Mail Service Pharmacy
|
|
|
- Lester Chase
- 5 years ago
- Views:
Transcription
1 Mail Service s At A Glance Disclaimer: reserves the right to update its measures and measure sets to maintain measure relevancy and to remedy any unintended consequences that may arise during implementation. Further, may add and/or align its measures with regulatory requirements of federal, state, and local governments. Updated: 11/01/ Page 1 of 12
2 Mandatory s (15) Mail Service Note: Mandatory measures are those measures that are a requirement of accreditation and must be reported to on an annual basis. # Name Description Numerator Denominator Source DM Proportion of Days Covered (PDC) ; PH Adherence to Long-Acting Inhaled Bronchodilator Agents in COPD Patients The percentage of patients 18 years and older who met the proportion of days covered (PDC) threshold of 80% during the A performance rate is calculated separately for the following medication categories: Betablockers (BB); Renin Angiotensin System Antagonists (RASA); Calcium Channel Blockers (CCB); Diabetes All Class (DR); Statins (STA); Anti-retrovirals (this measure has a threshold of 90% for at least 2 medications - ARV). The percentage of patients with COPD who met the Proportion of Days Covered (PDC) threshold of 80 percent during the period for longacting inhaled bronchodilator agents. A higher rate indicates better performance. Number of patients who met the PDC threshold for a target medication during the Number of patients who met the PDC threshold for a target medication during the Patients 18 years and older as of the last day of the year who filled at least two prescriptions for a target medication on two unique dates of service during the treatment Patients 18 years and older with a diagnosis of COPD and at least 2 prescription claims for any long-acting inhaled bronchodilator on unique dates of service during the treatment ; Disclaimer: reserves the right to update its measures and measure sets to maintain measure relevancy and to remedy any unintended consequences that may arise during implementation. Further, may add and/or align its measures with regulatory requirements of federal, state, and local governments. Updated: 11/01/ Page 2 of 12
3 Mail Service # Name PH Adherence to Non-Infused Biologic Agents to Treat Rheumatoid Arthritis (RA) PH Adherence to Non-Infused Disease- Modifying Agents Used to Treat Multiple Sclerosis (MS) Description Numerator Denominator Source The percentage of patients with 18 years and older with rheumatoid arthritis (RA) who met the Proportion of Days Covered (PDC) threshold of 80 percent during the period for biologic medications used to treat RA. A higher rate indicates better performance. The percentage of patients with 18 years and older who met the Proportion of Days Covered (PDC) threshold of 80 percent during the period for disease-modifying agents used to treat multiple sclerosis (MS). A higher rate indicates better performance. Number of patients who met the PDC threshold for a target medication during the Number of patients who met the PDC threshold for a target medication during the Patients 18 years and older with a rheumatoid arthritis (RA) diagnosis during the year AND two or more prescription claims for non-infused biologic medications used to treat RA on two unique dates of service, for which the sum of the days supply is 56 days or greater during the treatment Patients who filled at least two prescriptions for non-infused disease modifying agents used to treat MS on two unique dates of service in the treatment period AND who received at least 56 cumulative day s supply of the medication during the treatment ; ; Disclaimer: reserves the right to update its measures and measure sets to maintain measure relevancy and to remedy any unintended consequences that may arise during implementation. Further, may add and/or align its measures with regulatory requirements of federal, state, and local governments. Updated: 11/01/ Page 3 of 12
4 Mail Service # DTM Name Adherence to Non-Warfarin Oral Anticoagulants Description Numerator Denominator Source percentage of patients 18 years and older who met the Proportion of Days Covered (PDC) threshold of 80% during the period for non-warfarin oral anticoagulants. A higher rate indicates better performance. The number of patients who met the PDC threshold during the Patients who filled at least two prescriptions for a nonwarfarin oral anticoagulant on two unique dates of service at least 180 days apart during the treatment period AND who received greater than 60 days supply of the medication during the treatment ; DM Drug-Drug Interactions Safe Care percentage of patients who received a prescription for a target medication during the period and who were dispensed a concurrent prescription for a precipitant medication. The number of patients in the denominator who were dispensed a concurrent precipitant medication during the Patients who received a target medication. Claims; MP Generic Dispensing Rates Health Care Mgmt percentage of all prescriptions that were dispensed as generics, branded generics, or brands for which members paid the generic co-pay. Total number of prescriptions in the denominator as dispensed as generics. Total number of prescription claims available in generic form (i.e., multi-source) that were dispensed during the Disclaimer: reserves the right to update its measures and measure sets to maintain measure relevancy and to remedy any unintended consequences that may arise during implementation. Further, may add and/or align its measures with regulatory requirements of federal, state, and local governments. Updated: 11/01/ Page 4 of 12
5 Mail Service # DTM Name Call Center Performance Health Care Mgmt Description Numerator Denominator Source This measure has two parts: Part A evaluates the percentage of calls during normal business hours to the organization s call service center(s) during the period that were answered by a live voice within 30 seconds; Part B evaluates the percentage of calls made during normal business hours to the organization s call service center(s) during the reporting year that were abandoned by callers before being answered by a live customer service representative. Part A: The number of calls answered by a live customer service representative within 30 seconds of being placed in the organization's ACD call queue. Part B: The number of calls abandoned by callers after being placed in the ACD call queue and before being answered by a live customer service representative. Total number of calls received by the organization's call service center during normal business hours during the Automatic Call Distribution (ACD) [REMAINDER OF PAGE INTENTIONALLY LEFT BLANK] Disclaimer: reserves the right to update its measures and measure sets to maintain measure relevancy and to remedy any unintended consequences that may arise during implementation. Further, may add and/or align its measures with regulatory requirements of federal, state, and local governments. Updated: 11/01/ Page 5 of 12
6 # MP MP Name Dispensing Accuracy Distribution Accuracy Mail Service Description Numerator Denominator Source Safe Care This six-part measure and Part A: The number of drugs and Total number of composite roll-up assesses the products in the denominator in which drugs and percentage of prescriptions an incorrect drug and/or product was products that the organization dispensed. dispensed by the dispensed inaccurately. Part B: The number of drugs and organization to or products in the denominator that were on behalf of a parts include: (A) dispensed to an incorrect recipient. specific patient Incorrect Drug and/or Product Part C: The number of prescriptions in during the Dispensed; (B) Incorrect the denominator that were dispensed Recipient; (C) Incorrect at an incorrect strength. Strength; (D) Incorrect Dosage Part D: The number of prescriptions in Form; (E) Incorrect the denominator that were dispensed Instructions; (F) Incorrect in an incorrect dosage form. Quantity. Part E: The number of drugs and products in the denominator that were dispensed with incorrect patient instruction. Part F: The number of drugs and products in the denominator that were dispensed as an incorrect quantity. Roll up Methodology: Sum numerator from Parts A - F. Safe Care percentage of prescriptions delivered to the wrong recipient. Part A measures the percentage of prescriptions mailed with an incorrect address; Part B measures the percentage of prescriptions mailed with a correct address that were not delivered to the correct location. Part A: The number of drugs and products dispensed with an incorrect address. Part B: The number of drugs and products delivered to the wrong location despite having the correct address on the package. Total number of drugs and products dispensed by the organization to or on behalf of a specific patient during the ; Disclaimer: reserves the right to update its measures and measure sets to maintain measure relevancy and to remedy any unintended consequences that may arise during implementation. Further, may add and/or align its measures with regulatory requirements of federal, state, and local governments. Updated: 11/01/ Page 6 of 12
7 Mail Service # MP Name Turnaround Time for Prescriptions Health Care Mgmt Description Numerator Denominator Source This 3-part measure assesses the average speed with which the organization fills new and refill prescriptions. Part A measures prescription turnaround time for new and refill clean prescriptions; Part B measures prescription turnaround time for new and refill prescriptions that required intervention; and Part C measures prescription turnaround time for all new and refill prescriptions. Part A, B, C: The sum of business days to fill new and refill prescriptions in the denominator (n1 + n2 + nx, where n1 = the number of business days to schedule delivery of prescription 1, n2 = the number of business days to schedule delivery of prescription 2 nx = the number of business days to schedule delivery of prescription x). Part A: Total number of drugs and products that arrived clean and that the organization filled during the Part B: Total number of prescriptions that initially required intervention and that the organization filled during the Part C: Total number of prescriptions the organization filled during the Disclaimer: reserves the right to update its measures and measure sets to maintain measure relevancy and to remedy any unintended consequences that may arise during implementation. Further, may add and/or align its measures with regulatory requirements of federal, state, and local governments. Updated: 11/01/ Page 7 of 12
8 # Name PH Concurrent Use of Opioids & Benzodiazepines Patient Safety Mail Service Description Numerator Denominator Source The percentage of individuals 18 years and older with concurrent use of prescription opioids and benzodiazepines. A lower rate indicates better performance. The number of individuals from the denominator with two (2) or more prescription claims for any benzodiazepines on unique dates of service, AND concurrent use of opioids and benzodiazepines for 30 or more cumulative days. Individuals with 2 or more prescription claims for opioids on unique dates of service, for which the sum of the days supply is 15 or more days supply during the ; ; RxHCCs PH Polypharmacy: Use of Multiple Anticholinergic Medications in Older Adults Patient Safety The percentage of adults 65 years and older with concurrent use of 2 or more unique anticholinergic medications. A lower rate indicates better performance. The number of individuals from the denominator with concurrent use for 30 or more cumulative days of 2 or more unique anticholinergic medications, each with 2 or more prescription claims on unique dates of service during the Individuals with 2 or more prescription claims for the same anticholinergic medication on unique dates of service during the ; Disclaimer: reserves the right to update its measures and measure sets to maintain measure relevancy and to remedy any unintended consequences that may arise during implementation. Further, may add and/or align its measures with regulatory requirements of federal, state, and local governments. Updated: 11/01/ Page 8 of 12
9 Mail Service # Name Description Numerator Denominator Source PH Polypharmacy: Use of Multiple CNS-Active Medications in Older Adults Patient Safety The percentage of adults 65 years and older with concurrent use of 3 or more unique central-nervous system (CNS) active medications. A lower rate indicates better performance. The number of individuals from the denominator with concurrent use for 30 or more cumulative days of 3 or more unique CNS-active medications, each with 2 or more prescription claims on unique dates of service during the Individuals with 2 or more prescription claims for the same CNS-active medication on unique dates of service during the ; Disclaimer: reserves the right to update its measures and measure sets to maintain measure relevancy and to remedy any unintended consequences that may arise during implementation. Further, may add and/or align its measures with regulatory requirements of federal, state, and local governments. Updated: 11/01/ Page 9 of 12
10 Mail Service # Name Description Numerator Denominator Source PH Use of Opioids at High Dosage or from Multiple Providers in Persons Without Cancer Patient Safety Three (3) measures that examine high dosage opioid use from multiple providers, among individuals 18 years and older without cancer. 1: Use of Opioid at High Dosage in Persons Without Cancer (OHD): The proportion (XX out of 1,000) of individuals from the denominator receiving prescriptions for opioids with a daily dosage greater than 120 morphine milligram equivalents (MME) for 90 consecutive days or longer. 2: Use of Opioids from Multiple Providers in Persons Without Cancer (OMP): The proportion (XX out of 1,000) of individuals from the denominator receiving prescriptions for opioids from four (4) or more prescribers AND four (4) or more pharmacies. 3: Use of Opioids at High Dosage and from Multiple Providers in Persons Without Cancer (OHDMP): The proportion (XX out of 1,000) of individuals from the denominator receiving prescriptions for opioids with a daily dosage greater than 120 morphine milligram equivalents (MME) for 90 consecutive days or longer, AND who received opioid prescriptions from four (4) or more prescribers AND four (4) or more pharmacies. 1: The number of individuals from the denominator with greater than 120 MME for 90 or more consecutive days. 2: Any member in the denominator who received opioids from 4 or more prescribers AND 4 or more pharmacies. 3: Any member in the denominator with greater than 120 MME for 90 days or more consecutive days AND who received opioids from 4 or more prescribers AND 4 or more pharmacies. 1, 2, & 3: Individuals with 2 or more prescription claims for opioids on unique dates of service, for which the sum of the days supply is 15 or more days supply during the Medical Claims; ; RxHCCs; ICD-10s Disclaimer: reserves the right to update its measures and measure sets to maintain measure relevancy and to remedy any unintended consequences that may arise during implementation. Further, may add and/or align its measures with regulatory requirements of federal, state, and local governments. Updated: 11/01/ Page 10 of 12
11 Mail Service Exploratory s (6) Note: Exploratory measures are measures on the cutting edge, meaning that either the industry has not come to consensus on how to measure a particular concept or the measure is experimental or in development. In the case of exploratory measure, the organization has the option to report. # Name SP Fulfillment of Promise to Deliver Health Care Mgmt Description Numerator Denominator Source percentage of prescriptions that the organization delivered on time (i.e., the percentage of prescriptions that reached patients on the date scheduled for delivery). Number of prescriptions in the denominator that patients received on the date scheduled for delivery. Total number of prescriptions that the organization filled during the Administrative ; HIM PH Use of High-Risk Medications in the Elderly (HRM) Primary Medication Non-Adherence Safe Care percentage of patients 65 years of age and older who received two or more prescription fills for a high-risk medication during the Stratify by Commercial, Medicaid, and Medicare (i.e., report each product line separately). The percentage of prescriptions for chronic medications e- prescribed by a prescriber and not obtained by the patient in the following 30 days. This rate measures the level of primary medication non-adherence across a population of patients. The number of patients who received at least two prescription fills on different dates of service for the same high-risk medication during the The number of e- prescribing transactions in the denominator where there was no pharmacy dispensing event that matched the patient and the prescribed drug or appropriate alternative drug within 30 days following the e-prescribing event. Members 66 years or older on the last day of the year and continuously enrolled during the The number of e- prescriptions for newly initiated drug therapy for medications in Table A: Chronic Medications for PMN during the period and for the eligible population. Claims; Prescription Claims ; e- Prescribing Disclaimer: reserves the right to update its measures and measure sets to maintain measure relevancy and to remedy any unintended consequences that may arise during implementation. Further, may add and/or align its measures with regulatory requirements of federal, state, and local governments. Updated: 11/01/ Page 11 of 12
12 Mail Service # Name PH Use of Benzodiazepine Sedative Hypnotic Medications in the Elderly Safe Care Description Numerator Denominator Source percentage of individuals 65 years of age and older that received two or more prescription fills for any benzodiazepine sedative hypnotic for a cumulative period of more than 90 days. Individuals in the denominator who received two prescriptions for any benzodiazepine sedativehypnotic medication and a cumulative days supply of greater than 90 days for any benzodiazepine sedative hypnotic during the Members 65 years or older on the first day of the Claims; PH Statin Use in Persons with Diabetes Prevention & Treatment percentage of patients ages years who were dispensed a medication for diabetes that receive a statin medication. Stratify by Commercial, Medicaid, and Medicare (i.e., report each product line separately). The number of eligible patients who received one or more prescription fills for a statin during the The number of eligible patients who were dispensed two or more prescription fills for a diabetic medication during the Claims; PH Consumer Experience with Services Survey s: Staff Communication, Information about Medicine, Written Information, New Prescriptions, and About You. N/A N/A PQA Survey Disclaimer: reserves the right to update its measures and measure sets to maintain measure relevancy and to remedy any unintended consequences that may arise during implementation. Further, may add and/or align its measures with regulatory requirements of federal, state, and local governments. Updated: 11/01/ Page 12 of 12
Drug Therapy Management
 Measures At A Glance Updated: 11/01/2018 2018 Page 1 of 9 Mandatory Measures (10) Note: Mandatory measures are those measures that are a requirement of accreditation and must be reported to on an annual
Measures At A Glance Updated: 11/01/2018 2018 Page 1 of 9 Mandatory Measures (10) Note: Mandatory measures are those measures that are a requirement of accreditation and must be reported to on an annual
Note: Mandatory measures are those measures that are a requirement of accreditation and must be reported to URAC on an annual basis.
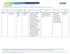 Note: Mandatory measures are those measures that are a requirement of accreditation and must be reported to URAC on an annual basis. DESCRIPTION NUMERATOR DENOMINATOR DM2012-12 Portion of Days Covered
Note: Mandatory measures are those measures that are a requirement of accreditation and must be reported to URAC on an annual basis. DESCRIPTION NUMERATOR DENOMINATOR DM2012-12 Portion of Days Covered
Pharmacy Benefit Management
 Benefit Management Measures At A Glance Updated: 10/8/2015 Page 1 of 7 Benefit Management Mandatory Measures (6) Note: Mandatory measures are those measures that are a requirement of accreditation and
Benefit Management Measures At A Glance Updated: 10/8/2015 Page 1 of 7 Benefit Management Mandatory Measures (6) Note: Mandatory measures are those measures that are a requirement of accreditation and
Note: Mandatory measures are those measures that are a requirement of accreditation and must be reported to URAC on an annual basis.
 COMMUNITY PHARMACY V1.1 MANDATORY S Note: Mandatory measures are those measures that are a requirement of accreditation and must be reported to URAC on an annual basis. # NAME DESCRIPTION NUMERATOR DENOMINATOR
COMMUNITY PHARMACY V1.1 MANDATORY S Note: Mandatory measures are those measures that are a requirement of accreditation and must be reported to URAC on an annual basis. # NAME DESCRIPTION NUMERATOR DENOMINATOR
MEASURE STEWARD Pharmacy Quality Alliance (PQA) D ATA SOURCE Enrollment; U R A C DOMAIN Engagement & Experience of Care
 MANDATORY S Note: Mandatory measures are those measures that are a requirement of accreditation and must be reported to URAC on an annual basis. DM2012-12 DTM2015-01 Portion of Days Covered (PDC) Adherence
MANDATORY S Note: Mandatory measures are those measures that are a requirement of accreditation and must be reported to URAC on an annual basis. DM2012-12 DTM2015-01 Portion of Days Covered (PDC) Adherence
Disease Management. Measures At A Glance
 s At A Glance Updated: 11/2/2017 Page 1 of 7 Cross Cutting Mandatory s (4) Note: Mandatory measures are those measures that are a requirement of accreditation and must be reported to URAC on an annual
s At A Glance Updated: 11/2/2017 Page 1 of 7 Cross Cutting Mandatory s (4) Note: Mandatory measures are those measures that are a requirement of accreditation and must be reported to URAC on an annual
2017 URAC PHARMACY BENEFIT MANAGEMENT PERFORMANCE MEASUREMENT: AGGREGATE SUMMARY PERFORMANCE REPORT
 2017 URAC PHARMACY BENEFIT MANAGEMENT PERFORMANCE MEASUREMENT: AGGREGATE SUMMARY PERFORMANCE REPORT December 2017 Table of Contents Executive Summary... 1 Pharmacy Benefit Management Organization Characteristics...
2017 URAC PHARMACY BENEFIT MANAGEMENT PERFORMANCE MEASUREMENT: AGGREGATE SUMMARY PERFORMANCE REPORT December 2017 Table of Contents Executive Summary... 1 Pharmacy Benefit Management Organization Characteristics...
Disease Management. Measures At A Glance
 s At A Glance Updated: 11/01/2018 URAC 2018 Page 1 of 7 Cross-Cutting Mandatory s (3) Note: Mandatory measures are those measures that are a requirement of accreditation and must be reported to URAC on
s At A Glance Updated: 11/01/2018 URAC 2018 Page 1 of 7 Cross-Cutting Mandatory s (3) Note: Mandatory measures are those measures that are a requirement of accreditation and must be reported to URAC on
PQS Summary of Pharmacy/ Medication-Related Updates in the CY 2020 Final Call Letter
 Managing Performance Information in a Quality Driven World PQS Summary of Pharmacy/ Medication-Related Updates in the CY 2020 Final Call Letter REGULATORY UPDATE PQS Summary of Pharmacy/ Medication-Related
Managing Performance Information in a Quality Driven World PQS Summary of Pharmacy/ Medication-Related Updates in the CY 2020 Final Call Letter REGULATORY UPDATE PQS Summary of Pharmacy/ Medication-Related
PATIENT-IMPACT SCORECARD
 UNDERSTANDING THE GSC PATIENT-IMPACT SCORECARD What is the GSC Patient-Impact Scorecard? The GSC Patient-Impact Scorecard shows pharmacy performance scores for GSC patients during the period indicated.
UNDERSTANDING THE GSC PATIENT-IMPACT SCORECARD What is the GSC Patient-Impact Scorecard? The GSC Patient-Impact Scorecard shows pharmacy performance scores for GSC patients during the period indicated.
PQA Measure Development Update: June 2017
 PQA Measure Development Update: June 2017 The Measure Development Teams and Task Forces have been meeting monthly via webinar, and the Stakeholder Advisory Panels had their first meetings in March. Additionally,
PQA Measure Development Update: June 2017 The Measure Development Teams and Task Forces have been meeting monthly via webinar, and the Stakeholder Advisory Panels had their first meetings in March. Additionally,
Understanding Your Patient Care Opportunity Report (PCOR)
 Understanding Your Patient Care Opportunity Report (PCOR) Use your January/February 208 PCOR to help improve performance on Medicare Part D Clinical Star Ratings measures. Your January/February 208 Patient
Understanding Your Patient Care Opportunity Report (PCOR) Use your January/February 208 PCOR to help improve performance on Medicare Part D Clinical Star Ratings measures. Your January/February 208 Patient
Proposed Changes to Existing Measure for HEDIS : Use of Opioids at High Dosage (UOD)
 Proposed Changes to Existing Measure for HEDIS 1 2020: Use of Opioids at High Dosage (UOD) NCQA seeks comments on proposed revisions to the Use of Opioids at High Dosage HEDIS measure. The current measure
Proposed Changes to Existing Measure for HEDIS 1 2020: Use of Opioids at High Dosage (UOD) NCQA seeks comments on proposed revisions to the Use of Opioids at High Dosage HEDIS measure. The current measure
9/25/15. Pharmacy Quality Measures: Financial Support. Learning Objectives. Speaker Disclosure. Access to Preferred Networks and Clinical Performance
 Pharmacy Quality Measures: Action Steps for Improvement Financial Support Financial support was provided for this activity through an unrestricted grant from Health Mart Systems, Inc. Christine Jacobson
Pharmacy Quality Measures: Action Steps for Improvement Financial Support Financial support was provided for this activity through an unrestricted grant from Health Mart Systems, Inc. Christine Jacobson
Telligen Quality Innovation Network Quality Improvement Organization
 Telligen Quality Innovation Network Quality Improvement Organization PQA Patient Safety Measures for Inappropriate Prescription Opioid Use August 29, 2017 Optimizing Patients' Health by Improving the Quality
Telligen Quality Innovation Network Quality Improvement Organization PQA Patient Safety Measures for Inappropriate Prescription Opioid Use August 29, 2017 Optimizing Patients' Health by Improving the Quality
Legislative & Regulatory Update Brad Young, RxPlus Government Affairs
 Legislative & Regulatory Update 2018 Brad Young, RxPlus Government Affairs Disclosures Brad Young reports no actual or potential conflicts of interest associated with this presentation. 2 Learning Objectives
Legislative & Regulatory Update 2018 Brad Young, RxPlus Government Affairs Disclosures Brad Young reports no actual or potential conflicts of interest associated with this presentation. 2 Learning Objectives
Coventry Health Care of Georgia, Inc.
 Coventry Health Care of Georgia, Inc. PRESCRIPTION DRUG RIDER (for High Deductible Health Plans) This Prescription Drug Rider is an attachment to the Coventry Health Care of Georgia, Inc. ( Health Plan
Coventry Health Care of Georgia, Inc. PRESCRIPTION DRUG RIDER (for High Deductible Health Plans) This Prescription Drug Rider is an attachment to the Coventry Health Care of Georgia, Inc. ( Health Plan
NM DRUG OVERDOSE PREVENTION QUARTERLY MEASURES REPORT THIRD QUARTER OF 2018 (2018Q3)
 NM DRUG OVERDOSE PREVENTION QUARTERLY MEASURES REPORT THIRD QUARTER OF 218 () Substance Abuse Epidemiology Section Prescription Drug Overdose Prevention Program Injury and Behavioral Epidemiology Bureau
NM DRUG OVERDOSE PREVENTION QUARTERLY MEASURES REPORT THIRD QUARTER OF 218 () Substance Abuse Epidemiology Section Prescription Drug Overdose Prevention Program Injury and Behavioral Epidemiology Bureau
Frequently Asked Questions (FAQ) IHA Align. Measure. Perform. (AMP) Programs January Audit Audit Roadmap Posted 1/23/19
 Frequently Asked Questions (FAQ) IHA Align. Measure. Perform. (AMP) Programs January 2019 Audit Audit Roadmap Question: For the AMP MY 2018 Roadmap, is the last part of question 5.2W in Section 5, Describe
Frequently Asked Questions (FAQ) IHA Align. Measure. Perform. (AMP) Programs January 2019 Audit Audit Roadmap Question: For the AMP MY 2018 Roadmap, is the last part of question 5.2W in Section 5, Describe
Medicare Part D Prescription Opioid Policies for 2019 Information for Pharmacists
 CENTERS FOR MEDICARE & MEDICAID SERVICES Medicare Part D Prescription Opioid Policies for 2019 Information for Pharmacists Background Opioid medications are effective at treating certain types of pain,
CENTERS FOR MEDICARE & MEDICAID SERVICES Medicare Part D Prescription Opioid Policies for 2019 Information for Pharmacists Background Opioid medications are effective at treating certain types of pain,
Alabama Medicaid Pharmacy Override
 Alabama Medicaid Pharmacy Override Therapeutic Duplication, Early Refill, Maximum Unit, Prescription Limit Switchover, Dispense as Written, Accumulation Edit, Maintenance Supply Opt Out, and Maximum Cost
Alabama Medicaid Pharmacy Override Therapeutic Duplication, Early Refill, Maximum Unit, Prescription Limit Switchover, Dispense as Written, Accumulation Edit, Maintenance Supply Opt Out, and Maximum Cost
Drug Use Evaluation: Low Dose Quetiapine
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
STARS SYSTEM 5 CATEGORIES
 TMG STARS 2018 1 2 STARS Program Implemented in 2008 by CMS. Tool to inform beneficiaries of quality of various health plans 5-star rating system Used to adjust payments to health plans (bonus to plans
TMG STARS 2018 1 2 STARS Program Implemented in 2008 by CMS. Tool to inform beneficiaries of quality of various health plans 5-star rating system Used to adjust payments to health plans (bonus to plans
April 26, New Mexico Board of Pharmacy Prescription Monitoring Program (PMP) New Mexico Board of Pharmacy Prescription Monitoring Program (PMP)
 New Mexico Board of Pharmacy Prescription Monitoring Program (PMP) New Mexico Nurse Practitioner Council New Mexico Board of Pharmacy Prescription Monitoring Program (PMP) Peter Ryba, PharmD PMP Director
New Mexico Board of Pharmacy Prescription Monitoring Program (PMP) New Mexico Nurse Practitioner Council New Mexico Board of Pharmacy Prescription Monitoring Program (PMP) Peter Ryba, PharmD PMP Director
Medication trends shaping workers compensation. A 2018 update of the prevailing industry influences impacting pharmacy outcomes
 Medication trends shaping workers compensation A 2018 update of the prevailing industry influences impacting pharmacy outcomes This page intentionally left blank Trends impacting workers compensation pharmacy
Medication trends shaping workers compensation A 2018 update of the prevailing industry influences impacting pharmacy outcomes This page intentionally left blank Trends impacting workers compensation pharmacy
Background. CMS finalized new policies for Medicare drug plans to follow starting on January 1,2019.
 Background CMS finalized new policies for Medicare drug plans to follow starting on January 1,2019. These policies involve further partnershipwith providers and prescription drug plans. Providers are in
Background CMS finalized new policies for Medicare drug plans to follow starting on January 1,2019. These policies involve further partnershipwith providers and prescription drug plans. Providers are in
USING MTM TO IMPROVE STAR RATINGS : CASE STUDIES
 USING MTM TO IMPROVE STAR RATINGS : CASE STUDIES Sept 13, 2015 Amanda Applegate, PharmD, BCACP Disclosures Nothing to disclose Learning Objectives Describe how MTM can improve star ratings Discuss strategies
USING MTM TO IMPROVE STAR RATINGS : CASE STUDIES Sept 13, 2015 Amanda Applegate, PharmD, BCACP Disclosures Nothing to disclose Learning Objectives Describe how MTM can improve star ratings Discuss strategies
PDMP Tools to Identify Red Flag Situations
 PDMP Tools to Identify Red Flag Situations Prescription Drug Monitoring Programs CBI Baltimore, MD February 7, 2017 Grant Carrow, Ph.D. Project Consultant The Heller School for Social Policy and Management
PDMP Tools to Identify Red Flag Situations Prescription Drug Monitoring Programs CBI Baltimore, MD February 7, 2017 Grant Carrow, Ph.D. Project Consultant The Heller School for Social Policy and Management
Proposed Revision to Med (i)
 Proposed Revision to Med 501.02 (i) I. Purpose This rule has been adopted to enable the Board to best protect public health and safety while providing a framework for licensees to effectively treat and
Proposed Revision to Med 501.02 (i) I. Purpose This rule has been adopted to enable the Board to best protect public health and safety while providing a framework for licensees to effectively treat and
Idaho DUR Board Meeting Minutes
 Idaho DUR Board Meeting Minutes Date: July 20, 2017 Time: 9am-12:30pm Location: Holiday Inn Boise Airport 2970 West Elder Street, Boise, Idaho, 83705 Moderator: David Agler, M.D. Committee Member Present:
Idaho DUR Board Meeting Minutes Date: July 20, 2017 Time: 9am-12:30pm Location: Holiday Inn Boise Airport 2970 West Elder Street, Boise, Idaho, 83705 Moderator: David Agler, M.D. Committee Member Present:
The STOP Measure. Safe and Transparent Opioid Prescribing to Promote Patient Safety and Reduced Risk of Opioid Misuse FEBRUARY 2018
 The STOP Measure Safe and Transparent Opioid Prescribing to Promote Patient Safety and Reduced Risk of Opioid Misuse FEBRUARY 2018 AHIP s Safe, Transparent Opioid Prescribing (STOP) Initiative Methodology
The STOP Measure Safe and Transparent Opioid Prescribing to Promote Patient Safety and Reduced Risk of Opioid Misuse FEBRUARY 2018 AHIP s Safe, Transparent Opioid Prescribing (STOP) Initiative Methodology
New Medicare Part D Prescription Opioid Policies for 2019 Information for Prescribers
 CENTERS FOR MEDICARE & MEDICAID SERVICES New Medicare Part D Prescription Opioid Policies for 2019 Information for Prescribers Background CMS understands the magnitude of the nation s opioid epidemic and
CENTERS FOR MEDICARE & MEDICAID SERVICES New Medicare Part D Prescription Opioid Policies for 2019 Information for Prescribers Background CMS understands the magnitude of the nation s opioid epidemic and
Supporting Documentation
 Supporting Documentation Technical Appendix for the Oregon Health Care Quality Reporting System Performance Reporting Portal (PRP) Version: Oregon Health Care Quality Corporation; Provider Organization
Supporting Documentation Technical Appendix for the Oregon Health Care Quality Reporting System Performance Reporting Portal (PRP) Version: Oregon Health Care Quality Corporation; Provider Organization
Prescription Drug Monitoring Program
 California Department of Justice Prescription Drug Monitoring Program May, 2016 Health and Safety Code section 11165. (a) To assist health care practitioners in their efforts to ensure appropriate prescribing,
California Department of Justice Prescription Drug Monitoring Program May, 2016 Health and Safety Code section 11165. (a) To assist health care practitioners in their efforts to ensure appropriate prescribing,
NM DRUG OVERDOSE PREVENTION QUARTERLY MEASURES REPORT THIRD QUARTER OF 2017 (2017Q3)
 NM DRUG OVERDOSE PREVENTION QUARTERLY MEASURES REPORT THIRD QUARTER OF 217 () Substance Abuse Epidemiology Section Prescription Drug Overdose Prevention Program Injury and Behavioral Epidemiology Bureau
NM DRUG OVERDOSE PREVENTION QUARTERLY MEASURES REPORT THIRD QUARTER OF 217 () Substance Abuse Epidemiology Section Prescription Drug Overdose Prevention Program Injury and Behavioral Epidemiology Bureau
2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY. MEASURE TYPE: Process
 Quality ID #52 (NQF 0102): Chronic Obstructive Pulmonary Disease (COPD): Long-Acting Inhaled Bronchodilator Therapy National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL
Quality ID #52 (NQF 0102): Chronic Obstructive Pulmonary Disease (COPD): Long-Acting Inhaled Bronchodilator Therapy National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL
HEDIS/QARR 2018 Quick Reference Guide ALL MEASURES
 2018 HEDIS Codes HEDIS/QARR 2018 Quick Reference Guide ALL MEASURES Code Age Band Denominator Event Numerator Requirement ADL AAB AAP ABA ADV Adolescent Preventive Care Avoidance of Antibiotic in Adults
2018 HEDIS Codes HEDIS/QARR 2018 Quick Reference Guide ALL MEASURES Code Age Band Denominator Event Numerator Requirement ADL AAB AAP ABA ADV Adolescent Preventive Care Avoidance of Antibiotic in Adults
Adherence to Non-Infused Biologic Medications Used to Treat Rheumatoid Arthritis (PDC-RA)
 Adherence to Non-Infused Biologic Medications Used to Treat Rheumatoid Arthritis (PDC-RA) Description The percentage of patients 18 years and older with rheumatoid arthritis (RA) who met the Proportion
Adherence to Non-Infused Biologic Medications Used to Treat Rheumatoid Arthritis (PDC-RA) Description The percentage of patients 18 years and older with rheumatoid arthritis (RA) who met the Proportion
SUBHEAD GOES HERE. Addressing Tennessee s Opioid Crisis. Natalie A. Tate, PharmD Vice President, Pharmacy
 SUBHEAD GOES HERE Addressing Tennessee s Opioid Crisis Natalie A. Tate, PharmD Vice President, Pharmacy Our opioids story Our approach Our response to neonatal abstinence syndrome Facts and faces of opioid
SUBHEAD GOES HERE Addressing Tennessee s Opioid Crisis Natalie A. Tate, PharmD Vice President, Pharmacy Our opioids story Our approach Our response to neonatal abstinence syndrome Facts and faces of opioid
PHARMACY BENEFITS MANAGER SELECTION FAQ FOR PRODUCERS
 PHARMACY BENEFITS MANAGER SELECTION FAQ FOR PRODUCERS For Producer Audience Only - Please Do Not Distribute Regence has selected Prime Therapeutics as the Pharmacy benefits manager for its health plans.
PHARMACY BENEFITS MANAGER SELECTION FAQ FOR PRODUCERS For Producer Audience Only - Please Do Not Distribute Regence has selected Prime Therapeutics as the Pharmacy benefits manager for its health plans.
Curbing Prescription Drug Abuse in Medicaid
 Curbing Prescription Drug Abuse in Medicaid Joint Legislative Health Care Oversight Committee October 12, 2010 Dr. Lisa Weeks, BSPharm, PharmD Pharmacy and Ancillary Services Division of Medical Assistance
Curbing Prescription Drug Abuse in Medicaid Joint Legislative Health Care Oversight Committee October 12, 2010 Dr. Lisa Weeks, BSPharm, PharmD Pharmacy and Ancillary Services Division of Medical Assistance
NM DRUG OVERDOSE PREVENTION QUARTERLY MEASURES REPORT FOURTH QUARTER OF 2017 (2017Q4)
 NM DRUG OVERDOSE PREVENTION QUARTERLY MEASURES REPORT FOURTH QUARTER OF 217 () Substance Abuse Epidemiology Section Prescription Drug Overdose Prevention Program Injury and Behavioral Epidemiology Bureau
NM DRUG OVERDOSE PREVENTION QUARTERLY MEASURES REPORT FOURTH QUARTER OF 217 () Substance Abuse Epidemiology Section Prescription Drug Overdose Prevention Program Injury and Behavioral Epidemiology Bureau
MEASURING CARE QUALITY
 MEASURING CARE QUALITY Region November 2016 For Clinical Effectiveness of Care Measures of Performance From: Healthcare Effectiveness Data and Information Set (HEDIS ) HEDIS is a set of standardized performance
MEASURING CARE QUALITY Region November 2016 For Clinical Effectiveness of Care Measures of Performance From: Healthcare Effectiveness Data and Information Set (HEDIS ) HEDIS is a set of standardized performance
Idaho DUR Board Meeting Minutes
 Idaho DUR Board Meeting Minutes Date: January 21, 2016 Time: 9am-1:30pm Location: Idaho Medicaid, 3232 Elder Street, Boise, Idaho, Conference Room D-West Moderator: Mark Turner, M.D. Committee Members
Idaho DUR Board Meeting Minutes Date: January 21, 2016 Time: 9am-1:30pm Location: Idaho Medicaid, 3232 Elder Street, Boise, Idaho, Conference Room D-West Moderator: Mark Turner, M.D. Committee Members
Amy Larrick Chavez-Valdez, Director, Medicare Drug Benefit and C & D Data Group
 DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services 7500 Security Boulevard Baltimore, Maryland 21244-1850 CENTER FOR MEDICARE TO: FROM: SUBJECT: All Part D Sponsors Amy Larrick
DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services 7500 Security Boulevard Baltimore, Maryland 21244-1850 CENTER FOR MEDICARE TO: FROM: SUBJECT: All Part D Sponsors Amy Larrick
General Providers. Tamper-Resistant Prescription Requirements
 September 2008 Provider Bulletin Number 8138 General Providers Tamper-Resistant Prescription Requirements Effective with processing dates on and after October 1, 2008, written prescriptions for Kansas
September 2008 Provider Bulletin Number 8138 General Providers Tamper-Resistant Prescription Requirements Effective with processing dates on and after October 1, 2008, written prescriptions for Kansas
6/18/2015. Disclosure. Objectives. Star Ratings. Understand the current climate of healthcare reform
 Star Ratings John A. Galdo, Pharm.D., BCPS, CGP (Jake) Assistant Professor of Pharmacy Practice Community Practice Residency Director jgaldo@samford.edu Disclosure I do not have (nor does any immediate
Star Ratings John A. Galdo, Pharm.D., BCPS, CGP (Jake) Assistant Professor of Pharmacy Practice Community Practice Residency Director jgaldo@samford.edu Disclosure I do not have (nor does any immediate
Clinical Policy: Opioid Analgesics Reference Number: CP.PMN.97 Effective Date: Last Review Date: 02.19
 Clinical Policy: Reference Number: CP.PMN.97 Effective Date: 02.11 Last Review Date: 02.19 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: Reference Number: CP.PMN.97 Effective Date: 02.11 Last Review Date: 02.19 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important regulatory
New Mexico Board of Pharmacy Prescription Monitoring Program (PMP)
 New Mexico Board of Pharmacy Prescription Monitoring Program (PMP) PDMP West Regional Meeting PMP Collaboration with the NM Department of Health April 26, 2018 New Mexico Board of Pharmacy Prescription
New Mexico Board of Pharmacy Prescription Monitoring Program (PMP) PDMP West Regional Meeting PMP Collaboration with the NM Department of Health April 26, 2018 New Mexico Board of Pharmacy Prescription
Medicare Star Ratings and the Shift to Quality- Based Payment Models. David Nau, RPh, PhD, FAPhA PQS President
 Medicare Star Ratings and the Shift to Quality- Based Payment Models David Nau, RPh, PhD, FAPhA PQS President The shift to Value-Driven Healthcare The U.S. health care system is rapidly moving to value-based
Medicare Star Ratings and the Shift to Quality- Based Payment Models David Nau, RPh, PhD, FAPhA PQS President The shift to Value-Driven Healthcare The U.S. health care system is rapidly moving to value-based
Pain: Assessment, Non-Opioid Treatment Approaches and Opioid Management
 Quality Improvement Support: Pain: Assessment, Non-Opioid Treatment Approaches and Opioid Management The Aims and Measures section is intended to provide protocol users with a menu of measures for multiple
Quality Improvement Support: Pain: Assessment, Non-Opioid Treatment Approaches and Opioid Management The Aims and Measures section is intended to provide protocol users with a menu of measures for multiple
Using the NC Controlled Substances Reporting System to Identify Providers Manifesting Unusual Prescribing Practices
 Using the NC Controlled Substances Reporting System to Identify Providers Manifesting Unusual Prescribing Practices Prevention Research Center Penn State December 2, 2015 Chris Ringwalt, DrPH* Sharon Schiro,
Using the NC Controlled Substances Reporting System to Identify Providers Manifesting Unusual Prescribing Practices Prevention Research Center Penn State December 2, 2015 Chris Ringwalt, DrPH* Sharon Schiro,
Medication Management When Caring for Seniors at Home
 Medication Management When Caring for Seniors at Home White Paper May 24, 2013 2013 Physician s Choice Private Duty http://private-duty.pchhc.com 1 Proper medication management for seniors who live at
Medication Management When Caring for Seniors at Home White Paper May 24, 2013 2013 Physician s Choice Private Duty http://private-duty.pchhc.com 1 Proper medication management for seniors who live at
The Morbidity and Mortality of Kansas Drug Epidemic
 The Morbidity and Mortality of Kansas Drug Epidemic Fan Xiong, MPH Senior Epidemiologist Kansas Board of Pharmacy Kansas Data-Driven Prevention Initiative Program Kansas Department of Health and Environment,
The Morbidity and Mortality of Kansas Drug Epidemic Fan Xiong, MPH Senior Epidemiologist Kansas Board of Pharmacy Kansas Data-Driven Prevention Initiative Program Kansas Department of Health and Environment,
Cynthia B. Jones, Director Department of Medical Assistance Services (DMAS)
 Department of Medical Assistance Services 600 East Broad Street, Suite 1300 Richmond, Virginia 23219 MEDICAID MEMO http://www.dmas.state.va.us TO: All Prescribing Providers, Pharmacists, and Managed Care
Department of Medical Assistance Services 600 East Broad Street, Suite 1300 Richmond, Virginia 23219 MEDICAID MEMO http://www.dmas.state.va.us TO: All Prescribing Providers, Pharmacists, and Managed Care
Medicare-Medicaid Data Integration (MMDI) Use Case: Profiling Potential Opioid Misuse among Dual Eligibles
 Medicare-Medicaid Data Integration (MMDI) Use Case: Profiling Potential Opioid Misuse among Dual Eligibles Contract: HHSN-316-2012-00139W Order: HHSM-500-2014-00114U December 12, 2017 Prepared by: FEi
Medicare-Medicaid Data Integration (MMDI) Use Case: Profiling Potential Opioid Misuse among Dual Eligibles Contract: HHSN-316-2012-00139W Order: HHSM-500-2014-00114U December 12, 2017 Prepared by: FEi
PHARMACY COMPLIANCE RISK AREAS FOR 2014
 PHARMACY COMPLIANCE RISK AREAS FOR 2014 2014 San Juan Puerto Rico Regional Compliance Conference Darrell W. Contreras, Esq., LHRM, CHC-F, CHPC, CHRC 5 Risk Areas 1. Overbilling of Herceptin 2. Quality
PHARMACY COMPLIANCE RISK AREAS FOR 2014 2014 San Juan Puerto Rico Regional Compliance Conference Darrell W. Contreras, Esq., LHRM, CHC-F, CHPC, CHRC 5 Risk Areas 1. Overbilling of Herceptin 2. Quality
Standard of Practice for Prescribing Opioids (Excluding Cancer, Palliative, and End-of-Life Care)
 Standard of Practice for Prescribing Opioids (Excluding Cancer, Palliative, and End-of-Life Care) Preamble This Standard establishes the standards of practice and ethical requirements of all physicians
Standard of Practice for Prescribing Opioids (Excluding Cancer, Palliative, and End-of-Life Care) Preamble This Standard establishes the standards of practice and ethical requirements of all physicians
4/24/15. New Mexico s Prescription Monitoring Program. Carl Flansbaum, RPh. PMP Director New Mexico Board of Pharmacy. New Mexico and the PMP
 New Mexico s Carl Flansbaum, RPh. PMP Director New Mexico Board of Pharmacy New Mexico and the PMP In 2012, New Mexico had the 3 nd Highest Overdose Death Rate in Nation.! 492 Deaths or a rate of 23.6
New Mexico s Carl Flansbaum, RPh. PMP Director New Mexico Board of Pharmacy New Mexico and the PMP In 2012, New Mexico had the 3 nd Highest Overdose Death Rate in Nation.! 492 Deaths or a rate of 23.6
SUMMARY TABLE OF MEASURES, PRODUCT LINES AND CHANGES
 Summary Table of Measures, Product Lines and Changes 1 SUMMARY TABLE OF MEASURES, PRODUCT LINES AND CHANGES General Guidelines for Data Collection and Reporting Guidelines for Calculations and Sampling
Summary Table of Measures, Product Lines and Changes 1 SUMMARY TABLE OF MEASURES, PRODUCT LINES AND CHANGES General Guidelines for Data Collection and Reporting Guidelines for Calculations and Sampling
Clinical Policy: Opioid Analgesics Reference Number: CP.PMN.97 Effective Date: Last Review Date: 02.18
 Clinical Policy: Reference Number: CP.PMN.97 Effective Date: 02.11 Last Review Date: 02.18 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: Reference Number: CP.PMN.97 Effective Date: 02.11 Last Review Date: 02.18 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important regulatory
PBMs: Impact on Cost and Quality of Pharmaceutical Care in the U.S.
 Speaker Brian K. Solow, MD, FAAFP Optum Life Sciences Irvine, CA, USA PBMs: Impact on Cost and Quality of Pharmaceutical Care in the U.S. Brian K. Solow, MD, FAAFP Chief Medical Officer, Optum Life Sciences
Speaker Brian K. Solow, MD, FAAFP Optum Life Sciences Irvine, CA, USA PBMs: Impact on Cost and Quality of Pharmaceutical Care in the U.S. Brian K. Solow, MD, FAAFP Chief Medical Officer, Optum Life Sciences
Pharmacy Audit Recovery Guidelines
 1/1E DAW 1 DAW 1 Error The prescription order does not indicate the prescriber ordered brand name. 2 /2E DAW 2 DAW 2 Not Documented The documentation does not specify the patient s request for brand. Pharmacy
1/1E DAW 1 DAW 1 Error The prescription order does not indicate the prescriber ordered brand name. 2 /2E DAW 2 DAW 2 Not Documented The documentation does not specify the patient s request for brand. Pharmacy
Section I. Short-acting opioid Prior Authorization Criteria
 Request for Prior Authorization for Opioid analgesics Website Form www.highmarkhealthoptions.com Submit request via: Fax - 1-855-476-4158 Requests for opioid analgesics may be subject to prior authorization
Request for Prior Authorization for Opioid analgesics Website Form www.highmarkhealthoptions.com Submit request via: Fax - 1-855-476-4158 Requests for opioid analgesics may be subject to prior authorization
Shining a Light on MEDs Understanding morphine equivalent dose
 Shining a Light on MEDs Understanding morphine equivalent dose In the workers compensation industry, 60.2 percent of claimants utilize opioid analgesics for the treatment of pain caused by a workplace
Shining a Light on MEDs Understanding morphine equivalent dose In the workers compensation industry, 60.2 percent of claimants utilize opioid analgesics for the treatment of pain caused by a workplace
Strengthening our global leadership in treatment of addiction. Morgan Stanley Global Healthcare Conference September 13 th and 14 th 2018
 Strengthening our global leadership in treatment of addiction Morgan Stanley Global Healthcare Conference September 13 th and 14 th 2018 Forward Looking Statements This presentation contains certain statements
Strengthening our global leadership in treatment of addiction Morgan Stanley Global Healthcare Conference September 13 th and 14 th 2018 Forward Looking Statements This presentation contains certain statements
Prescription Monitoring Program (PMP)
 06/15/2018 FACT SHEET Implementation of Enacted Prescribing Limits and Requirements and Relevant Opioid Prescribing Laws and Rules Background: The 2016 law (Chapter 488) makes five major changes to opioid
06/15/2018 FACT SHEET Implementation of Enacted Prescribing Limits and Requirements and Relevant Opioid Prescribing Laws and Rules Background: The 2016 law (Chapter 488) makes five major changes to opioid
Challenges for U.S. Attorneys Offices (USAO) in Opioid Cases
 Challenges for U.S. Attorneys Offices (USAO) in Opioid Cases Overview On August 2, 2017, U.S. Attorney General Jeff Sessions announced a pilot program whereby a new federal data analysis program is being
Challenges for U.S. Attorneys Offices (USAO) in Opioid Cases Overview On August 2, 2017, U.S. Attorney General Jeff Sessions announced a pilot program whereby a new federal data analysis program is being
Report to the Social Services Appropriations Subcommittee
 Report to the Social Services Appropriations Subcommittee Medicaid Coverage and Reimbursement for Outpatient Physical Therapy and Outpatient Occupational Therapy Prepared by the Division of Medicaid and
Report to the Social Services Appropriations Subcommittee Medicaid Coverage and Reimbursement for Outpatient Physical Therapy and Outpatient Occupational Therapy Prepared by the Division of Medicaid and
USE OF OPIODS AT HIGHER DOSES IN PERSONS WITHOUT CANCER: MORPHINE EQUIVALENT DOSE EDIT
 BACKGROUND: USE OF OPIODS AT HIGHER DOSES IN PERSONS WITHOUT CANCER: MORPHINE EQUIVALENT DOSE EDIT Approximately 10% of patients who are prescribed opioids and seek care from multiple doctors, are prescribed
BACKGROUND: USE OF OPIODS AT HIGHER DOSES IN PERSONS WITHOUT CANCER: MORPHINE EQUIVALENT DOSE EDIT Approximately 10% of patients who are prescribed opioids and seek care from multiple doctors, are prescribed
WHAT YOU NEED TO KNOW TO ABOUT AB 474
 WHAT YOU NEED TO KNOW TO ABOUT AB 474 PRESENTED BY: NEVADA STATE BOARD OF OSTEOPATHIC MEDICINE 2275 Corporate Circle, Suite 210 Henderson, NV 89074 702-732-2147 Fax 702-732-2079 Web Site: www.bom.nv.gov
WHAT YOU NEED TO KNOW TO ABOUT AB 474 PRESENTED BY: NEVADA STATE BOARD OF OSTEOPATHIC MEDICINE 2275 Corporate Circle, Suite 210 Henderson, NV 89074 702-732-2147 Fax 702-732-2079 Web Site: www.bom.nv.gov
Lindsay Erickson, Director, Value Based P4P, Integrated Healthcare Association. Measurement Years 2016 & 2017 Proposed Changes to the VBP4P Program
 To: From: Subject: Value Based Pay for Performance (VBP4P) Stakeholders Lindsay Erickson, Director, Value Based P4P, Integrated Healthcare Association Measurement Years 2016 & 2017 Proposed Changes to
To: From: Subject: Value Based Pay for Performance (VBP4P) Stakeholders Lindsay Erickson, Director, Value Based P4P, Integrated Healthcare Association Measurement Years 2016 & 2017 Proposed Changes to
VELTASSA (patiromer) oral suspension
 VELTASSA (patiromer) oral suspension Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy
VELTASSA (patiromer) oral suspension Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy
Addressing the Opioid Crisis Workgroup: Treatment and Overdose Prevention
 The Accountable Community for Health of King County Addressing the Opioid Crisis Workgroup: Treatment and Overdose Prevention May 7, 2018 1 Opiate Treatment & Overdose Prevention Project Goal Immediate:
The Accountable Community for Health of King County Addressing the Opioid Crisis Workgroup: Treatment and Overdose Prevention May 7, 2018 1 Opiate Treatment & Overdose Prevention Project Goal Immediate:
Policy Evaluation: Substance Use Disorders
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Safe Prescribing of Drugs with Potential for Misuse/Diversion
 College of Physicians and Surgeons of British Columbia Safe Prescribing of Drugs with Potential for Misuse/Diversion Preamble This document establishes both professional standards as well as guidelines
College of Physicians and Surgeons of British Columbia Safe Prescribing of Drugs with Potential for Misuse/Diversion Preamble This document establishes both professional standards as well as guidelines
Doctor Shopping Behavior and the Diversion of Opioid Analgesics:
 Doctor Shopping Behavior and the Diversion of Opioid Analgesics: 2008-2012 A report prepared for The Executive Office of The President Office of National Drug Control Policy Ron Simeone Simeone Associates,
Doctor Shopping Behavior and the Diversion of Opioid Analgesics: 2008-2012 A report prepared for The Executive Office of The President Office of National Drug Control Policy Ron Simeone Simeone Associates,
PCMH 2018 Enrollment and Update August 25, 2017
 PCMH 2018 Enrollment and Update August 25, 2017 Enrollment Requirements Anne Santifer HealthCare Innovations Department of Human Services 2018 Enrollment Requirements A physician practice that is enrolled
PCMH 2018 Enrollment and Update August 25, 2017 Enrollment Requirements Anne Santifer HealthCare Innovations Department of Human Services 2018 Enrollment Requirements A physician practice that is enrolled
Condition/Procedure Measure Compliance Criteria Reference Attribution Method
 Premium Specialty: Cardiology Credentialed Specialties include: Cardiac Diagnostic, Cardiology, Cardiovascular Disease, Clinical Cardiac Electrophysiology, and Interventional Cardiology This document is
Premium Specialty: Cardiology Credentialed Specialties include: Cardiac Diagnostic, Cardiology, Cardiovascular Disease, Clinical Cardiac Electrophysiology, and Interventional Cardiology This document is
REQUEST FOR MEDICARE PRESCRIPTION DRUG COVERAGE DETERMINATION This form may be sent to us by mail or fax:
 REQUEST FOR MEDICARE PRESCRIPTION DRUG COVERAGE DETERMINATION This form may be sent to us by mail or fax: Address: Fax Number: Anthem Blue Cross Cal MediConnect Plan 1-844-493-9213 Medicare Prior Authorization
REQUEST FOR MEDICARE PRESCRIPTION DRUG COVERAGE DETERMINATION This form may be sent to us by mail or fax: Address: Fax Number: Anthem Blue Cross Cal MediConnect Plan 1-844-493-9213 Medicare Prior Authorization
Executive Summary. Classes Under Review: Analysis:
 Outcome Report on MHQAC Edits Classes Under Review: Analysis: Atypical Antipsychotics, SSRIs, SNRIs, Antianxietals, Hypnotics, and Drugs to Treat ADD/ADHD Utilization and Cost Trends: Pre and Post Implementation
Outcome Report on MHQAC Edits Classes Under Review: Analysis: Atypical Antipsychotics, SSRIs, SNRIs, Antianxietals, Hypnotics, and Drugs to Treat ADD/ADHD Utilization and Cost Trends: Pre and Post Implementation
The University of Mississippi School of Pharmacy
 LONG TERM PERSISTENCE WITH ACEI/ARB THERAPY AFTER ACUTE MYOCARDIAL INFARCTION: AN ANALYSIS OF THE 2006-2007 MEDICARE 5% NATIONAL SAMPLE DATA Lokhandwala T. MS, Yang Y. PhD, Thumula V. MS, Bentley J.P.
LONG TERM PERSISTENCE WITH ACEI/ARB THERAPY AFTER ACUTE MYOCARDIAL INFARCTION: AN ANALYSIS OF THE 2006-2007 MEDICARE 5% NATIONAL SAMPLE DATA Lokhandwala T. MS, Yang Y. PhD, Thumula V. MS, Bentley J.P.
MEASURING CARE QUALITY
 MEASURING CARE QUALITY Region December 2013 For Clinical Effectiveness of Care Measures of Performance From: Healthcare Effectiveness Data and Information Set (HEDIS ) HEDIS is a set of standardized performance
MEASURING CARE QUALITY Region December 2013 For Clinical Effectiveness of Care Measures of Performance From: Healthcare Effectiveness Data and Information Set (HEDIS ) HEDIS is a set of standardized performance
Clinical Policy: Roflumilast (Daliresp) Reference Number: CP.PMN.46. Line of Business: Medicaid
 Clinical Policy: (Daliresp) Reference Number: CP.PMN.46 Effective Date: 11/11 Last Review Date: 08/17 Line of Business: Medicaid See Important Reminder at the end of this policy for important regulatory
Clinical Policy: (Daliresp) Reference Number: CP.PMN.46 Effective Date: 11/11 Last Review Date: 08/17 Line of Business: Medicaid See Important Reminder at the end of this policy for important regulatory
Connecticut Medical Assistance Pharmacy Program Drug Utilization Review (DUR) Program DUR Board Meeting
 March 2015 Minutes ATTENDEES Board Members Present: Kenneth Fisher, R.Ph. (Chair), Keith Lyke R.Ph., Bhupesh Mangla, MD, Richard Gannon, Pharm.D., Charles Caley Pharm.D. BCPP, Ram Illindala, MD, Carol
March 2015 Minutes ATTENDEES Board Members Present: Kenneth Fisher, R.Ph. (Chair), Keith Lyke R.Ph., Bhupesh Mangla, MD, Richard Gannon, Pharm.D., Charles Caley Pharm.D. BCPP, Ram Illindala, MD, Carol
Medicaid and the Opioid Crisis
 Medicaid and the Opioid Crisis Erica Floyd Thomas Bureau Chief of Medicaid Policy Agency for Health Care Administration Presented to: Medical Care Advisory Committee March 20, 2018 1 Florida Medicaid Covers
Medicaid and the Opioid Crisis Erica Floyd Thomas Bureau Chief of Medicaid Policy Agency for Health Care Administration Presented to: Medical Care Advisory Committee March 20, 2018 1 Florida Medicaid Covers
Commercial HMO/POS Effectiveness of Care Measure
 Commercial HMO/POS Effectiveness of Care Measure HEDIS 2017 NCQA Quality Compass National Average Adult BMI Assessment 91.85% 76.17% Weight Assessment and Counseling for Nutrition and Physical Activity
Commercial HMO/POS Effectiveness of Care Measure HEDIS 2017 NCQA Quality Compass National Average Adult BMI Assessment 91.85% 76.17% Weight Assessment and Counseling for Nutrition and Physical Activity
Greenbrier County. West Virginia Board of Pharmacy Prescription Opioid Problematic Prescribing Indicators County Report
 West Virginia Board of Pharmacy Prescription Opioid Problematic Prescribing Indicators Report The West Virginia Violence and Injury Prevention Program ( VIPP), in collaboration with the West Virginia Board
West Virginia Board of Pharmacy Prescription Opioid Problematic Prescribing Indicators Report The West Virginia Violence and Injury Prevention Program ( VIPP), in collaboration with the West Virginia Board
Amal AL-Anazi, BSc.(Pharm) Medication Safety Officer In Eastern Region
 Risks Of Polypharmacy Amal AL-Anazi, BSc.(Pharm) Medication Safety Officer In Eastern Region What is Polypharmacy? Polypharmacy means many drugs. In practice, polypharmacy refers to the use of more medication
Risks Of Polypharmacy Amal AL-Anazi, BSc.(Pharm) Medication Safety Officer In Eastern Region What is Polypharmacy? Polypharmacy means many drugs. In practice, polypharmacy refers to the use of more medication
OCCUPATIONAL AND PROFESSIONAL LICENSING MEDICINE AND SURGERY PRACTITIONERS MANAGEMENT OF PAIN AND OTHER CONDITIONS WITH CONTROLLED SUBSTANCES
 TITLE 16 CHAPTER 10 PART 14 OCCUPATIONAL AND PROFESSIONAL LICENSING MEDICINE AND SURGERY PRACTITIONERS MANAGEMENT OF PAIN AND OTHER CONDITIONS WITH CONTROLLED SUBSTANCES 16.10.14.1 ISSUING AGENCY: New
TITLE 16 CHAPTER 10 PART 14 OCCUPATIONAL AND PROFESSIONAL LICENSING MEDICINE AND SURGERY PRACTITIONERS MANAGEMENT OF PAIN AND OTHER CONDITIONS WITH CONTROLLED SUBSTANCES 16.10.14.1 ISSUING AGENCY: New
Medicare Part D Opioid Policies for 2019 Information for Patients
 CENTERS FOR MEDICARE & MEDICAID SERVICES Medicare Part D Opioid Policies for 2019 Information for Patients Introduction Prescription opioid pain medications like oxycodone (OxyContin ), hydrocodone (Vicodin
CENTERS FOR MEDICARE & MEDICAID SERVICES Medicare Part D Opioid Policies for 2019 Information for Patients Introduction Prescription opioid pain medications like oxycodone (OxyContin ), hydrocodone (Vicodin
Opioid prescription drug patterns in diagnosed and non-diagnosed opioid use disorder populations
 Opioid prescription drug patterns in diagnosed and non-diagnosed opioid use disorder populations Exploring prescription patterns among commercially insured patients with diagnosed opioid use disorder and
Opioid prescription drug patterns in diagnosed and non-diagnosed opioid use disorder populations Exploring prescription patterns among commercially insured patients with diagnosed opioid use disorder and
Rule Governing the Prescribing of Opioids for Pain
 Rule Governing the Prescribing of Opioids for Pain 1.0 Authority This rule is adopted pursuant to Sections 14(e) and 11(e) of Act 75 (2013) and Sections 2(e) and 2a of Act 173 (2016). 2.0 Purpose This
Rule Governing the Prescribing of Opioids for Pain 1.0 Authority This rule is adopted pursuant to Sections 14(e) and 11(e) of Act 75 (2013) and Sections 2(e) and 2a of Act 173 (2016). 2.0 Purpose This
Rethink. Adherence. David D. Pope, PharmD, CDE Editor-in-Chief, CreativePharmacist.com Brands
 Rethink. Adherence. David D. Pope, PharmD, CDE Editor-in-Chief, CreativePharmacist.com Brands Objectives Identify patient barriers to medication adherence. Describe the clinical and economic impact of
Rethink. Adherence. David D. Pope, PharmD, CDE Editor-in-Chief, CreativePharmacist.com Brands Objectives Identify patient barriers to medication adherence. Describe the clinical and economic impact of
Maine s New Opioid Prescribing Law & the Opioid Crisis: Implications for Providers
 Maine s New Opioid Prescribing Law & the Opioid Crisis: Implications for Providers Gordon H. Smith, Esq., Executive Vice President Maine Medical Association May 3, 2016 Facing the Opioid Crisis Today Opioid
Maine s New Opioid Prescribing Law & the Opioid Crisis: Implications for Providers Gordon H. Smith, Esq., Executive Vice President Maine Medical Association May 3, 2016 Facing the Opioid Crisis Today Opioid
Quality ID #438: Statin Therapy for the Prevention and Treatment of Cardiovascular Disease National Quality Strategy Domain: Effective Clinical Care
 Quality ID #438: Statin Therapy for the Prevention and Treatment of Cardiovascular Disease National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS F INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE
Quality ID #438: Statin Therapy for the Prevention and Treatment of Cardiovascular Disease National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS F INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE
Modernized Reference Drug Program
 Modernized Reference Drug Program Monitoring Report For the period ending May 31, 2017 Issued: November 2017 Published by: Pharmaceutical Services Division and Health Sector Information, Analysis and Reporting
Modernized Reference Drug Program Monitoring Report For the period ending May 31, 2017 Issued: November 2017 Published by: Pharmaceutical Services Division and Health Sector Information, Analysis and Reporting
HEDIS Quick Reference Guide Updated to reflect NCQA HEDIS 2016 Technical Specifications
 HEDIS Quick Reference Guide Updated to reflect NCQA HEDIS 2016 Technical Specifications Fidelis SecureCare strives to provide quality healthcare to our membership as measured through HEDIS quality metrics.
HEDIS Quick Reference Guide Updated to reflect NCQA HEDIS 2016 Technical Specifications Fidelis SecureCare strives to provide quality healthcare to our membership as measured through HEDIS quality metrics.
URINE DRUG TESTING FOR SUBSTANCE ABUSE TREATMENT AND CHRONIC PAIN MANAGEMENT
 Status Active Medical and Behavioral Health Policy Section: Laboratory Policy Number: VI-47 Effective Date: 07/21/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members
Status Active Medical and Behavioral Health Policy Section: Laboratory Policy Number: VI-47 Effective Date: 07/21/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members
