Future Formulary Changes
|
|
|
- Georgia Jackson
- 6 years ago
- Views:
Transcription
1 Future Formulary Changes Upd 05/16/2018 Applies to: Employer group plan 2018 open formularies with specialty tiers KEY: PA=prior authorization; ST=step therapy; QL=quantity limit Generic name Brand name Change Effective Bexarotene Targretin Ancillary charge applies Testosterone cypionate IM Depo-Testosterone Ancillary charge applies Pegvisomant Somavert Limited to Kaiser Guselkumab Tremfya Add QL of 1 syringe per 56 Brodalumab Siliq Add QL of 2 syringes per 28 Empagliflozin/metformin ER Synjardy XR Add QL of 30 Codeine sulfate; hydromorphone; levorphanol; morphine sulfate; oxycodone IR; oxycodone/acetaminophen; oxycodone/aspirin; tramadol; tramadol/acetaminophen; buprenorphine sublingual; acetaminophen/codeine; hydrocodone/acetaminophen; hydrocodone/ibuprofen; meperidine; oxymorphone; tapentadol; oxycodone/ibuprofen; butalbital/acetaminophen/caffeine/codeine; butalbital/aspirin/caffeine/codeine; acetaminophen/caffeine/dihydrocodeine Endocet; Lorcet; Lorcet Plus; Lortab; Dilaudid; Demerol; Roxicodone; Opana; Nucynta; Ultram; Percocet; Primlev; Tylenol #3; Tylenol #4; Capital/codeine; Fioricet; Fiorinal; Panlor; Synalgos- DC; Trezix; Norco; Vicodin; Vicodin ES; Vicodin HP; Verdrocet; Xodol; Hycet; Zamicet; Ibudone; Reprexain; Vicoprofen; Xylon; Ultracet; Oxaydo Up short acting opioid QL for 7 history of use. Maximum quantity now: 42 units or 210mLs for age >21 years; 18 units or 90mLs for age <21 years Atazanavir sulfate Sustiva Move to Tier 3 Efavirenz Reyataz capsules Move to Tier 5 Tenofovir disoproxil fumarate Viread Move to Tier 3 Upd 05/16/2018
2 Codeine/chlorpheniramine Tuzistra XR Add PA Dapagliflozin/saxagliptin Qtern Add QL of 30 Doxylamine/pyridoxine Diclegis Add PA Diclofenac Cambia; Zorvolex Add PA Dextromethorphan/quinidine Nuedexta Add PA Icatibant acetate Firazyr Limited to Kaiser Immune globulin Hizentra Limited to Kaiser Fluticasone propionate Flonase Move to Tier 3, add PA Beclomethasone dipropionate Beconase AQ; Qnasl Add PA Ciclesonide Omnaris; Zetonna Add PA Budesonide Rhinocort Aqua Add PA Mometasone furoate Nasonex Add PA Promethazine/codeine Phenergan with codeine Move to Tier 3; add PA Promethazine/phenylephrine/codeine Phenergan VC with codeine Move to Tier 3; add PA Enoxaparin sodium Lovenox Ancillary charge applies Tofacitinib citrate ER Xeljanz XR Limited to Kaiser Prasugrel Effient Move to Tier 3 Moxifloxacin Vigamox Move to Tier 3 Fosamprenavir Lexiva Move to Tier 5 01/01/ /13/2017 Upd 05/16/2018
3 Abacavir Ziagen Move to Tier 3 Sevelamer Renvela Move to Tier 3 Estradiol Climara Move to Tier 3 Enoxaparin Lovenox Move to Tier 3 Clidinium/chlordiazepoxide Librax Move to Tier 3 Scopolamine Transderm Scop Ancillary charge applies (generic available) Zileuton ER Zyflo CR Move to Tier 5 Becaplermin Regranex gel Move to Tier 5 Lurasidone Latuda Move to Tier 5 Dichlorphenamide Keveyis Move to Tier 5 Lanthanum carbonate Fosrenol Move to Tier 5 Felbamate Felbatol Move to Tier 5 Rufinamide Banzel Move to Tier 5 Alosetron Lotronex Move to Tier 5 Adefovir dipivoxil Hepsera Move brand to Tier 5; Move generic to Tier 4 Vancomycin Vancocin Move brand to Tier 5; Move generic to Tier 4 Erythromycin ethylsuccinate suspension Eryped; E.E.S. Move to Tier 3 Calcitriol Vectical ointment Move to Tier 2 Oxycodone Oxycontin CR Move to Tier 2 Mesalamine Asacol HD Move to Tier 2 Upd 05/16/2018
4 Fluorouracil Carac cream Move to Tier 2 Colchicine Mitigare Move to Tier 2 Lenvatinib Lenvima Limited to Kaiser Codeine sulfate; acetaminophen/codeine; promethazine/codeine; promethazine/phenylephrine/codeine; chlorpheniramine/codeine Tylenol with codeine; Phenergan with codeine; Lexuss; Tuzistra XR; Codar AR Cyclobenzaprine ER Amrix Add PA; add QL of 21 capsules per 180 Methylpheni ER Cotempla Add QL of 30 Amphetamine/dextroamphetamine Mydayis Add QL of 30 capsules per 30 Fentanyl Actiq; Subsys; Abstral; Fentora; Lazanda Add PA Add QL of 4 dosing units per day Daclizumab Zinbryta Add QL of 1 syringe per 28 Mometasone/formoterol Dulera Move to Tier 3 Ethacrynic acid Edecrin Add PA Ethacrynic acid Edecrin Move to Tier 3 Dupilumab Dupixent Add QL of 2 syringes (600mg) per 30 (exception for initial loading dose) Crisaborole Eucrisa Add QL of 120 grams per 30 Droxidopa Northera Limited to Kaiser 01/01/ /11/2017 Upd 05/16/2018
5 Morphine sulfate ER Morphabond ER Add QL of 60 Morphine sulfate ER Arymo ER Add QL of 90 Sacrosidase Sucraid Add PA Carisoprodol Soma Add QL of 45 Chlorzoxazone Lorzone, Parafon Forte Add QL of 60 Metaxalone Skelaxin, Metaxall Add QL of 60 Orphenadrine Norflex Add QL of 30 Methocarbamol Robaxin Add QL of 60 Cyclobenzaprine Flexeril, Fexmid Add QL of 45 Olorol Striverdi Add PA Quetiapine fumarate ER Seroquel XR Ancillary charge applies Melphalan Alkeran Move to Tier 3 Penicillamine Cuprimine; Depen Add PA; Limited to Kaiser Upd 05/16/2018
6 Ixekizumab Taltz Add QL of 2 syringes/pens (160 mg) at week 0 and 1 syringe/pen (80 mg) at week 2, 4, 6, 8, 10 and 12. Maintenance phase: QL of 1 syringe/pen (80 mg) per 28. Ibuprofen/famotidine Duexis Add coverage restriction; Move to Tier 5 Diclofenac potassium Zipsor Add coverage restriction Prednisone Rayos Add coverage restriction Adapalene Differin gel Move to Tier 3 Atomoxetine Strattera Move to Tier 3 Ezetimibe Zetia Move to Tier 3 Acyclovir Zovirax Move to Tier 3 Norethindrone/ethinyl estradiol Norinyl; Ortho-Novum Remove brand from ACA list Gabapentin oral capsules and lidocaine/menthol gel; diclofenac oral tablets, ranitidine oral tablets, lidocaine topical cream; diclofenac oral tablets, ranitidine oral tablets, lidocaine/prilocaine topical cream; cyclobenzaprine oral tablet, capsaicin/menthol topical patch Active-PAC with Gabapentin; Flexizol Pak Combipak; Inflammation Pak Reduction; Cyclobenzaprine Pak, Flexpax Remove from formulary, excluded product Conjugated estrogens Premarin Add ST Idelalisib; interferon alfa-2b; entacavir oral solution; pyrimethamine; auranofin; cobimetinib; alectinib; osimertinib; olaparib; peginterferon alfa-2b; trifluridine/tipiracil; alitretinoin; lenvatinib; aripiprazole lauroxil; cholic acid; dihydroergotamine mesylate; osalazine; ibuprofen/famotidine; atazanavir/cobicistat; alosetron; primidone; Zydelig; Intron-A; Baraclude oral solution; Daraprim; Ridaura; Cotellic; Alecensa; Targrisso; Lynparza; Sylatron; Lonsurf; Panretin; Lenvima; Aristada; Cholbam; D.H.E; Limited to a 30 day supply per fill Upd 05/16/2018
7 posaconazole; alirocumab; evolocumab; riluzole; acitretin; asfotase alfa; naproxen/esomeprazole; elvitegravir; selegeline; somatropin; lomitapide; ombitasvir/paritarevir/ritonavir; oxymetholone; paliperidone palmitate; parathyroid hormone; sapropterin; tolcapone; tolvaptan; zileuton; zileuton CR; ivacaftor packet; miltefosine Dipentum; Duexis; Evotaz; Lotronex; Mysoline; Noxafil; Praluent; Repatha; Rilutek; Soriatane; Strensiq; Vimovo; Viteka; Zelapar; Genotropin; Zomacton; Juxtapid; Technivie; Anadrol-50; Invega Trinzia; Natpara; Kuvan; Tasmar; Samsca; Zyflo, Zyflo CR; Kalydeco Packet; Impavido Lopinavir/ritonavir solution Kaletra solution Move to Tier 3 Oseltamivir Tamiflu Move to Tier 3 Rasagiline Azilect Move to Tier 3 Erythromycin ethylsuccinate suspension Eryped; E.E.S. Move to Tier 3 Glyburide Diabeta Move to Tier 3 Calcitonin nasal spray; Fortical Move to Tier 3 Pentamidine Nebupent Move to Tier 3 Estradiol valerate Desestrogen Add PA Flucytosine Ancobon Move to Tier 5 Flucytosine Generic Move to Tier 4 Aripiprazole lauroxil Aristrada Move to Tier 5 Entecavir oral solution Baraclude Move to Tier 4 Cholic acid Cholbam Move to Tier 5 Dihydroergotamine mesylate D.H.E Move to Tier 5 Pyrimethamine Daraprim Move to Tier 4 Fidaxomicin Dificid Move to Tier 5 Osalazine Dipentum Move to Tier 5 Atazanavir/cobicistat Evotaz Move to Tier 5 Felbamate Felbatol Move to Tier 5 Alosetron Lotronex Move to Tier 5 Primidone Mysoline Move to Tier 5 Alirocumab Praluent Move to Tier 5 Evolocumab Repatha Move to Tier 5 Auranofin Ridaura Move to Tier 4 Riluzole Rilutek Move to Tier 5 Upd 05/16/2018
8 Acitretin Soriatane Move to Tier 5 Asfotase alfa Strensiq Move to Tier 5 Naproxen/esomeprazole Vimovo Move to Tier 5 Elvitegravir Viteka Move to Tier 5 Selegiline Zelapar Move to Tier 5 Somatropin Genotropin; Zomacton Move to Tier 5 Entecavir Baraclude Move to Tier 5 Lomitapide Juxtapid Move to Tier 5 Ombitasvir/paritarevir/ritonavir Technivie Move to Tier 5 Oxymetholone Anadrol-50 Move to Tier 5 Paliperidone palmitate Invega Trinzia Move to Tier 5 Parathyroid hormone Natpara Move to Tier 5 Sapropterin Kuvan Move to Tier 5 Tolcapone Tasmar Move to Tier 5 Tolvaptan Samsca Move to Tier 5 Zileuton; zileuton CR Zyflo, Zyflo CR Move to Tier 5 Ivacaftor packet Kalydeco Packet Move to Tier 5 Miltefosine Impavido Move to Tier 5 Linezolid Zyvox Move to Tier 5 Upd 05/16/2018
Future Formulary Changes
 Future Formulary Changes Upd 09/11/2018 Applies to: Employer group plan 2018 open formularies KEY: =prior authorization; ST=step therapy; QL=quantity limit Generic name Brand name Change Effective Hydroxyprogesterone
Future Formulary Changes Upd 09/11/2018 Applies to: Employer group plan 2018 open formularies KEY: =prior authorization; ST=step therapy; QL=quantity limit Generic name Brand name Change Effective Hydroxyprogesterone
Future Formulary Changes
 Future Formulary Changes Applies to: Employer group plan 2019 open formularies with specialty tiers KEY: PA=prior authorization; ST=step therapy; QL=quantity limit Obeticholic acid Ocaliva Limited to Kaiser
Future Formulary Changes Applies to: Employer group plan 2019 open formularies with specialty tiers KEY: PA=prior authorization; ST=step therapy; QL=quantity limit Obeticholic acid Ocaliva Limited to Kaiser
Future Formulary Changes
 Future Formulary Changes Upd 11/21/2018 Applies to: Employer group plan 2018 2019 open formularies KEY: PA=prior authorization; ST=step therapy; QL=quantity limit Generic name Brand name Change Effective
Future Formulary Changes Upd 11/21/2018 Applies to: Employer group plan 2018 2019 open formularies KEY: PA=prior authorization; ST=step therapy; QL=quantity limit Generic name Brand name Change Effective
Capital BlueCross Open/Closed Formulary Update (1 st Quarter 2017)
 Capital BlueCross Open/Closed Formulary Update (1 st Quarter 2017) The Capital BlueCross formulary is a reference list of prescription drugs that contains a wide range of generic and brand drugs that have
Capital BlueCross Open/Closed Formulary Update (1 st Quarter 2017) The Capital BlueCross formulary is a reference list of prescription drugs that contains a wide range of generic and brand drugs that have
Cigna Drug and Biologic Coverage Policy
 Cigna Drug and Biologic Coverage Policy Subject Controlled Substance Analgesic and Narcotic Antagonist Quantity Limitations Table of Contents Coverage Policy... 1 General Background... 6 Coding/Billing
Cigna Drug and Biologic Coverage Policy Subject Controlled Substance Analgesic and Narcotic Antagonist Quantity Limitations Table of Contents Coverage Policy... 1 General Background... 6 Coding/Billing
Opioid Analgesic/Opioid Combination Products
 Market DC Opioid Analgesic/Opioid Combination Products Override(s) Quantity Limit Approval Duration 1 year Generic Name Brand Name Quantity Limit APAP/Caf/Dihydrocodeine 320.5mg/30mg/16mg APAP/Caf/Dihydrocodeine
Market DC Opioid Analgesic/Opioid Combination Products Override(s) Quantity Limit Approval Duration 1 year Generic Name Brand Name Quantity Limit APAP/Caf/Dihydrocodeine 320.5mg/30mg/16mg APAP/Caf/Dihydrocodeine
Opioid Analgesic/Opioid Combination Products
 Opioid Analgesic/Opioid Combination Products Override(s) Quantity Limit Approval Duration 1 year Generic Name Brand Name Quantity Limit 320.5mg/30mg/16mg 356.4mg/30mg/16mg 325mg/30mg/16mg Trezix (new formulation)
Opioid Analgesic/Opioid Combination Products Override(s) Quantity Limit Approval Duration 1 year Generic Name Brand Name Quantity Limit 320.5mg/30mg/16mg 356.4mg/30mg/16mg 325mg/30mg/16mg Trezix (new formulation)
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Opioid Immediate Release Page 1 of 13 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Opioid Immediate Release Prime Therapeutics will review Prior Authorization
Opioid Immediate Release Page 1 of 13 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Opioid Immediate Release Prime Therapeutics will review Prior Authorization
Prior Authorization Opioid Overutilization 2017
 Drugs Requiring Prior Authorization Label Name ACETAMINOPHEN/CAFFEINE/DIHYDROCODEINE CAPSULE ACETAMINOPHEN/CODEINE SOLUTION ACETAMINOPHEN/CODEINE TABLET ASCOMP/CODEINE CAPSULE BUTALBITAL/CAFFEINE/ACETAMINOPHEN/CODEINE
Drugs Requiring Prior Authorization Label Name ACETAMINOPHEN/CAFFEINE/DIHYDROCODEINE CAPSULE ACETAMINOPHEN/CODEINE SOLUTION ACETAMINOPHEN/CODEINE TABLET ASCOMP/CODEINE CAPSULE BUTALBITAL/CAFFEINE/ACETAMINOPHEN/CODEINE
PRESCRIPTION DRUG PROGRAM FORMULARY UPDATES Select Formulary April 1, 2018 Updates. Formulary Alternatives
 PRESCRIPTION DRUG PROGRAM FORMULARY UPDATES Select April 1, 2018 Updates Drug Name adapalene-benzoyl-peroxide Gel 0.1-2.5% (Brand = Epiduo ) prasugrel hcl (Brand = Effient ) vigabatrin pak 500 mg (Brand
PRESCRIPTION DRUG PROGRAM FORMULARY UPDATES Select April 1, 2018 Updates Drug Name adapalene-benzoyl-peroxide Gel 0.1-2.5% (Brand = Epiduo ) prasugrel hcl (Brand = Effient ) vigabatrin pak 500 mg (Brand
Generic Label Name Drug Strength Dosage Form Example Product (s) MME/Unit ACETAMINOPHEN WITH CODEINE
 STATE OF TENNESSEE DEPARTMENT OF FINANCE AND ADMINISTRATION DIVISION OF HEALTH CARE FINANCE AND ADMINISTRATION BUREAU OF TENNCARE 3 Great Circle Road NASHVILLE, TENNESSEE 37243 This notice is to advise
STATE OF TENNESSEE DEPARTMENT OF FINANCE AND ADMINISTRATION DIVISION OF HEALTH CARE FINANCE AND ADMINISTRATION BUREAU OF TENNCARE 3 Great Circle Road NASHVILLE, TENNESSEE 37243 This notice is to advise
PRESCRIPTION DRUG PROGRAM FORMULARY UPDATES Select Formulary April 1, 2018 Updates. Formulary Alternatives
 PRESCRIPTION DRUG PROGRAM FORMULARY UPDATES Select April 1, 2018 Updates Drug Name adapalene-benzoyl-peroxide Gel 0.1-2.5% (Brand = Epiduo ) prasugrel hcl (Brand = Effient ) vigabatrin pak 500 mg (Brand
PRESCRIPTION DRUG PROGRAM FORMULARY UPDATES Select April 1, 2018 Updates Drug Name adapalene-benzoyl-peroxide Gel 0.1-2.5% (Brand = Epiduo ) prasugrel hcl (Brand = Effient ) vigabatrin pak 500 mg (Brand
Opiate/Benzodiazepine/Muscle Relaxant Combinations
 Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Opiate/Benzodiazepine/Muscle Relaxant Combinations Clinical Edit Information Included in this Document Drugs requiring prior authorization:
Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Opiate/Benzodiazepine/Muscle Relaxant Combinations Clinical Edit Information Included in this Document Drugs requiring prior authorization:
PRESCRIPTION DRUG PROGRAM FORMULARY UPDATES Select Formulary October 1, 2018 Updates. Formulary. Alternatives
 PRESCRIPTION DRUG PROGRAM FORMULARY UPDATES Select October 1, 2018 Updates Drug Name efavirenz 600mg (Brand = Sustiva ) trientine (Brand = Syprine ) hydrocortisone lot 0.1% (Brand = Locoid ) sumatriptan-naproxen
PRESCRIPTION DRUG PROGRAM FORMULARY UPDATES Select October 1, 2018 Updates Drug Name efavirenz 600mg (Brand = Sustiva ) trientine (Brand = Syprine ) hydrocortisone lot 0.1% (Brand = Locoid ) sumatriptan-naproxen
APPROVED PA CRITERIA. Initial Approval: January 10, 2018 Revised Dates: April 11, 2018 CRITERIA FOR PRIOR AUTHORIZATION
 Initial Approval: January 10, 2018 Revised Dates: April 11, 2018 CRITERIA FOR PRIOR AUTHORIZATION PROVIDER GROUP Pharmacy Opioid Products Indicated for Pain Management MANUAL GUIDELINES All dosage forms
Initial Approval: January 10, 2018 Revised Dates: April 11, 2018 CRITERIA FOR PRIOR AUTHORIZATION PROVIDER GROUP Pharmacy Opioid Products Indicated for Pain Management MANUAL GUIDELINES All dosage forms
Opioid Management Program October 2018
 Opioid Management Program October 2018 What Is the Opioid Management Program? This program is based on guidelines developed by the U.S. Centers for Disease Control and Prevention (CDC). It consists of
Opioid Management Program October 2018 What Is the Opioid Management Program? This program is based on guidelines developed by the U.S. Centers for Disease Control and Prevention (CDC). It consists of
FORMULARY UPDATES TO DENVER HEALTH MEDICAID CHOICE (DHMC) AND CHILD HEALTH PLAN PLUS (CHP+) PLANS
 FORMULARY UPDATES TO DENVER HEALTH MEDICAID CHOICE (DHMC) AND CHILD HEALTH PLAN PLUS (CHP+) PLANS DHMC/CHP+ may add or remove drugs from the formulary or make changes to restrictions on formulary drugs
FORMULARY UPDATES TO DENVER HEALTH MEDICAID CHOICE (DHMC) AND CHILD HEALTH PLAN PLUS (CHP+) PLANS DHMC/CHP+ may add or remove drugs from the formulary or make changes to restrictions on formulary drugs
PRESCRIPTION DRUG LIST CHANGES
 PRESCRIPTION DRUG LIST CHANGES Cigna Pharmacy Management The medications listed below are changing coverage (or cost levels) on Cigna s Prescription Drug List. Changes are listed by drug list and by the
PRESCRIPTION DRUG LIST CHANGES Cigna Pharmacy Management The medications listed below are changing coverage (or cost levels) on Cigna s Prescription Drug List. Changes are listed by drug list and by the
** Fee-For-Service Pharmacy Provider Notice #229 May 2018 PDL Changes **
 ** Fee-For-Service Pharmacy Provider Notice #229 May 2018 PDL Changes ** August 03, 2018 Please be advised that the Department for Medicaid Services (DMS) is making changes to the Kentucky Medicaid Fee-For-Service
** Fee-For-Service Pharmacy Provider Notice #229 May 2018 PDL Changes ** August 03, 2018 Please be advised that the Department for Medicaid Services (DMS) is making changes to the Kentucky Medicaid Fee-For-Service
FORMULARY UPDATES TO DENVER HEALTH MEDICAID CHOICE (DHMC) AND CHILD HEALTH PLAN PLUS (CHP+) PLANS
 FORMULARY UPDATES TO DENVER HEALTH MEDICAID CHOICE (DHMC) AND CHILD HEALTH PLAN PLUS (CHP+) PLANS DHMC/CHP+ may add or remove drugs from the formulary or make changes to restrictions on formulary drugs
FORMULARY UPDATES TO DENVER HEALTH MEDICAID CHOICE (DHMC) AND CHILD HEALTH PLAN PLUS (CHP+) PLANS DHMC/CHP+ may add or remove drugs from the formulary or make changes to restrictions on formulary drugs
Opioid Management Program May 2018
 Opioid Management Program May 2018 What Is the Opioid Management Program? This program is based on guidelines developed by the U.S. Centers for Disease Control and Prevention (CDC). It consists of daily
Opioid Management Program May 2018 What Is the Opioid Management Program? This program is based on guidelines developed by the U.S. Centers for Disease Control and Prevention (CDC). It consists of daily
2017 Formulary Addendum Notice of Change (Medicare Advantage Plans)
 207 Formulary Addendum Notice of Change (Medicare Advantage Plans) Easy Choice Health Plan Easy Choice Plus Plan (HMO) H5087-002, H5087-07 This is a listing of the changes that have occurred in our formulary.
207 Formulary Addendum Notice of Change (Medicare Advantage Plans) Easy Choice Health Plan Easy Choice Plus Plan (HMO) H5087-002, H5087-07 This is a listing of the changes that have occurred in our formulary.
2017 Formulary Addendum Notice of Change (Medicare Advantage Plans)
 2017 Formulary Addendum Notice of Change (Medicare Advantage Plans) Easy Choice Health Plan Easy Choice Best Plan (HMO) H5087-005 This is a listing of the changes that have occurred in our formulary. Please
2017 Formulary Addendum Notice of Change (Medicare Advantage Plans) Easy Choice Health Plan Easy Choice Best Plan (HMO) H5087-005 This is a listing of the changes that have occurred in our formulary. Please
Xyrem (Sodium Oxybate)
 Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Clinical Criteria Information Included in this Document Drugs requiring prior authorization: the list of drugs requiring prior authorization
Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Clinical Criteria Information Included in this Document Drugs requiring prior authorization: the list of drugs requiring prior authorization
CHANGES TO YOUR DRUG LIST
 CHANGES TO YOUR DRUG LIST More generics and lower-cost brands to help you stay healthy and save money At Cigna, it s our goal to offer you access to coverage for safe, effective and affordable medications.
CHANGES TO YOUR DRUG LIST More generics and lower-cost brands to help you stay healthy and save money At Cigna, it s our goal to offer you access to coverage for safe, effective and affordable medications.
Pharmacy Medical Necessity Guidelines: Opioid Analgesics
 Pharmacy Medical Necessity Guidelines: Effective: January 1, 2019 Prior Authorization Required Type of Review Care Management Not Covered Type of Review Clinical Review Pharmacy (RX) or Medical (MED) Benefit
Pharmacy Medical Necessity Guidelines: Effective: January 1, 2019 Prior Authorization Required Type of Review Care Management Not Covered Type of Review Clinical Review Pharmacy (RX) or Medical (MED) Benefit
: Opioid Quantity Limits
 March 7, 2017 2017-09: Opioid Quantity Limits The Louisiana Department of Health (LDH), in conjunction with the Louisiana Medicaid Drug Utilization Review (DUR) Board, has revised quantity limits for selected
March 7, 2017 2017-09: Opioid Quantity Limits The Louisiana Department of Health (LDH), in conjunction with the Louisiana Medicaid Drug Utilization Review (DUR) Board, has revised quantity limits for selected
ADDITIONAL 2017 FORMULARY CHANGES CIGNA COMMERCIAL CUSTOMERS
 ADDITIONAL 2017 FORMULARY CHANGES CIGNA COMMERCIAL CUSTOMERS Effective 7/1/2017, Cigna will be making additional formulary changes that may impact customers at your pharmacy. We have included a list of
ADDITIONAL 2017 FORMULARY CHANGES CIGNA COMMERCIAL CUSTOMERS Effective 7/1/2017, Cigna will be making additional formulary changes that may impact customers at your pharmacy. We have included a list of
ADDITIONAL 2017 FORMULARY CHANGES CIGNA COMMERCIAL CUSTOMERS
 ADDITIONAL 2017 FORMULARY CHANGES CIGNA COMMERCIAL CUSTOMERS Cigna will be making additional formulary changes that may impact customers at your pharmacy. We have included a list of drugs by drug class
ADDITIONAL 2017 FORMULARY CHANGES CIGNA COMMERCIAL CUSTOMERS Cigna will be making additional formulary changes that may impact customers at your pharmacy. We have included a list of drugs by drug class
New Hampshire Healthy Families CLINICAL POLICY
 New Hampshire Healthy Families CLINICAL POLICY DEPARTMENT: Pharmacy DOCUMENT NAME: Opioid Analgesics PAGE: 1 o f 6 REFERENCE NUMBER: NH.PPA.13 EFFECTIVE DATE: 6/1/2016 REPLACES DOCUMENT: N/A RETIRED: REVIEWED:
New Hampshire Healthy Families CLINICAL POLICY DEPARTMENT: Pharmacy DOCUMENT NAME: Opioid Analgesics PAGE: 1 o f 6 REFERENCE NUMBER: NH.PPA.13 EFFECTIVE DATE: 6/1/2016 REPLACES DOCUMENT: N/A RETIRED: REVIEWED:
Texas Prior Authorization Program Clinical Edit Criteria
 Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Drugs requiring prior authorization: the list of drugs requiring prior authorization
Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Drugs requiring prior authorization: the list of drugs requiring prior authorization
Alprazolam 0.25mg, 0.5mg, 1mg tablets
 Presbyterian Senior Care (HMO) / Presbyterian MediCare PPO Quantity Limits Effective November 1, 2014 For the most recent list of drugs or other questions, please contact the Presbyterian Customer Service
Presbyterian Senior Care (HMO) / Presbyterian MediCare PPO Quantity Limits Effective November 1, 2014 For the most recent list of drugs or other questions, please contact the Presbyterian Customer Service
Medicare Part D 2016 Formulary Changes Service To Senior and OC Preferred
 Medicare Part D 2016 Formulary s Service To Senior and OC Preferred Inter Valley Health Plan may add or remove drugs from our formulary during the year. If we remove a drug from our formulary, add prior
Medicare Part D 2016 Formulary s Service To Senior and OC Preferred Inter Valley Health Plan may add or remove drugs from our formulary during the year. If we remove a drug from our formulary, add prior
2017 Formulary Changes Year to Date
 2017 Formulary Changes Year to Date Health Choice Arizona may add or remove drugs from our formulary during the year. If we remove drugs from our formulary, add prior authorization, quantity limits and/or
2017 Formulary Changes Year to Date Health Choice Arizona may add or remove drugs from our formulary during the year. If we remove drugs from our formulary, add prior authorization, quantity limits and/or
Updates to your prescription benefits
 Updates to your prescription benefits Effective July 1, 2018 Update Summary Within the Prescription Drug List (PDL), medications are grouped by tier. The tier indicates the amount you pay when you fill
Updates to your prescription benefits Effective July 1, 2018 Update Summary Within the Prescription Drug List (PDL), medications are grouped by tier. The tier indicates the amount you pay when you fill
used for dealing with anxiety. Both of the drugs are also given to patients for dealing with pain activated by damaged or hypersensitive nerves that i
 Aspirin (also classified under NSAIDs or acetylsalicylic acid). Morphine and morphine sustained release (MS-Contin, Avinza, Kadian) Oxymorphone (Opana, Opana ER) Hydrocodone with acetaminophen (Lortab
Aspirin (also classified under NSAIDs or acetylsalicylic acid). Morphine and morphine sustained release (MS-Contin, Avinza, Kadian) Oxymorphone (Opana, Opana ER) Hydrocodone with acetaminophen (Lortab
Updates to your prescription benefits
 Updates to your prescription benefits Effective July 1, 2018 Update Summary Within the Prescription Drug List (PDL), medications are grouped by tier. The tier indicates the amount you pay when you fill
Updates to your prescription benefits Effective July 1, 2018 Update Summary Within the Prescription Drug List (PDL), medications are grouped by tier. The tier indicates the amount you pay when you fill
2017 Formulary Addendum Notice of Change (Medicare Advantage Plans)
 2017 Formulary Addendum Notice of Change (Medicare Advantage Plans) Easy Choice Health Plan Easy Choice Freedom Plan (HMO SNP) H5087-001 This is a listing of the changes that have occurred in our formulary.
2017 Formulary Addendum Notice of Change (Medicare Advantage Plans) Easy Choice Health Plan Easy Choice Freedom Plan (HMO SNP) H5087-001 This is a listing of the changes that have occurred in our formulary.
High-Cost Drug Exclusions
 PHARMACY SERVICES High-Cost Exclusions The high cost medications listed below are excluded from coverage because lower cost similar alternatives are available. To help you get the best health benefit at
PHARMACY SERVICES High-Cost Exclusions The high cost medications listed below are excluded from coverage because lower cost similar alternatives are available. To help you get the best health benefit at
Exclusion Reasons Presumption of Long- Term Non-Acute Administration C9399 Unclassified Drugs or
 Noridian Healthcare Solutions, LLC Jurisdiction F Part B Self-Administered Drug (SAD) Exclusion List (A53033); Effective 8/7/2017 The following medications are considered self-administered and are not
Noridian Healthcare Solutions, LLC Jurisdiction F Part B Self-Administered Drug (SAD) Exclusion List (A53033); Effective 8/7/2017 The following medications are considered self-administered and are not
Beneficiary Advisory Panel Handout Uniform Formulary Decisions 24 June 2010
 Beneficiary Advisory Panel Handout Uniform Formulary Decisions 24 June 2010 PURPOSE: The purpose of this handout is to provide BAP Committee members with a reference document for the relative clinical
Beneficiary Advisory Panel Handout Uniform Formulary Decisions 24 June 2010 PURPOSE: The purpose of this handout is to provide BAP Committee members with a reference document for the relative clinical
Cigna Drug and Biologic Coverage Policy
 Cigna Drug and Biologic Coverage Policy Subject Opioid Therapy Table of Contents Coverage Policy... 1 General Background... 4 Coding/Billing Information... 7 References... 7 Effective Date..1/1/2018 Next
Cigna Drug and Biologic Coverage Policy Subject Opioid Therapy Table of Contents Coverage Policy... 1 General Background... 4 Coding/Billing Information... 7 References... 7 Effective Date..1/1/2018 Next
Changes to the 2018 BlueCross Secure SM (HMO) & BlueCross Total SM (PPO) Formularies
 Changes to the 2018 BlueCross Secure SM (HMO) & BlueCross Total SM (PPO) Formularies BlueCross BlueShield of South Carolina may add or remove drugs from the formulary during the year. If we remove drugs
Changes to the 2018 BlueCross Secure SM (HMO) & BlueCross Total SM (PPO) Formularies BlueCross BlueShield of South Carolina may add or remove drugs from the formulary during the year. If we remove drugs
Alameda Alliance for Health Pharmacy & Therapeutics (P&T) Committee Decisions
 Alameda Alliance for Health FORMULARY UPDATE Effective: February 15, 2018. Drugs notated with an * have an undetermined implementation date Alameda Alliance for Health Pharmacy & Therapeutics (P&T) Committee
Alameda Alliance for Health FORMULARY UPDATE Effective: February 15, 2018. Drugs notated with an * have an undetermined implementation date Alameda Alliance for Health Pharmacy & Therapeutics (P&T) Committee
2017 Formulary Addendum Notice of Change
 2017 Formulary Addendum Notice of Change (Prescription Drug Plans) WellCare Prescription Insurance, Inc. WellCare Classic (PDP) WellCare Extra (PDP) This is a listing of the changes that have occurred
2017 Formulary Addendum Notice of Change (Prescription Drug Plans) WellCare Prescription Insurance, Inc. WellCare Classic (PDP) WellCare Extra (PDP) This is a listing of the changes that have occurred
PRESCRIPTION DRUG BENEFITS. open/closed formulary. Capital BlueCross is an Independent Licensee of the BlueCross BlueShield Association
 GUIDE TO PRESCRIPTION DRUG BENEFITS open/closed formulary Capital BlueCross is an Independent Licensee of the BlueCross BlueShield Association TABLE OF CONTENTS 2 Contact Us Phone Number Website 3 Using
GUIDE TO PRESCRIPTION DRUG BENEFITS open/closed formulary Capital BlueCross is an Independent Licensee of the BlueCross BlueShield Association TABLE OF CONTENTS 2 Contact Us Phone Number Website 3 Using
2017 Formulary Addendum Notice of Change
 017 Formulary Addendum Notice of Change (Medicare Advantage Plans) WellCare Health Plans WellCare Choice (HMO), WellCare Essential (HMO-POS), WellCare Value (HMO) This is a listing of the changes that
017 Formulary Addendum Notice of Change (Medicare Advantage Plans) WellCare Health Plans WellCare Choice (HMO), WellCare Essential (HMO-POS), WellCare Value (HMO) This is a listing of the changes that
Medicare Part D 2016 Formulary Changes Desert Preferred Choice
 Medicare Part D 2016 Formulary s Desert Preferred Choice Inter Valley Health Plan may add or remove drugs from our formulary during the year. If we remove a drug from our formulary, add prior authorization,
Medicare Part D 2016 Formulary s Desert Preferred Choice Inter Valley Health Plan may add or remove drugs from our formulary during the year. If we remove a drug from our formulary, add prior authorization,
Presbyterian Health Plan, Inc. Presbyterian Insurance Company, Inc. NOTIFICATION OF FORMULARY CHANGES
 NOTIFICATION OF FORMULARY CHANGES The following summary describes changes to the Presbyterian Commercial Large Group Plans (Non-Metal Plans) Formularies effective 2018. For the most recent list of drugs,
NOTIFICATION OF FORMULARY CHANGES The following summary describes changes to the Presbyterian Commercial Large Group Plans (Non-Metal Plans) Formularies effective 2018. For the most recent list of drugs,
2018 Formulary Notice of Change Prescription Drug Plans
 2018 Formulary Notice of Change Prescription Drug Plans WellCare Prescription Insurance, Inc. Plans in all states: WellCare Classic (PDP) WellCare may add or remove drugs from our formulary during the
2018 Formulary Notice of Change Prescription Drug Plans WellCare Prescription Insurance, Inc. Plans in all states: WellCare Classic (PDP) WellCare may add or remove drugs from our formulary during the
Supplementary Online Content
 Supplementary Online Content Lin DH, Jones CM, Compton WM, et al. Prescription drug coverage for treatment of low back pain among US Medicaid, Medicare Advantage, and commercial insurers. JAMA Netw Open.
Supplementary Online Content Lin DH, Jones CM, Compton WM, et al. Prescription drug coverage for treatment of low back pain among US Medicaid, Medicare Advantage, and commercial insurers. JAMA Netw Open.
Medicare Part D 2016 Formulary Changes Service To Senior and OC Preferred
 Medicare Part D 2016 Formulary s Service To Senior and OC Preferred Inter Valley Health Plan may add or remove drugs from our formulary during the year. If we remove a drug from our formulary, add prior
Medicare Part D 2016 Formulary s Service To Senior and OC Preferred Inter Valley Health Plan may add or remove drugs from our formulary during the year. If we remove a drug from our formulary, add prior
10 mg hydrocodone equals how much oxycodone
 Cari untuk: Cari Cari 10 mg hydrocodone equals how much oxycodone Posts about dilaudid 8 vs oxycodone 30 written by buyprescriptionmedication. Can you help me with the conversion of Oxycodone IR (5mg tab)
Cari untuk: Cari Cari 10 mg hydrocodone equals how much oxycodone Posts about dilaudid 8 vs oxycodone 30 written by buyprescriptionmedication. Can you help me with the conversion of Oxycodone IR (5mg tab)
Pharmacy Formulary Updates for January 2019
 Pharmacy Formulary Updates for January 2019 To offer a pharmacy benefit that is clinically appropriate and cost effective, we constantly review how we cover prescription medications. Periodic adjustments
Pharmacy Formulary Updates for January 2019 To offer a pharmacy benefit that is clinically appropriate and cost effective, we constantly review how we cover prescription medications. Periodic adjustments
2017 Formulary Addendum Notice of Change (Prescription Drug Plans)
 2017 Formulary Addendum Notice of Change (Prescription Drug Plans) WellCare Prescription Insurance, Inc. WellCare Classic (PDP) WellCare Extra (PDP) This is a listing of the changes that have occurred
2017 Formulary Addendum Notice of Change (Prescription Drug Plans) WellCare Prescription Insurance, Inc. WellCare Classic (PDP) WellCare Extra (PDP) This is a listing of the changes that have occurred
Presbyterian Health Plan, Inc. Presbyterian Insurance Company, Inc. NOTIFICATION OF FORMULARY CHANGES
 NOTIFICATION OF FORMULARY CHANGES The following summary describes changes to the 2017 Presbyterian Individual and Family Metal Plan/Employer Group Metal Plan Formularies effective 2018. For the most recent
NOTIFICATION OF FORMULARY CHANGES The following summary describes changes to the 2017 Presbyterian Individual and Family Metal Plan/Employer Group Metal Plan Formularies effective 2018. For the most recent
FORMULARY LIST OF COVERED DRUGS
 FORMULARY LIST OF COVERED DRUGS 2018 PLEASE READ: THIS DOCUMENT CONTAINS INFORMATION ABOUT THE DRUGS WE COVER IN THIS PLAN. HPMS Approved Formulary File Submission ID 00018248, Version Number #3 This formulary
FORMULARY LIST OF COVERED DRUGS 2018 PLEASE READ: THIS DOCUMENT CONTAINS INFORMATION ABOUT THE DRUGS WE COVER IN THIS PLAN. HPMS Approved Formulary File Submission ID 00018248, Version Number #3 This formulary
2017 Formulary Addendum Notice of Change
 2017 Formulary Addendum Notice of Change (Medicare Advantage Plans) WellCare Health Plans WellCare Access (HMO SNP), WellCare Liberty (HMO SNP), WellCare Reserve (HMO), WellCare Rx (HMO), WellCare Select
2017 Formulary Addendum Notice of Change (Medicare Advantage Plans) WellCare Health Plans WellCare Access (HMO SNP), WellCare Liberty (HMO SNP), WellCare Reserve (HMO), WellCare Rx (HMO), WellCare Select
ICP Formulary Updates
 ICP Formulary Updates July 2017 TRADE NAME (generic name) adapalene cream 0.1% 2017-07-01 Removal adapalene gel 0.3% 2017-07-01 Removal adefovir dipivoxil tab 10 mg 2017-07-01 Removal ADVAIR DISKUS (fluticasone-salmeterol
ICP Formulary Updates July 2017 TRADE NAME (generic name) adapalene cream 0.1% 2017-07-01 Removal adapalene gel 0.3% 2017-07-01 Removal adefovir dipivoxil tab 10 mg 2017-07-01 Removal ADVAIR DISKUS (fluticasone-salmeterol
Drug Name (specify drug) Quantity Frequency Strength
 Prior Authorization Form GEHA FEDERAL - STANDARD OPTION 1363-M Opioids IR MME Limit and Post Limit This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information,
Prior Authorization Form GEHA FEDERAL - STANDARD OPTION 1363-M Opioids IR MME Limit and Post Limit This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information,
2018 Formulary Update
 MEDICARE ADVANTAGE BlueShield of Northeastern New York 2018 Formulary Update BlueShield of Northeastern New York has updated its formulary (drug list) since its original publication in January 2018. This
MEDICARE ADVANTAGE BlueShield of Northeastern New York 2018 Formulary Update BlueShield of Northeastern New York has updated its formulary (drug list) since its original publication in January 2018. This
Aetna Better Health of Illinois Medicaid Formulary Updates
 October 2017 o DOXYLAMINE SUCCINATE 25mg-QL o DULOXETINE CAP 40MG DR-QL o GUANFACIN ER TABS (all strengths)-ql o TOBRAMYCIN NEBU SOLUTION- PA August 2017 Aetna Better Health of Illinois Medicaid 2017 Formulary
October 2017 o DOXYLAMINE SUCCINATE 25mg-QL o DULOXETINE CAP 40MG DR-QL o GUANFACIN ER TABS (all strengths)-ql o TOBRAMYCIN NEBU SOLUTION- PA August 2017 Aetna Better Health of Illinois Medicaid 2017 Formulary
Drug Name (specify drug) Quantity Frequency Strength
 Prior Authorization Form MEDICA HEALTH PLAN IA EXCHANGE 1362-M Opioids IR Labeling Post Limit (HMF) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information,
Prior Authorization Form MEDICA HEALTH PLAN IA EXCHANGE 1362-M Opioids IR Labeling Post Limit (HMF) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information,
Pequot Health Care Opioid Analgesic Quantity Program*
 Pequot Health Care 1 Annie George Drive Mashantucket, CT 06338 Phone: 1-888-779-6638 Fax: 1-860-396-6494 Pequot Health Care Opioid Analgesic Quantity Program* Effective January 2018 *Quantity Program limits
Pequot Health Care 1 Annie George Drive Mashantucket, CT 06338 Phone: 1-888-779-6638 Fax: 1-860-396-6494 Pequot Health Care Opioid Analgesic Quantity Program* Effective January 2018 *Quantity Program limits
Opioid Capture in the AHSQC Can we reduce use by measuring it? Michael Reinhorn MD, MBA, FACS Dept of Surgery, Newton Wellesley Hospital
 Opioid Capture in the AHSQC Can we reduce use by measuring it? Michael Reinhorn MD, MBA, FACS Dept of Surgery, Newton Wellesley Hospital Disclosure Davol/Bard Consultant Medtronic Physician Advisory Honorarium
Opioid Capture in the AHSQC Can we reduce use by measuring it? Michael Reinhorn MD, MBA, FACS Dept of Surgery, Newton Wellesley Hospital Disclosure Davol/Bard Consultant Medtronic Physician Advisory Honorarium
Drug Name Description of Change Formulary Coverage Formulary Alternative(s)
 NOTIFICATION OF FORMULARY CHANGES The following summary describes changes to the Presbyterian Centennial Care Formulary effective 2018. For the most recent list of drugs, information on asking for a prior
NOTIFICATION OF FORMULARY CHANGES The following summary describes changes to the Presbyterian Centennial Care Formulary effective 2018. For the most recent list of drugs, information on asking for a prior
Medication Policy Manual. Topic: Immediate-release (IR) Opioid Medication Products for Pain. Date of Origin: January 1, 2018
 Independent licensees of the Blue Cross and Blue Shield Association Medication Policy Manual Topic: Immediate-release (IR) Opioid Medication Products for Pain Policy No: dru516 Date of Origin: January
Independent licensees of the Blue Cross and Blue Shield Association Medication Policy Manual Topic: Immediate-release (IR) Opioid Medication Products for Pain Policy No: dru516 Date of Origin: January
Drug Formulary Update, January 2018 Commercial and State Programs
 Drug Formulary Update, January 2018 Commercial and State Programs Updates to the HealthPartners Commercial and State Program Drug Formularies are listed below. Updates apply to all Commercial groups (PreferredRx,
Drug Formulary Update, January 2018 Commercial and State Programs Updates to the HealthPartners Commercial and State Program Drug Formularies are listed below. Updates apply to all Commercial groups (PreferredRx,
ALABAMA S ADAP FORMULARY OFFERS 117 MEDICATIONS
 ALABAMA S ADAP FORMULARY OFFERS 117 MEDICATIONS - 2014 Alabama s ADAP formulary offers a minimum of one medication from each HIV antiretroviral class approved by the U.S. Food and Drug Administration (FDA).
ALABAMA S ADAP FORMULARY OFFERS 117 MEDICATIONS - 2014 Alabama s ADAP formulary offers a minimum of one medication from each HIV antiretroviral class approved by the U.S. Food and Drug Administration (FDA).
High-Cost Drug Exclusions
 Pharmacy Services High-Cost Exclusions The high cost medications listed below are excluded from coverage because lower cost similar alternatives are available. To help you get the best health benefit at
Pharmacy Services High-Cost Exclusions The high cost medications listed below are excluded from coverage because lower cost similar alternatives are available. To help you get the best health benefit at
Drug Class Preferred Agents Non-Preferred Agents
 Commissioner for the Department for Medicaid Services Selections for Preferred Products This is a summary of the final Preferred Drug List (PDL) selections made by the Commissioner of the Department for
Commissioner for the Department for Medicaid Services Selections for Preferred Products This is a summary of the final Preferred Drug List (PDL) selections made by the Commissioner of the Department for
Rationale for Decision Excluded Generic OTC equivalent available (Flonase Allergy Relief) Medicare status (if differs)
 BLUE SHIELD OF CALIFORNIA FIRST QUARTER 2015 FORMULARY AND MEDICATION POLICY UPDATES EFFECTIVE MARCH 19, 2015 The Blue Shield of California (BSC) Pharmacy and Therapeutics (P&T) Committee, consisting of
BLUE SHIELD OF CALIFORNIA FIRST QUARTER 2015 FORMULARY AND MEDICATION POLICY UPDATES EFFECTIVE MARCH 19, 2015 The Blue Shield of California (BSC) Pharmacy and Therapeutics (P&T) Committee, consisting of
May 2016 P & T Updates
 Commercial Triple Tier 4th Tier Applicabl e Traditional Detailed s 2 films - DESCOVY 2 2 1 tablet NARCAN 2 2 ODEFSEY 2 2 1 tablet REPATHA 2 2 HoFH: 3 ml per 28 ROSUVASTATIN 1 1 - UPTRAVI 3 2 - Alternatives
Commercial Triple Tier 4th Tier Applicabl e Traditional Detailed s 2 films - DESCOVY 2 2 1 tablet NARCAN 2 2 ODEFSEY 2 2 1 tablet REPATHA 2 2 HoFH: 3 ml per 28 ROSUVASTATIN 1 1 - UPTRAVI 3 2 - Alternatives
Specialty Drugs. The specialty drug list below is effective June 5, 2018 and is subject to change at any time.
 Specialty Drugs The following is a list of medications that are considered specialty drugs. Specialty drugs include self-administered injectables, medications that are high cost, and/or medications that
Specialty Drugs The following is a list of medications that are considered specialty drugs. Specialty drugs include self-administered injectables, medications that are high cost, and/or medications that
Updates to your prescription benefits
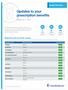 Updates to your prescription benefits Effective Jan. 1, 2018 Update Summary Within the Prescription Drug List (PDL), medications are grouped by tier. The tier indicates the amount you pay when you fill
Updates to your prescription benefits Effective Jan. 1, 2018 Update Summary Within the Prescription Drug List (PDL), medications are grouped by tier. The tier indicates the amount you pay when you fill
Formulary Changes. One mission: you TABLE A. FORMULARY CHANGES 7/1/2018: Commercial 3-Tier Formulary. Commercial 4-Tier Formulary
 One mission: you Changes July 1, 2018 Blue Cross of Idaho reviews its formularies (covered drug lists) periodically to allow members access to new drugs and to provide safe, cost effective options for
One mission: you Changes July 1, 2018 Blue Cross of Idaho reviews its formularies (covered drug lists) periodically to allow members access to new drugs and to provide safe, cost effective options for
Quarterly pharmacy formulary change notice
 Quarterly pharmacy formulary change notice Summary of change: The Pharmacy and Therapeutics Committee (P&T) reviewed and approved the formulary changes listed in the table below on March 29, 2016. What
Quarterly pharmacy formulary change notice Summary of change: The Pharmacy and Therapeutics Committee (P&T) reviewed and approved the formulary changes listed in the table below on March 29, 2016. What
Step Therapy Group. Atypical Antipsychotic Agents
 Atypical Antipsychotic Agents Any beneficiary newly enrolled into Community Care, Inc. currently receiving aripiprazole, aripiprazole ODT, risperidone, risperidone ODT, olanzapine, olanzapine ODT, quetiapine,
Atypical Antipsychotic Agents Any beneficiary newly enrolled into Community Care, Inc. currently receiving aripiprazole, aripiprazole ODT, risperidone, risperidone ODT, olanzapine, olanzapine ODT, quetiapine,
Drug Formulary Update, October 2016 Commercial and State Programs
 Drug Formulary Update, October 2016 Commercial and State Programs Updates to the HealthPartners Commercial and State Program Drug Formularies are listed below. Updates apply to all Commercial groups (PreferredRx,
Drug Formulary Update, October 2016 Commercial and State Programs Updates to the HealthPartners Commercial and State Program Drug Formularies are listed below. Updates apply to all Commercial groups (PreferredRx,
ALOGLIPTIN STEP. Details. Step Therapy Requirements Effective November 1, 2017
 ALOGLIPTIN STEP alogliptin 12.5 mg tablet alogliptin 12.5 mg-metformin 1,000 mg tablet alogliptin 12.5 mg-metformin 500 mg tablet alogliptin 12.5 mg-pioglitazone 15 mg tablet alogliptin 12.5 mg-pioglitazone
ALOGLIPTIN STEP alogliptin 12.5 mg tablet alogliptin 12.5 mg-metformin 1,000 mg tablet alogliptin 12.5 mg-metformin 500 mg tablet alogliptin 12.5 mg-pioglitazone 15 mg tablet alogliptin 12.5 mg-pioglitazone
Available Strengths. Cost per Rx 325 mg tablet - $ mg tablet - $ mg ER tablet - $ mg capsule - $ mg chewable tablet
 MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY Non-Opioids LAST REVIEW 5/9/2017 THERAPEUTIC CLASS Pain REVIEW HISTORY 2/16, 5/15 LOB AFFECTED Medi-Cal (MONTH/YEAR) This
MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY Non-Opioids LAST REVIEW 5/9/2017 THERAPEUTIC CLASS Pain REVIEW HISTORY 2/16, 5/15 LOB AFFECTED Medi-Cal (MONTH/YEAR) This
Step Therapy Medications
 Step Therapy Medications Step Therapy Group APTIOM Step-2: APTIOM 200 MG TABLET or APTIOM 400 MG TABLET or APTIOM 600 MG TABLET or APTIOM 800 MG TABLET Step 1 Drug(s): Oxcarbazepine immediate-release,
Step Therapy Medications Step Therapy Group APTIOM Step-2: APTIOM 200 MG TABLET or APTIOM 400 MG TABLET or APTIOM 600 MG TABLET or APTIOM 800 MG TABLET Step 1 Drug(s): Oxcarbazepine immediate-release,
Specialty Drugs. The following is a list of medications that are considered to be specialty drugs. Specialty drugs
 Specialty Drugs The following is a list of medications that are considered to be specialty drugs. Specialty drugs include self-administered injectables, medications that are high cost, and/or medications
Specialty Drugs The following is a list of medications that are considered to be specialty drugs. Specialty drugs include self-administered injectables, medications that are high cost, and/or medications
Superior Select Health Plans: Tribute-1 Tier May 2018 Formulary Addendum
 Superior Select Health Plans: Tribute-1 Tier May 2018 Formulary Addendum Below is a list formulary changes for the benefit year 2018. This is not a complete list of drugs covered by the Part D plan. The
Superior Select Health Plans: Tribute-1 Tier May 2018 Formulary Addendum Below is a list formulary changes for the benefit year 2018. This is not a complete list of drugs covered by the Part D plan. The
March 2018 P & T Updates
 March 2018 P & T Updates Commercial Triple Tier 4th Tier Applicable Traditional Prior Auth AURYXIA 3 2 12 tablets per BAXDELA TABLETS 3 2 2 tablets per Depending on your specific benefits and in which
March 2018 P & T Updates Commercial Triple Tier 4th Tier Applicable Traditional Prior Auth AURYXIA 3 2 12 tablets per BAXDELA TABLETS 3 2 2 tablets per Depending on your specific benefits and in which
July 2017 P&T Updates
 July 2017 P&T Updates Commercial Triple Tier 4th Tier Applicable Traditional Prior Auth Alternatives ALUNBRIG 3 2 DUPIXENT Pharmacy 3 2 6 tablets per day, 30 day One-week auth for QL of 3 syringes per
July 2017 P&T Updates Commercial Triple Tier 4th Tier Applicable Traditional Prior Auth Alternatives ALUNBRIG 3 2 DUPIXENT Pharmacy 3 2 6 tablets per day, 30 day One-week auth for QL of 3 syringes per
PAIN. TREATMENT TABLES Analgesics. NON-OPIOID ANALGESICS Generic Name Trade Names (Examples) Duration Initial Dose
 NON-OPIOID SHORT-ACTING LONG-ACTING **** O PAIN TREATMENT TABLES Analgesics NON-OPIOID ANALGESICS Generic Name Trade Names (Examples) Duration Initial Dose Tramadol 50 mg Ultram Every 4 hours 1-2 tabs,
NON-OPIOID SHORT-ACTING LONG-ACTING **** O PAIN TREATMENT TABLES Analgesics NON-OPIOID ANALGESICS Generic Name Trade Names (Examples) Duration Initial Dose Tramadol 50 mg Ultram Every 4 hours 1-2 tabs,
Medicare Part D 2017 Formulary Changes Service To Senior
 Medicare Part D 2017 Formulary Changes Service To Senior Inter Valley Health Plan may add or remove drugs from our formulary during the year. If we remove a drug from our formulary, add prior authorization,
Medicare Part D 2017 Formulary Changes Service To Senior Inter Valley Health Plan may add or remove drugs from our formulary during the year. If we remove a drug from our formulary, add prior authorization,
Pharmacy Benefit Coverage Updates Jan. 1, 2018
 Pharmacy Benefit Coverage Updates Jan. 1, 2018 UnitedHealthcare routinely evaluates prescription benefit coverage to help ensure we offer members affordable and effective medication options. Medications
Pharmacy Benefit Coverage Updates Jan. 1, 2018 UnitedHealthcare routinely evaluates prescription benefit coverage to help ensure we offer members affordable and effective medication options. Medications
You ll find the most up-to-date comprehensive version of our formulary on our website, Click on Drug Finder.
 3/1/2018 Medicare Part D Formulary Change In an effort to cover the most needed, cost-effective prescriptions, the AlohaCare Advantage Plus (HMO SNP) Formulary is updated monthly. The following are drugs
3/1/2018 Medicare Part D Formulary Change In an effort to cover the most needed, cost-effective prescriptions, the AlohaCare Advantage Plus (HMO SNP) Formulary is updated monthly. The following are drugs
Legacy Pain Management Center New Patient Questionnaire
 Legacy Pain Management Center New Patient Questionnaire Please complete this form prior to your visit to allow us to make the best use of our time together. Primary Care Provider: Referring Physician:
Legacy Pain Management Center New Patient Questionnaire Please complete this form prior to your visit to allow us to make the best use of our time together. Primary Care Provider: Referring Physician:
Section 2 Class III, IV & V Pharmaceuticals Page 13
 Section 2 Class III, IV & V Pharmaceuticals Page 13 ACETAMINOPHEN W/ CODEINE #3 30MG TABS (GENERIC TYLENOL) #100 41.99 ACETAMINOPHEN W/ CODEINE #3 30MG TABS #1000 298.99 ACETAMINOPHEN W/ CODEINE #4 60MG
Section 2 Class III, IV & V Pharmaceuticals Page 13 ACETAMINOPHEN W/ CODEINE #3 30MG TABS (GENERIC TYLENOL) #100 41.99 ACETAMINOPHEN W/ CODEINE #3 30MG TABS #1000 298.99 ACETAMINOPHEN W/ CODEINE #4 60MG
BLUE SHIELD OF CALIFORNIA MARCH 2016 STANDARD DRUG FORMULARY CHANGES
 BLUE SHIELD OF CALIFORNIA MARCH 2016 STANDARD DRUG FORMULARY CHANGES Blue Shield is committed to covering safe, effective and affordable medications, so we regularly review and update our drug formularies.
BLUE SHIELD OF CALIFORNIA MARCH 2016 STANDARD DRUG FORMULARY CHANGES Blue Shield is committed to covering safe, effective and affordable medications, so we regularly review and update our drug formularies.
WVCH Formulary Additions Effective 01/01/2016 Name Strength Dosage Form Route Formulary Restrictions
 WVCH Formulary Additions Effective 01/01/2016 Name Strength Dosage Form Route Formulary Restrictions ANORO ELLIPTA 62.5-25MCG BLST W/DEV INHALATION ARCAPTA NEOHALER 75 MCG CAP W/DEV INHALATION CALCIPOTRIENE
WVCH Formulary Additions Effective 01/01/2016 Name Strength Dosage Form Route Formulary Restrictions ANORO ELLIPTA 62.5-25MCG BLST W/DEV INHALATION ARCAPTA NEOHALER 75 MCG CAP W/DEV INHALATION CALCIPOTRIENE
No Pain, No Gain Pharmacy Patient Pain Counseling Competition
 No Pain, No Gain Pharmacy Patient Pain Counseling Competition Offered by the Maine Pharmacy Association as part of the 2010 MPA Fall Conference Sponsored by an educational grant by NASPA and Purdue Pharma,
No Pain, No Gain Pharmacy Patient Pain Counseling Competition Offered by the Maine Pharmacy Association as part of the 2010 MPA Fall Conference Sponsored by an educational grant by NASPA and Purdue Pharma,
Pharmacy and Therapeutics (P&T) Committee Provider Update
 Pharmacy and Therapeutics (P&T) Committee Provider Update THIRD QUARTER 2018 P&T Committee Decisions Effective September 1, 2018 Dear Healthcare Practitioner: The Presbyterian Health Plan, Inc., and Presbyterian
Pharmacy and Therapeutics (P&T) Committee Provider Update THIRD QUARTER 2018 P&T Committee Decisions Effective September 1, 2018 Dear Healthcare Practitioner: The Presbyterian Health Plan, Inc., and Presbyterian
Subject: HPN/SHL COMMERCIAL PDL UPDATES EFFECTIVE JANUARY 1, 2018
 Date: November 7, 2017 To: From: HPN/SHL Contracted Pharmacies and Providers Ryan Bitton, PharmD, MBA Senior Director, Pharmacy Subject: HPN/SHL COMMERCIAL PDL UPDATES EFFECTIVE JANUARY 1, 2018 Effective
Date: November 7, 2017 To: From: HPN/SHL Contracted Pharmacies and Providers Ryan Bitton, PharmD, MBA Senior Director, Pharmacy Subject: HPN/SHL COMMERCIAL PDL UPDATES EFFECTIVE JANUARY 1, 2018 Effective
PAIN CONTROL IN THE PATIENT WITH ACUTE/CHRONIC PAIN JAY E. OLSSON,DO AOBPMR DCAQPM ABPMR ABPM
 PAIN CONTROL IN THE PATIENT WITH ACUTE/CHRONIC PAIN JAY E. OLSSON,DO AOBPMR DCAQPM ABPMR ABPM Disclaimers Speaker Bureau Avanir Pharmaceuticals Nuedexta Pfizer Inc. Embeda Lyrica FOCUS Pharmacologic interventions
PAIN CONTROL IN THE PATIENT WITH ACUTE/CHRONIC PAIN JAY E. OLSSON,DO AOBPMR DCAQPM ABPMR ABPM Disclaimers Speaker Bureau Avanir Pharmaceuticals Nuedexta Pfizer Inc. Embeda Lyrica FOCUS Pharmacologic interventions
2015 Formulary. List of Covered Drugs. HMSA Akamai Advantage Enhanced and Prime Group Drug Plans. Formulary ID , version 17
 HMSA Akamai Advantage Enhanced and Prime Group Drug Plans 2015 Formulary PLEASE READ: THIS DOCUMENT CONTAINS INFORMATION ABOUT THE DRUGS WE COVER IN THIS PLAN. List of Covered Drugs Formulary ID 00015358,
HMSA Akamai Advantage Enhanced and Prime Group Drug Plans 2015 Formulary PLEASE READ: THIS DOCUMENT CONTAINS INFORMATION ABOUT THE DRUGS WE COVER IN THIS PLAN. List of Covered Drugs Formulary ID 00015358,
