Cubicin A Guide to Dosing
|
|
|
- Angelica Woods
- 5 years ago
- Views:
Transcription
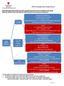
1 Cubicin A Guide to Dosing Cubicin (Daptomycin) powder for solution for injection or infusion Indications (see SmPC) 1 : Cubicin is indicated for the treatment of the following infections (see sections 4.4 and 5.1 of the SmPC) Adult and paediatric (1 to 17 years of age) patients with complicated skin and softtissue infections (cssti). Adult patients with right-sided infective endocarditis (RIE) due to Staphylococcus aureus. It is recommended that the decision to use daptomycin should take into account the antibacterial susceptibility of the organism and should be based on expert advice. See sections 4.4 and 5.1. Adult and paediatric (1 to 17 years of age) patients with Staphylococcus aureus bacteraemia (SAB). In adults, use in bacteraemia should be associated with RIE or with cssti; while in paediatric patients, use in bacteraemia should be associated with cssti. Bactericidal activity 2 against a broad range of Gram-positive bacteria 3, 4 In mixed infections where Gram-negative and/or certain types of anaerobic bacteria are suspected, Cubicin should be co-administered with appropriate antibacterial agent(s) Administered once daily Recommendations (see SmPC) Increases in plasma creatine phosphokinase (CPK) levels associated with musculoskeletal adverse events have been reported during Cubicin therapy Concomitant administration of Cubicin and other medicinal products associated with myopathy (e.g. statins, fibrates and cyclosporine) should be avoided, unless the benefit outweighs the risk CPK should be measured at baseline and at regular intervals (at least once weekly) during therapy in all patients
2 More frequent monitoring of CPK levels (e.g. every 2-3 days at least during the first two weeks of treatment) should be carried out in patients who are at higher risk of developing myopathies: o o Those with any degree renal insufficiency (creatinine clearance <80 ml/min) Patients taking other medicinal products known to be associated with myopathy Cases of interference between Cubicin and particular reagents (recombinant thromboplastin) used in some coagulation assays (Prothrombin Time [PT]; International Normalized Ratio [INR] have been reported. The interference leads to false results, with an apparent prolongation of PT and evaluation of INR Drawing samples near the time of Cubicin trough plasma concentrations may minimize the potential for erroneous results Results from the most recent studies 1, 5 in paediatric populations indicated that compared with adults, children show progressively higher daptomycin clearance and higher volume of distribution with decreasing age. Hence, higher doses will be required in children and will vary by age groups in order to produce exposures equivalent to those seen for efficacy in adults In the paediatric population, daptomycin administered at doses of 5 mg/kg (12 to 17 years), 7 mg/kg (7 to 11 years), 9 mg/kg (2 to 6 years) and 10 mg/kg (1 to < 2 years) for up to 14 days has a positive risk-benefit profile in the treatment of cssti caused by Gram-positive pathogens In the paediatric population, daptomycin administered at doses of 7 mg/kg (12 to 17 years), 9 mg/kg (7 to 11 years), or 12 mg/kg (1 to 6 years) for up to 42 days has a positive risk-benefit profile in the treatment of Staphylococcus aureus bacteraemia associated with cssti. Because higher clearance of daptomycin was observed in previous single-dose paediatric PK studies 6, 7, 8 and in the most recent paediatric studies described above 1, 5 Age-adjusted daptomycin doses were given once daily up to 14 days in order to achieve exposures equivalent to those documented in adult cssti studies Age-adjusted daptomycin doses were given once daily up to 42 days in order to achieve exposures equivalent to those documented in adult SAB study. Dosing is age-dependent and weight-dependent Both safety and efficacy results are consistent with those from adult studies and with data from the literature
3 Cubicin 4 mg/kg INDICATION cssti in adult patients without Staphylococcus aureus bacteraemia (see SmPC) DOSAGE Cubicin 4 mg/kg administered as a once-daily 2-minute intravenous (IV) injection or 30- minute IV infusion Cubicin should be reconstituted to a 50 mg/ml solution with: 350 mg vial: 7 ml of 9 mg/ml (0.9%) sodium chloride solution (injection or infusion) 500 mg vial: 10 ml of 9 mg/ml (0.9%) sodium chloride solution (injection or infusion) Volume of Cubicin 50 mg/ml solution required: Volume in ml = Bodyweight x 4/50 This volume may be injected intravenously over 2 minutes or diluted with 0.9% sodium chloride (typical volume 50 ml) for infusion over 30 minutes
4 Adjustment for Renal Impairment in Adults Indication for use cssti without S. aureus bacteraemia Creatinine clearance recommendation Comments 30 ml/min 4 mg/kg once daily <30 ml/min 4 mg/kg every 48 hours (1,2) (1) The safety and efficacy of the dose interval adjustment have not been evaluated in controlled clinical trials and the recommendation is based on pharmacokinetic studies and modelling results (2) The same dose adjustments, which are based on pharmacokinetic data in volunteers, including PK modelling results, are recommended for adult patients on haemodialysis (HD) or continuous ambulatory peritoneal dialysis. Whenever possible, Cubicin should be administered following the completion of dialysis on dialysis days Response to treatment, renal function and plasma CPK should be closely monitored in all patients with renal impairment.
5 Cubicin 6 mg/kg INDICATIONS RIE due to Staphylococcus aureus in adult patients (see SmPC) Staphylococcus aureus bacteraemia when associated with RIE or with cssti in adult patients (see SmPC) DOSAGE Cubicin 6 mg/kg administered as a once-daily 2-minute IV injection or 30-minute i.v. infusion Cubicin should be reconstituted to a 50 mg/ml solution with: 350 mg vial: 7 ml of 9 mg/ml (0.9%) sodium chloride solution (injection or infusion) 500 mg vial: 10 ml of 9 mg/ml (0.9%) sodium chloride solution (injection or infusion) Volume of Cubicin 50 mg/ml solution required: Volume in ml = Bodyweight x 6/50 This volume may be injected intravenously over 2 minutes or diluted with 0.9% sodium chloride (typical volume 50 ml) for infusion over 30 minutes
6 Adjustment in Renal Impairment in Adults Indication for use RIE or cssti associated with S. aureus bacteraemia Creatinine clearance recommendation Comments 30 ml/min 6 mg/kg once daily <30 ml/min 6 mg/kg every 48 hours (1,2) (1) The safety and efficacy of the dose interval adjustment have not been evaluated in controlled clinical trials and the recommendation is based on pharmacokinetic studies and modelling results (2) The same dose adjustments, which are based on pharmacokinetic data in volunteers including PK modelling results, are recommended for adult patients on haemodialysis (HD) or continuous ambulatory peritoneal dialysis. Whenever possible, Cubicin should be administered following the completion of dialysis on dialysis days Response to treatment, renal function and plasma CPK should be closely monitored in all patients with renal impairment.
7 Cubicin Dosing in Paediatric Patients for Treatment of cssti, without Staphylococcus aureus bacteraemia DOSAGE In the treatment of paediatric patients (1 to 17 years of age) with complicated skin and soft-tissue infections (cssti) without associated S. aureus bacteraemia, Cubicin is given by intravenous (IV) infusion over a 30 or 60-minute period depending on the age of the patient (see table below) also dosing and administration section for Paediatric Patients in the SmPC). Ages Daptomycin Infusion Time Treatment Duration 12 to 17 years 5 mg/kg once daily 30 minutes 7 to 11 years 7 mg/kg once daily 30 minutes 2 to 6 years 9 mg/kg once daily 60 minutes Up to 14 days 1 to <2 years 10 mg/kg once daily 60 minutes Cubicin Dosing in Paediatric Patients for Treatment of Staphylococcus aureus bacteraemia, when associated with cssti DOSAGE In the treatment of paediatric patients (1 to 17 years of age) with S. aureus bacteraemia when associated with complicated skin and soft-tissue infections (cssti), Cubicin is given by intravenous (IV) infusion over a 30 or 60-minute period depending on the age of the patient (see also dosing and administration section for Paediatric Patients in the SmPC): Ages Daptomycin Infusion Time Treatment Duration 12 to 17 years 7 mg/kg once daily 30 minutes 7 to 11 years 9 mg/kg once daily 30 minutes Up to 42 days 1 to 6 years 12 mg/kg once daily 60 minutes
8 Paediatric patients below the age of one year should not be given Cubicin due to the risk of potential effects on muscular, neuromuscular, and/or nervous systems (either peripheral and/or central) that were observed in neonatal dogs. The dosage regimen for CUBICIN in paediatric patients with renal impairment has not been established. Please refer to the Summary of Product Characteristics (SmPC) before prescribing Cubicin. REFERENCES 1 Clinical Study Report: An Evaluation of the Safety, Efficacy and Pharmacokinetics of Daptomycin in Paediatric Subjects Aged one to Seventeen Years with Complicated Skin and Skin Structure Infections Caused by Grampositive Pathogens (Protocol 017). 2 Kirkpatrick P, Raja A, LaBonte J, Lebbos J. Daptomycin. Nat Rev Drug Discov Dec;2(12): Rybak MJ, Hershberger E, Moldovan T, Grucz RG. In vitro activities of daptomycin, vancomycin, linezolid, and quinupristindalfopristin against Staphylococci and Enterococci, including vancomycinintermediate and -resistant strains. Antimicrob Agents Chemother Apr;44(4): Tedesco KL, Rybak MJ. Daptomycin. Pharmacotherapy Jan;24(1): Clinical Study Report, Multicenter Study: A Comparative Evaluation of the Safety and Efficacy of Daptomycin Versus Standard of Care in Paediatric Subjects One - Seventeen Years of Age with Bacteraemia Caused by Staphylococcus aureus (Protocol 005).
9 7 Clinical Study Report, Multicenter Study: An Evaluation of the Pharmacokinetic Profile and Safety of a Single of Daptomycin in Paediatric Subjects Aged Two to Six Years Who are Concurrently Receiving Standard Antibiotic Therapy for Proven or Suspected Gram-positive Infection (Protocol 023). 8 Clinical Study Report, Multicenter Study: An Evaluation of the Pharmacokinetic Profile and Safety of a Single of Daptomycin in Paediatric Subjects Aged 3 Months to Twentyfour Months Who are Concurrently Receiving Standard Antibiotic Therapy for Proven or Suspected Bacterial Infection (Protocol 018). Útg.: 10/2018
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Cubicin 350 mg powder for solution for injection or infusion Cubicin 500 mg powder for solution for injection or infusion 2.
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Cubicin 350 mg powder for solution for injection or infusion Cubicin 500 mg powder for solution for injection or infusion 2.
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 18 October 2006
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 18 October 2006 CUBICIN 350 mg (daptomycin), powder for perfusion solution Box of 1 bottle (CIP code: 567 219-3) CUBICIN
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 18 October 2006 CUBICIN 350 mg (daptomycin), powder for perfusion solution Box of 1 bottle (CIP code: 567 219-3) CUBICIN
Daptomycin (CUBICIN) 500 mg Lyophilized Powder for IV Infusion. CUBICIN contains daptomycin, a cyclic lipopeptide antibacterial agent.
 DAPTOMYCIN 0500778/2 CUBICIN TM 500 mg Lyophilized Powder for IV Infusion Antibacterial/Cyclic Lipopeptide Antibiotic 1. Product Description 1.1. Name of Pharmaceutical Product Daptomycin (CUBICIN) 500
DAPTOMYCIN 0500778/2 CUBICIN TM 500 mg Lyophilized Powder for IV Infusion Antibacterial/Cyclic Lipopeptide Antibiotic 1. Product Description 1.1. Name of Pharmaceutical Product Daptomycin (CUBICIN) 500
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT CUBICIN, 350 mg powder for concentrate for solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial contains
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT CUBICIN, 350 mg powder for concentrate for solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial contains
Trust Guideline for the Use of Parenteral Vancomycin and Teicoplanin in Adults
 A clinical guideline recommended for use: In: By: For: Division responsible for document: Key words: Names of document authors: Job titles of document authors: Name of document author s Line Manager: Job
A clinical guideline recommended for use: In: By: For: Division responsible for document: Key words: Names of document authors: Job titles of document authors: Name of document author s Line Manager: Job
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Cubicin 350 mg powder for solution for injection or infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial contains
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Cubicin 350 mg powder for solution for injection or infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial contains
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Daptomycin Hospira 350 mg powder for solution for injection/infusion Daptomycin Hospira 500 mg powder for solution for injection/infusion
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Daptomycin Hospira 350 mg powder for solution for injection/infusion Daptomycin Hospira 500 mg powder for solution for injection/infusion
12 to 17 years. 7 to 11 years
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use CUBICIN safely and effectively. See full prescribing information for CUBICIN. CUBICIN (daptomycin
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use CUBICIN safely and effectively. See full prescribing information for CUBICIN. CUBICIN (daptomycin
(telavancin) Healthcare Professional s Guide. Version 2, 4 November 2014
 VIBATIV (telavancin) Healthcare Professional s Guide Version 2, 4 November 2014 1 Table of Contents Introduction... 3 About Vibativ / Therapeutic indications... 3 Antimicrobial spectrum of activity for
VIBATIV (telavancin) Healthcare Professional s Guide Version 2, 4 November 2014 1 Table of Contents Introduction... 3 About Vibativ / Therapeutic indications... 3 Antimicrobial spectrum of activity for
Clinical Guidelines for Use of Antibiotics. VANCOMYCIN (Adult)
 VANCOMYCIN (Adult) Please always prescribe VANCOMYCIN in the Variable Dose Antibiotic section of the EPMA SUPPLEMENTARY drug chart (and add a placeholder on the electronic drug chart). 1 Background Vancomycin
VANCOMYCIN (Adult) Please always prescribe VANCOMYCIN in the Variable Dose Antibiotic section of the EPMA SUPPLEMENTARY drug chart (and add a placeholder on the electronic drug chart). 1 Background Vancomycin
Full title of guideline INTRAVENOUS VANCOMYCIN PRESCRIBING AND MONITORING GUIDELINE FOR ADULT PATIENTS. control
 Full title of guideline Author: Contact Name and Job Title Division and specialty Scope Explicit definition of patient group to which it applies (e.g. inclusion and exclusion criteria, diagnosis) Changes
Full title of guideline Author: Contact Name and Job Title Division and specialty Scope Explicit definition of patient group to which it applies (e.g. inclusion and exclusion criteria, diagnosis) Changes
Therapeutic drug monitoring of daptomycin: a retrospective monocentric analysis
 Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2015 Therapeutic drug monitoring of daptomycin: a retrospective monocentric
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2015 Therapeutic drug monitoring of daptomycin: a retrospective monocentric
Mesporin TM. Ceftriaxone sodium. Rapid onset, sustained action, for a broad spectrum of infections
 Ceftriaxone sodium Rapid onset, sustained action, for a broad spectrum of infections 1, 2, 3 Antibiotic with a broad spectrum of activity Broad spectrum of activity against gram-positive* and gram-negative
Ceftriaxone sodium Rapid onset, sustained action, for a broad spectrum of infections 1, 2, 3 Antibiotic with a broad spectrum of activity Broad spectrum of activity against gram-positive* and gram-negative
ROSOBAC-1GM / ROSOBAC-FORT
 ROSOBAC-1GM / ROSOBAC-FORT ROSOBAC - 1GM. COMPOSITION : Each vial contains Sterile Cefoperazone Sodium IP Eq. to Anhydrous Cefoperazone - Sterile Sulbactam Sodium USP Eq. to Anhydrous Sulbactam - ROSOBAC
ROSOBAC-1GM / ROSOBAC-FORT ROSOBAC - 1GM. COMPOSITION : Each vial contains Sterile Cefoperazone Sodium IP Eq. to Anhydrous Cefoperazone - Sterile Sulbactam Sodium USP Eq. to Anhydrous Sulbactam - ROSOBAC
CONTRAINDICATIONS Known hypersensitivity to daptomycin (4)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use CUBICIN safely and effectively. See full prescribing information for CUBICIN. CUBICIN (daptomycin
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use CUBICIN safely and effectively. See full prescribing information for CUBICIN. CUBICIN (daptomycin
Joint Trust Guideline for the Use of Intravenous Vancomycin in Paediatrics
 A clinical guideline recommended For use in: All clinical areas where vancomycin is prescribed for Children aged 1 month to 16 years By: All medical, nursing, pharmacy, microbiology and phlebotomy paediatric
A clinical guideline recommended For use in: All clinical areas where vancomycin is prescribed for Children aged 1 month to 16 years By: All medical, nursing, pharmacy, microbiology and phlebotomy paediatric
PRODUCT INFORMATION TARGOCID
 NAME OF THE MEDICINE Non-proprietary Name teicoplanin PRODUCT INFORMATION TARGOCID DESCRIPTION Teicoplanin is a glycopeptide-antibiotic produced by Actinoplanes teichomyceticus. It is presented as a sterile,
NAME OF THE MEDICINE Non-proprietary Name teicoplanin PRODUCT INFORMATION TARGOCID DESCRIPTION Teicoplanin is a glycopeptide-antibiotic produced by Actinoplanes teichomyceticus. It is presented as a sterile,
EDUCATIONAL COMMENTARY VANCOMYCIN MONITORING
 EDUCATIONAL COMMENTARY VANCOMYCIN MONITORING Commentary provided by: Julie Hall, MHS, MT (ASCP) Assistant Dean, College of Health Professions Assistant Professor, Medical Laboratory Science Grand Valley
EDUCATIONAL COMMENTARY VANCOMYCIN MONITORING Commentary provided by: Julie Hall, MHS, MT (ASCP) Assistant Dean, College of Health Professions Assistant Professor, Medical Laboratory Science Grand Valley
Pharmacokinetics of daptomycin in a patient with severe renal failure not under dialysis
 AAC Accepts, published online ahead of print on 1 April 2013 Antimicrob. Agents Chemother. doi:10.1128/aac.00230-13 Copyright 2013, American Society for Microbiology. All Rights Reserved. 1 Pharmacokinetics
AAC Accepts, published online ahead of print on 1 April 2013 Antimicrob. Agents Chemother. doi:10.1128/aac.00230-13 Copyright 2013, American Society for Microbiology. All Rights Reserved. 1 Pharmacokinetics
PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION
 PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION PR CUBICIN /CUBICIN RF (Daptomycin for Injection) Lyophilized Powder for Solution, For Intravenous Use Only 10 ml vial, 500 mg/vial Antibacterial
PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION PR CUBICIN /CUBICIN RF (Daptomycin for Injection) Lyophilized Powder for Solution, For Intravenous Use Only 10 ml vial, 500 mg/vial Antibacterial
Daptomycin for injection is not indicated for the treatment. Daptomycin for injection is not indicated for the treatment of
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use DAPTOMYCIN FOR INJECTION safely and effectively. See full prescribing information for DAPTOMYCIN
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use DAPTOMYCIN FOR INJECTION safely and effectively. See full prescribing information for DAPTOMYCIN
Drug dosing in patients with acute kidney injury
 Drug dosing in patients with acute kidney injury They don t know what they are doing Jan Jan T. T. Kielstein Department of of Nephrology and and Hypertension Medical School School Hannover Drug dosing
Drug dosing in patients with acute kidney injury They don t know what they are doing Jan Jan T. T. Kielstein Department of of Nephrology and and Hypertension Medical School School Hannover Drug dosing
TRANSPARENCY COMMITTEE OPINION. 15 October Date of Marketing Authorisation (national procedure): 16 April 1997, variation of 18 February 2008
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 15 October 2008 MERONEM 1 g, powder for solution for IV Injection Box of 10 vials (CIP: 387 830-6) Applicant: ASTRAZENECA
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 15 October 2008 MERONEM 1 g, powder for solution for IV Injection Box of 10 vials (CIP: 387 830-6) Applicant: ASTRAZENECA
VANCOMYCIN DOSING AND MONITORING GUIDELINES
 VANCOMYCIN DOSING AND MONITORING GUIDELINES NB Provincial Health Authorities Anti-Infective Stewardship Committee Approved: May 2017 GENERAL COMMENTS Vancomycin is a glycopeptide antibiotic with bactericidal
VANCOMYCIN DOSING AND MONITORING GUIDELINES NB Provincial Health Authorities Anti-Infective Stewardship Committee Approved: May 2017 GENERAL COMMENTS Vancomycin is a glycopeptide antibiotic with bactericidal
Ceftomax TM S (Cefoperazone Sodium plus Sulbactam Sodium Injection)
 COMPOSITION Ceftomax TM S (Cefoperazone Sodium plus Sulbactam Sodium Injection) CEFTOMAX - S Injection 1.5 gm Each vial contains: Cefoperazone Sodium equivalent to Cefoperazone IP. 1,000 mg Sulbactam Sodium
COMPOSITION Ceftomax TM S (Cefoperazone Sodium plus Sulbactam Sodium Injection) CEFTOMAX - S Injection 1.5 gm Each vial contains: Cefoperazone Sodium equivalent to Cefoperazone IP. 1,000 mg Sulbactam Sodium
SCIENTIFIC DISCUSSION
 European Medicines Agency London, 25 July 2007 Product name: Cubicin PROCEDURE NO. EMEA/H/637/II/05 SCIENTIFIC DISCUSSION 7 Westferry Circus, Canary Wharf, London E14 4HB, UK Tel. (44-20) 74 18 84 00 Fax
European Medicines Agency London, 25 July 2007 Product name: Cubicin PROCEDURE NO. EMEA/H/637/II/05 SCIENTIFIC DISCUSSION 7 Westferry Circus, Canary Wharf, London E14 4HB, UK Tel. (44-20) 74 18 84 00 Fax
Outpatient parenteral antibiotic therapy with daptomycin: insights from a patient registry
 doi: 10.1111/j.1742-1241.2008.01824.x ORIGINAL PAPER Outpatient parenteral antibiotic therapy with daptomycin: insights from a patient registry W. J. Martone, K. C. Lindfield, D. E. Katz OnlineOpen: This
doi: 10.1111/j.1742-1241.2008.01824.x ORIGINAL PAPER Outpatient parenteral antibiotic therapy with daptomycin: insights from a patient registry W. J. Martone, K. C. Lindfield, D. E. Katz OnlineOpen: This
Daptomycin Pharmacokinetics and Safety following Administration of Escalating Doses Once Daily to Healthy Subjects
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Apr. 2003, p. 1318 1323 Vol. 47, No. 4 0066-4804/03/$08.00 0 DOI: 10.1128/AAC.47.4.1318 1323.2003 Copyright 2003, American Society for Microbiology. All Rights Reserved.
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Apr. 2003, p. 1318 1323 Vol. 47, No. 4 0066-4804/03/$08.00 0 DOI: 10.1128/AAC.47.4.1318 1323.2003 Copyright 2003, American Society for Microbiology. All Rights Reserved.
Vancomycin: Class: Antibiotic.
 Vancomycin: Class: Antibiotic. Indications: Treatment of patients with infections caused by staphylococcal species and streptococcal Species. Available dosage form in the hospital: 1G VIAL, 500MG VIAL.
Vancomycin: Class: Antibiotic. Indications: Treatment of patients with infections caused by staphylococcal species and streptococcal Species. Available dosage form in the hospital: 1G VIAL, 500MG VIAL.
ESCMID Online Lecture Library. by author
 Hospital Universitario Virgen Macarena, Seville New drugs against MRSA and VRE L. Eduardo López Cortés Seville, 8th July Tedizolid Oxazolidinone Ceftaroline // Ceftobiprole 5 th gen cephalosporin Overview
Hospital Universitario Virgen Macarena, Seville New drugs against MRSA and VRE L. Eduardo López Cortés Seville, 8th July Tedizolid Oxazolidinone Ceftaroline // Ceftobiprole 5 th gen cephalosporin Overview
Aciphin Ceftriaxone Sodium
 Aciphin Ceftriaxone Sodium Only for the use of Medical Professionals Description Aciphin is a bactericidal, long-acting, broad spectrum, parenteral cephalosporin preparation, active against a wide range
Aciphin Ceftriaxone Sodium Only for the use of Medical Professionals Description Aciphin is a bactericidal, long-acting, broad spectrum, parenteral cephalosporin preparation, active against a wide range
INTRAVENOUS VANCOMYCIN PRESCRIBING AND MONITORING GUIDELINE FOR ADULT PATIENTS
 Title of guideline (must include the word Guideline (not protocol, policy, procedure etc) INTRAVENOUS VANCOMYCIN PRESCRIBING AND MONITORING GUIDELINE FOR ADULT PATIENTS Author: Contact Name and Job Title
Title of guideline (must include the word Guideline (not protocol, policy, procedure etc) INTRAVENOUS VANCOMYCIN PRESCRIBING AND MONITORING GUIDELINE FOR ADULT PATIENTS Author: Contact Name and Job Title
Use ideal body weight (IBW) unless actual body weight is less. Use the following equation to calculate IBW:
 Amikacin is a partially restricted (amber) antibiotic for the treatment of infections due to gentamicin resistant Gram negative bacilli or as advised by microbiology. As with other aminoglycosides, therapeutic
Amikacin is a partially restricted (amber) antibiotic for the treatment of infections due to gentamicin resistant Gram negative bacilli or as advised by microbiology. As with other aminoglycosides, therapeutic
CRIFOS 4 GM Injection (Fosfomycin sodium)
 Published on: 30 Sep 2016 CRIFOS 4 GM Injection (Fosfomycin sodium) Composition CRIFOS 4 GM Each vial contains: Fosfomycin Sodium BP equivalent to Fosfomycin..4 g Excipients q.s. Dosage Form Powder for
Published on: 30 Sep 2016 CRIFOS 4 GM Injection (Fosfomycin sodium) Composition CRIFOS 4 GM Each vial contains: Fosfomycin Sodium BP equivalent to Fosfomycin..4 g Excipients q.s. Dosage Form Powder for
Essential Shared Care Agreement (South Staffordshire): Aciclovir Administration in the Community
 E088 Essential Shared Care Agreement (South Staffordshire): Aciclovir Administration in the Community Patient s name: DOB NHS Number Patient s address: Consultant Note: Shared care agreement sets out a
E088 Essential Shared Care Agreement (South Staffordshire): Aciclovir Administration in the Community Patient s name: DOB NHS Number Patient s address: Consultant Note: Shared care agreement sets out a
Therapeutic drug monitoring of daptomycin: a retrospective monocentric analysis
 Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2015 Therapeutic drug monitoring of daptomycin: a retrospective monocentric
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2015 Therapeutic drug monitoring of daptomycin: a retrospective monocentric
Development of Drugs for Bacteremia
 Development of Drugs for Bacteremia Charles Knirsch, MD, MPH VP, Clinical Research Pfizer Inc 1 Bacteremia Guidance Issues EMA guidance suggests that bacteremia is not a primary diagnosis but represents
Development of Drugs for Bacteremia Charles Knirsch, MD, MPH VP, Clinical Research Pfizer Inc 1 Bacteremia Guidance Issues EMA guidance suggests that bacteremia is not a primary diagnosis but represents
VANLID Capsules (Vancomycin hydrochloride)
 Published on: 22 Sep 2014 VANLID Capsules (Vancomycin hydrochloride) Composition VANLID Capsules Each capsule contains: Vancomycin Hydrochloride IP equivalent to Vancomycin.. 250 mg Approved colours used
Published on: 22 Sep 2014 VANLID Capsules (Vancomycin hydrochloride) Composition VANLID Capsules Each capsule contains: Vancomycin Hydrochloride IP equivalent to Vancomycin.. 250 mg Approved colours used
PRESCRIBING INFORMATION Including Patient Medication Information. Colistimethate for Injection USP (colistimethate sodium)
 PRESCRIBING INFORMATION Including Patient Medication Information Pr Colistimethate for Injection USP (colistimethate sodium) Powder for Solution (equivalent to 150 mg colistin base) Antibiotic SteriMax
PRESCRIBING INFORMATION Including Patient Medication Information Pr Colistimethate for Injection USP (colistimethate sodium) Powder for Solution (equivalent to 150 mg colistin base) Antibiotic SteriMax
Annex III. Amendments to relevant sections of the summary of product characteristics and the package leaflets
 Annex III Amendments to relevant sections of the summary of product characteristics and the package leaflets 22 Changes agreed by the CHMP to the product information of CMS-containing products for injection
Annex III Amendments to relevant sections of the summary of product characteristics and the package leaflets 22 Changes agreed by the CHMP to the product information of CMS-containing products for injection
DOSAGE AND ADMINISTRATION
 Pr COLISTIMETHATE FOR INJECTION USP Colistimethate for Injection contains the sodium salt of colistimethate which is a polypeptide antibiotic with an approximate molecular weight of 1 750. The empirical
Pr COLISTIMETHATE FOR INJECTION USP Colistimethate for Injection contains the sodium salt of colistimethate which is a polypeptide antibiotic with an approximate molecular weight of 1 750. The empirical
Annex III. Summary of product characteristics, labelling and package leaflet
 Annex III Summary of product characteristics, labelling and package leaflet Note: This SmPC, labelling and packages leaflet is the version valid at the time of Commission decision. After the Commission
Annex III Summary of product characteristics, labelling and package leaflet Note: This SmPC, labelling and packages leaflet is the version valid at the time of Commission decision. After the Commission
PRODUCT INFORMATION. Colistin Link. Colistin 150 mg/2 ml (as colistimethate sodium) powder for injection vial
 PRODUCT INFORMATION Colistin Link Colistin 150 mg/2 ml (as colistimethate sodium) powder for injection vial For Intramuscular and Intravenous use. NAME OF THE MEDICINE Colistimethate sodium for injection,
PRODUCT INFORMATION Colistin Link Colistin 150 mg/2 ml (as colistimethate sodium) powder for injection vial For Intramuscular and Intravenous use. NAME OF THE MEDICINE Colistimethate sodium for injection,
Vancomycin Drug Class 1
 Drug Class 1 Antibiotic glycopeptide Spectrum 1 Cross Sensitivities / Allergies 1 Refer to product monograph for complete spectrum Gram positive pathogens (e.g., S. aureus, Enterococcus, S. viridans, methicillinresistant
Drug Class 1 Antibiotic glycopeptide Spectrum 1 Cross Sensitivities / Allergies 1 Refer to product monograph for complete spectrum Gram positive pathogens (e.g., S. aureus, Enterococcus, S. viridans, methicillinresistant
Colistimethate FOR INJECTION, USP 10 DIGIT NDC STRENGTH SIZE WHOLESALE NUMBERS WEB LISTING. 150 mg*/vial 12
 Colistimethate FOR INJECTION, USP 10 DIGIT NDC STRENGTH SIZE WHOLESALE NUMBERS WEB LISTING 39822-0617-1 150 mg*/vial 1 ABC 10167618 Cardinal 5276001 11 DIGIT NDC Colistin base activity vial McKesson 3574043
Colistimethate FOR INJECTION, USP 10 DIGIT NDC STRENGTH SIZE WHOLESALE NUMBERS WEB LISTING 39822-0617-1 150 mg*/vial 1 ABC 10167618 Cardinal 5276001 11 DIGIT NDC Colistin base activity vial McKesson 3574043
DRUG UTILIZATION EVALUATION
 DRUG UTILIZATION EVALUATION Prescribing Trends with Daptomycin (Cubicin) For the Treatment of Gram-Positive Infections Noreen H. Chan Tompkins, PharmD, and Stephen J. Harnicar, PharmD INTRODUCTION Daptomycin
DRUG UTILIZATION EVALUATION Prescribing Trends with Daptomycin (Cubicin) For the Treatment of Gram-Positive Infections Noreen H. Chan Tompkins, PharmD, and Stephen J. Harnicar, PharmD INTRODUCTION Daptomycin
Policy: Created: 2/11/2015; Approved: Adult Pharmacokinetic Dosing and Monitoring- Vancomycin Dosing
 ProMedica Health System Clinical Interdepartmental Policy and Procedure: Section: Policy: Date: Subject: Pharmacy Created: 2/11/2015; Approved: Adult Pharmacokinetic Dosing and Monitoring- Vancomycin Dosing
ProMedica Health System Clinical Interdepartmental Policy and Procedure: Section: Policy: Date: Subject: Pharmacy Created: 2/11/2015; Approved: Adult Pharmacokinetic Dosing and Monitoring- Vancomycin Dosing
PRESCRIBING INFORMATION Including Patient Medication Information USP
 PRESCRIBING INFORMATION Including Patient Medication Information Pr COLISTIMETHATE FOR INJECTION USP Sterile Colistimethate sodium Equivalent to 150 mg colistin base Parenteral Powder for Solution Antibiotic
PRESCRIBING INFORMATION Including Patient Medication Information Pr COLISTIMETHATE FOR INJECTION USP Sterile Colistimethate sodium Equivalent to 150 mg colistin base Parenteral Powder for Solution Antibiotic
Abhijit Chakraborty 1 *, Sandip Roy 1, Juergen Loeffler 2 and Ricardo L. Chaves 2. CH-4056 Basel, Switzerland. Introduction
 Journal of Antimicrobial Chemotherapy (29) 64, 151 158 doi:1.193/jac/dkp155 Advance Access publication 22 April 29 Comparison of the pharmacokinetics, safety and tolerability of in healthy adult volunteers
Journal of Antimicrobial Chemotherapy (29) 64, 151 158 doi:1.193/jac/dkp155 Advance Access publication 22 April 29 Comparison of the pharmacokinetics, safety and tolerability of in healthy adult volunteers
Vancomycin (as hydrochloride)
 Vancomycin (as hydrochloride) Vancocin CP 500mg Powder for Injection (I.V.) PRODUCT DESCRIPTION Vancomycin hydrochloride, 500 mg, lyophilisate for solution for infusion: Each vial contains vancomycin 500
Vancomycin (as hydrochloride) Vancocin CP 500mg Powder for Injection (I.V.) PRODUCT DESCRIPTION Vancomycin hydrochloride, 500 mg, lyophilisate for solution for infusion: Each vial contains vancomycin 500
Clinical Safety & Effectiveness Cohort # 10
 1 Clinical Safety & Effectiveness Cohort # 10 Improving Weight-Based Vancomycin Dosing and Monitoring DATE Educating for Quality Improvement & Patient Safety 2 Financial Disclosure lizabeth A. Walter,
1 Clinical Safety & Effectiveness Cohort # 10 Improving Weight-Based Vancomycin Dosing and Monitoring DATE Educating for Quality Improvement & Patient Safety 2 Financial Disclosure lizabeth A. Walter,
Cubicin (Daptomycin) Priv.Doz. Dr. med. Markus Rothenburger
 Cubicin (Daptomycin) Priv.Doz. Dr. med. Markus Rothenburger Cubicin (Daptomycin): A new generation of antibiotics First-in-class cyclic natural lipopeptide Activity against major gram positive pathogens
Cubicin (Daptomycin) Priv.Doz. Dr. med. Markus Rothenburger Cubicin (Daptomycin): A new generation of antibiotics First-in-class cyclic natural lipopeptide Activity against major gram positive pathogens
XYLISTIN 4.5 MIU Injection (Colistimethate sodium)
 Published on: 28 Jun 2016 XYLISTIN 4.5 MIU Injection (Colistimethate sodium) Composition XYLISTIN 4.5 MIU Injection Each vial contains: Colistimethate Sodium IP.45,00,000 IU (IU: International Units) As
Published on: 28 Jun 2016 XYLISTIN 4.5 MIU Injection (Colistimethate sodium) Composition XYLISTIN 4.5 MIU Injection Each vial contains: Colistimethate Sodium IP.45,00,000 IU (IU: International Units) As
Adult Institutional Pharmacokinetics Protocol
 Adult Institutional Pharmacokinetics Protocol Policy Title: Clinical Pharmacokinetics (PK) Service Policy Policy Statement: It is the policy of UMHC that PK consult orders (for vancomycin or aminoglycosides)
Adult Institutional Pharmacokinetics Protocol Policy Title: Clinical Pharmacokinetics (PK) Service Policy Policy Statement: It is the policy of UMHC that PK consult orders (for vancomycin or aminoglycosides)
Evaluation of Vancomycin Continuous Infusion in Trauma Patients
 OBJECTIVES Evaluation of Vancomycin Continuous Infusion in Trauma Patients Brittany D. Bissell, Pharm.D. PGY-2 Critical Care Pharmacy Resident Jackson Memorial Hospital Miami, Florida Evaluate the potential
OBJECTIVES Evaluation of Vancomycin Continuous Infusion in Trauma Patients Brittany D. Bissell, Pharm.D. PGY-2 Critical Care Pharmacy Resident Jackson Memorial Hospital Miami, Florida Evaluate the potential
Aminoglycosides John A. Bosso, Pharm.D.
 AMINOGLYCOSIDES Therapeutics/PHRMP-73 Aminoglycoside Mechanism of Action Aminoglycosides bind to 30s ribosomal subunit resulting in mistranslation of mrna thus disrupting protein synthesis. They are rapidly
AMINOGLYCOSIDES Therapeutics/PHRMP-73 Aminoglycoside Mechanism of Action Aminoglycosides bind to 30s ribosomal subunit resulting in mistranslation of mrna thus disrupting protein synthesis. They are rapidly
D DAVID PUBLISHING. 1. Introduction. Kathryn Koliha 1, Julie Falk 1, Rachana Patel 1 and Karen Kier 2
 Journal of Pharmacy and Pharmacology 5 (2017) 607-615 doi: 10.17265/2328-2150/2017.09.001 D DAVID PUBLISHING Comparative Evaluation of Pharmacist-Managed Vancomycin Dosing in a Community Hospital Following
Journal of Pharmacy and Pharmacology 5 (2017) 607-615 doi: 10.17265/2328-2150/2017.09.001 D DAVID PUBLISHING Comparative Evaluation of Pharmacist-Managed Vancomycin Dosing in a Community Hospital Following
Pharmacokinetics and Tolerability of Daptomycin at Doses up to 12 Milligrams per Kilogram of Body Weight Once Daily in Healthy Volunteers
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Oct. 2006, p. 3245 3249 Vol. 50, No. 10 0066-4804/06/$08.00 0 doi:10.1128/aac.00247-06 Copyright 2006, American Society for Microbiology. All Rights Reserved. Pharmacokinetics
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Oct. 2006, p. 3245 3249 Vol. 50, No. 10 0066-4804/06/$08.00 0 doi:10.1128/aac.00247-06 Copyright 2006, American Society for Microbiology. All Rights Reserved. Pharmacokinetics
Infections Amenable to OPAT. (Nabin Shrestha + Ajay Mathur)
 3 Infections Amenable to OPAT (Nabin Shrestha + Ajay Mathur) Decisions regarding outpatient treatment of infections vary with the institution, the prescribing physician, the individual patient s condition
3 Infections Amenable to OPAT (Nabin Shrestha + Ajay Mathur) Decisions regarding outpatient treatment of infections vary with the institution, the prescribing physician, the individual patient s condition
PACKAGE LEAFLET: INFORMATION FOR THE USER. Erpizon Lyophilisate for solution for infusion 250 mg/vial Aciclovir
 PACKAGE LEAFLET: INFORMATION FOR THE USER Erpizon Lyophilisate for solution for infusion 250 mg/vial Aciclovir 1. DESCRIPTION OF THE MEDICINAL PRODUCT 1.1 Name: ERPIZON 250 mg/vial 1.2 Qualitative composition:
PACKAGE LEAFLET: INFORMATION FOR THE USER Erpizon Lyophilisate for solution for infusion 250 mg/vial Aciclovir 1. DESCRIPTION OF THE MEDICINAL PRODUCT 1.1 Name: ERPIZON 250 mg/vial 1.2 Qualitative composition:
TARGOCID 200mg DATA SHEET
 פורמט עלון זה נקבע ע"י משרד הבריאות ותוכנו נבדק ואושר על ידו עלון מאושר 11.1010 TARGOCID 200mg DATA SHEET 1. Trade NAME OF THE MEDICINAL PRODUCT Targocid 200mg Powder for solution for Injection 2. QUALITATIVE
פורמט עלון זה נקבע ע"י משרד הבריאות ותוכנו נבדק ואושר על ידו עלון מאושר 11.1010 TARGOCID 200mg DATA SHEET 1. Trade NAME OF THE MEDICINAL PRODUCT Targocid 200mg Powder for solution for Injection 2. QUALITATIVE
PACKAGE INSERT USP ANTIBIOTIC
 Pr AMPICILLIN for Injection USP ANTIBIOTIC ACTIONS AND CLINICAL PHARMACOLOGY Ampicillin has a broad spectrum of bactericidal activity against many gram-positive and gramnegative aerobic and anaerobic bacteria.
Pr AMPICILLIN for Injection USP ANTIBIOTIC ACTIONS AND CLINICAL PHARMACOLOGY Ampicillin has a broad spectrum of bactericidal activity against many gram-positive and gramnegative aerobic and anaerobic bacteria.
Colistimethate sodium for injection and inhalation COLISTIN COMPOSITION
 Colistin, Colistimethate sodium, injection, cyclic polypeptide antibiotic, manufacturers, India, infusion, inhalation, Taj Products Pharmaceuticals, Allopathic Products Manufacturer,exporter,Supplier,india,formulations,medicines,injections,insulin,free
Colistin, Colistimethate sodium, injection, cyclic polypeptide antibiotic, manufacturers, India, infusion, inhalation, Taj Products Pharmaceuticals, Allopathic Products Manufacturer,exporter,Supplier,india,formulations,medicines,injections,insulin,free
Bassett Healthcare Clinical Laboratory
 Therapeutic Drug Level Collection Guidelines Anti-epileptic drugs (carbamazepine, phenobarbital, phenytoin, primidone, valproic acid) Consider collecting after steady state conditions are reached, i.e.
Therapeutic Drug Level Collection Guidelines Anti-epileptic drugs (carbamazepine, phenobarbital, phenytoin, primidone, valproic acid) Consider collecting after steady state conditions are reached, i.e.
WARNING: FETAL RISK See full prescribing information for complete boxed warning.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use VIBATIV safely and effectively. See full prescribing information for VIBATIV. VIBATIV (telavancin)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use VIBATIV safely and effectively. See full prescribing information for VIBATIV. VIBATIV (telavancin)
SBUH Aminoglycoside Dosing Protocol
 Adult Aminoglycoside Dosing for Gram negative infections prior to available serum levels (Excludes patients with cystic fibrosis, OB GYN patients and surgical prophylaxis) Cr Cl 40 ml/min 5 7 mg/kg INT
Adult Aminoglycoside Dosing for Gram negative infections prior to available serum levels (Excludes patients with cystic fibrosis, OB GYN patients and surgical prophylaxis) Cr Cl 40 ml/min 5 7 mg/kg INT
PRESCRIBING INFORMATION. BaciJect. Bacitracin for injection U.S.P. Powder for Solution. 50,000 Units/Vial. Antibiotic
 PRESCRIBING INFORMATION BaciJect Bacitracin for injection U.S.P. Powder for Solution 50,000 Units/Vial Antibiotic SteriMax Inc. 2770 Portland Drive, Oakville, ON L6H 6R4 Date of Revision: July 30, 2018
PRESCRIBING INFORMATION BaciJect Bacitracin for injection U.S.P. Powder for Solution 50,000 Units/Vial Antibiotic SteriMax Inc. 2770 Portland Drive, Oakville, ON L6H 6R4 Date of Revision: July 30, 2018
ORIGINAL ARTICLE DOI: (e) ISSN Online: (p) ISSN Print: Anand Kumar Singh 1, Poonam Verma 2. Sciences, Dehradun
 ORIGINAL ARTICLE CLINICAL ASSESSMENT OF NEPHROTOXICITY ASSOCIATED WITH VANCOMYCIN TROUGH CONCENTRATIONS DURING TREATMENT OF DEEP-SEATED INFECTIONS: A RETROSPECTIVE ANALYSIS Anand Kumar Singh 1, Poonam
ORIGINAL ARTICLE CLINICAL ASSESSMENT OF NEPHROTOXICITY ASSOCIATED WITH VANCOMYCIN TROUGH CONCENTRATIONS DURING TREATMENT OF DEEP-SEATED INFECTIONS: A RETROSPECTIVE ANALYSIS Anand Kumar Singh 1, Poonam
PART VI. SUMMARY OF THE RISK MANAGEMENT PLAN Summary of risk management plan for ZINFORO
 PART VI. SUMMARY OF THE RISK MANAGEMENT PLAN Summary of risk management plan for ZINFORO This is a summary of the risk management plan (RMP) for ZINFORO. The RMP details important risks of ZINFORO, how
PART VI. SUMMARY OF THE RISK MANAGEMENT PLAN Summary of risk management plan for ZINFORO This is a summary of the risk management plan (RMP) for ZINFORO. The RMP details important risks of ZINFORO, how
Summary of Product Characteristics
 1 NAME OF THE MEDICINAL PRODUCT Summary of Product Characteristics Vancocin 1 g powder for concentrate for solution for infusion and powder for oral solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
1 NAME OF THE MEDICINAL PRODUCT Summary of Product Characteristics Vancocin 1 g powder for concentrate for solution for infusion and powder for oral solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
CLINICAL USE OF GLYCOPEPTIDES. Herbert Spapen Intensive Care Department University Hospital Vrije Universiteit Brussel
 CLINICAL USE OF GLYCOPEPTIDES Herbert Spapen Intensive Care Department University Hospital Vrije Universiteit Brussel Glycopeptides Natural Vancomycin introduced in 1958 Teicoplanin introduced in Europe
CLINICAL USE OF GLYCOPEPTIDES Herbert Spapen Intensive Care Department University Hospital Vrije Universiteit Brussel Glycopeptides Natural Vancomycin introduced in 1958 Teicoplanin introduced in Europe
AXITAB-CV TAB. COMPOSITION :
 AXITAB-CV TAB. COMPOSITION : Each film coated tablet contains: Cefuroxime Axetil I.P. Eq. to Anhydrous 500mg. Potassium Clavulanate Diluted I.P. Eq. to Clavulanic Acid 125mg DESCRIPTION : Cefuroxime Axetil
AXITAB-CV TAB. COMPOSITION : Each film coated tablet contains: Cefuroxime Axetil I.P. Eq. to Anhydrous 500mg. Potassium Clavulanate Diluted I.P. Eq. to Clavulanic Acid 125mg DESCRIPTION : Cefuroxime Axetil
VIBATIV - telavancin hydrochlorideâ injection, powder, lyophilized, for solutionâ Theravance, Inc
 VIBATIV - telavancin hydrochloride injection, powder, lyophilized, for solution Theravance, Inc. ---------- HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed
VIBATIV - telavancin hydrochloride injection, powder, lyophilized, for solution Theravance, Inc. ---------- HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed
Daptomycin Pharmacokinetics in Adult Oncology Patients with Neutropenic Fever
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Feb. 2009, p. 428 434 Vol. 53, No. 2 0066-4804/09/$08.00 0 doi:10.1128/aac.00943-08 Copyright 2009, American Society for Microbiology. All Rights Reserved. Daptomycin
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Feb. 2009, p. 428 434 Vol. 53, No. 2 0066-4804/09/$08.00 0 doi:10.1128/aac.00943-08 Copyright 2009, American Society for Microbiology. All Rights Reserved. Daptomycin
Cefazolin for Injection, USP
 Cefazolin for Injection, USP Only To reduce the development of drug-resistant bacteria and maintain the effectiveness of Cefazolin for injection and other antibacterial drugs, Cefazolin for injection should
Cefazolin for Injection, USP Only To reduce the development of drug-resistant bacteria and maintain the effectiveness of Cefazolin for injection and other antibacterial drugs, Cefazolin for injection should
%T MIC MIC. Pharmacokinetics PK: Cmax AUC T1/2 Pharmacodynamics PD: MIC: minimum inhibitory concentration time-killing-curve 1990.
 THE JAPANESE JOURNAL OF ANTIBIOTICS 58 2 159( 55 ) ( 2 15 ) %T MIC MIC 2002 30%T MIC 50%T MIC 1000 mg 3 3 /day Pharmacokinetics PK: Cmax AUC T1/2 Pharmacodynamics PD: MIC: minimum inhibitory concentration
THE JAPANESE JOURNAL OF ANTIBIOTICS 58 2 159( 55 ) ( 2 15 ) %T MIC MIC 2002 30%T MIC 50%T MIC 1000 mg 3 3 /day Pharmacokinetics PK: Cmax AUC T1/2 Pharmacodynamics PD: MIC: minimum inhibitory concentration
Daptomycin in Clinical Practice. Paolo Grossi
 Clinica delle Malattie Infettive e Tropicali Università degli Studi dell Insubria Ospedale di Circolo e Fondazione Macchi, Varese Second Opinion Infettivologica Centro Nazionale Trapianti, ISS, Roma Daptomycin
Clinica delle Malattie Infettive e Tropicali Università degli Studi dell Insubria Ospedale di Circolo e Fondazione Macchi, Varese Second Opinion Infettivologica Centro Nazionale Trapianti, ISS, Roma Daptomycin
Continuous Infusion of Antibiotics In The ICU: What Is Proven? Professor of Medicine Vice-Chairman, Department of Medicine SUNY at Stony Brook
 Continuous Infusion of Antibiotics In The ICU: What Is Proven? Michael S. Niederman, M.D. Chairman, Department of Medicine Winthrop-University Hospital Mineola, NY Professor of Medicine Vice-Chairman,
Continuous Infusion of Antibiotics In The ICU: What Is Proven? Michael S. Niederman, M.D. Chairman, Department of Medicine Winthrop-University Hospital Mineola, NY Professor of Medicine Vice-Chairman,
ZINEX. Composition Each tablet contains Cefuroxime (as axetil) 250 or 500 mg
 ZINEX Composition Each tablet contains Cefuroxime (as axetil) 250 or 500 mg Tablets Action Cefuroxime axetil owes its bactericidal activity to the parent compound cefuroxime. Cefuroxime is a well-characterized
ZINEX Composition Each tablet contains Cefuroxime (as axetil) 250 or 500 mg Tablets Action Cefuroxime axetil owes its bactericidal activity to the parent compound cefuroxime. Cefuroxime is a well-characterized
New Zealand Data Sheet COLISTIN-LINK
 New Zealand Data Sheet COLISTIN-LINK Colistimethate sodium equivalent to colistin 150mg powder for injection For intramuscular and intravenous use. Description Colistimethate sodium for injection, USP
New Zealand Data Sheet COLISTIN-LINK Colistimethate sodium equivalent to colistin 150mg powder for injection For intramuscular and intravenous use. Description Colistimethate sodium for injection, USP
Risk Management Plan Linezolid solution for infusion
 VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Linezolid is used to treat pneumonia and some infections in the skin or under the skin, caused by a group of bacteria called the
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Linezolid is used to treat pneumonia and some infections in the skin or under the skin, caused by a group of bacteria called the
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Doribax 250 mg powder for solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial contains doripenem monohydrate
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Doribax 250 mg powder for solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial contains doripenem monohydrate
PHARMACOKINETICS SMALL GROUP II:
 PHARMACOKINETICS SMALL GROUP II: Question 1 Why are some drug therapies initiated with a loading dose? Emphasize that LD establishes initial therapeutic level quickly. The time to reach the steady-state
PHARMACOKINETICS SMALL GROUP II: Question 1 Why are some drug therapies initiated with a loading dose? Emphasize that LD establishes initial therapeutic level quickly. The time to reach the steady-state
Infective Endocarditis Empirical therapy Antibiotic Guidelines. Contents
 Infective Endocarditis Empirical therapy Antibiotic Guidelines Classification: Clinical Guideline Lead Author: Antibiotic Steering Group Additional author(s): as above Authors Division: Division of Clinical
Infective Endocarditis Empirical therapy Antibiotic Guidelines Classification: Clinical Guideline Lead Author: Antibiotic Steering Group Additional author(s): as above Authors Division: Division of Clinical
NHS Grampian Staff Guidance for the Administration of Intravenous Vancomycin in Adults via Intermittent (pulsed) Infusion
 Acute Sector NHS Grampian Staff Guidance for the Administration of Intravenous Vancomycin in Adults via Intermittent (pulsed) Infusion Co-ordinators: Gillian Macartney Fiona McDonald Specialist Antibiotic
Acute Sector NHS Grampian Staff Guidance for the Administration of Intravenous Vancomycin in Adults via Intermittent (pulsed) Infusion Co-ordinators: Gillian Macartney Fiona McDonald Specialist Antibiotic
Rifampin Resistance. Charlottesville, Virginia i0w organisms in Trypticase soy broth (BBL Microbiology
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Apr. 1980, p. 658-662 0066-4804/80/04-0658/05$02.00/0 Vol. 17, No. 14 Treatment of Experimental Staphylococcal Infections: Effect of Rifampin Alone and in Combination
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Apr. 1980, p. 658-662 0066-4804/80/04-0658/05$02.00/0 Vol. 17, No. 14 Treatment of Experimental Staphylococcal Infections: Effect of Rifampin Alone and in Combination
CHMP extension of indication variation assessment report
 23 April 2015 EMA/CHMP/245949/2015 adopted Committee for Medicinal Products for Human Use (CHMP) Invented name: Tygacil International non-proprietary name: TIGECYCLINE Procedure No. EMEA/H/C/000644/II/0092
23 April 2015 EMA/CHMP/245949/2015 adopted Committee for Medicinal Products for Human Use (CHMP) Invented name: Tygacil International non-proprietary name: TIGECYCLINE Procedure No. EMEA/H/C/000644/II/0092
M0BCore Safety Profile
 M0BCore Safety Profile Active substance: Aciclovir Pharmaceutical form(s)/strength: Tablets 200, 400 or 800 mg Dispersible tablets 200, 400 or 800 mg Oral suspensions 200 mg or 400 mg per 5 ml. Freeze
M0BCore Safety Profile Active substance: Aciclovir Pharmaceutical form(s)/strength: Tablets 200, 400 or 800 mg Dispersible tablets 200, 400 or 800 mg Oral suspensions 200 mg or 400 mg per 5 ml. Freeze
Renal Unit. Catheter Related Bacteraemia Guidelines
 Renal Unit Policy Manager Drew Henderson Policy Group Renal Unit Policy Established 21/01/2014 Policy Review Period/Expiry 21/01/2015 Last Updated 21/01/2014 This policy does apply to Medical/Dental Staff
Renal Unit Policy Manager Drew Henderson Policy Group Renal Unit Policy Established 21/01/2014 Policy Review Period/Expiry 21/01/2015 Last Updated 21/01/2014 This policy does apply to Medical/Dental Staff
ICU Volume 11 - Issue 3 - Autumn Series
 ICU Volume 11 - Issue 3 - Autumn 2011 - Series Impact of Pharmacokinetics of Antibiotics in ICU Clinical Practice Introduction The efficacy of a drug is mainly dependent on its ability to achieve an effective
ICU Volume 11 - Issue 3 - Autumn 2011 - Series Impact of Pharmacokinetics of Antibiotics in ICU Clinical Practice Introduction The efficacy of a drug is mainly dependent on its ability to achieve an effective
Received 30 March 2005; returned 16 June 2005; revised 8 September 2005; accepted 12 September 2005
 Journal of Antimicrobial Chemotherapy (2005) 56, 1047 1052 doi:10.1093/jac/dki362 Advance Access publication 20 October 2005 Evaluation of PPI-0903M (T91825), a novel cephalosporin: bactericidal activity,
Journal of Antimicrobial Chemotherapy (2005) 56, 1047 1052 doi:10.1093/jac/dki362 Advance Access publication 20 October 2005 Evaluation of PPI-0903M (T91825), a novel cephalosporin: bactericidal activity,
Adult Inpatient Antibiogram. Antimicrobial Susceptibilities of Frequently Recovered Clinical Isolates. January to December 2016
 Adult Inpatient Antibiogram Antimicrobial Susceptibilities of Frequently Recovered Clinical Isolates January to December 2016 Department of Pathology Camille Hamula, PhD Director, Clinical Microbiology
Adult Inpatient Antibiogram Antimicrobial Susceptibilities of Frequently Recovered Clinical Isolates January to December 2016 Department of Pathology Camille Hamula, PhD Director, Clinical Microbiology
KEFZOL (cephazolin, as cephazolin sodium)
 PRODUCT INFORMATION KEFZOL (cephazolin, as cephazolin sodium) DESCRIPTION Kefzol (cephazolin sodium) is a semisynthetic cephalosporin for parenteral administration. It is the sodium salt of 3-[[(5-methyl-1,3,4-thiadiazol-2-yl)-thio]
PRODUCT INFORMATION KEFZOL (cephazolin, as cephazolin sodium) DESCRIPTION Kefzol (cephazolin sodium) is a semisynthetic cephalosporin for parenteral administration. It is the sodium salt of 3-[[(5-methyl-1,3,4-thiadiazol-2-yl)-thio]
NEW ZEALAND DATA SHEET
 1 PRODUCT NAME Linezolid Kabi solution for intravenous infusion 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Linezolid 2 mg/ml For the full list of excipients, see section 6.1. 3 PHARMACEUTICAL FORM Solution
1 PRODUCT NAME Linezolid Kabi solution for intravenous infusion 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Linezolid 2 mg/ml For the full list of excipients, see section 6.1. 3 PHARMACEUTICAL FORM Solution
1. QUALITATIVE AND QUANTITATIVE COMPOSITION ZOVIRAX 250 mg: The sodium ion content is approximately 26 mg per vial.
 ZOVIRAX IV Aciclovir POWDER FOR I.V. INFUSION 1. QUALITATIVE AND QUANTITATIVE COMPOSITION ZOVIRAX 250 mg: The sodium ion content is approximately 26 mg per vial. 2. PHARMACEUTICAL FORM Freeze dried powder
ZOVIRAX IV Aciclovir POWDER FOR I.V. INFUSION 1. QUALITATIVE AND QUANTITATIVE COMPOSITION ZOVIRAX 250 mg: The sodium ion content is approximately 26 mg per vial. 2. PHARMACEUTICAL FORM Freeze dried powder
Skin and soft tissue (SSTI) sepsis (surgery, antimicrobial therapy and more)
 Skin and soft tissue (SSTI) sepsis (surgery, antimicrobial therapy and more) Christian Eckmann Antibiotic Stewardship Expert ECDC Chief of Staff Department of General, Visceral and Thoracic Surgery Klinikum
Skin and soft tissue (SSTI) sepsis (surgery, antimicrobial therapy and more) Christian Eckmann Antibiotic Stewardship Expert ECDC Chief of Staff Department of General, Visceral and Thoracic Surgery Klinikum
AMINOGLYCOSIDES DOSING AND MONITORING GUIDELINES
 Approved: September 2017 AMINOGLYCOSIDES DOSING AND MONITORING GUIDELINES NB Provincial Health Authorities Anti-Infective Stewardship Committee GENERAL COMMENTS Aminoglycosides (AG) include gentamicin,
Approved: September 2017 AMINOGLYCOSIDES DOSING AND MONITORING GUIDELINES NB Provincial Health Authorities Anti-Infective Stewardship Committee GENERAL COMMENTS Aminoglycosides (AG) include gentamicin,
Package leaflet: Information for the patient. Daptomycin 350 mg powder for solution for injection/infusion daptomycin
 Package leaflet: Information for the patient Daptomycin 350 mg powder for solution for injection/infusion daptomycin Read all of this leaflet carefully before you start using this medicine because it contains
Package leaflet: Information for the patient Daptomycin 350 mg powder for solution for injection/infusion daptomycin Read all of this leaflet carefully before you start using this medicine because it contains
