Recent Progress in Heme Synthesis and Metabolism
|
|
|
- Virginia Shepherd
- 5 years ago
- Views:
Transcription
1 Recent Progress in Heme Synthesis and Metabolism Shigeru Sassa The Rockefeller University, New York, New York, USA Key Words. 6-Aminolevulinic acid synthase 6-Aminolevulinic acid dehydratase Porphobilinogen deaminase Ferrochelatase Heme oxygenase Tissue-specific expression Porphyrias Abstract. Heme serves as the prosthetic group of various hemoproteins that carry out many essential functions for cells. For example, the transport of oxygen is carried out by hemoglobin and myoglobin; electron transport depends on the function of various mitochondrial cytochromes; oxidative metabolism in a number of xenobiotic substances and endogenous steroid hormones, vitamins and fatty acids are catalyzed by the activity of the microsomal cytochrome P450. In addition, heme is involved in the translation of proteins, and is required in certain aspects of cell development and differentiation. Inherited and acquired enzymatic defects in heme biosynthesis result in clinical conditions termed the porphyrias. Patients with porphyria express various difficulties in these functions as neurological disturbances, abnormal drug metabolism, and/or skin photosensitivity. Recent advances in this field have shed much light on the genetic, enzymological and clinical aspects of heme synthesis, catabolism and inherited defects of these enzymes. Introduction Heme, i.e., ferroprotoporphyrin IX, is the prosthetic group of all hemoproteins, which are required for the function of all aerobic cells. It serves as the prosthetic group of hemoglobin, mitochondria1 and microsornal cytochromes, catalase, peroxidases, tryptophan pyrrolase, prostaglandin H synthetase and NO synthase. Heme is involved in the transport of oxygen and electrons, in the oxidative metabolism of various endogenous and exogenous chemicals, in the decomposition of hydrogen peroxide or Correspondence: Dr. Shigeru Sassa, The Rockefeller University, 1230 York Avenue, New York, N.Y , USA. OAlphaMed Press ISBN X activation of peroxides, and in the oxidation of tryptophan. Alterations or defects of enzymatic activities in the heme biosynthetic pathway result in profound disturbances in these functions, which lead to the development of clinical conditions termed the porphyrias. Recent advances in the molecular studies of these enzymes have contributed not only to an understanding of the pathogenesis of these diseases, but also to the elucidation of hitherto unrecognized heme-mediated processes in normal cells. Biosynthesis of heme requires eight enzymes, while catabolism of heme requires four enzymes. As shown in Figure 1, the first step and the last three steps of heme biosynthesis take place in mitochondria, while the first two enzymes in heme catabolism are found in endoplasmic reticulum, and all the remaining steps of reactions occur in cytosol. Genetic deficiencies leading to the development of the porphyrias have been detected in seven out of the eight enzymes in the biosynthetic pathway, while genetic deficiencies of enzymes in the heme catabolic pathway have been found only in the last step: bilirubin-udpglucuronosyltransferase. In this chapter, important progress in heme synthesis and metabolism will be reviewed. Tissue-specific Regulation of Heme Synthesis Recent studies suggest that there is a copsiderable difference in the regulation of heme synthesis between erythroid and non-erythroid cells [ This distinctive tissue-specific regulation of heme synthesis occurs on the level of at least three enzymatic steps in the heme biosynthetic pathway, which are discussed below.
2 Uro'l p, +ljrod Copro'l - ALA 1 ALAD PBG 1 PBGD Uro'lll Copro'lll ALAS Mitochondrion Succinyl COA + Glycine - Heme T +Fe Fe-C Prot ol X t Proto 'Ox + Proto'lX :opro'ox. Recent Progress in Heme Synthesis and Metabolism Cytosol 'Free Heme", Endoplasmic Reticulum +NADPH * Biliverdin - CR-HO Bilirubin ~ glucuronide +NADPH * Bil BR tudp - GT ubin, Fe Excretion into bile Fig. 1. Subcellular localization of enzymes and intermediates in heme biosynthesis and catabolism [23]. Free heme is considered as heme which is either synthesized very recently and not yet bound, as the prosthetic group of hemoproteins, or heme which has just been released from hemoproteins. Free heme turns over very rapidly, and is involved in the repression of the synthesis of ALAS-N and in the induction of HO. ALA: 8-Aminolevulinic acid; ALAS: ALA synthase; ALAD: ALA dehydratase; PBG: Porphobilinogen; PBGD: PBG deaminase; HMB: Hydroxymethylbilane; Uro': Uroporphyrinogen; Uro'CoS: Uro'II1 cosynthase; Copro': Coproporphyrinogen; Copro'Ox: Copro' oxidase; Proto': Protoporphyrinogen; Proto'Ox: Proto' oxidase; Fe-C: Ferrochelatase; CR: NADPH-cytochrome c reductase; HO: Heme oxygenase; BR: Biliverdin reductase; GT: Bilirubin-UDP- Glucuronosyltransferase. b-ai~zinole~~srlitzute Sytzthase (ALAS) (EC In the liver, the rate of heme formation is determined by the level of ALAS, the first enzyme of the heme biosynthetic pathway [4], and the level of ALAS is in turn controlled by intracellular free heme concentration [5]. For example, in the liver, a) only ALAS activity is increased, while the activity of other heme pathway enzymes remains unchanged when heme synthesis is increased [6, 71, and b) heme suppresses the synthesis of hepatic ALAS [5,6]. An excessive amount of free heme in the liver induces microsomal heme oxygenase (HO) resulting in the breakdown of free heme [S], thus serving as a useful control mechanism to maintain a critical free heme concentration [5]. These findings suggest a negative feedback control of ALAS by heme, i.e., the end-product of the biosynthetic pathway. It has generally been believed that the synthesis of heme in non-hepatic cells, including erythroid cells in the bone marrow, may also be regulated in a similar manner. However, recent studies including our own [9-111 suggest that there may be a distinctive regulatory feature of heme biosynthesis in erythroid cells [lo].,
3 Sassa 3 Specifically, in erythroid precursor cells which can undergo erythroid cell differentiation such as murine Friend virus transformed erythroleukemia (MEL) cells, or K562 human erythroleukemia cells, a) there is an up-regulation of all heme pathway enzyme genes during cell differentiation [ 12, 131; b) hemin stimulates, rather than inhibits, the synthesis of ALAS-E [14, 151, ferrochelatase [16], heme [ll], globin mrna [17,18], and hemoglobin [18]; and c) heme oxygenase mrna [19] and its enzyme activity are down-regulated when cells are induced to undergo erythroid differentiation [20]. The positive effect of heme on its own synthesis in erythroid cells is in contrast to that in the liver. In in vitro erythroid colony culture systems, hemin also stimulates the growth and differentiation of erythroid colonies [21, 221. Thus heme up-regulates its own synthesis in erythroid cells, as well as erythroid cell differentiation. Distinctive aspects in the regulation of heme synthesis between hepatic and erythroid cells can be largely accounted for by the existence of tissue-specific ALAS isozymes, which are encoded by two different genes [23]. The original findings on two ALAS genes were made in avian cells [24], and similar findings were made subsequently in mammalian cells [14,25,26]. For example, the transcript encoding the non-specific ALAS (ALAS-N, or ALAS1) in mice was shown to be present in all tissues, while that encoding the erythroid-specific ALAS (ALAS-E, or ALAS2) was expressed only in erythroid cells [27]. During erythroid cell differentiation of MEL cells, ALAS-E mrna was found to increase dramatically [14], while ALAS-N mrna was rapidly down-regulated [12, 191. In humans, the ALAS-E gene spans 22 kb and is organized into 11 exons [28]. As in avian and murine cells, the human ALAS-E gene is expressed exclusively in erythroid cells. An openreading frame of 1,761 nucleotides from the first ATG codon encodes a precursor of 587 amino acids with a M, of 64.6 kda. Nucleotide sequences for ALAS-E and ALAS-N are = 60% similar, with the longest stretch of identical sequence being 21 nucleotides. There is no homology, however, in the amino-terminal region, while there is a high homology (= 73%) after hepatic residue 197 [29]. From their structural organization, it can be speculated that the two human ALAS genes may have evolved by duplication of a common ancestral gene which encoded a primitive catalytic peptide, with subsequent addition of DNA sequences encoding variable functions [28]. The human ALAS-E gene has been assigned to a distal subregion of Xp11.21 [30], while the ALAS-N gene is on chromosome 3p21 [26, 29, 311. The X-chromosomal localization of the erythroid gene is interesting in view of the fact that this enzyme activity is known to be markedly reduced in erythroid cells of patients with sideroblastic anemia [32], which often occurs as an X-linked disorder [33]. Recently, it has been shown in a patient with a pyridoxineresponsive X-linked sideroblastic anemia that there was a single T4714A transition in a highly conserved region of exon 9, resulting in an Ile +-Am substitution [34]. The amino acid substitution occurred in the putative pyridoxal5'- phosphate binding site, and the mutant enzyme which was expressed in a prokaryotic system showed low enzyme activity, which required a higher concentration of pyridoxal5'-phosphate than the wild-type enzyme for maximal activity. Thus the decreased enzyme activity appears to be due to a decreased affinity of the mutant enzyme to this cofactor. The promoter in the human ALAS-E contains several putative erythroid-specific cis-acting elements including both GATA-1 and an NF-E2 binding site [28]. Both GATA-1 and NF-E2 are erythroid-specific transcription factors which have been shown to bind to multiple DNA sites such as the promoter of the human P-globin gene, and the erythroid porphobilinogen deaminase (PBGD) promoter [35,36]. In addition to the transcriptional control described above, ALAS-E expression may also be controlled at the translational level. It is known that the synthesis of ferritin and transferrin receptor, two major proteins involved in iron homeostasis, is regulated post-transcriptionally by iron-responsive elements (IRES), which are located in the untranslated region of their mrna [37, 381. A functional IRE has also been identified [27, 291 in the 5'-untranslated region of the human ALAd-E mrna, but it is absent in ALAS-N mrna. This finding suggests that expression'of ALAS-E, but not that of ALAS-N, may also be translationally controlled by iron, or heme (as an iron donor), during the development of erythroid cells [15, 391.
4 4 Recent Progress in Heme Synthesis and Metabolism Substances or chemicals that induce ALAS activity in erythroid and non-erythroid cells are also distinct. For example, ALAS activity in the liver is strongly induced by treatment of animals [40] or hepatocytes in culture [4, 61 with 3,5-dicarbethoxy- 1,4-dihydrocollidine, while this chemical does not influence ALAS activity in erythroid cells [40]. Conversely, hypoxia or erythropoietin treatment increases ALAS activity in erythroid cells without affecting hepatic enzyme activity [40]. These findings indicate that the genes encoding the two ALAS isozymes are under separate controls and suggest that, while ALAS-N in erythroid cells is under feedback control by heme similar to that in the liver, the ALAS-E gene is up-regulated by heme and is responsible for increased heme synthesis in developing erythroid cells. b-a tn irzolevu /inate De hyd ratase (ALAD) [EC There are two alternative transcripts for ALAD, which contain either exon 1A or 1B, that are spliced to exon 2 where the coding region begins [41]. Exon 1A is transcribed in all tissues, while exon 1B is transcribed only in erythroid tissues. This is an interesting finding since the ALAD proteins encoded by the two-tissue specific transcripts are identical, and yet the regulation of mrna expression is under tissue-specific control. These findings indicate that there are erythroid-specific and non-specific transcripts of ALAD, as in the case of ALAS and PBGD, that they are likely be regulated in a tissue-specific manner, and that up-regulation of the erythroid-specific genes is probably accomplished via the transcription factor, GATA-1. Porplzobilinogeri Deaminase (PBGD) [EC In addition to ALAS, two distinct molecular forms of PBGD have been identified [42, 431. The larger form (44 kda) corresponds to a nonerythroid isoform of PBGD (PBGD-N), while the smaller form (42 kda) represents an erythroid-specific isoform of the enzyme (PBGD- E). Analysis of cell-free translation products directed by mrna from human erythropoietic spleen and from human liver showed that the two isoforms of PBGD were encoded by distinct mrna. Comparison of the sequences from human PBGD-E mrna [44] to that of PBGD-N mrna [43] revealed a 1,320 bp stretch of perfect identity, but with a mismatch in the first exon at their 5 extremities. An additional inframe AUG codon was found 51 bp upstream from the initiating codon of PBGD-E cdna. Thus an additional 17 amino acid residues occurred at the N-terminus of PBGD-N, which accounted for its higher molecular mass. It was also shown that the expression of PBGD-E mrna is exclusive to erythroid cells. Further studies of the cloned human PBGD gene demonstrated that the gene is split into 15 exons spread over 10 kb of DNA [45]. The two distinct mrna were produced through alternative splicing of two primary transcripts arising from two promoters. The upstream promoter was active in all tissues, and thus the enzyme encoded by the larger transcript was termed the housekeeping PBGD. The other promoter, located 3 kb downstream, was active only in erythroid cells. It displayed structural homology with the p- globin gene promoter, suggesting that some common trans-acting factors may coregulate the transcription of these genes during erythroid development [46]. It was found that two erythroid-specific trans-acting factors recognized sequences in the PBGD erythroid promoter [35, 361. One of these factors was GATA-1, which bound three sites located upstream (-180 bp and -70 bp) and downstream (+45 bp) of the initiation site. A sequence which matched the consensus CAC box found upstream of the P-globin gene cap site in multiple species was found twenty bases upstream from the GATA-1 binding site. The second erythroid-specific factor (NF-E2) recognized a sequence located at -160 bp. Point mutation and deletion studies suggested that GATA-1 and NF-E2 are necessary for correct regulation of this promoter in erythroid cells. Tissue specific expression of the molecular defect of acute intermittent porphyria (AIP) has been recognized. Studies of the PBGD gene locus showed that AIP mutations were associated with a different restriction haplotype [47- SO]. In the majority of patients with AIP, PBGD activity is decreased by 50% compared with normals. However, in some families, the enzyme activity is decreased in non-erythroid cells, but not in erythroid cells. In one of these families, a G - A transition was observed at the first position of the first intron. This modified the normal splice consensus sequence CGGTGAGT to CGATGAGT. In another family, a different
5 Sassa 5 point mutation, i.e., a single base substitution (G + T), was found within the non-erythropoietic exon [51], at the last position of exon 1, which also led to a splicing defect. Other Heme Pathway Enzymes Situations with respect to other enzymes in the heme biosynthetic pathway are less clear. No erythroid-specific transcript has been identified for uroporphyrinogen (Uro ) 111 cosynthase and Uro decarboxylase (Uro D). However, the level of Uro D mrna in erythroid cells appears to be higher than in the liver [52]. The human gene encoding ferrochelatase, the terminal enzyme in the heme biosynthetic pathway, contains a potential binding site for Spl, NF-E2 and GATA-1 [53]. Consistent with this finding, some erythroid-specific expression, characterized by the preferential utilization of a polyadenylation signal, has been suggested in MEL cells [54]. Coproporphyrinogen oxidase cdna has recently been cloned from a MEL cell library [55]. A single transcript of the oxidase is expressed both in erythroid and in non-erythroid cells. cdna cloning for the remaining enzyme in the heme biosynthetic pathway, i.e., protoporphyrinogen oxidase, has not been reported. Hepatic Versus Erythropoietic Porphyrias Inherited gene defects of heme pathway enzymes, except ALAS, are collectively called the porphyrias. Porphyrias are classified as either hepatic or erythropoietic, depending on the principal site of expression of the effect of the specific gene defect. Although the ALAS defect is not called a porphyria (since it does not accompany increased synthesis, nor excretion of porphyrins, nor their precursors), a defective ALAS-E gene has been described in patients with X-linked sideroblastic anemia [34]. It has never been sufficiently explained, however, why patients with a hepatic porphyria, e.g., AIP, have normal erythroid heme synthesis. For example, patients with AIP or hereditary coproporphyria had normal hemoglobin synthesis, despite the fact that their hepatic heme synthesis was markedly deranged [7]. As discussed above, many of these distinctive features of heme synthesis can now be ascribed to tissue-specific isozymes and their tissue-specific regulation [23]. Evidence also suggests that expression of the erythroid-specific heme pathway enzyme genes is critical in erythroid heme formation. For example, point mutations in the ALAS-E gene were described in patients with X-linked sideroblastic anemia, which appear to account for the disturbances of heme synthesis only in erythroid cells in this disorder [34]. In MEL cells, the ALAS-E gene is up-regulated, whereas the ALAS-N gene is down-regulated when cells are induced to undergo erythroid cell differentiation. A dimethyl sulfoxide (DMS0)-resistant mutant clone of MEL cells, termed DR, is defective in hemoglobin synthesis and in erythroid cell differentiation [ 141. The DR defect appears to be restricted to the lack of expression of ALAS-E mrna, since other heme pathway enzymes are all expressed and up-regulatable by DMSO [14]. In DR cells, ALAS-N mrna is also expressed, but is down-regulated by DMSO treatment as it is in DMSO-sensitive cells [12, 14, 561. These findings suggest that the expression and the up-regulation of erythroid-specific heme pathway enzymes are critical to increased heme formation during erythroid cell differentiation. The isozymic nature of certain enzymes also suggests that there may be tissue-specific expression of their gene defects. Recently a spontaneous form of erythropoietic protoporphyria (EPP) in the house mouse has been described [57]. The mouse form of EPP is a viable autosomal recessive mutation causing jaundice, hemolytic anemia and photosensitivity. Ferrochelatase activity is % of normal. The availability of an animal model of porphyria which is amenable to various experimental manipulations should be very useful for a trial of gene therapy. The Role of Heme Oxygenase in Heme Catabolism, Host Defense and cgmp Production Enzymes in the heme catabolic pathway consist of reduced nicotinamide adenine dinucleotide phosphate (NADPH)-cytochrome c reductase (CR), microsomal heme oxygenase (HO) and biliverdin reductase (BR). In animal cells, heme is converted to biliverdin IXa by the combined enzymatic actions of CR and HO. Biliverdin IXa is then reduced to bilirubin IXa by BR. This sequence of reactions requires NADPH and molecular oxygen. cdna for all these enzymes have been cloned and characterized. Microsomal heme oxygenase (HO) [EC is the key enzyme in the heme
6 6 Recent Progress in Heme Synthesis and Metabolism catabolic sequence in that it determines the mode of heme cleavage at the a-methene bridge. and it is the rate-limiting enzyme in this sequence [58]. There are two isozymes of HO, i.e., HO-1 and HO-2. HO-1 activity can be induced in many cell types by treatment with hemin, is., the substrate for the enzyme, as well as with non-heme substances [59]. For example. the enzyme is known to be inducible with treatment of animals or cells by various trace metals [60-631, and by oxidative stress such as UVA radiation, hydrogen peroxide and sodium arsenite [64]. cdna for HO-1 has been cloned from a rat spleen expression library by immunoscreening [65]. The primary structure of rat HO-1 deduced from the nucleotide sequence of cdna consisted of a protein with 289 amino acids with a M, of 33,000. Human HO-1 cdna has also been cloned and characterized [66]. Human HO-1 was found to be composed of 288 amino acids with a M, of 32,800. The degree of sequence homology between the rat and the human HO- I was = 80%. Both rat and human HO-1 contained a putative membrane segment at the carboxyl-terminus which was enriched in hydrophobic amino acids. The rat HO-1 gene was composed of 6,830 nucleotides and organized into four introns and five exons [67]. Its 5'-flanking region contained a number of DNA sequences of potential regulatory significance, including a heat shock element (HSE) [68]. By studying the expression of a chimeric gene which contained the promoter of rat HO-1 gene and Escherichia coli gene gpt, Slzibahara et al. [69] demonstrated that the 5'-flanking region bearing a HSE of the rat HO- 1 gene confers heat inducibility on the expression of gpt. In contrast, treatment with hemin did not increase gpt transcript expression, suggesting that heat and hemin may induce HO-1 by two different mechanisms. Although the human HO-1 gene also contained a potential HSE, it was originally thought that the human HO-1 gene was not activated by heat [66]. However, more recently, HO-1 mrna was shown to be inducible in human fibroblasts [64], and in human Hep3B hepatoma cells [70], suggesting that the human HO-1 may behave as a heat shock protein (HSP) in certain human cell lines. In Hep3B cells, but not in HepG2 hepatoma cells that do not show a heat-mediated HO-1 induction, a transcriptional activation of a heat-inducible nuclear factor was demonstrated, which binds specifically to the HSE in the human HO-1 gene [71]. This factor may therefore be involved, via binding to the HSE, in the activation of the human HO-1 gene in response to heat treatment. In addition to the HSE, the 5'-flanking region of the rat HO-1 gene contains GCN4- binding sites. GCN4 is a positive regulator for the gene for amino acid biosynthesis in yeast. It has been shown that there are certain functional similarities among the jun oncoproteins, AP-1 (a mammalian activating protein) and GCN4 [67]. Thus, it is possible that HSE and GCN4-binding sites in the rat HO-1 gene may function in activating transcription during stress reactions including heat shock and amino acid deprivation. In this respect, it is also interesting to note that HO-1 mrna has been shown to increase in human hepatoma cells in response to interleukin-6 (hil-6), an acute-phase inducer [70, 721. A nuclear factor was also demonstrated both in untreated and in the hil-6 treated Hep3B cells, which binds specifically to the IL-6 responsive element (IL6-RE) of the human HO- 1 gene. In human hepatoma cells, DMSO also elicited an acute-phase-like reaction [73], followed by an induction of HO-1 mrna [70]. These findings suggest that HO-1 is a positive acute-phase reactant which may be involved in host defense mechanisms [72, 741, and that the nuclear factor specific to the IL6-RE may be involved in the activation of the HO-1 gene during acute inflammation. In support of this conclusion, NFkB and AP-2-like binding sites, important regulatory sites in inflammation, have recently been reported in the 5'-untranslated region of the human HO-1 gene [75]. In contrast to the liver-derived cells, HO-1 mrna in erythroid cells, such as differentiating MEL cells, was found to rapidly decrease after DMSO treatment. Since differentiated MEL cells contain a considerable amount of heme, this finding may suggest that the regulation of HO-1 in erythroid cells is different. The downregulation of HO-1 mrna, as well as HSP70 mrna, another housekeeping protein, proceeded significantly earlier than the up-regulation of erythroid-specific genes [19]. Thus it was speculated that HO-1 may be involved in the cellular events that determine the onset of terminal cell differentiation [19].,,
7 Sassa 7 HO-2 cdna has also been isolated and characterized. The cdna for HO-2 encoded a protein with 315 amino acids corresponding to a M, of 35,757 and was reported to show a high HO activity when expressed in E. coli [76,77]. One of the products of the heme oxygenase reaction is carbon monoxide (CO), and this enzymatic reaction is the only known source of CO production in mammalian cells [78]. CO has recently been proposed to be a potential regulator of cyclic guanosine monophosphate (cgmp) production in the brain [79]. This hypothesis is based on a) the chemical interaction of CO with the heme moiety of guanylate cyclase, the enzyme which produces cgmp upon binding of its heme moiety to NO (or possibly to CO), b) the colocalization of guanylate cyclase with HO-1 and HO-2, c) the correlation between HO activity and cgmp levels, and d) the ability of CO to modulate cgmp levels [80]. Although this hypothesis has yet to be tested, it is interesting to note that CO may act as a retrograde message for long-term potentiation (LTP) in the hippocampus [81, 821. Thus it is reasonable to suggest that the role of HO is not only for the catabolism of heme, but also for the regulation of other fundamental functions such as heat-shock and acute-phase responses and, perhaps, cgmp production in the brain. Conclusion Recent progress in the molecular biology of heme pathway enzyme genes has elucidated a number of new aspects in the regulation of these genes in normal cells and in abnormal conditions such as the porphyrias. The availability of molecular probes, both normal and abnormal, has not only contributed significantly to a better understanding of the pathogenesis of the human porphyrias, but also opened up the possibility of gene therapy of the inherited porphyrias. Acknowledgments This work was supported in part by grants from U.S.P.H.S. DK and DK References 1 Sassa S. Heme stimulation of cellular growth and differentiation. Semin Hematol 1988;25: Abraham NG, Levere RD, Lutton JD. Eclectic mechanism of heme regulation of hematopoiesis. Int J Cell Cloning 1991;9: Ponka P, Schulman HM. Regulation of heme biosynthesis: distinct regulatory features in erythroid cells. Stem Cells 1993;11(suppl 1): Granick S. The induction in vitro of the synthesis of 6-aminolevulinic acid synthetase in chemical porphyria: a response to certain drugs, sex hormones, and foreign chemicals. J Biol Chem 1966;241: Granick S, Sinclair P, Sassa S, Grieninger G. Effects by heme, insulin, and serum albumin on heme and protein synthesis in chick embryo liver cells cultured in a chemically defined medium, and a spectrofluorometric assay for porphyrin composition. J Biol Chem 1975;250: Sassa S, Granick S. Induction of 6-aminolevulinic acid synthetase in chick embryo liver cells in culture. Proc Natl Acad Sci USA 1970;67: Kappas A, Sassa S, Galbraith RA, Nordmann Y. The Porphyrias. In: Scriver CR, Beaudet AL, Sly WS, Valle D, eds. The Metabolic Basis of Inherited Disease. Ed 6. New York: McGraw- Hill Book Co., 1989: Sassa S, Kappas A, Bernstein SE, Alvares AP. Heme biosynthesis and drug metabolism in mice with hereditary hemolytic anemia. J Biol Chem 1979;254: Sassa S, Takaku F, Nakao K. Regulation of erythropoiesis in the Friend leukemia mouse. Blood 1968;31: Sassa S. Control of heme biosynthesis in erythroid cells. In: Rossi GB, ed. In Vitro and In Vivo Erythropoiesis: The Friend System. Amsterdam: Elsevier/North-Holland Biochem Press, 1980: Granick JL, Sassa S. Hemin control of heme biosynthesis in mouse Friend virus-transformed erythroleukemia cells in culture. J Biol Chem 1978;253: Fujita H, Yamamoto M, Yamagami T, Hayashi N, Bishop TR, de Verneuil H, Yoshinaga T, Shibahara S, Morimoto R, Sassa S. Sequential activation of genes for heme pathway enzymes during erythroid differentiation of mouse Friend virus-transformed erythroleukemia cells. Biochim Biophys Acta 1991;1090: Hoffman R, Ibrahim N, Murnane MJ, Diamond A, Forget BG, Levere RD. Hemin control of heme biosynthesis and catabolism in a human leukemia cell line. Blood 1980;56:
8 8 Recent Progress in Heme Synthesis and Metabolism 14 Fujita H. Yamamoto M, Yamagami T, Hayashi N, Sassa S. Erythroleukemia differentiation. Distinctive responses of the erythroid-specific and the nonspecific 8-aniinolevulinate synthase mrna. J Biol Chem 1991;266: Melefors 0, Goossen B, Johansson HE, Stripecke R, Gray NK, Hentze MW. Translational control of 5-aminolevulinate synthase mrna by ironresponsive elements in erythroid cells. J Biol Chem 1993;268: Fukuda Y, Fujita H, Taketani S, Sassa S. Dimethyl sulfoxide and haemin induce ferrochelatase mrna by different mechanisms in murine erythroleukaemia cells. Br J Haematol 1993;83: Ross J, Sautner D. Induction of globin mrna accumulation by hemin in cultured erythroleukemic cells. Cell 1976;8: Dabney BJ, Beaudet AL. Increase in globin chains and globin mrna in erythroleukemia cells in response to hemin. Arch Biochem Biophys 1977: 179:1O Fujita H, Sassa S. The rapid and decremental change in haem oxygenase mrna during erythroid differentiation of murine erythroleukemia cells. Br J Haematol 1989;73: Sassa S. Heme biosynthesis in erythroid cells: the distinctive aspects of the regulatory mechanism. In: Goldwasser E, ed. Regulation of Hemoglobin Biosynthesis. Harvard, MA: ElsevieriNorth Holland, 1983: Porter PN, Meints RH, Mesner K. Enhancement of erythroid colony growth in culture by hemin. Exp Hematol 1979;7: Holden SA, Steinberg HN, Matzinger EA, Monette FC. Further characterization of the hemin-induced enhancement of primitive erythrnid progenitor cell growth in vitro. Exp Hematol 1983:11: Sassa S. Regulation of heme pathway enzyme genes in mammalian cells. In: Murphy MJ Jr, ed. Concise Reviews in Clinical and Experimental Hematology. Dayton, OH: AlphaMed Press, 1992: Riddle RD, Yamamoto M, Engel JD. Expression of b-aminolevulinate synthase in avian cells: separate genes encode erythroid-specific and nonspecific isozymes. Proc Natl Acad Sci USA 1989;86: Cox TC, Bawden MJ, Abraham NG, Bottomley SS, May BK, Baker E, Chen LZ, Sutherland GR. Erythroid 5-aminolevulinate synthase is located on the X chromosome. Am J Hum Genet 1990;46:1O Bishop DF, Henderson AS, Astrin KH. Human 6-aminolevulinate synthase: assignment of the housekeeping gene to 3p21 and the erythroidspecific gene to the X chromosome. Genomics 1990;7: Dierks P. Molecular biology of eukaryotic 5- aminolevulinate synthase. In: Dailey HA, ed. Biosynthesis of Heme and Chlorophylls. New York, NY: McGraw-Hill Publishing Company, 1990; Cox TC, Bawden MJ, Martin A, May BK. Human erythroid 5-aminolevulinate synthase: promoter analysis and identification of an ironresponsive element in the mrna. EMBO J 1991;10: Bishop DF. Two different genes encode S-aminolevulinate synthase in humans: nucleotide sequences of cdnas for the housekeeping and erythroid genes. Nucl Acids Res 1990;18: Cotter PD, Willard HF, Gorski JL, Bishop DF. Assignment of human erythroid 6-aminolevulinate synthase (ALAS2) to a distal subregion of band Xp11.21 by PCR analysis of somatic cell hybrids containing X autosome translocations. Genomics 1992;13: Sutherland GR, Baker E, Callen DF, Hyland VJ, May BK, Bawden MJ, Healy HM, Borthwick IA. 5-Aminolevulinate synthase is at 3p21 and thus not the primary defect in X-linked sideroblastic anemia. Am J Hum Genet 1988;43: Aoki Y, Muranaka S, Nakabayashi K, Ueda Y. 8-Aminolevulinic acid synthetase in erythroblasts of patients with pyridoxine-responsive anemia. Hypercatabolism caused by the increased susceptibility to the controlling protease. J Clin Invest 19 79;64: Bottomley SS, Healy HM, May BK. 5- Aminolevulinic acid synthase in sideroblastic anemia. Blood 1989;74:103a. 34 Cotter PD, Baumann M, Bishop DF. Enzymatic defect in X-linked sideroblastic anemia: molecular evidence for erythroid 6-aminolevulinate synthase deficiency. Proc Natl Acad Sci USA 1992;89: Mignotte V, Eleouet JF, Raich N, Romeo P-H. Cis- and trans-acting elements involved in the regulation of the erythroid promotor of the human porphobilinogen deaminase gene. Proc Natl Acad Sci USA 1989;86: Mignotte V, Wall L, deboer E, Grosveld F, Romeo P-H. Two tissue-specific factors bind the erythroid promoter of the human porphobilinogen deaminase gene. Nucl Acids Res 1989;17:37-54.
9 Sassa 9 37 Klausner RD, Harford JB. cis-trans models for post-transcriptional gene regulation. Science 1989;246: Theil EC. Regulation of ferritin and transferrin receptor mrnas. J Biol Chem 1990;265: Dandekar T, Stripecke R, Gray NK, Goossen B, Constable A, Johansson HE, Hentze MW. Identification of a novel iron-responsive element in murine and human erythroid 6-aminolevulinic acid synthase mrna. EMBO J 1991;10: Wada 0, Sassa S, Takaku F, Yano Y, Urata G, Nakao K. Different responses of the hepatic and erythropoietic 6-aminolevulinic acid synthetase of mice. Biochim Biophys Acta 1967;148: Bishop TR, Miller MW, Frelin LP, Dierks P, Boyer SH. The gene encoding the second enzyme of the heme biosynthetic pathway, aminolevulinate dehydratase, has two transcriptional promoters. Am J Hum Genet 1991;49(suppl):425a. 42 Grandchamp B, Beaumont C, de Verneuil H, Walter 0, Nordmann Y. Genetic expression of porphobilinogen deaminase and uroporphyrinogen decarboxylase during the erythroid differentiation of mouse erythroleukemic cells. In: Nordmann Y, ed. Porphyrins and Porphyrias. Colloque INSERM. London: John Libbey Eurotext, Ltd., 1986;134: Grandchamp B, de Verneuil H, Beaumont C, Chretien S, Walter 0, Nordmann Y. Tissue-specific expression of porphobilinogen deaminase. Two isoenzymes from a single gene. Eur J Biochem 1987;162: Romeo P-H, Raich N, Dubart A, Beaupain D, Mattei MG, Goossens M. Molecular cloning and tissue-specific expression analysis of human porphobilinogen deaminase and uroporphyrinogen decarboxylase. In: Nordmann Y, ed. Porphyrins and Porphyrias. London: John Libbey Eurotext, Ltd., 1986;134: Chretien S, Dubart A, Beaupain D, Raich N, Grandchamp B, Rosa J, Goossens M, Romeo P-H. Alternative transcription and splicing of the human porphobilinogen deaminase gene result either in tissue-specific or in housekeeping expression. Proc Natl Acad Sci USA 1988;85: Grandchamp B. Nordmann Y. Enzymes of the heme biosynthesis pathway: recent advances in molecular genetics. Semin Hematol 1988;25: Lee JS, Anvret M. A PstI polymorphism for the human porphobilinogen deaminase gene (PBG). Nucl Acids Res 1987;15: Llewellyn DH, Kalsheker NA, Elder GH, Harrison PR, Chretien S, Goossens M. A MspI polymorphism for the human porphobilinogen deaminase gene. Nucl Acids Res 1987;lS: Lee JS, Anvret M, Lindsten J, Lannfelt L, Gellerfors P, Wetterberg L, Floderus Y, Thunell S. DNA polymorphisms within the porphobilinogen deaminase gene in two Swedish families with acute intermittent porphyria. Hum Genet 1988;79: Lee JS, Lindsten J, Anvret M. Haplotyping of the human porphobilinogen deaminase gene in acute intermittent porphyria by polymerase chain reaction. Hum Genet 1990;84: Grandchamp B, Picat C, Kauppinen R, Mignotte V, Peltonen L, Mustajoki P, Romeo P-H, Goossens M, Nordmann Y. Molecular analysis of acute intermittent porphyria in a Finnish family with normal erythrocyte porphobilinogen deaminase. Eur J Clin Invest 1989;19: Romeo P-H, Raich N, Dubart A, Beaupain D, Pryor M, Kushner J, Cohen-Solal M, Goossens M. Molecular cloning and nucleotide sequence of a complete human uroporphyrinogen decarboxylase cdna. J Biol Chem 1986;261: Taketani S, Inazawa J, Nakahashi Y, Abe T, Tokunaga R. Structure of the human ferrochelatase gene: exon/intron gene organization and location of the gene to chromosome 18. Eur J Biochem 1992;205: Chan RY, Schulman HM, Ponka P. Expression of ferrochelatase mrna in erythroid and non-erythroid cells. Biochem J 1993;292:t2)343-t2) Kohno H, Furukawa T, Yoshinaga T, Tokunaga R, Taketani S. Coproporphyrinogen oxidase: purification, molecular cloning, and induction of mrna during erythroid differentiation. J Biol Chem 1993;268: Mitani K, Fujita H, Hayashi N, Yamamoto M, Sassa S. Differential induction responses of 6- aminolevulinate synthase mrnas during erythroid differentiation: use of nonradioactive in situ hybridization. Am J Hematol 1991;39: Tutois S, Montagutelli X, Da Silva V, Jouault H, Rouyer-Fessard P, Leroy-Viard K, Guenet J-L, Nordmann Y, Beuzard Y, Deybach J-C. Erythropoietic protoporphyria in the house mouse. A recessive inherited ferrochelatase deficiency with anemia, photosensitivity, and liver disease. J Clin Invest 1991;88: Yoshinaga T, Sassa S, Kappas A. A comparative study of heme degradation by NADPH-cytochrome c reductase alone and by the complete
10 10 Recent Progress in Heme Synthesis and Metabolism heme oxygenase system. Distinctive aspects of heme degradation by NADPH-cytochrome c reductase. J Biol Chem 1982;257: Kikuchi G, Yoshida T. Function and induction of the microsomal heme oxygenase. Mol Cell Biochem 1983;53/54: Maines MD, Kappas A. Metals as regulators of heme metabolism. Science 1977;198: Maines MD, Kappas A. The induction of heme oxidation in various tissues by trace metals: evidence for the catabolism of endogenous heme by hepatic heme oxygenase. Ann Clin Res 1976;8: Maines MD, Kappas A. Cobalt induction of hepatic heme oxygenase; with evidence that cyto?hrome P-450 is not essential for this enzyme activity. Proc Natl Acad Sci USA 1974;71: Lin JH-C, Villalon P, Martasek P, Abraham NG. Regulation of heme oxygenase gene expression by cobalt in rat liver and kidney. EUJ J Biochem 1990;192: Keyse SM, Tyrrell RM. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. PJOC Natl Acad Sci USA 1989;86: Shibahara S, Muller RM, Taguchi H, Yoshida T. Cloning and expression of cdna for rat heme oxygenase. PJOC Natl Acad Sci USA 1985;82: Yoshida T, Biro P, Cohen T, Muller RM, Shibahara S. Human heme oxygenase cdna and induction of its mrna by hemin. Eur J Biochem 1988;171: Muller RM, Taguchi H, Shibahara S. Nucleotide sequence and organization of the rat heme oxygenase gene. J Biol Chem 1987;262: Shibahara S. Regulation of heme oxygenase gene expression. Semin Hematol 1988;25: Shibahara S, Muller RM, Taguchi H. Transcriptional control of rat heme oxygenase by heat shock. J Biol Chem 1987;262: Sassa S, Fujita H, Mitani K, Shibahara S, Bishop TR, Yoshinaga T, de Verneuil H, Romeo P-H, Kappas A. Studies of gene expression of microsoma1 heme oxygenase in mouse erythroid and human hepatic cells. In: Sachs L, Abraham NG, Wiedermann CJ, Levine AS, Konwalinka G, eds. Molecular Biology of Haematopoiesis. Andover, Hampshire, U.K.: Intercept, Ltd., 1990: Mitani K, Fujita H, Sassa S, Kappas A. A heatinducible nuclear factor that binds to the heatshock element of the human haem oxygenase gene. Biochem J 1991;277: Mitani K, Fujita H, Kappas A, Sassa S. Heme oxygenase is a positive acute-phase reactant in human Hep3B hepatoma cells. Blood 1992;79: Iwasa F, Galbraith RA, Sassa S. Effects of dimethyl sulphoxide on the synthesis of plasma proteins in the human hepatoma HepG2. Induction of an acute-phase-like reaction. Biochem J 1988;253: Stocker R. Induction of haem oxygenase as a defense against oxidative stress. Free Radical Research Communications 1990; 9: Lavrovsky Y, Schwartzman ML, Abraham NG. Novel regulatory sites of the human heme oxygenase-1 promoter region. Biochem Biophys Res Commun 1993;196: Rotenberg MO, Maines MD. Isolation, characterization, and expression in Escherichia coli of a cdna encoding rat heme oxygenase-2. J Biol Chem 1990;265: Shibahara S, Yoshizawa M, Suzuki H, Takeda K, Meguro K, Endo K. Functional analysis of cdnas for two types of human heme oxygenase and evidence for their separate regulation. J Biochem 1993;113: Sassa S. Inhibition of carbon monoxide production by tin-protoporphyrin. J Pediatr Gastroenterol Nutr 1987;6: Maines MD. Carbon monoxide: an emerging regulator of cgmp in the brain. Mol Cell Neurosci 1993;4: Maines MD, Mark JA, Ewing JF. Heme oxygenase, a likely regulator of cgmp production in the brain: induction in vivo of HO-1 compensates for depression in NO synthase activity. Mol Cell Neurosci 1993;4: Zhuo M, Small SA, Kandel ER, Hawkins RD. Nitric oxide and carbon monoxide produce activity-dependent long-term synaptic enhancement in hippocampus. Science 1993;260: Stevens CF, Wang Y. Reversal of long-term potentiation by inhibitors of haem oxygenase. Nature 1993;364:
الفريق االكاديمي الطبي HLS/ Biochemistry Sheet Porphyrin and Heme metabolism By: Shatha Khtoum
 الفريق االكاديمي الطبي HLS/ Biochemistry Sheet Porphyrin and Heme metabolism By: Shatha Khtoum اا Today we will take about heme metabolism: -Heme is iron (Fe +2 ) with 4 pyrrole rings. -Its function it
الفريق االكاديمي الطبي HLS/ Biochemistry Sheet Porphyrin and Heme metabolism By: Shatha Khtoum اا Today we will take about heme metabolism: -Heme is iron (Fe +2 ) with 4 pyrrole rings. -Its function it
ENZYMES OF HEME METABOLISM IN THE KIDNEY Regulation by Trace Metals Which Do Not Form Heme Complexes*
 Published Online: 1 November, 1977 Supp Info: http://doi.org/10.1084/jem.146.5.1286 Downloaded from jem.rupress.org on October 2, 2018 ENZYMES OF HEME METABOLISM IN THE KIDNEY Regulation by Trace Metals
Published Online: 1 November, 1977 Supp Info: http://doi.org/10.1084/jem.146.5.1286 Downloaded from jem.rupress.org on October 2, 2018 ENZYMES OF HEME METABOLISM IN THE KIDNEY Regulation by Trace Metals
number Done by Corrected by Doctor Diala
 number 36 Done by Baraa Ayed Corrected by Moath Darweesh Doctor Diala 1 P a g e Today we are going to cover these concepts: Porphyrin structure Biosynthesis of Heme Regulation of heme synthesis A clinical
number 36 Done by Baraa Ayed Corrected by Moath Darweesh Doctor Diala 1 P a g e Today we are going to cover these concepts: Porphyrin structure Biosynthesis of Heme Regulation of heme synthesis A clinical
PORPHYRINS AND PORPHYRIN DISORDERS
 1 SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY ` CLINICAL BIOCHEMISTRY FOR BMLT 3 & BDS 4 PORPHYRINS AND PORPHYRIN DISORDERS
1 SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY ` CLINICAL BIOCHEMISTRY FOR BMLT 3 & BDS 4 PORPHYRINS AND PORPHYRIN DISORDERS
Regulation of the Genes for Heme Pathway Enzymes in Erythroid and in Non-Erythroid Cells
 Concise Review International Journal of Cell Cloning 8:lO-26 (1990) Regulation of the Genes for Heme Pathway Enzymes in Erythroid and in Non-Erythroid Cells Shigeru Sassa The Rockefeller University Hospital,
Concise Review International Journal of Cell Cloning 8:lO-26 (1990) Regulation of the Genes for Heme Pathway Enzymes in Erythroid and in Non-Erythroid Cells Shigeru Sassa The Rockefeller University Hospital,
Doaa Kotkot. Somaya Alkiswani. Nayef
 6 Doaa Kotkot Somaya Alkiswani Nayef Heme Synthesis In the previous lecture we talked about heme synthesis and we said that the rate limiting step is the condensation of Glycine + Succinyl CoA to produce
6 Doaa Kotkot Somaya Alkiswani Nayef Heme Synthesis In the previous lecture we talked about heme synthesis and we said that the rate limiting step is the condensation of Glycine + Succinyl CoA to produce
Regulation of Heme Biosynthesis: Distinct Regulatory Features in Erythroid Cells
 Regulation of Heme Biosynthesis: Distinct Regulatory Features in Erythroid Cells Preriz PonkaaVb, Herbert M. SchuZmana Lady Davis Institute for Medical Research, Sir Mortimer B. Davis Jewish General Hospital,
Regulation of Heme Biosynthesis: Distinct Regulatory Features in Erythroid Cells Preriz PonkaaVb, Herbert M. SchuZmana Lady Davis Institute for Medical Research, Sir Mortimer B. Davis Jewish General Hospital,
BIOCHEMISTRY LECTURE BY OJEMEKELE O. BLOOD CHEMISTRY; BLOOD AS A TISSUE AND PORPHYRINS
 BIOCHEMISTRY LECTURE BY OJEMEKELE O. BLOOD CHEMISTRY; BLOOD AS A TISSUE AND PORPHYRINS BLOOD AS A TISSUE Blood is a liquid connective tissue The total blood volume makes up about 6-8 percent of the body
BIOCHEMISTRY LECTURE BY OJEMEKELE O. BLOOD CHEMISTRY; BLOOD AS A TISSUE AND PORPHYRINS BLOOD AS A TISSUE Blood is a liquid connective tissue The total blood volume makes up about 6-8 percent of the body
PORPHYRIAS (Vampires and Crazy Kings)
 PORPHYRIAS (Vampires and Crazy Kings) Urs A. Meyer Biozentrum of the University of Basel CH-4056 Basel, Switzerland www.biozentrum.unibas.ch/meyer.html Falk Symposium No.156, Genetics in Liver Diseases
PORPHYRIAS (Vampires and Crazy Kings) Urs A. Meyer Biozentrum of the University of Basel CH-4056 Basel, Switzerland www.biozentrum.unibas.ch/meyer.html Falk Symposium No.156, Genetics in Liver Diseases
Goodridge - Heme Synthesis
 Goodridge - Heme Synthesis Study online at quizlet.com/_8r779 1. Acquired Porphyrias (lead poisoning) -inactivates ALA DHD -ALA appears in urine -inhibits protoporphyrinogen oxidase -free protoporphyrinogen
Goodridge - Heme Synthesis Study online at quizlet.com/_8r779 1. Acquired Porphyrias (lead poisoning) -inactivates ALA DHD -ALA appears in urine -inhibits protoporphyrinogen oxidase -free protoporphyrinogen
Biologic Oxidation BIOMEDICAL IMPORTAN
 Biologic Oxidation BIOMEDICAL IMPORTAN Chemically, oxidation is defined as the removal of electrons and reduction as the gain of electrons. Thus, oxidation is always accompanied by reduction of an electron
Biologic Oxidation BIOMEDICAL IMPORTAN Chemically, oxidation is defined as the removal of electrons and reduction as the gain of electrons. Thus, oxidation is always accompanied by reduction of an electron
Metabolism of porphyrins and bile pigments. Mária Sasvári 2017
 Metabolism of porphyrins and bile pigments Mária Sasvári 2017 1 The biological role of porphyrins rotoporphyrin IX + Fe 2+ heme the prosthetic group of several proteins, such as: hemoglobin, myoglobin
Metabolism of porphyrins and bile pigments Mária Sasvári 2017 1 The biological role of porphyrins rotoporphyrin IX + Fe 2+ heme the prosthetic group of several proteins, such as: hemoglobin, myoglobin
INTRODUCTION. Chapter. Overview. Li Zhang
 Chapter 1 INTRODUCTION Li Zhang Overview Heme, iron protoporphyrin IX (Fig. 1), is arguably one of life s most central molecules. Most of us know about heme because of hemoglobin the molecule that transports
Chapter 1 INTRODUCTION Li Zhang Overview Heme, iron protoporphyrin IX (Fig. 1), is arguably one of life s most central molecules. Most of us know about heme because of hemoglobin the molecule that transports
Porphyrins: Chemistry and Biology
 Porphyrins: Chemistry and Biology 20.109 Lecture 6 24 February, 2011 Goals Explore some essential roles of heme in biology Appreciate how ature has used the same cofactor to achieve diverse functions Gain
Porphyrins: Chemistry and Biology 20.109 Lecture 6 24 February, 2011 Goals Explore some essential roles of heme in biology Appreciate how ature has used the same cofactor to achieve diverse functions Gain
adepartment of Medicine, New York Medical College, Valhalla, New York, USA; bthe Rockefeller University, New York, New York, USA
 Concise Review International Journal of Cell Cloning 9:185-210 (1991) Eclectic Mechanisms of Heme Regulation of Hematopoiesis N.G. Abrahamanb, R.D. Levere",b, J.D. Lution" adepartment of Medicine, New
Concise Review International Journal of Cell Cloning 9:185-210 (1991) Eclectic Mechanisms of Heme Regulation of Hematopoiesis N.G. Abrahamanb, R.D. Levere",b, J.D. Lution" adepartment of Medicine, New
Last time we finished the abnormal hemoglobins. We have some hemoglobin. 5 Abdel-muez siyam Abdullah nimer Dr. nayef. Page 1
 Last time we finished the abnormal hemoglobins. We have some hemoglobin 5 Abdel-muez siyam Abdullah nimer Dr. nayef Page 1 derivatives (mostly outside the book) that are of importance: 1. Normal derivatives:
Last time we finished the abnormal hemoglobins. We have some hemoglobin 5 Abdel-muez siyam Abdullah nimer Dr. nayef Page 1 derivatives (mostly outside the book) that are of importance: 1. Normal derivatives:
H emel is a complex of iron with protoporphyrin. Cell Biology of Heme PREM PONKA, MD, PHD
 PREM PONKA, MD, PHD ABSTRACT: Heme is a complex of iron with protoporphyrin IX that is essential for the function of all aerobic cells. Heme serves as the prosthetic group of numerous hemoproteins (eg,
PREM PONKA, MD, PHD ABSTRACT: Heme is a complex of iron with protoporphyrin IX that is essential for the function of all aerobic cells. Heme serves as the prosthetic group of numerous hemoproteins (eg,
Definition of bilirubin Bilirubin metabolism
 Definition of bilirubin Bilirubin metabolism obilirubin formation otransport of bilirubin in plasma ohepatic bilirubin transport oexcretion through intestine Other substances conjugated by glucuronyl transferase.
Definition of bilirubin Bilirubin metabolism obilirubin formation otransport of bilirubin in plasma ohepatic bilirubin transport oexcretion through intestine Other substances conjugated by glucuronyl transferase.
Amino acid oxidation and the production of urea
 Seminar 10 Urea cycle Amino acid oxidation and the production of urea Oxidation Waste or Reuse Ammonia has to be eliminated ammonia originates in the catabolism of amino acids that are primarily produced
Seminar 10 Urea cycle Amino acid oxidation and the production of urea Oxidation Waste or Reuse Ammonia has to be eliminated ammonia originates in the catabolism of amino acids that are primarily produced
Introduction and II. Blood Cells A. Introduction
 Chapter 14: Blood 1. Blood is three to four times more viscous than water. Introduction and II. Blood Cells A. Introduction 2. Most blood cells form in red bone marrow. 3. Types of blood cells are red
Chapter 14: Blood 1. Blood is three to four times more viscous than water. Introduction and II. Blood Cells A. Introduction 2. Most blood cells form in red bone marrow. 3. Types of blood cells are red
Iron deficiency anemia and porphyrias
 Iron deficiency anemia and porphyrias Fleur Wolff¹, Frédéric Cotton¹, Axelle Gilles² ¹Department of clinical chemistry, Hôpital Erasme, ULB ²Department of hematology, Hôpital Erasme, ULB BHS, November
Iron deficiency anemia and porphyrias Fleur Wolff¹, Frédéric Cotton¹, Axelle Gilles² ¹Department of clinical chemistry, Hôpital Erasme, ULB ²Department of hematology, Hôpital Erasme, ULB BHS, November
Cytochrome P 450 Unique family of heme proteins present in bacteria, fungi, insects, plants, fish, mammals and primates. Universal oxygenases (oxygen-
 Cytochrome P 450 Biochemistry Department Cytochrome P 450 Unique family of heme proteins present in bacteria, fungi, insects, plants, fish, mammals and primates. Universal oxygenases (oxygen-utilizing
Cytochrome P 450 Biochemistry Department Cytochrome P 450 Unique family of heme proteins present in bacteria, fungi, insects, plants, fish, mammals and primates. Universal oxygenases (oxygen-utilizing
BCH 471 Experiment (7) HEMOGIOBIN AND ANEMIA, ERYTHROCYTE SEDIMENTATION RATE (ESR) AND HEMATOCRIT (HCT)
 BCH 471 Experiment (7) HEMOGIOBIN AND ANEMIA, ERYTHROCYTE SEDIMENTATION RATE (ESR) AND HEMATOCRIT (HCT) OBJECTIVES 1) Quantitative determination of hemoglobin in a blood sample. 2) Determination of erythrocyte
BCH 471 Experiment (7) HEMOGIOBIN AND ANEMIA, ERYTHROCYTE SEDIMENTATION RATE (ESR) AND HEMATOCRIT (HCT) OBJECTIVES 1) Quantitative determination of hemoglobin in a blood sample. 2) Determination of erythrocyte
Around million aged erythrocytes/hour are broken down.
 Anemia Degradation ofheme Around 100 200 million aged erythrocytes/hour are broken down. The degradation process starts in reticuloendothelial cells in the spleen, liver, and bone marrow. [1] The tetrapyrrole
Anemia Degradation ofheme Around 100 200 million aged erythrocytes/hour are broken down. The degradation process starts in reticuloendothelial cells in the spleen, liver, and bone marrow. [1] The tetrapyrrole
The citric acid cycle
 Integration of metabolism pathways Mitochondria The citric acid cycle Biosynthesis of haem Biochemistry I Lecture 10 2008 (J.S.) Stages in the extraction of energy from foodstuffs The first stage of catabolism
Integration of metabolism pathways Mitochondria The citric acid cycle Biosynthesis of haem Biochemistry I Lecture 10 2008 (J.S.) Stages in the extraction of energy from foodstuffs The first stage of catabolism
REVIEWS. Clinical, Biochemical and Molecular Characteristics of the Main Types of Porphyria. Heme Biosynthesis
 REVIEWS Adv Clin Exp Med 2016, 25, 2, 361 368 DOI: 10.17219/acem/58955 Copyright by Wroclaw Medical University ISSN 1899 5276 Urszula Szlendak 1, 2, D F, Ksenia Bykowska 2, A, D F, Agnieszka Lipniacka
REVIEWS Adv Clin Exp Med 2016, 25, 2, 361 368 DOI: 10.17219/acem/58955 Copyright by Wroclaw Medical University ISSN 1899 5276 Urszula Szlendak 1, 2, D F, Ksenia Bykowska 2, A, D F, Agnieszka Lipniacka
H 2 S: Synthesis and functions
 H 2 S: Synthesis and functions 1 Signaling gas molecules: O 2, NO and CO Then, H 2 S - Fourth singling gas molecule after O 2, NO and CO 2 Nothing Rotten About Hydrogen Sulfide s Medical Promise Science
H 2 S: Synthesis and functions 1 Signaling gas molecules: O 2, NO and CO Then, H 2 S - Fourth singling gas molecule after O 2, NO and CO 2 Nothing Rotten About Hydrogen Sulfide s Medical Promise Science
Acute porphyrias : Diagnosis, complications and treatment options
 Porphyrins & Porphyrias / Patients Day Lucerne, May 18 th, 2013 Acute porphyrias : Diagnosis, complications and treatment options Pr Jean-Charles Deybach, MD, PhD Centre National Maladies Rares Porphyries
Porphyrins & Porphyrias / Patients Day Lucerne, May 18 th, 2013 Acute porphyrias : Diagnosis, complications and treatment options Pr Jean-Charles Deybach, MD, PhD Centre National Maladies Rares Porphyries
Hemoglobin and anemia BCH 471
 Hemoglobin and anemia BCH 471 OBJECTIVES Quantitative determination of hemoglobin in a blood sample. Hemoglobin structure Hemoglobin (Hb) is a porphyrin iron (II) protein in RBCs that transport oxygen
Hemoglobin and anemia BCH 471 OBJECTIVES Quantitative determination of hemoglobin in a blood sample. Hemoglobin structure Hemoglobin (Hb) is a porphyrin iron (II) protein in RBCs that transport oxygen
Hemoglobin is recognized for its brilliant red
 Erythroid Heme Biosynthesis and Its Disorders Harry A. Dailey 1 and Peter N. Meissner 2 1 Department of Microbiology, Department of Biochemistry and Molecular Biology, Biomedical and Health Sciences Institute,
Erythroid Heme Biosynthesis and Its Disorders Harry A. Dailey 1 and Peter N. Meissner 2 1 Department of Microbiology, Department of Biochemistry and Molecular Biology, Biomedical and Health Sciences Institute,
INTERMEDIARY METABOLISM
 INTERMEDIARY METABOLISM Porphyrin Metabolism Dr Puneet Kumar Nigam M-117, Greater Kailash Part II New Delhi 110048 31 Mar 2007 (Revised 15 Oct 2007) CONTENTS Porphyrins Porphyrias Erythropoietic Porphyrias
INTERMEDIARY METABOLISM Porphyrin Metabolism Dr Puneet Kumar Nigam M-117, Greater Kailash Part II New Delhi 110048 31 Mar 2007 (Revised 15 Oct 2007) CONTENTS Porphyrins Porphyrias Erythropoietic Porphyrias
Chapter 4. Drug Biotransformation
 Chapter 4 Drug Biotransformation Drug Biotransformation 1 Why is drug biotransformation necessary 2 The role of biotransformation in drug disposition 3 Where do drug biotransformation occur 4 The enzymes
Chapter 4 Drug Biotransformation Drug Biotransformation 1 Why is drug biotransformation necessary 2 The role of biotransformation in drug disposition 3 Where do drug biotransformation occur 4 The enzymes
Introduction to Metabolism Cell Structure and Function
 Introduction to Metabolism Cell Structure and Function Cells can be divided into two primary types prokaryotes - Almost all prokaryotes are bacteria eukaryotes - Eukaryotes include all cells of multicellular
Introduction to Metabolism Cell Structure and Function Cells can be divided into two primary types prokaryotes - Almost all prokaryotes are bacteria eukaryotes - Eukaryotes include all cells of multicellular
STIMULATION OF HEMOGLOBIN SYNTHESIS IN CHICK
 STIMULATION OF HEMOGLOBIN SYNTHESIS IN CHICK BLASTODERMS BY CERTAIN 56 ANDROSTANE AND 50 PREGNANE STEROIDS* BY RICHARD D. LEVERE, ATTALLAH KAPPAS, AND S. GRANICK DEPARTMENT OF MEDICINE, STATE UNIVERSITY
STIMULATION OF HEMOGLOBIN SYNTHESIS IN CHICK BLASTODERMS BY CERTAIN 56 ANDROSTANE AND 50 PREGNANE STEROIDS* BY RICHARD D. LEVERE, ATTALLAH KAPPAS, AND S. GRANICK DEPARTMENT OF MEDICINE, STATE UNIVERSITY
number Done by Corrected by Doctor Nayef Karadsheh
 number 17 Done by Abdulrahman Alhanbali Corrected by Lara Abdallat Doctor Nayef Karadsheh 1 P a g e Pentose Phosphate Pathway (PPP) Or Hexose Monophosphate Shunt In this lecture We will talk about the
number 17 Done by Abdulrahman Alhanbali Corrected by Lara Abdallat Doctor Nayef Karadsheh 1 P a g e Pentose Phosphate Pathway (PPP) Or Hexose Monophosphate Shunt In this lecture We will talk about the
George R. Honig Junius G. Adams III. Human Hemoglobin. Genetics. Springer-Verlag Wien New York
 George R. Honig Junius G. Adams III Human Hemoglobin Genetics Springer-Verlag Wien New York George R. Honig, M.D., Ph.D. Professor and Head Department of Pediatrics, College of Medicine University of Illinois
George R. Honig Junius G. Adams III Human Hemoglobin Genetics Springer-Verlag Wien New York George R. Honig, M.D., Ph.D. Professor and Head Department of Pediatrics, College of Medicine University of Illinois
Moh Tarek + Faisal Massad. Tala Saleh ... Naif
 19 Moh Tarek + Faisal Massad Tala Saleh... Naif Last lecture we ve talked about the main antioxidant system which are the enzymes found in our body, mainly: 1. Glutathione peroxidase 2. Super oxide dismutase(sod)
19 Moh Tarek + Faisal Massad Tala Saleh... Naif Last lecture we ve talked about the main antioxidant system which are the enzymes found in our body, mainly: 1. Glutathione peroxidase 2. Super oxide dismutase(sod)
Insulin Resistance. Biol 405 Molecular Medicine
 Insulin Resistance Biol 405 Molecular Medicine Insulin resistance: a subnormal biological response to insulin. Defects of either insulin secretion or insulin action can cause diabetes mellitus. Insulin-dependent
Insulin Resistance Biol 405 Molecular Medicine Insulin resistance: a subnormal biological response to insulin. Defects of either insulin secretion or insulin action can cause diabetes mellitus. Insulin-dependent
Metabolism. Chapter 8 Microbial Metabolism. Metabolic balancing act. Catabolism Anabolism Enzymes. Topics. Metabolism Energy Pathways Biosynthesis
 Chapter 8 Microbial Metabolism Topics Metabolism Energy Pathways Biosynthesis Catabolism Anabolism Enzymes Metabolism 1 2 Metabolic balancing act Catabolism and anabolism simple model Catabolism Enzymes
Chapter 8 Microbial Metabolism Topics Metabolism Energy Pathways Biosynthesis Catabolism Anabolism Enzymes Metabolism 1 2 Metabolic balancing act Catabolism and anabolism simple model Catabolism Enzymes
Enzymes what are they?
 Topic 11 (ch8) Microbial Metabolism Topics Metabolism Energy Pathways Biosynthesis 1 Catabolism Anabolism Enzymes Metabolism 2 Metabolic balancing act Catabolism Enzymes involved in breakdown of complex
Topic 11 (ch8) Microbial Metabolism Topics Metabolism Energy Pathways Biosynthesis 1 Catabolism Anabolism Enzymes Metabolism 2 Metabolic balancing act Catabolism Enzymes involved in breakdown of complex
Omar Jabaiti+Saif Yamin
 34 Saif Yamin + Omar Jabaiti Omar Jabaiti+Saif Yamin ABDALLAH FAHMAWI DIALA NOTE : ask OMAR about the first 5 pages and SAIF about last 5 Phenylketonuria: (PKU) Amino Acid Metabolism Disorders Phenylketonuria
34 Saif Yamin + Omar Jabaiti Omar Jabaiti+Saif Yamin ABDALLAH FAHMAWI DIALA NOTE : ask OMAR about the first 5 pages and SAIF about last 5 Phenylketonuria: (PKU) Amino Acid Metabolism Disorders Phenylketonuria
Amino Acid Metabolism: Conversion of Amino Acids to Specialized Products
 Amino Acid Metabolism: Conversion of Amino Acids to Specialized Products Dr. Diala Abu-Hassan, DDS, PhD Medical students-first semester All images are taken from Lippincott s Biochemistry textbook except
Amino Acid Metabolism: Conversion of Amino Acids to Specialized Products Dr. Diala Abu-Hassan, DDS, PhD Medical students-first semester All images are taken from Lippincott s Biochemistry textbook except
Molecular Biology (BIOL 4320) Exam #2 May 3, 2004
 Molecular Biology (BIOL 4320) Exam #2 May 3, 2004 Name SS# This exam is worth a total of 100 points. The number of points each question is worth is shown in parentheses after the question number. Good
Molecular Biology (BIOL 4320) Exam #2 May 3, 2004 Name SS# This exam is worth a total of 100 points. The number of points each question is worth is shown in parentheses after the question number. Good
Red Blood Cells (Erythrocytes) Lecture-2
 Red Blood Cells (Erythrocytes) Lecture-2 Functions Transport hemoglobin, which in turn carries oxygen from the lungs to the tissues. RBCs contain a large quantity of carbonic anhydrase, an enzyme that
Red Blood Cells (Erythrocytes) Lecture-2 Functions Transport hemoglobin, which in turn carries oxygen from the lungs to the tissues. RBCs contain a large quantity of carbonic anhydrase, an enzyme that
Heme is an essential molecule for living aerobic organisms
 Role of Heme in Cardiovascular Physiology and Disease Konrad Teodor Sawicki, BS;* Hsiang-Chun Chang, PhD;* Hossein Ardehali, MD, PhD Heme is an essential molecule for living aerobic organisms and is involved
Role of Heme in Cardiovascular Physiology and Disease Konrad Teodor Sawicki, BS;* Hsiang-Chun Chang, PhD;* Hossein Ardehali, MD, PhD Heme is an essential molecule for living aerobic organisms and is involved
XENOBIOTIC REGULATION OF THE ATP BINDING CASSETTE TRANSPORTER ABCB6 AND ITS SIGNIFICANCE TO HEPATIC HEME HOMEOSTASIS
 XENOBIOTIC REGULATION OF THE ATP BINDING CASSETTE TRANSPORTER ABCB6 AND ITS SIGNIFICANCE TO HEPATIC HEME HOMEOSTASIS BY HEMANTKUMAR DILIP CHAVAN Submitted to the graduate degree program in Pharmacology,
XENOBIOTIC REGULATION OF THE ATP BINDING CASSETTE TRANSPORTER ABCB6 AND ITS SIGNIFICANCE TO HEPATIC HEME HOMEOSTASIS BY HEMANTKUMAR DILIP CHAVAN Submitted to the graduate degree program in Pharmacology,
Examination III PHRM 836 Biochemistry for Pharmaceutical Sciences II December 19, 2014
 Examination III PHRM 836 Biochemistry for Pharmaceutical Sciences II December 19, 2014 Name: Instructions PHRM 836 Exam III - 1 1. Check your exam to make certain that it has 8 pages including this cover
Examination III PHRM 836 Biochemistry for Pharmaceutical Sciences II December 19, 2014 Name: Instructions PHRM 836 Exam III - 1 1. Check your exam to make certain that it has 8 pages including this cover
In glycolysis, glucose is converted to pyruvate. If the pyruvate is reduced to lactate, the pathway does not require O 2 and is called anaerobic
 Glycolysis 1 In glycolysis, glucose is converted to pyruvate. If the pyruvate is reduced to lactate, the pathway does not require O 2 and is called anaerobic glycolysis. If this pyruvate is converted instead
Glycolysis 1 In glycolysis, glucose is converted to pyruvate. If the pyruvate is reduced to lactate, the pathway does not require O 2 and is called anaerobic glycolysis. If this pyruvate is converted instead
number Done by Corrected by Doctor
 number 18 Done by Mahmoud Harbi Corrected by حسام أبو عوض Doctor Nayef Karadsheh Sources of Reactive Oxygen Species (ROS) 1 P a g e 1- Oxidases: there are some that produce hydrogen peroxide (H₂O₂) 2-
number 18 Done by Mahmoud Harbi Corrected by حسام أبو عوض Doctor Nayef Karadsheh Sources of Reactive Oxygen Species (ROS) 1 P a g e 1- Oxidases: there are some that produce hydrogen peroxide (H₂O₂) 2-
Concise Review for Primary-Care Physicians
 Concise Review for Primary-Care Physicians Acute Porphyrias: Diagnosis and Management AYALEW TEFFERI, M.D., JOSEPH P. COLGAN, M.D., AND LAWRENCE A. SOLBERG, JR., M.D. To summarize recent information about
Concise Review for Primary-Care Physicians Acute Porphyrias: Diagnosis and Management AYALEW TEFFERI, M.D., JOSEPH P. COLGAN, M.D., AND LAWRENCE A. SOLBERG, JR., M.D. To summarize recent information about
Metabolism. Topic 11&12 (ch8) Microbial Metabolism. Metabolic Balancing Act. Topics. Catabolism Anabolism Enzymes
 Topic 11&12 (ch8) Microbial Metabolism Topics Metabolism Energy Pathways Biosynthesis 1 Catabolism Anabolism Enzymes Metabolism 2 Metabolic Balancing Act Catabolism Enzymes involved in breakdown of complex
Topic 11&12 (ch8) Microbial Metabolism Topics Metabolism Energy Pathways Biosynthesis 1 Catabolism Anabolism Enzymes Metabolism 2 Metabolic Balancing Act Catabolism Enzymes involved in breakdown of complex
Problem Set #5 4/3/ Spring 02
 Question 1 Chloroplasts contain six compartments outer membrane, intermembrane space, inner membrane, stroma, thylakoid membrane, and thylakoid lumen each of which is populated by specific sets of proteins.
Question 1 Chloroplasts contain six compartments outer membrane, intermembrane space, inner membrane, stroma, thylakoid membrane, and thylakoid lumen each of which is populated by specific sets of proteins.
Chapter 9 Overview. Aerobic Metabolism I: The Citric Acid Cycle. Live processes - series of oxidation-reduction reactions. Aerobic metabolism I
 n n Chapter 9 Overview Aerobic Metabolism I: The Citric Acid Cycle Live processes - series of oxidation-reduction reactions Ingestion of proteins, carbohydrates, lipids Provide basic building blocks for
n n Chapter 9 Overview Aerobic Metabolism I: The Citric Acid Cycle Live processes - series of oxidation-reduction reactions Ingestion of proteins, carbohydrates, lipids Provide basic building blocks for
Metabolism Energy Pathways Biosynthesis. Catabolism Anabolism Enzymes
 Topics Microbial Metabolism Metabolism Energy Pathways Biosynthesis 2 Metabolism Catabolism Catabolism Anabolism Enzymes Breakdown of complex organic molecules in order to extract energy and dform simpler
Topics Microbial Metabolism Metabolism Energy Pathways Biosynthesis 2 Metabolism Catabolism Catabolism Anabolism Enzymes Breakdown of complex organic molecules in order to extract energy and dform simpler
III. 6. Test. Respiració cel lular
 III. 6. Test. Respiració cel lular Chapter Questions 1) What is the term for metabolic pathways that release stored energy by breaking down complex molecules? A) anabolic pathways B) catabolic pathways
III. 6. Test. Respiració cel lular Chapter Questions 1) What is the term for metabolic pathways that release stored energy by breaking down complex molecules? A) anabolic pathways B) catabolic pathways
Published on Second Faculty of Medicine, Charles University (http://www.lf2.cuni.cz )
 Published on Second Faculty of Medicine, Charles University (http://www.lf2.cuni.cz ) Biochemistry Submitted by Marie Havlová on 8. February 2012-0:00 Syllabus of Biochemistry Mechanisms of enzyme catalysis.
Published on Second Faculty of Medicine, Charles University (http://www.lf2.cuni.cz ) Biochemistry Submitted by Marie Havlová on 8. February 2012-0:00 Syllabus of Biochemistry Mechanisms of enzyme catalysis.
CHAPTER 27. Haem biosynthesis and the porphyrias. Jean-Charles Deybach, Laurent Gouya, Hervé Puy
 IRO2009_CAP.27(624-641):EBMT2008 4-12-2009 16:46 Pagina 624 * CAPTER 27 aem biosynthesis and the porphyrias Jean-Charles Deybach, Laurent Gouya, ervé Puy IRO2009_CAP.27(624-641):EBMT2008 4-12-2009 16:46
IRO2009_CAP.27(624-641):EBMT2008 4-12-2009 16:46 Pagina 624 * CAPTER 27 aem biosynthesis and the porphyrias Jean-Charles Deybach, Laurent Gouya, ervé Puy IRO2009_CAP.27(624-641):EBMT2008 4-12-2009 16:46
Figure 1 Original Advantages of biological reactions being catalyzed by enzymes:
 Enzyme basic concepts, Enzyme Regulation I III Carmen Sato Bigbee, Ph.D. Objectives: 1) To understand the bases of enzyme catalysis and the mechanisms of enzyme regulation. 2) To understand the role of
Enzyme basic concepts, Enzyme Regulation I III Carmen Sato Bigbee, Ph.D. Objectives: 1) To understand the bases of enzyme catalysis and the mechanisms of enzyme regulation. 2) To understand the role of
Variegate porphyria: an unusual cause of skin blistering
 H.K. Dermatol. Venereol. Bull. (2003) 11, 20-24 Case Report Variegate porphyria: an unusual cause of skin blistering Variegate porphyria (VP) is an autosomal dominant disorder caused by a partial deficiency
H.K. Dermatol. Venereol. Bull. (2003) 11, 20-24 Case Report Variegate porphyria: an unusual cause of skin blistering Variegate porphyria (VP) is an autosomal dominant disorder caused by a partial deficiency
Clinical diagnosis? AIP (Acute Intermittent Porphyria)
 Case 1 18 yo woman came to ER with a 5-day history of severe abdominal pain Localized, intermittent, sharp, epigastric and periumbilical pain associated with mild nausea but no vomiting for the past 6
Case 1 18 yo woman came to ER with a 5-day history of severe abdominal pain Localized, intermittent, sharp, epigastric and periumbilical pain associated with mild nausea but no vomiting for the past 6
BIOL 158: BIOLOGICAL CHEMISTRY II
 BIOL 158: BIOLOGICAL CHEMISTRY II Lecture 5: Vitamins and Coenzymes Lecturer: Christopher Larbie, PhD Introduction Cofactors bind to the active site and assist in the reaction mechanism Apoenzyme is an
BIOL 158: BIOLOGICAL CHEMISTRY II Lecture 5: Vitamins and Coenzymes Lecturer: Christopher Larbie, PhD Introduction Cofactors bind to the active site and assist in the reaction mechanism Apoenzyme is an
Globular proteins Proteins globular fibrous
 Globular proteins Globular proteins Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form in a biologically functional way. Globular
Globular proteins Globular proteins Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form in a biologically functional way. Globular
Recent Advances in Erythroid Iron Homeostasis: Implications for Pathophysiology of Microcytic Anemias
 Recent Advances in Erythroid Iron Homeostasis: Implications for Pathophysiology of Microcytic Anemias Prem Ponka Department of Physiology Lady Davis Institute, Jewish General Hospital McGill University,
Recent Advances in Erythroid Iron Homeostasis: Implications for Pathophysiology of Microcytic Anemias Prem Ponka Department of Physiology Lady Davis Institute, Jewish General Hospital McGill University,
Ch. 18 Regulation of Gene Expression
 Ch. 18 Regulation of Gene Expression 1 Human genome has around 23,688 genes (Scientific American 2/2006) Essential Questions: How is transcription regulated? How are genes expressed? 2 Bacteria regulate
Ch. 18 Regulation of Gene Expression 1 Human genome has around 23,688 genes (Scientific American 2/2006) Essential Questions: How is transcription regulated? How are genes expressed? 2 Bacteria regulate
Chemical and Biochemical Mechanism Of Cell Injury.
 Chemical and Biochemical Mechanism Of Cell Injury. Professor Dr. M. Tariq Javed Dept. of Pathology Faculty of Vet. Science The University Of Agriculture Faisalabad Cell Injury When the cell is exposed
Chemical and Biochemical Mechanism Of Cell Injury. Professor Dr. M. Tariq Javed Dept. of Pathology Faculty of Vet. Science The University Of Agriculture Faisalabad Cell Injury When the cell is exposed
Amino Acid Metabolism: Conversion of Amino Acids to Specialized Products
 Amino Acid Metabolism: Conversion of Amino Acids to Specialized Products Dr. Diala Abu-Hassan, DDS, PhD Medical students-first semester All images are taken from Lippincott s Biochemistry textbook except
Amino Acid Metabolism: Conversion of Amino Acids to Specialized Products Dr. Diala Abu-Hassan, DDS, PhD Medical students-first semester All images are taken from Lippincott s Biochemistry textbook except
Glycogen Metabolism Dr. Mohammad Saadeh
 Glycogen Metabolism Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry II Philadelphia University Faculty of pharmacy I. overview Glucose is energy source for Brain.
Glycogen Metabolism Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry II Philadelphia University Faculty of pharmacy I. overview Glucose is energy source for Brain.
Topics of this lecture : RBC. Structural characteristics Hemoglobin Erythropoiesis Erythrocytes destruction
 Topics of this lecture : RBC Structural characteristics Hemoglobin Erythropoiesis Erythrocytes destruction Structural characteristics Its small size and biconcave shape provides more surface area than
Topics of this lecture : RBC Structural characteristics Hemoglobin Erythropoiesis Erythrocytes destruction Structural characteristics Its small size and biconcave shape provides more surface area than
Cell Signaling (part 1)
 15 Cell Signaling (part 1) Introduction Bacteria and unicellular eukaryotes respond to environmental signals and to signaling molecules secreted by other cells for mating and other communication. In multicellular
15 Cell Signaling (part 1) Introduction Bacteria and unicellular eukaryotes respond to environmental signals and to signaling molecules secreted by other cells for mating and other communication. In multicellular
Chapter 9. Biotransformation
 Chapter 9 Biotransformation Biotransformation The term biotransformation is the sum of all chemical processes of the body that modify endogenous or exogenous chemicals. Focus areas of toxicokinetics: Biotransformation
Chapter 9 Biotransformation Biotransformation The term biotransformation is the sum of all chemical processes of the body that modify endogenous or exogenous chemicals. Focus areas of toxicokinetics: Biotransformation
Hemoglobin. Each alpha subunit has 141 amino acids, and each beta subunit has 146 amino acids.
 In the previous lecture we talked about erythropoiesis and its regulation by many vitamins like vitamin B12 and folic acid, proteins, iron and trace elements copper and cobalt. Also we talked about pernicious
In the previous lecture we talked about erythropoiesis and its regulation by many vitamins like vitamin B12 and folic acid, proteins, iron and trace elements copper and cobalt. Also we talked about pernicious
Chemistry 107 Exam 4 Study Guide
 Chemistry 107 Exam 4 Study Guide Chapter 10 10.1 Recognize that enzyme catalyze reactions by lowering activation energies. Know the definition of a catalyst. Differentiate between absolute, relative and
Chemistry 107 Exam 4 Study Guide Chapter 10 10.1 Recognize that enzyme catalyze reactions by lowering activation energies. Know the definition of a catalyst. Differentiate between absolute, relative and
Electron Transport Chain and Oxidative phosphorylation
 Electron Transport Chain and Oxidative phosphorylation So far we have discussed the catabolism involving oxidation of 6 carbons of glucose to CO 2 via glycolysis and CAC without any oxygen molecule directly
Electron Transport Chain and Oxidative phosphorylation So far we have discussed the catabolism involving oxidation of 6 carbons of glucose to CO 2 via glycolysis and CAC without any oxygen molecule directly
6.5 Enzymes. Enzyme Active Site and Substrate Specificity
 180 Chapter 6 Metabolism 6.5 Enzymes By the end of this section, you will be able to: Describe the role of enzymes in metabolic pathways Explain how enzymes function as molecular catalysts Discuss enzyme
180 Chapter 6 Metabolism 6.5 Enzymes By the end of this section, you will be able to: Describe the role of enzymes in metabolic pathways Explain how enzymes function as molecular catalysts Discuss enzyme
Metabolism of Nucleotides
 Metabolism of Nucleotides Outline Nucleotide degradation Components of Nucleobases Purine and pyrimidine biosynthesis Hyperuricemia Sources Nucleotide degradation The nucleotides are among the most complex
Metabolism of Nucleotides Outline Nucleotide degradation Components of Nucleobases Purine and pyrimidine biosynthesis Hyperuricemia Sources Nucleotide degradation The nucleotides are among the most complex
Research Institute, NC, USA. Journal of Exploratory Research in Pharmacology 2017 vol
 Review Article Pathophysiology, Pharmacology and Treatment of Acute Intermittent Porphyria: A Patient Case Description and Recommendations from the Current Literature Teminioluwa Ajayi 1, Rachael Ward
Review Article Pathophysiology, Pharmacology and Treatment of Acute Intermittent Porphyria: A Patient Case Description and Recommendations from the Current Literature Teminioluwa Ajayi 1, Rachael Ward
Heme-based CO sensors. CooA. Cystathionine b-synthase (CBS)
 Heme-based CO sensors CooA Cystathionine b-synthase (CBS) 1 Carbon monoxide (CO): Simple! Heme Fe(II) complex, Metal complex NO, H 2 S : Complicated! Heme iron complex, Protein modification, Other molecules
Heme-based CO sensors CooA Cystathionine b-synthase (CBS) 1 Carbon monoxide (CO): Simple! Heme Fe(II) complex, Metal complex NO, H 2 S : Complicated! Heme iron complex, Protein modification, Other molecules
Regulation of Gene Expression in Eukaryotes
 Ch. 19 Regulation of Gene Expression in Eukaryotes BIOL 222 Differential Gene Expression in Eukaryotes Signal Cells in a multicellular eukaryotic organism genetically identical differential gene expression
Ch. 19 Regulation of Gene Expression in Eukaryotes BIOL 222 Differential Gene Expression in Eukaryotes Signal Cells in a multicellular eukaryotic organism genetically identical differential gene expression
7.012 Problem Set 6 Solutions
 Name Section 7.012 Problem Set 6 Solutions Question 1 The viral family Orthomyxoviridae contains the influenza A, B and C viruses. These viruses have a (-)ss RNA genome surrounded by a capsid composed
Name Section 7.012 Problem Set 6 Solutions Question 1 The viral family Orthomyxoviridae contains the influenza A, B and C viruses. These viruses have a (-)ss RNA genome surrounded by a capsid composed
Erythrocytes. Dr. MOHAMED SAAD DAOUD BCH 471 1
 Red blood cells Erythrocytes Circulating erythrocytes are derived from erythropoietic cells (the precursors of erythrocytes). RBCs arise from mesenchymal cells present in bone marrow. RBCs lack nucleus
Red blood cells Erythrocytes Circulating erythrocytes are derived from erythropoietic cells (the precursors of erythrocytes). RBCs arise from mesenchymal cells present in bone marrow. RBCs lack nucleus
Structure and Regulation of Vertebrate 6-Aminolevulinate Synthases
 Structure and Regulation of Vertebrate 6-Aminolevulinate Synthases Masayuki Yamamoto,a Kim-Chew Lim,b Tadashi Nagai, S Kazumichi Furuyamaja James Douglas EngeP ~ Department of Biochemistry, Tohoku University
Structure and Regulation of Vertebrate 6-Aminolevulinate Synthases Masayuki Yamamoto,a Kim-Chew Lim,b Tadashi Nagai, S Kazumichi Furuyamaja James Douglas EngeP ~ Department of Biochemistry, Tohoku University
Vocabulary. Chapter 20: Electron Transport and Oxidative Phosphorylation
 Vocabulary ATP Synthase: the enzyme responsible for production of ATP in mitochondria Chemiosmotic Coupling: the mechanism for coupling electron transport to oxidative phosphorylation; it requires a proton
Vocabulary ATP Synthase: the enzyme responsible for production of ATP in mitochondria Chemiosmotic Coupling: the mechanism for coupling electron transport to oxidative phosphorylation; it requires a proton
Amino Acid Metabolism
 Amino Acid Metabolism Last Week Most of the Animal Kingdom = Lazy - Most higher organisms in the animal kindom don t bother to make all of the amino acids. - Instead, we eat things that make the essential
Amino Acid Metabolism Last Week Most of the Animal Kingdom = Lazy - Most higher organisms in the animal kindom don t bother to make all of the amino acids. - Instead, we eat things that make the essential
ANSC/NUTR 618 LIPIDS & LIPID METABOLISM. Fatty Acid Elongation and Desaturation
 ANSC/NUTR 618 LIPIDS & LIPID METABOLISM I. Fatty acid elongation A. General 1. At least 60% of fatty acids in triacylglycerols are C18. 2. Free palmitic acid (16:0) synthesized in cytoplasm is elongated
ANSC/NUTR 618 LIPIDS & LIPID METABOLISM I. Fatty acid elongation A. General 1. At least 60% of fatty acids in triacylglycerols are C18. 2. Free palmitic acid (16:0) synthesized in cytoplasm is elongated
This amount has to be newly synthesized each day because:
 Hemoglubin synthesis The circulation blood of normal adult contain about 750 mg of hemoglobin and of this about 7 8 gm are degraded daily. This amount has to be newly synthesized each day because: 1) The
Hemoglubin synthesis The circulation blood of normal adult contain about 750 mg of hemoglobin and of this about 7 8 gm are degraded daily. This amount has to be newly synthesized each day because: 1) The
P450 CYCLE. All P450s follow the same catalytic cycle of;
 P450 CYCLE All P450s follow the same catalytic cycle of; 1. Initial substrate binding 2. First electron reduction 3. Oxygen binding 4. Second electron transfer 5 and 6. Proton transfer/dioxygen cleavage
P450 CYCLE All P450s follow the same catalytic cycle of; 1. Initial substrate binding 2. First electron reduction 3. Oxygen binding 4. Second electron transfer 5 and 6. Proton transfer/dioxygen cleavage
Porphyria in Japan : The Past, Present, and Future
 Porphyrins 009: 8(-3),-6 Mini Review Porphyria in Japan : The Past, Present, and Future Masao KONDO ) and Tetsuya KUBO,3) ) Faculty of Human Life Sciences, Musashi Institute of Technology, 8-9-8, Todoroki,
Porphyrins 009: 8(-3),-6 Mini Review Porphyria in Japan : The Past, Present, and Future Masao KONDO ) and Tetsuya KUBO,3) ) Faculty of Human Life Sciences, Musashi Institute of Technology, 8-9-8, Todoroki,
Rama Nada. -Ensherah Mokheemer. 1 P a g e
 - 3 - Rama Nada -Ensherah Mokheemer - 1 P a g e Don t forget to refer to page index wherever you see * Quick revision: In the previous lecture we said that: - your body contains 4-5g of iron (4g in females
- 3 - Rama Nada -Ensherah Mokheemer - 1 P a g e Don t forget to refer to page index wherever you see * Quick revision: In the previous lecture we said that: - your body contains 4-5g of iron (4g in females
Syllabus for BASIC METABOLIC PRINCIPLES
 Syllabus for BASIC METABOLIC PRINCIPLES The video lecture covers basic principles you will need to know for the lectures covering enzymes and metabolism in Principles of Metabolism and elsewhere in the
Syllabus for BASIC METABOLIC PRINCIPLES The video lecture covers basic principles you will need to know for the lectures covering enzymes and metabolism in Principles of Metabolism and elsewhere in the
PETER L. LUTZ. Red-eared slider Trachemys scripta elegans
 www.carleton.ca/~kbstorey PETER L. LUTZ Red-eared slider Trachemys scripta elegans Lutz PL, Storey KB. 1997. Handbook of Physiology (Dantzler WH, ed) Oxford Univ. Press, Vol. 2, pp. 1479-1522. 1 Relative
www.carleton.ca/~kbstorey PETER L. LUTZ Red-eared slider Trachemys scripta elegans Lutz PL, Storey KB. 1997. Handbook of Physiology (Dantzler WH, ed) Oxford Univ. Press, Vol. 2, pp. 1479-1522. 1 Relative
Eukaryotic Gene Regulation
 Eukaryotic Gene Regulation Chapter 19: Control of Eukaryotic Genome The BIG Questions How are genes turned on & off in eukaryotes? How do cells with the same genes differentiate to perform completely different,
Eukaryotic Gene Regulation Chapter 19: Control of Eukaryotic Genome The BIG Questions How are genes turned on & off in eukaryotes? How do cells with the same genes differentiate to perform completely different,
Glycolysis - Plasmodium
 Apicomplexan Biochemistry Basics Toxoplasma Good cell biology model Genome sequencing not completed Virtual pathways Cryptosporidium The strange one Genome sequence completed Virtual pathways Plasmodium
Apicomplexan Biochemistry Basics Toxoplasma Good cell biology model Genome sequencing not completed Virtual pathways Cryptosporidium The strange one Genome sequence completed Virtual pathways Plasmodium
RNA Processing in Eukaryotes *
 OpenStax-CNX module: m44532 1 RNA Processing in Eukaryotes * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section, you
OpenStax-CNX module: m44532 1 RNA Processing in Eukaryotes * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section, you
The hemoglobin (Hb) can bind a maximum of 220 ml O2 per liter.
 Hemoglobin Hemoglobin The most important function of the red blood cells is totransport (O2) from the lungs into the tissues, and carbon dioxide (CO2) from the tissues back into the lungs. O2 is poorly
Hemoglobin Hemoglobin The most important function of the red blood cells is totransport (O2) from the lungs into the tissues, and carbon dioxide (CO2) from the tissues back into the lungs. O2 is poorly
How Cells Harvest Energy. Chapter 7. Respiration
 How Cells Harvest Energy Chapter 7 Respiration Organisms classified on how they obtain energy: autotrophs: produce their own organic molecules through photosynthesis heterotrophs: live on organic compounds
How Cells Harvest Energy Chapter 7 Respiration Organisms classified on how they obtain energy: autotrophs: produce their own organic molecules through photosynthesis heterotrophs: live on organic compounds
The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein
 THESIS BOOK The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein Orsolya Buzás-Bereczki Supervisors: Dr. Éva Bálint Dr. Imre Miklós Boros University of
THESIS BOOK The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein Orsolya Buzás-Bereczki Supervisors: Dr. Éva Bálint Dr. Imre Miklós Boros University of
Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment
 Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment In multicellular eukaryotes, gene expression regulates development
Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment In multicellular eukaryotes, gene expression regulates development
Thioredoxin-interacting (TXNIP) protein regulates the differentiation of erythroid precursors
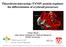 Thioredoxin-interacting (TXNIP) protein regulates the differentiation of erythroid precursors Volker Blank Lady Davis Institute for Medical Research McGill University www.scientificimages.co.uk INSTITUT
Thioredoxin-interacting (TXNIP) protein regulates the differentiation of erythroid precursors Volker Blank Lady Davis Institute for Medical Research McGill University www.scientificimages.co.uk INSTITUT
Biology 638 Biochemistry II Exam-2
 Biology 638 Biochemistry II Exam-2 Biol 638, Exam-2 (Code-1) 1. Assume that 16 glucose molecules enter into a liver cell and are attached to a liner glycogen one by one. Later, this glycogen is broken-down
Biology 638 Biochemistry II Exam-2 Biol 638, Exam-2 (Code-1) 1. Assume that 16 glucose molecules enter into a liver cell and are attached to a liner glycogen one by one. Later, this glycogen is broken-down
BCH 471. Experiment (5) Hemoglobin Estimation, Erythrocyte Sedimentation Rate (ESR) and Hematocrit (HCT)
 BCH 471 Experiment (5) Hemoglobin Estimation, Erythrocyte Sedimentation Rate (ESR) and Hematocrit (HCT) Objectives Quantitative determination of hemoglobin in a blood sample. Determination of erythrocyte
BCH 471 Experiment (5) Hemoglobin Estimation, Erythrocyte Sedimentation Rate (ESR) and Hematocrit (HCT) Objectives Quantitative determination of hemoglobin in a blood sample. Determination of erythrocyte
