter 4: Research and Future Directions Introduction Many of the future research needs listed in the 1994 Agency for Health Care Policy and Research (AHCPR) clinical practice guideline Benign Prostatic
PROSTATIC EMBOLIZATION FOR BENIGN HYPERPLASIA
 St. Louis Hospital PROSTATIC EMBOLIZATION FOR BENIGN HYPERPLASIA INITIAL CLINICAL RESULTS Faculty of Medical Sciences New University of Lisbon JOÃO PISCO LUÍS CAMPOS PINHEIRO TIAGO BILHIM HUGO RIO TINTO
St. Louis Hospital PROSTATIC EMBOLIZATION FOR BENIGN HYPERPLASIA INITIAL CLINICAL RESULTS Faculty of Medical Sciences New University of Lisbon JOÃO PISCO LUÍS CAMPOS PINHEIRO TIAGO BILHIM HUGO RIO TINTO
Chapter 2: Methodology
 Chapter 2: Methodology TABLE OF CONTENTS Introduction... 2 Study Selection and Data Abstraction... 2 Data Synthesis... 8 Guideline Development and Approvals... 9 Conflict of Interest... 10 Copyright 2010
Chapter 2: Methodology TABLE OF CONTENTS Introduction... 2 Study Selection and Data Abstraction... 2 Data Synthesis... 8 Guideline Development and Approvals... 9 Conflict of Interest... 10 Copyright 2010
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Prostatic Urethral Lift Page 1 of 19 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Prostatic Urethral Lift Professional Institutional Original Effective Date:
Prostatic Urethral Lift Page 1 of 19 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Prostatic Urethral Lift Professional Institutional Original Effective Date:
Prostatic Urethral Lift
 Prostatic Urethral Lift Policy Number: 7.01.151 Last Review: 10/2018 Origination: 10/2015 Next Review: 10/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Prostatic
Prostatic Urethral Lift Policy Number: 7.01.151 Last Review: 10/2018 Origination: 10/2015 Next Review: 10/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Prostatic
Name of Policy: Speculoscopy
 Name of Policy: Speculoscopy Policy #: 095 Latest Review Date: September 2011 Category: Medicine/OB Gyn Policy Grade: C Background/Definitions: As a general rule, benefits are payable under Blue Cross
Name of Policy: Speculoscopy Policy #: 095 Latest Review Date: September 2011 Category: Medicine/OB Gyn Policy Grade: C Background/Definitions: As a general rule, benefits are payable under Blue Cross
Prostate Artery Embolization
 Prostate Artery Embolization in the office interventional suite Robert J. Kennedy, M.D. Interventional & Vascular Center Melbourne, Florida The speaker has no financial conflicts of interest to disclose.
Prostate Artery Embolization in the office interventional suite Robert J. Kennedy, M.D. Interventional & Vascular Center Melbourne, Florida The speaker has no financial conflicts of interest to disclose.
Benign Prostatic Hyperplasia (BPH):
 Benign Prostatic Hyperplasia (BPH): Evidence Based Guidelines for Primary Care Providers Jeanne Martin, DNP, ANP-BC Objectives 1. Understand the pathophysiology and prevalence of BPH 2. Select the appropriate
Benign Prostatic Hyperplasia (BPH): Evidence Based Guidelines for Primary Care Providers Jeanne Martin, DNP, ANP-BC Objectives 1. Understand the pathophysiology and prevalence of BPH 2. Select the appropriate
Medical Coverage Policy Prostatic Urethral Lifts
 Medical Coverage Policy Prostatic Urethral Lifts EFFECTIVE DATE:01 01 2017 POLICY LAST UPDATED: 10 03 2017 OVERVIEW Benign prostatic hyperplasia is a common condition in older men that can lead to increased
Medical Coverage Policy Prostatic Urethral Lifts EFFECTIVE DATE:01 01 2017 POLICY LAST UPDATED: 10 03 2017 OVERVIEW Benign prostatic hyperplasia is a common condition in older men that can lead to increased
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: January 15, 2019 Related Policies: None Prostatic Urethral Lift Description Benign prostatic hyperplasia (BPH) is a common condition in older individuals that
FEP Medical Policy Manual Effective Date: January 15, 2019 Related Policies: None Prostatic Urethral Lift Description Benign prostatic hyperplasia (BPH) is a common condition in older individuals that
Management of Voiding Problems in Older Men. Dr. John Fenn Consultant, QEH 10 th October, 2005
 Management of Voiding Problems in Older Men Dr. John Fenn Consultant, QEH 10 th October, 2005 Voiding Problems Poor stream Hesitancy Straining Incomplete emptying Intermittent micturition Terminal dribbling
Management of Voiding Problems in Older Men Dr. John Fenn Consultant, QEH 10 th October, 2005 Voiding Problems Poor stream Hesitancy Straining Incomplete emptying Intermittent micturition Terminal dribbling
Name of Policy: Sensory Integration Therapy, Auditory Integration Therapy and Facilitated Communication
 Name of Policy: Sensory Integration Therapy, Auditory Integration Therapy and Facilitated Communication Policy #: 333 Latest Review Date: December 2013 Category: Therapy Policy Grade: B Background/Definitions:
Name of Policy: Sensory Integration Therapy, Auditory Integration Therapy and Facilitated Communication Policy #: 333 Latest Review Date: December 2013 Category: Therapy Policy Grade: B Background/Definitions:
50% of men. 90% of men PATIENT FACTSHEET: BPH CONDITION AND TREATMENTS. Want more information? What are the symptoms?
 PATIENT FACTSHEET: BPH CONDITION AND TREATMENTS What is Benign Prostatic Hyperplasia (enlarged prostate)? Benign prostatic hyperplasia (BPH) is a noncancerous enlargement of the prostate, the gland that
PATIENT FACTSHEET: BPH CONDITION AND TREATMENTS What is Benign Prostatic Hyperplasia (enlarged prostate)? Benign prostatic hyperplasia (BPH) is a noncancerous enlargement of the prostate, the gland that
Managing urinary morbidity after brachytherapy. Kieran O Flynn Department of Urology, Salford Royal Foundation Trust, Manchester
 Managing urinary morbidity after brachytherapy Kieran O Flynn Department of Urology, Salford Royal Foundation Trust, Manchester Themes Can we predict urinary morbidity? Prevention of urinary morbidity
Managing urinary morbidity after brachytherapy Kieran O Flynn Department of Urology, Salford Royal Foundation Trust, Manchester Themes Can we predict urinary morbidity? Prevention of urinary morbidity
OVER 70% OF MEN IN THEIR 60s HAVE SYMPTOMS OF BPH 1
 PATIENT INFORMATION BPH affects more than 500 million men worldwide, with many men suffering from symptoms of enlarged prostate. 1 You no longer have to be one of them! OVER 70% OF MEN IN THEIR 60s HAVE
PATIENT INFORMATION BPH affects more than 500 million men worldwide, with many men suffering from symptoms of enlarged prostate. 1 You no longer have to be one of them! OVER 70% OF MEN IN THEIR 60s HAVE
Safety and efficacy of photoselective vaporization of the prostate using the 180-W GreenLight XPS laser system in patients taking oral anticoagulants
 Clinical Report Safety and efficacy of photoselective vaporization of the prostate using the 180-W GreenLight XPS laser system in patients taking oral anticoagulants Journal of International Medical Research
Clinical Report Safety and efficacy of photoselective vaporization of the prostate using the 180-W GreenLight XPS laser system in patients taking oral anticoagulants Journal of International Medical Research
Development of Feedback Microwave Thermotherapy in Symptomatic Benign Prostatic Hyperplasia.
 Development of Feedback Microwave Thermotherapy in Symptomatic Benign Prostatic Hyperplasia. Schelin, Sonny 2006 Link to publication Citation for published version (APA): Schelin, S. (2006). Development
Development of Feedback Microwave Thermotherapy in Symptomatic Benign Prostatic Hyperplasia. Schelin, Sonny 2006 Link to publication Citation for published version (APA): Schelin, S. (2006). Development
Last Review Status/Date: December Summary
 Section: Surgery Effective Date: January 15, 2016 Subject: Prostatic Urethral Lift Page: 1 of 9 Last Review Status/Date: December 2015 Summary Benign prostatic hyperplasia (BPH) is a common condition in
Section: Surgery Effective Date: January 15, 2016 Subject: Prostatic Urethral Lift Page: 1 of 9 Last Review Status/Date: December 2015 Summary Benign prostatic hyperplasia (BPH) is a common condition in
Posterior Tibial Nerve Stimulation for Voiding Dysfunction
 Posterior Tibial Nerve Stimulation for Voiding Dysfunction Corporate Medical Policy File name: Posterior Tibial Nerve Stimulation for Voiding Dysfunction File code: UM.NS.05 Origination: 8/2011 Last Review:
Posterior Tibial Nerve Stimulation for Voiding Dysfunction Corporate Medical Policy File name: Posterior Tibial Nerve Stimulation for Voiding Dysfunction File code: UM.NS.05 Origination: 8/2011 Last Review:
Indications for Use Targis System Description Contraindications for Targis Treatment Warnings Precautions
 Instructions for Use Targis System Control Unit, Model 4000 and Model 5000 Series Targis Microwave Delivery System (MDS) Rectal Thermosensing Unit (RTU) Coolant Bag CAUTION: Federal (U.S.A.) law restricts
Instructions for Use Targis System Control Unit, Model 4000 and Model 5000 Series Targis Microwave Delivery System (MDS) Rectal Thermosensing Unit (RTU) Coolant Bag CAUTION: Federal (U.S.A.) law restricts
Corporate Medical Policy Transanal Endoscopic Microsurgery (TEMS)
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: transanal_endoscopic_microsurgery_(tems) 6/2008 11/2018 11/2019 11/2018 Description of Procedure or Service
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: transanal_endoscopic_microsurgery_(tems) 6/2008 11/2018 11/2019 11/2018 Description of Procedure or Service
Prostatic Urethral Lift. Summary
 Section: Surgery Effective Date: January 15, 2017 Subject: Prostatic Urethral Lift Page: 1 of 10 Last Review Status/Date: December 2016 Prostatic Urethral Lift Summary Benign prostatic hyperplasia (BPH)
Section: Surgery Effective Date: January 15, 2017 Subject: Prostatic Urethral Lift Page: 1 of 10 Last Review Status/Date: December 2016 Prostatic Urethral Lift Summary Benign prostatic hyperplasia (BPH)
Name of Policy: Cellular Immunotherapy for Prostate Cancer
 Name of Policy: Cellular Immunotherapy for Prostate Cancer Policy #: 432 Latest Review Date: July 2014 Category: Medical Policy Grade: A Background/Definitions: As a general rule, benefits are payable
Name of Policy: Cellular Immunotherapy for Prostate Cancer Policy #: 432 Latest Review Date: July 2014 Category: Medical Policy Grade: A Background/Definitions: As a general rule, benefits are payable
Medifocus, Inc. OTCQX: MDFZF TSXV: MFS
 Medifocus, Inc. OTCQX: MDFZF TSXV: MFS www.medifocusinc.com Develops and commercializes minimally invasive focused heat thermotherapy systems for the treatment of cancer and other diseases January 2014
Medifocus, Inc. OTCQX: MDFZF TSXV: MFS www.medifocusinc.com Develops and commercializes minimally invasive focused heat thermotherapy systems for the treatment of cancer and other diseases January 2014
Public Statement: Medical Policy Statement:
 Medical Policy Title: Radiofrequency Ablation ARBenefits Approval: 10/26/2011 of Tumors Effective Date: 01/01/2012 Document: ARB0300 Revision Date: Code(s): 20982 Ablation, bone tumor(s), radiofrequency,
Medical Policy Title: Radiofrequency Ablation ARBenefits Approval: 10/26/2011 of Tumors Effective Date: 01/01/2012 Document: ARB0300 Revision Date: Code(s): 20982 Ablation, bone tumor(s), radiofrequency,
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Prostatic Urethral Lift Page 1 of 17 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Prostatic Urethral Lift Professional Institutional Original Effective Date:
Prostatic Urethral Lift Page 1 of 17 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Prostatic Urethral Lift Professional Institutional Original Effective Date:
Voiding Dysfunction. Joon Seok Kwon, Jung Woo Lee 1, Seung Wook Lee, Hong Yong Choi, Hong Sang Moon. DOI: /kju
 www.kjurology.org DOI:10.4111/kju.2011.52.4.269 Voiding Dysfunction Comparison of Effectiveness of Monopolar and Bipolar Transurethral Resection of the Prostate and Open Prostatectomy in Large Benign Prostatic
www.kjurology.org DOI:10.4111/kju.2011.52.4.269 Voiding Dysfunction Comparison of Effectiveness of Monopolar and Bipolar Transurethral Resection of the Prostate and Open Prostatectomy in Large Benign Prostatic
Related Policies None
 Medical Policy MP 7.01.151 BCBSA Ref. Policy: 7.01.151 Last Review: 12/27/2017 Effective Date: 03/01/2018 Section: Surgery End Date: 08/19/2018 Related Policies None DISCLAIMER Our medical policies are
Medical Policy MP 7.01.151 BCBSA Ref. Policy: 7.01.151 Last Review: 12/27/2017 Effective Date: 03/01/2018 Section: Surgery End Date: 08/19/2018 Related Policies None DISCLAIMER Our medical policies are
Medical Coverage Policy Prostatic Urethral Lifts
 Medical Coverage Policy Prostatic Urethral Lifts EFFECTIVE DATE:12 01 2018 POLICY LAST UPDATED: 10 03 2017 OVERVIEW Benign prostatic hyperplasia is a common condition in older men that can lead to increased
Medical Coverage Policy Prostatic Urethral Lifts EFFECTIVE DATE:12 01 2018 POLICY LAST UPDATED: 10 03 2017 OVERVIEW Benign prostatic hyperplasia is a common condition in older men that can lead to increased
Transurethral incision versus transurethral resection of the prostate in small prostatic adenoma: Long-term follow-up
 African Journal of Urology (2012) 18, 29 33 Pan African Urological Surgeons Association African Journal of Urology www.ees.elsevier.com/afju www.sciencedirect.com Transurethral incision versus transurethral
African Journal of Urology (2012) 18, 29 33 Pan African Urological Surgeons Association African Journal of Urology www.ees.elsevier.com/afju www.sciencedirect.com Transurethral incision versus transurethral
Related Policies None
 Medical Policy MP 7.01.151 Original Policy Date: August 2015 Last Review: 12/27/2017 Effective Date: 03/01/2018 Section: Surgery Related Policies None DISCLAIMER Our medical policies are designed for informational
Medical Policy MP 7.01.151 Original Policy Date: August 2015 Last Review: 12/27/2017 Effective Date: 03/01/2018 Section: Surgery Related Policies None DISCLAIMER Our medical policies are designed for informational
Comparison of outpatient versus inpatient transurethral prostate resection for benign prostatic hyperplasia: Comparative, prospective bi-centre study
 original research Comparison of outpatient versus inpatient transurethral prostate resection for benign prostatic hyperplasia: Comparative, prospective bi-centre study Jae Heon Kim, MD; * Jae Young Park,
original research Comparison of outpatient versus inpatient transurethral prostate resection for benign prostatic hyperplasia: Comparative, prospective bi-centre study Jae Heon Kim, MD; * Jae Young Park,
Surgical Treatment of LUTS in Men with BPE
 Patient Information English 35 Surgical Treatment of LUTS in Men with BPE The underlined terms are listed in the glossary. You have been diagnosed with benign prostatic enlargement (BPE) and your doctor
Patient Information English 35 Surgical Treatment of LUTS in Men with BPE The underlined terms are listed in the glossary. You have been diagnosed with benign prostatic enlargement (BPE) and your doctor
SURGICAL MANAGEMENT OF BPH IN GHANA: A NEED TO IMPROVE ACCESS TO TRANSURETHRAL RESECTION OF THE PROSTATE
 July 2012 East African Medical Journal 241 East African Medical Journal Vol. 89 No. 7 July 2012 SURGICAL MANAGEMENT OF BPH IN GHANA: A NEED TO IMPROVE ACCESS TO TRANSURETHRAL RESECTION OF THE PROSTATE
July 2012 East African Medical Journal 241 East African Medical Journal Vol. 89 No. 7 July 2012 SURGICAL MANAGEMENT OF BPH IN GHANA: A NEED TO IMPROVE ACCESS TO TRANSURETHRAL RESECTION OF THE PROSTATE
Transurethral Hot Water Balloon Thermotherapy for Benign Prostatic Hyperplasia
 Transurethral Hot Water Balloon Thermotherapy for Benign Prostatic Hyperplasia Lance A. Mynderse, MD* and Thayne R. Larson, MD Address *Department of Urology, Mayo Foundation, 200 First Street, SW, Rochester,
Transurethral Hot Water Balloon Thermotherapy for Benign Prostatic Hyperplasia Lance A. Mynderse, MD* and Thayne R. Larson, MD Address *Department of Urology, Mayo Foundation, 200 First Street, SW, Rochester,
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Medical technology guidance SCOPE The UroLift system for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia 1
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Medical technology guidance SCOPE The UroLift system for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia 1
Changes in Surgical Strategy for Patients with Benign Prostatic Hyperplasia: 12-Year Single-Center Experience
 www.kjurology.org DOI:10.4111/kju.2011.52.3.189 Voiding Dysfunction Changes in Surgical Strategy for Patients with Benign Prostatic Hyperplasia: 12-Year Single-Center Experience Yu Seob Shin 1, Jong Kwan
www.kjurology.org DOI:10.4111/kju.2011.52.3.189 Voiding Dysfunction Changes in Surgical Strategy for Patients with Benign Prostatic Hyperplasia: 12-Year Single-Center Experience Yu Seob Shin 1, Jong Kwan
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: April 15, 2018 Related Policies: None Prostatic Urethral Lift Description Benign prostatic hyperplasia (BPH) is a common condition in older individuals that can
FEP Medical Policy Manual Effective Date: April 15, 2018 Related Policies: None Prostatic Urethral Lift Description Benign prostatic hyperplasia (BPH) is a common condition in older individuals that can
Prostatic Urethral Lift Corporate Medical Policy
 Prostatic Urethral Lift Corporate Medical Policy File Name: Prostatic Urethral Lift File Code: UM.SURG.19 Origination: 05/2018 Last Review: 01/2019 Next Review: 01/2020 Effective Date: 04/01/2019 Description/Summary
Prostatic Urethral Lift Corporate Medical Policy File Name: Prostatic Urethral Lift File Code: UM.SURG.19 Origination: 05/2018 Last Review: 01/2019 Next Review: 01/2020 Effective Date: 04/01/2019 Description/Summary
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of transurethral water jet ablation for lower urinary tract symptoms caused by benign
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of transurethral water jet ablation for lower urinary tract symptoms caused by benign
PATIENT INFORMATION 2017 NeoTract, Inc. All rights reserved. Printed in the USA. MAC Rev A
 PATIENT INFORMATION OVER 70% OF MEN IN THEIR 60s HAVE SYMPTOMS OF BPH 1 BPH affects more than 500 million men worldwide, with many men suffering from symptoms of enlarged prostate. 1 You no longer have
PATIENT INFORMATION OVER 70% OF MEN IN THEIR 60s HAVE SYMPTOMS OF BPH 1 BPH affects more than 500 million men worldwide, with many men suffering from symptoms of enlarged prostate. 1 You no longer have
Cooled ThermoTherapy TM
 Are BPH Medications Weighing You Down? Think Outside the Pillbox Cooled ThermoTherapy TM A non-surgical, 30 minute in-office treatment that provides long-term relief from BPH symptoms and urinary obstruction
Are BPH Medications Weighing You Down? Think Outside the Pillbox Cooled ThermoTherapy TM A non-surgical, 30 minute in-office treatment that provides long-term relief from BPH symptoms and urinary obstruction
European Urology 44 (2003) 89 93
 European Urology European Urology 44 (2003) 89 93 Long-Term Evaluation of Transurethral Needle Ablation of the Prostate (TUNA) fortreatment of Symptomatic Benign Prostatic Hyperplasia: Clinical Outcome
European Urology European Urology 44 (2003) 89 93 Long-Term Evaluation of Transurethral Needle Ablation of the Prostate (TUNA) fortreatment of Symptomatic Benign Prostatic Hyperplasia: Clinical Outcome
PATIENT SELECTION GUIDE. finding the right fit
 PATIENT SELECTION GUIDE finding the right fit IDENTIFYING PATIENTS Rezūm, a new minimally invasive treatment, is now available to offer patients as a first-line BPH therapy. BPH TREATMENT OPTIONS Behavior
PATIENT SELECTION GUIDE finding the right fit IDENTIFYING PATIENTS Rezūm, a new minimally invasive treatment, is now available to offer patients as a first-line BPH therapy. BPH TREATMENT OPTIONS Behavior
Handheld Radiofrequency Spectroscopy for Intraoperative Assessment of Surgical Margins During Breast-Conserving Surgery
 7.01.140 Handheld Radiofrequency Spectroscopy for Intraoperative Assessment of Surgical Margins During Breast-Conserving Surgery Section 7.0 Surgery Subsection Description Effective Date November 26, 2014
7.01.140 Handheld Radiofrequency Spectroscopy for Intraoperative Assessment of Surgical Margins During Breast-Conserving Surgery Section 7.0 Surgery Subsection Description Effective Date November 26, 2014
What is Benign Prostatic Hyperplasia (BPH)?
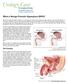 What is Benign Prostatic Hyperplasia (BPH)? Benign prostatic hyperplasia (BPH) is an enlarged prostate. The prostate goes through two main growth periods as a man ages. The first occurs early in puberty,
What is Benign Prostatic Hyperplasia (BPH)? Benign prostatic hyperplasia (BPH) is an enlarged prostate. The prostate goes through two main growth periods as a man ages. The first occurs early in puberty,
Name of Policy: Hematopoietic Stem-Cell Transplantation for Epithelial Ovarian Cancer
 Name of Policy: Hematopoietic Stem-Cell Transplantation for Epithelial Ovarian Cancer Policy #: 401 Latest Review Date: November 2013 Category: Surgery Policy Grade: A Background/Definitions: As a general
Name of Policy: Hematopoietic Stem-Cell Transplantation for Epithelial Ovarian Cancer Policy #: 401 Latest Review Date: November 2013 Category: Surgery Policy Grade: A Background/Definitions: As a general
How Do New Data from Clinical Trials Allow Us to Optimise the Assessment and Treatment of Patients with Benign Prostatic Hyperplasia?
 available at www.sciencedirect.com journal homepage: www.europeanurology.com How Do New Data from Clinical Trials Allow Us to Optimise the Assessment and Treatment of Patients with Benign Prostatic Hyperplasia?
available at www.sciencedirect.com journal homepage: www.europeanurology.com How Do New Data from Clinical Trials Allow Us to Optimise the Assessment and Treatment of Patients with Benign Prostatic Hyperplasia?
PROSTATIC ARTERY EMBOLISATION (PAE) FOR BENIGN PROSTATIC HYPERPLASIA. A Minimally Invasive Innovative Treatment
 PROSTATIC ARTERY EMBOLISATION (PAE) FOR BENIGN PROSTATIC HYPERPLASIA A Minimally Invasive Innovative Treatment What is the prostate? The prostate is an accessory organ of the male reproductive system.
PROSTATIC ARTERY EMBOLISATION (PAE) FOR BENIGN PROSTATIC HYPERPLASIA A Minimally Invasive Innovative Treatment What is the prostate? The prostate is an accessory organ of the male reproductive system.
What should we consider before surgery? BPH with bladder dysfunction. Inje University Sanggye Paik Hospital Sung Luck Hee
 What should we consider before surgery? BPH with bladder dysfunction Inje University Sanggye Paik Hospital Sung Luck Hee Diagnostic tests in three categories Recommendation: there is evidence to support
What should we consider before surgery? BPH with bladder dysfunction Inje University Sanggye Paik Hospital Sung Luck Hee Diagnostic tests in three categories Recommendation: there is evidence to support
BENIGN PROSTATIC HYPERPLASIA (BPH) affects 70%
 JOURNAL OF ENDOUROLOGY Volume 14, Number 2, March 2000 Mary Ann Liebert, Inc. Holmium Laser Enucleation of the Prostate With Tissue Morcellation: Initial United States Experience JEFFREY A. MOODY, M.D.,
JOURNAL OF ENDOUROLOGY Volume 14, Number 2, March 2000 Mary Ann Liebert, Inc. Holmium Laser Enucleation of the Prostate With Tissue Morcellation: Initial United States Experience JEFFREY A. MOODY, M.D.,
All about the Prostate
 MEN S HEALTH Dr Nick Pendleton January 16 th 2018 All about the Prostate 1 What does it do? Functions of the Prostate 1. Secretes Prostatic Fluid slightly alkaline fluid, 30% of volume of seminal fluid,
MEN S HEALTH Dr Nick Pendleton January 16 th 2018 All about the Prostate 1 What does it do? Functions of the Prostate 1. Secretes Prostatic Fluid slightly alkaline fluid, 30% of volume of seminal fluid,
