Tumour necrosis factor a inhibitors in the treatment of childhood uveitis
|
|
|
- Beatrice Norton
- 5 years ago
- Views:
Transcription
1 Rheumatology 2006;45: Advance Access publication 3 February 2006 Tumour necrosis factor a inhibitors in the treatment of childhood uveitis R. K. Saurenmann, A. V. Levin 1, J. B. Rose, S. Parker, T. Rabinovitch 3, P. N. Tyrrell, B. M. Feldman, R. M. Laxer 2, R. Schneider and E. D. Silverman doi: /rheumatology/kel030 Objective. To describe the efficacy of anti-tnf- agents in the treatment of childhood uveitis. Methods. We performed a retrospective chart review of all children with uveitis treated with TNF-a blockers at The Hospital for Sick Children, Toronto. Results. Twenty-one children with uveitis were treated with the anti-tnf-a agents etanercept (11 patients) and infliximab (13 patients), resulting in 24 treatment courses. All patients had persistently active uveitis despite treatment with at least one standard immunosuppressive drug before the start of anti-tnf-a therapy. Six of 21 patients (29%) had idiopathic uveitis. In the other 15 patients, the underlying disease was juvenile idiopathic arthritis in 12 (57%), Behc et disease in two (9%) and sarcoidosis in one (5%). Response to etanercept treatment was good in 27%, moderate in 27% and poor in 45% of patients. Response to infliximab treatment was good in 38%, moderate in 54% and poor in 8% of patients. The difference in the percentage of patients with a moderate or good response was statistically significant (P^0.0481). We also observed a lower rate of complications, such as new-onset or worsening or cataract in the infliximab-treated group. Conclusion. Anti-TNF-a treatment was beneficial in a high percentage of patients with childhood uveitis refractory to standard immunosuppressive treatment. Infliximab resulted in better clinical responses with less ocular complications than etanercept. KEY WORDS: Biological agents, Eye diseases, Juvenile idiopathic arthritis, Outcome assessment. Non-infectious uveitis of childhood is rare, with an annual incidence in Western countries of 4 7/ children/yr [1 3]. Unlike what is seen in adults, juvenile idiopathic arthritis (JIA) is the most common underlying disease in children with uveitis [4 7]. The prognosis of paediatric uveitis is poor as a high percentage will eventually develop complications such as cataracts and. It is estimated that up to 45% of patients will end up with visual impairment [1, 5]. Treatment of uveitis relies mainly on local and systemic corticosteroids to control inflammation, dilating agents to prevent synechia formation, and treatment of, cataract and band keratopathy [8, 9]. To reduce corticosteroid use and prevent side-effects of this treatment, immunosuppressive agents have been used with variable success. Published experience with numerous immunosuppressive drugs used to control uveitis both in adults and in children consists largely of case reports and case series, while prospective studies and randomized controlled trials are rare [10 17]. More recently, biological agents, including tumour necrosis factor- (TNF-)-blocking agents, have been used to treat uveitis including the uveitis associated with JIA [18, 19]. However, there have also been reports of worsening or new-onset uveitis during treatment with anti-tnf- agents [20 22]. The aim of our study was to describe the efficacy of two TNF- inhibitors, etanercept and infliximab, in the treatment of children with uveitis in a retrospective setting. Methods The charts of all 102 patients who were started on an anti-tnf- agent (etanercept or infliximab) in the Division of Rheumatology at The Hospital for Sick Children in Toronto, Canada, between 1 January 1997 and 31 December 2003 were reviewed. Twenty-one patients with active uveitis at onset of anti-tnf- treatment were identified. All patients had persistently active uveitis despite treatment with at least one standard immunosuppressive drug before the start of anti-tnf- therapy. The following data were collected: age at initiation of therapy, date of diagnosis of underlying disease (if applicable), date of diagnosis of uveitis, autoantibody status, start and end date of all medications used, and date of diagnosis of uveitis complications (synechiae, cataract, band keratopathy, macula oedema, legal blindness), start date and end date of anti-tnf- treatment and specific anti-tnf- agent used. At each visit while receiving anti-tnf- treatment, data on the patients height, weight and medication doses were collected. Ophthalmological examination (visual acuity, slit lamp examination, intraocular pressure, fundus examination with or without pharmacological mydriasis as indicated) was performed periodically on a schedule customized to the needs of the patients but not less frequently than every 3 months (for well-controlled uveitis). From 1997 until 2000, etanercept was the only TNF- blocker available for use in children. After October 2000, the choice of drug as well as dose and dose increase of infliximab was an Division of Rheumatology, 1 Department of Ophthalmology and Vision Sciences, 2 Departments of Paediatrics and Medicine, Hospital for Sick Children, Toronto, Canada and 3 North Toronto Eye specialists, Toronto, Canada. Received 20 September 2005; revised version accepted 6 January Correspondence to: R. Saurenmann, Zurich University Children s Hospital, Postal Box 8032, Zu rich, Switzerland. traudel.saurenmann@kispi.unizh.ch 982 ß The Author Published by Oxford University Press on behalf of the British Society for Rheumatology. All rights reserved. For Permissions, please journals.permissions@oxfordjournals.org
2 Anti-TNF treatment for childhood uveitis 983 opinion-based decision of the treating ophthalmologist and rheumatologist in collaboration. It was hospital policy that anti-tnf- therapy was never used as a first-line treatment but would only be started after failure of at least one immunosuppressive drug. Doses routinely used for etanercept treatment were mg/kg administered twice weekly as subcutaneous injections. Infliximab infusions at 3 10 mg/kg were given at weeks 0, 2, 6 and then every 4 8 weeks. Infusions were given in a day therapy unit in hospital. The dose of methotrexate for uveitis treatment was mg/kg either orally or subcutaneously once weekly. On infliximab, the methotrexate dose could be reduced to mg/kg by the same route and frequency. All patients treated with infliximab routinely received prophylactic medication with diphenhydramine (1.25 mg/kg) and acetaminophen (paracetamol; 15 mg/kg) 30 min prior to the start of infliximab infusion. Patients were followed by different ophthalmologists, all with expertise in paediatric uveitis, who used best clinical judgement in reducing medications. We used reduction of concomitant medication to assess the response to treatment as an objective measure because of the possibility of interpreter variability in the assessment of anterior chamber cell count. Decisions to discontinue or reduce systemic immunosuppressive agents were at the discretion of the treating physicians based on clinical assessment for both etanercept and infliximab patients. Immunosuppressive drugs were only discontinued if the uveitis had improved or was quiescent and therefore discontinuation of immunosuppressive agent without flare of uveitis was used as indicative of response to anti-tnf therapy. The study was approved by the Research Ethics Board of The Hospital for Sick Children. Written informed consent and authorization for release of information was obtained from patients who were followed by ophthalmologists outside The Hospital for Sick Children. Outcome definitions Treatment response. A good response was defined as a decrease of 50% in both corticosteroid use and immunosuppressive agent (if on immunosuppressive drug at study entry). A moderate response was a decrease of 50% in either corticosteroid or immunosuppressive agent but not both. A poor response was a decrease of <50% in both corticosteroid and immunosuppressive agent. A decrease in corticosteroid dose was defined as either a decrease in the number of drops per eye or a decreased oral prednisone dose. Visual acuity. Snellen linear letters or appropriate-for-age equivalent were used. Normal acuity was defined as best corrected visual acuity 20/40 or better; impaired acuity was best corrected visual acuity between 20/50 and 20/100; blind was defined as best corrected visual acuity 20/200 or worse. TABLE 1. Patient characteristics These groups were chosen in accordance with the values commonly used for granting a driver s licence (20/40) and for legal blindness (20/200). Worsening visual acuity was defined as a change from one of these categories to a category with less best corrected visual acuity. Improving visual acuity was defined as a change to a better category. Glaucoma. Worsening was defined as the need for an increased number of different anti- medications to control intra-ocular pressure, or the need for surgery. Improved was defined as the ability to reduce the number of different medications without surgery. Statistics Fisher s exact test was used to assess differences in response between the groups. Results For the patients characteristics, see Table 1. All 21 patients had severe disease refractory to at least one standard immunosuppressive agent. In 18/21 patients, severe refractory uveitis was the indication for treatment with TNF- blockers. In the remaining 3/21 patients, TNF- blockers were started because of severe refractory joint disease. All three had persistently active uveitis requiring local steroid drops despite systemic immunosuppressive treatment, and all three had an excellent response of their joint disease to anti-tnf- therapy. Of the 21 patients, 12 had JIA (57%), six had idiopathic uveitis (29%), two had Behc et disease (9%) and one had sarcoidosis (5%) (Table 2). At study entry, 39/42 eyes had active uveitis with normal visual acuity in 23 (59%), impaired visual acuity in four (10%) and legal blindness in 12 (31%). In three patients (14%), legal blindness was bilateral. Ten of the 21 patients (48%), with 19 affected eyes, had normal best corrected visual acuity in both eyes. In 5/12 eyes, the legal blindness was due to cataract, in 4/12 it was due to cystoid macular oedema, in 2/12 it was due to retinal detachment and in one patient the exact reason for blindness caused by the refractory panuveitis was not clearly determinable. In 22 of the 39 eyes with uveitis (56% of eyes, 14 patients), cataract was diagnosed before study entry. In two of the 22 eyes with cataract (one patient), visual acuity was normal despite the cataract. Thirteen eyes (eight patients) had cataract surgery prior to anti-tnf- treatment and in six eyes (five patients) surgery was performed during anti- TNF- treatment. One patient had cataract surgery in one eye performed before and in the other eye during anti-tnf treatment. One patient had one eye cataract extraction performed only after discontinuing unsuccessful etanercept treatment. Indication for anti-tnf therapy Characteristic Uveitis (n ¼ 18) Joints (n ¼ 3) All (n ¼ 21) female patients (percentage) 13 (72%) 3 (100%) 16 (76%) patients with positive ANA (percentage) 13 (72%) 2 (67%) 15 (71%) Mean age at diagnosis of primary disease, yr (range) 6.5 ( ) 1.8 ( ) 5.9 ( ) Mean duration of uveitis before study, yr (range) 4.5 ( ) 5.6 ( ) 4.6 ( ) Mean total number of immunosuppressive drugs before study (range) 2.2 (1 6) 2 (1 3) 2.2 (1 6) Mean number of immunosuppressive drugs used at study entry (range) 1.5 (1 4) 1.3 (0 3) 1.5 (0 4) Mean dose of oral prednisone used at study entry, mg/kg (range) 0.25 (0 1.2) 0.16 (0 0.33) 0.24 (0 1.2) Mean dose of oral prednisone used at end of study, mg/kg (range) 0.04 (0 0.25) (0 0.25) Mean time to reach moderate or good response, months (range) 3.4 (1 11) 8.2 (1.5 19) 4.2 (1 19) Mean observation time on anti-tnf therapy, yr (range) 1.7 ( ) 2.0 ( ) 1.75 ( )
3 984 R. K. Saurenmann et al. Fourteen patients (22/39 eyes, 56%) had at the time of initiation of anti-tnf- treatment, eight of whom (12 eyes) had previous surgery (Table 3). Thirteen of the 21 patients were treated with infliximab (10 as initial therapy and three following etanercept) and 11 with etanercept (all as initial treatment), resulting in 24 treatment courses. In 18/24 treatment courses, additional immunosuppressive drugs (other than local and/or systemic corticosteroids) were continued at study entry (13 methotrexate, three hydroxychloroquine, three azathioprine, one sulphasalazine, one chlorambucil, one intravenous immunoglobulins). In four of the 18 patients, a combination of two or three immunosuppressive drugs was used in addition to the TNF- inhibitor. During the study period, six patients were able to completely discontinue eight additional immunosuppressive agents (two methotrexate, two azathioprine, one chlorambucil, one sulphasalazine, one hydroxychloroquine, one intravenous immunoglobulins). However, two patients required addition of methotrexate after initiation of TNF- inhibitor treatment to control their uveitis. In both, immunosuppressive therapy with methotrexate or sulphasalazine, respectively, had been discontinued at initiation of etanercept treatment. Another patient on etanercept experienced a flare of uveitis after discontinuation of methotrexate during the study and had to restart methotrexate. Methotrexate was used as a concomitant immunosuppressive drug during 7/11 (64%) treatment courses with etanercept and 10/13 (77%) treatment courses with infliximab (P ¼ 0.66). Outcome Primary outcome variable: uveitis response to treatment. The 21 patients (39 involved eyes) accounted for 24 treatment courses for 44 involved eyes (Table 2). Overall, 38% of patients treated with infliximab had a good response, 54% a moderate response and 8% a poor response. By comparison, 27% of patients treated with etanercept had a good response, 27% a moderate response and 45% a poor response (Fig. 1). The three patients who failed etanercept and were then treated with infliximab all had a moderate response to infliximab (mean dose of 5.0 mg/kg with a range of mg/kg). Overall, 18/24 (75%) treatment courses resulted in a moderate or good response. For duration of treatment, see Table 1. The mean duration of anti-tnf- treatment needed to achieve the criterion for a moderate response was 4.2 months (range 1 19 months) for all patients, with 3.4 months for infliximab-treated patients and 5.4 months for etanercept-treated patients. For response rates according to the underlying disease, see Table 2. When the response of patients to therapy was divided into only two categories TABLE 2. Response to treatment according to underlying disease Anti-TNF- agent rather than the initial three [poor vs moderate or good response (moderate/good)], there was a statistically significant difference in the percentage of patients with a moderate/good response to infliximab compared with the percentage of patients with a moderate/good response to etanercept (92 vs 55%; P ¼ ). Four of the eight patients with a good response (three on infliximab and one on etanercept) and 5/10 patients with a moderate response (three on infliximab and two on etanercept) were able to discontinue systemic corticosteroid treatment. However, only two patients were able to completely discontinue all corticosteroid treatment (topical and systemic): one patient with polyarticular JIA and one patient with severe uveitis and psoriatic arthritis, who had complete remission of psoriasis, arthritis and uveitis. Both patients were maintained on low-dose methotrexate and infliximab. The mean dose of infliximab in the patients who responded was 5.4 mg/kg (range mg/kg). The infusions were given at 4-week intervals in eight patients (62%) and at 8-week intervals in three patients (23%). In two patients (15%), the initial interval of 4 weeks was gradually increased to 6 weeks after improvement of disease. The etanercept dose routinely used was between mg/kg and the dose was increased to 0.8 mg/kg in only one patient (this patient, with severe uveitis, had shown some response to the standard dose of etanercept and had additional improvement when the dose was increased). Three patients experienced a flare of their uveitis after the interval between infliximab infusions was increased from 8 to 12, from 8 to 9 and from 4 to 6 weeks, respectively. This relapse was controlled again in all of them upon return to the previous infusion interval. Secondary outcome variables. Eleven patients (16 eyes) had decreased visual acuity at study entry. Best corrected visual acuity improved in 5/11 patients (7/16 eyes, 43%). Four patients (5/16 eyes, 31%) improved as a result of cataract surgery. In two eyes (two patients, both on infliximab), improvement of visual acuity from 20/400 to 20/70 and from 20/400 to 20/20, respectively, was unrelated to cataract surgery. The difference in improvement of visual acuity between etanercept- and infliximab-treated patients was not statistically significant (P ¼ 0.15). At study entry, best corrected visual acuity was better than legal blindness in 27 eyes (18 patients). Vision decreased in 2/18 patients (2/27 eyes, 7%) from 20/100 to 20/200 and from 20/60 to 20/200 respectively, both on etanercept (13% of etanercept treated eyes) due to uncontrolled inflammation, and remained unchanged in 16 patients (25 eyes) during the study (difference between etanercept and infliximab treated group, P ¼ 0.48). For changes in visual acuity during the study, see Fig. 2. Response Underlying disease Etanercept Infliximab Good Moderate Poor eyes involved JIA subtype Persistent oligoarticular (n ¼ 3) Extended oligoarticular (n ¼ 2) Polyarticular, RF-negative (n ¼ 4) Psoriatic (n ¼ 2) Enthesitis-related arthritis (n ¼ 1) Idiopathic uveitis Anterior (n ¼ 3) Intermediate (n ¼ 1) Panuveitis (n ¼ 2) Behc et s (n ¼ 2) Sarcoidosis (n ¼ 1) Total (21 patients with 24 treatment courses) RF, rheumatoid factor.
4 Anti-TNF treatment for childhood uveitis 985 TABLE 3. Cataract and before and after start of anti-tnf- treatment Before start of treatment with TNF inhibitor After start of treatment with TNF inhibitor medications used surgeries cataract extractions eyes with new cataracts eyes with new medications used surgeries cataract extractions eyes with cataracts eyes with Underlying disease JIA (21 eyes) Idiopathic uveitis (12 eyes) Behcet s disease/sarcoidosis (6 eyes) Total (39 eyes) Time to complication, yr (range) 1.1 ( ) 1.22 ( ) 0.77 ( ) 0.59 ( ) In 14/21 patients (22/39 eyes), cataracts were diagnosed before anti-tnf- treatment. New cataracts were diagnosed in 4/7 of the remaining patients (5/17 eyes, 29%) during the study. Two patients (three eyes) were on etanercept and two patients (two eyes) were on infliximab. However, these newly diagnosed cataracts did not affect visual acuity and did not lead to cataract surgery during the study period. Pre-existing improved in 4/22 eyes (18%) of 4/14 patients during the study: three on infliximab and one on etanercept (difference between etanercept- and infliximab-treated groups, P ¼ 0.61). In three of these eyes, all medications were stopped without the requirement for surgery (one etanercept, two infliximab). One patient on infliximab required surgery in one eye; in the other eye improved without surgery. New-onset during anti-tnf- treatment was diagnosed in two patients (4/17 eyes, 24%), both on etanercept, despite a moderate response of the uveitis to this treatment. Pre-existing worsened in 7/22 eyes (32%) of five patients: two treated with etanercept, two treated with infliximab and one treated with both etanercept and infliximab consecutively, of which five eyes (four patients) required surgery (Fig. 3). Overall, we observed new-onset or worsening in 7/20 (35%) eyes in the etanercept-treated group and 4/24 (17%) eyes in the infliximab-treated group (difference between etanercept and infliximab treated group, P ¼ 0.29) Five patients (six eyes) had cataract surgery during the course of the study. In four of the five patients, the extraction of a cataract which was present before initiation of anti-tnf- treatment was only possible because the previously heavy inflammation was well controlled. In four patients (five eyes), surgery (total, 14 procedures) was performed during the study period. One patient had both cataract surgery (two eyes) and surgery (seven surgeries) after initiation of anti-tnf- treatment. Adverse reactions One of the 21 patients (5%) had an infection (cellulitis of the forearm) requiring hospitalization and intravenous antibiotic treatment. Etanercept was temporarily withheld. No other adverse reactions were seen in any of the remaining 20 patients, including infusion reactions, infections requiring hospitalization or any new-onset autoimmune disease. We did not measure the financial or psychosocial impact of the all day visits to the hospital for infliximab infusion vs the home injections of etanercept. Discussion Non-infectious uveitis in childhood can be either idiopathic or associated with a systemic autoimmune disease. Although many patients respond to topical therapy, recalcitrant uveitis leads to significant ocular morbidity despite topical and systemic therapy with immunosuppressive agents and corticosteroids. In this study, we have shown that the majority of patients (86%) with active, recalcitrant uveitis had a favourable response to treatment with TNF- inhibitors after failing other immunosuppressive agents. The response to anti-tnf- therapy was independent of the underlying cause of the uveitis. Although the majority of patients responded to therapy, we found a statistically significant difference in the response rate between etanercept- and infliximab-treated patients, favouring infliximab. There was a trend for fewer complications and higher rate of improvement of both and visual acuity in the infliximab-treated group compared with the etanercept-treated group. Interestingly, we also found that three patients responded to infliximab following failure of etanercept. The one patient with a poor response to infliximab did not have a trial on etanercept.
5 986 R. K. Saurenmann et al Percent of patients Etanercept (n=11) Infliximab (n=13) FIG. 1. Response to anti-tnf- therapy in patients with active uveitis. Treatment is shown on the horizontal axis and the percentage of patients who responded on the vertical axis. A good response is shown by the medium grey box, a moderate response by the dark box and poor response by light grey box. Visual acuity At start of anti-tnfa treatment Last value during anti-tnfa treatment FIG. 2. Changes in visual acuity during the study in nine eyes of seven patients. Patients treated with etanercept are shown with triangles and dotted lines and patients treated with infliximab are shown with squares and bold lines. An open triangle or square indicates cataract surgery during study. Snellen chart values for visual acuity are transformed into a single number dividing the numerator by the denominator. A differential response to etanercept and infliximab therapy has been reported in studies of patients with Crohn s disease, but not in patients with inflammatory arthritis [18, 23, 24]. Although all TNF- inhibitors work by binding to and inhibiting the effect of TNF-, they are usually divided into two groups: monoclonal antibodies and soluble receptors. It has been hypothesized that the beneficial effect of infliximab in Crohn s disease is the result of apoptosis of monocytes and/or T lymphocytes [25, 26]. However, the effect of etanercept on colonic T lymphocytes has not been studied. In addition, if infliximab is superior to etanercept in causing apoptosis of monocytes, it is not clear why this should be important in Crohn s disease and not in inflammatory arthritis. TNF- has been demonstrated to play an important role in the development of uveitis [27, 28]. However, it may also have a protective effect in some situations [29]. To date, there have been only two prospective studies of anti-tnf- therapy, both using etanercept, in children with uveitis [19, 30]. The two trials, one controlled and one uncontrolled, showed similar response rates of 57 and 50%, respectively. However, the rate of response to therapy in the controlled trial was not statistically significant different from that in the control group. Despite the different methods we used
6 Anti-TNF treatment for childhood uveitis Percent of eyes for assessing improvement, our etanercept response rate (54%) is consistent with the previous studies. In our study, we found that the response to anti-tnf- was independent of the underlying disease as 12 patients had JIA, six had idiopathic uveitis, two had Behc et disease and one had sarcoidosis. These results are similar to the results of the open-label trial in which patients with idiopathic uveitis and uveitis secondary to JIA were studied [19]. All patients in the placebo-controlled trial had JIA [30]. Systemic corticosteroid treatment was discontinued in 9/16 of our patients after the start of TNF- blocker therapy (six on infliximab, three on etanercept). This effect was also described in 5/6 patients in the study of Richards et al. [34], whereas only one patient in Reiff s group [19] and four patients in Smith s study [30] had systemic corticosteroids at study entry and any further systemic corticosteroid requirement of these patients was not described. Treatment with TNF- blockers allowed the discontinuation of concomitant immunosuppressive medication in 6/18 patients in our study, an effect that was also observed in 3/6 patients in Richards study with infliximab. However, in three of our patients etanercept alone was not able to control uveitis. This finding is in accordance with results published by Foster et al. regarding the efficacy of etanercept to maintain uveitis remission achieved with methotrexate [31]. Case reports and small series exist describing infliximab in the treatment of children with uveitis also show a positive effect [32 34]. For adult patients, striking improvement of both inflammation and best corrected visual acuity with infliximab have been reported for the treatment of uveitis related to a variety of underlying diseases, most of them for Behc et disease and for HLA B27-related uveitis [35 37]. No direct comparison of the two most commonly used anti- TNF- agents, etanercept and infliximab, for the treatment of uveitis have been previously performed to our knowledge. We observed changes in visual acuity unrelated to cataract surgery in four patients, with improvement in two eyes on infliximab and worsening in two eyes on etanercept. Previous paediatric studies have reported both improvement and worsening of visual acuity [19, 30]. In a recently published prospective trial of infliximab in refractory uveitis of 23 patients, one of them a child with idiopathic uveitis, Suhler et al. [37] also described Cataract Glaucoma Decreased visual acuity FIG. 3. New complications encountered during anti-tnf- therapy. Individual complications are shown on the horizontal axis and the percentage of eyes with the complication is shown on the vertical axis. Eyes treated with etanercept (n ¼ 20) are shown in the light boxes and the eyes treated with infliximab (n ¼ 24) in the dark boxes. Visual acuity refers to best corrected visual acuity. improvement of visual acuity in 10/44 eyes, whereas worsening visual acuity occurred in both eyes of one patient experiencing a uveitis flare after 8 weeks of treatment with infliximab. In a group of adult patients, resolution of cystoid macular oedema has been described during treatment with infliximab; however, a similar response to treatment with etanercept has not been looked for to our knowledge [38]. Although macular thickness was not measured routinely in our patients, this effect might be a possible explanation for improvement of VA in our infliximab treated patients. In neither of the two prospective studies were new-onset cataracts observed, a complication we observed in 5/17 eyes without prior cataract. However, previous work has shown that development of cataract in patients with paediatric uveitis is strongly correlated with the duration of uveitis [39]. Our patients had a mean duration of uveitis of 4.6 yr at study entry and a mean observation time on TNF- inhibitor treatment of 1.75 yr, whereas both the mean disease duration and follow-up duration were shorter in the previous paediatric studies [19, 30]. The mean duration from the start of anti-tnf- treatment to the diagnosis of new-onset cataract in our patients was 1.2 yr. Therefore, the difference in observation time on anti-tnf- treatment might be one possible explanation for this previously undescribed complication. However, very little is known about the role of TNF- in the development of cataract, and therefore careful long-term monitoring is required to determine the role of anti-tnf- therapy in cataract formation. Glaucoma is a common complication in children with chronic uveitis. Treatment consists of topical anti- agents with oral carbonic anhydrase inhibitors and/or surgery for more severe cases [40]. Goniotomy provides good results in children with sight-threatening related to uveitis, but in most cases topical therapy still has to be continued [41]. An unexpected finding in our study was an improvement of without surgery in four patients. Both drugs were associated with this outcome. However, two patients had new-onset (both on etanercept) despite the improvement of uveitis, and five patients (two each on etanercept and on infliximab and one on both etanercept and infliximab consecutively) had worsening despite a moderate to good response for their uveitis.
7 988 R. K. Saurenmann et al. In Richards study of six patients with JIA-associated uveitis treated with infliximab, improvement of without surgery was observed in 2/6 patients [34]. Reiff and co-workers did not observe changes in intraocular pressure during their prospective open-label study with etanercept [19], and in the masked placebo-controlled study of Smith [30] and co-workers intraocular pressure was not one of the outcome variables. Like cataract, development of is correlated with duration of uveitis and therefore also for this complication the longer overall disease duration of our patients may have played a role [39]. The role of TNF- in the development of is not clear and our results, showing both improvement and worsening of, do not allow any insight into the role of TNF- in the development of. Our study is limited by a small sample size, retrospective data and heterogeneity of the underlying diseases and patients. Furthermore, we did not control for any difference in concomitant use of immunosuppressive agents other than methotrexate between the two groups. Patients treated with infliximab were allowed to increase the dose while patients on etanercept did not have a similar dose escalation. Yet, to our knowledge, this is the largest number of patients with refractory childhood uveitis treated with TNF- inhibitors reported and the only study that has compared the effect of the two TNF- inhibitors currently available for treatment in children. In conclusion, treatment for recalcitrant uveitis with anti- TNF- medications was associated with a favourable response in the majority of cases. Our results suggest that infliximab is superior to etanercept not only by decreasing the number of concomitant medications required to control the uveitis but also due to a lower rate of new-onset and cataract. Both medications had excellent short-term side-effect profiles. We suggest that a large, prospective, multicentre, interdisciplinary (rheumatology and ophthalmology) controlled study be conducted to confirm our findings. Rheumatology Key messages Anti-TNF- treatment was beneficial in a high percentage of patients with severe refractory childhood uveitis. Infliximab resulted in better clinical responses than etanercept. Treatment with infliximab seemed to lead to less ocular complications Acknowledgements This work would not have been possible without the contributions of the involved ophthalmologists throughout Ontario and the contribution of the Rheumatology Volunteers of The Hospital for Sick Children (HSC). We wish to thank Dr L. Spiegel and Dr B. Cameron, Division of Rheumatology, HSC, for their support and contribution, Dr D. Derzko-Dzulynski, Dr A. Budning and Dr Amy for sharing their patients ophthalmology records, and Clinical Coordinator Katarzyna Gogol and Research Assistants Enza Perruzza and Erica Bell from the Department of Ophthalmology and Vision Sciences for their support. Grant support was received from the Swiss Arthritis Society (Schweizerische Rheumaliga), Stiftung Sanitas (Switzerland) and Brandan s Eye Research Fund. R.K.S. has served as a paid consultant for Centocor. The other authors have declared no conflicts of interest. References 1. Edelsten C, Reddy MA, Stanford MR, Graham EM. Visual loss associated with pediatric uveitis in English primary and referral centers. Am J Ophthalmol 2003;135: Paivonsalo-Hietanen T, Tuominen J, Saari KM. Uveitis in children: population-based study in Finland. Acta Ophthalmol Scand 2000; 78: Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California: the Northern California Epidemiology of Uveitis Study. Ophthalmology 2004;111: Tugal-Tutkun I, Havrlikova K, Power WJ, Foster CS. Changing patterns in uveitis of childhood. Ophthalmology 1996;103: Cunningham ET Jr. Uveitis in children. Ocul Immunol Inflamm 2000;8: Stoffel PB, Sauvain MJ, von Vigier RO, Beretta-Piccoli BC, Ramelli GP, Bianchetti MG. Non-infectious causes of uveitis in 70 Swiss children. Acta Paediatr 2000;89: Kadayifcilar S, Eldem B, Tumer B. Uveitis in childhood. J Pediatr Ophthalmol Strabismus 2003;40: Smith JR. Management of uveitis in pediatric patients: special considerations. Paediatr Drugs 2002;4: Holland GN, Stiehm ER. Special considerations in the evaluation and management of uveitis in children. Am J Ophthalmol 2003; 135: Kilmartin DJ, Forrester JV, Dick AD. Cyclosporin A therapy in refractory non-infectious childhood uveitis. Br J Ophthalmol 1998;82: Hesselink DA, Baarsma GS, Kuijpers RW, van Hagen PM. Experience with cyclosporine in endogenous uveitis posterior. Transplant Proc 2004;36:372S 377S. 12. Weiss AH, Wallace CA, Sherry DD. Methotrexate for resistant chronic uveitis in children with juvenile rheumatoid arthritis. J Pediatr 1998;133: Samson CM, Waheed N, Baltatzis S, Foster CS. Methotrexate therapy for chronic noninfectious uveitis: analysis of a case series of 160 patients. Ophthalmology 2001;108: Baltatzis S, Tufail F, Yu EN, Vredeveld CM, Foster CS. Mycophenolate mofetil as an immunomodulatory agent in the treatment of chronic ocular inflammatory disorders. Ophthalmology 2003;110: Lau CH, Comer M, Lightman S. Long-term efficacy of mycophenolate mofetil in the control of severe intraocular inflammation. Clin Exp Ophthalmol 2003;31: Durrani K, Papaliodis GN, Foster CS. Pulse IV cyclophosphamide in ocular inflammatory disease: efficacy and short-term safety. Ophthalmology 2004;111: Miserocchi E, Baltatzis S, Ekong A, Roque M, Foster CS. Efficacy and safety of chlorambucil in intractable noninfectious uveitis: the Massachusetts Eye and Ear Infirmary experience. Ophthalmology 2002;109: Lovell D. Biologic agents for the treatment of juvenile rheumatoid arthritis: current status. Paediatr Drugs 2004;6: Reiff A, Takei S, Sadeghi S et al. Etanercept therapy in children with treatment-resistant uveitis. Arthritis Rheum 2001;44: Smith JR, Levinson RD, Holland GN et al. Differential efficacy of tumor necrosis factor inhibition in the management of inflammatory eye disease and associated rheumatic disease. Arthritis Rheum 2001;45: Reddy AR, Backhouse OC. Does etanercept induce uveitis? Br J Ophthalmol 2003;87: De Vos AF, Van Haren MA, Verhagen C, Hoekzema R, Kijlstra A. Systemic anti-tumor necrosis factor antibody treatment exacerbates endotoxin-induced uveitis in the rat. Exp Eye Res 1995;61: Sandborn WJ, Hanauer SB, Katz S et al. Etanercept for active Crohn s disease: a randomized, double-blind, placebo-controlled trial. Gastroenterology 2001;121:
8 Anti-TNF treatment for childhood uveitis Akobeng AK, Zachos M. Tumor necrosis factor-alpha antibody for induction of remission in Crohn s disease. Cochrane Database Syst Rev 2004:CD Lugering A, Schmidt M, Lugering N, Pauels HG, Domschke W, Kucharzik T. Infliximab induces apoptosis in monocytes from patients with chronic active Crohn s disease by using a caspasedependent pathway. Gastroenterology 2001;121: ten Hove T, van Montfrans C, Peppelenbosch MP, van Deventer SJ. Infliximab treatment induces apoptosis of lamina propria T lymphocytes in Crohn s disease. Gut 2002;50: Sartani G, Silver PB, Rizzo LV et al. Anti-tumor necrosis factor alpha therapy suppresses the induction of experimental autoimmune uveoretinitis in mice by inhibiting antigen priming. Invest Ophthalmol Vis Sci 1996;37: Santos Lacomba M, Marcos Martin C et al. Aqueous humor and serum tumor necrosis factor-alpha in clinical uveitis. Ophthalmic Res 2001;33: Kasner L, Chan CC, Whitcup SM, Gery I. The paradoxical effect of tumor necrosis factor alpha (TNF-alpha) in endotoxin-induced uveitis. Invest Ophthalmol Vis Sci 1993;34: Smith JA, Thompson DJ, Whitcup SM et al. A randomized, placebocontrolled, double-masked clinical trial of etanercept for the treatment of uveitis associated with juvenile idiopathic arthritis. Arthritis Rheum 2005;53: Foster CS, Tufail F, Waheed NK et al. Efficacy of etanercept in preventing relapse of uveitis controlled by methotrexate. Arch Ophthalmol 2003;121: Mangge H, Heinzl B, Grubbauer HM, El-Shabrawi Y, Schauenstein K. Therapeutic experience with infliximab in a patient with polyarticular juvenile idiopathic arthritis and uveitis. Rheumatol Int 2003;23: Murphy CC, Ayliffe WH, Booth A, Makanjuola D, Andrews PA, Jayne D. Tumor necrosis factor alpha blockade with infliximab for refractory uveitis and scleritis. Ophthalmology 2004;111: Richards JC, Tay-Kearney ML, Murray K, Manners P. Infliximab for juvenile idiopathic arthritis-associated uveitis. Clin Exp Ophthalmol 2005;33: El-Shabrawi Y, Hermann J. Anti-tumor necrosis factoralpha therapy with infliximab as an alternative to corticosteroids in the treatment of human leukocyte antigen B27-associated acute anterior uveitis. Ophthalmology 2002;109: Sfikakis PP, Kaklamanis PH, Elezoglou A et al. Infliximab for recurrent, sight-threatening ocular inflammation in Adamantiades- Behcet disease. Ann Intern Med 2004;140: Suhler EB, Smith JR, Wertheim MS et al. A prospective trial of infliximab therapy for refractory uveitis: preliminary safety and efficacy outcomes. Arch Ophthalmol 2005;123: Markomichelakis NN, Theodossiadis PG, Pantelia E, Papaefthimiou S, Theodossiadis GP, Sfikakis PP. Infliximab for chronic cystoid macular edema associated with uveitis. Am J Ophthalmol 2004;138: Rosenberg KD, Feuer WJ, Davis JL. Ocular complications of pediatric uveitis. Ophthalmology 2004;111: Foster CS, Havrlikova K, Baltatzis S, Christen WG, Merayo-Lloves J. Secondary in patients with juvenile rheumatoid arthritis-associated iridocyclitis. Acta Ophthalmol Scand 2000;78: Freedman SF, Rodriguez-Rosa RE, Rojas MC, Enyedi LB. Goniotomy for secondary to chronic childhood uveitis. Am J Ophthalmol 2002;133:
Methotrexate for uveitis associated with juvenile idiopathic arthritis: Value and requirement for additional anti-inflammatory medication
 European Journal of Ophthalmology / Vol. 17 no. 5, 2007 / pp. 743-748 Methotrexate for uveitis associated with juvenile idiopathic arthritis: Value and requirement for additional anti-inflammatory medication
European Journal of Ophthalmology / Vol. 17 no. 5, 2007 / pp. 743-748 Methotrexate for uveitis associated with juvenile idiopathic arthritis: Value and requirement for additional anti-inflammatory medication
Course, complications, and outcome of juvenile arthritis related uveitis
 Major Articles Course, complications, and outcome of juvenile arthritis related uveitis Kourosh Sabri, MB, ChB, FRCOphth, a Rotraud K. Saurenmann, MD, b,a Earl D. Silverman, MD, FRCPC, b,c and Alex V.
Major Articles Course, complications, and outcome of juvenile arthritis related uveitis Kourosh Sabri, MB, ChB, FRCOphth, a Rotraud K. Saurenmann, MD, b,a Earl D. Silverman, MD, FRCPC, b,c and Alex V.
Loss of efficacy during long-term infliximab therapy for sight-threatening childhood uveitis
 Rheumatology 08;47:10 14 Advance Access publication 1 August 08 doi:10.1093/rheumatology/ken298 Concise Report Loss of efficacy during long-term infliximab therapy for sight-threatening childhood uveitis
Rheumatology 08;47:10 14 Advance Access publication 1 August 08 doi:10.1093/rheumatology/ken298 Concise Report Loss of efficacy during long-term infliximab therapy for sight-threatening childhood uveitis
New Drugs for Uveitis. Medical Eye Unit St Thomas Hospital
 New Drugs for Uveitis Miles Stanford Medical Eye Unit St Thomas Hospital x Epithelium x x Antigen Y Y Y Y IgG m cd4 IL-2 Y m + IL-12 Cytotoxic T B pmn Ig s PG s. LTB4 O - IL-6 TNFα IFNγγ IL-2 Th1 IL-10
New Drugs for Uveitis Miles Stanford Medical Eye Unit St Thomas Hospital x Epithelium x x Antigen Y Y Y Y IgG m cd4 IL-2 Y m + IL-12 Cytotoxic T B pmn Ig s PG s. LTB4 O - IL-6 TNFα IFNγγ IL-2 Th1 IL-10
Paediatric rheumatology. Epidemiology of uveitis in children over a 10-year period
 Paediatric rheumatology Epidemiology of uveitis in children over a 10-year period L.A.L. Clarke 1, Y. Guex-Crosier 2, M. Hofer 1 1 Immunoallergology and Rheumatology Unit, Department of Paediatrics (DMCP),
Paediatric rheumatology Epidemiology of uveitis in children over a 10-year period L.A.L. Clarke 1, Y. Guex-Crosier 2, M. Hofer 1 1 Immunoallergology and Rheumatology Unit, Department of Paediatrics (DMCP),
Nausheen Khuddus, MD Melissa Elder, MD, PhD
 Nausheen Khuddus, MD Melissa Elder, MD, PhD Nausheen Khuddus, MD Pediatric Ophthalmologist and Strabismus Specialist Accent Physicians Gainesville, Florida What Is Uveitis? Uveitis is caused by inflammatory
Nausheen Khuddus, MD Melissa Elder, MD, PhD Nausheen Khuddus, MD Pediatric Ophthalmologist and Strabismus Specialist Accent Physicians Gainesville, Florida What Is Uveitis? Uveitis is caused by inflammatory
Infliximab for the treatment of refractory scleritis
 1 Massachussets Eye Research and Surgery Institution, Cambridge, Massachusetts, USA 2 BayView Clinic, Mumbai, India 3 Department of Ophthalmology, Harvard Medical School, Boston, Massachusetts, USA 4 Brigham
1 Massachussets Eye Research and Surgery Institution, Cambridge, Massachusetts, USA 2 BayView Clinic, Mumbai, India 3 Department of Ophthalmology, Harvard Medical School, Boston, Massachusetts, USA 4 Brigham
HARVARD PILGRIM HEALTH CARE RECOMMENDED MEDICATION REQUEST GUIDELINES HUMIRA PEDIATRIC
 Generic Brand HICL GCN Exception/Other ADALIMUMAB HUMIRA 24800 HUMIRA PEDIATRIC GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Is the patient currently taking Humira? If
Generic Brand HICL GCN Exception/Other ADALIMUMAB HUMIRA 24800 HUMIRA PEDIATRIC GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Is the patient currently taking Humira? If
Uveitis unplugged: systemic therapy
 Uveitis unplugged: systemic therapy Hobart 2017 Peter McCluskey Save Sight Institute Sydney Eye Hospital Sydney Medical School University of Sydney Sydney Australia No financial or proprietary interest
Uveitis unplugged: systemic therapy Hobart 2017 Peter McCluskey Save Sight Institute Sydney Eye Hospital Sydney Medical School University of Sydney Sydney Australia No financial or proprietary interest
Approach to Pediatric Uveitis. Paris Tranos PhD,ICO,FRCS OPHTHALMICA Vitreoretinal & Uveitis Service
 Approach to Pediatric Uveitis Paris Tranos PhD,ICO,FRCS OPHTHALMICA Vitreoretinal & Uveitis Service Epidemiology Uveitis is the 3 rd leading cause of blindness in USA 5-10% of uveitis cases involve children
Approach to Pediatric Uveitis Paris Tranos PhD,ICO,FRCS OPHTHALMICA Vitreoretinal & Uveitis Service Epidemiology Uveitis is the 3 rd leading cause of blindness in USA 5-10% of uveitis cases involve children
Evolving therapies for posterior uveitis. Infliximab (Remicade) Infliximab: pharmacology. FDA-approved monoclonal antibody therapy Target
 Evolving therapies for posterior uveitis Sam Dahr, M.D. September 17, 2005 Midwest Ophthalmology Conference Infliximab (Remicade) FDA approved for Crohn s disease, rheumatoid arthritis, and psoriatic arthritis
Evolving therapies for posterior uveitis Sam Dahr, M.D. September 17, 2005 Midwest Ophthalmology Conference Infliximab (Remicade) FDA approved for Crohn s disease, rheumatoid arthritis, and psoriatic arthritis
The Hospital for Sick Children Technology Assessment at SickKids (TASK)
 The Hospital for Sick Children Technology Assessment at SickKids (TASK) THE USE OF BIOLOGIC RESPONSE MODIFIERS IN POLYARTICULAR-COURSE JUVENILE IDIOPATHIC ARTHRITIS Report No. 2010-01 Date: January 11,
The Hospital for Sick Children Technology Assessment at SickKids (TASK) THE USE OF BIOLOGIC RESPONSE MODIFIERS IN POLYARTICULAR-COURSE JUVENILE IDIOPATHIC ARTHRITIS Report No. 2010-01 Date: January 11,
Adalimumab and dexamethasone for treating non-infectious uveitis [ID763]
![Adalimumab and dexamethasone for treating non-infectious uveitis [ID763] Adalimumab and dexamethasone for treating non-infectious uveitis [ID763]](/thumbs/82/86218480.jpg) Adalimumab and dexamethasone for treating non-infectious uveitis [ID763] Multiple Technology Appraisal 2 nd meeting: 12 th April 2017 Committee C Slides for Committee, projector and public [NoACIC] 1 The
Adalimumab and dexamethasone for treating non-infectious uveitis [ID763] Multiple Technology Appraisal 2 nd meeting: 12 th April 2017 Committee C Slides for Committee, projector and public [NoACIC] 1 The
Surgery in patients with uveitis. Lyndell Lim and Anthony Hall
 Surgery in patients with uveitis Lyndell Lim and Anthony Hall Disclosures Off label treatments Paid advisory board Bayer Paid research support Allergan (makers of Ozurdex) Paid research support B and L
Surgery in patients with uveitis Lyndell Lim and Anthony Hall Disclosures Off label treatments Paid advisory board Bayer Paid research support Allergan (makers of Ozurdex) Paid research support B and L
The Future Is Now: Biologics for Non-Infectious Pediatric Anterior Uveitis
 Pediatr Drugs (2015) 17:283 301 DOI 10.1007/s40272-015-0128-2 REVIEW ARTICLE The Future Is Now: Biologics for Non-Infectious Pediatric Anterior Uveitis Melissa A. Lerman 1 C. Egla Rabinovich 2 Published
Pediatr Drugs (2015) 17:283 301 DOI 10.1007/s40272-015-0128-2 REVIEW ARTICLE The Future Is Now: Biologics for Non-Infectious Pediatric Anterior Uveitis Melissa A. Lerman 1 C. Egla Rabinovich 2 Published
Horizon Scanning Technology Summary. Adalimumab (Humira) for juvenile idiopathic arthritis. National Horizon Scanning Centre.
 Horizon Scanning Technology Summary National Horizon Scanning Centre Adalimumab (Humira) for juvenile idiopathic arthritis June 2007 This technology summary is based on information available at the time
Horizon Scanning Technology Summary National Horizon Scanning Centre Adalimumab (Humira) for juvenile idiopathic arthritis June 2007 This technology summary is based on information available at the time
Regional vs. Systemic Therapy. Corticosteroids. Regional vs. Systemic Therapy for Uveitis. Considerations
 Regional vs. Systemic Therapy for Uveitis Nisha Acharya,, M.D., M.S. Director, Uveitis Service F.I. Proctor Foundation University of California, San Francisco December 4, 2010 No financial disclosures
Regional vs. Systemic Therapy for Uveitis Nisha Acharya,, M.D., M.S. Director, Uveitis Service F.I. Proctor Foundation University of California, San Francisco December 4, 2010 No financial disclosures
Ophthalmology Subcommittee of PTAC Meeting held 30 October (minutes for web publishing)
 Ophthalmology Subcommittee of PTAC Meeting held 30 October 2014 (minutes for web publishing) Ophthalmology Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
Ophthalmology Subcommittee of PTAC Meeting held 30 October 2014 (minutes for web publishing) Ophthalmology Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
iologic Agents in the Treatment of Non-Infectious Uveitis
 What is New B iologic Agents in the Treatment of Non-Infectious Uveitis Amala George DNB The treatment of non-infectious uveitis is a challenge to clinicians. The treatment involves suppressing the deleterious
What is New B iologic Agents in the Treatment of Non-Infectious Uveitis Amala George DNB The treatment of non-infectious uveitis is a challenge to clinicians. The treatment involves suppressing the deleterious
Clinical Course of Uveitis in Children in a Tertiary Ophthalmology Center in Northwest Iran
 http://www.cjmb.org Open Access Original Article Crescent Journal of Medical and Biological Sciences Vol. 4, No. 4, October 2017, 200 204 eissn 2148-9696 Clinical Course of Uveitis in Children in a Tertiary
http://www.cjmb.org Open Access Original Article Crescent Journal of Medical and Biological Sciences Vol. 4, No. 4, October 2017, 200 204 eissn 2148-9696 Clinical Course of Uveitis in Children in a Tertiary
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 3 October 2012
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 3 October 2012 REMICADE 100 mg, powder for concentrate for solution for infusion B/1 vial (CIP code: 562 070-1) Applicant:
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 3 October 2012 REMICADE 100 mg, powder for concentrate for solution for infusion B/1 vial (CIP code: 562 070-1) Applicant:
C. Assess clinical response after the first three months of treatment.
 Government Health Plan (GHP) of Puerto Rico Authorization Criteria Tumor Necrosis Factor Alpha (TNFα) Adalimumab (Humira ) Managed by MCO Section I. Prior Authorization Criteria A. Physician must submit
Government Health Plan (GHP) of Puerto Rico Authorization Criteria Tumor Necrosis Factor Alpha (TNFα) Adalimumab (Humira ) Managed by MCO Section I. Prior Authorization Criteria A. Physician must submit
Uveitis literature 2014: the year in review. Russell N. Van Gelder, MD, PhD Department of Ophthalmology University of Washington Seattle, WA
 Uveitis literature 2014: the year in review Russell N. Van Gelder, MD, PhD Department of Ophthalmology University of Washington Seattle, WA Disclosures RVG serves as Associate Editor of IOVS Editorial
Uveitis literature 2014: the year in review Russell N. Van Gelder, MD, PhD Department of Ophthalmology University of Washington Seattle, WA Disclosures RVG serves as Associate Editor of IOVS Editorial
University of Bristol - Explore Bristol Research
 Hawkins, M. J., Dick, A. D., Lee, R. W. J., Ramanan, A. V., Carreño, E., Guly, C., & Ross, A. H. (2016). Managing juvenile idiopathic arthritisassociated uveitis. Survey of Ophthalmology, 61(2), 197-210.
Hawkins, M. J., Dick, A. D., Lee, R. W. J., Ramanan, A. V., Carreño, E., Guly, C., & Ross, A. H. (2016). Managing juvenile idiopathic arthritisassociated uveitis. Survey of Ophthalmology, 61(2), 197-210.
Update on management of Anterior Uveitis
 Update on management of Anterior Uveitis Parthopratim Dutta Majumder Senior Consultant, Department of Uvea & Intraocular Inflammation Medical Research Foundation, Sankara Nethralaya ABCD of Treating a
Update on management of Anterior Uveitis Parthopratim Dutta Majumder Senior Consultant, Department of Uvea & Intraocular Inflammation Medical Research Foundation, Sankara Nethralaya ABCD of Treating a
Abbreviated Drug Evaluation: Fluocinolone acetonide intravitreal implant (Retisert )
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
APPLICATION FOR SPECIAL AUTHORITY. Subsidy for Infliximab
 APPLICATION FOR SPECIAL AUTHORITY Fm SA1778 Subsidy f Infliximab Application Categy Page Graft vs host disease - Initial application... 2 Pulmonary sarcoidosis - Initial application... 2 Previous use -
APPLICATION FOR SPECIAL AUTHORITY Fm SA1778 Subsidy f Infliximab Application Categy Page Graft vs host disease - Initial application... 2 Pulmonary sarcoidosis - Initial application... 2 Previous use -
ADALIMUMAB Generic Brand HICL GCN Exception/Other ADALIMUMAB HUMIRA GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW)
 Generic Brand HICL GCN Exception/Other ADALIMUMAB HUMIRA 24800 GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Does the patient have a diagnosis of moderate to severe rheumatoid
Generic Brand HICL GCN Exception/Other ADALIMUMAB HUMIRA 24800 GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Does the patient have a diagnosis of moderate to severe rheumatoid
INFLIXIMAB Remicade (infliximab), Inflectra (infliximab-dyyb), Renflexis (infliximab-abda)
 RATIONALE FOR INCLUSION IN PA PROGRAM Background Remicade, Renflexis and Inflectra are tumor necrosis factor (TNFα) blockers. Tumor necrosis factor is an endogenous protein that regulates a number of physiologic
RATIONALE FOR INCLUSION IN PA PROGRAM Background Remicade, Renflexis and Inflectra are tumor necrosis factor (TNFα) blockers. Tumor necrosis factor is an endogenous protein that regulates a number of physiologic
Intravitreal Triamcinolone Acetonide for Macular Edema in HLA-B27 Negative Ankylosing Spondylitis
 105 This is an Open Access article licensed under the terms of the Creative Commons Attribution- NonCommercial-NoDerivs 3.0 License (www.karger.com/oa-license), applicable to the online version of the
105 This is an Open Access article licensed under the terms of the Creative Commons Attribution- NonCommercial-NoDerivs 3.0 License (www.karger.com/oa-license), applicable to the online version of the
The effect of a single intravitreal implantation of dexamethasone on the fellow eye in bilateral non-infectious uveitis case report
 European Review for Medical and Pharmacological Sciences The effect of a single intravitreal implantation of dexamethasone on the fellow eye in bilateral non-infectious uveitis case report J. CISZEWSKA,
European Review for Medical and Pharmacological Sciences The effect of a single intravitreal implantation of dexamethasone on the fellow eye in bilateral non-infectious uveitis case report J. CISZEWSKA,
Clinical Features and Prognosis of HLA-B27 Positive and Negative Anterior Uveitis in a Korean Population
 J Korean Med Sci 2009; 24: 722-8 ISSN 1011-8934 DOI: 10.3346/jkms.2009.24.4.722 Copyright The Korean Academy of Medical Sciences Clinical Features and Prognosis of HLA-B27 Positive and Negative Anterior
J Korean Med Sci 2009; 24: 722-8 ISSN 1011-8934 DOI: 10.3346/jkms.2009.24.4.722 Copyright The Korean Academy of Medical Sciences Clinical Features and Prognosis of HLA-B27 Positive and Negative Anterior
Coverage Criteria: Express Scripts, Inc. monograph dated 12/15/ months or as otherwise noted by indication
 BENEFIT DESCRIPTION AND LIMITATIONS OF COVERAGE ITEM: PRODUCT LINES: COVERED UNDER: DESCRIPTION: CPT/HCPCS Code: Company Supplying: Setting: Kineret (anakinra subcutaneous injection) Commercial HMO/PPO/CDHP
BENEFIT DESCRIPTION AND LIMITATIONS OF COVERAGE ITEM: PRODUCT LINES: COVERED UNDER: DESCRIPTION: CPT/HCPCS Code: Company Supplying: Setting: Kineret (anakinra subcutaneous injection) Commercial HMO/PPO/CDHP
Review Article The Role of Gender in Juvenile Idiopathic Arthritis-Associated Uveitis
 Ophthalmology, Article ID 461078, 7 pages http://dx.doi.org/10.1155/2014/461078 Review Article The Role of Gender in Juvenile Idiopathic Arthritis-Associated Uveitis Ahmadreza Moradi, 1 Rowayda M. Amin,
Ophthalmology, Article ID 461078, 7 pages http://dx.doi.org/10.1155/2014/461078 Review Article The Role of Gender in Juvenile Idiopathic Arthritis-Associated Uveitis Ahmadreza Moradi, 1 Rowayda M. Amin,
Juvenile Idiopathic Arthritis with Associated Bilateral Anterior Uveitis in a Four Year- Old Girl
 Juvenile Idiopathic Arthritis with Associated Bilateral Anterior Uveitis in a Four Year- Old Girl Pavlina S. Kemp, MD, Susannah Q. Longmuir, MD August 14, 2012 Chief complaint: Central posterior synechiae
Juvenile Idiopathic Arthritis with Associated Bilateral Anterior Uveitis in a Four Year- Old Girl Pavlina S. Kemp, MD, Susannah Q. Longmuir, MD August 14, 2012 Chief complaint: Central posterior synechiae
CLINICAL SCIENCES. A Prospective Trial of Infliximab Therapy for Refractory Uveitis
 CLINICAL SCIENCES A Prospective Trial of Infliximab Therapy for Refractory Uveitis Preliminary Safety and Efficacy Outcomes Eric B. Suhler, MD; Justine R. Smith, MBBS, PhD; Michael S. Wertheim, MBChB,
CLINICAL SCIENCES A Prospective Trial of Infliximab Therapy for Refractory Uveitis Preliminary Safety and Efficacy Outcomes Eric B. Suhler, MD; Justine R. Smith, MBBS, PhD; Michael S. Wertheim, MBChB,
The Leeds Teaching Hospitals NHS Trust Uveitis in children and young people
 n The Leeds Teaching Hospitals NHS Trust Uveitis in children and young people A guide for young people and parents/carers This leaflet is for parents who have a child with uveitis and for young people
n The Leeds Teaching Hospitals NHS Trust Uveitis in children and young people A guide for young people and parents/carers This leaflet is for parents who have a child with uveitis and for young people
Combined Infliximab and Rituximab in Necrotising Scleritis
 This is an Open Access article licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 License (www.karger.com/oa-license), applicable to the online version of the article
This is an Open Access article licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 License (www.karger.com/oa-license), applicable to the online version of the article
Interim Clinical Commissioning Policy: Adalimumab for Children with Severe Refractory Uveitis
 Interim Clinical Commissioning Policy: Adalimumab for Children with Severe Refractory Uveitis Reference: D12X02 NHS England INFORMATION READER BOX Directorate Medical Commissioning Operations Patients
Interim Clinical Commissioning Policy: Adalimumab for Children with Severe Refractory Uveitis Reference: D12X02 NHS England INFORMATION READER BOX Directorate Medical Commissioning Operations Patients
Uveitis. Pt Info Brochure. Q: What is Uvea?
 Pt Info Brochure Uveitis Q: What is Uvea? A: Uvea is the middle layer of the eye. It is the most vascular structure of the eye. It provides nutrition to the other parts of the eye. The uvea is made up
Pt Info Brochure Uveitis Q: What is Uvea? A: Uvea is the middle layer of the eye. It is the most vascular structure of the eye. It provides nutrition to the other parts of the eye. The uvea is made up
Prior Authorization Conditions for Approval of Humira (adalimumab) Website Form Submit request via: Fax
 Prior Authorization Conditions for Approval of Humira (adalimumab) Website Form www.highmarkhealthoptions.com Submit request via: Fax - 1-855-476-4158 All requests for Humira (adalimumab) require a prior
Prior Authorization Conditions for Approval of Humira (adalimumab) Website Form www.highmarkhealthoptions.com Submit request via: Fax - 1-855-476-4158 All requests for Humira (adalimumab) require a prior
Interim Clinical Commissioning Policy Statement: Adalimumab for Severe Refractory Uveitis. Reference: NHS England: /PS
 Interim Clinical Commissioning Policy Statement: Adalimumab for Severe Refractory Uveitis Reference: NHS England: 170010/PS Publications Gateway Reference: Document Purpose Policy 05527s Document Name
Interim Clinical Commissioning Policy Statement: Adalimumab for Severe Refractory Uveitis Reference: NHS England: 170010/PS Publications Gateway Reference: Document Purpose Policy 05527s Document Name
1 P a g e. Systemic Juvenile Idiopathic Arthritis (SJIA) (1.3) Patients 2 years of age and older with active systemic juvenile idiopathic arthritis.
 LENGTH OF AUTHORIZATION: Initial: 3 months for Crohn s or Ulcerative Colitis; 1 year for all other indications. Renewal: 1 year dependent upon medical records supporting response to therapy and review
LENGTH OF AUTHORIZATION: Initial: 3 months for Crohn s or Ulcerative Colitis; 1 year for all other indications. Renewal: 1 year dependent upon medical records supporting response to therapy and review
Technology appraisal guidance Published: 26 July 2017 nice.org.uk/guidance/ta460
 Adalimumab and dexamethasone for treating non-infectious uveitis Technology appraisal guidance Published: 26 July 2017 nice.org.uk/guidance/ta460 NICE 2017. All rights reserved. Subject to Notice of rights
Adalimumab and dexamethasone for treating non-infectious uveitis Technology appraisal guidance Published: 26 July 2017 nice.org.uk/guidance/ta460 NICE 2017. All rights reserved. Subject to Notice of rights
and eyes and have also looked at histocompatibility and 1980 were identified as having had either rate (ESR) and all ANA results were noted. ANA.
 Archives of Disease in Childhood, 1986, 61, 168-172 Antinuclear antibody studies in juvenile chronic arthritis A M LEAK, B M ANSELL, AND S J BURMAN Division of Rheumatology, Canadian Red Cross Memorial
Archives of Disease in Childhood, 1986, 61, 168-172 Antinuclear antibody studies in juvenile chronic arthritis A M LEAK, B M ANSELL, AND S J BURMAN Division of Rheumatology, Canadian Red Cross Memorial
Sarcoidosis: is there a role for anti-tnf-α?
 Sarcoidosis: is there a role for anti-tnf-α? Abstract In severe cases of sarcoidosis treatment can be very difficult. The common treatment strategies might be failing. Tumour necrosis factor (TNF) α therapy
Sarcoidosis: is there a role for anti-tnf-α? Abstract In severe cases of sarcoidosis treatment can be very difficult. The common treatment strategies might be failing. Tumour necrosis factor (TNF) α therapy
Horizon Scanning Technology Summary. Abatacept (Orencia) for juvenile idiopathic arthritis. National Horizon Scanning Centre.
 Horizon Scanning Technology Summary National Horizon Scanning Centre Abatacept (Orencia) for juvenile idiopathic arthritis June 2007 This technology summary is based on information available at the time
Horizon Scanning Technology Summary National Horizon Scanning Centre Abatacept (Orencia) for juvenile idiopathic arthritis June 2007 This technology summary is based on information available at the time
Review Article Gender and Spondyloarthropathy-Associated Uveitis
 Ophthalmology Volume 2013, Article ID 928264, 6 pages http://dx.doi.org/10.1155/2013/928264 Review Article Gender and Spondyloarthropathy-Associated Uveitis Wendy M. Smith MayoClinic,200FirstStreetSW,Rochester,MN55905,USA
Ophthalmology Volume 2013, Article ID 928264, 6 pages http://dx.doi.org/10.1155/2013/928264 Review Article Gender and Spondyloarthropathy-Associated Uveitis Wendy M. Smith MayoClinic,200FirstStreetSW,Rochester,MN55905,USA
Primary Results Citation 2
 Table S1. Adalimumab clinical trials 1 ClinicalTrials.gov Rheumatoid Arthritis 3 NCT00195663 Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study. A multicenter, randomized, double-blind clinical
Table S1. Adalimumab clinical trials 1 ClinicalTrials.gov Rheumatoid Arthritis 3 NCT00195663 Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study. A multicenter, randomized, double-blind clinical
Technology appraisal guidance Published: 16 December 2015 nice.org.uk/guidance/ta373
 Abatacept, adalimumab, etanercept and tocilizumab for treating juvenile idiopathic arthritis Technology appraisal guidance Published: 16 December 2015 nice.org.uk/guidance/ta373 NICE 2017. All rights reserved.
Abatacept, adalimumab, etanercept and tocilizumab for treating juvenile idiopathic arthritis Technology appraisal guidance Published: 16 December 2015 nice.org.uk/guidance/ta373 NICE 2017. All rights reserved.
Importance of Recognizing and Preventing Blindness From Juvenile Idiopathic Arthritis Associated Uveitis
 Arthritis Care & Research Vol. 64, No. 5, May 2012, pp 653 657 DOI 10.1002/acr.21599 2012, American College of Rheumatology REVIEW ARTICLE Importance of Recognizing and Preventing Blindness From Juvenile
Arthritis Care & Research Vol. 64, No. 5, May 2012, pp 653 657 DOI 10.1002/acr.21599 2012, American College of Rheumatology REVIEW ARTICLE Importance of Recognizing and Preventing Blindness From Juvenile
Management of uveitis
 Management of uveitis DR. ANUPAMA KARANTH Anti-inflammatory agents -itis = inflammation Treatment : stop inflammation Use anti-inflammatory drugs Most potent of such agents : Corticosteroids Corticosteroids
Management of uveitis DR. ANUPAMA KARANTH Anti-inflammatory agents -itis = inflammation Treatment : stop inflammation Use anti-inflammatory drugs Most potent of such agents : Corticosteroids Corticosteroids
Acute Retinal Necrosis Secondary to Varicella Zoster Virus in an Immunosuppressed Post-Kidney Transplant Patient
 CM&R Rapid Release. Published online ahead of print September 20, 2012 as Aperture Acute Retinal Necrosis Secondary to Varicella Zoster Virus in an Immunosuppressed Post-Kidney Transplant Patient Elizabeth
CM&R Rapid Release. Published online ahead of print September 20, 2012 as Aperture Acute Retinal Necrosis Secondary to Varicella Zoster Virus in an Immunosuppressed Post-Kidney Transplant Patient Elizabeth
Ontario Public Drug Programs. Inflectra (infliximab) Frequently Asked Questions
 Ontario Public Drug Programs Inflectra (infliximab) Frequently Asked Questions 1. What is the funding status of Inflectra (infliximab)? Effective February 25 2016, Inflectra (infliximab) will be added
Ontario Public Drug Programs Inflectra (infliximab) Frequently Asked Questions 1. What is the funding status of Inflectra (infliximab)? Effective February 25 2016, Inflectra (infliximab) will be added
Humira. (adalimumab) Drug Update Slideshow NEW INDICATION
 Humira (adalimumab) NEW INDICATION Drug Update Slideshow Introduction Brand name: Humira Generic name: Adalimumab Pharmacological class: Tumor necrosis factor (TNF) blocker Strength and Formulation: 10mg/0.2mL,
Humira (adalimumab) NEW INDICATION Drug Update Slideshow Introduction Brand name: Humira Generic name: Adalimumab Pharmacological class: Tumor necrosis factor (TNF) blocker Strength and Formulation: 10mg/0.2mL,
Outcomes of non-infectious Paediatric uveitis in the era of biologic therapy
 Cann et al. Pediatric Rheumatology (2018) 16:51 https://doi.org/10.1186/s12969-018-0266-5 RESEARCH ARTICLE Outcomes of non-infectious Paediatric uveitis in the era of biologic therapy Open Access Megan
Cann et al. Pediatric Rheumatology (2018) 16:51 https://doi.org/10.1186/s12969-018-0266-5 RESEARCH ARTICLE Outcomes of non-infectious Paediatric uveitis in the era of biologic therapy Open Access Megan
Choroidal Neovascularization in Sympathetic Ophthalmia
 Choroidal Neovascularization in Sympathetic Ophthalmia Lucia Sobrin, Miguel Cordero Coma, C. Stephen Foster Case Report A 49-year-old man presented after a ruptured globe repair of his left eye status
Choroidal Neovascularization in Sympathetic Ophthalmia Lucia Sobrin, Miguel Cordero Coma, C. Stephen Foster Case Report A 49-year-old man presented after a ruptured globe repair of his left eye status
Literature Review. Biological therapy in treatment of uveitis. Contemporary trends. A.V. Zborovskaia, Dr Sc (Med), A.E. Dorokhova, Cand Sc (Med)
 Literature Review Biological therapy in treatment of uveitis. Contemporary trends A.V. Zborovskaia, Dr Sc (Med), A.E. Dorokhova, Cand Sc (Med) Filatov Institute of Eye Diseases and Tissue Therapy of NAMS
Literature Review Biological therapy in treatment of uveitis. Contemporary trends A.V. Zborovskaia, Dr Sc (Med), A.E. Dorokhova, Cand Sc (Med) Filatov Institute of Eye Diseases and Tissue Therapy of NAMS
Amjevita (adalimumab-atto) CG-DRUG-64, CG-DRUG-65
 Market DC Amjevita (adalimumab-atto) CG-DRUG-64, CG-DRUG-65 Override(s) Prior Authorization Quantity Limit Medications Amjevita 20 mg/0.4 ml prefilled syringe Amjevita (adalimumab-atto) 40 mg/0.8 ml 2
Market DC Amjevita (adalimumab-atto) CG-DRUG-64, CG-DRUG-65 Override(s) Prior Authorization Quantity Limit Medications Amjevita 20 mg/0.4 ml prefilled syringe Amjevita (adalimumab-atto) 40 mg/0.8 ml 2
o White dot syndromes pattern recognition o Activity and damage o Quality of life o Key points o Idiopathic o Sarcoidosis o Multiple sclerosis
 Introduction Clinical Assessment of Posterior Uveitis Philip I. Murray Centre for Translational Inflammation Research University of Birmingham Birmingham and Midland Eye Centre o Classification of uveitis
Introduction Clinical Assessment of Posterior Uveitis Philip I. Murray Centre for Translational Inflammation Research University of Birmingham Birmingham and Midland Eye Centre o Classification of uveitis
Scleritis LEN V KOH OD
 Scleritis LEN V KOH OD 2014 PUCO 1 Introduction A painful, destructive, and potentially blinding disorder Highly symptomatic High association with systemic disease Immunosuppresssive agents 2014 PUCO 2
Scleritis LEN V KOH OD 2014 PUCO 1 Introduction A painful, destructive, and potentially blinding disorder Highly symptomatic High association with systemic disease Immunosuppresssive agents 2014 PUCO 2
Amjevita (adalimumab-atto)
 *- Florida Healthy Kids Amjevita (adalimumab-atto) Override(s) Prior Authorization Quantity Limit Medications Amjevita 20 mg/0.4 ml prefilled syringe Amjevita (adalimumab-atto) 40 mg/0.8 ml 2 #* ^ prefilled
*- Florida Healthy Kids Amjevita (adalimumab-atto) Override(s) Prior Authorization Quantity Limit Medications Amjevita 20 mg/0.4 ml prefilled syringe Amjevita (adalimumab-atto) 40 mg/0.8 ml 2 #* ^ prefilled
1. Background: Infliximab is administered parenterally; therefore, it is not covered under retail pharmacy benefits.
 Subject: Infliximab (Remicade ) Original Original Committee Approval: October 13, 2006 Revised Last Committee Approval: December 3, 2008 Last Review: October 19, 2007 1. Background: Infliximab is a genetically
Subject: Infliximab (Remicade ) Original Original Committee Approval: October 13, 2006 Revised Last Committee Approval: December 3, 2008 Last Review: October 19, 2007 1. Background: Infliximab is a genetically
Moncef Khairallah, MD
 Moncef Khairallah, MD Department of Ophthalmology, Fattouma Bourguiba University Hospital Faculty of Medicine, University of Monastir Monastir, Tunisia INTRODUCTION IU: anatomic form of uveitis involving
Moncef Khairallah, MD Department of Ophthalmology, Fattouma Bourguiba University Hospital Faculty of Medicine, University of Monastir Monastir, Tunisia INTRODUCTION IU: anatomic form of uveitis involving
Remicade. Remicade (infliximab), Inflectra (infliximab-dyyb) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Remicade Page: 1 of 9 Last Review Date: June 22, 2017 Remicade Description Remicade (infliximab),
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Remicade Page: 1 of 9 Last Review Date: June 22, 2017 Remicade Description Remicade (infliximab),
ISPUB.COM. An Atypical Presentation of Posterior Scleritis. A Ramanathan, A Gaur CASE REPORT
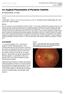 ISPUB.COM The Internet Journal of Ophthalmology and Visual Science Volume 8 Number 2 A Ramanathan, A Gaur Citation A Ramanathan, A Gaur.. The Internet Journal of Ophthalmology and Visual Science. 2009
ISPUB.COM The Internet Journal of Ophthalmology and Visual Science Volume 8 Number 2 A Ramanathan, A Gaur Citation A Ramanathan, A Gaur.. The Internet Journal of Ophthalmology and Visual Science. 2009
E.V. Gaidar, M.M. Kostik, M.F. Dubko, V.V. Masalova, L.S. Snegireva, E.A. Isupova, T.N. Nikitina, E.D. Serogodskaya, O.V. Kalashnikova, V.G.
 E.V. Gaidar, M.M. Kostik, M.F. Dubko, V.V. Masalova, L.S. Snegireva, E.A. Isupova, T.N. Nikitina, E.D. Serogodskaya, O.V. Kalashnikova, V.G. Chasnyk St. Petersburg State Pediatric Medical University, St.
E.V. Gaidar, M.M. Kostik, M.F. Dubko, V.V. Masalova, L.S. Snegireva, E.A. Isupova, T.N. Nikitina, E.D. Serogodskaya, O.V. Kalashnikova, V.G. Chasnyk St. Petersburg State Pediatric Medical University, St.
TRANSPARENCY COMMITTEE. Opinion. 29 November 2006
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 29 November 2006 HEXATRIONE 2% suspension for injection (intra-articular) Box containing one 2-ml vial - CIP code:
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 29 November 2006 HEXATRIONE 2% suspension for injection (intra-articular) Box containing one 2-ml vial - CIP code:
Remicade. Remicade (infliximab), Inflectra (infliximab-dyyb) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.02 Subsection: Gastrointestinal nts Original Policy Date: May 20, 2011 Subject: Remicade Page: 1 of
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.02 Subsection: Gastrointestinal nts Original Policy Date: May 20, 2011 Subject: Remicade Page: 1 of
Humira (adalimumab) DRUG.00002
 Humira (adalimumab) DRUG.00002 Override(s) Prior Authorization Quantity Limit Approval Duration 1 year Medications Humira 10 mg/0.2 ml syringe Humira pediatric Crohn s Disease starter pack 40 mg/0.8 ml
Humira (adalimumab) DRUG.00002 Override(s) Prior Authorization Quantity Limit Approval Duration 1 year Medications Humira 10 mg/0.2 ml syringe Humira pediatric Crohn s Disease starter pack 40 mg/0.8 ml
ACTEMRA (tocilizumab)
 RATIONALE FOR INCLUSION IN PA PROGRAM Background Actemra is an agent in the class of drugs known as biologic disease modifiers. It is used to treat adult onset rheumatoid (RA) arthritis, polyarticular
RATIONALE FOR INCLUSION IN PA PROGRAM Background Actemra is an agent in the class of drugs known as biologic disease modifiers. It is used to treat adult onset rheumatoid (RA) arthritis, polyarticular
Pharmacy Prior Authorization
 Pharmacy Prior Authorization AETA BETTER HEALTH PESLVAIA & AETA BETTER HEALTH KIDS Humira (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information,
Pharmacy Prior Authorization AETA BETTER HEALTH PESLVAIA & AETA BETTER HEALTH KIDS Humira (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information,
4. Behçet s - Treatment
 Registered Charity No: 326679 Caring for those with a rare, complex and lifelong disease www.behcets.org.uk 4. Behçet s - Treatment Introduction Because Behçet s Syndrome/Disease is a multisystem disorder,
Registered Charity No: 326679 Caring for those with a rare, complex and lifelong disease www.behcets.org.uk 4. Behçet s - Treatment Introduction Because Behçet s Syndrome/Disease is a multisystem disorder,
ETANERCEPT Generic Brand HICL GCN Exception/Other ETANERCEPT ENBREL GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW)
 Generic Brand HICL GCN Exception/Other ETANERCEPT ENBREL 18830 GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Does the patient have a diagnosis of moderate to severe rheumatoid
Generic Brand HICL GCN Exception/Other ETANERCEPT ENBREL 18830 GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Does the patient have a diagnosis of moderate to severe rheumatoid
Prednisolone-2 I -stearoylglycolate in scleritis
 Brit. j. Ophthal. (1970) 54, 394 Prednisolone- -stearoylglycolate in scleritis S. S. HAYREH* AND P. G. WATSON Scleritis Clinic, Moorfields Eye Hospital, City Road, London, E.C. i Scleritis is one of the
Brit. j. Ophthal. (1970) 54, 394 Prednisolone- -stearoylglycolate in scleritis S. S. HAYREH* AND P. G. WATSON Scleritis Clinic, Moorfields Eye Hospital, City Road, London, E.C. i Scleritis is one of the
Remicade (infliximab) DRUG.00002
 Applicability/Effective Date *- Florida Healthy Kids Remicade (infliximab) DRUG.00002 Override(s) Prior Authorization Step Therapy Medications Remicade (infliximab) Approval Duration 1 year Comment Intravenous
Applicability/Effective Date *- Florida Healthy Kids Remicade (infliximab) DRUG.00002 Override(s) Prior Authorization Step Therapy Medications Remicade (infliximab) Approval Duration 1 year Comment Intravenous
Overview of Paediatric Investigation Plan (PIP) in Paediatric Rheumatology
 Overview of Paediatric Investigation Plan (PIP) in Paediatric Rheumatology Paediatric Rheumatology Expert Meeting, London 4 th December 29 Dr. Richard Veselý, Dr. Emma Sala Soriano Paediatric Investigation
Overview of Paediatric Investigation Plan (PIP) in Paediatric Rheumatology Paediatric Rheumatology Expert Meeting, London 4 th December 29 Dr. Richard Veselý, Dr. Emma Sala Soriano Paediatric Investigation
CIBMTR Center Number: CIBMTR Recipient ID: RETIRED. Today s Date: Date of HSCT for which this form is being completed:
 Juvenile Idiopathic Arthritis Pre-HSCT Data Sequence Number: Date Received: Registry Use Only Today s Date: Date of HSCT for which this form is being completed: HSCT type: autologous allogeneic, allogeneic,
Juvenile Idiopathic Arthritis Pre-HSCT Data Sequence Number: Date Received: Registry Use Only Today s Date: Date of HSCT for which this form is being completed: HSCT type: autologous allogeneic, allogeneic,
ERROR CORRECTION FORM
 Juvenile Idiopathic Arthritis Pre-HSCT Data Sequence Number: Registry Use Only Date of HSCT for which this form is being completed: HSCT type: autologous allogeneic, allogeneic, syngeneic unrelated related
Juvenile Idiopathic Arthritis Pre-HSCT Data Sequence Number: Registry Use Only Date of HSCT for which this form is being completed: HSCT type: autologous allogeneic, allogeneic, syngeneic unrelated related
HLA-B27-related anterior Uveitis
 HLA-B27-related anterior Uveitis Nicholas Jones Manchester Uveitis Clinic The Royal Eye Hospital Manchester Anterior means anterior only IUSG classification: Anterior uveitis = Iris & pars plicata AU
HLA-B27-related anterior Uveitis Nicholas Jones Manchester Uveitis Clinic The Royal Eye Hospital Manchester Anterior means anterior only IUSG classification: Anterior uveitis = Iris & pars plicata AU
I ntermediate uveitis is a chronic relapsing condition
 412 EXTENDED REPORT Systemic CD4 + T cell phenotype and activation status in intermediate uveitis C C Murphy, L Duncan, J V Forrester, A D Dick... See end of article for authors affiliations... Correspondence
412 EXTENDED REPORT Systemic CD4 + T cell phenotype and activation status in intermediate uveitis C C Murphy, L Duncan, J V Forrester, A D Dick... See end of article for authors affiliations... Correspondence
Clinical Commissioning Policy: Infliximab (Remicade) as Anti-TNF Alpha Treatment Option for Paediatric Patients with Severe Refractory Uveitis
 Clinical Commissioning Policy: Infliximab (Remicade) as Anti-TNF Alpha Treatment Option for Paediatric Patients with Severe Refractory Uveitis Reference: NHS England D12/P/b NHS England INFORMATION READER
Clinical Commissioning Policy: Infliximab (Remicade) as Anti-TNF Alpha Treatment Option for Paediatric Patients with Severe Refractory Uveitis Reference: NHS England D12/P/b NHS England INFORMATION READER
Juvenile idiopathic arthritis managed in the new millennium: one year outcomes of an inception cohort of Australian children
 Tiller et al. Pediatric Rheumatology (2018) 16:69 https://doi.org/10.1186/s12969-018-0288-z RESEARCH ARTICLE Open Access Juvenile idiopathic arthritis managed in the new millennium: one year outcomes of
Tiller et al. Pediatric Rheumatology (2018) 16:69 https://doi.org/10.1186/s12969-018-0288-z RESEARCH ARTICLE Open Access Juvenile idiopathic arthritis managed in the new millennium: one year outcomes of
American Journal of Therapeutics
 American Journal of Therapeutics Golimumab may induce exacerbation of inflammatory bowel disease when it is used for the treatment of ankylosing spondylitis: A case report with a review of literature.
American Journal of Therapeutics Golimumab may induce exacerbation of inflammatory bowel disease when it is used for the treatment of ankylosing spondylitis: A case report with a review of literature.
Screening for Uveitis in Children
 Information for patients and parents Manchester Royal Eye Hospital Paediatric Uveitis Service Screening for Uveitis in Children What is uveitis? Uveitis is inflammation of a layer of the eye, called the
Information for patients and parents Manchester Royal Eye Hospital Paediatric Uveitis Service Screening for Uveitis in Children What is uveitis? Uveitis is inflammation of a layer of the eye, called the
Effective Health Care Program
 Comparative Effectiveness Review Number 28 Effective Health Care Program Disease-Modifying Antirheumatic Drugs (DMARDs) in Children With Juvenile Idiopathic Arthritis (JIA) Executive Summary Background
Comparative Effectiveness Review Number 28 Effective Health Care Program Disease-Modifying Antirheumatic Drugs (DMARDs) in Children With Juvenile Idiopathic Arthritis (JIA) Executive Summary Background
Report on the Deliberation Results
 Report on the Deliberation Results September 14, 216 Pharmaceutical Evaluation Division, Pharmaceutical Safety and Environmental Health Bureau Ministry of Health, Labour and Welfare Brand Name (a) Humira
Report on the Deliberation Results September 14, 216 Pharmaceutical Evaluation Division, Pharmaceutical Safety and Environmental Health Bureau Ministry of Health, Labour and Welfare Brand Name (a) Humira
Uveitis / Iritis. Introduction. Other formats
 Other formats Introduction Uveitis / Iritis If you need this information in another format such as audio tape or computer disk, Braille, large print, high contrast, British Sign Language or translated
Other formats Introduction Uveitis / Iritis If you need this information in another format such as audio tape or computer disk, Braille, large print, high contrast, British Sign Language or translated
Case 1 History. William Tremaine, M.D. CP
 Extraintestinal Manifestations of IBD Case Studies William Tremaine, M.D. Case 1 History 18 year-old woman with Crohn s disease Onset at age 5: colonic & perianal Sulfasalazine, prednisone, mercaptopurine
Extraintestinal Manifestations of IBD Case Studies William Tremaine, M.D. Case 1 History 18 year-old woman with Crohn s disease Onset at age 5: colonic & perianal Sulfasalazine, prednisone, mercaptopurine
Certolizumab pegol (Cimzia) for psoriatic arthritis second line
 Certolizumab pegol (Cimzia) for psoriatic arthritis second line This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
Certolizumab pegol (Cimzia) for psoriatic arthritis second line This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
Uveitis is a common cause of preventable blindness
 Treatment of Uveitic Cystoid Macular Edema Three case reports discuss the management of inflammatory CME. BY PRANJAL THAKURIA, MD; AND C. STEPHEN FOSTER, MD Uveitis is a common cause of preventable blindness
Treatment of Uveitic Cystoid Macular Edema Three case reports discuss the management of inflammatory CME. BY PRANJAL THAKURIA, MD; AND C. STEPHEN FOSTER, MD Uveitis is a common cause of preventable blindness
UnitedHealthcare Pharmacy Clinical Pharmacy Programs
 UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2017 P 1041-8 Program Prior Authorization/Notification Medication Humira (adalimumab) P&T Approval Date 1/2007, 6/2008, 4/2009, 6/2009,
UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2017 P 1041-8 Program Prior Authorization/Notification Medication Humira (adalimumab) P&T Approval Date 1/2007, 6/2008, 4/2009, 6/2009,
London, 1 June 2006 Product name: REMICADE Procedure number: Remicade-H-240-II-73-AR SCIENTIFIC DISCUSSION 1/8
 London, 1 June 2006 Product name: REMICADE Procedure number: Remicade-H-240-II-73-AR SCIENTIFIC DISCUSSION 1/8 1. Introduction Infliximab is a chimeric human-murine IgG1κ monoclonal antibody, which binds
London, 1 June 2006 Product name: REMICADE Procedure number: Remicade-H-240-II-73-AR SCIENTIFIC DISCUSSION 1/8 1. Introduction Infliximab is a chimeric human-murine IgG1κ monoclonal antibody, which binds
Infliximab/Infliximab-dyyb DRUG.00002
 Infliximab/Infliximab-dyyb DRUG.00002 Override(s) Prior Authorization Step Therapy Medications Remicade (infliximab) Inflectra (inflectra-dyyb) Approval Duration 1 year Comment Intravenous administration
Infliximab/Infliximab-dyyb DRUG.00002 Override(s) Prior Authorization Step Therapy Medications Remicade (infliximab) Inflectra (inflectra-dyyb) Approval Duration 1 year Comment Intravenous administration
Inflectra (infliximab-dyyb), Remicade (infliximab), Renflexis (infliximab-abda) DRUG CG-DRUG-64
 Inflectra (infliximab-dyyb), Remicade (infliximab), Renflexis (infliximab-abda) DRUG.00002 CG-DRUG-64 Override(s) Prior Authorization *Washington Medicaid See State Specific Mandates Medications Inflectra
Inflectra (infliximab-dyyb), Remicade (infliximab), Renflexis (infliximab-abda) DRUG.00002 CG-DRUG-64 Override(s) Prior Authorization *Washington Medicaid See State Specific Mandates Medications Inflectra
Understanding Myositis Medications
 Understanding Myositis Medications 2015 TMA Annual Patient Conference Orlando, Florida Chester V. Oddis, MD University of Pittsburgh Director, Myositis Center Disclosures Mallinckrodt: Research Grant Genentech:
Understanding Myositis Medications 2015 TMA Annual Patient Conference Orlando, Florida Chester V. Oddis, MD University of Pittsburgh Director, Myositis Center Disclosures Mallinckrodt: Research Grant Genentech:
What prescribers need to know
 HUMIRA Citrate-free presentations in an Electronic Medical Record (EMR) What prescribers need to know 2 / This is your guide to identifying HUMIRA Citrate-free presentations in your Electronic Medical
HUMIRA Citrate-free presentations in an Electronic Medical Record (EMR) What prescribers need to know 2 / This is your guide to identifying HUMIRA Citrate-free presentations in your Electronic Medical
What you can expect with OZURDEX
 Important Information About Noninfectious Uveitis Affecting the Back Segment of the Eye and Treatment What you can expect with OZURDEX Approved Use OZURDEX (dexamethasone intravitreal implant) is a prescription
Important Information About Noninfectious Uveitis Affecting the Back Segment of the Eye and Treatment What you can expect with OZURDEX Approved Use OZURDEX (dexamethasone intravitreal implant) is a prescription
Scottish Medicines Consortium
 Scottish Medicines Consortium infliximab 100mg powder for intravenous infusion (Remicade ) No. (364/07) Schering-Plough UK Ltd 6 April 2007 The Scottish Medicines Consortium (SMC) has completed its assessment
Scottish Medicines Consortium infliximab 100mg powder for intravenous infusion (Remicade ) No. (364/07) Schering-Plough UK Ltd 6 April 2007 The Scottish Medicines Consortium (SMC) has completed its assessment
