Oral Dexamethasone versus Oral Prednisolone in Acute Asthma: A New Randomized Controlled Trial and Updated Meta-analysis.
|
|
|
- Elwin Newman
- 5 years ago
- Views:
Transcription
1 J O U R N A L C L U B Oral Dexamethasone versus Oral Prednisolone in Acute Asthma: A New Randomized Controlled Trial and Updated Meta-analysis. SOURCE CITATION: Paniagua N, Lopez R, Muñoz N, Tames M, Mojica E, Arana-Arri E, et al. Randomized trial of dexamethasone versus prednisone for children with acute asthma exacerbations. J Pediatr. 2017;191: SECTION EDITOR: ABHIJEET SAHA SUMMARY This randomized, noninferiority trial included patients aged 1-14 years who presented to the emergency department (ED) with acute asthma. Primary objective was to compare the efficacy of two doses of dexamethasone (0.6 mg/kg/dose, experimental treatment) and a 5-day course of prednisolone/prednisone (1.5 mg/ kg/d, followed by 1 mg/kg/d on days 2-5, conventional treatment). The primary outcome measures were the percentage of patients with asthma symptoms and quality of life at day 7. Secondary outcomes were unscheduled returns, admissions, adherence, and vomiting. During the study period, 710 children who met the inclusion criteria were invited to participate and 590 agreed. Primary outcome data were available in 557 patients. At day 7, experimental and conventional groups did not show differences related to persistence of symptoms (56.6%, 95% CI 50.6 to 62.6 vs 58.3%, 95% CI 52.3 to 64.2, respectively), quality of life score (80.0 vs 77.7), admission rate (23.9% vs 21.7%), unscheduled ED return visits (4.6% vs 3.3%), and vomiting (2.1% vs 4.4%). Adherence was greater in the dexamethasone group (99.3% vs 96.0%, P<0.05). Authors concluded that two doses of dexamethasone may be an effective alternative to a 5-day course of prednisone/prednisolone for asthma exacerbations, as measured by persistence of symptoms and quality of life at day 7. COMMENTARIES Evidence-based Medicine Viewpoint Relevance: Administration of parenteral corticosteroids is a standard of care for acute asthma exacerbations in children and adults. This is reflected in most evidencebased guidelines, irrespective of whether initial management is started at home, primary health-care facilities or hospitals [1-4]. In fact, these guidelines recommend initiation of steroid therapy within the first hour of management in all except mild exacerbations. Oral administration has been shown to be as effective as intravenous or intramuscular administration. Thus, oral prednisone/prednisolone in the dose of 1-2 mg/kg per day has been recommended for a total of 5-7 days; although some studies have examined shorter courses and/or lower doses. A limited number of studies also compared prednisone versus dexamethasone with the goal of evaluating whether the duration of therapy and/or number of doses could be reduced. There are four reasonably well-designed trials comparing oral prednisone versus oral dexamethasone in children [5-8]. A relatively recent Cochrane systematic review [9] reported that both medications had comparable efficacy in terms of hospital admission frequency (OR 1.08; 95% CI 0.74, 1.58; 3 trials; 1007 participants), re-admission to hospital (OR 0.44; 95% CI 0.15, 1.33; 3 trials; 985 participants), new exacerbations during the follow-up period necessitating unplanned visits to health-care providers (OR 0.85; 95% CI 0.54, 1.34; 4 trials; 981 participants), new exacerbations requiring additional oral steroids (OR % CI 0.10, 0.81; 1 trial; 242 participants), and prevalence of vomiting (OR 3.05; CI 0.88, 10.55; 3 trials; 867 participants). Two of the four trials in the review reported comparable symptom scores between the two groups. Overall, these data suggest that dexamethasone has comparable (but not superior) efficacy and safety to prednisone. However, the trials had differences in terms of dose and/or duration of medications, outcomes studied, timing of outcome assessment, and methodological quality. Table I summarizes the characteristics of the trials and their differences. One more trial comparing prednisone and dexamethasone has been recently published [10]. The trial characteristics are compared to the previous trials in INDIAN PEDIATRICS 155 VOLUME 55 FEBRUARY 15, 2018
2 TABLE I CHARACTERISTICS OF TRIALS COMPARING ORAL DEXAMETHASONE AND PREDNISONE IN ACUTE ASTHMA Study Qureshi 2001 Altamimi 2006 Greenberg 2008 Cronin 2015 Paniagua 2017 Design Open-label RCT DB, RCT DB, RCT Open-label RCT Open-label, non-inferiority RCT Setting USA Canada USA Ireland Spain Participants 2-18 y with exacerbation 2-16 y with mild to 2-18 y with 2-16 y with exacerbation 1-14 y with exacerbation (ED (worsening symptoms or increased moderate exacerbation (PIS exacerbation (episode prompting ED visit with any of: dyspnea, breathing difficulty with decline in score <9 and PEFR >60%). (criteria unclear) visit with any of: dyspnea, wheezing, cough, increased PEFR). Severe asthma excluded. Severity criteria for wheezing, cough, increased work of breathing, increased exclusion not work of breathing, increased need of bronchodilator). Critical mentioned. use of β-2 agonist or SpO 2 or life-threatening episode <95%). Critical or life- excluded. threatening episode excluded. Intervention 0.6 mg/kg/d 2 days 0.6 mg/kg single dose 0.6 mg/kg/d 2 days 0.3 mg/kg single dose 0.6 mg/kg/d 2 days (Dexamethasone) Comparison 2 mg/kg initial + 2 mg/kg/d (in 2 divided 2 mg/kg/d (in 2 1 mg/kg/d 3 d 1.5 mg/kg initial + (Prednisolone) 1 mg/kg/d 5 d doses) 5 d divided doses) 5 d 1mg/kg/d 4 d Outcomes Relapse within 10 d (i.e. unscheduled No. of days for return of PSAS Relapse within 10 d PRAM score on d4, change in Persistence of symptoms on d7, visit of persistent/ worse/ recurrent score to baseline or PEFR (i.e. need for PRAM score, PRAM score at QoL score on d7, vomiting, symptoms), vomiting, compliance, 80%, adverse events, hospitalization or discharge, hospital admission treatment adherence, hospital symptom persistence school/work rescue medication, unscheduled unscheduled visit), on d1, ED LOS, unscheduled admission, unscheduled visits, absence visits, oxygen saturation, vital vomiting visits, re-admission, additional hospital re-admissions, school/ signs, compliance systemic steroids, vomiting work absence within 30 minutes, school/ work absence.. Sample size I = 309; C = 319 I = 67; C = 67 Total 167 I = 127 episodes; C = 123 I = 294; C = 296 episodes Risk of bias High Low Moderate Moderate Moderate DB: Double blind; RCT: Randomized controlled trial; ED: emergency department;pis: Pulmonary index score; PEFR: Peak expiratory flow rate; PRAM: Pediatric Respiratory Assessment Measure; PSAS = Patient Self Assessment Sheet; QoL: Quality of life; SpO2: oxygen saturation; I: intervention group; C: Control group. INDIAN PEDIATRICS 156 VOLUME 55 FEBRUARY 15, 2018
3 Table I. The results showed that dexamethasone was comparable to prednisone with respect to persistence of symptoms (OR 0.93; 95% CI 0.67, 1.30), Quality of Life (QoL) score (mean difference 2.30 percentiles; 95% CI , 5.24), admission to an observation unit (OR 1.07; 95% CI 0.71, 1.63), hospital admission (1.42; 95% CI 0.53, 3.78), stay in the Emergency (mean difference 0.10 hours; 95% CI -0.82, 1.02), unscheduled visits (OR 1.44; 95% CI 0.60, 3.42), hospital re-admission (0.49; 95% CI 0.04, 5.43), vomiting (0.48; 95% CI 0.18, 1.30), and use of additional steroids (1.38; 95% CI 0.71, 2.69). Even the number of days of school/work missed were comparable. The only significant difference observed was that failure of adherence to treatment was lower with dexamethasone (OR 0.17; 95% CI 0.04, 0.79). The authors also reported a high degree of parental satisfaction on day 7 but this could not be compared (see below for details). The additional data from this trial [10] facilitates an updated meta-analysis for major outcomes. This showed comparable results for initial hospital admission (OR 0.98; 95% CI 0.69, 1.40; 4 trials; 1564 participants; I 2 = 0%), hospital re-admission (OR 1.75; 95% CI 0.67, 4.54; 4 trials; 1542 episodes; I 2 = 0%), and unscheduled visits (OR 1.25; 95% CI 0.84, 1.85; 5 trials; 1538 participants; I 2 = 0%). However, vomiting was significantly reduced with dexamethasone (OR 0.34; 95% CI 0.19, 0.60; 4 trials; 1424 participants; I 2 = 28%). Critical appraisal: Methodological critical appraisal using the Cochrane Risk of Bias tool [11] is presented in Table II. The trial included several methodological refinements. It was designed as a non-inferiority trial necessitating a larger sample size. In fact, this is the only non-inferiority trial comparing dexamethasone versus prednisone. The trial included fairly robust definitions/ criteria for asthma, exacerbations, and the scores used to calculate symptom persistence and quality of life. Although most outcomes were based on parental report, some of the data were retrieved from electronic records reducing the risk of bias in parental reporting. However, there are some significant limitations worth mentioning. The investigators chose two patient-centric measures (viz symptom persistence and QoL score on day 7) as the primary outcomes rather than the conventional objective measures of improvement (such as symptom scores, need for admission, step-up of therapy/care, etc). The authors attempted to justify this decision, but the arguments are weak. Since three of the four previous trials were already available when this trial was started, it would have been better to align the outcomes to facilitate comparison with existing data. Further, both the primary outcomes were based on parental report, and that too, obtained over telephone, rather than a face-to-face interview. This makes it difficult to assess the reliability of these outcome measures. Despite this, the investigators did not choose to ensure blinding of the parents/children and personnel collecting data over the phone. In a trial of this nature, this would have been relatively easy using the double-dummy design. The impact of absence of blinding of these groups is difficult to assess in this trial. One of the major challenges in this trial is a very high proportion of children with persistence of symptoms at the end-point (7 days). In fact, 57% children who received dexamethasone and 58% of those receiving prednisone continued to be symptomatic well after therapy was discontinued. This suggests a high rate of treatment failure (even though it was comparable between the two groups). No clear explanation was offered by the authors for this; although the doses and durations in both groups Participant baseline characteristics Randomization Allocation concealment Blinding of participants and personnel Blinding of outcome assessors Incomplete outcome data Selective outcome reporting Other sources of bias Overall assessment TABLE II CRITICAL APPRAISAL OF THE RCT The participants in both groups were comparable in terms of age, gender distribution, characteristics of the exacerbation, and symptom persistence. Adequate; A computer program was used to generate the allocation sequence. Adequate; The allocation of individual children was concealed in serially numbered opaque envelopes. Inadequate; This was not done. Inadequate; This was not done. Unclear; The trial randomized 590 children, but despite planning an intention-to-treat analysis, results were reported only in 557. The proportion of children whose data were missing was 4.4% in dexamethasone group and 6.8% in prednisone group. Adequate; All the outcomes planned, were measured and reported. No obvious bias Moderate risk of bias INDIAN PEDIATRICS 157 VOLUME 55 FEBRUARY 15, 2018
4 were in line with standard recommendations. Further careful analysis of the primary outcome measures shows that the proportion of children with symptom persistence (in both groups) was higher at the end-point than at the baseline (56.6% compared to 43.8% for dexamethasone; and 58.3% compared to 37.7% for prednisone). This seems strange, but no explanation has been offered. In fact, each of the components of the symptom persistence scoring system also showed worsening after therapy (than at baseline) in both groups. Further, the QoL score remained unchanged after therapy (80.0 vs 79.4 for dexamethasone; and 77.7 vs 79.5 for prednisone). This is also difficult to explain. It is possible that these apparently unusual observations could be related to data being obtained by direct interview at baseline, but by telephone at the end-point. This again highlights that the primary outcome measures in this study were not ideal. In contrast, most of the secondary outcome measures did not suggest that the apparent treatment failure necessitated medical attention to the same extent. For example, there was very low hospitalization rate, hospital re-admission rate, unscheduled visits to healthcare provider(s), need for additional steroids, and school absenteeism. However, one outcome designated admission to observation unit was present in nearly one-fifth of all children. Unfortunately, the details have not been described, but it could suggest need for further care in a significant proportion of the children (although the rate was comparable between the groups). These observations suggest that dexamethasone and prednisone had a very high (through comparable) lack of efficacy in this trial. The only outcome tilted in favor of dexamethasone was lack of adherence; although, it was extremely low in both groups. However, as this was also based purely on parental report, the veracity is questionable. The investigators suggested very high parental satisfaction at the end-point; however, this outcome does not appear to have been measured in all the children. Strangely, parental satisfaction is reported in 99.3% and 96.0% in the dexamethasone and prednisone groups, respectively, whereas the respective absolute numbers are only 210 and 179. This makes it difficult to interpret this outcome. Last but not the least, the investigators planned intention-to-treat analysis. In its purest sense this implies that all randomized participants should appear in the analysis, and not only those for whom data are available, or those who complete the trial per protocol [12]. In this trial, 590 children were randomized (and should have constituted the denominator), whereas data were analyzed using only 557 children. Extendibility: Both medications used in this trial are easily available in our setting in various formulations and packaging, making it easy to administer. Further, the possibility of reducing treatment duration from 5 days to 2 days (by using dexamethasone instead of prednisone) makes it an attractive proposition. However, the data from this trial do not provide compelling evidence to switch from the current standard of care to an alternative strategy. This is because there are several concerns with the internal validity of the trial (highlighted above). Further, the four previous trials also demonstrated only comparability, but not superiority of dexamethasone over prednisone. Conclusion: This randomized trial showed that the treatment of acute asthma (except severe cases) with oral dexamethasone had comparable efficacy to oral prednisone, although a high rate of treatment failure was observed in both groups. Updated meta-analysis confirmed comparable efficacy outcomes, but vomiting was significantly lower with dexamethasone. This is a new finding not identified in the previous meta-analyses [9]. Funding: None; Competing interests: None stated. REFERENCES JOSEPH L MATHEW Department of Pediatrics, PGIMER, Chandigarh, India. dr.joseph.l.mathew@gmail.com 1. National Institute for Health and Care Excellence (NICE). Asthma Quality Standard Available from: Accessed January 15, Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention. Available from: /ginasthma.org/2017-gina-report-global-strategy-forasthma-management-and-prevention/. Accessed January 15, British Thoracic Society and Scottish Intercollegiate Guidelines Network. British Guideline on the Management of Asthma. Available from: asthma/btssign-asthma-guideline-2016/. Accessed January 15, Arakawa H, Hamasaki Y, Kohno Y, Ebisawa M, Kondo N, Nishima S, et al. Japanese guidelines for childhood asthma Allergol Int. 2017;66: Qureshi F, Zaritsky A, Poirier MP. Comparative efficacy of oral dexamethasone versus oral prednisone in acute pediatric asthma. J Pediatr. 2001;139: Altamimi S, Robertson G, Jastaniah W, Davey A, Dehghani INDIAN PEDIATRICS 158 VOLUME 55 FEBRUARY 15, 2018
5 N, Chen R, et al. Single-dose oral dexamethasone in the emergency management of children with exacerbations of mild to moderate asthma. Pediatr Emerg Care. 2006;22: Greenberg RA, Kerby G, Roosevelt GE. A comparison of oral dexamethasone with oral prednisone in pediatric asthma exacerbations treated in the emergency department. Clin Pediatr (Phila). 2008;47: Cronin JJ, McCoy S, Kennedy U, An Fhailí SN,Wakai A, Hayden J, et al. A randomized trial of single-dose oral dexamethasone versus multi dose prednisolone for acute exacerbations of asthma in children who attend the emergency department. Ann Emerg Med. 2016;67: Normansell R, Kew KM, Mansour G. Different oral corticosteroid regimens for acute asthma. Cochrane Database Syst Rev. 2016;5:CD Paniagua N, Lopez R, Muñoz N, Tames M, Mojica E, Arana-Arri E, et al. Randomized trial of dexamethasone versus prednisone for children with acute asthma exacerbations. J Pediatr. 2017;191: Cochrane Risk of Bias Tool (modified) for Quality Assessment of Randomized Controlled Trials. Available from: rct.crit.pdf. Accessed November 20, Cochrane Community. Glossary. Available from: community.cochrane.org/glossary#letter-i. Accessed January 15, Pediatric Pulmonologist s Viewpoint The investigators in this randomized, non-inferiority trial compared two doses of dexamethasone (0.6 mg/kg/dose) with 5 day oral prednisone (1-1.5 mg/kg/day) in children 1-14 years of age with acute asthma presentation to emergency department (ED). At day 7, no difference between groups was noted for persistent symptoms, quality of life (QoL) score, admission rate, unscheduled ED return and vomiting. Adherence was greater in the dexamethasone group. Previous studies that have explored this question have chosen to compare either single or two days of dexamethasone (0.3 or 0.6 mg/kg/dose) vs prednisone (1-2 mg/kg/day) for 3 or 5 days. A meta-analysis in 2014, including six randomized controlled trials in children with acute asthma, comparing dexamethasone (oral or IM) and oral prednisone (5 days) indicated no difference in relative risk (RR) of relapse between the groups at any time point (5 days RR 0.90, 95% CI 0.46, 1.78; days RR 1.14, 95% CI 0.77, 1.67). Dexamethasone group were less likely to experience vomiting in either the ED (RR 0.29, 95% CI 0.12, 0.69) or home (RR 0.32, 95% CI 0.14, 0.74) [1]. International and Indian Academy of Pediatrics (IAP) asthma Guidelines are uniform in recommending systemic corticosteroids for asthma exacerbations, except the mildest severity, for speedy resolution and prevention of relapse and hospital admission. Traditionally oral prednisone/prednisolone has been the most commonly recommended corticosteroid. BTS-SIGN 2016 guidance recommends oral prednisolone for 3 days, or IV hydrocortisone when unable to tolerate oral medication [2]. GINA 2017 guideline recommends either oral prednisolone for 3-5 days or oral dexamethasone for 2 days [3]. Dexamethasone is associated with metabolic adverse effects if continued beyond 2 days. Oral dexamethasone has the advantage of longer biological half life compared to prednisone (36-72 h vs h), good bioavailability, and better palatability. Oral prednisone is bitter in taste and patients report vomiting leading to poor adherence. Dexamethasone is more palatable and the two day course ensures better compliance. Cost-effective analysis model indicates 2 days dexamethasone compared to 5 days prednisone was cost-saving on both direct and indirect measures(missed parental work days, parental salary) based on US and Canadian cost estimates [4]. These comparisons and recent studies suggest that a 2-day dexamethasone course can be explored as an alternative option to the longer prednisolone regimen in management of acute asthma in children. Funding: None; Competing interests: None stated. MANDEEP KAUR WALIA Clinical Fellow, Respiratory Division, BC Children s Hospital, University of British Columbia, Canada. mkwalia2000@yahoo.co.in REFERENCES 1. Randolph C. Dexamethasone for acute asthma exacerbations in children: A meta-analysis. Pediatrics. 2014;134 (Suppl 3):S BTS/SIGN British Guideline for the management of asthma, 2016, SIGN 153. Available from: January 10, Global Initiative for Asthma, GINA, Available from: Accessed January 10, Andrews AL, Wong KA, Heine D, Scott Russell W. A costeffectiveness analysis of dexamethasone versus prednisone in pediatric acute asthma exacerbations. Acad Emerg Med. 2012;19: INDIAN PEDIATRICS 159 VOLUME 55 FEBRUARY 15, 2018
Office of Evidence Based Practice Specific Care Question: Dexamethasone vs. Prednisone for a Pediatric Asthma Exacerbation
 Specific Care Question : Is dexamethasone better than prednisolone/prednisone for a pediatric asthma exacerbation Question Originator: Jeff Michael, DO, FAAP Plain Language Summary from The Office of Evidence
Specific Care Question : Is dexamethasone better than prednisolone/prednisone for a pediatric asthma exacerbation Question Originator: Jeff Michael, DO, FAAP Plain Language Summary from The Office of Evidence
Systematic Review on Efficacy of Magnesium (Intravenous or Nebulized) for Acute Asthma Episodes in Children
 J O U R N A L C L U B Systematic Review on Efficacy of Magnesium (Intravenous or Nebulized) for Acute Asthma Episodes in Children Source Citation: Su Z, Li R, Gai Z. Intravenous and nebulized magnesium
J O U R N A L C L U B Systematic Review on Efficacy of Magnesium (Intravenous or Nebulized) for Acute Asthma Episodes in Children Source Citation: Su Z, Li R, Gai Z. Intravenous and nebulized magnesium
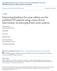
Dex single dose Pred- 5 day course Mean Difference Mean Difference Mean 3.5. Weight 100.0% IV, Fixed, 95% CI [-1.97, 0.37] 100.
![Dex single dose Pred- 5 day course Mean Difference Mean Difference Mean 3.5. Weight 100.0% IV, Fixed, 95% CI [-1.97, 0.37] 100. Dex single dose Pred- 5 day course Mean Difference Mean Difference Mean 3.5. Weight 100.0% IV, Fixed, 95% CI [-1.97, 0.37] 100.](/thumbs/90/101418001.jpg) Question : In the child with asthma exacerbation in the ED should prednisolone/prednisone vs. dexamethasone be used to prevent hospitalization, decrease time in the ED or improve pulmonary function? Forest
Question : In the child with asthma exacerbation in the ED should prednisolone/prednisone vs. dexamethasone be used to prevent hospitalization, decrease time in the ED or improve pulmonary function? Forest
Is Oral Dexamethasone Safe and Effective for Treating Asthma Exacerbations In Pediatric Patients?
 Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2014 Is Oral Dexamethasone Safe and Effective
Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2014 Is Oral Dexamethasone Safe and Effective
Children suffering from asthma symptoms present
 Original Article Single-Dose Oral Dexamethasone in the Emergency Management of Children With Exacerbations of Mild to Moderate Asthma Saleh Altamimi, MD, FRCP(C), FAAP, Glenn Robertson, MD, FRCP(C), UBC,
Original Article Single-Dose Oral Dexamethasone in the Emergency Management of Children With Exacerbations of Mild to Moderate Asthma Saleh Altamimi, MD, FRCP(C), FAAP, Glenn Robertson, MD, FRCP(C), UBC,
Dexamethasone for Acute Asthma Exacerbations in Children: A Meta-analysis
 Dexamethasone for Acute Asthma Exacerbations in Children: A Meta-analysis AUTHORS: Grant E. Keeney, MD, a Matthew P. Gray, MD, a Andrea K. Morrison, MD, MS, a Michael N. Levas, MD, a Elizabeth A. Kessler,
Dexamethasone for Acute Asthma Exacerbations in Children: A Meta-analysis AUTHORS: Grant E. Keeney, MD, a Matthew P. Gray, MD, a Andrea K. Morrison, MD, MS, a Michael N. Levas, MD, a Elizabeth A. Kessler,
Predicting, Preventing and Managing Asthma Exacerbations. Heather Zar Department of Paediatrics & Child Health University of Cape Town South Africa
 Predicting, Preventing and Managing Asthma Exacerbations Heather Zar Department of Paediatrics & Child Health University of Cape Town South Africa Asthma exacerbations Predicting exacerbation recognising
Predicting, Preventing and Managing Asthma Exacerbations Heather Zar Department of Paediatrics & Child Health University of Cape Town South Africa Asthma exacerbations Predicting exacerbation recognising
Ivax Pharmaceuticals UK Sponsor Submission to the National Institute for Health and Clinical Excellence
 Ivax Pharmaceuticals UK Sponsor Submission to the National Institute for Health and Clinical Excellence Clinical and cost-effectiveness of QVAR for the treatment of chronic asthma in adults and children
Ivax Pharmaceuticals UK Sponsor Submission to the National Institute for Health and Clinical Excellence Clinical and cost-effectiveness of QVAR for the treatment of chronic asthma in adults and children
Dexamethasone for Acute Asthma Exacerbations in Children: A Meta-analysis. abstract REVIEW ARTICLE
 REVIEW ARTICLE Dexamethasone for Acute Asthma Exacerbations in Children: A Meta-analysis AUTHORS: Grant E. Keeney, MD, a Matthew P. Gray, MD, a Andrea K. Morrison, MD, MS, a Michael N. Levas, MD, a Elizabeth
REVIEW ARTICLE Dexamethasone for Acute Asthma Exacerbations in Children: A Meta-analysis AUTHORS: Grant E. Keeney, MD, a Matthew P. Gray, MD, a Andrea K. Morrison, MD, MS, a Michael N. Levas, MD, a Elizabeth
Acute Wheezing Emergencies: From Young to Old! Little Wheezers in the ED: Managing Acute Pediatric Asthma
 Acute Wheezing Emergencies: From Young to Old! Little Wheezers in the ED: Managing Acute Pediatric Asthma Talk Outline Case Delivery of bronchodilators Meter-dose inhalers and spacers Continuous nebulization
Acute Wheezing Emergencies: From Young to Old! Little Wheezers in the ED: Managing Acute Pediatric Asthma Talk Outline Case Delivery of bronchodilators Meter-dose inhalers and spacers Continuous nebulization
Is Dexamethasone an Effective Alternative to Oral Prednisone in the Treatment of Pediatric Asthma Exacerbations?
 CLINICAL QUESTION REVIEW CQR is a recurring section in Hospital Pediatrics where authors start with a relevant clinical question, find and synthesize the recent literature and provide their best answer
CLINICAL QUESTION REVIEW CQR is a recurring section in Hospital Pediatrics where authors start with a relevant clinical question, find and synthesize the recent literature and provide their best answer
Outcomes for Pediatric Asthmatic Inpatients After Implementation of an Emergency Department Dexamethasone Treatment Protocol
 RESEARCH ARTICLE Outcomes for Pediatric Asthmatic Inpatients After Implementation of an Emergency Department Dexamethasone Treatment Protocol Amy Tyler, MD, MSCS, a,b Jillian M. Cotter, MD, a,b Angela
RESEARCH ARTICLE Outcomes for Pediatric Asthmatic Inpatients After Implementation of an Emergency Department Dexamethasone Treatment Protocol Amy Tyler, MD, MSCS, a,b Jillian M. Cotter, MD, a,b Angela
PEDIATRIC ACUTE ASTHMA SCORE (P.A.A.S.) GUIDELINES. >97% 94% to 96% 91%-93% <90% Moderate to severe expiratory wheeze
 Inclusion: Children experiencing acute asthma exacerbation 24 months to 18 years of age with a diagnosis of asthma Patients with a previous history of asthma (Consider differential diagnosis for infants
Inclusion: Children experiencing acute asthma exacerbation 24 months to 18 years of age with a diagnosis of asthma Patients with a previous history of asthma (Consider differential diagnosis for infants
Asthma: Evaluate and Improve Your Practice
 Potential Barriers and Suggested Ideas for Change Key Activity: Initial assessment and management Rationale: The history and physical examination obtained from the patient and family interviews form the
Potential Barriers and Suggested Ideas for Change Key Activity: Initial assessment and management Rationale: The history and physical examination obtained from the patient and family interviews form the
Diagnosis and Management of Asthma in Children based on the British Thoracic Society and Scottish Intercollegiate Guidelines Network September 2016
 Diagnosis and Management of Asthma in Children based on the British Thoracic Society and Scottish Intercollegiate Guidelines Network September 2016 Diagnosis: There is no lower limit to the age at which
Diagnosis and Management of Asthma in Children based on the British Thoracic Society and Scottish Intercollegiate Guidelines Network September 2016 Diagnosis: There is no lower limit to the age at which
Supplementary Medications during asthma attack. Prof. Dr Finn Rasmussen PhD. DrMedSc. Near East University Hospital North Cyprus
 Supplementary Medications during asthma attack Prof. Dr Finn Rasmussen PhD. DrMedSc. Near East University Hospital North Cyprus Conflicts of Interest None Definition of Asthma Airway narrowing that is
Supplementary Medications during asthma attack Prof. Dr Finn Rasmussen PhD. DrMedSc. Near East University Hospital North Cyprus Conflicts of Interest None Definition of Asthma Airway narrowing that is
10/6/2014. Tommy s Story: An Overview of Asthma Mangement. Disclosure. Objectives for this talk.
 Tommy s Story: An Overview of Asthma Mangement Clifton C. Lee, MD, FAAP, FHM Associate Professor of Pediatrics Chief, Pediatric Hospital Medicine Children s Hospital of Richmond at VCU Disclosure Obviously,
Tommy s Story: An Overview of Asthma Mangement Clifton C. Lee, MD, FAAP, FHM Associate Professor of Pediatrics Chief, Pediatric Hospital Medicine Children s Hospital of Richmond at VCU Disclosure Obviously,
Management of Acute Exacerbation of Asthma Presenting to the Emergency Department or Urgent Care
 December 1, 2018 Management of Acute Exacerbation of Asthma Presenting to the Emergency Department or Urgent Care INTRODUCTION / BACKGROUND Discharge data for pediatric patients who had presented to the
December 1, 2018 Management of Acute Exacerbation of Asthma Presenting to the Emergency Department or Urgent Care INTRODUCTION / BACKGROUND Discharge data for pediatric patients who had presented to the
Original Article. Kishore Kumar K 1, Sai Samrat K 2, Sony Reddy S 3 INTRODUCTION
 Original Article A Comparative Study between Intramuscular and Oral Methylprednisolone Acetate in the Treatment of Asthma Exacerbation Following Discharge from the Hospital Kishore Kumar K 1, Sai Samrat
Original Article A Comparative Study between Intramuscular and Oral Methylprednisolone Acetate in the Treatment of Asthma Exacerbation Following Discharge from the Hospital Kishore Kumar K 1, Sai Samrat
Asthma self management. Duncan MacIntyre & Christine Bucknall August 2010
 Asthma self management Duncan MacIntyre & Christine Bucknall August 2010 Health Belief Model These beliefs make it more likely that patients will follow preventive or therapeutic recommendations I am susceptible
Asthma self management Duncan MacIntyre & Christine Bucknall August 2010 Health Belief Model These beliefs make it more likely that patients will follow preventive or therapeutic recommendations I am susceptible
Pediatric Respiratory Disease: A Model for the Future of Emergency Medicine Research
 Pediatric Respiratory Disease: A Model for the Future of Emergency Medicine Research Joseph J. Zorc, MD, MSCE Mark Fishman Professor, Department of Pediatrics Perelman School of Medicine, University of
Pediatric Respiratory Disease: A Model for the Future of Emergency Medicine Research Joseph J. Zorc, MD, MSCE Mark Fishman Professor, Department of Pediatrics Perelman School of Medicine, University of
roflumilast 500 microgram tablets (Daxas ) SMC No. (635/10) Nycomed Ltd
 roflumilast 500 microgram tablets (Daxas ) SMC No. (635/10) Nycomed Ltd 06 August 2010 (Issued 10 September 2010) The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
roflumilast 500 microgram tablets (Daxas ) SMC No. (635/10) Nycomed Ltd 06 August 2010 (Issued 10 September 2010) The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
Improving timeliness for acute asthma care for paediatric ED patients using a nurse driven intervention: an interrupted time series analysis.
 Himmelfarb Health Sciences Library, The George Washington University Health Sciences Research Commons Emergency Medicine Faculty Publications Emergency Medicine 1-1-2016 Improving timeliness for acute
Himmelfarb Health Sciences Library, The George Washington University Health Sciences Research Commons Emergency Medicine Faculty Publications Emergency Medicine 1-1-2016 Improving timeliness for acute
Cost effectiveness of fluticasone and budesonide in patients with moderate asthma Steinmetz K O, Volmer T, Trautmann M, Kielhorn A
 Cost effectiveness of fluticasone and budesonide in patients with moderate asthma Steinmetz K O, Volmer T, Trautmann M, Kielhorn A Record Status This is a critical abstract of an economic evaluation that
Cost effectiveness of fluticasone and budesonide in patients with moderate asthma Steinmetz K O, Volmer T, Trautmann M, Kielhorn A Record Status This is a critical abstract of an economic evaluation that
The Use of Dexamethasone vs Prednisone in the Treatment of Asthma Exacerbation
 The Use of Dexamethasone vs Prednisone in the Treatment of Asthma Exacerbation Abstract Asthma accounts for 2.1 million unscheduled ED visits annually, with a prevalence of 8.4% of the US population. 2
The Use of Dexamethasone vs Prednisone in the Treatment of Asthma Exacerbation Abstract Asthma accounts for 2.1 million unscheduled ED visits annually, with a prevalence of 8.4% of the US population. 2
Commissioning for Better Outcomes in COPD
 Commissioning for Better Outcomes in COPD Dr Matt Kearney Primary Care & Public Health Advisor Respiratory Programme, Department of Health General Practitioner, Runcorn November 2011 What are the Commissioning
Commissioning for Better Outcomes in COPD Dr Matt Kearney Primary Care & Public Health Advisor Respiratory Programme, Department of Health General Practitioner, Runcorn November 2011 What are the Commissioning
ADULT ASTHMA GUIDE SUMMARY. This summary provides busy health professionals with key guidance for assessing and treating adult asthma.
 ADULT ASTHMA GUIDE SUMMARY This summary provides busy health professionals with key guidance for assessing and treating adult asthma. Its source document Asthma and Respiratory Foundation NZ Adult Asthma
ADULT ASTHMA GUIDE SUMMARY This summary provides busy health professionals with key guidance for assessing and treating adult asthma. Its source document Asthma and Respiratory Foundation NZ Adult Asthma
SYNOPSIS THIS IS A PRINTED COPY OF AN ELECTRONIC DOCUMENT. PLEASE CHECK ITS VALIDITY BEFORE USE.
 Drug product: Drug substance(s): Document No.: Edition No.: 1 Study code: Accolate Zafirlukast (ZD9188) 9188IL/0138 Date: 02 May 2007 SYNOPSIS A Multicenter, Randomized, Double-blind, -controlled, Parallel
Drug product: Drug substance(s): Document No.: Edition No.: 1 Study code: Accolate Zafirlukast (ZD9188) 9188IL/0138 Date: 02 May 2007 SYNOPSIS A Multicenter, Randomized, Double-blind, -controlled, Parallel
Respiratory Subcommittee of PTAC Meeting held 4 March 2015
 Respiratory Subcommittee of PTAC Meeting held 4 March 2015 (minutes for web publishing) Respiratory Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology and
Respiratory Subcommittee of PTAC Meeting held 4 March 2015 (minutes for web publishing) Respiratory Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology and
KDIGO GN Guideline update Evidence summary. Steroid-sensitive nephrotic syndrome. Corticosteroid therapy for nephrotic syndrome in children
 KDIGO GN Guideline update Evidence summary Steroid-sensitive nephrotic syndrome Corticosteroid therapy for nephrotic syndrome in children PICO question In children (aged 3 to 18 years of age) with steroid-sensitive
KDIGO GN Guideline update Evidence summary Steroid-sensitive nephrotic syndrome Corticosteroid therapy for nephrotic syndrome in children PICO question In children (aged 3 to 18 years of age) with steroid-sensitive
RESPIRATORY CARE IN GENERAL PRACTICE
 RESPIRATORY CARE IN GENERAL PRACTICE Definitions of Asthma and COPD Asthma is due to inflammation of the air passages in the lungs and affects the sensitivity of the nerve endings in the airways so they
RESPIRATORY CARE IN GENERAL PRACTICE Definitions of Asthma and COPD Asthma is due to inflammation of the air passages in the lungs and affects the sensitivity of the nerve endings in the airways so they
Nguyen Tien Dung A/Prof. PhD. MD Head of Pediatric Department - Bach Mai Hospital
 Nguyen Tien Dung A/Prof. PhD. MD Head of Pediatric Department - Bach Mai Hospital A girl patient 11 years old admitted to Bach mai Hospital at 4h15, 12nd November because of difficult breathing She has
Nguyen Tien Dung A/Prof. PhD. MD Head of Pediatric Department - Bach Mai Hospital A girl patient 11 years old admitted to Bach mai Hospital at 4h15, 12nd November because of difficult breathing She has
Allwin Mercer Dr Andrew Zurek
 Allwin Mercer Dr Andrew Zurek 1 in 11 people are currently receiving treatment for asthma (5.4 million people in the UK) Every 10 seconds, someone is having a potentially life-threatening asthma attack
Allwin Mercer Dr Andrew Zurek 1 in 11 people are currently receiving treatment for asthma (5.4 million people in the UK) Every 10 seconds, someone is having a potentially life-threatening asthma attack
Type of intervention Treatment. Economic study type Cost-effectiveness analysis.
 Cost-effectiveness of salmeterol/fluticasone propionate combination product 50/250 micro g twice daily and budesonide 800 micro g twice daily in the treatment of adults and adolescents with asthma Lundback
Cost-effectiveness of salmeterol/fluticasone propionate combination product 50/250 micro g twice daily and budesonide 800 micro g twice daily in the treatment of adults and adolescents with asthma Lundback
Meenu Singh, Joseph L. Mathew, Prabhjot Malhi, B.R. Srinivas and Lata Kumar
 Comparison of Improvement in Quality of Life Score with Objective Parameters of Pulmonary Function in Indian Asthmatic Children Receiving Inhaled Corticosteroid Therapy Meenu Singh, Joseph L. Mathew, Prabhjot
Comparison of Improvement in Quality of Life Score with Objective Parameters of Pulmonary Function in Indian Asthmatic Children Receiving Inhaled Corticosteroid Therapy Meenu Singh, Joseph L. Mathew, Prabhjot
Asthma in Pregnancy, Labour and Postnatal Guidelines
 Asthma in Pregnancy, Labour and Postnatal Guidelines N.B. Staff should be discouraged from printing this document. This is to avoid the risk of out of date printed versions of the document. The Intranet
Asthma in Pregnancy, Labour and Postnatal Guidelines N.B. Staff should be discouraged from printing this document. This is to avoid the risk of out of date printed versions of the document. The Intranet
BEST in MH clinical question-answering service
 1 BEST.awp.nhs.uk Best Evidence Summaries of Topics in Mental Healthcare BEST in MH clinical question-answering service Question In adult patients experiencing a mental health crisis, which service model
1 BEST.awp.nhs.uk Best Evidence Summaries of Topics in Mental Healthcare BEST in MH clinical question-answering service Question In adult patients experiencing a mental health crisis, which service model
Cost-effectiveness of telephone or surgery asthma reviews: economic analysis of a randomised controlled trial Pinnock H, McKenzie L, Price D, Sheikh A
 Cost-effectiveness of telephone or surgery asthma reviews: economic analysis of a randomised controlled trial Pinnock H, McKenzie L, Price D, Sheikh A Record Status This is a critical abstract of an economic
Cost-effectiveness of telephone or surgery asthma reviews: economic analysis of a randomised controlled trial Pinnock H, McKenzie L, Price D, Sheikh A Record Status This is a critical abstract of an economic
DATE: 09 December 2009 CONTEXT AND POLICY ISSUES:
 TITLE: Tiotropium Compared with Ipratropium for Patients with Moderate to Severe Chronic Obstructive Pulmonary Disease: A Review of the Clinical Effectiveness DATE: 09 December 2009 CONTEXT AND POLICY
TITLE: Tiotropium Compared with Ipratropium for Patients with Moderate to Severe Chronic Obstructive Pulmonary Disease: A Review of the Clinical Effectiveness DATE: 09 December 2009 CONTEXT AND POLICY
BRONCHIAL THERMOPLASTY
 BRONCHIAL THERMOPLASTY UnitedHealthcare Community Plan Medical Policy Policy Number: CS014.E Effective Date: July 1, 2017 Table of Contents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS... 1 COVERAGE
BRONCHIAL THERMOPLASTY UnitedHealthcare Community Plan Medical Policy Policy Number: CS014.E Effective Date: July 1, 2017 Table of Contents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS... 1 COVERAGE
Peak Expiratory Flow Rate (PEFR) for ED Management of Acute Asthma Exacerbation
 Peak Expiratory Flow Rate (PEFR) for ED Management of Acute Asthma Exacerbation PI: Brian Driver, MD Checklist Reviewed Inclusion and Exclusion Criteria Confirm pertinent exclusion criteria with PMP Engage
Peak Expiratory Flow Rate (PEFR) for ED Management of Acute Asthma Exacerbation PI: Brian Driver, MD Checklist Reviewed Inclusion and Exclusion Criteria Confirm pertinent exclusion criteria with PMP Engage
Out of date SUGGESTIONS FOR CLINICAL CARE (Suggestions are based on level III and IV evidence)
 Membranous nephropathy role of cyclosporine therapy Date written: July 2005 Final submission: September 2005 Author: Merlin Thomas GUIDELINES a. The use of cyclosporine therapy alone to prevent progressive
Membranous nephropathy role of cyclosporine therapy Date written: July 2005 Final submission: September 2005 Author: Merlin Thomas GUIDELINES a. The use of cyclosporine therapy alone to prevent progressive
GRADE. Grading of Recommendations Assessment, Development and Evaluation. British Association of Dermatologists April 2018
 GRADE Grading of Recommendations Assessment, Development and Evaluation British Association of Dermatologists April 2018 Previous grading system Level of evidence Strength of recommendation Level of evidence
GRADE Grading of Recommendations Assessment, Development and Evaluation British Association of Dermatologists April 2018 Previous grading system Level of evidence Strength of recommendation Level of evidence
INDIAN PEDIATRICS 149 VOLUME 53 FEBRUARY 15, 2016
 J O U R N A L C L U B Placebo-controlled Randomized Trial Evaluating Efficacy of Ondansetron in Children with Diarrhea and Vomiting: Critical Appraisal and Updated Meta-analysis SOURCE CITATION: Danewa
J O U R N A L C L U B Placebo-controlled Randomized Trial Evaluating Efficacy of Ondansetron in Children with Diarrhea and Vomiting: Critical Appraisal and Updated Meta-analysis SOURCE CITATION: Danewa
UPDATE IN HOSPITAL MEDICINE
 UPDATE IN HOSPITAL MEDICINE FLORIDA CHAPTER ACP MEETING 2016 Himangi Kaushal, M.D., F.A.C.P. Program Director Memorial Healthcare System Internal Medicine Residency DISCLOSURES None OBJECTIVES Review some
UPDATE IN HOSPITAL MEDICINE FLORIDA CHAPTER ACP MEETING 2016 Himangi Kaushal, M.D., F.A.C.P. Program Director Memorial Healthcare System Internal Medicine Residency DISCLOSURES None OBJECTIVES Review some
JOINT CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD) MANAGEMENT GUIDELINES
 JOINT CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD) MANAGEMENT GUIDELINES Authors Dr Ian Benton Respiratory Consultant COCH Penny Rideal Respiratory Nurse COCH Kirti Burgul Respiratory Pharmacist COCH Pam
JOINT CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD) MANAGEMENT GUIDELINES Authors Dr Ian Benton Respiratory Consultant COCH Penny Rideal Respiratory Nurse COCH Kirti Burgul Respiratory Pharmacist COCH Pam
BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE
 BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE December 2014 Review Date: December 2017 Bulletin 206 : DuoResp Spiromax 160 / 4.5 and 320 / 9 budesonide & formoterol dry powder inhaler JPC Recommendations
BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE December 2014 Review Date: December 2017 Bulletin 206 : DuoResp Spiromax 160 / 4.5 and 320 / 9 budesonide & formoterol dry powder inhaler JPC Recommendations
TORCH: Salmeterol and Fluticasone Propionate and Survival in COPD
 TORCH: and Propionate and Survival in COPD April 19, 2007 Justin Lee Pharmacy Resident University Health Network Outline Overview of COPD Pathophysiology Pharmacological Treatment Overview of the TORCH
TORCH: and Propionate and Survival in COPD April 19, 2007 Justin Lee Pharmacy Resident University Health Network Outline Overview of COPD Pathophysiology Pharmacological Treatment Overview of the TORCH
SCREENING AND PREVENTION
 These protocols are designed to implement standard guidelines, based on the best evidence, that provide a consistent clinical experience for AHC II Integrated Clinical Delivery Network patients and allow
These protocols are designed to implement standard guidelines, based on the best evidence, that provide a consistent clinical experience for AHC II Integrated Clinical Delivery Network patients and allow
GRADE. Grading of Recommendations Assessment, Development and Evaluation. British Association of Dermatologists April 2014
 GRADE Grading of Recommendations Assessment, Development and Evaluation British Association of Dermatologists April 2014 Previous grading system Level of evidence Strength of recommendation Level of evidence
GRADE Grading of Recommendations Assessment, Development and Evaluation British Association of Dermatologists April 2014 Previous grading system Level of evidence Strength of recommendation Level of evidence
Setting The setting was outpatient departments of referral hospitals. The economic analysis was conducted in India.
 Three day versus five day treatment with amoxicillin for non-severe pneumonia in young children: a multicentre randomised controlled trial ISCAP study group Record Status This is a critical abstract of
Three day versus five day treatment with amoxicillin for non-severe pneumonia in young children: a multicentre randomised controlled trial ISCAP study group Record Status This is a critical abstract of
HCT Medical Policy. Bronchial Thermoplasty. Policy # HCT113 Current Effective Date: 05/24/2016. Policy Statement. Overview
 HCT Medical Policy Bronchial Thermoplasty Policy # HCT113 Current Effective Date: 05/24/2016 Medical Policies are developed by HealthyCT to assist in administering plan benefits and constitute neither
HCT Medical Policy Bronchial Thermoplasty Policy # HCT113 Current Effective Date: 05/24/2016 Medical Policies are developed by HealthyCT to assist in administering plan benefits and constitute neither
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Lead team presentation: Roflumilast for treating chronic obstructive pulmonary disease [ID984]
![Lead team presentation: Roflumilast for treating chronic obstructive pulmonary disease [ID984] Lead team presentation: Roflumilast for treating chronic obstructive pulmonary disease [ID984]](/thumbs/85/91889043.jpg) Lead team presentation: Roflumilast for treating chronic obstructive pulmonary disease [ID984] 1 st Appraisal Committee meeting Background & Clinical Effectiveness John McMurray 11 th January 2016 For
Lead team presentation: Roflumilast for treating chronic obstructive pulmonary disease [ID984] 1 st Appraisal Committee meeting Background & Clinical Effectiveness John McMurray 11 th January 2016 For
TRIALS. Cronin et al. Trials 2012, 13:141
 Cronin et al. Trials 2012, 13:141 TRIALS STUDY PROTOCOL Open Access Single dose oral dexamethasone versus multi-dose prednisolone in the treatment of acute exacerbations of asthma in children who attend
Cronin et al. Trials 2012, 13:141 TRIALS STUDY PROTOCOL Open Access Single dose oral dexamethasone versus multi-dose prednisolone in the treatment of acute exacerbations of asthma in children who attend
Royal College of Surgeons in Ireland John Cronin Our Lady's Children's Hospital, Dublin Una Kennedy St. James's Hospital, Dublin
 Royal College of Surgeons in Ireland e-publications@rcsi General Practice Articles Department of General Practice 21-8-2012 Single dose oral dexamethasone versus multi-dose prednisolone in the treatment
Royal College of Surgeons in Ireland e-publications@rcsi General Practice Articles Department of General Practice 21-8-2012 Single dose oral dexamethasone versus multi-dose prednisolone in the treatment
Dose: Metoclopramide -0.1 mg/kg (max 10 mg) IV, over 15 minutes
 Metoclopramide for Refractory Migraine in the ED Specific Care Question : In the pediatric patient diagnosed with refractory migraine, is metoclopramide an effective treatment? Question Originator: Migraine
Metoclopramide for Refractory Migraine in the ED Specific Care Question : In the pediatric patient diagnosed with refractory migraine, is metoclopramide an effective treatment? Question Originator: Migraine
Pathway diagrams Annex F
 Pathway diagrams Annex F Fig 1 Asthma: The patient journey Asthma is diagnosed Making the diagnosis of asthma Confirming the diagnosis may depend on history, response to treatment, measurement of airflow
Pathway diagrams Annex F Fig 1 Asthma: The patient journey Asthma is diagnosed Making the diagnosis of asthma Confirming the diagnosis may depend on history, response to treatment, measurement of airflow
ARCHE Risk of Bias (ROB) Guidelines
 Types of Biases and ROB Domains ARCHE Risk of Bias (ROB) Guidelines Bias Selection Bias Performance Bias Detection Bias Attrition Bias Reporting Bias Other Bias ROB Domain Sequence generation Allocation
Types of Biases and ROB Domains ARCHE Risk of Bias (ROB) Guidelines Bias Selection Bias Performance Bias Detection Bias Attrition Bias Reporting Bias Other Bias ROB Domain Sequence generation Allocation
Wheezy? Easy Peasy! The Emergent Management of Asthma & Bronchiolitis. Maneesha Agarwal MD Assistant Professor of Pediatrics & Emergency Medicine
 Wheezy? Easy Peasy! The Emergent Management of Asthma & Bronchiolitis Maneesha Agarwal MD Assistant Professor of Pediatrics & Emergency Medicine Asthma Defined National Asthma Education and Prevention
Wheezy? Easy Peasy! The Emergent Management of Asthma & Bronchiolitis Maneesha Agarwal MD Assistant Professor of Pediatrics & Emergency Medicine Asthma Defined National Asthma Education and Prevention
J O U R N A L C L U B
 J O U R N A L C L U B Immunogenicity and Safety of a Heptavalent (Diphtheria, Tetanus, Pertussis, Hepatitis B, Poliomyelitis, Haemophilus influenzae b, and Meningococcal Serogroup C) Vaccine SOURCE CITATION:
J O U R N A L C L U B Immunogenicity and Safety of a Heptavalent (Diphtheria, Tetanus, Pertussis, Hepatitis B, Poliomyelitis, Haemophilus influenzae b, and Meningococcal Serogroup C) Vaccine SOURCE CITATION:
Subject: Emflaza (deflazacort) Original Effective Date: 7/7/2017. Policy Number: MCP-298 Revision Date(s): 1/12/2018. Review Date(s): DISCLAIMER
 Subject: Emflaza (deflazacort) Original Effective Date: 7/7/2017 Policy Number: MCP-298 Revision Date(s): 1/12/2018 Review Date(s): DISCLAIMER This Molina Clinical Policy (MCP) is intended to facilitate
Subject: Emflaza (deflazacort) Original Effective Date: 7/7/2017 Policy Number: MCP-298 Revision Date(s): 1/12/2018 Review Date(s): DISCLAIMER This Molina Clinical Policy (MCP) is intended to facilitate
AT TRIAGE. Alberta Acute Childhood Asthma Pathway: Evidence based* recommendations For Emergency / Urgent Care
 1 1 Should the child be placed into the Pathway? Asthma Clinical Score (PRAM) Inclusion Children 1 year and 18 years of age who present with wheezing and respiratory distress, and have been diagnosed by
1 1 Should the child be placed into the Pathway? Asthma Clinical Score (PRAM) Inclusion Children 1 year and 18 years of age who present with wheezing and respiratory distress, and have been diagnosed by
Sample. Affix patient label within this box.
 Instructions for completing orders Complete pages 1-3 for General Inpatient Orders. All pathway compatible orders (indicated by ) within the General Inpatient Orders will be followed automatically. Optional
Instructions for completing orders Complete pages 1-3 for General Inpatient Orders. All pathway compatible orders (indicated by ) within the General Inpatient Orders will be followed automatically. Optional
Title Protocol for the Management of Asthma
 Document Control Title Protocol for the Management of Asthma Author Author s job title Professional Lead, Minor Injuries Unit Directorate Emergency Services, Logistics and Resilience Department Version
Document Control Title Protocol for the Management of Asthma Author Author s job title Professional Lead, Minor Injuries Unit Directorate Emergency Services, Logistics and Resilience Department Version
Quick Literature Searches
 Quick Literature Searches National Pediatric Nighttime Curriculum Written by Leticia Shanley, MD, FAAP Institution: University of Texas Southwestern Medical Center Case 1 It s 1:00am and you have just
Quick Literature Searches National Pediatric Nighttime Curriculum Written by Leticia Shanley, MD, FAAP Institution: University of Texas Southwestern Medical Center Case 1 It s 1:00am and you have just
Alcohol interventions in secondary and further education
 National Institute for Health and Care Excellence Guideline version (Draft for Consultation) Alcohol interventions in secondary and further education NICE guideline: methods NICE guideline Methods
National Institute for Health and Care Excellence Guideline version (Draft for Consultation) Alcohol interventions in secondary and further education NICE guideline: methods NICE guideline Methods
aclidinium 322 micrograms inhalation powder (Eklira Genuair ) SMC No. (810/12) Almirall S.A.
 aclidinium 322 micrograms inhalation powder (Eklira Genuair ) SMC No. (810/12) Almirall S.A. 05 October 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and
aclidinium 322 micrograms inhalation powder (Eklira Genuair ) SMC No. (810/12) Almirall S.A. 05 October 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
Is reslizumab effective in improving quality of life and asthma control in adolescent and adult patients with poorly controlled eosinophilic asthma?
 Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2018 Is reslizumab effective in improving
Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2018 Is reslizumab effective in improving
Healthy People 2010 Asthma Objectives December 2009 Update
 December 2009 Update Healthy People 2010 is a set of national health goals focusing on disease prevention and health promotion to be reached by the year 2010. The following are the Healthy People 2010
December 2009 Update Healthy People 2010 is a set of national health goals focusing on disease prevention and health promotion to be reached by the year 2010. The following are the Healthy People 2010
Specific Care Question : Question Originator: Plain Language Summary from The Office of Evidence Based Practice: Conditional Recommendation
 Ketorolac for Refractory Migraine in the ED Specific Care Question : In the pediatric patient diagnosed with refractory migraine is ketorolac an effective treatment? Question Originator: Migraine Therapy
Ketorolac for Refractory Migraine in the ED Specific Care Question : In the pediatric patient diagnosed with refractory migraine is ketorolac an effective treatment? Question Originator: Migraine Therapy
fluticasone furoate / vilanterol 92/22, 184/22 micrograms inhalation powder (Relvar Ellipta ) SMC No. (966/14) GlaxoSmithKline UK
 fluticasone furoate / vilanterol 92/22, 184/22 micrograms inhalation powder (Relvar Ellipta ) SMC No. (966/14) GlaxoSmithKline UK 09 May 2014 The Scottish Medicines Consortium (SMC) has completed its assessment
fluticasone furoate / vilanterol 92/22, 184/22 micrograms inhalation powder (Relvar Ellipta ) SMC No. (966/14) GlaxoSmithKline UK 09 May 2014 The Scottish Medicines Consortium (SMC) has completed its assessment
EPSDT. EPSDT-Acute Visit & Well Visit EPSDT Documentation. Acute Visit & Well Visit L. Chana Spearmon, MD, FAAP Neighborhood Health Pediatrics
 EPSDT Acute Visit & Well Visit L. Chana Spearmon, MD, FAAP Neighborhood Health Pediatrics EPSDT Documentation Comprehensive Health and Developmental History Screenings for Vision/Hearing Developmental
EPSDT Acute Visit & Well Visit L. Chana Spearmon, MD, FAAP Neighborhood Health Pediatrics EPSDT Documentation Comprehensive Health and Developmental History Screenings for Vision/Hearing Developmental
TREAMENT OF RECURRENT VIRUS-INDUCED WHEEZING IN YOUNG CHILDREN. Dr Lại Lê Hưng Respiratory Department
 TREAMENT OF RECURRENT VIRUS-INDUCED WHEEZING IN YOUNG CHILDREN Dr Lại Lê Hưng Respiratory Department Literature review current through: Feb 2013. This topic last updated: Aug 14, 2012 INTRODUCTION Wheezing
TREAMENT OF RECURRENT VIRUS-INDUCED WHEEZING IN YOUNG CHILDREN Dr Lại Lê Hưng Respiratory Department Literature review current through: Feb 2013. This topic last updated: Aug 14, 2012 INTRODUCTION Wheezing
US max daily dose i Food and Drug Administration [2]
![US max daily dose i Food and Drug Administration [2] US max daily dose i Food and Drug Administration [2]](/thumbs/88/115964542.jpg) APPENDIX 2 etable 1. Regulatory limits on total daily dose for asthma medications Drugs Canadian max daily dose i Health Canada [1] US max daily dose i Food and Drug Administration [2] European max daily
APPENDIX 2 etable 1. Regulatory limits on total daily dose for asthma medications Drugs Canadian max daily dose i Health Canada [1] US max daily dose i Food and Drug Administration [2] European max daily
Steroid in Paediatric Sepsis. Dr Pon Kah Min Hospital Pulau Pinang
 Steroid in Paediatric Sepsis Dr Pon Kah Min Hospital Pulau Pinang Contents Importance of steroid in sepsis Literature Review for adult studies Literature Review for paediatric studies Conclusions. Rationale
Steroid in Paediatric Sepsis Dr Pon Kah Min Hospital Pulau Pinang Contents Importance of steroid in sepsis Literature Review for adult studies Literature Review for paediatric studies Conclusions. Rationale
PEDIATRIC ASTHMA INPATIENT CARE MAP
 DATE PATIENT PEDIATRIC ASTHMA INPATIENT CARE MAP DOB HSC NO. PHIN Approved by the Winnipeg Regional Health Authority This Care Map is to be used as a guideline and in no way replaces sound clinical judgment
DATE PATIENT PEDIATRIC ASTHMA INPATIENT CARE MAP DOB HSC NO. PHIN Approved by the Winnipeg Regional Health Authority This Care Map is to be used as a guideline and in no way replaces sound clinical judgment
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE 1 Guideline title SCOPE Bronchiolitis: diagnosis and management of bronchiolitis in children. 1.1 Short title Bronchiolitis in children 2 The remit The
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE 1 Guideline title SCOPE Bronchiolitis: diagnosis and management of bronchiolitis in children. 1.1 Short title Bronchiolitis in children 2 The remit The
ASH Draft Recommendations for Immune Thrombocytopenia
 ASH Draft Recommendations for Immune Thrombocytopenia INTRODUCTION American Society of Hematology (ASH) guidelines are based on a systematic review of available evidence. Through a structured process,
ASH Draft Recommendations for Immune Thrombocytopenia INTRODUCTION American Society of Hematology (ASH) guidelines are based on a systematic review of available evidence. Through a structured process,
Management of Acute Asthma Exacerbations in Children 2012 Update. Sharon Kling Dept Paediatrics & Child Health University of Stellenbosch
 Management of Acute Asthma Exacerbations in Children 2012 Update Sharon Kling Dept Paediatrics & Child Health University of Stellenbosch Acknowledgements BTS/SIGN guidelines GINA guidelines NAEPP guidelines
Management of Acute Asthma Exacerbations in Children 2012 Update Sharon Kling Dept Paediatrics & Child Health University of Stellenbosch Acknowledgements BTS/SIGN guidelines GINA guidelines NAEPP guidelines
Type: Clinical Guideline Register No: Status: Public MANAGEMENT OF ACUTE ASTHMA IN CHILDREN MORE THAN 2 YEARS IN HOSPITAL
 MANAGEMENT OF ACUTE ASTHMA IN CHILDREN MORE THAN 2 YEARS IN HOSPITAL Type: Clinical Guideline Register No: 09055 Status: Public Developed in Response to: Best practice CQC Fundamental Standard: 9, 12,
MANAGEMENT OF ACUTE ASTHMA IN CHILDREN MORE THAN 2 YEARS IN HOSPITAL Type: Clinical Guideline Register No: 09055 Status: Public Developed in Response to: Best practice CQC Fundamental Standard: 9, 12,
Critical Review Form Therapy
 Critical Review Form Therapy Does End-tidal Carbon Dioxide Monitoring Detect Respiratory Events Prior to Current Sedation Monitoring Practices, Acad Emerg Med 2006; 13:500-504 Objective: To determine the
Critical Review Form Therapy Does End-tidal Carbon Dioxide Monitoring Detect Respiratory Events Prior to Current Sedation Monitoring Practices, Acad Emerg Med 2006; 13:500-504 Objective: To determine the
On completion of this chapter you should be able to: discuss the stepwise approach to the pharmacological management of asthma in children
 7 Asthma Asthma is a common disease in children and its incidence has been increasing in recent years. Between 10-15% of children have been diagnosed with asthma. It is therefore a condition that pharmacists
7 Asthma Asthma is a common disease in children and its incidence has been increasing in recent years. Between 10-15% of children have been diagnosed with asthma. It is therefore a condition that pharmacists
A Systematic Review of the Efficacy and Clinical Effectiveness of Group Analysis and Analytic/Dynamic Group Psychotherapy
 A Systematic Review of the Efficacy and Clinical Effectiveness of Group Analysis and Analytic/Dynamic Group Psychotherapy Executive summary Aims of the review The main aim of the review was to assess the
A Systematic Review of the Efficacy and Clinical Effectiveness of Group Analysis and Analytic/Dynamic Group Psychotherapy Executive summary Aims of the review The main aim of the review was to assess the
TARGET POPULATION Eligibility Inclusion Criterion Exclusion Criterion RECOMMENDATIONS
 TARGET POPULATION Eligibility Inclusion Criterion Exclusion Criterion RECOMMENDATIONS Recommendation PULMONARY FUNCTION TESTING (SPIROMETRY) Conditional: The Expert Panel that spirometry measurements FEV1,
TARGET POPULATION Eligibility Inclusion Criterion Exclusion Criterion RECOMMENDATIONS Recommendation PULMONARY FUNCTION TESTING (SPIROMETRY) Conditional: The Expert Panel that spirometry measurements FEV1,
GINA. At-A-Glance Asthma Management Reference. for adults, adolescents and children 6 11 years. Updated 2017
 GINA At-A-Glance Asthma Management Reference for adults, adolescents and children 6 11 years Updated 2017 This resource should be used in conjunction with the Global Strategy for Asthma Management and
GINA At-A-Glance Asthma Management Reference for adults, adolescents and children 6 11 years Updated 2017 This resource should be used in conjunction with the Global Strategy for Asthma Management and
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
COPD. Breathing Made Easier
 COPD Breathing Made Easier Catherine E. Cooke, PharmD, BCPS, PAHM Independent Consultant, PosiHleath Clinical Associate Professor, University of Maryland School of Pharmacy This program has been brought
COPD Breathing Made Easier Catherine E. Cooke, PharmD, BCPS, PAHM Independent Consultant, PosiHleath Clinical Associate Professor, University of Maryland School of Pharmacy This program has been brought
Not available 100/6mcg 2 BD formoterol (Fostair MDI) 100/6mcg 33
 COMMISSIONING POLICY RECOMMENDATION TREATMENT ADVISORY GROUP FLUTICASONE FUROATE/VILANTEROL COMBINATION INHALER - ASTHMA Policy agreed by Vale of York CCG (date) Drug, Treatment, Device name Fluticasone
COMMISSIONING POLICY RECOMMENDATION TREATMENT ADVISORY GROUP FLUTICASONE FUROATE/VILANTEROL COMBINATION INHALER - ASTHMA Policy agreed by Vale of York CCG (date) Drug, Treatment, Device name Fluticasone
BRONCHIAL THERMOPLASTY
 BRONCHIAL THERMOPLASTY UnitedHealthcare Oxford Clinical Policy Policy Number: RESPIRATORY 009.11 T2 Effective Date: August 1, 2017 Table of Contents Page BRONCHIAL THERMOPLASTY... 1 INSTRUCTIONS FOR USE...
BRONCHIAL THERMOPLASTY UnitedHealthcare Oxford Clinical Policy Policy Number: RESPIRATORY 009.11 T2 Effective Date: August 1, 2017 Table of Contents Page BRONCHIAL THERMOPLASTY... 1 INSTRUCTIONS FOR USE...
Asthma/wheeze management plan
 Asthma/wheeze management plan Name of Patient Date of Birth NHS Number GP surgery Telephone Next appointment Children s Assessment unit/ward telephone Out of hours call 111 Open access Y/N Until date Some
Asthma/wheeze management plan Name of Patient Date of Birth NHS Number GP surgery Telephone Next appointment Children s Assessment unit/ward telephone Out of hours call 111 Open access Y/N Until date Some
DR REBECCA THOMAS CONSULTANT RESPIRATORY PHYSICIAN YORK DISTRICT HOSPITAL
 DR REBECCA THOMAS CONSULTANT RESPIRATORY PHYSICIAN YORK DISTRICT HOSPITAL Definition Guidelines contact complicated definitions Central to this is Presence of symptoms Variable airflow obstruction Diagnosis
DR REBECCA THOMAS CONSULTANT RESPIRATORY PHYSICIAN YORK DISTRICT HOSPITAL Definition Guidelines contact complicated definitions Central to this is Presence of symptoms Variable airflow obstruction Diagnosis
Rapid Effects of Inhaled Corticosteroids in Acute Asthma Gustavo J. Rodrigo, MD.
 Rapid Effects of Inhaled Corticosteroids in Acute Asthma Gustavo J. Rodrigo, MD WWW.chestjournal.org Background: Current reviews on the use of inhaled corticosteroids (ICS) for acute asthma underestimated
Rapid Effects of Inhaled Corticosteroids in Acute Asthma Gustavo J. Rodrigo, MD WWW.chestjournal.org Background: Current reviews on the use of inhaled corticosteroids (ICS) for acute asthma underestimated
BRONCHIAL THERMOPLASTY
 BRONCHIAL THERMOPLASTY UnitedHealthcare of California (HMO) UnitedHealthcare Benefits Plan of California (IEX EPO, IEX PPO) UnitedHealthcare of Oklahoma, Inc. UnitedHealthcare of Oregon, Inc. UnitedHealthcare
BRONCHIAL THERMOPLASTY UnitedHealthcare of California (HMO) UnitedHealthcare Benefits Plan of California (IEX EPO, IEX PPO) UnitedHealthcare of Oklahoma, Inc. UnitedHealthcare of Oregon, Inc. UnitedHealthcare
Interventions to improve adherence to inhaled steroids for asthma. Respiratory department
 Interventions to improve adherence to inhaled steroids for asthma Respiratory department Content Overview Research References Overview Asthma is a chronic breathing condition that affects more than 300
Interventions to improve adherence to inhaled steroids for asthma Respiratory department Content Overview Research References Overview Asthma is a chronic breathing condition that affects more than 300
Use of Sacubitril/Valsartan in Heart Failure
 Use of Sacubitril/Valsartan in Heart Failure & the PARADIGM-HF trial Sarah Mackenzie, PharmD student, University of Toronto Presentation Outline Overview of: Entresto PARADIGM-HF trial Critical Appraisal
Use of Sacubitril/Valsartan in Heart Failure & the PARADIGM-HF trial Sarah Mackenzie, PharmD student, University of Toronto Presentation Outline Overview of: Entresto PARADIGM-HF trial Critical Appraisal
REVIEW ARTICLE. Systematic Review of Randomized Controlled Trials Examining Written Action Plans in Children
 REVIEW ARTICLE Systematic Review of Randomized Controlled Trials Examining Written Action Plans in Children What Is the Plan? Roger L. Zemek, MD; Sanjit Kaur Bhogal, MSc; Francine M. Ducharme, MD Objectives:
REVIEW ARTICLE Systematic Review of Randomized Controlled Trials Examining Written Action Plans in Children What Is the Plan? Roger L. Zemek, MD; Sanjit Kaur Bhogal, MSc; Francine M. Ducharme, MD Objectives:
Clinical Practice Guideline: Asthma
 Clinical Practice Guideline: Asthma INTRODUCTION A critical aspect of the diagnosis and management of asthma is the precise and periodic measurement of lung function both before and after bronchodilator
Clinical Practice Guideline: Asthma INTRODUCTION A critical aspect of the diagnosis and management of asthma is the precise and periodic measurement of lung function both before and after bronchodilator
