Final Results of the Phase 3 ENDEAR Study Assessing the Efficacy and Safety of Nusinersen in Infants With Spinal Muscular Atrophy (SMA)
|
|
|
- Phillip Welch
- 5 years ago
- Views:
Transcription
1 69th Annual Meeting of the American Academy of Neurology April 22-28, 2017 Boston, MA, USA Final Results of the Phase 3 ENDEAR Study Assessing the Efficacy and Safety of Nusinersen in Infants With Spinal Muscular Atrophy (SMA) Nancy Kuntz, MD April 24, 2017 Kuntz N, 1 Finkel RS, 2 Mercuri E, 3 Chiriboga CA, 4 Darras BT, 5 Topaloglu H, 6 Montes J, 4 Su J, 7 Zhong ZJ, 8 Gheuens S, 8 Bennett CF, 7 Schneider E, 7 Farwell W, 8 on behalf of the ENDEAR Study Group 1 Ann & Robert H. Lurie Children s Hospital of Chicago, Chicago, IL, USA; 2 Nemours Children s Hospital, Orlando, FL, USA; 3 Università Cattolica del Sacro Cuore, Rome, Italy; 4 Columbia University, New York, NY, USA; 5 Boston Children s Hospital, Boston, MA, USA; 6 Hacettepe University, Ankara, Turkey; 7 Ionis Pharmaceuticals, Inc., Carlsbad, CA, USA; 8 Biogen, Cambridge, MA, USA
2 Disclosures NK: advisory boards for Biogen; outside of the submitted work, advisory boards and consulting fees for AveXis, Catalyst, Cytokinetics, Marathon, PTC, and Sarepta; advisory capacity to CureSMA and MGFA. RSF: grants and personal fees from Ionis Pharmaceuticals during ENDEAR and CHERISH; grants and advisor fees from Biogen, grants from Cytokinetics and advisor to Roche outside the submitted work; advisory capacity to non-profit organizations: the SMA Foundation, Cure SMA, SMA Reach (UK) and SMA Europe; serves on the Data Safety Monitoring Board for the AveXis gene transfer study. EM: advisory boards for SMA studies for Avexis, Biogen, Ionis Pharmaceuticals, Inc., Novartis and Roche; principal investigator for ongoing Ionis/Biogen and Roche clinical trials; receives funding from Famiglie SMA Italy, Italian Telethon and SMA Europe CAC: advisory boards for Roche, Ionis Pharmaceuticals, Inc., Biogen, and AveXis for SMA studies; grants from Ionis Pharmaceuticals, Biogen and the SMA Foundation. BTD: scientific advisory board consultant for AveXis, Biogen, Marathon Pharmaceuticals, Sarepta, Cytokinetics, PTC Therapeutics and Roche; grants from Ionis Pharmaceuticals during ENDEAR, CHERISH, CS12, CS11 studies, Cytokinetics, Sarepta, PTC, Summit and advisor to Ionis Pharmaceuticals, Inc., Roche, Sarepta, AveXis, Biogen, Marathon and BMS HT: nothing to disclose JM: support from Eunice Kennedy Shriver National Institute for Child Health and Human Development (1K01HD A1); consultant for Ionis Pharmaceuticals, Inc.; advisory boards for Biogen and Roche JS, CFB and ES: employees of and hold stock/stock options in Ionis Pharmaceuticals, Inc. ZJZ, SG and WF: employees of and hold stock/stock options in Biogen This study was sponsored by Ionis Pharmaceuticals, Inc. (Carlsbad, CA, USA) and Biogen (Cambridge, MA, USA) Writing and editorial support for the preparation of this presentation was provided by Excel Scientific Solutions (Southport, CT, USA): funding was provided by Biogen 2
3 Introduction Spinal muscular atrophy (SMA) - SMA is a rare, debilitating, autosomal recessive neuromuscular disorder 1 - Caused by insufficient levels of SMN protein 2 Nusinersen: an antisense oligonucleotide - Modulates splicing of SMN2 pre-mrna to promote increased production of full-length SMN protein 3,4,5 Phase 2 study (CS3A) interim results a in infants with SMA 6 - Demonstrated increased SMN protein levels in motor neurons - Showed promising safety and efficacy ENDEAR interim results b in infants with SMA - 41% of nusinersen-treated versus 0% of control infants were motor milestone responders (P<0.0001) mrna = messenger RNA; SMN = survival of motor neuron. a Interim analysis data cut conducted 26 January b Interim analysis data cut conducted on 15 June Lunn MR, Wang CH. Lancet. 2008;371(9630): Prior TW. Curr Opin Pediatr. 2010;22(6): Hua Y, et al. Genes Dev. 2010;24(15): Passini MA, et al. Sci Transl Med. 2011;3(72):72ra Darras B, et al. Neuromusc Disord. 2014;24(9-10): Finkel RS, et al. Lancet. 2016; 388(10063):
4 ENDEAR Study Design Phase 3, randomized, double-blind, sham-procedure controlled study to assess the clinical efficacy, safety, and tolerability of intrathecal nusinersen in infants with SMA Key eligibility criteria: genetic diagnosis of SMA, 2 copies of the SMN2 gene, onset of SMA symptoms at age 6 months and age 7 months with no hypoxemia at screening Screening 21-day screening Randomization a Double-blind treatment period Nusinersen 12-mg scaled equivalent dose (n=80) 2:1 randomization; 13 months of treatment and follow-up Sham procedure control (n=41) Pre-specified interim efficacy analysis D183 onwards: ~80 participants b Study participants transferred to SHINE open-label extension c Dosing schedule: D1 D15 D29 D64 D183 D302 D394 Loading dose Maintenance dose ITT and safety population: randomized and received 1 dose of study drug Interim efficacy set (IES): ITT participants who received nusinersen dose/sham procedure control 6 months before cutoff date for interim analysis and/or were assessed at any of the Day 183, 302 or 394 visits Efficacy set: All infants who received nusinersen dose/sham procedure control 6 months before cutoff date for final analysis and/or were assessed at any of the Day 183, 302 or 394 visits ITT = intention-to-treat. a Randomization was stratified by disease duration during screening (age at screening minus age at symptom onset): 12 vs. >12 weeks. b Interim efficacy analysis was conducted on 15 June 2016, once ~80 participants had the opportunity to be assessed at the Day 183 visit. c All infants completing the end of study visit for ENDEAR had the opportunity to enrol in SHINE. ClinicalTrials.gov, NCT
5 ENDEAR Hierarchical Endpoints Primary endpoints a - Proportion of motor milestone responders (IES population) Assessed from Day 183 onwards using modified section 2 of the HINE 1 Interim efficacy analysis conducted once ~80 participants had the opportunity to be assessed at the Day 183 visit» Only endpoint with formal statistical testing at interim - Event-free survival, i.e., time to death or permanent ventilation (ITT population at end of study) Permanent ventilation: tracheostomy or 16 hours ventilatory support per day for >21 days Events adjudicated by a blinded, central, independent EAC Secondary endpoints a - CHOP INTEND responders 4-point improvement from Baseline in total score from Day 183 onwards - Survival rate - Participants (%) not requiring permanent ventilation - Proportion of CMAP responders (peroneal nerve) Maintenance or increase by 1 mv vs. Baseline from Day 183 onwards - Time to death or permanent ventilation in the subgroups of participants below the study median disease duration - Time to death or permanent ventilation in the subgroups of participants above the study median disease duration CHOP INTEND = Children s Hospital of Philadelphia Infant Test of Neuromuscular Disorders; CMAP = compound muscle action potential; EAC = endpoint adjudication committee; HINE = Hammersmith Infant Neurological Exam; IES = interim efficacy set. a Primary analyses must reach statistical significance before inferential conclusions can be drawn from the remaining secondary and tertiary endpoints. 1. Haataja L, et al. J Pediatr. 1999;135(2 pt 1):
6 Improvement ENDEAR Primary Endpoint: Definition of HINE Motor Milestone Responders Modified section 2 of the HINE 1 Motor function Voluntary grasp Ability to kick (supine) Head control No grasp No kicking Unable to maintain upright Milestone progression score Uses whole hand Kick horizontal, legs do not lift Wobbles Index finger and thumb but immature grasp Pincer grasp Upward (vertical) Touches leg Touches toes All the time upright Improvement: 2-point improvement in ability to kick (or maximal score), or 1-point improvement in any other milestone, excluding voluntary grasp Rolling No rolling Rolling to side Prone to supine Supine to prone Sitting Cannot sit Sit with support at hips Crawling Does not lift head On elbow Standing Does not support weight Supports weight Walking No walking Bouncing Props Stable sit Pivots (rotates) On outstretched hand Stands with support Cruising (walks holding on) Crawling flat on abdomen Stands unaided Walking independently On hands and knees Worsening: 2-point worsening in ability to kick (or zero score), or 1-point worsening in any other milestone, excluding voluntary grasp Improvement Motor milestone responder definition a : more HINE categories with improvement than worsening - Participants who die or withdraw are counted as non-responders a Study participants on permanent ventilation will be assessed. 1. Haataja L, et al. J Pediatr. 1999;135(2 pt 1):
7 Baseline Disease Characteristics: ITT Population Characteristic Sham procedure control n=41 Nusinersen n=80 Female, n (%) 24 (59) 43 (54) Median (range) age at first dose, d 205 (30, 262) 165 (52, 242) Median (range) age at symptom onset, wk 8.0 (1, 20) 6.5 (2, 18) Median (range) age at SMA diagnosis, wk 20.0 (2, 30) 11.0 (0, 29) Median (range) disease duration, wk 12.7 (0, 23) 13.1 (0, 26) SMA symptoms, n (%) Hypotonia 41 (100) 80 (100) Developmental motor delay 39 (95) 71 (89) Paradoxical breathing 27 (66) 71 (89) Pneumonia or respiratory symptoms 9 (22) 28 (35) Limb weakness 41 (100) 79 (99) Swallowing or feeding difficulties 12 (29) 41 (51) Other 14 (34) 20 (25) Participants requiring ventilation support, n (%) 6 (15) 21 (26) 7
8 Motor milestone Motor Milestone Responders at End of Study Highly clinically and statistically significant percentage of motor milestone responders responders (%) a % Sham procedure control P< % Nusinersen Motor milestone responders, % Sham procedure control Nusinersen P value 6-month visit (n=110) 5 41 < month visit (n=77) 0 45 < month visit (n=58) 0 54 < a Interim endpoint re-evaluated with final study data with no alpha spending. Infants with opportunity for at least a 6-month (Day 183) assessment were included in the analysis; last available assessment of 6-month (Day 183), 10-month (Day 302), or 13-month (Day 394) was used. n=110. 8
9 Motor milestone Motor Milestone Responders: Interim vs. End of Study Nusinersen-treated infants demonstrated continued improvement over the previous interim analysis 60 Interim analysis b End of study c % responders (%) a % 41% 0% 0% Sham control Nusinersen Sham control Nusinersen a Interim endpoint re-evaluated with final study data with no alpha spending. b The interim efficacy analysis was conducted on June 15, 2016, once ~80 participants had the opportunity to be assessed at the Day 183 visit; n=78. c The end of study analysis was conducted on November 21, Infants with opportunity for at least a Day 183 assessment were included; n=110. 9
10 Total motor milestone change from baseline Improvement in Total Motor Milestone Score (HINE Section 2) at End of Study Infants treated with nusinersen had greater improvement in total motor milestone score a vs. sham procedure control 20 N=78 Treatment Nusinersen 15 Sham procedure control a Total motor milestone change from baseline to later of Day 183, 302, 394. Shortest bars indicate zero value. Of the 110 infants in the efficacy set, 29 died (nusinersen, n=13; sham procedure control, n=16) and 3 withdrew for a reason other than death (nusinersen, n=2; sham procedure control, n=1) and were not included in this analysis. 10
11 Efficacy set, % Quality of Motor Responses at End of Study Infants treated with nusinersen achieved motor milestones unexpected for infants with SMA Type I a Nusinersen-treated infants achieved: Supine to prone rolling: 10% Stable sit: 5% Pivots (rotate) sit: 3% Full head control b 10 Supine to prone rolling 0 8 Independent sitting c 0 Standing d 1 Sham procedure control (n=37) Nusinersen (n=73) a HINE motor milestone achievement in infants at the later of Days 183, 302 and 394. b Full head control was defined as all the time upright (HINE score = 2). c Independent sitting includes HINE score categories: stable sit and pivots (rotates). d Standing includes HINE score categories: stands with support and stands unaided. 11
12 Change in HINE Motor Milestone Scores Across Studies NURTURE (N=18) CS3A (N=20) ENDEAR (CS3B)-nusinersen (N=73) ENDEAR (CS3B)-control (N=37) Mean (±SE) total milestone score a NURTURE (presymptomatic infantile-onset SMA; 2 or 3 SMN2 copies) b Scheduled visit day CS3A (infantile-onset SMA) c Nusinersen vs. Sham procedure control in ENDEAR final analysis (infantile-onset SMA; 2 SMN2 copies) d NURTURE CS3A CS3B-nusinersen CS3B-control Populations: NURTURE (232SM201) = interim efficacy set, CS3A = all dosed infants; ENDEAR (CS3B) = interim efficacy set. For each study, visits with n<5 are not plotted. a Maximum total milestone score = 26. b Median (range) age at first dose: 19.0 (3 42) days. c Median (range) age at enrolment: = 155 (36 210) days. d Median (range) age at first dose: (30 262) days. 12
13 Event-Free Survival at End of Study Significantly prolonged event-free survival a in nusinersen-treated infants (HR, 0.53; P= b ) Outcome Sham procedure control Nusinersen Death or permanent ventilation, n (%) 28 (68%) 31 (39%) Alive and no permanent ventilation, n (%) 13 (32%) 49 (61%) Probability of Ventilation Free Survival c Median time to death or permanent ventilation: Nusinersen: not reached Sham procedure control: 22.6 weeks Nusinersen Sham procedure control Nusinersen: 55% Sham procedure control: 26% Time, weeks Sham procedure control Nusinersen All infants randomized who received at least one dose of nusinersen or sham procedure were included in the analysis. a Event-free survival = time to death or permanent ventilation (permanent ventilation was defined as tracheostomy or 16 hours ventilatory support per day for >21 days in the absence of acute reversible event in the determination of an independent endpoint adjudication committee). b Log-rank statistical test stratified by disease duration. c Estimated from the Kaplan-Meier method. HR = hazard ratio. 13
14 Overall Survival at End of Study Significantly prolonged overall survival in nusinersen-treated infants a (HR, 0.372; P= b ) Outcome Sham procedure control Nusinersen Death, n (%) 16 (39%) 13 (16%) Alive, n (%) 25 (61%) 67 (84%) 1.0 Probability of Survival c Nusinersen: 83% Sham procedure control: 58% 0.0 Sham procedure control Nusinersen Time, weeks Sham procedure control Nusinersen All infants randomized who received at least one dose of nusinersen or sham procedure were included in the analysis a Versus sham control-treated patients. HR = hazard ratio. b Log-rank statistical test stratified by disease duration. c Estimated from the Kaplan-Meier method. 14
15 Patients, % CHOP INTEND Motor Function Scores at End of Study More improvement and less worsening in nusinersen-treated patients a A significantly greater proportion of nusinersen-treated patients a were CHOP INTEND responders ( 4-point improvement; b 71% vs. 3%; P<0.0001) Worsening in CHOP- INTEND total scores No change in CHOP-INTEND total scores Improvement in CHOP- INTEND total scores points 1 points No Change 1 point 4 points Sham procedure control (n=37) Nusinersen (n=73) a Versus sham control-treated patients. Infants with opportunity for at least a 6-month (Day 183) assessment were included in the analysis; last available assessment of 6-month (Day 183), 10-month (Day 302), or 13-month (Day 394) was used. b The proportion of infants with 4 point increase from Baseline in CHOP INTEND score at the later of the Day 183, 302, or 394 study visit assessment. 15
16 CHOP INTEND change from baseline Date of presentation: 28 Apr 2017 CHOP INTEND Motor Function Scores at End of Study More improvement and less worsening in motor function assessment (CHOP INTEND) in nusinersen-treated patients a 40 N=78 30 Treatment Nusinersen Sham procedure control Infants with opportunity for at least a 6-month (Day 183) assessment were included in the analysis; last available assessment of 6-month (Day 183), 10-month (Day 302), or 13- month (Day 394) was used. Shortest bars indicate zero value. Of the 110 infants in the efficacy set, 29 died (nusinersen, n=13; sham procedure control, n=16) and 3 withdrew for a reason other than death (nusinersen, n=2; sham procedure control, n=1) and were not included in this analysis. a Versus sham-control treated infants. 16
17 Date of presentation: 28 Apr 2017 Permanent Ventilation Requirement at End of Study The risk of permanent ventilation was 34% lower in nusinersen-treated infants vs. sham procedure control Estimated % of patients who required permanent ventilation b Sham procedure control Nusinersen Day Day Day Day Day HR for nusinersen vs. sham procedure control 0.66 c All infants randomized who received at least one dose of nusinersen or sham procedure were included in the analysis a Versus sham control-treated patients. b Based on the Kaplan- Meier product limit method. c P= based on log-rank test stratified by disease duration. HR=hazard ratio. 17
18 Patients, % Peroneal CMAP Amplitude at End of Study More improvement was observed in nusinersen-treated patients a A significantly greater proportion of nusinersen-treated patients a were peroneal CMAP responders b (36% vs. 5%; nominal P=0.0004) Worsening in peroneal CMAP amplitude No change in peroneal CMAP amplitude Improvement in peroneal CMAP amplitude mv 0.5 mv No Change 0.5 mv 1 mv Sham procedure control (n=37) Nusinersen (n=73) a Versus sham control-treated patients. Infants with opportunity for at least a 6-month (Day 183) assessment were included in the analysis; last available assessment of 6-month (Day 183), 10-month (Day 302), or 13-month (Day 394) was used. b CMAP responder was defined as an infant with peroneal CMAP amplitude increasing to or maintained at 1mV compared to Baseline at the later of the Day 183, 302, or 394 study assessments. 18
19 Peroneal amplitude change from baseline Date of presentation: 28 Apr 2017 Peroneal CMAP Amplitude at End of Study More improvement and less worsening in nusinersen-treated patients a Similar improvements in ulnar CMAP amplitude were observed 3.0 N= Treatment Nusinersen Sham procedure control Infants with opportunity for at least a 6-month (Day 183) assessment were included in the analysis; last available assessment of 6-month (Day 183), 10-month (Day 302), or 13- month (Day 394) was used. Shortest bars indicate zero value. Of the 110 infants in the efficacy set, 29 died (nusinersen, n=13; sham procedure control, n=16), 3 withdrew for a reason other than death (nusinersen, n=2; sham procedure control, n=1), and 8 had missing data and were not included in this analysis. a Versus sham-control treated infants. 19
20 AE Summary: End of Study Analysis No AEs were considered related to treatment by the investigator All AEs that led to discontinuation were AEs with fatal outcomes AE, n (%) Sham procedure control n=41 Nusinersen n=80 Any AE 40 (98) 77 (96) AEs leading to discontinuation 16 (39) 13 (16) Treatment-related AE a 0 0 Possibly treatment-related AE a 6 (15) 9 (11) Severe AE 33 (80) 45 (56) Serious AE 39 (95) 61 (76) Serious AE with fatal outcome 16 (39) 13 (16) Respiratory, thoracic and mediastinal disorders 12 (29) 7 (9) Cardiac disorders 3 (7) 2 (3) General disorders 1 (2) 2 (3) Nervous system disorders 0 2 (3) AE = adverse event. a Investigators assessed whether the AE was related to study drug. A serious AE was any untoward medical occurrence that resulted in death/risk of death, hospitalisation/prolonged hospitalisation, persistent or significant disability/incapacity or that resulted in a congenital anomaly/birth defect. Severe AEs were defined as symptoms causing severe discomfort, incapacitation or significant impact on daily life; participants reporting >1 AE were counted once for total incidence, using the highest severity. 20
21 AE Summary: End of Study Analysis (cont) AE, n (%) Sham procedure control n=41 Nusinersen n=80 Common AEs ( 20% in either treatment group) Pyrexia 24 (59) 45 (56) Constipation 9 (22) 28 (35) Upper respiratory tract infection 9 (22) 24 (30) Pneumonia 7 (17) 23 (29) Respiratory distress 12 (29) 21 (26) Respiratory failure 16 (39) 20 (25) Atelectasis 12 (29) 18 (23) Vomiting 8 (20) 14 (18) Acute respiratory failure 10 (24) 11 (14) Gastroesophageal reflux disease 8 (20) 10 (13) Oxygen saturation decreased 10 (24) 10 (13) Cough 8 (20) 9 (11) Dysphagia 9 (22) 9 (11) Most frequent serious AEs ( 10% in either treatment group) Respiratory distress 8 (20) 21 (26) Respiratory failure 16 (39) 20 (25) Pneumonia 5 (12) 19 (24) Atelectasis 4 (10) 14 (18) Acute respiratory failure 9 (22) 11 (14) Pneumonia aspiration 5 (12) 8 (10) Cardiorespiratory arrest 5 (12) 5 (6) Respiratory arrest 4 (10) 5 (6) Viral upper respiratory tract infection 6 (15) 3 (4) Bronchial secretion retention 5 (12) 1 (1) 21
22 Conclusions Nusinersen-treated infants demonstrated a clinically and statistically significantly greater percentage of motor milestone responders vs. sham procedure control - Greater improvements in CHOP INTEND scores and CMAP amplitude were displayed by nusinersen-treated infants vs. sham procedure control Nusinersen-treated infants demonstrated statistically significant increases in event-free survival and overall survival vs. sham procedure control Nusinersen was well tolerated and no safety concerns were identified - Commonly reported AEs were consistent with those expected in the general population of infants with SMA Participants from ENDEAR have been transitioned to the SHINE open-label extension 1 - SHINE is enrolling all participants with SMA who were previously entered into and completed nusinersen investigational studies - Safety, efficacy and tolerability will be assessed 1. ClinicalTrials.gov, NCT
23 Acknowledgements The authors thank the patients who are participating in this study and their parents/guardians and family members, without whom this effort cannot succeed The authors thank the ENDEAR study investigators The authors also thank all the contributors to the ENDEAR study, including the clinical monitors, study coordinators, physical therapists, pharmacists and laboratory technicians Patient advocacy groups assisted in promoting awareness of this study 23
24 Backup
25 Participant Eligibility Criteria Key inclusion criteria Key exclusion criteria Onset of clinical signs and symptoms consistent with SMA at 6 months of age Genetic diagnosis of 5q SMA homozygous gene deletion or mutation or compound heterozygous mutation 7 months of age at screening a 2 SMN2 copies Hypoxaemia (oxygen saturation of <96% awake or asleep without ventilation support) Signs or symptoms of SMA present at birth or within 1 week after birth Untreated or treated active infection Previous use of an investigational drug for the treatment of SMA a Additionally, a gestational age of weeks was required. 25
26 Baseline Disease Characteristics: ITT Population Characteristic Sham procedure control n=41 Nusinersen n=80 Median (range) gestational age, wk 40 (37, 42) 39 (36, 41) Geographic region, n (%) North America 22 (54) 38 (48) Europe 17 (41) 30 (38) Asia-Pacific 2 (5) 12 (15) Ethnicity, n (%) Hispanic or Latin American 4 (10) 12 (15) Not Hispanic or Latin American 37 (90) 68 (85) Median (range) age at screening, d 190 (20, 211) 152 (32, 210) Age at symptom onset, wk, n (%) (78) 72 (90) >12 9 (22) 8 (10) Disease duration, wk, n (%) (44) 34 (43) >12 23 (56) 46 (58) Mean (SD) time on ventilation support at baseline, a h 6.8 (4.2) 8.4 (4.3) a Based on those infants requiring ventilator support at baseline. 26
Nusinersen Demonstrates Efficacy in Infants With and Without Permanent Ventilation: Final Results From the ENDEAR Study
 2017 Annual Spinal Muscular Atrophy Conference June 29 July 2, 2017 Orlando, FL Nusinersen Demonstrates Efficacy in Infants With and Without Permanent Ventilation: Final Results From the ENDEAR Study Richard
2017 Annual Spinal Muscular Atrophy Conference June 29 July 2, 2017 Orlando, FL Nusinersen Demonstrates Efficacy in Infants With and Without Permanent Ventilation: Final Results From the ENDEAR Study Richard
Nusinersen in Pre-symptomatic Infants With Spinal Muscular Atrophy (SMA): Interim Efficacy and Safety Results From the Phase 2 NURTURE Study
 21 st International Congress of the World Muscle Society -8 October, 2016 Granada, Spain Nusinersen in Pre-symptomatic Infants With Spinal Muscular Atrophy (SMA): Interim Efficacy and Safety Results From
21 st International Congress of the World Muscle Society -8 October, 2016 Granada, Spain Nusinersen in Pre-symptomatic Infants With Spinal Muscular Atrophy (SMA): Interim Efficacy and Safety Results From
Interim Efficacy and Safety Results from the Phase 2 NURTURE Study Evaluating Nusinersen in Presymptomatic Infants With Spinal Muscular Atrophy
 American Academy of Neurology 2017 69th Annual Meeting April 22 28, 2017 Boston, MA Interim Efficacy and Safety Results from the Phase 2 NURTURE Study Evaluating Nusinersen in Presymptomatic Infants With
American Academy of Neurology 2017 69th Annual Meeting April 22 28, 2017 Boston, MA Interim Efficacy and Safety Results from the Phase 2 NURTURE Study Evaluating Nusinersen in Presymptomatic Infants With
Thomas O. Crawford, MD July 1, Annual Spinal Muscular Atrophy Conference June 29 July 2, 2017 Orlando, FL
 2017 Annual Spinal Muscular Atrophy Conference June 29 July 2, 2017 Orlando, FL Efficacy and Safety of Nusinersen in Genetically Diagnosed Infants With Presymptomatic Spinal Muscular Atrophy (SMA): Results
2017 Annual Spinal Muscular Atrophy Conference June 29 July 2, 2017 Orlando, FL Efficacy and Safety of Nusinersen in Genetically Diagnosed Infants With Presymptomatic Spinal Muscular Atrophy (SMA): Results
Spinal Muscular Atrophy in 2017
 Spinal Muscular Atrophy in 2017 Leigh Maria Ramos-Platt, MD Children s Hospital of Los Angeles University of Southern California Keck School of Medicine Disclosures MDA Care Center Grant In this presentation,
Spinal Muscular Atrophy in 2017 Leigh Maria Ramos-Platt, MD Children s Hospital of Los Angeles University of Southern California Keck School of Medicine Disclosures MDA Care Center Grant In this presentation,
Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy
 The new england journal of medicine Original Article versus Sham in Infantile-Onset Spinal Muscular Atrophy R.S. Finkel, E. Mercuri, B.T. Darras, A.M. Connolly, N.L. Kuntz, J. Kirschner, C.A. Chiriboga,
The new england journal of medicine Original Article versus Sham in Infantile-Onset Spinal Muscular Atrophy R.S. Finkel, E. Mercuri, B.T. Darras, A.M. Connolly, N.L. Kuntz, J. Kirschner, C.A. Chiriboga,
Letter of Medical Necessity The Use of SPINRAZA (nusinersen) for Spinal Muscular Atrophy
 TEMPLATE Letter of Medical Necessity The Use of SPINRAZA (nusinersen) for Spinal Muscular Atrophy Date: [Insert Name of Medical Director] RE: Patient Name [ ] [Insurance Company] Policy Number [ ] [Address]
TEMPLATE Letter of Medical Necessity The Use of SPINRAZA (nusinersen) for Spinal Muscular Atrophy Date: [Insert Name of Medical Director] RE: Patient Name [ ] [Insurance Company] Policy Number [ ] [Address]
Spinraza (nusinersen)
 Spinraza (nusinersen) Policy Number: 5.02.540 Last Review: 04/2018 Origination: 03/2017 Next Review: 04/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Spinraza
Spinraza (nusinersen) Policy Number: 5.02.540 Last Review: 04/2018 Origination: 03/2017 Next Review: 04/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Spinraza
Clinical Policy Bulletin: Nusinersen (Spinraza)
 Clinical Policy Bulletin: Nusinersen (Spinraza) Number: 0915 Policy *Pleasesee amendment forpennsylvaniamedicaidattheendofthiscpb. Note: REQUIRES PRECERTIFICATION.Footnotes for Precertification of nusinersen
Clinical Policy Bulletin: Nusinersen (Spinraza) Number: 0915 Policy *Pleasesee amendment forpennsylvaniamedicaidattheendofthiscpb. Note: REQUIRES PRECERTIFICATION.Footnotes for Precertification of nusinersen
SPINRAZA (NUSINERSEN)
 SPINRAZA (NUSINERSEN) UnitedHealthcare Commercial Medical Benefit Drug Policy Policy Number: 2018D0059D Effective Date: April 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS...
SPINRAZA (NUSINERSEN) UnitedHealthcare Commercial Medical Benefit Drug Policy Policy Number: 2018D0059D Effective Date: April 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS...
Cigna Drug and Biologic Coverage Policy
 Cigna Drug and Biologic Coverage Policy Subject Nusinersen Table of Contents Coverage Policy... 1 General Background... 2 Coding/Billing Information... 5 References... 5 Effective Date... 10/15/2017 Next
Cigna Drug and Biologic Coverage Policy Subject Nusinersen Table of Contents Coverage Policy... 1 General Background... 2 Coding/Billing Information... 5 References... 5 Effective Date... 10/15/2017 Next
Spinraza (Nusinersen) Drug Prior Authorization Protocol (Medical Benefit & Part B Benefit)
 Line of Business: All Lines of Business Effective Date: August 16, 2017 Spinraza (Nusinersen) Drug Prior Authorization Protocol (Medical Benefit & Part B Benefit) This policy has been developed through
Line of Business: All Lines of Business Effective Date: August 16, 2017 Spinraza (Nusinersen) Drug Prior Authorization Protocol (Medical Benefit & Part B Benefit) This policy has been developed through
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: nusinersen_spinraza 03/2017 10/2017 10/2018 10/2017 Description of Procedure or Service Spinal muscular atrophy
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: nusinersen_spinraza 03/2017 10/2017 10/2018 10/2017 Description of Procedure or Service Spinal muscular atrophy
CADTH Canadian Drug Expert Committee Recommendation
 CADTH COMMON DRUG REVIEW CADTH Canadian Drug Expert Committee Recommendation (FINAL) Nusinersen (Spinraza Biogen Canada Inc.) Indication: Treatment of 5q Spinal Muscular Atrophy RECOMMENDATION: The CADTH
CADTH COMMON DRUG REVIEW CADTH Canadian Drug Expert Committee Recommendation (FINAL) Nusinersen (Spinraza Biogen Canada Inc.) Indication: Treatment of 5q Spinal Muscular Atrophy RECOMMENDATION: The CADTH
Title: Developmental milestones in type I spinal muscular atrophy
 Accepted Manuscript Title: Developmental milestones in type I spinal muscular atrophy Author: Roberto De Sanctis, Giorgia Coratti, Amy Pasternak, Jacqueline Montes, Marika Pane, Elena S. Mazzone, Sally
Accepted Manuscript Title: Developmental milestones in type I spinal muscular atrophy Author: Roberto De Sanctis, Giorgia Coratti, Amy Pasternak, Jacqueline Montes, Marika Pane, Elena S. Mazzone, Sally
Clinical Policy: Nusinersen (Spinraza) Reference Number: CP.PHAR.327
 Clinical Policy: (Spinraza) Reference Number: CP.PHAR.327 Effective Date: 03/17 Last Review Date: 03/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: (Spinraza) Reference Number: CP.PHAR.327 Effective Date: 03/17 Last Review Date: 03/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Spinraza) Reference Number: CP.PHAR.327 Effective Date: 11.28.17 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder
Clinical Policy: (Spinraza) Reference Number: CP.PHAR.327 Effective Date: 11.28.17 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: nusinersen_spinraza 03/2017 10/2018 10/2019 10/2018 Description of Procedure or Service Spinal muscular atrophy
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: nusinersen_spinraza 03/2017 10/2018 10/2019 10/2018 Description of Procedure or Service Spinal muscular atrophy
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Nusinersen (Spinraza) Reference Number: CP.PHAR.327 Effective Date: 10.01.18 Last Review Date: 07.13.18 Line of Business: Oregon Health Plan Coding Implications Revision Log See Important
Clinical Policy: Nusinersen (Spinraza) Reference Number: CP.PHAR.327 Effective Date: 10.01.18 Last Review Date: 07.13.18 Line of Business: Oregon Health Plan Coding Implications Revision Log See Important
Final Phase 3 Study Data Show SPINRAZA (nusinersen) Significantly Improved Motor Function in Children with Later-Onset Spinal Muscular Atrophy
 MEDIA CONTACT: Biogen Ligia Del Bianco Ph: +1 781 464 3260 public.affairs@biogen.com INVESTOR CONTACT: Biogen Ben Strain Ph: +1 781 464 2442 IR@biogen.com Final Phase 3 Study Data Show SPINRAZA (nusinersen)
MEDIA CONTACT: Biogen Ligia Del Bianco Ph: +1 781 464 3260 public.affairs@biogen.com INVESTOR CONTACT: Biogen Ben Strain Ph: +1 781 464 2442 IR@biogen.com Final Phase 3 Study Data Show SPINRAZA (nusinersen)
Related Policies None
 Medical Policy MP 5.01.28 BCBSA Ref. Policy: 5.01.28 Last Review: 04/30/2018 Effective Date: 07/30/2018 Section: Prescription Drug Related Policies None DISCLAIMER Our medical policies are designed for
Medical Policy MP 5.01.28 BCBSA Ref. Policy: 5.01.28 Last Review: 04/30/2018 Effective Date: 07/30/2018 Section: Prescription Drug Related Policies None DISCLAIMER Our medical policies are designed for
New Drug Evaluation: Nusinersen Injection, Intrathecal
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
INDUSTRY PERSPECTIVE ON DRUG DEVELOPMENT FOR TYPE 1 SMA
 INDUSTRY PERSPECTIVE ON DRUG DEVELOPMENT FOR TYPE 1 SMA Kathie M. Bishop, PhD Disclosures: Currently, CSO of Tioga Pharmaceuticals, no activities in SMA; 2009-2015, full-time employee of Ionis Pharmaceuticals,
INDUSTRY PERSPECTIVE ON DRUG DEVELOPMENT FOR TYPE 1 SMA Kathie M. Bishop, PhD Disclosures: Currently, CSO of Tioga Pharmaceuticals, no activities in SMA; 2009-2015, full-time employee of Ionis Pharmaceuticals,
Clinical Review Report
 CADTH COMMON DRUG REVIEW Clinical Review Report Nusinersen (Spinraza) (Biogen Canada Inc.) Indication: Treatment of patients with 5q SMA Service Line: CADTH Common Drug Review Version: Version 1.0 Publication
CADTH COMMON DRUG REVIEW Clinical Review Report Nusinersen (Spinraza) (Biogen Canada Inc.) Indication: Treatment of patients with 5q SMA Service Line: CADTH Common Drug Review Version: Version 1.0 Publication
NEW HORIZONS IN TREATING SMA. Dr. Huda Mussaffi Schneider Children s Medical Center of Israel
 NEW HORIZONS IN TREATING SMA Dr. Huda Mussaffi Schneider Children s Medical Center of Israel WHAT IS SMA? Rare and debilitating autosomal recessive neuromuscular disease characterized by degeneration of
NEW HORIZONS IN TREATING SMA Dr. Huda Mussaffi Schneider Children s Medical Center of Israel WHAT IS SMA? Rare and debilitating autosomal recessive neuromuscular disease characterized by degeneration of
SPINRAZA (nusinersen) CLINICAL OVERVIEW
 SPINRAZA (nusinersen) CLINICAL OVERVIEW IN BOTH CONTROLLED AND OPEN-LABEL STUDIES, SOME PATIENTS WHO HAD OR WERE LIKELY TO DEVELOP SPINAL MUSCULAR ATROPHY (SMA) TYPE 1, 2, OR 3 TREATED WITH SPINRAZA 1
SPINRAZA (nusinersen) CLINICAL OVERVIEW IN BOTH CONTROLLED AND OPEN-LABEL STUDIES, SOME PATIENTS WHO HAD OR WERE LIKELY TO DEVELOP SPINAL MUSCULAR ATROPHY (SMA) TYPE 1, 2, OR 3 TREATED WITH SPINRAZA 1
Evolving therapeutic landscape for inherited neurologic disorders. Kathryn Swoboda, MD HMS Child Neurology Course September
 Evolving therapeutic landscape for inherited neurologic disorders Kathryn Swoboda, MD HMS Child Neurology Course September 5 2017 Disclosures I ve received funding as a consultant for Biogen for clinical
Evolving therapeutic landscape for inherited neurologic disorders Kathryn Swoboda, MD HMS Child Neurology Course September 5 2017 Disclosures I ve received funding as a consultant for Biogen for clinical
2018 F. Hoffmann-La Roche Ltd. All rights reserved.
 PRELIMINARY EVIDENCE FOR PHARMACODYNAMIC EFFECTS OF RG7916 IN JEWELFISH A STUDY IN PATIENTS WITH SPINAL MUSCULAR ATROPHY WHO PREVIOUSLY PARTICIPATED IN A STUDY WITH ANOTHER SMN2-SPLICING TARGETING THERAPY
PRELIMINARY EVIDENCE FOR PHARMACODYNAMIC EFFECTS OF RG7916 IN JEWELFISH A STUDY IN PATIENTS WITH SPINAL MUSCULAR ATROPHY WHO PREVIOUSLY PARTICIPATED IN A STUDY WITH ANOTHER SMN2-SPLICING TARGETING THERAPY
Nusinersen Use in Spinal Muscular Atrophy
 Nusinersen Use in Spinal Muscular Atrophy Report by: Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology Slide 1 Evidence in Focus Endorsement and
Nusinersen Use in Spinal Muscular Atrophy Report by: Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology Slide 1 Evidence in Focus Endorsement and
Roche leads the clinical development of risdiplam as part of a collaboration with the SMA Foundation and PTC Therapeutics.
 Media Release PRIME designation granted by European Medicines Agency for Roche s risdiplam for treatment of spinal muscular atrophy (SMA) Risdiplam has the potential to be the first oral medicine for the
Media Release PRIME designation granted by European Medicines Agency for Roche s risdiplam for treatment of spinal muscular atrophy (SMA) Risdiplam has the potential to be the first oral medicine for the
Panel II: SMA Drugs in Development
 Panel II: SMA Drugs in Development Jinsy Andrews MD, Director of Clinical Research and Development, Cytokinetics Thomas Blaettler MD, Global Clinical Development Team Leader, F. Hoffmann-La Roche Jerry
Panel II: SMA Drugs in Development Jinsy Andrews MD, Director of Clinical Research and Development, Cytokinetics Thomas Blaettler MD, Global Clinical Development Team Leader, F. Hoffmann-La Roche Jerry
Diagnosis, management and new treatments for Spinal Muscular Atrophy Special Focus: SMA Type 1
 Diagnosis, management and new treatments for Spinal Muscular Atrophy Special Focus: SMA Type 1 17 th April 2018 Adnan Manzur Consultant Paediatric Neurologist Dubowitz Neuromuscular Centre, GOSH & ICH,
Diagnosis, management and new treatments for Spinal Muscular Atrophy Special Focus: SMA Type 1 17 th April 2018 Adnan Manzur Consultant Paediatric Neurologist Dubowitz Neuromuscular Centre, GOSH & ICH,
COMPANY OVERVIEW. June 2016
 COMPANY OVERVIEW June 2016 Disclaimers Forward-Looking Statements This presentation contains forward-looking statements, including: statements about: the timing, progress and results of preclinical studies
COMPANY OVERVIEW June 2016 Disclaimers Forward-Looking Statements This presentation contains forward-looking statements, including: statements about: the timing, progress and results of preclinical studies
PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION
 PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION PR SPINRAZA TM (nusinersen injection) Solution for intrathecal injection 2.4 mg/ml nusinersen as nusinersen sodium Other drugs for disorders of
PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION PR SPINRAZA TM (nusinersen injection) Solution for intrathecal injection 2.4 mg/ml nusinersen as nusinersen sodium Other drugs for disorders of
Spinal muscular atrophy 5Q Treatment with nusinersen
 GUIDELINES IN FOCUS Spinal muscular atrophy 5Q Treatment with nusinersen Author: Brazilian Medical Association Participants: Antonio Silvinato; Wanderley M Bernardo Final version: May 5, 2018 1. Brazilian
GUIDELINES IN FOCUS Spinal muscular atrophy 5Q Treatment with nusinersen Author: Brazilian Medical Association Participants: Antonio Silvinato; Wanderley M Bernardo Final version: May 5, 2018 1. Brazilian
Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study
 Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase, open-label, dose-escalation study Richard S Finkel, Claudia A Chiriboga, Jiri Vajsar, John W Day, Jacqueline Montes, Darryl
Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase, open-label, dose-escalation study Richard S Finkel, Claudia A Chiriboga, Jiri Vajsar, John W Day, Jacqueline Montes, Darryl
Spinal Muscular Atrophy Newborn Screening
 10.2018 Spinal Muscular Atrophy Newborn Screening Melissa Gibbons, MS, CGC Erica Wright, MS, CGC Clinical Genetics and Metabolism Department of Pediatrics Children s Hospital Colorado/ University of Colorado
10.2018 Spinal Muscular Atrophy Newborn Screening Melissa Gibbons, MS, CGC Erica Wright, MS, CGC Clinical Genetics and Metabolism Department of Pediatrics Children s Hospital Colorado/ University of Colorado
Prior Authorization Update: Nusinersen
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
MEASURES OF MOBILITY AND PHYSICAL ABILITY EVALUATED IN CLINICAL TRIALS
 MEASURES OF MOBILITY AND PHYSICAL ABILITY EVALUATED IN CLINICAL TRIALS HAMMERSMITH INFANT NEUROLOGICAL EXAMINATION (HINE) SECTION 2 CHILDREN S HOSPITAL OF PHILADELPHIA INFANT TEST OF NEUROMUSCULAR DISORDERS
MEASURES OF MOBILITY AND PHYSICAL ABILITY EVALUATED IN CLINICAL TRIALS HAMMERSMITH INFANT NEUROLOGICAL EXAMINATION (HINE) SECTION 2 CHILDREN S HOSPITAL OF PHILADELPHIA INFANT TEST OF NEUROMUSCULAR DISORDERS
Family Health, Nottingham Children s Hospital Date of submission February 2018
 Nusinersen Title of Guideline Guideline for the prescribing and administration of Nusinersen to patients with Spinal Muscular Atrophy Type 1 Contact Name and Job Title (author) Dr Gabriel Chow- Consultant
Nusinersen Title of Guideline Guideline for the prescribing and administration of Nusinersen to patients with Spinal Muscular Atrophy Type 1 Contact Name and Job Title (author) Dr Gabriel Chow- Consultant
The first and only treatment for spinal muscular atrophy (SMA)
 Discover SPINRAZA The first and only treatment for spinal muscular atrophy (SMA) INDICATION SPINRAZA is a prescription medicine used to treat spinal muscular atrophy (SMA) in pediatric and adult patients.
Discover SPINRAZA The first and only treatment for spinal muscular atrophy (SMA) INDICATION SPINRAZA is a prescription medicine used to treat spinal muscular atrophy (SMA) in pediatric and adult patients.
AVXS-101 and Nusinersen for Spinal Muscular Atrophy: Effectiveness and Value
 AVXS-101 and Nusinersen for Spinal Muscular Atrophy: Effectiveness and Value Background Draft Background and Scope August 22, 2018 Spinal muscular atrophy (SMA) is a rare, genetic neuromuscular disease
AVXS-101 and Nusinersen for Spinal Muscular Atrophy: Effectiveness and Value Background Draft Background and Scope August 22, 2018 Spinal muscular atrophy (SMA) is a rare, genetic neuromuscular disease
PRODUCT INFORMATION SPINRAZA nusinersen heptadecasodium solution for injection.
 PRDUCT IFRMATI SPIRAZA nusinersen heptadecasodium solution for injection. AME F THE MEDICIE usinersen is a fully modified 2 --2-methoxyethyl antisense oligonucleotide designed to bind to a specific sequence
PRDUCT IFRMATI SPIRAZA nusinersen heptadecasodium solution for injection. AME F THE MEDICIE usinersen is a fully modified 2 --2-methoxyethyl antisense oligonucleotide designed to bind to a specific sequence
SMA Treatments and Clinical Trials. Kenneth Hobby, President Mary Schroth, MD, Chief Medical Officer Jill Jarecki, PhD, Chief Scientific Officer
 SMA Treatments and Clinical Trials Kenneth Hobby, President Mary Schroth, MD, Chief Medical Officer Jill Jarecki, PhD, Chief Scientific Officer Agenda Introduction Kenneth Hobby, President Spinraza Update
SMA Treatments and Clinical Trials Kenneth Hobby, President Mary Schroth, MD, Chief Medical Officer Jill Jarecki, PhD, Chief Scientific Officer Agenda Introduction Kenneth Hobby, President Spinraza Update
SMA Therapeutics: A Comparative Overview of Drugs Approved and in Development. Sponsored By:
 SMA Therapeutics: A Comparative Overview of Drugs Approved and in Development Sponsored By: August 8, 2017 Targets for Therapeutic Intervention in SMA Decrease in SMN protein due to SMN1 gene deletion
SMA Therapeutics: A Comparative Overview of Drugs Approved and in Development Sponsored By: August 8, 2017 Targets for Therapeutic Intervention in SMA Decrease in SMN protein due to SMN1 gene deletion
Clinical Policy Title: Spinraza
 Clinical Policy Title: Spinraza Clinical Policy Number: 02.02.03 Effective Date: July 1, 2018 Initial Review Date: May 19, 2017 Most Recent Review Date: June 5, 2018 Next Review Date: June 2019 Related
Clinical Policy Title: Spinraza Clinical Policy Number: 02.02.03 Effective Date: July 1, 2018 Initial Review Date: May 19, 2017 Most Recent Review Date: June 5, 2018 Next Review Date: June 2019 Related
Nusinersen versus Sham Control in Later-Onset Spinal Muscular Atrophy
 Original Article Nusinersen versus Sham Control in Later-Onset Spinal Muscular Atrophy E. Mercuri, B.T. Darras, C.A. Chiriboga, J.W. Day, C. Campbell, A.M. Connolly, S.T. Iannaccone, J. Kirschner, N.L.
Original Article Nusinersen versus Sham Control in Later-Onset Spinal Muscular Atrophy E. Mercuri, B.T. Darras, C.A. Chiriboga, J.W. Day, C. Campbell, A.M. Connolly, S.T. Iannaccone, J. Kirschner, N.L.
Association of motor milestones and SMN2 copy and outcome in spinal muscular. atrophy types 0 4
 jnnp-2016-314292 1 - SUPPLEMENTARY FILE - Methods and additional data on clinical characteristics and motor development Association of motor milestones and SMN2 copy and outcome in spinal muscular atrophy
jnnp-2016-314292 1 - SUPPLEMENTARY FILE - Methods and additional data on clinical characteristics and motor development Association of motor milestones and SMN2 copy and outcome in spinal muscular atrophy
The 2017 Update of the Standard of Care Recommendations for Spinal Muscular Atrophy
 Richard Finkel, MD Nemours Children s Hospital Orlando, FL, USA Thomas Crawford, MD Johns Hopkins Hospital Baltimore, MD, USA The 2017 Update of the Standard of Care Recommendations for Spinal Muscular
Richard Finkel, MD Nemours Children s Hospital Orlando, FL, USA Thomas Crawford, MD Johns Hopkins Hospital Baltimore, MD, USA The 2017 Update of the Standard of Care Recommendations for Spinal Muscular
SMA IS A SEVERE NEUROLOGICAL DISORDER [1]
![SMA IS A SEVERE NEUROLOGICAL DISORDER [1] SMA IS A SEVERE NEUROLOGICAL DISORDER [1]](/thumbs/72/67086153.jpg) SMA OVERVIEW SMA IS A SEVERE NEUROLOGICAL DISORDER [1] Autosomal recessive genetic inheritance 1 in 50 people (approximately 6 million Americans) are carriers [2] 1 in 6,000 to 1 in 10,000 children born
SMA OVERVIEW SMA IS A SEVERE NEUROLOGICAL DISORDER [1] Autosomal recessive genetic inheritance 1 in 50 people (approximately 6 million Americans) are carriers [2] 1 in 6,000 to 1 in 10,000 children born
DOSAGE FORMS AND STRENGTHS Injection: 12 mg/5 ml (2.4 mg/ml) in a single-dose vial (3)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use SPINRAZA safely and effectively. See full prescribing information for SPINRAZA. SPINRAZA (nusinersen)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use SPINRAZA safely and effectively. See full prescribing information for SPINRAZA. SPINRAZA (nusinersen)
Evaluation of Nusinersen (SPINRAZA TM ) in Medicinrådet
 Evaluation of Nusinersen (SPINRAZA TM ) in Medicinrådet Contents 1. Executive summary... 3 2. Natural history of the disease... 4 3. Medicine Council Questions... 6 4. Clinical added value of nusinersen
Evaluation of Nusinersen (SPINRAZA TM ) in Medicinrådet Contents 1. Executive summary... 3 2. Natural history of the disease... 4 3. Medicine Council Questions... 6 4. Clinical added value of nusinersen
SMA Treatment Access and Clinical Trials Webinar. February 15, 2018
 SMA Treatment Access and Clinical Trials Webinar February 15, 2018 Treatment Access Update Current Dosing Metrics December 2017: ~1,900 patients been dosed in US Including those in extension trials and
SMA Treatment Access and Clinical Trials Webinar February 15, 2018 Treatment Access Update Current Dosing Metrics December 2017: ~1,900 patients been dosed in US Including those in extension trials and
Chemotherapy administration, into CNS (eg, intrathecal), requiring and including spinal puncture
 COMPANY: Biogen PRODUCT TRADE NAME: SPINRAZA GENERIC NAME: nusinersen INDICATION: SPINRAZA is a survival motor neuron-2 (SMN2)-directed antisense oligonucleotide indicated for the treatment of spinal muscular
COMPANY: Biogen PRODUCT TRADE NAME: SPINRAZA GENERIC NAME: nusinersen INDICATION: SPINRAZA is a survival motor neuron-2 (SMN2)-directed antisense oligonucleotide indicated for the treatment of spinal muscular
Spinal Muscular Atrophy: Case Study. Spinal muscular atrophy (SMA) is a fairly common genetic disorder, affecting
 Spinal Muscular Atrophy: Case Study Spinal muscular atrophy (SMA) is a fairly common genetic disorder, affecting approximately one in 6,000 babies. It is estimated that one in every 40 Americans carries
Spinal Muscular Atrophy: Case Study Spinal muscular atrophy (SMA) is a fairly common genetic disorder, affecting approximately one in 6,000 babies. It is estimated that one in every 40 Americans carries
Objectives. What is SMA? Pathophysiologic and genetic mechanisms How to identify a case of SMA
 Objectives What is SMA? Pathophysiologic and genetic mechanisms How to identify a case of SMA What can be done? Review of advances in standards of care and treatment Detailed review of treatment available
Objectives What is SMA? Pathophysiologic and genetic mechanisms How to identify a case of SMA What can be done? Review of advances in standards of care and treatment Detailed review of treatment available
ISIS-SMN Rx Background and Current Status. C. Frank Bennett Sr. VP, Research Isis Pharmaceuticals
 ISIS-SMN Rx Background and Current Status C. Frank Bennett Sr. VP, Research Isis Pharmaceuticals ISIS-SMN Rx : Modulating Splicing of SMN2 to Increase Normal SMN Protein 2 Uniformly 2 -O-methoxyethyl modified
ISIS-SMN Rx Background and Current Status C. Frank Bennett Sr. VP, Research Isis Pharmaceuticals ISIS-SMN Rx : Modulating Splicing of SMN2 to Increase Normal SMN Protein 2 Uniformly 2 -O-methoxyethyl modified
Pharmacoeconomic Review Report
 CADTH COMMON DRUG REVIEW Pharmacoeconomic Review Report NUSINERSEN (SPINRAZA) (Biogen Canada Inc.) Indication: Treatment of patients with 5q SMA Service Line: CADTH Common Drug Review Version: Final Publication
CADTH COMMON DRUG REVIEW Pharmacoeconomic Review Report NUSINERSEN (SPINRAZA) (Biogen Canada Inc.) Indication: Treatment of patients with 5q SMA Service Line: CADTH Common Drug Review Version: Final Publication
DOSAGE FORMS AND STRENGTHS Injection: 12 mg/5 ml (2.4 mg/ml) in a single-dose vial (3)
 HIGHLIGHTS F PRESCRIBIG IFRMATI These highlights do not include all the information needed to use SPIRAZA safely and effectively. See full prescribing information for SPIRAZA. SPIRAZA (nusinersen) injection,
HIGHLIGHTS F PRESCRIBIG IFRMATI These highlights do not include all the information needed to use SPIRAZA safely and effectively. See full prescribing information for SPIRAZA. SPIRAZA (nusinersen) injection,
Update on Pulmonary Management in Spinal Muscular Atrophy type 1
 Update on Pulmonary Management in Spinal Muscular Atrophy type 1 David Zielinski, MD FRCPC, FCCP Associate Professor McGill University Montreal Children s Hospital None Disclosures Objectives Review genetic
Update on Pulmonary Management in Spinal Muscular Atrophy type 1 David Zielinski, MD FRCPC, FCCP Associate Professor McGill University Montreal Children s Hospital None Disclosures Objectives Review genetic
You ve found a treatment center. What might be next?
 You ve found a treatment center. What might be next? The next step is to get acquainted with the process for receiving SPINRAZA by talking to the point of contact at the treatment center and the administering
You ve found a treatment center. What might be next? The next step is to get acquainted with the process for receiving SPINRAZA by talking to the point of contact at the treatment center and the administering
ABSTRACT ORIGINAL RESEARCH
 https://doi.org/10.1007/s12325-019-00923-8 ORIGINAL RESEARCH Survival, Motor Function, and Motor Milestones: Comparison of AVXS-101 Relative to Nusinersen for the Treatment of Infants with Spinal Muscular
https://doi.org/10.1007/s12325-019-00923-8 ORIGINAL RESEARCH Survival, Motor Function, and Motor Milestones: Comparison of AVXS-101 Relative to Nusinersen for the Treatment of Infants with Spinal Muscular
SPINRAZA READINESS KIT
 SPINRAZA READINESS KIT Please see full Prescribing Information for Important Safety Information. MANAGEMENT OF THE REIMBURSEMENT PROCESS FOR SPINRAZA 1 SELECT TREATMENT AFTER DISCUSSING BENEFITS AND RISKS
SPINRAZA READINESS KIT Please see full Prescribing Information for Important Safety Information. MANAGEMENT OF THE REIMBURSEMENT PROCESS FOR SPINRAZA 1 SELECT TREATMENT AFTER DISCUSSING BENEFITS AND RISKS
SMA TYPE I ASSESSMENT - EAP
 Name of Patient DOB GC n. NHS n. Clinical assessment Weight: kg ength: cm Pre-dose SMA TYPE I ASSESSMENT - EAP DOA Consent date Visit Age (days) Diagnosis Genetic confirmation NIV YES/NO if YES needs to
Name of Patient DOB GC n. NHS n. Clinical assessment Weight: kg ength: cm Pre-dose SMA TYPE I ASSESSMENT - EAP DOA Consent date Visit Age (days) Diagnosis Genetic confirmation NIV YES/NO if YES needs to
Normal development & reflex
 Normal development & reflex Definition of Development : acquisition & refinement of skills 1 대근육운동발달 2 소근육운동발달 3 대인관계및사회성발달 4 적응능력혹은비언어성발달 5 의사소통및언어발달 6 학습, 청각, 시각의발달 Department of Rehabilitation Medicine,
Normal development & reflex Definition of Development : acquisition & refinement of skills 1 대근육운동발달 2 소근육운동발달 3 대인관계및사회성발달 4 적응능력혹은비언어성발달 5 의사소통및언어발달 6 학습, 청각, 시각의발달 Department of Rehabilitation Medicine,
Assessing the Infant and Child with Spinal Muscular Atrophy. Why Assessment Matters. Course Objectives. Introduction to SMA 10/3/2017
 Assessing the Infant and Child with Spinal Muscular Atrophy Becca Oschwald, PT, DPT Oct. 14, 2017 Why Assessment Matters New drug therapies for SMA Spinraza (nusinersen) First FDA approved treatment for
Assessing the Infant and Child with Spinal Muscular Atrophy Becca Oschwald, PT, DPT Oct. 14, 2017 Why Assessment Matters New drug therapies for SMA Spinraza (nusinersen) First FDA approved treatment for
SUMMARY OF RELEVANT CODES FOR SPINRAZA
 SUMMARY OF RELEVANT CODES FOR SPINRAZA Please see full Prescribing Information for additional Important Safety Information. SUMMARY OF RELEVANT CODES FOR SPINRAZA ICD-10-CM CODE EXAMPLES ICD-10-CM Code
SUMMARY OF RELEVANT CODES FOR SPINRAZA Please see full Prescribing Information for additional Important Safety Information. SUMMARY OF RELEVANT CODES FOR SPINRAZA ICD-10-CM CODE EXAMPLES ICD-10-CM Code
Kathryn J. Swoboda, MD, FACMG NBSTRN Meeting
 Kathryn J. Swoboda, MD, FACMG NBSTRN Meeting SPOT SMA LPDR resource: overview of clinical research forms and overall database Feasibility and utility of a minimal common set of data elements vs. a more
Kathryn J. Swoboda, MD, FACMG NBSTRN Meeting SPOT SMA LPDR resource: overview of clinical research forms and overall database Feasibility and utility of a minimal common set of data elements vs. a more
Using Biomarkers to Increase the Efficiency of Early Stage Drug Development
 Using Biomarkers to Increase the Efficiency of Early Stage Drug Development Personal consulting compensation received from Cytokinetics, Biogen, MT Pharma, Lilly, Karyopharm, Denali Goals of Talk Describe
Using Biomarkers to Increase the Efficiency of Early Stage Drug Development Personal consulting compensation received from Cytokinetics, Biogen, MT Pharma, Lilly, Karyopharm, Denali Goals of Talk Describe
GENERAL INFORMATION. Adverse Event (AE) Definition (ICH GUIDELINES E6 FOR GCP 1.2):
 Make copies of the blank SAE report form as needed. Retain originals with confirmation of all information faxed to DMID Pharmacovigilance Group Clinical Research Operations and Management Support (CROMS
Make copies of the blank SAE report form as needed. Retain originals with confirmation of all information faxed to DMID Pharmacovigilance Group Clinical Research Operations and Management Support (CROMS
DEVELOPMENT OF THE MOTOR SYSTEM
 DEVELOPMENT OF THE MOTOR SYSTEM HDP1: Fall 2007 Joan Stiles Department of Cognitive Science University of California, San Diego Motor system development begins during the Prenatal period Thalamocortical
DEVELOPMENT OF THE MOTOR SYSTEM HDP1: Fall 2007 Joan Stiles Department of Cognitive Science University of California, San Diego Motor system development begins during the Prenatal period Thalamocortical
When a Threshold Crossing approach may and may not be appropriate: A Case Study in SMA
 When a Threshold Crossing approach may and may not be appropriate: A Case Study in SMA EFSPI Regulatory Statistics Workshop, 24-25 th September 2018 Carol Reid and Uli Burger picture placeholder Outline
When a Threshold Crossing approach may and may not be appropriate: A Case Study in SMA EFSPI Regulatory Statistics Workshop, 24-25 th September 2018 Carol Reid and Uli Burger picture placeholder Outline
1/28/2019. OSF HealthCare INI Care Center Team. Neuromuscular Disease: Muscular Dystrophy. OSF HealthCare INI Care Center Team: Who are we?
 Neuromuscular Disease: Muscular Dystrophy Muscular Dystrophy Association (MDA) and OSF HealthCare Illinois Neurological Institute (INI) Care Center Team The Neuromuscular clinic is a designated MDA Care
Neuromuscular Disease: Muscular Dystrophy Muscular Dystrophy Association (MDA) and OSF HealthCare Illinois Neurological Institute (INI) Care Center Team The Neuromuscular clinic is a designated MDA Care
Disclosures Arthur Burghes. SAB AveXis. Performed studies funded by AveXis. Consulted for Novartis
 Disclosures Arthur Burghes SAB AveXis Performed studies funded by AveXis Consulted for Novartis Type of Biomarker Prognostic Disease Progression Predictive Pharmacodynamic Surrogate Endpoint Definition
Disclosures Arthur Burghes SAB AveXis Performed studies funded by AveXis Consulted for Novartis Type of Biomarker Prognostic Disease Progression Predictive Pharmacodynamic Surrogate Endpoint Definition
Screening for spinal muscular atrophy External review against programme appraisal criteria for the UK National Screening Committee (UK NSC)
 Screening for spinal muscular atrophy External review against programme appraisal criteria for the UK National Screening Committee (UK NSC) Version: Third Draft Consultation Author: Costello Medical Date:
Screening for spinal muscular atrophy External review against programme appraisal criteria for the UK National Screening Committee (UK NSC) Version: Third Draft Consultation Author: Costello Medical Date:
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked
SUMMARY OF RELEVANT CODES FOR SPINRAZA
 SUMMARY OF RELEVANT CODES FOR SPINRAZA Please see accompanying full Prescribing Information for additional Important Safety Information. SUMMARY OF RELEVANT CODES FOR SPINRAZA ICD-10-CM CODE EXAMPLES ICD-10-CM
SUMMARY OF RELEVANT CODES FOR SPINRAZA Please see accompanying full Prescribing Information for additional Important Safety Information. SUMMARY OF RELEVANT CODES FOR SPINRAZA ICD-10-CM CODE EXAMPLES ICD-10-CM
Infant Reflexes and Stereotypies. Chapter 9
 Infant Reflexes and Stereotypies Chapter 9 Infant reflexes and stereotypies are very important in the process of development Importance of Infant Reflexes Reflexive movements occur during the last 4 months
Infant Reflexes and Stereotypies Chapter 9 Infant reflexes and stereotypies are very important in the process of development Importance of Infant Reflexes Reflexive movements occur during the last 4 months
Learn the steps to identify pediatric muscle weakness and signs of neuromuscular disease.
 Learn the steps to identify pediatric muscle weakness and signs of neuromuscular disease. Listen Observe Evaluate Test Refer Guide for primary care providers includes: Surveillance Aid: Assessing Weakness
Learn the steps to identify pediatric muscle weakness and signs of neuromuscular disease. Listen Observe Evaluate Test Refer Guide for primary care providers includes: Surveillance Aid: Assessing Weakness
ASPIRO Phase 1/2 Gene Therapy Trial in X-Linked Myotubular Myopathy (XLMTM): Preliminary Safety and Efficacy Findings
 ASPIRO Phase 1/2 Gene Therapy Trial in X-Linked Myotubular Myopathy (XLMTM): Preliminary Safety and Efficacy Findings Safe Harbor Except for statements of historical fact, any information contained in
ASPIRO Phase 1/2 Gene Therapy Trial in X-Linked Myotubular Myopathy (XLMTM): Preliminary Safety and Efficacy Findings Safe Harbor Except for statements of historical fact, any information contained in
Clinical Trial Results Database Page 1
 Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Corporation Generic Drug Name Therapeutic Area of Trial Major Depressive Disorder (MDD) Approved Indication Treatment of major depressive
Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Corporation Generic Drug Name Therapeutic Area of Trial Major Depressive Disorder (MDD) Approved Indication Treatment of major depressive
Course Handouts & Disclosure
 ALS: DISEASE TRAJECTORY AND HOSPICE ELIGIBILITY Terri L. Maxwell PhD, APRN VP, Strategic Initiatives Weatherbee Resources Inc Hospice Education Network Inc Course Handouts & Disclosure To download presentation
ALS: DISEASE TRAJECTORY AND HOSPICE ELIGIBILITY Terri L. Maxwell PhD, APRN VP, Strategic Initiatives Weatherbee Resources Inc Hospice Education Network Inc Course Handouts & Disclosure To download presentation
ClinicalTrials.gov "Basic Results" Data Element Definitions (DRAFT)
 ClinicalTrials.gov "Basic Results" Data Element Definitions (DRAFT) January 9, 2009 * Required by ClinicalTrials.gov [*] Conditionally required by ClinicalTrials.gov (FDAAA) May be required to comply with
ClinicalTrials.gov "Basic Results" Data Element Definitions (DRAFT) January 9, 2009 * Required by ClinicalTrials.gov [*] Conditionally required by ClinicalTrials.gov (FDAAA) May be required to comply with
Challenges in measuring QoL in rare conditions (1)
 Biogen-01490, October 2018 HOW SHOULD WE MEASURE QUALITY OF LIFE IMPACT IN RARE DISEASE? RECENT LEARNINGS IN SPINAL MUSCULAR ATROPHY Introduction of the symposium Martina Garau ISPOR Europe 2018. Sponsored
Biogen-01490, October 2018 HOW SHOULD WE MEASURE QUALITY OF LIFE IMPACT IN RARE DISEASE? RECENT LEARNINGS IN SPINAL MUSCULAR ATROPHY Introduction of the symposium Martina Garau ISPOR Europe 2018. Sponsored
Roche/Genentech Anti-Myostatin Adnectin RG6206 Development Program in Duchenne
 Roche/Genentech Anti-Myostatin Adnectin RG6206 Development Program in Duchenne Michelle Krishnan MD, PhD. Translational Medicine Leader, Roche information is presented only for purposes of providing a
Roche/Genentech Anti-Myostatin Adnectin RG6206 Development Program in Duchenne Michelle Krishnan MD, PhD. Translational Medicine Leader, Roche information is presented only for purposes of providing a
TEST INFORMATION Test: CarrierMap GEN (Genotyping) Panel: CarrierMap Expanded Diseases Tested: 311 Genes Tested: 299 Mutations Tested: 2647
 Ordering Practice Jane Smith John Smith Practice Code: 675 Miller MD 374 Broadway New York, NY 10000 Physician: Dr. Frank Miller Report Generated: 2016-02-03 DOB: 1973-02-19 Gender: Female Ethnicity: European
Ordering Practice Jane Smith John Smith Practice Code: 675 Miller MD 374 Broadway New York, NY 10000 Physician: Dr. Frank Miller Report Generated: 2016-02-03 DOB: 1973-02-19 Gender: Female Ethnicity: European
SYNOPSIS. ER OROS Paliperidone: Clinical Study Report R SCH-301
 SYNOPSIS Protocol No.: R076477-SCH-301 Title of Study: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study With an Open-Label Extension Evaluating Extended Release OROS Paliperidone in
SYNOPSIS Protocol No.: R076477-SCH-301 Title of Study: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study With an Open-Label Extension Evaluating Extended Release OROS Paliperidone in
Summary 1. Comparative effectiveness of ataluren Study 007
 Cost-effectiveness of Ataluren (Transarna TM ) for the treatment of Duchenne muscular dystrophy resulting from a nonsense mutation in the dystrophy gene in ambulatory patients aged 5 years and older The
Cost-effectiveness of Ataluren (Transarna TM ) for the treatment of Duchenne muscular dystrophy resulting from a nonsense mutation in the dystrophy gene in ambulatory patients aged 5 years and older The
ClinicalTrials.gov Protocol and Results Registration System (PRS) Receipt Release Date: 09/30/2015. ClinicalTrials.gov ID: NCT
 ClinicalTrials.gov Protocol and Results Registration System (PRS) Receipt Release Date: 09/30/2015 ClinicalTrials.gov ID: NCT01378962 Study Identification Unique Protocol ID: ML25514 Brief Title: A Study
ClinicalTrials.gov Protocol and Results Registration System (PRS) Receipt Release Date: 09/30/2015 ClinicalTrials.gov ID: NCT01378962 Study Identification Unique Protocol ID: ML25514 Brief Title: A Study
Genotype phenotype studies in infantile spinal muscular atrophy (SMA) type I in Germany: implications for clinical trials and genetic counselling
 Clin Genet 2009: 76: 168 178 Printed in Singapore. All rights reserved Short Report 2009 John Wiley & Sons A/S CLINICAL GENETICS doi: 10.1111/j.1399-0004.2009.01200.x Genotype phenotype studies in infantile
Clin Genet 2009: 76: 168 178 Printed in Singapore. All rights reserved Short Report 2009 John Wiley & Sons A/S CLINICAL GENETICS doi: 10.1111/j.1399-0004.2009.01200.x Genotype phenotype studies in infantile
Patient L.L. KRISTEN ARREDONDO, MD CHILD NEUROLOGY PGY5, UT SOUTHWESTERN
 Patient L.L. KRISTEN ARREDONDO, MD CHILD NEUROLOGY PGY5, UT SOUTHWESTERN Birth History LL was born to a healthy first time mother with an uncomplicated pregnancy Delivered at 38 weeks via C-section due
Patient L.L. KRISTEN ARREDONDO, MD CHILD NEUROLOGY PGY5, UT SOUTHWESTERN Birth History LL was born to a healthy first time mother with an uncomplicated pregnancy Delivered at 38 weeks via C-section due
Case History Report Form
 Child details ( * required) Name (first name only) or *Code John *Chronological age (CA) Actual age since birth *Diagnosis and/or presenting problems 18-27 mos Joubert Syndrome - a rare brain malformation
Child details ( * required) Name (first name only) or *Code John *Chronological age (CA) Actual age since birth *Diagnosis and/or presenting problems 18-27 mos Joubert Syndrome - a rare brain malformation
Industry Relationships and Institutional Affiliations
 Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated daily statin: results from the ODYSSEY COMBO II study Christopher
Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated daily statin: results from the ODYSSEY COMBO II study Christopher
Below are photos from a 6 month old child with Werdnig-Hoffman s disease.
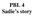 PBL 4 Sadie s story Sadie s Story Objectives of PBL: 1) Review and be able to answer questions about normal skeletal muscle organization and structure. 2) Review and be able to answer questions about spinal
PBL 4 Sadie s story Sadie s Story Objectives of PBL: 1) Review and be able to answer questions about normal skeletal muscle organization and structure. 2) Review and be able to answer questions about spinal
Raxone (idebenone) and pulmonary care in Duchenne Muscular Dystrophy (DMD)
 Raxone (idebenone) and pulmonary care in Duchenne Muscular Dystrophy (DMD) Thomas Meier, PhD February 2018 Agenda Medical need for effective treatment of respiratory illness in DMD Understanding respiratory
Raxone (idebenone) and pulmonary care in Duchenne Muscular Dystrophy (DMD) Thomas Meier, PhD February 2018 Agenda Medical need for effective treatment of respiratory illness in DMD Understanding respiratory
SALSA MLPA KIT P060-B2 SMA
 SALSA MLPA KIT P6-B2 SMA Lot 111, 511: As compared to the previous version B1 (lot 11), the 88 and 96 nt DNA Denaturation control fragments have been replaced (QDX2). Please note that, in contrast to the
SALSA MLPA KIT P6-B2 SMA Lot 111, 511: As compared to the previous version B1 (lot 11), the 88 and 96 nt DNA Denaturation control fragments have been replaced (QDX2). Please note that, in contrast to the
Bringing Differentiated Therapies to Duchenne Patients Stuart Peltz, PhD
 Bringing Differentiated Therapies to Duchenne Patients Stuart Peltz, PhD Jul-18 1 Main Objectives Translarna TM (ataluren) Update FDA pathway forward for NDA Ongoing clinical trials EMFLAZA (deflazacort)
Bringing Differentiated Therapies to Duchenne Patients Stuart Peltz, PhD Jul-18 1 Main Objectives Translarna TM (ataluren) Update FDA pathway forward for NDA Ongoing clinical trials EMFLAZA (deflazacort)
Chapter 16 Moving and Positioning Patients
 Chapter 16 Moving and Positioning Patients Terminology Related to Movement Contractures Shortening and tightening of muscles due to disuse Dorsiflexion Bending of the foot in an upward direction Plantar
Chapter 16 Moving and Positioning Patients Terminology Related to Movement Contractures Shortening and tightening of muscles due to disuse Dorsiflexion Bending of the foot in an upward direction Plantar
