@ Int. CI *: C 08 F 114/06 C 08 F 2/18, C 08 F 4/40. Applicant: THE GOODYEAR TIRE &RUBBER COMPANY 1144 East Market Street Akron, Ohio (US)
|
|
|
- Garry Nelson
- 5 years ago
- Views:
Transcription
1 Europaisches Patentamt European Patent Office Office europeen des Publication number: A2 EUROPEAN PATENT APPLICATION Application number: Date of filing: Int. CI *: C 08 F 114/06 C 08 F 2/18, C 08 F 4/40 ( ) Priority: US Date of publication of application: Bulletin Designated Contracting States: DE ES FR GB IT NL Applicant: THE GOODYEAR TIRE &RUBBER COMPANY 1144 East Market Street Akron, Ohio (US) Inventor: Marshall, Richard Allen 1651 Arrowhead Drive Oxford Ohio (US) Hershberger, James William 1007 Arrowhead Drive Oxford Ohio (US) Hershberger, Susan Ann 1007 Arrowhead Drive Oxford Ohio (US) (74) Representative: Weyland, Joseph Jean Pierre Goodyear International Tire Technical Center Patent Department Avenue Gordon Smith L-7750 Colmar-Berg Micro-suspension polymerization of vinyl chloride. (g) Micro-suspension polymerization is a technique that it is widely used on a commercial basis for the polymerization of vinyl chloride monomer into polyvinyl chloride. This invention reveals a greatly improved redox initiator system for use in such polymerizations. The redox initiator systems of this invention are comprised of (a) a free radical generator, such as a peroxide, and (b) a reducing agent selected from the group consisting of ascorbic acid, isoascorbic acid, and certain derivatives of ascorbic acid. The initiator systems of this invention do not require the utilization of water-soluble metal salts. However, water-soluble metal salts can be utilized in such initiator systems in catalytic amounts wherein the ratio of the water-soluble metal salt to the free radical generator is less than Bundesdruckerei Berlin
2 Description MICRO-SUSPENSION POLYMERIZATION OF VINYL CHLORIDE Background of the Invention 5 Polyvinyl chloride (PVC) is widely used in a variety of applications. PVC is utilized in making rigid articles such as pipe, house siding, phonograph records, automotive parts, and household appliances. PVC is also utilized in manufacturing flexible items, such as packaging films, gaskets, furniture upholstery, wall coverings, garden hoses, shower curtains, car tops, floor mats, and detergent bottles. PVC can be made by utilizing 10 suspension polymerization, mass polymerization, emulsion polymerization, or solution polymerization. Suspension polymerization is the most widely used technique for preparing PVC. In suspension polymerizations monomer droplets are suspended in an aqueous polymerization medium by a combination of vigorous agitation and protective colloids. Such polymerizations are generally initiated by free radicals which are produced by the thermal decomposition of peroxides. 15 United States Patent 3,879,364 discloses the utilization of a seeding product in the polymerization of vinyl chloride monomer into polyvinyl chloride. The seeding product contains all of the initiator which is required for the main polymerization. United States Patent 4,091,197 suggests activating the initiator with a metal complex which is formed by the reaction between a water-soluble metal salt and a complexing agent. U.S. Patent 4,091,197 states that these complexes are utilized in proportions such that the molar ratio of metallic complex 20 to initiator is between 0.1 and 10. Summary of the Invention This invention is based upon the discovery that ascorbic acid, isoascorbic acid and certain derivatives of 25 ascorbic acid can be utilized to activate free radical generators without the need for any water-soluble metal salts. In fact, it has been determined that numerous benefits can be realized by conducting such polymerization in the presence of little or no metal salt complexes. This invention more specifically reveals a process for the micro-suspension polymerization of vinyl chloride monomer into polyvinyl chloride in the presence of water and an emulsifier; the improvement which comprises 30 utilizing as the initiator a redox system which is comprised of (a) a free radical generator and (b) a reducing agent selected from the group consisting of ascorbic acid, isoascorbic acid, and ascorbic acid derivatives having the structural formula: wherein P. is an alkyl group containing from 1 to 30 carbon atoms, and wherein said polymerization is 45 conducted at a ph which is within the range of about 8.5 to about 11, with the proviso that said polymerization is conducted in the absence of any water-soluble metal salts of iron, copper, cobalt, nickel, zinc, tin, titanium, vanadium, manganese, chromium or silver. The present invention also discloses a process for the micro-suspension polymerization of vinyl chloride monomer into polyvinyl chloride in the presence of water and an emulsifier; the improvement which comprises 50 utilizing as the initiator a redox system which is comprised of (a) a free radical generator, (b) a reducing agent selected from the group consisting of ascorbic acid, isoascorbic acid, and ascorbic acid derivatives having the structural formula: 55 HO OH 60 2
3 =P A2 vherein R is an alkyl group containing from 1 to 30 carbon atoms, and (c) a water-soluble metal salt of iron, ;opper, cobalt, nickel, tin, titanium, vanadium, manganese, chromium or silver; wherein the molar ratio of said water-soluble metal salt to said free radical generator is less than This invention further discloses a process for preparing polyvinyl chloride in micro-suspension Dolymerization which comprises polymerizing vinyl chloride monomer in micro-suspension in the presence of 5 i seeding product in the form of a dispersion of particles of polyvinyl chloride prepared beforehand in nicro-suspension wherein said seeding product contains all of the free radical generator necessary for Dolymerization and wherein said free radical generator is activated with a reducing agent selected from the group consisting of ascorbic acid, isoascorbic acid, and ascorbic acid derivatives having the structural brmula: 10 wherein R is an alkyl group containing from 1 to 30 carbon atoms, and wherein said polymerization is conducted at a ph which is within the range of about 8.5 to about 1 1, with the proviso that said polymerization is conducted in the absence of any water-soluble metal salts of iron, copper, cobalt, nickel, zinc, tin, titanium, vanadium, manganese, chromium or silver. Detailed Description of the Invention The term polyvinyl chloride (PVC) as used herein is meant to include homopolymers which are comprised of repeat units which are derived from vinyl chloride monomer as well as copolymers containing at least 50% by weight repeat units which are derived from vinyl chloride monomer and at least one additional monomer which is copolymerizable with the vinyl chloride. In many cases such copolymers will contain at least 90% by weight repeat units which are derived from vinyl chloride. Such copolymerizable monomers are those generally employed in conventional methods of copolymerizing vinyl chloride. Some examples are vinyl ester of monoand polycarboxylic acids, such as vinyl acetate, propionate and benzoate, unsaturated mono- and polycarboxylic acids such as acrylic, methacrylic, maleic, fumaric and itaconic acid as well as their aliphatic, cycloaliphatic and aromatic esters, their amides and their nitriles; vinyl and vinylidene halides; alkyl vinyl ethers and olefins. PVC is prepared in accordance with this invention utilizing a micro-suspension polymerization technique. Such polymerizations are conducted in an aqueous medium which contains water, vinyl chloride monomer, at least one emulsifier, and the redox system of this invention. The redox systems of this invention are comprised of a free radical generator and a reducing agent selected from the group consisting of ascorbic acid, isoascorbic acid, and ascorbic acid derivatives having the structural formula: wherein R is an alkyl group containing from 1 to 30 carbon atoms. Such redox systems can be utilized in the absence of any water-soluble metal salts of iron, copper, cobalt, nickel, zinc, tin, titanium, vanadium, manganese, chromium or silver. However, in cases where such redox systems are utilized in the absence of water-soluble metal salts, it is critical to maintain a ph throughout the polymerization which is within the range of about 8.5 to about 11. In such cases, it is preferable to maintain a ph which is within the range of 9 to It is most preferred to maintain a ph which is within the range of 9.5 to 10. The redox initiator systems of the present invention can also be utilized in conjunction with catalytic amounts of water-soluble metal salts of iron, copper, cobalt, nickel, tin, titanium, vanadium, manganese, chromium or silver. In such cases, the molar ratio of the water-soluable metal salt to the free radical generator is less than In most cases, the molar ratio of the water-soluble metal salt to the free radical generator will be within the range of to It is normally preferred for the ratio of the water-soluble metal salt to the free radical generator to be within the range of to 0.01 with the ratio of to being most 3
4 preferred. It is not as critical to control the ph in polymerizations which are conducted in the presence of a catalytic amount of a water-soluble metal salt. For this reason, it is advantageous in many cases to utilize a catalytic amount of such a water-soluble metal salt in the redox system. The free radical generators which are utilized in the redox initiator systems of this invention are well known 5 to persons skilled in the art. Some representative examples of suitable free radical generators include the various peroxygen compounds such as potassium persulfate, ammonium persulfate, benzoyl peroxide, hydrogen peroxide, di-t-butyl peroxide, dicumyl peroxide, 2,4-dichlorobenzoyl peroxide, decanoyl peroxide, lauroyl peroxide, cumene hydroperoxide, p-menthane hydroperoxide, t-butyl hydroperoxide, acetyl acetone peroxide, methyl ethyl ketone peroxide, succinic acid peroxide, dicetyl peroxydicarbonate, t-butyl 10 peroxyacetate, t-butyl peroxymaleic acid, t-butyl peroxybenzoate, acetyl cyclohexyl sulfonyl peroxide, and the like; and the various alkyl perketals, such as 2,2-bis-(t-butylperoxy)butane, ethyl 3,3-bis(t-butylperoxy)butyrate, 1,1-di-(t-butylperoxy) cyclohexane, and the like. The reducing agent utilized in the redox initiator systems of this invention can be ascorbic acid, isoascorbic acid, or an ascorbic acid derivative having the structural formula: wherein R is an alkyl group containing from 1 to 30 carbon atoms. In most cases R will be an alkyl group containing from 10 to 20 carbon atoms. Ascorbic acid 6-palmitate is an example of such an ascorbic acid derivative which is highly preferred. In fact, the 6-palmitate derivative of ascorbic acid is 2 to 3 times more reactive than ascorbic acid or isoascorbic acid as a reducing agent for lauroyl peroxide. 30 In most cases from to phm of the free radical generator will be utilized in the redox systems of this invention. It will normally be preferred for the amount of free radical generator used to be within the range of phm to phm with it being more preferred for to phm of the free radical generator to be used. The reducing agent is normally added incrementally at a rate of to 0.1 phm per hour. It is preferred for the reducing agent to be added at a rate of to 0.01 phm per hour with a rate of to phm per hour being more preferred. To increase the stability of the micro-suspension, it may be advantageous to add prior to and/or during the course of polymerization, an anionic emulsifier in proportion of up to 2 /o by weight of the monomer or monomers. This emulsifier may be the same as that or those used in the preparation of any seeding product that may be utilized. It may be selected from conventional emulsifiers such as fatty acid soaps, alkyl sulfates, 40 alkyl sulphonates, alkyl aryl sulphonates, alkyl sulphosuccinates and alkyl phosphates. There may be associated with this emulsifier, a nonionic surface-active agent, such as for example the condensates of ethylene oxide or propylene oxide on various hydroxylated organic compounds. The quantity of water to be used in the polymerization method of this invention will be such that the ratio of the sum of the weight of the monomer or monomers and the polymer of the seeding product to that of the 45 water (including that of the seeding product) is between 0.3 and 1.5. The size of the particles is regulated by the usual methods suitable for polymerization in emulsion, e.g. by carefully choosing the nature and quantity of emulsifier used, by using seeds and by adjusting the agitating speed. The reaction mixture is generally heated under autogenous pressure and with moderate agitation to a 50 temperature from 30" to 65 C. When the pressure has dropped, the reaction is stopped and any unconverted monomer is degassed. It is highly preferred for the polymerizations of this invention to be carried out utilizing a seeding product which contain all of the free radical generator necessary for carrying out the polymerization. The seeding product is in the form of a dispersion of particles of polyvinyl chloride prepared in advance which contains all of 55 the free radical generator needed for the polymerization. The preparation of seeding products is described in detail in U.S. Patent 3,879,364 which is incorporated by reference herein in its entirety. In fact, the redox system of the present invention can be used in conjunction with the technique disclosed in U.S. Patent 3, by simply utilizing ascorbic acid, isoascorbic acid, or an ascorbic acid derivative to activate the initiator system. This can be accomplished by simply adding the ascorbic acid, isoascorbic acid or ascorbic qo acid derivative to the reaction zone (polymerization medium). It is also highly preferred to carry out the polymerizations of this invention in the presence of one or more other seeding products in the form of dispersions of particles of vinyl polymer, in which the sizes of the particles differ from one another and differ from that of the first seeding product. Such a technique is described in detail in United States Patent 4,245,070 which is incorporated herein by reference in its entirety. The redox system of the present invention can be 65 used in conjunction with the technique disclosed in U.S. Patent 4,245,070 by simply activating the initiator with 4
5 i reducing agent selected from the group consisting of ascorbic acid, isoascorbic acid, or an ascorbic acid derivative. However, such polymerizations cannot be conducted in the presence of a molar ratio of /vater-soluble metal salt to the free radical generator used of greater than However, it is generally desirable to conduct such polymerizations in the presence of a catalytic amount of such a water-soluble metal salt of iron, copper, cobalt, nickel, tin, titanium, vanadium, manganese, chromium or silver. In the alternative, 5 such polymerizations can be conducted in the absence of any water-soluble metal salts. However, when such Dolymerizations are carried out in the absence of a water-soluble metal salt, it is essential to maintain the ph of :he polymerization medium within the range of 8.5 to 11. The PVC made in accordance with the process of this invention can be recovered from the aqueous Dolymerization medium utilizing any one of numerous techniques known to persons skilled in the art. For 10 nstance, the PVC can be recovered from the polymerization medium by filtration, drying by coagulation, laking, centrifugal decantation or atomization. The PVC can then be compounded with the desired Dombination of chemical agents. In most cases, it will be desirable to add a thermal stabilizer to the PVC in arder to prevent degradation processing. In some cases it may also be desirable to include lubricants, plasticizers, impact modifiers, processing aids, antidegradants, blowing agents, flame retardants, fungicides, 15 antistatic agents, antiblocking agents and/or colorants in the compounding formulation depending upon the properties being sought. The PVC can then be processed by extrusion, injection molding, blow molding, calendaring or powder coating. This invention is illustrated by the following examples which are merely for the purpose of illustration and are not to be regarded as limiting the scope of the invention or the manner in which it can be practiced. Unless 20 specifically indicated otherwise, part and percentages are given by weight. Examples 1 & 2 25 In these examples PVC was prepared in a 200 gallon (757 liter) stainless steel autoclave which was equipped with a jacketed cooling system. Example 1 was carried out utilizing the process of the present invention. Example 2 was done as a comparative experiment. In these examples, the reactor was initially charged with 150 kg of water, 1.2 g of copper sulfate ( phm), 0.6 g of nitric acid, 50 g of monopotassium phosphate, g of an anionic emulsifier and 10.5 kg of an emulsion PVC latex (as a seeding product). The aqueous medium in the reactor was agitated for about 10 minutes at 30 rpm and was then double evacuated to remove any oxygen that may have been present. The free radical generator was then introduced by adding 8.5 kg of seeding product which contained about 1.9 of lauroyl peroxide. The aqueous medium was agitated for 10 minutes and then 250 kg of vinyl chloride was 35 added which resulted in a pressure of 140 psi (9.65 x 105 Pa s). The temperature was raised to 62 C and phm (parts per hundred parts of monomer) of ascorbic acid was added per hour. Additional emulsifier was also added after 2 hours, 4 hours, and 6 hours. After a polymerization time of about 7.25 hours, the pressure dropped to 60 psi (4.14 x 105 Pa- s). The system was then degassed and the PVC produced was recovered. In Example 1, phm of copper sulfate and phm of nitric acid was added to the aqueous 40 polymerization system. This resulted in only 1 lb. (0.43 kg) of berries being produced. Berries are solid granular matter that is recovered from latex by filtration which must be disposed of as waste material. Thus, it is highly desirable to reduce the amount of berries being produced. Example 2 was conducted in a manner identical to the one utilized in Example 1 except that phm of copper sulfate and phm of nitric acid was added to the aqueous polymerization medium. In other 45 words, 6 times as much copper sulfate was added in Example 2 as was added in Example 1. This resulted in 7.2 lbs. (3.27 kg) of berries being produced. This experiment clearly shows the superiority of utilizing only catalytic amounts of copper sulfate in the redox initiator system. The presence of the additional metal salt and additional acid clearly destabilizes the latex being formed and consequently results in berry formation being greatly increased. 50 Examples 3-9 The procedure utilized in this series of experiments was essentially the same as the technique described in Examples 1 and 2, except that a slightly different anionic emulsifier system was utilized. In this series of experiments, the amount of copper sulfate, nitric acid, ascorbic acid and sodium bicarbonate added was varied. The amount of each of these constituents added is shown in Table I. In Examples 3 and 4, catalytic amounts of copper sulfate were added. In Examples 5-8, no copper sulfate was added. Since no copper sulfate 60 was added in Examples 5-8, it was not necessary to utilize nitric acid in order to maintain the solubility of the copper salt. Example 9 was conducted as a control and utilized phm of copper sulfate and phm of nitric acid. 5
6 TABLE I Ascorbic* Sodium* Example CuSQ^* HNO^* Acid Bicarbonate Berries: * Phm ** pounds As can be determined by examining Table I, the amount of berries produced was reduced dramatically by eliminating copper sulfate and nitric acid from the polymerization medium. In Examples 5-8, which did not utilize copper sulfate in the redox system, the amount of berries produced was reduced by approximately ten fold. In Examples 3 and 4 wherein a catalytic amount of copper sulfate was utilized in the redox systems, the amount of berries produced was reduced by almost 50%. This benefit was realized without experiencing any detrimental effects. This series of experiments clearly shows that the amount of berries produced can be dramatically decreased by utilizing the redox initiator system of the present invention. Example " This example is included in order to show that the benefits of the redox initiator systems of this invention can be realized in commercial scale polymerizations. A 10,000 gallon (37,850 liter) stainless steel autoclave which was equipped with a jacketed cooling system was charged with 33,730 lbs. (15,295 kg) of water, 12.8 lbs. (5.8 kg) of sodium bicarbonate, 0.2 lbs. (0.09 kg) of copper sulfate, 36.4 lbs. (16.5 kg) of an anionic emulsifier, 4,786 lbs. (2,171 kg) of an emulsion PVC latex as a seeding product, and 4,171 lbs. (1,892 kg) of a seeding product containing lauroyl peroxide. The temperature was set to 70 C and 36,376 lbs. (16,500 kg) of vinyl chloride monomer was charged into the aqueous polymerization medium. The reaction medium was maintained at 60.5C C with 2.6 lbs. (1.2 kg) of ascorbic acid being incrementally added throughout the polymerization. After 2 hours, 4 hours and 6 hours additional emulsifier totaling 90.9 lbs. (41.2 kg) was added. Three runs were made utilizing the redox initiator system of the subject invention. These runs had an average polymerization time (until a pressure drop was experienced) of about 8 hours. The amount of berries produced was reduced to 2.22 drums per batch. This compares favorably to runs which are made utilizing conventional initiator systems which utilize much larger amounts of a water-soluble metal salt and acid. For instance, a series of runs utilizing the same technique but which employed 0.5 kg of zinc sulfate and 0.50 kg of nitric acid in the initiator system resulted in the production of an average of 2.64 drums of berries per batch. In commercial scale quantities an additional benefit is realized by utilizing the redox initiator system of this invention. More specifically, the amount of time that it takes to degas the aqueous medium after the polymerization has been completed is greatly reduced. By reducing the time required for degassing the throughput of a reactor can be greatly increased. In the case at hand, the utilization of the redox initiator system if this invention resulted in an average degas time of about 1.9 hours. This compares very favorably with an average degas time of about 2.8 hours which is experienced utilizing conventional initiator systems which utilize 0.5 kg of zinc sulfate and 0.5 kg of nitric acid. By practicing the process of the present invention, several advantages can be realized in the commercial synthesis of PVC. More specifically, the utilization of the redox initiator systems of the present invention 6
7 esults in the formation of more stable latices, the production of fewer berries, and the formation of less xjagulum. Additionally, the utilization of the process of the present invention results in shorter degas times md consequently greater reactor throughputs. The problem of discoloration which is sometimes caused by :he water-soluble metal salt can also be eliminated. While certain representative embodiments and details have been shown for the purpose of illustrating the Dresent invention, it will be apparent to those skilled in this art that various changes and modifications can be Tiade without departing from the scope of the present invention. Claims 1. A process for the micro-suspension polymerization of vinyl chloride monomer into polyvinyl chloride in the aresence of water and an emulsifier which utilizes as the initiator a redox system which is comprised of (a) a free radical generator and (b) a reducing agent selected from the group consisting of ascorbic acid, isoascorbic acid, and ascorbic acid derivatives having the structural formula: HO DH wherein R is an alkyl group containing from 1 to 30 carbon atoms, and wherein said polymerization is conducted at a ph which is within the range of about 8.5 to about 1 1, characterized in that said polymerization is conducted in the absence of any water-soluble metal salts of iron, copper, cobalt, nickel, zinc, tin, titanium, vanadium, manganese, chromium or silver. 2. A process for the micro-suspension polymerization of vinyl chloride monomer into polyvinyl chloride in the presence of water and an emulsifier which utilizes as the initiator a redox system which is comprised of (a) a free radical generator, (b) a reducing agent selected from the group consisting of ascorbic acid, isoascorbic acid, and ascorbic acid derivatives having the structural formula: wherein R is an alkyl group containing from 1 to 30 carbon atoms, and (c) a water-soluble metal salt ot iron, copper, cobalt, nickel, tin, titanium, vanadium, manganese, chromium or silver; characterized in that the molar ratio of said water-soluble metal salt to said free radical generator is less than A process as specified in claim 1 or 2 which is characterized by polymerizing vinyl chloride monomer in micro-suspension in the presence of a seeding product in the form of a dispersion of particles of polyvinyl chloride prepared beforehand in micro-suspension wherein said seeding product contains all of the free radical generator necessary for polymerization. 4. A process as specified in Claim 1, 2, or 3 characterized in that said reducing agent is selected from the group consisting of ascorbic acid, isoascorbic acid and ascorbic acid 6-palmitate. 5. A process as specified in Claim 2 or Claim 4 when dependent upon Claim 2 characterized in that the molar ratio of said water-soluble metal salt to said free radical generator is within the range of to A process as specified in Claim 2 or 5 characterized in that from phm to phm of said free radical generator is present. 7. A process as specified in any of the preceding claims characterized in that said free radical generator is a peroxygen compound. 8. A process as specified in Claim 2 characterized in that said reducing agent is selected from the group consisting of ascorbic acid and isoascorbic acid; characterized in that the molar ratio of said water-soluble metal salt to said free radical generator is within the range of to 0.01 ; characterized in that from
8 to phm of said free radical generator is present and characterized in that the reducing agent is added at a rate of to 0.01 phm per hour. 9. A process as specified in Claim 1, 2, 3, 5 or 6 characterized in that said free radical generator is selected from the group consisting of potassium persulfate, ammonium persulfate, benzoyl peroxide, hydrogen peroxide, di-t-butyl peroxide, dicumyl peroxide, 2,4-dichlorobenzoyl peroxide, decanoyl peroxide, lauroyl peroxide, cumene hydroperoxide, p-menthane hydroperoxide, t-butyl hydroperoxide, acetyl acetone peroxide, methyl ethyl ketone peroxide, succinic acid peroxide, dicetyl peroxydicarbonate, t-butyl peroxyacetate, t-butyl peroxymaleic acid, t-butyl peroxybenzoate, and acetyl cyclohexyl sulfonyl peroxide. 10. A process as specified in Claim 2 or 5 characterized in that the water-soluble metal salt is a copper salt or copper sulfate.
US A United States Patent (19) 11 Patent Number: 5,583,173 Gujarathi et al. (45) Date of Patent: Dec. 10, 1996
 IIII III IIII US0083173A United States Patent (19) 11 Patent Number: Gujarathi et al. () Date of Patent: Dec., 1996 (54) PROCESS FOR PREPARING OTHER PUBLICATIONS STYRENE-BUTADIENE RUBBER The Vanderbilt
IIII III IIII US0083173A United States Patent (19) 11 Patent Number: Gujarathi et al. () Date of Patent: Dec., 1996 (54) PROCESS FOR PREPARING OTHER PUBLICATIONS STYRENE-BUTADIENE RUBBER The Vanderbilt
TEPZZ 76 5Z A_T EP A1 (19) (11) EP A1 (12) EUROPEAN PATENT APPLICATION
 (19) TEPZZ 76 Z A_T (11) EP 2 762 02 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: 06.08.14 Bulletin 14/32 (21) Application number: 1410886.1 (1) Int Cl.: C08F 2/18 (06.01) C09D 133/08
(19) TEPZZ 76 Z A_T (11) EP 2 762 02 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: 06.08.14 Bulletin 14/32 (21) Application number: 1410886.1 (1) Int Cl.: C08F 2/18 (06.01) C09D 133/08
Part I Short Answer Choose a letter to fill in the blanks. Use choices as many times as you wish. Only one choice is needed per blank.
 Part I Short Answer Choose a letter to fill in the blanks. Use choices as many times as you wish. Only one choice is needed per blank. 1. (3 points each) First set functional groups A. ether D. amine B.
Part I Short Answer Choose a letter to fill in the blanks. Use choices as many times as you wish. Only one choice is needed per blank. 1. (3 points each) First set functional groups A. ether D. amine B.
ACRYLAMIDE AND POLYACRYLAMIDE
 Report No. 99 ACRYLAMIDE AND POLYACRYLAMIDE by YEN-CHEN YEN June 1976 A private report by the PROCESS ECONOMICS PROGRAM STANFORD RESEARCH INSTITUTE I MENLO PARK. CALIFORNIA CONTENTS 1 INTRODUCTION......,..................
Report No. 99 ACRYLAMIDE AND POLYACRYLAMIDE by YEN-CHEN YEN June 1976 A private report by the PROCESS ECONOMICS PROGRAM STANFORD RESEARCH INSTITUTE I MENLO PARK. CALIFORNIA CONTENTS 1 INTRODUCTION......,..................
PACKAGING MIGRATION AND HARMFUL ELEMENT COMPLIANCES. By : Mr. Dilip Singh National Manager SGS India Pvt. Ltd.
 PACKAGING MIGRATION AND HARMFUL ELEMENT COMPLIANCES By : Mr. Dilip Singh National Manager SGS India Pvt. Ltd. CONCEPT OF MIGRATION TESTING How do you know if the food packaging you wrap your Food in is
PACKAGING MIGRATION AND HARMFUL ELEMENT COMPLIANCES By : Mr. Dilip Singh National Manager SGS India Pvt. Ltd. CONCEPT OF MIGRATION TESTING How do you know if the food packaging you wrap your Food in is
PERP/PERP ABSTRACTS Acrylic Acid
 PERP/PERP ABSTRACTS 2010 Acrylic Acid Technology and Cost Estimates for Production Via Propylene Oxidation, 3HP Fermentation, (Glycerol Derived) Acrolein, (Ethylene-Oxide Derived) β- Propiolactone, Acetylene
PERP/PERP ABSTRACTS 2010 Acrylic Acid Technology and Cost Estimates for Production Via Propylene Oxidation, 3HP Fermentation, (Glycerol Derived) Acrolein, (Ethylene-Oxide Derived) β- Propiolactone, Acetylene
Speciality additives for PVC
 Reasons to buy Wide ranging effects Internal and external lubricants Technical expertise Speciality additives for PVC At the heart of better plastics Speciality additives for PVC Croda offers a comprehensive
Reasons to buy Wide ranging effects Internal and external lubricants Technical expertise Speciality additives for PVC At the heart of better plastics Speciality additives for PVC Croda offers a comprehensive
SURFACE COATINGS. Paint, Ink, Adhesives, Elastomers, Performance Coatings, UV Curing, Latex Emulsion
 SURFACE COATINGS Paint, Ink, Adhesives, Elastomers, Performance Coatings, UV Curing, Latex Emulsion Harcros Chemicals-Introduction 1 Since our inception in 1917, we have been committed to safety, quality
SURFACE COATINGS Paint, Ink, Adhesives, Elastomers, Performance Coatings, UV Curing, Latex Emulsion Harcros Chemicals-Introduction 1 Since our inception in 1917, we have been committed to safety, quality
EUROPEAN PATENT APPLICATION. <S) mt. a*: C 05 D 9/02
 3 Europaisches Patentamt European Patent Office Office europeen des brevets @ Publication number: 0 284 339 A2 EUROPEAN PATENT APPLICATION @ Application number: 882487.9
3 Europaisches Patentamt European Patent Office Office europeen des brevets @ Publication number: 0 284 339 A2 EUROPEAN PATENT APPLICATION @ Application number: 882487.9
Functional Additive Masterbatches for Plastic Packaging: An Overview. Dean Dodaro Polyvel, Inc 100 Ninth St Hammonton, NJ 08037
 Functional Additive Masterbatches for Plastic Packaging: An Overview Dean Dodaro Polyvel, Inc 100 Ninth St Hammonton, NJ 08037 Outline About Us Additive Functions: Antiblocks Antifogs Antimicrobials Antistats
Functional Additive Masterbatches for Plastic Packaging: An Overview Dean Dodaro Polyvel, Inc 100 Ninth St Hammonton, NJ 08037 Outline About Us Additive Functions: Antiblocks Antifogs Antimicrobials Antistats
DISPONIL AES 60 is an emulsifier for the manufacture of finely dispersed emulsion polymers, in particular for
 DISPONIL AES 60 Use DISPONIL AES 60 is an emulsifier for the manufacture of finely dispersed emulsion polymers, in particular for styrene homo and copolymers acrylate homo and copolymers. Composition Alkylaryl
DISPONIL AES 60 Use DISPONIL AES 60 is an emulsifier for the manufacture of finely dispersed emulsion polymers, in particular for styrene homo and copolymers acrylate homo and copolymers. Composition Alkylaryl
TECHNICAL BULLETIN. MAGNESIUM METHYLATE MGME-8 Liquid Magnesium Methoxide (8% Solution) DESCRIPTION TYPICAL PROPERTIES APPLICATIONS
 TECHNICAL BULLETIN MAGNESIUM METHYLATE MGME-8 Liquid Magnesium Methoxide (8% Solution) DESCRIPTION MGME-8 is a new liquid form of Magnesium Methylate (8% solution). Unlike solid Magnesium Methylate, MGME-8
TECHNICAL BULLETIN MAGNESIUM METHYLATE MGME-8 Liquid Magnesium Methoxide (8% Solution) DESCRIPTION MGME-8 is a new liquid form of Magnesium Methylate (8% solution). Unlike solid Magnesium Methylate, MGME-8
Versatic. Acids and Derivatives. Product Overview
 Versatic Acids and Derivatives Product verview Hexion is the number one global supplier of Versatic acids and derivatives, primarily to the polymer, paints, coatings, construction and adhesives markets.
Versatic Acids and Derivatives Product verview Hexion is the number one global supplier of Versatic acids and derivatives, primarily to the polymer, paints, coatings, construction and adhesives markets.
Ulllted States Patent [19] [11] Patent Number: 4,997,881
![Ulllted States Patent [19] [11] Patent Number: 4,997,881 Ulllted States Patent [19] [11] Patent Number: 4,997,881](/thumbs/91/105614517.jpg) Ulllted States Patent [19] [11] Patent Number: 4,997,881 Patel et a1. [45] Date of Patent: Mar. 5, 1991 [54] POLY(ALKYL VINYL ETHER)POLYOLEFIN [56] References Cited COPOLYMERS U.S. PATENT DOCUMENTS [75]
Ulllted States Patent [19] [11] Patent Number: 4,997,881 Patel et a1. [45] Date of Patent: Mar. 5, 1991 [54] POLY(ALKYL VINYL ETHER)POLYOLEFIN [56] References Cited COPOLYMERS U.S. PATENT DOCUMENTS [75]
Oy Publication number:
 Europaisches Patentamt J European Patent Office Office europeen des brevets Oy Publication number: 0 468 564 A2 EUROPEAN PATENT APPLICATION Application number: 91201723.3 Date of filing: 04.07.91 int.
Europaisches Patentamt J European Patent Office Office europeen des brevets Oy Publication number: 0 468 564 A2 EUROPEAN PATENT APPLICATION Application number: 91201723.3 Date of filing: 04.07.91 int.
Chemicals Based on Ethylene
 Chemicals Based on Ethylene Ethylene is sometimes known as the king of petrochemicals because more commercial chemicals are produced from ethylene than from any other intermediate. This unique position
Chemicals Based on Ethylene Ethylene is sometimes known as the king of petrochemicals because more commercial chemicals are produced from ethylene than from any other intermediate. This unique position
Additives for polyolefines: chemistry involved and innovative effects
 Additives for polyolefines: chemistry involved and innovative effects Abstract Because many synthetic resins suffer from photo degradation, the investigation of ways to inhibit or at least retard this
Additives for polyolefines: chemistry involved and innovative effects Abstract Because many synthetic resins suffer from photo degradation, the investigation of ways to inhibit or at least retard this
(51) Int Cl.: A61K 9/20 ( ) A61K 31/41 ( )
 (19) TEPZZ 94677_A_T (11) (12) EUROPEAN PATENT APPLICATION (43) Date of publication: 2.11.1 Bulletin 1/48 (1) Int Cl.: A61K 9/ (06.01) A61K 31/41 (06.01) (21) Application number: 116790.3 (22) Date of
(19) TEPZZ 94677_A_T (11) (12) EUROPEAN PATENT APPLICATION (43) Date of publication: 2.11.1 Bulletin 1/48 (1) Int Cl.: A61K 9/ (06.01) A61K 31/41 (06.01) (21) Application number: 116790.3 (22) Date of
Carboxylic Acids and their Derivatives I
 2302272 Org Chem II Part I Lecture 5 Carboxylic Acids and their Derivatives I Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 20 in Organic Chemistry,
2302272 Org Chem II Part I Lecture 5 Carboxylic Acids and their Derivatives I Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 20 in Organic Chemistry,
» Croscarmellose Sodium is a cross linked polymer of carboxymethylcellulose sodium.
 BRIEFING Croscarmellose Sodium, NF 22 page 2856 and page 702 of PF 30(2) [Mar. Apr. 2004]. A modification is made in the test for Degree of substitution to correct the endpoint color to agree with the
BRIEFING Croscarmellose Sodium, NF 22 page 2856 and page 702 of PF 30(2) [Mar. Apr. 2004]. A modification is made in the test for Degree of substitution to correct the endpoint color to agree with the
Emulsion Polymerization Product Catalog
 Leadership. Service. Flexibility. Success. We re going further. Emulsion Polymerization Catalog Leadership. Service. Flexibility. Success. We re going further. Charting Your Course with Innovative Chemical
Leadership. Service. Flexibility. Success. We re going further. Emulsion Polymerization Catalog Leadership. Service. Flexibility. Success. We re going further. Charting Your Course with Innovative Chemical
PURE BRAZIL BRAND PRODUCTS
 PURE BRAZIL BRAND PRODUCTS WHAT ARE THE PURE BRAZIL BRAND PRODUCTS? PURE BRAZIL BRAND Essential Micronutrients contains eight micronutrient elements essential to plant growth and health. Five (calcium,
PURE BRAZIL BRAND PRODUCTS WHAT ARE THE PURE BRAZIL BRAND PRODUCTS? PURE BRAZIL BRAND Essential Micronutrients contains eight micronutrient elements essential to plant growth and health. Five (calcium,
 www.dongeunchemical.com DISPERSING AGENT DISPERGATOR DF-908A DISPERGATOR DF-908B BLEND OF SPECIAL FATTY ACID ESTERS AND METAL SOAPS. ESTER OF SPECIAL FATTY ACID CONTAINING POLAR GROUPS. Processing aid
www.dongeunchemical.com DISPERSING AGENT DISPERGATOR DF-908A DISPERGATOR DF-908B BLEND OF SPECIAL FATTY ACID ESTERS AND METAL SOAPS. ESTER OF SPECIAL FATTY ACID CONTAINING POLAR GROUPS. Processing aid
Introduction to Adhesives
 Introduction to Adhesives UPACO Division of Worthen Industries Barbara Strickland April 11, 2013 20,000 BC Cavemen Beeswax, Pine Sap feathers on arrows 1500 BC Egyptians Animal glues repair Asphalt mosaics
Introduction to Adhesives UPACO Division of Worthen Industries Barbara Strickland April 11, 2013 20,000 BC Cavemen Beeswax, Pine Sap feathers on arrows 1500 BC Egyptians Animal glues repair Asphalt mosaics
Material Safety Data Sheet
 Material Safety Data Sheet Date Reviewed and Issued: February 16, 2013 Section 1. Identification: Proven Winners Controlled Release Fertilizer Product Names: Sunshine Proven Winners Premium Continuous
Material Safety Data Sheet Date Reviewed and Issued: February 16, 2013 Section 1. Identification: Proven Winners Controlled Release Fertilizer Product Names: Sunshine Proven Winners Premium Continuous
USER SPECIFICATIONS FOR QUINTOLUBRIC 888 Series DESCRIPTION OF THE MOST IMPORTANT PROPERTIES AND THE POSSIBLE VARIATIONS AND TOLERANCES
 USER SPECIFICATIONS FOR QUINTOLUBRIC 888 Series OVERVIEW The QUINTOLUBRIC 888 Series of fluids are based on a synthetic organic ester, which is the main base component, plus a number of selected additives
USER SPECIFICATIONS FOR QUINTOLUBRIC 888 Series OVERVIEW The QUINTOLUBRIC 888 Series of fluids are based on a synthetic organic ester, which is the main base component, plus a number of selected additives
(12) United States Patent (10) Patent No.: US 6,451,893 B1
 USOO6451893B1 (12) United States Patent (10) Patent No.: Tao (45) Date of Patent: Sep. 17, 2002 (54) SOFT NITRILE ZINCOXIDE FREE RE35,616 E 9/1997 Tillotson et al.... 2/168 MEDICAL, GLOVES 5,872,173 A
USOO6451893B1 (12) United States Patent (10) Patent No.: Tao (45) Date of Patent: Sep. 17, 2002 (54) SOFT NITRILE ZINCOXIDE FREE RE35,616 E 9/1997 Tillotson et al.... 2/168 MEDICAL, GLOVES 5,872,173 A
APPLIED CHEMISTRY SURFACE TENSION, SURFACTANTS TYPES OF SURFACTANTS & THEIR USES IN TEXTILE PROCESSING
 APPLIED CHEMISTRY SURFACE TENSION, SURFACTANTS TYPES OF SURFACTANTS & THEIR USES IN TEXTILE PROCESSING Lecture No. 13 & 14 2 Surface Tension This property of liquids arises from the intermolecular forces
APPLIED CHEMISTRY SURFACE TENSION, SURFACTANTS TYPES OF SURFACTANTS & THEIR USES IN TEXTILE PROCESSING Lecture No. 13 & 14 2 Surface Tension This property of liquids arises from the intermolecular forces
Chapter 23. Functional Groups. Halogen Side Chains What is a halocarbon? How are organic compounds classified?
 23.1 Chapter 23 From a distance, the musicians in an orchestra may look alike, but each musician contributes a unique sound. In a similar way, one hydrocarbon is nearly identical to another until it picks
23.1 Chapter 23 From a distance, the musicians in an orchestra may look alike, but each musician contributes a unique sound. In a similar way, one hydrocarbon is nearly identical to another until it picks
Fatty acid product guide
 Fatty acid product guide Kraton Corporation s Sylfat and Century fatty acids are useful in a wide range of industrial applications. These fatty acids (tall oil fatty acid, monomer acid, stearic acid and
Fatty acid product guide Kraton Corporation s Sylfat and Century fatty acids are useful in a wide range of industrial applications. These fatty acids (tall oil fatty acid, monomer acid, stearic acid and
Purity Tests for Modified Starches
 Residue Monograph prepared by the meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), 82 nd meeting 2016 Purity Tests for Modified Starches This monograph was also published in: Compendium
Residue Monograph prepared by the meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), 82 nd meeting 2016 Purity Tests for Modified Starches This monograph was also published in: Compendium
AROLON 6436 Product Code: Acrylic Polyol
 March 216 Product Code: 9162- Acrylic Polyol DESCRIPTION is an acrylic polyol resin designed for high solids, high performance polyurethane coatings. Low VOC (42 grams per liter) polyurethane coatings
March 216 Product Code: 9162- Acrylic Polyol DESCRIPTION is an acrylic polyol resin designed for high solids, high performance polyurethane coatings. Low VOC (42 grams per liter) polyurethane coatings
HPHP- A Component for Enviornmentally Advanced Coatings
 HPHP- A Component for Enviornmentally Advanced Coatings m 2 fd- Don Morris Texas Eastman Division, P.O. Box 7444 Longview, Texas 75607 The coating s industry is undergoing a dramatic shift from solvent
HPHP- A Component for Enviornmentally Advanced Coatings m 2 fd- Don Morris Texas Eastman Division, P.O. Box 7444 Longview, Texas 75607 The coating s industry is undergoing a dramatic shift from solvent
Working with Peroxide Formers. Ethers, Acetals, and Ketals, especially Cyclic Ethers and those with primary and Secondary Alkyl Groups
 Peroxide Formers Peroxide Formers Organic peroxides are a dangerous fire hazard if allowed to react with reducing agents. They are potent oxidizers and are a severe explosion hazard when shocked, when/if
Peroxide Formers Peroxide Formers Organic peroxides are a dangerous fire hazard if allowed to react with reducing agents. They are potent oxidizers and are a severe explosion hazard when shocked, when/if
(12) United States Patent (10) Patent No.: US 6, B1
 USOO6187829B1 (12) United States Patent (10) Patent No.: Sellers () Date of Patent: Feb. 13, 2001 (54) HEAT GELLABLE LATEX COMPOSITION 4,172,067 10/1979 Benton... 521/ AND METHOD OF MAKING SAME 4,214,053
USOO6187829B1 (12) United States Patent (10) Patent No.: Sellers () Date of Patent: Feb. 13, 2001 (54) HEAT GELLABLE LATEX COMPOSITION 4,172,067 10/1979 Benton... 521/ AND METHOD OF MAKING SAME 4,214,053
Wastewater Treatment: Reducing Salts Generated During Treatment to Promote Water Re-Use. By: David Calnan Cherokee Chemical Inc, (CCI)
 Wastewater Treatment: Reducing Salts Generated During Treatment to Promote Water Re-Use By: David Calnan Cherokee Chemical Inc, (CCI) Objective Cost-effective effluent recovery utilizing chemical pre-treatment
Wastewater Treatment: Reducing Salts Generated During Treatment to Promote Water Re-Use By: David Calnan Cherokee Chemical Inc, (CCI) Objective Cost-effective effluent recovery utilizing chemical pre-treatment
Acids. Chemical GS HT UT UR XP HD
 STONCLAD STONCLAD CHEMICAL RESISTANCE GUIDE The purpose of this guide is to aid in determining the potential value of the Stonclad family surfacers when exposed to the damaging effects of corrosive chemical
STONCLAD STONCLAD CHEMICAL RESISTANCE GUIDE The purpose of this guide is to aid in determining the potential value of the Stonclad family surfacers when exposed to the damaging effects of corrosive chemical
*EP A1* EP A1 (19) (11) EP A1 (12) EUROPEAN PATENT APPLICATION. (43) Date of publication: Bulletin 2002/03
 (19) Europäisches Patentamt European Patent Office Office européen des brevets *EP001172143A1* (11) EP 1 172 143 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: 16.01.02 Bulletin 02/03 (1)
(19) Europäisches Patentamt European Patent Office Office européen des brevets *EP001172143A1* (11) EP 1 172 143 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: 16.01.02 Bulletin 02/03 (1)
(12) Patent Application Publication (10) Pub. No.: US 2017/ A1
 (19) United States US 2017.0099889A1 (12) Patent Application Publication (10) Pub. No.: US 2017/0099889 A1 LOU (43) Pub. Date: (54) HIGH STRESS RETENTION NITRILE (52) U.S. Cl. GLOVE CPC... A41D 19/0082
(19) United States US 2017.0099889A1 (12) Patent Application Publication (10) Pub. No.: US 2017/0099889 A1 LOU (43) Pub. Date: (54) HIGH STRESS RETENTION NITRILE (52) U.S. Cl. GLOVE CPC... A41D 19/0082
CORROSION INHIBITORS CATALOGUE
 CORROSION INHIBITORS CATALOGUE PETROSTEP CORROSION INHIBITORS: PROTECT AND PRESERVE Corrosive agents can be found throughout the oilfield, from pipelines to production and injection wells, to storage
CORROSION INHIBITORS CATALOGUE PETROSTEP CORROSION INHIBITORS: PROTECT AND PRESERVE Corrosive agents can be found throughout the oilfield, from pipelines to production and injection wells, to storage
Product Focus Customer Commitment Performance Flexibility
 NAXCAT Acid Catalysts Product Focus Customer Commitment Performance Flexibility The Industry Leader for the Dependable Quality Difference Cost Effective Solutions-When Quality Counts NEASE Co manufactures
NAXCAT Acid Catalysts Product Focus Customer Commitment Performance Flexibility The Industry Leader for the Dependable Quality Difference Cost Effective Solutions-When Quality Counts NEASE Co manufactures
Official Journal of the European Union REGULATIONS
 L 30/2 6.2.2015 REGULATIONS COMMISSION REGULATION (EU) 2015/174 of 5 February 2015 amending and correcting Regulation (EU) No 10/2011 on plastic materials and articles intended to come into contact with
L 30/2 6.2.2015 REGULATIONS COMMISSION REGULATION (EU) 2015/174 of 5 February 2015 amending and correcting Regulation (EU) No 10/2011 on plastic materials and articles intended to come into contact with
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1)
 Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 7. CARBOXYLIC ACIDS AND THEIR
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 7. CARBOXYLIC ACIDS AND THEIR
METOLOSE: CONTENTS PAGE
 METOLOSE: CONTENTS PAGE 2 Preface What is Metolose Substitution types Specifications 1) Available grades & viscosity 2) Nomenclature 3) Packaging Characteristics of Metolose Properties of Metolose 1) Powder
METOLOSE: CONTENTS PAGE 2 Preface What is Metolose Substitution types Specifications 1) Available grades & viscosity 2) Nomenclature 3) Packaging Characteristics of Metolose Properties of Metolose 1) Powder
(12) United States Patent (10) Patent No.: US 8,207,277 B2
 US008207277B2 (12) United States Patent () Patent No.: Beran et al. (45) Date of Patent: Jun. 26, 2012 (54) MODIFYING TUBULAR LDPE WITH FREE 2002/0169238 A1* 11/2002 Caronia et al.... 524/0 RADICAL INITIATOR
US008207277B2 (12) United States Patent () Patent No.: Beran et al. (45) Date of Patent: Jun. 26, 2012 (54) MODIFYING TUBULAR LDPE WITH FREE 2002/0169238 A1* 11/2002 Caronia et al.... 524/0 RADICAL INITIATOR
Chapter 18. Carboxylic Acids and Their Derivatives. Nucleophilic Addition-Elimination at the Acyl Carbon
 Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition-Elimination at the Acyl Carbon Carboxylic Acids Organic compounds characterized by their acidity Contains COOH group (must be at
Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition-Elimination at the Acyl Carbon Carboxylic Acids Organic compounds characterized by their acidity Contains COOH group (must be at
Quiz 6 Introduction to Polymers
 100518 Quiz 6 Introduction to Polymers 1) Suspension polymerization is similar to emulsion polymerization. a) Describe the importance of water to emulsion and suspension polymerization. Does water play
100518 Quiz 6 Introduction to Polymers 1) Suspension polymerization is similar to emulsion polymerization. a) Describe the importance of water to emulsion and suspension polymerization. Does water play
KURARAY POVAL & EXCEVAL
 Characteristics Polyvinyl alcohol (PVOH) having varying degree of polymerization and. Recommended Uses Ranging from emulsion polymerization aid to binder for pigments in paper applications. Form supplied
Characteristics Polyvinyl alcohol (PVOH) having varying degree of polymerization and. Recommended Uses Ranging from emulsion polymerization aid to binder for pigments in paper applications. Form supplied
by Luis Cruz, Ben Labovitz, and Eric Farone
 by Luis Cruz, Ben Labovitz, and Eric Farone Liquid Stabilizers for Flexible Vinyl Barium-Cadmium-Zinc some still in use Barium-Zinc most common today worldwide Barium-Calcium-Zinc and Calcium Zinc up and
by Luis Cruz, Ben Labovitz, and Eric Farone Liquid Stabilizers for Flexible Vinyl Barium-Cadmium-Zinc some still in use Barium-Zinc most common today worldwide Barium-Calcium-Zinc and Calcium Zinc up and
Mini-emulsion polymers as binder for fire protection coatings
 Mini-emulsion polymers as binder for fire protection coatings Barbora Deppe Dirk Kruse, Benjamin Becker, Deppe laf Structural Engineering and Construction Fraunhofer Institute for Wood Research Wilhelm-Klauditz-Institute
Mini-emulsion polymers as binder for fire protection coatings Barbora Deppe Dirk Kruse, Benjamin Becker, Deppe laf Structural Engineering and Construction Fraunhofer Institute for Wood Research Wilhelm-Klauditz-Institute
Troubleshooting the. Update on. PVC Extrusion Process. ismithers. Natami Subramanian Muralisrinivasan
 Update on Troubleshooting the PVC Extrusion Process Natami Subramanian Muralisrinivasan Smithers ismithers /Smithers - A Smithers Group Company Shawbury, Shrewsbury, Shropshire, SY4 4NR, United Kingdom
Update on Troubleshooting the PVC Extrusion Process Natami Subramanian Muralisrinivasan Smithers ismithers /Smithers - A Smithers Group Company Shawbury, Shrewsbury, Shropshire, SY4 4NR, United Kingdom
Name the ester produced when methanol and pentanoic acid react. methyl pentanoate. Name the type of reaction used to make an ester
 1 Name the ester produced when methanol and pentanoic acid react methyl pentanoate 2 Name the type of reaction used to make an ester condensation reaction 3 Name the by-product of the reaction used to
1 Name the ester produced when methanol and pentanoic acid react methyl pentanoate 2 Name the type of reaction used to make an ester condensation reaction 3 Name the by-product of the reaction used to
EUDRAGIT L 100 and EUDRAGIT S 100
 Technical Information EUDRAGIT L 100 and EUDRAGIT S 100 Specification and Test Methods Ph. Eur. Methacrylic Acid - Methyl Methacrylate Copolymer (1:1) Methacrylic Acid - Methyl Methacrylate Copolymer (1:2)
Technical Information EUDRAGIT L 100 and EUDRAGIT S 100 Specification and Test Methods Ph. Eur. Methacrylic Acid - Methyl Methacrylate Copolymer (1:1) Methacrylic Acid - Methyl Methacrylate Copolymer (1:2)
Example: Ammonium Sulphate (also called Sulphate of Ammonia) is composed of the following:
 Atoms are made up of smaller particles that are held together by electrical or magnetic forces. Each atom is, in effect, like a mini solar system with a cluster of particles called electrons orbiting it.
Atoms are made up of smaller particles that are held together by electrical or magnetic forces. Each atom is, in effect, like a mini solar system with a cluster of particles called electrons orbiting it.
Depending on individual compound, between -34 ๐ C and -57 ๐ C
 Acrylonitrile-Butadiene (NBR) Nitrile rubber (NBR) is the general term for acrylonitrile butadiene copolymer. The acrylonitrile content of nitrile sealing compounds varies considerably (18% to 50%) and
Acrylonitrile-Butadiene (NBR) Nitrile rubber (NBR) is the general term for acrylonitrile butadiene copolymer. The acrylonitrile content of nitrile sealing compounds varies considerably (18% to 50%) and
 You should ALWAYS store persulfates: The Right Chemistry When Handled Right In a cool, dry area with temperatures below 77ºF (25ºC) for best stability. In a well-ventilated space. Away from incompatible
You should ALWAYS store persulfates: The Right Chemistry When Handled Right In a cool, dry area with temperatures below 77ºF (25ºC) for best stability. In a well-ventilated space. Away from incompatible
Can Wetting and Dispersing Additives Improve the Durability of Coatings? János Hajas. Technical support manager Coating additives
 Lehet-e nedvesitö-és diszpergálószerekkel a festékek tartósságát javítani? Wetting, Dispersion and Stabilization of Titanium Dioxide in Organic Coatings Can Wetting and Dispersing Additives Improve the
Lehet-e nedvesitö-és diszpergálószerekkel a festékek tartósságát javítani? Wetting, Dispersion and Stabilization of Titanium Dioxide in Organic Coatings Can Wetting and Dispersing Additives Improve the
(12) United States Patent
 (12) United States Patent Teoh et al. USOO65664B1 (10) Patent No.: () Date of Patent: May 20, 2003 (54) ELASTOMERIC GLOVES (75) Inventors: Seng Chin Teoh, Penang (MY); Seong Fong Chen, Penang (MY) (73)
(12) United States Patent Teoh et al. USOO65664B1 (10) Patent No.: () Date of Patent: May 20, 2003 (54) ELASTOMERIC GLOVES (75) Inventors: Seng Chin Teoh, Penang (MY); Seong Fong Chen, Penang (MY) (73)
4 Types of Organic Polar Reactions
 Objective 12 Apply Reactivity Principles to Electrophilic Addition Reactions 1: Alkenes Identify structural features (pi bond) and electrophiles Use curved arrows to predict product 4 Types of Organic
Objective 12 Apply Reactivity Principles to Electrophilic Addition Reactions 1: Alkenes Identify structural features (pi bond) and electrophiles Use curved arrows to predict product 4 Types of Organic
Flexible Reinforced Hoses from soft PVC
 Flexible Reinforced Hoses from soft PVC 137 137 FLEXIBLE HOSES FASOPLEX The flexible FASOPLEX hoses are made from plasticized (soft) PVC, enriched with appropriate additives that are necessary to ensure
Flexible Reinforced Hoses from soft PVC 137 137 FLEXIBLE HOSES FASOPLEX The flexible FASOPLEX hoses are made from plasticized (soft) PVC, enriched with appropriate additives that are necessary to ensure
ACUSOLTM PRODUCT SELECTION GUIDE DISPERSANT POLYMERS FOR HOUSEHOLD PRODUCTS AND INDUSTRIAL & INSTITUTIONAL CLEANERS
 ACUSOLTM DISPERSANT POLYMERS PRODUCT SELECTION GUIDE DISPERSANT POLYMERS FOR HOUSEHOLD PRODUCTS AND INDUSTRIAL & INSTITUTIONAL CLEANERS Recommended product applications Product properties 1 2 3 4 Why choose
ACUSOLTM DISPERSANT POLYMERS PRODUCT SELECTION GUIDE DISPERSANT POLYMERS FOR HOUSEHOLD PRODUCTS AND INDUSTRIAL & INSTITUTIONAL CLEANERS Recommended product applications Product properties 1 2 3 4 Why choose
New stabilisers for polyvinyl chloride mixed salts of calcium carboxylates
 Plasticheskie Massy,, 2000, p. 19 New stabilisers for polyvinyl chloride mixed salts of calcium carboxylates R.F. Nafikova, E.I. Nagumanova, Ya.M. Abdrashitov and K.S. Minsker Translation submitted by
Plasticheskie Massy,, 2000, p. 19 New stabilisers for polyvinyl chloride mixed salts of calcium carboxylates R.F. Nafikova, E.I. Nagumanova, Ya.M. Abdrashitov and K.S. Minsker Translation submitted by
Alehydes, Ketones and Carboxylic Acid
 Alehydes, Ketones and Carboxylic Acid Aldehydes and Ketones: Introduction Aldedydes and ketones are organic compounds that contain carbon-oxygen doule bonds. The general formula for aldehydes is O C R
Alehydes, Ketones and Carboxylic Acid Aldehydes and Ketones: Introduction Aldedydes and ketones are organic compounds that contain carbon-oxygen doule bonds. The general formula for aldehydes is O C R
Patentamt JEuropaisches. European Patent Off ice Publication number: Office europeen des brevets EUROPEAN PATENT APPLICATION
 Patentamt JEuropaisches European Patent Off ice Publication number: 0038 0 1 3 Office europeen des brevets ^2 EUROPEAN PATENT APPLICATION Application number: 81102618.6 Int. CI.3. A 61 K 9/00 Date of filing:
Patentamt JEuropaisches European Patent Off ice Publication number: 0038 0 1 3 Office europeen des brevets ^2 EUROPEAN PATENT APPLICATION Application number: 81102618.6 Int. CI.3. A 61 K 9/00 Date of filing:
THERMALLY OXIDIZED SOYA BEAN OIL interacted with MONO- and DIGLYCERIDES of FATTY ACIDS
 THERMALLY OXIDIZED SOYA BEAN OIL interacted with MONO- and DIGLYCERIDES of FATTY ACIDS Prepared at the 39th JECFA (1992), published in FNP 52 Add 1 (1992). Metals and arsenic specifications revised at
THERMALLY OXIDIZED SOYA BEAN OIL interacted with MONO- and DIGLYCERIDES of FATTY ACIDS Prepared at the 39th JECFA (1992), published in FNP 52 Add 1 (1992). Metals and arsenic specifications revised at
L 113/18 EN Official Journal of the European Union COMMISSION REGULATION (EU) 2017/752 of 28 April 2017 amending and correcting Regulation (
 L 113/18 EN 29.4.2017 COMMISSION REGULATION (EU) 2017/752 of 28 April 2017 amending and correcting Regulation (EU) No 10/2011 on plastic materials and articles intended to come into contact with food (Text
L 113/18 EN 29.4.2017 COMMISSION REGULATION (EU) 2017/752 of 28 April 2017 amending and correcting Regulation (EU) No 10/2011 on plastic materials and articles intended to come into contact with food (Text
GB Translated English of Chinese Standard: GB NATIONAL STANDARD OF THE
 Translated English of Chinese Standard: GB5009.268-2016 www.chinesestandard.net Buy True-PDF Auto-delivery. Sales@ChineseStandard.net GB NATIONAL STANDARD OF THE PEOPLE S REPUBLIC OF CHINA GB 5009.268-2016
Translated English of Chinese Standard: GB5009.268-2016 www.chinesestandard.net Buy True-PDF Auto-delivery. Sales@ChineseStandard.net GB NATIONAL STANDARD OF THE PEOPLE S REPUBLIC OF CHINA GB 5009.268-2016
The four levels of protein structure are: primary structure, secondary structure, tertiary structure, and quaternary structure.
 Proteins Proteins are organic complex nitrogenous compounds of high molecular weight, formed of C, H, O and N. They are formed of a number of amino acids linked together by peptide linkage [-CO-NH-]. Proteins
Proteins Proteins are organic complex nitrogenous compounds of high molecular weight, formed of C, H, O and N. They are formed of a number of amino acids linked together by peptide linkage [-CO-NH-]. Proteins
Review of literature Previously there is lot of work done on Sertraline hydrochloride a psychotic drugs undesired isomer recycling
 Review of literature Previously there is lot of work done on Sertraline hydrochloride a psychotic drugs undesired isomer recycling In the above mentioned scheme sertraline have four different isomers possible.
Review of literature Previously there is lot of work done on Sertraline hydrochloride a psychotic drugs undesired isomer recycling In the above mentioned scheme sertraline have four different isomers possible.
Pierre-Marie Baudoin Vandeputte Oleochemicals
 Pierre-Marie Baudoin Vandeputte leochemicals Atelier MPR, AG de ValBiom, Gembloux, 25 avril 2012 1 Your linseed oil & vegetable oils derivatives specialist 2 1887: Foundation of the company by the Vandeputte
Pierre-Marie Baudoin Vandeputte leochemicals Atelier MPR, AG de ValBiom, Gembloux, 25 avril 2012 1 Your linseed oil & vegetable oils derivatives specialist 2 1887: Foundation of the company by the Vandeputte
POLYTONE UA 810. Aldehyde Resin
 An Aldehyde Resin which is yellowing resistant, practically soluble in almost all paint solvents and compatible with almost all coating raw material. Aldehyde Resin POLYOLS & POLYMERS PVT.LTD. C-1/58-59,
An Aldehyde Resin which is yellowing resistant, practically soluble in almost all paint solvents and compatible with almost all coating raw material. Aldehyde Resin POLYOLS & POLYMERS PVT.LTD. C-1/58-59,
IMPRESSION MATERIALS
 IMPRESSION MATERIALS Function of Impression Materials To make a negative copy of an oral structure single tooth, quadrant, or arch edentulous or dentulous Negative copy must be accurate to produce an accurate
IMPRESSION MATERIALS Function of Impression Materials To make a negative copy of an oral structure single tooth, quadrant, or arch edentulous or dentulous Negative copy must be accurate to produce an accurate
Compatibility Nonwoven Oil And Universal Sorbents
 Compatibility Nonwoven Oil And Universal Sorbents Pads Rolls Pillows Drum Top Covers Socks Flakes ADR Strips Spillosens sorbents rapidly and effectively remove liquid spills and leaks. As a precautionary
Compatibility Nonwoven Oil And Universal Sorbents Pads Rolls Pillows Drum Top Covers Socks Flakes ADR Strips Spillosens sorbents rapidly and effectively remove liquid spills and leaks. As a precautionary
Purpose. NPRI Identification Number 2065
 , a wholly owned subsidiary of The Dow Chemical Company Toxic Substance Reduction Plan Summary Ammonia (Total) Purpose Rohm and Haas Canada LP, a wholly owned subsidiary of The Dow Chemical Company is
, a wholly owned subsidiary of The Dow Chemical Company Toxic Substance Reduction Plan Summary Ammonia (Total) Purpose Rohm and Haas Canada LP, a wholly owned subsidiary of The Dow Chemical Company is
Cable Ties-Material Selection Ordering Guide
 Introduction Thomas & Betts offers TY-RAP cable ties and accessories in a wide variety of materials, each suited for specific environments. The purpose of this document, therefore, is to assist you in
Introduction Thomas & Betts offers TY-RAP cable ties and accessories in a wide variety of materials, each suited for specific environments. The purpose of this document, therefore, is to assist you in
(12) Patent Application Publication (10) Pub. No.: US 2004/ A1
 (19) United States US 2004O156999A1 (12) Patent Application Publication (10) Pub. No.: US 2004/0156999 A1 Biddulph et al. (43) Pub. Date: (54) BLACK TRIVALENT CHROMIUM CHROMATE CONVERSION COATING (75)
(19) United States US 2004O156999A1 (12) Patent Application Publication (10) Pub. No.: US 2004/0156999 A1 Biddulph et al. (43) Pub. Date: (54) BLACK TRIVALENT CHROMIUM CHROMATE CONVERSION COATING (75)
11/5/ Oxidation of Alkenes: Cleavage to Carbonyl Compounds. Oxidation of Alkenes: Cleavage to Carbonyl Compounds
 8.8 Oxidation of Alkenes: Cleavage to Carbonyl Compounds Ozone (O 3 ) is useful double-bond cleavage reagent Ozone is generated by passing a stream of oxygen through a highvoltage electrical discharge
8.8 Oxidation of Alkenes: Cleavage to Carbonyl Compounds Ozone (O 3 ) is useful double-bond cleavage reagent Ozone is generated by passing a stream of oxygen through a highvoltage electrical discharge
Continuous process of detergents production on the basis of alkylarylsulfonic acids
 MATERIAL FOR EXPERIMENT NO. 09 Continuous process of detergents production on the basis of alkylarylsulfonic acids based on: Podręcznik do ćwiczeń z technologii chemicznej (Ed. T. Kasprzycka-Guttman),
MATERIAL FOR EXPERIMENT NO. 09 Continuous process of detergents production on the basis of alkylarylsulfonic acids based on: Podręcznik do ćwiczeń z technologii chemicznej (Ed. T. Kasprzycka-Guttman),
6/9/2015. Unit 15: Organic Chemistry Lesson 15.2: Substituted Hydrocarbons & Functional Groups
 1-chloropropane 2-methylpropane 1-iodobutane Ethanoic Acid Unit 15: Organic Chemistry Lesson 15.2: Substituted Hydrocarbons & Functional Groups 43 It Ain t Just Hydrocarbons There are all sorts of organic
1-chloropropane 2-methylpropane 1-iodobutane Ethanoic Acid Unit 15: Organic Chemistry Lesson 15.2: Substituted Hydrocarbons & Functional Groups 43 It Ain t Just Hydrocarbons There are all sorts of organic
Downloaded from
 Question 16.1: Why do we need to classify drugs in different ways? The classification of drugs and the reasons for classification are as follows: (i) On the basis of pharmacological effect: This classification
Question 16.1: Why do we need to classify drugs in different ways? The classification of drugs and the reasons for classification are as follows: (i) On the basis of pharmacological effect: This classification
Carbopol Aqua SF-1 Polymer Technical Data Sheet
 *** D R A F T *** 18 Dec 2 (Draft 6) The Specialty Chemicals Innovator TDS-294 December 2 Carbopol Aqua SF-1 Technical Data Sheet Introduction Noveon, Inc. is pleased to introduce Carbopol Aqua SF-1 polymer,
*** D R A F T *** 18 Dec 2 (Draft 6) The Specialty Chemicals Innovator TDS-294 December 2 Carbopol Aqua SF-1 Technical Data Sheet Introduction Noveon, Inc. is pleased to introduce Carbopol Aqua SF-1 polymer,
Usable in formulations. Active content [%] Flash point [ C] Delivery form. Chemical description. Product. Solvent. Solvent free UV curing.
![Usable in formulations. Active content [%] Flash point [ C] Delivery form. Chemical description. Product. Solvent. Solvent free UV curing. Usable in formulations. Active content [%] Flash point [ C] Delivery form. Chemical description. Product. Solvent. Solvent free UV curing.](/thumbs/95/122775499.jpg) Product Chemical description Active content [%] Solvent ph-value Flash point [ C] Delivery form Usable in formulations Solvent free UV curing Solvent based Water based LUCRAMUL 1820 LIQ. LUCRAMUL AP LUCRAMUL
Product Chemical description Active content [%] Solvent ph-value Flash point [ C] Delivery form Usable in formulations Solvent free UV curing Solvent based Water based LUCRAMUL 1820 LIQ. LUCRAMUL AP LUCRAMUL
EUROPEAN QUALIFYING EXAMINATION 2006
 EUROPEAN QUALIFYING EXAMINATION 2006 PAPER A CHEMISTRY This paper comprises: * Letter from the applicant 2006/A(Ch)/e/1-6 * Document 1 2006/A(Ch)/e/7 * Document 2 2006/A(Ch)/e/8-10 2006/A(Ch)/e - 1 - LETTER
EUROPEAN QUALIFYING EXAMINATION 2006 PAPER A CHEMISTRY This paper comprises: * Letter from the applicant 2006/A(Ch)/e/1-6 * Document 1 2006/A(Ch)/e/7 * Document 2 2006/A(Ch)/e/8-10 2006/A(Ch)/e - 1 - LETTER
EH1008 Biomolecules. Inorganic & Organic Chemistry. Water. Lecture 2: Inorganic and organic chemistry.
 EH1008 Biomolecules Lecture 2: Inorganic and organic chemistry limian.zheng@ucc.ie 1 Inorganic & Organic Chemistry Inorganic Chemistry: generally, substances that do not contain carbon Inorganic molecules:
EH1008 Biomolecules Lecture 2: Inorganic and organic chemistry limian.zheng@ucc.ie 1 Inorganic & Organic Chemistry Inorganic Chemistry: generally, substances that do not contain carbon Inorganic molecules:
Properties of Alcohols and Phenols Experiment #3
 Properties of Alcohols and Phenols Experiment #3 Objectives: To observe the solubility of alcohols relative to their chemical structure, to perform chemical tests to distinguish primary, secondary and
Properties of Alcohols and Phenols Experiment #3 Objectives: To observe the solubility of alcohols relative to their chemical structure, to perform chemical tests to distinguish primary, secondary and
Protective Equipment: Hazards Identification: ZINC OXIDE POWDER - COA - MSDS.
 ZINC OXIDE POWDER - COA - MSDS http://www.essentialdepot.com/msds/zincoxide.pdf TECHNICAL DATA Representative Physical Properties Mean Surface Particle Diam., μ... 0.12 Specific Surface, m 2 /g... 9.0
ZINC OXIDE POWDER - COA - MSDS http://www.essentialdepot.com/msds/zincoxide.pdf TECHNICAL DATA Representative Physical Properties Mean Surface Particle Diam., μ... 0.12 Specific Surface, m 2 /g... 9.0
FROM SOLVENT TO AQUEOUS COATINGS. (Out of the Frying Pan...) Ralph E. Pondell. Coating Place, Inc. P.O. Box
 84-1 FROM SOLVENT TO AQUEOUS COATINGS (Out of the Frying Pan...) Ralph E. Pondell Coating Place, Inc. P.O. Box 930310 Verona, WI 53593 A number of reasons exist for the current high level of interest in
84-1 FROM SOLVENT TO AQUEOUS COATINGS (Out of the Frying Pan...) Ralph E. Pondell Coating Place, Inc. P.O. Box 930310 Verona, WI 53593 A number of reasons exist for the current high level of interest in
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 CONSUMER TESTING LABORATORIES (FAR EAST), LTD. Unit 703-704 7 th Floor, Riley House 88 Lei Muk Road Kwai Chung, Hong Kong David Chung Phone: 852-2423-7161 CHEMICAL
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 CONSUMER TESTING LABORATORIES (FAR EAST), LTD. Unit 703-704 7 th Floor, Riley House 88 Lei Muk Road Kwai Chung, Hong Kong David Chung Phone: 852-2423-7161 CHEMICAL
PUTTING ON PAPER. Dow Paper Coatings
 PUTTING ON PAPER Dow Paper Coatings www.dow.com/papercoatings Dow Paper Coatings offers access to a broad portfolio of technologies and chemistries for coated paperboard, SBS paperboard, coated freesheet,
PUTTING ON PAPER Dow Paper Coatings www.dow.com/papercoatings Dow Paper Coatings offers access to a broad portfolio of technologies and chemistries for coated paperboard, SBS paperboard, coated freesheet,
*EP A1* EP A1 (19) (11) EP A1. (12) EUROPEAN PATENT APPLICATION published in accordance with Art.
 (19) Europäisches Patentamt European Patent Office Office européen des brevets *EP00147089A1* (11) EP 1 47 089 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 18(3) EPC (43) Date
(19) Europäisches Patentamt European Patent Office Office européen des brevets *EP00147089A1* (11) EP 1 47 089 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 18(3) EPC (43) Date
Radicals. Structure and Stability of Radicals. Radicals are formed from covalent bonds by adding energy in the form of heat (Δ) or light (hν).
 Radicals Chapter 15 A small but significant group of reactions involve radical intermediates. A radical is a reactive intermediate with a single unpaired electron, formed by homolysis of a covalent bond.
Radicals Chapter 15 A small but significant group of reactions involve radical intermediates. A radical is a reactive intermediate with a single unpaired electron, formed by homolysis of a covalent bond.
EXPERIMENT 8 (Organic Chemistry II) Carboxylic Acids Reactions and Derivatives
 EXPERIMENT 8 (rganic Chemistry II) Carboxylic Acids Reactions and Derivatives Pahlavan/Cherif Materials Medium test tubes (6) Test tube rack Beakers (50, 150, 400 ml) Ice Hot plate Graduated cylinders
EXPERIMENT 8 (rganic Chemistry II) Carboxylic Acids Reactions and Derivatives Pahlavan/Cherif Materials Medium test tubes (6) Test tube rack Beakers (50, 150, 400 ml) Ice Hot plate Graduated cylinders
(Non-legislative acts) REGULATIONS
 29.8.2013 Official Journal of the European Union L 230/1 II (Non-legislative acts) REGULATIONS COMMISSION REGULATION (EU) No 816/2013 of 28 August 2013 amending Annex II to Regulation (EC) No 1333/2008
29.8.2013 Official Journal of the European Union L 230/1 II (Non-legislative acts) REGULATIONS COMMISSION REGULATION (EU) No 816/2013 of 28 August 2013 amending Annex II to Regulation (EC) No 1333/2008
Designing a Non-Soap Cleansing Bar
 Designing a Non-Soap Cleansing Bar Michael I. Hill M Hill & Associates 31 Winding Trail Mahwah, NJ, 07430 Dove Beauty Bar was developed by Lever Brothers Co., a subsidiary of Unilever in the United States,
Designing a Non-Soap Cleansing Bar Michael I. Hill M Hill & Associates 31 Winding Trail Mahwah, NJ, 07430 Dove Beauty Bar was developed by Lever Brothers Co., a subsidiary of Unilever in the United States,
supersaturated, yellow, opaque solution
 Initiatior BK Properties Initiator BK has been developed for the initiation of radical polymerisation reactions. The product can also be used as a reactive retarding agent for peroxides. Initiator BK is
Initiatior BK Properties Initiator BK has been developed for the initiation of radical polymerisation reactions. The product can also be used as a reactive retarding agent for peroxides. Initiator BK is
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 CONSUMER TESTING LABORATORIES (FAR EAST), LTD. Unit 703-704 7 th Floor, Riley House 88 Lei Muk Road Kwai Chung, Hong Kong David Chung Phone: 852-2423-7161 CHEMICAL
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 CONSUMER TESTING LABORATORIES (FAR EAST), LTD. Unit 703-704 7 th Floor, Riley House 88 Lei Muk Road Kwai Chung, Hong Kong David Chung Phone: 852-2423-7161 CHEMICAL
Chemical Effect on Concrete Concrete Treated with Penetron. Acetic Acid to 30% Disintegrates Slowly S/E. Acetone Liquid loss by penetration N/E
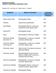 PENETRON SYSTEMS CHEMICAL RESISTANCE/CORROSION CHART Symbols: = No Effect = Slight Effect E = Effected Chemical Effect on Concrete Concrete Treated with Penetron Acetic Acid to 30% Disintegrates Slowly
PENETRON SYSTEMS CHEMICAL RESISTANCE/CORROSION CHART Symbols: = No Effect = Slight Effect E = Effected Chemical Effect on Concrete Concrete Treated with Penetron Acetic Acid to 30% Disintegrates Slowly
HyProBlack Hexavalent chrome free RoHS and ELV compliant. HyPro System
 HyProBlack 2020 HyPro System HyProBlack 2020 is trivalent black passivate for zinc that provides 120 hours to white corrosion when sealed with HyPro 200 or HyPro 280 COF Provides glossy, uniform, deep
HyProBlack 2020 HyPro System HyProBlack 2020 is trivalent black passivate for zinc that provides 120 hours to white corrosion when sealed with HyPro 200 or HyPro 280 COF Provides glossy, uniform, deep
EP A1 (19) (11) EP A1 (12) EUROPEAN PATENT APPLICATION. (43) Date of publication: Bulletin 2008/26
 (19) (12) EUROPEAN PATENT APPLICATION (11) EP 1 93 941 A1 (43) Date of publication: 2.06.08 Bulletin 08/26 (21) Application number: 0724886.0 (1) Int Cl.: C08L 43/02 (06.01) B60R 13/08 (06.01) C08F 2/02
(19) (12) EUROPEAN PATENT APPLICATION (11) EP 1 93 941 A1 (43) Date of publication: 2.06.08 Bulletin 08/26 (21) Application number: 0724886.0 (1) Int Cl.: C08L 43/02 (06.01) B60R 13/08 (06.01) C08F 2/02
EBB 220/3 ELASTOMER & LATEX
 EBB 220/3 ELASTOMER & LATEX Introduction The words rubber come from the materials from the rubber tree name Havea Brasiliensis The different between raw rubber and vulcanized rubber or elastomer: 1. Raw
EBB 220/3 ELASTOMER & LATEX Introduction The words rubber come from the materials from the rubber tree name Havea Brasiliensis The different between raw rubber and vulcanized rubber or elastomer: 1. Raw
