THRANE (enflurane, USP)
|
|
|
- Christiana Doyle
- 5 years ago
- Views:
Transcription
1 Ethrane (enflurane, USP) Page 1 of 9 THRANE (enflurane, USP) Liquid For Inhalation R x only DESCRIPTION THRANE (enflurane, USP), a nonflammable liquid administered by vaporizing, is a general inhalation anesthetic drug. It is 2-chloro-1,1,2-trifluoroethyl difluoromethyl ether (CHF 2 OCF 2 CHFCl). The boiling point is 56.5ºC at 760 mm Hg, and the vapor pressure (in mm Hg) is 175 at 20ºC, 218 at 25ºC, and 345 at 36ºC. Vapor pressures can be calculated using the equation: A = log 10 P vap = A + B/T B = T = ºC (Kelvin) The specific gravity (25º/25ºC) is The refractive index at 20ºC is The blood/gas coefficient is 1.91 at 37ºC and the oil/gas coefficient is 98.5 at 37ºC. Enflurane is a clear, colorless, stable liquid whose purity exceeds 99.9% (area percent by gas chromatography). No stabilizers are added as these have been found, through controlled laboratory tests, to be unnecessary even in the presence of ultraviolet light. Enflurane is stable to strong base, does not decompose in contact with soda lime (at normal operating temperatures), and does not react with aluminum, tin, brass, iron or copper. The partition coefficients of enflurane at 25ºC are 74 in conductive rubber and 120 in polyvinyl chloride. CLINICAL PHARMACOLOGY THRANE (enflurane, USP) is an inhalation anesthetic. The MAC (minimum alveolar concentration) in man is 1.68% in pure oxygen, 0.57 in 70% nitrous oxide, 30% oxygen, and 1.17 in 30% nitrous oxide, 70% oxygen. Induction of and recovery from anesthesia with enflurane are rapid. Enflurane has a mild, sweet odor. Enflurane may provide a mild stimulus to salivation or tracheobronchial secretions. Pharyngeal and laryngeal reflexes are readily obtunded. The level of anesthesia can be changed rapidly by changing the inspired enflurane concentration. Enflurane reduces ventilation as depth of anesthesia increases. High PaCO 2 levels can be obtained at deeper levels of anesthesia if ventilation is not supported. Enflurane provokes a sigh response reminiscent of that seen with diethyl ether. There is a decrease in blood pressure with induction of anesthesia, followed by a return to near normal with surgical stimulation. Progressive increases in depth of anesthesia produce 1
2 Ethrane (enflurane, USP) Page 2 of 9 corresponding increases in hypotension. Heart rate remains relatively constant without significant bradycardia. Electrocardiographic monitoring or recordings indicate that cardiac rhythm remains stable. Elevation of the carbon dioxide level in arterial blood does not alter cardiac rhythm. Studies in man indicate a considerable margin of safety in the administration of epinephrinecontaining solutions during enflurane anesthesia. Enflurane anesthesia has been used in excision of pheochromocytoma in man without ventricular arrhythmias. On the basis of studies in patients anesthetized with enflurane and injected with epinephrine-containing solutions to achieve hemostasis in a highly vascular area (transsphenoidal surgery), up to 2 micrograms per kilogram (2 g/kg) of epinephrine may be injected subcutaneously over a 10 minute period in patients judged to have ordinary tolerance to epinephrine administration. This would represent up to 14 ml of 1:100,000 epinephrine-containing solution (10 g/ml), or the equivalent quantity, in a 70 kilogram patient. This may be repeated up to 3 times per hour (total 42 ml per hour). The concomitant administration of lidocaine enhances the safety of the use of epinephrine during enflurane anesthesia. This effect of lidocaine is dose related. All customary precautions in the use of vasoconstrictor substances should be observed. Muscle relaxation may be adequate for intra-abdominal operations at normal levels of anesthesia. Muscle relaxants may be used to achieve greater relaxation and all commonly used muscle relaxants are compatible with enflurane. THE NON-DEPOLARIZING MUSCLE RELAXANTS ARE POTENTIATED. In the normal 70 kg adult, 6 to 9 mg of d-tubocurarine or 1.0 to 1.5 mg of pancuronium will produce a 90% or greater depression of twitch height. Neostigmine does not reverse the direct effect of enflurane. Enflurane 0.25 to 1.0% (average 0.5%) provides analgesia equal to that produced by 30 to 60% (average 40%) nitrous oxide for vaginal delivery. With either agent, patients remain awake, cooperative and oriented. Maternal blood losses are comparable. These clinical approaches produce normal Apgar scores. Serial neurobehavioral testing of the newborn during the first 24 hours of life reveals that neither enflurane nor nitrous oxide analgesia is associated with obvious neurobehavioral alterations. Neither enflurane nor nitrous oxide when used for obstetrical analgesia alters BUN, creatinine, uric acid or osmolality. The only difference in the use of these two agents for obstetrical analgesia appears to be higher inspired oxygen concentration that may be used with enflurane. Analgetic doses of enflurane, up to approximately 1.0%, do not significantly depress the rate or force of uterine contraction during labor and delivery. A slowing of the rate of uterine contraction and a diminution of the force of uterine contraction is noted between the administration of 1.0 to 2.0% delivered enflurane; concentrations somewhere between 2.0 and 3.0% delivered enflurane may abolish uterine contractions. Enflurane displaces the myometrial response curve to oxytocin so that at lower concentrations of enflurane oxytocin will restore uterine contractions; however, as the 2
3 Ethrane (enflurane, USP) Page 3 of 9 dose of enflurane progresses (somewhere between 1.5 and 3.0% delivered enflurane) the response to oxytocin is diminished and then abolished. Uterine bleeding may be increased when enflurane is used in higher concentrations for vaginal delivery or to facilitate delivery by Cesarean section; however, this has not been demonstrated within the recommended dosage range (see DOSAGE AND ADMINISTRATION section). Mean estimated blood loss in patients anesthetized for therapeutic termination of pregnancy with 1.0% enflurane in 70% nitrous oxide with oxygen is approximately twice that noted following therapeutic termination of pregnancy performed with the use of a local anesthetic technique (40 ml versus 20 ml). Pharmacokinetics Biotransformation of enflurane in man results in low peak levels of serum fluoride averaging 15 mol/l. These levels are well below the 50 mol/l threshold level which can produce minimal renal damage in normal subjects. However, patients chronically ingesting isoniazid or other hydrazine-containing compounds may metabolize greater amounts of enflurane. Although no significant renal dysfunction has been found thus far in such patients, peak serum fluoride levels can exceed 50 mol/l, particularly when anesthesia goes beyond 2 MAC hours. Depression of lymphocyte transformation does not follow prolonged enflurane anesthesia in man in the absence of surgery. Thus enflurane does not depress this aspect of the immune response. INDICATIONS AND USAGE THRANE (enflurane, USP) may be used for induction and maintenance of general anesthesia. Enflurane may be used to provide analgesia for vaginal delivery. Low concentrations of enflurane (see DOSAGE AND ADMINISTRATION) may also be used to supplement other general anesthetic agents during delivery by Cesarean section. Higher concentrations of enflurane may produce uterine relaxation and an increase in uterine bleeding. CONTRAINDICATIONS Seizure disorders (see WARNINGS). Known sensitivity to THRANE (enflurane, USP) or other halogenated anesthetics. Known or suspected genetic susceptibility to malignant hyperthermia. WARNINGS Perioperative Hyperkalemia Use of inhaled anesthetic agents has been associated with rare increases in serum potassium levels that have resulted in cardiac arrhythmias and death in pediatric patients during the postoperative period. Patients with latent as well as overt neuromuscular disease, particularly Duchenne 3
4 Ethrane (enflurane, USP) Page 4 of 9 muscular dystrophy, appear to be most vulnerable. Concomitant use of succinylcholine has been associated with most, but not all, of these cases. These patients also experienced significant elevations in serum creatinine kinase levels and, in some cases, changes in urine consistent with myoglobinuria. Despite the similarity in presentation to malignant hyperthermia, none of these patients exhibited signs or symptoms of muscle rigidity or hypermetabolic state. Early and aggressive intervention to treat the hyperkalemia and resistant arrhythmias is recommended, as is subsequent evaluation for latent neuromuscular disease. Malignant Hyperthermia In susceptible individuals, enflurane anesthesia may trigger a skeletal muscle hypermetabolic state leading to high oxygen demand and the clinical syndrome known as malignant hyperthermia. The syndrome includes nonspecific features such as muscle rigidity, tachycardia, tachypnea, cyanosis, arrhythmias and unstable blood pressure. (It should also be noted that many of these nonspecific signs may appear with light anesthesia, acute hypoxia, etc. The syndrome of malignant hyperthermia secondary to enflurane appears to be rare; by March 1980, 35 cases had been reported in North America for an approximate incidence of 1:725,000 enflurane anesthetics.) An increase in overall metabolism may be reflected in an elevated temperature (which may rise rapidly early or late in the case, but usually is not the first sign of augmented metabolism) and an increased usage of CO 2 absorption system (hot cannister). PaO 2 and ph may decrease, and hyperkalemia and a base deficit may appear. Treatment includes discontinuance of triggering agents (e.g., enflurane), administration of intravenous dantrolene sodium, and application of supportive therapy. Such therapy includes vigorous efforts to restore body temperature to normal, respiratory and circulatory support as indicated, and management of electrolyte-fluid-acid-base derangement. (Consult prescribing information for dantrolene sodium intravenous for additional information on patient management.) Renal failure may appear later, and urine flow should be sustained if possible. Increasing depth of anesthesia with THRANE (enflurane, USP) may produce a change in the electroencephalogram characterized by high voltage, fast frequency, progressing through spikedome complexes alternating with periods of electrical silence to frank seizure activity. The latter may or may not be associated with motor movement. Motor activity, when encountered, generally consists of twitching or jerks of various muscle groups; it is self-limiting and can be terminated by lowering the anesthetic concentration. This electroencephalographic pattern associated with deep anesthesia is exacerbated by low arterial carbon dioxide tension. A reduction in ventilation and anesthetic concentrations usually suffices to eliminate seizure activity. Cerebral blood flow and metabolism studies in normal volunteers immediately following seizure activity show no evidence of cerebral hypoxia. Mental function testing does not reveal any impairment of performance following prolonged enflurane anesthesia associated with or not associated with seizure activity. 4
5 Ethrane (enflurane, USP) Page 5 of 9 Since levels of anesthesia may be altered easily and rapidly, only vaporizers producing predictable concentrations should be used. Hypotension and respiratory exchange can serve as a guide to depth of anesthesia. Deep levels of anesthesia may produce marked hypotension and respiratory depression. When previous exposure to a halogenated anesthetic is known to have been followed by evidence of unexplained hepatic dysfunction, consideration should be given to use of an agent other than enflurane. PRECAUTIONS General THRANE (enflurane, USP) should be used with caution in patients who by virtue of medical or drug history could be considered more susceptible to cortical stimulation produced by the drug. THRANE (enflurane, USP), like some other inhalational anesthetics, can react with desiccated carbon dioxide (CO 2 ) absorbents to produce carbon monoxide which may result in elevated levels of carboxyhemoglobin in some patients. Case reports suggest that barium hydroxide lime and soda lime become desiccated when fresh gases are passed through the CO 2 absorber cannister at high flow rates over many hours or days. When a clinician suspects that CO 2 absorbent may be desiccated, it should be replaced before the administration of THRANE (enflurane, USP). Information to Patients Enflurane, as well as other general anesthetics, may cause a slight decrease in intellectual function for 2 to 3 days following anesthesia. As with other anesthetics, small changes in moods and symptoms may persist for several days following administration. Laboratory Tests Bromsulfthalein (BSP) retention is mildly elevated postoperatively in some cases. This may relate to the effect of surgery since prolonged anesthesia (5 to 7 hours) in human volunteers does not result in BSP elevation. There is some elevation of glucose and white blood count intraoperatively. Glucose elevation should be considered in diabetic patients. Drug Interactions The action of nondepolarizing relaxants is augmented by enflurane. Less than the usual amounts of these drugs should be used. If the usual amounts of nondepolarizing relaxants are given, the time for recovery from neuromuscular blockade will be longer in the presence of enflurane than when halothane or nitrous oxide with a balanced technique are used. 5
6 Ethrane (enflurane, USP) Page 6 of 9 Carcinogenesis/Mutagenesis Swiss ICR mice were given enflurane to determine whether such exposure might induce neoplasia. Enflurane was given at 1/2, 1/8 and 1/32 MAC for four in-utero exposures and for 24 exposures to the pups during the first nine weeks of life. The mice were killed at 15 months of age. The incidence of tumors in these mice was the same as in untreated control mice who were given the same background gases, but not the anesthetic. Exposure of mice to 20 hours of 1.2% enflurane causes a small (about 1/2 of 1.0%) but statistically significant increase in sperm abnormalities. In contrast to these results, in vitro approaches to the study of mutagenesis (Ames test, sister chromatid exchange test, and the 8-azaguanine system) have not shown a mutagenic effect of enflurane. Pregnancy Category B Reproduction studies have been performed in rats and rabbits at doses up to four times the human dose and have revealed no evidence of impaired fertility or harm to the fetus due to enflurane. There are, however, no adequate and well controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. Nursing Mothers It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when enflurane is administered to a nursing woman. ADVERSE REACTIONS 1. Malignant hyperthermia (see WARNINGS). 2. Motor activity exemplified by movements of various muscle groups and/or seizures may be encountered with deep levels of THRANE (enflurane, USP) anesthesia, or light levels with hypocapnia. 3. Hypotension, respiratory depression, and hypoxia have been reported. 4. Arrhythmias, shivering, nausea and vomiting have been reported. 5. Elevation of the white blood count has been observed. 6. Mild, moderate and severe liver injury, including hepatic failure, may rarely follow anesthesia with enflurane. Serum transaminases may be increased and histologic evidence of injury may be found. The histologic changes are neither unique nor consistent. In several of these cases, it has not been possible to exclude enflurane as the cause or as a contributing cause to liver injury. The incidence of unexplained hepatotoxicity following the administration of enflurane is unknown, but it appears to be rare and not dose related. 6
7 Ethrane (enflurane, USP) Page 7 of 9 THRANE (enflurane, USP) has also been associated with perioperative hyperkalemia (see WARNINGS). Post-Marketing Events The following adverse events have been identified during post-approval use of THRANE (enflurane, USP). Due to the spontaneous nature of these reports, the actual incidence and relationship of THRANE (enflurane, USP) to these events cannot be established with certainty. Cardiac Disorders: Cardiac arrest Hepatobiliary Disorders: Hepatic necrosis, Hepatic failure OVERDOSAGE In the event of overdosage, or what may appear to be overdosage, the following action should be taken: Stop drug administration, establish a clear airway and initiate assisted or controlled ventilation with pure oxygen. DOSAGE AND ADMINISTRATION The concentration of THRANE (enflurane, USP) being delivered from a vaporizer during anesthesia should be known. This may be accomplished by using: a. vaporizers calibrated specifically for enflurane; b. vaporizers from which delivered flows can easily and readily be calculated. Preanesthetic Medication Preanesthetic medication should be selected according to the need of the individual patient, taking into account that secretions are weakly stimulated by enflurane and that enflurane does not alter heart rate. The use of anticholinergic drugs is a matter of choice. Surgical Anesthesia Induction may be achieved using enflurane alone with oxygen or in combination with oxygennitrous oxide mixtures. Under these conditions some excitement may be encountered. If excitement is to be avoided, a hypnotic dose of a short-acting barbiturate should be used to induce unconsciousness, followed by the enflurane mixture. In general, inspired concentrations of 2.0 to 4.5% enflurane produce surgical anesthesia in 7 to 10 minutes. 7
8 Ethrane (enflurane, USP) Page 8 of 9 Maintenance Surgical levels of anesthesia may be maintained with 0.5 to 3.0% enflurane. Maintenance concentrations should not exceed 3.0%. If added relaxation is required, supplemental doses of muscle relaxants may be used. Ventilation to maintain the tension of carbon dioxide in arterial blood in the 35 to 45 mm Hg range is preferred. Hyperventilation should be avoided in order to minimize possible CNS excitation. The level of blood pressure during maintenance is an inverse function of enflurane concentration in the absence of other complicating problems. Excessive decreases (unless related to hypovolemia) may be due to depth of anesthesia and in such instances should be corrected by lightening the level of anesthesia. Analgesia Enflurane 0.25 to 1.0% provides analgesia for vaginal delivery equal to that produced by 30 to 60% nitrous oxide. These concentrations normally do not produce amnesia. See also the information on the effects of enflurane on uterine contraction contained in the CLINICAL PHARMACOLOGY section. Cesarean Section Enflurane should ordinarily be administered in the concentration range of 0.5 to 1.0% to supplement other general anesthetics. See also the information on the effects of enflurane on uterine contraction contained in the CLINICAL PHARMACOLOGY section. HOW SUPPLIED THRANE (enflurane, USP) is packaged in 125 and 250 ml amber-colored bottles. 125 ml NDC ml NDC Safety and Handling Occupational Caution There is no specific work exposure limit established for THRANE (enflurane, USP). However, the National Institute for Occupational Safety and Health Administration (NIOSH) recommends that no worker should be exposed at ceiling concentrations greater than 2 ppm of any halogenated anesthetic agent over a sampling period not to exceed one hour. 8
9 Ethrane (enflurane, USP) Page 9 of 9 The predicted effects of acute overexposure by inhalation of THRANE (enflurane, USP) include headache, dizziness or (in extreme cases) unconsciousness. There are no documented adverse effects of chronic exposure to halogenated anesthetic vapors (Waste Anesthetic Gases or WAGs) in the workplace. Although results of some epidemiological studies suggest a link between exposure to halogenated anesthetics and increased health problems (particularly spontaneous abortion), the relationship is not conclusive. Since exposure to WAGs is one possible factor in the findings for these studies, operating room personnel, and pregnant women in particular, should minimize exposure. Precautions include adequate general ventilation in the operating room, the use of a well-designed and well-maintained scavenging system, work practices to minimize leaks and spills while the anesthetic agent is in use, and routine equipment maintenance to minimize leaks. Storage Store at room temperature 15º-30ºC (59º-86ºF). Enflurane contains no additives and has been demonstrated to be stable at room temperature for periods in excess of five years. Baxter and THRANE are trademarks of Baxter International Inc. registered in the United States Patent and Trademark Office. Manufactured for Baxter Healthcare Corporation Deerfield, IL USA For Product Inquiry ANA DRUG ( ) MLT 00088/3.0 Revised: January
ISPUB.COM. Review Of Currently Used Inhalation Anesthetics: Part II. O Wenker SIDE EFFECTS OF INHALED ANESTHETICS CARDIOVASCULAR SYSTEM
 ISPUB.COM The Internet Journal of Anesthesiology Volume 3 Number 3 O Wenker Citation O Wenker.. The Internet Journal of Anesthesiology. 1998 Volume 3 Number 3. Abstract SIDE EFFECTS OF INHALED ANESTHETICS
ISPUB.COM The Internet Journal of Anesthesiology Volume 3 Number 3 O Wenker Citation O Wenker.. The Internet Journal of Anesthesiology. 1998 Volume 3 Number 3. Abstract SIDE EFFECTS OF INHALED ANESTHETICS
General anesthesia. No single drug capable of achieving these effects both safely and effectively.
 General anesthesia General anesthesia is essential to surgical practice, because it renders patients analgesic, amnesia, and unconscious reflexes, while causing muscle relaxation and suppression of undesirable
General anesthesia General anesthesia is essential to surgical practice, because it renders patients analgesic, amnesia, and unconscious reflexes, while causing muscle relaxation and suppression of undesirable
Chapter 25. General Anesthetics
 Chapter 25 1. Introduction General anesthetics: 1. Analgesia 2. Amnesia 3. Loss of consciousness 4. Inhibition of sensory and autonomic reflexes 5. Skeletal muscle relaxation An ideal anesthetic: 1. A
Chapter 25 1. Introduction General anesthetics: 1. Analgesia 2. Amnesia 3. Loss of consciousness 4. Inhibition of sensory and autonomic reflexes 5. Skeletal muscle relaxation An ideal anesthetic: 1. A
Fentanyl Citrate Injection, USP CII
 Fentanyl Citrate Injection, USP CII R x only DESCRIPTION Fentanyl Citrate Injection is a sterile, non-pyrogenic solution for intravenous or intramuscular use as a potent narcotic analgesic. Each ml contains
Fentanyl Citrate Injection, USP CII R x only DESCRIPTION Fentanyl Citrate Injection is a sterile, non-pyrogenic solution for intravenous or intramuscular use as a potent narcotic analgesic. Each ml contains
COMPOSITION Each bottle contains the active ingredient, sevoflurane, only. No additive or stabilizer is present.
 PROPRIETARY NAME (and dosage form) ULTANE TM liquid COMPOSITION Each bottle contains the active ingredient, sevoflurane, only. No additive or stabilizer is present. PHARMACOLOGICAL CLASSIFICATION N01AB08
PROPRIETARY NAME (and dosage form) ULTANE TM liquid COMPOSITION Each bottle contains the active ingredient, sevoflurane, only. No additive or stabilizer is present. PHARMACOLOGICAL CLASSIFICATION N01AB08
Inhalational Anesthesia. Munir Gharaibeh, MD, PhD, MHPE School of Medicine The University of Jordan February, 2018
 Inhalational Anesthesia School of Medicine The University of Jordan February, 2018 mgharaib@ju.edu.jo Inhalational Anesthesia n Gases or volatile liquids n Administration and Elimination is by the lungs
Inhalational Anesthesia School of Medicine The University of Jordan February, 2018 mgharaib@ju.edu.jo Inhalational Anesthesia n Gases or volatile liquids n Administration and Elimination is by the lungs
General anesthetics. Dr. Shamil AL-Noaimy Lecturer of Pharmacology Dept. of Pharmacology College of Medicine
 General anesthetics Dr. Shamil AL-Noaimy Lecturer of Pharmacology Dept. of Pharmacology College of Medicine Rationale General anesthesia is essential to surgical practice, because it renders patients analgesic,
General anesthetics Dr. Shamil AL-Noaimy Lecturer of Pharmacology Dept. of Pharmacology College of Medicine Rationale General anesthesia is essential to surgical practice, because it renders patients analgesic,
LIDOCAINE HYDROCHLORIDE TOPICAL SOLUTION USP 4%
 LIDOCAINE HYDROCHLORIDE TOPICAL SOLUTION USP 4% PRODUCT OVERVIEW: Lidocaine Hydrochloride SOLUTION Rx only DESCRIPTION Lidocaine Hydrochloride Topical Solution USP 4% contains a local anesthetic agent
LIDOCAINE HYDROCHLORIDE TOPICAL SOLUTION USP 4% PRODUCT OVERVIEW: Lidocaine Hydrochloride SOLUTION Rx only DESCRIPTION Lidocaine Hydrochloride Topical Solution USP 4% contains a local anesthetic agent
DBL NALOXONE HYDROCHLORIDE INJECTION USP
 Name of medicine Naloxone hydrochloride Data Sheet New Zealand DBL NALXNE HYDRCHLRIDE INJECTIN USP Presentation DBL Naloxone Hydrochloride Injection USP is a sterile, clear, colourless solution, free from
Name of medicine Naloxone hydrochloride Data Sheet New Zealand DBL NALXNE HYDRCHLRIDE INJECTIN USP Presentation DBL Naloxone Hydrochloride Injection USP is a sterile, clear, colourless solution, free from
General Anesthesia. Mohamed A. Yaseen
 General Anesthesia Mohamed A. Yaseen M.S,c Surgery Before Anesthesia General Anesthesia ( GA ) Drug induced absence of perception of all sensation allowing surgery or other painful procedure to be carried
General Anesthesia Mohamed A. Yaseen M.S,c Surgery Before Anesthesia General Anesthesia ( GA ) Drug induced absence of perception of all sensation allowing surgery or other painful procedure to be carried
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
 PACKAGE LEAFLET Package leaflet: Information for the patient ISOFLURANE 100% Inhalation Vapour, Liquid Read all of this leaflet carefully before you start using this medicine because it contains important
PACKAGE LEAFLET Package leaflet: Information for the patient ISOFLURANE 100% Inhalation Vapour, Liquid Read all of this leaflet carefully before you start using this medicine because it contains important
General and Local Anesthetics TURNING POINT PHARM THURSDAY IMC606 Neuroscience Module
 General and Local Anesthetics TURNING POINT PHARM THURSDAY IMC606 Neuroscience Module Peter Bradford, PhD pgb@buffalo.edu, JSMBS 3204 13-December-2018 Disclosures NO SIGNIFICANT FINANCIAL, GENERAL, OR
General and Local Anesthetics TURNING POINT PHARM THURSDAY IMC606 Neuroscience Module Peter Bradford, PhD pgb@buffalo.edu, JSMBS 3204 13-December-2018 Disclosures NO SIGNIFICANT FINANCIAL, GENERAL, OR
NEUROMUSCULAR BLOCKING AGENTS
 NEUROMUSCULAR BLOCKING AGENTS Edward JN Ishac, Ph.D. Associate Professor, Pharmacology and Toxicology Smith 742, 828-2127, Email: eishac@vcu.edu Learning Objectives: 1. Understand the physiology of the
NEUROMUSCULAR BLOCKING AGENTS Edward JN Ishac, Ph.D. Associate Professor, Pharmacology and Toxicology Smith 742, 828-2127, Email: eishac@vcu.edu Learning Objectives: 1. Understand the physiology of the
Core Safety Profile. Pharmaceutical form(s)/strength: 5mg/ml and 25 mg/ml, Solution for injection, IM/IV FI/H/PSUR/0010/002 Date of FAR:
 Core Safety Profile Active substance: Esketamine Pharmaceutical form(s)/strength: 5mg/ml and 25 mg/ml, Solution for injection, IM/IV P-RMS: FI/H/PSUR/0010/002 Date of FAR: 29.05.2012 4.3 Contraindications
Core Safety Profile Active substance: Esketamine Pharmaceutical form(s)/strength: 5mg/ml and 25 mg/ml, Solution for injection, IM/IV P-RMS: FI/H/PSUR/0010/002 Date of FAR: 29.05.2012 4.3 Contraindications
PRODUCT MONOGRAPH. Pr SEVOFLURANE. sevoflurane, USP % v/v sevoflurane (on anhydrous basis) Liquid for Inhalation. Inhalation Anesthetic
 PRODUCT MONOGRAPH Pr SEVOFLURANE sevoflurane, USP 99.97% v/v sevoflurane (on anhydrous basis) Liquid for Inhalation Inhalation Anesthetic Baxter Corporation Mississauga, Ontario L5N 0C2 Date of Revision:
PRODUCT MONOGRAPH Pr SEVOFLURANE sevoflurane, USP 99.97% v/v sevoflurane (on anhydrous basis) Liquid for Inhalation Inhalation Anesthetic Baxter Corporation Mississauga, Ontario L5N 0C2 Date of Revision:
PROSTIGMIN (neostigmine bromide)
 PROSTIGMIN (neostigmine bromide) TABLETS DESCRIPTION: Prostigmin (neostigmine bromide), an anticholinesterase agent, is available for oral administration in 15 mg tablets. Each tablet also contains gelatin,
PROSTIGMIN (neostigmine bromide) TABLETS DESCRIPTION: Prostigmin (neostigmine bromide), an anticholinesterase agent, is available for oral administration in 15 mg tablets. Each tablet also contains gelatin,
ADVERSE REACTIONS Most common adverse reactions during treatment: nausea, vomiting, and tachycardia. (6)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use AKOVAZ safely and effectively. See full prescribing information for AKOVAZ. AKOVAZ (ephedrine sulfate
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use AKOVAZ safely and effectively. See full prescribing information for AKOVAZ. AKOVAZ (ephedrine sulfate
Environmental Health & Safety Policy Manual
 Environmental Health & Safety Policy Manual Issue Date: 5/31/2017 Policy # EHS-400.17 Isoflurane Use and Exposure Control Procedures 1.0 PURPOSE: LSUHSC is committed to keeping all exposures to hazardous
Environmental Health & Safety Policy Manual Issue Date: 5/31/2017 Policy # EHS-400.17 Isoflurane Use and Exposure Control Procedures 1.0 PURPOSE: LSUHSC is committed to keeping all exposures to hazardous
ALFENTANIL INJECTION, USP Ampul
 ALFENTANIL INJECTION, USP Ampul R x only DESCRIPTION Alfentanil Injection, USP is an opioid analgesic chemically designated as N-[1-[2-(4-ethyl-4,5- dihydro-5-oxo-1h-tetrazol-1-yl)ethyl]-4-(methoxymethyl)-4-piperidinyl]-n-phenylpropanamide
ALFENTANIL INJECTION, USP Ampul R x only DESCRIPTION Alfentanil Injection, USP is an opioid analgesic chemically designated as N-[1-[2-(4-ethyl-4,5- dihydro-5-oxo-1h-tetrazol-1-yl)ethyl]-4-(methoxymethyl)-4-piperidinyl]-n-phenylpropanamide
Salapin: Salbutamol BP 2mg as sulphate in each 5mL of a raspberry cola flavoured, sugar free syrup.
 Salapin Salbutamol Syrup 2mg/5mL Qualitative and quantitative composition Salapin: Salbutamol BP 2mg as sulphate in each 5mL of a raspberry cola flavoured, sugar free syrup. Clinical particulars Therapeutic
Salapin Salbutamol Syrup 2mg/5mL Qualitative and quantitative composition Salapin: Salbutamol BP 2mg as sulphate in each 5mL of a raspberry cola flavoured, sugar free syrup. Clinical particulars Therapeutic
SODIUM POLYSTYRENE SULFONATE Suspension, USP
 SODIUM POLYSTYRENE SULFONATE Suspension, USP DESCRIPTION Sodium Polystyrene Sulfonate Suspension, USP can be administered orally or in an enema. It is a cherryflavored suspension containing 15 grams of
SODIUM POLYSTYRENE SULFONATE Suspension, USP DESCRIPTION Sodium Polystyrene Sulfonate Suspension, USP can be administered orally or in an enema. It is a cherryflavored suspension containing 15 grams of
Pharmacology: Inhalation Anesthetics
 Pharmacology: Inhalation Anesthetics This is an edited and abridged version of: Pharmacology: Inhalation Anesthetics by Jch Ko, DVM, MS, DACVA Oklahoma State University - Veterinary Medicine, February
Pharmacology: Inhalation Anesthetics This is an edited and abridged version of: Pharmacology: Inhalation Anesthetics by Jch Ko, DVM, MS, DACVA Oklahoma State University - Veterinary Medicine, February
May 2013 Anesthetics SLOs Page 1 of 5
 May 2013 Anesthetics SLOs Page 1 of 5 1. A client is having a scalp laceration sutured and is to be given Lidocaine that contains Epinephrine. The nurse knows that this combination is desgined to: A. Cause
May 2013 Anesthetics SLOs Page 1 of 5 1. A client is having a scalp laceration sutured and is to be given Lidocaine that contains Epinephrine. The nurse knows that this combination is desgined to: A. Cause
NEW ZEALAND DATA SHEET 1. SEVORANE VOLATILE LIQUID FOR INHALATION 100% 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 NEW ZEALAND DATA SHEET 1. VOLATILE LIQUID FOR INHALATION 100% 2. QUALITATIVE AND QUANTITATIVE COMPOSITION is comprised only of the active ingredient sevoflurane 100%. 3. PHARMACEUTICAL FORM Volatile liquid
NEW ZEALAND DATA SHEET 1. VOLATILE LIQUID FOR INHALATION 100% 2. QUALITATIVE AND QUANTITATIVE COMPOSITION is comprised only of the active ingredient sevoflurane 100%. 3. PHARMACEUTICAL FORM Volatile liquid
PARACOD Tablets (Paracetamol + Codeine phosphate)
 Published on: 22 Sep 2014 PARACOD Tablets (Paracetamol + Codeine phosphate) Composition PARACOD Tablets Each effervescent tablet contains: Paracetamol IP...650 mg Codeine Phosphate IP... 30 mg Dosage Form/s
Published on: 22 Sep 2014 PARACOD Tablets (Paracetamol + Codeine phosphate) Composition PARACOD Tablets Each effervescent tablet contains: Paracetamol IP...650 mg Codeine Phosphate IP... 30 mg Dosage Form/s
INHALATIONAL ANESTHETICS Nitrous Oxide (N 2 O)
 INHALATIONAL ANESTHETICS Nitrous Oxide (N 2 O) -low molecular weight, clear, odorless, inert, inorganic gas -non-flammable but does support combustion -gas at room temperature, kept as liquid under pressure
INHALATIONAL ANESTHETICS Nitrous Oxide (N 2 O) -low molecular weight, clear, odorless, inert, inorganic gas -non-flammable but does support combustion -gas at room temperature, kept as liquid under pressure
3 DOSAGE FORMS AND STRENGTHS
 PATADAY- olopatadine hydrochloride solution/ drops Alcon Laboratories, Inc. ---------- HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PATADAY safely
PATADAY- olopatadine hydrochloride solution/ drops Alcon Laboratories, Inc. ---------- HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PATADAY safely
ISOFLURANE SAFETY GUIDELINES
 ISOFLURANE SAFETY GUIDELINES Isoflurane is a halogenated anesthetic gas commonly used in university animal research facilities and individual laboratories for inhalational anesthesia. The name for Isoflurane
ISOFLURANE SAFETY GUIDELINES Isoflurane is a halogenated anesthetic gas commonly used in university animal research facilities and individual laboratories for inhalational anesthesia. The name for Isoflurane
PRODUCT MONOGRAPH. Sevoflurane volatile liquid ( % v/v sevoflurane on anhydrous basis) Inhalation Anesthetic
 PRODUCT MONOGRAPH Pr SEVORANE AF Sevoflurane volatile liquid (99.9875% v/v sevoflurane on anhydrous basis) Inhalation Anesthetic Date of Preparation: September 18, 1995 Date of Previous Revision: December
PRODUCT MONOGRAPH Pr SEVORANE AF Sevoflurane volatile liquid (99.9875% v/v sevoflurane on anhydrous basis) Inhalation Anesthetic Date of Preparation: September 18, 1995 Date of Previous Revision: December
SEVOFLURANE. Sevoflurane, USP Liquid for Inhalation. Volatile Liquid 99.97% w/w sevoflurane (on anhydrous basis)
 PRODUCT MONOGRAPH Pr SOJOURN SEVOFLURANE Sevoflurane, USP Liquid for Inhalation Volatile Liquid 99.97% w/w sevoflurane (on anhydrous basis) Inhalation Anesthetic Piramal Critical Care Inc. 3950 Schelden
PRODUCT MONOGRAPH Pr SOJOURN SEVOFLURANE Sevoflurane, USP Liquid for Inhalation Volatile Liquid 99.97% w/w sevoflurane (on anhydrous basis) Inhalation Anesthetic Piramal Critical Care Inc. 3950 Schelden
Inhalation anesthesia. Somchai Wongpunkamol,MD Anes., CMU
 Inhalation anesthesia Somchai Wongpunkamol,MD nes., CMU Inhalation anesthetics is agent that possess anaesthetic qualities that are administered by breathing through an anaesthesia mask or ET tube connected
Inhalation anesthesia Somchai Wongpunkamol,MD nes., CMU Inhalation anesthetics is agent that possess anaesthetic qualities that are administered by breathing through an anaesthesia mask or ET tube connected
[Version 8.1,01/2017] ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
![[Version 8.1,01/2017] ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS [Version 8.1,01/2017] ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS](/thumbs/89/100763391.jpg) [Version 8.1,01/2017] ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Sevotek 1000 mg/g inhalation vapour, liquid for dogs and cats 2. QUALITATIVE AND QUANTITATIVE
[Version 8.1,01/2017] ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Sevotek 1000 mg/g inhalation vapour, liquid for dogs and cats 2. QUALITATIVE AND QUANTITATIVE
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Comfora 595 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One film-coated tablet contains: glucosamine sulphate
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Comfora 595 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One film-coated tablet contains: glucosamine sulphate
PHENTOLAMINE MESYLATE INJECTION SANDOZ STANDARD 5 mg/ ml THERAPEUTIC CLASSIFICATION Alpha-adrenoreceptor Blocker
 PACKAGE INSERT Pr PHENTOLAMINE MESYLATE INJECTION SANDOZ STANDARD 5 mg/ ml THERAPEUTIC CLASSIFICATION Alpha-adrenoreceptor Blocker ACTIONS AND CLINICAL PHARMACOLOGY Phentolamine produces an alpha-adrenergic
PACKAGE INSERT Pr PHENTOLAMINE MESYLATE INJECTION SANDOZ STANDARD 5 mg/ ml THERAPEUTIC CLASSIFICATION Alpha-adrenoreceptor Blocker ACTIONS AND CLINICAL PHARMACOLOGY Phentolamine produces an alpha-adrenergic
PACKAGE INSERT TEMPLATE FOR SALBUTAMOL TABLET & SALBUTAMOL SYRUP
 PACKAGE INSERT TEMPLATE FOR SALBUTAMOL TABLET & SALBUTAMOL SYRUP Brand or Product Name [Product name] Tablet 2mg [Product name] Tablet 4mg [Product name] Syrup 2mg/5ml Name and Strength of Active Substance(s)
PACKAGE INSERT TEMPLATE FOR SALBUTAMOL TABLET & SALBUTAMOL SYRUP Brand or Product Name [Product name] Tablet 2mg [Product name] Tablet 4mg [Product name] Syrup 2mg/5ml Name and Strength of Active Substance(s)
50% Concentrated Injection
 NAME OF THE MEDICINE. The molecular weight of the compound is 246.5 and the CAS registry number is 10034-99-8. The molecular formula is MgSO4, 7H2O. DESCRIPTION MAGNESIUM SULFATE HEPTAHYDRATE 50% CONCENTRATED
NAME OF THE MEDICINE. The molecular weight of the compound is 246.5 and the CAS registry number is 10034-99-8. The molecular formula is MgSO4, 7H2O. DESCRIPTION MAGNESIUM SULFATE HEPTAHYDRATE 50% CONCENTRATED
MATERIAL SAFETY DATA SHEET
 Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 Emergency telephone number:
Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 Emergency telephone number:
CHAPTER 11. General and Local Anesthetics. Anesthetics. Anesthesia. Eliza Rivera-Mitu, RN, MSN NDEG 26 A
 CHAPTER 11 General and Local Anesthetics Eliza Rivera-Mitu, RN, MSN NDEG 26 A Anesthetics Agents that depress the central nervous system (CNS) Depression of consciousness Loss of responsiveness to sensory
CHAPTER 11 General and Local Anesthetics Eliza Rivera-Mitu, RN, MSN NDEG 26 A Anesthetics Agents that depress the central nervous system (CNS) Depression of consciousness Loss of responsiveness to sensory
DBL MAGNESIUM SULFATE CONCENTRATED INJECTION
 DBL MAGNESIUM SULFATE CONCENTRATED INJECTION NAME OF MEDICINE Magnesium Sulfate BP DESCRIPTION DBL Magnesium Sulfate Concentrated Injection is a clear, colourless, sterile solution. Each ampoule contains
DBL MAGNESIUM SULFATE CONCENTRATED INJECTION NAME OF MEDICINE Magnesium Sulfate BP DESCRIPTION DBL Magnesium Sulfate Concentrated Injection is a clear, colourless, sterile solution. Each ampoule contains
SUCRALFATE TABLETS, USP
 1234567890 10 210002 SUCRALFATE TABLETS, USP DESCRIPTION Sucralfate is an -D-glucopyranoside, -D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. It has the following structural formula:
1234567890 10 210002 SUCRALFATE TABLETS, USP DESCRIPTION Sucralfate is an -D-glucopyranoside, -D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. It has the following structural formula:
What is MH? Malignant Hyperthermia (MH)! Malignant Hyperthermia (MH) Malignant Hyperthermia (MH) ! The underlying physical mechanismintracellular
 10/2/13 What is MH? Libby Morse BSN RN CPAN An inherited disorder of skeletal muscle triggered in susceptible humans in most instances by inhalation agents and/ or succinylcholine, resulting in hypermetabolism,
10/2/13 What is MH? Libby Morse BSN RN CPAN An inherited disorder of skeletal muscle triggered in susceptible humans in most instances by inhalation agents and/ or succinylcholine, resulting in hypermetabolism,
Chapter 18 Neuromuscular Blocking Agents Study Guide and Application Exercise
 Chapter 18 Neuromuscular Blocking Agents Study Guide and Application Exercise 1. Read chapter 2. Review objectives (p.305) 3. Review key terms and definitions (p.305) Add: Cholinesterase inhibitor Vagal
Chapter 18 Neuromuscular Blocking Agents Study Guide and Application Exercise 1. Read chapter 2. Review objectives (p.305) 3. Review key terms and definitions (p.305) Add: Cholinesterase inhibitor Vagal
Drugs used in obstetrics
 Drugs used in obstetrics Drugs used in obstetrics Drugs may be used to modify uterine contractions. These include oxytocic drugs used to stimulate uterine contractions both in induction of labour and to
Drugs used in obstetrics Drugs used in obstetrics Drugs may be used to modify uterine contractions. These include oxytocic drugs used to stimulate uterine contractions both in induction of labour and to
ABBVIE. Inhalation Anaesthetic NAME OF THE MEDICINAL PRODUCT. Sevoflurane
 08-15 Sevoflurane ABBVIE Inhalation Anaesthetic NAME OF THE MEDICINAL PRODUCT Sevoflurane QUALITATIVE AND QUANTITATIVE COMPOSITION The finished product is comprised only of the active ingredient (Sevoflurane).
08-15 Sevoflurane ABBVIE Inhalation Anaesthetic NAME OF THE MEDICINAL PRODUCT Sevoflurane QUALITATIVE AND QUANTITATIVE COMPOSITION The finished product is comprised only of the active ingredient (Sevoflurane).
SUCRALFATE TABLETS, USP
 1234567890 10 210002-01 SUCRALFATE TABLETS, USP DESCRIPTION Sucralfate is an -D-glucopyranoside, -D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. It has the following structural formula:
1234567890 10 210002-01 SUCRALFATE TABLETS, USP DESCRIPTION Sucralfate is an -D-glucopyranoside, -D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. It has the following structural formula:
Neosynephrine. Name of the Medicine
 Name of the Medicine Neosynephrine Phenylephrine hydrochloride 1% injection Neosynephrine Presentation Neosynephrine is a clear, colourless, aqueous solution, free from visible particulates, in sterile
Name of the Medicine Neosynephrine Phenylephrine hydrochloride 1% injection Neosynephrine Presentation Neosynephrine is a clear, colourless, aqueous solution, free from visible particulates, in sterile
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Citanest with Octapressin Dental 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains Prilocaine Hydrochloride 30 mg (54 mg/1.8
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Citanest with Octapressin Dental 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains Prilocaine Hydrochloride 30 mg (54 mg/1.8
Rx only. wen-4121v03 Page 1 of 9
 Fentanyl Citrate Injection, USP 50 mcg (0.05 mg) fentanyl/ml FOR INTRAVENOUS OR INTRAMUSCULAR USE ONLY Ampul Fliptop Vial Protect From Light. Retain In Carton Until Time Of Use. Rx only DESCRIPTION Fentanyl
Fentanyl Citrate Injection, USP 50 mcg (0.05 mg) fentanyl/ml FOR INTRAVENOUS OR INTRAMUSCULAR USE ONLY Ampul Fliptop Vial Protect From Light. Retain In Carton Until Time Of Use. Rx only DESCRIPTION Fentanyl
3% Sorbitol Urologic Irrigating Solution in UROMATIC Plastic Container
 3% Sorbitol Urologic Irrigating Solution in UROMATIC Plastic Container Description 3% Sorbitol Urologic Irrigating Solution is a sterile, nonpyrogenic, nonhemolytic, electrically nonconductive solution
3% Sorbitol Urologic Irrigating Solution in UROMATIC Plastic Container Description 3% Sorbitol Urologic Irrigating Solution is a sterile, nonpyrogenic, nonhemolytic, electrically nonconductive solution
ULTANE (sevoflurane) volatile liquid for inhalation NOVAPLUS
 ULTANE (sevoflurane) volatile liquid for inhalation NOVAPLUS DESCRIPTION ULTANE (sevoflurane), volatile liquid for inhalation, a nonflammable and nonexplosive liquid administered by vaporization, is a
ULTANE (sevoflurane) volatile liquid for inhalation NOVAPLUS DESCRIPTION ULTANE (sevoflurane), volatile liquid for inhalation, a nonflammable and nonexplosive liquid administered by vaporization, is a
Volatile Anaesthetic Agents (Basic Principles)
 Volatile Anaesthetic Agents (Basic Principles) KSS School of Anaesthesia Basic Science Course South Coast Training Group Dr S M Walton Consultant Anaesthetist Eastbourne What do you need to know about
Volatile Anaesthetic Agents (Basic Principles) KSS School of Anaesthesia Basic Science Course South Coast Training Group Dr S M Walton Consultant Anaesthetist Eastbourne What do you need to know about
NERVOUS SYSTEM NERVOUS SYSTEM. Somatic nervous system. Brain Spinal Cord Autonomic nervous system. Sympathetic nervous system
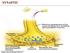 SYNAPTIC NERVOUS SYSTEM NERVOUS SYSTEM CENTRAL NERVOUS SYSTEM PERIPHERAL NERVOUS SYSTEM Brain Spinal Cord Autonomic nervous system Somatic nervous system Sympathetic nervous system Parasympathetic nervous
SYNAPTIC NERVOUS SYSTEM NERVOUS SYSTEM CENTRAL NERVOUS SYSTEM PERIPHERAL NERVOUS SYSTEM Brain Spinal Cord Autonomic nervous system Somatic nervous system Sympathetic nervous system Parasympathetic nervous
PACKAGE LEAFLET: INFORMATION FOR THE USER. Active substance: enoximone
 PACKAGE LEAFLET 1 PACKAGE LEAFLET: INFORMATION FOR THE USER Perfan Injection 100 mg/20 ml Concentrate for Solution for Injection Active substance: enoximone Read all of this leaflet carefully before you
PACKAGE LEAFLET 1 PACKAGE LEAFLET: INFORMATION FOR THE USER Perfan Injection 100 mg/20 ml Concentrate for Solution for Injection Active substance: enoximone Read all of this leaflet carefully before you
MATERIAL SAFETY DATA SHEET
 MATERIAL SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE AND THE COMPANY Material Divalproex Sodium Delayed-Release Tablets USP 125 mg, 250 mg & 500 mg Manufacturer Lupin Limited Mumbai 400 098 INDIA
MATERIAL SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE AND THE COMPANY Material Divalproex Sodium Delayed-Release Tablets USP 125 mg, 250 mg & 500 mg Manufacturer Lupin Limited Mumbai 400 098 INDIA
INHALATION AGENTS 2013/05/28 1
 INHALATION AGENTS 2013/05/28 1 2013/05/28 Isn t it romantic? 2 Administration 3 Physics Critical temperature Vapour vs. Gas Vapour pressure Blood Gas Partition Coefficient BGPC MAC 2013/05/28 4 Critical
INHALATION AGENTS 2013/05/28 1 2013/05/28 Isn t it romantic? 2 Administration 3 Physics Critical temperature Vapour vs. Gas Vapour pressure Blood Gas Partition Coefficient BGPC MAC 2013/05/28 4 Critical
PHARMACEUTICAL INFORMATION AZILSARTAN
 AZEARLY Tablets Each Tablet Contains Azilsartan 20/40/80 mg PHARMACEUTICAL INFORMATION AZILSARTAN Generic name: Azilsartan Chemical name: 2-Ethoxy-1-{[2'-(5-oxo-2,5-dihydro-1,2,4-oxadiazol-3-yl)-4-biphenylyl]methyl}-
AZEARLY Tablets Each Tablet Contains Azilsartan 20/40/80 mg PHARMACEUTICAL INFORMATION AZILSARTAN Generic name: Azilsartan Chemical name: 2-Ethoxy-1-{[2'-(5-oxo-2,5-dihydro-1,2,4-oxadiazol-3-yl)-4-biphenylyl]methyl}-
Pharmacokinetics. Inhalational Agents. Uptake and Distribution
 Pharmacokinetics Inhalational Agents The pharmacokinetics of inhalational agents is divided into four phases Absorption Distribution (to the CNS Metabolism (minimal Excretion (minimal The ultimate goal
Pharmacokinetics Inhalational Agents The pharmacokinetics of inhalational agents is divided into four phases Absorption Distribution (to the CNS Metabolism (minimal Excretion (minimal The ultimate goal
DESCRIPTION Bethanechol chloride, USP, a cholinergic agent, is a synthetic ester which is structurally and pharmacologically related to acetylcholine.
 BETHANECHOL CHLORIDE TABLETS, USP 5 mg, 10 mg, 25 mg and 50 mg Rx only DESCRIPTION Bethanechol chloride, USP, a cholinergic agent, is a synthetic ester which is structurally and pharmacologically related
BETHANECHOL CHLORIDE TABLETS, USP 5 mg, 10 mg, 25 mg and 50 mg Rx only DESCRIPTION Bethanechol chloride, USP, a cholinergic agent, is a synthetic ester which is structurally and pharmacologically related
Safety Data Sheet. Product Name: Product Number: Product Identity. 2. Hazardous Ingredients. Formaldehyde: CAS: Methanol: CAS:
 Olathe, KS Tel: 913-390-6184 Safety Data Sheet Solution (Formalin, Formol, Methanol, Formaldehyde containing solutions, Formalith) Emergency phone: 800 424 9300 (Chemtrec) NFPA Rating: Health 3, Flammability
Olathe, KS Tel: 913-390-6184 Safety Data Sheet Solution (Formalin, Formol, Methanol, Formaldehyde containing solutions, Formalith) Emergency phone: 800 424 9300 (Chemtrec) NFPA Rating: Health 3, Flammability
Draft Labeling Package Insert Not Actual Size. BRAINTREE LABORATORIES, INC. PhosLo Capsules (Calcium Acetate)
 Draft Labeling Package Insert Not Actual Size BRAINTREE LABORATORIES, INC. PhosLo Capsules (Calcium Acetate) DESCRIPTION: Full Size: Each opaque capsule with a white cap and white body is spin printed
Draft Labeling Package Insert Not Actual Size BRAINTREE LABORATORIES, INC. PhosLo Capsules (Calcium Acetate) DESCRIPTION: Full Size: Each opaque capsule with a white cap and white body is spin printed
Chapter 19. Media Directory. Topical (Surface) Anesthesia. Spinal Anesthesia. Nerve-Block Anesthesia. Infiltration (Field-Block) Anesthesia
 Chapter 19 Drugs for Local and General Anesthesia Slide 18 Media Directory Lidocaine Animation Upper Saddle River, New Jersey 07458 All rights reserved. Topical (Surface) Anesthesia Creams, sprays, suppositories
Chapter 19 Drugs for Local and General Anesthesia Slide 18 Media Directory Lidocaine Animation Upper Saddle River, New Jersey 07458 All rights reserved. Topical (Surface) Anesthesia Creams, sprays, suppositories
PRODUCT INFORMATION. (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate
![PRODUCT INFORMATION. (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate PRODUCT INFORMATION. (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate](/thumbs/81/82665370.jpg) NORGESIC Orphenadrine citrate and paracetamol PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Chemical name: CAS number: 4682-36-4 Chemical structure: Orphenadrine citrate (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine
NORGESIC Orphenadrine citrate and paracetamol PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Chemical name: CAS number: 4682-36-4 Chemical structure: Orphenadrine citrate (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine
Neuromuscular Junction
 Muscle Relaxants Neuromuscular Junction Cholinergic antagonists Neuromuscular-blocking agents (mostly nicotinic antagonists): interfere with transmission of efferent impulses to skeletal muscles. These
Muscle Relaxants Neuromuscular Junction Cholinergic antagonists Neuromuscular-blocking agents (mostly nicotinic antagonists): interfere with transmission of efferent impulses to skeletal muscles. These
HIGHLIGHTS OF PRESCRIBING INFORMATION
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use epinastine hydrochloride ophthalmic solution safely and effectively. See full prescribing information
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use epinastine hydrochloride ophthalmic solution safely and effectively. See full prescribing information
General Anesthesia. My goal in general anesthesia is to stop all of these in the picture above (motor reflexes, pain and autonomic reflexes).
 General Anesthesia General anesthesia is essential to surgical practice, because it renders patients analgesic, amnesia and unconscious reflexes, while causing muscle relaxation and suppression of undesirable
General Anesthesia General anesthesia is essential to surgical practice, because it renders patients analgesic, amnesia and unconscious reflexes, while causing muscle relaxation and suppression of undesirable
Package leaflet: Information for the patient. Morphine Unimedic 1 mg/ml solution for injection morphine hydrochloride trihydrate
 Package leaflet: Information for the patient Morphine Unimedic 1 mg/ml solution for injection morphine hydrochloride trihydrate Read all of this leaflet carefully before you start using this medicine because
Package leaflet: Information for the patient Morphine Unimedic 1 mg/ml solution for injection morphine hydrochloride trihydrate Read all of this leaflet carefully before you start using this medicine because
Core Safety Profile. Pharmaceutical form(s)/strength: Solution for Injection CZ/H/PSUR/0005/002 Date of FAR:
 Core Safety Profile Active substance: Rocuronium bromide Pharmaceutical form(s)/strength: Solution for Injection P-RMS: CZ/H/PSUR/0005/002 Date of FAR: 31.05.2012 4.3 Contraindications Hypersensitivity
Core Safety Profile Active substance: Rocuronium bromide Pharmaceutical form(s)/strength: Solution for Injection P-RMS: CZ/H/PSUR/0005/002 Date of FAR: 31.05.2012 4.3 Contraindications Hypersensitivity
Cadila Healthcare Ltd. Ahmedabad, India. Sarkhej Bavla. N.H. 8A, Moraiya. Tal. Sanand. Dist. Ahmedabad State: Gujarat.
 EMERGENCY OVERVIEW Etomidate Injection intended for Intravenous injection contains Etomidate and excipients generally considered to be non- toxic and non-hazardous in small quantities and under conditions
EMERGENCY OVERVIEW Etomidate Injection intended for Intravenous injection contains Etomidate and excipients generally considered to be non- toxic and non-hazardous in small quantities and under conditions
ISPUB.COM. Review Of Currently Used Inhalation Anesthetics: Part I. O Wenker INTRODUCTION HISTORY
 ISPUB.COM The Internet Journal of Anesthesiology Volume 3 Number 2 O Wenker Citation O Wenker.. The Internet Journal of Anesthesiology. 1998 Volume 3 Number 2. Abstract INTRODUCTION Inhalation anesthetics
ISPUB.COM The Internet Journal of Anesthesiology Volume 3 Number 2 O Wenker Citation O Wenker.. The Internet Journal of Anesthesiology. 1998 Volume 3 Number 2. Abstract INTRODUCTION Inhalation anesthetics
Product Type: Adhesive Emergency Contact: Chemtrec Product Name: Multi Purpose Cement Phone (24 hours): (800) Part Number(s): AA-74 AA-74-4
 MATERIAL SAFETY DATA SHEET Complies with OSHA Hazard Communication Standard 29 CFR 1910.1200 Product Type: Adhesive Emergency Contact: Chemtrec Phone (24 hours): (800) 424-9300 Part Number(s): AA-74 AA-74-4
MATERIAL SAFETY DATA SHEET Complies with OSHA Hazard Communication Standard 29 CFR 1910.1200 Product Type: Adhesive Emergency Contact: Chemtrec Phone (24 hours): (800) 424-9300 Part Number(s): AA-74 AA-74-4
RYANODEX (dantrolene sodium) for injectable suspension, for intravenous use. Initial U.S. Approval: 1974
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use RYANODEX safely and effectively. See full prescribing information for RYANODEX. RYANODEX (dantrolene
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use RYANODEX safely and effectively. See full prescribing information for RYANODEX. RYANODEX (dantrolene
Physical Characteristics: Desflurane is a colourless, mobile liquid, practically odourless and tasteless at below 23 C.
 SUPRANE NAME OF THE MEDICINE Australian Approved Name: Chemical Structure: Desflurane CAS Number: 57041-67-5 Molecular formula: C 3 H 2 F 6 O Molecular Weight: 168.04 DESCRIPTION Chemical Name: (±) 2-difluoromethyl-1,2,2,2-tetrafluoroethyl
SUPRANE NAME OF THE MEDICINE Australian Approved Name: Chemical Structure: Desflurane CAS Number: 57041-67-5 Molecular formula: C 3 H 2 F 6 O Molecular Weight: 168.04 DESCRIPTION Chemical Name: (±) 2-difluoromethyl-1,2,2,2-tetrafluoroethyl
Multi-center (5 centers); United States and Canada. September 10, 1992 to April 9, 1993
 vi STUDY SYNOPSIS Study Number: Title: Investigator: GHBA-534 A Phase III, Randomized, Open-Label Study To Compare The Safety, Tolerability And Recovery Characteristics of Sevoflurane Versus Halothane
vi STUDY SYNOPSIS Study Number: Title: Investigator: GHBA-534 A Phase III, Randomized, Open-Label Study To Compare The Safety, Tolerability And Recovery Characteristics of Sevoflurane Versus Halothane
SUMMARY OF PRODUCT CHARACTERISTICS 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT SCANDONEST 3% PLAIN BIOCAINE 3% PLAIN, solution for injection 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Mepivacaine hydrochloride 30.00
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT SCANDONEST 3% PLAIN BIOCAINE 3% PLAIN, solution for injection 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Mepivacaine hydrochloride 30.00
SAFETY DATA SHEET SECTION 1: PRODUCT IDENTIFICATION PRODUCT: Hand Sanitizer Product Label Name:
 SECTION 1: PRODUCT IDENTIFICATION PRODUCT: Hand Sanitizer Product Label Name: Dawn Mist Alcohol Gel Hand Sanitizer Company Name and Address: Dukal Corporation 2 Fleetwood Court Ronkonkoma, NY 11779 Emergency
SECTION 1: PRODUCT IDENTIFICATION PRODUCT: Hand Sanitizer Product Label Name: Dawn Mist Alcohol Gel Hand Sanitizer Company Name and Address: Dukal Corporation 2 Fleetwood Court Ronkonkoma, NY 11779 Emergency
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Magnesium Trisilicate Mixture B.P. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Magnesium Trisilicate B.P. 250 mg/5 ml Light Magnesium
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Magnesium Trisilicate Mixture B.P. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Magnesium Trisilicate B.P. 250 mg/5 ml Light Magnesium
NEW ZEALAND DATA SHEET
 1. PRODUCT NAME Sudomyl, Tablet, 60 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Name and strength of the active substance Pseudoephedrine Hydrochloride 60mg Excipient(s) with known effect For the full
1. PRODUCT NAME Sudomyl, Tablet, 60 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Name and strength of the active substance Pseudoephedrine Hydrochloride 60mg Excipient(s) with known effect For the full
Quelicin TM SUCCINYLCHOLINE CHLORIDE INJECTION, USP
 Quelicin TM SUCCINYLCHOLINE CHLORIDE INJECTION, USP A short-acting depolarizing skeletal muscle relaxant. Fliptop Vial Rx only WARNING RISK OF CARDIAC ARREST FROM HYPERKALEMIC RHABDOMYOLYSIS There have
Quelicin TM SUCCINYLCHOLINE CHLORIDE INJECTION, USP A short-acting depolarizing skeletal muscle relaxant. Fliptop Vial Rx only WARNING RISK OF CARDIAC ARREST FROM HYPERKALEMIC RHABDOMYOLYSIS There have
NEW ZEALAND DATA SHEET
 1 Sevoflurane (100%, volatile liquid for inhalation) 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Active ingredient Sevoflurane 100%. Non proprietary name Fluoromethyl 2,2,2, trifluoro 1 (trifluoromethyl)
1 Sevoflurane (100%, volatile liquid for inhalation) 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Active ingredient Sevoflurane 100%. Non proprietary name Fluoromethyl 2,2,2, trifluoro 1 (trifluoromethyl)
DUODOTE AUTO-INJECTOR
 Hazardous Material (WMD) use only Indications The DuoDote may be administered by the EMT or Paramedic who have had adequate training in the on-site recognition and treatment of nerve agent exposure. Chemical
Hazardous Material (WMD) use only Indications The DuoDote may be administered by the EMT or Paramedic who have had adequate training in the on-site recognition and treatment of nerve agent exposure. Chemical
PRODUCT MONOGRAPH. Isoflurane USP. (Isoflurane, 99.9%) Inhalation Anesthetic
 PRODUCT MONOGRAPH Pr Isoflurane USP (Isoflurane, 99.9%) Inhalation Anesthetic Fresenius Kabi Canada Ltd. 165 Galaxy Blvd, Suite 100 Toronto, ON M9W 0C8 Date of Revision: May 23, 2018 Control No.: 215732
PRODUCT MONOGRAPH Pr Isoflurane USP (Isoflurane, 99.9%) Inhalation Anesthetic Fresenius Kabi Canada Ltd. 165 Galaxy Blvd, Suite 100 Toronto, ON M9W 0C8 Date of Revision: May 23, 2018 Control No.: 215732
Reference ID:
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LYSODREN safely and effectively. See full prescribing information for LYSODREN. LYSODREN (mitotane)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LYSODREN safely and effectively. See full prescribing information for LYSODREN. LYSODREN (mitotane)
MATERIAL SAFETY DATA SHEET
 MATERIAL SAFETY DATA SHEET 1. COMPANY AND PRODUCT IDENTIFICATION DUNCAN ENTERPRISES EMERGENCY TELEPHONE NUMBERS 5673 East Shields Avenue Health Emergency: 559-291-4444 7:00 am 3:30 pm Fresno, CA 93727
MATERIAL SAFETY DATA SHEET 1. COMPANY AND PRODUCT IDENTIFICATION DUNCAN ENTERPRISES EMERGENCY TELEPHONE NUMBERS 5673 East Shields Avenue Health Emergency: 559-291-4444 7:00 am 3:30 pm Fresno, CA 93727
PCTH 400 Systematic Pharmacology
 Objectives At the end of this session, you will be able to: 1. Define the components of general anesthesia; PCTH 400 Systematic Pharmacology Inhaled s and Amnestic Agents Dr. Peter Choi (peter.choi@ubc.ca)
Objectives At the end of this session, you will be able to: 1. Define the components of general anesthesia; PCTH 400 Systematic Pharmacology Inhaled s and Amnestic Agents Dr. Peter Choi (peter.choi@ubc.ca)
For topical use only. Not for oral, ophthalmic, or intravaginal use.
 DESOXIMETASONE Ointment USP, 0.25% For topical use only. Not for oral, ophthalmic, or intravaginal use. Rx only DESCRIPTION Desoximetasone ointment USP, 0.25% contains the active synthetic corticosteroid
DESOXIMETASONE Ointment USP, 0.25% For topical use only. Not for oral, ophthalmic, or intravaginal use. Rx only DESCRIPTION Desoximetasone ointment USP, 0.25% contains the active synthetic corticosteroid
Active ingredients: Metoclopramide Hydrochloride mg Equivalent to metoclopramide hydrochloride anhydrous mg
 Name Primperan 10 mg / 2 ml Metoclopramide hydrochloride anhydrous Solution for I.M. or I.V. injection (Ampoules) Composition Each 2 ml ampoule contains: Active ingredients: Metoclopramide Hydrochloride.
Name Primperan 10 mg / 2 ml Metoclopramide hydrochloride anhydrous Solution for I.M. or I.V. injection (Ampoules) Composition Each 2 ml ampoule contains: Active ingredients: Metoclopramide Hydrochloride.
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Addaven concentrate for solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Addaven contains: 1 ml 1 ampoule (10 ml) Chromic
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Addaven concentrate for solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Addaven contains: 1 ml 1 ampoule (10 ml) Chromic
Malignant Hyperthermia: What the ICU Needs to Know
 Malignant Hyperthermia: What the ICU Needs to Know Objectives 1. Compare the pathophysiology of malignant hyperthermia (MH) with presenting signs/symptoms in a critical care environment. 2. Identify critical,
Malignant Hyperthermia: What the ICU Needs to Know Objectives 1. Compare the pathophysiology of malignant hyperthermia (MH) with presenting signs/symptoms in a critical care environment. 2. Identify critical,
NITROGLYCERIN INJECTION 50 mg/ml Department of Pharmacy Duke University Medical Center Box 3089 Durham, NC
 Page 1 of 6 1. IDENTIFICATION OF SUBSTANCE Name: Manufacturer: NITROGLYCERIN INJECTION 50 mg/ml Department of Pharmacy Duke University Medical Center Box 3089 Durham, NC 27710 919-684-5125 Information
Page 1 of 6 1. IDENTIFICATION OF SUBSTANCE Name: Manufacturer: NITROGLYCERIN INJECTION 50 mg/ml Department of Pharmacy Duke University Medical Center Box 3089 Durham, NC 27710 919-684-5125 Information
(PP XI) Dr. Samir Matloob
 DRUGS ACTING ON THE CHOLINERGIC SYSTEM AND THE NEUROMUSCULAR BLOCKING DRUGS IV (NICOTINIC ANTAGONISTS) (PP XI) Dr. Samir Matloob Dept. of Pharmacology Baghdad College of Medicine Drugs acting on the cholinergic
DRUGS ACTING ON THE CHOLINERGIC SYSTEM AND THE NEUROMUSCULAR BLOCKING DRUGS IV (NICOTINIC ANTAGONISTS) (PP XI) Dr. Samir Matloob Dept. of Pharmacology Baghdad College of Medicine Drugs acting on the cholinergic
DRUGS THAT ACT IN THE CNS
 DRUGS THAT ACT IN THE CNS Anxiolytic and Hypnotic Drugs Dr Karamallah S. Mahmood PhD Clinical Pharmacology 1 OTHER ANXIOLYTIC AGENTS/ A. Antidepressants Many antidepressants are effective in the treatment
DRUGS THAT ACT IN THE CNS Anxiolytic and Hypnotic Drugs Dr Karamallah S. Mahmood PhD Clinical Pharmacology 1 OTHER ANXIOLYTIC AGENTS/ A. Antidepressants Many antidepressants are effective in the treatment
PRODUCT INFORMATION. Colistin Link. Colistin 150 mg/2 ml (as colistimethate sodium) powder for injection vial
 PRODUCT INFORMATION Colistin Link Colistin 150 mg/2 ml (as colistimethate sodium) powder for injection vial For Intramuscular and Intravenous use. NAME OF THE MEDICINE Colistimethate sodium for injection,
PRODUCT INFORMATION Colistin Link Colistin 150 mg/2 ml (as colistimethate sodium) powder for injection vial For Intramuscular and Intravenous use. NAME OF THE MEDICINE Colistimethate sodium for injection,
PRESCRIBING INFORMATION
 PRESCRIBING INFORMATION pdp-pyrazinamide Pyrazinamide Tablets, USP 500 mg Antimycobacterial / Antituberculosis Agent PENDOPHARM, Division of Pharmascience Inc.. 6111, Royalmount Ave, Suite 100 Montréal,
PRESCRIBING INFORMATION pdp-pyrazinamide Pyrazinamide Tablets, USP 500 mg Antimycobacterial / Antituberculosis Agent PENDOPHARM, Division of Pharmascience Inc.. 6111, Royalmount Ave, Suite 100 Montréal,
ANESTHETIZING DISEASED PATIENTS: URINARY; NEUROLOGICAL; TRAUMATIZED
 ANESTHETIZING DISEASED PATIENTS: URINARY; NEUROLOGICAL; TRAUMATIZED Lyon Lee DVM PhD DACVA Patients with Urinary Tract Diseases General considerations Three main factors to consider in anesthetizing urinary
ANESTHETIZING DISEASED PATIENTS: URINARY; NEUROLOGICAL; TRAUMATIZED Lyon Lee DVM PhD DACVA Patients with Urinary Tract Diseases General considerations Three main factors to consider in anesthetizing urinary
CLINICAL PHARMACOLOGY
 robaxin / robaxin -7 50 (methocarbamol tablets, USP) Rx Only DESCRIPT ION robaxin /robaxin -750 (methocarbamol tablets, USP), a carbamate derivative of guaifenesin, is a central nervous system (CNS) depressant
robaxin / robaxin -7 50 (methocarbamol tablets, USP) Rx Only DESCRIPT ION robaxin /robaxin -750 (methocarbamol tablets, USP), a carbamate derivative of guaifenesin, is a central nervous system (CNS) depressant
Metabolism Paracetamol is metabolised in the liver and excreted in the urine mainly as glucuronide and sulphate conjugates.
 FEBRAMOL Composition Febramol 150 Suppositories Each suppository contains Paracetamol 150 mg. Suppositories, Tablets & Syrup Febramol 300 Suppositories Each suppository contains Paracetamol 300 mg. Each
FEBRAMOL Composition Febramol 150 Suppositories Each suppository contains Paracetamol 150 mg. Suppositories, Tablets & Syrup Febramol 300 Suppositories Each suppository contains Paracetamol 300 mg. Each
MATERIAL SAFETY DATA SHEET
 MATERIAL SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE AND THE COMPANY Material Manufacturer Distributor Amlodipine Besylate Tablets 2.5 mg, 5 mg and 10 mg Lupin Limited Mumbai 400 098 INDIA Lupin
MATERIAL SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE AND THE COMPANY Material Manufacturer Distributor Amlodipine Besylate Tablets 2.5 mg, 5 mg and 10 mg Lupin Limited Mumbai 400 098 INDIA Lupin
Anesthesia and Mitochondrial Cytopathies Bruce H. Cohen, MD, John Shoffner, MD, Glenn DeBoer, MD United Mitochondrial Disease Foundation, 1998
 Introduction Anesthesia and Mitochondrial Cytopathies Bruce H. Cohen, MD, John Shoffner, MD, Glenn DeBoer, MD United Mitochondrial Disease Foundation, 1998 This article will outline some basic aspects
Introduction Anesthesia and Mitochondrial Cytopathies Bruce H. Cohen, MD, John Shoffner, MD, Glenn DeBoer, MD United Mitochondrial Disease Foundation, 1998 This article will outline some basic aspects
Chapter 16 Nutrition, Fluids and Electrolytes, and Acid-Base Balance Nutrition Nutrients Water o Functions Promotes metabolic processes Transporter
 Chapter 16 Nutrition, Fluids and Electrolytes, and Acid-Base Balance Nutrition Nutrients Water o Functions Promotes metabolic processes Transporter for nutrients and wastes Lubricant Insulator and shock
Chapter 16 Nutrition, Fluids and Electrolytes, and Acid-Base Balance Nutrition Nutrients Water o Functions Promotes metabolic processes Transporter for nutrients and wastes Lubricant Insulator and shock
SAFETY DATA SHEET. 2 Fleetwood Court Ronkonkoma, NY Skin Irritant 3. Causes mild skin irritation (H316)
 SECTION 1: PRODUCT IDENTIFICATION PRODUCT: BZK Prep Pads and Towelette Product Label Name: BZK Prep Pads and Towelette Company Name and Address: Dukal Corporation 2 Fleetwood Court Ronkonkoma, NY 11779
SECTION 1: PRODUCT IDENTIFICATION PRODUCT: BZK Prep Pads and Towelette Product Label Name: BZK Prep Pads and Towelette Company Name and Address: Dukal Corporation 2 Fleetwood Court Ronkonkoma, NY 11779
