AETNA BETTER HEALTH Prior Authorization guideline for Injectable Osteoporosis Agents
|
|
|
- Agnes Benson
- 6 years ago
- Views:
Transcription
1 AETNA BETTER HEALTH Prior Authorization guideline for Injectable Osteoporosis Agents Injectable Osteoporosis Agents Forteo (teriparatide); zoledronic acid Prolia (denosumab)] Authorization guidelines For patients who have the following: For the treatment of osteoporosis in members who meet the following criteria(zoledronic acid, Prolia, and Forteo) 1. Diagnosis of osteoporosis (T-score <-2.5 OR fragility fracture at the hip, spine, wrist, arm, rib, or pelvis) 2. Patient s 25-hydroxyvitamin D level is >20 ng/ml. Patients who are vitamin D deficient should have vitamin D replaced (i.e., 50,000 IU weekly) before starting treatment with an injectable osteoporosis agent. 3. Patient has ONE of the following: a. Therapeutic failure on oral bisphosphonate despite compliance (including new b. Contraindication or severe intolerance to oral bisphosphonate (e.g., current upper GI symptoms, inability to swallow, or inability to remain in an upright position after oral bisphosphonate administration for the required length of 4. In addition for men: Testosterone level is normal. If patient is hypogonadal, testosterone replacement therapy should be prescribed before starting treatment with an injectable osteoporosis agent unless the patient has a history of prostate cancer. Prevention of Osteoporosis in Postmenopausal Women: (Zoledronic acid) 1. Diagnosis of osteopenia (T-score between -1.0 and -2.5) and at high risk for OP fracture (FRAX risk 3.0% for hip fracture or 20% for any major OP-related fracture OR multiple risk factors for fracture) *See Additional information for details 2. Patient s 25-hydroxyvitamin D level is >20 ng/ml. Patients who are vitamin D deficient should have vitamin D replaced (i.e., 50,000 IU weekly) before starting treatment with an injectable osteoporosis agent. 3. Patient has ONE of the following: a. Therapeutic failure on oral bisphosphonate despite compliance (including new
2 b. Contraindication or severe intolerance to oral bisphosphonate (e.g., current upper GI symptoms, inability to swallow, or inability to remain in an upright position after oral bisphosphonate administration for the required length of 4. For the treatment of corticosteroid-induced osteoporosis (Zoledronic acid, Forteo): a. Patient meets ONE of the following: o Postmenopausal woman or a man >50 years old and has received, or is expected to receive, prednisone >7.5mg/day (or equivalent) for >3 months o Postmenopausal woman or a man >50 years old who has received, or is expected to receive, any dose of corticosteroid for any duration IF they are at high risk for OP fracture (FRAX risk 3.0% for hip fracture or 20% for any major OP-related fracture OR multiple risk factors for fracture) o Premenopausal woman or a man <50 years old WITH a history of fragility fracture and has received, or is expected to receive, prednisone >7.5mg/day (or equivalent) for >3 months b. Patient s 25-hydroxyvitamin D level is >20 ng/ml. Patients who are vitamin D c. Patient has ONE of the following: o o Therapeutic failure on oral bisphosphonate despite compliance (including new Contraindication or severe intolerance to oral bisphosphonate (e.g., current 5. For the treatment of Paget s disease of bone in men and women (zoledronic acid) a. Patient has bone specific alkaline phosphatase >2x ULN OR has symptoms related to active Paget s (i.e., pain at the site of the pagetic lesion) b. Patient has normal serum calcium, phosphorus, and 25-hydroxyvitamin D. Abnormalities should be treated before starting IV bisphosphonates c. Patient meets ONE of the following: o Contraindication or severe intolerance to oral bisphosphonate (e.g., current 6. Bone Metastases of Cancer of Multiple Myeloma: (, zoledronic acid) a. Patient has ONE of the following diagnoses: o Solid tumor with bone metastases o Castration-resistant prostate cancer with bone metastases o Multiple myeloma with osteolytic lesions o Multiple myeloma and osteoporosis or osteopenia b. Patient s 25-hydroxyvitamin D level is >20 ng/ml. Patients who are vitamin D
3 7. Increase of Bone Mass in Men on Androgen Deprivation Therapy for Prostate Cancer WITHOUT Bone Metastases: (Prolia, zoledronic acid) 8. Patient is at high risk for OP fracture (FRAX risk of 3.0% for hip fracture or 20% for any major OP-related fracture, or multiple risk factors for fracture) *See Additional information for details 9. Patient s 25-hydroxyvitamin D level is >20 ng/ml. Patients who are vitamin D 10. Patient has ONE of the following: a. Therapeutic failure on oral bisphosphonate despite compliance (including new b. Contraindication or severe intolerance to oral bisphosphonate (e.g., current 11. Increase of Bone Mass in Women on Aromatase Inhibitory therapy for Breast Cancer WITHOUT Bone Metastases: (Prolia, zoledronic acid,) 1. Patient is postmenopausal OR premenopausal with a higher risk of fracture (T-score <-2 OR FRAX risk of 3.0% for hip fracture or 20% for any major OP-related fracture OR previous hip or vertebral fracture)) 2. Patient s 25-hydroxyvitamin D level is >20 ng/ml. Patients who are vitamin D 3. Patient has ONE of the following: o Therapeutic failure on oral bisphosphonate despite compliance (including new o Contraindication or severe intolerance to oral bisphosphonate (e.g., current 12. Hypercalcemia of Malignancy: (zoledronic acid) 1. Patient has moderate or severe hypercalcemia (refer to additional information for details) associated with malignancy 2. Patient is receiving vigorous saline hydration with a goal of increasing urine output to about 2 L/day Authorization and Limitations Initial Approval: Paget s Disease: 1 treatment Hypercalcemia from Malignancy: 1 treatment Osteoporosis: 5 years (except Forteo, 2yrs) All other indications: 2 years
4 Extended Approval: Paget s Disease: 1 treatment o If bone specific alkaline phosphatase rises after initial treatment OR if patient has symptoms o Bisphosphonates usually induce remission, therefore long-term approval is usually NOT appropriate Hypercalcemia from Malignancy: Retreatment not recommended unless new occurrence Osteoporosis: Patients with stable BMD without fractures on treatment may be appropriate for a drug holiday after 4-5 years of treatment. Continue treatment if BMD has worsened or if patient had fractures on treatment All other indications: 2 years if patient meets criteria for initial approval Note: Forteo is NOT recommended for longer than 2 years due to the risk of osteosarcoma Additional Information: Quantity Limits: Forteo: 1 pen per 28 days Prolia: 1 vial/syringe per 112 to 168 (6 months) Zoledronic Acid: o For Treatment of Osteoporosis and GIOP: 1, 5mg vial per year o For Prevention of Osteoporosis: 1, 5mg vial every 2 years o For MM or Bone Metastases: 1, 4mg vial per 21 days Injectable Osteoporosis Agents are NOT covered for members with the following criteria: Use not approved by the FDA; AND The use is unapproved and not supported by the literature or evidence as an accepted off-label use. 1. It is recommended that all patients should receive >1200 mg of elemental calcium and >1000 mg of vitamin D from diet and/or supplements to improve effectiveness of the medications and to prevent hypocalcemia. 2. FRAX Calculator: 3. Severe Hypercalcemia = albumin-corrected calcium (cca) >12 mg/dl [3.0 mmol/l] Formula: cca in mg/dl=ca in mg/dl (4.0 g/dl - patient albumin [g/dl]). 4. Major Risk factors for Osteoporotic Fractures: a. low body mass index b. previous fragility fracture c. parental history of hip fracture d. glucocorticoid treatment (refer to specific criteria above for this indication) e. current smoking f. alcohol intake of 3 or more units per day g. rheumatoid arthritis h. secondary causes of osteoporosis
5 Medically Necessary A service or benefit is Medically Necessary if it is compensable under the MA Program and if it meets any one of the following standards: The service or benefit will, or is reasonably expected to, prevent the onset of an illness, condition or disability. The service or benefit will, or is reasonably expected to, reduce or ameliorate the physical, mental or developmental effects of an illness, condition, injury or disability. The service or benefit will assist the Member to achieve or maintain maximum functional capacity in performing daily activities, taking into account both the functional capacity of the Member and those functional capacities that are appropriate for Members of the same age. Determination of Medical Necessity for covered care and services, whether made on a Prior Authorization, Concurrent Review, Retrospective Review, or exception basis, must be documented in writing. The determination is based on medical information provided by the Member, the Member s family/caretaker and the Primary Care Practitioner, as well as any other Providers, programs, agencies that have evaluated the Member. All such determinations must be made by qualified and trained Health Care Providers. A Health Care Provider who makes such determinations of Medical Necessity is not considered to be providing a health care service under this Agreement. References: 1. Forteo [package insert]. Indianapolis, IN: Eli Lilly and Company; Revised February Boniva Injection [package insert]. Roche Laboratories, Inc., Nutley, NJ; Accessed Sept 2, Reclast [package insert].novartis Pharmaceuticals Corporation, East Hanover, NJ; Accessed Sept 2, Prolia [package insert]. Amgen Manufacturing Limited, Thousand Oaks, CA; Accessed Sept 2, American association of clinical endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of postmenopausal osteoporosis (AACE guidelines): Endocrine Practice. 2010; Vol 16 (Suppl 3). Accessed on Sept 2, Shapiro C.L, Pant S. (August 2015) Evaluation and management of aromatase inhibitor-induced bone loss. UpToDate. (C.J. Rosen, J.E. Mulder, Eds.). Waltham, MA. Retrieved September 15, 2015 from 7. Smith M.R., Crawford E.D. (August 2015) Side effects of androgen deprivation therapy. UpToDate. (N. Vogelzang, W.R Lee, J.P. Richie, M.E. Ross, Eds.). Waltham, MA. Retrieved
6 September 15, 2015 from H Singer FR, Bone HG, Hosking DJ, et al. Paget s disease of bone: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99: National Osteoporosis Foundation. Clinician s guide to prevention and treatment of osteoporosis. Washington, DC: National Osteoporosis Foundation; Grossman JM, Gordon R, Ranganath VK, et al. American college of rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res. 2010;62(11) NCCN: National Comprehensive Cancer Network. NCCN Clinical Practice Guideline in Oncology: Prostate Cancer. Version Accessed August 28, NCCN: National Comprehensive Cancer Network. NCCN Clinical Practice Guideline in Oncology: Multiple Myeloma. Version Accessed October 5, 2016.
[If no, skip to question 10.] Y N. 2. Does the member have a diagnosis of Paget s disease of bone? Y N. [If no, skip to question 4.
![[If no, skip to question 10.] Y N. 2. Does the member have a diagnosis of Paget s disease of bone? Y N. [If no, skip to question 4. [If no, skip to question 10.] Y N. 2. Does the member have a diagnosis of Paget s disease of bone? Y N. [If no, skip to question 4.](/thumbs/86/94943423.jpg) Pharmacy Prior Authorization AETA BETTER HEALTH EW JERSE (MEDICAID) Zoledronic Acid (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information,
Pharmacy Prior Authorization AETA BETTER HEALTH EW JERSE (MEDICAID) Zoledronic Acid (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information,
3. Has bone specific alkaline phosphatase level increased OR does the member have symptoms related to active Paget s?
 Pharmacy Prior Authorization AETA BETTER HEALTH VIRGIIA CCC PLUS and MEDALLIO/FAMIS 4.0 Zoledronic Acid (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review
Pharmacy Prior Authorization AETA BETTER HEALTH VIRGIIA CCC PLUS and MEDALLIO/FAMIS 4.0 Zoledronic Acid (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review
Osteoporosis Agents Drug Class Prior Authorization Protocol
 Osteoporosis Agents Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review of
Osteoporosis Agents Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review of
HARVARD PILGRIM HEALTH CARE RECOMMENDED MEDICATION REQUEST GUIDELINES
 Generic Brand HICL GCN Exception/Other PROLIA, XGEVA 37012 If the caller wishes to initiate a request then a MRF must be completed. This drug requires a written request for prior authorization. All requests
Generic Brand HICL GCN Exception/Other PROLIA, XGEVA 37012 If the caller wishes to initiate a request then a MRF must be completed. This drug requires a written request for prior authorization. All requests
This Coverage Policy applies to Individual Health Insurance Marketplace benefit plans only.
 This Coverage Policy applies to Individual Health Insurance Marketplace benefit plans only. INJECTABLE OSTEOPOSIS AGENTS SUBJECT Pharmacologic Agents: Bisphosphonates: Boniva IV (ibandronate) Reclast (zoledronic
This Coverage Policy applies to Individual Health Insurance Marketplace benefit plans only. INJECTABLE OSTEOPOSIS AGENTS SUBJECT Pharmacologic Agents: Bisphosphonates: Boniva IV (ibandronate) Reclast (zoledronic
Approval of a drug under this criteria document does not ensure full coverage of the drug.
 Criteria Document: Reference #: PC/A011 Page 1 of 8 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community
Criteria Document: Reference #: PC/A011 Page 1 of 8 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community
Parathyroid Hormone Analogs
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.36 Subject: Parathyroid Hormone Analogs Page: 1 of 6 Last Review Date: September 15, 2017 Parathyroid
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.36 Subject: Parathyroid Hormone Analogs Page: 1 of 6 Last Review Date: September 15, 2017 Parathyroid
Pharmacy Management Drug Policy
 SUBJECT: - Forteo (teriparatide), Prolia (denosumab), Tymlos (abaloparatide) POLICY NUMBER: Pharmacy-35 EFFECTIVE DATE: 9/07 LAST REVIEW DATE: 9/29/2017 If the member s subscriber contract excludes coverage
SUBJECT: - Forteo (teriparatide), Prolia (denosumab), Tymlos (abaloparatide) POLICY NUMBER: Pharmacy-35 EFFECTIVE DATE: 9/07 LAST REVIEW DATE: 9/29/2017 If the member s subscriber contract excludes coverage
Pharmacy Management Drug Policy
 Clinical criteria used to make utilization review decisions are based on credible scientific evidence published in peer reviewed medical literature generally recognized by the medical community. Guidelines
Clinical criteria used to make utilization review decisions are based on credible scientific evidence published in peer reviewed medical literature generally recognized by the medical community. Guidelines
Forteo (teriparatide) Prior Authorization Program Summary
 Forteo (teriparatide) Prior Authorization Program Summary FDA APPROVED INDICATIONS DOSAGE 1 FDA Indication 1 : Forteo (teriparatide) is indicated for: the treatment of postmenopausal women with osteoporosis
Forteo (teriparatide) Prior Authorization Program Summary FDA APPROVED INDICATIONS DOSAGE 1 FDA Indication 1 : Forteo (teriparatide) is indicated for: the treatment of postmenopausal women with osteoporosis
Prior Authorization Required: Yes as shown below
 PROLIA, XGEVA (denosumab) MB9409 Covered Service: Prior Authorization Required: Additional Information Medicare Policy: BadgerCare Plus Policy: Yes when meets criteria below Yes as shown below Must be
PROLIA, XGEVA (denosumab) MB9409 Covered Service: Prior Authorization Required: Additional Information Medicare Policy: BadgerCare Plus Policy: Yes when meets criteria below Yes as shown below Must be
Pharmacy Management Drug Policy
 SUBJECT: - Forteo (teriparatide), Prolia (denosumab), Tymlos (abaloparatide), Boniva injection (Ibandronate) POLICY NUMBER: Pharmacy-35 EFFECTIVE DATE: 9/07 LAST REVIEW DATE: 10/15/2018 If the member s
SUBJECT: - Forteo (teriparatide), Prolia (denosumab), Tymlos (abaloparatide), Boniva injection (Ibandronate) POLICY NUMBER: Pharmacy-35 EFFECTIVE DATE: 9/07 LAST REVIEW DATE: 10/15/2018 If the member s
Parathyroid Hormone Analog for Osteoporosis Prior Authorization with Quantity Limit Criteria Program Summary
 Parathyroid Hormone Analog for Osteoporosis Prior Authorization with Quantity Limit Criteria Program Summary This prior authorization program applies to Commercial, NetResults A series, NetResults F series
Parathyroid Hormone Analog for Osteoporosis Prior Authorization with Quantity Limit Criteria Program Summary This prior authorization program applies to Commercial, NetResults A series, NetResults F series
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Reclast, Zometa) Reference Number: CP.PHAR.59 Effective Date: 03.11 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder
Clinical Policy: (Reclast, Zometa) Reference Number: CP.PHAR.59 Effective Date: 03.11 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder
Clinical Policy: Denosumab (Prolia, Xgeva) Reference Number: CP.PHAR.58
 Clinical Policy: (Prolia, Xgeva) Reference Number: CP.PHAR.58 Effective Date: 03/11 Last Review Date: 08/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Prolia, Xgeva) Reference Number: CP.PHAR.58 Effective Date: 03/11 Last Review Date: 08/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Prolia, Xgeva) Reference Number: CP.PHAR.58 Effective Date: 03.01.11 Last Review Date: 02.19 Line of Business: Commercial, HIM, Medicaid Coding Implications Revision Log See Important
Clinical Policy: (Prolia, Xgeva) Reference Number: CP.PHAR.58 Effective Date: 03.01.11 Last Review Date: 02.19 Line of Business: Commercial, HIM, Medicaid Coding Implications Revision Log See Important
Tymlos (abaloparatide)
 Tymlos (abaloparatide) Policy Number: 5.01.638 Last Review: 11/2018 Origination: 10/2017 Next Review: 11/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Tymlos
Tymlos (abaloparatide) Policy Number: 5.01.638 Last Review: 11/2018 Origination: 10/2017 Next Review: 11/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Tymlos
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Prolia, Xgeva) Reference Number: CP.PHAR.58 Effective Date: 03.01.11 Last Review Date: 05.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder
Clinical Policy: (Prolia, Xgeva) Reference Number: CP.PHAR.58 Effective Date: 03.01.11 Last Review Date: 05.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder
FYI ONLY Generic Name. Generics available. zoledronic acid N/A
 Criteria Document: Reference #: PC/A011 Page 1 of 5 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community
Criteria Document: Reference #: PC/A011 Page 1 of 5 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community
Xgeva. Xgeva (denosumab) Description. Section: Prescription Drugs Effective Date: January 1, 2016
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.07.18 Subject: Xgeva Page: 1 of 5 Last Review Date: December 3, 2015 Xgeva Description Xgeva (denosumab)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.07.18 Subject: Xgeva Page: 1 of 5 Last Review Date: December 3, 2015 Xgeva Description Xgeva (denosumab)
PROLIA: Medical Coverage Policy Denosumab (Prolia and. Xgeva) EFFECTIVE DATE: POLICY LAST UPDATED:
 Medical Coverage Policy Denosumab (Prolia and Xgeva) EFFECTIVE DATE: 11 01 2016 POLICY LAST UPDATED: 12 19 2017 OVERVIEW Prolia (denosumab) is indicated for the treatment of postmenopausal women with osteoporosis
Medical Coverage Policy Denosumab (Prolia and Xgeva) EFFECTIVE DATE: 11 01 2016 POLICY LAST UPDATED: 12 19 2017 OVERVIEW Prolia (denosumab) is indicated for the treatment of postmenopausal women with osteoporosis
1
 www.osteoporosis.ca 1 2 Overview of the Presentation Osteoporosis: An Overview Bone Basics Diagnosis of Osteoporosis Drug Therapies Risk Reduction Living with Osteoporosis 3 What is Osteoporosis? Osteoporosis:
www.osteoporosis.ca 1 2 Overview of the Presentation Osteoporosis: An Overview Bone Basics Diagnosis of Osteoporosis Drug Therapies Risk Reduction Living with Osteoporosis 3 What is Osteoporosis? Osteoporosis:
Xgeva. Xgeva (denosumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.18 Subject: Xgeva Page: 1 of 5 Last Review Date: March 16, 2018 Xgeva Description Xgeva (denosumab)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.18 Subject: Xgeva Page: 1 of 5 Last Review Date: March 16, 2018 Xgeva Description Xgeva (denosumab)
TYMLOS (abaloparatide)
 TYMLOS (abaloparatide) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
TYMLOS (abaloparatide) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: denosumab_prolia_xgeva 3/2011 9/2017 9/2018 9/2017 Description of Procedure or Service Receptor activator
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: denosumab_prolia_xgeva 3/2011 9/2017 9/2018 9/2017 Description of Procedure or Service Receptor activator
Bone Densitometry Pathway
 Bone Densitometry Pathway The goal of the Bone Densitometry pathway is to manage our diagnosed osteopenic and osteoporotic patients, educate and monitor the patient population at risk for bone density
Bone Densitometry Pathway The goal of the Bone Densitometry pathway is to manage our diagnosed osteopenic and osteoporotic patients, educate and monitor the patient population at risk for bone density
denosumab (Prolia ) Policy # Original Effective Date: 07/21/2011 Current Effective Date: 04/19/2017
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Based on review of available data, the Company may consider the use of denosumab (Prolia) for the
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Actonel, Atelvia) Reference Number: CP.PMN.100 Effective Date: 03.01.18 Last Review Date: 02.19 Line of Business: Commercial, HIM, Medicaid Revision Log See Important Reminder at the
Clinical Policy: (Actonel, Atelvia) Reference Number: CP.PMN.100 Effective Date: 03.01.18 Last Review Date: 02.19 Line of Business: Commercial, HIM, Medicaid Revision Log See Important Reminder at the
Pathway from Fracture or Risk Factor to Treatment
 Appendix 6A - Guidance on Diagnosis and Management of Osteoporosis Pathway from Fracture or Risk Factor to Treatment Fragility Fracture = fracture sustained from a low energy fall from standing height
Appendix 6A - Guidance on Diagnosis and Management of Osteoporosis Pathway from Fracture or Risk Factor to Treatment Fragility Fracture = fracture sustained from a low energy fall from standing height
DENOSUMAB (PROLIA & XGEVA )
 DENOSUMAB (PROLIA & XGEVA ) UnitedHealthcare Oxford Clinical Policy Policy Number: PHARMACY 306.3 T2 Effective Date: July 2, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 CONDITIONS OF COVERAGE...
DENOSUMAB (PROLIA & XGEVA ) UnitedHealthcare Oxford Clinical Policy Policy Number: PHARMACY 306.3 T2 Effective Date: July 2, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 CONDITIONS OF COVERAGE...
Osteoporosis Management
 Osteoporosis Management Lisa Voss PA C, CCD Laura Frontiero NP C, CCD Kaiser Healthy Bones Program San Diego Disclosures: Nothing to disclose www.zazzle.com 1 Overview How to diagnose Osteoporosis FRAX
Osteoporosis Management Lisa Voss PA C, CCD Laura Frontiero NP C, CCD Kaiser Healthy Bones Program San Diego Disclosures: Nothing to disclose www.zazzle.com 1 Overview How to diagnose Osteoporosis FRAX
John J. Wolf, DO Family Medicine
 John J. Wolf, DO Family Medicine Objectives: 1. Review incidence & Risk of Osteoporosis 2.Review indications for testing 3.Review current pharmacologic & Non pharmacologic Tx options 4.Understand & Utilize
John J. Wolf, DO Family Medicine Objectives: 1. Review incidence & Risk of Osteoporosis 2.Review indications for testing 3.Review current pharmacologic & Non pharmacologic Tx options 4.Understand & Utilize
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Forteo) Reference Number: CP.PHAR.188 Effective Date: 11.15.17 Last Review Date: 02.19 Line of Business: Commercial* (Exchange Plans), HIM, Medicaid Coding Implications Revision Log See
Clinical Policy: (Forteo) Reference Number: CP.PHAR.188 Effective Date: 11.15.17 Last Review Date: 02.19 Line of Business: Commercial* (Exchange Plans), HIM, Medicaid Coding Implications Revision Log See
Osteoporosis Treatment Overview. Colton Larson RFUMS October 26, 2018
 Osteoporosis Treatment Overview Colton Larson RFUMS October 26, 2018 Burden of Disease Most common bone disease 9.9 million Americans + 43.1 million Americans have low bone mineral density (BMD) Stealthy
Osteoporosis Treatment Overview Colton Larson RFUMS October 26, 2018 Burden of Disease Most common bone disease 9.9 million Americans + 43.1 million Americans have low bone mineral density (BMD) Stealthy
Men and Osteoporosis So you think that it can t happen to you
 Men and Osteoporosis So you think that it can t happen to you Jonathan D. Adachi MD, FRCPC Alliance for Better Bone Health Chair in Rheumatology Professor, Department of Medicine Michael G. DeGroote School
Men and Osteoporosis So you think that it can t happen to you Jonathan D. Adachi MD, FRCPC Alliance for Better Bone Health Chair in Rheumatology Professor, Department of Medicine Michael G. DeGroote School
Medication Prior Authorization Form
 Reclast (Zoledronic Acid) Policy Number: 1050 Policy History Approve Date: 12/11/2015 Revise Dates: 8/22/2016 Next Review: 12/11/2016 Review Dates: Preauthorization All Plans Benefit plans vary in coverage
Reclast (Zoledronic Acid) Policy Number: 1050 Policy History Approve Date: 12/11/2015 Revise Dates: 8/22/2016 Next Review: 12/11/2016 Review Dates: Preauthorization All Plans Benefit plans vary in coverage
Osteoporosis Clinical Guideline. Rheumatology January 2017
 Osteoporosis Clinical Guideline Rheumatology January 2017 Introduction Osteoporosis is a condition of low bone mass leading to an increased risk of low trauma fractures. The prevalence of osteoporosis
Osteoporosis Clinical Guideline Rheumatology January 2017 Introduction Osteoporosis is a condition of low bone mass leading to an increased risk of low trauma fractures. The prevalence of osteoporosis
What is Osteoporosis?
 What is Osteoporosis? 2000 NIH Definition A skeletal disorder characterized by compromised bone strength predisposing a person to an increased risk of fracture. Bone strength reflects the integration of
What is Osteoporosis? 2000 NIH Definition A skeletal disorder characterized by compromised bone strength predisposing a person to an increased risk of fracture. Bone strength reflects the integration of
Prolia /Xgeva (denosumab) Document Number: IC-0098
 / (denosumab) Document Number: IC-0098 Last Review Date: 5/30/2017 Date of Origin: 11/28/2011 Dates Reviewed: 12/2011, 03/2012, 06/2012, 09/2012, 12/2012, 03/2013, 06/2013, 03/2014, 06/2014, 09/2014, 12/2014,
/ (denosumab) Document Number: IC-0098 Last Review Date: 5/30/2017 Date of Origin: 11/28/2011 Dates Reviewed: 12/2011, 03/2012, 06/2012, 09/2012, 12/2012, 03/2013, 06/2013, 03/2014, 06/2014, 09/2014, 12/2014,
Using the FRAX Tool. Osteoporosis Definition
 How long will your bones remain standing? Using the FRAX Tool Gary Salzman M.D. Director Banner Good Samaritan/ Hayden VAMC Internal Medicine Geriatric Fellowship Program Phoenix, Arizona Using the FRAX
How long will your bones remain standing? Using the FRAX Tool Gary Salzman M.D. Director Banner Good Samaritan/ Hayden VAMC Internal Medicine Geriatric Fellowship Program Phoenix, Arizona Using the FRAX
CASE 1 WHY IS IT IMPORTANT TO TREAT? FACTS CONCERNS
 4:30-5:15pm Ask the Expert: Osteoporosis SPEAKERS Silvina Levis, MD OSTEOPOROSIS - FACTS 1:3 older women and 1:5 older men will have a fragility fracture after age 50 After 3 years of treatment, depending
4:30-5:15pm Ask the Expert: Osteoporosis SPEAKERS Silvina Levis, MD OSTEOPOROSIS - FACTS 1:3 older women and 1:5 older men will have a fragility fracture after age 50 After 3 years of treatment, depending
Name of Policy: Zoledronic Acid (Reclast ) Injection
 Name of Policy: Zoledronic Acid (Reclast ) Injection Policy #: 355 Latest Review Date: May 2011 Category: Pharmacy Policy Grade: Active Policy but no longer scheduled for regular literature reviews and
Name of Policy: Zoledronic Acid (Reclast ) Injection Policy #: 355 Latest Review Date: May 2011 Category: Pharmacy Policy Grade: Active Policy but no longer scheduled for regular literature reviews and
Osteoporosis/Fracture Prevention
 Osteoporosis/Fracture Prevention NATIONAL GUIDELINE SUMMARY This guideline was developed using an evidence-based methodology by the KP National Osteoporosis/Fracture Prevention Guideline Development Team
Osteoporosis/Fracture Prevention NATIONAL GUIDELINE SUMMARY This guideline was developed using an evidence-based methodology by the KP National Osteoporosis/Fracture Prevention Guideline Development Team
Osteoporosis: A Tale of 3 Task Forces!
 Osteoporosis: A Tale of 3 Task Forces! Robert A. Adler, MD McGuire Veterans Affairs Medical Center Virginia Commonwealth University Richmond, Virginia, USA Disclosures The opinions are those of the speaker
Osteoporosis: A Tale of 3 Task Forces! Robert A. Adler, MD McGuire Veterans Affairs Medical Center Virginia Commonwealth University Richmond, Virginia, USA Disclosures The opinions are those of the speaker
OSTEOPOROSIS AND WHAT TO DO AFTER A VERTEBRAL FRACTURE. Lydia Au Geriatrics Ng Teng Fong Hospital
 OSTEOPOROSIS AND WHAT TO DO AFTER A VERTEBRAL FRACTURE Lydia Au Geriatrics Ng Teng Fong Hospital LET S START WITH WHAT YOU WANT TO KNOW AND DO WITH A VERT FRACTURE Vertebral fractures Most common (550K
OSTEOPOROSIS AND WHAT TO DO AFTER A VERTEBRAL FRACTURE Lydia Au Geriatrics Ng Teng Fong Hospital LET S START WITH WHAT YOU WANT TO KNOW AND DO WITH A VERT FRACTURE Vertebral fractures Most common (550K
Osteoporosis challenges
 Osteoporosis challenges Osteoporosis challenges Who should have a fracture risk assessment? Who to treat? Drugs, holidays and unusual adverse effects Fracture liaison service? The size of the problem 1
Osteoporosis challenges Osteoporosis challenges Who should have a fracture risk assessment? Who to treat? Drugs, holidays and unusual adverse effects Fracture liaison service? The size of the problem 1
4.7 Studies of Quality Holy Cross Hospital Bone Health Early Stage I ER/PR Positive Breast Cancer Patients December 13, 2017
 4.7 Studies of Quality Holy Cross Hospital 2017 Bone Health Early Stage I ER/PR Positive Breast Cancer Patients December 13, 2017 Bone Health in Stage I ER/PR Positive Breast Cancer Patients To review
4.7 Studies of Quality Holy Cross Hospital 2017 Bone Health Early Stage I ER/PR Positive Breast Cancer Patients December 13, 2017 Bone Health in Stage I ER/PR Positive Breast Cancer Patients To review
FRAX Based Lebanese Osteoporosis Guidelines Second Update for Lebanese Guidelines for Osteoporosis Assessment and Treatment
 These guidelines are endorsed by the following Lebanese Scientific Societies and Associations: Lebanese Society of Endocrinology Diabetes and Lipids, Lebanese Society of Rheumatology, Lebanese Society
These guidelines are endorsed by the following Lebanese Scientific Societies and Associations: Lebanese Society of Endocrinology Diabetes and Lipids, Lebanese Society of Rheumatology, Lebanese Society
Disclosures. Diagnostic Challenges in Osteoporosis: Whom To Treat 9/25/2014
 Disclosures Diagnostic Challenges in Osteoporosis: Whom To Treat Ethel S. Siris, MD Columbia University Medical Center New York, NY Consultant on scientific issues for: AgNovos Amgen Eli Lilly Merck Novartis
Disclosures Diagnostic Challenges in Osteoporosis: Whom To Treat Ethel S. Siris, MD Columbia University Medical Center New York, NY Consultant on scientific issues for: AgNovos Amgen Eli Lilly Merck Novartis
Osteoporosis: Are your bones at risk of fracturing? Rachel Wallwork, MD Internal medicine resident Massachusetts General Hospital
 Osteoporosis: Are your bones at risk of fracturing? Rachel Wallwork, MD Internal medicine resident Massachusetts General Hospital What is Osteoporosis? Osteoporosis causes bones to lose density, become
Osteoporosis: Are your bones at risk of fracturing? Rachel Wallwork, MD Internal medicine resident Massachusetts General Hospital What is Osteoporosis? Osteoporosis causes bones to lose density, become
Osteoporosis/Fracture Prevention Clinician Guide SEPTEMBER 2017
 Kaiser Permanente National CLINICAL PRACTICE GUIDELINES Osteoporosis/Fracture Prevention Clinician Guide SEPTEMBER 2017 Introduction This Clinician Guide was developed to assist Primary Care physicians
Kaiser Permanente National CLINICAL PRACTICE GUIDELINES Osteoporosis/Fracture Prevention Clinician Guide SEPTEMBER 2017 Introduction This Clinician Guide was developed to assist Primary Care physicians
AETNA BETTER HEALTH Prior Authorization guideline for Erythropoiesis Stimulating Agents (ESA)
 AETNA BETTER HEALTH Prior Authorization guideline for Erythropoiesis Stimulating Agents (ESA) Drugs Covered Procrit Epogen Aranesp Authorization guidelines For patients who meet all of the following: Does
AETNA BETTER HEALTH Prior Authorization guideline for Erythropoiesis Stimulating Agents (ESA) Drugs Covered Procrit Epogen Aranesp Authorization guidelines For patients who meet all of the following: Does
BMD: A Continuum of Risk WHO Bone Density Criteria
 Pathogenesis of Osteoporosis Osteoporosis Diagnosis: BMD, FRAX and Assessment of Secondary Osteoporosis AGING MENOPAUSE OTHER RISK FACTORS RESORPTION > FORMATION Bone Loss LOW PEAK BONE MASS Steven T Harris
Pathogenesis of Osteoporosis Osteoporosis Diagnosis: BMD, FRAX and Assessment of Secondary Osteoporosis AGING MENOPAUSE OTHER RISK FACTORS RESORPTION > FORMATION Bone Loss LOW PEAK BONE MASS Steven T Harris
Name of Policy: Boniva (Ibandronate Sodium) Infusion
 Name of Policy: Boniva (Ibandronate Sodium) Infusion Policy #: 266 Latest Review Date: April 2010 Category: Pharmacology Policy Grade: Active Policy but no longer scheduled for regular literature reviews
Name of Policy: Boniva (Ibandronate Sodium) Infusion Policy #: 266 Latest Review Date: April 2010 Category: Pharmacology Policy Grade: Active Policy but no longer scheduled for regular literature reviews
Osteoporosis. Treatment of a Silently Developing Disease
 Osteoporosis Treatment of a Silently Developing Disease Marc K. Drezner, MD Senior Associate Dean Emeritus Professor of Medicine Emeritus University of Wisconsin-Madison Auditorium The Forest at Duke October
Osteoporosis Treatment of a Silently Developing Disease Marc K. Drezner, MD Senior Associate Dean Emeritus Professor of Medicine Emeritus University of Wisconsin-Madison Auditorium The Forest at Duke October
Kristen M. Nebel, DO PENN/ LGHP Geriatrics. Temple Family Medicine Review
 Kristen M. Nebel, DO PENN/ LGHP Geriatrics 10/3/17 Temple Family Medicine Review OBJECTIVES Define Revised 2017 American College of Physician Recommendations Screening, Prevention and Treatment Application
Kristen M. Nebel, DO PENN/ LGHP Geriatrics 10/3/17 Temple Family Medicine Review OBJECTIVES Define Revised 2017 American College of Physician Recommendations Screening, Prevention and Treatment Application
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Reference Number: HIM.PA.51 Effective Date: 12/14 Last Review Date: 08/17 Line of Business: Health Insurance Marketplace Revision Log See Important Reminder at the end of this policy for
Clinical Policy: Reference Number: HIM.PA.51 Effective Date: 12/14 Last Review Date: 08/17 Line of Business: Health Insurance Marketplace Revision Log See Important Reminder at the end of this policy for
BONIVA (ibandronate sodium)
 BONIVA (ibandronate sodium) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices
BONIVA (ibandronate sodium) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices
Bone Mass Measurement BONE MASS MEASUREMENT HS-042. Policy Number: HS-042. Original Effective Date: 8/25/2008
 Easy Choice Health Plan, Inc. Harmony Health Plan of Illinois, Inc. Missouri Care, Inc. Ohana Health Plan, a plan offered by WellCare Health Insurance of Arizona, Inc. WellCare Health Insurance of Illinois,
Easy Choice Health Plan, Inc. Harmony Health Plan of Illinois, Inc. Missouri Care, Inc. Ohana Health Plan, a plan offered by WellCare Health Insurance of Arizona, Inc. WellCare Health Insurance of Illinois,
Chau Nguyen, D.O. Rheumatologist Clinical Assistant Professor of Internal Medicine at Western University of Health Sciences
 Chau Nguyen, D.O Rheumatologist Clinical Assistant Professor of Internal Medicine at Western University of Health Sciences I do not have any relationship with the manufacturer of any commercial products
Chau Nguyen, D.O Rheumatologist Clinical Assistant Professor of Internal Medicine at Western University of Health Sciences I do not have any relationship with the manufacturer of any commercial products
FORTEO (teriparatide) INJECTION
 FORTEO (teriparatide) INJECTION Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage
FORTEO (teriparatide) INJECTION Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage
Pharmacy Medical Policy Bisphosphonates and Monoclonal Antibodies, Infusion/Injection
 Pharmacy Medical Policy Bisphosphonates and Monoclonal Antibodies, Infusion/Injection Table of Contents Policy: Commercial Policy History References Coding Information Information Pertaining to All Policies
Pharmacy Medical Policy Bisphosphonates and Monoclonal Antibodies, Infusion/Injection Table of Contents Policy: Commercial Policy History References Coding Information Information Pertaining to All Policies
Disclosure and Conflicts of Interest Steven T Harris MD Osteoporosis Diagnosis: BMD, FRAX and Assessment of Secondary Osteoporosis
 Osteoporosis Diagnosis: BMD, FRAX and Assessment of Secondary Osteoporosis Steven T Harris MD FACP Clinical Professor of Medicine University of California, San Francisco Disclosure and Conflicts of Interest
Osteoporosis Diagnosis: BMD, FRAX and Assessment of Secondary Osteoporosis Steven T Harris MD FACP Clinical Professor of Medicine University of California, San Francisco Disclosure and Conflicts of Interest
Overview. Bone Biology Osteoporosis Osteomalacia Paget s Disease Cases. People Centred Positive Compassion Excellence
 Overview Osteoporosis and Metabolic Bone Disease Dr Chandini Rao Consultant Rheumatologist Bone Biology Osteoporosis Osteomalacia Paget s Disease Cases Bone Biology Osteoporosis Increased bone remodelling
Overview Osteoporosis and Metabolic Bone Disease Dr Chandini Rao Consultant Rheumatologist Bone Biology Osteoporosis Osteomalacia Paget s Disease Cases Bone Biology Osteoporosis Increased bone remodelling
AACE/ACE Osteoporosis Treatment Decision Tool
 AACE/ACE Osteoporosis Treatment Decision Tool What is Osteoporosis? OSTEOPOROSIS is defined as reduced bone strength leading to an increased risk of fracture. Osteoporosis, or porous bones, occurs when
AACE/ACE Osteoporosis Treatment Decision Tool What is Osteoporosis? OSTEOPOROSIS is defined as reduced bone strength leading to an increased risk of fracture. Osteoporosis, or porous bones, occurs when
OSTEOPOROSIS IN MEN. Nelson B. Watts, MD OSTEOPOROSIS AND BONE HEALTH SERVICES CINCINNATI, OHIO
 OSTEOPOROSIS IN MEN Nelson B. Watts, MD OSTEOPOROSIS AND BONE HEALTH SERVICES CINCINNATI, OHIO DISCLOSURES Speakers Bureau: Amgen, Radius Consultant: Abbvie, Amgen, Janssen, Radius, Sanofi Watts NB et
OSTEOPOROSIS IN MEN Nelson B. Watts, MD OSTEOPOROSIS AND BONE HEALTH SERVICES CINCINNATI, OHIO DISCLOSURES Speakers Bureau: Amgen, Radius Consultant: Abbvie, Amgen, Janssen, Radius, Sanofi Watts NB et
Dumfries and Galloway. Treatment Protocol for Osteoporosis
 Dumfries and Galloway Treatment Protocol for Osteoporosis DIAGNOSIS OF OSTEOPOROSIS 2 Diagnostic Criteria 2 REFERRAL CRITERIA FOR DEXA 3 TREATMENT 4 Non-Drug Therapy : for all 4 Non-Drug Therapy : in the
Dumfries and Galloway Treatment Protocol for Osteoporosis DIAGNOSIS OF OSTEOPOROSIS 2 Diagnostic Criteria 2 REFERRAL CRITERIA FOR DEXA 3 TREATMENT 4 Non-Drug Therapy : for all 4 Non-Drug Therapy : in the
Breast Cancer and Bone Loss. One in seven women will develop breast cancer during a lifetime
 Breast Cancer and Bone Loss One in seven women will develop breast cancer during a lifetime Causes of Bone Loss in Breast Cancer Patients Aromatase inhibitors Bil Oophorectomy Hypogonadism Steroids Chemotherapy
Breast Cancer and Bone Loss One in seven women will develop breast cancer during a lifetime Causes of Bone Loss in Breast Cancer Patients Aromatase inhibitors Bil Oophorectomy Hypogonadism Steroids Chemotherapy
Case Finding and Risk Assessment for Osteoporosis
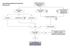 Case Finding and Risk Assessment for Osteoporosis Patient may present as a fragility fracture or risk fracture Fragility fracture age 50 Clinical risk factors aged 50 Very strong clinical risk factors
Case Finding and Risk Assessment for Osteoporosis Patient may present as a fragility fracture or risk fracture Fragility fracture age 50 Clinical risk factors aged 50 Very strong clinical risk factors
Vol. 19, Bulletin No. 108 August-September 2012 Also in the Bulletin: Denosumab 120mg for Bone Metastases
 ה מ ר א פ הביטאון לענייני תרופות ISRAEL DRUG BULLETIN 19 years of unbiased and independent drug information P H A R x M A Vol. 19, Bulletin No. 108 August-September 2012 Also in the Bulletin: Denosumab
ה מ ר א פ הביטאון לענייני תרופות ISRAEL DRUG BULLETIN 19 years of unbiased and independent drug information P H A R x M A Vol. 19, Bulletin No. 108 August-September 2012 Also in the Bulletin: Denosumab
BAD TO THE BONE. Peter Jones, Rheumatologist QE Health, Rotorua. GP CME Conference Rotorua, June 2008
 BAD TO THE BONE Peter Jones, Rheumatologist QE Health, Rotorua GP CME Conference Rotorua, June 2008 Agenda Osteoporosis in Men Vitamin D and Calcium Long-term treatment with Bisphosphonates Pathophysiology
BAD TO THE BONE Peter Jones, Rheumatologist QE Health, Rotorua GP CME Conference Rotorua, June 2008 Agenda Osteoporosis in Men Vitamin D and Calcium Long-term treatment with Bisphosphonates Pathophysiology
Osteoporosis: An Overview. Carolyn J. Crandall, MD, MS
 Osteoporosis: An Overview Carolyn J. Crandall, MD, MS Osteoporosis: An Overview Carolyn J. Crandall, MD, MS Professor of Medicine David Geffen School of Medicine at UCLA Objectives Review osteoporosis
Osteoporosis: An Overview Carolyn J. Crandall, MD, MS Osteoporosis: An Overview Carolyn J. Crandall, MD, MS Professor of Medicine David Geffen School of Medicine at UCLA Objectives Review osteoporosis
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 : assessing the risk of fragility fracture bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new
: assessing the risk of fragility fracture bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new
Update on Osteoporosis 2016
 WELCOME! Update on Osteoporosis 2016 Jennifer J. Kelly, D.O., F.A.C.E. Associate Professor of Medicine Division of Endocrinology, Diabetes and Metabolism Upstate Medical University Director of the Clinical
WELCOME! Update on Osteoporosis 2016 Jennifer J. Kelly, D.O., F.A.C.E. Associate Professor of Medicine Division of Endocrinology, Diabetes and Metabolism Upstate Medical University Director of the Clinical
Clinician s Guide to Prevention and Treatment of Osteoporosis
 Clinician s Guide to Prevention and Treatment of Osteoporosis Published: 15 August 2014 committee of the National Osteoporosis Foundation (NOF) Tipawan khiemsontia,md outline Basic pathophysiology screening
Clinician s Guide to Prevention and Treatment of Osteoporosis Published: 15 August 2014 committee of the National Osteoporosis Foundation (NOF) Tipawan khiemsontia,md outline Basic pathophysiology screening
nogg Guideline for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK
 nogg NATIONAL OSTEOPOROSIS GUIDELINE GROUP Guideline for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK Produced by J Compston, A Cooper,
nogg NATIONAL OSTEOPOROSIS GUIDELINE GROUP Guideline for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK Produced by J Compston, A Cooper,
Osteoporosis in Men Wendy Rosenthal PharmD. This program has been brought to you by PharmCon
 Osteoporosis in Men Wendy Rosenthal PharmD This program has been brought to you by PharmCon Osteoporosis in Men Speaker: Dr. Wendy Rosenthal, President of MedOutcomes, will be the presenter for this webcast.
Osteoporosis in Men Wendy Rosenthal PharmD This program has been brought to you by PharmCon Osteoporosis in Men Speaker: Dr. Wendy Rosenthal, President of MedOutcomes, will be the presenter for this webcast.
Subject: Denosumab (Prolia ; Xgeva ) Injection
 09-J1000-25 Original Effective Date: 08/15/10 Reviewed: 03/14/18 Revised: 12/15/18 Next Review: 01/09/19 Subject: Denosumab (Prolia ; Xgeva ) Injection THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION,
09-J1000-25 Original Effective Date: 08/15/10 Reviewed: 03/14/18 Revised: 12/15/18 Next Review: 01/09/19 Subject: Denosumab (Prolia ; Xgeva ) Injection THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION,
Fragile Bones and how to recognise them. Rod Hughes Consultant physician and rheumatologist St Peter s hospital Chertsey
 Fragile Bones and how to recognise them Rod Hughes Consultant physician and rheumatologist St Peter s hospital Chertsey Osteoporosis Osteoporosis is a skeletal disorder characterised by compromised bone
Fragile Bones and how to recognise them Rod Hughes Consultant physician and rheumatologist St Peter s hospital Chertsey Osteoporosis Osteoporosis is a skeletal disorder characterised by compromised bone
Guidelines for the Pharmaceutical Management of Osteoporosis in Adult WA Public Hospitals
 WA.DRUG EVALUATION PANEL Guidelines for the Pharmaceutical Management of Osteoporosis in Adult WA Public Hospitals Introduction Osteoporotic fracture-related hospitalisations impose a substantial financial
WA.DRUG EVALUATION PANEL Guidelines for the Pharmaceutical Management of Osteoporosis in Adult WA Public Hospitals Introduction Osteoporotic fracture-related hospitalisations impose a substantial financial
Summary. Background. Diagnosis
 March 2009 Management of post-menopausal osteoporosis This bulletin focuses on the pharmacological management of patients with post-menopausal osteoporosis both those with clinically evident disease (e.g.
March 2009 Management of post-menopausal osteoporosis This bulletin focuses on the pharmacological management of patients with post-menopausal osteoporosis both those with clinically evident disease (e.g.
Advanced medicine conference. Monday 20 Tuesday 21 June 2016
 Advanced medicine conference Monday 20 Tuesday 21 June 2016 Osteoporosis: recent advances in risk assessment and management Juliet Compston Emeritus Professor of Bone Medicine Cambridge Biomedical Campus
Advanced medicine conference Monday 20 Tuesday 21 June 2016 Osteoporosis: recent advances in risk assessment and management Juliet Compston Emeritus Professor of Bone Medicine Cambridge Biomedical Campus
Bone Health in Celiac Disease. Partha S. Sinha MD, PhD October 29 th, 2017
 Bone Health in Celiac Disease Partha S. Sinha MD, PhD October 29 th, 2017 No Disclosures Objectives Recognize the mechanisms by which celiac disease can affect bone health Review what diagnostic tests
Bone Health in Celiac Disease Partha S. Sinha MD, PhD October 29 th, 2017 No Disclosures Objectives Recognize the mechanisms by which celiac disease can affect bone health Review what diagnostic tests
Download slides:
 Download slides: https://www.tinyurl.com/m67zcnn https://tinyurl.com/kazchbn OSTEOPOROSIS REVIEW AND UPDATE Boca Raton Regional Hospital Internal Medicine Conference 2017 Benjamin Wang, M.D., FRCPC Division
Download slides: https://www.tinyurl.com/m67zcnn https://tinyurl.com/kazchbn OSTEOPOROSIS REVIEW AND UPDATE Boca Raton Regional Hospital Internal Medicine Conference 2017 Benjamin Wang, M.D., FRCPC Division
Module 5 - Speaking of Bones Osteoporosis For Health Professionals: Fracture Risk Assessment. William D. Leslie, MD MSc FRCPC
 Module 5 - Speaking of Bones Osteoporosis For Health Professionals: Fracture Risk Assessment William D. Leslie, MD MSc FRCPC Case #1 Age 53: 3 years post-menopause Has always enjoyed excellent health with
Module 5 - Speaking of Bones Osteoporosis For Health Professionals: Fracture Risk Assessment William D. Leslie, MD MSc FRCPC Case #1 Age 53: 3 years post-menopause Has always enjoyed excellent health with
MAKE NO BONES ABOUT IT: UNDERSTANDING THE PHARMACIST S ROLE IN OSTEOPOROSIS MANAGEMENT. Jill Hiers, Pharm.D., BCPS
 MAKE NO BONES ABOUT IT: UNDERSTANDING THE PHARMACIST S ROLE IN OSTEOPOROSIS MANAGEMENT Jill Hiers, Pharm.D., BCPS Outline Definition of osteoporosis/osteopenia Disease prevalence/burden Risk Factors AACE
MAKE NO BONES ABOUT IT: UNDERSTANDING THE PHARMACIST S ROLE IN OSTEOPOROSIS MANAGEMENT Jill Hiers, Pharm.D., BCPS Outline Definition of osteoporosis/osteopenia Disease prevalence/burden Risk Factors AACE
Learning Objectives. Controversies in Osteoporosis Prevention and Management. Definition. Presenter Disclosure Information.
 4 4:45 pm Controversies in Osteoporosis Prevention and Management SPEAKER Carolyn Crandall, MD, MS Presenter Disclosure Information The following relationships exist related to this presentation: Carolyn
4 4:45 pm Controversies in Osteoporosis Prevention and Management SPEAKER Carolyn Crandall, MD, MS Presenter Disclosure Information The following relationships exist related to this presentation: Carolyn
Bisphosphonates. Making intelligent drug choices
 Making intelligent drug choices Bisphosphonates are a first choice for treating osteoporosis, according to Kedrin E. Van Steenwyk, DO, an obstetrician/gynecologist at Sycamore Women s Center, Miamisburg,
Making intelligent drug choices Bisphosphonates are a first choice for treating osteoporosis, according to Kedrin E. Van Steenwyk, DO, an obstetrician/gynecologist at Sycamore Women s Center, Miamisburg,
Bone Health for Women: Current Research, Initiatives and Recommendations
 Page 1 BONE HEALTH FOR WOMEN: CURRENT RESEARCH, INITIATIVES AND RECOMMENDATIONS Dr. Melissa Kagarise This program has been brought to you by PharmCon PharmCon is accredited by the Accreditation Council
Page 1 BONE HEALTH FOR WOMEN: CURRENT RESEARCH, INITIATIVES AND RECOMMENDATIONS Dr. Melissa Kagarise This program has been brought to you by PharmCon PharmCon is accredited by the Accreditation Council
Osteoporosis and Lupus. Andrew Ruthberg, MD University Rheumatologists
 Osteoporosis and Lupus Andrew Ruthberg, MD University Rheumatologists 1 Forget the medical terminology (osteoporosis, osteopenia, low bone mass, DEXA, DXA, T score etc) The bottom line is that you don
Osteoporosis and Lupus Andrew Ruthberg, MD University Rheumatologists 1 Forget the medical terminology (osteoporosis, osteopenia, low bone mass, DEXA, DXA, T score etc) The bottom line is that you don
Skeletal Manifestations
 Skeletal Manifestations of Metabolic Bone Disease Mishaela R. Rubin, MD February 21, 2008 The Three Ages of Women Gustav Klimt 1905 1 Lecture Outline Osteoporosis epidemiology diagnosis secondary causes
Skeletal Manifestations of Metabolic Bone Disease Mishaela R. Rubin, MD February 21, 2008 The Three Ages of Women Gustav Klimt 1905 1 Lecture Outline Osteoporosis epidemiology diagnosis secondary causes
Page 1
 Osteoporosis Osteoporosis is a condition characterised by weakened bones that fracture easily. After menopause many women are at risk of developing osteoporosis. Peak bone mass is usually reached during
Osteoporosis Osteoporosis is a condition characterised by weakened bones that fracture easily. After menopause many women are at risk of developing osteoporosis. Peak bone mass is usually reached during
Osteoporosis as a Focus for Practice Improvement
 Osteoporosis as a Focus for Practice Improvement Karen E. Hansen, M.D. Assistant Professor of Medicine Rheumatology and Endocrine Sections University of Wisconsin Madison, WI Postmenopausal Osteoporosis
Osteoporosis as a Focus for Practice Improvement Karen E. Hansen, M.D. Assistant Professor of Medicine Rheumatology and Endocrine Sections University of Wisconsin Madison, WI Postmenopausal Osteoporosis
Clinical Practice. Presented by: Internist, Endocrinologist
 Clinical Practice Management of Osteoporosis Presented by: SaeedBehradmanesh, h MD Internist, Endocrinologist Iran, Isfahan, Feb. 2017 Definition: A disease characterized by low bone mass and microarchitectural
Clinical Practice Management of Osteoporosis Presented by: SaeedBehradmanesh, h MD Internist, Endocrinologist Iran, Isfahan, Feb. 2017 Definition: A disease characterized by low bone mass and microarchitectural
Learning Objectives. Controversies in Osteoporosis Prevention and Management. Etiology. Presenter Disclosure Information. Epidemiology.
 12:45 1:30pm Controversies in Osteoporosis Prevention and Management SPEAKER Carolyn Crandall, MD, MS Presenter Disclosure Information The following relationships exist related to this presentation: Carolyn
12:45 1:30pm Controversies in Osteoporosis Prevention and Management SPEAKER Carolyn Crandall, MD, MS Presenter Disclosure Information The following relationships exist related to this presentation: Carolyn
S H A R E D C A R E G U I D E L I N E Drug: Denosumab 60mg injection Indication: treatment of osteoporosis in postmenopausal women
 S H A R E D C A R E G U I D E L I N E Drug: Denosumab 60mg injection Indication: treatment of osteoporosis in postmenopausal women Introduction Indication: Denosumab (Prolia ) is recommended in NICE TA204
S H A R E D C A R E G U I D E L I N E Drug: Denosumab 60mg injection Indication: treatment of osteoporosis in postmenopausal women Introduction Indication: Denosumab (Prolia ) is recommended in NICE TA204
Medication Policy Manual. Topic: Prolia, denosumab Date of Origin: August 11, 2010
 Independent licensees of the Blue Cross and Blue Shield Association Medication Policy Manual Policy No: dru223 Topic: Prolia, denosumab Date of Origin: August 11, 2010 Committee Approval Date: August 11,
Independent licensees of the Blue Cross and Blue Shield Association Medication Policy Manual Policy No: dru223 Topic: Prolia, denosumab Date of Origin: August 11, 2010 Committee Approval Date: August 11,
Prior treatment with non-biologic Disease- Modifying Antirheumatic. Not to be used in combination with another biologic DMARD
 Abatacept (Orencia) 1, 2, 7, 11, 13, 14, 18, 24, 31, 44, 48, 49, 51, 53, 55, 57 J0129 Alpha 1 - Proteinase inhibitor (Prolastin-C) 5, 6, 10, 12, 40 Medically Necessary (if all the following criteria apply):
Abatacept (Orencia) 1, 2, 7, 11, 13, 14, 18, 24, 31, 44, 48, 49, 51, 53, 55, 57 J0129 Alpha 1 - Proteinase inhibitor (Prolastin-C) 5, 6, 10, 12, 40 Medically Necessary (if all the following criteria apply):
