CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE
|
|
|
- William Marshall
- 5 years ago
- Views:
Transcription
1 CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts this device to sale by or on the order of a physician. CONTRAINDICATIONS Presence of an active infection at the implantation site. Patients known or suspected to have an allergy to PLA or absorbable materials. WARNINGS Intended for single use only. Do NOT re-sterilize and/or reuse. Do not use the device if the integrity of the sterile packaging has been compromised or a loss of sterility is suspected. Improper patient selection, surgical site preparation, or implantation may potentially cause device failure and/or adverse reactions. The LATERA Absorbable Nasal Implant is not intended to replace normal healthy bone or cartilage. Subsequent infection may require Implant removal. Do not expose the LATERA Absorbable Nasal Implant to high temperatures and do not use if package temperature indicator shows exposure to temperature above 38 C. POSSIBLE ADVERSE EFFECTS Adverse reactions typical to surgically implanted materials may occur. These include: Inflammatory foreign body reaction, foreign body sensation, pain or discomfort, infection, and extrusion. Excessive activity, trauma, or loading may lead to bending, fracture, loosening, and/or migration of the Implant. Implants placed near the skin surface may be palpable or cause skin irritation. Temporary hematoma from cannula insertion. PACKAGING STERILE: The LATERA Absorbable Nasal Implant is sterilized with electron beam radiation. Do not use if the package is open or damaged. The Accessory Delivery Device and Implant Positioning Guide are sterilized with electron beam radiation. Do not use if the package is open or damaged. IMPLANT STORAGE: Store in a cool, dry location at or below 30 C. ACCESSORY DELIVERY DEVICE STORAGE: Store in a cool dry place. SINGLE USE: The LATERA Absorbable Nasal Implant, Accessory Delivery Device and Implant Positioning Guide are intended for single patient use only. The Accessory Delivery Device may be used to deliver multiple LATERA Absorbable Nasal Implants to a single patient in a single clinical setting. Do NOT re-sterilize and/or reuse. Effective Date: 2/23/2018 Page 1 of 9
2 DEVICE DESCRIPTION The Spirox LATERA Absorbable Nasal Implant system is composed of the LATERA Absorbable Nasal Implant (Implant) and the Accessory Delivery Device (Delivery Device). An Implant Positioning Guide is provided to serve as an external visual planning aid prior to Implant placement. The Implant is predominantly cylindrical in shape with a diameter of 1 mm and an overall length of 24 mm with a forked distal end for anchoring and features on the proximal end for increased flexibility. The Implant is composed of Poly (L-lactide-co-D-L-lactide) 70:30 copolymer which is absorbed in the body over a period of approximately 18 months. The Implant is provided in a plastic tray with a slidable lid. The Implant is depicted in Figure 1 below. Forked Tip Atraumatic Flex Tip Figure 1: LATERA Absorbable Nasal Implant and Packaging The Delivery Device is a single use device composed of an inner shaft, an outer handle with a push rod, a deploy button, an open button and a 16 gauge delivery cannula with a protective cover. The inner shaft includes an implant loading port which enables the loading of the Implant and may include graphics to indicate the open position. The inner shaft transitions between the open position and the cannula to collapse the implant forks within the cannula inner lumen and prepare the Implant for deployment. The outer handle includes deploy and open buttons that lock and release the handle from these respective positions. The outer handle also includes a push rod that shuttles the implant from the implant loading port to a ready position for deployment. The Implant Positioning Guide is packaged with the Delivery Device and is provided as an aid to the physician for planning the procedure and identifying the target Implant location. The Delivery Device and the Implant Positioning Guide are shown in Figure 2 below. Effective Date: 2/23/2018 Page 2 of 9
3 Fork Orientation Features Ready Position Deploy Button Open Button Delivery Cannula (Cover removed) Green Ring Open Position Implant Loading Port Outer Handle Inner Shaft* Implant Positioning Guide Distal and Proximal Marking Holes Figure 2: Delivery Device and Implant Positioning Guide *NOTE: Some Delivery Devices will not include the graphics shown on the Inner Shaft. COMPATIBILITY The LATERA Absorbable Nasal Implant is compatible with the Accessory Delivery Device. The Accessory Delivery Device should be used for proper insertion of the Absorbable Nasal Implant. Effective Date: 2/23/2018 Page 3 of 9
4 INSTRUCTIONS FOR USE Implant Target Location and Device Preparation: 1. Standard surgical procedures should be used to prepare the site for implantation (e.g. cleaning, disinfection, anesthetic, etc.) 2. Prior to implantation, identify the target Implant location and cannula insertion trajectory. The forked distal tip of the Implant should be positioned adjacent and across the maxilla bone and the cylindrical portion of the Implant should be positioned to support the upper and lower lateral cartilage as shown in Figure 3. Figure 3: Example Implant location showing support of upper and lower lateral cartilage and position of Implant forks across maxilla bone to cartilage transition (dashed line). 3. Use the Implant Positioning Guide to mark the surgical trajectory as shown in Figure 4 using a standard surgical pen. The holes provided on the Implant Positioning Guide allow for marking the base of Implant forked tip and the spherical end of the atraumatic proximal tip. The distal mark correlates to the final position of the cannula tip prior to Implant delivery. Figure 4: Implant Positioning Guide superimposed on upper and lower lateral cartilage 4. Retract the outer handle of the Delivery Device by gripping the distal flange of the inner shaft, holding the open button in the depressed position and gently pulling until the push rod is clear of the implant loading port. On devices with inner shaft graphics, the green ring will be proximal to the solid black line graphic when the push rod is clear of the implant loading port. Continue to retract the outer handle fully until the pushrod is clear of the implant loading port. Effective Date: 2/23/2018 Page 4 of 9
5 5. Using sterile surgical forceps transfer the Implant from the plastic tray to the implant loading port of the Delivery Device as shown in Figure 5. Figure 5: Delivery Device with Implant Loaded 6. Slowly advance the outer handle until the outer handle locks into the ready position to advance the Implant into the delivery cannula. While advancing, watch the implant load into cannula inner lumen. This positions the Implant at the tip of the cannula in the ready position. Proper implant loading and handle positioning is shown in Figure 6 below. Note: If the deploy button is pressed prematurely and the outer handle is advanced too far, the Implant may exit the delivery cannula. If this should happen, completely advance the outer handle so the forks exit the cannula. Carefully remove the Implant from the cannula and repeat the setup process from Step 4. Proper Cannula Advancement and Implant loading Ready Position (a) Fully Deployed Position (b) (c) Figure 6: Images showing (a) Proper Cannula advancement and Implant Loading, (b) Delivery Device Ready Position, (c) Delivery Device Deployed Position Effective Date: 2/23/2018 Page 5 of 9
6 Implant Delivery: 7. Under direct visualization, the delivery cannula is inserted through the nasal vestibular lining of the lateral wall within the nasal cavity near the margin of the nostril. A small conventional scalpel incision at the target cannula entry location may be optionally created to ease cannula puncture. Approximate Delivery Device orientation and cannula trajectory is shown in Figure 7 below. Figure 7: Approximate Delivery Device orientation relative to nasal anatomy 8. Identify the cannula insertion point, Figure 8(a) below, to provide the maximum distance between the cannula insertion point and the target position of the proximal tip of the Implant to ensure the Implant is fully embedded within the tissue, Figure 8(b) below. (a) (b) Figure 8: Images showing (a) approximate caudal cannula pierce position in the vestibular lining at the margin of the nostril and (b) the distance intended to be maximized between cannula pierce point and the proximal tip of the Implant 9. The cannula should pass along the center of the thickness of the lateral wall to avoid piercing medial through the mucosa or lateral through the skin as it traverses the wall to the target location. 10. When the cannula reaches the bony cartilaginous junction, the cannula is passed over the maxillary bone to the target depth. 11. If the nasal tissue has compressed or bunched-up during cannula insertion, relax the tissue to its native position. Verify that the cannula is inserted deep enough such that the tip of the cannula is positioned over the maxilla bone. An example of appropriate cannula position within the nasal lateral wall is shown in Figure 9 below. Effective Date: 2/23/2018 Page 6 of 9
7 1. Instructions For Use Figure 9: Cannula at target depth mid-thickness within the lateral wall structure. The intended position of the cannula tip and cannula are shown in the magnified views 12. The Implant forks will expand to their original shape as they exit the cannula tip in the orientation they are loaded. Using the Implant fork orientation features on the distal end of the Delivery Device as a reference for fork orientation, verify that the Delivery Device rotation about its axis is appropriate to deliver the forks parallel to the underlying bone. CAUTION: The Implant forks diverge as they engage with the tissue; orientation of the forks must be controlled by orienting the Delivery Device to prevent forks from piercing towards skin surface. 13. When the cannula is in the appropriate location and orientation, the deploy button can be depressed and released and the outer handle can be carefully advanced to the deployed position. Keep fingers proximal to the green ring when deploying the implant. Cannula may be stabilized with off-hand while deploying, if desired. The Implant forks are driven approximately 4 mm into the tissue beyond the distal tip of the cannula when deployed. The Implant delivery process is shown in Figure 10 below. Effective Date: 2/23/2018 Page 7 of 9
8 Depress Deploy Button Fully Deployed The cannula may be stabilized during deployment Slowly advance outer handle to deploy Implant Figure 10a Figure 10b Figure 10: Images of the Delivery Device proximal end and cannula positioned within the lateral wall showing Implant deployment process including: (a) Actuation of the Delivery Device outer handle and (b) Implant forks expanded approximately 4 mm distal to the cannula tip after deployment. CAUTION: The forks will advance approximately 4 mm beyond the tip of the cannula when deployed. This should be accounted for in determining the extent of cannula advancement. CAUTION: Do not hold the inner shaft of the delivery device while deploying. Applying all distal pressure to the outer handle ensures minimal Delivery Device movement during deployment. Effective Date: 2/23/2018 Page 8 of 9
9 14. Following deployment, slowly withdraw the cannula from the tissue. Take care to not alter the angle or rotational orientation of the Delivery Device while withdrawing or the Implant could be dislodged. 15. After complete withdrawal, visually examine the insertion site to ensure the Implant is not exposed and is fully embedded within the tissue. Do not compress or fold the lateral wall to visualize the insertion site. The insertion site may be optionally closed by conventional suture techniques. 16. If multiple implantation attempts are required, a second insertion should utilize a different pierce point within the mucosa and follow a different cannula trajectory. 17. Counsel the patient to avoid manipulation of the nose during the acute healing period (e.g., Week 1: do not pinch or blow nose; Weeks 1-2: avoid strenuous activity; Weeks 1-4: do not place objects inside of nose). DISPOSAL The Accessory Delivery Device should be disposed of in a biohazard sharps disposal container. The Implant Positioning Guide and the LATERA Absorbable Nasal Implant container may be disposed of along with standard medical waste. GRAPHIC SYMBOLS CONTAINED IN DEVICE LABELING STERILE R Sterilized using Irradiation Keep Away from Sunlight Batch Code Do Not Re-Use Catalog Number On Order of Physician Only Manufactured For Date of Manufacture Use By Consult Instructions For Use Contents of Package/Box Upper Limit of Temperature Keep dry Do not use if package is damaged TRADEMARKS AND PATENTS Spirox and LATERA are trademarks or registered trademarks of Spirox Incorporated in the U.S. and other countries. Consult a list of patents covering this product at Effective Date: 2/23/2018 Page 9 of 9
The Ceterix NovoStitch Disposable Suture Passer. Do Not Reuse
 The Ceterix NovoStitch Disposable Suture Passer Ceterix Orthopaedics 959 Hamilton Avenue Menlo Park, CA 94025 Customer Service: (650) 241-1748 IK Sterile D Do Not Reuse i See Instructions for Use THE CETERIX
The Ceterix NovoStitch Disposable Suture Passer Ceterix Orthopaedics 959 Hamilton Avenue Menlo Park, CA 94025 Customer Service: (650) 241-1748 IK Sterile D Do Not Reuse i See Instructions for Use THE CETERIX
Peel-Apart Percutaneous Introducer Kits for
 Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Visit Linvatec.com today to learn more. Surgical Technique: Sequential Meniscal Running Stitch
 ORDERING INFORMATION Sequent Meniscal Repair System MR004S MR007S MR004C MR007C SC047D Straight Needle, 4 Implants Straight Needle, 7 Implants Curved Needle, 4 Implants Curved Needle, 7 implants Sequent
ORDERING INFORMATION Sequent Meniscal Repair System MR004S MR007S MR004C MR007C SC047D Straight Needle, 4 Implants Straight Needle, 7 Implants Curved Needle, 4 Implants Curved Needle, 7 implants Sequent
Endorectal Balloon (ERB) Endorectal Balloon (ERB) Instructions for Use
 Endorectal Balloon (ERB) Instructions for Use GRF-0004-00 Rev. 08 Page 1 of 9 Contents Instructions for Use... 3 Indications for Use... 3 Contraindications... 3 Storage... 4 Warnings... 4 Accessories Provided...
Endorectal Balloon (ERB) Instructions for Use GRF-0004-00 Rev. 08 Page 1 of 9 Contents Instructions for Use... 3 Indications for Use... 3 Contraindications... 3 Storage... 4 Warnings... 4 Accessories Provided...
Single Use Curlew TM Multiple Biopsy Forceps
 Single Use Curlew TM Multiple Biopsy Forceps 13 SPECIMEN WITH METAL STORAGE CYLINDER With In Situ Fixation, Batch or Single Specimen Collection US Patents 5,782,747; 5,980,468; 6,071,248; foreign patents
Single Use Curlew TM Multiple Biopsy Forceps 13 SPECIMEN WITH METAL STORAGE CYLINDER With In Situ Fixation, Batch or Single Specimen Collection US Patents 5,782,747; 5,980,468; 6,071,248; foreign patents
mild Devices Kit - Instructions for Use
 INDICATION FOR USE The Vertos mild Devices are specialized surgical instruments intended to be used to perform lumbar decompressive procedures for the treatment of various spinal conditions. CONTENTS AND
INDICATION FOR USE The Vertos mild Devices are specialized surgical instruments intended to be used to perform lumbar decompressive procedures for the treatment of various spinal conditions. CONTENTS AND
Technique Guide KISSloc Suture System
 Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Technique Guide Small Bone Fusion System
 Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
with Blom-Singer Gel Cap Insertion System
 with Blom-Singer Gel Cap Insertion System MEDICAL PROFESSIONAL Instructions For Use R2 37269-02G 37269-02G Effective January 2016 Blom-Singer and InHealth Technologies are registered trademarks of Freudenberg
with Blom-Singer Gel Cap Insertion System MEDICAL PROFESSIONAL Instructions For Use R2 37269-02G 37269-02G Effective January 2016 Blom-Singer and InHealth Technologies are registered trademarks of Freudenberg
Instructions for Use io-flex Probe
 DEVICE DESCRIPTION: The is an accessory of the Amendia io-flex System, which includes the MicroBlade Shaver device. The io-flex Probe is designed to access the neural foramen. It is comprised of an outer
DEVICE DESCRIPTION: The is an accessory of the Amendia io-flex System, which includes the MicroBlade Shaver device. The io-flex Probe is designed to access the neural foramen. It is comprised of an outer
TABLE OF CONTENTS. 2 (8144 Rev 2)
 1 (8144 Rev 2) TABLE OF CONTENTS Introduction Conventus CAGE TM - Proximal Humerus...3 Indications and Contraindications...4 Surgical Summary...5 Patient Positioning & Approach...6 Surgical Technique Plate
1 (8144 Rev 2) TABLE OF CONTENTS Introduction Conventus CAGE TM - Proximal Humerus...3 Indications and Contraindications...4 Surgical Summary...5 Patient Positioning & Approach...6 Surgical Technique Plate
Device Preparation (all steps to be performed per standard interventional technique)
 sertera Biopsy Device Instructions for Use Please read all information carefully. Failure to properly follow the instructions may lead to unintended consequences. Important: This package insert is designed
sertera Biopsy Device Instructions for Use Please read all information carefully. Failure to properly follow the instructions may lead to unintended consequences. Important: This package insert is designed
Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter
 Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. DEVICE DESCRIPTION
Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. DEVICE DESCRIPTION
Quick Reference Guide
 Quick Reference Guide Indications for Use The AFX Endovascular AAA System is indicated for endovascular treatment in patients with AAA. The devices are indicated for patients with suitable aneurysm morphology
Quick Reference Guide Indications for Use The AFX Endovascular AAA System is indicated for endovascular treatment in patients with AAA. The devices are indicated for patients with suitable aneurysm morphology
Surgical Technique. CONQUEST FN Femoral Neck Fracture System
 Surgical Technique CONQUEST FN Femoral Neck Fracture System Table of Contents Introduction... 3 Indications... 3 Product Overview... 4 Surgical Technique... 5 Patient Positioning... 5 Reduce the Fracture...
Surgical Technique CONQUEST FN Femoral Neck Fracture System Table of Contents Introduction... 3 Indications... 3 Product Overview... 4 Surgical Technique... 5 Patient Positioning... 5 Reduce the Fracture...
JuggerLoc Bone-to-Bone System for Ankle Syndesmosis Fixation. Surgical Technique
 JuggerLoc Bone-to-Bone System for Ankle Syndesmosis Fixation Surgical Technique Table of Contents Position and Preparation... 2 Incision... 2 Fracture Reduction... 2 Drill Fibula and Tibia... 3 JuggerLoc
JuggerLoc Bone-to-Bone System for Ankle Syndesmosis Fixation Surgical Technique Table of Contents Position and Preparation... 2 Incision... 2 Fracture Reduction... 2 Drill Fibula and Tibia... 3 JuggerLoc
GENERAL TECHNIQUE GUIDE
 GENERAL TECHNIQUE GUIDE PRODUCT OVERVIEW The Eclipse Soft Tissue Anchor employs MedShape s shape memory PEEK Altera technology for soft tissue fixation procedures in the shoulder, elbow, knee, hand & wrist,
GENERAL TECHNIQUE GUIDE PRODUCT OVERVIEW The Eclipse Soft Tissue Anchor employs MedShape s shape memory PEEK Altera technology for soft tissue fixation procedures in the shoulder, elbow, knee, hand & wrist,
Initial placement 20FR Guidewire PEG kit REORDER NO:
 Initial placement 20FR Guidewire PEG kit REORDER NO: 00710802 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 20FR Guidewire
Initial placement 20FR Guidewire PEG kit REORDER NO: 00710802 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 20FR Guidewire
Cardiva Catalyst III INSTRUCTIONS FOR USE
 Cardiva Catalyst III INSTRUCTIONS FOR USE IFU 2469 Rev. G CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician. For single use only GRAPHICAL SYMBOLS ON THE CARDIVA
Cardiva Catalyst III INSTRUCTIONS FOR USE IFU 2469 Rev. G CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician. For single use only GRAPHICAL SYMBOLS ON THE CARDIVA
O.S.2-C & O.S.2-V. Static Staple - Stainless steel
 O.S.2-C & O.S.2-V Compression Staple PEEK Static Staple - Stainless steel 2 O.S.2 - TABLE OF CONTENTS O.S.2-C 3 Product description 4 Indications 8 Surgical technique 9 O.S.2-V 13 Product description 14
O.S.2-C & O.S.2-V Compression Staple PEEK Static Staple - Stainless steel 2 O.S.2 - TABLE OF CONTENTS O.S.2-C 3 Product description 4 Indications 8 Surgical technique 9 O.S.2-V 13 Product description 14
Pinit Plate Small Bone Fusion System Bone Plate & Screw System
 Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
ER REBOA Catheter. Instructions for Use
 ER REBOA Catheter Instructions for Use Prytime Medical Devices, Inc. 229 N. Main Street Boerne, TX 78006, USA feedback@prytimemedical.com www.prytimemedical.com US 1 210 340 0116 U.S. and Foreign Patents
ER REBOA Catheter Instructions for Use Prytime Medical Devices, Inc. 229 N. Main Street Boerne, TX 78006, USA feedback@prytimemedical.com www.prytimemedical.com US 1 210 340 0116 U.S. and Foreign Patents
Conventus CAGE PH Surgical Techniques
 Conventus CAGE PH Surgical Techniques Conventus Orthopaedics The Conventus CAGE PH (PH Cage) is a permanent implant comprised of an expandable scaffold, made from nitinol and titanium, which is deployed
Conventus CAGE PH Surgical Techniques Conventus Orthopaedics The Conventus CAGE PH (PH Cage) is a permanent implant comprised of an expandable scaffold, made from nitinol and titanium, which is deployed
Initial placement 24FR Pull PEG kit REORDER NO:
 Initial placement 24FR Pull PEG kit REORDER NO: 00710805 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 24FR Pull PEG kit is
Initial placement 24FR Pull PEG kit REORDER NO: 00710805 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 24FR Pull PEG kit is
Title: EZ-IO. Effective Date: January SOG Number: EMS Rescinds:
 S O G Title: EZ-IO Effective Date: January 2010 SOG Number: EMS - 25 Rescinds: Scope: Providers Authorized are AIC s in the following certifications EMT-I and EMT-P who have been trained and cleared by
S O G Title: EZ-IO Effective Date: January 2010 SOG Number: EMS - 25 Rescinds: Scope: Providers Authorized are AIC s in the following certifications EMT-I and EMT-P who have been trained and cleared by
LCP Medial Distal Tibia Plate, without Tab. The Low Profile Anatomic Fixation System with Angular Stability and Optimal Screw Orientation.
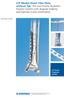 LCP Medial Distal Tibia Plate, without Tab. The Low Profile Anatomic Fixation System with Angular Stability and Optimal Screw Orientation. Technique Guide LCP Small Fragment System Table of Contents Introduction
LCP Medial Distal Tibia Plate, without Tab. The Low Profile Anatomic Fixation System with Angular Stability and Optimal Screw Orientation. Technique Guide LCP Small Fragment System Table of Contents Introduction
Per-Q-Cath* PICC Catheters with Excalibur Introducer* System
 Bard Access Systems Per-Q-Cath* PICC and Catheters with Excalibur Introducer* System Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description, Indications & Contraindications
Bard Access Systems Per-Q-Cath* PICC and Catheters with Excalibur Introducer* System Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description, Indications & Contraindications
DISTAL RADIUS. Instructions for Use
 DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
3. PATIENT POSITIONING & FRACTURE REDUCTION 3 8. DISTAL GUIDED LOCKING FOR PROXIMAL NAIL PROXIMAL LOCKING FOR LONG NAIL 13
 Contents IMPLANT FEATURES 2 1. INDICATIONS 3 2. PRE-OPERATIVE PLANNING 3 3. PATIENT POSITIONING & FRACTURE REDUCTION 3 4. INCISION 4 5. ENTRY POINT 4-6 6. PROXIMAL NAIL INSERTION 6-7 7. PROXIMAL LOCKING
Contents IMPLANT FEATURES 2 1. INDICATIONS 3 2. PRE-OPERATIVE PLANNING 3 3. PATIENT POSITIONING & FRACTURE REDUCTION 3 4. INCISION 4 5. ENTRY POINT 4-6 6. PROXIMAL NAIL INSERTION 6-7 7. PROXIMAL LOCKING
AXS Catalyst Distal Access Catheter
 AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
AXIOS Stent and Electrocautery Enhanced Delivery System. Quick Reference Guide
 AXIOS Stent and Electrocautery Enhanced Delivery System Quick Reference Guide AXIOS Stent (front and side views) UPN Codes Flange Diameter (mm) Lumen Diamter (mm) Saddle Length (mm) Catheter OD (Fr) Catheter
AXIOS Stent and Electrocautery Enhanced Delivery System Quick Reference Guide AXIOS Stent (front and side views) UPN Codes Flange Diameter (mm) Lumen Diamter (mm) Saddle Length (mm) Catheter OD (Fr) Catheter
Integra. Endoscopic Gastrocnemius Release System SURGICAL TECHNIQUE
 Integra Endoscopic Gastrocnemius Release System SURGICAL TECHNIQUE Table of Contents Indications... 2 Contraindications... 2 System Description... 2 Features and Benefits... 2 Surgical Site Preparation...3
Integra Endoscopic Gastrocnemius Release System SURGICAL TECHNIQUE Table of Contents Indications... 2 Contraindications... 2 System Description... 2 Features and Benefits... 2 Surgical Site Preparation...3
CLARIVEIN INFUSION CATHETER
 CLARIVEIN INFUSION CATHETER General Product Description Overview The ClariVein Infusion Catheter (ClariVein -IC) is an infusion catheter system designed to introduce physician-specified medicaments into
CLARIVEIN INFUSION CATHETER General Product Description Overview The ClariVein Infusion Catheter (ClariVein -IC) is an infusion catheter system designed to introduce physician-specified medicaments into
O.S.2-C. Compression staple PEEK
 3 O.S.2-C Compression staple PEEK 4 O.S.2 -C - PRODUCT DESCRIPTION (1/4) The O.S.2 staples are indicated for fixation of arthrodesis, osteotomies and fractures in hand or foot surgery. The size of the
3 O.S.2-C Compression staple PEEK 4 O.S.2 -C - PRODUCT DESCRIPTION (1/4) The O.S.2 staples are indicated for fixation of arthrodesis, osteotomies and fractures in hand or foot surgery. The size of the
EXACTECH SPINE. Operative Technique. Cervical Spacer System. Surgeon focused. Patient driven. TM
 EXACTECH SPINE Operative Technique Cervical Spacer System Surgeon focused. Patient driven. TM ACAPELLA ONE Acapella One Cervical Spacer System is an anterior cervical discectomy and fusion device with
EXACTECH SPINE Operative Technique Cervical Spacer System Surgeon focused. Patient driven. TM ACAPELLA ONE Acapella One Cervical Spacer System is an anterior cervical discectomy and fusion device with
SURGICAL TECHNIQUE GUIDE: JONES FRACTURE USING THE PRECISION JONES FRACTURE SCREW SYSTEM
 PRODUCT DESCRIPTION The PRECISION Jones Fracture Screw System offers extensive options of Type II Anodized Titanium screws. System-specific instrumentation is designed to address procedural challenges
PRODUCT DESCRIPTION The PRECISION Jones Fracture Screw System offers extensive options of Type II Anodized Titanium screws. System-specific instrumentation is designed to address procedural challenges
The following languages are included in this packet:
 OSTEOSET XR BONE VOID FILLER 150841-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
OSTEOSET XR BONE VOID FILLER 150841-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
Stratis INSTRUCTIONS FOR USE. Needle-free Injection System. 0.5mL volume (+/-5%)
 Stratis Needle-free Injection System INSTRUCTIONS FOR USE 0.5mL volume (+/-5%) Stratis English Symbols Glossary (Note: All symbols are derived from ISO 15223-1, Medical Devices - Symbols to be used with
Stratis Needle-free Injection System INSTRUCTIONS FOR USE 0.5mL volume (+/-5%) Stratis English Symbols Glossary (Note: All symbols are derived from ISO 15223-1, Medical Devices - Symbols to be used with
Technique Guide. 3.5 mm LCP Low Bend Medial Distal Tibia Plates. Part of the Synthes locking compression plate (LCP) system.
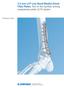 Technique Guide 3.5 mm LCP Low Bend Medial Distal Tibia Plates. Part of the Synthes locking compression plate (LCP) system. Table of Contents Introduction 3.5 mm LCP Low Bend Medial Distal Tibia Plates
Technique Guide 3.5 mm LCP Low Bend Medial Distal Tibia Plates. Part of the Synthes locking compression plate (LCP) system. Table of Contents Introduction 3.5 mm LCP Low Bend Medial Distal Tibia Plates
Corex HOSPITAL OVERVIEW AND TECHNICAL ASSESSMENT TECHNOLOGY OVERVIEW NEW TECHNOLOGY REFERENCE GUIDE. Minimally Invasive Bone Harvester
 NEW TECHNOLOGY REFERENCE GUIDE Corex Minimally Invasive Bone Harvester HOSPITAL OVERVIEW AND TECHNICAL ASSESSMENT The Corex Bone Harvester is a supplied sterile, single patient use, manually operated trephine
NEW TECHNOLOGY REFERENCE GUIDE Corex Minimally Invasive Bone Harvester HOSPITAL OVERVIEW AND TECHNICAL ASSESSMENT The Corex Bone Harvester is a supplied sterile, single patient use, manually operated trephine
RD180 SP TECHNOLOGY GUIDE READ THIS PRODUCT INSERT THOROUGHLY BEFORE USE
 RD180 SP TECHNOLOGY GUIDE READ THIS PRODUCT INSERT THOROUGHLY BEFORE USE FIG. 1 RD180 SP - THE RUNNING DEVICE 1 2 6 3 7 4 5 1 NEEDLE 2 FERRULE 3 SUTURE 4 DEVICE TIP 5 ANGLED SHAFT 6 HANDLE 7 PINK LEVER
RD180 SP TECHNOLOGY GUIDE READ THIS PRODUCT INSERT THOROUGHLY BEFORE USE FIG. 1 RD180 SP - THE RUNNING DEVICE 1 2 6 3 7 4 5 1 NEEDLE 2 FERRULE 3 SUTURE 4 DEVICE TIP 5 ANGLED SHAFT 6 HANDLE 7 PINK LEVER
nva Anterior Lumbar Interbody Fusion System
 nva Anterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
nva Anterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
System. Humeral Nail. Surgical Technique
 System Humeral Nail Surgical Technique Contents IMPLANT FEATURES 2 1. INDICATIONS 3 2. PRE-OPERATIVE PLANNING 3 3. PATIENT POSITIONING & FRACTURE REDUCTION 3 4. INCISION 4 5. ENTRY POINT 4-6 6. PROXIMAL
System Humeral Nail Surgical Technique Contents IMPLANT FEATURES 2 1. INDICATIONS 3 2. PRE-OPERATIVE PLANNING 3 3. PATIENT POSITIONING & FRACTURE REDUCTION 3 4. INCISION 4 5. ENTRY POINT 4-6 6. PROXIMAL
Balloon in Balloon (BIB )
 Balloon in Balloon (BIB ) Stent Placement Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed
Balloon in Balloon (BIB ) Stent Placement Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed
OptiFix Absorbable Fixation System
 OptiFix Absorbable Fixation System Absorbable Fixation Redefined Advancing the Fixation Experience. SOFT TISSUE REPAIR Right Procedure. Right Product. Right Outcome. Hernia Repair Fixation Challenges and
OptiFix Absorbable Fixation System Absorbable Fixation Redefined Advancing the Fixation Experience. SOFT TISSUE REPAIR Right Procedure. Right Product. Right Outcome. Hernia Repair Fixation Challenges and
Step by step description - Use of Bonopty Bone Biopsy System, 12 gauge
 Step by step description - Use of Bonopty Bone Biopsy System, 12 gauge Before use, please read the Instructions For Use which accompany the product for indications, contraindications, warnings and precautions.
Step by step description - Use of Bonopty Bone Biopsy System, 12 gauge Before use, please read the Instructions For Use which accompany the product for indications, contraindications, warnings and precautions.
SURGICAL TECHNIQUE GUIDE
 DANGER indicates an imminently hazardous situation which, if not avoided, will result in death or serious injury. WARNING indicates a potentially hazardous situation which, if not avoided, could result
DANGER indicates an imminently hazardous situation which, if not avoided, will result in death or serious injury. WARNING indicates a potentially hazardous situation which, if not avoided, could result
Altus Product description / Instructions for Use IFU 6 Rev. 1
 Content: 1 Introduction... 2 1.1 Legend... 2 1.2 Indication... 2 1.3 Contraindication... 2 1.4 Field of Application... 2 1.5 Sterilization... 2 1.6 Storage... 2 1.7 Allergies... 2 2 SYSTEM DESCRIPTION...
Content: 1 Introduction... 2 1.1 Legend... 2 1.2 Indication... 2 1.3 Contraindication... 2 1.4 Field of Application... 2 1.5 Sterilization... 2 1.6 Storage... 2 1.7 Allergies... 2 2 SYSTEM DESCRIPTION...
Headless Compession Screw 2.5 / 3.0
 SURGICAL TECHNIQUE Headless Compession Screw 2.5 / 3.0 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
SURGICAL TECHNIQUE Headless Compession Screw 2.5 / 3.0 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
EXCELLA ll. Spinal System
 EXCELLA ll Spinal System Excella II Spinal System INDICATIONS FOR USE The Innovasis Excella II Spinal System is intended for use in the non-cervical area of the spine. WARNING: The safety and effectiveness
EXCELLA ll Spinal System Excella II Spinal System INDICATIONS FOR USE The Innovasis Excella II Spinal System is intended for use in the non-cervical area of the spine. WARNING: The safety and effectiveness
Directions For Use. All directions should be read before use
 Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
FastFrame External Fixation System. Damage Control Surgical Technique
 FastFrame External Fixation System Damage Control Surgical Technique 1 FastFrame External Fixation System Damage Control Surgical Technique Table of Contents Introduction... 2 Indications and Contradictions...
FastFrame External Fixation System Damage Control Surgical Technique 1 FastFrame External Fixation System Damage Control Surgical Technique Table of Contents Introduction... 2 Indications and Contradictions...
FAST1 Intraosseous Infusion System. Training Session
 FAST1 Intraosseous Infusion System Training Session Why IO? Peripheral IV is often difficult to obtain Requires an average of 3-12 minutes Failure rate ranges between 10-40% AHA & ILCOR guidelines now
FAST1 Intraosseous Infusion System Training Session Why IO? Peripheral IV is often difficult to obtain Requires an average of 3-12 minutes Failure rate ranges between 10-40% AHA & ILCOR guidelines now
Arthroscopic Shoulder Repair Using the Smith & Nephew KINSA Suture Anchor
 Shoulder Series Technique Guide *smith&nephew KINSA Suture Anchor Arthroscopic Shoulder Repair Using the Smith & Nephew KINSA Suture Anchor Reviewed by: Larry D. Field, MD Mississippi Sports Medicine and
Shoulder Series Technique Guide *smith&nephew KINSA Suture Anchor Arthroscopic Shoulder Repair Using the Smith & Nephew KINSA Suture Anchor Reviewed by: Larry D. Field, MD Mississippi Sports Medicine and
CATHETER ACCESS KIT. For use with Prometra Programmable Infusion Systems
 CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
EndoRelease ENDOSCOPIC CUBITAL TUNNEL RELEASE SYSTEM
 EndoRelease ENDOSCOPIC CUBITAL TUNNEL RELEASE SYSTEM SURGICAL TECHNIQUE Up p e r Ex t r e m i t y So l u t i o n s ENDOSCOPIC CUBITAL TUNNEL RELEASE SYSTEM Description: The EndoRelease Endoscopic Cubital
EndoRelease ENDOSCOPIC CUBITAL TUNNEL RELEASE SYSTEM SURGICAL TECHNIQUE Up p e r Ex t r e m i t y So l u t i o n s ENDOSCOPIC CUBITAL TUNNEL RELEASE SYSTEM Description: The EndoRelease Endoscopic Cubital
Integra SURGICAL TECHNIQUE. MemoFix Super Elastic Nitinol Staple System. Super Elastic Nitinol Staple System
 Integra MemoFix Super Elastic Nitinol Staple System SURGICAL TECHNIQUE Super Elastic Nitinol Staple System Table of Contents Design Rationale...2 System Features...2 Indications...2 Contraindications...2
Integra MemoFix Super Elastic Nitinol Staple System SURGICAL TECHNIQUE Super Elastic Nitinol Staple System Table of Contents Design Rationale...2 System Features...2 Indications...2 Contraindications...2
Surgical Technique Guide
 Sacroiliac Joint Fusion System Surgical Technique Guide Moving Life Forward Table of Contents SiCure Implant Overview...2 SiCure System Information...3 X-ray Basics...4 Patient Positioning....5 Surgical
Sacroiliac Joint Fusion System Surgical Technique Guide Moving Life Forward Table of Contents SiCure Implant Overview...2 SiCure System Information...3 X-ray Basics...4 Patient Positioning....5 Surgical
Step by step description - Use of Bonopty Bone Biopsy System, 14 gauge
 Step by step description - Use of Bonopty Bone Biopsy System, 14 gauge Before use, please read the Instructions For Use which accompany the product for indications, contraindications, warnings and precautions.
Step by step description - Use of Bonopty Bone Biopsy System, 14 gauge Before use, please read the Instructions For Use which accompany the product for indications, contraindications, warnings and precautions.
Cervical Spacer System surgical technique
 Blackhawk TM Cervical Spacer System surgical technique Blackhawk TM The BLACKHAWK Cervical Spacer System is designed to provide biomechanical stabilization as an adjunct to fusion. Spinal fixation should
Blackhawk TM Cervical Spacer System surgical technique Blackhawk TM The BLACKHAWK Cervical Spacer System is designed to provide biomechanical stabilization as an adjunct to fusion. Spinal fixation should
RAPIDSORB RAPID ReSORBABLe CRANIAL CLAMP
 RAPIDSORB RAPID ReSORBABLe CRANIAL CLAMP Resorbable fixation of cranial bone flaps SURGICAL TeCHNIQUe RAPIDSORB Rapid Resorbable CRANIAL CLAMP Indication DePuy Synthes Companies of Johnson & Johnson RAPIDSORB
RAPIDSORB RAPID ReSORBABLe CRANIAL CLAMP Resorbable fixation of cranial bone flaps SURGICAL TeCHNIQUe RAPIDSORB Rapid Resorbable CRANIAL CLAMP Indication DePuy Synthes Companies of Johnson & Johnson RAPIDSORB
Headless Compession Screw 4.5 / 6.5 SURGICAL TECHNIQUE. Titanium or Stainless Steel. Cannulated Headless Design. Multiple Thread Options.
 Headless Compession Screw SURGICAL TECHNIQUE 4.5 / 6.5 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
Headless Compession Screw SURGICAL TECHNIQUE 4.5 / 6.5 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
HawkeyeTM Peek. surgical technique
 HawkeyeTM Peek surgical technique Introduction The ChoiceSpine HAWKEYE Vertebral Body Replacement (VBR) System is intended for use in the thoracolumbar spine (T1 - L5) to replace a collapsed, damaged,
HawkeyeTM Peek surgical technique Introduction The ChoiceSpine HAWKEYE Vertebral Body Replacement (VBR) System is intended for use in the thoracolumbar spine (T1 - L5) to replace a collapsed, damaged,
FLEXIC ATH LTD. Peripherally Inserted. Instructions n For Use.
 FLEXIC ATH LTD * M/29M Peripherally Inserted Catheter Instructions n For Use This leaflet contains instructions for both stan- dard needle-introducer er and protection con- tained M/29 models, i.e., with
FLEXIC ATH LTD * M/29M Peripherally Inserted Catheter Instructions n For Use This leaflet contains instructions for both stan- dard needle-introducer er and protection con- tained M/29 models, i.e., with
Integra. DigiFuse Cannulated Intramedullary Fusion System SURGICAL TECHNIQUE
 Integra DigiFuse Cannulated Intramedullary Fusion System SURGICAL TECHNIQUE Table of Contents Design Rationale... 2 System Features... 2 Indications... 2 Contraindications... 2 Surgical Technique...3 Step
Integra DigiFuse Cannulated Intramedullary Fusion System SURGICAL TECHNIQUE Table of Contents Design Rationale... 2 System Features... 2 Indications... 2 Contraindications... 2 Surgical Technique...3 Step
DEVELOPED BY MEDSHAPE, INC. IN CONJUNCTION WITH PATRICK ST. PIERRE, M.D. BICEPS TENODESIS ARTHROSCOPIC AND SUBPECTORAL SURGICAL TECHNIQUE
 ! SURGICAL TECHNIQUE! DEVELOPED BY MEDSHAPE, INC. IN CONJUNCTION WITH PATRICK ST. PIERRE, M.D. ARTHROSCOPIC AND SUBPECTORAL BICEPS TENODESIS SURGICAL TECHNIQUE BICEPS TENODESIS Indications Tenodesis of
! SURGICAL TECHNIQUE! DEVELOPED BY MEDSHAPE, INC. IN CONJUNCTION WITH PATRICK ST. PIERRE, M.D. ARTHROSCOPIC AND SUBPECTORAL BICEPS TENODESIS SURGICAL TECHNIQUE BICEPS TENODESIS Indications Tenodesis of
100 Interpace Parkway Parsippany, NJ
 100 Interpace Parkway Parsippany, NJ 07054 www.biometspine.com 800-526-2579 All trademarks are the property of Biomet, Inc. or one of its subsidiaries, unless otherwise indicated. Rx Only. 2009 EBI, LLC.
100 Interpace Parkway Parsippany, NJ 07054 www.biometspine.com 800-526-2579 All trademarks are the property of Biomet, Inc. or one of its subsidiaries, unless otherwise indicated. Rx Only. 2009 EBI, LLC.
Foot & Ankle. Smart Toe II. Intramedullary Implant. Operative Technique. Foot & Ankle
 Foot & Ankle Smart Toe II Intramedullary Implant Operative Technique Foot & Ankle Smart Toe This publication sets forth detailed recommended procedures for using Stryker Osteosynthesis devices and instruments.
Foot & Ankle Smart Toe II Intramedullary Implant Operative Technique Foot & Ankle Smart Toe This publication sets forth detailed recommended procedures for using Stryker Osteosynthesis devices and instruments.
nvp Posterior Lumbar Interbody Fusion System
 nvp Posterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
nvp Posterior Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine following
nvt Transforaminal Lumbar Interbody Fusion System
 nvt Transforaminal Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine
nvt Transforaminal Lumbar Interbody Fusion System 1 IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND/OR STAFF The nv a, nv p, and nv t are an intervertebral body fusion device used in the lumbar spine
Lapidus Arthrodesis System Instructions for Use
 Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
Low Bend Distal Tibia Plates
 Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Low Bend Medial Distal Tibia Plates Surgical Technique Table of Contents Introduction 3.5 mm LCP Low Bend Medial Distal Tibia
Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Low Bend Medial Distal Tibia Plates Surgical Technique Table of Contents Introduction 3.5 mm LCP Low Bend Medial Distal Tibia
COR. Precision Targeting Cartilage Repair System. Arthroscopic Technique for Repair of Osteochondral Defects
 COR Precision Targeting Cartilage Repair System Arthroscopic Technique for Repair of Osteochondral Defects Planning the Procedure An 18-gauge spinal needle is initially used to plan a perpendicular approach
COR Precision Targeting Cartilage Repair System Arthroscopic Technique for Repair of Osteochondral Defects Planning the Procedure An 18-gauge spinal needle is initially used to plan a perpendicular approach
Radux StandTall Instructions for Use Sheath Extender and Securement Clasp
 Radux StandTall Instructions for Use Sheath Extender and Securement Clasp USA Caution Federal (USA) law restricts this device to sale by or on the order of a health care professional. Caution The Radux
Radux StandTall Instructions for Use Sheath Extender and Securement Clasp USA Caution Federal (USA) law restricts this device to sale by or on the order of a health care professional. Caution The Radux
Home Health Foundation, Inc. To create more permanent IV access for patients undergoing long term IV therapy.
 PROCEDURE ORIGINAL DATE: 06/99 Revised Date: 09/02 Home Health Foundation, Inc. SUBJECT: PURPOSE: MIDLINE CATHETER INSERTION To create more permanent IV access for patients undergoing long term IV therapy.
PROCEDURE ORIGINAL DATE: 06/99 Revised Date: 09/02 Home Health Foundation, Inc. SUBJECT: PURPOSE: MIDLINE CATHETER INSERTION To create more permanent IV access for patients undergoing long term IV therapy.
Technique Guide. 3.5 mm LCP Low Bend Medial Distal Tibia Plate Aiming Instruments. Part of the 3.5 mm LCP Percutaneous Instrument System.
 Technique Guide 3.5 mm LCP Low Bend Medial Distal Tibia Plate Aiming Instruments. Part of the 3.5 mm LCP Percutaneous Instrument System. Table of Contents Introduction 3.5 mm LCP Low Bend Medial Distal
Technique Guide 3.5 mm LCP Low Bend Medial Distal Tibia Plate Aiming Instruments. Part of the 3.5 mm LCP Percutaneous Instrument System. Table of Contents Introduction 3.5 mm LCP Low Bend Medial Distal
CHANGING THE WAY YOU LOOK AT THINGS
 CHANGING THE WAY YOU LOOK AT THINGS At ConMed Linvatec, we re constantly challenging ourselves to develop truly innovative arthroscopic products that not only complement your technique, but also lead to
CHANGING THE WAY YOU LOOK AT THINGS At ConMed Linvatec, we re constantly challenging ourselves to develop truly innovative arthroscopic products that not only complement your technique, but also lead to
The AperFix II System
 The AperFix II System A Complete Anatomic Solution Transtibial Surgical Technique 2 AperFix II System Transtibial Surgical Technique Figure 1 A Complete Anatomic Solution The Cayenne Medical AperFix and
The AperFix II System A Complete Anatomic Solution Transtibial Surgical Technique 2 AperFix II System Transtibial Surgical Technique Figure 1 A Complete Anatomic Solution The Cayenne Medical AperFix and
Technique Guide. Midface Distractor System. For the temporary stabilization and gradual lengthening of the cranial or midfacial bones.
 Technique Guide Midface Distractor System. For the temporary stabilization and gradual lengthening of the cranial or midfacial bones. Table of Contents Introduction Midface Distractor System 2 Indications
Technique Guide Midface Distractor System. For the temporary stabilization and gradual lengthening of the cranial or midfacial bones. Table of Contents Introduction Midface Distractor System 2 Indications
Surgical Technique Carpal Fusion
 Carpal Fusion Patent and Patent Pending CAUTION: Federal Law (USA) restricts this device to sale by or on the order of a physician. INDICATIONS FOR USE The Extremity Medical Lag Screw and X-Post System
Carpal Fusion Patent and Patent Pending CAUTION: Federal Law (USA) restricts this device to sale by or on the order of a physician. INDICATIONS FOR USE The Extremity Medical Lag Screw and X-Post System
1 Description. 2 Indications. 3 Warnings ASPIRATION CATHETER
 Page 1 of 5 ASPIRATION CATHETER Carefully read all instructions prior to use, observe all warnings and precautions noted throughout these instructions. Failure to do so may result in complications. STERILE.
Page 1 of 5 ASPIRATION CATHETER Carefully read all instructions prior to use, observe all warnings and precautions noted throughout these instructions. Failure to do so may result in complications. STERILE.
4766 Research Dr. San Antonio, TX insightdentalsystems.com
 OVERVIEW OF THE INSIGHT DENTAL IMPLANT DELIVERY SYSTEM The IDS system comes in a unit dose implant system where its advantage provides sterile instrumentation in one single-use kit. It is organized to
OVERVIEW OF THE INSIGHT DENTAL IMPLANT DELIVERY SYSTEM The IDS system comes in a unit dose implant system where its advantage provides sterile instrumentation in one single-use kit. It is organized to
EVER Pharma D-mine Pen Pen injector for Apomorphine 10 mg/ml
 EVER Pharma D-mine Pen Pen injector for Apomorphine 10 mg/ml Instructions for Use with Dacepton 3 ml Cartridges Apomorphine hydrochloride hemihydrate solution for injection Subcutaneous Use TABLE OF CONTENTS
EVER Pharma D-mine Pen Pen injector for Apomorphine 10 mg/ml Instructions for Use with Dacepton 3 ml Cartridges Apomorphine hydrochloride hemihydrate solution for injection Subcutaneous Use TABLE OF CONTENTS
Vascu-PICC WITH CUFF PERIPHERALLY INSERTED CENTRAL VEIN ACCESS CATHETER INSTRUCTIONS FOR USE
 Vascu-PICC WITH CUFF PERIPHERALLY INSERTED CENTRAL VEIN ACCESS CATHETER INSTRUCTIONS FOR USE INDICATIONS FOR USE: The Vascu-PICC with cuff Peripherally Inserted Central Vein Catheters are designed for
Vascu-PICC WITH CUFF PERIPHERALLY INSERTED CENTRAL VEIN ACCESS CATHETER INSTRUCTIONS FOR USE INDICATIONS FOR USE: The Vascu-PICC with cuff Peripherally Inserted Central Vein Catheters are designed for
Surgical Technique. Calcaneal Locking Plate
 Surgical Technique Calcaneal Locking Plate PERI-LOC Locked Plating System Calcaneal Locking Plate Surgical TechniqueCatalog Infor Table of Contents Introduction...2 Indications...3 Plate Features...3 Patient
Surgical Technique Calcaneal Locking Plate PERI-LOC Locked Plating System Calcaneal Locking Plate Surgical TechniqueCatalog Infor Table of Contents Introduction...2 Indications...3 Plate Features...3 Patient
Absorbable Woven Polyglycolic Acid Mesh Tube (Absorbable Nerve Conduit Tube) INSTRUCTIONS FOR USE 2 6
 Absorbable Woven Polyglycolic Acid Mesh Tube (Absorbable Nerve Conduit Tube) INSTRUCTIONS FOR USE 2 6 1 0086 SYMBOL DEFINITIONS ENGLISH Do not Reuse Consult Instructions For Use Ethylene Oxide Sterilized
Absorbable Woven Polyglycolic Acid Mesh Tube (Absorbable Nerve Conduit Tube) INSTRUCTIONS FOR USE 2 6 1 0086 SYMBOL DEFINITIONS ENGLISH Do not Reuse Consult Instructions For Use Ethylene Oxide Sterilized
St. Jude Medical 8-Channel Adapter. Clinician's Manual
 St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
Document No. BMB/IFU/40 Rev No. & Date 00 & 15/11/2017 Issue No & Date 01 & 15/11/2017
 Central Venous Catheter Device Description Multi-lumen catheters incorporate separate, non-communicating vascular access lumens within a single catheter body. Minipunctur Access Sets And Trays: Used for
Central Venous Catheter Device Description Multi-lumen catheters incorporate separate, non-communicating vascular access lumens within a single catheter body. Minipunctur Access Sets And Trays: Used for
Integra. ADVANSYS Plating System SURGICAL TECHNIQUE
 Integra ADVANSYS Plating System SURGICAL TECHNIQUE Table of Contents Dorsal Lisfranc Plate Indications...2 Contraindications...2 Description...2 Surgical Technique...3 Surgical Site Preparation...3 Step
Integra ADVANSYS Plating System SURGICAL TECHNIQUE Table of Contents Dorsal Lisfranc Plate Indications...2 Contraindications...2 Description...2 Surgical Technique...3 Surgical Site Preparation...3 Step
AFX. Femoral Implant. System. The AperFix. AM Portal Surgical Technique Guide. with the. The AperFix System with the AFX Femoral Implant
 The AperFix System AFX with the Femoral Implant AM Portal Surgical Technique Guide The Cayenne Medical AperFix system with the AFX Femoral Implant is the only anatomic system for soft tissue ACL reconstruction
The AperFix System AFX with the Femoral Implant AM Portal Surgical Technique Guide The Cayenne Medical AperFix system with the AFX Femoral Implant is the only anatomic system for soft tissue ACL reconstruction
Correction System. Surgical Technique
 Re+Line Bunion Correction System Surgical Technique Bunion Correction System Easy insertion and medial placement accuracy using Landmark Guide technology 1 mm compression slot and fixed tines to encourage
Re+Line Bunion Correction System Surgical Technique Bunion Correction System Easy insertion and medial placement accuracy using Landmark Guide technology 1 mm compression slot and fixed tines to encourage
BACTISEAL Endoscopic Ventricular Catheter (REF & )
 BACTISEAL Endoscopic Ventricular Catheter (REF 82-3087 & 82-3088) LCN 206966-001/E 2011 2017 DePuy Synthes. All rights reserved. Revised 07/17 206966-001-E.indd 1 ENGLISH IMPORTANT INFORMATION Please Read
BACTISEAL Endoscopic Ventricular Catheter (REF 82-3087 & 82-3088) LCN 206966-001/E 2011 2017 DePuy Synthes. All rights reserved. Revised 07/17 206966-001-E.indd 1 ENGLISH IMPORTANT INFORMATION Please Read
EXACTECH KNEE. Operative Technique ADDENDUM. Metaphyseal Cones. Surgeon focused. Patient driven. TM
 EXACTECH KNEE Operative Technique ADDENDUM Metaphyseal Cones Surgeon focused. Patient driven. TM TABLE OF CONTENTS INTRODUCTION...1 DESCRIPTION...1 DETAILED OPERATIVE TECHNIQUE Initial Preparation and
EXACTECH KNEE Operative Technique ADDENDUM Metaphyseal Cones Surgeon focused. Patient driven. TM TABLE OF CONTENTS INTRODUCTION...1 DESCRIPTION...1 DETAILED OPERATIVE TECHNIQUE Initial Preparation and
PediLoc 3.5mm and 4.5mm Contour Femur Plate Surgical Technique
 PediLoc 3.5mm and 4.5mm Contour Femur Plate Surgical Technique Surgical Technique Contour Femur Plate The technique description herein is made available to the healthcare professional to illustrate the
PediLoc 3.5mm and 4.5mm Contour Femur Plate Surgical Technique Surgical Technique Contour Femur Plate The technique description herein is made available to the healthcare professional to illustrate the
INSTRUCTIONS FOR USE FOR:
 INSTRUCTIONS FOR USE FOR: en English INSTRUCTIONS FOR USE GORE ENFORM PREPERITONEAL BIOMATERIAL Carefully read all instructions prior to use. Observe all instructions, warnings, and precautions noted throughout.
INSTRUCTIONS FOR USE FOR: en English INSTRUCTIONS FOR USE GORE ENFORM PREPERITONEAL BIOMATERIAL Carefully read all instructions prior to use. Observe all instructions, warnings, and precautions noted throughout.
CrossFix II. All-Suture, All-Inside Meniscal Repair System. Surgical Technique
 CrossFix II All-Suture, All-Inside Meniscal Repair System Surgical Technique 3 CrossFix II All- Suture Repair System Surgical Technique All-Inside Repair with Inside-Out Results The CrossFix II Meniscal
CrossFix II All-Suture, All-Inside Meniscal Repair System Surgical Technique 3 CrossFix II All- Suture Repair System Surgical Technique All-Inside Repair with Inside-Out Results The CrossFix II Meniscal
ASEPT. Pleural Drainage System INSTRUCTIONS FOR USE REF LOT STERILE EO. Manufactured for: 824 Twelfth Avenue Bethlehem, PA
 ASEPT Pleural Drainage System LS-00116-01-AB 017-11 INSTRUCTIONS FOR USE Do not reuse STERILIZE Do not resterilize 30 C Rx only 15 C REF Store at controlled room temperature 15-30 C (59-86 F) Keep away
ASEPT Pleural Drainage System LS-00116-01-AB 017-11 INSTRUCTIONS FOR USE Do not reuse STERILIZE Do not resterilize 30 C Rx only 15 C REF Store at controlled room temperature 15-30 C (59-86 F) Keep away
Flexible Fragment Fixation. Surgical Technique
 Flexible Fragment Fixation Surgical Technique 2 F 3 Flexible Fragment Fixation The F 3 Fragment Plating System offers low profile, yet strong fixation in a locked plating construct that can be contoured
Flexible Fragment Fixation Surgical Technique 2 F 3 Flexible Fragment Fixation The F 3 Fragment Plating System offers low profile, yet strong fixation in a locked plating construct that can be contoured
