CHAPTER 3 Amino Acids, Peptides, Proteins
|
|
|
- Winifred Greer
- 5 years ago
- Views:
Transcription
1 CHAPTER 3 Amino Acids, Peptides, Proteins Learning goals: Structure and naming of amino acids Structure and properties of peptides Ionization behavior of amino acids and peptides Methods to characterize peptides and proteins
2 Proteins: Main Agents of Biological Function Catalysis enolase (in the glycolytic pathway) DNA polymerase (in DNA replication) Transport hemoglobin (transports O 2 in the blood) lactose permease (transports lactose across the cell membrane) Structure collagen (connective tissue) keratin (hair, nails, feathers, horns) Motion myosin (muscle tissue) actin (muscle tissue, cell motility)
3 Proteins serve a wide range of biological functions
4 Amino Acids: Building Blocks of Protein Proteins are linear heteropolymers of α-amino acids Amino acids have properties that are well-suited to carry out a variety of biological functions Capacity to polymerize Useful acid-base properties Varied physical properties Varied chemical functionality
5 Amino acids share many features, differing only at the R substituent
6 Most α-amino acids are chiral The α-carbon always has four substituents and is tetrahedral All (except proline) have: an acidic carboxyl group a basic amino group an α-hydrogen connected to the α-carbon The fourth substituent (R) is unique In glycine, the fourth substituent is also hydrogen
7 Amino Acids: Atom Naming Organic nomenclature: start from one end Biochemical designation: start from α-carbon and go down the R-group
8 All amino acids are chiral (except glycine) Proteins only contain L amino acids
9 Amino Acids: Classification Common amino acids can be placed in five basic groups depending on their R substituents: Nonpolar, aliphatic (7) Aromatic (3) Polar, uncharged (5) Positively charged (3) Negatively charged (2)
10
11 These amino acid side chains absorb UV light at nm
12 These amino acids side chains can form hydrogen bonds. Cysteine can form disulfide bonds.
13
14
15 Uncommon Amino Acids in Proteins Not incorporated by ribosomes except for Selenocysteine Arise by post-translational modifications of proteins Reversible modifications, especially phosphorylation, are important in regulation and signaling
16 Modified Amino Acids Found in Proteins
17 Reversible Modifications of Amino Acids
18 Ionization of Amino Acids At acidic ph, the carboxyl group is protonated and the amino acid is in the cationic form. At neutral ph, the carboxyl group is deprotonated but the amino group is protonated. The net charge is zero; such ions are called Zwitterions. At alkaline ph, the amino group is neutral NH 2 and the amino acid is in the anionic form.
19 Cation Zwitterion Anion
20 Chemical Environment Affects pk a Values α-carboxy group is much more acidic than in carboxylic acids α-amino group is slightly less basic than in amines
21 Amino acids can act as buffers Amino acids with uncharged side chains, such as glycine, have two pk a values: The pk a of the α-carboxyl group is 2.34 The pk a of the α-amino group is 9.6 It can act as a buffer in two ph regimes.
22 Buffer Regions
23 Amino acids carry a net charge of zero at a specific ph (the pi) Zwitterions predominate at ph values between the pk a values of the amino and carboxyl groups For amino acids without ionizable side chains, the Isoelectric Point (equivalence point, pi) is pk 1 + pk pi = 2 2 At this point, the net charge is zero AA is least soluble in water AA does not migrate in electric field
24 Ionizable side chains can show up in titration curves Ionizable side chains can be also titrated Titration curves are now more complex pk a values are discernable if two pk a values are more than two ph units apart Why is the side chain pk a so much higher?
25
26 How to Calculate the pi When the Side Chain is Ionizable Identify species that carries a net zero charge Identify pk a value that defines the acid strength of this zwitterion: (pk 2 ) Identify pk a value that defines the base strength of this zwitterion: (pk 1 ) Take the average of these two pk a values What is the pi of histidine?
CHAPTER 3 Amino Acids, Peptides, Proteins
 CHAPTER 3 Amino Acids, Peptides, Proteins Learning goals: Structure and naming of amino acids Structure and properties of peptides Ionization behavior of amino acids and peptides Methods to characterize
CHAPTER 3 Amino Acids, Peptides, Proteins Learning goals: Structure and naming of amino acids Structure and properties of peptides Ionization behavior of amino acids and peptides Methods to characterize
Amino acids. (Foundation Block) Dr. Essa Sabi
 Amino acids (Foundation Block) Dr. Essa Sabi Learning outcomes What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino acid configuration. Non-standard amino
Amino acids (Foundation Block) Dr. Essa Sabi Learning outcomes What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino acid configuration. Non-standard amino
Chemical Nature of the Amino Acids. Table of a-amino Acids Found in Proteins
 Chemical Nature of the Amino Acids All peptides and polypeptides are polymers of alpha-amino acids. There are 20 a- amino acids that are relevant to the make-up of mammalian proteins (see below). Several
Chemical Nature of the Amino Acids All peptides and polypeptides are polymers of alpha-amino acids. There are 20 a- amino acids that are relevant to the make-up of mammalian proteins (see below). Several
Biomolecules: amino acids
 Biomolecules: amino acids Amino acids Amino acids are the building blocks of proteins They are also part of hormones, neurotransmitters and metabolic intermediates There are 20 different amino acids in
Biomolecules: amino acids Amino acids Amino acids are the building blocks of proteins They are also part of hormones, neurotransmitters and metabolic intermediates There are 20 different amino acids in
Amino Acids and Proteins Hamad Ali Yaseen, PhD MLS Department, FAHS, HSC, KU Biochemistry 210 Chapter 22
 Amino Acids and Proteins Hamad Ali Yaseen, PhD MLS Department, FAHS, HSC, KU Hamad.ali@hsc.edu.kw Biochemistry 210 Chapter 22 Importance of Proteins Main catalysts in biochemistry: enzymes (involved in
Amino Acids and Proteins Hamad Ali Yaseen, PhD MLS Department, FAHS, HSC, KU Hamad.ali@hsc.edu.kw Biochemistry 210 Chapter 22 Importance of Proteins Main catalysts in biochemistry: enzymes (involved in
Ionization of amino acids
 Amino Acids 20 common amino acids there are others found naturally but much less frequently Common structure for amino acid COOH, -NH 2, H and R functional groups all attached to the a carbon Ionization
Amino Acids 20 common amino acids there are others found naturally but much less frequently Common structure for amino acid COOH, -NH 2, H and R functional groups all attached to the a carbon Ionization
3. AMINO ACID AND PEPTIDES
 3. AMINO ACID AND PEPTIDES 3.1 Amino Acids and Peptides General structure - Only 20 amino-acids are found in proteins - Amino group and carboxyl group - α-carbon and side chain group 3.1 Amino Acids and
3. AMINO ACID AND PEPTIDES 3.1 Amino Acids and Peptides General structure - Only 20 amino-acids are found in proteins - Amino group and carboxyl group - α-carbon and side chain group 3.1 Amino Acids and
Chapter 3: Amino Acids and Peptides
 Chapter 3: Amino Acids and Peptides BINF 6101/8101, Spring 2018 Outline 1. Overall amino acid structure 2. Amino acid stereochemistry 3. Amino acid sidechain structure & classification 4. Non-standard
Chapter 3: Amino Acids and Peptides BINF 6101/8101, Spring 2018 Outline 1. Overall amino acid structure 2. Amino acid stereochemistry 3. Amino acid sidechain structure & classification 4. Non-standard
Lecture 3: 8/24. CHAPTER 3 Amino Acids
 Lecture 3: 8/24 CHAPTER 3 Amino Acids 1 Chapter 3 Outline 2 Amino Acid Are Biomolecules and their Atoms Can Be Visualized by Two Different Ways 1) Fischer projections: Two dimensional representation of
Lecture 3: 8/24 CHAPTER 3 Amino Acids 1 Chapter 3 Outline 2 Amino Acid Are Biomolecules and their Atoms Can Be Visualized by Two Different Ways 1) Fischer projections: Two dimensional representation of
Amino acids. Dr. Mamoun Ahram Summer semester,
 Amino acids Dr. Mamoun Ahram Summer semester, 2017-2018 Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure (Chiral carbon) The amino acids that occur in
Amino acids Dr. Mamoun Ahram Summer semester, 2017-2018 Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure (Chiral carbon) The amino acids that occur in
Metabolic Classification of the Amino Acids
 Metabolic Classification of the Amino Acids *Essential and Non-essential * Glucogenic and Ketogenic 1 Essential Amino Acids Of the 20 amino acids that make up proteins 10 of them can be synthesized by
Metabolic Classification of the Amino Acids *Essential and Non-essential * Glucogenic and Ketogenic 1 Essential Amino Acids Of the 20 amino acids that make up proteins 10 of them can be synthesized by
PHAR3316 Pharmacy biochemistry Exam #2 Fall 2010 KEY
 1. How many protons is(are) lost when the amino acid Asparagine is titrated from its fully protonated state to a fully deprotonated state? A. 0 B. 1 * C. 2 D. 3 E. none Correct Answer: C (this question
1. How many protons is(are) lost when the amino acid Asparagine is titrated from its fully protonated state to a fully deprotonated state? A. 0 B. 1 * C. 2 D. 3 E. none Correct Answer: C (this question
O H 2 N. α H. Chapter 4 - Amino Acids
 hapter 4 - Amino Acids Introduction Several amino acids were produced in the electrical discharge in the reducing, primordial atmosphere that gave rise to the first biomolecules (see chapter 1). The importance
hapter 4 - Amino Acids Introduction Several amino acids were produced in the electrical discharge in the reducing, primordial atmosphere that gave rise to the first biomolecules (see chapter 1). The importance
Structure of -amino acids. Stereoisomers of -amino acids. All amino acids in proteins are L-amino acids, except for glycine, which is achiral.
 amino acids Any of a large number of compounds found in living cells that contain carbon, oxygen, hydrogen, and nitrogen, and join together to form proteins. Amino acids contain a basic amino group (NH
amino acids Any of a large number of compounds found in living cells that contain carbon, oxygen, hydrogen, and nitrogen, and join together to form proteins. Amino acids contain a basic amino group (NH
Biochemistry Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology Kharagpur. Lecture -02 Amino Acids II
 Biochemistry Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology Kharagpur Lecture -02 Amino Acids II Ok, we start off with the discussion on amino acids. (Refer Slide Time: 00:48)
Biochemistry Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology Kharagpur Lecture -02 Amino Acids II Ok, we start off with the discussion on amino acids. (Refer Slide Time: 00:48)
Understand how protein is formed by amino acids
 Identify between fibrous and globular proteins Understand how protein is formed by amino acids Describe the structure of proteins using specific examples Functions of proteins Fibrous proteins Globular
Identify between fibrous and globular proteins Understand how protein is formed by amino acids Describe the structure of proteins using specific examples Functions of proteins Fibrous proteins Globular
Amino acids. Dr. Mamoun Ahram and Dr. Diala Abu-Hassan Summer semester,
 Amino acids Dr. Mamoun Ahram and Dr. Diala Abu-Hassan Summer semester, 2017-2018 dr.abuhassand@gmail.com Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure
Amino acids Dr. Mamoun Ahram and Dr. Diala Abu-Hassan Summer semester, 2017-2018 dr.abuhassand@gmail.com Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure
Protiens and Amino Acids 2
 Protiens and Amino Acids 2 By Alaa J. Mahrath, Biochemistry Department / College of Medicine/ Babylon University 2 Amino Acid Stereoisomers Protiens and Amino Acids All the a-amino acids except for glycine
Protiens and Amino Acids 2 By Alaa J. Mahrath, Biochemistry Department / College of Medicine/ Babylon University 2 Amino Acid Stereoisomers Protiens and Amino Acids All the a-amino acids except for glycine
Amino Acids االحماض األمينية
 Amino Acids االحماض األمينية Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Cell Structure Amino Acids The study of
Amino Acids االحماض األمينية Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Cell Structure Amino Acids The study of
Chemistry 20 Chapter 14 Proteins
 Chapter 14 Proteins Proteins: all proteins in humans are polymers made up from 20 different amino acids. Proteins provide structure in membranes, build cartilage, muscles, hair, nails, and connective tissue
Chapter 14 Proteins Proteins: all proteins in humans are polymers made up from 20 different amino acids. Proteins provide structure in membranes, build cartilage, muscles, hair, nails, and connective tissue
AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS
 AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine
AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine
2018/9/24 AMINO ACIDS. Important. 436 Notes Original slides. 438 notes Extra information
 2018/9/24 AMINO ACIDS Important. 436 Notes Original slides. 438 notes Extra information Objectives: What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino
2018/9/24 AMINO ACIDS Important. 436 Notes Original slides. 438 notes Extra information Objectives: What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino
BCH302 [Practical] 1
![BCH302 [Practical] 1 BCH302 [Practical] 1](/thumbs/78/76808179.jpg) BCH302 [Practical] 1 Amino acids play a central role: i. As building blocks of proteins. ii. As intermediates in metabolism, converted to specialized products. There are 20 natural amino acids that are
BCH302 [Practical] 1 Amino acids play a central role: i. As building blocks of proteins. ii. As intermediates in metabolism, converted to specialized products. There are 20 natural amino acids that are
AMINO ACIDS. Qualitative Tests
 AMINO ACIDS Qualitative Tests AMINO ACIDS Amino acid play A central role as building block of proteins. Amino acids also converted to specialized products. More than 300 different amino acids have been
AMINO ACIDS Qualitative Tests AMINO ACIDS Amino acid play A central role as building block of proteins. Amino acids also converted to specialized products. More than 300 different amino acids have been
Lecture 4. Grouping Amino Acid 7/1/10. Proteins. Amino Acids. Where Are Proteins Located. Nonpolar Amino Acids
 Proteins Lecture 4 Proteins - Composition of Proteins (Amino Acids) Chapter 21 ection 1-6! Proteins are compounds of high molar mass consisting almost entirely of amino acid chain(s)! Molar masses range
Proteins Lecture 4 Proteins - Composition of Proteins (Amino Acids) Chapter 21 ection 1-6! Proteins are compounds of high molar mass consisting almost entirely of amino acid chain(s)! Molar masses range
Model 2: Aldohexoses, aldopentoses, ketohexoses and ketopentoses
 Model 1: D and L Sugars CHEM1405 Worksheet 13: Sugars and Amino Acids The simplest sugar is glyceraldehyde. This is a chiral molecule and the two enantiomers are shown opposite with wedges and dashes and
Model 1: D and L Sugars CHEM1405 Worksheet 13: Sugars and Amino Acids The simplest sugar is glyceraldehyde. This is a chiral molecule and the two enantiomers are shown opposite with wedges and dashes and
Molecular Biology. general transfer: occurs normally in cells. special transfer: occurs only in the laboratory in specific conditions.
 Chapter 9: Proteins Molecular Biology replication general transfer: occurs normally in cells transcription special transfer: occurs only in the laboratory in specific conditions translation unknown transfer:
Chapter 9: Proteins Molecular Biology replication general transfer: occurs normally in cells transcription special transfer: occurs only in the laboratory in specific conditions translation unknown transfer:
Reactions and amino acids structure & properties
 Lecture 2: Reactions and amino acids structure & properties Dr. Sameh Sarray Hlaoui Common Functional Groups Common Biochemical Reactions AH + B A + BH Oxidation-Reduction A-H + B-OH + energy ª A-B + H
Lecture 2: Reactions and amino acids structure & properties Dr. Sameh Sarray Hlaoui Common Functional Groups Common Biochemical Reactions AH + B A + BH Oxidation-Reduction A-H + B-OH + energy ª A-B + H
Molecular and Cellular Biology. 2. Bio-Chemical Foundations & Key Molecules of a Cell
 Molecular and Cellular Biology 2. Bio-Chemical Foundations & Key Molecules of a Cell Prof. Dr. Klaus Heese Cell Function & Chemistry Interaction 1 Molecular Bonds Define Cellular Functions Interactions
Molecular and Cellular Biology 2. Bio-Chemical Foundations & Key Molecules of a Cell Prof. Dr. Klaus Heese Cell Function & Chemistry Interaction 1 Molecular Bonds Define Cellular Functions Interactions
Properties of amino acids in proteins
 Properties of amino acids in proteins one of the primary roles of DNA (but far from the only one!!!) is to code for proteins A typical bacterium builds thousands types of proteins, all from ~20 amino acids
Properties of amino acids in proteins one of the primary roles of DNA (but far from the only one!!!) is to code for proteins A typical bacterium builds thousands types of proteins, all from ~20 amino acids
Amino Acids. Lecture 4: Margaret A. Daugherty. Fall Swiss-prot database: How many proteins? From where?
 Lecture 4: Amino Acids Margaret A. Daugherty Fall 2004 Swiss-prot database: How many proteins? From where? 1986 Use http://us.expasy.org to get to swiss-prot database Proteins are the workhorses of the
Lecture 4: Amino Acids Margaret A. Daugherty Fall 2004 Swiss-prot database: How many proteins? From where? 1986 Use http://us.expasy.org to get to swiss-prot database Proteins are the workhorses of the
Human Biochemistry Option B
 Human Biochemistry Option B A look ahead... Your body has many functions to perform every day: Structural support, genetic information, communication, energy supply, metabolism Right now, thousands of
Human Biochemistry Option B A look ahead... Your body has many functions to perform every day: Structural support, genetic information, communication, energy supply, metabolism Right now, thousands of
Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids.
 Today we begin our discussion of the structure and properties of proteins. Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids. Conjugated proteins contain
Today we begin our discussion of the structure and properties of proteins. Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids. Conjugated proteins contain
UNIT 2 Amino acids and Proteins
 UNIT 2 Amino acids and Proteins Significance of Proteins 1. Keep the cells and tissues growing, renewing and mending 2. Take part in some kinds of important physiological activities 3. Oxidation and supply
UNIT 2 Amino acids and Proteins Significance of Proteins 1. Keep the cells and tissues growing, renewing and mending 2. Take part in some kinds of important physiological activities 3. Oxidation and supply
1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids
 Amino acids 1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids 5-To understand amino acids synthesis Amino
Amino acids 1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids 5-To understand amino acids synthesis Amino
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges
 Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 9. AMINO ACIDS, PEPTIDES AND
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 9. AMINO ACIDS, PEPTIDES AND
Proteins with similar functions often have αα s can act as both weak acids & weak bases similar αα sequences
 Chapter 5: Amino Acids, Peptides & Proteins Amino acids share common functional groups Amino, Carboxyl & H bonded to C Distinct chemistry of s result of side chains Proteins & polypeptides are polymers
Chapter 5: Amino Acids, Peptides & Proteins Amino acids share common functional groups Amino, Carboxyl & H bonded to C Distinct chemistry of s result of side chains Proteins & polypeptides are polymers
7/11/17. Cell Function & Chemistry. Molecular and Cellular Biology. 2. Bio-Chemical Foundations & Key Molecules of a Cell
 Molecular and Cellular Biology Cell Function & Chemistry 2. Bio-Chemical Foundations & Key Molecules of a Cell Prof. Dr. Klaus Heese Interaction Molecular Bonds Define Cellular Functions Water H 2 O Interactions
Molecular and Cellular Biology Cell Function & Chemistry 2. Bio-Chemical Foundations & Key Molecules of a Cell Prof. Dr. Klaus Heese Interaction Molecular Bonds Define Cellular Functions Water H 2 O Interactions
Defense Antibodies, interferons produced in response to infection Coordination and growth (signaling) Hormones (e.g. insulin, growth hormone) Communic
 Proteins Chapter 3 An Introduction to Organic Compounds Most varied of the biomolecules Also called polypeptides Make up more than half the dry weight of cells Categorized by function Lecture 3: Proteins
Proteins Chapter 3 An Introduction to Organic Compounds Most varied of the biomolecules Also called polypeptides Make up more than half the dry weight of cells Categorized by function Lecture 3: Proteins
Tala Saleh. Tamer Barakat. Diala Abu-Hassan
 13 Tala Saleh Tamer Barakat Diala Abu-Hassan Biochemical application of monosodium glutamate MSG MSG is a glutamic acid derivative, used as a flavor enhancer in Asian food. Chinese restaurant syndrome
13 Tala Saleh Tamer Barakat Diala Abu-Hassan Biochemical application of monosodium glutamate MSG MSG is a glutamic acid derivative, used as a flavor enhancer in Asian food. Chinese restaurant syndrome
Proteins are sometimes only produced in one cell type or cell compartment (brain has 15,000 expressed proteins, gut has 2,000).
 Lecture 2: Principles of Protein Structure: Amino Acids Why study proteins? Proteins underpin every aspect of biological activity and therefore are targets for drug design and medicinal therapy, and in
Lecture 2: Principles of Protein Structure: Amino Acids Why study proteins? Proteins underpin every aspect of biological activity and therefore are targets for drug design and medicinal therapy, and in
Amino acids. You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures.
 Amino acids You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures. If you wanna make any classification in the world, you have to find what
Amino acids You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures. If you wanna make any classification in the world, you have to find what
استاذ الكيمياءالحيوية
 قسم الكيمياء الحيوية د.دولت على سالمه استاذ الكيمياءالحيوية ٢٠١٥-٢٠١٤ الرمز الكودي : ٥١٢ المحاضرة األولى ١ Content : Definition of proteins Definition of amino acids Definition of peptide bond General
قسم الكيمياء الحيوية د.دولت على سالمه استاذ الكيمياءالحيوية ٢٠١٥-٢٠١٤ الرمز الكودي : ٥١٢ المحاضرة األولى ١ Content : Definition of proteins Definition of amino acids Definition of peptide bond General
Different types of proteins. The structure and properties of amino acids. Formation of peptide bonds.
 Introduction to proteins and amino acids Different types of proteins. The structure and properties of amino acids. Formation of peptide bonds. Introduction We tend to think of protein as a mass noun: a
Introduction to proteins and amino acids Different types of proteins. The structure and properties of amino acids. Formation of peptide bonds. Introduction We tend to think of protein as a mass noun: a
Biochemistry - I. Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I
 Biochemistry - I Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I Hello, welcome to the course Biochemistry 1 conducted by me Dr. S Dasgupta,
Biochemistry - I Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I Hello, welcome to the course Biochemistry 1 conducted by me Dr. S Dasgupta,
BIO 311C Spring Lecture 15 Friday 26 Feb. 1
 BIO 311C Spring 2010 Lecture 15 Friday 26 Feb. 1 Illustration of a Polypeptide amino acids peptide bonds Review Polypeptide (chain) See textbook, Fig 5.21, p. 82 for a more clear illustration Folding and
BIO 311C Spring 2010 Lecture 15 Friday 26 Feb. 1 Illustration of a Polypeptide amino acids peptide bonds Review Polypeptide (chain) See textbook, Fig 5.21, p. 82 for a more clear illustration Folding and
130327SCH4U_biochem April 09, 2013
 Option B: B1.1 ENERGY Human Biochemistry If more energy is taken in from food than is used up, weight gain will follow. Similarly if more energy is used than we supply our body with, weight loss will occur.
Option B: B1.1 ENERGY Human Biochemistry If more energy is taken in from food than is used up, weight gain will follow. Similarly if more energy is used than we supply our body with, weight loss will occur.
Chapter 21 Lecture Outline
 Chapter 21 Lecture Outline Amino Acids, Proteins, and Enzymes! Introduction! Proteins are biomolecules that contain many amide bonds, formed by joining amino acids. Prepared by Andrea D. Leonard University
Chapter 21 Lecture Outline Amino Acids, Proteins, and Enzymes! Introduction! Proteins are biomolecules that contain many amide bonds, formed by joining amino acids. Prepared by Andrea D. Leonard University
9/6/2011. Amino Acids. C α. Nonpolar, aliphatic R groups
 Amino Acids Side chains (R groups) vary in: size shape charge hydrogen-bonding capacity hydrophobic character chemical reactivity C α Nonpolar, aliphatic R groups Glycine (Gly, G) Alanine (Ala, A) Valine
Amino Acids Side chains (R groups) vary in: size shape charge hydrogen-bonding capacity hydrophobic character chemical reactivity C α Nonpolar, aliphatic R groups Glycine (Gly, G) Alanine (Ala, A) Valine
Gentilucci, Amino Acids, Peptides, and Proteins. Peptides and proteins are polymers of amino acids linked together by amide bonds CH 3
 Amino Acids Peptides and proteins are polymers of amino acids linked together by amide bonds Aliphatic Side-Chain Amino Acids - - H CH glycine alanine 3 proline valine CH CH 3 - leucine - isoleucine CH
Amino Acids Peptides and proteins are polymers of amino acids linked together by amide bonds Aliphatic Side-Chain Amino Acids - - H CH glycine alanine 3 proline valine CH CH 3 - leucine - isoleucine CH
Ali Yaghi مها أبو عجمية. Mamon Ahram
 13 Ali Yaghi مها أبو عجمية Mamon Ahram 0 P a g e This sheet is the last sheet we are supposed to study for the midterm exam. Let s begin. Biological significance of amino acids: Amino acids are used for
13 Ali Yaghi مها أبو عجمية Mamon Ahram 0 P a g e This sheet is the last sheet we are supposed to study for the midterm exam. Let s begin. Biological significance of amino acids: Amino acids are used for
Chemistry B11 Chapters 16 Proteins and Enzymes
 Chapters 16 Proteins and Enzymes Proteins: all proteins in humans are polymers made up from 20 different amino acids. Proteins provide structure in membranes, build cartilage, muscles, hair, nails, and
Chapters 16 Proteins and Enzymes Proteins: all proteins in humans are polymers made up from 20 different amino acids. Proteins provide structure in membranes, build cartilage, muscles, hair, nails, and
COO - l. H 3 N C a H l R 1
 COO - l + H 3 N C a H l R 1 Amino acids There are 20 standard amino acids. All proteins are built from the same amino acids. The most important criteria for classification is affinity to water: hydrophilic
COO - l + H 3 N C a H l R 1 Amino acids There are 20 standard amino acids. All proteins are built from the same amino acids. The most important criteria for classification is affinity to water: hydrophilic
Chapter 2 Biosynthesis of Enzymes
 Chapter 2 Biosynthesis of Enzymes 2.1 Basic Enzyme Chemistry 2.1.1 Amino Acids An amino acid is a molecule that has the following formula: The central carbon atom covalently bonded by amino, carboxyl,
Chapter 2 Biosynthesis of Enzymes 2.1 Basic Enzyme Chemistry 2.1.1 Amino Acids An amino acid is a molecule that has the following formula: The central carbon atom covalently bonded by amino, carboxyl,
Lecture 11 AMINO ACIDS AND PROTEINS
 Lecture 11 AMINO ACIDS AND PROTEINS The word "Protein" was coined by J.J. Berzelius in 1838 and was derived from the Greek word "Proteios" meaning the first rank. Proteins are macromolecular polymers composed
Lecture 11 AMINO ACIDS AND PROTEINS The word "Protein" was coined by J.J. Berzelius in 1838 and was derived from the Greek word "Proteios" meaning the first rank. Proteins are macromolecular polymers composed
Proteins and their structure
 Proteins and their structure Proteins are the most abundant biological macromolecules, occurring in all cells and all parts of cells. Proteins also occur in great variety; thousands of different kinds,
Proteins and their structure Proteins are the most abundant biological macromolecules, occurring in all cells and all parts of cells. Proteins also occur in great variety; thousands of different kinds,
WHY IS THIS IMPORTANT?
 CHAPTER 2 FUNDAMENTAL CHEMISTRY FOR MICROBIOLOGY WHY IS THIS IMPORTANT? An understanding of chemistry is essential to understand cellular structure and function, which are paramount for your understanding
CHAPTER 2 FUNDAMENTAL CHEMISTRY FOR MICROBIOLOGY WHY IS THIS IMPORTANT? An understanding of chemistry is essential to understand cellular structure and function, which are paramount for your understanding
AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy
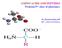 AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy Dr. Basima Sadiq Jaff PhD. Clinical Biochemistry MEDICAL AND BIOLOGICAL IMPORTANCE 1. building blocks of proteins. Some amino acids are found in
AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy Dr. Basima Sadiq Jaff PhD. Clinical Biochemistry MEDICAL AND BIOLOGICAL IMPORTANCE 1. building blocks of proteins. Some amino acids are found in
(5) 1. List five unusual properties of water resulting from its hydrogen bonded structure
 BCH 4053 June 1, 2001 Points HOUR TEST 1 NAME (5) 1. List five unusual properties of water resulting from its hydrogen bonded structure. Page Points 1 2 3 4 5 Total (5) 2. Draw a diagram to show how water
BCH 4053 June 1, 2001 Points HOUR TEST 1 NAME (5) 1. List five unusual properties of water resulting from its hydrogen bonded structure. Page Points 1 2 3 4 5 Total (5) 2. Draw a diagram to show how water
I) Choose the best answer: 1- All of the following amino acids are neutral except: a) glycine. b) threonine. c) lysine. d) proline. e) leucine.
 1- All of the following amino acids are neutral except: a) glycine. b) threonine. c) lysine. d) proline. e) leucine. 2- The egg white protein, ovalbumin, is denatured in a hard-boiled egg. Which of the
1- All of the following amino acids are neutral except: a) glycine. b) threonine. c) lysine. d) proline. e) leucine. 2- The egg white protein, ovalbumin, is denatured in a hard-boiled egg. Which of the
AMINO ACIDS AND PROTEINS. HLeeYu Jsuico Junsay Department of Chemistry School of Science and Engineering Ateneo de Manila University
 AMINO ACIDS AND PROTEINS HLeeYu Jsuico Junsay Department of Chemistry School of Science and Engineering Ateneo de Manila University 1 Proteins serves as the cell s machinery as well as an organism s other
AMINO ACIDS AND PROTEINS HLeeYu Jsuico Junsay Department of Chemistry School of Science and Engineering Ateneo de Manila University 1 Proteins serves as the cell s machinery as well as an organism s other
Ch5: Macromolecules. Proteins
 Ch5: Macromolecules Proteins Essential Knowledge 4.A.1 The subcomponents of biological molecules and their sequence determine the properties of that molecule A. Structure and function of polymers are derived
Ch5: Macromolecules Proteins Essential Knowledge 4.A.1 The subcomponents of biological molecules and their sequence determine the properties of that molecule A. Structure and function of polymers are derived
! Proteins are involved functionally in almost everything: " Receptor Proteins - Respond to external stimuli. " Storage Proteins - Storing amino acids
 Proteins Most structurally & functionally diverse group! Proteins are involved functionally in almost everything: Proteins Multi-purpose molecules 2007-2008 Enzymatic proteins - Speed up chemical reactions!
Proteins Most structurally & functionally diverse group! Proteins are involved functionally in almost everything: Proteins Multi-purpose molecules 2007-2008 Enzymatic proteins - Speed up chemical reactions!
Charges on amino acids and proteins. ph 1. ph 7. Acidic side chains: glutamate and aspartate
 harges on amino acids and proteins Acidic side chains: glutamate and aspartate A A- + + + - + Basic side chains: arginine, lysine & histidine Glycine @ p 1 B+ B + + + The amino group, pka 9.6 3 N+ The
harges on amino acids and proteins Acidic side chains: glutamate and aspartate A A- + + + - + Basic side chains: arginine, lysine & histidine Glycine @ p 1 B+ B + + + The amino group, pka 9.6 3 N+ The
Organic Chemistry - Problem Drill 23: Amino Acids, Peptides, and Proteins
 rganic Chemistry - Problem Drill 23: Amino Acids, Peptides, and Proteins No. 1 of 10 1. Which amino acid does not contain a chiral center? (A) Serine (B) Proline (C) Alanine (D) Phenylalanine (E) Glycine
rganic Chemistry - Problem Drill 23: Amino Acids, Peptides, and Proteins No. 1 of 10 1. Which amino acid does not contain a chiral center? (A) Serine (B) Proline (C) Alanine (D) Phenylalanine (E) Glycine
CHM333 LECTURE 6: 1/25/12 SPRING 2012 Professor Christine Hrycyna AMINO ACIDS II: CLASSIFICATION AND CHEMICAL CHARACTERISTICS OF EACH AMINO ACID:
 AMINO ACIDS II: CLASSIFICATION AND CHEMICAL CHARACTERISTICS OF EACH AMINO ACID: - The R group side chains on amino acids are VERY important. o Determine the properties of the amino acid itself o Determine
AMINO ACIDS II: CLASSIFICATION AND CHEMICAL CHARACTERISTICS OF EACH AMINO ACID: - The R group side chains on amino acids are VERY important. o Determine the properties of the amino acid itself o Determine
THE UNIVERSITY OF MANITOBA. DATE: Oct. 22, 2002 Midterm EXAMINATION. PAPER NO.: PAGE NO.: 1of 6 DEPARTMENT & COURSE NO.: 2.277/60.
 PAPER NO.: PAGE NO.: 1of 6 GENERAL INSTRUCTIONS You must mark the answer sheet with pencil (not pen). Put your name and enter your student number on the answer sheet. The examination consists of multiple
PAPER NO.: PAGE NO.: 1of 6 GENERAL INSTRUCTIONS You must mark the answer sheet with pencil (not pen). Put your name and enter your student number on the answer sheet. The examination consists of multiple
BCMB 3100 Chapter 3 (part 1)
 BCMB 3100 Chapter 3 (part 1) Diversity of protein function Complete definition of amino acids Memorize complete structure of 20 common amino acids!!! pka s of amino and carboxyl groups Amino acids with
BCMB 3100 Chapter 3 (part 1) Diversity of protein function Complete definition of amino acids Memorize complete structure of 20 common amino acids!!! pka s of amino and carboxyl groups Amino acids with
Page 1 of 5 Biochemistry I Fall 2017 Practice for Exam2 Dr. Stone Name
 Page 1 of 5 Biochemistry I Fall 2017 Practice for Exam2 Dr. Stone ame o answers will be provided. ere are some constants and equations that may be useful: K a = [+][A-]/[A] p = pka + log [A-]/[A] K a for
Page 1 of 5 Biochemistry I Fall 2017 Practice for Exam2 Dr. Stone ame o answers will be provided. ere are some constants and equations that may be useful: K a = [+][A-]/[A] p = pka + log [A-]/[A] K a for
CHY2026: General Biochemistry. Unit 4:Amino Acid Chemistry
 CHY2026: General Biochemistry Unit 4:Amino Acid Chemistry http://www.hcc.mnscu.edu/programs/dept/chem/v.27/amino_acid_structure_2.jpg Hydrogen Amino group Carboxyl Group Unique side chain (R-group) R Central
CHY2026: General Biochemistry Unit 4:Amino Acid Chemistry http://www.hcc.mnscu.edu/programs/dept/chem/v.27/amino_acid_structure_2.jpg Hydrogen Amino group Carboxyl Group Unique side chain (R-group) R Central
9/11/18. Cell Function & Chemistry. Molecular and Cellular Biology. 2. Bio-Chemical Foundations & Key Molecules of a Cell
 Molecular and Cellular Biology Cell Function & Chemistry 2. Bio-Chemical Foundations & Key Molecules of a Cell Prof. Dr. Klaus Heese Interaction Molecular Bonds Define Cellular Functions Water H 2 O Interactions
Molecular and Cellular Biology Cell Function & Chemistry 2. Bio-Chemical Foundations & Key Molecules of a Cell Prof. Dr. Klaus Heese Interaction Molecular Bonds Define Cellular Functions Water H 2 O Interactions
Proteins. AP Biology. Proteins. Proteins. Proteins. Effect of different R groups: Nonpolar amino acids. Amino acids H C OH H R. Structure.
 2008-2009 Most structurally & functionally diverse group : involved in almost everything (pepsin, DNA polymerase) (keratin, collagen) (hemoglobin, aquaporin) (insulin & other hormones) (antibodies) (actin
2008-2009 Most structurally & functionally diverse group : involved in almost everything (pepsin, DNA polymerase) (keratin, collagen) (hemoglobin, aquaporin) (insulin & other hormones) (antibodies) (actin
Organic Compounds: Carbohydrates
 Organic Compounds: Carbohydrates Carbohydrates include sugars and starches Contain the elements C,H,O (H & O ratio like water, 2 H s to 1O), ex. glucose C 6 H 12 O 6 Word means hydrated carbon Classified
Organic Compounds: Carbohydrates Carbohydrates include sugars and starches Contain the elements C,H,O (H & O ratio like water, 2 H s to 1O), ex. glucose C 6 H 12 O 6 Word means hydrated carbon Classified
Chapter 20 and GHW#10 Questions. Proteins
 Chapter 20 and GHW#10 Questions Proteins Proteins Naturally occurring bioorganic polyamide polymers containing a sequence of various combinations of 20 amino acids. Amino acids contain the elements carbon,
Chapter 20 and GHW#10 Questions Proteins Proteins Naturally occurring bioorganic polyamide polymers containing a sequence of various combinations of 20 amino acids. Amino acids contain the elements carbon,
BCMB Chapter 3 (part1)
 Chapter 3 (with parts of 4 and 5) Amino Acids and Primary Structures of Proteins BCMB 3100 - Chapter 3 (part1) Diversity of protein function Complete definition of amino acids Memorize complete structure
Chapter 3 (with parts of 4 and 5) Amino Acids and Primary Structures of Proteins BCMB 3100 - Chapter 3 (part1) Diversity of protein function Complete definition of amino acids Memorize complete structure
Macromolecules of Life -3 Amino Acids & Proteins
 Macromolecules of Life -3 Amino Acids & Proteins Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ Amino Acids Proteins
Macromolecules of Life -3 Amino Acids & Proteins Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ Amino Acids Proteins
Biomolecules Amino Acids & Protein Chemistry
 Biochemistry Department Date: 17/9/ 2017 Biomolecules Amino Acids & Protein Chemistry Prof.Dr./ FAYDA Elazazy Professor of Biochemistry and Molecular Biology Intended Learning Outcomes ILOs By the end
Biochemistry Department Date: 17/9/ 2017 Biomolecules Amino Acids & Protein Chemistry Prof.Dr./ FAYDA Elazazy Professor of Biochemistry and Molecular Biology Intended Learning Outcomes ILOs By the end
Protein Structure and Function
 Protein Structure and Function Protein Structure Classification of Proteins Based on Components Simple proteins - Proteins containing only polypeptides Conjugated proteins - Proteins containing nonpolypeptide
Protein Structure and Function Protein Structure Classification of Proteins Based on Components Simple proteins - Proteins containing only polypeptides Conjugated proteins - Proteins containing nonpolypeptide
The Structure and Func.on of Macromolecules Proteins GRU1L6
 The Structure and Func.on of Macromolecules Proteins GRU1L6 Proteins Proteins Most structurally & functionally diverse group Function: involved in almost everything enzymes (pepsin, DNA polymerase) structure
The Structure and Func.on of Macromolecules Proteins GRU1L6 Proteins Proteins Most structurally & functionally diverse group Function: involved in almost everything enzymes (pepsin, DNA polymerase) structure
Proteins. Proteins. Proteins. Proteins. Effect of different R groups: Nonpolar amino acids. Amino acids H C OH H R. Multipurpose molecules.
 Multipurpose molecules 2008-2009 Most structurally & functionally diverse group Function: involved in almost everything enzymes (pepsin, DNA polymerase) structure (keratin, collagen) carriers & transport
Multipurpose molecules 2008-2009 Most structurally & functionally diverse group Function: involved in almost everything enzymes (pepsin, DNA polymerase) structure (keratin, collagen) carriers & transport
Chemistry 121 Winter 17
 Chemistry 121 Winter 17 Introduction to Organic Chemistry and Biochemistry Instructor Dr. Upali Siriwardane (Ph.D. Ohio State) E-mail: upali@latech.edu Office: 311 Carson Taylor Hall ; Phone: 318-257-4941;
Chemistry 121 Winter 17 Introduction to Organic Chemistry and Biochemistry Instructor Dr. Upali Siriwardane (Ph.D. Ohio State) E-mail: upali@latech.edu Office: 311 Carson Taylor Hall ; Phone: 318-257-4941;
T.Y.B.Sc. SemIII Paper VI CH-336C Introduction to Biochemistry and Molecular Biology. Lecture Notes
 Lecture Notes Chapter-I: Amino Acids and Proteins 1. Introduction Amino acids are biologically important organic compounds containing amine (-NH 2 ) and carboxylic acid (-COOH) functional groups, usually
Lecture Notes Chapter-I: Amino Acids and Proteins 1. Introduction Amino acids are biologically important organic compounds containing amine (-NH 2 ) and carboxylic acid (-COOH) functional groups, usually
Q1: Circle the best correct answer: (15 marks)
 Q1: Circle the best correct answer: (15 marks) 1. Which one of the following incorrectly pairs an amino acid with a valid chemical characteristic a. Glycine, is chiral b. Tyrosine and tryptophan; at neutral
Q1: Circle the best correct answer: (15 marks) 1. Which one of the following incorrectly pairs an amino acid with a valid chemical characteristic a. Glycine, is chiral b. Tyrosine and tryptophan; at neutral
BIOB111 - Tutorial activity for Session 14
 BIOB111 - Tutorial activity for Session 14 General topics for week 7 Session 14 Amino acids and proteins Students review the concepts learnt and answer the selected questions from the textbook. General
BIOB111 - Tutorial activity for Session 14 General topics for week 7 Session 14 Amino acids and proteins Students review the concepts learnt and answer the selected questions from the textbook. General
Organic Molecules: Proteins
 Organic Molecules: Proteins Proteins Most structurally & functionally diverse group Function: involved in almost everything enzymes (pepsin, DNA polymerase) structure (keratin, collagen) carriers & transport
Organic Molecules: Proteins Proteins Most structurally & functionally diverse group Function: involved in almost everything enzymes (pepsin, DNA polymerase) structure (keratin, collagen) carriers & transport
MBB 694:407, 115:511. Please use BLOCK CAPITAL letters like this --- A, B, C, D, E. Not lowercase!
 MBB 694:407, 115:511 First Test Severinov/Deis Tue. Sep. 30, 2003 Name Index number (not SSN) Row Letter Seat Number This exam consists of two parts. Part I is multiple choice. Each of these 25 questions
MBB 694:407, 115:511 First Test Severinov/Deis Tue. Sep. 30, 2003 Name Index number (not SSN) Row Letter Seat Number This exam consists of two parts. Part I is multiple choice. Each of these 25 questions
PAPER No. : 16, Bioorganic and biophysical chemistry MODULE No. : 22, Mechanism of enzyme catalyst reaction (I) Chymotrypsin
 Subject Paper No and Title 16 Bio-organic and Biophysical Module No and Title 22 Mechanism of Enzyme Catalyzed reactions I Module Tag CHE_P16_M22 Chymotrypsin TABLE OF CONTENTS 1. Learning outcomes 2.
Subject Paper No and Title 16 Bio-organic and Biophysical Module No and Title 22 Mechanism of Enzyme Catalyzed reactions I Module Tag CHE_P16_M22 Chymotrypsin TABLE OF CONTENTS 1. Learning outcomes 2.
The Structure and Function of Large Biological Molecules Part 4: Proteins Chapter 5
 Key Concepts: The Structure and Function of Large Biological Molecules Part 4: Proteins Chapter 5 Proteins include a diversity of structures, resulting in a wide range of functions Proteins Enzymatic s
Key Concepts: The Structure and Function of Large Biological Molecules Part 4: Proteins Chapter 5 Proteins include a diversity of structures, resulting in a wide range of functions Proteins Enzymatic s
KEY NAME (printed very legibly) UT-EID
 BIOLOGY 311C - Brand Spring 2007 KEY NAME (printed very legibly) UT-EID EXAMINATION II Before beginning, check to be sure that this exam contains 7 pages (including front and back) numbered consecutively,
BIOLOGY 311C - Brand Spring 2007 KEY NAME (printed very legibly) UT-EID EXAMINATION II Before beginning, check to be sure that this exam contains 7 pages (including front and back) numbered consecutively,
A Chemical Look at Proteins: Workhorses of the Cell
 A Chemical Look at Proteins: Workhorses of the Cell A A Life ciences 1a Lecture otes et 4 pring 2006 Prof. Daniel Kahne Life requires chemistry 2 amino acid monomer and it is proteins that make the chemistry
A Chemical Look at Proteins: Workhorses of the Cell A A Life ciences 1a Lecture otes et 4 pring 2006 Prof. Daniel Kahne Life requires chemistry 2 amino acid monomer and it is proteins that make the chemistry
Introduction to Proteomics Dr. Sanjeeva Srivastava Department of Biosciences and Bioengineering Indian Institute of Technology - Bombay
 Introduction to Proteomics Dr. Sanjeeva Srivastava Department of Biosciences and Bioengineering Indian Institute of Technology - Bombay Lecture 01 Introduction to Amino Acids Welcome to the proteomic course.
Introduction to Proteomics Dr. Sanjeeva Srivastava Department of Biosciences and Bioengineering Indian Institute of Technology - Bombay Lecture 01 Introduction to Amino Acids Welcome to the proteomic course.
AA s are the building blocks of proteins
 Chamras Chemistry 106 Lecture otes Chapter 24: Amino Acids, Peptides, and Proteins General Formula: () n (') α-amino Acids: (n = 1) Example: Amino Acids and Proteins: Glycine Alanine Valine AA s are the
Chamras Chemistry 106 Lecture otes Chapter 24: Amino Acids, Peptides, and Proteins General Formula: () n (') α-amino Acids: (n = 1) Example: Amino Acids and Proteins: Glycine Alanine Valine AA s are the
Section 1 Proteins and Proteomics
 Section 1 Proteins and Proteomics Learning Objectives At the end of this assignment, you should be able to: 1. Draw the chemical structure of an amino acid and small peptide. 2. Describe the difference
Section 1 Proteins and Proteomics Learning Objectives At the end of this assignment, you should be able to: 1. Draw the chemical structure of an amino acid and small peptide. 2. Describe the difference
Chapter 18. Carboxylic Acids and Their Derivatives. Nucleophilic Addition-Elimination at the Acyl Carbon
 Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition-Elimination at the Acyl Carbon Carboxylic Acids Organic compounds characterized by their acidity Contains COOH group (must be at
Chapter 18 Carboxylic Acids and Their Derivatives. Nucleophilic Addition-Elimination at the Acyl Carbon Carboxylic Acids Organic compounds characterized by their acidity Contains COOH group (must be at
Proteins are linear polymers built of monomer units called amino acids. Proteins contain a wide range of functional groups.
 Chapter 2: Protein Structure and Function Proteins arevery versatile with regards to functions for the cell Uses? Proteins are linear polymers built of monomer units called amino acids. One dimensional
Chapter 2: Protein Structure and Function Proteins arevery versatile with regards to functions for the cell Uses? Proteins are linear polymers built of monomer units called amino acids. One dimensional
Page 8/6: The cell. Where to start: Proteins (control a cell) (start/end products)
 Page 8/6: The cell Where to start: Proteins (control a cell) (start/end products) Page 11/10: Structural hierarchy Proteins Phenotype of organism 3 Dimensional structure Function by interaction THE PROTEIN
Page 8/6: The cell Where to start: Proteins (control a cell) (start/end products) Page 11/10: Structural hierarchy Proteins Phenotype of organism 3 Dimensional structure Function by interaction THE PROTEIN
Chemistry 107 Exam 3 Study Guide
 Chapter 7 Chemistry 107 Exam 3 Study Guide 7.1 Recognize the aldehyde, ketone and hydroxyl (-OH) functional groups found in carbohydrates. Differentiate between mono-, di-, and polysaccharides. Master
Chapter 7 Chemistry 107 Exam 3 Study Guide 7.1 Recognize the aldehyde, ketone and hydroxyl (-OH) functional groups found in carbohydrates. Differentiate between mono-, di-, and polysaccharides. Master
AP Biology. Proteins. Proteins. Proteins. Amino acids H C OH H R. Effect of different R groups: Polar amino acids polar or charged & hydrophilic
 Most structurally & functionally diverse group : involved in almost everything enzymes (pepsin, DNA polymerase) structure (keratin, collagen) carriers & transport (, aquaporin) cell communication signals
Most structurally & functionally diverse group : involved in almost everything enzymes (pepsin, DNA polymerase) structure (keratin, collagen) carriers & transport (, aquaporin) cell communication signals
Chapter 5 Overview. Amino Acids, Peptides, and Proteins. Proteins molecular tools of life. Functions
 Chapter 5 Overview Amino Acids, Peptides, and Proteins Proteins molecular tools of life Functions n n n n n Structural cell shape, connective tissue (cartilage, bond) Catalysis enzymes Metabolic regulation
Chapter 5 Overview Amino Acids, Peptides, and Proteins Proteins molecular tools of life Functions n n n n n Structural cell shape, connective tissue (cartilage, bond) Catalysis enzymes Metabolic regulation
Lecture 5. Secondary Structure of Proteins. "-Pleated Sheet. !-Helix. Examples of Protein Structures
 econdary tructure of Proteins Lecture 5 Proteins- tructure and Properties Chapter 21 ections 7-11! There are two main aspects of 2 o structure!the type of fold or bend in the protein chain!the types of
econdary tructure of Proteins Lecture 5 Proteins- tructure and Properties Chapter 21 ections 7-11! There are two main aspects of 2 o structure!the type of fold or bend in the protein chain!the types of
