DNA and Protein Synthesis Practice
|
|
|
- Barnaby Woods
- 5 years ago
- Views:
Transcription
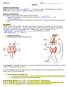
1 Biology 12 DNA and Protein Synthesis Practice Name: 1. DNA is often called the "code of life". Actually it contains the code for a) the sequence of amino acids in a protein b) the sequence of base pairs c) producing mutations d) making a recipe 2. a) Name the molecule indicated by X. b) Where in a human cell does the process shown occur? c) What is the result of the process shown? d) After this process, cell division will occur. Why does the process need to occur before cell division? 3. Fill in the following chart that compares DNA and RNA: # of strands type of sugar location in cell bases types DNA ONLY ONE TYPE OF DNA RNA 4. Protein Synthesis using the DNA instructions to make a protein Location in cell where it occurs Types of RNA associated with this Transcription Translation Product
2 Metabolism Practice Section 1: What is an enzyme? 1. Which of the following statements is true about enzymes? a) 3D shape can vary and still be active b) they may catalyze only 1 specific reaction c) boiling temps do not affect activity d) they are unaffected by changes in ph 2. Enzymes a) give energy to metabolic reactions b) speed up metabolic reactions c) change the direction of reactions d) act as a buffer in metabolic reactions 3. Enzymes are specific. This means that they a) have a preferred ph b) have a preferred temperature c) have a particular substrate d) are only in certain cells 4. Most enzymes a) keep the speed of the chemical reaction constant b) inhibit the speed of the chemical reaction c) speed up the chemical reaction d) have no effect on the speed of the chemical reaction 5. Most enzymes are a) lipids b) carbohydrates c) proteins d) nucleic acids 6. One enzyme a) allows a chemical reaction in a cell to occur at moderate temperatures b) raises the energy of activation of a reaction c) speeds up 2 or more reactions in a cell d) cannot be used after the reaction 7. Each enzyme has a particular substrate because enzymes a) increase the energy of activation b) decrease the productivity of the cell c) always require coenzymes d) have active sites complementary in shape 8. Which of the following can be used to determine the rate of enzyme-catalyzed reactions? a) increase in activation energy b) increase in enzyme over time c) increase in product over time d) increase in coenzyme over time
3 Section 2: How Do Enzymes Work Lock and Key Theory 9. During enzymatic action, the enzyme a) becomes the product b) and substrate form a permanent complex c) is used up d) and substrate form a temporary complex 10. The maximum enzymatic rate occurs when of the active site is filled with substrate. a) 0% b) 25% c) 50% d) 100% 11. The place where the substrate fits onto the enzyme is called the a) active site b) inhibitor site c) receptor site d) E - S complex 12. Which of the following can be used to determine the rate of enzyme-catalyzed reactions? a) increase in activation energy b) increase in enzyme over time c) increase in product over time d) increase in coenzyme over time 13. a) If the following enzyme is a dehydration synthesis enzyme, which order would the diagrams have to be placed? b.) If the diagram represents a hydrolytic reaction, which order would the diagrams have to be placed? c.) In the diagrams above, what name would you give to the darker colored square?
4 14. If peptidase were added to side A, what would occur? (Peptidase is an enzyme that breaks peptides into amino acids) Section 3: Factors That Affect the Rate of Enzyme Catalyzed Reactions 15. Initially, as the substrate concentration is increased and there is a lot of enzyme, the reaction rate a) slows down b) speeds up c) stays the same d) speeds up then decreases 16. The graph shows the effect of ph on three different enzyme-catalyzed reactions. Which of the following would best describe the effect of ph on enzyme-catalyzed reactions? a) enzyme action increases as ph increases b) enzyme action decreases as ph increases c) enzymes work best in an acid environment d) each enzyme works best within a specific ph range 17. The diagram below represents the shapes of reacting molecules in a living system. When molecule X was added to the system, the amount of product decreased. Molecule X must be acting a) as a coenzyme. b) to denature the reactants. c) as a competitive inhibitor. d) to synthesize more of molecule W.
5 18. An experiment was performed to determine the effect of changing temperature on the speed enzyme activity. Substrate was placed in test tubes. The tubes were then placed in water baths of various temperatures. The substrate concentration in each tube was then measured. The results are graphed below. 10 C 40 C 50 C Give the amount of substrate in the tube at 10 C, 40 C and 50 C and explain why these substrate concentrations are different at each temperature. 19. The following data show the rate of an enzyme catalyzed reaction at various temperatures. a. Graph the data on the grid provided. b. Use the graphed data to describe the effect of temperature on the rate of enzyme activity. i. From C ii. at 38 C iii C
6 c. Describe what would happen if the ph of the enzymes was changed from 7 to 2 d. Describe what would happen if a heavy metal was added to the enzyme 20. The graph shows the change in the rate of an enzyme-catalyzed reaction over time. a) Explain why the rate became constant at time X. b) Which labeled line correctly illustrates what would occur if more enzyme was added at time Y. Explain your answer. Section 4: Metabolism and Metabolic Pathways 21. If represents a metabolic pathway, then letter E would be a) a substrate b) a product c) an end product d) an enzyme 22. In a metabolic pathway a) product of 1 reaction becomes substrate of next reaction b) ATP is always released c) the same enzyme is used for all reactions d) the end product is never formed 23. Which of the following statements is false? a) metabolism is the sum of all chemical activities inside a cell b) a metabolic pathway involves only one chemical reaction c) one metabolic pathway may lead into another metabolic pathway d) the reactants in an enzymatic reaction are called substrates 24. An experiment investigating enzyme activity was carried out. A test tube was prepared containing substrate solution W and enzyme solutions 1 and 2. The reactions that occur in the test tube are summarized below: The letters represent substrates and products and the numbers represent enzymes. a. State two ways to increase the rate of production of product Y. b. A substance is added to the test tube containing substrate W. As a result, no product is formed. Suggest what this substance may be and explain how it achieves these results.
7 25. An experiment investigating enzyme activity is carried out. A test tube is prepared containing substrate solution W and enzyme solutions 1, 2 and 3. The reactions that occur in the test tube are summarized to the right. a) Describe two ways in which the rate of production of product Y can be increased. b) A substance is added to the test tube. As a result, no product Y is formed, but product Z is still formed. Explain why. Section 5: Cellular Respiration and ATP 26. ATP is the energy currency of cells because it a) is a large molecule b) carries a positive charge c) contains high energy phosphate bonds d) is made from glucose 27. What is the common energy currency in cells for the synthesis of molecules? a) glucose b) proteins c) lipids d) ATP 28. ATP contains a) 1 phosphate b) 2 phosphates c) 3 phosphates d) no phosphates 29. The high energy bond in ATP is found in or between a) adenine base b) cytosine base c) adenosine d) the phosphate groups 30. When ATP is broken down a) ADP is formed b) ADP + phosphate is formed c) ADP + phosphate + energy is formed d) Lactic acid is formed 31. Which of the following would be considered normal energy sources for ATP production? a) Carbohydrates, fats and proteins b) Carbohydrates and fats c) Carbohydrates only d) Carbohydrates and proteins 32. Which of the following statements is the most likely to be incorrect? A breakdown of protein to produce ATP may occur under the following conditions. a) The individual is exercising at an extreme level b) The individual has a low body fat percentage c) The individual is not very fit and works out too hard d) An individual does not do any exercise
8 33. During exercise, most ATP is made a) During aerobic cellular respiration b) During anaerobic cellular respiration c) When muscle cells contain low numbers of mitochondria d) When muscle cells contains low concentrations of mitochondrial enzymes 34. Which substrate can provide the highest theoretical ATP production? a) Carbohydrates b) Triglycerides (fats) c) Nucleic acids d) Proteins 35. The diagram below represents a molecule found in all living cells. a) What is this molecule called? b) What occurs if one phosphate is removed? Why? c) Name one cellular function that requires the above molecule. Section 6: Higher Level Thinking Question (application of knowledge) 36. The cells of the thyroid gland are able to take in iodine atoms and may contain iodine concentrations up to 25 times that of the surrounding tissue fluid. In an experiment designed to study factors affecting the rate of iodine intake, thyroid cells were cultured and placed in a medium containing normal blood concentrations of iodine. Temperature and glucose concentrations were varied and the effects recorded. The results of the study are shown in the table below. a) Explain the observed results for sample A. b) Explain the observed results for sample B. c) Explain how the movement of amino acids into the cells would be affected by the conditions in sample B
1) DNA unzips - hydrogen bonds between base pairs are broken by special enzymes.
 Biology 12 Cell Cycle To divide, a cell must complete several important tasks: it must grow, during which it performs protein synthesis (G1 phase) replicate its genetic material /DNA (S phase), and physically
Biology 12 Cell Cycle To divide, a cell must complete several important tasks: it must grow, during which it performs protein synthesis (G1 phase) replicate its genetic material /DNA (S phase), and physically
Name: Date: Block: Biology 12
 Name: Date: Block: Biology 12 Provincial Exam Review: Cell Processes and Applications January 2003 Use the following diagram to answer questions 1 and 2. 1. Which labelled organelle produces most of the
Name: Date: Block: Biology 12 Provincial Exam Review: Cell Processes and Applications January 2003 Use the following diagram to answer questions 1 and 2. 1. Which labelled organelle produces most of the
Chapter 5 Metabolism: Energy and Enzymes
 Biology 12 Name: Cell Biology Per: Date: Chapter 5 Metabolism: Energy and Enzymes Complete using BC Biology 12, pages 154-175 Diagnostic Questions (mark using the answer key on page 533) 1. B 2. B 3. C
Biology 12 Name: Cell Biology Per: Date: Chapter 5 Metabolism: Energy and Enzymes Complete using BC Biology 12, pages 154-175 Diagnostic Questions (mark using the answer key on page 533) 1. B 2. B 3. C
Living Environment. Scientific Inquiry Exam
 Name: Class: 1. Which elements are present in all organic compounds? 1) nitrogen and carbon 3) hydrogen and oxygen 2) nitrogen and oxygen 4) hydrogen and carbon 2. Which substances are inorganic compounds?
Name: Class: 1. Which elements are present in all organic compounds? 1) nitrogen and carbon 3) hydrogen and oxygen 2) nitrogen and oxygen 4) hydrogen and carbon 2. Which substances are inorganic compounds?
Jeopardy Q $100 Q $100 Q $100 Q $100 Q $100 Q $200 Q $200 Q $200 Q $200 Q $200 Q $300 Q $300 Q $300 Q $300 Q $300 Q $400 Q $400 Q $400 Q $400 Q $400
 Jeopardy Proteins Carbohydrates Lipids Nucleic Acids Energy & Reactions Q $100 Q $200 Q $300 Q $400 Q $500 Q $100 Q $100 Q $100 Q $100 Q $200 Q $200 Q $200 Q $200 Q $300 Q $300 Q $300 Q $300 Q $400 Q $400
Jeopardy Proteins Carbohydrates Lipids Nucleic Acids Energy & Reactions Q $100 Q $200 Q $300 Q $400 Q $500 Q $100 Q $100 Q $100 Q $100 Q $200 Q $200 Q $200 Q $200 Q $300 Q $300 Q $300 Q $300 Q $400 Q $400
Energy Production In A Cell (Chapter 25 Metabolism)
 Energy Production In A Cell (Chapter 25 Metabolism) Large food molecules contain a lot of potential energy in the form of chemical bonds but it requires a lot of work to liberate the energy. Cells need
Energy Production In A Cell (Chapter 25 Metabolism) Large food molecules contain a lot of potential energy in the form of chemical bonds but it requires a lot of work to liberate the energy. Cells need
Chemistry 107 Exam 4 Study Guide
 Chemistry 107 Exam 4 Study Guide Chapter 10 10.1 Recognize that enzyme catalyze reactions by lowering activation energies. Know the definition of a catalyst. Differentiate between absolute, relative and
Chemistry 107 Exam 4 Study Guide Chapter 10 10.1 Recognize that enzyme catalyze reactions by lowering activation energies. Know the definition of a catalyst. Differentiate between absolute, relative and
WHY IS THIS IMPORTANT?
 CHAPTER 2 FUNDAMENTAL CHEMISTRY FOR MICROBIOLOGY WHY IS THIS IMPORTANT? An understanding of chemistry is essential to understand cellular structure and function, which are paramount for your understanding
CHAPTER 2 FUNDAMENTAL CHEMISTRY FOR MICROBIOLOGY WHY IS THIS IMPORTANT? An understanding of chemistry is essential to understand cellular structure and function, which are paramount for your understanding
Objectives. Carbon Bonding. Carbon Bonding, continued. Carbon Bonding
 Biochemistry Table of Contents Objectives Distinguish between organic and inorganic compounds. Explain the importance of carbon bonding in biological molecules. Identify functional groups in biological
Biochemistry Table of Contents Objectives Distinguish between organic and inorganic compounds. Explain the importance of carbon bonding in biological molecules. Identify functional groups in biological
BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2.
 BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2. A possible explanation for an event that occurs in nature is
BIOLOGICAL MOLECULES REVIEW-UNIT 1 1. The factor being tested in an experiment is the A. data. B. variable. C. conclusion. D. observation. 2. A possible explanation for an event that occurs in nature is
Do Now #1. Name: Enzymes & ph. 1. Enzymes, hormones and cell receptors are examples of which type of macromolecule?
 Name: Do Now #1 Enzymes & ph 1. Enzymes, hormones and cell receptors are examples of which type of macromolecule? 2. What do you think enzymes do for the body? Chemical reactions with enzymes are used
Name: Do Now #1 Enzymes & ph 1. Enzymes, hormones and cell receptors are examples of which type of macromolecule? 2. What do you think enzymes do for the body? Chemical reactions with enzymes are used
Chapter 3. Table of Contents. Section 1 Carbon Compounds. Section 2 Molecules of Life. Biochemistry
 Biochemistry Table of Contents Section 1 Carbon Compounds Section 2 Molecules of Life Section 1 Carbon Compounds Objectives Distinguish between organic and inorganic compounds. Explain the importance of
Biochemistry Table of Contents Section 1 Carbon Compounds Section 2 Molecules of Life Section 1 Carbon Compounds Objectives Distinguish between organic and inorganic compounds. Explain the importance of
c. Reaction will drive Reaction in a reaction. d. Which statement (A or B) has more energy in products than reactants?
 Energy and Enzymes (32 questions) 1. Chemical reactions involve a. Formation of chemical bonds b. Breakage of chemical bonds c. Both formation and breakage of chemical bonds d. Neither formation and breakage
Energy and Enzymes (32 questions) 1. Chemical reactions involve a. Formation of chemical bonds b. Breakage of chemical bonds c. Both formation and breakage of chemical bonds d. Neither formation and breakage
Do Now Makeups. 4. In which organelle would water and dissolved materials be stored? A. 1 B. 2 C. 3 D. 5. A. mitochondria B.
 Do Now Makeups Name: Date: 1. Which organelle is primarily concerned with the conversion of potential energy of organic compounds into suitable form for immediate use by the cell? A. mitochondria B. centrosomes
Do Now Makeups Name: Date: 1. Which organelle is primarily concerned with the conversion of potential energy of organic compounds into suitable form for immediate use by the cell? A. mitochondria B. centrosomes
The Chemical Building Blocks of Life. Chapter 3
 The Chemical Building Blocks of Life Chapter 3 Biological Molecules Biological molecules consist primarily of -carbon bonded to carbon, or -carbon bonded to other molecules. Carbon can form up to 4 covalent
The Chemical Building Blocks of Life Chapter 3 Biological Molecules Biological molecules consist primarily of -carbon bonded to carbon, or -carbon bonded to other molecules. Carbon can form up to 4 covalent
Biological Molecules
 The Chemical Building Blocks of Life Chapter 3 Biological molecules consist primarily of -carbon bonded to carbon, or -carbon bonded to other molecules. Carbon can form up to 4 covalent bonds. Carbon may
The Chemical Building Blocks of Life Chapter 3 Biological molecules consist primarily of -carbon bonded to carbon, or -carbon bonded to other molecules. Carbon can form up to 4 covalent bonds. Carbon may
The Structure and Function of Biomolecules
 The Structure and Function of Biomolecules The student is expected to: 9A compare the structures and functions of different types of biomolecules, including carbohydrates, lipids, proteins, and nucleic
The Structure and Function of Biomolecules The student is expected to: 9A compare the structures and functions of different types of biomolecules, including carbohydrates, lipids, proteins, and nucleic
How Cells Release Chemical Energy. Chapter 7
 How Cells Release Chemical Energy Chapter 7 7.1 Overview of Carbohydrate Breakdown Pathways All organisms (including photoautotrophs) convert chemical energy of organic compounds to chemical energy of
How Cells Release Chemical Energy Chapter 7 7.1 Overview of Carbohydrate Breakdown Pathways All organisms (including photoautotrophs) convert chemical energy of organic compounds to chemical energy of
Dalkeith High School Higher Human Biology Homework 3
 Dalkeith High School Higher Human Biology Homework 3 1. During which of the following chemical conversions is A T P produced? A B C Amino acids protein Glucose pyruvic acid Haemoglobin oxyhaemoglobin energy
Dalkeith High School Higher Human Biology Homework 3 1. During which of the following chemical conversions is A T P produced? A B C Amino acids protein Glucose pyruvic acid Haemoglobin oxyhaemoglobin energy
2.3: Carbon-Based Molecules Notes
 2.3: Carbon-Based Molecules Notes Carbon-based molecules are the of life. Bonding Properties of Carbon Carbon forms bonds with up to other atoms, including other carbon atoms. QUESTION: What types of elements
2.3: Carbon-Based Molecules Notes Carbon-based molecules are the of life. Bonding Properties of Carbon Carbon forms bonds with up to other atoms, including other carbon atoms. QUESTION: What types of elements
3.7.1 Define cell respiration [Cell respiration is the controlled release of energy from organic compounds in cells to form ATP]
![3.7.1 Define cell respiration [Cell respiration is the controlled release of energy from organic compounds in cells to form ATP] 3.7.1 Define cell respiration [Cell respiration is the controlled release of energy from organic compounds in cells to form ATP]](/thumbs/72/66350985.jpg) 3.7 Cell respiration ( Chapter 9 in Campbell's book) 3.7.1 Define cell respiration [Cell respiration is the controlled release of energy from organic compounds in cells to form ATP] Organic compounds store
3.7 Cell respiration ( Chapter 9 in Campbell's book) 3.7.1 Define cell respiration [Cell respiration is the controlled release of energy from organic compounds in cells to form ATP] Organic compounds store
An example of a carbohydrate A) 1 B) 2 C) 3 D) 4
 1. Which chemical formula represents a carbohydrate? A) CH4 B) C3H7O2N C) Cl2H22O11 D) CO2 2. Base your answer to the following question on the diagram below. For each of the following phrases, select
1. Which chemical formula represents a carbohydrate? A) CH4 B) C3H7O2N C) Cl2H22O11 D) CO2 2. Base your answer to the following question on the diagram below. For each of the following phrases, select
Name Class Date. Write the correct letter in the blank before each numbered term. a. forms large molecules from smaller. ones
 Name lass Date Assessment hapter Test B Biochemistry Write the correct letter in the blank before each numbered term. 1. nucleotide 2. hydrolysis 3. steroid 4. amino acid 5. condensation reaction 6. glucose
Name lass Date Assessment hapter Test B Biochemistry Write the correct letter in the blank before each numbered term. 1. nucleotide 2. hydrolysis 3. steroid 4. amino acid 5. condensation reaction 6. glucose
ADP, ATP and Cellular Respiration
 ADP, ATP and Cellular Respiration What Is ATP? Energy used by all Cells Adenosine Triphosphate Organic molecule containing highenergy Phosphate bonds Chemical Structure of ATP Adenine Base 3 Phosphates
ADP, ATP and Cellular Respiration What Is ATP? Energy used by all Cells Adenosine Triphosphate Organic molecule containing highenergy Phosphate bonds Chemical Structure of ATP Adenine Base 3 Phosphates
Background knowledge
 Background knowledge This is the required background knowledge: State three uses of energy in living things Give an example of an energy conversion in a living organism State that fats and oils contain
Background knowledge This is the required background knowledge: State three uses of energy in living things Give an example of an energy conversion in a living organism State that fats and oils contain
Assignment #1: Biological Molecules & the Chemistry of Life
 Assignment #1: Biological Molecules & the Chemistry of Life A. Important Inorganic Molecules Water 1. Explain why water is considered a polar molecule. The partial negative charge of the oxygen and the
Assignment #1: Biological Molecules & the Chemistry of Life A. Important Inorganic Molecules Water 1. Explain why water is considered a polar molecule. The partial negative charge of the oxygen and the
Biochemistry Name: Practice Questions
 Name: Practice Questions 1. Carbohydrate molecules A and B come in contact with the cell membrane of the same cell. Molecule A passes through the membrane readily, but molecule B does not. It is most likely
Name: Practice Questions 1. Carbohydrate molecules A and B come in contact with the cell membrane of the same cell. Molecule A passes through the membrane readily, but molecule B does not. It is most likely
BIOCHEMISTRY. There are 4 major types of organic compounds each with unique characteristics: A. CARBOHYDRATES Contain,, and. Ratio of H:O is always
 BIOCHEMISTRY All organic compounds must contain and Are the following organic? Why or why not? H2O CO2 CH4 There are 4 major types of organic compounds each with unique characteristics: A. CARBOHYDRATES
BIOCHEMISTRY All organic compounds must contain and Are the following organic? Why or why not? H2O CO2 CH4 There are 4 major types of organic compounds each with unique characteristics: A. CARBOHYDRATES
Macromolecules. SC.912.L.18.1 Describe the basic molecular structures and primary functions of the four major categories of biological macromolecules.
 Macromolecules SC.912.L.18.1 Describe the basic molecular structures and primary functions of the four major categories of biological macromolecules. FOUR MAJOR BIOLOGICAL MACROMOLECULES 1.Carbohydrates
Macromolecules SC.912.L.18.1 Describe the basic molecular structures and primary functions of the four major categories of biological macromolecules. FOUR MAJOR BIOLOGICAL MACROMOLECULES 1.Carbohydrates
Digestion and Human Health
 Digestion and Human Health The Molecules of Living Systems There are three main fluid components in your body Cytoplasm in your cells Fluid between your cells Fluid in your blood The also contain many
Digestion and Human Health The Molecules of Living Systems There are three main fluid components in your body Cytoplasm in your cells Fluid between your cells Fluid in your blood The also contain many
Carbon. p Has four valence electrons p Can bond with many elements p Can bond to other carbon atoms
 Organic Compounds Carbon p Has four valence electrons p Can bond with many elements p Can bond to other carbon atoms n Gives carbon the ability to form chains that are almost unlimited in length. p Organic
Organic Compounds Carbon p Has four valence electrons p Can bond with many elements p Can bond to other carbon atoms n Gives carbon the ability to form chains that are almost unlimited in length. p Organic
MUSCLE METABOLISM. Honors Anatomy & Physiology
 MUSCLE METABOLISM Honors Anatomy & Physiology ROLE OF ATP ATP binds to myosin heads and upon hydrolysis into ADP and Pi, transfers its energy to the cross bridge, energizing it. ATP is responsible for
MUSCLE METABOLISM Honors Anatomy & Physiology ROLE OF ATP ATP binds to myosin heads and upon hydrolysis into ADP and Pi, transfers its energy to the cross bridge, energizing it. ATP is responsible for
Biological Molecules
 Chemical Building Blocks of Life Chapter 3 Biological Molecules Biological molecules consist primarily of -carbon bonded to carbon, or -carbon bonded to other molecules. Carbon can form up to 4 covalent
Chemical Building Blocks of Life Chapter 3 Biological Molecules Biological molecules consist primarily of -carbon bonded to carbon, or -carbon bonded to other molecules. Carbon can form up to 4 covalent
10. The diagram below shows two different kinds of substances, A and B, entering a cell.
 1. In the binomial system of nomenclature, which two classification groups provide the scientific name of an organism? A) kingdom and phylum B) phylum and species C) kingdom and genus D) genus and species
1. In the binomial system of nomenclature, which two classification groups provide the scientific name of an organism? A) kingdom and phylum B) phylum and species C) kingdom and genus D) genus and species
Biochemistry 7/11/ Bio-Energetics & ATP. 5.1) ADP, ATP and Cellular Respiration OVERVIEW OF ENERGY AND METABOLISM
 Biochemistry 5. Bio-Energetics & ATP 5.1) ADP, ATP and Cellular Respiration Prof. Dr. Klaus Heese OVERVIEW OF ENERGY AND METABOLISM 1. The food we eat, (carbohydrates/ glucose /sugar, lipids/fat, proteins),
Biochemistry 5. Bio-Energetics & ATP 5.1) ADP, ATP and Cellular Respiration Prof. Dr. Klaus Heese OVERVIEW OF ENERGY AND METABOLISM 1. The food we eat, (carbohydrates/ glucose /sugar, lipids/fat, proteins),
CP Biology Chapter 2: Molecules of Life Name Amatuzzi #1: Carbohydrates pp Period Homework
 Amatuzzi #1: Carbohydrates pp. 46-47 Period 1. Which elements make up carbohydrates? a. In which ratio? 2. How do living things use most of their carbohydrates? 3. How do cells get energy from carbs? a.
Amatuzzi #1: Carbohydrates pp. 46-47 Period 1. Which elements make up carbohydrates? a. In which ratio? 2. How do living things use most of their carbohydrates? 3. How do cells get energy from carbs? a.
Name: Date: Per: Enzymes Review & Using text to understand ATP & Cellular Respiration
 Name: Date: Per: Enzymes Review & Using text to understand ATP & Cellular Respiration Read this: Digestive enzymes are protein-based biological catalysts that play important roles in our lives. They help
Name: Date: Per: Enzymes Review & Using text to understand ATP & Cellular Respiration Read this: Digestive enzymes are protein-based biological catalysts that play important roles in our lives. They help
2.5 Dehydration Synthesis and Hydrolysis Activity Name Date Block
 2.5 Dehydration Synthesis and Hydrolysis Activity Name Date Block Instructions: Complete the printout of this activity sheet with your lab partner. Show your work to your instructor when completed. Switch
2.5 Dehydration Synthesis and Hydrolysis Activity Name Date Block Instructions: Complete the printout of this activity sheet with your lab partner. Show your work to your instructor when completed. Switch
Types of macromolecules. Proteins. Amino acids 9/15/2010. Carbohydrates. Lipids. Proteins. Nucleic acids
 Types of macromolecules Carbohydrates Lipids Proteins Nucleic acids Proteins Chief building blocks of life 1000s of proteins Lots of different functions, but all built the same way & from the same raw
Types of macromolecules Carbohydrates Lipids Proteins Nucleic acids Proteins Chief building blocks of life 1000s of proteins Lots of different functions, but all built the same way & from the same raw
3. Hydrogen bonds form between which atoms? Between an electropositive hydrogen and an electronegative N, O or F.
 Chemistry of Life Answers 1. Differentiate between an ionic and covalent bond. Provide an example for each. Ionic: occurs between metals and non-metals, e.g., NaCl Covalent: occurs between two non-metals;
Chemistry of Life Answers 1. Differentiate between an ionic and covalent bond. Provide an example for each. Ionic: occurs between metals and non-metals, e.g., NaCl Covalent: occurs between two non-metals;
OVERVIEW OF ENERGY AND METABOLISM
 Biochemistry 5. Bio-Energetics & ATP 5.1) ADP, ATP and Cellular Respiration OVERVIEW OF ENERGY AND METABOLISM 1. The food we eat, (carbohydrates/ glucose /sugar, lipids/fat, proteins), are our only source
Biochemistry 5. Bio-Energetics & ATP 5.1) ADP, ATP and Cellular Respiration OVERVIEW OF ENERGY AND METABOLISM 1. The food we eat, (carbohydrates/ glucose /sugar, lipids/fat, proteins), are our only source
P ROGRESSIONS: P EER-LED TEAM LEARNING
 The Workshop Project Newsletter P ROGRESSIONS: P EER-LED TEAM LEARNING Volume 7, Issue 3 Spring 2006 Module 4: Metabolism and Cellular Respiration Nichole McDaniel, Ph.D. I. Introduction One of the characteristics
The Workshop Project Newsletter P ROGRESSIONS: P EER-LED TEAM LEARNING Volume 7, Issue 3 Spring 2006 Module 4: Metabolism and Cellular Respiration Nichole McDaniel, Ph.D. I. Introduction One of the characteristics
Bio 12 Important Organic Compounds: Biological Molecules NOTES Name:
 Bio 12 Important Organic Compounds: Biological Molecules NOTES Name: Many molecules of life are.(means many molecules joined together) Monomers: that exist individually Polymers: Large organic molecules
Bio 12 Important Organic Compounds: Biological Molecules NOTES Name: Many molecules of life are.(means many molecules joined together) Monomers: that exist individually Polymers: Large organic molecules
Unit 7 ~ Learning Guide
 Unit 7 ~ Learning Guide Name: INSTRUCTIONS Complete the following notes and questions as you work through the related lessons. You are required to have this package completed BEFORE you write your unit
Unit 7 ~ Learning Guide Name: INSTRUCTIONS Complete the following notes and questions as you work through the related lessons. You are required to have this package completed BEFORE you write your unit
Chapter 2: The Chemical Level of. Organization. Copyright 2009, John Wiley & Sons, Inc.
 Chapter 2: Organization The Chemical Level of Question Of the following functions, the major propose of RNA is to A. Function in the synthesis of protein. B. Transmit genetic information to offspring.
Chapter 2: Organization The Chemical Level of Question Of the following functions, the major propose of RNA is to A. Function in the synthesis of protein. B. Transmit genetic information to offspring.
What is a Biomolecule?
 Biology Unit 03 What is a Biomolecule? Organic molecule made by living organisms Consists mostly of carbon (C), hydrogen (H), and oxygen (O) But wait What is an Organic Molecule? Organic Molecules: Contain
Biology Unit 03 What is a Biomolecule? Organic molecule made by living organisms Consists mostly of carbon (C), hydrogen (H), and oxygen (O) But wait What is an Organic Molecule? Organic Molecules: Contain
October 13, Biochemistry.notebook. Nov 10 12:03 AM. Nov 22 9:45 AM. Nov 22 8:57 AM. Nov 22 8:34 AM. Aim: What are the molecules of life?
 Aim: What are the molecules of life? LE1 & 3 11/22/10 LE3 11/22 Class Notes Do Now: List the elements & compounds cycled through ecosystems. Homework: Read pp. 59 63 P. 63 # 1,2,3,4,5 Vocabulary: Carbohydrate,
Aim: What are the molecules of life? LE1 & 3 11/22/10 LE3 11/22 Class Notes Do Now: List the elements & compounds cycled through ecosystems. Homework: Read pp. 59 63 P. 63 # 1,2,3,4,5 Vocabulary: Carbohydrate,
Chapter 1-2 Review Assignment
 Class: Date: Chapter 1-2 Review Assignment Multiple Choice dentify the choice that best completes the statement or answers the question. Corn seedlings A student wanted to design an investigation to see
Class: Date: Chapter 1-2 Review Assignment Multiple Choice dentify the choice that best completes the statement or answers the question. Corn seedlings A student wanted to design an investigation to see
Bioenergetics. Finding adequate sources of energy is a constant challenge for all living organisms, including this bear.
 33 Bioenergetics Finding adequate sources of energy is a constant challenge for all living organisms, including this bear. Introduction to General, Organic, and Biochemistry, 10e John Wiley & Sons, Inc
33 Bioenergetics Finding adequate sources of energy is a constant challenge for all living organisms, including this bear. Introduction to General, Organic, and Biochemistry, 10e John Wiley & Sons, Inc
9. At about 0 C., most enzymes are (1.) inactive (2.) active (3.) destroyed (4.) replicated
 Study Guide 1. Which of the following enzymes would digest a fat? (1.) sucrase (2.) fatase (3.) protease (4.) lipase 2. At high temperatures, the rate of enzyme action decreases because the increased heat
Study Guide 1. Which of the following enzymes would digest a fat? (1.) sucrase (2.) fatase (3.) protease (4.) lipase 2. At high temperatures, the rate of enzyme action decreases because the increased heat
Cellular Respiration
 Cellular Respiration 1. To perform cell work, cells require energy. a. A cell does three main kinds of work: i. Mechanical work, such as the beating of cilia, contraction of muscle cells, and movement
Cellular Respiration 1. To perform cell work, cells require energy. a. A cell does three main kinds of work: i. Mechanical work, such as the beating of cilia, contraction of muscle cells, and movement
MIDDLETOWN HIGH SCHOOL SOUTH BIOLOGY
 MIDDLETOWN HIGH SCHOOL SOUTH BIOLOGY BOOKLET 10 NAME: CLASS: 1 S.Tagore Middletown South High School March 2013 LEARNING OUTCOMES The role and production of ATP (a) Importance, role and structure of ATP
MIDDLETOWN HIGH SCHOOL SOUTH BIOLOGY BOOKLET 10 NAME: CLASS: 1 S.Tagore Middletown South High School March 2013 LEARNING OUTCOMES The role and production of ATP (a) Importance, role and structure of ATP
D. glycerol and fatty acids 4. Which is an example of an inorganic compound?
 Name: ate: 1. Glucose and maltose are classified as organic compounds because they are both 3. Which process is most directly responsible for the production of O 2 in these sugar solutions?. carbon-containing
Name: ate: 1. Glucose and maltose are classified as organic compounds because they are both 3. Which process is most directly responsible for the production of O 2 in these sugar solutions?. carbon-containing
Chapter Three (Biochemistry)
 Chapter Three (Biochemistry) 1 SECTION ONE: CARBON COMPOUNDS CARBON BONDING All compounds can be classified in two broad categories: organic compounds and inorganic compounds. Organic compounds are made
Chapter Three (Biochemistry) 1 SECTION ONE: CARBON COMPOUNDS CARBON BONDING All compounds can be classified in two broad categories: organic compounds and inorganic compounds. Organic compounds are made
OCR (A) Biology GCSE. Topic 1: Cell Level Systems
 OCR (A) Biology GCSE Topic 1: Cell Level Systems Notes (Content in bold is for higher tier only) Cell structures Microscopes (1.1a and c) Light (optical) microscopes The specimen is placed onto a slide,
OCR (A) Biology GCSE Topic 1: Cell Level Systems Notes (Content in bold is for higher tier only) Cell structures Microscopes (1.1a and c) Light (optical) microscopes The specimen is placed onto a slide,
WHY IS THIS IMPORTANT?
 CHAPTER 3 ESSENTIALS OF METABOLISM WHY IS THIS IMPORTANT? It is important to have a basic understanding of metabolism because it governs the survival and growth of microorganisms The growth of microorganisms
CHAPTER 3 ESSENTIALS OF METABOLISM WHY IS THIS IMPORTANT? It is important to have a basic understanding of metabolism because it governs the survival and growth of microorganisms The growth of microorganisms
Chapter 6. Metabolism & Enzymes. AP Biology
 Chapter 6. Metabolism & Enzymes Flow of energy through life Life is built on chemical reactions Chemical reactions of life Metabolism forming bonds between molecules dehydration synthesis anabolic reactions
Chapter 6. Metabolism & Enzymes Flow of energy through life Life is built on chemical reactions Chemical reactions of life Metabolism forming bonds between molecules dehydration synthesis anabolic reactions
Higher Biology. Unit 2: Metabolism and Survival Topic 2: Respiration. Page 1 of 25
 Higher Biology Unit 2: Metabolism and Survival Topic 2: Respiration Page 1 of 25 Sub Topic: Respiration I can state that: All living cells carry out respiration. ATP is the energy currency of the cell
Higher Biology Unit 2: Metabolism and Survival Topic 2: Respiration Page 1 of 25 Sub Topic: Respiration I can state that: All living cells carry out respiration. ATP is the energy currency of the cell
Chapter 2 The Chemistry of Life Part 2
 Chapter 2 The Chemistry of Life Part 2 Carbohydrates are Polymers of Monosaccharides Three different ways to represent a monosaccharide Carbohydrates Carbohydrates are sugars and starches and provide
Chapter 2 The Chemistry of Life Part 2 Carbohydrates are Polymers of Monosaccharides Three different ways to represent a monosaccharide Carbohydrates Carbohydrates are sugars and starches and provide
AP Biology Protein Structure and Enzymes
 AP Biology Protein Structure and Enzymes Connection to the Nitrogen-cycle Amino acids (protein) Nucleic acids (RNA and DNA) ATP 78% 1. Assimilation of nitrate by photosynthetic eukaryotes 2. Nitrogen fixation
AP Biology Protein Structure and Enzymes Connection to the Nitrogen-cycle Amino acids (protein) Nucleic acids (RNA and DNA) ATP 78% 1. Assimilation of nitrate by photosynthetic eukaryotes 2. Nitrogen fixation
Notes 2-4. Chemical Reactions and Enzymes
 Notes 2-4 Chemical Reactions and Enzymes Chemical Reaction: A process that changes one set of chemicals into another set of chemicals Reactants: Elements entered into the reaction Products: Elements or
Notes 2-4 Chemical Reactions and Enzymes Chemical Reaction: A process that changes one set of chemicals into another set of chemicals Reactants: Elements entered into the reaction Products: Elements or
2.2 Properties of Water
 2.2 Properties of Water I. Water s unique properties allow life to exist on Earth. A. Life depends on hydrogen bonds in water. B. Water is a polar molecule. 1. Polar molecules have slightly charged regions
2.2 Properties of Water I. Water s unique properties allow life to exist on Earth. A. Life depends on hydrogen bonds in water. B. Water is a polar molecule. 1. Polar molecules have slightly charged regions
Enzymes. Ch 3: Macromolecules
 Enzymes Ch 3: Macromolecules Living things use different chemical reactions to get the energy needed for life Chemical Reactions Reactants = substance that is changed Products = new substance that forms
Enzymes Ch 3: Macromolecules Living things use different chemical reactions to get the energy needed for life Chemical Reactions Reactants = substance that is changed Products = new substance that forms
Analysis & Interpretation. Analysis Questions answer questions on a separate sheet of paper. Name(s): Period: Date:
 Name(s): Period: Date: Dehydration Synthesis and Hydrolysis The chemical reactions that bond together macromolecules are similar and require water. When macromolecules are consumed, they must be broken
Name(s): Period: Date: Dehydration Synthesis and Hydrolysis The chemical reactions that bond together macromolecules are similar and require water. When macromolecules are consumed, they must be broken
Biology 2201 Unit 1 Matter & Energy for Life
 Biology 2201 Unit 1 Matter & Energy for Life 3.3 Cellular Respiration 3.4 The Carbon Cycle What is cellular respiration? Cellular respiration all of the chemical reactions needed to break down (metabolize)
Biology 2201 Unit 1 Matter & Energy for Life 3.3 Cellular Respiration 3.4 The Carbon Cycle What is cellular respiration? Cellular respiration all of the chemical reactions needed to break down (metabolize)
Carbon. Has four valence electrons Can bond with many elements. Can bond to other carbon atoms. Hydrogen, Oxygen, Phosphorus, Sulfur, and Nitrogen
 Organic Compounds Carbon Has four valence electrons Can bond with many elements Hydrogen, Oxygen, Phosphorus, Sulfur, and Nitrogen Can bond to other carbon atoms Gives carbon the ability to form chains
Organic Compounds Carbon Has four valence electrons Can bond with many elements Hydrogen, Oxygen, Phosphorus, Sulfur, and Nitrogen Can bond to other carbon atoms Gives carbon the ability to form chains
1. What substance could be represented by the letter X in the diagram below?
 1. What substance could be represented by the letter X in the diagram below? A) carbohydrates B) ozone C) carbon dioxide D) water 2. Base your answer to the following question on the diagram below. For
1. What substance could be represented by the letter X in the diagram below? A) carbohydrates B) ozone C) carbon dioxide D) water 2. Base your answer to the following question on the diagram below. For
Aerobic vs Anaerobic Respiration. 1. Glycolysis 2. Oxidation of Pyruvate and Krebs Cycle
 CELLULAR RESPIRATION Student Packet SUMMARY ALL LIVING SYSTEMS REQUIRE CONSTANT INPUT OF FREE ENERGY Cellular respiration is a catabolic pathway in which glucose and other organic fuels (such as starch,
CELLULAR RESPIRATION Student Packet SUMMARY ALL LIVING SYSTEMS REQUIRE CONSTANT INPUT OF FREE ENERGY Cellular respiration is a catabolic pathway in which glucose and other organic fuels (such as starch,
LAB 3: Biomolecules and Digestion
 Page 3.1 LAB 3: Biomolecules and Digestion Food taken into our bodies must first be broken down by mechanical and chemical digestion before it can be absorbed and used as an energy source. The chemical
Page 3.1 LAB 3: Biomolecules and Digestion Food taken into our bodies must first be broken down by mechanical and chemical digestion before it can be absorbed and used as an energy source. The chemical
Cellular Respiration
 Cellular Respiration C 6 H 12 O 6 + 6O 2 -----> 6CO 2 + 6H 2 0 + energy (heat and ATP) 1. Energy Capacity to move or change matter Forms of energy are important to life include Chemical, radiant (heat
Cellular Respiration C 6 H 12 O 6 + 6O 2 -----> 6CO 2 + 6H 2 0 + energy (heat and ATP) 1. Energy Capacity to move or change matter Forms of energy are important to life include Chemical, radiant (heat
Cell Compounds and Biological Molecules. Biology 12 Unit 2 Cell Compounds and Biological Molecules Inquiry into Life pages 20-44
 Cell Compounds and Biological Molecules Biology 12 Unit 2 Cell Compounds and Biological Molecules Inquiry into Life pages 20-44 Basic Chemistry Matter anything that has mass and volume Element comprises
Cell Compounds and Biological Molecules Biology 12 Unit 2 Cell Compounds and Biological Molecules Inquiry into Life pages 20-44 Basic Chemistry Matter anything that has mass and volume Element comprises
CHAPTER 5 MICROBIAL METABOLISM
 CHAPTER 5 MICROBIAL METABOLISM I. Catabolic and Anabolic Reactions A. Metabolism - The sum of all chemical reactions within a living cell either releasing or requiring energy. (Overhead) Fig 5.1 1. Catabolism
CHAPTER 5 MICROBIAL METABOLISM I. Catabolic and Anabolic Reactions A. Metabolism - The sum of all chemical reactions within a living cell either releasing or requiring energy. (Overhead) Fig 5.1 1. Catabolism
Review for Test #1: Biochemistry
 Review for Test #1: Biochemistry 1. Know and understand the definitions and meanings of the following terms. Be able to write complete definitions for the terms in BOLD: Biology triglyceride metabolism
Review for Test #1: Biochemistry 1. Know and understand the definitions and meanings of the following terms. Be able to write complete definitions for the terms in BOLD: Biology triglyceride metabolism
AP Biology. Metabolism & Enzymes
 Metabolism & Enzymes From food webs to the life of a cell energy energy energy Flow of energy through life Life is built on chemical reactions transforming energy from one form to another organic molecules
Metabolism & Enzymes From food webs to the life of a cell energy energy energy Flow of energy through life Life is built on chemical reactions transforming energy from one form to another organic molecules
Chapter 8.4, 8.5. Enzymes. AP Biology
 Chapter 8.4, 8.5 Enzymes Activation energy Breaking down large molecules requires an initial input of energy activation energy large biomolecules are stable must absorb energy to break bonds cellulose
Chapter 8.4, 8.5 Enzymes Activation energy Breaking down large molecules requires an initial input of energy activation energy large biomolecules are stable must absorb energy to break bonds cellulose
Ms. Golub & Ms. Sahar Date: Unit 2- Test #1
 Name Ms. Golub & Ms. Sahar Date: Unit 2- Test #1 1. The interaction between guard cells and a leaf opening would not be involved in A) diffusion of carbon dioxide B) maintaining homeostasis C) heterotrophic
Name Ms. Golub & Ms. Sahar Date: Unit 2- Test #1 1. The interaction between guard cells and a leaf opening would not be involved in A) diffusion of carbon dioxide B) maintaining homeostasis C) heterotrophic
Copy into Note Packet and Return to Teacher Section 3 Chemistry of Cells
 Copy into Note Packet and Return to Teacher Section 3 Chemistry of Cells Objectives Summarize the characteristics of organic compounds. Compare the structures and function of different types of biomolecules.
Copy into Note Packet and Return to Teacher Section 3 Chemistry of Cells Objectives Summarize the characteristics of organic compounds. Compare the structures and function of different types of biomolecules.
Unit 2 - Characteristics of Living Things
 Living Environment Answer Key to Practice Exam- Parts A and B-1 1. A fully functioning enzyme molecule is arranged in a complex three-dimensional shape. This shape determines the A) specific type of molecule
Living Environment Answer Key to Practice Exam- Parts A and B-1 1. A fully functioning enzyme molecule is arranged in a complex three-dimensional shape. This shape determines the A) specific type of molecule
Chapter 8 Microbial Metabolism: The Chemical Crossroads of Life
 Chapter 8 Microbial Metabolism: The Chemical Crossroads of Life / Building Your Knowledge 1) What are the two branches of metabolism? a. b. Which branch synthesizes large molecules from small subunits?
Chapter 8 Microbial Metabolism: The Chemical Crossroads of Life / Building Your Knowledge 1) What are the two branches of metabolism? a. b. Which branch synthesizes large molecules from small subunits?
Chemistry Regents Review A. A B. B C. C D. D
 hemistry Regents Review Name: ate: 1. Nitrogenous wastes result from the metabolism of 4. Which sugar solution was the first to liberate a measurable volume of O 2?. amino acids. glucose molecules. fatty
hemistry Regents Review Name: ate: 1. Nitrogenous wastes result from the metabolism of 4. Which sugar solution was the first to liberate a measurable volume of O 2?. amino acids. glucose molecules. fatty
Organic Molecules. 8/27/2004 Mr. Davenport 1
 Organic Molecules 8/27/2004 Mr. Davenport 1 Carbohydrates Commonly called sugars and starches Consist of C, H, O with H:O ration 2:1 Usually classified as to sugar units Monosaccharide are single sugar
Organic Molecules 8/27/2004 Mr. Davenport 1 Carbohydrates Commonly called sugars and starches Consist of C, H, O with H:O ration 2:1 Usually classified as to sugar units Monosaccharide are single sugar
3.7 CELLULAR RESPIRATION. How are these two images related?
 3.7 CELLULAR RESPIRATION How are these two images related? CELLULAR RESPIRATION Cellular respiration is the process whereby the body converts the energy that we get from food (glucose) into an energy form
3.7 CELLULAR RESPIRATION How are these two images related? CELLULAR RESPIRATION Cellular respiration is the process whereby the body converts the energy that we get from food (glucose) into an energy form
The building blocks for this molecule are A) amino acids B) simple sugars C) fats D) molecular bases
 1. Base your answer to the following question on the diagram below and on your knowledge of biology. The diagram represents a portion of a starch molecule. The building blocks for this molecule are A)
1. Base your answer to the following question on the diagram below and on your knowledge of biology. The diagram represents a portion of a starch molecule. The building blocks for this molecule are A)
Biochemistry.notebook. October 18, Oct 4 10:25 AM. Nov 23 8:14 AM. Aim: What are the molecules that make up living organisms?
 Aim: What are the molecules that make up living organisms? Do Now: Locate Oxygen, carbon, Nitrogen, Hydrogen, and water on the Periodic Table : Study for test Thursday Read pp. 51 54 Copy & answer p. 54
Aim: What are the molecules that make up living organisms? Do Now: Locate Oxygen, carbon, Nitrogen, Hydrogen, and water on the Periodic Table : Study for test Thursday Read pp. 51 54 Copy & answer p. 54
Organic Molecules. 1. The structural formulas shown represent certain organic compounds found in living cells.
 Name: ate: 1. The structural formulas shown represent certain organic compounds found in living cells. 1. (1) () (3) Which formula represents a monosaccharide? (4) (5). 1.. 3. 5. Which formula represents
Name: ate: 1. The structural formulas shown represent certain organic compounds found in living cells. 1. (1) () (3) Which formula represents a monosaccharide? (4) (5). 1.. 3. 5. Which formula represents
GRU3L1 Metabolism & Enzymes. AP Biology
 GRU3L1 Metabolism & Enzymes From food webs to the life of a cell energy energy energy Flow of energy through life Life is built on chemical reactions u transforming energy from one form to organic molecules
GRU3L1 Metabolism & Enzymes From food webs to the life of a cell energy energy energy Flow of energy through life Life is built on chemical reactions u transforming energy from one form to organic molecules
Unit 2: Metabolic Processes
 How is energy obtained biologically? Recall: Red Ox Reactions Unit 2: Metabolic Processes Oxidation Is the chief mechanism by which chemical potential energy is released This energy comes from reduced
How is energy obtained biologically? Recall: Red Ox Reactions Unit 2: Metabolic Processes Oxidation Is the chief mechanism by which chemical potential energy is released This energy comes from reduced
Chapter 5-7, 10. Read P , , and
 Chapter 5-7, 10 Read P. 75-82, 91-100, 107-117 and 173-185 Introduction to Metabolism and Enzymes Catabolic reactions (also called catabolism ) break down larger, more complex molecules into smaller molecules
Chapter 5-7, 10 Read P. 75-82, 91-100, 107-117 and 173-185 Introduction to Metabolism and Enzymes Catabolic reactions (also called catabolism ) break down larger, more complex molecules into smaller molecules
The chemistry of life
 The chemistry of life All living organisms are comprised of organic molecules. Organic molecules contain CARBON and HYDROGEN which is not true of inorganic molecules. Carbon is central to life on Earth
The chemistry of life All living organisms are comprised of organic molecules. Organic molecules contain CARBON and HYDROGEN which is not true of inorganic molecules. Carbon is central to life on Earth
Section 9 2 The Krebs Cycle and Electron Transport (pages )
 Section 9 2 The Krebs Cycle and Electron Transport (pages 226 232) This section describes what happens during the second stage of cellular respiration, called the Krebs cycle. It also explains how high-energy
Section 9 2 The Krebs Cycle and Electron Transport (pages 226 232) This section describes what happens during the second stage of cellular respiration, called the Krebs cycle. It also explains how high-energy
Concept 8.3: ATP powers cellular work by coupling exergonic reactions to endergonic reactions
 Concept 8.3: ATP powers cellular work by coupling exergonic reactions to endergonic reactions A cell does three main kinds of work: Chemical Transport Mechanical To do work, cells manage energy resources
Concept 8.3: ATP powers cellular work by coupling exergonic reactions to endergonic reactions A cell does three main kinds of work: Chemical Transport Mechanical To do work, cells manage energy resources
BACKGROUND INFORMATION:
 BIOLOGY 12 ENZYMES NAME: BACKGROUND INFORMATION: Energy: is defined as the ability to do or bring about change. A living organism must constantly perform work in order to maintain its organization, to
BIOLOGY 12 ENZYMES NAME: BACKGROUND INFORMATION: Energy: is defined as the ability to do or bring about change. A living organism must constantly perform work in order to maintain its organization, to
Activity: Biologically Important Molecules
 Activity: Biologically Important Molecules AP Biology Introduction We have already seen in our study of biochemistry that the molecules that comprise living things are carbon-based, and that they are thought
Activity: Biologically Important Molecules AP Biology Introduction We have already seen in our study of biochemistry that the molecules that comprise living things are carbon-based, and that they are thought
Examples. Chapter 8. Metabolism & Enzymes. Flow of energy through life. Examples. Chemical reactions of life. Chemical reactions & energy
 WH Examples dehydration synthesis Chapter 8 Metabolism & Enzymes + H 2 O hydrolysis + H 2 O Flow of energy through life Life is built on chemical reactions Examples dehydration synthesis hydrolysis 2005-2006
WH Examples dehydration synthesis Chapter 8 Metabolism & Enzymes + H 2 O hydrolysis + H 2 O Flow of energy through life Life is built on chemical reactions Examples dehydration synthesis hydrolysis 2005-2006
A cell s metabolism is all the organism s chemical reactions. Metabolism manages the material and energy resources of the cell.
 Enzymes Metabolism Metabolism A cell s metabolism is all the organism s chemical reactions. Metabolism manages the material and energy resources of the cell. Energy is the capacity to do work. Metabolism
Enzymes Metabolism Metabolism A cell s metabolism is all the organism s chemical reactions. Metabolism manages the material and energy resources of the cell. Energy is the capacity to do work. Metabolism
An organic catalysts that enhances the speed or likelihood of a bio-chemical reaction by lowering the energy of activation.
 Micro 260 Fall 2009 Name: Allan Keys Tools: You may use your notes and or book for this assignment 1) What is an enzyme? (4 pts) An organic catalysts that enhances the speed or likelihood of a bio-chemical
Micro 260 Fall 2009 Name: Allan Keys Tools: You may use your notes and or book for this assignment 1) What is an enzyme? (4 pts) An organic catalysts that enhances the speed or likelihood of a bio-chemical
Physiology 12. Metabolism. Metabolism. Cellular metabolism. The synthesis and Breakdown of organic molecules required for cell structure and function
 Physiology 12 Cellular metabolism Germann Ch3 Metabolism The synthesis and Breakdown of organic molecules required for cell structure and function Metabolism Anabolism = Synthesis Catabolism = Breaking
Physiology 12 Cellular metabolism Germann Ch3 Metabolism The synthesis and Breakdown of organic molecules required for cell structure and function Metabolism Anabolism = Synthesis Catabolism = Breaking
I. ROLE OF CARBON IN ORGANISMS:
 Name: Period: Date: I. ROLE OF CARBON IN ORGANISMS: = compounds that contain carbon Ex: Carbohydrates, lipids, proteins = compounds that DO NOT contain carbon Ex: Vitamins, minerals, water Carbon forms
Name: Period: Date: I. ROLE OF CARBON IN ORGANISMS: = compounds that contain carbon Ex: Carbohydrates, lipids, proteins = compounds that DO NOT contain carbon Ex: Vitamins, minerals, water Carbon forms
LAB 6 Fermentation & Cellular Respiration
 LAB 6 Fermentation & Cellular Respiration INTRODUCTION The cells of all living organisms require energy to keep themselves alive and fulfilling their roles. Where does this energy come from? The answer
LAB 6 Fermentation & Cellular Respiration INTRODUCTION The cells of all living organisms require energy to keep themselves alive and fulfilling their roles. Where does this energy come from? The answer
Good Afternoon! 11/30/18
 Good Afternoon! 11/30/18 1. The term polar refers to a molecule that. A. Is cold B. Has two of the same charges C. Has two opposing charges D. Contains a hydrogen bond 2. Electrons on a water molecule
Good Afternoon! 11/30/18 1. The term polar refers to a molecule that. A. Is cold B. Has two of the same charges C. Has two opposing charges D. Contains a hydrogen bond 2. Electrons on a water molecule
