Re: Docket No. FDA-2013-N-0521, Menthol in Cigarettes, Tobacco Products; Request for Comments
|
|
|
- David Reynolds
- 5 years ago
- Views:
Transcription
1 November 22, 2013 Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, rm Rockville, MD Re: Docket No. FDA-2013-N-0521, Menthol in Cigarettes, Tobacco Products; Request for Comments To Whom It May Concern: The American Association for Cancer Research (AACR) and the American Society of Clinical Oncology (ASCO) appreciate the opportunity to provide input on the U.S. Food and Drug Administration s (FDA s) Advance Notice of Proposed Rulemaking regarding menthol in cigarettes. As the nation s leading scientific and professional organizations representing cancer researchers and oncology care professionals, the AACR and ASCO strongly support the development of evidence-based tobacco control policies aimed at reducing the burden of death and disease caused by tobacco use, which is the leading preventable cause of death in the United States and around the world. Consistent with the scientific evidence demonstrating that menthol cigarettes have an adverse impact on public health, the AACR and ASCO urge the FDA to ban the addition of menthol as a characterizing flavor to cigarettes and other combustible tobacco products. We also recommend that the FDA evaluate the public health impact of tobacco products containing menthol and other flavorants at sub-characterizing levels and the impact that banning these flavorants altogether would have on public health. As the FDA s Tobacco Products Scientific Advisory Committee (TPSAC) stated in its comprehensive review of the issue, menthol cigarettes have an adverse impact on public health in the United States. Moreover, multiple lines of investigation support a role for menthol cigarettes in increasing experimentation and progression to regular smoking. Youth who initiated smoking with menthol cigarettes are more likely to become daily, regular, or established smokers than those who initiated with non menthol cigarettes, and adolescent menthol cigarette smokers have a higher prevalence of nicotine dependence and more severe nicotine addiction than those who smoke non menthol cigarettes.
2 Moreover, the danger of menthol-flavored cigarettes falls disproportionately on African Americans. For example, African Americans are more likely to smoke menthol cigarettes, and African-American menthol smokers are also less likely to quit smoking successfully than are non-menthol smokers. Banning menthol flavoring would be an important step toward reducing tobacco-associated harm to this population and would curtail a longstanding tobacco industry marketing approach targeting African Americans, including youth. In the pages that follow we have provided additional input on the questions posed in the FDA s request for comments on menthol in cigarettes. A. Tobacco Product Standards 1. Should FDA consider establishing a tobacco product standard for menthol in menthol cigarettes? If so, what allowable level of menthol (e.g., maximum or minimum) would be appropriate for the protection of the public health? The AACR and ASCO urge FDA to establish a tobacco product standard for menthol in cigarettes. Specifically, and in light of the evidence showing that menthol cigarettes have an adverse impact on public health, increase experimentation and progression to regular smoking, and disproportionately affect African-Americans, we strongly recommend that the FDA ban the addition of menthol to cigarettes and other combustible tobacco products as a characterizing flavor. Our organizations are also concerned that the addition of menthol and other flavorants to combustible tobacco products at levels below characterizing levels could facilitate smoking initiation (particularly among youth), dependence, and addiction. Indeed, the TPSAC review cites at least one report showing that the addition of menthol at concentrations below those used in non menthol cigarettes (and undetectable by taste and aroma) is likely to make smoke smoother and less harsh. We strongly recommend that the FDA evaluate the public health impact of cigarettes and other combustible products containing menthol at sub-characterizing levels and the impact that banning these constituents would have on public health. 2. Rather than a tobacco product standard for menthol in menthol cigarettes, should FDA consider a tobacco product standard for any additive, constituent, artificial or natural flavor, or other ingredient that produces a characterizing flavor of menthol in the tobacco product or its smoke? The AACR and ASCO support a ban on the inclusion of any additive, constituent, artificial or natural flavor, or other ingredient that produces a characterizing flavor of menthol in combustible tobacco products or their smoke or any ingredients that, like menthol, reduce the harshness and irritation of smoke and that may increase smoking initiation, dependence, and addiction.
3 3. If a tobacco product standard for menthol in menthol cigarettes were to be established, should FDA consider issuing regulations to address menthol in other tobacco products besides cigarettes? If so, what other tobacco products with menthol should be regulated: All tobacco products, just all combusted tobacco products, or some other category or group of tobacco products? If not, what distinctions should be made between products? The AACR and ASCO support a ban on the addition of any ingredient that produces a characterizing flavor of menthol in combustible tobacco products or their smoke including in cigarettes, cigars, and roll-your-own, pipe, and hookah tobacco. 4. If a product standard prohibiting or limiting menthol were to be established, what length of time should manufacturers be provided to achieve compliance with the standard? If a product standard prohibiting or limiting menthol were to be established, would a stepped approach in which the level of menthol was gradually reduced be appropriate for the protection of the public health? The AACR and ASCO recommend an immediate ban on the addition of ingredients that produce a characterizing flavor of menthol to combustible tobacco products or their smoke. An immediate ban on flavors would improve public health more quickly than a stepped approach to reducing the level of menthol in these products. The sooner we can reduce smoking initiation and the development of dependence in adolescents, the greater the probability of reducing tobacco-related morbidity and mortality in the future. 5. If a product standard limiting menthol were to be established, are there alternatives that could be substituted by manufacturers to maintain the effect or appeal of menthol to menthol cigarette smokers and potential initiators? If so, what are these substitutes? Should they be regulated if menthol is regulated; and if so, how should they be regulated? If not, what distinctions should be made between menthol and potential substitutes? In its report, TPSAC notes that tobacco companies have explored adding chemicals with menthol like cooling effects to cigarettes. The AACR and ASCO support a ban on the addition of any ingredient that imparts a menthol-like cooking effect to combustible tobacco products or that otherwise reduces the harshness and irritation of tobacco smoke. B. Sale and Distribution Restrictions 1. Should FDA consider establishing restrictions on the sale and/or distribution of menthol cigarettes? If so, what restrictions would be appropriate and what would be the impact on youth or adult smoking behavior, initiation, and cessation? The evidence evaluated by TPSAC demonstrates that the availability of menthol cigarettes has an adverse impact on public health by increasing the numbers of smokers with resulting premature death and avoidable morbidity. In light of these data, TPSAC stated that removal of menthol cigarettes from the marketplace would benefit public health in the
4 United States. To achieve this goal, we urge FDA to implement a full ban, including prohibiting the manufacture, sale, and distribution, of cigarettes and other combustible tobacco products containing menthol as a characterizing flavor. 2. Should FDA consider establishing restrictions on the advertising and promotion of menthol cigarettes? If so, what restrictions would be appropriate and what would be the impact on youth or adult smoking behavior, initiation, and cessation? The manufacture, sale, and distribution of combustible tobacco products containing menthol as a characterizing flavor should be banned, obviating the need for advertising bans on these products. The FDA should, however, also consider the implications of implementing a ban on products intended to be used in conjunction with non-mentholated cigarettes in order to impart the flavor of menthol to the product or otherwise reduce the harshness or irritation associated with tobacco smoke. The sale and marketing of such products could make it possible to evade the ban on mentholated products and undermine FDA efforts to safeguard public health. C. Other Actions and Considerations 1. Are there other tobacco product standards, regulatory, or other actions that FDA could implement that would more effectively reduce the harms caused by menthol cigarette smoking and better protect the public health than the tobacco product standards or regulatory actions discussed in the preceding questions? The most effective action the FDA could take to reduce the harms caused by menthol cigarettes is to implement a full ban on cigarettes and other combustible tobacco products containing menthol as a characterizing flavor. 2. Is compliance with the tobacco product standard or other regulatory action you identified technically achievable? Compliance with a ban on the addition of menthol as a characterizing flavor to cigarettes and other combustible tobacco products is technically achievable. Menthol is an additive to tobacco and, therefore, it is possible to limit the amount of menthol added to tobacco products to levels below those known to produce characterizing flavors. The FDA has successfully banned the addition of other characterizing flavors to cigarettes and roll-yourown tobacco, demonstrating the feasibility of a similar ban on menthol. 4. What additional information and research beyond that described in the evaluation is there on the potential impact of sale and distribution restrictions of menthol cigarettes on specific subpopulations, such as those based on racial, ethnic, socioeconomic status, and sexuality/gender identity? As we discussed above, menthol cigarette smoking disproportionately harms African- Americans and youths. African Americans are especially likely to smoke menthol cigarettes and African-American menthol smokers are less likely than non-menthol smokers to quit
5 successfully. At the same time, African Americans are more likely to develop lung cancer, more likely to be diagnosed with the disease at an advanced stage, less likely to seek treatment, and more likely to die of lung cancer than their white counterparts. Banning the addition of menthol as a characterizing flavor to cigarettes and other combustible tobacco products would be an important step forward in protecting the health of these populations. Thank you for very much for considering our input on this important issue. If the AACR or ASCO can provide any additional information or assistance to the FDA, please do not hesitate to contact Jennifer A. Hobin, Ph.D., Director of Government Relations for AACR at (202) or jennifer.hobin@aacr.org or Courtney Tyne, M.P.H., Health Policy Manager for ASCO at (571) or courtney.tyne@asco.org. Sincerely, Roy S. Herbst, M.D., Ph.D. Chair, Tobacco and Cancer Subcommittee American Association for Cancer Research Margaret Foti, Ph.D., M.D. (h.c.) Chief Executive Officer American Association for Cancer Research Frank L. Meyskens, Jr., M.D., FACP Chair, Cancer Prevention Committee American Society of Clinical Oncology Allen S. Lichter, M.D. Chief Executive Officer American Society of Clinical Oncology
RE: Docket No. FDA-2014-N , Electronic Cigarettes and the Public Health
 VIA ELECTRONIC SUBMISSION June 30, 2015 Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 RE: Docket No. FDA-2014-N-1936-0001, Electronic
VIA ELECTRONIC SUBMISSION June 30, 2015 Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 RE: Docket No. FDA-2014-N-1936-0001, Electronic
Menthol cigarettes are a public health problem
 Menthol cigarettes are a public health problem ANDREA VILLANTI, PHD MPH JUNE 22, 2016 disclosures Andrea Director The Adjunct Department Johns Villanti, PhD, MPH, CHES for Regulatory Science and Policy
Menthol cigarettes are a public health problem ANDREA VILLANTI, PHD MPH JUNE 22, 2016 disclosures Andrea Director The Adjunct Department Johns Villanti, PhD, MPH, CHES for Regulatory Science and Policy
FDA s Action Agenda to Reduce Tobacco Related-Cancer Incidence and Mortality
 FDA s Action Agenda to Reduce Tobacco Related-Cancer Incidence and Mortality Lawrence Deyton, M.S.P.H., M.D. Director, FDA Center for Tobacco Products June 11, 2012 FDA s Vision To make tobaccorelated
FDA s Action Agenda to Reduce Tobacco Related-Cancer Incidence and Mortality Lawrence Deyton, M.S.P.H., M.D. Director, FDA Center for Tobacco Products June 11, 2012 FDA s Vision To make tobaccorelated
STATE OF NEW JERSEY. ASSEMBLY, No th LEGISLATURE. Sponsored by: Assemblyman HERB CONAWAY, JR. District 7 (Burlington)
 ASSEMBLY, No. STATE OF NEW JERSEY th LEGISLATURE INTRODUCED JANUARY, 0 Sponsored by: Assemblyman HERB CONAWAY, JR. District (Burlington) SYNOPSIS Prohibits sale of menthol cigarettes. CURRENT VERSION OF
ASSEMBLY, No. STATE OF NEW JERSEY th LEGISLATURE INTRODUCED JANUARY, 0 Sponsored by: Assemblyman HERB CONAWAY, JR. District (Burlington) SYNOPSIS Prohibits sale of menthol cigarettes. CURRENT VERSION OF
Menthol Cigarette Report
 Menthol Cigarette Report Tobacco Products Scientific Advisory Committee Meeting July 15, 2010 Corinne G. Husten, MD, MPH Senior Medical Advisor Center for Tobacco Products, FDA 1 Menthol Cigarette Report
Menthol Cigarette Report Tobacco Products Scientific Advisory Committee Meeting July 15, 2010 Corinne G. Husten, MD, MPH Senior Medical Advisor Center for Tobacco Products, FDA 1 Menthol Cigarette Report
Mitch Zeller, Director, Center for Tobacco Products, FDA September 19, 2013 Kansas Public Health Association
 Regulatory Public Laws Compliance & Education Policies Science & Enforcement & Communications The FDA Center for Tobacco Products (CTP): Its Role in Reducing Tobacco Use Mitch Zeller, Director, Center
Regulatory Public Laws Compliance & Education Policies Science & Enforcement & Communications The FDA Center for Tobacco Products (CTP): Its Role in Reducing Tobacco Use Mitch Zeller, Director, Center
TOBACCO USE AMONG AFRICAN AMERICANS
 TOBACCO USE AMONG AFRICAN AMERICANS Each year, approximately 45,000 African Americans die from smoking-related disease. 1 Smoking-related illnesses are the number one cause of death in the African-American
TOBACCO USE AMONG AFRICAN AMERICANS Each year, approximately 45,000 African Americans die from smoking-related disease. 1 Smoking-related illnesses are the number one cause of death in the African-American
consistent with the industry documents we note in our September letter.
 February 18, 2010 Division of Dockets Management (HFA-305) Food and Drug Administration Department of Health and Human Services 5630 Fishers Lane, Room 1061 Rockville, MD 20852 RE: Docket Number FDA-2010-N-0020
February 18, 2010 Division of Dockets Management (HFA-305) Food and Drug Administration Department of Health and Human Services 5630 Fishers Lane, Room 1061 Rockville, MD 20852 RE: Docket Number FDA-2010-N-0020
RADM Patrick O Carroll, MD, MPH Senior Advisor, Assistant Secretary for Health, US DHSS
 Ending the Tobacco Epidemic RADM Patrick O Carroll, MD, MPH Senior Advisor, Assistant Secretary for Health, US DHSS Tim McAfee, MD, MPH Senior Medical Officer, Office on Smoking and Health, CDC www.nwcphp.org/hot-topics
Ending the Tobacco Epidemic RADM Patrick O Carroll, MD, MPH Senior Advisor, Assistant Secretary for Health, US DHSS Tim McAfee, MD, MPH Senior Medical Officer, Office on Smoking and Health, CDC www.nwcphp.org/hot-topics
Subject: Regulation of Flavors in Tobacco Products (Docket No. FDA N-6565)
 Via Electronic Submission July 19, 2018 Scott Gottlieb, MD Commissioner U.S. Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, Maryland 20993 Subject: Regulation of Flavors in Tobacco
Via Electronic Submission July 19, 2018 Scott Gottlieb, MD Commissioner U.S. Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, Maryland 20993 Subject: Regulation of Flavors in Tobacco
Ordinance amending the Health Code to prohibit tobacco retailers from selling flavored
 FILE NO. ORDINANCE NO. 1 [Health Code - Banning the Sale of Flavored Tobacco Products] Ordinance amending the Health Code to prohibit tobacco retailers from selling flavored tobacco products, including
FILE NO. ORDINANCE NO. 1 [Health Code - Banning the Sale of Flavored Tobacco Products] Ordinance amending the Health Code to prohibit tobacco retailers from selling flavored tobacco products, including
REPORT OF THE BOARD OF TRUSTEES. Subject: Annual Update on Activities and Progress in Tobacco Control: March 2017 through February 2018
 REPORT OF THE BOARD OF TRUSTEES B of T Report -A- Subject: Annual Update on Activities and Progress in Tobacco Control: March 0 through February 0 Presented by: Gerald E. Harmon, MD, Chair 0 0 0 This report
REPORT OF THE BOARD OF TRUSTEES B of T Report -A- Subject: Annual Update on Activities and Progress in Tobacco Control: March 0 through February 0 Presented by: Gerald E. Harmon, MD, Chair 0 0 0 This report
Nebraska Youth Tobacco Survey 2015/2017
 Nebraska Youth Tobacco Survey 2015/2017 TABLE OF CONTENTS Introduction... 1 Background... 1 Method... 1 Sampling Frame and Response Rates... 1 Weighting Data... 2 Terms and Definitions... 3 Executive Summary...
Nebraska Youth Tobacco Survey 2015/2017 TABLE OF CONTENTS Introduction... 1 Background... 1 Method... 1 Sampling Frame and Response Rates... 1 Weighting Data... 2 Terms and Definitions... 3 Executive Summary...
Three-Month Extension of Certain Tobacco Product Compliance Deadlines Related to the
 This document is scheduled to be published in the Federal Register on 05/15/2017 and available online at https://federalregister.gov/d/2017-09754, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 05/15/2017 and available online at https://federalregister.gov/d/2017-09754, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
ELECTRONIC CIGARETTES WHAT S THE BOTTOM LINE?
 ELECTRONIC CIGARETTES WHAT S THE BOTTOM LINE? E-cigarettes have the potential to benefit adult smokers who are not pregnant if used as a complete substitute for regular cigarettes and other smoked tobacco
ELECTRONIC CIGARETTES WHAT S THE BOTTOM LINE? E-cigarettes have the potential to benefit adult smokers who are not pregnant if used as a complete substitute for regular cigarettes and other smoked tobacco
National Conference of State Legislatures Legislative Summit: Clearing the Air About E-Cigarettes
 National Conference of State Legislatures Legislative Summit: Clearing the Air About E-Cigarettes Jack Henningfield Ph.D. Vice President, Research and Health Policy Pinney Associates And Professor, Adjunct,
National Conference of State Legislatures Legislative Summit: Clearing the Air About E-Cigarettes Jack Henningfield Ph.D. Vice President, Research and Health Policy Pinney Associates And Professor, Adjunct,
FDA S CENTER FOR TOBACCO PRODUCTS: AN UPDATE ON REGULATORY ACTIVITIES AND PRIORITIES
 FDA S CENTER FOR TOBACCO PRODUCTS: AN UPDATE ON REGULATORY ACTIVITIES AND PRIORITIES Mitch Zeller, J.D. (Director, FDA CTP) Matthew R. Holman, Ph.D. (Director, Office of Science, FDA CTP) Kathy Crosby
FDA S CENTER FOR TOBACCO PRODUCTS: AN UPDATE ON REGULATORY ACTIVITIES AND PRIORITIES Mitch Zeller, J.D. (Director, FDA CTP) Matthew R. Holman, Ph.D. (Director, Office of Science, FDA CTP) Kathy Crosby
SUMMARY: The Food and Drug Administration (FDA) is issuing this advance notice of
 This document is scheduled to be published in the Federal Register on 03/16/2018 and available online at https://federalregister.gov/d/2018-05345, and on FDsys.gov 4164-01-P DEPARTMENT
This document is scheduled to be published in the Federal Register on 03/16/2018 and available online at https://federalregister.gov/d/2018-05345, and on FDsys.gov 4164-01-P DEPARTMENT
UNITED STATES REGULATION OF TOBACCO PRODUCTS. Presented by Mitch Zeller Center Director FDA Center for Tobacco Products
 UNITED STATES REGULATION OF TOBACCO PRODUCTS Presented by Mitch Zeller Center Director FDA Center for Tobacco Products May 24, 2016 OVERVIEW OF TODAY S PRESENTATION Highlights of the Deeming Final Rule
UNITED STATES REGULATION OF TOBACCO PRODUCTS Presented by Mitch Zeller Center Director FDA Center for Tobacco Products May 24, 2016 OVERVIEW OF TODAY S PRESENTATION Highlights of the Deeming Final Rule
Roll Call. Board Members Present:
 Meeting Summary of the Regular Meeting of the Chicago Board of Health CDPH Board Room, 2 nd Floor DePaul Center, 333 S. State Street Wednesday, August 21, 2013, 9:00 AM Roll Call Board Members Present:
Meeting Summary of the Regular Meeting of the Chicago Board of Health CDPH Board Room, 2 nd Floor DePaul Center, 333 S. State Street Wednesday, August 21, 2013, 9:00 AM Roll Call Board Members Present:
Use of Light, Mild, Low, or Similar Descriptors in the Label, Labeling, or Advertising of Tobacco Products
 Guidance for Industry and FDA Staff Use of Light, Mild, Low, or Similar Descriptors in the Label, Labeling, or Advertising of Tobacco Products June 2010 For questions regarding this guidance, contact the
Guidance for Industry and FDA Staff Use of Light, Mild, Low, or Similar Descriptors in the Label, Labeling, or Advertising of Tobacco Products June 2010 For questions regarding this guidance, contact the
Problem Which option Additional option Additional comments definition Yes No change No further observations.
 Department of Health, United Kingdom electronic contribution rec. 317 - by Mr Lee McGill lee.mcgill@dh.gsi.gov.uk Question 1 - scope Problem Which option Recommend option Additional comments Yes No change
Department of Health, United Kingdom electronic contribution rec. 317 - by Mr Lee McGill lee.mcgill@dh.gsi.gov.uk Question 1 - scope Problem Which option Recommend option Additional comments Yes No change
SENATE, No. 298 STATE OF NEW JERSEY. 217th LEGISLATURE PRE-FILED FOR INTRODUCTION IN THE 2016 SESSION
 SENATE, No. STATE OF NEW JERSEY th LEGISLATURE PRE-FILED FOR INTRODUCTION IN THE 0 SESSION Sponsored by: Senator JOSEPH F. VITALE District (Middlesex) Senator RICHARD J. CODEY District (Essex and Morris)
SENATE, No. STATE OF NEW JERSEY th LEGISLATURE PRE-FILED FOR INTRODUCTION IN THE 0 SESSION Sponsored by: Senator JOSEPH F. VITALE District (Middlesex) Senator RICHARD J. CODEY District (Essex and Morris)
FDA Center for Tobacco Products: Tobacco Research and the Population Assessment of Tobacco and Health (PATH) Study
 FDA Center for Tobacco Products: Tobacco Research and the Population Assessment of Tobacco and Health (PATH) Study Regulatory Public Laws Compliance & Education Policies Science & Enforcement & Communications
FDA Center for Tobacco Products: Tobacco Research and the Population Assessment of Tobacco and Health (PATH) Study Regulatory Public Laws Compliance & Education Policies Science & Enforcement & Communications
AN UPDATE ON FDA S COMPREHENSIVE PLAN ON TOBACCO AND NICOTINE
 AN UPDATE ON FDA S COMPREHENSIVE PLAN ON TOBACCO AND NICOTINE Mitch Zeller, J.D. Director, FDA May 3, 2018 CENTER FOR TOBACCO PRODUCTS HOW CAN WE MAKE THE GREATEST IMPACT? We truly find ourselves at a
AN UPDATE ON FDA S COMPREHENSIVE PLAN ON TOBACCO AND NICOTINE Mitch Zeller, J.D. Director, FDA May 3, 2018 CENTER FOR TOBACCO PRODUCTS HOW CAN WE MAKE THE GREATEST IMPACT? We truly find ourselves at a
Are Menthol Cigarettes a Starter Product for Youth?
 Posted For Public Comment May 7, 2010 Are Menthol Cigarettes a Starter Product for Youth? By: James C. Hersey, Shu Wen Ng, James M. Nonnemaker, Paul Mowery, Kristin Y. Thomas, My-Charllins Vilsaint, Jane
Posted For Public Comment May 7, 2010 Are Menthol Cigarettes a Starter Product for Youth? By: James C. Hersey, Shu Wen Ng, James M. Nonnemaker, Paul Mowery, Kristin Y. Thomas, My-Charllins Vilsaint, Jane
April 28, The President The White House 1600 Pennsylvania Avenue, N.W. Washington, DC Dear Mr. President:
 April 28, 2015 The The White House 1600 Pennsylvania Avenue, N.W. Washington, DC 20500 Dear Mr. : It has been more than a year since the Food and Drug Administration (FDA) proposed a regulation to extend
April 28, 2015 The The White House 1600 Pennsylvania Avenue, N.W. Washington, DC 20500 Dear Mr. : It has been more than a year since the Food and Drug Administration (FDA) proposed a regulation to extend
How to Regulate E-Cigarettes? Are we asking the right questions?
 How to Regulate E-Cigarettes? Are we asking the right questions? Eric N. Lindblom Director, Tobacco Control and Food & Drug Law O Neill Institute for National & Global Health Law Georgetown University
How to Regulate E-Cigarettes? Are we asking the right questions? Eric N. Lindblom Director, Tobacco Control and Food & Drug Law O Neill Institute for National & Global Health Law Georgetown University
UPDATES FROM THE FDA CENTER FOR TOBACCO PRODUCTS (CTP)
 UPDATES FROM THE FDA CENTER FOR TOBACCO PRODUCTS (CTP) Mitch Zeller, J.D. Director, FDA May 4, 2017 CENTER FOR TOBACCO PRODUCTS AGENDA Update on Regulations and Guidances: Deeming Update on Science Update
UPDATES FROM THE FDA CENTER FOR TOBACCO PRODUCTS (CTP) Mitch Zeller, J.D. Director, FDA May 4, 2017 CENTER FOR TOBACCO PRODUCTS AGENDA Update on Regulations and Guidances: Deeming Update on Science Update
Tobacco Data, Prevention Spending, and the Toll of Tobacco Use in North Carolina
 Tobacco Data, Prevention Spending, and the Toll of Tobacco Use in North Carolina North Carolina Alliance for Health 2017 0 Table of Contents Highlights from the Surgeon General s Report on E-Cigarette
Tobacco Data, Prevention Spending, and the Toll of Tobacco Use in North Carolina North Carolina Alliance for Health 2017 0 Table of Contents Highlights from the Surgeon General s Report on E-Cigarette
The Voice of Local Public Health in New York State. May 12, 2014
 The Voice of Local Public Health in New York State May 12, 2014 Testimony before the Senate Standing Committee on Health To consider including electronic cigarettes in the existing Clean Indoor Air Act
The Voice of Local Public Health in New York State May 12, 2014 Testimony before the Senate Standing Committee on Health To consider including electronic cigarettes in the existing Clean Indoor Air Act
COMMENT PREPARED AFTER THE TPSAC MEETIING ON CAMEL SNUS. significantly reduce harm to individuals or benefit population health.
 COMMENT PREPARED AFTER THE TPSAC MEETIING ON CAMEL SNUS RJR failed to demonstrate that Camel Snus, as actually used by consumers, will significantly reduce harm to individuals or benefit population health.
COMMENT PREPARED AFTER THE TPSAC MEETIING ON CAMEL SNUS RJR failed to demonstrate that Camel Snus, as actually used by consumers, will significantly reduce harm to individuals or benefit population health.
IMPACT OF MENTHOL CIGARETTES ON YOUTH SMOKING INITIATION AND HEALTH DISPARITIES
 IMPACT OF MENTHOL CIGARETTES ON YOUTH SMOKING INITIATION AND HEALTH DISPARITIES Cigarettes with specific characterizing flavors were prohibited in the U.S. on September 22, 2009, as part of the Family
IMPACT OF MENTHOL CIGARETTES ON YOUTH SMOKING INITIATION AND HEALTH DISPARITIES Cigarettes with specific characterizing flavors were prohibited in the U.S. on September 22, 2009, as part of the Family
Electronic Cigarettes and the Medical Society
 Electronic Cigarettes and the Medical Society Michele Coleman MPH, MPA 2014 WI Medical Society Health Policy Intern LRB 1844 Exempting electronic smoking devices from the types of smoking devices that
Electronic Cigarettes and the Medical Society Michele Coleman MPH, MPA 2014 WI Medical Society Health Policy Intern LRB 1844 Exempting electronic smoking devices from the types of smoking devices that
Smoking Cessation: A readily modifiable risk factor
 Robert L. Keith MD FCCP Associate Professor of Medicine Medical Director of Respiratory Therapy Eastern Colorado Health Care System University of Colorado Denver Smoking Cessation: A readily modifiable
Robert L. Keith MD FCCP Associate Professor of Medicine Medical Director of Respiratory Therapy Eastern Colorado Health Care System University of Colorado Denver Smoking Cessation: A readily modifiable
Flavors Hook Kids. The Tobacco Industry s Kids Menu MARIPOSA COUNTY HEALTH DEPARTMENT
 Flavors Hook Kids The Tobacco Industry s Kids Menu Mariposa County Tobacco Education Program We engage with the community to: Create smoke free environments Counter the aggressive marketing practices of
Flavors Hook Kids The Tobacco Industry s Kids Menu Mariposa County Tobacco Education Program We engage with the community to: Create smoke free environments Counter the aggressive marketing practices of
Challenges and Opportunities: Implementing the Tobacco Control Act
 Challenges and Opportunities: Implementing the Tobacco Control Act Lawrence R. Deyton, M.S.P.H., M.D. Director, Center for Tobacco Products April 5, 2011 1 To make tobaccorelated death and disease part
Challenges and Opportunities: Implementing the Tobacco Control Act Lawrence R. Deyton, M.S.P.H., M.D. Director, Center for Tobacco Products April 5, 2011 1 To make tobaccorelated death and disease part
The Latest on Vaping Among U.S. Teens
 The Latest on Vaping Among U.S. Teens Jon Macy, PhD, MPH Indiana University School of Public Health Bloomington Presentation Outline Overview of Electronic Nicotine Delivery Systems (ENDS) New Prevalence
The Latest on Vaping Among U.S. Teens Jon Macy, PhD, MPH Indiana University School of Public Health Bloomington Presentation Outline Overview of Electronic Nicotine Delivery Systems (ENDS) New Prevalence
A REPORT ON THE INCIDENCE AND PREVALENCE OF YOUTH TOBACCO USE IN DELAWARE
 A REPORT ON THE INCIDENCE AND PREVALENCE OF YOUTH TOBACCO USE IN DELAWARE RESULTS FROM THE ADMINISTRATION OF THE DELAWARE YOUTH TOBACCO SURVEY IN SPRING 00 Delaware Health and Social Services Division
A REPORT ON THE INCIDENCE AND PREVALENCE OF YOUTH TOBACCO USE IN DELAWARE RESULTS FROM THE ADMINISTRATION OF THE DELAWARE YOUTH TOBACCO SURVEY IN SPRING 00 Delaware Health and Social Services Division
Menthol and Cigarettes
 Menthol and Cigarettes What is Menthol and How is it Used? Menthol is a naturally occurring compound derived from mint plants and is also synthetically produced. [1] Because of its cool, minty candy-like
Menthol and Cigarettes What is Menthol and How is it Used? Menthol is a naturally occurring compound derived from mint plants and is also synthetically produced. [1] Because of its cool, minty candy-like
Menthol and the Tobacco Products Scientific Advisory Committee
 Tobacco, Menthol and the Tobacco Products Scientific Advisory Committee 2010 CBC Spring Health Braintrust/NMQF 7 th Annual Leadership Meeting The Ritz Carlton Hotel Washington, DC April 20, 2009 NAATPN
Tobacco, Menthol and the Tobacco Products Scientific Advisory Committee 2010 CBC Spring Health Braintrust/NMQF 7 th Annual Leadership Meeting The Ritz Carlton Hotel Washington, DC April 20, 2009 NAATPN
Communicating the Risk of Nicotine Delivery Products
 Communicating the Risk of Nicotine Delivery Products August 10, 2014 ACS National Meeting Jim Solyst June 24, 2014 1 Nicotine Background Key Points The popularity of e-cigarettes has increased the need
Communicating the Risk of Nicotine Delivery Products August 10, 2014 ACS National Meeting Jim Solyst June 24, 2014 1 Nicotine Background Key Points The popularity of e-cigarettes has increased the need
FDLI s Enforcement, Litigation, and Compliance Conference. Center for Tobacco Products Office of Compliance and Enforcement 2017 Update
 FDLI s Enforcement, Litigation, and Compliance Conference Center for Tobacco Products Office of Compliance and Enforcement 2017 Update Ann Simoneau, Director Office of Compliance and Enforcement Center
FDLI s Enforcement, Litigation, and Compliance Conference Center for Tobacco Products Office of Compliance and Enforcement 2017 Update Ann Simoneau, Director Office of Compliance and Enforcement Center
A REPORT ON THE INCIDENCE AND PREVALENCE OF YOUTH TOBACCO USE IN DELAWARE :
 A REPORT ON THE INCIDENCE AND PREVALENCE OF YOUTH TOBACCO USE IN DELAWARE : RESULTS FROM ADMINISTRATION OF THE DELAWARE YOUTH TOBACCO SURVEY IN SPRING 2000 Delaware Health and Social Services Division
A REPORT ON THE INCIDENCE AND PREVALENCE OF YOUTH TOBACCO USE IN DELAWARE : RESULTS FROM ADMINISTRATION OF THE DELAWARE YOUTH TOBACCO SURVEY IN SPRING 2000 Delaware Health and Social Services Division
Sacramento City College Smoke/Tobacco/Vape (STV) Free Environmental Standard
 Sacramento City College Smoke/Tobacco/Vape (STV) Free Environmental Standard In the interest of promoting a healthy learning and working environment for its students and employees, Sacramento City College
Sacramento City College Smoke/Tobacco/Vape (STV) Free Environmental Standard In the interest of promoting a healthy learning and working environment for its students and employees, Sacramento City College
Texas Bill to Regulate Vapor Products. Over the past few years, electronic cigarettes, also commonly referred to as e-cigarettes
 Texas Bill to Regulate Vapor Products Over the past few years, electronic cigarettes, also commonly referred to as e-cigarettes or vapor, have been trending all over the state of Texas as a tobacco alternative,
Texas Bill to Regulate Vapor Products Over the past few years, electronic cigarettes, also commonly referred to as e-cigarettes or vapor, have been trending all over the state of Texas as a tobacco alternative,
Electronic Nicotine Delivery Systems: Patterns of Use and Disparities
 Electronic Nicotine Delivery Systems: Patterns of Use and Disparities Daniel P. Giovenco, PhD, MPH Assistant Professor of Sociomedical Sciences Columbia University Mailman School of Public Health Outline
Electronic Nicotine Delivery Systems: Patterns of Use and Disparities Daniel P. Giovenco, PhD, MPH Assistant Professor of Sociomedical Sciences Columbia University Mailman School of Public Health Outline
Dissolvable Tobacco Products
 Dissolvable Tobacco Products James E. Dillard Senior Vice President, Regulatory Affairs Altria Client Services 1 l Altria Client Services (on behalf of PM USA and USSTC) I Presentation to the Tobacco Products
Dissolvable Tobacco Products James E. Dillard Senior Vice President, Regulatory Affairs Altria Client Services 1 l Altria Client Services (on behalf of PM USA and USSTC) I Presentation to the Tobacco Products
Modified Risk Tobacco Product Applications A Succinct ENDS Industry Perspective
 Modified Risk Tobacco Product Applications A Succinct ENDS Industry Perspective Patricia I. Kovacevic General Counsel, Chief Compliance Officer Presentation to the Food and Drug Law Institute Tobacco Conference,
Modified Risk Tobacco Product Applications A Succinct ENDS Industry Perspective Patricia I. Kovacevic General Counsel, Chief Compliance Officer Presentation to the Food and Drug Law Institute Tobacco Conference,
Introduction. Principles
 NHS Health Scotland s position statement on Electronic Nicotine Delivery Systems ENDS - e-cigarettes and other smoking simulator products 31 October 2014 Introduction NHS Health Scotland is the national
NHS Health Scotland s position statement on Electronic Nicotine Delivery Systems ENDS - e-cigarettes and other smoking simulator products 31 October 2014 Introduction NHS Health Scotland is the national
Should FDA try to move smokers to e-cigarettes or other less harmful tobacco-nicotine products and, if so, how?
 Should FDA try to move smokers to e-cigarettes or other less harmful tobacco-nicotine products and, if so, how? Jeff Weiss General Counsel/EVP of Gov. Affairs of NJOY, LLC FDLI - October 20, 2017 FDA s
Should FDA try to move smokers to e-cigarettes or other less harmful tobacco-nicotine products and, if so, how? Jeff Weiss General Counsel/EVP of Gov. Affairs of NJOY, LLC FDLI - October 20, 2017 FDA s
MDQuit Best Practices Conference January 26, Presented by William C. Tilburg Deputy Director
 MDQuit Best Practices Conference January 26, 2017 Presented by William C. Tilburg Deputy Director Founded in 2001 Partnership between UM School of Law, DHMH, and Maryland Office of the Attorney General
MDQuit Best Practices Conference January 26, 2017 Presented by William C. Tilburg Deputy Director Founded in 2001 Partnership between UM School of Law, DHMH, and Maryland Office of the Attorney General
Can a system like the U.S. OTC monographs work for ENDS?
 Can a system like the U.S. OTC monographs work for ENDS? Jack Henningfield, PhD Vice President, Research and Health Policy PinneyAssociates and Professor, Behavioral Biology, Adjunct Department of Psychiatry
Can a system like the U.S. OTC monographs work for ENDS? Jack Henningfield, PhD Vice President, Research and Health Policy PinneyAssociates and Professor, Behavioral Biology, Adjunct Department of Psychiatry
Policy Options for the Regulation of Electronic Cigarettes
 Policy Options for the Regulation of Electronic Cigarettes Consultation submission Your details This submission was completed by: (name) Philip Hope / Assoc Prof Chris Atkinson Address: (street/box number)
Policy Options for the Regulation of Electronic Cigarettes Consultation submission Your details This submission was completed by: (name) Philip Hope / Assoc Prof Chris Atkinson Address: (street/box number)
Electronic cigarettes: A new era for tobacco harm reduction Adapted for SW Specialist Nurses for Children in Care meeting 17 January 2017
 Electronic cigarettes: A new era for tobacco harm reduction Adapted for SW Specialist Nurses for Children in Care meeting 17 January 2017 Russ Moody Health & Wellbeing Programme Lead Public Health England
Electronic cigarettes: A new era for tobacco harm reduction Adapted for SW Specialist Nurses for Children in Care meeting 17 January 2017 Russ Moody Health & Wellbeing Programme Lead Public Health England
Centers for Disease Control and Prevention
 This document is scheduled to be published in the Federal Register on 12/11/2018 and available online at https://federalregister.gov/d/2018-26708, and on govinfo.gov Billing Code: 4163-18-P DEPARTMENT
This document is scheduled to be published in the Federal Register on 12/11/2018 and available online at https://federalregister.gov/d/2018-26708, and on govinfo.gov Billing Code: 4163-18-P DEPARTMENT
16851 Mount Wolfe Road Caledon ON L7E 3P or 1 (855)
 2 Copyright 2015, Canadian Network for Respiratory Care 16851 Mount Wolfe Road Caledon ON L7E 3P6 905 880-1092 or 1 (855) 355-4672 www.cnrchome.net www.cnrchome.net Contents 1 Health Promotion and Tobacco
2 Copyright 2015, Canadian Network for Respiratory Care 16851 Mount Wolfe Road Caledon ON L7E 3P6 905 880-1092 or 1 (855) 355-4672 www.cnrchome.net www.cnrchome.net Contents 1 Health Promotion and Tobacco
OMA Submission on Health Canada s Proposed Regulations for Additional Cannabis Products. February 2019
 OMA Submission on Health Canada s Proposed Regulations for Additional Cannabis Products February 2019 OMA Submission on Health Canada s Proposed Regulations for Additional Cannabis Products The Ontario
OMA Submission on Health Canada s Proposed Regulations for Additional Cannabis Products February 2019 OMA Submission on Health Canada s Proposed Regulations for Additional Cannabis Products The Ontario
THE IMPORTANCE OF POINT OF SALE
 THE IMPORTANCE OF POINT OF SALE Counter Tobacco Allison E. Myers, MPH Kurt M. Ribisl, PhD Adapted from a presentation given January 16, 2013 Office of Smoking and Health Centers for Disease Control and
THE IMPORTANCE OF POINT OF SALE Counter Tobacco Allison E. Myers, MPH Kurt M. Ribisl, PhD Adapted from a presentation given January 16, 2013 Office of Smoking and Health Centers for Disease Control and
FDA Tobacco Retailers Compliance Program Presentation
 Arizona Department of Health Services FDA Tobacco Retailers Compliance Program Presentation June 29, 2011 Revised 06/27/11 FDA Retail Tobacco Compliance Program In contract with Arizona Department of Health
Arizona Department of Health Services FDA Tobacco Retailers Compliance Program Presentation June 29, 2011 Revised 06/27/11 FDA Retail Tobacco Compliance Program In contract with Arizona Department of Health
The Family Smoking Prevention and Tobacco Control Act s Grant of Authority to the FDA and the Impact on the Grower
 The Family Smoking Prevention and Tobacco Control Act s Grant of Authority to the FDA and the Impact on the Grower Victor L. Moldovan McGuireWoods, LLP The Act The Act governs all manufacturers and importers
The Family Smoking Prevention and Tobacco Control Act s Grant of Authority to the FDA and the Impact on the Grower Victor L. Moldovan McGuireWoods, LLP The Act The Act governs all manufacturers and importers
FDA should prohibit of use of flavors in deemed tobacco products as part of the current rulemaking
 FDA should prohibit of use of flavors in deemed tobacco products as part of the current rulemaking Docket No. FDA-2014-N-0189 Elizabeth Couch, RDH, MS, Benjamin Chaffee DDS, MPH, PhD, Gwen Essex, RDH,
FDA should prohibit of use of flavors in deemed tobacco products as part of the current rulemaking Docket No. FDA-2014-N-0189 Elizabeth Couch, RDH, MS, Benjamin Chaffee DDS, MPH, PhD, Gwen Essex, RDH,
Effective and Compliance Dates Applicable to Retailers, Manufacturers, Importers, and Distributors of Newly Deemed Tobacco Products
 Effective and Compliance Dates Applicable to Retailers, Manufacturers, Importers, and Distributors of Newly Deemed Tobacco Quick Facts Retailers that mix and prepare e-liquids or create or modify vaporizers
Effective and Compliance Dates Applicable to Retailers, Manufacturers, Importers, and Distributors of Newly Deemed Tobacco Quick Facts Retailers that mix and prepare e-liquids or create or modify vaporizers
News From the Front: State & Local Regulation of E-Cigarettes
 News From the Front: State & Local Regulation of E-Cigarettes We will begin shortly after 12:00pm Central. Until then you will not hear any audio. Legislation and Advocacy Susan Weisman & Mark Meaney,
News From the Front: State & Local Regulation of E-Cigarettes We will begin shortly after 12:00pm Central. Until then you will not hear any audio. Legislation and Advocacy Susan Weisman & Mark Meaney,
Tobacco Use in Adolescents
 Tobacco Use in Adolescents Joycelyn Lawrence, MD Leonard Miller School of Medicine at the University of Miami Department of Family Medicine 1 Overview Description: This section will introduce you to the
Tobacco Use in Adolescents Joycelyn Lawrence, MD Leonard Miller School of Medicine at the University of Miami Department of Family Medicine 1 Overview Description: This section will introduce you to the
SIPP Phase II: Vaping and Vapor Devices. Subcommittee Briefing and Discussion
 SIPP Phase II: Vaping and Vapor Devices Subcommittee Briefing and Discussion August 19, 2015 Welcome and INTRODUCTIONS Subcommittee and Staff Sam Low (Lake Stevens City Council) Linda Grafer (Mukilteo
SIPP Phase II: Vaping and Vapor Devices Subcommittee Briefing and Discussion August 19, 2015 Welcome and INTRODUCTIONS Subcommittee and Staff Sam Low (Lake Stevens City Council) Linda Grafer (Mukilteo
The Changing Landscape of Tobacco: E-Cigarettes, E-Hookahs and More
 38th Annual Family Medicine Intensive Review Course The Changing Landscape of Tobacco: E-Cigarettes, E-Hookahs and More Debbie Rushing, LADAC, CTTS-M Associate Branch Chief Tobacco Prevention and Cessation
38th Annual Family Medicine Intensive Review Course The Changing Landscape of Tobacco: E-Cigarettes, E-Hookahs and More Debbie Rushing, LADAC, CTTS-M Associate Branch Chief Tobacco Prevention and Cessation
PUBLIC CONSULTATION DOCUMENT
 EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL Directorate C Public Health and Risk Assessment POSSIBLE REVISION OF THE TOBACCO PRODUCTS DIRECTIVE 2001/37/EC PUBLIC CONSULTATION DOCUMENT
EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL Directorate C Public Health and Risk Assessment POSSIBLE REVISION OF THE TOBACCO PRODUCTS DIRECTIVE 2001/37/EC PUBLIC CONSULTATION DOCUMENT
Evaluating implementation of Chicago s city ordinance restricting sales of flavored tobacco products near schools
 Evaluating implementation of Chicago s city ordinance restricting sales of flavored tobacco products near schools Sandy Slater, PhD, MS Dianne C. Barker, MHS Anita Bontu, MPH Frank Chaloupka, PhD @tobacconomics
Evaluating implementation of Chicago s city ordinance restricting sales of flavored tobacco products near schools Sandy Slater, PhD, MS Dianne C. Barker, MHS Anita Bontu, MPH Frank Chaloupka, PhD @tobacconomics
Understanding the Tobacco Control Act
 Understanding the Tobacco Control Act An Overview of the Tobacco Control Act and Center for Tobacco Products for the National Native Network CAPT Gail Cherry-Peppers, Tribal Liaison Heather Althouse, Senior
Understanding the Tobacco Control Act An Overview of the Tobacco Control Act and Center for Tobacco Products for the National Native Network CAPT Gail Cherry-Peppers, Tribal Liaison Heather Althouse, Senior
Compensation: money, gratuity, privilege, or benefit received from an Employer in return for work performed or services rendered.
 Chapter 217. Health and Sanitation Article IV. Tobacco and Nicotine Delivery Product Control 217-11. Findings and purpose There exists substantial evidence that tobacco smoke causes cancer, heart disease,
Chapter 217. Health and Sanitation Article IV. Tobacco and Nicotine Delivery Product Control 217-11. Findings and purpose There exists substantial evidence that tobacco smoke causes cancer, heart disease,
TUPAC Five-Year Action Plan
 TUPAC Five-Year Action Plan 2015-2020 New Mexico Department of Health Tobacco Use Prevention and Control Program 5301 Central Avenue NE, Suite 800, Albuquerque, NM 87108 505.841.5845 nmtupac.com TUPAC
TUPAC Five-Year Action Plan 2015-2020 New Mexico Department of Health Tobacco Use Prevention and Control Program 5301 Central Avenue NE, Suite 800, Albuquerque, NM 87108 505.841.5845 nmtupac.com TUPAC
Where We Are: State of Tobacco Control and Prevention
 Where We Are: State of Tobacco Control and Prevention Corinne Husten, MD, MPH Acting Director CDC Office on Smoking and Health Nova Scotia, Canada October 2006 Tobacco Impact Background Tobacco is leading
Where We Are: State of Tobacco Control and Prevention Corinne Husten, MD, MPH Acting Director CDC Office on Smoking and Health Nova Scotia, Canada October 2006 Tobacco Impact Background Tobacco is leading
POSSIBLE REVISION OF THE TOBACCO PRODUCTS DIRECTIVE 2001/37/EC PUBLIC CONSULTATION DOCUMENT
 POSSIBLE REVISION OF THE TOBACCO PRODUCTS DIRECTIVE 2001/37/EC DG SANCO 2010 PUBLIC CONSULTATION DOCUMENT Answer given by the General Direction of Public Health of the Spanish Ministry of Health and Social
POSSIBLE REVISION OF THE TOBACCO PRODUCTS DIRECTIVE 2001/37/EC DG SANCO 2010 PUBLIC CONSULTATION DOCUMENT Answer given by the General Direction of Public Health of the Spanish Ministry of Health and Social
Menthol Cigarettes, Smoking Cessation, Atherosclerosis and Pulmonary Function
 Center for Regulatory Effectiveness (CRE) assessment of the following research report: Menthol Cigarettes, Smoking Cessation, Atherosclerosis and Pulmonary Function By: Mark J. Pletcher, MD, MPH; Benjamin
Center for Regulatory Effectiveness (CRE) assessment of the following research report: Menthol Cigarettes, Smoking Cessation, Atherosclerosis and Pulmonary Function By: Mark J. Pletcher, MD, MPH; Benjamin
Tobacco Use: Epidemiology and Determinants Andrea Villanti, PhD, MPH Vermont Center on Behavior and Health University of Vermont November 1, 2017
 Tobacco Use: Epidemiology and Determinants Andrea Villanti, PhD, MPH Vermont Center on Behavior and Health University of Vermont November 1, 2017 2 1 What is public health? Public health promotes and protects
Tobacco Use: Epidemiology and Determinants Andrea Villanti, PhD, MPH Vermont Center on Behavior and Health University of Vermont November 1, 2017 2 1 What is public health? Public health promotes and protects
TOBACCO PRODUCT OR MEDICAL PRODUCT?
 TOBACCO PRODUCT OR MEDICAL PRODUCT? Priscilla Callahan-Lyon, MD Deputy Director Division of Individual Health Science Office of Science, CTP Grail Sipes, JD Director Office of Regulatory Policy, CDER Disclaimer:
TOBACCO PRODUCT OR MEDICAL PRODUCT? Priscilla Callahan-Lyon, MD Deputy Director Division of Individual Health Science Office of Science, CTP Grail Sipes, JD Director Office of Regulatory Policy, CDER Disclaimer:
Global E-Cigarette Market- Industry Analysis & Outlook ( )
 Global E-Cigarette Market- Industry Analysis & Outlook (2016-2020) ----------------------------------------- Executive Summary Electronic cigarettes, also known as E-cigarettes, electronic nicotine delivery
Global E-Cigarette Market- Industry Analysis & Outlook (2016-2020) ----------------------------------------- Executive Summary Electronic cigarettes, also known as E-cigarettes, electronic nicotine delivery
Maryland Tobacco Control Program Successes. Donald Shell, MD, MA Interim Director DHMH, Center for Health Promotion Education, Tobacco Use Prevention
 Maryland Tobacco Control Program Successes Donald Shell, MD, MA Interim Director DHMH, Center for Health Promotion Education, Tobacco Use Prevention Monitoring Changing Tobacco Use Behaviors 2000-2010
Maryland Tobacco Control Program Successes Donald Shell, MD, MA Interim Director DHMH, Center for Health Promotion Education, Tobacco Use Prevention Monitoring Changing Tobacco Use Behaviors 2000-2010
Tobacco-related risk perceptions in the regulation of tobacco products at the FDA Center for Tobacco Products
 Tobacco-related risk perceptions in the regulation of tobacco products at the FDA Center for Tobacco Products David B. Portnoy, PhD, MPH Conrad J. Choiniere, PhD Office of Science FDA, Center for Tobacco
Tobacco-related risk perceptions in the regulation of tobacco products at the FDA Center for Tobacco Products David B. Portnoy, PhD, MPH Conrad J. Choiniere, PhD Office of Science FDA, Center for Tobacco
E-Cigarettes: A Game Changer. Dr. Blake Brown Hugh C. Kiger Professor Agriculture and Resource Economics College of Agriculture & Life Sciences
 E-Cigarettes: A Game Changer Dr. Blake Brown Hugh C. Kiger Professor Agriculture and Resource Economics College of Agriculture & Life Sciences What is an e-cigarette? Battery Cartridge Indicator Light
E-Cigarettes: A Game Changer Dr. Blake Brown Hugh C. Kiger Professor Agriculture and Resource Economics College of Agriculture & Life Sciences What is an e-cigarette? Battery Cartridge Indicator Light
Re: Docket No. FDA-2009-N-0294 Regulation of Tobacco Products; Request for Comments
 VIA Electronic Submission to http://www.regulations.gov September 29, 2009 Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, rm. 1061 Rockville, MD 20852 Re: Docket
VIA Electronic Submission to http://www.regulations.gov September 29, 2009 Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, rm. 1061 Rockville, MD 20852 Re: Docket
MARKETING STANDARDS FOR MEMBERSHIP
 MARKETING STANDARDS FOR MEMBERSHIP The Vapor Technology Association (VTA) is a leading national trade association in the electronic cigarette and vapor product industry. VTA represents the manufacturers,
MARKETING STANDARDS FOR MEMBERSHIP The Vapor Technology Association (VTA) is a leading national trade association in the electronic cigarette and vapor product industry. VTA represents the manufacturers,
DRAFT FOR COMMENT ( ) All Tobacco Products are Deadly and Addictive: Product Diversity Challenges Regulation and Communication
 DRAFT FOR COMMENT (24.01.06) All Tobacco Products are Deadly and Addictive: Product Diversity Challenges Regulation and Communication Jack E. Henningfield, PhD Conference of the Parties Technical Briefing
DRAFT FOR COMMENT (24.01.06) All Tobacco Products are Deadly and Addictive: Product Diversity Challenges Regulation and Communication Jack E. Henningfield, PhD Conference of the Parties Technical Briefing
City and County of San Francisco Department of Public Health
 City and County of San Francisco Department of Public Health Population Health and Prevention TOBACCO FREE PROJECT Community Health Education Section Community Health Promotion & Prevention Branch November
City and County of San Francisco Department of Public Health Population Health and Prevention TOBACCO FREE PROJECT Community Health Education Section Community Health Promotion & Prevention Branch November
Discussion points on Bill S-5
 Disclaimer: The following document was developed to provide a brief set of discussion points for individuals wishing to discuss Bill S-5 with policy makers, politicians, senators or their advisers. It
Disclaimer: The following document was developed to provide a brief set of discussion points for individuals wishing to discuss Bill S-5 with policy makers, politicians, senators or their advisers. It
Name Organization Committees. Topic Discussion Action/Follow-up
 Mission: To protect the residents of Wichita/Sedgwick County from the adverse effects of tobacco use. Date/Time Location Type of Meeting January 28, 2019 11:00 a.m. 12:00 p.m. Kansas Food Bank (1919 E.
Mission: To protect the residents of Wichita/Sedgwick County from the adverse effects of tobacco use. Date/Time Location Type of Meeting January 28, 2019 11:00 a.m. 12:00 p.m. Kansas Food Bank (1919 E.
Population impacts of snus tobacco initiation and cessation
 Population impacts of snus tobacco initiation and cessation Karl Lund, Ph.d Research Director, Tobacco Polarcircle Norway Registered sales of tobacco products in Norway 1909-2014 World war II Cigarettes
Population impacts of snus tobacco initiation and cessation Karl Lund, Ph.d Research Director, Tobacco Polarcircle Norway Registered sales of tobacco products in Norway 1909-2014 World war II Cigarettes
Tobacco Use among Year Old Students in the Philippines, Authors. Nathan R. Jones CDC Office on Smoking and Health
 Tobacco Use among 13-15 Year Old Students in the Philippines, 2000-2003 Authors Nathan R. Jones CDC Office on Smoking and Health Marina Miguel-Baquilod Ministry of Health - Philippines Burke Fishburn WHO
Tobacco Use among 13-15 Year Old Students in the Philippines, 2000-2003 Authors Nathan R. Jones CDC Office on Smoking and Health Marina Miguel-Baquilod Ministry of Health - Philippines Burke Fishburn WHO
Tobacco use among african-americans in Minnesota :
 As the leading cause of preventable death and disease in the United States,1 tobacco use poses a serious health threat. The impact of smoking within African-American communities is even more devastating
As the leading cause of preventable death and disease in the United States,1 tobacco use poses a serious health threat. The impact of smoking within African-American communities is even more devastating
The Modified Risk Products. or Protectionism?
 The Modified Risk Products Provisions of the FDA Tobacco Act: Science Based Policy or Protectionism? Michael Siegel, MD, MPH Professor Boston University School of Public Health Who Am I? Testified in more
The Modified Risk Products Provisions of the FDA Tobacco Act: Science Based Policy or Protectionism? Michael Siegel, MD, MPH Professor Boston University School of Public Health Who Am I? Testified in more
Document Number: HR Title: Tobacco-Free Campus Approved: 07/03/2014 Effective Date: 08/01/2014 Revised Dated:
 Document Number: HR 6.024 Title: Tobacco-Free Campus Approved: 07/03/2014 Effective Date: 08/01/2014 Revised Dated: Tobacco-Free Campus Policy Policy Statement Pursuant to Act 211 (also known as Senate
Document Number: HR 6.024 Title: Tobacco-Free Campus Approved: 07/03/2014 Effective Date: 08/01/2014 Revised Dated: Tobacco-Free Campus Policy Policy Statement Pursuant to Act 211 (also known as Senate
Regulatory Priorities
 Lawrence Deyton, M.S.P.H., M.D. Director, Center for Tobacco Products U.S. Food and Drug Administration 9200 Corporate Boulevard Rockville, MD 20850 Dear Dr. Deyton: On behalf of our 157,000 member dentists,
Lawrence Deyton, M.S.P.H., M.D. Director, Center for Tobacco Products U.S. Food and Drug Administration 9200 Corporate Boulevard Rockville, MD 20850 Dear Dr. Deyton: On behalf of our 157,000 member dentists,
Welcome Superheros! YOUR. Winter Awareness Workshop 2017 BE A HERO IN COMMUNITY!
 Welcome Superheros! BE A HERO IN YOUR COMMUNITY! Winter Awareness Workshop 2017 WHAT ARE E-CIGARETTES /VAPING DEVICES? Devices that allow users to inhale aerosol containing nicotine or other substances.
Welcome Superheros! BE A HERO IN YOUR COMMUNITY! Winter Awareness Workshop 2017 WHAT ARE E-CIGARETTES /VAPING DEVICES? Devices that allow users to inhale aerosol containing nicotine or other substances.
VAPING WHAT YOU NEED TO KNOW. Presented By: THE WINCHESTER COALITION FOR A SAFER COMMUNITY
 VAPING WHAT YOU NEED TO KNOW. Presented By: THE WINCHESTER COALITION FOR A SAFER COMMUNITY WHAT ARE WE TALKING ABOUT? Vapes come in all sizes. They can be filled with nicotine based juice, flavored glycerine
VAPING WHAT YOU NEED TO KNOW. Presented By: THE WINCHESTER COALITION FOR A SAFER COMMUNITY WHAT ARE WE TALKING ABOUT? Vapes come in all sizes. They can be filled with nicotine based juice, flavored glycerine
Mr José Manuel Barroso President of the European Commission Rue de la Loi 200 B-1049 Brussels. Courtesy translation
 Mr José Manuel Barroso President of the European Commission Rue de la Loi 200 B-1049 Brussels Courtesy translation Parliament of Denmark International Secretariat Christiansborg DK-1240 Copenhagen K Phone:
Mr José Manuel Barroso President of the European Commission Rue de la Loi 200 B-1049 Brussels Courtesy translation Parliament of Denmark International Secretariat Christiansborg DK-1240 Copenhagen K Phone:
Tobacco 21 in Oregon 7,000. Leading Causes of Preventable Death in Oregon. Most addiction to tobacco starts in adolescence.
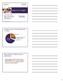 Tobacco 21 in Oregon Luci Longoria, MPH Health Promotion Manager Health Promotion and Chronic Disease Prevention Oregon Health Authority www.nwcphp.org/hot-topics Leading Causes of Preventable Death in
Tobacco 21 in Oregon Luci Longoria, MPH Health Promotion Manager Health Promotion and Chronic Disease Prevention Oregon Health Authority www.nwcphp.org/hot-topics Leading Causes of Preventable Death in
E-Cigs, Etc.: Policy Options for Regulating Nicotine Delivery Devices. Indiana Local Boards of Health Webinar Feb. 12, 2015
 E-Cigs, Etc.: Policy Options for Regulating Nicotine Delivery Devices Indiana Local Boards of Health Webinar Feb. 12, 2015 How to Use Webex If you can hear us through your computer, you do not need to
E-Cigs, Etc.: Policy Options for Regulating Nicotine Delivery Devices Indiana Local Boards of Health Webinar Feb. 12, 2015 How to Use Webex If you can hear us through your computer, you do not need to
