USERS HANDBOOK Version 4.0
|
|
|
- Kelley Dickerson
- 6 years ago
- Views:
Transcription
1 USERS HANDBOOK Version 4.0 BIOCHEMICAL GENETICS UNIT ADDENBROOKE S HOSPITAL CAMBRIDGE PLEASE DO NOT USE AFTER JUNE 2010
2 CONTENTS CONTENTS 2 1 CONTACT DETAILS AND ENQUIRIES 4 2 GENERAL INFORMATION 5 3 SPECIMEN COLLECTION 6 4 ABBREVIATIONS 7 5 INDICATIONS FOR REQUESTING A METABOLIC SCREEN 8 6 FREQUENT ENQUIRIES GLUTARIC ACIDURIA TYPE HELLP MEDIUM CHAIN ACYL-COA DEHYDROGENASE DEFICIENCY SULPHITE OXIDASE / MOLYBDENUM COFACTOR DEFICIENCIES TRIMETHYLAMINE INVESTIGATION FOR SUSPECTED MITOCHONDRIAL DISORDERS INVESTIGATION FOR A METABOLIC CAUSE OF RHABDOMYOLYSIS 11 7 IN-HOUSE METABOLIC INVESTIGATIONS ACYLCARNITINES AMINO ACIDS a Plasma Amino Acids b Urine Amino Acids c CSF Amino Acids BIOTINIDASE CHITOTRIOSIDASE CREATINE AND GUANIDINOACETATE GALACTOSE-1-PHOSPHATE URIDYLTRANSFERASE GLYCOSAMINOGLYCANS HOMOCYSTEINE ORGANIC ACIDS OROTATE VERY LONG CHAIN FATTY ACIDS, PRISTANATE AND PHYTANATE MONITORING TREATMENT OF PHENYLKETONURIA SWEAT TESTS OTHER ASSAYS a Ammonia b Lactate 22 8 REFERRED METABOLIC ASSAYS HYDROXYBUTYRATE AND FREE FATTY ACIDS DEHYDROCHOLESTEROL BILE ACID METABOLITES BIOPTERINS AND DIHYDROPTERIDINE REDUCTASE CARNITINE (TOTAL AND FREE) NEUROTRANSMITTERS OLIGOSACCHARIDES AND SIALIC ACID PLASMALOGENS PURINES AND PYRIMIDINES TRANSFERRIN ISOELECTRIC FOCUSSING 26 BGU Handbook version 4.0 June
3 8.11 WHITE CELL ENYZMES 26 9 IN-HOUSE NON-METABOLIC ASSAYS URINE VANILLYLMANDELIC ACID AND HOMOVANILLIC ACID URINE REDUCING SUBSTANCES FAECAL REDUCING SUBSTANCES IMMUNOSUPPRESSANT DRUGS QUALITY ASSURANCE SCHEMES REFERENCES AND FURTHER INFORMATION 30 INDEX 31 BGU Handbook version 4.0 June
4 1 CONTACT DETAILS AND ENQUIRIES Contact Details Biochemical Genetics Unit Box 247 Addenbrooke s Hospital Hills Road Cambridge CB2 0QQ Hospital Switchboard: Departmental Fax: Clinical Enquiries Dr Jacqui Calvin, Consultant Clinical Scientist Tel: jacqui.calvin@addenbrookes.nhs.uk Ms Sarah Hogg, Principal Clinical Scientist Tel: sarah.hogg@addenbrookes.nhs.uk Laboratory Enquiries Tel: Newborn Screening & Biochemical Genetics Unit Ms Jackie Udall, Chief Biomedical Scientist Tel: jackie.udall@addenbrookes.nhs.uk Mr Kieran McIntee, Senior Biomedical Scientist Tel: kieran.mcintee@addenbrookes.nhs.uk Immunosuppressant Drug Service Mr Eamonn O Driscoll, Chief Biomedical Scientist Tel: eamonn.odriscoll@addenbrookes.nhs.uk BGU Handbook version 4.0 June
5 2 GENERAL INFORMATION The Biochemical Genetics Unit (BGU) is based at Addenbrooke s Hospital in Cambridge and serves East Anglia, providing laboratory services for the investigation and monitoring of inborn errors of metabolism and newborn screening. Approximately 25,000 babies are born in East Anglia each year and they are screened for phenylketonuria (PKU), congenital hypothyroidism (CHT), cystic fibrosis (CF), sickle cell disease (SCD) and medium chain acyl- CoA dehydrogenase deficiency (MCADD). The laboratory also provides core metabolic tests supporting the metabolic (adult and paediatric), genetic and neurology clinics both locally at Addenbrooke s and across the region. The laboratory has full CPA accreditation. The biochemical basis of inherited disorders includes many metabolic pathways and there is a vast array of specialist assays available throughout the UK. Please contact one of the clinical scientists if you require information about assays or diseases not covered in this booklet. Opening Times The core opening hours of the BGU are 08:00 to 16:00 Monday to Friday. There is no formal out of hours service offered by this laboratory. If urgent assays / advice is required out of hours, please contact the on call biomedical scientist via switchboard ( , bleep ) who will forward the request to the Duty Biochemist. BGU Handbook version 4.0 June
6 3 SPECIMEN COLLECTION In patients with episodic illness it is necessary to collect specimens at a time when they are symptomatic, therefore it is important that the date and time of sampling are included on the sample and request form. Diagnoses may be missed if the patient is well or if changes in treatment or diet are initiated prior to specimen collection. Drugs may interfere in the analytical processes or by in vivo alteration of metabolic pathways; details of any drug treatments or special diets should be included on the request form. Exchange transfusions / blood transfusions may affect analytes measured in blood, especially enzymes in erythrocytes. Sample Identification & Policy on Unlabelled Specimens Each specimen must be clearly labelled with the patient s demographic details. Unidentifiable samples are not suitable for analysis. All samples and request forms must have 3 points of identification. Sample Volumes Ideal sample volumes are quoted for each analyte. However it may be possible to use a smaller volume, for urine samples the volume is dependent on the creatinine concentration. It may also be possible to analyse samples on dilution where it is not possible to obtain another specimen. If in doubt please contact the laboratory. Request Forms A request form must accompany each sample (the exception to this rule is dried blood spot cards sent for routine newborn screening). In addition to the demographic details, please provide: 1. Date and time of sample collection particularly important where the patient is undergoing active (and changing) treatment. 2. Clinical details important for selecting the method of analysis (eg qualitative vs quantitative urine amino acids) and interpretation of the results, particularly where there are mild or apparently non-specific abnormalities. Please include any suspected diagnosis so that the presence or absence of pathognomic metabolites and any further tests required can be included in the report. 3. Details of any drug therapy. 4. GP details we are required to inform the genetics purchasing consortium of the number of tests requested by each PCT. Transport of Samples Urgent samples should be sent by courier but only after discussion with one of the clinical scientists. Non-urgent samples should be sent directly to the BGU by 1 st class post or hospital transport. All samples should be packaged in accordance with UN3373 and Packaging Instruction 602. Turnaround Times The usual turnaround times are detailed with each test. If urgent results are required, please contact one of the clinical scientists to discuss. Result Reporting Reports are printed daily and dispatched by first class post. BGU Handbook version 4.0 June
7 4 ABBREVIATIONS AA AIP BH4 CDG CF FAOD GAGS GC-MS GE HPLC LCHADD LSD MCADD MMA MPS MSUD NKH OA PKU TLC VLCADD Amino acids Acute Intermittent Porphyria Tetrahydrobiopterin Congenital Disorders of Glycosylation Cystic Fibrosis Fatty Acid Oxidation Defect Glycosaminoglycans (mucopolysaccharides) Gas Chromatography Mass Spectrometry Glycine Encephalopathy (Non-Ketotic Hyperglycinaemia) High Performance Liquid Chromatography Long Chain Hydroxy Acyl CoA Dehydrogenase Deficiency Lysosomal Storage Disorder Medium Chain Acyl CoA Dehydrogenase Deficiency Methylmalonic Aciduria Mucopolysaccharides Maple Syrup Urine Disease Non Ketotic Hyperglycinaemia (Glycine encephalopathy) Organic Acids Phenylketonuria Thin Layer Chromatography Very Long Chain Acyl CoA Dehydrogenase Deficiency BGU Handbook version 4.0 June
8 5 INDICATIONS FOR REQUESTING A METABOLIC SCREEN It is difficult to provide an exhaustive list of the indications for screening a child (or adult) for an inborn error of metabolism. There are many diseases, most of which are very rare, associated with a variety of clinical signs and symptoms. These may be vague and non specific and may not always be present. Other considerations include family history (e.g. infant deaths), parental consanguinity, previous unexplained episodes and regression. The presence or absence of other signs and symptoms and the age of the child should also always be taken into account. The presence of strange odours or coloured urine may provide important clues to the presence of metabolic disease; however these characteristic odours are not always present. First-line (core) metabolic tests should usually include: Urine organic acids Blood spot acylcarnitines Plasma amino acids Urine amino acid screening has a fairly low yield but should be undertaken where a transport disorder (e.g. cystinuria, Hartnup disease) or renal tubular disorder is suspected. However urine organic acids and amino acids are undertaken on all samples on which a urine metabolic screen has been requested. Urine Metabolic Screen Initially urine samples will be screened for the following: Glucose, ketones, ph - Multistix Sulphur containing amino acids - nitroprusside test Amino Acids - desalted urine 2D TLC with ninhydrin detection Organic Acids GC-MS In addition urine glycosaminoglycans may be added depending on the clinical details provided. Indications: See section 5.1 Sample Type: 10 ml Urine (plain) Transport: Freeze urine on receipt and dispatch frozen (1 st class post or hospital transport) Turn Around Time: 5 working days Depending upon the results of the qualitative amino acid screen, samples may be referred for quantitative amino acid analysis. BGU Handbook version 4.0 June
9 6 FREQUENT ENQUIRIES 6.1 GLUTARIC ACIDURIA TYPE 1 Glutaric aciduria type 1 (GA-1) is due to deficiency of the enzyme glutaryl-coa dehydrogenase, which leads to the build up of glutarate and 3- hydroxyglutarate (excreted in the urine). Clinical features include macrocephaly, subdural haematoma, seizures and dystonia, often following an encephalopathic episode. It is an important diagnosis since GA-I can mimic non-accidental injury. Tests to request: Urine organic acids (glutarate and 3-hydroxyglutarate) and blood spot acylcarnitines (glutarylcarnitine). It is stated in the literature that organic acids and acylcarnitines can be normal in GA-I. If acylcarnitines and organic acids are normal but there is a strong clinical suspicion of this disorder fibroblast glutaryl-coa dehydrogenase activity should be measured (skin biopsy), review by a paediatric neurologist is recommended. Please phone to discuss. 6.2 HELLP Haemolysis, elevated liver enzymes and low platelets (HELLP) during the last trimester of pregnancy is associated with long chain hydroxyacyl-coa dehydrogenase deficiency (LCHADD) in the infant. The incidence of HELLP in mothers whose babies have LCHADD is relatively high. Conversely, LCHADD is a rare cause of HELLP. Tests to request: Blood spot acylcarnitines. 6.3 MEDIUM CHAIN ACYL-CoA DEHYDROGENASE DEFICIENCY MCADD is the most common of the fatty acid oxidation defects and affects fatty acids of carbon chain length C6 to C10. Clinical features include hypoketotic hypoglycaemia, however ketosis does not exclude a fatty acid oxidation defect. Note: newborn screening started in East Anglia on 1st June Tests to request: Blood spot acylcarnitine analysis (octanoylcarnitine) and urine organic acids (dicarboxylic aciduria, hexanoylglycine, suberylglycine). Mutation analysis is available as a confirmatory test (5-10 ml EDTA whole blood or a dried blood spot). 6.4 SULPHITE OXIDASE / MOLYBDENUM COFACTOR DEFICIENCIES Sulphite oxidase deficiency is a cause of intractable seizures in infancy. Biochemical abnormalities include increased sulphite, thiosulphite, taurine and sulphocysteine with a low plasma total homocysteine. Molydenum cofactor deficiency is clinically indistinguishable from sulphite oxidase deficiency. In BGU Handbook version 4.0 June
10 addition to the biochemical abnormalities described above patients have raised urine xanthine and low uric acid in plasma and urine. Dip stick testing of urine sulphite is unreliable and is not recommended. Urine thiosulphate is also unsatisfactory with non-specific increases in acutely ill infants. Tests to request: Sulphocysteine is a stable metabolite and an excellent indicator of sulphite oxidase deficiency. This compound can be detected at the beginning of the quantitative amino acid trace. Unfortunately interfering substances in plasma and urine can make interpretation difficult, however CSF often provides a cleaner sample. If sulphite oxidase/molydenum cofactor is suspected clinically please contact the laboratory to discuss the relevant investigations. 6.5 TRIMETHYLAMINE Patients with primary trimethylaminuria have defective trimethylamine N-oxide synthetase activity. This deficiency does not produce disease but the strong, unpleasant fishy odour can lead to social ostracism and psychological disorders. Secondary trimethylaminuria also occurs and is caused by enterobacterial overproduction of trimethylamine. A diet low in fish, liver and egg yolks usually improves the odour. Tests to request: Urine samples for trimethylamine are referred to Sheffield Children s Hospital for analysis. Please phone to discuss. 6.6 INVESTIGATION FOR SUSPECTED MITOCHONDRIAL DISORDERS Mitochondrial disorders are a clinically and genetically heterogeneous group of disorders which can present in any system, at any age with any pattern of inheritance. Investigation of these disorders is complex; Dr Alasdair Parker (Consultant Paediatric Neurologist) has developed investigation protocols for suspected mitochondrial disease. Included are a number of non-laboratory investigations (e.g. ophthalmology, audiology etc) as well as biochemistry, histopathology and genetic investigations. Biochemical investigations are mainly used to provide further clues to a possible mitochondrial disorder (e.g evidence of a tubulopathy) and to exclude other metabolic diseases which may mimic a mitochondrial disorder. The cardinal investigation for mitochondrial disease is measurement of plasma and CSF lactate, however it should be borne in mind that even in proven mitochondrial disease plasma lactate can be normal, slightly raised or only intermittently raised. CSF lactate may be more useful, a normal CSF lactate concentration in a fitting child is reassuring. Please phone one of the clinical scientists or paediatric neurologists ( / ext. 2662) to discuss. BGU Handbook version 4.0 June
11 6.7 INVESTIGATION FOR A METABOLIC CAUSE OF RHABDOMYOLYSIS Once acquired causes of rhabdomyolysis have been excluded, metabolic causes should be considered. These include glycogen storage diseases and fatty acid oxidation defects. In the first instance acylcarnitines (blood spot and plasma) and urine organic acids should be analysed. For further information please contact one of the BGU Clinical Scientists 7 IN-HOUSE METABOLIC INVESTIGATIONS 7.1 ACYLCARNITINES Analysis of the acylcarnitine profile is a powerful tool in the investigation of FAOD (eg MCADD, LCHADD, VLCADD) and classical organic acidurias. β- oxidation of long chain fatty acids has an important role in energy production, a process that becomes critical during prolonged fasting. The clinical presentation of FAODs is variable but they typically present in early childhood with hypoketotic hypoglycaemia. Analysis of plasma total and free carnitine may be indicated in the investigation of carnitine transporter defects and plasma acylcarnitines for carnitine palmitoyltransferase 2 deficiency (see section 8.5). NB: Ketonuria does not exclude a FAOD Diagnostic abnormalities may not be present in carnitine deficient individuals. Indications: Hypoglycaemia (usually hypoketotic) Cardiomyopathy Hepatomegaly Hyperammonaemia Hypotonia Muscle weakness Rhabdomyolysis Method: Tandem Mass Spectrometry (underivatised) Sample Type: Dried blood spot Transport: Store at room temperature in glassine envelope. Send by 1 st class post or hospital transport. Turn Around Time: 5 working days Table 7.1: Blood Spot Acylcarnitine Reference Ranges Acylcarnitine* (µmol/l) Acylcarnitine* (µmol/l) Free 8 35 Hexanoyl < 0.2 Acetyl 5 27 Octanoyl < 0.2 Propionyl Decanoyl < 0.2 Butyryl < 0.5 Myristoyl < 0.5 Isovaleryl < 0.3 Palmitoyl * Please note: the above acylcarnitines are quantitated. Any other acylcarnitines present in abnormal amounts will be reported qualitatively. BGU Handbook version 4.0 June
12 7.2 AMINO ACIDS 7.2.a Plasma Amino Acids Plasma amino acids may be abnormal in a variety of amino acid disorders, including urea cycle defects and some organic acidurias. Investigations should be carried out, as far as possible, on samples taken when the patient is symptomatic. Dietary restrictions may cause characteristic patterns to disappear and result in false negative results. Plasma amino acids fluctuate depending on the protein intake and whether the patient is in a fed or fasted state. Patients receiving an intravenous amino acid mixture may have an abnormal amino acid pattern. Information on the type of diet and the timing of the sample in relation to meals will aid interpretation. For the investigation of epileptic encephalopathy a paired plasma sample must accompany any CSF. Indications: Hyperammonaemia Lethargy progressing to coma, overwhelming illness in first few days of life Unexplained seizures Episodic vomiting Microcephaly Epileptic encephalopathy Method: Cation exchange chromatography with post-column derivatisation (ninhydrin) and spectrophotometric detection Sample Type: 0.5 ml Lithium heparin plasma. The sample should be separated immediately after collection and the plasma stored frozen until dispatch. Transport: Dispatch frozen (1 st class post or hospital transport) Turn Around Time: 5 working days Table 7.2a: Plasma Amino Acid Reference Ranges Please note the paediatric reference ranges relate to fasting samples Amino Acid µmol/l Amino Acid µmol/l Taurine Methionine Aspartate 0 14 Isoleucine Threonine Leucine Serine Tyrosine Glutamate 8 64 Phenylalanine Glutamine Ornithine Proline Lysine Glycine Histidine Alanine Arginine Citrulline 8 47 (Free) Homocystine Less than 5 Valine Tryptophan For information about sulphocysteine see 6.4 Sulphite oxidase deficiency / molybdenum cofactor deficiency. BGU Handbook version 4.0 June
13 7.2.b Urine Amino Acids Indications: Qualitative urine amino acid analysis is undertaken as part of the urine metabolic screen. Any samples showing abnormal patterns plus requests relating to specific disorders (eg?homocystinuria) will undergo quantitative analysis. The most sensitive test for the investigation of suspected amino acidopathies is quantitation of plasma amino acids. However urine should be analysed if a transport defect is suspected (e.g. cystinuria or Hartnup s disease) or where assessment of renal tubular function is required. In addition urine is also required for the measurement of phosphoethanolamine where hypophosphatasia is suspected (skeletal problems with a low alkaline phosphatase activity). Please indicate on the request form that this is required as phosphoethanolamine is not normally reported. Method: Quantitative Analysis cation exchange chromatography with postcolumn derivatisation (ninhydrin) and spectrophotometric detection Sample Type: 2 ml Urine (plain) Transport: freeze urine on receipt and dispatch frozen (1 st class post or hospital transport) Turn Around Time: 5 working days Table 7.2b: Urine Amino Acid Reference Ranges Amino Acid µmol/mmol creatinine Amino Acid µmol/mmol creatinine Threonine < 300 Tyrosine < 50 Serine < 390 Phenylalanine < 30 Proline < 190 Histidine < 190 Glycine < 1050 Ornithine < 30 Alanine < 250 Lysine < 190 Valine < 30 Histidine < 310 Cystine* < 20 Arginine < 30 Leucine < 20 Homocystine < 5 *NB Cystine reference range in those under a year of age is less than 50 µmol/mmol creatinine. Monitoring Cystinuria In patients with cystinuria only cystine, ornithine, arginine and lysine will be reported. The cystine concentration will also be given in µmol/l, the solubility of cystine in urine is approximately 1000 µmol/l. At concentrations above this, the patient is at high risk of stone formation. 7.2.c CSF Amino Acids Indications: Intractable seizures. CSF amino acid analysis is required for the diagnosis of glycine encephalopathy (GE) (also known as non-ketotic hyperglycinaemia or NKH) and 3-phosphoglycerate dehydrogenase deficiency. BGU Handbook version 4.0 June
14 It may also be useful in the investigation of sulphite oxidase deficiency. A paired plasma sample must always accompany the CSF. Method: Quantitative analysis cation exchange chromatography with postcolumn derivatisation (ninhydrin) and spectrophotometric detection Sample Type: 0.5 ml CSF (plain, fluoride oxalate may also be used) with a paired lithium heparin plasma sample (0.5 ml plasma). Note blood-stained CSF is not suitable for analysis. Transport: freeze CSF and plasma on receipt and dispatch frozen (1 st class post or hospital transport) Turn Around Time: 5 working days CSF Amino Acid Reference Ranges CSF serine µmol/l CSF glycine < 10 µmol/l CSF:plasma glycine ratio < BIOTINIDASE Biotin is a cofactor for multiple carboxylases and the recycling of biotin requires the activity of the enzyme biotinidase. Typically biotinidase deficiency presents between 3-6 months of life with a variety of symptoms. Treatment is with biotin replacement, and should be initiated when biotinidase deficiency is suspected, prior to confirmation of the diagnosis. Biotinidase is a relatively unstable enzyme. Low results should be checked on a fresh sample if clinically indicated. Indications: Seizures Ataxia Hypotonia Alopecia Skin rashes Method: Spectrophotometry Sample Type: 250 µl Lithium heparin plasma. Separate and freeze ASAP Transport: Dispatch frozen (1 st class post or hospital transport) Turn Around Time: 5 working days Table 7.3 Plasma Biotinidase Reference Ranges Biotinidase Activity (nmol/ml/min) Normal Partial deficiency Deficiency < 0.7 Obligate heterozygote BGU Handbook version 4.0 June
15 7.4 CHITOTRIOSIDASE Gaucher disease is a lysosomal storage disorder resulting from an inherited deficiency of the enzyme beta-glucosidase. This deficiency results in impaired breakdown of the lipid glucocerebrosidase and its subsequent accumulation in cells. Gaucher disease is characterised by markedly elevated chitotriosidase activity; symptomatic Gaucher patients typically exhibit concentrations 100 times greater than the reference range. However, chitotriosidase may be mildly increased in a number of other lysosomal storage disorders and other illnesses, such as sarcoidosis. Benign deficiency of chitotriosidase occurs in approximately 6% of Caucasians. Indications: Diagnosis and monitoring of Gaucher disease Method: Fluorimetric Sample Type: 100 µl Lithium heparin plasma or serum Transport: Store frozen, dispatch 1 st class post or hospital transport Turn Around Time: 7 working days Reference Range: µmol/l/hour 7.5 CREATINE AND GUANIDINOACETATE This relatively new group of disorders is characterised by cerebral creatine deficiency, the main symptoms of which are learning disability and speech delay, and, in some patients intractable seizures. Of the three disorders described; arginine:glycine amidinotransferase (AGAT) deficiency and guanidinoacetate methyltransferase (GAMT) deficiency show decreased creatine in the urine and plasma. In addition, in GAMT deficiency there is an increase in the excretion of guanidinoacetate. Defects in the creatine transporter (an X-linked disorder) result in an increase in the urine creatine/creatinine ratio. Guanidinoacetate is stable in urine and plasma. Whilst creatine is also stable in plasma, it is unstable in urine and concentrations increase within 1-2 hours of collection, leading to potentially false positive results for the creatine transporter defect or spuriously normal results in AGAT and GAMT. Indications: Mental retardation Absent / delayed speech Seizures Movement disorder Method: Tandem mass spectrometry Sample Type: 1 ml urine (plain) frozen as soon as possible after collection (e.g. 1 to 2 hours) and transported on dry ice. 100 µl Lithium heparin plasma (or serum), sent by 1st class post Turn Around Time: 1 month BGU Handbook version 4.0 June
16 Table 7.5 Creatine and Guanidinoacetate Reference Ranges Creatine Guanidinoacetate Age Urine Plasma µmol/mmol creatinine µmol/l All ages years years Older than 12 years All ages years Older than 15 years GALACTOSE-1-PHOSPHATE URIDYLTRANSFERASE Classical galactosaemia is caused by a deficiency of the enzyme galactose-1- phosphate uridyl transferase. Galactose is produced in the small intestine from the breakdown of dietary lactose into galactose and glucose. The presence of reducing substances in urine may be an important clue to diagnosis but equally can be misleading. False positives can occur in severe liver disease and false negatives can occur if lactose is not present in the diet. Carriers of galactosaemia cannot be detected by this screening method. NB. Results are invalid if the sample has been collected within 6 weeks of a blood transfusion. Indications: Hepatomegaly Prolonged jaundice with abnormal liver function tests Presence of reducing substances in urine (negative for glucose) Method: Beutler screening test Sample Type: Dried blood spot Transport: 1 st class post Turn Around Time: 5 working days. Reference Range: Qualitative result only Normal results are reported as: Galactose-1-phosphate uridyl transferase activity measured by the Beutler screening test appeared to be within normal limits. Action taken on the strength of this result should recognise that it is a screening test. Deficient results are reported as: There was no detectable galactose-1- phosphate uridyl transferase activity when measured by the Beutler screening test. This enzyme is relatively unstable, particularly if the dried blood spot is subjected to hot or humid conditions. The screening test also requires the presence and activity of endogenous blood glucose-6-phosphate dehydrogenase. Action taken on the strength of this result should recognise that it is a screening test. Deficient results should be confirmed - the laboratory will contact you to arrange this. BGU Handbook version 4.0 June
17 7.7 GLYCOSAMINOGLYCANS The mucopolysaccharidoses are a group of inherited disorders characterised by the accumulation of glycosaminoglycans in the lysosomes. Children may appear normal at birth but later develop progressive skeletal abnormalities, coarse facies and hepatomegaly. Normal urine contains mostly chondroitin sulphate with traces of heparan and dermatan sulphates. Mucopolysaccharidoses are characterised by abnormal patterns of glycosaminoglycans in urine. Initially, samples are screened for total glycosaminoglycan concentration. False positive results are common, particularly in young infants. Positive results will be referred for typing by electrophoresis if clinical details suggestive of a mucopolysaccharidosis are given. False negative results may also be encountered. Please specifically request GAG typing if there is a strong clinical suspicion. Indications: Hepatomegaly Skeletal deformities Abnormal facies Behavioural problems Inguinal and umbilical hernias Loss of developmental skills Method: GAG quantitation: dimethylmethylene blue dye binding method with spectrophotometric detection GAG typing: 2D electrophoresis Sample Type: 5 ml Urine (plain) Transport: Store frozen, dispatch frozen (1 st class post or hospital transport) Turn Around Time: 10 working days Table 7.7: Urine GAG Reference Ranges Age Glycosaminoglycans (mg/mmol creatinine) 1 week 2 months < 70 2 months 1 year < years < years < years < years < 21 > 12 years < 13 All results within the reference range will be reported with the following comment: This assay is a screening test only. If there is strong clinical suspicion of a mucopolysaccharidosis, please contact Dr Jacqui Calvin or Sarah Hogg to discuss further investigations. If urine glycosaminoglycan typing and white cell enzymes are normal and a storage disorder is still suspected clinically, urinary oligosaccharide and sialic acid analysis should be considered (see section 8.7). BGU Handbook version 4.0 June
18 7.8 HOMOCYSTEINE Measurement of total homocysteine is offered for the diagnosis and monitoring of inherited defects in homocystine metabolism, such as classical homocystinuria and methionine synthase deficiency. Free homocystine is not recommended as it is only detectable in plasma when the binding capacity of plasma proteins has been exceeded. The binding of homocystine to plasma protein, mainly albumin, seems to be saturable with a maximal capacity of about 140 µmol/l total homocysteine. Likewise urine homocystine will only be detectable when the binding capacity is exceeded. Indications: Marfanoid appearance Early onset vascular occlusive disease Lens dislocation (usually downward) Early onset osteoporosis Method: tandem mass spectrometry Sample Type: 0.5 ml Lithium heparin plasma. The sample should be separated within one hour of collection and the plasma stored frozen until analysis. Please contact your local laboratory prior to sample collection, to arrange. Transport: Freeze on receipt, despatch frozen by 1st class post Turn around time: 1 month Total homocysteine reference ranges: males females < 18 µmol/l < 16 µmol/l 7.9 ORGANIC ACIDS Analysis of organic acids in urine can assist in the diagnosis of a number of disorders including those of amino acid metabolism (e.g. MSUD, urea cycle defects). Indications: [Note: (+) indicates occurring with other features ] Recurrent episodic ketosis, acidosis, vomiting and dehydration Reye-like syndrome Hypoglycaemia Hyperammonaemia Seizures (+) Seizures, ataxia, hypotonia Macrocephaly, dystonia, seizures, neurodegeneration Cardiomyopathy Unexplained lactic acidaemia Alopecia (+) Failure to thrive (+) Developmental Delay (+) Method: Solvent extraction followed by GC-MS of silylesters (qualitative) BGU Handbook version 4.0 June
19 Sample Type: Urine (plain). Volume is dependent on the urine creatinine concentration (the more dilute the urine the larger the volume required). Usually 5 ml is sufficient for analysis. Transport: Freeze urine on receipt and dispatch frozen (1 st class post or hospital transport) Turn Around Time: 5 working days Table 7.9: Organic Acids Sometimes Requested Individually Organic Acid N-Acetylaspartate Disease Canavan s Disease Glutarate, 3-hydroxyglutarate Glutaric aciduria type 1 Homogentisate 4-Hydroxybutyrate Orotate* Methylmalonate** Mevalonate Suberylglycine, hexanoylglycine Succinylacetone Alkaptonuria 4 hydroxybutyric aciduria Urea cycle defects MMA Mevalonic aciduria MCADD Tyrosinaemia type I * Orotate is quantitated using d3-orotate internal standard see below for more information. ** Methylmalonate is quantitated using d3-methylmalonate internal standard. Methylmalonate Reference range 0-1 yrs < 20 µmol/mmol creatinine 1 yr to adult < 10 µmol/mmol creatinine Mild increases (up to 100 µmol/mmol creatinine) in methylmalonate are not uncommon, they are not believed to be significant unless the patient is a breast fed infant where there is maternal vitamin B12 deficiency. The advice is usually to repeat in one month, although an earlier repeat is recommended if there is evidence of acidosis, lethargy, hypotonia or developmental delay OROTATE Orotic acid is an intermediate in the synthesis of pyrimidine nucleotides. In most defects of the urea cycle carbamoyl phosphate accumulates. This feeds into the pyrimidine biosynthetic pathway resulting in an excess of orotic acid. Mildly raised values have also been reported in mitochondrial disease. Indications: Differential diagnosis of urea cycle defects Disorders of pyrimidine metabolism Method: Tandem Mass Spectrometry Sample Type: 1 ml Urine (plain) Transport: Store frozen, dispatch frozen (1st class post or hospital transport) BGU Handbook version 4.0 June
20 Turn Around Time: 20 working days Reference Range: 0-2 years: 0 6 µmol/mmol creatinine Older than 2 years: 0-3 µmol/mmol creatinine NB. Samples collected following an allopurinol load test will be referred to Guy s Hospital for measurement of orotic acid and orotidine. (The Guy s protocol for this test is available from one of the BGU Clinical Scientists) VERY LONG CHAIN FATTY ACIDS, PRISTANATE AND PHYTANATE Peroxisomes are responsible for β oxidation of very long chain fatty acids (fatty acids with a carbon length more than 22), bile acid metabolism and plasmalogen synthesis. Peroxisomal disorders can be classified into 2 categories; defects in peroxisomal biogenesis disorders (eg Zellweger syndrome, infantile Refsum s disease) and defects in specific peroxisomal enzymes. Very long chain fatty acids are very sensitive for the diagnosis of X- linked adrenoleukodystrophy in males. However approximately 15% of symptomatic female carriers have normal very long chain fatty acids. Indications: [Note: (+) indicates occurring with other features ] Idiopathic adrenal insufficiency Hypotonia in males Ocular abnormalities Neurological abnormalities Skeletal abnormalities Leukodystrophy Dysmorphic features Ataxia Liver dysfunction (+) Seizures (+) Hepatomegaly Method: GC-MS Sample Type: 0.5 ml EDTA plasma. Separate plasma from cells within 2 hours of collection. Transport: Plasma is stable at room temp dispatch samples by 1 st class post or hospital transport. Turn Around Time: 10 working days Table 7.11: Plasma VLCFA Reference Ranges VLCFA (µmol/l) < 1 yr 1 10 yrs > 10 yrs C22 (docosanoate) C24 (tetracosanoate) C26 (hexacosanoate) C24/C22 ratio C26/C22 ratio Phytanate Pristanate BGU Handbook version 4.0 June
21 Phytanate and Pristanate Phytanate and pristanate are assayed as part of the plasma very long chain fatty acid profile (see above). They are useful in the diagnosis of Refsum s disease, α-methyl-acylcoa racemase deficiency and rhizomelic chondrodysplasia punctata (depending on the age of the patient). Pristanate and phytanate may be normal in young infants with peroxisomal biogenesis defects as both compounds are derived from exogenous, dietary sources MONITORING TREATMENT OF PHENYLKETONURIA Phenylketonuria is an autosomal recessive condition with an incidence of about 1 in 12,000. It is caused by a deficiency of phenylalanine hydroxylase which results in a marked increase in blood phenylalanine. Untreated, severe learning disability and spasticity ensues. Treatment is effective and consists of dietary phenylalanine restriction and supplementation of essential amino acids. Dried blood spot samples are used for monitoring and adjustment of the diet. The frequency of testing and the target concentration depends on the age of the patient. Method: Phenylalanine and tyrosine by tandem mass spectrometry Sample Type: Dried blood spot Transport: 1 st class post Turn Around Time: 1 working day 7.13 SWEAT TESTS The determination of sweat chloride concentration is useful in the diagnosis of cystic fibrosis. Sweat testing can be performed after 2 weeks of age on infants greater than 3 kg that are normally hydrated and without significant systemic illness. If clinically important, sweat testing can be attempted after one week of age but will need repeating if insufficient sweat is collected. A repeat test is recommended when the result is abnormal or borderline and the genotype is not confirmatory. Indications: Phenotype suggestive of CF (respiratory infection, exocrine pancreatic insufficiency) Positive newborn screening test Method: Pilocarpine, transported by iontophoresis is used to induce sweating. Sweat is collected via the capillary macroduct system. After collection the tube is sealed at both ends to prevent evaporation. The concentration of chloride ions is determined using an ion selective electrode. National guidelines for sweat collection are available. 2 Sweat collection can be arranged with the CF Clinical Nurse Specialist. Sample Type: Sweat collected into a Wescor Macroduct tube. National guidelines state not less than 1g/m 2 /min. A minimum sweat volume of 60 µl is required to enable duplicate analysis. Turn Around Time: 1 working day 21 BGU Handbook version 4.0 June 2009
22 Table 7.13: Sweat chloride Reference Range Sweat Chloride (mmol/l) Interpretation Less than 40 Low Probability of CF Equivocal Greater than 60 Supports diagnosis of CF 7.14 OTHER ASSAYS 7.14.a Ammonia Ammonia is primarily produced from the catabolism of amino acids. It is neurotoxic and is converted to urea in the liver via the urea cycle. Hyperammonaemia can present as an acute overwhelming crisis in the neonatal period or as a more insidious, episodic illness in children and adults. Indications: any one of the following: Neonate unexplained neurological deterioration Infant unexplained illness, particularly if male, history of sibling death or parental consanguinity Infant or child failure to thrive, feeding problems, vomiting, unexplained seizures chronic neurological problems (including developmental delay or regression or ataxia) Child or adult unexplained episodic illness (lethargy, cyclical vomiting, ataxia, seizures) particularly if precipitated by protein intake unexplained encephalopathy encephalitis, behavioural problems, psychosis unexplained progressive quadriplegia and learning disability Sample Type: 2 ml EDTA blood (keep air space in tube to a minimum) Transport: Sample must be sent immediately to the laboratory on ice and separated within 30 minute of collection. Turn Around Time: 1 working day 7.14.b Lactate Indications: Hypoglycaemia Hepatomegaly Neurodegeneration Encephalopathy Muscle disease Suspected mitochondrial disorder* Unexplained metabolic acidosis *See section 6.6 Sample Type: 1 ml fluoride oxalate plasma or CSF Transport: Send to the lab on ice. Plasma must be separated promptly. BGU Handbook version 4.0 June
23 Turn Around Time: 1 working day 8 REFERRED METABOLIC ASSAYS HYDROXYBUTYRATE AND FREE FATTY ACIDS These intermediary metabolites may be useful in the investigation of unexplained hypoglycaemia. (Please request a simultaneous lab glucose analysis.) Interpretation depends on the fed or fasted state of the patient. If hypoglycaemic, suppression of both 3OHB and FFA is consistent with hyperinsulinism whereas in FAOD the ratio of FFA to 3OHB is typically greater than 3. However these two diagnoses may be more easily made by analysing insulin at the time of hypoglycaemia and blood spot acylcarnitines. Sample: 1 ml fluoride tube on ice, separated and frozen within 30 minutes of venepuncture Transport: Store frozen, dispatch frozen 1 st class post Referral laboratory: Chemical Pathology, Sheffield Children s hospital DEHYDROCHOLESTEROL The Smith-Lemli-Opitz syndrome (SLO) is an autosomal recessive disorder with multiple congenital malformations (microcephaly, 2,3 syndactyly, cleft palate, congenital heart defects). There is a deficiency of sterol delta-7-reductase that causes an increase in the cholesterol precursor 7-DHC. Typically the total cholesterol is below 1.5 mmol/l (measured by GC-MS), with an increase in the 7DHC:cholesterol ratio. Sample Type: 0.5 ml lithium heparin or EDTA plasma, or serum Transport: 1 st Class post Referral laboratory: Chemical Pathology, Sheffield Children s Hospital 8.3 BILE ACID METABOLITES Inherited defects in bile acid biosynthesis cause cholestasis and malabsoption (due to bile acid deficiency), with progressive neurological dysfunction and xanthomas (due to deposition of precursors). Bile acids are partly synthesised in the peroxisome. Analysis is indicated in the workup of peroxisomal biogenesis disorders; however VLCFA remains the firstline test for this group of disorders. Sample type: 10 ml plain urine and/or 1 ml lithium heparin or EDTA plasma Sample storage: Store frozen prior to dispatch first class post Referral laboratory: Chemical Pathology, Sheffield Children s Hospital 23 BGU Handbook version 4.0 June 2009
24 8.4 BIOPTERINS AND DIHYDROPTERIDINE REDUCTASE The conversion of phenylalanine to tyrosine by phenylalanine hydroxylase requires a cofactor, tetrahydrobiopterin (BH4). This cofactor is also required for the synthesis of the neurotransmitters, serotonin and dopamine. Genetic defects in the synthesis or metabolism of biopterin cause hyperphenylalaninaemia and are associated with severe learning disability and a neurodegenerative course. All babies identified as presumptive positives on newborn PKU screening will be tested for biopterin defects. Interpretation depends on the phenylalanine concentration at time of sampling. For newborn screening samples this is organised by the laboratory and does not need to be requested separately. Please note that DHPR results are not valid within 6 weeks of a blood transfusion. Sample type: at least 2 dried blood spots (6 & 8 mm in diameter) Sample storage: Room temperature Referral laboratory: Newborn Screening Laboratory, Birmingham Children s Hospital 8.5 CARNITINE (TOTAL AND FREE) Plasma total and free carnitine is not recommended for the first line investigation of fatty acid oxidation defects as it gives less information than an acylcarnitine profile. However plasma carnitine and acylcarnitine analysis may be useful in the investigation of suspected carnitine transporter defects and carnitine palmitoyltransferase 2 deficiency, please phone one of the Clinical Scientists to discuss. NB: valproate treatment can cause low levels. Sample Type: 0.5 ml lithium heparin plasma. Sample storage: Store frozen if not sent immediately, 1 st class post Referral laboratory: BGU, Royal Victoria Infirmary, Newcastle 8.6 NEUROTRANSMITTERS The following metabolites may be analysed in CSF, depending on the clinical details provided: HVA, 5HIAA, 5-methyltetrahydrofolate (5MTHF), neopterin, dihydrobiopterin, tetrahydrobiopterin and pyridoxal phosphate. Clinical indications include oculogyric crises, temperature instability, ptosis, parkinsonian features and dystonia. This test is usually requested after the child has been reviewed by a paediatric neurologist. Sample Type: CSF collected into a set of three 1 ml tubes containing special preservatives and frozen on dry ice at the bedside. (Tubes and collection instructions are obtained from the Neurometabolic Unit, the National Hospital, Queen Square, London. Tel: ext 3844).) Sample storage: Once collected CSF should be stored at 70 o C and can be kept for more than a year. BGU Handbook version 4.0 June
25 Transport: Must be transported on dry ice. Referral laboratory: Neurometabolic Unit, National Hospital, London 8.7 OLIGOSACCHARIDES AND SIALIC ACID Oligosaccharides are low molecular weight carbohydrate polymers made up of at least 3 monosaccharide subunits. The oligosaccharides in urine are derived from the incomplete breakdown of carbohydrate side chains of complex glycoproteins. Abnormal oligosaccharides accumulate in a range of lysosomal storage diseases. Unfortunately this screening test is insensitive with poor specificity. The oligosaccharide excretion in glycoproteinoses may be variable and/or the abnormality subtle. Spurious results may be seen in patients infused with large amounts of complex carbohydrates (eg dextran). Neonates and breast fed infants show patterns which would be considered abnormal in older children. For these reasons urine oligosaccharide chromatography is not offered as a first line test by the BGU. If a lysosomal storage disorder is suspected clinically WCE analysis is recommended. If the WCE analyses and urine GAGS are normal and a storage disease is still suspected urines will be referred to the Willink, Manchester for urine oligosaccharides and sialic acid, to investigate the possibility of sialidosis or galactosialidosis. 8.8 PLASMALOGENS Plasmalogens are synthesized by peroxisomes and low values are encountered in a range of peroxisomal disorders. The plasmalogen assay is technically demanding and therefore its availability is limited. This assay is only available for cases of suspected Rhizomelic Chondrodysplasia Punctata (RCDP). Unlike Zellweger syndrome and other peroxisomal disorders plasma VLCFA are normal in patients with RCDP. Sample: 1 ml EDTA whole blood (RBC s washed x3 with 0.9% saline) Transport: Post special delivery Referral laboratory: Chemical Pathology, Sheffield Children s hospital 8.9 PURINES AND PYRIMIDINES Over 20 different disorders of purine and pyrimidine metabolism are known, of which several cause significant clinical disease. Three organ systems are prominently affected: kidneys (renal stones), bone marrow (immunodeficiency ± megaloblastic anaemia) and brain (neurological problems, e.g. abnormal muscle tone, dystonia, autistic-like behavioural problems), some patients may have more than one organ system affected. This test is often undertaken in children with unexplained neurological problems when first line investigations have drawn a blank. 25 BGU Handbook version 4.0 June 2009
26 Sample Type: 5 ml random urine, plain universal Sample storage: Store frozen until sending to lab by 1st class post Referral laboratory: Purine Research Laboratory, Guy s Hospital, London 8.10 TRANSFERRIN ISOELECTRIC FOCUSSING The initial screening test for congenital defects of glycosylation (CDG) is isoelectric focusing of transferrin to detect abnormal patterns of glycosylation. Enzyme analysis is available for confirmatory testing of some subtypes. The molecular basis of more than 20 defects have been identified however CDG Type Ia is the most common, accounting for approximately 80% of cases. Please note this test is unreliable in the first 3 weeks of life (reflects maternal transferrin). Indications: [Note: (+) indicates occurring with other features ] Psychomotor retardation Seizures (+) Strabismus Cerebellar hypoplasia Dysmorphy (fat pads, inverted nipples) Coagulopathy Protein-losing enteropathy (type 1b) In view of the extremely broad clinical spectrum of CDG patients, it is recommended that transferrin glycoform analysis is considered in any unexplained multisystem disorder. Sample Type: 1 ml serum or lithium heparin plasma Storage: Store frozen prior to dispatch by 1st Class Post Referral Laboratory: Neurometabolic Unit, National Hospital, London 8.11 WHITE CELL ENYZMES Lysosomal storage disorders result from a deficiency of a specific lysosomal enzyme, activator or transport protein, causing an accumulation of undegraded substrates within the lysosomes. Characteristically patients develop normally during the neonatal period but present with progressive deterioration in early childhood. Unlike small molecule metabolic disorders, presentation tends to be slow and more insidious. Symptoms include bone deformities and short stature, heart and respiratory difficulties, coarse facial features, an enlarged head, tongue, liver and spleen, and, in many patients, neurological degeneration. There are no reliable screening tests for these disorders (apart from urine GAGS in suspected mucopolysaccharidoses) and white cell enzyme analysis is often the first line test when there is a strong clinical suspicion of an LSD. Indications for analysis include hepatosplenomegaly, developmental regression, neurological deterioration, dysmorphic features, cherry red spot, leukodystrophy and angiokeratoma. Please contact the laboratory to discuss before taking samples. BGU Handbook version 4.0 June
27 Sample Type: 10 ml EDTA whole blood Sample storage: For Addenbrooke s patients please send whole blood (at room temperature) to the BGU urgently. Samples must be received before 2pm to enable same day despatch to Manchester. We are unable to accept samples on a Friday. Please phone in advance to enable the laboratory to arrange transport. Referral laboratory: Willink Laboratory, Manchester Children s Hospital Table 8.11: The following white cell enzymes (by disease) are offered as a battery of tests by the Willink laboratory. Enzyme Acid Esterase α-fucosidase α-galactosidase α-mannosidase α-n-acetylgalactosaminidase Arylsulphatase A β-galactosidase β-glucosidase β Glucuronidase β-hexosaminidase β-mannosidase Chitotriosidase Galactocerebrosidase Glycoaspariginase MUGS (methylumbelliferyl-nacetylglucosamine-6-sulphate substrate) Sphingomyelinase Disease Wolman's disease Fucosidosis Fabry's disease Alpha-mannosidosis Schindler disease Metachromatic leukodystrophy GM1 gangliosidosis Gaucher disease Sly's disease Sandhoff's Disease Beta-Mannosidosis Non-specific storage marker Krabbe leukodystrophy Aspartylglucosaminuria Tay-Sachs Niemann-Pick A&B Other enzymes are available on an individual basis (eg α-glucosidase for Pompe disease) please phone to discuss. 27 BGU Handbook version 4.0 June 2009
28 9 IN-HOUSE NON-METABOLIC ASSAYS 9.1 URINE VANILLYLMANDELIC ACID AND HOMOVANILLIC ACID Indications: Suspected neuroblastoma Hypertension Tachycardia Pallor Headache Method: Gas chromatography mass spectrometry Sample Type: Spot urine (plain) acidified with chcl (ph<2) in local laboratory within 12 hours of collection Transport: 1 st class post Turn Around Time: 3 working days Reference Ranges: VMA (µmol/mmol creatinine) HVA (µmol/mmol creatinine) Less than 2 years years years years URINE REDUCING SUBSTANCES Indications: Prolonged jaundice Method: Clinitest Sample Type: Spot urine (plain), refrigerate if not analysed within 2 hours of collection Turn Around Time: 2 days Reference Ranges: Reported as negative or percentage positive. If the sample is positive it will be tested for glucose (dipstick) 9.3 FAECAL REDUCING SUBSTANCES Indications: Unexplained diarrhoea Method: Clinitest Sample Type: At least 3 ml fresh faecal sample (must be frozen within 1 hour of collection) Turn Around Time: 2 days Reference Ranges: Reported as negative or percentage positive. BGU Handbook version 4.0 June
BIOCHEMICAL GENETICS UNIT METABOLIC ASSAYS
 BIOCHEMICAL GENETICS UNIT METABOLIC ASSAYS USERS HANDBOOK Version 5.0 PLEASE DO NOT USE AFTER JUNE 2011 BGU Handbook version 5.0 June 2010 1 CONTENTS CONTENTS 2 1 CONTACT DETAILS AND ENQUIRIES 3 2 GENERAL
BIOCHEMICAL GENETICS UNIT METABOLIC ASSAYS USERS HANDBOOK Version 5.0 PLEASE DO NOT USE AFTER JUNE 2011 BGU Handbook version 5.0 June 2010 1 CONTENTS CONTENTS 2 1 CONTACT DETAILS AND ENQUIRIES 3 2 GENERAL
BIOCHEMICAL GENETICS UNIT METABOLIC ASSAYS
 BIOCHEMICAL GENETICS UNIT METABOLIC ASSAYS USERS HANDBOOK Version 9.0 PLEASE DO NOT USE AFTER JUNE 2015 BGU Handbook version 9.0 July 2014 CONTENTS 1 CONTACT DETAILS AND ENQUIRIES...3 2 GENERAL INFORMATION...4
BIOCHEMICAL GENETICS UNIT METABOLIC ASSAYS USERS HANDBOOK Version 9.0 PLEASE DO NOT USE AFTER JUNE 2015 BGU Handbook version 9.0 July 2014 CONTENTS 1 CONTACT DETAILS AND ENQUIRIES...3 2 GENERAL INFORMATION...4
BIOCHEMICAL GENETICS UNIT METABOLIC ASSAYS
 BIOCHEMICAL GENETICS UNIT METABOLIC ASSAYS USERS HANDBOOK Version 10 PLEASE DO NOT USE AFTER JUNE 2016 BGU Handbook version 10 July 2015 CONTENTS CONTENTS... 2 1 CONTACT DETAILS AND ENQUIRIES... 3 2 GENERAL
BIOCHEMICAL GENETICS UNIT METABOLIC ASSAYS USERS HANDBOOK Version 10 PLEASE DO NOT USE AFTER JUNE 2016 BGU Handbook version 10 July 2015 CONTENTS CONTENTS... 2 1 CONTACT DETAILS AND ENQUIRIES... 3 2 GENERAL
INBORN ERRORS OF METABOLISM (IEM) IAP UG Teaching slides
 INBORN ERRORS OF METABOLISM (IEM) 1 OBJECTIVES What are IEMs? Categories When to suspect? History and clinical pointers Metabolic presentation Differential diagnosis Emergency and long term management
INBORN ERRORS OF METABOLISM (IEM) 1 OBJECTIVES What are IEMs? Categories When to suspect? History and clinical pointers Metabolic presentation Differential diagnosis Emergency and long term management
Dysmorphology Guy Besley
 Dysmorphology Guy Besley Willink Biochemical Genetics Unit, Manchester Children s s Hospital Dysmorphic presentation Congenital malformation Disorder of embryogenesis Intrauterine insult infection; chromosomal/genetic
Dysmorphology Guy Besley Willink Biochemical Genetics Unit, Manchester Children s s Hospital Dysmorphic presentation Congenital malformation Disorder of embryogenesis Intrauterine insult infection; chromosomal/genetic
National Metabolic Biochemistry Network Best Practice Guidelines. The Biochemical Investigation of Fits and Seizures for Inherited Metabolic Disorders
 Introduction National Metabolic Biochemistry Network Best Practice Guidelines The Biochemical Investigation of Fits and Seizures for Inherited Metabolic Disorders These guidelines describe the differential
Introduction National Metabolic Biochemistry Network Best Practice Guidelines The Biochemical Investigation of Fits and Seizures for Inherited Metabolic Disorders These guidelines describe the differential
Summary. Syndromic versus Etiologic. Definitions. Why does it matter? ASD=autism
 Summary It is becoming clear that multiple genes with complex interactions underlie autism spectrum (ASD). A small subset of people with ASD, however, actually suffer from rare single-gene Important to
Summary It is becoming clear that multiple genes with complex interactions underlie autism spectrum (ASD). A small subset of people with ASD, however, actually suffer from rare single-gene Important to
Training Syllabus CLINICAL SYLLABUS
 Training Syllabus CLINICAL SYLLABUS SYLLABUS FOR TRAINING IN CLINICAL PAEDIATRIC METABOLIC MEDICINE Updated July 2006 This syllabus is intended as a guide. Whilst the training should be comprehensive,
Training Syllabus CLINICAL SYLLABUS SYLLABUS FOR TRAINING IN CLINICAL PAEDIATRIC METABOLIC MEDICINE Updated July 2006 This syllabus is intended as a guide. Whilst the training should be comprehensive,
HA Convention 2016 Master course How to Handle Abnormal Newborn Metabolic Screening Results Causes, Management and Follow up
 HA Convention 2016 Master course How to Handle Abnormal Newborn Metabolic Screening Results Causes, Management and Follow up Dr. Josephine Chong Clinical Professional Consultant Centre of Inborn Errors
HA Convention 2016 Master course How to Handle Abnormal Newborn Metabolic Screening Results Causes, Management and Follow up Dr. Josephine Chong Clinical Professional Consultant Centre of Inborn Errors
The role of the laboratory in diagnosing lysosomal disorders
 The role of the laboratory in diagnosing lysosomal disorders Dr Guy Besley, formerly Willink Biochemical Genetics Unit, Manchester Children s Hospital, Manchester M27 4HA, UK. Lysosomal disorders What
The role of the laboratory in diagnosing lysosomal disorders Dr Guy Besley, formerly Willink Biochemical Genetics Unit, Manchester Children s Hospital, Manchester M27 4HA, UK. Lysosomal disorders What
Lab Guide 2019 Metabolic Section Lab Guide
 Lab Guide 2019 Metabolic Section Lab Guide Quantitative Amino acids Plasma Plasma. Container/Tube: Preferred EDTA, Place immediately in ice. Acceptable: lithium heparin, sodium heparin. Patient preparation:
Lab Guide 2019 Metabolic Section Lab Guide Quantitative Amino acids Plasma Plasma. Container/Tube: Preferred EDTA, Place immediately in ice. Acceptable: lithium heparin, sodium heparin. Patient preparation:
METABOLIC TEST REPERTOIRE
 METABOLIC TEST REPERTOIRE Department of Medical Biochemistry and Immunology University Hospital of Wales Heath Park Cardiff CF14 4XW Metabolic section open Monday to Friday 08:45 to 17:15 For Laboratory
METABOLIC TEST REPERTOIRE Department of Medical Biochemistry and Immunology University Hospital of Wales Heath Park Cardiff CF14 4XW Metabolic section open Monday to Friday 08:45 to 17:15 For Laboratory
National Metabolic Biochemistry Network Guidelines for the investigation of hypoglycaemia in infants and children
 National Metabolic Biochemistry Network Guidelines for the investigation of hypoglycaemia in infants and children Aim To provide guidance on the biochemical investigation of hypoglycaemia in infants and
National Metabolic Biochemistry Network Guidelines for the investigation of hypoglycaemia in infants and children Aim To provide guidance on the biochemical investigation of hypoglycaemia in infants and
CSF Investigations in patients with seizures. Dr Simon Olpin Sheffield Children s Hospital
 CSF Investigations in patients with seizures Dr Simon Olpin Sheffield Children s Hospital Background Epileptic seizures common feature in many inherited metabolic disorders particularly those involving
CSF Investigations in patients with seizures Dr Simon Olpin Sheffield Children s Hospital Background Epileptic seizures common feature in many inherited metabolic disorders particularly those involving
Newborn Screening & Methods for Diagnosing Inborn Errors of Metabolism
 Newborn Screening & Methods for Diagnosing Inborn Errors of Metabolism Patricia Jones, PhD DABCC FACB UT Southwestern Medical Center Children s Medical Center Dallas, Texas Learning Objectives Justify
Newborn Screening & Methods for Diagnosing Inborn Errors of Metabolism Patricia Jones, PhD DABCC FACB UT Southwestern Medical Center Children s Medical Center Dallas, Texas Learning Objectives Justify
e-learning Fatty Acid Oxidation Defects Camilla Reed and Dr Simon Olpin Sheffield Children s Hospital
 e-learning Fatty Acid Oxidation Defects Camilla Reed and Dr Simon Olpin Sheffield Children s Hospital Fatty Acids Fatty acids are a major source of energy and body fat is an energy dense material. They
e-learning Fatty Acid Oxidation Defects Camilla Reed and Dr Simon Olpin Sheffield Children s Hospital Fatty Acids Fatty acids are a major source of energy and body fat is an energy dense material. They
Module : Clinical correlates of disorders of metabolism Block 3, Week 2
 Module : Clinical correlates of disorders of metabolism Block 3, Week 2 Department of Paediatrics and Child Health University of Pretoria Tutor : Prof DF Wittenberg : dwittenb@medic.up.ac.za Aim of this
Module : Clinical correlates of disorders of metabolism Block 3, Week 2 Department of Paediatrics and Child Health University of Pretoria Tutor : Prof DF Wittenberg : dwittenb@medic.up.ac.za Aim of this
Inborn Error Of Metabolism :
 Inborn Error Of Metabolism : Inborn Error Of Metabolism inborn error of metabolism are a large group of hereditary biochemical diseases in which specific gene mutation cause abnormal or missing proteins
Inborn Error Of Metabolism : Inborn Error Of Metabolism inborn error of metabolism are a large group of hereditary biochemical diseases in which specific gene mutation cause abnormal or missing proteins
Routine Newborn Screening, Testing the Newborn Inherited Metabolic Disorders Update August 2015
 Routine Newborn Screening, Testing the Newborn Inherited Metabolic Disorders Update August 2015 Metabolic birth defects can cause physical problems, mental retardation and, in some cases, death. It is
Routine Newborn Screening, Testing the Newborn Inherited Metabolic Disorders Update August 2015 Metabolic birth defects can cause physical problems, mental retardation and, in some cases, death. It is
Biochemical Genetics
 Biochemical Genetics Laboratory Handbook 20010/11 Block 20 St James s Hospital Beckett Street Leeds LS9 7TF Introduction The Biochemical Genetics service is comprised of two regional laboratories, screening
Biochemical Genetics Laboratory Handbook 20010/11 Block 20 St James s Hospital Beckett Street Leeds LS9 7TF Introduction The Biochemical Genetics service is comprised of two regional laboratories, screening
Syllabus for Training in Inborn Errors of Metabolism for Scientists and Medically Qualified Laboratory Staff
 Training Syllabus LABORATORY SYLLABUS Syllabus for Training in Inborn Errors of Metabolism for Scientists and Medically Qualified Laboratory Staff This syllabus is intended as a guide. Whilst the training
Training Syllabus LABORATORY SYLLABUS Syllabus for Training in Inborn Errors of Metabolism for Scientists and Medically Qualified Laboratory Staff This syllabus is intended as a guide. Whilst the training
Childhood epilepsy: the biochemical epilepsies. Dr Colin D Ferrie Consultant Paediatric Neurologist Leeds General Infirmary
 Childhood epilepsy: the biochemical epilepsies Dr Colin D Ferrie Consultant Paediatric Neurologist Leeds General Infirmary Definitions Epileptic Seizure Manifestation(s) of epileptic (excessive and/or
Childhood epilepsy: the biochemical epilepsies Dr Colin D Ferrie Consultant Paediatric Neurologist Leeds General Infirmary Definitions Epileptic Seizure Manifestation(s) of epileptic (excessive and/or
Urea Cycle Defects. Dr Mick Henderson. Biochemical Genetics Leeds Teaching Hospitals Trust. MetBioNet IEM Introductory Training
 Urea Cycle Defects Dr Mick Henderson Biochemical Genetics Leeds Teaching Hospitals Trust The Urea Cycle The urea cycle enables toxic ammonia molecules to be converted to the readily excreted and non toxic
Urea Cycle Defects Dr Mick Henderson Biochemical Genetics Leeds Teaching Hospitals Trust The Urea Cycle The urea cycle enables toxic ammonia molecules to be converted to the readily excreted and non toxic
Metabolic Disorders. Chapter Thomson - Wadsworth
 Metabolic Disorders Chapter 28 1 Metabolic Disorders Inborn errors of metabolism group of diseases that affect a wide variety of metabolic processes; defective processing or transport of amino acids, fatty
Metabolic Disorders Chapter 28 1 Metabolic Disorders Inborn errors of metabolism group of diseases that affect a wide variety of metabolic processes; defective processing or transport of amino acids, fatty
The spectrum and outcome of the. neonates with inborn errors of. metabolism at a tertiary care hospital
 The spectrum and outcome of the neonates with inborn errors of metabolism at a tertiary care hospital Dr. Sevim Ünal Neonatology Division, Ankara Children s Hematology Oncology Research Hospital, Ankara,
The spectrum and outcome of the neonates with inborn errors of metabolism at a tertiary care hospital Dr. Sevim Ünal Neonatology Division, Ankara Children s Hematology Oncology Research Hospital, Ankara,
Information for health professionals
 Introduction of a new screening test for newborn babies in Wales Newborn bloodspot screening for Medium chain acyl-coa dehydrogenase deficiency (MCADD) Newborn bloodspot screening for MCADD is being introduced
Introduction of a new screening test for newborn babies in Wales Newborn bloodspot screening for Medium chain acyl-coa dehydrogenase deficiency (MCADD) Newborn bloodspot screening for MCADD is being introduced
Newborn Screen & Development Facts about the genetic diseases new since March 2006 (Excluding Cystic Fibrosis)
 Newborn Screen & Development Facts about the genetic diseases new since March 2006 (Excluding Cystic Fibrosis) 1) Argininosuccinic acidemia (ASA) a) Incidence: ~1 in 70,000 b) Deficiency in an enzyme of
Newborn Screen & Development Facts about the genetic diseases new since March 2006 (Excluding Cystic Fibrosis) 1) Argininosuccinic acidemia (ASA) a) Incidence: ~1 in 70,000 b) Deficiency in an enzyme of
Clinical Approach to Diagnosis of Lysosomal Storage Diseases
 Clinical Approach to Diagnosis of Lysosomal Storage Diseases M. Rohrbach, MD, PhD FMH Pädiatrie und FMH Medizinische Genetik Abteilung Stoffwechsel Universitätskinderklinik Zürich Lysosomal storage disorders
Clinical Approach to Diagnosis of Lysosomal Storage Diseases M. Rohrbach, MD, PhD FMH Pädiatrie und FMH Medizinische Genetik Abteilung Stoffwechsel Universitätskinderklinik Zürich Lysosomal storage disorders
Presentation and investigation of mitochondrial disease in children
 Presentation and investigation of mitochondrial disease in children Andrew Morris Willink Unit, Manchester Mitochondrial function Carbohydrate Fat Respiratory chain Energy Mitochondria are the product
Presentation and investigation of mitochondrial disease in children Andrew Morris Willink Unit, Manchester Mitochondrial function Carbohydrate Fat Respiratory chain Energy Mitochondria are the product
Newborn Screening in Manitoba. Information for Health Care Providers
 Newborn Screening in Manitoba Information for Health Care Providers Newborn screening: a healthy start leads to a healthier life Health care professionals have provided newborn screening for phenylketonuria
Newborn Screening in Manitoba Information for Health Care Providers Newborn screening: a healthy start leads to a healthier life Health care professionals have provided newborn screening for phenylketonuria
SYLLABUS FOR TRAINING IN CLINICAL PAEDIATRIC METABOLIC MEDICINE
 SYLLABUS FOR TRAINING IN CLINICAL PAEDIATRIC METABOLIC MEDICINE Updated December 2014: Vassili Valayannopoulos and Andrew Morris Paediatrics is an independent medical specialty based on the knowledge and
SYLLABUS FOR TRAINING IN CLINICAL PAEDIATRIC METABOLIC MEDICINE Updated December 2014: Vassili Valayannopoulos and Andrew Morris Paediatrics is an independent medical specialty based on the knowledge and
Inborn Errors of Metabolism Clinical Approach to Diagnosis and Treatment
 Case Scenario: 15 year old girl presented in ED with aggressive behaviour and hallucinations. No associated fever, vomiting, seizures or developmental concerns Previously well Inborn Errors of Metabolism
Case Scenario: 15 year old girl presented in ED with aggressive behaviour and hallucinations. No associated fever, vomiting, seizures or developmental concerns Previously well Inborn Errors of Metabolism
Newborn Bloodspot Screening (NBS) Training for Health Visitors. December 2017
 Newborn Bloodspot Screening (NBS) Training for Health Visitors www.newbornbloodspotscreening.wales.nhs.uk December 2017 Aims To enable you to gain a clear understanding of the following: Aim and rationale
Newborn Bloodspot Screening (NBS) Training for Health Visitors www.newbornbloodspotscreening.wales.nhs.uk December 2017 Aims To enable you to gain a clear understanding of the following: Aim and rationale
Newborn Screening: Blood Spot Disorders
 Newborn Screening: Blood Spot Disorders Arizona s Newborn Screening Program Program Overview Panel of Disorders Disorder Descriptions Program Components Hospitals ADHS Lab ADHS Follow-up ADHS Billing Medical
Newborn Screening: Blood Spot Disorders Arizona s Newborn Screening Program Program Overview Panel of Disorders Disorder Descriptions Program Components Hospitals ADHS Lab ADHS Follow-up ADHS Billing Medical
Peroxisomal Disorders
 Peroxisomal Disorders George Gray Birmingham Childrens Hospital Peroxisomal Disorders Peroxisomes are large single membrane bound organelles that are present in the cytoplasm of all cells. They are formed
Peroxisomal Disorders George Gray Birmingham Childrens Hospital Peroxisomal Disorders Peroxisomes are large single membrane bound organelles that are present in the cytoplasm of all cells. They are formed
Introduction to Organic Acidemias. Hilary Vernon, MD PhD Assistant Professor of Genetic Medicine Johns Hopkins University 7.25.
 Introduction to Organic Acidemias Hilary Vernon, MD PhD Assistant Professor of Genetic Medicine Johns Hopkins University 7.25.2014 A Brief Historical Overview Garrod, Archibald E. 1902. The Incidence of
Introduction to Organic Acidemias Hilary Vernon, MD PhD Assistant Professor of Genetic Medicine Johns Hopkins University 7.25.2014 A Brief Historical Overview Garrod, Archibald E. 1902. The Incidence of
Amino Acid Oxidation and the Urea Cycle
 Amino Acid Oxidation and the Urea Cycle Amino Acids: Final class of biomolecules whose oxidation contributes significantly to the generation of energy Undergo oxidation in three metabolic circumstances
Amino Acid Oxidation and the Urea Cycle Amino Acids: Final class of biomolecules whose oxidation contributes significantly to the generation of energy Undergo oxidation in three metabolic circumstances
UK NATIONAL METABOLIC BIOCHEMISTRY NETWORK GUIDELINES FOR THE INVESTIGATION OF HYPERAMMONAEMIA
 UK NATIONAL METABOLIC BIOCHEMISTRY NETWORK GUIDELINES FOR THE INVESTIGATION OF HYPERAMMONAEMIA Hyperammonaemia results from defective catabolism of amino acids to urea. Recognition and treatment of hyperammonaemia,
UK NATIONAL METABOLIC BIOCHEMISTRY NETWORK GUIDELINES FOR THE INVESTIGATION OF HYPERAMMONAEMIA Hyperammonaemia results from defective catabolism of amino acids to urea. Recognition and treatment of hyperammonaemia,
Neurodegenerative disorders: an approach to investigation. Robert Robinson Practical Paediatric Neurology Study Days April 2018
 Neurodegenerative disorders: an approach to investigation Robert Robinson Practical Paediatric Neurology Study Days April 2018 Aims An approach to investigating and diagnosing young children with progressive
Neurodegenerative disorders: an approach to investigation Robert Robinson Practical Paediatric Neurology Study Days April 2018 Aims An approach to investigating and diagnosing young children with progressive
Acylcarnitine measurement in blood spots: methodological aspects, problems and pitfalls with reference to the ERNDIM QA scheme
 Acylcarnitine measurement in blood spots: methodological aspects, problems and pitfalls with reference to the ERNDIM QA scheme Charles Turner Laboratory Guy s Hospital (Evelina Childrens Hospital, St Thomas
Acylcarnitine measurement in blood spots: methodological aspects, problems and pitfalls with reference to the ERNDIM QA scheme Charles Turner Laboratory Guy s Hospital (Evelina Childrens Hospital, St Thomas
Metabolic Disorders Screened Overseas but not Screened in Australia Condition Features Inherited Diagnosis Treatment Newborn Screen
 Metabolic Disorders ed Overseas but not ed in Australia Biotinidase Deficiency Severe form causes seizures & delay Biotin can prevent complications NZ, USA Tyrosinaemia Type I Coma & death before age 10
Metabolic Disorders ed Overseas but not ed in Australia Biotinidase Deficiency Severe form causes seizures & delay Biotin can prevent complications NZ, USA Tyrosinaemia Type I Coma & death before age 10
Inborn Errors of Metabolism. Metabolic Pathway. Digestion and Fasting. How is Expanded Newborn Screening Different? MS/MS. The body is a factory.
 Inborn Errors of Metabolism The body is a factory. Inborn errors of metabolism are rare genetic disorders in which the body cannot properly turn food into energy. The disorders are usually caused by defects
Inborn Errors of Metabolism The body is a factory. Inborn errors of metabolism are rare genetic disorders in which the body cannot properly turn food into energy. The disorders are usually caused by defects
SHEFFIELD CHILDREN S NHS FOUNDATION TRUST CLINICAL CHEMISTRY & SHEFFIELD DIAGNOSTIC GENETICS SERVICE USER S HANDBOOK FOR METABOLIC INVESTIGATIONS
 SHEFFIELD CHILDREN S NHS FOUNDATION TRUST CLINICAL CHEMISTRY & SHEFFIELD DIAGNOSTIC GENETICS SERVICE USER S HANDBOOK FOR METABOLIC INVESTIGATIONS APRIL 2019 Do Not Use This Edition after March 2020 103002
SHEFFIELD CHILDREN S NHS FOUNDATION TRUST CLINICAL CHEMISTRY & SHEFFIELD DIAGNOSTIC GENETICS SERVICE USER S HANDBOOK FOR METABOLIC INVESTIGATIONS APRIL 2019 Do Not Use This Edition after March 2020 103002
Best Practice Guidelines for the Biochemical Investigation of Patients with Foetal and Neonatal Hydrops
 National Metabolic Biochemistry Network Best Practice Guidelines for the Biochemical Investigation of Patients with Foetal and Neonatal Hydrops INTRODUCTION Hydrops is defined as the presence of skin oedema
National Metabolic Biochemistry Network Best Practice Guidelines for the Biochemical Investigation of Patients with Foetal and Neonatal Hydrops INTRODUCTION Hydrops is defined as the presence of skin oedema
Lecture 10 - Protein Turnover and Amino Acid Catabolism
 Lecture 10 - Protein Turnover and Amino Acid Catabolism Chem 454: Regulatory Mechanisms in Biochemistry University of Wisconsin-Eau Claire 1 Introduction 2 Proteins are degraded into amino acids. Protein
Lecture 10 - Protein Turnover and Amino Acid Catabolism Chem 454: Regulatory Mechanisms in Biochemistry University of Wisconsin-Eau Claire 1 Introduction 2 Proteins are degraded into amino acids. Protein
D evelopmental disabilities occur in approximately
 1128 REVIEW Developmental delay: when to suspect and how to investigate for an inborn error of metabolism M A Cleary, A Green... The purpose of this review is to provide a practical guideline on the suspicion
1128 REVIEW Developmental delay: when to suspect and how to investigate for an inborn error of metabolism M A Cleary, A Green... The purpose of this review is to provide a practical guideline on the suspicion
So Much More Than The PKU Test
 Newborn Metabolic Screening So Much More Than The PKU Test Sarah Viall, MSN, PPCNP BC Newborn Screening Program Coordinator Division of Genetics & Metabolism Conflicts of Interest I have no conflicts of
Newborn Metabolic Screening So Much More Than The PKU Test Sarah Viall, MSN, PPCNP BC Newborn Screening Program Coordinator Division of Genetics & Metabolism Conflicts of Interest I have no conflicts of
Medical Foods for Inborn Errors of Metabolism
 Medical Foods for Inborn Errors of Metabolism Policy Number: Original Effective Date: MM.02.014 02/18/2000 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 08/23/2013 Section: Medicine Place(s)
Medical Foods for Inborn Errors of Metabolism Policy Number: Original Effective Date: MM.02.014 02/18/2000 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 08/23/2013 Section: Medicine Place(s)
What s New in Newborn Screening?
 What s New in Newborn Screening? Funded by: Illinois Department of Public Health Information on Newborn Screening Newborn screening in Illinois is mandated and administered by the Illinois Department of
What s New in Newborn Screening? Funded by: Illinois Department of Public Health Information on Newborn Screening Newborn screening in Illinois is mandated and administered by the Illinois Department of
Newborn screening for congenital metabolic diseases Optional out-of-pocket tests Information Sheet
 Newborn screening for congenital metabolic diseases Optional out-of-pocket tests Information Sheet Website:www.tipn.org.tw Telephone:(02)85962050 Ext. 401-403 Service line:(02)85962065 Fax:(02)85962067
Newborn screening for congenital metabolic diseases Optional out-of-pocket tests Information Sheet Website:www.tipn.org.tw Telephone:(02)85962050 Ext. 401-403 Service line:(02)85962065 Fax:(02)85962067
The breakdown of fats to provide energy occurs in segregated membrane-bound compartments
 CPT1a deficiency The breakdown of fats to provide energy occurs in segregated membrane-bound compartments of the cell known as mitochondria. Carnitine palmitoyltransferase Ia (CPT1a) is a protein that
CPT1a deficiency The breakdown of fats to provide energy occurs in segregated membrane-bound compartments of the cell known as mitochondria. Carnitine palmitoyltransferase Ia (CPT1a) is a protein that
NEWBORN METABOLIC SCREEN, MINNESOTA
 Lab Dept: Test Name: Chemistry NEWBORN METABOLIC SCREEN, MINNESOTA General Information Lab Order Codes: Synonyms: CPT Codes: Test Includes: PKUN Newborn Screen for Hyopothyroidism, Phenylketonuria (PKU),
Lab Dept: Test Name: Chemistry NEWBORN METABOLIC SCREEN, MINNESOTA General Information Lab Order Codes: Synonyms: CPT Codes: Test Includes: PKUN Newborn Screen for Hyopothyroidism, Phenylketonuria (PKU),
Inborn Errors of Metabolism (IEM)
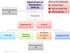 Clinical Presentation Inborn Errors of Metabolism (IEM) Click on the following: - Clinical Pearl - link to movie clip - link to picture Investigations Blood Work Urine No Acidosis NH 4 + Metabolic Acidosis
Clinical Presentation Inborn Errors of Metabolism (IEM) Click on the following: - Clinical Pearl - link to movie clip - link to picture Investigations Blood Work Urine No Acidosis NH 4 + Metabolic Acidosis
MetBioNet Best Practice Guidelines. Investigation of an inherited metabolic cause of cardiomyopathy
 Introduction MetBioNet Best Practice Guidelines Investigation of an inherited metabolic cause of Inborn errors of metabolism (IEM) account for at least 5% of cases of. In some disorders, is the major cause
Introduction MetBioNet Best Practice Guidelines Investigation of an inherited metabolic cause of Inborn errors of metabolism (IEM) account for at least 5% of cases of. In some disorders, is the major cause
Most common is the congenital adrenogenital syndrome (AGS) or congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency.
 Newborn Screening Examination parameters: TSH-neonatal (hypothyreosis), 17-OH progesterone (AGS), galactose (galactosemia), galactose-uridyl transferase (galacto semia), biotinidase (biotinidase ), phenylalanine
Newborn Screening Examination parameters: TSH-neonatal (hypothyreosis), 17-OH progesterone (AGS), galactose (galactosemia), galactose-uridyl transferase (galacto semia), biotinidase (biotinidase ), phenylalanine
Neonatal Biochemistry Investigation for Inherited Metabolic Disorders (IMDs)
 Neonatal Biochemistry Investigation for Inherited Metabolic Disorders (IMDs) Anne Green Birmingham Children s Hospital Leeds May 2005 Overview of how IMDs present cases Range of Disorders & Incidence disorders
Neonatal Biochemistry Investigation for Inherited Metabolic Disorders (IMDs) Anne Green Birmingham Children s Hospital Leeds May 2005 Overview of how IMDs present cases Range of Disorders & Incidence disorders
Inborn Errors of Metabolism in the Emergency Department. Will Davies June 2014
 Inborn Errors of Metabolism in the Emergency Department Will Davies June 2014 Inborn Errors of Metabolism in the Emergency Department Overview Although individually rare, altogether they are 1:1000-2500
Inborn Errors of Metabolism in the Emergency Department Will Davies June 2014 Inborn Errors of Metabolism in the Emergency Department Overview Although individually rare, altogether they are 1:1000-2500
Paediatric Clinical Chemistry
 Paediatric Clinical Chemistry Dr N Oosthuizen Dept Chemical Pathology UP 2011 Paediatric biochemistry The child is not a miniature adult Physiological development Immature organ systems Growing individual
Paediatric Clinical Chemistry Dr N Oosthuizen Dept Chemical Pathology UP 2011 Paediatric biochemistry The child is not a miniature adult Physiological development Immature organ systems Growing individual
For the Ongoing Management of Babies under 1 year old.
 Pathway Author: Nicy Turney, Senior Nurse Professional Lead, Health Visiting 19.06.17 For the Ongoing Management of Babies under 1 year old. Check Health Records (CHR) to undertake daily checks to identify:
Pathway Author: Nicy Turney, Senior Nurse Professional Lead, Health Visiting 19.06.17 For the Ongoing Management of Babies under 1 year old. Check Health Records (CHR) to undertake daily checks to identify:
What s New in Newborn Screening?
 What s New in Newborn Screening? Funded by: Illinois Department of Public Health Information on Newborn Screening Newborn screening in Illinois is administered by the Illinois Department of Public Health.
What s New in Newborn Screening? Funded by: Illinois Department of Public Health Information on Newborn Screening Newborn screening in Illinois is administered by the Illinois Department of Public Health.
Carnitine palmitoyl transferase 2 deficiency (CPT2) is a rare inherited disorder that occurs when
 CPT2 Deficiency Carnitine palmitoyl transferase 2 deficiency (CPT2) is a rare inherited disorder that occurs when the last step in the entry of fats into sac-like bodies called mitochondria is blocked.
CPT2 Deficiency Carnitine palmitoyl transferase 2 deficiency (CPT2) is a rare inherited disorder that occurs when the last step in the entry of fats into sac-like bodies called mitochondria is blocked.
A Guide for Prenatal Educators
 A Guide for Prenatal Educators Why Teach Newborn Screening This booklet is designed to make it easy for you, a prenatal educator, to effectively inform expectant parents about newborn screening. All of
A Guide for Prenatal Educators Why Teach Newborn Screening This booklet is designed to make it easy for you, a prenatal educator, to effectively inform expectant parents about newborn screening. All of
CLINICAL SIGNS SUGGESTIVE OF A NEUROMETABOLIC DISEASE. Bwee Tien Poll-The Amsterdam UMC The Netherlands
 CLINICAL SIGNS SUGGESTIVE OF A NEUROMETABOLIC DISEASE Bwee Tien Poll-The Amsterdam UMC The Netherlands FRAMEWORK OF PRINCIPALS 1. Problem-oriented clinical approach 2. Biomarkers in plasma, urine, CSF
CLINICAL SIGNS SUGGESTIVE OF A NEUROMETABOLIC DISEASE Bwee Tien Poll-The Amsterdam UMC The Netherlands FRAMEWORK OF PRINCIPALS 1. Problem-oriented clinical approach 2. Biomarkers in plasma, urine, CSF
Biochemistry: A Short Course
 Tymoczko Berg Stryer Biochemistry: A Short Course Second Edition CHAPTER 30 Amino Acid Degradation and the Urea Cycle 2013 W. H. Freeman and Company Chapter 30 Outline Amino acids are obtained from the
Tymoczko Berg Stryer Biochemistry: A Short Course Second Edition CHAPTER 30 Amino Acid Degradation and the Urea Cycle 2013 W. H. Freeman and Company Chapter 30 Outline Amino acids are obtained from the
Methylmalonic aciduria
 Methylmalonic aciduria Introductory information Written by: F. Hörster, S. Kölker & P. Burgard Reviewed & Revised for North America by: S. van Calcar Methylmalonic aciduria MMA 2 Methylmalonic aciduria
Methylmalonic aciduria Introductory information Written by: F. Hörster, S. Kölker & P. Burgard Reviewed & Revised for North America by: S. van Calcar Methylmalonic aciduria MMA 2 Methylmalonic aciduria
ERNDIM QC Schemes. Diagnostic Proficiency Scheme. Practice at Reporting and Interpretation
 ERNDIM QC Schemes Diagnostic Proficiency Scheme Practice at Reporting and Interpretation ERNDIM Proficiency Scheme: Design Clinical picture Lab results and interpretation Pre-investigations Amino acid
ERNDIM QC Schemes Diagnostic Proficiency Scheme Practice at Reporting and Interpretation ERNDIM Proficiency Scheme: Design Clinical picture Lab results and interpretation Pre-investigations Amino acid
Current Management and Future Developments in Metabolic Disease
 Current Management and Future Developments in Metabolic Disease APAGBI Annual Scientific Meeting Friday 15 th May 2015 Dr Saikat Santra Birmingham Children s Hospital, UK Outline Metabolic disorders in
Current Management and Future Developments in Metabolic Disease APAGBI Annual Scientific Meeting Friday 15 th May 2015 Dr Saikat Santra Birmingham Children s Hospital, UK Outline Metabolic disorders in
ERNDIM DPT Schemes. Common sample Sialidosis type I. Istanbul, 31 August Dr Christine Vianey-Saban, CHU Lyon
 ERNDIM DPT Schemes Common sample 2010 Sialidosis type I Dr Christine Vianey-Saban, CHU Lyon christine.saban@chu-lyon.fr Patient information on form at referral 43 year old woman who presented progressive
ERNDIM DPT Schemes Common sample 2010 Sialidosis type I Dr Christine Vianey-Saban, CHU Lyon christine.saban@chu-lyon.fr Patient information on form at referral 43 year old woman who presented progressive
A rough guide to Acylcarnitines
 A rough guide to Acylcarnitines Roy Talbot & Nigel Manning Roy.Talbot@sch.nhs.uk Dept. of Clinical Chemistry, Sheffield Children s Hospital Menu Acylcarnitines Basic Tandem MS theory SCADD MCADD LCHADD
A rough guide to Acylcarnitines Roy Talbot & Nigel Manning Roy.Talbot@sch.nhs.uk Dept. of Clinical Chemistry, Sheffield Children s Hospital Menu Acylcarnitines Basic Tandem MS theory SCADD MCADD LCHADD
HEREDITARY METABOLIC DISEASES
 HEREDITARY METABOLIC DISEASES Particular risk factors are: Advanced maternal age (e.g. Down's syndrome) Family history of inherited diseases (e.g. fragile X syndrome, Huntington's chorea) Previous child
HEREDITARY METABOLIC DISEASES Particular risk factors are: Advanced maternal age (e.g. Down's syndrome) Family history of inherited diseases (e.g. fragile X syndrome, Huntington's chorea) Previous child
7 Medical Genetics. Hemoglobinopathies. Hemoglobinopathies. Protein and Gene Structure. and Biochemical Genetics
 SESSION 7 Medical Genetics Hemoglobinopathies and Biochemical Genetics J a v a d F a s a J a m s h i d i U n i v e r s i t y o f M e d i c a l S c i e n c e s, N o v e m b e r 2 0 1 7 Hemoglobinopathies
SESSION 7 Medical Genetics Hemoglobinopathies and Biochemical Genetics J a v a d F a s a J a m s h i d i U n i v e r s i t y o f M e d i c a l S c i e n c e s, N o v e m b e r 2 0 1 7 Hemoglobinopathies
The laboratory investigation of lactic acidaemia. J Bonham/T Laing
 The laboratory investigation of lactic acidaemia J Bonham/T Laing Reference range Typical ranges for blood lactate are: Newborn 0.3-2.2 mmol/l Nielsen J et al1 1994 1-12mo 0.9-1.8 mmol/l Bonnefont et al
The laboratory investigation of lactic acidaemia J Bonham/T Laing Reference range Typical ranges for blood lactate are: Newborn 0.3-2.2 mmol/l Nielsen J et al1 1994 1-12mo 0.9-1.8 mmol/l Bonnefont et al
COMMON INHERITED METABOLIC CONDITIONS IN SOUTH AFRICA DIAGNOSING RARE DISEASE IN GENETICALLY UNIQUE AND UNDERSTUDIED POPULATION GROUPS
 COMMON INHERITED METABOLIC CONDITIONS IN SOUTH AFRICA DIAGNOSING RARE DISEASE IN GENETICALLY UNIQUE AND UNDERSTUDIED POPULATION GROUPS S MELDAU, G VAN DER WATT INHERITED METABOLIC DISEASES GROUP UCT /
COMMON INHERITED METABOLIC CONDITIONS IN SOUTH AFRICA DIAGNOSING RARE DISEASE IN GENETICALLY UNIQUE AND UNDERSTUDIED POPULATION GROUPS S MELDAU, G VAN DER WATT INHERITED METABOLIC DISEASES GROUP UCT /
Newborn Screening in Washington State Saving lives with a simple blood spot
 Newborn Screening in Washington State Saving lives with a simple blood spot Ashleigh Ragsdale, MPH Gauri Gupta, MScPH Objectives Newborn Screening Overview Process and Law Completing Collection Cards Video
Newborn Screening in Washington State Saving lives with a simple blood spot Ashleigh Ragsdale, MPH Gauri Gupta, MScPH Objectives Newborn Screening Overview Process and Law Completing Collection Cards Video
Newborn Bloodspot Screening Information for parents
 Newborn Bloodspot Screening Information for parents You will be offered a bloodspot screening test for your newborn baby for several rare but serious conditions. The sample for this test is usually taken
Newborn Bloodspot Screening Information for parents You will be offered a bloodspot screening test for your newborn baby for several rare but serious conditions. The sample for this test is usually taken
Organic acidaemias (OAs) & Urea cycle disorders (UCDs) PRESENTATION & MANAGEMENT
 Great Ormond Street Hospital London 20/04/2018 Organic acidaemias (OAs) & Urea cycle disorders (UCDs) PRESENTATION & MANAGEMENT Spyros P. Batzios, MD, MSc, PhD OAs & UCDs How do they present? neonatal
Great Ormond Street Hospital London 20/04/2018 Organic acidaemias (OAs) & Urea cycle disorders (UCDs) PRESENTATION & MANAGEMENT Spyros P. Batzios, MD, MSc, PhD OAs & UCDs How do they present? neonatal
TITLE: NURSING GUIDELINES FOR THE MANAGEMENT OF CHILDREN WITH METHYLMALONIC ACIDURIA. Eilish O Connell, Clinical Education Facilitator, NCIMD
 NO. OF PAGES: Page 1 of 17 SUPERCEDES: N/A NURSING GUIDELINES FOR THE MANAGEMENT OF CHILDREN WITH METHYLMALONIC ACIDURIA NAME/ Eilish O Connell, Clinical Education Facilitator, NCIMD SIGNATURE: DATE: NAME/
NO. OF PAGES: Page 1 of 17 SUPERCEDES: N/A NURSING GUIDELINES FOR THE MANAGEMENT OF CHILDREN WITH METHYLMALONIC ACIDURIA NAME/ Eilish O Connell, Clinical Education Facilitator, NCIMD SIGNATURE: DATE: NAME/
Newborn Bloodspot Screening Information for parents
 Newborn Bloodspot Screening Information for parents You will be offered a bloodspot screening test for your newborn baby for several rare but serious conditions. The sample for this test is usually taken
Newborn Bloodspot Screening Information for parents You will be offered a bloodspot screening test for your newborn baby for several rare but serious conditions. The sample for this test is usually taken
Metabolic diseases of the liver
 Metabolic diseases of the liver Central role in metabolism Causes and mechanisms of dysfunction Clinical patterns of metabolic disease Clinical approach to problem-solving Specific disorders Liver s central
Metabolic diseases of the liver Central role in metabolism Causes and mechanisms of dysfunction Clinical patterns of metabolic disease Clinical approach to problem-solving Specific disorders Liver s central
Test de aminoácidos. Orina.
 SPECIMEN VALIDITY per creatinine INTERVAL 2.5 th 16 th 50 th 84 th 97.5 th Creatinine 57 mg/dl 15-120 Glutamine/Glutamate 4.3 5-160 Ammonia Level (NH 4 ) 42800 μm/g 18000-100000 Specimen Validity Index
SPECIMEN VALIDITY per creatinine INTERVAL 2.5 th 16 th 50 th 84 th 97.5 th Creatinine 57 mg/dl 15-120 Glutamine/Glutamate 4.3 5-160 Ammonia Level (NH 4 ) 42800 μm/g 18000-100000 Specimen Validity Index
The Role of Organic Acids in the Diagnosis of Peroxisomal Biogenesis Disorders
 The Role of Organic Acids in the Diagnosis of Peroxisomal Biogenesis Disorders Catherine Dibden Northern General Hospital Sheffield Children s Hospital Peroxisomes Small sub-cellular organelles Present
The Role of Organic Acids in the Diagnosis of Peroxisomal Biogenesis Disorders Catherine Dibden Northern General Hospital Sheffield Children s Hospital Peroxisomes Small sub-cellular organelles Present
When users open the application interface, the starting page presents a disclaimer. Upon agreeing to the
 Walkthrough of IEMbase When users open the application interface, the starting page presents a disclaimer. Upon agreeing to the disclaimer, users are directed to the main page, which presents a search
Walkthrough of IEMbase When users open the application interface, the starting page presents a disclaimer. Upon agreeing to the disclaimer, users are directed to the main page, which presents a search
Suspected Metabolic Disease in the Newborn Period Acute Management "What do I do?" Barbara Marriage, PhD RD Abbott Nutrition
 Suspected Metabolic Disease in the Newborn Period Acute Management "What do I do?" Barbara Marriage, PhD RD Abbott Nutrition Introduction Review clinical findings that may be suspicious of a metabolic
Suspected Metabolic Disease in the Newborn Period Acute Management "What do I do?" Barbara Marriage, PhD RD Abbott Nutrition Introduction Review clinical findings that may be suspicious of a metabolic
Age-related reference ranges
 Authoriser: Peter Beresford Page 1 of 6 Age-related reference ranges Alkaline Phosphatase (ALP) IU/L Both less than 14 days 90 273 Both 14 days
Authoriser: Peter Beresford Page 1 of 6 Age-related reference ranges Alkaline Phosphatase (ALP) IU/L Both less than 14 days 90 273 Both 14 days
Beyond the case for NBS in South Africa. Chris Vorster 28/05/2016
 Beyond the case for NBS in South Africa Chris Vorster 28/05/2016 The case for NBS in SA Economic justification Cost Utility analysis = Cost/QALY GDP/Capita Immediate implementation (WHO) Political justification
Beyond the case for NBS in South Africa Chris Vorster 28/05/2016 The case for NBS in SA Economic justification Cost Utility analysis = Cost/QALY GDP/Capita Immediate implementation (WHO) Political justification
PROTEIN METABOLISM: SPECIFIC WAYS OF AMINO ACIDS CATABOLISM AND SYNTHESIS
 PROTEIN METABOLISM: SPECIFIC WAYS OF AMINO ACIDS CATABOLISM AND SYNTHESIS SPECIFIC WAYS OF AMINO ACID CATABOLISM After removing of amino group the carbon skeletons of amino acids are transformed into metabolic
PROTEIN METABOLISM: SPECIFIC WAYS OF AMINO ACIDS CATABOLISM AND SYNTHESIS SPECIFIC WAYS OF AMINO ACID CATABOLISM After removing of amino group the carbon skeletons of amino acids are transformed into metabolic
OVERVIEW M ET AB OL IS M OF FR EE FA TT Y AC ID S
 LIPOLYSIS LIPOLYSIS OVERVIEW CATABOLISM OF FREE FATTY ACIDS Nonesterified fatty acids Source:- (a) breakdown of TAG in adipose tissue (b) action of Lipoprotein lipase on plasma TAG Combined with Albumin
LIPOLYSIS LIPOLYSIS OVERVIEW CATABOLISM OF FREE FATTY ACIDS Nonesterified fatty acids Source:- (a) breakdown of TAG in adipose tissue (b) action of Lipoprotein lipase on plasma TAG Combined with Albumin
Positive Newborn Screens: What do you do next?
 Positive Newborn Screens: What do you do next? James B. Gibson, MD, Ph.D. Biochemical Geneticist at Specially for Children Clinical Associate Professor of Pediatrics UTHSCSA Carla R. Scott, MD Pediatric
Positive Newborn Screens: What do you do next? James B. Gibson, MD, Ph.D. Biochemical Geneticist at Specially for Children Clinical Associate Professor of Pediatrics UTHSCSA Carla R. Scott, MD Pediatric
Attachment 1. Newborn Screening Program Description
 Attachment 1 Newborn Screening Program Description The core mission of Texas Newborn Screening Program is to save children s lives through the early detection of life-threatening disorders. 34 Newborn
Attachment 1 Newborn Screening Program Description The core mission of Texas Newborn Screening Program is to save children s lives through the early detection of life-threatening disorders. 34 Newborn
Genética e Hígado: Cómo contribuye la genética en el algoritmo diagnóstico de la enfermedad hepática pediátrica? Nicholas Ah Mew, MD
 Genética e Hígado: Cómo contribuye la genética en el algoritmo diagnóstico de la enfermedad hepática pediátrica? Nicholas Ah Mew, MD April 24, 2017 SAP 2017 Buenos Aires Genetic disorders are rare why
Genética e Hígado: Cómo contribuye la genética en el algoritmo diagnóstico de la enfermedad hepática pediátrica? Nicholas Ah Mew, MD April 24, 2017 SAP 2017 Buenos Aires Genetic disorders are rare why
N.I.R.M.A.N. Dr. ANIL B. JALAN (MD DCH MCPS) Test list Oct 2017
 Tests and Profiles Tests Method Sample Needed TAT Genetic Consultation / - All samples to be sent at 2 8 C. Counseling 1 Urine MRST - 15 ml Urine 24 hrs 2 Sr. Ammonia, Lactate, Sugar Fuji Dry Chem 2 ml
Tests and Profiles Tests Method Sample Needed TAT Genetic Consultation / - All samples to be sent at 2 8 C. Counseling 1 Urine MRST - 15 ml Urine 24 hrs 2 Sr. Ammonia, Lactate, Sugar Fuji Dry Chem 2 ml
CHRONIC MYELOGENOUS LEUKEMIA
 CHRONIC MYELOGENOUS LEUKEMIA SHUFFLING THE GENETIC DECK IN CML 9 9 (q+) 22 Ph (22q-) bcr bcr-abl abl Fusion protein causes cancer GLEEVEC AND BCR-ABL FUSION PROTEIN GENETIC MEDICINE A. Genetic diseases
CHRONIC MYELOGENOUS LEUKEMIA SHUFFLING THE GENETIC DECK IN CML 9 9 (q+) 22 Ph (22q-) bcr bcr-abl abl Fusion protein causes cancer GLEEVEC AND BCR-ABL FUSION PROTEIN GENETIC MEDICINE A. Genetic diseases
Proficiency Testing Centre Czech Republic Annual Report 2017
 ERNDIM DPT Center Czech Republic Department of Pediatrics and Adolescent Medicine General Faculty Hospital and Charles University 1 st Faculty of Medicine Ke Karlovu 2, 128 08 Prague 2, Czech Republic
ERNDIM DPT Center Czech Republic Department of Pediatrics and Adolescent Medicine General Faculty Hospital and Charles University 1 st Faculty of Medicine Ke Karlovu 2, 128 08 Prague 2, Czech Republic
Date of commencement: February Principal Investigator Dr. Jayesh J. Sheth CASE RECORD FORM
 ICMR-FRIGE-MULTICENTRIC LSDs Project Foundation for Research in Genetics & Endocrinology [FRIGE], FRIGE House, Jodhpur Gam road, Satellite, Ahmedabad-380015 Tel no: 079-26921414, Fax no: 079-26921415 E-mail:
ICMR-FRIGE-MULTICENTRIC LSDs Project Foundation for Research in Genetics & Endocrinology [FRIGE], FRIGE House, Jodhpur Gam road, Satellite, Ahmedabad-380015 Tel no: 079-26921414, Fax no: 079-26921415 E-mail:
Genetic Testing FOR DISEASES OF INCREASED FREQUENCY IN THE ASHKENAZI JEWISH POPULATION
 Carrier Screening and Diagnostic Testing for the Ashkenazi Jewish Population Genetic Testing FOR DISEASES OF INCREASED FREQUENCY IN THE ASHKENAZI JEWISH POPULATION Our Science. Your Care. An extensive
Carrier Screening and Diagnostic Testing for the Ashkenazi Jewish Population Genetic Testing FOR DISEASES OF INCREASED FREQUENCY IN THE ASHKENAZI JEWISH POPULATION Our Science. Your Care. An extensive
Analysis of Neurotransmitters. Simon Heales
 Analysis of Neurotransmitters Simon Heales HealeS@gosh.nhs.uk Tyrosine O 2 L-Dopa Dopamine HVA Tryptophan 5-HTP PLP Serotonin 5-HIAA Phenylalanine Tyrosine BH4 qbh2 BH2 CSF Sample Requirements Tube 1 Tube
Analysis of Neurotransmitters Simon Heales HealeS@gosh.nhs.uk Tyrosine O 2 L-Dopa Dopamine HVA Tryptophan 5-HTP PLP Serotonin 5-HIAA Phenylalanine Tyrosine BH4 qbh2 BH2 CSF Sample Requirements Tube 1 Tube
Proficiency Testing Centre Basel Annual Report 2007
 ERNDIM DPT Center Basel University Children s Hospital Metabolic Unit Roemergasse 8, 4005 Basel, Switzerland phone: +41 61 685 62 75 or 61 685 63 23 fax: +41 61 685 60 16 Proficiency Testing Centre Basel
ERNDIM DPT Center Basel University Children s Hospital Metabolic Unit Roemergasse 8, 4005 Basel, Switzerland phone: +41 61 685 62 75 or 61 685 63 23 fax: +41 61 685 60 16 Proficiency Testing Centre Basel
ISOVALERIC ACIDAEMIA -ACUTE DECOMPENSATION (standard version)
 Contact Details Name: Hospital Telephone: This protocol has 5 pages ISOVALERIC ACIDAEMIA -ACUTE DECOMPENSATION (standard version) Please read carefully. Meticulous treatment is very important as there
Contact Details Name: Hospital Telephone: This protocol has 5 pages ISOVALERIC ACIDAEMIA -ACUTE DECOMPENSATION (standard version) Please read carefully. Meticulous treatment is very important as there
THE ED APPROACH OF THE CHILD WITH SUSPECTED METABOLIC DISEASE
 THE ED APPROACH OF THE CHILD WITH SUSPECTED METABOLIC DISEASE Dr. Nadeem Qureshi M.D, FAAP, FCCM Associate Professor Pediatrics School of Medicine. St Louis University Attending Physician Pediatric Emergency
THE ED APPROACH OF THE CHILD WITH SUSPECTED METABOLIC DISEASE Dr. Nadeem Qureshi M.D, FAAP, FCCM Associate Professor Pediatrics School of Medicine. St Louis University Attending Physician Pediatric Emergency
Diagnostic Proficiency Testing Survey Annual Report
 ERNDIM DPT Centre Basel University Children s Hospital Metabolic Unit Postfach, CH-4005 Basel, Switzerland phone: ++41 61 685 62 75 or ++41 61 685 63 23 Diagnostic Proficiency Testing Survey 2009 Annual
ERNDIM DPT Centre Basel University Children s Hospital Metabolic Unit Postfach, CH-4005 Basel, Switzerland phone: ++41 61 685 62 75 or ++41 61 685 63 23 Diagnostic Proficiency Testing Survey 2009 Annual
