Patient-specific immunotherapy T-cell product ATIR
|
|
|
- Hector Dennis
- 5 years ago
- Views:
Transcription
1 Patient-specific immunotherapy T-cell product ATIR Unlock full potential of haploidentical hematopoietic stem cell transplantations (HSCT) Company presentation, July 20, 2018 Amsterdam, The Netherlands Euronext (KDS) 1
2 Disclaimer These slides and the accompanying oral presentation contain forward-looking statements and information. Forward-looking statements are subject to known and unknown risks, uncertainties, and other factors that may cause our or our industry s actual results, levels or activity, performance or achievements to be materially different from those anticipated by such statements. The use of words such as may, might, will, should, could, expect, plan, anticipate, believe, estimate, project, intend, future, potential or continue, and other similar expressions are intended to identify forward looking statements. For example, all statements we make regarding (i) the initiation, timing, cost, progress and results of our preclinical and clinical studies and our research and development programs, (ii) our ability to advance product candidates into, and successfully complete, clinical studies, (iii) the timing or likelihood of regulatory filings and approvals, (iv) our ability to develop, manufacture and commercialize our product candidates and to improve the manufacturing process, (v) the rate and degree of market acceptance of our product candidates, (vi) the size and growth potential of the markets for our product candidates and our ability to serve those markets, and (vii) our expectations regarding our ability to obtain and maintain intellectual property protection for our product candidates, are forward looking. All forward-looking statements are based on current estimates, assumptions and expectations by our management that, although we believe to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that we expected. Any forward-looking statement speaks only as of the date on which it was made. We undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law. This presentation is not, and nothing in it should be construed as, an offer, invitation or recommendation in respect of our securities, or an offer, invitation or recommendation to sell, or a solicitation of an offer to buy, any of our securities in any jurisdiction. Neither this presentation nor anything in it shall form the basis of any contract or commitment. This presentation is not intended to be relied upon as advice to investors or potential investors and does not take into account the investment objectives, financial situation or needs of any investor. 2
3 Kiadis: Patient specific immunotherapy T-cell product ATIR UNIQUE HAPLO- IDENTICAL HSCT STRONG PHASE 2 DATA CLOSE TO MARKET WORLDWIDE RIGHTS, PREPARING EU LAUNCH LARGE TARGET POPULATION CELL THERAPY & HSCT PLATFORM T-cell product ATIR: haploidentical donor T-cells, depleted of hostreactive T-cells, given after T-cell depleted haploidentical HSCT Clinically meaningful OS benefits over historical control, potential for clinically meaningful benefits over PTCy/Baltimore protocol EMA opinion expected 4Q18; Potential first EU country launch 2H19; FDA RMAT breakthrough designation; Phase 3 vs PTCy enrolling Building own commercialization infrastructure in EU (small number of transplantation sites, worldwide rights retained) 50,000 currently eligible HSCT patients per year; Potential to expand to inherited blood disorders and autoimmune disease Leverage patient specific cell therapy and HSCT platform to expand suite of products HSCT: Hematopoietic Stem Cell Transplantation; GVHD: Graft Versus Host Disease; Haplo: Haploidentical; OS: Overall Survival; 3
4 Kiadis ATIR product: regulatory status (adult blood cancers) Region Phase 1 Phase 2 Phase 3 Filing Potential catalysts Commercial Rights Regulatory Status EU Orphan Drug Designation CHMP opinion (Q4 2018) EU approval (Q1 2019) EU launch (H2 2019) EMA Day 180 List of Issues received (5/2018) USA Orphan Drug & RMAT Designations Phase 3 interim read out (2020; 2/3 of events) RMAT breakthrough designation (9/2017; FDA access, priority review, support) 4
5 Allogeneic Hematopoietic Stem Cell Transplantations (HSCT) Potentially curative: destroy patient s blood/immune system (chemotherapy/radiation), replace with healthy system from donor Inherent risk of Graft versus Host disease (GVHD): donor immune system attacks patient Lack of donors: many patients cannot find matched donor (US 2012: 13,500) Mostly blood cancers (~85%) and adults (84%): potentially also for inherited disorders and autoimmune disease Expensive procedure: around $500,000 (1 year cost; US) Maarten van der Weijden, swimmer: After acute leukemia and HSCT in 2001: Olympic Gold 10 km open water (2008) UK-France-UK double Channel crossing >70km (19 hours; 2017) World distance record 103km (24 hours; 2018) Sources: Broder 2017; Passweg 2018; CIBMTR 2017 summary slides; Besse
6 Graft Versus Host Disease (GVHD): terrible disease Subpopulation of T-cells from donor (i.e. graft) recognize patient tissue (i.e. host) as foreign and attack, mostly due to genetic differences in MHC Class II proteins (major mismatches) Disease Treatment Skin, eye, mouth or GI tract disease Immunodeficiency (infections) Muscle constriction, bone loss, pulmonary disease, thyroid disfunction, solid tumors Sleep deprivation, depression Immunosuppression (steroids, MMF, MTX) The holy grail of HSCT: Give donor T-cells that protect Remove donor T-cells that attack Avoid immunosuppressants Quality of life QoL loss: 27% for severe GVHD (up to 68%) and 20% for moderate GVHD Quality of life with severe GVHD worse than vision impairment, MS, loss of arm/leg, diabetes Impact on patients, donors, families, physicians MMF: mycophenolate mofetil Sources: Jones 2016; Koreth 2013; Lewalle 2003 MTX: methotrexate 6
7 Severe Graft Versus Host Disease (GVHD): high mortality Chronic GVHD severe Acute GVHD grade III/IV 5 year survival: 22% 1 year survival: 24-51% Acute Grade Survival at year 1 Grade III 51% Grade IV 24% Sources: Pidala et al Haematologica (2011); 96(11)
8 HSCT: continued growth due to PTCy/Baltimore protocol (US) Matched Related Donors (MRD) Matched Unrelated Donor (MUD; registries) 13,500 patients could not find matched donor Haploidentical donors Cord blood Matched Donors: Between 25% (White European) and 80% (African American) cannot find a matched donor Haploidentical (half matched) Donors: Motivated donors almost always available (e.g., parent/child); Haploidentical enabled by Post Transplant Cyclophosphamide (PTCy or Baltimore) protocol: Trigger immediate GVHD attack by infusing all T-cells of a haploidentical (half matched) donor, then deplete host-reactive donor T-cells with chemo (cyclophosphamide, day 3 and 5) and immunosuppression Source: CIBMTR 2017 Summary slides; Fuchs 2017; Gragert 2014; Besse
9 PTCy: benefits over MUD, yet still unmet need relapse & GVHD PTCy vs MUD 53% PTCy (up to 3 yr) 51% PTCy (1 year) Average 49% 45% MUD Overall Survival 60% 38% 38% Relapse 29% 26% 27% Relapse Related Mortality (RRM) 18% Acute GVHD II-IV Chronic GVHD Relapse Survival Weighted average of 5 PTCy/MUD comparison publications in the review paper by Fuchs E 2017 that reported AML/MDS/NHL/HL (Ciurea 2015; Rashidi 2016; Burroughs 2008; Kanate 2016; Garciaz 2015); n=463 PTCy and n=2647 MUD patients Non Relapse Mortality (NRM) 22% Acute GVHD grade III/IV 5% Chronic GVHD 24% Chronic GVHD - severe 8% Weigthed average in PTCy publications that reported >50% AML/ALL (Ciurea 2015; Piemontese 2017, Solomon 2012, Ciurea 2012; Devillier 2016; Di Stasi 2014; Esquirol 2016; Sugita 2015); n=571; Chronic GVHD confirmed with other publications PTCy has many advantages over MUD: donor faster available, lower GVHD Still significant unmet medical need: relapse and chronic GVHD, secondary malignancies, toxicity, immunosuppression 9
10 PTCy vs MUD: GVHD-free and Relapse-Free survival (GRFS) McCurdy 2017 (Johns Hopkins, Baltimore) Solh 2016 (Northside, Atlanta) * Haplo and HIDT: both PTCy haploidentical HSCT GVHD-free and Relapse Free Survival (GRFS): Survival without: Acute GVHD grade III/IV Chronic GVHD requiring systemic therapy Relapse 10
11 Haploidentical HSCT: PTCy/Baltimore protocol versus ATIR PTCy/ Baltimore protocol (All T-cells) Conditioning of patient Apheresis of donor, graft infusion Engraftment of donor stem cells Cyclophosphamide and immunosuppression Treat GVHD: kill attacking T-cells in the patient (with chemo and immunosuppression) Haplo HSCT with T-cells (T-cell replete) Haplo HSCT plus ATIR: (Subset of T- cells, depleted of host-reactive T-cells) Apheresis of patient and donor Central ATIR production Produce ATIR 5 days (total 14 days till interim release) Conditioning of patient Apheresis of donor, graft infusion Haplo HSCT without T-cells (T-cell depleted, CD34+) Engraftment of donor stem cells ATIR Infuse ATIR ~30 days after graft infusion Prevent GVHD: kill attacking T-cells outside the patient (without immunosuppression) 11
12 Healthy donor donor ATIR: safe subset of T-cells, aiming to protect, not to attack ATIR TM MANUFACTURING Mix haplo donor T- PROCESS Add TH9402*, which cells with patient accumulates only in cells: host-reactive Step 1 (Day 1 4) activated donor T- Healthy T-cells become cells (MDR pump is donor ATIR ATIR TM MANUFACTURING TM Immune cells are collected and mixed MANUFACTURING activated PROCESS (1-way PROCESS switched off in Mixed Lymphocyte activated T-cells) Step 1 (Day 1 4) Step 2 (Day 5) Step 1 (Day 1 4) Step 2 (Day ` 5) Reaction) ` donor Healthy ATIRPatient TM MANUFACTURING cells PROCESS Healthy Patient cells donor Patient cells inactivated Patient inactivated by radiation by radiation inactivated Immune cells are collected and mixed Immune cells are collected and mixed by radiation Step 1 (Day 1 4) Immune cells are collected and mixed Step 2 (Day 5) Step 2 (Day 5) Expose to green light: TH9402* induces apoptosis: activated and thus host-reactive donor T-cells are killed Step Step 3 (Day 3 (Day 5) ` 5) FEBRUARY Step 3 (Day 5) Step 3 (Day 5) ATIR: remaining potent non-hostreactive donor T- cells (2 million T- cells/kg) FEBR Final ATIR TM FEBR Final Final Step Step - Infusion - Infusion of ATIR101 of ATIR101 Healthy donor Fina Patient Patient patient Patient Patient cells inactivated by radiation Certain T-cells from the donor are activated by the TH9402 is introduced. Mixture is exposed to light. presence of the foreign patient Protect: cells. If not eliminated, Retain protective TH9402 is retained T-cells ONLY in the GvHD-causing to fight T-cells relapse and TH9402 infections is activated by light, causing the these cells would cause GvHD in the patient stained GvHD-causing T-cells to self-destruct Certain T-cells from the donor are activated by the TH9402 introduced. Mixture is exposed to light. The remaining product is infused back Certain T-cells from the donor are activated by the presence of the foreign patient & Not Mixture is exposed to light. cells. If not attack: eliminated, Reduce TH9402 is retained risk TH9402 ONLY in of is introduced. the GvHD-causing GVHD T-cells by depleting TH9402 is activated host-reactive by light, causing the T-cells ex The vivo remaining product is infused back presence of the foreign patient cells. If not eliminated, TH9402 is retained ONLY in the GvHD-causing T-cells TH9402 is activated by light, causing the and helps rebuild their immune system these cells would cause GvHD in the patient stained GvHD-causing T-cells to self-destruct and helps rebuild their immune system these cells would cause GvHD in the patient stained GvHD-causing T-cells to self-destruct to f ght infections and eliminate to f ght infections and eliminate remaining tumor cells remaining tumor cells Patient Pati inac by r The r and h *TH9402 proprietary selective rhodamine derivative, modified to become cytotoxic under green light MANUFACTURING 12
13 ATIR: simplified patient specific supply chain Attractive production process (as compared to CAR-T) 5 day process (total 14 days to interim product release) No genetic engineering (no viral vector production, no BL2) Cleanrooms with LAF cabinets (no bioreactors) Supply chain fits with routine HSCT procedures Ingoing (patient/donor materials): Routine apheresis and fresh shipment (formulated in hypothermosol; 42 hour hold time) Outgoing (final product): Routine frozen shipment and infusion Control over (own) manufacturing Contract manufacturing in Germany, adding CMO capacity Own commercial manufacturing in Netherlands (starting up) 13
14 Kiadis trials: haploidentical HSCT in adult blood cancers Phase 1/2 Phase 2 Phase 3 CR-GVH-001: Single dose ATIR Dose escalation (completed) 19 patients Advanced malignancies AML/ALL 5 year follow up Single site in Canada (CA) Historical controls CR-AIR-004: No ATIR Open label single arm (completed) 44 patients (29 matched to CR-AIR-007*) AML/ALL/MDS Sites in BE, CA, GE, NL, UK, US CR-AIR-006: No ATIR Observational cohort, EBMT registry (completed) 35 patients (all matched to CR-AIR-007*) AML/ALL/MDS 1 year follow up CR-AIR-007: Single dose ATIR Open label single arm (completed) 23/26 patients (MITT/ITT**) AML/ALL/MDS, median age 41 2 year follow up Sites in BE, CA, GE, UK CR-AIR-008: Single dose ATIR and two doses ATIR*** Open label single arm (enrolled, ongoing) 9/11 single dose; 6/6 patients two doses (MITT/ITT**) AML/ALL/MDS 1 year follow up Sites in CA, BE, GE, UK CR-AIR-009: Single dose ATIR Randomized/controlled (ATIR versus PTCy) 2017 (enrolling) 250 patients AML/ALL/MDS Event driven primary endpoint (GRFS) ~50 sites planned CA, Europe, US, Israel All trials: Patients: adult AML/ALL/MDS Conditioning: Myeloablative Graft source: haplo PBMCs HSCT: T-cell depleted (CD34+) ATIR: ~30 days after HSCT * Same in/exclusion criteria and HSCT and overlapping sites; control in accordance with regulatory feedback ** MITT: Modified Intent to Treat (transplanted and ATIR); ITT: Intent to Treat (transplanted) *** CR-AIR-008 was designed to test safety of second dose, but due to higher then expected GVHD it was decided to stop infusing second dose (in accordance with protocol) 14
15 ATIR: improved Overall Survival (OS; ITT, single dose; 1yr) T-cell depleted (CD34+) with ATIR (n=37)* T-cell depleted (CD34+) without ATIR (n=64)* OS 1 yr: 58% [44-77**] *ITT (intention to treat): all patients undergoing T-cell depleted CD34+ HSCT; SD: single dose; All clinical studies have similar patient characteristics and treatment sites; CR-AIR-008 status 1 June 2018 (3 patients at risk) ** 95% confidence interval [ ] OS 1 yr: 23% [14-37**] P=0.005 ( vs ) 15
16 ATIR: improved OS & NRM, low GVHD & relapse (ITT, single dose) T-cell depleted (CD34+) with ATIR (n=37)* T-cell depleted (CD34+) without ATIR (n=64)* CR-AIR-007 CR-AIR CR-AIR-006 CR-AIR single dose single dose single dose control control control 1 year post HSCT (ITT n=26) (ITT n=11) (ITT n=37) (n=35) (n=29) (n=64) Overall survival 58% [42-80] 64% [41-100] 58% [44-77] 20% [10-39] 27% [13-54] 23% [14-37] Non-relapse mortality 35% 27% 33% 66% 59% 63% Relapse-related mortality 8% 9% 8% 15% 14% 14% Relapse 8% 9% 8% NA NA NA ATIR after T-cell depleted HSCT: Improve OS and NRM, Retain low chronic GVHD & relapse Acute GVHD grade II-IV 19% 27% 21% 20% 18% 19% Acute GVHD grade III-IV 0% 18% 5% 6% 7% 6% Chronic GVHD 4% 0% 3% 11% 5% 8% Chronic GVHD severe 0% 0% 0% 9% 5% 7% 6 months post HSCT (MITT n=23) (MITT n=9) (MITT n=32) (n=35) (n=29) (n=64) P-values for vs : OS (0-12 month) p=0.005 NRM (0-6 month) p= 0.02 Non-relapse mortality 13% [0-27] 11% [0-29] 13% [0-24] 37% [19-51] 35% [15-51] 36% [23-47] Notes: NRM at 6 months primary endpoint of CR-AIR-007; Kaplan-Meier estimates for OS and NRM at 6 months; all other estimates cumulative incidence analyses; ITT: patients receiving HSCT; MITT: patients receiving HSCT and ATIR; OS: overall survival; CR-AIR-008 status 1 June 2018 (3 patients at risk) 16
17 Phase 3 (CR-AIR-009) study design: ATIR and PTCy GRFS GRFS in PTCy literature: 30-40% Solh 2016 (n=126) McCurdy 2017 (n=372) Santoro 2017 (n=208) GRFS with ATIR (ITT): 53% [39-72] Sites included Atlanta (single site) Johns Hopkins (single site) 69 EBMT sites Patients AML/ALL/MDS/ NHL/HL, CML AML/ALL/MDS/ NHL/HL, MM ALL GRFS 1-yr [95% CI] Disease risk index (DRI)* 33% [25,41] 20% low 40% intermediate 40% high/v. high 45% [40,50] 14% low 67% intermediate 19% high/v. high 33% (average) Not available 1-year estimates [95% CI]: % [38-77] 008 SD 55% [32-94] SD 53% [39-72] Normalized GRFS 1-yr* 30% 40% Not available 250 patients in CR-AIR-009: 80% power to detect 16% GRFS difference * Normalized by adjusting based on the average DRI of the CR-AIR-007/008 patient population (57% intermediate risk; 43% high/very high risk); using the hazard rates per DRI as reported in the Solh/McCurdy publications (2 patients in Solh for which DRI not known excluded); DRI is an important prognostic factor for OS,: e.g., NHL patient in CR1 is low risk, AML in CR2 is very high risk (Armand 2014); CR-AIR-008 status 1 June 2018 (3 patients at risk) 17
18 Phase 3 (CR-AIR-009): ATIR vs. PTCy/Baltimore protocol Objectives: demonstrate superior clinical benefit and collect pharmacoeconomic data Randomized Controlled (1:1) R HSCT plus ATIR: T-cell deplete CD34+ HSCT plus single dose ATIR 2 mln cells/kg at ~30 days 250 adult patients with AML, ALL or MDS PTCy/Baltimore protocol: T-cell replete HSCT with 50 mg/kg cyclophosphamide at days 3 and 5 post HSCT & prophylactic immunosuppressants Primary endpoint: GVHD-Free and Relapse- Free Survival (GRFS); Interim analysis: at 2/3 of events (17,6% GRFS treatment effect, hazard ratio 0.61) Primary analysis: at 156 events (11,4% GRFS treatment effect, hazard ratio 0.73) Secondary endpoints: Overall Survival (OS), Progression Free Survival (PFS), Relapse, Non Relapse Mortality (NRM) Other: Randomization at enrollment; Balanced conditioning regimens in ATIR/PTCy arms; Stratification for Disease Risk Index, underlying disease and treatment site 50 sites in US, Canada, EU & Israel; multiple open in Canada and EU; Enrolling patients (since December 2017) 18
19 Allogeneic Potential future Hematopoietic studies with Stem ATIR Cell Transplantations (HSCT) Pediatric blood cancers (EMA Pediatric Investigation Plan deferral obtained) ATIR as adjunctive after other haploidentical HSCT protocols, e.g. PTCy/Baltimore protocol α/β T-cell depleted protocol Other (RIC/MA) conditioning regimens Other HSCT indications, e.g. Inherited blood disorders (e.g. thalassemia or sickle cell anemia) Inherited immune disorders (e.g. severe combined immunodeficiency) Autoimmune disease (e.g. multiple sclerosis or lupus) 19
20 Potential: 50,000 annual patients US/EU (based on 2016) Continuation of current growth of haploidentical (3-fold in US in ) 33,500 50,500 20,000 30,800 EU US 13,200* 13, , Haploidentical now (2016) Cannibalize Matched Unrelated & cord blood donors (2016) Haplo donor available to almost all eligible patients** Total potential (based on current number of eligible patients) * 20% of MUDs are partially matched or Mismatched MUDs (MMUD) with worse outcomes & thus first candidates for cannibalization by Haplo ** US: 13,500 patients that could not find matched donor in 2012 (Besse 2015); EU rough estimation by extrapolation from US based on EU population Source: CIBMTR summary slides 2017, Passweg 2017, Besse
21 Potential: > 50% adoption based on GRFS benefit (survey) Market survey among 100 US/EU clinicians (2013): In what percent of your haplo s would you use ATIR given strong Phase 3 data and formal approval? EU US 58% 55% Potential adoption of >50% in US based on GRFS benefit confirmed by 2018 market survey: 9 clinicians representing 27% of US HSCTs ATIR product profile presented: 18% GRFS benefit over PTCy (with or without 5% OS benefit) Source: ATIR Assessment, September 2013 by Defined Health, based on Phase 1 data: 100 transplant physicians/kols (50/50 US/EU; eg Harvard, Johns Hopkins, MD Anderson, Stanford, Dresden, Saint Antoine); July 2018 by Simon Kucher 21
22 Potential: independent commercialization Europe: 63 sites in EU4 US: Top 25 sites perform 65% of HSCTs GE IT FR UK Shortened dossier to G-BA via AMNOG (until sales > 50M) L-648 (named patient basis); P&R Dossier to AIFA/CTS at MAA ATU (named patient basis); HAS/CEPS filing at MAA NICE evaluation; Interim funding potentially via Cancer Drug Fund Source: Simon Kucher survey Building medical affairs, market access, commercial, supply chain and manufacturing infrastructure (EU first) 22
23 Protection: patents, orphan drug designation, know how Patents (owned/ licensed) Orphan drug designation Proprietary know how and infrastructure Methods for reducing GVHD (expiring October 2021) More rhodamine derivatives (expiring January 2024) Improved photodynamic process (expiring February 2036 if granted) US (7 years from launch): for prevention of GVHD or TRM EU (10 years from launch): for treatment in HSCT regardless of disease, for treatment of AML, for prevention of GVHD Manufacturing critical process parameters Release assays based on critical quality attributes Cell handling, storage, formulation and shipment Patient specific supply chain with HSCT clinics (IT, relationships, service) Multiple barriers to entry for competition, many similarities with biologics/biosimilars 23
24 Kiadis: company at a glance ORGANIZATION SHAREHOLDERS FINANCIALS (July 13, 2018) Management team: track record in building biotech and in haemonc and cell therapy late stage development and commercialization from Crucell, Medivation, Dendreon, J&J, AstraZeneca, McKinsey Supervisory board: Novartis ExCom; Actelion COO; Prosensa/Jerini CFO; Dana Farber chief of HSCT & haemonc and board member of NMDP/BeTheMatch Euronext Amsterdam/Brussels, IPO in 2015 Major shareholders (>5%): Life Sciences Partners, Lenildis Holding, Esprit Market cap: ~$240M / 200M 20,1 million shares outstanding Raised > 60M in equity & debt since June 2017 Cash 47.7M end March 18; Cash runway into Q
25 Kiadis: track record late stage development and commercial Arthur Lahr (April 2017) Chief Executive Officer Chief Strategy Officer Crucell (BD, M&A, M&S); Supervisory Board Sanquin; McKinsey; Unilever Andrew Sandler (Oct 2017) Chief Medical Officer SVP Medical Affairs Medivation; CMO Dendreon and Spectrum Pharma; Bayer; Berlex; Seattle Genetics; Board certified medical oncologist Robbert van Heekeren Chief Financial Officer Head Global Finance & Control Akzo/Organon Jan Feijen (April 2017) Chief Operations Officer EVP Operations J&J Vaccines and VP Manufacturing and Ops J&J Vaccines & Advanced Therapies; VP Crucell Asia; Managing Director Operations Avebe; Gist-Brocades Karl Hård (Sept 2017) Head IR & communications Head investor relations AstraZeneca; 10 years investor relations; 10 years pharma R&D; Assistant Professor chemistry James Joy (July 2018) General Counsel Group General Counsel TomTom; VP Legal and Compliance Royal Ahold; General Counsel Navigator Asset Management; Norton Rose; Latham & Watkins; Member New York State Bar 25
26 Kiadis key milestones and upcoming catalysts EMA submission of ATIR for marketing authorization approval First patient enrolled for ATIR Phase 3 FDA Regenerative Medicine Advanced Therapy (RMAT) designation Secured own commercial manufacturing facility Completion of enrollment of second Phase 2 (CR-AIR-008) Submission of answers to EMA Day 120 questions (End Q1) Submission of answers to EMA Day 180 List of Issues (Q3) Potential EMA CHMP opinion (Q4) Updates Phase 2 data and Phase 3 enrollment Potential approval of ATIR by the European Commission (Q1) Potential initial commercial launch of ATIR in first EU countries (H2) Initiate trial with ATIR as adjunctive to PTCy (H2) Potential reimbursement and commercial roll out across EU countries (H1) Potential interim read out Phase 3 (at 2/3 of GRFS events) (H2) 26
27 Practice two things in your dealings with disease: either help or do not harm the patient Epidemics, Book I, of the Hippocratic school I will prevent disease whenever I can but I will always look for a path to a cure for all diseases Hippocratic oath, Louis Lasagna, Academic Dean of the School of Medicine at Tufts University (1964) 27
28 Attachments 28
29 Haploidentical HSCT approaches Haplo HSCT Haplo T-cell Product (after HSCT) GVHD Treatment/ Prophylaxis Approach ATIR (Kiadis) T-cell depleted (CD34+) Subset of T-cells, depleted of hostreactive T-cells No immunosuppressant Prevent GVHD Zalmoxis (MolMed) BPX-501 (Bellicum) T-cell depleted (CD34+ and αβt-cell depleted) All T-cells (including host-reactive T-cells), engineered with suicide gene Eliminate host-reactive T-cells by infusing suicide agent, if GVHD occurs Treat GVHD PTCy or Baltimore protocol T-cell replete (All T-cells, including alloreactive T-cells) Post Transplant Cyclophosphamide & immunosuppressants Treat GVHD Table provided for illustrative purposes, not for direct comparison 29
30 Molmed/Bellicum: January 2018 (1 year) Patients Survival* Relapse NRM Status EMA Zalmoxis** (MolMed) Adult Approval Q BPX-501 (Bellicum) Pediatric Submission 2019 Product (Matched) Historical Control Zalmoxis pricing/dose*** (1-4 doses per patient): Germany: 163,900 Italy: 149,000 Comparison provided for illustrative purposes, based on literature comparison, NOT based on randomized controlled trials * Leukemia Free Survival for BPX-501 (BPX-501 Overall Survival is 89%, Overall Survival not reported for controls); ** CD34+ HSCT; 74% AML; 10% ALL; 16%MDS/NHL/HD; patients receiving Zalmoxis (MITT= 36 patients) *** Prices as at 16 January 2018 and 13 December 2017, respectively; subject to negotiation with GBA in Germany Source: CHMP Assessment report (Zalmoxis); Merli EHA 2017 (BPX-501); Locatelli 2017 (BPX-501) 30
31 CR-GVH-001: Overall Survival (5 years) Patients: 19 with advanced hematological malignancies (14 not in remission at transplant) ATIR101 doses: 10k cells/kg to 5 mln cells/kg; median of 31 days after HSCT Results: 67% Overall Survival at middle dose level after 5 years No acute GVHD grade III/IV related to ATIR101 at any dose 100% 80% 60% 40% 20% 0% Overall Survival (OS) 9 patients, 320k- 2M cells/kg Dose L4-L6 3 patients; 2,6-5M cells/kg) Dose L7 7 patients, 10- Dose 130k cells/kg L1-L Time after HSCT (months) Note: an unmanipulated donor lymphocyte infusion from a haploidentical donor Source: Lewalle 2003 that still contains all T-cells may cause GVHD at significantly lower doses of 10,000 cells/kg 31
32 CR-AIR-007: Outcomes (2 years, MITT) primary and secondary efficacy endpoints in CR-AIR-007, MITT Kaplan Meijer estimates 6 months 12 months 24 months... Non Relapse Mortality 13% 32% 48%... Relapse Related Mortality 5% 10% 25%... Progression Free Survival 78% 61% 39%... Overall Survival 83% 61% 39% Outcome No. of pts Cause of death < 6 months 6-12 months months RRM 1 TRM Infections 2 Adenovirus and JC virus infections TRM Other 1 Pulmonary embolism RRM 1 TRM Infections 3 Respiratory/pulmonary infections/distress TRM Other 1 Multi-organ failure RRM 2 TRM-Infections 3 * Pneumonia/sepsis/septic shock * All 3 patients immunosuppressed, subsequently contracted infections, leading to death: 2 patients who received un-manipulated DLI s in 2 nd year after HSCT and subsequently developed severe acute GVHD; 1 patient with chronic GVHD 32
33 Proliferation Index (PI) ATIR: alloreactive T-cells depleted, potency retained 12 6 Donor ATIR * Autologous Recipient 3 rd Party CD3/28 Functional release assay based on Quality Target Product Profile & Critical Quality Attributes Control: no donor reactivity Safety: depleted allo-reactivity Potency: other reactivity retained Source: Bonig ISCT
34 Healthcare costs of allogeneic HSCTs and complications (US) Total HSCT costs* $402,000 (MA) $301,000 (RIC) $549,000 (MA) $432,000 (RIC) Period / Source 100 days; Broder year; Broder 2017 $893, days; Milliman 2017** HSCT complications Cancer death $165,000 Relapse $69,000*** Hemorrhagic cystitis Acute GVHD $324,000 Chronic GVHD moderate/mild Chronic GVHD severe Costs to healthcare system $242,000***/**** $124,000 ($14,400 per year*****) $322,000 ($37,400 per year*****) * Includes Inpatient/Outpatient/pharmacy costs: MA: myeloablative conditioning; RIC: reduced intensity conditioning ** Includes different physician charges, graft procurement costs *** Cost based on Broder total cost and cost multiplier Khera **** Side effect of PTCy protocol **** 10 years, discounted Sources: Mariotto 2011; Yu 2017; Broder 2017; Khera 2014; Milliman 2017; Svan 2006; literature PTCy analysis 34
35 so that many more patients with otherwise incurable diseases will have a reasonable chance of long survival and cure Dr. E. Donnall Thomas established bone marrow transplantation as a treatment for leukemia Nobel Lecture
Annual Results 2017 & Business Update 13 April 2018
 Annual Results 2017 & Business Update 13 April 2018 1 Disclaimer These slides and the accompanying oral presentation contain forward-looking statements and information. Forward-looking statements are subject
Annual Results 2017 & Business Update 13 April 2018 1 Disclaimer These slides and the accompanying oral presentation contain forward-looking statements and information. Forward-looking statements are subject
Patient-specific immunotherapy T-cell product ATIR
 Patient-specific immunotherapy T-cell product ATIR Unlock full potential of haploidentical hematopoietic stem cell transplantations (HSCT) Company presentation, October 16, 2018 Amsterdam, The Netherlands
Patient-specific immunotherapy T-cell product ATIR Unlock full potential of haploidentical hematopoietic stem cell transplantations (HSCT) Company presentation, October 16, 2018 Amsterdam, The Netherlands
Patient-specific immunotherapy designed to reduce the risk of GVHD and relapse in stem cell transplantations
 Patient-specific immunotherapy designed to reduce the risk of GVHD and relapse in stem cell transplantations Unlock full potential of haploidentical hematopoietic stem cell transplantations (HSCT), with
Patient-specific immunotherapy designed to reduce the risk of GVHD and relapse in stem cell transplantations Unlock full potential of haploidentical hematopoietic stem cell transplantations (HSCT), with
Help patients with immunotherapy designed to reduce risk of GVHD in stem cell transplantations
 Help patients with immunotherapy designed to reduce risk of GVHD in stem cell transplantations Unlock full potential of haploidentical hematopoietic stem cell transplantations (HSCT), with allo-depleted
Help patients with immunotherapy designed to reduce risk of GVHD in stem cell transplantations Unlock full potential of haploidentical hematopoietic stem cell transplantations (HSCT), with allo-depleted
Making bone marrow transplantations safer and more effective
 Making bone marrow transplantations safer and more effective Patient specific cell-based immunotherapy products, as adjunctive treatment to haploidentical hematopoietic stem cell transplantation (HSCT),
Making bone marrow transplantations safer and more effective Patient specific cell-based immunotherapy products, as adjunctive treatment to haploidentical hematopoietic stem cell transplantation (HSCT),
Help patients by aiming to prevent severe GVHD in stem cell transplantations
 Help patients by aiming to prevent severe GVHD in stem cell transplantations Unlock full potential of haploidentical hematopoietic stem cell transplantations (HSCT), with allo-depleted T-cell product ATIR
Help patients by aiming to prevent severe GVHD in stem cell transplantations Unlock full potential of haploidentical hematopoietic stem cell transplantations (HSCT), with allo-depleted T-cell product ATIR
Cell-based immunotherapy products for the treatment of blood cancers and inherited blood disorders. Company Presentation June 2016
 Cell-based immunotherapy products for the treatment of blood cancers and inherited blood disorders Company Presentation June 2016 Disclaimer These slides and the accompanying oral presentation contain
Cell-based immunotherapy products for the treatment of blood cancers and inherited blood disorders Company Presentation June 2016 Disclaimer These slides and the accompanying oral presentation contain
Making hematopoietic stem cell transplantation (HSCT) safer and more effective
 Making hematopoietic stem cell transplantation (HSCT) safer and more effective Cell-based immunotherapy products for the treatment of blood cancers and inherited blood disorders Company presentation, April
Making hematopoietic stem cell transplantation (HSCT) safer and more effective Cell-based immunotherapy products for the treatment of blood cancers and inherited blood disorders Company presentation, April
The National Marrow Donor Program. Graft Sources for Hematopoietic Cell Transplantation. Simon Bostic, URD Transplant Recipient
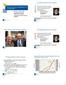 1988 199 1992 1994 1996 1998 2 22 24 26 28 21 212 214 216 218 Adult Donors Cord Blood Units The National Donor Program Graft Sources for Hematopoietic Cell Transplantation Dennis L. Confer, MD Chief Medical
1988 199 1992 1994 1996 1998 2 22 24 26 28 21 212 214 216 218 Adult Donors Cord Blood Units The National Donor Program Graft Sources for Hematopoietic Cell Transplantation Dennis L. Confer, MD Chief Medical
Haploidentical Transplantation: The Answer to our Donor Problems? Mary M. Horowitz, MD, MS CIBMTR, Medical College of Wisconsin January 2017
 Haploidentical Transplantation: The Answer to our Donor Problems? Mary M. Horowitz, MD, MS CIBMTR, Medical College of Wisconsin January 2017 Allogeneic Transplant Recipients in the US, by Donor Type 9000
Haploidentical Transplantation: The Answer to our Donor Problems? Mary M. Horowitz, MD, MS CIBMTR, Medical College of Wisconsin January 2017 Allogeneic Transplant Recipients in the US, by Donor Type 9000
An Introduction to Bone Marrow Transplant
 Introduction to Blood Cancers An Introduction to Bone Marrow Transplant Rushang Patel, MD, PhD, FACP Florida Hospital Medical Group S My RBC Plt Gran Polycythemia Vera Essential Thrombocythemia AML, CML,
Introduction to Blood Cancers An Introduction to Bone Marrow Transplant Rushang Patel, MD, PhD, FACP Florida Hospital Medical Group S My RBC Plt Gran Polycythemia Vera Essential Thrombocythemia AML, CML,
Allogeneic Hematopoietic Stem Cell Transplantation: State of the Art in 2018 RICHARD W. CHILDS M.D. BETHESDA MD
 Allogeneic Hematopoietic Stem Cell Transplantation: State of the Art in 2018 RICHARD W. CHILDS M.D. BETHESDA MD Overview: Update on allogeneic transplantation for malignant and nonmalignant diseases: state
Allogeneic Hematopoietic Stem Cell Transplantation: State of the Art in 2018 RICHARD W. CHILDS M.D. BETHESDA MD Overview: Update on allogeneic transplantation for malignant and nonmalignant diseases: state
MUD SCT. Pimjai Niparuck Division of Hematology, Department of Medicine Ramathibodi Hospital, Mahidol University
 MUD SCT Pimjai Niparuck Division of Hematology, Department of Medicine Ramathibodi Hospital, Mahidol University Outlines Optimal match criteria for unrelated adult donors Role of ATG in MUD-SCT Post-transplant
MUD SCT Pimjai Niparuck Division of Hematology, Department of Medicine Ramathibodi Hospital, Mahidol University Outlines Optimal match criteria for unrelated adult donors Role of ATG in MUD-SCT Post-transplant
Reduced-intensity Conditioning Transplantation
 Reduced-intensity Conditioning Transplantation Current Role and Future Prospect He Huang M.D., Ph.D. Bone Marrow Transplantation Center The First Affiliated Hospital Zhejiang University School of Medicine,
Reduced-intensity Conditioning Transplantation Current Role and Future Prospect He Huang M.D., Ph.D. Bone Marrow Transplantation Center The First Affiliated Hospital Zhejiang University School of Medicine,
The future of HSCT. John Barrett, MD, NHBLI, NIH Bethesda MD
 The future of HSCT John Barrett, MD, NHBLI, NIH Bethesda MD Transplants today Current approaches to improve SCT outcome Optimize stem cell dose and source BMT? PBSCT? Adjusting post transplant I/S to minimize
The future of HSCT John Barrett, MD, NHBLI, NIH Bethesda MD Transplants today Current approaches to improve SCT outcome Optimize stem cell dose and source BMT? PBSCT? Adjusting post transplant I/S to minimize
Company Overview. January 2019
 Company Overview January 2019 1 Disclaimer This Presentation includes certain projections and forward-looking statements as of the date of this Presentation provided by Gamida Cell Ltd (the Company ).
Company Overview January 2019 1 Disclaimer This Presentation includes certain projections and forward-looking statements as of the date of this Presentation provided by Gamida Cell Ltd (the Company ).
What s new in Blood and Marrow Transplant? Saar Gill, MD PhD Jan 22, 2016
 What s new in Blood and Marrow Transplant? Saar Gill, MD PhD Jan 22, 2016 Division of Hematology-Oncology University of Pennsylvania Perelman School of Medicine 1 Who should be transplanted and how? Updates
What s new in Blood and Marrow Transplant? Saar Gill, MD PhD Jan 22, 2016 Division of Hematology-Oncology University of Pennsylvania Perelman School of Medicine 1 Who should be transplanted and how? Updates
Dynavax Corporate Presentation
 Dynavax Corporate Presentation Forward-Looking Statements This presentation contains forward-looking statements, including statements regarding our HEPLISAV-B TM regulatory submissions, product profile,
Dynavax Corporate Presentation Forward-Looking Statements This presentation contains forward-looking statements, including statements regarding our HEPLISAV-B TM regulatory submissions, product profile,
Actinium Pharmaceuticals Highlights Analysis of Pivotal Iomab-B Phase 3 SIERRA Trial Presented in Oral Session at ASH Annual Meeting
 December 4, 2018 Actinium Pharmaceuticals Highlights Analysis of Pivotal Iomab-B Phase 3 SIERRA Trial Presented in Oral Session at ASH Annual Meeting - Key highlights include near universal engraftment
December 4, 2018 Actinium Pharmaceuticals Highlights Analysis of Pivotal Iomab-B Phase 3 SIERRA Trial Presented in Oral Session at ASH Annual Meeting - Key highlights include near universal engraftment
Corporate Overview June 2014 Jefferies Healthcare Conference NASDAQ: GLYC
 Corporate Overview June 2014 Jefferies Healthcare Conference NASDAQ: GLYC Forward-Looking Statements To the extent that statements contained in this presentation are not descriptions of historical facts
Corporate Overview June 2014 Jefferies Healthcare Conference NASDAQ: GLYC Forward-Looking Statements To the extent that statements contained in this presentation are not descriptions of historical facts
Corporate Presentation. December, 2018
 Corporate Presentation December, 2018 Forward Looking Statement This presentation contains estimates, projections and other forward-looking statements, concerning, among other things: our research and
Corporate Presentation December, 2018 Forward Looking Statement This presentation contains estimates, projections and other forward-looking statements, concerning, among other things: our research and
Bone Marrow Transplantation and the Potential Role of Iomab-B
 Bone Marrow Transplantation and the Potential Role of Iomab-B Hillard M. Lazarus, MD, FACP Professor of Medicine, Director of Novel Cell Therapy Case Western Reserve University 1 Hematopoietic Cell Transplantation
Bone Marrow Transplantation and the Potential Role of Iomab-B Hillard M. Lazarus, MD, FACP Professor of Medicine, Director of Novel Cell Therapy Case Western Reserve University 1 Hematopoietic Cell Transplantation
Business Update & Financial Results for Q1 2018
 Business Update & Financial Results for Q1 2018 May 15, 2018 Disclaimer The statements made in this presentation may include forward-looking statements regarding the future operations of ERYTECH Pharma
Business Update & Financial Results for Q1 2018 May 15, 2018 Disclaimer The statements made in this presentation may include forward-looking statements regarding the future operations of ERYTECH Pharma
Company Overview. October 2018
 Company Overview October 2018 1 Disclaimer This presentation, the information contained herein and the materials accompanying it (collectively, this Presentation ) does not purport to contain all of the
Company Overview October 2018 1 Disclaimer This presentation, the information contained herein and the materials accompanying it (collectively, this Presentation ) does not purport to contain all of the
Advancing Cell Therapies
 Take Control of Life Take Control of Life Jefferies 2016 Global Healthcare Conference Advancing Cell Therapies by giving physicians control over cells inside the body June 8, 2016 Forward Looking Statements
Take Control of Life Take Control of Life Jefferies 2016 Global Healthcare Conference Advancing Cell Therapies by giving physicians control over cells inside the body June 8, 2016 Forward Looking Statements
Company presentation. Jefferies Global Healthcare Conference New York, June 6, Claudio Bordignon Chairman and CEO
 Company presentation Jefferies Global Healthcare Conference New York, June 6, 2013 Claudio Bordignon Chairman and CEO Forward-looking statements The presentation contains certain forward-looking statements.
Company presentation Jefferies Global Healthcare Conference New York, June 6, 2013 Claudio Bordignon Chairman and CEO Forward-looking statements The presentation contains certain forward-looking statements.
ZIOPHARM / Intrexon Graft-Versus-Host Disease Exclusive Channel Collaboration SEPTEMBER 28, 2015
 ZIOPHARM / Intrexon Graft-Versus-Host Disease Exclusive Channel Collaboration SEPTEMBER 28, 2015 1 Forward-looking Statements This presentation contains certain forward-looking information about ZIOPHARM
ZIOPHARM / Intrexon Graft-Versus-Host Disease Exclusive Channel Collaboration SEPTEMBER 28, 2015 1 Forward-looking Statements This presentation contains certain forward-looking information about ZIOPHARM
Quarterly Update & ASH 2017 Abstract Conference Call
 Quarterly Update & ASH 2017 Abstract Conference Call November 1, 2017 Nasdaq : BLUE 1 Forward Looking Statements These slides and the accompanying oral presentation contain forward-looking statements and
Quarterly Update & ASH 2017 Abstract Conference Call November 1, 2017 Nasdaq : BLUE 1 Forward Looking Statements These slides and the accompanying oral presentation contain forward-looking statements and
5/9/2018. Bone marrow failure diseases (aplastic anemia) can be cured by providing a source of new marrow
 5/9/2018 or Stem Cell Harvest Where we are now, and What s Coming AA MDS International Foundation Indianapolis IN Luke Akard MD May 19, 2018 Infusion Transplant Conditioning Treatment 2-7 days STEM CELL
5/9/2018 or Stem Cell Harvest Where we are now, and What s Coming AA MDS International Foundation Indianapolis IN Luke Akard MD May 19, 2018 Infusion Transplant Conditioning Treatment 2-7 days STEM CELL
Haplo vs Cord vs URD Debate
 3rd Annual ASBMT Regional Conference for NPs, PAs and Fellows Haplo vs Cord vs URD Debate Claudio G. Brunstein Associate Professor University of Minnesota Medical School Take home message Finding a donor
3rd Annual ASBMT Regional Conference for NPs, PAs and Fellows Haplo vs Cord vs URD Debate Claudio G. Brunstein Associate Professor University of Minnesota Medical School Take home message Finding a donor
One Day BMT Course by Thai Society of Hematology. Management of Graft Failure and Relapsed Diseases
 One Day BMT Course by Thai Society of Hematology Management of Graft Failure and Relapsed Diseases Piya Rujkijyanont, MD Division of Hematology-Oncology Department of Pediatrics Phramongkutklao Hospital
One Day BMT Course by Thai Society of Hematology Management of Graft Failure and Relapsed Diseases Piya Rujkijyanont, MD Division of Hematology-Oncology Department of Pediatrics Phramongkutklao Hospital
Investor Presentation
 Investor Presentation February 2018 2 FORWARD-LOOKING INFORMATION The following presentation contains statements that are considered forward-looking information ( FLI ) within the meaning of securities
Investor Presentation February 2018 2 FORWARD-LOOKING INFORMATION The following presentation contains statements that are considered forward-looking information ( FLI ) within the meaning of securities
Sunesis Pharmaceuticals Reports Third Quarter 2014 Financial Results and Recent Highlights
 Sunesis Pharmaceuticals Reports Third Quarter 2014 Financial Results and Recent Highlights November 10, 2014 7:00 AM ET Announces Submission of Letter of Intent to File MAA for Vosaroxin in Relapsed or
Sunesis Pharmaceuticals Reports Third Quarter 2014 Financial Results and Recent Highlights November 10, 2014 7:00 AM ET Announces Submission of Letter of Intent to File MAA for Vosaroxin in Relapsed or
Haploidentical Transplantation today: and the alternatives
 Haploidentical Transplantation today: and the alternatives Daniel Weisdorf MD University of Minnesota February, 2013 No matched sib: where to look? URD donor requires close HLA matching and 3-12 weeks
Haploidentical Transplantation today: and the alternatives Daniel Weisdorf MD University of Minnesota February, 2013 No matched sib: where to look? URD donor requires close HLA matching and 3-12 weeks
Company presentation. Claudio Bordignon, Chairman and CEO. Jefferies Global Healthcare Conference New York, 7 June 2012
 Company presentation Claudio Bordignon, Chairman and CEO Jefferies Global Healthcare Conference New York, 7 June 2012 Forward-looking statements The presentation contains certain forward-looking statements.
Company presentation Claudio Bordignon, Chairman and CEO Jefferies Global Healthcare Conference New York, 7 June 2012 Forward-looking statements The presentation contains certain forward-looking statements.
Current Status of Haploidentical Hematopoietic Stem Cell Transplantation
 Current Status of Haploidentical Hematopoietic Stem Cell Transplantation Annalisa Ruggeri, MD, PhD Hematology and BMT Unit Hôpital Saint Antoine, Paris, France #EBMTITC16 www.ebmt.org Hematopoietic SCT
Current Status of Haploidentical Hematopoietic Stem Cell Transplantation Annalisa Ruggeri, MD, PhD Hematology and BMT Unit Hôpital Saint Antoine, Paris, France #EBMTITC16 www.ebmt.org Hematopoietic SCT
Full Year 2017 Financial Results. February 14, 2018
 Full Year 2017 Financial Results February 14, 2018 Agios Conference Call Participants Prepared Remarks Introduction KENDRA ADAMS, Sr. Director, Investor Relations 2018 Vision & Key Milestones DAVID SCHENKEIN,
Full Year 2017 Financial Results February 14, 2018 Agios Conference Call Participants Prepared Remarks Introduction KENDRA ADAMS, Sr. Director, Investor Relations 2018 Vision & Key Milestones DAVID SCHENKEIN,
Asterias Biotherapeutics NYSE American: AST
 Clinical-Stage Cell Therapy Programs Addressing Significant Unmet Medical Needs in Neurology and Oncology Asterias Biotherapeutics NYSE American: AST November 2017 Forward-Looking Statements Statements
Clinical-Stage Cell Therapy Programs Addressing Significant Unmet Medical Needs in Neurology and Oncology Asterias Biotherapeutics NYSE American: AST November 2017 Forward-Looking Statements Statements
Haploidentical Transplants for Lymphoma. Andrea Bacigalupo Universita Cattolica Policlinico Gemelli Roma - Italy
 Haploidentical Transplants for Lymphoma Andrea Bacigalupo Universita Cattolica Policlinico Gemelli Roma - Italy HODGKIN NON HODGKIN Non Myelo Ablative Regimen Luznik L et al BBMT 2008 Comparison of Outcomes
Haploidentical Transplants for Lymphoma Andrea Bacigalupo Universita Cattolica Policlinico Gemelli Roma - Italy HODGKIN NON HODGKIN Non Myelo Ablative Regimen Luznik L et al BBMT 2008 Comparison of Outcomes
Presentation to 2019 JP Morgan Healthcare Conference
 For immediate release 10 January 2019 Presentation to 2019 JP Morgan Healthcare Conference Please find attached CSL Limited s presentation at the 2019 JP Morgan Healthcare Conference. For further information,
For immediate release 10 January 2019 Presentation to 2019 JP Morgan Healthcare Conference Please find attached CSL Limited s presentation at the 2019 JP Morgan Healthcare Conference. For further information,
Federica Galaverna, 1 Daria Pagliara, 1 Deepa Manwani, 2 Rajni Agarwal-Hashmi, 3 Melissa Aldinger, 4 Franco Locatelli 1
 Administration of Rivogenlecleucel (Rivo-cel, BPX-501) Following αβ T- and B-Cell Depleted Haplo-HSCT in Children With Transfusion-Dependent Thalassemia Federica Galaverna, 1 Daria Pagliara, 1 Deepa Manwani,
Administration of Rivogenlecleucel (Rivo-cel, BPX-501) Following αβ T- and B-Cell Depleted Haplo-HSCT in Children With Transfusion-Dependent Thalassemia Federica Galaverna, 1 Daria Pagliara, 1 Deepa Manwani,
What s a Transplant? What s not?
 What s a Transplant? What s not? How to report the difference? Daniel Weisdorf MD University of Minnesota Anti-cancer effects of BMT or PBSCT [HSCT] Kill the cancer Save the patient Restore immunocompetence
What s a Transplant? What s not? How to report the difference? Daniel Weisdorf MD University of Minnesota Anti-cancer effects of BMT or PBSCT [HSCT] Kill the cancer Save the patient Restore immunocompetence
February Company Overview. Curative Treatments for Cancer and Orphan Genetic Diseases
 February 2017 Company Overview Curative Treatments for Cancer and Orphan Genetic Diseases Curative Treatments for Orphan Indications NiCord - a bone marrow transplantation treatment for patients with high
February 2017 Company Overview Curative Treatments for Cancer and Orphan Genetic Diseases Curative Treatments for Orphan Indications NiCord - a bone marrow transplantation treatment for patients with high
Two-stage study designed to evaluate tolerability and efficacy of pracinostat combined with azacitidine in patients with high and very high risk MDS
 Helsinn Group and MEI Pharma Announce First Patient Dosed in Phase 2 Dose-Optimization Study of Pracinostat and Azacitidine in Myelodysplastic Syndrome Two-stage study designed to evaluate tolerability
Helsinn Group and MEI Pharma Announce First Patient Dosed in Phase 2 Dose-Optimization Study of Pracinostat and Azacitidine in Myelodysplastic Syndrome Two-stage study designed to evaluate tolerability
PLEO-CMT Top-line Results. Presentation October 16, 2018
 PLEO-CMT Top-line Results Presentation October 16, 2018 Disclaimer References herein to this presentation (the Presentation ) shall mean and include this document, any oral presentation accompanying this
PLEO-CMT Top-line Results Presentation October 16, 2018 Disclaimer References herein to this presentation (the Presentation ) shall mean and include this document, any oral presentation accompanying this
Committed to Transforming the Treatment Paradigm for Migraine Prevention
 June 14, 2018 Committed to Transforming the Treatment Paradigm for Migraine Prevention September 6, 2018 Forward-Looking Statements This presentation and the accompanying commentary contains certain forward-looking
June 14, 2018 Committed to Transforming the Treatment Paradigm for Migraine Prevention September 6, 2018 Forward-Looking Statements This presentation and the accompanying commentary contains certain forward-looking
INVESTOR PRESENTATION
 INVESTOR PRESENTATION May 2018 2 FORWARD-LOOKING INFORMATION The following presentation contains statements that are considered forward-looking information ( FLI ) within the meaning of securities regulation.
INVESTOR PRESENTATION May 2018 2 FORWARD-LOOKING INFORMATION The following presentation contains statements that are considered forward-looking information ( FLI ) within the meaning of securities regulation.
High dose cyclophosphamide in HLAhaploidentical
 High dose cyclophosphamide in HLAhaploidentical stem cell transplantation Ephraim J. Fuchs, M.D., M.B.A. Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins fuchsep@jhmi.edu Alternative Donor Transplantation:
High dose cyclophosphamide in HLAhaploidentical stem cell transplantation Ephraim J. Fuchs, M.D., M.B.A. Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins fuchsep@jhmi.edu Alternative Donor Transplantation:
A Biopharmaceutical Company Focused on Controlled Immunotherapies and Point-of-Care Solutions
 A Biopharmaceutical Company Focused on Controlled Immunotherapies and Point-of-Care Solutions Second Quarter 2017 Company Update and Financial Results July 31, 2017 2 Forward-Looking Statements This presentation
A Biopharmaceutical Company Focused on Controlled Immunotherapies and Point-of-Care Solutions Second Quarter 2017 Company Update and Financial Results July 31, 2017 2 Forward-Looking Statements This presentation
Corporate Presentation May Transforming Immuno-Oncology Using Next-Generation Immune Cell Engagers
 Corporate Presentation May 2016 Transforming Immuno-Oncology Using Next-Generation Immune Cell Engagers Forward-looking statements / safe harbor This presentation and the accompanying oral commentary contain
Corporate Presentation May 2016 Transforming Immuno-Oncology Using Next-Generation Immune Cell Engagers Forward-looking statements / safe harbor This presentation and the accompanying oral commentary contain
Introduction to Clinical Hematopoietic Cell Transplantation (HCT) George Chen, MD Thursday, May 03, 2018
 Introduction to Clinical Hematopoietic Cell Transplantation (HCT) George Chen, MD Thursday, May 03, 2018 The transfer of hematopoietic progenitor and stem cells for therapeutic purposes Hematopoietic Cell
Introduction to Clinical Hematopoietic Cell Transplantation (HCT) George Chen, MD Thursday, May 03, 2018 The transfer of hematopoietic progenitor and stem cells for therapeutic purposes Hematopoietic Cell
Making Hope A Reality December 10, Nasdaq : BLUE
 Making Hope A Reality December 10, 2014 Nasdaq : BLUE Forward Looking Statement These slides and the accompanying oral presentation contain forward-looking statements and information. The use of words
Making Hope A Reality December 10, 2014 Nasdaq : BLUE Forward Looking Statement These slides and the accompanying oral presentation contain forward-looking statements and information. The use of words
Calliditas Therapeutics Q2 Report Webcast August 16, 2018, 10:00 Presenters: Renée Aguiar-Lucander, CEO Fredrik Johansson, CFO
 Calliditas Therapeutics Q2 Report 2018 Webcast August 16, 2018, 10:00 Presenters: Renée Aguiar-Lucander, CEO Fredrik Johansson, CFO Disclaimer Important information This presentation may contain certain
Calliditas Therapeutics Q2 Report 2018 Webcast August 16, 2018, 10:00 Presenters: Renée Aguiar-Lucander, CEO Fredrik Johansson, CFO Disclaimer Important information This presentation may contain certain
Inducing Tumor-Specific Ischemic Necrosis to Enhance the Efficacy of Checkpoint Inhibitors and Chemotherapy
 Inducing Tumor-Specific Ischemic Necrosis to Enhance the Efficacy of Checkpoint Inhibitors and Chemotherapy Company Overview, September 2018 Safe Harbor Statement This presentation contains forward-looking
Inducing Tumor-Specific Ischemic Necrosis to Enhance the Efficacy of Checkpoint Inhibitors and Chemotherapy Company Overview, September 2018 Safe Harbor Statement This presentation contains forward-looking
Advancing Mitochondrial Medicine. Thomas Meier, PhD CEO
 Advancing Mitochondrial Medicine Thomas Meier, PhD CEO Disclaimer 2 This presentation is not and under no circumstances to be construed as a solicitation, offer, or recommendation, to buy or sell securities
Advancing Mitochondrial Medicine Thomas Meier, PhD CEO Disclaimer 2 This presentation is not and under no circumstances to be construed as a solicitation, offer, or recommendation, to buy or sell securities
IMMUNOMEDICS, INC. Advanced Antibody-Based Therapeutics
 IMMUNOMEDICS, INC. Advanced Antibody-Based Therapeutics Oncology Autoimmune Diseases Rodman & Renshaw 19 th Annual Global Investment Conference Michael R. Garone, Principal Executive Officer and CFO Forward-Looking
IMMUNOMEDICS, INC. Advanced Antibody-Based Therapeutics Oncology Autoimmune Diseases Rodman & Renshaw 19 th Annual Global Investment Conference Michael R. Garone, Principal Executive Officer and CFO Forward-Looking
Programmed Cellular Immunotherapies
 Better Cells For Better Therapies Programmed Cellular Immunotherapies Corporate Overview August 2017-1 - Forward-Looking Statements This presentation contains "forward-looking statements" within the meaning
Better Cells For Better Therapies Programmed Cellular Immunotherapies Corporate Overview August 2017-1 - Forward-Looking Statements This presentation contains "forward-looking statements" within the meaning
Investor presentation. Bioshares Biotech Summit July 2017
 Investor presentation Bioshares Biotech Summit 2017 22 July 2017 1 Disclaimer Some of the information in this presentation may refer to Dimerix Limited ( Dimerix or the Company ) based on information available
Investor presentation Bioshares Biotech Summit 2017 22 July 2017 1 Disclaimer Some of the information in this presentation may refer to Dimerix Limited ( Dimerix or the Company ) based on information available
Bank of America Merrill Lynch 2016 Health Care Conference
 Bank of America Merrill Lynch 2016 Health Care Conference Dr. Steven Stein Chief Medical Officer David Gryska Chief Financial Officer May 11, 2016 Forward Looking Statements Except for the historical information
Bank of America Merrill Lynch 2016 Health Care Conference Dr. Steven Stein Chief Medical Officer David Gryska Chief Financial Officer May 11, 2016 Forward Looking Statements Except for the historical information
CytoDyn Announces Initiation of Metastatic Triple Negative Breast Cancer Trial and Reiterates Phase 3 Goal in Cancer
 November 26, 2018 CytoDyn Announces Initiation of Metastatic Triple Negative Breast Cancer Trial and Reiterates Phase 3 Goal in Cancer VANCOUVER, Washington, Nov. 26, 2018 (GLOBE NEWSWIRE) -- CytoDyn Inc.
November 26, 2018 CytoDyn Announces Initiation of Metastatic Triple Negative Breast Cancer Trial and Reiterates Phase 3 Goal in Cancer VANCOUVER, Washington, Nov. 26, 2018 (GLOBE NEWSWIRE) -- CytoDyn Inc.
Back to the Future: The Resurgence of Bone Marrow??
 Back to the Future: The Resurgence of Bone Marrow?? Thomas Spitzer, MD Director. Bone Marrow Transplant Program Massachusetts General Hospital Professor of Medicine, Harvard Medical School Bone Marrow
Back to the Future: The Resurgence of Bone Marrow?? Thomas Spitzer, MD Director. Bone Marrow Transplant Program Massachusetts General Hospital Professor of Medicine, Harvard Medical School Bone Marrow
A G E N D A CIBMTR WORKING COMMITTEE FOR GRAFT SOURCES & MANIPULATION Salt Lake City, UT Thursday, February 22, 2018, 2:45 4:45 pm
 2. Accrual 3. Presentations, Not for publication or presentation A G E N D A CIBMTR WORKING COMMITTEE FOR GRAFT SOURCES & MANIPULATION Salt Lake City, UT Thursday, February 22, 2018, 2:45 4:45 pm Co-Chair:
2. Accrual 3. Presentations, Not for publication or presentation A G E N D A CIBMTR WORKING COMMITTEE FOR GRAFT SOURCES & MANIPULATION Salt Lake City, UT Thursday, February 22, 2018, 2:45 4:45 pm Co-Chair:
Stem Cell Transplantation
 Stem Cell Transplantation Evelyne Willems Centre Hospitalier Universitaire, ULg, Liège Post-ASH meeting, January 11, 2012, Brussels Plan 1. Select the patient: validation of HCT-CI 2. Select the donor
Stem Cell Transplantation Evelyne Willems Centre Hospitalier Universitaire, ULg, Liège Post-ASH meeting, January 11, 2012, Brussels Plan 1. Select the patient: validation of HCT-CI 2. Select the donor
[ NASDAQ: MEIP ] Cowen and Company Health Care Conference March 2015
![[ NASDAQ: MEIP ] Cowen and Company Health Care Conference March 2015 [ NASDAQ: MEIP ] Cowen and Company Health Care Conference March 2015](/thumbs/90/104469787.jpg) [ NASDAQ: MEIP ] Cowen and Company Health Care Conference March 2015 Forward-Looking Statements These slides and the accompanying oral presentation contain forward-looking statements. Actual events or
[ NASDAQ: MEIP ] Cowen and Company Health Care Conference March 2015 Forward-Looking Statements These slides and the accompanying oral presentation contain forward-looking statements. Actual events or
Oncology Therapeutics without Compromise APRIL 2011
 Oncology Therapeutics without Compromise APRIL 2011 Forward Looking Statements This presentation contains forward-looking statements that involve substantial risks and uncertainties, including among other
Oncology Therapeutics without Compromise APRIL 2011 Forward Looking Statements This presentation contains forward-looking statements that involve substantial risks and uncertainties, including among other
1Q2018 EARNINGS CALL MAY 7, 2018
 1Q2018 EARNINGS CALL MAY 7, 2018 Forward-Looking Statements These slides and the accompanying oral presentation contain forward-looking statements and information within the meaning of the Private Securities
1Q2018 EARNINGS CALL MAY 7, 2018 Forward-Looking Statements These slides and the accompanying oral presentation contain forward-looking statements and information within the meaning of the Private Securities
LION. Corporate Presentation June 2016 BIOTECHNOLOGIES. Leadership & Innovation in Oncology
 LION BIOTECHNOLOGIES Leadership & Innovation in Oncology Corporate Presentation June 2016 Forward-Looking Statements This presentation contains forward-looking statements within the meaning of the Private
LION BIOTECHNOLOGIES Leadership & Innovation in Oncology Corporate Presentation June 2016 Forward-Looking Statements This presentation contains forward-looking statements within the meaning of the Private
Sunesis Pharmaceuticals Reports Second Quarter 2011 Financial Results
 Investor and Media Inquiries: David Pitts Argot Partners 212-600-1902 Eric Bjerkholt Sunesis Pharmaceuticals Inc. 650-266-3717 Sunesis Pharmaceuticals Reports Second Quarter 2011 Financial Results SOUTH
Investor and Media Inquiries: David Pitts Argot Partners 212-600-1902 Eric Bjerkholt Sunesis Pharmaceuticals Inc. 650-266-3717 Sunesis Pharmaceuticals Reports Second Quarter 2011 Financial Results SOUTH
An Overview of Blood and Marrow Transplantation
 An Overview of Blood and Marrow Transplantation October 24, 2009 Stephen Couban Department of Medicine Dalhousie University Objectives What are the types of blood and marrow transplantation? Who may benefit
An Overview of Blood and Marrow Transplantation October 24, 2009 Stephen Couban Department of Medicine Dalhousie University Objectives What are the types of blood and marrow transplantation? Who may benefit
Actinium Pharmaceuticals, Inc.
 , Inc. Actimab-A MRD Consolidation Strategy in MRD+ AML July 10, 2018 1 Disclaimer and Safe Harbor Some of the information presented herein may contain projections or other forward-looking statements regarding
, Inc. Actimab-A MRD Consolidation Strategy in MRD+ AML July 10, 2018 1 Disclaimer and Safe Harbor Some of the information presented herein may contain projections or other forward-looking statements regarding
ASCO Analyst & Investor Webcast. June 1, 2018
 ASCO Analyst & Investor Webcast June 1, 2018 June 1, 2018 NASDAQ: BLUE Forward Looking Statements These slides and the accompanying oral presentation contain forward-looking statements and information
ASCO Analyst & Investor Webcast June 1, 2018 June 1, 2018 NASDAQ: BLUE Forward Looking Statements These slides and the accompanying oral presentation contain forward-looking statements and information
World leader in navigated, non-invasive brain stimulation therapy and diagnosis
 World leader in navigated, non-invasive brain stimulation therapy and diagnosis Martin Jamieson CEO & Chairman of the Board Nexstim Plc Mikko Karvinen CFO Nexstim Plc Corporate Presentation, BioTrinity,
World leader in navigated, non-invasive brain stimulation therapy and diagnosis Martin Jamieson CEO & Chairman of the Board Nexstim Plc Mikko Karvinen CFO Nexstim Plc Corporate Presentation, BioTrinity,
IMMUNOMEDICS, INC. Advanced Antibody-Based Therapeutics. Jefferies 2014 Global Healthcare Conference Cynthia L. Sullivan, President and CEO
 IMMUNOMEDICS, INC. Advanced Antibody-Based Therapeutics Oncology Autoimmune Diseases Jefferies 2014 Global Healthcare Conference Cynthia L. Sullivan, President and CEO Forward-Looking Statements This presentation,
IMMUNOMEDICS, INC. Advanced Antibody-Based Therapeutics Oncology Autoimmune Diseases Jefferies 2014 Global Healthcare Conference Cynthia L. Sullivan, President and CEO Forward-Looking Statements This presentation,
Revista Cubana de Hematología, Inmunología y Hemoterapia. 2017; 36 (Suplemento).
 Depletion of TCR alpha/beta+ T-lymphocytes from grafts for haplo haematopoietic CELL transplantation (HCT) in children Heilmann C, Ifversen M, Haastrup E, Fischer-Nielsen A. Haematopoietic Cell Transplantation
Depletion of TCR alpha/beta+ T-lymphocytes from grafts for haplo haematopoietic CELL transplantation (HCT) in children Heilmann C, Ifversen M, Haastrup E, Fischer-Nielsen A. Haematopoietic Cell Transplantation
Committed to Transforming the Treatment Paradigm for Migraine Prevention
 Committed to Transforming the Treatment Paradigm for Migraine Prevention 36th Annual J.P. Morgan Healthcare Conference January 8, 2018 Forward-Looking Statements This presentation and the accompanying
Committed to Transforming the Treatment Paradigm for Migraine Prevention 36th Annual J.P. Morgan Healthcare Conference January 8, 2018 Forward-Looking Statements This presentation and the accompanying
AVEO and Astellas Report Final Overall Survival Results from TIVO-1
 AVEO and Astellas Report Final Overall Survival Results from TIVO-1 - Median Overall Survival of 28.8 Months Reported for Tivozanib in Patients with Advanced Kidney Cancer - CAMBRIDGE, Mass. and TOKYO,
AVEO and Astellas Report Final Overall Survival Results from TIVO-1 - Median Overall Survival of 28.8 Months Reported for Tivozanib in Patients with Advanced Kidney Cancer - CAMBRIDGE, Mass. and TOKYO,
LJPC-401 Phase 1 Results and Development Update. September 7, 2016
 LJPC-401 Phase 1 Results and Development Update September 7, 2016 Forward-Looking Statements These slides contain "forward-looking" statements within the meaning of the Private Securities Litigation Reform
LJPC-401 Phase 1 Results and Development Update September 7, 2016 Forward-Looking Statements These slides contain "forward-looking" statements within the meaning of the Private Securities Litigation Reform
Trends in Hematopoietic Cell Transplantation. AAMAC Patient Education Day Oct 2014
 Trends in Hematopoietic Cell Transplantation AAMAC Patient Education Day Oct 2014 Objectives Review the principles behind allogeneic stem cell transplantation Outline the process of transplant, some of
Trends in Hematopoietic Cell Transplantation AAMAC Patient Education Day Oct 2014 Objectives Review the principles behind allogeneic stem cell transplantation Outline the process of transplant, some of
NASDAQ: ZGNX. Company Presentation. October 2017
 NASDAQ: ZGNX Company Presentation October 2017 2 Forward Looking Statement Zogenix cautions you that statements included in this presentation that are not a description of historical facts are forward-looking
NASDAQ: ZGNX Company Presentation October 2017 2 Forward Looking Statement Zogenix cautions you that statements included in this presentation that are not a description of historical facts are forward-looking
J.P. Morgan Healthcare Conference
 J.P. Morgan Healthcare Conference Shire plc January 10, 2012 Angus Russell Chief Executive Officer Our purpose We enable people with life-altering conditions to lead better lives. THE SAFE HARBOR STATEMENT
J.P. Morgan Healthcare Conference Shire plc January 10, 2012 Angus Russell Chief Executive Officer Our purpose We enable people with life-altering conditions to lead better lives. THE SAFE HARBOR STATEMENT
March Corporate Presentation
 March 2017 Corporate Presentation Disclaimer This presentation contains forward-looking statements, as that term is defined under the Private Securities Litigation Reform Act of 1995 (PSLRA), which statements
March 2017 Corporate Presentation Disclaimer This presentation contains forward-looking statements, as that term is defined under the Private Securities Litigation Reform Act of 1995 (PSLRA), which statements
AVEO and Astellas Announce Positive Findings from TIVO-1 Superiority Study of Tivozanib in First-Line Advanced RCC
 FOR IMMEDIATE RELEASE AVEO and Astellas Announce Positive Findings from TIVO-1 Superiority Study of Tivozanib in First-Line Advanced RCC - Tivozanib is the First Agent to Demonstrate Greater than One Year
FOR IMMEDIATE RELEASE AVEO and Astellas Announce Positive Findings from TIVO-1 Superiority Study of Tivozanib in First-Line Advanced RCC - Tivozanib is the First Agent to Demonstrate Greater than One Year
Le infezioni fungine nel trapianto di cellule staminali emopoietiche. Claudio Viscoli Professor of Infectious Disease University of Genova, Italy
 Le infezioni fungine nel trapianto di cellule staminali emopoietiche Claudio Viscoli Professor of Infectious Disease University of Genova, Italy Potential conflicts of interest Received grants as speaker/moderator
Le infezioni fungine nel trapianto di cellule staminali emopoietiche Claudio Viscoli Professor of Infectious Disease University of Genova, Italy Potential conflicts of interest Received grants as speaker/moderator
Disclosures. Franco Locatelli Advisory Board, Bellicum Pharmaceuticals, Inc. Lakshmanan Krishnamurti No disclosures. David Jacobsohn.
 Administration of Rivogenlecleucel (rivo-cel; BPX-51) Cells Following αβ-t and B-cell-Depleted HLA Haploidentical HSCT (haplo-hsct) in Children With Acute Leukemias Franco Locatelli, 1 Annalisa Ruggeri,
Administration of Rivogenlecleucel (rivo-cel; BPX-51) Cells Following αβ-t and B-cell-Depleted HLA Haploidentical HSCT (haplo-hsct) in Children With Acute Leukemias Franco Locatelli, 1 Annalisa Ruggeri,
3.1 Clinical safety of chimeric or humanized anti-cd25 (ch/anti-cd25)
 3 Results 3.1 Clinical safety of chimeric or humanized anti-cd25 (ch/anti-cd25) Five infusions of monoclonal IL-2 receptor antibody (anti-cd25) were planned according to protocol between day 0 and day
3 Results 3.1 Clinical safety of chimeric or humanized anti-cd25 (ch/anti-cd25) Five infusions of monoclonal IL-2 receptor antibody (anti-cd25) were planned according to protocol between day 0 and day
Transplantation - Challenges for the future. Dr Gordon Cook S t James s Institute of Oncology, Leeds Teaching Hospitals Trust
 Transplantation - Challenges for the future Dr Gordon Cook S t James s Institute of Oncology, Leeds Teaching Hospitals Trust Bone Marrow Transplantation Timeline, 1957-2006 Appelbaum F. N Engl J Med 2007;357:1472-1475
Transplantation - Challenges for the future Dr Gordon Cook S t James s Institute of Oncology, Leeds Teaching Hospitals Trust Bone Marrow Transplantation Timeline, 1957-2006 Appelbaum F. N Engl J Med 2007;357:1472-1475
Third Quarter 2018 Financial Results. November 1, 2018
 Third Quarter 2018 Financial Results November 1, 2018 Agios Conference Call Participants Prepared Remarks Introduction RENEE LECK, Associate Director, Investor Relations Business Highlights & 2018 Key
Third Quarter 2018 Financial Results November 1, 2018 Agios Conference Call Participants Prepared Remarks Introduction RENEE LECK, Associate Director, Investor Relations Business Highlights & 2018 Key
Corporate Overview. July 2016 NASDAQ: CYTR
 Corporate Overview July 2016 NASDAQ: CYTR CytRx Safe Harbor Statement THIS PRESENTATION CONTAINS FORWARD-LOOKING STATEMENTS THAT INVOLVE CERTAIN RISKS AND UNCERTAINTIES ASSOCIATED WITH A DEVELOPMENT-STAGE
Corporate Overview July 2016 NASDAQ: CYTR CytRx Safe Harbor Statement THIS PRESENTATION CONTAINS FORWARD-LOOKING STATEMENTS THAT INVOLVE CERTAIN RISKS AND UNCERTAINTIES ASSOCIATED WITH A DEVELOPMENT-STAGE
Bobby W. Sandage, Jr., PhD President & Chief Executive Officer. Lazard Capital Markets 8 th Annual Healthcare Conference
 Bobby W. Sandage, Jr., PhD President & Chief Executive Officer Lazard Capital Markets 8 th Annual Healthcare Conference Statements in this presentation that are not descriptions of historical facts are
Bobby W. Sandage, Jr., PhD President & Chief Executive Officer Lazard Capital Markets 8 th Annual Healthcare Conference Statements in this presentation that are not descriptions of historical facts are
Jefferies 2016 Healthcare Conference. Reid Huber, PhD Chief Scientific Officer
 Jefferies 2016 Healthcare Conference Reid Huber, PhD Chief Scientific Officer June 8, 2016 Forward-looking Statements Except for the historical information set forth herein, the matters set forth in this
Jefferies 2016 Healthcare Conference Reid Huber, PhD Chief Scientific Officer June 8, 2016 Forward-looking Statements Except for the historical information set forth herein, the matters set forth in this
February 23, Q4 and Year-End 2016 Financial Results
 February 23, 2017 Q4 and Year-End 2016 Financial Results 2 RETHINKING CNS Agenda Today s Speakers Paul Cox, Senior Director, Investor Relations Jeff Jonas, M.D., Chief Executive Officer Jim Doherty, Ph.D.,
February 23, 2017 Q4 and Year-End 2016 Financial Results 2 RETHINKING CNS Agenda Today s Speakers Paul Cox, Senior Director, Investor Relations Jeff Jonas, M.D., Chief Executive Officer Jim Doherty, Ph.D.,
Inarigivir ACHIEVE Trial Results and HBV Clinical Program Update. August 2, 2018
 Inarigivir ACHIEVE Trial Results and HBV Clinical Program Update August 2, 2018 FORWARD LOOKING STATEMENT This presentation includes forward-looking statements within the meaning of the Private Securities
Inarigivir ACHIEVE Trial Results and HBV Clinical Program Update August 2, 2018 FORWARD LOOKING STATEMENT This presentation includes forward-looking statements within the meaning of the Private Securities
Tumor Antigens in the Age of Engineered T cell Therapies
 Tumor Antigens in the Age of Engineered T cell Therapies September 30 th 2016 ESMO Preceptorship Course Amsterdam Carsten Linnemann, PhD Senior Scientist Kite Pharma EU B.V. Amsterdam Forward Looking Statements/Safe
Tumor Antigens in the Age of Engineered T cell Therapies September 30 th 2016 ESMO Preceptorship Course Amsterdam Carsten Linnemann, PhD Senior Scientist Kite Pharma EU B.V. Amsterdam Forward Looking Statements/Safe
Transforming patients lives through cellular immunotherapy. Next Generation Cellular Immunotherapy June 2017
 Transforming patients lives through cellular immunotherapy Next Generation Cellular Immunotherapy June 2017 1 Overview of Cell Medica Mission: Transform the treatment of cancer with cellular immunotherapy
Transforming patients lives through cellular immunotherapy Next Generation Cellular Immunotherapy June 2017 1 Overview of Cell Medica Mission: Transform the treatment of cancer with cellular immunotherapy
Q4 Report Webcast February 7, 2019 Presenters: Renée Aguiar-Lucander, CEO Fredrik Johansson, CFO
 Q4 Report 2018 Webcast February 7, 2019 Presenters: Renée Aguiar-Lucander, CEO Fredrik Johansson, CFO Disclaimer Important information This presentation has been prepared by Calliditas Therapeutics AB
Q4 Report 2018 Webcast February 7, 2019 Presenters: Renée Aguiar-Lucander, CEO Fredrik Johansson, CFO Disclaimer Important information This presentation has been prepared by Calliditas Therapeutics AB
Dawson James Conference
 Dawson James Conference October 2018 Forward-looking Statements Except for historical information, this presentation contains forward-looking statements, which reflect IMV s current expectations regarding
Dawson James Conference October 2018 Forward-looking Statements Except for historical information, this presentation contains forward-looking statements, which reflect IMV s current expectations regarding
Valneva Reports Strong 2017 Revenues Driven by Double Digit Product Sales Growth
 Valneva Reports Strong 2017 Revenues Driven by Double Digit Product Sales Growth Strong sales performance in 2017 (unaudited) Total revenues of 109.8 million in 2017 (2016-97.9 million) representing year-on-year
Valneva Reports Strong 2017 Revenues Driven by Double Digit Product Sales Growth Strong sales performance in 2017 (unaudited) Total revenues of 109.8 million in 2017 (2016-97.9 million) representing year-on-year
