The comparison or control group may be allocated a placebo intervention, an alternative real intervention or no intervention at all.
|
|
|
- Jocelin Shepherd
- 6 years ago
- Views:
Transcription
1 1. RANDOMISED CONTROLLED TRIALS (Treatment studies) (Relevant JAMA User s Guides, Numbers IIA & B: references (3,4) Introduction: The most valid study design for assessing the effectiveness (both the benefits and harms) of therapeutic or preventive interventions is the randomised controlled trial (RCT). This is an experiment in which the investigator controls the random allocation of participants or study communities in the study population to the interventions of interest (i.e. exposure or intervention subgroup/s) or a comparison subgroup (i.e. the control group). Trials are considered the purest type of epidemiological study because the investigator has control over exposure allocation. If the investigator randomises individual participants or communities to intervention and comparison subgroups, it is possible to minimise differences in baseline characteristics between the groups that might influence the outcome of interest (i.e. it minimises confounding). The comparison or control group may be allocated a placebo intervention, an alternative real intervention or no intervention at all. If randomisation is successful and the groups are similar at baseline, the investigator can be more confident that observed differences in outcomes between the groups are related to the intervention rather than confounding factors. Trials have a number of potential limitations compared with other designs. For practical and ethical reasons some important questions cannot be investigated using an experimental design. Moreover when trials are possible, they are often conducted in artificial environments and with highly motivated volunteers. This may limit their generalisability to populations of interest. 4
2 GATE Checklist for Randomised Controlled Trials (Intervention: benefit or harm) Study author, title, publication reference Key 5 part study question (PECOT). Was it focussed? source pop: Study Population Participant selection Exposure (intervention) D E N E Outcome + - randomly allocated D C N C Comparison (control) D E = Denominator (D) for exposure (intervention) group, D C = D for comparison (control) group N E = Numerator (N) for exposure group, N C = N for comparison group SECTION 1: STUDY VALIDITY Evaluation criterion Exposures & Comparison Outcomes What were the key selection (inclusion & exclusion) criteria? Were they well defined? Were they replicable? Were inclusion & exclusion criteria appropriate given study question? What were the exposures (interventions) & comparison? Well defined? Replicable? Was assignment to exposure & comparison groups randomised? Was randomisation concealed? Was randomisation successful: were exposure & comparison groups similar at start of study? Were all participants analysed in groups to which randomised? Were participants, health workers, researchers blind to interventions? Apart from study interventions, were groups treated equally? Was compliance with interventions measured? Was it sufficient? What outcome measures were used? Well defined? Replicable? How complete was follow up? Was it sufficient? How many dropouts? Was outcome assessment blind? Appraised by: How well was this criterion addressed? Was follow up time sufficiently long to detect important effects on outcomes of interest? QUALITY OF STUDY DESIGN: How successfully do you think the study minimised bias? Very well = +, okay =, poorly = - 5
3 SECTION 2: STUDY RESULTS: MAGNITUDE & PRECISION OF EFFECTS What measures of occurrence (incidence / prevalence) & intervention effects (RR /RD /NNTs) were reported? What measures of precision of effects were reported (CIs, p-values)? THE NUMBERS TABLE: OCCURRENCE, EFFECT ESTIMATES & PRECISION Outcomes* & (T) Exposure event rate (EER=N E/D E/T) or mean* Comparison event rate (CER=N C/D C/T) or mean* Relative Risk* (RR = EER/CER) ± (95% CI) Risk difference or mean difference (RD = CER-EER) ± (95% CI) Number Needed to Treat* (NNT = 1/RD) ± (95% CI) * if outcomes continuous, can calculate means, mean differences, but not NNTs (don t usually calculate relative means) D E = Denominator (D) for exposure (intervention) group(s), D C = D for comparison (control) group N E = Numerator (N) for exposure group(s), N C = N for comparison group Could useful effect estimates (e.g. RR, RDs or mean differences, NNTs) be calculated? For benefits & harm? What was the magnitude and direction of the effect estimates? Was the precision of the effect estimates sufficient? If no statistically significant effects detected, was there sufficient power? If multi-centred RCT - were the results homogeneous between sites? QUALITY OF STUDY RESULTS: Useful, precise +/or sufficient power? Very good = +, okay =, poor = - SECTION 3: STUDY APPLICABILITY Exposures & Comparison Outcomes Was the source population for participants well described? Were participants representative of source population? Can the relevance / similarity of the participants to a specific target group(s) be determined? Were the characteristics of the study setting well described? e.g. rural, urban, inpatient, primary care Can the applicability of interventions be determined? Can the relevance of the comparison group management be determined? Were all important outcomes considered: benefits? harms? costs? Are likely benefits greater than potential harms & costs (or vice versa)? In what target group(s)? QUALITY OF STUDY APPLICABILITY: (a) Was it possible to determine applicability? Very well = +, okay =, poorly = - (b) Are findings applicable in your practice/setting? Very well = +, okay =, poorly = - 6
4 USERS GUIDE for GATE Checklist for Randomised Controlled Trials Study author, title, publication reference Key 5 part study question (PECOT). Was it focussed? source pop: Study Population Participant selection Exposure (intervention) D E N E Outcome + - randomly allocated D C N C Comparison (control) D E = Denominator (D) for exposure (intervention) group, D C = D for comparison (control) group N E = Numerator (N) for exposure group, N C = N for comparison group SECTION 1: STUDY VALIDITY Evaluation criterion Exposures & Comparison What were the key selection (inclusion & exclusion) criteria? Were they well defined? Were they replicable? Were inclusion & exclusion criteria appropriate given study question? What were the exposures (interventions) & comparison? Well defined? Replicable? Was assignment to exposure & comparison groups randomised? Was randomisation concealed? Appraised by: How well was this criterion addressed? List important selection criteria; e.g. age group, gender, risk profile, medical history. Usually in Methods section. There should be sufficient information in the paper (or referenced) to allow the reader to theoretically select a similar population Are the participants a relevant group to apply the study intervention to? (e.g. diagnostic tests are not very helpful in people with a very high probability of disease). Examples include: dosage of drugs, description of surgical procedure, number of faecal occult blood tests in a screening study, management in comparison group (e.g. check what care the comparison (placebo group) receive). Random allocation of interventions is fundamental to this type of study. The method of randomisation should be described (e.g. using a computer algorithm). If the description of the randomisation method is poor, or the process used is not truly random (e.g. allocation by date, alternating between one group and another) or can otherwise be seen as flawed, the study should be given a lower quality rating. This is an important quality issue as it has been shown that RCTs that failure to conceal allocation typically lead to an overestimate of the treatment effect. The ideal concealment process would involve an independent group that registered each new participant, determined the allocation and then informed the caregivers of the allocation. This can be done relatively simply by phone or fax or by using an automatic webbased system. This reduces the chance of the care giver influencing allocation. 7
5 Outcomes Was randomisation successful: were exposure & comparison groups similar at start of study? Were all participants analysed in groups to which randomised? Were participants, health workers, researchers blind to interventions? Apart from study interventions, were groups treated equally? Was compliance with interventions measured? Was it sufficient? What outcome measures were used? Well defined? Replicable? How complete was the follow up? How many dropouts were there? Was outcome assessment blind? Was follow up time sufficiently long to detect important effects on outcomes of interest? This can be judged by examining the similarity between baseline characteristics of the groups in each arm of the study. Successful randomisation will produce similar groups. The study should report significant differences in the composition of the study groups in relation to gender mix, age, stage of disease (if appropriate), social background, ethnic origin, or comorbid conditions. Of note, statistically significant differences are not always important in practice; consider if the differences are likely to be meaningful given the study questions. It is rarely that all participants allocated to the intervention group receive the intervention throughout the trial, or that all those in the comparison group do not. may refuse treatment, or contra-indications may arise. If the comparability of groups through randomisation is to be maintained, however, patient outcomes should be analysed according to the group to which they were originally allocated irrespective of the treatment they actually received. (This is known as intention to treat analysis.) If analysis were not on an intention to treat basis, the study validity could be compromised. Bias can be quantified by attempting both on-treatment and intention-to-treat analyses. Blinding can be carried out on up to three levels. Single blinding is where participants are unaware of which intervention they are receiving; in double blind studies neither the care giver nor the patient know which intervention is being given; in triple blind studies neither patients, care givers, nor those conducting the analysis are aware of which participants received which intervention. Most studies described as double blind studies are usually triple blind. The higher the level of blinding, the lower the risk of bias in the study. Although the nature of the intervention may unblind the allocation in some cases (eg. Surgical trials) the allocation to intervention & comparison groups should be blind. If some participants received additional interventions, even if of a minor nature or consisting of advice and counselling rather than a physical intervention, this can introduce confounding and may invalidate the results. If there is unequal intervention (apart from the study intervention) the study results should be interpreted with caution and given a low quality rating. Compliance is often a problem in studies involving ongoing interventions such as daily medication or behaviour change. Pill counts and blood levels of drugs are examples of objective methods of measuring compliance, although self-reports are more common but less reliable. Criteria for assessing outcomes such as diagnostic algorithms should be well described or referenced. It should be theoretically possible for the reader to replicate the process. The number of participants who drop out of a study is a concern if the number is very high. Conventionally, a 20% drop out rate is regarded as acceptable, but this depends on the study question. Some regard should be paid to why participants dropped out, as well as how many. It should be noted that the drop out rate may be expected to be higher in studies conducted over a long period of time. A higher drop out rate will normally lead to downgrading, rather than rejection of a study. Ideally the assessors who measure & record outcomes should be blind to participant allocation. This is more important for assessing outcomes that are not clear cut & where knowledge of the intervention may influence the diagnostic assessment. This is specific to the study intervention and outcomes assessed QUALITY OF STUDY DESIGN: How successfully do you think the study minimised bias? Very well = +, okay =, poorly = - 8
6 SECTION 2: STUDY RESULTS: MAGNITUDE & PRECISION OF EFFECTS What measures of occurrence (incidence / prevalence) & intervention effects (RR /RD /NNTs) were reported? What measures of precision of effects were reported (CIs, p-values)? Some studies do not provide the relevant number of participants (D) in the exposure and comparison groups, the number of outcomes (N), the event rates / proportions with outcomes (N/D) in each study group, or the relevant measures of effect (RR, etc). If they are not reported or cannot be calculated, it is not possible to ascertain the accuracy of the effect estimates such as relative risk (RR), risk difference (RD) or mean differences (if continuous measures of outcome are given) and numbers needed to treat (NNT) see definitions below in the Numbers Table below. Either confidence intervals or p values for the estimates of effect should be reported or be possible to calculate. THE NUMBERS TABLE: OCCURRENCE, EFFECT ESTIMATES & PRECISION Outcomes* & (T) Exposure event rate (EER=N E/D E/T) or mean* Comparison event rate (CER=N C/D C/T) or mean* Relative Risk* (RR = EER/CER) ± (95% CI) Risk difference or mean difference (RD = CER-EER) ± (95% CI) Number Needed to Treat* (NNT = 1/RD) ± (95% CI) complete complete complete complete complete complete * if outcomes continuous, can calculate means, mean differences, but not NNTs (don t usually calculate relative means) D E = Denominator (D) for exposure (intervention) group(s), D C = D for comparison (control) group N E = Numerator (N) for exposure group(s), N C = N for comparison group Could useful effect estimates (e.g. RR, RDs or mean differences, NNTs) be calculated? For benefits & harm? What was the magnitude and direction of the effect estimates?(rr, RD, mean differences, NNTs) These numbers should be reported or able to be calculated in the Numbers Table (above). To be useful, they need to have some meaning in practice. For example a change of one point on a visual analogue scale of symptoms may have little meaning unless clearly linked to a symptom description. These numbers are the bottom line of every study. All other appraisal questions relate to the validity, precision and applicability of these numbers. The importance of these numbers in practice depends on the group to which they are applied (see Applicability - next section). Was the precision of the effect estimates sufficient? If no statistically significant effects detected, was there sufficient power? If multi-centred RCT - were effects homogeneous between sites? If 95% confidence intervals are wide and include the no effect point (e.g. RR=1, RD=0) or p-values are >> 0.05, then the precision of the estimates is likely to be poor & insufficient If an effect estimate is not significantly different from no effect and the confidence interval is wide, the study is probably not large enough to detect a real difference between treatment and comparison groups (i.e. a low power study). A non significant effect associated with a tight CI suggests there is no effect and that the study has adequate power. Look for a power calculation in the methods section. In multi-site studies, confidence in the results should be increased if it can be shown that similar results were obtained at the different participating centres. QUALITY OF STUDY RESULTS: Useful, precise +/or sufficient power? Very good = +, okay =, poor = - 9
7 SECTION 3: STUDY APPLICABILITY Exposures & Comparison Outcomes Was the source population for participants well described? Were participants representative of source population? Can the relevance / similarity of the participants to a specific target group(s) be determined? Were the characteristics of the study setting well described? e.g. rural, urban, inpatient, primary care Can the applicability of interventions be determined? Can the relevance of the comparison group management be determined? Were all important outcomes considered: benefits? harms? costs? Are likely benefits greater than potential harms & costs (or vice versa)? In what target group(s)? If the source population is not well described it is not easy to assess the generalisability of the study findings to a target group or whether the study participants are a typical or atypical subset of the source population. As above As above This helps determine the applicability of the interventions These should be described in some detail in the paper or referenced. It should be theoretically possible for the reader to replicate the process. It is important to determine whether the comparison group receive no interventions (e.g.placebo only) or whether they receive usual care. As usual care may differ in different settings, it is important to determine what usual care involves Many studies only report data on benefits of interventions. Decisions to intervene need to balance benefits, harms and costs. The benefits, harms and costs of interventions may differ between different groups of people due to severity, comorbidities etc. Ideally studies should describe the overall balance of risks, benefits and costs in different subgroups. QUALITY OF STUDY APPLICABILITY: (a) Was it possible to determine applicability? Very well = +, okay =, poorly = - (b) Are findings applicable in your practice/setting? Very well = +, okay =, poorly = - 10
STUDIES OF THE ACCURACY OF DIAGNOSTIC TESTS: (Relevant JAMA Users Guide Numbers IIIA & B: references (5,6))
 STUDIES OF THE ACCURACY OF DIAGNOSTIC TESTS: (Relevant JAMA Users Guide Numbers IIIA & B: references (5,6)) Introduction: The most valid study design for assessing the accuracy of diagnostic tests is a
STUDIES OF THE ACCURACY OF DIAGNOSTIC TESTS: (Relevant JAMA Users Guide Numbers IIIA & B: references (5,6)) Introduction: The most valid study design for assessing the accuracy of diagnostic tests is a
GATE CAT Intervention RCT/Cohort Studies
 GATE: a Graphic Approach To Evidence based practice updates from previous version in red Critically Appraised Topic (CAT): Applying the 5 steps of Evidence Based Practice Using evidence about interventions
GATE: a Graphic Approach To Evidence based practice updates from previous version in red Critically Appraised Topic (CAT): Applying the 5 steps of Evidence Based Practice Using evidence about interventions
1. Draft checklist for judging on quality of animal studies (Van der Worp et al., 2010)
 Appendix C Quality appraisal tools (QATs) 1. Draft checklist for judging on quality of animal studies (Van der Worp et al., 2010) 2. NICE Checklist for qualitative studies (2012) 3. The Critical Appraisal
Appendix C Quality appraisal tools (QATs) 1. Draft checklist for judging on quality of animal studies (Van der Worp et al., 2010) 2. NICE Checklist for qualitative studies (2012) 3. The Critical Appraisal
Critical Appraisal Series
 Definition for therapeutic study Terms Definitions Study design section Observational descriptive studies Observational analytical studies Experimental studies Pragmatic trial Cluster trial Researcher
Definition for therapeutic study Terms Definitions Study design section Observational descriptive studies Observational analytical studies Experimental studies Pragmatic trial Cluster trial Researcher
How to use this appraisal tool: Three broad issues need to be considered when appraising a case control study:
 CASP Checklist: 11 questions to help you make sense of a Case Control Study How to use this appraisal tool: Three broad issues need to be considered when appraising a case control study: Are the results
CASP Checklist: 11 questions to help you make sense of a Case Control Study How to use this appraisal tool: Three broad issues need to be considered when appraising a case control study: Are the results
Randomized Controlled Trial
 Randomized Controlled Trial Training Course in Sexual and Reproductive Health Research Geneva 2016 Dr Khalifa Elmusharaf MBBS, PgDip, FRSPH, PHD Senior Lecturer in Public Health Graduate Entry Medical
Randomized Controlled Trial Training Course in Sexual and Reproductive Health Research Geneva 2016 Dr Khalifa Elmusharaf MBBS, PgDip, FRSPH, PHD Senior Lecturer in Public Health Graduate Entry Medical
GLOSSARY OF GENERAL TERMS
 GLOSSARY OF GENERAL TERMS Absolute risk reduction Absolute risk reduction (ARR) is the difference between the event rate in the control group (CER) and the event rate in the treated group (EER). ARR =
GLOSSARY OF GENERAL TERMS Absolute risk reduction Absolute risk reduction (ARR) is the difference between the event rate in the control group (CER) and the event rate in the treated group (EER). ARR =
CONSORT 2010 checklist of information to include when reporting a randomised trial*
 CONSORT 2010 checklist of information to include when reporting a randomised trial* Section/Topic Title and abstract Introduction Background and objectives Item No Checklist item 1a Identification as a
CONSORT 2010 checklist of information to include when reporting a randomised trial* Section/Topic Title and abstract Introduction Background and objectives Item No Checklist item 1a Identification as a
GATE: Graphic Appraisal Tool for Epidemiology picture, 2 formulas & 3 acronyms
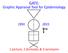 GATE: Graphic Appraisal Tool for Epidemiology 1991-2015 1 picture, 2 formulas & 3 acronyms 1 GATE: Graphic Appraisal Tool for Epidemiology Graphic Architectural Tool for Epidemiology Graphic Approach To
GATE: Graphic Appraisal Tool for Epidemiology 1991-2015 1 picture, 2 formulas & 3 acronyms 1 GATE: Graphic Appraisal Tool for Epidemiology Graphic Architectural Tool for Epidemiology Graphic Approach To
CRITICAL APPRAISAL SKILLS PROGRAMME Making sense of evidence about clinical effectiveness. 11 questions to help you make sense of case control study
 CRITICAL APPRAISAL SKILLS PROGRAMME Making sense of evidence about clinical effectiveness 11 questions to help you make sense of case control study General comments Three broad issues need to be considered
CRITICAL APPRAISAL SKILLS PROGRAMME Making sense of evidence about clinical effectiveness 11 questions to help you make sense of case control study General comments Three broad issues need to be considered
Critical Appraisal of Evidence
 Critical Appraisal of Evidence A Focus on Intervention/Treatment Studies Bernadette Mazurek Melnyk, PhD, CPNP/PMHNP, FNAP, FAAN Associate Vice President for Health Promotion University Chief Wellness Officer
Critical Appraisal of Evidence A Focus on Intervention/Treatment Studies Bernadette Mazurek Melnyk, PhD, CPNP/PMHNP, FNAP, FAAN Associate Vice President for Health Promotion University Chief Wellness Officer
Implementing scientific evidence into clinical practice guidelines
 Evidence Based Dentistry Implementing scientific evidence into clinical practice guidelines Asbjørn Jokstad University of Oslo, Norway 15/07/2004 1 PRACTICE GUIDELINES IN DENTISTRY (Medline) 2100 1945
Evidence Based Dentistry Implementing scientific evidence into clinical practice guidelines Asbjørn Jokstad University of Oslo, Norway 15/07/2004 1 PRACTICE GUIDELINES IN DENTISTRY (Medline) 2100 1945
Guidelines for Reporting Non-Randomised Studies
 Revised and edited by Renatus Ziegler B.C. Reeves a W. Gaus b a Department of Public Health and Policy, London School of Hygiene and Tropical Medicine, Great Britain b Biometrie und Medizinische Dokumentation,
Revised and edited by Renatus Ziegler B.C. Reeves a W. Gaus b a Department of Public Health and Policy, London School of Hygiene and Tropical Medicine, Great Britain b Biometrie und Medizinische Dokumentation,
RATING OF A RESEARCH PAPER. By: Neti Juniarti, S.Kp., M.Kes., MNurs
 RATING OF A RESEARCH PAPER RANDOMISED CONTROLLED TRIAL TO COMPARE SURGICAL STABILISATION OF THE LUMBAR SPINE WITH AN INTENSIVE REHABILITATION PROGRAMME FOR PATIENTS WITH CHRONIC LOW BACK PAIN: THE MRC
RATING OF A RESEARCH PAPER RANDOMISED CONTROLLED TRIAL TO COMPARE SURGICAL STABILISATION OF THE LUMBAR SPINE WITH AN INTENSIVE REHABILITATION PROGRAMME FOR PATIENTS WITH CHRONIC LOW BACK PAIN: THE MRC
Critical Appraisal of RCT
 Critical Appraisal of RCT What is critical appraisal? Definition Process of systematically examining research evidence to assess its reliability (validity/internal validity), results and relevance (external
Critical Appraisal of RCT What is critical appraisal? Definition Process of systematically examining research evidence to assess its reliability (validity/internal validity), results and relevance (external
GATE: Graphic Appraisal Tool for Epidemiology picture, 2 formulas & 3 acronyms
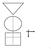 1 GATE: Graphic Appraisal Tool for Epidemiology 1991-2016 1 picture, 2 formulas & 3 acronyms 2 GATE: Graphic Appraisal Tool for Epidemiology Graphic Architectural Tool for Epidemiology Graphic Approach
1 GATE: Graphic Appraisal Tool for Epidemiology 1991-2016 1 picture, 2 formulas & 3 acronyms 2 GATE: Graphic Appraisal Tool for Epidemiology Graphic Architectural Tool for Epidemiology Graphic Approach
Checklist for appraisal of study relevance (child sex offenses)
 Appendix 3 Evaluation protocols. [posted as supplied by author] Checklist for appraisal of study relevance (child sex offenses) First author, year, reference number Relevance Yes No Cannot answer Not applicable
Appendix 3 Evaluation protocols. [posted as supplied by author] Checklist for appraisal of study relevance (child sex offenses) First author, year, reference number Relevance Yes No Cannot answer Not applicable
Module 5. The Epidemiological Basis of Randomised Controlled Trials. Landon Myer School of Public Health & Family Medicine, University of Cape Town
 Module 5 The Epidemiological Basis of Randomised Controlled Trials Landon Myer School of Public Health & Family Medicine, University of Cape Town Introduction The Randomised Controlled Trial (RCT) is the
Module 5 The Epidemiological Basis of Randomised Controlled Trials Landon Myer School of Public Health & Family Medicine, University of Cape Town Introduction The Randomised Controlled Trial (RCT) is the
11 questions to help you make sense of a case control study
 Critical Appraisal Skills Programme (CASP) making sense of evidence 11 questions to help you make sense of a case control study How to use this appraisal tool Three broad issues need to be considered when
Critical Appraisal Skills Programme (CASP) making sense of evidence 11 questions to help you make sense of a case control study How to use this appraisal tool Three broad issues need to be considered when
Evidence-based practice tutorial Critical Appraisal Skills
 Evidence-based practice tutorial Critical Appraisal Skills Earlier evidence based practice tutorials have focussed on skills to search various useful sites on the internet for evidence. Anyone who has
Evidence-based practice tutorial Critical Appraisal Skills Earlier evidence based practice tutorials have focussed on skills to search various useful sites on the internet for evidence. Anyone who has
Critical Appraisal of Evidence A Focus on Intervention/Treatment Studies
 Critical Appraisal of Evidence A Focus on Intervention/Treatment Studies Bernadette Mazurek Melnyk, PhD, CPNP/PMHNP, FAANP, FAAN Associate Vice President for Health Promotion University Chief Wellness
Critical Appraisal of Evidence A Focus on Intervention/Treatment Studies Bernadette Mazurek Melnyk, PhD, CPNP/PMHNP, FAANP, FAAN Associate Vice President for Health Promotion University Chief Wellness
Journal Club: Fairfax Internal Medicine. Stephanie Shin PGY-2 Bianca Ummat PGY-2 July 27, 2012
 Journal Club: Fairfax Internal Medicine Stephanie Shin PGY-2 Bianca Ummat PGY-2 July 27, 2012 S Objective Review EBM basic principles Learn how to apply the principles in critically appraising the article.
Journal Club: Fairfax Internal Medicine Stephanie Shin PGY-2 Bianca Ummat PGY-2 July 27, 2012 S Objective Review EBM basic principles Learn how to apply the principles in critically appraising the article.
Clinical research in AKI Timing of initiation of dialysis in AKI
 Clinical research in AKI Timing of initiation of dialysis in AKI Josée Bouchard, MD Krescent Workshop December 10 th, 2011 1 Acute kidney injury in ICU 15 25% of critically ill patients experience AKI
Clinical research in AKI Timing of initiation of dialysis in AKI Josée Bouchard, MD Krescent Workshop December 10 th, 2011 1 Acute kidney injury in ICU 15 25% of critically ill patients experience AKI
Determinants of quality: Factors that lower or increase the quality of evidence
 Determinants of quality: Factors that lower or increase the quality of evidence GRADE Workshop CBO, NHG and Dutch Cochrane Centre CBO, April 17th, 2013 Outline The GRADE approach: step by step Factors
Determinants of quality: Factors that lower or increase the quality of evidence GRADE Workshop CBO, NHG and Dutch Cochrane Centre CBO, April 17th, 2013 Outline The GRADE approach: step by step Factors
Protocol Development: The Guiding Light of Any Clinical Study
 Protocol Development: The Guiding Light of Any Clinical Study Susan G. Fisher, Ph.D. Chair, Department of Clinical Sciences 1 Introduction Importance/ relevance/ gaps in knowledge Specific purpose of the
Protocol Development: The Guiding Light of Any Clinical Study Susan G. Fisher, Ph.D. Chair, Department of Clinical Sciences 1 Introduction Importance/ relevance/ gaps in knowledge Specific purpose of the
Appendix G: Methodology checklist: the QUADAS tool for studies of diagnostic test accuracy 1
 Appendix G: Methodology checklist: the QUADAS tool for studies of diagnostic test accuracy 1 Study identification Including author, title, reference, year of publication Guideline topic: Checklist completed
Appendix G: Methodology checklist: the QUADAS tool for studies of diagnostic test accuracy 1 Study identification Including author, title, reference, year of publication Guideline topic: Checklist completed
GRADE. Grading of Recommendations Assessment, Development and Evaluation. British Association of Dermatologists April 2018
 GRADE Grading of Recommendations Assessment, Development and Evaluation British Association of Dermatologists April 2018 Previous grading system Level of evidence Strength of recommendation Level of evidence
GRADE Grading of Recommendations Assessment, Development and Evaluation British Association of Dermatologists April 2018 Previous grading system Level of evidence Strength of recommendation Level of evidence
Journal Club Critical Appraisal Worksheets. Dr David Walbridge
 Journal Club Critical Appraisal Worksheets Dr David Walbridge The Four Part Question The purpose of a structured EBM question is: To be directly relevant to the clinical situation or problem To express
Journal Club Critical Appraisal Worksheets Dr David Walbridge The Four Part Question The purpose of a structured EBM question is: To be directly relevant to the clinical situation or problem To express
Psychology of Dysfunctional Behaviour RESEARCH METHODS
 Psychology of Dysfunctional Behaviour RESEARCH METHODS The history of abnormal psychology shows that theories and treatment procedures may seem effective in some cases but prove useless and even harmful
Psychology of Dysfunctional Behaviour RESEARCH METHODS The history of abnormal psychology shows that theories and treatment procedures may seem effective in some cases but prove useless and even harmful
Critical Appraisal. Dave Abbott Senior Medicines Information Pharmacist
 Critical Appraisal Dave Abbott Senior Medicines Information Pharmacist Aims Identify key components of clinical trial design and apply these to a critical appraisal of the literature Be able to work out
Critical Appraisal Dave Abbott Senior Medicines Information Pharmacist Aims Identify key components of clinical trial design and apply these to a critical appraisal of the literature Be able to work out
CHECK-LISTS AND Tools DR F. R E Z A E I DR E. G H A D E R I K U R D I S TA N U N I V E R S I T Y O F M E D I C A L S C I E N C E S
 CHECK-LISTS AND Tools DR F. R E Z A E I DR E. G H A D E R I K U R D I S TA N U N I V E R S I T Y O F M E D I C A L S C I E N C E S What is critical appraisal? Critical appraisal is the assessment of evidence
CHECK-LISTS AND Tools DR F. R E Z A E I DR E. G H A D E R I K U R D I S TA N U N I V E R S I T Y O F M E D I C A L S C I E N C E S What is critical appraisal? Critical appraisal is the assessment of evidence
Controlled Trials. Spyros Kitsiou, PhD
 Assessing Risk of Bias in Randomized Controlled Trials Spyros Kitsiou, PhD Assistant Professor Department of Biomedical and Health Information Sciences College of Applied Health Sciences University of
Assessing Risk of Bias in Randomized Controlled Trials Spyros Kitsiou, PhD Assistant Professor Department of Biomedical and Health Information Sciences College of Applied Health Sciences University of
Glossary of Practical Epidemiology Concepts
 Glossary of Practical Epidemiology Concepts - 2009 Adapted from the McMaster EBCP Workshop 2003, McMaster University, Hamilton, Ont. Note that open access to the much of the materials used in the Epi-546
Glossary of Practical Epidemiology Concepts - 2009 Adapted from the McMaster EBCP Workshop 2003, McMaster University, Hamilton, Ont. Note that open access to the much of the materials used in the Epi-546
Cochrane Pregnancy and Childbirth Group Methodological Guidelines
 Cochrane Pregnancy and Childbirth Group Methodological Guidelines [Prepared by Simon Gates: July 2009, updated July 2012] These guidelines are intended to aid quality and consistency across the reviews
Cochrane Pregnancy and Childbirth Group Methodological Guidelines [Prepared by Simon Gates: July 2009, updated July 2012] These guidelines are intended to aid quality and consistency across the reviews
PLS 506 Mark T. Imperial, Ph.D. Lecture Notes: Reliability & Validity
 PLS 506 Mark T. Imperial, Ph.D. Lecture Notes: Reliability & Validity Measurement & Variables - Initial step is to conceptualize and clarify the concepts embedded in a hypothesis or research question with
PLS 506 Mark T. Imperial, Ph.D. Lecture Notes: Reliability & Validity Measurement & Variables - Initial step is to conceptualize and clarify the concepts embedded in a hypothesis or research question with
Clinical problems and choice of study designs
 Evidence Based Dentistry Clinical problems and choice of study designs Asbjørn Jokstad University of Oslo, Norway Nov 21 2001 1 Manipulation with intervention Yes Experimental study No Non-experimental
Evidence Based Dentistry Clinical problems and choice of study designs Asbjørn Jokstad University of Oslo, Norway Nov 21 2001 1 Manipulation with intervention Yes Experimental study No Non-experimental
Quantitative research Quiz Answers
 Quantitative research Quiz Answers 1. What is a clinical trial? A. A clinical trial is an experiment where patients volunteer to test new ways of screening for, preventing, diagnosing or treating a disease.
Quantitative research Quiz Answers 1. What is a clinical trial? A. A clinical trial is an experiment where patients volunteer to test new ways of screening for, preventing, diagnosing or treating a disease.
GRADE. Grading of Recommendations Assessment, Development and Evaluation. British Association of Dermatologists April 2014
 GRADE Grading of Recommendations Assessment, Development and Evaluation British Association of Dermatologists April 2014 Previous grading system Level of evidence Strength of recommendation Level of evidence
GRADE Grading of Recommendations Assessment, Development and Evaluation British Association of Dermatologists April 2014 Previous grading system Level of evidence Strength of recommendation Level of evidence
CHAMP: CHecklist for the Appraisal of Moderators and Predictors
 CHAMP - Page 1 of 13 CHAMP: CHecklist for the Appraisal of Moderators and Predictors About the checklist In this document, a CHecklist for the Appraisal of Moderators and Predictors (CHAMP) is presented.
CHAMP - Page 1 of 13 CHAMP: CHecklist for the Appraisal of Moderators and Predictors About the checklist In this document, a CHecklist for the Appraisal of Moderators and Predictors (CHAMP) is presented.
Lecture 5 Conducting Interviews and Focus Groups
 Lecture 5 Conducting Interviews and Focus Groups Talking to participants enables in-depth information about the experiences of health and illness; and of factors that influence health and illness behaviour
Lecture 5 Conducting Interviews and Focus Groups Talking to participants enables in-depth information about the experiences of health and illness; and of factors that influence health and illness behaviour
The role of Randomized Controlled Trials
 The role of Randomized Controlled Trials Dr. Georgia Salanti Lecturer in Epidemiology University of Ioannina School of Medicine Outline Understanding study designs and the role of confounding Observational
The role of Randomized Controlled Trials Dr. Georgia Salanti Lecturer in Epidemiology University of Ioannina School of Medicine Outline Understanding study designs and the role of confounding Observational
University of Wollongong. Research Online. Australian Health Services Research Institute
 University of Wollongong Research Online Australian Health Services Research Institute Faculty of Business 2011 Measurement of error Janet E. Sansoni University of Wollongong, jans@uow.edu.au Publication
University of Wollongong Research Online Australian Health Services Research Institute Faculty of Business 2011 Measurement of error Janet E. Sansoni University of Wollongong, jans@uow.edu.au Publication
Quality of Clinical Practice Guidelines
 Evidence Based Dentistry Quality of Clinical Practice Guidelines Asbjørn Jokstad University of Oslo, Norway 07/04/2005 1 Justification for developing guidelines Demand for effectiveness and efficacy studies
Evidence Based Dentistry Quality of Clinical Practice Guidelines Asbjørn Jokstad University of Oslo, Norway 07/04/2005 1 Justification for developing guidelines Demand for effectiveness and efficacy studies
ACR OA Guideline Development Process Knee and Hip
 ACR OA Guideline Development Process Knee and Hip 1. Literature searching frame work Literature searches were developed based on the scenarios. A comprehensive search strategy was used to guide the process
ACR OA Guideline Development Process Knee and Hip 1. Literature searching frame work Literature searches were developed based on the scenarios. A comprehensive search strategy was used to guide the process
The RoB 2.0 tool (individually randomized, cross-over trials)
 The RoB 2.0 tool (individually randomized, cross-over trials) Study design Randomized parallel group trial Cluster-randomized trial Randomized cross-over or other matched design Specify which outcome is
The RoB 2.0 tool (individually randomized, cross-over trials) Study design Randomized parallel group trial Cluster-randomized trial Randomized cross-over or other matched design Specify which outcome is
CRITICAL APPRAISAL WORKSHEET 1
 For Residents CRITICAL APPRAISAL WORKSHEET 1 AN ARTICLE ON THERAPY This worksheet should be submitted along with the completed Submission Form to your supervisor. It is based on the articles on therapy
For Residents CRITICAL APPRAISAL WORKSHEET 1 AN ARTICLE ON THERAPY This worksheet should be submitted along with the completed Submission Form to your supervisor. It is based on the articles on therapy
Evaluating and Interpreting Clinical Trials
 Article #2 CE Evaluating and Interpreting Clinical Trials Dorothy Cimino Brown, DVM, DACVS University of Pennsylvania ABSTRACT: For the practicing veterinarian, selecting the best treatment for patients
Article #2 CE Evaluating and Interpreting Clinical Trials Dorothy Cimino Brown, DVM, DACVS University of Pennsylvania ABSTRACT: For the practicing veterinarian, selecting the best treatment for patients
Tammy Filby ( address: 4 th year undergraduate occupational therapy student, University of Western Sydney
 There is evidence from one RCT that an energy conservation course run by an occupational therapist decreased the impact of fatigue by 7% in persons with multiple sclerosis Prepared by; Tammy Filby (email
There is evidence from one RCT that an energy conservation course run by an occupational therapist decreased the impact of fatigue by 7% in persons with multiple sclerosis Prepared by; Tammy Filby (email
In this second module in the clinical trials series, we will focus on design considerations for Phase III clinical trials. Phase III clinical trials
 In this second module in the clinical trials series, we will focus on design considerations for Phase III clinical trials. Phase III clinical trials are comparative, large scale studies that typically
In this second module in the clinical trials series, we will focus on design considerations for Phase III clinical trials. Phase III clinical trials are comparative, large scale studies that typically
Study design continued: intervention studies. Outline. Repetition principal approaches. Gustaf Edgren, PhD Karolinska Institutet.
 Study design continued: intervention studies Gustaf Edgren, PhD Karolinska Institutet Background Outline Uncontrolled trials Non randomized, controlled trials Randomized controlled trials (with an overview
Study design continued: intervention studies Gustaf Edgren, PhD Karolinska Institutet Background Outline Uncontrolled trials Non randomized, controlled trials Randomized controlled trials (with an overview
GATE CAT Case Control Studies
 GATE: a Graphic Approach To Evidence based practice updates from previous version in red Critically Appraised Topic (CAT): Applying the 5 steps of Evidence Based Practice Using evidence about aetiology/risk/interventions
GATE: a Graphic Approach To Evidence based practice updates from previous version in red Critically Appraised Topic (CAT): Applying the 5 steps of Evidence Based Practice Using evidence about aetiology/risk/interventions
Critical Appraisal Practicum. Fabio Di Bello Medical Implementation Manager
 Critical Appraisal Practicum Fabio Di Bello Medical Implementation Manager fdibello@ebsco.com What we ll talk about today: DynaMed process for appraising randomized trials and writing evidence summaries
Critical Appraisal Practicum Fabio Di Bello Medical Implementation Manager fdibello@ebsco.com What we ll talk about today: DynaMed process for appraising randomized trials and writing evidence summaries
ISPOR Task Force Report: ITC & NMA Study Questionnaire
 INDIRECT TREATMENT COMPARISON / NETWORK META-ANALYSIS STUDY QUESTIONNAIRE TO ASSESS RELEVANCE AND CREDIBILITY TO INFORM HEALTHCARE DECISION-MAKING: AN ISPOR-AMCP-NPC GOOD PRACTICE TASK FORCE REPORT DRAFT
INDIRECT TREATMENT COMPARISON / NETWORK META-ANALYSIS STUDY QUESTIONNAIRE TO ASSESS RELEVANCE AND CREDIBILITY TO INFORM HEALTHCARE DECISION-MAKING: AN ISPOR-AMCP-NPC GOOD PRACTICE TASK FORCE REPORT DRAFT
Learning objectives. Examining the reliability of published research findings
 Examining the reliability of published research findings Roger Chou, MD Associate Professor of Medicine Department of Medicine and Department of Medical Informatics and Clinical Epidemiology Scientific
Examining the reliability of published research findings Roger Chou, MD Associate Professor of Medicine Department of Medicine and Department of Medical Informatics and Clinical Epidemiology Scientific
Issues to Consider in the Design of Randomized Controlled Trials
 Issues to Consider in the Design of Randomized Controlled Trials Jay Wilkinson, MD, MPH Professor of Pediatrics & Epidemiology Miller School of Medicine Seminar Purpose To facilitate an interactive discussion
Issues to Consider in the Design of Randomized Controlled Trials Jay Wilkinson, MD, MPH Professor of Pediatrics & Epidemiology Miller School of Medicine Seminar Purpose To facilitate an interactive discussion
Critical Appraisal for Research Papers. Appraisal Checklist & Guide Questions
 Critical Appraisal for Research Papers Appraisal Checklist & Guide Questions EBP@NUHS Critically Appraised Topic (CAT) Checklist and Guide Questions Sullivan 2008 2 Critical Appraisal for Research Papers
Critical Appraisal for Research Papers Appraisal Checklist & Guide Questions EBP@NUHS Critically Appraised Topic (CAT) Checklist and Guide Questions Sullivan 2008 2 Critical Appraisal for Research Papers
Systematic reviews and meta-analyses of observational studies (MOOSE): Checklist.
 Systematic reviews and meta-analyses of observational studies (MOOSE): Checklist. MOOSE Checklist Infliximab reduces hospitalizations and surgery interventions in patients with inflammatory bowel disease:
Systematic reviews and meta-analyses of observational studies (MOOSE): Checklist. MOOSE Checklist Infliximab reduces hospitalizations and surgery interventions in patients with inflammatory bowel disease:
Database of Abstracts of Reviews of Effects (DARE) Produced by the Centre for Reviews and Dissemination Copyright 2017 University of York.
 A comparison of the cost-effectiveness of five strategies for the prevention of non-steroidal anti-inflammatory drug-induced gastrointestinal toxicity: a systematic review with economic modelling Brown
A comparison of the cost-effectiveness of five strategies for the prevention of non-steroidal anti-inflammatory drug-induced gastrointestinal toxicity: a systematic review with economic modelling Brown
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) POINTS TO CONSIDER ON MISSING DATA
 The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 15 November 2001 CPMP/EWP/1776/99 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) POINTS TO
The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 15 November 2001 CPMP/EWP/1776/99 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) POINTS TO
Greg Pond Ph.D., P.Stat.
 Statistics for Clinical Trials: Basics of a Phase III Trial Design Greg Pond Ph.D., P.Stat. Ontario Clinical Oncology Group Escarpment Cancer Research Institute Department of Oncology, McMaster University
Statistics for Clinical Trials: Basics of a Phase III Trial Design Greg Pond Ph.D., P.Stat. Ontario Clinical Oncology Group Escarpment Cancer Research Institute Department of Oncology, McMaster University
CRITICAL APPRAISAL OF MEDICAL LITERATURE. Samuel Iff ISPM Bern
 CRITICAL APPRAISAL OF MEDICAL LITERATURE Samuel Iff ISPM Bern siff@ispm.unibe.ch Contents Study designs Asking good questions Pitfalls in clinical studies How to assess validity (RCT) Conclusion Step-by-step
CRITICAL APPRAISAL OF MEDICAL LITERATURE Samuel Iff ISPM Bern siff@ispm.unibe.ch Contents Study designs Asking good questions Pitfalls in clinical studies How to assess validity (RCT) Conclusion Step-by-step
Experimental Design. Terminology. Chusak Okascharoen, MD, PhD September 19 th, Experimental study Clinical trial Randomized controlled trial
 Experimental Design Chusak Okascharoen, MD, PhD September 19 th, 2016 Terminology Experimental study Clinical trial Randomized controlled trial 1 PHASES OF CLINICAL TRIALS Phase I: First-time-in-man studies
Experimental Design Chusak Okascharoen, MD, PhD September 19 th, 2016 Terminology Experimental study Clinical trial Randomized controlled trial 1 PHASES OF CLINICAL TRIALS Phase I: First-time-in-man studies
Dr. Engle has received an unrestricted educational grant for the MD Anderson Pain Medicine Fellowship.
 Mitchell Engle, MD, PhD Dr. Mitchell Engle earned his medical degree and a Ph.D. in Pharmacology from the University Of Iowa Carver College Of Medicine. His Ph.D. research examined the role of the spinal
Mitchell Engle, MD, PhD Dr. Mitchell Engle earned his medical degree and a Ph.D. in Pharmacology from the University Of Iowa Carver College Of Medicine. His Ph.D. research examined the role of the spinal
CLINICAL DECISION USING AN ARTICLE ABOUT TREATMENT JOSEFINA S. ISIDRO LAPENA MD, MFM, FPAFP PROFESSOR, UPCM
 CLINICAL DECISION USING AN ARTICLE ABOUT TREATMENT JOSEFINA S. ISIDRO LAPENA MD, MFM, FPAFP PROFESSOR, UPCM Principles of Decision Making Knowing the patient s true state is often unnecessary Does my patient
CLINICAL DECISION USING AN ARTICLE ABOUT TREATMENT JOSEFINA S. ISIDRO LAPENA MD, MFM, FPAFP PROFESSOR, UPCM Principles of Decision Making Knowing the patient s true state is often unnecessary Does my patient
GATE CAT Diagnostic Test Accuracy Studies
 GATE: a Graphic Approach To Evidence based practice updates from previous version in red Critically Appraised Topic (CAT): Applying the 5 steps of Evidence Based Practice Using evidence from Assessed by:
GATE: a Graphic Approach To Evidence based practice updates from previous version in red Critically Appraised Topic (CAT): Applying the 5 steps of Evidence Based Practice Using evidence from Assessed by:
Critical Review Form Therapy Objectives: Methods:
 Critical Review Form Therapy Clinical Trial Comparing Primary Coronary Angioplasty with Tissue-Plasminogen Activator for Acute Myocardial Infarction (GUSTO-IIb), NEJM 1997; 336: 1621-1628 Objectives: To
Critical Review Form Therapy Clinical Trial Comparing Primary Coronary Angioplasty with Tissue-Plasminogen Activator for Acute Myocardial Infarction (GUSTO-IIb), NEJM 1997; 336: 1621-1628 Objectives: To
GRADE, Summary of Findings and ConQual Workshop
 GRADE, Summary of Findings and ConQual Workshop To discuss Introduction New JBI Levels of Evidence and Grades of Recommendation Moving towards GRADE Summary of Findings tables Qualitative Levels Conclusion
GRADE, Summary of Findings and ConQual Workshop To discuss Introduction New JBI Levels of Evidence and Grades of Recommendation Moving towards GRADE Summary of Findings tables Qualitative Levels Conclusion
Critical Appraisal Istanbul 2011
 Critical Appraisal Istanbul 2011 The Conviction with which many Nephrologists hold an opinion varies inversely with the Evidence Ed Lewis The Good Old Times. The Google Generation. ASN Kidney Daily LEADING
Critical Appraisal Istanbul 2011 The Conviction with which many Nephrologists hold an opinion varies inversely with the Evidence Ed Lewis The Good Old Times. The Google Generation. ASN Kidney Daily LEADING
Recent developments for combining evidence within evidence streams: bias-adjusted meta-analysis
 EFSA/EBTC Colloquium, 25 October 2017 Recent developments for combining evidence within evidence streams: bias-adjusted meta-analysis Julian Higgins University of Bristol 1 Introduction to concepts Standard
EFSA/EBTC Colloquium, 25 October 2017 Recent developments for combining evidence within evidence streams: bias-adjusted meta-analysis Julian Higgins University of Bristol 1 Introduction to concepts Standard
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Howard R, McShane R, Lindesay J, et al. Donepezil and memantine
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Howard R, McShane R, Lindesay J, et al. Donepezil and memantine
Overview. Goals of Interpretation. Methodology. Reasons to Read and Evaluate
 Overview Critical Literature Evaluation and Biostatistics Ahl Ashley N. Lewis, PharmD, BCPS Clinical Specialist, Drug Information UNC Hospitals Background Review of basic statistics Statistical tests Clinical
Overview Critical Literature Evaluation and Biostatistics Ahl Ashley N. Lewis, PharmD, BCPS Clinical Specialist, Drug Information UNC Hospitals Background Review of basic statistics Statistical tests Clinical
Rapid appraisal of the literature: Identifying study biases
 Rapid appraisal of the literature: Identifying study biases Rita Popat, PhD Clinical Assistant Professor Division of Epidemiology Stanford University School of Medicine August 7, 2007 What is critical
Rapid appraisal of the literature: Identifying study biases Rita Popat, PhD Clinical Assistant Professor Division of Epidemiology Stanford University School of Medicine August 7, 2007 What is critical
Outline. What is Evidence-Based Practice? EVIDENCE-BASED PRACTICE. What EBP is Not:
 Evidence Based Practice Primer Outline Evidence Based Practice (EBP) EBP overview and process Formulating clinical questions (PICO) Searching for EB answers Trial design Critical appraisal Assessing the
Evidence Based Practice Primer Outline Evidence Based Practice (EBP) EBP overview and process Formulating clinical questions (PICO) Searching for EB answers Trial design Critical appraisal Assessing the
Checklist for Randomized Controlled Trials. The Joanna Briggs Institute Critical Appraisal tools for use in JBI Systematic Reviews
 The Joanna Briggs Institute Critical Appraisal tools for use in JBI Systematic Reviews Checklist for Randomized Controlled Trials http://joannabriggs.org/research/critical-appraisal-tools.html www.joannabriggs.org
The Joanna Briggs Institute Critical Appraisal tools for use in JBI Systematic Reviews Checklist for Randomized Controlled Trials http://joannabriggs.org/research/critical-appraisal-tools.html www.joannabriggs.org
How to Interpret a Clinical Trial Result
 How to Interpret a Clinical Trial Result Edward V. Loftus, Jr., M.D. Professor of Medicine Division of Gastroenterology and Hepatology Mayo Clinic College of Medicine Rochester MN CP123456-1 Are results
How to Interpret a Clinical Trial Result Edward V. Loftus, Jr., M.D. Professor of Medicine Division of Gastroenterology and Hepatology Mayo Clinic College of Medicine Rochester MN CP123456-1 Are results
Evidence Based Practice
 Evidence Based Practice RCS 6740 7/26/04 Evidence Based Practice: Definitions Evidence based practice is the integration of the best available external clinical evidence from systematic research with individual
Evidence Based Practice RCS 6740 7/26/04 Evidence Based Practice: Definitions Evidence based practice is the integration of the best available external clinical evidence from systematic research with individual
CHL 5225 H Advanced Statistical Methods for Clinical Trials. CHL 5225 H The Language of Clinical Trials
 CHL 5225 H Advanced Statistical Methods for Clinical Trials Two sources for course material 1. Electronic blackboard required readings 2. www.andywillan.com/chl5225h code of conduct course outline schedule
CHL 5225 H Advanced Statistical Methods for Clinical Trials Two sources for course material 1. Electronic blackboard required readings 2. www.andywillan.com/chl5225h code of conduct course outline schedule
CEU screening programme: Overview of common errors & good practice in Cochrane intervention reviews
 CEU screening programme: Overview of common errors & good practice in Cochrane intervention reviews Since September 2013, the CEU has been responsible for pre-publication screening of new intervention
CEU screening programme: Overview of common errors & good practice in Cochrane intervention reviews Since September 2013, the CEU has been responsible for pre-publication screening of new intervention
Basic Biostatistics. Dr. Kiran Chaudhary Dr. Mina Chandra
 Basic Biostatistics Dr. Kiran Chaudhary Dr. Mina Chandra Overview 1.Importance of Biostatistics 2.Biological Variations, Uncertainties and Sources of uncertainties 3.Terms- Population/Sample, Validity/
Basic Biostatistics Dr. Kiran Chaudhary Dr. Mina Chandra Overview 1.Importance of Biostatistics 2.Biological Variations, Uncertainties and Sources of uncertainties 3.Terms- Population/Sample, Validity/
EBM: Therapy. Thunyarat Anothaisintawee, M.D., Ph.D. Department of Family Medicine, Ramathibodi Hospital, Mahidol University
 EBM: Therapy Thunyarat Anothaisintawee, M.D., Ph.D. Department of Family Medicine, Ramathibodi Hospital, Mahidol University How to appraise therapy literature Are the result valid? What are the result?
EBM: Therapy Thunyarat Anothaisintawee, M.D., Ph.D. Department of Family Medicine, Ramathibodi Hospital, Mahidol University How to appraise therapy literature Are the result valid? What are the result?
Principles and Methods of Intervention Research
 Principles and Methods of Intervention Research NVVO, February 2, 2009 Jan G.P. Tijssen, Ph.D. Academic Medical Center - University of Amsterdam Introduction Pathophysiologic and pharmacological insight
Principles and Methods of Intervention Research NVVO, February 2, 2009 Jan G.P. Tijssen, Ph.D. Academic Medical Center - University of Amsterdam Introduction Pathophysiologic and pharmacological insight
Purpose. Study Designs. Objectives. Observational Studies. Analytic Studies
 Purpose Study Designs H.S. Teitelbaum, DO, PhD, MPH, FAOCOPM AOCOPM Annual Meeting Introduce notions of study design Clarify common terminology used with description and interpretation of information collected
Purpose Study Designs H.S. Teitelbaum, DO, PhD, MPH, FAOCOPM AOCOPM Annual Meeting Introduce notions of study design Clarify common terminology used with description and interpretation of information collected
Evaluating Effectiveness of Treatments. Elenore Judy B. Uy, M.D.
 Evaluating Effectiveness of Treatments Elenore Judy B. Uy, M.D. OUTLINE 1. Why evaluate effectiveness 2. How to acquire evidence 3. How to appraise evidence Directness Validity Results 4. Our Case WALA
Evaluating Effectiveness of Treatments Elenore Judy B. Uy, M.D. OUTLINE 1. Why evaluate effectiveness 2. How to acquire evidence 3. How to appraise evidence Directness Validity Results 4. Our Case WALA
HOW TO CRITIQUE AN ARTICLE ON THERAPY
 HOW TO CRITIQUE AN ARTICLE ON THERAPY DR. BRUCE F. D.C., M.P.H.* Abstract: The ability to critique the literature and source out relevant information to answer a clinical question is a skill that is being
HOW TO CRITIQUE AN ARTICLE ON THERAPY DR. BRUCE F. D.C., M.P.H.* Abstract: The ability to critique the literature and source out relevant information to answer a clinical question is a skill that is being
Lecture 2. Key Concepts in Clinical Research
 Lecture 2 Key Concepts in Clinical Research Outline Key Statistical Concepts Bias and Variability Type I Error and Power Confounding and Interaction Statistical Difference vs Clinical Difference One-sided
Lecture 2 Key Concepts in Clinical Research Outline Key Statistical Concepts Bias and Variability Type I Error and Power Confounding and Interaction Statistical Difference vs Clinical Difference One-sided
About Reading Scientific Studies
 About Reading Scientific Studies TABLE OF CONTENTS About Reading Scientific Studies... 1 Why are these skills important?... 1 Create a Checklist... 1 Introduction... 1 Abstract... 1 Background... 2 Methods...
About Reading Scientific Studies TABLE OF CONTENTS About Reading Scientific Studies... 1 Why are these skills important?... 1 Create a Checklist... 1 Introduction... 1 Abstract... 1 Background... 2 Methods...
aps/stone U0 d14 review d2 teacher notes 9/14/17 obj: review Opener: I have- who has
 aps/stone U0 d14 review d2 teacher notes 9/14/17 obj: review Opener: I have- who has 4: You should be able to explain/discuss each of the following words/concepts below... Observational Study/Sampling
aps/stone U0 d14 review d2 teacher notes 9/14/17 obj: review Opener: I have- who has 4: You should be able to explain/discuss each of the following words/concepts below... Observational Study/Sampling
Copyright GRADE ING THE QUALITY OF EVIDENCE AND STRENGTH OF RECOMMENDATIONS NANCY SANTESSO, RD, PHD
 GRADE ING THE QUALITY OF EVIDENCE AND STRENGTH OF RECOMMENDATIONS NANCY SANTESSO, RD, PHD ASSISTANT PROFESSOR DEPARTMENT OF CLINICAL EPIDEMIOLOGY AND BIOSTATISTICS, MCMASTER UNIVERSITY Nancy Santesso,
GRADE ING THE QUALITY OF EVIDENCE AND STRENGTH OF RECOMMENDATIONS NANCY SANTESSO, RD, PHD ASSISTANT PROFESSOR DEPARTMENT OF CLINICAL EPIDEMIOLOGY AND BIOSTATISTICS, MCMASTER UNIVERSITY Nancy Santesso,
A Systematic Review of the Efficacy and Clinical Effectiveness of Group Analysis and Analytic/Dynamic Group Psychotherapy
 A Systematic Review of the Efficacy and Clinical Effectiveness of Group Analysis and Analytic/Dynamic Group Psychotherapy Executive summary Aims of the review The main aim of the review was to assess the
A Systematic Review of the Efficacy and Clinical Effectiveness of Group Analysis and Analytic/Dynamic Group Psychotherapy Executive summary Aims of the review The main aim of the review was to assess the
Κριτική αξιολόγηση τυχαιοποιημένης κλινικής δοκιμής Άρης Λιάκος, MD MSc Ειδικευόμενος Παθολογίας, Υποψήφιος Διδάκτωρ
 Αριστοτέλειο Πανεπιστήμιο Θεσσαλονίκης Σχολή Επιστημών Υγείας Τμήμα Ιατρικής Πρόγραμμα Μεταπτυχιακών Σπουδών «Ερευνητική Μεθοδολογία στην Ιατρική και τις Επιστήμες Υγείας» Μάθημα: Ιατρική Βασισμένη στην
Αριστοτέλειο Πανεπιστήμιο Θεσσαλονίκης Σχολή Επιστημών Υγείας Τμήμα Ιατρικής Πρόγραμμα Μεταπτυχιακών Σπουδών «Ερευνητική Μεθοδολογία στην Ιατρική και τις Επιστήμες Υγείας» Μάθημα: Ιατρική Βασισμένη στην
How to carry out health technology appraisals and guidance. Learning from the Scottish experience Richard Clark, Principal Pharmaceutical
 The Managed Introduction of New Medicines How to carry out health technology appraisals and guidance. Learning from the Scottish experience Richard Clark, Principal Pharmaceutical Analyst July 10 th 2009,
The Managed Introduction of New Medicines How to carry out health technology appraisals and guidance. Learning from the Scottish experience Richard Clark, Principal Pharmaceutical Analyst July 10 th 2009,
Bandolier. Professional. Independent evidence-based health care ON QUALITY AND VALIDITY. Quality and validity. May Clinical trial quality
 Bandolier Professional Independent evidence-based health care ON QUALITY AND VALIDITY If studies are not done properly, any results they produce will be worthless. We call this validity. What constitutes
Bandolier Professional Independent evidence-based health care ON QUALITY AND VALIDITY If studies are not done properly, any results they produce will be worthless. We call this validity. What constitutes
Problem solving therapy
 Introduction People with severe mental illnesses such as schizophrenia may show impairments in problem-solving ability. Remediation interventions such as problem solving skills training can help people
Introduction People with severe mental illnesses such as schizophrenia may show impairments in problem-solving ability. Remediation interventions such as problem solving skills training can help people
Experiments in the Real World
 Experiments in the Real World Goal of a randomized comparative experiment: Subjects should be treated the same in all ways except for the treatments we are trying to compare. Example: Rats in cages given
Experiments in the Real World Goal of a randomized comparative experiment: Subjects should be treated the same in all ways except for the treatments we are trying to compare. Example: Rats in cages given
Can we teach how to discriminate between good & bad evidence? the GATE approach
 Can we teach how to discriminate between good & bad evidence? in 20 minutes! the GATE approach Rod Jackson June 2010 GATE: a Graphic Appraisal Tool for Epidemiology A picture, 2 acronyms & 2 formulas A
Can we teach how to discriminate between good & bad evidence? in 20 minutes! the GATE approach Rod Jackson June 2010 GATE: a Graphic Appraisal Tool for Epidemiology A picture, 2 acronyms & 2 formulas A
Study Designs in Epidemiology
 Study Designs in Epidemiology Dr. Sireen Alkhaldi, BDS, MPH, DrPH First semester 2017/ 2018 Department of Family and Community Medicine School of Medicine/ The University of Jordan Epidemiologic Study
Study Designs in Epidemiology Dr. Sireen Alkhaldi, BDS, MPH, DrPH First semester 2017/ 2018 Department of Family and Community Medicine School of Medicine/ The University of Jordan Epidemiologic Study
Contents. Version 1.0: 01/02/2010 Protocol# ISRCTN Page 1 of 7
 Contents 1. INTRODUCTION... 2 2. STUDY SYNOPSIS... 2 3. STUDY OBJECTIVES... 2 3.1. Primary Objective... 2 3.2. Secondary Objectives... 2 3.3. Assessment of Objectives... 3 3.4. Change the Primary Objective
Contents 1. INTRODUCTION... 2 2. STUDY SYNOPSIS... 2 3. STUDY OBJECTIVES... 2 3.1. Primary Objective... 2 3.2. Secondary Objectives... 2 3.3. Assessment of Objectives... 3 3.4. Change the Primary Objective
Epidemiological study design. Paul Pharoah Department of Public Health and Primary Care
 Epidemiological study design Paul Pharoah Department of Public Health and Primary Care Molecules What/why? Organelles Cells Tissues Organs Clinical medicine Individuals Public health medicine Populations
Epidemiological study design Paul Pharoah Department of Public Health and Primary Care Molecules What/why? Organelles Cells Tissues Organs Clinical medicine Individuals Public health medicine Populations
Glossary. for the General Methods Version 1.0 of Contact:
 Glossary for the General Methods 3.0 1 Version 1.0 of 27.05.2008 Contact: Institute for Quality and Efficiency in Health Care (IQWiG) Dillenburger Str. 27 51105 Cologne Germany Tel: +49 (0)221 / 35685-0
Glossary for the General Methods 3.0 1 Version 1.0 of 27.05.2008 Contact: Institute for Quality and Efficiency in Health Care (IQWiG) Dillenburger Str. 27 51105 Cologne Germany Tel: +49 (0)221 / 35685-0
Are the likely benefits worth the potential harms and costs? From McMaster EBCP Workshop/Duke University Medical Center
 CRITICAL REVIEW FORM FOR THERAPY STUDY Did experimental and control groups begin the study with a similar prognosis? Were patients randomized? Was randomization concealed? Were patients analyzed in the
CRITICAL REVIEW FORM FOR THERAPY STUDY Did experimental and control groups begin the study with a similar prognosis? Were patients randomized? Was randomization concealed? Were patients analyzed in the
