New Zealand Bone Marrow Donor Registry
|
|
|
- Thomas Miller
- 5 years ago
- Views:
Transcription
1 Title: SECTION 4.0 NZBMDR STANDARDS DONOR RECRUITMENT, ENROLMENT & INFORMATION Authorised by: Executive Officer Contributing Authors: ABMDR Dr Hilary Blacklock Raewyn Fisher NZBMDR-Guidelines Date Effective: 10/5/2017 Page 1
2 Table of Contents SECTION 4.0 NZBMDR STANDARDS DONOR RECRUITMENT, ENROLMENT & INFORMATION RECRUITMENT POLICY DONOR AGE CRITERIA ELIGIBILITY FOR ENROLMENT AS A BONE MARROW DONOR Infectious Disease Markers (see Section 6.0) NZBMDR Policy on variant CJD (vcjd) and Donor Deferral Other Causes for Ineligibility PROTECTION OF DONORS FAMILY DONOR RECRUITMENT Process PLATELET DONOR RECRUITMENT DONOR INFORMATION Stage 1 - At RECRUITMENT Stage 2 - At RECALL for repeat blood sampling Stage 3 - At FINAL ASSESSMENT for donation Information concerning requests for Second or Subsequent Donations International Movement of Donors... 7 Criteria for American Society for Anaesthesiology (ASA) Forms / Work Instructions New Donor Consent Form Donor Consent Form for previously Tissue typed donors DR Consent Form VT Consent Form VT Information Workup Information Consent Form A Consent Form TR Consent Form DR Consent Form VT Donor VT Information Donor Workup Information NZBMDR Guidelines Date Effective: 10/5/2017 Page 2
3 SECTION 4.0 NZBMDR STANDARD DONOR RECRUITMENT, ENROLMENT & INFORMATION 4.0 NZBMDR is funded to recruit males with Maori or Pacific Island ancestry. Other NZ Ethnic minority groups may also be recruited. People who have been tissue typed in another capacity such as platelet donors, or family members who have been tissue typed in the search for a family donor may also be approached to join the NZBMDR. A signed consent form must be obtained at the time of recruitment Refer: Consent Form A Unrelated Donor 4.1 DONOR AGE CRITERIA New unrelated donors should only be enrolled by the NZBMDR if they are between the ages of 18 and 40 years. Previously tissue typed donors such as platelet donors, or family members who have been tissue typed in the search for a family donor will be enrolled up to the age of 59 years. Donors will be removed from the NZBMDR on their 60 th birthday. Younger male donors are the preferred group for recruitment. 4.2 ELIGIBILITY FOR ENROLMENT AS A BONE MARROW DONOR For the protection of recipients, criteria regarding infectious disease markers and the diagnosis of certain medical conditions as per NZBS A-Z Collection Standards should be adhered to regarding the donation of stem cells. However given that there may be only one acceptable HLA matched donor available for an individual patient the medical condition of an otherwise ineligible donor may be acceptable. Whether or not the transplant proceeds is at the discretion of the transplant physician in consultation with the patient. Donors should be eligible and willing to donate blood. Those unable to meet the blood donation criteria will usually not be enrolled on the NZBMDR. For those wishing to join the Registry but who are unable to meet the blood donation criteria due to the CJD restriction, the Donor Centre Director (via the NZBMDR Executive Officer) may give approval for them to enrol if this would enhance the diversity of the Registry. NZBMDR Guidelines Date Effective: 10/5/2017 Page 3
4 4.2.1 Specific tests for infectious disease markers are defined in NZBMDR Standard - Infectious Disease Markers (refer: Section 6.0). Contraindications to enrolment are: the risk or confirmation of HIV1/2 or the confirmation of HTLV I/II; Donors with a diagnosis of Creutzfeldt-Jakob Disease (CJD), Gerstmann Straussler Scheinker Syndrome (GSS), Fatal Familial Insomnia (FFI) or at risk of developing CJD, GSS or FFI must be deferred permanently; Donors with a family member with CJD, GSS or FFI must be deferred permanently. Donors with haemophilia or a related clotting disorder who have received treatment with clotting factor concentrate at any time must be permanently deferred NZBMDR Policy on variant CJD (vcjd) and Donor Deferral Blood donors who have lived in or visited the United Kingdom (UK) (England, Scotland, Wales, Northern Ireland, Isle of Man and the Channel islands) or in France or the Republic of Ireland for a total period of 6 months or longer, between 1980 and 1996, are deferred from donating blood and should not be recruited as a new donor to the NZBMDR. Donors who received a blood transfusion in the United Kingdom, France or the republic of Ireland from 1980 onwards are deferred from donating blood and should not be recruited to the NZBMDR as new donors. Existing donors already on the registry, or donors who have transferred from overseas bone marrow donor registries and are deferred from donating blood because of the NZBS policy on vcjd, should have this information recorded on the MATCHPOINT database and will remain on the NZBMDR. Donors who have previously been regular blood donors but are now excluded from donating blood because of the vcjd policy may be considered by the NZBMDR, if their joining will enhance the diversity of the registry. This information should be recorded on the MATCHPOINT database Other Causes for Ineligibility Prospective donors who have had successfully treated basal cell carcinoma and squamous cell carcinoma or successful therapy for carcinoma in situ of the cervix, should be evaluated by a medical officer before being accepted as donors see NZBS Collection Standards manual Donors with a history of any other diseases of malignant origin especially leukaemia, lymphoma or myeloma or other haematological conditions must be deferred permanently. A donor with a diagnosis of one of the following must be removed from the Registry: NZBMDR Guidelines Date Effective: 10/5/2017 Page 4
5 Myeloproliferative diseases including polycythemia vera, thrombocythaemia and myelofibrosis. Other evidence of serious bone marrow dysfunction (e.g. refractory anaemias, chronic neutropenias, thrombocytopenia). Thalassaemia major, HbS and severe coagulopathy. The Donor Centres must advise the Transplant Centres of any significant medical conditions affecting the donor. Instances where exceptions occur, such as residence in the UK between the years of 1980 and 1996, must be documented and communicated to the Transplant Centre. 4.3 PROTECTION OF DONORS Potential unrelated donors who have a condition which would place them at risk if undergoing a general anaesthetic, as defined by the American Society for Anaesthesiology Class I standards will only be available for a PBSC collection. Refer Attachment Section 4: Criteria for American Society for Anaesthesiology (ASA) This will be established at the time of the donor's physical examination by a third party haematologist and communicated to the Transplant Centre as this may negate a marrow collection if insufficient HPA, apheresis cells are collected. 4.4 FAMILY DONOR RECRUITMENT Individuals who have been tissue typed as part of an extended family search may be approached by the relevant search centre to consider enrolling with NZBMDR. This is particularly important for those family members tissue typed for patients of minority ethnic groups, particularly those who do not have a Bone Marrow Donor Registry in their country of origin. Apart from the obvious advantage of having these individuals on the registry, as genotyping and in some cases Class II typing have been performed, often many family members wish to make a commitment to the Registry Process The Information Brochure and Form TR may be sent to the family members aged 18 to 59 by agreement with the Transplant Centre. Refer: Information brochure and Transfer Consent Form TR NZBMDR Guidelines Date Effective: 10/5/2017 Page 5
6 PLATELET and PLASMA DONOR RECRUITMENT NZBS platelet and plasma donors, up to the age of 59, who have been tissue typed may be approached by NZ BS to consider joining the NZBMDR. Donors must have read the Information brochure. Form TR must be completed and forwarded to the NZBMDR. DRB1* tissue typing may be required. Refer: Consent Form TR- Transfer of Previously tissue typed donors onto NZBMDR 4.5 DONOR INFORMATION A potential donor must be aware of the following before joining the registry NZBMDR donors are volunteer donors Donors must be willing to donate to any patient in need, from any part of the world Donors will not be paid for their donation but may be reimbursed for expenses such as travel and accommodation associated with the workup and collection. Expenses for one companion may also be reimbursed. Donors must be informed of their potential role in the donation of HPC, the risks involved in the donation and the tests required during the donor workup.. Donors must be informed about the use of any medical intervention such as a general anaesthetic or the administration of GCSF and the known risks and side effects Donors must be informed about the possibility of a request for subsequent donations Donors must be informed that there is a follow-up process to ensure their complete recovery Donors must be informed that they can withdraw from the registry at any time but also be made aware of the implications of this action at certain periods of the search process Donors must be made aware of the privacy policy of the registry It is essential that donors receive accurate and up to date information on the donation process. This will take place in three stages: Stage 1 - At RECRUITMENT The NZBMDR New Donor information brochure must be provided to potential donors at recruitment. Only after this has been read and understood should Consent Form A be signed. Most NZBMDR donors join the registry when they donate a unit of blood at an NZBS collection site. Enrolment will be performed under the direction of NZBS staff that have training and experience in donor enrolment and donor management activities including Informed consent, confidentiality, and health screening. All NZBS staff will have an information session with a staff member from NZBMDR at which they will be given information on the donation process. NZBMDR Guidelines Date Effective: 10/5/2017 Page 6
7 Refer: Unrelated Donor Consent Form A Donor Information Brochure Stage 2 - At RECALL for repeat blood sampling NZBMDR staff will give donors information regarding the search process by phone and send the Donor VT information (3 page document) before a VT sample is collected. Donors should be contacted no later than eight weeks after a verification testing sample was drawn, to give them an update on the progress of this testing. Refer: SOP 601 DR Consent SOP 503 VT Consent SOP 502 Collection of VT samples Donor VT Information Stage 3 - At FINAL ASSESSMENT for donation NZBMDR staff will give donors information by phone and send the Donor Workup Information Document (4 pages) when workup is requested. Prior to donation, donors must meet with an independent third party haematologist for an information session, medical assessment and to consent to the release of non identifying details to the Transplant Centre. Potential donors with learning difficulties should be excluded from donating stem cells. Refer: section 5 All women of child bearing age must have a pregnancy test at workup. Female donors must be given full disclosure of risks associated with GCSF including the possibility of foetal malformation and/or miscarriage if the donation proceeds whilst pregnant. The result must be documented on Form 43 Refer: Interpretation of Third Party Physical Exam at Workup Form 43 Donor Workup Information, HPC, apheresis Information, HPC SOP 802 Donor Contact Call Information concerning requests for Second or Subsequent Donations At the time of the final assessment of the donor (Work up), donors must be informed that they may be asked to donate stem cells (either HPC marrow or HPC apheresis) on a second or subsequent occasion if the patient has experienced graft failure. Alternatively they may be asked to donate lymphocytes if the recipient has had disease relapse. This information is contained in the donor workup information sheet which becomes part of the consent form signed by the donor at the medical assessment. The transplant centre will be informed both from Form 43 and Form WU2 as to the willingness of donors to be contacted should a subsequent donation be requested NZBMDR Guidelines Date Effective: 10/5/2017 Page 7
8 For further information concerning this process refer Section INTERNATIONAL TRANSFER OF DONORS NZBMDR donors, who travel and reside overseas for an extended period of time, may transfer to the Registry of their country of residence. Likewise, international donors residing for any length of time in New Zealand may be transferred either permanently or temporarily to the NZBMDR. To join the NZBMDR they must be able to complete the Blood Donor Declaration Form and Consent Form TR. Their recruitment to the NZBMDR would be subject to the Guidelines of the NZBMDR. NZBMDR Guidelines Date Effective: 10/5/2017 Page 8
9 CRITERIA FOR AMERICAN SOCIETY FOR ANAESTHESIOLOGY (ASA) CLASS I ANAESTHETIC RISK "The donor has no organic, physiological, biochemical or psychiatric disturbance." Minimum Criteria for acceptance at Medical Director's discretion are those of ASA CLASS II ANAESTHETIC RISK "Mild to moderate systemic disturbance caused by pathophysiological processes such as nonlimiting organic heart disease; mild diabetes; essential hypertension; anaemia; chronic bronchitis; morbid obesity; smoking" as described below. Suggested criteria for exclusion of patients from the Bone Marrow Donor Register due to increased risks from anaesthesia: 1 Personal or family history of severe reactions to general anaesthesia. Include known susceptibility to malignant hyperpyrexia, porphyria etc. 2 Cardiovascular disease: Any history of angina, myocardial infarction, atrial fibrillation or ventricular arrhythmia Hypertension that is not well controlled Congenital and valvular heart disease Any episodes of congestive heart failure 3 Pulmonary disease: Asthma leading to hospitalisation in the past year Chronic airways disease with FVC or FEV <70% of predicted value, secondary polycythemia 4 Haematologic function: Chronic anaemia Polycythemia Coagulation disorders, including history of DVT in past year. 5 Psychiatric disorders: Treatment with anti-depressant drugs or major tranquillisers in the past year. 6 Endocrine and Renal function: Diabetes - all insulin dependent diabetics; those requiring hospital for control, or with history of hypoglycaemia episodes in the past six months Thyroid disease - unless stable on thyroid replacement Steroid treatment in the past year Renal impairment with elevated serum creatinine 7 Gastrointestinal function: Hiatus hernia Peptic ulcer, unless endoscopic evidence of healing or asymptomatic for 12 months History of hepatitis or jaundice in past year. Hep B Ag or HIV positive, abnormal liver function tests Obesity, >40% over ideal weight NZBMDR Guidelines Date Effective: 10/5/2017 Page 9
Title: SECTION 7.0 NZBMDR STANDARDS PROCESS FOR DONOR IDENTIFICATION
 Title: SECTION 7.0 NZBMDR STANDARDS PROCESS FOR DONOR IDENTIFICATION Authorised by: Executive Officer Contributing Authors: ABMDR Scientific Expert Advisory Committee Sally Gordon Dr Hilary Blacklock Raewyn
Title: SECTION 7.0 NZBMDR STANDARDS PROCESS FOR DONOR IDENTIFICATION Authorised by: Executive Officer Contributing Authors: ABMDR Scientific Expert Advisory Committee Sally Gordon Dr Hilary Blacklock Raewyn
Ontario s Referral and Listing Criteria for Adult Heart Transplantation
 Ontario s Referral and Listing Criteria for Adult Heart Transplantation Version 3.0 Trillium Gift of Life Network Adult Heart Transplantation Referral & Listing Criteria PATIENT REFERRAL CRITERIA: The
Ontario s Referral and Listing Criteria for Adult Heart Transplantation Version 3.0 Trillium Gift of Life Network Adult Heart Transplantation Referral & Listing Criteria PATIENT REFERRAL CRITERIA: The
Donor Registration and Consent for HLA Typing
 Place NMDP Bar Code label here Jackie (left), donated to save the life of Paizley (right) Randy (left), donated to save the life of Luke (right) Tobias (left), donated to save the life of Betsy (right)
Place NMDP Bar Code label here Jackie (left), donated to save the life of Paizley (right) Randy (left), donated to save the life of Luke (right) Tobias (left), donated to save the life of Betsy (right)
HEALTH SCREENING QUESTIONNAIRE CT Work-up. Donor ID EdgeCell #:
 HEALTH SCREENING QUESTIONNAIRE CT Work-up Héma-Québec Registre des Donneurs de Cellules Souches 4045 Côte-vertu, St-Laurent, QC, Canada, H4R 2W7 Tél : + 514-832-1031 Fax : + 514-832-0266 www.hema-quebec.qc.ca
HEALTH SCREENING QUESTIONNAIRE CT Work-up Héma-Québec Registre des Donneurs de Cellules Souches 4045 Côte-vertu, St-Laurent, QC, Canada, H4R 2W7 Tél : + 514-832-1031 Fax : + 514-832-0266 www.hema-quebec.qc.ca
Ontario s Referral and Listing Criteria for Adult Kidney Transplantation
 Ontario s Referral and Listing Criteria for Adult Kidney Transplantation Version 3.0 Trillium Gift of Life Network Adult Kidney Transplantation Referral & Listing Criteria PATIENT REFERRAL CRITERIA: The
Ontario s Referral and Listing Criteria for Adult Kidney Transplantation Version 3.0 Trillium Gift of Life Network Adult Kidney Transplantation Referral & Listing Criteria PATIENT REFERRAL CRITERIA: The
Overview of Organ Donation and Transplantation
 2 Overview of Organ Donation and Transplantation Overview of Organ Donation and Transplantation A summary of organ donation and transplantation activity in the UK during the financial year from April 207
2 Overview of Organ Donation and Transplantation Overview of Organ Donation and Transplantation A summary of organ donation and transplantation activity in the UK during the financial year from April 207
Cord Blood Medical History/ Health Assessment Questionnaire
 OTT BRM EDM VAN Date: YYYY / MM / DD Phone Interview (if applicable) Informed Consent on file * Mandatory fields GENERAL INFORMATION *Last Name As per government ID *First Name As per government ID *DOB
OTT BRM EDM VAN Date: YYYY / MM / DD Phone Interview (if applicable) Informed Consent on file * Mandatory fields GENERAL INFORMATION *Last Name As per government ID *First Name As per government ID *DOB
HEALTH SCREENING QUESTIONNAIRE. Work-up. Donor ID EdgeCell #:
 HEALTH SCREENING QUESTIONNAIRE Héma-Québec Registre des Donneurs de Cellules Souches 4045 Côte-vertu, St-Laurent, QC, Canada, H4R 2W7 Tél : + 514-832-1031 Fax : + 514-832-0266 www.hema-quebec.qc.ca CT
HEALTH SCREENING QUESTIONNAIRE Héma-Québec Registre des Donneurs de Cellules Souches 4045 Côte-vertu, St-Laurent, QC, Canada, H4R 2W7 Tél : + 514-832-1031 Fax : + 514-832-0266 www.hema-quebec.qc.ca CT
Ontario s Referral and Listing Criteria for Adult Pancreas-After- Kidney Transplantation
 Ontario s Referral and Listing Criteria for Adult Pancreas-After- Kidney Transplantation Version 2.0 Trillium Gift of Life Network Adult Pancreas-After-Kidney Transplantation Referral & Listing Criteria
Ontario s Referral and Listing Criteria for Adult Pancreas-After- Kidney Transplantation Version 2.0 Trillium Gift of Life Network Adult Pancreas-After-Kidney Transplantation Referral & Listing Criteria
DHQ Flowcharts v2.0 eff. February 2016
 Question: 1. Are you feeling healthy and well today? Donor Eligibility: A person should be free of infectious diseases, including colds, on the day of donation. A person who is not in good health should
Question: 1. Are you feeling healthy and well today? Donor Eligibility: A person should be free of infectious diseases, including colds, on the day of donation. A person who is not in good health should
TESTS: Baseline tests: - FBC, U&Es, LFTs, creatinine. - Physical exam including splenic measurement by palpation - Weight - ECG, blood pressure.
 INDICATIONS FOR USE: Ruxolitinib Monotherapy INDICATION ICD10 Protocol Code Treatment of disease-related splenomegaly or symptoms in adult patients with: Primary myelofibrosis (chronic idiopathic myelofibrosis)
INDICATIONS FOR USE: Ruxolitinib Monotherapy INDICATION ICD10 Protocol Code Treatment of disease-related splenomegaly or symptoms in adult patients with: Primary myelofibrosis (chronic idiopathic myelofibrosis)
BLOOD DONATION Northern Ireland Blood Tranfusion Service
 FREQUENTLY ASKED QUESTIONS BLOOD DONATION Northern Ireland Blood Tranfusion Service Why do we need donated blood? Everyone knows blood is literally a lifesaver for those who have been in an accident or
FREQUENTLY ASKED QUESTIONS BLOOD DONATION Northern Ireland Blood Tranfusion Service Why do we need donated blood? Everyone knows blood is literally a lifesaver for those who have been in an accident or
Aquila Smoldering Multiple Myeloma
 Inklusionskriterier: Ja Nej 1. At least 18 years of age or at least the legal age of consent in the jurisdiction in which the study is taking place, whichever is the older age. 2. Diagnosis of SMM for
Inklusionskriterier: Ja Nej 1. At least 18 years of age or at least the legal age of consent in the jurisdiction in which the study is taking place, whichever is the older age. 2. Diagnosis of SMM for
TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING October2010. Issue Summary
 TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING 28-29October2010 Issue Summary Informational Topic: FDA s Geographic Donor Deferral Policy to Reduce the Possible Risk of Transmission
TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING 28-29October2010 Issue Summary Informational Topic: FDA s Geographic Donor Deferral Policy to Reduce the Possible Risk of Transmission
Specific Requirements
 Specific Requirements AIMS Specific requirements your patients have for transfusion and how this is managed Classify which patients require: Irradiated components CMV negative components Washed components
Specific Requirements AIMS Specific requirements your patients have for transfusion and how this is managed Classify which patients require: Irradiated components CMV negative components Washed components
OneMatch Stem Cell and Marrow Network. Training Guide
 OneMatch Stem Cell and Marrow Network Training Guide What is OneMatch all about? OneMatch is a Canadian Program that matches and coordinates the collection of stem cells from potential donors to help save
OneMatch Stem Cell and Marrow Network Training Guide What is OneMatch all about? OneMatch is a Canadian Program that matches and coordinates the collection of stem cells from potential donors to help save
Guidelines for Gamma Irradiation of Blood Components
 AUSTRALIAN & NEW ZEALAND SOCIETY OF BLOOD TRANSFUSION INC. AUSTRALIAN RED CROSS BLOOD SERVICE NEW ZEALAND BLOOD SERVICE Guidelines for Gamma Irradiation of Blood Components Revised 2003 AN ZS B T Australian
AUSTRALIAN & NEW ZEALAND SOCIETY OF BLOOD TRANSFUSION INC. AUSTRALIAN RED CROSS BLOOD SERVICE NEW ZEALAND BLOOD SERVICE Guidelines for Gamma Irradiation of Blood Components Revised 2003 AN ZS B T Australian
Comorbidity or medical history Existing diagnoses between 1 January 2007 and 31 December 2011 AF management care AF symptoms Tachycardia
 Supplementary Table S1 International Classification of Disease 10 (ICD-10) codes Comorbidity or medical history Existing diagnoses between 1 January 2007 and 31 December 2011 AF management care I48 AF
Supplementary Table S1 International Classification of Disease 10 (ICD-10) codes Comorbidity or medical history Existing diagnoses between 1 January 2007 and 31 December 2011 AF management care I48 AF
Online Supplementary Data. Country Number of centers Number of patients randomized
 A Randomized, Double-Blind, -Controlled, Phase-2B Study to Evaluate the Safety and Efficacy of Recombinant Human Soluble Thrombomodulin, ART-123, in Patients with Sepsis and Suspected Disseminated Intravascular
A Randomized, Double-Blind, -Controlled, Phase-2B Study to Evaluate the Safety and Efficacy of Recombinant Human Soluble Thrombomodulin, ART-123, in Patients with Sepsis and Suspected Disseminated Intravascular
For practical information about using Revlimid, patients should read the package leaflet or contact their doctor or pharmacist.
 EMA/113870/2017 EMEA/H/C/000717 EPAR summary for the public lenalidomide This is a summary of the European public assessment report (EPAR) for. It explains how the Agency assessed the medicine to recommend
EMA/113870/2017 EMEA/H/C/000717 EPAR summary for the public lenalidomide This is a summary of the European public assessment report (EPAR) for. It explains how the Agency assessed the medicine to recommend
Donatore HLA identico di anni o MUD giovane?
 Donatore HLA identico di 60-70 anni o MUD giovane? Stella Santarone Dipartimento di Ematologia, Medicina Trasfusionale e Biotecnologie Pescara AGENDA 1. Stem Cell Donation: fatalities and severe events
Donatore HLA identico di 60-70 anni o MUD giovane? Stella Santarone Dipartimento di Ematologia, Medicina Trasfusionale e Biotecnologie Pescara AGENDA 1. Stem Cell Donation: fatalities and severe events
Living Donor Paired Exchange (LDPE)
 Living Donor Paired Exchange (LDPE) Why do we need Living Donation? 3,796 patients waiting for an organ transplant 2,679 (71%) waiting for a kidney transplant 249 people died while waiting for an organ
Living Donor Paired Exchange (LDPE) Why do we need Living Donation? 3,796 patients waiting for an organ transplant 2,679 (71%) waiting for a kidney transplant 249 people died while waiting for an organ
Organ Donation and Transplantation. Activity Report 2017/18
 Organ Donation and Transplantation Activity Report 2017/18 Preface This report has been produced by Statistics and Clinical Studies, NHS Blood and Transplant. All figures quoted in this report are as reported
Organ Donation and Transplantation Activity Report 2017/18 Preface This report has been produced by Statistics and Clinical Studies, NHS Blood and Transplant. All figures quoted in this report are as reported
Elements for a Public Summary
 VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Chronic lymphocytic leukaemia 1 Chronic lymphocytic leukemia (CLL) is a condition characterized by a progressive accumulation
VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Chronic lymphocytic leukaemia 1 Chronic lymphocytic leukemia (CLL) is a condition characterized by a progressive accumulation
Not Under Document Control If Printed. Donor Educational Materials. If you are deferred
 Jul 2009 (yellow version) Donor Educational Materials Thank you for coming in today! These educational materials explain how you can help us make the donation process safe for you and patients who might
Jul 2009 (yellow version) Donor Educational Materials Thank you for coming in today! These educational materials explain how you can help us make the donation process safe for you and patients who might
Blood Donor Counselling
 Blood Donor Counselling A presentation for the Nepal Red Cross Society Blood Transfusion Service Dr Che Kit Lin On behalf of GAP and Hong Kong Red Cross 26 th August 2014 Purpose and Outcomes of Workshop
Blood Donor Counselling A presentation for the Nepal Red Cross Society Blood Transfusion Service Dr Che Kit Lin On behalf of GAP and Hong Kong Red Cross 26 th August 2014 Purpose and Outcomes of Workshop
Question: 1. Are you currently taking an antibiotic?
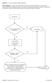 Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The number of patients on the active liver transplant list at 31 March 2017 was 530, a fall of 8% from 2016
 8 Liver Activity Liver Activity Key messages The number of patients on the active liver transplant list at 31 March 2017 was 530, a fall of 8% from 2016 The number of liver donors after brain death increased
8 Liver Activity Liver Activity Key messages The number of patients on the active liver transplant list at 31 March 2017 was 530, a fall of 8% from 2016 The number of liver donors after brain death increased
Organ Donation Activity
 3 Organ Donation Activity Organ Donation Activity Key messages There has been a 11% increase in deceased donors (to 1,574) and a
3 Organ Donation Activity Organ Donation Activity Key messages There has been a 11% increase in deceased donors (to 1,574) and a
A trial looking at selumetinib for thyroid cancer that has stopped taking up radioactive iodine (SEL-I-METRY)
 Page 1 of 6 A trial looking at selumetinib for thyroid cancer that has stopped taking up radioactive iodine (SEL-I-METRY) Cancer type: Thyroid cancer Status: Open Phase: Phase 2 This trial is for people
Page 1 of 6 A trial looking at selumetinib for thyroid cancer that has stopped taking up radioactive iodine (SEL-I-METRY) Cancer type: Thyroid cancer Status: Open Phase: Phase 2 This trial is for people
HEALTH SERVICES POLICY & PROCEDURE MANUAL
 PAGE 1 of 6 PURPOSE To establish basic understanding of indications and contraindications for transplantation of various organs. POLICY The N.C. Department of Correction, Division of Prisons, Health Services
PAGE 1 of 6 PURPOSE To establish basic understanding of indications and contraindications for transplantation of various organs. POLICY The N.C. Department of Correction, Division of Prisons, Health Services
Blood Cancers. Blood Cells. Blood Cancers: Progress and Promise. Bone Marrow and Blood. Lymph Nodes and Spleen
 Blood Cancers: Progress and Promise Mike Barnett & Khaled Ramadan Division of Hematology Department of Medicine Providence Health Care & UBC Blood Cancers Significant health problem Arise from normal cells
Blood Cancers: Progress and Promise Mike Barnett & Khaled Ramadan Division of Hematology Department of Medicine Providence Health Care & UBC Blood Cancers Significant health problem Arise from normal cells
CHAPTER 4 SECTION 24.2 HEART TRANSPLANTATION TRICARE POLICY MANUAL M, AUGUST 1, 2002 SURGERY. ISSUE DATE: December 11, 1986 AUTHORITY:
 SURGERY CHAPTER 4 SECTION 24.2 ISSUE DATE: December 11, 1986 AUTHORITY: 32 CFR 199.4(e)(5) I. CPT 1 PROCEDURE CODES 33940-33945, 33975-33980 II. POLICY A. Benefits are allowed for heart transplantation.
SURGERY CHAPTER 4 SECTION 24.2 ISSUE DATE: December 11, 1986 AUTHORITY: 32 CFR 199.4(e)(5) I. CPT 1 PROCEDURE CODES 33940-33945, 33975-33980 II. POLICY A. Benefits are allowed for heart transplantation.
CREUTZFELDT-JAKOB DISEASE (CJD)
 Cause/Epidemiology CREUTZFELDT-JAKOB DISEASE (CJD) The agent causing CJD and other human transmissible spongiform encephalopathy (TSE) has not yet been definitively identified. It was originally thought
Cause/Epidemiology CREUTZFELDT-JAKOB DISEASE (CJD) The agent causing CJD and other human transmissible spongiform encephalopathy (TSE) has not yet been definitively identified. It was originally thought
PATIENT SELECTION FOR DECEASED DONOR KIDNEY ONLY TRANSPLANTATION
 PATIENT SELECTION FOR DECEASED DONOR KIDNEY ONLY TRANSPLANTATION This policy has been created by the Kidney Advisory Group on behalf of NHSBT. The policy has been considered and approved by the Organ Donation
PATIENT SELECTION FOR DECEASED DONOR KIDNEY ONLY TRANSPLANTATION This policy has been created by the Kidney Advisory Group on behalf of NHSBT. The policy has been considered and approved by the Organ Donation
Question: 1. Are you currently taking an antibiotic?
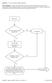 Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
EZER MIZION PROCEDURES FOR TRANSPLANT CENTERS AND COOPERATIVE REGISTRIES
 EZER MIZION PROCEDURES FOR TRANSPLANT CENTERS AND COOPERATIVE REGISTRIES Table of content 1. Purpose and Scope... 2 2. Introduction... 2 3. Procedure for Requesting a Search... 3 4. The Search Process...
EZER MIZION PROCEDURES FOR TRANSPLANT CENTERS AND COOPERATIVE REGISTRIES Table of content 1. Purpose and Scope... 2 2. Introduction... 2 3. Procedure for Requesting a Search... 3 4. The Search Process...
Organ Donation and Transplantation. Activity Report 2015/16
 Organ Donation and Transplantation Activity Report 2015/16 Preface This report has been produced by Statistics and Clinical Studies, NHS Blood and Transplant. All figures quoted in this report are as reported
Organ Donation and Transplantation Activity Report 2015/16 Preface This report has been produced by Statistics and Clinical Studies, NHS Blood and Transplant. All figures quoted in this report are as reported
Information for patients with Sickle Cell Disease who may need a blood transfusion. Patient information
 Information for patients with Sickle Cell Disease who may need a blood transfusion Patient information This information leaflet answers some of the questions you may have about having a blood transfusion
Information for patients with Sickle Cell Disease who may need a blood transfusion Patient information This information leaflet answers some of the questions you may have about having a blood transfusion
Appendix 6: Indications for adult allogeneic bone marrow transplant in New Zealand
 Appendix 6: Indications for adult allogeneic bone marrow transplant in New Zealand This list provides indications for the majority of adult BMTs that are performed in New Zealand. A small number of BMTs
Appendix 6: Indications for adult allogeneic bone marrow transplant in New Zealand This list provides indications for the majority of adult BMTs that are performed in New Zealand. A small number of BMTs
Organ Procurement and Transplantation Network
 OPTN Organ Procurement and Transplantation Network POLICIES This document provides the policy language approved by the OPTN/UNOS Board at its meeting in June 2015 as part of the Operations and Safety Committee
OPTN Organ Procurement and Transplantation Network POLICIES This document provides the policy language approved by the OPTN/UNOS Board at its meeting in June 2015 as part of the Operations and Safety Committee
MANAGEMENT OF OVERANTICOAGULATION AND PREOPERATIVE MANAGEMENT OF WARFARIN DOSE 1. GUIDELINES FOR THE MANAGEMENT OF AN ELEVATED INR
 MANAGEMENT OF OVERANTICOAGULATION AND PREOPERATIVE MANAGEMENT OF WARFARIN DOSE 1. GUIDELINES FOR THE MANAGEMENT OF AN ELEVATED INR 1.1 Time to lower INR Prothrombinex-VF - 15 minutes Fresh Frozen Plasma
MANAGEMENT OF OVERANTICOAGULATION AND PREOPERATIVE MANAGEMENT OF WARFARIN DOSE 1. GUIDELINES FOR THE MANAGEMENT OF AN ELEVATED INR 1.1 Time to lower INR Prothrombinex-VF - 15 minutes Fresh Frozen Plasma
D7.1 Report summarising results of survey of EU countries to identify volumes and trends in relation to the import and export of stem cells
 Disclaimer: The content of this Deliverable represents the views of the author only and is his/her sole responsibility; it cannot be considered to reflect the views of the European Commission and/or the
Disclaimer: The content of this Deliverable represents the views of the author only and is his/her sole responsibility; it cannot be considered to reflect the views of the European Commission and/or the
D7.1 Report summarising results of survey of EU countries to identify volumes and trends in relation to the import and export of stem cells
 Disclaimer: The content of this Deliverable represents the views of the author only and is his/her sole responsibility; it cannot be considered to reflect the views of the European Commission and/or the
Disclaimer: The content of this Deliverable represents the views of the author only and is his/her sole responsibility; it cannot be considered to reflect the views of the European Commission and/or the
FREQUENTLY ASKED QUESTIONS BONE MARROW / STEM CELL DONATION
 FREQUENTLY ASKED QUESTIONS BONE MARROW / STEM CELL DONATION About Anthony Nolan Anthony Nolan is a pioneering charity that saves the lives of people with blood cancer who need a bone marrow, or stem cell,
FREQUENTLY ASKED QUESTIONS BONE MARROW / STEM CELL DONATION About Anthony Nolan Anthony Nolan is a pioneering charity that saves the lives of people with blood cancer who need a bone marrow, or stem cell,
Seventy percent of people who are candidates for allogeneic hematopoietic
 274 7th Biennial Symposium on Minorities, the Medically Underserved and Cancer Supplement to Cancer The National Marrow Donor Program Meeting the Needs of the Medically Underserved Dennis L. Confer, M.D.
274 7th Biennial Symposium on Minorities, the Medically Underserved and Cancer Supplement to Cancer The National Marrow Donor Program Meeting the Needs of the Medically Underserved Dennis L. Confer, M.D.
Chapter 4 Section Combined Heart-Kidney Transplantation (CHKT)
 Surgery Chapter 4 Section 24.3 Issue Date: May 7, 1999 Authority: 32 CFR 199.4(e)(5) 1.0 POLICY 1.1 is a TRICARE benefit that requires preauthorization. 1.1.1 A TRICARE Prime enrollee must have a referral
Surgery Chapter 4 Section 24.3 Issue Date: May 7, 1999 Authority: 32 CFR 199.4(e)(5) 1.0 POLICY 1.1 is a TRICARE benefit that requires preauthorization. 1.1.1 A TRICARE Prime enrollee must have a referral
This report has been produced by Statistics and Clinical Audit, NHS Blood and Transplant.
 Preface This report has been produced by Statistics and Clinical Audit, NHS Blood and Transplant. All figures quoted in this report are as reported to NHS Blood and Transplant by 20 May 2013 for the UK
Preface This report has been produced by Statistics and Clinical Audit, NHS Blood and Transplant. All figures quoted in this report are as reported to NHS Blood and Transplant by 20 May 2013 for the UK
INFECTED BLOOD INQUIRY TERMS OF REFERENCE
 INFECTED BLOOD INQUIRY TERMS OF REFERENCE What happened and why? 1. To examine the circumstances in which men, women and children 1 treated by national Health Services in the United Kingdom (collectively,
INFECTED BLOOD INQUIRY TERMS OF REFERENCE What happened and why? 1. To examine the circumstances in which men, women and children 1 treated by national Health Services in the United Kingdom (collectively,
The Blood Donor: Demographics, Donor Selection and Tests on Donor Blood. Liz Caffrey, Patricia Hewitt and John Barbara
 CHAPTER 1 The Blood Donor: Demographics, Donor Selection and Tests on Donor Blood Liz Caffrey, Patricia Hewitt and John Barbara 0.3 OVERVIEW Total 0.254 0.247 A safe and sufficient blood supply depends
CHAPTER 1 The Blood Donor: Demographics, Donor Selection and Tests on Donor Blood Liz Caffrey, Patricia Hewitt and John Barbara 0.3 OVERVIEW Total 0.254 0.247 A safe and sufficient blood supply depends
TRANSFUSION ASSOCIATED DISEASE, RECALL, OR COMPLICATION INVESTIGATION POLICY I. FATALITIES AND COMPLICATIONS ASSOCIATED WITH TRANSFUSION:
 I. FATALITIES AND COMPLICATIONS ASSOCIATED WITH TRANSFUSION: A. TRANSFUSION RELATED FATALITY: FDA and MEDIC must be notified immediately, and subsequently in writing, when a possible transfusion related
I. FATALITIES AND COMPLICATIONS ASSOCIATED WITH TRANSFUSION: A. TRANSFUSION RELATED FATALITY: FDA and MEDIC must be notified immediately, and subsequently in writing, when a possible transfusion related
Outline Pretransplant Essential data Why comorbidities are important? For patients with cancer For patients given allogeneic HCT
 Comorbidities before Allogeneic Hematopoietic Cell Transplantation (HCT) The HCT-specific Comorbidity Index (HCT-CI) Mohamed Sorror, M.D., M.Sc. FHCRC Seattle, WA Outline Pretransplant Essential data Why
Comorbidities before Allogeneic Hematopoietic Cell Transplantation (HCT) The HCT-specific Comorbidity Index (HCT-CI) Mohamed Sorror, M.D., M.Sc. FHCRC Seattle, WA Outline Pretransplant Essential data Why
Who and When to Refer for a Heart Transplant
 Who and When to Refer for a Heart Transplant Dr Jayan Parameshwar Consultant Cardiologist Papworth Hospital BSH 24 th November 2017 BSH Annual Autumn Meeting 2017 Presentation title: Who and when to refer
Who and When to Refer for a Heart Transplant Dr Jayan Parameshwar Consultant Cardiologist Papworth Hospital BSH 24 th November 2017 BSH Annual Autumn Meeting 2017 Presentation title: Who and when to refer
PATHOLOGY & PATHOPHYSIOLOGY
 PATHOLOGY & PATHOPHYSIOLOGY DISORDERS OF BLOOD DISORDERS OF BLOOD Disorders of Blood Infections Tumours Nutritional disorders Coagulation disorders Congenital disorders Septicaemia Leukemia Iron deficiency
PATHOLOGY & PATHOPHYSIOLOGY DISORDERS OF BLOOD DISORDERS OF BLOOD Disorders of Blood Infections Tumours Nutritional disorders Coagulation disorders Congenital disorders Septicaemia Leukemia Iron deficiency
PREOPERATIVE PATIENT PREPARATION PROTOCOL
 PREOPERATIVE PATIENT PREPARATION PROTOCOL Each surgical discipline should have a standard set of published guidelines for the preparation of its patients for theatre procedures. These should be readily
PREOPERATIVE PATIENT PREPARATION PROTOCOL Each surgical discipline should have a standard set of published guidelines for the preparation of its patients for theatre procedures. These should be readily
Subject ID: I N D # # U A * Consent Date: Day Month Year
 IND Study # Eligibility Checklist Pg 1 of 15 Instructions: Check the appropriate box for each Inclusion and Exclusion Criterion below. Each criterion must be marked and all protocol criteria have to be
IND Study # Eligibility Checklist Pg 1 of 15 Instructions: Check the appropriate box for each Inclusion and Exclusion Criterion below. Each criterion must be marked and all protocol criteria have to be
Blood transfusion. Dr. J. Potgieter Dept. of Haematology NHLS - TAD
 Blood transfusion Dr. J. Potgieter Dept. of Haematology NHLS - TAD General Blood is collected from volunteer donors >90% is separated into individual components and plasma Donors should be: healthy, have
Blood transfusion Dr. J. Potgieter Dept. of Haematology NHLS - TAD General Blood is collected from volunteer donors >90% is separated into individual components and plasma Donors should be: healthy, have
BLOOD COMPONENT SUPPORT OF RhD NEGATIVE INDIVIDUALS
 REASON FOR ISSUE: Review of section 4 along with changes in requirement to inform TMS in respect of authorisation process (in Appendix A) DCR16969. 1. INTRODUCTION of RhD positive blood components to an
REASON FOR ISSUE: Review of section 4 along with changes in requirement to inform TMS in respect of authorisation process (in Appendix A) DCR16969. 1. INTRODUCTION of RhD positive blood components to an
INFORMATION ONLY Changes to Requesting HLA/HPA Selected Apheresis Platelets Customer Letter #
 INFORMATION ONLY Changes to Requesting HLA/HPA Selected Apheresis Platelets Customer Letter # 2015-21 Head Office / Siège social 2015-08-11 Dear Customer, We are writing to inform you of changes to the
INFORMATION ONLY Changes to Requesting HLA/HPA Selected Apheresis Platelets Customer Letter # 2015-21 Head Office / Siège social 2015-08-11 Dear Customer, We are writing to inform you of changes to the
SUBCUTANEOUS Bortezomib + Thalidomide +Dexamethasone Available for Routine Use in
 SUBCUTANEOUS Bortezomib + Thalidomide +Dexamethasone Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby
SUBCUTANEOUS Bortezomib + Thalidomide +Dexamethasone Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby
OneMatch : A Closer Look. Gail Morris Principal Case Manager April 2017
 OneMatch : A Closer Look Gail Morris Principal Case Manager April 2017 Donor Recruitment Recruitment Managed by the CBS Donor Relations Team staff across Canada Danny, OneMatch Registrant Canadians register
OneMatch : A Closer Look Gail Morris Principal Case Manager April 2017 Donor Recruitment Recruitment Managed by the CBS Donor Relations Team staff across Canada Danny, OneMatch Registrant Canadians register
Inspections, Compliance, Enforcement, and Criminal Investigations. Central Texas Regional Blood & Tissue Center 07-Nov-03
 1 of 7 6/10/2009 2:20 PM Inspections, Compliance, Enforcement, and Criminal Investigations Department of Health and Human Services Public Health Service Food and Drug Administration Dallas District 4040
1 of 7 6/10/2009 2:20 PM Inspections, Compliance, Enforcement, and Criminal Investigations Department of Health and Human Services Public Health Service Food and Drug Administration Dallas District 4040
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
CODING GUIDELINES No. 3, June, 1999
 CODING GUIDELINES No. 3, June, 1999 Please note that the Coding Advisory Service Telephone Number is 0131-552-7325 The number is manned Tuesday to Thursday from 09.00 to 17.00 hrs. Coding Guidelines -
CODING GUIDELINES No. 3, June, 1999 Please note that the Coding Advisory Service Telephone Number is 0131-552-7325 The number is manned Tuesday to Thursday from 09.00 to 17.00 hrs. Coding Guidelines -
Medical Declaration Form. Important information to read before completing the form:
 Administered by Medical Declaration Form Important information to read before completing the form: Pre-Existing Medical conditions Travel insurance only provides cover for emergency medical events that
Administered by Medical Declaration Form Important information to read before completing the form: Pre-Existing Medical conditions Travel insurance only provides cover for emergency medical events that
DHQ v2.0, February 2016 Table Detailing Changes from v1.3 v1.3 Revised language in v2.0 Rationale Question 1: Are you
 DHQ v2.0, February 2016 Table Detailing s from v1.3 v1.3 Revised language in v2.0 Rationale Question 1: Are you Q 1, No Feeling healthy and well today? Question 2: Are you Q 2, No change Currently taking
DHQ v2.0, February 2016 Table Detailing s from v1.3 v1.3 Revised language in v2.0 Rationale Question 1: Are you Q 1, No Feeling healthy and well today? Question 2: Are you Q 2, No change Currently taking
Chapter 4 Section 24.2
 Surgery Chapter 4 Section 24.2 Issue Date: December 11, 1986 Authority: 32 CFR 199.4(e)(5) 1.0 CPT 1 PROCEDURE CODES 33940-33945, 33975-33980 2.0 POLICY 2.1 Benefits are allowed for heart transplantation.
Surgery Chapter 4 Section 24.2 Issue Date: December 11, 1986 Authority: 32 CFR 199.4(e)(5) 1.0 CPT 1 PROCEDURE CODES 33940-33945, 33975-33980 2.0 POLICY 2.1 Benefits are allowed for heart transplantation.
CHAPTER 3 SECTION 1.6B HEART-LUNG AND LUNG TRANSPLANTATION TRICARE POLICY MANUAL M, MARCH 15, 2002 SURGERY AND RELATED SERVICES
 TRICARE POLICY MANUAL 6010.47-M, MARCH 15, 2002 SURGERY AND RELATED SERVICES CHAPTER 3 SECTION 1.6B ISSUE DATE: October 27, 1995 AUTHORITY: 32 CFR 199.4(e)(5) I. CODES A. CPT 1 Procedure Codes 33930, 33935,
TRICARE POLICY MANUAL 6010.47-M, MARCH 15, 2002 SURGERY AND RELATED SERVICES CHAPTER 3 SECTION 1.6B ISSUE DATE: October 27, 1995 AUTHORITY: 32 CFR 199.4(e)(5) I. CODES A. CPT 1 Procedure Codes 33930, 33935,
Guideline scope Hypertension in adults (update)
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Guideline scope Hypertension in adults (update) This guideline will update the NICE guideline on hypertension in adults (CG127). The guideline will be
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Guideline scope Hypertension in adults (update) This guideline will update the NICE guideline on hypertension in adults (CG127). The guideline will be
Sperm Donation Patient Information Becoming a sperm donor
 Sperm Donation Patient Information Becoming a sperm donor b Contents Introduction 2 General information 2 What is sperm donation? 2 Who can use donated sperm? 2 Who can be a sperm donor? 2 Can I be paid
Sperm Donation Patient Information Becoming a sperm donor b Contents Introduction 2 General information 2 What is sperm donation? 2 Who can use donated sperm? 2 Who can be a sperm donor? 2 Can I be paid
Chapter 10. Cancer. ANZDATA Registry 39th Annual Report. Data to 31-Dec-2015
 Chapter Cancer 216 ANZDATA Registry 39th Annual Report Data to 31-Dec-215 Incidence of Cancer on Renal Replacement Therapy Figures.1-.6 and table.1 show the cumulative incidence of non-skin cancer in patients
Chapter Cancer 216 ANZDATA Registry 39th Annual Report Data to 31-Dec-215 Incidence of Cancer on Renal Replacement Therapy Figures.1-.6 and table.1 show the cumulative incidence of non-skin cancer in patients
NRG ONCOLOGY NRG-CC003
 NRG ONCOLOGY NRG-CC003 A Randomized Phase II/III Trial of Prophylactic Cranial Irradiation with or without Hippocampal Avoidance for Small Cell Lung Cancer SCHEMA Histologic proof or unequivocal cytologic
NRG ONCOLOGY NRG-CC003 A Randomized Phase II/III Trial of Prophylactic Cranial Irradiation with or without Hippocampal Avoidance for Small Cell Lung Cancer SCHEMA Histologic proof or unequivocal cytologic
BMTCN REVIEW COURSE PRE-TRANSPLANT CARE
 BMTCN REVIEW COURSE PRE-TRANSPLANT CARE Jennifer Shamai MS, RN, AOCNS, BMTCN Professional Practice Leader Department of Clinical Practice And Professional Education Click How to edit the Master Experts
BMTCN REVIEW COURSE PRE-TRANSPLANT CARE Jennifer Shamai MS, RN, AOCNS, BMTCN Professional Practice Leader Department of Clinical Practice And Professional Education Click How to edit the Master Experts
More Fun Than Giving Blood CLINIC RECRUITED
 More Fun Than Giving Blood CLINIC RECRUITED Are you thinking of becoming a sperm donor? Choosing to become a sperm donor is a generous act, and by entering our sperm donor program you can help make someone
More Fun Than Giving Blood CLINIC RECRUITED Are you thinking of becoming a sperm donor? Choosing to become a sperm donor is a generous act, and by entering our sperm donor program you can help make someone
You can save even more lives. Join the British Bone Marrow Registry
 You can save even more lives. Join the British Bone Marrow Registry What is bone marrow? Bone marrow is a remarkable factory. It s the soft, spongy tissue found at the centre of certain bones in your body
You can save even more lives. Join the British Bone Marrow Registry What is bone marrow? Bone marrow is a remarkable factory. It s the soft, spongy tissue found at the centre of certain bones in your body
Once you have phoned in and registered, all you need to do is take the forms to any of the following Pathcares:
 Sarah Stuart was recently diagnosed with aplastic anemia but tragically and with great sadness she passed away before we could find her a donor. Many people find themselves in the same situation as Sarah
Sarah Stuart was recently diagnosed with aplastic anemia but tragically and with great sadness she passed away before we could find her a donor. Many people find themselves in the same situation as Sarah
Plerixafor use for Peripheral Blood Stem Cell Mobilisation
 use for Peripheral Blood Stem Cell Mobilisation 1.0 Purpose The purpose of this protocol is to ensure standardised practice for the use of 1 (Mozobil ) in peripheral blood stem cell (PBSC) mobilisation.
use for Peripheral Blood Stem Cell Mobilisation 1.0 Purpose The purpose of this protocol is to ensure standardised practice for the use of 1 (Mozobil ) in peripheral blood stem cell (PBSC) mobilisation.
USER INSTRUCTIONS HPC, Cord Blood Donor History Questionnaire. Table of Contents
 Page 1 of 9 USER INSTRUCTIONS HPC, Cord Blood Donor History Questionnaire Table of Contents Purpose Introduction Capture Questions Methods of Administration DHQ Materials Structure and Content Reformatting
Page 1 of 9 USER INSTRUCTIONS HPC, Cord Blood Donor History Questionnaire Table of Contents Purpose Introduction Capture Questions Methods of Administration DHQ Materials Structure and Content Reformatting
OPERATIONS AND PATIENT SERVICES USER GUIDE. Procedures for transplant centres and international establishments
 OPERATIONS AND PATIENT SERVICES USER GUIDE Procedures for transplant centres and international establishments 1. INTRODUCTION 9 1.1. Introduction 9 1.2. User Guide 9 1.3. Services Provided by Anthony Nolan
OPERATIONS AND PATIENT SERVICES USER GUIDE Procedures for transplant centres and international establishments 1. INTRODUCTION 9 1.1. Introduction 9 1.2. User Guide 9 1.3. Services Provided by Anthony Nolan
Etanercept for Treatment of Hidradenitis
 Home Search Browse Resources Help What's New About Purpose Etanercept for Treatment of Hidradenitis This study is currently recruiting patients. Sponsors and Collaborators: University of Pennsylvania Amgen
Home Search Browse Resources Help What's New About Purpose Etanercept for Treatment of Hidradenitis This study is currently recruiting patients. Sponsors and Collaborators: University of Pennsylvania Amgen
The number of patients waiting on the pancreas transplant list fell by 7% during the year, to 252 at 31 March 2015
 6 Pancreas Activity Pancreas Activity Key messages The number of patients waiting on the pancreas transplant list fell by 7% during the year, to 252 at 31 March 2015 The number of pancreas donors after
6 Pancreas Activity Pancreas Activity Key messages The number of patients waiting on the pancreas transplant list fell by 7% during the year, to 252 at 31 March 2015 The number of pancreas donors after
YEREVAN STATE MEDICAL UNIVERSITY DEPARTMENT OF HEMATOLOGY COURSE DESCRIPTION HEMATOLOGY
 1. Module/unit Code II. 1.2. 2. Module/unit Title Hematology 3. Subject Field Internal Diseases Group 4. Faculty/Department General Medicine, Department of Hematology 5. Programme(s) to which the Doctor
1. Module/unit Code II. 1.2. 2. Module/unit Title Hematology 3. Subject Field Internal Diseases Group 4. Faculty/Department General Medicine, Department of Hematology 5. Programme(s) to which the Doctor
Impact of the Human Transplantation Act (Wales)
 NHSBT BOARD 28th September 2017 Impact of the Human Transplantation Act (Wales) 1. Status Public 2. Executive Summary This paper summarises organ donation activity and s in Wales since the introduction
NHSBT BOARD 28th September 2017 Impact of the Human Transplantation Act (Wales) 1. Status Public 2. Executive Summary This paper summarises organ donation activity and s in Wales since the introduction
Contents SECTION 1: PHYSIOLOGY OF BLOOD
 Contents SECTION 1: PHYSIOLOGY OF BLOOD Chapter 1: Overview of Physiology of Blood 1 Normal Haematopoiesis 1 Red Blood Cells 6 White Blood Cells 15 Immune System 27 Megakaryopoiesis 32 Normal Haemostasis
Contents SECTION 1: PHYSIOLOGY OF BLOOD Chapter 1: Overview of Physiology of Blood 1 Normal Haematopoiesis 1 Red Blood Cells 6 White Blood Cells 15 Immune System 27 Megakaryopoiesis 32 Normal Haemostasis
Scottish Infected Blood Support Scheme. Payments for People Infected With Hepatitis C or HIV or both from Infected Blood
 Scottish Infected Blood Support Scheme Payments for People Infected With Hepatitis C or HIV or both from Infected Blood Introduction The Scottish Infected Blood Support Scheme (SIBSS) is managed by NHS
Scottish Infected Blood Support Scheme Payments for People Infected With Hepatitis C or HIV or both from Infected Blood Introduction The Scottish Infected Blood Support Scheme (SIBSS) is managed by NHS
Skin Pathway Group Alemtuzumab in Cutaneous Lymphoma
 Skin Pathway Group Alemtuzumab in Cutaneous Lymphoma Indication: Treatment of patients with Cutaneous Lymphoma (Unlicensed use) Disease control prior to Reduced Intensity Conditioning Stem Cell Transplant
Skin Pathway Group Alemtuzumab in Cutaneous Lymphoma Indication: Treatment of patients with Cutaneous Lymphoma (Unlicensed use) Disease control prior to Reduced Intensity Conditioning Stem Cell Transplant
a resource for physicians Recommended Referral Timing for Stem Cell Transplant Evaluation
 a resource for physicians Recommended Referral Timing for Stem Cell Transplant Evaluation This resource has been developed to help guide you regarding the appropriate timing and conditions for a referral
a resource for physicians Recommended Referral Timing for Stem Cell Transplant Evaluation This resource has been developed to help guide you regarding the appropriate timing and conditions for a referral
An introduction to organ and tissue donation
 Student resource Lesson 1: An introduction to organ and tissue donation Lesson outcomes To be able to give a definition of organ and tissue donation, a transplant, a recipient and the NHS Organ Donor Register
Student resource Lesson 1: An introduction to organ and tissue donation Lesson outcomes To be able to give a definition of organ and tissue donation, a transplant, a recipient and the NHS Organ Donor Register
Chapter 4 Section 24.1
 Surgery Chapter 4 Section 24.1 Issue Date: October 27, 1995 Authority: 32 CFR 199.4(e)(5) 1.0 CPT 1 PROCEDURE CODES 32850-32854, 33930-33935 2.0 DIAGNOSTIC RELATED GROUPS (DRGs) 495 for lung transplant.
Surgery Chapter 4 Section 24.1 Issue Date: October 27, 1995 Authority: 32 CFR 199.4(e)(5) 1.0 CPT 1 PROCEDURE CODES 32850-32854, 33930-33935 2.0 DIAGNOSTIC RELATED GROUPS (DRGs) 495 for lung transplant.
Special Requirements Lab Matters. 21 st June Barrie Ferguson
 Special Requirements Lab Matters. 21 st June 2017 Barrie Ferguson Special requirements Patients requiring Methylene Blue or Solvent Detergent treated Fresh Frozen Plasma Patients requiring apheresis platelets
Special Requirements Lab Matters. 21 st June 2017 Barrie Ferguson Special requirements Patients requiring Methylene Blue or Solvent Detergent treated Fresh Frozen Plasma Patients requiring apheresis platelets
End Stage Kidney Disease Among Indigenous Peoples of Australia and New Zealand
 Chapter 12 End Stage Kidney Disease Among Indigenous Peoples of and New Zealand 216 ANZDATA Registry 39th Annual Report Data to 31-Dec-215 Introduction In this chapter, rates of end-stage kidney disease
Chapter 12 End Stage Kidney Disease Among Indigenous Peoples of and New Zealand 216 ANZDATA Registry 39th Annual Report Data to 31-Dec-215 Introduction In this chapter, rates of end-stage kidney disease
The number of patients waiting on the pancreas transplant list fell by 1% during the year, to 224 at 31 March 2017
 6 Pancreas Activity Pancreas Activity Key messages The number of patients waiting on the pancreas transplant list fell by 1% during the year, to 224 at 31 March 2017 The number of pancreas donors after
6 Pancreas Activity Pancreas Activity Key messages The number of patients waiting on the pancreas transplant list fell by 1% during the year, to 224 at 31 March 2017 The number of pancreas donors after
Living Kidney Donation. Dr. Joseph Keith Melancon
 Living Kidney Donation Dr. Joseph Keith Melancon Thanks to our speaker! Keith Melancon, MD Chief Transplant Institute And Division Of Transplant Surgery; Medical Director GW/ Ron And Joy Paul Kidney Center
Living Kidney Donation Dr. Joseph Keith Melancon Thanks to our speaker! Keith Melancon, MD Chief Transplant Institute And Division Of Transplant Surgery; Medical Director GW/ Ron And Joy Paul Kidney Center
An Approach to the Patient Refractory to Platelets Transfusion. Harold Alvarez, MD
 Harold Alvarez, MD Objectives Explain the etiology of platelet refractoriness Discuss the different types of platelet refractoriness Describe how platelet refractoriness is diagnosed Discuss different
Harold Alvarez, MD Objectives Explain the etiology of platelet refractoriness Discuss the different types of platelet refractoriness Describe how platelet refractoriness is diagnosed Discuss different
Clinical & Laboratory Assessment
 Clinical & Laboratory Assessment Dr Roger Pool NHLS & University of Pretoria Clinical Assessment (History) Anaemia ( haemoglobin) Dyspnoea (shortness of breath) Tiredness Angina Headache Clinical Assessment
Clinical & Laboratory Assessment Dr Roger Pool NHLS & University of Pretoria Clinical Assessment (History) Anaemia ( haemoglobin) Dyspnoea (shortness of breath) Tiredness Angina Headache Clinical Assessment
Internal Medicine Certification Examination Blueprint
 Internal Medicine Certification Examination Blueprint What Does the Examination Cover? The exam is designed to evaluate the extent of the candidate s knowledge and clinical judgment in the areas in which
Internal Medicine Certification Examination Blueprint What Does the Examination Cover? The exam is designed to evaluate the extent of the candidate s knowledge and clinical judgment in the areas in which
SUMMARY OF THE RISK MANAGEMENT PLAN FOR. BESPONSA 1mg (INOTUZUMAB OZOGAMICIN) Powder for concentrate for solution for infusion.
 SUMMARY OF THE RISK MANAGEMENT PLAN FOR BESPONSA 1mg (INOTUZUMAB OZOGAMICIN) Powder for concentrate for solution for infusion. This RMP Summary is based on Part VI of the EU RMP for BESPONSA (Inotuzumab
SUMMARY OF THE RISK MANAGEMENT PLAN FOR BESPONSA 1mg (INOTUZUMAB OZOGAMICIN) Powder for concentrate for solution for infusion. This RMP Summary is based on Part VI of the EU RMP for BESPONSA (Inotuzumab
