HARVARD PILGRIM HEALTH CARE RECOMMENDED MEDICATION REQUEST GUIDELINES
|
|
|
- Rhoda Francis
- 5 years ago
- Views:
Transcription
1 Generic Brand HICL GCN Exception/Other PEGLOTICASE KRYSTEXXA GUIDELINES FOR USE 1. Does the patient have a diagnosis of symptomatic chronic gout (prior to initiating Krystexxa therapy) with clinical features that may include the following? Tophi Gouty arthropathy Radiographic changes of gout Multiple joint involvement Associated uric acid nephrolithiasis If yes, continue to #2. only covered when prescribed for symptomatic chronic gout. Your physician did not indicate that you have a diagnosis of symptomatic chronic gout and therefore your request was not approved. 2. Has the provider confirmed that Krystexxa will NOT be used concomitantly with oral urate-lowering therapies? If yes, continue to #3. only covered if your provider confirms you will not be taking Krystexxa at the same time with oral urate-lowering therapies. Your physician did not confirm you will not be taking this medication with oral urate-lowering therapies and therefore your request was not approved. 3. Is the request for a renewal where the patient has had a previous prior authorization for Krystexxa and received at least a three-month supply (i.e., six doses) of Krystexxa with paid claims through the pharmacy or medical benefit within the previous 120 days? If yes, continue to #4. If no, continue to #5. Page 1
2 4. Did the provider submit documentation demonstrating that the patient has not had two consecutive uric acid levels above 6mg/dL since starting treatment with Krystexxa? If yes, approve for 6 months by HICL for two 1mL vials (2mL) per 28 days. Please use status code #056 and the following approval language: APPROVAL TEXT: Your request for Krystexxa has been approved two vials per 28 days for a 6-month period. DENIAL TEXT: Per your health plan's Krystexxa (pegloticase) guideline, authorization for renewal requires documentation showing that you did not have two consecutive uric acid levels above 6mg/dL since starting treatment with Krystexxa. Your physician [did not provide this information//or//indicated that you do not meet this criteria] and therefore your request was not approved. 5. Has the patient had an inadequate response or is there a clinical reason for not completing at least a three-month trial of allopurinol at the medically appropriate maximum dose as defined by ONE of the following? The patient experienced a severe allergic reaction to allopurinol The patient experienced toxicity with allopurinol The patient could not tolerate allopurinol The patient's current medication regimen has a significant drug interaction with allopurinol The patient has severe renal dysfunction If yes, continue to #6. a 3-month trial with allopurinol at the medically appropriate maximal dose as defined by one of the following: You experienced a severe allergic reaction to allopurinol You experienced toxicity with allopurinol You could not tolerate allopurinol Your current medication regimen has a significant drug interaction with allopurinol You have severe renal dysfunction Your physician did not indicate that you previously had a 3-month trial with allopurinol or there is a clinical reason why you cannot try it and therefore your request was not approved. Page 2
3 6. Has the patient had an inadequate response or is there a clinical reason for not completing at least a three-month trial of febuxostat (Uloric) at the medically appropriate maximum dose as defined by ONE of the following? The patient experienced a severe allergic reaction to Uloric (febuxostat) The patient experienced toxicity with Uloric (febuxostat) The patient could not tolerate Uloric (febuxostat) The patient's current medication regimen has a significant drug interaction with Uloric (febuxostat) If yes, continue to #7. a 3-month trial with febuxostat (Uloric) at the medically appropriate maximal dose as defined by one of the following: You experienced a severe allergic reaction to Uloric (febuxostat) You experienced toxicity with Uloric (febuxostat) You could not tolerate Uloric (febuxostat) Your current medication regimen has a significant drug interaction with Uloric (febuxostat) Your physician did not indicate that you previously had a 3-month trial with febuxostat (Uloric) or there is a clinical reason why you cannot try it and therefore your request was not approved. Page 3
4 7. Has the patient had an inadequate response or is there a clinical reason for not completing at least a three-month trial of probenecid alone, or in combination with allopurinol or febuxostat, at the medically appropriate maximum dose as defined by ONE of the following? The patient experienced a severe allergic reaction to probenecid The patient experienced toxicity with probenecid The patient could not tolerate probenecid The patient has known blood dyscrasias or uric acid kidney stones The patient's current medication regimen has a significant drug interaction with probenecid The patient have severe renal dysfunction (i.e., glomerular filtration rate of 30mL/minute or less) If yes, approve by HICL two 1mL vials (2mL) per 28 days for 6 months. Please use status code #056 and the following approval language: APPROVAL TEXT: Your request for Krystexxa has been approved two vials per 28 days for a 6-month period. a 3-month trial with probenecid alone, or in combination with allopurinol or febuxostat, at the medically appropriate maximal dose as defined by one of the following: You experienced a severe allergic reaction probenecid You experienced toxicity with probenecid You could not tolerate probenecid You have known blood dyscrasias or uric acid kidney stones Your current medication regimen has a significant drug interaction with probenecid You have severe renal dysfunction (i.e., glomerular filtration rate of 30mL/minute or less) Your physician did not indicate that you previously had a 3-month trial with probenecid or there is a clinical reason why you cannot try it and therefore your request was not approved. Page 4
5 RATIONALE Ensure appropriate utilization of Krystexxa for the treatment of chronic gout. FDA APPROVED INDICATIONS Krystexxa is a pegylated uric acid specific enzyme indicated for the treatment of chronic gout in adult patient's refractory to conventional therapy. REFERENCES Krystexxa [package insert]. Bridgewater, NJ: Savient Pharmaceuticals, Inc.; December DRUGDEX System (electronic version). Truven Health Analytics, Ann Arbor, Michigan. Available at Accessed May 4, Khanna D, Fitzgerald JD, Khanna PP, et al American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. 2012;64(10): Zhang W, Doherty M, Bardin T, et al. EULAR evidence based recommendations for gout. Part II: management. Report of a task force of the EULAR standing committee for international clinical studies including therapeutics (ESCISIT). Ann Rheum Dis. 2006;65: Khanna D, Khanna PP, Fitzgerald JD, et al American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res. 2012;64(10): Jordan KM, Cameron JS, Snaith M, et al. British Society of Rheumatology and British Health Professionals in Rheumatology guideline for the management of gout. Rheumatology. 2007;46(8): Sivera F, Andres M, Carmona L, et al. Multinational evidence-based recommendations for the diagnosis and management of gout: integrating systemic literature review and expert opinion of a broad panel of rheumatologists in the 3e initiative. Ann Rheum Dis. 2014;73(2): Clinical Consult: CVS Caremark Clinical Programs Review. Focus on Rheumatology Clinical Programs. July Probenecid [package insert]. Morgantown, WV: Mylan Pharmaceuticals Inc.; March Clinical Consult: CVS Caremark Clinical Programs Review. Focus on Rheumatology Clinical Programs. July Created: 06/17 Effective: 07/14/17 Client Approval: 06/30/17 P&T Approval: 09/11/17 Page 5
Krystexxa. Krystexxa (pegloticase) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.14 Subject: Krystexxa Page: 1 of 5 Last Review Date: March 16, 2018 Krystexxa Description Krystexxa
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.14 Subject: Krystexxa Page: 1 of 5 Last Review Date: March 16, 2018 Krystexxa Description Krystexxa
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Krystexxa) Reference Number: CP.PHAR.115 Effective Date: 06.01.13 Last Review Date: 02.19 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder
Clinical Policy: (Krystexxa) Reference Number: CP.PHAR.115 Effective Date: 06.01.13 Last Review Date: 02.19 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder
Krystexxa (pegloticase) Document Number: IC-0158
 Krystexxa (pegloticase) Document Number: IC-0158 Last Review Date: 06/27/2017 Date of Origin: 02/07/20103 Dates Reviewed: 11/2013, 08/2014, 07/2015, 07/2016, 09/2016, 12/2016, 03/2017, 06/2017 I. Length
Krystexxa (pegloticase) Document Number: IC-0158 Last Review Date: 06/27/2017 Date of Origin: 02/07/20103 Dates Reviewed: 11/2013, 08/2014, 07/2015, 07/2016, 09/2016, 12/2016, 03/2017, 06/2017 I. Length
Limitations of Use: (1) Duzallo is not recommended for the treatment of asymptomatic hyperuricemia.
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.63 Subject: Duzallo Page: 1 of 5 Last Review Date: December 8, 2017 Duzallo Description Duzallo (lesinurad
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.63 Subject: Duzallo Page: 1 of 5 Last Review Date: December 8, 2017 Duzallo Description Duzallo (lesinurad
MEDICAL POLICY PEGLOTICASE (KRYSTEXXA ) POLICY NUMBER MP POLICY TITLE. Original Issue Date (Created): January 1, 2011
 Original Issue Date (Created): January 1, 2011 Most Recent Review Date (Revised): September 24, 2013 Effective Date: November 1, 2013 I. POLICY PREAUTHORIZATION REQUIRED Note: Requests for pegloticase
Original Issue Date (Created): January 1, 2011 Most Recent Review Date (Revised): September 24, 2013 Effective Date: November 1, 2013 I. POLICY PREAUTHORIZATION REQUIRED Note: Requests for pegloticase
Uloric Step Therapy Program
 Uloric Step Therapy Program Policy Number: 5.01.584 Last Review: 7/2017 Origination: 7/2014 Next Review: 7/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for brand
Uloric Step Therapy Program Policy Number: 5.01.584 Last Review: 7/2017 Origination: 7/2014 Next Review: 7/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for brand
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Krystexxa) Reference Number: CP.CPA.57 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this policy
Clinical Policy: (Krystexxa) Reference Number: CP.CPA.57 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this policy
pegloticase (Krystexxa )
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Case presentation. serum uric acid = 11.5 mg/dl 24-hour uric acid excretion = 300 mg
 GOUT 55 y/o male 12 hours pain in my big toe & ankle went to bed last night feeling fine felt as if had broken toe this morning similar problems in right ankle & left wrist Case presentation lab studies
GOUT 55 y/o male 12 hours pain in my big toe & ankle went to bed last night feeling fine felt as if had broken toe this morning similar problems in right ankle & left wrist Case presentation lab studies
Subject: Krystexxa (pegloticase) Original Effective Date: 06/26/13. Policy Number: MCP-138. Revision Date(s):
 Subject: Krystexxa (pegloticase) Original Effective Date: 06/26/13 Policy Number: MCP-138 Revision Date(s): Review Date(s): 12/16/15; 6/15/2016; 3/21/2017 DISCLAIMER This Molina Clinical Policy (MCP) is
Subject: Krystexxa (pegloticase) Original Effective Date: 06/26/13 Policy Number: MCP-138 Revision Date(s): Review Date(s): 12/16/15; 6/15/2016; 3/21/2017 DISCLAIMER This Molina Clinical Policy (MCP) is
New Drug Evaluation: lesinurad tablet, oral
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Zurampic) Reference Number: CP.CPA.174 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this policy
Clinical Policy: (Zurampic) Reference Number: CP.CPA.174 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this policy
OBJECTIVES GOUT GOUTY INFLAMMATION 6/10/2016 GOUT INCIDENCE AND PREVALENCE MONOSODIUM URATE CRYSTAL DEPOSITION DISEASE
 GOUT Lisa Talbert, MD Family Medicine Update June 15, 2016 OBJECTIVES To be familiar with the clinical presentation and pathophysiology of gouty arthritis Be able to incorporate current guidelines when
GOUT Lisa Talbert, MD Family Medicine Update June 15, 2016 OBJECTIVES To be familiar with the clinical presentation and pathophysiology of gouty arthritis Be able to incorporate current guidelines when
Zurampic, Duzallo (lesinurad Products)
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
ZURAMPIC (lesinurad) oral tablet
 ZURAMPIC (lesinurad) oral tablet Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy Coverage
ZURAMPIC (lesinurad) oral tablet Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy Coverage
An update on the management of gout
 An update on the management of gout 8 The management of gout involves treatment of an acute attack, lifestyle modification and urate lowering treatment to achieve a target serum urate level. Recent evidence
An update on the management of gout 8 The management of gout involves treatment of an acute attack, lifestyle modification and urate lowering treatment to achieve a target serum urate level. Recent evidence
Clinical Policy: Colchicine (Colcrys) Reference Number: CP.PMN.123 Effective Date: Last Review Date: 05.18
 Clinical Policy: (Colcrys) Reference Number: CP.PMN.123 Effective Date: 05.01.11 Last Review Date: 05.18 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Colcrys) Reference Number: CP.PMN.123 Effective Date: 05.01.11 Last Review Date: 05.18 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
Literature Scan: Analgesics for Gout. Month/Year of Review: April 2015 Date of Last Review: January 2014
 Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301 1079 Phone 503 947 5220 Fax 503 947 1119 Copyright 2012 Oregon State University. All Rights
Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301 1079 Phone 503 947 5220 Fax 503 947 1119 Copyright 2012 Oregon State University. All Rights
Long-term Treatment of Gout: New Opportunities for Improved Outcomes
 Long-term Treatment of Gout: New Opportunities for Improved Outcomes Paul P. Doghramji, MD, FAAFP CONTINUING MEDICAL EDUCATION LEARNING OBJECTIVES Make a presumptive diagnosis of gout based on history
Long-term Treatment of Gout: New Opportunities for Improved Outcomes Paul P. Doghramji, MD, FAAFP CONTINUING MEDICAL EDUCATION LEARNING OBJECTIVES Make a presumptive diagnosis of gout based on history
HARVARD PILGRIM HEALTH CARE RECOMMENDED MEDICATION REQUEST GUIDELINES
 Generic Brand HICL GCN Exception/Other INSULIN REGULAR, HUMAN AFREZZA 37619, 37622, 37623, 38923, 37624, 42833, 38918, 37621 GUIDELINES FOR USE 1. Is the member currently taking the requested medication
Generic Brand HICL GCN Exception/Other INSULIN REGULAR, HUMAN AFREZZA 37619, 37622, 37623, 38923, 37624, 42833, 38918, 37621 GUIDELINES FOR USE 1. Is the member currently taking the requested medication
lamedicaid.com or calling the Recipient Eligibility Verification System (REVS) at
 Louisiana Medicaid Provider UPDATE Volume 29, Issue 4 July/August 2011 Medicaid Eligibility Card Changes Louisiana Medicaid has stopped issuing the pink Take Charge Medicaid eligibility card (MEC) for
Louisiana Medicaid Provider UPDATE Volume 29, Issue 4 July/August 2011 Medicaid Eligibility Card Changes Louisiana Medicaid has stopped issuing the pink Take Charge Medicaid eligibility card (MEC) for
HARVARD PILGRIM HEALTH CARE RECOMMENDED MEDICATION REQUEST GUIDELINES
 Generic Brand HICL GCN Exception/Other ONABOTULINUMTOXINA BOTOX 04867 BRAND BOTOX COSMETIC ABOBOTULINUMTOXINA DYSPORT 36477 RIMABOTULINUMTOXINB MYOBLOC 21869 INCOBOTULINUMTOXINA XEOMIN 36687 Please use
Generic Brand HICL GCN Exception/Other ONABOTULINUMTOXINA BOTOX 04867 BRAND BOTOX COSMETIC ABOBOTULINUMTOXINA DYSPORT 36477 RIMABOTULINUMTOXINB MYOBLOC 21869 INCOBOTULINUMTOXINA XEOMIN 36687 Please use
Rheumatoid arthritis, seronegative spondylarthritides and gout. György Nagy
 Rheumatoid arthritis, seronegative spondylarthritides and gout György Nagy Dec 4, 2017 Rheumatoid arthritis Rheumatoid arthritis Chronic, progressive, autoimmune disorder of the joints with extra-articular
Rheumatoid arthritis, seronegative spondylarthritides and gout György Nagy Dec 4, 2017 Rheumatoid arthritis Rheumatoid arthritis Chronic, progressive, autoimmune disorder of the joints with extra-articular
Retreatment with Pegloticase after a Gap in Therapy in Patients with Gout: A Report of Four Cases
 Rheumatol Ther (2018) 5:583 594 https://doi.org/10.1007/s40744-018-0111-9 CASE SERIES Retreatment with Pegloticase after a Gap in Therapy in Patients with Gout: A Report of Four Cases Allan H. Morton.
Rheumatol Ther (2018) 5:583 594 https://doi.org/10.1007/s40744-018-0111-9 CASE SERIES Retreatment with Pegloticase after a Gap in Therapy in Patients with Gout: A Report of Four Cases Allan H. Morton.
Long-term safety of pegloticase in chronic gout refractory to conventional treatment
 University of Massachusetts Medical School escholarship@umms Meyers Primary Care Institute Publications and Presentations Meyers Primary Care Institute 11-10-2012 Long-term safety of pegloticase in chronic
University of Massachusetts Medical School escholarship@umms Meyers Primary Care Institute Publications and Presentations Meyers Primary Care Institute 11-10-2012 Long-term safety of pegloticase in chronic
HARVARD PILGRIM HEALTH CARE RECOMMENDED MEDICATION REQUEST GUIDELINES PROCRIT METHOXY PEG-EPOETIN BETA MIRCERA 35005
 Generic Brand HICL GCN Exception/Other DARBEPOETIN ALFA IN ARANESP 22890 POLYSORBATE EPOETIN ALFA EPOGEN, 04553 PROCRIT METHOXY PEG-EPOETIN BETA MIRCERA 35005 GUIDELINES FOR USE NOTE: Requirements regarding
Generic Brand HICL GCN Exception/Other DARBEPOETIN ALFA IN ARANESP 22890 POLYSORBATE EPOETIN ALFA EPOGEN, 04553 PROCRIT METHOXY PEG-EPOETIN BETA MIRCERA 35005 GUIDELINES FOR USE NOTE: Requirements regarding
HARVARD PILGRIM HEALTH CARE RECOMMENDED MEDICATION REQUEST GUIDELINES
 Generic Brand HICL GCN Exception/Other EFINACONAZOLE JUBLIA 41184 TAVABOROLE KERYDIN 41353 GUIDELINES FOR USE 1. Does the patient have a diagnosis of onychomycosis of the fingernail or toenail? If yes,
Generic Brand HICL GCN Exception/Other EFINACONAZOLE JUBLIA 41184 TAVABOROLE KERYDIN 41353 GUIDELINES FOR USE 1. Does the patient have a diagnosis of onychomycosis of the fingernail or toenail? If yes,
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Lesinurad (Zurampic), Lesinurad/Allopurinol (Duzallo) Reference Number: CP.PMN.150 Effective Date: 11.16.16 Last Review Date: 08.18 Line of Business: Commercial, Medicaid See Important
Clinical Policy: Lesinurad (Zurampic), Lesinurad/Allopurinol (Duzallo) Reference Number: CP.PMN.150 Effective Date: 11.16.16 Last Review Date: 08.18 Line of Business: Commercial, Medicaid See Important
Clinical Policy: Colchicine (Colcrys) Reference Number: CP.PPA.11. Line of Business: Medicaid
 Clinical Policy: (Colcrys) Reference Number: CP.PPA.11 Effective Date: 05/11 Last Review Date: 05/176 Line of Business: Medicaid Coding Implications Revision Log See Important Reminder at the end of this
Clinical Policy: (Colcrys) Reference Number: CP.PPA.11 Effective Date: 05/11 Last Review Date: 05/176 Line of Business: Medicaid Coding Implications Revision Log See Important Reminder at the end of this
New Drug Evaluation: lesinurad tablet, oral
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
HARVARD PILGRIM HEALTH CARE RECOMMENDED MEDICATION REQUEST GUIDELINES
 Generic Brand HICL GCN Exception/Other GOLIMUMAB SIMPONI 22533, 22536, 34697, 35001 ROUTE = SUBCUTANE. GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Is the request for a
Generic Brand HICL GCN Exception/Other GOLIMUMAB SIMPONI 22533, 22536, 34697, 35001 ROUTE = SUBCUTANE. GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Is the request for a
HARVARD PILGRIM HEALTH CARE RECOMMENDED MEDICATION REQUEST GUIDELINES
 Generic Brand HICL GCN Exception/Other CERTOLIZUMAB PEGOL CIMZIA 35554 GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Is the request for a patient with a diagnosis of moderate
Generic Brand HICL GCN Exception/Other CERTOLIZUMAB PEGOL CIMZIA 35554 GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Is the request for a patient with a diagnosis of moderate
Drugs Used to Treat Gout. Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia
 Drugs Used to Treat Gout Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Gout is a metabolic disease characterized by recurrent episodes of acute arthritis
Drugs Used to Treat Gout Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia Gout is a metabolic disease characterized by recurrent episodes of acute arthritis
HARVARD PILGRIM HEALTH CARE RECOMMENDED MEDICATION REQUEST GUIDELINES
 Generic Brand HICL GCN Exception/Other DEFLAZACORT EMFLAZA 11668 If the caller wishes to initiate a request then a MRF must be completed. This drug requires a written request for prior authorization. All
Generic Brand HICL GCN Exception/Other DEFLAZACORT EMFLAZA 11668 If the caller wishes to initiate a request then a MRF must be completed. This drug requires a written request for prior authorization. All
SUMMACARE COMMERCIAL MEDICATION REQUEST GUIDELINES
 INTRON A () Generic Brand HICL GCN Exception/Other, RECOMB. INTRON A 04528 This drug requires a written request for prior authorization. All requests for medications used to treat hepatitis C require review
INTRON A () Generic Brand HICL GCN Exception/Other, RECOMB. INTRON A 04528 This drug requires a written request for prior authorization. All requests for medications used to treat hepatitis C require review
Clinical Study Urate Lowering Therapy with Febuxostat in Daily Practice A Multicentre, Open-Label, Prospective Observational Study
 International Rheumatology, Article ID 123105, 6 pages http://dx.doi.org/10.1155/2014/123105 Clinical Study Urate Lowering Therapy with Febuxostat in Daily Practice A Multicentre, Open-Label, Prospective
International Rheumatology, Article ID 123105, 6 pages http://dx.doi.org/10.1155/2014/123105 Clinical Study Urate Lowering Therapy with Febuxostat in Daily Practice A Multicentre, Open-Label, Prospective
COPYRIGHT. Update in Internal Medicine December 4, 2016
 Update in Internal Medicine December 4, 2016 Fadi Badlissi, MD, MSc Director of the Musculoskeletal Medicine Unit The Orthopedic Department & Rheumatology Division Beth Israel Deaconess Medical Center
Update in Internal Medicine December 4, 2016 Fadi Badlissi, MD, MSc Director of the Musculoskeletal Medicine Unit The Orthopedic Department & Rheumatology Division Beth Israel Deaconess Medical Center
Class Update: Drugs for Gout
 Copyright 2012 Oregon State University. ll Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. ll Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Professional organisation statement template
 Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can provide a unique perspective on the technology
Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can provide a unique perspective on the technology
ACP Rheumatology Pearls. Adam Q Carlson MD Assistant Professor UVA Rheumatology
 ACP Rheumatology Pearls Adam Q Carlson MD Assistant Professor UVA Rheumatology Disclosures I have no personal or professional disclosures Case #1 27 yo woman with a history of systemic lupus complicated
ACP Rheumatology Pearls Adam Q Carlson MD Assistant Professor UVA Rheumatology Disclosures I have no personal or professional disclosures Case #1 27 yo woman with a history of systemic lupus complicated
A Patient s Guide to Gout. Foot and Ankle Center of Massachusetts, P.C.
 A Patient s Guide to Gout Welcome to Foot and Ankle Center of Massachusetts, where we believe in accelerating your learning curve with educational materials that are clearly written and professionally
A Patient s Guide to Gout Welcome to Foot and Ankle Center of Massachusetts, where we believe in accelerating your learning curve with educational materials that are clearly written and professionally
Urate Lowering Efficacy of Febuxostat Versus Allopurinol in Hyperuricemic Patients with Gout
 Philippine Journal of Internal Medicine Meta-Analysis Urate Lowering Efficacy of Febuxostat Versus Allopurinol in Hyperuricemic Patients with Gout Erika Bianca S. Villazor-Isidro, M.D.*; John Carlo G.
Philippine Journal of Internal Medicine Meta-Analysis Urate Lowering Efficacy of Febuxostat Versus Allopurinol in Hyperuricemic Patients with Gout Erika Bianca S. Villazor-Isidro, M.D.*; John Carlo G.
A Pharmacist-Staffed, Virtual Gout Management Clinic for Achieving Target Serum Uric Acid Levels: A Randomized Clinical Trial
 A Pharmacist-Staffed, Virtual Gout Management Clinic for Achieving Target Serum Uric Acid Levels: A Randomized Clinical Trial Robert Goldfien, MD; Alice Pressman, PhD, MS; Alice Jacobson, MS; Michele Ng,
A Pharmacist-Staffed, Virtual Gout Management Clinic for Achieving Target Serum Uric Acid Levels: A Randomized Clinical Trial Robert Goldfien, MD; Alice Pressman, PhD, MS; Alice Jacobson, MS; Michele Ng,
HARVARD PILGRIM HEALTH CARE RECOMMENDED MEDICATION REQUEST GUIDELINES HUMIRA PEDIATRIC
 Generic Brand HICL GCN Exception/Other ADALIMUMAB HUMIRA 24800 HUMIRA PEDIATRIC GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Is the patient currently taking Humira? If
Generic Brand HICL GCN Exception/Other ADALIMUMAB HUMIRA 24800 HUMIRA PEDIATRIC GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Is the patient currently taking Humira? If
Novel uricosurics RHEUMATOLOGY. Thomas Bardin 1,2 and Pascal Richette 1,2. Abstract. Introduction REVIEW
 RHEUMATOLOGY Rheumatology 2018;57:i42 i46 doi:10.1093/rheumatology/kex433 Novel uricosurics Thomas Bardin 1,2 and Pascal Richette 1,2 REVIEW Abstract Objective. According to recent guidelines, the mainstay
RHEUMATOLOGY Rheumatology 2018;57:i42 i46 doi:10.1093/rheumatology/kex433 Novel uricosurics Thomas Bardin 1,2 and Pascal Richette 1,2 REVIEW Abstract Objective. According to recent guidelines, the mainstay
Gout- Treatment Updates. Harinder Singh, MD Rheumatology Mercy Internal Medicine Clinic Mason City, IA
 Gout- Treatment Updates Harinder Singh, MD Rheumatology Mercy Internal Medicine Clinic Mason City, IA Gout Outline of purine metabolism: (1) amidophosphoribosyltransferase (2) hypoxanthine-guanine phosphoribosyltransferase
Gout- Treatment Updates Harinder Singh, MD Rheumatology Mercy Internal Medicine Clinic Mason City, IA Gout Outline of purine metabolism: (1) amidophosphoribosyltransferase (2) hypoxanthine-guanine phosphoribosyltransferase
Treatment with Allopurinol is Associated with Lower Risk of Acute Kidney Injury in Patients with Gout: A Retrospective Analysis of a Nested Cohort
 Rheumatol Ther (2017) 4:419 425 DOI 10.1007/s40744-017-0082-2 ORIGINAL RESEARCH Treatment with Allopurinol is Associated with Lower Risk of Acute Kidney Injury in Patients with Gout: A Retrospective Analysis
Rheumatol Ther (2017) 4:419 425 DOI 10.1007/s40744-017-0082-2 ORIGINAL RESEARCH Treatment with Allopurinol is Associated with Lower Risk of Acute Kidney Injury in Patients with Gout: A Retrospective Analysis
1. To review the diagnosis of gout and its differential. 2. To understand the four stages of gout
 Objectives 1. To review the diagnosis of gout and its differential GOUT 2. To understand the four stages of gout 3. To develop an approach for the acute treatment of gout Anthony Lim 9/13/12 Cycle 3 4.
Objectives 1. To review the diagnosis of gout and its differential GOUT 2. To understand the four stages of gout 3. To develop an approach for the acute treatment of gout Anthony Lim 9/13/12 Cycle 3 4.
Practice research in the field of gout - clinical pharmacology of antihyperuricemic drugs Reinders, Mattheus Karsien
 University of Groningen Practice research in the field of gout - clinical pharmacology of antihyperuricemic drugs Reinders, Mattheus Karsien IMPORTANT NOTE: You are advised to consult the publisher's version
University of Groningen Practice research in the field of gout - clinical pharmacology of antihyperuricemic drugs Reinders, Mattheus Karsien IMPORTANT NOTE: You are advised to consult the publisher's version
The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters.
 Flare frequency, healthcare resource utilisation and costs among patients with gout in a managed care setting: a retrospective medical claims-based analysis The Harvard community has made this article
Flare frequency, healthcare resource utilisation and costs among patients with gout in a managed care setting: a retrospective medical claims-based analysis The Harvard community has made this article
ADALIMUMAB Generic Brand HICL GCN Exception/Other ADALIMUMAB HUMIRA GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW)
 Generic Brand HICL GCN Exception/Other ADALIMUMAB HUMIRA 24800 GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Does the patient have a diagnosis of moderate to severe rheumatoid
Generic Brand HICL GCN Exception/Other ADALIMUMAB HUMIRA 24800 GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Does the patient have a diagnosis of moderate to severe rheumatoid
Lesinurad in Combination With a Xanthine Oxidase Inhibitor for Treatment of Hyperuricemia Associated With Gout
 Lesinurad in Combination With a Xanthine Oxidase Inhibitor for Treatment of Hyperuricemia Associated With Gout Briefing Document for the Arthritis Advisory Committee Meeting Date: 23 October 215 Ardea
Lesinurad in Combination With a Xanthine Oxidase Inhibitor for Treatment of Hyperuricemia Associated With Gout Briefing Document for the Arthritis Advisory Committee Meeting Date: 23 October 215 Ardea
Gout Hanan Abdel Rehim
 Review article 35 Gout Hanan Abdel Rehim Department of Internal Medicine, Kasr-Al Aini School of Medicine, Cairo University, Cairo, Egypt Correspondence to Hanan Abdel Rehim, MD, 11 Ismaiel Wahby Street,
Review article 35 Gout Hanan Abdel Rehim Department of Internal Medicine, Kasr-Al Aini School of Medicine, Cairo University, Cairo, Egypt Correspondence to Hanan Abdel Rehim, MD, 11 Ismaiel Wahby Street,
HARVARD PILGRIM HEALTH CARE RECOMMENDED MEDICATION REQUEST GUIDELINES
 Generic Brand HICL GCN Exception/Other SOMATROPIN HUMATROPE GENOTROPIN NORDITROPIN NORDITROPIN FLEXPRO NORDITROPIN NORDIFLEX NUTROPIN NUTROPIN AQ OMNITROPE SAIZEN ZOMACTON 02824 BRAND ZORBTIVE BRAND SEROSTIM
Generic Brand HICL GCN Exception/Other SOMATROPIN HUMATROPE GENOTROPIN NORDITROPIN NORDITROPIN FLEXPRO NORDITROPIN NORDIFLEX NUTROPIN NUTROPIN AQ OMNITROPE SAIZEN ZOMACTON 02824 BRAND ZORBTIVE BRAND SEROSTIM
Essence of the Revised Guideline for the Management of Hyperuricemia and Gout
 Research and Reviews Essence of the Revised Guideline for the Management of Hyperuricemia and Gout JMAJ 55(4): 324 329, 2012 Hisashi YAMANAKA,* 1 The Guideline Revising Committee of Japanese Society of
Research and Reviews Essence of the Revised Guideline for the Management of Hyperuricemia and Gout JMAJ 55(4): 324 329, 2012 Hisashi YAMANAKA,* 1 The Guideline Revising Committee of Japanese Society of
Gout: A Clinical Update. Why talk about Gout? Why talk about Gout? Populations at risk: Why is Gout Less Common in Women? US Gout Population: 2009
 Gout: A Clinical Update Peng Thim Fan, MD, FACP Clinical Professor of Medicine Division of Rheumatology David Geffen School of Medicine at UCLA Why talk about Gout? Large increase in gout in the last 20
Gout: A Clinical Update Peng Thim Fan, MD, FACP Clinical Professor of Medicine Division of Rheumatology David Geffen School of Medicine at UCLA Why talk about Gout? Large increase in gout in the last 20
Gout 2.0. Scott Vogelgesang, M.D. Division of Immunology: Rheumatology & Allergy
 Gout 2.0 Scott Vogelgesang, M.D. Division of Immunology: Rheumatology & Allergy Case 48 year old man presents with swollen, painful left toe that started overnight. Didn t hurt when he went to bed. No
Gout 2.0 Scott Vogelgesang, M.D. Division of Immunology: Rheumatology & Allergy Case 48 year old man presents with swollen, painful left toe that started overnight. Didn t hurt when he went to bed. No
Gout affects approximately 8.3 million people in the
 RESEARCH Benefit Restrictions and Gout Treatment Sharon M. Wang, PharmD, MS; Gustavus A. Aranda, Jr., PharmD, MS; Sara Gao, MS; and Bimal V. Patel, PharmD, MS ABSTRACT BACKGROUND: Gout is a chronic rheumatic
RESEARCH Benefit Restrictions and Gout Treatment Sharon M. Wang, PharmD, MS; Gustavus A. Aranda, Jr., PharmD, MS; Sara Gao, MS; and Bimal V. Patel, PharmD, MS ABSTRACT BACKGROUND: Gout is a chronic rheumatic
ETANERCEPT Generic Brand HICL GCN Exception/Other ETANERCEPT ENBREL GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW)
 Generic Brand HICL GCN Exception/Other ETANERCEPT ENBREL 18830 GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Does the patient have a diagnosis of moderate to severe rheumatoid
Generic Brand HICL GCN Exception/Other ETANERCEPT ENBREL 18830 GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Does the patient have a diagnosis of moderate to severe rheumatoid
Febuxostat now subsidised on Special Authority
 Gout update: Febuxostat now subsidised on Special Authority 38 Febuxostat was added to the New Zealand Pharmaceutical Schedule on 1 June, 2014. It is now available as a third-line preventive treatment
Gout update: Febuxostat now subsidised on Special Authority 38 Febuxostat was added to the New Zealand Pharmaceutical Schedule on 1 June, 2014. It is now available as a third-line preventive treatment
3/2/2014. Got Gout? Get a Plumber. Objectives. Disclosures
 Got Gout? Get a Plumber. Heidi Garcia, PA-C Department of Rheumatology Division of Internal Medicine Mayo Clinic Arizona 2013 MFMER slide-1 Objectives Recall some of the history of Gout. Describe the pathophysiology
Got Gout? Get a Plumber. Heidi Garcia, PA-C Department of Rheumatology Division of Internal Medicine Mayo Clinic Arizona 2013 MFMER slide-1 Objectives Recall some of the history of Gout. Describe the pathophysiology
Gout: Update in therapeutics
 Summary Gout: Update in therapeutics 29/11/14 Caroline van Durme CHU de Liège Maastricht University Medical Centre+ Why treating gout? Guidelines: ACR 12 Drugs: Colchicine Allopurinol: what about the kidney?
Summary Gout: Update in therapeutics 29/11/14 Caroline van Durme CHU de Liège Maastricht University Medical Centre+ Why treating gout? Guidelines: ACR 12 Drugs: Colchicine Allopurinol: what about the kidney?
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 24 June 2009
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 24 June 2009 ADENURIC 80 mg, film-coated tablets B/28 (CIP code: 385 724-4) B/84 (CIP code: 572 820-3) ADENURIC 120
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 24 June 2009 ADENURIC 80 mg, film-coated tablets B/28 (CIP code: 385 724-4) B/84 (CIP code: 572 820-3) ADENURIC 120
4/1/2011. New Developments in Gout. Conflict of Interest Declaration. Objectives
 New Developments in Gout Tatum N. Mead, Pharm.D. Clinical Assistant Professor UMKC SOP meadt@umkc.edu April 16, 2011 1 Conflict of Interest Declaration I have no actual or potential conflict of interest
New Developments in Gout Tatum N. Mead, Pharm.D. Clinical Assistant Professor UMKC SOP meadt@umkc.edu April 16, 2011 1 Conflict of Interest Declaration I have no actual or potential conflict of interest
Clinical Policy Bulletin: Gout
 Gout Page 1 of 13 Clinical Policy Bulletin: Gout Number: 0810 Policy Aetna considers the following tests medically necessary for the diagnosis of gout: Measurement of blood uric acid levels Measurement
Gout Page 1 of 13 Clinical Policy Bulletin: Gout Number: 0810 Policy Aetna considers the following tests medically necessary for the diagnosis of gout: Measurement of blood uric acid levels Measurement
CARDIOVASCULAR SAFETY OF FEBUXOSTAT OR ALLOPURINOL IN PATIENTS WITH GOUT AND CARDIOVASCULAR DISEASE (The CARES Trial)
 CARDIOVASCULAR SAFETY OF FEBUXOSTAT OR ALLOPURINOL IN PATIENTS WITH GOUT AND CARDIOVASCULAR DISEASE (The CARES Trial) William B. White, MD for the CARES Investigators Calhoun Cardiology Center University
CARDIOVASCULAR SAFETY OF FEBUXOSTAT OR ALLOPURINOL IN PATIENTS WITH GOUT AND CARDIOVASCULAR DISEASE (The CARES Trial) William B. White, MD for the CARES Investigators Calhoun Cardiology Center University
Febuxostat: a new treatment for hyperuricaemia in gout
 Rheumatology 2009;48:ii15 ii19 doi:10.1093/rheumatology/kep088 Febuxostat: a new treatment for hyperuricaemia in gout N. Lawrence Edwards 1 Febuxostat is a new non-purine xanthine oxidase inhibitor that
Rheumatology 2009;48:ii15 ii19 doi:10.1093/rheumatology/kep088 Febuxostat: a new treatment for hyperuricaemia in gout N. Lawrence Edwards 1 Febuxostat is a new non-purine xanthine oxidase inhibitor that
Implementing AHRQ Effective Health Care Reviews Helping Clinicians Make Better Treatment Choices
 Implementing AHRQ Effective Health Care Reviews Helping Clinicians Make Better Treatment Choices Gout: Diagnosis and Management Practice Pointers by MATTHEW R. NOSS, DO, MSEd, U.S. Army Health Clinic,
Implementing AHRQ Effective Health Care Reviews Helping Clinicians Make Better Treatment Choices Gout: Diagnosis and Management Practice Pointers by MATTHEW R. NOSS, DO, MSEd, U.S. Army Health Clinic,
Rheumatology Review Update in Internal Medicine COPYRIGHT. Robert H. Shmerling, M.D. Beth Israel Deaconess Medical Center.
 Rheumatology Review Update in Internal Medicine Robert H. Shmerling, M.D. Beth Israel Deaconess Medical Center Boston MA Case #1 True statement(s) regarding etanercept and leflunomide, for the treatment
Rheumatology Review Update in Internal Medicine Robert H. Shmerling, M.D. Beth Israel Deaconess Medical Center Boston MA Case #1 True statement(s) regarding etanercept and leflunomide, for the treatment
USTEKINUMAB Generic Brand HICL GCN Exception/Other USTEKINUMAB STELARA GUIDELINES FOR USE
 Generic Brand HICL GCN Exception/Other USTEKINUMAB STELARA 36187 GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Does the patient have a diagnosis of psoriatic arthritis (PsA)
Generic Brand HICL GCN Exception/Other USTEKINUMAB STELARA 36187 GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Does the patient have a diagnosis of psoriatic arthritis (PsA)
Effectiveness of a pharmacist-based gout care management programme in a large integrated health plan: results from a pilot study
 Research Effectiveness of a pharmacist-based gout care management programme in a large integrated health plan: results from a pilot study Robert D Goldfien, 1 Michele S Ng, 2 Goldie Yip, 2 Alice Hwe, 2
Research Effectiveness of a pharmacist-based gout care management programme in a large integrated health plan: results from a pilot study Robert D Goldfien, 1 Michele S Ng, 2 Goldie Yip, 2 Alice Hwe, 2
Zurampic. (lesinurad) New Product Slideshow
 Zurampic (lesinurad) New Product Slideshow Introduction Brand name: Zurampic Generic name: Lesinurad Pharmacological class: URAT1 inhibitor Strength and Formulation: 200mg; tablets Manufacturer: Ironwood
Zurampic (lesinurad) New Product Slideshow Introduction Brand name: Zurampic Generic name: Lesinurad Pharmacological class: URAT1 inhibitor Strength and Formulation: 200mg; tablets Manufacturer: Ironwood
Cost-effectiveness of lesinurad (Zurampic ) for the treatment of adult patients with gout
 Cost-effectiveness of lesinurad (Zurampic ) for the treatment of adult patients with gout The NCPE has issued a recommendation regarding the cost-effectiveness of Lesinurad (Zurampic ) in combination with
Cost-effectiveness of lesinurad (Zurampic ) for the treatment of adult patients with gout The NCPE has issued a recommendation regarding the cost-effectiveness of Lesinurad (Zurampic ) in combination with
SUMMACARE COMMERCIAL MEDICATION REQUEST GUIDELINES
 Generic Brand HICL GCN Exception/Other TELAPREVIR INCIVEK 37629 This drug requires a written request for prior authorization. All requests for hepatitis C medications require review by a pharmacist prior
Generic Brand HICL GCN Exception/Other TELAPREVIR INCIVEK 37629 This drug requires a written request for prior authorization. All requests for hepatitis C medications require review by a pharmacist prior
Effectiveness of a pharmacist-based gout care management program in a large integrated health plan: Results from a pilot study For peer review only
 BMJ Open Effectiveness of a pharmacist-based gout care management program in a large integrated health plan: Results from a pilot study Journal: BMJ Open Manuscript ID: bmjopen--00 Article Type: Research
BMJ Open Effectiveness of a pharmacist-based gout care management program in a large integrated health plan: Results from a pilot study Journal: BMJ Open Manuscript ID: bmjopen--00 Article Type: Research
HARVARD PILGRIM HEALTH CARE RECOMMENDED MEDICATION REQUEST GUIDELINES
 Generic Brand HICL GCN Exception/Other BLOOD SUGAR DIAGNOSTIC DIABETIC TEST STRIPS 25200 NOTE: Requests for preferred blood glucose (diabetic) test strips manufactured by Abbott Diabetes Care (such as
Generic Brand HICL GCN Exception/Other BLOOD SUGAR DIAGNOSTIC DIABETIC TEST STRIPS 25200 NOTE: Requests for preferred blood glucose (diabetic) test strips manufactured by Abbott Diabetes Care (such as
Session 2: Ambulatory Care and Critical Care Answer Explanations
 Session 2: Ambulatory Care and Critical Care Answer Explanations Ambulatory Care 1. Answer C: Not well controlled. Her nighttime symptoms fall under the not well controlled category, even though her FEV
Session 2: Ambulatory Care and Critical Care Answer Explanations Ambulatory Care 1. Answer C: Not well controlled. Her nighttime symptoms fall under the not well controlled category, even though her FEV
Current treatment options for acute and chronic gout
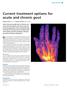 DRUG REVIEW n Current treatment options for acute and chronic gout Kelsey Jordan FRCP and Andrew Jeffries PGDip, MRCP Gout is the only curable form of arthritis, yet only a third of patients with chronic
DRUG REVIEW n Current treatment options for acute and chronic gout Kelsey Jordan FRCP and Andrew Jeffries PGDip, MRCP Gout is the only curable form of arthritis, yet only a third of patients with chronic
GOUT & PSEUDOGOUT OPSC 2018 HOWARD L. FEINBERG, D.O., F.A.C.O.I.., F.A.C.R.
 GOUT & PSEUDOGOUT OPSC 2018 HOWARD L. FEINBERG, D.O., F.A.C.O.I.., F.A.C.R. Everything in excess is opposed by nature Eunuchs do not take the gout, nor become bald. GOUT Hyperuricemia is not gout Gout
GOUT & PSEUDOGOUT OPSC 2018 HOWARD L. FEINBERG, D.O., F.A.C.O.I.., F.A.C.R. Everything in excess is opposed by nature Eunuchs do not take the gout, nor become bald. GOUT Hyperuricemia is not gout Gout
School of Health and Related Research (ScHARR), The University of Sheffield. Produced by
 Pegloticase for the treatment of hyperuricaemia in people with symptomatic gout whose disease is refractory to conventional uratelowering therapy, or in whom conventional urate-lowering therapy is contraindicated
Pegloticase for the treatment of hyperuricaemia in people with symptomatic gout whose disease is refractory to conventional uratelowering therapy, or in whom conventional urate-lowering therapy is contraindicated
Chronic gout: Barriers to effective management
 Chronic gout: Barriers to effective management Sylvie Rogenmoser, Mark H Arnold Background Gout is one of the most common inflammatory arthropathies, and the pathogenesis is well understood. In Australia,
Chronic gout: Barriers to effective management Sylvie Rogenmoser, Mark H Arnold Background Gout is one of the most common inflammatory arthropathies, and the pathogenesis is well understood. In Australia,
New and emerging therapies for gout
 New and emerging therapies for gout Clin. Invest. (2011) 1(11), 1563 1575 After nearly 50 years, new drugs are now available or in development for gout. Febuxostat (approved 2009) selectively inhibits
New and emerging therapies for gout Clin. Invest. (2011) 1(11), 1563 1575 After nearly 50 years, new drugs are now available or in development for gout. Febuxostat (approved 2009) selectively inhibits
Rheumatology Subcommittee of PTAC Meeting held 7 October (minutes for web publishing)
 Rheumatology Subcommittee of PTAC Meeting held 7 October 2014 (minutes for web publishing) Rheumatology Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
Rheumatology Subcommittee of PTAC Meeting held 7 October 2014 (minutes for web publishing) Rheumatology Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
BioCryst Pharmaceuticals
 BioCryst Pharmaceuticals BCX4208 Results Presented at ACR 2011 Dr. William Sheridan Senior Vice President & Chief Medical Officer Jon Stonehouse President & Chief Executive Officer November 8, 2011 Forward-Looking
BioCryst Pharmaceuticals BCX4208 Results Presented at ACR 2011 Dr. William Sheridan Senior Vice President & Chief Medical Officer Jon Stonehouse President & Chief Executive Officer November 8, 2011 Forward-Looking
Rheumatology Cases for the Internist
 Rheumatology Cases for the Internist Marc C. Hochberg, MD, MPH Professor of Medicine Head, Division of Rheumatology and Clinical Immunology Vice Chair, Department of Medicine University of Maryland School
Rheumatology Cases for the Internist Marc C. Hochberg, MD, MPH Professor of Medicine Head, Division of Rheumatology and Clinical Immunology Vice Chair, Department of Medicine University of Maryland School
D espite reasonable understanding of its pathogenesis
 1312 EXTENDED REPORT EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee For International Clinical Studies Including Therapeutics
1312 EXTENDED REPORT EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee For International Clinical Studies Including Therapeutics
HARVARD PILGRIM HEALTH CARE RECOMMENDED MEDICATION REQUEST GUIDELINES. SEDATIVE HYPNOTIC AGENTS Generic Brand HICL GCN Exception/Other ZOLPIDEM
 Generic Brand HICL GCN Exception/Other ZOLPIDEM AMBIEN 07842 GENERIC IS UNRESTRICTED TARTRATE AMBIEN CR EDLUAR INTERMEZZO ZOLPIMIST ESZOPICLONE LUNESTA 26791 GENERIC IS UNRESTRICTED RAMELTEON ROZEREM 33126
Generic Brand HICL GCN Exception/Other ZOLPIDEM AMBIEN 07842 GENERIC IS UNRESTRICTED TARTRATE AMBIEN CR EDLUAR INTERMEZZO ZOLPIMIST ESZOPICLONE LUNESTA 26791 GENERIC IS UNRESTRICTED RAMELTEON ROZEREM 33126
Latest Press Release. eagle scout thank you letter to donor
 corp@stantec.com Latest Press Release eagle scout thank you letter to donor S Elitek (rasburicase) is an extremely effective but expensive medicine that can quickly lower uric acid blood levels and prevent
corp@stantec.com Latest Press Release eagle scout thank you letter to donor S Elitek (rasburicase) is an extremely effective but expensive medicine that can quickly lower uric acid blood levels and prevent
Podcast (Video Recorded Lecture Series): Gout for the USMLE Step One Exam. Howard J. Sachs, MD
 Podcast (Video Recorded Lecture Series): Gout for the USMLE Step One Exam Howard J. Sachs, MD www.12daysinmarch.com Email: Howard@12daysinmarch.com Podcast (Video Recorded Lecture Series): Gout for the
Podcast (Video Recorded Lecture Series): Gout for the USMLE Step One Exam Howard J. Sachs, MD www.12daysinmarch.com Email: Howard@12daysinmarch.com Podcast (Video Recorded Lecture Series): Gout for the
Gout Treatment Guidelines
 Gout Treatment Guidelines Gout is a disorder that manifests as a spectrum of clinical and pathologic features built on a foundation of an excess body burden of uric acid, manifested in part by hyperuricemia,
Gout Treatment Guidelines Gout is a disorder that manifests as a spectrum of clinical and pathologic features built on a foundation of an excess body burden of uric acid, manifested in part by hyperuricemia,
Gout. Edward Roddy, 1 Christian D Mallen, 1 Michael Doherty 2 CLINICAL REVIEW
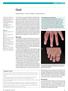 Gout Edward Roddy, 1 Christian D Mallen, 1 Michael Doherty 2 1 Arthritis Research UK Primary Care Centre, Primary Care Sciences, Keele University, Keele ST5 5BG, UK 2 Academic Rheumatology, University
Gout Edward Roddy, 1 Christian D Mallen, 1 Michael Doherty 2 1 Arthritis Research UK Primary Care Centre, Primary Care Sciences, Keele University, Keele ST5 5BG, UK 2 Academic Rheumatology, University
Treating to target: a strategy to cure gout
 Rheumatology 2009;48:ii9 ii14 doi:10.1093/rheumatology/kep087 Treating to target: a strategy to cure gout Fernando Perez-Ruiz 1 Acute gout attacks and the long-term complications of gout are associated
Rheumatology 2009;48:ii9 ii14 doi:10.1093/rheumatology/kep087 Treating to target: a strategy to cure gout Fernando Perez-Ruiz 1 Acute gout attacks and the long-term complications of gout are associated
HARVARD PILGRIM HEALTH CARE RECOMMENDED MEDICATION REQUEST GUIDELINES
 Generic Brand HICL GCN Exception/Other SELEXIPAG UPTRAVI 42922 TREPROSTINIL ORENITRAM ER 40827 If the caller wishes to initiate a request then a MRF must be completed. This drug requires a written request
Generic Brand HICL GCN Exception/Other SELEXIPAG UPTRAVI 42922 TREPROSTINIL ORENITRAM ER 40827 If the caller wishes to initiate a request then a MRF must be completed. This drug requires a written request
Benlysta (belimumab) Prior Authorization Criteria Program Summary
 Benlysta (belimumab) Prior Authorization Criteria Program Summary This prior authorization applies to Commercial, NetResults A series, NetResults F series and Health Insurance Marketplace formularies.
Benlysta (belimumab) Prior Authorization Criteria Program Summary This prior authorization applies to Commercial, NetResults A series, NetResults F series and Health Insurance Marketplace formularies.
Therapy for Gout: The Past
 Advances in Therapy for Gout: 2011 The Past, Present, and Future Therapy for Gout: The Past May 22, 1997 Pity a Tyrannosaur? Sue Had Gout By MALCOLM W. BROWNE Jonathan Graf, M.D. Associate Professor of
Advances in Therapy for Gout: 2011 The Past, Present, and Future Therapy for Gout: The Past May 22, 1997 Pity a Tyrannosaur? Sue Had Gout By MALCOLM W. BROWNE Jonathan Graf, M.D. Associate Professor of
A randomised controlled trial of the efficacy and safety of allopurinol dose escalation to achieve target serum urate in people with gout
 1/5 This site uses cookies. More info Home / Online First Article Text Article menu Clinical and epidemiological research Extended report A randomised controlled trial of the efficacy and safety of allopurinol
1/5 This site uses cookies. More info Home / Online First Article Text Article menu Clinical and epidemiological research Extended report A randomised controlled trial of the efficacy and safety of allopurinol
Opinion 23 July 2014
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 23 July 2014 IMUREL 50 mg, film-coated tablet (B/100) (CIP: 34009 364 149 0 7) IMUREL 25 mg, film-coated tablet (B/50)
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 23 July 2014 IMUREL 50 mg, film-coated tablet (B/100) (CIP: 34009 364 149 0 7) IMUREL 25 mg, film-coated tablet (B/50)
Pennsylvania Academy of Family Physicians Foundation & UPMC 43rd Refresher Course in Family Medicine CME Conference March 10-13, 2016
 Pennsylvania Academy of Family Physicians Foundation & UPMC 43rd Refresher Course in Family Medicine CME Conference March 10-13, 2016 Disclosures: Gout and Pseudogout Wayne Blount MD Speaker has no disclosures
Pennsylvania Academy of Family Physicians Foundation & UPMC 43rd Refresher Course in Family Medicine CME Conference March 10-13, 2016 Disclosures: Gout and Pseudogout Wayne Blount MD Speaker has no disclosures
