The MDI project in Mexico a V.A.R.I & Co. perspective. Gabriele Marchetti
|
|
|
- Brett McKinney
- 6 years ago
- Views:
Transcription
1 The MDI project in Mexico a V.A.R.I & Co. perspective. Gabriele Marchetti
2 A GAP in the market For the budget made available from the Multilateral fund, none of the traditional inhaler development companies were interested, partly due to the cost and partly due to the terms of reference.
3 V.A.R.I. S.p.A recognised an opportunity To integrate specialist service providers to create a highly responsive synergistic group, drawing on industry leading expertise, without incurring the overheads of a larger organization. And so was born V.A.R.I & Co.
4 VARI VARI, part of the LINDAL Group, is one of the world s leading manufacturer of valves and actuators for pharmaceutical aerosol systems. VARI have manufactured 20mm metering valves for CFC propellants for a wide range of pharmaceuticals applications for over 25 years, supplying pharmaceutical companies all around the world. VARI has an extensive experience in a range of territories including South America, Cuba, Africa, Europe, Russia and Asia and is the leading supplier with more than 90% of the pmdi valve market share in some of these regions.
5 VARI VARI facility is located in Oggiono (LC), in the North of Italy. The manufacturing area has 6000 square meters: 1500 Square metre cleanroom 300 Square metre laboratories 900 Square metre offices VARI services provided: Pharma components moulding (ISO8) pmdis valves assembling (ISO7) Other valves production (Nasal, Oral, Topical) Analytical support Engineering and technical support pmdi Formulation support (i2c partnership)
6 VARI FACILITY VARI new facility has been built from greenfield in 2009 with a state of the art positively pressurized cleanroom. VARI s cleanrooms are equipped with HEPA filters that allows both moulding and assembly of products in a tightly controlled environment, which with our associated procedures greatly reduces risks associated with product contamination The cleanroom injection moulding equipment are fully electric machines with clamping forces up to 110 tonnes. These machines include a dedicated clean air module above the clamping unit with HEPA filter (H14). All servomotors are fanless and ecapsulated. Full encasement of the discharge area in stainless steel. VARI assembling lines make in-process controls of 100% of the valves produced (components presence, dimensions, functionality, and dose checks). All valves are laser coded on the ferrule to allow full traceability of each valve.
7 VARI QUALITY SYSTEM VARI operates its systems to ensure full compliance of external regulatory expectations, although not a manufacturer of finished product VARI s approaches and procedures are consistent with the aims of cgmp. VARI maintains full traceable records of all inputs and outputs throughout the manufacturing process. VARI have implemented and maintains a quality system in compliance with ISO 9001:2008 and ISO 13485:2004. ISO 13485:2004 specifies requirements for a quality management system where an organisation needs to demonstrate its ability to provide medical devices and related services that consistently meet customer and regulatory requirements
8 VARI MARKET PRESENCE United Kingdom Poland Russia Mexico Cuba Italy Iran Syria Iraq Egypt Pakistan Bangladesh India Taiwan Brasil Argentina
9 i 2 C UK Location London Heathrow and Birmingham Airports are ~ 2 hours by rail or car from Cardiff. Cardiff London London Birmingham Bristol Bristol Airport is ~ 1 hour from Cardiff. i2c history 9
10 i 2 C : History 1980 s Pharmaceutics research group at Welsh School of Pharmacy, Cardiff University, pioneer uses of gamma scintigraphy to investigate lung deposition and gastrointestinal transit of novel dosage forms 1992 Cardiff Scintigraphics Limited (CSL) formed as an independent spin-off company from Cardiff University 2001: Simbec Scintigraphics (SS) joint venture formed between CSL and Simbec Research Limited (SRL) to promote clinical scintigraphy services 2005: SS included in Wales top 100 most enterprising new companies present: CSL relocated in Cardiff to promote in vitro evaluation of inhaled pharmaceuticals trading as i2c Pharmaceutical Services SS continues with clinical scintigraphy trials provided via an alliance between i 2 C and SRL i2c history 10
11 i 2 C : Facilities Unit 8 11
12 i2c: Facilities Projected Plan Unit 6 i2c history 12
13 i2c Pharm Aerosol Testing Equipment 13
14 Scintigraphic Analysis: Comparators Kr Vent DPI MDI MDI + Spacer Spacer 14
15 Who or What is PharmaDelivery Solutions Ltd? An independent consultancy (Founded July 2003) service focusing on Drug Delivery Device Technology. It draws actively, both on an extensive experience base and ongoing links to the drug delivery market. It has formed strategic associations that will allow us to offer an even broader compliment of inputs to accelerate the development process of products relying on device technology.
16 VARI HFA VALVE The VARI HFA valve is a new generation product based on a tried and tested design used in pharmaceuticals aerosol application worldwide for over twenty five years. It has been specifically developed for use with HydroFluoro Alkane propellants systems and incorporates the latest innovations and thinking in materials engineering to offer reliability, performance and clean product profile. The VARI HFA valve components are: FERRULE: Aluminum Silver Anodized INNER GASKET: Chlorobutyle STEM PBT / POM METERING CHAMBER TPE SPRING AISI 316 Stainless steel HOUSING PBT / POM OUTER GASKET Chlorobutyle OUTER RING LDPE Standard dosages are 25µl, 40µl, 50µl, 55µl, 63µl, other dosages available upon requests The valve is NOW commercially available in two versions: 3.00 mm stem (VARI Standard stem size) The new 3.16 mm stem diameter Detailed Type III DMF lodged with FDA (Nr April 2008)
17 Metering chamber refills following valve operation
18 Metering chamber empties to atmosphere PROPELLANT BOILS AND EXPANDS
19 Existing supplier Enhanced performance Coated Make and fill Time Temperature Homogenization Many considerations when Own CFC Brand CFC Brand HFA Other None None Water Acetic Acid Citric Acid Other Stabilizer Predicate product Packaging components Number of actuations Canister size Dose Volume developing an MDI Formulation preparation Product Profile Replacement HFA product Basic Approach Purging Filling approach Post fill clean Surfactant Solution Suspension None Vacuum Propellant in can Propellant through valve Single stage Two stage Cold Fill None Vacuum Propellant fill None Oleic acid Sorbitan tri-oleate Lecithin PEG Novel
20 Simplified outline of the programme
21 Original agreed products Salbutamol 200 dose, 100 µg/ dose label claim of Salbutamol Base (equivalent) from the valve (may be formulated using Salbutamol Sulphate and/ or specified in an acceptable manner as the Dose ex mouthpiece if agreed). Unless otherwise agreed the reference product for determination of equivalence shall be, Laboratorios Salus, ASSAL Beclamethasone dipropionate (High strength ) 200 Dose, 250 µg/ dose label claim ex valve of Beclamethasone dipropionate( in the form of a recognised, salt, solvate etc. If required) and/ or specified in an acceptable manner as the Dose ex mouthpiece if agreed) Unless otherwise agreed the reference product for determination of equivalence shall be, Laboratorios Salus DOBIPRO 250
22 Basic outline for provided technology Formulation Development Pilot Batches Pilot Stability Tech Transfer Validation M anufacture Industrial Stability Batches Equipment M anufacture Factory Acceptance Test Shipping, Site Acceptance Tests 24 M onths Stability 6 Months Accelerated Stability Regulatory Submission
23 General Objectives To develop HFA MDIs which as a minimum may not be considered inferior to the current local marketed HFA and CFC products. To develop HFA MDIs which (where appropriate) may not be considered inferior to equivalent international HFA MDIs which are (or may become) available in the local market. To develop HFA MDIs applying quality standards equivalent to international regulatory expectations.
24 General Objectives (continued) Draw upon formulation approaches which have been approved by multiple regulatory bodies with experience of inhaled therapeutics. Avoid the use of novel excipients where possible. Specification of commercially advantageous products through the avoidance of proprietary and/ or prohibitively expansive packaging components.
25 General Objectives (continued) Wherever possible draw on international clinical opinion on products to reduce the risk of generating clinical concerns. Reduce the Risk of IP infringement as far as is possible.
26 An example of the development process
27 Step 1 Literature review/ commercial products Ventolin Evohaler ProAir HFA Airomir/ Proventil HFA
28 Which reference product Current CFC product OR
29 Don t have to look the same to be equivalent
30 Risks of referencing a current CFC product May differ in specification to internationally approved HFA brands, which are the current competitor products. Significant clinical review of the internationally approved HFA brands has been undertaken, a different therapeutic profile would need to be considered locally. May un-necessarily and unfairly expose historic CFC products to scrutiny, using more current analytical techniques than were in place during original approval.
31 Ventolin Evohaler
32 Proair HFA
33 Airomir/ Proventil HFA
34 Overview All three of the international products are suspension of Salbutamol (as sulphate) Ventolin HFA only uses HFA 134a propellant Proair and Airomir contain Ethanol
35 Ethanol widely used Trade Name API No. Doses Label Claim mcg Dose of Active* mcg Dose of Salt* mcg Metered Volume mcl Alvesco Ciclesonide Alvesco Ciclesonide Alvesco Ciclesonide Atrovent HFA Ipratropium Bromide Combivent Ipratropium Bromide/ Salbutamol Sulphate /100 21/100 21/120 Flixotide 50 Fluticasone Propionate Flixotide 125 Fluticasone Propionate Flixotide 250 Fluticasone Propionate Ventolin EVOhaler Salbutamol Sulphate Seretide 50 EVOhaler Salmeterol xinafoate/ Fluticasone Propionate /50 25/50 25/50 63 Salmeterol xinafoate/ Seretide 125 EVOhaler Fluticasone Propionate /100 25/100 25/ Salmeterol xinafoate/ Fluticasone Propionate /250 25/250 25/ Seretide 250 EVOhaler Serevent Evohaler Salmeterol xinafoate Tilade CFC free nedocromil sodium Proventil HFA Salbutamol Sulphate QVAR 50 Beclomethasone dipropionate QVAR 100 Beclomethasone dipropionate Those shaded in green contain ethanol
36 Solubility aspects OK API WATER ETHANOL Salbutamol Sulphate Freely Soluble Practically Insoluble Salbutamol Base Sparingly soluble Soluble (96%) Levalbuterol HCL 180 mg/ml (Freely) Practically Insoluble Formoterol fumarate Slightly soluble Sparingly soluble Fluticasone Propionate practically insoluble slightly soluble 95% ethanol Ipratropium Bromide freely soluble Slightly soluble Mometasone furoate monohydrate practically insoluble Slightly soluble Beclamethosone Dipropianate Very slightly soluble Freely soluble/ Sparingly soluble (96%) Salmeterol Xinofoate sparingly soluble slightly soluble Salmeterol Base Slightly soluble Sparingly soluble Fenoterol hydrobromide Soluble Soluble Tiotropium Bromide sparingly soluble Soluble Nedocromil sodium Soluble Triamcinolone Acetonide practically insoluble Sparingly soluble SCG Soluble Practically Insoluble Bambuterol hydrochloride Freely Soluble Soluble Budesonide practically insoluble Sparingly soluble Terbutaline Sulphate 1 g / 1-5 ml (Freely) 1 g / 250 ml (Slightly)
37 Step 2 patent literature review The following patents were reviewed amongst many others MX A US 5,225,183 EP EP US 5,439,670 US 5,695,743 US 5,776,434
38 Step 3 SWOT analysis conducted on formulation approaches
39 Ethanol Containing Lower cost of goods (plain cans) Lower cost of goods higher outputs Lower GWP than propellant only formulations Improved moisture stability Simplified processing steps Contains ethanol More competitive for export markets S W O T Flammability in manufacturing
40 Ethanol Free No Ethanol More expensive Coated Cans More Expensive Lower output rate More complex to manufacture Higher GWP than ethanol containing formulation. Batch size may be limited at benificiary by equipment available. Closer to GSK product S W O T Known Stability issues at high humidity Closer to GSK patents May require Foil overwrap.
41 Impact of choosing the Propellant only approach Vs. ethanol containing Coated cans Foil overwrap Cost of goods as much as 50% greater
42 Orifice size 0,25 0,30 0,30 0,35 Vent Airomir Metering vol µl
43 Step 4 Tentative development specification Based on literature and product review ethanol contents in the range 5 to 10% appear to give acceptable performance. To achieve acceptable particle size distributions with HFA/ ethanol formulations spray orifii in the range 0,25 to 0,35 preferred. 25 or 50µl metering valves used.
44 Step 5 preliminary screening A number of trial formulations were prepared and evaluated against commercial innovator products.
45 Issue Ventolin and Airomir do not have equivalent performance, a balance between actual performance and most probable registered specification was developed.
46 Orifice size 0,25 0,30 0,30 0,35 Metering vol µl
47 Active % Deposition Active % Deposition NGI Distribution of Ventolin Ventolin 1 Ventolin 2 Ventolin 3 NGI NGI Distribution using 50 µl valve & 0.25 mm Nemo Actuator 7.35 % EtOH 8 % EtOH 10 % EtOH NGI
48 Active % Deposition Active % Deposition NGI Distribution of Airomir Airomir Airomir 2 Airomir 3 NGI NGI Distribution using 50 µl valve & 0.25 mm Nemo Actuator 7.35 % EtOH 8 % EtOH 10 % EtOH NGI
49 Step 6 Product specification based on optimum overall performance selected.
50 AGREE THE SPECIFICATION BASED ON THE Terms Of Reference (with a reality check)
51 The sensible approach To agree a target specification(s) based on published data (reviewed) and in-vitro assessment of internationally launched HFA products (where in existence). Where an internationally launched HFA products does not exist. To agree a target specification based on published data (reviewed) and in-vitro assessment of both the local and international CFC products.
52 Proposed approach Label claim Containing 108 µg per actuation (ex actuator) of Salbutamol sulphate (Equivalent to 90µg Salbutamol base) from the mouthpiece. Formulation contents containing HFA 134a ethanol and oleic acid. Reference product: 3M Qvar (Or an equivalent Brand Name) Justification: This is an internationally approved HFA MDI version of this product approved in over 55 countries, including UK, USA, Australia, New Zealand, France, Germany and Japan.
53 Ventolin/ Airomir as declared In can Salb Sulp Salb base μg/ puff μg/ puff From valve No loss Salb Sulp Salb base μg/ puff μg/ puff From Act 10% loss Salb Sulp Salb base μg/ puff μg/ puff
54 Typical published actuator loss Declared Ex Company Product Declared Ex- Valve µg/ puff Mouthpiece µg/ puff Loss % GSK Ventolin M Airomir Boehringer Ingelheim M Qvar M Qvar GSK Flovent GSK Flovent GSK Flovent GSK Serevent AstraZeneca *1 Symbicort 80/ AstraZeneca *1 Symbicort 160/ GSK Seretide As per the individual losses above 12 and 16
55 Stability at pilot scale was primarily to demonstrate the robustness of the formulation filled at pilot scale, not for registration.
56 Basic outline for provided technology Formulation Development Pilot Batches Pilot Stability Tech Transfer Validation M anufacture Industrial Stability Batches Equipment M anufacture Factory Acceptance Test Shipping, Site Acceptance Tests 24 M onths Stability 6 Months Accelerated Stability Regulatory Submission
57 A significant undertaking
58 The output 1075 pages of data 1620 pages of data
59 A mammoth undertaking
60 The story now becomes that of the industrialization Thank You
First to Market or 505 (b)2 CMC Considerations IPAC-RS/UF Orlando Inhalation Conference Orlando, Florida
 First to Market or 505 (b)2 CMC Considerations IPAC-RS/UF Orlando Inhalation Conference Orlando, Florida Prasad Peri, Ph.D., Branch Chief, ONDQA, FDA March 19, 2014 1 Topics for discussion Introduction
First to Market or 505 (b)2 CMC Considerations IPAC-RS/UF Orlando Inhalation Conference Orlando, Florida Prasad Peri, Ph.D., Branch Chief, ONDQA, FDA March 19, 2014 1 Topics for discussion Introduction
Inhalers containing CFCs. CFC-free inhalers
 Propellants used in medical metered dose inhalers and aerosol-based breath activated devices in New Zealand August to October 2002 (most recent period for which data is available) (February 2003) Inhalers
Propellants used in medical metered dose inhalers and aerosol-based breath activated devices in New Zealand August to October 2002 (most recent period for which data is available) (February 2003) Inhalers
Diagnosis and Management of Asthma
 Supporting Evidence: Diagnosis and Management of Asthma The subdivision of this section is: Appendix B Tables Copyright 2016 by 1 Eleventh Edition/December 2016 Appendix B Asthma Summary Tables Class:
Supporting Evidence: Diagnosis and Management of Asthma The subdivision of this section is: Appendix B Tables Copyright 2016 by 1 Eleventh Edition/December 2016 Appendix B Asthma Summary Tables Class:
040716_ANDI_VENTIS WEBSITE(new) Printout 1. HOME PAGE. Welcome to our world of Dry Powder Inhalers!
 1. HOME PAGE Welcome to our world of Dry Powder Inhalers! Andi-Ventis Ltd, established in 2001 is a joint venture between Medochemie Ltd, Cyprus and Miat SpA Italy, founded to carry out the pharmaceutical
1. HOME PAGE Welcome to our world of Dry Powder Inhalers! Andi-Ventis Ltd, established in 2001 is a joint venture between Medochemie Ltd, Cyprus and Miat SpA Italy, founded to carry out the pharmaceutical
Drug/Device Combination Products: Bioequivalence
 Drug/Device Combination Products: Bioequivalence Three stories:. The story of Nasal and Inhalation Product BE 2. The story of the Generic Auto-Injector 3. The story of User Interface Considerations Bioequivalence
Drug/Device Combination Products: Bioequivalence Three stories:. The story of Nasal and Inhalation Product BE 2. The story of the Generic Auto-Injector 3. The story of User Interface Considerations Bioequivalence
What You Need to Know about Metered-Dose Inhalers and the HFA Propellant
 What You Need to Know about Metered- Inhalers and the HFA Propellant There are a number ways to deliver inhaled medication. They include: Metered-dose inhaler () Metered-dose inhaler with spacer/holding
What You Need to Know about Metered- Inhalers and the HFA Propellant There are a number ways to deliver inhaled medication. They include: Metered-dose inhaler () Metered-dose inhaler with spacer/holding
Guide to Inhaled Treatment Choices
 Guide to Inhaled Treatment Choices Note: this is guidance only, it is important to consider which device is best suited to the patient. This may NOT be the first line choice (but should be on the joint
Guide to Inhaled Treatment Choices Note: this is guidance only, it is important to consider which device is best suited to the patient. This may NOT be the first line choice (but should be on the joint
Guide to Inhaled Treatment Choices
 Guide to Inhaled Treatment Choices Note: this is guidance only, it is important to consider which device is best suited to the patient. This may NOT be the first line choice (but should be on the joint
Guide to Inhaled Treatment Choices Note: this is guidance only, it is important to consider which device is best suited to the patient. This may NOT be the first line choice (but should be on the joint
The Montreal Protocol and the Phase-out of CFC-based Metered Dose Inhalers. Paul Krajnik UNIDO, Montreal Protocol Branch
 The Montreal Protocol and the Phase-out of CFC-based Metered Dose Inhalers Paul Krajnik UNIDO, Montreal Protocol Branch 1 The Montreal Protocol Binding international agreement for the preservation and
The Montreal Protocol and the Phase-out of CFC-based Metered Dose Inhalers Paul Krajnik UNIDO, Montreal Protocol Branch 1 The Montreal Protocol Binding international agreement for the preservation and
National Transition Strategy to Replace CFC-based. MDIs with in the Commonwealth of Independent States. Paul Krajnik UNIDO, Montreal Protocol Branch
 National Transition Strategy to Replace CFC-based MDIs with HFA-MDIs in the Commonwealth of Independent States Paul Krajnik UNIDO, Montreal Protocol Branch 1 Starting point: total CFC phase-out CFC phase-out
National Transition Strategy to Replace CFC-based MDIs with HFA-MDIs in the Commonwealth of Independent States Paul Krajnik UNIDO, Montreal Protocol Branch 1 Starting point: total CFC phase-out CFC phase-out
Caption: The equipment required for testing Fluticasone Propionate (FP) Inhalation Powder in line with a new product-specific monograph (USP36-NF31).
 Product-specific FDA guidance, and product-specific pharmacopeial monographs, point to the use of test equipment, some of which isn t included in the general USP/Ph. Eur. chapters for orally inhaled products
Product-specific FDA guidance, and product-specific pharmacopeial monographs, point to the use of test equipment, some of which isn t included in the general USP/Ph. Eur. chapters for orally inhaled products
Respiratory Inhalers. Identification Guide Version 3
 Respiratory Inhalers Identification Guide Version 3 This booklet has been prepared by NHSGGC Medicines Information. Endorsed by NHSGGC Respiratory Managed Clinical Network, February 2017. Designed by Medical
Respiratory Inhalers Identification Guide Version 3 This booklet has been prepared by NHSGGC Medicines Information. Endorsed by NHSGGC Respiratory Managed Clinical Network, February 2017. Designed by Medical
BREEZHALER. Medications available: Onbrez (indacaterol maleate) Seebri (glycopyrronium bromide) Ultibro (glycopyrronium bromide/ (indacaterol maleate)
 Medications available: Onbrez (indacaterol maleate) Seebri (glycopyrronium bromide) Ultibro (glycopyrronium bromide/ (indacaterol maleate) BREEZHALER Please date initial after you have directly observed
Medications available: Onbrez (indacaterol maleate) Seebri (glycopyrronium bromide) Ultibro (glycopyrronium bromide/ (indacaterol maleate) BREEZHALER Please date initial after you have directly observed
BREEZHALER. Medications available: Onbrez (indacaterol maleate) Seebri (glycopyrronium bromide) Ultibro (glycopyrronium bromide/ (indacaterol maleate)
 Medications available: Onbrez (indacaterol maleate) Seebri (glycopyrronium bromide) Ultibro (glycopyrronium bromide/ (indacaterol maleate) BREEZHALER Please date initial after you have directly observed
Medications available: Onbrez (indacaterol maleate) Seebri (glycopyrronium bromide) Ultibro (glycopyrronium bromide/ (indacaterol maleate) BREEZHALER Please date initial after you have directly observed
Inhaled bronchodilators relax constricted airways and treat the noisy part of asthma: coughing, wheezing, choking and shortness of breath.
 Inhaled bronchodilators relax constricted airways and treat the noisy part of asthma: coughing, wheezing, choking and shortness of breath. AccuNeb inhalation 0.021% solution: 0.63mg/3mL 3-4 times solution
Inhaled bronchodilators relax constricted airways and treat the noisy part of asthma: coughing, wheezing, choking and shortness of breath. AccuNeb inhalation 0.021% solution: 0.63mg/3mL 3-4 times solution
TRELEGY ELLIPTA (fluticasone-umeclidinium-vilanterol) aerosol powder
 TRELEGY ELLIPTA (fluticasone-umeclidinium-vilanterol) aerosol powder Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific
TRELEGY ELLIPTA (fluticasone-umeclidinium-vilanterol) aerosol powder Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific
BREEZHALER. Medications available: Onbrez (indacaterol maleate) Seebri (glycopyrronium bromide) Ultibro (glycopyrronium bromide/ (indacaterol maleate)
 Medications available: Onbrez (indacaterol maleate) Seebri (glycopyrronium bromide) Ultibro (glycopyrronium bromide/ (indacaterol maleate) BREEZHALER Please date initial after you have directly observed
Medications available: Onbrez (indacaterol maleate) Seebri (glycopyrronium bromide) Ultibro (glycopyrronium bromide/ (indacaterol maleate) BREEZHALER Please date initial after you have directly observed
Ferris State University College of Pharmacy MPA CE Symposium 2016 Paul Thill, PharmD, BCPS
 Ferris State University College of Pharmacy MPA CE Symposium 2016 Paul Thill, PharmD, BCPS Objectives Categorize the new asthma and COPD inhalers in to existing or newly created categories Discuss the
Ferris State University College of Pharmacy MPA CE Symposium 2016 Paul Thill, PharmD, BCPS Objectives Categorize the new asthma and COPD inhalers in to existing or newly created categories Discuss the
Device Change Management for Inhaled Products. Loy Britto, Ph.D. GlaxoSmithKline ISAM Congress Munich 2015
 Device Change Management for Inhaled Products Loy Britto, Ph.D. GlaxoSmithKline ISAM Congress Munich 2015 Topics to be covered Update on ISO/TC 084/WG 15: Guidelines for development of drug products- Quality
Device Change Management for Inhaled Products Loy Britto, Ph.D. GlaxoSmithKline ISAM Congress Munich 2015 Topics to be covered Update on ISO/TC 084/WG 15: Guidelines for development of drug products- Quality
Metered Dose Inhalers (MDIs) In Vitro Measures to Confirm Patient Perceptions: HFA vs. CFC
 Metered Dose Inhalers (MDIs) In Vitro Measures to Confirm Patient Perceptions: HFA vs. CFC William H. Doub, Ph.D. Division of Pharmaceutical Analysis (DPA) US FDA/CDER/OPS/OTR The information presented
Metered Dose Inhalers (MDIs) In Vitro Measures to Confirm Patient Perceptions: HFA vs. CFC William H. Doub, Ph.D. Division of Pharmaceutical Analysis (DPA) US FDA/CDER/OPS/OTR The information presented
Common Inhaled Asthma Medications Dose Comparison and Tips for Use
 Detail-Document #210303 This Detail-Document accompanies the related article published in PHARMACIST S LETTER / PRESCRIBER S LETTER March 2005 ~ Volume 21 ~ Number 210303 Common Inhaled Asthma Medications
Detail-Document #210303 This Detail-Document accompanies the related article published in PHARMACIST S LETTER / PRESCRIBER S LETTER March 2005 ~ Volume 21 ~ Number 210303 Common Inhaled Asthma Medications
Respiratory Health. Asthma and COPD
 Respiratory Health Asthma and COPD Definition of asthma Working definition by AAH 2014: Chronic lung disease Can be controlled not cured Large variation in lung function Large variation in respiratory
Respiratory Health Asthma and COPD Definition of asthma Working definition by AAH 2014: Chronic lung disease Can be controlled not cured Large variation in lung function Large variation in respiratory
Metering Valve Design and Material Selection; How can this be chosen to maximize product performance?
 Metering Valve Design and Material Selection; How can this be chosen to maximize product performance? B. Grosjean Medical Plastics 2007 Inhalation Devices for Medicine Delivery 2007, 1-2 October 2007,
Metering Valve Design and Material Selection; How can this be chosen to maximize product performance? B. Grosjean Medical Plastics 2007 Inhalation Devices for Medicine Delivery 2007, 1-2 October 2007,
The Medical Letter. on Drugs and Therapeutics
 The Medical Letter publications are protected by US and international copyright laws. Forwarding, copying or any other distribution of this material is strictly prohibited. For further information call:
The Medical Letter publications are protected by US and international copyright laws. Forwarding, copying or any other distribution of this material is strictly prohibited. For further information call:
NEBULIZERS, METERED DOSE INHALERS, AND DRY POWDER INHALERS
 NEBULIZERS, METERED DOSE INHALERS, AND DRY POWDER INHALERS Douglas S. Gardenhire, Ed.D, RRT-NPS MODULE 1 Manipulate Small Volume Nebulizers by Order or Protocol 1 Objectives for Module 1 At the end of
NEBULIZERS, METERED DOSE INHALERS, AND DRY POWDER INHALERS Douglas S. Gardenhire, Ed.D, RRT-NPS MODULE 1 Manipulate Small Volume Nebulizers by Order or Protocol 1 Objectives for Module 1 At the end of
Delivering Aerosol Medication in ICU
 Delivering Aerosol Medication in ICU 18th Aug 2017 Lau Chee Lan Pharmacist HCTM PPUKM ASMIC 2017 Aerosol Therapy Part of the treatment for a variety of respiratory disease * asthma and chronic obstructive
Delivering Aerosol Medication in ICU 18th Aug 2017 Lau Chee Lan Pharmacist HCTM PPUKM ASMIC 2017 Aerosol Therapy Part of the treatment for a variety of respiratory disease * asthma and chronic obstructive
Formulation Considerations for Inhaled Products
 Formulation Considerations for Inhaled Products Formulation Considerations of Inhaled Products Inhalation Therapy Nebulizers and Formulations Dry Powder Inhalers and Formulations Metered Dose Inhalers
Formulation Considerations for Inhaled Products Formulation Considerations of Inhaled Products Inhalation Therapy Nebulizers and Formulations Dry Powder Inhalers and Formulations Metered Dose Inhalers
Partner with the Global Leader in Drug Delivery Systems.
 3M DRUG DELIVERY SYSTEMS Partner with the Global Leader in Drug Delivery Systems. Hurlingham, Argentina Manufacturing Facility Experts at Commercializing Innovation 3M is a global innovation company that
3M DRUG DELIVERY SYSTEMS Partner with the Global Leader in Drug Delivery Systems. Hurlingham, Argentina Manufacturing Facility Experts at Commercializing Innovation 3M is a global innovation company that
Partner with the Global Leader in Drug Delivery Systems.
 3M DRUG DELIVERY SYSTEMS Partner with the Global Leader in Drug Delivery Systems. Hurlingham, Argentina Manufacturing Facility Experts at Commercializing Innovation Drug Delivery Systems: It s in the detail
3M DRUG DELIVERY SYSTEMS Partner with the Global Leader in Drug Delivery Systems. Hurlingham, Argentina Manufacturing Facility Experts at Commercializing Innovation Drug Delivery Systems: It s in the detail
Metered Dose Inhaler Propellants
 Metered Dose Inhaler Propellants The driving force behind inhaled medications for 60 years Dr Tim Noakes Dr Stuart Corr 7 th December 2016 Acknowledgements Mexichem is extremely grateful to Professor Rob
Metered Dose Inhaler Propellants The driving force behind inhaled medications for 60 years Dr Tim Noakes Dr Stuart Corr 7 th December 2016 Acknowledgements Mexichem is extremely grateful to Professor Rob
Correct Use of Inhaler Devices
 PL Detail-Document #300206 This PL Detail-Document gives subscribers additional insight related to the Recommendations published in PHARMACIST S LETTER / PRESCRIBER S LETTER February 2014 Correct Use of
PL Detail-Document #300206 This PL Detail-Document gives subscribers additional insight related to the Recommendations published in PHARMACIST S LETTER / PRESCRIBER S LETTER February 2014 Correct Use of
Dose. Route. Units. Given. Dose. Route. Units. Given
 Chapter 4 Respiratory Andrew Stanton SALBUTAMOL (in acute asthma) 5 in acute asthma Nebulised (driven by oxygen not air) 4 6 hourly In acute severe asthma not responding to initial treatment or in life-threatening
Chapter 4 Respiratory Andrew Stanton SALBUTAMOL (in acute asthma) 5 in acute asthma Nebulised (driven by oxygen not air) 4 6 hourly In acute severe asthma not responding to initial treatment or in life-threatening
Considerations for Evaluation of Bioequivalence and Interchangeability of Orally Inhaled Products
 Considerations for Evaluation of Bioequivalence and Interchangeability of Orally Inhaled Products Sven Stegemann Sept 16, 2015 Institute for Process and Particle Engineering, Pharmaceutical Engineering
Considerations for Evaluation of Bioequivalence and Interchangeability of Orally Inhaled Products Sven Stegemann Sept 16, 2015 Institute for Process and Particle Engineering, Pharmaceutical Engineering
APPENDIX 1 Printable point-of-care tables Asthma Action Plan Yellow Zone Formulation Table Region: Europe
 APPENDIX 1 Printable point-of-care tables Asthma Action Plan Yellow Zone Formulation Table Region: Europe Instructions: Print on 8.5 x14 (216 x 279 mm) paper (Legal size) Medication in Green Zone Change
APPENDIX 1 Printable point-of-care tables Asthma Action Plan Yellow Zone Formulation Table Region: Europe Instructions: Print on 8.5 x14 (216 x 279 mm) paper (Legal size) Medication in Green Zone Change
COMMENTS. Submitted by The International Pharmaceutical Aerosol Consortium
 COMMENTS on a draft Guidance for Industry Nasal Spray and Inhalation Solution, Suspension, and Spray Drug Products Chemistry, Manufacturing, and Controls Documentation (Docket No. 99D-1454) Submitted by
COMMENTS on a draft Guidance for Industry Nasal Spray and Inhalation Solution, Suspension, and Spray Drug Products Chemistry, Manufacturing, and Controls Documentation (Docket No. 99D-1454) Submitted by
How to Use Inhaled Medications for Asthma and COPD
 How to Use Inhaled Medications for Asthma and COPD This information is not intended to diagnose health problems or to take the place of medical advice or care you receive from your physician or other health
How to Use Inhaled Medications for Asthma and COPD This information is not intended to diagnose health problems or to take the place of medical advice or care you receive from your physician or other health
COPD Medicine. No one ever showed me how to use this. Wendy Happel; RRT, COPD Educator Krystal Fedoris; RRT-NPS, BA, COPD Educator
 Medicine. No one ever showed me how to use this. Wendy Happel; RRT, Educator Krystal Fedoris; RRT-NPS, BA, Educator 1 Taking prescriptions correctly Taking prescriptions can be a challenge Busy schedules
Medicine. No one ever showed me how to use this. Wendy Happel; RRT, Educator Krystal Fedoris; RRT-NPS, BA, Educator 1 Taking prescriptions correctly Taking prescriptions can be a challenge Busy schedules
CLINICAL RELEVANCE OF IN VITRO PARTICLE SIZING DATA. Steve Newman, PhD Nottingham, UK November 2006
 IPAC-RS Conference 6-8 November 2006 CLINICAL RELEVANCE OF IN VITRO PARTICLE SIZING DATA Steve Newman, PhD Nottingham, UK November 2006 IPAC-RS Conference 6-8 November 2006 1 EFFECT OF PARTICLE SIZE ON
IPAC-RS Conference 6-8 November 2006 CLINICAL RELEVANCE OF IN VITRO PARTICLE SIZING DATA Steve Newman, PhD Nottingham, UK November 2006 IPAC-RS Conference 6-8 November 2006 1 EFFECT OF PARTICLE SIZE ON
12/18/2017. Disclosures. Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing
 Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
Select Inhaled Respiratory Agents
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Process Drift and it s Resolution in the Manufacture of Drug Products. MDI s and DPI s Metered Dose Inhalations and Dry Powder Inhalations
 Process Drift and it s Resolution in the Manufacture of Drug Products MDI s and DPI s Metered Dose Inhalations and Dry Powder Inhalations Ed Warner, Merck MMD December 2, 2010 PQRI-FDA Workshop on Process
Process Drift and it s Resolution in the Manufacture of Drug Products MDI s and DPI s Metered Dose Inhalations and Dry Powder Inhalations Ed Warner, Merck MMD December 2, 2010 PQRI-FDA Workshop on Process
UNITED NATIONS INDUSTRIAL DEVELOPMENT ORGANIZATION
 UNITED NATIONS INDUSTRIAL DEVELOPMENT www.unido.org ORGANIZATION EXPERT GROUP MEETING ON ELIMINATION OF CFCs CONTAINED IN AEROSOL METERED DOSE INHALERS (MDI) IN THE COMMONWELATH OF INDEPENDENT STATES (CIS)
UNITED NATIONS INDUSTRIAL DEVELOPMENT www.unido.org ORGANIZATION EXPERT GROUP MEETING ON ELIMINATION OF CFCs CONTAINED IN AEROSOL METERED DOSE INHALERS (MDI) IN THE COMMONWELATH OF INDEPENDENT STATES (CIS)
The clinical effectiveness and costeffectiveness. treatment of chronic asthma in children under the age of 12 years
 The clinical effectiveness and costeffectiveness of corticosteroids for the treatment of chronic asthma in children under the age of 12 years Submission of evidence from AstraZeneca UK Ltd regarding the
The clinical effectiveness and costeffectiveness of corticosteroids for the treatment of chronic asthma in children under the age of 12 years Submission of evidence from AstraZeneca UK Ltd regarding the
Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing
 Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON THE PHARMACEUTICAL QUALITY OF INHALATION AND NASAL PRODUCTS
 European Medicines Agency Inspections London, 16 February 2005 Doc Ref.: EMEA/CHMP/QWP/49313/2005 corr. COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON THE PHARMACEUTICAL QUALITY OF
European Medicines Agency Inspections London, 16 February 2005 Doc Ref.: EMEA/CHMP/QWP/49313/2005 corr. COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON THE PHARMACEUTICAL QUALITY OF
What Is. Is the combination of two or more things to produce something that neither component could produce alone.
 What Is Is the combination of two or more things to produce something that neither component could produce alone. 1 Our Skills Synergy Swiss Pharma has an experienced team with collective knowledge and
What Is Is the combination of two or more things to produce something that neither component could produce alone. 1 Our Skills Synergy Swiss Pharma has an experienced team with collective knowledge and
Testing Inhaled Generics
 Generic Bioequivalence Testing Inhaled Generics By Mark Copley at Copley Scientific New product-specific FDA guidance and USP monographs support the development of popular inhaled products. This article
Generic Bioequivalence Testing Inhaled Generics By Mark Copley at Copley Scientific New product-specific FDA guidance and USP monographs support the development of popular inhaled products. This article
Sub-components in Nicotine cessation products?
 Sub-components in Nicotine cessation products? Mr Arun Sarda, Director of Quality and Regulatory Affairs Member of the HEITKAMP & THUMANN GROUP Regulatory approval of sub-components in nicotine cessation
Sub-components in Nicotine cessation products? Mr Arun Sarda, Director of Quality and Regulatory Affairs Member of the HEITKAMP & THUMANN GROUP Regulatory approval of sub-components in nicotine cessation
Tamsulosin Hydrochloride 0.4 mg Capsule
 Tamsulosin Hydrochloride 0.4 mg Capsule, Tamsulosin Hydrochloride 0.4 mg Capsule India, Tamsulosin Hydrochloride 0.4 mg Capsule manufacturers India, side effects Tamsulosin Hydrochloride 0.4 mg Capsule
Tamsulosin Hydrochloride 0.4 mg Capsule, Tamsulosin Hydrochloride 0.4 mg Capsule India, Tamsulosin Hydrochloride 0.4 mg Capsule manufacturers India, side effects Tamsulosin Hydrochloride 0.4 mg Capsule
ASTRAZENECA v GLAXOSMITHKLINE
 CASE AUTH/1986/4/07 ASTRAZENECA v GLAXOSMITHKLINE Symbicort and Seretide cost comparisons AstraZeneca complained about cost comparisons made by GlaxoSmithKline between AstraZeneca s Symbicort (budesonide/formoterol)
CASE AUTH/1986/4/07 ASTRAZENECA v GLAXOSMITHKLINE Symbicort and Seretide cost comparisons AstraZeneca complained about cost comparisons made by GlaxoSmithKline between AstraZeneca s Symbicort (budesonide/formoterol)
Medical Developments International
 Medical Developments International Vision Medical Developments International (MDI) is a leading Emergency Medicine Company. Our aim is to 1. Dominate the analgesic trauma and minor surgical procedures
Medical Developments International Vision Medical Developments International (MDI) is a leading Emergency Medicine Company. Our aim is to 1. Dominate the analgesic trauma and minor surgical procedures
Disclosure. Case. Objectives. Case Continued. Inhalers. Asthma: A GINA Update to the NAEPP 2007 Guidelines 1/20/2015
 Disclosure Asthma: A GINA Update to the NAEPP 2007 Guidelines Robert (RC) Hellinga, Pharm.D. PGY 1 Pharmacy Resident Wolfson Children s Hospital/Baptist Health I do not have a vested interest in or affiliation
Disclosure Asthma: A GINA Update to the NAEPP 2007 Guidelines Robert (RC) Hellinga, Pharm.D. PGY 1 Pharmacy Resident Wolfson Children s Hospital/Baptist Health I do not have a vested interest in or affiliation
Significance of Leachables and Extractables to Pharmaceutical Quality
 Significance of Leachables and Extractables to Pharmaceutical Quality Gordon Hansen, MS Vice President Analytical Development Ridgefield Boehringer Ingelheim Pharmaceuticals, Inc. Presentation Outline
Significance of Leachables and Extractables to Pharmaceutical Quality Gordon Hansen, MS Vice President Analytical Development Ridgefield Boehringer Ingelheim Pharmaceuticals, Inc. Presentation Outline
Foundations of Pharmacology
 Pharmacologic Management of Asthma Objectives: 1. Review the physiological basis for asthma therapy 2. Discuss the differences between SABA and LABA 3. Discuss the role of inhaled and oral systemic corticosteroids
Pharmacologic Management of Asthma Objectives: 1. Review the physiological basis for asthma therapy 2. Discuss the differences between SABA and LABA 3. Discuss the role of inhaled and oral systemic corticosteroids
ASTHMA PRESCRIBING GUIDELINES FOR ADULTS AND CHILDREN OVER 12
 North Hampshire CCG Asthma Prescribing Guidelines June 2015 ASTHMA PRESCRIBING GUIDELINES FOR ADULTS AND CHILDREN OVER 12 These guidelines are based on the British Thoracic Society (BTS) and Scottish Intercollegiate
North Hampshire CCG Asthma Prescribing Guidelines June 2015 ASTHMA PRESCRIBING GUIDELINES FOR ADULTS AND CHILDREN OVER 12 These guidelines are based on the British Thoracic Society (BTS) and Scottish Intercollegiate
2008 IPAC-RS Conference Doing the Right Thing Science, Quality and Patient Focus
 2008 IPAC-RS Conference Doing the Right Thing Science, Quality and Patient Focus Patient Perspective Alpha-1 1 Foundation John W. Walsh Patient Focus Personal perspective Challenges and lessons Importance
2008 IPAC-RS Conference Doing the Right Thing Science, Quality and Patient Focus Patient Perspective Alpha-1 1 Foundation John W. Walsh Patient Focus Personal perspective Challenges and lessons Importance
A Visual Approach to Simplifying Respiratory Drug Regimens
 A Visual Approach to Simplifying Respiratory Drug Regimens Stephanie Cheng, PharmD, MPH, BCGP 3 Main Categories Inhaled Respiratory Drugs Binds to beta-2 receptors Relaxation of smooth muscles in the lung
A Visual Approach to Simplifying Respiratory Drug Regimens Stephanie Cheng, PharmD, MPH, BCGP 3 Main Categories Inhaled Respiratory Drugs Binds to beta-2 receptors Relaxation of smooth muscles in the lung
Public Assessment Report Scientific discussion. Flumetor (salmeterol xinafoate/fluticasone propionate) SE/H/1068/01-02/DC
 Public Assessment Report Scientific discussion Flumetor (salmeterol xinafoate/fluticasone propionate) SE/H/1068/01-02/DC This module reflects the scientific discussion for the approval of Flumetor. The
Public Assessment Report Scientific discussion Flumetor (salmeterol xinafoate/fluticasone propionate) SE/H/1068/01-02/DC This module reflects the scientific discussion for the approval of Flumetor. The
Understanding Regulatory Global Requirements for Nasal Drug Products. Julie D. Suman, Ph.D. April 8, 2016
 Understanding Regulatory Global Requirements for Nasal Drug Products Julie D. Suman, Ph.D. April 8, 2016 AGENDA NDA vs ANDA Regulatory Approaches for Bioequivalence (BE) FDA Drug Specific Guidances FDA,
Understanding Regulatory Global Requirements for Nasal Drug Products Julie D. Suman, Ph.D. April 8, 2016 AGENDA NDA vs ANDA Regulatory Approaches for Bioequivalence (BE) FDA Drug Specific Guidances FDA,
Breakout Session # A 12:45 1:25. New Methods of Medication Delivery to the Lungs: Say Goodbye to the MDI as we know it
 Breakout Session # A 12:45 1:25 New Methods of Medication Delivery to the Lungs: Say Goodbye to the MDI as we know it Michael J. Welch MD, FAAP, FAAAAI, CPI Co-director, Allergy & Asthma Medical Group
Breakout Session # A 12:45 1:25 New Methods of Medication Delivery to the Lungs: Say Goodbye to the MDI as we know it Michael J. Welch MD, FAAP, FAAAAI, CPI Co-director, Allergy & Asthma Medical Group
Dry Powder Inhaler. Developing an Efficient. 3M Conix DPI. White Paper / Spring Proven Solutions that Enable Your Success
 3M Drug Delivery Systems Developing an Efficient Dry Powder Inhaler 3M Conix DPI White Paper / Spring 2011 Proven Solutions that Enable Your Success Introduction introduction Inhalation drug delivery has
3M Drug Delivery Systems Developing an Efficient Dry Powder Inhaler 3M Conix DPI White Paper / Spring 2011 Proven Solutions that Enable Your Success Introduction introduction Inhalation drug delivery has
AIRDUO RESPICLICK (fluticasone-salmeterol) aerosol DULERA (mometasone furoate and formoterol fumarate dihydrate) aerosol
 DULERA (mometasone furoate and formoterol fumarate dihydrate) aerosol Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific
DULERA (mometasone furoate and formoterol fumarate dihydrate) aerosol Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific
NATIVA GROUP. Inspired by Innovation and Technology
 NATIVA GROUP Inspired by Innovation and Technology INNOVATION & TECHNOLOGY IS OUR PASSION Nativa is a leading group of pharmaceutical companies in Russia. It develops and produces worldclass complex medicines
NATIVA GROUP Inspired by Innovation and Technology INNOVATION & TECHNOLOGY IS OUR PASSION Nativa is a leading group of pharmaceutical companies in Russia. It develops and produces worldclass complex medicines
Stepping down asthma treatment guidelines
 Stepping down asthma treatment guidelines The potential for inhaled corticosteroids (ICS) to cause dose-related side-effects has led to asthma management guidelines recommending a dose reduction once asthma
Stepping down asthma treatment guidelines The potential for inhaled corticosteroids (ICS) to cause dose-related side-effects has led to asthma management guidelines recommending a dose reduction once asthma
COPD and Asthma Devices Market by Inhalers Type (Drug powder, Metered Dose, Soft Mist), Nebulizers (Compressor, Ultrasonic, Mesh) - Global
 COPD and Asthma Devices Market by Inhalers Type (Drug powder, Metered Dose, Soft Mist), Nebulizers (Compressor, Ultrasonic, Mesh) - Global Opportunity Analysis and Industry Forecasts, 2015-2022 COPD and
COPD and Asthma Devices Market by Inhalers Type (Drug powder, Metered Dose, Soft Mist), Nebulizers (Compressor, Ultrasonic, Mesh) - Global Opportunity Analysis and Industry Forecasts, 2015-2022 COPD and
10/18/2012. Penn State University Children s Hospital JODIE STABINSKI CRNP MSN AE-C
 Penn State University Children s Hospital JODIE STABINSKI CRNP MSN AE-C Daily: Long-Term Control Corticosteroids (inhaled and systemic) Long-acting beta 2 -agonists (Serevent, Foradil) Methylxanthines
Penn State University Children s Hospital JODIE STABINSKI CRNP MSN AE-C Daily: Long-Term Control Corticosteroids (inhaled and systemic) Long-acting beta 2 -agonists (Serevent, Foradil) Methylxanthines
Patricia KP Burnell Inhalation Product Development
 Patricia KP Burnell Inhalation Product Development Inhaled products: types, development The critical parameters In-vitro testing Ex-vivo testing What dose? Product Development: drug medicine Safety and
Patricia KP Burnell Inhalation Product Development Inhaled products: types, development The critical parameters In-vitro testing Ex-vivo testing What dose? Product Development: drug medicine Safety and
Position within the Organisation
 ASTHMA TREATMENT GUIDELINES Document Description Document Type Service Application Guidelines All healthcare professionals(hcps) caring for patients with asthma Version 4.0 Ratification date September
ASTHMA TREATMENT GUIDELINES Document Description Document Type Service Application Guidelines All healthcare professionals(hcps) caring for patients with asthma Version 4.0 Ratification date September
Inhaled Corticosteroids for the Treatment of Chronic Asthma in Adults & Adolescents aged 12 years & over
 Manufacturer Submission To The National Institute for Health and Clinical Excellence By GlaxoSmithKline UK Inhaled Corticosteroids for the Treatment of Chronic Asthma in Adults & Adolescents aged 12 years
Manufacturer Submission To The National Institute for Health and Clinical Excellence By GlaxoSmithKline UK Inhaled Corticosteroids for the Treatment of Chronic Asthma in Adults & Adolescents aged 12 years
GMMMG Asthma Formulary Inhaler Options August 2017
 Regular Preventer Beclometasone Beclometasone Beclometasone Brand name Qvar Easi-Breathe Clenil Modulite Easyhaler Device MDI MDI DPI Strengths 50 microgram 100 microgram 200 microgram Adult asthma 2 inhalations
Regular Preventer Beclometasone Beclometasone Beclometasone Brand name Qvar Easi-Breathe Clenil Modulite Easyhaler Device MDI MDI DPI Strengths 50 microgram 100 microgram 200 microgram Adult asthma 2 inhalations
Go With the Flow REGULATORY LANDSCAPE. Mark Copley at Copley Scientific
 Go With the Flow Image: Guzel Studio shutterstock.com Increasing global requirements for efficacious, inexpensive products to treat respiratory illnesses are driving the development of inhaled generics.
Go With the Flow Image: Guzel Studio shutterstock.com Increasing global requirements for efficacious, inexpensive products to treat respiratory illnesses are driving the development of inhaled generics.
PHARMACY COVERAGE GUIDELINES ORIGINAL EFFECTIVE DATE: 11/16/17 SECTION: DRUGS LAST REVIEW DATE: 11/16/17 LAST CRITERIA REVISION DATE: ARCHIVE DATE:
 LEVALBUTEROL HFA (levalbuterol tartrate) inhalation aerosol Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit
LEVALBUTEROL HFA (levalbuterol tartrate) inhalation aerosol Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit
MDI Bonanza. Dwayne Griffin, DO
 MDI Bonanza Dwayne Griffin, DO Bonanza 3. A MDI costing $200 - $500 per month SISYPHUS MDI Griffin Mountain Evolution of Deliver Systems for COPD in the US 2003 2009 2011 2013 2004 2012 2014 Prescribing
MDI Bonanza Dwayne Griffin, DO Bonanza 3. A MDI costing $200 - $500 per month SISYPHUS MDI Griffin Mountain Evolution of Deliver Systems for COPD in the US 2003 2009 2011 2013 2004 2012 2014 Prescribing
Public Assessment Report. Scientific discussion. Ipratropiumbromide Newline Pharma 20 microgram per actuation, pressurised inhalation solution
 Public Assessment Report Scientific discussion Ipratropiumbromide Newline Pharma 20 microgram per actuation, pressurised inhalation solution (ipratropium bromide) NL/H/3507/001/DC Date: 19 December 2016
Public Assessment Report Scientific discussion Ipratropiumbromide Newline Pharma 20 microgram per actuation, pressurised inhalation solution (ipratropium bromide) NL/H/3507/001/DC Date: 19 December 2016
Delay Between Actuation and Shaking of a Hydrofluoroalkane Fluticasone Pressurized Metered-Dose Inhaler
 Delay Between Actuation and Shaking of a Hydrofluoroalkane Fluticasone Pressurized Metered-Dose Inhaler Ariel Berlinski MD, Dirk von Hollen, John N Pritchard PhD, and Ross HM Hatley PhD BACKGROUND: Inhaled
Delay Between Actuation and Shaking of a Hydrofluoroalkane Fluticasone Pressurized Metered-Dose Inhaler Ariel Berlinski MD, Dirk von Hollen, John N Pritchard PhD, and Ross HM Hatley PhD BACKGROUND: Inhaled
Public Assessment Report. Scientific discussion. Ipratropiumbromide Sandoz 20 microgram per actuation, pressurised inhalation solution
 Public Assessment Report Scientific discussion Ipratropiumbromide Sandoz 20 microgram per actuation, pressurised inhalation solution (ipratropium bromide) NL/H/3040/001/DC Date: 21 October 2015 This module
Public Assessment Report Scientific discussion Ipratropiumbromide Sandoz 20 microgram per actuation, pressurised inhalation solution (ipratropium bromide) NL/H/3040/001/DC Date: 21 October 2015 This module
For personal use only
 AusCann Makes Major Progress Towards Production of Cannabinoid Medicines During FY18 Highlights Undertook comprehensive pharmaceutical development project to create an optimal dosage form cannabinoid medicine
AusCann Makes Major Progress Towards Production of Cannabinoid Medicines During FY18 Highlights Undertook comprehensive pharmaceutical development project to create an optimal dosage form cannabinoid medicine
Orally Inhaled Corticosteroids to 2022
 Greystone Research Associates 1+603-595-4340 April 2015 Orally Inhaled Corticosteroids to 2022 Drugs, Devices, Markets and Forecasts Contents A Comprehensive Market Analysis Report Scope & Overview 2 Table
Greystone Research Associates 1+603-595-4340 April 2015 Orally Inhaled Corticosteroids to 2022 Drugs, Devices, Markets and Forecasts Contents A Comprehensive Market Analysis Report Scope & Overview 2 Table
AEROSOL THERAPY: THE PRACTICALITIES
 AEROSOL THERAPY: THE PRACTICALITIES Lester I. Harrison, PhD Section Head, Clinical Pharmacokinetics, 3M Pharmaceuticals, 3M Center 270-3S-05, St. Paul, MN, USA 55144 liharrison@mmm.com Introduction: Horses,
AEROSOL THERAPY: THE PRACTICALITIES Lester I. Harrison, PhD Section Head, Clinical Pharmacokinetics, 3M Pharmaceuticals, 3M Center 270-3S-05, St. Paul, MN, USA 55144 liharrison@mmm.com Introduction: Horses,
Qualifying Container Closure Systems for OINDP: Current & Future Regulatory Expectations. Julie D. Suman, Ph.D. November 14, 2014
 Qualifying Container Closure Systems for OINDP: Current & Future Regulatory Expectations Julie D. Suman, Ph.D. November 14, 2014 Objectives Container-Closure System Attributes Extractables Regulatory Expectations
Qualifying Container Closure Systems for OINDP: Current & Future Regulatory Expectations Julie D. Suman, Ph.D. November 14, 2014 Objectives Container-Closure System Attributes Extractables Regulatory Expectations
Aerospan (flunisolide)
 STRENGTH DOSAGE FORM ROUTE GPID 80mcg/actuation HFA aerosol inhaler w/ Inhaled 35718 8.9 g/canister adapter MANUFACTURER Meda Pharmaceuticals INDICATION Aerospan Inhalation Aerosol is indicated for the
STRENGTH DOSAGE FORM ROUTE GPID 80mcg/actuation HFA aerosol inhaler w/ Inhaled 35718 8.9 g/canister adapter MANUFACTURER Meda Pharmaceuticals INDICATION Aerospan Inhalation Aerosol is indicated for the
No.1, Xianyao Road, Xianju, Zhejiang, China,
 No.1, Xianyao Road, Xianju, Zhejiang, China, 317300 Xianju Pharma Outline Outline I. Brief Introduction II. Quality Unit III. Production System IV. EHS System I. Brief Introduction Xianju Pharma Zhejiang
No.1, Xianyao Road, Xianju, Zhejiang, China, 317300 Xianju Pharma Outline Outline I. Brief Introduction II. Quality Unit III. Production System IV. EHS System I. Brief Introduction Xianju Pharma Zhejiang
Study Summary Updated: September, 2017
 Study Summary Updated: September, 2017 Foreword The AeroChamber* Brand of Valved Holding Chamber (VHC) has been continually updated and improved upon since it was first introduced in 1983. The product
Study Summary Updated: September, 2017 Foreword The AeroChamber* Brand of Valved Holding Chamber (VHC) has been continually updated and improved upon since it was first introduced in 1983. The product
CFCs in inhalers for asthma and COPD
 CFCs in inhalers for asthma and COPD What s happening? 2009 United Nations Environment Programme Developed in association with the National Asthma Council Australia Supported by the Multilateral Fund for
CFCs in inhalers for asthma and COPD What s happening? 2009 United Nations Environment Programme Developed in association with the National Asthma Council Australia Supported by the Multilateral Fund for
Question I was one of the first dry power devices available in the US Flovent, Serevent and Advair are all available in this device
 What Device am I Class Side Effects History Potpourri Monitoring Tools 10 10 10 10 10 20 20 20 20 20 30 30 30 30 30 40 40 40 40 40 50 50 50 50 50 WHAT KIND OF DEVICE AM I? I was one of the first dry power
What Device am I Class Side Effects History Potpourri Monitoring Tools 10 10 10 10 10 20 20 20 20 20 30 30 30 30 30 40 40 40 40 40 50 50 50 50 50 WHAT KIND OF DEVICE AM I? I was one of the first dry power
beclometasone 100 MDI 2 puffs twice a day (recently changed to non CFC (Clenil Modulite))
 Case 1 Mr Thomson, a 32 year old asthmatic who is well known to you comes into your pharmacy. He is known to have a best peak flow of 640 L/min. He tells you that over the last few weeks he has been wakening
Case 1 Mr Thomson, a 32 year old asthmatic who is well known to you comes into your pharmacy. He is known to have a best peak flow of 640 L/min. He tells you that over the last few weeks he has been wakening
A Visual Approach to Simplifying Respiratory Drug Regimens
 A Visual Approach to Simplifying Respiratory Drug Regimens Stephanie Cheng, PharmD, MPH, BCGP October 23, 2017 Learning Objectives Be able to list at least 3 major adverse effects of inhaled medications
A Visual Approach to Simplifying Respiratory Drug Regimens Stephanie Cheng, PharmD, MPH, BCGP October 23, 2017 Learning Objectives Be able to list at least 3 major adverse effects of inhaled medications
A Visual Approach to Simplifying Respiratory Drug Regimens
 Adverse Effects of Inhaled Medications A Visual Approach to Simplifying Respiratory Drug Regimens Stephanie Cheng, PharmD, MPH, BCGP June 28, 2017 Drug Category Beta 2 agonists antagonists Adverse Effects
Adverse Effects of Inhaled Medications A Visual Approach to Simplifying Respiratory Drug Regimens Stephanie Cheng, PharmD, MPH, BCGP June 28, 2017 Drug Category Beta 2 agonists antagonists Adverse Effects
Beclometasone dipropionate (BDP) Prophylactic management of mild, moderate or severe asthma in adults
 CLENIL MODULITE Beclometasone dipropionate (BDP) Prophylactic management of mild, moderate or severe asthma in adults Corticosteroids for the treatment of chronic asthma in adults and children aged 12
CLENIL MODULITE Beclometasone dipropionate (BDP) Prophylactic management of mild, moderate or severe asthma in adults Corticosteroids for the treatment of chronic asthma in adults and children aged 12
A NEW, ADVANCED HIGH- THROUGHPUT SYSTEM FOR AUTOMATED INHALER TESTING
 A NEW, ADVANCED HIGH- THROUGHPUT SYSTEM FOR AUTOMATED INHALER TESTING Two years ago, Novi Systems Ltd set out to shake up the inhaler automation market. On December 8th, 2015, at the Drug Delivery to the
A NEW, ADVANCED HIGH- THROUGHPUT SYSTEM FOR AUTOMATED INHALER TESTING Two years ago, Novi Systems Ltd set out to shake up the inhaler automation market. On December 8th, 2015, at the Drug Delivery to the
SAMPLE. mg by mouth every day for day(s) Prednisolone. Other Medicine: Medicine Dose How long Directions
 Pediatric Asthma Discharge Prescription and Short-Term Plan The doctor will fill out this form before your child is discharged. Please follow this plan until you see your usual doctor in 3 to 7 days. Hospital
Pediatric Asthma Discharge Prescription and Short-Term Plan The doctor will fill out this form before your child is discharged. Please follow this plan until you see your usual doctor in 3 to 7 days. Hospital
Metered Dose Inhaler Technology
 Metered Dose Inhaler Technology Edited by Tol S. Purewal and David J. W. Grant Interpharm Press, Inc. Buffalo Grove, Illinois Contents 1. INTRODUCTION 1 T. S. Purewal Definition of the Metered Dose Inhaler
Metered Dose Inhaler Technology Edited by Tol S. Purewal and David J. W. Grant Interpharm Press, Inc. Buffalo Grove, Illinois Contents 1. INTRODUCTION 1 T. S. Purewal Definition of the Metered Dose Inhaler
Reference Guide for Caring for Pediatric Patients with Asthma
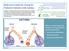 Reference Guide for Caring for Pediatric Patients with Asthma Co-Chair: Nancy Cantey Banasiak, DNP, PPCNP-BC, APRN Co-Chair: Deborah Hickman, DNP, APRN-CNP, CPNP-PC, NNP-BC Asthma and Allergy SIG Members
Reference Guide for Caring for Pediatric Patients with Asthma Co-Chair: Nancy Cantey Banasiak, DNP, PPCNP-BC, APRN Co-Chair: Deborah Hickman, DNP, APRN-CNP, CPNP-PC, NNP-BC Asthma and Allergy SIG Members
IVIVC in Pediatric OIPs
 IPAC-RS/UF Orlando Conference 2014 March 20, 2014 IVIVC in Pediatric OIPs Herbert Wachtel Declaration of Conflicts of Interest H. Wachtel is employee of Boehringer Ingelheim Pharma GmbH & Co. KG, Germany.
IPAC-RS/UF Orlando Conference 2014 March 20, 2014 IVIVC in Pediatric OIPs Herbert Wachtel Declaration of Conflicts of Interest H. Wachtel is employee of Boehringer Ingelheim Pharma GmbH & Co. KG, Germany.
Everything for Inhalation
 Everything for Inhalation Everything for Inhalation Inhalation drug product development at Hovione has a strong focus on formulation for Dry Powder Inhalers (DPI), particularly for capsule-based and reservoir-based
Everything for Inhalation Everything for Inhalation Inhalation drug product development at Hovione has a strong focus on formulation for Dry Powder Inhalers (DPI), particularly for capsule-based and reservoir-based
Include patients: with a confirmed diagnosis of asthma who have been free of asthma symptoms for 3 months or more.
 Corby Clinical Commissioning Group Kettering General Hospital NHS Trust Nene Clinical Commissioning Group rthampton General Hospital NHS Trust rthamptonshire Healthcare Foundation Trust Stepping down asthma
Corby Clinical Commissioning Group Kettering General Hospital NHS Trust Nene Clinical Commissioning Group rthampton General Hospital NHS Trust rthamptonshire Healthcare Foundation Trust Stepping down asthma
YES = formulary NO = non-formulary NO BENEFIT = excluded. Beat4 Beat1 Beat 3 Pace2 Beat2 Pace1 Pace3 Pace4 B BECLOMETHASONE BECEZE 50MCG INHALER
 Benefits are subject to the following: Pre-authorisation Bestmed guidelines Bestmed protocols Mediscor Reference Price (MRP) This price represents the reasonable price in the market place for a particular
Benefits are subject to the following: Pre-authorisation Bestmed guidelines Bestmed protocols Mediscor Reference Price (MRP) This price represents the reasonable price in the market place for a particular
Report generated from BNF provided by FormularyComplete (www.pharmpress.com). Accessed Formulary Status. TA Number. Section.
 Report generated from BNF provided by FormularyComplete (www.pharmpress.com). Accessed 16 02 2017 Title Formulary Status Section TA Number TA Link Annotation ACLIDINIUM BROMIDE bronchodilators ADRENALINE/EPINEPHRINE
Report generated from BNF provided by FormularyComplete (www.pharmpress.com). Accessed 16 02 2017 Title Formulary Status Section TA Number TA Link Annotation ACLIDINIUM BROMIDE bronchodilators ADRENALINE/EPINEPHRINE
RDD Europe 2011 Workshop 4 May 2011
 RDD Europe 2011 Workshop 4 May 2011 This file represents the slides presented on May 4, 2011 by 3M Drug Delivery Systems at the RDD Europe 2011 Conference in Berlin, Germany. Slides have been modified
RDD Europe 2011 Workshop 4 May 2011 This file represents the slides presented on May 4, 2011 by 3M Drug Delivery Systems at the RDD Europe 2011 Conference in Berlin, Germany. Slides have been modified
