Infliximab for the treatment of refractory scleritis
|
|
|
- Richard Scott Pearson
- 5 years ago
- Views:
Transcription
1 1 Massachussets Eye Research and Surgery Institution, Cambridge, Massachusetts, USA 2 BayView Clinic, Mumbai, India 3 Department of Ophthalmology, Harvard Medical School, Boston, Massachusetts, USA 4 Brigham & Women s Hospital, Department of Medicine, Division of Preventative Medicine, Boston, Massachusetts, USA 5 Ocular Immunology and Uveitis Foundation, Cambridge, Massachusetts, USA Correspondence to Dr C Stephen Foster, Massachusetts Eye Research and Surgery Institution, 5 Cambridge Center, Cambridge, MA 02142, USA; sfoster@mersi.us Accepted 30 September 2009 Published Online First 2 December 2009 This paper is freely available online under the BMJ Journals unlocked scheme, see bjo.bmj.com/site/about/ unlocked.xhtml. Infliximab for the treatment of refractory scleritis Priyanka Doctor, 1,2 Amyna Sultan, 1 Sana Syed, 1 William Christen, 3,4 Pooja Bhat, 1 C Stephen Foster 1,3,5 ABSTRACT Background Scleritis is a potentially blinding inflammatory disorder. Standard care consists of systemic corticosteroids and immunosuppresants. The authors describe a series of 10 patients suffering from scleritis treated with the TNF inhibitor because this scleritis was refractory to standard. Methods The authors reviewed the medical records of patients with scleritis at the Massachusetts Eye Research and Surgery Institution, treated with. All cases had non-infectious scleritis refractory to traditional immunomodulatory and received 5 mg/kg of at 4e8-weekly intervals. The main outcome measures evaluated were clinical response, reduction in concomitant immunomodulatory and adverse effects. Inflammation control and visual acuity were assessed using life-table methods. Results A favourable clinical response to was seen in 100% of the patients, with six (60%) of them achieving remission and cessation of concomitant immunosuppression. A clinical response to occurred within weeks on average. Based on clinical response, the authors found that repeat monthly infusions were required to maintain remission. One (10%) patient developed a lupus-like reaction necessitating discontinuation of. Conclusion Infliximab may be considered in the treatment of non-infectious scleritis refractory to other treatment. BACKGROUND Scleral inflammation is associated with systemic autoimmune disorders in 50% of cases, and is often associated with significant morbidity. 1 Ocular complications include keratitis, uveitis, and glaucoma with anterior scleritis and exudative detachments or other posterior segment complications with posterior scleritis. 1 2 Immunosuppressive has proved to be successful in the treatment of autoimmune disorders. 3 4 Infliximab, a humanised, chimeric monoclonal antibody directed against the proinflammatory cytokine tumour necrosis factor a (TNF-a), has been approved and marketed for the treatment of rheumatoid arthritis and Crohn disease. 56 While there have been reports of the efficacy of in the treatment of uveitis, there is little known about the efficacy and tolerability of for the treatment of scleritis. We review our experience with this drug in the treatment of scleritis refractory to conventional treatment. METHODS The medical records of 10 patients with scleritis who received (Remicade, Centocor,, Clinical science Horsham, Pennsylvania) from September 2003 to October 2007 were reviewed. All of the patients were seen by the same physician (CSF). Scleritis was defined as oedema in the episcleral and scleral tissues with both superficial and deep episcleral vessel injection accompanied by pain and tenderness to palpation. It was classified as anterior (diffuse, sectoral or necrotising) or posterior, as proposed by Watson and Hayreh. 7 Posterior scleritis was diagnosed on the basis of clinical and ultrasonography findings. Scleritis was graded and scored according to the grading system defined by Foster and Vitaledsclera injection and inflammation 0 to 4 in 0.5 gradations; these findings were documented by drawings, photography or both. Treatment with was considered on an off-label basis after failure of alternative immunosuppression. Infliximab was initiated as 5 mg/kg infusions over 120 min (180 min for the first infusion). A loading dose was infused at zero and 2 weeks, and then maintenance was administered at intervals of approximately 1 month. The intervals between infusions and dose of were adjusted depending on disease activity and tolerance of the medications. Ophthalmic assessment was performed every 4e6 weeks. Serum biochemical and haematological profiles were monitored at each clinic visit. Remission was defined as control of inflammation while on without use of corticosteroid. Outcome variables evaluated included inflammation recurrence, treatment response and decrease in ocular and systemic adjuvant. Statistical analysis was performed using PROC LIFETEST in PC_SAS (version 6.08; SAS Institute, Cary, North Carolina). Because eyes were not examined independently and because disease progression and response to are highly correlated between eyes, the data for left and right eyes were analysed separately. RESULTS The clinical data for each patient are summarised in table 1. The ocular diagnoses included diffuse scleritis (n¼4), nodular scleritis (n¼2), sclerouveitis (n¼2) and scleritis associated with keratitis (n¼2). Seven patients had underlying systemic diagnoses, which included rheumatoid arthritis, Crohn disease and Behçet disease. The mean patient age was (range 35 to 70). The mean number of infusions was (range 7 to 24), and the mean followup time while on was 16.4 months (range 6e48). Nine patients received primarily for treatment of scleritis, whereas patient no 6 received primarily for control of uveitis associated with scleritis. Br J Ophthalmol 2010;94:579e583. doi: /bjo
2 Table 1 Clinical data of patients treated with No Ocular diagnoses Systemic diagnoses Therapy Current No of infusion(s) Follow-up (months) Visual acuity Visual acuity after Inflammation Inflammation after (at last visit) Status at last follow-up 1 Sclerouveitis OU, CMO OU, OAG OU Crohn disease Sirolimus, CHLOR, CYCLO, MMF 2 Scleritis OU Crohn disease, RA CHLOR, MMF, AZA, Pred 3 Scleritis OU RA AZA, CYCLOP, MTX Lost to follow-up, MMF at last visit 4 Scleritis with PUK OU Crohn disease, RA intravenous MP INF, MTX, Methylprednisolone 5 Scleritis OU Crohn disease intravenous MP, Pred INF, MMF, Sirolimus OD 20/20, OS 20/25 OD 20/20, OS 20/40 1 injection OU Quiet OU Responder, unable to reduce concurrent IMT INF OU 20/15 OU 20/15 Quiet OU OU 20/20 OU 20/20 1 injection OU Quiet OU Responder, control of inflammation with INF but developed lupus-like reaction OD 20/25, OS 20/20 OU 20/20 2 injection OD, 1 Quiet OU Partial responder, required concurrent antimetabolite and steroid INF OU 20/20 OU 20/20 1 injection OU discontinue methylprednisolone infusions 6 Sclerouveitis OU RA MTX, Pred INF, MTX, Pred OU 20/20 OU 20/20 1 injection OU Quiet OU Responder, unable to reduce concurrent IMT 7 Nodular scleritis OU None MMF, CYCLO INF OU 20/20 OU 20/20 Quiet OU 8 Scleritis OU None MMF, CYCLO, MTX INF 8 6 OU 20/40 OD 20/60, OS 20/20 2 injection OU, 1 9 Nodular scleritis OU None MTX, CYCLO None OU 20/20 OU 20/20 2 injection OU, 1 10 Scleritis OS Behçet disease Pred, Celecoxib, MMF, MTX,intravenous MP, Chlor, Napr INF, Napr 7 6 OU 20/40 OD 20/60, OS 20/20 2 injection OD, 1 discontinue all IMT (discontinued INF use because of remission) ; use of concomitant naproxen required AZA, azathioprine; CHLOR, chlorambucil; CMO, cystoid macular oedema; CYCLO, cyclophosphamide; INF, ; intravenous MP, intravenous methylprednisolone; MMF, mycophenolate mofetil; MTX, methotrexate; Napr, naproxen; OAG, open-angle glaucoma; OD, right eye; OS, left eye; OU, both eyes; PUK, peripheral ulcerative keratitis; RA, rheumatoid arthritis. 580 Br J Ophthalmol 2010;94:579e583. doi: /bjo
3 The medications in table 1 reflect all IMT regimes used prior to. Lifetime tables for control of inflammation are reported in table 2, and visual acuity in table 3. Case reports Patient 1 had a long history of Crohn disease with bilateral sclerouveitis, cystoid macular oedema and open-angle glaucoma. This patient had initially been treated with chlorambucil which had been discontinued due to leucopenia, and subsequently with mycophenolate mofetil with sirolimus, which proved inadequate for control of ocular inflammation. A marked clinical improvement occurred within days of the first infusion. Mycophenolate mofetil and sirolimus were coadministered to maintain remission of inflammation. The patient underwent bilateral glaucoma surgeries and unilateral cataract surgery while on this combination with no reactivation of scleritis postoperatively. Patient 2 with Crohn disease initially presented with right eye (OD) pain, redness and photophobia. The patient was diagnosed to have nodular scleritis and was treated with subconjunctival triamcinolone acetonide 4 mg and oral Prednisone on a gradual taper. After an initial improvement, the symptoms recurred 2 months following steroid taper. There was no clinical improvement with oral diclofenac, and the patient developed fatigue and leucopenia following treatment with mycophenolate mofetil, which was therefore discontinued. Chlorambucil along with topical prednisolone acetate eye-drops were instituted, but the patient continued to have pain in the right eye and developed new symptoms in the left eye (OS). Cholrambucil 6 mg/day was started becasue of prior failed treatments with good clinical response, though the dosage needed to be reduced to 2 mg every alternate day due to leucopenia. This dosage proved to be inadequate for control of ocular inflammation, and a decision to add was made. The patient s leucocyte count initially rose but subsequently fell on this regime, necessitating discontinuation of chlorambucil. Infliximab was withheld, as the liver function tests were noted to be abnormal. The patient was evaluated and diagnosed as having rheumatoid arthritis with possible autoimmune hepatitis. Liver biopsy was inconclusive, and was resumed. The ocular inflammation and arthritis-related symptoms are currently in control with 4-weekly infusions of. Patient 3 had a history of rheumatoid arthritis-associated bilateral diffuse anterior scleritis treated with azathioprine and chlorambucil in the past and uncontrolled while on subcutaneous methotrexate. The patient responded well to monthly infusions of with resolution of ocular and jointrelated symptoms. After 12 infusions, the patient developed a rash with antinuclear antibody (ANA) titres of 1:640 and skin biopsy-proven systemic lupus erythematosus (SLE). Infliximab was therefore discontinued. On treatment with mycophenolate Table 2 Life-table estimate of relapse occurrence Follow-up month Relapse rate Table 3 Life table estimate of visual acuity change Minus 1 line Minus 2 lines Plus 1 line Plus 2 lines Follow-up (months) OD OS OD OS OD OS OD OS OD, right eye; OS, left eye. mofetil, the patient developed nausea, fatigue and gastritis, and raised erythrocyte sedimentation rate with normal blood counts and liver function tests. We suspected a reactivation of SLE, and the patient was referred for evaluation but subsequently lost to follow-up. Patient 4 was diagnosed as having rheumatoid arthritis and Crohn disease with bilateral diffuse scleritis and unilateral peripheral ulcerative keratitis. The patient reported recurrent episodes of scleritis while on oral diclofenac and topical prednisolone acetate necessitating frequent intravenous corticosteroid infusions. In an attempt to achieve control with a steroid-sparing regime, 4-weekly infusions of were started. Suboptimal resolution of symptoms led us to add weekly subcutaneous methotrexate (20 mg) injections. After 4 months, resolution of inflammation was achieved and has been maintained since. The patient has been controlled on this regime in the absence of systemic or topical corticosteroids. Patient 5, with Crohn disease and bilateral sclerouveitis with interstitial keratitis, was inadequately controlled with oral prednisone and topical prednisolone acetate. An intravenous course of methylprednisone for 3 days was administered, with topical prednisolone acetate eye-drops, concomitant to a regime of 4-weekly while tapering off the oral prednisone. Following a mild reactivation of inflammation (trace injection OU) noted after 9 months of treatment, during a reportedly stressful period, the patient is currently well controlled on monthly infusions. Patient 6, on subcutaneous methotrexate for rheumatoid arthritis, had a history of bilateral sclerouveitis. Methotrexate had recently been increased to 25 mg/week with the addition of oral prednisone 5 mg for control of her joint symptoms. Despite this, the patient s symptoms persisted and were accompanied by an increase in ocular pain. We noted scleral inflammation and anterior uveitis OS. Topical prednisolone was started along with, in addition to prior. While the ocular pain and scleritis resolved, the patient noted repeated reactivation of anterior uveitis on this regime necessitating episodic topical predisolone acetate to keep the associated anterior uveitis in check. Patient 7 had bilateral nodular scleritis, uncontrolled initially on mycophenolate mofetil and subsequently on cyclophosphamide. The patient was started on monthly infusions. The infusions had to be temporarily withheld due to development of herpes zoster 3 months after initiating, which was treated with oral valacyclovir. Infliximab was resumed after a month and took 9 months for achieving control of inflammation with the concomitant use of diflunisal. Patient 8 developed bilateral diffuse anterior scleritis which had in the past been unresponsive to a combination of subcutaneous methotrexate with oral prednisone 5 mg. Following this, replacement of methotrexate with mycophenolate mofetil was attempted in vain. Ciclosporin A had been added and later Br J Ophthalmol 2010;94:579e583. doi: /bjo
4 withdrawn due to raised liver enzymes. Subsequent had included intravenous methyl prednisone, regional triamcinolone acetonide and oral prednisone. A month later, ocular symptoms recurred, and we instituted a regime of intravenous methotrexate 50 mg with methylprednisone 1000 mg while we obtained insurance approval for. The patient responded well to infusions administered 4-weekly with control of ocular inflammation, except for one episode of reactivation following a missed dose of. While on, the patient underwent cataract surgery with no reactivation of inflammation postoperatively, and is currently well controlled on this regime. Patient 9 had a history of bilateral nodular scleritis unresponsive initially to methotrexate and later cyclophosphamide. The patient responded well to, initially at 4- weekly intervals. Topical prednisolone acetate was tapered over the initial 3 months. After 1 year of monthly 400 mg infusions, we gradually reduced the dosage and were eventually able to wean the patient off over a period of 2.5 years. The patient is off all systemic and topical medication with no activation of inflammation. Patient 10 had a history of unilateral anterior scleritis OS, dependent on corticosteroid. The patient was HLA-B51- positive and was diagnosed as having Behçet disease, using the 1990 International criteria. The patient was initially unsuccessfully treated with oral celecoxib, mycophenolate mofetil and subsequently methotrexate. The patient achieved symptomatic relief with subcutaneous methotrexate and oral prednisone but needed to change to chlorambucil due to side effects. With this new agent, we were able to discontinue oral prednisone, but the patient developed leucopenia and unresolving pharyngitis. Two months later, the patient presented with uncontrolled ocular inflammation and joint pain, for which three infusions of 50e100 mg intravenous methotrexate combined with 1 g of intravenous methyl prednisolone were administered while obtaining insurance approval for. The patient was initially treated with 300 mg infusions every 4 weeks and was steroid-free, and without inflammation, for 4 months. Joint pain and mouth ulcers resolved after initiation of. The patient missed one infusion and developed mild eye pain and injection that resolved with combined infusion of 300 mg and 1 g of methylprednisolone. The patient did not need additional steroid after this episode and reports no side effects while on. The patient is currently well-controlled on 6-weekly infusions. DISCUSSION Infliximab, a monoclonal antibody to TNF-a, is a novel immunomodulatory agent which has proven to be effective in a number of autoimmune diseases, in particular Crohn disease, rheumatoid arthritis, psoriasis and systemic vasculitis Its use in the treatment of scleritis has been reported in a small number of patients This report describes a relatively large case series of patients with scleritis to be treated with. A favourable response to was seen in 90% of the patients, with six (60%) of them achieving remission and cessation of concomitant immunosuppression. A clinical response to occurred within weeks on average. Based on clinical response, we found that repeat monthly infusions were required to maintain remission. Eight (80%) patients continue to receive on a regularly scheduled basis. The appropriate length of treatment for these patients is unclear and may be indefinite. Life-table analysis further supports the efficacy of for controlling ocular inflammation. One patient (patient 9) with idiopathic nodular scleritis had inflammation resolution after 24 infusions and has remained relapse-free without IMT for 27 months. While biological therapies such as are generally felt not to be curative, it is possible some patients may achieve durable remission without the need for ongoing treatment. One patient (patient 4) who initially had inflammation recurrence while receiving required the addition of methotrexate with good inflammation control thereafter. In two patients (patients 1 and 6) we were unable to reduce concomitant immunomodulatory. In five patients (patients 2, 7, 8, 9 and 10), we were able to stop concomitant systemic immunomodulatory medication with successful control of inflammation. One patient (patient 6) had persistant uveitis with resolution of scleral inflammation while on, necessitating the use of prednisolone acetate topically. Two patients underwent cataract or glaucoma surgery with no exacerbation of scleritis postoperatively. In each instance, ocular surgery was timed to occur within a week of administration. Postoperative topical medications were as prescribed after routine ocular surgeries. Two patients developed streptococcal upper respiratory infection while on, necessitating cessation of until resolution with antibiotics was achieved. In contrast with the recent finding of a relatively high rate of adverse events in a prospective trial of, 12 our review included only one adverse event necessitating discontinuation. Other studies have shown low rates of adverse events similar to those in our report. Drug-induced lupus reaction is the one significant adverse event that we observed; this has been previously reported in the ophthalmic and rheumatological literature with use of. 13 All the reported cases of lupus-like syndrome have improved with discontinuation. Another patient (patient 2) developed possible autoimmune hepatitis which was not confirmed, and liver function was subsequently normal after resuming with. Autoimmune hepatitis has been noted to occur with a lupus-like reaction to. 14 Five of our patients are continuing to receive without any concomitant IMT. Typically, patients are maintained on low-dose immunosuppressive to prevent antibody production to. The patients who opted for mono were all informed of possible risks. Their desire to avoid immunosuppressive and their good inflammation control while taking led them to make this choice. All these patients have maintained an excellent response to, having received between two and 22 infusions. Mono may be offered to patients with scleritis and may be efficacious over the long term. In our experience, the optimal maintenance dosing interval for patients with scleritis is shorter than the 8 weeks that has been reported for patients with diseases such as Crohn disease, 15 and is usually 4 weeks. We have found that everymonth dosing in these patients is well tolerated and eliminates escape of inflammation between treatments. Infliximab is a highly targeted with a rapid action that avoids many of the adverse effects associated with other immunosuppressive agents. Furthermore, immunosuppressants that target T cell activation, such as ciclosporin A and mycophenolate mofetil, may act synergistically with, because they target the T cell effector response through 582 Br J Ophthalmol 2010;94:579e583. doi: /bjo
5 a different mechanism. Disadvantages of include short duration of effect and its high cost. All 10 of these patients with scleritis had inflammation control while taking and may be a subgroup of patients who respond well to and who are more likely to achieve remission. A larger randomised trial would be necessary to further test this hypothesis. This report demonstrates the efficacy of TNFa blockade with in treatment-resistant scleritis. This study, however, is limited by its retrospective nature, lack of a control group, relatively small number of patients and limited follow-up period for some patients. Further clinical studies, in particular randomised clinical trials, are needed to investigate the potential impact of anti-tnf-a in scleritis. Competing interest None. Ethics approval Ethics approval was provided by the institutional review board of the Massachusetts Eye and Ear Infirmary. Patient consent Obtained. Provenance and peer review Not commissioned; externally peer reviewed. REFERENCES 1. Benson WE. Posterior scleritis. Surv Ophthalmol 1988;32:297e Sainz de la Maza M, Jabbur NS, Foster CS. An analysis of therapeutic decision for scleritis. Ophthalmology 1993;100:1372e6. 3. Soukiasian SH, Foster CS, Raizman MB. Treatment strategies for scleritis and uveitis associated with inflammatory bowel disease. Am J Ophthalmol 1994;118:601e Kim EC, Foster CS. Immunomodulatory for the treatment of ocular inflammatory disease: evidence-based medicine recommendations for use. Int Ophthalmol Clin 2006;46:141e Onrust SV, Lamb HM. Infliximab: a review of its use in Crohn s disease and rheumatoid arthritis. BioDrugs 1998;10:397e Valesini G, Iannuccelli C, Marocchi E, et al. Biological and clinical effects of anti- TNFalpha treatment. Autoimmun Rev 2007;7:35e Watson PG, Hayreh SS. Scleritis and episcleritis. Br J Ophthalmol 1976;60: 163e Mease P. Infliximab (Remicade) in the treatment of psoriatic arthritis. Ther Clin Risk Manag 2006;2:389e Lamprecht P, Till A, Steinmann J, et al. Current state of biologicals in the management of systemic vasculitis. Ann N Y Acad Sci 2007;1110:261e Sobrin L, Kim EC, Christen W, et al. Infliximab for the treatment of refractory ocular inflammatory disease. Arch Ophthalmol 2007;125:895e Theodossiadis PG, Markomichelakis NN, Sfikakis PP. Tumor necrosis factor antagonists: preliminary evidence for an emerging approach in the treatment of ocular inflammation. Retina 2007;27:399e Suhler EB, Smith JR, Wertheim MS, et al. A prospective trial of for refractory uveitis: preliminary safety and efficacy outcomes. Arch Ophthalmol 2005;123:903e Costa MF, Said NR, Zimmermann B. Drug-induced lupus due to anti-tumor necrosis factor alpha agents. Semin Arthritis Rheum 2008;37:381e Marques M, Magro F, Cardoso H, et al. Infliximab-induced lupus-like syndrome associated with autoimmune hepatitis. Inflamm Bowel Dis 2007; 14:723e Behm BW, Bickston SJ. Tumor necrosis factor-alpha antibody for maintenance of remission in Crohn s disease. Cochrane Database Syst Rev 2008;1: CD Br J Ophthalmol: first published as /bjo on 2 December Downloaded from on 21 January 2019 by guest. Protected by copyright. Br J Ophthalmol 2010;94:579e583. doi: /bjo
Prednisolone-2 I -stearoylglycolate in scleritis
 Brit. j. Ophthal. (1970) 54, 394 Prednisolone- -stearoylglycolate in scleritis S. S. HAYREH* AND P. G. WATSON Scleritis Clinic, Moorfields Eye Hospital, City Road, London, E.C. i Scleritis is one of the
Brit. j. Ophthal. (1970) 54, 394 Prednisolone- -stearoylglycolate in scleritis S. S. HAYREH* AND P. G. WATSON Scleritis Clinic, Moorfields Eye Hospital, City Road, London, E.C. i Scleritis is one of the
Scleritis LEN V KOH OD
 Scleritis LEN V KOH OD 2014 PUCO 1 Introduction A painful, destructive, and potentially blinding disorder Highly symptomatic High association with systemic disease Immunosuppresssive agents 2014 PUCO 2
Scleritis LEN V KOH OD 2014 PUCO 1 Introduction A painful, destructive, and potentially blinding disorder Highly symptomatic High association with systemic disease Immunosuppresssive agents 2014 PUCO 2
New Drugs for Uveitis. Medical Eye Unit St Thomas Hospital
 New Drugs for Uveitis Miles Stanford Medical Eye Unit St Thomas Hospital x Epithelium x x Antigen Y Y Y Y IgG m cd4 IL-2 Y m + IL-12 Cytotoxic T B pmn Ig s PG s. LTB4 O - IL-6 TNFα IFNγγ IL-2 Th1 IL-10
New Drugs for Uveitis Miles Stanford Medical Eye Unit St Thomas Hospital x Epithelium x x Antigen Y Y Y Y IgG m cd4 IL-2 Y m + IL-12 Cytotoxic T B pmn Ig s PG s. LTB4 O - IL-6 TNFα IFNγγ IL-2 Th1 IL-10
Regional vs. Systemic Therapy. Corticosteroids. Regional vs. Systemic Therapy for Uveitis. Considerations
 Regional vs. Systemic Therapy for Uveitis Nisha Acharya,, M.D., M.S. Director, Uveitis Service F.I. Proctor Foundation University of California, San Francisco December 4, 2010 No financial disclosures
Regional vs. Systemic Therapy for Uveitis Nisha Acharya,, M.D., M.S. Director, Uveitis Service F.I. Proctor Foundation University of California, San Francisco December 4, 2010 No financial disclosures
Evolving therapies for posterior uveitis. Infliximab (Remicade) Infliximab: pharmacology. FDA-approved monoclonal antibody therapy Target
 Evolving therapies for posterior uveitis Sam Dahr, M.D. September 17, 2005 Midwest Ophthalmology Conference Infliximab (Remicade) FDA approved for Crohn s disease, rheumatoid arthritis, and psoriatic arthritis
Evolving therapies for posterior uveitis Sam Dahr, M.D. September 17, 2005 Midwest Ophthalmology Conference Infliximab (Remicade) FDA approved for Crohn s disease, rheumatoid arthritis, and psoriatic arthritis
CLINICAL SCIENCES. Antineutrophil Cytoplasmic Antibody Associated Active Scleritis
 CLINICAL SCIENCES Antineutrophil Cytoplasmic Antibody Associated Active Scleritis Lani T. Hoang, MD; Lyndell L. Lim, MBBS, FRANZCO; Brian Vaillant, MD; Dongseok Choi, PhD; James T. Rosenbaum, MD Objective:
CLINICAL SCIENCES Antineutrophil Cytoplasmic Antibody Associated Active Scleritis Lani T. Hoang, MD; Lyndell L. Lim, MBBS, FRANZCO; Brian Vaillant, MD; Dongseok Choi, PhD; James T. Rosenbaum, MD Objective:
Combined Infliximab and Rituximab in Necrotising Scleritis
 This is an Open Access article licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 License (www.karger.com/oa-license), applicable to the online version of the article
This is an Open Access article licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 License (www.karger.com/oa-license), applicable to the online version of the article
1. Background: Infliximab is administered parenterally; therefore, it is not covered under retail pharmacy benefits.
 Subject: Infliximab (Remicade ) Original Original Committee Approval: October 13, 2006 Revised Last Committee Approval: December 3, 2008 Last Review: October 19, 2007 1. Background: Infliximab is a genetically
Subject: Infliximab (Remicade ) Original Original Committee Approval: October 13, 2006 Revised Last Committee Approval: December 3, 2008 Last Review: October 19, 2007 1. Background: Infliximab is a genetically
Nausheen Khuddus, MD Melissa Elder, MD, PhD
 Nausheen Khuddus, MD Melissa Elder, MD, PhD Nausheen Khuddus, MD Pediatric Ophthalmologist and Strabismus Specialist Accent Physicians Gainesville, Florida What Is Uveitis? Uveitis is caused by inflammatory
Nausheen Khuddus, MD Melissa Elder, MD, PhD Nausheen Khuddus, MD Pediatric Ophthalmologist and Strabismus Specialist Accent Physicians Gainesville, Florida What Is Uveitis? Uveitis is caused by inflammatory
Ophthalmology Subcommittee of PTAC Meeting held 30 October (minutes for web publishing)
 Ophthalmology Subcommittee of PTAC Meeting held 30 October 2014 (minutes for web publishing) Ophthalmology Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
Ophthalmology Subcommittee of PTAC Meeting held 30 October 2014 (minutes for web publishing) Ophthalmology Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
Uveitis unplugged: systemic therapy
 Uveitis unplugged: systemic therapy Hobart 2017 Peter McCluskey Save Sight Institute Sydney Eye Hospital Sydney Medical School University of Sydney Sydney Australia No financial or proprietary interest
Uveitis unplugged: systemic therapy Hobart 2017 Peter McCluskey Save Sight Institute Sydney Eye Hospital Sydney Medical School University of Sydney Sydney Australia No financial or proprietary interest
Scottish Medicines Consortium
 Scottish Medicines Consortium infliximab 100mg powder for intravenous infusion (Remicade ) No. (364/07) Schering-Plough UK Ltd 6 April 2007 The Scottish Medicines Consortium (SMC) has completed its assessment
Scottish Medicines Consortium infliximab 100mg powder for intravenous infusion (Remicade ) No. (364/07) Schering-Plough UK Ltd 6 April 2007 The Scottish Medicines Consortium (SMC) has completed its assessment
HARVARD PILGRIM HEALTH CARE RECOMMENDED MEDICATION REQUEST GUIDELINES HUMIRA PEDIATRIC
 Generic Brand HICL GCN Exception/Other ADALIMUMAB HUMIRA 24800 HUMIRA PEDIATRIC GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Is the patient currently taking Humira? If
Generic Brand HICL GCN Exception/Other ADALIMUMAB HUMIRA 24800 HUMIRA PEDIATRIC GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Is the patient currently taking Humira? If
Sarcoidosis Case. Robert P. Baughman Interstitial Lung Disease and Sarcoidosis Clinic University of Cincinnati, USA. WASOG: educational material
 Sarcoidosis Case Robert P. Baughman Interstitial Lung Disease and Sarcoidosis Clinic University of Cincinnati, USA WASOG: educational material Sarcoidosis Case patient is a Caucasian male age 46 was diagnosed
Sarcoidosis Case Robert P. Baughman Interstitial Lung Disease and Sarcoidosis Clinic University of Cincinnati, USA WASOG: educational material Sarcoidosis Case patient is a Caucasian male age 46 was diagnosed
Coverage Criteria: Express Scripts, Inc. monograph dated 12/15/ months or as otherwise noted by indication
 BENEFIT DESCRIPTION AND LIMITATIONS OF COVERAGE ITEM: PRODUCT LINES: COVERED UNDER: DESCRIPTION: CPT/HCPCS Code: Company Supplying: Setting: Kineret (anakinra subcutaneous injection) Commercial HMO/PPO/CDHP
BENEFIT DESCRIPTION AND LIMITATIONS OF COVERAGE ITEM: PRODUCT LINES: COVERED UNDER: DESCRIPTION: CPT/HCPCS Code: Company Supplying: Setting: Kineret (anakinra subcutaneous injection) Commercial HMO/PPO/CDHP
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 3 October 2012
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 3 October 2012 REMICADE 100 mg, powder for concentrate for solution for infusion B/1 vial (CIP code: 562 070-1) Applicant:
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 3 October 2012 REMICADE 100 mg, powder for concentrate for solution for infusion B/1 vial (CIP code: 562 070-1) Applicant:
Doncaster & Bassetlaw Medicines Formulary
 Doncaster & Bassetlaw Medicines Formulary Section 1.5 Chronic Bowel Disorders (including IBD) Aminosalicylates: Mesalazine 400mg and 800mg MR Tablets (Octasa) Mesalazine 1.2g MR Tablets (Mezavant XL) Mesalazine
Doncaster & Bassetlaw Medicines Formulary Section 1.5 Chronic Bowel Disorders (including IBD) Aminosalicylates: Mesalazine 400mg and 800mg MR Tablets (Octasa) Mesalazine 1.2g MR Tablets (Mezavant XL) Mesalazine
Update on management of Anterior Uveitis
 Update on management of Anterior Uveitis Parthopratim Dutta Majumder Senior Consultant, Department of Uvea & Intraocular Inflammation Medical Research Foundation, Sankara Nethralaya ABCD of Treating a
Update on management of Anterior Uveitis Parthopratim Dutta Majumder Senior Consultant, Department of Uvea & Intraocular Inflammation Medical Research Foundation, Sankara Nethralaya ABCD of Treating a
TOCILIZUMAB FOR DIFFICULT TO TREAT IDIOPATHIC RETROPERITONEAL FIBROSIS. A PILOT TRIAL
 TOCILIZUMAB FOR DIFFICULT TO TREAT IDIOPATHIC RETROPERITONEAL FIBROSIS. A PILOT TRIAL Dr Augusto Vaglio, Dr Federica Maritati Unit of Nephrology, University Hospital of Parma, Via Gramsci 14, 43126 Parma
TOCILIZUMAB FOR DIFFICULT TO TREAT IDIOPATHIC RETROPERITONEAL FIBROSIS. A PILOT TRIAL Dr Augusto Vaglio, Dr Federica Maritati Unit of Nephrology, University Hospital of Parma, Via Gramsci 14, 43126 Parma
Uveitis literature 2014: the year in review. Russell N. Van Gelder, MD, PhD Department of Ophthalmology University of Washington Seattle, WA
 Uveitis literature 2014: the year in review Russell N. Van Gelder, MD, PhD Department of Ophthalmology University of Washington Seattle, WA Disclosures RVG serves as Associate Editor of IOVS Editorial
Uveitis literature 2014: the year in review Russell N. Van Gelder, MD, PhD Department of Ophthalmology University of Washington Seattle, WA Disclosures RVG serves as Associate Editor of IOVS Editorial
Evidence review for Surrey Prescribing Clinical Network SUMMARY
 East Surrey CCG, Guildford & Waverley CCG, North West Surrey CCG, Surrey Downs CCG, Surrey Heath CCG, Crawley CCG, Horsham & Mid-Sussex CCG Evidence review for Surrey Prescribing Clinical Network Medicine
East Surrey CCG, Guildford & Waverley CCG, North West Surrey CCG, Surrey Downs CCG, Surrey Heath CCG, Crawley CCG, Horsham & Mid-Sussex CCG Evidence review for Surrey Prescribing Clinical Network Medicine
REFRESHER: ANTERIOR UVEITIS
 REFRESHER: ANTERIOR UVEITIS 2. SAoO Kongress 28.2.2018 Messe Luzern Dr. med. Christian Böni Augenklinik Universitätsspital Zürich Christian Böni Seite 1 Anterior Uveitis: Clinical Issues Diagnostics: yes
REFRESHER: ANTERIOR UVEITIS 2. SAoO Kongress 28.2.2018 Messe Luzern Dr. med. Christian Böni Augenklinik Universitätsspital Zürich Christian Böni Seite 1 Anterior Uveitis: Clinical Issues Diagnostics: yes
Case 1 History. William Tremaine, M.D. CP
 Extraintestinal Manifestations of IBD Case Studies William Tremaine, M.D. Case 1 History 18 year-old woman with Crohn s disease Onset at age 5: colonic & perianal Sulfasalazine, prednisone, mercaptopurine
Extraintestinal Manifestations of IBD Case Studies William Tremaine, M.D. Case 1 History 18 year-old woman with Crohn s disease Onset at age 5: colonic & perianal Sulfasalazine, prednisone, mercaptopurine
Tumor necrosis factor-alpha antibody for maintenace of remission in Crohn s disease (Review)
 Tumor necrosis factor-alpha antibody for maintenace of remission in Crohn s disease (Review) Behm BW, Bickston SJ This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration
Tumor necrosis factor-alpha antibody for maintenace of remission in Crohn s disease (Review) Behm BW, Bickston SJ This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration
New Evidence reports on presentations given at EULAR Rituximab for the Treatment of Rheumatoid Arthritis and Vasculitis
 New Evidence reports on presentations given at EULAR 2011 Rituximab for the Treatment of Rheumatoid Arthritis and Vasculitis Report on EULAR 2011 presentations Anti-TNF failure and response to rituximab
New Evidence reports on presentations given at EULAR 2011 Rituximab for the Treatment of Rheumatoid Arthritis and Vasculitis Report on EULAR 2011 presentations Anti-TNF failure and response to rituximab
Additional file 2: Details of cohort studies and randomised trials
 Reference Randomised trials Ye et al. 2001 Abstract 274 R=1 WD=0 Design, numbers, treatments, duration Randomised open comparison of: (45 patients) 1.5 g for 3, 1 g for 3, then 0.5 to 0.75 g IV cyclophosphamide
Reference Randomised trials Ye et al. 2001 Abstract 274 R=1 WD=0 Design, numbers, treatments, duration Randomised open comparison of: (45 patients) 1.5 g for 3, 1 g for 3, then 0.5 to 0.75 g IV cyclophosphamide
London, 1 June 2006 Product name: REMICADE Procedure number: Remicade-H-240-II-73-AR SCIENTIFIC DISCUSSION 1/8
 London, 1 June 2006 Product name: REMICADE Procedure number: Remicade-H-240-II-73-AR SCIENTIFIC DISCUSSION 1/8 1. Introduction Infliximab is a chimeric human-murine IgG1κ monoclonal antibody, which binds
London, 1 June 2006 Product name: REMICADE Procedure number: Remicade-H-240-II-73-AR SCIENTIFIC DISCUSSION 1/8 1. Introduction Infliximab is a chimeric human-murine IgG1κ monoclonal antibody, which binds
Abatacept (Orencia) for active rheumatoid arthritis. August 2009
 Abatacept (Orencia) for active rheumatoid arthritis August 2009 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
Abatacept (Orencia) for active rheumatoid arthritis August 2009 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
UNFOLDING NATURE S ORIGAMI: MEDICAL TREATMENT OF TAKAYASU ARTERITIS AND GIANT CELL ARTERITIS
 UNFOLDING NATURE S ORIGAMI: MEDICAL TREATMENT OF TAKAYASU ARTERITIS AND GIANT CELL ARTERITIS CanVasc meeting Montreal Nov 22 2012 Patrick Liang Service de rhumatologie Centre Hospitalier Universitaire
UNFOLDING NATURE S ORIGAMI: MEDICAL TREATMENT OF TAKAYASU ARTERITIS AND GIANT CELL ARTERITIS CanVasc meeting Montreal Nov 22 2012 Patrick Liang Service de rhumatologie Centre Hospitalier Universitaire
Management of uveitis
 Management of uveitis DR. ANUPAMA KARANTH Anti-inflammatory agents -itis = inflammation Treatment : stop inflammation Use anti-inflammatory drugs Most potent of such agents : Corticosteroids Corticosteroids
Management of uveitis DR. ANUPAMA KARANTH Anti-inflammatory agents -itis = inflammation Treatment : stop inflammation Use anti-inflammatory drugs Most potent of such agents : Corticosteroids Corticosteroids
iologic Agents in the Treatment of Non-Infectious Uveitis
 What is New B iologic Agents in the Treatment of Non-Infectious Uveitis Amala George DNB The treatment of non-infectious uveitis is a challenge to clinicians. The treatment involves suppressing the deleterious
What is New B iologic Agents in the Treatment of Non-Infectious Uveitis Amala George DNB The treatment of non-infectious uveitis is a challenge to clinicians. The treatment involves suppressing the deleterious
Adalimumab and dexamethasone for treating non-infectious uveitis [ID763]
![Adalimumab and dexamethasone for treating non-infectious uveitis [ID763] Adalimumab and dexamethasone for treating non-infectious uveitis [ID763]](/thumbs/82/86218480.jpg) Adalimumab and dexamethasone for treating non-infectious uveitis [ID763] Multiple Technology Appraisal 2 nd meeting: 12 th April 2017 Committee C Slides for Committee, projector and public [NoACIC] 1 The
Adalimumab and dexamethasone for treating non-infectious uveitis [ID763] Multiple Technology Appraisal 2 nd meeting: 12 th April 2017 Committee C Slides for Committee, projector and public [NoACIC] 1 The
Choroidal Neovascularization in Sympathetic Ophthalmia
 Choroidal Neovascularization in Sympathetic Ophthalmia Lucia Sobrin, Miguel Cordero Coma, C. Stephen Foster Case Report A 49-year-old man presented after a ruptured globe repair of his left eye status
Choroidal Neovascularization in Sympathetic Ophthalmia Lucia Sobrin, Miguel Cordero Coma, C. Stephen Foster Case Report A 49-year-old man presented after a ruptured globe repair of his left eye status
The Future Is Now: Biologics for Non-Infectious Pediatric Anterior Uveitis
 Pediatr Drugs (2015) 17:283 301 DOI 10.1007/s40272-015-0128-2 REVIEW ARTICLE The Future Is Now: Biologics for Non-Infectious Pediatric Anterior Uveitis Melissa A. Lerman 1 C. Egla Rabinovich 2 Published
Pediatr Drugs (2015) 17:283 301 DOI 10.1007/s40272-015-0128-2 REVIEW ARTICLE The Future Is Now: Biologics for Non-Infectious Pediatric Anterior Uveitis Melissa A. Lerman 1 C. Egla Rabinovich 2 Published
Optical coherence tomography findings in a child with posterior scleritis
 European Journal of Ophthalmology / Vol. 18 no. 6, 2008 / pp. 1007-1010 SHORT OMMUNITIONS & SE REPORTS Optical coherence tomography findings in a child with posterior scleritis H. ERDÖL, M. KOL,. TÜRK
European Journal of Ophthalmology / Vol. 18 no. 6, 2008 / pp. 1007-1010 SHORT OMMUNITIONS & SE REPORTS Optical coherence tomography findings in a child with posterior scleritis H. ERDÖL, M. KOL,. TÜRK
Rayos Prior Authorization Program Summary
 Rayos Prior Authorization Program Summary FDA APPROVED INDICATIONS AND DOSAGE FDA-Approved Indications: 1 Agent Indication Dosage Rayos (prednisone delayedrelease tablet) as an anti-inflammatory or immunosuppressive
Rayos Prior Authorization Program Summary FDA APPROVED INDICATIONS AND DOSAGE FDA-Approved Indications: 1 Agent Indication Dosage Rayos (prednisone delayedrelease tablet) as an anti-inflammatory or immunosuppressive
Moncef Khairallah, MD
 Moncef Khairallah, MD Department of Ophthalmology, Fattouma Bourguiba University Hospital Faculty of Medicine, University of Monastir Monastir, Tunisia INTRODUCTION IU: anatomic form of uveitis involving
Moncef Khairallah, MD Department of Ophthalmology, Fattouma Bourguiba University Hospital Faculty of Medicine, University of Monastir Monastir, Tunisia INTRODUCTION IU: anatomic form of uveitis involving
Sarcoidosis: is there a role for anti-tnf-α?
 Sarcoidosis: is there a role for anti-tnf-α? Abstract In severe cases of sarcoidosis treatment can be very difficult. The common treatment strategies might be failing. Tumour necrosis factor (TNF) α therapy
Sarcoidosis: is there a role for anti-tnf-α? Abstract In severe cases of sarcoidosis treatment can be very difficult. The common treatment strategies might be failing. Tumour necrosis factor (TNF) α therapy
Efficacy and Safety of Rituximab in the Treatment of Rheumatoid Arthritis and ANCA-associated Vasculitis
 New Evidence reports on presentations given at ACR/ARHP 2010 Efficacy and Safety of Rituximab in the Treatment of Rheumatoid Arthritis and ANCA-associated Vasculitis Report on ACR/ARHP 2010 presentations
New Evidence reports on presentations given at ACR/ARHP 2010 Efficacy and Safety of Rituximab in the Treatment of Rheumatoid Arthritis and ANCA-associated Vasculitis Report on ACR/ARHP 2010 presentations
H.P. Acthar Gel (repository corticotropin) Prior Authorization Criteria Program Summary
 OBJECTIVE The intent of the H.P. Acthar Gel (repository corticotropin) Prior Authorization (PA) Criteria is to appropriately select patients for therapy according to product labeling and/or clinical studies
OBJECTIVE The intent of the H.P. Acthar Gel (repository corticotropin) Prior Authorization (PA) Criteria is to appropriately select patients for therapy according to product labeling and/or clinical studies
C. Assess clinical response after the first three months of treatment.
 Government Health Plan (GHP) of Puerto Rico Authorization Criteria Tumor Necrosis Factor Alpha (TNFα) Adalimumab (Humira ) Managed by MCO Section I. Prior Authorization Criteria A. Physician must submit
Government Health Plan (GHP) of Puerto Rico Authorization Criteria Tumor Necrosis Factor Alpha (TNFα) Adalimumab (Humira ) Managed by MCO Section I. Prior Authorization Criteria A. Physician must submit
Methotrexate for uveitis associated with juvenile idiopathic arthritis: Value and requirement for additional anti-inflammatory medication
 European Journal of Ophthalmology / Vol. 17 no. 5, 2007 / pp. 743-748 Methotrexate for uveitis associated with juvenile idiopathic arthritis: Value and requirement for additional anti-inflammatory medication
European Journal of Ophthalmology / Vol. 17 no. 5, 2007 / pp. 743-748 Methotrexate for uveitis associated with juvenile idiopathic arthritis: Value and requirement for additional anti-inflammatory medication
APPLICATION FOR SPECIAL AUTHORITY. Subsidy for Infliximab
 APPLICATION FOR SPECIAL AUTHORITY Fm SA1778 Subsidy f Infliximab Application Categy Page Graft vs host disease - Initial application... 2 Pulmonary sarcoidosis - Initial application... 2 Previous use -
APPLICATION FOR SPECIAL AUTHORITY Fm SA1778 Subsidy f Infliximab Application Categy Page Graft vs host disease - Initial application... 2 Pulmonary sarcoidosis - Initial application... 2 Previous use -
Definitions. Clinical remission: Resolution of symptoms (stool frequency 3/day, no bleeding and no urgency)
 CROHN S DISEASE Definitions Clinical remission: Resolution of symptoms (stool frequency 3/day, no bleeding and no urgency) Recurrence: The reappearance of lesions after surgical resection Endoscopic remission:
CROHN S DISEASE Definitions Clinical remission: Resolution of symptoms (stool frequency 3/day, no bleeding and no urgency) Recurrence: The reappearance of lesions after surgical resection Endoscopic remission:
Uveitis. Pt Info Brochure. Q: What is Uvea?
 Pt Info Brochure Uveitis Q: What is Uvea? A: Uvea is the middle layer of the eye. It is the most vascular structure of the eye. It provides nutrition to the other parts of the eye. The uvea is made up
Pt Info Brochure Uveitis Q: What is Uvea? A: Uvea is the middle layer of the eye. It is the most vascular structure of the eye. It provides nutrition to the other parts of the eye. The uvea is made up
SCIENTIFIC DISCUSSION
 European Medicines Agency London, 20 September 2007 Product name: Remicade Procedure number: EMEA/H/C/240/II/95 SCIENTIFIC DISCUSSION 7 Westferry Circus, Canary Wharf, London, E14 4HB, UK Tel. (44-20)
European Medicines Agency London, 20 September 2007 Product name: Remicade Procedure number: EMEA/H/C/240/II/95 SCIENTIFIC DISCUSSION 7 Westferry Circus, Canary Wharf, London, E14 4HB, UK Tel. (44-20)
Infliximab: A Treatment Option for Ulcerative Pyoderma Gangrenosum
 To Print: Click your browser's PRINT button. NOTE: To view the article with Web enhancements, go to: http://www.medscape.com/viewarticle/449955 Infliximab: A Treatment Option for Ulcerative Pyoderma Gangrenosum
To Print: Click your browser's PRINT button. NOTE: To view the article with Web enhancements, go to: http://www.medscape.com/viewarticle/449955 Infliximab: A Treatment Option for Ulcerative Pyoderma Gangrenosum
DRUG THERAPY
 www.pediatric-rheumathology.printo.it DRUG THERAPY NSAIDS NON-STEROIDAL ANTI-INFLAMMATORY DRUGS They are symptomatic anti-inflammatory, anti febrile (fever) and analgesic (pain reducing) medications. Symptomatic
www.pediatric-rheumathology.printo.it DRUG THERAPY NSAIDS NON-STEROIDAL ANTI-INFLAMMATORY DRUGS They are symptomatic anti-inflammatory, anti febrile (fever) and analgesic (pain reducing) medications. Symptomatic
INFLIXIMAB Remicade (infliximab), Inflectra (infliximab-dyyb), Renflexis (infliximab-abda)
 RATIONALE FOR INCLUSION IN PA PROGRAM Background Remicade, Renflexis and Inflectra are tumor necrosis factor (TNFα) blockers. Tumor necrosis factor is an endogenous protein that regulates a number of physiologic
RATIONALE FOR INCLUSION IN PA PROGRAM Background Remicade, Renflexis and Inflectra are tumor necrosis factor (TNFα) blockers. Tumor necrosis factor is an endogenous protein that regulates a number of physiologic
Medical Management of Rheumatoid Arthritis (RA)
 Medical Management of Rheumatoid Arthritis (RA) Dr Lee-Suan Teh Rheumatologist Royal Blackburn Hospital Educational objectives ABC Appreciate the epidemiology of RA Be able to diagnosis of RA Competent
Medical Management of Rheumatoid Arthritis (RA) Dr Lee-Suan Teh Rheumatologist Royal Blackburn Hospital Educational objectives ABC Appreciate the epidemiology of RA Be able to diagnosis of RA Competent
Mucosal Healing in Crohn s Disease. Geert D Haens MD, PhD University Hospital Gasthuisberg University of Leuven Leuven, Belgium
 Mucosal Healing in Crohn s Disease Geert D Haens MD, PhD University Hospital Gasthuisberg University of Leuven Leuven, Belgium Mucosal Lesions in CD: General Features CD can affect the entire GI tract
Mucosal Healing in Crohn s Disease Geert D Haens MD, PhD University Hospital Gasthuisberg University of Leuven Leuven, Belgium Mucosal Lesions in CD: General Features CD can affect the entire GI tract
What prescribers need to know
 HUMIRA Citrate-free presentations in an Electronic Medical Record (EMR) What prescribers need to know 2 / This is your guide to identifying HUMIRA Citrate-free presentations in your Electronic Medical
HUMIRA Citrate-free presentations in an Electronic Medical Record (EMR) What prescribers need to know 2 / This is your guide to identifying HUMIRA Citrate-free presentations in your Electronic Medical
Horizon Scanning Technology Summary. Adalimumab (Humira) for juvenile idiopathic arthritis. National Horizon Scanning Centre.
 Horizon Scanning Technology Summary National Horizon Scanning Centre Adalimumab (Humira) for juvenile idiopathic arthritis June 2007 This technology summary is based on information available at the time
Horizon Scanning Technology Summary National Horizon Scanning Centre Adalimumab (Humira) for juvenile idiopathic arthritis June 2007 This technology summary is based on information available at the time
Bilateral acute retinal necrosis in a patient with multiple sclerosis on natalizumab
 Bilateral acute retinal necrosis in a patient with multiple sclerosis on natalizumab Arjun B. Sood, Emory University Gokul Kumar, Emory University Joshua Robinson, Emory University Journal Title: Journal
Bilateral acute retinal necrosis in a patient with multiple sclerosis on natalizumab Arjun B. Sood, Emory University Gokul Kumar, Emory University Joshua Robinson, Emory University Journal Title: Journal
A role for methotrexate in the management of non-infectious orbital inflammatory disease
 1220 Casey Eye Institute, Oregon Health Sciences University, Portland, Oregon, USA J R Smith J T Rosenbaum Correspondence to: Dr Justine R Smith, Casey Eye Institute, Oregon Health Sciences University,
1220 Casey Eye Institute, Oregon Health Sciences University, Portland, Oregon, USA J R Smith J T Rosenbaum Correspondence to: Dr Justine R Smith, Casey Eye Institute, Oregon Health Sciences University,
Horizon Scanning Technology Summary. Abatacept (Orencia) for juvenile idiopathic arthritis. National Horizon Scanning Centre.
 Horizon Scanning Technology Summary National Horizon Scanning Centre Abatacept (Orencia) for juvenile idiopathic arthritis June 2007 This technology summary is based on information available at the time
Horizon Scanning Technology Summary National Horizon Scanning Centre Abatacept (Orencia) for juvenile idiopathic arthritis June 2007 This technology summary is based on information available at the time
infliximab, 100mg, powder for concentrate for solution for infusion (Inflectra ) SMC No. (1007/14) Hospira UK Ltd.
 infliximab, 100mg, powder for concentrate for solution for infusion (Inflectra ) SMC No. (1007/14) Hospira UK Ltd. 07 November 2014 (Issued 06 March 2015) The Scottish Medicines Consortium (SMC) has completed
infliximab, 100mg, powder for concentrate for solution for infusion (Inflectra ) SMC No. (1007/14) Hospira UK Ltd. 07 November 2014 (Issued 06 March 2015) The Scottish Medicines Consortium (SMC) has completed
Recent advances in management of Pulmonary Vasculitis. Dr Nita MB
 Recent advances in management of Pulmonary Vasculitis Dr Nita MB 23-01-2015 Overview of the seminar Recent classification of Vasculitis What is new in present classification? Trials on remission induction
Recent advances in management of Pulmonary Vasculitis Dr Nita MB 23-01-2015 Overview of the seminar Recent classification of Vasculitis What is new in present classification? Trials on remission induction
Moderately to severely active ulcerative colitis
 Adalimumab in the Treatment of Moderate-to-Severe Ulcerative Colitis: ULTRA 2 Trial Results Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients
Adalimumab in the Treatment of Moderate-to-Severe Ulcerative Colitis: ULTRA 2 Trial Results Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients
13. Behçet s - In Children
 Registered Charity No: 326679 Caring for those with a rare, complex and lifelong disease www.behcets.org.uk 13. Behçet s - In Children How are children affected by Behçet s disease? Behçet s disease (also
Registered Charity No: 326679 Caring for those with a rare, complex and lifelong disease www.behcets.org.uk 13. Behçet s - In Children How are children affected by Behçet s disease? Behçet s disease (also
Serologic Markers CONVENTIONAL ANTIBODIES ANTIBODIES UNCONVENTIONAL. AIH Type I
 Autoimmune Hepatitis By Thomas Frazier Objective What we need to know about AIH Diagnosis Treatment Difficulties in both Liver transplantation concerns AASLD Guidelines: Hepatology. 2010 Jun;51(6):2193-213.
Autoimmune Hepatitis By Thomas Frazier Objective What we need to know about AIH Diagnosis Treatment Difficulties in both Liver transplantation concerns AASLD Guidelines: Hepatology. 2010 Jun;51(6):2193-213.
Abbreviated Drug Evaluation: Fluocinolone acetonide intravitreal implant (Retisert )
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Understanding Myositis Medications
 Understanding Myositis Medications 2015 TMA Annual Patient Conference Orlando, Florida Chester V. Oddis, MD University of Pittsburgh Director, Myositis Center Disclosures Mallinckrodt: Research Grant Genentech:
Understanding Myositis Medications 2015 TMA Annual Patient Conference Orlando, Florida Chester V. Oddis, MD University of Pittsburgh Director, Myositis Center Disclosures Mallinckrodt: Research Grant Genentech:
Intravitreal Triamcinolone Acetonide for Macular Edema in HLA-B27 Negative Ankylosing Spondylitis
 105 This is an Open Access article licensed under the terms of the Creative Commons Attribution- NonCommercial-NoDerivs 3.0 License (www.karger.com/oa-license), applicable to the online version of the
105 This is an Open Access article licensed under the terms of the Creative Commons Attribution- NonCommercial-NoDerivs 3.0 License (www.karger.com/oa-license), applicable to the online version of the
Pharmacy Management Drug Policy
 SUBJECT: : Nucala (mepolizumab), Cinqair (reslizumab), & Fasenra (benralizumab) POLICY NUMBER: Pharmacy-62 EFFECTIVE DATE: 12/15 LAST REVIEW DATE: 3/5/2018 If the member s subscriber contract excludes
SUBJECT: : Nucala (mepolizumab), Cinqair (reslizumab), & Fasenra (benralizumab) POLICY NUMBER: Pharmacy-62 EFFECTIVE DATE: 12/15 LAST REVIEW DATE: 3/5/2018 If the member s subscriber contract excludes
Drugs and Applicable Coding: J-code: Enbrel-J1438; Humira-J0135; Remicade-J1745; Inflectra-Q5102; Cimzia-J0718; Simponi-J1602 Renflexis - pending
 Policy Subject: Anti-TNF Agents Policy Number: SHS PBD16 Category: Rheumatology & Autoimmune Policy Type: Medical Pharmacy Department: Pharmacy Product (check all that apply): Group HMO/POS Individual
Policy Subject: Anti-TNF Agents Policy Number: SHS PBD16 Category: Rheumatology & Autoimmune Policy Type: Medical Pharmacy Department: Pharmacy Product (check all that apply): Group HMO/POS Individual
Arthritis & Rheumatology Clinics of Kansas PATIENT EDUCATION SYSTEMIC LUPUS ERYTHEMATOSUS
 Arthritis & Rheumatology Clinics of Kansas PATIENT EDUCATION SYSTEMIC LUPUS ERYTHEMATOSUS Introduction: There is perhaps no rheumatic disease that evokes so much fear and confusion among both patients
Arthritis & Rheumatology Clinics of Kansas PATIENT EDUCATION SYSTEMIC LUPUS ERYTHEMATOSUS Introduction: There is perhaps no rheumatic disease that evokes so much fear and confusion among both patients
ISPUB.COM. An Atypical Presentation of Posterior Scleritis. A Ramanathan, A Gaur CASE REPORT
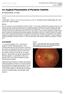 ISPUB.COM The Internet Journal of Ophthalmology and Visual Science Volume 8 Number 2 A Ramanathan, A Gaur Citation A Ramanathan, A Gaur.. The Internet Journal of Ophthalmology and Visual Science. 2009
ISPUB.COM The Internet Journal of Ophthalmology and Visual Science Volume 8 Number 2 A Ramanathan, A Gaur Citation A Ramanathan, A Gaur.. The Internet Journal of Ophthalmology and Visual Science. 2009
4. Behçet s - Treatment
 Registered Charity No: 326679 Caring for those with a rare, complex and lifelong disease www.behcets.org.uk 4. Behçet s - Treatment Introduction Because Behçet s Syndrome/Disease is a multisystem disorder,
Registered Charity No: 326679 Caring for those with a rare, complex and lifelong disease www.behcets.org.uk 4. Behçet s - Treatment Introduction Because Behçet s Syndrome/Disease is a multisystem disorder,
Course instructors (2 hour lecture) 1. Barbara Caffery OD, PhD, FAAO Diplomate, Cornea, Contact Lenses, and Refractive Technologies
 Course instructors (2 hour lecture) 1. Barbara Caffery OD, PhD, FAAO Diplomate, Cornea, Contact Lenses, and Refractive Technologies 2. Danielle Robertson, OD, PhD, FAAO, FBCLA Diplomate, Cornea, Contact
Course instructors (2 hour lecture) 1. Barbara Caffery OD, PhD, FAAO Diplomate, Cornea, Contact Lenses, and Refractive Technologies 2. Danielle Robertson, OD, PhD, FAAO, FBCLA Diplomate, Cornea, Contact
Uveitis / Iritis. Introduction. Other formats
 Other formats Introduction Uveitis / Iritis If you need this information in another format such as audio tape or computer disk, Braille, large print, high contrast, British Sign Language or translated
Other formats Introduction Uveitis / Iritis If you need this information in another format such as audio tape or computer disk, Braille, large print, high contrast, British Sign Language or translated
HLA-B27-related anterior Uveitis
 HLA-B27-related anterior Uveitis Nicholas Jones Manchester Uveitis Clinic The Royal Eye Hospital Manchester Anterior means anterior only IUSG classification: Anterior uveitis = Iris & pars plicata AU
HLA-B27-related anterior Uveitis Nicholas Jones Manchester Uveitis Clinic The Royal Eye Hospital Manchester Anterior means anterior only IUSG classification: Anterior uveitis = Iris & pars plicata AU
Chronic Granulomatous Disease Managing GI Issues
 Chronic Granulomatous Disease Managing GI Issues N I C H O L A S H A R T O G, M D D i r e c t o r o f P e d i a t r i c / A d u l t P r i m a r y I m m u n o d e f i c i e n c y C l i n i c A s s i s t
Chronic Granulomatous Disease Managing GI Issues N I C H O L A S H A R T O G, M D D i r e c t o r o f P e d i a t r i c / A d u l t P r i m a r y I m m u n o d e f i c i e n c y C l i n i c A s s i s t
Case Report Pulmonary Sarcoidosis following Etanercept Treatment
 Case Reports in Rheumatology Volume 2012, Article ID 724013, 4 pages doi:10.1155/2012/724013 Case Report Pulmonary Sarcoidosis following Etanercept Treatment Kuljeet Bhamra and Richard Stevens Department
Case Reports in Rheumatology Volume 2012, Article ID 724013, 4 pages doi:10.1155/2012/724013 Case Report Pulmonary Sarcoidosis following Etanercept Treatment Kuljeet Bhamra and Richard Stevens Department
Announcing HUMIRA. Psoriasis Starter Package
 Announcing HUMIRA (adalimumab) Psoriasis Starter Package HUMIRA is indicated for the treatment of adult patients with moderate to severe chronic plaque psoriasis who are candidates for systemic therapy
Announcing HUMIRA (adalimumab) Psoriasis Starter Package HUMIRA is indicated for the treatment of adult patients with moderate to severe chronic plaque psoriasis who are candidates for systemic therapy
Ustekinumab (Stelara) for psoriatic arthritis second line after disease modifying anti rheumatic drugs (DMARDs)
 Ustekinumab (Stelara) for psoriatic arthritis second line after disease modifying anti rheumatic drugs (DMARDs) January 2010 This technology summary is based on information available at the time of research
Ustekinumab (Stelara) for psoriatic arthritis second line after disease modifying anti rheumatic drugs (DMARDs) January 2010 This technology summary is based on information available at the time of research
Committee Approval Date: May 9, 2014 Next Review Date: May 2015
 Medication Policy Manual Policy No: dru248 Topic: Benlysta, belimumab Date of Origin: May 13, 2011 Committee Approval Date: May 9, 2014 Next Review Date: May 2015 Effective Date: June 1, 2014 IMPORTANT
Medication Policy Manual Policy No: dru248 Topic: Benlysta, belimumab Date of Origin: May 13, 2011 Committee Approval Date: May 9, 2014 Next Review Date: May 2015 Effective Date: June 1, 2014 IMPORTANT
EudraCT number: Page 8 of 42
 EudraCT number: 2012-001102-14 Page 8 of 42 5 Trial Synopsis Title Sponsor name North American Sponsor Disease Under Investigation An international, open label, randomised, controlled trial comparing rituximab
EudraCT number: 2012-001102-14 Page 8 of 42 5 Trial Synopsis Title Sponsor name North American Sponsor Disease Under Investigation An international, open label, randomised, controlled trial comparing rituximab
Indications for use of Infliximab
 Indications for use of Infliximab Moscow, June 10 th 2006 Prof. Dr. Dr. Gerhard Rogler Klinik und Poliklinik für Innere Medizin I Universität Regensburg Case report 1989: Diagnosis of Crohn s disease of
Indications for use of Infliximab Moscow, June 10 th 2006 Prof. Dr. Dr. Gerhard Rogler Klinik und Poliklinik für Innere Medizin I Universität Regensburg Case report 1989: Diagnosis of Crohn s disease of
Technology appraisal guidance Published: 26 August 2015 nice.org.uk/guidance/ta352
 Vedolizumab for treating moderately to severely erely active Crohn's disease after prior therapy Technology appraisal guidance Published: 26 August 2015 nice.org.uk/guidance/ta352 NICE 2017. All rights
Vedolizumab for treating moderately to severely erely active Crohn's disease after prior therapy Technology appraisal guidance Published: 26 August 2015 nice.org.uk/guidance/ta352 NICE 2017. All rights
Masquerade Scleritis CASE REPORT INTRODUCTION
 Ocular Immunology and Inflammation, 13:479 482, 2005 Copyright c Taylor & Francis Inc. ISSN: 0927-3948 print; 1744-5078 online DOI: 10.1080/09273940591004133 CASE REPORT Masquerade Scleritis Chrysanthi
Ocular Immunology and Inflammation, 13:479 482, 2005 Copyright c Taylor & Francis Inc. ISSN: 0927-3948 print; 1744-5078 online DOI: 10.1080/09273940591004133 CASE REPORT Masquerade Scleritis Chrysanthi
Tumour necrosis factor a inhibitors in the treatment of childhood uveitis
 Rheumatology 2006;45:982 989 Advance Access publication 3 February 2006 Tumour necrosis factor a inhibitors in the treatment of childhood uveitis R. K. Saurenmann, A. V. Levin 1, J. B. Rose, S. Parker,
Rheumatology 2006;45:982 989 Advance Access publication 3 February 2006 Tumour necrosis factor a inhibitors in the treatment of childhood uveitis R. K. Saurenmann, A. V. Levin 1, J. B. Rose, S. Parker,
Clinical Commissioning Policy: Rituximab for the treatment of idiopathic membranous nephropathy in adults
 Clinical Commissioning Policy: Rituximab for the treatment of idiopathic membranous nephropathy in adults Reference: NHS England: 16047/P NHS England INFORMATION READER BOX Directorate Medical Operations
Clinical Commissioning Policy: Rituximab for the treatment of idiopathic membranous nephropathy in adults Reference: NHS England: 16047/P NHS England INFORMATION READER BOX Directorate Medical Operations
Surgery in patients with uveitis. Lyndell Lim and Anthony Hall
 Surgery in patients with uveitis Lyndell Lim and Anthony Hall Disclosures Off label treatments Paid advisory board Bayer Paid research support Allergan (makers of Ozurdex) Paid research support B and L
Surgery in patients with uveitis Lyndell Lim and Anthony Hall Disclosures Off label treatments Paid advisory board Bayer Paid research support Allergan (makers of Ozurdex) Paid research support B and L
Pharmacy Prior Authorization
 Pharmacy Prior Authorization AETA BETTER HEALTH PESLVAIA & AETA BETTER HEALTH KIDS Humira (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information,
Pharmacy Prior Authorization AETA BETTER HEALTH PESLVAIA & AETA BETTER HEALTH KIDS Humira (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information,
A case of scleritis associated rheumatoid arthritis accompanying an intraocular elevated lesion
 Kobayashi et al. BMC Ophthalmology (2018) 18:129 https://doi.org/10.1186/s12886-018-0797-z CASE REPORT Open Access A case of scleritis associated rheumatoid arthritis accompanying an intraocular elevated
Kobayashi et al. BMC Ophthalmology (2018) 18:129 https://doi.org/10.1186/s12886-018-0797-z CASE REPORT Open Access A case of scleritis associated rheumatoid arthritis accompanying an intraocular elevated
Neuropsychiatric Systemic Lupus Erythematosus (NPSLE) Case presentations and topic discussion The Rheumatology Unit UMMC experience
 Neuropsychiatric Systemic Lupus Erythematosus (NPSLE) Case presentations and topic discussion The Rheumatology Unit UMMC experience References Sanna G, Bertolaccini ML. Neuropsychiatric manifestations
Neuropsychiatric Systemic Lupus Erythematosus (NPSLE) Case presentations and topic discussion The Rheumatology Unit UMMC experience References Sanna G, Bertolaccini ML. Neuropsychiatric manifestations
Tumor Necrosis Factor-alpha Antagonists in the Treatment of Secondary Amyloidosis. Gulen Hatemi Istanbul University
 Tumor Necrosis Factor-alpha Antagonists in the Treatment of Secondary Amyloidosis Gulen Hatemi Istanbul University TNF-alpha blockage in AA amyloidosis TNF induces SAA production in hepatocytes during
Tumor Necrosis Factor-alpha Antagonists in the Treatment of Secondary Amyloidosis Gulen Hatemi Istanbul University TNF-alpha blockage in AA amyloidosis TNF induces SAA production in hepatocytes during
Report on the Deliberation Results
 Report on the Deliberation Results September 14, 216 Pharmaceutical Evaluation Division, Pharmaceutical Safety and Environmental Health Bureau Ministry of Health, Labour and Welfare Brand Name (a) Humira
Report on the Deliberation Results September 14, 216 Pharmaceutical Evaluation Division, Pharmaceutical Safety and Environmental Health Bureau Ministry of Health, Labour and Welfare Brand Name (a) Humira
Corporate Medical Policy
 Corporate Medical Policy Measurement of Serum Antibodies to Infliximab, Adalimumab and File Name: Origination: Last CAP Review: Next CAP Review: Last Review: measurement_of_serum_antibodies_to_infliximab_and_adalimumab
Corporate Medical Policy Measurement of Serum Antibodies to Infliximab, Adalimumab and File Name: Origination: Last CAP Review: Next CAP Review: Last Review: measurement_of_serum_antibodies_to_infliximab_and_adalimumab
Juvenile Idiopathic Arthritis with Associated Bilateral Anterior Uveitis in a Four Year- Old Girl
 Juvenile Idiopathic Arthritis with Associated Bilateral Anterior Uveitis in a Four Year- Old Girl Pavlina S. Kemp, MD, Susannah Q. Longmuir, MD August 14, 2012 Chief complaint: Central posterior synechiae
Juvenile Idiopathic Arthritis with Associated Bilateral Anterior Uveitis in a Four Year- Old Girl Pavlina S. Kemp, MD, Susannah Q. Longmuir, MD August 14, 2012 Chief complaint: Central posterior synechiae
Remicade. Remicade (infliximab), Inflectra (infliximab-dyyb) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.02 Subsection: Gastrointestinal nts Original Policy Date: May 20, 2011 Subject: Remicade Page: 1 of
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.02 Subsection: Gastrointestinal nts Original Policy Date: May 20, 2011 Subject: Remicade Page: 1 of
To help protect your privacy, PowerPoint prevented this external picture from being automatically downloaded. To download and display this picture,
 To help protect your privacy, PowerPoint prevented this external picture from being automatically downloaded. To download and display this picture, click Options in the Message Bar, and then click Enable
To help protect your privacy, PowerPoint prevented this external picture from being automatically downloaded. To download and display this picture, click Options in the Message Bar, and then click Enable
Use of mycophenolate mofetil in steroid-dependent and -resistant nephrotic syndrome
 Pediatr Nephrol (2003) 18:833 837 DOI 10.1007/s00467-003-1175-4 BRIEF REPORT Gina-Marie Barletta William E. Smoyer Timothy E. Bunchman Joseph T. Flynn David B. Kershaw Use of mycophenolate mofetil in steroid-dependent
Pediatr Nephrol (2003) 18:833 837 DOI 10.1007/s00467-003-1175-4 BRIEF REPORT Gina-Marie Barletta William E. Smoyer Timothy E. Bunchman Joseph T. Flynn David B. Kershaw Use of mycophenolate mofetil in steroid-dependent
Certolizumab pegol (Cimzia) for psoriatic arthritis second line
 Certolizumab pegol (Cimzia) for psoriatic arthritis second line This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
Certolizumab pegol (Cimzia) for psoriatic arthritis second line This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
SWL Drug Pathway Ulcerative Colitis Version 3 (Oct 2018) (based on NICE ulcerative colitis commissioning algorithm - with local adaptation)
 Adult with active ulcerative colitis Does the adult have moderately to severely active ulcerative colitis managed in outpatients with no need for hospitalisation/surgery? Moderately to severely active
Adult with active ulcerative colitis Does the adult have moderately to severely active ulcerative colitis managed in outpatients with no need for hospitalisation/surgery? Moderately to severely active
10/25/2018. Autoimmunity and how to treat it. Disclosure. Why do we get autoimmunity? James Verbsky MD/PhD Pediatric Rheumatology/Immunology
 Autoimmunity and how to treat it James Verbsky MD/PhD Pediatric Rheumatology/Immunology Disclosure None I will mention drug names and some brand names but I have no financial interest or any other ties
Autoimmunity and how to treat it James Verbsky MD/PhD Pediatric Rheumatology/Immunology Disclosure None I will mention drug names and some brand names but I have no financial interest or any other ties
Scottish Medicines Consortium
 Scottish Medicines Consortium ustekinumab, 45mg solution for injection (Stelara ) No. (572/09) Janssen-Cilag Ltd 15 January 2010 The Scottish Medicines Consortium (SMC) has completed its assessment of
Scottish Medicines Consortium ustekinumab, 45mg solution for injection (Stelara ) No. (572/09) Janssen-Cilag Ltd 15 January 2010 The Scottish Medicines Consortium (SMC) has completed its assessment of
Emerging therapies for the treatment of uveitis: clinical trial observations
 Review: Clinical Trial Outcomes Emerging therapies for the treatment of uveitis: clinical trial observations Clin. Invest. (2013) 3(10), 951 966 Uveitis, a leading cause of blindness in the USA and western
Review: Clinical Trial Outcomes Emerging therapies for the treatment of uveitis: clinical trial observations Clin. Invest. (2013) 3(10), 951 966 Uveitis, a leading cause of blindness in the USA and western
