PROPOSED TECHNICAL REQUIREMENTS BLOOD AND BLOOD COMPONENTS. File 1 DRAFT FOR CONSULTATION PURPOSES ONLY
|
|
|
- Barnard Blaze Carr
- 5 years ago
- Views:
Transcription
1 PROPOSED TECHNICAL REQUIREMENTS BLOOD AND BLOOD COMPONENTS File 1 DRAFT FOR CONSULTATION PURPOSES ONLY
2 ANNEX I INFORMATION REQUIREMENTS A. INFORMATION TO BE PROVIDED TO DONORS 1. Accurate but generally understandable educational materials about the essential nature of blood, the products derived from it, and the important benefits to patients of blood and plasma donations; 2. The reasons for requiring a medical history, physical examination, and the testing of donations; information on the risk of infectious diseases that may be transmitted by blood and blood products; the signs and symptoms of AIDS, and the significance of informed consent, self-deferral, and temporary and permanent deferral; 3. Information about protection of personal data: No unauthorised disclosure of the name of the donor, of information concerning his health, and of the results of the tests performed; 4. The reasons why they should not donate which may be detrimental to their own health; 5. The reasons why they should not donate which put recipients at risk, such as unsafe sexual behaviour, HIV /AIDS, hepatitis, drug addiction and the use and abuse of drugs; 6. The option of changing their mind about donating prior to proceeding further without any undue embarrassment or discomfort; 7. Information on the possibility of withdrawing or self-deferring at any time during the donation process; 8. The opportunity to ask questions at any time; 9. The undertaking that if test results show evidence of any pathology, they will be contacted by the blood collection centre; 10. Specific information on the nature of the procedures involved in the donation process and associated risks for those willing to participate in apheresis programmes, whether for plasma or cellular components. 2
3 B. INFORMATION TO BE OBTAINED FROM DONORS 1. Identification Appropriate means of identification, providing name (first and surname), address, date of birth, or alternative means allowing the donor to be uniquely identified. 2. Health history Health and medical history any relevant factors that may assist in identifying and screening out persons whose donation could present a health risk to themselves or a risk of transmitting diseases to others, by way of a written questionnaire addressing the criteria listed in Annex II and a personal interview with a trained health care staff member. Abnormal conditions should be referred to the physician-in-charge who should have the final say on whether blood should be collected from a donor. If the physician is in doubt, the donor should be deferred. 3. Signature Signature, on the donor questionnaire, countersigned by the health care staff member conducting the interview under the responsibility of the responsible person, or subject to the approval of this responsible person; Signature on a separate attestation, to acknowledge that educational materials provided have been read and understood, that opportunity to ask questions has been presented, and that satisfactory responses have been received. to agree that his / her blood or plasma donation could be used for patients needing transfusion or blood products in the country where the donation is made or in another country, to which it would be transferred in accordance with the provisions of the legislation of the country where the donation is made, particularly with regard to the destination of the donation; and to indicate his / her informed consent of the wish to proceed with the donation process. 3
4 ANNEX II REQUIREMENTS CONCERNING THE SUITABILITY OF BLOOD AND PLASMA DONORS AND THE SCREENING OF DONATED BLOOD 1. REQUIREMENTS FOR THE PROTECTION OF BLOOD AND PLASMA DONORS a) Physical acceptance criteria Age Body weight Blood pressure Pulse Haemoglobin (or haematocrit) Haematocrit Protein years 50kg for either whole blood or plasma Systolic 180 mm of mercury beats per minute and regular for females 12.5 g/100 ml for females 38% For plasmapheresis 60 g/litre years (first-time donor) - at discretion of responsible physician Diastolic 100 mm of mercury < 50 beats per minute Accepted if undergoes intensive sport training for males 13.5 g/100 ml for males 40% 17 years and not legally classified as a minor; otherwise written consent according to law For apheresis plasma: males and females 12.5 g/100 ml For apheresis plasma 38% + 65 years - with permission of responsible physician given annually b) Donation criteria Time interval For whole blood > 8weeks Maximum 6 donations per year for males, 4 for females Per whole blood donation 500 ml For apheresis plasma Normally > 2 weeks 4
5 2. DEFERRAL CRITERIA FOR BLOOD AND PLASMA DONORS 2.1 Permanent Deferral Criteria Prospective donors who have, or have a history of, any of the following: Auto-immune diseases if more than one organ is affected Cardiovascular diseases Central nervous system diseases Malignant diseases except after successful treatment for non-invasive cervical cancer and rodent ulcer Abnormal bleeding tendency Fainting spells (syncope) or convulsions Severe or chronic gastrointestinal, haematological, metabolic, respiratory, or renal disease not included in preceding categories Infectious diseases - persons suffering or having suffered from Babesiosis Hepatitis B (HBsAg confirmed positive) Hepatitis C Hepatitis, infectious (of unexplained aetiology) HIV/AIDS HTLV I/II Leprosy Kala Azar (leishmaniasis) Q fever Syphilis Trypanosoma cruzi (Chagas disease) the blood of residents in an endemic area associated with poor living conditions may be used only for plasma fractionated products TSEs (or history thereof in genetic family) Alcoholism, chronic Cornea/dura mater transplantation recipient Diabetes, if treated with insulin Intravenous (IV) drug use Pituitary hormone of human origin (e.g. growth hormone) recipient Sexual behaviour that places them at high risk of transmitting infectious diseases, including (a) persons who have had sex in return for money or drugs (b) current sexual partners of people with HIV (c) current sexual partners of people with HBV unless demonstrated to be immune Xenotransplant recipients Allergy individuals with a documented history of anaphylaxis Malaria if test results positive for individual who lived in endemic area for first five years of life, reject as a cellular donor. 5
6 2.2 Temporary Deferral Criteria Ineligible for five years Acute glomerulonephritis (following complete recovery) Ineligible for three years Epilepsy (off-treatment and without an attack) Ineligible for two years Tuberculosis (after declared cured) Osteomyelitis (after declared cured) Toxoplasmosis (after recovery and absence of IgM antibodies) Brucellosis (after full recovery) Rheumatic fever (after an attack if no evidence of chronic heart disease) Ineligible for one year Accidental exposure to blood or blood contaminated instruments Endoscopic examination Treatment involving use of catheters Transfusion with blood or blood components Tissue or cell transplant Major surgery Acupuncture (if not performed by a qualified practitioner) Tattoo Body piercing Drug allergy, in particular allergy to penicillin (after last exposure) Close contact with a case of hepatitis B or C Rabies vaccine (if post exposure) Tick-borne encephalitis vaccine (if post exposure) Ineligible for nine months Pregnancy (after delivery) Abortion Ineligible for six months Infectious mononucleosis (after recovery) Malaria (after return from last visit to endemic area and symptom free) Ineligible for at least two weeks Prophylactic immunisations (following administration of vaccines with attenuated bacteria and viruses (four weeks) Minor infectious diseases (two weeks) Fever above 38º C, flu-like illness (following cessation of symptoms) Ineligible for at least one week Minor surgery (without complications) Ineligible for 72 hours Following administration of vaccines (desensitising) 6
7 Ineligible for 48 hours Treatment by dentist or dental hygienist Following administration of killed/inactivated viral/bacterial and rickettsial vaccines Rabies vaccine (prophylactic administration) 7
8 ANNEX III STORAGE AND FREEZING A. STORAGE Blood product Storage Temperature Cryoprecipitate -18 ºC -25 C Below -25 ºC Granulocytes Plasma, cryoprecipitatedepleted Plasma, fresh frozen Plasma, thawed Platelets (single unit, concentrate recovered, buffy coat pool, apheresis) Platelets, cryopreserved: apheresis Not suitable for storage. If unavoidable: +20 C +24 C -18 C -25 C Below -25 ºC -18 C -25 C Below -25 ºC Thawed between +30 C +37 C +20 C +24 C Frozen platelets: maintained at: -80 C (in electrical freezer); -150 C (in vapour phase liquid nitrogen). Thawed platelets To be stored at +20 C to +24 C with adequate agitation, if short term storage required. Red cells +2 C +6 C Red cells in additive solution +2 C +6 C Length of storage 3 months 24 months Administered as soon as possible after collection, with maximum storage of 24 hours. 3 months 24 months 3 months 24 months Transfused as soon as possible 5days(with continuous gentle agitation) < 6 hours (after open system manipulation) Up to 12 months Beyond 12 months To be used immediately after thawing. 35 days (in adenine supplemented anticoagulant) According to anticoagulant and additive solution Transportation Similar to storage Similar to storage Similar to storage Similar to storage Similar to storage (with continuous gentle agitation) Similar to storage Transportation time +2 C +10 C 24 hours +2 C + 10 C 24 hours 8
9 Blood product Red cells in additive solution, buffy coat removed Red cells, leukocytereduced Red cells, frozen by low glycerol method Red cells, frozen by high glycerol method Red cells, washed Whole blood (for transfusion as whole blood) Whole blood (for component preparation) Storage Temperature +2 C +6 C +2 C +6 C -140 ºC -150 ºC in vapour phase of liquid nitrogen +2 ºC +6 ºC, after thawing 60 ºC 80 ºC in an electrical freezer +2 ºC +6 ºC, after thawing +2 C +6 C +2 C +6 C +1 C +6 C +20 ºC +24 ºC (iftobeusedforthe preparation of platelets) Length of storage According to anticoagulant and additive solution. Normally 35 days. 35 days with adenine supplemented anticoagulant. < 24 hours if prepared in open system 10 years < 24 hours, use as soon as possible after thawing 10 years < 24 hours, use as soon as possible after thawing < 24 hours if prepared at low < 6 hours if prepared at room < 35 days with adenine supplemented anticoagulant Upto8hours before use Up to 24 hours before use Transportation Transportation time +2 C + 10 C 24 hours +1 C +10 C Storage conditions should be maintained Storage conditions should be maintained 24 hours Frozen red cells: as short as possible. Thawed red cells: to be transfused within 24 hours of thawing Frozen red cells: as short as possible. Thawed red cells: to be transfused within 24 hours of thawing +2 C +6 C Limited by the appropriate storage time +2 C +10 C 24 hours 9
10 B. FREEZING Blood product Plasma A Plasma B Plasma C Platelets Red cells Time of freezing Frozen within 6 hours of phlebotomy Frozen within 24 hours of phlebotomy Frozen after 24 hours of phlebotomy Frozen within 24 hours Frozen within 7 days 10
11 Component Cryoprecipitate Granulocytes, apheresis Plasma, cryoprecipitatedepleted Plasma, fresh frozen ANNEX IV QUALITY REQUIREMENTS FOR BLOOD COMPONENTS Properties Contains a major portion of Factor VIII, von Willebrand factor, fibrinogen, Factor XIII and fibronectin present in freshly drawn and separated plasma. Principal function is phagocytosis of bacteria. Content of albumin, immunoglobulins and coagulation factors comparable to fresh frozen plasma. Reduced levels of Factors V, VIII, XIII, von Willebrand factor, fibrinogen, & fibronectin. Contains normal plasma levels of stable coagulation factors, albumin & immunoglobulins; at least 70% of original Factor VIIIc, other labile coagulation factors, & naturally occurring inhibitors. European Community legislation applies if source material for fractionated products. Parametertobecheckedon all units (unless otherwise indicated) Factor VIIIc Sampling -1% of all units. Every two months: a) pool of 6 units of mixed blood groups during first month of storage; b) pool of 6 units of mixed blood groups during last month of storage Fibrinogen Sampling -1%ofallunits; with a minimum of 4 units per month Granulocytes. (unless plasma itself is the source) Sampling - all units (unless plasma itself is the source) Quality requirements ml > 70 I.U./unit > 140 mg/unit < 500 ml > 10 X 10 9 /unit Stated volume ±10% Stated volume ±10% Appearance Clear 11
12 Component Properties Parametertobecheckedon all units (unless otherwise indicated) Red Cells Leukocytes Platelets Quality requirements <6X10 9 /l <0.1X10 9 /l <50X10 9 /l Platelets, apheresis Platelets, cryopreserved: apheresis Platelets, recovered from single unit by PRP Platelet content per procedure variable depending on method of preparation and machine used. Same applies to leukocyte and red cell contamination of product. Standard unit = 5-6 single units by PRP. Reconstituted unit of cryopreserved platelets is practically free of red cells and granulocytes. Amount of platelets in adult standard dose equivalent to that obtained from 4-6 units of whole blood. Sampling: 1% of all units with minimum of 4 units per month Factor VIIIc Sampling: every 2 months a) pool of 6 units of mixed blood groups during first month of storage b) pool of 6 units of mixed blood groups during last month of storage.. Platelet content Sampling 1% of all units. minimum of 10 units per month. (90% of units sampled should fall within specified values). Residual leukocytes - after leukocyte depletion Sampling 1% of all units. minimum of 10 units per month. (90% of units sampled should fall within specified values.) Swirling Sampling - all units HLA or HPA (when & as required) ph measured at the end of the recommended shelf life Sampling 1% of all units, minimum of 4 units per month Platelet content Residual leukocytes (before freezing) Minimum 70% of original value > 40 ml/60 X 10 9 platelets > 200 X 10 9 platelets / donation <1.0X10 6 / standard unit +1 (score) Typing ml > 40% of original pre-frozen platelet content <0.2X10 6 per 60 X 10 9 platelets 12
13 Component Properties Parametertobecheckedon all units (unless otherwise indicated) HLA or HPA (when & as required) Platelet content Sampling -1%ofallunits: 10 units/month (75% of units sampled should fall within values specified). Residual leukocyte content - before leukocyte depletion Quality requirements Typing ml 60 X 10 9 platelets / single unit equivalent < 0.2 X 10 9 / single unit equivalent Platelet pool from buffy coat - after leukocyte depletion Sampling -1%ofallunits; 10 units / month (75% of units sampled should fall within values specified). ph (at end of recommended shelf life) Sampling -1%ofallunits; minimum 10 units per month HLA or HPA (when & as required) Platelet content Sampling -1%ofallunits. Minimum 10 units / month (75% of units sampled should fall within values specified) Residual leukocyte content. - before leukocyte depletion <0.2X10 6 / single unit equivalent n.s. >60X10 9 platelets / single unit equivalent <0.05X10 9 /single unit equivalent Red cells Red cells, buffy coat removed Contains all red cells from donated unit after centrifugation. No procedures taken to remove leukocytes or platelets. All red cells from donated unit, except ml, remain after centrifugation. - after leukocyte depletion Sampling - 1% of all units; 10 units / month (75% of units sampled should fall within values specified). ph measured at the end of the recommended shelf life Sampling 1% of all units Haematocrit (Hct) Haemoglobin Haemolysis at the end of storage. <0.2X10 6 / single unit equivalent ±50 ml 65 75% 45 g / unit < 0.8% of red cell mass 13
14 Component Red cells in additive solution Red cells in additive solution, buffy coat removed Red cells, cryopreserved Properties All red cells from donated unit remain after centrifugation. No procedures taken to remove leukocytes or platelets. All red cells from donated unit, except ml, remain after centrifugation. Parametertobecheckedon all units (unless otherwise indicated) Sampling 1% of all units Haematocrit (Hct) Haemoglobin Leukocyte content (75% of units sampled should fall within specified values) : to be defined for the system used. Sampling - 1% of all units Haematocrit (Hct). Haemoglobin.. Haemolysis at the end of storage.. Sampling 1% of all units Haematocrit (Hct) Haemoglobin.. Haemolysis at the end of storage Sampling: 4 units per month Leukocyte content Sampling: 4 units per month (75% of units sampled should fall within specified values) Hb (supernatant) (Final suspending solution) Quality requirements 250 ±50 ml 65 75% > 43 g / unit <1.2X10 9 cells /unit Defined volume ±10% 50 70% (depending on additive solution, method of centrifugation, & quantity of remaining plasma) 45 g / unit <0.8% of red cell mass To be defined for the system used 50 70% (depending on nature of additive solution, method of centrifugation, & amount of remaining plasma) 43 g / unit < 0.8% of red cell mass <1.2X10 9 cells /unit > 185 ml < 0.2 g / unit Haematocrit (Hct) 65 75% 14
15 Component Red cells, leukocytereduced Red cells, washed Whole blood Properties Amountofresidualplasma depends upon washing protocol. Parametertobecheckedon all units (unless otherwise indicated) Haemoglobin Osmolarity. Sampling - 1% of all units with a minimum of 4 units per month Leukocytes Sampling - 1% of all units with a minimum of 4 units per month. (75% of units sampled fall within values specified) Sterility. Sampling -1%ofallunits: Residual leukocyte content Sampling - 1% of all units with a minimum of 4 units per month (validation with 100 filtrations for each kind of filter) Haematocrit (Hct) Haemoglobin. Sampling - 1% of all units with a minimum of 4 units per month. Haemolysis at the end of storage Sampling 4 units per month Quality requirements 36 g / unit < 340 mosm/l <0.1X10 9 cells /unit Sterile 250 ±50 ml <1X10 6 cells /unit 65 75% 40 g / unit < 0.8% of red cell mass To be defined for the system used Haematocrit (Hct) 65 75% Haemoglobin 40 g / unit Haemolysis at the end of the < 0.8% of red cell process mass Residual protein of final supernatant. Sampling 1% of all units with a minimum of 4 units per month Haemoglobin Haemolysis at end of storage. <5mg/unit (To ensure IgA content < 0.2 mg / unit) ml excluding anticoagulant 45 g/unit < 0.8% of red cell mass 15
Blood Components & Indications for Transfusion. Neda Kalhor
 Blood Components & Indications for Transfusion Neda Kalhor Blood products Cellular Components: Red blood cells - Leukocyte-reduced RBCs - Washed RBCs - Irradiated RBCs Platelets - Random-donor platelets
Blood Components & Indications for Transfusion Neda Kalhor Blood products Cellular Components: Red blood cells - Leukocyte-reduced RBCs - Washed RBCs - Irradiated RBCs Platelets - Random-donor platelets
Blood transfusion. Dr. J. Potgieter Dept. of Haematology NHLS - TAD
 Blood transfusion Dr. J. Potgieter Dept. of Haematology NHLS - TAD General Blood is collected from volunteer donors >90% is separated into individual components and plasma Donors should be: healthy, have
Blood transfusion Dr. J. Potgieter Dept. of Haematology NHLS - TAD General Blood is collected from volunteer donors >90% is separated into individual components and plasma Donors should be: healthy, have
HEALTH SCREENING QUESTIONNAIRE CT Work-up. Donor ID EdgeCell #:
 HEALTH SCREENING QUESTIONNAIRE CT Work-up Héma-Québec Registre des Donneurs de Cellules Souches 4045 Côte-vertu, St-Laurent, QC, Canada, H4R 2W7 Tél : + 514-832-1031 Fax : + 514-832-0266 www.hema-quebec.qc.ca
HEALTH SCREENING QUESTIONNAIRE CT Work-up Héma-Québec Registre des Donneurs de Cellules Souches 4045 Côte-vertu, St-Laurent, QC, Canada, H4R 2W7 Tél : + 514-832-1031 Fax : + 514-832-0266 www.hema-quebec.qc.ca
UNIVERSITY OF WASHINGTON MEDICAL CENTER Men s Health Center and Male Fertility Laboratory Sperm & Testis Cryopreservation Program Patient NAME and ID
 UNIVERSITY OF WASHINGTON MEDICAL CENTER Men s Health Center and Male Fertility Laboratory Sperm & Testis Cryopreservation Program Patient NAME and ID CONSENT FOR MINOR s SPERM OR TESTIS CRYOPRESERVATION
UNIVERSITY OF WASHINGTON MEDICAL CENTER Men s Health Center and Male Fertility Laboratory Sperm & Testis Cryopreservation Program Patient NAME and ID CONSENT FOR MINOR s SPERM OR TESTIS CRYOPRESERVATION
Reporting from Council of Europe member states on the collection, testing and use of blood and blood components in Europe The 2006 Survey
 Reporting from Council of Europe member states on the collection, testing and use of blood and blood components in Europe The 2006 Survey This questionnaire consists of three sections: A. Collection and
Reporting from Council of Europe member states on the collection, testing and use of blood and blood components in Europe The 2006 Survey This questionnaire consists of three sections: A. Collection and
HEALTH SCREENING QUESTIONNAIRE. Work-up. Donor ID EdgeCell #:
 HEALTH SCREENING QUESTIONNAIRE Héma-Québec Registre des Donneurs de Cellules Souches 4045 Côte-vertu, St-Laurent, QC, Canada, H4R 2W7 Tél : + 514-832-1031 Fax : + 514-832-0266 www.hema-quebec.qc.ca CT
HEALTH SCREENING QUESTIONNAIRE Héma-Québec Registre des Donneurs de Cellules Souches 4045 Côte-vertu, St-Laurent, QC, Canada, H4R 2W7 Tél : + 514-832-1031 Fax : + 514-832-0266 www.hema-quebec.qc.ca CT
Pretransfusion Testing
 Pretransfusion Testing Blood transfusion services The immunohematologic testing needed for By proper patient blood typing, component Dr. selection Reham and Talaat compatibility testing. Which Blood ensure
Pretransfusion Testing Blood transfusion services The immunohematologic testing needed for By proper patient blood typing, component Dr. selection Reham and Talaat compatibility testing. Which Blood ensure
DHQ Flowcharts v2.0 eff. February 2016
 Question: 1. Are you feeling healthy and well today? Donor Eligibility: A person should be free of infectious diseases, including colds, on the day of donation. A person who is not in good health should
Question: 1. Are you feeling healthy and well today? Donor Eligibility: A person should be free of infectious diseases, including colds, on the day of donation. A person who is not in good health should
Database on Blood Safety 2009 in ECO Member States
 Database on Blood Safety 2009 in ECO Member States Section 1: Administrative Information Information provided by: 1.1 Name: 1.2 Title: 1.3 Position: 1.4 Organization: 1.5 Address: 1.6 Country: 1.7 Tel.
Database on Blood Safety 2009 in ECO Member States Section 1: Administrative Information Information provided by: 1.1 Name: 1.2 Title: 1.3 Position: 1.4 Organization: 1.5 Address: 1.6 Country: 1.7 Tel.
Irish Blood Transfusion Service Seirbhís Fuilaistriúcháin na héireann
 Document Detail Seirbhís Fuilaistriúcháin na héireann Type: Document No.: Title: PMF IBTS SPEC IBTS/PMF/SPEC/0219[1] PLATELETS, ADULT DOSE WITH PLASMA / PAS, WASHED, IRRADIATED Owner: 1895 REBECCA WALDEN
Document Detail Seirbhís Fuilaistriúcháin na héireann Type: Document No.: Title: PMF IBTS SPEC IBTS/PMF/SPEC/0219[1] PLATELETS, ADULT DOSE WITH PLASMA / PAS, WASHED, IRRADIATED Owner: 1895 REBECCA WALDEN
The establishment of well-organized, nationally-coordinated blood transfusion services with quality systems in all areas
 Blood Safety Global Database on Blood Safety (GDBS) 2013 The World Health Organization (WHO) programme on Blood Transfusion Safety would appreciate your kind cooperation in completing this data collection
Blood Safety Global Database on Blood Safety (GDBS) 2013 The World Health Organization (WHO) programme on Blood Transfusion Safety would appreciate your kind cooperation in completing this data collection
Blood Transfusion. What is blood transfusion? What are blood banks? When is a blood transfusion needed? Who can donate blood?
 What is blood transfusion? A blood transfusion is a safe, common procedure in which blood is given through an intravenous (IV) line in one of the blood vessels. A blood transfusion usually takes two to
What is blood transfusion? A blood transfusion is a safe, common procedure in which blood is given through an intravenous (IV) line in one of the blood vessels. A blood transfusion usually takes two to
Crossmatching and Issuing Blood Components; Indications and Effects.
 Crossmatching and Issuing Blood Components; Indications and Effects. Alison Muir Blood Transfusion, Blood Sciences, Newcastle Trust Topics Covered Taking the blood sample ABO Group Antibody Screening Compatibility
Crossmatching and Issuing Blood Components; Indications and Effects. Alison Muir Blood Transfusion, Blood Sciences, Newcastle Trust Topics Covered Taking the blood sample ABO Group Antibody Screening Compatibility
TRANSFUSION ASSOCIATED DISEASE, RECALL, OR COMPLICATION INVESTIGATION POLICY I. FATALITIES AND COMPLICATIONS ASSOCIATED WITH TRANSFUSION:
 I. FATALITIES AND COMPLICATIONS ASSOCIATED WITH TRANSFUSION: A. TRANSFUSION RELATED FATALITY: FDA and MEDIC must be notified immediately, and subsequently in writing, when a possible transfusion related
I. FATALITIES AND COMPLICATIONS ASSOCIATED WITH TRANSFUSION: A. TRANSFUSION RELATED FATALITY: FDA and MEDIC must be notified immediately, and subsequently in writing, when a possible transfusion related
Question: 1. Are you currently taking an antibiotic?
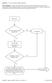 Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Document for Obstetric Patients Who Refuse Consent for Blood and Blood Product Administration, Including Jehovah s Witnesses
 Document for Obstetric Patients Who Refuse Consent for Blood and Blood Product Administration, Including Jehovah s Witnesses Patient addressograph Label This form must only be used for the refusal of blood
Document for Obstetric Patients Who Refuse Consent for Blood and Blood Product Administration, Including Jehovah s Witnesses Patient addressograph Label This form must only be used for the refusal of blood
Breastfeeding Center Milk Connection Questionnaire and Release for Donor
 Breastfeeding Center Milk Connection Questionnaire and Release for Donor All of us at The Breastfeeding Center are deeply grateful to you for your important gift to infants in our community. Your donated
Breastfeeding Center Milk Connection Questionnaire and Release for Donor All of us at The Breastfeeding Center are deeply grateful to you for your important gift to infants in our community. Your donated
CORD BLOOD TRANSPLANTATION STUDY CBU EXCLUSION AND QUARANTINE RELEASE FORM
 (Any answer of Yes indicates exclusion) 1. Informed Consent and Maternal/Infant Demographics a. Lack of confirmation of signed and witnessed informed consent 1 Yes 2 No Is the following information missing
(Any answer of Yes indicates exclusion) 1. Informed Consent and Maternal/Infant Demographics a. Lack of confirmation of signed and witnessed informed consent 1 Yes 2 No Is the following information missing
The testing of Donated Blood and Components at NHSBT
 The testing of Donated Blood and Components at NHSBT NHSBT is responsible for collecting all donated blood and platelets in England, then processing into components before issuing to client hospitals.
The testing of Donated Blood and Components at NHSBT NHSBT is responsible for collecting all donated blood and platelets in England, then processing into components before issuing to client hospitals.
DHQ v2.0, February 2016 Table Detailing Changes from v1.3 v1.3 Revised language in v2.0 Rationale Question 1: Are you
 DHQ v2.0, February 2016 Table Detailing s from v1.3 v1.3 Revised language in v2.0 Rationale Question 1: Are you Q 1, No Feeling healthy and well today? Question 2: Are you Q 2, No change Currently taking
DHQ v2.0, February 2016 Table Detailing s from v1.3 v1.3 Revised language in v2.0 Rationale Question 1: Are you Q 1, No Feeling healthy and well today? Question 2: Are you Q 2, No change Currently taking
BLOOD DONATION OR BLOOD DONOR DEFERRAL BASED ON DATA ON NONCONFORMITIES DETECTED IN POST-SELECTION PERIOD (DURING AND AFTER BLOOD COLLECTION)
 BLOOD DONATION OR BLOOD DONOR DEFERRAL BASED ON DATA ON NONCONFORMITIES DETECTED IN POST-SELECTION PERIOD (DURING AND AFTER BLOOD COLLECTION) Tomislav Vuk Croatian Institute of Transfusion Medicine Zagreb
BLOOD DONATION OR BLOOD DONOR DEFERRAL BASED ON DATA ON NONCONFORMITIES DETECTED IN POST-SELECTION PERIOD (DURING AND AFTER BLOOD COLLECTION) Tomislav Vuk Croatian Institute of Transfusion Medicine Zagreb
HONG KONG RED CROSS BLOOD TRANSFUSION SERVICE BLOOD DONATION REGISTRATION FORM
 I Bleed no. HONG KONG RED CROSS BLOOD TRANSFUSION SERVICE
I Bleed no. HONG KONG RED CROSS BLOOD TRANSFUSION SERVICE
CELLULAR THERAPY QUIZ. Correct answers and rationale
 CELLULAR THERAPY QUIZ Correct answers and rationale In January 2018, registered cellular therapy (CT) facilities were asked to participate in the quiz to determine the educational needs of the users regarding
CELLULAR THERAPY QUIZ Correct answers and rationale In January 2018, registered cellular therapy (CT) facilities were asked to participate in the quiz to determine the educational needs of the users regarding
Question: 1. Are you currently taking an antibiotic?
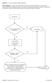 Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Irish Blood Transfusion Service Seirbhís Fuilaistriúcháin na héireann
 Document Detail Irish Blood Transfusion Service Seirbhís Fuilaistriúcháin na héireann Type: Document No.: Title: PMF IBTS SPEC IBTS/PMF/SPEC/0214[2] PLATELETS, ADULT DOSE WITH PLASMA / PAS, IRRADIATED
Document Detail Irish Blood Transfusion Service Seirbhís Fuilaistriúcháin na héireann Type: Document No.: Title: PMF IBTS SPEC IBTS/PMF/SPEC/0214[2] PLATELETS, ADULT DOSE WITH PLASMA / PAS, IRRADIATED
Jovona Powelson, B.S. MLT (ASCP) Director of Laboratories
 Jovona Powelson, B.S. MLT (ASCP) Director of Laboratories Blood Products From the Donor to You Objectives Deliver a brief virtual tour of a blood center. Describe the number of donors needed to meet the
Jovona Powelson, B.S. MLT (ASCP) Director of Laboratories Blood Products From the Donor to You Objectives Deliver a brief virtual tour of a blood center. Describe the number of donors needed to meet the
Primary Care Services for blood borne viral hepatitis prevention, treatment and care
 Primary Care Services for blood borne viral hepatitis prevention, treatment and care Author: Josie Smith, Marion Lyons Page 1 of 13 October 2006 Status : Final Contents: Page: Executive Summary 3 Introduction
Primary Care Services for blood borne viral hepatitis prevention, treatment and care Author: Josie Smith, Marion Lyons Page 1 of 13 October 2006 Status : Final Contents: Page: Executive Summary 3 Introduction
Surveillance Report 2014
 Surveillance Report 2014 Executive summary We are pleased to present the third report for our stakeholders describing infectious disease surveillance. High quality and timely surveillance is key to the
Surveillance Report 2014 Executive summary We are pleased to present the third report for our stakeholders describing infectious disease surveillance. High quality and timely surveillance is key to the
Guidelines for Use of Canine Blood Components
 Guidelines for Use of Canine Blood Components Cryoprecipitate This product is prepared by a controlled thaw of fresh frozen plasma, resulting in a concentration of Factor VIII, Factor XIII, vwf and some
Guidelines for Use of Canine Blood Components Cryoprecipitate This product is prepared by a controlled thaw of fresh frozen plasma, resulting in a concentration of Factor VIII, Factor XIII, vwf and some
SAFE BLOOD SAVE LIVES
 DIN BLOOD DONATION REGISTRATION FORM PART I: PRE-DONATION INFORMATION Thank you for coming to give blood today. Your donation could save and change the lives of the recipients. We sincerely request you
DIN BLOOD DONATION REGISTRATION FORM PART I: PRE-DONATION INFORMATION Thank you for coming to give blood today. Your donation could save and change the lives of the recipients. We sincerely request you
BLOOD COMPONENT SUPPORT OF RhD NEGATIVE INDIVIDUALS
 REASON FOR ISSUE: Review of section 4 along with changes in requirement to inform TMS in respect of authorisation process (in Appendix A) DCR16969. 1. INTRODUCTION of RhD positive blood components to an
REASON FOR ISSUE: Review of section 4 along with changes in requirement to inform TMS in respect of authorisation process (in Appendix A) DCR16969. 1. INTRODUCTION of RhD positive blood components to an
BLOOD DONOR INTERVIEW AND PHYSICAL
 EXERCISE : BLOOD DONOR INTERVIEW AND PHYSICAL Skills: 20 points Objectives:. Determine if an individual is eligible to donate blood by proper performance of the donor interview. 2. Identify the conditions
EXERCISE : BLOOD DONOR INTERVIEW AND PHYSICAL Skills: 20 points Objectives:. Determine if an individual is eligible to donate blood by proper performance of the donor interview. 2. Identify the conditions
UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM. A Pilot Study for Collection of Anti-Zika Immune Plasma
 A Pilot Study for Collection of Anti-Zika Immune Plasma CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Your contacts for this study at the Hospital of the University
A Pilot Study for Collection of Anti-Zika Immune Plasma CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Your contacts for this study at the Hospital of the University
More Fun Than Giving Blood CLINIC RECRUITED
 More Fun Than Giving Blood CLINIC RECRUITED Are you thinking of becoming a sperm donor? Choosing to become a sperm donor is a generous act, and by entering our sperm donor program you can help make someone
More Fun Than Giving Blood CLINIC RECRUITED Are you thinking of becoming a sperm donor? Choosing to become a sperm donor is a generous act, and by entering our sperm donor program you can help make someone
The Blood Donor: Demographics, Donor Selection and Tests on Donor Blood. Liz Caffrey, Patricia Hewitt and John Barbara
 CHAPTER 1 The Blood Donor: Demographics, Donor Selection and Tests on Donor Blood Liz Caffrey, Patricia Hewitt and John Barbara 0.3 OVERVIEW Total 0.254 0.247 A safe and sufficient blood supply depends
CHAPTER 1 The Blood Donor: Demographics, Donor Selection and Tests on Donor Blood Liz Caffrey, Patricia Hewitt and John Barbara 0.3 OVERVIEW Total 0.254 0.247 A safe and sufficient blood supply depends
BLOOD DONATION AFTER ACUPUNCTURE: A JUSTIFIED DEFERRAL?
 BLOOD DONATION AFTER ACUPUNCTURE: A JUSTIFIED DEFERRAL? What if your patients want to donate blood? Are you aware that patients undergoing Acupuncture treatment, according to international guidelines,
BLOOD DONATION AFTER ACUPUNCTURE: A JUSTIFIED DEFERRAL? What if your patients want to donate blood? Are you aware that patients undergoing Acupuncture treatment, according to international guidelines,
Non-reproductive tissues and cells Recommending authority/ association
 Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent - legy binding More stringent - recommended Not legy binding and not recommended Non-reproductive tissues and cells VIRAL
Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent - legy binding More stringent - recommended Not legy binding and not recommended Non-reproductive tissues and cells VIRAL
TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING October2010. Issue Summary
 TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING 28-29October2010 Issue Summary Informational Topic: FDA s Geographic Donor Deferral Policy to Reduce the Possible Risk of Transmission
TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING 28-29October2010 Issue Summary Informational Topic: FDA s Geographic Donor Deferral Policy to Reduce the Possible Risk of Transmission
Screening donors and donations for transfusion transmissible infectious agents. Alan Kitchen
 Screening donors and donations for transfusion transmissible infectious agents Alan Kitchen Aim Not to teach you microbiology To provide and awareness of the big picture To provide an understanding of
Screening donors and donations for transfusion transmissible infectious agents Alan Kitchen Aim Not to teach you microbiology To provide and awareness of the big picture To provide an understanding of
Non-reproductive tissues and cells
 Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent testing - legally binding on national level More stringent testing - recommended on national level Not legally binding
Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent testing - legally binding on national level More stringent testing - recommended on national level Not legally binding
GUIDELINES FOR THE TRANSFUSION OF BLOOD COMPONENTS
 CHILDREN S HOSPITALS AND CLINICS OF MINNESOTA Introduction: GUIDELINES FOR THE TRANSFUSION OF BLOOD COMPONENTS These guidelines have been developed in conjunction with the hospital Transfusion Committee.
CHILDREN S HOSPITALS AND CLINICS OF MINNESOTA Introduction: GUIDELINES FOR THE TRANSFUSION OF BLOOD COMPONENTS These guidelines have been developed in conjunction with the hospital Transfusion Committee.
PLASMA EXCHANGE J MANION NEPEAN HOSPITAL
 PLASMA EXCHANGE J MANION NEPEAN HOSPITAL PLASMA The fluid portion of blood Normally approx 5% body weight or 3.5L in 70kg male Clots on standing unless anticoagulated Common plasma proteins are albumin,
PLASMA EXCHANGE J MANION NEPEAN HOSPITAL PLASMA The fluid portion of blood Normally approx 5% body weight or 3.5L in 70kg male Clots on standing unless anticoagulated Common plasma proteins are albumin,
Blood Component Information. CIRCULAR OF INFORMATION An extension of blood component labels
 Blood Component Information CIRCULAR OF INFORMATION An extension of blood component labels 2009 Copyright Australian Red Cross Blood Service 2009 Last updated April 2009 Apart from any fair dealing for
Blood Component Information CIRCULAR OF INFORMATION An extension of blood component labels 2009 Copyright Australian Red Cross Blood Service 2009 Last updated April 2009 Apart from any fair dealing for
CAUTION: Refer to the Document Library for the most recent version of this document. Cryoprecipitate Transfusion Guideline for Practice.
 Directorate Department Year Version Number Central Index Number Endorsing Committee Date Endorsed Approval Committee Date Approved Author Name and Job Title Key Words (for search purposes) Date Published
Directorate Department Year Version Number Central Index Number Endorsing Committee Date Endorsed Approval Committee Date Approved Author Name and Job Title Key Words (for search purposes) Date Published
UKGS TRANSFUSION SERVICE PRODUCTS AND AVAILABILITY
 Lexington, KY Page 1 of 13 Affected Sites: Enterprise Chandler X Good Samaritan I. PRINCIPLE: The UK Good Samaritan Hospital is dedicated to serve the patients with safe, high quality blood products and
Lexington, KY Page 1 of 13 Affected Sites: Enterprise Chandler X Good Samaritan I. PRINCIPLE: The UK Good Samaritan Hospital is dedicated to serve the patients with safe, high quality blood products and
Confirmed (Laboratory Tests) Serum positive for IgM anti-hbc or, hepatitis B surface antigen (HbsAg).
 Hepatitis B Hepatitis B is a liver disease that results from infection with the Hepatitis B virus. It can range in severity from a mild illness lasting a few weeks to a serious, lifelong illness. Hepatitis
Hepatitis B Hepatitis B is a liver disease that results from infection with the Hepatitis B virus. It can range in severity from a mild illness lasting a few weeks to a serious, lifelong illness. Hepatitis
Non-reproductive tissues and cells
 Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent - legy binding on national level More stringent - recommended on national level Not legy binding and not recommended on
Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent - legy binding on national level More stringent - recommended on national level Not legy binding and not recommended on
Patient Name: MRN: DOB: Treatment Location:
 Page 1 of 5 I. TO (Required) This Section is required to be completed by all patients who undergo kidney transplant surgery. I hereby consent to and authorize Dr. and his/her assistant(s), including supervised
Page 1 of 5 I. TO (Required) This Section is required to be completed by all patients who undergo kidney transplant surgery. I hereby consent to and authorize Dr. and his/her assistant(s), including supervised
Red cell antigens and blood group antibodies
 Hematology Blood transfusion د.ميسم مؤيد علوش Objectives: O Identify the most important blood group systems? O List types of antibodies and the main features of each type? O Define the ABO system and its
Hematology Blood transfusion د.ميسم مؤيد علوش Objectives: O Identify the most important blood group systems? O List types of antibodies and the main features of each type? O Define the ABO system and its
Guide to the preparation, use and quality assurance of blood components
 Contents Foreword...3 Members of the European Committee (Partial Agreement) on Blood Transfusion... 8 Members of the GTS working group... 22 Members of the TS066 working group... 30 Recommendation No.
Contents Foreword...3 Members of the European Committee (Partial Agreement) on Blood Transfusion... 8 Members of the GTS working group... 22 Members of the TS066 working group... 30 Recommendation No.
Biology 3201 Unit 2 Reproduction: Sexually Transmitted Infections (STD s/sti s)
 Biology 3201 Unit 2 Reproduction: Sexually Transmitted Infections (STD s/sti s) STI s once called venereal diseases More than 20 STIs have now been identified most prevalent among teenagers and young adults.
Biology 3201 Unit 2 Reproduction: Sexually Transmitted Infections (STD s/sti s) STI s once called venereal diseases More than 20 STIs have now been identified most prevalent among teenagers and young adults.
Presentation Created by; Shana Chiborak RN CNCC (C) Nurse Coordinator Blood Management Service May 2016
 Presentation Created by; Shana Chiborak RN CNCC (C) Nurse Coordinator Blood Management Service May 2016 What is Cryoprecipitate? Cryoprecipitate contains factor VIII (8), fibrinogen, and von Willebrand
Presentation Created by; Shana Chiborak RN CNCC (C) Nurse Coordinator Blood Management Service May 2016 What is Cryoprecipitate? Cryoprecipitate contains factor VIII (8), fibrinogen, and von Willebrand
HEALTH EXAMINATION GUIDELINES
 HEALTH EXAMINATION GUIDELINES 1. PLEASE READ THE INSTRUCTIONS CAREFULLY BEFORE FILLING IN THE FORM. 2. PLEASE FILL IN THE FORM IN ENGLISH LANGUAGE. 3. PLEASE WRITE IN CAPITAL LETTERS. 4. THIS FORM HAS
HEALTH EXAMINATION GUIDELINES 1. PLEASE READ THE INSTRUCTIONS CAREFULLY BEFORE FILLING IN THE FORM. 2. PLEASE FILL IN THE FORM IN ENGLISH LANGUAGE. 3. PLEASE WRITE IN CAPITAL LETTERS. 4. THIS FORM HAS
BLOOD TRANSFUSION. Dr Lumka Ntabeni
 BLOOD TRANSFUSION Dr Lumka Ntabeni Blood transfusion definition SAFE transfer of BLOOD COMPONENTS from a DONOR to a RECEPIENT CONTENT Brief history of blood transfusion How is safety guaranteed? How do
BLOOD TRANSFUSION Dr Lumka Ntabeni Blood transfusion definition SAFE transfer of BLOOD COMPONENTS from a DONOR to a RECEPIENT CONTENT Brief history of blood transfusion How is safety guaranteed? How do
Transfusion Medicine Best Practices: Indications for Blood Components
 Transfusion Medicine Best Practices: 1.0 Policy Statements 1.1 Regional Health Authorities (RHAs) shall develop policies, processes and procedures for ordering, distribution, storage, transfusion and administration
Transfusion Medicine Best Practices: 1.0 Policy Statements 1.1 Regional Health Authorities (RHAs) shall develop policies, processes and procedures for ordering, distribution, storage, transfusion and administration
Donor Risk Assessment Interview (Donor >12 yrs old)
 Your logo Donor Risk Assessment Interview (Donor >12 yrs old) Your address Donor Name: First Middle Last Person Interviewed: Name Relationship Contact Information: ( ) Phone Address City State Zip The
Your logo Donor Risk Assessment Interview (Donor >12 yrs old) Your address Donor Name: First Middle Last Person Interviewed: Name Relationship Contact Information: ( ) Phone Address City State Zip The
What is an Apheresis Donation?
 What are Platelets? Platelets are colorless, irregularly shaped bodies found in blood. The primary role of platelets is to prevent bleeding in injured blood vessel walls by forming an aggregate at the
What are Platelets? Platelets are colorless, irregularly shaped bodies found in blood. The primary role of platelets is to prevent bleeding in injured blood vessel walls by forming an aggregate at the
L 256/32 Official Journal of the European Union
 L 256/32 Official Journal of the European Union 1.10.2005 COMMISSION DIRECTIVE 2005/61/EC of 30 September 2005 implementing Directive 2002/98/EC of the European Parliament and of the Council as regards
L 256/32 Official Journal of the European Union 1.10.2005 COMMISSION DIRECTIVE 2005/61/EC of 30 September 2005 implementing Directive 2002/98/EC of the European Parliament and of the Council as regards
New Advances in Transfusion EM I LY CO BERLY, M D
 New Advances in Transfusion EM I LY CO BERLY, M D TRANSFUSI ON M EDI CI NE FELLO W VANDERBI LT UNI VERSITY Objectives To discuss the terminology, components, transfusion risks, and dosing guidelines for
New Advances in Transfusion EM I LY CO BERLY, M D TRANSFUSI ON M EDI CI NE FELLO W VANDERBI LT UNI VERSITY Objectives To discuss the terminology, components, transfusion risks, and dosing guidelines for
RESPONSES TO OPEN CONSULTATION on Draft Technical Requirements for blood and blood components
 RESPONSES TO OPEN CONSULTATION on Draft Technical Requirements for blood and blood components Annex II Requirements concerning the suitability of blood and plasma donors and the screening of donated blood
RESPONSES TO OPEN CONSULTATION on Draft Technical Requirements for blood and blood components Annex II Requirements concerning the suitability of blood and plasma donors and the screening of donated blood
Non-reproductive tissues and cells Recommending authority/ association
 Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent - legy binding More stringent - recommended Not legy binding and not recommended Tested pathogen Donor test/ technique
Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent - legy binding More stringent - recommended Not legy binding and not recommended Tested pathogen Donor test/ technique
Name of Recipient: Recipient s DOB (if known) Relationship to Recipient: (Example: mother, father, sister, brother, friend, etc)
 The Christ Hospital Health Network DONOR REGISTRATION INFORMATION Phone: 513-585-2493 Fax: 513-585-0433 (Please be advised donor information is needed ONLY to register donor in the Christ Hospital system.
The Christ Hospital Health Network DONOR REGISTRATION INFORMATION Phone: 513-585-2493 Fax: 513-585-0433 (Please be advised donor information is needed ONLY to register donor in the Christ Hospital system.
Making Your Blood Donation Safe
 BLOOD DONOR EDUCATIONAL MATERIALS Making Your Blood Donation Safe Pete Lawson Burned in a wildfire, LifeStream blood donations saved his life. WWW.LSTREAM.ORG NEW! Important Information on Iron! Recent
BLOOD DONOR EDUCATIONAL MATERIALS Making Your Blood Donation Safe Pete Lawson Burned in a wildfire, LifeStream blood donations saved his life. WWW.LSTREAM.ORG NEW! Important Information on Iron! Recent
New Zealand Bone Marrow Donor Registry
 Title: SECTION 4.0 NZBMDR STANDARDS DONOR RECRUITMENT, ENROLMENT & INFORMATION Authorised by: Executive Officer Contributing Authors: ABMDR Dr Hilary Blacklock Raewyn Fisher NZBMDR-Guidelines-004-13 Date
Title: SECTION 4.0 NZBMDR STANDARDS DONOR RECRUITMENT, ENROLMENT & INFORMATION Authorised by: Executive Officer Contributing Authors: ABMDR Dr Hilary Blacklock Raewyn Fisher NZBMDR-Guidelines-004-13 Date
Non-reproductive tissues and cells
 Ministry of Health: Institute for Transplantation and Biomedicine / Colour key VIRAL HIV 1 and HIV 2 Hepatitis B Minimum requirements as set out in Directive 2004/23/EC More stringent - legy binding More
Ministry of Health: Institute for Transplantation and Biomedicine / Colour key VIRAL HIV 1 and HIV 2 Hepatitis B Minimum requirements as set out in Directive 2004/23/EC More stringent - legy binding More
15. Procuring, processing and transporting gametes and
 15. Procuring, processing and transporting gametes and embryos Version 6.0 On this page: : Extracts from the HFE Act Directions HFEA guidance: Documented procedures: general Patient selection and procurement
15. Procuring, processing and transporting gametes and embryos Version 6.0 On this page: : Extracts from the HFE Act Directions HFEA guidance: Documented procedures: general Patient selection and procurement
RISK OF DISEASE TRANSMISSION FROM ORGAN DONORS PATIENT INFORMATION GUIDE
 Receiving an organ transplant carries many risks, including the risk of getting a disease from the June 2017 donor. This is true for every organ we transplant. BC Transplant makes every effort to minimize
Receiving an organ transplant carries many risks, including the risk of getting a disease from the June 2017 donor. This is true for every organ we transplant. BC Transplant makes every effort to minimize
Surveillance Report 2015
 Surveillance Report 2015 Executive summary We are pleased to present the fourth report for our stakeholders describing infectious disease surveillance. High quality and timely surveillance is key to the
Surveillance Report 2015 Executive summary We are pleased to present the fourth report for our stakeholders describing infectious disease surveillance. High quality and timely surveillance is key to the
2/2/2011. Blood Components and Transfusions. Why Blood Transfusion?
 Blood Components and Transfusions Describe blood components Identify nursing responsibilities r/t blood transfusion Discuss factors r/t blood transfusion including blood typing, Rh factor, and cross matching
Blood Components and Transfusions Describe blood components Identify nursing responsibilities r/t blood transfusion Discuss factors r/t blood transfusion including blood typing, Rh factor, and cross matching
PATIENT DETAILS. NHS PRIVATE NHS No: UCLH Hospital No: Surname. Date of Birth. Forename(s) Full Address: Post Code: REFERRING PRACTITIONER
 Semen Cryopreservation Referral Form Please return to contact details accompanied by laboratory reports for HIV, HepB and HepC testing. Enclose letter of guarantee if private patient. Fertility & Reproductive
Semen Cryopreservation Referral Form Please return to contact details accompanied by laboratory reports for HIV, HepB and HepC testing. Enclose letter of guarantee if private patient. Fertility & Reproductive
UNIVERSITY OF PENNSYLVANIA HEALTH SYSTEM
 White Blood Cell Collection by Leukapheresis in HIV-infected Individuals On Chemotherapy and Controls Not on Chemotherapy: A Study of HIV Reservoir Eradication CONSENT TO PARTICIPATE IN A RESEARCH STUDY
White Blood Cell Collection by Leukapheresis in HIV-infected Individuals On Chemotherapy and Controls Not on Chemotherapy: A Study of HIV Reservoir Eradication CONSENT TO PARTICIPATE IN A RESEARCH STUDY
Egg and Sperm Bank Edinburgh Fertility & Reproductive Endocrine Centre (EFREC) DONOR INFORMATION Screening Tests
 Egg and Sperm Bank Edinburgh Fertility & Reproductive Endocrine Centre (EFREC) DONOR INFORMATION Screening Tests Introduction As a potential donor, it is important you are aware of the screening tests
Egg and Sperm Bank Edinburgh Fertility & Reproductive Endocrine Centre (EFREC) DONOR INFORMATION Screening Tests Introduction As a potential donor, it is important you are aware of the screening tests
Surveillance Report 2016
 Surveillance Report 2016 Executive summary We are pleased to present the fifth report for our stakeholders describing infectious disease surveillance. High quality and timely surveillance is key to the
Surveillance Report 2016 Executive summary We are pleased to present the fifth report for our stakeholders describing infectious disease surveillance. High quality and timely surveillance is key to the
UNIVERSITY OF PENNSYLVANIA RESEARCH SUBJECT INFORMED CONSENT AND HIPAA AUTHORIZATION FORM
 UNIVERSITY OF PENNSYLVANIA RESEARCH SUBJECT INFORMED CONSENT AND HIPAA AUTHORIZATION FORM Protocol Title: Principal Investigator: 24 hr. Emergency Contact: Evaluating the HIV-1 Reservoir: BEAT HIV Delaney
UNIVERSITY OF PENNSYLVANIA RESEARCH SUBJECT INFORMED CONSENT AND HIPAA AUTHORIZATION FORM Protocol Title: Principal Investigator: 24 hr. Emergency Contact: Evaluating the HIV-1 Reservoir: BEAT HIV Delaney
Frequently Asked Questions on Blood Donation
 Frequently Asked Questions on Blood Donation SEA-HLM-418 Frequently Asked Questions (FAQs) on Blood Donation Q 1: What is blood? How much blood does a human body contain? 2 Q 2: What is the composition
Frequently Asked Questions on Blood Donation SEA-HLM-418 Frequently Asked Questions (FAQs) on Blood Donation Q 1: What is blood? How much blood does a human body contain? 2 Q 2: What is the composition
Human Hepatitis B Immunoglobulin, solution for intramuscular injection.
 New Zealand Data Sheet Hepatitis B Immunoglobulin-VF NAME OF THE MEDICINE Human Hepatitis B Immunoglobulin, solution for intramuscular injection. DESCRIPTION Hepatitis B Immunoglobulin-VF is a sterile,
New Zealand Data Sheet Hepatitis B Immunoglobulin-VF NAME OF THE MEDICINE Human Hepatitis B Immunoglobulin, solution for intramuscular injection. DESCRIPTION Hepatitis B Immunoglobulin-VF is a sterile,
Guidelines for Gamma Irradiation of Blood Components
 AUSTRALIAN & NEW ZEALAND SOCIETY OF BLOOD TRANSFUSION INC. AUSTRALIAN RED CROSS BLOOD SERVICE NEW ZEALAND BLOOD SERVICE Guidelines for Gamma Irradiation of Blood Components Revised 2003 AN ZS B T Australian
AUSTRALIAN & NEW ZEALAND SOCIETY OF BLOOD TRANSFUSION INC. AUSTRALIAN RED CROSS BLOOD SERVICE NEW ZEALAND BLOOD SERVICE Guidelines for Gamma Irradiation of Blood Components Revised 2003 AN ZS B T Australian
Blood Product Modifications: Leukofiltration, Irradiation and Washing
 1. Leukocyte Reduction Definitions and Standards: o Process also known as leukoreduction, or leukofiltration o Applicable AABB Standards, 25th ed. Leukocyte-reduced RBCs At least 85% of original RBCs
1. Leukocyte Reduction Definitions and Standards: o Process also known as leukoreduction, or leukofiltration o Applicable AABB Standards, 25th ed. Leukocyte-reduced RBCs At least 85% of original RBCs
Juventas SHARING LIFE CONSENT FOR PLASMA INFUSION
 Juventas SHARING LIFE Print Name Date of Birth CONSENT FOR PLASMA INFUSION To the Patient or Guardian of the Patient: The purpose of this document is to provide written information as of (month and day),
Juventas SHARING LIFE Print Name Date of Birth CONSENT FOR PLASMA INFUSION To the Patient or Guardian of the Patient: The purpose of this document is to provide written information as of (month and day),
Version for the Silent Procedure 29 April Agenda item January Hepatitis
 Version for the Silent Procedure 29 April 2014 134th session EB134.R18 Agenda item 10.5 25 January 2014 Hepatitis The Executive Board, Having considered the report on hepatitis, 1 RECOMMENDS to the Sixty-seventh
Version for the Silent Procedure 29 April 2014 134th session EB134.R18 Agenda item 10.5 25 January 2014 Hepatitis The Executive Board, Having considered the report on hepatitis, 1 RECOMMENDS to the Sixty-seventh
HEALTH EXAMINATION GUIDELINES
 HEALTH EXAMINATION GUIDELINES 1. PLEASE READ THE INSTRUCTIONS CAREFULLY BEFORE FILLING IN THE FORM. 2. PLEASE FILL IN THE FORM IN ENGLISH LANGUAGE. 3. PLEASE WRITE IN CAPITAL LETTERS. 4. THIS FORM HAS
HEALTH EXAMINATION GUIDELINES 1. PLEASE READ THE INSTRUCTIONS CAREFULLY BEFORE FILLING IN THE FORM. 2. PLEASE FILL IN THE FORM IN ENGLISH LANGUAGE. 3. PLEASE WRITE IN CAPITAL LETTERS. 4. THIS FORM HAS
Non-reproductive tissues and cells Recommending authority/ association
 Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent - legally binding More stringent - recommended Not legally binding and not recommended Non-reproductive tissues and cells
Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent - legally binding More stringent - recommended Not legally binding and not recommended Non-reproductive tissues and cells
Non-reproductive tissues and cells
 Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent testing - legally binding on national level More stringent testing - recommended on national level Not legally binding
Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent testing - legally binding on national level More stringent testing - recommended on national level Not legally binding
Risk of ID transmission. Patient Blood Management - Blood Safety and Component Utilization. Transfusion and Cancer 4/9/2014
 Patient Blood Management - Blood Safety and Component Utilization Lowell Tilzer M.D. Pathology and Lab Medicine Kansas University Med Center Risk of ID transmission Pre NAT Post NAT HIV 1:607,000 ~1:2.5
Patient Blood Management - Blood Safety and Component Utilization Lowell Tilzer M.D. Pathology and Lab Medicine Kansas University Med Center Risk of ID transmission Pre NAT Post NAT HIV 1:607,000 ~1:2.5
HEALTH EXAMINATION GUIDELINES FOR ENTRY INTO MALAYSIAN HIGHER EDUCATIONAL INSTITUTIONS
 HEALTH EXAMINATION GUIDELINES FOR ENTRY INTO MALAYSIAN HIGHER EDUCATIONAL INSTITUTIONS 1. PLEASE READ THE INSTRUCTIONS CAREFULLY BEFORE FILLING IN THE FORM. 2. PLEASE FILL IN THE FORM IN ENGLISH LANGUAGE.
HEALTH EXAMINATION GUIDELINES FOR ENTRY INTO MALAYSIAN HIGHER EDUCATIONAL INSTITUTIONS 1. PLEASE READ THE INSTRUCTIONS CAREFULLY BEFORE FILLING IN THE FORM. 2. PLEASE FILL IN THE FORM IN ENGLISH LANGUAGE.
Privigen solution for infusion
 Privigen solution for infusion Haematology Patient Information What is is Privigen? Privigen? Privigen belongs in a class of medicines called human normal immunoglobins. Immunoglobulins are also called
Privigen solution for infusion Haematology Patient Information What is is Privigen? Privigen? Privigen belongs in a class of medicines called human normal immunoglobins. Immunoglobulins are also called
Fecal microbial transplantation
 Version 09/16 Consent Form For Fecal microbial transplantation Fecal transplant is an operation in which liquid produced from a healthy human being (as per a survey questionnaire and blood and feces tests)
Version 09/16 Consent Form For Fecal microbial transplantation Fecal transplant is an operation in which liquid produced from a healthy human being (as per a survey questionnaire and blood and feces tests)
PATIENT INFORMATION LEAFLET: INFORMATION FOR THE USER
 Verified with regard to factual content 13.12.2011 PATIENT INFORMATION LEAFLET: INFORMATION FOR THE USER GAMMA anty-hbs 1000 Immunoglobulinum humanum hepatitidis B Human immunoglobulin against Hepatitis
Verified with regard to factual content 13.12.2011 PATIENT INFORMATION LEAFLET: INFORMATION FOR THE USER GAMMA anty-hbs 1000 Immunoglobulinum humanum hepatitidis B Human immunoglobulin against Hepatitis
BLOOD DONATION Northern Ireland Blood Tranfusion Service
 FREQUENTLY ASKED QUESTIONS BLOOD DONATION Northern Ireland Blood Tranfusion Service Why do we need donated blood? Everyone knows blood is literally a lifesaver for those who have been in an accident or
FREQUENTLY ASKED QUESTIONS BLOOD DONATION Northern Ireland Blood Tranfusion Service Why do we need donated blood? Everyone knows blood is literally a lifesaver for those who have been in an accident or
Special Requirements Lab Matters. 21 st June Barrie Ferguson
 Special Requirements Lab Matters. 21 st June 2017 Barrie Ferguson Special requirements Patients requiring Methylene Blue or Solvent Detergent treated Fresh Frozen Plasma Patients requiring apheresis platelets
Special Requirements Lab Matters. 21 st June 2017 Barrie Ferguson Special requirements Patients requiring Methylene Blue or Solvent Detergent treated Fresh Frozen Plasma Patients requiring apheresis platelets
BMTCN REVIEW COURSE PRE-TRANSPLANT CARE
 BMTCN REVIEW COURSE PRE-TRANSPLANT CARE Jennifer Shamai MS, RN, AOCNS, BMTCN Professional Practice Leader Department of Clinical Practice And Professional Education Click How to edit the Master Experts
BMTCN REVIEW COURSE PRE-TRANSPLANT CARE Jennifer Shamai MS, RN, AOCNS, BMTCN Professional Practice Leader Department of Clinical Practice And Professional Education Click How to edit the Master Experts
MEDICAL RECORD. Last and given name(s)... Personal Identification Number PESEL.. Residence address... Phone number..
 MEDICAL RECORD Last and given name(s)... Personal Identification Number PESEL.. Residence address... Phone number The person authorised by the patient to be contacted and receive information concerning
MEDICAL RECORD Last and given name(s)... Personal Identification Number PESEL.. Residence address... Phone number The person authorised by the patient to be contacted and receive information concerning
Class 9 th Why do we fall ill?
 Class 9 th Why do we fall ill? Health: health is a state of physical, mental and social well being. The health of all individuals is dependent on their physical environment, social environment, and their
Class 9 th Why do we fall ill? Health: health is a state of physical, mental and social well being. The health of all individuals is dependent on their physical environment, social environment, and their
GUIDELINES TO FILL IN HEALTH EXAMINATION REPORT
 GUIDELINES TO FILL IN HEALTH EXAMINATION REPORT 1. PLEASE READ THE INSTRUCTIONS CAREFULLY BEFORE FILLING IN THE FORM. 2. PLEASE FILL IN THE FORM IN ENGLISH LANGUAGE. 3. PLEASE WRITE IN CAPITAL LETTERS.
GUIDELINES TO FILL IN HEALTH EXAMINATION REPORT 1. PLEASE READ THE INSTRUCTIONS CAREFULLY BEFORE FILLING IN THE FORM. 2. PLEASE FILL IN THE FORM IN ENGLISH LANGUAGE. 3. PLEASE WRITE IN CAPITAL LETTERS.
A Guide To Safe Blood Transfusion Practice
 A Guide To Safe Blood Transfusion Practice Marie Browett, Pavlina Sharp, Fiona Waller, Hafiz Qureshi, Malcolm Chambers (on behalf of the UHL Blood Transfusion Team) A Guide To Safe Blood Transfusion Practice
A Guide To Safe Blood Transfusion Practice Marie Browett, Pavlina Sharp, Fiona Waller, Hafiz Qureshi, Malcolm Chambers (on behalf of the UHL Blood Transfusion Team) A Guide To Safe Blood Transfusion Practice
Organ Procurement and Transplantation Network
 OPTN Organ Procurement and Transplantation Network POLICIES This document provides the policy language approved by the OPTN/UNOS Board at its meeting in June 2015 as part of the Operations and Safety Committee
OPTN Organ Procurement and Transplantation Network POLICIES This document provides the policy language approved by the OPTN/UNOS Board at its meeting in June 2015 as part of the Operations and Safety Committee
Hot Topics in Tissue Safety
 Hot Topics in Tissue Safety 37 th Annual AATB Meeting National Harbor, Maryland 03 October 2013 Scott A. Brubaker, CTBS Chief Policy Officer PHS Guideline for Reducing Human Immunodeficiency Virus, Hepatitis
Hot Topics in Tissue Safety 37 th Annual AATB Meeting National Harbor, Maryland 03 October 2013 Scott A. Brubaker, CTBS Chief Policy Officer PHS Guideline for Reducing Human Immunodeficiency Virus, Hepatitis
CIRCULAR. of information. For the use of Labile Blood Products. Edition
 For the use of Labile Blood Products CIRCULAR of information july 2009 Edition WARNING: The risk of transmitting known and unknown infectious disease agents is present in the transfusion of labile blood
For the use of Labile Blood Products CIRCULAR of information july 2009 Edition WARNING: The risk of transmitting known and unknown infectious disease agents is present in the transfusion of labile blood
Medical and Dental Health History Form Getting to Know You As Our Patient
 Medical and Dental Health History Form Getting to Know You As Our Patient Account number: Date: Patient name (first and last): Name of previous dentist/location: Date of last dental examination: Date of
Medical and Dental Health History Form Getting to Know You As Our Patient Account number: Date: Patient name (first and last): Name of previous dentist/location: Date of last dental examination: Date of
Blood Products & Transfusion. Karim Rafaat, M.D.
 Blood Products & Transfusion Karim Rafaat, M.D. Compatibility Testing Compatibility testing involves three separate procedures involving both donor and recipient blood. 1. ABO & Rh blood type identification
Blood Products & Transfusion Karim Rafaat, M.D. Compatibility Testing Compatibility testing involves three separate procedures involving both donor and recipient blood. 1. ABO & Rh blood type identification
