Preparation and cellular uptake of PLGA particles loaded with lamivudine
|
|
|
- Agnes Harris
- 5 years ago
- Views:
Transcription
1 Article SPECIAL TOPIC: Multifunctional Nanocarriers for Biomedical Applications November 2012 Vol.57 No.31: doi: /s Preparation and cellular uptake of PLGA particles loaded with lamivudine WANG Bing, CHEN GuanQun, MAO ZhengWei *, ZHANG YuYing, YU DaHai & GAO ChangYou * Key Laboratory of Macromolecular Synthesis and Functionalization (Ministry of Education), Department of Polymer Science and Engineering, Zhejiang University, Hangzhou , China Received January 14, 2012; accepted May 30, 2012; published online September 1, 2012 Poly(D,L-lactide-co-glycolide) (PLGA) nanoparticles loaded with lamivudine and coated with bovine serum albumin (BSA) were prepared via a double emulsion method. The influences of experiments parameters such as volume of inner aqueous phase, concentration of organic phase and ultrasonication time on the particle size and drug entrapment efficiency were investigated, obtaining PLGA particles with a diameter of ~260 nm and drug entrapment efficiency of ~35%. The particles were observed by scanning electron microscopy and transmittance electron microscopy, showing a core-shell structure. BCA assay found that 58 mg BSA was present on/in 1 g LPB particles. The loaded lamivudine showed a burst release at beginning and sustained release until 24 h in physiological conditions. Low ph could accelerate the release of lamivudine from PLGA particles, making the PLGA particles potential intelligent intracellular drug carriers. The PLGA particles were readily internalized into the human liver cells within a short time and increased gradually with the prolongation of incubation time regardless of the loading of lamivudine. The particles either resided within lysosomes or transferred to cytoplasm, but could not enter into the cell nucleus. The cell viability was not significantly influenced in the presence of the particles regardless of lamivudine encapsulation, suggesting that this kind of particles may be a good candidate for the intracellular anti-hepatitis B drug delivery. PLGA, lamivudine, cell uptake, intracellular distribution, drug delivery Citation: Wang B, Chen G Q, Mao Z W, et al. Preparation and cellular uptake of PLGA particles loaded with lamivudine. Chin Sci Bull, 2012, 57: , doi: /s Hepatitis B caused by hepatitis B virus (HBV) is an infectious inflammatory illness of the liver, and is also considered as a causative factor of the development of hepatocellular carcinoma [1]. HBV infection is one of the most common infections over the world and especially severe in China. Of the million individuals worldwide infected with the HBV, one-third resides in China, and about 5% of them are chronically infected [2,3]. Every year, estimated people die from HBV-related diseases in China, including patients with hepatocellular carcinoma. Currently, medications for treatment of hepatitis B infection are mainly divided into antiviral drugs (nucleoside analogues) and immune system modulators (interferon). Although none of these drugs can clear the infection, they can *Corresponding authors ( zwmao@zju.edu.cn; cygao@mail.hz.zj.cn) stop the virus from replicating, thus minimizing liver damage. Among the antiviral drugs, lamivudine (LAM, Figure 1(a)), a cytosine nucleoside analogue, has shown good results in the treatment of HBV infection in human beings [4,5]. Once phosphorylated to active metabolites, lamivudine can inhibit the HBV reverse transcriptase enzyme competitively and act as a chain terminator of DNA synthesis. However, this drug exhibits very short physiological half-life, and therefore, requires a specially designed dosage everyday, which may cause high level resistance and other side effects [6]. Thus, protection in physiological environment and increase of residence time of lamivudine inside cells would greatly improve the treatment efficiency. To achieve this aim, lamivudine has been encapsulated into various colloidal carriers such as biodegradable polymer spheres [7] and micelles [8], and conjugated to polysaccharide The Author(s) This article is published with open access at Springerlink.com csb.scichina.com
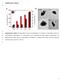
2 3986 Wang B, et al. Chin Sci Bull November (2012) Vol.57 No.31 Figure 1 (a) Chemical structure of lamivudine; (b) preparation process of LPB particles. and dendrimers [9,10]. Poly(lactide-co-glycolide) (PLGA) nanoparticles are widely used as carriers for drug, gene and vaccine delivery because of its good biocompatibility and biodegradability, low cytotoxicity and easy preparation [11,12]. The PLGA particles may protect the drug, gene and vaccine against degradation and ensure their transport and delivery [13,14], and also can control the release rate and achieve targeting [15 18]. Among the method for preparation of PLGA particles, the emulsification-solvent evaporation is the most frequently employed one [19]. The water/oil/water (W/O/W) double emulsion technique has been successfully developed and used to incorporate hydrophilic molecules such as hydrophilic drugs, proteins and nucleotide into the lumen of biodegradable particles [20 23]. In our previous study, PLGA particles are used to encapsulate hydrophobic dyes and anti-cancer drug by oil/water emulsion employing bovine serum albumin (BSA) as stabilizers, which are simultaneously loaded onto the particles during the formulation process. The surface immobilized macromolecules enable further covalent grafting of poly(ethylene glycol) (PEG) and folic acid (FA), or physical modification via layer-by-layer (LBL) assembly [24 26]. Herein we shall use W/O/W double emulsion to encapsulate water soluble lamivudine with BSA as macromolecular surfactant. The preparation parameters are optimized with respect to the particle size and drug encapsulation efficiency. The physiochemical properties of resultant particles (the so-called LAM- PLGA-BSA, LPB) are characterized in terms of chemical composition, morphology, surface charge property and drug release kinetics. Finally, cellular uptake kinetics and intracellular distribution of the LPB particles by human liver cells and their influences on the cell viability shall be explored. 1 Materials and methods 1.1 Reagents Poly(lactide-co-glycolide) (PLGA, LA:GA=75:25, M W = 130 kd), bovine serum albumin (BSA) and 4,6-diamidino- 2-phenylindole (DAPI) were obtained from Sigma-Aldrich. MitoTracker Green and LysoTracker Green were purchased from Invitrogen Co., Ltd. All other chemicals were analytical grade and used without further treatment. Milli-Q water was used throughout the experiments. 1.2 Particles preparation PLGA particles were prepared by means of a double emulsion-solvent evaporation method (Figure 1(b)). Briefly, ml lamivudine (Figure 1(a)) aqueous solution (10 mg/ml, internal aqueous phase) or pure water (blank PLGA/BSA particles) was emulsified in 1 ml PLGA dichloromethane solution (oil phase) using an ultrasonicator (MISONIX Ultrasonic liquid Processors) for desired time ( s). The power of ultrasonic was set as 6 W. Four different PLGA concentrations (2%, 4%, 6%, 8%; w/v) were employed in the oil phase. This primary W/O emulsion was rapidly added into 4 ml 3% (w/v) BSA aqueous solution (external aqueous phase) and emulsified again for the same time period. The resultant double emulsion was then poured into 50 ml Milli-Q water, stirred for 1 h at room temperature to allow evaporation of the organic solvent. The PLGA particles were collected by centrifugation at r/min for 15 min, washed with Milli-Q water for three times, and freezedried overnight under vacuum. PLGA particles containing Nile red or coumarin were similarly prepared by addition of 0.2 mg/ml Nile red or coumarin into PLGA solution before mixing with lamivudine solution. 1.3 Particles characterization (1) Morphology. The morphology of the PLGA particles was analyzed by scanning electron microscopy (SEM, HITACHI S-520) and transmission electron microscopy (TEM, Philips TECNAL-10). A drop of the PLGA particles suspension was added onto a clean glass and copper grid with carbon membrane for SEM and TEM observation,
3 Wang B, et al. Chin Sci Bull November (2012) Vol.57 No respectively. (2) Size and surface charge measurements. The size and surface charge of the particles were determined using Beckman Delsa TM Nano (Beckman Coulter). The data was averaged from three parallel experiments. (3) BSA content assay. To determine the BSA amount on the particles, a certain amount PLGA particles was dissolved in a solution of 5% (w/v) sodium dodecyl sulfate (SDS) in NaOH (ph 13). The system was sonicated at 40 C for 40 min and centrifuged at r/min for 15 min. The amount of BSA was measured from the supernatant by a BCA protein assay kit. (4) Drug loading determination. The drug content was determined by a high-pressure liquid chromatography (HPLC) method. Briefly, a certain amount of particles was dissolved in 1 mol/l NaOH solution, and the suspension was stirred at 40 C for 30 min until complete dissolution of the polymer spheres. The resultant transparent solution was neutralized with 1 mol/l HCl and diluted to a certain volume. Then the solution was filtered and injected into the HPLC equipment to determine drug content. Samples were passing through a C18 pre-column and a Jupiter C18 reverse-phase ( mm (I.D.)) with 5 mm packing particles (Phenomenex, Torrance, CA). The mobile phase was 0.02 mol/l phosphate buffer saline (ph 7.2), acetonitrile and methanol (91:0.1:8.9). The mobile phase was degassed and filtered before use. Flow rate was set at 1.5 ml/min and the UV-vis detector was set at 274 nm. The 20 L sample was injected and the measurement was repeated in triplicate and averaged. In this study, drug loading (DL) and entrapment efficiency (EE) are expressed as [27]: Amount of drug DL(%) 100, (1) Amount of (polymer drug) Drug loading amount EE(%) 100. (2) Drug feeding amount (5) Release of lamivudine from the LPB particles [28,29]. The release behavior of lamivudine encapsulated inside the LPB particles was studied at 37 C. Briefly, 50 mg LPB particles were suspended in 2 ml PBS, and sealed into a dialysis bag. The dialysis bag was then immersed into 48 ml PBS, and stirred for designed time intervals with a magnetic stirrer. At each time interval, 1 ml of PBS outside the dialysis bag was taken out from the beaker and the content of lamivudine was measured with a UV-vis spectrometer. The 1 ml of fresh PBS was added to the beaker to keep the constant volume. Each data was averaged from 3 parallel experiments. 1.4 In vitro cell culture experiments (1) Cell culture. HepLL cells are immortalized human liver cells [30] which kindly donated by Dr. Jun Li at First Affiliated Hospital, Zhejiang University, and cultured with regular growth medium consisting of high-glucose DMEM (Gibco) containing 100 g/ml streptomycin, 100 U/mL penicillin, and 10% fetal bovine serum, and incubated in a 5% CO 2 incubator at 37 C with 100% humidity. (2) Cellular uptake of PLGA particles. HepLL cells were seeded on a 24-well plate at a density of cells per well and allowed to attach for 16 h. To determine the particles uptake rate and amount as a function of incubation time, the cells were incubated with 100 g/ml of coumarin-labeled PLGA particles for different time. Then the cells were washed three times with PBS to remove free PLGA particles and detached by trypsin. Finally the uptake of coumarinlabeled particles was determined by flow cytometry (FACS Calibur, Becton Dickinson BD). (3) Intracellular distribution. Fluorescent staining of lysosomes, mitochondria, and cell nuclei was carried out and confocal laser scanning microscopy (CLSM, LSM 510, Carl Zeiss) was used to observe the intracellular distribution of PLGA particles. Briefly, after incubation with 100 g/ml of Nile red-labeled particles for desired time, the HepLL cells were carefully washed with PBS for three times, then incubated with LysoTracker Green and MitoTracker Green at 37 C for 30 min, respectively. Finally the cells were stained with DAPI and observed under CLSM. (4) Cell viability. The influences of PLGA particles on cell viability were assessed using 3-(4,5-dimethylthiazol-2- yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Briefly, 10 4 HepLL cells in 100 L medium were seeded into a well of a 96-well plate and incubated overnight. To determine the cell viability as a function of particles concentration and incubation time, the cells were incubated with various concentrations of particles for 24 h and incubated with 100 g/ml of particles for different time, respectively. After removal of the particles, the cells were cultured in fresh medium supplemented with 0.5 mg/ml MTT at 37 C for another 4 h. The blue crystals generated by the mitochondria dehydrogenase were dissolved with 150 L dimethyl sulfoxide (DMSO) and the absorbance at 570 nm was measured by a microplate reader (Model 550, Bio-Rad). The data was normalized to the particle free control group. 2 Results and discussion 2.1 Optimization of fabrication parameters The LPB particles were prepared by a W/O/W double emulsion-solvent evaporation method. The particle size and the drug loading and entrapment efficiency of LPB particles were influenced by parameters such as the volume of inner aqueous phase and organic phase, concentration of organic phase, ultrasonication time, and so on [31]. (1) Effect of volume of inner aqueous phase. Firstly, in-
4 3988 Wang B, et al. Chin Sci Bull November (2012) Vol.57 No.31 fluence of the volume of inner aqueous phase on the physiochemical properties of the as-prepared PLGA particles in terms of particle size, surface charge, drug loading and entrapment efficiency was investigated. Table 1 shows no significant change in the particle size along with increase of the volume of inner aqueous phase when it was below 0.3 ml. However, the particle size significantly increased with further increase of the volume, which should be resulted from the less stable W/O emulsion and thereby the coalescence of suspended droplets. Meanwhile, the drug loading and entrapment efficiency increased with the volume of the inner aqueous phase, reached 1.92% and 40.35% for the batch with 0.4 ml internal aqueous phase, respectively. The surface zeta potential was around 30 mv due to the adsorption of negatively charged BSA and not obviously influenced by the polymer concentration. Considering the balance between the particle size and the drug encapsulation efficiency, 0.3 ml inner aqueous phase was used for the following experiments. (2) Effect of the PLGA concentration of organic phase. The influence of PLGA concentration in organic phase on the particle size and drug loading and encapsulation efficiency was then evaluated. The results are summarized in Table 2. As the concentration of polymer increased from 2% to 8%, the particle diameter increased from 257 to 567 nm. It can be explained by enhancement of viscosity of the PLGA solution at higher concentration, leading to poorer dispersion. On the other hand, with the increase of the PLGA concentration in the oil phase, the drug encapsulation efficiency decreased from 34.9% to 24.4%, and the drug loading efficiency decreased as well from 1.66% to 1.16%. The surface charge property was not obviously influenced by the volume of inner aqueous phase. (3) Effect of ultrasonication time. During preparation of particles with the double emulsion method, the second emulsification critically influences the particle size. Hence, the intensity and time of emulsification need to be finely tuned. The particle size and drug loading efficiency as a function of ultrasonication time are summarized in Table 3. With the increase of the ultrasonication time, the particle size decreased remarkably in the first 40 s, i.e. from 380 to 280 nm, and then reached a plateau. Meanwhile, both the drug loading efficiency (from 0.68% at 10 s to 1.68% at 60 s) and drug entrapment efficiency (from 13% at 10 s to ~35% at 60 s) were sharply improved at longer ultrasonication time, and then leveled off at still longer time. Therefore, 60 s would be necessary to form well dispersed emulsion, preventing droplet coalescence during the second emulsion process and subsequent drug leakage into the outer aqueous phase. Surface zeta potential of the particles was almost constant in the experiments, suggesting that the surface charge property of the particles mainly depends on the selected macromolecular surfactant instead of the preparation parameters. Taking all the results into consideration, the optimal fabrication conditions are 0.3 ml of inner aqueous phase, 2% (w/v) PLGA in dichloromethane and double-60 s ultrasonication. 2.2 Characterization of LPB nanoparticles The morphology of the LPB particles was characterized by SEM and TEM (Figure 2). The LPB particles show a spherical morphology with relative narrow size distribution and Table 1 Influence of volume of inner aqueous phase on particle size, surface charge and drug loading (DL) and entrapment efficiency (EE) of the LPB particles a) Volume of inner phase (ml) Size (nm) Zeta potential (mv) DL (%) EE (%) ± ± ± ± ± ± ± ± ± ± ± ± ± ± ±5.9 a) All batches were emulsified using an ultrasonicator (6 W) for 60 s. The 1 ml PLGA dichloromethane solution (2% (w/v), oil phase) was employed. The volume of the BSA aqueous solution (3% (w/v), external aqueous phase) was 4 ml. Table 2 Influence of concentration of organic phase on particle size, surface charge and drug loading and entrapment efficiency of the LPB particles a) Concentration of the organic phase (%, w/v) Size (nm) Zeta potential (mv) DL (%) EE (%) 2 257± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ±4.4 a) All batches were emulsified using an ultrasonicator (6 W) for 60 s. The 0.3 ml lamivudine aqueous solution (10 mg/ml, internal aqueous phase) was employed. The volume of the BSA aqueous solution (3% (w/v), external aqueous phase) was 4 ml.
5 Wang B, et al. Chin Sci Bull November (2012) Vol.57 No Table 3 Influence of ultrasonication time on particle size, surface charge and drug loading and entrapment efficiency of the LPB particles a) Ultrasonication time (s) Size (nm) Zeta potential (mv) DL (%) EE (%) ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ±2.7 a) All batches were emulsified using an ultrasonicator (6 W) for desired time. The 0.3 ml 10 mg/ml lamivudine aqueous solution and 1 ml 2% (w/v) PLGA dichloromethane solution were employed as internal aqueous phase and oil phase, respectively. The volume of the BSA aqueous solution (3% (w/v), external aqueous phase) was 4 ml. Figure 2 SEM (a) and TEM (b) images of LPB particles. good dispersity (Figure 2(a)). As shown in Figure 2(b), a thick shell was found around the LPB particles, confirming the existence of BSA layers. According to the results of BCA assay, 58 mg BSA was present on/in 1 g LPB particles. The average diameter of the LPB particles was 257 nm in the wet state (as measured by DLS) and 212 nm in the dry state (as measured by TEM and image-analysis software). The difference of the measured size is attributed to the hydrophilic BSA molecules on the particle surface. Furthermore, the surface zeta potentials of the LPB particles in 10 mmol/l NaCl solution was 29.3±4.2 mv, which was improved to 10.9±2.4 mv in cell culture medium with 10% FCS due to adsorption of serum proteins. 2.3 Drug release in vitro The release behavior of lamivudine from the LPB particles was characterized in PBS with different ph values to mimic physiological and intra-lysosome environment at 37 C (Figure 3). At ph 7.4, an obvious burst release was observed during the first 2 h, and then the release was slowed down and reached to a plateau after 8 h, during which about 60% of the drug was released. The burst effect is caused by lamivudine molecules adsorbed or located near the particle Figure 3 Release profile of lamivudine from LPB particles at 37 C in different mediums. surface. At ph 5.5 (similar as in lysosomes), the release became faster, and more than 60% of the drug was released at first 4 h. The release became much slower afterwards and reached a plateau after 48 h, during which 90% of the encapsulated lamivudine was released. The faster release in acidic environment might be attributed to protonation of the amine group in lamivudine, leading to the change of charge balance and a larger repulsion between the molecules. Nev-
6 3990 Wang B, et al. Chin Sci Bull November (2012) Vol.57 No.31 ertheless, the LPB particles might protect the encapsulated drug in physiological condition and release quickly when they are internalized by cells, and particularly transported into lysosomes. 2.4 Cellular uptake To make the PLGA particles detectable via fluorescence microscopy and flow cytometry, hydrophobic dyes, coumarin and Nile red were pre-loaded during the particle fabrication, respectively. Due to their very poor solubility in aqueous medium, no detectable release from the particles was found after incubation in the cell culture medium at least for 24 h at 37 C (data not shown). Loading of the trace amount of coumarin/nile red did not bring significant influences on the particle size and morphology as well as surface charge, colloidal stability and protein adsorption property. Normalization of the fluorescence intensity of each type of the coumarin loaded particles was performed for the quantitative measurements by FCM. To investigate the uptake kinetics, the HepLL cells were incubated with 100 g/ml coumarin-labeled LPB particles and blank PLGA (PB) particles for different time intervals, respectively, and then quantified with FCM by determining the green fluorescence-emitting cells (Figure 4). For the FCM study, the logarithmic fluorescence intensity of particle-free control cells was set below 10. Therefore, the cells possessing fluorescence intensity larger than 10 were considered as positive ones [32,33]. As shown in Figure 4, the cellular uptake amount of both particles monotonously increased along with the prolongation of the culture time. For both types of the particles, the fluorescence intensity was already quite high after incubation for 4 h, indicating that internalization of both types of PLGA particles is very fast. During the next 20 h, the fluorescence intensity increased monotonously, and then leveled off, revealing that the uptake process was completed in 24 h. There is no significant difference in the uptake kinetics between the two types of particles, suggesting Figure 4 Uptake of LPB particles by HepLL cells as a function of culture time with a particle concentration of 100 µg/ml. Data was measured by flow cytometry and averaged to each cell. the encapsulation of lamivudine does not influence on the cellular uptake process. 2.5 Intracellular distribution During the process of cellular uptake, the exogenous particles are initially enclosed into endosomes, which mature into multivesicular bodies or late endosomes and eventually fuse with lysosomes [34]. Mitochondria are important intracellular energy metabolism places, and are closely related to the signals which induce cell apoptosis. The internalization process and the cellular distribution of the Nile redlabeled LPB particles were microscopically monitored by staining the lysosomal compartments with LysoTracker Green, the mitochondria with MitoTracker Green, and the cell nuclei with DAPI, respectively. Figure 5 shows that some LPB particles were internalized after 12 h incubation and further increased after 24 h. Only a few particles could be observed and colocalized with lysosomes after 12 h incubation. After 24 h incubation, more LPB particles were overlapped with lysosomes (yellow) while many LPB particles were located in the cytoplasm (red) rather than lysosomes. This result implies that many LPB particles can escape from the lysosomes. Although many particles were found around nucleus, no colocalization signals of the particles and cell nucleus could be found even after 24 h coincubation, suggesting that the particles could not penetrate into cell nucleus. As shown in Figure 6, very a few Nile red-labeled LPB particles overlapped with the mitochondria after 12 h incubation. When the incubation time was extended to 24 h, a few LPB particles were colocalized with the mitochondria, indicating the possible interaction of the particles with cell organelles such as mitochondria. 2.6 Cytotoxicity MTT assay was used to assess the viability of the HepLL cells after incubation with various concentrations of PB and LPB particles for 24 h and incubated with 100 g/ml of particles for different time, respectively. Figure 7(a) shows that in general the cell viability was largely maintained after incubation with both types of particles. The cell viability slightly decreased along with the increase of particles concentration, which decreased to 79.2% and 81.3% of particles free controls when the cells were exposed to 200 μg/ml PLGA-BSA (PB) and LPB particles, the highest concentration tested. As shown in Figure 7(b), both PB and LPB particles showed very low cytotoxicity to HepLL cells during the 48 h culture period. Thus, LPB particles are considered to be safe as potential drug carriers and encapsulation of lamivudine does not bring additional toxicity to normal cells. In next step, the cell model with hepatitis B virus infection would be created and the anti-virus effect of the LPB particles would be studied to elucidate the potential application
7 Wang B, et al. Chin Sci Bull November (2012) Vol.57 No Figure 5 CLSM images of HepLL cells incubated with 100 g/ml LPB particles for 12 h (a) or 24 h (b), respectively. The cell nuclei were stained by DAPI (a1, b1), the lysosomes were stained by LysoTracker (a2, b2) and the LPB particles were labeled by Nile red (a3, b3). (a4, b4) The merge images of nucleus (blue), lysosomes (green) and LPB particles (red). Scale bar: 10 m. Figure 6 CLSM images of HepLL cells incubated with 100 g/ml LPB particles for 12 h (a) or 24 h (b), respectively. The cell nuclei were stained by DAPI (a1, b1), the mitochondria were stained by MitoTracker (a2, b2) and the LPB particles were labeled by Nile red (a3, b3). (a4, b4) The merge images of nucleus (blue), mitochondria (green) and LPB particles (red). Scale bar: 10 m. of this kind of drug carriers. 3 Conclusion The lamivudine-loaded PLGA particles were prepared by a W/O/W double emulsion-solvent evaporation method with BSA as macromolecular surfactant. By varying the experimental conditions such as volume of inner aqueous phase, concentration of organic phase and sonication parameters, the particle size and drug encapsulation efficiency were optimized and finally the LPB particles with ~260 nm in diameter, ~35% drug loading efficiency and negative surface charge were obtained. The lamivudine inside LPB particles showed a burst release at beginning and sustained release until 24 h in physiological conditions. The release of lamivudine from LPB particles was accelerated in acidic medium, making the LPB particles potential intelligent intracellular drug carriers. The LPB particles were readily internalized into human liver cells within a short time and
8 3992 Wang B, et al. Chin Sci Bull November (2012) Vol.57 No.31 Figure 7 Relative cell viability after incubation of HepLL cells with the PB or LPB particles as a function of (a) concentration with a culture time of 24 h, and (b) culture time with a particle concentration of 100 g/ml. increased gradually with the prolongation of incubation. After ingested into the cells, the LPB particles would either reside within the lysosomes or transfer to cytoplasm, but could not enter into the cell nucleus. The cell viability was not significantly influenced by the ingestion of the particles regardless of lamivudine encapsulation. This work was supported by the National Natural Science Foundation of China ( ), Department of Education of Zhejiang Province (Z ), and the National Basic Research Program of China (2011CB606203). 1 Hildt E, Hofschneider P H, Urban S. The role of hepatitis B virus (HBV) in the development of hepatocellular carcinoma. Semin Virol, 1996, 7: Lai C L, Ratziu V, Yuen M F, et al. Viral hepatitis B. Lancet, 2003, 362: Ye L, Zhang Y, Mei Y, et al. Detecting putative recombination events of hepatitis B virus: An updated comparative genome analysis. Chin Sci Bull, 2010, 55: Doong S L, Tsai C H, Schinazi R F. Inhibition of the replication of hepatitis B virus in vitro by 2,3 -dideoxy-3 -thiacytidine and related analogues. Proc Natl Acad Sci USA, 1991, 88: Bartholomew M M, Jansen R W, Jeffers L J. Hepatitis-B-virus resistance to lamivudine given for recurrent infection after orthotopic liver transplantation. Lancet, 1997, 349: Fox Z, Dragsted U B, Gerstoft J. A randomized trial to evaluate continuation versus discontinuation of lamivudine in individuals failing a lamivudine-containing regimen: The COLATE trial. Antivir Ther, 2006, 11: Kuo Y C, Chen H H. Effect of nanoparticulate polybutylcyanoacrylate and methylmethacrylate sulfopropylmethacrylate on the permeability of zidovudine and lamivudine across the in vitro blood-brain barrier. Int J Pharm, 2006, 327: Li Q, Du Y Z, Yuan H. Synthesis of lamivudine stearate and antiviral activity of stearic acid-g-chitosan oligosaccharide polymeric micelles delivery system. Eur J Pharm Sci, 2010, 41: Chimalakonda K C, Agarwal H K, Kumar A. Synthesis, analysis, in vitro characterization, and in vivo disposition of a lamivudine-dextran conjugate for selective antiviral delivery to the liver. Bioconj Chem, 2007, 18: Dutta T, Jain N K. Targeting potential and anti-hiv activity of lamivudine loaded mannosylated poly(propyleneimine) dendrimer. BBA-General Subjects, 2007, 1770: Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliver Rev, 2003, 55: Zhang Y, Hu L, Gao C. Effect of cellular uptake of SiO 2 particles on adhesion and migration of HepG2 cells. Acta Polym Sin, 2009, Brannon-Peppas L, Blanchette J O. Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliver Rev, 2004, 56: Hu L, Zhang Y, Gao C. Influence of structures and properties of polymer nanoparticles on their cellular uptake and cell functions. Prog Chem, 2009, 21: Yang H, Miyoshi H, Lou C, et al. Preparation, characterization and release of methyl viologen from a novel nanoparticle delivery system with double shells of silica and PLGA. Chin Sci Bull, 2010, 55: Faisant N, Akiki J, Siepmann F. Effect of the type of release medium on drug release from PLGA-based microparticles: Experiment and theory. Int J Pharm, 2006, 314: Siepmann J, Faisant N, Akiki J. Effect of the size of biodegradable microparticles on drug release: Experiment and theory. J Control Release, 2004, 96: Hu L, Mao Z, Gao C. Colloidal particles for cellular uptake and delivery. J Mater Chem, 2009, 19: Mundargi R C, Babu V R, Vidhya R. Nano/micro technologies for delivering macromolecular therapeutics using poly(d,l-lactide-coglycolide) and its derivatives. J Control Release, 2008, 125: Cu Y, LeMoellic C, Caplan M J. Ligand-modified gene carriers increased uptake in target cells but reduced DNA release and transfection efficiency. Nanomed: Nanotechnol Biol Med, 2009, 6: Zhou S, Sun J, Sun L. Preparation and characterization of interferonloaded magnetic biodegradable microspheres. J Biomed Mater Res B, 2008, 87B: Zambaux M F. Preparation and characterization of protein C-loaded PLA nanoparticles. J Control Release, 1999, 60: Gorner T, Gref R, Michenot D. Lidocaine-loaded biodegradable nanospheres. I. Optimization of the drug incorporation into the polymer matrix. J Control Release, 1999, 57: Zhou J, Moya S, Gao C. Folic acid modified poly(lactide-co-glycolide) nanoparticles, layer-by-layer surface engineered for targeted delivery. Macromol Chem Phys, 2010, 211: Zhou J, Moya S, Gao C. Layer by layer chitosan/alginate coatings on poly(lactide-co-glycolide) nanoparticles for antifouling protection and Folic acid binding to achieve selective cell targeting. J Colloid Interf Sci, 2010, 345: Zhou J, Moya S, Gao C. Polyelectrolyte coated PLGA nanoparticles:templation and release behavior. Macromol Biosci, 2009, 9: Bilati U, Mann E A, Doelker E. Poly(D,L-lactide-co-glycolide) proteinloaded nanoparticlesprepared by the double emulsion method Processing and formulation issues for enhanced entrapment efficiency. J Microencapsul, 2005, 22: Xie L, Tong W, Xu J, et al. Multilayers and poly(allylamine hydro-
9 Wang B, et al. Chin Sci Bull November (2012) Vol.57 No chloride)-graft-poly(ethylene glycol) modified bovine serum albumin nanoparticles: Improved stability and ph-responsive drug delivery. Chin J Polym Sci, 2012, 30: Feng Z, Gao C, Shen J. Fabrication and characterization of gold nanoparticles loaded microcapsules. Acta Polym Sin, 2008, 8: Li J, Li L, Cao H. Establishment of highly differentiated immortalized human hepatocyte line with simian virus 40 large tumor antigen for liver based cell therapy. ASAIO J, 2005, 51: Bilati U, Allemann E, Doelke E. Sonication parameters for the preparation of biodegradable nanocapsules of controlled size by the double emulsion method. Pharm Dev Technol, 2003, 8: Chung T H, Wu S, Yao M. The effect of surface charge on the uptake and biological function of mesoporous silica nanoparticles in 3T3-L1 cells and human mesenchymal stem cells. Biomaterials, 2007, 28: Zhang Y, Hu L, Yu D, et al. Influence of silica particle internalization on adhesion and migration of human dermal fibroblasts. Biomaterials, 2010, 31: Hillaireau H, Couvreur P. Nanocarriers entry into the cell: Relevance to drug delivery. Cell Mol Life Sci, 2009, 66: Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Dual-Responsive Polymer Micelles for. Target-Cell-Specific Anticancer Drug Delivery
 Dual-Responsive Polymer Micelles for Target-Cell-Specific Anticancer Drug Delivery Xing Guo, Chunli Shi, Guang Yang, Jie Wang, Zhenghong Cai, and Shaobing Zhou,, * Key Laboratory of Advanced Technologies
Dual-Responsive Polymer Micelles for Target-Cell-Specific Anticancer Drug Delivery Xing Guo, Chunli Shi, Guang Yang, Jie Wang, Zhenghong Cai, and Shaobing Zhou,, * Key Laboratory of Advanced Technologies
Supporting Information. Maximizing the Supported Bilayer Phenomenon: LCP Liposomes Comprised Exclusively
 S-1 Supporting Information Maximizing the Supported Bilayer Phenomenon: LCP Liposomes Comprised Exclusively of PEGylated Phospholipids for Enhanced Systemic and Lymphatic Delivery Matthew T. Haynes and
S-1 Supporting Information Maximizing the Supported Bilayer Phenomenon: LCP Liposomes Comprised Exclusively of PEGylated Phospholipids for Enhanced Systemic and Lymphatic Delivery Matthew T. Haynes and
Dense and Dynamic Polyethylene Glycol Shells Cloak Nanoparticles. from Uptake by Liver Endothelial Cells for Long Blood Circulation
 Dense and Dynamic Polyethylene Glycol Shells Cloak Nanoparticles from Uptake by Liver Endothelial Cells for Long Blood Circulation Hao Zhou, Zhiyuan Fan, Peter Y. Li, Junjie Deng,, Dimitrios C. Arhontoulis,
Dense and Dynamic Polyethylene Glycol Shells Cloak Nanoparticles from Uptake by Liver Endothelial Cells for Long Blood Circulation Hao Zhou, Zhiyuan Fan, Peter Y. Li, Junjie Deng,, Dimitrios C. Arhontoulis,
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION DOI: 10.1038/NNANO.2012.80 Protein-Inorganic Hybrid Nanoflowers Jun Ge, Jiandu Lei, and Richard N. Zare Supporting Online Material Materials Proteins including albumin from bovine
SUPPLEMENTARY INFORMATION DOI: 10.1038/NNANO.2012.80 Protein-Inorganic Hybrid Nanoflowers Jun Ge, Jiandu Lei, and Richard N. Zare Supporting Online Material Materials Proteins including albumin from bovine
Characterization and Modification of Low Molecular Water-Soluble Chitosan for Pharmaceutical Application
 Characterization and Modification of Low Molecular Water-Soluble Chitosan Bull. Korean Chem. Soc. 2003, Vol. 24, No. 9 1303 Characterization and Modification of Low Molecular Water-Soluble Chitosan for
Characterization and Modification of Low Molecular Water-Soluble Chitosan Bull. Korean Chem. Soc. 2003, Vol. 24, No. 9 1303 Characterization and Modification of Low Molecular Water-Soluble Chitosan for
Supplementary Information for
 Supplementary Information for Acid-Resistant Mesoporous Metal Organic Framework Toward Oral Insulin Delivery: Protein Encapsulation, Protection & Release Yijing Chen, Peng Li, Justin A. Modica, Riki J.
Supplementary Information for Acid-Resistant Mesoporous Metal Organic Framework Toward Oral Insulin Delivery: Protein Encapsulation, Protection & Release Yijing Chen, Peng Li, Justin A. Modica, Riki J.
EFFECT OF COMPRESSED CO 2 ON THE PROPERTIES OF AOT IN ISOOCTANE REVERSE MICELLAR SOLUTION AND ITS APPLICATION TO RECOVER NANOPARTICLES
 EFFECT OF COMPRESSED CO 2 ON THE PROPERTIES OF AOT IN ISOOCTANE REVERSE MICELLAR SOLUTION AND ITS APPLICATION TO RECOVER NANOPARTICLES Dongxia Liu, Jianling Zhang, Tiancheng Mu, Jing Chen, Weize Wu, Jun
EFFECT OF COMPRESSED CO 2 ON THE PROPERTIES OF AOT IN ISOOCTANE REVERSE MICELLAR SOLUTION AND ITS APPLICATION TO RECOVER NANOPARTICLES Dongxia Liu, Jianling Zhang, Tiancheng Mu, Jing Chen, Weize Wu, Jun
Enhanced delivery methods for greater efficacy
 On-Line Formulation Training - Anywhere In The World - Enhanced delivery methods for greater efficacy Belinda Carli Director, Institute of Personal Care Science Image showing absorbance in the outer stratum
On-Line Formulation Training - Anywhere In The World - Enhanced delivery methods for greater efficacy Belinda Carli Director, Institute of Personal Care Science Image showing absorbance in the outer stratum
Study on Targeting and in vitro Anti-oxidation of Baicalin Solid Lipid Nanoparticles
 Ping Y et al. Chinese Herbal Medicines, 212, 4(4): 335-339 335 Study on Targeting and in vitro Anti-oxidation of Baicalin Solid Lipid Nanoparticles PING Yang 1, YU Lian 1, HU Yan-qiu 1, MA Li-na 1, CAO
Ping Y et al. Chinese Herbal Medicines, 212, 4(4): 335-339 335 Study on Targeting and in vitro Anti-oxidation of Baicalin Solid Lipid Nanoparticles PING Yang 1, YU Lian 1, HU Yan-qiu 1, MA Li-na 1, CAO
Protein-Mediated Anti-Adhesion Surface against Oral Bacteria
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2018 Supporting Information Protein-Mediated Anti-Adhesion Surface against Oral Bacteria Xi Liu a,b,
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2018 Supporting Information Protein-Mediated Anti-Adhesion Surface against Oral Bacteria Xi Liu a,b,
General and Facile Surface Functionalization of Hydrophobic Nanocrystals with Poly(amino acid) for Cell Luminescence Imaging
 General and Facile Surface Functionalization of Hydrophobic Nanocrystals with Poly(amino acid) for Cell Luminescence Imaging Sheng Huang, Min Bai, Leyu Wang* State Key Laboratory of Chemical Resource Engineering,
General and Facile Surface Functionalization of Hydrophobic Nanocrystals with Poly(amino acid) for Cell Luminescence Imaging Sheng Huang, Min Bai, Leyu Wang* State Key Laboratory of Chemical Resource Engineering,
Supporting Information
 Supporting Information ph- and Amylase-Responsive Carboxymethyl Starch/Poly(2-Isobutyl-acrylic acid) Hybrid Microgels as Effective Enteric Carriers for Oral Insulin Delivery Liang Liu, a,b Ying Zhang,
Supporting Information ph- and Amylase-Responsive Carboxymethyl Starch/Poly(2-Isobutyl-acrylic acid) Hybrid Microgels as Effective Enteric Carriers for Oral Insulin Delivery Liang Liu, a,b Ying Zhang,
Thiol-Activated gem-dithiols: A New Class of Controllable. Hydrogen Sulfide (H 2 S) Donors
 Thiol-Activated gem-dithiols: A New Class of Controllable Hydrogen Sulfide (H 2 S) Donors Yu Zhao, Jianming Kang, Chung-Min Park, Powell E. Bagdon, Bo Peng, and Ming Xian * Department of Chemistry, Washington
Thiol-Activated gem-dithiols: A New Class of Controllable Hydrogen Sulfide (H 2 S) Donors Yu Zhao, Jianming Kang, Chung-Min Park, Powell E. Bagdon, Bo Peng, and Ming Xian * Department of Chemistry, Washington
Paclitaxel-loaded biodegradable nanoparticles developed by direct dialysis and electrodydrodynamic atomization methods
 Paclitaxel-loaded biodegradable nanoparticles developed by direct dialysis and electrodydrodynamic atomization methods Jingwei Xie 1, Chi-Hwa Wang 1,2 1 Department of Chemical and Biomolecular Engineering,
Paclitaxel-loaded biodegradable nanoparticles developed by direct dialysis and electrodydrodynamic atomization methods Jingwei Xie 1, Chi-Hwa Wang 1,2 1 Department of Chemical and Biomolecular Engineering,
Supporting information for the manuscript
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2014 Supporting information for the manuscript Toward enhanced photoactivity
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2014 Supporting information for the manuscript Toward enhanced photoactivity
Supplementary Data. Different volumes of ethanol or calcium solution were slowly added through one of four
 Supplementary Data METHODS Liposome preparation Different volumes of ethanol or calcium solution were slowly added through one of four methods: Method I, no ethanol or calcium solution; Method II, exactly
Supplementary Data METHODS Liposome preparation Different volumes of ethanol or calcium solution were slowly added through one of four methods: Method I, no ethanol or calcium solution; Method II, exactly
Supporting Information
 Supporting Information Cancer Cell Membrane-Biomimetic Nanoprobes with Two-Photon Excitation and Near-Infrared Emission for Intravital Tumor Fluorescence Imaging Yanlin Lv 1,2,, Ming Liu 3,4,, Yong Zhang
Supporting Information Cancer Cell Membrane-Biomimetic Nanoprobes with Two-Photon Excitation and Near-Infrared Emission for Intravital Tumor Fluorescence Imaging Yanlin Lv 1,2,, Ming Liu 3,4,, Yong Zhang
The effect of insulin on chemotherapeutic drug sensitivity in human esophageal and lung cancer cells
 The effect of insulin on chemotherapeutic drug sensitivity in human esophageal and lung cancer cells Published in: Natl Med J China, February 10, 2003; Vol 83, No 3, Page 195-197. Authors: JIAO Shun-Chang,
The effect of insulin on chemotherapeutic drug sensitivity in human esophageal and lung cancer cells Published in: Natl Med J China, February 10, 2003; Vol 83, No 3, Page 195-197. Authors: JIAO Shun-Chang,
Supporting Information Nitric oxide releasing photoresponsive nanohybrids as excellent therapeutic agent for cervical cancer cell lines
 upporting Information itric oxide releasing photoresponsive nanohybrids as excellent therapeutic agent for cervical cancer cell lines Priya udhesh a, Kaviyarasan Tamilarasan a, Palaniappan Arumugam a and
upporting Information itric oxide releasing photoresponsive nanohybrids as excellent therapeutic agent for cervical cancer cell lines Priya udhesh a, Kaviyarasan Tamilarasan a, Palaniappan Arumugam a and
A study on the effects of different surfactants on Ethylcellulose microspheres
 International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 0974-4304 Vol.1, No.4, pp 966-971, Oct-Dec 2009 A study on the effects of different surfactants on Ethylcellulose microspheres Lalduhsanga
International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 0974-4304 Vol.1, No.4, pp 966-971, Oct-Dec 2009 A study on the effects of different surfactants on Ethylcellulose microspheres Lalduhsanga
8. CHAPTER IV. ANTICANCER ACTIVITY OF BIOSYNTHESIZED SILVER NANOPARTICLES
 8. CHAPTER IV. ANTICANCER ACTIVITY OF BIOSYNTHESIZED SILVER NANOPARTICLES 8.1. Introduction Nanobiotechnology, an emerging field of nanoscience, utilizes nanobased-systems for various biomedical applications.
8. CHAPTER IV. ANTICANCER ACTIVITY OF BIOSYNTHESIZED SILVER NANOPARTICLES 8.1. Introduction Nanobiotechnology, an emerging field of nanoscience, utilizes nanobased-systems for various biomedical applications.
Self-assembled ZnO nanoparticle capsules for carrying and delivering isotretinoin to cancer cells
 Supporting Information Self-assembled ZnO nanoparticle capsules for carrying and delivering isotretinoin to cancer cells Wei Zhao, Ji-Shi Wei, Peng Zhang, Jie Chen, Ji-Lie Kong,,, * Lian-Hua Sun, Huan-Ming
Supporting Information Self-assembled ZnO nanoparticle capsules for carrying and delivering isotretinoin to cancer cells Wei Zhao, Ji-Shi Wei, Peng Zhang, Jie Chen, Ji-Lie Kong,,, * Lian-Hua Sun, Huan-Ming
Supporting Information. Silver Nanoparticle-Gated Mesoporous Silica-Coated Gold Nanorods. Low Premature Release and Multifunctional
 Supporting Information Silver Nanoparticle-Gated Mesoporous Silica-Coated Gold Nanorods (AuNR@MS@AgNPs): Low Premature Release and Multifunctional Cancer Theranostic Platform Zhehua Zhang, a, Changhui
Supporting Information Silver Nanoparticle-Gated Mesoporous Silica-Coated Gold Nanorods (AuNR@MS@AgNPs): Low Premature Release and Multifunctional Cancer Theranostic Platform Zhehua Zhang, a, Changhui
Plasmonic blood glucose monitor based on enzymatic. etching of gold nanorods
 Plasmonic blood glucose monitor based on enzymatic etching of gold nanorods Xin Liu, Shuya Zhang, Penglong Tan, Jiang Zhou, Yan Huang, Zhou Nie* and Shouzhuo Yao State Key Laboratory of Chemo/Biosensing
Plasmonic blood glucose monitor based on enzymatic etching of gold nanorods Xin Liu, Shuya Zhang, Penglong Tan, Jiang Zhou, Yan Huang, Zhou Nie* and Shouzhuo Yao State Key Laboratory of Chemo/Biosensing
Formulation and Evaluation of Solid lipid nanoparticles: Isoniazid
 129 Research Article Formulation and Evaluation of Solid lipid nanoparticles: Isoniazid Umatiya Imran*, Chintan Aundhia, A.K.Seth, Sachin Chauhan, Nirmal Shah Department of pharmacy, Sumandeep Vidhyapeeth
129 Research Article Formulation and Evaluation of Solid lipid nanoparticles: Isoniazid Umatiya Imran*, Chintan Aundhia, A.K.Seth, Sachin Chauhan, Nirmal Shah Department of pharmacy, Sumandeep Vidhyapeeth
IJEScA. Protein-Benign Microencapsulation with a Water/Oil Microemulsion formed by a Biodegradable Polymer Surfactant
 Protein-Benign Microencapsulation with a Water/il Microemulsion formed by a Biodegradable Polymer Surfactant S. Nishino 1, H. Yoshizawa & K. Shiomori 2 1 Department of Chemical Engineering, Nara National
Protein-Benign Microencapsulation with a Water/il Microemulsion formed by a Biodegradable Polymer Surfactant S. Nishino 1, H. Yoshizawa & K. Shiomori 2 1 Department of Chemical Engineering, Nara National
Supplementary data. High-Performance Ultrafiltration Membranes Based on Polyethersulfone/Graphene Oxide Composites
 Supplementary data High-Performance Ultrafiltration Membranes Based on Polyethersulfone/Graphene Oxide Composites Fengmin Jin a, Wei Lv b,a, Chen Zhang a, Zhengjie Li a, Rongxin Su a, Wei Qi a, Quan-Hong
Supplementary data High-Performance Ultrafiltration Membranes Based on Polyethersulfone/Graphene Oxide Composites Fengmin Jin a, Wei Lv b,a, Chen Zhang a, Zhengjie Li a, Rongxin Su a, Wei Qi a, Quan-Hong
Engineering the Growth of TiO 2 Nanotube Arrays on Flexible Carbon Fibre Sheets
 Engineering the Growth of TiO 2 Nanotube Arrays on Flexible Carbon Fibre Sheets Peng Chen, a Li Gu, b Xiudong Xue, a Mingjuan Li a and Xuebo Cao* a a Key Lab of Organic Synthesis of Jiangsu Province and
Engineering the Growth of TiO 2 Nanotube Arrays on Flexible Carbon Fibre Sheets Peng Chen, a Li Gu, b Xiudong Xue, a Mingjuan Li a and Xuebo Cao* a a Key Lab of Organic Synthesis of Jiangsu Province and
5 Application of the ESR online-method for the monitoring of nanocapsule digestion
 5 Application of the ESR online-method for the monitoring of nanocapsule digestion 5.1 Introduction The oral use of nanocapsules has received considerable attention in recent years because the bioavailability
5 Application of the ESR online-method for the monitoring of nanocapsule digestion 5.1 Introduction The oral use of nanocapsules has received considerable attention in recent years because the bioavailability
Measuring Lipid Composition LC-MS/MS
 Project: Measuring Lipid Composition LC-MS/MS Verification of expected lipid composition in nanomedical controlled release systems by liquid chromatography tandem mass spectrometry AUTHORED BY: DATE: Sven
Project: Measuring Lipid Composition LC-MS/MS Verification of expected lipid composition in nanomedical controlled release systems by liquid chromatography tandem mass spectrometry AUTHORED BY: DATE: Sven
Design and Development of Surface Modification and Synthesis Strategies to Reduce Toxicity of Nanoparticles
 Design and Development of Surface Modification and Synthesis Strategies to Reduce Toxicity of Nanoparticles Seda Keleştemur Genetics and Bioengineering Department Yeditepe University Istanbul, Turkey Outline
Design and Development of Surface Modification and Synthesis Strategies to Reduce Toxicity of Nanoparticles Seda Keleştemur Genetics and Bioengineering Department Yeditepe University Istanbul, Turkey Outline
Fluorescent Carbon Dots as Off-On Nanosensor for Ascorbic Acid
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Fluorescent Carbon Dots as Off-On Nanosensor for Ascorbic Acid Jun Gong, Xin Lu, Xueqin An*
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Fluorescent Carbon Dots as Off-On Nanosensor for Ascorbic Acid Jun Gong, Xin Lu, Xueqin An*
Title Revision n date
 A. THIN LAYER CHROMATOGRAPHIC TECHNIQUE (TLC) 1. SCOPE The method describes the identification of hydrocortisone acetate, dexamethasone, betamethasone, betamethasone 17-valerate and triamcinolone acetonide
A. THIN LAYER CHROMATOGRAPHIC TECHNIQUE (TLC) 1. SCOPE The method describes the identification of hydrocortisone acetate, dexamethasone, betamethasone, betamethasone 17-valerate and triamcinolone acetonide
Electronic Supplementary Information (ESI)
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information (ESI) One-Step Encapsulation and Triggered Release Based on
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information (ESI) One-Step Encapsulation and Triggered Release Based on
International Conference on Biomedical and Biological Engineering (BBE 2016)
 International Conference on Biomedical and Biological Engineering (BBE 2016) Injury Mechanism of Sub-Micron Calcium Oxalate Monohydrate and Dihydrate Crystals on Renal Epithelial Cells Poonam BHADJA, Kai
International Conference on Biomedical and Biological Engineering (BBE 2016) Injury Mechanism of Sub-Micron Calcium Oxalate Monohydrate and Dihydrate Crystals on Renal Epithelial Cells Poonam BHADJA, Kai
THE INFLUENCE OF OILS AND SURFACTANTS ON THE FORMATION OF SELF-NANOEMULSIFYING DRUG DELIVERY SYSTEMS (SNEDDS) CONTAINING THERAPEUTIC PROTEIN
 MATERIALS SCIENCE and TECHNOLOGY Edited by Evvy Kartini et.al. THE INFLUENCE OF OILS AND SURFACTANTS ON THE FORMATION OF SELF-NANOEMULSIFYING DRUG DELIVERY SYSTEMS (SNEDDS) CONTAINING THERAPEUTIC PROTEIN
MATERIALS SCIENCE and TECHNOLOGY Edited by Evvy Kartini et.al. THE INFLUENCE OF OILS AND SURFACTANTS ON THE FORMATION OF SELF-NANOEMULSIFYING DRUG DELIVERY SYSTEMS (SNEDDS) CONTAINING THERAPEUTIC PROTEIN
Supporting Information
 Supporting Information Polymer Micelles Stabilization-n-Demand through Reversible Photocrosslinking 1. Synthesis and Characterization of Diblock Copolymers Materials. Cu(I)Br, 2-Bromo-2-methylpropionyl
Supporting Information Polymer Micelles Stabilization-n-Demand through Reversible Photocrosslinking 1. Synthesis and Characterization of Diblock Copolymers Materials. Cu(I)Br, 2-Bromo-2-methylpropionyl
ENHANCEMENT OF SOLUBILITY OF BICALUTAMIDE DRUG USING SOLID DISPERSION TECHNIQUE
 PHARMA SCIENCE MONITOR AN INTERNATIONAL JOURNAL OF PHARMACEUTICAL SCIENCES ENHANCEMENT OF SOLUBILITY OF BICALUTAMIDE DRUG USING SOLID DISPERSION TECHNIQUE Kantilal B. Narkhede *1, R. B. Laware 2, Y. P.
PHARMA SCIENCE MONITOR AN INTERNATIONAL JOURNAL OF PHARMACEUTICAL SCIENCES ENHANCEMENT OF SOLUBILITY OF BICALUTAMIDE DRUG USING SOLID DISPERSION TECHNIQUE Kantilal B. Narkhede *1, R. B. Laware 2, Y. P.
CHARACTERIZATION OF NANO CARRIERS FOR DRUG DELIVERY SYSTEMS: THE LIPIDOTS NanoSafe 2016 Amandine Arnould 07-11/11/2016
 CHARACTERIZATION OF NANO CARRIERS FOR DRUG DELIVERY SYSTEMS: THE LIPIDOTS NanoSafe 2016 Amandine Arnould 07-11/11/2016 CONTEXT : NANOPARTICLES IN SENSITIVE MEDIA Nano-safety issue Environment Medicine
CHARACTERIZATION OF NANO CARRIERS FOR DRUG DELIVERY SYSTEMS: THE LIPIDOTS NanoSafe 2016 Amandine Arnould 07-11/11/2016 CONTEXT : NANOPARTICLES IN SENSITIVE MEDIA Nano-safety issue Environment Medicine
PREPARATION OF POLYSTYRENE HOLLOW MICROSPHERES FROM WASTE FOAMED POLYSTYRENE PLASTICS BY MICROENCAPSULATION METHOD
 PREPARATION OF POLYSTYRENE HOLLOW MICROSPHERES FROM WASTE FOAMED POLYSTYRENE PLASTICS BY MICROENCAPSULATION METHOD Wei Bin* 1, Wang Shujun 1, Liu Hongyan 2, Liu Ning 1 1 State Key Laboratory of Heavy Oil
PREPARATION OF POLYSTYRENE HOLLOW MICROSPHERES FROM WASTE FOAMED POLYSTYRENE PLASTICS BY MICROENCAPSULATION METHOD Wei Bin* 1, Wang Shujun 1, Liu Hongyan 2, Liu Ning 1 1 State Key Laboratory of Heavy Oil
Coordination-responsive Selenium-containing Polymer Micelles for. Supporting information
 Electronic Supplementary Material (ESI) for Chemical Science Coordination-responsive Selenium-containing Polymer Micelles for Controlled Drug Release Wei Cao, a Yang Li, b Yu Yi, a Shaobo Ji, a Lingwu
Electronic Supplementary Material (ESI) for Chemical Science Coordination-responsive Selenium-containing Polymer Micelles for Controlled Drug Release Wei Cao, a Yang Li, b Yu Yi, a Shaobo Ji, a Lingwu
Biodegradable Zwitterionic Nanogels with Long. Circulation for Antitumor Drug Delivery
 Supporting Information Biodegradable Zwitterionic Nanogels with Long Circulation for Antitumor Drug Delivery Yongzhi Men, Shaojun Peng, Peng Yang, Qin Jiang, Yanhui Zhang, Bin Shen, Pin Dong, *, Zhiqing
Supporting Information Biodegradable Zwitterionic Nanogels with Long Circulation for Antitumor Drug Delivery Yongzhi Men, Shaojun Peng, Peng Yang, Qin Jiang, Yanhui Zhang, Bin Shen, Pin Dong, *, Zhiqing
Supporting Information
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Supporting Information Enzyme-activatable Probe with a Self-immolative Linker for Rapid and Sensitive
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Supporting Information Enzyme-activatable Probe with a Self-immolative Linker for Rapid and Sensitive
Proteomic profiling of small-molecule inhibitors reveals dispensability of MTH1 for cancer cell survival
 Supplementary Information for Proteomic profiling of small-molecule inhibitors reveals dispensability of MTH1 for cancer cell survival Tatsuro Kawamura 1, Makoto Kawatani 1, Makoto Muroi, Yasumitsu Kondoh,
Supplementary Information for Proteomic profiling of small-molecule inhibitors reveals dispensability of MTH1 for cancer cell survival Tatsuro Kawamura 1, Makoto Kawatani 1, Makoto Muroi, Yasumitsu Kondoh,
Liquid-Liquid Extraction Prof. Mukesh Doble Department Of Biotechnology Indian Institute Of Technology, Madras. Lecture - 19
 Liquid-Liquid Extraction Prof. Mukesh Doble Department Of Biotechnology Indian Institute Of Technology, Madras Lecture - 19 Liquid-Liquid Extraction Let us continue with the Liquid- Liquid Extraction.
Liquid-Liquid Extraction Prof. Mukesh Doble Department Of Biotechnology Indian Institute Of Technology, Madras Lecture - 19 Liquid-Liquid Extraction Let us continue with the Liquid- Liquid Extraction.
Blocking the expression of the hepatitis B virus S gene in hepatocellular carcinoma cell lines with an anti-gene locked nucleic acid in vitro
 Blocking the expression of the hepatitis B virus S gene in hepatocellular carcinoma cell lines with an anti-gene locked nucleic acid in vitro Y.-B. Deng, H.-J. Qin, Y.-H. Luo, Z.-R. Liang and J.-J. Zou
Blocking the expression of the hepatitis B virus S gene in hepatocellular carcinoma cell lines with an anti-gene locked nucleic acid in vitro Y.-B. Deng, H.-J. Qin, Y.-H. Luo, Z.-R. Liang and J.-J. Zou
Supporting Information
 Supporting Information For Intracellularly Acid-Switchable Multifunctional Micelles for Combinational Photo/Chemotherapy of the Drug Resistant Tumor Tingting Wang +,#, Dangge Wang +,#, Haijun Yu +, *,
Supporting Information For Intracellularly Acid-Switchable Multifunctional Micelles for Combinational Photo/Chemotherapy of the Drug Resistant Tumor Tingting Wang +,#, Dangge Wang +,#, Haijun Yu +, *,
Cytosolar delivery of large proteins using nanoparticlestabilized
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2016 Supporting Information Cytosolar delivery of large proteins using nanoparticlestabilized nanocapsules
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2016 Supporting Information Cytosolar delivery of large proteins using nanoparticlestabilized nanocapsules
SUPPLEMENTAL INFORMATION
 SUPPLEMENTAL INFORMATION EXPERIMENTAL PROCEDURES Tryptic digestion protection experiments - PCSK9 with Ab-3D5 (1:1 molar ratio) in 50 mm Tris, ph 8.0, 150 mm NaCl was incubated overnight at 4 o C. The
SUPPLEMENTAL INFORMATION EXPERIMENTAL PROCEDURES Tryptic digestion protection experiments - PCSK9 with Ab-3D5 (1:1 molar ratio) in 50 mm Tris, ph 8.0, 150 mm NaCl was incubated overnight at 4 o C. The
CytoPainter Lysosomal Staining Kit - Blue Fluorescence
 ab112135 CytoPainter Lysosomal Staining Kit - Blue Fluorescence Instructions for Use For staining Lysosomes in suspension and adherent cells by using our proprietary blue fluorescence probe This product
ab112135 CytoPainter Lysosomal Staining Kit - Blue Fluorescence Instructions for Use For staining Lysosomes in suspension and adherent cells by using our proprietary blue fluorescence probe This product
Chapter 3. Need for Present Study
 Need for Present Study Chapter 3 Chemotherapy, the use of cytotoxic drugs to kill cancerous cells remains the most common approach for cancer therapy. In conventional chemotherapy most of the anticancer
Need for Present Study Chapter 3 Chemotherapy, the use of cytotoxic drugs to kill cancerous cells remains the most common approach for cancer therapy. In conventional chemotherapy most of the anticancer
A Long-Term and Slow-Releasing Hydrogen Sulfide Donor Protects against Myocardial. Ischemia/Reperfusion Injury
 Supporting Information A Long-Term and Slow-Releasing Hydrogen Sulfide Donor Protects against Myocardial Ischemia/Reperfusion Injury Xiaotian Sun 1 *, Wenshuo Wang 2, Jing Dai 3, Sheng Jin 4, Jiechun Huang
Supporting Information A Long-Term and Slow-Releasing Hydrogen Sulfide Donor Protects against Myocardial Ischemia/Reperfusion Injury Xiaotian Sun 1 *, Wenshuo Wang 2, Jing Dai 3, Sheng Jin 4, Jiechun Huang
Reagent Set DAS ELISA, Alkaline phosphatase label
 List of contents Lot number Item 96 wells 500 wells 1000 wells 5000 wells Capture antibody 0.150 ml 0.275 ml 0.525 ml 2.525 ml Alkaline phosphatase enzyme conjugate 0.150 ml 0.275 ml 0.525 ml 2.525 ml
List of contents Lot number Item 96 wells 500 wells 1000 wells 5000 wells Capture antibody 0.150 ml 0.275 ml 0.525 ml 2.525 ml Alkaline phosphatase enzyme conjugate 0.150 ml 0.275 ml 0.525 ml 2.525 ml
Human Carbamylated LDL ELISA Kit (CBL-LDL Quantitation)
 Product Manual Human Carbamylated LDL ELISA Kit (CBL-LDL Quantitation) Catalog Number MET-5032 96 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Lipoproteins are submicroscopic
Product Manual Human Carbamylated LDL ELISA Kit (CBL-LDL Quantitation) Catalog Number MET-5032 96 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Lipoproteins are submicroscopic
Anti-cancer activity of Aya Thambira Chendooram (ATC) in in-vitro cell line against Breast Carcinoma
 International Journal of Advanced Research in Biological Sciences ISSN: 2348-8069 www.ijarbs.com DOI: 10.22192/ijarbs Coden: IJARQG(USA) Volume 5, Issue 1-2018 Research Article DOI: http://dx.doi.org/10.22192/ijarbs.2018.05.01.010
International Journal of Advanced Research in Biological Sciences ISSN: 2348-8069 www.ijarbs.com DOI: 10.22192/ijarbs Coden: IJARQG(USA) Volume 5, Issue 1-2018 Research Article DOI: http://dx.doi.org/10.22192/ijarbs.2018.05.01.010
Supporting Information
 Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2017 Supporting Information Cancer-mitochondria-targeted photodynamic therapy with supramolecular
Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2017 Supporting Information Cancer-mitochondria-targeted photodynamic therapy with supramolecular
Hosta virus X ELISA KIT
 Hosta virus X ELISA KIT Cat. No.:DEIA9256 Pkg.Size:96T Intended use The Hosta virus X ELISA KIT is used to detect HVX in both leaves and roots of hosta plants. Reagents And Materials Provided 1. Capture
Hosta virus X ELISA KIT Cat. No.:DEIA9256 Pkg.Size:96T Intended use The Hosta virus X ELISA KIT is used to detect HVX in both leaves and roots of hosta plants. Reagents And Materials Provided 1. Capture
Fabrication of Bio-based Polyelectrolyte Capsules and Their Application for Glucose-Triggered Insulin Delivery
 Supporting Information for Fabrication of Bio-based Polyelectrolyte Capsules and Their Application for Glucose-Triggered Insulin Delivery Dongjian Shi a*, Maoshuang Ran a, Li Zhang a, He Huang a, Xiaojie
Supporting Information for Fabrication of Bio-based Polyelectrolyte Capsules and Their Application for Glucose-Triggered Insulin Delivery Dongjian Shi a*, Maoshuang Ran a, Li Zhang a, He Huang a, Xiaojie
Optimization of the Fuse-It-mRNA Protocol for L929 Cells in the µ-plate 24 Well
 Optimization of the Fuse-It-mRNA Protocol for L929 Cells in the µ-plate 24 Well 1. General Information... 1 2. Background... 1 3. Material and Equipment Required... 2 4. Experimental Procedure and Results...
Optimization of the Fuse-It-mRNA Protocol for L929 Cells in the µ-plate 24 Well 1. General Information... 1 2. Background... 1 3. Material and Equipment Required... 2 4. Experimental Procedure and Results...
FORMULATION AND CHARACTERIZATION OF TELMISATAN SOLID DISPERSIONS
 International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 974-434 Vol.2, No.1, pp 341-347, Jan-Mar 1 FORMULATION AND CHARACTERIZATION OF TELMISATAN SOLID DISPERSIONS Kothawade S. N. 1 *, Kadam
International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 974-434 Vol.2, No.1, pp 341-347, Jan-Mar 1 FORMULATION AND CHARACTERIZATION OF TELMISATAN SOLID DISPERSIONS Kothawade S. N. 1 *, Kadam
Encapsulation techniques
 Loughborough University Institutional Repository Encapsulation techniques This item was submitted to Loughborough University's Institutional Repository by the/an author. Citation: VLADISAVLJEVIC, G.T.,
Loughborough University Institutional Repository Encapsulation techniques This item was submitted to Loughborough University's Institutional Repository by the/an author. Citation: VLADISAVLJEVIC, G.T.,
Reagent Set DAS ELISA, Alkaline phosphatase label SRA 22001, SRA 23203, SRA 27703, SRA & SRA ToRSV, ArMV, GFLV, AnFBV and PDV
 List of contents Lot number Reagent Set Item 96 wells 500 wells 1000 wells 5000 wells Capture antibody 0.150 ml 0.275 ml 0.525 ml 2.525 ml Alkaline phosphatase enzyme conjugate 0.150 ml 0.275 ml 0.525
List of contents Lot number Reagent Set Item 96 wells 500 wells 1000 wells 5000 wells Capture antibody 0.150 ml 0.275 ml 0.525 ml 2.525 ml Alkaline phosphatase enzyme conjugate 0.150 ml 0.275 ml 0.525
Gladstone Institutes, University of California (UCSF), San Francisco, USA
 Fluorescence-linked Antigen Quantification (FLAQ) Assay for Fast Quantification of HIV-1 p24 Gag Marianne Gesner, Mekhala Maiti, Robert Grant and Marielle Cavrois * Gladstone Institutes, University of
Fluorescence-linked Antigen Quantification (FLAQ) Assay for Fast Quantification of HIV-1 p24 Gag Marianne Gesner, Mekhala Maiti, Robert Grant and Marielle Cavrois * Gladstone Institutes, University of
Supplementary Figures
 Supplementary Figures 7 8 Supplementary Figure Intracellular fusion and degradation. (a) Changes of intracellular metal ion concentrations. Intracellular ion concentrations were normalized by total protein
Supplementary Figures 7 8 Supplementary Figure Intracellular fusion and degradation. (a) Changes of intracellular metal ion concentrations. Intracellular ion concentrations were normalized by total protein
Potato leafroll virus (PLRV) Reagent Set DAS ELISA, Alkaline phosphatase label Catalog number: SRA List of contents
 Potato leafroll virus (PLRV) Reagent Set List of contents Lot number Item 96 wells 500 wells 1000 wells 5000 wells Capture antibody 0.150 ml 0.275 ml 0.525 ml 2.525 ml Alkaline phosphatase enzyme conjugate
Potato leafroll virus (PLRV) Reagent Set List of contents Lot number Item 96 wells 500 wells 1000 wells 5000 wells Capture antibody 0.150 ml 0.275 ml 0.525 ml 2.525 ml Alkaline phosphatase enzyme conjugate
Determination of Tetracyclines in Chicken by Solid-Phase Extraction and High-Performance Liquid Chromatography
 Determination of Tetracyclines in Chicken by Solid-Phase Extraction and High-Performance Liquid Chromatography Application ote Food Safety Authors Chen-Hao Zhai and Yun Zou Agilent Technologies Co. Ltd.
Determination of Tetracyclines in Chicken by Solid-Phase Extraction and High-Performance Liquid Chromatography Application ote Food Safety Authors Chen-Hao Zhai and Yun Zou Agilent Technologies Co. Ltd.
Solid-in-oil peptide nanocarriers for transcutaneous cancer vaccine delivery. against melanoma
 Supplementary Information for Solid-in-oil peptide nanocarriers for transcutaneous cancer vaccine delivery against melanoma Rie Wakabayashi,,a,b Masato Sakuragi,,a Shuto Kozaka, a Yoshiro Tahara, a Noriho
Supplementary Information for Solid-in-oil peptide nanocarriers for transcutaneous cancer vaccine delivery against melanoma Rie Wakabayashi,,a,b Masato Sakuragi,,a Shuto Kozaka, a Yoshiro Tahara, a Noriho
IN VITRO ANTICANCER ACTIVITY OF FLOWER EXTRACTS OF COUROUPITA GUIANENSIS
 CHAPTER 3 IN VITRO ANTICANCER ACTIVITY OF FLOWER EXTRACTS OF COUROUPITA GUIANENSIS 3. INTRODUCTION Plants are the basic source of knowledge of modern medicine. Almost all the parts of the plant, namely
CHAPTER 3 IN VITRO ANTICANCER ACTIVITY OF FLOWER EXTRACTS OF COUROUPITA GUIANENSIS 3. INTRODUCTION Plants are the basic source of knowledge of modern medicine. Almost all the parts of the plant, namely
Anti-Aging Activity Of Cucurbita moschata Ethanolic Extract Towards NIH3T3 Fibroblast Cells Induced By Doxorubicin
 Indonesian Journal of Cancer Chemoprevention, 2016, 7(2): 49-53 ISSN: 2088 0197 Anti-Aging Activity Of Cucurbita moschata Ethanolic Extract Towards NIH3T3 Fibroblast Cells Induced By Doxorubicin Laeli
Indonesian Journal of Cancer Chemoprevention, 2016, 7(2): 49-53 ISSN: 2088 0197 Anti-Aging Activity Of Cucurbita moschata Ethanolic Extract Towards NIH3T3 Fibroblast Cells Induced By Doxorubicin Laeli
Instructions. Fuse-It-mRNA easy. Shipping and Storage. Overview. Kit Contents. Specifications. Note: Important Guidelines
 Membrane fusion is a highly efficient method for transfecting various molecules and particles into mammalian cells, even into sensitive and primary cells. The Fuse-It reagents are cargo-specific liposomal
Membrane fusion is a highly efficient method for transfecting various molecules and particles into mammalian cells, even into sensitive and primary cells. The Fuse-It reagents are cargo-specific liposomal
Preparation and Evaluation of Ethyl Cellulose Coated Microcapsules of Carbamazepine for Controlled Release
 Asian Journal of Chemistry Vol. 20, No. 8 (2008), 5901-5907 Preparation and Evaluation of Ethyl Cellulose Coated Microcapsules of Carbamazepine for Controlled Release K.P.R. CHOWDARY* and MALLURU SUBBA
Asian Journal of Chemistry Vol. 20, No. 8 (2008), 5901-5907 Preparation and Evaluation of Ethyl Cellulose Coated Microcapsules of Carbamazepine for Controlled Release K.P.R. CHOWDARY* and MALLURU SUBBA
Nature Protocols: doi: /nprot Supplementary Figure 1. Fluorescent titration of probe CPDSA.
 Supplementary Figure 1 Fluorescent titration of probe CPDSA. Fluorescent titration of probe CPDSA (10 um) upon addition of GSH in HEPES (10 mm, ph = 7.4) containing 10% DMSO. Each spectrum was recorded
Supplementary Figure 1 Fluorescent titration of probe CPDSA. Fluorescent titration of probe CPDSA (10 um) upon addition of GSH in HEPES (10 mm, ph = 7.4) containing 10% DMSO. Each spectrum was recorded
CHAPTER - IV OBJECTIVES OF THE STUDY
 CHAPTER - IV OBJECTIVES OF THE STUDY 4.1 BACKGROUND Human immunodeficiency virus (HIV) infection and acquired immune deficiency syndrome (AIDS) commonly referred to as HIV & AIDS have emerged as being
CHAPTER - IV OBJECTIVES OF THE STUDY 4.1 BACKGROUND Human immunodeficiency virus (HIV) infection and acquired immune deficiency syndrome (AIDS) commonly referred to as HIV & AIDS have emerged as being
Supporting Information
 Supporting Information Wiley-VCH 2006 69451 Weinheim, Germany Stepwise Directing Nanocrystals to Self-Assemble at Water/Oil Interfaces Jing Wang, Dayang Wang, Nelli S. Sobal, Michael Giersig, Ming Jiang,
Supporting Information Wiley-VCH 2006 69451 Weinheim, Germany Stepwise Directing Nanocrystals to Self-Assemble at Water/Oil Interfaces Jing Wang, Dayang Wang, Nelli S. Sobal, Michael Giersig, Ming Jiang,
Novel ph-responsive nanovectors for controlled release of ionisable drugs
 Supplementary Information Novel ph-responsive nanovectors for controlled release of ionisable drugs Francesca Mastrotto, a Stefano Salmaso a, Cameron Alexander, b Giuseppe Mantovani b and Paolo Caliceti
Supplementary Information Novel ph-responsive nanovectors for controlled release of ionisable drugs Francesca Mastrotto, a Stefano Salmaso a, Cameron Alexander, b Giuseppe Mantovani b and Paolo Caliceti
OxiSelect Human Oxidized LDL ELISA Kit (OxPL-LDL Quantitation)
 Product Manual OxiSelect Human Oxidized LDL ELISA Kit (OxPL-LDL Quantitation) Catalog Number STA-358 96 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Lipoproteins are submicroscopic
Product Manual OxiSelect Human Oxidized LDL ELISA Kit (OxPL-LDL Quantitation) Catalog Number STA-358 96 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Lipoproteins are submicroscopic
Cell type- and sizedependent in vitro toxicity of silica particles in human skin cells
 Cell type- and sizedependent in vitro toxicity of silica particles in human skin cells 22 October 2014 Claudia Moia Early Stage Researcher, Cranfield University (UK) Presentation Overview Introduction
Cell type- and sizedependent in vitro toxicity of silica particles in human skin cells 22 October 2014 Claudia Moia Early Stage Researcher, Cranfield University (UK) Presentation Overview Introduction
Supporting Information
 Supporting Information The Effects of Spacer Length and Composition on Aptamer-Mediated Cell-Specific Targeting with Nanoscale PEGylated Liposomal Doxorubicin Hang Xing +, [a] Ji Li +, [a] Weidong Xu,
Supporting Information The Effects of Spacer Length and Composition on Aptamer-Mediated Cell-Specific Targeting with Nanoscale PEGylated Liposomal Doxorubicin Hang Xing +, [a] Ji Li +, [a] Weidong Xu,
INTERNATIONAL RESEARCH JOURNAL OF PHARMACY
 INTERNATIONAL RESEARCH JOURNAL OF PHARMACY www.irjponline.com ISSN 2230 8407 Research Article FORMULATION AND CHARACTERIZATION OF DOXORUBICIN HYDROCHLORIDE LIPOSOMES BY DOUBLE EMULSION METHOD Shah Sumit
INTERNATIONAL RESEARCH JOURNAL OF PHARMACY www.irjponline.com ISSN 2230 8407 Research Article FORMULATION AND CHARACTERIZATION OF DOXORUBICIN HYDROCHLORIDE LIPOSOMES BY DOUBLE EMULSION METHOD Shah Sumit
SUPPORTING INFORMATION. For. ACS Applied Materials & Interfaces
 SUPPRTIG IFRMATI For ACS Applied Materials & Interfaces S-1 Specific Fluorescence Probes for Lipid Droplets Based on Simple AIEgens Zhiming Wang,,,, # Chen Gui,,, Engui Zhao,, Jing Wang, # Xiaodong Li,
SUPPRTIG IFRMATI For ACS Applied Materials & Interfaces S-1 Specific Fluorescence Probes for Lipid Droplets Based on Simple AIEgens Zhiming Wang,,,, # Chen Gui,,, Engui Zhao,, Jing Wang, # Xiaodong Li,
Biopharmaceutics Dosage form factors influencing bioavailability Lec:5
 Biopharmaceutics Dosage form factors influencing bioavailability Lec:5 Ali Y Ali BSc Pharmacy MSc Industrial Pharmaceutical Sciences Dept. of Pharmaceutics School of Pharmacy University of Sulaimani 09/01/2019
Biopharmaceutics Dosage form factors influencing bioavailability Lec:5 Ali Y Ali BSc Pharmacy MSc Industrial Pharmaceutical Sciences Dept. of Pharmaceutics School of Pharmacy University of Sulaimani 09/01/2019
Oncolytic Adenovirus Complexes Coated with Lipids and Calcium Phosphate for Cancer Gene Therapy
 Oncolytic Adenovirus Complexes Coated with Lipids and Calcium Phosphate for Cancer Gene Therapy Jianhua Chen, Pei Gao, Sujing Yuan, Rongxin Li, Aimin Ni, Liang Chu, Li Ding, Ying Sun, Xin-Yuan Liu, Yourong
Oncolytic Adenovirus Complexes Coated with Lipids and Calcium Phosphate for Cancer Gene Therapy Jianhua Chen, Pei Gao, Sujing Yuan, Rongxin Li, Aimin Ni, Liang Chu, Li Ding, Ying Sun, Xin-Yuan Liu, Yourong
Physical Pharmacy. Interfacial phenomena. Khalid T Maaroof MSc. Pharmaceutical sciences School of pharmacy Pharmaceutics department
 Physical Pharmacy Interfacial phenomena Khalid T Maaroof MSc. Pharmaceutical sciences School of pharmacy Pharmaceutics department 1 Introduction The boundary between two phases is generally described as
Physical Pharmacy Interfacial phenomena Khalid T Maaroof MSc. Pharmaceutical sciences School of pharmacy Pharmaceutics department 1 Introduction The boundary between two phases is generally described as
A Photostable AIE Luminogen for Specific Mitochondrial
 SUPPORTING INFORMATION A Photostable AIE Luminogen for Specific Mitochondrial Imaging and Tracking Chris Wai Tung Leung,,# Yuning Hong,,# Sijie Chen, Engui Zhao, Jacky Wing Yip Lam, and Ben Zhong Tang
SUPPORTING INFORMATION A Photostable AIE Luminogen for Specific Mitochondrial Imaging and Tracking Chris Wai Tung Leung,,# Yuning Hong,,# Sijie Chen, Engui Zhao, Jacky Wing Yip Lam, and Ben Zhong Tang
Supporting Information For
 Supporting Information For MicroRNA-Catalyzed Cancer Therapeutics Based on DNA-Programmed Nanoparticle Complex Xucheng Luo, 1 Zhi Li, 1 Ganglin Wang, 1 Xuewen He, 2,3 Xiaoqin Shen, 1 Quanhong Sun, 1 Li
Supporting Information For MicroRNA-Catalyzed Cancer Therapeutics Based on DNA-Programmed Nanoparticle Complex Xucheng Luo, 1 Zhi Li, 1 Ganglin Wang, 1 Xuewen He, 2,3 Xiaoqin Shen, 1 Quanhong Sun, 1 Li
Formulation and Evaluation of Acyclovir Liposomes
 Krishna Mohan Chinnala and Rabinarayan Panigrahy., 217/ Formulation and evaluation of acyclovir RESEARCH ARTICLE International Research Journal of Pharmaceutical and Biosciences Pri -ISSN: 2394-5826 http://www.irjpbs.com
Krishna Mohan Chinnala and Rabinarayan Panigrahy., 217/ Formulation and evaluation of acyclovir RESEARCH ARTICLE International Research Journal of Pharmaceutical and Biosciences Pri -ISSN: 2394-5826 http://www.irjpbs.com
Mitochondrial Trifunctional Protein (TFP) Protein Quantity Microplate Assay Kit
 PROTOCOL Mitochondrial Trifunctional Protein (TFP) Protein Quantity Microplate Assay Kit DESCRIPTION Mitochondrial Trifunctional Protein (TFP) Protein Quantity Microplate Assay Kit Sufficient materials
PROTOCOL Mitochondrial Trifunctional Protein (TFP) Protein Quantity Microplate Assay Kit DESCRIPTION Mitochondrial Trifunctional Protein (TFP) Protein Quantity Microplate Assay Kit Sufficient materials
RESEARCH INTEREST INTRODUCTION:
 RESEARCH INTEREST INTRODUCTION: Cancer is regarded as one among the dreadful diseases. Despite of much advancement in the theranostics of cancer, the cure rate for this terrible disease without any side
RESEARCH INTEREST INTRODUCTION: Cancer is regarded as one among the dreadful diseases. Despite of much advancement in the theranostics of cancer, the cure rate for this terrible disease without any side
Gold Tripod Nanoparticles Effectiveness for Killing PC-3 Prostate Cancer Cells in Vitro. Deirdre O Sullivan. Nyack High School
 Gold Tripod Nanoparticles Effectiveness for Killing PC-3 Prostate Cancer Cells in Vitro Deirdre O Sullivan Nyack High School Abstract: Gold Tripod Nanoparticles Effectiveness of Killing PC-3 Prostate Cancer
Gold Tripod Nanoparticles Effectiveness for Killing PC-3 Prostate Cancer Cells in Vitro Deirdre O Sullivan Nyack High School Abstract: Gold Tripod Nanoparticles Effectiveness of Killing PC-3 Prostate Cancer
UV Tracer TM Maleimide NHS ester
 UV Tracer TM Maleimide HS ester Product o.: 1020 Product ame: UV-Tracer TM Maleimide-HS ester Chemical Structure: Chemical Composition: C 41 H 67 5 18 Molecular Weight: 1014.08 Appearance: Storage: Yellow
UV Tracer TM Maleimide HS ester Product o.: 1020 Product ame: UV-Tracer TM Maleimide-HS ester Chemical Structure: Chemical Composition: C 41 H 67 5 18 Molecular Weight: 1014.08 Appearance: Storage: Yellow
Single and combined cytotoxicity research of propiconazole and nano-zinc oxide on the NIH/3T3 cell
 Available online at www.sciencedirect.com Procedia Environmental Sciences 18 ( 2013 ) 100 105 2013 International Symposium on Environmental Science and Technology (2013 ISEST) Single and combined cytotoxicity
Available online at www.sciencedirect.com Procedia Environmental Sciences 18 ( 2013 ) 100 105 2013 International Symposium on Environmental Science and Technology (2013 ISEST) Single and combined cytotoxicity
Studies on the cyclosporin A loaded stearic acid nanoparticles
 International Journal of Pharmaceutics 200 (2000) 153 159 www.elsevier.com/locate/ijpharm Studies on the cyclosporin A loaded stearic acid Qiang Zhang a, Guoqing Yie a, Yie Li a, Qingsong Yang a, T. Nagai
International Journal of Pharmaceutics 200 (2000) 153 159 www.elsevier.com/locate/ijpharm Studies on the cyclosporin A loaded stearic acid Qiang Zhang a, Guoqing Yie a, Yie Li a, Qingsong Yang a, T. Nagai
Simultaneous Blood Brain Barrier Crossing and Protection for Stroke Treatment Based on Edaravone-Loaded Ceria Nanoparticles
 Supporting Information Simultaneous Blood Brain Barrier Crossing and Protection for Stroke Treatment Based on Edaravone-Loaded Ceria Nanoparticles Qunqun Bao, Ping Hu, * Yingying Xu, Tiansheng Cheng, Chenyang
Supporting Information Simultaneous Blood Brain Barrier Crossing and Protection for Stroke Treatment Based on Edaravone-Loaded Ceria Nanoparticles Qunqun Bao, Ping Hu, * Yingying Xu, Tiansheng Cheng, Chenyang
LDL Uptake Cell-Based Assay Kit
 LDL Uptake Cell-Based Assay Kit Catalog Number KA1327 100 assays Version: 07 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the Assay...
LDL Uptake Cell-Based Assay Kit Catalog Number KA1327 100 assays Version: 07 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the Assay...
Xianren Zhang ( 张现仁 )
 The interaction between nanoparticles and membranes: from cytotoxicity to drug delivery Xianren Zhang ( 张现仁 ) zhangxr@mail.buct.edu.cn State Key Laboratory of Organic-Inorganic Composites, Beijing University
The interaction between nanoparticles and membranes: from cytotoxicity to drug delivery Xianren Zhang ( 张现仁 ) zhangxr@mail.buct.edu.cn State Key Laboratory of Organic-Inorganic Composites, Beijing University
B16-F10 (Mus musculus skin melanoma), NCI-H460 (human non-small cell lung cancer
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 Experimental Methods Cell culture B16-F10 (Mus musculus skin melanoma), NCI-H460 (human non-small
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 Experimental Methods Cell culture B16-F10 (Mus musculus skin melanoma), NCI-H460 (human non-small
Comparison of Young and Old Cardiac Telocytes Using Atomic Force Microscopy
 Comparison of Young and Old Cardiac Telocytes Using Atomic Force Microscopy Jiali Luo 1, 2, 3, 4, a, Shanshan Feng 1, 2, 3, 4, b 1Key Laboratory of Regenerative Medicine, Ministry of Education, Jinan University,
Comparison of Young and Old Cardiac Telocytes Using Atomic Force Microscopy Jiali Luo 1, 2, 3, 4, a, Shanshan Feng 1, 2, 3, 4, b 1Key Laboratory of Regenerative Medicine, Ministry of Education, Jinan University,
IN VITRO HORMESIS EFFECTS OF SODIUM FLUORIDE ON KIDNEY CELLS OF THREE-DAY-OLD MALE RATS
 292 Tang, An, Du, Zhang, Zhou 292 IN VITRO HORMESIS EFFECTS OF SODIUM FLUORIDE ON KIDNEY CELLS OF THREE-DAY-OLD MALE RATS Qin-qing Tang, a Xiao-jing An, a Jun Du, a Zheng-xiang Zhang, a Xiao-jun Zhou a
292 Tang, An, Du, Zhang, Zhou 292 IN VITRO HORMESIS EFFECTS OF SODIUM FLUORIDE ON KIDNEY CELLS OF THREE-DAY-OLD MALE RATS Qin-qing Tang, a Xiao-jing An, a Jun Du, a Zheng-xiang Zhang, a Xiao-jun Zhou a
TRANSIL ASSAY KITS. Frequently Asked Questions (FAQs)
 TRANSIL ASSAY KITS Frequently Asked Questions (FAQs) 1. 2. 3. What is the principle of TRANSIL technology? What membrane is on the surface of the What are the key advantages of the TRANSIL technology?
TRANSIL ASSAY KITS Frequently Asked Questions (FAQs) 1. 2. 3. What is the principle of TRANSIL technology? What membrane is on the surface of the What are the key advantages of the TRANSIL technology?
Supporting information
 S1 Supporting information Biodegradable Injectable Polymer Systems Exhibiting Temperature-Responsive Irreversible Sol-to-Gel Transition by Covalent Bond Formation Yasuyuki YOSHIDA 1,2, Keisuke KAWAHARA
S1 Supporting information Biodegradable Injectable Polymer Systems Exhibiting Temperature-Responsive Irreversible Sol-to-Gel Transition by Covalent Bond Formation Yasuyuki YOSHIDA 1,2, Keisuke KAWAHARA
