Specificity of Enzyme Immunoassay for Serologic Coccidioidomycosis Diagnosis Compared to Immunodiffusion
|
|
|
- Clare Riley
- 6 years ago
- Views:
Transcription
1 Specificity of Enzyme Immunoassay for Serologic Coccidioidomycosis Diagnosis Compared to Immunodiffusion Item Type Thesis Authors Petein, Nathalie Rights Copyright is held by the author. Digital access to this material is made possible by the College of Medicine - Phoenix, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 25/04/ :29:53 Link to Item
2 Specificity of Enzyme Immunoassay for Serologic Coccidioidomycosis Diagnosis Compared to Immunodiffusion A Thesis submitted to The University of Arizona College of Medicine- Phoenix in partial fulfillment of the requirements for the Degree of Doctor of Medicine Nathalie Petein
3 Dedication For my mom, who suffered from disseminated coccidioidomycosis, which had taken months before an accurate diagnosis was made. 2
4 Acknowledgements Arizona Department of Health Services (ADHS) Laura Erhart Shoana Anderson Clarisse Tsang Sara Imholte Maricopa County Department of Public Health (MCDPH) Rebecca Sunenshine University of Arizona College of Medicine (UACOM) Doug Campos-Outcalt John Galgiani Lab Corp Frank Ryan Sonora Quest Mike Saubolle 3
5 Abstract BACKGROUND: Serologic testing for coccidioidomycosis challenges clinicians due to conflicting small studies regarding the sensitivity and specificity of newer enzyme immunoassay (EIA) tests and the lack of a true gold standard diagnostic test for comparison. METHODS: We analyzed all Lab Corp coccidioidomycosis serological test results from February 2008 through February 2009 and calculated the sensitivity, specificity, and positive/negative predictive values of EIA immunoglobulin (Ig)M and IgG. Immunodiffusion IgM and IgG (ID), complement fixation titers (CF), and tissue/culture diagnosis were used as tests for comparison. The comparison test (CT) was considered positive if any comparison test was positive the day of EIA collection or if tissue/culture diagnosis occurred during the time period. Cases required EIA IgM and IgG and 2 comparison tests performed the same day for inclusion. Medical records associated with positive EIA and negative comparison test results were reviewed for coccidioidomycosis symptoms, physician diagnosis, and subsequent positive comparison test results. Sensitivity, specificity, and predictive values were calculated, including those with subsequent positive comparison test results. RESULTS: A total of 1445 laboratory test sets were identified. EIA sensitivity and specificity were 83.8% and 92.6%, respectively. Positive and negative predictive values were 61.5% and 97.6%, respectively. Of 94 false positive EIA results, 92 (97.9%) were associated with documented coccidioidomycosis symptoms and 81% with coccidioidomycosis physician diagnosis. CONCLUSION: Based on the largest study of sensitivity and specificity calculated from laboratory surveillance data, EIA sensitivity and specificity for coccidioidomycosis diagnosis are lower than previously reported using only coccidioidomycosis laboratory tests as a comparison. However, association of false positive EIA results with coccidioidomycosis symptoms and physician diagnosis suggests that ID and CF laboratory tests alone are not a sufficient confirmation test for diagnosis. 4
6 Table of Contents Dedication.2 Acknowledgements.3 Abstract.4 Table of Contents.5 List of Figures and Tables.6 Introduction..7 Research Materials and Methods..12 Results...14 Discussion...16 Future Directions..19 Conclusions.19 References...21 Appendix.24 Poster
7 List of Figures and Tables Figure 1. Rates of Reported Valley Fever (VF) in Arizona, Table 1. Learning objectives and implementation plan Figure 2. Diagram of matching schema for public health reporting validation for laboratories A and B Figure 3. Summary of false positive enzyme immunoassay results Table 2. Enzyme immunoassay compared to comparison tests 6
8 Introduction Coccidioidomycosis, or Valley Fever, is an infection caused by the fungus Coccidioides immitis or Coccidioides posadasii. This fungus grows in the soil of hot, dry climates in the southern and central portions of California, Arizona, New Mexico, Texas, and the southern portions of Nevada and Utah (13). Valley Fever is also endemic in parts of Mexico, Central, and South America (10). Anyone who lives, visits, or travels through the areas where the fungus is endemic may acquire Valley Fever (18). People working in certain occupations such as construction, excavation, agriculture, archaeological digging, and other occupations, which disturb soil in endemic areas, may be at increased risk of exposure (5, 8). Various domestic animals such as dogs, cats, and horses, as well as wild animals are also susceptible. Valley Fever is acquired by inhaling one or more airborne spores of the fungus Coccidioides immitis or C. posadasii (5, 13). The spores are carried on dust particles by the wind when the desert soil is disturbed. Approximately 60% of infected individuals are asymptomatic or have mild respiratory symptoms (4, 5, 10, 13, 18). Those who develop symptoms usually develop them within 7-21 days after exposure (4). Most symptomatic people have a pneumonia-like illness, with symptoms of acute influenza-like illness (fever, cough, fatigue), which makes it difficult to distinguish from communityacquired pneumonia (CAP). About 1-4% of symptomatic individuals will develop disseminated disease (6), where the infection spreads to other parts of the body, such as the skin, joints, bones, lymph nodes, and central nervous system. Disseminated coccidioidomycosis always requires treatment and can lead to serious complications, including death. There is no known cure and no licensed vaccine available. Valley Fever is not spread from human to human, animal to animal, animal to human, or human to animal (13, 16). Because Coccidioides spp. are dimorphic fungi, the spores change form from infectious arthrospores in the soil to non-infectious spherules in tissues of the body. Coccidioides spp. are very contagious when grown in the laboratory setting and require laboratorians to take special precautions once the fungus is isolated (4). Additionally, since it is a select agent of bioterrorism, once the fungus is isolated in a laboratory, it must be either destroyed or transported to a biosafety level 4 laboratory. 7
9 Sixty percent of US reported coccidioidomycosis cases occur in Arizona. Therefore, of the estimated 150,000 U. S. coccidioidomycosis infections per year, approximately 90,000 are thought to occur in Arizona (predominantly Pima, Maricopa and Pinal Counties), making this region the focal point for investigation of the disease (2). Arizona has had mandated reporting of Valley Fever by both laboratories and physicians since Figure 1 shows the rates of reported Valley Fever (VF) in Arizona from Over the last decade, the reported rate of Valley Fever cases in Arizona has more than quadrupled. In 2007, there were 4815 cases (75/100,000 population) reported to the Arizona Department of Health Services (ADHS) (1), whereas only 958 cases (16/100,000 population) were reported to ADHS in Valley Fever is seasonal with the highest rates of infection in Arizona typically occurring from June through August and from October through November. For public health surveillance purposes, the Council of State and Territorial Epidemiologists (CSTE) clinical criteria for coccidioidomycosis diagnosis include at least one of the following: 1) Influenza-like signs and symptoms, including fever, chest pain, cough, myalgia, arthralgia, headache; 2) Pneumonia or other pulmonary lesion, by chest X-ray; 3) Rash, including erythema nodosum or erythema multiforme; 4) Involvement of bones, joints, or skin; 5) Meningitis or other CNS involvement; or 6) Involvement of viscera and lymph nodes (20). The CSTE case definition for a confirmed case of coccidioidomycosis also includes laboratory criteria for diagnosis, which require at least one of the following: 1) Cultural, histopathologic, or molecular evidence of presence of C. immitis or C. posadasii or 2) Immunologic evidence of infection (20). Immunologic evidence can be demonstrated by a) coccidioidal skin test conversion from negative to positive after the onset of clinical signs and symptoms (Note: the skin test reagent is not currently commercially 8
10 available in the US), or b) serologic (testing of serum, cerebrospinal fluid, or other body fluid) via 1) detection of coccidioidal immunoglobulin (Ig)M by immunodiffusion, enzyme immunoassay (EIA), latex agglutination, or tube precipitin, or 2) detection of any titer of coccidioidal IgG by immunodiffusion, enzyme immunoassay (EIA), or complement fixation (20). In Arizona, for public health surveillance purposes, a confirmed case is a case that is laboratory confirmed following the CSTE case definition; the clinical criteria are not required. This epidemiological case definition was validated by a study indicating that 95% of all reported laboratory diagnoses of coccidioidomycosis were associated with symptoms meeting the CSTE clinical criteria for coccidioidomycosis diagnosis (21). For the past half century, detecting anticoccidioidal antibodies has been an important means of establishing a diagnosis of coccidioidomycosis (19). The two major antigens used to detect anticoccidioidal antibodies are the tube precipitin-reacting (TP) antigen --- so called because of the precipitin button that formed in the bottom of the test tube in the originally described tube precipitin test --- and the complement-fixing (CF) antigen. During primary infections, IgM antibodies against the TP antigen are usually found in serum earlier than CF antibodies (IgG) and disappear sooner, although exceptions to this rule can occur. In contrast, TP antibodies (IgM) are not usually found in more chronic infections whereas CF antibodies persist. Dual immunodiffusion procedures for both IgM and IgG that can be more readily supplied commercially have been developed as surrogate procedures to detect antibodies of the same specificity as the original TP and CF tests (16). The immunodiffusion tests (ID) are considered to be highly specific and at least as sensitive as the earlier methods (16). Other tests such as the latex agglutination test are available for detecting coccidioidal infections but are at this time of more limited value. More recently, an enzyme-linked immunosorbent assay (EIA) has been introduced, which may be more specific than latex agglutination tests (9). Although easier to perform and faster than immunodiffusion, the results from these methods have not yet been extensively correlated with results from TP or CF tests. Thus, the sensitivity and specificity of the EIA test has not been clearly defined (7). Today, serologic testing includes qualitative tests (immunodiffusion, enzyme immunoassay, or latex particle 9
11 agglutination), which permit detection in the serum of the major antibody responses: coccidioidal IgM in early coccidioidomycosis and complement fixation (CF) IgG, which appears later and is more persistent (3, 12, 14, 23). Unlike other infectious diseases, a positive IgG, whether by immunodiffusion, CF, or EIA, indicates active disease, since IgG is thought to persist in the blood for less than one year in nondisseminated disease. Further, quantitation of the level (titer) of coccidioidal IgG is useful in prognosis and management of the disease but less for diagnosis. The CF titer is typically higher in more severe or disseminated disease and decreases with treatment response (7). Because the EIA test is the easiest and least expensive to perform of all serologic tests for coccidioidomycosis, it is one of the most widely used by commercial laboratories and thus is the basis for the majority of Arizona s coccidioidomycosis surveillance data. Like all the other serologic tests for coccidioidomycosis, the EIA test can be falsely negative early in disease because the body requires 1-2 weeks to mount an antibody response. However, some believe that EIA might actually become positive earlier in the course of disease than immunodiffusion, giving it a distinct advantage for diagnosis (3, 12, 14, 23). Likewise, false positives may occur, leading to additional diagnostic testing and unwelcome patient anxiety. Specifically, concerns have been raised among clinicians about false positive EIA IgM results. The literature on this issue is conflicting and difficult to interpret (3, 12, 14, 23). CF testing typically becomes positive after EIA tests but is more specific. It is recommended that the ID test be run in parallel with the CF test (11). It is also recommended when performing EIA tests for diagnosis, that both IgM and IgG be performed simultaneously, as this greatly increases the sensitivity and specificity of the test (12). Additionally, some literature suggests the use of EIA IgM and IgG as a screening test only (12), which when positive should be confirmed with ID. Subsequently, if the EIA test is positive and the ID is negative, laboratories that use EIA as a screening test report the test results as negative. This raises concerns about missed diagnoses if the EIA is either more sensitive or becomes positive earlier in the course of disease than the test used for confirmation. Other laboratories report any positive EIA result as positive. This inconsistency among laboratory reporting practices significantly affects the quality of Arizona s coccidioidomycosis surveillance data. Therefore, it is important for 10
12 public health purposes to take into account these variable laboratory reporting practices. The consistent reporting of positive laboratory results for reportable infectious diseases is critical to public health surveillance. Surveillance is used to identify changing trends in diseases, detect outbreaks, and control disease spread. However, if new cases are not reliably reported, the public health system cannot compile an accurate picture of disease in the community. For this reason, conducting validations of surveillance data periodically is an important way to determine how well the public health system is capturing the burden of disease in the community. Therefore, prior to utilization of Arizona coccidioidomycosis surveillance data for this investigation, a validation study was performed. The main research objective of this investigation is to define the specificity of enzyme immunoassay (EIA) for coccidioidomycosis diagnosis compared to immunodiffusion, complement fixation, or tissue/culture diagnosis, although it is acknowledged that there is not a true gold standard available. Secondary objectives are to define the sensitivity and positive/negative predictive values of enzyme immunoassay (EIA) for coccidioidomycosis diagnosis compared to immunodiffusion, complement fixation, or tissue/culture diagnosis, with the same caveat for the comparison tests. As mentioned previously, in order to accomplish the primary and secondary objectives, the public health reporting of coccidioidomycosis was evaluated during a selected period for two commercial Arizona laboratories and laboratory reporting of coccidioidomycosis results was validated from one major Arizona laboratory. A program plan was established with a goal to gain practical skills and experiences in analyzing diagnostic testing methods for coccidioidomycosis. The planned outcomes were 1) to determine the specificity and positive predictive value of EIA for coccidioidomycosis diagnosis, 2) validate Arizona s laboratory reporting of coccidioidomycosis from one major laboratory, and 3) provide information to clinicians, laboratories, and public health practitioners to maximize accuracy of coccidioidomycosis diagnosis and public health surveillance. An evaluation plan (Table 1) was designed to measure our success in achieving the project s goals in the following ways: report to a supervising mentor at ADHS, submit quarterly progress notes to the 11
13 supervising mentor, and meet monthly with staff to discuss the progress of the project and address any issues. Research Materials and Methods Validation of public health reporting in Arizona In 2007, two laboratories accounted for a combined total of 46% of the initial reports of coccidioidomycosis, Laboratory A (30%) and Laboratory B (16%). In 2008, both laboratories provided independent reports of positive coccidioidomycosis tests; these data were used to validate public health reporting of coccidioidomycosis from the time period of March 1, 2008, through May 31, The study examined data from the ADHS surveillance system from March 1, 2008 through May 31, 2008 to ensure that all positive test results from two major laboratories (Laboratories A and B) were reported to ADHS. Positive results from Laboratory A included results for immunodiffusion and complement fixation tests. At that time, enzyme immunoassays (EIAs) were used as a screening test and positive results were not reported to physicians or public health unless the EIA was confirmed by immunodiffusion or complement fixation. Positive results from Laboratory B included immunodiffusion, complement fixation, and EIA, even if no confirmatory test had been run. Surveillance data at ADHS, including all positive coccidioidomycosis reports, have been entered in the Medical Electronic Disease Surveillance Intelligence System (MEDSIS) since January When multiple positive tests of coccidioidomycosis are received for a single person, only the first report is counted. When data entry staff enter a new laboratory report, they first check that the case has not been previously entered in MEDSIS, and also check against a database with cases reported from 1998 through 2005 (the historic data ). Because the surveillance databases capture data on the person, not on the individual tests, multiple positive tests on one individual (based on patient name and date of birth) from the laboratory data were eliminated so that matching was done on only one positive test per person. Data from both laboratories were first matched with MEDSIS, using an exact match on first and last name and birth date. Laboratory 12
14 reports that did not match MEDSIS cases were then matched against the database of historic cases ( ) using the same criteria of an exact match on first and last name and date of birth. Laboratoryreported results that did not match either public health databases were then hand-matched to MEDSIS and the historic database to identify matches with possible misspellings or missing information. County of resident was sometimes used as a criterion for the hand-match to confirm or reject suspected matches on name and birth date, such as if the date of birth was missing or a close match. A positive match was considered to be a patient reported by the laboratory that was found in either database during the automated or hand-matching steps. Tests that did not match were reviewed for test type and date to look for trends among cases that may not have been reported. Specificity of EIA compared to immunodiffusion or complement fixation All coccidioidomycosis testing results including serological tests and cultures from February 2008 through February 2009 were obtained from Laboratory Corporation (Lab Corp). The data were cleaned and duplicates were removed. Duplicates were identified by last name, first name, middle initial when available, and date of birth. The tests were then categorized based on the test performed. Results were categorized as positive or negative. If a test result was listed as indeterminate or had an equivocal value as a result, it was considered negative. If a test result was listed as test not performed, it was excluded. A CF was considered positive at titer levels equal to or greater than 1:2. EIA was considered positive when the absorbance value was equal to or greater than and if at least one EIA test was positive (IgM or IgG). Individual test results were grouped together into sets by last name, first name, date of birth, and test date, so that each set represented all the tests run for a single patient from a single day. If a person had more than one set of tests done, only the first set of tests was included in the original calculation of the sensitivity, specificity, and positive/negative predictive values. The inclusion criterion for the analysis was: presence of two coccidioidomycosis serologic EIA tests (both IgM and IgG) and two or more ID/CF tests run on the same day (from the same blood sample). Sensitivity, specificity, and positive/negative predictive values (PPV or NPV) of EIA (IgM and IgG) were calculated using the following 13
15 comparison tests (CT): if any immunodiffusion IgM or IgG (ID) or complement fixation titers (CF) were positive the day of EIA collection, then the CT was considered positive. A false positive test set is one in which at least one EIA test was positive and all CT tests were negative. After the sensitivity, specificity, and positive/negative predictive values were calculated, medical records associated with test sets consisting of at least one positive EIA result and all negative ID/CF tests, referred to as false positives (FPs), were requested. Records were reviewed for coccidioidomycosis symptoms as defined by the CSTE coccidioidomycosis clinical case definition, physician diagnosis, and subsequent positive CT laboratory results. The subsequent positive laboratory results examined included tissue/culture diagnosis from any time during the test period if listed in the medical records, a thorough review of the all of the Lab Corp data used in the original analysis, and review of additional Lab Corp test results through December of After review of the medical records and additional laboratory data, test sets were re-classified as being positive for the CT if they had tissue or culture diagnosis from any time in the study period or positive ID or CF tests identified through any of these methods. Sensitivity, specificity, PPV, and NPV were then recalculated. The final analysis also included 1) the number of FP test sets that met the CSTE clinical case definition, 2) the number of FP test sets that were associated with a physician diagnosis of coccidioidomycosis, and 3) whether the test sets were positive for EIA IgM, IgG, or both. Results Validation of public health reporting in Arizona The results of the validation study are found in Figure 2. Laboratory A reported positive results for 664 patients during this time. Of those, 509 matched with MEDSIS data ( ) and 73 matched with the older surveillance data ( ). After examining the remaining 82 Laboratory A results by hand, 69 matched. The primary reasons for why results did not originally match the surveillance data included name misspellings, inclusion or exclusion of a middle initial in the name fields, and discrepant birthdates that appear to be typographical errors. The final validation for Laboratory A 14
16 showed a positive match for 651 of 664 (98%) patients with positive tests reported. Laboratory B reported positive results for 517 patients during this time. Of these, 360 matched with MEDSIS data ( ) and 36 matched with the older surveillance data ( ). After examining the remaining Laboratory B results by hand, 41 of 121 matched. The primary reasons for why lab results did not originally match the surveillance data were similar to those for Laboratory A and included name misspellings, inclusion or exclusion of a middle initial in the name fields, and discrepant birthdates that appear to be typographical errors. The final validation for Laboratory B showed a positive match for 427 of 517 (85%) patients with positive tests reported. Of the thirteen test results that did not match from Laboratory A, most were EIA tests, either IgM or IgG, and were mostly in the months of March and May. The unreported positive tests from Laboratory B, which had a greater number unmatched than Laboratory A, were spread evenly across the three months. However, the type of test among the unreported results was more likely to be EIA (89%) and less likely to be immunodiffusion (5%) compared to the matched tests (67% and 22%, respectively). Specificity of EIA compared to immunodiffusion or complement fixation There were 3942 CF tests, 3867 ID IgM tests, 6486 ID IgG tests, 18,750 EIA IgM tests, 18,698 EIA IgG tests, and 18,617 combined EIA IgM and IgG tests available for review. A total of 1445 lab test sets met the inclusion criteria. A total of 125 false positives (FPs) were identified and their associated clinical records were requested and reviewed. We received all 125 records that we requested. Of those, 31 (25%) were re-classified as true coccidioidomycosis cases with tissue/culture diagnosis and/or a positive serologic comparison test (CT) reported in the clinical record or identified in review of the additional laboratory data (Figure 3). The 94 remaining FPs were used to calculate sensitivity, specificity, PPV, and NPV (Table 2). EIA sensitivity equaled 83.8%; EIA specificity equaled 92.6%; positive predictive value equaled 61.5%; and negative predictive value equaled 97.6%. 15
17 Clinical chart review of the 94 FP results revealed that 92 (97.9%) were associated with documented coccidioidomycosis symptoms, and 76 (80.9%) were associated with documented physician-diagnosed disease. In the 18 cases without documented physician-diagnosed disease, the physician had not yet given the patient a diagnosis or it was not documented in the medical record. Further laboratory review of the 2009 data from Lab Corp revealed that 30/94 (32%) had subsequent coccidioidomycosis serology testing done at Lab Corp in Of those 30, 25 had at least one comparison test run, which were all negative, 3/30 (10%) had both positive EIA tests, 10/30 (33%) had one positive EIA test, and 17/30 (57%) had all negative tests. Further laboratory review of the 2010 data from Lab Corp revealed that only 9/94 (10%) FP had coccidioidomycosis serology testing done, including comparison tests run, at Lab Corp in Of those 9, none had any positive CT, 2/9 (22%) had one positive EIA test, and 7/9 (78%) had all negative tests. Discussion This is the largest investigation of EIA specificity for coccidioidomycosis diagnosis and the only validation of laboratory reporting of coccidioidomycosis in Arizona. Based on laboratory tests alone, the EIA specificity is calculated to be 93% with a PPV of 62%, and 85% of positive coccidioidomycosis laboratory tests performed at a major commercial laboratory were reported to public health as mandated by statute. The calculated EIA specificity and PPV from this investigation are lower than those previously reported in the literature, which range from 96%-100% and 96%-98% for specificity and PPV, respectively (3, 12, 14, 23). The lower EIA specificity and PPV from our investigation is likely due to the epidemiologic design of the study and the fact that there is no known gold standard for the diagnosis of coccidioidomycosis, especially in early disease. In our investigation, there were 18,617 specimens where both EIAs were run. A total of 7.7% are positive for IgM or IgG. In the final data set, however, there are 1445 specimens, and 16.9% are positive. Our methods apparently select for positive EIA tests, which might mean we are missing a lot from the negative EIA column, which would drive down specificity, since the positivity among 16
18 the CTs does not seem as much affected. Additionally, because all coccidioidomycosis serology tests from February 2008 through February 2009 were reviewed for inclusion in the analysis, rather than testing serum from selected patients with known disease and known absence of disease, it is much easier for misclassification bias to occur with an epidemiologic design than a laboratory design. Previous studies have been based on a laboratory design using serum from patients with known coccidioidomycosis diagnosis (confirmed by a serologic test, tissue or culture) as a gold standard. In our investigation, patients presenting to their clinicians early in the disease process are much more likely to have falsely negative serology results because it can take up to several weeks for the body to mount an immune response. These patients would have to return for a repeat test in order to be accurately identified as a case. Further, if the EIA does become positive earlier than ID, which is suggested by some studies (3, 22), any case, which is tested during this window period, would have a positive EIA and negative comparison test and be misclassified as a FP in our investigation. In a laboratory design, patients who are further along in the disease process can be selected to ensure they would test positive by ID. Additionally, because this was done as part of public health surveillance, patients with FP results could not be contacted and asked to obtain repeat testing to ensure they had not seroconverted. Due to the fact that no sensitive and specific gold standard laboratory test for coccidioidomycosis exists (other than culture, which is rarely obtained), we reviewed the medical records associated with all FP results to determine if subsequent coccidioidomycosis diagnostic testing had been performed and to determine if the patient had clinical illness consistent with coccidioidomycosis and/or a physician diagnosis of disease. This review revealed that 25% of false positive EIA results represented laboratory confirmed disease. Additionally, almost all patients with FP results had a clinical illness consistent with the disease and >80% had a physician s diagnosis of coccidioidomycosis. In the cases without documented physician-diagnosed disease, the physician had not yet given the patient a diagnosis or it was not documented in the medical record. Unfortunately, the latter information cannot be included in the specificity or PPV calculations because we do not know whether the physician diagnosed coccidioidomycosis on the basis of the FP EIA test result or based on some other clinical suspicion or a combination of both. 17
19 It is concerning that major commercial laboratories continue to use EIA as a screening test and report only those with positive ID results as positive, both to the ordering physician and to public health. It is also concerning that this practice is recommended by the EIA kit manufacturer (15). The result of this practice is that at least 1 in 4 individuals with a positive EIA and negative ID are actually infected with coccidioidomycosis and are likely misdiagnosed. Further, of the 94 remaining false positive EIA results in our investigation, 21 (22%) were positive for both IgM and IgG, which increases the likelihood that they represent true disease, since it is unlikely that both tests would be falsely positive (12). Of the 31 FPs that were subsequently confirmed as positive cases based on a comparison test, 26 (94%) had a positive EIA IgG and 8 (26%) were positive for both EIA IgG and IgM. EIA IgG has been shown to be more specific than EIA IgM when done in isolation (98.3% vs. 84.6%) (23). Based on the increased specificity of EIA IgG, the 54 (57%) false positives that were positive for EIA IgG are more likely to represent actual disease (probably early in the course of disease). Finally, Blair and Currier investigated 28 patients with isolated EIA IgM positive serology results and negative ID results among patients suspected of having coccidioidomycosis and found that all 28 eventually developed laboratory confirmed disease either by subsequent ID testing, or based on microbiologic or histopathologic evidence of disease (3). All of this information supports that the specificity calculated from our epidemiological dataset is likely an underestimate of the true EIA specificity for coccidioidomycosis diagnosis. Other than the limitation regarding a lack of CT described previously, our investigation has several additional limitations. First, upon reviewing the 2009 and 2010 data for repeat comparison tests, only 30 (in 2009) and 9 (in 2010) of 94 individuals with FP results had repeat testing done at Lab Corp during that time period. The absence of repeat serology data prevented a final determination of whether the FP tests represented true disease. This could be due to the fact that the patients stopped getting coccidioidomycosis serology testing done, or they were tested at a different laboratory. Secondly, serologic test results were only reviewed from one major laboratory in Arizona, and due to differences between laboratories in testing methodologies, correlation with other laboratories results is needed. Thus, these results may not be generalizable to all laboratories in Arizona. 18
20 For all of the reasons cited above, the calculated specificity and PPV of EIA for the diagnosis of coccidioidomycosis in this investigation likely represent underestimates of the true values. The findings of our investigation support those of Blair and Currier and suggest that serologic testing for coccidioidomycosis should always be performed in combination. Further, use of EIA as a screening test that is only reported as positive when confirmed with ID leads to missed diagnoses, which is a disservice to patients, physicians, and public health. For patients with clinical illness consistent with coccidioidomycosis and isolated EIA positivity, clinicians should consider close observation and repeat serologic testing or performance of other diagnostic methods such as culture or biopsy. Furthermore, clinicians should inform patients of positive EIA results irrespective of CF/ID results and explain that further laboratory testing and monitoring of the illness may be needed to establish a diagnosis. Future Directions Once completed, this project will not have to be repeated by the same researchers. Correlation with other laboratories results is needed along with an evaluation of the impact of the results within the community. Recommendations to laboratories about how to report coccidioidomycosis serological testing results along with instructions to physicians about how to interpret the reported results are also necessary. Conclusions Based on the largest study of sensitivity and specificity calculated from laboratory surveillance data, EIA sensitivity and specificity for coccidioidomycosis diagnosis are lower than previously reported using only coccidioidomycosis laboratory tests as comparison tests. However, 25% of positive EIA results with negative comparison tests were found to have subsequent confirmatory test results, suggesting that single immunodiffusion or complement fixation tests are not a sufficient comparison tests/gold standards for coccidioidomycosis diagnosis. Association of false positive EIA results 19
21 with coccidioidomycosis symptoms suggests that some of these may represent missed diagnoses. Repeat serologic or other coccidioidomycosis diagnostic testing for patients with isolated positive EIA test results may improve EIA diagnostic utility. 20
22 References 1. ADHS Office of Infectious Disease Services (2011). Infectious Disease Epidemiology, Coccidioidomycosis: Frequently Asked Questions. Retrieved from 2. ADHS Office of Infectious Disease (2008). Valley Fever Annual Report Retrieved from 3. Blair, J. E. & Currier, J. T. (2008). Significance of isolated positive IgM serologic results by enzyme immunoassay for coccidioidomycosis. Mycopathologia, 166, Centers for Disease Control and Prevention (CDC) Division of Foodborne, Bacterial and Mycotic Diseases (DFBMD) (2010). Coccidioidomycosis. Retrieved from 5. Centers for Disease Control and Prevention (CDC) (2003). Increase in coccidioidomycosis--arizona, MMWR - Morbidity & Mortality Weekly Report, 52(6), Chiller, T. M., Galgiani, J. N., & Stevens, D. A. (2003). Coccidioidomycosis. Infectious Disease Clinics of North America, 17, Crum, N. F., Lederman, E. R., Stafford, C. M., Parrish, J. S., & Wallace, M. R. (2004). Coccidioidomycosis: A descriptive survey of a reemerging disease. Clinical characteristics and current controversies. Medicine, 83(3), Crum, N. F., Potter, M., & Pappagianis, D. (2004). Seroincidence of coccidioidomycosis during military desert training exercises. Journal of Clinical Microbiology, 42(10), Gade, W., Ledman, D. W., Wethington, R., & Yi, A. (1992). Serological responses to various coccidioides antigen preparations in a 21
23 new enzyme immunoassay. Journal of Clinical Microbiology, 30(8), Galgiani, J. N. (1993). Coccidioidomycosis. The Western Journal of Medicine, 159(2), Kaufman, L., & Clark, M. J. (1974). Value of the concomitant use of complement fixation and immunodiffusion tests in the diagnosis of coccidioidomycosis. Applied Microbiology, 28(4), Kaufman, L., Sekhon, A.S., Moledina, N., Jalbert, M., & Pappagianis, D. (1995). Comparative evaluation of commercial premier EIA and microimmunodiffusion and complement fixation tests for Coccidioides immitis antibodies. Journal of Clinical Microbiology, 33(3), Kirkland, T. N., & Fierer, J. (1996). Coccidioidomycosis: A reemerging infectious disease. Emerging Infectious Diseases, 2(3), Martins, T. B., Jaskowski, T. D., Mouritsen, C. L., & Hill, H. R. (1995). Comparison of commercially available enzyme Immunoassay with traditional serological tests for detection of antibodies to Coccidioides immitis. Journal of Clinical Microbiology, 33(4), Meridian Bioscience Incorporated (2011). Package Insert: Premier Coccidioides EIA. Catalogue Number Pappagianis, D., & Zimmer, B. L. (1990). Serology of coccidioidomycosis. Clinical Microbiology Reviews, 3(3), Saubolle, M. A. (2007). Laboratory aspects in the diagnosis of coccidioidomycosis. Annals of the New York Academy of Sciences, 1111, Saubolle, M. A., McKellar, P. P., & Sussland, D. (2007). Epidemiologic, clinical, and diagnostic aspects of coccidioidomycosis. Journal of Clinical Microbiology, 45(1),
24 19. Smith, C. E., Saito, M. T., & Simons, S. A. (1956). Pattern of 39,500 serologic tests in coccidioidomycosis. Journal of the American Medical Association, 160(7), Sunenshine, R. H., Anderson, S., Erhart, L., Vossbrink, A., Kelly, P. C., Engelthaler, D., et al. (2007). Public health surveillance for coccidioidomycosis in Arizona. Annals of the New York Academy of Sciences, 1111, Tsang, C. A., Anderson, S. M., Imholte, S. B., Erhart, L. M., Chen, S., Park, B. J., Christ, C., Komatsu, K. K., Chiller, T., & Sunenshine, R. H. (2010). Enhanced Surveillance of Coccidioidomycosis, Arizona, USA, , Volume 16, Number 11, Wieden, M. A., Lundegan, L. L., Blum, J., Delgado, K. L., Coolbaugh, R., Howard, R., et al. (1996). Detection of coccidioidal antibodies by 33-kDa spherule antigen, Coccidiodes EIA and standard serologic tests in sera from patients evaluated for coccidioidomycosis. Journal of Infectious Diseases, 173, Zartarian, M., Peterson, E. M., & de la Maza, L. M. (1997). Detection of antibodies to Coccidioides immitis by enzyme immunoassay. American Journal of Clinical Pathology, 107(2),
25 Appendix Figure 1. Rates of Reported Valley Fever (VF) in Arizona, Reported cases per 100, Year of Onset or Diagnosis 24
26 Table 1. Learning objectives and implementation plan Learning objectives Implementation plan Describe the impact of coccidioidomycosis in AZ and why establishing a diagnosis is difficult. Establish the sensitivity and specificity of EIA for coccidioidomycosis diagnosis compared to ID and CF as the comparison tests. Determine the positive predictive value of EIA by comparing it to a combination of ID and CF, as well as clinical symptoms as the comparison tests. Specify the best way for laboratories to report diagnostic testing results for coccidioidomycosis to healthcare providers. Gain knowledge of the process and strengths and limitations in collecting and analyzing reported laboratory data to the state health department. Gain knowledge on the process and strengths and limitations in collecting and analyzing clinical data reported to the state health department. 1. Conduct a literature search. 2. Learn about the various diagnostic methods. 1. Request data from selected laboratories. 2. Clean, validate, and analyze the data. 3. Compare the results with published studies. 1. Create a clinical review form. 2. Extract data from selected records. 3. Recalculate the PPV using the results from the clinical review. 1. Report the results of the study to the participating laboratory directors with recommendations. 1. Request data from selected laboratories. 2. Clean, validate, and analyze the data. 3. Compare the results with published studies. 1. Request data from selected providers. 2. Create a form to extract the data from the records. 3. Analyze the data. 4. Compare the results with published studies. 25
27 Learning objectives Construct a database and create a statistical program to calculate true and false positives and true and false negatives to determine sensitivity, specificity, PPV, and NPV. Implementation plan 1. Analyze laboratory data to determine which variables need to be included in the database. 2. Meet with a biostatistician to create a statistical program. 3. Define sensitivity/specificity/ppv/npv. 26
28 Figure 2. Diagram of matching schema for public health reporting validation for laboratories A and B Laboratory A 517 persons with positive tests Laboratory B 664 persons with positive tests 360 MEDSIS matches 157 did not match MEDSIS 509 MEDSIS matches 155 did not match MEDSIS 36 historic data matches 121 did not match historic data 73 historic data matches 82 did not match historic data 41 hand matches 69 hand matches 80 did not match by hand 13 did not match by hand 27
29 Figure 3. Summary of false positive enzyme immunoassay results 125 FP based on one test set 94 (75%) FP 31 (25%) with confirmatory laboratory tests from medical record 21 (22%) IgG pos, IgM pos 40 (43%) IgG neg, IgM pos 33 (35%) IgG pos, IgM neg 8 (26%) IgG pos, IgM pos 2 (6%) IgG neg, IgM pos 21 (68%) IgG pos, IgM neg FP = false positive IgG = immunoglobulin G IgM = immunoglobulin M pos = positive neg = negative 28
30 Table 2. Enzyme immunoassay compared to comparison tests CT positive CT negative Total (CF/ID) (CF/ID) EIA positive EIA negative Total EIA = enzyme immunoassay CT = comparison test CF = complement fixation ID = immunodiffusion 29
31 30
Title: Public Health Reporting and National Notification for Coccidioidomycosis
 10-ID-04 Committee: Infectious Diseases Title: Public Health Reporting and National Notification for Coccidioidomycosis I. Statement of the Problem: In 1994, CSTE passed a resolution recommending key federal
10-ID-04 Committee: Infectious Diseases Title: Public Health Reporting and National Notification for Coccidioidomycosis I. Statement of the Problem: In 1994, CSTE passed a resolution recommending key federal
Overview of Coccidioidomycosis (Valley Fever) Stephen Munday, MD MPH Imperial County Public Health May 21, 2013
 Overview of Coccidioidomycosis (Valley Fever) Stephen Munday, MD MPH Imperial County Public Health May 21, 2013 Objectives Assess the epidemiology of Coccidioidomycosis (aka Valley Fever) Describe the
Overview of Coccidioidomycosis (Valley Fever) Stephen Munday, MD MPH Imperial County Public Health May 21, 2013 Objectives Assess the epidemiology of Coccidioidomycosis (aka Valley Fever) Describe the
Mike Olbinski. Mike Olbinski. Michael Olbinski
 Mike Olbinski Mike Olbinski Michael Olbinski Table of Contents List of Figures and Tables... 3 Executive Summary... 4 Introduction... 5 Epidemiology in Arizona... 7 Reporting Sources and Changes in Laboratory
Mike Olbinski Mike Olbinski Michael Olbinski Table of Contents List of Figures and Tables... 3 Executive Summary... 4 Introduction... 5 Epidemiology in Arizona... 7 Reporting Sources and Changes in Laboratory
Coccidioidomycosis, aka Valley Fever
 Coccidioidomycosis, aka Valley Fever Presented to the California Partnership for the San Joaquin Valley June 15, 2018 Karen Haught, MD, MPH Public Health Officer Tulare County Public Health Coccidioidomycosis
Coccidioidomycosis, aka Valley Fever Presented to the California Partnership for the San Joaquin Valley June 15, 2018 Karen Haught, MD, MPH Public Health Officer Tulare County Public Health Coccidioidomycosis
John Galgiani MD or Fariba Donovan MD PhD Banner University Valley Fever Program
 John Galgiani MD or Fariba Donovan MD PhD Banner University Valley Fever Program Drs. Galgiani and Donovan Have no conflicts of interest to disclose Caused by soil fungi Coccidioides immitis Coccidioides
John Galgiani MD or Fariba Donovan MD PhD Banner University Valley Fever Program Drs. Galgiani and Donovan Have no conflicts of interest to disclose Caused by soil fungi Coccidioides immitis Coccidioides
Title Coccidioidomycosis (Valley fever) case data for the southwestern United States
 Open Health Data: 1. Overview Title Coccidioidomycosis (Valley fever) case data for the southwestern United States Paper Authors 1. Gorris, Morgan E.; (Lead/corresponding author) 2. Cat, Linh A.; 3. Matlock,
Open Health Data: 1. Overview Title Coccidioidomycosis (Valley fever) case data for the southwestern United States Paper Authors 1. Gorris, Morgan E.; (Lead/corresponding author) 2. Cat, Linh A.; 3. Matlock,
Infectious Diseases-HAI, Infectious Diseases Arizona Department of Health Services, Public Health Services. Phoenix, Arizona. Assignment Description
 Infectious Diseases-HAI, Infectious Diseases Arizona Department of Health Services, Public Health Services Phoenix, Arizona Assignment Description The Fellow will be situated in the the Office of Infectious
Infectious Diseases-HAI, Infectious Diseases Arizona Department of Health Services, Public Health Services Phoenix, Arizona Assignment Description The Fellow will be situated in the the Office of Infectious
DRUG INFORMATION ALERT
 Paul Davis From: Sent: To: Subject: TSHP Friday, December 20, 2013 4:03 AM paul.davis@tshp.org 12-20-2013 Drug Info Alert - Unseen Danger: Dust-Born Disease DRUG INFORMATION ALERT
Paul Davis From: Sent: To: Subject: TSHP Friday, December 20, 2013 4:03 AM paul.davis@tshp.org 12-20-2013 Drug Info Alert - Unseen Danger: Dust-Born Disease DRUG INFORMATION ALERT
Coccidioidomycosis Update for Primary Care
 Coccidioidomycosis Update for Primary Care SW Conference on Medicine April 26, 2018 John N Galgiani MD University of Arizona What Is? Caused by soil fungi Coccidioides immitis Coccidioides posadasii Other
Coccidioidomycosis Update for Primary Care SW Conference on Medicine April 26, 2018 John N Galgiani MD University of Arizona What Is? Caused by soil fungi Coccidioides immitis Coccidioides posadasii Other
The Public Health Impact of Coccidioidomycosis in Arizona and California
 Int. J. Environ. Res. Public Health 2011, 8, 1150-1173; doi:10.3390/ijerph8041150 OPEN ACCESS Article International Journal of Environmental Research and Public Health ISSN 1660-4601 www.mdpi.com/journal/ijerph
Int. J. Environ. Res. Public Health 2011, 8, 1150-1173; doi:10.3390/ijerph8041150 OPEN ACCESS Article International Journal of Environmental Research and Public Health ISSN 1660-4601 www.mdpi.com/journal/ijerph
Coping with Valley Fever Information for Families
 Coping with Valley Fever Information for Families Valley Children s Healthcare & University of California, Merced 9300 Valley Children s Place Madera, CA 93636 Social Worker: Margie Arreguin-Zarate, LCSW
Coping with Valley Fever Information for Families Valley Children s Healthcare & University of California, Merced 9300 Valley Children s Place Madera, CA 93636 Social Worker: Margie Arreguin-Zarate, LCSW
California digging. Task 1 Interpret the chest radiograph. L. Hendriks
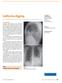 Case history An18-yr-old male presented at the emergency department with a 4-week history of fever, coughing and dyspnoea. Symptoms presented on a holiday in California (USA) searching the desert for fossils.
Case history An18-yr-old male presented at the emergency department with a 4-week history of fever, coughing and dyspnoea. Symptoms presented on a holiday in California (USA) searching the desert for fossils.
COCCIDIOIDES SPP. EXPOSURE/INJURY RESPONSE PROTOCOL
 PLEASE POST THIS PAGE IN AREAS WHERE COCCIDIOIDES IS USED IN RESEARCH LABORATORIES UNIVERSITY OF CALIFORNIA, SAN FRANCISCO ENVIRONMENTAL HEALTH AND SAFETY/BIOSAFETY COCCIDIOIDES SPP. EXPOSURE/INJURY RESPONSE
PLEASE POST THIS PAGE IN AREAS WHERE COCCIDIOIDES IS USED IN RESEARCH LABORATORIES UNIVERSITY OF CALIFORNIA, SAN FRANCISCO ENVIRONMENTAL HEALTH AND SAFETY/BIOSAFETY COCCIDIOIDES SPP. EXPOSURE/INJURY RESPONSE
Council of State and Territorial Epidemiologists Position Statement
 04-ID-01 Committee: Title: Infectious Disease Revision of the National Surveillance Case Definition of Diseases Caused by Neurotropic Domestic Arboviruses, Including the Addition to the NNDSS of Non-Neuroinvasive
04-ID-01 Committee: Title: Infectious Disease Revision of the National Surveillance Case Definition of Diseases Caused by Neurotropic Domestic Arboviruses, Including the Addition to the NNDSS of Non-Neuroinvasive
3/16/16 COCCIDIOIDOMYCOSIS COCCIDIOIDOMYCOSIS COCCIDIOIDOMYCOSIS COCCIDIOIDOMYCOSIS COCCIDIOIDOMYCOSIS COCCIDIOIDOMYCOSIS. Selected References
 10 MARCH 2006 Updated 5 June 2008 Updated 11 February 2011 Updated 4 March 2016 Herbert Boro, M.D., F.A.C.P. Selected References 1. Fiese, Marshall J. 1958. Coccidioidomycosis. Charles C. Thomas. 253 pp.
10 MARCH 2006 Updated 5 June 2008 Updated 11 February 2011 Updated 4 March 2016 Herbert Boro, M.D., F.A.C.P. Selected References 1. Fiese, Marshall J. 1958. Coccidioidomycosis. Charles C. Thomas. 253 pp.
Appendix B: Provincial Case Definitions for Reportable Diseases
 Infectious Diseases Protocol Appendix B: Provincial Case Definitions for Reportable Diseases Disease: Influenza Revised December 2014 Influenza 1.0 Provincial Reporting Confirmed cases of disease 2.0 Type
Infectious Diseases Protocol Appendix B: Provincial Case Definitions for Reportable Diseases Disease: Influenza Revised December 2014 Influenza 1.0 Provincial Reporting Confirmed cases of disease 2.0 Type
Rapid PCR TB Testing Results in 68.5% Reduction in Unnecessary Isolation Days in Smear Positive Patients.
 Rapid PCR TB Testing Results in 68.5% Reduction in Unnecessary Isolation Days in Smear Positive Patients. Item Type Thesis Authors Patel, Ravikumar Publisher The University of Arizona. Rights Copyright
Rapid PCR TB Testing Results in 68.5% Reduction in Unnecessary Isolation Days in Smear Positive Patients. Item Type Thesis Authors Patel, Ravikumar Publisher The University of Arizona. Rights Copyright
West Nile Virus in Maricopa County
 West Nile Virus in Maricopa County Culex larvae found collecting in standing water Image by CDC/James Gathany - License: Public Domain. Maricopa County Department of Public Health Office of Epidemiology
West Nile Virus in Maricopa County Culex larvae found collecting in standing water Image by CDC/James Gathany - License: Public Domain. Maricopa County Department of Public Health Office of Epidemiology
Validation of communicable disease reporting from hospitals using the hospital discharge database, Arizona,
 Validation of communicable disease reporting from hospitals using the hospital discharge database, Arizona, 2007 2009 (Poster is shared here as an 8.5 x11 document for easier viewing. All content is identical.)
Validation of communicable disease reporting from hospitals using the hospital discharge database, Arizona, 2007 2009 (Poster is shared here as an 8.5 x11 document for easier viewing. All content is identical.)
Early Treatment With Fluconazole May Abrogate the Development of IgG Antibodies in Coccidioidomycosis
 MAJOR ARTICLE Early Treatment With Fluconazole May Abrogate the Development of IgG Antibodies in Coccidioidomycosis George R. Thompson III, 1,2 Jennine M. Lunetta, 1 Suzanne M. Johnson, 1 Sandra Taylor,
MAJOR ARTICLE Early Treatment With Fluconazole May Abrogate the Development of IgG Antibodies in Coccidioidomycosis George R. Thompson III, 1,2 Jennine M. Lunetta, 1 Suzanne M. Johnson, 1 Sandra Taylor,
Alberta Health and Wellness Public Health Disease Under Surveillance Management Guidelines March 2011
 March 2011 Histoplasmosis Case Definition Confirmed Case Clinical illness [1] with laboratory confirmation of infection: Isolation of Histoplasma capsulatum from an appropriate clinical specimen (tissue
March 2011 Histoplasmosis Case Definition Confirmed Case Clinical illness [1] with laboratory confirmation of infection: Isolation of Histoplasma capsulatum from an appropriate clinical specimen (tissue
In the setting of measles elimination in the United States, the current measles case definition lacks specificity.
 12-ID-07 Committee: Infectious Disease Title: Public Health Reporting and ational otification for Measles I. Statement of the Problem In the setting of measles elimination in the United States, the current
12-ID-07 Committee: Infectious Disease Title: Public Health Reporting and ational otification for Measles I. Statement of the Problem In the setting of measles elimination in the United States, the current
LYME DISEASE Last revised May 30, 2012
 Wisconsin Department of Health Services Division of Public Health Communicable Disease Surveillance Guideline LYME DISEASE Last revised May 30, 2012 I. IDENTIFICATION A. CLINICAL DESCRIPTION: A multi-systemic
Wisconsin Department of Health Services Division of Public Health Communicable Disease Surveillance Guideline LYME DISEASE Last revised May 30, 2012 I. IDENTIFICATION A. CLINICAL DESCRIPTION: A multi-systemic
Incidence rate per 100,000 persons Year FIGURE 1. The incidence of coccidioidal infection in
 Coccidioidomycosis CONCISE REVIEW FOR COCCIDIOIDAL CLINICIANS INFECTION JAMES M. PARISH, MD, AND JANIS E. BLAIR, MD On completion of this article, you should be able to (1) describe the geographic areas
Coccidioidomycosis CONCISE REVIEW FOR COCCIDIOIDAL CLINICIANS INFECTION JAMES M. PARISH, MD, AND JANIS E. BLAIR, MD On completion of this article, you should be able to (1) describe the geographic areas
West Nile Virus in Maricopa County
 West Nile Virus in Maricopa County A Culex quinquefasciatus mosquito on a human finger. Image by James Gathany/ CDC gov/ public domain Maricopa County Department of Public Health Office of Epidemiology
West Nile Virus in Maricopa County A Culex quinquefasciatus mosquito on a human finger. Image by James Gathany/ CDC gov/ public domain Maricopa County Department of Public Health Office of Epidemiology
Management of Coccidioidomycosis in Rheumatic Patients on Biologic Response Modifiers
 Management of Coccidioidomycosis in Rheumatic Patients on Biologic Response Modifiers Dominick George Sudano, MD Assistant Professor of Medicine Department of Rheumatology University of Arizona Objectives
Management of Coccidioidomycosis in Rheumatic Patients on Biologic Response Modifiers Dominick George Sudano, MD Assistant Professor of Medicine Department of Rheumatology University of Arizona Objectives
Coccidioidomycosis in Patients with Diabetes Mellitus
 The American Journal of Medicine (2006) 119, 964-969 CLINICAL RESEARCH STUDY AJM Theme Issue: Infectious Disease Coccidioidomycosis in Patients with Diabetes Mellitus Ana C. Santelli, MD, a Janis E. Blair,
The American Journal of Medicine (2006) 119, 964-969 CLINICAL RESEARCH STUDY AJM Theme Issue: Infectious Disease Coccidioidomycosis in Patients with Diabetes Mellitus Ana C. Santelli, MD, a Janis E. Blair,
West Nile Virus in Maricopa County
 West Nile Virus in Maricopa County Maricopa County Department of Public Health Office of Epidemiology July 21 January 1, 29 December 31, 29 Commentary West Nile virus (WNV) is a mosquito-borne virus that
West Nile Virus in Maricopa County Maricopa County Department of Public Health Office of Epidemiology July 21 January 1, 29 December 31, 29 Commentary West Nile virus (WNV) is a mosquito-borne virus that
Value o f Immunodiffusion Tests in the Diagnosis o f Systemic M ycotic Diseases
 A n n a l s of C l i n i c a l L a b o r a t o r y S c i e n c e, Vol. 3, N o. 2 Copyright 1 9 7 3, Institute for Clinical Science Value o f Immunodiffusion Tests in the Diagnosis o f Systemic M ycotic
A n n a l s of C l i n i c a l L a b o r a t o r y S c i e n c e, Vol. 3, N o. 2 Copyright 1 9 7 3, Institute for Clinical Science Value o f Immunodiffusion Tests in the Diagnosis o f Systemic M ycotic
West Nile Virus in Maricopa County
 West Nile Virus in Maricopa County Maricopa County Department of Public Health Office of Epidemiology July 2009 January 1, 2008 December 31, 2008 Commentary West Nile virus (WNV) is a mosquito-borne virus
West Nile Virus in Maricopa County Maricopa County Department of Public Health Office of Epidemiology July 2009 January 1, 2008 December 31, 2008 Commentary West Nile virus (WNV) is a mosquito-borne virus
Coccidioidomycosis in Infants: A Retrospective Case Series
 Coccidioidomycosis in Infants: A Retrospective Case Series Jessica M. Lee, MD, 1,2 * Ana Lia Graciano, MD, 1,3 Lukasz Dabrowski, MD, 1,4 Brenik Kuzmic, PharmD, 5 and Mary Anne Tablizo, MD 1,6 Summary.
Coccidioidomycosis in Infants: A Retrospective Case Series Jessica M. Lee, MD, 1,2 * Ana Lia Graciano, MD, 1,3 Lukasz Dabrowski, MD, 1,4 Brenik Kuzmic, PharmD, 5 and Mary Anne Tablizo, MD 1,6 Summary.
09-ID-67. Committee: Infectious. Title: Public Health Reporting and National Notification for Typhoid Fever. I. Statement of the Problem
 09-ID-67 Committee: Infectious Title: Public Health Reporting and National Notification for Typhoid Fever I. Statement of the Problem CSTE position statement 07-EC-02 recognized the need to develop an
09-ID-67 Committee: Infectious Title: Public Health Reporting and National Notification for Typhoid Fever I. Statement of the Problem CSTE position statement 07-EC-02 recognized the need to develop an
STARK COUNTY INFLUENZA SNAPSHOT, WEEK 15 Week ending 18 April, With updates through 04/26/2009.
 STARK COUNTY INFLUENZA SNAPSHOT, WEEK 15 Week ending 18 April, 29. With updates through 4/26/29. During week 15, countywide, state and national indicators confirmed very low markers of seasonal influenza
STARK COUNTY INFLUENZA SNAPSHOT, WEEK 15 Week ending 18 April, 29. With updates through 4/26/29. During week 15, countywide, state and national indicators confirmed very low markers of seasonal influenza
ARIZONA INFLUENZA SUMMARY
 ARIZONA INFLUENZA SUMMARY Week 4 (1/21/2018 1/27/2018) Synopsis: Influenza activity is elevated. Arizona reported Widespread Activity for week 4. Subscribe to the Flu & RSV report at azhealth.gov/email.
ARIZONA INFLUENZA SUMMARY Week 4 (1/21/2018 1/27/2018) Synopsis: Influenza activity is elevated. Arizona reported Widespread Activity for week 4. Subscribe to the Flu & RSV report at azhealth.gov/email.
Infectious Disease Update
 Infectious Disease Update Karen Rose, RN BSN Communicable Disease Surveillance Nurse; Office of Epidemiology WeArePublicHealth.org twitter.com/maricopahealth facebook.com/mcdph Today s Updates: Influenza
Infectious Disease Update Karen Rose, RN BSN Communicable Disease Surveillance Nurse; Office of Epidemiology WeArePublicHealth.org twitter.com/maricopahealth facebook.com/mcdph Today s Updates: Influenza
Use of Treponemal Immunoassays for Screening and Diagnosis of Syphilis
 Use of Treponemal Immunoassays for Screening and Diagnosis of Syphilis Guidance for Medical Providers and Laboratories in California These guidelines were developed by the California Department of Public
Use of Treponemal Immunoassays for Screening and Diagnosis of Syphilis Guidance for Medical Providers and Laboratories in California These guidelines were developed by the California Department of Public
Rubella rev Jan 2018
 rev Jan 2018 Infectious Agent virus (family Togaviridae; genus Rubivirus) BASIC EPIDEMIOLOGY Transmission is spread from person to person via direct or droplet contact shed from nasopharyngeal secretions
rev Jan 2018 Infectious Agent virus (family Togaviridae; genus Rubivirus) BASIC EPIDEMIOLOGY Transmission is spread from person to person via direct or droplet contact shed from nasopharyngeal secretions
Disseminated Coccidioidomycosis of the Rearfoot: A case report
 Open Access Publication Disseminated Coccidioidomycosis of the Rearfoot: A case report by Robert Frykberg, DPM, MPH 1 4 Tierney, DPM 2 3, Beth Noe, DPM, Stephanie Michael, DPM, Edward The Foot & Ankle
Open Access Publication Disseminated Coccidioidomycosis of the Rearfoot: A case report by Robert Frykberg, DPM, MPH 1 4 Tierney, DPM 2 3, Beth Noe, DPM, Stephanie Michael, DPM, Edward The Foot & Ankle
Polio (Paralytic and Non-paralytic
 Polio (Paralytic and Non-paralytic Infection) rev Jan 2018 Infectious Agent Poliovirus (genus Enterovirus) types, 1, 2, and 3. BASIC EPIDEMIOLOGY Transmission Poliovirus is transmitted by person-to-person
Polio (Paralytic and Non-paralytic Infection) rev Jan 2018 Infectious Agent Poliovirus (genus Enterovirus) types, 1, 2, and 3. BASIC EPIDEMIOLOGY Transmission Poliovirus is transmitted by person-to-person
Using Hospitalization Data To Estimate The Public Health Burden of Coccidioidomycosis in California
 Using Hospitalization Data To Estimate The Public Health Burden of Coccidioidomycosis in California V. Flaherman, R. Hector, G. Rutherford University of California, San Francisco Valley Fever Vaccine Project
Using Hospitalization Data To Estimate The Public Health Burden of Coccidioidomycosis in California V. Flaherman, R. Hector, G. Rutherford University of California, San Francisco Valley Fever Vaccine Project
Fungal Diseases of the Respiratory System
 Fungal Diseases of the Respiratory System Histoplasmosis(cave disease) Dr. Hala Al Daghistani Histoplasmosis is a disease caused by the fungus Histoplasma capsulatum. Histoplasma capsulatum, is usually
Fungal Diseases of the Respiratory System Histoplasmosis(cave disease) Dr. Hala Al Daghistani Histoplasmosis is a disease caused by the fungus Histoplasma capsulatum. Histoplasma capsulatum, is usually
Uphill both ways: Fatigue and quality of life in valley fever
 Medical Mycology, 2016, 54, 310 317 doi: 10.1093/mmy/myv097 Advance Access Publication Date: 26 November 2015 Original Article Original Article Uphill both ways: Fatigue and quality of life in valley fever
Medical Mycology, 2016, 54, 310 317 doi: 10.1093/mmy/myv097 Advance Access Publication Date: 26 November 2015 Original Article Original Article Uphill both ways: Fatigue and quality of life in valley fever
Influenza-Associated Pediatric Mortality rev Jan 2018
 rev Jan 2018 Infectious Agent Influenza A, B or C virus BASIC EPIDEMIOLOGY Transmission Transmission occurs via droplet spread. After a person infected with influenza coughs, sneezes, or talks, influenza
rev Jan 2018 Infectious Agent Influenza A, B or C virus BASIC EPIDEMIOLOGY Transmission Transmission occurs via droplet spread. After a person infected with influenza coughs, sneezes, or talks, influenza
NCCID RAPID REVIEW. 1. What are the case definitions and guidelines for surveillance and reporting purposes?
 NCCID RAPID REVIEW 1. What are the case definitions and guidelines for surveillance and reporting purposes? Middle East Respiratory Syndrome Coronavirus: Ten Questions and Answers for Canadian Public Health
NCCID RAPID REVIEW 1. What are the case definitions and guidelines for surveillance and reporting purposes? Middle East Respiratory Syndrome Coronavirus: Ten Questions and Answers for Canadian Public Health
ARIZONA INFLUENZA SUMMARY
 ARIZONA INFLUENZA SUMMARY Week 47 (11/19/2017 11/25/2017) Synopsis: Influenza activity is increasing. Arizona reported Local Activity for week 47. Influenza activity highlights: 2017 2018 Influenza Season
ARIZONA INFLUENZA SUMMARY Week 47 (11/19/2017 11/25/2017) Synopsis: Influenza activity is increasing. Arizona reported Local Activity for week 47. Influenza activity highlights: 2017 2018 Influenza Season
April 26, Typical symptoms include: cough - 85% nasal congestion - 81% nasal discharge - 70% sore throat - 52% fever - 44% headache - 30%
 April 26, 2012 RUBELLA ALERT! A case of rubella has been serologically confirmed in an adult male from the City of Milwaukee. The patient s rash onset was 4/20/2012. Additional information about the case
April 26, 2012 RUBELLA ALERT! A case of rubella has been serologically confirmed in an adult male from the City of Milwaukee. The patient s rash onset was 4/20/2012. Additional information about the case
A horrible, painful disease
 October 17, 2016 House Majority Leader Kevin McCarthy Phoenix, UA Med School - Arizona Republic - Tucson Arizona Star A horrible, painful disease Raising the Standard of Care David Larwood, MS/JD/MBA,
October 17, 2016 House Majority Leader Kevin McCarthy Phoenix, UA Med School - Arizona Republic - Tucson Arizona Star A horrible, painful disease Raising the Standard of Care David Larwood, MS/JD/MBA,
ISPUB.COM. Coccidioidomycosis In A Cancer Hospital. A Huaringa INTRODUCTION
 ISPUB.COM The Internet Journal of Pulmonary Medicine Volume 11 Number 2 Coccidioidomycosis In A Cancer Hospital A Huaringa Citation A Huaringa. Coccidioidomycosis In A Cancer Hospital. The Internet Journal
ISPUB.COM The Internet Journal of Pulmonary Medicine Volume 11 Number 2 Coccidioidomycosis In A Cancer Hospital A Huaringa Citation A Huaringa. Coccidioidomycosis In A Cancer Hospital. The Internet Journal
OCCCUPATIONAL COCCIDIOIDOMYCOSIS TWO CASE STUDIES. Mark Nicas, PhD, CIH School of Public Health University of California, Berkeley
 OCCCUPATIONAL COCCIDIOIDOMYCOSIS TWO CASE STUDIES Mark Nicas, PhD, CIH School of Public Health University of California, Berkeley VALLEY FEVER = COCCIDIOIDOMYCOSIS Infection in lungs from inhaled Coccidioides
OCCCUPATIONAL COCCIDIOIDOMYCOSIS TWO CASE STUDIES Mark Nicas, PhD, CIH School of Public Health University of California, Berkeley VALLEY FEVER = COCCIDIOIDOMYCOSIS Infection in lungs from inhaled Coccidioides
Histoplasmosis. Disease Etiology: Disease Transmission: Reservoirs: Specific Microbial Characteristics: By: Ernest Aguilar
 Histoplasmosis By: Ernest Aguilar Disease Etiology: Histoplasmosis is an infection resulting from the inhalation of spores belonging to the fungus Histoplasma capsulatum. [1] This one of three fungi that
Histoplasmosis By: Ernest Aguilar Disease Etiology: Histoplasmosis is an infection resulting from the inhalation of spores belonging to the fungus Histoplasma capsulatum. [1] This one of three fungi that
Precision Health in Valley Fever Does Ancestry Matter? John N Galgiani MD Valley Fever Center for Excellence University of Arizona
 Precision Health in Does Ancestry Matter? John N Galgiani MD University of Arizona Coccidioidomycosis Spectrum of Disease 100 Infections 60 No Symptoms 40 Symptoms 37 Recover Life-Long Immunity 100,000-
Precision Health in Does Ancestry Matter? John N Galgiani MD University of Arizona Coccidioidomycosis Spectrum of Disease 100 Infections 60 No Symptoms 40 Symptoms 37 Recover Life-Long Immunity 100,000-
Peggy Leslie-Smith, RN
 Peggy Leslie-Smith, RN EMPLOYEE HEALTH DIRECTOR - AVERA TRAINING CONTENT 1. South Dakota Regulations 2. Iowa Regulations 3. Minnesota Regulations 4. Interferon Gamma Release Assay (IGRA)Testing 1 SOUTH
Peggy Leslie-Smith, RN EMPLOYEE HEALTH DIRECTOR - AVERA TRAINING CONTENT 1. South Dakota Regulations 2. Iowa Regulations 3. Minnesota Regulations 4. Interferon Gamma Release Assay (IGRA)Testing 1 SOUTH
INFLUENZA (Outbreaks; hospitalized or fatal pediatric cases)
 INFLUENZA (Outbreaks; hospitalized or fatal pediatric cases) 1. Agent: Influenza viruses A, B, and C. Only influenza A and B are of public health concern since they are responsible for epidemics. 2. Identification:
INFLUENZA (Outbreaks; hospitalized or fatal pediatric cases) 1. Agent: Influenza viruses A, B, and C. Only influenza A and B are of public health concern since they are responsible for epidemics. 2. Identification:
Measles and Measles Vaccine
 Measles and Measles Vaccine Epidemiology and Prevention of Vaccine- Preventable Diseases Note to presenters: Images of vaccine-preventable diseases are available from the Immunization Action Coalition
Measles and Measles Vaccine Epidemiology and Prevention of Vaccine- Preventable Diseases Note to presenters: Images of vaccine-preventable diseases are available from the Immunization Action Coalition
U.S. Human Cases of Swine Flu Infection (As of April 29, 2009, 11:00 AM ET)
 Swine Flu Call Center Script 4/29/2009 3:00 PM SWINE FLU QUESTIONS What is swine flu? Swine Influenza, also called swine flu, is a respiratory disease of pigs caused by type A influenza viruses. Outbreaks
Swine Flu Call Center Script 4/29/2009 3:00 PM SWINE FLU QUESTIONS What is swine flu? Swine Influenza, also called swine flu, is a respiratory disease of pigs caused by type A influenza viruses. Outbreaks
Mosquitoborne Viral Diseases
 Mosquitoborne Viral Diseases Originally prepared by Tom J. Sidwa, D.V.M, M.P.H State Public Health Veterinarian Zoonosis Control Branch Manager Texas Department of State Health Services 1 AGENT Viruses
Mosquitoborne Viral Diseases Originally prepared by Tom J. Sidwa, D.V.M, M.P.H State Public Health Veterinarian Zoonosis Control Branch Manager Texas Department of State Health Services 1 AGENT Viruses
How many students at St. Francis Preparatory School in New York City have become ill or been confirmed with swine flu?
 Swine Flu Call Center Script SWINE FLU QUESTIONS What is swine flu? Swine Influenza, also called swine flu, is a respiratory disease of pigs caused by type A influenza viruses. Outbreaks of swine flu happen
Swine Flu Call Center Script SWINE FLU QUESTIONS What is swine flu? Swine Influenza, also called swine flu, is a respiratory disease of pigs caused by type A influenza viruses. Outbreaks of swine flu happen
Texas Influenza Summary Report, Season (September 28, 2008 April 11, 2009)
 Texas Influenza Summary Report, 2008 2009 Season (September 28, 2008 April 11, 2009) Background Influenza and influenza-like illnesses (ILI) were last reportable by law in any county in Texas in 1993 (1).
Texas Influenza Summary Report, 2008 2009 Season (September 28, 2008 April 11, 2009) Background Influenza and influenza-like illnesses (ILI) were last reportable by law in any county in Texas in 1993 (1).
Epidemiologic, Clinical, and Diagnostic Aspects of Coccidioidomycosis
 JOURNAL OF CLINICAL MICROBIOLOGY, Jan. 2007, p. 26 30 Vol. 45, No. 1 0095-1137/07/$08.00 0 doi:10.1128/jcm.02230-06 Copyright 2007, American Society for Microbiology. All Rights Reserved. Epidemiologic,
JOURNAL OF CLINICAL MICROBIOLOGY, Jan. 2007, p. 26 30 Vol. 45, No. 1 0095-1137/07/$08.00 0 doi:10.1128/jcm.02230-06 Copyright 2007, American Society for Microbiology. All Rights Reserved. Epidemiologic,
Syphilis Testing in Northern California Kaiser
 Syphilis Testing in Northern California Kaiser Jen Shieh, MS, CLS Test Development Scientist Kaiser Permanente TPMG Regional Laboratory Microbiology Department Kaiser Permanente 3.3 million members 22
Syphilis Testing in Northern California Kaiser Jen Shieh, MS, CLS Test Development Scientist Kaiser Permanente TPMG Regional Laboratory Microbiology Department Kaiser Permanente 3.3 million members 22
Coccidioidomycosis in Rheumatologic Patients. Susan E. Hoover, MD
 Coccidioidomycosis in Rheumatologic Patients Susan E. Hoover, MD The problem Prevalence of rheumatoid arthritis is 1% Population of Arizona is 6 million At least 150,000 new cocci infections yearly DMARDs
Coccidioidomycosis in Rheumatologic Patients Susan E. Hoover, MD The problem Prevalence of rheumatoid arthritis is 1% Population of Arizona is 6 million At least 150,000 new cocci infections yearly DMARDs
Situation update pandemic (H1N1) 2009
 Situation update pandemic (H1N1) 2009 February 2010 USPACOM/COE Pandemic Influenza Workshop Overview Overview of Events Pandemic (H1N1) 2009 Issues/Concerns 2 Overview of events April 12: an outbreak of
Situation update pandemic (H1N1) 2009 February 2010 USPACOM/COE Pandemic Influenza Workshop Overview Overview of Events Pandemic (H1N1) 2009 Issues/Concerns 2 Overview of events April 12: an outbreak of
Teenage Female With Chronic Coccidioidomycosis and Left Wrist Pain
 http://www.hcplive.com/journals/family practice recertification/2015/may2015/teenage Female With Chronic Coccidioidomycosis and Left Wrist Pain Teenage Female With Chronic Coccidioidomycosis and Left Wrist
http://www.hcplive.com/journals/family practice recertification/2015/may2015/teenage Female With Chronic Coccidioidomycosis and Left Wrist Pain Teenage Female With Chronic Coccidioidomycosis and Left Wrist
Yersiniosis rev Apr 2017
 rev Apr 2017 BASIC EPIDEMIOLOGY Infectious Agent Yersinia species, a Gram negative bacilli. Y. enterocolitica is the species most commonly associated with human infection. Y. pseudotuberculosis infection
rev Apr 2017 BASIC EPIDEMIOLOGY Infectious Agent Yersinia species, a Gram negative bacilli. Y. enterocolitica is the species most commonly associated with human infection. Y. pseudotuberculosis infection
Mumps. Ellen Dorshow-Gordon, MPH Jackson County Health Department March Follow Us on Social Media:
 Mumps Ellen Dorshow-Gordon, MPH Jackson County Health Department March 2017 1 Epidemiology Mumps is a mild acute viral illness, often asymptomatic Etiologic agent is paramyxovirus, a member of the Rubulavirus
Mumps Ellen Dorshow-Gordon, MPH Jackson County Health Department March 2017 1 Epidemiology Mumps is a mild acute viral illness, often asymptomatic Etiologic agent is paramyxovirus, a member of the Rubulavirus
Measles: United States, January 1 through June 10, 2011
 Measles: United States, January 1 through June 10, 2011 Preeta K. Kutty, MD, MPH Measles, Mumps, Rubella and Polio Team Division of Viral Diseases Centers for Disease Control and Prevention Atlanta, GA
Measles: United States, January 1 through June 10, 2011 Preeta K. Kutty, MD, MPH Measles, Mumps, Rubella and Polio Team Division of Viral Diseases Centers for Disease Control and Prevention Atlanta, GA
Common Fungi. Catherine Diamond MD MPH
 Common Fungi Catherine Diamond MD MPH Birth Month and Day & Last Four Digits of Your Cell Phone # BEFORE: http://tinyurl.com/kvfy3ts AFTER: http://tinyurl.com/lc4dzwr Clinically Common Fungi Yeast Mold
Common Fungi Catherine Diamond MD MPH Birth Month and Day & Last Four Digits of Your Cell Phone # BEFORE: http://tinyurl.com/kvfy3ts AFTER: http://tinyurl.com/lc4dzwr Clinically Common Fungi Yeast Mold
Human Infection with Novel Influenza A Virus Case Report Form
 Human Infection with Novel Influenza A Virus Case Report Form Form Approved OMB No. 0920-0004 Exp. Date 6/30/2013 Reporter Information State: Date reported to state/local health department: / / (MM/DD/YYYY)
Human Infection with Novel Influenza A Virus Case Report Form Form Approved OMB No. 0920-0004 Exp. Date 6/30/2013 Reporter Information State: Date reported to state/local health department: / / (MM/DD/YYYY)
Title: National Surveillance for Severe Acute Respiratory Syndrome (SARS-CoV)
 09-ID-11 Committee: Infectious Title: National Surveillance for Severe Acute Respiratory Syndrome (SARS-CoV) I. Statement of the Problem CSTE position statement 07-EC-02 recognized the need to develop
09-ID-11 Committee: Infectious Title: National Surveillance for Severe Acute Respiratory Syndrome (SARS-CoV) I. Statement of the Problem CSTE position statement 07-EC-02 recognized the need to develop
Pediatric influenza-associated deaths in Arizona,
 Pediatric influenza-associated deaths in Arizona, 2004-2012 (Poster is shared here as an 8.5 x11 document for easier viewing. All content is identical, though graphs and tables are formatted differently.)
Pediatric influenza-associated deaths in Arizona, 2004-2012 (Poster is shared here as an 8.5 x11 document for easier viewing. All content is identical, though graphs and tables are formatted differently.)
Tuscarawas County Health Department
 Tuscarawas County Health Department 2017 Quarterly Report to the District Advisory Council Volume 1; Issue 4 www.tchdnow.org HOME SEWAGE OPERATION AND MAINTENANCE PROGRAM IMPLEMENTATION INFORMATIONAL SESSION
Tuscarawas County Health Department 2017 Quarterly Report to the District Advisory Council Volume 1; Issue 4 www.tchdnow.org HOME SEWAGE OPERATION AND MAINTENANCE PROGRAM IMPLEMENTATION INFORMATIONAL SESSION
Technical Bulletin No. 121
 CPAL Central Pennsylvania Alliance Laboratory Technical Bulletin No. 121 January 31, 2014 Lyme Blot, IgG and IgM - Now Performed at CPAL Contact: J. Matthew Groeller, 717.851.1416 Operations Manager, Clinical
CPAL Central Pennsylvania Alliance Laboratory Technical Bulletin No. 121 January 31, 2014 Lyme Blot, IgG and IgM - Now Performed at CPAL Contact: J. Matthew Groeller, 717.851.1416 Operations Manager, Clinical
Wisconsin physicians, other clinicians, infection control professionals, local health department directors in Wisconsin
 DIVISION OF PUBLIC HEALTH 1 WEST WILSON STREET P O BOX 2659 Jim Doyle MADISON WI 53701-2659 Governor State of Wisconsin 608-266-1251 Kevin R. Hayden FAX: 608-267-2832 Secretary TTY: 888-701-1253 Department
DIVISION OF PUBLIC HEALTH 1 WEST WILSON STREET P O BOX 2659 Jim Doyle MADISON WI 53701-2659 Governor State of Wisconsin 608-266-1251 Kevin R. Hayden FAX: 608-267-2832 Secretary TTY: 888-701-1253 Department
Vaccine Preventable Diseases in San Francisco. Susan Fernyak, MD MPH CDCP Section Director and Deputy Health Officer August 17, 2010
 Vaccine Preventable Diseases in San Francisco Susan Fernyak, MD MPH CDCP Section Director and Deputy Health Officer August 17, 2010 Section Responsibilities Communicable Disease Control Unit (CDCU): Communicable
Vaccine Preventable Diseases in San Francisco Susan Fernyak, MD MPH CDCP Section Director and Deputy Health Officer August 17, 2010 Section Responsibilities Communicable Disease Control Unit (CDCU): Communicable
HISTOPLASMOSIS - LABORATORY DIAGNOSIS IN VIETNAM
 HISTOPLASMOSIS - LABORATORY DIAGNOSIS IN VIETNAM National Institute of Hygiene and Epidemiology, Hanoi, Vietnam, National Institute of Infectious Diseases, Tokyo, Japan, Bach Mai hospital, Vietnam, Military
HISTOPLASMOSIS - LABORATORY DIAGNOSIS IN VIETNAM National Institute of Hygiene and Epidemiology, Hanoi, Vietnam, National Institute of Infectious Diseases, Tokyo, Japan, Bach Mai hospital, Vietnam, Military
It IS a Small World After All: The Public Health Impact and Immunologic Assessment of a Disneyland Measles Case in El Paso County, Colorado
 It IS a Small World After All: The Public Health Impact and Immunologic Assessment of a Disneyland Measles Case in El Paso County, Colorado Panel: Robyn Espy, M.P.H, Marigny Klaber, M.Sc., Shannon Rowe,
It IS a Small World After All: The Public Health Impact and Immunologic Assessment of a Disneyland Measles Case in El Paso County, Colorado Panel: Robyn Espy, M.P.H, Marigny Klaber, M.Sc., Shannon Rowe,
MEASLES HEALTH ALERT/ADVISORY. Date: February 5, Dear Colleague:
 MEASLES HEALTH ALERT/ADVISORY Date: February 5, 2019 Dear Colleague: Details of situation: Five measles cases, four between the ages of 12 to 21 months, have been reported in three different counties within
MEASLES HEALTH ALERT/ADVISORY Date: February 5, 2019 Dear Colleague: Details of situation: Five measles cases, four between the ages of 12 to 21 months, have been reported in three different counties within
Measles Update. March 16, 2015 Lisa Miller, MD, MSPH Communicable Disease Branch Chief Lynn Trefren MSN, RN Immunization Branch Chief
 Measles Update March 16, 2015 Lisa Miller, MD, MSPH Communicable Disease Branch Chief Lynn Trefren MSN, RN Immunization Branch Chief Colorado Department of Public Health and Environment Presenters have
Measles Update March 16, 2015 Lisa Miller, MD, MSPH Communicable Disease Branch Chief Lynn Trefren MSN, RN Immunization Branch Chief Colorado Department of Public Health and Environment Presenters have
Infection Prevention Prevention and Contr
 Infection Prevention and Control o What is an infection? An infection is an illness caused by microorganisms A disease producing micro organism is called a pathogen Most microorganisms are classified as:
Infection Prevention and Control o What is an infection? An infection is an illness caused by microorganisms A disease producing micro organism is called a pathogen Most microorganisms are classified as:
14-ID-06. Revision of the National Surveillance Case Definition for Meningococcal Disease
 14-ID-06 Committee: Infectious Disease Title: Revision of the National urveillance Case Definition for Meningococcal Disease I. tatement of the Problem The current case definition for meningococcal disease,
14-ID-06 Committee: Infectious Disease Title: Revision of the National urveillance Case Definition for Meningococcal Disease I. tatement of the Problem The current case definition for meningococcal disease,
HEALTH ADVISORY: MEASLES EXPOSURES IN NEW YORK STATE
 December 11, 2018 To: Health Care Providers, Hospitals, Emergency Departments, Dental Providers, and Local Health Departments From: New York State Department of Health, Bureau of Immunization HEALTH ADVISORY:
December 11, 2018 To: Health Care Providers, Hospitals, Emergency Departments, Dental Providers, and Local Health Departments From: New York State Department of Health, Bureau of Immunization HEALTH ADVISORY:
Royce Johnson, M.D., F.A.C.P. Disclosure Information Research Support/Consultant/Speaker
 Royce Johnson, M.D., F.A.C.P. Disclosure Information Research Support/Consultant/Speaker Astellas Enzon Pharmaceuticals Merck & Co, Inc. Ortho-McNeil, Inc. Pfizer, Inc. Sanofi Aventis Schering-Plough The
Royce Johnson, M.D., F.A.C.P. Disclosure Information Research Support/Consultant/Speaker Astellas Enzon Pharmaceuticals Merck & Co, Inc. Ortho-McNeil, Inc. Pfizer, Inc. Sanofi Aventis Schering-Plough The
Title: Public Health Reporting and National Notification for Trichinellosis
 09-ID-64 Committee: Infectious Title: Public Health Reporting and National Notification for Trichinellosis I. Statement of the Problem CSTE position statement 07-EC-02 recognized the need to develop an
09-ID-64 Committee: Infectious Title: Public Health Reporting and National Notification for Trichinellosis I. Statement of the Problem CSTE position statement 07-EC-02 recognized the need to develop an
Coccidioidomycosis, also called, is caused
 REVIEW Coccidioidomycosis in African Americans Barbara E. Ruddy, MD; Anita P. Mayer, MD; Marcia G. Ko, MD; Helene R. Labonte, DO; Jill A. Borovansky, MD; Erika S. Boroff, MD; and Janis E. Blair, MD Coccidioidomycosis
REVIEW Coccidioidomycosis in African Americans Barbara E. Ruddy, MD; Anita P. Mayer, MD; Marcia G. Ko, MD; Helene R. Labonte, DO; Jill A. Borovansky, MD; Erika S. Boroff, MD; and Janis E. Blair, MD Coccidioidomycosis
4.3.9 Pandemic Disease
 4.3.9 Pandemic Disease This section describes the location and extent, range of magnitude, past occurrence, future occurrence, and vulnerability assessment for the pandemic disease hazard for Armstrong
4.3.9 Pandemic Disease This section describes the location and extent, range of magnitude, past occurrence, future occurrence, and vulnerability assessment for the pandemic disease hazard for Armstrong
Chapter 4. Antibody detection methods for laboratory confirmation of measles, rubella, and CRS
 Chapter 4. Antibody detection methods for laboratory confirmation of measles, rubella, and CRS In this chapter: 4.1 Selection and comparison of EIAs for IgM detection 4.2 Interpretation of IgM results
Chapter 4. Antibody detection methods for laboratory confirmation of measles, rubella, and CRS In this chapter: 4.1 Selection and comparison of EIAs for IgM detection 4.2 Interpretation of IgM results
Infectious Diseases, Chronic Diseases District of Columbia Department of Health, Center for Policy, Planning & Evaluation Assignment Description
 Infectious Diseases, Chronic Diseases District of Columbia Department of Health, Center for Policy, Planning & Evaluation Washington, District Of Columbia Assignment Description The District of Columbia
Infectious Diseases, Chronic Diseases District of Columbia Department of Health, Center for Policy, Planning & Evaluation Washington, District Of Columbia Assignment Description The District of Columbia
Appendix B: Provincial Case Definitions for Reportable Diseases
 Infectious Diseases Protocol Appendix B: Provincial Case Definitions for Reportable Diseases Disease: Measles Revised August 2014 Measles 1.0 Provincial Reporting Confirmed and probable cases of disease
Infectious Diseases Protocol Appendix B: Provincial Case Definitions for Reportable Diseases Disease: Measles Revised August 2014 Measles 1.0 Provincial Reporting Confirmed and probable cases of disease
Influenza Season and EV-D68 Update. Johnathan Ledbetter, MPH
 2014-2015 Influenza Season and EV-D68 Update Johnathan Ledbetter, MPH 2014-2015 Influenza Season Influenza Reporting Individual cases are not reportable in the state of Texas Situations where influenza
2014-2015 Influenza Season and EV-D68 Update Johnathan Ledbetter, MPH 2014-2015 Influenza Season Influenza Reporting Individual cases are not reportable in the state of Texas Situations where influenza
During Influenza Season A Checklist for Residential Care Facilities
 During Influenza Season A Checklist for Residential Care Facilities Seasonal influenza is a serious cause of illness, disability and death in residents of care facilities. Each year, across Canada there
During Influenza Season A Checklist for Residential Care Facilities Seasonal influenza is a serious cause of illness, disability and death in residents of care facilities. Each year, across Canada there
Maricopa County Department of Public Health Outbreak Summary Report
 Maricopa County Department of Public Health 29 Outbreak Summary Report Office of Epidemiology April 2 Introduction The purpose of this report is to provide a general overview of the disease outbreak investigations
Maricopa County Department of Public Health 29 Outbreak Summary Report Office of Epidemiology April 2 Introduction The purpose of this report is to provide a general overview of the disease outbreak investigations
AGENT-BASED MODELING OF COCCIDIOIDOMYCOSIS. Carrie Marie Anderson. B.S., University of Minnesota, M.S., University of Minnesota, 1999
 AGENT-BASED MODELING OF COCCIDIOIDOMYCOSIS by Carrie Marie Anderson B.S., University of Minnesota, 1997 M.S., University of Minnesota, 1999 Submitted to the Graduate Faculty of Graduate School of Public
AGENT-BASED MODELING OF COCCIDIOIDOMYCOSIS by Carrie Marie Anderson B.S., University of Minnesota, 1997 M.S., University of Minnesota, 1999 Submitted to the Graduate Faculty of Graduate School of Public
Appendix B: Provincial Case Definitions for Reportable Diseases
 Ministry of Health and Long-Term Care Infectious Diseases Protocol Appendix B: Provincial Case Definitions for Reportable Diseases Disease: West Nile Virus Illness Revised March 2017 West Nile Virus Illness
Ministry of Health and Long-Term Care Infectious Diseases Protocol Appendix B: Provincial Case Definitions for Reportable Diseases Disease: West Nile Virus Illness Revised March 2017 West Nile Virus Illness
Avian influenza Avian influenza ("bird flu") and the significance of its transmission to humans
 15 January 2004 Avian influenza Avian influenza ("bird flu") and the significance of its transmission to humans The disease in birds: impact and control measures Avian influenza is an infectious disease
15 January 2004 Avian influenza Avian influenza ("bird flu") and the significance of its transmission to humans The disease in birds: impact and control measures Avian influenza is an infectious disease
ARIZONA INFLUENZA SUMMARY Week 1 (1/4/2015 1/10/2015)
 ARIZONA INFLUENZA SUMMARY Week 1 (1/4/2015 1/10/2015) 2014-2015 Season (9/28/2014 10/3/2015) Synopsis: Influenza activity is increasing in Arizona. Arizona reported Widespread activity for week 1. Influenza
ARIZONA INFLUENZA SUMMARY Week 1 (1/4/2015 1/10/2015) 2014-2015 Season (9/28/2014 10/3/2015) Synopsis: Influenza activity is increasing in Arizona. Arizona reported Widespread activity for week 1. Influenza
Q Fever What men and women on the land need to know
 Q Fever What men and women on the land need to know Dr. Stephen Graves Director, Australian Rickettsial Reference Laboratory Director, Division of Microbiology, Pathology North (Hunter) NSW Health Pathology,
Q Fever What men and women on the land need to know Dr. Stephen Graves Director, Australian Rickettsial Reference Laboratory Director, Division of Microbiology, Pathology North (Hunter) NSW Health Pathology,
Epidemiologic and Clinical Features of Measles and Rubella in a Rural Area in China
 ORIGINAL ARTICLE Epidemiologic and Clinical Features of Measles and Rubella in a Rural Area in China Youwang Yan* Health Bureau of Jingzhou District, Jingzhou City, Hubei Province, People s Republic of
ORIGINAL ARTICLE Epidemiologic and Clinical Features of Measles and Rubella in a Rural Area in China Youwang Yan* Health Bureau of Jingzhou District, Jingzhou City, Hubei Province, People s Republic of
WHO Technical Consultation on the severity of disease caused by the new influenza A (H1N1) virus infections
 WHO Technical Consultation on the severity of disease caused by the new influenza A (H1N1) virus infections Original short summary posted 6 May 2009. Revised full report posted May 9 2009. On 5 May 2009
WHO Technical Consultation on the severity of disease caused by the new influenza A (H1N1) virus infections Original short summary posted 6 May 2009. Revised full report posted May 9 2009. On 5 May 2009
Trichinosis Table of Contents
 Subsection: Trichinosis Page 1 of 9 Trichinosis Table of Contents Trichinosis Trichinosis (Trichinellosis) Fact Sheet Investigation of Trichinosis Surveillance Case Report (CDC 54.7) Subsection: Trichinosis
Subsection: Trichinosis Page 1 of 9 Trichinosis Table of Contents Trichinosis Trichinosis (Trichinellosis) Fact Sheet Investigation of Trichinosis Surveillance Case Report (CDC 54.7) Subsection: Trichinosis
Chapter 6: Measles. I. Disease description. II. Background
 Chapter 6, Measles: 6 1 Chapter 6: Measles Mark Papania, MD, MPH; Melinda Wharton, MD, MPH; Susan Redd I. Disease description Measles is an acute viral illness caused by a virus in the family paramyxovirus,
Chapter 6, Measles: 6 1 Chapter 6: Measles Mark Papania, MD, MPH; Melinda Wharton, MD, MPH; Susan Redd I. Disease description Measles is an acute viral illness caused by a virus in the family paramyxovirus,
