|
|
|
- Lindsey Shelton
- 6 years ago
- Views:
Transcription
1 Research articles Hepatitis C virus infection among pregnant women in Slovenia: study on 31,849 samples obtained in four screening rounds during 1999, 2003, 2009 and 2013 B Kopilović 1, M Poljak 2, K Seme 2, I Klavs (Irena.Klavs@nijz.si) 1 1. ational Institute of Public Health, Ljubljana, Slovenia 2. Institute of Microbiology and Immunology, Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia Citation style for this article: Kopilović B, Poljak M, Seme K, Klavs I. Hepatitis C virus infection among pregnant women in Slovenia: study on 31,849 samples obtained in four screening rounds during 1999, 2003, 2009 and Euro Surveill. 2015;20(22):pii= Available online: Article submitted on 01 October 2014 / published on 04 June 2015 The majority of people infected with hepatitis C virus (HCV) are unaware of their infection. Assessment of the prevalence of HCV infection in the general population and in key populations at increased risk is needed for evidence-based testing policies. Our objectives were to estimate the prevalence of antibodies to HCV (anti-hcv), the prevalence of HCV viraemia (HCV RA), and to describe HCV genotype distribution among pregnant women in Slovenia. Unlinked anonymous testing was performed on residual sera obtained from 31,849 pregnant women for routine syphilis screening during 1999, 2003, 2009, and Anti-HCV reactive specimens were tested for HCV RA and HCV genotypes were determined. Annual prevalence of anti-hcv ranged between % (95% confidence interval (CI): ) in 2009 and 0.21% (95% CI: ) in 2003 and HCV RA positivity between 0.06% (95% CI: ) in 2009 and 0.14% (95% CI: ) in We observed no statistically significant differences in anti-hcv or HCV RA prevalence between age groups (<20, and 30 years) in any year and no trend in time. Of 29 HCV active infections, 19 were with genotype 1 and 10 with genotype 3. HCV infection among pregnant women was rare suggesting a low burden in the Slovenian general population. Antenatal screening for HCV in Slovenia could not be recommended. Introduction Hepatitis C virus (HCV) is among the most common blood-borne viruses [1]. In ca 75% to 85% of cases of infection, HCV persists as a chronic infection and one third of chronically infected individuals is predicted to develop liver cirrhosis or hepatocellular carcinoma [2]. Although treatment success has substantially improved in recent years [3,4], most infected people are unaware of their infection and/or do not have access to treatment [5]. According to estimates published in 2013, by 2005, more than 185 million people around the world were infected with HCV, of whom 350,000 die annually [1]. The prevalence of antibodies to HCV (anti-hcv) in central Europe was estimated to be 2.4% (> 2.9 million people infected), in eastern Europe 2.9% (> 6.2 million people infected) and in western Europe 2.4% (> 10 million people infected) [1]. Compared with other geographical areas in the world these figures indicate a moderate prevalence (1.5% 3.5%) [1]. A recent review of available data from Europe indicated a wide variation in HCV infection prevalence between countries, ranging from 0.1% to 6.0% [6]. The lowest HCV prevalence ( 0.5%) estimates were from Scandinavian countries and the etherlands, and the highest ( 5%) from Romania [7-10]. As HCV shows great diversity in prevalence in different parts of the world, the 2010 World Health Assembly resolution urged Member States to generate reliable information as a foundation for building prevention and control measures that match the local epidemiological profile and health system capacities [11]. In 1998, the United States (US) Centers for Disease Control and Prevention had already recommended routine HCV testing for several population groups at increased risk for HCV, based on HCV risk factors ascertainment, but not for pregnant women and the general population [12]. In 2012, once per lifetime HCV testing for adults born between 1945 and 1965 without prior ascertainment of HCV risk factors was added as a recommendation since the prevalence of anti-hcv among the US population born during these years was estimated to be 3.25% (95% confidence interval (CI): ) and persons born during these years accounted for approximately three quarters of all chronic HCV infections among adults [13]. In 2014, the World Health Organisation (WHO) recommended to offer anti-hcv testing to individuals who are part of a population with high HCV prevalence or who have a history of HCV risk exposure or behaviour, and suggested that nucleic acid testing for the detection of 1
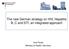
2 Figure Sentinel sites involved in collection of residual sera specimens from pregnant women that were used to test for antibodies to hepatitis C virus a, Slovenia, (n=7) ova Gorica Koper Kranj Ljubljana HCV RA be used following a positive HCV serological test to establish the diagnosis of chronic HCV infection as part of the assessment for starting treatment [14]. WHO identified the following populations at increased risk for HCV: persons who inject drugs, recipients of infected blood products or invasive procedures in healthcare facilities with inadequate infection control practices, children born to mothers infected with HCV, people with sexual partners who are HCV-infected, persons with human immunodeficiency virus (HIV) infection, in particular men who have sex with men, people who have used intranasal drugs, and people who have had tattoos or piercings. ational testing policies based on the best assessment of the prevalence of HCV infection in the general population and in key populations at increased risk are needed for evidencebased HCV control policy [14]. Due to under-ascertainment and under-reporting, Slovenian HCV surveillance data, which are based on mandatory reporting of new hepatitis C diagnoses, do not provide a full picture of the epidemiology of HCV infection [15]. In Slovenia, we have some anti-hcv prevalence estimates for groups at higher risk (haemodialysis patients, people who inject drugs, HIV infected individuals) and data about the distribution of HCV genotypes among patients with HCV infection [16-20]. During the period from 2009 to 2013, the prevalence of Celje ovo Mesto Maribor General hospital Maribor Institute of Blood Transfusion of the Republic of Slovenia, Ljubljana Institute of Public Health Celje Institute of Public Health Koper Institute of Public Health Kranj Institute of Public Health Maribor Institute of Public Health ova Gorica Institute of Public Health ovo mesto The site in Maribor comprised two participating laboratories. All other sites included one respective laboratory. a Sera used in this study to test for antibodies to hepatitis C virus had been originally collected for syphilis screening and subsequently systematically sampled for unlinked anonymous human immunodeficiency virus prevalence monitoring for surveillance purposes. anti-hcv among confidentially tested people who inject drugs entering or re-entering treatment within the network of Centres for the Prevention and Treatment of Illicit Drug Addiction ranged from the lowest 21.5% in 2010 to the highest 31.3% in These values were relatively low in comparison to a number of other countries in Europe where the prevalence among people who injected drugs during the period from 2011 to 2012 varied from 19% to 84%, with seven of the 11 countries with national data reporting a prevalence exceeding 50% (Austria, Cyprus, Greece, Latvia, orway, Portugal, Turkey) [21]. We also have data about anti- HCV prevalence among blood donors for the period from 2001 to 2010 with an average of % anti- HCV positive donations [22]. By 2013, we had neither reliable data about past and/or active HCV infection prevalence among pregnant women and in the general population nor about possible trends over time. To complement available information on the prevalence of HCV infection in different population groups in Slovenia, our objectives were to estimate the prevalence of anti-hcv, the prevalence of HCV viraemia (HCV RA), and to describe HCV genotype and subtype distribution among pregnant women in Slovenia for years 1999, 2003, 2009, and In addition, we wanted to explore whether there were any differences in anti-hcv and HCV RA prevalence between different age groups of pregnant women in any of these years and possible changes in anti-hcv and HCV RA prevalence through time. Methods Samples In Slovenia, syphilis screening for pregnant women is universal. For this study, 31,849 sera stored at the ational Institute of Public Health that were obtained from pregnant women for syphilis screening purposes and were systematically sampled for unlinked anonymous testing for HIV surveillance purposes during 1999, 2003, 2009, and 2013 were included. The sampling strategy for unlinked anonymous testing of pregnant women for HIV surveillance purposes was described previously [23,24]. Briefly, residual sera from specimens obtained from pregnant women for syphilis screening were continuously and consecutively sampled in eight participating laboratories. The eight laboratories were located at seven different sites across Slovenia, whereby one site comprised two laboratories (Figure). The second inclusion of specimens obtained from the same women during the same calendar year was prevented by keeping a separate list of identifying information on women whose sera had already been included into the sample during a particular year, which was checked before storing any new specimen. All specimens were labelled only with the information about the laboratory where samples were collected, sampling period (calendar year), and age group of the pregnant woman (<20, 20 24, 25 29, and 30 years) from whom the serum specimen had been obtained 2
3 for syphilis screening. They were frozen and stored at -20 C until testing [23]. Laboratory testing strategy All 31,849 specimens were initially tested for the presence of anti-hcv in pools of 12 specimens by using Ortho HCV Version 3.0 ELISA Test system. Individual specimens from screen reactive pools were retested with the same test. To identify pregnant women with active hepatitis C infection all screen repeatedly anti- HCV reactive specimens were further tested for the presence of HCV RA by reverse transcriptase polymerase chain reaction (RT-PCR)-based COBAS Amplicor HCV 2.0 (Roche Molecular Systems, Branchburg, J, US) test. Anti-HCV screen positive pregnant women with measurable HCV RA were considered as actively infected with hepatitis C. Anti-HCV screen positive pregnant women without measurable HCV RA were further tested with Hepatitis C Virus Encoded Antigen CHIRO RIBA HCV 3.0 Strip Immunoblot Assay (Chiron Corporation, Emeryville, US) to distinguish pregnant women with false positive anti-hcv screen test (negative with Immunoblot Assay) from those who spontaneously cleared hepatitis C in the past (positive with Immunoblot Assay). In all HCV RA positive specimens, HCV genotype was determined with InnoLiPa HCV 2.0 test (Innogenetics, Zwijndrecht, Belgium). Analyses Statistical analyses were performed using STATA package version 10.0 (Stata Statistical Software: release 10.0 College Station. TX: Stata Corporation). We estimated the overall and annual prevalence of anti-hcv and HCV RA together with 95% CIs, overall and according to age groups of pregnant women. Chi-squared test was used to assess the differences between the prevalence of anti-hcv and HCV RA in pregnant women of different ages for respective calendar years and for the differences between different calendar years. Ethical consent Ethical consent to unlinked anonymous testing of pregnant women screened for syphilis for HIV surveillance purposes (consent number 54/09/00) and ethical consent for HCV unlinked anonymous testing of specimens collected in 1999, 2003, 2009, and 2013 (consent number 86/04/13) were obtained from the Medical Ethics Committee at the Ministry of Health in Slovenia. Results Among a total of 31,849 sera specimens tested, 41 were anti-hcv positive, corresponding to the pooled prevalence estimate of anti-hcv of 0.13% (95% CI: 0.17). The 41 positive samples originated from all seven sentinel sites. Among 41 sera specimens positive for anti-hcv, 29 were positive for HCV RA, corresponding to the pooled prevalence estimate of HCV RA of % (95% Cl: ). Annual prevalence estimates for anti-hcv ranged between % (95% CI: ) in 2009 and 0.21% (95% CI: ) in 2003 and for HCV RA positivity between 0.06% (95% CI: ) in 2009 and 0.14% (95% CI: ) in 2003 (Table). We observed no statistically significant differences in anti- HCV or HCV RA prevalence between age groups (< 20, and 30 years) in any calendar year and no trend in time. Among a total of 29 pregnant women positive for HCV RA, 19 were infected with genotype 1 (12 with subtype 1b, 3 with subtype 1a, while in 4 cases subtype could not be determined) and 10 with genotype 3 (all subtype 3a). Infection with HCV genotypes 2, 4, 5 or 6 was not detected. Discussion The prevalence of antibodies to HCV and HCV viraemia among pregnant women in Slovenia was relatively low and we have not identified any changes during this 15 years period. In comparison to available data from other European countries, our estimates of prevalence of anti-hcv among pregnant women were more similar to published prevalence estimates among pregnant women in some western European countries (in the United Kingdom (UK): 0.2%, April 1997 June 1998; in Amsterdam, the etherlands: 0.3%, 2003). Our estimates were however lower than in some southern European countries (in northern Greece: 1.9%, March 1996 February 1997; in Bergamo, Italy: 2.4%, January 1995 December 1998) and eastern European countries (in Moldova: 2.3%, 1994) [25-29]. We should be cautious in comparing our results with the published results of similar studies, as different approaches were used for laboratory testing and for sampling/enrolling pregnant women into the studies (for example invitation to be voluntarily and confidentially tested accompanied with HCV related counselling in contrast to our unlinked anonymous testing of sera specimens obtained from a sera bank, which had been initially collected for syphilis screening purposes). Relatively low estimated anti-hcv and HCV RA prevalence among pregnant women in Slovenia in comparison to many other European countries may correspond to relatively low prevalence of anti-hcv among confidentially tested people who inject drugs [21]. Although some researchers have reported that anti- HCV prevalence among pregnant women increases with age, we did not found a statistically significant association between age group and prevalence in our study [25,30]. Only genotypes 1 and 3 were identified in our study which is consistent with the results of another Slovenian study in which chronic hepatitis C patients were enrolled and 93.8% of patients had genotypes 1 and 3 [20]. The fact that we did not find any patients with genotypes 4, 5 and 6, could be partly explained by the observation that the introduction of genotype 4, 3
4 Table Annual prevalence of antibodies to hepatitis C virus (HCV) and HCV viraemia among pregnant women, overall and by age group, 1999, 2003, 2009, and 2013, Slovenia (n=31,849) Age group in years < Total 0.27 ( ) ( ) 0.10 ( ) 0.12 ( ) Year HCV RA % 0.27 ( ) ( ) 0.05 ( ) ( ) 367 4,573 1,990 6,930 HCV RA % ( ) a ( ) a 0.27 ( ) 0.12 ( ) 0.21 ( ) 0.16 ( ) 0.12 ( ) 0.14 ( ) 4,475 2,547 7,281 HCV RA % 0.00 ( ) a 0.00 ( ) a ( ) ( ) ( ) 0.07 ( ) 0.05 ( ) 0.06 ( ) 4,185 3,745 8,064 HCV RA % ( ) a ( ) a ( ) 0.13 ( ) ( ) ( ) 0.08 ( ) 0.08 ( ) 4,645 4,760 9,574 Anti-HCV: antibodies to HCV; : number of sera collected from pregnant women for syphilis serology screening subjected to unlinked anonymous testing for anti-hcv or HCV RA; CI: confidence interval. a One-sided, 97.5% confidence interval. 5 and 6 in European countries has been related mostly to immigration from Africa and in Slovenia immigration from Africa has been relatively low [20]. Since the available estimates of HCV infection among pregnant women in Europe are generally relatively low, only two countries (orway and Spain) introduced antenatal screening programs for hepatitis C [31], while for example, in the UK, routine antenatal screening for hepatitis C virus infection was decided against [25]. Our approach to obtain estimates of anti-hcv and HCV RA prevalence by unlinked anonymous testing of rather large convenience samples of stored residual sera specimens obtained from pregnant women for syphilis screening in four calendar years spanning the period from 1999 to 2013, has proven to be logistically feasible. The strengths of such unlinked anonymous monitoring are minimised participation bias, noninvasive specimen collection and a very cost-efficient approach to collecting substantial number of specimens in laboratories. By repeating cross-sectional studies using the same methodology over time, we can monitor possible trends. As syphilis screening in Slovenia is universal and the numbers of residual sera tested corresponded to substantial proportions of pregnancies in respective calendar years (equivalent to 38% to 46% of deliveries), we believe that our prevalence estimates reflect quite accurately the true HCV prevalence among Slovenian pregnant women in those years. Pregnant women cannot be assumed to be representative of the general population. However, we believe that the estimated level of HCV infection prevalence among pregnant women may fairly well reflect the level of HCV infection prevalence in the Slovenian general population of reproductive ages, as suggested by others [32]. Other countries with constrained resources may consider using similar, logistically relatively simple and rather cost-effective, approaches to obtain better population HCV prevalence data. Our study had several limitations. We tested residual sera specimens from convenience and not probability samples of pregnant women in Slovenia during the respective years. We did not have information on additional risk behaviour for pregnant women (for example, information on possible history of sharing injecting equipment) and women whose sera specimens had not been included into our samples may have been at a different risk for HCV infection. Finally, we may have slightly underestimated the prevalence of HCV RA, as only screen repeatedly anti-hcv reactive specimens were tested for the presence of HCV RA and not all 31,849 sera specimens. However, we believe that because of very low anti-hcv prevalence and consequent extremely low probability for a specimen to be collected before seroconversion (recently infected person who is still anti-hcv negative while already HCV RA positive), little, if any, loss of sensitivity for ascertainment of HCV RA positivity would result from our testing algorithm. Thus we assumed that the estimation of HCV viraemia prevalence by our laboratory testing algorithm fairly well reflected the true prevalence of HCV viraemia. To conclude, our data represent the first reliable estimates of the relatively low burden of hepatitis C among pregnant women in Slovenia and suggest a relatively low burden of hepatitis C in the Slovenian general population. This suggests that the anti-hcv prevalence estimate for central Europe (2.4%) published in 2013 [1] may have been an overestimation and should be revised according to new information available. But it should be noted, that considerable heterogeneity in the HCV infection prevalence may exists among different countries of central Europe. Based on our results, opportunistic screening for HCV should not be recommended for pregnant women or the general population in Slovenia, however, voluntary HCV testing should be offered when there is a history of risk exposure or behaviour or a medical condition suggestive of HCV 4
5 infection. Opportunistic screening for HCV should only be targeted to groups at increased risk, such as people who inject drugs, persons with HIV infection, in particular men who have sex with men, and other groups at increased risk for HCV as defined by the WHO [14]. Acknowledgments We thank the personnel from the syphilis serology laboratories in the following healthcare institutions: General Hospital Maribor, Institute of Blood Transfusion of the Republic of Slovenia, Institute of Microbiology and Immunology, Faculty of Medicine, University of Ljubljana, Institute of Public Health Celje, Institute of Public Health Koper, Institute of Public Health Kranj, Institute of Public Health Maribor, Institute of Public Health ova Gorica, Institute of Public Health ovo mesto. We also thank Ms. Zdenka Kastelic for her dedicated support in coordination of the national unlinked anonymous HIV prevalence monitoring surveillance system and Robi Krošelj for his excellent support in laboratory testing. Conflict of interest one declared. Authors contributions BK contributed to the design of the study, analysed the data and drafted the manuscript. MP contributed to the design of the study, supervised the testing and commented on the final version of the manuscript. KS contributed to the supervision of testing and commented on the final version of the manuscript. IK designed the study, supervised analyses and contributed to drafting the manuscript. All authors participated in interpretation of the results and approved the final version of a manuscript. References 1. Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57(4): PMID: Ly K, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and Ann Intern Med. 2012;156(4): PMID: Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5 Pt 1): dx.doi.org/ / PMID: Backus LI, Boothroyd DB, Phillips BR, Belperio P, Halloran J, Mole LA. A sustained virologic response reduces risk of allcause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9(6): e1. cgh PMID: Lazarus JV, Sperle I, Matičič M, Wiessing L. A systematic review of Hepatitis C virus treatment uptake among people who inject drugs in the European Region. BMC Infect Dis. 2014;14(Suppl 6):S16. PMID: Esteban JI, Sauleda S, Quer J. The changing epidemiology of hepatitis C virus infection in Europe. J Hepatol. 2008;48(1): PMID: Rantala M, van de Laar MJ. Surveillance and epidemiology of hepatitis B and C in Europe - a review. Euro Surveill. 2008;13(21): PMID: Duberg A, Janzon R, Bäck E, Ekdahl K, Blaxhult A. The epidemiology of hepatitis C virus infection in Sweden. Euro Surveill. 2008;13(21): PMID: Cozzolongo R, Osella AR, Elba S, Petruzzi J, Buongiorno G, Giannuzzi V, et al.; UTRIHEP Collaborating Group. Epidemiology of HCV infection in the general population: a survey in a southern Italian town. Am J Gastroenterol. 2009;104(11): PMID: Delarocque-Astagneau E, Meffre C, Dubois F, Pioche C, Le Strat Y, Roudot-Thoraval F, et al.; Hepatitis C Surveillance System Committee; Scientific Committee for the ational Prevalence Survey of Hepatitis B and C Markers. The impact of the prevention programme of hepatitis C over more than a decade: the French experience. J Viral Hepat. 2010;17(6): dx.doi.org/111/j x PMID: World Health Organization (WHO). Global policy report on the prevention and control of viral hepatitis in WHO Member States. Geneva: WHO Press; p Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR-19):1-39. PMID: Smith BD, Morgan RL, Beckett GA, Falck-Ytter Y, Holtzman D, Teo CG, et al.; Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during MMWR Recomm Rep. 2012;61(RR-4):1-32. PMID: World Health Organization (WHO). Guidelines for the screening, care and treatment of persons with hepatitis C infection. Geneva: WHO Press; P Kraigher A, Sočan M, Klavs I, Frelih T, Grilc E, Grgič Vitek M, et al., editors. Epidemiološko spremljanje nalezljivih bolezni v Sloveniji v letu [Epidemiological surveillance of communicable diseases in Slovenia in 2012]. Ljubljana: Inštitut za varovanje zdravja; p Slovenian. 16. Seme K, Poljak M, Žužek-Resek S, Avšič-Zupanc T. High prevalence of hepatitis C virus infection in hemodialysis patients from one dialysis unit in Slovenia. ephron. 1995;71(1): PMID: Seme K, Poljak M, Lešničar G, Močivnik M. Dialysis unit without hepatitis C virus infection in Slovenia. ephron. 1996;73(2): PMID: Trišler Z, Seme K, Poljak M, Čelan-Lucu B, Sakoman S. Prevalence of hepatitis C and G virus infections among intravenous drug users in Slovenia and Croatia. Scand J Infect Dis. 1999;31(1): org/ / PMID: Seme K, Lunar MM, Tomažič J, Vidmar L, Karner P, Matičič M, et al. Low prevalence of hepatitis B and C infections among HIV-infected individuals in Slovenia: a nation-wide study, Acta Dermatovenerol Alp Pannonica Adriat. 2009;18(4): PMID: Seme K, Vrhovac M, Močilnik T, Matičič M, Lešničar G, Baklan Z, et al. Hepatitis C virus genotypes in 1,504 patients in Slovenia, J Med Virol. 2009;81(4): dx.doi.org/ /jmv PMID: Klavs I, Kustec T. Drug Related Infectious Diseases. In: Drev A, ed. Report on the Drug situation 2014 of the Republic of Slovenia. Ljubljana: Inštitut za varovanje zdravja; p Levičnik Stezinar S, Rahne Potokar U. Presejanje krvodajalcev na označevalce okužb v Sloveniji v obdobju [Screening of blood donors for markers of infection in Slovenia in the period ]. Zdrav Vestn. 2012;81(11): Slovenian. 23. Klavs I, Poljak M. Unlinked anonymous monitoring of human immunodeficiency virus prevalence in high- and low-risk groups in Slovenia, Croat Med J. 2003;44(5): PMID: Klavs I, Kustec T, Kastelic Z. Okužba s HIV v Sloveniji Letno poročilo [HIV infection in Slovenia Annual Report 2013]. Ljubljana: Inštitut za varovanje zdravja; p.25. Slovenian. 25. Ades AE, Parker S, Walker J, Cubitt WD, Jones R. HCV prevalence in pregnant women in the UK. Epidemiol Infect. 2000;125(2): S PMID: Urbanus AT, van Keep M, Matser AA, Rozenbaum MH, Weegink CJ, van den Hoek A, et al. Is adding HCV screening to the antenatal national screening program in Amsterdam, the etherlands, cost-effective? PLoS OE. 2013;8(8):e PMID:
6 27. Conte D, Fraquelli M, Prati D, Colucci A, Minola E. Prevalence and clinical course of chronic hepatitis C virus (HCV) infection and rate of HCV vertical transmission in a cohort of 15,250 pregnant women. Hepatology. 2000;31(3): org/ /hep PMID: Raptopoulou-Gigi M, Orphanou E, Lalla TH, Lita A, Garifallos A. Prevalence of hepatitis C virus infection in a cohort of pregnant women in northern Greece and transmission of HCV from mother to child. Eur J Epidemiol. 2001;17(3): dx.doi.org/ /a: PMID: Drobeniuc J, Hutin YJ, Harpaz R, Favorov M, Melnik A, Iarovoi P, et al. Prevalence of hepatitis B, D and C virus infections among children and pregnant women in Moldova: additional evidence supporting the need for routine hepatitis B vaccination of infants. Epidemiol Infect. 1999;123(3): org/ /s PMID: Costa ZB, Machado GC, Avelino MM, Gomes Filho C, Macedo Filho JV, Minuzzi AL, et al. Prevalence and risk factors for Hepatitis C and HIV-1 infections among pregnant women in Central Brazil. BMC Infect Dis. 2009;9(116): org/186/ PMID: European Centre for Disease Prevention and Control (ECDC). Surveillance and prevention of hepatitis B and C in Europe. Stockholm: ECDC; Urbanus AT, van de Laar TJW, van den Hoek A, Zuure FR, Speksnijder AGCL, Baaten GGG, et al. Hepatitis C in the general population of various ethnic origins living in the etherlands: should non-western migrants be screened? J Hepatol. 2011;55(6): jhep PMID:
Hepatitis C. Core slides
 Hepatitis C Core slides This material was prepared by the Viral Hepatitis Prevention Board The slides (or subsets) can be reproduced for educational use only, with reference to the original source and
Hepatitis C Core slides This material was prepared by the Viral Hepatitis Prevention Board The slides (or subsets) can be reproduced for educational use only, with reference to the original source and
Annual Epidemiological Report
 November 218 Annual Epidemiological Report 1 Hepatitis C in Ireland, 217 Key Facts Number of cases, 217: 62 Crude notification rate, 217: 13/1, population The number of notifications of hepatitis C decreased
November 218 Annual Epidemiological Report 1 Hepatitis C in Ireland, 217 Key Facts Number of cases, 217: 62 Crude notification rate, 217: 13/1, population The number of notifications of hepatitis C decreased
A national multidisciplinary healthcare network for treatment of hepatitis C in PWID in Slovenia Prof. Mojca Matičič, MD, PhD
 A national multidisciplinary healthcare network for treatment of hepatitis C in PWID in Slovenia Prof. Mojca Matičič, MD, PhD Clinic for Infectious Diseases and Febrile Illnesses University Medical Centre
A national multidisciplinary healthcare network for treatment of hepatitis C in PWID in Slovenia Prof. Mojca Matičič, MD, PhD Clinic for Infectious Diseases and Febrile Illnesses University Medical Centre
Screening programmes for Hepatitis B/C in Europe
 Programme STI, HIV/AIDS and viral hepatitis Screening programmes for Hepatitis B/C in Europe Mika Salminen, Ph.D. European Centre for Disease Prevention and Control Why might screening be needed for hepatitis
Programme STI, HIV/AIDS and viral hepatitis Screening programmes for Hepatitis B/C in Europe Mika Salminen, Ph.D. European Centre for Disease Prevention and Control Why might screening be needed for hepatitis
VIRAL HEPATITIS: SITUATION ANALYSIS AND PERSPECTIVES IN THE AFRICAN REGION. Report of the Secretariat. CONTENTS Paragraphs BACKGROUND...
 8 April 2014 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH PROGRAMME SUBCOMMITTEE Sixty-fourth session Brazzaville, Republic of Congo, 9 11 June 2014 Provisional agenda item 6 VIRAL HEPATITIS: SITUATION
8 April 2014 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH PROGRAMME SUBCOMMITTEE Sixty-fourth session Brazzaville, Republic of Congo, 9 11 June 2014 Provisional agenda item 6 VIRAL HEPATITIS: SITUATION
Lymphogranuloma venereum
 Annual Epidemiological Report for 2015 Lymphogranuloma venereum Key facts In 2015, 1 787 cases of Lymphogranuloma venereum (LGV) were reported in 23 countries. Three countries (France, the Netherlands
Annual Epidemiological Report for 2015 Lymphogranuloma venereum Key facts In 2015, 1 787 cases of Lymphogranuloma venereum (LGV) were reported in 23 countries. Three countries (France, the Netherlands
VIRAL HEPATITIS: SITUATION ANALYSIS AND PERSPECTIVES IN THE AFRICAN REGION. Report of the Secretariat. CONTENTS Paragraphs BACKGROUND...
 5 November 2014 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH Sixty-fourth session Cotonou, Republic of Benin, 3 7 November 2014 Provisional agenda item 11 VIRAL HEPATITIS: SITUATION ANALYSIS AND PERSPECTIVES
5 November 2014 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH Sixty-fourth session Cotonou, Republic of Benin, 3 7 November 2014 Provisional agenda item 11 VIRAL HEPATITIS: SITUATION ANALYSIS AND PERSPECTIVES
EDMA HIV-AIDS TEAM Fact Sheet November 2007
 EDMA HIV-AIDS TEAM Fact Sheet November 2007 1. HIV Facts AIDS epidemic update UNAIDS Epidemic Update, November 2007 (1) 760,000 people to be living with HIV in Western and Central Europe in 2007. 31,000
EDMA HIV-AIDS TEAM Fact Sheet November 2007 1. HIV Facts AIDS epidemic update UNAIDS Epidemic Update, November 2007 (1) 760,000 people to be living with HIV in Western and Central Europe in 2007. 31,000
Hepatitis A SURVEILLANCE REPORT. Annual Epidemiological Report for Key facts. Methods
 SURVEILLANCE REPORT Annual Epidemiological Report for 2015 Hepatitis A Key facts In 2015, 29 EU/EEA countries reported a total of 12 641 cases of hepatitis A, 12 527 (99.1%) of which were confirmed. The
SURVEILLANCE REPORT Annual Epidemiological Report for 2015 Hepatitis A Key facts In 2015, 29 EU/EEA countries reported a total of 12 641 cases of hepatitis A, 12 527 (99.1%) of which were confirmed. The
HEPATITIS C, ACUTE CRUDE DATA. Number of Cases 5 Annual Incidence a LA County 0.05 California b 0.10 United States b 0.68 Age at Diagnosis Mean 38
 2016 Annual Morbidity Report HEPATITIS C, ACUTE a Rates calculated based on less than 19 cases or events are considered unreliable b Calculated from: CDC. Notice to Readers: Final 2016 Reports of Nationally
2016 Annual Morbidity Report HEPATITIS C, ACUTE a Rates calculated based on less than 19 cases or events are considered unreliable b Calculated from: CDC. Notice to Readers: Final 2016 Reports of Nationally
HEV Update Blood Components. Dragoslav Domanović, European Centre for Disease Prevention and Control (Sweden)
 HEV Update Blood Components Dragoslav Domanović, European Centre for Disease Prevention and Control (Sweden) Outline ECDC activities started in 2015 to understand the epidemiology and burden of HEV infection
HEV Update Blood Components Dragoslav Domanović, European Centre for Disease Prevention and Control (Sweden) Outline ECDC activities started in 2015 to understand the epidemiology and burden of HEV infection
Digestive & Liver Disease Wellness
 Digestive & Liver Disease Wellness Colorectal Cancer Screening & Prevention According to the American Cancer Society, this year 136,830 people in the U.S. According will be to diagnosed the American with
Digestive & Liver Disease Wellness Colorectal Cancer Screening & Prevention According to the American Cancer Society, this year 136,830 people in the U.S. According will be to diagnosed the American with
Weekly Influenza Surveillance Report. Week 11
 Weekly Influenza Surveillance Report Week 11 Report produced: 22/03/2001 Influenza activity in Ireland For the week ending the 18/03/01, week 11, influenza activity has increased. Sentinel general practices
Weekly Influenza Surveillance Report Week 11 Report produced: 22/03/2001 Influenza activity in Ireland For the week ending the 18/03/01, week 11, influenza activity has increased. Sentinel general practices
Hepatitis C Seroprevalence Among HIV-Infected Childbearing Women in New York State in 2006
 DOI 10.1007/s10995-015-1853-4 Hepatitis C Seroprevalence Among HIV-Infected Childbearing Women in New York State in 2006 L. Ghazaryan 1 L. Smith 2 M. Parker 3 C. Flanigan 4 W. Pulver 2 T. Sullivan 5 A.
DOI 10.1007/s10995-015-1853-4 Hepatitis C Seroprevalence Among HIV-Infected Childbearing Women in New York State in 2006 L. Ghazaryan 1 L. Smith 2 M. Parker 3 C. Flanigan 4 W. Pulver 2 T. Sullivan 5 A.
Version for the Silent Procedure 29 April Agenda item January Hepatitis
 Version for the Silent Procedure 29 April 2014 134th session EB134.R18 Agenda item 10.5 25 January 2014 Hepatitis The Executive Board, Having considered the report on hepatitis, 1 RECOMMENDS to the Sixty-seventh
Version for the Silent Procedure 29 April 2014 134th session EB134.R18 Agenda item 10.5 25 January 2014 Hepatitis The Executive Board, Having considered the report on hepatitis, 1 RECOMMENDS to the Sixty-seventh
Commonly Asked Questions About Chronic Hepatitis C
 Commonly Asked Questions About Chronic Hepatitis C From the American College of Gastroenterology 1. How common is the hepatitis C virus? The hepatitis C virus is the most common cause of chronic viral
Commonly Asked Questions About Chronic Hepatitis C From the American College of Gastroenterology 1. How common is the hepatitis C virus? The hepatitis C virus is the most common cause of chronic viral
Recommendations for Hepatitis C Screening
 Hepatitis C Online PDF created June 16, 2018, 9:14 am Recommendations for Hepatitis C Screening This is a PDF version of the following document: Module 1: Screening and Diagnosis of Hepatitis C Infection
Hepatitis C Online PDF created June 16, 2018, 9:14 am Recommendations for Hepatitis C Screening This is a PDF version of the following document: Module 1: Screening and Diagnosis of Hepatitis C Infection
HIV, HBV and HCV testing policy experiences and lessons learned.
 HIV, HBV and HCV testing policy experiences and lessons learned. Contents HIV and viral hepatitis: distinct epidemics at different stages of evolution Epidemiology: transmission, prevalence, incidence
HIV, HBV and HCV testing policy experiences and lessons learned. Contents HIV and viral hepatitis: distinct epidemics at different stages of evolution Epidemiology: transmission, prevalence, incidence
Access to treatment and disease burden
 Access to treatment and disease burden Robert Flisiak Department of Infectious Diseases and Hepatology Medical University in Białystok, Poland Moulin de Vernègues, 27-29 August 2015 Disclosures Advisor
Access to treatment and disease burden Robert Flisiak Department of Infectious Diseases and Hepatology Medical University in Białystok, Poland Moulin de Vernègues, 27-29 August 2015 Disclosures Advisor
HIV SCREENING WORKSHOP Exercise
 HIV SCREENING WORKSHOP Exercise INTRODUCTION: Since its first discovery in 1981, AIDS became a pandemic. Worldwide, actually over 30 million people are living with HIV (i.e., are infected by the human
HIV SCREENING WORKSHOP Exercise INTRODUCTION: Since its first discovery in 1981, AIDS became a pandemic. Worldwide, actually over 30 million people are living with HIV (i.e., are infected by the human
Primary Care Services for blood borne viral hepatitis prevention, treatment and care
 Primary Care Services for blood borne viral hepatitis prevention, treatment and care Author: Josie Smith, Marion Lyons Page 1 of 13 October 2006 Status : Final Contents: Page: Executive Summary 3 Introduction
Primary Care Services for blood borne viral hepatitis prevention, treatment and care Author: Josie Smith, Marion Lyons Page 1 of 13 October 2006 Status : Final Contents: Page: Executive Summary 3 Introduction
Viral Hepatitis Burden and Policy Directions in the European Region of WHO
 Viral Hepatitis Burden and Policy Directions in the European Region of WHO Dr Nedret Emiroglu WHO Regional Office for Europe Brussels 14-15 October 2010 Global Burden of Chronic Viral Hepatitis 2.7% all
Viral Hepatitis Burden and Policy Directions in the European Region of WHO Dr Nedret Emiroglu WHO Regional Office for Europe Brussels 14-15 October 2010 Global Burden of Chronic Viral Hepatitis 2.7% all
Estimating the incidence and prevalence of chronic hepatitis C (HCV) in Ireland
 Estimating the incidence and prevalence of chronic hepatitis C (HCV) in Ireland Niamh Murphy & Lelia Thornton Health Protection Surveillance Centre RCPI, Faculty of Public Health Summer Scientific Meeting
Estimating the incidence and prevalence of chronic hepatitis C (HCV) in Ireland Niamh Murphy & Lelia Thornton Health Protection Surveillance Centre RCPI, Faculty of Public Health Summer Scientific Meeting
Epidemiology and Screening for Hepatitis C Infection
 Epidemiology and Screening for Hepatitis C Infection Atif Zaman, MD MPH Oregon Health & Science University Professor of Medicine Division of Gastroenterology and Hepatology Epidemiology/Screening for Hepatitis
Epidemiology and Screening for Hepatitis C Infection Atif Zaman, MD MPH Oregon Health & Science University Professor of Medicine Division of Gastroenterology and Hepatology Epidemiology/Screening for Hepatitis
Angelos Hatzakis. 10th Paris Hepatology Conference
 Angelos Hatzakis Professor of Epidemiology and Preventive Medicine Faculty of Medicine National and Kapodistrian University of Athens Co-Chair, Hepatitis B and C Public Policy Association 10th Paris Hepatology
Angelos Hatzakis Professor of Epidemiology and Preventive Medicine Faculty of Medicine National and Kapodistrian University of Athens Co-Chair, Hepatitis B and C Public Policy Association 10th Paris Hepatology
2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY. MEASURE TYPE: Process
 Quality ID #400 (NQF 3059): One-Time Screening for Hepatitis C Virus (HCV) for Patients at Risk National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS F INDIVIDUAL MEASURES: REGISTRY ONLY
Quality ID #400 (NQF 3059): One-Time Screening for Hepatitis C Virus (HCV) for Patients at Risk National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS F INDIVIDUAL MEASURES: REGISTRY ONLY
Viral Hepatitis. Dr Melissa Haines Gastroenterologist Waikato Hospital
 Viral Hepatitis Dr Melissa Haines Gastroenterologist Waikato Hospital Viral Hepatitis HAV HBV HCV HDV HEV Other viral: CMV, EBV, HSV Unknown Hepatitis A Hepatitis A Transmitted via the faecal-oral route
Viral Hepatitis Dr Melissa Haines Gastroenterologist Waikato Hospital Viral Hepatitis HAV HBV HCV HDV HEV Other viral: CMV, EBV, HSV Unknown Hepatitis A Hepatitis A Transmitted via the faecal-oral route
Hepatitis C Best Practice Guidelines For Local Health Departments
 Hepatitis C Best Practice Guidelines For Local Health Departments LHDs are responsible for investigating and reporting all physician reported cases of acute hepatitis C (HCV). For clients known to have
Hepatitis C Best Practice Guidelines For Local Health Departments LHDs are responsible for investigating and reporting all physician reported cases of acute hepatitis C (HCV). For clients known to have
Epidemiology of HCV infection among HD pts in Iran and prevention strategies
 Epidemiology of HCV infection among HD pts in Iran and prevention strategies B. Einollahi Professor of Internal Medicine/Nephrology Division Baqiyatallah University of Medical Sciences 4th International
Epidemiology of HCV infection among HD pts in Iran and prevention strategies B. Einollahi Professor of Internal Medicine/Nephrology Division Baqiyatallah University of Medical Sciences 4th International
National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention
 National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention Increased Hepatitis C Virus (HCV) Detection in Women of Childbearing Age and Potential Risk for Vertical Transmission - United States
National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention Increased Hepatitis C Virus (HCV) Detection in Women of Childbearing Age and Potential Risk for Vertical Transmission - United States
Monthly measles and rubella monitoring report
 SURVEILLANCE REPORT Monthly measles and rubella monitoring report December 2018 Period covered: 1 November 2017 to 31 October 2018 Introduction This monitoring report is based on measles and rubella data
SURVEILLANCE REPORT Monthly measles and rubella monitoring report December 2018 Period covered: 1 November 2017 to 31 October 2018 Introduction This monitoring report is based on measles and rubella data
Seroprevalence of Hepatitis B Surface Antigen (HBsAg) Among Patients at a Tertiary Care Hospital in Mumbai, India
 International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 6 Number 4 (2017) pp. 722-726 Journal homepage: http://www.ijcmas.com Original Research Article https://doi.org/10.20546/ijcmas.2017.604.088
International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 6 Number 4 (2017) pp. 722-726 Journal homepage: http://www.ijcmas.com Original Research Article https://doi.org/10.20546/ijcmas.2017.604.088
Summary of Key Points. WHO Position Paper on Vaccines against Hepatitis B, July 2017
 Summary of Key Points WHO Position Paper on Vaccines against Hepatitis B, July 2017 1 Background l HBV is transmitted by exposure of mucosal membranes or non-intact skin to infected blood, saliva, semen
Summary of Key Points WHO Position Paper on Vaccines against Hepatitis B, July 2017 1 Background l HBV is transmitted by exposure of mucosal membranes or non-intact skin to infected blood, saliva, semen
Detection of Hepatitis B and C in Primary Care
 Detection of Hepatitis B and C in Primary Care Presentation 2 January 2016 Quality Quality Education Education for for aa Healthier Healthier Scotland Scotland 1 1 Learning Outcomes Participants will be
Detection of Hepatitis B and C in Primary Care Presentation 2 January 2016 Quality Quality Education Education for for aa Healthier Healthier Scotland Scotland 1 1 Learning Outcomes Participants will be
The U.S. Preventive Services Task Force (USPSTF) makes
 Annals of Internal Medicine Clinical Guideline Screening for Hepatitis C Virus Infection in Adults: U.S. Preventive Services Task Force Recommendation Statement Virginia A. Moyer, MD, MPH, on behalf of
Annals of Internal Medicine Clinical Guideline Screening for Hepatitis C Virus Infection in Adults: U.S. Preventive Services Task Force Recommendation Statement Virginia A. Moyer, MD, MPH, on behalf of
SHOULD ANTENATAL SCREENING FOR HEPATITIS C VIRUS SHOULD BE MADE PART OF ROUTINE CARE IN THE UK?
 The West London Medical Journal 2010 Vol 2 1 pp 1-11 SHOULD ANTENATAL SCREENING FOR HEPATITIS C VIRUS SHOULD BE MADE PART OF ROUTINE CARE IN THE UK? Krupa Pitroda Screening has the potential to save lives
The West London Medical Journal 2010 Vol 2 1 pp 1-11 SHOULD ANTENATAL SCREENING FOR HEPATITIS C VIRUS SHOULD BE MADE PART OF ROUTINE CARE IN THE UK? Krupa Pitroda Screening has the potential to save lives
Prevent Hepatocellular Carcinoma through Screening, Vaccination, and Treatment of Viral Hepatitis Milena Gould Suarez, MD
 Prevent Hepatocellular Carcinoma through Screening, Vaccination, and Treatment of Viral Hepatitis Milena Gould Suarez, MD Hello, my name is Milena Gould Suarez, and today I will present "Prevent Hepatocellular
Prevent Hepatocellular Carcinoma through Screening, Vaccination, and Treatment of Viral Hepatitis Milena Gould Suarez, MD Hello, my name is Milena Gould Suarez, and today I will present "Prevent Hepatocellular
TEN YEARS OF SYPHILIS TRENDS IN THE NORTHERN CAPE PROVINCE, SOUTH AFRICA, UTILISING THE NHLS CORPORATE DATA WAREHOUSE
 TEN YEARS OF SYPHILIS TRENDS IN THE NORTHERN CAPE PROVINCE, SOUTH AFRICA, UTILISING THE NHLS CORPORATE DATA WAREHOUSE Introduction Ngormbu Ballah 1,2,3, Lazarus Kuonza 1,3, Gloria De Gita 2, Alfred Musekiwa
TEN YEARS OF SYPHILIS TRENDS IN THE NORTHERN CAPE PROVINCE, SOUTH AFRICA, UTILISING THE NHLS CORPORATE DATA WAREHOUSE Introduction Ngormbu Ballah 1,2,3, Lazarus Kuonza 1,3, Gloria De Gita 2, Alfred Musekiwa
Table 2.6. Cohort studies of HCV and lymphoid malignancies
 HIV-negative subjects Ohsawa et al. (1999) Japan 2162 patients with HCVrelated chronic hepatitis (1398 men, 834 women), admitted to 3 medical institutions in Osaka between 1957 and 1997; age range: 18
HIV-negative subjects Ohsawa et al. (1999) Japan 2162 patients with HCVrelated chronic hepatitis (1398 men, 834 women), admitted to 3 medical institutions in Osaka between 1957 and 1997; age range: 18
Viral Hepatitis. WHO Regional Office for Europe July 2013
 Viral Hepatitis WHO Regional Office for Europe July 2013 What is Hepatitis? Hepatitis is a viral infection that causes inflammation of the liver There are five main types of viral hepatitis: A, B, C, D,
Viral Hepatitis WHO Regional Office for Europe July 2013 What is Hepatitis? Hepatitis is a viral infection that causes inflammation of the liver There are five main types of viral hepatitis: A, B, C, D,
Hepatitis B: A Preventable Cause of Liver Cancer. Saira Khaderi MD, MPH Assistant Professor of Surgery Associate Director, Project ECHO June 17, 2016
 Hepatitis B: A Preventable Cause of Liver Cancer Saira Khaderi MD, MPH Assistant Professor of Surgery Associate Director, Project ECHO June 17, 2016 Overview Epidemiology HBV and cancer Screening, Diagnosis
Hepatitis B: A Preventable Cause of Liver Cancer Saira Khaderi MD, MPH Assistant Professor of Surgery Associate Director, Project ECHO June 17, 2016 Overview Epidemiology HBV and cancer Screening, Diagnosis
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hepatitis_c 1/1/2019 N/A 1/1/2020 1/1/2019 Description of Procedure or Service Description Hepatitis C is
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hepatitis_c 1/1/2019 N/A 1/1/2020 1/1/2019 Description of Procedure or Service Description Hepatitis C is
Guidance for Industry
 Guidance for Industry Lookback for Hepatitis C Virus (HCV): Product Quarantine, Consignee Notification, Further Testing, Product Disposition, and Notification of Transfusion Recipients Based on Donor Test
Guidance for Industry Lookback for Hepatitis C Virus (HCV): Product Quarantine, Consignee Notification, Further Testing, Product Disposition, and Notification of Transfusion Recipients Based on Donor Test
Viral hepatitis in a multi-ethnic neighborhood in the Netherlands: results of a community-based study in a low prevalence country
 International Journal of Infectious Diseases (2009) 13, e9 e13 http://intl.elsevierhealth.com/journals/ijid Viral hepatitis in a multi-ethnic neighborhood in the Netherlands: results of a community-based
International Journal of Infectious Diseases (2009) 13, e9 e13 http://intl.elsevierhealth.com/journals/ijid Viral hepatitis in a multi-ethnic neighborhood in the Netherlands: results of a community-based
World Health Organization Regional Office for Europe Surveillance of measles and rubella Data as of 15 March 2006
 World Health Organization Regional Office for Europe Surveillance of measles and rubella Data as of 15 March 2006 WHO Regional Office for Europe Vaccine-preventable Diseases and Immunization programme,
World Health Organization Regional Office for Europe Surveillance of measles and rubella Data as of 15 March 2006 WHO Regional Office for Europe Vaccine-preventable Diseases and Immunization programme,
Original article J Bas Res Med Sci 2017; 4(2):4-8.
 Prevalence of HIV, hepatitis B and C infections among volunteer blood donors at the blood transfusion center of Ilam city, Iran Hasan Boustani 1, Enayat Anvari 2, Sedighe Saiadi Sartang 2, Mehdi Omidi
Prevalence of HIV, hepatitis B and C infections among volunteer blood donors at the blood transfusion center of Ilam city, Iran Hasan Boustani 1, Enayat Anvari 2, Sedighe Saiadi Sartang 2, Mehdi Omidi
Guidance for Industry
 Guidance for Industry Use of Nucleic Acid Tests on Pooled and Individual Samples from Donors of Whole Blood and Blood Components (including Source Plasma and Source Leukocytes) to Adequately and Appropriately
Guidance for Industry Use of Nucleic Acid Tests on Pooled and Individual Samples from Donors of Whole Blood and Blood Components (including Source Plasma and Source Leukocytes) to Adequately and Appropriately
T h e e p i d e m i o l o g y o f h e pat i t i s C v i r u s i n f e c t i o n in
 Surveillance and outbreak reports T h e e p i d e m i o l o g y o f h e pat i t i s C v i r u s i n f e c t i o n in S w e d e n A Duberg (ann-sofi.duberg@orebroll.se) 1, R Janzon 2, E Bäck 1,3, Karl Ekdahl
Surveillance and outbreak reports T h e e p i d e m i o l o g y o f h e pat i t i s C v i r u s i n f e c t i o n in S w e d e n A Duberg (ann-sofi.duberg@orebroll.se) 1, R Janzon 2, E Bäck 1,3, Karl Ekdahl
E u r o p e a n m o n i t o r i n g o f n o t i f i c at i o n s o f h e pat i t i s C
 Rapid Communications E u r o p e a n m o n i t o r i n g o f n o t i f i c at i o n s o f h e pat i t i s C v i r u s i n f e c t i o n in t h e g e n e r al p o p u l at i o n a n d a m o n g injecting
Rapid Communications E u r o p e a n m o n i t o r i n g o f n o t i f i c at i o n s o f h e pat i t i s C v i r u s i n f e c t i o n in t h e g e n e r al p o p u l at i o n a n d a m o n g injecting
ECDC and Spanish Ministry of Health workshop:
 ECDC and Spanish Ministry of Health workshop: Improving the monitoring of HIV among migrant populations in Europe, Madrid, 3-4 October 2013 Teymur Noori EU Commission Thank Tank on HIV/AIDS Luxembourg,
ECDC and Spanish Ministry of Health workshop: Improving the monitoring of HIV among migrant populations in Europe, Madrid, 3-4 October 2013 Teymur Noori EU Commission Thank Tank on HIV/AIDS Luxembourg,
Emerging epidemics: Will they derail progress? Eastern/Central Europe
 Emerging epidemics: Will they derail progress? Eastern/Central Europe Marieta Simonova, MD Clinic of Gastroenterology, Military Medical Academy Sofia, Bulgaria Disclosures Speaker/Adviser for AbbVie, Gilead,
Emerging epidemics: Will they derail progress? Eastern/Central Europe Marieta Simonova, MD Clinic of Gastroenterology, Military Medical Academy Sofia, Bulgaria Disclosures Speaker/Adviser for AbbVie, Gilead,
Comparative evaluation of Immunochromatographic Assay for screening Hepatitis C among blood donors in a rural teaching hospital, Sangareddy
 Original Research Article Comparative evaluation of Immunochromatographic Assay for screening Hepatitis C among blood donors in a rural teaching hospital, Sangareddy Nagababu Pyadala 1*, Prudhvi Chand
Original Research Article Comparative evaluation of Immunochromatographic Assay for screening Hepatitis C among blood donors in a rural teaching hospital, Sangareddy Nagababu Pyadala 1*, Prudhvi Chand
Hepatitis C Virus. https://www.labcorp.com/wps/wcm/connect/labcorp+content/labcorp/education+and+re...
 Page 1 of 16 Hepatitis C Virus Data reflected in this report are based solely on the collection of samples submitted to LabCorp for testing. Refer to the limitations section of this report for additional
Page 1 of 16 Hepatitis C Virus Data reflected in this report are based solely on the collection of samples submitted to LabCorp for testing. Refer to the limitations section of this report for additional
SIXTY-SECOND WORLD HEALTH ASSEMBLY A62/22 Provisional agenda item April Viral hepatitis. Report by the Secretariat
 SIXTY-SECOND WORLD HEALTH ASSEMBLY A62/22 Provisional agenda item 12.17 16 April 2009 Viral hepatitis Report by the Secretariat THE DISEASES AND BURDEN 1. The group of viruses that cause acute and/or chronic
SIXTY-SECOND WORLD HEALTH ASSEMBLY A62/22 Provisional agenda item 12.17 16 April 2009 Viral hepatitis Report by the Secretariat THE DISEASES AND BURDEN 1. The group of viruses that cause acute and/or chronic
Notes Setting the Scene
 We need to know who is at risk of Hepatitis B and C infections so that we can identify them and offer them testing. Primary care has important roles in diagnosing patients but also after diagnosis, helping
We need to know who is at risk of Hepatitis B and C infections so that we can identify them and offer them testing. Primary care has important roles in diagnosing patients but also after diagnosis, helping
Who is a migrant? 12/10/2018. HIV and migrants. Heterogeneous groups of persons with different migration drivers
 HIV and migrants Julia del Amo has received research grants awarded to her institution from Companies BMS, Gilead, ViiV and epidemiology teaching fees from ViiV, Gilead and MSD Dr Julia del Amo National
HIV and migrants Julia del Amo has received research grants awarded to her institution from Companies BMS, Gilead, ViiV and epidemiology teaching fees from ViiV, Gilead and MSD Dr Julia del Amo National
Viral Hepatitis Diagnosis and Management
 Viral Hepatitis Diagnosis and Management CLINICAL BACKGROUND Viral hepatitis is a relatively common disease (25 per 100,000 individuals in the United States) caused by a diverse group of hepatotropic agents
Viral Hepatitis Diagnosis and Management CLINICAL BACKGROUND Viral hepatitis is a relatively common disease (25 per 100,000 individuals in the United States) caused by a diverse group of hepatotropic agents
The epidemiology of hepatitis C in Canada
 The epidemiology of hepatitis C in Canada FACT SHEET Published 2017 This fact sheet provides a snapshot of the hepatitis C epidemic in Canada. It is one of a series of fact sheets providing epidemiological
The epidemiology of hepatitis C in Canada FACT SHEET Published 2017 This fact sheet provides a snapshot of the hepatitis C epidemic in Canada. It is one of a series of fact sheets providing epidemiological
2014/LSIF/PD/025 Malaysia s Approach to Testing Strategies
 2014/LSIF/PD/025 Malaysia s Approach to Testing Strategies Submitted by: Malaysia Policy Dialogue and Workshop on Attaining a Safe and Sustainable Blood Supply Chain Manila, Philippines 30 September 1
2014/LSIF/PD/025 Malaysia s Approach to Testing Strategies Submitted by: Malaysia Policy Dialogue and Workshop on Attaining a Safe and Sustainable Blood Supply Chain Manila, Philippines 30 September 1
Giardiasis (lambliasis)
 SURVEILLANCE REPORT Annual Epidemiological Report for 2016 Giardiasis (lambliasis) Key facts In 2016, 18 985 confirmed giardiasis cases were reported in the EU/EEA, representing an increase of 5.3% from
SURVEILLANCE REPORT Annual Epidemiological Report for 2016 Giardiasis (lambliasis) Key facts In 2016, 18 985 confirmed giardiasis cases were reported in the EU/EEA, representing an increase of 5.3% from
SIXTY-SEVENTH WORLD HEALTH ASSEMBLY A67/13 Provisional agenda item March Hepatitis
 SIXTY-SEVENTH WORLD HEALTH ASSEMBLY A67/13 Provisional agenda item 12.3 28 March 2014 Hepatitis Improving the health of patients with viral hepatitis Report by the Secretariat 1. The Executive Board at
SIXTY-SEVENTH WORLD HEALTH ASSEMBLY A67/13 Provisional agenda item 12.3 28 March 2014 Hepatitis Improving the health of patients with viral hepatitis Report by the Secretariat 1. The Executive Board at
ECDC s role in the fight against HIV/AIDS in Europe
 European Centre for Disease Prevention and Control ECDC s role in the fight against HIV/AIDS in Europe Marita van de Laar, PhD Program Coordinator HIV/AIDS, STI, hepatitis HIV in Europe, Stockholm, 2 November
European Centre for Disease Prevention and Control ECDC s role in the fight against HIV/AIDS in Europe Marita van de Laar, PhD Program Coordinator HIV/AIDS, STI, hepatitis HIV in Europe, Stockholm, 2 November
Clinical Management of Hepatitis B WAN-CHENG CHOW DEPARTMENT OF GASTROENTEROLOGY & HEPATOLOGY SINGAPORE GENERAL HOSPITAL
 Clinical Management of Hepatitis B WAN-CHENG CHOW DEPARTMENT OF GASTROENTEROLOGY & HEPATOLOGY SINGAPORE GENERAL HOSPITAL The World Health Organisation recent initiatives on HBV infection Launching of the
Clinical Management of Hepatitis B WAN-CHENG CHOW DEPARTMENT OF GASTROENTEROLOGY & HEPATOLOGY SINGAPORE GENERAL HOSPITAL The World Health Organisation recent initiatives on HBV infection Launching of the
Situation update in the European Region: overview of influenza surveillance data week 40/2009 to week 07/2010.
 Situation update in the European Region: overview of influenza surveillance data week 40/2009 to week 07/2010. WHO/Europe publishes a weekly electronic bulletin on influenza activity in the Region 1 and
Situation update in the European Region: overview of influenza surveillance data week 40/2009 to week 07/2010. WHO/Europe publishes a weekly electronic bulletin on influenza activity in the Region 1 and
risk of Transfusion-Transmitted Transmitted Viral Infections (HIV, HCV and HBV)
 Prevention and ResidualR risk of Transfusion-Transmitted Transmitted Viral Infections (HIV, HCV and HBV) Syria Laperche L (Centre National de référence r rence pour les hépatites h B et C en Transfusion)
Prevention and ResidualR risk of Transfusion-Transmitted Transmitted Viral Infections (HIV, HCV and HBV) Syria Laperche L (Centre National de référence r rence pour les hépatites h B et C en Transfusion)
REVIEW OF THE ANALYSIS RELATED TO RABIES DIAGNOSIS AND FOLLOW-UP OF ORAL VACCINATION PERFORMED IN NRLS IN 2015
 European Union European Union WHO Collaborating OIE Reference Reference Centre Reference NANCY LABORATORY FOR RABIES AND WILDLIFE Laboratory for Rabies Institute for Rabies Serology for Research and Management
European Union European Union WHO Collaborating OIE Reference Reference Centre Reference NANCY LABORATORY FOR RABIES AND WILDLIFE Laboratory for Rabies Institute for Rabies Serology for Research and Management
We need to know who is at risk of Hepatitis B and C infections so that we can identify them and offer them testing. Primary care has important roles
 0 We need to know who is at risk of Hepatitis B and C infections so that we can identify them and offer them testing. Primary care has important roles in diagnosing patients but also after diagnosis, helping
0 We need to know who is at risk of Hepatitis B and C infections so that we can identify them and offer them testing. Primary care has important roles in diagnosing patients but also after diagnosis, helping
Criteria Grid Best Practices and Interventions for the Diagnosis and Treatment of Hepatitis C
 Criteria Grid Best Practices and Interventions for the Diagnosis and Treatment of Hepatitis C Best Practice/Intervention: Moyer VA. (2013) U.S.Preventive Services Task Force. Screening for hepatitis C
Criteria Grid Best Practices and Interventions for the Diagnosis and Treatment of Hepatitis C Best Practice/Intervention: Moyer VA. (2013) U.S.Preventive Services Task Force. Screening for hepatitis C
Activities in 2010, Priorities for 2011
 European Centre for Disease Prevention and Control Activities in 2010, Priorities for 2011 Presentation to ENVI Committee by Marc Sprenger, Director ECDC 27 October 2010 Part 1: Examples of ECDC s work
European Centre for Disease Prevention and Control Activities in 2010, Priorities for 2011 Presentation to ENVI Committee by Marc Sprenger, Director ECDC 27 October 2010 Part 1: Examples of ECDC s work
SEROPREVALENCE OF ANTI-HCV ANTIBODIES AMONG VOLUNTARY BLOOD DONORS IN FATEHABAD DISTRICT OF HARYANA
 SEROPREVALENCE OF ANTI-HCV ANTIBODIES AMONG VOLUNTARY BLOOD DONORS IN FATEHABAD DISTRICT OF HARYANA *Sangwan L., Arun P. 2 and Munjal A. 3 Haryana Civil Medical Service, Fatehabad, Haryana *Author for
SEROPREVALENCE OF ANTI-HCV ANTIBODIES AMONG VOLUNTARY BLOOD DONORS IN FATEHABAD DISTRICT OF HARYANA *Sangwan L., Arun P. 2 and Munjal A. 3 Haryana Civil Medical Service, Fatehabad, Haryana *Author for
Chronic viral hepatitis and liver disease in Belgium Pierre Deltenre
 Chronic viral hepatitis and liver disease in Belgium Pierre Deltenre Brussels, November 7, 2017 Hepatitis B and C in Belgium What we need to know 1. Who is at risk of infection? 2. What is the natural
Chronic viral hepatitis and liver disease in Belgium Pierre Deltenre Brussels, November 7, 2017 Hepatitis B and C in Belgium What we need to know 1. Who is at risk of infection? 2. What is the natural
Complicated viral infections
 Complicated viral infections Clinical case discussion Diagnostic dilemmas NSW State Reference Laboratory for HIV St Vincent s Hospital Sydney Diagnostic dilemmas Indeterminate or discordant serology (western
Complicated viral infections Clinical case discussion Diagnostic dilemmas NSW State Reference Laboratory for HIV St Vincent s Hospital Sydney Diagnostic dilemmas Indeterminate or discordant serology (western
HBV vaccination: Optimizing coverage and efficacy. Alex Vorsters, Pierre Van Damme Viral Hepatitis Prevention Board
 HBV vaccination: Optimizing coverage and efficacy. Alex Vorsters, Pierre Van Damme Viral Hepatitis Prevention Board 2 nd Hepatitis Cure and Eradication Meeting 11 12 November 2015 Declaration of Interest
HBV vaccination: Optimizing coverage and efficacy. Alex Vorsters, Pierre Van Damme Viral Hepatitis Prevention Board 2 nd Hepatitis Cure and Eradication Meeting 11 12 November 2015 Declaration of Interest
Hepatitis C in the WHO European Region
 Hepatitis C in the WHO European Region Antons Mozalevskis WHO Regional Office for Europe Drug-related infectious disease (DRID) annual expert meeting 15 16 June 2015, EMCDDA Lisbon Burden of viral hepatitis
Hepatitis C in the WHO European Region Antons Mozalevskis WHO Regional Office for Europe Drug-related infectious disease (DRID) annual expert meeting 15 16 June 2015, EMCDDA Lisbon Burden of viral hepatitis
How France is eliminating HCV and the role of screening strategies
 HCV Elimination Mini Policy Summit «Eliminating HCV in Romania» European Parliament, 27 September 2017 How France is eliminating HCV and the role of screening strategies Sylvie Deuffic-Burban, Inserm,
HCV Elimination Mini Policy Summit «Eliminating HCV in Romania» European Parliament, 27 September 2017 How France is eliminating HCV and the role of screening strategies Sylvie Deuffic-Burban, Inserm,
10/07/18. Dr. Otilia Mårdh, ECDC. No conflicts of interest.
 No conflicts of interest. Prevention and control of chlamydia in Europe from data to policies and testing recommendations Dr. Otilia Mårdh, ECDC 15th Congress of the European Society of Contraception and
No conflicts of interest. Prevention and control of chlamydia in Europe from data to policies and testing recommendations Dr. Otilia Mårdh, ECDC 15th Congress of the European Society of Contraception and
Hepatitis B vaccine alone or with HBIG in neonates of HBsAg+/HBeAg- mothers: a systematic review and meta-analysis.
 Hepatitis B vaccine alone or with HBIG in neonates of HBsAg+/HBeAg- mothers: a systematic review and meta-analysis. Vana Papaevangelou (Greece) National and Kapodistrian University of Athens Chang SemFNM
Hepatitis B vaccine alone or with HBIG in neonates of HBsAg+/HBeAg- mothers: a systematic review and meta-analysis. Vana Papaevangelou (Greece) National and Kapodistrian University of Athens Chang SemFNM
HIV in the United Kingdom: 2009 Report
 HIV in the United Kingdom: 2009 Report Key findings The number of people living with HIV in the UK continues to rise, with an estimated 83,000 infected at the end of 2008, of whom over a quarter (27%)
HIV in the United Kingdom: 2009 Report Key findings The number of people living with HIV in the UK continues to rise, with an estimated 83,000 infected at the end of 2008, of whom over a quarter (27%)
NIH Consensus Conference Statement. Management of Hepatitis C. March 24-26, NIH Web site. Available at:
 ABC s of Hepatitis C Treatment Today Elizabeth N. Britton, MSN, FNP-BC Hepatology Services Louisiana State University Health Sciences Center ebritt@lsuhsc.edu ANAC CONFERENCE -TUCSON NOV 2012 Hepatitis
ABC s of Hepatitis C Treatment Today Elizabeth N. Britton, MSN, FNP-BC Hepatology Services Louisiana State University Health Sciences Center ebritt@lsuhsc.edu ANAC CONFERENCE -TUCSON NOV 2012 Hepatitis
Draft resolution proposed by Brazil, Colombia, Costa Rica, Egypt, Republic of Moldova and South Africa
 Draft resolution proposed by Brazil, Colombia, Costa Rica, Egypt, Republic of Moldova and South Africa Resolutions WHA 63.18: Recognized viral hepatitis as a global public health problem; The need for
Draft resolution proposed by Brazil, Colombia, Costa Rica, Egypt, Republic of Moldova and South Africa Resolutions WHA 63.18: Recognized viral hepatitis as a global public health problem; The need for
Transfusion Risk for Hepatitis B, Hepatitis C and HIV in the State of Santa Catarina, Brazil,
 236 BJID 2004; 8 (June) Transfusion Risk for Hepatitis B, Hepatitis C and HIV in the State of Santa Catarina, Brazil, 1991-2001 Emil Kupek Department of Public Health, Federal University of Santa Catarina,
236 BJID 2004; 8 (June) Transfusion Risk for Hepatitis B, Hepatitis C and HIV in the State of Santa Catarina, Brazil, 1991-2001 Emil Kupek Department of Public Health, Federal University of Santa Catarina,
Monitoraggio delle epatiti virali in Europa
 Monitoraggio delle epatiti virali in Europa Pier Luigi Lopalco Università di Bari Roma, 17 Dicembre 2015 By courtesy of the Programme for HIV, STI and Viral Hepatitis B and C infections, ECDC Surveillance
Monitoraggio delle epatiti virali in Europa Pier Luigi Lopalco Università di Bari Roma, 17 Dicembre 2015 By courtesy of the Programme for HIV, STI and Viral Hepatitis B and C infections, ECDC Surveillance
ELPA S VISION OF SCREENING
 ELPA S VISION OF SCREENING By Nadine Piorkowsky President of the European Liver Patients Association, Budapest, 18-19 March 2010 ELPA s network The Patient s View a viral time bomb: Up to 9 out of 10 chronic
ELPA S VISION OF SCREENING By Nadine Piorkowsky President of the European Liver Patients Association, Budapest, 18-19 March 2010 ELPA s network The Patient s View a viral time bomb: Up to 9 out of 10 chronic
VIRUS VACCINES & CANCER a story with two chapters
 VIRUS VACCINES & CANCER a story with two chapters F X BOSCH Institut Catala d Oncologia Sant Pau 2017 Potential conflict of interest Research and educational institutional grants: GSK, SPMSD, Merck, Qiagen
VIRUS VACCINES & CANCER a story with two chapters F X BOSCH Institut Catala d Oncologia Sant Pau 2017 Potential conflict of interest Research and educational institutional grants: GSK, SPMSD, Merck, Qiagen
Shigellosis SURVEILLANCE REPORT. Annual Epidemiological Report for Key facts. Methods
 Annual Epidemiological Report for 2016 Shigellosis Key facts Shigellosis is a relatively uncommon disease in the EU/EEA, but remains of concern in some countries and for some population groups. In 2016,
Annual Epidemiological Report for 2016 Shigellosis Key facts Shigellosis is a relatively uncommon disease in the EU/EEA, but remains of concern in some countries and for some population groups. In 2016,
Estimating the treatment cascade of chronic hepatitis B and C in Greece using a telephone survey
 Journal of Viral Hepatitis, 2015, 22, 409 415 doi:10.1111/jvh.12314 Estimating the treatment cascade of chronic hepatitis B and C in Greece using a telephone survey G. Papatheodoridis, 1 V. Sypsa, 2 M.
Journal of Viral Hepatitis, 2015, 22, 409 415 doi:10.1111/jvh.12314 Estimating the treatment cascade of chronic hepatitis B and C in Greece using a telephone survey G. Papatheodoridis, 1 V. Sypsa, 2 M.
A SYSTEMATIC REVIEW OF HEPATITIS B AND C TESTING IN THE COUNTRIES OF THE WHO EUROPEAN REGION
 A SYSTEMATIC REVIEW OF HEPATITIS B AND C TESTING IN THE COUNTRIES OF THE WHO EUROPEAN REGION Jeffrey V. Lazarus* 1, Ida Sperle1, Jürgen K. Rockstroh2, Alexander Spina3, Lucas Wiessing4 1CHIP, Rigshospitalet,
A SYSTEMATIC REVIEW OF HEPATITIS B AND C TESTING IN THE COUNTRIES OF THE WHO EUROPEAN REGION Jeffrey V. Lazarus* 1, Ida Sperle1, Jürgen K. Rockstroh2, Alexander Spina3, Lucas Wiessing4 1CHIP, Rigshospitalet,
Key issues for HIV testing and
 Key issues for HIV testing and counselling in Europe Martin C Donoghoe Programme Manager HIV/AIDS, STI &Viral Hepatitis Programme WHO Europe Key issues for HIV testing & counselling in Europe HIV epidemics
Key issues for HIV testing and counselling in Europe Martin C Donoghoe Programme Manager HIV/AIDS, STI &Viral Hepatitis Programme WHO Europe Key issues for HIV testing & counselling in Europe HIV epidemics
Media centre. WHO Hepatitis B. Key facts. 1 of :12 AM.
 1 of 5 2013-08-02 7:12 AM Media centre Hepatitis B Share Print Fact sheet N 204 Updated July 2013 Key facts Hepatitis B is a viral infection that attacks the liver and can cause both acute and chronic
1 of 5 2013-08-02 7:12 AM Media centre Hepatitis B Share Print Fact sheet N 204 Updated July 2013 Key facts Hepatitis B is a viral infection that attacks the liver and can cause both acute and chronic
The Alphabet Soup of Viral Hepatitis Testing
 The Alphabet Soup of Viral Hepatitis Testing August 18, 2011 Patricia Slev, PhD, DABCC Medical Director, Serologic Hepatitis and Retrovirus Laboratory, ARUP Laboratories Assistant Professor of Pathology,
The Alphabet Soup of Viral Hepatitis Testing August 18, 2011 Patricia Slev, PhD, DABCC Medical Director, Serologic Hepatitis and Retrovirus Laboratory, ARUP Laboratories Assistant Professor of Pathology,
Improving NSP and Harm Reduction Services. Jason Farrell, European HCV and Drug Use Initiative
 Improving NSP and Harm Reduction Services Jason Farrell, European HCV and Drug Use Initiative Presentation Overview With the growing need to treat HCV, NSP programs have been identified in numerous stakeholder
Improving NSP and Harm Reduction Services Jason Farrell, European HCV and Drug Use Initiative Presentation Overview With the growing need to treat HCV, NSP programs have been identified in numerous stakeholder
UK Standards for Microbiology Investigations
 UK Standards for Microbiology Investigations Blood Borne Virus Testing in Dialysis Patients Issued by the Standards Unit, Microbiology Services, PHE Virology V 10 Issue no: 2 Issue date: 27.04.15 Page:
UK Standards for Microbiology Investigations Blood Borne Virus Testing in Dialysis Patients Issued by the Standards Unit, Microbiology Services, PHE Virology V 10 Issue no: 2 Issue date: 27.04.15 Page:
Trichinellosis SURVEILLANCE REPORT. Annual Epidemiological Report for Key facts. Methods
 Annual Epidemiological Report for 2015 Trichinellosis Key facts In 2015, a total of 156 confirmed cases of trichinellosis was reported from 29 EU/EEA countries. The overall notification rate was 0.03 cases
Annual Epidemiological Report for 2015 Trichinellosis Key facts In 2015, a total of 156 confirmed cases of trichinellosis was reported from 29 EU/EEA countries. The overall notification rate was 0.03 cases
Confirmed (Laboratory Tests) Serum positive for IgM anti-hbc or, hepatitis B surface antigen (HbsAg).
 Hepatitis B Hepatitis B is a liver disease that results from infection with the Hepatitis B virus. It can range in severity from a mild illness lasting a few weeks to a serious, lifelong illness. Hepatitis
Hepatitis B Hepatitis B is a liver disease that results from infection with the Hepatitis B virus. It can range in severity from a mild illness lasting a few weeks to a serious, lifelong illness. Hepatitis
The new German strategy on HIV, Hepatitis B, C and STI, an integrated approach. Ines Perea Ministry of Health, Germany
 The new German strategy on HIV, Hepatitis B, C and STI, an integrated approach Ines Perea Ministry of Health, Germany Reasons for a new strategy in 2016 New international agreements (SDG s) Renewed political
The new German strategy on HIV, Hepatitis B, C and STI, an integrated approach Ines Perea Ministry of Health, Germany Reasons for a new strategy in 2016 New international agreements (SDG s) Renewed political
Open Access Ongoing Care and Follow-up Behavior of Working Age Japanese with Hepatitis C Virus
 Send Orders for Reprints to reprints@benthamscience.net The Open Public Health Journal, 2014, 7, 1-5 1 Open Access Ongoing Care and Follow-up Behavior of Working Age Japanese with Hepatitis C Virus Koji
Send Orders for Reprints to reprints@benthamscience.net The Open Public Health Journal, 2014, 7, 1-5 1 Open Access Ongoing Care and Follow-up Behavior of Working Age Japanese with Hepatitis C Virus Koji
Hepatitis STARS Program. Geri Brown, M.D. Associate Professor Department of Internal Medicine October 4, 2003
 Hepatitis 2003 STARS Program Geri Brown, M.D. Associate Professor Department of Internal Medicine October 4, 2003 Outline n Hepatitis A Epidemiology and screening Transmission n Hepatitis B Epidemiology
Hepatitis 2003 STARS Program Geri Brown, M.D. Associate Professor Department of Internal Medicine October 4, 2003 Outline n Hepatitis A Epidemiology and screening Transmission n Hepatitis B Epidemiology
Weekly influenza surveillance overview
 SURVEILLANCE REPORT Weekly influenza surveillance overview 7 February 2014 Main surveillance developments in week 5/2014 (27 January 2014 2 February 2014) This first page contains the main developments
SURVEILLANCE REPORT Weekly influenza surveillance overview 7 February 2014 Main surveillance developments in week 5/2014 (27 January 2014 2 February 2014) This first page contains the main developments
Prevalence of hepatitis C virus infection among pregnant women in a rural district in Egypt
 Article Prevalence of hepatitis C virus infection among pregnant women in a rural district in Egypt Tropical Doctor 2016, Vol. 46(1) 21 27! The Author(s) 2014 Reprints and permissions: sagepub.co.uk/journalspermissions.nav
Article Prevalence of hepatitis C virus infection among pregnant women in a rural district in Egypt Tropical Doctor 2016, Vol. 46(1) 21 27! The Author(s) 2014 Reprints and permissions: sagepub.co.uk/journalspermissions.nav
New Brunswick Report on Sexually Transmitted and Blood Borne Infections, 2016
 New Brunswick Report on Sexually Transmitted and Blood Borne Infections, 6 Table of Contents. Introduction.... Methodology... 3. Data Limitations.... Definitions used... 3 5. Overview of STBBI epidemiology
New Brunswick Report on Sexually Transmitted and Blood Borne Infections, 6 Table of Contents. Introduction.... Methodology... 3. Data Limitations.... Definitions used... 3 5. Overview of STBBI epidemiology
