Clinical Commissioning Policy Proposition: Rituximab for anti- NMDAR autoimmune encephalitis (all ages)
|
|
|
- Melina Jones
- 5 years ago
- Views:
Transcription
1 Clinical Commissioning Policy Proposition: Rituximab for anti- NMDAR autoimmune encephalitis (all ages) Reference: NHS England 1625
2 First published: TBC Prepared by NHS England Specialised Services Clinical Reference Group for Paediatric Neurosciences Published by NHS England, in electronic format only 2
3 Contents 1 Executive Summary... 4 Equality Statement... 4 Plain Language Summary Introduction Proposed Intervention and Clinical Indication Definitions Aims and Objectives Epidemiology and Needs Assessment Evidence Base Proposed Criteria for Commissioning Proposed Patient Pathway Proposed Governance Arrangements Proposed Mechanism for Funding Proposed Audit Requirements Documents That Have Informed This Policy Proposition Date of Review References
4 1 Executive Summary Equality Statement Promoting equality and addressing health inequalities are at the heart of NHS England s values. Throughout the development of the policies and processes cited in this document, we have: Given due regard to the need to eliminate discrimination, harassment and victimisation, to advance equality of opportunity, and to foster good relations between people who share a relevant protected characteristic (as cited under the Equality Act 2010) and those who do not share it; and Given regard to the need to reduce inequalities between patients in access to, and outcomes from healthcare services and to ensure services are provided in an integrated way where this might reduce health inequalities. Plain Language Summary About Acute Anti-NMDAR Autoimmune Encephalitis Acute autoimmune encephalitis is a rare, debilitating neurological disorder with a significant burden to patients, families and society. It causes inflammation of the brain and in most cases it progresses rapidly into a severe syndrome including altered mental status, neurological and psychiatric symptoms or death. Acute anti N- methyl-d-aspartate brain cell-surface receptor (anti -NMDAR) autoimmune encephalitis (AE) is one of the commonest known types of autoimmune encephalitis, most often noted in children and young adults. It is characterised by abnormal behavioural and cognitive problems, seizures and movement disorders. If treatment does not work or the patient does not receive treatment, this may result in long term disabilities or death. About current treatments Second-line treatment is usually administered when the response to first-line therapy is inadequate or when the disease is known to be severe or relapsing. It typically includes immunomodulators such as; cyclophosphamide, azathioprine, mycophenolate mofetil or others. There are promising reports on use of rituximab for 4
5 this condition alone or alongside other immunomodulators. Patients are also treated with tumour resection as certain tumours may be associated with anti-nmdar AE (e.g. ovarian teratoma). About the new treatment Rituximab is a monoclonal antibody inducing B-cell depletion that has been approved by the National Institute of Health and Care Excellence (NICE) in England and Wales for use in suppressing the body s immune response in some autoimmune disorders. It is currently not licensed to treat neurological inflammatory disease. Rituximab use is known to be associated with some adverse events. What we have decided NHS England has carefully reviewed the evidence to treat anti-nmdar AE in children and adults with rituximab. We have concluded that there is enough evidence to consider making the treatment available to a well-defined group of children and adults suffering from acute anti- NMDAR AE who have not or have inadequately responded to the first-line therapy by four weeks of treatment initiation OR within six weeks of first symptoms. 2 Introduction This document describes the evidence that has been considered by NHS England in formulating a proposal to routinely commission rituximab for the treatment of acute anti- N-methyl-D-aspartate neuronal cell surface receptor autoimmune encephalitis (AE) when patients have not or have inadequately responded to first-line immunotherapies by four weeks of treatment initiation OR within six weeks of symptom/disease onset. This document also describes the proposed criteria for commissioning, proposed governance arrangements and proposed funding mechanisms. For the purpose of consultation NHS England invites views on the evidence and other information that has been taken into account as described in this policy 5
6 proposition. A final decision as to whether rituximab treatment for patients with acute anti- NMDAR AE will be routinely commissioned is planned to be made by NHS England following a recommendation from the Clinical Priorities Advisory Group. 3 Proposed Intervention and Clinical Indication Acute AE is a debilitating neurological disorder that causes brain inflammation and develops as a rapidly progressive encephalopathy syndrome presenting with altered mental status and a range of neurological and psychiatric symptoms (Graus et al., 2016, Nosadini et al., 2015). The death rate is estimated to range from 2% to 5% but can approach 20% in patients who fail first line therapy and do not receive second-line immunotherapies (Dale et al., 2014, Titulaer et al., 2013). One of the commonest known types of autoimmune encephalitis is associated with antibodies against NMDAR (anti-nmdar). Anti-NMDAR AE is characterised by abnormal behavioural and cognitive dysfunctions, seizures, movement disorder, reduced consciousness, speech disorder, autonomic dysfunction, hypoventilation and memory deficit ( Dalmau et. al, 2007; Dalmau et. al, 2008; Graus et al., 2016, Irani et. al 2010; Titulaer et al.,2013, Wright et al., 2015). The presence of tumour, most commonly ovarian teratoma in young women, is associated with anti-nmdar AE especially in young adults ( Florance et. al, 2009; Gong et. al, 2017;Graus et. al, 2016; Irani et al, 2010;Titulaer et al., 2013; Wright et. al, 2015). Most types of AE have significant clinical overlap (Nosadini et al, 2015). Impairment in many of these conditions appears to be associated with the degree of active inflammation, and therefore the mainstay of treatment is immunosuppression. Firstline immunotherapies generally consist of corticosteroids (intravenous and oral), intravenous immunoglobulin (IVIg) and/or plasma exchange (PLEX) (Nosadini et al., 2015). Second-line treatment is usually administered when the response to first-line therapy is inadequate or when the disease is known to be severe or relapsing. It typically includes cyclophosphamide, azathioprine, mycophenolate mofetil or others (Nosadini et al., 2015). Patients are also treated with tumour resection if one is present. 6
7 Current evidence suggests that the use of second-line immunotherapies for AE associated with antibodies to cell surface antigens is beneficial, more commonly associated with a better outcome and lower rates of relapses (Dale et al., 2014, Nosadini et al., 2015, Titulaer et al., 2013). Evidence also suggests that early commencement of immunotherapy favours a better neurological outcome and may prevent major disability (Dale et al., 2014; Nosardini et al., 2015; Titulaer et al., 2013). Rituximab is a chimeric monoclonal antibody against CD20-positive B lymphocytes (B cells) inducing B-cell depletion (Dale et al., 2014, Lee et al., 2016) that has been approved by the National Institute of Health and Care Excellence (NICE) in England and Wales for use in suppressing autoimmune disorders. It is currently not licensed to treat neurological inflammatory disorders, leading to off-label use as a secondline immunotherapy in patients with severe or refractory diseases who fail the firstline treatment (Dale et al., 2014; Nosadini et al., 2015). Rituximab is known to be associated with adverse events, particularly anaphylactic reactions, its interaction with live vaccines and infectious side effects (such as virus reactivations, bacteria in the blood (bacteraemia) and sepsis). Most evidence on the safety and efficacy of rituximab comes from studies in patients with lymphomas and rheumatoid arthritis. About 10% of patients with any autoimmune neurological disorders treated with rituximab experience hypotension and bronchospasm, usually at the first administration of the drug. Severe manifestations such as acute respiratory distress syndrome (ARDS), myocardial infarction, ventricular fibrillation and cardiogenic shock have been reported but these are uncommon (Kosmidis, 2010).These studies suggest close monitoring is mandatory in patients with poor general condition and pre-existing pulmonary and cardiac insufficiency (Waubant 2008). Caution is also required in patients that receive rituximab and other immunosuppressive therapies together to avoid progressive multifocal leukoencephalopathy (PML) mostly in patients with disorders characterised by proliferation of lymphoid tissue (lymphoproliferative disorders) (Carston et al.,2009). There are few case reports of posterior reversible encephalopathy syndrome 7
8 (PRES) / reversible posterior leukoencephalopathy syndrome (RPLS) in immunocompromised patients or patients receiving a combination of immunotherapy and/or chemotherapy. A diagnosis of PRES/RPLS requires confirmation by brain imaging. The best available evidence related to children suggests that the rate of infectious complications in children treated with rituximab varies based on underlying diagnosis and was estimated to be 7.2% in children with autoimmune disorders (Kavcic et al., 2013). 4 Definitions Autoimmune encephalitis (AE) is an acute inflammation of the brain resulting from body's own antibodies attacking brain tissue (e.g. neuronal cell-surface antigens such as extracellular epitopes of synaptic receptors) and impairing its function. It progresses rapidly into encephalopathy syndrome including altered mental status and a range of neurological and psychiatric symptoms. NMDAR Receptor is a synaptic receptor composed of two glutamate-binding GluN2 (NR2) subunits and two glycine/d-serine-binding GluN1 (NR1) subunits. It is critically involved in normal neural network formation, synaptic plasticity, and higher brain functions such as learning and memory. Relapse is defined as the new onset or worsening of symptoms occurring after at least 4 weeks of improvement or stabilisation. Adverse event is any unwanted experience associated with the use of a medical product in a patient. It is usually classified using Common Terminology Criteria for Adverse Events (CTCAE) v4.0. Ovarian Teratoma, also referred to as dermoid cyst of the ovary, is a tumour, usually benign and mature composed of tissues not normally present in the ovary, typically containing a diversity of tissues including hair, teeth, bone and thyroid. Modified Rankin Scale (mrs) is a scale for measuring the degree of neurological disability or dependence in daily activities modified for use in paediatric disorders. The scale ranges from 0 to 6 (0 No symptoms at all; 1 No significant disability 8
9 despite symptoms: able to carry out all usual duties and activities; 2 Slight disability: unable to carry out all previous activities but able to look after own affairs without assistance; 3 Moderate disability: requiring some help, but able to walk without assistance; 4 Moderately severe disability: unable to walk without assistance, and unable to attend to own bodily needs without assistance; 5 Severe disability: bedridden, incontinent, and requiring constant nursing care and attention; 6 Dead). P (p-value) is the level of marginal significance within a statistical hypothesis test representing the probability of the occurrence of a given event. It helps determine the significance of the results. Paraneoplastic refers to a syndrome or other systemic disturbance associated with but not directly related to a primary tumour or its metastases. Progressive multifocal leukoencephalopathy (PML) is a rare condition caused by reactivation of a virus, occurring particularly when patients are very immunosuppressed, resulting in life threatening and often fatal brain inflammation 5 Aims and Objectives This policy proposition considered NHS England s commissioning position for rituximab as second-line therapy for a well-defined cohort of patients with acute anti- NMDAR AE who have not or have inadequately responded to first-line immunotherapies. The objectives are to: Provide an overview of the current evidence for use of rituximab in patients with acute anti-nmdar autoimmune encephalitis to ensure evidence-based commissioning; Provide a rationale and propose criteria for commissioning of rituximab usage in paediatric and adult settings aiming at improving health and care outcomes. 9
10 6 Epidemiology and Needs Assessment AE is a rare debilitating neurological disorder that represents a significant burden to patients, families and society (Graus et al., 2016). Anti-NMDAR AE is noted most often in young adults and children ( Titulaer et al., 2013). The true incidence of AE is not known. Current evidence suggests that the incidence of anti-nmdar encephalitis, the commonest type of AE accounting for approximately 27% of all Autoimmune Encephalitis cases (Hacohen et al., 2013) per million children (aged under 18 years) per year (95% confidence interval 0.64 to 1.06) are estimated to have NMDAR AE (Wright et al., 2015). The paediatric presentation has been described as more neurological than the more psychiatric presentation in adults (Titulaer et al., 2013). As such, it is estimated that there are approximately 11 children (range 8-13) diagnosed with acute anti-nmdar encephalitis every year among 12.2 million children living in the UK. It should be noted that these figures may underestimate the true incidence. Furthermore, some additional cases with anti-nmdar AE emerge every year due to disease relapse occurring in 8% to 29% of patients (Zekeridou et al., 2015). The majority of patients (approximately 80%) are young women with an estimated median age of onset of 21 years; however this ranges from 8 months to 85 years. (Dalmau et al., 2008; Dalmau et al., 2011) In adults the proportion of paraneoplastic case varies ( %) but is higher than in paediatric cases ( %) (Dalmau et al., 2008; Nosardini et al., 2015 ;). Approximately 44% of patients with anti NMDAR AE do not respond to first line therapy (Titulaer et al., 2013), thus are likely to need second-line therapy, although tumour removal may influence this response (39% with tumours fail first line therapy versus 48% without tumour). Currently there are around 70 laboratory confirmed anti NMDAR AE cases annually based on the Oxford Autoimmune Neurology Group laboratory that process 60% of UK s requests. 10
11 7 Evidence Base NHS England has concluded that there is sufficient evidence to support a proposal for the routine commissioning of rituximab for the treatment of a well-defined cohort suffering from acute anti-nmdar AE who have not or have inadequately responded to the first-line therapy by four weeks of treatment initiation OR within six symptomatic weeks. Evidence summary A total of 11 studies were considered in the evidence review which fit the selection criteria. These studies provide some evidence on the efficacy and safety of rituximab used as a second-line immunotherapy. Rituximab was used alone or in combination with other first and second line immunosuppressive therapies. The retrospective observational multi-centre study of 144 children (Dale et al., 2014) provided the best available evidence for using rituximab in treatment of children with autoimmune and inflammatory CNS disorders. In the study, 87% of all patients and 97% of patients with anti-nmdar encephalitis had some form of benefit from rituximab treatment used as second-line, especially when received early. 17% of patients had modified Rankin Scale (mrs) of 0-2 (considered to be a good neurological disability score) at rituximab initiation compared to 74% at outcome. The change in mrs 0-2 was greater in patients given rituximab early compared to those treated later. The study reported a total of three deaths (2%), of which two occurred due to infectious adverse event. In addition, the large prospective cohort study of 577 all age patients (Titulaer et al., 2013) found that 78% of patients with anti-nmdar encephalitis who failed first-line and received second-line immunotherapy (with rituximab and/or cyclophosphamide) had good outcome at 24 months, compared to 55% of patients who failed first-line and did not receive second-line therapy. Early treatment was associated with good outcome (p<0.001). Furthermore the use of immunotherapy (p=0.038) and use of second-line immunotherapy in patients without tumour (p=0.007) were associated with fewer relapses. Overall 30 of 501 patients died, including 6 of 177 children. At 24 months follow-up, 10% mortality rate was estimated (24 deaths among
12 patients who were followed up at 24 months ). Rituximab used as second-line therapy was generally well tolerated with 2-3% of children reporting severe infusion and infectious adverse effects of grade 4 or more (Dale et al., 2014). It should be noted that all studies (including the large cohorts) presented in the evidence review are low grade studies and have significant limitations that affect generalisability of results and their application in clinical practice. There are no studies that compare the effects of individual immunotherapies, thus it is not possible to ascribe therapeutic benefits solely to rituximab. Furthermore, it is unclear if patients with anti-nmdar encephalitis are more likely to benefit, whether use of rituximab has any benefits over different second-line therapies such as cyclophosphamide, or at which stage of disease it should be used (acute, subacute or chronic). Better quality evidence is needed to investigate the safety and efficacy of rituximab monotherapy in patients with autoimmune CNS disorders (and anti-nmdar encephalitis in particular), to compare rituximab with other immunotherapies, to determine the most optimal dosage regimen and timing of rituximab therapy to yield maximum benefit, and to standardise diagnostics and safety monitoring. 8 Proposed Criteria for Commissioning Employing Rituximab as a second line agent is a key immunotherapeutic strategy in the acute treatment of anti-nmdar AE. Rituximab selectively targets B cells and provides sound biological basis for treatment of an antibody mediated disorder. Whilst other immunosuppression therapy is available for use as second line therapy in acute anti-nmdar autoimmune encephalitis, their slow pace of response (e.g. mycophenolate) and side effects (e.g. cyclophosphamide) in children and adults limits their utility. It is proposed to routinely commission rituximab for children and adults, including those with an identifiable tumour (e.g. ovarian teratoma) when all of the following 12
13 inclusion criteria have been met. Patients with a confirmed diagnosis of anti-nmdar autoimmune encephalitis 1 ; Patients have had adequate first line immunotherapy e.g. intravenous methylprednisolone (IVMP), intravenous immunoglobulin (IVIG), plasma exchange (PLEX) given either sequentially or in combination as detailed in the pathway figure on page 16 Patients have failed or not responded adequately to first line immunotherapy, defined as deterioration of less than 2-point scale improvement in mrs and/or not attained a minimum score of 2 by four weeks of treatment initiation (usually within 6 weeks of symptom onset). For patients aged 18 years and younger, they must have been reviewed by a Consultant Paediatric Neurologist and managed within a tertiary paediatric neurology service. In patients aged over 18 years of age, the use of rituximab must be discussed with a regional adult neurologist with expertise in neuroinflammation, but by agreement can be managed within a setting with experience of the use of rituximab. Patients aged years of age can be treated in either the paediatric or adult pathway. Rituximab should only be administered in an area where full resuscitation facilities and close monitoring are available; either in a day-case setting or in acute admissions wards depending on clinical requirements. A doctor should be present on the ward/unit while the infusion is commenced. The lowest acquisition cost of rituximab must be used. Exclusion criteria: Patients will be excluded from further treatment if they have had a severe life threatening infusion reaction to previous rituximab treatment, and or are considered by their clinician to be at higher risk due to contraindications as noted below. 1 A diagnosis of definite or highly probably anti-nmdar encephalitis based on the Graus criteria. A definite diagnosis can be made with a positive CSF and/or serum (definite) for NMDAR antibody. 13
14 Contraindications: As per the drug company information on contraindications (see Summary of Product Characteristics). Cautions: Patients with a history of cardiovascular disease or renal impairment should be appropriately reviewed prior to dosing Rituximab should be used carefully in patients with history of severe infections, particularly tuberculosis and viral hepatitis (particularly hepatitis B). Patient should have undergone specialist assessment and be on active treatment and have stable risk of infection prior to onset of rituximab therapy. The safety of vaccination, especially with live vaccines following treatment with rituximab is not known. Live vaccines are currently contraindicated post rituximab whilst B cells are depleted, and/or patients are on additional immunosuppressive therapy. Some patients may present with mono-symptomatic psychiatric presentations; and must meet the diagnosis of definite or highly probably anti-nmdar encephalitis based on the Graus criteria Dosage of rituximab: Paediatric patients: 375mg/m2 (capped at 500mg) x 4 doses at weekly intervals Adults: 1g x 2 doses two weeks apart. Response to the treatment must be monitored by modified Rankin Scale (mrs) score and improvement of neurological syndrome. Depletion of B cells can be monitored by CD19/20 levels in peripheral blood if clinically indicated (e.g. stopping criteria). A top up dose of rituximab during acute treatment in a patient who has not responded to one rituximab treatment course (from 4 weeks following completion of first treatment course) can be considered (Child: 375mg/m2 x2 doses at weekly intervals; Adult: 1g) if the patient has a higher clearance of rituximab which is 14
15 confirmed by demonstrating failure to achieve B cell depletion. A subsequent treatment course of rituximab treatment, often termed re-dosing should only be considered in a patient that has relapsed 2 who has previously responded (improved 2 mrs) to the first course of rituximab treatment; and have undergone adequate 1 st line treatment at relapse. For patients with severe life threatening inflammation, rituximab may be used in combination with another second-line immunotherapy, usually cyclophosphamide, to provide urgent (faster speed of action) and broader (targeting more components of the immune system) treatment to reduce brain inflammation. 2 Any recurrence of symptoms fulfilling the Graus clinical criteria following prior symptom remission 15
16 9 Proposed Patient Pathway Figure 1 1. Proposal is based on Graus et al., 2016 Lancet Neurol. 15(4): ; supported by the largest cohort of 577 patients, where only 1% of patients had monosymptomatic disease during the first month of illnesss (Titulaer et al., 2013 Lancet Neurol12:157-65) 2. As CSF and MRI more likely to be normal in children; care need to be given using this criteria; and that some of the results may not be available at time of initiating treatment. 3. First line treatment involves oral or IV corticosteroids which may be supplemented with IVIG/PLEX depending on clinical policies and patient circumstances 4. Diagnosis is confirmed (DEFINITE) on identification of NMDAR antibodies in serum and/or CSF. In patients who have predominantly monophasic presentation or do not have the full clinical picture as proposed by Graus et al., 2016, CSF NMDAR positivity is required to confirm diagnosis (highly PROBABLE). Patients may be advised of support from the Encephalitis Society 5. Patients with DEFINITE and in some circumstance highly PROBABLE disease who continue to deteriorate, do not improve of have inadequate improvement needs to be considered for Rituximab. 16
17 10 Proposed Governance Arrangements Rituximab will be available for paediatric cases following agreement by a Paediatric Neurology Consultant and treated in a tertiary paediatric neurology unit. The adult cases should be treated after discussion and approval by a regional adult neurologist with expertise in neuro-inflammation but by agreement can be managed within a setting with experience of the use of rituximab. Patients aged may be treated either in the paediatric or adult pathway. Any provider organisation treating patients with this intervention will be required to assure that the internal governance arrangements have been completed before the medicine is prescribed. These arrangements may be through the Trust s Drugs and Therapeutics committee (or similar) and NHS England may ask for assurance of this process. 11 Proposed Mechanism for Funding From April 2013 the NHS England has been responsible for commissioning specialised services in line with published policy on behalf of the population of England. The funding and commissioning will be managed through the relevant local NHS England Specialised Commissioning Team. 12 Proposed Audit Requirements All patients who receive rituximab for the treatment of AE must be entered onto an electronic patient registration system. Rituximab treatment will be available through tertiary paediatric/adult neurology units that agree to audit and publish their results. This should include rates of adverse events related to the use of rituximab. 13 Documents That Have Informed This Policy Proposition See Section 15 for references. 17
18 14 Date of Review This document will lapse upon publication by NHS England of a clinical commissioning policy for the proposed intervention that confirms whether it is routinely or non-routinely commissioned. 18
19 15 References Armangue T, Moris G, Cantarín-Extremera V, Conde CE, Rostasy K, Erro ME, et al. Autoimmune post-herpes simplex encephalitis of adults and teenagers. Neurology 2015; 85: Brenton, J. N., Kim, J. & Schwartz, R. H Approach to the management of pediatriconset anti-n-methyl-d-aspartate (anti-nmda) receptor encephalitis: a case series. J Child Neurol, 31, Carson KR, Evens AM, Richey EA, Habermann TM, Focosi D, Seymour JF, Laubach J, Bawn SD, Gordon LI, Winter JN, Furman RR, Vose JM, Zelenetz AD, Mamtani R, Raisch DW, Dorshimer GW, Rosen ST, Muro K, Gottardi-Littell NR, Talley RL, Sartor O, Green D, Major EO, Bennett CL Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood May 14; 113(20): Dale, R. C., Brilot, F., Duffy, l. V., Twilt, M., Waldman, A. T., Narula, S., Muscal, E., Deiva, K., Andersen, E., Eyre, M. R., Eleftheriou, D., Brogan, P. A., Kneen, R., Alper, G., Anlar, B., Wassmer, E., Heineman, K., Hemingway, C., Riney, C. J., Kornberg, A., Tardieu, M., Stocco, A., Banwell, B., Gorman, M. P., Benseler, S. M. & Lim, M Utility and safety of rituximab in pediatric autoimmune and inflammatory cns disease. Neurology, 83, Josep Dalmau, Amy J Gleichman, * Ethan G Hughes, * Jeffrey E Rossi, Xiaoyu Peng, Meizan Lai, Scott K Dessain, Myrna R Rosenfeld, Rita Balice-Gordon, and David R Lynch 2008 Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies Lancet Neurol. Dec; 7(12): Dalmau, J., Lancaster, E., Martinez-Hernandez, E., Rosenfeld, M. R. & Balice-Gordon, R Clinical experience and laboratory investigations in patients with anti-nmdar encephalitis. The Lancet Neurology, 10, Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008; 7: Dalmau J, Tüzün E, Wu H-Y, Masjuan J, Rossi JE, Voloschin A, et al. Paraneoplastic anti- 19
20 N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007; 61: Finke C, Prüss H, Heine J, Reuter S, Kopp UA, Wegner F, et al. Evaluation of Cognitive Deficits and Structural Hippocampal Damage in Encephalitis With Leucine-Rich, Glioma-Inactivated 1 Antibodies. JAMA Neurol 2017; 74: Florance NR, Davis RL, Lam C, Szperka C, Zhou L, Ahmad S, et al. Anti-N-methyl-Daspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol. 2009; 66: Graus, f., Titulaer, M. J., Balu, R., Benseler, S., Bien, C. G., Cellucci, T., Cortese, I., Dale, R. C., Gelfand, J. M., Geschwind, M., Glaser, C. A., Honnorat, J., Höftberger, R., Iizuka, T., Irani, S. R., Lancaster, E., Leypoldt, F., Prüss, H., Rae-Grant, A., Reindl, M., Rosenfeld, M. R., Rostásy, K., Saiz, A., Venkatesan, A., Vincent, A., Wandinger, K.- P., Waters, P. & Dalmau, J A clinical approach to diagnosis of autoimmune encephalitis. The Lancet Neurology, 15, Gabilondo I, Saiz A, Galan L, Gonzalez V, Jadraque R, Sabater L, et al. Analysis of relapses in anti-nmdar encephalitis. Neurology 2011; 77: Gong S, Zhou M, Shi G, Guo J, Chen N, Yang R, et al. Absence of NMDA receptor antibodies in patients with ovarian teratoma without encephalitis. Neurol Neuroimmunol Neuroinflamm 2017; 4: e Granerod J, Ambrose HE, Davies N. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis 2010; 10: Hacohen, Y., Wright, S., Waters, P., Agrawal, S., Carr, l., Cross, H., De sousa, C., Devile, C., Fallon, P., Gupta, R., Hedderly, T., Hughes, E., Kerr, T., Lascelles, k., lin, j. P., philip, s., pohl, k., Prabahkar, P., Smith, M., Williams, R., Clarke, A., Hemingway, C., Wassmer, E., Vincent, A. & Lim, M. J Paediatric autoimmune encephalopathies: clinical features, laboratory investigations and outcomes in patients with or without antibodies to known central nervous system autoantigens. J Neurol Neurosurg Psychiatry, 84, Irani S, Bera K, Waters P, Zuliani L, Maxwell S, Zandi M, Friese M, Kullman D, Beeson D, Lang B, Bien C and Vincent A N-Methyl-D-aspartate antibody enchephalitis: temporalprogression of clicnical paraclinical observations in predominantly non- 20
21 praneoplastic dsiorder of both sexes Brain133; Irani SR, Bera K, Waters P, Zuliani L, Maxwell S, Zandi MS, et al. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain 2010; 133: Irani SR, Stagg CJ, Schott JM, Rosenthal CR, Schneider SA, Pettingill P, et al. Faciobrachial dystonic seizures: the influence of immunotherapy on seizure control and prevention of cognitive impairment in a broadening phenotype. Brain 2013; 136: Helen Barry, Susan Byrne, Elizabeth Barrett, Kieran C. Murphy,and David R. Cotter List, 2015, Anti-N-methyl-d-aspartate receptor encephalitis: review of clinical presentation, diagnosis and treatment BJPsych Bull v.39(1); 2015 Feb Kavcic, M., Fisher, B. T., Seif, A. E., Li, Y., Huang, Y.-S., Walker, D. & Aplenc, R Leveraging administrative data to monitor rituximab use in 2875 patients at 42 freestanding children's hospitals across the united states. J Pediatr, 162, Kosmidis,M., Practical considerations on the use of rituximab in autoimmune neurological disorders Ther Adv Neurol Disord. Mar; 3(2): Lee, W.-J., Lee, S.-T., Byun, J.-I., Sunwoo, J.-S., Kim, T.-J., Lim, J.-a., moon, j., lee, h. S., shin, y.-w., lee, k.-j., kim, s., jung, k.-h., jung, k.-y., chu, k. & lee, s. K Rituximab treatment for autoimmune limbic encephalitis in an institutional cohort. Neurology, 86, Nosadini, M., Mohammad, S. S., Ramanathan, S., Brilot, F. & Dale, R. C Immune therapy in autoimmune encephalitis: a systematic review. Expert Rev Neurother, 15, Summary of Product Characteristics. Accessed April 2017: _Product_Information/human/000165/WC pdf Shin Y-W, Lee S-T, Shin J-W, Moon J, Lim J-A, Byun J-I, et al. VGKC-complex/LGI1- antibody encephalitis: clinical manifestations and response to immunotherapy. J. Neuroimmunol. 2013; 265:
22 Titulaer, M. J., Mccracken, l., Gabilondo, I., Armangué, T., Glaser, C., Iizuka, T., Honig, l. S., Benseler, S. M., Kawachi, I., Martinez-Hernandez, E., Aguilar, E., Gresa-Arribas, N., Ryan-Florance, N., Torrents, A., Saiz, A., Rosenfeld, M. R., Balice-Gordon, R., Graus, F. & Dalmau, J Treatment and prognostic factors for long-term outcome in patients with anti-nmda receptor encephalitis: an observational cohort study. The Lancet Neurology, 12, Waubant E Review Spotlight on anti-cd20.int MS J Mar; 15(1): Wright, S., Hacohen, Y., Jacobson, l., Agrawal, S., Gupta, R., Philip, S., Smith, M., Lim, M., Wassmer, E. & Vincent, A N-methyl-d-aspartate receptor antibody-mediated neurological disease: results of a uk-based surveillance study in children. Arch Dis Child, 100, Zekeridou, A., Karantoni, E., Viaccoz, A., Ducray, F., Gitiaux, C., Villega, F., Deiva, K., Rogemond, V., Mathias, E., Picard, G., Tardieu, M., Antoine, J. C., Delattre, J. Y. & Honnorat, J Treatment and outcome of children and adolescents with n- methyl-d-aspartate receptor encephalitis. J Neurol, 262,
Evidence review: Rituximab for Paediatric autoimmune encephalitis
 Use this style of front cover if the subject matter is sensitive e.g. abuse, sexual health and it would not be appropriate to use a person s photo. If you need to print/photocopy multiple copies of your
Use this style of front cover if the subject matter is sensitive e.g. abuse, sexual health and it would not be appropriate to use a person s photo. If you need to print/photocopy multiple copies of your
ANTI-NMDA-RECEPTOR ENCEPHALITIS
 ANTI-NMDA-RECEPTOR ENCEPHALITIS Joint Program for European Medical Studies Nantes, 17th October 2014 Felix Gassert - Universität Ulm, Germany Sara Mahmoud Université d'angers, France Sindy Sim Université
ANTI-NMDA-RECEPTOR ENCEPHALITIS Joint Program for European Medical Studies Nantes, 17th October 2014 Felix Gassert - Universität Ulm, Germany Sara Mahmoud Université d'angers, France Sindy Sim Université
Lancet Neurology 2016 Apr; 15(4): The estimated incidence is 5-10 patients per inhabitants per year.
 Lancet Neurology 2016 Apr; 15(4):391-404 Position Paper 1 A clinical approach to diagnosis of autoimmune encephalitis Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, Cortese I, Dale RC,
Lancet Neurology 2016 Apr; 15(4):391-404 Position Paper 1 A clinical approach to diagnosis of autoimmune encephalitis Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, Cortese I, Dale RC,
دمانس های اتوایمون دکتر رضائی طلب نورولوژیست آذر 95
 دمانس های اتوایمون دکتر رضائی طلب نورولوژیست آذر 95 Definition: Dementia According the DSM-5, dementia is defined as significant acquired cognitive impairment in one or more cognitive domains (eg, learning
دمانس های اتوایمون دکتر رضائی طلب نورولوژیست آذر 95 Definition: Dementia According the DSM-5, dementia is defined as significant acquired cognitive impairment in one or more cognitive domains (eg, learning
Autoimmune epilepsies:
 Autoimmune epilepsies: Syndromes and Immunotherapies Sarosh R Irani Associate Professor, Wellcome Trust Intermediate Fellow and Honorary Consultant Neurologist Nuffield Department of Clinical Neurosciences,
Autoimmune epilepsies: Syndromes and Immunotherapies Sarosh R Irani Associate Professor, Wellcome Trust Intermediate Fellow and Honorary Consultant Neurologist Nuffield Department of Clinical Neurosciences,
Anti-N-Methyl-D-Aspartate Receptor Encephalitis in Korea: Clinical Features, Treatment, and Outcome
 ORIGINAL ARTICLE J Clin Neurol 2014;10(2):157-161 Print ISSN 1738-6586 / On-line ISSN 2005-5013 http://dx.doi.org/10.3988/jcn.2014.10.2.157 Open Access Anti-N-Methyl-D-Aspartate Receptor Encephalitis in
ORIGINAL ARTICLE J Clin Neurol 2014;10(2):157-161 Print ISSN 1738-6586 / On-line ISSN 2005-5013 http://dx.doi.org/10.3988/jcn.2014.10.2.157 Open Access Anti-N-Methyl-D-Aspartate Receptor Encephalitis in
Clinical Commissioning Policy Proposition: Rituximab for cytopaenia complicating primary immunodeficiency
 Clinical Commissioning Policy Proposition: Rituximab for cytopaenia complicating primary immunodeficiency Reference: NHS England F06X02/01 Information Reader Box (IRB) to be inserted on inside front cover
Clinical Commissioning Policy Proposition: Rituximab for cytopaenia complicating primary immunodeficiency Reference: NHS England F06X02/01 Information Reader Box (IRB) to be inserted on inside front cover
Clinical Commissioning Policy: Rituximab for the treatment of idiopathic membranous nephropathy in adults
 Clinical Commissioning Policy: Rituximab for the treatment of idiopathic membranous nephropathy in adults Reference: NHS England: 16047/P NHS England INFORMATION READER BOX Directorate Medical Operations
Clinical Commissioning Policy: Rituximab for the treatment of idiopathic membranous nephropathy in adults Reference: NHS England: 16047/P NHS England INFORMATION READER BOX Directorate Medical Operations
AUTOIMMUNE ENCEPHALITIS
 AUTOIMMUNE ENCEPHALITIS Shruti Agnihotri, MD Assistant Professor Department of Neurology, UAB August 12, 2017 DISCLOSURES No financial disclosure Evolving evidence Page 2 OBJECTIVES Review the types of
AUTOIMMUNE ENCEPHALITIS Shruti Agnihotri, MD Assistant Professor Department of Neurology, UAB August 12, 2017 DISCLOSURES No financial disclosure Evolving evidence Page 2 OBJECTIVES Review the types of
Autoimmune encephalopathieslatest. Prof Belinda Lennox Department of Psychiatry, University of Oxford
 Autoimmune encephalopathieslatest advances Prof Belinda Lennox Department of Psychiatry, University of Oxford Belinda.lennox@psych.ox.ac.uk RCP Advanced Medicine 20 th June 2016 Declarations of Interest
Autoimmune encephalopathieslatest advances Prof Belinda Lennox Department of Psychiatry, University of Oxford Belinda.lennox@psych.ox.ac.uk RCP Advanced Medicine 20 th June 2016 Declarations of Interest
AUTOIMMUNE ENCEPHALITIS THE CELL SURFACE AND SYNAPTIC ANTIBODIES
 AUTOIMMUNE ENCEPHALITIS THE CELL SURFACE AND SYNAPTIC ANTIBODIES Josep Dalmau, MD, PhD University of Barcelona Barcelona, Spain University of Pennsylvania Philadelphia, PA Encephalitis The term encephalitis
AUTOIMMUNE ENCEPHALITIS THE CELL SURFACE AND SYNAPTIC ANTIBODIES Josep Dalmau, MD, PhD University of Barcelona Barcelona, Spain University of Pennsylvania Philadelphia, PA Encephalitis The term encephalitis
Clinical Commissioning Policy Proposition: Everolimus for subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex
 Clinical Commissioning Policy Proposition: Everolimus for subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex Reference: NHS England E09X04/01 Information Reader Box (IRB)
Clinical Commissioning Policy Proposition: Everolimus for subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex Reference: NHS England E09X04/01 Information Reader Box (IRB)
Clinical Commissioning Policy Proposition:
 Clinical Commissioning Policy Proposition: Rituximab for the treatment of dermatomyositis and polymyositis (Adults) Reference: NHS England A13X05/01 Information Reader Box (IRB) to be inserted on inside
Clinical Commissioning Policy Proposition: Rituximab for the treatment of dermatomyositis and polymyositis (Adults) Reference: NHS England A13X05/01 Information Reader Box (IRB) to be inserted on inside
Anti-NMDA receptor encephalitis presenting as an acute psychotic episode misdiagnosed as dissociative disorder: a case report
 Shimoyama et al. JA Clinical Reports (2016) 2:22 DOI 10.1186/s40981-016-0048-3 CASE REPORT Anti-NMDA receptor encephalitis presenting as an acute psychotic episode misdiagnosed as dissociative disorder:
Shimoyama et al. JA Clinical Reports (2016) 2:22 DOI 10.1186/s40981-016-0048-3 CASE REPORT Anti-NMDA receptor encephalitis presenting as an acute psychotic episode misdiagnosed as dissociative disorder:
Clinical Commissioning Policy Proposition: Intravenous immunoglobulin for acute disseminated encephalomyelitis and autoimmune encephalitis
 Clinical Commissioning Policy Proposition: Intravenous immunoglobulin for acute disseminated encephalomyelitis and autoimmune encephalitis Reference: NHS England F06X05/01 First published: March 2016 Prepared
Clinical Commissioning Policy Proposition: Intravenous immunoglobulin for acute disseminated encephalomyelitis and autoimmune encephalitis Reference: NHS England F06X05/01 First published: March 2016 Prepared
Long-term and Strong Immunotherapy to Treat Anti-N-Methyl- D-Aspartate Receptor Encephalitis with Refractory Status Epilepticus
 99 Long-term and Strong Immunotherapy to Treat Anti-N-Methyl- D-Aspartate Receptor Encephalitis with Refractory Status Epilepticus Lan-Hsin Lee, Chien-Jung Lu Abstract- Background: Anti-N-Methyl-D-Aspartate
99 Long-term and Strong Immunotherapy to Treat Anti-N-Methyl- D-Aspartate Receptor Encephalitis with Refractory Status Epilepticus Lan-Hsin Lee, Chien-Jung Lu Abstract- Background: Anti-N-Methyl-D-Aspartate
Non-paraneoplastic Anti-NMDAR Encephalitis: First Confirmed Adult Case Report in Latvia
 Journal of Pharmacy and Pharmacology 6 (2018) 123-129 doi: 10.17265/2328-2150/2018.02.002 D DAVID PUBLISHING Non-paraneoplastic Anti-NMDAR Encephalitis: First Confirmed Adult Case Report in Latvia Dace
Journal of Pharmacy and Pharmacology 6 (2018) 123-129 doi: 10.17265/2328-2150/2018.02.002 D DAVID PUBLISHING Non-paraneoplastic Anti-NMDAR Encephalitis: First Confirmed Adult Case Report in Latvia Dace
Application of the 2016 diagnostic approach for autoimmune encephalitis from Lancet Neurology to Chinese patients
 Li et al. BMC Neurology (2017) 17:195 DOI 10.1186/s12883-017-0974-3 RESEARCH ARTICLE Open Access Application of the 2016 diagnostic approach for autoimmune encephalitis from Lancet Neurology to Chinese
Li et al. BMC Neurology (2017) 17:195 DOI 10.1186/s12883-017-0974-3 RESEARCH ARTICLE Open Access Application of the 2016 diagnostic approach for autoimmune encephalitis from Lancet Neurology to Chinese
Reference: NHS England 1602
 Clinical Commissioning Policy Proposition: Clofarabine for refractory or relapsed acute myeloid leukaemia (AML) as a bridge to stem cell transplantation Reference: NHS England 1602 First published: TBC
Clinical Commissioning Policy Proposition: Clofarabine for refractory or relapsed acute myeloid leukaemia (AML) as a bridge to stem cell transplantation Reference: NHS England 1602 First published: TBC
ANTI-NMDA RECEPTOR ENCEPHALITIS: CASE SERIES AND LONG-TERM OUTCOMES
 ANTI-NMDA RECEPTOR ENCEPHALITIS: CASE SERIES AND LONG-TERM OUTCOMES Mongkol Chanvanichtrakool 1, Surachai Likasitwattanakul 1, Kanokpan Rongnoparat 1, Metha Apiwattanakul 2 and Sorawit Viravan 1 1 Division
ANTI-NMDA RECEPTOR ENCEPHALITIS: CASE SERIES AND LONG-TERM OUTCOMES Mongkol Chanvanichtrakool 1, Surachai Likasitwattanakul 1, Kanokpan Rongnoparat 1, Metha Apiwattanakul 2 and Sorawit Viravan 1 1 Division
Clinical Commissioning Policy: Gemcitabine and capecitabine following surgery for pancreatic cancer (all ages)
 Clinical Commissioning Policy: Gemcitabine and capecitabine following surgery for pancreatic cancer (all ages) NHS England Reference: 1711P 1 NHS England INFORMATION READER BOX Directorate Medical Operations
Clinical Commissioning Policy: Gemcitabine and capecitabine following surgery for pancreatic cancer (all ages) NHS England Reference: 1711P 1 NHS England INFORMATION READER BOX Directorate Medical Operations
Autoimmune Encephalitis
 Evaluation Approach for Suspected Autoimmune Encephalitis M.R ASHRAFI PROFESSOR OF PEDIATRIC NEUROLOGY CHILDREN S MEDICAL CENTER PEDIATRIC CENTER OF EXCELLENCE TEHRAN UNIVERSITY OF MEDICAL SCIENCES TEHRAN
Evaluation Approach for Suspected Autoimmune Encephalitis M.R ASHRAFI PROFESSOR OF PEDIATRIC NEUROLOGY CHILDREN S MEDICAL CENTER PEDIATRIC CENTER OF EXCELLENCE TEHRAN UNIVERSITY OF MEDICAL SCIENCES TEHRAN
DRAFT FOR POC BOARD Clinical Commissioning Policy Proposition: Rituximab in Connective Tissue Disease associated Interstitial Lung Disease (adults)
 Clinical Commissioning Policy Proposition: Rituximab in Connective Tissue Disease associated Interstitial Lung Disease (adults) Reference: NHS England A/14/X01 CHECK Information Reader Box (IRB) to be
Clinical Commissioning Policy Proposition: Rituximab in Connective Tissue Disease associated Interstitial Lung Disease (adults) Reference: NHS England A/14/X01 CHECK Information Reader Box (IRB) to be
Hodgkin Lymphoma Presenting with Anti-N-methyl-Daspartate Receptor Encephalitis
 Open Journal of Clinical & Medical Case Reports Volume 1 (2015) Issue 3 Hodgkin Lymphoma Presenting with Anti-N-methyl-Daspartate Receptor Encephalitis Benjamin W. Hoyt, BS Department of Surgery, Uniformed
Open Journal of Clinical & Medical Case Reports Volume 1 (2015) Issue 3 Hodgkin Lymphoma Presenting with Anti-N-methyl-Daspartate Receptor Encephalitis Benjamin W. Hoyt, BS Department of Surgery, Uniformed
NIH Public Access Author Manuscript Lancet Neurol. Author manuscript; available in PMC 2014 February 01.
 NIH Public Access Author Manuscript Published in final edited form as: Lancet Neurol. 2013 February ; 12(2): 157 165. doi:10.1016/s1474-4422(12)70310-1. Treatment and prognostic factors for long-term outcome
NIH Public Access Author Manuscript Published in final edited form as: Lancet Neurol. 2013 February ; 12(2): 157 165. doi:10.1016/s1474-4422(12)70310-1. Treatment and prognostic factors for long-term outcome
Clinical Commissioning Policy Proposition:
 Clinical Commissioning Policy Proposition: Rituximab for chronic inflammatory demyelinating polyradiculoneuropathy (CIDP), multifocal motor neuropathy (MMN), vasculitis of the peripheral nervous system
Clinical Commissioning Policy Proposition: Rituximab for chronic inflammatory demyelinating polyradiculoneuropathy (CIDP), multifocal motor neuropathy (MMN), vasculitis of the peripheral nervous system
Clinical Commissioning Policy Proposition: Bendamustine with rituximab for relapsed indolent non-hodgkin s lymphoma (all ages)
 Clinical Commissioning Policy Proposition: Bendamustine with rituximab for relapsed indolent non-hodgkin s lymphoma (all ages) Reference: NHS England 1607 1 First published: TBC Prepared by NHS England
Clinical Commissioning Policy Proposition: Bendamustine with rituximab for relapsed indolent non-hodgkin s lymphoma (all ages) Reference: NHS England 1607 1 First published: TBC Prepared by NHS England
EPILEPSY AND AUTOIMMUNE ENCEPHALITIS
 EPILEPSY AND AUTOIMMUNE ENCEPHALITIS Maarten J Titulaer, MD PhD Erasmus Medical Center, Erasmus University Rotterdam, THE NETHERLANDS Contents Introduction VGKC-complex antibodies o anti-lgi1 encephalitis
EPILEPSY AND AUTOIMMUNE ENCEPHALITIS Maarten J Titulaer, MD PhD Erasmus Medical Center, Erasmus University Rotterdam, THE NETHERLANDS Contents Introduction VGKC-complex antibodies o anti-lgi1 encephalitis
CASE REPORT. Abstract. Introduction. Case Report. Masato Kadoya, Hiroyuki Onoue, Akiko Kadoya, Katsunori Ikewaki and Kenichi Kaida
 CASE REPORT Refractory Status Epilepticus Caused by Anti-NMDA Receptor Encephalitis that Markedly Improved Following Combination Therapy with Rituximab and Cyclophosphamide Masato Kadoya, Hiroyuki Onoue,
CASE REPORT Refractory Status Epilepticus Caused by Anti-NMDA Receptor Encephalitis that Markedly Improved Following Combination Therapy with Rituximab and Cyclophosphamide Masato Kadoya, Hiroyuki Onoue,
Local Natalizumab Treatment Protocol
 Local Natalizumab Treatment Protocol 1. New medicine name: Natalizumab 300mg concentrate for solution for infusion (Natalizumab ) 2. Licensed indication(s): Natalizumab is indicated for single disease
Local Natalizumab Treatment Protocol 1. New medicine name: Natalizumab 300mg concentrate for solution for infusion (Natalizumab ) 2. Licensed indication(s): Natalizumab is indicated for single disease
Clinical Commissioning Policy: Immune Tolerance Induction (ITI) for haemophilia A (all ages)
 Clinical Commissioning Policy: Immune Tolerance Induction (ITI) for haemophilia A (all ages) Reference: NHS England: 16042/P NHS England INFORMATION READER BOX Directorate Medical Operations and Information
Clinical Commissioning Policy: Immune Tolerance Induction (ITI) for haemophilia A (all ages) Reference: NHS England: 16042/P NHS England INFORMATION READER BOX Directorate Medical Operations and Information
Supplementary information
 Study Supplementary information S1 (table) : Additional data of studies examining patients with anti- LGI1 antibodies Number of patients Male Age median (range) in years Tumour Tumour type Relapses Irani
Study Supplementary information S1 (table) : Additional data of studies examining patients with anti- LGI1 antibodies Number of patients Male Age median (range) in years Tumour Tumour type Relapses Irani
Paradox Lost: Exploring the Clinical-Radiologic Dissociation Seen in Anti-NMDA Receptor Encephalitis
 Current Literature In Clinical Science Paradox Lost: Exploring the Clinical-Radiologic Dissociation Seen in Anti-NMDA Receptor Encephalitis Functional and Structural Brain Changes in Anti N-Methyl-D-Aspartate
Current Literature In Clinical Science Paradox Lost: Exploring the Clinical-Radiologic Dissociation Seen in Anti-NMDA Receptor Encephalitis Functional and Structural Brain Changes in Anti N-Methyl-D-Aspartate
NCCP Chemotherapy Regimen. Obinutuzumab Maintenance Therapy following O-Bendamustine therapy
 Obinutuzumab following O-Bendamustine therapy INDICATIONS FOR USE: INDICATION Obinutuzumab maintenance therapy is indicated in patients with follicular lymphoma (FL) who have responded to induction treatment
Obinutuzumab following O-Bendamustine therapy INDICATIONS FOR USE: INDICATION Obinutuzumab maintenance therapy is indicated in patients with follicular lymphoma (FL) who have responded to induction treatment
JNNP Online First, published on November 22, 2012 as /jnnp Neuro-inflammation
 1 Department of Clinical Neurology, John Radcliffe Hospital, University of Oxford, Oxford, UK 2 Department of Paediatric Neurology, Birmingham Children s Hospital, Birmingham, UK 3 Department of Paediatric
1 Department of Clinical Neurology, John Radcliffe Hospital, University of Oxford, Oxford, UK 2 Department of Paediatric Neurology, Birmingham Children s Hospital, Birmingham, UK 3 Department of Paediatric
Case report of anti-n-methyl-d-aspartate receptor encephalitis in a middle-aged woman with a long history of major depressive disorder
 Rong et al. BMC Psychiatry (2017) 17:320 DOI 10.1186/s12888-017-1477-x CASE REPORT Case report of anti-n-methyl-d-aspartate receptor encephalitis in a middle-aged woman with a long history of major depressive
Rong et al. BMC Psychiatry (2017) 17:320 DOI 10.1186/s12888-017-1477-x CASE REPORT Case report of anti-n-methyl-d-aspartate receptor encephalitis in a middle-aged woman with a long history of major depressive
Summary of Risk Minimization Measures
 Table 6.1.4-1: Summary of Risk Minimization Measures Safety Concern Vaccination Hepatic and renal impairment Combination therapy Elderly Routine Risk Minimization Measures Specific subsection on vaccination
Table 6.1.4-1: Summary of Risk Minimization Measures Safety Concern Vaccination Hepatic and renal impairment Combination therapy Elderly Routine Risk Minimization Measures Specific subsection on vaccination
Case Report LGI1-antibody encephalitis with subsequent rapid progression of diffuse cerebral atrophy: a case report
 Int J Clin Exp Med 2016;9(3):7041-7045 www.ijcem.com /ISSN:1940-5901/IJCEM0020965 Case Report LGI1-antibody encephalitis with subsequent rapid progression of diffuse cerebral atrophy: a case report Fang
Int J Clin Exp Med 2016;9(3):7041-7045 www.ijcem.com /ISSN:1940-5901/IJCEM0020965 Case Report LGI1-antibody encephalitis with subsequent rapid progression of diffuse cerebral atrophy: a case report Fang
A score that predicts 1-year functional status in patients with anti-nmda receptor encephalitis
 ARTICLE Published Ahead of Print on December 21, 2018 as 10.1212/WNL.0000000000006783 OPEN ACCESS A score that predicts 1-year functional status in patients with anti-nmda receptor encephalitis Ramani
ARTICLE Published Ahead of Print on December 21, 2018 as 10.1212/WNL.0000000000006783 OPEN ACCESS A score that predicts 1-year functional status in patients with anti-nmda receptor encephalitis Ramani
N-Methyl-D-Aspartate Receptor Antibodies in Post Herpes Simplex Virus Encephalitis Neurological Relapse
 J_ID: MDS Customer A_ID: MDS25626 Cadmus Art: MDS25626 Ed. Ref. No.: 13-0201.R3 Date: 9-August-13 Stage: Page: 1 ID: jwweb3b2server Time: 17:07 I Path: //xinchnasjn/01journals/wiley/3b2/mds#/vol00000/130297/appfile/jw-mds#130297
J_ID: MDS Customer A_ID: MDS25626 Cadmus Art: MDS25626 Ed. Ref. No.: 13-0201.R3 Date: 9-August-13 Stage: Page: 1 ID: jwweb3b2server Time: 17:07 I Path: //xinchnasjn/01journals/wiley/3b2/mds#/vol00000/130297/appfile/jw-mds#130297
Gazyva. Gazyva (obinutuzumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.29 Subject: Gazyva Page: 1 of 7 Last Review Date: March 16, 2018 Gazyva Description Gazyva (obinutuzumab)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.29 Subject: Gazyva Page: 1 of 7 Last Review Date: March 16, 2018 Gazyva Description Gazyva (obinutuzumab)
Clinical Commissioning Policy: Selexipag in the treatment of Pulmonary Arterial Hypertension
 Clinical Commissioning Policy: Selexipag in the treatment of Pulmonary Arterial Hypertension Reference: NHS England: 16017/P NHS England INFORMATION READER BOX Directorate Medical Operations and Information
Clinical Commissioning Policy: Selexipag in the treatment of Pulmonary Arterial Hypertension Reference: NHS England: 16017/P NHS England INFORMATION READER BOX Directorate Medical Operations and Information
COPYRIGHT 2012 THE TRANSVERSE MYELITIS ASSOCIATION. ALL RIGHTS RESERVED
 The Transverse Myelitis Association...advocating for those with acute disseminated encephalomyelitis, neuromyelitis optica, optic neuritis and transverse myelitis ACUTE DISSEMINATED ENCEPHALOMYELITIS (ADEM)
The Transverse Myelitis Association...advocating for those with acute disseminated encephalomyelitis, neuromyelitis optica, optic neuritis and transverse myelitis ACUTE DISSEMINATED ENCEPHALOMYELITIS (ADEM)
Reference: NHS England 1605
 Clinical Commissioning Policy Proposition: Bendamustine with rituximab for first line treatment of advanced indolent non- Hodgkin s lymphoma (all ages) Reference: NHS England 1605 First published: TBC
Clinical Commissioning Policy Proposition: Bendamustine with rituximab for first line treatment of advanced indolent non- Hodgkin s lymphoma (all ages) Reference: NHS England 1605 First published: TBC
Autoimmune encephalitis: An emerging entity
 Leading Article Autoimmune encephalitis: An emerging entity Pyara Ratnayake 1 Sri Lanka Journal of Child Health, 2013: 42(1): 3-9 (Key words: Autoimmune encephalitis) Introduction The importance of immunology
Leading Article Autoimmune encephalitis: An emerging entity Pyara Ratnayake 1 Sri Lanka Journal of Child Health, 2013: 42(1): 3-9 (Key words: Autoimmune encephalitis) Introduction The importance of immunology
ISSN Multimodal Imaging for Characterizing Neoplasia in Anti- NMDA Receptor encephalitis *
 Open Journal of Clinical & Medical Case Reports Volume 2 (2016) Issue 5 Abstract ISSN 2379-1039 Multimodal Imaging for Characterizing Neoplasia in Anti- NMDA Receptor encephalitis * Michael J. Bradshaw,
Open Journal of Clinical & Medical Case Reports Volume 2 (2016) Issue 5 Abstract ISSN 2379-1039 Multimodal Imaging for Characterizing Neoplasia in Anti- NMDA Receptor encephalitis * Michael J. Bradshaw,
Rituximab treatment in autoimmune blistering diseases
 Rituximab treatment in autoimmune blistering diseases This leaflet explains more about having treatment with the medicine rituximab for an autoimmune blistering disease. It includes information about the
Rituximab treatment in autoimmune blistering diseases This leaflet explains more about having treatment with the medicine rituximab for an autoimmune blistering disease. It includes information about the
Clinical Commissioning Policy Proposition: Selexipag in the treatment of Pulmonary Arterial Hypertension
 Clinical Commissioning Policy Proposition: Selexipag in the treatment of Pulmonary Arterial Hypertension Reference: NHS England A11X04/01 Information Reader Box (IRB) to be inserted on inside front cover
Clinical Commissioning Policy Proposition: Selexipag in the treatment of Pulmonary Arterial Hypertension Reference: NHS England A11X04/01 Information Reader Box (IRB) to be inserted on inside front cover
Clinical Commissioning Policy Proposition:
 Clinical Commissioning Policy Proposition: Chemosaturation for liver metastases from ocular melanomas Reference: NHS England A02X05/01 Information Reader Box (IRB) to be inserted on inside front cover
Clinical Commissioning Policy Proposition: Chemosaturation for liver metastases from ocular melanomas Reference: NHS England A02X05/01 Information Reader Box (IRB) to be inserted on inside front cover
Hippocampal Sclerosis in LGI1 and CSPR2 Positive Limbic Encephalopathy: Case Report
 Hippocampal Sclerosis in LGI1 and CSPR2 Positive Limbic Encephalopathy: Ammar Taha Abdulaziz 1, Le Zhang 1, Dong Zhou 2, JinMei Li 3, Abstract Background: Limbic encephalopathy (LE) is a sub-acute neuropsychiatric
Hippocampal Sclerosis in LGI1 and CSPR2 Positive Limbic Encephalopathy: Ammar Taha Abdulaziz 1, Le Zhang 1, Dong Zhou 2, JinMei Li 3, Abstract Background: Limbic encephalopathy (LE) is a sub-acute neuropsychiatric
RiTUXimab 375 mg/m 2 Therapy-7 day
 RiTUXimab 375 mg/m 2 Therapy-7 day This regimen supercedes NCCP Regimen 00208 rituximab 375mg/m2 therapy-follicular lymphoma as of February 2019 INDICATIONS FOR USE: Regimen *Reimbursement INDICATION ICD10
RiTUXimab 375 mg/m 2 Therapy-7 day This regimen supercedes NCCP Regimen 00208 rituximab 375mg/m2 therapy-follicular lymphoma as of February 2019 INDICATIONS FOR USE: Regimen *Reimbursement INDICATION ICD10
Gazyva. Gazyva (obinutuzumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.29 Subject: Gazyva Page: 1 of 7 Last Review Date: September 15, 2016 Gazyva Description Gazyva (obinutuzumab)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.29 Subject: Gazyva Page: 1 of 7 Last Review Date: September 15, 2016 Gazyva Description Gazyva (obinutuzumab)
National Horizon Scanning Centre. Rituximab (MabThera) for chronic lymphocytic leukaemia. September 2007
 Rituximab (MabThera) for chronic lymphocytic leukaemia This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a definitive
Rituximab (MabThera) for chronic lymphocytic leukaemia This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a definitive
Clinical Commissioning Policy: Deep Brain Stimulation for Refractory Epilepsy
 Clinical Commissioning Policy: Deep Brain Stimulation for Refractory Epilepsy Reference: NHS England xxx/x/x 1 Clinical Commissioning Policy: Deep Brain Stimulation for Refractory Epilepsy First published:
Clinical Commissioning Policy: Deep Brain Stimulation for Refractory Epilepsy Reference: NHS England xxx/x/x 1 Clinical Commissioning Policy: Deep Brain Stimulation for Refractory Epilepsy First published:
Screening Autoimmune Anti-neuronal Antibodies in Pediatric Patients with Suspected Autoimmune Encephalitis
 Screening Autoimmune Anti-neuronal Antibodies in Pediatric Patients with Suspected Autoimmune Encephalitis Original Article Journal of Epilepsy Research pissn 2233-6249 / eissn 2233-6257 Soo Yeon Kim 1,
Screening Autoimmune Anti-neuronal Antibodies in Pediatric Patients with Suspected Autoimmune Encephalitis Original Article Journal of Epilepsy Research pissn 2233-6249 / eissn 2233-6257 Soo Yeon Kim 1,
CLINICAL GUIDELINES. Summary of Literature and Recommendations Concerning Immunization and Steroid Injections Thomas J. Gilbert M.D., M.P.P.
 CLINICAL GUIDELINES Summary of Literature and Recommendations Concerning Immunization and Steroid Injections Thomas J. Gilbert M.D., M.P.P. 11/2/15 Several practices routinely delay steroid injections
CLINICAL GUIDELINES Summary of Literature and Recommendations Concerning Immunization and Steroid Injections Thomas J. Gilbert M.D., M.P.P. 11/2/15 Several practices routinely delay steroid injections
Treatment of Autoimmune Brain Disorders: A Medical Perspective
 Treatment of Autoimmune Brain Disorders: A Medical Perspective Pediatric Rheumatology Duke Children s Hospital Director, Duke Autoimmune Brain Disorders Program NCCCAP Sept 29, 2018 DISCLOSURES Research
Treatment of Autoimmune Brain Disorders: A Medical Perspective Pediatric Rheumatology Duke Children s Hospital Director, Duke Autoimmune Brain Disorders Program NCCCAP Sept 29, 2018 DISCLOSURES Research
Autoimmune encephalopathies in children: diagnostic clues and therapeutic challenges
 Topic: Autoimmune neurological diseases associated with autoantibodies specific for synaptic antigens Autoimmune encephalopathies in children: diagnostic clues and therapeutic challenges Giorgia Olivieri,
Topic: Autoimmune neurological diseases associated with autoantibodies specific for synaptic antigens Autoimmune encephalopathies in children: diagnostic clues and therapeutic challenges Giorgia Olivieri,
Treatment of Autoimmune Brain Disorders: A Medical Perspective
 Treatment of Autoimmune Brain Disorders: A Medical Perspective Heather Van Mater, MD, MS Pediatric Rheumatology Duke Children s Hospital Director, Duke Autoimmune Brain Disorders Program NCCCAP Sept 29,
Treatment of Autoimmune Brain Disorders: A Medical Perspective Heather Van Mater, MD, MS Pediatric Rheumatology Duke Children s Hospital Director, Duke Autoimmune Brain Disorders Program NCCCAP Sept 29,
Neuronal antibodies in pediatric epilepsy: Clinical features and long-term outcomes of a historical cohort not treated with immunotherapy
 FULL-LENGTH ORIGINAL RESEARCH Neuronal antibodies in pediatric epilepsy: Clinical features and long-term outcomes of a historical cohort not treated with immunotherapy *Sukhvir Wright, Ada T. Geerts, Cornelia
FULL-LENGTH ORIGINAL RESEARCH Neuronal antibodies in pediatric epilepsy: Clinical features and long-term outcomes of a historical cohort not treated with immunotherapy *Sukhvir Wright, Ada T. Geerts, Cornelia
Clinical Commissioning Policy: Bendamustine with rituximab for relapsed and refractory mantle cell lymphoma (all ages)
 Clinical Commissioning Policy: Bendamustine with rituximab for relapsed and refractory mantle cell lymphoma (all ages) NHS England Reference: 170029P 1 NHS England INFORMATION READER BOX Directorate Medical
Clinical Commissioning Policy: Bendamustine with rituximab for relapsed and refractory mantle cell lymphoma (all ages) NHS England Reference: 170029P 1 NHS England INFORMATION READER BOX Directorate Medical
Prevalence and clinical characteristics of serum neuronal cell surface antibodies in first episode psychosis: a case-control comparison study
 Prevalence and clinical characteristics of serum neuronal cell surface antibodies in first episode psychosis: a case-control comparison study Belinda R. Lennox DM 1, Emma C. Palmer-Cooper PhD 1, Thomas
Prevalence and clinical characteristics of serum neuronal cell surface antibodies in first episode psychosis: a case-control comparison study Belinda R. Lennox DM 1, Emma C. Palmer-Cooper PhD 1, Thomas
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Single Technology Appraisal (STA)
 Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can provide a unique perspective on the technology
Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can provide a unique perspective on the technology
Urgent Clinical Commissioning Policy Statement: Pembrolizumab for drug-resistant gestational trophoblastic neoplasia. NHS England Reference: P
 Urgent Clinical Commissioning Policy Statement: Pembrolizumab for drug-resistant gestational trophoblastic neoplasia NHS England Reference: 170027P 1 Equality statement Promoting equality and addressing
Urgent Clinical Commissioning Policy Statement: Pembrolizumab for drug-resistant gestational trophoblastic neoplasia NHS England Reference: 170027P 1 Equality statement Promoting equality and addressing
NMDA Receptor Antibody Encephalitis
 Curr Neurol Neurosci Rep (2011) 11:298 304 DOI 10.1007/s11910-011-0186-y NMDA Receptor Antibody Encephalitis Sarosh R. Irani & Angela Vincent Published online: 18 February 2011 # Springer Science+Business
Curr Neurol Neurosci Rep (2011) 11:298 304 DOI 10.1007/s11910-011-0186-y NMDA Receptor Antibody Encephalitis Sarosh R. Irani & Angela Vincent Published online: 18 February 2011 # Springer Science+Business
BMC Research Notes. Open Access CASE REPORT. Maria Moura 1, Amilcar Silva dos Santos 2,3,4*, Joana Afonso 5 and Miguel Talina 2,6
 DOI 10.1186/s13104-016-2180-6 BMC Research Notes CASE REPORT Open Access First episode psychosis in a 15 year old female with clinical presentation of anti NMDA receptor encephalitis: a case report and
DOI 10.1186/s13104-016-2180-6 BMC Research Notes CASE REPORT Open Access First episode psychosis in a 15 year old female with clinical presentation of anti NMDA receptor encephalitis: a case report and
Reference: NHS England: 16022/P
 Clinical Commissioning Policy: The use of Stereotactic Ablative Radiotherapy (SABR) as a treatment option for patients with Hepatocellular carcinoma or Cholangiocarcinoma Reference: NHS England: 16022/P
Clinical Commissioning Policy: The use of Stereotactic Ablative Radiotherapy (SABR) as a treatment option for patients with Hepatocellular carcinoma or Cholangiocarcinoma Reference: NHS England: 16022/P
Clinical Commissioning Policy: Bendamustine with rituximab for relapsed and refractory mantle cell lymphoma (all ages) NHS England Reference: P
 Clinical Commissioning Policy: Bendamustine with rituximab for relapsed and refractory mantle cell lymphoma (all ages) NHS England Reference: 170054P 1 NHS England INFORMATION READER BOX Directorate Medical
Clinical Commissioning Policy: Bendamustine with rituximab for relapsed and refractory mantle cell lymphoma (all ages) NHS England Reference: 170054P 1 NHS England INFORMATION READER BOX Directorate Medical
FOR PUBLIC CONSULTATION ONLY. Evidence Review: Rituximab for immunoglobulin G4-related disease (IgG4-RD)
 Evidence Review: Rituximab for immunoglobulin G4-related disease (IgG4-RD) NHS England FOR PUBLIC CONSULTATION ONLY Evidence Review: Rituximab for immunoglobulin G4-related disease (IgG4- RD) First published:
Evidence Review: Rituximab for immunoglobulin G4-related disease (IgG4-RD) NHS England FOR PUBLIC CONSULTATION ONLY Evidence Review: Rituximab for immunoglobulin G4-related disease (IgG4- RD) First published:
NCCP Chemotherapy Regimen. Brentuximab vedotin Monotherapy
 Brentuximab INDICATIONS FOR USE: INDICATION ICD10 Regimen Code *Reimbursement Status Treatment of adult patients with relapsed or refractory CD30+ Hodgkin lymphoma (HL): Following autologous stem cell
Brentuximab INDICATIONS FOR USE: INDICATION ICD10 Regimen Code *Reimbursement Status Treatment of adult patients with relapsed or refractory CD30+ Hodgkin lymphoma (HL): Following autologous stem cell
Urgent Clinical Commissioning Policy Statement: Alemtuzumab for treating relapsingremitting. sclerosis third cycle (all ages)
 Urgent Clinical Commissioning Policy Statement: Alemtuzumab for treating relapsingremitting multiple sclerosis third cycle (all ages) NHS England Reference: 170075P 1 Equality statement Promoting equality
Urgent Clinical Commissioning Policy Statement: Alemtuzumab for treating relapsingremitting multiple sclerosis third cycle (all ages) NHS England Reference: 170075P 1 Equality statement Promoting equality
Clinical Commissioning Policy: Rituximab For ANCA Vasculitis. December Reference : NHSCB/ A3C/1a
 Clinical Commissioning Policy: Rituximab For ANCA Vasculitis December 2012 Reference : NHSCB/ A3C/1a NHS Commissioning Board Clinical Commissioning Policy: Rituximab For The Treatment Of Anti-Neutrophil
Clinical Commissioning Policy: Rituximab For ANCA Vasculitis December 2012 Reference : NHSCB/ A3C/1a NHS Commissioning Board Clinical Commissioning Policy: Rituximab For The Treatment Of Anti-Neutrophil
Anti-NMDAR Encephalitis in a 13-Year-Old Female: A 24-Month Clinical Follow-Up
 Anti-NMDAR Encephalitis in a 13-Year-Old Female: A 24-Month Clinical Follow-Up Eunsil Kim, MD 1, Eu Gene Park, MD 2, Jiwon Lee, MD 1, Munhyang Lee, MD, PhD 1, Jihye Kim, MD, PhD 3, Jeehun Lee, MD, PhD
Anti-NMDAR Encephalitis in a 13-Year-Old Female: A 24-Month Clinical Follow-Up Eunsil Kim, MD 1, Eu Gene Park, MD 2, Jiwon Lee, MD 1, Munhyang Lee, MD, PhD 1, Jihye Kim, MD, PhD 3, Jeehun Lee, MD, PhD
HHS Public Access Author manuscript Lancet Neurol. Author manuscript; available in PMC 2017 April 01.
 HHS Public Access Author manuscript Published in final edited form as: Lancet Neurol. 2016 April ; 15(4): 391 404. doi:10.1016/s1474-4422(15)00401-9. A clinical approach to diagnosis of autoimmune encephalitis
HHS Public Access Author manuscript Published in final edited form as: Lancet Neurol. 2016 April ; 15(4): 391 404. doi:10.1016/s1474-4422(15)00401-9. A clinical approach to diagnosis of autoimmune encephalitis
Case Report High Grade Glioma Mimicking Voltage Gated Potassium Channel Complex Associated Antibody Limbic Encephalitis
 Case Reports in Neurological Medicine, Article ID 458790, 4 pages http://dx.doi.org/10.1155/2014/458790 Case Report High Grade Glioma Mimicking Voltage Gated Potassium Channel Complex Associated Antibody
Case Reports in Neurological Medicine, Article ID 458790, 4 pages http://dx.doi.org/10.1155/2014/458790 Case Report High Grade Glioma Mimicking Voltage Gated Potassium Channel Complex Associated Antibody
Rituximab for the treatment of adults with idiopathic (immune) thrombocytopenic purpura (ITP)
 Bedfordshire and Luton Joint Prescribing Committee Date: September 2015 Review date: September 2018 Bullletin 221: Rituximab (MabThera ) for the treatment of adults with idiopathic (immune) thrombocytopenic
Bedfordshire and Luton Joint Prescribing Committee Date: September 2015 Review date: September 2018 Bullletin 221: Rituximab (MabThera ) for the treatment of adults with idiopathic (immune) thrombocytopenic
Downloaded from Medico Research Chronicles NMDA Encefalitis case report and literature review. NMDA ENCEFALITIS CASE REPORT AND LITERATURE REVIEW
 NMDA ENCEFALITIS CASE REPORT AND LITERATURE REVIEW Najada Como 1, Migena Qato 1, Dhimiter Kraja 1, Drini Dobi 2, Pellumb Pipero 1, Arjan Harxhi 1 1. Department of Infectious Disease Hospital, UHC Mother
NMDA ENCEFALITIS CASE REPORT AND LITERATURE REVIEW Najada Como 1, Migena Qato 1, Dhimiter Kraja 1, Drini Dobi 2, Pellumb Pipero 1, Arjan Harxhi 1 1. Department of Infectious Disease Hospital, UHC Mother
Regulatory Status FDA-approved indication: Tysabri is an integrin receptor antagonist indicated for treatment of (1):
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.60.13 Subject: Tysabri Page: 1 of 6 Last Review Date: June 22, 2017 Tysabri Description Tysabri (natalizumab)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.60.13 Subject: Tysabri Page: 1 of 6 Last Review Date: June 22, 2017 Tysabri Description Tysabri (natalizumab)
Autoimmune Epilepsy: Are We Seeing the Tip of the Iceberg... or the Whole Thing?
 Current Literature In Clinical Science Autoimmune Epilepsy: Are We Seeing the Tip of the Iceberg... or the Whole Thing? Autoimmune Epilepsy: Clinical Characteristics and Response to Immunotherapy. Quek
Current Literature In Clinical Science Autoimmune Epilepsy: Are We Seeing the Tip of the Iceberg... or the Whole Thing? Autoimmune Epilepsy: Clinical Characteristics and Response to Immunotherapy. Quek
Clinical Commissioning Policy Statement: Rituximab For Systemic Lupus Erythematosus (SLE) December Reference : NHSCB/A3C/1b
 Clinical Commissioning Policy Statement: Rituximab For Systemic Lupus Erythematosus (SLE) December 2012 Reference : NHSCB/A3C/1b NHS Commissioning Board Clinical Commissioning Policy Statement: Rituximab
Clinical Commissioning Policy Statement: Rituximab For Systemic Lupus Erythematosus (SLE) December 2012 Reference : NHSCB/A3C/1b NHS Commissioning Board Clinical Commissioning Policy Statement: Rituximab
Rituxan Hycela. Rituxan Hycela (rituximab and hyaluronidase human) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.96 Subject: Rituxan Hycela Page: 1 of 5 Last Review Date: September 15, 2017 Rituxan Hycela Description
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.96 Subject: Rituxan Hycela Page: 1 of 5 Last Review Date: September 15, 2017 Rituxan Hycela Description
Technology appraisal guidance Published: 11 April 2018 nice.org.uk/guidance/ta517
 Avelumab for treating metastatic Merkelel cell carcinoma Technology appraisal guidance Published: 11 April 2018 nice.org.uk/guidance/ta517 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Avelumab for treating metastatic Merkelel cell carcinoma Technology appraisal guidance Published: 11 April 2018 nice.org.uk/guidance/ta517 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Autoimmune Encephalitis Autoimmunity and acute neuropsychiatric disorders: the clinical challenge of seronegative but probable autoimmune psychosis
 Autoimmune Encephalitis Autoimmunity and acute neuropsychiatric disorders: the clinical challenge of seronegative but probable autoimmune psychosis Souhel Najjar, MD Professor & Chairman, Department of
Autoimmune Encephalitis Autoimmunity and acute neuropsychiatric disorders: the clinical challenge of seronegative but probable autoimmune psychosis Souhel Najjar, MD Professor & Chairman, Department of
Reference: NHS England B01X26
 Clinical Commissioning Policy Proposition: The use of Stereotactic Ablative Radiotherapy (SABR) as a treatment option for patients with Hepatocellular carcinoma or Cholangiocarcinoma Reference: NHS England
Clinical Commissioning Policy Proposition: The use of Stereotactic Ablative Radiotherapy (SABR) as a treatment option for patients with Hepatocellular carcinoma or Cholangiocarcinoma Reference: NHS England
Committee Approval Date: May 9, 2014 Next Review Date: May 2015
 Medication Policy Manual Policy No: dru248 Topic: Benlysta, belimumab Date of Origin: May 13, 2011 Committee Approval Date: May 9, 2014 Next Review Date: May 2015 Effective Date: June 1, 2014 IMPORTANT
Medication Policy Manual Policy No: dru248 Topic: Benlysta, belimumab Date of Origin: May 13, 2011 Committee Approval Date: May 9, 2014 Next Review Date: May 2015 Effective Date: June 1, 2014 IMPORTANT
Risk Evaluation and Mitigation Strategy (REMS): Cytokine release syndrome and neurological toxicities
 Risk Evaluation and Mitigation Strategy (REMS): Cytokine release syndrome and neurological toxicities A REMS is a program required by the FDA to manage known or potential serious risks associated with
Risk Evaluation and Mitigation Strategy (REMS): Cytokine release syndrome and neurological toxicities A REMS is a program required by the FDA to manage known or potential serious risks associated with
10/27/2013. Funding from the Autoimmune Encephalitis Alliance. Heather Van Mater, MD MS October 27, 2013
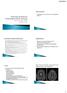 Funding from the Alliance Heather Van Mater, MD October 27, 2013 1 2 Aviv, et al. MR imaging and angiography of primary CNS of childhood. AJNR Am J Neuroradiol. 2006; 27: 192-199 Aviv, et al. of primary
Funding from the Alliance Heather Van Mater, MD October 27, 2013 1 2 Aviv, et al. MR imaging and angiography of primary CNS of childhood. AJNR Am J Neuroradiol. 2006; 27: 192-199 Aviv, et al. of primary
Rituximab (weekly) for Primary Cutaneous B cell Lymphoma
 Rituximab (weekly) for Primary Cutaneous B cell Lymphoma Indication: Palliative therapy for Low grade Primary Cutaneous B cell Lymphoma (Primary cutaneous Follicle centre cell Lymphoma and Primary cutaneous
Rituximab (weekly) for Primary Cutaneous B cell Lymphoma Indication: Palliative therapy for Low grade Primary Cutaneous B cell Lymphoma (Primary cutaneous Follicle centre cell Lymphoma and Primary cutaneous
The Laboratory Diagnosis of Autoimmune Encephalitis
 The Laboratory Diagnosis of Autoimmune Encephalitis Review Journal of Epilepsy Research pissn 2233-6249 / eissn 2233-6257 Sang Kun Lee, Soon-Tae Lee Department of Neurology, Seoul National University College
The Laboratory Diagnosis of Autoimmune Encephalitis Review Journal of Epilepsy Research pissn 2233-6249 / eissn 2233-6257 Sang Kun Lee, Soon-Tae Lee Department of Neurology, Seoul National University College
Obinutuzumab+Bendamustine followed by Obinutuzumab Maintenance Burton in-patient Derby in-patient Burton day-case Derby day-case
 Obinutuzumab+Bendamustine followed by Obinutuzumab Maintenance Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient Available
Obinutuzumab+Bendamustine followed by Obinutuzumab Maintenance Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient Available
Low back pain and sciatica in over 16s NICE quality standard
 March 2017 Low back pain and sciatica in over 16s NICE quality standard Draft for consultation This quality standard covers the assessment and management of non-specific low back pain and sciatica in young
March 2017 Low back pain and sciatica in over 16s NICE quality standard Draft for consultation This quality standard covers the assessment and management of non-specific low back pain and sciatica in young
NCCP Chemotherapy Regimen. Brentuximab vedotin Monotherapy
 INDICATIONS FOR USE: INDICATION Brentuximab Treatment of adult patients with relapsed or refractory CD30+ Hodgkin lymphoma (HL): Following autologous stem cell transplant (ASCT) or Following at least two
INDICATIONS FOR USE: INDICATION Brentuximab Treatment of adult patients with relapsed or refractory CD30+ Hodgkin lymphoma (HL): Following autologous stem cell transplant (ASCT) or Following at least two
Progressive Multifocal Leukoencephalopathy (PML) in Natalizumab-Treated Patients: Experience of the FDA Division of Neurology Products
 Progressive Multifocal Leukoencephalopathy (PML) in Natalizumab-Treated Patients: Experience of the FDA Division of Neurology Products M. Lisa Jones MD, MPH Russell Katz, M.D. Director Division of Neurology
Progressive Multifocal Leukoencephalopathy (PML) in Natalizumab-Treated Patients: Experience of the FDA Division of Neurology Products M. Lisa Jones MD, MPH Russell Katz, M.D. Director Division of Neurology
Welcome to todays Webinar
 Welcome to todays Webinar Your Presenter is: Lyndal Emery Your Facilitator is: Andrea Salmon Acknowledgement We acknowledge and pay respect to the traditional custodians past and present on whose lands
Welcome to todays Webinar Your Presenter is: Lyndal Emery Your Facilitator is: Andrea Salmon Acknowledgement We acknowledge and pay respect to the traditional custodians past and present on whose lands
Autoimmune Epilepsy : Encephalitis With Autoantibodies for Epileptologists
 Current Review In Clinical Science Autoimmune Epilepsy : Encephalitis With Autoantibodies for Epileptologists Christian G. Bien, 1* Martin Holtkamp 2 1 Epilepsy Centre Bethel, Krankenhaus Mara, Bielefeld,
Current Review In Clinical Science Autoimmune Epilepsy : Encephalitis With Autoantibodies for Epileptologists Christian G. Bien, 1* Martin Holtkamp 2 1 Epilepsy Centre Bethel, Krankenhaus Mara, Bielefeld,
Atypical presentation of anti-n-methyl-daspartate receptor encephalitis: two case reports
 Maggio et al. Journal of Medical Case Reports (2017) 11:225 DOI 10.1186/s13256-017-1388-y CASE REPORT Atypical presentation of anti-n-methyl-daspartate receptor encephalitis: two case reports Maria Cristina
Maggio et al. Journal of Medical Case Reports (2017) 11:225 DOI 10.1186/s13256-017-1388-y CASE REPORT Atypical presentation of anti-n-methyl-daspartate receptor encephalitis: two case reports Maria Cristina
Evidence review for Surrey Prescribing Clinical Network SUMMARY
 East Surrey CCG, Guildford & Waverley CCG, North West Surrey CCG, Surrey Downs CCG, Surrey Heath CCG, Crawley CCG, Horsham & Mid-Sussex CCG Evidence review for Surrey Prescribing Clinical Network Medicine
East Surrey CCG, Guildford & Waverley CCG, North West Surrey CCG, Surrey Downs CCG, Surrey Heath CCG, Crawley CCG, Horsham & Mid-Sussex CCG Evidence review for Surrey Prescribing Clinical Network Medicine
Autologous Hematopoietic Stem Cell Transplantation for the Treatment of Neuromyelitis Optica in Singapore
 Case Reports 26 Autologous Hematopoietic Stem Cell Transplantation for the Treatment of Neuromyelitis Optica in Singapore Koh Yeow Hoay, Pavanni Ratnagopal Abstract Introduction: Neuromyelitis optica (NMO)
Case Reports 26 Autologous Hematopoietic Stem Cell Transplantation for the Treatment of Neuromyelitis Optica in Singapore Koh Yeow Hoay, Pavanni Ratnagopal Abstract Introduction: Neuromyelitis optica (NMO)
Autoimmune Epilepsy:
 Autoimmune Epilepsy: More Than Just A Paraneoplastic Syndrome A newly recognized category of epilepsy caused by or associated with antibodies. By Lindsay M. Higdon, MD Introduction Approximately 30% of
Autoimmune Epilepsy: More Than Just A Paraneoplastic Syndrome A newly recognized category of epilepsy caused by or associated with antibodies. By Lindsay M. Higdon, MD Introduction Approximately 30% of
