Transmittal 86 Date: July 3, SUBJECT: Continuous Positive Airway Pressure (CPAP) Therapy for Obstructive Sleep Apnea (OSA)
|
|
|
- Rose O’Connor’
- 6 years ago
- Views:
Transcription
1 CMS Manual System Pub Medicare Natinal Cverage Determinatins Department f Health & Human Services (DHHS) Centers fr Medicare & Medicaid Services (CMS) Transmittal 86 Date: July 3, 2008 Change Request 6048 SUBJECT: Cntinuus Psitive Airway Pressure (CPAP) Therapy fr Obstructive Sleep Apnea (OSA) I. SUMMARY OF CHANGES: After recnsideratin, cverage fr CPAP therapy fr OSA is expanded t include a psitive diagnsis f OSA made using a hme sleep test under specified criteria. This revisin is a natinal cverage determinatin (NCD). NCDs are binding n all carriers, fiscal intermediaries, quality imprvement rganizatins, qualified independent cntractrs, the Medicare appeals cuncil, and administrative law judges (ALJs) (see 42 CFR sectin (a)(4) (2005)). An NCD that expands cverage is als binding n a Medicare advantage rganizatin. In additin, an ALJ may nt review an NCD. (See sectin 1869(f)(1)(A)(i) f the Scial Security Act.) NEW / REVISED MATERIAL EFFECTIVE DATE: March 13, 2008 IMPLEMENTATION DATE: August 4, 2008 Disclaimer fr manual changes nly: The revisin date and transmittal number apply nly t red italicized material. Any ther material was previusly published and remains unchanged. Hwever, if this revisin cntains a table f cntents, yu will receive the new/revised infrmatin nly, and nt the entire table f cntents. II. CHANGES IN MANUAL INSTRUCTIONS: (N/A if manual is nt updated) R=REVISED, N=NEW, D=DELETED R/N/D R CHAPTER / SECTION / SUBSECTION / TITLE 1/240.4/Cntinuus Psitive Airway Pressure (CPAP) Therapy fr Obstructive Sleep Apnea (OSA) (Effective March 13, 2008) III. FUNDING: SECTION A: Fr Fiscal Intermediaries and Carriers: N additinal funding will be prvided by CMS; cntractr activities are t be carried ut within their perating budgets. SECTION B: Fr Medicare Administrative Cntractrs (MACs): The Medicare administrative cntractr is hereby advised that this cnstitutes technical directin as defined in yur cntract. CMS des nt cnstrue this as a change t the MAC Statement f Wrk. The cntractr is nt bligated t incur csts in excess f the amunts alltted in yur cntract unless and until specifically authrized by the cntracting fficer. If the cntractr cnsiders anything prvided, as described abve, t be utside the current scpe f wrk, the cntractr shall withhld perfrmance n the part(s) in questin and immediately ntify the cntracting fficer, in writing r by , and request frmal directins regarding cntinued perfrmance requirements.
2 IV. ATTACHMENTS: Business Requirements Manual Instructin *Unless therwise specified, the effective date is the date f service.
3 Attachment - Business Requirements Pub Transmittal: 86 Date: July 3, 2008 Change Request: CR 6048 SUBJECT: Cntinuus Psitive Airway Pressure (CPAP) Therapy fr Obstructive Sleep Apnea (OSA) Effective Date: March 13, 2008 Implementatin Date: August 4, 2008 I. GENERAL INFORMATION A. Backgrund: The Centers fr Medicare & Medicaid Services (CMS) received a request frm the American Academy f Otlarynglgy-Head and Neck Surgery, t recnsider its 2005 Natinal Cverage Determinatin (NCD) fr CPAP Therapy fr OSA t allw fr cverage f CPAP based upn a diagnsis f OSA by hme sleep testing (HST). Medicare cvers the use f CPAP in beneficiaries wh have been diagnsed with mderate t severe OSA when rdered and prescribed by a licensed treating physician and cnfirmed by plysmngraphy (PSG) perfrmed in a sleep labratry in accrdance with sectin 240.4, f the NCD Manual. There are currently a number f prcedures used by physicians singly r in cmbinatin that cntribute t a diagnsis f OSA: clinical evaluatin, PSG perfrmed in a sleep labratry, HST using Type II, III, & IV devices, and ther services. After careful cnsideratin, CMS is revising its NCD n CPAP therapy fr OSA t mdify certain elements as well as allwing fr the cverage f CPAP based n a psitive diagnsis f OSA by HST as cntained in sectin 240.4, f Pub , f the NCD Manual. Als refer t chapter 20, sectin 30.5, f Pub , f the Medicare Claims Prcessing Manual, fr billing guidelines fr capped rental items, including CPAP. B. Plicy: Effective fr claims with dates f service n and after March 13, 2008, Medicare will allw fr cverage f CPAP therapy based upn a psitive diagnsis f OSA by HST. Refer t sectin 240.4, f Pub , f the NCD Manual. 1. Cverage f CPAP is initially limited t a 12-week perid fr beneficiaries diagnsed with OSA as subsequently described. CPAP is subsequently cvered fr thse beneficiaries diagnsed with OSA whse OSA imprved as a result f CPAP during this 12-week perid. NOTE: The CMS reminds the reader that durable medical equipment, prsthetics, rthtics, and supplies (DMEPOS) suppliers are required t prvide beneficiaries with necessary infrmatin and instructins n hw t use Medicare-cvered items safely and effectively. 42 CFR (c)(12). Failure t meet this standard may result in revcatin f the DMEPOS supplier s billing privileges. 42 CFR (d). 2. The CPAP fr adults is cvered when diagnsed using a clinical evaluatin and a psitive: a. plysmngraphy (PSG) perfrmed in a sleep labratry; r b. unattended hme sleep mnitring device f Type II; r c. unattended hme sleep mnitring device f Type III; r d. unattended hme sleep mnitring device f Type IV, measuring at least 3 channels
4 NOTE: The CMS reminds the reader that, in general, pursuant t 42 CFR (a) diagnstic tests that are nt rdered by the beneficiary s treating physician are nt cnsidered reasnable and necessary. Pursuant t 42 CFR (b) diagnstic tests payable under the physician fee schedule that are furnished withut the required level f supervisin by a physician are nt reasnable and necessary. 3. A psitive test fr OSA is established if either f the fllwing criterin using the Apnea-Hyppnea Index (AHI) r Respiratry Disturbance Index (RDI) are met: AHI r RDI greater than r equal t 15 events per hur, r AHI r RDI greater than r equal t 5 and less than r equal t 14 events per hur with dcumented symptms f excessive daytime sleepiness, impaired cgnitin, md disrders r insmnia, r dcumented hypertensin, ischemic heart disease, r histry f strke. The AHI is equal t the average number f episdes f apnea and hyppnea per hur. The RDI is equal t the average number f respiratry disturbances per hur. 4. If the AHI r RDI is calculated based n less than 2 hurs f cntinuus recrded sleep, the ttal number f recrded events t calculate the AHI r RDI during sleep testing is at least the number f events that wuld have been required in a 2-hur perid. 5. The CMS is deleting the distinct requirements that an individual have mderate t severe OSA and that surgery is a likely alternative. 6. The CPAP based n clinical diagnsis alne r using a diagnstic prcedure ther than PSG r Type II, Type III, r a Type IV HST measuring at least three channels is cvered nly when prvided in the cntext f a clinical study as specified in sectin 310.1, f Pub , f the NCD Manual. New HST Prtable Mnitring G Cdes Effective March 13, 2008: G0398: Hme sleep study test (HST) with type II prtable mnitr, unattended; minimum f 7 channels: EEG, EOG, EMG, ECG/heart rate, airflw, respiratry effrt and xygen saturatin. G0398 Shrt Descriptr: Hme sleep test/type 2 Prta G0399: Hme sleep test (HST) with type III prtable mnitr, unattended; minimum f 4 channels: 2 respiratry mvement/airflw, 1 ECG/heart rate and 1 xygen saturatin G0399 Shrt Descriptr: Hme sleep test/type 3 Prta G0400: Hme sleep test (HST) with type IV prtable mnitr, unattended; minimum f 3 channels G0400 Shrt Descriptr: Hme sleep test/type 4 Prta II. BUSINESS REQUIREMENTS TABLE Use Shall" t dente a mandatry requirement Number Requirement Respnsibility (place an X in each applicable clumn) Effective fr claims with dates f X X X X A/B DME FI CARRIER RHHI Shared-System Maintainers FISS MCS VMS CWF MAC MAC OTHER
5 Number Requirement Respnsibility (place an X in each applicable clumn) service n and after March 13, 2008, Medicare will allw fr cverage f CPAP therapy fr adult beneficiaries based upn clinical evaluatin and a psitive diagnsis f OSA by HST. Refer t sectin 240.4, f Pub , f the NCD Manual Fr cverage f rutine items/services prvided in the cntext f a clinical study, claims shall be prcessed accrding t CED/clinical trials criteria at sectin 310.1, f Pub , f the NCD Manual, sectin 240.4, f the NCD Manual, and chapter 32, sectins , f Pub f the Claims Prcessing Manual Cntractrs shall nt search files t adjust claims but shall adjust claims brught t their attentin. A/B MAC DME MAC X X X X X X X X FI CARRIER RHHI Shared-System Maintainers FISS MCS VMS CWF OTHER III. PROVIDER EDUCATION TABLE Number Requirement Respnsibility (place an X in each applicable clumn) A prvider educatin article related t this instructin will be available at shrtly after the CR is released. Yu will receive ntificatin f the article release via the established "MLN Matters" listserv. Cntractrs shall pst this article, r a direct link t this article, n their Web site and include infrmatin abut it in a listserv message within ne week f the availability f the prvider educatin article. In additin, the prvider educatin article shall be included in yur next regularly scheduled bulletin. Cntractrs are free t supplement MLN Matters articles with lcalized infrmatin that wuld benefit their prvider cmmunity in billing and administering the Medicare prgram crrectly. A/B MAC DME MAC X X X X FI CARRIER RHHI Shared-System Maintainers FISS MCS VMS CWF OTHER
6 IV. SUPPORTING INFORMATION Sectin A: Fr any recmmendatins and supprting infrmatin assciated with listed requirements, use the bx belw: N/A Use "Shuld" t dente a recmmendatin. X-Ref Requirement Number Recmmendatins r ther supprting infrmatin: Sectin B: Fr all ther recmmendatins and supprting infrmatin, use this space: N/A V. CONTACTS Pre-Implementatin Cntact(s): Jean Stiller (cverage), , jean.stiller@cms.hhs.gv, Pat Brcat-Simns (cverage), , patricia.brcatsimns@cms.hhs.gv Pst-Implementatin Cntact(s): Apprpriate RO. VI. FUNDING Sectin A: Fr Fiscal Intermediaries (FIs), Carriers, and Reginal Hme Health Carriers (RHHIs) use nly ne f the fllwing statements: N additinal funding will be prvided by CMS; cntractr activities are t be carried ut within their perating budgets. Sectin B: Fr Medicare Administrative Cntractrs (MACs), use the fllwing statement: The Medicare administrative cntractr is hereby advised that this cnstitutes technical directin as defined in yur cntract. CMS des nt cnstrue this as a change t the MAC Statement f Wrk. The cntractr is nt bligated t incur csts in excess f the amunts alltted in yur cntract unless and until specifically authrized by the cntracting fficer. If the cntractr cnsiders anything prvided, as described abve, t be utside the current scpe f wrk, the cntractr shall withhld perfrmance n the part(s) in questin and immediately ntify the cntracting fficer, in writing r by , and request frmal directins regarding cntinued perfrmance requirements.
7 Medicare Natinal Cverage Determinatins Manual Chapter 1, Part 4 (Sectins ) Cverage Determinatins Table f Cntents (Rev.86, ) Cntinuus Psitive Airway Pressure (CPAP) Therapy fr Obstructive Sleep Apnea (OSA) (Effective March 13, 2008)
8 Cntinuus Psitive Airway Pressure (CPAP) Therapy Fr Obstructive Sleep Apnea (OSA) (Effective March 13, 2008) (Rev.86, Issued: , Effective: , Implementatin: ) A. General Cntinuus Psitive Airway Pressure (CPAP) is a nn-invasive technique fr prviding single levels f air pressure frm a flw generatr, via a nse mask, thrugh the nares. The purpse is t prevent the cllapse f the rpharyngeal walls and the bstructin f airflw during sleep, which ccurs in bstructive sleep apnea (OSA). The apnea hyppnea index (AHI) is equal t the average number f episdes f apnea and hyppnea per hur. The respiratry disturbance index (RDI) is equal t the average number f respiratry disturbances per hur. Apnea is defined as a cessatin f airflw fr at least 10 secnds. Hyppnea is defined as an abnrmal respiratry event lasting at least 10 secnds with at least a 30% reductin in thracabdminal mvement r airflw as cmpared t baseline, and with at least a 4% xygen desaturatin. The AHI and/r RDI may be measured by plysmngraphy (PSG) in a facility-based sleep study labratry, r by a Type II hme sleep test (HST) mnitr, a Type III HST mnitr, r a Type IV HST mnitr measuring at least 3 channels. B. Natinally Cvered Indicatins Effective fr claims with dates f service n and after March 13, 2008, the Centers fr Medicare & Medicaid Services (CMS) determines that CPAP therapy when used in adult patients with OSA is cnsidered reasnable and necessary under the fllwing situatins: 1. The use f CPAP is cvered under Medicare when used in adult patients with OSA. Cverage f CPAP is initially limited t a 12-week perid t identify beneficiaries diagnsed with OSA as subsequently described wh benefit frm CPAP. CPAP is subsequently cvered nly fr thse beneficiaries diagnsed with OSA wh benefit frm CPAP during this 12-week perid. 2. The prvider f CPAP must cnduct educatin f the beneficiary prir t the use f the CPAP device t ensure that the beneficiary has been educated in the prper use f the device. A caregiver, fr example a family member, may be cmpensatry, if cnsistently available in the beneficiary's hme and willing and able t safely perate the CPAP device. 3. A psitive diagnsis f OSA fr the cverage f CPAP must include a clinical evaluatin and a psitive: a. attended PSG perfrmed in a sleep labratry; r
9 b. unattended HST with a Type II hme sleep mnitring device; r c. unattended HST with a Type III hme sleep mnitring device; r d. unattended HST with a Type IV hme sleep mnitring device that measures at least 3 channels. 4. The sleep test must have been previusly rdered by the beneficiary s treating physician and furnished under apprpriate physician supervisin. 5. An initial 12-week perid f CPAP is cvered in adult patients with OSA if either f the fllwing criterin using the AHI r RDI are met: a. AHI r RDI greater than r equal t 15 events per hur, r b. AHI r RDI greater than r equal t 5 events and less than r equal t 14 events per hur with dcumented symptms f excessive daytime sleepiness, impaired cgnitin, md disrders r insmnia, r dcumented hypertensin, ischemic heart disease, r histry f strke. 6. The AHI r RDI is calculated n the average number f events f per hur. If the AHI r RDI is calculated based n less than 2 hurs f cntinuus recrded sleep, the ttal number f recrded events t calculate the AHI r RDI during sleep testing must be at a minimum the number f events that wuld have been required in a 2-hur perid. 7. Apnea is defined as a cessatin f airflw fr at least 10 secnds. Hyppnea is defined as an abnrmal respiratry event lasting at least 10 secnds with at least a 30% reductin in thracabdminal mvement r airflw as cmpared t baseline, and with at least a 4% xygen desaturatin. 8. Cverage with Evidence Develpment (CED): Medicare prvides the fllwing limited cverage fr CPAP in adult beneficiaries wh d nt qualify fr CPAP cverage based n criteria 1-7 abve. A clinical study seeking Medicare payment fr CPAP prvided t a beneficiary wh is an enrlled subject in that study must address ne r mre f the fllwing questins a. In Medicare-aged subjects with clinically identified risk factrs fr OSA, hw des the diagnstic accuracy f a clinical trial f CPAP cmpare with PSG and Type II, III & IV HST in identifying subjects with OSA wh will respnd t CPAP? b. In Medicare-aged subjects with clinically identified risk factrs fr OSA wh have nt undergne cnfirmatry testing with PSG r Type II, III & IV HST, des CPAP cause clinically meaningful harm? The study must meet the fllwing additinal standards:
10 c. The principal purpse f the research study is t test whether a particular interventin ptentially imprves the participants health utcmes. d. The research study is well-supprted by available scientific and medical infrmatin r it is intended t clarify r establish the health utcmes f interventins already in cmmn clinical use. e. The research study des nt unjustifiably duplicate existing studies. f. The research study design is apprpriate t answer the research questin being asked in the study. g. The research study is spnsred by an rganizatin r individual capable f executing the prpsed study successfully. h. The research study is in cmpliance with all applicable Federal regulatins cncerning the prtectin f human subjects fund at 45 CFR Part 46. If a study is Fd and Drug Administratin-regulated, it als must be in cmpliance with 21 CFR Parts 50 and 56. i. All aspects f the research study are cnducted accrding t the apprpriate standards f scientific integrity. j. The research study has a written prtcl that clearly addresses, r incrprates by reference, the Medicare standards. k. The clinical research study is nt designed t exclusively test txicity r disease pathphysilgy in healthy individuals. Trials f all medical technlgies measuring therapeutic utcmes as ne f the bjectives meet this standard nly if the disease r cnditin being studied is life-threatening as defined in 21 CFR (a) and the patient has n ther viable treatment ptins. l. The clinical research study is registered n the ClinicalTrials.gv Web site by the principal spnsr/investigatr prir t the enrllment f the first study subject. m. The research study prtcl specifies the methd and timing f public release f all pre-specified utcmes t be measured, including release f utcmes if utcmes are negative r study is terminated early. The results must be made public within 24 mnths f the end f data cllectin. If a reprt is planned fr publicatin in a peer-reviewed jurnal, then that initial release may be an abstract that meets the requirements f the Internatinal Cmmittee f Medical Jurnal Editrs. Hwever, a full reprt f the utcmes must be made public n later than 3 years after the end f data cllectin. n. The research study prtcl must explicitly discuss subppulatins affected by the treatment under investigatin, particularly traditinally underrepresented grups in clinical studies, hw the inclusin and exclusin criteria affect enrllment f these
11 ppulatins, and a plan fr the retentin and reprting f said ppulatins in the trial. If the inclusin and exclusin criteria are expected t have a negative effect n the recruitment r retentin f underrepresented ppulatins, the prtcl must discuss why these criteria are necessary.. The research study prtcl explicitly discusses hw the results are r are nt expected t be generalizable t the Medicare ppulatin t infer whether Medicare patients may benefit frm the interventin. Separate discussins in the prtcl may be necessary fr ppulatins eligible fr Medicare due t age, disability, r Medicaid eligibility. C. Natinally Nn-cvered Indicatins Effective fr claims with dates f services n and after March 13, 2008, ther diagnstic tests fr the diagnsis f OSA ther than thse nted abve fr prescribing CPAP are nt cvered. D. Other N/A (This NCD last reviewed March 2008.)
Continuous Positive Airway Pressure (CPAP) and Respiratory Assist Devices (RADs) including Bi-Level PAP
 Cntinuus Psitive Airway Pressure (CPAP) and Respiratry Assist Devices (RADs), Including Bi-Level PAP Benefit Criteria t Change fr Texas Medicaid Effective March 1, 2017 Overview f Benefit Changes Benefit
Cntinuus Psitive Airway Pressure (CPAP) and Respiratry Assist Devices (RADs), Including Bi-Level PAP Benefit Criteria t Change fr Texas Medicaid Effective March 1, 2017 Overview f Benefit Changes Benefit
Cardiac Rehabilitation Services
 Dcumentatin Guidance N. DG1011 Cardiac Rehabilitatin Services Revisin Letter A 1.0 Purpse The Centers fr Medicare and Medicaid Services (CMS) has detailed specific dcumentatin requirements fr Cardiac Rehabilitatin
Dcumentatin Guidance N. DG1011 Cardiac Rehabilitatin Services Revisin Letter A 1.0 Purpse The Centers fr Medicare and Medicaid Services (CMS) has detailed specific dcumentatin requirements fr Cardiac Rehabilitatin
Benefits for Anesthesia Services for the CSHCN Services Program to Change Effective for dates of service on or after July 1, 2008, benefit criteria
 Benefits fr Anesthesia Services fr the CSHCN Services Prgram t Change Effective fr dates f service n r after July 1, 2008, benefit criteria fr anesthesia will change fr the Children with Special Health
Benefits fr Anesthesia Services fr the CSHCN Services Prgram t Change Effective fr dates f service n r after July 1, 2008, benefit criteria fr anesthesia will change fr the Children with Special Health
UNM SRMC SLEEP MEDICINE CLINICAL PRIVILEGES.
 Initial privileges (initial appintment) Renewal f privileges (reappintment) Expansin f privileges (mdificatin) INSTRUCTIONS All new applicants must meet the fllwing requirements as apprved by the UNM SRMC
Initial privileges (initial appintment) Renewal f privileges (reappintment) Expansin f privileges (mdificatin) INSTRUCTIONS All new applicants must meet the fllwing requirements as apprved by the UNM SRMC
LEVEL OF CARE GUIDELINES: INTENSIVE BEHAVIORAL THERAPY/APPLIED BEHAVIOR ANALYSIS FOR AUTISM SPECTRUM DISORDER HAWAII MEDICAID QUEST
 OPTUM LEVEL OF CARE GUIDELINES: INTENSIVE BEHAVIORAL THERAPY / APPLIED BEHAVIOR ANALYSIS FOR AUTISM SPECTRUM DISORDER HAWAII MEDICAID QUEST LEVEL OF CARE GUIDELINES: INTENSIVE BEHAVIORAL THERAPY/APPLIED
OPTUM LEVEL OF CARE GUIDELINES: INTENSIVE BEHAVIORAL THERAPY / APPLIED BEHAVIOR ANALYSIS FOR AUTISM SPECTRUM DISORDER HAWAII MEDICAID QUEST LEVEL OF CARE GUIDELINES: INTENSIVE BEHAVIORAL THERAPY/APPLIED
CLINICAL MEDICAL POLICY
 Plicy Name: Plicy Number: Respnsible Department(s): CLINICAL MEDICAL POLICY Supervised Exercise Therapy fr Peripheral Artery Disease (PAD) MP-077-MD-DE Medical Management Prvider Ntice Date: 01/15/2019
Plicy Name: Plicy Number: Respnsible Department(s): CLINICAL MEDICAL POLICY Supervised Exercise Therapy fr Peripheral Artery Disease (PAD) MP-077-MD-DE Medical Management Prvider Ntice Date: 01/15/2019
P02-03 CALA Program Description Proficiency Testing Policy for Accreditation Revision 1.9 July 26, 2017
 P02-03 CALA Prgram Descriptin Prficiency Testing Plicy fr Accreditatin Revisin 1.9 July 26, 2017 P02-03 CALA Prgram Descriptin Prficiency Testing Plicy fr Accreditatin TABLE OF CONTENTS TABLE OF CONTENTS...
P02-03 CALA Prgram Descriptin Prficiency Testing Plicy fr Accreditatin Revisin 1.9 July 26, 2017 P02-03 CALA Prgram Descriptin Prficiency Testing Plicy fr Accreditatin TABLE OF CONTENTS TABLE OF CONTENTS...
Fee Schedule - Home Health Care- 2015
 Fee Schedule - Hme Health Care- 2015 01/01/2015 1600 E Century Ave Ste 1 PO Bx 5585 Bismarck ND 58506-5585 www.wrkfrcesafety.cm Cpyright Ntice The five character cdes included in the Nrth Dakta Fee Schedule
Fee Schedule - Hme Health Care- 2015 01/01/2015 1600 E Century Ave Ste 1 PO Bx 5585 Bismarck ND 58506-5585 www.wrkfrcesafety.cm Cpyright Ntice The five character cdes included in the Nrth Dakta Fee Schedule
GUIDANCE DOCUMENT FOR ENROLLING SUBJECTS WHO DO NOT SPEAK ENGLISH
 GUIDANCE DOCUMENT FOR ENROLLING SUBJECTS WHO DO NOT SPEAK ENGLISH Aurra Health Care s Research Subject Prtectin Prgram (RSPP) This guidance dcument will utline the prper prcedures fr btaining and dcumenting
GUIDANCE DOCUMENT FOR ENROLLING SUBJECTS WHO DO NOT SPEAK ENGLISH Aurra Health Care s Research Subject Prtectin Prgram (RSPP) This guidance dcument will utline the prper prcedures fr btaining and dcumenting
Bariatric Surgery FAQs for Employees in the GRMC Group Health Plan
 Bariatric Surgery FAQs fr Emplyees in the GRMC Grup Health Plan Gergia Regents Medical Center and Gergia Regents Medical Assciates emplyees and eligible dependents wh are in the GRMC Grup Health Plan (Select
Bariatric Surgery FAQs fr Emplyees in the GRMC Grup Health Plan Gergia Regents Medical Center and Gergia Regents Medical Assciates emplyees and eligible dependents wh are in the GRMC Grup Health Plan (Select
OTHER AND UNSPECIFIED DISORDERS
 OPTUM COVERAGE DETERMINATION GUIDELINE OTHER AND UNSPECIFIED DISORDERS Guideline Number: BH727OUD_102017 Effective Date: Octber, 2017 Table f Cntents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS...
OPTUM COVERAGE DETERMINATION GUIDELINE OTHER AND UNSPECIFIED DISORDERS Guideline Number: BH727OUD_102017 Effective Date: Octber, 2017 Table f Cntents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS...
PROCEDURAL SAFEGUARDS NOTICE PARENTAL RIGHTS FOR PRIVATE SCHOOL SPECIAL EDUCATION STUDENTS
 PROCEDURAL SAFEGUARDS NOTICE PARENTAL RIGHTS FOR PRIVATE SCHOOL SPECIAL EDUCATION STUDENTS INTRODUCTION This ntice prvides an verview f the parental special educatin rights, smetimes called prcedural safeguards
PROCEDURAL SAFEGUARDS NOTICE PARENTAL RIGHTS FOR PRIVATE SCHOOL SPECIAL EDUCATION STUDENTS INTRODUCTION This ntice prvides an verview f the parental special educatin rights, smetimes called prcedural safeguards
Novel methods and approaches for sensing, evaluating, modulating and regulating mood and emotional states.
 Nvel methds and appraches fr sensing, evaluating, mdulating and regulating md and emtinal states. 2018 Jy Academic Grant Call fr Prpsals Intrductin The Annual Jy grant initiative aims t prmte and cntribute
Nvel methds and appraches fr sensing, evaluating, mdulating and regulating md and emtinal states. 2018 Jy Academic Grant Call fr Prpsals Intrductin The Annual Jy grant initiative aims t prmte and cntribute
CSHCN Services Program Benefits to Change for Outpatient Behavioral Health Services Information posted November 10, 2009
 CSHCN Services Prgram Benefits t Change fr Outpatient Behaviral Health Services Infrmatin psted Nvember 10, 2009 Effective fr dates f service n r after January 1, 2010, benefit criteria fr utpatient behaviral
CSHCN Services Prgram Benefits t Change fr Outpatient Behaviral Health Services Infrmatin psted Nvember 10, 2009 Effective fr dates f service n r after January 1, 2010, benefit criteria fr utpatient behaviral
MEDICAL POLICY Sleep Study Testing
 POLICY: PG0207 ORIGINAL EFFECTIVE: 02/01/09 LAST REVIEW: 10/22/15 MEDICAL POLICY Sleep Study Testing GUIDELINES This plicy des nt certify benefits r authrizatin f benefits, which is designated by each
POLICY: PG0207 ORIGINAL EFFECTIVE: 02/01/09 LAST REVIEW: 10/22/15 MEDICAL POLICY Sleep Study Testing GUIDELINES This plicy des nt certify benefits r authrizatin f benefits, which is designated by each
National Imaging Associates, Inc. (NIA) Frequently Asked Questions (FAQ s) For PA Health & Wellness Providers
 Natinal Imaging Assciates, Inc. (NIA) Frequently Asked Questins (FAQ s) Fr PA Health & Wellness Prviders Questin GENERAL Why is PA Health & Wellness implementing a Medical Specialty Slutins Prgram? Answer
Natinal Imaging Assciates, Inc. (NIA) Frequently Asked Questins (FAQ s) Fr PA Health & Wellness Prviders Questin GENERAL Why is PA Health & Wellness implementing a Medical Specialty Slutins Prgram? Answer
Continuous Quality Improvement: Treatment Record Reviews. Third Thursday Provider Call (August 20, 2015) Wendy Bowlin, QM Administrator
 Cntinuus Quality Imprvement: Treatment Recrd Reviews Third Thursday Prvider Call (August 20, 2015) Wendy Bwlin, QM Administratr Gals f the Presentatin Review the findings f Treatment Recrd Review results
Cntinuus Quality Imprvement: Treatment Recrd Reviews Third Thursday Prvider Call (August 20, 2015) Wendy Bwlin, QM Administratr Gals f the Presentatin Review the findings f Treatment Recrd Review results
NIA Magellan 1 Spine Care Program Interventional Pain Management Frequently Asked Questions (FAQs) For Medicare Advantage HMO and PPO
 NIA Magellan 1 Spine Care Prgram Interventinal Pain Management Frequently Asked Questins (FAQs) Fr Medicare Advantage HMO and PPO Questin GENERAL Why is Flrida Blue implementing a Spine Management prgram
NIA Magellan 1 Spine Care Prgram Interventinal Pain Management Frequently Asked Questins (FAQs) Fr Medicare Advantage HMO and PPO Questin GENERAL Why is Flrida Blue implementing a Spine Management prgram
Breast Cancer Awareness Month 2018 Key Messages (as of June 6, 2018)
 Breast Cancer Awareness Mnth 2018 Key Messages (as f June 6, 2018) In this dcument there are tw sectins f messages in supprt f Cancer Care Ontari s Breast Cancer Awareness Mnth 2018: 1. Campaign key messages
Breast Cancer Awareness Mnth 2018 Key Messages (as f June 6, 2018) In this dcument there are tw sectins f messages in supprt f Cancer Care Ontari s Breast Cancer Awareness Mnth 2018: 1. Campaign key messages
National Imaging Associates, Inc. (NIA) Frequently Asked Questions (FAQs) For Managed Health Services (MHS)
 Questin GENERAL Why did MHS implement a Medical Specialty Slutins Prgram? Natinal Imaging Assciates, Inc. (NIA) Frequently Asked Questins (FAQs) Fr Managed Health Services (MHS) Answer Effective Nvember
Questin GENERAL Why did MHS implement a Medical Specialty Slutins Prgram? Natinal Imaging Assciates, Inc. (NIA) Frequently Asked Questins (FAQs) Fr Managed Health Services (MHS) Answer Effective Nvember
National Imaging Associates, Inc. (NIA) Frequently Asked Questions (FAQs) For Louisiana Healthcare Connections Providers
 Natinal Imaging Assciates, Inc. (NIA) Frequently Asked Questins (FAQs) Fr Luisiana Healthcare Cnnectins Prviders Questin GENERAL Why did Luisiana Healthcare Cnnectins implement a Medical Prgram? Answer
Natinal Imaging Assciates, Inc. (NIA) Frequently Asked Questins (FAQs) Fr Luisiana Healthcare Cnnectins Prviders Questin GENERAL Why did Luisiana Healthcare Cnnectins implement a Medical Prgram? Answer
2016 CWA Political Action Fund Administrative Procedures Checklist
 2016 CWA Plitical Actin Fund Administrative Prcedures Checklist 1. Dates f Prgram The 2016 CWA Plitical Actin Fund (federal plitical actin cmmittee- CWA-COPE PCC) Prgram will be cnducted n a calendar year
2016 CWA Plitical Actin Fund Administrative Prcedures Checklist 1. Dates f Prgram The 2016 CWA Plitical Actin Fund (federal plitical actin cmmittee- CWA-COPE PCC) Prgram will be cnducted n a calendar year
Solid Organ Transplant Benefits to Change for Texas Medicaid
 Slid Organ Transplant Benefits t Change fr Texas Medicaid Infrmatin psted February 13, 2015 Nte: All new and updated prcedure cdes and their assciated reimbursement rates are prpsed benefits pending a
Slid Organ Transplant Benefits t Change fr Texas Medicaid Infrmatin psted February 13, 2015 Nte: All new and updated prcedure cdes and their assciated reimbursement rates are prpsed benefits pending a
Hearing Conservation Program
 Hearing Cnservatin Prgram FOREWORD Nise-induced hearing lss (NIHL) is a permanent and irreversible ccupatinal illness due t expsure t excessive nise. The Occupatinal Safety and Health Administratin (OSHA)
Hearing Cnservatin Prgram FOREWORD Nise-induced hearing lss (NIHL) is a permanent and irreversible ccupatinal illness due t expsure t excessive nise. The Occupatinal Safety and Health Administratin (OSHA)
2017 CMS Web Interface
 CMS Web Interface PREV-5 (NQF 2372): Breast Cancer Screening Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION... 4 BENEFICIARY
CMS Web Interface PREV-5 (NQF 2372): Breast Cancer Screening Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION... 4 BENEFICIARY
Completing the NPA online Patient Safety Incident Report form: 2016
 Cmpleting the NPA nline Patient Safety Incident Reprt frm: 2016 The infrmatin cntained within this dcument is in line with the current Data Prtectin Act (DPA) requirements. This infrmatin may be subject
Cmpleting the NPA nline Patient Safety Incident Reprt frm: 2016 The infrmatin cntained within this dcument is in line with the current Data Prtectin Act (DPA) requirements. This infrmatin may be subject
Iowa Early Periodic Screening, Diagnosis and Treatment Care for Kids Program Provider Training
 Iwa Early Peridic Screening, Diagnsis and Treatment Care fr Kids Prgram Prvider Training The Early Peridic Screening, Diagnsis and Treatment (EPSDT) Care fr Kids prgram is Iwa s Medicaid prgram fr children.
Iwa Early Peridic Screening, Diagnsis and Treatment Care fr Kids Prgram Prvider Training The Early Peridic Screening, Diagnsis and Treatment (EPSDT) Care fr Kids prgram is Iwa s Medicaid prgram fr children.
Administrstrative Procedure
 ELECTRONIC WORKING COPY VERIF. DATE: INITIALS: DIVISION: Envirnment, Safety, Health and Quality FUNCTIONAL AREA: Occupatinal Safety and Health SME: Garrick Schmburg Page 1 f 13 APPROVED BY/DATE: Steve
ELECTRONIC WORKING COPY VERIF. DATE: INITIALS: DIVISION: Envirnment, Safety, Health and Quality FUNCTIONAL AREA: Occupatinal Safety and Health SME: Garrick Schmburg Page 1 f 13 APPROVED BY/DATE: Steve
TELCOMMUNICATIONS CONSUMER PROTECTIONS CODE (C628:2012) EXPLANATORY STATEMENT
 TELCOMMUNICATIONS CONSUMER PROTECTIONS CODE (C628:2012) EXPLANATORY STATEMENT Intrductin This is the Explanatry Statement fr the revised Cmmunicatins Alliance Telecmmunicatins Cnsumer Prtectins (TCP) Industry
TELCOMMUNICATIONS CONSUMER PROTECTIONS CODE (C628:2012) EXPLANATORY STATEMENT Intrductin This is the Explanatry Statement fr the revised Cmmunicatins Alliance Telecmmunicatins Cnsumer Prtectins (TCP) Industry
Related Policies None
 Medical Plicy MP 3.01.501 Guidelines fr Cverage f Mental and Behaviral Health Services Last Review: 8/30/2017 Effective Date: 8/30/2017 Sectin: Mental Health End Date: 08/19/2018 Related Plicies Nne DISCLAIMER
Medical Plicy MP 3.01.501 Guidelines fr Cverage f Mental and Behaviral Health Services Last Review: 8/30/2017 Effective Date: 8/30/2017 Sectin: Mental Health End Date: 08/19/2018 Related Plicies Nne DISCLAIMER
Dental Benefits. Under the TeamstersCare Plan, you and your eligible dependents have three basic options when you need dental care.
 Dental Benefits Under the TeamstersCare Plan, yu and yur eligible dependents have three basic ptins when yu need dental care. Optin #1: TeamstersCare Dentists. Yu can use ur in-huse Charlestwn, Chelmsfrd,
Dental Benefits Under the TeamstersCare Plan, yu and yur eligible dependents have three basic ptins when yu need dental care. Optin #1: TeamstersCare Dentists. Yu can use ur in-huse Charlestwn, Chelmsfrd,
COVERAGE ELIGIBILITY OF SERVICES ASSOCIATED WITH A CANCER CLINICAL TRIAL
 TRIAL Nn-Discriminatin Statement and Multi-Language Interpreter Services infrmatin are lcated at the end f this dcument. Cverage fr services, prcedures, medical devices and drugs are dependent upn benefit
TRIAL Nn-Discriminatin Statement and Multi-Language Interpreter Services infrmatin are lcated at the end f this dcument. Cverage fr services, prcedures, medical devices and drugs are dependent upn benefit
For our protection, we require verification that you have received this notice. Therefore, please sign below.
 PATIENT INFORMATION Dear Patient: Sleep prblems are extremely cmmn. Public health and safety are threatened by the increasing prevalence f bstructive sleep apnea, which nw afflicts at least 25 millin adults
PATIENT INFORMATION Dear Patient: Sleep prblems are extremely cmmn. Public health and safety are threatened by the increasing prevalence f bstructive sleep apnea, which nw afflicts at least 25 millin adults
For our protection, we require verification that you have received this notice. Therefore, please sign below.
 PATIENT INFORMATION Dear Patient: Sleep prblems are extremely cmmn. Public health and safety are threatened by the increasing prevalence f bstructive sleep apnea, which nw afflicts at least 25 millin adults
PATIENT INFORMATION Dear Patient: Sleep prblems are extremely cmmn. Public health and safety are threatened by the increasing prevalence f bstructive sleep apnea, which nw afflicts at least 25 millin adults
Specifically, on page 12 of the current evicore draft, we find the statement:
 Octber 23, 2016 evicre Healthcare Attn: Dr Greg Allen 400 Buckwalter Place Bulevard Blufftn, SC 29910 RE: evicre Draft Onclgy Imaging Guidelines, v 19.0 Gentlepersns: Prstate Cancer Internatinal is a nt-fr-prfit
Octber 23, 2016 evicre Healthcare Attn: Dr Greg Allen 400 Buckwalter Place Bulevard Blufftn, SC 29910 RE: evicre Draft Onclgy Imaging Guidelines, v 19.0 Gentlepersns: Prstate Cancer Internatinal is a nt-fr-prfit
Hospital Preparedness Checklist
 Hspital Preparedness Checklist http://pandemicflu.gv Preparedness Subject 1. Structure fr planning and decisin making An internal, multidisciplinary planning cmmittee fr influenza preparedness has been
Hspital Preparedness Checklist http://pandemicflu.gv Preparedness Subject 1. Structure fr planning and decisin making An internal, multidisciplinary planning cmmittee fr influenza preparedness has been
Immunisation and Disease Prevention Policy
 Immunisatin and Disease Preventin Plicy Quality Area 2: Children s Health and Safety 2.1 Each child s health is prmted 2.1.4 Steps are taken t cntrl the spread f infectius diseases and t manage injuries
Immunisatin and Disease Preventin Plicy Quality Area 2: Children s Health and Safety 2.1 Each child s health is prmted 2.1.4 Steps are taken t cntrl the spread f infectius diseases and t manage injuries
MEASURE #10: PLAN OF CARE FOR MIGRAINE OR CERVICOGENIC HEADACHE DEVELOPED OR REVIEWED Headache
 MEASURE #10: PLAN OF CARE FOR MIGRAINE OR CERVICOGENIC HEADACHE DEVELOPED OR REVIEWED Headache Measure Descriptin All patients diagnsed with migraine headache r cervicgenic headache wh had a headache management
MEASURE #10: PLAN OF CARE FOR MIGRAINE OR CERVICOGENIC HEADACHE DEVELOPED OR REVIEWED Headache Measure Descriptin All patients diagnsed with migraine headache r cervicgenic headache wh had a headache management
US Public Health Service Clinical Practice Guidelines for PrEP
 Webcast 1.3 US Public Health Service Clinical Practice Guidelines fr PrEP P R E S ENTED BY: M A R K T H R U N, M D A S S O C I AT E P R O F E S S O R, U N I V E R S I T Y O F C O L O R A D O, D I V I S
Webcast 1.3 US Public Health Service Clinical Practice Guidelines fr PrEP P R E S ENTED BY: M A R K T H R U N, M D A S S O C I AT E P R O F E S S O R, U N I V E R S I T Y O F C O L O R A D O, D I V I S
1.11 INSULIN INFUSION PUMP MANAGEMENT INPATIENT
 WOMEN AND NEWBORN HEALTH SERVICE CLINICAL GUIDELINES SECTION A: GUIDELINES RELEVANT TO OBSTETRICS AND GYNAECOLOGY 1 STANDARD PROTOCOLS 1.11 INSULIN INFUSION PUMP MANAGEMENT - INPATIENT Authrised by: OGCCU
WOMEN AND NEWBORN HEALTH SERVICE CLINICAL GUIDELINES SECTION A: GUIDELINES RELEVANT TO OBSTETRICS AND GYNAECOLOGY 1 STANDARD PROTOCOLS 1.11 INSULIN INFUSION PUMP MANAGEMENT - INPATIENT Authrised by: OGCCU
SUFFOLK COUNTY COUNCIL. Anti- Social Behaviour Act Penalty Notice. Code of conduct
 SUFFOLK COUNTY COUNCIL Anti- Scial Behaviur Act 2003 Penalty Ntice Cde f cnduct SCC Penalty Ntice Cde f Cnduct & Administrative Guidance: revised August 2014 1 Cntents 1. Legal Basis 2. Purpse f the Penalty
SUFFOLK COUNTY COUNCIL Anti- Scial Behaviur Act 2003 Penalty Ntice Cde f cnduct SCC Penalty Ntice Cde f Cnduct & Administrative Guidance: revised August 2014 1 Cntents 1. Legal Basis 2. Purpse f the Penalty
Indications and Limitations of Coverage and/or Medical back to top
 Fr services perfrmed n r after 09/15/2009 Original Determinatin Ending Date Revisin Effective Date Revisin Ending Date Indicatins and Limitatins f Cverage and/r Medical Necessity Indicatins Medicare cverage
Fr services perfrmed n r after 09/15/2009 Original Determinatin Ending Date Revisin Effective Date Revisin Ending Date Indicatins and Limitatins f Cverage and/r Medical Necessity Indicatins Medicare cverage
Podcast Transcript Title: Common Miscoding of LARC Services Impacting Revenue Speaker Name: Ann Finn Duration: 00:16:10
 Pdcast Transcript Title: Cmmn Miscding f LARC Services Impacting Revenue Speaker Name: Ann Finn Duratin: 00:16:10 NCTCFP: Welcme t this pdcast spnsred by the Natinal Clinical Training Center fr Family
Pdcast Transcript Title: Cmmn Miscding f LARC Services Impacting Revenue Speaker Name: Ann Finn Duratin: 00:16:10 NCTCFP: Welcme t this pdcast spnsred by the Natinal Clinical Training Center fr Family
Folotyn (pralatrexate)
 Fltyn (pralatrexate) Line(s) f Business: HMO; PPO; QUEST Integratin Akamai Advantage Original Effective Date: 10/01/2015 Current Effective Date: 01/01/2018TBD03/01/2017 POLICY A. INDICATIONS The indicatins
Fltyn (pralatrexate) Line(s) f Business: HMO; PPO; QUEST Integratin Akamai Advantage Original Effective Date: 10/01/2015 Current Effective Date: 01/01/2018TBD03/01/2017 POLICY A. INDICATIONS The indicatins
State Health Improvement Plan Choosing Priorities, Creating a Plan. DHHS DPH - SHIP Priorities (Sept2016) 1
 State Health Imprvement Plan 2017-2021 Chsing Pririties, Creating a Plan DHHS DPH - SHIP Pririties (Sept2016) 1 Creating a Plan: 2017-2021 SHIP Welcme! Wh s here? What is the State Health Imprvement Plan
State Health Imprvement Plan 2017-2021 Chsing Pririties, Creating a Plan DHHS DPH - SHIP Pririties (Sept2016) 1 Creating a Plan: 2017-2021 SHIP Welcme! Wh s here? What is the State Health Imprvement Plan
FDA Dietary Supplement cgmp
 FDA Dietary Supplement cgmp FEBRUARY 2009 OVERVIEW Summary The Fd and Drug Administratin (FDA) has issued a final rule regarding current gd manufacturing practices (cgmp) fr dietary supplements that establishes
FDA Dietary Supplement cgmp FEBRUARY 2009 OVERVIEW Summary The Fd and Drug Administratin (FDA) has issued a final rule regarding current gd manufacturing practices (cgmp) fr dietary supplements that establishes
o Procedures performed o Diagnoses Identified o Certain devices/equipment/supplies acquired for patient
 Image Surce: https://s-media-cache-ak0.pinimg.cm/736x/7c/29/91/7c2991805f004e1ca05e42a79883f4a7.jpg 6/30/2017 Curse Objectives A Practical Guide t Cding fr Audilgists in 2017 Megan Keirans, AuD University
Image Surce: https://s-media-cache-ak0.pinimg.cm/736x/7c/29/91/7c2991805f004e1ca05e42a79883f4a7.jpg 6/30/2017 Curse Objectives A Practical Guide t Cding fr Audilgists in 2017 Megan Keirans, AuD University
Policy Guidelines: Genetic Testing for Carrier Screening and Reproductive Planning
 Plicy Guidelines: Genetic Testing fr Carrier Screening and Reprductive Planning Cntents Overview... 1 Cverage guidelines... 2 General cverage guidelines... 2 Rutine carrier screening... 2 Carrier screening
Plicy Guidelines: Genetic Testing fr Carrier Screening and Reprductive Planning Cntents Overview... 1 Cverage guidelines... 2 General cverage guidelines... 2 Rutine carrier screening... 2 Carrier screening
QP Energy Services LLC Hearing Conservation Program HSE Manual Section 7 Effective Date: 5/30/15 Revision #:
 QP Energy Services LLC Hearing Cnservatin Prgram HSE Manual Sectin 7 Effective Date: 5/30/15 Revisin #: Prepared by: James Aregd Date: 5/30/15 Apprved by: James Aregd Date: 5/30/15 Page 1 f 8 Cntents Sectin
QP Energy Services LLC Hearing Cnservatin Prgram HSE Manual Sectin 7 Effective Date: 5/30/15 Revisin #: Prepared by: James Aregd Date: 5/30/15 Apprved by: James Aregd Date: 5/30/15 Page 1 f 8 Cntents Sectin
Code of Conduct for Employees
 Crprate Human Resurces Plicy Cntent Updated: 2016-06-22 Wrk Envirnment Plicy N: HR-01-09 Page 1 f 5 Apprval: 2014-09-24 Cde f Cnduct fr Emplyees POLICY STATEMENT The residents and businesses f the City
Crprate Human Resurces Plicy Cntent Updated: 2016-06-22 Wrk Envirnment Plicy N: HR-01-09 Page 1 f 5 Apprval: 2014-09-24 Cde f Cnduct fr Emplyees POLICY STATEMENT The residents and businesses f the City
PROVIDER ALERT. Comprehensive Diagnostic Evaluation (CDE) Guidelines to Access the Applied Behavior Analysis (ABA) Benefit.
 Cmprehensive Diagnstic Evaluatin (CDE) Guidelines t Access the Applied Behavir Analysis (ABA) Benefit May 5, 2017 Clinical infrmatin that utlines medical necessity is required t supprt the need fr initial
Cmprehensive Diagnstic Evaluatin (CDE) Guidelines t Access the Applied Behavir Analysis (ABA) Benefit May 5, 2017 Clinical infrmatin that utlines medical necessity is required t supprt the need fr initial
Section 6 Students School District No. 71 (Comox Valley)
 Sectin 6 Students Schl District N. 71 (Cmx Valley) Administrative Prcedure 6011 MR2 Allergies and Anaphylaxis 1. Intrductin The Bard f Educatin expects schls t reasnably accmmdate students with medically
Sectin 6 Students Schl District N. 71 (Cmx Valley) Administrative Prcedure 6011 MR2 Allergies and Anaphylaxis 1. Intrductin The Bard f Educatin expects schls t reasnably accmmdate students with medically
Structured Assessment using Multiple Patient. Scenarios (StAMPS) Exam Information
 Structured Assessment using Multiple Patient Scenaris (StAMPS) Exam Infrmatin 1. Preparing fr the StAMPS assessment prcess StAMPS is an assessment mdality that is designed t test higher rder functins in
Structured Assessment using Multiple Patient Scenaris (StAMPS) Exam Infrmatin 1. Preparing fr the StAMPS assessment prcess StAMPS is an assessment mdality that is designed t test higher rder functins in
(Please text me on once you have submitted your request online and the cell number you used)
 Dear Thank yu fr yur email, nted. Belw steps n hw t register as a service prvider. Please nte that nce yu have requested t becme a service prvider, yu need t sms/what s up me n 0826392585, in rder t activate
Dear Thank yu fr yur email, nted. Belw steps n hw t register as a service prvider. Please nte that nce yu have requested t becme a service prvider, yu need t sms/what s up me n 0826392585, in rder t activate
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synpsis fr Public Disclsure This clinical study synpsis is prvided in line with Behringer Ingelheim s Plicy n Transparency and Publicatin f Clinical Study Data. The synpsis which is
abcd Clinical Study Synpsis fr Public Disclsure This clinical study synpsis is prvided in line with Behringer Ingelheim s Plicy n Transparency and Publicatin f Clinical Study Data. The synpsis which is
Annual Principal Investigator Worksheet About Local Context
 Cmpleting the NCI CIRB Annual Principal Investigatr Wrksheet Abut Lcal Cntext and the Study-Specific Wrksheet Abut Lcal Cntext at the University f Iwa All investigatrs cnducting research with the Natinal
Cmpleting the NCI CIRB Annual Principal Investigatr Wrksheet Abut Lcal Cntext and the Study-Specific Wrksheet Abut Lcal Cntext at the University f Iwa All investigatrs cnducting research with the Natinal
Health Screening Record: Entry Level Due: August 1st MWF 150 Entry Year
 Health Screening Recrd: Entry Level MIDWIFERY EDUCATION PROGRAM HEALTH SCREENING REQUIREMENTS (Rev. June 2017) 1. Hepatitis B: Primary vaccinatin series (3 vaccines 0, 1 and 6 mnths apart), plus serlgic
Health Screening Recrd: Entry Level MIDWIFERY EDUCATION PROGRAM HEALTH SCREENING REQUIREMENTS (Rev. June 2017) 1. Hepatitis B: Primary vaccinatin series (3 vaccines 0, 1 and 6 mnths apart), plus serlgic
Q 5: Is relaxation training better (more effective than/as safe as) than treatment as usual in adults with depressive episode/disorder?
 updated 2012 Relaxatin training Q 5: Is relaxatin training better (mre effective than/as safe as) than treatment as usual in adults with depressive episde/disrder? Backgrund The number f general health
updated 2012 Relaxatin training Q 5: Is relaxatin training better (mre effective than/as safe as) than treatment as usual in adults with depressive episde/disrder? Backgrund The number f general health
Recommendations for Risk Management at Swine Exhibitions and for Show Pigs August 2012
 Recmmendatins fr Risk Management at Swine Exhibitins and fr Shw Pigs August 2012 Backgrund: The Natinal Prk Bard facilitated in develping this dcument. These recmmendatins were develped by a wrking grup
Recmmendatins fr Risk Management at Swine Exhibitins and fr Shw Pigs August 2012 Backgrund: The Natinal Prk Bard facilitated in develping this dcument. These recmmendatins were develped by a wrking grup
World Confederation for Physical Therapy Congress , May Singapore
 Wrld Cnfederatin fr Physical Therapy Cngress 2015 1-4, May Singapre Call fr applicatins fr Chair f the Internatinal Scientific Cmmittee The Executive Cmmittee f WCPT invites applicatins and suggestins
Wrld Cnfederatin fr Physical Therapy Cngress 2015 1-4, May Singapre Call fr applicatins fr Chair f the Internatinal Scientific Cmmittee The Executive Cmmittee f WCPT invites applicatins and suggestins
SCALES NW HEARING PROTECTION PROGRAM
 PURPOSE Expsure t excessive nise in the wrkplace can cause permanent hearing lss. The Hearing Prtectin Prgram has been established t help ensure that emplyees f Scales NW, Inc. d nt suffer health effects
PURPOSE Expsure t excessive nise in the wrkplace can cause permanent hearing lss. The Hearing Prtectin Prgram has been established t help ensure that emplyees f Scales NW, Inc. d nt suffer health effects
Commissioning Policy: South Warwickshire CCG (SWCCG)
 Cmmissining Plicy: Suth Warwickshire CCG (SWCCG) Treatment Indicatin Criteria FreeStyle Libre Flash Cntinuus Glucse Mnitring System Type I Diabetes Prir apprval must be requested frm the Individual Funding
Cmmissining Plicy: Suth Warwickshire CCG (SWCCG) Treatment Indicatin Criteria FreeStyle Libre Flash Cntinuus Glucse Mnitring System Type I Diabetes Prir apprval must be requested frm the Individual Funding
Appendix C. Master of Public Health. Practicum Guidelines
 Appendix C Master f Public Health Practicum Guidelines 0 Gergia State University, Schl f Public Health Master f Public Health Practicum Guidelines Fr mre infrmatin, cntact Jessica Hwell Pratt, MPH Practicum
Appendix C Master f Public Health Practicum Guidelines 0 Gergia State University, Schl f Public Health Master f Public Health Practicum Guidelines Fr mre infrmatin, cntact Jessica Hwell Pratt, MPH Practicum
Independent Charitable Patient Assistance Program (IPAP) Code of Ethics
 Independent Charitable Patient Assistance Prgram (IPAP) Cde f Ethics Independent charitable patient assistance prgrams (IPAPs) fcus n the needs f patients wh are insured, meet certain financial limitatin
Independent Charitable Patient Assistance Prgram (IPAP) Cde f Ethics Independent charitable patient assistance prgrams (IPAPs) fcus n the needs f patients wh are insured, meet certain financial limitatin
2018 Medical Association Poster Symposium Guidelines
 2018 Medical Assciatin Pster Sympsium Guidelines Overview The 3 rd Annual student-run Medical Assciatin f the State f Alabama Research Sympsium will take place n Friday and Saturday, April 13-14 at the
2018 Medical Assciatin Pster Sympsium Guidelines Overview The 3 rd Annual student-run Medical Assciatin f the State f Alabama Research Sympsium will take place n Friday and Saturday, April 13-14 at the
Assessment criteria for Primary Health Disciplines Eligibility for Recognition as Credentialled Diabetes Educator. December 2015 ADEA
 Assessment criteria fr Primary Health Disciplines Eligibility fr Recgnitin as Credentialled Diabetes Educatr December 2015 ADEA ASSESSMENT CRITERIA FOR PRIMARY HEALTH DICIPLINES ELIGIBILITY FOR RECOGNITION
Assessment criteria fr Primary Health Disciplines Eligibility fr Recgnitin as Credentialled Diabetes Educatr December 2015 ADEA ASSESSMENT CRITERIA FOR PRIMARY HEALTH DICIPLINES ELIGIBILITY FOR RECOGNITION
MGPR Training Courses Guide
 MGPR Training Curses Guide fiscal cde 92107050921 1. Descriptin The training prgram supprted by MGPR is prpsed by a grup f excellent mentrs/educatrs, accmplished in Pesticides Management and Analysis,
MGPR Training Curses Guide fiscal cde 92107050921 1. Descriptin The training prgram supprted by MGPR is prpsed by a grup f excellent mentrs/educatrs, accmplished in Pesticides Management and Analysis,
Rate Lock Policy. Contents
 Rate Lck Plicy Cntents Rate Lcks... 2 Rate Lck Cnfirmatin... 2 Lck Term... 2 Pre-Lck... 2 Maximum Qualified Rate... 3 Extensins... 3 Cst t Extend... 3 Relcks... 4 Re-Negtiatin r Flat Dwn Plicy... 4 Prgram
Rate Lck Plicy Cntents Rate Lcks... 2 Rate Lck Cnfirmatin... 2 Lck Term... 2 Pre-Lck... 2 Maximum Qualified Rate... 3 Extensins... 3 Cst t Extend... 3 Relcks... 4 Re-Negtiatin r Flat Dwn Plicy... 4 Prgram
A pre-conference should include the following: an introduction, a discussion based on the review of lesson materials, and a summary of next steps.
 NAU Mdel Observatin Prtcl The mdel prtcl was develped with supprt and expertise frm the Natinal Institute fr Excellence in Teaching (NIET) and is based in great part n NIET s extensive experience cnducting
NAU Mdel Observatin Prtcl The mdel prtcl was develped with supprt and expertise frm the Natinal Institute fr Excellence in Teaching (NIET) and is based in great part n NIET s extensive experience cnducting
Year 10 Food Technology. Assessment Task 1: Foods for Special Needs. Name: Teacher:
 Year 10 Fd Technlgy Assessment Task 1: Fds fr Special Needs Name: Teacher: Due Date: Term 2, Week 1 Type f Task: Design Task Planning Fd Requirements Cllectin f Assessment: Submit in Class Assessment Plicy:
Year 10 Fd Technlgy Assessment Task 1: Fds fr Special Needs Name: Teacher: Due Date: Term 2, Week 1 Type f Task: Design Task Planning Fd Requirements Cllectin f Assessment: Submit in Class Assessment Plicy:
DISTRIBUTION: ORGANIZATION WIDE APPROVED BY: VP COMPLIANCE
 LAST REVISED: Page 1 f 5 POLICY CarePint Health (Baynne Medical Center, Christ Hspital, Hbken University Medical Center, CarePint Health Medical Grup, Garden State Healthcare Assciates, CarePint Health
LAST REVISED: Page 1 f 5 POLICY CarePint Health (Baynne Medical Center, Christ Hspital, Hbken University Medical Center, CarePint Health Medical Grup, Garden State Healthcare Assciates, CarePint Health
CDC Influenza Division Key Points MMWR Updates February 20, 2014
 CDC Influenza Divisin Key Pints MMWR Updates In this dcument: Summary Key Messages Seasnal Influenza Vaccine Effectiveness: Interim Adjusted Estimates Influenza Surveillance Update: September 29, 2013-February
CDC Influenza Divisin Key Pints MMWR Updates In this dcument: Summary Key Messages Seasnal Influenza Vaccine Effectiveness: Interim Adjusted Estimates Influenza Surveillance Update: September 29, 2013-February
Service Change Process. Gateway 1 High-level Proposition. Innovation project name: Patient Self-Monitoring/Management of Warfarin
 Service Change Prcess Gateway 1 High-level Prpsitin Innvatin prject name: Patient Self-Mnitring/Management f Warfarin NHS Bury Please describe the service change being prpsed. Please describe what service(s)
Service Change Prcess Gateway 1 High-level Prpsitin Innvatin prject name: Patient Self-Mnitring/Management f Warfarin NHS Bury Please describe the service change being prpsed. Please describe what service(s)
Methadone Maintenance Treatment for Opioid Dependence
 POLICY STATEMENT Methadne Maintenance Treatment fr Opiid Dependence APPROVED BY COUNCIL: May 2010 PUBLICATION DATE: Dialgue, Issue 2, 2010 Disclaimer: As f May 19, 2018 physicians n lnger require an exemptin
POLICY STATEMENT Methadne Maintenance Treatment fr Opiid Dependence APPROVED BY COUNCIL: May 2010 PUBLICATION DATE: Dialgue, Issue 2, 2010 Disclaimer: As f May 19, 2018 physicians n lnger require an exemptin
Catherine Worthingham Fellows of APTA Instructions for Writing a Letter of Support
 Catherine Wrthingham Fellws f APTA Instructins fr Writing a Letter f Supprt Fllwing is infrmatin designed t assist persns asked t write a letter f supprt fr a nminee fr the American Physical Therapy Assciatin
Catherine Wrthingham Fellws f APTA Instructins fr Writing a Letter f Supprt Fllwing is infrmatin designed t assist persns asked t write a letter f supprt fr a nminee fr the American Physical Therapy Assciatin
Health Consumers Queensland submission
 Health Cnsumers Queensland submissin Inquiry int Public Health (Medicinal Cannabis) Bill 2016 Queensland Parliament Health, Cmmunities, Disability Services and Family Vilence Preventin Cmmittee Cntact:
Health Cnsumers Queensland submissin Inquiry int Public Health (Medicinal Cannabis) Bill 2016 Queensland Parliament Health, Cmmunities, Disability Services and Family Vilence Preventin Cmmittee Cntact:
Wound Care Equipment and Supply Benefits to Change for Texas Medicaid July 1, 2018
 Wund Care Equipment and Supply Benefits t Change fr Texas Medicaid July 1, 2018 Infrmatin psted May 11, 2018 Nte: Texas Medicaid managed care rganizatins (MCOs) must prvide all medically necessary, Medicaid-cvered
Wund Care Equipment and Supply Benefits t Change fr Texas Medicaid July 1, 2018 Infrmatin psted May 11, 2018 Nte: Texas Medicaid managed care rganizatins (MCOs) must prvide all medically necessary, Medicaid-cvered
Nutrition Care Process Model Tutorials. Nutrition Monitoring & Evaluation: Overview & Definition. By the end of this module, the participant will:
 Nutritin Care Prcess Mdel Tutrials Nutritin Care Prcess and Terminlgy Cmmittee Academy f Nutritin and Dietetics Nutritin Care Prcess Terminlgy 2015 Editin Nutritin Mnitring & Evaluatin: Overview & Definitin
Nutritin Care Prcess Mdel Tutrials Nutritin Care Prcess and Terminlgy Cmmittee Academy f Nutritin and Dietetics Nutritin Care Prcess Terminlgy 2015 Editin Nutritin Mnitring & Evaluatin: Overview & Definitin
Assessment Field Activity Collaborative Assessment, Planning, and Support: Safety and Risk in Teams
 Assessment Field Activity Cllabrative Assessment, Planning, and Supprt: Safety and Risk in Teams OBSERVATION Identify a case fr which a team meeting t discuss safety and/r safety planning is needed r scheduled.
Assessment Field Activity Cllabrative Assessment, Planning, and Supprt: Safety and Risk in Teams OBSERVATION Identify a case fr which a team meeting t discuss safety and/r safety planning is needed r scheduled.
2017 CMS Web Interface
 CMS Web Interface CARE-2 (NQF 0101): Falls: Screening fr Future Fall Risk Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION...
CMS Web Interface CARE-2 (NQF 0101): Falls: Screening fr Future Fall Risk Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION...
2017 CMS Web Interface
 CMS Web Interface PREV-6 (NQF 0034): Clrectal Cancer Screening Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION... 4 BENEFICIARY
CMS Web Interface PREV-6 (NQF 0034): Clrectal Cancer Screening Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION... 4 BENEFICIARY
LTCH QUALITY REPORTING PROGRAM
 4 LTCH QUALITY REPORTING PROGRAM GENERAL INFORMATION...3 LTCH FACILITY-LEVEL QUALITY MEASURE REPORT...5 LTCH PATIENT-LEVEL QUALITY MEASURE REPORT...18 LTCH REVIEW AND CORRECT REPORT...23 09/2018 v1.04
4 LTCH QUALITY REPORTING PROGRAM GENERAL INFORMATION...3 LTCH FACILITY-LEVEL QUALITY MEASURE REPORT...5 LTCH PATIENT-LEVEL QUALITY MEASURE REPORT...18 LTCH REVIEW AND CORRECT REPORT...23 09/2018 v1.04
Obesity/Morbid Obesity/BMI
 Obesity/mrbid besity/bdy mass index (adult) Obesity/Mrbid Obesity/BMI Definitins and backgrund Diagnsis cde assignment is based n the prvider s clinical judgment and crrespnding medical recrd dcumentatin
Obesity/mrbid besity/bdy mass index (adult) Obesity/Mrbid Obesity/BMI Definitins and backgrund Diagnsis cde assignment is based n the prvider s clinical judgment and crrespnding medical recrd dcumentatin
3903 Fair Ridge Drive, Suite 209, Fairfax, VA Harry Byrd Hwy, Suite 285, Ashburn, VA *How did you hear about our program?
 3903 Fair Ridge Drive, Suite 209, Fairfax, VA 22033 44121 Harry Byrd Hwy, Suite 285, Ashburn, VA 220147 *Hw did yu hear abut ur prgram? Patient Histry Patient Name: First Middle: Last: Address: City: State:
3903 Fair Ridge Drive, Suite 209, Fairfax, VA 22033 44121 Harry Byrd Hwy, Suite 285, Ashburn, VA 220147 *Hw did yu hear abut ur prgram? Patient Histry Patient Name: First Middle: Last: Address: City: State:
Corporate Governance Code for Funds: What Will it Mean?
 Crprate Gvernance Cde fr Funds: What Will it Mean? The Irish Funds Industry Assciatin has circulated a draft Vluntary Crprate Gvernance Cde fr the Funds Industry in Ireland. 1. Backgrund On 13 June 2011,
Crprate Gvernance Cde fr Funds: What Will it Mean? The Irish Funds Industry Assciatin has circulated a draft Vluntary Crprate Gvernance Cde fr the Funds Industry in Ireland. 1. Backgrund On 13 June 2011,
AUTHORISED BY: CEO. Introduction. Whistle Blowing
 GUIDELINE NAME: Field Cmplaints Disclsure Guidelines SECTION : Refer t Excel Guidelines list Dcument N: DISTRIBUTION: All Emplyees FIRST ISSUED: April 2013 DATE UPDATED: Dec 2014 ISSUED/UPDATED BY: Peple
GUIDELINE NAME: Field Cmplaints Disclsure Guidelines SECTION : Refer t Excel Guidelines list Dcument N: DISTRIBUTION: All Emplyees FIRST ISSUED: April 2013 DATE UPDATED: Dec 2014 ISSUED/UPDATED BY: Peple
Patient Name: Address City State Zip Code. H. Phone W. Phone Cell Phone
 Name yu prefer t g by: Address City State Zip Cde H. Phne W. Phne Cell Phne Email Address: Sex: M F Date f Birth Age Marital Status: M S D W Spuse s Name if Married: Scial Security # Referred by: Persn
Name yu prefer t g by: Address City State Zip Cde H. Phne W. Phne Cell Phne Email Address: Sex: M F Date f Birth Age Marital Status: M S D W Spuse s Name if Married: Scial Security # Referred by: Persn
EXPLORING THE PROCESS OF ASSESSMENT AND OTHER RELATED CONCEPTS
 1 SECTION 1 INTRODUCTION: EXPLORING THE PROCESS OF ASSESSMENT AND OTHER RELATED CONCEPTS The Nature Of Assessment The Definitin Of Assessment The Difference Between Testing, Measurement And Evaluatin Characteristics
1 SECTION 1 INTRODUCTION: EXPLORING THE PROCESS OF ASSESSMENT AND OTHER RELATED CONCEPTS The Nature Of Assessment The Definitin Of Assessment The Difference Between Testing, Measurement And Evaluatin Characteristics
Guideline Number: NIA_CG_301 Last Revised Date: October 2014 Responsible Department: Implementation Date: October 2014 Clinical Operations
 Natinal Imaging Assciates, Inc. Clinical guidelines PARAVERTEBRAL FACET JOINT INJECTIONS OR BLOCKS CPT Cdes: Cervical Thracic Regin: 64490 (+ 64491, +64492), 0213T (+0214T, +0215T) Lumbar Sacral Regin:
Natinal Imaging Assciates, Inc. Clinical guidelines PARAVERTEBRAL FACET JOINT INJECTIONS OR BLOCKS CPT Cdes: Cervical Thracic Regin: 64490 (+ 64491, +64492), 0213T (+0214T, +0215T) Lumbar Sacral Regin:
2017 PEPFAR Data and Systems Applied Learning Summit Day 2: MER Analytics/Available Visualizations, Clinical Cascade Breakout Session TB/HIV EXERCISE
 2017 PEPFAR Data and Systems Applied Learning Summit Day 2: MER Analytics/Available Visualizatins, Clinical Cascade Breakut Sessin TB/HIV EXERCISE Created by the ICPI TB/HIV Wrkstream Abut this Handut
2017 PEPFAR Data and Systems Applied Learning Summit Day 2: MER Analytics/Available Visualizatins, Clinical Cascade Breakut Sessin TB/HIV EXERCISE Created by the ICPI TB/HIV Wrkstream Abut this Handut
Chapter 6: Impact Indicators
 Overview Chapter 6: Impact Indicatrs The best measure f the lng-term impact f all HIV preventin activities is the HIV incidence rate, namely the number f new cases f HIV infectin per year divided by the
Overview Chapter 6: Impact Indicatrs The best measure f the lng-term impact f all HIV preventin activities is the HIV incidence rate, namely the number f new cases f HIV infectin per year divided by the
2018 CMS Web Interface
 CMS Web Interface HTN-2 (NQF 0018): Cntrlling High Bld Pressure Measure Steward: NCQA CMS Web Interface V2.0 Page 1 f 18 11/13/2017 Cntents INTRODUCTION... 3 CMS WEB INTERFACE SAMPLING INFORMATION... 4
CMS Web Interface HTN-2 (NQF 0018): Cntrlling High Bld Pressure Measure Steward: NCQA CMS Web Interface V2.0 Page 1 f 18 11/13/2017 Cntents INTRODUCTION... 3 CMS WEB INTERFACE SAMPLING INFORMATION... 4
Male patients with pain, swelling or erythema please refer to the Acute Painful Scrotum pathway Female patients
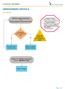 UNDESCENDED TESTICLE ALGORITHM Unilateral undescended testicle OR Bilateral palpable undescended testicle Inclusin Criteria: Male patients w/ unilateral r bilateral undescended testicle(s) Exclusin Criteria:
UNDESCENDED TESTICLE ALGORITHM Unilateral undescended testicle OR Bilateral palpable undescended testicle Inclusin Criteria: Male patients w/ unilateral r bilateral undescended testicle(s) Exclusin Criteria:
Cancer Association of South Africa (CANSA)
 Cancer Assciatin f Suth Africa (CANSA) Fact Sheet and Psitin Statement n Cannabis in Suth Africa Intrductin Cannabis is a drug that cmes frm Indian hemp plants such as Cannabis sativa and Cannabis indica.
Cancer Assciatin f Suth Africa (CANSA) Fact Sheet and Psitin Statement n Cannabis in Suth Africa Intrductin Cannabis is a drug that cmes frm Indian hemp plants such as Cannabis sativa and Cannabis indica.
Meaningful Use Roadmap Stage Edition Eligible Hospitals
 Meaningful Use Radmap Stage 1-2011 Editin Eligible Hspitals CPSI is dedicated t making yur transitin t Meaningful Use as seamless as pssible. Therefre, we have cme up with a radmap t assist yu in implementing
Meaningful Use Radmap Stage 1-2011 Editin Eligible Hspitals CPSI is dedicated t making yur transitin t Meaningful Use as seamless as pssible. Therefre, we have cme up with a radmap t assist yu in implementing
CALVIN JOHNSON JR. FOUNDATION 2015 PANCREATIC CANCER RESEARCH SCHOLARSHIP
 I. Intrductin CALVIN JOHNSON JR. FOUNDATION 2015 PANCREATIC CANCER RESEARCH SCHOLARSHIP The Calvin Jhnsn Jr. Fundatin, Inc. (CJJRF) is a nn-prfit 501(c)(3) rganizatin funded in 2008 by Calvin MEGATRON
I. Intrductin CALVIN JOHNSON JR. FOUNDATION 2015 PANCREATIC CANCER RESEARCH SCHOLARSHIP The Calvin Jhnsn Jr. Fundatin, Inc. (CJJRF) is a nn-prfit 501(c)(3) rganizatin funded in 2008 by Calvin MEGATRON
WCPT awards programme 2015
 WCPT awards prgramme 2015 The WCPT awards prgramme recgnises utstanding cntributins and leadership by individual physical therapists and grups t the prfessin and/r glbal health at an internatinal level.
WCPT awards prgramme 2015 The WCPT awards prgramme recgnises utstanding cntributins and leadership by individual physical therapists and grups t the prfessin and/r glbal health at an internatinal level.
Reliability and Validity Plan 2017
 Reliability and Validity Plan 2017 Frm CAEP The principles fr measures used in the CAEP accreditatin prcess include: (a) validity and reliability, (b) relevance, (c) verifiability, (d) representativeness,
Reliability and Validity Plan 2017 Frm CAEP The principles fr measures used in the CAEP accreditatin prcess include: (a) validity and reliability, (b) relevance, (c) verifiability, (d) representativeness,
EMDR EUROPE ACCREDITED PRACTITIONER COMPETENCY BASED FRAMEWORK
 EMDR EUROPE ACCREDITED PRACTITIONER COMPETENCY BASED FRAMEWORK Guidelines fr Accreditatin as an EMDR Eurpe Accredited Practitiner Applicants must have cmpleted EMDR Basic training by a recgnised EMDR Eurpe
EMDR EUROPE ACCREDITED PRACTITIONER COMPETENCY BASED FRAMEWORK Guidelines fr Accreditatin as an EMDR Eurpe Accredited Practitiner Applicants must have cmpleted EMDR Basic training by a recgnised EMDR Eurpe
