Chapters 1 & 2 A. Intro to Studies of the Human Body
|
|
|
- Arlene Morton
- 5 years ago
- Views:
Transcription
1 Chapters 1 & 2 A. Intro to Studies of the Human Body a. Anatomy the study of body structure -tomy means cutting or dissecting b. Physiology The study of body function; based on a Latin term meaning nature c. Pathology the study of diseases that affect the body
2 d. Levels of Organization Each level is built from the parts of the previous level. None of the systems can work alone; all are depended upon the other (life could not have evolved one system at a time). Psalm 139 You are fearfully and wonderfully made!
3 B. Body Systems Overview Protection and Support: 1. Integumentary System composed of skin; covers and protects body 2. Skeletal System Composed of bones; provides a framework for body 3. Muscular System Composed of muscles; produces movement
4 Body Systems Overview Coordination and Control: 4. Nervous System Composed of brain, spinal cords and nerves; controls and coordinates the whole body 5. Endocrine System Composed of glands that release hormones; regulate various body activities
5 Body Systems Overview Circulation 6. Cardiovascular System Composed of heart and blood vessels; supplies nutrients and takes away wastes 7. Lymphatic/Immune System Composed of lymphatic vessels and nodes; returns excess fluids from tissues back to blood, and protects against diseases.
6 Body Systems Overview Energy Needs and Fluid Balance 8. Respiratory System Composed of lungs; provides for the exchange of gases with the blood 9. Digestive System Composed of all organs that take in food; allows for absorption of nutrients into the body 10. Urinary System Composed of kidneys, bladder and urethra; rids the body of waste products
7 Body Systems Overview Production of Offspring 11. Reproductive System Composed of external and internal sex organs; provides for the creation of new life.
8 C. Metabolism & Its Regulation a. Metabolism all the life-sustaining reactions that go on within the body systems. b. 2 phases: Catabolism break down of nutrients Anabolism nutrients are used to build the body
9 C. Metabolism & Its Regulation c. Homeostasis - the state of internal balance required for the body to function well. 1. extracellular fluid surrounds and bathes cells; carries nutrients to and from cells into the blood 2. intracellular fluid fluid within the cell 3. Negative feedback mechanism of maintaining homeostasis by keeping systems in normal range
10 D. Directions in the Body a. Universal terms provide consistency for describing body position and direction. b. Anatomical Position: person is facing forward, arms at side, feet parallel, palms up.
11 E. Directional Terms Superior (above) / Inferior (below) ex. the heart is superior to the stomach Ventral or anterior (body front) / Dorsal or posterior (near the body back); Medial (midline of body) / Lateral (toward the side) Proximal (near the origin of a structure) / Distal (farther away from the origin); Ex. The finger tip is distal to the knuckle of the hand. Label the diagram using page 8 in your textbook.
12 F. Planes of division a. Frontal (coronal) divides the body into front and back b. Sagittal divides the body into left and right. (midsagittal) c. Transverse (horizontal) divides into upper and lower parts.
13 G. Body Cavities (Def.) Cavities large spaces containing organs Dorsal Cavity: Cranial the brain Spinal spinal cord
14 Body Cavities Ventral Cavity Thoracic cavity Heart, lungs Abdominal cavity Stomach, intestines, liver, gall bladder, pancreas, spleen, kidneys Pelvic cavity Urinary bladder, rectum, reproductive organs
15 Body Cavities - Thoracic
16 Abdominal regions
17 Ch 2 Chemistry of the Body A. The Importance of water: A. The most abundant compound in your body B. The ideal medium for carrying substances to and from your cells: why? 1. the universal solvent 2. very stable at ordinary temperatures 3. participates in almost all bodily chemical reactions
18 Ch 2 Chemistry of the Body A.Compounds: Acid, Bases and Salts (p 25) 1.Acid a chemical substance capable of donating a hydrogen ion (H+) a.example: HCl H+ + Cl- 2.Base a chemical substance (usually contains a OH-) capable of accepting a hydrogen ion. a.example: NaOH Na+ + OH- 3.A reaction between an acid and a base produces a salt. a.example: HCl + NaOH NaCl (salt) + H2O
19 C. The ph scale (0-14) 1. ph represents the concentration of hydrogen and hydroxide ions in solution a. ph scale acid range is 0-6 ; b. base/alkalinity range is 8-14 c. pure water is ph 7 (neutral)
20 ph scale d. Blood and other body fluids range between 7.35 and 7.45 and are therefore more alkaline e. Acidosis is when a patient s ph falls below 7.35, to 7.0 f. Alkalosis is when a patient s ph rises above g. Buffers are chemicals released into the blood to prevent sharp changes in body fluid ph.
21 D. Organic Compounds characterize living things; all contain the elements hydrogen, carbon, oxygen and nitrogen as their main ingredients. E. Four main types of organic compounds are: a. Carbohydrates b. Lipids, c. Proteins d. Nucleic acids (nucleotides)
22 F. Carbohydrates a. Basic unit of carbs are simple sugars called monosaccharides such as glucose (blood sugar). b. Two simple sugars link to form a disaccharide such as sucrose (table sugar). c. Many sugar linked together become polysaccharide, such as starch (in plants), and glycogen, found in the muscle cells. d. Carbohydrates are an important source of dietary energy.
23 G. Lipids a. b. c. Usually found in the body as fat. Provide insulation and protections for organs. Main source of energy in the body. d. Simple fats are made from glycerol and three fatty acids and are called triglycerides. e. Phospholipids contain the element phosphorus and are found in the membrane of all living cells. f. Steroids are lipids containing rings of carbon atoms, examples include cholesterol, cortisol, testosterone.
24 H. Proteins a. Contain the additional element nitrogen (and sometimes sulfur or phosphorus) b. They make up muscles, bones, skin c. All are composed of monomers, which link together to form amino acid chains, which are then folded into different proteins.
25 I. Enzymes a. Are proteins essential for metabolism b. Most are catalysts which increase the speed of reactions c. Enzyme shape is important to its action. Harsh conditions can alter the shape of the protein, called denaturation, making it ineffective. d. Most enzymes end with the suffix ase; such as lipase, protease, and oxidase.
26 J. Nucleotides A special class of organic compound composed of: A nitrogen base A sugar (usually ribose or deoxyribose) A phosphate group Examples: DNA and RNA involved in transmission of genetic traits ATP the cells high energy compound involved in most cellular activities
Essentials of Anatomy and Physiology, 9e (Marieb) Chapter 1 The Human Body: An Orientation. Short Answer. Figure 1.1
 Essentials of Anatomy and Physiology, 9e (Marieb) Chapter 1 The Human Body: An Orientation Short Answer Figure 1.1 Using Figure 1.1, identify the following: 1) Label A points to the cavity. 2) Label B
Essentials of Anatomy and Physiology, 9e (Marieb) Chapter 1 The Human Body: An Orientation Short Answer Figure 1.1 Using Figure 1.1, identify the following: 1) Label A points to the cavity. 2) Label B
Introduction. Study detail of structure - - Gross Anatomy. Study all structures in one part of body Study of internal structures as relate to skin
 Introduction What is Anatomy and Physiology? Anatomy study of the shape and structure of body parts and their relationships to one another Physiology study of how the body functions individually and cooperatively
Introduction What is Anatomy and Physiology? Anatomy study of the shape and structure of body parts and their relationships to one another Physiology study of how the body functions individually and cooperatively
Chapter 2 Part 3: Organic and Inorganic Compounds
 Chapter 2 Part 3: Organic and Inorganic Compounds Objectives: 1) List the major groups of inorganic chemicals common in cells. 2) Describe the functions of various types of inorganic chemicals in cells.
Chapter 2 Part 3: Organic and Inorganic Compounds Objectives: 1) List the major groups of inorganic chemicals common in cells. 2) Describe the functions of various types of inorganic chemicals in cells.
Chapter 1 The Human Body: An Orientation
 Chapter 1 The Human Body: An Orientation 1 Anatomy Study of the body Structure what something looks like where something is located how big or small it is Ex- what the heart looks like Gross Anatomy structures
Chapter 1 The Human Body: An Orientation 1 Anatomy Study of the body Structure what something looks like where something is located how big or small it is Ex- what the heart looks like Gross Anatomy structures
Human Anatomy and Physiology (ANAT 5) Mrs. Fulton Phone: ext. 6049
 Human Anatomy and Physiology (ANAT 5) Mrs. Fulton Phone: 645-1300 ext. 6049 Please, read your green sheets. 1 Lecture #1 A. Definitions: anatomy, physiology B. Requirements of an Organism C. Homeostasis
Human Anatomy and Physiology (ANAT 5) Mrs. Fulton Phone: 645-1300 ext. 6049 Please, read your green sheets. 1 Lecture #1 A. Definitions: anatomy, physiology B. Requirements of an Organism C. Homeostasis
Human Anatomy Key Points Unit 1/ Study Guide
 Human Anatomy Key Points Unit 1/ Study Guide I. Anatomy and Physiology a. Anatomy 1. Means cutting apart (dissection) 2. Study of the body and the relationships of its parts to each other. 3. Dissection
Human Anatomy Key Points Unit 1/ Study Guide I. Anatomy and Physiology a. Anatomy 1. Means cutting apart (dissection) 2. Study of the body and the relationships of its parts to each other. 3. Dissection
Chapter 1 An Introduction to the Human Body
 1-1 Chapter 1 An Introduction to the Human Body Anatomy science of structure relationships revealed by dissection (cutting apart) Physiology science of body functions Levels of Organization Chemical Cellular
1-1 Chapter 1 An Introduction to the Human Body Anatomy science of structure relationships revealed by dissection (cutting apart) Physiology science of body functions Levels of Organization Chemical Cellular
Chapter 1- An Orientation to the Human Body
 Chapter 1- An Orientation to the Human Body Overview of Anatomy and Physiology: -Anatomy- of body parts and their relationships to one another. -Gross or Macroscopic= large and easily observable -Microscopic=
Chapter 1- An Orientation to the Human Body Overview of Anatomy and Physiology: -Anatomy- of body parts and their relationships to one another. -Gross or Macroscopic= large and easily observable -Microscopic=
Molecule - two or more atoms held together by covalent bonds. Ex. = water, H O
 ORGANIC CHEMISTRY NOTES Why study carbon? ORGANIC CHEMISTRY NOTES Why study carbon? * All of life is built on carbon * Cells are made up of about 72% water 3% salts (NaCl, and K) 25% carbon compounds which
ORGANIC CHEMISTRY NOTES Why study carbon? ORGANIC CHEMISTRY NOTES Why study carbon? * All of life is built on carbon * Cells are made up of about 72% water 3% salts (NaCl, and K) 25% carbon compounds which
Homework Packet. The branch of biological science that studies and describes how body parts. The study of the shape and structure of body parts
 Anatomy & Physiology Chap. 1: The Human Body Name Block: P/W Homework Packet ANATOMY & PHYSIOLOGY DISTINCTIONS 1. Match the term on the right to the appropriate description on the left. Enter the correct
Anatomy & Physiology Chap. 1: The Human Body Name Block: P/W Homework Packet ANATOMY & PHYSIOLOGY DISTINCTIONS 1. Match the term on the right to the appropriate description on the left. Enter the correct
Introduction to The Human Body
 1 Introduction to The Human Body FOCUS: The human organism is often examined at seven structural levels: chemical, organelle, cell, tissue, organ, organ system, and the organism. Anatomy examines the structure
1 Introduction to The Human Body FOCUS: The human organism is often examined at seven structural levels: chemical, organelle, cell, tissue, organ, organ system, and the organism. Anatomy examines the structure
Biochemistry. Chapter 6
 Biochemistry Chapter 6 Game Plan for Today. - Collect your papers - Hand back quests - Go over Amoeba Sister Chart - Biochem Notes - Video Carbohydrate Lab Food Label Lab! Testing For Carbohydrates Benedict's
Biochemistry Chapter 6 Game Plan for Today. - Collect your papers - Hand back quests - Go over Amoeba Sister Chart - Biochem Notes - Video Carbohydrate Lab Food Label Lab! Testing For Carbohydrates Benedict's
Introduction to A & P Medical Terminology
 Human Anatomy & Physiology PHA322.10 D. Matesic, Spring, 2012 Class Notes Introduction to A & P Medical Terminology Levels of Structural Organization Anatomy the study of the structure of body parts and
Human Anatomy & Physiology PHA322.10 D. Matesic, Spring, 2012 Class Notes Introduction to A & P Medical Terminology Levels of Structural Organization Anatomy the study of the structure of body parts and
The Human Body An Overview
 The Human Body An Overview An Overview of Anatomy OAnatomy - The study of the structure of the human body OPhysiology - The study of body function The Hierarchy of Structural Organization O Chemical level
The Human Body An Overview An Overview of Anatomy OAnatomy - The study of the structure of the human body OPhysiology - The study of body function The Hierarchy of Structural Organization O Chemical level
Unit 1: Intro to Physiology
 Unit 1: Intro to Physiology Anatomy The branch of science that studies body parts. Physiology! The branch of science that describes HOW the body works, or functions.! Example - how the heart pumps blood
Unit 1: Intro to Physiology Anatomy The branch of science that studies body parts. Physiology! The branch of science that describes HOW the body works, or functions.! Example - how the heart pumps blood
Bi100 Chapter 1 Introduction to Human Anatomy and Physiology
 Bi100 Chapter 1 Introduction to Human Anatomy and Physiology Anatomy and Physiology A. Anatomy deals with the structure (morphology) of the body and its parts; in other words, what are things called? B.
Bi100 Chapter 1 Introduction to Human Anatomy and Physiology Anatomy and Physiology A. Anatomy deals with the structure (morphology) of the body and its parts; in other words, what are things called? B.
The Structure and Function of Biomolecules
 The Structure and Function of Biomolecules The student is expected to: 9A compare the structures and functions of different types of biomolecules, including carbohydrates, lipids, proteins, and nucleic
The Structure and Function of Biomolecules The student is expected to: 9A compare the structures and functions of different types of biomolecules, including carbohydrates, lipids, proteins, and nucleic
Anatomy & Physiology Ch 1: The Human Body Worksheet
 Anatomy & Physiology Ch 1: The Human Body Worksheet 1. The structures of the body are organized in successively larger and more complex structures. Fill in the blanks with the correct terms for these increasingly
Anatomy & Physiology Ch 1: The Human Body Worksheet 1. The structures of the body are organized in successively larger and more complex structures. Fill in the blanks with the correct terms for these increasingly
Introduction in human anatomy
 Introduction in human anatomy Overview of Anatomy Anatomy is the study of the body structure and the relationships of the various parts of the body Gross or macroscopic (visible structures) Microscopic
Introduction in human anatomy Overview of Anatomy Anatomy is the study of the body structure and the relationships of the various parts of the body Gross or macroscopic (visible structures) Microscopic
2.2 Properties of Water
 2.2 Properties of Water I. Water s unique properties allow life to exist on Earth. A. Life depends on hydrogen bonds in water. B. Water is a polar molecule. 1. Polar molecules have slightly charged regions
2.2 Properties of Water I. Water s unique properties allow life to exist on Earth. A. Life depends on hydrogen bonds in water. B. Water is a polar molecule. 1. Polar molecules have slightly charged regions
CHAPTER 2 Terms Pertaining to the Body as a Whole
 CHAPTER 2 Terms Pertaining to the Body as a Whole OBJECTIVES 1. Define terms that apply to the structural organization of the body. 2. Identify the body cavities and the organs contained within the cavities.
CHAPTER 2 Terms Pertaining to the Body as a Whole OBJECTIVES 1. Define terms that apply to the structural organization of the body. 2. Identify the body cavities and the organs contained within the cavities.
Macromolecules Chapter 2.3
 Macromolecules Chapter 2.3 E.Q. What are the 4 main macromolecues found in living things and what are their functions? Carbon-Based Molecules Why is carbon called the building block of life? Carbon atoms
Macromolecules Chapter 2.3 E.Q. What are the 4 main macromolecues found in living things and what are their functions? Carbon-Based Molecules Why is carbon called the building block of life? Carbon atoms
Anatomical Terminology
 Anatomical Terminology Dr. A. Ebneshahidi Anatomy Anatomy : is the study of structures or body parts and their relationships to on another. Anatomy : Gross anatomy - macroscopic. Histology - microscopic.
Anatomical Terminology Dr. A. Ebneshahidi Anatomy Anatomy : is the study of structures or body parts and their relationships to on another. Anatomy : Gross anatomy - macroscopic. Histology - microscopic.
THE HUMAN BODY. study of the structure of living organisms. Physiologythe study of how the body works. Ex: studying the structure of the heart.
 HUMAN BODY SYSTEMS Anatomythe study of the structure of living organisms. Ex: studying the structure of the heart. Physiologythe study of how the body works. Ex: how the heart works to pump blood, etc.
HUMAN BODY SYSTEMS Anatomythe study of the structure of living organisms. Ex: studying the structure of the heart. Physiologythe study of how the body works. Ex: how the heart works to pump blood, etc.
CP Biology: Basic Biochemistry
 CP Biology: Basic Biochemistry Organic Chemistry Organic chemistry is the study of carbon compounds. Organic compounds are compounds composed primarily of a carbon skeleton. All living things are composed
CP Biology: Basic Biochemistry Organic Chemistry Organic chemistry is the study of carbon compounds. Organic compounds are compounds composed primarily of a carbon skeleton. All living things are composed
Anatomy & Physiology. An Introduction
 Anatomy & Physiology An Introduction An Overview of Anatomy Anatomy - The study of the structure of the human body Physiology - The study of body function Branches of Anatomy Surface anatomy Gross anatomy
Anatomy & Physiology An Introduction An Overview of Anatomy Anatomy - The study of the structure of the human body Physiology - The study of body function Branches of Anatomy Surface anatomy Gross anatomy
What is an atom? An atom is the smallest component of all living and nonliving materials.
 What is an atom? An atom is the smallest component of all living and nonliving materials. It is composed of protons (+), neutrons (0), and electrons (-). The Periodic Table Elements are composed of all
What is an atom? An atom is the smallest component of all living and nonliving materials. It is composed of protons (+), neutrons (0), and electrons (-). The Periodic Table Elements are composed of all
What is an atom? An atom is the smallest component of all living and nonliving materials.
 What is an atom? An atom is the smallest component of all living and nonliving materials. It is composed of protons (+), neutrons (0), and electrons (-). The Periodic Table Elements are composed of all
What is an atom? An atom is the smallest component of all living and nonliving materials. It is composed of protons (+), neutrons (0), and electrons (-). The Periodic Table Elements are composed of all
Anatomy & Physiology. Advanced Biology Mrs. Layton-Krout
 Anatomy & Physiology Advanced Biology Mrs. Layton-Krout A & P Anatomy - study of structure Physiology - study of function Structure is always related to function Levels of Organization Atom Chemical Molecule
Anatomy & Physiology Advanced Biology Mrs. Layton-Krout A & P Anatomy - study of structure Physiology - study of function Structure is always related to function Levels of Organization Atom Chemical Molecule
2.3 Carbon-Based Molecules. KEY CONCEPT Carbon-based molecules are the foundation of life.
 KEY CONCEPT Carbon-based molecules are the foundation of life. Carbon atoms have unique bonding properties. Carbon forms covalent bonds with up to four other atoms, including other carbon atoms. Carbon-based
KEY CONCEPT Carbon-based molecules are the foundation of life. Carbon atoms have unique bonding properties. Carbon forms covalent bonds with up to four other atoms, including other carbon atoms. Carbon-based
Biology 12. Biochemistry. Water - a polar molecule Water (H 2 O) is held together by covalent bonds.
 Biology 12 Biochemistry Water - a polar molecule Water (H 2 O) is held together by covalent bonds. Electrons in these bonds spend more time circulating around the larger Oxygen atom than the smaller Hydrogen
Biology 12 Biochemistry Water - a polar molecule Water (H 2 O) is held together by covalent bonds. Electrons in these bonds spend more time circulating around the larger Oxygen atom than the smaller Hydrogen
CHAPTER 1: THE HUMAN BODY A & P 8/20/13
 CHAPTER 1: THE HUMAN BODY A & P 8/20/13 ANATOMY The study of the Structure and Shapes of the body and their relationships to one another. Gross Anatomy: The study of large easily observable structures
CHAPTER 1: THE HUMAN BODY A & P 8/20/13 ANATOMY The study of the Structure and Shapes of the body and their relationships to one another. Gross Anatomy: The study of large easily observable structures
BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2.
 BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2. A possible explanation for an event that occurs in nature is
BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2. A possible explanation for an event that occurs in nature is
Carbon. Has four valence electrons Can bond with many elements. Can bond to other carbon atoms. Hydrogen, Oxygen, Phosphorus, Sulfur, and Nitrogen
 Organic Compounds Carbon Has four valence electrons Can bond with many elements Hydrogen, Oxygen, Phosphorus, Sulfur, and Nitrogen Can bond to other carbon atoms Gives carbon the ability to form chains
Organic Compounds Carbon Has four valence electrons Can bond with many elements Hydrogen, Oxygen, Phosphorus, Sulfur, and Nitrogen Can bond to other carbon atoms Gives carbon the ability to form chains
Carbon Compounds (2.3) (Part 1 - Carbohydrates)
 Carbon Compounds (2.3) (Part 1 - Carbohydrates) The Chemistry of Carbon (Organic Chemistry) Organic Chemistry: The study of compounds that contain bonds between carbon atoms. Carbon can bond with many
Carbon Compounds (2.3) (Part 1 - Carbohydrates) The Chemistry of Carbon (Organic Chemistry) Organic Chemistry: The study of compounds that contain bonds between carbon atoms. Carbon can bond with many
Basic Body Structure
 Basic Body Structure The Cell All life consists of microscopic living structures called cells. They perform various functions throughout the body. All cells are similar in structure, but not identical.
Basic Body Structure The Cell All life consists of microscopic living structures called cells. They perform various functions throughout the body. All cells are similar in structure, but not identical.
Carbon. p Has four valence electrons p Can bond with many elements p Can bond to other carbon atoms
 Organic Compounds Carbon p Has four valence electrons p Can bond with many elements p Can bond to other carbon atoms n Gives carbon the ability to form chains that are almost unlimited in length. p Organic
Organic Compounds Carbon p Has four valence electrons p Can bond with many elements p Can bond to other carbon atoms n Gives carbon the ability to form chains that are almost unlimited in length. p Organic
Anatomy & Physiology Chapter 1 Study Guide
 Name Pd Date Anatomy & Physiology Chapter 1 Study Guide AN OVERVIEW OF ANATOMY & PHYSIOLOGY 1. Match the terms in Column B to the appropriate descriptions provided in Column A. Enter the correct letter
Name Pd Date Anatomy & Physiology Chapter 1 Study Guide AN OVERVIEW OF ANATOMY & PHYSIOLOGY 1. Match the terms in Column B to the appropriate descriptions provided in Column A. Enter the correct letter
Chapter 2: Biochemistry
 Chapter 2: Biochemistry Biochemistry Biochemistry is the study of chemical makeup and reactions of living matter All chemicals in the body are either organic & inorganic Organic compounds contain carbon
Chapter 2: Biochemistry Biochemistry Biochemistry is the study of chemical makeup and reactions of living matter All chemicals in the body are either organic & inorganic Organic compounds contain carbon
2 3 Carbon Compounds (Macromolecules)
 2 3 Carbon Compounds (Macromolecules) Slide 1 of 37 Organic Chemistry Organic chemistry is the study of all compounds that contain bonds between carbon atoms. Slide 2 of 37 Carbon Living organisms are
2 3 Carbon Compounds (Macromolecules) Slide 1 of 37 Organic Chemistry Organic chemistry is the study of all compounds that contain bonds between carbon atoms. Slide 2 of 37 Carbon Living organisms are
1.45_Internet Assignment #1: The Human Body: An Orientation
 1.45_Internet Assignment #1: The Human Body: An Orientation Go to the following website to complete the following Activities, Quizzes, and Reading: http://wps.aw.com/bc_marieb_hap_9_oa/218/55856/14299219.cw/index.html
1.45_Internet Assignment #1: The Human Body: An Orientation Go to the following website to complete the following Activities, Quizzes, and Reading: http://wps.aw.com/bc_marieb_hap_9_oa/218/55856/14299219.cw/index.html
Water: 1. The bond between water molecules is a(n) a. ionic bond b. covalent bond c. polar covalent bond d. hydrogen bond
 Biology 12 - Biochemistry Practice Exam KEY Water: 1. The bond between water molecules is a(n) a. ionic bond b. covalent bond c. polar covalent bond d. hydrogen bond 2. The water properties: good solvent,
Biology 12 - Biochemistry Practice Exam KEY Water: 1. The bond between water molecules is a(n) a. ionic bond b. covalent bond c. polar covalent bond d. hydrogen bond 2. The water properties: good solvent,
The Human Body: An Orientation
 1 The Human Body: An Orientation Yong Jeong, MD, PhD Department of Bio and Brain Engineering The Human Body An Orientation Anatomy Study of the structure and shape of the body and its parts Physiology
1 The Human Body: An Orientation Yong Jeong, MD, PhD Department of Bio and Brain Engineering The Human Body An Orientation Anatomy Study of the structure and shape of the body and its parts Physiology
B. Element - each different kind of atom is a different element 1. Examples: C = carbon H = hydrogen
 I. Chemistry study of what substances are made of and how they change and combine Structural Formula A. Atom fundamental unit of matter 1. Subatomic particles: n o = neutron p + = proton e - = electron
I. Chemistry study of what substances are made of and how they change and combine Structural Formula A. Atom fundamental unit of matter 1. Subatomic particles: n o = neutron p + = proton e - = electron
Organic Molecules. 8/27/2004 Mr. Davenport 1
 Organic Molecules 8/27/2004 Mr. Davenport 1 Carbohydrates Commonly called sugars and starches Consist of C, H, O with H:O ration 2:1 Usually classified as to sugar units Monosaccharide are single sugar
Organic Molecules 8/27/2004 Mr. Davenport 1 Carbohydrates Commonly called sugars and starches Consist of C, H, O with H:O ration 2:1 Usually classified as to sugar units Monosaccharide are single sugar
Lesson Overview. Carbon Compounds. Lesson Overview. 2.3 Carbon Compounds
 Lesson Overview 2.3 The Chemistry of Carbon What elements does carbon bond with to make up life s molecules? Carbon can bond with many elements, including Hydrogen, Oxygen, Phosphorus, Sulfur, and Nitrogen
Lesson Overview 2.3 The Chemistry of Carbon What elements does carbon bond with to make up life s molecules? Carbon can bond with many elements, including Hydrogen, Oxygen, Phosphorus, Sulfur, and Nitrogen
Biology. Slide 1 of 37. End Show. Copyright Pearson Prentice Hall
 Biology 1 of 37 2 of 37 The Chemistry of Carbon The Chemistry of Carbon Organic chemistry is the study of all compounds that contain bonds between carbon atoms. 3 of 37 Macromolecules Macromolecules Macromolecules
Biology 1 of 37 2 of 37 The Chemistry of Carbon The Chemistry of Carbon Organic chemistry is the study of all compounds that contain bonds between carbon atoms. 3 of 37 Macromolecules Macromolecules Macromolecules
Ch 1.1 An Overview of Anatomy and Physiology. Copyright 2009 Pearson Education, Inc., publishing as Benjamin Cummings
 Ch 1.1 An Overview of Anatomy and Physiology The Human Body An Orientation Anatomy Study of the structure and shape of the body and its parts Physiology Study of how the body and its parts work or function
Ch 1.1 An Overview of Anatomy and Physiology The Human Body An Orientation Anatomy Study of the structure and shape of the body and its parts Physiology Study of how the body and its parts work or function
Systemic The study of anatomy by body systems
 Chapter 1: Introduction to Structural Units Video Worksheet Anatomy Terms: What should you do to learn the terms in this class??? Buy notecards Anatomy The study of the body o Identification of the body
Chapter 1: Introduction to Structural Units Video Worksheet Anatomy Terms: What should you do to learn the terms in this class??? Buy notecards Anatomy The study of the body o Identification of the body
Biological Chemistry. Is biochemistry fun? - Find it out!
 Biological Chemistry Is biochemistry fun? - Find it out! 1. Key concepts Outline 2. Condensation and Hydrolysis Reactions 3. Carbohydrates 4. Lipids 5. Proteins 6. Nucleic Acids Key Concepts: 1. Organic
Biological Chemistry Is biochemistry fun? - Find it out! 1. Key concepts Outline 2. Condensation and Hydrolysis Reactions 3. Carbohydrates 4. Lipids 5. Proteins 6. Nucleic Acids Key Concepts: 1. Organic
The Human Body: An Orientation
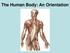 The Human Body: An Orientation Body standing upright Anatomical Position feet slightly apart palms facing forward thumbs point away from body Directional Terms Superior and inferior toward and away from
The Human Body: An Orientation Body standing upright Anatomical Position feet slightly apart palms facing forward thumbs point away from body Directional Terms Superior and inferior toward and away from
Microscopic Anatomy Cytology study of the cell Histology study of tissues
 Introduction to Anatomy and Physiology Dr. Gary Mumaugh Overview of Anatomy and Physiology Anatomy the study of the structure of body parts and their relationships to one another o Gross or macroscopic
Introduction to Anatomy and Physiology Dr. Gary Mumaugh Overview of Anatomy and Physiology Anatomy the study of the structure of body parts and their relationships to one another o Gross or macroscopic
The HUMAN BODY. Concepts of ANATOMY and PHYSIOLOGY
 The HUMAN BODY Concepts of ANATOMY and PHYSIOLOGY 1 1 ANATOMY The scientific study of structures and the relationship of structures to each other. FORM Other terms include shape, structure, and appearance.
The HUMAN BODY Concepts of ANATOMY and PHYSIOLOGY 1 1 ANATOMY The scientific study of structures and the relationship of structures to each other. FORM Other terms include shape, structure, and appearance.
Chapter One: Introduction to Human Anatomy and Physiology
 Chapter One: Introduction to Human Anatomy and Physiology Anatomy is the scientific study of structure or form (morphology) Physiology is the scientific study of function Functional role of a body part
Chapter One: Introduction to Human Anatomy and Physiology Anatomy is the scientific study of structure or form (morphology) Physiology is the scientific study of function Functional role of a body part
Testbank Chapter 1. An Introduction to the Human Body
 Testbank Chapter 1. An Introduction to the Human Body Multiple Choice 1. This is the study of the functions of body structures. a. Anatomy b. Physiology c. Dissection d. Histology e. Immunology Ans: B
Testbank Chapter 1. An Introduction to the Human Body Multiple Choice 1. This is the study of the functions of body structures. a. Anatomy b. Physiology c. Dissection d. Histology e. Immunology Ans: B
Assignment #1: Biological Molecules & the Chemistry of Life
 Assignment #1: Biological Molecules & the Chemistry of Life A. Important Inorganic Molecules Water 1. Explain why water is considered a polar molecule. The partial negative charge of the oxygen and the
Assignment #1: Biological Molecules & the Chemistry of Life A. Important Inorganic Molecules Water 1. Explain why water is considered a polar molecule. The partial negative charge of the oxygen and the
Proteins. Biomolecules. Nucleic Acids. The Building Blocks of Life
 Proteins Biomolecules Nucleic Acids The Building Blocks of Life Carbohydrates Lipids Biomolecules are Organic Molecules 1. Organic molecules that are Carbon based (at least 1 Carbon molecule and often
Proteins Biomolecules Nucleic Acids The Building Blocks of Life Carbohydrates Lipids Biomolecules are Organic Molecules 1. Organic molecules that are Carbon based (at least 1 Carbon molecule and often
BIOMOLECULES. Ms. Bosse Fall 2015
 BIOMOLECULES Ms. Bosse Fall 2015 Biology Biology is the study of the living world. Bio = life Major Molecules of Life Macromolecules giant molecules found in living cells; made from thousands of smaller
BIOMOLECULES Ms. Bosse Fall 2015 Biology Biology is the study of the living world. Bio = life Major Molecules of Life Macromolecules giant molecules found in living cells; made from thousands of smaller
Most life processes are a series of chemical reactions influenced by environmental and genetic factors.
 Biochemistry II Most life processes are a series of chemical reactions influenced by environmental and genetic factors. Metabolism the sum of all biochemical processes 2 Metabolic Processes Anabolism-
Biochemistry II Most life processes are a series of chemical reactions influenced by environmental and genetic factors. Metabolism the sum of all biochemical processes 2 Metabolic Processes Anabolism-
Chapter 1 - The Human Body: An Orientation
 Chapter 1 - The Human Body: An Orientation A. Terminology I. AN OVERVIEW OF ANATOMY AND PHYSIOLOGY 1. Anatomy: To Cut. No analyzing involved. - where things are, how connect to each other - Gross vs. microscopic
Chapter 1 - The Human Body: An Orientation A. Terminology I. AN OVERVIEW OF ANATOMY AND PHYSIOLOGY 1. Anatomy: To Cut. No analyzing involved. - where things are, how connect to each other - Gross vs. microscopic
Chapter 3 The Molecules of Life
 Chapter 3 The Molecules of Life State Standards Standard 1.h. Standard 5.a. Standard 4.e. Organic Molecules A cell is mostly water. The rest of the cell consists mostly of carbon based molecules organic
Chapter 3 The Molecules of Life State Standards Standard 1.h. Standard 5.a. Standard 4.e. Organic Molecules A cell is mostly water. The rest of the cell consists mostly of carbon based molecules organic
Cell Chemistry - Intro
 Cell Chemistry - Intro SBI 3C Cell Chemistry All things are made of atoms, including living things. As we explore the cell we need to have a basic understanding of the chemistry and molecules that make
Cell Chemistry - Intro SBI 3C Cell Chemistry All things are made of atoms, including living things. As we explore the cell we need to have a basic understanding of the chemistry and molecules that make
Introduction to Anatomical Terms. Packet #3
 Introduction to Anatomical Terms Packet #3 Directional Terms Directional terms describe the positions of structures relative to other structures or locations in the body. Introduction Superior vs. Inferior
Introduction to Anatomical Terms Packet #3 Directional Terms Directional terms describe the positions of structures relative to other structures or locations in the body. Introduction Superior vs. Inferior
Chapter 3: Biochemistry Adapted from PPT by S. Edwards. By PresenterMedia.com
 Chapter 3: Biochemistry Adapted from PPT by S. Edwards By PresenterMedia.com CARBON COMPOUNDS CHAPTER 3 SECTION 1 By PresenterMedia.com Compounds LOOK NO Carbon!!! ORGANIC COMPOUNDS Compounds that contain
Chapter 3: Biochemistry Adapted from PPT by S. Edwards By PresenterMedia.com CARBON COMPOUNDS CHAPTER 3 SECTION 1 By PresenterMedia.com Compounds LOOK NO Carbon!!! ORGANIC COMPOUNDS Compounds that contain
Carbon Compounds. Lesson Overview. Lesson Overview. 2.3 Carbon Compounds
 Lesson Overview Carbon Compounds Lesson Overview 2.3 THINK ABOUT IT In the early 1800s, many chemists called the compounds created by organisms organic, believing they were fundamentally different from
Lesson Overview Carbon Compounds Lesson Overview 2.3 THINK ABOUT IT In the early 1800s, many chemists called the compounds created by organisms organic, believing they were fundamentally different from
Organic compounds. Lipids, Carbohydrates, Proteins, and Nucleic Acids
 Organic compounds Lipids, Carbohydrates, Proteins, and Nucleic Acids Essential for life Organic compounds: Contain carbon Most are covalently bonded Example: C 6 H 12 O 6 (Glucose) Inorganic Compounds:
Organic compounds Lipids, Carbohydrates, Proteins, and Nucleic Acids Essential for life Organic compounds: Contain carbon Most are covalently bonded Example: C 6 H 12 O 6 (Glucose) Inorganic Compounds:
Lesson Overview. Carbon Compounds. Lesson Overview. 2.3 Carbon Compounds
 Lesson Overview 2.3 THINK ABOUT IT In the early 1800s, many chemists called the compounds created by organisms organic, believing they were fundamentally different from compounds in nonliving things. We
Lesson Overview 2.3 THINK ABOUT IT In the early 1800s, many chemists called the compounds created by organisms organic, believing they were fundamentally different from compounds in nonliving things. We
The Atoms of Life. What are other elements would you expect to be on this list? Carbon Hydrogen Nitrogen Oxygen Phosphorous Sulfur (sometimes)
 Macromolecules The Atoms of Life The most frequently found atoms in the body are Carbon Hydrogen Nitrogen Oxygen Phosphorous Sulfur (sometimes) What are other elements would you expect to be on this list?
Macromolecules The Atoms of Life The most frequently found atoms in the body are Carbon Hydrogen Nitrogen Oxygen Phosphorous Sulfur (sometimes) What are other elements would you expect to be on this list?
Biochemistry. Biome. & Compound. Macromolecules
 Biochemistry Biome Macromolecules & Compound 1 ATOMS the smallest unit of an element. Ex: Carbon- C MOLECULE A molecule is formed when two or more atoms join together chemically. EX: O 2 (Oxygen Gas) 2
Biochemistry Biome Macromolecules & Compound 1 ATOMS the smallest unit of an element. Ex: Carbon- C MOLECULE A molecule is formed when two or more atoms join together chemically. EX: O 2 (Oxygen Gas) 2
Unit 2: Cellular Chemistry, Structure, and Physiology Module 2: Cellular Chemistry
 Unit 2: Cellular Chemistry, Structure, and Physiology Module 2: Cellular Chemistry NC Essential Standard: 1.2.1 Explain how cells use buffers to regulate cell ph 4.1.1 Compare the structure and functions
Unit 2: Cellular Chemistry, Structure, and Physiology Module 2: Cellular Chemistry NC Essential Standard: 1.2.1 Explain how cells use buffers to regulate cell ph 4.1.1 Compare the structure and functions
CHAPTER 1 THE HUMAN BODY
 HPTER 1 THE HUMN OY TRUE/FLSE 1. midsagittal plane vertically divides the body through the midline into two equal left and right portions or halves. NS: T NOT: midsagittal plane vertically divides the
HPTER 1 THE HUMN OY TRUE/FLSE 1. midsagittal plane vertically divides the body through the midline into two equal left and right portions or halves. NS: T NOT: midsagittal plane vertically divides the
Macromolecules. Molecules of Life
 Macromolecules Molecules of Life Learning Objectives know the difference between a dehydration synthesis reaction and a hydrolysis reaction know the different types of biological macromolecules be able
Macromolecules Molecules of Life Learning Objectives know the difference between a dehydration synthesis reaction and a hydrolysis reaction know the different types of biological macromolecules be able
Biological Molecules. Carbohydrates, Proteins, Lipids, and Nucleic Acids
 Biological Molecules Carbohydrates, Proteins, Lipids, and Nucleic Acids Organic Molecules Always contain Carbon (C) and Hydrogen (H) Carbon is missing four electrons Capable of forming 4 covalent bonds
Biological Molecules Carbohydrates, Proteins, Lipids, and Nucleic Acids Organic Molecules Always contain Carbon (C) and Hydrogen (H) Carbon is missing four electrons Capable of forming 4 covalent bonds
Anatomy and Physiology Unit 1 Review Sheet
 Anatomy and Physiology Unit 1 Review Sheet Chapter 1 Name Date Hour 1. investigates the body's structure, whereas investigates the processes or functions of living things. A. Physiology, cytology B. Physiology,
Anatomy and Physiology Unit 1 Review Sheet Chapter 1 Name Date Hour 1. investigates the body's structure, whereas investigates the processes or functions of living things. A. Physiology, cytology B. Physiology,
2-2 Properties of Water
 2-2 Properties of Water 1 A. The Water Molecule o o o Water is polar Hydrogen bonds form between water molecules Properties of Water: cohesion adhesion capillary action high specific heat ice floats good
2-2 Properties of Water 1 A. The Water Molecule o o o Water is polar Hydrogen bonds form between water molecules Properties of Water: cohesion adhesion capillary action high specific heat ice floats good
Introduction to Human Anatomy (Chapter 1)
 Name Date Period Introduction to Human Anatomy (Chapter 1) 1. Anatomy is the study of structures making up an organism. 2. Physiology is the study of how an organism functions. 3. The major organ systems
Name Date Period Introduction to Human Anatomy (Chapter 1) 1. Anatomy is the study of structures making up an organism. 2. Physiology is the study of how an organism functions. 3. The major organ systems
Biology 12 - Biochemistry Practice Exam
 Biology 12 - Biochemistry Practice Exam Name: Water: 1. The bond between water molecules is a (n) a. ionic bond b. covalent bond c. polar covalent bond d. hydrogen bond 2. The water properties: good solvent,
Biology 12 - Biochemistry Practice Exam Name: Water: 1. The bond between water molecules is a (n) a. ionic bond b. covalent bond c. polar covalent bond d. hydrogen bond 2. The water properties: good solvent,
The Chemistry of Life
 The Chemistry of Life Biomolecules Warm-up List the percentages of each: Total Fats Saturated Fats 25% Carbohydrates 10% Protein 7% 20% What Biomolecule would cholesterol be classified as? Lipids (fats)
The Chemistry of Life Biomolecules Warm-up List the percentages of each: Total Fats Saturated Fats 25% Carbohydrates 10% Protein 7% 20% What Biomolecule would cholesterol be classified as? Lipids (fats)
INTRODUCTION TO ANATOMY & PHYSIOLOGY
 INTRODUCTION TO ANATOMY & PHYSIOLOGY Introduction Anatomy Deals with the structures (morphology) of body parts Physiology Deals with the functions of body parts. What they do and how they do it. Structure
INTRODUCTION TO ANATOMY & PHYSIOLOGY Introduction Anatomy Deals with the structures (morphology) of body parts Physiology Deals with the functions of body parts. What they do and how they do it. Structure
Chapter 3- Organic Molecules
 Chapter 3- Organic Molecules CHNOPS Six of the most abundant elements of life (make up 95% of the weight of all living things)! What are they used for? Structures, enzymes, energy, hormones, DNA How do
Chapter 3- Organic Molecules CHNOPS Six of the most abundant elements of life (make up 95% of the weight of all living things)! What are they used for? Structures, enzymes, energy, hormones, DNA How do
BIOCHEMISTRY NOTES Pre AP
 BIOCHEMISTRY NOTES Pre AP I. Chemistry study of what are made of and how they (text pages 35 43) A. Atom fundamental unit of matter 1. Subatomic particles: n o = neutron p + = proton e - = electron B.
BIOCHEMISTRY NOTES Pre AP I. Chemistry study of what are made of and how they (text pages 35 43) A. Atom fundamental unit of matter 1. Subatomic particles: n o = neutron p + = proton e - = electron B.
Bio 5/6
 Name: THE HUMAN BODY: AN ORIENTATION 1 An Introduction to Anatomy and Physiology Study Guide Period: Most of us have a natural curiosity about our bodies, and a study of anatomy and physiology elaborates
Name: THE HUMAN BODY: AN ORIENTATION 1 An Introduction to Anatomy and Physiology Study Guide Period: Most of us have a natural curiosity about our bodies, and a study of anatomy and physiology elaborates
Chapter 1. Introduction to Human. Anatomy and Physiology 8/16/2012. believe are the major requirements
 Anatomy and Physiology Chapter 1 Introduction to Human Anatomy and Physiology Anatomy deals with the structure (morphology) of the body and its parts, in other words, what things are called. Physiology
Anatomy and Physiology Chapter 1 Introduction to Human Anatomy and Physiology Anatomy deals with the structure (morphology) of the body and its parts, in other words, what things are called. Physiology
NOTE: For studying for the final, you only have to worry about those with an asterix (*)
 NOTE: For studying for the final, you only have to worry about those with an asterix (*) (*)1. An organic compound is one that: a. contains carbon b. is slightly acidic c. forms long chains d. is soluble
NOTE: For studying for the final, you only have to worry about those with an asterix (*) (*)1. An organic compound is one that: a. contains carbon b. is slightly acidic c. forms long chains d. is soluble
Lesson 2. Biological Molecules. Introduction to Life Processes - SCI 102 1
 Lesson 2 Biological Molecules Introduction to Life Processes - SCI 102 1 Carbon in Biological Molecules Organic molecules contain carbon (C) and hydrogen (H) Example: glucose (C 6 H 12 O 6 ) Inorganic
Lesson 2 Biological Molecules Introduction to Life Processes - SCI 102 1 Carbon in Biological Molecules Organic molecules contain carbon (C) and hydrogen (H) Example: glucose (C 6 H 12 O 6 ) Inorganic
2.3: Carbon-Based Molecules Notes
 2.3: Carbon-Based Molecules Notes Carbon-based molecules are the of life. Bonding Properties of Carbon Carbon forms bonds with up to other atoms, including other carbon atoms. QUESTION: What types of elements
2.3: Carbon-Based Molecules Notes Carbon-based molecules are the of life. Bonding Properties of Carbon Carbon forms bonds with up to other atoms, including other carbon atoms. QUESTION: What types of elements
Chapter Overview. Chapter 1. Anatomy. Physiology
 Chapter Overview Chapter 1 An Introduction to the Human Body Define Anatomy and Physiology Levels of Organization Characteristics of Living Things Homeostasis Anatomical Terminology 1 2 Anatomy Describes
Chapter Overview Chapter 1 An Introduction to the Human Body Define Anatomy and Physiology Levels of Organization Characteristics of Living Things Homeostasis Anatomical Terminology 1 2 Anatomy Describes
Digestive and Excretory Systems
 Digestive and Excretory Systems Homeostasis Q: How are the materials that enter and leave your body related to the processes that maintain homeostasis? 30.1 How is the human body organized and regulated?
Digestive and Excretory Systems Homeostasis Q: How are the materials that enter and leave your body related to the processes that maintain homeostasis? 30.1 How is the human body organized and regulated?
Essential Components of Food
 Essential Components of Food The elements of life living things are mostly (98%) made of 6 elements: C carbon H hydrogen O oxygen P phosphorus N nitrogen S sulphur -each element makes a specific number
Essential Components of Food The elements of life living things are mostly (98%) made of 6 elements: C carbon H hydrogen O oxygen P phosphorus N nitrogen S sulphur -each element makes a specific number
Chapter 1-2 Review Assignment
 Class: Date: Chapter 1-2 Review Assignment Multiple Choice dentify the choice that best completes the statement or answers the question. Corn seedlings A student wanted to design an investigation to see
Class: Date: Chapter 1-2 Review Assignment Multiple Choice dentify the choice that best completes the statement or answers the question. Corn seedlings A student wanted to design an investigation to see
All living things are mostly composed of 4 elements: H, O, N, C honk Compounds are broken down into 2 general categories: Inorganic Compounds:
 Organic Chemistry All living things are mostly composed of 4 elements: H, O, N, C honk Compounds are broken down into 2 general categories: Inorganic Compounds: Do not contain carbon Organic compounds
Organic Chemistry All living things are mostly composed of 4 elements: H, O, N, C honk Compounds are broken down into 2 general categories: Inorganic Compounds: Do not contain carbon Organic compounds
Refresher: What do we remember about CARBON? What makes it special? Nickname? Where do we find it?
 2.3: Carbon Based Molecules Situation: You are tasked with making Chicken Parm and ziti for you entire family (aunts, uncles, cousins, etc). There are 92 different ingredients you have access to in the
2.3: Carbon Based Molecules Situation: You are tasked with making Chicken Parm and ziti for you entire family (aunts, uncles, cousins, etc). There are 92 different ingredients you have access to in the
is the study of the body s structure. a. Histology b. Anatomy c. Embryology d. Physiology
 is the study of the body s structure. a. Histology b. Anatomy c. Embryology d. Physiology is the study of the body s function. a. Histology b. Anatomy c. Embryology d. Physiology When the anatomy of a
is the study of the body s structure. a. Histology b. Anatomy c. Embryology d. Physiology is the study of the body s function. a. Histology b. Anatomy c. Embryology d. Physiology When the anatomy of a
Welcome to ANAT 10A! What is Anatomy? Different levels of Anatomy The Language of Anatomy Pearson Education, Inc.
 Welcome to ANAT 10A! What is Anatomy? Different levels of Anatomy The Language of Anatomy Introduction Anatomy means to dissect: (ANAT 10A) The study of internal & external body structures The study of
Welcome to ANAT 10A! What is Anatomy? Different levels of Anatomy The Language of Anatomy Introduction Anatomy means to dissect: (ANAT 10A) The study of internal & external body structures The study of
Macromolecules Carbohydrates A COMPLEX COLORING EXPERIENCE
 Macromolecules Carbohydrates A COMPLEX COLORING EXPERIENCE Name: Per: Date: All plants, animals and microorganisms use carbohydrates as sources of energy. Carbohydrates are also used as structural building
Macromolecules Carbohydrates A COMPLEX COLORING EXPERIENCE Name: Per: Date: All plants, animals and microorganisms use carbohydrates as sources of energy. Carbohydrates are also used as structural building
Biology Chapter 5. Biological macromolecules
 Biology Chapter 5 Biological macromolecules Small molecules (like water and NaCl) have certain properties that arise from the bonds which hold atoms together in a particular arrangement. Many of the molecules
Biology Chapter 5 Biological macromolecules Small molecules (like water and NaCl) have certain properties that arise from the bonds which hold atoms together in a particular arrangement. Many of the molecules
B i o c h e m i s t r y N o t e s
 14 P a g e Carbon Hydrogen Nitrogen Oxygen Phosphorus Sulfur ~Major ~Found in all ~Found in most ~Found in all component of all organic organic molecules. molecules. ~Major structural atom in all organic
14 P a g e Carbon Hydrogen Nitrogen Oxygen Phosphorus Sulfur ~Major ~Found in all ~Found in most ~Found in all component of all organic organic molecules. molecules. ~Major structural atom in all organic
½ cup of CHEX MIX contains 13 g of carbs = 4% daily value. How much more can you have the rest of the day??? _4_ = X X= 325 g
 BIOCHEMISTRY ½ cup of CHEX MIX contains 13 g of carbs = 4% daily value. How much more can you have the rest of the day??? _4_ = 13 100 X X= 325 g These spinach imposters contain less than 2 percent of
BIOCHEMISTRY ½ cup of CHEX MIX contains 13 g of carbs = 4% daily value. How much more can you have the rest of the day??? _4_ = 13 100 X X= 325 g These spinach imposters contain less than 2 percent of
Good Afternoon! 11/30/18
 Good Afternoon! 11/30/18 1. The term polar refers to a molecule that. A. Is cold B. Has two of the same charges C. Has two opposing charges D. Contains a hydrogen bond 2. Electrons on a water molecule
Good Afternoon! 11/30/18 1. The term polar refers to a molecule that. A. Is cold B. Has two of the same charges C. Has two opposing charges D. Contains a hydrogen bond 2. Electrons on a water molecule
