A randomized, double blind, controlled study of adult human. mesenchymal stem cells delivered via intra-articular injection to the
|
|
|
- Sheryl Joseph
- 5 years ago
- Views:
Transcription
1 A randomized, double blind, controlled study of adult human mesenchymal stem cells delivered via intra-articular injection to the knee joint following meniscectomy Authors/Study Investigators C. Thomas Vangsness Jr., MD David Fox, MD David Dellaero, MD David Griffin, MD Jack Farr, MD Joel Boyd, MD John O Donnell, MD University of Southern California Orthopaedic Surgery Associates Unlimited Research Triangle Orthopaedic Associates, PA Orthopaedic Center of Vero Beach OrthoIndy TRIA Orthopaedic Center Greater Chesapeake Orthopaedic Associates, LLC 1
2 Abstract Introduction: In a double blind, controlled study, the safety and exploratory efficacy of an allogeneic mesenchymal stem cell (MSC) drug injection was investigated in patients after medial meniscectomy surgery. The purpose was to evaluate the effect of an MSC injection on meniscus regeneration and osteoarthritis (OA) progression. Methods: 55 patients at 7 institutions underwent a randomized, prospective, doubleblind trial. Under IRB approval, a single superior lateral knee injection was given 7-10 days after meniscectomy and aspiration of the knee. Patients were randomized to one of three single injection groups: low dose (50 million MSCs), high dose (150 million MSCs) and a hyaluronic acid (HA) vehicle control. Patients were followed for safety, pain, meniscus regeneration and overall knee joint condition. Timed intervals out to 2 years evaluated these parameters along with sequential MRI evaluations reviewed by a team of independent blinded musculoskeletal radiologists. Results: In the two MSC treatment groups, there were patients who exhibited increased meniscal volume (reaching the 15% predefined threshold based on MRI quantitative analysis, 23.5% in the 50 million cells group and 6% in the 150 million cells group) at the 12 month time point. There were no patients in the vehicle control group who exhibited the minimum 15% volume increase in the meniscus. Patients with osteoarthritic changes in their joint receiving MSCs experienced a statistically significant reduction in pain compared to those receiving the control, based in visual analog scale assessments. Recipients receiving the control were 3.5 times more likely to experience degenerative bone changes associated with OA as compared to those receiving MSCs 2
3 at 2 years. The OA effects were dose dependent and pain scores improved following MSC treatment. Discussion and conclusion: The single MSC injections were found to be well tolerated at 2 years following surgery with no ectopic tissue formation. Meniscus regeneration was statistically significant at the 12 month timepoint but not at 6 months and 2 years. An improvement in knee pain was noted in the MSC group suggesting a decrease in knee OA progression and better joint condition with MSC treatment. Key words: Articular Cartilage, Knee Arthroscopy, Meniscal repair, Meniscal Transplants, Meniscus, MRI, Mesenchymal Stem Cells 3
4 Background Arthroscopy of the knee is one of the most common surgical procedures today. These procedures are often to treat soft tissue injury of cartilage or the meniscus. It is estimated that 1.3 million meniscal pathology-associated procedures were performed in the United States in 2005 (Datamonitor 2000). Injuries to the meniscus or other soft tissue structures such as the ligaments of the knee often lead to degeneration of the joint and progression to osteoarthritis (OA) (Gillquist 1999). Isolated meniscus tears and subsequent repair increase the risk of OA 10-fold (15%-20% incidence) and meniscectomy in a joint with intact ligaments further doubles the risk (30%-40% incidence). OA is the most prevalent form of arthritis and is the leading cause of chronic disability, functional loss, and morbidity in the United States (Dunlop 2003). OA is a degenerative joint disease characterized by the progressive deterioration of cartilage with subsequent joint space narrowing, osteophyte formation, and subchondral sclerosis (Kijowski 2006). These structural changes can manifest clinically as joint pain, stiffness, swelling, and limited mobility. Symptomatic OA of the knee impairs an individual s ability to stand, walk, climb, and engage in other physical activities, thereby reducing overall quality of life. There are few options apart from surgical intervention for patients with knee disorders, and the available therapies for pain are not disease-modifying therapies. Mesenchymal stem cells are cells of mesodermal origin that have the capacity to differentiate into connective tissues such as bone, cartilage, tendon, ligament and fat (Pittenger 1999). They also have been observed to have potent anti-inflammatory and immune 4
5 modulatory effects (Aggarwal, Newman). In a series of preclinical meniscectomy animal studies MSCs were shown to have a potential for neomeniscal tissue formation and joint preservation (Murphy 2003, Murphy 2005). Mechanical instability was induced in the knee joint of goats by surgical resection of the anterior cruciate ligament and/or meniscus. Subsequently, MSCs were delivered to the joint by direct intra-articular injection. The injected cells became associated with the injured connective tissues. Safety and retention studies demonstrated that MSCs were concentrated on soft tissues within the joint and viable at 7 days post injection. Twelve-month safety studies in the normal knee indicated no significant negative findings on pathological or histological examination. Based upon preclinical studies in goats, it was believed that a single injection of hmscs delivered to the joint intra-articularly could initiate meniscal regeneration with the potential for long-term chondroprotection. Substantial evidence now exists that preparations of these cells can be delivered allogeneically without adverse immune effects (Kebriaei 2009, Hare 2009). Allogeneic therapy allows for immediate treatment in acute settings with a good source of cells that have been well qualified. Together, these findings led to a clinical investigation of MSCs as a potential treatment option following injury to the knee. The goal was to prevent the expected consequences of partial meniscectomy surgery, including increasing meniscal tissue formation and improving overall joint condition. The objectives were to evaluate the safety of intraarticular injection of MSCs to the knee joint, their ability to promote meniscus repair following surgery and limit changes characteristic of OA to the joint. 5
6 Methods Trial Design and Disposition This trial was a phase I/II, multicenter, randomized, controlled, double-blind, multipledose study of MSCs delivered intra-articularly after meniscectomy. Sixty male or female patients 18 to 60 years old, inclusive, meeting the main criteria for inclusion were randomly assigned to each of the 3 treatment groups (50 million hmscs-group 1, 150 million hmscs-group 2, or vehicle control-control). The trial was conducted in compliance with current Good Clinical Practice (cgcp) standards and in accordance with the principles set forth under the Declaration of Helsinki (1989). Institutional Review Board (IRB) approval of the treatment protocol was obtained at all centers prior to the initiation of patient enrollment. All patients entering the trial agreed to and signed an IRB approved statement of informed consent. Patients who required partial medial meniscectomy and were otherwise healthy were enrolled in the study. A total of 55 were treated: 18 patients were in Group 1(50 million hmscs), 18 patients were in Group 2 (150 million hmscs), and 19 patients received control. A total of 8 patients were discontinued from the study, of which 5 were discontinued before treatment with the investigational agent. Patients received the treatment injection 1 week after meniscectomy and were assessed for treatment safety and efficacy at different time points during the 2-year study duration. Fifty-four patients completed the study through 1 year and 52 patients completed the study visits through 2 years. 6
7 Composition of Active Investigational Agent and Control The active investigational agent was human mesenchymal stem cells, hmscs, isolated from bone marrow aspirates obtained from donors that are unrelated and not HLA matched to recipients. All donors are tested according to the FDA Donor Suitability Guidance prior to donation. The phenotype of the hmsc population was characterized by a cell surface profile of CD105+, CD166+, and CD45. The formulated treatment injection consisted of 1 injection of either 50 million or 150 million donor-derived hmscs suspended in 5 ml of diluted sodium hyaluronan or 1 injection of vehicle control delivered 7-10 days after meniscectomy. The active agent was composed of donor-derived hmscs suspended in 5 ml of sodium hyaluronan (20 mg)/plasmalyte/human serum albumin (1.2%). The vehicle control was composed of 5-mL sodium hyaluronan (20 mg)/plasmalyte/human serum albumin (1.2%). Release testing of the cells included measurement of cell-surface markers CD105 and CD166, absence of CD45, and karyotyping to exclude chromosomal abnormalities. (Osiris Therapeutics, Inc., Columbia, MD), a highly purified preparation of ex-vivo cultured adult hmscs. Patient Population Diagnosis for inclusion was made during the arthroscopic procedure (partial medial meniscectomy surgery). To be eligible, patients must have undergone partial medial meniscectomy requiring removal of at least 50% of the meniscus. The affected joint was required to have normal axial alignment. Any previous ligament construction must have been determined to the stable. Patients had to be willing to follow standard 7
8 postoperative rehabilitation and to participate in follow-up for 2 years from the time of meniscectomy surgery. Exclusion criteria of interest included confirmation at surgery of anterior cruciate ligament injury other support structure damage or Grade 3 or 4 cartilage damage. Also, if a patient had hyaluronic acid, steroid, or corticosteroid injections in the preceding 3 months, diffuse synovitis at the time of arthroscopy, or inflammatory arthritis, they were deemed ineligible. Patient enrollment and randomization Patients were enrolled by study investigators once they confirmed eligibility. When it was established that the patient met all inclusion criteria, randomization was performed. Randomization was conducted in a centralized manner. Manual randomization was performed using sealed envelopes at a 1:1:1 distribution across the three treatment groups. Prior and Concomitant Therapy Concomitant medications were recorded continuously during and after the investigational agent injection. All medications were coded according to preferred terms using the World Health Organization drug dictionary. Concomitant medications were summarized by each preferred term for the safety population (ie, all randomly assigned patients who received any study therapy). In the first 6 months of the study evaluation period, the following were not permitted by the study: corticosteroid injections, hyaluronic acid injections other than those prescribed by the protocol, oral glucosamine/chondroitin or oral corticosteroids. In addition, patients were required to 8
9 avoid running and/or repetitive impact activity during the first 6 weeks after surgery and strenuous activities or prolonged weight bearing for 48 hours after study injection. Safety - Clinical Assessments Physicians and other clinical personnel remained blinded to treatment assignment for all patients throughout the study period. Primary safety assessments included monitoring and recording all adverse events (AEs), and serious adverse events (SAEs). Safety laboratory and urine values, regular vital sign measurements and physical examination results were recorded as well. In addition to a standard physical examination covering major systems, an examination of the knee was performed to inspect for redness, swelling, deformity, abnormal tissue presentation and/or skin changes. The MRI images were reviewed for any signs of abnormal tissue formation (ectopic tissue) inside the joint capsule and in the surrounding tissues. The images were also reviewed for any abnormal bony changes. The expression of various immune cell markers for T cells, natural killer cells, and B cells in the peripheral blood was analyzed to detect general changes in the immune system relative to Baseline (preoperative screening). This provided an indication of any cell-mediated or humoral immune response to the investigational agent. Safety - Adverse Events Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events. The AEs were graded on a 5-point intensity scale ranging from 1 (mild) to 5 (death). The assessment of severity was made independent of the relationship to investigational agent administration or the 9
10 seriousness of the event. The relationship of an AE to investigational agent was assessed by the investigator. Magnetic Resonance Imaging Magnetic resonance imaging was performed on a clinical grade unit using a transmit/receive, phased array knee coil. A cartilage-sensitive pulse sequence using moderate cho time, fast spin-echo techniques was performed, which accentuated inherent magnetization transfer, yielding differential contrast between fluid, articular cartilage, and subchondral bone/calcified cartilage. In addition, the normal meniscus appeared hypointense on this pulse sequence because of its highly ordered structure that yielded relative restriction of water. This was effective in differentiating regenerated meniscal tissue from the native remnant. Preoperative MRIs were assessed for cartilage degeneration. The subchondral bone and plate were also assessed for areas of thickening and sclerosis. Meniscal changes were monitored with MRI throughout the study. Magnetic resonance imaging was performed as a preoperative screening measure (within 30 days before the surgery); after the meniscectomy at Visit 1 (baseline, before injection, Visit 2 (6 weeks), Visit 4 (6 months), Visit 5 (12 months), and Visit 7 (2 years). The meniscus was assessed for the size of the remnant (as judged from the zone of resection), the signal characteristics of the remnant (which reflected the degree of degeneration), and fraying of the inner edge. At the before injection and follow-up MRIs, all of the previously mentioned criteria for joint changes were assessed, and any additional alterations in signal intensity and size of the meniscal remnant were measured. 10
11 Field of view (FOV), matrix, and slice thickness constant throughout the examination and on subsequent visits. Scans were performed in the axial plane, sagittal plane, and coronal plane. The axial plane scans had contiguous coverage from above patella to below the tibial tubercle. The sagittal plane scans were aligned parallel to the lateral femoral condyle covering the entire knee. The coronal plane scans were perpendicular to the lateral femoral condyle and also covered the entire knee. Computational analysis of meniscal volume was performed by two independent university based musculosketal radiologists. To assure blinding in the early stages of the study, random knee images were instated in the sequence of the analyses. Slice-omatic v 4.3 software (TomoVision, 4559 Pontiac, Montreal, Quebec, CANADA, H2J 2T2) was used to digitize the DICOM images. Images of each 2 mm slice were digitized and the 3D volume reconstructed in each plane of MR image acquisition. Meniscal boundaries of each slice will be determined in two consecutive steps. First, the outline of the body of the meniscus was determined though automatic signal analysis using threshold segmentation. This was followed by visual inspection of signal intensity of border pixels in step two. Each border pixel was individually screened using the threshold segmentation mode and either included or excluded from the meniscal volume. Pixel size, determined by the FOV and resolution, was 0.42 x 0.42 mm. A qualitative analysis of the meniscal remnant and any new tissue that was present, as well as analysis of the cartilage and joint structures was performed. Changes in the extent of cartilage degeneration, osteophyte formation, and joint edema were tracked. The joint was examined for evidence of ectopic tissue formation. Data was analyzed sequentially. 11
12 The volume of the meniscus was determined for each of the postoperative time points using volume analysis postprocessing techniques. The volume of the meniscus was also compared with the postoperative volume of the meniscal remnant. This allowed for quantification of the degree of meniscal regeneration. The evaluation of MRI volume was challenging due to boundary detection and registration issues, so an additional over-read was performed. Also whole-organ evaluation of the knee in osteoarthritis (OA) was performed based on magnetic resonance imaging (MRI) findings, according to a validated method (Peterfy 2003). This is a semi-quantitative scoring method for overall evaluation of the osteoarthritic condition of the knee joint. This was performed by a contracted team of independent radiologists. Visual Analog Scale At the planned intervals, patients assessed their own perceptions of knee pain using a visual analog scale (VAS). The VAS was scored by measuring the distance of the mark along the scale from 0 (no pain) to 10 units (worst imaginable pain) and reporting a percentage value. Statistical Analysis Two-sided tests were used at a type I error rate of 5% for comparison between any 2 of the treatment groups or control and overall among the 3 treatment groups. Paired tests were used for significant changes from Baseline for any 1 of the 3 treatment groups. No adjustment for type I error was made for multiple treatment comparisons and testing multiple study endpoints. 12
13 Continuous data were described using descriptive statistics: n, mean, standard deviation, median, minimum, and maximum. For each study endpoint only observed data were used for analysis and no imputation of missing values was employed. All analyses were conducted using SAS software Version 8.2 (SAS Institute, Inc; Cary, NC) or higher. The completed population was defined as all randomly assigned patients who had the scheduled 6-month MRI evaluation, analyzed according to the treatment the patient actually received. The safety population was defined as all randomly assigned patients who received any study therapy, analyzed according to the treatment that the patient actually received. Categorical measures in ordinal scale were analyzed at each evaluation visit using the Mantel-Haenszel correlation test for pairwise treatment group comparisons and overall comparisons for the 3 treatment groups. Categorical measures in nominal scales were tested on general association between treatment group and outcomes at each visit using the Mantel-Haenszel test for general association. If any cell in a contingency table had a count less than 5, a Fisher exact test was used for nominal outcomes and exact P values of Mantel-Haenszel correlation tests were used for ordinal outcomes. Safety Analysis All safety data able to be verified against source documentation were used in safety analyses irrespective of the timing of adverse events (AEs), laboratory findings, or physical examinations. All AEs reported for this study were treatment emergent. Adverse events were coded according to Medical Dictionary for Regulatory Activities (MedDRA), Version
14 Adverse events were presented by system organ class and preferred term. The incidence of AEs table included only 1 occurrence of a preferred term per patient. If a patient reported the same preferred term multiple times, then that preferred term was counted only once, because patient counts (not the event counts) were presented. As with the preferred term, if a patient reported multiple AEs within the same system organ class, then that system organ class was counted only once. Percentages were calculated out of the number of patients in the safety analysis population. A summary of AEs by relationship to study drug was presented by incidence of occurrence. The event s relationship to study drug was classified as unrelated, possible, or probable. In the AE relationship table, if a patient reported multiple occurrences of the same AE, only the most closely related occurrence was presented. Results Basic demographics are presented in Table 1. The overall mean age for patients was 46.0 years with a minimum age of 18 years and maximum age of 60 years, consistent with the study eligibility criteria. Patients had a mean height of 174 cm, a mean weight of 87.0 kg, and a mean body mass index of 28.6 kg/m 2. No patients were unblinded prematurely during the study. Safety Evaluation No deaths were reported during the study and no AEs led to treatment discontinuation or study termination. A total of 427 AEs were experienced by the study subjects in the safety population, comprising patients who received any investigational agent. There were 118 in the vehicle control group, 158 in the Chondrogen 50 group, and 151 in the Chondrogen 150 group. Of the total number of AEs, 272 were reported as mild, 126 as 14
15 moderate, and 28 as severe. Adverse events that had an occurrence of at least 10% in any study group are summarized in Table 2. Nine SAEs occurred in 8 patients (Table 3), and all were determined by the blinded investigator to be unlikely related to investigational agent. Fifty-two of the 55 patients (94.5%) in the safety population reported at least 1 AE. The most frequently reported AEs by system organ class were musculoskeletal and connective tissue disorders (50/55, 90.9%), followed by general disorders and administration site conditions (27/55, 49.1%). Clinical laboratory testing There were no unexpected adverse trends identified in routine immunology, hematology, blood chemistry, and urinalysis analyses after study drug injection. No patients experienced a clinically significant abnormal hematology laboratory result. One patient experienced a clinically significant abnormal urinalysis laboratory result listed as crystals, however this patient had a history of nonclinically significant calcium oxalate crystals. The number of patients with shifts in laboratory values from normal at Baseline to values outside the normal range (low or high) or with shifts from normal to abnormal for categorical urinalysis analyses was generally low ( 4 patients per parameter). Vital signs, physical examination, and MRI safety. Two patients had vital sign measurements that were clinically significant and were reported as AEs. One patient experienced elevated blood pressure after injection of the study drug and 1 patient experienced an increase in body temperature. The 2 events were resolved. For physical examinations, there were no notable changes from Baseline in physical examination findings for all of the major organ system classes. There was no evidence of ectopic tissue formation from MRI evaluation. 15
16 MRI Evaluation The comparison of the Chondrogen groups versus the vehicle control group was analyzed for the percentage of patients with greater than 15% improvement in MRIbased meniscal volume from Baseline (post meniscectomy surgery and prior to investigation agent injection). The results of the analysis are shown in Table 4. At the 6 month evaluation, two patients (1 in each of the Chondrogen treatment groups) showed a change in meniscus volume greater than 15%. At 12 months, four patients in the Chondrogen 50 group showed a change in meniscus volume greater than 15%. Also, at Visit 7, three patients in the Chondrogen 50 treatment group showed a change in meniscus volume greater than 15%. At no time point was there the specific volume increase in any of the control group patients. At 12 months both the vehicle control versus Chondrogen 50 (P=0.040) and the overall group comparison (P=0.022) were statistically significant. At 2 years the overall group comparison (P=0.029) was statistically significant. The evaluation of MRI volume was challenging due to boundary detection and registration issues, so an additional second read was performed. The joint evaluation indicated 8 patients (44%) in the Chondrogen 50 group, 13 patients (72%) in the Chondrogen 150 group, and 9 patients (47%) in the Control group had degenerative changes consistent with OA. Bony changes associated with osteoarthritis, such as subchrondral sclerosis and osteophyte formation, were reported in 21% of patients receiving the control, but only 6% of Chondrogen-treated patients. 16
17 VAS Patients assessed perceptions of knee pain using a VAS. Overall, VAS scores decreased for patients post-surgery. The within-group P value revealed significance (P<0.001) in the reduction in pain when compared with baseline values as assessed by the VAS score for all treatment groups (Table 5). In patients with osteoarthritic-changes at the time of surgery, a statistically significant 20 mm reduction in pain, as measured by the visual analog scale (VAS), was observed in patients receiving a single injection of Chondrogen over patients receiving an injection of the control, HA, at one year (Chondrogen 150 : 47.6 mm vs. Chondrogen 50: 35.1 mm Control: 28.1 mm, p=0.05). The reduction in pain increased further with more severe osteoarthritic changes in the joint (p=0.004, Chondrogen 150: 55.7 mm vs. Chondrogen 50: 25.5 vs Control: 19.5 mm). These data are shown graphically in Figures 1 and 2. Discussion This was a Phase I/II, randomized, controlled double-blind study to evaluate the safety and preliminary efficacy of hmscs delivered by intra-articular injection after partial meniscectomy. In the study 55 male and female patients who required medial meniscectomy and were otherwise healthy were treated. Patients were followed for 2 years from the meniscectomy surgery. Overall, the study injections were well tolerated with minimal adverse effects. There were no deaths or AEs that led to treatment discontinuation or study termination. The most frequently reported AEs by preferred term were arthralgia, joint swelling, joint stiffness, injection site joint pain, joint effusion, headache, and peripheral edema. Many of the listed AEs such as injection site joint pain, joint swelling/effusion, and headache 17
18 were commonly reported with the use of sodium hyaluronan, the vehicle control used in this study. Eight patients reported SAEs, and all were considered by the investigator as unlikely to be related to study drug. Sequential immunologic, hematologic, and urine testing showing no unexpected adverse trends. A few patients experienced clinically significant abnormal laboratory results; however, none of these were considered to be related to study drug. In addition, there was no evidence of ectopic tissue formation in the knee joint. This study demonstrated that MSCs can be delivered in a concentrated manner to an enclosed space, here the joint capsule, with signs of abnormal tissue formation. This finding is consistent with other results of systemic administration of MSCs that have shown no evidence of unwanted tissue formation from imaging studies of patients receiving these cells. (Hare 2009, Kebriaei 2009). The results of this study suggest that MSCs have to potential to improve the overall joint condition. This study investigated only a single treatment of stem cells; multiple or serial stem cell injection protocols might bring greater clinical changes. For some patients receiving MSCs, there was an increase in meniscal volume indicating de novo tissue regeneration. Perhaps more importantly for the patients, a higher proportion of those with osteoarthritic changes experienced a reduction in pain. The reduction in pain was relative to an active control, hyaluronic acid. In fact, hyaluronic acid is often a treatment for the pain of osteoarthritis itself and possibly was providing some benefit to the patients in the control group. The magnitude of effect relative to placebo was clinically meaningful. The observed effect on a patient-reported outcome was 18
19 compelling given that this was a double blind study, which eliminates the possible bias from knowing that one received stem cells. The ability to modify the course of OA, especially in patients following removal of the meniscus, which serves to protect the joint, is a tantalizing possibility that requires further investigation. Currently no available therapies have been proven to be disease modifying. Standard therapy for OA of the knee begins with over-the-counter pharmacologic agents such as acetaminophen and nonsteroidal anti-inflammatory drugs. Treatment then progresses to agents including codeine and propoxyphene and COX-2 specific inhibitors. The last line of therapy before a final outcome of total knee replacement is often intra-articular injection of corticosteroid or hyaluronic acid into the knee joint. Difficulties with consistency of MRI scans from different centers, visit to visit, and edge detection were some of the challenges encountered with attempting quantitative MRI analysis of the knee. Further work is needed to validate quantitative assessment of meniscal volumes. While evaluation of meniscal volumes was initiated in an effort to demonstrate the mechanism of MSCs in knee, further studies would need to be designed around and focus on the patient reported outcomes, which are required for products/drugs under development in this area. Conclusion While further research will further elucidate the effects of MSC administration on the condition of the knee joint, this study provided the first controlled human data on allogeneic MSCs. In terms of safety there were no adverse effects from these cells. 19
20 Whether providing additional treatments (i.e. injections) enhances the effect on pain or regeneration remains to be evaluated. Many joint treatments are given repeatedly and MSCs multiply dosed might take advantage of the anti-inflammatory effects of MSC administration. 20
21 Acknowledgments This study was sponsored by Osiris Therapeutics, Inc. (Baltimore, MD). The investigators would like to thank the people who agreed to participate in this trial and the study contributors, especially Dr. Wendy Burke. 21
22 Table 1: Patient Disposition and Demographics Vehicle Control (n=20) Chondrogen 50 (n=20) Chondrogen 150 (n=20) Total (N=60) Number of Patients Randomized 20 (100.0%) 20 (100.0%) 20 (100.0%) 60 (100.0%) Completed 17 (85.0%) 17 (85.0%) 18 (90.0%) 52 (86.7%) Discontinued 3 (15.0%) 3 (15.0%) 2 (10.0%) 8 (13.3%) Completed Population 19 (95.0%) 17 (85.0%) 18 (90.0%) 54 (90.0%) Age (years) N Mean (SD) 47.8 (8.00) 44.6 (9.82) 45.6 (12.42) 46.0 (10.16) Median Min, Max 24, 60 24, 57 18, 60 18, 60 Sex - n (%) Male 13 (65.0) 11 (55.0) 14 (70.0) 38 (63.3) Height (cm) N Mean (SD) (11.894) (9.021) (11.190) (10.734) Median Min, Max 153.2, , , , Weight (kg) N Mean (SD) (17.292) (21.422) (22.614) (20.331) Median Min, Max 49.5, , , , Body-Mass Index (kg/m 2 ) N Mean (SD) (4.049) (7.943) (5.909) (6.201) Median Min, Max 19.1, , , , 45.5 Abbreviations: Max = maximum; Min = minimum. Note: Percentages are based on the number of patients in each treatment group. 22
23 Table 2: Adverse Events Occurring in 10% of Patients System Organ Class Preferred Term Control (n=19) Chondrogen 50 (n=18) Chondrogen 150 (n=18) Total number of adverse events Number of patients with at least 1 AE 17 (89.5%) 18 (100.0%) 17 (94.4%) Gastrointestinal disorders 2 (10.5%) 2 (11.1%) 1 (5.6%) Nausea 0 2 (11.1%) 1 (5.6%) General disorders/administration site conditions 6 (31.6%) 10 (55.6%) 11 (61.1%) Injection site joint pain 5 (26.3%) 3 (16.7%) 6 (33.3%) Injection site joint swelling 0 2 (11.1%) 3 (16.7%) Injection site pain 0 2 (11.1%) 2 (11.1%) Oedema peripheral 2 (10.5%) 4 (22.2%) 3 (16.7%) Infections and infestations 3 (15.8%) 3 (16.7%) 3 (16.7%) Nasopharyngitis 1 (5.3%) 0 2 (11.1%) Sinusitis 1 (5.3%) 1 (5.6%) 2 (11.1%) Injury, poisoning, procedural complications 6 (31.6%) 8 (44.4%) 8 (44.4%) Contusion 2 (10.5%) 0 2 (11.1%) Excoriation 1 (5.3%) 2 (11.1%) 0 Meniscus lesion 1 (5.3%) 2 (11.1%) 2 (11.1%) Muscle strain 1 (5.3%) 0 2 (11.1%) Joint sprain 1 (5.3%) 2 (11.1%) 2 (11.1%) Procedural pain 1 (5.3%) 0 3 (16.7%) Investigations 3 (15.8%) 6 (33.3%) 2 (11.1%) Blood triglycerides increased 1 (5.3%) 2 (11.1%) 1 (5.6%) Musculoskeletal and connective tissue disorders 15 (78.9%) 18 (100.0%) 17 (94.4%) Arthralgia 13 (68.4%) 17 (94.4%) 15 (83.3%) Back pain 0 2 (11.1%) 1 (5.6%) Joint crepitation 0 1 (5.6%) 3 (16.7%) Joint effusion 3 (15.8%) 4 (22.2%) 2 (11.1%) Joint range of motion decreased (27.8%) Joint stiffness 4 (21.1%) 7 (38.9%) 5 (27.8%) Joint swelling 8 (42.1%) 8 (44.4%) 10 (55.6%) Muscle atrophy 2 (10.5%) 1 (5.6%) 0 Muscular weakness 0 2 (11.1%) 2 (11.1%) Musculoskeletal pain 1 (5.3%) 1 (5.6%) 2 (11.1%) Myalgia 0 2 (11.1%) 0 Osteoarthritis 1 (5.3%) 2 (11.1%) 3 (16.7%) Pain in extremity 1 (5.3%) 1 (5.6%) 3 (16.7%) Rotator Cuff Syndrome 2 (10.5%) 0 0 Synovial Cyst 0 3 (16.7%) 1 (5.6%) Tendonitis 2 (10.5%) 2 (11.1%) 0 23
24 24
25 Table 3: Serious Adverse Events Event Control (n=19) Chondrogen 50 x 10 6 cells (n=18) Chondrogen 150 x 10 6 cells (n=18) Acute myocardial infarction Ileus Small intestinal obstruction Femur fracture Fibula fracture Hand fracture Meniscus lesion Osteoarthritis
26 Table 4: Magnetic Resonance Imaging Results for Meniscal Volume Control (n=19) Chondrogen 50 (n=17) Chondrogen 150 (n=18) >15% Change in Meniscus Volume Relative to Baseline 6 months n (%) 0 1 (5.9%) 1 (5.6%) 95% CI for Percentage (0.0, 17.6) (0.1, 28.7) (0.1, 27.3) P Value* for Vehicle Versus Vehicle Versus Versus 50 >0.999 Overall months n (%) 0 4 (23.5) 1 (5.6) 95% CI for Percentage (0.0, 17.6) (6.8, 49.9) (0.1, 27.3) P Value* for Vehicle Versus Vehicle Versus Versus Overall years n (%) 0 3 (17.6) 0 95% CI for Percentage (0.0, 19.5) (4.0, 45.6) (0.0, 19.5) P Value* for Vehicle Versus 150 >0.999 Vehicle Versus Versus Overall Abbreviations: CI = confidence interval. * P values are for the 3 pairwise treatment group actual result comparisons and the overall 3 treatment group comparison using the Mantel-Haenszel general association test. 26
27 Table 5: Absolute improvement in VAS relative to baseline Overall n 6 weeks 3 months 6 months 1 year Chondrogen Chondrogen Control OA n 6 weeks 3 months 6 months 1 year Chondrogen Chondrogen Control More severe OA 6 weeks 3 months 6 months 1 year Chondrogen Chondrogen Control
28 Figure 1: Improvement in Pain Score relative to vehicle control in the presence of osteoarthritic changes based on MRI. 28
29 Figure 2: Improvement in Pain Score relative to vehicle control in the presence of moderate to severe osteoarthritic changes based on MRI. 29
ClinialTrials.gov Identifier: Sponsor/company: sanofi-aventis
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
This presentation is the intellectual property of the author. Contact them for permission to reprint and/or distribute.
 MRI of the Knee Jennifer Swart, M.D. Musculoskeletal Radiology South Texas Radiology Group Outline Coils, Patient Positioning Acquisition Parameters, Planes and Pulse Sequences Knee Arthrography Normal
MRI of the Knee Jennifer Swart, M.D. Musculoskeletal Radiology South Texas Radiology Group Outline Coils, Patient Positioning Acquisition Parameters, Planes and Pulse Sequences Knee Arthrography Normal
This presentation is the intellectual property of the author. Contact them at for permission to reprint and/or distribute.
 MRI of the Knee Jennifer Swart, M.D. Musculoskeletal Radiology South Texas Radiology Group Financial Disclosure Dr. Jennifer Swart has no relevant financial relationships with commercial interests to disclose.
MRI of the Knee Jennifer Swart, M.D. Musculoskeletal Radiology South Texas Radiology Group Financial Disclosure Dr. Jennifer Swart has no relevant financial relationships with commercial interests to disclose.
Evaluation and Treatment of Knee Arthritis Classification of Knee Arthritis Osteoarthritis Osteoarthritis Osteoarthritis of Knee
 1 2 Evaluation and Treatment of Knee Arthritis John Zebrack, MD Reno Orthopaedic Clinic Classification of Knee Arthritis Non-inflammatory Osteoarthritis Primary Secondary Post-traumatic, dysplasia, neuropathic,
1 2 Evaluation and Treatment of Knee Arthritis John Zebrack, MD Reno Orthopaedic Clinic Classification of Knee Arthritis Non-inflammatory Osteoarthritis Primary Secondary Post-traumatic, dysplasia, neuropathic,
MRI KNEE WHAT TO SEE. Dr. SHEKHAR SRIVASTAV. Sr.Consultant KNEE & SHOULDER ARTHROSCOPY
 MRI KNEE WHAT TO SEE Dr. SHEKHAR SRIVASTAV Sr.Consultant KNEE & SHOULDER ARTHROSCOPY MRI KNEE - WHAT TO SEE MRI is the most accurate and frequently used diagnostic tool for evaluation of internal derangement
MRI KNEE WHAT TO SEE Dr. SHEKHAR SRIVASTAV Sr.Consultant KNEE & SHOULDER ARTHROSCOPY MRI KNEE - WHAT TO SEE MRI is the most accurate and frequently used diagnostic tool for evaluation of internal derangement
MR imaging of the knee in marathon runners before and after competition
 Skeletal Radiol (2001) 30:72 76 International Skeletal Society 2001 ARTICLE W. Krampla R. Mayrhofer J. Malcher K.H. Kristen M. Urban W. Hruby MR imaging of the knee in marathon runners before and after
Skeletal Radiol (2001) 30:72 76 International Skeletal Society 2001 ARTICLE W. Krampla R. Mayrhofer J. Malcher K.H. Kristen M. Urban W. Hruby MR imaging of the knee in marathon runners before and after
What is the most effective MRI specific findings for lateral meniscus posterior root tear in ACL injuries
 What is the most effective MRI specific findings for lateral meniscus posterior root tear in ACL injuries Kazuki Asai 1), Junsuke Nakase 1), Kengo Shimozaki 1), Kazu Toyooka 1), Hiroyuki Tsuchiya 1) 1)
What is the most effective MRI specific findings for lateral meniscus posterior root tear in ACL injuries Kazuki Asai 1), Junsuke Nakase 1), Kengo Shimozaki 1), Kazu Toyooka 1), Hiroyuki Tsuchiya 1) 1)
Priorities Forum Statement GUIDANCE
 Priorities Forum Statement Number 21 Subject Knee Arthroscopy including arthroscopic knee washouts Date of decision November 2016 Date refreshed March 2017 Date of review November 2018 Osteoarthritis of
Priorities Forum Statement Number 21 Subject Knee Arthroscopy including arthroscopic knee washouts Date of decision November 2016 Date refreshed March 2017 Date of review November 2018 Osteoarthritis of
New Directions in Osteoarthritis Research
 New Directions in Osteoarthritis Research Kananaskis October 22, 2015 Nick Mohtadi MD MSc FRCSC No conflicts of interest related to this presentation 1 Osteoarthritis: Disease? Fact of Life? Strong family
New Directions in Osteoarthritis Research Kananaskis October 22, 2015 Nick Mohtadi MD MSc FRCSC No conflicts of interest related to this presentation 1 Osteoarthritis: Disease? Fact of Life? Strong family
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. PROPRIETARY DRUG NAME / GENERIC DRUG NAME: Advil / Ibuprofen
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. PROPRIETARY DRUG NAME / GENERIC DRUG NAME: Advil / Ibuprofen
Medical Practice for Sports Injuries and Disorders of the Knee
 Sports-Related Injuries and Disorders Medical Practice for Sports Injuries and Disorders of the Knee JMAJ 48(1): 20 24, 2005 Hirotsugu MURATSU*, Masahiro KUROSAKA**, Tetsuji YAMAMOTO***, and Shinichi YOSHIDA****
Sports-Related Injuries and Disorders Medical Practice for Sports Injuries and Disorders of the Knee JMAJ 48(1): 20 24, 2005 Hirotsugu MURATSU*, Masahiro KUROSAKA**, Tetsuji YAMAMOTO***, and Shinichi YOSHIDA****
SCALENE ASIA PACIFIC SDN BHD ROTATIONAL FIELD QUANTUM NUCLEAR MAGNETIC RESONANCE (RFQMR) IN TREATMENT OF OSTEOARTHRITIS OF THE KNEE JOINT
 SCALENE ASIA PACIFIC SDN BHD ROTATIONAL FIELD QUANTUM NUCLEAR MAGNETIC RESONANCE (RFQMR) IN TREATMENT OF OSTEOARTHRITIS OF THE KNEE JOINT ROTATIONAL FIELD QUANTUM NUCLEAR MAGNETIC RESONANCE (RFQMR) IN
SCALENE ASIA PACIFIC SDN BHD ROTATIONAL FIELD QUANTUM NUCLEAR MAGNETIC RESONANCE (RFQMR) IN TREATMENT OF OSTEOARTHRITIS OF THE KNEE JOINT ROTATIONAL FIELD QUANTUM NUCLEAR MAGNETIC RESONANCE (RFQMR) IN
Osteoarthritis. Dr Anthony Feher. With special thanks to Dr. Tim Williams and Dr. Bhatia for allowing me to use some of their slides
 Osteoarthritis Dr Anthony Feher With special thanks to Dr. Tim Williams and Dr. Bhatia for allowing me to use some of their slides No Financial Disclosures Number one chronic disability in the United States
Osteoarthritis Dr Anthony Feher With special thanks to Dr. Tim Williams and Dr. Bhatia for allowing me to use some of their slides No Financial Disclosures Number one chronic disability in the United States
SUPPLEMENTAL DIGITAL CONTENT (SDC)
 Orozco_Osteoarthritis_MSC_v4.3.doc Mar_1_2013 1 SUPPLEMENTAL DIGITAL CONTENT (SDC) Contents: SUPPLEMENTARY TABLES Supplementary Table S1. Antecedent history of the patients included in this trial. Supplementary
Orozco_Osteoarthritis_MSC_v4.3.doc Mar_1_2013 1 SUPPLEMENTAL DIGITAL CONTENT (SDC) Contents: SUPPLEMENTARY TABLES Supplementary Table S1. Antecedent history of the patients included in this trial. Supplementary
RECENT ADVANCES IN CLINICAL MR OF ARTICULAR CARTILAGE
 In Practice RECENT ADVANCES IN CLINICAL MR OF ARTICULAR CARTILAGE By Atsuya Watanabe, MD, PhD, Director, Advanced Diagnostic Imaging Center and Associate Professor, Department of Orthopedic Surgery, Teikyo
In Practice RECENT ADVANCES IN CLINICAL MR OF ARTICULAR CARTILAGE By Atsuya Watanabe, MD, PhD, Director, Advanced Diagnostic Imaging Center and Associate Professor, Department of Orthopedic Surgery, Teikyo
4 2 Osteoarthritis 1
 Osteoarthritis 1 Osteoarthritis ( OA) Osteoarthritis is a chronic disease and the most common of all rheumatological disorders. It particularly affects individuals over the age of 65 years. The prevalence
Osteoarthritis 1 Osteoarthritis ( OA) Osteoarthritis is a chronic disease and the most common of all rheumatological disorders. It particularly affects individuals over the age of 65 years. The prevalence
YOUR TOTAL KNEE REPLACEMENT
 YOUR TOTAL KNEE REPLACEMENT Dr. M.S. Barrow Barrow Physiotherapy MBBch (Wits), FCS (SA) Orth. Waterfall City Hospital Orthopaedic Surgeon Tel: 011 304 7829 Suite 5, East Wing, Sunninghill Hospital www.barrowphysiotherapy.co.za
YOUR TOTAL KNEE REPLACEMENT Dr. M.S. Barrow Barrow Physiotherapy MBBch (Wits), FCS (SA) Orth. Waterfall City Hospital Orthopaedic Surgeon Tel: 011 304 7829 Suite 5, East Wing, Sunninghill Hospital www.barrowphysiotherapy.co.za
CASE REPORT GIANT OSTEOCHONDRAL LOOSE BODY OF THE KNEE JOINT
 Journal of Musculoskeletal Research, Vol. 4, No. 2 (2000) 145 149 World Scientific Publishing Company ORIGINAL CASE REPORT ARTICLES GIANT OSTEOCHONDRAL LOOSE BODY OF THE KNEE JOINT Mustafa Yel *,, Mustafa
Journal of Musculoskeletal Research, Vol. 4, No. 2 (2000) 145 149 World Scientific Publishing Company ORIGINAL CASE REPORT ARTICLES GIANT OSTEOCHONDRAL LOOSE BODY OF THE KNEE JOINT Mustafa Yel *,, Mustafa
Conservative surgical treatments for osteoarthritis: A Finite Element Study
 Conservative surgical treatments for osteoarthritis: A Finite Element Study Diagarajen Carpanen, BEng (Hons), Franziska Reisse, BEng(Hons), Howard Hillstrom, PhD, Kevin Cheah, FRCS, Rob Walker, PhD, Rajshree
Conservative surgical treatments for osteoarthritis: A Finite Element Study Diagarajen Carpanen, BEng (Hons), Franziska Reisse, BEng(Hons), Howard Hillstrom, PhD, Kevin Cheah, FRCS, Rob Walker, PhD, Rajshree
MY PATIENT HAS KNEE PAIN. David Levi, MD Chief, Division of Musculoskeletal l limaging Atlantic Medical Imaging
 MY PATIENT HAS KNEE PAIN David Levi, MD Chief, Division of Musculoskeletal l limaging Atlantic Medical Imaging Causes of knee pain Non traumatic Trauma Osteoarthritis Patellofemoral pain Menisci or ligaments
MY PATIENT HAS KNEE PAIN David Levi, MD Chief, Division of Musculoskeletal l limaging Atlantic Medical Imaging Causes of knee pain Non traumatic Trauma Osteoarthritis Patellofemoral pain Menisci or ligaments
NEW DEVELOPMENTS IN MENISCAL SURGERY
 NEW DEVELOPMENTS IN MENISCAL SURGERY Written by Peter Verdonk, Belgium and Francesco Perdisa, Italy Meniscectomy is one of the most common procedures in orthopaedic surgery, capable of returning the knee
NEW DEVELOPMENTS IN MENISCAL SURGERY Written by Peter Verdonk, Belgium and Francesco Perdisa, Italy Meniscectomy is one of the most common procedures in orthopaedic surgery, capable of returning the knee
W. Dilworth Cannon, M.D. Professor of Clinical Orthopaedic Surgery University of California San Francisco
 Knee Pain And Injuries In Adults W. Dilworth Cannon, M.D. Professor of Clinical Orthopaedic Surgery University of California San Francisco Pain Control Overview Narcotics rarely necessary after 1 st 1-2
Knee Pain And Injuries In Adults W. Dilworth Cannon, M.D. Professor of Clinical Orthopaedic Surgery University of California San Francisco Pain Control Overview Narcotics rarely necessary after 1 st 1-2
FAI syndrome with or without labral tear.
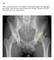 Case This 16-year-old female, soccer athlete was treated for pain in the right groin previously. Now has acute onset of pain in the left hip. The pain was in the groin that was worse with activities. Diagnosis
Case This 16-year-old female, soccer athlete was treated for pain in the right groin previously. Now has acute onset of pain in the left hip. The pain was in the groin that was worse with activities. Diagnosis
MRI of Cartilage. D. BENDAHAN (PhD)
 MRI of Cartilage D. BENDAHAN (PhD) Centre de Résonance Magnétique Biologique et Médicale UMR CNRS 7339 Faculté de Médecine de la Timone 27, Bd J. Moulin 13005 Marseille France david.bendahan@univ-amu.fr
MRI of Cartilage D. BENDAHAN (PhD) Centre de Résonance Magnétique Biologique et Médicale UMR CNRS 7339 Faculté de Médecine de la Timone 27, Bd J. Moulin 13005 Marseille France david.bendahan@univ-amu.fr
IAIABC 2003 Lower Extremity Impairment Guides Part 4 of the Supplemental Impairment Rating Guides
 IAIABC 2003 Lower Extremity Impairment Guides Part 4 of the Supplemental Impairment Rating Guides Draft 11-03 IAIABC Executive Office 5610 Medical Circle, Suite 24 Madison, WI 53719 Phone: (608) 663-6355
IAIABC 2003 Lower Extremity Impairment Guides Part 4 of the Supplemental Impairment Rating Guides Draft 11-03 IAIABC Executive Office 5610 Medical Circle, Suite 24 Madison, WI 53719 Phone: (608) 663-6355
JMSCR Vol 05 Issue 01 Page January
 www.jmscr.igmpublication.org Impact Factor 5.244 Index Copernicus Value: 83.27 ISSN (e)-2347-176x ISSN (p) 2455-0450 DOI: https://dx.doi.org/10.18535/jmscr/v5i1.28 Diagnostic Accuracy of Magnetic Resonance
www.jmscr.igmpublication.org Impact Factor 5.244 Index Copernicus Value: 83.27 ISSN (e)-2347-176x ISSN (p) 2455-0450 DOI: https://dx.doi.org/10.18535/jmscr/v5i1.28 Diagnostic Accuracy of Magnetic Resonance
Sponsor / Company: sanofi-aventis and Proctor & Gamble Drug substance(s): Risedronate (HMR4003)
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: sanofi-aventis and
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: sanofi-aventis and
ORIGINAL ARTICLE. ROLE OF MRI IN EVALUATION OF TRAUMATIC KNEE INJURIES Saurabh Chaudhuri, Priscilla Joshi, Mohit Goel
 ROLE OF MRI IN EVALUATION OF TRAUMATIC KNEE INJURIES Saurabh Chaudhuri, Priscilla Joshi, Mohit Goel 1. Associate Professor, Department of Radiodiagnosis & imaging, Bharati Vidyapeeth Medical College and
ROLE OF MRI IN EVALUATION OF TRAUMATIC KNEE INJURIES Saurabh Chaudhuri, Priscilla Joshi, Mohit Goel 1. Associate Professor, Department of Radiodiagnosis & imaging, Bharati Vidyapeeth Medical College and
2.0 Synopsis. Choline fenofibrate capsules (ABT-335) M Clinical Study Report R&D/06/772. (For National Authority Use Only) Name of Study Drug:
 2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: Choline Fenofibrate (335) Name of Active Ingredient:
2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: Choline Fenofibrate (335) Name of Active Ingredient:
Prevalence of Meniscal Radial Tears of the Knee Revealed by MRI After Surgery
 Downloaded from www.ajronline.org by 46.3.207.114 on 12/22/17 from IP address 46.3.207.114. Copyright RRS. For personal use only; all rights reserved Thomas Magee 1 Marc Shapiro David Williams Received
Downloaded from www.ajronline.org by 46.3.207.114 on 12/22/17 from IP address 46.3.207.114. Copyright RRS. For personal use only; all rights reserved Thomas Magee 1 Marc Shapiro David Williams Received
Osteochondritis Dissecans
 Osteochondritis Dissecans Introduction Osteochondritis dissecans (OCD) is a problem that affects the knee, mostly at the end of the big bone of the thigh (the femur). A joint surface damaged by OCD doesn't
Osteochondritis Dissecans Introduction Osteochondritis dissecans (OCD) is a problem that affects the knee, mostly at the end of the big bone of the thigh (the femur). A joint surface damaged by OCD doesn't
Clinical Trial Results Summary Study EN3409-BUP-305
 Title of Study: A 52-Week, Open-Label, Long-Term Treatment Evaluation of the Safety and Efficacy of BEMA Buprenorphine in Subjects with Moderate to Severe Chronic Pain Coordinating Investigator: Martin
Title of Study: A 52-Week, Open-Label, Long-Term Treatment Evaluation of the Safety and Efficacy of BEMA Buprenorphine in Subjects with Moderate to Severe Chronic Pain Coordinating Investigator: Martin
Treatment of meniscal lesions and isolated lesions of the anterior cruciate ligament of the knee in adults
 QUICK REFERENCE GUIDE Treatment of meniscal s and isolated s of the anterior cruciate ligament of the knee in adults June 2008 AIM OF THE GUIDELINES To encourage good practices in the areas of meniscal
QUICK REFERENCE GUIDE Treatment of meniscal s and isolated s of the anterior cruciate ligament of the knee in adults June 2008 AIM OF THE GUIDELINES To encourage good practices in the areas of meniscal
Rehabilitation Guidelines for Knee Arthroscopy
 Rehabilitation Guidelines for Knee Arthroscopy The knee is the body's largest joint, and the place where the femur, tibia, and patella meet to form a hinge-like joint. These bones are supported by a large
Rehabilitation Guidelines for Knee Arthroscopy The knee is the body's largest joint, and the place where the femur, tibia, and patella meet to form a hinge-like joint. These bones are supported by a large
Mr Aslam Mohammed FRCS, FRCS (Orth) Consultant Orthopaedic Surgeon Specialising in Lower Limb Arthroplasty and Sports Injury
 Mr Aslam Mohammed FRCS, FRCS (Orth) Consultant Orthopaedic Surgeon Specialising in Lower Limb Arthroplasty and Sports Injury I qualified from the Welsh National School of Medicine in Cardiff in 1984. I
Mr Aslam Mohammed FRCS, FRCS (Orth) Consultant Orthopaedic Surgeon Specialising in Lower Limb Arthroplasty and Sports Injury I qualified from the Welsh National School of Medicine in Cardiff in 1984. I
(For National Authority Use Only) Name of Study Drug: to Part of Dossier:
 2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: ABT-335 Name of Active Ingredient: Page: ABT-335, A-7770335.115
2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: ABT-335 Name of Active Ingredient: Page: ABT-335, A-7770335.115
WORKPLACE SAFETY AND INSURANCE APPEALS TRIBUNAL DECISION NO. 2023/14
 WORKPLACE SAFETY AND INSURANCE APPEALS TRIBUNAL DECISION NO. 2023/14 BEFORE: A. T. Patterson: Vice-Chair HEARING: November 3, 2014 at Toronto Oral DATE OF DECISION: April 17, 2015 NEUTRAL CITATION: 2015
WORKPLACE SAFETY AND INSURANCE APPEALS TRIBUNAL DECISION NO. 2023/14 BEFORE: A. T. Patterson: Vice-Chair HEARING: November 3, 2014 at Toronto Oral DATE OF DECISION: April 17, 2015 NEUTRAL CITATION: 2015
Hydrocodone/Acetaminophen Extended-Release Tablets M Clinical Study Report R&D/09/1109
 2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: ABT-712 Volume: Hydrocodone/Acetaminophen Extended-Release Name
2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: ABT-712 Volume: Hydrocodone/Acetaminophen Extended-Release Name
Ampion TM. Management Presentation 2018
 TM Management Presentation 2018 2 Forward-Looking Statement These slides and materials, including any accompanying oral presentation, contain forward-looking statements about our business. You should not
TM Management Presentation 2018 2 Forward-Looking Statement These slides and materials, including any accompanying oral presentation, contain forward-looking statements about our business. You should not
The Aging Athletes Knee
 The Aging Athletes Knee Douglas P Tewes Orthopedic Sports Medicine Lincoln Orthopedic Center 1 Common Sports Knee Injuries Cartilage defects/chondromalacia Meniscal tears ACL tear Articular Cartilage injury/chondromalacia
The Aging Athletes Knee Douglas P Tewes Orthopedic Sports Medicine Lincoln Orthopedic Center 1 Common Sports Knee Injuries Cartilage defects/chondromalacia Meniscal tears ACL tear Articular Cartilage injury/chondromalacia
Anterior Cruciate Ligament Injuries
 Anterior Cruciate Ligament Injuries One of the most common knee injuries is an anterior cruciate ligament sprain or tear.athletes who participate in high demand sports like soccer, football, and basketball
Anterior Cruciate Ligament Injuries One of the most common knee injuries is an anterior cruciate ligament sprain or tear.athletes who participate in high demand sports like soccer, football, and basketball
D. Doré 1, C. Ding 1,2, J.P. Pelletier 3, J. Martel-Pelletier 3, F. Cicuttini 2, G. Jones 1.
 Responsiveness of qualitative and quantitative MRI measures over 2.7 years D. Doré 1, C. Ding 1,2, J.P. Pelletier 3, J. Martel-Pelletier 3, F. Cicuttini 2, G. Jones 1. 1 Menzies Research Institute Tasmania,
Responsiveness of qualitative and quantitative MRI measures over 2.7 years D. Doré 1, C. Ding 1,2, J.P. Pelletier 3, J. Martel-Pelletier 3, F. Cicuttini 2, G. Jones 1. 1 Menzies Research Institute Tasmania,
Anterior Cruciate Ligament (ACL) Injuries
 Anterior Cruciate Ligament (ACL) Injuries Mark L. Wood, MD The anterior cruciate ligament (ACL) is one of the most commonly injured ligaments of the knee. The incidence of ACL injuries is currently estimated
Anterior Cruciate Ligament (ACL) Injuries Mark L. Wood, MD The anterior cruciate ligament (ACL) is one of the most commonly injured ligaments of the knee. The incidence of ACL injuries is currently estimated
Meniscus Tears. Three bones meet to form your knee joint: your thighbone (femur), shinbone (tibia), and kneecap (patella).
 Meniscus Tears Information on meniscus tears is also available in Spanish: Desgarros de los meniscus (topic.cfm?topic=a00470) and Portuguese: Rupturas do menisco (topic.cfm?topic=a00754). Meniscus tears
Meniscus Tears Information on meniscus tears is also available in Spanish: Desgarros de los meniscus (topic.cfm?topic=a00470) and Portuguese: Rupturas do menisco (topic.cfm?topic=a00754). Meniscus tears
Medial Knee Osteoarthritis Precedes Medial Meniscal Posterior Root Tear with an Event of Painful Popping
 Medial Knee Osteoarthritis Precedes Medial Meniscal Posterior Root Tear with an Event of Painful Popping Dhong Won Lee, M.D, Ji Nam Kim, M.D., Jin Goo Kim, M.D., Ph.D. KonKuk University Medical Center
Medial Knee Osteoarthritis Precedes Medial Meniscal Posterior Root Tear with an Event of Painful Popping Dhong Won Lee, M.D, Ji Nam Kim, M.D., Jin Goo Kim, M.D., Ph.D. KonKuk University Medical Center
Meniscal Tears with Fragments Displaced: What you need to know.
 Meniscal Tears with Fragments Displaced: What you need to know. Poster No.: C-1339 Congress: ECR 2015 Type: Authors: Keywords: DOI: Educational Exhibit M. V. Ferrufino, A. Stroe, E. Cordoba, A. Dehesa,
Meniscal Tears with Fragments Displaced: What you need to know. Poster No.: C-1339 Congress: ECR 2015 Type: Authors: Keywords: DOI: Educational Exhibit M. V. Ferrufino, A. Stroe, E. Cordoba, A. Dehesa,
CLINICAL PRESENTATION AND RADIOLOGY QUIZ QUESTION
 Donald L. Renfrew, MD Radiology Associates of the Fox Valley, 333 N. Commercial Street, Suite 100, Neenah, WI 54956 12/01/2012 Radiology Quiz of the Week # 101 Page 1 CLINICAL PRESENTATION AND RADIOLOGY
Donald L. Renfrew, MD Radiology Associates of the Fox Valley, 333 N. Commercial Street, Suite 100, Neenah, WI 54956 12/01/2012 Radiology Quiz of the Week # 101 Page 1 CLINICAL PRESENTATION AND RADIOLOGY
DECISION NO. 2870/16
 Counsel: H.K., for Worker No one for Employer 2016 ONWSIAT 3235 Ontario Workplace Safety and Insurance Appeals Tribunal Decision No. 2870/16 2016 CarswellOnt 19003, 2016 ONWSIAT 3235 DECISION NO. 2870/16
Counsel: H.K., for Worker No one for Employer 2016 ONWSIAT 3235 Ontario Workplace Safety and Insurance Appeals Tribunal Decision No. 2870/16 2016 CarswellOnt 19003, 2016 ONWSIAT 3235 DECISION NO. 2870/16
Page: 17 December 2012 (Study M13-692) 22 October 2013 (Study M13-692)
 2.0 Synopsis AbbVie Inc. Name of Study Drug: Adalimumab Name of Active Ingredient: D2E7 Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Title of Studies:
2.0 Synopsis AbbVie Inc. Name of Study Drug: Adalimumab Name of Active Ingredient: D2E7 Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Title of Studies:
Life. Uncompromised. The KineSpring Knee Implant System Surgeon Handout
 Life Uncompromised The KineSpring Knee Implant System Surgeon Handout 2 Patient Selection Criteria Patient Selection Criteria Medial compartment degeneration must be confirmed radiographically or arthroscopically
Life Uncompromised The KineSpring Knee Implant System Surgeon Handout 2 Patient Selection Criteria Patient Selection Criteria Medial compartment degeneration must be confirmed radiographically or arthroscopically
CLINICAL PRESENTATION AND RADIOLOGY QUIZ QUESTION
 Donald L. Renfrew, MD Radiology Associates of the Fox Valley, 333 N. Commercial Street, Suite 100, Neenah, WI 54956 7/28/2012 Radiology Quiz of the Week # 83 Page 1 CLINICAL PRESENTATION AND RADIOLOGY
Donald L. Renfrew, MD Radiology Associates of the Fox Valley, 333 N. Commercial Street, Suite 100, Neenah, WI 54956 7/28/2012 Radiology Quiz of the Week # 83 Page 1 CLINICAL PRESENTATION AND RADIOLOGY
Diagnosis & Nonoperative Treatment of the Osteoarthritic Knee. Randall R Wroble MD Orthopedic One Columbus OH
 Diagnosis & Nonoperative Treatment of the Osteoarthritic Knee Randall R Wroble MD Orthopedic One Columbus OH There are 2 things a good doctor does First Step: Finds out what's wrong Second step Makes the
Diagnosis & Nonoperative Treatment of the Osteoarthritic Knee Randall R Wroble MD Orthopedic One Columbus OH There are 2 things a good doctor does First Step: Finds out what's wrong Second step Makes the
THE KNEE JOINT. At a glance. 1. Introduction
 THE KNEE JOINT At a glance As explained in more detail below, the knee is a very complex joint. Owing to its anatomical structure, it is extremely prone not only to injury, but to wear and tear as well.
THE KNEE JOINT At a glance As explained in more detail below, the knee is a very complex joint. Owing to its anatomical structure, it is extremely prone not only to injury, but to wear and tear as well.
Comparative study of high resolusion ultrasonography and magnetic resonance imaging in diagnosing traumatic knee injuries & pathologies
 Original article: Comparative study of high resolusion ultrasonography and magnetic resonance imaging in diagnosing traumatic knee injuries & pathologies Dr. Rakesh Gujjar*, Dr. R. P. Bansal, Dr. Sandeep
Original article: Comparative study of high resolusion ultrasonography and magnetic resonance imaging in diagnosing traumatic knee injuries & pathologies Dr. Rakesh Gujjar*, Dr. R. P. Bansal, Dr. Sandeep
ACL Athletic Career. ACL Rupture - Warning Features Intensive pain Immediate swelling Locking Feel a Pop Dead leg Cannot continue to play
 FIMS Ambassador Tour to Eastern Europe, 2004 Belgrade, Serbia Montenegro Acute Knee Injuries - Controversies and Challenges Professor KM Chan OBE, JP President of FIMS Belgrade ACL Athletic Career ACL
FIMS Ambassador Tour to Eastern Europe, 2004 Belgrade, Serbia Montenegro Acute Knee Injuries - Controversies and Challenges Professor KM Chan OBE, JP President of FIMS Belgrade ACL Athletic Career ACL
Anterior Cruciate Ligament (ACL)
 Anterior Cruciate Ligament (ACL) The anterior cruciate ligament (ACL) is one of the 4 major ligament stabilizers of the knee. ACL tears are among the most common major knee injuries in active people of
Anterior Cruciate Ligament (ACL) The anterior cruciate ligament (ACL) is one of the 4 major ligament stabilizers of the knee. ACL tears are among the most common major knee injuries in active people of
CLINICAL PRESENTATION AND RADIOLOGY QUIZ QUESTION
 Donald L. Renfrew, MD Radiology Associates of the Fox Valley, 333 N. Commercial Street, Suite 100, Neenah, WI 54956 11/24/2012 Radiology Quiz of the Week # 100 Page 1 CLINICAL PRESENTATION AND RADIOLOGY
Donald L. Renfrew, MD Radiology Associates of the Fox Valley, 333 N. Commercial Street, Suite 100, Neenah, WI 54956 11/24/2012 Radiology Quiz of the Week # 100 Page 1 CLINICAL PRESENTATION AND RADIOLOGY
Relieving Arthritis Knee Pain Michael J. Repine MD Boulder Medical Center Orthopedics
 Arthritis is COMMON Relieving Arthritis Michael J. Repine MD Boulder Medical Center Orthopedics 303-622-3172 More than 43 million people have some form of arthritis. It is estimated that the number of
Arthritis is COMMON Relieving Arthritis Michael J. Repine MD Boulder Medical Center Orthopedics 303-622-3172 More than 43 million people have some form of arthritis. It is estimated that the number of
Immediate-release Hydrocodone/Acetaminophen M Abbreviated Clinical Study Report R&D/08/1020
 2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: Hydrocodone Bitartrate- Acetaminophen (NORCO ) Name of
2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: Volume: Hydrocodone Bitartrate- Acetaminophen (NORCO ) Name of
Case 27 Clinical Presentation
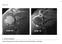 53 Case 27 Clinical Presentation 40-year-old man presents with acute shoulder pain and normal findings on radiographs. 54 RadCases Musculoskeletal Radiology Imaging Findings (,) Coronal images of the shoulder
53 Case 27 Clinical Presentation 40-year-old man presents with acute shoulder pain and normal findings on radiographs. 54 RadCases Musculoskeletal Radiology Imaging Findings (,) Coronal images of the shoulder
Epidemiology. Meniscal Injury & Repair. Meniscus Anatomy. Meniscus Anatomy
 Epidemiology 60-70/100,000 per year Meniscal Injury & Repair Arthroscopic Mensiscectomy One of the most common orthopaedic procedures 20% of all surgeries at some centers Male:Female ratio - 2-4:1 Younger
Epidemiology 60-70/100,000 per year Meniscal Injury & Repair Arthroscopic Mensiscectomy One of the most common orthopaedic procedures 20% of all surgeries at some centers Male:Female ratio - 2-4:1 Younger
Patellofemoral Replacement
 Patellofemoral Replacement During knee replacement surgery, damaged bone and cartilage is resurfaced with metal and plastic components. Patellofemoral replacement is a type of "partial" knee replacement
Patellofemoral Replacement During knee replacement surgery, damaged bone and cartilage is resurfaced with metal and plastic components. Patellofemoral replacement is a type of "partial" knee replacement
American College of Physicians 2013 Ohio Chapter Scientific Meeting Columbus, OH October 11, 2013
 American College of Physicians 2013 Ohio Chapter Scientific Meeting Columbus, OH October 11, 2013 Paul J. Gubanich, MD, MPH Assistant Professor of Internal Medicine/Sports Medicine Team Physician, Ohio
American College of Physicians 2013 Ohio Chapter Scientific Meeting Columbus, OH October 11, 2013 Paul J. Gubanich, MD, MPH Assistant Professor of Internal Medicine/Sports Medicine Team Physician, Ohio
SYNOPSIS. Study center(s) This study was conducted in the United States (128 centers).
 Drug product: Drug substance(s): Document No.: Edition No.: Study code: Date: SYMBICORT pmdi 160/4.5 µg Budesonide/formoterol SD-039-0725 17 February 2005 SYNOPSIS A Twelve-Week, Randomized, Double-blind,
Drug product: Drug substance(s): Document No.: Edition No.: Study code: Date: SYMBICORT pmdi 160/4.5 µg Budesonide/formoterol SD-039-0725 17 February 2005 SYNOPSIS A Twelve-Week, Randomized, Double-blind,
Arthritis of the Knee
 Arthritis of the Knee There are three basic types of arthritis that may affect the knee joint. Osteoarthritis Osteoarthritis (OA) is the most common form of knee arthritis. OA is usually a slowly progressive
Arthritis of the Knee There are three basic types of arthritis that may affect the knee joint. Osteoarthritis Osteoarthritis (OA) is the most common form of knee arthritis. OA is usually a slowly progressive
Original Date: December 2015 Page 1 of 8 FOR CMS (MEDICARE) MEMBERS ONLY
 National Imaging Associates, Inc. Clinical guidelines TOTAL JOINT ARTHROPLASTY -Total Hip Arthroplasty -Total Knee Arthroplasty -Replacement/Revision Hip or Knee Arthroplasty CPT4 Codes: Please refer to
National Imaging Associates, Inc. Clinical guidelines TOTAL JOINT ARTHROPLASTY -Total Hip Arthroplasty -Total Knee Arthroplasty -Replacement/Revision Hip or Knee Arthroplasty CPT4 Codes: Please refer to
Study Number CAIN457C2302 (core study) and CAIN457C2302E1 (extension study)
 Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Uveitis Approved Indication Investigational Study Number CC2302 (core study) and CC2302E1
Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Uveitis Approved Indication Investigational Study Number CC2302 (core study) and CC2302E1
Study No. 178-CL-008 Report Final Version, 14 Dec 2006 Reissued Version, 18 Jul 2011 Astellas Pharma Europe B.V. Page 13 of 122
 Page 13 of 122 3 SYNOPSIS Title of study: (International) Study No: A randomized, double-blind, parallel group, proof-of-concept study of in comparison with placebo and tolterodine in patients with symptomatic
Page 13 of 122 3 SYNOPSIS Title of study: (International) Study No: A randomized, double-blind, parallel group, proof-of-concept study of in comparison with placebo and tolterodine in patients with symptomatic
New Evidence Suggests that Work Related Knee Pain with Degenerative Complications May Not Require Surgery
 4 th Quarter 2017 New Evidence Suggests that Work Related Knee Pain with Degenerative Complications May Not Require Surgery By: Michael Erdil MD, FACOEM Introduction It is estimated there are approximately
4 th Quarter 2017 New Evidence Suggests that Work Related Knee Pain with Degenerative Complications May Not Require Surgery By: Michael Erdil MD, FACOEM Introduction It is estimated there are approximately
Evaluation and Management of Knee Pain. Michael Cassat, MD University of Arkansas for Medical Sciences
 Evaluation and Management of Knee Pain Michael Cassat, MD University of Arkansas for Medical Sciences Disclosure I have no actual or potential conflict of interest in relation to this program/presentation.
Evaluation and Management of Knee Pain Michael Cassat, MD University of Arkansas for Medical Sciences Disclosure I have no actual or potential conflict of interest in relation to this program/presentation.
2.0 Synopsis. ABT-711 M Clinical Study Report R&D/06/054. (For National Authority Use Only) Referring to Part of Dossier: Volume: Page:
 2.0 Synopsis Abbott Laboratories Name of Study Drug: Depakote ER Name of Active Ingredient: Divalproex Sodium Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority
2.0 Synopsis Abbott Laboratories Name of Study Drug: Depakote ER Name of Active Ingredient: Divalproex Sodium Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority
2.0 Synopsis. Adalimumab R&D/04/118. (For National Authority Use Only) Referring to Part of Dossier: Volume:
 2.0 Synopsis Abbott Laboratories Name of Study Drug: Adalimumab Name of Active Ingredient: Adalimumab Title of Study: Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority
2.0 Synopsis Abbott Laboratories Name of Study Drug: Adalimumab Name of Active Ingredient: Adalimumab Title of Study: Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
A Patient s Guide to Osteochondritis Dissecans of the Knee
 A Patient s Guide to Osteochondritis Dissecans of the Knee 2350 Royal Boulevard Suite 200 Elgin, IL 60123 Phone: 847.931.5300 Fax: 847.931.9072 DISCLAIMER: The information in this booklet is compiled from
A Patient s Guide to Osteochondritis Dissecans of the Knee 2350 Royal Boulevard Suite 200 Elgin, IL 60123 Phone: 847.931.5300 Fax: 847.931.9072 DISCLAIMER: The information in this booklet is compiled from
Clinical Trial Results Summary Study AUX-CC-854
 Name of Sponsor/Company: Auxilium Pharmaceuticals, Inc. Name of Finished Product: AA4500 Name of Active Ingredient: Collagenase clostridium histolyticum Title of Study: A Phase 3, Open-Label Study of the
Name of Sponsor/Company: Auxilium Pharmaceuticals, Inc. Name of Finished Product: AA4500 Name of Active Ingredient: Collagenase clostridium histolyticum Title of Study: A Phase 3, Open-Label Study of the
SM04690: Potential first-in-class disease modifying treatment for knee osteoarthritis. Nancy Lane, MD
 SM04690: Potential first-in-class disease modifying treatment for knee osteoarthritis Nancy Lane, MD 1 Disclosures Yusuf Yazici Timothy McAlindon Allan Gibofsky Nancy Lane Daniel Clauw Christopher Swearingen
SM04690: Potential first-in-class disease modifying treatment for knee osteoarthritis Nancy Lane, MD 1 Disclosures Yusuf Yazici Timothy McAlindon Allan Gibofsky Nancy Lane Daniel Clauw Christopher Swearingen
Overview Ligament Injuries. Anatomy. Epidemiology Very commonly injured joint. ACL Injury 20/06/2016. Meniscus Tears. Patellofemoral Problems
 Overview Ligament Injuries Meniscus Tears Pankaj Sharma MBBS, FRCS (Tr & Orth) Consultant Orthopaedic Surgeon Manchester Royal Infirmary Patellofemoral Problems Knee Examination Anatomy Epidemiology Very
Overview Ligament Injuries Meniscus Tears Pankaj Sharma MBBS, FRCS (Tr & Orth) Consultant Orthopaedic Surgeon Manchester Royal Infirmary Patellofemoral Problems Knee Examination Anatomy Epidemiology Very
Full Novartis CTRD Results Template
 Full Novartis CTRD Results Template Sponsor Novartis Generic Drug Name vildagliptin Therapeutic Area of Trial Type 2 diabetes Approved Indication Type 2 diabetes Protocol Number CLAF237A23138E1 Title A
Full Novartis CTRD Results Template Sponsor Novartis Generic Drug Name vildagliptin Therapeutic Area of Trial Type 2 diabetes Approved Indication Type 2 diabetes Protocol Number CLAF237A23138E1 Title A
A Comparative Study of Ultrasonographic Findings with Clinical and Radiological Findings of Painful Osteoarthritis of the Knee Joint
 Med. J. Cairo Univ., Vol. 84, No. 3, December: 97-, www.medicaljournalofcairouniversity.net A Comparative Study of Ultrasonographic Findings with Clinical and Radiological Findings of Painful Osteoarthritis
Med. J. Cairo Univ., Vol. 84, No. 3, December: 97-, www.medicaljournalofcairouniversity.net A Comparative Study of Ultrasonographic Findings with Clinical and Radiological Findings of Painful Osteoarthritis
The posterolateral corner of the knee: the normal and the pathological
 The posterolateral corner of the knee: the normal and the pathological Poster No.: P-0104 Congress: ESSR 2014 Type: Educational Poster Authors: M. Bartocci 1, C. Dell'atti 2, E. Federici 1, V. Martinelli
The posterolateral corner of the knee: the normal and the pathological Poster No.: P-0104 Congress: ESSR 2014 Type: Educational Poster Authors: M. Bartocci 1, C. Dell'atti 2, E. Federici 1, V. Martinelli
A Guide to Common Ankle Injuries
 A Guide to Common Ankle Injuries Learn About: Common ankle injuries Feet and Ankle Diagnosis and Treatment Ankle exercises Beginning your recovery Frequently asked questions Do s and Don t s Arthroscopy
A Guide to Common Ankle Injuries Learn About: Common ankle injuries Feet and Ankle Diagnosis and Treatment Ankle exercises Beginning your recovery Frequently asked questions Do s and Don t s Arthroscopy
Anterior Cruciate Ligament (ACL) Injuries
 Anterior Cruciate Ligament (ACL) Injuries This article is also available in Spanish: Lesiones del ligamento cruzado anterior (topic.cfm?topic=a00697) and Portuguese: Lesões do ligamento cruzado anterior
Anterior Cruciate Ligament (ACL) Injuries This article is also available in Spanish: Lesiones del ligamento cruzado anterior (topic.cfm?topic=a00697) and Portuguese: Lesões do ligamento cruzado anterior
Corporate Medical Policy
 Corporate Medical Policy Continuous Passive Motion in the Home Setting File Name: Origination: Last CAP Review: Next CAP Review: Last Review: continuous_passive_motion_in_the_home_setting 9/1993 6/2018
Corporate Medical Policy Continuous Passive Motion in the Home Setting File Name: Origination: Last CAP Review: Next CAP Review: Last Review: continuous_passive_motion_in_the_home_setting 9/1993 6/2018
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
FieldStrength. Achieva 3.0T enables cutting-edge applications, best-in-class MSK images
 FieldStrength Publication for the Philips MRI Community Issue 33 December 2007 Achieva 3.0T enables cutting-edge applications, best-in-class MSK images Palo Alto Medical Clinic Sports Medicine Center employs
FieldStrength Publication for the Philips MRI Community Issue 33 December 2007 Achieva 3.0T enables cutting-edge applications, best-in-class MSK images Palo Alto Medical Clinic Sports Medicine Center employs
BASELINE QUESTIONNAIRE (SURGEON)
 SECTION A: STUDY INFORMATION Subject ID: - - Study Visit: Baseline Site Number: Date: / / Surgeon ID: SECTION B: INITIAL SURGEON HISTORY B1. Previous Knee Surgery: Yes No Not recorded B2. Number of Previous
SECTION A: STUDY INFORMATION Subject ID: - - Study Visit: Baseline Site Number: Date: / / Surgeon ID: SECTION B: INITIAL SURGEON HISTORY B1. Previous Knee Surgery: Yes No Not recorded B2. Number of Previous
Effectiveness of True Acupuncture as an Adjunct to Standard Care or Electro-Physiotherapy in Osteoarthritis of the Knee
 Cronicon OPEN ACCESS ORTHOPAEDICS Research article Effectiveness of True Acupuncture as an Adjunct to Standard Care or Electro-Physiotherapy in Osteoarthritis of Dimitar Tonev 1 *, Stoyka Radeva 2 and
Cronicon OPEN ACCESS ORTHOPAEDICS Research article Effectiveness of True Acupuncture as an Adjunct to Standard Care or Electro-Physiotherapy in Osteoarthritis of Dimitar Tonev 1 *, Stoyka Radeva 2 and
Case study #11 Rt. knee
 The patient is a 55 year old female who presents with bilateral knee pain. Patient is a collegiate softball coach and has a very active lifestyle and career that is hampered by her chronic knee pain. She
The patient is a 55 year old female who presents with bilateral knee pain. Patient is a collegiate softball coach and has a very active lifestyle and career that is hampered by her chronic knee pain. She
Medical Policy Original Effective Date: Revised Date: 07/26/17 Page 1 of 9
 Page 1 of 9 Disclaimer Description Coverage Determination/ Clinical Indications Refer to the member s specific benefit plan and Schedule of Benefits to determine coverage. This may not be a benefit on
Page 1 of 9 Disclaimer Description Coverage Determination/ Clinical Indications Refer to the member s specific benefit plan and Schedule of Benefits to determine coverage. This may not be a benefit on
MRI grading of postero-lateral corner and anterior cruciate ligament injuries
 MRI grading of postero-lateral corner and anterior cruciate ligament injuries Poster No.: C-2533 Congress: ECR 2012 Type: Educational Exhibit Authors: J. Lopes Dias, J. A. Sousa Pereira, L. Fernandes,
MRI grading of postero-lateral corner and anterior cruciate ligament injuries Poster No.: C-2533 Congress: ECR 2012 Type: Educational Exhibit Authors: J. Lopes Dias, J. A. Sousa Pereira, L. Fernandes,
DISEASES AND DISORDERS
 DISEASES AND DISORDERS 9. 53 10. Rheumatoid arthritis 59 11. Spondyloarthropathies 69 12. Connective tissue diseases 77 13. Osteoporosis and metabolic bone disease 95 14. Crystal arthropathies 103 15.
DISEASES AND DISORDERS 9. 53 10. Rheumatoid arthritis 59 11. Spondyloarthropathies 69 12. Connective tissue diseases 77 13. Osteoporosis and metabolic bone disease 95 14. Crystal arthropathies 103 15.
2.0 Synopsis. ABT-711 M Clinical Study Report R&D/06/573. (For National Authority Use Only) to Part of Dossier: Volume:
 2.0 Synopsis Abbott Laboratories Name of Study Drug: Depakote ER Name of Active Ingredient: Divalproex sodium (ABT-711) Individual Study Table Referring to Part of Dossier: Volume: Page: (For National
2.0 Synopsis Abbott Laboratories Name of Study Drug: Depakote ER Name of Active Ingredient: Divalproex sodium (ABT-711) Individual Study Table Referring to Part of Dossier: Volume: Page: (For National
UNUSUAL ACL CASE: Tibial Eminence Fracture in a Female Collegiate Basketball Player
 UNUSUAL ACL CASE: Tibial Eminence Fracture in a Female Collegiate Basketball Player Cheri Drysdale, MEd,, ATC Margot Putukian,, MD Jeffery Bechler,, MD Princeton University How many of you have done an
UNUSUAL ACL CASE: Tibial Eminence Fracture in a Female Collegiate Basketball Player Cheri Drysdale, MEd,, ATC Margot Putukian,, MD Jeffery Bechler,, MD Princeton University How many of you have done an
Comparison of effects of Mckenzie exercises and conventional therapy in ACL reconstruction on knee range of motion and functional ability
 2018; 4(4): 415-420 ISSN Print: 2394-7500 ISSN Online: 2394-5869 Impact Factor: 5.2 IJAR 2018; 4(4): 415-420 www.allresearchjournal.com Received: 25-02-2018 Accepted: 26-03-2018 Riya Sadana BPTh Student,
2018; 4(4): 415-420 ISSN Print: 2394-7500 ISSN Online: 2394-5869 Impact Factor: 5.2 IJAR 2018; 4(4): 415-420 www.allresearchjournal.com Received: 25-02-2018 Accepted: 26-03-2018 Riya Sadana BPTh Student,
Medical Diagnosis for Michael s Knee
 Medical Diagnosis for Michael s Knee Introduction The following report mainly concerns the diagnosis and treatment of the patient, Michael. Given that Michael s clinical problem surrounds an injury about
Medical Diagnosis for Michael s Knee Introduction The following report mainly concerns the diagnosis and treatment of the patient, Michael. Given that Michael s clinical problem surrounds an injury about
A Patient s Guide to Partial Knee Resurfacing
 A Patient s Guide to Partial Knee Resurfacing Surgical Outcomes System (SOS ) www.orthoillustrated.com OrthoIllustrated is a leading Internet-based resource for patient education. Please visit this website
A Patient s Guide to Partial Knee Resurfacing Surgical Outcomes System (SOS ) www.orthoillustrated.com OrthoIllustrated is a leading Internet-based resource for patient education. Please visit this website
Clinical Trial Synopsis TL-OPI-518, NCT#
 Clinical Trial Synopsis, NCT# 00225264 Title of Study: A Double-Blind, Randomized, Comparator-Controlled Study in Subjects With Type 2 Diabetes Mellitus Comparing the Effects of Pioglitazone HCl vs Glimepiride
Clinical Trial Synopsis, NCT# 00225264 Title of Study: A Double-Blind, Randomized, Comparator-Controlled Study in Subjects With Type 2 Diabetes Mellitus Comparing the Effects of Pioglitazone HCl vs Glimepiride
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Meniscal Root Tears: Evaluation, Imaging, and Repair Techniques
 Meniscal Root Tears: Evaluation, Imaging, and Repair Techniques R O B E R T N A S C I M E N TO, M D, M S C H I E F OF S P O RT S M E D I C I N E & SH O U L D E R S U R G E RY N E W TO N- W E L L E S L
Meniscal Root Tears: Evaluation, Imaging, and Repair Techniques R O B E R T N A S C I M E N TO, M D, M S C H I E F OF S P O RT S M E D I C I N E & SH O U L D E R S U R G E RY N E W TO N- W E L L E S L
