Objectives. What is SMA? Pathophysiologic and genetic mechanisms How to identify a case of SMA
|
|
|
- Griffin Stokes
- 5 years ago
- Views:
Transcription
1
2 Objectives What is SMA? Pathophysiologic and genetic mechanisms How to identify a case of SMA What can be done? Review of advances in standards of care and treatment Detailed review of treatment available regionally What to do if you have a suspected case? How to refer a patient? How to counsel a patient/ family? Urgency of referral
3 What is Spinal Muscular Atrophy? Autosomal recessive pediatric onset neurodegenerative disease Deletions (mutation) in 5q13 SMN1 gene Survival of the motor neuron gene SMN protein is important for motor neuron health and survival Progressive loss of alpha motor neurons in the anterior horn cell of spinal cord Incidence 1:10,000 Carrier frequency about 1:35 in the Caucasian population ** ** May be even higher because we may miss: Asymptomatic individuals Embryonic lethal subjects 3 Images:
4 SMN Genetics C to T transition: Affects correct splicing of exon 7 SMN1 and SMN2 nearly identical genes Exon 7 not recognized by splicing machinery Get SMN2Δ7 transcripts Truncated SMN protein (only 282 aa) is unstable and nonfunctional SMN2 gene allows for rescue (from embryonic lethality) Less efficient = each copy produces about 10-15% full length protein compared to SMN1 gene 4
5 SMA Subtypes The more SMN2 copies you have, the better. 5
6 SMA Type 1 (Werdnig-Hoffman Disease) Disease onset within first 6 months of life Muscle weakness, hypotonia, areflexia in limbs and trunk Clinical course: Impaired head control (neck weakness) Unable to sit or walk Weak cry and cough Difficulty with swallowing, feeding, and handling of oral secretions (before 1 year of age) Die (or require > 16 hrs respiratory support) within first 2 years of life due to bulbar dysfunction or pulmonary complications 6
7 Intermediate Form 7 SMA Type 2 Symptom onset after 6 months old Clinical Course: Achieve sitting, but never able to walk unaided Bulbar weakness; swallowing difficulties can lead to poor weight gain Intercostal muscle weakness weak cough, difficulty clearing secretions Fine tremors with extended fingers or when attempting hand grips Kyphoscoliosis develops requiring bracing or spinal surgery Joint contractures over years Lack of DTRs in about 70% of patients Survival > 2 years Wang C, et al. J Child Neurol 2007.
8 SMA Type 3 (Kugelberg-Welander Disease) Able to sit and walk (some lose ability to walk in childhood) Presenting Features: Difficulties ascending and descending stairs at 2-3 years of age Proximal Muscle weakness Lower extremities more severely affected than upper extremities Reduced or absent DTRs Onset < 3 years Type 3a 44 % maintained walking by age 20 years 22% maintained walking by age 40 years Onset > 3 years Type 3b 90% maintained walking by age 20 year 58% maintained walking by age 40 years Scoliosis can develop Swallowing, cough, and nocturnal hypoventilation (may occur) Muscle aches and joint overuse symptoms are common 8 Wang C, et al. J Child Neurol 2007.
9 Spinal Muscular Atrophy: Making the Diagnosis 9
10 DNA Testing for SMA 1 st line SMN gene deletion test (Athena, Quest, Invitae) Via molecular genetic PCR-based testing (2-3 weeks for result; now quicker) 95% sensitivity, 100% specificity 95% will have homozygous deletions of SMN1 90% homozygous absence of exons 7 and 8 10% show homozygous absence of exon 7 but not 8 ~ 4% of SMA patients exhibit intragenic SMN1 mutations instead of deletion EMG less used as first line; possibly more in later onset cases Prenatal diagnosis: Carrier testing/ screening in expectant mother Via CVS (10-12 th week GA) or Amniocentesis (14-16 th week GA) 10
11 Spinal Muscular Atrophy: Updates in Management 11
12 History 12 Kolb S. Arch Neurol. 2011
13 First line: Management Supportive Care Clinicians can improve survival by optimal management of respiratory, nutritional, orthopedic health Even in era of new drugs available This has dramatically improved since 2007 standard of care document by Wang et al. Referral for care to a specialized neuromuscular clinical program (Muscular Dystrophy Association/ MDA Clinical Program) 13
14 14 Drug Development
15 Elevate endogenous FL- SMN protein levels generated by SMN2 Transcriptional SMN2 activation via gene promotor Restore correct splicing of SMN2 pre-mrna Translational activation and stabilization of the FL-SMN protein Suppression of the SMN2 stop codon to elongate SMN2Δ7 protein HDAC inhibitors Na butyrate, Valproic Acid, 4-phenylbutyrate, SAHA, M344 Regulating DNA methylation of SMN2 gene promotor VPA, 5- Aza-2 deoxicytidine Others: interferon, hydroxyurea, indoprofen HDAC inhibitors Small antisense RNA molecules Nusinersen Other aclarubicin, Na vanadate Phosphatases and kinases SMA modifying factors - albuterol Aminoglycosides Therapeutic Approaches Improve Motor Neuron Viability Gene Replacement Neurotrophic factors - cardiotrophin Neuroprotective compounds - Riluzole Regular exercise 15
16 Drug Development Pipeline 16
17 Clinical Trials Olesoxime: Cholesterol-oxime: NCT Targets mitochondrial integrity in stressed cells promote motor neuron survival Safe and well tolerated 2 year study patients with Type 2 and nonambulant Type 3 ages 3-25 yrs Primary endpoint not met, secondary endpoint suggests this may maintain motor function in patients with Type 2 or Type 3 SMA over 24 month period Roche/ PTC: RG7800/ Selectively modulates inclusion of SMN2 exon 7 orally bioavailable Phase I safe in HV Phase Ib/IIa randomized placebo control trial in adults and pediatric SMA patients suspended due to unexpected eye condition Modified compound: RG7916/ R Phase I/II studies in infants with: Type 1 SMA (NCT ) Type 2 and Type 3 SMA patients (NCT )
18 Clinical Trials Cytokinetics/ Astellas: CK-107/CK : NCT Skeletal muscle troponin activator slow calcium release increased skeletal muscle contractility enhance performance Completed Phase I study in HV In Phase 2 DB/PC/ multi-dose study in patients with Types 2,3 and 4 SMA Avexis: Gene Replacement: AVXS101 (AAV9) Strong preclinical data in mice (improved motor function, survival, weight, gene expression) Strong phase I clinical data: Type 1 SMA (2 copies SMN2) Mean age treatment 6.3 months Outcomes: 11 sat unassisted 9 rolled over 11 fed orally and could speak 2 walked independently 18
19 Clinical Trials: Gene Replacement: Enrolling Pre-Symptomatic Study of Intravenous AVXS-101 in Spinal Muscular Atrophy (SMA) for Patients With Multiple Copies of SMN2 (SPR1NT): NCT Pre-symptomatic Type 1, 2 or 3 SMA (2,3 or 4 copies SMN2); intravenous Study of Intrathecal Administration of AVXS-101 for Spinal Muscular Atrophy (STRONG): NCT Type 2 SMA (3 copies SMN2); intrathecal Gene Replacement Therapy Clinical Trial for Patients With Spinal Muscular Atrophy Type 1 (STR1VE): NCT Type 1 SMA (2 copies SMN2); intravenous A rapidly evolving space for research and therapeutic development 19
20 20 Approved Therapeutics
21 Antisense Oligonucleotide ( ASO ) (Nusinersen Biogen/ Ionis) Goal Manipulate RNA sequences to increase exon-7 incorporation during SMN2 RNA processing therefore increase FL-SMN Need drug that can have effect within the CNS ASO s do not cross BBB With IT delivery can get ASO s distributed into neurons, microglial cells, and astrocytes 21
22 Trials Double blind controlled clinical trial in 121 patients with SMA type 1 dosed < 7 months of age: IT administration Analysis in 82 patients showed motor improvement in 40% of patients on treatment vs none in sham group Trial halted, all patients rolled into open label extension Study in presymptomatic patients with 2 or 3 copies of SMN2 showed favorable results FDA approval December
23 Spinraza (nusinersen) Antisense oligonucleotide Modifies the transcription of SMN2 to produce a full-length SMN protein. Only effective for SMA caused by deletions/ point mutations of SMN1 Approved for use in patients of all ages with 5q SMA Given via intrathecal injection, 12 mg (in 5 ml solution) single dose vial Induction phase, then maintenance every 4 months for life 2 wks 2 wks 30 days 4 months 4 months... INDUCTION MAINTENANCE 23
24 Treatment Genetic Testing Baseline evaluation with laboratory testing Clinical documentation and consent for treatment/financial review Payer authorization White bag process for drug acquisition Scheduling (!) Loading doses Weeks 1,3,5,9 Safety labs Biobanking Multidisciplinary f/u Maintenance dosing every 4 months 24
25 Spinraza (nusinersen) at Children s National 32 patients on drug (8 Type 1, 10 Type 2, 14 Type 3 SMA patients) 28 in maintenance, 3 in loading, 1 awaiting first loading dose 1 international patient One of largest injecting sites in region (total 164 injections) One of earliest sites to initiate clinical dosing of Spinraza in 3/2017 Tracking motor function, respiratory function, speech/ communication, and biomarkers All patients showing subjective and objective functional gains; better tolerance to respiratory infections; increased energy; improved motor milestones 1 prenatally diagnosed patient prenatal referral baby seen immediately postnatally, predicted type 2 or type 3 SMA Dosed by 5 weeks of life 2 wks 2 wks 30 days 4 months 4 months... INDUCTION MAINTENANCE 25
26 Cure SMA Center Designation
27 Patient Management Medical treatment: Spinraza Nutrition: swallow, feeding, fluids, calories Respiratory: adequate ventilation and pulmonary toilet Communication: dysarthria, phonation, devices Self Care: occupational therapy, adaptive equipment Mobility: physical therapy, mobility devices +/- power Positioning: joint integrity, scoliosis 27
28 When to Treat? Based on electrodiagnostic studies in pre-symptomatic patients (Finkel R. 2012, Swaboda K. 2005): Early preservation of the motor unit Precipitous drop Then more gradual decline There may be a critical window for treatment based upon natural history and timing of motor neuron loss This is further reinforced by trial data EARLIER IS BETTER!!! - Benchmark for prenatally/ NBS neonatally identified cases: - Predicted Type 1 SMA: Dose within 3-4 weeks of life - Predicted Type 2 or Type 3 SMA: Dose within 2 months of life 28
29 Take Home Points When to suspect SMA?. Weakness, hypotonia, areflexia, tongue fasciculations, fine tremor (on reaching), relative facial sparing What to do?... Referral to neuromuscular specialist Children s National Health System: Neuromuscular Coordinator: Kathleen Smart: ksmart@childrensnational.org Counseling/ information?... When to treat?... Earlier is better!! 29
30 Thanks! ***1 st contact**** Neuromuscular/ MDA/ Cure SMA coordinator Neuromuscular Neurology, Co-director Cure SMA Center Chief, Physical Medicine & Rehabilitation, Co-director Cure SMA Center 30
31 References Bosboom WMJ, Vrancken AFJE, van den Berg LH, Wokke JHJ, Iannaccone ST. Drug treatment for spinal muscular atrophy types II and III. The Cochrane Collaboration and Foust KD, Wang X, McGovern VL, et al. Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nature Biotechnology. 2010;28: Kaufmann P, McDermott MP, Darras BT, et al. Prospective cohort study of spinal musuclar atrophy types 2 and 3. Neurology 2012;79: Kissel JT, Scott CB, Reyna SP, et al. SMA CARNI-VAL Trial Part II: A prospective, single-armed trial of L-carnitine and valproic acid in ambulatory children with Spinal muscular atrophy. Plos ONE 2011; 6 (7):e Kolb SJ and Kissel JT. Spinal muscular atrophy. Arch Neurol. 2011; 68: Porensky PN and Burghes AHM. Antisense oligonucleotides for the treatment of spinal muscular atrophy. Human Gene Therapy Rigo F, Hua Y, Krainer AR, Bennett CF. Antisense-based therapy for the treatment ofspinal muscular atrophy. J Cell Biol. 2012;199: Sproule DM and Kaufmann P. Therapeutic developments in spinal muscular atrophy. Ther Adv Neurol Disord. 2010; 3: Swaboda KJ, Scott CB, Crawford TO, et al. SMA CARNI-VAL Trial Part I: Double-blind, randomized, placebocontrolled trial of L-carnitine and valproic acid in spinal muscular atrophy. Plos ONE. 2010;5:e Wirth B, Brichta L, and Hahnen E. Spinal muscular atrophy: from gene to therapy. Semin Pediatr Neurol. 2006; 13: Zhou H, Janghra N, Mitrpant C, et al. A novel morpholino oligomer targeting ISS-N1 improves rescue of severe spinal muscular atrophy transgenic mice. Human Gene Therapy. 2013;24:
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: nusinersen_spinraza 03/2017 10/2018 10/2019 10/2018 Description of Procedure or Service Spinal muscular atrophy
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: nusinersen_spinraza 03/2017 10/2018 10/2019 10/2018 Description of Procedure or Service Spinal muscular atrophy
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: nusinersen_spinraza 03/2017 10/2017 10/2018 10/2017 Description of Procedure or Service Spinal muscular atrophy
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: nusinersen_spinraza 03/2017 10/2017 10/2018 10/2017 Description of Procedure or Service Spinal muscular atrophy
Cigna Drug and Biologic Coverage Policy
 Cigna Drug and Biologic Coverage Policy Subject Nusinersen Table of Contents Coverage Policy... 1 General Background... 2 Coding/Billing Information... 5 References... 5 Effective Date... 10/15/2017 Next
Cigna Drug and Biologic Coverage Policy Subject Nusinersen Table of Contents Coverage Policy... 1 General Background... 2 Coding/Billing Information... 5 References... 5 Effective Date... 10/15/2017 Next
SMA Therapeutics: A Comparative Overview of Drugs Approved and in Development. Sponsored By:
 SMA Therapeutics: A Comparative Overview of Drugs Approved and in Development Sponsored By: August 8, 2017 Targets for Therapeutic Intervention in SMA Decrease in SMN protein due to SMN1 gene deletion
SMA Therapeutics: A Comparative Overview of Drugs Approved and in Development Sponsored By: August 8, 2017 Targets for Therapeutic Intervention in SMA Decrease in SMN protein due to SMN1 gene deletion
Letter of Medical Necessity The Use of SPINRAZA (nusinersen) for Spinal Muscular Atrophy
 TEMPLATE Letter of Medical Necessity The Use of SPINRAZA (nusinersen) for Spinal Muscular Atrophy Date: [Insert Name of Medical Director] RE: Patient Name [ ] [Insurance Company] Policy Number [ ] [Address]
TEMPLATE Letter of Medical Necessity The Use of SPINRAZA (nusinersen) for Spinal Muscular Atrophy Date: [Insert Name of Medical Director] RE: Patient Name [ ] [Insurance Company] Policy Number [ ] [Address]
Clinical Policy Bulletin: Nusinersen (Spinraza)
 Clinical Policy Bulletin: Nusinersen (Spinraza) Number: 0915 Policy *Pleasesee amendment forpennsylvaniamedicaidattheendofthiscpb. Note: REQUIRES PRECERTIFICATION.Footnotes for Precertification of nusinersen
Clinical Policy Bulletin: Nusinersen (Spinraza) Number: 0915 Policy *Pleasesee amendment forpennsylvaniamedicaidattheendofthiscpb. Note: REQUIRES PRECERTIFICATION.Footnotes for Precertification of nusinersen
Spinraza (Nusinersen) Drug Prior Authorization Protocol (Medical Benefit & Part B Benefit)
 Line of Business: All Lines of Business Effective Date: August 16, 2017 Spinraza (Nusinersen) Drug Prior Authorization Protocol (Medical Benefit & Part B Benefit) This policy has been developed through
Line of Business: All Lines of Business Effective Date: August 16, 2017 Spinraza (Nusinersen) Drug Prior Authorization Protocol (Medical Benefit & Part B Benefit) This policy has been developed through
Spinal Muscular Atrophy in 2017
 Spinal Muscular Atrophy in 2017 Leigh Maria Ramos-Platt, MD Children s Hospital of Los Angeles University of Southern California Keck School of Medicine Disclosures MDA Care Center Grant In this presentation,
Spinal Muscular Atrophy in 2017 Leigh Maria Ramos-Platt, MD Children s Hospital of Los Angeles University of Southern California Keck School of Medicine Disclosures MDA Care Center Grant In this presentation,
Spinal Muscular Atrophy Newborn Screening
 10.2018 Spinal Muscular Atrophy Newborn Screening Melissa Gibbons, MS, CGC Erica Wright, MS, CGC Clinical Genetics and Metabolism Department of Pediatrics Children s Hospital Colorado/ University of Colorado
10.2018 Spinal Muscular Atrophy Newborn Screening Melissa Gibbons, MS, CGC Erica Wright, MS, CGC Clinical Genetics and Metabolism Department of Pediatrics Children s Hospital Colorado/ University of Colorado
Spinraza (nusinersen)
 Spinraza (nusinersen) Policy Number: 5.02.540 Last Review: 04/2018 Origination: 03/2017 Next Review: 04/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Spinraza
Spinraza (nusinersen) Policy Number: 5.02.540 Last Review: 04/2018 Origination: 03/2017 Next Review: 04/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Spinraza
SMA Treatments and Clinical Trials. Kenneth Hobby, President Mary Schroth, MD, Chief Medical Officer Jill Jarecki, PhD, Chief Scientific Officer
 SMA Treatments and Clinical Trials Kenneth Hobby, President Mary Schroth, MD, Chief Medical Officer Jill Jarecki, PhD, Chief Scientific Officer Agenda Introduction Kenneth Hobby, President Spinraza Update
SMA Treatments and Clinical Trials Kenneth Hobby, President Mary Schroth, MD, Chief Medical Officer Jill Jarecki, PhD, Chief Scientific Officer Agenda Introduction Kenneth Hobby, President Spinraza Update
SPINRAZA (NUSINERSEN)
 SPINRAZA (NUSINERSEN) UnitedHealthcare Commercial Medical Benefit Drug Policy Policy Number: 2018D0059D Effective Date: April 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS...
SPINRAZA (NUSINERSEN) UnitedHealthcare Commercial Medical Benefit Drug Policy Policy Number: 2018D0059D Effective Date: April 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS...
Spinal Muscular Atrophy: Case Study. Spinal muscular atrophy (SMA) is a fairly common genetic disorder, affecting
 Spinal Muscular Atrophy: Case Study Spinal muscular atrophy (SMA) is a fairly common genetic disorder, affecting approximately one in 6,000 babies. It is estimated that one in every 40 Americans carries
Spinal Muscular Atrophy: Case Study Spinal muscular atrophy (SMA) is a fairly common genetic disorder, affecting approximately one in 6,000 babies. It is estimated that one in every 40 Americans carries
Spinal Muscular Atrophy as a Focus Indication for Biomarker Development. Meg Winberg, PhD Spinal Muscular Atrophy Foundation February 26, 2007
 Spinal Muscular Atrophy as a Focus Indication for Biomarker Development Meg Winberg, PhD Spinal Muscular Atrophy Foundation February 26, 2007 Why SMA? p Low incidence, but a large orphan indication p Scientifically
Spinal Muscular Atrophy as a Focus Indication for Biomarker Development Meg Winberg, PhD Spinal Muscular Atrophy Foundation February 26, 2007 Why SMA? p Low incidence, but a large orphan indication p Scientifically
Panel II: SMA Drugs in Development
 Panel II: SMA Drugs in Development Jinsy Andrews MD, Director of Clinical Research and Development, Cytokinetics Thomas Blaettler MD, Global Clinical Development Team Leader, F. Hoffmann-La Roche Jerry
Panel II: SMA Drugs in Development Jinsy Andrews MD, Director of Clinical Research and Development, Cytokinetics Thomas Blaettler MD, Global Clinical Development Team Leader, F. Hoffmann-La Roche Jerry
SMA IS A SEVERE NEUROLOGICAL DISORDER [1]
![SMA IS A SEVERE NEUROLOGICAL DISORDER [1] SMA IS A SEVERE NEUROLOGICAL DISORDER [1]](/thumbs/72/67086153.jpg) SMA OVERVIEW SMA IS A SEVERE NEUROLOGICAL DISORDER [1] Autosomal recessive genetic inheritance 1 in 50 people (approximately 6 million Americans) are carriers [2] 1 in 6,000 to 1 in 10,000 children born
SMA OVERVIEW SMA IS A SEVERE NEUROLOGICAL DISORDER [1] Autosomal recessive genetic inheritance 1 in 50 people (approximately 6 million Americans) are carriers [2] 1 in 6,000 to 1 in 10,000 children born
Diagnosis, management and new treatments for Spinal Muscular Atrophy Special Focus: SMA Type 1
 Diagnosis, management and new treatments for Spinal Muscular Atrophy Special Focus: SMA Type 1 17 th April 2018 Adnan Manzur Consultant Paediatric Neurologist Dubowitz Neuromuscular Centre, GOSH & ICH,
Diagnosis, management and new treatments for Spinal Muscular Atrophy Special Focus: SMA Type 1 17 th April 2018 Adnan Manzur Consultant Paediatric Neurologist Dubowitz Neuromuscular Centre, GOSH & ICH,
SMA Treatment Access and Clinical Trials Webinar. February 15, 2018
 SMA Treatment Access and Clinical Trials Webinar February 15, 2018 Treatment Access Update Current Dosing Metrics December 2017: ~1,900 patients been dosed in US Including those in extension trials and
SMA Treatment Access and Clinical Trials Webinar February 15, 2018 Treatment Access Update Current Dosing Metrics December 2017: ~1,900 patients been dosed in US Including those in extension trials and
The 2017 Update of the Standard of Care Recommendations for Spinal Muscular Atrophy
 Richard Finkel, MD Nemours Children s Hospital Orlando, FL, USA Thomas Crawford, MD Johns Hopkins Hospital Baltimore, MD, USA The 2017 Update of the Standard of Care Recommendations for Spinal Muscular
Richard Finkel, MD Nemours Children s Hospital Orlando, FL, USA Thomas Crawford, MD Johns Hopkins Hospital Baltimore, MD, USA The 2017 Update of the Standard of Care Recommendations for Spinal Muscular
Nusinersen in Pre-symptomatic Infants With Spinal Muscular Atrophy (SMA): Interim Efficacy and Safety Results From the Phase 2 NURTURE Study
 21 st International Congress of the World Muscle Society -8 October, 2016 Granada, Spain Nusinersen in Pre-symptomatic Infants With Spinal Muscular Atrophy (SMA): Interim Efficacy and Safety Results From
21 st International Congress of the World Muscle Society -8 October, 2016 Granada, Spain Nusinersen in Pre-symptomatic Infants With Spinal Muscular Atrophy (SMA): Interim Efficacy and Safety Results From
COMPANY OVERVIEW. June 2016
 COMPANY OVERVIEW June 2016 Disclaimers Forward-Looking Statements This presentation contains forward-looking statements, including: statements about: the timing, progress and results of preclinical studies
COMPANY OVERVIEW June 2016 Disclaimers Forward-Looking Statements This presentation contains forward-looking statements, including: statements about: the timing, progress and results of preclinical studies
Final Phase 3 Study Data Show SPINRAZA (nusinersen) Significantly Improved Motor Function in Children with Later-Onset Spinal Muscular Atrophy
 MEDIA CONTACT: Biogen Ligia Del Bianco Ph: +1 781 464 3260 public.affairs@biogen.com INVESTOR CONTACT: Biogen Ben Strain Ph: +1 781 464 2442 IR@biogen.com Final Phase 3 Study Data Show SPINRAZA (nusinersen)
MEDIA CONTACT: Biogen Ligia Del Bianco Ph: +1 781 464 3260 public.affairs@biogen.com INVESTOR CONTACT: Biogen Ben Strain Ph: +1 781 464 2442 IR@biogen.com Final Phase 3 Study Data Show SPINRAZA (nusinersen)
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Nusinersen (Spinraza) Reference Number: CP.PHAR.327 Effective Date: 10.01.18 Last Review Date: 07.13.18 Line of Business: Oregon Health Plan Coding Implications Revision Log See Important
Clinical Policy: Nusinersen (Spinraza) Reference Number: CP.PHAR.327 Effective Date: 10.01.18 Last Review Date: 07.13.18 Line of Business: Oregon Health Plan Coding Implications Revision Log See Important
Nusinersen Use in Spinal Muscular Atrophy
 Nusinersen Use in Spinal Muscular Atrophy Report by: Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology Slide 1 Evidence in Focus Endorsement and
Nusinersen Use in Spinal Muscular Atrophy Report by: Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology Slide 1 Evidence in Focus Endorsement and
Related Policies None
 Medical Policy MP 5.01.28 BCBSA Ref. Policy: 5.01.28 Last Review: 04/30/2018 Effective Date: 07/30/2018 Section: Prescription Drug Related Policies None DISCLAIMER Our medical policies are designed for
Medical Policy MP 5.01.28 BCBSA Ref. Policy: 5.01.28 Last Review: 04/30/2018 Effective Date: 07/30/2018 Section: Prescription Drug Related Policies None DISCLAIMER Our medical policies are designed for
Emerging Therapies for SMA. Francesco Muntoni
 Emerging Therapies for SMA Francesco Muntoni TREAT-NMD Alliance Conference 2013 Newcastle Dubowitz Neuromuscular Centre UCL Institute of Child Health & Great Ormond Street Hospital London Therapeutic targets
Emerging Therapies for SMA Francesco Muntoni TREAT-NMD Alliance Conference 2013 Newcastle Dubowitz Neuromuscular Centre UCL Institute of Child Health & Great Ormond Street Hospital London Therapeutic targets
Roche leads the clinical development of risdiplam as part of a collaboration with the SMA Foundation and PTC Therapeutics.
 Media Release PRIME designation granted by European Medicines Agency for Roche s risdiplam for treatment of spinal muscular atrophy (SMA) Risdiplam has the potential to be the first oral medicine for the
Media Release PRIME designation granted by European Medicines Agency for Roche s risdiplam for treatment of spinal muscular atrophy (SMA) Risdiplam has the potential to be the first oral medicine for the
NEW HORIZONS IN TREATING SMA. Dr. Huda Mussaffi Schneider Children s Medical Center of Israel
 NEW HORIZONS IN TREATING SMA Dr. Huda Mussaffi Schneider Children s Medical Center of Israel WHAT IS SMA? Rare and debilitating autosomal recessive neuromuscular disease characterized by degeneration of
NEW HORIZONS IN TREATING SMA Dr. Huda Mussaffi Schneider Children s Medical Center of Israel WHAT IS SMA? Rare and debilitating autosomal recessive neuromuscular disease characterized by degeneration of
Association of motor milestones and SMN2 copy and outcome in spinal muscular. atrophy types 0 4
 jnnp-2016-314292 1 - SUPPLEMENTARY FILE - Methods and additional data on clinical characteristics and motor development Association of motor milestones and SMN2 copy and outcome in spinal muscular atrophy
jnnp-2016-314292 1 - SUPPLEMENTARY FILE - Methods and additional data on clinical characteristics and motor development Association of motor milestones and SMN2 copy and outcome in spinal muscular atrophy
Interim Efficacy and Safety Results from the Phase 2 NURTURE Study Evaluating Nusinersen in Presymptomatic Infants With Spinal Muscular Atrophy
 American Academy of Neurology 2017 69th Annual Meeting April 22 28, 2017 Boston, MA Interim Efficacy and Safety Results from the Phase 2 NURTURE Study Evaluating Nusinersen in Presymptomatic Infants With
American Academy of Neurology 2017 69th Annual Meeting April 22 28, 2017 Boston, MA Interim Efficacy and Safety Results from the Phase 2 NURTURE Study Evaluating Nusinersen in Presymptomatic Infants With
2018 F. Hoffmann-La Roche Ltd. All rights reserved.
 PRELIMINARY EVIDENCE FOR PHARMACODYNAMIC EFFECTS OF RG7916 IN JEWELFISH A STUDY IN PATIENTS WITH SPINAL MUSCULAR ATROPHY WHO PREVIOUSLY PARTICIPATED IN A STUDY WITH ANOTHER SMN2-SPLICING TARGETING THERAPY
PRELIMINARY EVIDENCE FOR PHARMACODYNAMIC EFFECTS OF RG7916 IN JEWELFISH A STUDY IN PATIENTS WITH SPINAL MUSCULAR ATROPHY WHO PREVIOUSLY PARTICIPATED IN A STUDY WITH ANOTHER SMN2-SPLICING TARGETING THERAPY
Clinical Policy: Nusinersen (Spinraza) Reference Number: CP.PHAR.327
 Clinical Policy: (Spinraza) Reference Number: CP.PHAR.327 Effective Date: 03/17 Last Review Date: 03/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: (Spinraza) Reference Number: CP.PHAR.327 Effective Date: 03/17 Last Review Date: 03/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
New Drug Evaluation: Nusinersen Injection, Intrathecal
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Clinical Review Report
 CADTH COMMON DRUG REVIEW Clinical Review Report Nusinersen (Spinraza) (Biogen Canada Inc.) Indication: Treatment of patients with 5q SMA Service Line: CADTH Common Drug Review Version: Version 1.0 Publication
CADTH COMMON DRUG REVIEW Clinical Review Report Nusinersen (Spinraza) (Biogen Canada Inc.) Indication: Treatment of patients with 5q SMA Service Line: CADTH Common Drug Review Version: Version 1.0 Publication
22nd Annual SMA Researcher Meeting
 22nd Annual SMA Researcher Meeting Thursday June 14, 2018 8:30 AM Welcoming Remarks Jill Jarecki PhD, Cure SMA Chief Scientific Officer Special Session: Clinical and Basic Questions of SMA in the Era of
22nd Annual SMA Researcher Meeting Thursday June 14, 2018 8:30 AM Welcoming Remarks Jill Jarecki PhD, Cure SMA Chief Scientific Officer Special Session: Clinical and Basic Questions of SMA in the Era of
Orofacial function of persons having. Report from questionnaires. Spinal muscular atrophy
 27-2-8 Orofacial function of persons having Spinal muscular atrophy Report from questionnaires The survey comprises questionnaires. Synonyms: SMA I (Werdnig-Hoffmann disease, SMA II, SMA III (Kugelberg-Welander
27-2-8 Orofacial function of persons having Spinal muscular atrophy Report from questionnaires The survey comprises questionnaires. Synonyms: SMA I (Werdnig-Hoffmann disease, SMA II, SMA III (Kugelberg-Welander
Nusinersen Demonstrates Efficacy in Infants With and Without Permanent Ventilation: Final Results From the ENDEAR Study
 2017 Annual Spinal Muscular Atrophy Conference June 29 July 2, 2017 Orlando, FL Nusinersen Demonstrates Efficacy in Infants With and Without Permanent Ventilation: Final Results From the ENDEAR Study Richard
2017 Annual Spinal Muscular Atrophy Conference June 29 July 2, 2017 Orlando, FL Nusinersen Demonstrates Efficacy in Infants With and Without Permanent Ventilation: Final Results From the ENDEAR Study Richard
Thomas O. Crawford, MD July 1, Annual Spinal Muscular Atrophy Conference June 29 July 2, 2017 Orlando, FL
 2017 Annual Spinal Muscular Atrophy Conference June 29 July 2, 2017 Orlando, FL Efficacy and Safety of Nusinersen in Genetically Diagnosed Infants With Presymptomatic Spinal Muscular Atrophy (SMA): Results
2017 Annual Spinal Muscular Atrophy Conference June 29 July 2, 2017 Orlando, FL Efficacy and Safety of Nusinersen in Genetically Diagnosed Infants With Presymptomatic Spinal Muscular Atrophy (SMA): Results
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Spinraza) Reference Number: CP.PHAR.327 Effective Date: 11.28.17 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder
Clinical Policy: (Spinraza) Reference Number: CP.PHAR.327 Effective Date: 11.28.17 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder
1/28/2019. OSF HealthCare INI Care Center Team. Neuromuscular Disease: Muscular Dystrophy. OSF HealthCare INI Care Center Team: Who are we?
 Neuromuscular Disease: Muscular Dystrophy Muscular Dystrophy Association (MDA) and OSF HealthCare Illinois Neurological Institute (INI) Care Center Team The Neuromuscular clinic is a designated MDA Care
Neuromuscular Disease: Muscular Dystrophy Muscular Dystrophy Association (MDA) and OSF HealthCare Illinois Neurological Institute (INI) Care Center Team The Neuromuscular clinic is a designated MDA Care
Spinal muscular atrophy 5Q Treatment with nusinersen
 GUIDELINES IN FOCUS Spinal muscular atrophy 5Q Treatment with nusinersen Author: Brazilian Medical Association Participants: Antonio Silvinato; Wanderley M Bernardo Final version: May 5, 2018 1. Brazilian
GUIDELINES IN FOCUS Spinal muscular atrophy 5Q Treatment with nusinersen Author: Brazilian Medical Association Participants: Antonio Silvinato; Wanderley M Bernardo Final version: May 5, 2018 1. Brazilian
Evolving therapeutic landscape for inherited neurologic disorders. Kathryn Swoboda, MD HMS Child Neurology Course September
 Evolving therapeutic landscape for inherited neurologic disorders Kathryn Swoboda, MD HMS Child Neurology Course September 5 2017 Disclosures I ve received funding as a consultant for Biogen for clinical
Evolving therapeutic landscape for inherited neurologic disorders Kathryn Swoboda, MD HMS Child Neurology Course September 5 2017 Disclosures I ve received funding as a consultant for Biogen for clinical
Clinical Policy Title: Spinraza
 Clinical Policy Title: Spinraza Clinical Policy Number: 02.02.03 Effective Date: July 1, 2018 Initial Review Date: May 19, 2017 Most Recent Review Date: June 5, 2018 Next Review Date: June 2019 Related
Clinical Policy Title: Spinraza Clinical Policy Number: 02.02.03 Effective Date: July 1, 2018 Initial Review Date: May 19, 2017 Most Recent Review Date: June 5, 2018 Next Review Date: June 2019 Related
Screening for spinal muscular atrophy External review against programme appraisal criteria for the UK National Screening Committee (UK NSC)
 Screening for spinal muscular atrophy External review against programme appraisal criteria for the UK National Screening Committee (UK NSC) Version: Third Draft Consultation Author: Costello Medical Date:
Screening for spinal muscular atrophy External review against programme appraisal criteria for the UK National Screening Committee (UK NSC) Version: Third Draft Consultation Author: Costello Medical Date:
Measurement of SMN protein in Blood samples Biomarker test for SMA. Thesis. By Hongyang Pi Chemical and Biomolecular Engineering
 Measurement of SMN protein in Blood samples Biomarker test for SMA Thesis Presented in Partial Fulfillment of the Requirements for the Honors Degree Bachelor of Science in the undergraduate college of
Measurement of SMN protein in Blood samples Biomarker test for SMA Thesis Presented in Partial Fulfillment of the Requirements for the Honors Degree Bachelor of Science in the undergraduate college of
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name Amyotrophic Lateral Sclerosis 10 (ALS10) and Amyotrophic Lateral Sclerosis 6 (ALS6)
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name Amyotrophic Lateral Sclerosis 10 (ALS10) and Amyotrophic Lateral Sclerosis 6 (ALS6)
CADTH Canadian Drug Expert Committee Recommendation
 CADTH COMMON DRUG REVIEW CADTH Canadian Drug Expert Committee Recommendation (FINAL) Nusinersen (Spinraza Biogen Canada Inc.) Indication: Treatment of 5q Spinal Muscular Atrophy RECOMMENDATION: The CADTH
CADTH COMMON DRUG REVIEW CADTH Canadian Drug Expert Committee Recommendation (FINAL) Nusinersen (Spinraza Biogen Canada Inc.) Indication: Treatment of 5q Spinal Muscular Atrophy RECOMMENDATION: The CADTH
AVXS-101 and Nusinersen for Spinal Muscular Atrophy: Effectiveness and Value
 AVXS-101 and Nusinersen for Spinal Muscular Atrophy: Effectiveness and Value Background Draft Background and Scope August 22, 2018 Spinal muscular atrophy (SMA) is a rare, genetic neuromuscular disease
AVXS-101 and Nusinersen for Spinal Muscular Atrophy: Effectiveness and Value Background Draft Background and Scope August 22, 2018 Spinal muscular atrophy (SMA) is a rare, genetic neuromuscular disease
ISIS PHARMACEUTICALS. ISIS-SMN Rx Investor Event. March 21, Slides available for download at:
 ISIS PHARMACEUTICALS ISIS-SMN Rx Investor Event March 21, 2013 Slides available for download at: http://www.isisph.com Forward Looking Language Statement 2 This presentation includes forward-looking statements
ISIS PHARMACEUTICALS ISIS-SMN Rx Investor Event March 21, 2013 Slides available for download at: http://www.isisph.com Forward Looking Language Statement 2 This presentation includes forward-looking statements
INDUSTRY PERSPECTIVE ON DRUG DEVELOPMENT FOR TYPE 1 SMA
 INDUSTRY PERSPECTIVE ON DRUG DEVELOPMENT FOR TYPE 1 SMA Kathie M. Bishop, PhD Disclosures: Currently, CSO of Tioga Pharmaceuticals, no activities in SMA; 2009-2015, full-time employee of Ionis Pharmaceuticals,
INDUSTRY PERSPECTIVE ON DRUG DEVELOPMENT FOR TYPE 1 SMA Kathie M. Bishop, PhD Disclosures: Currently, CSO of Tioga Pharmaceuticals, no activities in SMA; 2009-2015, full-time employee of Ionis Pharmaceuticals,
Final Results of the Phase 3 ENDEAR Study Assessing the Efficacy and Safety of Nusinersen in Infants With Spinal Muscular Atrophy (SMA)
 69th Annual Meeting of the American Academy of Neurology April 22-28, 2017 Boston, MA, USA Final Results of the Phase 3 ENDEAR Study Assessing the Efficacy and Safety of Nusinersen in Infants With Spinal
69th Annual Meeting of the American Academy of Neurology April 22-28, 2017 Boston, MA, USA Final Results of the Phase 3 ENDEAR Study Assessing the Efficacy and Safety of Nusinersen in Infants With Spinal
Disclosures Arthur Burghes. SAB AveXis. Performed studies funded by AveXis. Consulted for Novartis
 Disclosures Arthur Burghes SAB AveXis Performed studies funded by AveXis Consulted for Novartis Type of Biomarker Prognostic Disease Progression Predictive Pharmacodynamic Surrogate Endpoint Definition
Disclosures Arthur Burghes SAB AveXis Performed studies funded by AveXis Consulted for Novartis Type of Biomarker Prognostic Disease Progression Predictive Pharmacodynamic Surrogate Endpoint Definition
Spinal muscular atrophy Report from observation charts
 Orofacial function of persons having Spinal muscular atrophy Report from observation charts The survey comprises 49 observation charts. Synonyms: SMA I (Werdnig-Hoffmann disease, SMA II, SMA III (Kugelberg-Welander
Orofacial function of persons having Spinal muscular atrophy Report from observation charts The survey comprises 49 observation charts. Synonyms: SMA I (Werdnig-Hoffmann disease, SMA II, SMA III (Kugelberg-Welander
Neuromuscular in the Pediatric Clinic: Recognition and Referral
 Neuromuscular in the Pediatric Clinic: Recognition and Referral Matthew Harmelink, MD Assistant Professor, Pediatric Neurology Medical College of Wisconsin Objectives: 1. Understand common presentations
Neuromuscular in the Pediatric Clinic: Recognition and Referral Matthew Harmelink, MD Assistant Professor, Pediatric Neurology Medical College of Wisconsin Objectives: 1. Understand common presentations
Pharmacoeconomic Review Report
 CADTH COMMON DRUG REVIEW Pharmacoeconomic Review Report NUSINERSEN (SPINRAZA) (Biogen Canada Inc.) Indication: Treatment of patients with 5q SMA Service Line: CADTH Common Drug Review Version: Final Publication
CADTH COMMON DRUG REVIEW Pharmacoeconomic Review Report NUSINERSEN (SPINRAZA) (Biogen Canada Inc.) Indication: Treatment of patients with 5q SMA Service Line: CADTH Common Drug Review Version: Final Publication
Assessing the Needs of the SMA Population: Survey Results of Health Care Providers and Families
 559018SGOXXX10.1177/2158244014559018SAGE OpenHalanski et al. research-article2014 Article Assessing the Needs of the SMA Population: Survey Results of Health Care Providers and Families SAGE Open October-December
559018SGOXXX10.1177/2158244014559018SAGE OpenHalanski et al. research-article2014 Article Assessing the Needs of the SMA Population: Survey Results of Health Care Providers and Families SAGE Open October-December
Learn the steps to identify pediatric muscle weakness and signs of neuromuscular disease.
 Learn the steps to identify pediatric muscle weakness and signs of neuromuscular disease. Listen Observe Evaluate Test Refer Guide for primary care providers includes: Surveillance Aid: Assessing Weakness
Learn the steps to identify pediatric muscle weakness and signs of neuromuscular disease. Listen Observe Evaluate Test Refer Guide for primary care providers includes: Surveillance Aid: Assessing Weakness
Genetic diagnosis of limb girdle muscular dystrophy type 2A, A Case Report
 Genetic diagnosis of limb girdle muscular dystrophy type 2A, A Case Report Roshanak Jazayeri, MD, PhD Assistant Professor of Medical Genetics Faculty of Medicine, Alborz University of Medical Sciences
Genetic diagnosis of limb girdle muscular dystrophy type 2A, A Case Report Roshanak Jazayeri, MD, PhD Assistant Professor of Medical Genetics Faculty of Medicine, Alborz University of Medical Sciences
Neonatal Hypotonia. Encephalopathy acute No encephalopathy. Neurology Chapter of IAP
 The floppy infant assumes a frog legged position. On ventral suspension, the baby can not maintain limb posture against gravity and assumes the position of a rag doll. Encephalopathy acute No encephalopathy
The floppy infant assumes a frog legged position. On ventral suspension, the baby can not maintain limb posture against gravity and assumes the position of a rag doll. Encephalopathy acute No encephalopathy
Olesoxime for amyotrophic lateral sclerosis first line
 Olesoxime for amyotrophic lateral sclerosis first line May 2011 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
Olesoxime for amyotrophic lateral sclerosis first line May 2011 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
Orofacial function of persons having. Spinal muscular atrophy
 Orofacial function of persons having Report from questionnaires 31 questionnaires Synonym SMA I (Werdnig-Hoffmann disease, SMA II, SMA III (Kugelberg-Welander disease). ICD-10 G12.0 Estimated occurance
Orofacial function of persons having Report from questionnaires 31 questionnaires Synonym SMA I (Werdnig-Hoffmann disease, SMA II, SMA III (Kugelberg-Welander disease). ICD-10 G12.0 Estimated occurance
SALSA MLPA KIT P060-B2 SMA
 SALSA MLPA KIT P6-B2 SMA Lot 111, 511: As compared to the previous version B1 (lot 11), the 88 and 96 nt DNA Denaturation control fragments have been replaced (QDX2). Please note that, in contrast to the
SALSA MLPA KIT P6-B2 SMA Lot 111, 511: As compared to the previous version B1 (lot 11), the 88 and 96 nt DNA Denaturation control fragments have been replaced (QDX2). Please note that, in contrast to the
DOSAGE FORMS AND STRENGTHS Injection: 12 mg/5 ml (2.4 mg/ml) in a single-dose vial (3)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use SPINRAZA safely and effectively. See full prescribing information for SPINRAZA. SPINRAZA (nusinersen)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use SPINRAZA safely and effectively. See full prescribing information for SPINRAZA. SPINRAZA (nusinersen)
Evaluation of the Hypotonic Infant and Child
 Evaluation of the Hypotonic Infant and Child Basil T. Darras, M.D. Neuromuscular Program Boston Children s Hospital Harvard Medical School Boston, MA, USA Classification and General Clinical Evaluation
Evaluation of the Hypotonic Infant and Child Basil T. Darras, M.D. Neuromuscular Program Boston Children s Hospital Harvard Medical School Boston, MA, USA Classification and General Clinical Evaluation
Drug treatment for spinal muscular atrophy types II and III (Review)
 Drug treatment for spinal muscular atrophy types II and III (Review) Wadman RI, Bosboom WMJ, van der Pol WL, van den Berg LH, Wokke JHJ, Iannaccone ST, Vrancken AFJE This is a reprint of a Cochrane review,
Drug treatment for spinal muscular atrophy types II and III (Review) Wadman RI, Bosboom WMJ, van der Pol WL, van den Berg LH, Wokke JHJ, Iannaccone ST, Vrancken AFJE This is a reprint of a Cochrane review,
The Floppy Baby. Clare Betteridge
 The Floppy Baby Clare Betteridge The floppy baby Identification Evaluation Investigation Diagnosis Examples What is a floppy baby? Elbows and knees loosely extended. Head control is usually poor or absent.
The Floppy Baby Clare Betteridge The floppy baby Identification Evaluation Investigation Diagnosis Examples What is a floppy baby? Elbows and knees loosely extended. Head control is usually poor or absent.
CARE CONSIDERATIONS FOR DUCHENNE MUSCULAR DYSTROPHY
 IMPORTANT NEW UPDATE A Summary of the Report of the DMD Care Considerations Working Group Intended for US healthcare professionals only. CARE CONSIDERATIONS FOR DUCHENNE MUSCULAR DYSTROPHY Full article
IMPORTANT NEW UPDATE A Summary of the Report of the DMD Care Considerations Working Group Intended for US healthcare professionals only. CARE CONSIDERATIONS FOR DUCHENNE MUSCULAR DYSTROPHY Full article
MOTOR NEURONE DISEASE
 MOTOR NEURONE DISEASE Dr Arun Aggarwal Department of Rehabilitation Medicine, RPAH Department of Neurology, Concord Hospital. Motor Neurone Disease Umbrella term in UK and Australia (ALS in USA) Neurodegenerative
MOTOR NEURONE DISEASE Dr Arun Aggarwal Department of Rehabilitation Medicine, RPAH Department of Neurology, Concord Hospital. Motor Neurone Disease Umbrella term in UK and Australia (ALS in USA) Neurodegenerative
Muscular Dystrophy. Biol 405 Molecular Medicine
 Muscular Dystrophy Biol 405 Molecular Medicine Duchenne muscular dystrophy Duchenne muscular dystrophy is a neuromuscular disease that occurs in ~ 1/3,500 male births. The disease causes developmental
Muscular Dystrophy Biol 405 Molecular Medicine Duchenne muscular dystrophy Duchenne muscular dystrophy is a neuromuscular disease that occurs in ~ 1/3,500 male births. The disease causes developmental
A Study of valproic acid for patients with spinal muscular atrophy
 doi:10.1111/ncn3.140 ORIGINAL ARTICLE A Study of valproic acid for patients with spinal muscular atrophy Toshio Saito, 1 Dian K Nurputra, 2,3 Nur Imma F Harahap, 2,3 Indra Sari K Harahap, 2,3 Hiroshi Yamamoto,
doi:10.1111/ncn3.140 ORIGINAL ARTICLE A Study of valproic acid for patients with spinal muscular atrophy Toshio Saito, 1 Dian K Nurputra, 2,3 Nur Imma F Harahap, 2,3 Indra Sari K Harahap, 2,3 Hiroshi Yamamoto,
Neuroscience 410 Huntington Disease - Clinical. March 18, 2008
 Neuroscience 410 March 20, 2007 W. R. Wayne Martin, MD, FRCPC Division of Neurology University of Alberta inherited neurodegenerative disorder autosomal dominant 100% penetrance age of onset: 35-45 yr
Neuroscience 410 March 20, 2007 W. R. Wayne Martin, MD, FRCPC Division of Neurology University of Alberta inherited neurodegenerative disorder autosomal dominant 100% penetrance age of onset: 35-45 yr
Small Molecules in Development for the Treatment of Spinal Muscular Atrophy
 Small Molecules in Development for the Treatment of Spinal Muscular Atrophy Alyssa N. Calder, a Elliot J. Androphy, b Kevin J. Hodgetts a* a Laboratory for Drug Discovery in Neurodegeneration, Brigham
Small Molecules in Development for the Treatment of Spinal Muscular Atrophy Alyssa N. Calder, a Elliot J. Androphy, b Kevin J. Hodgetts a* a Laboratory for Drug Discovery in Neurodegeneration, Brigham
Update on Pulmonary Management in Spinal Muscular Atrophy type 1
 Update on Pulmonary Management in Spinal Muscular Atrophy type 1 David Zielinski, MD FRCPC, FCCP Associate Professor McGill University Montreal Children s Hospital None Disclosures Objectives Review genetic
Update on Pulmonary Management in Spinal Muscular Atrophy type 1 David Zielinski, MD FRCPC, FCCP Associate Professor McGill University Montreal Children s Hospital None Disclosures Objectives Review genetic
9/30/2017. SMA: Spinal Muscular Atrophy Learning Outcomes: SMA. Prevalence. Review of the diagnosis. Here are the odds
 SMA: Spinal Muscular Atrophy Learning Outcomes: 1 in 6,000 to 1 in 10,000 but 100% to CRT Kay Koch, OTR/L, ATP The van Halem Group, A Division of VGM kkotrchoa@yahoo.com The participant will be able to
SMA: Spinal Muscular Atrophy Learning Outcomes: 1 in 6,000 to 1 in 10,000 but 100% to CRT Kay Koch, OTR/L, ATP The van Halem Group, A Division of VGM kkotrchoa@yahoo.com The participant will be able to
Genotype phenotype studies in infantile spinal muscular atrophy (SMA) type I in Germany: implications for clinical trials and genetic counselling
 Clin Genet 2009: 76: 168 178 Printed in Singapore. All rights reserved Short Report 2009 John Wiley & Sons A/S CLINICAL GENETICS doi: 10.1111/j.1399-0004.2009.01200.x Genotype phenotype studies in infantile
Clin Genet 2009: 76: 168 178 Printed in Singapore. All rights reserved Short Report 2009 John Wiley & Sons A/S CLINICAL GENETICS doi: 10.1111/j.1399-0004.2009.01200.x Genotype phenotype studies in infantile
MRC-Holland MLPA. Description version 19;
 SALSA MLPA probemix P6-B2 SMA Lot B2-712, B2-312, B2-111, B2-511: As compared to the previous version B1 (lot B1-11), the 88 and 96 nt DNA Denaturation control fragments have been replaced (QDX2). SPINAL
SALSA MLPA probemix P6-B2 SMA Lot B2-712, B2-312, B2-111, B2-511: As compared to the previous version B1 (lot B1-11), the 88 and 96 nt DNA Denaturation control fragments have been replaced (QDX2). SPINAL
When a Threshold Crossing approach may and may not be appropriate: A Case Study in SMA
 When a Threshold Crossing approach may and may not be appropriate: A Case Study in SMA EFSPI Regulatory Statistics Workshop, 24-25 th September 2018 Carol Reid and Uli Burger picture placeholder Outline
When a Threshold Crossing approach may and may not be appropriate: A Case Study in SMA EFSPI Regulatory Statistics Workshop, 24-25 th September 2018 Carol Reid and Uli Burger picture placeholder Outline
Motor neuron diseases
 Motor neuron diseases What are motor neuron diseases? The motor neuron diseases (MNDs) are a group of progressive neurological disorders that destroy motor neurons, the cells that control essential voluntary
Motor neuron diseases What are motor neuron diseases? The motor neuron diseases (MNDs) are a group of progressive neurological disorders that destroy motor neurons, the cells that control essential voluntary
Evaluation of Nusinersen (SPINRAZA TM ) in Medicinrådet
 Evaluation of Nusinersen (SPINRAZA TM ) in Medicinrådet Contents 1. Executive summary... 3 2. Natural history of the disease... 4 3. Medicine Council Questions... 6 4. Clinical added value of nusinersen
Evaluation of Nusinersen (SPINRAZA TM ) in Medicinrådet Contents 1. Executive summary... 3 2. Natural history of the disease... 4 3. Medicine Council Questions... 6 4. Clinical added value of nusinersen
Edinburgh Research Explorer
 Edinburgh Research Explorer Advances in therapy for spinal muscular atrophy: promises and challenges Citation for published version: Groen, E, Talbot, K & Gillingwater, T 2018, 'Advances in therapy for
Edinburgh Research Explorer Advances in therapy for spinal muscular atrophy: promises and challenges Citation for published version: Groen, E, Talbot, K & Gillingwater, T 2018, 'Advances in therapy for
The first and only treatment for spinal muscular atrophy (SMA)
 Discover SPINRAZA The first and only treatment for spinal muscular atrophy (SMA) INDICATION SPINRAZA is a prescription medicine used to treat spinal muscular atrophy (SMA) in pediatric and adult patients.
Discover SPINRAZA The first and only treatment for spinal muscular atrophy (SMA) INDICATION SPINRAZA is a prescription medicine used to treat spinal muscular atrophy (SMA) in pediatric and adult patients.
Patient L.L. KRISTEN ARREDONDO, MD CHILD NEUROLOGY PGY5, UT SOUTHWESTERN
 Patient L.L. KRISTEN ARREDONDO, MD CHILD NEUROLOGY PGY5, UT SOUTHWESTERN Birth History LL was born to a healthy first time mother with an uncomplicated pregnancy Delivered at 38 weeks via C-section due
Patient L.L. KRISTEN ARREDONDO, MD CHILD NEUROLOGY PGY5, UT SOUTHWESTERN Birth History LL was born to a healthy first time mother with an uncomplicated pregnancy Delivered at 38 weeks via C-section due
they have one or two SMN1 genes. In most places, this test will only be done when an individual is an adult or of child bearing age.
 Families of Spinal Muscular Atrophy Transcript of Chat Live with an Expert December 1, 2004 Topic: Genetics and SMA Our expert is Louise Simard, PhD, and SMA researcher at Montreal s Saint Justine Hospital.
Families of Spinal Muscular Atrophy Transcript of Chat Live with an Expert December 1, 2004 Topic: Genetics and SMA Our expert is Louise Simard, PhD, and SMA researcher at Montreal s Saint Justine Hospital.
Ambulant & non ambulant (Types 2 & 3) Spinal Muscular Atrophy
 Ambulant & non ambulant (Types 2 & 3) Spinal Muscular Atrophy Mário Miguel Rosa, MD, PhD SAWP, CNSWP (EMA) FMUL, INFARMED Lisbon - Portugal SMA EMA workshop An agency of the European Union Content Relevant
Ambulant & non ambulant (Types 2 & 3) Spinal Muscular Atrophy Mário Miguel Rosa, MD, PhD SAWP, CNSWP (EMA) FMUL, INFARMED Lisbon - Portugal SMA EMA workshop An agency of the European Union Content Relevant
Gene therapy and genome editing technologies for the study and potential treatment of :
 WORKSHOP ON GENOME EDITING Gene therapy and genome editing technologies for the study and potential treatment of : Duchenne Muscular Dystrophy by Dr France Piétri-Rouxel, Institut de Myologie Centre de
WORKSHOP ON GENOME EDITING Gene therapy and genome editing technologies for the study and potential treatment of : Duchenne Muscular Dystrophy by Dr France Piétri-Rouxel, Institut de Myologie Centre de
The clinical landscape for SMA in a new therapeutic era
 OPEN Gene Therapy (2017) 24, 529 533 www.nature.com/gt REVIEW The clinical landscape for SMA in a new therapeutic era K Talbot 1,4 and EF Tizzano 2,3,4 Despite significant advances in basic research, the
OPEN Gene Therapy (2017) 24, 529 533 www.nature.com/gt REVIEW The clinical landscape for SMA in a new therapeutic era K Talbot 1,4 and EF Tizzano 2,3,4 Despite significant advances in basic research, the
NIH Public Access Author Manuscript J Child Neurol. Author manuscript; available in PMC 2012 January 17.
 NIH Public Access Author Manuscript Published in final edited form as: J Child Neurol. 2007 August ; 22(8): 957 966. doi:10.1177/0883073807305665. Perspectives on Clinical Trials in Spinal Muscular Atrophy
NIH Public Access Author Manuscript Published in final edited form as: J Child Neurol. 2007 August ; 22(8): 957 966. doi:10.1177/0883073807305665. Perspectives on Clinical Trials in Spinal Muscular Atrophy
Nemaline (rod) myopathies
 Nemaline (rod) myopathies Nemaline, or rod, myopathies are a group of conditions which fall under the umbrella of congenital myopathies. They are characterised by rod-like structures in the muscle cells,
Nemaline (rod) myopathies Nemaline, or rod, myopathies are a group of conditions which fall under the umbrella of congenital myopathies. They are characterised by rod-like structures in the muscle cells,
LIMP BABIES - WHAT MAY THAT MEAN?
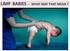 LIMP BABIES - WHAT MAY THAT MEAN? ARE WE LOOKING WHERE WE SHOULD? FLOPPY = LIMP = FLACCID = WEAK Poor head control when pulled C-posture sitting, or in ventral susp. Frog-leg lying Dropping arms Casey
LIMP BABIES - WHAT MAY THAT MEAN? ARE WE LOOKING WHERE WE SHOULD? FLOPPY = LIMP = FLACCID = WEAK Poor head control when pulled C-posture sitting, or in ventral susp. Frog-leg lying Dropping arms Casey
Title: Developmental milestones in type I spinal muscular atrophy
 Accepted Manuscript Title: Developmental milestones in type I spinal muscular atrophy Author: Roberto De Sanctis, Giorgia Coratti, Amy Pasternak, Jacqueline Montes, Marika Pane, Elena S. Mazzone, Sally
Accepted Manuscript Title: Developmental milestones in type I spinal muscular atrophy Author: Roberto De Sanctis, Giorgia Coratti, Amy Pasternak, Jacqueline Montes, Marika Pane, Elena S. Mazzone, Sally
Spinal Muscular Atrophy: What s new in the management of pediatric neuromuscular disease
 Spinal Muscular Atrophy: What s new in the management of pediatric neuromuscular disease Mary Schroth MD Professor, Pediatric Pulmonology University of Wisconsin School of Medicine and Public Health, Madison
Spinal Muscular Atrophy: What s new in the management of pediatric neuromuscular disease Mary Schroth MD Professor, Pediatric Pulmonology University of Wisconsin School of Medicine and Public Health, Madison
Pompe. Lysosomal Disorders. Introduction
 Introduction disease is an inherited, genetic disorder which results in the lack of an enzyme 'acid alpha-glucosidase. disease is also known as acid maltase deficiency or glycogen storage disease type
Introduction disease is an inherited, genetic disorder which results in the lack of an enzyme 'acid alpha-glucosidase. disease is also known as acid maltase deficiency or glycogen storage disease type
Using Biomarkers to Increase the Efficiency of Early Stage Drug Development
 Using Biomarkers to Increase the Efficiency of Early Stage Drug Development Personal consulting compensation received from Cytokinetics, Biogen, MT Pharma, Lilly, Karyopharm, Denali Goals of Talk Describe
Using Biomarkers to Increase the Efficiency of Early Stage Drug Development Personal consulting compensation received from Cytokinetics, Biogen, MT Pharma, Lilly, Karyopharm, Denali Goals of Talk Describe
Genetics and Reproductive Options for SMA Families Annual SMA Conference Dallas, Texas Friday, June 15, 2017
 Genetics and Reproductive Options for SMA Families 2018 Annual SMA Conference Dallas, Texas Friday, June 15, 2017 Part 1: SMA and Genetics Louise R Simard, PhD Part 2: SMA Carrier Screening Melissa Gibbons,
Genetics and Reproductive Options for SMA Families 2018 Annual SMA Conference Dallas, Texas Friday, June 15, 2017 Part 1: SMA and Genetics Louise R Simard, PhD Part 2: SMA Carrier Screening Melissa Gibbons,
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Proposed Highly Specialised Technology Evaluation
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Highly Specialised Technology Evaluation Drisapersen for treating Duchenne muscular Draft scope (pre-referral) Draft remit/evaluation objective
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Highly Specialised Technology Evaluation Drisapersen for treating Duchenne muscular Draft scope (pre-referral) Draft remit/evaluation objective
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE. PATIENT MEDICATION INFORMATION SPINRAZA nusinersen injection
 READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION SPINRAZA nusinersen injection Read this carefully before you or your child start receiving SPINRAZA and before each
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION SPINRAZA nusinersen injection Read this carefully before you or your child start receiving SPINRAZA and before each
Kathryn J. Swoboda, MD, FACMG NBSTRN Meeting
 Kathryn J. Swoboda, MD, FACMG NBSTRN Meeting SPOT SMA LPDR resource: overview of clinical research forms and overall database Feasibility and utility of a minimal common set of data elements vs. a more
Kathryn J. Swoboda, MD, FACMG NBSTRN Meeting SPOT SMA LPDR resource: overview of clinical research forms and overall database Feasibility and utility of a minimal common set of data elements vs. a more
Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy
 The new england journal of medicine Original Article versus Sham in Infantile-Onset Spinal Muscular Atrophy R.S. Finkel, E. Mercuri, B.T. Darras, A.M. Connolly, N.L. Kuntz, J. Kirschner, C.A. Chiriboga,
The new england journal of medicine Original Article versus Sham in Infantile-Onset Spinal Muscular Atrophy R.S. Finkel, E. Mercuri, B.T. Darras, A.M. Connolly, N.L. Kuntz, J. Kirschner, C.A. Chiriboga,
SPINRAZA READINESS KIT
 SPINRAZA READINESS KIT Please see full Prescribing Information for Important Safety Information. MANAGEMENT OF THE REIMBURSEMENT PROCESS FOR SPINRAZA 1 SELECT TREATMENT AFTER DISCUSSING BENEFITS AND RISKS
SPINRAZA READINESS KIT Please see full Prescribing Information for Important Safety Information. MANAGEMENT OF THE REIMBURSEMENT PROCESS FOR SPINRAZA 1 SELECT TREATMENT AFTER DISCUSSING BENEFITS AND RISKS
Corporate Medical Policy
 Corporate Medical Policy Genetic Testing for Duchenne and Becker Muscular Dystrophy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_duchenne_and_becker_muscular_dystrophy
Corporate Medical Policy Genetic Testing for Duchenne and Becker Muscular Dystrophy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_duchenne_and_becker_muscular_dystrophy
